|
Survey of Estrogenic Activity in the Danish Aquatic Environment 1 Pre-survey testing of procedures and equipment1.1 Assessment of loss of conjugates during analysis 1.1 Assessment of loss of conjugates during analysisEstrogens are primarily excreted from humans and animals in conjugated form. The conjugants are either sulphate or glucuronide and each of them can bind to the estrogens on either the 3 and 17 positions (see Box 1 below) or on both positions. The di-conjugated estrogens are however chemically unstable and are readily cleaved to mono-conjugates. This cleavage occurs almost instantly [1] and the di-conjugated estrogens will therefore not occur in sewage and are therefore irrelevant in the current context. The current section discusses the importance of the conjugated estrogens with regard to interpretation of the results and to the assessment of the potential “delayed” release of estrogens to the environment when conjugated estrogens are cleaved in the environment.
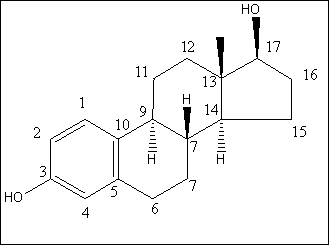
Box 1.1: Structure and numbering of 17β-estradiol In the project, the conjugated estrogens have only been measured indirectly, by measuring the total estrogen concentration after enzymatic de-conjugation. As will be discussed in the following, there is a risk that this determination of conjugated estrogens underestimates the actual amount of these substances. Two factors may have impact on this result. The first occur if the conjugated estrogens not are cleaved completely during the enzymatic deconjugation procedure. Here, it was shown that the cleavage was quantitative with the exception of E2-17S of which only approximately 9% was cleaved (appendix 1.5). A number of studies has however, shown that E2-17S is not excreted from humans and therefore this insufficient cleavage is unimportant in the current context [1-3]. The second reason for a potential underestimation of the concentration of conjugated estrogens is if the loss of conjugated estrogens during the analytical steps is significantly different than that of the parent compounds. This reduction is not taken into account in the calculation of the concentrations which is based on the use of deuterated internal standards (E1, E2 and EE2). Investigations were therefore made to quantify any on loss of conjugated estrogens due to:
Each of these experiments is described in details in the subsequent sections. Our results show that there is no reduction in the amount of conjugated estrogens in the samples during storage. The loss during solid phase extraction is independent of the compound in question and range from 2 to 27%. These findings are consistent with the observations made for the non-conjugated estrogens (the parent compounds) and consequently it can be assumed that the amount of conjugated estrogens is determined with precision which is close to that of the parent compounds. Reference List
1.2 Stability of mono-conjugated steroid estrogensAn experiment was conducted with the aim of documenting the stability of the conjugated estrogens from sampling until storage on SPE-cartridges. Both, the concentration of the conjugated estrogens as well as free estrogens produced during the deconjugation of the conjugated estrogens, are monitored during this experiment. The experiment was performed with seven conjugated estrogens: E1-3G, E2-3G, E2-17G, E1-3S, E2-3S, 17-E2-3S, and E2-17S. More than 6 litres of sewage effluent (from Lynetten Sewage Treatment Plant, Copenhagen) were pH-adjusted to 3.0 using diluted H2SO4. The sewage effluent was divided into three replicate samples each of 2 litres. Each sample was spiked with conjugated estrogens (E-G 125 ng/L and E-S 12.5 ng/L). The samples were stored at 4°C. Two subsamples of 250 mL were taken after 0, 5, 24, 48 and 168 hours from each 2 litre sample. The six subsamples were dived in two subsets (E and EC) each consisting of 3 subsamples. The E subset was reserved for analysis of free estrogens, while the EC subset was prepared for analysis of the conjugated estrogens. The collected subsamples were filtered through a glass fiber filter (GF/C). Subset E was spiked with an internal standard mixture of deuterium marked estrogens (d4E1, d5E2 and d4EE2 at 20 ng/L), whereas subset EC was spiked with a conjugated estrogen Eq-3S (120 ng/L) used as internal standard. The subsamples were pre-concentrated at pre-conditioned SPE cartridges. The cartridges were dried for approximately one hour by N2 and stored at –18°C until analysis. After elution from the cartridges with acetone, the subsamples of subset E was clean-up using silica. All samples were analyzed for free estrogens using the GC-MS-MS method and for conjugated estrogens using HPLC-MS-MS. Results The results of the samples analysed for conjugated estrogens show that the conjugated estrogens are stable during a period of 7 days when the acidity of the effluent samples are adjusted to pH 3 and stored at 4°C (see Tabel 1.1). Calculation of the reduction of the conjugated estrogens in percent is shown in Table 1.2, at it gives a more clear picture of the stability of the substances and confirms their high stability. The analyses of the free estrogens confirm that degradation products of the conjugated estrogens (the free estrogens) are not formed during the storage period. The content of the free estrogens around 2- 6 ng/L is probably the natural content of the sewage effluent used in the current experiment (see Table 1.2). Conclusion Table 1.1: Mean concentration in ng/L of the different conjugated and free estrogens in the spiked effluent samples after 0, 5, 24, 48 and 168 hours of storage. Numbers in brackets represent the STD.
