|
Release of chemical substances from tents and tunnels for children 5 Assessment of problematic substances
5.1 Method of assessmentThe purpose of assessing the substances is to decide whether children may be affected by using the analysed products. The assessment is based on a worst case scenario. The principles for the assessment of health effects are based on EU's revised Technical Guidance Document (TGD) for risk assessments. The results of the analyses show the quantities of the substances in question, which are released from each product. The quantity that a child may be exposed to by inhalation is assessed. For those substances for which it has been possible to retrieve data for NOAEC (No Observed Adverse Effect Concentration) and/or LOAEC (Lowest Observed Adverse Effect Concentration), a direct comparison has been made with the measured concentrations. The quantity that a child may absorb has been estimated. Then the estimated value is compared with the data on the substance in question concerning the NOAEL level – No Observed Adverse Effect Level and LOAEL – Lowest Observed Adverse Effect Level or other relevant data indicating permanent effects or other relevant data as far as it has been possible to retrieve it. The quantity of the substance that a child may absorb is estimated based on the following conditions. It is assumed that a child may have a body weight as low as 10 kg. The assumption is conservative has been retrieved from the TGD. It is assumed that a child is exposed one hour per day and the focus is on absorption by inhaling the substance. An assessment is made based on the highest measured concentration occurring three hours after the product has been unpacked. If it gives reason to assess the substance further the other analysis results measured at 3, 10 and 28 days after the product has been unpacked will be taken into account. The quantity of the inhaled substance can be determined according to the guidelines in TGD (2002) using the formula:
where:
The part of absorbed or respirable fractions, Fresp, is fixed at a given fraction if data are available. If not the fraction is fixed at 1. The inhalation rate, Qinh, is fixed at 1.9 m³/hour indicating the inhalation rate at high activity (TGD, 2002). The duration of exposure is fixed at 1 hour (Tcontact) and the number of times per day (Nevent) is also fixed at 1. The body weight of a child is fixed at 10 kg. Based on the given assumptions [formula 1] can be reduced to: Iinh [ mg/kg BW/day] = 0.19 • Fresp • Cinh [ mg/m³] 5.2 Assessment of each substance5.2.1 2-Butoxyethanol5.2.1.1 Identity
The boiling point of the substance is 171-172C (Budavari, S., 1989) and its melting point is -70C (American Conference of Governmental Industrial Hygienists; 1986). The vapour pressure of the substance is 0.88 mm Hg at 25C (Dow Chemical Company; 1990). 5.2.1.2 Detected quantitiesAfter three hours significant quantities of 2-butoxyethanol - 153 g/m³ - have been found in sample D. Regarding the other samples the concentration is below 10 g/m³ after three hours. For sample D concentration of the substance falls relatively quickly so that after 3, 10 and 28 days it is about 10 g/m³. It is therefore assessed that only during the first day of using the tent concentrations above 100 g/m³ may occur. 5.2.1.3 Classification2-butoxyethanol is included on the list on dangerous substances and is classified under EU index no 603-014-00-0 (List over dangerous substances, Miljøministeriet 2002):
5.2.1.4 Health conditionsIn an animal experiment it is shown, that the substance is absorbed quickly by inhalation, if swallowed or in contact with skin and that it is decomposed to 2-butoxyacetaldehyd and 2-butoxy acetic acid. The primary effect is that the substance and its metabolites are hematoxic (affect the haemoglobin in blood). In rats the effects detected in the central nervous system, liver and kidneys are permanent at high concentrations (the source does not mention the quantity). The substance does not cause sensitisation and no data indicate that the substance is mutagenic (World Health Organization, 1998). In a 13 weeks experiment prepared by NTP (1993) rats were tested. Damages on the blood and the bone marrow were observed and the LOAEL was determined to be between 69 and 82 mg/kg/day. A value for NOAEL was not determined. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a value for TDI (tolerable daily intake) of 0.05 mg/kg is stated. 5.2.1.5 ExposureAs for 2-butoxyethanol it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.19 • 1 • 0.153 mg/m³ = 0.03 mg/kg BW/day. The determined LOAEL is more than 1000 times the estimated inhaled quantity. The TDI-value is a little above the estimated value. It is therefore assessed that exposure of the substance in the mentioned concentrations has a minimal influence on the health. 5.2.2 2-Ethoxyethanol5.2.2.1 Identity
