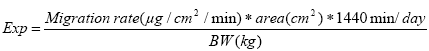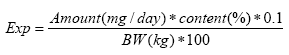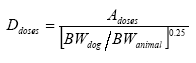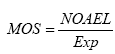|
Survey of Chemical Substances in Consumer Products no. 74, 2006 Evaluation of the health risk to animals playing with phthalate containing toysContents
SammenfatningPhthalater anvendes bl.a. som blødgørere i forskellige polymerer (f.eks. PVC) og findes derfor i en lang række forbrugerprodukter. En undersøgelse igangsat af Miljøstyrelsen viste, at der var store mængder af de to phthalater - DEHP og DINP - i PVC legetøj til dyr. I Danmark er brugen af phthalater i legetøj til børn reguleret, hvilket ikke er tilfældet for legetøj til dyr. Der er således en risiko for at dyr, der leger med legetøjet, kan blive udsat for disse stoffer. Formålet med denne rapport er derfor at evaluere sundhedsrisikoen for dyr, der leger med phthalatholdigt legetøj. Evalueringen er foretaget på baggrund af effekt vurderingerne fra EU's Risikovurderingsrapporter af DEHP og DINP samt på baggrund af eksponeringsberegninger for hunde, der leger med legetøjet og i den forbindelse spiser dele af legetøjet. De kritiske effekter af DINP og DEHP i dyreforsøg inkluderer lever og nyre toksicitet samt effekter på udviklingen af reproduktions systemet. Eksponeringerne er beregnet for hunde af forskellig størrelse (1-40 kg), der spiser forskellige mængder af legetøj (1-50 g eller 1-50 cm²) indeholdende enten den gennemsnitlige eller den højest målte koncentration af stofferne i legetøjet. Samtidig er beregningerne foretaget både ved at anvende en migrationsrate målt i spyt samt udfra en antagelse om at 10% af det totale indhold migrerer, mens legetøjet opholder sig i tarmsystemet. Med hensyn til DEHP viste eksponeringsberegningerne både ved anvendelsen af den målte migrationsrate samt ved antagelsen om 10%’s migration, at eksponeringen for størstedelen af alle scenarierne oversteg det kritiske niveau, hvor toksisk effekter er vurderet at kunne opstå. For DEHP er NOAEL fastsat på baggrund af effekter på udviklingen af reproduktions systemet. Det mest kritiske tidspunkt for eksponering er derfor en relativ kort periode primært i forbindelse med at det reproduktive system i fostre differentieres og udvikles. På baggrund af eksponeringsscenarierne samt det faktum at den kritiske eksponerings periode er relativ kort, er det sandsynligt, at DEHP i legetøj kan udgøre en sundhedsrisiko for dyr, der leger med legetøjet og i den forbindelse spiser dele af det. Med hensyn til DINP viste eksponeringsberegningerne, at det primært var ved anvendelsen af den relativt konservative antagelse om, at 10% af det totale indhold af DINP migrerer fra legetøjet over i dyret, at eksponeringen oversteg de niveauer, hvor toksiske effekter er vurderet at kunne opstå. Ved anvendelse af den målte migrationsrate oversteg de estimerede eksponeringer kun det kritiske niveau for små hunde, der spiser relativt store mængder legetøj, hvilket er mindre sandsynligt. For DINP er NOAEL fastsat på baggrund af et kronisk toksicitets studie, hvor der blev observeret effekter på leveren. Længere tids eksponering er derfor nødvendig, for at de pågældende toksiske effekter kan opstå. Umiddelbart forekommer det ikke særlig sandsynligt, at dyr kontinuerligt får legetøj blot for at bide det i stykker og spise det. Sammenholdt med eksponeringsvurderingerne er det derfor mindre sandsynligt at DINP i legetøj kan udgøre en sundhedsrisiko for dyr. Nyere forskning tyder på at DINP ligesom DEHP muligvis også påvirker udviklingen af reproduktions systemet dog blot ved betydelig højere doser end DEHP. Derfor vurderes risikoen for skader på dyrenes reproduktionssystem på grund af tilstedeværelse af DINP i legetøj for lav. Vores husdyr bliver udsat for phthalater fra andre kilder end legetøj. Ligesom mennesker eksponeres de formodentlig også fra miljø, fra foder samt andre produkter, hvorfor den samlede eksponering af dyrene antagelig er højere. Det kan dreje sig både om DEHP og DINP, der er behandlet i denne rapport, men også andre phthalater f.eks. DBP hvor kombinationseffekter er sandsynlige. 