|
Survey of Chemical Substances in Consumer Products, no. 46, 2004 Release of chemical substances from tents and tunnels for childrenContents
5 Assessment of problematic substances
PrefaceThe present report brings the results from the project "Release of chemical substances from tents and tunnels for children" (J.nr. M 7041-0123), which has been carried out for the Danish Environmental Protection Agency based on a tender of March 2003. The project is part of a special action for mapping of chemical substances in consumer products as granted in the Appropriation Act. The project has been carried out by the Danish Technological Institute through the centres Textile, Wood Technology and Chemistry and Water Technology. Evaluation of chemicals has been carried out by Kirsten Pommer and quality assurance by Ole Christian Hansen. The project has had a steering committee consisting of Shima Dobel and Annette Orloff from the Danish Environmental Protection Agency and John Hansen, Danish Technological Institute, Textile. Summary and conclusionsA survey has been carried out regarding which tents and tunnels for children exist on the Danish market. Figures for the turnover has been collected, and it is investigated, which materials are typically used for such products. Among the products on the market six products were selected for testing. It is estimated that the six products represent 56-62% of the products annually sold. On the six selected products tests have been performed to determine their release of chemical substances, partly at room temperature, partly at elevated temperature. The analyses have been followed by an assessment of the health aspects. The objective has been to pinpoint the risks that might exist when children are playing and staying in tents and tunnels. A total of 46 substances or groups of substances were identified in the analyses. For most of the substances the concentration declines with time as would be expected. For some of the products, however, a higher concentration has been determined for certain substances after 28 days than after 10 days. This was the case for formaldehyde, dimethylformamide, xylenes and acetone. Health assessment A screening of the 46 identified substances or groups of substances has been carried out based on classifications and limit values of the substances. Out of the 46 substances 14 were chosen for a closer assessment. For the 14 substances an assessment of the impact on children during playing has been carried out. The background was the possible absorption by inhalation by a child of 10 kg, based on the maximum measured concentrations. The estimated amount of absorbed substance has then been compared to the data for NOAEL/LOAEL. The substances 2-butoxyethanol, phenol, toluene, and xylene do not cause long-term effect in the measured concentrations. These have been found in quantities that are more than a factor 100 below the levels that may cause damages to the health. The substances ethoxyethanol and dimethyl formamide are teratogenic. Ethoxyethanol has been found in quantities that are considerably below the TDI-value. Dimethyl formamide has been found in quantities that are considerably below the levels that may cause damages to the health. Acetaldehyde, formaldehyde, tetrachloroethylene, and trimethylcyclohexen-1-on are under suspicion of causing cancer (Carc3). Formaldehyde has been found in one measure series in concentrations that are above the indoor limit value. In a supplementary test relatively low values were detected. Acetaldehyde and tetrachlorethylen have been found in very small quantities and it is assessed that the risk of health effects will be insignificant. The substances 3-carene, α-pinene, 2,6-di-tert-butyl-p-cresol, and formaldehyde may cause allergy in contact with skin. There is a risk that formaldehyde may cause health effects while the other substances appear in very small quantities and it is assessed that the health effects will be insignificant. The measurement showed that the different aliphatic hydrocarbons with the carbon chains of C10 to C16 are emitted. It is assessed that this group of substances resembles turpentine, which is carcinogenic. It has not been possible to determine a NOAEL. In stead the TLV of aliphatic hydrocarbons and turpentine has been compared. The measured concentrations represent less than 0.4 % of the TLV for turpentine. A comparison with the B-values has also been made. The highest measured concentration represents 50% of the B-value. Based on these comparisons it is assessed that the health risk will be insignificant. Summing up several of the substances, which are present in the tents and tunnels for children, are under suspicion of having carcinogenic, teratogenic, mutagenic, and allergic effects. Formaldehyde is present in one product in a concentration above the indoor limit value while two of the other products are close to the value. In a supplementary three days test of the emission of formaldehyde no concentrations above the limit values were detected. As the measured concentrations of formaldehyde decreased over time, it will be during the first hours of using the tents that the largest emission of the substance will occur. As for the other substances the emitted quantities are relatively small. None of these substances are present in concentrations, which will cause a potential health risk for children to play with the tested products. Sammenfatning og konklusionerDer er gennemført en kortlægning af, hvilke telte og tunneler til børn der findes på det danske marked. Samtidig er der fundet tal for omsætningen, ligesom det er undersøgt, hvilke materialer der typisk anvendes. Blandt de på markedet værende produkter er udvalgt 6 produkter til analyse. De 6 produkter anslås skønsmæssigt at repræsentere 56 - 62% af de produkter, der årligt sælges. På de 6 udvalgte produkter er der foretaget bestemmelser af afgivelse af kemiske stoffer, dels ved stuetemperatur, dels ved forhøjet temperatur. Analyserne er fulgt op af en sundhedsmæssig vurdering. Formålet hermed har været at få afklaret de risici, der måtte være ved børns leg og ophold i telte og tunneler. Der er blevet identificeret 46 stoffer/stofgrupper ved analyserne. For langt de fleste stoffer falder koncentrationen over tid, således som det også må forventes. For nogle af produkterne er der dog målt en højere koncentration for visse stoffer efter 28 døgn end efter 10 døgn. Det drejer sig om formaldehyd, dimethylformamid, xylener og acetone. Sundhedsmæssig vurdering Der er foretaget en screening af de 46 identificerede stoffer/stofgrupper på baggrund af stoffernes klassificering og grænseværdier. Af de 46 stoffer blev 14 udvalgt for en nærmere vurdering. For de 14 stoffer blev der gennemført en vurdering af påvirkningen af børn ved leg. Der blev taget udgangspunkt i, hvor meget et barn på 10 kg kan optage ved indånding, baseret på de maksimalt målte koncentrationer. Den beregnede mængde optaget stof blev derefter sammenlignet med data for NOAEL/LOAEL. Stofferne 2-butoxyethanol, phenol, toluen og xylen medfører ikke langtidseffekter i de målte koncentrationer og forekommer i mængder, der ligger mere end en faktor 100 under de niveauer, der kan give skader. Stofferne ethoxyethanol og dimethylformamid er reprotoksiske. Ethoxy-ethanol forekommer i mængder, der ligger væsentligt under TDI-værdien. Dimethylformamid forekommer i mængder, der ligger væsentligt under de niveauer, der giver skader. Acetaldehyd, formaldehyd, tetrachlorethylen og trimethylcyclohexen-1-on er mistænkt for at være kræftfremkaldende (Carc3). Formaldehyd forekommer i en måleserie i betænkeligt høje koncentrationer, mens der i et supplerende forsøg blev konstateret relativt lave værdier. Acetaldehyd og tetrachlorethylen forekommer i meget små mængder, og det er vurderet, at risikoen for sundhedsmæssige effekter vil være minimal. Stofferne 3-caren, α-pinen, 2,6-di-tert-butyl-p-cresol og formaldehyd kan give allergi ved hudkontakt. Der er risiko for at formaldehyd kan give sundhedsmæssige problemer, mens de øvrige stoffer forekommer i meget små mængder, og det er vurderet at risikoen for sundhedsmæssige effekter vil være minimal. Målingerne viste, at der afgives forskellige alifatiske kulbrinter med kulstofkæder på C10 til C16. Det er vurderet, at denne stofgruppe ligner terpentin, der er kræftfremkaldende. Det har ikke været muligt at fastlægge en NOAEL. I stedet er der foretaget en sammenligning med grænseværdien for terpentin. De målte koncentrationer udgør mindre end 0,4 % af grænseværdien. Der er ligeledes foretaget en sammenligning med B-værdien. Den højeste målte koncentration udgør 50% af B-værdien. På baggrund af dette vurderes det, at den sundhedsmæssige risiko vil være minimal. Sammenfattende kan det konstateres, at flere af stofferne, der findes i telte og tunneler til børn, er mistænkt for at være kræftfremkaldende, reproduktionsskadende og allergifremkaldende. Formaldehyd kan give anledning til betænkeligheder i de målte koncentrationer. For de øvrige stoffer gælder det, at de afgivne mængder er relativt små. Ingen af disse stoffer forekommer i koncentrationer, der gør, at det vil være betænkeligt, at børn leger med de undersøgte produkter. For produkterne tre af produkterne er formaldehyd målt i relativt høje koncentrationer. Formaldehydafgivelsen falder med tiden, og det vil derfor være ved starten af brugen af teltene, at den største potentielle sundhedsfare forekommer. 1 Introduction1.1 BackgroundTents and tunnels exist on the market, with which children can play indoor as well as outside. A German survey (Öko-Test, 2002) identified a long list of chemical substances in tents and tunnels, and children can thus be exposed to these substances. The German survey analysed for total VOC, chloro-organic substances, tin compounds, dyestuffs, lead and antimony. One would also expect to find substances such as formaldehyde. It is expected that tents and tunnels for children are produced from textile materials, such as woven fabric made by cotton, polyester, nylon or polypropylene. The materials can probably be coated with synthetic polymers like polyurethanes (PUR), or polyvinyl chloride (PVC). Further the products will normally be dyed and/or printed, and it is likely that they have been subjected to various kinds of finishing to avoid water penetration, soiling, rot and fungi as well as ignition. Finally the products probably will include non-textile materials such as wood, leather, metal and plastic. Children can be exposed to the chemical substances in various ways, but this project is limited to exposure due to inhalation. This means that the substances, which have been looked for, must be able to be released from the material to the air, which in turn can be inhaled by the children. 1.2 PurposeThe purpose of the project has been to clarify the possible risks that might be linked to the staying in tents and tunnels for children, and possibly give recommendations as to what could be done to avoid or counteract such risks. Further it should be clarified whether possible release of chemical substances would decline with time. 1.3 ProcedureThe project has be divided into three phases: Phase 1: Survey of products on the Danish market. Phase 2: Determination of release of chemical substances from selected products, partly at room temperature, partly at elevated temperature. Phase 3: Screening of health effects and evaluation health risks. 