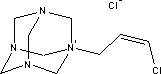|
Survey of Chemical Substances in Consumer Products, no. 59, 2005 Survey and assessmens of chemical substances in glass and porcelain coloursContents2 Composition of and typical constituent substances in hobby colours 3 Survey of suppliers and producers 4 Survey of constituent substances in glass and porcelain colours 6 Selection of substances and data search 7 Toxicological profile of selected substances
Appendix A Extractable substances PrefaceList of Abbreviations The Danish Environment Protection Agency (DEPA) has taken an initiative to illustrate the consumers' exposure to chemical substances in consumer products. Several product categories, e.g. hair styling products, hygiene products and glass and porcelain colours for hobby use are covered by the process. The aim of the project is to give an overview of substances in both CE labelled and non-CE labelled glass and porcelain colours. The survey is based on information from producers, suppliers and other relevant sources. It has furthermore been the intention to make a human and ecotoxicological assessment of selected substances in the products and to estimate the volume of the impact the consumers and the environment are exposed to if possible. The human and eco toxic assessment is carried out in view of giving input to a possible future risk assessment with special focus on the human toxicological impact and children's possible exposure to the substances. At the same time it is the intention to document the content of selected chemical substances through chemical analysis of a number of products available on the market. Finally, the need for additional chemical analysis has been assessed based on the results from this survey. The project report "Survey and assessment of chemical substances in glass and porcelain colours" includes an overview of the manufacturers whose products are on the Danish market, the most important suppliers of the products and the products on the Danish market. In an overview form, the substances of the glass and porcelain colours are categorised as pigments, binders and additives. The chemical name of the substance, formula, CAS no. and selected physical properties are listed. The project began in October 2001. Phase 1 ended in December 2001 and Phase 2 in June 2003. However a few analyses were repeated on 4 new products in September 2004. The report was sent in hearing in May 2005 and verification that the ingredients in P1 and P2 have been changed so that they no longer contain 2-butanonoxim has been obtained by the Danish EPA. Furthermore we have been informed that product P12 is no longer on the marked. Product P6 contains methylpyrrolidone but the large content has been ascribed to analysis uncertainty as the manufacturer has provided proof of a lower content based on the recipe and therefore hazard labelling is not required. The project was carried out by COWI A/S. MILJØ-KEMI, Dansk Miljø Center A/S (now Eurofins Denmark A/S) has made the analysis of selected glass and porcelain colours. The project consisted of Sonja Hagen Mikkelsen (project manager), Sven Havelund, Anders Skibsted Mogensen and Frank Stuer-Lauridsen (quality assurance). Contact persons in DEPA were Shima Dobel, Annette Orloff and Lise Emmy Møller, Consumer Section, Chemicals Division. List of Abbreviations
Hazard symbols and R-phrases used
Summary and conclusionsSurvey SurveyGlass and porcelain colours are hobby products for children and adults. CE labelled products are intended for children under 14 years. Products with the CE mark must conform to the prevailing requirements for toys and are restricted in regard to content and release of certain substances dangerous to health. As all private consumers are not familiar with the CE mark, the Danish Environmental Protection Agency expects that children below 14 years will have a risk of getting into contact with glass and porcelain colours that are not CE marked and do not conform with the safety requirements for toys. Exposure to substances in the products can occur in the form of direct contact with fingers, hands and face and by inhalation. Exposure of the environment is possible when brushes and pencils are cleaned and disposed of after use. Therefore there is a need to examine the substances the consumers and the environment may be exposed to during use of the products. There are about 15 producers of glass and porcelain colour and window colour worldwide and most products from these producers are on the Danish market. There are fewer glass and porcelain colours which are CE labeled or carry some other labelling than window colours. 17 pigments, of which two are intermediates, including both inorganic and organic substances are identified in the survey. Among the organic pigments, azo-pigments and polycyclic pigments have been identified. Only one pigment, copper phthalocyanine, containing heavy metal has been identified. None of the inorganic pigments identified in the survey contain heavy metals. All glass and porcelain colours except one included in the survey and test are water based. Binders are primarily acrylates, often thermoplastic (meth)acrylates. Acrylates consist of acrylic acid and methacrylic acid and their methyl-, ethyl- and butyl esters. Additives include among others thickeners, surfactants, biocides, anti foaming agents and solvents (co-solvents). In glass and porcelain colour, biocides like isothiazolones and bronopol are used. These biocides are also used in CE marked products. A range of glycols and alcohols are also found in this type of products. Chemical analysisScreening analysis of substances in glass and porcelain colours show in general that it is alcohols, glycols, ketones, esters and simple hydrocarbons, which are the main constituents among the extractable substances. Some of these are identified by chemical name and some as substance groups. The levels in the tested products vary between few mg/kg to 150,000 mg/kg in one product for methylpyrrolidone corresponding to 15% in the product (P6). In the safety data sheet it is declared that the content is 1-1.5%. Butanoneoxim is found in concentrations up to 2.9%. A hydrocarbon mixture (C6-C12) of alifatic, alicyclic and aromatic hydrocarbons in concentrations of 17% is found in one product (P12) corresponding to the information in the safety data sheet on the white spirit content. Examples of other substances identified in relatively high concentrations in a single product are buthoxyethanol (2.4-2.5%) (P9) and phthalic acid anhydride (2.3-3.0%) (P12). Methoxy(propenyl)phenol (Eugenol) was found in two products in concentrations between 21 and 26 mg/kg (0.0021 and 0.0026%). This substance is considered sensitising by skin contact and is covered by the amendment to the Cosmetic Directive of 27 February 2003 with a requirement to indicate the presence of the substance in the ingredients list when its concentrations exceeds 0.001% in leave-on products, and 0.01% in rinse-off products. Other substances are found in concentrations ranging from about 0.0004% up to 0.5%, most in the lower end. This is also the case for the substances selected for toxicological evaluation based on the results from the chemical analysis. Most of the concentrations are therefore below the general minimum limits stated in Statutory Order no. 329 of 16 May 2002 from the Ministry of the Environment on classification, packaging, labelling, sale and storage of chemical substances and products (classification order). Among the metals, lead, zinc, copper, cobalt and titanium have been analyzed. Lead is found in three products in concentrations corresponding to 0.0026 and 0.0027% in the products. Consequently the concentration levels are below 0.15% requires special labelling of lead containing products to be used as paint or lacquers according to the classification order. Health evaluationBased on the survey and the results from the chemical analyses, 9 substances have been selected for toxicological evaluation. The substances are:
