|
Toxicological evaluation and limit values for Methyl-tertiary-butyl ether (MTBE), Formaldehyde, Glutaraldehyde, Furfural
1. General description
1.1 Identity
Molecular formula: C5H12O
Structural formula:
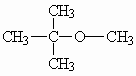
| Molecular weight: | 88.15 |
| CAS-no. | 1634-04-4 |
| Synonyms: | tert-Butoxymethane tert-Butyl methyl ether 1,1-Dimethylethyl methyl ether 2-Methoxy-2-methyl propane Methyl-1,1-dimethyl ethyl ether 2-Methyl-2-methoxypropane (2-Methyl-2-propyl)methyl ether MTBE |
1.2 Physical / chemical properties
| Description: | Colourless liquid with characteristic terpene-like odour. |
| Purity: | - |
| Melting point: | -109 ° C |
| Boiling point: | 55.2° C |
| Density: | 0.75 g/ml (at 20° C) |
| Vapour pressure: | 245 mmHg (326 hPa) at 25° C |
| Concentration of saturated vapours: | 322.000 ppm (calculated) (20° C, 760 mmHg) |
| Vapour density: | 3.1 (air = 1) |
| Conversion factor: | 1 ppm = 3.60 mg/m3 20° C 1 mg/m3 = 0.278 ppm 1 atm |
| Flash point: | -28 ° C |
| Flammable limits: | - |
| Autoignition temp.: | - |
| Solubility: | Water: 48 g/100 ml (at 20° C) Very soluble in other ethers, in alcohols and in gasoline. |
| logPoctanol/water: | 0.8 - 1.3 |
| Henry’s constant: | 5.87 x 10-4 (atm x m3)/mole |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | 0.19 mg/m3 |
| Odour threshold, water: | 0.18 mg/l |
| References: | IPCS (1996), Wibowo (1994). |
1.3 Production and use
In 1994, 6.2 million tonnes was produced in U.S.A. and 3.3 million tonnes in Europe. MTBE is used as an additive in gasoline for increasing the octane content and for enhancing the complete burning of the fuel in order to reduce carbon monoxide and ozone-forming emissions. Typical MTBE concentrations in gasoline are in the range of 7-11 vol%. (IPCS 1996, Wibowo 1994).
In medicine MTBE can be used as an agent to dissolve gallstones (IPCS 1996).
1.4 Environmental occurrence
Air
Due to its high vapour pressure, MTBE will be distributed mainly to the air when released into the environment.
Exposure during refuelling at gas stations is in the range of 0.3-0.5 ppm (1.1-1.8 mg/m3) (Drew 1995). In Finland mean short term exposure of 6.0-7.5 mg/m3 was measured for consumers during tank filling of gasoline containing about 11% MTBE (ECETOC 1997). An average annual exposure level of 0.03-0.05 mg/m3 for maximally exposed persons in the general public has been calculated for areas where gasoline with 15% MTBE content is used during winter (Lucier et al 1995, IPCS 1996). In Fairbanks, Alaska where high levels of MTBE (15 vol%) was used during winter time, the indoor and outdoor level averaged about 0.020 mg/m3 (IPCS 1996).
A geometric mean value of 0.021 mg/m3 was found for air inside cars during an one-hour drive (with a 13-15% MTBE content in gasoline) (IPCS 1996).
For the occupational environment an 8h-average exposure level of up to 31 mg/m3 has been estimated for service stations attendants (Wibowo 1994).
Water
In Denmark MTBE has been found in sub-surface groundwater underneath gasoline stations in a concentration range of 1 µg/l - 500 mg/l, however most of the measurements were in the range of 1-10 mg/l. MTBE has not been found in the deeper primary groundwater (Jensen 1997).
In a study conducted by the United States Geological Survey, detectable concentrations of MTBE was found in 27 % of shallow wells. Of 60 volatile chemicals MTBE was the second most frequently detected chemical. In urban areas the concentration ranged from 0.2 to 23 000 mg/l with a median concentration of 0.6 mg/l (IPCS 1996).
Soil
No data available.
1.5 Environmental fate
Air
Atmospheric MTBE undergoes photooxidation mainly through reaction with photochemically produced hydroxyl radicals. The major initial products are t-butyl formate and 2-methoxy-2-methyl propanal. Further formaldehyde, acetone and CO2 is generated (IPCS 1996).
Soil and water
In surface soil MTBE is due to the high vapour pressure expected to evaporate. As MTBE is of moderate water solubility (the most water soluble constituent in gasoline) wash out with the rain water may be an important elimination route for the deeper soil layer where the evaporation process is suppressed (Larsen 1993).
In conventional tests, MTBE has shown poor biodegradation in soil and water under aerobic as well as anaerobic conditions (IPCS 1996).
No biodegradation of MTBE was found after 60 days in experiments using aquifer soil material as inoculum. After two types of activated sludge as inoculum, no degradation of MTBE occurred after 40 days (Møller Jensen & Arvin 1990 in IPCS 1996).
One study found degradation of MTBE to t-butyl alcohol and CO2 under aerobic conditions using bacterial culture from an industrial sludge sample (Salnitro et al. 1994 in IPCS 1996).
Bioaccumulation
For Japanese carp a log bioconcentration factor of 1.5 has been determined (IPCS 1996).
1.6 Human exposure
The primary route of exposure to humans is through inhalation, as people are exposed to MTBE from air. If a level of 5-10 mg/m3 is anticipated as an average level for the population in the Danish cities, a daily dose of about 100-200 mg is considered a realistic average dose for an adult person with a daily inhalation volume of 20 m3 air. (A level of 5-10 mg/m3 for Danish cities is predicted to be about half the levels of North American cities where 15% MTBE in gasoline were used, see section 1.4).
Further the public may be exposed through the drinking water. As no measurement of MTBE in drinking water have been performed in Denmark it is difficult to make an estimate of the exposure, however the daily doses from drinking water for the general public is considered significantly lower than from inhalation exposure.
[Front page] [Contents] [Previous] [Next] [Top] |