|
Pesticides Research no. 83, 2004 The Effect of Esfenvalerate and Prochloraz on Amphibians with special reference to Xenopus laevis and Bombina bombinaContents
PrefaceThis report is a part of the research programm “Effects of Pesticides on Ponds”. The projects were funded by the Danish Environmental Protection Agency' s Research programme on Environmental effects of pesticides. The aim of the project was:
The project consisted of four subprojects with individual objectives. The sub-projects are listed in Table 1. Table 1. Sub-projects of ” Effects of Pesticides on Ponds”. Tabel 1. Oversigt over delprojekter i ”Effekter af pesticider i vandhuller”.
The reports produced by the projects are:
The project was overseen by a steering committee. The members have made valuable contributions to the project. The committee consisted of:
For this particular report we especially wish to express our gratitude to Dr. Per Rosenkilde, University of Copenhagen, for the use of facilities for maintaining adult Xenopus, and to Mrs. Inger Sørensen for excellent skilled technical assistance. Moreover Linda Gertz is thanked for the preparation of the manuscript. The financial support of the Danish EPA is gratefully acknowledged. SummaryThe South African clawed frog, Xenopus laevis is the test organism used in the only internationally recognised test with amphibians. However, this test has a number of deficiencies, which make it less suited for ecotoxicological risk assessment in Denmark. The purpose of this study has therefore been to develop and to test a new test guideline with the fire-bellied toads Bombina since this organism has widespread wild living species represented in Europe. When choosing Bombina bombina as a test organism, it has become possible to make an important comparison between laboratory tests and field test. The results from the present study have revealed that it is possible to breed Bombina bombina throughout the year and get a sufficient number of healthy embryos for ecotoxicological testing. A new test guideline using Bombina was tested with two pesticides (the insecticide esfenvalerate and the fungicide prochloraz). We also conducted standard tests with Xenopus with the two pesticides to compare their sensitivity as bio-indicators. Esfenvalerate has only a small effect on the mortality on both Bombina and Xenopus embryos and the LC50 values were higher than 150 µg/l in both species. However, when the number of malformations were used as an end point it was demonstrated that the EC50 values for malformations were only 3 µg/l and 29 µg/l for Xenopus and Bombina, respectively. It should be noted that Xenopus and Bombina responded to esfenvalerate in the same way and that the change in sensitivity was less than a factor of 10. Such a difference can easily be ascribed to normal differences in species sensitivity. Furthermore, it is interesting that the in vivo observation revealed an effect as low as 1 µg/l in both species. These effects are twisting and apparent partial paralysis of the embryos. For Xenopus and Bombina, no significant mortality was seen at prochloraz concentrations below 3 mg/l and the LC50 values were found to be 4.5 mg/l and more than 10 mg/l for Xenopus and Bombina, respectively. As for esfenvalerate the most sensitive end point which can easily be quantified is the malformation where an EC50 value of 1.4 and 1.5 mg/l were calculated for Xenopus and Bombina, respectively. Many embryos possess multiple malformations, and the same malformations were seen in both Bombina and Xenopus. The present study has also demonstrated that the jelly coat has no significant influence on the effect of prochloraz on the embryos of Xenopus. Even though the LC50 value is higher in Bombina as compared with the value found in Xenopus it should be noted that the difference in effect concentration was only about a factor of 2. Thus, it can be concluded that prochloraz has the same effect on Bombina and Xenopus and at the same concentrations as well. If mortality was our only measure of response to pesticide exposure, we would conclude that these amphibians were relatively tolerant to these pesticides. However, the sublethal effects that we witnessed are likely to have serious implications on the long-term success of the exposed individuals. Additional research on environmental concentrations, more chemicals, effects of temperatures, and life-stage sensitivity should be investigated before the toxicity of pesticides to amphibians can be evaluated properly. Dansk sammendragFETAX testen, der anvender den sydafrikanske sporefrø, Xenopus laevis, er den eneste internationalt anerkendte testmetode til vurdering af kemiske stoffers effekt på amfibier. Da denne testmetode på en række områder vanskeliggør en relation til danske forhold, er et af formålene med denne undersøgelse derfor at udarbejde en ny test guideline med en for europæiske forhold relevant testorganisme. Klokkefrøen Bombina bombina er blevet udvalgt som testorganisme i denne undersøgelse, da den er udbredt over hele Europa og derudover er grundigt undersøgt her i Danmark. Ved at anvende denne frø er det muligt at lave en sammenligning mellem de udviklede laboratorieforsøg og de feltforsøg, der udføres i Fyns amt. Resultaterne fra denne undersøgelse har vist, at det er lykkedes at udarbejde en metode, således at vi kan få klokkefrøen til at yngle hele året og få den til på et på forhånd fastlagt tidspunkt at give et tilstrækkeligt antal sunde embryoner, hvilket er en forudsætning for at kunne anvende denne organisme som testorganisme til økotoksikologiske test. Den udviklede testmetode blev undersøgt med to pesticider (esfenvalerat og prochloraz). Derudover blev der udført standard forsøg med Xenopus med de samme to pesticider for herved at kunne sammenligne følsomheden hos de to forskellige arter. Esfenvalerat har kun en lille effekt på embryonernes dødelighed, og dette gælder både for Xenopus og Bombina. Således er LC50 værdien større end 150 µg/l for begge arter. Hvis man derimod betragtet misdannelser som et end point, findes EC50 værdier nede på 3 og 29 µg/l for henholdsvis Xenopus og Bombina. Det skal dog bemærkes, at de to arter reagerer på esfenvalerat på samme måde, samt at forskellen i følsomhed er under en faktor 10. En sådan forskel i følsomhed betragtes ikke som værende markant. Det skal bemærked, at der ved in vivo observationer blev iagttaget en markant effekt ved en stofkoncentration på helt ned til 1 µg/l og det hos begge arter. Effekten ved disse lave koncentrationer bestod i, at embryonerne havde krampeagtige vridninger, en effekt der forøges i intensitet med forøget eksponeringstid og dosis. Der ses ingen forøget dødelighed, når Xenopus og Bombina eksponeres for prochloraz i stofkoncentrationer på op til 3 mg/l. LC50 værdierne blev bestemt til at være 4,5 mg/l hos Xenopus og mere end 10 mg/l hos Bombina. Det mest følsomme end point, der let kunne kvantificeres, var dog, som tilfældet var ved esfenvalerat, registreringen af misdannelser. Således fandtes EC50 værdier på 1,4 mg/l hos Xenopus og 1,5 mg/l hos Bombina. Flere af embryonerne havde mange misdannelser, og samme type af misdannelser blev observeret hos de to forskellige arter. Denne undersøgelse viste, at gelékappen omkring frøæggene ikke havde nogen virkning på effekten af prochloraz. Derudover viser resultaterne fra både esfenvalerat og prochloraz, at der ikke er en markant forskel i følsomheden overfor disse to stoffer hos de to undersøgte arter. Undersøgelsen viser endvidere, at hvis man alene bygger sin vurdering over pesticidernes toksicitet på en registrering af dødelighed efter en given eksponeringsperiode, vil man antage, at disse amfibier er relativt tolerante overfor de undersøgte pesticider. Det er dog af væsentlig betydning også at tage de sublethale effekter med i sin vurdering af stofferne. Baseret på de misdannelser, som de valgte pesticider forårsager, er det klart, at forkerte konklusioner ville være draget, hvis man ikke tog hensyn til de in vivo observationer og de øvrige sublethale effekter, som stofferne medfører. Før en endelig vurdering af risikoen vedrørende forskellige pesticiders toksiske effekter overfor amfibier i danske vandhuller kan foretages, er det nødvendigt at foretage yderligere undersøgelser. Disse undersøgelser bør omfatte en måling af pesticid-koncentrationer i miljøet, inddragelse af flere pesticider til effektvurderinger, undersøgelse af forskellige temperaturers indflydelse på effekten af pesticider samt en analyse af forskellige livscyklus stadiers følsomhed overfor pesticider. 1 IntroductionThere is strong evidence that many amphibian species from a variety of taxa are declining at a drastic rate world-wide (Blaustein and Wake, 1990). Although much of the present decline may be due to loss of suitable habitats or due to swamp drainage, predation and disease, and local development, aquatic contaminants are likely to be involved (Cooke, 1972; Harte and Hoffman, 1989; Bradford, 1991). The increasing use of pesticides in modern agriculture has been mentioned as one of the reasons for the decline of the amphibian fauna stated over the last 40 years. A number of tests of field water ponds/streams in intensive grown farm lands have shown content of pesticides coming directly from spraying, wind drift, surface runoff/drain flow and washing of spraying equipment (Gomme et al. 1991; Berrill et al. 1993). Amphibians are potentially sensitive indicator organisms of environmental stress because of their permeable skins and bi-phasic life cycle (Duellman and Trueb, 1986). Amphibians are not likely to be killed by normal exposures, due to low concentrations that are likely to occur in aquatic systems. Recognition of a need to identify sublethal effects of contamination in order to assess sensitivity is therefore growing. It is important to measure embryo mortality, malformation and growth inhibition because they can often occur at concentrations far less than those required to affect adult organisms. The idea of the present study is to investigate the effect of two pesticides with different mode of action on embryos of two different amphibian species. The selected pesticides are
We use an in vitro standard biological test (FETAX) that is designed to use on embryos of the South African clawed frog Xenopus laevis. This bioassay gives data on the lethal, teratogenic and growth retardation effects caused by xenobiotic exposure. It is possible to test pure chemicals or complex mixtures (even with an unknown composition) that are soluble in water. The test can be realised in a short time and with low costs. Because of the sensitivity of embryonic and early life stages, FETAX provides information that may be useful when estimating the chronic toxicity of a test material to aquatic organisms. However, the sensitivity of embryo and tadpole stages of a particular species may vary significantly. Thus, the effect of many pesticides, e.g. pyrethroids, is found to be highly dependent on the temperatures, and therefore varies with latitude and season (Coats et al., 1989; Materna et al., 1995). The effect of pesticides on populations in northern latitudes as well as spring-breeding species may therefore be different from the effect found in test with an African frog. A new standardised toxicity test with Bombina bombina, which has a European distribution and an aquatic lifestyle outside the hibernation season, was therefore developed. The species has a wide distribution in nature, from Denmark in the West to the Urals in the East and Greece in the South (Corbett, 1989). B. bombina inhabits lowlands of Northern and Central Europe, and breeds most typically in lakes, for example ox-bows, fish ponds or flooded areas alongside larger rivers (Madej, 1973). It is declining in especially the north-western part of its range. Acquired knowledge from the laboratory tests in the present study is validated with field and semi-field data from Funen's County, the National Forest and Nature Agency, the University of Roskilde (RUC), and the adjoining projects by the Danish National Environmental Research Institute. The Danish Technological Institute's part of the total pond project will thus make up the necessary laboratory counterpart for the field test of the direct and indirect effects of pesticides. This makes the important comparison between laboratory tests and field tests possible. 2 Materials and methods2.1 Principles and methods for maintaining and breeding the South African clawed frog, Xenopus laevis and the fire-bellied toads, Bombina bombina 2.1 Principles and methods for maintaining and breeding the South African clawed frog, Xenopus laevis and the fire-bellied toads, Bombina bombina2.1.1 Facilities for maintaining adults XenopusAdult Xenopus were kept in an animal room without any light-affection from outside, in such a way that a photoperiod of 12-h day/12-h night could be maintained. By keeping this photoperiod it was possible to breed Xenopus year-round. Adults were kept in large aquaria or in fibreglass raceways at densities of less than 6 per 1800 cm² of water surface area. The water depth was between 7 and 14 cm and the water was aerated by the use of air stones. pH of the water was between 6.5 and 9. Xenopus males were between 7.5 and 10 cm in crown-rump length and more than 2 years old. Adult males had dark pads on both side of every forearms and did not have cloacal labra. Adult females were between 10 and 12.5 cm in length and at least 3 years old. Females were larger than males and easy to identify by the presence of prominent cloacal labra. Diet The adults were fed three times per week with beef liver. Liquid multiple vitamins were added to the ground beef liver (AMSTM standard E 1439-91). Temperature Adults were kept at 23±3°C. 2.1.2 Facilities for breeding XenopusMales and females were bred as a single pair in a FETAX solution. FETAX solution was composed of 625 mg NaCl, 96 mg NaHCO3, 30 mg KCl, 15 mg CaCl2, 60 mg CaSO4. 2H2O, and 75 mg MgSO4 per litre of deionised or distilled water. The pH of the final solution should be 7.6 to 7.9. All chemicals was reagent-grade or better. A 45l glass aquarium fitted with a 1 cm mesh suspended about 3 cm above the bottom of the aquarium was used. This ensured that deposited eggs could lie undisturbed on the bottom of the aquarium and could be scraped into Petri dishes. The sides of the breeding aquarium were opaque and a bubbler fitted to oxygenate the water. The top of the aquarium was covered with opaque porous material. Water temperature was adjusted to 23±0.5°C . Breeding To mature the oocyttes, the females received about 200 IU of Pregnant Mare Gonadotropin (PMG) 2 days before the animals were moved to the breeding aquarium. To induce breeding, the males and the females received 250 to 500 and 500 to 1000 IU, respectively, of human chorionic gonadotropin by way of injection into the dorsal lymph sac. The hormone concentration was about 1000 IU/ml in sterile 0.9% NaCl. The amount of human chorionic gonadotropin injected depended on the time of year and condition of the adults. Lower doses were usually used in spring and higher doses in autumn. Amplexus normally ensues within 2 to 6 h and eggs deposition about 9 to 12 h after injection. The eggs were immediately inspected for fertility and quality. The fertility rate should be > 75% before a toxicity test was performed. Eggs laid in “strings” or not perfectly round was not used because they develop abnormally. 2.1.3 Principles and methods for maintaining adults Bombina bombinaAdult Bombina was at least 2 years of age and weighed about 6.5 to 8.5 g (males and females are about the same size). 