|
Pesticides Research no. 84 2004 The Effects of Selected Pyrethroids on Embryos of Bombina bombina during different Culture and Semi-field ConditionsContentsPART I : The effects of esfenvalerate on embryos of Bombina bombina during different culture conditions and prolonged studies for identifying effects after recovery of a short embryological sublethal exposure
PART II : The effects of selected pyrethroids on embryo development of Bombina bombina under semi-field conditions
Appendix A: Score sheet of malformations after 96 hours Appendix B: The composition of the culture media Appendix C: The effect of esfenvalerate on malformation, growth inhibition, and mortality PrefaceThis report is a part of the research programm "Effects of Pesticides on Ponds". The projects were funded by the Danish Environmental Protection Agency' s Research programme on Environmental effects of pesticides. The aim of the project was: To develop a model-based tool for evaluation of risk related to pesticide exposure in surface water. The tool must be directly applicable by the Danish Environmental Protection Agency (DEPA) in their approval procedure. As part of this goal, the project had to:
The project consisted of four subprojects with individual objectives. The sub-projects are listed in Table 1. Table 1. Sub-projects of " Effects of Pesticides on Ponds". Tabel i. Oversigt over delprojekter i "Effekter af pesticider i vandhuller".
The reports produced by the projects are:
The project was overseen by a steering committee. The members have made valuable contributions to the project. The committee consisted of:
For this report Betty Mogensen, Frank Stuer-Lauridsen, Peter Sørensen and Pia Lassen from National Environmental Research Institute are thanked for their great work with the mesocosms. Louise Schlütter is thanked for the great help to finish the report. Moreover Linda Gertz is thanked for the preparation of the manuscript. SummaryPart IIn the present study, the developmental effects of the pesticide esfenvalerate on the fire-bellied toad Bombina bombina were studied at different temperatures and in different media. Additionally, the effect studies were prolonged in order to include recovery experiments of the tadpoles after transfer to fresh medium without the pesticide for the purpose of studying the possible effects occurring up to 6-7 weeks after the exposure to esfenvalerate was ended as well. Spasmodic twisting was the most obvious effect on embryos of exposure to esfenvalerate, and at the end of the experiment after 216 h this effect was seen in the embryos exposed to as low as 1 μg/l esfenvalerate. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and blistering and oedema were often seen. At concentrations above 100 μg/l, blistering and oedema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were seen at 100, 150 and 300 g/l. The most significant malformations observed included cardiac malformations, severe lateral flexure, oedema, brain and gut malformations. Neither growth nor mortality was distinctly affected by the concentrations of esfenvalerate used in this study (1-300 μg/l). When pond water was used instead of the FETAX solution used in standard FETAX tests few effects of esfenvalerate treatment were seen. It is suggested that difference in ion composition and content of dissolved organic matter may cause the observed difference. Apparently, the pond water used in the study was more appropriate in ion composition and concentration than the FETAX solution, since the EC50 values for malformations were 43.7 and 24.7 μg/l in pond water and FETAX solution, respectively. It is well-known that pyrethroids strongly absorb to organic matter and the bioavaliability may be reduced in this way. Since no organic substance are added to the FETAX solution it is evident that the low toxicity in pondwater may be due to high contain of dissolved organic matter. The effects of esfenvalerate added in different concentrations were almost comparable at two different temperatures, although displaced in time. The same pattern of malformations was found at both temperatures, however, the embryos at 20°C had more malformations and low EC50 (32.7 and 24.7 μg/l at 24 and 20 °C, respectively). In the group pre-exposed to 20 μg/l esfenvalerate about 70% of the organisms recovered after 24 h in pesticide free medium, while only 20% of the embryos pre-exposed to 125 μg/l esfenvalerate recovered. After two days all living larvae were swimming around and eating algae like the controls. A distinct effect of esfenvalerate was, however, seen six weeks after the transfer to the fresh medium without esfenvalerate. During the metamorphosis it got evident that esfenvalerate at both concentrations induced malformed forelimbs of Bombina bombina still six weeks after the exposure was ended. The many malformations recorded in the study as well as the above mentioned results of the recovery experiment as a consequence of exposure to sublethal concentrations of esfenvalerate emphasize the severe impact that this toxicant can have on populations of amphibians in natural environments. From the present study it can be concluded that Bombina bombina is very sensitive to sublethal exposure of pyrethroids and will probably die as a consequence of the developmental abnormalities caused by even a few days' short exposure to rather low concentrations of pyrethroids. Part IIDevelopmental toxicity effects of four pyrethroids, sprayed simultaneously to an artificial pond, on caged embryos of Bombina bombina were studied for the purpose of examining the possibility of using natural conditions when monitoring for effects of toxicants on embryos of amphibians in the environment. A new toxicity test using Bombina bombina as test organism (Larsen & Sørensen, 2004) was used. The embryos were inspected for in vivo effects of pyrethroids every day during the experimental period until day 9. Malformations, growth inhibition and mortality were recorded at the end of the test. Spasmodic twisting was seen as one of the effects of the pyrethroid treatment, and it caused immobilisation of more than half of the embryos. The effect decreased when the water concentration of pyrethroids decreased due to absorption by sediment and macrophytes. Furthermore, the pyrethroids caused the mortality to increase from 5-10% in the controls to 51%, and had also obvious developmental effects, since 85% of the embryos possessed multiple malformations by the end of the experiments. The growth of the embryos was, however, not affected. From this study it can be concluded that the new toxicity test guideline using Bombina bombina as test organism (Larsen & Sørensen, 2004) can be modified and used under in situ conditions for testing developmental toxicity of toxicants, thereby increasing the realism of toxicity tests by including natural food webs, decomposers, sediment, natural physical conditions, etc., in the tests. Dansk sammendragDel IUdviklingsmæssige effekter af pesticidet esfenvalerat på klokkefrøen Bombina bombina blev i dette studie undersøgt ved forskellige temperaturer og i forskellige medier. Endvidere blev effektstudierne forlænget til også at inkludere recovery-forsøg af haletudserne efter overførsel til frisk medium uden pesticid med det formål at undersøge de mulige effekter, der kunne opstå ved metamorfosen 6-7 uger, efter embryoner var blevet udsat for en kortvarig eksponering med esfenvalerat. Spasmoide vridninger var den mest iøjefaldende effekt af esfenvalerat, og efter 216 timer ved slutningen af forsøget blev denne effekt fundet i embryoner udsat til så lave koncentrationer som 1 μg/l esfenvalerat. Ved koncentrationer over 50 μg/l blev embryoner fastlåst på samme sted på grund af konstante spasmoider vridninger, og blister og ødemer blev observeret. Ved koncentrationer over 100 μg/l var blister og ødemer talrige, og hoved- og hjernemisdannelser blev observeret. Hjerteslaget var langsomt og alvorlige hjertemisdannelser blev set ved 100, 150 og 300 μg/l. De mest signifikante misdannelser var hjertemisdannelser, alvorlige sidebøjninger af kroppen, ødemer, hjerne- og tarmmisdannelser. Hverken væksten eller overlevelsen blev påvirket af de koncentrationer af esfenvalerat der var anvendt i denne undersøgelse (1-300 μg/l). Når der i forsøget blev anvendt vand fra et vandhul i stedet for FETAX-opløsningen, der anvendes i standard FETAX-testen, blev der set færre effekter af esfenvalerat. Forskelle i ionsammensætningen og indholdet af opløst organisk stof kan være mulige forklaringer på denne forskel. Tilsyneladende har det anvendte vandhulsvand en mere passende ionsammensætning og koncentration end FETAX-opløsningen, da EC50-værdien for misdannelser var henholdsvis 43,7 og 24,7 μg/l i vandhulsvand og FETAX-opløsning. Det er et velkendt fænomen, at pyretroider absorberes stærkt til organisk stof, og at dette kan reducere biotilgængligheden. Da der ikke er tilsat organiske stoffer til FETAX-opløsningen, er det sandsynligt, at indholdet af opløst organisk stof kan forklare den mindre effekt i vandhulsvandet. Effekterne af esfenvalerat tilført i de forskellige koncentrationer, var næsten sammenlignelige ved to forskellige temperaturer, men forskudt i tid. Det samme mønster af misdannelser blev fundet ved begge temperaturer, men embryoner ved 20°C havde flere misdannelser og lavere EC50 (henholdsvis, 32,7 og 24,7 μg/l ved 24 og 20°C). Omkring 70% af embryoner, der var præ-eksponeret til 20 μg/l esfenvalerat, kom sig i løbet af 24 timer efter overførslen til pesticidfrit medium, mens kun 20% af embryonerne præ-eksponeret til 125 μg/l esfenvalerat kom sig. Efter to dage svømmede alle haletudserne rundt og spiste alger. En udtalt effekt af esfenvalerat blev dog fundet 6 uger efter overførsel til nyt medie uden esfenvalerat. I metamorfosen blev det tydeligt, at esfenvalerat ved begge koncentrationer inducerede misdannede forlemmer på Bombina bombina hele 6 uger efter behandlingen var afsluttet. De mange misdannelser, som er fundet i denne undersøgelse, understreger den alvorlige indvirkning, som dette toksiske stof kan have på populationer af amphibier i naturen. Ud fra denne undersøgelse kan det konkluderes, at Bombina bombina er meget følsom overfor sublethale koncentrationer af pyrethroider og vil sandsynligvis dø som følge af de udviklingsmæssige abnormaliteter forårsaget af kortvarig kontakt til endda ret lave pyrethroid-koncentrationer. Del IIUdviklingsmæssige effekter af fire pyrethroider, sprøjtet samtidigt på et kunstigt vandhul, på Bombina bombina embryoer anbragt i åbne kamre i vandhullet, blev studeret for at undersøge muligheden for at anvende naturlige forhold ved monitering af effekter af giftige stoffer på amphibier i vandmiljøet. En ny toksicitetstest med Bombina bombina som forsøgsorganisme (Larsen & Sørensen, 2004) blev anvendt. Embryoerne blev undersøgt for in vivo effekter af pyrethroider hver dag i forsøgsperioden indtil dag 9. Misdannelser, væksthæmning og mortalitet blev undersøgt ved forsøgets slutning. En af effekterne af pyrethroid-behandlingen var spasmoide vridninger af embryoerne, og det forårsagede, at mere end halvdelen af embryoerne blev fastlåst på samme sted. Denne effekt aftog, da vandkoncentrationen af pyrethroid gik ned som følge af absorbtion i sedimentet og på makrofytterne. Endvidere forårsagede pyrethroiderne, at mortaliteten steg fra 5-10% i kontrollerne til 51% i testvandhullet og havde synlig udviklingsmæssige effekter, da 85% af embryoerne udviste flere forskellige misdannelser ved slutningen af forsøget. Væksten af embryoerne blev dog ikke påvirket. Fra dette studie kan det konkluderes, at den nye toksicitetstest med Bombina bombina som forsøgsorganisme (Larsen & Sørensen, 2004) også kan anvendes under in situ forhold for at teste udviklingsmæssige effekter af giftige stoffer og dermed øge realismen af toxicitetstests ved at inkludere naturlige fødekæder, nedbrydere, sediment, fysiske forhold, etc., i forsøgene. PART I : The effects of esfenvalerate on embryos of Bombina bombina during different culture conditions and prolonged studies for identifying effects after recovery of a short embryological sublethal exposure1 IntroductionPopulations of many amphibians have declined in and some species have disappeared from, certain localities around the world. The phenomenon appears to have accelerated recently. While evidence for widespread reduction in numbers of amphibians is rapidly accumulating, the reasons for the declines are less clear. Complicating matters are the apparent decline of many species in relatively unaltered habitats (e.g. parts of the rain forest of Costa Rica (Crump et al. 1992) and the difficulty of appropriately monitoring and censoring these highly variable populations (Pechmann et al. 1991; Blaustein et al. 1994). Possible causes include loss of suitable habitats (Cooke, 1970), acid rain (Phillips, 1990), climate changes (Pounds and Crump, 1994), Wyman 1990), ultraviolet radiation (Wyman, 1990), and other environmental contaminants. Due to their thin and permeable skins, and prolonged exposure first to the aquatic environment and then to the terrestrial, amphibians may be particularly sensitive to environmental stress. Embryonic and larval anurans have shown sensitivity to a number of environmental pollutants, such as hydrazine and its methylated derivatives (Greenhouse, 1976; Greenhouse, 1976), phthalate esters (Larsson and Thuren, 1987), and metals (Miller and Mackay, 1987; Birge et al., 1985). Agricultural pesticides in particular may contribute to the decline in amphibian populations (Phillips, 1990; Dutta and Mohanty-Hejmadi, 1978; Berrill et al., 1994). Much of the amphibian life cycle occurs in ponds, streams, and temporary pools that are often associated with agricultural areas receiving pesticide applications. In addition, breeding and larval development of amphibians occur in spring and summer at the same time that heavy application of pesticides on agricultural lands occurs. In the present study the possible role of one of the widely used synthetic pyrethroid pesticides, esfenvalerat, on amphibian declines has been investigated. Synthetic pyrethroids are increasing in number and used because they have retained the high insecticidal activity and low avian and mammalian toxicity characteristic of natural pyrethrins. Effects on amphibians are less well-known, but some sensitivity has been observed. Berrill et al. (1993) and Phillips (1990) exposed five species of amphibians to permethrin and fenvalerate at concentrations between 10 and 200 μg/l with no mortality resulting but with a notable reduction in growth rates. In the present study the main goal has been to test whether amphibians are sensitive to low levels of contaminants and whether gross sublethal effects do occur. The only internationally recognized test with amphibians is FETAX (Frog Embryo Teratogenesis Assay-Xenopus) using embryos of Xenopus laevis, the South African clawed frog. As this species is not ecological relevant for Denmark and the rest of Europe a new toxicity test guideline with the fire-bellied toad Bombina bombina as test organism has been developed (Larsen & Sørensen 2004). The present work is a further application of this new test with Bombina bombina where the developmental effects of esfenvalerat at different temperatures and in different media have been investigated. Additionally, the effect studies were prolonged in the present study to examine the recovery of the organisms after transfer to a fresh medium without pesticide 6-7 weeks after the exposure was ended. 2 Materials and methods