The boiling point of the substance is 135°C (Lide, D.R., 1994-1995) and its melting point is -70°C (Kirk-Othmer Encyclopedia of Chemical Technology, 1980). The vapour pressure of the substance is 5.31 mm Hg at 25°C (eksperimentally)(Daubert, T.E., R.P. Danner, 1989). 5.2.2.2 Detected quantitiesOnly in sample F 23 µg/m³ of the substance was detected after three hours. After three days the concentration was as low as 6 µg/m³ and after 10 and 28 days concentrations of 2 µg/m³ or less were measured. 5.2.2.3 ClassificationEthoxy ethanole is included in the list on dangerous substances and classified under EU index no 603-012-00-X (List on dangerous substances, Miljøministeriet 2002):
5.2.2.4 Health conditions2-ethoxyethanol may be slightly irritating in contact with eyes and mucous membrane, however, there are no indications saying that it may be irritating in contact with skin (Clayton, G. D. and F. E. Clayton (eds.)1981-1982). The source does not mentioned at which concentrations of the substance the assessment is related to. The lethal dose for humans is about 1.4 ml/kg conforming with about 100 ml for a human weighing 70 kg (Amdur et al., 1991). The effects of 2-ethoxyethanol, which influence the central nervous system, are headache, fatigue, dizziness, grogginess, slurred speech and behaviour changes. (Hamilton, A., and H. L. Hardy, 1974). An experiment shows that humans exposed to up to 88 mg/m³ of 2-ethoxyethanol had significantly lower production of sperm than the control group. This occurred even though the exposed test persons and the control group had a lower quantity of sperm than other groups of employees (American Conference of Governmental Industrial Hygienists, 1991). In the IRIS-database it is stated that data on inhalation toxicity is assessed with some reservations (Medium confidence), as the most significant experiment was only of short duration. Subchronic inhalation tests indicate that teratogenic toxicity is the most sensitive endpoint. However, no chronic tests have been retrieved in the available literature. Barbee et al., 1984, has carried out a subchronic inhalation test with rats and rabbits. The NOAEL for rats was determined to be 265 mg/m³, based on observed modifications in the weight of the hypophysis in male rats and diminished spleen in female rats. In experiments with rabbits the NOAEL was determined to be 68 mg/m³ and the LOAEL to be 265 mg/m³, based on the damages such as modifications in the content of haemoglobin and the haematocrit values and weight loss. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a value for TDI (tolerable daily intake) of 0.05 mg/kg is stated. 5.2.2.5 ExposureThe measured concentration of 0.023 mg/m³ can directly be compared with a NOAEC of 68 mg/m³. As for 2-ethoxyethanol it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.19 • 1 • 0.023 mg/m³ = 0.004 mg/kg BW/day The potentially absorbed quantity of 0.004 mg/kg is considerably lower than the TDI value of 0.05 mg/kg. It is therefore assessed that there are no significant effects on the health under the present circumstances, even though the substance is known to have teratogenic effects on humans. 5.2.3 3-Carene5.2.3.1 Identity
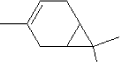
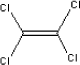
It has not been possible to retrieve many data on 3-carene. Monoterpenes, which among others include limonene and pinene as well as carenes, are characterised as turpentine with CAS-no 8006-64-2. Turpentine consists chemically of 58-65% γ-pinene together with β-pinene and other isomer terpenes. Turpentine from wood, extracted from waste wood or sawdust, consists of 80% γ-pinene, 15% monocyclic terpenes, 1.5% terpen alcohol and other terpenes (Bingham et al., 2001). The boiling point of terpenes is about 154-170°C (Lewis, R.J., 1999), the melting point is about -50 to -60°C (Clayton, G.D., F.E. Clayton (eds.), 1993-1994) and the vapour pressure is about 5 mm Hg at 25°C for an unspecified mixture (National Fire Protection Association, 1978). 5.2.3.2 Detected quantitiesThe substance 3-carene has been detected in two of the samples, A and B in quantities of 23 µg/m³ and 11 µg/m³, respectively, after three hours. After 3 and 10 days the concentration of 3-carene had fallen considerably so that the concentration emitted from sample A had fallen from 23 to 4 µg/m³, and the concentration emitted from sample B had fallen from 11 µg/m³ to less than 1 µg/m³. 5.2.3.3 ClassificationThe substance itself is not classified. The classification is based on the group of turpentine, which is classified under EU index no 650-002-00-6 (List on over dangerous substances, Miljøministeriet 2002):
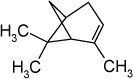
5.2.3.4 Health conditionsVapours are irritating in contact with eyes and respiratory passages. If vapours are inhaled they may cause head aches, vomiting, dizziness and faint. The fluid irritates the skin and if swallowed it will irritate the entire alimentary system and it may cause damages to the kidneys. If the fluid enters the lungs it will cause severe attack of pneumonia (Prager, J.C., 1996). The lethal dose of for turpentine if swallowed may be as low as 110 g. However, survival has been observed after having swallowed 120 g. Thus, no more than 15 g of the substance has been fatal for a child (Bingham, E. et al., 2001). In an experiment with male and female volunteers the following observations have been reported. People with an average age of 35 years were exposed to 0 or 450 mg/m³ of a mixture consisting of 10 parts α-pinene, 1 part β-pinene and 5 parts 3-carene (synthetic turpentine) for 12 hours 4 times over a two-week period. Acute damages to the lungs were observed. The male volunteers, exposed for two hours with 450 mg/m³ during easy workout, experienced their respiratory passages were influenced by the exposure and they had breathing difficulties after the termination of the exposure, E. et al., 2001). LCI (Lowest Concentration of Interest) is 250 µg/m³ for most of the turpentines, based on an inhalation study on humans with a NOEC for lung symptoms on 25 mg/m³, that is LCI = NOEC/11010 (Larsen et al., 1999). 