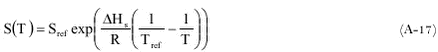Dry deposition and spray drift of pesticides to nearby water bodies
Appendix A. Calculation of the fraction of the pesticide in the gas phase in the soilDeriviation of the equation for the fraction in the gas phaseDerivation of Kd from Kom , Koc or Kow Derivation of KH from molecular weight, vapour pressure and solubility Temperature dependence of the vapour pressure Temperature dependence of the solubility Temperature dependence of the Henry’s law coefficient References Deriviation of the equation for the fraction in the gas phaseThe following set of equations is necessary to find the fraction of the pesticide in the gas phase (Smit et al., 1997). The Henry’s law coefficient gives the relation between the concentration of the pesticide in the gas and water phase:
where: Henry’s law coefficient can be determined directly or can be determined from the molecular weight, vapour pressure and the solubility in water of the pesticide (see section 1.3). Both the measured or calculated values can be uncertain. It is not unusual that for one compound Henry’s law coefficients are reported in the literature, which differ an order of magnitude. Henry’s law coefficient is rather temperature dependent (see section 1.4). The solid-liquid partitioning coefficient Kd gives the relation between the mass of pesticide adsorbed to the soil particles and the concentration in the water phase in the soil. If a linear sorption isotherm is assumed Kd the following equation is found:
where: Often the sorption is not linear and Kd is decreasing with increasing concentration in the water phase increases (Green and Karickhoff, 1990). Kd is not very temperature dependent (F. van den Berg, Alterra, Wageningen, personal communication, 2001). The total concentration of pesticide in the soil (in all phases) can now be described by:
where: Equation (3) can also be written as:
with the (dimensionless) capacity factor Q:
The dimensionless fraction of the pesticide in the gas phase is then:
KH and Kd should be known, or can be derived from other properties of the pesticide and/or the soil. θ air and θ water are usually not given, but have to be derived from the following parameters:
Dry soil consists of organic matter and mineral parts. The density of the solid part of the soil ρ soil,solid (kg m-3) is calculated from the information on the organic matter content and the densities of the organic and mineral parts of the soil:
As an intermediate step θair+water, the volume fraction of air and water together in the moist soil, can be found from:
When deriving this equation one should note that the difference between dry soil and moist soil is that part of the volume fraction of air of the dry soil is replaced by water in the moist soil. This means that the volume fraction of air in the dry soil is equal to the volume fraction of air and water together in the moist soil. The volume fraction of air θ air can then be found from:
Derivation of Kd from Kom , Koc or KowMany pesticides adsorb to organic matter in the soil, but not all. If the soil contains organic matter and the pesticide adsorbs mainly to organic matter, Kd can be calculated from Kom and the organic matter content (in %):
where: Kom = coefficient for sorption to soil organic matter (m3 kg-1). It should be noted that Kom is often given in (l kg-1) in the literature. If Kom is not known it can be estimated from Koc (Chiou, 1989):
where:
If no value of Kd is not availabe, it may be estimated with the methods presented here, but in that case the uncertainty in Kd becomes larger. Derivation of KH from molecular weight, vapour pressure and solubilityThe Henry’s law coefficient as defined by (A-1) can be found from the molecular weight, vapour pressure and solubility given in units that are often used:
where: Temperature dependence of the vapour pressureThe Clausius-Clapeyron equation describes the temperature dependence of the vapour pressure:
where: If it is assumed that Hv is constant with changes in temperature the following equation can be obtained (Lyman et al., 1990):
where: There are methods to estimate Hv if it is not known, but these methods are rather uncertain (Lyman et al., 1990). The heat of vaporisation has only been determined for a limited number of pesticides. Smit et al. (1997) found an average value for Hv of 95000 J mol-1 for about 15 pesticides. The values range from 58000 to 146000 J mol-1. In PESTDEP model a default value for Hv of 95000 J mol-1 is used if no values are known. Temperature dependence of the solubilityThe solubility as a function of the temperature can be found from:
where: Smit et al. (1997) found an average value for Hs of 27000 J mol-1 for about 11 pesticides. The values range from -17380 to 54350 J mol-1. In the PESTDEP model a default value for Hv of 27000 J mol-1 is used if no values for Hs are known. Temperature dependence of the Henry’s law coefficientThe relation for the temperature dependence of the Henry’s law coefficient is given by (Seinfeld and Pandis, 1998):
where: The relation between Henry’s law coefficients KH and H that are defined in different ways is:
From (A-15) and (A-16) the following function for KH as a function of temperature can be found:
KH generally increases with temperature. HA will be different for different compounds. ReferencesChiou, C.T. (1989) Theoretical considerations of the partition uptake of nonionic organic compounds by soil organic matter. In Sawhney, B.L., Brown, K.: Reactions and movement of organic chemicals in soils. SSSA special publication 22, Soil Science Society of America, Madison, Wisconsin, USA, 1-30. Green, R.E., Karickhoff, S.W. (1990) Sorption estimates for modeling. In: Cheng, H.H. (ed.) Pesticides in the soil environment: processes, impacts, and modeling. Soil Science Society of America Inc., Madison, Wisconsin, USA, 79-101. Lyman, W.J., Reehl, W.F., Rosenblatt, D.H. (eds.) (1990) Handbook of chemical property estimation methods, American Chemical Society, Washington DC, USA. Rao, P.S.C., Davidson, J.M. (1980) Estimation of pesticide retention and transformation parameters required in nonpoint source pollution models. In: Overcash, M.R., Davidson, J.M. (eds.): Environmental impact of nonpoint source pollution. Ann Arbor Sci. Publ., Ann Arbor, USA, 23-67. Seinfeld, J.H., Pandis, S.N. (1998) Atmospheric chemistry and physics. John Wiley, New York, USA, 342. Smit, A.A.M.F.R., van den Berg, F., Leistra, M. (1997) Estimation method for the volatilization of pesticides from fallow soil. Report Environmental Planning Bureau Series 2, DLO Winand Staring Centre, Wageningen, the Netherlands. |