1 IntroductionPhthalates are used as plasticisers in various polymers (e.g., PVC) that are found in a wide range of products. While the use of phthalates in toys for children is regulated in Denmark, there is no regulation of the use of phthalates in toys for pets. An investigation of chemicals in toys for cats and dogs available on the Danish market and on the Internet (Nielsen et al. 2005 A) revealed that investigated toys made of PVC contained large amounts of 2 phthalates – di(2-ethylhexyl) phthalate (DEHP) and di-isononyl-phthalate (DINP). Since the phthalates are not chemically bound in the material, they may be released from the products during their lifetime. Therefore cats and dogs may be exposed to these substances while playing with the toy. Some of the critical effects of the phthalates include effects on liver and kidney as well as effects on the development of the reproductive system of animals. The aim of the present report is therefore to evaluate the health risk to dogs and cats playing with phthalate containing toys. In order to evaluate the risk both exposure- and effect assessments will be carried out. Established exposure as well as effect levels will be used to estimate margin of safety (MOS). The effect assessment will be in concordance with the EU Risk Assessment Reports on DINP (2001) and DEHP (2001). The exposure assessment will focus on a relevant scenario and deterministic estimations of exposure will be made using a range of values for certain parameters. 2 Animal exposure
2.1 The relevant exposure scenarioOn the Internet, safety information about toys to dogs and cats can be found (e.g. http://www.hunde-info.dk/farlege.shtml). Animal doctors experience that dogs often swallow toys (and many other products as well) and due to the physical properties of the products they can pose a risk to the animals. It is also experienced that those items that were soft plastic when e.g. the dog played with it turns out to be hard and sharp when in the gastrointestinal tract indicating that the softeners present in the product leach out. Furthermore, information such as “går legetøjet i stykker skal det fjernes fra dyret” is written on some of the analysed toys (Nielsen et al. 2005A). When the animals bite in the toy, the physical chewing and the presence of saliva result in the extraction of the phthalates. Furthermore, if the toy is swallowed the phthalates can migrate from the piece of toy as long as it is retained in the gastrointestinal tract. Therefore, oral exposure during chewing and biting in the toy as well as the swallowing of parts of the toys is of concern. The playing behaviour of dogs and cats are somewhat different. In general dogs bite and chew in the toy whereas cats are more likely to play with the toy with the paws. Dermal exposure during the animals playing with the toy is of little concern because the dermal absorption measured on skin is relatively low (Deisinger et al. 1998) and it will be even more reduced because of the fur of the animal. Therefore, in the present risk assessment the relevant and realistic worst-case exposure scenario will be a dog that bite, chew and swallow a piece of toy that continuously emits phthalates while in the gastrointestinal tract. Dermal exposure will not be considered in the present assessment and it will be assumed that the estimated exposure to dogs will also cover the exposure to cats as it in general is expected to be lower. The different parameters that will be used in the estimation of oral exposure to dogs chewing and eating the toy will be presented in the following sections. 2.2 Occurrence of phthalates in toysAs mentioned in the introduction the content of DEHP and DINP in 13 PVC containing toys for cats and dogs available on the Danish marked and on the Internet has been analysed. The investigated phthalates were present in all products in concentrations ranging from 6.9 to 54% (v/v) (Table 1). Exposures will be estimated for minimum, mean and maximum content of DEHP and DINP in the toy. Table 1. The amount of DINP and DEHP in 13 products of toys for animals
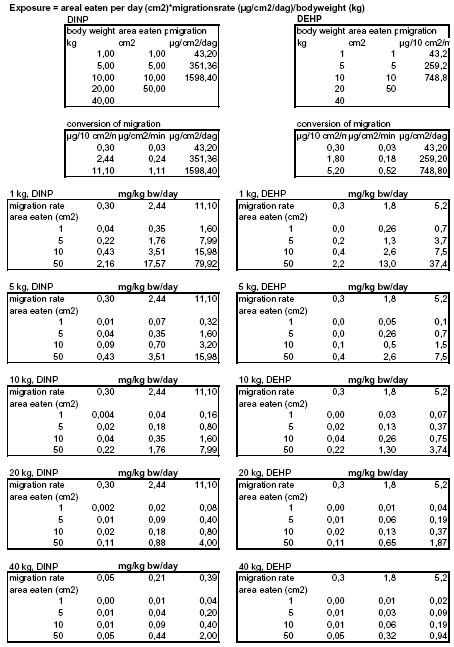
(Nielsen et al. 2005A) 2.3 Migration of phthalates from toySeveral investigations of the migration of phthalates from toys have been performed with both in vivo as well as in vitro extraction methods. In an investigation by Kùnemann (1998), the release of DINP from PVC samples was measured in 10 test persons both sucking and biting on three different DINP containing (38-43%) specimens (10 cm²). The release ranged from 0.3 - 8.9 μg/10 cm²/min with an average release for each of the three specimens of 1.38, 2.44 and 1.63 μg/10 cm²/min, respectively. In the experiment pH, protein content and volume of saliva were also measured (values not given) and according to authors there was no influence from pH and protein content of the saliva on the migration rate. Steiner et al. (1998) found average migration rates of DINP (1.4 μg/10 cm²/min) and DEHP (0.4 μg/10 cm²/min) released during sucking on PVC sheets containing 32% DEHP or 36% DINP. Chen (1998) measured the migration of DINP from disks of five different toys in two test persons for 4 time periods. The average migration rate of DINP for all time periods for individuals ranged from 1.1 to 9.9 μg/10 cm²/min. It should be mentioned that the released phthalates from the toy might be hydrolysed by saliva forming monoesters of released phthalates. These monoesters are not included since only the parent compounds (diesters) in the saliva have been measured. 