|
Biological Control of Weevils (Strophosoma melanogrammum and S. capitatum) in Greenery Plantations in Denmark 2 Virulence of hyphomycete fungi against Strophosoma melanogrammum and S.capitatum2.1 Definitions and terms used in laboratory bioassays with entomopathogenic fungi The first step toward utilisation of insect pathogenic fungi as a biological control agent is to select biologically and ecologically fit pathogens. In order to compare the efficacy of pathogens, assays are normally carried out under laboratory conditions to maintain the maximum control over variability that might affect the results of the test. Comparative studies must be conducted under standardised conditions, which permit normal or close to normal behaviour and avoid abnormally high mortality among the untreated insects (Lacey, 1997b). In this chapter emphasis is given to bioassay methodology and data obtained in the projects. 2.1 Definitions and terms used in laboratory bioassays with entomopathogenic fungiPathogenicity of an insect pathogen is defined as the ability to produce disease in insects (Lacey, 1997a). The proof of pathogenicity is the first step towards studies on virulence. The virulence of an insect pathogen is defined as the quality or property of being virulent or the disease-producing power of a micro-organism (Lacey, 1997a). Assessment of the virulence of an insect pathogen requires quantitative studies. In studies of time-response relationships the terms average survival time (AST) and median lethal time (LT50) are the most common expressions of the time required to kill a given insect. LT50 is defined as the time period required to kill 50 % of the test insect population when subjected to a given concentration or dose of an insect pathogen. AST is the average lifetime of the population of interest. The terms are often used as a quantitative expression of the virulence of fungi. The shorter the lethal time or survival time is for the test insect population subjected to a fungal isolate, the higher the virulence. Furthermore, the term LT50 or AST provides much of the information needed to understand the dispersal of the disease in the insect population and the dynamics of the host-pathogen system (Goettel and Inglis, 1997). In studies of dose-response relationships, the terms LC50 and LD50 are the most common expressions of virulence. LC50 is the concentration of a given insect pathogen required to kill 50 % of the test insect population within a given period of time, whereas LD50 expresses the dose required to kill 50% of the population. With respect to hyphomycete fungi, LC50 is the appropriate term since the methodology only admits of an estimate of the concentration used and not of the dose actually received by the test insects (Goettel and Inglis, 1997). 2.2 Bioassay methodologyStandardised bioassay systems are not available for entomopathogenic Hyphomycetes. This is mostly due to the wide range of hosts, which vary in their requirements. Therefore, specific bioassays must be developed for most host-pathogen combinations. However, the most common quantitative bioassay method for insects feeding above the soil surface is the `dipping method' (Goettel and Inglis, 1997, Eilenberg et al., 2003). One or a series of aqueous suspensions are prepared with a known concentration of conidia. Insects are immersed singly or in cohorts into the suspension. After a specified time the suspension is quickly drained off by suction (Hall, 1976). Another possibility is to present inoculum via a secondary substrate. The most common method is to dip the substrate, usually the food source into the suspension and then transfer the insects onto it. The insects will then pick up the inoculum by contact with the substrate as they feed or move on it (Goettel and Inglis, 1997). A study comparing the effect of the two dipping methods described above showed that the median lethal time was prolonged when dipping the substrate rather than the insect (Eilenberg et al., 2003). For soil dwelling insect larvae such as, for example, larvae of Strophosoma spp. bioassays are often performed by incorporation of the inoculum into the soil substrate (Goettel and Inglis, 1997). 2.3 Analysis of bioassay dataThe most used response in bioassays is the categories “dead” or “alive” and the response is thus binary having only two possibilities. 