|
Mapping and release of chemical substances from products made of chloroprene 7 Assessment of Health Effects
7.1 Initial health screening of the identified substances in chloroprene rubberFor the identified substances in the screenings analysis an initial assessment has been carried out. In the initial assessment focus has been towards classification of the substances and potential health effects. The assessment was carried out by combining the CAS-numbers of the substances with information from the list of hazardous substances and other assessable sources. In cases where information was not available for the chemical substance were the properties judged from similar chemical substances. Further to a certain extent information from new investigations has been inclused in the assessment. In table 7.1 one has listed the classification of the substances which are included in the list of hazardous substances. For substances not classified comparison has been made to classified substances. In case this has been made it has been marked in Italics. Table 7.1 Screening of possible health effects
7.2 Assessment of health effects and risk based on migration analysisThe principles for the assessment of health risks are based on EU's Technical Guidance Document (TGD). The assessment is based on the exposure of an adult with a body weight of 70 kg. For calculation of the of the human uptake the following exposure areas are assumed:
The uptake is calculated by:
In the calculation is assumed the exposure happens no more than once a day in the amount of hours that are given for each product. Further, it is assumed that 100 % of the substance is absorbed. The results of the migration tests and the diving test are calculated to amount of the substance that might be present in the body after exposure. The calculations are based on data from table 5.1 and table 6.1. Tabel 7.2 Substances that potentialy can be uptaken in the body
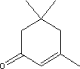
The possible uptake where 100 % of the substance is assumed absorbed is compared with information from the chemical substance under assessment in relation to NOAEL (No observed Adverse effect Level), LOAEL (Lowest Observed Adverse Effect Level) or other relevant sources available. 7.3 Assessment of selected substancesIn the following, a description of the substances potential health effects is described. In the assessment is focused on the properties, that are relevant for skin contact and exposure to skin (dermal uptake). Primarily data on skin penetration, dermal uptake and irritation is given. When is has been possible measured concentrations and the calculated amounts of uptake is compared to NOAEL, No Adverse Effect Levels. For substances, where evaporation is relevant, data for NOAEC, No Adverse Effect Concentrations, is included. 7.3.1 Isophorone7.3.1.1 Identity
Isophorone has a boiling point of 215°C and a melting point of 8.1°C (Lide, DR). The substance is soluble in ether, acetone and alcohol and is used as a solvent for substances that has a very low solubility in other solvents. The substance is soluble in water, 12 gram/litre (Kirk-Othmer). The ration between solubility in octanole and water for the substance, log KOW = 1,7, which means that the substance is more soluble in organic solvents than in water (Veith, 1980) Isophorone has a vapour pressure of 0.438 mm Hg at 25°C (Daubert, 1989). 7.3.1.2 Amount of the substance in the tested productsIsophorone is found in the two tests of diving suits. In the lab-test a concentration of 0.15 mg/litre was found equivalent to a potential uptake of 3 µg/kg based on 100% uptake. In the diving test an amount of 0.1 µg/kg was found based on 100% uptake. Both amounts are based on the earlier mentioned assumptions of contact time per day. 7.3.1.3 Function of the substanceIsophorone has a technical function as highboiling solvent for adhesive and ovendrying laquers. Technical applications include PVC joint sealants. It is judged that in relation to chloroprene based products it is used in relation to the use of adhesive or lamination. 7.3.1.4 ClassificationIsophorone is included in the list of hazardous substances and classified as EU index no. 201-126-0 (Listen over farlige stoffer, Miljøministeriet 2002):
7.3.1.5 Health effectsIsophorone is describes in IUCLID's dataset from 2000 (IUCLID, isophorone). From this comes the following:
Isophorone is included in Environmental Health Criteria 174. From this comes the following information:
7.3.1.6 AssessmentBecause the substance can be absorbed through the skin it is reasonable to assume 100 percent uptake. The potential uptake is 0.003 mg/kg and 0.001 mg/kg. The lowest found value for NOAEL is 150 mg/kg by intake. No information regarding NOAEL data for dermal uptake. LD50 for rabits is 1.200 mg/kg. It is judged that dermal uptake will not be a health risk. It is also assumed that the relative low observed concentrations at 0.15 mg pr. litre will cause no irritations. Based on the above described assessment it is concluded that the observed amounts of isophorone will cause no health effects. 7.3.2 Toluene7.3.2.1 Identity
