|
Evironmental project no. 986, 2005 Background Report on Pre-validation of an OECD Springtail Test GuidelineContents
OECD Guidelines for the Testing of Chemicals List of participants Summary and conclusionsThe aim of the present project was to develop a draft proposal for an OECD test guideline for long-term toxicity-testing of Collembola (see Annex A). The draft guideline was based on two existing standardised test methods, one for Folsomia fimetaria (Wiles & Krogh, 1998) and one for Folsomia candida (ISO, 1999), which were combined and updated. The test on F. candida employs an asexually reproducing species typically living in the organic matter of compost heaps or pot-plants, whereas the test on F. fimetaria employs a sexually reproducing species living in garden and agricultural soils. Accordingly for the test on F. fimetaria the effect on sexual reproduction is obtained. This latter may be seen as a more ecologically relevant endpoint than effects on asexual reproduction, as sexual reproduction is the most frequent reproductive strategy of soil dwelling organisms. In addition, effects on the sexual reproduction of F. fimetaria would include effects caused by chemicals that act via a disturbance of the sex-determination system in arthropods. In the present project the possibility of including a simple test for effects on the sex-determination was investigated, but this was not successful. Given distinct life strategies (and sizes) initial studies were performed investigating possible general sensitivity differences between the two species. The results indicate a difference in chemical sensitivity both according to species and to life-stage, but the differences could only partly be explained by size-differences. To prepare for a ring-test of the draft test guideline three compounds were tested fipronil, 3,5-dichlorophenol and boric acid. Future test concentration ranges were identified for these compounds. Sammendrag og konklusionerFormålet med projektet var at udvikle et udkast til en OECD test-guideline for langtids toksicitetstest af springhaler (se Annex A). Udkastet til test-guidelinen blev baseret på to eksisterende testmetoder, en for Folsomia fimetaria (Wiles & Krogh, 1997) og en for Folsomia candida (ISO 1999), hvilke blev kombineret og opdateret. Testen med F. candida baseres på en aseksuel reproducerende art, der typisk lever i organisk materiale som kompost eller potteplante muld, hvorimod testen med F. fimetaria benytter en seksuelt reproducerende art, der lever i have- og landbrugsjorde. Deraf følger, at testen med F. fimetaria vil medtage effekter på den seksuelle reproduktion, hvilket synes at være et mere økologisk relevant mål, idet seksuel reproduktion er den mest forekommende strategi for jordlevende organismer. Ydermere vil effekter på F. fimetarias seksuelle reproduktion inkludere effekter forårsaget af kemikalier, der virker via en forstyrrelse af kønsbestemmelses-systemet hos leddyr. I det nuværende projekt blev det undersøgt, hvorvidt det var muligt at inkludere en simpel test for effekter på kønsbestemmelsen, men dette lykkedes ikke. Givet de to forskellige livsstrategier (og størrelser) for de to arter blev det undersøgt, om der var en generel forskel i følsomheden imellem arterne. Resultaterne indikerede en forskel i kemikaliefølsomheden som følge af arten og som følge af livsstadiet, men forskellen kunne delvist forklares ved hjælp af størrelsesforskellen. For at forberede en ring-test til test-guidelineudkastet blev tre kemiske stoffer testet fipronil, 3,5-dichlorophenol og borsyre. Testkoncentrations områder til brug i ringtesten blev identificeret for disse stoffer. 1 IntroductionThis report provides a background report for a draft OECD test guideline for long-term toxicity to Collembola, commonly known as springtails (see Annex A). This report aim at providing a background for the draft guideline and to discuss relevant questions related to this guideline. Collembolans are the most numerous and widely occurring insects in terrestrial ecosystems. This is the one of the main reasons for why they have been widely used as bioindicators and test organisms for detecting the effects of environmental pollutants and different agricultural (especially pesticide) regimes on biodiversity in soils (e.g. Paoletti et al. 1991; Crossley et al. 1992; Filser 1995; Filser et al. 1995; Frampton 1997; Paoletti and Bressan 1996). A variety of species have been employed in terrestrial ecotoxicological test. For the present draft OECD guideline Folsomia candida and Folsomia fimetaria have been select as previous standard tests are available (for further discussion on this see "results and discussion"). A springtail test employing the species Folsomia candida (ISO, 19993) is already in use for pesticide evaluation within Europe and USA. It is used where Environmental Agencies require an evaluation of the soil quality, for example when evaluating the potential problems related to spreading sewage-sludge or plant protection products on agricultural land. The test is well suited as a standard-test as the test organisms are easily cultured, the test easily accomplished and the demand for equipment is minimal. The springtail is representative for arthropods that is characterised by an aerobiontic cuticle compared to the soft-bodied semi-aquatic organisms viz. the earthworm test. On these grounds the springtail test is recommended for development in the OECD TG in OECD Monograph no. 11 (Detailed review Paper on Aquatic Testing methods for Pesticides and Industrial Chemicals (Paris 1998) [1]). The method is also recommended by SETAC [2] and in the EU Guidance Document for risk assessment for the soil environment for pesticides [3], biocides and industrial chemicals [4]. An alternative to the F. candida test is a standard test with the near relative Folsomia fimetaria (Wiles & Krogh, 1998). The two species differ in their habitat choice, F. candida resides primarily in habitats rich in organic matter such as pot-plants and compost heaps, whereas F. fimetaria is common in agricultural soils. This make the toxicity effects estimated based on F. fimetaria more applicable to agricultural land and nature areas. Further, Folsomia fimetaria has a sexual mode of reproduction, which is in contrast to F. candida consist of females only with a parthenogenetic (asexual) reproductive mode. It is expected that when utilising a sexually reproducing species the results will have a broader application, since the majority of species are assumed to have sexual reproduction. The two species can appear alike from a visual point-of-view, but they differ in size with F. candida being the larger. It is unknown whether this difference is important for their general sensitivity toward chemicals. The literature is inconclusive on whether the two species differ in sensitivity. For example, Krogh (1995) exposed the two species to dimethoate under identical conditions and observed significant (P<0.05) differences in the EC10 values with regard to reproduction. On the other hand, no significant differences were observed for the copper EC50 values for the two species when exposed under identical conditions (Pedersen et al 1999). Form a pure theoretical point-of-view it could be argued that the two species are likely to differ in sensitivity as the surface-to-volume ratio [this is probably important for toxicity of chemicals that have a passive uptake] is related to the size. As F. fimetaria have a sexual mode of reproduction it may in theory be possible to identify chemicals with sex-determination effects. There are several ways in which to identify a compound that can influence the sex-determination, these includes biochemical assays, morphological tests and sex-ratio tests (deFur et al 1999). Since, the mode-of-action is not known a test of sex-ratio of the juvenile outcome seem the most appropriate test. In addition, a determination of the sex-ratio of the offspring, if successful, could be a simple addition to the overall test design of the draft guideline. There is at present no clear evidence for which compounds that primarily influence sex-determination of invertebrates. The vertebrate sex hormones have been suggested, but this is uncertain. Testosterone has been reported to have some androgenlike activity in some crustaceans (LeBlanc 1999) and endogenous concentrations of testosterone and other vertebrate-type androgens have been reported in Daphnia magna and Neomysis integer (LeBlanc and MacLachlan 2000, Verslycke et al 2002, 2004). Estradiol has been recorded in the silk moth Bombyx mori (Keshan and Ray 2001). However, neither estradiol nor testosterone affected the sex-ratio in exposed Daphnia magna (Kashian and Dodson 2004). Nevertheless, two chemicals, methyl-testosterone and ethynyl-estradiol, have been suggested as reference chemicals for evaluating potential sex-endocrine-disrupting compounds in invertebrates by the participants of "Workshop on Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment (EDIETA)", held in The Netherlands in 1997 (Ingersoll et al 1999). 1.1 AimsTo support the draft guideline two main activities were pursued.
