|
Environmental Project nr. 944, 2004 Evaluation of Health Hazards by exposure to Triazines and Degradation ProductsContents
7 Risk characterisation via drinking water exposure 1 General description
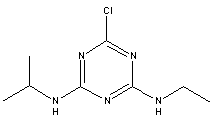
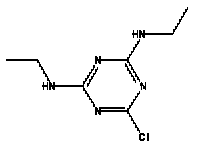
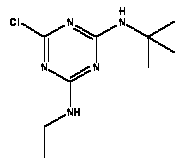
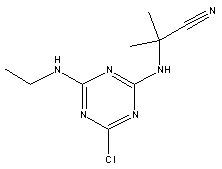
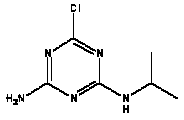
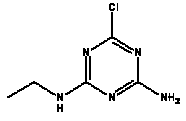
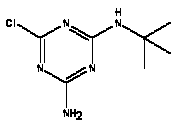
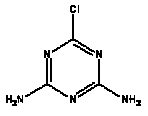
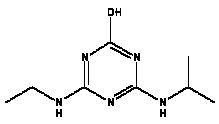
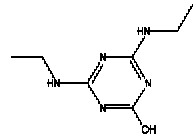
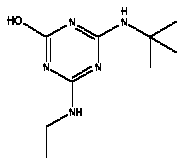
Atrazine, simazine, terbutylazine and cyanazine are triazines, which have been used (atrazine, cyanazine) or still are in use (simazine, terbutylazine) in agriculture as herbicides in Denmark (MST 2003b). Desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyl terbutylazine, desethyldesisopropyl atrazine (DACT), hydroxyatrazine, hydroxysimazine, and hydroxyterbutylazine are some of the degradation products of these triazines (IARC 1999a,b, Jørgensen 2002, WHO 1996a,b, WHO 1998a,b). This document does not necessarily include all studies that have been performed with the triazines and their degradation products. The included studies are viewed as representative for the toxicology of these chemical substances. In this and in subsequent chapters, no data were found for a triazine or its degradation products if the chemical is not mentioned. 1.1 IdentityName: Molecular formula: Structural formula: a) b) c) d) e) f) g) h) i) j) k) Molecular weight: CAS-no.:
a) 1912-24-9
Synonyms: b) 6-Chloro-N,N'-diethyl-1,3,5-triazine-2,4-diamine; 2-Chloro-4,6-bis(ethylamino)-s-triazine; 2-Chloro-4,6-bis(ethylamino)-1,3,5-triazine; 1-Chloro-3,5-bis(ethylamino)-2,4,6-triazine c) 6-Chloro-N-(1,1-dimethylethyl)-N'-ethyl-1,3,5-triazine-2,4-diamine; 2-tert-Butylamino-4-chloro-6-ethylamino-1,3,5-triazine d) 2-Chloro-4-ethylamino-6-(cyano-1-methyl)-(ethylamino)-1,3,5-triazine; 2-[[4-Chloro-6-ethylamino-1,3,5-triazine-2-yl]amino]-2-methylpropanenitrile e) 6-Chloro-N-(1-methylethyl)-1,3,5-triazine-2,4-diamine; 2-Amino-4-isopropylamino-6-chloro-1,3,5-triazine f) 6-Chloro-N-ethyl-1,3,5-triazine-2,4-diamine; 2-Amino-4-chloro-6-ethylamino-1,3,5-triazine g) 6-Chloro-N-(1,1-dimethylethyl)-1,3,5-triazine-2,4-diamine; 2-Amino-4-tert-butylamino-6-chloro-1,3,5-triazine h) 6-Chloro-1,3,5-triazine-2,4-diamine; 2-Chloro-4,6-diamino-1,3,5-triazine; diaminochlorotriazine i) 4-ethylamino-6-[(1-methylethyl)amino]-1,3,5-triazin-2 (1H)-one; 2-hydroxy-4-ethylamino-6-isopopylamino-s-triazine 1.2 Physical / chemical propertiesDescription: Purity: Melting point: Boiling point: Density: Vapour pressure: Flash point: Flammable limits: Autoignition temp.: Solubility: Considerably more soluble (110-183.000 mg/l) in organic solvents (acetone, chloroform, dichloromethan, diethyl ether, dimethyl sulfoxide, ethanol, ether, ethyl acetate, n-hexane, methanol, n-octanol, toluene) than in water b) Water: 5 mg/l (at °C) c) Water: 8.5 mg/l (at 20°C) d) Water: 171 mg/l (at 25°C) Considerably more soluble (15.000-210.000 mg/l) in organic solvents (benzene, chloroform, ethanol, hexane) than in water logPoctanol/water: pKa-value: Stability: a,b,d) Relatively resistant to decomposition by ultraviolet radiation Incompatibilities: No data were found Odour threshold, air: Odour threshold, water: Taste threshold, water: References: 1.3 Production and useAtrazine, simazine, terbutylazine and cyanazine are produced by the reaction of 2,4,6-trichloro-1,3,5-triazine with appropriate intermediates (US-EPA 2002). For instance, atrazine is produced by a reaction between 2,4,6-trichloro-1,3,5-triazine and isopropyl amine under basic conditions followed by a reaction with monoethyl amine and dilute caustic (IARC 1999a). Atrazine, simazine, terbutylazine and cyanazine are used in agriculture, in forestry and/or in nurseries as herbicides. Atrazine has also been used as a soil sterilant for airfields, parking lots and industrial sites and as an algaecide in swimming pools. In the European Union, where a limit of 0.1 μg/l has been set for all pesticide residues in drinking- and groundwater, the use of atrazine-containing herbicides has been limited mainly to agricultural uses on corn and on sorghum. (IARC 1999a,b, WHO 1996a,b, WHO 1998a,b). In Denmark, atrazine has not been sold since 1994 (Brüsch 2002). Today, both atrazine and cyanazine are forbidden to sell or use in Denmark. Simazine is allowed for use in Denmark in forestry and nurseries on a limited amount of plants, bushes and trees. Terbutylazine is allowed for use in Denmark in forestry and in corn for fodder. (MST 2003b). But simazine-containing products will be withdrawn in EU in 2004-5 and products containing terbutylazine are being reviewed. 1.4 Environmental occurrenceThere are no known natural sources of atrazine. Virtually the entire production volume is released to the environment, primarily soils, mainly as a result of agricultural and other weed-control practices. (ATSDR 2001). 1.4.1 AirAtrazine has been detected in the atmosphere both nearby and distant from areas where it has been applied as a pesticide and has been detected up to 300 km from the closest application area. The concentration of atrazine in air will vary with application season and measured concentrations have ranged from just above the detection limit (about 0.03 ng/m3) to more typical concentrations of 0.2-0.3 μg/m3 (measured in regions in and around Paris, France). Once in the air, atrazine will exist in both the particulate and vapour phases, but with a tendency to exist more in the particulate phase than in the vapour phase. Atrazine has been found in the vapour phase in the atmosphere in association with fog and rainwater and is commonly found in rainwater in the seasons following agricultural applications; a concentration of up to 1 g/l has been found in Ohio, USA. (ATSDR 2001, IARC 1999a). Simazine has been detected at low concentrations in ambient air and rainwater (IARC 1999b). 1.4.2 WaterAtrazine is found in surface and groundwater and in drinking water wells as a result of its application to crop fields as a herbicide as well as from its disposal. Table 1 contains data from 1993 to 2001on triazine concentrations measured in Danish ground water and in Danish ground water abstraction wells. The triazines and their degradation products are found both in ground water rich in and deficient in oxygen and also in ground water containing high concentrations of nitrate. (Jørgensen 2002). Results from an investigation of 628 small water supply plants in 4 different counties (København, Sønderjylland, Storstrøm, Viborg) in Denmark also included analyses of selected triazines, Table 1. Small water supply plants are defined as wells or borings providing water for 1-9 households. The data are based on double water samples from all 628 plants. Analysis was not performed for desethyldesisopropyl atrazine (DACT) and for the hydroxy triazines, which were found in the national ground water monitoring and in the ground water abstraction wells. (Brüsch et al. 2004). Atrazine, cyanazine, simazine and terbutylazine have also been detected in surface- and/or groundwater at concentrations above 0.1 g/l in other European countries (WHO 1996a,b, WHO 1998a,b). The concentration of atrazine varies during the season in waters receiving runoff from agricultural lands with the highest concentrations generally being found during the 1½ to 2 months after application. Typically, atrazine is found more frequently and usually at lower concentrations in groundwater than in surface water. Simazine and its degradation products are detected less frequently and at lower concentrations than atrazine. (IARC 1999a,b). Table 1. Triazine herbicides or degradation products in Danish water.
1.4.3 SoilThe triazines are commonly found in agricultural soils following their application to crop fields as herbicides. They can also be detected in soils that have been impacted by runoff or by atmospheric deposition. Following application to crop soils, most atrazine is found at the highest concentrations in the upper layers of soil, as a result of sorption. Atrazine has been shown to be relatively mobile in soils and it can leach through the soil column and contaminate groundwater. Transport through soil occurs via macro pores, along roots and through earthworm burrows. The rate of transport is dependent on many soil factors including the soil type, the amount of water that is applied to the soil, the presence of crop residues, and the types of fertilisers used. Atrazine has been reported to move more rapidly through soils than its breakdown products. (ATSDR 2001). An investigation of the dry soil concentration of triazines and their degradations products in a limited number of point sources (gardening, machine pools, feedstuff businesses, and nurseries) in Denmark has shown the following results (AVJ 2001):
1.4.4 FoodstuffsSee chapter 5 for maximum residue limits (MRLs) in foods. No residues (< 6 μg/kg) of atrazine were found in 1339 samples of fruit and vegetables in Denmark in 2002. Out of 1339 fruit and vegetable samples, simazine was found in only one (a lemon from Spain) at a concentration of 9 μg/kg. Analysis was not performed for cyanazine, terbutylazine, and any of the degradation products. (Andersen et al. 2003). No residues (< 50 μg/kg) of atrazine, simazine or cyanazine were found in more than 75000 food samples in USA. Uptake of atrazine in corn and sorghum is relatively low and the metabolism is rapid. (IARC 1999a,b). However, hydroxy metabolites of atrazine have been found in plants grown in soil treated with atrazine (WHO 1996a). No residues (< 500 μg/kg) of terbutylazine were found in almost 7000 vegetable and fruit samples in USA (WHO 1998b). 1.5 Environmental fateTriazines can be degraded by biological or chemical (e.g., photolysis) processes via N-dealkylation and hydrolysis of the chloro substituent. Atrazine is degraded slowly in most environmental compartments, whether by biological or chemical processes. Chemical (abiotic) degradation of atrazine occurs by hydrolysis to hydroxyatrazine; no direct photolytic degradation has been detected. Biodegradation of atrazine occurs primarily by dealkylation resulting in the formation of desethyl atrazine (DEA), desisopropyl atrazine (DIA) and desethyldesisopropyl atrazine (DACT). Hydroxyatrazine can also be formed by biodegradation. Biodegradation may lead to a complete degradation of atrazine, but this is not always observed. In some cases, atrazine residues become incorporated into unextractable residues, which are considered to be less bioavailable than the free parent or metabolite compounds. (ATSDR 2001). Terbutylazine is degraded to e.g. hydroxyterbutylazine, desethyl terbutylazine, desisopropyl atrazine (DIA), and desethyldesisopropyl atrazine (DACT). (Brüsch 2002, WHO 1998b). 1.5.1 AirIn the atmosphere, atrazine will exist in both the particulate and vapour phases. Particulate phase atrazine will be removed from the atmosphere by wet and dry deposition. Vapour phase atrazine can be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; a half-life of 14 hours has been estimated. Atrazine has not been observed to undergo direct photolytic degradation in the atmosphere. (ATSDR 2001). Terbutylazine in air is degraded by reactions with hydroxyl radicals with a half-life of about 1.5 days (WHO 1998b). 1.5.2 WaterAtrazine is stable to abiotic hydrolysis at pH 5, 7 and 9. The photolysis in water is very slow with an estimated half-life of 805 days. In controlled aerobic water-sediment systems atrazine disappeared from the water phase with a half-life of 28 - 134 days, while the degradation half-life was found to be 45 - 253 days for the whole system. (EC 1996a). Simazine is stable to abiotic hydrolysis at pH 5, 7 and 9. The photolysis in water is described with contrasting half-lives from 0.7 to 382 days. In controlled aerobic water-sediment systems the dissipation half-life for the water phase was 12 - > 77 days, while the degradation half-life was 26 - 201 days for the whole system. (EC 1996b). The abiotic hydrolysis of terbutylazine occurs only slowly with a half-life of 73 days (pH 5), 205 days (pH 7) and 194 days (pH 9). The photolysis in water also seems to be slow with reported half-lives of > 40 to 70 days and 172 days in the presence of photosensitisers. Terbutylazine shall be classified not ready biodegradable. In two controlled aerobic water-sediment systems terbutylazine disappeared from the water phase with half-lives of 6 and 7 days, while the degradation half-lives were 33 and 80 days for the whole system, respectively. High microbial activity enhanced the degradation. (MST 2003a). 1.5.3 SoilUnder aerobic conditions at 20°C in the laboratory the half-life of atrazine was in the range of 41 – 146 days with an average of 81 days. The mineralisation was very slow with a reported CO2 production of 6 % in 300 days. An anaerobic half-life of 160 days has been reported. Field studies carried out in Switzerland, Austria, France and USA showed half-lives in the range of 5 - 60 days with an average of 29 days. Regarding ground water contamination atrazine and the three metabolites desethyl atrazine (DEA), desisopropyl atrazine (DIA) and hydroxyatrazine are in risk to possess an unacceptable leaching. (EC 1996a). The aerobic half-life of simazine at 20°C in the laboratory was in the range of 20 – 270 days with an average of 53 days. The anaerobic degradation rate was comparable to the aerobic rate. The mineralisation accounted for 19 % in 120 days. Field studies conducted in Switzerland, UK, Germany, Italy, Netherlands and Sweden showed half-lives in the range of 14 - 146 days, average rates were 64 and 130 days at spring and fall application, respectively. Regarding ground water contamination the highest risk comes from the metabolite desisopropyl atrazine (DIA). (EC 1996b). Degradation of terbutylazine under aerobic conditions at 20°C in laboratory studies occurred with half-lives in the range of 61 – 169 days with a median value of 79 days (n=10). High microbial activity enhanced the degradation. Ten metabolites with the triazine structure intact have been identified. The mineralisation was very slow with an average CO2 production of about 5 % in half a year. Under anaerobic conditions the degradation was somewhat slower and no mineralisation was observed. Field studies carried out in Germany and Switzerland showed half-lives of terbutylazine in the range of 10 - 36 days with a median of 22 days (n=7). Regarding ground water contamination the most important metabolites from terbutylazine were hydroxyterbutylazine and desethyl terbutylazine, the former having the highest leaching potential. The leaching potential of terbutylazine is less than the potential of the two metabolites mentioned. Hydroxyterbutylazine can be produced both abiotic and biotic, while desethyl terbutylazine is only produced under biotic conditions. Therefore, risk to groundwater from hydroxyterbutylazine is highest when terbutylazine is applied under conditions not favourable for the microbial processes. The lowest risk to ground water pollution in Denmark was found to be from soils with a high amount of organic matter and a high biological activity as exemplified by cornfields receiving manure every year. (MST 2003a). The half-life is commonly 14-98 days for cyanazine (WHO 1998a). 