|
Toxicological evaluation and limit values for Methyl-tertiary-butyl ether (MTBE), Formaldehyde, Glutaraldehyde, Furfural
4. Toxicity, animal data
4.1 Short term toxicity
Inhalation
In rats, the LC50-value for four-hour inhalation exposure to MTBE is about 85 mg/l. Toxicity signs following exposure were: hyperactivity, eye irritation, salivation, ataxia, weakness, tremors tachypnoea, loss of righting reflex, and unconsciousness. Surviving animal appeared to recover within 24 hours. At necropsy pulmonary hyperaemia was noted (IPCS 1996).
In rats, six hours exposure to 2880 and 14400 mg/m3 resulted in motor activity changes, which also appeared within 10 minutes at 28800 mg/m3. At the two highest dose levels reversible CNS sedation occurred. Survivors killed after a 14 days recovery period had slight to mild lung hyperaemia (IPCS 1996).
In mice, an RD50-value (the dose level that decreases respiratory rate 50%) was found to 16600 mg/m3 (Tepper et al. 1994).
Oral administration
The LD50-value in rats after oral exposure is about 3800 mg/kg. Signs of intoxication were: hypoactivity, muscular weakness, Hyperpnoea, lachrymation, prostration, and death. Recovery was complete at sublethal doses (IPCS 1996).
Repeated gavage administration with doses of 1200 mg/kg/d and higher to rats produced anaesthesia for about 2 h following the administration (Robinson et al. 1990).
In a 28-day oral study a significant increase in mean corpuscular haemoglobin was found down to a dose level of 90 mg/kg/d in female rats. Further a dose-related trend (dose levels of 0, 90, 440 and 1750 mg/kg/day) of increased absolute kidney and liver (primarily female) weight was found. Histopathology showed hyaline droplet formation in the proximal convoluted tubules in the kidneys of mid- and high-dose males (IPCS 1990). LOAEL in this study was 90 mg/kg/day.
Dermal contact
In rabbit the acute dermal LD50-value is > 10200 mg/kg.
Skin irritation
Adverse local effects in a 14 days observation period following 24 hours exposure of rabbits to 6800 and 10200 mg/kg included erythema, oedema, fissuring, and necrosis (IPCS 1996).
Eye irritation
In two separate studies MTBE was judged to cause eye irritation in rabbits after instillation of 0.05 or 0.1 ml of the undiluted liquid. In one study were the irritation was found to be mild a score of 20 out of a max. score of 110 was reached. The effects were reversible (IPCS 1996; the studies were conducted in 1969 and 1980 and thus is difficult to interpret in relation to modern guideline studies).
Eye irritation occurred in rats exposed to vapours at 14400 mg/m3 (IPCS 1996).
In long term studies where mice and rats were exposed to 0, 1440, 10800, and 28 800 mg/m3 the two highest exposure levels produced swollen periocular tissue and spasm of the eyelids (Bird et al. 1997).
Skin sensitisation
MTBE did not induce dermal sensitisation in ten guinea pigs following intradermal injections of 0.1% MTBE every second day for three weeks, with challenge injection of 0.1% MTBE two weeks later (IPCS 1996). [The study is conducted in 1980 and is considered of limited relevance as the study design is not in accordance with guideline studies for skin sensitisation].
4.2 Long term toxicity
Inhalation
In a 13-week vapour inhalation study rats were exposed to 0, 2880, 14400, and 28800 mg MTBE/m3 6h/d, 5d/week. At the two highest dose levels changes in motor activity and body temperature were observed, while further ataxia, depressed body weight gain and increased cortisone levels occurred in the highest dose group. All treatment groups showed increased absolute and relative liver and kidney weights and also increased weight of adrenal glands (primarily males), however, without treatment related microscopic changes in the organs (IPCS 1996). A LOAEL of 2880 mg/m3 could be set from this study.
See also section 4.5.
Oral administration
In a 90-day oral study using dose levels of 0, 100, 300, 900, and 1200 mg/kg/day the relative kidney weight was significantly increased at and above 300 mg/kg/day in female rats and the relative liver, thymic, and cardiac weights showed dose-related increases, statistically significant at 900 mg/kg. In male rats the mean absolute kidney weight was significantly elevated at the two highest dose levels. Microscopic findings in kidneys in high dose male rats were comparable to a2m-globulin nephropathy, otherwise no histopathological findings were noted (Robinson et al. 1990). NOAEL in this study was 100 mg/kg/day.
See also section 4.5.
Dermal contact
No data available.
4.3 Reproductive and developmental effects
In an one-generation study with rats exposed by inhalation to 0, 1080, 4680, or 12240 mg/m3 MTBE no adverse effects on reproduction were registered at the highest dose level (IPCS 1996).
In a two-generation study in which rats were exposed 0, 1440, 10800, or 28800 mg/m3 MTBE a NOEL for reproductive effects was 28800 mg/m3. LOEL for toxicity towards adults and offspring was 10800 mg/m3 (IPCS 1996).