2.3.1 Concentration response bioassays (LC50)The only explanatory variable considered in concentration response bioassays is the concentration. The response of the test subject is assumed to be functionally related to the dose or concentration level so that as the concentration or dose of the pathogen increases more test subjects respond by dying. The equation of a binary response with a single explanatory variable (concentration) is given by: Pi = F (a+ bxi), where Pi is the probability of response, xi is the ith concentration or a function of that dose (eg. logarithm of concentration), a is the intercept of the regression line, b is the slope of the regression line, and F is the distribution function. The most used functions are either probit or logit functions (Robertson and Preisler, 1992). Data for concentration-response relationships (LC50) presented in this report was calculated using probit as the distribution function. 2.3.2 Correlated observations over time at one concentrationStandard probit or logit analysis techniques are, however, not applicable to serial time mortality data because observations made on the same group of insects at different times are correlated (Robertson and Preisler, 1992, Throne et al., 1995). Basically two statistical methods have been used for analysing bioassay data where test insects have been subjected to a pathogen followed by regular recording of mortality of the same group of insects. At present mainly two methods are used for analysis of serial time mortality data. This is the Kaplan-Meier Survival Analysis to calculate the average survival time in a given population and logistic regression to calculate the medium lethal time (Kessler and Nielsen, 2000). In this report the virulence of a given isolate is calculated and given as average survival time (AST). 2.4 Bioassays with adult Strophosoma melanogrammum2.4.1 Determination of average survival time at different temperatures2.4.1.1 Materials and methodsTest insect: Adults of S. melanogrammum collected from the field in spring 2001 were used as test insects. Before use, weevils were in quarantine for three weeks to ensure healthy test material. Isolates: Fourteen isolates were tested in laboratory bioassays (Table 2.1) and included two V. lecanii isolates (KVL 98-6 and 98-8); seven B. bassiana isolates (KVL 98-20, 98-35, 99-117, 00-125, 00-126, 00-127, 00-128) and three P. farinosus isolates (KVL 99-28, 00-88, 00-124) which all originated from naturally infected adult Curculionidae collected in Denmark. Furthermore, two M. anisopliae isolates (BIPESCO 5, KVL 00-31) were selected based on earlier records of successful control of other insect pests (Vestergaard et al., 1995; 2002). The two M. anisopliae isolates originated from a naturally infected Cydia pomonella L. collected in Austria by Dr. Gisbert Zimmermann and from Danish soil, respectively. Table 2.1. Insect host and geographical origin of isolates of entomopathogenic fungal isolates tested in laboratory bioassay.
* BIPESCO 5 is a descendant of isolate 275-86 from Horticulture Research International culture collection. Bioassay procedure: In initial bioassays infectivity and virulence of all fourteen isolates were tested against S. melanogrammum at 20°C. The conidia were harvested from cultures grown on 1.5% Sabouraud dextrose agar (SDA) plates by flooding the plates with sterile 0.05% aqueous (w/v) Triton X-100 and gently agitated with a glass rod. Conidia were separated from agar and hyphae by centrifugation for 5 min at 3000 rpm. The pellet was washed twice with sterile 0.05% Triton X-100 with intervening centrifugation steps. The conidial concentration was determined using a haemocytometer and adjusted to 107 conidia/ml with 0.05% Triton X-100. The viability of the conidia was assessed on 1.5% SDA plates after 24 hours of incubation at 23°C by counting the number of germinating conidia out of 300. The germination rate ranged from 94 to 100%. Ten weevils were inoculated by immersion for 10 sec in 10 ml conidial suspension. The suspension and the weevils were poured onto filter paper in a Buchner funnel and excess suspension was removed by vacuum. Insects were carefully transferred individually to medicine cups (30 ml) containing 5 ml of 3% water agar and a small twig of A. procera. The medicine cups were sealed with polyethylene (PE) cling film, a semi-permeable membrane that ensures humid conditions without condensation. Finally, cups were closed with a plastic ring, and incubated at 20°C with a 16:8 h photoperiod. Controls consisted of weevils treated with 0.05% Triton X-100 only. Three times 10 weevils were used per treatment and mortality was recorded every day for five weeks. During the incubation period medicine cups and twigs were renewed whenever they appeared dry. After the initial bioassays two B. bassiana (KVL 98-20, 00-125), two P. farinosus (KVL 00-88, 00-124) and one M. anisopliae (BIPESCO 5) isolate were selected for further testing at lower temperature (12°C and 15°C) against S. melanogrammum and against females of S. capitatum at 20C. Finally, B. bassiana (KVL 00-125) and M. anisopliae (BIPESCO 5) were tested against males of S. capitatum. Data-analysis: The LIFETEST procedure in SAS vers. 8.2 (SAS Institute, 1999) was used to compute nonparametric estimates of the survivor function by the product-limit method. Pair-wise comparisons for differences in average survival time (AST) between populations subjected to different treatments or between Strophosoma species were tested for significance using a log-rank chi-square test. 2.4.1.2 ResultsIn none of the bioassays performed control mortality exceeded 10% and the test methods used are therefore regarded as reliable. All tested isolates were able to infect the target it was tested against, and average survival time (AST) for all the tested isolates is given in table 2.2. Among the tested isolates a huge variation was found with AST for S. melanogrammum ranging from 13.1 ± 0.7 to 22.6 ± 0.6 days when weevils were incubated at 20°C. Neither original host insect of the isolate nor fungal species did influence the AST of the weevils. Three B. bassiana isolates (KVL 98-20; KVL 00-125 and KVL 00-126) and one M. anisopliae isolate (BIPESCO 5) caused AST below 15 days which were significantly lower than all other isolates tested against S. melanogrammum at 20°C (P-values from log-rank chi-square test all ≤0.0181) but not mutually different (P-values from log-rank ? chi-square test all ≥ 0.5027). At lower temperatures the AST were prolonged but all tested isolates were able to infect and cause mycosis. For all isolates tested against both S. melanogrammum and S. capitatum AST were significantly longer for S. capitatum (P-values from log-rank chi-square test all < 0.0001). Table 2.2: Average survival time (AST) ± S.E. for field collected adults of Strophosoma melanogrammum and S. capitatum after inoculation with hyphomycete fungi. After subjection to the fungi S. melanogrammum was incubated at 20, 15 and 12°C and S. capitatum incubated at 20°C . –: Isolate not tested.
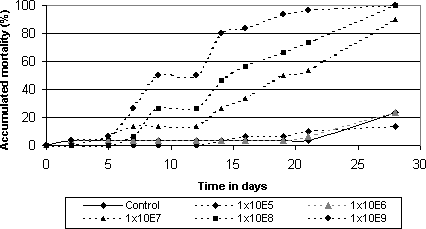
2.4.2 Determination of lethal concentration of M. anisopliae BIPESCO 5On the basis of the results of the bioassays described above as well as the ability to sporulate under large scale conditions on rice (C. Lomer, pers. com) one isolate, M. anisopliae BIPESCO 5, was chosen for further test in a concentration-response bioassay as well as field experiments (Chapter 5). 2.4.2.1 Materials and methodsThe bioassay procedure was the same as described above. Concentrations were adjusted to following concentrations: 1x105, 1x106, 1x107, 1x108 and 1x109 conidia/ml. Test insects were collected from the field in spring 2000. Data analysis: The computer program Polo-PC (LeOra Software, 1987) was used to compute the concentration-response relationships (LC50) and probit was used as the distribution function. 2.4.2.2 ResultsResults of concentration-response bioassay with BIPESCO 5 against spring-collected adults of S. melanogrammum are shown in Fig 2.1. The LC50 value was estimated to 2.1x107 conidia per ml (95 % confidence limit: 0.8 – 4.5x107) after 21 days at 20°C; 16:8 L:D.
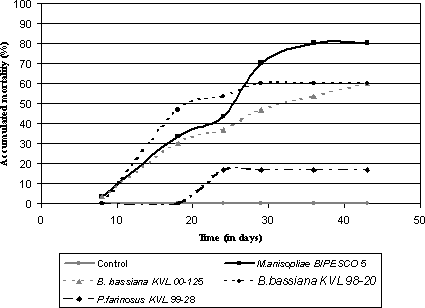
Figure 2.1: Concentration-response bioassay with Metarhizium anisopliae (Isolate no. BIPESCO 5) against adults of Strophosoma melanogrammum collected in spring. 2.5 Bioassays with Strophosoma spp. larvae2.5.1 Determination of lethal time2.5.1.1 Material and methodsTest insect: Larvae (3. and 4. instar) of Strophosoma spp. collected from the soil in a Noble fir plantation at `Storskoven' Bidstrup, Denmark, during the summer of 2001 were used as test insects. Fungal material: Two isolates of B. bassiana (KVL 98-20, Boverol®); one P. farinosus (KVL 99-28) and one M. anisopliae (BIPESCO 5) isolates were tested against Strophosoma spp. larvae. Conidial suspensions were prepared as described in paragraph 2.4.1. Bioassay procedure: For each isolate tested 3 ml of a conidia suspension adjusted to 108 conidia/ml or 3 ml Triton-X (control) were mixed into 30 g of field collected soil corresponding to the concentration obtained in the field experiments just after spraying (see chapter 5). The soil was transferred to 30 ml cups and ten larvae were introduced into each cup. All isolates were tested in constant darkness at 15°C. Three times 10 larvae were used per treatment. Mortality was recorded weekly for six weeks. 2.5.1.2 ResultsNo larvae died in the control treatment and all tested isolates were able to infect and cause mycosis in larvae of Strophosoma spp. The results are shown in fig. 2.2. Among the tested isolates the most virulent isolate was M. anisopliae BIPESCO 5, which resulted in 80% mortality and mycosis. The average survival time ranged between 20 and 30 days at 15°C for B. bassiana and M. anisopliae. The average survival time for P. farinosus (KVL 99-28) was not calculated since the mortality never exceeded 18%.