5.2.3.5 ExposureComparing the measure concentration of 23 µg/m³ at the maximum with the detected LCI-value of 250 µg/m³ it shows that the observed level is 10 times less than the establish limit. It indicates that the liberated quantity of the substance does not have any influence on the health. 5.2.4 Alfaα-pinene5.2.4.1 Identity
The melting point of the substance is –62.5°C. The boiling point is 156°C (Furia og Bellanca, 1975). Vapour pressure is 633 Pa at 25°C (4.75 mm Hg) (Daubert og Danner, 1989). The water solubility is 0.65 mg/l at 250°C (FFHPVC, 2002). The distribution coefficient log Kow is experimentally detected to be 4.83 (Li og Perdue, 1995). 5.2.4.2 Detected quantitiesThe substance α-pinene is detected in the four samples, A, B, E, and F. The detected quantities is between 7 and 16 µg/m³ after three hours. The analysis results show that the emitted quantity of α-pinene is declining over time. The highest concentration of 16 µg/m³ was detected after three hours and reduced to 6 µg/m³ after 10 days. As for the other samples the emission was reduced to below 5 µg/m³ after 10 days. 5.2.4.3 Classificationα-pinene is primarily extracted from tree oils and other biological materials, but may also occur in mineral oil products. The substance is not classified under its own name, but belongs to the group of turpentine's, which are classified under EU index no 650-002-00-6 (List on dangerous substances, Miljøministeriet 2002):
5.2.4.4 Health conditionsα-pinene is primarily toxic if inhaled (Lewis, 1992) and severely irritating in contact with eyes, mucous membranes, and skin (Budavari, 1996; Lewis, 1992). Examples on effect levels are stated below. α-pinene is known to be contact allergen (Thomsen, 1990). Acute toxicity:
In the description of 3-carene (subsection 5.2.3) tests and experiments with turpentine are mentioned in which α-pinene is the main ingredient. LCI is 250 µg/m³ for most types of turpentine, based on a inhalation study on humans with a NOEC for lunge symptoms of 25 mg/m³, that is LCI = NOEC/1×10×10 (Larsen et al., 1999). 5.2.4.5 ExposureCompared with the mentioned inhalation study (LCI=0.25 mg/m³) the quantity of the substance emitted from the samples in the present study (maximum 0.016 mg/m³) does not give any reason for health risks. 5.2.5 Acetaldehyde5.2.5.1 Identity
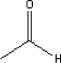
Acetaldehyde has a boiling point of 21°C and a melting point of -123.5°C (Budavari, 1996). The vapour pressure is 902 mm Hg at 25°C (Boublik et al., 1984). 5.2.5.2 Detected quantitiesAcetaldehyde has been detected in sample A, C, D, and E in concentrations between 2 and 12 µg/m³ after three hours. Sample C, causing the highest concentration of 12 µg/m³ after three hours, was at 2 µg/m³ after three days and was not analysed anymore. As for the other samples the level was very low after three days. 5.2.5.3 ClassificationAcetaldehyde is included in the list of dangerous substances and is classified under EU index no 605-003-00-6 (List on dangerous substances, Miljøministeriet 2002):
5.2.5.4 Health conditionsThe TLV for acetaldehyde for working environment is 45 mg/m³ (At-vejledning C.0.1). The database IRIS retrieves two studies carried out by Appleman et al., 1986 and 1982, that determine NOAEC and LOAEC based on short-term experiments. The results showed a NOAEC of 150 ppm corresponding to 273 mg/m³ and a LOAEC of 400 ppm corresponding to 728 mg/m³. The experiments are based on damages to the lungs, which also occur at chronic experiments. Acetaldehyde is placed in group 2B by IARC, which may cause cancer in humans. It has been proven experimentally that the substance is carcinogenic in animals but the evidence is insufficient as far as the effects on humans are concerned (IARC, 1999). 5.2.5.5 ExposureIf the maximum measured concentration of 0.012 mg/m³ is compared with the detected values for NOAEC of 273 mg/m³ and LOAEC of 728 mg/m³ it appears that the quantity of substance liberated from the samples do not cause any health risk. 5.2.6 2,6-Di-tert-butyl-p-cresolThe substance has previously been researched in the project "Kortlægning af hygiejnebind" (Hazardous substances in sanitary towels) (2002). If nothing else is stated the data from that project have been used. 5.2.6.1 Identity