24 DINP containing toys (12.9 to 39.4% DINP) have been tested in a dynamic in vitro method (“head-over-heels”) and migration rates ranged from 1 to 11.1 μg/10 cm²/min with a mean migration rate of 4.1μg/10 cm²/min (Simoneau et al. 2001 – as quoted in Babich et al. 2004). In another “head-over-heels” study, 14 DEHP containing products (3-45%) were included. The migration rate of DEHP ranged from 0.3 – 5.2 μg/10 cm²/min with a mean of 1.8 μg/10 cm²/min. The migration of DINP was at the same level (Bouma et al. 2002). The content of DINP and DEHP in the products investigated is at the same level as the content found in pet toys (Nielsen et al. 2005A). However, not only plasticizer content but also other parameters of the product (e.g. surface roughness, coating type, thickness) influence the release (Bouma et al. 2002). It is also very likely that dogs chewing and biting result in more mechanical agitation of the products compared to humans and thereby to a higher release of phthalates. Differences in saliva (quality, amount and destiny (ingestion vs slavering)) could also influence the migration rate. Furthermore, we have no information on the migration of phthalates in contact with gastrointestinal fluid that e.g. has a lower pH, a higher fat content and contains other enzymes than saliva. However, it will be necessary to assume that the migration rate measured by either in vivo or in vitro extraction methods resemble the migration in dogs during biting and chewing on the toy as well as the migration from the toy while in the gastrointestinal tract. Overall, the migration rate of DINP range from 0.3 μg/10 cm²/min to 11.1 μg/10 cm²/min. To cover the whole range of migration rates the minimum, an average value from Kùnemann (1998) as well as the maximum migration rate (0.3, 2.44 and 11.1 μg/10 cm²/min respectively) will be used as examples in the exposure estimations. Regarding the migration of DEHP there are fewer investigations than of DINP. However minimum, mean and maximum migration rates (0.3, 1.8 and 5.2 μg/10 cm²/min, respectively) measured by the in vitro extraction method will be used in the assessment. 2.4 Duration of gastrointestinal passage in dogsThe duration of the gastrointestinal passage in dogs is not a fixed parameter as it differs with e.g. the composition of food and food particle size. However, in general it ranges from 12-30 hours. A value of 24 hours will be used as the duration of the gastrointestinal passage, i.e., the exposure duration. The exposure duration of 24 hours includes both the time where the dog chews and bites in the toy as well as the time pieces of toy is retained in the gastrointestinal tract. 2.5 Body weights of dogsThe body weight of dogs depends off course on strain and age. In general it ranges from 1 kg to 40 kg and values of 1, 5, 10, 20 and 40 kg will be used in the exposure estimations. 2.6 Estimating the exposureTwo different ways to estimate the exposure will be performed. The estimations will either be based on A) a measured migration rate or B) the assumption that 10% of the phthalate present in the toy migrate while in the gastrointestinal tract. 2.6.1 Estimation A - based on measured migration rateIt will be assumed that a dog chews and bites and swallows a piece of toy with a given area (1, 5, 10 and 50 cm²) and as mentioned previously that the duration of chewing and biting as well as time the product is retained in the gastrointestinal tract is 24 hours in total. The exposure can be estimated as:
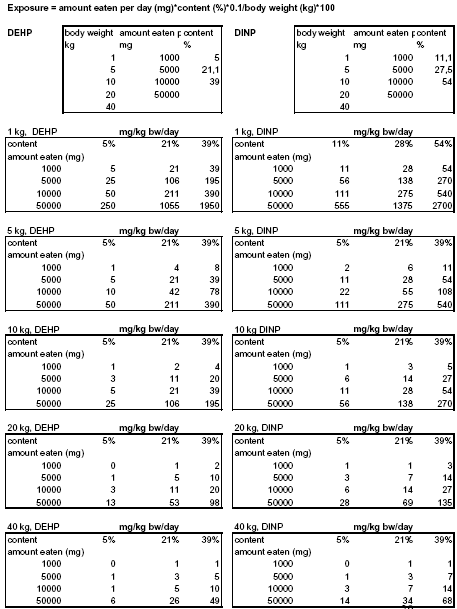
Worst-case exposure estimations are based on the maximum migration rate (5.2 and 11.1 μg/10 cm²/min for DEHP and DINP, respectively) and average exposure estimations on the average rate (1.8 and 2.44 μg/10 cm²/min for DEHP and DINP, respectively).The estimated exposures of DEHP and DINP for dogs weighing from 1 to 40 kg, eating from 1 to 50 cm² of toy is presented in Table 2A and 3A. See Appendix 2 for further details. 2.6.2 Estimation B - based on 10% (w/w) migration of phthalateIt will be assumed that a dog has swallowed a piece of toy with a given mass (1, 5, 10 and 50 g) and that 10 % of the phthalates present in the product migrate while in the gastrointestinal tract (24 h). The exposure can be estimated as:
Worst-case exposure estimations are based on maximum contents (39% DEHP and 54% DINP) and average exposure estimations on average contents (21% DEHP and 27.5 % DINP). The estimated exposures of DEHP and DINP for dogs weighing from 1 to 40 kg, eating from 1 to 50 g of toy is presented in Table 2B and 3B. See Appendix 1 for further details. Table 2. Estimated exposures of DEHP for dogs weighing from 1 to 40 kg, eating 1, 5, 10 or 50 g or cm² of product and using either migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B) to estimate exposure.