Of the 9 evaluated substances, three are on the List of Dangerous Substances and one substance is on the Danish EPA's guidance list for self classification. For these substances toxicological literature has been available which supports and to some extent supplements the information expressed through the classification. For the five other substances more limited information or no information on toxicological properties has been available in the searched literature. This is also the situation regarding NOAEL values, and exposure data in relation to human exposure. Critical effects in relation to the evaluated substances for the intended use are acute toxicity (1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride, 2-naphthol, hexamethylene diisocyanate and 5-isocyanate-1-(isocyanatemethyl)-1,3,3-trimethyl-cyclohexane), possible carcinogenic effects (anthraquinone and 2-butanone oxime) and sensitising effects (anthraquinone, 2-butanone oxime, 2-naphtol, 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride, diarylide, hexamethylene diisocyanate and chloroisocyanate benzene). Seven of the 9 substances which are evaluated with regard to health and environmental properties have more or less well documented sensitising potential and four are classified as sensitisers. In the product P12 phthalic acid anhydride has been identified in concentrations of 2.3 - 3%. This substance must be classified as sensitising by skin contact and by inhalation in concentrations of 1% or more. The substance must be expected to come from alkyd resin in the product. The substance will therefore exist in chemically combined form in the product, but can be released by thermal decomposition. Methylpyrrolidone is found in concentrations between 0.8 and 15%. The substance must be classified as irritating to skin and eyes in concentrations of 10% or more. In total, three of the 10 analysed products (P3, P4 and P8) are CE labelled. Based on information from the suppliers and results from the analyses, all three products are considered to comply with the rules. Environmental evaluationWith regard to the environmental properties, only few data have been found in the literature about the substances. For two of the substances it is known that they are both very dangerous to aquatic organisms and also very persistent in the environment. Hence it is important to minimise the discharge to the water environment. 1 IntroductionGlass and porcelain colours, as well as window colours, are hobby products for children and adults. The products are used for decoration of glass, bowls etc. with following hardening and burning in an ordinary oven for 40 minutes at 160°C. Some products are CE labelled and as such meant for play use for children below the age of 14. CE labelled products must observe current regulations for toys and are submitted to restrictions related to content and emission of certain substances harmful to health. As not all consumers are familiar with the labelling, DEPA assesses that children below the age of 14 have a risk of getting in contact with glass and porcelain colours which are not CE labelled. The CE labeling indicates that the toy meets the safety requirements, set out the in Danish Consumer Agency's Statutory Order no. 329 of 23 May 1995 on safety requirements to toy and products which, due to their outward appearance, may be mistaken with food products. Considering the chemical properties of the toy, the requirements are that the toy is produced so that it does not present any health risk. It is the responsibility of the toy manufacturer to observe this. The rules apply both when the toy is:
In general, the toy must not contain dangerous substances in amounts that represents a health risk, i.e. substances that are classified as dangerous and on the List of Dangerous Substances, or which meet the criteria for classification according to the Statutory Order no. 329 of the Ministry of the Environment dated 16 May 2002 on classification, packing, labelling, sale and storage of chemical substances and products. Chemical toys, as defined in DS/EN 71-4 on experimental sets for chemistry experiments and equivalent activities and DS/EN 71-5 about chemical toys (sets) other than sets for chemistry experiments, can be marketed if the content of dangerous substances are below a certain low maximum concentration limit if the chemical substances are necessary for the functioning of the toy. DEPA's assessment is that CE marked hobby paint products must contain maximum 1% substances that are harmful to health, local irritant or corrosive, where 1% is the so-called de minimis limit. However, this is not relevant if the products are comprised by the standards on sets for chemical use or other chemical toy. The CE labelling also implies that the toy meets the requirements of maximum labelling of substances with a considerable health impact. Such substances as lead, cadmium, mercury, selenium, chromium, barium, arsenic and antimony. Exposure to the product substances may occur in form of direct contact with fingers, hands and face and by inhalation. Exposure to the environment is possible when the brushes are cleaned after usage and when the product is disposed of. A survey of which substances the consumers and the environment may be exposed to by using glass and porcelain colours is thus relevant and necessary. The products are sold for private use and have a chemical composition that typically does not require application to the product register. It is thus necessary to obtain information of the content from suppliers and producers. Furthermore, a literature search was carried out for typical constituent substances. The survey was partly based on literature information, available information from the Internet and partly on information from safety data sheets, suppliers, producers and applicators of chemical products. The project team also contacted the Danish Council for Creative and Hobby Materials (Fællesråd for formnings- og hobbymaterialer). From a consultant, Chemtox, working with the assessment of chemical products in view of their registration in the product register and elaboration of safety data sheets, a list of constituents in glass and porcelain colours was supplied. For confidentiality reasons there is no information about amounts or relation to product and producer names. Finally, a number of stores were visited to examine the product range and product information on the package. 10 different glass and porcelain colours in different colours were purchased in view of chemical analysis. Subsequently, 9 chemical substances were selected and based on the survey and on results from the chemical analysis of these substances; a toxicological profile was made covering both human toxicological and eco toxicological data. In agreement with the importers the analyses of 2-butanonoxime were repeated in 2004 on new versions of four products. 2 Composition of and typical constituent substances in hobby colours2.1 Pigments Colours and paint contain four basic components. There is a large difference between the selected basic components depending on the function of the colour and requirement to appearance and durability. The four basic components are:
A short description is given in the following of the four types of content. 2.1 PigmentsPrimary pigments add whiteness and colour to the product. TiO2, titandioxid is typically used as white pigment. Colour pigments add colour by selective absorption of light. Organic colour pigments are used (bright/sparkling colours) and inorganic colour pigments (earth colours). Of organic pigments can be mentioned phthalo blue. Inorganic pigments could for example be metal oxides (iron oxide). The actual pigments are normally powder form and they are mixed with the binder and thinned with a dilutent in order to obtain the actual colour. 2.2 BindersBinders are used to bind the pigment and act as glue. There are oil based and latex based binders. The oil based binder dries/oxidises when exposed to air. Oil based binders may consist of linseed oil or soya oil. Also alkyd can be used in oil based binders. Latex based binders are used in water based paints. The binder is a solid, plastic like material. The particles are microscopic and contained in the paint. Latex based binders may consist of acrylic or vinyle acrylic (poly vinyl acetate, PVA) or styrene acrylic. Polyurethanpolymerer may also act as a binder. 2.3 DiluentFor oil based paint and alkyd paint a diluter is used. Typically an organic solvent and for latex based paint, water is used as vehicle. 2.4 AdditivesAdditives are for example:
Thickeners are used to give the paint the right consistency when used. Surface active substances stabilise the paint so it does not divide and give increased dispersion of pigments. Preservatives prevent undesired bacteria in the paint when stored or after being used. Anti-foam materials prevent foam at blending and applied. Co-solvents are used for non-water based paint and are typically an organic solvent that improves the dissolution of one or several of the components. 3 Survey of suppliers and producersGlass and porcelain colours are sold in many stores but do not seem to be as prevalent as for example window colours for hobby use. In Denmark the products mainly come from European and American producers. A short overview presenting producers, suppliers and products in the market is shown in the following. 3.1 ProducersThe European producers are dominant on the Danish market. Worldwide there are approx. 15 producers of hobby paint /23/. The producers of glass and porcelain colours also make window colours. Table 3.1 presents an overview of the main producers on the Danish market. Table 3.1 Producers of glass and porcelain colours on the Danish market.
