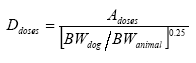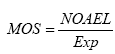|
Evaluation of the health risk to animals playing with phthalate containing toys 4 Risk Assessment
In the present report, the risk characterisation is carried out by quantitatively comparing the outcome of the effects assessment to the outcome of the exposure assessment. The ratio resulting from this comparison is called Margin of Safety (MOS). In judging the sufficiency of the MOS, several parameters regarding the NOAEL/LOAEL have to be considered in terms of uncertainties and variabilities e.g. related to the extrapolation from experimental data to the actual situation; in the present case the dog situation. Therefore in the interpretation of the MOS, default assessment factors are often used to account for these uncertainties and variabilities. The factors are combined to form the so-called “minimal-MOS”, which can be interpreted as an “overall” uncertainty factor. 4.1 Uncertainty factors or establishing minimal MOSWhen a risk characterisation is made for humans based on experimental animal studies, uncertainty factors are applied in order to cover 1) intraspecies variability in susceptibility, 2) interspecies variability in susceptibility and 3) other parameters that have to be taken into account e.g. quality of study, LOAEL instead of NOAEL etc. The uncertainty factor accounting for intraspecies variability in susceptibility is relevant also for dogs as it is expected that the dog population show a broad range of biological sensitivity. An uncertainty factor of 10 is usually applied to account for variability in susceptibility within the human population (Nielsen et al. 2005B) and it is assumed that a factor of 10 will also be adequate in order to account for the variability in susceptibility within the dog population. The uncertainty factor accounting for interspecies variability in susceptibility is relevant when extrapolating from data in rodents to dogs as dogs may be more susceptible to a given effect than rodents. The factor is usually 10 when extrapolating from experimental animals to humans (Nielsen et al. 2005B). The factor can be divided into two parameters; one parameter accounting for species differences in toxicokinetics (absorption, distribution, metabolism and excretion) and the other parameter accounting for other differences, e.g toxicodynamic. Generally, factors of 4 and 2.5 have been suggested for differences in toxicokinetics and toxicodynamics, respectively (Renwick 1993 and WHO 1994 – quoted in Nielsen et al. 2005B). However, it is a general point of view that regarding interspecies differences in toxicokinetics, the difference in metabolism is a key factor (Nielsen et al. 2005B). Therefore, the factor of 4 can be replaced by a correction of doses due to differences in metabolism estimated by allometric scaling, where a biological parameter e.g. metabolism can be expressed as a function of bodyweight. The correction of doses by allometric scaling on the basis of differences in body weight and metabolism can be estimated as follows:
where Adose is the dose in the animal study, BWdog is the body weight of dogs and BWanimal is the body weight of the test animal. The denominator It is assumed that the body weight of an adult rat is 400 g, and the correction factors for different sizes of dogs are presented in Table 5. These correction factors predominantly take toxicokinetic differences into account, predominantly in form of differences in the metabolism due to differences in body size. To account for other differences as e.g the toxicodynamic differences between rats and dogs the factor of 2.5 will also be applied. The total interspecies uncertainty factor can be seen in Table 5, as well as the overall uncertainty factor - the minimal MOS - where a factor of 10 for intraspecies variability has also been applied. The uncertainty factor accounting for the quality and relevance of the data is not relevant in the present assessment as the NOAELs taken forward to the risk characterisation are derived from well-performed and relevant toxicity studies. Table 5. Correction factors for extrapolation of doses in rats (400 g) to doses in dogs derived by allometric scaling based on metabolism as well as the total interspecies uncertainty factor, the intraspecies uncertainty factor and the overall uncertainty factor (minimal MOS) covering both inter- and intraspecies variation.
4.2 MOSThe margin of safety (MOS) can be calculated as follows:
In the calculation of MOS, the NOAEL of DEHP is 4.9 mg/kg b.w./day, the NOAEL of DINP is 88 mg/kg b.w./day and the exposures have been estimated for different sizes of dogs as well as amount of toy eaten and by using either a migration rate or the assumption that 10% of the phthalate migrate. The estimated MOS of DEHP and DINP is presented in Table 6 and Table 7, respectively, where also the minimal MOS is included. In the tables, exposure scenarios where MOS is below the minimal MOS have been marked with grey. As can be seen in the Tables, many of the calculated MOS values are below the minimal MOS. The MOS values for DEHP derived from the most conservative way of estimating the exposure – assuming 10% of the phthalate migrate while in the gastrointestinal tract – is in all cases below the minimal MOS; even the largest dog eating only 1 g of toy/day that contain an average content of DEHP is exposed to doses of DEHP where toxic effects can occur. Also for DINP that have higher NOAEL than DEHP, the main part of the MOS values are below the minimal MOS except for the larger dogs (20-40 kg) eating the smallest amount of toy (1g) with an average content of DINP. Table 6. MOS for DEHP has been estimated for dogs weighing from 1 to 40 kg with exposure estimations either based on migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B). Average and worst-case migration is 1.8 and 5.2 μg/10 cm2/min, respectively and average content is 21.1 and 39 %, respectively.