1-2 pairs were kept together in a 50 x 30 cm vivarium, the floor area of which was 60% water, about 8 cm deep and the land area was dark-coloured with hiding and a feeding place. The vivarium was fitted with a mesh to prevent escape. Artificial lighting was switched on from 7 am to 7 pm daily, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Slow growing natural aquatic plants and plants of plastic and broken pots were provided to create hiding places. Diet Food consisted of crickets, meal worms, small earthworms, flies (with crumpled wings) and other suitable invertebrates supplemented with a special vitamin supplement. Feeding was continued throughout the year, although at a reduced rate (about 2 times a week) during November-February when the animals showed minimal activity. Temperature Adults were kept at 19-21°C in the winter and at room temperature in the summer period. 2.1.4 Principles and methods for breeding Bombina bombinaMales and females were bred as a single pair and the animals were moved to a 45 l glass aquarium with a water depth of about 20 cm. The aquarium was richly furnished with both natural and plastic plants. In addition, 2 mm round sticks of wood were placed in the aquarium, and 1 to 2 islands of floating cork enabled the animals to leave the water if desired. Artificial lighting was switched on from 5 am to 9 pm daily, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Water temperature was adjusted to 22±0.5°C . Breeding To mature the oocyttes, the females received about 14 IU of Pregnant Mare Gonadotropin (PMG) 2 days before the animals were moved to the breeding aquarium. Just before the animals were moved to the breeding aquarium both males and the females received 55 to 100 IU, of human chorionic gonadotropin to induce breeding. The amount of hormones injected depended on the size of the animals and the time of year. Lower doses were usually used in spring and higher doses in autumn. Amplexus normally ensued within 2 to 6 h and eggs deposition about 9 to 12 h after injection. The eggs were immediately inspected for fertility and quality. The fertility rate should be > 75% before a toxicity test was performed. Examination of the aquarium showed that the eggs were often found on vegetation and on the round sticks of wood, which were placed in the breeding aquarium to imitate straw and stems. 2.2 Toxicity tests using embryos of Xenopus laevis and Bombina bombina2.2.1 Principle and design of the toxicity testFETAX is a 96 h renewal whole embryo assay that can be used to evaluate the developmental toxicity of a test material. Exposure is continuous throughout the test. For each concentration, two dishes each containing 25 embryos and 10 ml of test solution are used. For each control, four dishes of 25 embryos each are used in the standard experiments with Xenopus, however, in experiments with Bombina only 5 embryos are placed in each dish. Embryos must be randomly assigned to test dishes. Dishes must be randomly assigned to their positions in the incubator. In order to evaluate developmental toxicity, mortality, malformation, and growth inhibition properly, data must be collected. In most tests, it will be possible to generate concentration-response curves for mortality, malformation, and growth inhibition. The mortality and malformation concentration-response curves should then be used to estimate the concentration that would affect 50% of the exposed embryos. At least 90% of the FETAX solution controls must have attained stage 46 at 96 h (Nieuwkoop and Faber, 1975). Test organisms The aim was to compare the results obtained from embryos of the South African clawed frog, Xenopus laevis with another species, which has a wide distribution in Europe, the fire-bellied toads, Bombina bombina. Test chemicals The aim was to determine the effect of two different pesticides with different mechanisms of toxic action and with different in physico-chemical properties. Egg manipulation The aim was to assess the influence of the jelly coat on the toxicity of pesticides. This part of the work was carried out because the jelly coat is normally removed before the starting on an experiment in an ordinary FETAX test. 2.2.2 Test substancesTwo pesticides with different mechanism of toxic action and physicochemical properties were selected:
Table 2-a Udvalgte fysisk-kemiske egenskaber for de to pesticider
* Aqueous photolysis studies with esfenvalerate and prochloraz indicated that the half-lives of both pesticides are about 10 days, however, the half-lives are of course dependent on the intensity of the light. Data from The Pesticide Manual, tenth edition and the Danish EPA. Esfenvalerate is an insecticide with contact and stomach action and is a voltage dependent sodium channel agonist. It is used as a potent contact and ingested insecticide with a very broad range of activity, especially effective against Coleoptera, Diptera, Hemiptera, Lepidoptera, and Orthoptera on cotton, fruit, vegetables, and other crops at 5-25 g a.i./ha. It is effective against strains resistant to organochlorine, organophosphorus and carbamate insecticides. Prochloraz is an ergosterol biosynthesis inhibitor. Prochloraz is a protectant and eradicant fungicide effective against a wide range of diseases affecting field crops, fruit, turf, and vegetables. An EC is normally recommended for use in cereals (400-600 g. a.i./ha). 2.2.3 Preparation of test solutionsThe test material was reagent-grade or better unless for the commercial product, Sumi alfa (contains 5% esfenvalerate; active ingredients). Stock solutions were prepared daily. The pH of the stock solutions was 7.5±0.5. Dimethyl sulfoxide and acetone were used for preparing stock solutions. Concentrations of dimethyl sulfoxide and acetone in test solutions were < 1.1% v/v. At these concentrations, no effect in the FETAX standard test has been observed (Fort et al., 1991). 2.2.4 EmbryosRemoval of jelly coat Dejellying of embryos should begin immediately after the end of egg laying. Dejellying of embryos was carried out by gentle swirling for 1 to 3 min in a 2% w/v L-cysteine (CAS #52-90-4) solution prepared in FETAX solution. The cysteine solution was adjusted to pH 8.1 with 1 N NaOH. The solution was made up immediately prior to use. Dejellying was monitored continuously and the process stopped just after all jelly was removed. Care should be taken not to treat the embryos too long because survival may be reduced. Staging of embryos Staging of embryos was done according to Nieuwkoop and Faber (1975). Embryo selection Normally cleaving embryos were selected for use in testing. Two levels of selection were used. In double selection, normally cleaving embryos were first sorted into dishes containing fresh FETAX solution. After a short period during which cleavage continues, embryos were sorted again to ensure that only normal embryos were selected. Abnormal pigmentation was viewed as an indicator of bad embryos. Both Nieuwkoop and Faber (1975) and the “Atlas of Abnormalities” [1] were used as a reference to determine whether the cleavage pattern was normal. Midblastula (stage 8) to early gastrula (stage 11) was used to start the test. By this stage, normal cleavage and development can be ascertained. Embryos chosen prior to stage 8 might develop abnormal cleavage patterns later whereas embryos selected after stage 11 have commenced organogenesis. A large bore blood bank Pasteur pipette was used to transfer embryos at this stage without any harm. The sorting was done in Petri dishes. 2.2.5 Culture mediumThe culture medium used for the test was FETAX solution. This medium was used for embryos of both Xenopus and Bombina. 2.2.6 Experimental designAll tests with embryos of Xenopus and Bombina were conducted in an incubator at 24±0.5°C. The tests chambers were covered 60 mm glass Petri dishes (before use all glass wares were treated with silylation reagent solution and thoroughly washed in water) or in a few cases in disposable 55 mm polystyrene Petri dishes with an initial culture volume of 10 ml. If a solvent other than dilution-water or FETAX solution was used for preparing test solutions, the concentration of solvent was the same in all test solutions that contained the test material and a solvent control was incorporated in the test which contained the same concentration of solvent. The Petri dishes in the incubator were randomised by rows. A binocular dissection microscope capable of magnifications up to 30 × was used to count and evaluate abnormal embryos. The embryo length (head-tail length measurements) was measured through the use of a map measurer or an ocular micrometer. Maintenance of separate clutches It is necessary to keep clutches separate because embryos of a particular mating pair might develop poorly although they initially appear acceptable. This would cause all the embryos to be discarded if embryos are mixed from different mating pairs. Renewal Renewal of the medium was performed every 24 h during the test. The renewal procedure entails fresh replacement of test material every 24 h during the test. Just prior to this change the pH was measured of the control and the highest test concentrations in order to determine if significant changes occurred. The pH of the test solutions was 7.5 +/- 0.5. Renewal is accomplished by removing the test solution with a Pasteur pipette. The orifice of the Pasteur pipette should be enlarged and fire-polished to accommodate embryos without damage in case the embryos are accidentally picked up. This is standard procedure for FETAX. Duration of the test The standard exposure time for FETAX is 96 h and the attainment of stage 46 in controls. Deviations from this standard exposure time must be reported as deviating from standard FETAX conditions. Thus in the Bombina experiments, the exposure time was 120 h. 2.2.7 Determination of the effects on embryo of Xenopus and Bombina bombinaIn vivo observations In vivo observations were performed every 24 h during the test. A binocular dissection microscope capable of magnifications up to 30 was used to evaluate the abnormal embryos. Mortality Dead embryos was removed at the end of each 24 h period when the solutions were renewed and the mortality data were recorded. If dead embryos were not removed microbial growth could occur which might kill living embryos. Death at 24 h (stage 27) was ascertained by the embryo's skin pigmentation, structural integrity, and irritability (measured on lack of response on physical stimulations). At 48 h (stage 35), 72 h (stage 42), and 96 h (stage 46) the lack of heartbeat serves as an unambiguous sign of death. At 96 h of exposure or stage 46 of controls, the total number of dead embryos was recorded during the test (mortality was registered after 24, 48, 72, and 96 h of exposure). Dead embryos were removed and the remaining living embryos were fixed in 3% formaldehyde solution. Malformation Malformation and other sublethal end points are normally more sensitive than mortality and are therefore included in the present test protocol. As abnormal embryos rarely survive in nature this test end point may also be used to estimate chronic toxicity to aquatic organisms. Malformation was recorded at the end of 96 h. Embryos exposed to the test material was compared with appropriate controls. The number of malformations in each category was reported in standard format to ease interlaboratory comparison (cf. Appendix 1). Growth inhibition The ability of a material to inhibit embryonic growth is often the most sensitive indicator of developmental toxicity. Thus, Berrill et al. (1993) exposed five species of amphibians to pyrethroid insecticide at concentrations between 10 and 200 µg/l with no results of mortality, but with a notable reduction in growth rates. Head-tail length data (growth) were likewise tested at the end of each test. If the embryo was curved or kinked, the measurement was made as if the embryo was straight. Measurement was made after embryos were fixed in 3% formaldehyde solution. Teratogenic Index Teratogenic Index (TI) is determined after 96 h of exposure. TI is defined as 96 h LC50 (mortality) divided with 96 h EC50 (malformations). TI values higher than 1.5 signify large separation of mortality and malformation concentration ranges and, therefore, a large potential for all embryos to be malformed in the absence of significant embryo mortality (ASTM 1991). 2.2.8 Replicates and controlsThe test design includes three independent tests (on different days). For each concentration, two dishes each containing 25 embryos of Xenopus and 5 embryos of Bombina and 10 ml of test solution are used. For each control, four dishes of 25 or 5 embryos are used. Thus, a range-finding and three replicate tests are performed on each test material. A control, in which no test material has been added, is used to provide
If a solvent other than dilution-water or FETAX solution is used, at least one solvent control, using solvent from the same batch used to make the stock solution, must be included in the test. Range-finding The range-finding test consists of a series of at least seven concentrations that differ by a factor of ten. This should be adequate to delineate the concentration range needed to establish the 96 h LC50 and EC50 (malformation). The more similar the range-finding and replicate-definitive test are, the more useful the range-finding test will be. Growth inhibition data are not collected from range-finding tests. Replicate-definitive tests - number of tests and data collection Three definitive tests were conducted on each test material in a random block design. Because it is necessary to acquire data on mortality, malformation, and growth inhibition, the concentration series needs to be adjusted to the expected 96 h LC50, 96 h EC50 (malformation), and the minimum concentration needed to inhibit growth (MCIG). To ensure an adequate supply of normal embryos for each test, two mating pairs were induced and clutches harvested, separately. Embryos were sorted to ensure viability prior to testing. Each test used early embryos derived from a single mating pair. Each individual test will yield data that will be used to generate concentration-response curves for mortality, malformation, and growth inhibition. Experimental dilutions Each test consisted of at least five concentrations for determining concentrations-response curves for both mortality and malformation. Reference toxicant For a positive control or reference toxicant, 6-aminonicotinamide presents a mortality and malformation database convenient for reference purpose. For each test, the positive control consisted of two dishes of 25 or 5 embryos each exposed to 2500 mg 6-aminonicotinamide/l and two dishes of 25 or 5 embryos each exposed to 5.5 mg 6-aminonicotina-mide/l. Only the biological responses related to mortality and malformation were considered in the analysis. Growth inhibition was not considered in regard to responses to 6-aminonicotinamide. 2.2.9 Physical - analysisThe temperature of the incubator was measured daily by a thermometer. The pH was daily checked before and after the addition of the test substance. The pH examination was made with a pH electrode. The pH of the stock and test solutions should be 7.5. 2.2.10 Chemical - analysisRenewal of the medium was performed every 24 h during the test. Renewal was accomplished by removing the approximately 10 ml test solution (exact amount known) with a Pasteur pipette to a 10 ml glass flask. Determination of esfenvalerate and prochloraz in these solutions were carried out in samples after 24 h, 72 h, and after 96 h of exposure. Esfenvalerate The 10 ml sample was spiked with standard solutions of phenanthrene-d10 and cyhalothrin in acetone. The spiked sample was then extracted with 1 ml toluene by shaking for 30 minutes on an “end-over-end” shaker. Finally, the organic phase was transferred to 1 ml glass vial for analysis by GC-MS in SCAN-mode and SIM-mode, respectively. Prochloraz 20 µg of propiconazol (internal standard) in acetone (1036 ng/µl) was added to the 10 ml sample, corresponding to an aqueous concentration of approximately 2.1 µg/ml. The aqueous samples were then extracted (shaken mechanically for 20 minutes) with 1 ml pentane. The pentane phase was transferred to 1 ml glass vials. 