The results listed in the tables below leads to the conclusion that conjugated estrogens present in sewage effluent water are stable under the conditions used for sample storage in the current project. Table 1.2: The mean concentration of the different conjugated and free estrogens in the spiked effluent samples after 0, 5, 24, 48 and 168 hours of storage, expressed in percentage of the initial concentration at 0 hours. Numbers in brackets represent the relative standard deviation.
1.3 Adsorption of conjugated estrogens to glass equipmentThe binding of estrogens to glassware has previously been investigated by Fürhacker et al., who found that E2 does not absorb significantly to glass bottles [1]. As conjugated estrogens are more hydrophilic than the free compounds they may absorb more easily to glassware which has a polar surface. Therefore, in order to find the best suitable equipment for sampling and transportation of the conjugated estrogens, a study was conducted with the aim of testing the sorption of conjugated estrogens to different types of glass ware. Three different flasks were tested, Duran glass laboratory flasks (Schott) that has been washed and heated (450°C), disposable glass bottles (Identipak), and Duran glass laboratory-flasks that has been silanized with dimethyldichlorosilane (in order to reduce the number of negative charges on the glass surface). For each type of glass, duplicate sets of 1 liter of Milli-Q water which had been added a mix of cojugated estrogens (200 ng/l), were prepared. The spiked test solutions were adjusted to pH=3 with 4 M H2SO4. All flasks were stored at 4°C for two days. After storage, the content from each flask, were eluted through SPE columns and dried with N2-gas and stored at -18°C until analysis. After storage, 5 mL of acetone was used to extract the analytes from the SPE-cartridge. Samples representative of no absorption of estrogens to glassware (t=0) was prepared before adding the solutions to the flasks as described above. From these solutions, 1000 mL was eluted through dried with N2-gas and stored at -18°C until analysis. Samples were analyzed for their content of conjugated estrogens using HPLC-MS-MS. Results Recoveries of the conjugated estrogens are calculated as the amount detected in flasks stored for two days divided by the amount found in control samples (t=0). Table 1.3 below lists the recovery obtained for the three different types of flasks. Table 1.3 Recoveries obtained for steroid estrogens in three types of flasks
Conclusion As seen, the recovery was close to 100 for all substances in all types of glassware. The deviations from 100% recovery are due to the uncertainty of the HPLC-MS-MS method used. In conclusion it can be stated that among the types of glassware tested here, there is no absorption of conjugated estrogens to the glass ware. References: Fürhacker, M., Breithofer, A., Jungbauer, A. (1999). 17beta-estradiol: Behavior during waste water analyses. Chemosphere vol. 39, 1903-1909. 1.4 Solid Phase extraction (SPE) testingThis section describes the SPE pre-concentration step The SPE cartridges used was from Varian® (C18 Bond Elut®, 1 g/6 ml). Briefly, filtered samples were acidified with 3.5 M H2SO4 until pH=3, then sample volumes of two litres were spiked with the internal standards (d4E1, d5E1, d4EE2) in concentrations of 10 ng/L. The samples were eluted through the preconditioned SPE cartridge and the analytes were extracted from the cartridge using 5 ml of acetone.
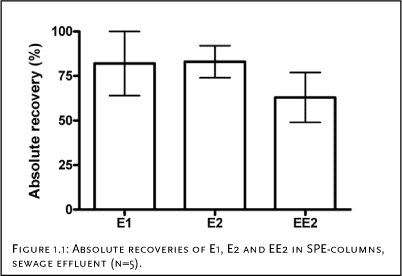
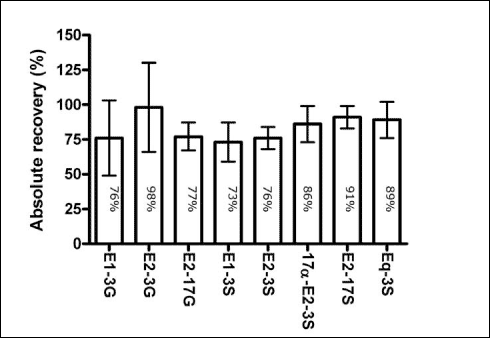
Preliminary experiments The recovery of free estrogens on SPE columns has been studied by several authors and it is generally accepted that high recovery (> 60%) is achieved when using a method as the current (see e.g. [1,4,5]). In a preliminary study, five different cartridges (Varian® C18 Bond Elut® (6 ml/1 g); Isolute® C18 (6 ml/500 mg); Waters Oasis™ HLB (6 cc/200 mg);Isolute® ENV+ (6 ml/1 g); Waters Porapak™ Rdx (6 cc/ mg)) were tested using spiked tap water to 100 ng/L. The highest recovery was obtained using the Varian cartridges, which subsequently has been applied though out the project. This column material has previously been used for analysis of steroid estrogens [2]. SPE recovery (Conjugated estrogens) The recovery of the SPE cartridges (Varian® C18 Bond Elut® (6 ml/1 g) was evaluated using STP effluent samples (2 L) spiked with 20 ng/L for the glucuronidated compounds (E1-3G, E2-3G, E2-17G) and 8 ng/L for the sulphate conjugates (E1-3S, E2-3S, E2-17S, 17-E2-3S). After filtration and pH adjustment, the samples were spiked with