Table 3. Estimated exposures of DEHP for dogs weighing from 1 to 40 kg, eating 1, 5, 10 or 50 g or cm² of product and using either migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B) to estimate exposure.
3 Toxicity
Within the EU, specific programs on risk assessment for new and existing chemicals are on going. For both DEHP and DINP individual Risk Assessment Reports have been produced and agreed. The adverse effects of the individual phthalates will only be summarized shortly and the no observed adverse effect levels (NOAEL) used in the present risk assessment will be in concordance with the NOAELs presented in the agreed reports. The Risk Assessment Reports are published at http://ecb.jrc.it/existing-chemicals/. Most studies regarding the toxicological effect of DEHP and DINP are made in rats and NOAELs for DINP and DEHP have been established based on studies in rats. However, few studies in dogs have been reported and these studies will be described shortly. 3.1 Critical effects of DEHPThe effects of DEHP on testis, development of the reproductive system, fertility and kidney (repeated dose toxicity) are considered to be the critical effects. Severe and irreversible testicular injury was induced in rats exposed to low oral doses of DEHP in a three generation reproductive toxicity study by Wolfe et al. (2003 – quoted in DEHP 2001) with a NOAEL of 4.9 mg/kg b.w./day. The testes injury was much more severe in the F1 and F2 generation than in F0. Also severe developmental effects were observed in mice in the absence of maternal toxicity with a NOAEL of 20 mg/kg b.w./day. Effects on male fertility have been observed in mice and rats. In mice, DEHP adversely affected the number of fertile matings. In a continuous breeding study in mice, an oral NOAEL of 20 mg/kg b.w./day was identified for fertility (Lamb et al, 1987 – quoted in DEHP 2001). In rats, the oral NOAEL for body weight, testis, epididymis, and prostate weights and for endocrine and gonadal effects in male rats was considered to be 69 mg/kg b.w. per day in a 60 day study (Agarwal et al. 1986 – quoted in DEHP 2001). The NOAEL for kidney toxicity is considered to be 500 ppm DEHP in the diet (corresponding to 28.9 mg/kg b.w./day in the males and 36.1 mg/kg/day in the females) derived from a 2-year study in rats and based on increased absolute and relative kidney weight in both sexes at the next higher dose level (2500 ppm) (Moore 1996 – quoted in DEHP 2001). Based on all available studies, an overall oral NOAEL of 4.9 mg/kg b.w./day for developmental toxicity is established for DEHP. The NOAEL is derived from the three-generation study in rats (Wolfe et al. (2003) – quoted in DEHP 2001) and it is based on small male reproductive organs (testis, epididymes and seminal vesicles) and testis atrophy. 3.1.1 Studies in dogsThe effects of DEHP have been studied in groups of dogs (2 of each sex) receiving 59 mg DEHP /kg b.w./day in gelatine capsules 5 times weekly for one year. No significant differences were observed between treated and control dogs in several parameters: mortality, body weight gain, blood counts and gross and microscopic examination of several organs and tissues (e.g. liver, kidney, spleen, adrenal and ovary or testes) (Union Carbide 1951 – quoted in DEHP 2001). 3.2 Critical effects of DINPThe effects of DINP on the liver and kidney (repeated dose toxicity) are considered to be the critical effects. Testicular toxicity is also observed. However, DINP seems to be less potent in inducing hormonal and testicular effects than DEHP. Effects on the liver consisting of hepatic biochemical changes (increased ALT, AST) and of increased liver weights in both sexes (absolute and relative liver weights) concurrently with histopathological findings have been observed in rats with a NOAEL of 88 mg/kg b.w./day being established from a 2-year study (Aristech 1994 – quoted in DINP 2001). This NOAEL is established for liver effects, which are not related to peroxisome proliferation. For effects on the kidney, a NOAEL of 88 mg/kg b.w./day can be established from the same study based on increased kidney weights in both sexes (absolute and relative kidney weights) at higher dose levels. For testicular effects (decreased testicular weight without histological changes), a NOAEL of 276 mg/kg b.w./day can be derived from a 2-year mouse study (Aristech Chemical Corporation – quoted in DINP 2001). Based on the available studies, an overall oral NOAEL of 88 mg/kg b.w./day is established for DINP. The NOAEL is derived from the chronic study (2 years) in rats for effects on the liver and kidneys. This NOAEL also covers the reproductive effects of DINP as they were seen at higher doses e.g. a NOAEL of 276 mg/kg b.w./day for testicular effects (DINP 2001). 