The substance has a boiling point of 265°C and a melting point of 71°C. The vapour pressure of the substance is 0.015 mm Hg at 20°C. 5.2.6.2 Detected quantitiesThe substance 2,6-di-tert-butyl-p-cresol has only been detected in sample A in a concentration of 50 g/m³. The concentration is slowly decreasing over time so that after 10 days it is 42 µg/m³ and after 28 days 30 µg/m³. 5.2.6.3 Health conditionsThe substance 2,6-di-tert-butyl-p-cresol is not classified and there is no TLV for the substance. In "Kortlægning af hygiejnebind" (2002) it is stated that the substance seems to be moderately toxic at oral intake and it has shown to cause allergic effects in humans in several cases. Furthermore, it is stated that the substance is under the suspicion of being a cancer promotor in skin. The survey concludes that the critical acute effect in humans is irritation in contact with skin and it is stated that NOAEL = 0.1 % in ethanol The critical acute effect in mice is breathing disorders and enlarged lungs after application on the skin. The results are: NOAEL = 145 mg/kg BW 5.2.6.4 ExposureFor 2,6-di-tert-butyl-p-cresol it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). Iinh [mg/kg BW/day] = 0.09 × 1 × 0.050 mg/m³ = 0.01 mg/kg BW/day or 10 µg/kg BW/day. Assessing absorption by inhalation of the substance, it is seen that the potential absorbed amount is only a fraction of the establish limits. It should be noted that the circumstance about skin irritation is not assessed, as the quantity of the substance has not been detected in the actual toy. 5.2.7 Dimethyl formamide5.2.7.1 Identity
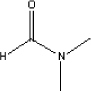
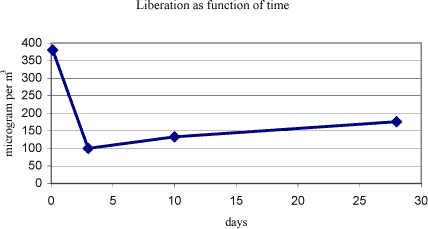
Dimethyl formamide has a boiling point of 153°C and a melting point of 61°C (Budavari S., 1996). The vapour pressure is 3.87 mm Hg at 25°C (Daubert T.E., 1989). 5.2.7.2 Detected quantitiesDimethyl formamide has been detected in all the samples. In sample A the concentration was high, 380 g/m³, in samples D and E the concentration was at a mean level between 20 and 25 µg/m³. For the other three samples, B, C, and F, the concentration was at low level between 1 and 5 µg/m³. The emitted concentration over time evolved quite unexpectedly regarding sample A. It is illustrated in figure 5.1. Figure 5.1 Concentration of dimethyl formamide in sample A as function of time
For sample D and E the concentration decreased rather quickly and was for both samples between 1 and 5 µg/m³ after three days. 5.2.7.3 ClassificationDimetyl formamide is included in the list on dangerous substances and is classified under EU index no 616-001-0X (List on dangerous substances, Miljøministeriet 2002):
5.2.7.4 Health conditionsThe TLV for dimethyl formamide in the working environment is 30 mg/m³ (At-vejledning C.0.1). Several tests on humans exposed to dimethyl formamide indicate that the substance may contribute in developing cancer in the testicles. Tests on animals have not proven that the substance is mutagenic or carcinogenic (Ellenhorn et al., 1997). It has been proven that the substance cause damages to the liver. In IRIS-database two experiments have been retrieved for determination of NOAEC and LOAEC. One experiment was carried out by Cirla et al., 1984, and the other by Catenacci et al., 1984, and both experiments indicate damages to the liver. In the description of the experiments it is stated that it has not been possible to determine NOAEC. On the other hand LOAEC is determined to be 22 mg/m³. 5.2.7.5 ExposureIt is expected that NOAEC is below the value of LOAEC. The highest measured concentration is at 0.38 mg/m³ and is somewhat below LOAEC (and NOAEC). 5.2.8 FormaldehydeFormaldehyde is described in the project "Kortlægning af kemiske stoffer i tekstilmetervarer" (Hazardous substances in textiles), survey report no 23, 2003. If nothing else is stated data from that report have been used. 5.2.8.1 Identity
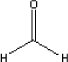
Formaldehyde is a gas at ambient temperature. The melting point is -92°C and the boiling point is -19°C. 5.2.8.2 Detected quantitiesFormaldehyde has been detected in all the samples. Three of the samples, B, C, and D, the concentrations are between 100 and 200 µg/m³ after three hours. For the other samples the level is between 15 and 25 µg/m³. For sample C, in which the concentration after three hours is determined to be 163 µg/m³, the concentration is halved after three days. The same effect was seen in sample B and D. For the other samples the level is also reduced to about the half of the concentration after three days compared with the level after three hours. Supplementary analyses have been carried out for two of the tents, namely sample B and C. The supplementary measurements include continuous measurement of the concentration of formaldehyde over three days inside and outside the tents. A reference measurement and two measurements of each of the two tents have been carried out. The measured concentrations are relatively constant during the three days and vary between:
These supplementary measurements show that the highest concentration is about 37 µg/m³ with a background concentration of about 25 µg/m³. 5.2.8.3 ClassificationFormaldehyde is included in the list on dangerous substances and is classified under EU index no 605-001-00-5 (List on dangerous substances, Miljøministeriet 2002):