2 Survey of tents and tunnelsA survey has been carried out of tents and tunnels for children on the Danish market, partly regarding the amounts sold, partly from which the products are produced and which finishes they are subjected to. As we are dealing with toys, they carry a CE-label. By way of introduction it must be mentioned that it has been very difficult to get information of these toy products, tents and tunnels. It has thus not been possible to find literature, which specifically deals with these products. The survey of the materials and contents in the products thus originates from a general knowledge about textile products and their manufacture, and partly from the product information, which could be obtained from the importers about the products selected for the analyses. Further it has not been possible through official statistics to find figures of turnover about these specific products. Such degree of detail does not exist in material from Statistics Denmark. The figures of the turnover thus originates from kind suppliers and retailers, and the summation and conclusions thus must be read with all possible reservations. 2.1 Trade conditionsTents and tunnels for children are primarily sold in toyshops, but they have also been identified in certain department stores for furniture as well as other major grocery department stores. In the toyshops they are normally standard goods, which are always there in spite of a certain seasonal variation. This is true for the furniture department stores as well, whereas in the grocery department stores they more have the character as special offers during the season. The trade has been contacted in various ways in order to illustrate the questions about the concrete products and their manufacture. The Association of Danish Toy Traders (Danmarks Legetøjshandlerforening) was contacted without success, and later information has made it likely that the activities of the association have ceased. The Common Council of the Toy Trade (Legetøjsbranchens Fællesråd) formerly represented both manufacturers, wholesalers and retailers. Following a recent restructure the Council is now linked with Danish Commerce & Services (Dansk Handel & Service) and today only represents wholesalers and retailers. Toy manufacturers are today organised in the Association of Toy Manufacturers in Denmark (Foreningen af Legetøjsfabrikanter i Danmark (FLD)) with secretariat at European Advisers (Europa-Konsulenterne). None of these organisations has, however, been able to supply further information for this project. According to the telephone directory seven toy factories exist in Denmark. After contact to each of them it became clear that none of them produces tents and tunnels. Later information has confirmed that all these kind of products are imported form foreign countries. According to the telephone directory under the category "Toys, wholesale" 39 companies exist. By looking at the names one can identify a part, which obviously do not supply tents and tunnels. The remaining 28 were contacted, and out of these 28 answers were received from 12, of which 10 indicated that they do not supply tents and tunnels. Only two companies supply such products, and they readily gave information about their products and turnover. 2.2 Figures of turnoverBased of information mentioned in the previous chapter and information from a furniture department store the following picture can be drawn of the turnover of tents and tunnels for children in Denmark. The three companies together sell a total of about 30 different products with total annual sale of 40 - 44,000 pieces. Assuming that these thre companies cover 80% of the market it leads to a total annual sale of 50 - 55,000 pieces. The figures must as mentioned earlier be read with all possible reservations, as they are based on information from only a few suppliers. The products can roughly be divided into three different types as indicated in table 2.1 below. Table 2.1. Types of tents and tunnels and their turnover
The prices of the products vary from 125 to 140 DKK for the most wide spread igloo and Indian tents. Tunnels, which can be linked to the tents, cost between 115 and 120 DKK. The more special playhouses and pop-up tents cost from 200 to 350 DKK. The total annual turnover is supposed to be between 6.8 and 7.2 million DKK. 2.3 Production2.3.1 ManufactureAs mentioned all products within the category are imported goods, and typically the information given about the products are quite scarce. The products typically consist of a tent canvas, which makes up the largest part of the product both in area and weight. In the products identified on the market the tent canvas consists of either cotton, nylon or polyester, where nylon is the predominant type. In many of the products there will be a bottom, which can consist of the same material as the canvas, but more often will be made from another material. In the products identified on the market the bottom consist of either nylon, polyethene (polyethylene, PE) or polyurethane (PUR). In many products finally poles, ropes, pegs etc. will be present. Area wise they will, however, constitute a very small part, so it must be presumed that with regard to release of chemicals, which is the main objective of the study, the canvas and the bottom must be by far the most important source. The products are most often dyed and/or printed in bright and strong colours, and it is presumed that dye auxiliaries and print paste may have contained chemicals, which are subject to release. Further a part of the products are impregnated, primarily to make them water repelling or water proof. Such impregnation might cause chemical release. A couple of the products are allegedly equipped with a UV-filter, the nature of this filter not being revealed in the first place. 2.3.2 Materials2.3.2.1 CottonCotton is a vegetable fibre, which is grown in tropical and sub-tropical regions. It is a crop, which demands large amounts of water and fertiliser, and to ensure a good yield often quite large amounts of pesticides are used during growing, as the plant easily gets attacked from pests and diseases. In the EU eco-label, the Flower, requirements for the maximum content of pesticides in cotton exist, but it is not very likely that there will be traces of pesticides in a finished fabric, which could degas from the final product. 2.3.2.2 Nylon (PA)Nylon or polyamide is a synthetic fibre, which is manufactured on the basis of crude oil. Two types exist, PA 6 and PA 6.6; but for the use in this connection it is not likely to play a role, which type of fibre we are dealing with. In the EU eco-label, the Flower, requirements for the maximum emission of N2O during monomer production, but this requirement is not relevant for the use of nylon in products in this study. 2.3.2.3 Polyester (PES)Polyester is a synthetic fibre, which is manufactured on the basis of crude oil. Traditionally antimony compounds are used as catalysts during polymerisation, and a VOC emission is also taking place during polymerisation. In the EU eco-label, the Flower, requirements regarding both of these aspects exist, but they are not presumed to have any importance in relation to degassing of chemicals from tents and tunnels. 2.3.2.4 Polyethene (PE)Polyethene or polyethylene is a synthetic plastic material, which can exist either as fibres or as foil. PE might contain plasticisers, which might degas from the final products. 2.3.2.5 Polyurethane (PUR)Polyurethane is a synthetic plastic material, which might contain starting materials from the synthesis (cyanates) or plasticisers, which might degas from the final products. 2.3.2.6 Dye auxiliariesCertain dye auxiliaries might be present in the finished fabric and might degas at a later stage. This might be the case for the so-called carriers in polyester dyeing, which might be chlorinated organic compounds. 2.3.2.7 Printing auxiliariesCertain print pastes for textile printing may contain volatile organic substances. The print paste itself may be based on white spirit, or certain auxiliaries can have a certain content of VOC's. Print which is based on white spirit is no longer common in the western world, but can exist in Eastern Europe or the Far East. 2.3.3 FinishingA large number of finishing products for textile products exists. Their purpose is to equip the textile product with a specific property. Regarding tents and tunnels it seems that water repellents are quite common. Water repellent impregnation can be based on wax or paraffin emulsions or silicones (polysiloxanes). During curing of some of these products formaldehyde might be produced or cleaved off, which the in turn can degas. Some products are equipped with UV filter, the chemical nature of which is not revealed. Such impregnations can be based upon heterocyclic compounds or dispersions of substituted benzotriazol compounds. Certain fibres, e.g. viscose, may be equipped with special pigments, which give a UV protecting effect. Some products are allegedly fire retarding. A large number of fire retarding products for textile or plastic materials exist. It may often be phosphorous compounds or various metal salts. Certain synthetic textile fibres have already during fibre production been equipped with fire retarding chemicals. Common for most of these fire retarding chemicals is that they do not consist of volatile compounds, but during curing of some of these products formaldehyde might be produced or cleaved off, which the in turn can degas. Tents and tunnels might also have been impregnated against rot and fungi or other microbial attacks. Such products could be coppernaphtenate, copperoxichinolate, cadmiumselenide, pentachloro compounds, dimethyl dithiocarbamate, dichloro diphenylmethane, tetramethyl thiuramidsulphide, salicylanilide, trialkyltin compounds or organic mercury compounds. Also quaternary ammonium compounds have been used. There is, however, nothing that indicates that the selected products have been equipped with such finishes. 2.4 Choice of products for analysesApproximately 30 products were identified on the market within the category tents and tunnels. From those the following six products were selected for analyses. The information in the table originates from the suppliers. Table 2.2 Products for analyses
The products have been selected to cover the most sold types and the most used materials. Further products are included both woth and without alleged impregnation. The Danish suppliers had no immediate detailed knowledge of the chemical products used for the impregnations mentioned in the table. The selected products cover about 31,000 pieces of the 50 - 55,000 pieces sold annually, or 56 - 62% of the annual turnover. Further most of the products, which were not selected, consist of the same or similar materials. 3 Emission testing