3.2.1 Studies in dogsThe effect of DINP has been studied in beagle dogs in a 13 week feeding study. Groups of dogs (4 of each sex) were fed approximately 37, 160 and 2000 mg DINP/kg/day. As an indication of liver damage, a slight to moderate elevation of serum glutamic oxaloacetic transaminase (SGOT or ALT) was observed at week 4 at all doses in both sexes (no statistical data available) and the effect showed dose-dependent relationship in females. These biochemical changes were associated with an increase of absolute and relative liver weights from 160 mg/kg b.w./day as well as liver histopathology at highest dose. At the highest dose, changes in relative and absolute weight of kidney and spleen were also observed. No NOAEL can be identified in this dog study. Instead, a LO(A)EL of 37 mg/kg b.w./day based on an increase in SGOT (ALT) is identified. However, according to DINP (2001), there was absence of statistical data and inconsistency between tables and text which weakens the relevance of this study (Hazleton et al. 1971 – quoted in DINP 2001). 3.3 NOAEL or LOAEL of DEHP and DINPThe critical effect(s) identified for each phthalate as well as the NOAEL (or LOAEL in the case where a NOAEL has not been identified) have been compiled in Table 4. Table 4. NOAELs (or LOAELs) for DEHP and DINP
(DEHP 2001 and DINP 2001) 3.4 Relevance of effects observed in rats for effects in dogs3.4.1 DEHPDifferences in DEHP-induced toxicity between rats and dogs and cats can be caused by e.g. toxicokinetic differences between species. MEHP is believed to be the active metabolite of DEHP and therefore knowledge about the ability of a species to form MEHP is crucial when considering the relevance of the DEHP-induced testicular effects observed in rats to e.g. dogs. In one study, the toxicokinetics of DEHP in rats and dogs have been compared. DEHP were administrated in the diet in doses of 50 mg/kg b.w./day for 21-28 days before a single dose of 14C-labelled DEHP. The distribution and excretion of the radioactivity were analysed. The fraction of radioactivity excreted in faeces and urine was relatively similar in rats and dogs with faecal excretion as predominant. The excretion was virtually complete in 4 days. However, the elimination was slightly more prolonged in dogs compared to rats. There were 4 radioactive metabolites in rat urine and 3 in the dog urine. Metabolites in dog urine were indistinguishable from those in the rat urine. In both rats and dogs, one of the metabolites present in bile was indistinguishable from MEHP. Substantial radioactivity was found in both rats and dogs in the gastro-intestinal tract. In remaining organs the highest level of radioactivity was found in the liver of rats and in bile samples from dogs (Ikeda et al. 1980). The possible role of other metabolites in the testicular toxicity of DEHP is not fully elucidated and therefore the importance of species differences in the formation of other metabolites is not known. Other parameters e.g. altered zinc homeostatsis or protein-content in diet also seems to influence the susceptibility to the toxic effects (DEHP 2001), but no data are available with regard to these differences between rats and dogs. The limited toxicokinetic data in dogs, indicate that MEHP is formed following exposure to DEHP and that metabolism of DEHP are relatively similar between rats and dogs. Therefore, DEHP induced effects on testes and reproductive functions in rats are also considered relevant for dogs and cats. 3.4.2 DINPAlthough limited reported, the toxicity study in dogs reveals effects on the liver at lower doses than the rat study used to establish the overall NOAEL. Therefore it is obvious that the effects on liver in rats are also relevant to dogs. No data is available for cats, but it will be assumed that the observed effects in rats and dogs also are relevant to cats. 4 Risk Assessment
In the present report, the risk characterisation is carried out by quantitatively comparing the outcome of the effects assessment to the outcome of the exposure assessment. The ratio resulting from this comparison is called Margin of Safety (MOS). In judging the sufficiency of the MOS, several parameters regarding the NOAEL/LOAEL have to be considered in terms of uncertainties and variabilities e.g. related to the extrapolation from experimental data to the actual situation; in the present case the dog situation. Therefore in the interpretation of the MOS, default assessment factors are often used to account for these uncertainties and variabilities. The factors are combined to form the so-called “minimal-MOS”, which can be interpreted as an “overall” uncertainty factor. 