5.2.8.4 Health conditionsRegarding acute toxicity, inhalation tests with rats and mice show that LC50 is about 0.5 mg/litre. Inhalation tests varying from three days to two years showed NOAEC of 1.2 mg/m³ in rats with lesions of the nose epithelium. Contact with formaldehyde causes allergic reactions (allergy). The substance provokes especially allergic reactions by skin contact and by inhalation. Formaldehyde is under suspicion of causing cancer by inhalation in animal experiments. 5.2.8.5 ExposureNOAEC for lesions of the nose epithelium in rats is determined to be 1.2 mg/m³. In the first set of measurement the level for sample B, C, and D is between 0.1 and 0.2 mg/m³ and is therefore less than a factor 10 under NOAEC. This result indicates a potential health risk. For the supplementary measurement the NOAEC is about a factor 30 over the highest measured value of 0.037 mg/m³. 5.2.9 Phenol5.2.9.1 Identity
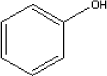
The boiling point of phenol is 182C and its melting point is 41°C. The substance has a vapour pressure of 0.35 mm Hg at 25°C. 5.2.9.2 Detected quantitiesPhenol has only been detected in sample D. The maximum concentration detected is 18 µg/m³ after three hours. After three days the concentration has been measured to 15 µg/m³ and after 10 days to 7 µg/m³. 5.2.9.3 ClassificationPhenol is included in the list on dangerous substances and is classified under EU index no 604-001-00-2 (List on dangerous substances, Miljøministeriet 2002):
5.2.9.4 Health conditionsFor phenol the TLV in the working environment is 4 mg/m³ (At-vejledning C.0.1). In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a TLV of 2 ppm (8.4 mg/m³) in the working environment is mentioned and an indoor value of 0.1 ppm (0.4 mg/m³) Phenol is toxic with a lethal dose of 50-500 mg/kg for humans. Some people may be hypersensitive, causing death or very serious effects after exposure to low doses. Phenol penetrates the skin and is quickly absorbed. Effects on the central nervous system, the hart, the blood stream, lungs and kidneys have been observed. The observed effects after short-term exposure may include shock, coma, delirium and death. Long-term or repeated exposure may result in damages to liver, kidneys and eyes. Changes in skin pigment have been observed. Inhalation may cause lung irritations and oedemas. Phenol is described in an IUCLID-data sheet from 2000. Among others the following it stated. Tests show that phenol does not cause sensitisation. In a 28-days test with mice oral intake show effects on the red corpuscles and the level of anti-substances in the blood. LOAEL is determined to 1.8 mg/kg body weight. A study of phenol in rats was performed (Argus Research Laboratories, 1997). The effects on the development of the offspring were tested and NOAEL was determined to be 60 mg/kg per day. A benchmark dose was calculated to be 93 mg/kg per day, and with a safety factor of 300 the reference dose was determined to be 0.1 mg/kg/day. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) a TDI value of 1.5 mg/kg BW is stated. 5.2.9.5 ExposureThe maximum concentration observed is 0.018 mg/m³. It is considerably below the Danish TLV in the working environment and the mentioned value for concentrations indoors of 0.4 mg/m³. For phenol it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). The exposure can therefore be calculated to be as follows: Iinh [mg/kg BW/day] = 0.09 × 1 × 0.018 mg/m³ = 0.0034 mg/kg BW/day or 3.4 µg/kg BW/day. The estimated amount of phenol is considerably below the stated values for NOAEL and TDI (1.5 mg/kg) and there is therefore no indications that the potential observed uptake of phenol from sample D causes any effect on the health. 5.2.10 Tetrachloroethylene5.2.10.1Identity
Tetrachloroethylen has a boiling point o 121.3°C and a melting point of -22.3°C. The substance has a vapour pressure of 18.5 mm Hg at 25°C. 5.2.10.2 Detected quantitiesLow concentrations of the substance have been observed in sample C and D. The maximum concentrations after three hours have been measured to be 1 µg/m³ in sample C and 2 µg/m³ in sample D. After three days the concentration in both for samples are below 1 µg/m³. 5.2.10.3 ClassificationTetrachloroethylene is included in the list on dangerous substances and is classified under EU index no 602-028-00-4 (List on dangerous substances, Miljøministeriet 2002):
5.2.10.4 Health conditionsThe TLV for tetrachloroethylene in the working environment is 70 mg/m³ (At-vejledning C.0.1). IARC, 1995, has assessed the carcinogenic effect of tetrachloroethylen and has reached to the conclusion that the substance may be carcinogenic. Heavy exposure of the substance has resulted in effects on the central nervous system, eyes, and skin, and to a lesser extent lungs, liver, and kidneys. The effects on the central nervous system have often been unconsciousness, dizziness, headache, hypersensitivity towards light, and depressions. Damages on human liver have been observed, normally after exposure to more than 100 ppm. At repeated exposures of humans with more than 200 ppm early signs of depressions have occurred while no effects have been observed on males and females exposed to 100 ppm for 7 hours per day. Clinical analyses indicate no damages to the liver or kidneys at these concentrations. In IUCLID (2000) a 28-days test with rats is described. NOAEL was stated to be 16 mg/kg and LOAEL to be 405 mg/kg, based