This chapter brings the results of the analyses of degassing, which was carried out with the six selected products. The selected tests and methods of analysis are described below. The methods have been selected based upon the future toy standards. 3.1 Methods of analysis3.1.1 Headspace analysesThe release at elevated temperature has been determined by static headspace analysis. The sample was received in a Rilsan bag. A weighed sample (1 - 2 g) was transferred to headspace glass (22 ml). The sample was heated to 100oC for 1 hour. A gas sample (0.5 ml) was taken with a gas squirt and analysed by gas chromatography combined with mass spectrometry (GC-MS). The components were identified by comparing the actual mass spectres with spectres from the NIST 98 Library. The fraction of each component of the total VOC content is given as area percentage, assuming that all detected components have the same response for the same amount. Detection limit: 0.2 - 1 ng/l. Standard deviation is assumed to be 10 - 20% 3.1.2 Climate chamber analysesThe release at room temperature was been carried out in a climate chamber with controlled atmosphere and air change. The general principle of emission measurements in climate chambers is that the sample, from which the emission is to be analysed, is placed in a climate chamber at standard test conditions. Gasses and vapours released from the sample are mixed with the chamber air. Air samples are taken at fixed times and analysed by means of chemical analysis techniques. On a warm summer's day with temperatures above room temperature the emission will often be larger. On the other hand the tents will often be used outdoor resulting in a larger air change. It is thus difficult to say anything more concrete about the importance for the concentration of the compounds identified. Air samples have been taken on Tenax tubes (VOC) and dinitrophenylhydrazine tubes (aldehydes). The selected tents are placed in complete, unfolded but not pitched condition with all poles in the climate chamber. Samples are taken after 1½ hours, 3 hours and 3 days. Then the samples are folded and kept in original packaging until the 9th day after start. Samples are then taken after 10 days. Then the samples are folded and kept in original packaging until the 27th day. Samples are taken after 28 days. In this way one can partly simulate the immediate impact on a child, and partly find out how the emission changes with time. Usually accepted standard conditions for emission testing have been applied (prEN 717-1, 2002):
3.1.2.1 VOCThe Tenax filters (Tenax TA - approx. 200 mg) were analysed by thermal desorption at 300oC followed by analysis on GC-MS-SCAN (29-450 amu screening analysis) according to ISO/DIS 16000-6.2. The components were identified by comparing the actual mass spectres with spectres from the NIST 98 Library. The amount of each component was determined towards external standards of corresponding components (chemical composition and boiling point). Detection limit: 2 - 5 ng per component, corresponding to 0.3 - 1 mg/m³. Standard deviation is assumed to be 10 - 15%. 3.1.2.2 AldehydesDNHP-filters (Supelco LP DNPH S10) were extracted with acetonitrile and the extract was analysed with HPLC-UV. The aldehydes were identified and quantified towards external standards. Detection limit: 0.03 µg per component, corresponding to 1 µg/m³. Standard deviation is assumed to be 10 - 15%. Blind values for the empty chamber were analysed before testing, and unexposed tubes have been analysed together with test tubes. 3.1.3 Testing of formaldehyde release inside tentAs it showed that some of the tents (product B, C and D) released larger amounts of formaldehyde than others during the tests in the climate chamber, it was decided to test product B and C again. The tents were pitched in a room with known atmosphere, and the formaldehyde concentration was measured inside the tent during a period of 3 days by means of an automatic formaldehyde analyser (Skalar Monitor 9000). The concentration in the reference air was measured during the same period. Through this one can simulate the impact on a child during a longer period of stay in a tent pitched for 3 days. 3.2 Results of headspace analysesResults of analyses for products A - F are shown in table 3.1 to 3.6. 3.2.1 Product ATable 3.1 Results of analyses for product A
3.2.2 Product BTable 3.2 Results of analyses for product B
3.2.3 Product CTable 3.3 Results of analyses for product C
3.2.4 Product DTable 3.4 Results of analyses for product D
3.2.5 Product ETable 3.5 Results of analyses for product E
3.2.6 Product FTable 3.6 Results of analyses for product F
3.3 Results from climate chamber study3.3.1 Product ATable 3.7 Results of analyses for product A in µg/m³
3.3.2 Product BTable 3.8 Results of analyses for product B in µg/m³
3.3.3 Product CTable 3.9 Results of analyses for product C in µg/m³
3.3.4 Product DTable 3.10 Results of analyses for product D in µg/m³
3.3.5 Product ETable 3.11 Results of analyses for product E in µg/m³
3.3.6 Product FTable 3.12 Results of analyses for product F in µg/m³
For most of the substances the concentration decreases with time, such as would be expected. For some of the products a higher concentration has been measured for certain substances after 28 days than after 10 days. This is the case for formaldehyde (product B, C, D and E), dimethylformamide (product A), xylenes (product B, D, E and F) and acetone (product F). Regarding formaldehyde the concentration is higher after 28 days than after 10 days for sample B, C, D and E, where the content is already relatively high. The reason for this could be that during the period between analyses the tents are packed, and during this period formaldehyde can be released ready for degassing, which is then measured after 28 days. For the other substances there is no immediate explanation. The results are further subject to comments in chapters 4 and 5. 3.4 Results from the study of release of formaldehyde inside tentTwo tents were studied in the way described in chapter 3.1.3, i.e. product B and product C. The concentration of formaldehyde was measured from the time the tents were pitched and during 3 days. As the concentration was neither decreasing nor increasing systematically, the table only shows the interval in which the concentrations were found. Table 3.13 Results of formaldehyde release inside tent µg/m³
The measurements show that the concentration inside the tents at no point of time is significantly higher than in the surrounding room (reference). The limit for indoor climate for formaldehyde is 0.15 mg/m³ (or 150 µg/m³). The results are subject to further comment in chapters 4 and 5. 4 Screening of health effects
In the present chapter a screening is presented for the health effects of the substances, which have been detected in the test programme. The results of the analysis clearly show that the maximum concentration of the emitted substances from the test material has been measured after three hours. Regarding the time-depending emissions, the concentrations are lower both before and after the three hours. Therefore, the screening is based on the three-hour results. Not all the substances have been found in the same tests. Table 4.1 shows an outline of the substances and the concentrations that have been found in all the six tests measured three hours after the tents and tunnels have been unpacked. 4.1 Method for screeningThe object of the screening is to point out the substances that may cause a potential health risk for children when playing with the products. Each substance is found in more than one product. In the screening, the highest measured concentration of each substance has been used for a conservative evaluation. It has been checked whether the substances are included in the list on dangerous substances (the Danish statutory order no 439, 2002) or the guideline of self-classification of dangerous substances of the Danish EPA. As for the substances for which threshold limit values (TLV) in the working environment have been determined, the values have also been used (At-vejledning C.0.1, 2002). The substances for which the highest concentration constitutes less than 1 % of the TLV and for which no long-term effects have been detected sorted out at first. For a number of the substances no classification or no TLV has been determined. In table 4.1 these substances are registered with "*". It indicates that there is no TLV for substance or the substance is not classified or both the priors mentioned. The screening of these substances is discussed in section 4.2. Table 4.1 Outline on the measured substances and their concentrations after three hours in the climatic chamber
4.2 Screening of substancesAll the substances in table 4.1 have been screened. In table 4.1 the classification of the substances appearing on the list on dangerous substances is listed. Furthermore, it has been monitored whether a TLV has been determined. If neither a TLV nor a classification of a substance have been determined a screening has been carried out in anticipation of finding supplementary data on substances that, in structure, are similar to the substance in question. The substances, for which supplementary data have been retrieved, are marked with an "*" in table 4.1. 4.2.1 Supplementary data4.2.1.1 1,2-PropandiolThe substance 1,2-propandiol with CAS-no 4254-15-3 is not classified and no TLV has been established. CAS-no 4254-15-3 represents (S)-(+)-1,2-Propanediol. The CAS-no 57-55-6, representing 1,2-Propanediol or propylene glycol, has been used in the additional search. Propylene glycol has no limit value; but an average eight hour mean limit of eight hours at 50 ppm has been retrieved (AIHA 1999) (Workplace Environmental Exposure Level). The limit corresponds to 155 mg/m³. Ethylene glycol has a limit value of 26 mg/m³. In the present screening it is assumed that propylene glycol has a TLV at a level corresponding to 50 mg/m³. 4.2.1.2 1-Butoxy-2-propanolThe substance 1-butoxy-2-propanol with CAS-no 5131-66-8 is classified to be irritating to eyes and skin (Xi;R36/38). No TLV has been established for the substance. Its structure resembles the substance butoxyethanol (CAS-no 111-76-2), that has a TLV of 98 mg/m³ and with the comment ”may penetrate the skin”. In the present screening it is therefore assumed that 1-butoxy-2-propanol has a TLV at a level corresponding to 100 mg/m³. 4.2.1.3 2-Ethyl-1-hexanolThe substance 2-ethyl-1-hexanol has CAS-no 104-76-7. Official classification of the substance does not exist nor has the substance a TLV. The substance is a C8-alcohol. To a certain extent the alcohol 1-octanol with CAS-no 111-87-5 is similar to the substance. As for 1-octanol AIHA stated in 1999 an average eight hours mean value to be 50 ppm per workday (8 hours)(Workplace Environmental Exposure Level). Such a limit corresponds to 266 mg/m³. The substance 1-hexanol (CAS-no 111-27-3) is classified Xn; R22. In Gosselin et al., 1976, it is stated that 2-ethylhexanol is similar to butyl alcohol regarding toxicity. As a conservative preliminary assessment of the substance the following classification is therefore used for butanol (R10 Xn;R22 Xi;R37/38-41 R67). 4.2.1.4 3-Caren and α-pineneThe substance 3-caren (CAS-no 13466-78-9) is a double-cyclic compound with seven carbon atoms and three methyl groups. α-pinene is also a double-cyclic compound with seven carbon atoms and three methyl groups. The substances are described as terpenes. Both compounds are very rarely described in the literature. aα-pinene is classified according to the guideline of the Danish EPA as hazardous to the environment with N; R51/53, toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Carene and pinene resembles in their structure to a certain extent to turpentine. Turpentine has CAS-no 8006-64-2 and is classified as hazardous to the health and hazardous to the environment (R10 Xn; R20/R21/22-65 Xi; R36/38 R43 N;R51/53). Turpentine has a limit value of 140 mg/m³. The terpene limonene (CAS-no 138-86-3) is also classified as R10 Xi; R38 R43 N; R50/53. It is therefore assumed that carene and pinene are hazardous to the health, may cause allergic reactions and are hazardous to the environment. Data on turpentine are used in the following assessment. 