Glass and porcelain colours are popular and can be purchased in both special shops for hobby products, book shops, toy shops and supermarkets and the colours are also sold directly to institutions. You can also buy the colours on-line from suppliers on the Internet. 3.2 SuppliersTable 3.2 shows a selection of suppliers of glass and porcelain colours in Denmark. Normally, the suppliers only have products from one or two producers. Table 3.2 Selected Danish suppliers of glass and porcelain colours.
3.3 Glass and porcelain coloursTable 3.3 shows the main part of glass and porcelain colours on the Danish market. The label on the package is also listed. Table 3.3 Glass and porcelain colours on the Danish market
Apart from the above there are a number of contour paints for drawing the outline. 4 Survey of constituent substances in glass and porcelain colours4.1 Pigments The survey of constituent substances is based on information from producers, suppliers and other relevant sources. As a starting point available information has been obtained from safety data sheets and other product information from suppliers and producers. Furthermore it has been tried, after agreement with the suppliers, to obtain additional information from the producers. All producers have, as a minimum, been contacted by letter, either via the Danish supplier or directly. With this contact the producers have also received an accompanying letter from DEPA with product information and reference to the responsible in DEPA. The request has been followed up by phone and in writing. Two producers have given additional information on constituent substances which is not in the safety data sheets. However, the survey has not been so extensive that it allowed a very comprehensive collection of data. Furthermore, a list of constituent substances in a number of glass and porcelain colours has been obtained from the consultant Chemtox. The information covers products in relation to possible registration in the Product Register and the elaboration of safety data sheets. It is thus safety data sheets on glass and porcelain colours, product information from suppliers and producers and the previously mentioned gross list which are the basis of the survey. All together it has been assessed that this information gives a satisfactory overview of the constituent substances in glass and porcelain colours. As a minimum, safety data sheets must contain information on dangerous substances as defined in the Statutory Order no. 559 of the Danish Working Environment Service dated 4 July 2002 on special requirements to producers, suppliers and importers etc. of substances and materials according to law on working environment. In some cases, the data sheets also have information about other substances in the product. In total, data sheets or other information from eight products have been used in this survey. For two products there is no information. The result of the survey has also been presented to the Danish Council for Creative and Hobby Materials. The Council has confirmed conformity with the Council's information and the result of the survey. The constituent substances are divided into the categories:
4.1 PigmentsSeveral of the used pigments are both used in other paints and other product types for colouring of e.g. textiles. It is shown in Table 4.1 that both inorganic and organic pigments are used in the glass and porcelain colours. Of organic pigments azopigments and polyclyclic pigments have been found. There has only been found one pigment, copper phthalocyanine, which contains a dangerous heavy metal. None of the inorganic pigments contain environmentally hazardous heavy metals. Table 4.1 Pigments in glass and porcelain colours and their physical-chemical properties (data from 26, 16 and 17). "-": the value is unknown. "ir": irrelevant.
1) The numbers in this column shows how the pigments may be used in cosmetic products. * May be used in cosmetics, but not as a pigment. Of pigments and medium products, only 2-napthol is on the List of Dangerous Substances in the danger classes harmful to health and dangerous to the environment and with the classification Xn;R29/22, N;R50. Isoindolinon is on the recommended list for self classification in the danger class dangerous to the environment and with N;R51/53. 4.2 BindersAcrylate and polyurethanpolymer are used as binders in glass and porcelain colours. Acrylates and metacrylates are used broadly as binders in both water soluble colours and solvent middle based colours. Poly(met)acrylates represent a large group of substances and the composition may vary depending on the desired properties. /25/. Termoplastic (met)acrylates are often used as binders. Acrylates consist of acryl acid and metaacryl acid ant their methyl, ethyl and botylesters (2). Table 4.2 shows a list of binders in glass and porcelain colours. Table 4.2 Binders and residual monomers in glass and porcelain colours and their physical-chemical properties (data from 26, 16 and 17). "-": the value is unknown.
4.3 Fluid/VehicleAll of the found glass and porcelain colours are water based - with the exception of one product. 4.4 AdditivesAccording to the information there is a number of additives in glass and porcelain colours. In Table 4.3 is shown the additives used in glass and porcelain colours and selected physical-chemical properties. Table 4.3 Additives in glass and porcelain colours and their physical-chemical properties (data from 26, 16 og 17) and classification. "-" : the value is unknown, "ir": irrelevant.