Footnotes [1] Danish publication (sponsored by DG ENV, Danish EPA 14th Office and the Danish EPA Pesticide Research Programme) [2] Cf. ”Test Methods to determine hazards of Sparing Soluble Metal Compounds in Soils” 2002 [3] Cf. EU Guidance Document on terrestrial Ecotoxicology Under Council Directive 91/414/EEC, SANCO/10329/2002 rev.2 final [4] jfr. EU’s rev. TGD: ”Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances and Commission Regulation (EC) No. 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council Concerning the placing of Biocidal Products on the Market” (May 2002) 2 Materials and methods2.1 Background - species identification and comparison According to the aims the studies were divided in two parts. The first part deals with studies on the importance of ecological, biological and physiological for choice of species. These issues will be dealt with though specific aspects (see 2.1). Second, to identify test-concentration ranges for future positive control compounds three compounds will be tested based on previous ring-test programmes and test guidelines (see 2.2). 2.1 Background - species identification and comparison2.1.1 Literature search - Ecology and biology of speciesBased on a literature search and experience a short description of the ecology and biology was performed. Emphasis was put on background for choice of the species included in the guideline (see 3.1). 2.2 Sensitivity comparisonThe two selected species differ in various aspects and may hence in theory differ in sensitivity to chemical toxicity. This issue was studied via two approaches. 2.2.1 General sensitivity comparisonTo study the general chemical sensitivity of the species a literature survey was performed. In this review two compounds were selected for further analysis. The first compound was an choline-esterase inhibiting pesticide, dimethoate. For this compound there was already considerable knowledge available. The second compound was a heavy metal, copper, with a more broad mode-of-action. For this group relevant information is also available in the published literature. Hence, in the present report a comparison was made between the two species, F. fimetaria and F. candida, with regard to the toxicity of Copper and Dimethoate. The data has been obtained via searches in international databases such as WOS, BIOSIS, SCIC and ECOTOX. Only original literature has been cited (see 3.2.1). 2.2.2 Laboratory experiments - Comparison of age/size and toxicityOne of the notable differences between the two species is the size difference. In theory the two species may differ in sensitivity to chemicals due to such a size difference, i.e. the surface area compared to volume is larger for smaller animals (F. fimetaria), which will influence uptake chemicals taken up via outer cuticle. In accordance, an experiment was set up to study whether the toxicity differed between the two species and to see if this could be attributed to size differences. The experiment was based on exposing a range of age-classes (hence also size classes) to one concentration of a chemical and observing differences in mortality between the age-classes (see Table 1). Due to the limited time-span of the project it was decided to use a compound, dimethoate, already tested for both species and to use the LC50 for Folsomia fimetaria reported by Krogh (1995) (see 3.2.2). 2.2.2.1 Test compound
Table 1. Age of animals in size classes employed for both Folsomia fimetaria and Folsomia candida.
Hence, the mortality after exposure for one week to dimethoate 2 mg kg-1 and control was tested for 9 size/age-classes of Folsomia fimetaria and Folsomia candida in 5% OECD soil. They were fed 15 mg Baker's yeast in the beginning of the experiment. Ten individuals were added to each replicate container and extracted after 1 week. There were 2 replicates per species and size-class and treatment. 2.2.2.2 Test soil, counting and statisticsSee section 2.2.1.3-2.2.1-5 2.2.3 Sex-ratio effects on offspringExperiments were conducted to see if it was possible to include offspring sex-ratio as an effect parameter in the draft guideline. To study this adult Folsomia fimetaria were exposed for 42 days to soil contaminated with two potential sex-ratio disrupting compounds, ethynyl-estradiol and methyl-testosterone. The experiment was continued for a total of 42 days to allow the first clutch of the juveniles to become adult (Table 2). Allowing the first clutch of the juveniles to become adult made it potentially possible to identify the sexes on the offspring. The maximum concentrations used were 1000 mg a.s. kg-1 soil corresponding to the limit-testing for plant protection products (see 3.2.3). Since the compounds may bind to the soil particles and hence not be available to the organism a water- exposure test was conducted to ensure exposure. Hence, adult Collembola were exposed for 48 hours to 12 mg testosterone L-1 or 2 mg estradiol L-1 after which the adults were allowed to produce eggs over a period of 7 days. The hatched juveniles were allowed to growth for 23 days prior to size measuring. Table 2. Test conditions of the sex-determination toxicity tests with Folsomia candida.
2.2.3.1 Test compounds
2.2.3.2 Test soil, counting and statisticsSee section 2.2.1.3-2.2.1-5 2.3 Identifying test concentration ranges for ring-testingExperiments were performed in order to identify the test concentrations used in a future ring-test programme and to identify test concentrations used for a positive control. The experiments included three compounds fipronil, 3,5-dichlorophenol and boric acid. The experiments were performed according to conditions stated in table 3. 2.3.1 Test conditions2.3.1.1 Test organismsThe Collembolans Folsomia fimetaria and Folsomia candida (Collembola, Isotomidae) were used as test organisms. For fipronil and 3,5-dichlorophenol tests were performed with F. fimetaria and for boric acid tests were performed with both species. Cultures of these species were kept on moist substrate of plaster of Paris and pulverised activated charcoal (as described in the guideline). Granulated yeast was added weekly as a food source. The cultures were maintained at the Department of Terrestrial Ecology, National Environmental Research Institute. Table 3: Test conditions of the range determination toxicity tests with Folsomia candida and Folsomia fimetaria.
2.3.1.2 Test compoundsAll compound used were of analytic grade and obtained from Sigma-Aldrich (Germany) or Cheminova.
2.3.1.3 Test soilThe test soil was an artificial mixed soil according to OECD guideline. Compared to the "standard" OECD soil, which has a 10% organic matter content, it was chosen in the present test to use a 5% organic matter soil. This latter choice was made to mimic field condition and reduce binding of test chemicals. Soil composition: Organic matter - 5%; clay - 21% and sand - 74% [the clay and sand content has been changed, compared to the original OECD soil, to keep the relative composition the same]. 2.3.1.4 CountingAt the end of each experiment the animals were heat-extracted and counted with a computerised system employing a digital image processing (DIP) approach (Krogh et al. 1998). The guideline alternatively suggests to extract the animals by flotation and to manually count the organisms floating on the surface. It was chosen in this context to use the heat extraction and the automated counting since this is considered more precise than the flotation and manually counting. 2.3.1.5 Statisticsthe data were checked for normality using a 2 test, and for homogeneity of variance by Barlett's test. Effect concentrations, EC10 and EC50, and confidence intervals were estimated by fitting a logistic model to the data (Lacey and Mallet 1991). The formulae were reparametrized by incorporation of the EC10 and EC50 into the equation (Krogh 1995). The estimates with a 95% confidence interval were performed with the SAS procedure PROC NLIN (Enterprise Guide 1.3) 3 Results and Discussion3.1 Background 3.1 Background3.1.1 Ecology and Biology of SpeciesThe following summary is based on the chapter written by Wiles and Krogh (1998). The Collembola, commonly known as springtails, are the most numerous and widely occurring insects in terrestrial ecosystems. This is one of the main reasons why they have been widely used as bioindicators for detecting the effects of environmental pollutants and different agricultural (especially pesticide) regimes on biodiversity in soils (e.g. Paoletti et al. 1991; Crossley et al. 1992; Filser 1995; Filser et al. 1995; Frampton 1997; Paoletti and Bressan 1996, Fountain and Hopkin 2004 a, b). A wealth of literature exists concerning the systematics, evolution, physiology, behaviour and ecology of these organisms (see Hale 1967; Wallwork 1970; Fjellberg 1985; Eisenbeis and Wichard 1987; Greenslade 1991; Christiansen 1990; Curry 1994 and Hopkin 1997). Population densities of Collembola commonly reach 105 m-2 (e.g. Burges and Raw 1967; Peterson and Luxton 1982) in soil and leaf litter layers in many terrestrial ecosystems. Because of their small size however, adults typically measure 0.5 - 5 mm long, their contribution to total soil animal biomass and respiration is low, estimated at being between 1 and 5% (see Petersen 1994). Their most important role may therefore be as potential regulators of processes through microbivory and microfauna predation. Springtails are prey animals for a wide variety of endogeic and epigeic invertebrates, such as mites, centipedes, spiders and carabid and rove beetles. The role of Collembola in humus formation is not well known, but it is commonly accepted that they may contribute significantly to the decomposition processes on acidic stands, where earthworms and diplopods are absent. 