Assessment of DHA in self-tanning creams applied in spray booths
5 Health assessment
- 5.1 TOXOCOLOGICAL PROFILE OF DIHYDROXYACETONE (DHA) (CAS NO. 96-26-4)
- 5.2 BRIEF HEALTH ASSESSMENT OF ETHOXYDIGLYCOL (CAS NO. 111-90-0)
- 5.3 BRIEF HEALTH ASSESSMENT OF PHENOXYETHANOL (CAS NO.122-99-6)
- 5.4 BRIEF HEALTH ASSESSMENT OF GLYCERINE (CAS NO. 56-81-5)
- 5.5 BRIEF HEALTH ASSESSMENT OF POLYSORBATES AND SORBITAN ESTERS
- 5.6 BRIEF HEALTH ASSESSMENT OF ERYTHRULOSE (CAS NO. 40031-31-0)
- 5.7 BRIEF HEALTH ASSESSMENT OF PARABENS (METHYLPARABEN CAS NO. 99-76-3, ETHYLPARABEN, CAS NO. 120-47-8 AND PROPYLPARABEN CAS NO. 94-13-3)
- 5.8 BRIEF HEALTH ASSESSMENT OF PCA (CAS NO. 98-79-3 and 149-87-1) AND SODIUM PCA (CAS NO. 28874-51-3 AND 54571-67-4)
The health assessment focuses on dihydroxyacetone (DHA) as the active ingredient in self-tanning agents. The other ingredients in self-tanning products vary from manufacturer to manufacturer. However, some ingredients are prevalent in many products, for example ethoxydiglycol, phenoxyethanol, glycerine, polysorbates/sorbitan esters, parabens and PCA/Sodium PCA. The following is a brief health profile of these. Only limited data is available for several of the substances/substance groups.
Meetings and other contact with representatives from some of the large manufacturers of booths and products demonstrated that none of them was able to document that DHA does not pose a health risk from exposure of the eyes and mucous membranes, or through inhalation. The US manufacturer Magic Tan has completed studies of the effects of self-tanning products from exposure via inhalation, the eyes and the mucous membranes, but they would not provide information on the results when requested (5).
5.1 TOXOCOLOGICAL PROFILE OF DIHYDROXYACETONE (DHA) (CAS NO. 96-26-4)
Occurrence and use
DHA is a molecule with three carbon atoms and it is the most commonly used active ingredient in self-tanning preparations. DHA was first used to treat diabetics, as some patients were more tolerant to DHA than to glucose. In 1957 the skin-colouring properties of DHA were discovered at a children's hospital. DHA was administered orally to treat childhood glycogen storage disease. A doctor noticed that when the children spat out some of the DHA preparation, they developed brown marks where the DHA had hit their skin. The first scientific article on DHA was published in 1960. Since then, the physical-chemical and tanning properties of DHA, as well as its reaction on the skin have been studied (1).
The skin-colouring properties of DHA come through a reaction with the amino acids and amino groups found on the outer layer of the skin (stratum corneum) during the formation of high-molecular pigments (melanoids). This occurs in a Maillard-like reaction, where pyruvate and other hydroxycarbonyle compounds are formed from dihydroxyacetone.
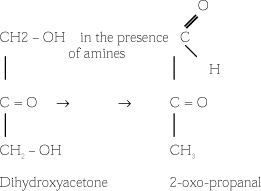
The subsequent reaction steps have not yet been fully explained as they seem extremely complicated. We know, however, that keto or aldo compounds react with an amine (the amino acids in the skin) during the formation of a ketoimine or an aldoimine. Furthermore, we know that the resulting compounds are cyclical and linier polymers with yellow-brown colouring and they are assumed to follow the course below:

DHA does not colour other surfaces than the stratum corneum, i.e. not the mucous membranes. On the other hand, there is stronger colouring of skin areas where the stratum corneum is thicker, for example on the palms, soles of feet, knees and ankles. (6).