Briefly, the present study includes work related to maintaining and breeding the fire-bellied toad, Bombina bombina, toxicity test in 120 h or 216 h (depending on the temperature), a study of the recovery of the embryos and larvae after transferring to fresh pesticide free medium, and a prolongation of the study to include possibly effects up to 6-7 weeks after the exposure was ended. 2.1 Principles and methods for maintaining and breeding the fire-bellied toads, Bombina bombina2.1.1 Principles and methods for maintaining adults Bombina bombinaAdults Bombina bombina were at least two years of age and weighed about 6.5 to 8.5 g (males and females are about the same size). 1-2 pairs were kept together in a 50 30 cm vivarium. The floor area consisted of 60% water, with a depth of 8 cm, and the land area was dark-coloured with hiding areas and a feeding place. The vivarium was fitted with a mesh to prevent escape. Artificial lighting was switched on from 7 am to 7 pm daily, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Slowly growing natural aquatic plants and plants of plastic and broken pots were creating hiding places. Diet Food consisted of crickets, meal worms, small earthworms, flies (with crumpled wings) and other suitable invertebrates supplemented with a special vitamin supplement. Feeding was continued throughout the year, although at a reduced rate (about two times a week) during November-February when the animals showed minimal activity. Temperature Adults were kept at 19-21°C in the winter and at room temperature in the summer period. 2.1.2 Principles and methods for breeding Bombina bombinaMales and females were bred as a single pair and the animals were moved to a 45 l glass aquarium with a water depth of about 20 cm. The aquarium was richly furnished with both natural and plastic plants. In addition, 2 mm round sticks of wood were placed in the aquarium, and 1 to 2 islands of floating cork enabled the animals to leave the water if desired. Artificial lighting was switched on from 5 am to 9 pm daily, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Water temperature was adjusted to 22 ±0.5°C. Breeding To mature the oocyttes, the females received about 14 IU of Pregnant Mare Gonadotropin (PMG) two days before the animals were moved to the breeding aquarium. Just before the animals were moved to the breeding aquarium both males and the females received 55 to 100 IU, of human chorionic gonadotropin to induce breeding. The amount of hormones injected depended on the size of the animals and the time of year. Low doses were usually used in spring and higher doses in autumn. Amplexus normally ensued within 2 to 6 h and eggs deposition about 9 to 12 h after injection. The eggs were immediately inspected for fertility and quality. The fertility rate should be > 75% before a toxicity test was performed. Examination of the aquarium showed that the eggs were often found on vegetation and on the round sticks of wood, which were placed in the breeding aquarium to imitate straw and stems from aquatic plants. 2.2 Toxicity tests using embryos of Bombina bombina2.2.1 Principle and design of the toxicity testThe new Bombina bombina test is a 120 h or 216 h, (depending on the temperature) renewal whole embryo assay that can be used to evaluate the developmental toxicity of a test material. Exposure was continuous throughout the test. For each concentration, two dishes each containing 5 embryos and 10 ml test solution were used. For each control, four dishes of 5 embryos each were used in the standard experiments with Bombina bombina. Embryos were randomly assigned to test dishes. Dishes were randomly assigned to positions in the incubator. Data were recorded to evaluate developmental toxicity, mortality, malformation, and growth inhibition properly. In most tests, it was possible to generate concentration-response curves for mortality, malformation, and growth inhibition. The mortality and malformation concentration-response curves were then used to estimate the concentration that affected 50% of the exposed embryos. It was assured that according to Nieuwkoop and Faber (1975) at least 90% of the controls had attained stage 46 at the end of the experiments. Test organisms The fire-bellied toad, Bombina bombina has been kept at laboratory facilities for more than two years. Egg manipulation To assess the influence of esfenvalerate on the embryos of Bombina bombina as realistic as possible the jelly coats were not removed. The jelly coat is normally removed before starting an experiment in an ordinary FETAX test. 2.2.2 Test substancesEsfenvalerate (CAS no. 66230-04-4), a pyrethroid insecticide, was added as the formulated product Sumi Alfa 5 FW (contains 5% esfenvalerate as the active ingredient). Selected physico-chemical properties are shown in table 2.1. Table 2.1 Udvalgte fysisk-kemiske egenskaber for esfenvalerat.
* Aqueous photolysis studies with esfenvalerate indicated that the half-lives are about 10 days, however, the half-lives are of course dependent on the intensity of the light. Data from The Pesticide Manual, tenth edition and the Danish EPA. Esfenvalerate is an insecticide with contact and stomach action and is a voltage dependent sodium channel agonist. It is used as a potent contact and ingested insecticide with a very broad range of activity, especially effective against Coleoptera, Diptera, Hemiptera, Lepidoptera, and Orthoptera on cotton, fruit, vegetables, and other crops at 5-25 g a.i./ha. It is effective against strains resistant to organochlorine, organophosphorus and carbamate insecticides. 2.2.3 Preparation of test solutionsStock solutions of esfenvalerate were prepared daily. The pH of the stock solutions was adjusted to 7.5 ±0.5. 2.2.4 EmbryosStaging of embryos Staging of embryos was done according to Gosner (1960) and Nieuwkoop and Faber (1975). Embryo selection Normally cleaving embryos were selected for use in testing. Two levels of selection were used. Normally cleaving embryos were first sorted into dishes containing fresh water. After a short period during which cleavage continued, embryos were sorted again to ensure that only normal embryos were selected. Abnormal pigmentation was viewed as an indicator of bad embryos. Both Nieuwkoop and Faber (1975) and the "Atlas of Abnormalities" [1] were used as references to determine whether the cleavage patterns were normal. Midblastula (stage 8) to early gastrula (stage 11) was used to start the test. At this stage, normal cleavage and development can be ascertained. Embryos, chosen prior to stage 8, might develop abnormal cleavage patterns later, whereas embryos selected after stage 11 have commenced organogenesis. The sorting was done in Petri dishes. 2.2.5 Culture mediumThe culture medium used for the test was FETAX solution or pond water. FETAX solution FETAX solution was composed of 625 mg NaCl, 96 mg NaHCO3, 30 mg KCl, 15 mg CaCl2, 60 mg CaSO4 2H2O, and 75 mg MgSO4 per litre of deionized or distilled water. The pH of the final solution was controlled to be between 7.6 and 7.9. All chemicals were reagent-grade or better. Pond water Pond water was collected in experimental ponds at National Environment Research Institute. GF/C filtrated water was stored at 4°C. Before use the water was warmed up to test temperature. 2.2.6 Experimental designTests with embryos of Bombina bombina were conducted in an incubator at 20 or 24 ±0.5°C. The tests chambers were covered by 60 mm glass Petri dishes (before use all glass wares were treated with silylation reagent solution and thoroughly washed in water) with an initial culture volume of 10 ml. A binocular dissection microscope capable of magnifications up to 30 was used to count and evaluate abnormal embryos. The embryo length (head-tail length measurements) was measured with a map measurer or an ocular micrometer. Maintenance of separate clutches It is necessary to keep clutches separate because embryos of a particular mating pair may develop poorly although they initially appear acceptable. This would cause all the embryos to be discarded if embryos were mixed from different mating pairs. Esfenvalerate concentrations The effect of esfenvalerate was tested at 1, 2.5, 5, 10, 50, 100, 150, and 300 μg/l (a.i.) and the results were compared with the control group. Renewal Renewal of the medium was performed every 24 h during the test. Just prior to medium renewal the pH was measured in the control and in the highest test concentrations to determine if significant changes had occurred. The test solution was removed with a Pasteur pipette. The orifice of the Pasteur pipette was enlarged and fire-polished to accommodate embryos without damage in case the embryos were accidentally picked up. This is standard procedure for FETAX. New medium was added immediately after. Duration of the test The standard exposure time for Bombina bombina was 120 h at 24°C and 216 h at 20°C, at which time stage 46 was attained. 2.2.7 Determination of the effects of esfenvalerate on embryos of Bombina bombinaIn vivo observations Dead embryos were removed at the end of each 24 h period when the solutions were renewed and the mortality data were recorded. If dead embryos were not removed, microbial growth could occur which may kill living embryos. Death during the first 24-72 h, depending on the temperature and exposure, was ascertained by the embryo's skin pigmentation, structural integrity, and irritability (measured as lack of response on physical stimulations). After 72 h the lack of heartbeat served as an unambiguous sign of death. At 120 h (at 24°C) or 216 h (at 20°C) of exposure or on stage 46 of controls, the total number of dead embryos was recorded during the test (mortality was registered after 24, 48, 72, 96,120, 144, 168, 192, and 216 h of exposure). Dead embryos were removed while the remaining living embryos were fixed in 3% formaldehyde solution. Malformations were recorded at the end of the test. Embryos exposed to the test material were compared with appropriate controls. The number of malformations in each category was reported in standard format to ease interlaboratory comparison (cf. Appendix A). Malformation Head-tail length (growth) was measured at the end of each test. If the embryo was curved or kinked, the measurement was made as if the embryo was straight. The embryos were measured after fixation in 3% formaldehyde solution. Teratogenic Index Teratogenic Index (TI) was determined after 120 h or 216 h, depending of temperature and of exposure. TI is defined as 120 h or 216 h LC50 (mortality) divided with 120 h or 216 h EC50 (malformations). TI values higher than 1.5 signify large separation of mortality and malformation concentration ranges and therefore a large potential for all embryos to be malformed in the absence of significant embryo mortality (ASTM 1991). 2.2.8 Replicates and controlsNumber of tests and data collection Three definitive tests were conducted in a random block design. Because it is necessary to acquire data on mortality, malformation, and growth inhibition, the concentration series were adjusted to the expected 120 or 216 h LC50, 120 h or 216 h EC50 (malformation) values, and the minimum concentration needed to inhibit growth (MCIG). Reference toxicant For a positive control or reference toxicant, 6-aminonicotinamide presented a mortality and malformation database convenient for reference purpose. For each test, the positive control consisted of two dishes of five embryos each exposed to 2500 mg 6-aminonicotinamide/l and two dishes of five embryos each exposed to 5.5 mg 6-aminonicotinamide/l. The mortality and malformation observed should be between 40 and 60%. For example, at 2500 mg/l 4 to 6 of the 10 embryos should have died by 120 or 216 h (depending on the temperature). Only biological responses related to mortality and malformation were considered in this analysis. Growth inhibition was not considered with regard to aminonicotinamide. 2.2.9 Data treatment and reportingWith the Probit analysis it was possible to obtain concentration-response curves to determine the values of 120 h or 216 h LC50 and 120 h or 216 h EC50. The comparison of measurement results (head-tail length) between controls and treated embryos was obtained with the ANOVA statistical analysis. The minimum concentration to inhibit growth (MCIG) is the minimum concentration of test material that significantly inhibits growth as determined by measurement of head-tail length. A significant difference in growth should be determined by the t-Test for grouped observations at the p = 0.05 level (Dawson et al. 1989). 2.3 Principle and design of recovery experiments using embryos of Bombina bombina2.3.1 Exposure of the embryos to esfenvalerateThe recovery test included two independent tests (on different days). The test consisted of two sublethal concentrations of the test substance, 20 and 125 μg/l esfenvalerate, and an untreated control group. The selected concentrations were based on concentrations-response curves for both mortality and malformation determined in a toxicity test with Bombina bombina. For each concentration, at least two dishes each containing five embryos of Bombina bombina and 10 ml of test solution were used. For each control, at least four dishes of 5 embryos were used. Each test used early embryos derived from a single mating pair. The toxicity test were otherwise carried out exactly as described previously in FETAX solution at 20°C and according to Nieuwkoop and Faber (1975) the embryos were at stage 46 at the end of this part of the test. 2.3.2 Principles and methods for maintaining embryos and larvae (tadpoles) of Bombina bombina during the recovery periodTransfer of embryos to fresh medium At the end of the toxicity test (after 216 h) dead embryos were removed and the remaining living embryos were transferred to three different glass dishes each containing 200 ml FETAX solution without pesticides. The lack of heartbeat served as an unambiguous sign of death. All the control embryos were transferred to one glass dish, and embryos, which previously had been exposed to the same concentration of pesticide, were collected in the same dish. The maximum number of embryos in each glass dish was about 20. 