3.1.2 EcotoxicologyCollembola possess many attributes of the ideal experimental organism for ecotoxicological research. They are ecologically important, widely distributed, often highly abundant, easily sampled in the field, can be cultured or maintained in the laboratory and have a relatively rapid life-cycle with a high reproductive rate (Spahr 1981). They lend themselves to studies over a wide range of spatio-temporal scales including field monitoring of population trends (e.g. Filser 1995), field bioassays (e.g. Wiles and Frampton 1996), meso- and microcosm studies (e.g. Parmelee et al. 1993) and a wide variety of laboratory investigations (e.g. Crommentuijn et al. 1993; Krogh 1995, Scott-Fordsmand et al 2000; van Gestel and Koolhaas 2004, da Luz et al 2004). They have been used in studies to investigate the environmental effects of a wide range of environmental contaminants. One of the earliest laboratory studies was undertaken by Sheals (1956) who studied effects of organochlorine compounds on microarthropod communities and used supplementary tests with filter paper to screen for species differences in susceptibility to DDT. In a later study Scopes and Lichtenstein (1967) used 6 week old F. fimetaria in an acute test, also using exposure through filter paper. Their results showed that the LD50 for adults was greater than twice the LD50 for juveniles, a fact that has since been used to explain interspecific differences between collembolan species of different sizes. In 1972 Thompson and Gore described an acute test on filter paper and in soil for a sexual population of F. candida. They were one of the first authors to promote the use of F. candida as a test species for laboratory bioassays of pesticides. A large number of laboratory studies with Collembola followed in the 1970's, 80's and early 90's. In these studies a range of ten to fifteen different species of Collembola were used, of which four species have been by far the most common; Folsomia candida, Folsomia fimetaria, Onychiurus armatus (Protaphorura armata), Orchesella cincta. Some of the first standard acute toxicity tests with Collembola were suggested by Huang (1992) and Kiss and Bakonyi (1992) and more recently by Houx et al. (1996). The first initiative to develop a standard sub-lethal test was taken at the Institute for Chemical Examination of the German Federal Biological Research Centre for Agriculture and Forestry. A description of the background for the development of the resulting draft test method of sub-lethal testing with F. candida has been given by Riepert and Kula (1996). The development of a test with F. fimetaria was started in Denmark in 1990 in a project on side-effects of pesticides funded by the Danish Ministry of the Environment (Løkke 1995). The choice of F. fimetaria was based on its wide distribution in Danish agricultural soil and the ease with which it can be cultured in the laboratory (Petersen and Gjelstrup 1995). The procedures for culturing and conducting tests with F. fimetaria were described in Krogh and Pedersen (1995) and Krogh (1995). Based on the above consideration these two species, F. fimetaria and F. candida, were chosen for the draft OECD guideline. 3.1.3 Species inclusion in draft guidelineThe draft OECD guideline proposal includes a choice between two species (see Annex A). The most prominent reason for choosing a particular species for the toxicity test is the reason of relevance to the compartment or life-characteristic which this toxicity study is supposed to represent, e.g. use a species present in agricultural soils when the test is used for agricultural risk assessment. F. fimetaria is widely distributed and common in several soil types ranging from sandy to loamy soils and from mull to mor soils. It has been recorded in agricultural soils in Denmark (Christensen et al. 1987; Krogh 1994; Petersen and Gjelstrup 1995), Sweden (Andrén and Lagerlöf 1983), Finland (Huldén 1984), and Germany (Heimann-Detlefsen et al. 1994). It has an omnivorous feeding habit, including fungal hyphae, bacteria, protozoa and detritus in its food. It interacts through grazing with infections of plant pathogenic fungi (Ulber 1983) and may influence mychorrhiza, as is known to be the case for F. candida. Folsomia fimetaria has a sexual mode of reproduction, whereas F. candida consists of females only with a parthenogenetic (asexual) reproductive mode. It is expected that when utilising a sexually reproducing species the results will have a broader application, since the majority of species are assumed to have sexual reproduction. F. candida is widely distributed throughout Europe. Although it is not common in most natural soils, it often occurs in very high numbers in humus rich sites. It is a blind, unpigmented collembolan which reproduces parthenogenetically. It has a well developed furca (spring organ) and an active running movement and springs readily if disturbed. 3.2 Sensitivity of species3.2.1 Ecotoxicology - General sensitivity differences between the two speciesWhen deciding which of the two species have to be tested a relevant question is whether one of the two species proposed in the guideline can be assumed generally more sensitive than the other species. There has hitherto been few studies dealing with differences in sensitivity between F. fimetaria and F. candida, and it is unknown whether this difference is important for their general sensitivity toward chemicals. To study this a literature was performed and an array of experiments performed (see also 3.2.2). With regard to the literature study two compounds were selected, i.e. the specific acting dimethoate (acetylcholinesterase inhibitor) and the toxicologically more broad acting metal Copper. Based on the literature data for the two compounds no differences in sensitivity between the two species could be detected (see Table 4). For dimethoate the mean EC50 for F. fimetaria was 1.8 [±1.1] dimethoate kg-1 and for F. candida it was 4.0 [±2.6] dimethoate kg-1, but a t-test showed no significant difference (P>0.05). As observed the means were more than 2-fold different, although not significant, which indicate a possible trend in F. fimetaria being more sensitive. This is in line with the observation by Krogh (1995) who exposed the two species under identical conditions and observed significant (P<0.05) differences in the EC10 values with regard to reproduction. They observed that for F. fimetaria the EC50 (reproduction) was 0.3 [0.14-0.47, 95% C.I.] mg kg-1 soil and for F. candida the EC50 (reproduction) was 0.5 [0.43-0.57, 95% C.I.] mg kg-1 soil. Significant differences were also observed on the mortality level, with F. fimetaria being the most sensitive. However, curiously the current laboratory data (see Fig 1) indicate that F. candida is more sensitive than F. fimetaria on a mortality level. Studying the pooled literature data (Table 3) in detail reveals that much of the variation between the different reporting can probably be explained by differences in experimental conditions, such as differences in the soil organic matter content. Differences in the exposure conditions, such as variations in soil type, may render the "bioavailable" fraction different under such conditions. To eliminate differences due to exposure condition (especially soil type) the soil-water concentration could be calculated for KOW (Martikainen and Krogh 1999). Calculating the soil-water concentration by the equation reported by Martikainen and Krogh (1995) it is observed that the ECx values are relatively closer for the two species, when expressed on mg dimethoate L-1 soil water (see Table 4) than when based on soil concentrations. Table 4. The EC50 values (reproduction) for Copper and dimethoate exposure of Folsomia fimetaria and Folsomia candida based on studies reported in the literature and recalculated to soil water concentrations after Martikainen and Krogh 1999).
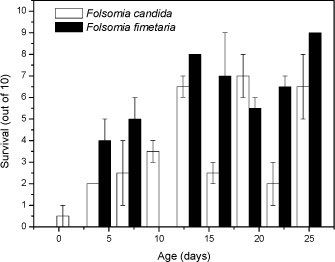
For Cu the t-test comparison between the two species also exhibited no difference between the means with regard to reproduction (P>0.05). The mean for F. fimetaria was 722 [±496] Cu kg-1 and F. candida was 814 [±299] Cu kg-1. This is in line with observations by Pedersen et al (1999) who observed no differences in the copper EC50 values for the two species when exposed under identical conditions. As for dimethoate soil type models could be considered resolving the differences between the soil types, for example the biological ligand-model (BLM). The latter conversion was not performed as the models are presently under development and analysis of the experiments showed that for example temperature and pH may be as important for toxicity as soil type (Sandifer and Hopkin 1996, 1997). In conclusion no difference in toxicity between the two species could be identified, but a possible difference in toxicity may be blurred by confounding factors. 3.2.2 Age- and size-toxicity relationships using dimethoateFolsomia fimetaria and F. candida can appear quite alike from a visual point-of-view, but they differ in size with F. candida being the larger. From a pure theoretical point-of-view it could be argued that the two species are likely to differ in sensitivity as the surface-to-volume ratio is related to the size. Such sensitivity differences could be very relevant when choosing which species to test. Based on an experimental study (see 2.1.2.2) growth of F. candida was faster than F. fimetaria as would be expected from previous knowledge of this species (data not shown). The toxicity decreased with age with juveniles being most sensitive and it decreased faster for juvenile F. fimetaria than for F. candida (Fig 1). Furthermore, there is a clear indication of increased sensitivity of the reproductive instars about age 15 and 22 days (every second instar is reproductive and occurs approx. weekly). Thus, juvenile F. fimetaria was the least sensitive to dimethoate, which is different from a previous studying showing adult F. fimetaria to be the most sensitivity (Krogh 1995). Hence, it seems that the sensitivity is life-stage dependent as confirmed on a refined level in this study (Fig 1). In fact it is seen that both species have very contrasting sensitivities in the days around the age for which they are selected in the draft OECD test. For example, in the F. candida test the start age should be 10-12 days, but if the organisms tend to rather be 9-11 days than 12-14 days the may change the sensitivity two fold (see Fig 1). It is important to realise such small differences may not only be due to a selection based on number of days (age) for the organisms, but a slight increase or drop in temperature may also cause the organisms to be in a different life-stage at the start of the test. In fact it is seen that both species have very contrasting sensitivities around the age for with they are selected for the OECD test. For example, in the F. candida test the start age should be 10-12 days, but if the organisms tend to be 9-11 days rather than 12-14 days this may increase the sensitivity two fold (see Fig 1). It is important to realise such small differences may not only be due to a selection based on number of days (age) for the organisms, but a slight increase or drop in temperature may also cause the organisms to be in a different life-stage.