1) According to List of dangerous substances (in bold) or from the recommended list for own classification (italic). The most widespread preservative in glass and porcelain colours are isothiazolinones, 2-bromo-2-nitropropane-1, 3-diol (bronopol) and formaldehyde, but also other preservatives are used as listed in the table. For example the parabenes benzo acid p-hydroxy methylester and benzo acid p-hydroxy propylester and the formaldehyde releaser, 1-(3-chloroallyl)-3,5,7-triasa-1-azoniaadamantan chloride (Quartenium-15). These substances have also been found in CE labelled products. The two isothiazolinones 5-chloro-2-methyl-4-isothiazol-3-one (cas no. 26172-55-4) and 2-methyl-4-isothiazol-3-one (Cas no. 2682-20-4) which are marketed under the name Kathon, are often found in glass and porcelain colours. This also applies for 2-bromo-2-nitropropane-1,3-diol and formaldehyde, which often derives from impurities in the used raw materials or from release of formaldehyde releasers (preservatives). The two isothiazolinone derivatives are both in a low concentration in glass and porcelain colours and are under the de minimis limit according to the safety data sheets for the examined products. The substances are also present in for example cosmetic products, where the permitted limit is 0,0015%. 5 Chemical analyses5.1 Test products 5.1 Test productsTable 5.1 shows the products included in the project. Table 5.1 Test products selected for qualitative analysis
5.2 Methods of analysis5.2.1 GC/MS screening (extractable substances)Approximately 5 g of the product was taken and extracted with dichloor-methane added internal standard by using Soxhlet extraction for 16 hours. A part sample of the extract was taken and analysed directly as well as concentrated at combined gas chromatography and mass spectrometry (GC/MS) by scanning over a large mass area. The content is calculated compared to internal standards. The analyses were carried out as genuine double specifications. The detection limit is 1-5mg/kg for components that are semi-quantified. The uncertainty is 10-20% RSD. The analyses of 2-butanoneoxime were repeated in 2004 on four new versions of the products P1, P2, P10 and P11. The detection limit is 50 mg/kg. The uncertainty is 15% RSD. 5.2.2 X-rayA part sample was examined using x-ray technique to discover the content of 40 elements. This analysis was outsourced. The analyses were carried out as genuine double specifications. The detection limit is 10 mg/kg. The uncertainty of the analysis is 5-10% RSD. 5.2.3 Water content by Karl Fisher titrationThe water content of the sample is determined by automatic titration using the Karl Fisher titration method (KF). The analyses are made as genuine double specifications. The uncertainty of the analysis is 10% RSD. 5.2.4 IR-screeningA part sample was taken which was pressed into a potassium bromide tablet to be analysed by the FT-IR analysis to determine content of organic main parts by comparison with data library spectra. The analyses were carried out as genuine double specifications. The analysis will not be reported separately. The result of the screenings has been used to support the result of the GC/MS screening. 5.2.5 Identification of azo pigmentsA part sample test is refluxed with alkaline sodium dithionite and the azo pigment is transformed to arylamine by a reductive decomposition. An analysis for arylamines is made by use of high pressure chromatography (HPLC). The detection limit is 5 mg/kg and the uncertainty is 10% RSD. The analysis includes the following amines: 4-Aminodiphenyl, Benzidine, 4-Chloro-o-toluidin, 2-Naphthylamine, p-Chloraniline, 2,4'-Diaminoanisol, 4,4'-Diaminodiphenylmethan, 3,3'-Dichlorbenzidin, 3,3'-Dimethoxybenzidine, 3,3'-Dimethylbenzidine, 3,3'-Dimethyl-4,4'-diaminodiphenylmethane, p-Cresidine, 4,4'-Methylene-bis-(2-chloraniline), 4,4'-Oxydianiline, 4,4'-Thiodianiline, o-Toluidine, 2,4-Toluendiamine, 2,4,5-Trimethylaniline, o-Aminoazotoluene and 2-Amino-4-nitrotoluene. The analysed amines are all covered by restrictions in the Statutory Order no. 755 of the Ministry of the Environment on prohibition of import, sale and use of certain pigments dated 15 August 2003. Azo pigments, which can emit these amines in concentrations above 30 ppm in the final products or in the coloured parts hereof, are not allowed to be used in textile or leather colours which may be in direct contact with human skin or mouth in for a longer period. 5.3 Test results5.3.1 GC/MS screening (extractable substances)The results from the GC/MS screening are shown below. The two results indicate the double specification. The substances were identified from the mass spectre by comparing with the mass specter in a data library. Specters, which represent the best match, are in each case assessed by "scientific judgment". In those cases where identification is not possible, the components are part of a group designation with a total sum. The detection limit is 1-5 mg/kg. The DEPA requested that standards for each component were included, some were detected in the GC/MC screening and others were found at the survey. These components are thus calculated compared to the equivalent external reference substances and marked by a ^ in the table. For all other organic components a semi quantative calculation has been made against internal standards. The detection limit for components compared to external standard is 1 mg/kg and the uncertainty is 10-15% RSD. For a few components the content was so large that it was necessary to dilute the sample in order to calculate the content correctly. Those components are listed by Because of findings in the survey DEPA wished to include the pigment diarylide (C.I. pigment yellow 12) as external standard. This component could not be obtained as standard why it was decided to analyse for a number of azo pigments in the two relevant samples of porcelain colours (yellow colours). The analysis for azo pigments is made by changing possible azo pigments into arylamines and then analyse for a total of 20 arylamines. The results of the repeated analyses on the four products P1, P2, P10 and P11 are presented in table 5.4. Table 5.2. Analysis results of extractable substances in glass and porcelain colours. The two results refer to the double specification. The results are in mg/kg. The detection limit is described above. Table 5.3 Analysis results of extractable substances in glass and porcelain colours. The two results refer to the double specification. The results are in mg/kg. The detection limit is described above. Table 5.4 Analysis results of repeated analyses of 2-butanoneoxime in four products. The two results refer to the double specification. The results are in mg/kg. The detection limit is described above.
^: betyder at komponenter er regnet overfor ekstern standard. The new analyses in 2004 show concentrations of 2-butanonoxime in the range from 1.4 to 2.1%. The substances in table 5.2, table 5.3 and table 5.4 are listed in Appendix A with CAS numbers. 5.3.2 X-ray analysesTable 5.4 shows the results of the x-ray analyses. The elements not listed in the table were not detected at the analysis. The analysis is made by a single specification for reliability reasons. The detection limit is 10 mg/kg. Table 5.4 The results from the x-ray analyses of glass and porcelain colours. The results are in mg/kg.
-: below the detection limit. 5.3.3 Water contentTable 5.5 and 5.6 show the results of water content analyses. The two values refer to the double specifications. Table 5.5 Results of water analyses of glass and porcelain colours. The results are in %.
Table 5.6 Results of water analyses of glass and porcelain colours. The results are in %.
5.4 Azo pigmentsTable 5.7 shows the results of the analysis of azo pigments. The analysis was only carried out in two yellow porcelain colours as agreed between COWI and DEPA. Table 5.7 The results for arylmines of two selected glass and porcelain colours. The results are in mg/kg.