4.1 Uncertainty factors or establishing minimal MOSWhen a risk characterisation is made for humans based on experimental animal studies, uncertainty factors are applied in order to cover 1) intraspecies variability in susceptibility, 2) interspecies variability in susceptibility and 3) other parameters that have to be taken into account e.g. quality of study, LOAEL instead of NOAEL etc. The uncertainty factor accounting for intraspecies variability in susceptibility is relevant also for dogs as it is expected that the dog population show a broad range of biological sensitivity. An uncertainty factor of 10 is usually applied to account for variability in susceptibility within the human population (Nielsen et al. 2005B) and it is assumed that a factor of 10 will also be adequate in order to account for the variability in susceptibility within the dog population. The uncertainty factor accounting for interspecies variability in susceptibility is relevant when extrapolating from data in rodents to dogs as dogs may be more susceptible to a given effect than rodents. The factor is usually 10 when extrapolating from experimental animals to humans (Nielsen et al. 2005B). The factor can be divided into two parameters; one parameter accounting for species differences in toxicokinetics (absorption, distribution, metabolism and excretion) and the other parameter accounting for other differences, e.g toxicodynamic. Generally, factors of 4 and 2.5 have been suggested for differences in toxicokinetics and toxicodynamics, respectively (Renwick 1993 and WHO 1994 – quoted in Nielsen et al. 2005B). However, it is a general point of view that regarding interspecies differences in toxicokinetics, the difference in metabolism is a key factor (Nielsen et al. 2005B). Therefore, the factor of 4 can be replaced by a correction of doses due to differences in metabolism estimated by allometric scaling, where a biological parameter e.g. metabolism can be expressed as a function of bodyweight. The correction of doses by allometric scaling on the basis of differences in body weight and metabolism can be estimated as follows:
where Adose is the dose in the animal study, BWdog is the body weight of dogs and BWanimal is the body weight of the test animal. The denominator It is assumed that the body weight of an adult rat is 400 g, and the correction factors for different sizes of dogs are presented in Table 5. These correction factors predominantly take toxicokinetic differences into account, predominantly in form of differences in the metabolism due to differences in body size. To account for other differences as e.g the toxicodynamic differences between rats and dogs the factor of 2.5 will also be applied. The total interspecies uncertainty factor can be seen in Table 5, as well as the overall uncertainty factor - the minimal MOS - where a factor of 10 for intraspecies variability has also been applied. The uncertainty factor accounting for the quality and relevance of the data is not relevant in the present assessment as the NOAELs taken forward to the risk characterisation are derived from well-performed and relevant toxicity studies. Table 5. Correction factors for extrapolation of doses in rats (400 g) to doses in dogs derived by allometric scaling based on metabolism as well as the total interspecies uncertainty factor, the intraspecies uncertainty factor and the overall uncertainty factor (minimal MOS) covering both inter- and intraspecies variation.
4.2 MOSThe margin of safety (MOS) can be calculated as follows:
In the calculation of MOS, the NOAEL of DEHP is 4.9 mg/kg b.w./day, the NOAEL of DINP is 88 mg/kg b.w./day and the exposures have been estimated for different sizes of dogs as well as amount of toy eaten and by using either a migration rate or the assumption that 10% of the phthalate migrate. The estimated MOS of DEHP and DINP is presented in Table 6 and Table 7, respectively, where also the minimal MOS is included. In the tables, exposure scenarios where MOS is below the minimal MOS have been marked with grey. As can be seen in the Tables, many of the calculated MOS values are below the minimal MOS. The MOS values for DEHP derived from the most conservative way of estimating the exposure – assuming 10% of the phthalate migrate while in the gastrointestinal tract – is in all cases below the minimal MOS; even the largest dog eating only 1 g of toy/day that contain an average content of DEHP is exposed to doses of DEHP where toxic effects can occur. Also for DINP that have higher NOAEL than DEHP, the main part of the MOS values are below the minimal MOS except for the larger dogs (20-40 kg) eating the smallest amount of toy (1g) with an average content of DINP. Table 6. MOS for DEHP has been estimated for dogs weighing from 1 to 40 kg with exposure estimations either based on migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B). Average and worst-case migration is 1.8 and 5.2 μg/10 cm2/min, respectively and average content is 21.1 and 39 %, respectively.
Table 7. MOS for DINP has been estimated for dogs weighing from 1 to 40 kg with exposure estimations either based on migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B). Average and worst-case migration is 2.4 and 11.1 μg/10 cm2/min, respectively and average content is 27.5 and 54 %, respectively.