100 µl was transferred to another glass vial, evaporated to dryness, and redissolved in 100 µl of hexane. The hexane extract was subsequently analysed by capillary gas chromatography combined with mass spectrometry. The mass spectrometer was operated in the selective ion monitoring mode (GC-MS-SIM). 2.2.11 Data treatment and reportingWith the Probit analysis it was possible to obtain concentrations-response curves to determine the values of 96 h LC50 and 96 h EC50. The ratio between these two values gives the Teratogenic Index (TI). The comparison of measurement results (head-tail length) between controls and treated embryos was obtained with the ANOVA statistical analysis. The minimum concentration to inhibit growth (MCIG) was the minimum concentration of test material that significantly inhibits growth as determined by measurement of head-tail length. A significant difference in growth should be determined by the t-Test for grouped observations at the p = 0.05 level (Dawson D.A. et al. 1989). Fodnoter[1] Availabe from John A. Bantle, Dept. of Zoology, 430 LSW, Oklahoma State University, Stillwater OK 74078. 3 Results3.1 The effects of vehicles and 6-aminonicotinamide on embryo development of Xenopus laevis and Bombina bombina Egg deposition of both Xenopus laevis and Bombina bombina was about 9-12 h after injection of human chorionic gonadotropin. About 1,000 eggs could be found in the breeding aquarium with Xenopus. However, only about 75-400 eggs were found in the breeding aquarium with Bombina. As for Bombina, the eggs were found on the aquatic vegetation and plastic plants as well as on the small sticks in the aquarium. 3.1 The effects of vehicles and 6-aminonicotinamide on embryo development of Xenopus laevis and Bombina bombina3.1.1 Embryo development in FETAX solutionsControls Only the best fertilised eggs from a given breeding aquarium were used and only normally cleaving embryos were used in these tests. Control mortality could be kept to less than 5% when this procedure was followed. After 96 h, the control group of Xenopus was in the stage 46. A stage 46 larva was recognised by the appearance of the hind limb bud, the coiling of the gut, and the shape of the operculum covering the gills. The best indicator that the larva had attained at stage 46 was the appearance of the hind limb bud. Gut coiling was also easily observed at stage 46 (at stage 45 embryo does not display complete tight gut coiling). At stage 46, the larva was about 9.8 mm in length. After 24 h, the controls of Xenopus were at stage 27, at 48 h (stage 35), 72 h (stage 42), and 96 h (stage 46). Deviations from this standard exposure time were necessary in test with Bombina because the development of the embryos was slower under the same experimental conditions. Thus at 96 h, the embryos of Bombina were at stage 42 corresponding to 72 h in the standard Xenopus test. In embryos of Bombina, the attainment of stage 46 of the controls occurred after 120 h at 24°C. In experiments with Xenopus in which the jelly coat had not been removed, most of the embryos were free of the jelly coat after 24 h at 24°C. Embryos of Bombina, kept at the same temperature, were still in the jelly coat after 24 h and were not free until after about 72 h. Vehicle control The results showed no significant effects of DMSO at a concentration of 1 % v/v, compared with the controls. The mortality and the number of embryos with malformations were less than 5% for both Bombina and Xenopus. The results revealed no reduction of growth rate. At stage 46, the mean length of the embryos of Xenopus and Bombina was 9.8 mm and 9.4 mm, respectively. Vehicle control The results showed no significant effects of acetone at a concentration of 1% v/v, compared with the controls. The mortality and the number of embryos with malformations were less than 5% for both Bombina and Xenopus. The results revealed no reduction of growth rate. At stage 46, the mean length of the embryos was 9.5 and 9.6 for Xenopus and Bombina, respectively. Effect of positive control The results revealed that the effect of 6-aminonicotinamide in the FETAX solutions with Xenopus was comparable with the effect described in the ASTM standards. The EC50 value for malformations after 96 h was 5.3 mg/l and the LC50 value was 2250 mg/l (mean values of 10 experiments; SD of the mean less than 10%). For Bombina, the EC50 value for malformations after 120 h was 4.8 mg/l and the LC50 value was 2450 mg/l (mean values of 10 experiments; SD of the mean less than 10%). For both Xenopus and Bombina, the most conspicuous malformations caused by 6-aminonicotinamide were severe optic cup rupture, displays edema and facial malformations such as ocular edema and foreshortened facial features. In addition, severe heart malformations were also seen. 3.2 Effects of esfenvalerate on Xenopus laevisThe effect of esfenvalerate on malformation, growth inhibition and mortality in Xenopus laevis were tested in the FETAX solution during 96 h of exposure. The effect of esfenvalerate was tested at 1, 2.5, 5, 10, 50, 100 and 150 µg/l and the results were compared with the control group and a DMSO control. The measured concentrations exhibited large variations relative to the nominal values. Since the renewal procedure entailed fresh replacement of the test material every 24 h during the test and the chemical measurement did not reveal a decrease of the pesticide during the 96 h exposure period, the results are expressed as nominal concentrations. The experiments were normally carried out in glass Petri dishes, however, the effect of esfenvalerate was also investigated at concentrations of 1, 10 and 100 µg/l in polystyrene Petri dishes. The results from these experiments were not significantly different from those found in glass Petri dishes. Sumi-Alfa 5 FW The effect of Sumi-Alfa 5 FW was tested at 1, 5, 10 and 100 µg/l (a.i.) to see if the effect was larger when esfenvalerate was added as a formulated product. The results revealed no significant increase in the effect when compared with the experiments where the active ingredient was added with DMSO as a vehicle. 3.2.1 In vivo observationsEsfenvalerate Esfenvalerate affected embryos gradually during the 96 h exposure period and a dose-response relationship was seen. After 24 h, no effect was seen at concentrations up to 5 µg/l, compared with the controls. At 10 µg/l, beginning twisting of the embryos was seen whereas the movements of the controls were more moderate. The intensity of the twisting increased with increasing concentrations of esfenvalerate and at 150 µg/l pronounced twisting was seen in all embryos. After 48 h, no effect was seen at 1 µg/l, however, at 2.5 and 5 µg/l beginning twisting of the embryos was seen. At 10 and 50 µg/l the embryos had constant spasmodic twisting and in 50 µg/l some of the embryos were immobilised. At 100 µg/l, all embryos were immobilised by spasmodic twisting and at 150 µg/l, haemorrhage near the notochord was seen in some of the embryos. After 72 h, beginning twisting was seen in most of the embryos at 1 µg/l and at concentrations above 2.5 µg/l periodic spasmodic twisting was seen. At 10 µg/l, the embryos were immobilised caused by constant spasmodic twisting and at 50, 100 and 150 µg/l many of the embryos had haemorrhage near the notochord and severe eye abnormalities. After 96 h at 1, 2.5 and 5 µg/l, periodic spasmodic twisting was seen in most of the embryos. The intensity increased with increasing concentrations of esfenvalerate. At 10, 50, 100 and 150 µg/l, the embryos were immobilised caused by constant spasmodic twisting and the heartbeat was slow. Many of the embryos had haemorrhage near the notochord, severe eye abnormalities and cardiac edema (the intensity increased with increasing concentrations). 3.2.2 Recordings of malformation, growth inhibition and mortality at the end of the test (96 h)Malformation Most abnormal embryos possessed multiple malformations. However, at 1 and 2.5 µg/l no malformations were seen and the larva were at the normal stage 46 and comparable with the controls in all respects. At a concentration above 2.5 µg/l, the axial malformations increased with increasing concentrations of esfenvalerate. Thus at 5 and 10 µg/l, a relatively mild lateral flexure of the tail was seen. At concentrations above 5 µg/l, optica cup rupture was often seen. Abnormalities of the head and face may be less obvious than other types of malformations, however, at concentrations above 5 µg/l embryos with a sloping forehead and distended mouth were often seen. The head and face regions of these embryos were significantly malformed. The face was extremely flattened and the forebrain was deflected downward along the front of the brow. Heart malformations were seen at concentrations above 5 µg/l. Increasing concentrations of esfenvalerate resulted in increasingly severe heart malformations. Often the heart showed an abnormal expansion of the ventricle just in front of the gut. Gut abnormalities were also seen at concentrations above 5 µg/l where incomplete gut coiling was common. Various degrees of undeveloped gills were also seen at concentrations above 5 µg/l. At higher concentrations (50, 100 and 150 µg/l), severe lateral flexure of the tail was seen. At 50 µg/l, edema was a frequent occurrence. Edema was easily identified and appeared as transparent, swollen, fluid-filled areas. Many embryos exhibited edema in the cardiac region. With increasing concentrations of esfenvalerate embryos displayed increasingly severe abdominal and cardiac edema and at concentrations of 100 and at 150 µg/l optic edema was common.
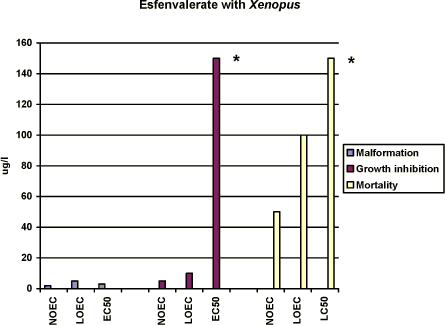
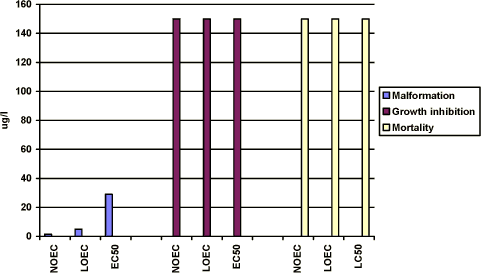
Figure 3-a Effekten af esfenvalerat på Xenopus laevis udtrykt som sublethale defekter, væksthæmning og dødelighed. Testen er udført i overensstemmelse med FETAX standardtesten, og resultaterne udtrykkes ved
angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 96 timers eksponering. Resultaterne er et gennemsnit af 3 forsøg, og SD af middeltallene er mindre end 10%. For yderligere detaljer, jf. Bilag 4. No effects was seen at 2.5 µg/l, however, more than 80% of the embryos had malformations at 5 µg/l and all of the embryos exposed to 10 µg/l and higher concentrations had malformations. The NOEC, LOEC, and EC50 values for malformations were calculated to be 2.0 µg/l, 5 µg/l, and 3 µg/l, respectively (cf. Appendix 4). The most significant malformation observed was cardiac which more than 80% of the embryos had in 5 µg/l. At concentrations above 10 µg/l cardiac, notochord, brain and gut malformations were also common. Growth inhibition After 96 h of exposure to 2.5 µg/l, the mean length of the embryos was measured to 9.7 mm which was comparable with that of the control group. At 5 µg/l and 10 µg/l, the mean length of the embryos was measured to 8.9 and 8.4 mm, respectively. However, no further reduction of the length of the embryos was seen with increasing concentrations of esfenvalerate. Thus, the results revealed that for growth inhibition NOEC was 5 µg/l, LOEC was 10 µg/l and the EC50 was higher than 150 µg/l. (cf. Appendix 4). Mortality No significant mortality was seen at concentrations below 100 µg/l. However, at 100 and 150 µg/l a mortality of about 15% was seen. No significant difference in the mortality rate in the 100 and 150 µg/l samples was observed. The NOEC value for mortality was 50 µg/l and the LOEC value was 100. The LC50 value was higher than 150 µg/l, the highest concentration tested. 3.3 Effects of Prochloraz on Xenopus laevisThe effect of prochloraz on Xenopus was tested at 0.1; 1.0; 1.5; 2.0 ; 3.0, 5.0 and 10 mg/l in FETAX solutions. Acetone was used as a vehicle The measured concentrations exhibited large variations relative to the nominal values. Since the renewal procedure entailed fresh replacement of the test material every 24 h during the test and the chemical measurement did not revealed a decrease of the pesticide during the 96 h exposure period, the results are expressed as nominal concentrations. 3.3.1 In vivo observationsAfter 24 h of exposure, no effect was seen at prochloraz concentrations below 10 mg/l. However, various degrees of edema and blistering were seen at 10 mg/l. After 48 h, some embryos exposed to 1.5 mg/l exhibited mild edema in the cardiac region. With increasing concentrations of prochloraz more and more embryos displayed increasingly severe edema especially optic and cardiac edema. As for embryos exposed to 10 mg/l, the most characteristic malformations were severe optic and cardiac edema. After 72 h, the most characteristic malformation caused by 1.5 mg/l prochloraz was mild edema especially in the cardiac region. In 2 mg/l, embryos with a sloping forehead and edema in especially cardiac and optic region were seen. The heartbeat was slower and decreased when increasing the concentration of prochloraz. At concentrations above 5 mg/l, it seemed like the internal organs of the embryos had more or less loosen from the notochord. Malformations of the gut were also seen and often the gut had coiled into a single loop. Furthermore, severe heart malformations were typical and often the heart was composed of a single beating tube. In 10 mg/l, many embryos (about 20%) were dead and the surviving embryos were immobilised. It was nearly impossible to register the heartbeat in the surviving embryos at 10 mg/l. Edema was a frequent occurrence and may be general (somatic) or regional, e.g., eye (optic), abdominal, cranial or mallar. Many of the embryos also had various degrees of blistering. Blisters were most frequently observed along the dorsal mid-line (fin) area or ventrally near the ventral fin. After 96 h, the above mentioned malformations were seen at concentrations above 3 mg/l. About 60% of the embryos were dead in 5 mg/l and all were dead in 10 mg/l. 3.3.2 Recordings of malformation, growth inhibition and mortality at the end of the test (96 h)Malformation No effect was seen at 0.1 mg/l. However, at 1 and 1.5 mg/l mild rupture in the eye and mild edema in the optic and heart region were seen. Only a few embryos were affected (about 10% increase compared with the controls) in 1.0 mg/l. However, 57% of the embryos had malformations at 1.5 mg/l and all of the embryos exposed to 2 mg/l and higher concentrations had malformations. At 2 mg/l, the above mentioned malformations were normally seen and some embryos had sloping foreheads and their gut were coiled but not to the extent normally expected by stage 46. At concentrations above 3 mg/l most abnormal embryos possessed multiple malformations. The most significant malformations were heart malformations where the heart was often composed of a single straight tube. Often severe abdominal and cardiac edema caused failure to achieve normal gut development. In this case, an almost total failure of the gut to undergo normal coiling was observed. Facial abnormalities generally, though not always, appeared in conjunction with edema of the eyes and head. The most characteristic malformations caused by prochloraz were severe edema, especially optic and cardiac edema. At 5 and 10 mg/l, the surviving embryos normally had edema which may be general (somatic) or regional, e.g., eye (optic), abdominal, cranial, or mallar. Many of the embryos also had various degrees of blistering. Blisters were most frequently observed along the dorsal mid-line (fin) area or ventrally near the ventral fin. In the surviving embryos at concentrations above 5 mg/l it seemed like the internal organs of the embryos were more or less free and loosen from the notochord. Furthermore, severe heart malformations were typical and the heart was often composed of a single tube. Various degrees of incomplete gut coiling were also typical malformations caused by this toxicant. The NOEC value was found to be 0.1 mg/l and the LOEC value was 1.0 mg/l, and the EC50 value was calculated to 1.4 mg/l, cf. Appendix 4.