Toluene has a boiling point of 110.6°C and a melting point of -94.9°C. The substance is miscible with most solvents. Toluene has solubility in water of 526 mg/litre. It has a log KOW of 2.73, which indicate that it is far more soluble in organic solvents than in water. Toluene has a vapour pressure of 28.4 mm Hg at 25°C. 7.3.2.2 Amount of the substance in the tested productsToluene was found in test of waders. The test showed a concentration of 0.12 µg/gram, which is equivalent to an uptake of 0.418 µg/kg, based on 100 percent uptake. Toluene was not found in the other tested products. 7.3.2.3 Function of the substanceToluene is a technical solvent. In relation to chloroprene products it is judged that toluene is used by gluing and lamination of rubber/textile. 7.3.2.4 ClassificationToluene is on the list of hazardous substances and classified under EU index nr. 601-021-00-3 (Listen over farlige stoffer, Miljøministeriet 2002):
A new classification has been proposed for toluene (EU's Risk Assessment report nr. 30). This upcomming classification is:
7.3.2.5 Health effectsIn the Risk Assessment report no. 30 from the EU (EU's Risk Assessment report no. 30). From this report the following information is found. Toluene is very rapidly absorbed by inhalation. The substance is able to penetrate through the skin and will be absorbed by skin contact. Toluene is distributed in the whole body and is primarily found in fatty tissue. Toluene has a low toxicity. Humans exposed to toluene at concentrations of 285 mg/m³ and higher will experience headache, dizziness and fatigue. A value for NOAEC of 150 mg/m³ is estimated based on these findings. Liquid toluene irritates the eyes and vapours in concentrations of around and above 150 mg/m³ causes eye irritations in humans. Based on this a NOAEC for eye irritations is estimated to 150 mg/m³. In relation to inhalation a value of NOAEC is estimated to 1,125 mg/m³. Long term exposure of high concentrations of toulene has caused serious brain damage. It has not been possible to estimate values for NOAEC or LOAEC for long tern exposure in respect to brain damage. There are very limited tests for oral intake and skin contact. In a 13-day test with rats a NOAEL of 625 mg/kg was estimated based on brain damage. In another test with mice a NOAEL of 625 mg/kg was estimated based on damage of the liver. It is stated an LOAEC 330 mg/m³ value with risk for spontaneous abortion and a NOAEC value of 2.250 mg/m³ for low birth weight and retardation. 7.3.2.6 AssessmentWhile toluene is able to penetrate the skin and be absorbed in the body it is assumed that 100 percent will be absorbed. A part of the toluene might evaporate, while the substance is volatile and will be absorbed by inhalation. If it is assumed that 100 percent is absorbed though the skin, the level in the body will be about 0.0004 mg/kg, which is considerable lower than the value of NOAEL of 625 mg/kg. Toluene is present in the product in concentration measured to 0,0046 µg/cm³. This result in a calculated amount in the product corresponding to 0,0029 mg. This amount can in theory evaporate as toluene is rather volatile. However the concentration in the air will be so low that no health risk exist. Although a part of the toluene will evaporate, at no time the concentration in the air will come close to the value of NOAEC at 150 mg/m³. Based on the above-described assessment it is concluded that the observed amounts of toluene will cause no health effects of significance. 7.3.3 Phenol7.3.3.1 Identity
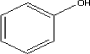
The boiling point of phenol is182°C and the melting point is 41°C. The substance has a vapour pressure of 0,35 mm Hg at 25°C. Phenol is soluble in most organic solvents. The water solubility is 66 gram per litre. At temperatures above 65°C the substance is 100 percent mixable with water. 7.3.3.2 Amount of the substance in the tested productsPhenol is found in dive gloves no. 7. An amount of 0.9 µg/gram was found equivalent to a potential uptake of 0.744 µg/kg. 7.3.3.3 Function of the substancePhenol is not used for technical reasons in chloroprene products. It is judged that phenol originate from inpurities in phenolic resins. Phenolic resins are used for priming of textile for better adhesion to the rubber. 7.3.3.4 ClassificationPhenol is on the list of hazardous chemical substances and classified under EU index no. 203-632-7 (Listen over farlige stoffer, Miljøministeriet 2002):