4.2.1.5 4-Methyl-1-pentanolThe substance 4-methyl-1-pentanol has CAS-no 626-89-1. The substance is not classified and it is assumed in the present connection to have properties similar to those of 4-methyl-2-pentanol (108-11-2). This substance is classified R10 Xi; R37, and the classification is used in the present screening. Pentanol has as a TLV of 360 mg/m³. As methyl pentanol is not likely to have a lower limit value than pentanol, the limit value of pentanol is therefore used in the present screening. 4.2.1.6 BenzaldehydeBenzaldehyde (100-52-7) is classified as Dangerous to the Health, Xn; R22. The substance has no Danish TLV, but AIHA 1999 indicates an average eight hours mean value of 2 ppm (Workplace Environmental Exposure Level). Such a limit corresponds to 8 mg/m³. This TLV is used in the present screening. 4.2.1.7 2,6-Di-tert-butyl-p-cresolThe substance 2,6-di-tert-butyl-p-cresol also called BHT has CAS-no 128-37-0. As the substance is not classified the substance is assessed as cresol in the present screening. Cresol is toxic and corrosive and is classified T; C, R24/25-34. The limit value for cresol is 22 mg/m³. 4.2.1.8 Aliphatic carbon hydridesIn the analyses a number of not further identified aliphatic carbon hydrides have been detected with chain lengths of C10 to C16. They can be linear or branched and have one or more double bonds. The analysis showed that the carbons hydrides do not consist of aromatic compounds. On the list on dangerous substances the group alkanes, C12-26, is mentioned. The group is given the CAS-no 90622-53-0 and index-no 649-242-00-4. Group is classified Carc2; R45. It is also stated that the category described as hydrocarbons, C9-16, hydrogen treated and de-aromatised. The CAS-no 93763-35-0 is stated and an index-no 649-429-00-0. The classification is Xn; R65. As the analyses show the hydrocarbon fraction does not contain aromates but whether or not the substances may be carcinogenic, cannot be determined. At present it is therefore assessed that substances may be carcinogenic. As it has not been possible to distinguish between the different substances, the fraction in question, C10-C16, is assessed as one compound. The substance tetramethyl octane may be 2,2,7,7-tetramethyloctan. The molecular formula is C12H26 and can therefore be described as being part of the group of aliphates C12-16. The compound of tetramethyl octane is therefore assessed along with the other aliphatic carbon hydrides. Solvent naphtha is a hydrocarbon with a boiling interval 150-205°C. It resembles aliphatic hydrocarbons with a carbon chain of about C10-16. Solvent naphtha has CAS-no 8052-41-3, index-no 649-345-00-4 and the classification is R10 Carc2; R45 Xn; R48/20-65. Solvent naphtha has a TLV of 145 mg/m³. In the present report these data are used as the basis for the assessment. 4.2.1.9 AldehydeIn the analyses a number of aldehydes have been detected. They are shown in table 4.2. Table 4.2 Aldehydes and their properties
Aldehydes are generally irritating to skin and eyes and may be irritating to the respiratory system. A part of the aldehydes can be used as additives for food. As for propanal, no risk occur to the central nervous system and the blood after inhalation of concentration less than 0.5 mg/m³ (Tokanova, 1982). The substance butanal is classified as very combustible with F; R11. The substance has no Danish TLV, but AIHA 2001 indicates an average eight hours mean limit of 25 ppm (Workplace Environmental Exposure Level) Such a limit corresponds to 74 mg/m³. Pentanal is also described as valeraldehyde and has a TLV of 175 mg/m³. Saturated aldehydes as pentanal and hexanal are not toxic (Kaneko, 1988). No TLVs for hexanal, heptanal, octanal, nonal and decanal have been detected. Nonal and decanal is included in the guideline for dangerous substance of the Danish EPA and is classified as Dangerous to the Environment. Nonal is classified N; R50, and decanal is classified N; R50/53. For the preliminary screening a TLV of 0.5 mg/m³ for propanal, 74 mg/m³ for butanal, and 175 mg/m³ for other aldehydes is used. 4.3 Potentially problematic substances in the screening4.3.1 Selection of potentially problematic substancesThe substances, detected in the analyses, which are described as potentially problematic substances, are shown in table 4.3. The data, emphasised by a grey colour in table 4.3, are estimated data. The arguments for the estimations are described in subsection 4.2.1. Table 4.3 includes the substances, known for having or under suspicion of causing long-term effect and/or where the measured concentration constitutes more than 1 % of the TLV. An estimated TLV for aliphatic hydrocarbons C10-C16 is shown in table 4.3. The hydrocarbons are solely assessed based on the fact that they may be carcinogenic. At the same time they appear in the analyses in a relatively high concentration. Table 4.3 List on potentially problematic substances
As shown in table 4.3 the detected concentrations for dimethyl formamide, formaldehyde and hydrocarbons C10-C16 and phenol are relatively high. Compared with the TLVs the measured concentrations represent more than 1% of the TLV of
Among the selected substances the group of hydrocarbons, aliphates with carbon chains at C10-16 are classified as carcinogenic in category 2 (Carc2). Ethoxyethanole is teratogenic (Rep2; R60-61). Dimethyl formamide is also teratogenic (Rep2; R61). Among the selected substances the following substance are under the suspicion of causing cancer (Carc3; R40):
The substances α-pinene and 3-caren may cause sensitisation by skin contact. (Xn; R43). Phenol is toxic and corrosive. The substance 2,6-di-tert-butyl-p-crecol has probably the same properties and therefore both substances are mentioned. In addition the following substances are selected as being significant as agreed with the Danish EPA:
4.3.2 The existence of potentially problematic substancesThe preliminary screening is based on the highest measured values. Table 4.4 shows in which products the substances are found and in which concentrations. In analyses of 3-caren and α-pinene blind values (background values) between 1 and 3 µg/m³ have been measured. It indicates that the measured values of these substances of less than 5 µg/m³ may be considered uncertainty/pollutants As for the hydrocarbons C10-C16 blind values between 10 and 20 µg/m³ have been measured implying that the blind values do no significant influence in the interpretation of the results. The blind values of formaldehyde, dimethyl formamide and 2-butoxyethanol are below 1 µµg/m³. The results of analyses shown in table 4.4 are adjusted according to the blind values. Table 4.4 Overview of results of the analyses on selected substances.
In sample A, the substances detected are primarily dimethyl formamide, 2,6-di-tert-butyl-p-cresol, formaldehyde and aliphatic hydrocarbons. In addition acetaldehyde has been detected, which is under the suspicion of causing cancer, and 3-caren and α-pinene, which cause sensitisation by skin contact. In sample B a high concentration of formaldehyde and aliphatic hydrocarbons have been detected. In addition 3-caren and α-pinene have been detected as well, which may cause sensitisation by skin contact. In sample C high concentrations of formaldehyde and aliphatic hydrocarbons have been detected. In addition acetaldehyde has been detected, which is under suspicion of causing cancer. Sample D is characterised by high concentrations of 2-Butoxyethanol, formaldehyde and aliphatic hydrocarbons. Dimethyl formamide, which is present in relatively small quantities, is teratogenic. Likewise small quantities of acetaldehyde and tetrachloroethylen, which are under the suspicion of causing cancer, have been detected. Sample E is characterised by a certain concentration of aliphatic hydrocarbons, which are carcinogenic, and of dimethyl formamide, which is teratogenic. Likewise formaldehyde and trimethylcyclohexen-1-on, which are under suspicion of causing cancer, have been detected. Sample F is primarily characterised by high concentrations of aliphatic hydrocarbons, which are carcinogenic. 2-Ethoxyethanol, which is present in a certain quantity, and dimethyl formamide, which is present in a rather low concentration, are both teratogenic. Formaldehyde, which is under the suspicion of causing cancer, appears in a relatively low concentration. In addition α-pinene, which may cause sensitization by skin contact, is present. 4.4 Non problematic substances in the screening of effects on the healthThe substances that have been detected as insignificant in the screening among all the identified substances are shown in table 4.5. The values based on assessments are emphasised with a grey background in table 4.5. The assessment is mentioned in subsection 4.2.1. Table 4.5 Overview on less significant substances detected in the screening
As shown in table 4.5, none of the listed substances are known or under suspicion of having any long-term effects on the health. In addition, the highest measured concentrations pose less than 1% of the limit value. The substances shown in table 4.5 will not be assessed any further. 5 Assessment of problematic substances
5.1 Method of assessmentThe purpose of assessing the substances is to decide whether children may be affected by using the analysed products. The assessment is based on a worst case scenario. The principles for the assessment of health effects are based on EU's revised Technical Guidance Document (TGD) for risk assessments. The results of the analyses show the quantities of the substances in question, which are released from each product. The quantity that a child may be exposed to by inhalation is assessed. For those substances for which it has been possible to retrieve data for NOAEC (No Observed Adverse Effect Concentration) and/or LOAEC (Lowest Observed Adverse Effect Concentration), a direct comparison has been made with the measured concentrations. The quantity that a child may absorb has been estimated. Then the estimated value is compared with the data on the substance in question concerning the NOAEL level – No Observed Adverse Effect Level and LOAEL – Lowest Observed Adverse Effect Level or other relevant data indicating permanent effects or other relevant data as far as it has been possible to retrieve it. The quantity of the substance that a child may absorb is estimated based on the following conditions. It is assumed that a child may have a body weight as low as 10 kg. The assumption is conservative has been retrieved from the TGD. It is assumed that a child is exposed one hour per day and the focus is on absorption by inhaling the substance. An assessment is made based on the highest measured concentration occurring three hours after the product has been unpacked. If it gives reason to assess the substance further the other analysis results measured at 3, 10 and 28 days after the product has been unpacked will be taken into account. The quantity of the inhaled substance can be determined according to the guidelines in TGD (2002) using the formula:
