[Front page] [Contents] [Previous] [Next] |
Cross-flow filtration of fruit juice
5. Microfiltration
5.1 Microfiltration of Cherry Juice with Polymeric Membranes
5.1.1 Objectives
5.1.2 Materials and Methods
5.1.2.1 Sour cherry juice
5.1.2.2 Membrane modules
5.1.2.3 Experimental set-up
5.1.2.4 Equipment
5.1.2.5 Tests and procedures
5.1.2.5.1 Leakage test or Forward flow test (FF)
5.1.2.5.2 Water permeability
5.1.2.6 Start and filtration procedure
5.1.2.7 Cleaning procedure
5.1.2.8 Analyses
5.1.2.9 Data treatment
5.1.3 Experiments & Results
5.1.3.1 Maximum permeate flux
5.1.3.2 Backshock effect
5.1.3.3 Effect of backshock frequency
5.1.3.4 Pore size
5.1.3.5 Study of flux decline for MF05
5.1.4 Study of flux decline for MF08
5.1.5 Conclusions
5.2 Microfiltration of Cherry Juice with Ceramic Membranes
5.2.1 Objectives
5.2.2 Materials & methods
5.2.2.1 Filtration procedure
5.2.2.2 Cleaning procedure
5.2.2.3 Filtration test
5.2.2.4 Juice analysis
5.2.2.5 Analysis of the fouled membrane
5.2.3 Results and discussion
5.2.3.1 Permeate fluxes and permeabilities
5.2.3.2 Permeate quality
5.2.3.3 Membrane foulants
5.2.4 Conclusions
5.1 Microfiltration of Cherry Juice with Polymeric Membranes
5.1.1 Objectives
The aim of this work was to find out the suitability of polymeric membranes for the filtration of ultrafiltered sour cherry juice.
5.1.2 Materials and Methods
5.1.2.1 Sour cherry juice
The experiments were performed using UF-sour cherry juice supplied by Vallø Saft A/S (Denmark). This juice was produced, according to the flow diagram shown in the previous section on Juice Production, from sour cherries (Prunus cerasus L.) named ‘Stevnsbær’. The cherries were harvested in Denmark in August 1998.
5.1.2.2 Membrane modules
Membrane modules of 0.0094 m2 with reverse asymmetric structure (RAS) were used to run the experiments

Figure 5.1. Membrane module (Department of Biotechnology, Technical University of Denmark). Membrane module (Department of Biotechnology, Technical University of Denmark). Figure 5.1. Membrane module (Department of Biotechnology, Technical University of Denmark).
These modules were assembled at the Department of Biotechnology of the Technical University of Denmark using hollow fibres and epoxy glue. The characteristics of the membrane modules are shown in Table 5.1.
Table 5.1. Characteristics of the membrane modules. Characteristics of the membrane modules. Characteristics of the membrane modules.
| Number of hollow fibres NHF | 8 |
| Length of the hollow fibres LHF | 25 cm |
| Diameter of the hollow fibres DHF | 1.5 mm |
| Membrane Area A | 0.00942 m2 |
| Cross-flow Area AC | 1.1413 x 10-5 m2 |
Each module consisted of 8 hollow fibres made of polyethersulfone-polyvinylpyrrolidone (PES-PVP) and supplied by X-Flow (Almelo, The Netherlands). The pore sizes of the fibres used in this experiments are shown in Table 5.2.
Table 5.2. Characteristics of the hollow fibres. M2 is an abbreviation for hydrophobic.
Name |
Batch |
Pore size |
||
Min. |
Mean |
Max. |
||
MF05 M2 RAS |
091096-1 |
0.203 |
0.315 |
0.383 |
MF08 M2 RAS |
box |
0.487 |
0.602 |
|
5.1.2.3 Experimental set-up
The microfiltrations tests were run in a mini-flexible rig built and assembled at the Department of Biotechnology of the Technical University of Denmark. The experimental set-up is illustrated in Figure 5.2.
Figure 5.2. Schematic drawing of the experimental set-up.
5.1.2.4 Equipment
The following equipment was used during the experiments:
| 394Manometer | |
| 384Manometer | |
| 481Pt-100 sensor (temperature) | |
| 482Pt-100 sensor (temperature) | |
| 483Pt-100 sensor (temperature) | |
| 485Flow-meter | |
| 490Heat exchanger | |
| 491Heat exchanger | |
| 493Manometer | |
| 494Manometer | |
| 496Manometer | |
| 497Centrifugal pump | |
| 498Frequency converter | |
| 499Transmitter | |
| 5013-way valve | |
| 5023-way valve | |
| 504Magnet valve | |
| 505Magnet valve | |
| 507Transmitter (pressure) | |
| 508Transmitter (pressure) | |
| 509Transmitter (pressure) | |
| 623Reduction valve | |
| 6243-way valve | |
| 625Pump | |
| 626Scale | |
| 627Security valve | |
| 6283-way valve | |
| 6293-way valve | |
| 775Scale | |
| 1107Pump |
5.1.2.5 Tests and procedures
5.1.2.5.1 Leakage test or Forward flow test (FF)
The membrane module was tested before use for a possible leakage and pore characterisation. The test was accomplished by using the Palltronic FFE03 system.
The leakage test is based on the forward flow principle and performed in the form of a pressure hold test. Pressurised air was applied to the wetted filter (800 mbar in 120 seconds) and the test equipment measured the decrease on pressure as a result of airflow through the filter. The test was used as an indication for a damaged membrane, ineffective seals, or a leak in the system.