8 h after the embryos were transferred to pesticide free medium, half of the FETAX solution was withdrawn and replaced with aerated tap water supplemented with green algae. After 24 h the medium was exchanged with pure tap water containing algae. Culture conditions After transfer to a pesticide free medium, the embryos and larvae were kept in an animal room at 21-22°C so that a photoperiod of 15 h day/9 h night could be maintained. During the recovery period the embryos and larvae were moved from a smaller aquaria to a larger aquaria serveral times to maintain a sufficient amount of food and space. After 1 week each of the three groups were transferred from the 200 ml glass dishes to 3 l aquaria containing aquatic plants and small stones on the bottom. After two weeks of recovery the tadpoles were transferred to 11 l aquaria with more aquatic plants. After four weeks of recovery the tadpoles were transferred to 45 l aquria each containing 25 l of water and many aquatic plants and stones on the bottom where they were maintained for additionally another week. The water was in all cases aerated by the use of air stones, and the pH of the water was about 7. A suspension of green algae like Mougeotia and Oedogonium was added every day to each glass dishes and aquaria together with Spirulina discs (algae pills from Wordley) to ensure a sufficient food supply. Renewal of the water was performed every day during the first two weeks (in the glass dishes and the 3 l aquaria) and twice a week in the 11 l and 45 l aquaria. Determination of the effects on embryos and larvae In vivo observations were performed every day during the first two weeks and twice a week during the rest of the test. Dead, malformation, and development stages were followed. Growth was measured during the period. 2.3.3 Principles and methods for maintaining Bombina bombina just before and during metamorphosisCulture conditions Five weeks after the embryos of Bombina bombina were transferred to a pesticide free medium, the tadpoles were transferred to a 50 30 cm vivarium with 70% water, about 8 cm deep, and 1 to 2 islands of floating cork enabling the animals to leave the water if desired. The land area was dark-coloured with hiding places and a feeding place. The vivarium was fitted with a mesh to prevent escape and was placed in the room as previously described. Natural aquatic plants and broken pots created hiding places. Food consisted primarily of flies (with crumpled wings) and later, mealworms, small earthworms, and other suitable invertebrates supplemented with a special vitamin supplement. Duration of the test This part of the test had duration of three weeks starting from five weeks after the embryos were transferred to a pesticide free medium to about eight weeks after this transfer, or until the metamorphosis of most of the tadpoles had been completed. At the end of the test all animals, which had previously been in contact with the test substance, were anaesthetized with benzokain, killed and fixed in 25% glutaraldehyde. The same number tadpoles of the control group was also killed and fixed using the same procedure whereas the rest of the control groups were kept in laboratory facilities. Determination of the effects on larvae In vivo observations were performed five times a week. Dead, malformation, and development stages were followed. In addition the head-tail length data (growth) were recorded every week. Different stages of the Bombina bombina are illustrated figure 2.1. Figure 2.1 Forskellige stadier af Bombina bombina. 3 Results
Egg deposition of Bombina bombina occurred 9-12 h after injection of human chorionic gonadotropin. About 75-400 eggs were found in the breeding aquarium, on the aquatic vegetation and plastic plants as well as on the small sticks. 3.1 Embryo development in control groups at different culture conditionOnly the best-fertilised eggs from a given breeding aquarium were used and only normally cleaving embryos were used in the tests. Control mortality could be kept to less than 5% when this procedure was followed. 3.1.1 Embryo development in FETAX solutions at two different temperaturesControls At 24°C, the control group of Bombina bombina reached stage 46 after 120 h. A stage 46 larvae were recognised by the appearance of the hind limb bud, the coiling of the gut, and the shape of the operculum covering the gills. The best indicator that the larvae had attained at stage 46 was the appearance of the hind limb bud. Gut coiling was also easily observed at stage 46 (at stage 45 embryos do not display complete tight gut coiling). At stage 46, the larvae were about 9.5 mm in length. Embryos of Bombina bombina, kept at 24°C, were still in the jelly coat after 24 h and were not free until after about 72 h. At 20°C, the control group reached stage 46 according to Nieuwkoop and Faber (1975) after 216 h. At stage 46, the larvae were about 9.5 mm in length. Most of embryos, kept at 20°C, were still in the jelly coat after 96 h. After 120 h most of the embryos were free, however, a few were not free until after 144 h. The development of the control groups of Bombina bombina at the two different temperatures is illustrated in table 3.1. For comparison with the development of Xenopus normally used for the standard FETAX test, selected staging according to Nieuwkoop and Faber (1975) is also included. Table 3.1 Effekten af temperaturen på udviklingen af Bombina bombina embryoer. Embryostadiet blev bestemt ved hjælp af Gosner (1960).
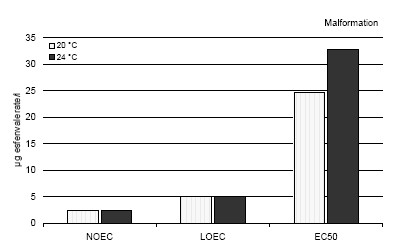
* Stage according to Nieuwkoop and Faber (1975) for comparison with Xenopus. 3.1.2 Embryo development in two different mediaControls The results revealed that the embryo development was identical in the two media, FETAX and pond water. After 216 h, the control group reached stage 46 in both media at 20°C and all embryos were normal and showed no malformations. At stage 46, the larvae were about 9.5 mm in length in both media. 3.2 Tests with reference substanceEffect of 6-aminonicotinamide The results revealed that the effect of 6-aminonicotinamide in both pond water and the FETAX solutions with Bombina bombina were comparable with the effect described for Xenopus in the ASTM standards. The EC50 value for malformations after 120 h and 216 h at 24°C and 20°C, respectively, was 4.9 mg/l in FETAX (at both temperatures) and 5.2 mg/l in pond water. The LC50 value was about 25000 mg/l in FETAX and 26000 in pond water (mean values of 9 experiments; SD of the mean less than 10%). The results revealed that 55% and 58% of the embryos had malformation at 5.5 mg/l 6-aminonicotinamide at 24°C and 20°C, respectively. A mortality of 44% and 51% was found at a concentration of 2500 mg/l aminonicotinamide at 24°C and 20°C, respectively. The most conspicuous malformations caused by 6-aminonicotinamide were severe optic cup rupture, edema and facial malformations such as ocular edema and foreshortened facial features. In addition, severe heart malformations were also seen. 3.3 Effects of esfenvalerate on Bombina bombina at two different temperaturesSince the renewal procedure entailed fresh replacement of the test material every 24 h during the test and the chemical measurement from previous experiments did not reveal a decrease of the pesticide concentration during the exposure period (Larsen and Sørensen, 2004), the results of the present study are expressed as nominal concentrations. 3.3.1 In vivo observationsEsfenvalerate Esfenvalerate affected the embryos gradually during the exposure in a dose depended manner at both temperatures. After 24 h, no effect of esfenvalerate was seen on the embryos even at the highest concentration compared with the controls at both temperatures. After 48 h, no effect of esfenvalerate was seen on the embryos even at the highest concentration compared with the controls at 20°C. At 24°C, no effect was seen at concentrations up to 5 μg/l, however, at 5 g/l beginning twisting of the embryos was seen now and then. The spasmodic twisting increased in intensity with increasing concentrations of esfenvalerate and at 150 and 300μg/l, the embryos had constant spasmodic twisting. At concentrations from 50 μg/l, the spasmodic twisting of the embryos caused that some of the embryos had already left the jelly coat at this stage. In the control group, only a few embryos had begun to leave the jelly coat after 48 h. At 100, 150, and 300 μg/l, edema was seen in some of the embryos. After 72 h, no effect of esfenvalerate was seen on the embryos at 20°C even at the highest concentration compared with the controls, and all embryos were in the jelly coat. At 24°C, several of the embryos in the control group had begun to leave the jelly coat and all the embryos exposed to esfenvalerate concentrations above 50 μg/l were free. Periodic spasmodic twisting was seen in most of the embryos above 5 μg/l and in 50 μg/l constant spasmodic twisting was seen. At concentrations above 100 μg/l, occasionally blistering and cardiac edema appeared. In 150 and 300 μg/l, blistering and cardiac edema were frequently seen. The embryos were immobilised by constant spasmodic twisting. After 96 h, the first effects of esfenvalerate were seen at 20°C. No effect was seen at concentrations up to 5 μg/l, however, at 5 μg/l beginning twisting of the embryos was seen now and then. The spasmodic twisting increased in intensity with increasing concentrations of esfenvalerate and at 300 μg/l the embryos had constant spasmodic twisting. At concentrations from 10 μg/l, the spasmodic twisting of the embryos caused that some of the embryos had left the jelly coat at this stage. In the control group, only a few embryos had begun to leave the jelly coat after 96 h. At 100 μg/l, most of the embryos had left the jelly coat. At 24°C all embryos in the control group were free from the jelly coat and beginning twisting was seen at 1 μg/l. The intensity of these spasmodic twisting increased when the concentrations of esfenvalerate were increased. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and occasionally blistering and cardiac edema appeared. At concentrations above 100 μg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were normally seen. After 120 h, several of the embryos in the control group began to leave the jelly coat at 20°C, and all the embryos exposed to esfenvalerate concentrations above 10 μg/l were free. Periodic spasmodic twisting was seen in most of the embryos above 2.5 μg/l and in 50 μg/l constant spasmodic twisting was seen. At concentrations above 100 μg/l, occasionally blistering and cardiac edema appeared. In 150 and 300 μg/l, blistering and cardiac edema were frequently seen. The embryos were immobilised by constant spasmodic twisting. At 24°C, spasmodic twisting was seen at 1 μg/l. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and blistering and edema were often seen. At concentrations above 100 μg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were seen at 100, 150 and 300 μg/l. At this point the embryos had reached stage 46 and were killed and fixed as previously described. After 144, 168, and 192 the same picture was seen at 20°C. All embryos in the control group were free from the jelly coat and beginning twisting was seen at 2.5 μg/l. The intensity of these spasmodic twisting increased when the concentrations of esfenvalerate were increased. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and occasionally blistering and cardiac edema appeared. At concentrations above 100 μg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were normally seen. After 216 h, spasmodic twisting was seen at 1 μg/l. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and blistering and edema were often seen. At concentrations above 100 μg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were seen at 100, 150 and 300 μg/l. At this point the embryos had reached stage 46 and were killed and fixed as previously described. 3.3.2 Recordings of malformation, growth inhibition and mortality at the end of the test (120 h and 216 h for 24°C and 20°C, respectively)Malformation Most abnormal embryos possessed multiple malformations. However, at 1 and 2.5 μg/l no malformations were seen and the larvae were at the normal stage 46 according to Nieuwkoop and Faber (1975) and comparable with the controls in all respects. Above a concentration of 5 μg/l, gut abnormalities were often seen indicated by a loose gut coiling. At 10 μg/l in addition to gut abnormalities, a relatively mild flexure of the tail was normally seen. Embryos with a sloping forehead and distended mouth were often seen. The head and face region of these embryos was malformed. The face was flattened and the forebrain was deflected downward along the front of the brow. Heart malformations were also observed at concentrations above 10 μg/l. Increasing concentrations of esfenvalerate resulted in increasingly severe heart malformations. Often the heart showed an abnormal expansion of the ventricle just in front of the gut. At 50 μg/l, blistering and edema were common and various degrees of undeveloped gills were also seen. At 24°C, about 20% of the embryos had malformations at 10 μg/l, and more than 75% of the embryos exposed to 100 μg/l and higher concentrations had malformations. At 300 μg/l, all embryos had malformations. At 20°C, about 30% of the embryos had malformations at 10 μg/l and about 90% of the embryos exposed to 100 μg/l and higher concentrations had malformations. At 300 μg/l all embryos had malformations. The EC50 value for malformations was calculated to be 32.7 and 24.7 μg/l for 24°C and 20°C, respectively (further details cf. Appendix C). The most significant malformation observed included cardiac malformations, severe lateral flexure, edema, notochord, brain and gut malformations. Effect concentrations are shown in figure 3.1. Growth inhibition After 120 h and 216 h (for 24°C and 20°C, respectively) of exposure to concentrations up to 300 g/l, the mean length of the embryos was at both temperatures comparable with the length of the control group. The mean length of embryos in the control group was 9.5 mm (S.D. 0.08) and 9.3 mm. (S.D. 0.3), in 24°C and 20°C, respectively. The NOEC, LOEC, and LC50 values for growth inhibition were all higher than 300 μg/l. Mortality No significant mortality was seen at concentrations up to 300 μg/l at both temperatures. The NOEC, LOEC, and LC50 values were all higher than 300 μg/l. Teratogen index (TI) The effects of esfenvalerate on TI on Bombina bombina at different temperatures are depicted in table 3.2. Table 3.2 Teratogen index (TI) for esfenvalerat ved to forskellige temperaturer.