Fig 1. Survival (out of 10, mean ± standard error) of nine age-/size-classes for Folsomia candida and Folsomia fimetaria exposed for 7 days to dimethoate, 2 mg kg-1 in 5% OECD soil. In conclusion, difference in toxicity between the species could be detected, and a pronounce life-stage toxicity dependency was present for both species. 3.2.3 Additional differences: Sex-ratio of off-springGiven that F. fimetaria is a sexually reproducing species it is possible that compounds which introduces changes in the sex-ratio of the offspring can be identified when using this species. Measurements of the offspring sex-ratio, if successful, would be a simple addition to, and involve a minor change of, the overall test design of the general guideline for long-term Collembola toxicity tests. Hence, based on these considerations it was decided to attempt the sex-ratio approach. The simplest way, and the approach taken, to identify a bias in the offspring sex-ratio was to extend the exposure period ensuring that the offspring would be of sufficient age (19-23 days) for sex identification. Given the excessive man-power that would be required if each individual should be sexed under the microscope it was decided to use the size characteristics to class animals into sexes, this latter could be done by employing the data produced by the digital image processing counting (see Fig 2). In performing this it is necessary to have an synchronous culture partly enabling the addition for females and males at the beginning of the experiment, partly to ensure synchronised clutches and thus avoiding overlap of size classes. An overlap in size-classes will make sex-determination extremely difficult by the termination of the experiment. It was decided to extend the exposure period to 42 days, which would allow for the first clutch to become adult, hence allowing for sex-ratio determination of this.
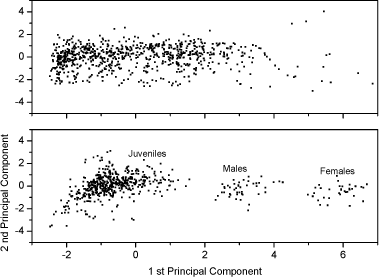
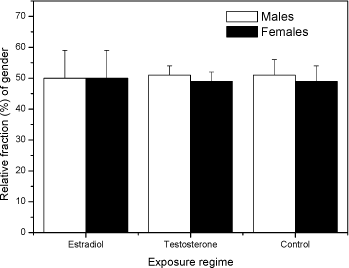
Fig 2. PCA plot of the first and second principal component scores of the original 5 dimension data: area, length, width, slimness and grey density. Lower Fig: three Folsomia fimetaria clusters can be identified: females, males and juveniles following exposure to copper (21, days of exposure, Scott-Fordsmand et al 1997). Upper Fig: No clusters can be identified following exposure to testosterone (42 days of exposure). Two chemicals, methyl-testosterone and ethynyl-estradiol, are suggested as reference chemicals for evaluating potential endocrine-disrupting compounds in invertebrates (Ingersoll et al 1999). There is at present no clear evidence for whether these compounds are involved in the sex-determination of invertebrates. Testosterone has been reported to have some androgen-like activity in some crustaceans LeBlanc (1999) and endogenous concentrations of testosterone and other vertebrate-type androgens have been reported in Daphnia magna and Neomysis integer (LeBlanc and MacLachlan 2000, Verslycke et al 2002, 2004). Estradiol has been recorded in the silk moth Bombyx mori (Keshan and Ray 2001). However, neither estradiol nor testosterone affected the sex-ratio in exposed Daphnia magna (Kashian and Dodson 2004). No studies have previously been reported for Collembola. Upon counting and sizing the offspring from the conducted soil-tests it was realised that with the employed approach it was not possible to identify the sex-ratio of the offspring. The problem seemed to be that the juvenile growth was not sufficient synchronous under the present conditions and the individual clutches overlapped, hence a "small" male from the first clutch had the same size as a "large" female from the second clutch (see Fig 2). It could then have been resolved to identifying the sex of the individual animals, but as the tests resulted in more than 18,000 animals per test this would have been an almost impossible task. The present result indicates that the sex-ratio identification is not applicable with the present approach (see Fig 2). Other approaches may be considered in the future. In fact another approach was partly followed using short-term exposure to an aquatic solution. Based on aquatic solutions adult Collembola were exposed for 24 hours to 12 mg testosterone L-1 or 2,0 mg estradiol L-1 after which the adults were allowed to produce eggs over a period of 7 days. The hatched juveniles were allowed to growth for 23 days prior to size measuring. No bias in the sex-ratio could be identified (Fig 3). No comparable literature data was available.
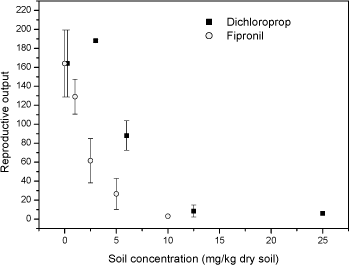
Fig 3 Relative fraction of gender Folsomia fimetaria following juvenile exposed to water, estradiol (12 mg L-1) and testosterone (2 mg L-1) following exposure of parent generation for 48 hours in an aqueous solution. Columns represent mean ± standard deviation. 3.3 Test concentration ranges of possible ring-test compoundsIn connection to the test-guideline and a ring-test programme "standardized" test compounds should be available. Such compounds can be selected on a wide range of criteria, such as how non-toxic it is to humans, how easy it is to store etc. In the present project three compounds were tested, fipronil, 3,5-Dichlorophenol and boric acid. The three compounds were selected on basis of the inclusion in similar test programmes. Two of these compounds, fipronil and 3,5-DCP are currently used in the Copepod ring-test and included here upon the request from the Danish Environmental Protection Agency. Boric acid is suggested as positive control in soil test in North America. Previously, dimethoate has been suggested as a positive control in Collembola tests, but considering that this compound may be taken off the marked it is suggested to avoid using this. As the above sensitivity studies indicated that the difference between the two species would be less than what is important for determining future test ranges, it was decided to test two of the compounds on F. fimetaria only. In other words, the F. fimetaria test ranges is supposed also to cover the F. candida test ranges. For boric acid both species were tested, confirming that the test range covering F. fimetaria also covered F. candida. 3.3.1 FipronilFipronil has been suggested as a positive control in the Copepod ring-test programme. It is an insecticide, mainly used on pets for flea elimination (for a detailed review on this see Tingle et al 2003). As such it should be highly toxic to arthropods, as also confirmed with the present results. For F. fimetaria exposed through the soil fipronil had EC10 and EC50 (based on nominal concentrations) values of 0.58 mg fipronil kg-1 and 1.89 mg fipronil kg-1, respectively. Based on these tests, the ring-test test ranges should be 0-10 mg fipronil kg-1 (see Fig 4). Many data are available with regard to effects of fipronil on invertebrates, but few with soil exposed organisms. Soil toxicity data were only available for earthworms showing LC50 values above 8550 mg kg-1 (Mostert et al 2002). As fipronil is an insecticide it is of little surprise that it is relatively non-toxic to earthworms. No soil toxicity data were found for Collembolans. In conclusion: Based on these tests, the ring-test test ranges should be 0-15 mg kg-1 for fipronil.
Fig 4. Number of juveniles (mean ± standard deviation) Folsomia fimetaria, following exposure of adult Collembola to fipronil or 3,5-chlorophenol for 21 days. 3.3.2 3,5-dichlorophenol3,5-Dichlorophenol (3,5-DCP) has been suggested as a positive control in the Copepod ring-test programme. For F. fimetaria exposed through the soil 3,5-DCP had EC10 and EC50 (based on nominal concentrations) values of 3.07 mg 3,5-DCP kg-1 and 5.53 mg 3,5-DCP kg-1, respectively (see Fig 5). Many data are available with regard to chlorophenol effects on invertebrates, but no 3,5-DCP toxicity data for soil organisms were available. For F. candida Weigmann et al (1985) reported an LC50 value (24 hr exposure) for PCP at 32 ppm. Being weak acids, chlorophenol dissociate at a given pH in accordance with their pKa values. Hence, it can be inferred that the solubility alters much with environmental changes in pH if the pKa is close to the environmental pH. The pKa of 3,5-DCP is 8.54 indicating that at a soil pH around 6 (as in the present test) more than 99% is on the non-ionized state. In conclusion: Based on these tests, the ring-test test ranges should be 0-15 mg 3,5-DCP kg-1 using the reproduction test. 3.3.3 Boric acidBoric acid is suggested as positive control in North America and has been used in many plant toxicity studies. The toxicity of boric acid was studied for both species, first in a short-term test (F. fimetaria) and second in a reproduction study (F. fimetaria and F. candida). According to the guideline the two test should last 21 and 28 days respectively. It was, however, chosen to use a 21-day exposure for both species in order to be able to compare the sensitivity between the two species. The present studies revealed LC10 and LC50 values of 394 mg boric acid kg-1 and 648 mg boric acid kg-1 for F. fimetaria in a 14-day experiment (see table 5). Based on this it was decided to run the reproduction test with concentrations ranging for 0 to 1000 mg boric acid kg-1 for both species. In the reproduction test, which lasted 7 days more than the preliminary test, the mortality proved higher resulting in lower LCx values. The LC10 was below 200 mg boric acid kg-1 for both species. Applying dose-response modelling the EC10 and EC50 values were 24 and 117 mg boric acid kg-1 for F. fimetaria and 22 and 111 mg boric acid kg-1 for F. candida. The latter ECx values should be considered with great care since no test concentration below 200 mg boric acid kg-1 was employed. Time did not allow for a repetition of the experiment, besides the experiment is valid for a test-range identification. Becker-van Slooten (2004) reports ECx values corresponded with results obtained in the present study (see Table 5). Table 5. LCx and ECx concentrations (mg kg-1) for Folsomia fimetaria (14-days and 21-day) and Folsomia candida (21-day) exposed to boric acid in soil.