5.1.2.5.2 Water permeability
As mentioned in the previous section on Pre-treatment, the water permeability (Wp) gives an indication of the membrane condition, therefore it should be measured before and after each experiment as well as after each cleaning step.
The water permeability was measured at dead-end mode. This means that the direction of the water is from the retentate side to the permeate side. Ultrafiltered water obtained within 1 minute was collected from the permeate side (permeate water flow). If the membrane is new or clean, the TMP has to be as low as 0.1 bar (max. 0.2 bar) when measuring the Wp in order to get the proper value, but not lower than this value in order to be out of the range of the manometer’s error (+/- 0.04 bar). If the membrane is partly or completely blocked, the TMP should not be below 0.4-0.8 bar.
The FF test was performed and the Wp was measured according to the following procedure:
| The filter was inserted into the housing | |
| The filter housing was placed in a tank filled with water in order to wet the filter | |
| The filter housing was closed and connected to the Palltronic system | |
| The FF test was performed | |
| The housing containing the filter was assembled in the system | |
| Tank 489 was filled up with UF-water | |
| Valves D-2, D-4, D-5, D-11, D-7, D-8, D-10 and valve number 624 were closed | |
| Valves D-1, D-3 and D-6 were opened | |
| The pipe connecting valve D-9 and pump number 1107 was disconnected and the valve was opened | |
| Pump 497 was started | |
| When the system and the backshock valve were filled up with distilled UF water, the clamps that held the manometers in the filtration system and the valves were slightly opened in order to eliminate air bubbles and afterwards these were closed | |
| When the pressure in the membrane had stabilised, the flow in valve D-9 was adjusted and the pressure in the manometers retentate in (494), retentate out (493) and permeate (349) was noted. Valve D-9 was slightly closed and the flow and the pressure were measured again. This should be run for 3 different permeate fluxes since the WP should be the average of at least three measurements |
The Wp was calculated by using the equation shown in the section on Membrane Processes.
5.1.2.6 Start and filtration procedure
Before filling the tank with juice, the system was run at the decided parameters with UF-water until the system had been stabilised. When the system was stable, the juice was added to the feeding tank and recirculated without coming in contact with the filter until its temperature was 2°C. At this point the filtration could be started.
- Tank nr 489 was filled with UF-water
- Valves D-1, D-3, D-6, D-10, D-9, D-13 and nr 629 were opened
- Valves D-2, D-4, D-5, D-7, D-8, D-11, D-12 and nr 628 were closed
- Pumps nr 497, 625 and 1107 were started
- The filtration system was set to the running parameters with the help of a computer, and the backshock was adjusted with the help of a 3-way valve
- The regulation was stopped (via PC) and pumps nr 497, 625 and 1107 were stopped
- Tank nr 489 was emptied for water
- Tank nr 489 was filled with the juice
- Valves nr D-6, D-10 and D-13 were closed
- .Valves nr 501, D-11 and D-12 were opened
- .Pump nr. 497 was turned on. The system was emptied for water through valve D-12, and after that valves D-13 and D-12 were opened. The juice was cooled down to approximately 2°C
- .When the temperature of the juice was 2° C, valves D-6 and D-10 were opened and valve D-11 was closed. The programme on the computer was turned on again and in this way the filtration was started
5.1.2.7 Cleaning procedure
After each filtration the system was rinsed with distilled UF water and the Wp
measured.
A new filter was used for each experiment. However, the filtration system and the filter
were cleaned with distilled UF water at 50°C and with different cleaning agents and
disinfectants.
All the valves in the cleaning system were closed and the cleaning procedure described below was followed:
| Pressurised air was let into the system through a regulation valve | |
| The steam valve was opened | |
| A valve on the UF-water system was connected to a valve on the cleaning station, so UF-water was at disposal | |
| The tank was filled with 10 l of UF-water | |
| Some more valves were opened in order to make the UF-water circulate and the pump was started | |
| 100 ml P3-Ultrasil 65 were added at 50°C (cleaning time: 60 min.) | |
| 2 drops of P3-Ultrasil 91 and 200 ml of P3-Ultrasil 05 were added when the temperature of the cleaning water was 20°C and the pH is 9.6 (cleaning time 30 min.) | |
| 100 ml P3-Ultrasil 75 were added at 60° C (cleaning time 30 min.) | |
| 100 ml P3-Ultrasil 91 and 200 ml P3-Ultrasil 05 were added when the temperature was 60°C (cleaning time 15 min.) | |
| 50 ml p3-Ultrasil 75 were added at 60°C (cleaning time 15 min.) | |
| 20 ml P3-oxonia aktiv were added at 20°C (disinfection time 60 min.) | |
| The valves were closed in a way so the pressure at the permeate side reached 1.1 bar | |
| The tank was half emptied through valve 11 | |
| Valve 11 was closed | |
| Valve 7 was closed in a way so the pressure at the permeate side reached 1 bar | |
| Valve 9 was opened and valve 8 was closed when the cleaning time was over | |
| The pump was turned off when the tank was empty | |
| The filter and the system were rinsed after each cleaning step | |
| All valves on the filtration system were closed in order to rinse the filter | |
| Valves 1, 2, 4, 6, 8 and 10 were opened | |
| Valve 10 was closed and the tank was manually rinsed | |
| The tank was fully emptied | |
| Valves 2 and 4 were closed | |
| Valve 10 was opened | |
| Valves 6, 8 and 10 were closed when the tank was filled up | |
| Valves 3 and 9 were closed in order to rinse the by-pass | |
| The pump was turned on for 4 seconds | |
| Valves 5 and 7 were opened and valve 3 was closed in order to rinse the retentate side | |
| The pump was turned on for 15 seconds | |
| Valves 4 and 6 were opened and valves 5 and 7 closed in order to rinse the permeate side | |
| The pump was turned on for 40 seconds | |
| Valves 5 and 7 were opened and 4 and 6 closed | |
| The pump was turned on while opening valve 11 | |
| The tank was filled with water in order to start the next cleaning step | |
| After the last cleaning step the system was emptied for water by opening valves 1 and 2 |
The pH of the flushed water after cleaning was determined to ensure that the cleaning solutions had been completely removed from the filtration system.