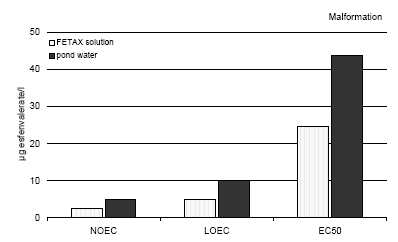
Figure 3.1 En sammenligning af misdannelserne forårsaget af esfenvalerat på Bombina bombina ved to forskellige temperaturer. Resultaterne er udtrykt ved angivelse af NOEC, LOEC og EC50/LC50 værdierne efter 120 og 216 timers eksponering ved henholdsvis 24 og 20°C. Efter denne eksponeringstid var embryonerne i de respektive kontrolgrupper nået til stadie 46. Resultaterne er gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene var mindre end 10%. 3.4 Effects of esfenvalerate on Bombina bombina in different culture mediaThe effect of esfenvalerate on malformation, growth inhibition, and mortality in Bombina bombina was tested in pond water and the effects were compared with that found in the FETAX solution. After 216 h of exposure at 20°C, the embryos in the control group were at stage 46 corresponding to the stage of the controls at the end of the test in the standard FETAX test with Xenopus in both media. 3.4.1 In vivo observationsEsfenvalerate Esfenvalerate affected the embryos gradually during the 216 h exposure in a dose depended manner in both media. The same effects of esfenvalerate were seen in both media; however, the effects were more distinct in the FETAX solution. No effect of esfenvalerate was seen on the embryos even at the highest concentration compared with the controls during the first 72 h of exposure. After 96 h, the first effects of esfenvalerate were seen in the FETAX medium at 5 μg/l where beginning twisting of the embryos was seen now and then. The spasmodic twisting increased in intensity with increasing concentrations of esfenvalerate and at 300 μg/l the embryos had constant spasmodic twisting. At concentrations from 10 μg/l some of the embryos had left the jelly coat and at 100 μg/l most of the embryos had left the jelly coat. The same pattern was seen in pond water, however, in this medium no effect was seen at concentrations up to 10 μg/l. In pond water beginning twisting was first seen at 50 μg/l. At concentrations from 50 μg/l some of the embryos had left the jelly coat. In the control group, only a few embryos had left the jelly coat after 96 h in both media. After 120 h, several of the embryos in the control group began to leave the jelly coat in both media, and all the embryos exposed to esfenvalerate concentrations above 10 μg/l were free. Periodic spasmodic twisting was seen in most of the embryos above 2.5 μg/l and 10 μg/l in FETAX and pond water, respectively. At concentrations above 100 μg/l spasmodic twisting was significant and occasionally blistering and cardiac edema appeared in both media. In 150 and 300 μg/l, blistering and cardiac edema were frequently seen. The embryos were immobilised by constant spasmodic twisting in both media at concentrations of 150 and 300 μg/l. After 144, 168, and 192 the same picture was seen in both media. All embryos in the control group were free from the jelly coat. The intensity of the spasmodic twisting increased with increasing concentrations of esfenvalerate. Periodic spasmodic twisting was seen in most of the embryos at concentrations above 50 μg/l in the pond water. In FETAX, the embryos were immobilised caused by constant spasmodic twisting at these concentrations and occasionally blistering and cardiac edema appeared. At concentrations above 100 μg/l, blistering and edema were frequently seen in both media as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were normally seen. After 216 h, spasmodic twisting was seen at 1 μg/l and 5 μg/l in FETAX and pond water, respectively. At concentrations above 50 μg/l and above 100μg/l, in FETAX and pond water, respectively, the embryos were immobilised caused by constant spasmodic twisting as well as blistering and edema were often seen. At concentrations above 100 μg/l, blistering and edema were frequently seen in both media as well as head and brain malformations. Furthermore, the heartbeat was very slow and severe heart malformations were seen at 100, 150 and 300 μg/l. 3.4.2 Recordings of malformation, growth inhibition, and mortality at the end of the testMalformation Most abnormal embryos possessed multiple malformations. However, at 1 and 2.5 μg/l no malformations were seen and the larvae were at the normal stage 46 according to Nieuwkoop and Faber (1975) and comparable with the controls in all respects. At concentrations above 5 μg/l, gut abnormalities were often seen in FETAX indicated by a loose gut coiling, however, no significant effects was found in embryos in pond water. At 10 μg/l in addition to gut abnormalities, a relatively mild flexure of the tail was normally seen. Embryos with a sloping forehead and distended mouth were often seen. The head and face region of these embryos was malformed. The face was flattened and the forebrain was deflected downward along the front of the brow. Heart malformations were also observed at concentrations above 10 g/l. Increasing concentrations of esfenvalerate resulted in increasingly severe heart malformations. The heart often showed an abnormal expansion of the ventricle just in front of the gut. At 50 μg/l, blistering and edema were common and various degrees of undeveloped gills were seen, too. In FETAX solution about 30% of the embryos had malformations at 10 μg/l and about 90% of the embryos exposed to 100 μg/l and higher concentrations had malformations. In pond water a significant effect of esfenvalerate was seen at 50 μg/l where about 15% of the embryos had malformations. In pond water at 100 μg/l and at 300 μg/l nearly all embryos had malformations in both media. The EC50 value for malformations was calculated to be 24.7 and 43.7 μg/l in FETAX solution and pond water, respectively (Further detail cf. Appendix C). The most significant malformation observed included cardiac, severe lateral flexure, edema, notochord, brain, and gut malformations. Effect concentrations for malformation are shown in figure 3.2 Growth inhibition After 216 h of exposure to concentrations up to 300 μg/l, the mean length of the embryos was in both media comparable with the length of the control group. The mean length of embryos in the control group was 9.3 mm. (S.D. 0.3) and 9.5 mm (S.D. 0.2) in FETAX and pond water, respectively. Thus, the results revealed that for growth inhibition NOEC, LOEC and the EC50 valued were higher than 300 μg/l in both media. Mortality No significant mortality was seen at concentrations up to 300 μg/l in both media. The NOEC, LOEC, and LC50 values were all higher than 300 μg/l. Teratogen index (TI) The effects of esfenvalerate on IT on Bombina bombina in different media were comparable. The TI was >12.1 and > 6.9 in FETAX and pond water, respectively.
Figure 3.2 En sammenligning af effekterne af esfenvalerat på Bombina bombina i to forskellige medier udtrykt ved sublethale misdannelser. Resultaterne er udtrykt ved angivelse af NOEC, LOEC og EC50 værdierne efter 216 timers eksponering i henholdsvis FETAX opløsning og i damvand. Efter denne eksponeringstid var embryoerne i de respektive kontrolgrupper nået til stadie 46. Resultaterne er et gennemsnit af 3 forsøg. Standardafvigelsen af middeltallene var mindre end 10%. 3.5 Recovery experiments using embryos of Bombina bombina3.5.1 The effects of esfenvalerate on Bombina bombina during the toxicity testControls During the 216 h toxicity assay the embryo development of the control group was normal. After 216 h, the embryos were at stage 46, and the head-tail length was 9.0 mm (S.D. = 0.1). Malformation was seen in 5.9% (S.D. = 1.7) of the surviving embryos. The mortality was 9.3%, which corresponds to the normal range in these tests. Embryos exposed to 20 μg/l esfenvalerate No effect of esfenvalerate was seen on the embryos during the first 96 h. After 120 h all embryos were free from the jelly coat and periodic spasmodic twisting was seen. The intensity of these spasmodic twisting was increased with increasing time of exposure. Malformations were seen in 38.9% (S.D. = 4.1) of the embryos at the end of the exposure. Most abnormal embryos possessed multiple malformations including gut abnormalities as loose gut coiling, mild flexure of the tail, sloping forehead, and heart malformation. At the end of the test, a mortality of 7.3% was found and the surviving embryos were at stage 46 with a length of 9.2 (S.D. = 0.2) mm. Embryos exposed to 125 μg/l esfenvalerate During the first 72 h of exposure no effect was seen; however, after 96 h, the first effects of esfenvalerate were seen. These effects were spasmodic twisting and most of the embryos had left the jelly coat at this stage. After 120 h, constant spasmodic twisting was seen in many embryos, furthermore blistering and cardiac edema were frequently seen. After 144-216 h, all embryos were immobilised caused by constant spasmodic twisting. Blisting and edema were frequently seen as well as gut abnormalities, flexure of the tail, head and brain malformations. 83.2% (S.D. = 0.2) of the embryos had malformations at the end of the exposure. The mortality was 6% and the mean length of the surviving embryos was 8.8 mm (S.D. = 0.2) at the end of the exposure. 3.5.2 Embryo and larval development during the first part of the recovery periodImmediately after transferring the embryos to fresh medium without pesticides most of the embryos in the control group started to eat algae and moved around searching for food. About 70% of the embryos previously exposed to 20 μg/l esfenvalerate gradually recovered and were comparable with the controls. 24 h after the transfer to fresh medium, however, about 30% of this group did not ingest algae and spasmodic twisting was seen now and then. After 24 h about 80% of the embryos previously exposed to 125 μg/l esfenvalerate had still spasmodic twisting and the heartbeat of these embryos was very slow; however, about 20% of the embryos in this group were swimming around now and then. Two days after transferring the embryos to fresh medium all living larvae were swimming around and eating algae revealed by the green content in the gut. During the following weeks there was no difference in larval development in the three groups. After three weeks the larvae were at stage 38-45 and hind limps were developing. Five weeks after the transfer to fresh medium free hind limps were seen in all larvae and the hind limps were about one-third to half the length of the tail. No significant difference in development was found in the three different groups. The fore limps were not free even though they were pronounced. The larvae started to develop darker drawings on the skin than normal. Growth inhibition and mortality The effects of a short exposure to esfenvalerate on growth were not significant. All embryos were about 9 mm in length when they were transferred to the pesticide free medium, and all the larvae had grown to about 20 and 30 mm in length after two and three weeks, respectively. All larvae grew quickly and reached a maximum length within the metamorphosis of about 50 mm after about 5 weeks (after the transfer to fresh medium with food). The variation of the length was significantly within each group, however, not among the three groups (controls, previously exposed to 20 μg/l and 125 μg/l esfenvalerate, respectively). Thus, after five weeks the mean length (head-tail length) of the control group in the first experiment was 48 mm (S.D. 4.7), however, the smallest larvae in this group were 41 mm and the largest 58 mm. The length of larvae previously exposed to 20 μg/l esfenvalerate was in average 49 mm (S.D. 4.0) varying from 42-58 mm, and 49 mm (S.D. 5.3), varying from 42-58 mm for larvae which were previously exposed to 125 μg/l. In the second experiment the same pattern of variation was seen. In the two independent experiments the mean head-tail length after five weeks was 49.6 mm (S.D. 1.6), for the control group, 49.4 mm (S.D. 0.2) and 49.6 mm (S.D. 0.6) for larvae previously exposed for 20 and 125 μg/l esfenvalerate, respectively.
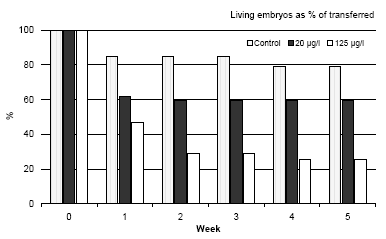
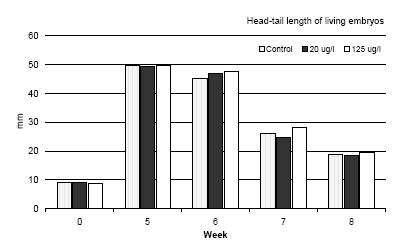
Figure 3.3 Dødeligheden af Bombina bombina i løbet af de første fem uger efter at embryonerne er blevet overført til et frisk medium uden esfenvalerat. Resultaterne er udtrykt i procent af levende embryoner, der er overført til et frisk medium (uge 0). Ved uge 0 er alle embryonerne på stadie 46. Resultaterne er gennemsnittet af to eksperimenter. Standardafvigelsen af gennemsnitsværdien var mindre end 10%. The results revealed that a high mortality was found during the first week after the larvae were transferred to the pesticide free medium and a concentration-response was found (cf. figure 3.3). Even in the control group a mortality of about 23% was found. Furthermore, it was also seen that after the first week no further mortality was found during the following period until the metamorphosis. 3.5.3 Development before and during the metamorphosis of Bombina bombinaIn vivo observations During the beginning of metamorphosis (week 6) the forelimbs became free and the notochord began to degenerate posteriorly but still normal in appearance anteriorly. At this stage the head-tail length was about 45 mm and no significant differences were seen between the three groups. The hind limb had the same length as the tail and about 60% of the larvae had free forelimbs at this stage. However, the forelimbs seemed to be bent in over the body in some of the larvae previously exposed to 20 μg/l. In this case the amphibians were not using the forelimbs and were tumbling around when they were trying to jump. All larvae previously exposed to 125 μg/l had problems with the forelimbs, which were bent in over the body and could not be used. The degradation of the tail proceeded rapidly so that the basis of the tail was affected too and after seven weeks the head-tail length was about 25 mm in all amphibians (the tail was only about 3-5 mm). A comparison of the head-tail length of Bombina bombina during metamorphosis is shown in figure 3.4. At this stage all amphibians had forelimbs; however, 50% of the amphibians previously exposed to 20 μg/l had defect forelimbs and all the amphibians previously exposed to 125 μg/l had defect forelimbs. No problems were identified in the control group. The major problem for amphibians with defect forelimbs was that they tumbled around when trying to jump on land. At the end of week 7 some of the amphibians in the control had no longer any tail and they were jumping around on land eating flies. After eight weeks all frogs in the control group had no tail and were jumping around eating flies. Amphibians previously exposed to 20 μg/l had no tail and about 20% of these animals were also jumping around and eating flies like the control group. However, about 80% of these amphibians, which were at the same developmental stage and of the same size as the control group, had problems with the forelimbs, which could not support the animals. These animals were tumbling around when they tried to jump and had difficulties catching flies. All animals previously exposed to 125 μg/l were at the same developmental stage and had the same size as the control group, but all animals had problems with their forelimbs and were tumbling around when they tried to jump on land.
