|
| Front page | | Contents | | Previous | | Next |
The Influence of Sorption on the Degradation of Pesticides and other Chemicals in Soil
11 Discussion
Whether it is a matter of chemicals mainly bound to the soil organic matter or to the clay minerals, it is apparently a matter of basically the same model for the influence of sorption on the degradation as illustrated in Figure 24: Sorption and degradation take place in two dominating compartments, the aqueous phase and the soil phase. A part of the xenobiotic chemical may be degraded relatively rapidly in the first compartment, the aqueous phase, possibly through the formation of identifiable metabolites, and can be completely mineralised to CO2. If the substance is not transformed sufficiently rapidly, it may be leached. Both the xenobiotic chemical or metabolites from this may be adsorbed to soil particles through a number of different binding mechanisms, and the substances may partly be desorbed from this again and go back into the aqueous phase where they may be degraded or leached. From being more "loosely" bound to the soil particles, the substances may be more "firmly" bound whereby it becomes a matter of "bound residues". "Bound residues" appear in various forms: The entire molecule of the xenobiotic or parts of it may have entered a new molecular structure as a part of the organic matter, it may be sequestrated in inaccessible places in the soil aggregates or it may be strongly sorbed. There is a difference on what can be extracted by chemical methods and what can be activated purely biologically. The dotted lines (A) in Figure 24 represent substances that can be extracted with extraction agents that do not change the chemical structure of the substances, the soild lines (B) represent the bound residues that can be extracted with stronger extraction agents, and the dashed lines (C) represent the bound residues that cannot be extracted chemically. In each of the three groups, there may be subgroups that may be/become bioavailable to both plants, animals, and degrading microorganisms, as these subgroups are substances that can be reactivated as the original molecule or a metabolite from this after which it can be degraded or leached (process (1)). Other parts of the substances may, in the strongly bound structure in which they form part, be directly transformed to CO2 by the microorganisms as a constant transformation of the organic pool takes place (process (2) in Figure 24). The fundamental principles – the model according to which the substances are degraded – are as mentioned before the same for all types of substances. The variations that are caused by the chemical structure of the substances and the soil texture are seen in the distribution of the amounts of substance in the two compartments, in the distribution of the amounts of substance in the individual subgroups A, B, and C, and in how much of the amounts in A, B, and C that can be transformed according to processes 1 and 2, respectively. When the substances are bound to the clay minerals, they will not be transformed according to process 2 but only according to process 1. If the part that is transformed according to process 1 is small it will result in larger amounts remaining in the soil as bound residues. The crucial question here is whether there may be changes in the soil that can lead to an increased reactivation and whether this reactivation may lead to the occurrence of biologically active reactivated amounts of the substance that may have an adverse effect on the soil environment or may be leached before it is degraded.
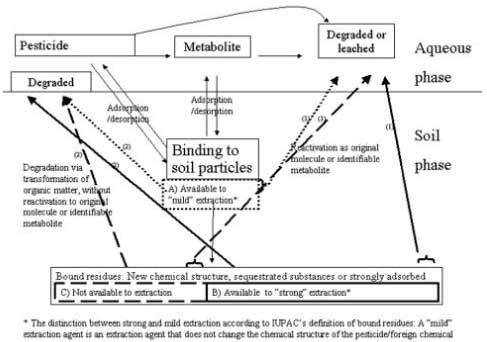
Figure 24. Outline of the problems concerning the influence of sorption on the degradation of pesticides and other chemical in soil.
Figur 24. Oversigt over problematikken omkring sorptionens indflydelse på nedbrydningen af pesticider og andre kemiske stoffer i jord.
The degradation in the second slow compartment is considerably slower than the average degradation rate that usually can be calculated from a simple first-order process, but at the same time the degradation is also considerably quicker in the first compartment than it is when a simple first-order process is used. The choice of model may not be particularly crucial for some substances, but for others where the course is special in one compartment it may be crucial. As far back as 25 years ago, Hamaker and Goring (1976) described that the degradation of pesticides depends very much on the extent to which the substance is bound to the soil particles and that the degradation therefore can be described as taking place in two compartments. At that time, results had to be marked and calculated on semilogarithmic paper, so the simple first-order kinetics has often been assumed for the calculation of degradation rates. The parameters in more complex models can now be calculated by means of a computer, so the time should have come where the importance of using one or the other model for the evaluation of the tendency towards leaching of substances is assessed. It is therefore highly relevant that a number of comparisons are made of the calculated remaining amounts of substances after certain intervals during the application of the relevant simple first-order model and the two-compartment model, so that there is a basis for assessing whether the approval system for pesticides should be changed to allow for this fact, and that the assessment of the persistence of the substances according to a new approval system is carried out on the basis of calculations in which the two-compartment model has been used. It is necessary to take a position on the biphasic system for degradation and identify the different results of the most frequently used models in leaching scenarios.