5.1.2.8 Analyses
The different responses were analysed or measured following the same procedure as shown in the section on Pre-treatment.
5.1.2.9 Data treatment
Noted data and data from the PC logging were compared, selected, adjusted and the TMP and the permeate flux were calculated. TMP and permeate versus time was presented in a graph (Excel 5.0 for Windows) for each experiment.
5.1.3 Experiments & Results
Several experiments were run in order to optimise the microfiltration efficiency and this was done by:
| Building and testing the optimal filtration system | |
| Calibrating the filtration system | |
| Calibrating the equipment used for the analyses | |
| Studying the microfiltration performance | |
| Determining the maximum permeate flux, | |
| Studying the backshock effect | |
| Determining the optimal backshock frequency | |
| Studying the pore size of the membrane | |
| Studying the effect of some filtration parameters | |
| Studying the phenomena that lead to a decrease on the permeate fluxes during filtration |
A selection of these experiments will be presented in this section.
5.1.3.1 Maximum permeate flux
Seven experiments were run in order to study the microfiltration performance and to determine the maximum permeate flux when filtering UF sour cherry juice with a MF08 membrane. The most representative experiment will be presented in this section.
| Medium | UF sour cherry juice |
| Filtration | Microfiltration combined with backshock |
| Volume | 25l |
| Membrane | MF08 RAS |
| Temperature | 3-5° C |
| Pressure at the tanks | 0.3 bar |
| Permeate flux | 50-350 l/h/m2 |
| Retentate flow | 0.5 m/s |
| Backshock duration | 0.01 s |
| Backshock frequency | 3 s |
The permeate flux was set at 50 l/h/m2 at the beginning of the experiments and it was increased 50 l/h/m2 every 20 minutes until reaching a maximum. After that, the flux was decreased in the same way to 50 l/h/m2 in order to determine whether fouling is stopping the filter.
The TMP was 0.06 bar at the beginning of the experiment and this was 1.21 bar at the end of the experiment. This means that the membrane had been fouled. A layer might have been deposited on top of the membrane resulting in an increase on the pressure difference.
The maximum flux achieved was 350 l/h/m2. However, fluxes up to 500 l/h/m2 were reached in other experiments run at the same conditions with the same type of membrane. The Wp was 24500 l/m2/h/bar and 830 l/m2/h/bar at the end of the experiment.
Further experiments should be run in order to determine whether the decrease on the flux is caused by fouling or concentration polarisation phenomena.
5.1.3.2 Backshock effect
No backshock
Several experiments were run in order to determine whether backshock had a positive effect on the microfiltration performance when filtering UF sour cherry juice with a MF08 RAS membrane. The three most relevant experiments will be presented in this section.
| Medium | UF sour cherry juice |
| Filtration | Microfiltration combined / not combined with backshock |
| Volume | 25 l |
| Membrane | MF08 RAS |
| Temperature | 3-5° C |
| Pressure at the tanks | 0.3 bar |
| Permeate flux | 50-350 l/h/m2 |
| Retentate flow | 0.5 m/s |
The first experiment was run without backshock for 20 minutes, and at that moment the TMP had already increased to 0.46 bar (Figure 5.3).
Figure 5.3. Permeate flux and TMP versus time for the experiment run without backshock.
This means that the membrane was getting fouled rapidly in these conditions. Therefore, backshock was applied from that moment and every 20 minutes for 0.01 seconds during 5 minutes.
Backshock every 20 min.
The results for these experiments are shown in Table 5.3.
Table 5.3. TMP, permeate flux, and duration for the experiments.
Experiment |
TMP start |
TMP end |
Duration |
Max. flux |
Without backshock |
0.19 |
0.46 |
20 |
50 |
BS every 20 min |
0.19 |
0.18 |
65 |
100 |
BS every 3 s |
0.07 |
0.35 |
550 |
200 |
As it is observed in Figure 5.4, a significant decrease on the TMP was observed when backshock was applied, which means that backshock partly removed the layer that had been formed on top of the membrane. This experiment was stopped after 65 minutes and the TMP was 0.18 bar at that moment. The permeate flux was 50 l/h/m2 during the first 20 minutes and it increased to approximately 100 l/h/m2 when the first backshock was applied staying stable on that level for the rest of the experiment.

Figure 5.4. Permeate flux and TMP versus time for the experiment run with backshock every 20 minutes.
Constant backshock
The permeate flux for the experiment run with frequent backshock (every 3 seconds for 0.01 s) was approximately 200 l/h/m2. However, it was quite unstable during the whole experiment. The experiment was running for 550 minutes and the TMP was 0.35 bar at the end of the experiment (Figure 5.5).

Figure 5.5. Permeate flux and TMP versus time for the experiment run with frequent backshock.
These experiments have shown that backshock has a positive effect on the microfiltration performance. This technique cleans periodically the membrane avoiding and / or reducing further deposition of compounds on top of the membrane. Furthermore, backshock results in higher fluxes.