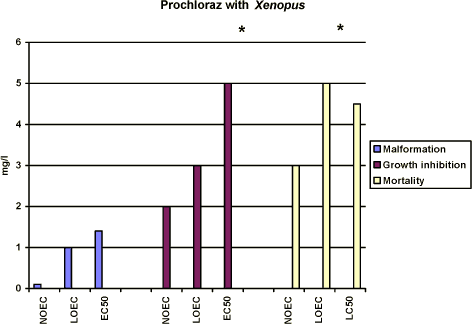
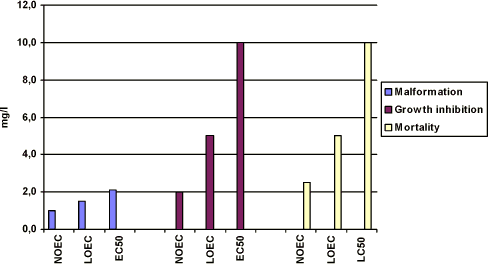
Figure 3-b Effekten af prochloraz på Xenopus laevis udtrykt som sublethale defekter, væksthæmning og dødelighed udført i overensstemmelse med FETAX standard testen. Resultaterne udtrykkes ved angivelse af
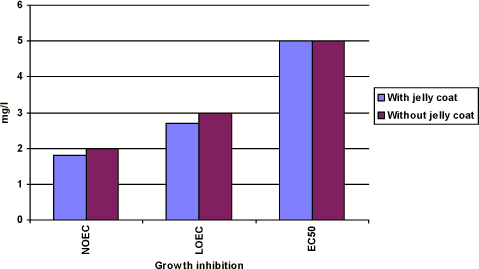
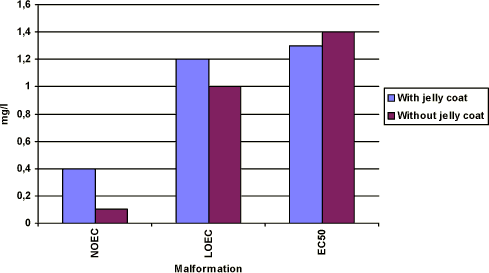
NOEC, LOEC og EC50/LC50 værdierne efter 96 timers eksponering og er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%. Yderligere detaljer, jf. Bilag 4. Growth inhibition After 96 h of exposure, no effect on the mean length of the embryos was found at concentrations up to 2 mg/l. At 3 mg/l, the mean length of the embryos was measured to 7.7 mm corresponding to about 80% of the controls. At 5 mg/l, the mean length of the embryos was about 76% of the controls. Thus, the results revealed that for growth inhibition NOEC value was 2 mg/l, LOEC value was 3 mg/l and the EC50 value was higher than 5 mg/l. Further details cf. Appendix 4. Mortality No significant mortality was seen at concentrations below 3 mg/l. However at 5 mg/l, a mortality of about 60% was seen. The mortality at this concentration of prochloraz occurred primarily on the last day of exposure. At 10 mg/l, a mortality of 20% was seen after 72 h of exposure and all embryos were dead after 96 h. The NOEC value for mortality was 3 mg/l, the LOEC value was 5 mg/l and the LC50 value was calculated to 4.5 (Further details, cf. Appendix 4). 3.3.3 Comparison of the effects of prochloraz on Xenopus with and without jelly coatIn vivo observations The embryos were free of the jelly coat at all concentrations of prochloraz after 48 h, which is comparable with the control group. No significant difference in either the effects or the time at which the effects were seen at the different concentrations of prochloraz was found when comparing the effect of prochloraz on Xenopus with and without jelly coat. No difference in response in the two groups of embryos was either seen in the control group, acetone control or in the positive control group exposed to 6-nicotinamide. Recordings of malforma-tion, growth inhibition, and mortality at the end of the test (96 h) The results revealed the same effect of prochloraz on growth inhibition, malformation, and mortality, when expressed as NOEC, LOEC and EC50/LC50 values in embryos with and without jelly coat after 96 h of exposure as depicted in figure 3-c to figure 3-e (Further details cf. Appendix 4). Furthermore, the most obvious abnormalities, which were induced by prochloraz in embryos, still retaining their jelly coats, were identical with those seen in embryos whose jelly coats had been removed. Thus, no difference in either the type or seriousness of the malformations was found compared with the effect found in embryos from eggs in which the jelly coat had been removed and then exposed to the same concentrations of prochloraz.
Figure 3-c Effekten af prochloraz på væksthæmningen hos Xenopus laevis. Figuren sammenligner forsøgsresultaterne fra forsøg, hvor man har fjernet gelékappen fra æggene, og hvor man ikke har fjernet gelékappen. Ellers er forsøgene udført i overensstemmelse med FETAX standard testen. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50 værdierne efter 96 timers eksponering og er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%.
Figure 3-d Effekten af prochloraz på sublethale deformasiteter hos Xenopus laevis. Figuren sammenligner forsøgsresultaterne fra forsøg, hvor man har fjernet gelékappen fra æggene, og hvor man ikke har fjernet gelékappen. Ellers er forsøgene udført i overensstemmelse med FETAX standard testen. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50 værdierne efter 96 timers eksponering og er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%.
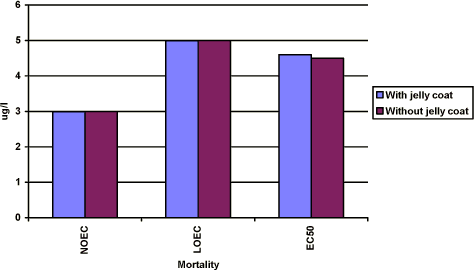
Figure 3-e Effekten af prochloraz på dødeligheden hos Xenopus laevis. Figuren sammenligner forsøgsresultaterne fra forsøg, hvor man har fjernet gelékappen fra æggene, og hvor man ikke har fjernet gelékappen. Ellers er forsøgene udført i overensstemmelse med FETAX standard testen. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og LC50 værdierne efter 96 timers eksponering og er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%. 3.4 Effects of esfenvalerate on Bombina bombinaThe effect of esfenvalerate on malformation, growth inhibition and mortality in Bombina bombina was tested in the FETAX solution. After 120 h of exposure at 24°C, the embryos in the control group were at stage 46 corresponding to the stage of the controls at the end of the test in the standard FETAX test with Xenopus. The effect of esfenvalerate was tested at 1, 2.5, 5, 10, 50, 100, and 150 µg/l and the results were compared with the control group and a DMSO control. These experiments were made with embryos from eggs where the jelly coat had not been removed. Positive controls experiments with two concentrations of 6-aminonicotinamide were also included in the tests. The results revealed that no effect was seen in the DMSO control group compared with the untreated controls and 56% of the embryos had malformation at 5.5 mg/l 6-aminonicotinamide. A mortality of 43% was found at a concentration of 2500 mg/l. 3.4.1 In vivo observationsEsfenvalerate Esfenvalerate affected embryos gradually during the 120 h exposure in a dose depended manner. After 24 h, no effect of esfenvalerate was seen on the embryos even at the highest concentration compared with the controls. After 48 h, no effect was seen at concentrations up to 5 µg/l, however, at 5 µg/l beginning twisting of the embryos was seen now and then. The spasmodic twisting increased in intensity with increasing concentrations of esfenvalerate and at 150 µg/l the embryos had constant spasmodic twisting. At concentrations from 50 µg/l, the spasmodic twisting of the embryos caused that some of the embryos had already left the jelly coat at this stage. In the control group, only a few embryos had begun to leave the jelly coat after 48 h. At 100 and 150 µg/l, edema was seen in some of the embryos. After 72 h, several of the embryos in the control group began to leave the jelly coat and all the embryos exposed to esfenvalerate concentrations above 50 µg/l were free. Periodic spasmodic twisting was seen in most of the embryos above 5 µg/l and in 50 µg/l constant spasmodic twisting was seen. At concentrations above 100 µg/l, occasionally blistering and cardiac edema appeared. In 150 µg/l, blistering and cardiac edema were frequently seen. The embryos were immobilised by constant spasmodic twisting. After 96 h, all embryos in the control group were free from the jelly coat and beginning twisting was seen at 1 µg/l. The intensity of these spasmodic twisting increased when increasing the concentrations of esfenvalerate. At concentrations above 50 µg/l, the embryos were immobilised caused by constant spasmodic twisting and occasionally blistering and cardiac edema appeared. At concentrations above 100 g/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were normally seen. After 120 h, spasmodic twisting was seen at 1 µg/l. At concentrations above 50 µg/l, the embryos were immobilised caused by constant spasmodic twisting and blistering and edema were often seen. At concentrations above 100 µg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were seen at 100 and 150 µg/l. 3.4.2 Recordings of malformation, growth inhibition and mortality at the end of the test (120 h)Malformation At concentrations up to 2.5 µg/l, no malformations were seen and the larva were at the normal stage 46 and comparable with the controls in all aspects. Above a concentration of 5 µg/l, gut abnormalities were often seen indicated by a loose gut coiling. At 10 µg/l in addition to gut abnormalities, a relatively mild flexure of the tail was normally seen. Embryos with a sloping forehead and distended mouth were often seen. The head and face region of these embryos were malformed. The face was flattened and the forebrain was deflected downward along the front of the brow. Heart malformations were also observed at concentrations above 10 µg/l. Increasing concentrations of esfenvalerate resulted in increasingly severe heart malformations. Often the heart showed an abnormal expansion of the ventricle just in front of the gut. At 50 µg/l, blistering and edema were common and various degrees of undeveloped gills were seen, too. About 10% of the embryos had malformations at 10 µg/l and all of the embryos exposed to 100 µg/l and higher concentrations had malformations. The EC50 value for malformations was calculated to be 29 µg/l (Further details cf. Appendix 4). The most significant malformation observed was flexure of the tail and cardiac.