Table 7. MOS for DINP has been estimated for dogs weighing from 1 to 40 kg with exposure estimations either based on migration rate (A) or the assumption that 10% of the phthalate in the toy migrate while in the gastrointestinal tract (B). Average and worst-case migration is 2.4 and 11.1 μg/10 cm2/min, respectively and average content is 27.5 and 54 %, respectively.
Regarding the MOS values derived from the exposure estimations based on measured migration rates, they are also in several cases especially for DEHP below the minimal MOS. The MOS for DEHP is below the minimal MOS for dogs weighing 1 kg eating 1 cm² of toy, for dogs weighing 5 and 10 kg eating 5 cm² of toy, for dogs weighing 20 kg eating 10 cm² of toy or for dogs weighing 40 kg and eating 50 cm² of toy. The MOS for DINP is only below the minimal MOS for dogs weighing 1 kg eating 10 cm² of toy and for dogs weighing 5 and 10 kg eating 50 cm² of toy. The MOS values are derived based on the NOAEL from DINP (2001) (88 mg/kg b.w./day). If instead the LO(A)EL from the available dog study although badly reported but never the less a toxicity study in dogs is used (37 mg/kg b.w./day), the estimated MOS values for DINP are lower. However, the minimal MOS is also lower (20 for all sizes of dogs). The uncertainty factor for interspecies variability is not relevant. Instead a factor of 2 is applied accounting for the use of a LO(A)EL instead of a NOAEL. Therefore, although the MOS values are lower, it is exactly the same exposure scenarios that are below the minimal MOS (see appendix 3 for details) compared to when the MOS values are estimated based on the NOAEL from DINP (2001) (Table 7). 4.3 Critical period of exposureIt may seem unrealistic that eating a piece of toy should be an “every day” scenario for a dog. However, this is not necessary as effects of DEHP are seen also following exposure during a relatively limited but critical period of time; that is in utero and post-natal. Developing and prepubetal rats have been found to be much more sensitive to exposure to DEHP than adults (DEHP 2001). Therefore, the most critical period of exposure of dogs to DEHP is also expected to be in utero and as pups. The critical in utero exposure period can even be more defined; the most critical period of exposure is the period of sexual differentiation which is during the last half of pregnancy e.g. in rats from gestation day 11 – 21 and with the differentiation of testes occurring around gestation day 14-15 (Rogers and Kavlock, 1998). Therefore, the dogs only need to eat pieces of toy at some relatively short but critical periods of time for critical effects to occur. Off course if the exposure period is extended e.g. in pups the effects will increase further. Regarding DINP the critical effects are not developmental toxicity and therefore not especially related to in utero and post-natal exposure. Continued exposure during longer period of time is therefore necessary to cause the liver effects seen at the NOAEL. 4.4 Exposure to animals from other sourcesThe human population is exposed to phthalates via the environment, via food and via consumer products (Müller et al. 2003). This is likely also to be the case for dogs and cats as they live in association with humans. Therefore the exposure via toys may be a major source to phthalates, but it is important to remember that probably it is not the only one. 4.5 Uncertainty and limitationsThe products are continuously emitting phthalates even though the intensity of emission is not expected to be linear over the product’s lifetime. New products are expected to release higher amounts of phthalates compared with older products. In the present assessment, no differences in release during ageing of the product have been considered. The bioavailability is not included in the exposure estimations and therefore, the estimates can only be considered as the external exposures. However, the established NOAELs are also expressed as an external dose and therefore, the estimated exposures and the NOAELs can be compared. In the first estimation (A), the migration rates used to estimate the exposure is measured in either saliva or in a saliva simulant. It is likely that the migration of phthalates from products in the gastrointestinal tract is different due to both chemical (lower pH, higher content of fat), physical as well as biological differences. In estimation A, the exposure time off course also influences the outcome significantly. In general the time for food items to pass through the gastrointestinal tract is 12-30 hours depending on e.g. size and composition of food particles. It is very likely that pieces of plastic pass through the gastrointestinal tract relatively slow as they in general are considered to be relatively large particles, hard as well as indigestible. In the second estimation (B), the migration of 10% of the phthalates in the product is a qualified guess off the size of migration which of course influences the level of estimated exposure significantly. As mentioned previously, it may seem unrealistic that the scenario of eating a piece of toy leads to chronic exposure of DEHP and DINP to dogs. However, this will probably depend on the individual dog (age, strain, nature) as well as on the availability of phthalate containing products for the dog. Whether phthalate containing products are available for the dog depends on the owner of the dog and the likelihood of the owner continuously to provide the dog with toys which it tears into pieces. Furthermore, as also mentioned the effects of DEHP on the reproductive system does not only occur following long-term exposure as the most critical time of exposure is a relatively short period in utero.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||