-: below the detection limit. 5.5 Summary of analysesThe screening analyses of constituent substances in glass and porcelain colours generally show that it is especially alcohols, glycols, ketones, esters, and simple hydrocarbons. Some of them are identified by name and others as substance groups. The levels in the different products vary from a few mg/kg to 150,000 mg/kg equivalent to 15%. Methylpyrrolidone which is found in the highest concentration among these substances, are at a double specification measured to be between 8,000 and 150,000 mg/kg equivalent to 0.8-15.0% in the three products (P1, P2, P6, P10 and P11). 2-butanone oxime has been found in five products in concentrations between 1.4 and 2.9% (P11) and butoxyethanol in a concentration 2.4-2.5%. Among the other substances it is a hydrocarbon composition (C6-C12) of alifatic, alicyclic and aromatic hydrocarbons that are found in the highest concentration (17%). Furthermore phthalic acid anhydride (2.3-3.0%) is an example of another substance and group of substances that by double specification has been found in relatively high concentrations in one product. Toluene diisocyanate has been measured to 3.0-7.4% in one product. Methoxy(propenyl)phenol (Eugenol) has been found in 2 products in concentrations between 21 and 26 mg/kg (0.0021 and 0.0026%). Other substances have been found in concentrations from approx. 4 mg/kg (0.0004%) and up to approx. 5000 mg/kg (0,5%), most in the lower end. This also applies for the substances selected for toxicological assessment based on the chemical analyses. The substances are listed in Table 5.8. Anthraquinone and 2-butanoneoxime were selected in the first place on the basis of the survey. 2-butanonoxime was at the first analyses found in concentrations in the range from 1.7 to 9.2%. The repeated analysis in 2004 has shown concentration levels from 1.4 to 2.1% for the four products P1, P2, P10 and P11. Table 5.8 Selected substances for environmental and health assessment
Of metals lead, zinc, copper, cobalt and titanium have been found. Lead has been found in concentrations of 26 and 27 mg/kg equivalent to 0.0026 and 0.0027% in the products. In this way the concentration limits below 0.15% which requires special labelling of lead products to be used for chemical products as paint or varnish according to the Statutory Order no. 329 of the Ministry of the Environment dated 16 May 2002 on classification, packing, labelling, sale and storage of chemical substances and products. The adjusted analytical result for lead in accordance with Danish Standard, DS/EN 71-3:1995 on safety requirements to toys and migration of particular materials is maximum 18.9 mg/kg (calculated based on the maximum measured concentration of 27 mg/kg). The adjusted analytical result is considerably below the standards requirements to the marginal value for migration of lead from toys of 90 mg/kg. The marginal value for migration of lead has been calculated based on the requirement of set out in the Statutory Order no. 1161 dated 12. December on safety requirements to toy. Copper has been found in concentrations of up to 0.14%, cobalt in one product with a concentration of 0.056% and zinc also in one product in a concentration of 0.098%. Two yellow porcelain colours have been examined for content of azo pigments with a negative result. The analyses have thus determined approx. 50% (weight) of the content of all the products. Other constituent substance components are primarily binders and fillers. 6 Selection of substances and data search6.1 Background for selection of substances 6.1 Background for selection of substancesAt the initial survey 17 substances were identified in the pigment group (including two intermediate products), two substances in the group of binders and their residual monomers and 22 substances in the group of additives. Among these substances which both has been identified based on general information about content in glass and porcelain colours and concrete information about the test products only 3 substances have been found in connection with the chemical analyses. In this connection it should be underlined that the screening analyses do not specify all substances. A number of substances are grouped under the designations alkanes, alkenes, alcohols, cycloalkanes/-alkenes, glycols and oxy compounds, aromatic compounds and other unidentified substances. Many of the substances found during the survey must be assumed to be covered by these unit designations. This also applies for the additives which are identified in the survey and shown in Table 6.1. The survey also includes a number of pigments which you would not expect to find at screening analysis of only 10 products. Based on the results from the survey and the chemical analysis of the test products, DEPA has selected a number of test products which should be assessed in view of their health and environmental profile. For these 10 substances a toxicological profile has been elaborated based on available literature. At the selection the concentration of the substances has been considered and existing knowledge on/or assessments of the substances in connection with consumer products. The selected substances thus represent substances that are found in the highest concentrations in the test products and the substances which DEPA requested to be analysed. 6.2 Selected substancesTable 6.1 gives an overview of selected substances to be assessed for health and environmental properties: Table 6.1 Overview of selected substances to be assessed for environment and health properties
6.3 Data searchData for the substances' physical-chemical properties were collected from a number of internet based databases.
There will be a certain overlap between several of these databases. Table 6.2 gives an overview of the result of the data search. The overview shows whether the 14 databases contained data for each substance. Table 6.2. Overview of the result of the data search. The number of the databases refer to: 1. Chembank, 2. Chemfinder, 3. Ullmann, 4. NTP, 5. SAX, 6. TOXLINE, 7. MEDLINE, 8. HSDB, 9. IRIS, 10. CCRIS, 11. GENETOX, 12. IUCLID, 13. PHYSPROP, 14. BIODEG, 15. BIOLOG, 16. ECoTOX. +: data was found. -: data was not found.
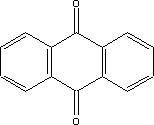
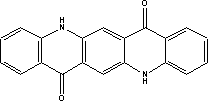
It can be seen from the above that for some substances, only limited data are available in the searched literature. 7 Toxicological profile of selected substances7.1 Toxicological profile of anthraquinone 7.1 Toxicological profile of anthraquinone7.1.1 Identification of the substance and physical-chemical properties7.1.1.1 Identification
Applications Synonyms
Regulation
7.1.1.2 Physico/chemical propertiesTable 7.1. Physico-chemical properties of anthraquinone.
7.1.2 Toxicological propertiesAbsorption Metabolism 7.1.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness The application of the substance in powder form to the eyes of rabbits has caused irritation. This effect is assumed to be principally mechanical in nature, as the substance cannot be dissolved in the eye. Other tests with the application of the substance in dissolved form showed no signs of eye irritation /16/. 7.1.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Daily oral dosing of rats with 1, 10 or 100 mg/kg for three months resulted in a decline in food intake and bodyweight, liver weight increases and changes in clinical-chemical parameters. NOAEL was determined at 1 mg/kg /16/. Daily dosing of rats via stomach pump with 2, 10, 20, 50 or 250 mg/kg for 28 days resulted in a decline in bodyweight and increases in the weight of certain organs including the liver, kidneys, spleen, thyroid gland, heart, testes and ovaries. NOAEL was determined at 2 mg/kg /16/. The literature makes reference to acute and chronic effects on the nervous system /9/. Mentioned as acute effects are convulsive fits, paralysis of the spinal cord, while chronic effects mentioned include visual disorders. Genetic damage Cancer In equivalent tests on mice exposed to daily doses of 90, 265 or 825 mg/kg for males and 80, 235 or 745 mg/kg for females, non-neoplastic damage to liver, bladder and spleen was observed in both males and females and to thyroid gland and kidneys in males. It was concluded that there was clear evidence of carcinogenic activity in both males and females /18/. In other tests involving dermal and oral administration of the substance to mice no carcinogenic activity was observed /16/. Damage to the reproductive process and the foetus 7.1.3 Ecotoxicological propertiesThe table below summarises the effects of anthraquinone on aquatic organisms. Table 7.2. Ecotoxicological data for anthraquinone (16, 20). - No data
7.1.4 Fate in the environment7.1.4.1 DegradationTable 7.3. Reported minimum and maximum degradation for anthraquinone (14). - No data. Length of test is stated in parentheses immediately after the degradation ratio.