* Effect concentrations, EC10 and EC50, and bootstrapping intervals (equivalent to 95 % confidence intervals, ICP Nordberg-King, 1993). The studies by Becker van Slooten et al. (2004) is based on a 10% OECD soil In conclusion: Based on the given data the following test concentration ranges are suggested for a ring-test: 0 to 300 mg boric acid kg-1 dry soil. The EC50 (reproduction) should be in the range of 100-150 mg boric acid kg-1 and the LC50 needs to be better defined in the ring test. 3.3.4 Proposed test ranges for a ring-testBased on the three above tests, the ring-test test ranges should be 0-10 mg kg-1 for fipronil, 0-15 mg kg-1 for 3,5-dichlorophenol and 0-300 mg kg-1 for boric acid using reproductive output as a test endpoint. 4 ConclusionSearching the literature a variety of collembolan species have been used in terrestrial ecology. For the draft OECD guideline two collembolan species, Folsomia fimetaria and Folsomia candida, were chosen as these two species have been much used in terrestrial ecotoxicology and standard tests are available. The two selected species represents two ecological and biological approaches. F. candida's habitat is primarily high organic soils, whereas F. fimetaria is present in most agricultural soils. F. candida is parthenogenetic (asexual) reproducing, whereas F. fimetaria is sexually reproducing as are most other species. It was attempted to include juvenile sex-determination as an additional endpoint for the test, but due to overlapping juvenile clutches (within one test) this was not possible. Given the differences between the two species these were reviewed with respect to differences in sensitivity to chemicals, with particular emphasis on possible alterations caused by differences in their size. Neither the literature study nor the laboratory studies resulted in conclusive differences in sensitivity between the species. A comparison in size-class related differences between the two species revealed that there was a slight difference in sensitivity for animals of the same size, but a pronounced stage related difference in toxicity. Hence, the life-stage at which the organisms are exposed determines the sensitivity of the test. In connection with the test guideline (and a ring-test) it will be necessary to have a positive control to ensure the stability of the test-system. Three compounds were tested in order to identify future test ranges. The compounds were fipronil, 3,5-dichlorophenol and boric acid. The tests performed with these chemicals indicated that the test range for fipronil should be 0 to 10 mg fipronil kg-1, for 3,5-Dichlorophenol 0 to 15 mg 3,5-dichlorophenol kg-1 soil and for Boric acid 0 to 300 mg boric acid kg-1. 5 ReferencesAndrén O and Lagerlöf J (1983) Succession of soil microarthopods in decomposing barley straw. In New Trends in Soil Biology. Proc VI Intl Coll Soil Zool, Lebrun Ph, André HM, De Medts A, Gregoire-Wibo and Wauthy G (eds), Louvain-la-Neuve (Belgium), August 30-September 2, 1982, I. Dieu-Brichart, Ottignies-Louvain-la-Neuve, pp. 644-646. Becker-van Slooten K, Campiche S, Feisthauer N, Stephenson G, Tarrasellas and Scroggings R (2004) Research in support of quality assurance requirements for new soil toxicity tests with Collembola. Poster presented at SETAC 2004, Prague, Czeck. Burges A and Raw F (eds) (1967) Soil Biology, Academic Press Inc., London. Christiansen KA (1990) Insecta: Collembola. In Soil Biology Guide, Dindal DL (ed), John Wiley & Sons, New York, pp. 965-995. Christensen BT, Boye Jensen M and Søndergård Klausen P (1987b) Springtails and mites (microarthropods) in different soil types with continuous spring barley and mulching of straw (In Danish with an English summary), Statens Planteavlsforsøg, Tidsskrift for Planteavls Specialserie, Beretning nr. S 1884, Copenhagen. Curry JP (1994) Grassland Invertebrates: Ecology, Influence on Soil Fertility and the Effects on Plant Growth, Chapman and Hall, 437 pp. Crommentuijn T, Brils J and Van Straalen NM (1993) Influence of cadmium onnlife-history characteristics of Folsomia candida (Willem) in an artificial soil substrate. Ecotoxicol. Environ. Safety 26: 216-227. Crossley DA, Mueller BR and Perdue JC (1992) Biodiversity of microarthropods in agricultural soils: relation to processes. Agricul. Ecosyst. Environ. 40: 37-46. deFur PL, Crane M, Ingersoll C and Tattersfield L (1999) Endocrine disruption in invertebrates: Endocrinology, testing, and assessment. SETAC A technical publication. Eisenbeis G and Wichard W (1987) Atlas on the Biology of Soil Arthropods, Springer Verlag, Berlin, 437 pp. Filser J (1995) Collembola as indicators for long-term effects of intensive management. Acta Zool. Fennica 196: 326-328. Filser J, Fromm H, Nagel RF and Winter K (1995) Effects of previous intensive agricultural management on microorganisms and the biodiversity of soil fauna. Plant and Soil 170: 123-129. Fjellberg A (1985) Recent advances and future needs in the study of Collembola biology and systematics. Quaestiones Entomologicae 21: 559-570. Fountain MT and Hopkin SP (2004a) Biodiversity of Collembola in urban soils and the use of Folsomia candida to assess soil 'quality'. Ecotox. 13:555-572. Fountain MT and Hopkin SP (2004b) A comparative study of the effects of metal contamination on collembola in the field and in the laboratory. Ecotox. 13:573-587. Frampton GK (1997) The potential of Collembola as indicators of pesticide usage: evidence and methods from the UK arable ecosystem. Pedobiologia 41:179-184. Greenslade PJ (1991) Collembola (Springtails). In The Insects of Australia: A textbook for students and research workers, 2nd Edition, Division of Entomology, CSIRO, Melbourne University Press, Melbourne, pp. 252-264. Hale WG (1967) Collembola. In Soil Biology, Burgess A and Raw F (eds), Academic Press, London, pp. 397-411. Heimann-Detlefsen D, Theiss S and Heimbach U (1994) Auswirkungen Unterschiedlich Intensiver Bewirtschaftungsintensitäten auf die Collembolenfauna des Ackerbodens. In: Bartels G and Kampmann T (eds). Auswirkungen von Langjährigen Einsatzes von Pflanzenschutzmitteln bei unterschiedlichen Intensitätsstufen und Entwicklung von Bewertungskriterien. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft. Berlin Dahlem. Blackwell Scientific Publishers Heft 295: 230-273. Herbert IN, Svendsen C, Hankard PK and Spurgeon DJ (2004) Comparison of instantaneous rate of population increase and critical-effect estimates in Folsomia candida exposed to four toxicants. Ecotox. Environ. Safety 57:175-183. Hopkin SP (1997) Biology of the Springtails, Insecta: Collembola, Oxford University Press, Oxford, 330 pp. Houx NWH, Dekker A, van Kammen-Polman and Ronday R (1996) Acute toxicity test for terrestrial hazard assessment with exposure of Folsomia candida to pesticides in an aqueous medium. Arch. Environ. Contam. Toxicol 30: 9-14. Huang P (1992) Draft guideline on testing the effects of plant protection agents on Isotoma tigrina Nicolet (Collembola). Bulletin of the International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palaeartic Regional Section (IOBC/WPRS) 15: 122-130. Huldén L (1984) A check list of the Finnish insects. Small orders. Notulae Entomologicae 64: 1-29. Ingersoll GG et al. (1999) Laboratory toxicity tests for evaluating potential effects of endocrine-disrupting compounds. In: deFur, P.L., Crane M, Ingersoll C, Tattersfield L. (1999) Endocrine disruption in invertebrates: Endocrinology, testing, and assessment. SETAC A technical publication. ISO (1999) Soil Quality - Effects of soil pollutants on Collembola (Folsomia candida):method for determination of effects on reproduction. No. 11267. International Organisation for Standardisation, Geneve. Kashian DR and Dodson SI (2004) Effects of vertebrate hormones on development and sex determination in Daphnia magna. Environ. Toxicol. Chem. 23: 1282-1288. Keshan B and Ray AK (2001) The presence of Estradiol-17 and its specific binding sites in posterior silk gland of Bombyx mori. Gen. Comp. Endocrin. 