5.1.3.3 Effect of backshock frequency
Several experiments were run in order to study the effect of the backshock frequency on the microfiltration performance when filtering UF sour cherry juice with a MF08 membrane. The three most representative experiments will be presented in this section.
| Medium | UF sour cherry juice |
| Filtration | Microfiltration combined with backshock |
| Volume | 25 l |
| Membrane | MF08 RAS |
| Temperature | 3-5° C |
| Permeate flux | 300 l/h/m2 |
| Retentate flow | 0.5 m/s |
| Backshock duration | 0.01 s |
| Backshock frequency | 1, 3 or 5 s |
The results for these experiments are shown in Table 5.4 and Figures 5.6, 5.7 and 5.8.
Table 5.4. TMP and duration for the experiments run at different backshock frequencies.
Experiment |
TMP start |
TMP end |
Duration |
Backshock every 1 s |
0.13 |
0.53 |
360 |
Backshock every 3 s |
0.07 |
0.33 |
360 |
Backshock every 5 s |
0.04 |
0.70 |
200 |
The two first experiments were running for 360 minutes, whereas the experiment run with backshock every 5 s was only running for 200 minutes.
1 sec. backshock interval
As it can be seen in 5.6, the permeate flux was quite unstable for the experiment run with
backshock every second. The TMP increased slowly at the beginning of the experiment, but
this changed after 190 minutes.
Figure 5.6. Permeate flux and TMP versus time for the experiment run with backshock every second.
3 sec. backshock interval
The permeate flux was more stable for the experiment run with backshock every 3 s and the TMP increased constantly during the whole experiment from 0.07 to 0.33 bar.
Figure 5.7. Permeate flux and TMP versus time for the experiment runwith backshock every 3 seconds.
5 sec. backshock interval
The permeate was quite unstable for the experiment run with backsock every 5 s and the TMP
increased from 0.04 to 0.7 bar in 200 minutes.
Figure 5.8. Permeate flux and TMP versus time for the experiment run with backshock every 5 seconds.
It has been demonstrated that the backshock frequency has a significant effect on the filtration duration. The backshock frequency should be between 1 and 3 seconds. However, both flux and TMP were more stable when applying backshock every 3 seconds.
5.1.3.4 Pore size
Several experiments were run in order to test MF05 RAS and to compare the microfiltration performace with the one obtained when running with a MF08 RAS membrane. The three most representative experiments will be presented in this section.
| Medium | UF sour cherry juice |
| Filtration | Microfiltration combined with backshock |
| Volume | 25 l |
| Membrane | MF05 RAS |
| Temperature | 3-5° C |
| Pressure at the tanks | 0.3 bar |
| Retentate flow | 0.5 m/s |
| Backshock duration | 0.01 s |
| Backshock frequency | 3 s |
Two concentration experiments were run; one at a flux of 150 l/h/m2 and the other at 300 l/h/m2. The third experiment was run in order to determine the maximum permeate flux. This was done by setting the flux at 50 l/h/m2 at the beginning of the experiment and increasing it 50 l/h/m2 every 20 minutes until a maximum was reached. After that, the flux was decreased in the same way to 50 l/h/m2 in order to determine whether fouling was stopping the filter.
The results of these experiments are shown in Table 5.5 and Figures 5.9, 5.10 and 5.11.
Table 5.5. TMP and duration for the experiments on MF 05.
Experiment |
TMP start |
TMP end |
Max. flux |
Duration |
| Concentration at 300 l/h/m2 | 0.21 |
0.49 |
300 |
50 |
Determination of max.flux |
0.06 |
0.48 |
200 |
140 |
| Concentration at 150 l/h/m2 | 0.12 |
0.47 |
150 |
150 |
The TMP increased to 0.49 bar in 50 minutes when filtering UF sour cherry juice through a MF05 membrane at a flux of 300 l/h/m2, whereas it was 0.33 bar after 360 minutes when filtering under the same conditions with a MF08 membrane.
Figure 5.9. Permeate flux and TMP versus time for the concentration experiment at 300 l/h/m2.
As it can be seen in Figure 5.10, the TMP increased pronouncedly after 60 minutes (flux 150 l/h/m2), and this increase became even more obvious when the flux was increased to 200 l/h/m2. This indicates that the maximum permeate flux for this membrane has been reached. The maximum permeate flux is a lot lower than the one found for the MF08 membrane.
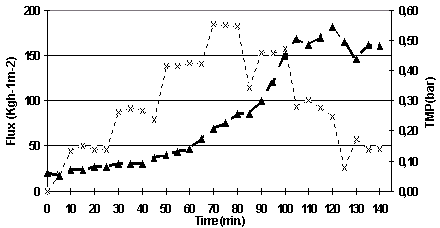
Figure 5.10. Permeate flux and TMP versus time for the determination of maximum flux experiment.
When running at 150 l/h/m2, the concentration experiment could be run for 150 minutes (three times more than at 300 l/h/m2). The TMP increased significantly after 90 minutes, but this was due to some problems in controlling the permeate pump via computer.

Figure 5.11. Permeate flux and TMP versus time for the concentration experiment at 150 l/h/m2.
It can be concluded that significant lower fluxes are achieved when filtering UF sour cherry juice with MF05 than with MF08. MF05 can be used at the same conditions as MF08. However, more experiments should be run in order to find the optimal conditions.
5.1.3.5 Study of flux decline for MF05
Aim
The aim of these experiments was to find out at which ° Brix the MF05 membrane could run for four different permeate fluxes. This study will lead to the cause for flux decline (concentration polarisation or fouling).