7.3.3.5 Heath effectsPhenol is toxic with a lethal dose of 50-500 mg/kg for humans. Some persons can be hyper sensitive with serious effects or death caused by exposure to even lower doses. Repeated exposure to drinking water contaminated by phenol during several weeks is reported. The estimated amount of uptake was around 10-240 mg/day and caused skin damage in the mouth, diarré and dark urine. There was no permanent effects six month after the exposure (IARC, 1999). Phenol is able to penetrate the skin and is absorbed quickly to the body. Effects on the body are damage to the CNS, the hart, blood, lungs and kidneys. Observed effects from short-term exposure can include chock, coma, delirium and death. Long term or repeated exposure can cause damage to the liver, kidneys and eyes. Changes in the pigmentation of the skin are reported. Inhalation can cause irritations and oedema. Phenol is included in the IUCLID database from 2000. From the data set comes the following information. Tests show that phenol is not a sensitiser. In a 28-days test with mice were shown that oral intake cause effects on red blood cells and on the level of antibodies in the blood. LOAEL was estimated to 1.8 mg/kg body weight. In a test with rats (Argus Research Laboratories, 1997) the effects on the development of the offspring was analysed. A NOAEL of 60 mg/kg per day was estimated. A benchmark dose of 93 mg/kg was calculated and by including a safety factor of 300 the reference dose was estimated to 0,1 mg/kg/day. Phenol is not recognised as a carcinogen (IARC, group 3) based on insufficient evidence for both humans and animals (IARC, 1999). 7.3.3.6 AssessmentWhile phenol is able to penetrate skin it is assumed that 100 percent of substance is absorbed. The reference dose of 100 µg/kg/day is substantially higher than the calculated amount of 0.7 µg/kg, which might be the daily possible uptake. The calculated amount 0,7 µg/kg is also in relation to the LOAEL of 1800 µg/kg on an acceptable level. Based on the above-described assessment it is concluded that the observed amount of phenol will cause no health effects of significance. 7.3.4 Dibutylformamide7.3.4.1 Identity
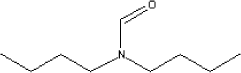
7.3.4.2 Amount of the substance in the tested productsThe substance dibutylformamide was found in knee bandages, diving hut and diving suit. From the knee bandages can be absorbed an amount of 1.3 µg/kg per day if 100 percent is absorbed. From the diving hut can be absorbed an amount of 0.4 µg/kg per day if 100 percent is absorbed. Tests for diving suits were carried out twice, - in the laboratorium and in practice. In the laboratorium an amount of 5.7 µg/kg per day was found and in practise an amount of 0.7 µg/kg per day. The value found in the practical experiment is judged to be the most reliable. 7.3.4.3 Function of the substanceDibutylformamide has no direct technical function in relation to chloroprene products. It is a decomposition product from the accelerators used for crosslinking. 7.3.4.4 ClassificationThe substance is not classified by EU (Listen over farlige stoffer, Miljøministeriet 2002). The substance dimethylformamid which has a similar chemical structure is classified by EU under index no. 616-001-00-X:
7.3.4.5 Health effectsThere is a very limited amount of information regarding the substance dibutylformamide. The substance is described in a few articles in the database TOXLINE, but these articles are primarily about analysis methods. In an article by Chang P-H et al. (1973) is dimethyl-, diethyl-, dipropyl- and dibutylformide briefly described. For both acute and long term toxicity it was shown that the toxicity was decreasing as the molecular weight was increasing. Dipropyl- and dibutylformamide caused liver damage, but did not cause damage to the testicles as the two other formamides did. After daily doses of the four formamides damages of the liver was observed as well as biochemical changes in blood and urine. The most serious effects was seen with lowest molecular weight. Stula og Krauss (1977) have conducted a test for reproductive effects. Both dibutyl- and dimethylformamide were reported causing damages to the foetuses in test with rats. In the following, it is decided to base further information on dimethylfor-mamide while dibutyl- and dimethylformamide in the two references are described as having similar effects or that dibutylfomamide has less health effects than dimethylformamide. In a test with rats lasting 104 days NOAEL was estimated to 210-235 mg/kg based on observations of liver damages (Becci et al, 1983). With respect to absorption through the skin is found a 60-day test with rats, where the animals 4 hours daily had their tails dripped with the substance. Observations were changes in the liver and damages on the CNS. NOAEL was estimated to 60 mg/kg (Medyankin, 1975). Reprotoxic effects have been studied for dimethylformamide. The experiments were all based on inhalation studies. LOAEC for rabbits was determined to 448 mg/m³. The effect of the dimethylformamide was a reduction of birth weight. In the same study NOAEC was found to 150 mg/m³ (Praetorius, W. 1989). In another study pregnant rats were exposed to dimethylformamide vapours in the period from 6. to 15. day of pregnancy. The effect of the inhalation of the substance was low birth weight and fewer new-born young ones. LOAEC was found to 900 mg/m³ and NOAEC to 90 mg/m³ (Bio/Dynamics, Inc. 1978). 