123:23-30. Kiss I and Bakonyi G (1992) Guideline for testing the effects of pesticides on Folsomia candida Willem (Collembola): Laboratory tests. Bulletin of the International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palaeartic Regional Section (IOBC/WPRS) 15: 131-137. Krogh PH (1994) Microarthropods as Bioindicators - a Study of Disturbed Populations, PhD thesis, University of Aarhus. National Environmental Research Institute, Silkeborg, Denmark, 96 pp. Krogh PH (1995) Does a heterogenous distribution of food or pesticide affect the outcome of toxicity tests with Collembola? Ecotoxcol. Environ. Saf. 30:158-163. Krogh PH and Bruus Pedersen M (1995) Laboratory toxicity testing with Collembola. In Effects of Pesticides on Meso- and Microfauna in Soil, Løkke H (ed) (1995), Ministry of the Environment and Energy, Bekæmpelsesmiddelforskning fra Miljøstyrelsen, No. 8, pp. 39-58. Krogh PH, Johansen K and Holmstrup M (1998) Automatic counting of collembolans for laboratory experiments. Appl. Soil Ecol. 7: 201-205. LeBlanc GA (1999) Screening approaches for the evaluation of endocrine disruption in invertebrates. In Henshel DS, Black MC, Harrass MC, eds, Environmental Toxicology and Risk Assessment: Standardization of Biomarkers for Endocrine Disruption and Environmental Assessment, Vol 8. STP 1364. American Society for Testing and Materials, Philadelphia, PA, pp 3–23. LeBlanc GA and McLachlan JB (2000) Changes in the elimination profile of testosterone following exposure of the crustacean Daphnia magna to tributyltin. Ecotoxicol Environ Saf 45:296–303. Lacey RF and Mallett MJ (1991) Further statistical analysis of the EEC ruing test of a method for determining the effect of chemicals on growth rate of fish. OECD Ad hoc Meeting of experts on aquatic toxicology. WRC Medmenham, 10-12 December 1991. Room Document 3. Løkke H (ed) (1995) Effects of pesticides on meso- and microfauna in soil, Ministry of the Environment and Energy, Bekæmpelsesmiddelforskning fra Miljøstyrelsen, No. 8, 185 pp. da Luz TN, Ribero R, Sousa JP (2004) Avoidance test with Collembola and earthworms as early screening tools for site-specific assessment of polluted soils. Environ. Toxicol. Chem. 23:2188-2193. Martikainen EAT and Krogh PH (1999) Effect of sol organic matter and temperature on toxicity of dimethoate to Folsomia fimetaria (Colembola:Isotomiidae)? Ecotoxcol. Environ. Safety 33:128-136. Martikainen EAT (1996) Toxicity of dimethoate to some animal species in different soil types. Ecotoxcol. Toxicol. Chem. 18:865-872. Mostert MA, Schoeman As, van der Merwe M (2000) The toxicity of five insecticides to earthworms of the Pheretima group, using an artificial soil test. Pest Manage. Science 56: 1093-1097. Norberg-King TJ (1993) A liniear Interpolation Method for Sublethal Toxicity: The inhibition concentration (Icp) Approach (Version 2.0). and Dunnett Program (Version 1.5), NETAC Technical Report 03-93, U.S. Environmental Protection Agency, Duluth, MN, USA. OECD Monograph no. 11 (Detailed review Paper on Aquatic Testing methods for Pesticides and Industrial Chemicals (Paris 1998). Paoletti MG and Bressan M (1996) Soil invertebrates as bioindicators of human disturbance. Critical Reviews in Plant Sciences 15: 21-62. Paoletti MG, Favretto MR, Stinner BR, Purrington FF and Bater JE (1991) Invertebrates as bioindicators of soil use. In Modern Techniques in Soil Ecology, Crossley DA Jr., Coleman DC, Hendrix PF, Cheng W, Wright DH, Beare MH and Edwards CA (eds), Agricul. Ecosyst. Environ. 34: 305-313. Parmelee RW, Wentsel RS, Phillips CT, Simini M and Checkai RT (1993) Soil microcosm for testing the effects of chemical pollutants on soil fauna communities and trophic structure. Environ. Toxicol. Chem. 12: 1477-1486. Pedersen MB, Axelsen JA, Strandberg B, Jensen J and Attril MJ (1999) The impact of a copper gradient on a microarthropod field community. Ecotoxocol. 8:467-483. Pedersen MB, van Gestel and Elmegaard N (2000) Effects of copper on reproduction of two Collembola species exposed through soil, food, and water. Environ. Toxicol. Chem. 19:2579-2588. Pedersen MB and van Gestel CAM (2001) Toxicity to the Collembolan Folsomia fimetaria in relation to the age of soil contamination. Ecotox. Environ. Safety 49:54-59. Petersen H (1994) A review of collembolan ecology in ecosystem context. Acta Zool. Fennica 195: 111-118. Petersen H and Gjelstrup P (1995) Development of a semi-field method for evaluation of laboratory tests as compared with field tests. In Effects of Pesticides on Meso- and Microfauna in Soil, Løkke H (ed), Ministry of the Environment and Energy, Bekæmpelsesmiddelforskning fra Miljøstyrelsen No. 8, pp. 67-142. Petersen H and Luxton M (1982) A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39: 287-388. Riepert F and Kula C (1996) Development of Laboratory Methods for Testing Effects of Chemicals and Pesticides on Collembola and Earthworms, Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, Berlin-Dahlem, Heft 320, Parey Buchverlag, Berlin 1996, 82 pp. Sandifer RD and Hopkin SP (1996) Effects of pH on the toxicity of cadmium, copper, lead and zinc to Folsomia candida Willem, 1902 (Collembola) in a standard laboratory test system. Chemosphere 33: 2475-2486. Sandifer RD and Hopkin SP (1997) Effects of temperature on the relative toxicity of Cd, Cu, Pb, abd Zn to Folsomia candida (Collembola). Ecotox. Environ. Safety 37: 125-130 Scopes NEA and Lichtenstein EP (1967) The use of F. fimetaria and Drosophila melanogaster as test insects for the detection of insecticide residues. J.Economic Entomol 60: 1539-1541. Sheals JG (1956) Soil population studies. I. The effects of cultivation and treatment with insecticides. Bull Entomoll Research 47: 803-822. Scott-Fordsmand JJ, Krogh PH and Weeks JM (1997) Sublethal toxicity of copper to a soil-dwelling springtail (Folsomia fimetaria) (Collembola: Isotomidae). Environ. Toxicol. Chem. 16:2538-2542. Scott-Fordsmand JJ, Weeks JM and Krogh PH (2000) Responses of Folsomia fimetaria (Collembola: isotomidae) to copper under different soil copper contamination histories, in relation to risk assessment. Environ. Toxicol. Chem. 19:1297-1303. Spahr HJ (1981) Die Bodenbiologische Bedeutung von Collembolen und Ihre Eignung als Testorganismem für die Ökotoxikologie. Anzeiger für Schadlingskunde Pflanzenschutz Umweltschutz b 27-29. Thompson AR and Gore FL (1972) Toxicity of twenty-nine insecticides to Folsomia candida: Laboratory studies. J. Econom. Entomol 65: 1255-1260. Tingle CCD, Rother JA, Dewhurst CF, Lauer S and King WJ (2003) Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 176:1-66. van Gestel CAM and Koolhaas JE (2004) Water-extractibility, free ion activity, and pH explain sorption and toxicity to Folsomia candida (Collembola) in seven soil-pH combinations. Environ. Toxicol. Chem. 23: 1822-1833. Verslycke T, Poelmans S, de Wasch K, de Brabander HF and Janssen CR (2004) Testosterone and energy metabolism in estuarine mysid Neomysis integer (Crustacea:Mysidacea) following exposure to endocrine disruptors. Environ. Toxicol. Chem. 23: 1289-1296. Verslycke T, De Wasch K, De Brabander F, Janssen CR (2002) Testosterone metabolism in the estuarine mysid Neomysis integer (Crustacea; Mysidacea): Identification of testosterone metabolites and endogenous vertebrate-type steroids. Gen. Comp. Endocrinol. 126:190–199. Wallwork JA (1970) Ecology of Soil Animals, McGraw-Hill, London, 283 pp. Weigmann G, Papenhausen U, Kratz W, Gruttke H (1985) Die wirkung chemisher belastungen auf tier- und pflanzengesellschaften städtischer brachflächen. Specielle Berichte Berlin, Kern Forshung Anlage Jülich, vol 296. 121-129. Wiles JA and Frampton GK (1996) A field bioassay approach to assess the toxicity of insecticide residues on soil to Collembola. Pesticide Science 47: 273-285. Wiles JA and Krogh PH (1998) Testing with the collembolans I. viridis, F. candida and F. fimetaria. In Handbook of soil invertebrate toxicity tests (eds Løkke H and Van Gestel CAM), pp. 131-156. John Wiley & Sons, Ltd., Chichester. OECD GUIDELINES FOR THE TESTING OF CHEMICALS
|
| Purpose | Range-finding test | Definitive Reproduction test - 1 | Definitive Reproduction test - 2 |
| Species | F. fimetaria/F. candida | F. candida | F. fimetaria |
| Aim | Mortality | Reproduction | Reproduction |
| Time (day) | |||
| -9-12 (F. candida) -13-26 (F. fimetaria) |
Preparation of synchronous culture | Preparation of synchronous culture | Preparation of synchronous culture |
| Day 5 or earlier | - Prepare artificial soil (mixing of dry constituents) | - Prepare artificial soil (mixing of dry constituents) | - Prepare artificial soil (mixing of dry constituents) |
| Day 3 | - Check pH of artificial soil - Measure max WHC of soil |
- Check pH of artificial soil - Measure max WHC of soil |
- Check pH of artificial soil - Measure max WHC of soil |
| Day 2 to 1 | - Sort Collembola for testing | Sort Collembola for testing | - Sort Collembola for testing |
| Day 1 | - Pre-moisten artificial soil and distribute into batches - Prepare stock solutions - Apply test substance if solvent required |
- Pre-moisten artificial soil and distribute into batches - Prepare stock solutions - Apply test substance if solvent required |
- Pre-moisten artificial soil and distribute into batches - Prepare stock solutions - Apply test substance if solvent required |
| Day 0 | - Prepare stock solutions - Apply test substance - Weigh test substrate into test vessels - Mix in food - Introduce Collembola - Measure soil pH and moisture content |
- Prepare stock solutions - Apply test substance - Weigh test substrate into test vessels - Mix in food - Introduce Collembola - Measure soil pH and moisture content |
- Prepare stock solutions - Apply test substance - Weigh test substrate into test vessels - Mix in food - Introduce Collembola - Measure soil pH and moisture content |
| Day 7 | - Check soil moisture content | - Check soil moisture content | - Check soil moisture content |
| Day 14 | - Determine adult mortality - Estimate number of juveniles - Measure soil pH and moisture content |
- Check soil moisture content - Feed |
- Check soil moisture content -Feed |
| Day 21 | - Check soil moisture content | -Extract all individuals - Check soil moisture content -Measure pH |
|
| Day 28 | - Extract all individuals - Check soil moisture content -Measure pH |
DATA AND REPORTING
Treatment of results
35. An overview is in the following but more detailed statistical guidance for analysing test results is given in the "OECD draft statistical report 2003".
