|
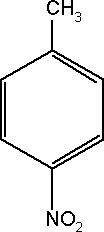
Substance Flow Analysis of 4-nitrotoluene 1 Introduction1.1 Purpose of the analysis 1.1 Purpose of the analysisThe purpose of this substance flow analysis has been to investigate the use, consumption and dissemination of 4-nitrotoluene in Denmark. The report presents the existing (and limited) knowledge about the use of 4-nitrotoluene in Denmark. It includes import/export, use, consumption, content in products and waste flows as well as the exposure of humans and the environment. A human and ecotoxicological assessment has been carried out as part of the project and included in the report. 1.2 Methodology and limitationsThe investigation is made as an overview analysis of the flows of 4-nitrotoluene in Denmark. The investigation was supposed to be carried out according to the guidelines for substance flow analysis presented by the Danish EPA (Lassen & Hansen, 2000), identifying the main fields of application and the sources of environmental exposure related to 4-nitrotoluene. However, lack of data has made it unsuitable for a full substance flow analysis. Therefore, only this short report has been prepared. The investigation was carried out during autumn 2003. The information and figures reported are in general from 2000 to 2002, but there are few exceptions, which have been specified in the text. 1.3 What is 4-nitrotoluene?4-nitrotoluene [Cas. No. 99-99-0] is one of the three structural isomers of nitrotoluene. The isomers are produced commercially, as a mixture, by nitration of toluene. Figure 1-1 illustrates the molecular structure of 4-nitrotoluene.
Figure 1-1. The molecular structure of 4-nitrotoluene. All three isomeric mononitrotoluenes are industrially important products obtained from the sequential nitration of toluene. After distillation of the meta fraction from the isomer mixture, the still residues are cooled in a crystallizer to separate technical quality 4-nitrotoluene. Further distillation of nitrotoluene residues and fractions should be implemented with great care because this has been reported to cause explosions. Holding residues at 150-200 °C results in an undefined “aging” process that can lead to unpredictable evolution of heat, especially if air is introduced (Iuclid, 2000). 1.3.1 SynonymsVarious synonyms and abbreviations of 4-nitrotoluene are used in the literature. In this report the chemical name 4-nitrotoluene is used. Some of the synonyms used include methyl nitrobenzene; 1-methyl-4-nitrobenzene; 4-methylnitrobenzene; p-methylnitrobenzene; p-nitrophenylmethane; 4-nitrotoluol; PNT (IUCLID, 2000). 1.3.2 Physical and Chemical Properties4-nitrotoluene is a mass of colourless or yellowish crystals/rhombic needles with the following properties (NTP, 2003):
1.4 International market and Trends in consumptionApproximately 20,000 tons of 4-nitrotoluene is produced annually in the EU (European Commission, 2001). The global production is expected to be between 50,000 – 100,000 tons/year (Iuclid, 2000). Worldwide 4-nitrotoluene is produced in Germany, Belgium, Italy, United Kingdom and the United States (Iuclid, 2000). 4-nitrotoluene is an important commercial chemical used to synthesize agricultural and rubber chemicals, azo and sulfur dyes, and dyes for cotton, wool, silk, leather, paper and explosives. 4-nitrotoluene is used as an intermediate for plastic foams, dyestuffs, paints and pharmaceuticals (Dunnick, 1992). 4-nitrotoluene derivatives are used primarily as intermediates for colorants and related products; for example, p-toluidine, 4-nitrobenzoic acid (by oxidation of 4-nitrotoluene with 15 % HNO3 at 175 °C), 4-amino-2-chlorotoluene (by reduction of 2-chloro-4-nitrotoluene), and 4-nitrotoluene-2-sulfonic acid, which is of great importance in forming stilbene intermediates for fluorescent whitening agents (Ullmann's, 2002).
|
||||||||||||||||||||