where:
The part of absorbed or respirable fractions, Fresp, is fixed at a given fraction if data are available. If not the fraction is fixed at 1. The inhalation rate, Qinh, is fixed at 1.9 m³/hour indicating the inhalation rate at high activity (TGD, 2002). The duration of exposure is fixed at 1 hour (Tcontact) and the number of times per day (Nevent) is also fixed at 1. The body weight of a child is fixed at 10 kg. Based on the given assumptions [formula 1] can be reduced to: Iinh [ mg/kg BW/day] = 0.19 • Fresp • Cinh [ mg/m³] 5.2 Assessment of each substance5.2.1 2-Butoxyethanol5.2.1.1 Identity
The boiling point of the substance is 171-172C (Budavari, S., 1989) and its melting point is -70C (American Conference of Governmental Industrial Hygienists; 1986). The vapour pressure of the substance is 0.88 mm Hg at 25C (Dow Chemical Company; 1990). 5.2.1.2 Detected quantitiesAfter three hours significant quantities of 2-butoxyethanol - 153 g/m³ - have been found in sample D. Regarding the other samples the concentration is below 10 g/m³ after three hours. For sample D concentration of the substance falls relatively quickly so that after 3, 10 and 28 days it is about 10 g/m³. It is therefore assessed that only during the first day of using the tent concentrations above 100 g/m³ may occur. 5.2.1.3 Classification2-butoxyethanol is included on the list on dangerous substances and is classified under EU index no 603-014-00-0 (List over dangerous substances, Miljøministeriet 2002):
5.2.1.4 Health conditionsIn an animal experiment it is shown, that the substance is absorbed quickly by inhalation, if swallowed or in contact with skin and that it is decomposed to 2-butoxyacetaldehyd and 2-butoxy acetic acid. The primary effect is that the substance and its metabolites are hematoxic (affect the haemoglobin in blood). In rats the effects detected in the central nervous system, liver and kidneys are permanent at high concentrations (the source does not mention the quantity). The substance does not cause sensitisation and no data indicate that the substance is mutagenic (World Health Organization, 1998). In a 13 weeks experiment prepared by NTP (1993) rats were tested. Damages on the blood and the bone marrow were observed and the LOAEL was determined to be between 69 and 82 mg/kg/day. A value for NOAEL was not determined. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a value for TDI (tolerable daily intake) of 0.05 mg/kg is stated. 5.2.1.5 ExposureAs for 2-butoxyethanol it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.19 • 1 • 0.153 mg/m³ = 0.03 mg/kg BW/day. The determined LOAEL is more than 1000 times the estimated inhaled quantity. The TDI-value is a little above the estimated value. It is therefore assessed that exposure of the substance in the mentioned concentrations has a minimal influence on the health. 5.2.2 2-Ethoxyethanol5.2.2.1 Identity
The boiling point of the substance is 135°C (Lide, D.R., 1994-1995) and its melting point is -70°C (Kirk-Othmer Encyclopedia of Chemical Technology, 1980). The vapour pressure of the substance is 5.31 mm Hg at 25°C (eksperimentally)(Daubert, T.E., R.P. Danner, 1989). 5.2.2.2 Detected quantitiesOnly in sample F 23 µg/m³ of the substance was detected after three hours. After three days the concentration was as low as 6 µg/m³ and after 10 and 28 days concentrations of 2 µg/m³ or less were measured. 5.2.2.3 ClassificationEthoxy ethanole is included in the list on dangerous substances and classified under EU index no 603-012-00-X (List on dangerous substances, Miljøministeriet 2002):
5.2.2.4 Health conditions2-ethoxyethanol may be slightly irritating in contact with eyes and mucous membrane, however, there are no indications saying that it may be irritating in contact with skin (Clayton, G. D. and F. E. Clayton (eds.)1981-1982). The source does not mentioned at which concentrations of the substance the assessment is related to. The lethal dose for humans is about 1.4 ml/kg conforming with about 100 ml for a human weighing 70 kg (Amdur et al., 1991). The effects of 2-ethoxyethanol, which influence the central nervous system, are headache, fatigue, dizziness, grogginess, slurred speech and behaviour changes. (Hamilton, A., and H. L. Hardy, 1974). An experiment shows that humans exposed to up to 88 mg/m³ of 2-ethoxyethanol had significantly lower production of sperm than the control group. This occurred even though the exposed test persons and the control group had a lower quantity of sperm than other groups of employees (American Conference of Governmental Industrial Hygienists, 1991). In the IRIS-database it is stated that data on inhalation toxicity is assessed with some reservations (Medium confidence), as the most significant experiment was only of short duration. Subchronic inhalation tests indicate that teratogenic toxicity is the most sensitive endpoint. However, no chronic tests have been retrieved in the available literature. Barbee et al., 1984, has carried out a subchronic inhalation test with rats and rabbits. The NOAEL for rats was determined to be 265 mg/m³, based on observed modifications in the weight of the hypophysis in male rats and diminished spleen in female rats. In experiments with rabbits the NOAEL was determined to be 68 mg/m³ and the LOAEL to be 265 mg/m³, based on the damages such as modifications in the content of haemoglobin and the haematocrit values and weight loss. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a value for TDI (tolerable daily intake) of 0.05 mg/kg is stated. 5.2.2.5 ExposureThe measured concentration of 0.023 mg/m³ can directly be compared with a NOAEC of 68 mg/m³. As for 2-ethoxyethanol it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.19 • 1 • 0.023 mg/m³ = 0.004 mg/kg BW/day The potentially absorbed quantity of 0.004 mg/kg is considerably lower than the TDI value of 0.05 mg/kg. It is therefore assessed that there are no significant effects on the health under the present circumstances, even though the substance is known to have teratogenic effects on humans. 5.2.3 3-Carene5.2.3.1 Identity
It has not been possible to retrieve many data on 3-carene. Monoterpenes, which among others include limonene and pinene as well as carenes, are characterised as turpentine with CAS-no 8006-64-2. Turpentine consists chemically of 58-65% γ-pinene together with β-pinene and other isomer terpenes. Turpentine from wood, extracted from waste wood or sawdust, consists of 80% γ-pinene, 15% monocyclic terpenes, 1.5% terpen alcohol and other terpenes (Bingham et al., 2001). The boiling point of terpenes is about 154-170°C (Lewis, R.J., 1999), the melting point is about -50 to -60°C (Clayton, G.D., F.E. Clayton (eds.), 1993-1994) and the vapour pressure is about 5 mm Hg at 25°C for an unspecified mixture (National Fire Protection Association, 1978). 5.2.3.2 Detected quantitiesThe substance 3-carene has been detected in two of the samples, A and B in quantities of 23 µg/m³ and 11 µg/m³, respectively, after three hours. After 3 and 10 days the concentration of 3-carene had fallen considerably so that the concentration emitted from sample A had fallen from 23 to 4 µg/m³, and the concentration emitted from sample B had fallen from 11 µg/m³ to less than 1 µg/m³. 5.2.3.3 ClassificationThe substance itself is not classified. The classification is based on the group of turpentine, which is classified under EU index no 650-002-00-6 (List on over dangerous substances, Miljøministeriet 2002):
5.2.3.4 Health conditionsVapours are irritating in contact with eyes and respiratory passages. If vapours are inhaled they may cause head aches, vomiting, dizziness and faint. The fluid irritates the skin and if swallowed it will irritate the entire alimentary system and it may cause damages to the kidneys. If the fluid enters the lungs it will cause severe attack of pneumonia (Prager, J.C., 1996). The lethal dose of for turpentine if swallowed may be as low as 110 g. However, survival has been observed after having swallowed 120 g. Thus, no more than 15 g of the substance has been fatal for a child (Bingham, E. et al., 2001). In an experiment with male and female volunteers the following observations have been reported. People with an average age of 35 years were exposed to 0 or 450 mg/m³ of a mixture consisting of 10 parts α-pinene, 1 part β-pinene and 5 parts 3-carene (synthetic turpentine) for 12 hours 4 times over a two-week period. Acute damages to the lungs were observed. The male volunteers, exposed for two hours with 450 mg/m³ during easy workout, experienced their respiratory passages were influenced by the exposure and they had breathing difficulties after the termination of the exposure, E. et al., 2001). LCI (Lowest Concentration of Interest) is 250 µg/m³ for most of the turpentines, based on an inhalation study on humans with a NOEC for lung symptoms on 25 mg/m³, that is LCI = NOEC/11010 (Larsen et al., 1999). 5.2.3.5 ExposureComparing the measure concentration of 23 µg/m³ at the maximum with the detected LCI-value of 250 µg/m³ it shows that the observed level is 10 times less than the establish limit. It indicates that the liberated quantity of the substance does not have any influence on the health. 5.2.4 Alfaα-pinene5.2.4.1 Identity
The melting point of the substance is –62.5°C. The boiling point is 156°C (Furia og Bellanca, 1975). Vapour pressure is 633 Pa at 25°C (4.75 mm Hg) (Daubert og Danner, 1989). The water solubility is 0.65 mg/l at 250°C (FFHPVC, 2002). The distribution coefficient log Kow is experimentally detected to be 4.83 (Li og Perdue, 1995). 5.2.4.2 Detected quantitiesThe substance α-pinene is detected in the four samples, A, B, E, and F. The detected quantities is between 7 and 16 µg/m³ after three hours. The analysis results show that the emitted quantity of α-pinene is declining over time. The highest concentration of 16 µg/m³ was detected after three hours and reduced to 6 µg/m³ after 10 days. As for the other samples the emission was reduced to below 5 µg/m³ after 10 days. 5.2.4.3 Classificationα-pinene is primarily extracted from tree oils and other biological materials, but may also occur in mineral oil products. The substance is not classified under its own name, but belongs to the group of turpentine's, which are classified under EU index no 650-002-00-6 (List on dangerous substances, Miljøministeriet 2002):
5.2.4.4 Health conditionsα-pinene is primarily toxic if inhaled (Lewis, 1992) and severely irritating in contact with eyes, mucous membranes, and skin (Budavari, 1996; Lewis, 1992). Examples on effect levels are stated below. α-pinene is known to be contact allergen (Thomsen, 1990). Acute toxicity:
In the description of 3-carene (subsection 5.2.3) tests and experiments with turpentine are mentioned in which α-pinene is the main ingredient. LCI is 250 µg/m³ for most types of turpentine, based on a inhalation study on humans with a NOEC for lunge symptoms of 25 mg/m³, that is LCI = NOEC/1×10×10 (Larsen et al., 1999). 5.2.4.5 ExposureCompared with the mentioned inhalation study (LCI=0.25 mg/m³) the quantity of the substance emitted from the samples in the present study (maximum 0.016 mg/m³) does not give any reason for health risks. 5.2.5 Acetaldehyde5.2.5.1 Identity