the conjugated estrogens and an extraction standard (Equlin-3-Sulphate, Eg-3S, 12.5 ng/L) was added. The samples were then eluted through SPE cartridges as described above [3]. These samples containing conjugated estrogens were not cleaned up using silica gel. Conjugated estrogens are rather polar and will become adsorbed to the gel instead of being washed through. Therefore, the acetone extract was evaporated under a gentle stream of N2-gas. The dry extract was re-dissolved in 200 µL MeOH:MilliQ water (1:1) containing an instrument standard (deuterium labelled estrone sulphate, E1-3S-d4, 25 ng) and analysed using LC-MS-MS. Results and conclusion Figure 1.1 gives an example of absolutes recoveries obtained for E1, E2 and EE2. It should be stressed that that the recovery is dependent of the matrix samples and is generally higher when the matrix is clean (e.g. tap water). In the full method this variability is compensated be the use of the deutorated standards. In Figure 1.2, the absolute recoveries of the conjugated estrogens are presented. As seen, the recoveries range from 73 to 98%. The differences observed between the different compounds are assumed to insignificant and therefore it can be concluded that there is no correlation between the recovery and
A comparison of the data in Figure 1.1 and Figure 1.2 leads to the conclusion that there is no difference between the extraction efficiency of conjugated and free estrogens.
Figure 1.1: Absolute recoveries of E1, E2 and EE2 in SPE-columns, sewage effluent (n=5).
Figure 1.2: Absolute recoveries of the conjugated estrogens (n=3). Error-bars indicate the standard deviations and the numbers on the bars indicate the recovery-values Reference List
1.5 DeconjugationThe acetone extract from the SPE column (only half of the 5 mL is used for the 01 sample) is evaporated to dryness under N2-gas. The samples are dissolved in 2 mL 0.1 M acetic acid buffer (pH=5) containing -glucuronidase enzyme with minimum 2000 units glucuronidase activity and 150 units sulfatase activity (HP-2 from Helix pomatia (Sigma, Germany)) and incubated for 24 h at 40°C. The incubation is ended by addition of 5 mL acidified water (pH=3). The incubation mixture is then extracted on preconditioned C18-columns as described previously. For evaluation of the procedure three issues have to be addressed:
Results and conclusions Three experiments were performed to test these points:
The overall conclusion on the three experiments is that the procedure performed satisfactory for the purpose of the analytical method. All glucoronide and aryl-sulphate conjugates of estrogens appear to be hydrolysed quantitatively to the parent estrogens. Alkylsulphate estrogens (E2-17S and E2-3,17S) which are not expected to be found in human sewage are almost not cleaved with the enzyme preparation. This is consistent with the description by the manufacturer that the enzyme preparation contains â-gluconidase and arylsulfatase activity, but not alkylsulfatase activity. Alkylsulfates are easily hydrolysed with other procedures, but given that no alkylsulphates are expected to occur in sewage, this was not included in the method. Table 1.4: Recovery of steroid estrogens after treatment
Table 1.5: Recovery of conjugates after treatment
Table 1.6: Recovery of E2 after cleavage of conjugated estrogens
1.6 Silica gel clean-upIn order to remove substances with interference on the chemical analyses a cleaning procedure using silica gel was used for all samples for chemical analyses. Briefly, the procedure was as follows: the samples in the acetone eluate from the SPE cartridge were evaporated to dryness with N2-gas. The samples were then redissolved in 200 µl of hexane:acetone (65:35 vol:vol). Columns with silicagel were packed de novo for each sample in 3 mL glass SPE colums using kieselgel 60 from Merck. The kieselgel were weighted to 1.0 g and suspended in the hexane-acetone mixture and stirred to remove bubbles before pouring it into the column. The redissolved samples were then loaded onto the column and eluted with the hexane-acetone mixture until 5 ml eluate were collected. Recovery experiments The absolute recoveries of the analyte for the step were evaluated for each analyte as shown in Table 7. The results are reported on basis of experiments with six replicates. It is seen that the clean-up step is almost quantitative for each analyte. In the full method the small loss and contribution to variation from the clean-up step was fully compensated by use of the deuterated standards. Table 1.7: Recoveries of analytes for the silicagel cleanup step with 95% confidence intervals.
|