Figure 3-f Effekten af esfenvalerat på Bombina bombina udtrykt som sublethale defekter, væksthæmning og dødelighed udført i overensstemmelse med FETAX standardtesten. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 120 timers eksponering og er et gennemsnit af 3 forsøg. For yderligere detaljer, se bilag 4. Growth inhibition After 120 h of exposure to concentrations up to 150 µg/l, the mean length of the embryos was measured to about 9.4 mm which was comparable with the length of the control group. Thus, the results revealed that for growth inhibition NOEC, LOEC and the EC50 valued were higher than 150 µg/l. Mortality No significant mortality was seen at concentrations up to 150 µg/l. The NOEC, LOEC, and LC50 values were all higher than 150 µg/l. 3.5 Effects of prochloraz on Bombina bombinaThe effect of prochloraz on Bombina was tested at 1.0, 1.5, 2.0, 5.0 and 10 mg/l in FETAX solution at 24°C. Acetone was used as a vehicle and an acetone control was therefore included in the test. The results revealed no significant effect in either growth inhibition, malformation or mortality when the acetone control was compared with the control group. Addition of 5.5 mg/l 6-aminonicotin-amide induced 57% malformation of the embryos and addition of 2500 mg/l induced 42% mortality after 120 h of exposure. 3.5.1 In vivo observationsAfter 24 h of exposure, no effect was seen at any prochloraz concentrations. After 48 h, no effect was seen at prochloraz concentrations up to 2 mg/l. However, embryos exposed to 5 and 10 mg/l showed signs of mild edema, especially in the optic and cardiac region. After 72 h, the embryos began to leave the jelly coat both in the control group and in samples exposed to prochloraz. However, at 10 mg/l prochloraz only a few embryos were free. No effect was seen at concentrations up to 2 mg/l. The most characteristic malformations caused by 2 mg/l prochloraz were mild edema, especially in the cardiac region. In 5 and 10 mg/l, embryos with a sloping forehead and edema especially in cardiac and optic region were seen. After 96 h, all embryos were free. Embryos exposed to 1.5 and 2 mg/l showed signs of edema especially in the cardiac region. In 5 and 10 mg/l, embryos with a sloping forehead and edema in especially cardiac and optic region were seen. Severe heart malformations were typical and the heart was often composed of a single beating tube. The heartbeat was slower at these concentrations and at 10 mg/l it was nearly impossible to observe heartbeat. The surviving embryos in 5 and 10 mg/l were immobilised (did not react on mechanical stimuli). Edema was a frequent occurrence and may be general (somatic) or regional, e.g., eye (optic), abdominal, cranial, or mallar. Many of the embryos had also various degrees of blistering. Blisters were most frequently observed along the dorsal middling (fin) area or ventrally near the ventral fin. Malformations of the gut were also seen. After 120 h, no effect was seen at 1 mg/l but at 1.5 mg/l edema was seen and at 2 mg/l the embryos were immobilised and edema was a frequent occurrence especially in eye (optic), abdominal, cranial, and heart. In 5 and 10 mg/l, the above (after 96 h) mentioned malformations were seen in a more pronounced manner. About 10% of the embryos were dead in 5 mg/l and 25% at 10 mg/l. 3.5.2 Recordings of malformation, growth inhibition and mortality at the end of the test (120 h)Malformation No effect was seen at 1 mg/l. However, at 1.5 mg/l mild edema in the heart region was seen. Only a slight effect (20% increase compared with the controls) was found at 1.5 mg/l, however, about 50% of the embryos had malformations at 2 mg/l and all of the embryos exposed to 5 and 10 mg/l had malformations. At 2 mg/l, the edema especially in the heart region was often seen and some embryos had a sloping forehead. At 5 and 10 mg/l, most embryos possessed multiple malformations, where the most significant was heart malformations. Often severe abdominal and cardiac edema caused failure to achieve normal gut development. Facial abnormalities generally, though not always, appeared in conjunction with edema of the eyes. The most characteristic malformations caused by prochloraz were severe edema especially optic and cardiac edema. Many of the embryos also had various degrees of blistering. Blisters were most frequently observed along the dorsal middling (fin) area. It seemed that the internal organs of the embryos were more or less free and loosen from the notochord. The NOEC value was found to be 1 mg/l and the LOEC value was 1.5 mg/l. The EC50 value for malformations was calculated to be 2.1 mg/l (Further details cf. Appendix 4). The most significant malformations observed were cardiac, brain, edema, and gut malformations.
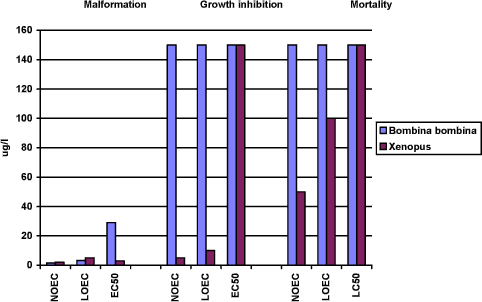
Figure 3-g Effekten af prochloraz på Bombina bombina udtrykt som sublethale defekter, væksthæmning og dødelighed. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 120 timers eksponering og er et gennemsnit af 3 forsøg. Standard afvigelsen af middeltallene er mindre end 10%. For yderligere information se Bilag 4. Growth inhibition After 120 h of exposure, no effect on the mean length of the embryos was found at concentrations up to 2 mg/l. At 5 mg/l, the mean length of the embryos was measured to 7.4 mm corresponding to about 80% of the controls. At 10 mg/l, the mean length of the embryos was about 70% of the controls. Thus, the results revealed that for growth inhibition NOEC was 2 mg/l, LOEC was 5 mg/l, and the EC50 was higher than 10 mg/l. Mortality No significant mortality was seen at concentrations below 5 mg/l. However, at 5 mg/l a mortality of about 10 % was seen. The mortality at this concentration of prochloraz occurred primarily on the last day of exposure. At 10 mg/l, a mortality of 25% was seen after 120 h of exposure. The NOEC value for mortality was 2.5 mg/l, the LOEC value was 5 mg/l and the LC50 value was more than 10 mg/l. 3.6 Comparison of the effects of esfenvalerate and prochloraz on Xenopus and Bombina3.6.1 Comparison of the effects of esfenvalerate on Xenopus and BombinaWhen a comparison of the effects of esfenvalerate on malformation in Xenopus and Bombina was made the results revealed that the NOEC and LOEC value in both species were 2.5 µg/l and 5 µg/l, respectively. However, a significant difference between the two species was found at 5 µg/l. In tests with Xenopus, more than 80% of the embryos had malformations at 5 µg/l and all of the embryos exposed to 10 µg/l had malformations while only about 10% of the embryos exposed to 10 µg/l had malformation in tests with Bombina. However, all embryos exposed to 100 µg/l had malformations. The EC50 value was calculated to be 3 µg/l and 29 µg/l for Xenopus and Bombina, respectively. However, an effect of esfenvalerate was seen at a concentration of 1 µg/l in the in vivo observations in both Xenopus and Bombina. The most characteristic effect of esfenvalerate on living embryos of both species was the spasmodic twisting which at the higher concentrations caused a complete immobilisation of the embryos. The most significant malformations observed after the end of the tests and fixation of the embryos were also identical in the two species and included cardiac, severe lateral flexure, edema, notochord, brain and gut malformations. The effect of esfenvalerate on growth seems to be different in the two species. The results revealed that in Xenopus addition of 10 g esfenvalerate only caused a reduction of about 15% of the embryos' mean length compared with the controls. No further reduction of the length of the embryos was seen when increasing the concentrations of esfenvalerate up to 150 µg/l. Thus, the results revealed that for growth inhibition NOEC was 5 µg/l, LOEC was 10 µg/l and the EC50 value was higher than 150 µg/l. In experiments with Bombina, esfenvalerate did not affect the mean length of the embryos at any concentrations up to 150 µg/l. Thus, the NOEC, LOEC and EC50 values were all higher than 150 µg/l. In Xenopus, a significant mortality of about 15% was seen both at 100 and 150 µg/l compared with the control group, but the LC50 value was higher than 150 µg/l. For Bombina, the results revealed that no significant mortality was seen at concentrations up to 150 µg/l compared with the controls.
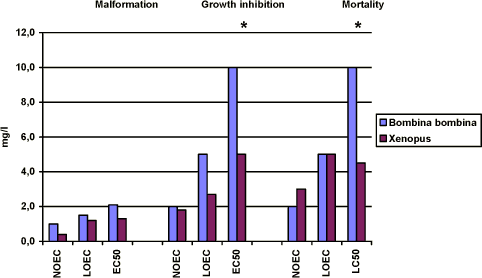
Figure 3-h En sammenligning af effekterne af esfenvalerat på Xenopus og Bombina udtrykt ved sublethale defekter, væksthæmning og dødelighed. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 96 og 120 timers eksponering for henholdsvis Xenopus og Bombina. Efter denne eksponeringstid er embryonerne i de respektive kontrolgrupper nået til stadie 46. Resultaterne er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%. 3.6.2 Comparison of the effects of prochloraz on Xenopus and BombinaWhen a comparison of the effect of prochloraz on malformation in Xenopus and Bombina was made, the results revealed that no effect was found at concentrations below 1 mg/l. However at 1 mg/l, about 10% of the embryos of Xenopus had malformations while no effect was seen in Bombina. A significant difference between the two species was found at 1.5 and 2 mg/l. Thus in tests with Xenopus, about 60% of the embryos had malformations at 1.5 mg/l and all of the embryos exposed to 2 mg/l and higher had malformations while only about 20% of the embryos exposed to 1.5 mg/l had malformation in tests with Bombina and about 50% of the embryos had malformations at 2 mg/l. All embryos exposed to 5 mg/l and higher concentrations had malformations. The EC50 value was calculated to be 1.3 and 2.9 mg/l for Xenopus and Bombina, respectively. The most characteristic effect of prochloraz on living embryos of both species was severe heart malformations. At the higher concentrations, the heartbeat was nearly impossible to observe and the surviving embryos were so damaged that they did not react on mechanical stimuli. After the end of the test, the most significant malformations observed were also identical in both species and included cardiac, brain, edema, and gut malformations. The effect of prochloraz on growth in both species seemed to be comparable and no effect was seen at concentrations below 2 mg/l. However, in Xenopus addition of 3 mg/l prochloraz caused a reduction of the mean length of the embryos of about 20% as compared with the controls and at 5 mg/l the mean length was reduced with about 25%. In experiments with Bombina 5 mg/l prochloraz caused a reduction of about 20% as found in experiments with Xenopus at 3 mg/l. About 75% of the embryos were still alive after 120 h exposure to 10 mg/l prochloraz and a reduction of the mean length of the surviving embryos was about 30% compared with the controls. In Xenopus, no significant mortality was seen at 3 mg/l. However, about 56% of the embryos were dead at 5 mg/l and all embryos were dead after 96 h exposure to 10 mg/l. For Bombina the results revealed that no significant mortality was seen at concentrations up to 2 mg/l. At 5 mg/l, a mortality of about 10% was seen and at 10 mg/l about 25% of the embryos were dead at the end of the experiments.
Figure 3-i En sammenligning af effekterne af prochloraz på Xenopus og Bombina udtrykt ved sublethale defekter, væksthæmning og dødelighed. Resultaterne udtrykkes ved angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 96 og 120 timers eksponering for henholdsvis Xenopus og Bombina. Efter denne eksponeringstid er embryonerne i de respektive kontrolgrupper nået til stadie 46. Resultaterne er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene er mindre end 10%. 3.6.3 The effects of esfenvalerate and prochloraz on TI on Bombina and XenopusThe TI is defined as the 96 h LC50 divided by the 96 h EC50 (malformation) and is a measurement of developmental hazard. Table 3-a Teratogen index (TI) for de to pesticider esfenvalerate og prochloraz.