1.5.4 BioaccumulationAtrazine has a slight to moderate tendency to bioconcentrate in microorganisms, algae, aquatic invertebrates, worms, snails, or fish (ATSDR 2001). 1.6 Human exposureOccupational exposure to triazines may occur through dermal contact or inhalation during the manufacture, formulation or application of the herbicides. (IARC 1999a,b). The general population may be exposed to the triazines and their degradation products through their widespread occurrence in the environment especially in drinking water. No significant exposure is expected to occur via foodstuffs. 2 Toxicokinetics2.1 Absorption, distribution and excretion2.1.1 InhalationNo data were found. 2.1.2 Oral intakeRats dosed orally with radioactively marked atrazine excreted 65-67% of the dose in urine within 72 hours indicating an extensive absorption by this route. About 20% of the dose was found in faeces, 4-16% was found in tissues, and only 0.1% was found in expired air at 72 hours following dosing. In tissues, the highest concentrations were found in erythrocytes, liver, spleen, kidneys and lungs. The plasma concentration of atrazine in rats peaked 8-10 hours after dosing. The elimination half-time was 11 hours. (Studies quoted from ACGIH 1991, ATSDR 2001, IARC 1999a, US-EPA 2002b, WHO 1996a). Rats dosed orally with radioactively marked simazine excreted 49% of the dose in urine within 96 hours. The highest tissue concentrations were found in spleen, liver, and kidney in mice and rats. (Studies quoted from US-EPA 2002b, WHO 1996b). At least 60% of an oral dose of terbutylazine was absorbed in rats, completely metabolised, and excreted via the urine and bile with a half-life of 16-17 hours. The highest tissue concentrations were found in kidney, liver and blood. (Studies quoted from WHO 1998b). Cyanazine was rapidly absorbed from the gastrointestinal tract of rats, dogs and cows. In rats, 80-88% of the administered dose was eliminated within 4 days with almost equal amounts in urine and faeces. Only 3% was found in tissues after 4 days. Cyanazine was detected in cows' milk. (Studies quoted from ACGIH 1991, WHO 1998a). 2.1.3 Dermal contactIn humans, 90-94% of an applied dose of technical atrazine (by dermal patches) remained on the skin 24 hours following application, but only 0.3-5.1% of the applied dose was recovered in the urine and faeces within 7 days after application. An in vitro study using human skin samples showed that about 16% of atrazine was absorbed in a 24-hour period but most of the absorbed atrazine (12% of the applied dose) remained in the skin. (Studies quoted from ATSDR 2001). In one study, absorption of atrazine through rat skin was limited amounting to less than 2% after a 10 hour exposure. In other rat studies, an inversely dose-dependent absorption (3-26%) of atrazine through skin was demonstrated. In some of these studies atrazine was in an aqueous formulation. In one of the studies it was stated that the majority of the "absorbed" dose was found in the skin application site after washing and up to 5% was detected as systemically absorbed. More than 50% of atrazine was absorbed through rat skin when it was administered as a solution in ethanol or tetrahydrofuran. (Studies quoted from ATSDR 2001, IARC 1999a, EC 1996a, WHO 1996a). Following dermal application of simazine to rats, less than 1% of the applied dose was absorbed through skin after a 24 hour exposure period. 20-40% of the applied dose was found in the skin application site. (Study quoted from EC 1996b). Following dermal application of terbutylazine to rats, 30% of the applied dose was found in urine and faeces (Study quoted from WHO 1998b). 2.2 MetabolismThe main biotransformation pathways for atrazine, cyanazine, simazine and terbutylazine in rats are N-dealkylation by the hepatic cytochrome P450 system, and glutathione conjugation of either the parent compound or the N-dealkylated metabolite to the ultimately excreted mercapturic acid conjugate (US-EPA 2002b). Atrazine is extensively metabolised. In urine, unchanged atrazine accounted for less than 2% of all atrazine-related compounds after dermal exposure in humans or oral exposure in rats. Figure 1 shows the metabolism for atrazine. Several studies have shown that the major urinary metabolite in rats dosed orally with radioactively labelled atrazine was desethyldesisopropyl atrazine (DACT), which accounted for more than half of the total urinary radioactivity. The other reported urinary metabolites in rats were desethyl atrazine mercapturate, desethyldesisopropyl atrazine mercapturate (diaminotriazine mercapturate in Figure 1), desethyl atrazine (DEA), desisopropyl atrazine (DIA), and ammeline. In humans, the same urinary metabolites have been detected except for ammeline following dermal exposure. In addition, desisopropyl atrazine mercapturate and atrazine mercapturate have been found in human urine. In humans, desethyldesisopropyl atrazine (DACT) and desisopropyl atrazine (DIA) seems to be the major metabolites. Rats and humans produced the same type of metabolites following exposure to atrazine, but species-specific differences in the metabolite ratios were found. (Several studies quoted from ATSDR 2001, IARC 1999a, US-EPA 2002b). Desethyldesisopropyl atrazine (DACT) and desisopropyl atrazine (DIA) has been detected as urinary metabolites in rats following oral dosing with simazine. Like for atrazine, conjugated mercapturates have also been detected (Studies quoted from US-EPA 2002b, WHO 1996b). The main metabolic degradation of terbutylazine occurs through N-dealkylation (creating desethyl terbutylazine), oxidation of one methyl group of the tert-butyl group, and subsequent conjugation of the alcohol with glucuronic acid. Minor pathways have been described with glutathione conjugation like for atrazine and simazine, with the formation of hydroxytriazine metabolites, and with the formation of sulphate esters of the alcohol derivative. (Study quoted from WHO 1998b). The major route of metabolic degradation of cyanazine occurs through N-dealkylation (creating desethyl cyanazine) followed by conjugation with glutathione (Studies quoted from WHO 1998a). Figure 1. Metabolism for atrazine (Copied from US-EPA 2002). 2.3 Mode of actionTreatment of laboratory animals with atrazine (and some of the other triazines and metabolites) results in toxic effects such as attenuation of the luteinizing hormone (LH) surge, and as a consequence alteration of the oestrous cycle, altered pregnancy outcome, delayed pubertal development, and mammary gland tumours. The mammary gland tumours are only found in female Sprague-Dawley rats (and not in Fischer 344 rats, CD-1 mice or ovariectomised Sprague-Dawley rats). Several non-guideline studies have been performed to elucidate the mechanism behind those effects. The primary proposed mode of action for these effects involves disruption of the hypothalamic-pituitary-gonadal axis in a manner very similar to the known mechanism of reproductive senescence in some strains of rats. According to this hypothesis, atrazine affects the hypothalamus leading to a decreased secretion of hypothalamic norepinephrine (NE). Decreased NE levels result in decreased release of gonadotrophin releasing hormone (GnRH) from the hypothalamus. GnRH stimulates release of follicle stimulating hormone (FSH) and LH from the anterior pituitary. Thus, a decreased GnRH level leads to a low serum level of LH (and FSH). LH normally provides a signal to the ovaries promoting ovulation, but low serum levels leads to anovulation. Under these circumstances, the ovarian follicles continue to secrete oestrogen leading to a physiological state of prolonged or persistent oestrus. Increased oestrogen stimulates the pituitary leading to hypertrophy and consequently an increased secretion of prolactin. A constant elevated serum level of prolactin and oestrogen may result in tumour formation in sensitive tissues such as the mammary glands. (Several studies quoted from ATSDR 2001, IARC 1999a, US-EPA 2002b). Alternative modes of action for the neuroendocrine effects following exposure to triazines have been suggested. Although several studies have found that the estrogenic effects associated with the triazines are not oestrogen receptor-mediated, these effects may be explained partly by their ability to induce aromatase, the enzyme responsible for converting androgens to estrogens. Recent studies demonstrated that atrazine and simazine and the metabolites desethyl atrazine (DEA) and desisopropyl atrazine (DIA) but not desethyldesisopropyl atrazine (DACT) induced aromatase activity in various cell lines. It has also been suggested that the anorexic effects of atrazine could account for most of atrazine's effects on LH since reduced food intake and weight loss is a potent stimulus for reduced LH. However, in pair-fed studies in both males and females, decreased food consumption and body weight could not account for the adverse effects of atrazine on the oestrous cycle and pubertal development. (Sanderson et al. 2001, several studies quoted from US-EPA 2002b). IARC and US-EPA have concluded that the mechanism by which atrazine increases the incidence of mammary gland tumours in Sprague-Dawley rats is not relevant to humans because of critical interspecies differences in hormonal changes associated with reproductive senescence. In women, reproductive senescence is characterized by ovarian depletion, declining oestrogen levels, and, eventually, dioestrus. While the pattern of reproductive senescence in female Fischer 344 rats is not identical to that of women, Fischer 344 rats share the following features with women, in contrast to female Sprague-Dawley rats: later onset of senescence, low oestrogen concentrations during late life and an ability to control the luteinizing hormone secretion during reproductive senescence. (US-EPA 2002b, several studies quoted from IARC 1999a). Nevertheless, US-EPA has also concluded that it is not unreasonable to expect that atrazine might cause adverse effects on hypothalamic-pituitary function in humans and that the same endocrine perturbations that induce tumours also appear to play a role in at least some reproductive developmental effects which may be relevant to humans. (US-EPA 2002b). Because of a common mode of action and for purposes of cumulative risk assessment, US-EPA has grouped atrazine, simazine, propazine and their degradation products desethyl atrazine (DEA), desisopropyl atrazine (DIA), and desethyldesisopropyl atrazine (DACT). Although hydroxyatrazine has been shown to alter pregnancy and delay puberty in males it was not included in this group based on the absence of mammary gland tumour induction and inconclusive data on its effect on the LH surge and/or LH-dependent events. Terbutylazine and cyanazine was not considered for grouping because they did not have uses that resulted in exposure to the general public. (US-EPA 2002b). 3 Human toxicity
3.1 Single dose toxicityNo data were found. 3.2 Skin irritationA case-report exist of a male farmer who was diagnosed with acute contact dermatitis on his hands and forearms on the same day as he had been exposed dermally to atrazine and cyanazine (Schlicher and Beat 1972 – quoted from ATSDR 2001). A total of 124 cases of contact dermatitis were noted in the former USSR among workers manufacturing simazine and propazine. Serious cases lasting 7-10 days involved erythema, oedema, and a vesiculopapular reaction that sometimes progressed to the formation of bullae. (Elizarov 1972 - quoted from IARC 1999b, WHO 1996b). 3.3 SensitisationA 0.5% suspension of an atrazine or simazine formulation did not cause skin sensitisation on repeated application to 50 humans (Shelanski and Gittes 1965 - quoted from EC 1996a,b). 3.4 Repeated dose toxicityNo data were found. 3.5 Toxicity to reproductionThe use of a number of pesticides and chemicals, including atrazine, in the three-month period preceding a pregnancy was assessed from a questionnaire completed by 1898 couples on farms in Ontario, Canada. The use of atrazine itself was not associated with increased odds ratios for miscarriage, pre-term delivery, or babies who were small for gestational age. In some instances, combined exposure to a variety of chemicals including atrazine generated odds ratios of 2 or greater. (Savitz et al. 1997 – quoted from ATSDR 2001, IARC 1999a). Atrazine was not associated with any decrease in fecundity in a survey (in which a number of confounders were controlled for) of 1048 couples on farms in Ontario, Canada. Pesticide exposure was defined as pesticide use on the farm during the month of trying to conceive or at any time during the prior 2 months. (Curtis et al. 1999 – quoted from ATSDR 2001). An ecological study in Iowa, USA, that examined the association of triazines in the 856 municipal drinking water supplies with intrauterine growth retardation, prematurity, and low birth weight found a greater risk of intrauterine growth retardation in live births by women in 13 communities served by a water system containing elevated levels of triazines. Multiple linear regression analysis showed that the levels of atrazine, cyanazine, and metalachlor were each significant predictors of community intrauterine growth retardation rates in the exposed communities. No definite causal relationship between any single water contaminant and risk of intrauterine growth retardation could be determined due to lack of individual exposure data and the limited ability to control for confounding factors. (Munger et al. 1997 – quoted from ATSDR 2001). 