Conditions
10 litres of UF water were added to the feed tank and cooled down to 3° C, and the permeate pump was set to a flux of 50, 100, 200 or 300 l/h/m2. After that, 1 litre of sour cherry concentrate with a °Brix of 64% was added and the °Brix was measured after 15 minutes at the permeate and the retentate sides. 0.5 l of the same concentrate was added every 15 minutes and the sugar concentration was measured. The experiments were stopped when the TMP was approximately 0.5 bar.
| Medium | Sour cherry concentrate and water |
| Filtration | Microfiltration combined with backshock |
| Membrane | MF05 RAS |
| Temperature | 3-5° C |
| Pressure at the tanks | 0.3 bar |
| Permeate flux | 50, 100, 200 or 300 l/h/m2 |
| Retentate flow | 0.5 m/s |
| Backshock duration | 0.01 s |
| Backshock frequency | 3 s |
The TMP and the °Brix results for the four different fluxes are shown in Figure 4.12.
The TMP increased slowly for 105 minutes when running at 50 l/h/m2
(23°Brix). The TMP increased more significantly after that and the filter blocked after
165 minutes.
The TMP increased significantly after 30 minutes when running at 100 l/h/m2. At
that time the °Brix concentration was 11.4%, and the filter blocked after 90 minutes.
The TMP increased significantly after 30 minutes when running at 200 l/h/m2. At
that time the °Brix concentration was 11.4%, and after that the filter blocked.
The TMP increased significantly after 15 minutes when running at 300 l/h/m2. At
that time the °Brix concentration was 7.4%, and after that the filter blocked.
Figure 5.12. TMP and °Brix versus time for the different permeate fluxes.
It can be concluded that MF05 can filter at 23°Brix when running at 50 l/h/m2, at 11.4°Brix when running at 100 and 200 l/h/m2, and at 7°Brix when running at 300 l/h/m2. These results showed that the cause for filter blocking was not the °Brix concentration but the amount of juice going through the membrane. No concentration polarisation phenomena were observed.
5.1.4 Study of flux decline for MF08
Aim
The aim of these experiments was to find out at which ° Brix the MF08 membrane could run for four different permeate fluxes. This study will lead to the cause for flux decline (concentration polarisation or fouling).
Conditions
10 litres of UF water were added to the feed tank and cooled down to 3° C, and the permeate pump was set to a flux of 50, 100, 200 or 300 l/h/m2. After that, 1 litre of sour cherry concentrate with a °Brix of 64% was added and the °Brix was measured after 15 minutes at the permeate and the retentate sides. 0.5 l of the same concentrate was added every 15 minutes and the sugar concentration was measured. The experiments were stopped when the TMP was approximately 0.5 bar.
| Medium | Sour cherry concentrate and water |
| Filtration | Microfiltration combined with backshock |
| Membrane | MF08 RAS |
| Temperature | 3-5° C |
| Pressure at the tanks | 0.3 bar |
| Permeate flux | 50, 100, 200 or 300 l/h/m2 |
| Retentate flow | 0.5 m/s |
| Backshock duration | 0.01 s |
| Backshock frequency | 3 s |
The TMP and the °Brix results for the four different fluxes are shown in Figure 5.13.
The TMP increased slowly for 45 minutes when running at 50 l/h/m2 and at 100
l/h/m2 (14.2°Brix). The TMP increased more significantly after 165 minutes
when running at 50 l/h/m2 and after 180 minutes when running at 100 l/h/m2.
The filters blocked at that time.
The TMP increased significantly after 30 minutes when running at 100 l/h/m2. At
that time the °Brix concentration was 11.4%, and the filter blocked after 90 minutes.
The TMP increased significantly after 30 minutes when running at 200 l/h/m2. At
that time the °Brix concentration was 12%, and after that the filter blocked.
The TMP increased significantly after 15 minutes when running at 300 l/h/m2. At
that time the °Brix concentration was 7%, and after that the filter blocked.
Figure 5.13. TMP and °Brix versus time for the different permeate fluxes.
It can be concluded that MF08 can filter at 14.2°Brix when running at 50 and 100 l/h/m2, at 12°Brix when running at 200 l/h/m2, and at 7°Brix when running at 300 l/h/m2. These results showed that the cause for filter blocking was not the °Brix concentration but the amount of juice going through the membrane. No concentration polarisation phenomena were observed.
5.1.5 Conclusions
The conclusions drawn from these experiments are:
| Good performance of the X-flow membranes was proved when filtering UF sour cherry juice. |
| Higher fluxes (200-300 l/h/m2) for 10 hours at 2°C compared to
Ultrafiltration (40 l/h/m2 at 40°C). It is recommended to filter at low
temperatures since this avoids precipitation of polyphenols. |
| The backshock technique had a positive effect on the microfiltration performance
reducing fouling and resulting in higher fluxes. |
| The backshock frequency has a significant effect on the filtration duration, and the
optimal value was 3 seconds. |
| Significant lower fluxes were achieved when filtering UF sour cherry juice with MF05
than with MF08. |
| The cause for filter blocking was the amount of juice going through the membrane and not the concentration. |
5.2 Microfiltration of Cherry Juice with Ceramic Membranes
5.2.1 Objectives
The aim of this work was to find out the suitability of new ceramic membranes for the microfiltration of ultrafiltered sour cherry juice. The effect of backshock during filtration was tested and some of the substances fouling the membrane were analysed.
5.2.2 Materials & methods
In the following sections, the filtration and cleaning procedures, the conditions used in different filtration tests and the analyses run on the juice samples as well as the analyses made on a used membrane will be presented.
The filtration equipment used was a membrane filtration rig with backshock possibilities built at the Department of Biotechnology of The Technical University of Denmark (description found in the section on Microfiltration of Cherry Juice with Polymeric Membranes).