The results from table 3-a revealed that in this test esfenvalerate as well as prochloraz can be considered as a teratogen according to this test. 4 Discussions4.1 The use of amphibians as an ecotoxicological test organism 4.1 The use of amphibians as an ecotoxicological test organismDue to their thin and permeable skins, and prolonged exposure first to the aquatic environment and then to the terrestrial, amphibians may be particularly sensitive to environmental contaminants. Populations of many amphibians have declined and some species have disappeared from certain regions around the world, a phenomenon which appears to have accelerated during the last years. Agricultural pesticides may contribute to the decline in amphibian populations (Phillips, 1990; Berrill et al. 1994). Much of the amphibian life cycle occurs in pons, streams, and temporary pools that are often associated with agricultural areas receiving pesticide applications. In addition, breeding and larval development of amphibians occur in spring and summer at the same time that heavy application of pesticides on agricultural lands occurs. Thus, the purpose of this part of the project is to provide a broader knowledge in handling the problems concerning the effects of pesticides on the ecology in small ponds in intensively farmed agricultural land. It is the idea to collect knowledge of amphibians' tolerance of pesticides from the literature and develop a new laboratory test system with Bombina bombina (test guideline development) for future testing of pesticides on amphibians living in Europe. The new test system is evaluated using esfenvalerate and prochloraz, two pesticides with different action and physico-chemical properties. 4.1.1 The effects of aquatic contaminants on amphibians compared with the effects of other aquatic test organismsThe toxicity of 11 organic compounds considered hazardous to water resources was evaluated using embryo-larva stages of up to eight fish and amphibian species. The animal test species exhibited varying degrees of sensitivity to the selected toxicant. For some test chemicals, the most sensitive species was amphibians, in other cases the fish was the most sensitive species. In most instances, higher LC50 values were obtained in tests with B. fowleri (fowler’s toad), R. palustris (pickerel frog), Xenopus laevis (African clawed frog), and the fathead minnow. The species which exhibited the largest susceptibility to organic compounds were the rainbow trout, R. pipiens (leopard frog), and the European common frog R.temporaria (Black and Wesley, 1982). Howe et al. (1989) investigated the effect of atrazine and alachlor to the Northern leopard frog and American toads and compared their sensitivity with rainbow trout and channel catfish. Overall, rainbow trout and channel catfish appeared to be less sensitive than amphibian larvae. However, in some specific tests with atrazine or tests with early-stage larvae, the toxicity was roughly similar. The 96 h LC50 values for later-stage amphibian larvae were approximately two to four times lower than those observed in fish. This stresses the importance of using different test organisms representing various trophic levels, taxa, and functions in the environment in harzard and risk assessment. 4.1.2 The effects of pesticides on different kinds of amphibiansBerrill et al. (1993) found differences in sensitivity among five species of amphibian embryos and tadpoles (the frogs Rena sylvatica, Rena pipiens, Rena clamitans, the toad Bufo americanus, the salamander Ambystoma madeculatum) exposed to permethrin and efenvalerate at concentrations between 10 and 200 µg/l. Even though no significant mortality of embryos, anuran tadpoles, or salamander larvae occurred either during or after exposure to these two pyrethroids, growth was delayed after exposure. Furthermore, newly hatched R. clamitans tadpoles recovered slowlier than R. pipiens following exposure to low levels of both pyrethroids. Older tadpoles of B. americanus were also more sensitive than R. pipiens tadpoles at approximately the same stage. Larvae of the salamander A. maculatum, the most sensitive of the organisms tested, either recovered far more slowly or failed to recover at all. This indicates that different amphibian species have different tolerance to pesticides. Black and Wesley (1982) tried to determine whether species tolerance in different amphibian species varies in a predictable manner with ecological adaptations or with other criteria which could be applied in extrapolating test results to natural aquatic ecosystems. The evaluated amphibian species were Ambystoma gracile (north-western salamander), Rena pipiens (leopard frog), Rena temporaria (European common frog), Rena palustris (pickerel frog), Bufo fowleri (fowler's toad), and Xenopus laevis (African clawed frog). These species were selected to represent different patterns of reproduction and variations in ecological habitat and geographical distribution to determine whether such factors correlate with susceptibility to organic toxicants. The toxicity of 11 organic compounds and two metals was evaluated using embryo-larva stages of the different species. The animals exhibited varying degrees of sensitivity to the selected toxicants. The most sensitive amphibian species were generally those which normally are restricted to aquatic or moist terrestrial habitats, whereas the most tolerant amphibians included those semi-aquatic and terrestrial species which appear to be more broadly adapted ecologically. However, Xenopus laevis (the African clawed frog) was an exception, since the results revealed that this aquatic organism was generally less sensitive to organic chemicals than other amphibian species. This was surprising and may be of some concern since Xenopus laevis is the test organism used in the only internationally recognised ecotoxicological test with amphibians. 4.2 A standardised toxicity test using embryos of amphibians4.2.1 Toxicity tests using embryos of amphibiansFETAX [2] (Frog Embryo Teratogenesis Assay-Xenopus) is a standard methodology for toxicity tests of chemicals using embryos of amphibians and is designed to use embryos of the South African clawed frog Xenopus laevis. Although the FETAX toxicity test is designed explicitly for the use of Xenopus laevis, the procedure has been found useful for conducting developmental toxicity tests with other species of frogs, however, with some necessary modifications. The procedure is applicable to all chemicals either individually or in formulations, commercial products or mixtures. With appropriate modification these procedures can also be used to conduct tests on temperature, dissolved oxygen, pH, physical agents and on materials such as aqueous extracts of water-insoluble materials and sediment. Methodologies for the evaluation of amphibian toxicity have normally been based on mortality, malformation and growth inhibition. Teratogenic screening The amphibian embryo has been and remains a classical model for experimental embryological studies. It is an intact developing system that undergoes events as cleavage, gastrulation, morphogenesis and organogenesis, comparable with those of other vertebrates. We therefore incorporated this aspect into the present study. A developmental toxicant is a test material that affects any developmental process. Therefore, a developmental toxicant affects embryo mortality and malformation. It is important to measure developmental toxicity because embryo mortality, malformation, and growth inhibition can often occur at concentrations far less than those required to affect adult organisms. A teratogen is a test material that causes abnormal morphogenesis (malformation). The Teratogenic Index or TI is a measure of developmental hazard (Dumont et al. 1983). FETAX is a potential rapid test for identifying developmental toxicant. Data may be extrapolated to other species including mammals. FETAX might be used to prioritise samples for further tests which use mammals. Validation studies using compounds with known mammalian or human developmental toxicity, or both, suggest that the predictive accuracy will approach or exceed 85% (Dawson and Bantle, J.A. 1987; Courchesne, and Bantle, 1985; Dumont et al. 1983; Sabourin et al. 1985, Sabourin and Faulk 1987). The accuracy rate compares favourably with other currently available “in vitro Teratogenesis screening” (Schuler et al. 1982; Greenberg, 1982; Kitchin et al. 1981). 4.2.2 Influence of the test protocols and the developmental stages of the amphibians on the ecotoxicological resultsSensitivity of different stages For many organic compounds, amphibian embryos have proved to be considerably more sensitive than larvae of the same species (Black and Wesley, 1982). Considering the limited data available from chronic life-cycle studies and the low cost of short-term embryo-larval bioassays, such tests may provide a useful means of quantifying the toxicity of aquatic contaminants. In some cases, older and more developed embryos were, however, found to be more sensitive to toxicants. Thus, older embryos of R. clamitans were found to be more sensitive to pyrethroid exposure than younger, less developed embryos (Berrill et al., 1993). Ecotoxicity data on the Northern leopard frogs and the American toads exposed to atrazine and alachlor also indicated that late-stage amphibian larvae may be more sensitive to some herbicides than earlier stage larvae (Howe et al. 1998). In a comparison of the effect of a given pesticide on different amphibians, it is therefore important to take the stage of development of the amphibians into consideration. Thus, acute toxicity data presented only for early-stage larvae should be interpreted with caution because they may, in some cases, underestimate the hazards to later life stages. Media Two principally different kinds of media have been employed in different studies with amphibian embryos: A “synthetic” salt solution (FETAX medium) with known composition (Appendix 2) and a medium, normally composed of naturally filtrated water (water from pond, streams and temporary pools). Several ecotoxicity studies indicate the influence of organic content and medium formulation on the toxic effects of different chemicals on both aquatic and terrestrial organisms (Di Toro et al. 1991; Berhin et al. 1981; Larsen and Nilsson 1983). This has especially been shown for complexed agents and reactive chemicals. A reduction of bioavailability of fenvalerate, by sorption to particulate and dissolve organic matter was demonstrated by Strawbridge et al. (1992) and Coats et al. (1989). Thus, fenvalerate was 6 times less toxic to mosquito larvae at 50 mg/l dissolved humic acid compared with the toxicity without humic acid. Temperatures Furthermore, the effect of many pesticides e.g. pyrethroids may be temperature dependent. Thus, according to Coats et al. (1989) pyrethroids are usually more effective at colder temperatures. On the contrary, Materna et al. (1995) found that the effect of esfenvalerate on larval leopard frogs increased with increasing temperature (the mortality was higher at 22°C compared with that at 18°C). Even though there is a significant uncertainty whether the effect of a given pesticide increases or decreases with increasing temperature, it seems clear that the temperature may influence the effect concentration of a pesticide. This factor should be taken into consideration when evaluating the toxicity of a pesticide. 4.2.3 Why introduce a new test with Bombina bombinaDevelopment of a new test guideline for ecotoxicological tests with Bombina will enable completion of the already recognised frog embryo teratogenesis assay with the South African clawed frog Xenopus laevis (FETAX test). This test has a number of deficiencies which make its use for ecotoxicological risk assessment in Denmark less suited.
There is therefore a requirement to introduce a new test with amphibians from Europe for ecotoxicological risk assessment of pesticides. The fire-bellied toad is particularly in focus because it is essential to develop a new amphibian test with an easily breeding frog/toad which, like Bombina sp., is living in nature in Europe. Such a test method will make it possible to compare laboratory tests with field tests. This is important in order to carry out an ecotoxicological risk assessment in accordance with the EU Commission's new suggestion for approval of plant protection measures in "Uniform Principles". Especially when using pesticides near sensitive small amphibian biotopes, toleration criteria limits must be made to ensure that the amphibian populations will survive. Bombina bombina, Bombina variegata and Bombina orientalis are animals belonging to the Discoglossidae family (Amphibia, Anura) which is spread out in all Europe and Asia. The two European fire-bellied toads B. bombina and B. varigata hybridize easily in nature and form fully viable and fertile hybride mutually (a natural phenomenon in Eastern Europe). So far studies have shown that wherever their ranges meet a zone of hybridisation is formed, separating areas inhabited by the pure forms. Such hybrid zones have already been discovered in Poland, Austria, Hungary and Czechoslovakia (Rafinska, 1991). Therefore a new standardised toxicity test with Bombina bombina was developed. This type of frog is chosen due to the considerable geographical distribution. Another reason is that the Bombina bombina is relevant in Denmark, as their population are dynamically and ecologically well-tested in among others Funen's County. Furthermore, it has an aquatic lifestyle outside the hibernation season. 4.2.4 Facilities for maintaining adults laboratory populations of Xenopus and BombinaThe facilities for maintaining adults laboratory populations of the South African clawed frog Xenopus laevis are well-described in the FETAX test guideline and information regarding the basic biology and development of this species has been reported by Deuchar (1972, 1975). For long there has, however, been a need for a reliable method whereby other laboratory populations of anurans could be both maintained and perpetuated at modest costs and with minimal time expenditure. The availability of reliable methods for the breeding and maintenance of anurans in a time-efficient operation and at modest costs is important to biologists in many disciplines. The results from the present study showed that it was rather easy to keep adults laboratory populations of Bombina bombina. However in contrast to Xenopus which can be kept in large aquaria, Bombina requires a vivarium with both water and a land area with hiding and feeding place. It is possible to keep the animals active the whole year and to prevent an hibernation period by keeping a constant temperature, a photo period of 12 h light, and constant feeding. Furthermore, Bombina bombina can be bred year-round as demonstrated in the present study, a requirement which is absolute necessary for a test organism used for ecotoxicological tests. The only problem in keeping adults populations of Bombina is that these organisms only eat food (crickets, meal worms, small earthworms, flies, and other suitable invertebrates) that is moving. This means that the animals must be fed by hand frequently which is a very time consuming procedure and therefore a disadvantage in connection with ecotoxicological testing. 