36. In the range finding test, the main endpoint is mortality. Probit analysis [14] should normally be applied to determine the LC50. However, in cases where this method of analysis is unsuitable (e.g., if less than three concentrations with partial kills are available), alternative methods can be used. These methods could include moving averages [14], the trimmed Spearman-Karber method [15] or simple interpolation (e.g., geometrical mean of LC0 and LC100, as computed by the square root of LC0 multiplied by LC100).
ECx estimation (preferred method)
37. To compute any ECx value, the per-treatment means data are used for regression analysis (linear or non-linear), after an appropriate dose-response function has been selected [16]. Among suitable functions are the normal sigmoid, logistic or Weibull functions, containing two to four parameters, some of which can also model hormetic responses. If a dose-response function was fitted by linear regression analysis a significant r² (coefficient of determination) and/or slope should be found with the regression analysis before estimating the ECx by inserting a value corresponding to x% of the control mean into the equation found by regression analysis. 95%-confidence limits are calculated according to [17].
38. Alternatively, one may express the treatment results as percentages of the control result or as percent inhibitions relative to control. In these cases, the normal (logistic, Weibull) sigmoid curve can often be easily fitted to the results using the probit regression procedure [17]. In these cases the weighting function has to be adjusted for metric responses as given by [18]. However, if hormesis has been observed, regression analysis should be replaced by a four-parameter logistic or Weibull function, fitted by a non-linear regression procedure [19]. If a suitable dose-response function cannot be fitted to the data, one may use alternative methods to estimate the ECx, and its confidence limits, such as Moving Averages after Thompson [14] and the Trimmed Spearman-Karber procedure [15].
NOEC estimation (alternative method)
39. If an analysis of variance has been performed, the standard deviation, s, and the degrees of freedom, df, may be replaced by the pooled variance estimate obtained from the ANOVA and by its degrees of freedom, respectively – provided variance does not depend on the concentration. In this case, use the single variances of control and treatments. Those values are usually calculated by commercial statistical software using the per-vessel results as replicates. If pooling data for the negative and solvent controls appears reasonable rather than testing against one of those, they should be tested to see that they are not significantly different (for the appropriate test consider Annex 4). Further statistical analysis and inferences depend on whether the replicate values are normally distributed and are homogeneous with regard to their variance.
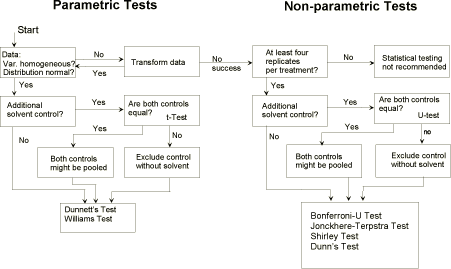
41. NOEC estimation: Kolmogoroff-Smirnov's [20] and Bartlett's-test procedures [21] are used respectively for testing for normality and homogeneity of variance homogeneity. With normally distributed and homogeneous data, multiple t-tests such as Dunnett’s test [22, 23] or William’s test (a = 0.05, one-sided) [24, 25] should be performed. It should be noted that, in the case of unequal replication, the tabulated t-values must be corrected as suggested by Dunnett and Williams. Sometimes, because of large variation the responses do not increase/decrease regularly. In this case Dunnett’s test does not lead to reasonable NOEC/LOEC values and analysis by Williams-test is to be preferred. Alternatively, Otherwise, a multiple U-test, e.g. the Bonferroni-U-test according to Holm [26], could be used.
42. If a limit test has been performed and the prerequisites of parametric test procedures (normality, homogeneity) are fulfilled, the pair-wise Student t-test can be used or otherwise the Mann-Whitney-U-test procedure [20].
40. The test report should include the following information:
40.a. Test substance:
- physical nature and, where relevant physical-chemical properties (e.g. water solubility, vapour pressure);
- chemical identification of the test substance according to IUPAC nomenclature, CAS-number, batch, lot, structural formula and purity;
- expiry date of sample.
40.b. Test species:
- test animals used: species, scientific name, source of organisms and breeding conditions.
40.c. Test conditions:
- ingredients and preparation details of the artificial soil; (minimum: C/N ratio, pH, WHC, CEC)
- method of application of the test substance (data verifying homogeneity of application);
- description of the test conditions, including temperature, moisture content, pH, etc.;
- full description of the experimental design and procedures.
40.d. Test results:
- Actual concentration of the tested compound in the test media (at minimum for control exposures)
- mortality of adult Collembola after two weeks and the number of juveniles at the end of the range-finding test;
- mortality of adult Collembola after three weeks exposure and the full record of juveniles at the end of the definitive test;
- any observed physical or pathological symptoms and behavioural changes in the test organisms;
- the LC50, the NOEC and/or ECx (e.g. EC50, EC10) for reproduction if some of them are applicable with confidence intervals, and a graph of the fitted model used for its calculation, the slope of the dose-response curve and its standard error;
- all information and observations helpful for the interpretation of the results.
- Power of the actual test.
Deviations from procedures described in this guideline and any unusual occurrences during the test.
LITERATURE
(1) Wiles JA and Krogh PH (1998) Testing with the collembolans I. viridis, F. candida and F. fimetaria. In Handbook of soil invertebrate toxicity tests (ed. H. Løkke and C. A. M. Van Gestel), pp. 131-156. John Wiley & Sons, Ltd., Chichester.
(2) ISO (1999) Soil Quality - Effects of soil pollutants on Collembola (Folsomia candida):method for determination of effects on reproduction. No. 11267. International Organisation for Standardisation, Geneve.
(3) Burges A and Raw F (Eds) (1967) Soil Biology. Academic Press. London
(4) Petersen H and Luxton M (1982) A comparative analysis of soil fauna popyulations and their role in decomposition processes. Oikos 39: 287-388
(5) Petersen H (1994) A review of collembolan ecology in ecosystem context. Acta Zoologica Fennnica 195: 111-118
(6) Hopkin SP (1997). Biology of the Springtails (Insecta : Collembola). Oxford University Press. 330pp (ISBN 0-19-854084-1).