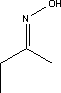
7.1.4.2 BioaccumulationNo data was found on anthraquinone's bioaccumulation potential. Based on Log Kow, the bioconcentration factor can be calculated using the following equation (21): log BCF = 0.76 log Kow – 0.23 Based on this equation, log BCF for anthraquinone comes out at 2.35. The BCF value is not considered to be critical with regard to the risk of accumulation in the food chain of living organisms until it reaches 100. 7.1.5 ConclusionAnthraquinone is of limited acute toxicity through inhalation, ingestion and skin contact and has not been found to irritate skin and eyes. The substance is described as possibly sensitizing, but additional documentation of this effect was not found. The lowest identified NOAEL in connection with repeated exposure was determined to be 1 mg/kg after 3 months' administration of the substance in food. At higher doses, effects observed included weight increase in liver and other organs. In tests, the substance showed carcinogenic activity in female rats and in both male and female mice. However, there is insufficient information on cancer studies to constitute the basis for self-classification. No data was found on reproduction toxic effects. In the light of the data gathered on anthraquinone's environmental properties, the substance would be classified as a hazard to the environment due to its high acute toxicity relative to aquatic organisms and its bioaccumulation potential. The analysis results show that the substance is contained in one of the analysed products in concentrations of 0.0034 and 0.0055% respectively. At such low concentrations it is unlikely the substance will constitute an environmental or health hazard. However, there are grounds for being attentive to the substance's carcinogenic properties, which may be considered to be the critical effect. 7.2 Toxicological profile of 2-butanoneoxime7.2.1 Identification of the substance and physical-chemical properties7.2.1.1 Identification
Applications Synonyms
Regulation
7.2.1.2 Physico/chemical propertiesTable 7.4. Physico-chemical properties of 2-butanoneoxime.
7.2.2 Toxicological propertiesAbsorption Metabolism 7.2.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness The substance has been found to be highly irritating to the eyes of rabbits and is classified as Irritant with R41, Risk of serious damage to eyes, on the List of Dangerous Substances. 7.2.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Oral dosing (stomach pump) of rats over 90 days with 25, 75 and 225 mg/kg/day resulted in dose-related changes to blood parameters and a change in the weight of spleen and liver. No neurological effects were observed and there were no fatalities. Based on the results NOEL was extrapolated to be 10 mg/kg. The blood is the primary target organ with effects such as haemolytic anaemia and the compensatory formation of blood cells (hematopoiesis). Changes were observed to blood parameters at all in dose groups in a 90-day neurotoxicity study using oral (stomach pump) dosing of rats with 40, 125 and 400 mg/kg respectively. Transitory behavioural changes were observed at 400 mg/kg. NOEL for neurotoxicity was established at 125 mg/kg/day /16/. Genetic damage Cancer This is confirmed by inhalation studies on rats and mice where the substance proved to be a liver oncogen at 75 ppm in male rats and at 374 ppm in male mice /16/. Damage to the reproductive process and the foetus Rats administered 2-butanoneoxime in drinking water (stomach pump) in doses of 60, 200 and 600 mg/kg/day showed no treatment-related effects on the foetuses and NOAEL was established at 600 mg/kg/day /16/. Corresponding tests on rabbits dosed with 8, 14, 24 and 40 mg/kg also failed to cause any treatment-related effects on the foetuses /16/. 7.2.3 Ecotoxicological propertiesThe table below summarises the effects of 2-butanoneoxime on aquatic organisms. Table 7.5. Ecotoxicological data for 2-butanoneoxime (16, 20). - No data
7.2.4 Fate in the environmentStandard tests for determining the aerobic biodegradation of 2-butanoneoxime show that the substance is relatively easily degradable, cf. Table 7.6. No data was found on 2-butanoneoxime's degradability under anaerobic conditions. Table 7.6. Reported minimum and maximum degradation for 2-butanoneoxime (16). - No data.
7.2.4.1 Bioaccumulation2-butanoneoxime's bioaccumulation potential is low. Based on Log Kow, Log BCF may be calculated at 0.25 (see section 7.1.4.2). The bioconcentration factor for freshwater fish has been established at 0.5-0.6 /14/. 7.2.5 Conclusion2-butanoneoxime is rapidly metabolised and does not accumulate in the tissues. The substance is classified as Harmful with R phrase R21, Harmful in contact with skin, Carcinogenic in Category 3 with R40, Possible risk of cancer, Local Irritant with R41, Risk of Serious Eye Damage, and Sensitizing with R43, May cause sensitization by skin contact. The blood system (the red blood cells) is the primary target organ given repeated exposure. Based on the data on 2-butanoneoxime's environmental properties the substance does not require classification as being an environmental hazard. Chemical analyses detected 2-butanoneoxime in concentrations of between 1.8 and 9.2% in five products in the first analyses. The second analyses of 2-butanonoxime detected concentrations between 1.4 and 2.1% for the products P1, P2, P10 and P11. Glass and porcelain paints containing such high concentrations of the substance must be considered unsuitable as hobby products for children and adults both by virtue of the sensitizing and the carcinogenic properties. 7.3 Toxicological profile of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride7.3.1 Identification of the substance and physical-chemical properties7.3.1.1 Identification
Applications Synonyms
Regulation
7.3.1.2 Physico/chemical propertiesTable 7.7. The physico-chemical properties of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride -no data was found
7.3.2 Toxicological propertiesAbsorption Metabolism 7.3.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness 7.3.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.3.3 Ecotoxicological propertiesTable 7.8 shows data on the aquatic toxicity of the substance. No EC50 value was found for algae or NOEC for any of the organism groups. Table 7.8. Ecotoxicological data for 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride /20/. Length of test (in hours (h)) is stated in parentheses after the concentration.
7.3.4 Fate in the environmentTable 7.9. Reported minimum and maximum degradation of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride /14/ - no data.
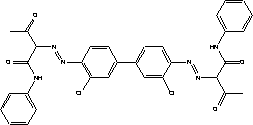
The little data found on aerobic degradability of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride shows that the substance degrades relatively rapidly. The minimum and maximum values given in Table 7.9 have been determined for a substance concentration of 10 mg/l with active sludge as inoculum. At 50 mg/l the layer phase was longer, but the degradation ratio after 7 days corresponded to that of the lower concentration. 7.3.4.1 BioaccumulationNo experimental data was found on the bioaccumulation potential of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride. Based on Log Kow the bioconcentration factor (log BCF) may be calculated to – 4.7 (see paragraph 7.1.4.2), and the substance is not expected to bioaccumulate in aquatic organisms. 7.3.5 ConclusionThe literature describes the substance as moderately toxic depending on animal species, concentration and exposure pathway. Only one oral LD50 value was found for rats and one dermal LD50 value for rabbits. According to the classification criteria both values would require classification as Harmful to Health, but they constitute too flimsy a basis for classification. The substance has displayed sensitizing potential, which is confirmed by clinical experience. Cross reaction with formaldehyde can occur. The substance does not appear to possess significant mutagenic activity. Foetal damage effects have been observed in rabbits following oral administration. One test established NOAEL for foetal-damaging effects at 5 mg/kg/day. However, in general the data found does not provide sufficient basis for a classification or its health hazard properties. Based on the data collected on 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride's environmental properties the substance does not require classification relative to environmental hazards. Reservations stem from uncertainty about the degradability profile and rate. Based on the results of the survey, the substance is expected to occur in concentrations of less than 0.5%. The substance is permitted in cosmetics and thus considered safe in concentrations up to 0.2%. At higher concentrations the risk of sensitization cannot be ruled out. However, no data has been found that would throw light on the substance's sensitization potential. There is too little information on the reproduction toxic properties of the substance for ingestion and skin contact to draw conclusions about any risks to children and pregnant women at the concentration levels used. 7.4 Toxicological profile of diarylide7.4.1 Identification of the substance and physical-chemical properties7.4.1.1 Identification