3.6 Mutagenic and genotoxic effectsNo statistically significant increase in micronucleus formation and sister chromatid exchange of peripheral blood lymphocytes was found in 34 males from Spain exposed to simazine in the drinking water at levels of 10 to 30 ppm compared to controls drinking water with no detectable level of simazine. A statistically significant increase was found in high frequency cells, HFC (the percent of lymphocytes with more than 11 sister chromatid exchanges). The HFC sample could contain a subpopulation of more sensitive cells or a subpopulation of long-living lymphocytes that accumulated DNA-lesions in vivo. The authors conclude that their findings indicate the lack of potential cytogenetic hazard due to simazine exposure. (Suárez et al. 2003). 3.7 Carcinogenic effectsA combined analysis of the results of two cohort studies of agricultural chemical production workers (4917 male persons) in the USA showed decreased mortality from cancers at all sites combined among the subset of workers (55%) who had definite or probable exposure to triazines. Site-specific cancer analysis in this subset of workers yielded no significant findings. A non-significant increase in the number of deaths from non-Hodgkin lymphoma was seen, but was based on only 3 observed cases. Two of these three men had had less than one year of employment involving exposure to triazines. Exposure to pesticides other than triazine herbicides was not controlled for in the analysis. (Sathiakumar et al 1996 – quoted from IARC 1999a). In a cohort study of American workers (2213 persons) at a plant that mainly made atrazine and other triazines, decreased mortality was observed. The total number of deaths from cancer was not significantly different from controls. A significant increase (standardized mortality ratio (SMR): 372, 95% confidence interval (CI): 101-952) in the number of deaths from non-Hodgkin lymphoma was seen, but the 4 observed cases were not concentrated in the subgroup with long duration of employment and many years since hire. The study was limited by its small size, by the relative young age and short follow-up of the workers, and by the lack of exposure data. In a companion study of cancer incidence in the same group of workers, a 1.8-fold increase in prostate cancer incidence was found. Bias could not be ruled out as a reason for this increase because the excess of prostate cancer occurred primarily in company employees, who had been screened annually with prostate-specific antigen tests. (MacLennan et al. 2003). A pooled analysis of the results of three population-based case-control studies of men in Kansas, eastern Nebraska and Iowa-Minnesota, USA, (933 cases, 2918 controls) in which the risk for non-Hodgkin lymphoma in relation to atrazine and other herbicides on farms were evaluated, showed a significant age and state adjusted association (odds ratio: 1.4, CI: 1.1-1.8).The association was weaker (odds ratio: 1.2, CI: 0.9-1.7) when adjustment was made for reported use of phenoxyacetic acid herbicides or organophosphate insecticides. A sub-analysis of results for farmers in Nebraska, the state in which the most detailed information on atrazine use was available, showed no excess risk for non-Hodgkin lymphoma among farmers who had used atrazine for at least 15 years after adjustment for use of other pesticides. In a case-control study of non-Hodgkin lymphoma among women (134 cases, 707 controls) in eastern Nebraska, a slight, non-significant increase in risk (odds ratio: 1.2, CI: 0.6-2.6) was seen among women who had ever used triazines on farms. The odds ratio was not adjusted for use of other types of pesticides. (Zahm et al. 1993a,b - quoted from ATSDR 2001, IARC 1999a). A small but statistically significant association (odds ratio: 1.7, CI: 1.0-2.8) was found between atrazine exposure and the non-Hodgkin lymphoma subtype defined by t(14;18) chromosomal translocation in a case-control study of a subgroup of the men from Iowa-Minnesota mentioned in the previous study. (Schroeder et al. 2001). Case-control studies of Hodgkin's lymphoma, soft-tissue sarcoma, and colon cancer in Kansas, leukaemia in Iowa-Minnesota, and multiple myeloma in Iowa showed no significant excess risk among persons handling triazine herbicides. (Hoar et al. 1985,1986, Brown et al. 1990,1993 – quoted from IARC 1999a). In a case-control study in Italy (65 cases, 126 controls), the odds ratios for primary malignant epithelial tumours of the ovary, adjusted for age, number of live births, and use of contraceptives were 2.7 (90% CI: 1.0-6.9) for definitely exposed and 1.8 (90% CI: 0.9-3.5) for possibly exposed. The odds ratios were slightly higher among women with at least 10 years of occupational contact with triazines when compared with those with fewer than 10 years of contact. The odds ratios were not adjusted for exposure to other types of pesticides. (Donna et al. 1989 - quoted from IARC 1999a, WHO 1996a,b). In a nested case-control study (222 cases, 1110 controls) in California, USA, within a cohort of a predominantly Hispanic labour union, an elevated risk of prostate cancer was found in farm workers with relative high exposure to simazine compared to workers with lower levels of exposure. Increased risk was also experienced with other specific pesticides. (Mills and Yang 2003). An ecological study that assessed the correlation of the amount of pesticides used in California counties to the incidence rates of each of several cancer types (non-Hodgkin's lymphoma, leukaemia, soft-tissue sarcoma, brain cancer, prostate cancer, and testicular cancer) found a correlation between atrazine use and brain and testis cancers and leukaemia in Hispanic males, and prostate cancer in black males. These segments of the population had traditionally been employed as farm workers and had had the greatest potential for exposure to pesticides. No individual exposure data were available. (Mills 1998 – quoted from ATSDR 2001). An ecological study in Kentucky, USA, that examined the association of atrazine exposure indices with breast and ovarian cancer incidence rates found an inverse association (with increasing exposure linked to decreasing incidence rates) between atrazine levels and ovarian cancer and no association between atrazine levels and breast cancer. Exposure indices to atrazine were derived based on public water measurements, acres of corn planted, and pounds of atrazine sold. No individual exposure data were available. (Hopenhayn et al. 2002). An ecological study in Ontario, Canada, that examined the association of atrazine in the drinking water supply with cancer incidence rates found a positive association between atrazine levels and stomach cancer but a negative association between atrazine levels and colon cancer. The average atrazine contamination level was 163 ng/l. No individual exposure data were available. Potential confounding variables were considered. (Van Leewen et al. 1999 – quoted from ATSDR 2001). IARC have concluded that there is inadequate evidence in humans for the carcinogenicity of atrazine and simazine (IARC 1999a,b). 4 Animal toxicity
4.1 Single dose toxicity4.1.1 InhalationThe reported inhalation LC50-value for rats exposed to atrazine was greater than 5800 mg/m3 (2 studies reported) (Studies quoted in EC 1996a, US-EPA 2002a). The reported inhalation LC50-value for rats exposed to simazine was greater than 5600 mg/m3 (2 studies reported) (Studies quoted in EC 1996b). The reported inhalation LC50-value for rats exposed to terbutylazine was greater than 5300 mg/m3 (1 study reported) (Study quoted in US-EPA 1995, WHO 1998b). 4.1.2 Oral intakeThe reported oral LD50-values for atrazine ranged from 670 to 3100 mg/kg for rats (6 studies reported) and from 1750 to 4000 mg/kg for mice (2 studies reported). Weanling male rats had a higher LD50-value than older male rats. (Studies quoted in ATSDR 2001, IARC 1999, EC 1996a, US-EPA 2002a, WHO 1996a). The reported oral LD50-values for simazine was greater than 5000 mg/kg for rats, mice and rabbits (unknown number of studies) (Studies quoted in WHO 1996b). The reported oral LD50-values for terbutylazine was 1000->2000 mg/kg for rats (2 studies reported), 7700 mg/kg for mice (1 study reported), and >3000 mg/kg for hamsters (1 study reported) (Studies quoted in NRA 2001, US-EPA 1995, WHO 1998b). The reported oral LD50-values for cyanazine ranged from 150 to 840 mg/kg for rats (4 studies reported). In these studies, the percentage of active ingredient in the tested products was not clearly identified. Studies with technical cyanazine in rats, mice, and rabbits showed LD50-values of 180, 380, and 140 mg/kg, respectively (1 study reported). (Studies quoted in WHO 1998a). The reported oral LD50-value for desethyl atrazine (DEA) was 670 mg/kg for female rats and 1890 mg/kg for male rats (1 study reported) (Kuhn 1991c – quoted from EC 1996a). The reported oral LD50-value for desisopropyl atrazine (DIA) was 810 mg/kg for female rats and 2290 mg/kg for male rats (1 study reported) (Kuhn 1991d – quoted from EC 1996a). The reported oral LD50-value for desethyldesisopropyl atrazine (DACT) ranged from 2360 to 5460 mg/kg for rats (3 studies reported) (Studies quoted in EC 1996a). The reported oral LD50-value for hydroxyatrazine was greater than 5050 mg/kg for rats (1 study reported) (Kuhn 1991e – quoted from EC 1996a). The reported oral LD50-value for hydroxysimazine was greater than 5000 mg/kg for rats (1 study reported) (Pels Rijcken 1994b – quoted from EC 1996b). 4.1.3 Dermal contactThe dermal LD50-value for rats and rabbits exposed to atrazine was >2000 mg/kg (3 studies reported) and 7500 mg/kg (1 study reported), respectively (Studies quoted in ATSDR 2001, IARC 1999, EC 1996a, US-EPA 2002a, WHO 1996a). The dermal LD50-value for rats and rabbits exposed to simazine was >2000 mg/kg (3 studies reported) (Studies quoted in EC 1996b). The dermal LD50-value for rats and rabbits exposed to terbutylazine was >2000 mg/kg (1 study reported) and >4000 mg/kg (1 study reported), respectively (Studies quoted in NRA 2001, US-EPA 1995, WHO 1998b). 4.2 Irritation4.2.1 Skin irritationAtrazine was none to moderately irritating to the rabbit skin (Studies quoted from EC 1996a, US-EPA 2002a, WHO 1996a). Simazine was none to slightly irritating to the rabbit skin (Studies quoted from EC 1996b). Terbutylazine was slightly irritating to the rabbit skin (Hazleton France 1990 – quoted from US-EPA 1995). Cyanazine caused slight skin irritation at 2000 mg in rabbits (Shell Chemical Co. 1979 – quoted from WHO 1998a). Desethyldesisopropyl atrazine (DACT) caused slight skin irritation in rabbits (Cannelongo 1979 – quoted from EC 1996a). 4.2.2 Eye irritationAtrazine was not (appreciable) irritating to the rabbit eye (Studies quoted from EC 1996a, US-EPA 2002a, WHO 1996a). Simazine was not (appreciable) irritating to the rabbit eye (Studies quoted from EC 1996b). Terbutylazine was minimal to moderately irritating to the rabbit eye (Hazleton France 1990, Ciba-Geigy 1989 – quoted from US-EPA 1995). Cyanazine caused mild eye irritation at 100 mg in rabbits (Shell Chemical Co. 1979 – quoted from WHO 1998a). Desethyldesisopropyl atrazine (DACT) caused eye irritation in rabbits (Metha 1979 – quoted from EC 1996a). 4.3 SensitisationAtrazine caused dermal sensitisation in the guinea pig maximization test of Magnusson and Kligman and in the optimisation test (Maurer 1983, Schoch 1985 – quoted from EC 1996a) but not in another maximization test where results were poorly presented (Wandrag 1994c – quoted from EC 1996a). Simazine caused dermal sensitisation (a weak 10% response) in one well-performed guinea pig maximization test of Magnusson and Kligman (Marty 1995 – quoted from EC 1996b) but not in several other tests including a well-performed Buehler test (Several studies quoted in EC 1996b). Terbutylazine was not a sensitiser in the guinea pig maximization test (Hazleton France 1991 – quoted from US-EPA 1995). A skin sensitisation test in guinea pigs with cyanazine was negative (Walker et al. 1974, Shell Chemical Co. 1979 – quoted from WHO 1998a). 4.4 Repeated dose toxicity4.4.1 InhalationNo data were found on repeated dose toxicity with inhalation of triazines. 4.4.2 Oral intakeSee Table 4 for repeated dose toxicity animal studies with oral exposure to triazines. For atrazine, Table 4 contains data from 8 studies with rats (5 with Sprague-Dawley rats, and 2 with Fischer rats), 1 study with mice, 1 study with dogs, and 1 study with pigs. In almost all studies decreased body weight and/or food consumption was observed. Several studies focused on the ability of atrazine to cause neuroendocrine effects. It was shown in rats that atrazine attenuated the luteinizing hormone surge, disrupted the oestrous cycle, increased the serum level of oestrogen and prolactin, increased the relative pituitary weights, and "thickened" mammary glands. In pigs, a disruption of the oestrous cycle was also observed but the serum level of oestrogen was decreased. See Table 10 for the lowest NOAELs/LOAELs for some of these effects as well as for other neuroendocrine effects that will be described in subsequent chapters. In addition to the neuroendocrine effects, haematological changes were observed (anaemia, increased myeloid hyperplasia in the bone marrow, extramedullary haematopoiesis and haemosiderin pigment in the spleen) in rats, mice and dogs. The haematological changes seem to be more severe in the rats than in the other species tested. In 2-year studies, the Sprague-Dawley rat seems to be more sensitive to the hormonal as well as the haematological changes than the Fischer 344 rat in which these effects were absent at the doses tested. Generally at higher doses than the haematological changes, kidney toxicity was detected in some of the studies in the form of decreased kidney weight and histopathological changes in rats, mice and pigs. Cardiac toxicity was detected in dogs and pigs but not in rats. In the dog, the cardiac toxicity was quite severe with clinical signs, ECG alterations, and macroscopic and histopathological findings. See Table 12 for the lowest NOAELs/LOAELs for haematological, kidney, and cardiac toxicity. Other more sporadic changes were observed – see Table 4 for more details. For simazine, Table 4 contains data from 4 studies with Sprague-Dawley rats, and 2 studies with dogs. In general decreased body weight gain was observed. It was shown in rats that simazine attenuated the luteinizing hormone surge, disrupted the oestrous cycle, decreased the serum level of oestrogen, increased the serum level of prolactin, decreased the ovarian and uterine weights, and caused cystic glandular hyperplasia of the mammary gland. The