7.3.4.6 AssessmentThe amounts found in the test are all lower than 0.002 mg/kg per day. The lowest NOAEL, which has been found for dimethylformamide, is based on skin absorption and estimated to 60 mg/kg per day. It is assumed that NOAEL for dibutylformamide wil have approximately the same value or higher. Even if a safety factor of 1000 is included the observed amounts will still be less than the no-effect-level. Dibutylformamide is like dimethylformamide toxic for reproduction. Data for NOAEC in tests with rabbits and rats where the animals had been exposed during pregnancy showed a lower birth weight for the exposed animals. The lowest NOAEC was determined to 90 mg/m³. It is judged that the amount (less than 0,002 mg/kg per day) which potentially can be inhaled will not give rise to damage to the unborn child. Based on the above describes assessment it is concluded that the observed amounts of dibutylformamide will cause no health effects. 7.3.5 Diethylthiourea7.3.5.1 Identity
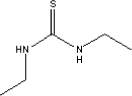
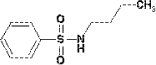
The substance is solid and has a melting point of approximately 70°C. Diethylthiourea is soluble in water, methanol, ether, benzene and ethylacetate. The substance is insoluble in oil. The solubility in water is estimated to 4555 mg per litre. Log KOW is 0.57 (Govers H et al; 1986). 7.3.5.2 Amounts of the substance in the tested productsThe substance diethylthiourea was found in waders and in the test with the diving suit in practice (“real life”). In the test with waders a concentration of 1.8 µg/ml was observed equivalent to a potential uptake of 6.7 µg/kg. In the test with the diving suit a concentration of 0.36 µg/ml was observed equivalent to a potential uptake of 0.8 µg/kg. 7.3.5.3 Function of the substanceDiethylthiourea is used as accelerator by the vulcanisation of chloroprene rubber goods. 7.3.5.4 ClassificationThe substance is not included in the List of hazardous substances. It is included in the consultative list from the Danish EPA )Miljøstyrelsens vejledende liste til selvklassificering af farlige stoffer 2001). In this list diethylthiourea is found as EINICS no. 203-308-5 and classified as Xn; R22, Harmfull and harmfull by ingestion. 7.3.5.5 Health effectsIn Sax (1984) is described 2 short-term test based on oral intake. For rats LD50 was estimated to 316 mg/kg and for mice LD50 was estimated to 681 mg/kg. In a test for potential cancer effects diethylthiourea was given to mice and rats by ingestion over a period of 103 weeks. Rats were daily given 125 mg and 250 mg and mice were given 250 mg and 500 mg. Changes in cells in the thyroid were observed in rats. The test was negative for mice (Bioassay, 1979). IARC (2001) has classified the substance in group 3, not carcinogenic for humans. There is inadequate evidence in humans and only very limited test with animals. Tests show that dimethylthiourea can cause allergy and sensitivity. Dooms-Goossens (1998) describes a study of 4 patients, where 3 developed dermatitis. I Ugeskrift for læger (Medical journal, in Danish) did Buus and Andersen (2002) describes the substance as causing allergic contact dermatitis. It has not been possible to find any data regarding NOAEL. 7.3.5.6 AssessmentThe highest value that was observed for potential uptake was 6.7 µg/kg for waders. No data was possible to retrieve for skin adsorption. While the substance is both water soluble and soluble in fats, it is assumed that the substance can be absorbed. The very limited data regarding toxicity shows LD50 values by oral ingestion as low as 300 mg/kg. Including a safety factor of 1000 this correspond to an acceptable limit of 300 µg/kg. It has been shown that the substance causes a risk for allergic dermatitis, but no information is available of which amount, that causes this effect. Based on the above-described assessment it is concluded that diethylthiourea probably not is toxic in the observed amounts, but there can be a risk of allergic dermatitis. 7.3.6 N-Butylbenzenesulfonamide7.3.6.1 Identity