Acetaldehyde has a boiling point of 21°C and a melting point of -123.5°C (Budavari, 1996). The vapour pressure is 902 mm Hg at 25°C (Boublik et al., 1984). 5.2.5.2 Detected quantitiesAcetaldehyde has been detected in sample A, C, D, and E in concentrations between 2 and 12 µg/m³ after three hours. Sample C, causing the highest concentration of 12 µg/m³ after three hours, was at 2 µg/m³ after three days and was not analysed anymore. As for the other samples the level was very low after three days. 5.2.5.3 ClassificationAcetaldehyde is included in the list of dangerous substances and is classified under EU index no 605-003-00-6 (List on dangerous substances, Miljøministeriet 2002):
5.2.5.4 Health conditionsThe TLV for acetaldehyde for working environment is 45 mg/m³ (At-vejledning C.0.1). The database IRIS retrieves two studies carried out by Appleman et al., 1986 and 1982, that determine NOAEC and LOAEC based on short-term experiments. The results showed a NOAEC of 150 ppm corresponding to 273 mg/m³ and a LOAEC of 400 ppm corresponding to 728 mg/m³. The experiments are based on damages to the lungs, which also occur at chronic experiments. Acetaldehyde is placed in group 2B by IARC, which may cause cancer in humans. It has been proven experimentally that the substance is carcinogenic in animals but the evidence is insufficient as far as the effects on humans are concerned (IARC, 1999). 5.2.5.5 ExposureIf the maximum measured concentration of 0.012 mg/m³ is compared with the detected values for NOAEC of 273 mg/m³ and LOAEC of 728 mg/m³ it appears that the quantity of substance liberated from the samples do not cause any health risk. 5.2.6 2,6-Di-tert-butyl-p-cresolThe substance has previously been researched in the project "Kortlægning af hygiejnebind" (Hazardous substances in sanitary towels) (2002). If nothing else is stated the data from that project have been used. 5.2.6.1 Identity
The substance has a boiling point of 265°C and a melting point of 71°C. The vapour pressure of the substance is 0.015 mm Hg at 20°C. 5.2.6.2 Detected quantitiesThe substance 2,6-di-tert-butyl-p-cresol has only been detected in sample A in a concentration of 50 g/m³. The concentration is slowly decreasing over time so that after 10 days it is 42 µg/m³ and after 28 days 30 µg/m³. 5.2.6.3 Health conditionsThe substance 2,6-di-tert-butyl-p-cresol is not classified and there is no TLV for the substance. In "Kortlægning af hygiejnebind" (2002) it is stated that the substance seems to be moderately toxic at oral intake and it has shown to cause allergic effects in humans in several cases. Furthermore, it is stated that the substance is under the suspicion of being a cancer promotor in skin. The survey concludes that the critical acute effect in humans is irritation in contact with skin and it is stated that NOAEL = 0.1 % in ethanol The critical acute effect in mice is breathing disorders and enlarged lungs after application on the skin. The results are: NOAEL = 145 mg/kg BW 5.2.6.4 ExposureFor 2,6-di-tert-butyl-p-cresol it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.09 × 1 × 0.050 mg/m³ = 0.01 mg/kg BW/day or 10 µg/kg BW/day. Assessing absorption by inhalation of the substance, it is seen that the potential absorbed amount is only a fraction of the establish limits. It should be noted that the circumstance about skin irritation is not assessed, as the quantity of the substance has not been detected in the actual toy. 5.2.7 Dimethyl formamide5.2.7.1 Identity
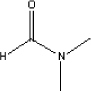
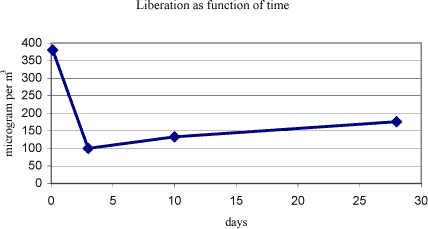
Dimethyl formamide has a boiling point of 153°C and a melting point of 61°C (Budavari S., 1996). The vapour pressure is 3.87 mm Hg at 25°C (Daubert T.E., 1989). 5.2.7.2 Detected quantitiesDimethyl formamide has been detected in all the samples. In sample A the concentration was high, 380 g/m³, in samples D and E the concentration was at a mean level between 20 and 25 µg/m³. For the other three samples, B, C, and F, the concentration was at low level between 1 and 5 µg/m³. The emitted concentration over time evolved quite unexpectedly regarding sample A. It is illustrated in figure 5.1. Figure 5.1 Concentration of dimethyl formamide in sample A as function of time
For sample D and E the concentration decreased rather quickly and was for both samples between 1 and 5 µg/m³ after three days. 5.2.7.3 ClassificationDimetyl formamide is included in the list on dangerous substances and is classified under EU index no 616-001-0X (List on dangerous substances, Miljøministeriet 2002):
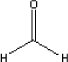
5.2.7.4 Health conditionsThe TLV for dimethyl formamide in the working environment is 30 mg/m³ (At-vejledning C.0.1). Several tests on humans exposed to dimethyl formamide indicate that the substance may contribute in developing cancer in the testicles. Tests on animals have not proven that the substance is mutagenic or carcinogenic (Ellenhorn et al., 1997). It has been proven that the substance cause damages to the liver. In IRIS-database two experiments have been retrieved for determination of NOAEC and LOAEC. One experiment was carried out by Cirla et al., 1984, and the other by Catenacci et al., 1984, and both experiments indicate damages to the liver. In the description of the experiments it is stated that it has not been possible to determine NOAEC. On the other hand LOAEC is determined to be 22 mg/m³. 5.2.7.5 ExposureIt is expected that NOAEC is below the value of LOAEC. The highest measured concentration is at 0.38 mg/m³ and is somewhat below LOAEC (and NOAEC). 5.2.8 FormaldehydeFormaldehyde is described in the project "Kortlægning af kemiske stoffer i tekstilmetervarer" (Hazardous substances in textiles), survey report no 23, 2003. If nothing else is stated data from that report have been used. 5.2.8.1 Identity
Formaldehyde is a gas at ambient temperature. The melting point is -92°C and the boiling point is -19°C. 5.2.8.2 Detected quantitiesFormaldehyde has been detected in all the samples. Three of the samples, B, C, and D, the concentrations are between 100 and 200 µg/m³ after three hours. For the other samples the level is between 15 and 25 µg/m³. For sample C, in which the concentration after three hours is determined to be 163 µg/m³, the concentration is halved after three days. The same effect was seen in sample B and D. For the other samples the level is also reduced to about the half of the concentration after three days compared with the level after three hours. Supplementary analyses have been carried out for two of the tents, namely sample B and C. The supplementary measurements include continuous measurement of the concentration of formaldehyde over three days inside and outside the tents. A reference measurement and two measurements of each of the two tents have been carried out. The measured concentrations are relatively constant during the three days and vary between:
These supplementary measurements show that the highest concentration is about 37 µg/m³ with a background concentration of about 25 µg/m³. 5.2.8.3 ClassificationFormaldehyde is included in the list on dangerous substances and is classified under EU index no 605-001-00-5 (List on dangerous substances, Miljøministeriet 2002):
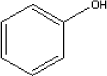
5.2.8.4 Health conditionsRegarding acute toxicity, inhalation tests with rats and mice show that LC50 is about 0.5 mg/litre. Inhalation tests varying from three days to two years showed NOAEC of 1.2 mg/m³ in rats with lesions of the nose epithelium. Contact with formaldehyde causes allergic reactions (allergy). The substance provokes especially allergic reactions by skin contact and by inhalation. Formaldehyde is under suspicion of causing cancer by inhalation in animal experiments. 5.2.8.5 ExposureNOAEC for lesions of the nose epithelium in rats is determined to be 1.2 mg/m³. In the first set of measurement the level for sample B, C, and D is between 0.1 and 0.2 mg/m³ and is therefore less than a factor 10 under NOAEC. This result indicates a potential health risk. For the supplementary measurement the NOAEC is about a factor 30 over the highest measured value of 0.037 mg/m³. 5.2.9 Phenol5.2.9.1 Identity
The boiling point of phenol is 182C and its melting point is 41°C. The substance has a vapour pressure of 0.35 mm Hg at 25°C. 5.2.9.2 Detected quantitiesPhenol has only been detected in sample D. The maximum concentration detected is 18 µg/m³ after three hours. After three days the concentration has been measured to 15 µg/m³ and after 10 days to 7 µg/m³. 5.2.9.3 ClassificationPhenol is included in the list on dangerous substances and is classified under EU index no 604-001-00-2 (List on dangerous substances, Miljøministeriet 2002):
5.2.9.4 Health conditionsFor phenol the TLV in the working environment is 4 mg/m³ (At-vejledning C.0.1). In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a TLV of 2 ppm (8.4 mg/m³) in the working environment is mentioned and an indoor value of 0.1 ppm (0.4 mg/m³) Phenol is toxic with a lethal dose of 50-500 mg/kg for humans. Some people may be hypersensitive, causing death or very serious effects after exposure to low doses. Phenol penetrates the skin and is quickly absorbed. Effects on the central nervous system, the hart, the blood stream, lungs and kidneys have been observed. The observed effects after short-term exposure may include shock, coma, delirium and death. Long-term or repeated exposure may result in damages to liver, kidneys and eyes. Changes in skin pigment have been observed. Inhalation may cause lung irritations and oedemas. Phenol is described in an IUCLID-data sheet from 2000. Among others the following it stated. Tests show that phenol does not cause sensitisation. In a 28-days test with mice oral intake show effects on the red corpuscles and the level of anti-substances in the blood. LOAEL is determined to 1.8 mg/kg body weight. A study of phenol in rats was performed (Argus Research Laboratories, 1997). The effects on the development of the offspring were tested and NOAEL was determined to be 60 mg/kg per day. A benchmark dose was calculated to be 93 mg/kg per day, and with a safety factor of 300 the reference dose was determined to be 0.1 mg/kg/day. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a TDI value of 1.5 mg/kg BW is stated. 5.2.9.5 ExposureThe maximum concentration observed is 0.018 mg/m³. It is considerably below the Danish TLV in the working environment and the mentioned value for concentrations indoors of 0.4 mg/m³. For phenol it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). The exposure can therefore be calculated to be as follows: Iinh [mg/kg BW/day] = 0.09 × 1 × 0.018 mg/m³ = 0.0034 mg/kg BW/day or 3.4 µg/kg BW/day. The estimated amount of phenol is considerably below the stated values for NOAEL and TDI (1.5 mg/kg) and there is therefore no indications that the potential observed uptake of phenol from sample D causes any effect on the health. 5.2.10 Tetrachloroethylene5.2.10.1Identity
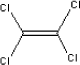
Tetrachloroethylen has a boiling point o 121.3°C and a melting point of -22.3°C. The substance has a vapour pressure of 18.5 mm Hg at 25°C. 5.2.10.2 Detected quantitiesLow concentrations of the substance have been observed in sample C and D. The maximum concentrations after three hours have been measured to be 1 µg/m³ in sample C and 2 µg/m³ in sample D. After three days the concentration in both for samples are below 1 µg/m³. 5.2.10.3 ClassificationTetrachloroethylene is included in the list on dangerous substances and is classified under EU index no 602-028-00-4 (List on dangerous substances, Miljøministeriet 2002):