Sprague-Dawley rat seemed to be more sensitive to the hormonal changes than the Fischer 344 rat. See Table 10 for the lowest NOAEL/LOAEL for the attenuation of LH surge as well as for other neuroendocrine effects that will be described in subsequent chapters. In addition to the neuroendocrine effects, haematological changes were observed (anaemia) in rats and dogs. Generally at higher doses than the haematological changes, kidney toxicity was detected in some of the studies in the form of increased kidney weight and histopathological changes in rats. See Table 12 for the lowest NOAELs/LOAELs for the haematological changes and the kidney toxicity. Cardiac toxicity was not evident in either the rat or the dog. Other more sporadic changes were observed – see Table 4 for more details. For terbutylazine, Table 4 contains data from 4 studies with rats, 2 studies with rabbits, 1 study with mice, and 1 study with dogs. In almost all studies decreased body weight and/or food consumption was observed. None of the studies focused on the ability of terbutylazine to cause neuroendocrine effects. However, in one of the rabbit studies it was shown that terbutylazine decreased the testes weight. See Table 10 for the lowest NOAEL/LOAEL for this effect as well as for other neuroendocrine effects that will be described in subsequent chapters. In addition to the neuroendocrine effects, haematological changes were observed (anaemia) in rats, rabbits and dogs. Cardiac toxicity was only observed in rabbits in the form of decreased heart weight. See Table 12 for the lowest NOAELs/LOAELs for haematological, and cardiac toxicity. Other more sporadic changes were observed – see Table 4 for more details. For cyanazine, Table 4 contains data from 3 studies with rats, 2 studies with mice, and 3 studies with dogs. In almost all studies decreased body weight gain was observed. None of the studies focused on the ability of cyanazine to cause neuroendocrine effects. Haematological changes were observed (increased myeloid hyperplasia in the bone marrow, extramedullary haematopoiesis in the spleen) in rats. Kidney toxicity was detected in rats, mice and dogs in some of the studies in the form of alterations in kidney function tests and increased kidney weight. Cardiac toxicity was not evident in any of the studies. See Table 12 for the lowest NOAELs/LOAELs for haematological and kidney toxicity. Other more sporadic changes were observed – see Table 4 for more details. For desethyl atrazine (DEA), Table 4 contains data from 1 study with rats, and 1 study with dogs. In both studies decreased body weight was observed. None of the studies focused on the ability of DEA to cause neuroendocrine effects. Haematological changes (anaemia) as well as cardiac (decreased heart weight, atrial fibrillation, inflammation and hyperplasia of the atrial wall) and kidney (tubular hyperplasia) toxicity were observed only in dogs. See Table 12 for the lowest NOAELs/LOAELs for haematological, kidney, and cardiac toxicity. For desisopropyl atrazine (DIA), Table 4 contains data from 1 study with rats, and 1 study with dogs. In both studies decreased body weight gain and/or food consumption was observed. None of the studies focused on the ability of DIA to cause neuroendocrine effects. However, in the dog study it was shown that DIA decreased the testes and prostate weight and in the rat study hypertrophy of pituitary cells occurred. See Table 10 for the lowest NOAEL/LOAEL for the effect on the testes and prostate as well as for other neuroendocrine effects that will be described in subsequent chapters. Haematological changes (extramedullary haematopoiesis in the spleen and liver) as well as kidney toxicity (increased weight) were observed only in rats. Cardiac toxicity (decreased heart weight) only occurred in the dog. See Table 12 for the lowest NOAELs/LOAELs for haematological, kidney, and cardiac toxicity. Other more sporadic changes were observed – see Table 4 for more details. For desethyldesisopropyl atrazine (DACT), Table 4 contains data from 2 studies with Sprague-Dawley rats, and 1 study with dogs. Decreased body weight gain was observed in both the rat and dog studies. The rat studies focused on the ability of DACT to cause neuroendocrine effects. It was shown that DACT attenuated the luteinizing hormone surge, and disrupted the oestrous cycle. See Table 10 for the lowest NOAELs/LOAELs for these effects as well as for other neuroendocrine effects that will be described in subsequent chapters. The primary treatment-related effect in dogs was impairment of heart function resulting in moribund sacrifice of several dogs. In addition to the cardiac effects, haematological changes (anaemia) as well as kidney toxicity (increased weight) were observed in dogs. See Table 12 for the lowest NOAELs/LOAELs for haematological, kidney, and cardiac toxicity. Other more sporadic changes were observed – see Table 4 for more details. For hydroxyatrazine, Table 4 contains data from 2 studies with Sprague-Dawley rats, and 1 study with dogs. In all studies decreased body weight gain was observed. None of the studies focused on the ability of hydroxyatrazine to cause neuroendocrine effects. The most severe effect both in rats and dogs was kidney toxicity (changes in clinical signs, in haematology, clinical chemical, and urinalysis parameters, in kidney weight, and in macroscopy and histopathology). At a dose of 17 mg/kg bw/day excessive mortality predominantly caused by renal failure occurred in one of the rat studies. In addition to the kidney toxicity, haematological changes were observed (anaemia). Cardiac toxicity was not evident in any of the studies. See Table 12 for the lowest NOAELs/LOAELs for haematological, and kidney toxicity. No studies have been found on the repeated dose toxicity with oral exposure of desethyl terbutylazine, hydroxysimazine, and hydroxyterbutylazine. 4.4.3 Dermal contactSee Table 5 for repeated dose toxicity animal studies with dermal contact to triazines. Table 5 contains data from only four studies (one with atrazine, one with simazine, and two with terbutylazine) all performed in rabbits. In all studies decreased body weight gain and food consumption was observed. Haematological changes were observed (anaemia, increased relative spleen weight) in the study with atrazine but at much higher doses (1000 mg/kg bw/day) than in the studies with oral exposure. Kidney and cardiac toxicity was not evident in any of the studies. The exposed rabbits had slight to moderate irritation of the skin. Other more sporadic changes were observed – see Table 5 for more details. 4.5 Toxicity to reproduction4.5.1 InhalationIn one study reported as an abstract, no developmental toxicity was seen in rats exposed by inhalation to concentrations up to 317 mg/m3 of simazine on days 7-14 of gestation. (Dilley et al. 1977 – quoted from IARC 1999b). 4.5.2 Oral intakeTwo studies have been performed with mixed exposure to the triazines. In the first study reported in the form of two abstracts, pregnant Long-Evans rats (more than 8/group) were dosed on gestational day 15-19 with a mixture of atrazine and its metabolites in doses estimated to be 50-10000 times the adult exposure (presuming that adults are drinking water with a concentration of 25 g/l of chlorotriazines). This concentration was based on combined maximum atrazine and degradation product concentrations detected in ground and surface water. The rats were gavaged with 0, 0.044, 0.087, 0.44, 0.87, 4.4, and 8.7 mg/kg bw/day of a mixture of atrazine (25%), desethyl atrazine (DEA)(15%), desisopropyl atrazine (DIA) (5%), desethyldesisopropyl atrazine (DACT) (35%), and hydroxyatrazine (20%). Only the male offspring were studied. On postnatal days 4, 21, or 120, the body weights of offspring of dosed rats were not significantly different from controls. Preputial separation (a marker of male puberty in the rat) was not statistically different from control according to Fenton et al. 2002, but according to Enoch et al. 2003 preputial separation was statistically delayed (not stated at what doses). At post-natal day 120, the males exposed to 0.087, 0.87, and 8.7 mg/kg bw/day had significantly larger anterior pituitary gland weights than controls. The ventral prostate weight was significantly smaller in the 4.4 and 8.7 mg/kg bw/day groups when compared to controls while the lateral prostate, testes, and seminal vesicle weights were unaffected by treatment at post-natal day 120. A subset of exposed males exhibited a dose-dependent increase in the concentration of serum testosterone (significant at 0.044, 0.44, and 4.4 mg/kg bw/day) at post-natal day 120. The concentration of serum oestrone was significantly increased (not stated at what doses) while the concentrations of serum and pituitary prolactin, serum oestradiol, and thyroid stimulating hormone were unaffected by treatment at post-natal day 120. A dose-dependent increase in the incidence of lipomatous masses nested in epididymal fat pads was noted. Lateral prostate inflammation was observed in several of the dose groups. However, the incidence and severity remained to be quantified via histopathology and immunohistochemistry. (Fenton et al. 2002, Enoch et al. 2003). In the second study, atrazine, simazine, and/or cyanazine were administered in two mixtures containing several other pesticides, fertilizers and other organic substances commonly found in groundwater in California or Iowa, USA, in a continuous breeding protocol to Swiss CD-1 mice, and in a developmental study to pregnant Sprague-Dawley rats on day 6-20 of gestation. The individual triazines were dosed at concentrations of about 0, 0.3-0.5, 3-5, and 30-50 g/l of drinking water (equivalent to about 0, 0.075-0.13, 0.75-1.3, and 7.5-13 g/kg bw/day). In the mice study, no effects on reproductive performance of F0 or F1 individuals, spermatogenesis, epididymal sperm concentration, percentage of motile sperm, percentage of abnormal sperm or testicular tissues were found. In the rat study, no evidence of developmental toxicity was observed. (Heindel et al. 1994 – quoted from BCERF 1998a,b, IARC 1999a). See Table 6 for toxicity to reproduction animal studies with oral exposure to individual triazines. For atrazine, 1 reproductive toxicity study in Charles River CD rats, 5 prenatal (4 in various strains of rats, 1 in rabbits) and 6 postnatal (in Wistar and Sprague-Dawley rats) developmental toxicity studies as well as a special study on neurobehavioral effects in pups are included in Table 6. In many of the studies decreased body weight gain in parental animals as well as in pups was observed. Atrazine was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites in foetuses was observed both in Sprague-Dawley and Charles River CD rats as well as in rabbits. Mild neurobehavioral effects were observed in pups of Fischer 344 dams, which had been dosed with atrazine a month before mating. See Table 11 for the lowest NOAELs/LOAELs for some of these effects. Several studies focused on the ability of atrazine to cause neuroendocrine effects. It was shown in prenatal studies in rats and rabbits that atrazine might cause pre- and postimplantation loss, and full litter resorption. Preimplantation loss was observed in Fischer 344 rats while the postimplantation loss was observed in Holtzman rats, Charles River CD rats, and rabbits. The Fischer 344 rats were more sensitive than the Sprague-Dawley and Long-Evans rats to full litter resorption in dams dosed on gestation days 6-10. No full litter resorption occurred when the rats were dosed on gestation days 11-15 suggesting that the full litter resorption is maternally mediated and consistent with loss of luteinizing hormone support of the corpora lutea. Holtzman, Sprague-Dawley, Long-Evans, and Fischer 344 dams participating in the prenatal studies all had a decreased serum level of luteinizing hormone. However, only Sprague-Dawley dams had an increased serum level of oestrogen. It was shown in postnatal studies in rats that atrazine decreased the serum testosterone level, and the ventral prostate, testes, and seminal vesicle weights. An in vitro experiment demonstrated that atrazine directly inhibited Leydig cell testosterone production. Atrazine also delayed the preputial separation of male rats and delayed the vaginal opening of female rats dosed postnatally indicating a delayed puberty of the rats. The Sprague-Dawley rat seems to be more sensitive to delayed vaginal opening than the Wistar rat. Atrazine suppressed suckling-induced prolactin release in dams at postnatal days 1-4 and 6-9. This suppression resulted in an increased incidence and severity of prostate inflammation in the male offspring. See Table 10 for the lowest NOAELs/LOAELs for some of these effects as well as for other neuroendocrine effects that have been described in precedent chapters. Other more sporadic changes were observed – see Table 6 for more details. For simazine, 2 reproductive toxicity study in rats, and 3 prenatal (2 in Sprague-Dawley rats, 1 in rabbits) developmental toxicity studies are included in Table 6. In most of the studies decreased body weight gain in parental animals as well as in pups was observed. Simazine was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites in foetuses was observed both in rats and rabbits. See Table 11 for the lowest NOAELs/LOAELs for these effects. In one of the prenatal studies in rats, hypoplasia of the lungs in association with malposition of the heart was observed in foetuses at a much higher dose than the other effects. For terbutylazine, 1 reproductive toxicity study in Sprague-Dawley rats, and 3 prenatal (1 in rats, 2 in rabbits) developmental toxicity studies are included in Table 6. In most of the studies decreased body weight gain in parental animals as well as in pups was observed. Slightly higher number of infertile pairings occurred in the reproductive study. Terbutylazine was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites in foetuses was observed in rats but not in rabbits, which were exposed to lower doses than the rats. See Table 11 for the lowest NOAELs/LOAELs for these effects. For cyanazine, 2 reproductive toxicity study in Long-Evans and Sprague-Dawley rats, and 4 prenatal (2 in Fischer 344 rats, 1 in Sprague-Dawley rats, 1 in rabbits) developmental toxicity studies are included in Table 6. In many of the studies decreased body weight gain in parental animals as well as in pups was observed. Cyanazine was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites in foetuses was observed in rabbits and in Fischer 