A very short data set from the UICLID database has been used. From this comes the following information. Butylbenzensulfonamides melting point is –30°C and the boiling point is higher than 250°C. The solubility in water is 1.02 at 20°C. There is no information about solubility in other media's as well as log KOW. 7.3.6.2 Amount of the substance in the tested productsButylbenzensulfonamid is found in knee bandages and in waders. For knee bandages the concentration was measured to 0.50 µg/ml equivalent to a potential uptake of 0.13 µg/kg. In waders the concentration was measured to 0.64 µg/ml equivalent to a potential uptake of 2.4 µg/kg. 7.3.6.3 Function of the substanceIt is judged that butylbenzenesulfonamide is a secundary decomposition product froman accelerator. Accelerators for rubber is e.g. sulfenamides, and formation of sulphoneamides can occur by oxidation of the divalent sulfur in the sulfenamide. 7.3.6.4 ClassificationThe substance is neither included in the List of hazardous substances nor on the consultative list from the Danish EPA. 7.3.6.5 Health effectsIn IUCLID two tests of short term toxicity is reported. The tests are tests on rats and oral ingestion. The reported results range from 1725 to 2050 mg/kg for LD50. For skin absorption is described two test with rabbits, where LD50 was estimated to be higher than 1150 mg/kg. A test with guinea pigs and skin irritation showed a negative result. In a test with rabbits irritations of the eyes were observed. No information regarding allergy is given. In a 28-days study with cats functional disorders were observed by oral ingestion of 57.5 mg/kg. No NOAEL was given. An Ames test with salmonella typhimurium gave a negative result. Besides this IUCLID has not included any further information regarding health aspects. Hashimoto et al (1991) did test the substance in mice to determine weather the substance is toxic of reproduction. Mice got the substance with the feed in doses of 500 mg/kg per day and 750 mg/kg per day. In both groups effects in terms of smaller foetuses were found. The test gave no explanation of weather the damage was caused directly to the foetuses or by effects from the mother. 7.3.6.6 AssessmentBased on the available data it is concluded that NOAEL will be lower 57.5 mg/kg. If it is assumed that NOAEL is about a level of 10 mg/kg and if a safety factor of 1000 included, amounts of 10 µg/kg will be acceptable. The observed values are around 2.4 µg/kg and less. N-butylbezensulfonamid will probably not cause any health effects based on the observed amounts, - but it has to be emphasised that the data is insufficient. Some data indicate that the substance can be toxic to reproduction. 7.3.7 NickelNickel is found in one of the tested products. It is assumed that dimethyl-dithiocarbamate, nickel salt is added to the chloroprene. The nickel ion is present as Ni 2+ and can migrate to the surroundings. In the following, the nickel ion is assessed based on information on nickel oxide and nickel sulphate. 7.3.7.1 Amount of the substance in the tested productsIn the test of the diving suit in practise a content of nickel is measured to 0.095 µg/ml equivalent to a potential uptake of 0.2 µg/kg. 7.3.7.2 Function of the substanceNickel salts from dithiocarbamic acid are effective to inhibit ageing of rubber goods due to exposure to ozone. 7.3.7.3 ClassificationNickeloxide has CAS-no. 1313-99-1 and EU index-n. 1313-99-1. The substance is classified:
Nickelsulfat has CAS-no. 7786-81-4 and EU index-no. 232-104-9. The substance is classified:
7.3.7.4 Health risksIn IUCLID’s data set for nickel sulphate the short-term toxicity in test with rats and oral ingestion showed an LD50 of 275-350 mg/kg. No data for cancerogenic and reproductive effects were found. In a two-generation test with rats (RTI, 1987) nickel chloride was given oral by drinking water. NOAEL was estimated to 30.5 mg/kg/day (250 ppm). Damage at high doses caused decreased birth weight and liver damage. Allergy caused by contact to nickel has been reported both among the general population and in the working environment (Environmental Health Criteria 1991). In IARC's assessment of nickel compounds (IARC, 1990) both nickel sulphate and nickel oxide are characterised as carcinogens, group 1. 7.3.7.5 AssessmentClassification of nickel oxide is irrelevant with respect to inhalation i this study. Based on a NOAEL of 30.5 mg/kg and a safety factor of 1000 a level of approximately 30 µg/kg will be an acceptable level. The observed amount in this study is 0,2 µg/kg and will therefore represent no concern. It should be mentioned that nickel ions can result in allergic reactions by contact with the skin. However it is not possible to judge if this effect can be expressed at the very low level 0,2 µg/kg. 7.4 ConclusionIn the study of the selected chloroprene products a number of chemical substances were found. The study includes identification of 46 chemical substances for which an initial screening was carried out. In the migration tests were identified 7 chemical substances and these are selected for a further assessment. The result of this assessment is presented in table 7.3. The "Analysed amounts" in table 7.3 are the amounts, which potentially can be adsorbed per kg body weight. Table 7.3 Summary of the assessment for substances identified in the migration test.
From table 7.3 can be seen that:
Overall it must be concluded that the found chemicalsubstances will not contribute to a health risk for the investigated products of chloroprene.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||