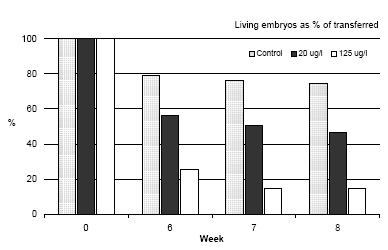
Figure 3.4 En sammenligning af hoved til hale længden af Bombina bombina under metamorfosen. Resultaterne er sammenlignet med længden af de embryoer der lige er blevet overført i et frisk medium (uge 0) og maksimum længden lige før metamopfosen (uge 5). Resultaterne er middelværdier af de to eksperimenter. Middelstandardafvigelsen er mindre end 10%. Mortality During metamorphosis only a slight mortality was found in the control group as shown in figure 3.5. A slight increase in mortality of Bombina bombina previously exposed to 20 μg/l was found. 55.3% of the transferred embryos were still alive just before the beginning of metamorphosis (week 5) whereas this number was reduced to 43.4% after the metamorphosis. The tendency was even more pronounced when Bombina bombina had been exposed to 125 μg/l. In this case 24.5% of the organisms were still alive before the metamorphosis, however, only 14% survived the metamorphosis and as previously described it is assumed that the surviving frogs will only survive for a very short period of time in natural environment.
Figure 3.5 Dødligheden af Bombina bombina under metamorfosen 6,7 and 8 uger efter at embryoer blev overført til pesticid frit medie. Resultaterne er udtryk i % af levende embryoer overført til friskt medie i uge 0. Resultaterne er gennemsnitsværdier af 2 eksperimenter. Standardafvigelsen af gennemsnit er mindre end 10%. 4 Discussions
4.1 The use of amphibians as an ecotoxicological test organismPopulations of many amphibians have declined and some species have disappeared from certain regions around the world, a phenomenon that appears to have accelerated during the last years (Blaustein & Wake, 1990). While there is evidence for widespread reduction in numbers of amphibians, the reasons for the declines are less clear. Much of the amphibian life cycle occurs in ponds, streams, and temporary pools that are often associated with agricultural areas receiving pesticide applications. In addition, breeding and larval development of amphibian occur in spring and summer at the same time that heavy application of pesticides on agricultural lands occurs. At present it is suggested that agricultural pesticides may contribute to the decline in the amphibian population (Phillips, 1990; Berrill et al. 1994). Therefore effects of the pyrethroid insecticide esfenvalerate on amphibians have been investigated in the present study. The main objectives in the project have been to establish data for evaluating the possible effects of esfenvalerate on amphibians in Danish aquatic environments. Bombina bombina as test organism In the present study effects of esfenvalerate on the fire-bellied toad Bombina bombina have been studied. A new developed test guideline for ecotoxicologcal tests with Bombina bombina (Larsen and Sørensen 2004) have been used. In relation to the standard FETAX (Frog Embryo Teratogenesis Assay-Xenopus) test the present test have been prolonged to include recovery up to 6-7 weeks after the exposure by esfenvalerate has been ended. In FETAX the South African clawed frog (Xenopus laevis) has been used as test organism. Since Xenopus laevis only live in water and are resticted to only very special limited ecosystems in Africa it makes it less suited for ecotoxicological risk assessment in Denmark and other European countries. However, it is found that Bombina bombina and Xenopus laevis have almost the same sensitivity for esfenvalerate (Larsen and Sørensen, 2004). Successful tests In the present study, the test with Bombina bombina has been very successful and realistic. After injection of human chorionic gonadotropin each female bred between 75-400 eggs, which is within the normal range. By selection the best fertilised eggs and only normally cleaving embryos the mortality in control systems could be kept below 5%, which is satisfactory low in ecotoxicology tests with amphibians (ASTM 1991). Also the embryo development was satisfactory. Developmental stage 46 was reached after 120 h at 24°C and after 216 h at 20 °C. At both temperatures the larvae were about 9.5 mm in length at stage 46. In addition the test with a reference substance (6-aminonicotinamid) resulted in the effects expected from the ASTM standard with Xenopus laevis at both temperatures and in both pond water and FETAX media. 4.2 Effects of esfenvalerate during the exposure periodAs found in others studies with embryos of amphibians, esfenvalerate affected the embryos gradually during the exposure period (Larsen & Sørensen 2004, ref). Very fine concentration-response relationships were found in the present study. The initial response was a decrease in activity followed by spasmodic twisting and immobilisation at the highest concentrations. At the end of the exposure period after 216 h spasmodic twisting was seen in the embryos exposed to concentration as low as 1 μg/l esfenvalerate. At concentrations above 50 μg/l, the embryos were immobilised caused by constant spasmodic twisting and blistering and edema were often seen. At concentrations above 100 μg/l, blistering and edema were frequently seen as well as head and brain malformations. The heartbeat was very slow and severe heart malformations were seen at 100, 150, and 300 μg/l. Neither growth nor mortality was significant affected by esfenvalerate at any of the tested concentrations. This corresponds to the results of other studies where it has been found that mortality and growth inhibition did not seem to be sensitive effect parameters (e.g. Materna et al., 1995, Larsen & Sørensen, 2004). Detection of malformations during the development revealed effects at concentrations as low as 2.5 μg/l esfenvalerate. However, in vivo observations revealed effect as low as 1 μg/l esfenvalerate, which indicates the importance of in vivo observation in test with amphibians. Such sublethal effects are likely to have serious implications on the long-term success of the exposed individuals and such effects need to be included in risk evaluation of toxicants in the environment. However, such risk evaluation is not straightforward since exposure condition in toxicity test and in the environment may differ. Normally is the concentration of the toxicant kept constant in standard test. In the present study, the medium was renewed every day keeping the concentration of esfenvalerate constant. Especially for toxicants with fast decline in the environment it is uncertain how risk evaluation should be done. Typically it is found that esfenvalerate will decline relatively fast in pond after spraying (Mogensen et al., 2004). At the moment our knowledge on the relation between toxicity and duration of exposure is not adequate. Analogous the usability of time weighted average concentration in risk assessment as suggested by EU is uncertain and currently insufficient tested. 4.3 Recovery and delayed effectsAfter the end of the toxicity test (after 216 h) the surviving embryos treated with 20 and 125 μg/l esfenvalerate, and untreated controls were transferred to fresh medium without esfenvalerate. At the same time algae was supplied to feed the larvae. Immediately after transfer, the embryos in the control group started to eat algae and moved around searching for food. In the groups pre-exposed to 20 μg/l esfenvalerate about 70% of the organisms had recovered after 24 h, while only 20% of the embryos pre-exposed to 125 μg/l esfenvalerate had recovered. After two days all surviving larvae in all the three groups were swimming around eating algae. The recovery seemed completed and fast. And the conclusion could be that the effect of esfenvalerate on amphibians in the environment is insignificant as the concentrations of esfenvalerate typically decline relatively fast after spraying. However, such a conclusion does not lay open all the life stages of an amphibian and if the ecological realism of the test is low as well, it is necessary to be very causious when evaluating such ecotoxicological tests. A distinct effect of esfenvalerate was seen six weeks after transfer to the fresh medium without esfenvalerate. During the development it got more and more evident that esfenvalerate at both concentrations induced malformed forelimbs still six weeks after the exposure was ended. The malformed forelimbs was fatal because the toads tumbled around when they tried to jump. This malformation is detrimental, since these toads will not be able to escape from predators, catch flies, and probably not reproduce themselves. The present study is special in the context to its thoroughness and in duration. The majority of the tests with amphibians are limited to test effects during a few days (ref) and prolonged studies as the present are uncommon. There may exist studies on effects of esfenvalerate tested in prolonged experiments although it has not been possible to find such references for the present study. In this connection especially one study deserves to be mentioned. In the study delayed effects of pre- and early-life time exposure to PCB on tadpoles of Xenopus laevis and Rana temporaria were investigated (Gutleb et al., 1999). The study is unique in extent and it includes effects of PCB exposure in amphibians, such as mortality, number and pattern of malformations, or body weight and successful metamorphosis into tadpoles, depended exposure route, the point of time of exposure during the complex life cycle of amphibians, and the length of the observation period. The conclusion in the study by Gutleb et al. (1999) is that presently used early-life test systems such as the FETAX assay may underestimate toxic effects of compounds due to long-term effects such as PCBs on amphibians. This conclusion agrees with the conclusion of the present study. 4.4 Effects of different media usedThe results revealed that the embryo development was almost identical in the two media, FETAX and pond water. The embryos reached stage 46 according to Nieuwkoop and Faber (1975) after 120 h and 216 h of exposure at 24°C and 20°C, respectively, in both media, and this correspond to the development in the standard FETAX test with Xenopus laevis. It indicates that the test design with Bombina bombina was optimal, as previously concluded (Larsen & Sørensen, 2004; Larsen, Sørensen & Gustavson, 2004), and that the jelly coat, which was not removed in the experiments, did not effect the embryo development. Effects of esfenvalerate The effects of esfenvalerate in the two different media, although comparable, occurred at different concentration of esfenvalerate. The first spasmodic twisting started in FETAX solution at 5 μg/l and in higher concentrations after 96 h, while in pond water spasmodic twisting started at 50 μg/l. The same pattern of malformations was found at the end of the experiments in both media, however, the embryos in FETAX medium had more malformations and these malformations were also more distinct. In FETAX solution about 30% of the embryos had malformations at 10 μg/l and about 90% of the embryos exposed to 100 μg/l and higher concentrations had malformations. In pond water a significant effect of esfenvalerate was seen at 50 μg/l where about 15% of the embryos had malformations. Ionic characteristic of the water It has previously been shown that differences in water hardness alter the toxicity of pyrethroids to aquatic organisms (Coats et al., 1989). Besides affecting the nervous system (Tippe, 1987), pyrethroids also cause an osmoregulatory imbalance and ionic characteristics of the water have been demonstrated to influence the toxicity of fenvalerate to bluegill (Coats et al., 1989). Dissolved organic matter Another explanation to the low toxicity of esfenvalerate in the pond water may be that the dissolved organic matter strongly absorb the esfenvalerate and thereby reduce the bioavaliability. FETAX solution is not added any organic substance so it is evident that it may be an explanation. It is well-known that pyrethroids strongly adsorb to the organic matters causing a decrease in bioavalaibility. Using natural water as medium Too low ion concentrations can, however, severely affect embryo survival, growth and occurrence of malformations (Tietge et al., 2000). This was found to be the case in the Northern USA, where three different species of frogs developed limb abnormalities which was found to be caused by low ion concentrations in the lake used in the study (Tietge et al., 2000). The occurrence of common metals as Al and Fe in high concentrations may, however, also be toxic to amphibians (Horne & Dunson, 1995). Furthermore, some acidic lakes and ponds are unsuitable for amphibian breeding, since amphibians embryos and larvae are highly sensitive to low pH (Freda, 1986). Careful consideration should therefore be taken before applying the FETAX protocol to natural samples when using water from ponds of unknown water chemistry, and initial tests should preferable be run prior to testing for e.g. toxicity of substances. The present study shows that test medium FETAX prepared in laboratory facilities with a controlled content of ions and free of toxicants is very suitable for Bombina bombina breeding and can be used without further testing. Furthermore, it shows that the FETAX medium produces reliable results that perhaps shows low effects concentrations for some soft water lakes, but takes into account that some surface waters have high concentrations of ions. Effects in sprayed ponds and lakes In the present study the media with esfenvalerate was renewed every day. In natural environments the insecticides which end up in aquatic environments are reduced in concentration in the water phase quite dramatically within a few days especially due to absorption by the sediments and aquatic plants (Mogensen et al., 2004, Larsen, Sørensen & Gustavson, 2004), since pyrethroids are highly hydrophobic. The tadpoles, however, feed on phytoplankton, epiphytes and filamentous algae, and may therefore increase their exposure to and uptake of pyrethroids through their feeding behaviour even though the concentration of pyrethroid in the water column is undetectable. The procedure of renewing the media containing the test substance is therefore sensible, for the purpose of detecting reliable effects of exposure to toxic substances. 4.5 Effects of different temperaturesIt is well-known that effects of toxic substances strongly depend on the temperature. In the present study the embryo development was tested in FETAX solution at 24°C corresponding to the temperature used in the normal FETAX test with Xenopus laevis and at 20°C corresponding to the temperature in the new guideline and more often found in Danish ponds. Since amphibians absorb heat from the environment and an increase in temperature increases their metabolic activity, it was expected that the embryos reached stage 46 faster at 24°C than embryos reared at 20°C. Accordingly, they reached stage 46 only 120 h after the start of the test at 24°C, which was 96 h earlier than the embryos at 20°C (Table 3.1). Effects of esfenvalerate The effects of esfenvalerate added in different concentrations were comparable in the two different temperatures, although displaced in time. Spasmodic twisting of embryos was seen down to 1 μg/l esfenvalerate and all embryos were immobilised at concentrations above 50 μg/l at 120 and 216 h at 24°C and 20°C, respectively. The same pattern of malformations was found at both temperatures, however, the embryos at 20°C had more malformations and lower EC50 (32.7 and 24.7 μg/l at 24 and 20°C, respectively) and these malformations were also more distinct. This agrees with the effects of temperature found in the study of Materna et al. (1994), where lower EC50 was found for tadpoles of Leopard frogs (Rana spp) exposed to esfenvalerate at 18°C compared with 22°C (3.40 and 6.14 μg/l, respectively). The lower EC50 values found for Leopard frogs compared to this study with Bombina bombina can be due to the use of tadpoles in the toxicity tests of Materna et al. (1994). There appears to be differences in sensitivity between embryonic stages, where older embryos show higher sensitivity to pyrethroids than younger stages (Berrill et al., 1993), and tadpoles could possibly be more sensitive than embryos. Furthermore, there is also interspecies variation in tolerance towards pyrethroids and toxic substances, since some amphibians are more sensitive than others are (Berrill et al., 1993; Berrill et al., 1995; Freda, 1986). Pyrethroids have generally been found to be more toxic at low temperatures for several aquatic organisms (After Coats et al., 1989 and Berrill et al. 1993). The sensitivity of embryos and larvae of amphibians in natural environments may therefore vary with the season and latitude. The temperature in Danish ponds will most likely not exceed 20°C. In spring, which is the breeding season for amphibians, the temperature is most likely 10-15°C, this will apparently affect the development of embryos even more than the effects observed in the present study if they are exposed to pyrethroids. In addition it should be noticed that the exposure time in the present study is almost twice as long at 20°C (216 h) than at 24°C (120 h). 