In developmental studies with inhalation exposure of rabbits, rats and mice during gestation, no foetotoxic or developmental effects were noted at exposure levels below the maternal toxicity level. In rabbits NOAEL for maternal toxicity was determined to 3600 mg/m3 and NOAEL for developmental toxicity to >28 800 mg/m3. In mice NOAEL for both maternal and developmental toxicity was found to 3600 mg/m3, and maternal and developmental LOAEL was found to 14 400 mg/m3 due to clinical signs of maternal toxicity and reduced foetal body weight. In rats NOAEL for developmental toxicity was found to 9000 mg/m3, the highest exposure level in the study (IPCS 1996).
4.4 Mutagenic and genotoxic effects
In vitro studies with Salmonella typhimurium and, primary hepatocytes from rats and in vivo studies with Drosophila melanogaster have resulted in negative results. In a forward mutation test using mouse lymphoma cells with metabolic activation MTBE produced a positive and dose-dependent response. The generation of formaldehyde in this special designed test was shown to be the cause of the mutagenic activity. (IPCS 1996).
No positive response was found in an in vivo test with inhalation exposure to mice (2880 - 28800 mg/m3 MTBE 6h/d for 5 days) from which hepatocytes were sampled and examined for DNA repair activity. Nor did MTBE induce micronuclei in bone marrow cells from mice exposed by inhalation (1440 - 28800 mg/m3 MTBE, 6h/d for two days). (IPCS 1996).
4.5 Carcinogenic effects
Inhalation
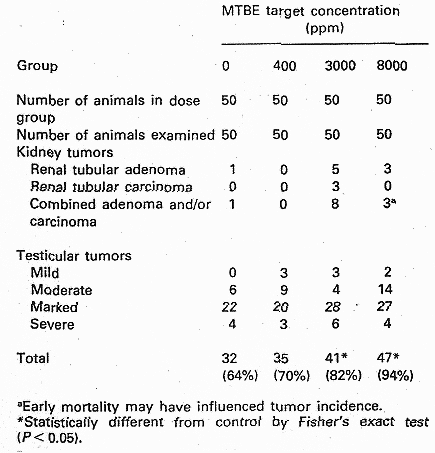
Fischer-344 rats (50 animals/ sex/ exposure level) were exposed up to 24 months to MTBE levels of 0, 1440 , 10800, and 28800 mg/m3 for 6 hours/day, 5 days/week. Increased mortality and decreased mean survival time were observed for male rats from all exposure groups. At the two highest dose levels clinical signs such as hypoactivity, ataxia, lack of startle reflex, swollen periocular tissue, spasm of the eye lids, and salivation were observed in both sexes. In female rats concentration related increases in liver and kidney weight (both absolute and relative) were observed at the two highest dose levels (due to the decrease in survival, statistical analysis could not be performed on organ weights in males for the two highest dose groups). An exposure related increased frequency of chronic nephropathy was observed at all dose levels in males and at the two highest dose levels in female rats. The males were more severely affected than females and nephropathy was the most common cause of death among the males. In males increased incidence of renal tubular cell adenomas and carcinomas was noted at the two highest dose levels (see table 4.1). In mid- and high dose males there was a dose-related increase of interstitial cell adenomas of the testes, see Table 2. (The incidence in the control group was considered low compared to historical data with incidences in the range of 83-88%). The authors suggested a NOEL of 1440 mg/m3 for males and females concerning general toxicity, however, this value may be debated as increased relative kidney weight was observed in male rats at this level. The NOEL for kidney tumours in males was set to 1440 mg/m3 (Bird et al. 1997).
Table 4.1, from Bird et al. (1997).
In CD-1 mice exposed to MTBE levels of 0, 1440 , 10800, and 28800 mg/m3 (50 animals/sex/exposure level) for 6 hours/day, 5 days/week for 18 months increased mortality was found only in males at the highest dose level. Clinical signs at the two highest dose levels were: ataxia, hypoactivity, prostration, lack of startle reflex, stereotypy and spasms of the eye lids. Increased relative liver weight was found in females in the two highest exposure groups. A significant, however not dose-related increase in kidney weight was observed in males from all dose groups and in females at the highest dose level. There was an increased (not significant) frequency of hepatic adenomas and carcinomas in male mice at the highest dose level, and in females, there was a significant increased incidence of hepatocellular adenomas at the highest dose level, see Table 4.2.
Table 4.2, from Bird et al. (1997)
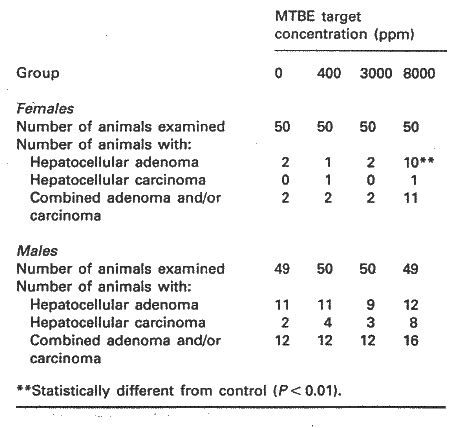
The NOEL for chronic toxicity was by the authors set to 1440 mg/m3 for both male and female mice. With respect to liver tumours the NOEL was set to 10,800 mg/m3 (Bird et al. 1997). Contradictory to this Rudo (1995) also considered the increased incidence of liver carcinomas in male mice as statistically significant.