Figure 2.2: Accumulated mortality of larvae of Strophosoma spp. after subjection to entomopathogenic fungi. 2.6 DiscussionThe bioassay showed that under laboratory conditions all of the tested isolates were able to infect and cause mycosis in adults of S. melanogrammum and S. capitatum even at low temperature comparable to the temperatures found in activity periods of the adult weevils in Denmark (average mean temperature in May 11°C, June 15°C, August 16°C, September 13°C, October 10°C (DMI, 2004). Average survival times for adults ranged between 16 and 20 days at 15°C depending on isolate. The survival time seen for S. melanogrammum and S. capitatum subjected to conidial suspensions of entomopathogenic fungi are in the same range as reported for many other adults in the family Curculionidae. For example, Chikwenhere and Vestergaard (2001) reported median lethal times between 9 and 15 days for Neochetina bruchi Hustache when subjected to B. bassiana and incubated at 25°C and Kaaya et al. (1993) reported median lethal times from 8 days and up to more than 35 days for Cosmopolites sordidus (Germar) when subjected to B. bassiana or M. anisopliae. Compared to survival times usually seen for weevils treated with chemical pesticides, the survival time is much longer and no immediate effect would be expected of treatment against adult Strophosoma spp. Nevertheless, due to an initial feeding period up to several weeks in fall and again in spring before the eggs are laid and the long period of time over which eggs are laid (see chapter 4) it is believed that a fungal treatment in either spring or fall will have a potential to prevent population increase in the next generation. Whether adults emerging from the soil in spring or in autumn have different susceptibility to entomopathogenic fungi so far remains unexplored. As concerns the Strophosoma larvae, it was possible to infect them in the laboratory. Larvae were, however, not identified to species level since it is not possible to identify them in a non-destructive way (see chapter 3), so whether both species can be infected by entomopathogens cannot be determined for sure. However, due to the following facts we believe that both species are susceptible: (1) 30 larvae were treated for each isolate, (2) The percentage of fungal infection ranged between 60 and 80 % for most isolates tested (3) at the time of the year when larvae were collected the ratio between the two Strophosoma species were the following year found to be approximately fifty-fifty (see chapter 4). Other reports from studies on lethal time of coleopteran larvae have shown much difference, mostly shorter than ours. The following studies did, however, all refer to dipping of larvae in a conidial suspension, a highly `quicker' method than ours, which more closely reflects the situation in the field upon treatment. Kershaw et al. (1999) reported LT50 values between 6 and 12 days for the curculionid Otiorhynchus sulcatus F. treated with M. anisopliae. Kassa et al . (2002) reported a median survival time between 3.6 and 6.3 days for the curculionid Sitophilus zeamais Motschulsky subjected to B. bassiana. White grubs in Mexico (Phyllophaga spp.) subjected to M. anisopliae and B. bassiana had a LT50 of 4.9 days and 6.1 days, respectively (Flores et al., 2002). Long average survival times were, however, reported by Keller et al. (1999) for Melolontha melolontha L. treated with Beauveria brongniartii (Saccardo) Petch: between 22.9 and 56.4 days. Two different populations showed different susceptibility. 2.7 Conclusions
|