Such model runs will, however, only be a step on the way to a better description. Soil that has a higher content of organic matter will in some cases delay the degradation and the leaching of chemicals due to the stronger binding. At the same time the larger amount of organic matter may result in a higher microbiological activity, which increases the degradation rate for pesticides. It is imperative that more studies are carried out in which measurements of sorption and measurements of the degradation are linked, e.g. through model descriptions of individual measuring results of sorption and degradation, respectively, or through practical experiments, in which the sorption and the degradation are measured in the same experiment process and a model is developed that describes the results. The latter, so-called reverse modelling, must be assumed to be the best solution. The published examples, in which such models have been developed, have naturally concentrated on individual substances. A test of these different models on a large and varied data material would provide the best basis for assessing which of these models allow for the varying soil conditions to the greatest extent. Such a model might then be built into the most frequently used leaching models as a substitute for the existing sorption and degradation modules, which use Kd-value determinations for sorption specifications and simple first-order half-lives for specification of degradation rates.
The error that is made by using a simple first-order model as the basis for calculations of half-lives varies according to the other circumstances in the soil. From Figure 10 is seen that the half-life is estimated at 15 days according to the simple first-order process whereas it is just 4.2 days for the first halving according the two-compartment model. If the part of the substance that is transformed in the second compartment is assumed only to have been extracted due to the application of an organic extraction agent and otherwise will not be available to the biology of the soil or to leaching to the groundwater, then the substance has been assessed harder than what could be regarded as reasonable at the application of the simple first-order process. If, on the other hand, the amount that is transformed in the second compartment in reality is bioavailable or can be leached to the groundwater, the substance is judged too mildly at the application of the simple first-order process as three halvings are estimated at just 45 days while it takes 62 days according to the two-compartment process. Thus, it is not just a question of a need to find the right model for describing the sorption-degradation process but also the question of a need to throw light upon the type of binding and the bioavailability of the amounts of substance that are transformed in the second compartment.
If the interesting factor is how much of the pesticide present in the soil that may have a toxic effect on soil organisms, a traditional chemical analysis will provide an overestimated result because the chemical analysis is traditionally carried out with extraction agents that extract as much of the substance as possible while after all a toxic effect on soil organisms only occurs if the substance is present in the aqueous phase. In the same way a traditional chemical method of analysis will often overestimate the amount of chemical that might be a threat to the groundwater.
If the two-compartment model is taken into account in connection with approvals and in model runs, the problem concerning bound residues must also be taken into account as it has been extremely much in fOC us during the last 10 years.
There are already a large number of studies of binding mechanisms for both desorbable xenobiotics and bound residues. However, it is necessary to couple these studies to studies of bioavailability to determine to which extent the substances can be reactivated in the natural system. Adsorption/desorption is a balance that over time may be disturbed and adjusted in a new balance where parts of bound substance will possibly be released. It would be very appropriate if there were chemical methods of analysis able to extract the amounts of the xenobiotics from the soil that could be correlated with the bioavailable amounts that are the results of biological studies of bioavailability. For the time being the biological studies are necessary to measure bioavailability, but they are expensive and difficult. The work on developing such methods should be intensified. There has been much fOC us on developing chemical methods of analysis that can extract everything, so the chemical laboratories needs to adapt themselves to fOC us on measurements of bioavailable amounts.
It is important to emphasize that laboratory experiments that are intended to be used for describing the complex connection between the binding and the degradation must allow for the extreme variation of the processes. There is already a large variation, which is merely caused by the heterogeneity of the soil. The part of the variation, which may be caused by the method, must be eliminated. Here, the first step must be to carry out the studies under conditions that resemble the natural conditions as much as possible, that is by application of the concentrations of pesticides that will be relevant according to normal farming practice, incubation at temperatures, water content, and oxygen conditions that conform to the climate and the soil where the pesticides are used. Further, it is important to establish to which extent the degrading microorganisms are influenced by the sieving and mixing of the soil that is frequently carried out before the experiments. If the microorganisms and with them the degradation process are significantly affected by this, experiments can be carried out in microcosmos setups with undisturbed soil columns where the conditions of the microorganisms are not changed.
In theory, one might imagine that the evaluation of the persistence of pesticides by the approval system required studies of degradation and sorption by methods closer to reality than the ones used at present and that these studies had to be carried out in a number of different soils with a varying content of organic matter and clay. At the same time it might be required that the degradation process was described by the two-compartment model. Different limit values for the degradation rate of the pesticides in the two compartments might be introduced. It may be accepted that the degradation rate in the second compartment (where the substance is bound) has a lower value, but this value must be dependent on the quantitative distribution of the pesticide in the two compartments. A demand for supplementary measurements of microbiological activity in the soil could also be attached to the system to ensure that the microbiological activity in the used soil samples was within a standard area. Both from a legal and a scientific point of view it is important to differentiate between free pesticide residues and bound pesticide residues just as it is important to distinguish between biological persistence and chemical persistence when persistence is in question, as these do not always go together. Therefore, it might be assumed that the approval system required a distinction between different types of extraction agents in the analysis of degradation and sorption measurements, so it was possible to predict the possibility of reactivation of the remaining amounts of substances.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2004, © Danish Environmental Protection Agency
|