(7) Ulber B (1983) Einfluss von Onychirurus fimatus Gisin (Collembola, Onychiuridae) und Folsomia fimetaria L. (Collembola, Isotomidae) auf Pythium ultimum trow. einen Erreger des Wurzelbrandes der Zuruckrübe. In New trends in soil Biology (Lebrun Ph, André HM, De Medts A, Grégoire-Wibo, Wauthy G (Eds), Proceedings of the VI international colloquium on soil zoology, Louvain-la-neuve (Belgium), 30 August-2 September 1982, I. Dieu-Brichart, Ottignies-Louvain.la.neuve, pp. 261-268.
(8) Scott-Fordsmand JJ and Krogh PH (2004). Background report on prevalidation of an OECD springtail test guideline. MST in press.
(9) Becker-van Slooten K, Campiche S, Feisthauer N, Stephenson G, Tarrasellas and Scroggings R (2004) Research in support of quality assurance requirements for new soil toxicity tests with Collembola. Poster presented at SETAC 2004, Prague, Czeck.
(10) OECD (Organisation for Economic Cooperation and Development) (1984). Earthworm, Acute Toxicity Tests, Guideline No. 207. OECD, Paris.
(11) Edwards (1955) Simple techniques for raraing Collembola, Symphyla and other small soil inhabiting arthropods. In Soil Zoology (Kevan DKMcE, Ed). Butterworths, London, pp. 412-416.
(12) Goto HE (1960) Simple techniques for the rearing of Collembola and a not on the use of a fungistatic substance in the cultures. Entomologists' Monthly Magazine 96:138-140
(13) Krogh PH, Johansen K and Holmstrup M (1998) Automatic counting of collembolans for laboratory experiments. Appl. Soil Ecol. 7, 201-205.
(14) Finney DJ (1978) Statistical Method in Biological Assay. - Charles Griffin & Company Ltd, London.
(15) Hamilton MA, Russo RC and Thurston RV (1977) Trimmed Spearman-Karber Method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11(7), 714-719; Correction Environ. Sci. Technol. 12(1998), 417.
(16) OECD (2003) Draft Guidance Document for on the Statistical Analysis of Ecotoxicity Data. Environment Directorate, ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT, Paris 2003.
(17) Finney DJ (1971) Probit Analysis (3rd ed.), pp. 19-76. Cambridge Univ. Press.
(18) Christensen ER (1984) Dose-response functions in aquatic toxicity testing and the Weibull model. Water Research 18, 213-221.
(19) Van Ewijk PH and Hoekstra JA (1993) Calculation of the EC50 and its confidence interval when sub-toxic stimulus is present. Ecotox, Environ. Safety. 25, 25-32.
(20) Sokal, R.R. and F.J. Rohlf. (1981) Biometry. The principles and practice of statistics in
biological research. 2nd edition. W.H. Freeman and Company. New York.
(21) Miller, R.G., Jr. (1986). Beyond ANOVA, basics of applied statistics. John Wiley & Sons. New York.
(22) Dunnett, C.W. (1955). A multiple comparison procedure for comparing several treatments with a control. Amer. Statist. Ass. J. 50, 1096-1121.
(23) Dunnett, C.W. (1964). New tables for multiple comparisons with a control. Biometrics 20, 482-491.
(24) Williams, D.A. (1971). A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics 27, 103-117.
(25) Williams DA. (1972). The comparison of several dose levels with a zero dose control. Biometrics 28, 519-531.
(26) Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scand. J. Statist. 6, 65-70.
ANNEX 1
The following definitions are applicable to this Guideline
LC50 (Median lethal concentration) is the concentration of a test substance that is statistically likely to kill 50% of exposed test organisms within a given time period. In this test the LC50 is expressed as a mass of test substance per dry mass of the test soil or as a mass of test substance per unit area of soil.
LOEC (Lowest Observed Effect Concentration) is the lowest test substance concentration that has a statistically significant effect (p < 0.05) In this test the LOEC is expressed as a mass of test substance per dry mass of the test soil or as a mass of test substance per unit area of soil. All test concentrations above the LOEC should normally show an effect that is statistically different from the control. Any deviations from the above must be justified in the test report.
NOEC (No Observed Effect Concentration) is the highest test substance concentration immediately below the LOEC at which no effect is observed. In this test, the concentration corresponding to the NOEC, has no statistically significant effect (p < 0.05) within a given exposure period when compared with the control.
ECx (Effect concentration for x% effect) is the concentration that causes an x% of an effect on test organisms within a given exposure period when compared with a control. For example, an EC50 is a concentration estimated to cause an effect on a test end point in 50% of an exposed population over a defined exposure period. In this test the effect concentrations are expressed as a mass of test substance per dry mass of the test soil or as a mass of the test substance per unit area of the soil.
Reproduction rate: Mean number of juvenile Collembola produced per a number of adults over the test period.
ANNEX 2
DETERMINATION OF THE MAXIMUM WATER HOLDING CAPACITY OF THE SOIL
The following method for determining the maximum water holding capacity of the soil has been found to be appropriate. It is described in Annex C of the ISO DIS 11268-2 (Soil Quality - Effects of pollutants on earthworms (Eisenia fetida). Part 2: Determination of effects on reproduction (3)).
Collect a defined quantity (e.g. 5 g) of the test soil substrate using a suitable sampling device (auger tube etc.). Cover the bottom of the tube with a piece of filter paper fill with water and then place it on a rack in a water bath. The tube should be gradually submerged until the water level is above to the top of the soil. It should then be left in the water for about three hours. Since not all water absorbed by the soil capillaries can be retained, the soil sample should be allowed to drain for a period of two hours by placing the tube onto a bed of very wet finely ground quartz sand contained within a covered vessel (to prevent drying). The sample should then be weighed, dried to constant mass at 105°C . The water holding capacity (WHC) can then be calculated as follows:
WHC (in % of dry mass) = ![]()
Where:
S = water-saturated substrate + mass of tube + mass of filter paper
T = tare (mass of tube + mass of filter paper)
D = dry mass of substrate
ANNEX 3
DETERMINATION OF SOIL pH
The following method for determining the pH of a soil is based on the description given in ISO DIS 10390: Soil Quality – Determination of pH (15).
A defined quantity of soil is dried at room temperature for at least 12 h. A suspension of the soil (containing at least 5 grams of soil) is then made up in five times its volume of either a 1 M solution of analytical grade potassium chloride (KCl) or a 0.01 M solution of analytical grade calcium chloride (CaCl2). The suspension is then shaken thoroughly for five minutes and then left to settle for at least 2 hours but not for longer than 24 hours. The pH of the liquid phase is then measured using a pH-meter that has been calibrated before each measurement using an appropriate series of buffer solutions (e.g. pH 4.0 and 7.0).
ANNEX 4
OVERVIEW OF THE STATISTICAL ASSESSMENT OF DATA (NOEC DETERMINATION)
List of participants
(15 countries and 26 laboratories):
Canada:
Dr. G. Stephenson /Dr. Feisthauer, ESG International, Ontario, Canada
Dr. J. Princz, Environment Canada, Quebec, Canada
Czech:
Dr. J. Haufman, Masaryk University, Czech
Denmark:
Dr. P.H. Krogh, Danmarks Miljøundersøgelser, Danmark
Dr. J.J. Scott-Fordsmand, Danmarks Miljøundersøgelser, Danmark
France:
Prof. P. Vasseur, Faculty of Sciences, Univeristy of Metz, France
Germany:
Dr. S. Knaebe, GAB-Biotech, Germany
Dr. J. Römbke, ECT Oekotoxikologie, Germany
Dr. B.M. Wilke, Berlin University of Technology, Germany
Dr. F. Riepert, BBA, Germany
Hungary:
Prof. G. Bakonyi, Department of Zoology and Ecology, Hungary
Japan:
Prof. N. Kaneko, Yokohama National University, Japan
Nigeria:
Prof. M.A. Badejo, Obafemi Awolowo University, Nigeria.
Norway:
Dr. H. Stubberup, Jordfrorsk, Norway
The Netherlands:
Dr. C.A.M. van Gestel, Vrije Universitet, The Netherlands
Portugal:
Dr. J-P. Sousa, University of Coimbra, Portugal
Prof. A. Soares/Dr. M. Amorim, University de Aveiro, Portugal
Russia:
Dr. A Pokarzhevskii, Russian Academy of Science, Russia
Switzerland:
Dr. K. Becker van Slooten, Ecole PolyTechnique federale de Lausanne, Switzerland
Dr. M. Candolfi, RCC Ltd, Itingen, Switzerland
Thailand:
Dr. C.B. Iwai, Khon Kaen University, Thailand
United Kingdom
Dr. G. Frampton, University of Southampton, UK.
Dr. M. Riches, CEMAS,UK
Dr. M. Coulson, Syngenta, UK
USA
Dr. R. Kuperman, US Army, Edgewood Chemical Biological Center, USA
Dr. R. Lanno, University of Ohio, USA
Version 1.0 March 2005, © Danish Environmental Protection Agency