Regarding the MOS values derived from the exposure estimations based on measured migration rates, they are also in several cases especially for DEHP below the minimal MOS. The MOS for DEHP is below the minimal MOS for dogs weighing 1 kg eating 1 cm² of toy, for dogs weighing 5 and 10 kg eating 5 cm² of toy, for dogs weighing 20 kg eating 10 cm² of toy or for dogs weighing 40 kg and eating 50 cm² of toy. The MOS for DINP is only below the minimal MOS for dogs weighing 1 kg eating 10 cm² of toy and for dogs weighing 5 and 10 kg eating 50 cm² of toy. The MOS values are derived based on the NOAEL from DINP (2001) (88 mg/kg b.w./day). If instead the LO(A)EL from the available dog study although badly reported but never the less a toxicity study in dogs is used (37 mg/kg b.w./day), the estimated MOS values for DINP are lower. However, the minimal MOS is also lower (20 for all sizes of dogs). The uncertainty factor for interspecies variability is not relevant. Instead a factor of 2 is applied accounting for the use of a LO(A)EL instead of a NOAEL. Therefore, although the MOS values are lower, it is exactly the same exposure scenarios that are below the minimal MOS (see appendix 3 for details) compared to when the MOS values are estimated based on the NOAEL from DINP (2001) (Table 7). 4.3 Critical period of exposureIt may seem unrealistic that eating a piece of toy should be an “every day” scenario for a dog. However, this is not necessary as effects of DEHP are seen also following exposure during a relatively limited but critical period of time; that is in utero and post-natal. Developing and prepubetal rats have been found to be much more sensitive to exposure to DEHP than adults (DEHP 2001). Therefore, the most critical period of exposure of dogs to DEHP is also expected to be in utero and as pups. The critical in utero exposure period can even be more defined; the most critical period of exposure is the period of sexual differentiation which is during the last half of pregnancy e.g. in rats from gestation day 11 – 21 and with the differentiation of testes occurring around gestation day 14-15 (Rogers and Kavlock, 1998). Therefore, the dogs only need to eat pieces of toy at some relatively short but critical periods of time for critical effects to occur. Off course if the exposure period is extended e.g. in pups the effects will increase further. Regarding DINP the critical effects are not developmental toxicity and therefore not especially related to in utero and post-natal exposure. Continued exposure during longer period of time is therefore necessary to cause the liver effects seen at the NOAEL. 4.4 Exposure to animals from other sourcesThe human population is exposed to phthalates via the environment, via food and via consumer products (Müller et al. 2003). This is likely also to be the case for dogs and cats as they live in association with humans. Therefore the exposure via toys may be a major source to phthalates, but it is important to remember that probably it is not the only one. 4.5 Uncertainty and limitationsThe products are continuously emitting phthalates even though the intensity of emission is not expected to be linear over the product’s lifetime. New products are expected to release higher amounts of phthalates compared with older products. In the present assessment, no differences in release during ageing of the product have been considered. The bioavailability is not included in the exposure estimations and therefore, the estimates can only be considered as the external exposures. However, the established NOAELs are also expressed as an external dose and therefore, the estimated exposures and the NOAELs can be compared. In the first estimation (A), the migration rates used to estimate the exposure is measured in either saliva or in a saliva simulant. It is likely that the migration of phthalates from products in the gastrointestinal tract is different due to both chemical (lower pH, higher content of fat), physical as well as biological differences. In estimation A, the exposure time off course also influences the outcome significantly. In general the time for food items to pass through the gastrointestinal tract is 12-30 hours depending on e.g. size and composition of food particles. It is very likely that pieces of plastic pass through the gastrointestinal tract relatively slow as they in general are considered to be relatively large particles, hard as well as indigestible. In the second estimation (B), the migration of 10% of the phthalates in the product is a qualified guess off the size of migration which of course influences the level of estimated exposure significantly. As mentioned previously, it may seem unrealistic that the scenario of eating a piece of toy leads to chronic exposure of DEHP and DINP to dogs. However, this will probably depend on the individual dog (age, strain, nature) as well as on the availability of phthalate containing products for the dog. Whether phthalate containing products are available for the dog depends on the owner of the dog and the likelihood of the owner continuously to provide the dog with toys which it tears into pieces. Furthermore, as also mentioned the effects of DEHP on the reproductive system does not only occur following long-term exposure as the most critical time of exposure is a relatively short period in utero. 