The membranes used in the filtration tests were tubular ceramic membranes manufactured by Haldor Topsøe (Denmark). These were unmodified and modified a -alumina membranes with a maximum pore size of 0.5 µm. The membrane area was 52.6 cm² and the cross-section area tube was 35.2 mm².
5.2.2.1 Filtration procedure
Filter tests
Filtration tests were run according to the following procedure: Before each filtration test, a Forward Flow test was run with Palltronic equipment in order to check the integrity of the membrane. After that, the permeability of ultrafiltered water was measured at dead-end filtration and at three different pressures.
Filtration procedure
The actual filtration test was started with ultrafiltered water in order to get the pumps calibrated and to let the adjusted filtration conditions to stabilize. Water filtration took approximately 30 minutes. Then, the permeate and the retentate valves were closed in order to keep the membrane cell pressurized during the change from water to cherry juice. Pumps were stopped and the water was discharged into sewer. The feed tank was filled with juice and pressurized to 0.5 bar. Water and a small amount of juice was discharged from the retentate line, after which a feed sample was taken. Filtration was started by circulating the juice through the by-pass until this was cooled down to 3-4ºC. After that, the retentate and the permeate valves were opened and the filtration was started. During filtration, the permeate flux was measured every five minutes, and samples were taken from permeate and retentate every 20 minutes. The filtration test was stopped when the transmembrane pressure got too high.
After each filtration test, the equipment and the membrane were rinsed at a high linear velocity with ultrafiltered water. Usually, the pressure on the retentate side was around 1 bar during rinsing. The pressure on the permeate side depended on the degree of fouling on the membrane, and this was therefore different for each test. At the beginning, the rinsing was conducted through all the lines, but after the retentate line was cleaned, the rinsing was continued at dead-end mode in order to clean the membrane and permeate line. Rinsing was continued until the water coming from the permeate line appeared clean. After rinsing, the permeability of ultrafiltered water was measured following the same procedure as before the filtration test.
5.2.2.2 Cleaning procedure
After each filtration test, the membranes were chemically cleaned by using the
cleaning agents and at the condition presented in Table 5.6. After each treatment with
cleaning agents, the membrane was thoroughly rinsed with water. The water used in all
cleaning steps was ultrafiltered tap water. After filtration test nr.4, P3-Ultrasil 75 was
replaced with P3-Ultrasil 73, which does not contain phosphoric acid. Phosphoric acid is
known to harm at least g -alumina membranes (Trädgårdh,
1989), and since the same kind of behaviour could be expected when cleaning a -alumina membranes, this cleaning agent was replaced with another
acid.
Table 5.6. Cleaning agents and conditions used for the cleaning of membranes used in the microfiltration of cherry juice.
| Cleaning agent | Dosage | Temperature ( ºC) |
Time (min) |
| P3-Ultrasil 65 | 100 ml | 50 |
60 |
| P3-Ultrasil 91 + P3-Ultrasil 05 |
to pH 9.6/ 200 ml | 20 |
30 |
| P3-Ultrasil 75 | 100 ml | 60 |
30 |
| P3-Ultrasil 91 + P3-Ultrasil 05 |
100 ml/ 200 ml | 60 |
15 |
| P3-Ultrasil 75 | 50 ml | 60 |
15 |
| P3-oxonia | 20 ml | 20 |
60 |
5.2.2.3 Filtration test
A list of the filtration tests and the conditions applied is presented in Table 5.7. The temperature in the feed tank was 4 ºC, the pressure in feed tank 0.3 bar, the linear velocity 0.5 m/s, and the backshock flow 2.5 l/h for all tests. The filtration tests were run keeping the permeate flux constant. In tests 2, 3, 4, and 6, the permeate flow was increased step-wise every 20 minutes until the transmembrane pressure was too high, and then decreased step-wise every 10 minutes. The change on the transmembrane pressure during the filtration test was also measured. In test 5, the permeate flow was kept constant during the whole test, and the increase on the transmembrane pressure during filtration was also measured. Filtration test 6 was run without backshock. In filtration test 1, the membrane broke after 10 minutes of filtration and no results will be presented.
Table 5.7. Filtration conditions used in microfiltration tests with cherry juice.
Test no. |
Membrane used |
Feed volume (l) |
Permeate flow (kg/m² h) |
Duration of backshock (s) |
Frequency of backshock (s) |
1 |
Ceramic 1 |
2 |
89 |
0.010 |
- |
2 |
Ceramic 2 |
2 |
89-625 |
0.010 |
3 |
3 |
Ceramic 3 |
2 |
89-625 |
0.010-0.016 |
3 |
4 |
Ceramic 3 |
2 |
89-300 |
0.010-0.022 |
3 |
5 |
Ceramic 4 |
9 |
268 |
0.010-0.029 |
3 |
6 |
Ceramic 5 |
20 |
54-393 |
- |
- |
5.2.2.4 Juice analysis
Colour, turbidity, sugar content, and total phenols were analysed from feed,
retentate and permeate samples.
The colour was analysed with a spectophotometer at a wavelength of 520 nm. The turbidity
was analysed with a nepholometer, and the samples were diluted to a ºBrix of 3 before
measurement. The sugar content was analysed with a refractometer. The total phenol content
was measured with a colorimetric method, in which the phenolic compounds form a blue
complex with molybdenum and tungsten and the colour of this complex was measured with a
spectrophotometer at a wavelength of 765 nm.
5.2.2.5 Analysis of the fouled membrane
Membrane nr. 3 (tests 3 and 4) was analysed after running the filtration test for membrane foulants. Total organics were analysed from the membrane by incinerating this one at 550ºC for 2 h. The protein content was analysed by means of Liquid Chromatography after extraction with HCl.