4.2.5 Principles and methods for breeding amphibians with special focus on Bombina bombinaEven though it is generally assumed to be very difficult to breed Bombina bombina under captive conditions (Wilkinson, 1994), the results from the present study revealed that it is possible even to breed Bombina bombina throughout the year and get a sufficient number of healthy embryos. The success of the present study in breeding and getting a large number of healthy embryos of Bombina bombina may be caused to application of the right amount of hormone. To ensure a sufficient amount of HCG hormone we used the same procedure as described for Xenopus and adjusted the amount of hormones according to the weight of Bombina. The amount of HCG injected depended on the time of year and the condition of the adults. However, the most important reason for the success of the present study in breeding and getting a large number of healthy embryos at any time we wanted may be ascribed to the injection of PMG to the females 2 days before the animals were injected with HCG in order to mature the oocyttes. A large number of available embryos facilitate statistical analysis and allow the construction of concentrations-response curves with narrow confidence limits which is a fundamental issue in ecotoxicological testing. With minor modifications the system should be suitable for many other anuran species. In view of the apparent decline of the amphibian species in the nature, it would seem that the herpetologist could play a significant role in the conservation of this species by the proper maintenance and breeding of this species which may then be used for provision of toadlets for reintroduction programmes. The success of such activities would of course depend upon the retention and maintenance of suitable habitats in which reintroduction could take place. 4.2.6 Toxicity test using embryos of Xenopus laevis and Bombina bombinaThe toxicity test with embryos of Xenopus was performed according to the procedure described in the ASTM standard. All tests were conducted at 24±0.5°C. Temperatures higher than 26°C may cause malformation whereas lower temperatures may prolong the test. For Bombina bombina a slightly modification of the standard test was necessary due to the lower number of healthy embryos available from this species. Thus for each concentration, only 5 embryos was used instead of the 25 embryos normally used in the ASTM standard. Other test conditions and test procedure like temperature, medium, and the renewal procedure etc. followed the ASTM test guideline. We found it very important to make as few changes in the test design as possible, especially when the effect of different toxicants is compared in different species of amphibians. However, one significant change of the standard procedure was made in the test design with Bombina. According to the ASTM standard the jelly coat is removed from the embryos at the beginning of the test with a cystein treatment. In the present test with Bombina, the jelly coat is not removed. This deviation from the standard procedure was chosen because we were afraid that the cyctein treatment may influence the sensitivity of the embryos to a given toxicant. An important part of the present project was to link our laboratory test with the field investigations in Funen's Country (these investigations will be published in a separate report) and it was therefore important to maintain the jelly coat so that these experiments are more comparable with the natural conditions. The standard exposure time for the FETAX test is 96 h and the attainment of stage 46 in the controls of Xenopus at 24°C. However, deviations from this standard exposure time was necessary in the tests with Bombina because the development of the embryos was slower under comparable experimental conditions. In Bombina the attainment of stage 46 of the controls occurred after 120 h at 24°C. This means that embryos of Bombina are exposed to the test substance for a longer period of time than in the standard FETAX test. This should be taken into consideration when the toxicity of a given test substance is evaluated and the effects concentrations are compared. However, in an evaluation and comparison of the effects of a given test substances on different amphibian species we found it more important to ensure that the embryos went through the same developmental stages, rather than to maintain the same time of exposure. To ensure that different embryos had passed through the same developmental stages is of course especially important in an evaluation of the developmental toxicity of a test material. 6-aminonicotinamide was used as a reference toxicant to evaluate the Bombina test design. For 6-aminonicotinamide, a mortality and malformation database for reference purpose exists. From this published data base for 6-aminonicotinamide, the 96 h LC50 is 2500 mg/l (95% CI = 2350 to 2650) and the 96 h EC50 for malformation is 5.5 mg/l (95% CI = 3.9 to 6.9), or a TI of 455 (Dawson et al. 1989). The results from the present study revealed that the effect of the positive control 6-amino-nicotinamide on Bombina bombina was comparable with the one found with Xenopus. Furthermore, the effect of 6-aminonicotinamide on both malformation and mortality was comparable with those described in the database for Xenopus. The most conspicuous malformations caused by 6-aminonicotinamide in Bombina were the same as those found in Xenopus. Thus, no significant difference was found in the effect of 6-aminonicotinamide on embryos of Bombina bombina and Xenopus laevis. 4.2.7 The effects of esfenvalerate on embryos of amphibians with special reference to Xenopus and BombinaEsfenvalerate is an insecticide used against insect pest in agriculture, gardening, fruit farming and forestry. The compound is effective against several insect species. It is a pyrethroid insecticide which contains the ss-isomer of fenvalerate, the most toxic of the four stereoisomers. It is sold under the trade name Sumi Alfa which contains 5% a.i. 4.2.8 In vivo observationsEsfenvalerate affected the embryos gradually during the exposure period and a dose-response relationship was found in both Xenopus and Bombina. The initial response of embryos was a decrease in activity and the characteristic spasmodic twisting and at the highest concentrations some of the embryos were immobilised. After 96 h, corresponding to the end of the test with Xenopus periodic spasmodic twisting was seen in most of the embryos of both Xenopus and Bombina at a concentration as low as 1 µg/l. The intensity increased with increasing concentration of esfenvalerate and at 10 µg/l (for Xenopus) and 50 µg/l (for Bombina) the embryos were immobilised caused by constant spasmodic twisting. Furthermore the heartbeat was slow. The results from the present study are in agreement with the effect seen in leopard frog (Rena pipiens) exposed to esfenvalerate where a decrease in activity was found at concentrations of 1.3 µg/l and a twisting response was seen at concentrations of 3.6 µg/l (Materna et al., 1995). In this study, spasmodic twisting associated with an uncoordinated spiral swimming was also seen at concentrations of above 5 µg/l. Thus, the results from the in vivo observations revealed that esfenvalerate affected the embryos of Xenopus and Bombina in the same way even though the embryos of Xenopus seem to be more sensitive compared with the effects found in Bombina. 4.2.9 Recordings of malformation, growth inhibition, mortality and teratogenic screeningThe results revealed that at concentrations up to 2.5 µg/l no malformations were seen. At a concentration above 2.5 µg/l, the malformations increased with increasing concentrations of esfenvalerate. Most embryos possessed multiple malformations and the same malformations were seen in both Xenopus and Bombina. That the effect of esfenvalerate seems to be less pronounced in experiments with Bombina compared with that found with Xenopus (a factor of 10 less sensitive) can not be ascribed to a less developed embryos of Bombina, since the test with Bombina had been prolonged to ensure that the embryos of both species went through the same stages of development. This is especially important when the effects of pyrethroids are investigated since it has been shown that older embryos of some amphibians are more sensitive to pyrethroid exposure than younger embryos (Berrill et al., 1993). As pyrethroids apparently act primarily on sodium and calcium channels in nervous tissue, the higher sensitivity of the late-stage embryos probably reflects the more differentiated state of the nervous system. The higher effect concentration found in Bombina may be ascribed to the jelly coat which may have a protective effect, even though it does not prevent the penetration of esfenvalerate. However, it should be noted that Xenopus and Bombina responded to esfenvalerate alike and that the difference in sensitivity was less than a factor of 10. Such a difference can easily be ascribed to normal differences in species sensitivity. Thus, the effect of esfenvalerate on different fish is found to differ in the same range (cf. Appendix 3). In Xenopus, addition of 10 g esfenvalerate caused a slight reduction of the mean length of the embryos compared with the controls. However, no further reduction of the length of the embryos was seen with increasing concentrations of esfenvalerate, and esfenvalerate did not affect the mean length of the embryos of Bombina at any concentration tested. Thus, the growth inhibition does not seem to be a sensitive effect parameter in the evaluation of esfenvalerate in either Xenopus or Bombina. Based on the present study, it can be concluded that esfenvalerate has only a small effect on the mortality on embryos of both Bombina and Xenopus and that the LC50 values are higher than 150 µg/l in both species. These results are in agreement with the results found by Berrill et al. (1993) who tested the effect of fenvalerate on embryos and newly hatched tadpoles of Rena clamitans, Rena pipiens, and larvae of the salamander A. maculatum. In that study, embryos and newly hatched tadpoles did not die at concentrations up to 100 µg/l. However, a higher mortality was seen in leopard frog exposed to esfenvalerate at 22°C, and the LC50 value was 7.3 µg/l (Materana et al., 1995). When the effect of Sumi Alfa was tested no significant change in effect was seen compared with the samples where only the active ingredient was added with DMSO as a vehicle. Thus, it is not expected that the formulated product has a significantly higher toxicity on amphibians in the environment compared with the active ingredient dissolved in DMSO. It is interesting that the in vivo observation revealed an effect at 1 µg/l in both species while the EC50 value based on malformation was 3 and 30 µg/l for Xenopus and Bombina, respectively. The LC50 values were higher than 150 µg/l in both species. This indicates that wrong conclusions could be drawn if the effect of esfenvalerate on amphibian was only based on LC50 values. Such sublethal effects that we witnessed are likely to have serious implications on the long-term success of the exposed individuals, thus these organisms are more susceptible to predation. Furthermore, the twisting response is so obvious and so abnormal that it may prove to be a useful indicator-behaviour in the field work. The results of the present study also revealed that esfenvalerate has a teratogen index which indicates that the pesticide might be considered as a teratogen. The present study also revealed that the effect of esfenvalerate on amphibians based on the results from the standard toxicity test with Xenopus (FETAX) gives approximately the same results as those found with embryos of Bombina bombina. 4.3 The effects of prochloraz on embryos of amphibians with special reference to Xenopus and BombinaProchloraz is a contact fungicide of the imidazole group acting by inhibiting ergosterol synthesis in the target organisms and primarily used in cereals, grasses and rape against a number of important diseases. 4.3.1 In vivo observationsThe number of malformation increased with increasing concentrations of prochloraz and the time of exposure. The most characteristic malformation caused by prochloraz in both species was edema especially in the cardiac region. The heartbeat decreased with increasing concentrations of prochloraz. The lowest effect concentration found for prochloraz was 1.5 and 2 mg/l for Xenopus and Bombina, respectively, where some embryos exhibited edema especially in the cardiac region. 4.3.2 Recordings of malformation, growth inhibition and mortality at the end of the testThe result revealed that the effect concentrations were a little bit higher in Bombina compared with those found in Xenopus even though Bombina embryos had been exposed to the test substance one day more than embryos of Xenopus. However, the same malformation was seen in both Bombina and Xenopus. Many embryos had multiple malformations. The most characteristic malformations caused by prochloraz were severe edema especially optic and cardiac malformations where the heart often composed of a single straight tube but also brain, edema, and gut malformation were common. Growth inhibition did not seem to be a very sensitive effect parameter when we look at the effect of prochloraz. For Xenopus and Bombina, no significant mortality was seen at concentrations below 3 mg/l. At the end of the experiments, the LC50 values were found to be 4.5 mg/l and more than 10 mg/l for Xenopus and Bombina, respectively. This indicates that malformation is the most sensitive end point. Furthermore, the results of this test also revealed that prochloraz might be considered as a teratogen. The present study has also demonstrated that the jelly coat has no significant influence on the effect of prochloraz on the embryos of Bombina. Thus, the difference in response in the two groups of embryos can not be ascribed to the present of the jelly coat in the experiments with Bombina. Tt should be kept in mind that the only distinct difference in the effect concentration between Xenopus and Bombina was found for mortality and even in this case the difference at effect concentration was only about a factor of 2 which can easily be ascribed to interspecies differences in sensitivity. Thus, it can be concluded that prochloraz has the same effect on Bombina and Xenopus and at the same concentrations. Furthermore, it can be concluded that the most sensitive end point, which can easily be quantified, is the malformation where an EC50 value of 1.4 and 2.1 mg/l was calculated for Xenopus and Bombina, respectively. Limited data on the toxicity of herbicides to amphibian larvae are available for comparison with our results. Thus, we have been unable to find any data on prochloraz for comparison with our results on Xenopus and Bombina. 4.4 Comparison of the effects of esfenvalerate and prochloraz on amphibians with the results from the OECD standard testsIt is well-known that the toxicity of esfenvalerate is very high to fish with 96 h LC50 values for fathead minnows, bluegill, and killifish in the range 0.0007-0.002 mg/l. Likewise, the toxicity to Daphnia is very high with an EC50 (48 h) value = 0.00024 mg/l (cf. Appendix 3). The LC50 values for Xenopus and Bombina found in the present study are higher than 0.15 mg/l the highest concentration tested (the water solubility of esfenvalerate is 0.002 mg/l). Thus, amphibians seem to be less sensitive to esfenvalerate compared with both fish and Daphnia. However, when the effect of esfenvalerate is based on malformation of the amphibian embryos EC50 values of 0.003 and 0.029 mg/l were found in Xenopus and Bombina, respectively, which is comparable with the value found in the standard test. Furthermore, in vivo observation has shown an effect of esfenvalerate on embryos of both Xenopus and Bombina at concentrations as low as 0.001 mg/l. Prochloraz is toxic to fish with 96 h LC50 values for rainbow trout, harlequin fish and bluegill sunfish in the range 1.5-2.9 mg/l. Likewise, the toxicity to Daphnia is high (EC50 (48 h) = 2.6 mg/l) while it is very high to algae with an IC50 (96 h) of 0.073 mg/l. (cf. Appendix 3). The LC50 values for Xenopus and Bombina found in the present study range from 4.5 to more than 10 mg/l which indicate that prochloraz has a lower toxicity to amphibians than the normally used aquatic test organisms in the OECD standard test. However, when we look at the effect of prochloraz to induce malformation EC50 values of 1.4 and 2.1 mg/l were found for Xenopus and Bombina, respectively. Based on these values prochloraz is toxic to amphibians and the effect of prochloraz on amphibians is in the same range as that found in fish and Daphnia. 