on observation of damages to the liver. In a 13 weeks inhalation test with mice NOAEC was determined to be 100 ppm (740 mg/m³) and LOAEL to be 200 ppm (1480 mg/m³), based on observation on damages to the liver. In the IRIS-base two tests on determination of NOAEL are mentioned. In one study NOAEL is stated to be 14 mg/kg/day based on oral intake on rats, where damages on the blood as well as liver and kidneys have been observed (Hayes et al., 1986). In the other study with mice over a 6 weeks period NOAEL is stated to be 100 mg/kg where damages to the liver were observed (Buben and O'Flaherty, 1985). In both studies a safety factor of 1000 is used, leading to the lowest reference dose of 0.1 mg/kg. 5.2.10.5 ExposureThe maximum observed concentration is 0.002 mg/m³. This value is more than one factor 10,000 below the TLV in the working environment, which is 70 mg/m³ For tetrachloroethylene it is assumed that 100% of the vapours are inhaled and absorbed (Fresp. = 1). The exposure can therefore be estimated to be as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.002 mg/m³ = 0.0004 mg/kg BW/day or 0.38 µg/kg BW/day. The estimated uptake of tetrachlorethylene is more than one factor 100 below reference dose of 0.1 mg/kg. Therefore, the emitted substance from sample C and D do not cause any health risks. 5.2.11 Toluene5.2.11.1 Identity
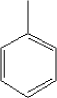
Toluene has a boiling point of 110.6°C and a melting point of -94.9°C. The substance has a vapour pressure of 28.4 mm Hg at 25°C. 5.2.11.2 Detected quantitiesToluene has been detected in all samples at a concentration between 10 and 20 µg/m³ after three hours. After 3 and 10 days the concentrations are at the same level or increased a little. Then, there is a tendency that the concentration decreases slowly. The blind values are about 10 µg/m³ and the measurements must therefore be considered quite unreliable. The highest measured concentration 27 µg/m³ was observed in sample A after 10 days and with a blind value of 12 µg/m³. The evaporation of toluene from the six samples is assessed to be at a level around max. 15 µg/m³. 5.2.11.3 ClassificationToluene is included in the list on dangerous substances and is classified under EU index no 601-021-00-3 (List on dangerous substances, Miljøministeriet 2002):
The substance is on 29th revised list of EU after the risk assessment (ECB) and is expected to get the following classification at the next revision of list on dangerous substances:
5.2.11.4 Health conditionsToluene has a TLV in the working environment of 94 mg/m³ (At-vejledning C.0.1). In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) the limit value in the working environment is fixed at 50 ppm (206 mg/m³). Two values for indoor limits are mentioned, one at 0.07 ppm (0.3 mg/m³) and the other at 0.7 ppm (3 mg/m³). Furthermore, a TDI of 0.223 mg/kg is mentioned. Toluene is described in the risk assessment report no 29 from EU (EU-RAR, no 29). From the report the following information has been retrieved. Toluene is quickly absorbed by inhalation and is absorbed in the body. The substance penetrates the skin and can be absorbed by skin contact. Toluene is distributed in the entire body and primarily absorbed in the fatty tissue. Toluene has a low acute toxicity. Humans exposed to toluene at concentrations of 285 mg/m³ will experience heavier headaches, dizziness, will be more irritable and tired. A NOAEC of 150 mg/m³ has been determined based on these facts. Liquid toluene irritates the eyes, and vapours in concentrations about and above 150 mg/m³ causes irritations of the eyes in humans. This determines a NOAEC of 150 mg/m³ for irritations of the eyes. Regarding inhalation a NOAEC of 1,125 mg/m³ has been stated. Long-term exposure of high concentrations of toluene has caused serious brain damages. However, it has not been possible to determined values for NOAEC or LOAEC for long-term exposure with reference to brain damages. Toluene is under suspicion of being reprotoxic causing damages to the foetus. In the following toluene will be assessed based on a NOAEC of 150 mg/m³. 5.2.11.5 ExposureThe measured concentration of toluene is 0.015 mg/m³. It is, thus, 20 times below the lowest stated limit of 0.3 mg/m³ and far below the other mentioned values. It is therefore assessed that evaporation of toluene will not cause any health risk. 5.2.12 Trimethylcyclohexen-1-on5.2.12.1 Identity
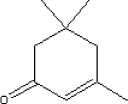
The substance has a boiling point of 215.32°C and a melting point of -8.1°C. The vapour pressure of the substance is 0.438 mm Hg at 25°C. 5.2.12.2 Detected quantitiesTrimethylcyclohexen-1-on has only been detected in sample E in a quantity of 24 µg/m³ after three hours. After three days the level is at 5 µg/m³. 5.2.12.3 ClassificationTrimethylcyclohexen-1-on is included in the list on dangerous substances and is classified under EU index no 606-012-00-8 (The list on dangerous substances, Miljøministeriet 2002):