5.2.10.4 Health conditionsThe TLV for tetrachloroethylene in the working environment is 70 mg/m³ (At-vejledning C.0.1). IARC, 1995, has assessed the carcinogenic effect of tetrachloroethylen and has reached to the conclusion that the substance may be carcinogenic. Heavy exposure of the substance has resulted in effects on the central nervous system, eyes, and skin, and to a lesser extent lungs, liver, and kidneys. The effects on the central nervous system have often been unconsciousness, dizziness, headache, hypersensitivity towards light, and depressions. Damages on human liver have been observed, normally after exposure to more than 100 ppm. At repeated exposures of humans with more than 200 ppm early signs of depressions have occurred while no effects have been observed on males and females exposed to 100 ppm for 7 hours per day. Clinical analyses indicate no damages to the liver or kidneys at these concentrations. In IUCLID (2000) a 28-days test with rats is described. NOAEL was stated to be 16 mg/kg and LOAEL to be 405 mg/kg, based on observation of damages to the liver. In a 13 weeks inhalation test with mice NOAEC was determined to be 100 ppm (740 mg/m³) and LOAEL to be 200 ppm (1480 mg/m³), based on observation on damages to the liver. In the IRIS-base two tests on determination of NOAEL are mentioned. In one study NOAEL is stated to be 14 mg/kg/day based on oral intake on rats, where damages on the blood as well as liver and kidneys have been observed (Hayes et al., 1986). In the other study with mice over a 6 weeks period NOAEL is stated to be 100 mg/kg where damages to the liver were observed (Buben and O'Flaherty, 1985). In both studies a safety factor of 1000 is used, leading to the lowest reference dose of 0.1 mg/kg. 5.2.10.5 ExposureThe maximum observed concentration is 0.002 mg/m³. This value is more than one factor 10,000 below the TLV in the working environment, which is 70 mg/m³ For tetrachloroethylene it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). The exposure can therefore be estimated to be as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.002 mg/m³ = 0.0004 mg/kg BW/day or 0.38 µg/kg BW/day. The estimated uptake of tetrachlorethylene is more than one factor 100 below reference dose of 0.1 mg/kg. Therefore, the emitted substance from sample C and D do not cause any health risks. 5.2.11 Toluene5.2.11.1 Identity
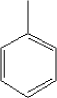
Toluene has a boiling point of 110.6°C and a melting point of -94.9°C. The substance has a vapour pressure of 28.4 mm Hg at 25°C. 5.2.11.2 Detected quantitiesToluene has been detected in all samples at a concentration between 10 and 20 µg/m³ after three hours. After 3 and 10 days the concentrations are at the same level or increased a little. Then, there is a tendency that the concentration decreases slowly. The blind values are about 10 µg/m³ and the measurements must therefore be considered quite unreliable. The highest measured concentration 27 µg/m³ was observed in sample A after 10 days and with a blind value of 12 µg/m³. The evaporation of toluene from the six samples is assessed to be at a level around max. 15 µg/m³. 5.2.11.3 ClassificationToluene is included in the list on dangerous substances and is classified under EU index no 601-021-00-3 (List on dangerous substances, Miljøministeriet 2002):
The substance is on 29th revised list of EU after the risk assessment (ECB) and is expected to get the following classification at the next revision of list on dangerous substances:
5.2.11.4 Health conditionsToluene has a TLV in the working environment of 94 mg/m³ (At-vejledning C.0.1). In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) the limit value in the working environment is fixed at 50 ppm (206 mg/m³). Two values for indoor limits are mentioned, one at 0.07 ppm (0.3 mg/m³) and the other at 0.7 ppm (3 mg/m³). Furthermore, a TDI of 0.223 mg/kg is mentioned. Toluene is described in the risk assessment report no 29 from EU (EU-RAR, no 29). From the report the following information has been retrieved. Toluene is quickly absorbed by inhalation and is absorbed in the body. The substance penetrates the skin and can be absorbed by skin contact. Toluene is distributed in the entire body and primarily absorbed in the fatty tissue. Toluene has a low acute toxicity. Humans exposed to toluene at concentrations of 285 mg/m³ will experience heavier headaches, dizziness, will be more irritable and tired. A NOAEC of 150 mg/m³ has been determined based on these facts. Liquid toluene irritates the eyes, and vapours in concentrations about and above 150 mg/m³ causes irritations of the eyes in humans. This determines a NOAEC of 150 mg/m³ for irritations of the eyes. Regarding inhalation a NOAEC of 1,125 mg/m³ has been stated. Long-term exposure of high concentrations of toluene has caused serious brain damages. However, it has not been possible to determined values for NOAEC or LOAEC for long-term exposure with reference to brain damages. Toluene is under suspicion of being reprotoxic causing damages to the foetus. In the following toluene will be assessed based on a NOAEC of 150 mg/m³. 5.2.11.5 ExposureThe measured concentration of toluene is 0.015 mg/m³. It is, thus, 20 times below the lowest stated limit of 0.3 mg/m³ and far below the other mentioned values. It is therefore assessed that evaporation of toluene will not cause any health risk. 5.2.12 Trimethylcyclohexen-1-on5.2.12.1 Identity
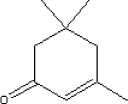
The substance has a boiling point of 215.32°C and a melting point of -8.1°C. The vapour pressure of the substance is 0.438 mm Hg at 25°C. 5.2.12.2 Detected quantitiesTrimethylcyclohexen-1-on has only been detected in sample E in a quantity of 24 µg/m³ after three hours. After three days the level is at 5 µg/m³. 5.2.12.3 ClassificationTrimethylcyclohexen-1-on is included in the list on dangerous substances and is classified under EU index no 606-012-00-8 (The list on dangerous substances, Miljøministeriet 2002):
5.2.12.4 Health conditionsTrimethylcyclohexen-1-on has a TLV of 25 mg/m³ (At-vejledning C.0.1). In Environmental Health Criteria 174 (1995) it is mentioned that trimethylcyclo-hexen-1-on irritates eyes, nose, and respiratory tract and may cause head aches, fatigue and faints. Acute effects on rats and rabbits in contact with skin varied from mild to severe irritation. Serious damages to the eyes have been observed after exposures in high concentrations. In acute and 90-days test on rodents damages to the liver and the central nervous system were observed at high doses. In a 90 days test with dogs no damages were detected at doses below 150 mg/kg per day. The substance does not cause mutations. In long-term experiments damages to the liver have been observed. In another long-term experiment where rats and rabbits inhaled the substance, irritation of the eyes and the respiratory tract were observed as well as damages to the lungs and the liver. In an IUCLID data sheet (2000) a number of NOAEC-values are reported. One of these tests is based on inhalation where NOAEC is stated to be 200 mg/m³ air with damages to the liver as the effect. In a 90 days experiment with dogs NOAEL was determined to be higher than 150 mg/kg per day as no damages were observed at 150 mg/kg. 5.2.12.5 ExposureFor trimethylcyclohexen-1-on it is assumed that100 % of the vapours are inhaled and absorbed (Fresp. = 1). The maximum observed concentration is 0.024 mg/m³. The exposure can therefore be estimated to be as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.024 mg/m³ = 0.0046 mg/kg BW/day or 4.6 µg/kg BW/day. The estimated exposure of trimethylcyclohexen-1-on is far below the stated NOAEL values and the measured concentration is below the NOAEC. Therefore, no indication is given that the observed content of the substance in the sample does have any influence on the health. 5.2.13 Xylene5.2.13.1 Identity
Xylene has a boiling point of 137-140C. The melting point varies according to the isomer forms from -48°C to 13°C. The vapour pressure of the substance is calculated to be 7.99 mm Hg at 25°C. 5.2.13.2 Detected quantitiesSmall quantities of xylene have been detected in all samples. After three hours the concentrations were measured to be between 3 and 9 µg/m³. For measurement carried out after 3 and 10 days the concentrations were at the same level or below. The concentrations measured after 28 days are considerably higher, which is likely to be due to an error, as it cannot be explained otherwise. 5.2.13.3 ClassificationToluene is included in the list on dangerous substances and is classified under EU index no 601-022-00-9 (The list on dangerous substances, Miljøministeriet 2002):
5.2.13.4 Health conditionsXylene has a TLV in the working environment of 109 mg/m³ (At-vejledning C.0.1). Xylene is described in Environmental Health Criteria 190 (1997). It is stated here that by inhaling the substance about 60% of the inhaled amount are retained in the lungs. Xylene metabolises effectively in the body. More than 90% is transformed to methyl hippuric acid, which will be liberated with the urine. Xylene does not accumulate in humans. Acute exposure to high concentrations irritates and effects the central nervous system. Chronic toxicity seems to be low in experimental animals. However, it indicates that the effects on the central nervous system may occur in animals exposed to moderate concentrations. It seems that xylene is not mutagenic or carcinogenic. The critical end-point is teratogenic effects. In an IUCLID data sheet NOAEC is stated to cause teratogenic effects. In an experiment with rabbits, in which the rabbits were exposed to the substance pregnant in their 7th to 20th day of pregnancy, NOAEL was determined to be 115 ppm or 544 mg/m³. In an experiment with mice exposed to the substance in their 6th to 15th day of pregnancy NOAEC was also determined to be 115 ppm. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) two limit values in the working environment is stated one at 50 ppm (237 mg/m³) and one at 100 ppm (473 mg/m³). As a value for indoor activities 2 ppm (9.5 mg/m³) is mentioned. Furthermore, TDI is stated to be 0.179 mg/kg. 5.2.13.5 ExposureThe maximum concentration observed is 0.040 mg/m³. The measured concentration is more than 200 times below this limit of 9.5 mg/m³ for indoor activities and far below the stated values for NOAEC. There is therefore no indication that the observed concentrations of the substance do have any influence on the health. 5.2.14 Aliphatic hydrocarbons C10-165.2.14.1 IdentityIn the present report, subsection 4.2.1.8, aliphatic hydrocarbons are described. Among others it is stated that hydrocarbons are linear or branched hydrocarbon chains, which may have one or several double bonds, and do not contain aromatic structures. The boiling points for some of the linear hydrocarbons are:
Substances with double bonds may have lower boiling points. It applies e.g. for 1-decene, which has a boiling point at 140C, and 1-hexadecene, which has a boiling point at 284C. Mineral turpentine has a boiling point interval from 150 to 205°C and resembles these hydrocarbons quite a lot. 5.2.14.2 Detected quantitiesAliphatic hydrocarbons have been detected in the samples. The detected concentrations are shown in table 5.1. Table 5.1 Measurements of hydrocarbons C10-C16