344 rats (but not in Sprague-Dawley rats at a similar dose). Microphtalmia/anophtalmia, diaphragmatic hernia associated with liver protrusion, and dilated brain ventricles were also observed in foetuses of Fischer 344 rat and rabbit dams dosed with cyanazine at the next dose level. See Table 11 for the lowest NOAELs/LOAELs for these effects. It was shown in prenatal studies in rats and rabbits that cyanazine might cause postimplantation loss and delayed parturition. See Table 10 for the lowest NOAELs/LOAELs for these effects as well as for other neuroendocrine effects that have been described in precedent chapters. Other more sporadic changes were observed – see Table 6 for more details. For desethyl atrazine (DEA), 2 prenatal (in Sprague-Dawley and Fischer 344 rats) and 1 postnatal (in Wistar rats) developmental toxicity studies are included in Table 6. In the prenatal studies decreased body weight gain in the dams was observed. DEA was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites and of fused sternebrae in foetuses was observed in the Sprague-Dawley rats. See Table 11 for the lowest NOAELs/LOAELs for these effects. It was shown in one of the prenatal studies in rats that DEA might cause delayed parturition and altered pregnancy maintenance. It was shown in the postnatal study in rats that DEA decreased the prostate, seminal vesicle, epididymal and anterior pituitary weights. DEA also delayed the preputial separation of male rats dosed postnatally. See Table 10 for the lowest NOAELs/LOAELs for most of these effects as well as for other neuroendocrine effects that have been described in precedent chapters. For desisopropyl atrazine (DIA), 2 prenatal (in Sprague-Dawley and Fischer 344 rats) and 1 postnatal (in Wistar rats) developmental toxicity studies are included in Table 6. In the prenatal studies decreased body weight gain in the dams was observed. DIA was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites and of fused sternebrae in foetuses was observed in the Sprague-Dawley rats. See Table 11 for the lowest NOAELs/LOAELs for these effects. It was shown in one of the prenatal studies in rats that DIA might cause delayed parturition and altered pregnancy maintenance. It was shown in the postnatal study in rats that DIA decreased the serum testosterone level and the prostate, seminal vesicle, epididymal and anterior pituitary weights. DIA also delayed the preputial separation of male rats dosed postnatally. See Table 10 for the lowest NOAELs/LOAELs for most of these effects as well as for other neuroendocrine effects that have been described in precedent chapters. For desethyldesisopropyl atrazine (DACT), 2 prenatal (in Sprague-Dawley and Fischer 344 rats) and 2 postnatal (in Wistar rats) developmental toxicity studies are included in Table 6. In the prenatal studies decreased body weight gain in the dams and foetuses was observed. DACT was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites and kidney toxicity in foetuses was observed in the Sprague-Dawley rats. See Table 11 for the lowest NOAELs/LOAELs for these effects. It was shown in the prenatal studies in rats that DACT might cause delayed parturition and altered pregnancy maintenance. It was shown in one of the postnatal studies in rats that DACT increased the serum oestrone level and decreased the ventral prostate, seminal vesicle, epididymal and anterior pituitary weights of the male offspring. DACT also delayed the preputial separation of male rats and delayed the vaginal opening of female rats dosed postnatally. See Table 10 for the lowest NOAELs/LOAELs for most of these effects as well as for other neuroendocrine effects that have been described in precedent chapters. For hydroxyatrazine, 2 prenatal (in Sprague-Dawley and Fischer 344 rats) and 2 postnatal (in Wistar rats) developmental toxicity studies are included in Table 6. In the prenatal studies decreased body weight gain and/or food consumption in the dams and foetuses was observed. Hydroxyatrazine was not a developmental toxicant when administered in doses that were not maternally toxic in the prenatal studies. An increased incidence of incomplete ossification sites in foetuses and kidney toxicity in dams was observed in the Sprague-Dawley rats. See Table 11 for the lowest NOAELs/LOAELs for most of these effects. It was shown in the prenatal studies in rats that hydroxyatrazine might cause altered pregnancy maintenance. It was shown in the postnatal studies in rats that hydroxyatrazine delayed the preputial separation of male rats but not the vaginal opening of female rats. See Table 10 for the lowest NOAELs/LOAELs for these effects as well as for other neuroendocrine effects that have been described in precedent chapters. No studies have been found on the reproductive toxicity of desethyl terbutylazine, hydroxysimazine, and hydroxyterbutylazine. 4.5.3 Dermal contactNo data were found. 4.5.4 Other routesAtrazine administered intraperitoneally twice a week for a period of 60 days to adult male Fischer 344 rats at 0, 60, or 120 mg/kg bw/day affected the spermatogenesis (increased testicular sperm numbers, decreased epididymal sperm numbers, decreased epididymal sperm motility) at both doses. Histological changes of the rat testis and degenerative changes in Leydig and Sertoli cells were observed. (Kniewald et al. 2000). 4.6 Mutagenic and genotoxic effectsSee Table 7 for mutagenic and genotoxic effects of triazines in vitro and Table 8 for mutagenic and genotoxic effects of triazines in vivo. In general, atrazine was mutagenic in Drosophila, yeast and plant cells but was not mutagenic in bacteria. Atrazine tested negative for most genotoxic effects in mammalian cells in vitro and in vivo. However, positive results have been found in some but not all studies in vitro for chromosomal aberrations and DNA damage in human lymphocytes, and in vivo for DNA damage in rats and mice and for micronucleus formation in mice. Simazine was mutagenic in Drosophila and plant cells but was not mutagenic in bacteria and yeast. Simazine tested negative for genotoxic effects in mammalian cells in vitro and in vivo. Terbutylazine was not mutagenic in Ames test. Terbutylazine tested negative for genotoxic effects in mammalian cells in vitro and in vivo. Cyanazine was not mutagenic in bacteria, yeast, and Drosophila. Cyanazine tested negative for most genotoxic effects in mammalian cells in vitro and in vivo. However, positive results have been found in some but not all studies in vitro for chromosomal aberrations in human lymphocytes and for repairable DNA damage in rat hepatocytes. Desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyldesisopropyl atrazine (DACT), and hydroxyatrazine were not mutagenic in Ames test. They tested negative for genotoxic effects in mammalian cells in vitro (repairable DNA damage) and in vivo (micronucleus formation). Hydroxysimazine was not mutagenic in Ames test. No studies have been found on the mutagenic and genotoxic effects of desethyl terbutylazine, and hydroxyterbutylazine. 4.7 Carcinogenic effectsNo data were found on carcinogenic effects following inhalation of or dermal contact with triazines. See Table 9 for carcinogenic effects in animal studies with oral exposure to triazines. For atrazine, Table 9 contains data from 5 studies with Sprague-Dawley rats, 2 studies with Fischer rats, and 1 study with mice. In general, atrazine caused an increased incidence and an earlier onset of mammary gland tumours (adenocarcinomas, fibroadenomas) in female Sprague-Dawley rats but not in Fischer 344 rats (in doses up to 38 mg/kg bw/day), CD-1 mice (in doses up to 483 mg/kg bw/day), or ovariectomised Sprague-Dawley rats (in doses up to 21 mg/kg bw/day). The lowest dose at which atrazine caused a statistically significant increase in mammary gland tumours in Sprague-Dawley rats was 3.1 mg/kg bw/day. The lowest dose at which atrazine caused an earlier onset of mammary gland tumours was 1.5 mg/kg bw/day. In one study in Sprague-Dawley rats an increased incidence of Leydig cell tumours were observed at a dose of 42 mg/kg bw/day. The incidence fell within historical control data and was attributed in part to the better survival of these animals. The NOAEL for carcinogenicity in Sprague-Dawley rats was 0.5 mg/kg bw/day. Simazine caused a statistically significant increase in the incidence of and an earlier onset of mammary gland tumours (adenocarcinomas, fibroadenomas) in females in one study with Sprague-Dawley rats dosed at 5.3 mg/kg bw/day for 2 years. The incidence of pituitary gland carcinomas was also significantly increased but within historical control data at 46 mg/kg bw/day in females. A small increase in renal tubular tumours was observed in both sexes at 46 mg/kg bw/day based on which the European Commission has classified simazine for carcinogenicity. The NOAEL for carcinogenicity was 0.52 mg/kg bw/day. Simazine was not carcinogenic in one study with mice dosed with up to 600 mg/kg bw/day for almost 2 years. Terbutylazine caused a statistically significant increase in the incidence of mammary gland carcinomas and a decrease in mammary gland fibroadenomas in females in one study with rats (probably Sprague-Dawley) dosed at 53 mg/kg bw/day for 2 years. At the same dose level an increased incidence of Leydig cell tumours were observed mainly in the old rats. The NOAEL for carcinogenicity was 7.0 mg/kg bw/day. Terbutylazine was not carcinogenic in one study with mice dosed with up to 89 mg/kg bw/day for 2 years. Cyanazine caused a statistically significant increase in the incidence of and an earlier onset of mammary gland carcinomas in females in one study with Sprague-Dawley rats dosed at 1.4 mg/kg bw/day for 2 years. The NOAEL for carcinogenicity was 0.26 mg/kg bw/day. Cyanazine was not carcinogenic in one study with mice dosed with up to 130 mg/kg bw/day for 2 years. Desethyldesisopropyl atrazine (DACT) caused a statistically significant increase in the incidence of mammary gland tumours in females in one study with Sprague-Dawley rats dosed at 10 mg/kg bw/day for 1 year. The NOAEL for carcinogenicity was 3.5 mg/kg bw/day. Hydroxyatrazine was not carcinogenic in one study with Sprague-Dawley rats dosed with up to 22 mg/kg bw/day for 2 years. No studies have been found on the carcinogenicity of desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyl terbutylazine, hydroxysimazine, and hydroxyterbutylazine. IARC have concluded that there is sufficient evidence in experimental animals for the carcinogenicity of atrazine and that there is limited evidence in experimental animals for the carcinogenicity of simazine. However, IARC has made an overall conclusion that atrazine is not classifiable as to its carcinogenicity to humans. In making this conclusion, the IARC working group concluded that the mammary tumours associated with exposure to atrazine involve a non-DNA-reactive, hormonally mediated mechanism. In reaching this conclusion, the following evidence was considered:
Therefore, there is strong evidence that the mechanism by which atrazine increases the incidence of mammary gland tumours in Sprague-Dawley rats is not relevant to humans. Nevertheless, US-EPA has also concluded that it is not unreasonable to expect that atrazine might cause adverse effects on hypothalamic-pituitary function in humans and that the same endocrine perturbations that induce tumours also appear to play a role in at least some reproductive developmental effects which may be relevant to humans. 5 Regulations
5.1 Ambient airNo data were found. 5.2 Drinking water
5.3 SoilNo data were found. 5.4 FoodsMaximum residue limits (MRL) in foods:
5.5 Occupational Exposure Limits
5.6 Classification
5.7 IARCAtrazine is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 1999a) Simazine is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 1999b) 5.8 US-EPAChronic oral reference dose (RfD) for atrazine/desethyldesisopropyl atrazine (DACT): 0.018 mg/kg/day (US-EPA 2002c) Carcinogenicity classification for atrazine/desethyldesisopropyl atrazine (DACT): Not likely to be carcinogenic to humans (Group D) (US-EPA 2002c) 6 Summary and evaluation
6.1 DescriptionAtrazine, simazine, terbutylazine and cyanazine are triazines, which have been used or still are in use in agriculture as herbicides in Denmark. Desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyl terbutylazine, desethyldesisopropyl atrazine (DACT), hydroxyatrazine, hydroxysimazine, and hydroxyterbutylazine are some of the degradation products of these triazines. The triazines are colourless to white powders with low water solubilities (5-170 mg/l) and vapour pressures lower than 10-6 mmHg. 6.2 EnvironmentThere are no known natural sources of atrazine. Virtually the entire production volume is released to the environment, primarily soils, mainly as a result of agricultural and other weed-control practices. In the atmosphere, atrazine will exist in both the particulate and vapour phases. Particulate phase atrazine will be removed from the atmosphere by wet and dry deposition. Vapour phase atrazine can be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; a half-life of 14 hours has been estimated. The triazines may be transported from where they are applied to soils by runoff into surface water and percolation into groundwater. Atrazine, simazine and terbutylazine tend to persist in surface and groundwater (no or slow abiotic hydrolysis and photolysis, and degradation half-lives of 26-253 days), with a moderate tendency to bind to sediments (dissipation half-lives for the water phase of 6-134 days in controlled aerobic water-sediment systems). When atrazine is degraded in aquatic systems, hydroxytriazine, desethyl atrazine (DEA) and desisopropyl atrazine (DIA) are the major products formed. Field studies in Europe showed average half-lives in soil of 29, 64-130, and 22 days for atrazine, simazine, and terbutylazine, respectively. For simazine, the half-life was shown to be higher following application at fall (130 days) than following application at spring (64 days). High microbial activity enhanced the degradation. Regarding ground water contamination, the most important metabolites from atrazine, simazine, and terbutylazine were desethyl atrazine (DEA), desisopropyl atrazine (DIA), and hydroxyatrazine; desisopropyl atrazine (DIA); and hydroxyterbutylazine, and desethyl terbutylazine, respectively. Atrazine has a slight to moderate tendency to bioconcentrate in microorganisms, algae, aquatic invertebrates, worms, snails, or fish. 6.3 Human exposureOccupational exposure to triazines may occur through dermal contact or inhalation during the manufacture, formulation or application of the herbicides. The general population may be exposed to the triazines and their degradation products through their widespread occurrence in the environment especially in drinking water. No significant exposure is expected to occur via foodstuffs. 