4.6 Developmental effects of esfenvalerate in relation to other studiesThe malformations found in the larvae at the end of the experiments in this study as a result of treatment with esfenvalerate were many. The most significant malformations observed included cardiac malformations, severe lateral flexure, oedema, notochord, brain and gut malformations. Especially lateral flexure or axial malformations have been often reported in developing amphibian larvae exposed to different toxicants in the laboratory (Cooke, 1981; Bantle et al., 1991; Hatch et al., 1998; Joffre et al., 1999). They have also been found in bullfrogs larvae in nature from coal ash-polluted sites, where lateral curvature of the spine occurred in high rates due to high concentrations of trace elements (As, Cd, Se, Cu, Cr, and V) (Hopkins et al., 2000). Axial malformation has a negative impact on the swimming performance, which is related to foraging, predator avoidance and thermoregulation and has accordingly serious implications for survival of the larvae. Uncoiling of the gut, brain and heart malformations, and edema were malformations also found in this study as effects of esfenvalerate. These malformations have been less often reported in other studies with amphibians and other toxicants than pyrethroids. Perkins et al. (2000) did not observe any malformations at any concentrations of two herbicides, glyphosate and triclopyt that were not also lethal to the embryos in Xenopus laevis. And edema was the only sublethal effect of the herbicide atrazine in two amphibians (Howe et al., 1998). Lordoscoliosis occurred as a malformation beside tail curvature in a FETAX experiment with lead (Sobotka & Rahwan, 1995). The many malformations recorded in this study as well as the above mentioned results of the recovery experiment as a consequence of exposure to sublethal concentrations of esfenvalerate emphasize the severe impact that this toxicant can have on populations of amphibians in natural environments. Other studies on toxic effects of pyrethroids on amphibians have also showed that several malformations and behavioural abnormalities developed in the larvae. Furthermore, the amphibians are generally very sensitive to sublethal exposures of pyrethroids and will probably die as a consequence of the developmental effects caused by pyrethroids (Berrill et al., 1993; Materna et al., 1995). 4.7 Further aspectMany different chemicals are applied to arable farmland and some are known to occur in freshwater ponds, perhaps at concentration that may have effects on amphibians. The present study shows that pesticides may have significant effect on amphibian and that prolongation of standard test are absolutely relevant and important in the future both in relation to risk and hazard evaluation and in monitoring for effect on amphibians in the environment. Trial is described in which the incidence of deformities was unusually high in caged tadpoles of Rana temporaria beside potato fields after application of oxamyl, carbamate nematicide and insecticide (Cook 1981). Effects on amphibians in the environment may be monitored by caging technique (e.g. Hopkins et al., 2000, Cook 1981 and Larsen, Sørensen & Gustavson 2004), releasing of tadpoles into the environment (Hopkins et al., 2000, Cook 1981), reciprocal transplanting or detecting e.g. malformation on natural populations if such effects occur. In view of the current world-wide decline in amphibian populations it has bring into focus to study the potential for environmental contaminants to act as endocrine disrupters of the amphibian reproductive system. Recently it has been shown that DDT induces endocrine disruption in Tiger Salamander (Clark et al., 1998). OECD has therefore recently called to some meeting inside test for endocrine disrupters in amphibian. At present no papers have yet been prepared on subject by OECD (pers. comment, Ms. Marie Chantal Huet, OECD). PART II : The effects of selected pyrethroids on embryo development of Bombina bombina under semi-field conditions5 IntroductionThere has been a worldwide trend of decreasing amphibian populations in different types of habitats and some species have disappeared from certain localities around the world. (Howe et al., 1998). Due to their thin and permeable skins, and prolonged exposure first to the aquatic environment and then to the terrestrial, from herbivorous tadpoles to carnivorous adults, amphibians may be particularly sensitive to toxic substances (Gutleb et al., 1999). Agricultural pesticides are under suspicion to cause or contribute to the decline in amphibian populations (Phillips, 1990; Dutta and Mohanty-Hejmadi, 1978; Berrill, et al., 1994). Much of the amphibian life cycle occurs in ponds, streams, and temporary pools that are often associated with agricultural areas receiving pesticide applications. In addition, breeding and larval development of amphibians occur in spring and summer at the same time that heavy application of pesticides on agricultural lands occurs. The only internationally standardized test with amphibians is FETAX (Frog Embryo Teratogenesis Assay-Xenopus, Dumont, 1983). FETAX is a standard method for developmental toxicity tests of chemicals using embryos of Xenopus laevis, the South African clawed frog. As Xenopus laevis has all its life stages only in water and only represents a limited African ecosystem, with restricted ecological relevance for e.g. Denmark, a new toxicity test guideline with the fire-bellied toad Bombina Bombina as test organism has been developed (Larsen & Sørensen, 2004). Bombina bombina is widespread throughout Europe and since several species are closely related to Bombina bombina this species is highly relevant as a test organism. Bombina bombina proved to be easy to maintain and breed throughout the year in the laboratory, provided that the adults are fed properly (only "moving" food, i.e., various invertebrates), and have access to both water and land area in the vivarium (Larsen & Sørensen, 2004). The new test guideline using Bombina bombina has been tested with two pesticides (the insecticide esfenvalerate and the fungicide prochloraz). Esfenvalerate had only a small effect on the mortality on Bombina bombina. However, when malformations in the embryos were examined, effects from as low as a concentration of 1g/l of esfenvalerate were seen (Larsen & Sørensen, 2004). Standard toxicity tests where chemical substances are tested for toxicity on one organism at a time have been criticized for not being realistic, since they are carried out in the laboratory under artificial conditions. There is a need for evaluating the validity of such toxicity tests by applying the tests under natural conditions, where food chains are intact and vegetation, sediment, decomposers etc. influence the behaviour of the test substances as well as the test organisms in the habitats. In the present study, effects of four pyrethroids, applied to an artificial pond, on caged embryos of Bombina bombina were studied for the purpose of examining the developmental toxicity effects under more realistic conditions. Furthermore, the potential of caging Bombina bombina embryoes/tadpoles under in situ conditions was evaluated with reference to monitoring for effects in the environment. 6 Materials and methods
6.1 Principles and methods for maintaining and breeding the fire-bellied toads, Bombina bombina6.1.1 Principles and methods for maintaining adults Bombina bombinaAdult Bombina bombina Adult Bombina bombina used in the present study were at least two years of age and weighed about 6.5 to 8.5 g (males and females are about the same size). 1-2 pairs were kept together in a 50 30 cm vivarium. The floor area consisted of 60% water, with a depth of 8 cm, and the land area was dark-coloured with hiding areas and a feeding place. The vivarium was fitted with a mesh to prevent escape. Artificial lighting was switched on daily from 7 am to 7 pm, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Slowly growing natural aquatic plants and plants of plastic and broken pots were creating hiding places. Diet Food consisted of crickets, meal worms, small earthworms, flies (with crumpled wings) and other suitable invertebrates supplemented with a special vitamin supplement. Feeding was continued throughout the year, although at a reduced rate (about 2 times a week) during November-February when the animals showed minimal activity. Temperature Adults were kept at 19-21°C in the winter period and at room temperature in the summer period. 6.1.2 Principles and methods for breeding Bombina bombinaMales and females were bred as a single pair and the animals were moved to a 45 l glass aquarium with a water depth of about 20 cm. The aquarium was richly furnished with both natural and plastic plants. In addition, 2 mm round sticks of wood were placed in the aquarium, and 1-2 islands of floating cork enabled the animals to leave the water if desired. Artificial lighting was switched on daily from 5 am to 9 pm, however, the vivarium was not protected from natural light so the animals were subjected to natural fluctuations in day length. Water temperature was adjusted to 22 ±0.5°C. Breeding To mature the oocyttes, the females received about 14 IU of Pregnant Mare Gonadotropin (PMG) two days before the animals were moved to the breeding aquarium. Just before the animals were moved to the breeding aquarium both males and females received 55 to 100 IU of human chorionic gonadotropin to induce breeding. The amount of hormones injected depended on the size of the animals and the time of year. Low doses were usually used in spring and high doses in autumn. Amplexus normally ensued within 2 to 6 h and eggs deposition about 9 to 12 h after injection. The eggs were immediately inspected for fertility and quality. The fertility rate should be > 75% before a toxicity test was performed. Examination of the aquarium showed that the eggs were often found on vegetation and on the round sticks of wood, which were placed in the breeding aquarium to imitate straw and stems. 6.2 Semi-field toxicity tests using embryos of Bombina bombina6.2.1 Principle and design of the semi-field toxicity testIn situ experiment The tests were carried out at semi-field conditions from 12 to 21 June 1996 in two artificial ponds, at the mesocosm facilities at the National Environmental Research Institute, Denmark. One pond was sprayed with pesticides and one pond served as a control. Air and water temperatures were measured daily. Just before spraying, 8 glass Pyrex dishes (9 cm in diameter and 5 cm high) each containing 10 embryos were placed in two baskets of stainless steel in the pond about 20 cm below the water surface at opposite banks before spraying with pesticides. Four dishes containing 25 embryos each were simultaneously placed in the control pond also about 20 cm below the water surface. The cages were placed in the ponds on June 11 at 23.00 pm. The test ponds were sprayed with pesticides on June 12 at 9.25 a.m. To evaluate developmental toxicity, mortality, malformation, and growth inhibition properly, data were collected every day until at least 90% of the controls had attained the development stage 46 according to Nieuwkoop and Faber (1975). In addition to the control group in the control pond, a control group grown under laboratory condition was included in the test design. For this purpose, four dishes of five embryos each were used. Test organisms and egg manipulation The fire-bellied toads, Bombina bombina had been kept in laboratory facilities for more than two years. This study was carried out with eggs in which the jelly coat had not been removed. The jelly coat is normally removed before starting an experiment in the standard FETAX test. Test chemicals The test chemicals were esfenvalerate, deltamethrine, fenpropathrin, which were added as formulated products, and permathrin, which was added as an analytical grade active ingredient. 6.2.2 Establishing of experimental ponds, mesocosms.Ponds The mesocosm facilities at National Environmental Research Institute (50 km west of Copenhagen, Denmark) were kindly provided for the present study. The facilities consist of four ponds with a bottom area of about 130 m2 and a depth of about 0.75 m, which were established in November-December 1994. The mesocosms were established in an area with heavy clay making it possible to retain water in the ponds without assistance of an artificial membrane. A pipe from each pond leads to a ditch outside the bank. In case of surplus rainfall it is possible to adjust the water level in all ponds to 1.5 m by draining surplus water into the ditch. From a natural pond in a landscape similar to the test facilities, natural sediment was transported to the mesocosms. The sludge was uniformly sprayed onto the bottom of the ponds in a layer of about 2 cm. The natural sediment was introduced to initiate aquatic flora and fauna in the ponds and a variety of plants, crustaceans and insects developed, which created an ecosystem resembling the ecosystem of a natural pond. For further details, cf. Mogensen et al. (2004). 6.2.3 Embryo selection for the testEmbryo selection Normally cleaving embryos were selected for the tests. Two levels of selection were used. Normally cleaving embryos were first sorted into dishes containing fresh water. After a short period during which cleavage continued embryos were sorted again to ensure that only normal embryos were used. Abnormal pigmentation was viewed as an indicator of bad embryos. Both Nieuwkoop and Faber (1975) and the "Atlas of Abnormalities" [2] were used as references to determine whether the cleavage pattern was normal. Midblastula (stage 8) to early gastrula (stage 11) was used to start the tests. By this stage, normal cleavage and development can be ascertained. Embryos chosen prior to stage 8 may develop abnormal cleavage patterns later whereas embryos selected after stage 11 have commenced organogenesis. The sorting was done in Petri dishes. Staging of embryos was done according to Gosner (1960) and Nieuwkoop and Faber (1975). After the last sorting the embryos were transferred to test chambers containing 40 ml of pond water. 6.2.4 Experimental design of the semi-field toxicity testIn the present study embryos from two mating pairs were used to ensure a sufficient number of embryos. Embryos from each mating pair were randomly divided in 3 groups: one group was used in the pond sprayed with the pyrethroid insecticides, the second group was placed in the control pond, and the third group was used as the laboratory control test. It was necessary to keep clutches separate because embryos of a particular mating pair may develop poorly although they initially appear acceptable. This would cause all the embryos to be discarded if embryos were mixed from different mating pairs. Determination of the effects on embryos To evaluate developmental toxicity, mortality, malformation, and growth inhibition properly, in vivo observations were performed 6 h after spraying and then every day after the start of the test. Each day the embryos were picked up from the test chamber using a Pasteur pipette, and malformation, mortality and growth inhibition data were recorded. After investigation all living embryos were taken back to the test chambers and returned to the respective ponds or to the incubator in the laboratory. Duration of the semi-field test The experimental period was 11 days (including the day of spraying) after which stage 46 was attained in the controls. Laboratory control experiments The test design of the laboratory control experiments was the same as previously described for toxicity bioassay with Bombina bombina (Larsen et al., 2004). The culture medium used for the laboratory control test was pond water taken from the control pond. Tests with embryos of Bombina bombina were conducted in an incubator at 20 ±0.5 °C. The tests chambers were covered with 60 mm glass Petri dishes and had a culture volume of 10 ml. The medium was renewed every 24 h during the laboratory tests. It took 216 h to attain stage 46 under these conditions. 6.2.5 Test substancesThe spraying liquid was prepared from three formulated products shown in table 6.1. Active ingredients are given in brackets: Decis from Hoechst (deltamethrin), Sumirody 10 FW from Du Pont (fenpropathrin) and Sumi-Alpha 5 FW from Du Pont (esfenvalerate). A formulated product with permethrin was not available so the active ingredient was dissolved in Decis prior to dilution with water. Table 6.1* Udvalgte fysisk-kemiske egenskaber for de anvendte forsøgsstoffer.