Phenol content
The total phenol content was extracted from the membrane with acetone, and the extract was analysed following the same method as described before for the juice samples.
SEM pictures
Pictures from the membrane surfaces and the cross-sections were taken with a Scanning Electron Microscope in order to see how the fouling layer looked like. Some pictures were also taken from membrane ceramic 4 (test 5) after cleaning, and these pictures were compared with those taken from membrane 3.
5.2.3 Results and discussion
This chapter presents the fluxes and the permeate quality data from microfiltration of cherry juice trials run with ceramic membranes, as well as a short description of the fouling substances analysed from a used membrane.
5.2.3.1 Permeate fluxes and permeabilities
Broken membranes
The ceramic membrane used in test 1 broke after 10 minutes of filtration, and therefore, no results from this test will be presented. The membrane used in test 2 broke after cleaning, and the membrane used in test 4 during the filtration test. The membrane used in test 5 also broke after cleaning. It seems that the membranes used in these filtrations were extremely fragile and broke down very easily either during assembling (gluing), during the filtration tests or cleaning, since they could not stand any kind of bending. One reason that might explain this phenomenon during backshock filtrations could be the distribution of the pressure on the membrane during backshock. It is likely that pressure shock exerts only to one part of the membrane causing the same effect as when the membrane is bent.
The transmembrane pressure versus flux curves for tests 2, 3, and 4 are presented in Figure 5.14. It is expected that when the permeate flow increases, the transmembrane pressure increases. If the membrane is fouled during filtration, the transmembrane pressures achieved when increasing the flux should be lower than those achieved when decreasing the flux. In test 2 and 3, when the permeate flux was increased, the transmembrane pressure increased. However, when the permeate flux was decreased, higher flux values resulted in lower transmembrane pressures than when the flux was increased. This is not in accordance with the theory. An explanation for this unexpected behavior could be the accuracy of the measurements or it could have something to do with the way the permeate is pumped into the backshock system. It is difficult to demonstrate the fouling on the membrane by looking at Fig. 5.15. However, the water permeabilities after the filtration tests were lower than those before the tests (Table 5.8.). This means that the membranes were partly fouled during filtration.

Figure 5.15. Permeate fluxes versus transmembrane pressures during microfiltration of cherry juice with ceramic membranes. Backshock of 0.010s every 3 s. Linear velocity 0.5 m/s.
Table 5.8. Water permeabilities before and after filtration.
Test |
Membrane | Permeability before test |
Permeability after test |
1 |
Ceramic 1 | 2511 |
3067 |
2 |
Ceramic 2 | 4670 |
4082 |
3 |
Ceramic 3 | 6325 |
4486 |
4 |
Ceramic 3 | 1710 |
- |
5 |
Ceramic 4 | 3334 |
2171 |
6 |
Ceramic 5 | 6911 |
635 |
The same membrane was used in tests 3 and 4. However, the membrane was cleaned with cleaning agents between these filtration tests. The permeability after cleaning was much lower than before cleaning, which indicates that the membrane was fouled already after cleaning. The reason for this behaviour was that the ultrafiltered water used for the integrity testing and the permeability measurements had been standing in a pool for a long period of time and some biological growth had taken place. Moreover, the ultrafiltration system used for the treatment of tap water had not been properly cleaned and particles that fouled the membrane leaked through the filter. Therefore, the membrane was already fouled before the actual test was started and this is confirmed by the higher transmembrane pressures in test 4 compared to those from tests 2 and 3.
In Figure 5.16. the behaviour of the transmembrane pressure during a 6 h filtration is presented. As it is observed, when the permeate flux was kept at approximately 236 l/m² h, the transmembrane pressure increased from 0.35 bar to 0.56 bar. This means that, although backshock was being applied, the membrane had fouled during the test, and that the backshocks could not totally stop the fouling. The variations in flux were probably caused by the backshocks.
 Figure 5.16. Permeate
flux and transmembrane pressure (TMP) during microfiltration of cherry juice. Filtration
conditions: backshock of 0.010 s every 3 s and linear velocity 0.5 m/s.
Figure 5.16. Permeate
flux and transmembrane pressure (TMP) during microfiltration of cherry juice. Filtration
conditions: backshock of 0.010 s every 3 s and linear velocity 0.5 m/s.
The effect of backshock on microfiltration of cherry juice can be seen in Figure 5.17. It is evident that when operating at transmembrane pressures higher than 0.5 bar, the filtration run with backshock resulted in better fluxes than the filtration run without backshock. In the filtration run without backshock and above a transmembrane pressure of 0.5 bar, the flux-pressure curve starts to deviate from linear and the increase in the pressure does not result in an increase on the flux as much as at lower pressures. It seems that there exists an optimum in the correlation flux-pressure, and that the highest flux without backshock can be achieved at a pressure of approximately 1.25 bar.

Figure 5.17. Effect of the backshock technique on the permeate flux as a
function of the transmembrane pressure in microfiltration of cherry juice with ceramic
membranes. Filtration conditions: backshock of 0.010 s every 3 s, linear velocity 0.5 m/s,
temperature 4ºC.
5.2.3.2 Permeate quality
The results of the different analysis for the feed, the retentate and the permeate samples are presented in Table 5.9. for all filtration tests. The data presented in the Table corresponds to samples collected at the end of each test for the permeate and the retentate and at the beginning of the test for the feed. Typically, the concentrations in the retentate and the permeate varied during the test, as it is observed in Figure 5.18., in which the colour of the retentate and the permeate samples collected during test 3 are presented as a function of the time. In most cases, at the beginning of the test, the concentrations in the permeate were much lower, and, therefore, the retentions were higher, since the ultrafiltered tap water used during the stabilisation of the filtration conditions diluted the permeate. At the end of the test, when the permeate side of the equipment was filled with permeate, the retentions were much lower, usually below 25%. In test 5, which was run for 6 h at a constant flux, the retentate and permeate values started to stabilise after 3 h of filtration.