6.4 ToxicokineticsIn general, triazines are well absorbed in rats by the oral route (50-70%). Following dermal exposure in humans only about 5% of the dose is absorbed. No data were found on the toxicokinetics following inhalation. The triazines are extensively metabolised and eliminated with a half-life of 10-20 hours via urine but also in faeces. The main biotransformation pathways for the triazines in rats are N-dealkylation by the hepatic cytochrome P450 system, and glutathione conjugation of either the parent compound or the N-dealkylated metabolite to the ultimately excreted mercapturic acid conjugate. Rats and humans produce the same type of metabolites following exposure to atrazine, but species-specific differences in the metabolite ratios are found. Desethyldesisopropyl atrazine (DACT) is a major metabolite of atrazine and simazine. Desisopropyl atrazine (DIA) is also a metabolite of atrazine and simazine. Desethyl atrazine (DEA) is a metabolite of atrazine, desethyl terbutylazine is a metabolite of terbutylazine, and desethyl cyanazine is a metabolite of cyanazine. 6.5 Mode of actionBecause of a common mode of action, US-EPA has grouped atrazine, simazine and their degradation products desethyl atrazine (DEA), desisopropyl atrazine (DIA), and desethyldesisopropyl atrazine (DACT). Hydroxyatrazine was not included in this group based on the absence of mammary gland tumour induction and inconclusive data on its effect on the luteinizing hormone. Treatment of laboratory animals with the triazines results in toxic effects such as mammary gland tumours in female Sprague-Dawley rats and reproductive and developmental alterations. The primary proposed mode of action for these tumours and some of the reproductive and developmental effects involves disruption of the hypothalamic-pituitary-gonadal axis resulting in a decreased serum level of luteinizing hormone, and as a consequence an increased serum level of oestrogen and prolactin. IARC and US-EPA have concluded that the mechanism by which atrazine increases the incidence of mammary gland tumours in Sprague-Dawley rats is not relevant to humans. 6.6 Human toxicity6.6.1 Single and repeated dose toxicityNo data were found. 6.6.2 Skin irritationCase-reports exist of contact dermatitis in workers exposed dermally to atrazine, simazine and/or cyanazine. 6.6.3 SensitisationAn 0.5% suspension of a formulation of atrazine or simazine did not cause skin sensitisation on repeated application to humans. 6.6.4 Toxicity to reproductionThe use of atrazine was not associated with any decrease in fecundity, increased odds ratios for miscarriage, pre-term delivery, or babies who were small for gestational age in two surveys of Canadian couples on farms. An ecological study in USA found a greater risk of intrauterine growth retardation in live births by women in 13 communities served by a water system containing elevated levels of triazines. No definite causal relationship could be determined. 6.6.5 Mutagenic and genotoxic effectsNo statistically significant increase in micronucleus formation and sister chromatid exchange of peripheral blood lymphocytes was found in 34 males exposed to simazine in the drinking water at levels of 10 to 30 ppm. 6.6.6 Carcinogenic effectsIn cohort studies of agricultural chemical workers, an increase in the number of deaths from non-Hodgkin lymphoma was observed. In one of the studies, the increase was significant but the 4 observed cases were not concentrated in the subgroup with long duration of employment and many years since hire. In a companion study of cancer incidence in the same group of workers, a 1.8-fold increase in prostate cancer incidence was found. Bias could not be ruled out as a reason for this increase. Case-control studies have shown an association between non-Hodgkin lymphoma and use of atrazine. When the odds ratios were adjusted for use of other pesticides, the association was not significant. Recently, a study has shown a small but statistically significant association between atrazine exposure and the non-Hodgkin lymphoma subtype defined by t(14;18) chromosomal translocation. In a nested case-control study in USA within a cohort of a predominantly Hispanic labour union, an elevated risk of prostate cancer was found in farm workers with relative high exposure to simazine compared to workers with lower levels of exposure. In a case-control study in Italy, the odds ratios for primary malignant epithelial tumours of the ovary were significantly increased for definitely exposed and non-significantly increased for possibly exposed. The odds ratios were not adjusted for exposure to other types of pesticides. Case-control studies of Hodgkin's lymphoma, soft-tissue sarcoma, colon cancer, leukaemia, and multiple myeloma showed no significant excess risk among persons handling triazine herbicides. Ecological studies in which no individual exposure data are available have found a correlation between atrazine use/levels and certain cancers (brain, stomach, testis, and prostate cancers, and leukaemia. For other cancers (non-Hodgkin's lymphoma, soft-tissue sarcoma, ovarian, breast, and colon cancer) no or inverse associations have been found. IARC have concluded that there is inadequate evidence in humans for the carcinogenicity of atrazine and simazine. 6.7 Animal toxicity6.7.1 Single dose toxicitySingle inhalatory, peroral or dermal administration of the triazines in general proved to be only slightly toxic (LC50-values for rats are greater than 5300 mg/m3 for atrazine, simazine, and terbutylazine; oral LD50-values for rats, mice, rabbits, and hamsters ranged from 670 to 7700 mg/kg for atrazine, simazine and terbutylazine; and dermal LD50-values for rats and rabbits are greater than 2000 mg/kg for atrazine, simazine, and terbutylazine). However, for cyanazine the oral LD50-values were lower than for the other triazines (ranged from 140 to 840 mg/kg for rats, mice, and rabbits). For the degradation products, oral LD50-values for rats were greater than 2360 mg/kg for desethyldesisopropyl atrazine (DACT), hydroxyatrazine, and hydroxysimazine. For desethyl atrazine (DEA) and desisopropyl atrazine (DIA), oral LD50-values for rats were lower for females (670-810 mg/kg) than for males (1890-2290 mg/kg). 6.7.2 IrritationThe triazines were none to moderately irritating to the rabbit skin and eye. 6.7.3 SensitisationAtrazine is classified for skin sensitisation based on positive results in the guinea pig maximization test and optimisation test . Simazine, terbutylazine and cyanazine did not cause appreciable skin sensitisation in the guinea pig. 6.7.4 Repeated dose toxicityNo data were found on repeated dose toxicity with inhalation of triazines. Most studies on repeated dose toxicity following oral exposure have been performed with atrazine but studies also exist for simazine, terbutylazine, cyanazine, desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyldesisopropyl atrazine (DACT), and hydroxyatrazine. See Table 10 and Table 12 for the lowest NOAELs/LOAELs for the repeated dose toxicity effects (following oral exposure) mentioned in this chapter. In general, the triazines and their degradation products decreased body weight and/or food consumption. Several studies mainly in Sprague-Dawley rats focused on the ability of the triazines (especially atrazine) to cause neuroendocrine effects. It was shown that they attenuated the luteinizing hormone surge, disrupted the oestrous cycle, increased the serum level of oestrogen and prolactin, increased the relative pituitary weights, decreased the testes and prostate weights, and "thickened" mammary glands at doses from 3.7 mg/kg bw/day and with a NOAEL of 1.8 mg/kg bw/day in rats and with a LOAEL of 1 mg/kg bw/day in pigs. In addition to the neuroendocrine effects, haematological changes were observed (anaemia, increased myeloid hyperplasia in the bone marrow, extramedullary haematopoiesis in the liver and spleen and haemosiderin pigment in the spleen) in several species. In 2-year studies, the Sprague-Dawley rat seems to be more sensitive to the hormonal as well as the haematological changes than the Fischer 344 rat in which these effects were absent at the doses tested. The haematological changes seem to be most severe in the rats dosed with cyanazine, atrazine, and desisopropyl atrazine (DIA) based on their ability to induce extramedullary haematopoiesis. With these three chemicals, the haematological changes also seem to be more severe in the rats than in the other species tested. In the rats dosed with the other triazines or degradation products, anaemia was the only sign of haematological toxicity. Cyanazine had the lowest LOAEL of 2.1 mg/kg bw/day and a NOAEL of 0.99 mg/kg bw/day for haematological changes. Generally at the same or higher doses than the haematological changes, kidney toxicity was detected in some of the studies in several species in the form of changes in mainly weight and/or histopathology of the kidney. The kidney toxicity seems to be most severe for hydroxyatrazine where a dose of 17 mg/kg bw/day to rats caused excessive mortality predominantly caused by renal failure. At the lower dose of 7.8 mg/kg bw/day, severe kidney toxicity was also observed while the NOAEL was 0.96 mg/kg bw/day. Cardiac toxicity was detected in dogs, pigs and rabbits but not in rats. In the dog, the cardiac toxicity was quite severe with clinical signs, ECG alterations, heart weight changes, and/or macroscopic and histopathological findings at doses from about 24 mg/kg bw/day and with a NOAEL of 3.4 mg/kg bw/day. For hydroxyatrazine, no cardiac toxicity was observed in the dog at doses up to 200 mg/kg bw/day. In the only study with pigs dosed with atrazine, the LOAEL (2 mg/kg bw/day) and the NOAEL (<2 mg/kg bw/day) were lower than in the dog studies. Only four studies (one with atrazine, one with simazine, and two with terbutylazine) were found on repeated dose toxicity following dermal exposure. All three studies were performed in rabbits and showed decreased body weight gain and food consumption. Haematological changes were observed in the study with atrazine but at much higher doses than in the studies with oral exposure. The exposed rabbits had slight to moderate irritation of the skin. 6.7.5 Toxicity to reproductionNo developmental toxicity was seen in rats exposed by inhalation to concentrations up to 317 mg/m3 of simazine on days 7-14 of gestation. Two studies have been performed with mixed oral exposure to the triazines. In the first study, groups of pregnant rats were gavaged on gestational day 15-19 with 0, 0.044, 0.087, 0.44, 0.87, 4.4, and 8.7 mg/kg bw/day of a mixture of atrazine (25%), desethyl atrazine (DEA)(15%), desisopropyl atrazine (DIA) (5%), desethyldesisopropyl atrazine (DACT) (35%), and hydroxyatrazine (20%). Only the male offspring were studied. It was unclear from the study abstracts if preputial separation (a marker of male puberty in the rat) was statistically significantly delayed. At some of the doses the male offspring had significantly larger anterior pituitary gland weights and the ventral prostate weight was significantly smaller while the lateral prostate, testes, and seminal vesicle weights were unaffected by treatment. At some of the doses the male offspring exhibited an increase in the concentration of serum testosterone and oestrone while the concentrations of serum and pituitary prolactin, serum oestradiol, and thyroid stimulating hormone were unaffected by treatment. A dose-dependent increase in the incidence of lipomatous masses nested in epididymal fat pads was noted. Lateral prostate inflammation was observed in several of the dose groups. However, the incidence and severity remained to be quantified via histopathology and immunohistochemistry. Based on the limited data from the study abstracts, it is impossible to set a NOAEL. In the second study, atrazine, simazine, and cyanazine were administered at individual maximal concentrations of about 30-50 g/l (equivalent to about 0.0075-0.013 mg/kg bw/day) of drinking water in a mixture containing several other pesticides, fertilizers and other organic substances commonly found in groundwater in California, USA. No effects on several reproductive parameters in mice and on developmental toxicity in rats were observed. Most studies on toxicity to reproduction following oral exposure to individual triazines and their degradation products have been performed with atrazine but studies also exist for simazine, terbutylazine, cyanazine, desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyldesisopropyl atrazine (DACT), and hydroxyatrazine. Reproductive as well as pre- and postnatal developmental studies have been performed mainly in rats but for a few of the prenatal studies also in rabbits. See Table 10 and Table 11 for the lowest NOAELs/LOAELs for the reproductive and developmental effects (following oral exposure) mentioned in this chapter. In general, the triazines and their degradation products decreased body weight gain in parental animals and sometimes in pups. In the prenatal studies, they were not developmental toxicants when administered in doses that were not maternally toxic (mainly decreased body weight gain). For all of the studied triazines and degradation products, an increased incidence of incomplete ossification sites in foetuses and/or fused sternebrae was observed with about equal sensitivity in rats and rabbits. Cyanazine seems to be more potent than the other triazines and degradation products in inducing this effect with a LOAEL of 5 mg/kg bw/day and 2 mg/kg bw/day for rats and rabbits, respectively, and a NOAEL of < 5 mg/kg bw/day and 1 mg/kg bw/day for rats and rabbits, respectively. However, the lower NOAEL/LOAEL for rats could possibly be due to the use of the Fischer 344 rat, which in the cyanazine studies seem to be more sensitive to this effect than the Sprague-Dawley rat. Cyanazine but not any of the other triazines or degradation products induced microphtalmia/anophtalmia, diaphragmatic hernia associated with liver protrusion, and dilated brain ventricles in foetuses of rats and rabbits although at a higher dose than required for induction of incomplete ossification sites. Mild neurobehavioral effects were observed in rat pups