* Aqueous photolysis studies with esfenvalerate and prochloraz indicated that the half-lives of both pesticides are about 10 days, however, the half-lives are of course dependent on the intensity of the light. Data from The Pesticide Manual, tenth edition and the Danish EPA. 6.2.6 Spraying method and estimated initial concentrationsSpecial equipment was constructed to make it possible to spray pesticides uniformly upon the water surface (Mogensen et al., 2004). Two-three persons carried the spraying boom during spraying. The amount of pesticide sprayed per ha depends on the pressure and walking speed. In the present study the pressure was 2.5 bar, spraying time 49 s. corresponding to application of approximately 5 L of spraying liquid according to Mogensen et al.; (2004). All four pyrethroids were sprayed simultaneously. Permethrin dissolved in Decis was mixed with Sumirody 10 FW and Sumi-Alpha 5 FW. Table 6.2 shows the composition of the spraying liquid and the approximate application rate. Table 6.2 Koncentrationen af pyrethroider i sprøjtevæsken og mængden af pyrethroid, der er udsprøjtet på overfladen af vandhullet, mg/m2 (Efter Mogensen et al. 2004).
6.2.7 Physical state of the pondsThe physical state of the ponds was controlled during the experiments by measuring a number of parameters like chlorophyll a, alkalinity, nitrogen and phosphorus, oxygen, temperature, pH, etc. The results from these measurements are reported in Mogensen et al. (2004). 6.2.8 Determination of the effects of pesticide application on embryos of Bombina bombina6 hours after spraying all baskets were carefully hoisted up from their respective ponds (by way of a fishing line attached to the handle of each basket) and the embryos were examined every day by picking up the embryos from the test chambers using a Pasteur pipette. The orifice of the Pasteur pipette was enlarged and fire-polished to accommodate embryos without damage. After examination, the embryos were placed in the test chambers and returned to the respective ponds or incubator in the laboratory. The dishes in the ponds were covered with a 500 μm plankton net. The plankton net was mounted on the dishes with a rubber band made of silicone 6 hours after the spraying to prevent the embryos to escape. In vivo observations A binocular dissection microscope capable of magnifications up to 30 was used to evaluate the abnormal embryos. The embryo lengths (head-tail length measurements) were measured by using a map measurer or an ocular micrometer. Staging of the embryos was done according to Gosner (1960) and Nieuwkoop and Faber (1975). Mortality Dead embryos were removed at the end of each 24 h period and the mortality data were recorded. Death during the first three days was ascertained by the embryo's skin pigmentation, structural integrity, and irritability (measured by lack of response on physical stimulations). After four days the lack of heartbeat served as an unambiguous sign of death. After nine days of exposure or at stage 46 for the controls, the total number of dead embryos was recorded during the test. Dead embryos were removed and the remaining living embryos were fixed in 3% formaldehyde solution. Malformation Malformation and other sublethal end points are normally more sensitive than mortality and are therefore included in the present test protocol. Malformation was recorded at the end of the test. Embryos exposed to the test material were compared with controls. Growth inhibition The ability of a material to inhibit embryonic growth is often the most sensitive indicator of developmental toxicity. Head-tail length data (growth) were likewise tested at the end of each test. If the embryo was curved or kinked, the measurement was made as if the embryo was straight. Measurements were made after the embryos were fixed in 3% formaldehyde solution. 7 Results
7.1 Embryo development of Bombina bombina.Egg deposition of Bombina bombina occurred about 9-12 h after injection of human chorionic gonadotropin. About 150-300 eggs were found in the breeding aquarium, and about 120–150 eggs were found normal cleavaged after selection. Laboratory control mortality was kept to less than 5% by only using the best eggs in the tests and only normally cleaving embryos. 7.1.1 Embryo development in laboratory controlLaboratory control Most of the embryos of Bombina bombina were still in the jelly coat after four days and were not free until after about six days. At 20°C, the control group of Bombina bombina reached stage 46 after nine days and all embryos were normal and showed no malformations. At stage 46, larvae were recognised by the appearance of the hind limb buds, the coiling of the gut, and the shape of the operculum covering the gills. The best indicator that the larva stage had been attained at stage 46 was the appearance of the hind limb buds. Gut coiling was also easily observed at stage 46 (at stage 45 embryos do not display complete tight gut coiling). At stage 46, the larvae were about 9.5 mm in length. 7.2 Effects of selected pyrethroids on Bombina bombina in a semi-field testControl pond The development of the control groups of Bombina bombina in the control pond is shown in table 7.1 (stage according to Gosner (1960) and selected stage according to Nieuwkoop and Faber (1975) are included), and corresponds to the development in the laboratory experiments. Table 7.1 Udviklingen af embryoer af Bombnina bombina i et kunstigt vandhul i Danmark. Embryo-stadier blev bestemt efter Gosner (1960).
* stage according to Nieuwkoop and Faber (1975) for comparison with Xenopus l 7.2.1 In vivo observationsTreated pond Pyrethroids affected gradually the embryos during the exposure. After 6h no effects of the pyrethroids were seen on the embryos compared with the controls. After 1 day, beginning twisting of the embryos was seen now and then. The spasmodic twisting increased in intensity with increased time of exposure and many embryos had constant spasmodic twisting two days after application of the pyrethroids. The spasmodic twisting of the embryos caused that some of the embryos had already left the jelly coat at this stage. In the control group, only a few embryos had begun to leave the jelly coat. After three days, all embryos exposed to pyrethroids were free of the jelly coat. Spasmodic twisting was seen in most of the embryos and constant spasmodic twisting was seen in many embryos. Occasionally, blistering and cardiac edema appeared. About 40% of the living embryos were immobilised by constant spasmodic twisting. Several of the embryos in the control group began to leave the jelly coat and move around. In the control group from the pond all embryos were free from the jelly coat and moved around after four days. After five days, blistering and cardiac edema were frequently seen in embryos exposed to pyrethroids. The embryos were often immobilised by constant spasmodic twisting. The heartbeat was very slow and severe heart malformations were often seen. After six, seven, eight and nine days the same picture was seen, however, the effects of pyrethroids seemed to be less pronounced. The intensity of the spasmodic twisting seemed to decrease and several of the surviving embryos started to swim around. All embryos in the control group were swimming around and had attained the development stage 46 according to Nieuwkoop and Faber (1975) after nine days. 7.2.2 Recordings of malformation, growth inhibition and mortality at the end of the test (nine days)Malformation Embryos from the control pond posed no malformations and the larvae were at the normal stage 46 according to Nieuwkoop and Faber (1975) and comparable with the laboratory controls in all respects. 9% of the embryos had malformation in the control pond whereas 5% of the embryos had malformation in the laboratory control culture. In embryos from the pond, sprayed with pesticides, gut abnormalities were often seen, indicated by a loose gut coiling. In addition to gut abnormalities, a relatively mild flexure of the tail was normally seen and heart malformations were also observed. 85% of the embryos had malformations. Most abnormal embryos possessed multiple malformations. The most significant malformations observed included cardiac, severe lateral flexure, edema, notochord, and gut malformations. Growth inhibition After nine days of exposure to pyrethroids, the mean length of the embryos was compared with the length of the embryos from the control pond. The mean length of embryos exposed to pyrethroids was 9.0 mm (S.D. 0.7) while in the control pond the mean length of the embryos was 10.3 mm. (S.D. 0.6). The mean length of embryos from the control cultures in the laboratory was 9.5 mm (S.D. 0.6). Mortality No significant mortality was seen in the control pond as compared with the laboratory experiments: the mortality of the embryos at the end of the experiments was 5 and 10%, respectively. A mortality of 51% was found for embryos exposed to pyrethroids. 8 Discussions
8.1 The use of amphibians as an ecotoxicological test organismFETAX was first developed by Dumont et al. (1983) and is useful for screening for the potential developmental toxicity of both single chemicals, and complex chemical mixtures (Bantle et al., 1991) on embryos of Xenopus laevis. Recently, a new test guideline, related to FETAX with the fire-bellied toad Bombina bombina, was developed (Larsen & Sørensen, 2004). Besides using Bombina bombina embryos instead of Xenopus laevis embryos, the new guideline differs by not removing the jelly coat before the start of the test. Previous experiments indicate that the removal of the jelly coat did not affect the development of the embryos (Larsen & Sørensen, 2004). However, since the jelly coat may influence the transport and exposure to toxic compounds from the water to the embryos it was chosen not to remove the jelly coat in the new guideline. Tests where the jelly coat is intact may, other things being equal, be more ecological relevant. Standard toxicity tests where chemical substances are tested for toxicity on one organism at a time have been criticized for not being realistic, since they are carried out in a laboratory under artificial conditions. In the present study, effects of four pyrethroids, applied to an artificial pond, on caged embryos of Bombina bombina, were studied for the purpose of examining the developmental toxicity effects under more realistic conditions and to establish a test protocol for using Bombina bombina in semi-field investigations. 8.2 Development in the controls and in the sprayed pondThe development of the embryos of Bombina bombina in the control pond corresponded to the embryo development in the laboratory control in which the embryos were kept under constant conditions. The develop-ment under these conditions from embryo to larvae stage took in both experiments nine days. Since the mortality of the embryos in the control was rather low, 5 and 10 % in the pond and in the laboratory, respectively, and the appearances of malformations were few, 9 and 5%, respectively, the development of the embryos was comparable with the two controls. It can therefore be concluded that the test ponds were very suitable for out-planting Bombina bombina embryos. The pesticides applied Four different pesticides were sprayed simultaneously on the surface of the test pond. This procedure does not mimic normal agricultural practice where usually only one pesticide is sprayed at the time. However, the present study was only a minor part of a large project where the objective was primarily to study the general fate of the pesticides and to generate data for calibration of a distribution model, and not to study effects to aquatic life (Mogensen et al. 2004). The results from the present experiment can therefore not be used to predict the effects of the individual pyrethroids used in this study on the development of embryos of Bombina bombina. The developmental abnormalities found by the end of the experiment are the results of the concerted actions of the four pyrethroids. In situ observations of the effects of the pyrethroids Spasmodic twisting was seen as an effect of the pyrethroids and caused the embryos exposed to pyrethroids to leave their jelly coats one day before the control group. The constant spasmodic twisting caused immobilisation of more than half of the embryos at day three, but this effect seemed to decrease after six days, where the surviving embryos started to swim around. The concentration of the total amount of the four pyrethroids was measured in an accompanying experiment by Mogensen et al. (2004) and was around 8 μgl-1 in the surface layer 2-4 h after spraying. The concentration then decreased to around 3 μgl-1 after 24 h, to concentration below 0.5 μgl-1 after six days. At the time where the surviving embryos started to swim around the concentration of the pyrethroids had therefore decreased to less than 1/10 of the water concentration 2-4 hours after spraying, due to absorption by the sediment and macrophytes (Mogensen et al., 2004). This might have caused the increased swimming activity. It is important to realize that the pyrethroid concentration used in the present study is almost 5 times higher than compared with the concentration that is expected to occur