Applications Synonyms
Regulation
7.4.1.2 Physico/chemical propertiesTable 7.10. Physico-chemical properties of diarylide - no data was found.
7.4.2 Toxicological propertiesAbsorption Metabolism 7.4.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness 7.4.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.4.3 Ecotoxicological propertiesTable 7.11. Ecotoxicological data for diarylide (16, 22). Length of test (in days (d) is stated in parentheses after the concentration.
Data on diarylide's acute ecotoxicity has only been found for fish (see Table 7.11). Two results indicate that the substance is potentially acutely toxic. The two other results indicate that the substance is not toxic to aquatic organisms. Note that the substance has a water-solubility of less than 1 µg/L. No conclusions can be drawn as to whether diarylide shows negative ecotoxicological effects due to a lack of data on diarylide's chronic toxicity and as data on acute toxicity only comprises a single group of organisms. 7.4.4 Fate in the environment7.4.4.1 DegradabilityThe little data on the degradability of diarylide shows that the substance is either not degradable or is slightly degradable with a degradation ratio of 81 per cent after 15 days /16, 22/. No data was encountered on the substance's anaerobic degradability. However, water solubility is so low that the substance must be considered to be non-degradable, as its bioaccessibility is very low. Table 7.12. Reported minimum and maximum degradation of diarylide /16, 22/. - No data. Test duration is given in parentheses immediately after the degradation ratio.
7.4.4.2 BioaccumulationAccording to the Danish EPA (1999), diarylide has a low bioconcentration factor (-0.42-0.51). Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 7.3 (see section 7.1.4.2). The large gap between the experimental and the calculated BCF may be due to the fact that azo pigments such as diarylide besides being of low water solubility also have very little potential for accumulating in fatty tissues /16/. 7.4.5 ConclusionDiarylide is not absorbed through the stomach and intestinal tract and is excreted mainly unconverted in the faeces. However, under certain circumstances the formation and excretion of 3,3'-dichlorobenzidine, which is an experimental carcinogen, is possible. The substance has limited acute toxicity by ingestion, but there is no information on other exposure pathways. The substance causes no or very little irritation of the skin and eyes. Cases of skin sensitization have been reported in humans. The substance has not been found to be mutagenic or carcinogenic in the tests referred to. No data was found on reproduction toxicity. The data found does not give cause for self-classification of the substance for the effects investigated. The data materials describing diarylide's fate in the environment is insufficient to make an accurate evaluation of the substance. It will primarily be found in solid form and water solubility is extremely low. The substance has been assessed to persist in the aquatic environment and its acute toxicity is low. Based on the data on diarylide's environmental properties the substance does not require classification as being an environmental hazard. Based on the results of the survey, the substance is expected to occur in concentrations of between 0 and 2%. In animal tests skin sensitization was seen at concentrations of 1% of the substance. 7.5 Toxicological profile of 2-naphthol7.5.1 Identification of the substance and physical-chemical properties7.5.1.1 Identification
Applications Synonyms
Regulation
7.5.1.2 Physico/chemical propertiesTable 7.13. Physico-chemical properties of 2-naphthol.
7.5.2 Toxicological propertiesAbsorption Metabolism 7.5.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness Turbidity of the cornea and damage to the conjunctiva have been reported in humans who have got the substance in their eyes /7/. 7.5.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Clinical studies have shown that chronic poisoning by the substance may be associated with lesions in the kidneys and effects on the gastro-intestinal tract and nervous system. Effects on kidney function seem to be the clearest sign of harmful effects /7/. Genetic damage Cancer Damage to the reproductive process and the foetus 7.5.3 Ecotoxicological propertiesData for 2-naphthol's ecotoxicity is shown in Table 7.14. No figures were found for NOEC. Table 7.14. Ecotoxicological data for 2-naphthol /15/. Length of test is stated in parentheses after the concentration.
In the List of Dangerous Substances the substance is classified as Hazardous to the Environment with R-phase R50, Highly toxic to aquatic organisms. No data was found in support of this classification in the literature examined. 7.5.4 Fate in the environment7.5.4.1 DegradabilityTable 7.15. Reported minimum and maximum degradation of 2-naphthol /16/. - No data Test duration is given in parentheses after the degradation ratio.
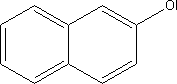
7.5.4.2 BioaccumulationNo data was found on 2-naphthol's bioaccumulation properties. Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 1.8 (see section 7.1.4.2). 7.5.5 Conclusion2-naphthol can be absorbed through the skin. The substance is classified as Harmful with R-phrases R20/22, Harmful by Inhalation and if Swallowed. The substance causes mild or no irritation of the skin and moderate eye irritation. The cornea and conjunctiva may be effected. Sensitization has been reported in animals and humans, but data does not indicate any significant effect. A number of studies have been made of the genotoxic properties of the substance, but no convincing evidence of genotoxic activity has been found. Nor has the substance shown tumour-promoting activity upon dermal application. The kidneys are the primary target organ given repeated exposure. In the light of 2-naphthol's relatively broad area of application, the environment will be exposed via a number of dispersal pathways. The substance is classified as Hazardous to the Environment with R phrase R50, Highly Toxic to Aquatic Organisms. 2-naphthol is an intermediate product in pigment manufacture and no information has been found on concentration of the substance in glass and porcelain paints. The substance was found in none of the ten analysed products. 7.6 Toxicological profile of quinacridone.7.6.1 Identification of the substance and physical-chemical properties7.6.1.1 Identification
Applications Synonyms
Regulation
7.6.1.2 Physico/chemical propertiesTable 7.16. Physico-chemical properties of quinacridone. - no data was found.
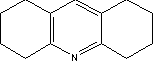
7.6.2 Toxicological propertiesAbsorption Metabolism 7.6.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness 7.6.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.6.3 Ecotoxicological propertiesNo data was found on quinacridone's ecotoxicological properties. 7.6.4 Fate in the environmentNo data was found on the degradability and bioaccumulation properties of quinacridone. Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 1.2 (see section 7.1.4.2). 7.6.5 ConclusionThe literature contains very little information on quinacridone. The LD50 values for ingestion and skin contact cannot be used for an evaluation of the toxicity, as there is insufficient information about the test conditions. Isolated items of information would indicate that the substance may cause skin inflammation when used in tattooing inks. Results of a single Ames' test were negative. Against this background it is not possible to assess the human toxicological properties of the substance. Insufficient data was found on quinacridone's environmental properties to assess the environmental hazardousness of the substance. Based on the results of the survey, the substance is expected to occur in concentrations of between 0 and 2%. The substance was not identified in conjunction with the chemical analysis of the selected products. 7.7 Toxicological profile of 1,2,3,4,5,6,7,8-octahydroacridine7.7.1 Identification of the substance and physical-chemical properties7.7.1.1 Identification
Applications Synonyms Regulation
7.7.1.2 Physico/chemical propertiesTable 7.17. Physico-chemical properties of 1,2,3,4,5,6,7,8-octahydroacridine. - no data was found.