4.5 ConclusionWhen the toxicity of different organic compounds was evaluated using different test organisms the animal test species exhibited varying degrees of sensitivity to the selected toxicant. For some test chemicals, the most sensitive species was amphibians in other cases the fish was the most sensitive species. This stresses the importance of using different test organisms representing various trophic levels, taxa, and functions in the environment in hazard and risk assessment. Much of the amphibian life cycle occurs in ponds, streams, and temporary pools that are often associated with agricultural areas receiving pesticide applications. Agricultural pesticides may contribute to the decline in amphibian populations and it is therefore important to investigate the effect on pesticides on amphibians. Xenopus laevis is the test organisms used in the only internationally recognised test with amphibians. However, this test has a number of deficiencies which make its use for ecotoxicological risk assessment in Denmark less suited. Thus, Xenopus laevis represents a very special limited ecosystem in Africa. Obviously, it is a disadvantage when evaulating pesticides' effects on amphibian in Europe. In addition, some results indicate that this organism is less sensitive to organic chemicals than other amphibians. Furthermore, the standard test involves removal of the jelly coat of the embryos which may influence the sensitivity of the test system. Therefore a new toxicity test with Bombina bombina was developed since this organism has widespread wild living and closely related species represented in Europe. This makes the important comparison between laboratory tests and field test possible. The results from the present study revealed that we are able to breed Bombina bombina throughout the year and get a sufficient number of healthy embryos for ecotoxicity testing. Esfenvalerate has only a small effect on the mortality on embryos of both Bombina and Xenopus during the present experimental conditions where the LC50 values were higher than 150 µg/l in both species. However, when the number of malformations were used as an end point it was demonstrated that the EC50 values for malformations were only 3 µg/l and 29 µg/l, for Xenopus and Bombina, respectively. It should be noted that Xenopus and Bombina responded to esfenvalerate in the same way and that the change in sensitivity was less than a factor of 10. Such a difference can easily be ascribed to normal differences in species sensitivity. Furthermore, it is interesting that the in vivo observation revealed effects at 1 µg/l in both species. The effects are twisting and apparent partial paralysis of the embryos. Such sublethal effects that we witnessed are likely to have serious implications on the long-term success of the exposed individuals, thus, these organisms are more susceptible to predation. It indicates that wrong conclusions could be drawn if the effect of esfenvalerate on amphibian was only based on LC50 values. For Xenopus and Bombina, no significant mortality was seen at prochloraz concentrations below 3 mg/l and the LC50 values were found to be 4.5 mg/l and more than 10 mg/l for Xenopus and Bombina, respectively. However as for esfenvalerate, the most sensitive end point which can easily be quantified is the malformation where EC50 values of 1.4 and 1.5 mg/l were calculated for Xenopus and Bombina, respectively. Many embryos possessed multiple malformations, and the same malformations were seen in both Bombina and Xenopus. The present study has also demonstrated that the jelly coat has no influence on the effect of prochloraz on the embryos of Xenopus. Even though the LC50 value is higher in Bombina compared with the one found in Xenopus it should be noted that even in this case the difference in effect concentration was only about a factor of 2 which can easily be ascribed to interspecies differences. It can be concluded that prochloraz has the same effect on Bombina and Xenopus and at the same concentrations. Embryos of Bombina and Xenopus are clearly unlikely to be killed by short-term exposure to low concentrations of esfenvalerate and prochloraz. Despite concentrations raised to environmentally unrealistic levels of 0.1 and more than 4 mg/l for esfenvalerate and prochloraz, respectively for 96 h, the embryos did not die. If mortality is our only measure of response to pesticide exposure, we would conclude that these amphibians were relatively tolerant of these pesticides. However, the sublethal effects that we witnessed are likely to have serious implications on the long-term success of the exposed individuals. Thus, when the effect of these pesticides is based on e.g. malformation of the amphibian embryos the EC50 values found in Xenopus and Bombina are comparable with those found in the standard aquatic tests with fish and Daphnia. Finally, the present study has demonstrated that the effect of pesticides on amphibians is highly dependent on several factors. Thus, several studies have found significant differences in sensitivity among different species of amphibian species and it is a well-known phenomenon that temperature may influence the effect concentration of pesticides. Furthermore, several studies indicate that the sensitivity of an amphibian to a given chemical is highly dependent on the different larvae stages of the amphibian. Therefore additional research with more chemicals, the sensitivity on different larval stage in several species, and the effects of temperature must be conducted to make definitive conclusions concerning hazards. Only with such information will it be possible to determine if pesticide use poses a serious threat to amphibian populations in Denmark. Fodnoter[2] This guide is under the jurisdiction of ASTM Committee E-47 on Biological Effects and Environmental Fate and is the direct responsibility of Subcommettee E47.01 on Aquatic Toxicology. Current edition approved Sept. 15, 1991, Published November 1991. 5 ReferencesArnold E.N. and Burton J.A. (1978). Reptiles and Amphibians of Britain and Europe. Collins, London. Bednarz T. (1981). The effect of pesticides on the growth of green and blue-green algae cultures. Acta Hydrobiol. 23,(2). pp. 155-172. Krakow. Berhin F., Houba C., Remacle J. (1984). Cadmium toxicity and accumulation by Tetrahymena in contaminated river waters. Environ Pollut Serv A; 35: 315 - 329. Berrill M., Bertram S., Wilson A., Louis S., Brigham D. and Stromberg C. (1993). Lethal and Sublethal impacts of Pyrethroid Insecticides on Amphibian embryos and tadpoles. Environmental Toxicology and Chemistry, Vol. 12, pp. 525 - 539. Berrill M.S., Bertram S., McGillivray L., Kolohon M. and Paull B. (1994). Effects of low concentrations of forest-use pesticides on frog embryos and tadpoles. Environ. Toxicol. Chem. 13: 657-664. Birge W.J. and Black J.A. (1981). In Situ Acute/chronic Toxicological Monitoring of Industrial Effluents for the NPDES Biomonitoring Program Using Fish and Amphibian Embryo-larval Stages as Test Organisms. OWEP-82-001. Office of Water Enforcement and Permits U.S.E.P.A., Washington DC, pp. 121. Black J.A. and Birge W.J. (1982). The Aquatic Toxicity of Organic Compounds to Embryo-Larval Stages of Fish and Amphibians. Research Report No. 133. Kentucky University, Lexington Water Resources Research Institute. pp 1-61. Bradford D.F. (1991). Mass mortality and extinction in a high elevation population of Rena muscosa. J. Herpetol. 25: 174-177. Brodie E.D. and Formanowicz D.R. (1987) Prey size preference of predators: Differential vulnerability of larval anurans. Herpetologica 39: 67-75 Christensen T. (1982). Alger i naturen og i laboratoriet. (Nucleus). Caldwell J.P., Thorp J.H. and Jervey T.O. (1980). Predator-prey relationships among larval dragonflies, salamanders, and frogs. Oecologia 46: 285-289. Coats J.R., Symonik D.M., Brandbuury S.P., Dyer S.D., Timson L.K., and Atchison G.J. (1989). Toxicology of synthetic pyrethroids in aquatic organisms: An overview. Environ. Toxicol. Chem. 8: 671-679. Cooke A.S. (1972). The effects of DDT, dieldrin and 2,4-D on amphibian spawn and tadpoles. Environ. Pollut. 3: 51-68. Corbett K. (1989). Conservation of the European Reptiles and Amphibians. Christopher Helm Ltd., Bromley. Courchesne C.L. and Bantle J.A. (1985). Analysis of the Activity of DNA, RNA, and the Protein Synthesis Inhibitors on Xenopus Embryo Development. Teratogenesis Carcinogenesis and Mutagenesis, Vol. 5, pp. 177-193. Dawson D.A., Fort D.J., Newell D.L., and Bantle J.A. (1989). Developmental Toxicity Testing with FETAX: Evaluation of Five Compounds. Drug and Chemical Toxicity, Vol. 12, pp. 67-75. Dawson D.A. and Bantle J.A. (1987). Development of a Reconstituted Water Medium and Preliminary Validation of the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Journal of Applied Toxicology, Vol 7, pp. 237-244. Di Toro D.M., Zarba C.S., Hansen D.J., Berry W.J., Swartz R.C., Cowan C.E., Pavlou S.P., Thomas H.A., and Paquin, P.R. (1991). Annual Review. Technical Basis for establishing sediment quality criteria for non-ionic organic chemicals using equilibrium partitioning. Environmental Toxicology and Chemistry, vol. 10: pp 1541 - 1583. Deuchar E.M. (1972). Xenopus laevis and Developmental Biology, Biological Reviews, Vol 47, pp. 37-112. Deuchar E. M. (1975). Xenopus: The South African Clawed Frog, Wiley, New York, NY. Duellman W.E. and Trueb L. (1986). Biology of Amphibians. McGraw-Hill, New York, N.Y. Dumont J., Schultz T.W., Buchanan M., and Kao G. (1983). Frog Embryo Teratogenesis Assay-Xenopus (FETAX)-A Short-term Assay Applicable to Complex Environmental Mixtures, In: Short-term Bioassays in the Analysis of Complex Environmental Mixtures III, Waters, Sandhu, Lewtas, Claxton, Chernoff and Nesnow, eds., Plenum, New York, NY, pp. 393-405. Dumont J., Schultz T.W., and Epler, R.G. (1983). The Response of the FETAX Model to Mammalian Teratogens. Teratology, Vol. 27, p. 39a. Fort D.J., James B.L., and Bantle J. (1991). Evaluation of the Developmental Toxicity of Five Compounds with the Frog Embryo Teratogenesis Assay: Xenopus (FETAX) and a Metabolic Activation System. Journal of Applied Toxicology. Gomme J.W. et al. (1991). Hydrology of Pesticides in a Chalk Catchment: Surface Waters. J. IWEM, pp. 546-552. Greenberg J. (1982).Detection of Teratogens by Differentiating Embryonic Neural Crest Cells in Culture: Evaluation as a Screening System. Teratogenesis Carcinogenesis and Mutagenesis, Vol. 2, 319-323. Harte J. and Hoffman E. (1989) Possible effects of acidic deposition on a rocky mountain population of the tiger salamander Ambystoma tigrinum. Conserv. Biol. 3: 149-158. Horning W.B. and Weber C.I. (1985). Short -term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms. EPA/600/4-89/001, pp. 105-162. Howe G.E., Gillis R., and Mowbray R.C. (1998). Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ. Toxicol. Chem. 17, no 3 519-525. Larsen J. and Nilsson J.R. (1983). Effect of nickel on the rate of enducytosis, mortality, and proliferation in Tetrahymena and determination on the cell content of the metal. Protoplasma. 118: 140-147. Kawamura T., Nishioka M. and Ueda H. (1972). Reproduction of the Oriental Fire-bellied Toad, Bombina orientalis, with special Reference to the Superiority of this Species as Laboratory Animal. Sci. Rep. Lab. Amphibian Biol., Hiroshima Univ. 1, 303-317. Kitchin K.T., Schmid B.P. and Sanyal M.K. (1981). A Coupled Microsomal-Activating/Embryo Culture System: Toxicity of Reduced Betanicotinamide Adenine Dinucleotide Phosphate (NADPH). Biochemical Pharmacology, Vol. 30, pp. 985-992. Madej Z. (1973). Ecology of European bellied toads. Przeglgd Zoologiczny, 17 pp 200-204. Materna E.J., Rabeni C.F. and LaPoint T.W. (1995) Effects of the synthetic pyrethroid insecticide, Esfenvalerate, on larval leopard frogs (Rena spp.) Environmental Toxicology and Chemistry, Vol 14, No 4 pp 613-622. Mattison C. (1993). Keeping and Breeding Amphibians. Blandford, London. Nieuwkoop P.D., and Faber (1975). Normal Tables of Xenopus laevis (Daudin), 2nd Ed., North Holland, Amsterdam. Obert H.-J. (1973). Untersuchungen zur hormonalen Steuerung der Ruf- und Paarungsaktivität bei der Rot-und Gelbbauchunke Bombina bombina (L.) und Bombina variegata (L.). Zool. jb. Physiol. 77, 166-198. Phillips, K. (1990). Where have all the frogs and toads gone? Bio-Science 40: 422-424. Pierce B.A. (1986). Acid Tolerance in Amphibians. EPA Water Quality Criteria for Water (EPA/440/5-86/001), Bioscience, Vol 35, pp. 239-243. Recker W. (1979). Gefangenschaftsnachzucht bei der Gelbbauchunke, Bombina variegata. Elaphe 1979 (3) 28 - 31. Rogner M. (1981). Durch Nachzucht erhalten: Gelbbauchunken. Aquarien Magazin 15 (11) 731 - 738. Sabourin T.D., Faulk R.T. and Goss L.B. (1985). The Efficacy of Three Non-mammalian Test Systems in the Identification of Chemical Teratogens. Journal of Applied Toxicology, Vol 5, pp. 225-233. Sabourin T.D. and Faulk R.T. (1987). Comparative Evaluation of a Short-term Test for Developmental Effects Using Frog Embryos. Banbury Report 26: Developmental Toxicology: Mechanisms and Risk, Cold Spring Harbor Laboratory, pp. 203-223. Schuller R., Hardin B.D., and Niemer R. (1982). Drosophila as a Tool for the Rapid Assessment of Chemicals for Teratogenicity. Teratogenesis Carcinogenesis and Mutagenesis, Vol. 2, pp. 293-301. Seale D.B. and Beckvar N. (1980). The Comparative Ability of Anuran Larvae (Genera: Hyla, Bufo, and Rana) to Ingest Suspended Blue-Green Algae. Copeia (3) pp. 495 - 503. Strawbridgs S., Coull B.C., and Chandler G.T. (1992). Reproductive output of a meiobenthic copepod exposed to sediment associated fenvalerate. Arch- Environ-Contam-Toxicol 23(3) pp 295-300. U.S. Environmental Protection Agency (1984) Federal Register, Vol 49, Feb. 7, 1984, pp. 4551-4554. Wilkinson J.W. (1994). An account of successful captive reproduction of Bombina bombina, the European fire-bellied toad. British Herpetological Society Bulletin, No. 49. Appendices 1-4Appendix 1 Score sheet of malformations after 96 hAppendix 2 The composition of the culture media FETAX solution water
per litre of deionised or distilled water. The pH of the final solution should be 7.6 to 7.9. All chemicals were reagent-grade or better. Appendix 3 Comparison of the LC50 values from the present amphibian test with the results from other tests with amphibians and the standard aquatic test organisms. Table A3.1
Table A3.2
Appendix 4 Table A4.1 Effeketen af esfenvalerat på Xenopus laevis illustreret ved sublethale defekter, væksthæmning og dødelighed. Middeltal af 3 forsøg.
Table A4.2 Effekten af esfenvalerat på Xenopus laevis illustreret ved sublethale defekter, væksthæmning og dødelighed. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 96 timers eksponering.
Table A4.3 Effekten af procloraz i Xenopus laevis med sublethale defekter, væksthæmning og dødelighed i eksperimenter, hvor gelékappen ikke er blevet fjernet. Middeltal af 3 forsøg.
Table A4.4 Effekten af prochloraz på Xenopus laevis illustreret ved sublethale defekter, væksthæmning og dødelighed i eksperimenter, hvor gelékappen ikke er blevet fjernet. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 96 timers eksponering.
Table A4.5 Effekten af procloraz på Xenopus laevis illustreret ved sublethale defekter, væksthæmning og dødelighed i eksperimenter, hvor gelékappen er blevet fjernet. Middeltal af 3 forsøg.
Table A4.6 Effekten af prochloraz på Xenopus laevis illustreret ved sublethale defekter, væksthæmning og dødelighed i eksperimenter, hvor gelékappen ikke er blevet fjernet. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 96 timers eksponering.
Table A4.7 Effekten af procloraz på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed. Middeltal af 3 forsøg.
Table A4.8 Effekten af prochloraz i Bombina bombina udtrykt ved sublethale defekter, væksthæmning og dødelighed. Resultaterne er udtrykt som NOEC, LOEC og EC50/LC50 efter 120 timers eksponering.
Table A4.9 Effekten af procloraz på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed. Middeltal af 3 forsøg.
Table A4.10 Effekten af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed. Resultaterne er udtrykt ved NOEC, LOEC og EC50/LC50 efter 120 timers eksponering.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||