Taking into account that the quality requirements for sour cherry juice are: a turbidity lower than 10 FNU, a sugar content of 11-15ºBrix, and a colour content between 0.70-1.50, the quality of the permeate was not good enough in many tests. As it is observed in Table 5.10, only the quality requirements set for sugar and colour were achieved except for a few exceptions, whereas the turbidity in most cases was too high (18-19 FNU).
Retentions of sugars were between 6 and 18% at the end of the filtration tests. Retentions of the phenols were negative in tests 3 and 5, which means that the concentrations of phenols were higher in the permeate than in the retentate. In tests 4 and 6, where the membranes were more fouled during the test, the retentions of phenols were 10-25%. This means that when backshock was applied, the membrane retained only small amounts of sugars and phenols. This did not have a significant effect on the quality of the juice. The retentions of colour were 0-11 % and those of turbidity 10-16%, except in test 6 (without backshock), where the retention of turbidity was 97%. The high retention of turbidity in test 6 was most likely due to the fouling of the membrane. Feed concentrations in test 5 were exceptionally low. One possible explanation for this is that the retentate line was not properly emptied from water when the feed sample was taken, and the feed sample was therefore diluted.
Table 5.9. Analysis data from test microfiltrations of cherry juice run with ceramic membranes.
| Feed | Retentate | Permeate | |
| Turbidity (FNU) | |||
| Test 3 | 22.6 | 20.5 | 18.4 |
| Test 4 | 20.6 | 22.4 | 18.9 |
| Test 5 | 9.9 | 22.5 | 19.5 |
| Test 6 | 13.3 | 7.3 | 0.23 |
| Colour | |||
| Test 3 | 1.17 | 1.22 | 1.09 |
| Test 4 | 1.17 | 1.15 | 1.07 |
| Test5 | 0.62 | 1.77 | 1.77 |
| Test 6 | 1.19 | 1.16 | 1.15 |
| Sugar content (ºBrix) | |||
| Test 3 | 12.0 | 11.0 | 10.0 |
| Test 4 | 11.2 | 11.0 | 9.0 |
| Test 5 | 12.0 | 16.1 | 15.2 |
| Test 6 | 14.0 | 14.0 | 12.0 |
| Total phenols (mg/l GAE) | |||
| Test 3 | 238 | 166 | 214 |
| Test 4 | 1207 | 1029 | 777 |
| Test 5 | 1255 | 2981 | 3246 |
| Test 6 | 2676 | 2659 | 2371 |

Figure 5.18. Colour of permeate and retentate during the microfiltration of cherry juice with ceramic membrane (Test 3). Filtration conditions: backshock of 0.010 s every 3s, linear velocity 0.5 m/s, and permeate flux 89-625 l/(m² h).
5.2.3.3 Membrane foulants
The pictures taken from the used, and the used and cleaned membrane showed that there was fouling at both sides of the membrane. The cleaning procedure could not remove all the foulants, and some of them could be seen still after cleaning forming a sponge like structure. Since the filtration was run from inside to outside (from the support layer to the skin layer), it is understandable that the foulants could be found at both sides of the membrane.
The amount of total organics in the ceramic membrane nr.3 was 1.93 mg/g incinerated membrane, whereas it was 0.13 mg/g incinerated membrane for the clean and unused membrane. The total amount of proteins in the used membrane was 217 m g/g dry membrane and 1.07 m g/g dry membrane for the unused membrane. In the chromatograms of the used membrane, the most abundant amino acids were glycine, aspartic acid, proline, serine and glutamic acid, whereas these were glycine, glutamic acid, serine, aspartic acid and histidine for the unused membrane. The peak of cysteine could be clearly detected for the used membrane, but not for the unused membrane. The total amount of phenols in the used membrane was 228 m g/g dry membrane and 72 m g/g dry membrane in the unused membrane. The total amount of proteins and phenols in the used membrane was only 0.45 mg/g, which was only 23% of the amount of total organics in the sample. Therefore, a large portion of the organic foulants stayed undefined. One of the main components of cherry juice, the sugars, were not analysed, and its role in the fouling formation is therefore unknown.
5.2.4 Conclusions
The membranes used proved to be extremely fragile. The brittle character of the membranes made them very difficult to handle, and they did not seem to suit to backshock filtration. However, it is obvious that the use of backshock improved the filtration performance, and much higher permeate fluxes could be achieved at lower transmembrane pressures than in filtration tests run without backshock. The 6 h filtration test run with backshock showed that the transmembrane pressure increased during filtration. This means that the membrane fouled during filtration. When backshock was not applied, an optimum in flux-pressure curve was observed, above which the increase on the transmembrane pressure resulted in a decrease on the flux.
Microfiltration of sour cherry juice did not have a significant effect on the colour, the sugar content, or the total phenols. The quality requirements for sugar content and colour were achieved except for a few exceptions, whereas turbidity was in most cases too high, and further treatment will therefore be needed.
The Scanning Electron Microscopy pictures taken from the used and the used and cleaned membrane showed that there was fouling on both sides of the membrane. The cleaning procedure could not remove all the foulants. Furthermore, most of the organic foulants stayed undefined.
[Front page] [Contents] [Previous] [Next] [Top] |