of dams, which had been dosed with atrazine a month before mating. This effect was not studied for the other triazines and degradation products. Several studies focused on the ability of the triazines and their degradation products to cause neuroendocrine effects. It was shown in prenatal studies in rats and rabbits that they might cause altered pregnancy outcome (pre- and postimplantation loss, full litter resorption, delayed parturition) with NOAELs of 17-40 mg/kg bw/day and LOAELs of 34-91 mg/kg bw/day for Fischer 344 rats. From the studies with cyanazine it seems that the NOAELs/LOAELs for the altered pregnancy outcome may be lower for rabbits than for rats. For atrazine, the preimplantation loss was observed in Fischer 344 rats while the postimplantation loss was observed in Holtzman rats, Charles River CD rats, and rabbits. The Fischer 344 rats were more sensitive than the Sprague-Dawley and Long-Evans rats to full litter resorption in dams dosed on gestation days 6-10. No full litter resorption occurred when the rats were dosed on gestation days 11-15 suggesting that the full litter resorption is maternally mediated and consistent with loss of luteinizing hormone support of the corpora lutea. Holtzman, Sprague-Dawley, Long-Evans, and Fischer 344 dams participating in the prenatal studies all had a decreased serum level of luteinizing hormone. However, only Sprague-Dawley dams had an increased serum level of oestrogen. It was shown in postnatal studies mainly in Wistar rats that the triazines and their degradation products decreased the serum testosterone level, and the prostate, testes, seminal vesicle, epididymal, and/or anterior pituitary weights. An in vitro experiment demonstrated that atrazine directly inhibited Leydig cell testosterone production. Generally at lower doses than the doses causing the effects on the serum testosterone level and on the weights of the male reproductive organs, the triazines and their degradation products delayed the preputial separation of male rats and delayed the vaginal opening of female rats dosed postnatally indicating a delayed puberty of the rats. These studies were mainly performed with Wistar rats. A study with atrazine indicated that the Sprague-Dawley rat was more sensitive to delayed vaginal opening than the Wistar rat. Male rats seem to be more sensitive than female rats to the delayed puberty. For male rats, DACT had the lowest NOAEL/LOAEL of 4.4/8.4 mg/kg bw/day. Atrazine at 13 mg/kg bw/day suppressed suckling-induced prolactin release in Wistar rat dams at postnatal days 1-4 and 6-9. The NOAEL was 6.3 mg/kg bw/day. This suppression resulted in an increased incidence and severity of prostate inflammation in the male offspring. This effect was not studied for any of the other triazines or degradation products. No data were found on toxicity to reproduction with dermal contact with triazines. Atrazine administered intraperitoneally to male rats affected the spermatogenesis. Histological changes of the rat testis and degenerative changes in Leydig and Sertoli cells were observed. 6.7.6 Mutagenic and genotoxic effectsOverall, the triazines and their degradation products tested negative for mutagenic and genotoxic effects in bacteria and mammalian cells (in vitro and in vivo), although a few positive results did occur. 6.7.7 Carcinogenic effectsNo data were found on carcinogenic effects following inhalation of or dermal contact with triazines. Most studies on carcinogenicity following oral exposure have been performed with atrazine but a few studies exist for simazine, terbutylazine, cyanazine, desethyldesisopropyl atrazine (DACT), and hydroxyatrazine. In general, the triazines and their degradation products seem to increase the incidence and cause an earlier onset of mammary gland tumours (fibroadenomas, adenocarcinomas) only in intact female Sprague-Dawley rats (and not in Fischer 344 rats, CD-1 mice or ovariectomised Sprague-Dawley rats). However, hydroxyatrazine was not carcinogenic in the carcinogenicity study with Sprague-Dawley rats. The lowest doses at which the triazines increased the incidence and/or caused an earlier onset of mammary gland tumours were 1.5, 5.3, 53, 1.4, and 10 mg/kg bw/day for atrazine, simazine, terbutylazine, cyanazine, and DACT, respectively. The NOAELs for carcinogenicity in Sprague-Dawley rats were 0.5, 0.52, 7.0, 0.26, and 3.5 mg/kg bw/day, respectively. An increased incidence of Leydig cell tumours was observed in one study with atrazine and in one with terbutylazine in Sprague-Dawley rats at doses of about 50 mg/kg bw/day. In one of the studies, the incidence fell within historical control data and was attributed in part to the better survival of these animals, and in the other study, the tumours were observed mainly in the old rats. An increased incidence of pituitary gland carcinomas was observed in one study with simazine in female Sprague-Dawley rats at doses of about 46 mg/kg bw/day. The incidence fell within historical control data. In the same study, a small increase in renal tubular tumours was observed in both sexes at the same dose based on which the European Commission has classified simazine for carcinogenicity. IARC have concluded that there is sufficient evidence in experimental animals for the carcinogenicity of atrazine and that there is limited evidence in experimental animals for the carcinogenicity of simazine. However, IARC and US-EPA have concluded that there is strong evidence that the mechanism by which atrazine increases the incidence of mammary gland tumours in Sprague-Dawley rats is not relevant to humans. 6.8 Evaluation6.8.1 Critical effect(s) and NOAEL(s)The overall critical effect in humans following exposure to triazines and their degradation products are considered to be the neuroendocrine effects (hormonal disturbances and reproductive and developmental effects caused by the disruption of the hypothalamic-pituitary-gonadal axis). This is based on the following: In humans, the available data on health effects are mainly focused on the possible carcinogenic effects. The usefulness of many of these studies are limited because mixed exposure often has occurred. IARC has concluded that there is inadequate evidence in humans for the carcinogenicity of atrazine and simazine. A few studies have focused on toxicity to reproduction without significant findings. In laboratory animals, the main effects observed is the neuroendocrine effects, haematological changes, cardiac toxicity, kidney toxicity, incomplete ossification sites and/or fused sternebrae in foetuses, and microphtalmia/anophtalmia, diaphragmatic hernia associated with liver protrusion, and dilated brain ventricles in foetuses. Overall, the hormonal disturbances occur at lower doses than the other effects. However, for hydroxyatrazine the critical effect seem to be kidney toxicity which occur at lower doses than the neuroendocrine effects and seem to be more severe than for the other triazines and degradation products for which data exist. In addition, hydroxyatrazine causes delayed puberty and altered pregnancy outcome (pre- and postimplantation loss, full litter resorption and delayed parturition) but mammary tumours in Sprague-Dawley rats does not occur suggesting that the main mode of action for hydroxyatrazine might be different from the other triazines and degradation products. However, since the NOAEL for kidney toxicity of hydroxyatrazine is higher than some of the NOAELs seen for the neuroendocrine effects in the other triazines and degradation products, setting an overall NOAEL for neuroendocrine effects will also cover the kidney toxicity caused by hydroxyatrazine. For cyanazine, decreased kidney function has been observed in one poorly reported 4 week rat study from 1968 at very low doses, but in a newer 2-year rat study from 1990, no kidney toxicity was observed at doses that were higher than the doses causing neuroendocrine effects. As the only triazine, cyanazine is causing microphtalmia/anophtalmia, diaphragmatic hernia associated with liver protrusion, and dilated brain ventricles in foetuses suggesting that cyanazine might have an additional mode of action compared to the other triazines and degradation products. However, cyanazine is also causing neuroendocrine effects (mammary gland tumours and altered pregnancy outcome) and at lower doses than the doses causing the developmental effects. Therefore, for cyanazine the critical effect is considered to be the neuroendocrine effects. The European Commission has classified simazine for carcinogenicity based on a small increase in renal tubular tumours at the highest dose tested in one rat study. Since simazine is not mutagenic and genotoxic and the renal tumours occur only in small numbers at a relatively high dose it is likely that a threshold exist for these tumours although the mechanism is unknown. Simazine is causing neuroendocrine effects at lower doses than the doses causing renal tumours. Therefore, for simazine the critical effect is considered to be the neuroendocrine effects. The neuroendocrine effects are mainly ascribed to a disruption of the hypothalamic-pituitary-gonadal axis resulting in a decreased serum level of luteinizing hormone, and an increased serum level of oestrogen and prolactin. In one prenatal study with atrazine, it was shown that atrazine was able to decrease the serum level of luteinizing hormone in several strains of rats but in the same study it was only the Sprague-Dawley rat that had an increased level of serum oestrogen suggesting that all rat strains are sensitive to triazine-induced hormonal disturbances but the Sprague-Dawley rat is more sensitive than the other rat strains to the effect on the serum oestrogen level. This will also explain why the mammary gland tumours (proposed to occur because of a constant elevated serum level of prolactin and oestrogen) were observed only in studies with Sprague-Dawley rats. IARC and US-EPA have concluded that there is strong evidence that the mechanism by which atrazine increases the incidence of mammary gland tumours in Sprague-Dawley rats is not relevant to humans. The mammary gland tumours are generally seen at lower doses than the other endocrine effects. Nevertheless in keeping to the proposed mode of action, the serum level of luteinizing hormone is expected to be decreased at the doses causing tumours. US-EPA has concluded that it is not unreasonable to expect that atrazine might cause adverse effects on hypothalamic-pituitary function in humans and that the same endocrine perturbations that induce tumours also appear to play a role in at least some reproductive developmental effects which may be relevant to humans. For rats it has e.g. been suggested that the full litter resorption is maternally mediated and consistent with loss of luteinizing hormone support of the corpora lutea. A decreased serum level of luteinizing hormone will most likely also influence the human reproduction and development. Therefore in selecting an overall NOAEL for the human risk characterisation of the triazines and their degradation products in drinking water it seems reasonable to select a NOAEL from the carcinogenicity studies. In general, cyanazine seems to be more toxic than the other triazines and their degradation product. This is also the case for the combined chronic toxicity/carcinogenicity study with cyanazine where a NOAEL of 0.20 mg/kg bw/day was established based on mammary tumours in female rats, decreased body weight gain in both sexes of rats, and increased hyperactivity in the male rats at the next dose level. Two reproductive and/or developmental studies have been performed with mixed exposure to the triazines. In the first study, where pregnant rats were exposed to a mixture of atrazine and its degradation products, neuroendocrine developmental effects might have occurred in the male offspring at doses lower than 0.20 mg/kg bw/day but based on the limited data from the study abstracts, it is impossible to set a NOAEL. In the second study, no effects on several reproductive parameters in mice and on developmental toxicity in rats were observed when atrazine, simazine, and cyanazine were administered at individual concentrations of maximal 0.013 mg/kg bw/day (the highest individual dose tested) in a mixture containing several other pesticides, fertilizers and other organic substances. The NOAEL of 0.20 mg/kg bw/day will be used as the overall NOAEL for the human risk characterisation of the triazines and their degradation products in drinking water. 7 Risk characterisation via drinking water exposureA NOAEL of 0.20 mg/kg bw/day is selected for the human risk characterisation of the triazines and their degradation products in drinking water. The general population is predominantly exposed to triazines and their degradation products from intake of contaminated drinking water. Table 2 shows data for selected intakes of triazines and their degradation products via drinking water. The intakes are calculated based on the assumption that an adult weighing 70 kg in average drinks 2 litre of water every day and a child weighing 10 kg in average drinks 0.8 litre of water every day. If, e.g., the water contains 0.1 μg/l, which is the administrative threshold limit in drinking water for individual pesticides, the intake will be 0.003 μg/kg bw/day and 0.008 μg/kg bw/day for adults and children, respectively. Table 2. Selected intakes of triazines and their degradation products via drinking water
Table 3 shows calculated margins of safety for triazines and their degradation products. The margin of safety (MOS) is calculated as: MOS = NOAEL / intake of triazines and their degradation products Table 3. Margin of safety (MOS)
When evaluating specific contaminations for the purpose of setting a MOS the following should be kept in mind. First the abovementioned theoretical MOS are based on a NOAEL which in fact may turn out to be a LOAEL, however the relevant study is not yet fully reported. Furthermore, any situation where setting of MOS for these substances is required should be seen in context with the possible effects of other contaminants. Finally the setting of MOS should always be related to the level of concern for the actual situation, e.g. impact of the contamination over time. 8 ReferencesACGIH (1991). Atrazine (and related symmetrical triazines). In: TLV's Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 1991-1992. Cincinnati, OH, 97-99. Andersen JH, Poulsen ME, Bille RLL, and Meyer O (2003). Pesticidrester i fødevarer 2002 – resultater fra den danske pesticidkontrol.