5 ConclusionThe risk to animals playing with DEHP and DINP containing toys has been evaluated based on effect assessment in concordance with the EU Risk Assessment Reports on DINP (2001) and DEHP (2001) and assessment of exposure to dogs eating pieces of toy. The exposure has been estimated for different sizes of dogs (1-40 kg) eating different amounts of toy (1-50 g or 1-50 cm²) and the estimations have been made either by the use of a measured migration rate or by assuming that 10% of the total content migrate while the toy is retained in the gastrointestinal tract. When DEHP exposure was estimated based on the relatively conservative assumption that 10% of the phthalate migrates while in the gastrointestinal tract, critical exposure levels were derived for all the scenarios, i.e. even the largest dog eating only 1 g of toy/day that contains a mean content of DEHP is exposed to doses of DEHP where toxic effects can occur. Also, when the exposure estimations of DEHP were based on measured migration rates of phthalates into saliva or saliva simulant, the major part of the scenarios results in an exposure level where toxic effects could be expected; not only when using the maximum migration rate but also when using the average migration rate. The NOAEL for DEHP is established for developmental toxicity. The most critical time of exposure to DEHP is a relatively short period especially during sexual differentiation but also during development. It seems realistic that dogs (including pregnant and/or nursing dogs) might be exposed to DEHP from toys during the relatively short critical period of exposure at levels, which can affect the reproductive system in dogs. Consequently, there is a concern for reproductive effects in dogs due to the presence of DEHP in toys. Also for DINP, the major part of the exposure estimations based on 10% migration of total DINP, result in exposures beyond the level where effects could be expected. An exception is the larger dogs (20-40 kg) eating the smallest amount of toy (1g) with an average content of DINP. When based on measured migration rates of DINP, critical exposure levels were only derived for the smaller dogs eating larger areas of toy. As the NOAEL for DINP is established for liver toxicity in a chronic toxicity study, a longer duration of exposure to DINP is necessary to cause the toxic effects. It does not seem very realistic that dog owners continuously should provide their dogs with toys even though the dogs continuously tear the toy apart. Therefore although possible, it seems less likely that the presence of DINP in toys can cause effects in the liver in dogs. Recent research has indicated that DINP affects the level of testosterone in the male reproductive system, an effect which is evident for DEHP. The available data are not sufficient in order to evaluate the risk for reproductive effects following exposure to DINP, but DINP seems to be much less potent than DEHP. Consequently, the concern for reproductive effects in dogs due to the presence of DINP in toys is considered as being low. The exposure via toys may be a major source of phthalate exposure to dogs. However as for humans, animals may also be exposed to phthalates via other sources (environment, food, consumer products). This includes exposure to DEHP and DINP, but also other phthalates e.g DBP where combined actions could be expected. As an advice to the animal owners, they can reduce the potential health risk to their animals by limiting the animal’s use of toys that potentially contain phthalates especially during pregnancy and as pups. 6 ReferencesBabich, M.A., Chen, S-B., Greene, M. A., Kiss, C. T., Porter, W. K., Smith, T. P., Wind, M. L. and Zamula, W. W. (2004). Risk assessment of oral exposure to diisononyl phthalate from childrens products. Regulatory Toxicology and Pharmacology 40: 151-167. Bouma, K. and Schakel, D.J. (2002) Migration of phthalates from PVC toys into saliva simulant by dynamic extraction. Food additives and contaminants 19: 602-610. Chen, S-B. (1998). Migration of DINP from Polyvinyl Chloride (PVC) childrens products. Directorate for Laboratory Sciences. U.S. Consumer Product Safety Commission. DEHP (2001). European Union Risk Assessment of bis(2-ethylhexyl)phthalate. CAS-No.:117-81-7. Consolidated Final Report : September 2001. http://ecb.jrc.it//existing-chemicals Deisinger, P. J., Perry, L. G. and Guest, D. (1998). In vivo Percutaneous Absorption of [14C]DEHP from [14C]DEHP-Plasticized Polyvinyl Chloride Film in Male Fischer 344 Rats. Food and Chemical Toxicology 36: 521-527. DINP (2001). European Union Risk Assessment of 1,2-Benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich and Di-“isononyl”phthalate. CAS-No.:68515-48-0 and CAS-No.:28553-12-0. Finalised report of May 2001. France. http://ecb.jrc.it//existing-chemicals Ikeda, G. J., Sapienza, P. P., Couvillion, J. L., Farber, T. M. and van Loon, E. J. (1980) Comparative distribution, excretion and metabolism of Di-(2-ethylhexyl) phthalate in rats, dogs and miniature pigs. Fd Cosmet Toxicol 18: 637-642. Kùnemann, W.H. (ed) (1998). Phthalate release from soft PVC baby toys. Report from a Dutch consensus group. RIVM report no. 613320 002. Müller, A,K., Nielsen, E. and Ladefoged, O (2003) Human exposure to selected phthalates in Denmark. Fødevaredirektoratet.FødevareRapport 2003:15. Nielsen, T. B., Bjarnov, E. and Bundgaard, O. (2005A) Kortlægning af kemiske stoffer i legetøj til dyr. Miljøstyrelsen. Kortlægning af kemiske stoffer i forbrugerprodukter, Nr. 56. Nielsen, E., Østergaard, G., Larsen, J. C. og Ladefoged, O. (2005B) Principper for sundhedsmæssig vurdering af kemiske stoffer med henblik på fastsættelse af kvalitetskriterier for luft, jord og vand. Miljøprojekt nr. 9742005. Rogers, J. M. and Kavlock, R. J. (1998) Developmental Toxicology. In Reproductive and developmental toxicology (Ed. Korach, K.), Marcel Dekker, Inc, USA Steiner, I., Kubesch, K. and Fiala, F. (1998; Preliminary summary). Migration of DEHP and DINP from PVC articles. CSTEE/97/1-Add.102. AbbreviationsBW – body weight DEHP – Di(2-ethylhexyl)phthalate DINP – Di-isononyl-phthalate NOAEL – No observed adverse effect level LOAEL – Lowest observed adverse effect level MOS – Margin of Safety PVC – Poly vinyl chloride Exp – Exposure AppendixAppendix 1Exposure estimations of DEHP and DINP based on the assumption that 10% of the phthalate present in the product migrate
Appendix 2Exposure estimations of DEHP and DINP based on measured migration rates
Appendix 3MOS values derived on the LOAEL (37 mg/kg bw/day) from the toxicity study in dogs (Hazleton et al. 1971 – quoted in DINP 2001). The minimal MOS is a product of a factor 10 for intraspecies variation and a factor 10 for using a LOAEL instead of a NOAEL.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||