5.2.12.4 Health conditionsTrimethylcyclohexen-1-on has a TLV of 25 mg/m³ (At-vejledning C.0.1). In Environmental Health Criteria 174 (1995) it is mentioned that trimethylcyclo-hexen-1-on irritates eyes, nose, and respiratory tract and may cause head aches, fatigue and faints. Acute effects on rats and rabbits in contact with skin varied from mild to severe irritation. Serious damages to the eyes have been observed after exposures in high concentrations. In acute and 90-days test on rodents damages to the liver and the central nervous system were observed at high doses. In a 90 days test with dogs no damages were detected at doses below 150 mg/kg per day. The substance does not cause mutations. In long-term experiments damages to the liver have been observed. In another long-term experiment where rats and rabbits inhaled the substance, irritation of the eyes and the respiratory tract were observed as well as damages to the lungs and the liver. In an IUCLID data sheet (2000) a number of NOAEC-values are reported. One of these tests is based on inhalation where NOAEC is stated to be 200 mg/m³ air with damages to the liver as the effect. In a 90 days experiment with dogs NOAEL was determined to be higher than 150 mg/kg per day as no damages were observed at 150 mg/kg. 5.2.12.5 ExposureFor trimethylcyclohexen-1-on it is assumed that100 % of the vapours are inhaled and absorbed (Fresp. = 1). The maximum observed concentration is 0.024 mg/m³. The exposure can therefore be estimated to be as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.024 mg/m³ = 0.0046 mg/kg BW/day or 4.6 µg/kg BW/day. The estimated exposure of trimethylcyclohexen-1-on is far below the stated NOAEL values and the measured concentration is below the NOAEC. Therefore, no indication is given that the observed content of the substance in the sample does have any influence on the health. 5.2.13 Xylene5.2.13.1 Identity
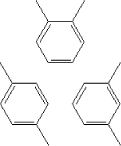
Xylene has a boiling point of 137-140C. The melting point varies according to the isomer forms from -48°C to 13°C. The vapour pressure of the substance is calculated to be 7.99 mm Hg at 25°C. 5.2.13.2 Detected quantitiesSmall quantities of xylene have been detected in all samples. After three hours the concentrations were measured to be between 3 and 9 µg/m³. For measurement carried out after 3 and 10 days the concentrations were at the same level or below. The concentrations measured after 28 days are considerably higher, which is likely to be due to an error, as it cannot be explained otherwise. 5.2.13.3 ClassificationToluene is included in the list on dangerous substances and is classified under EU index no 601-022-00-9 (The list on dangerous substances, Miljøministeriet 2002):
5.2.13.4 Health conditionsXylene has a TLV in the working environment of 109 mg/m³ (At-vejledning C.0.1). Xylene is described in Environmental Health Criteria 190 (1997). It is stated here that by inhaling the substance about 60% of the inhaled amount are retained in the lungs. Xylene metabolises effectively in the body. More than 90% is transformed to methyl hippuric acid, which will be liberated with the urine. Xylene does not accumulate in humans. Acute exposure to high concentrations irritates and effects the central nervous system. Chronic toxicity seems to be low in experimental animals. However, it indicates that the effects on the central nervous system may occur in animals exposed to moderate concentrations. It seems that xylene is not mutagenic or carcinogenic. The critical end-point is teratogenic effects. In an IUCLID data sheet NOAEC is stated to cause teratogenic effects. In an experiment with rabbits, in which the rabbits were exposed to the substance pregnant in their 7th to 20th day of pregnancy, NOAEL was determined to be 115 ppm or 544 mg/m³. In an experiment with mice exposed to the substance in their 6th to 15th day of pregnancy NOAEC was also determined to be 115 ppm. In the report on risk assessment of toys (CEN/TC 52/WG9, 2003) two limit values in the working environment is stated one at 50 ppm (237 mg/m³) and one at 100 ppm (473 mg/m³). As a value for indoor activities 2 ppm (9.5 mg/m³) is mentioned. Furthermore, TDI is stated to be 0.179 mg/kg. 5.2.13.5 ExposureThe maximum concentration observed is 0.040 mg/m³. The measured concentration is more than 200 times below this limit of 9.5 mg/m³ for indoor activities and far below the stated values for NOAEC. There is therefore no indication that the observed concentrations of the substance do have any influence on the health. 5.2.14 Aliphatic hydrocarbons C10-165.2.14.1 IdentityIn the present report, subsection 4.2.1.8, aliphatic hydrocarbons are described. Among others it is stated that hydrocarbons are linear or branched hydrocarbon chains, which may have one or several double bonds, and do not contain aromatic structures. The boiling points for some of the linear hydrocarbons are:
Substances with double bonds may have lower boiling points. It applies e.g. for 1-decene, which has a boiling point at 140C, and 1-hexadecene, which has a boiling point at 284C. Mineral turpentine has a boiling point interval from 150 to 205°C and resembles these hydrocarbons quite a lot. 5.2.14.2 Detected quantitiesAliphatic hydrocarbons have been detected in the samples. The detected concentrations are shown in table 5.1. Table 5.1 Measurements of hydrocarbons C10-C16