Table 5.1 shows that the concentrations over time are very different in the six samples. The level for sample E is considerably lower than the other samples. For sample B and F the concentrations are relatively high at the beginning and then decreases after 10 days to a low level. For sample A and C the concentrations are still at a rather high level after 28 days. 5.2.14.3 Health conditionsIt is extremely difficult to assess a compound of hydrocarbons when the substances in the compound are unknown. Mineral turpentine has a TLV of 145 mg/m³ in the working environment. There is a risk that substances in the hydrocarbon compound may cause cancer. Kjaergaard et al., 1989, describes a experiment with decane. 63 persons were tested for concentrations of n-decane of 0, 10, 35, and 100 mg/m³. Irritation of the mucous membranes was observed and the subjects experienced increased sensitivity towards odours and reduced air quality. Physiologically, reduced tear film stability was observed. In a laboratory experiment with rats the animals were exposed to non-aromatic White Spirit containing n-decane. The animals were exposed to the substance in concentrations of 0, 400 ppm (2290 mg/m³), and 800 ppm (4580 mg/m³) for six hours per day, five days per a week for three weeks. After a week changes in the concentration of neurotransmitters were observed, which was normalised after 2-3 week after the exposure. It has not been possible to retrieve relevant values for NOAEC. The B-value for mineral turpentine is stated to be 1 mg/m³ (Vejledning no 2, 2002). The stated B-value is based the on odour threshold and the observations in the guideline indicates that the limit for health risks may be 10 times higher. 5.2.14.4 ExposureFor hydrocarbons it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). The maximum observed concentration is 0.519 mg/m³. The exposure can therefore be calculated as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.519 mg/m³ = 0.099 mg/kg BW/day or approx. 100 µg/kg BW/day. Based on the limit value for turpentine it can be concluded that children are exposed to less than 0.4% of the TLV for product D in which the highest concentrations have been measured. The B-value is at least twice as high at the measured value. This indicates that the likelihood of causing health risk is minimal. However, it shall also be noted that the assessment is based on a very sparse data material. 5.3 Total assessmentThe result of the screening of de 14 substances/groups of substances has been brought together in the following overview. The substances 2-butoxyethanol, phenol, toluene, and xylene do not cause long-term effect in the measured concentrations. These have been found in quantities that are more than a factor 100 below the levels that may cause damages to the health. The substances ethoxyethanol and dimethyl formamide are teratogenic. Ethoxyethanol has been found in quantities that are considerably below the TDI-value. Dimethyl formamide has been found in quantities that are considerably below the levels that may cause damages to the health. Acetaldehyde, formaldehyde, tetrachloroethylene, and trimethylcyclohexen-1-on are under suspicion of causing cancer (Carc3). Formaldehyde has been found in one measure series in concentrations that are above the indoor limit value. In a supplementary test relatively low values were detected. Acetaldehyde and tetrachloroethylen have been found in very small quantities and it is assessed that the risk of health effects will be insignificant. The substances 3-carene, α-pinene, 2,6-di-tert-butyl-p-cresol, and formaldehyde may cause allergy in contact with skin. There is a risk that formaldehyde may cause health effects while the other substances appear in very small quantities and it is assessed that the health effects will be insignificant. The measurement showed that the different aliphatic hydrocarbons with the carbon chains of C10 to C16 are emitted. It is assessed that this group of substances resembles turpentine, which is carcinogenic. It has not been possible to determine a NOAEL. In stead the TLV of aliphatic hydrocarbons and turpentine has been compared. The measured concentrations represent less than 0.4 % of the TLV for turpentine. A comparison with the B-values has also been made. The highest measured concentration represents 50% of the B-value. Based on these comparisons it is assessed that the health risk will be insignificant. Summing up several of the substances, which are present in the tents and tunnels for children, are under suspicion of having carcinogenic, teratogenic, mutagenic, and allergic effects. Formaldehyde is present in one product in a concentration above the indoor limit value while two of the other products are close to the value. In a supplementary three days test of the emission of formaldehyde no concentrations above the limit values were detected. As the measured concentrations of formaldehyde decreased over time, it will be during the first hours of using the tents that the largest emission of the substance will occur. As for the other substances the emitted quantities are relatively small. None of these substances are present in concentrations, which will cause a potential health risk for children to play with the tested products.
ReferencesArgus Research Laboratories, Inc. (1997). Oral (gavage) developmental toxicity study of phenol in rats. Horsham, PA. Protocol number: 916-011. Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991. 703. American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991. 565. American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986. 71. American Industrial Hygiene Association. The AIHA 2001 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. AIHA Press, Fairfax, VA. 2001. 37. Appleman, L.M., R.A. Woutersen, and V.J. Feron. 1982. Inhalation toxicity of acetaldehyde in rats. I. Acute and subacute studies. Toxicology. 23: 293-297. Appleman, L.M., R.A. Woutersen, V.J. Feron, R.N. Hooftman and W.R.F. Notten. 1986. Effect of variable versus fixed exposure levels on the toxicity of acetaldehyde in rats. J. Appl. Toxicol. 6(5): 331-336. At-vejledning C.0.1, (2002) Grænseværdier for stoffer og materialer. Barbee, S.J., J.B. Terrill, D.J. DeSousa and C.C. Conaway. 1984. Subchronic inhalation toxicology of ethylene glycol monoethyl ether in the rat and rabbit. Environ. Health Perspect. 57: 157-163. Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001).V4 P209. Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001).V4 P211. Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001).V4 P212. Boublik, T., Fried, V., and Hala, E., The Vapour Pressures of Pure Substances. Second Revised Edition. Amsterdam: Elsevier, 1984. 125. Buben, J.A. and E.J. O'Flaherty. 1985. Delineation of the role of metabolism in the hepatotoxicity of trichloroethylene and perchloroethylene: a dose- effect study. Toxicol. Appl. Pharmacol. 78: 105-122. Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996. Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989. 239. Catenacci, G., D. Grampella, R. Terzi, A. Sala and G. Polline. 1984. Hepatic function in subjects exposed to environmental concentrations of DMF lower than the actually proposed TLV. G. Ital. Med. Lav. 6(3-4): 157-158. CEN/TC 52/WG 9: Risk Assessment, Safety of toys, January 2003. Cirla, A.M., G. Pisati, E. Invernizzi and P. Torricelli. 1984. Epidemiological study on workers exposed to low dimethylformamide concentrations. G. Ital. Med. Lav. 6(3-4): 149-156. Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982. 3920]. Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994. 1276. Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989. Dow Chemical Company; The Glycol Ethers Handbook. The Dow Chemical Company, Midland, MI 97 pp (1990)]. Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997. 1675. Environmental Health Criteria 174: Isophorone. Pp.1-22 (1995) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization. Environmental Health Criteria 190: Xylenes pp. 1-2 (1997) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization. EU-RAR no. 29: EU's Risk Assessment report no. 29 for stoffet toluen, udført af Danmark, 2003. Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976.,p. II-118. Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986. Hamilton, A., and H. L. Hardy. Industrial Toxicology. 3rd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1974. (301). Hayes, J.R., L.W. Condie, Jr. and J.F. Borzelleca. 1986. The subchronic toxicity of tetrachloroethylene (perchloroethylene) administered in the drinking water of rats. Fund. Appl. Toxicol. 7: 119-125. IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work).p. V71 1247 (1999). IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work).p. V71 331 (1999). IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume
work).p. 63 204 (1995). IUCLID dataset (2000) 3,5,5-trimethylcyclohex-2-enone. European Commision, European Chemical Bureau. IUCLID dataset (2000) tetrachlorethylen. European Commision, European Chemical Bureau. Kaneko T et al; Chem Biol Interact 67 (3-4): 295-304 (1988). Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984.,p. V11 944 (1980). Kjaergaard S et al; Environment International 15: 473-482 (1989). Kortlægning af kemiske stoffer i hygiejnebind, Kortlægning nr. 13, 2002. Jane Pors og René Fuhlendorff. Kortlægning af kemiske stoffer i tekstilmetervarer, kortlægningsrapport nr. 23, 2003. Lauersen SE, Hansen J, Drøjdahl A, Hansen OC, Pommer K, Pedersen E, Bernth N for Miljøstyrelsen. Lewis, R.J. Sax's Dangerous Properties of of Industrial Materials. 10th ed. Volumes 1-3 New York, NY: John Wiley & Sons Inc., 1999. 3637. Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 75th ed. Boca Raton, Fl: CRC Press Inc., 1994-1995.,p. 3-159. Listen over farlige stoffer. Miljøstyrelsens bekendtgørelse nr. 439, 2002. Lof A et al; Pharmacol Toxicol 85 (2): 92-7 (1999). National Fire Protection Association. Fire Protection Guide on Hazardous Materials. 7th ed. Boston, Mass.: National Fire Protection Association, 1978. 2. NTP. (1993) Technical report on toxicity studies of ethylene glycol ethers 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol administered in drinking water to F344/N rats and B6C3F1 mice. U.S. DHHS, PHS, NIH, Research Triangle Park, NC. NTP No. 26. NIH Publ. No. 93-3349. O'Donoghue, J.L. (ed.). Neurotoxicity of Industrial and Commercial Chemicals. Volume I. Boca Raton, FL: CRC Press, Inc., 1985. 129. Prager, J.C. Environmental Contaminant Reference Databook Volume 2. New York, NY: Van Nostrand Reinhold, 1996. 1067. The AIHA 1999 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. American Industrial Hygiene Association. Fairfax, VA 1999.40. Tokanova SE; Gig Sanit (4): 10-13 (1982). Vejledning nr. 2, 2002: B-værdivejledningen. Miljøstyrelsen. Vejledende liste til selvklassificering af stoffer. Miljøstyrelsen. World Health Organization/International Programme on Chemical Safety. Concise International Chemical Assessment Document No. 10. 2-Butoxyethanol p.4 (1998). Öko-Test nr. 12, 2002, p. 44-47.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||