Oral administration
In an oral study Sprague-Dawley rats (60 animals/sex/dose level) were administered 0, 250, or 1000 mg/kg/day of MTBE in virgin olive oil by gavage 4 days/week for 104 weeks (Belpoggi et al. 1995). High-dose male rats had a higher survival from treatment week 80 than controls (the animals were kept under observation to natural death). In females a treatment-related decrease in survival was observed from treatment-week 16. No evident behavioural changes were noted and no signs of general chronic toxicity were detected by gross and histopathological examination. With respect to oncogenic effects there was a statistically significant increased (p<0.05) incidence of testicular Leydig cell tumours in males in the highest dose group (11 of 32 animals surviving week 96) compared to 2 of 25 animals at the lowest dose level and 2 of 26 animals surviving week 96 in the control group. In female rats, a significant (p<0.01) and dose-related increase in the sum of lymphoma and leukaemia was found at both dose levels (in 12 of 47 animals in the highest dose group and in 6 of 51 animals at the lowest dose level compared to 2 of 58 animals in the control group).
Metabolites
tert-butyl alcohol
An oral carcinogenicity study has been conducted with tert-butyl alcohol (TBA) the metabolite of MTBE. Rats were through the drinking water exposed to average daily TBA doses of 0, 85, 195 and 420 mg/kg/day for males and 0, 175, 330, and 650 mg/kg/day for females. Exposure to TBA produced increased incidences of renal tubule adenoma and carcinoma in male rats, and transitional epithelial hyperplasia of the kidney in males and females.
Mice were exposed to average daily TBA doses of 0, 535, 1035, or 2065 mg/kg/day for males and 0, 510, 1015, or 2105 mg/kg/day for females. Exposure to TBA produced a significant increased incidence of follicular cell adenoma of the thyroid in female mice, while a slight increase was observed in males. Further, follicular cell hyperplasia of the thyroid and inflammation and hyperplasia of the urinary bladder in females and males were observed.
(Cirvello et al. 1995).
Formaldehyde
Formaldehyde is the other primary metabolite of MTBE. From experimental animal testing there is sufficient evidence for the carcinogenicity of formaldehyde. Clearest evidence was obtained from inhalation studies in which formaldehyde produced squamous-cell carcinomas of the nasal cavities in rats. In one oral study in which formaldehyde was administered to rats via the drinking water the dosing resulted in increased incidences of leukaemia. (IARC 1995).
Comments
The reporting and the conclusion from the oral MTBE study with rats has been criticised. Firstly, it is noted that the occurrence of Leydig cell tumours is age related, and therefore it may be expected that high dose male rats with a longer survival time than control rats turn out to have a higher incidence of tumours. Secondly, it is criticised that only the combined incidence of leukaemia and lymphoma for female rats is indicated. It is stated that there is little if any scientific reasons to group these two different kinds of tumours, and it is questioned whether the separate incidences of leukaemia and lymphoma would be significantly elevated in the dosed groups (Mennear 1995). Although the critique may be right in these points it seems very questionable that this should explain all the differences compared to the controls.
Further, it is has been argued that the increase in Leydig cell tumours in the inhalation study in rats could rather be explained by an unusual low occurrence in the control group, as the incidence in the dosed group was not elevated significantly compared to historical controls (Mennear 1996). Others however, have stated that more emphasis should be put on concurrent control than historical controls and that the incidence of Leydig cell tumours in the control group in fact should be considered as high. Further a clear dose-response relationship in the study should be recognised as evidence for a substance induced effect. It is considered supportive evidence that the induction of Leydig cell tumours has occurred in two different strains of rats with different background (historical) rates (Rudo 1995).
The exposure period in inhalation study with mice lasted for 18 months, and thus it has been speculated that the induction of hepatocellular adenoma in female mice may had become even more significant if a longer study duration of 24 months had been used for the study (Mennear 1996, Rudo 1995).
MTBE has been shown to bind to the male rats specific protein a2m-globulin and to accumulate in the kidney proximal tubule cells, however, it was found only to be a very mild inducer of a2m-globulin nephropathy. For other chemicals more severe a2m-globulin nephropathy has been shown to be responsible for the development of kidney tumours in male rats. Therefore in the case of MTBE it was suggested that the extra stress due to the a2m-globulin nephropathy may be a possible reason for the development of kidney tumours in the male rats. In females no kidney tumours were observed although MTBE exerted a chronic progressive nephropathy in females as well in males (Borghoff et al. 1996b, Prescott-Mathews et al. 1997).
[Front page] [Contents] [Previous] [Next] [Top] |