Table 5.1 shows that the concentrations over time are very different in the six samples. The level for sample E is considerably lower than the other samples. For sample B and F the concentrations are relatively high at the beginning and then decreases after 10 days to a low level. For sample A and C the concentrations are still at a rather high level after 28 days. 5.2.14.3 Health conditionsIt is extremely difficult to assess a compound of hydrocarbons when the substances in the compound are unknown. Mineral turpentine has a TLV of 145 mg/m³ in the working environment. There is a risk that substances in the hydrocarbon compound may cause cancer. Kjaergaard et al., 1989, describes a experiment with decane. 63 persons were tested for concentrations of n-decane of 0, 10, 35, and 100 mg/m³. Irritation of the mucous membranes was observed and the subjects experienced increased sensitivity towards odours and reduced air quality. Physiologically, reduced tear film stability was observed. In a laboratory experiment with rats the animals were exposed to non-aromatic White Spirit containing n-decane. The animals were exposed to the substance in concentrations of 0, 400 ppm (2290 mg/m³), and 800 ppm (4580 mg/m³) for six hours per day, five days per a week for three weeks. After a week changes in the concentration of neurotransmitters were observed, which was normalised after 2-3 week after the exposure. It has not been possible to retrieve relevant values for NOAEC. The B-value for mineral turpentine is stated to be 1 mg/m³ (Vejledning no 2, 2002). The stated B-value is based the on odour threshold and the observations in the guideline indicates that the limit for health risks may be 10 times higher. 5.2.14.4 ExposureFor hydrocarbons it is assumed that 100% of the vapour is inhaled and absorbed (Fresp. = 1). The maximum observed concentration is 0.519 mg/m³. The exposure can therefore be calculated as follows: Iinh [mg/kg BW/day] = 0.19 • 1 • 0.519 mg/m³ = 0.099 mg/kg BW/day or approx. 100 µg/kg BW/day. Based on the limit value for turpentine it can be concluded that children are exposed to less than 0.4% of the TLV for product D in which the highest concentrations have been measured. The B-value is at least twice as high at the measured value. This indicates that the likelihood of causing health risk is minimal. However, it shall also be noted that the assessment is based on a very sparse data material. 5.3 Total assessmentThe result of the screening of de 14 substances/groups of substances has been brought together in the following overview. The substances 2-butoxyethanol, phenol, toluene, and xylene do not cause long-term effect in the measured concentrations. These have been found in quantities that are more than a factor 100 below the levels that may cause damages to the health. The substances ethoxyethanol and dimethyl formamide are teratogenic. Ethoxyethanol has been found in quantities that are considerably below the TDI-value. Dimethyl formamide has been found in quantities that are considerably below the levels that may cause damages to the health. Acetaldehyde, formaldehyde, tetrachloroethylene, and trimethylcyclohexen-1-on are under suspicion of causing cancer (Carc3). Formaldehyde has been found in one measure series in concentrations that are above the indoor limit value. In a supplementary test relatively low values were detected. Acetaldehyde and tetrachloroethylen have been found in very small quantities and it is assessed that the risk of health effects will be insignificant. The substances 3-carene, α-pinene, 2,6-di-tert-butyl-p-cresol, and formaldehyde may cause allergy in contact with skin. There is a risk that formaldehyde may cause health effects while the other substances appear in very small quantities and it is assessed that the health effects will be insignificant. The measurement showed that the different aliphatic hydrocarbons with the carbon chains of C10 to C16 are emitted. It is assessed that this group of substances resembles turpentine, which is carcinogenic. It has not been possible to determine a NOAEL. In stead the TLV of aliphatic hydrocarbons and turpentine has been compared. The measured concentrations represent less than 0.4 % of the TLV for turpentine. A comparison with the B-values has also been made. The highest measured concentration represents 50% of the B-value. Based on these comparisons it is assessed that the health risk will be insignificant. Summing up several of the substances, which are present in the tents and tunnels for children, are under suspicion of having carcinogenic, teratogenic, mutagenic, and allergic effects. Formaldehyde is present in one product in a concentration above the indoor limit value while two of the other products are close to the value. In a supplementary three days test of the emission of formaldehyde no concentrations above the limit values were detected. As the measured concentrations of formaldehyde decreased over time, it will be during the first hours of using the tents that the largest emission of the substance will occur. As for the other substances the emitted quantities are relatively small. None of these substances are present in concentrations, which will cause a potential health risk for children to play with the tested products.
|