7.7.2 Toxicological propertiesAbsorption Metabolism 7.7.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness 7.7.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.7.3 Ecotoxicological propertiesNo data was found on octahydroacridine's ecotoxicological properties. 7.7.4 Fate in the environmentNo data was found on the degradability and bioaccumulation properties of octahydroacridine. 7.7.5 ConclusionInsufficient data has been found to allow an assessment of octahydroacridine's toxicological and ecotoxicological properties. During chemical analysis the substance was found in a single product in concentrations of 0.12 – 0.18%. 7.8 Toxicological profile of hexamethylene-1,6-diisocyanate7.8.1 Identification of the substance and physical-chemical properties7.8.1.1 Identification
Applications Synonyms
Regulation
7.8.1.2 Physico/chemical propertiesTable 7.18. Physico-chemical properties of hexamethylene-1,6-diisocyanate.
7.8.2 Toxicological propertiesAbsorption Metabolism 7.8.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness Studies involving 41 car painters employed for an average of seven years and exposed to HDI in concentrations of approx. 0.001 mg/m³ showed an increased incidence of eye, nose and throat irritation and chronic bronchitis /16/. 7.8.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.8.3 Ecotoxicological propertiesTable 7.19. Ecotoxicological data for hexamethylene-1,6-diisocyanate /16/. Test times are stated in parentheses immediately after the concentration.
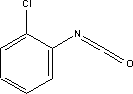
7.8.4 Fate in the environment7.8.4.1 DegradationData was only found from one test, which showed that the substance is not degradable under aerobic conditions over 28 days /16/. 7.8.4.2 BioaccumulationBased on Log Kow, the bioconcentration factor (log BCF) may be calculated at 2.2 (see section 7.1.4.2). 7.8.5 ConclusionHDI is classified as Toxic on the List of Dangerous Substances with R23, Toxic by inhalation, Irritant, with R36/37/38, Irritating to eyes, respiratory system and skin, and sensitizing with R42/43, May cause sensitisation by inhalation and skin contact. The LD50 values found also indicate that the substance should be classified as Harmful to Health R21/22, Harmful in contact with skin and if swallowed. The eyes are particular sensitive to isocyanates, which also have a primary irritating effect on the respiratory tract. No data was found to indicate that substance has mutagenic, carcinogenic or reproduction toxic effects. The information collected on hexamethylene-1,6-diisocyanate is insufficient to assess the extent to which the substance represents a hazard to the environment. The toxicity results found indicate a certain degree of toxicity to fish, and there may be a bioaccumulation potential. During chemical analysis the substance was found in four of the products in concentrations between 0.045 and 0.075%. No data was found to document sensitization at these concentration levels, but potent isocyanates can constitute a problem for sensitive people even at low concentrations. 7.9 Toxicological profile of chloroisocyanate benzene7.9.1 Identification of the substance and physical-chemical properties7.9.1.1 Identification
Applications Synonyms
Regulation
7.9.1.2 Physico/chemical propertiesTable 7.20. Physico-chemical properties of chloroisocyanate benzene /17/. -no data was found
7.9.2 Toxicological propertiesAbsorption Metabolism 7.9.2.1 Acute toxicityInhalation Ingestion Skin contact Irritation and corrosiveness 7.9.2.2 Subacute/chronic toxicityAllergy and hypersensitivity Organ damage Genetic damage Cancer Damage to the reproductive process and the foetus 7.9.3 Ecotoxicological propertiesNo data was found on chloroisocyanate benzene's ecotoxicological properties. 7.9.4 Fate in the environment7.9.4.1 DegradationNo data was found on chloroisocyanate benzene's degradability. 7.9.4.2 BioaccumulationBased on Log Kow, the bioconcentration factor (log BCF) may be calculated at 2.2 (see section 7.1.4.2). 7.9.5 ConclusionInsufficient data has been found to allow an assessment of chloroisocyanate benzene's toxicological and ecotoxicological properties. Upon chemical analysis the substance was found in just one of the ten products at a concentration of 0.015%. The critical effect of the substance is sensitization, but no documentation was found to show that concentrations of the magnitude of 0.015% could trigger allergies. 7.10 SummaryTable 7.21 summarises the substances' intrinsic toxicological properties with regard to the key parameters: acute effects, local effects, sensitization, the effects of repeated exposure, and carcinogenicity(C), mutagenicity (M), and reproduction toxicity (R). Moreover, the most highly critical effects of the substances have been listed based on the available data. Table 7.22 summarises information on the environmental effects of the substances and their environmental classification. Table 7.23 summarises information about regulatory requirements applying to the substances together with specification of concentrations of those substances identified through chemical analysis. In the cases of butanoneoxime, methylpyrrolidone and phthalatic acid anhydride the percentage contents found by chemical analysis are above the lower limits classification. With the exception of methylpyrrolidone all substances require classification as sensitizing. Both the first and the second analysis of 2- butanoneoxime require classification as category 3 carcinogens. All other detected compounds are present in concentrations below the classification limits. As regards the environmental properties of the substances, two of them (anthraquinone and 2-naphthol) were both extremely harmful to aquatic organisms and also persistent in the environment. As a consequence, there are grounds for limiting discharge into the aquatic environment. Table 7.21 List of toxicological properties and critical effects of the ten substances.
Table 7.22 Summary of environmental impact and classification
Table 7.23 Summary of regulations applying to the ten substances
1) Classifications from the List of Dangerous Substances are specified in bold type and classifications from the Danish EPA's guideline list for self-classification are specified in normal type. 8 References
Appendix A Extractable substancesIn table A-1 the chemical substances have been identified by analysis of extractable substances in the 20 porcelain coloures by a CAS no. For many of the substances it is not a unique identification and thus not a unique CAS no. The number listed is an example of one of the substances that may be covered by the chemical name. Tabel A-1 Chemical substances identified by analysis of extractable substances.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||