in ponds in agricultural areas. Fairchild et al. (1992) found that a pyrethroid concentration of 1.7 μg/l represents the worst case exposure condition caused by direct overspray or extensive drift during aerial application adjacent to a pond. Mortality The pyrethroids sprayed onto the pond had a direct effect by increasing the mortality from 5-10 % in the controls to 51% in ponds sprayed with the pyrethroids. However, this high mortality is not likely to occur under natural conditions, due to the high pyrethroid concentration applied in this study. Malformations The most significant malformations observed were heart malformations, severe lateral flexure, edema, notochord and gut malformations, and since 85 % of the embryos possessed multiple malformations the pyrethroids had severe effects. These results confirm the results found in other studies of toxic effects of pyrethroids on amphibians (Larsen & Sørensen, 2004; Berrill et al., 1993). Growth The ability of toxicants to inhibit embryonic growth is often a sensitive indicator of developmental toxicity. Thus, Berrill et al. (1993) exposed five species of amphibians to pyrethroid insecticide at concentrations between 10 and 200 g/l with no results of mortality, but with a notable reduction in growth rates. The applied pyrethroids did not inhibit the final length of the larvae in the present study. Although the larvae were not weighed after the experiments, the larvae which were exposed to pyrethroids did not appear smaller in any way compared with the control organisms, and the growth rates were therefore not notable affected by pyrethroids in this study. This agrees well with results of experiments on effects of pyrethroids on amphibian embryos (Larsen & Sørensen, 2004; Larsen, et al., 2004), but a reduction of the growth has been found in other studies (Berrill et al., 1993; Materna et al., 1995). While the growth of the surviving embryos was not affected by the pyrethroids, the embryos were seriously affected by the pyrethroid treatment. Although embryos were surviving the exposure to the initial high concentrations of pyrethroids, it does not indicate that the development of the embryos will result in surviving adult toads, capable of reproducing. Larsen et al. (2004) found in a accompanying study with recovery experiments where larvae, previously exposed to 20 or 125 μg/l esfenvalerate, were transferred to pure medium, that the larvae, which survived the treatment, developed severe malformations during metamorphosis. The most obvious deformity was their forelimbs which were bent in over the body and prevented them to jump. Instead they tumbled around when they tried to jump on land or catch flies and they were therefore unfit to survive in the nature. Survival and growth of embryos and larvae and progress of metamorphosis can therefore not be used as a success criterion in investigations on effects of toxic substances on amphibians. Furthermore, amphibians are not likely to be exposed to lethal concentrations of pyrethroids in the environment, due to short half-life and the low concentration likely to occur in ponds and lakes (Berrill et al., 1993). It is therefore important to identify sublethal effects. From the present study it can be concluded that the new toxicity test guideline using Bombina bombina as test organism can be used under in situ conditions for testing developmental toxicity of toxicants. When applying the test into natural ponds or lakes the realism of such tests increases by including natural food webs, decomposers, sediment and natural physical conditions. These kinds of caging experiments are therefore very suited for evaluating the fitness of a pond or lake to inhabit populations of amphibians as well as for evaluation of effects of toxicants such as insecticides sprayed adjacent to ponds or lakes. Compared with monitoring on natural population caging has several advantages. Time for out-planting is known, dispersion and predation are prevented, time-integrated monitoring can occur and a real control can be established. Time-integrated monitoring has the benefit that possibly effects of short-term pulses may be recorded. 9 ReferencesASTM (American Society of Testing and Materials), 1991. Standard Guide for Conducting the Frog Embryo Teratogenesis Assay-Xenopus (Fetax). Bantle, J.A., Dumont, J.N., Finch, R.A., Linder, G., 1991. Atlas of abnormalities: A guide for the performance of FETAX. Oklahoma State Publication Department, Stillwater, OK. Berrill, M., Bertram S., Wilson A., Louis S., Brigham D. and Stromberg C. (1993). Lethal and Sublethal impacts of Pyrethroid Insecticides on Ampfibian embryos and tadpoles. Environ.mental Toxicol. Chem., Vol. 12, pp. 525 - 539. Berrill, M., S. Bertram, B. Pauli, D. Coulson, M. Kolohon and D. Ostrander (1995). Comparative sensitivity of amphibian tadpoles to single and pulsed exposures of the forest-use insecticide fenitrothion. Environ. Toxicol. Chem., Vol 14, No. 6, pp. 1011-1018, 1995. Berrill, M.S., S. Bertram, L. McGillivray, M. Kolohon and B. Paull (1994). Effects of low concentrations of forest-use pesticides on frog embryos and tadpoles. Environ. Toxicol. Chem. 13: 657-664. Birge. W.J., Black J.A. and A.G. Westerman (1985). Short-term fish and amphibian embryo-larval tests for determining the effects of toxicant stress on early life stages and estimating chronic values for single compounds and complex effluents. Environ. Toxicol. Chem. 4: 807-821. Blaustein, A.R. & D.B. Wake, 1990. Declining Amphibian Populations: A Global Phenomenon ? TREE Vol. 5, No. 7, July 1990. Blaustein, A.R., D.B. Wake and W.P. Sousa (1994). Amphibian declines: Judging stability, persistence, and susceptibility of populations to local and global extinction. Conserv. Biol. 8: 60-71. Clark E.J, Norris D.O, Jones R.E. (1998) Interaction of Gonodal Steroids and Pesticides (DDT) on Growth in Larval Tiger Salamanders. General and Comparative Endocrinology. Vol. 109, 94-105. Coats, J.R., Symonik, D.M., Brandbuury, S.P., Dyer, S.D., Timson L.K., and Atchison G.J. (1989). Toxicology os synthetic pyrethroids in aquatic organisms: An overview. Environ. Toxicol. Chem. 8: 671-679. Cooke, A.S. (1970). The effect of pp'-DDT on tadpoles of the common frog (Rana temporaria). Environ. Pollut. 1: 57-71. Cooke, A.S. 1981. Tadpoles as indicators of harmful levels of pollution in the field. Env. Poll. (Series A) 25, 123-133. Crump, M.L., F.R. Hensley and K.L. Clark (1992). Apparent decline of the golden toad: Underground or extinct? Copeia, pp. 413-420. Dawson, D.A., Fort, D.J., Newell, D.L., Bantle, J.A. (1989). Developmental Toxicity Testing with FETAX: Evaluation of Five Compounds. Drug and Chemical Toxicity, Vol. 12, pp. 67-75. Dumont, J., Schultz, T.W., Buchanan, M., and Kao, G., (1983). Frog Embryo Teratoge.nesis Assay-Xenopus (FETAX)-A Short-term Assay Applicable to Complex En.vironmental Mixtures, In: Short-term Bioassays in the Analysis of Complex En.viron.mental Mixtures III, Waters, Sandhu, Lewtas, Claxton, Chernoff and Nes.now, eds., Plenum, New York, NY, pp. 393-405. Dutta, S.K. and P. Mohanty-Hejmadi (1978). Life history and pesticide susceptible embryonic stages of the Indian bull frog Rana tigrina Daudin. Indian J. Exp. Biol. 16: 727-729. Fairchild J.F., T.W La Point, J.L Zajicek, M.K. Nelson, F.J. Dwyer, P.A. lovely (1992). Population-, Community- and Ecosystem-level responses of Aquatic Mesocosms to pulsed Doses of a Pyretroid Insecticide. Environ Toxicol Chem 11:115-129. Freda, J 1986. The influence of acidic pond water on amphibians: A review. Water, Air and Soil Pollution 30, 439-450. Gosner K.L. (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183-190. Greenhouse, G. (1976). Effects of exposure to n-phenyl-naphthylamine, octyl-phenyl-naphthylamine, and dioctyl-diphenylamine on the development of frog embryos. Bull. Environ. Contam. Toxicol. 16: 626-629. Greenhouse, G. (1976). Evaluation of the teratogenic effects of hydrazine, methylhydrazine, and dimethylhydrazine on embryos of Xenopus laevis, South African clawed toad. Teratology 13: 167-178. Gutleb, A.C., J. Appelman, M.C. Bronkhorst, J.H.J. van den Berg, A. Spenkelink, A. Brouwer, A.J. Murk (1999). Delayed effects of pre- and early-life time exposure to polychlorinated biphenyls on tadpoles of two amphibian species (Xenopus laevis and Rana temporaria). Environ. Toxicol. Pharm. 8, 1999, 1-14. Hatch, A.C. & G. Allen Burton, Jr. 1998. Effects of photoinduced toxicity of fluoranthene on amphibian embryos and larvae. Environ. Toxicol. Chem., Vol 17, No. 9, pp. 1777-1785, 1985. Hopkins A.H., J. Congdon, J.K. Ray, 2000. Incidence and impact of axial malformations in larval bullfrogs (Rana catesbeiana) developing in sites polluted by coal-burning power plant. Environ Toxicol. Chem., Vol. 19, No. 4, pp. 862-868, 2000. Horne, M.T. & W.A. Durson, 1995. Effects of Low pH, Metals, and Water Hardness on Larval Amphibians. Arch. Environ. Contam. Toxicol. 29 500-505, 1995. Howe G.E., Gillis R., and Mowbray R.C. (1998). Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ. Toxicol. Chem. 17, no 3 519-525. Joffre M.B. & W. H. Karasov 1999. Direct effect of ammonia on three species of North American anuran amphibians. Environ. Toxocol. Chem., Vol. 18, No. 8, pp. 1806-1812, 1999. Larsen, J. and I. Sørensen (2004). The effect of esfenvalerate and prochloraz on amphibians with special reference to Xenopus laevis and Bombina bombina. Ministry of Environment, Danish Environmental Protection Agency, Pesticides Research No. 83. Larsen J, Sørensen I. and Gustavson K. (2004).The effects ofselected pyrethroids on embryos of Bombina bombina during different culture and semi-field conditions. Ministry of Environment, Danish Environmental Protection Agency, Pesticides Research No. 84. Larsson, P and A. Thuren (1987). Di-2-ethylhexylphthalate inhibits the hatching of frog eggs and is bioaccumulated by tadpoles. Environ. Toxicol. Chem. 6: 417-422. Materna, E.J., C.F. Rabeni and T.W. LaPoint (1995). Effects of the synthetic pyrethroid insecticide, esfenvalerate, on larval leopard frogs (0 spp.). Environ. Toxicol. Chem., Vol. 14, No. 4, pp. 613-622, 1995. Miller, T.G. and W.C. Mackay (1987). Evidence for an internal mechanism of copper toxicity in quatic animals. Comp. Biochem. Physiol. 76: 95-98. Nieuwkoop, P.D., and Faber (1975). Normal Tables of Xenopus laevis (Daudin), 2nd Ed., North Holland, Amsterdam. Pechmann, J.H.K. , D.E. Scott, R.D. Semlitsch, J.P. Caldwell, L.J. Vitt and J.W. Gibbons (1991). Declining amphibian population: The problems of separating human impacts from natural fluctuations. Science 253: 892-895. Perkins P.J., H.J. Boermans, G.R. Stephenson, 2000. Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay – Xenopus. Environ. Toxicol. Chem., Vol. 19, No. 4, 940-945, 2000. Phillips, K. (1990). Where have all the frogs and toads gone? Bio-Science 40: 422-424. Pounds, J.A. and M.L. Crump (1994). Amphibian declines and climate disturbance: The case of the golden toad and the harlequin frog. Conserv. Biol. 8:72-85. Sobotka, J.M. & R.G. Rahwan, 1995. Teratogenesis induced by short- and long-term exposure of Xenopus laevis progeny to lead. J. of Toxicol. & Environ. Health, 44:469-484, 1995. Tippe, A., 1987. Evidence for Different Mechanisms of Action of the Three Pyrethroids, Deltamethrin, Cypermethrin, and Fenvalerate, on the Excitation Threshold of Myelinate Nerve. Pesticide Bioichemistry and Physiology, 28, 67-74, 1987. Mogensen, B.B., Sørensen, P.B., Stuer-Lauridsen, F. & Lassen, P. (2004): Fate of pyrethroids in farmland ponds - Ministry of Environment, Danish Environmental Protection Agency, Pesticides Research No. 86. Wyman R.L. (1990). What's happening to the amphibians? Conserv. Biol. 4: 350-352. Appendix A Score sheet of malformations after 96 h
Appendix B The composition of the culture mediaFETAX solution water
per litre of deionised or distilled water. The pH of the final solution should be 7.6 to 7.9. All chemicals were reagent-grade or better. Appendix C The effect of esfenvalerate on malformation, growth inhibition, and mortalityTable C.1 Effeketen af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 20°C i FETAX opløsningen. Middeltal af 3 forsøg.
Table C.2 Effekten af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 20°C i FETAX opløsningen. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 216 timers eksponering.
Table C.3 Effeketen af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 24°C i FETAX opløsningen. Middeltal af 3 forsøg.
Table C.4 Effekten af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 24°C i FETAX opløsningen. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 120 timers eksponering.
Table C.5 Effeketen af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 20°C i vandhulds vand. Middeltal af 3 forsøg.
Table C.6 Effekten af esfenvalerat på Bombina bombina illustreret ved sublethale defekter, væksthæmning og dødelighed ved 20°C i vandhuldsvand. Resultaterne er udtrykt ved NOEC, LOEC, EC50/LC50 efter 216 timers eksponering.
Footnotes[1] Availabe from John A. Bantle, Dept. of Zoology, 430 LSW, Oklahoma State University, Stillwater OK 74078. [2] Availabe from John A. Bantle, Dept. of Zoology, 430 LSW, Oklahoma State University, Stillwater OK 74078.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||