http://www.foedevaredirektoratet.dk/fdir/publications Ashby J, Tinwell H, Stevens J, Pastoor T, and Breckenridge CB (2002). The effects of atrazine on sexual maturation of female rats. Regulatory Toxicol Pharmacol 35, 468-473. At (2002). Grænseværdier for stoffer og materialer. At-vejledning C.0.1, oktober 2002. ATSDR (2001). Toxicological Profile for Atrazine. Draft for public comment. U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. AVJ (2001). Erfaringsopsamling – amternes undersøgelser af pesticidpunktkilder. Teknik og Administration nr. 2. Amternes Videncenter for Jordforurening. BCERF (1998a). Critical evaluation of simazine's breast cancer risk. Cornell University. Program on Breast Cancer and Environmental Risk Factors in New York State.
http://envirocancer.cornell.edu/CriticalEval/ BCERF (1998b). Critical evaluation of cyanazine's breast cancer risk. Cornell University. Program on Breast Cancer and Environmental Risk Factors in New York State.
http://envirocancer.cornell.edu/CriticalEval/ Bogdanffy MS, O'Connor JC, Hansen JF, Gaddamidi V, Van Pelt CS, Green JW, and Cook JC (2000). Chronic toxicity and oncogenicity bioassay in rats with the chloro-s-triazine herbicide cyanazine. J Toxicol Environ Health A60, 567-586. Brüsch W (2002). Statusrapport 2002: Pesticidforurent vand i små vandforsyningsanlæg. Danmarks og Grønlands Geologiske Undersøgelse. Report number: 2002/87.
http://www.geus.dk/program-areas/water/denmark Brüsch W, Stockmarr J, Kelstrup N, von Platen-Hallermund G and Rosenberg P (2004). Pesticidforurenet vand i små vandforsyningsanlæg. Danmarks og Grønlands Geologiske
Undersøgelse, Rapport 2004/9. http://www.geus.dk/program-areas/water/denmark ChemFinder (2003a). Atrazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003b). Cyanazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003c). Simazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003d). Terbutylazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003e). Desethyl atrazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003f). Desisopropyl atrazine. In: http://chemfinder.cambridgesoft.com. ChemFinder (2003h). Deisopropyldesethylatrazine. In: http://chemfinder.cambridgesoft.com ChemFinder (2003i). Hydroxyatrazine. In: http://chemfinder.cambridgesoft.com Cooper RL, Stoker TE, Tyrey L, Goldman JM, and McElroy WK (2000). Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol sciences 53, 297-307. DMU (2000). Nationalt program for overvågning af vandmiljøet 1998-2003 "NOVA 2003". Datablade for stoffer der indgår i NOVA 2003. http://ovs.dmu.dk/2NOVA_2003_ov./4datablade/vejled~3.doc og http://ovs.dmu.dk/2NOVA_2003_ov./4datablade/pesticider.doc EC (1996a). European Commission Peer Review Programme: Atrazine (incl. addenda). EC (1996b). European Commission Peer Review Programme: Simazine (incl. addenda). Enoch R, Greiner S, Youngblood G, Davis C, and Fenton SE (2003). Effects from gestational exposure to a mixture of atrazine and its biological metabolites in male Long-Evans rats. Toxicologist 72 (Suppl 1), 275. EU (2003). EU MRLs for pesticides. Updated 28/10/2003. http://europa.eu.int/comm/food/fs/ph_ps/pest/index_en.htm Fenton SE, Greiner SN, Youngblood GL, and Davis CC (2002). Effects from gestational exposure to a mixture of atrazine and its biological metabolites in male Long-Evans rats. Biol Reprod 66 (Suppl 1), 199-200. FM (2003). Bekendtgørelse om pesticidrester i fødevarer og foderstoffer (med senere ændringer). Fødevareministeriets bekendtgørelse nr. 184 af 20. marts 2003. Friedmann A (2002). Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reproductive Toxicol 16, 275-279. Hopenhayn RC, Stump ML, and Browning SR (2002). Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Arch Environ Contam Toxicol 42, 127-136. IARC (1999a). Atrazine. In: IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans, Vol. 73, Lyon, 59-113. IARC (1999b). Simazine. In: IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans, Vol. 73, Lyon, 625-640. Iyer P, Gammon D, Gee J, and Pfeifer K (1999). Characterization of maternal influence on teratogenicity: An assessment of developmental toxicity studies for the herbicide cyanazine. Regulatory Toxicol Pharmacol 29, 88-95. Jørgensen LF (2002). Grundvandsovervågning 2002. Danmarks og Grønlands Geologiske Undersøgelse. http://www.geus.dk/publications/grundvandsovervaagning/g-o-2002.pdf. Kligerman AD, Doer CL, Tennant AH, and Peng B (2000a). Cytogenetic studies of three triazine herbicides. II. In vivo micronucleus studies in mouse bone marrow. Mutation Research 471, 107-112. Kligerman AD, Doer CL, Tennant AH, and Zucker RM (2000b). Cytogenetic studies of three triazine herbicides. I. In vitro studies. Mutation Research 465, 53-59. Kniewald J, Jakominiæ M, Tomljenoviæ A, Šimiæ B, Romac P, Vranešiæ Ð, and Kniewald Z (2000). Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol 20, 61-68. MacLennan PA, Delzell E, Sathiakumar N, and Myers SL (2003). Mortality among triazine herbicide manufacturing workers. J Toxicol Environ Health A66, 501-517. Merck Index (1996a). Atrazine. In: 12th. Ed., Rahway, New Jersey, Merck & Co., Inc., 147-148. Merck Index (1996b). Cyanazine. In: 12th. Ed., Rahway, New Jersey, Merck & Co., Inc., 452. Merck Index (1996c). Simazine. In: 12th. Ed., Rahway, New Jersey, Merck & Co., Inc., 1464. Mills PK and Yang R (2003). Prostate cancer risk in California farm workers. J Occup Environ Med 45, 249-258. MM (2001). Bekendtgørelse om vandkvalitet og tilsyn med vandforsyningsanlæg. Miljø- og Energiministeriets bekendtgørelse nr. 871 af 21. september 2001. MM (2002). The Statutory Order from the Ministry of the Environment no. 439 of June 3rd 2002, on the List of Chemical Substances. MST (2003a). Miljømæssig vurdering af terbutylazine, Bilag 1.a. MST (2003b). Oversigt over godkendte bekæmpelsesmidler. http://www.mst.dk/bekaemp/findbek.asp NRA (2001). Public release summary on evaluation of the active terbutylazine in the product Swimcare T swimming pool algaecide. National registration authority for agricultural and veterinary chemicals. Canberra, Australia. http://www.apvma.gov.au/publications/prster.pdf Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, and van der Berg M (2001). Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspectives 109, 1027-1031. Schroeder JC, Olshan AF, Baric R, Dent GA, Weinberg CR, Yount B, Cerhan JR, Lynch CF, Schuman LM, Tolbert PE, Rothman N, Cantor K, and Blair A (2001). Agricultural risk factors for t(14;18) subtypes of non-Hodgkin's lymphoma. Epidemiology 6, 701-709. Stoker TE, Guidici DL, Laws SC, and Cooper RL (2002). The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sciences 67, 198-206. Suárez S, Rubio A, Sueiro RA, and Garrida J (2003). Sister chromatid exchanges and micronuclei analysis in lymphocytes of men exposed to simazine through drinking water. Mutation Research 537, 141-149. Tennant AH, Peng B, and Kligerman AD (2001). Genotoxicity studies of three triazine herbicides: in vivo studies using the alkaline single cell gel (SCG) assay. Mutation Research 493, 1-10. US-EPA (1995). Re-registration eligibility decision (RED) Terbutylazine. http://www.epa.gov/oppsrrd1/REDs/2645.pdf US-EPA (2002a). Atrazine. Toxicology chapter of the re-registration eligibility decision. Second revision. April 11, 2002.
http://www.epa.gov/pesticides/reregistration US-EPA (2002b). The grouping of a series of triazine pesticides based on a common mechanism of toxicity.
http://www.epa.gov/pesticides/cumulative US-EPA (2002c). Atrazine/DACT. Fourth report of the hazard identification assessment review committees. April 5, 2002.
http://www.epa.gov/pesticides/reregistration/ WHO (1996a). Atrazine. In: Guidelines for drinking-water quality. Second edition, Vol. 2. World Health Organization, Geneva, 608-614. WHO (1996b). Simazine. In: Guidelines for drinking-water quality. Second edition, Vol. 2. World Health Organization, Geneva, 753-758. WHO (1998a). Cyanazine. In: Guidelines for drinking-water quality. Second edition, Vol. 2. World Health Organization, Geneva, 165-175. WHO (1998b). Terbutylazine. In: Guidelines for drinking-water quality. Second edition, Vol. 2. World Health Organization, Geneva, 245-253. Table 4. Repeated dose toxicity animal studies with oral exposure to triazines.
Table 5. Repeated dose toxicity animal studies with dermal contact to triazines.
Table 6. Toxicity to reproduction animal studies with oral exposure to triazines. Table 7. Mutagenic and genotoxic effects of triazines in vitro.
Table 8. Mutagenic and genotoxic effects of triazines in vivo.
Table 9. Carcinogenic effects in animal studies with oral exposure to triazines.
Table 10. Lowest NOAELs/LOAELs (mg/kg bw/day) for selected neuroendocrine effects in rats (unless otherwise stated) following oral exposure to the triazines or their degradation products. Table 11. Lowest NOAELs/LOAELs (mg/kg bw/day) for selected reproductive and developmental effects other than neuroendocrine effects in rats (unless otherwise stated) following oral exposure to the triazines or their degradation products.
Table 12. Lowest NOAELs/LOAELs (mg/kg bw/day) for selected repeated dose toxicity effects other than neuroendocrine effects in rats (unless otherwise stated) following oral exposure to the triazines or their degradation products.
Footnotes[3] - = No data or not able to determine a NOAEL/LOAEL from available data [4] Anaemia, increased myeloid hyperplasia in the bone marrow, extramedullary haematopoiesis in the liver and spleen, and/or haemosiderin pigment in the spleen [5] Clinical signs, ECG alterations, heart weight changes, and macroscopic and/or histopathological findings [6] Changes in clinical signs, in haematology, clinical chemical, and urinalysis parameters, in kidney weight, and/or in macroscopy and histopathology [7] - = No data or not able to determine a NOAEL/LOAEL from available data
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||