Identification
| Chemical name | 2-propanon, 1,3-dihydroxy-acetone |
| Synonyms | Dihydroxyacetone (DHA) |
| CAS No. | 96-26-4 |
| EINECS No. | 202-494-5 |
| Molecule formula | C3H6O3 |
| Molecule structure | |
| Legislation Classification in accordance with the list of hazardous substances (Statutory Order no. 439 of 3 June 2002) Statutory Order on Cosmetic Products (Statutory Order, no. 422 of 4 May 2006) |
Not classified Unlimited |
Physical-chemical properties (7,8)
| Physical state | Crystalline powder |
| Molecular weight (g/mol) | 90.08 |
| Boiling point, oC | 90 °C (68-71 °C) (9) |
| Vapour pressure | 0.021 mmHg (25 °C) |
| Henry's law constant | 1.21×10-3 atm m³/mol (25 °C) |
| Water solubility (mg/l) | 5.03×105 mg/l (25 °C) |
It has been demonstrated that pure DHA acts as a mixture of monomers and dimers where the dimers are dominant. Heating or melting converts the DHA to monomer form. The monomer is also formed after about 30 minutes in an aqueous solution. Only the monomer is active in colouring the skin (4).
In aqueous solution the DHA monomer can be gradually automerised to glyceraldehyde. As the equilibrium is shifted towards glyceraldehyde at higher pH values, the equilibrium depends on the pH of the solution.
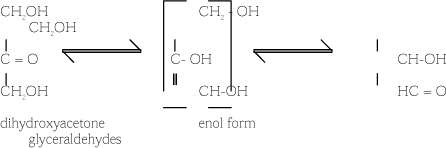
In alkaline conditions, starting in glyceraldehyde, different isomerisation and condensation reactions take place which lead to formation of brown-coloured oligomers (4).
The stability of DHA depends on the concentration of DHA, pH, and the presence of by-products. The pH value of DHA solutions falls over time to about pH 3 because of the formation of organic acids. DHA is slightly acidic in itself. Glyceraldehyde is an isomer of DHA (4).
DHA phosphate occurs naturally in the human body and is part of the Krebs cycle (citric acid cycle) (4). The pH of the stratum corneum is 4.2-5.6, and for the epidermic is 7.3-7.4. (10)
Acute toxicity
Table 5.1. Summary of toxicological data.
| Toxicological data (animals) | |
| LD50, (mg/kg BW), intraperitoneal, rat (United States Patent Document. Vol. #4049795) |
8750 |
| LD50, (mg/kg BW), intraperitoneal, rabbit (United States Patent Document. Vol. #4049795) |
8000 |
| LD50, (mg/kg BW), oral, rat (8) | >16000 |
| LD50, (mg/kg BW), intraperitoneal, rat (8) | 6400 |
A skin and mucous membrane test on rabbits exposed to DHA revealed no irritation (4,11).
A study by Goldman in the 1960s showed that ingestion of 18 g DHA, three times per day over 2-3 weeks did not produce any harmful effects on adult humans (12). No similar studies have been carried out more recently.
Ingestion of DHA has reduced amounts of body fat in rats (13). DHA is used as a food supplement together with pyruvate as a performance enhancer (14,15,16).
According to a more recent study by Kurz in 1994, DHA reacts with the skin's stratum corneum but it does not penetrate the skin (4). This conflicts with Goldman et al. who, in an older study from 1962, could demonstrate DHA in the blood shortly after applying DHA to the skin (17).
Long-term, repeated impacts
There is conflicting information about the health effects of DHA. Older studies by Akin et al. and Goldman et al. show that DHA has not demonstrated any toxic or carcinogenic effects. In the Akin study, 0.1 ml of aqueous 5 per cent and 40 per cent solutions of 97 per cent DHA were evenly applied to shaven Swiss-Webster mice once a week. The mice were divided into three groups, each with 50 males and 50 females. Treatment continued over 80 weeks. No significant carcinogenic effect could be demonstrated in any of the groups. Goldman refers to experience with the low toxicity of DHA without giving further information about channels of exposure and concentrations of DHA (18,19). According to Draelos et al., DHA has low toxicity, both for ingestion and for application to the skin, with only few reported cases of allergic contact dermatitis. The low toxic effect is not further documented in the article (20). Other in vitro studies show that DHA is suspected of causing damage to the DNA (21,22,23). DHA induced a significantly increased number of type 3 foci in 3T3 fibroblasts, indicating in vitro neoplastic cell transformation (24). DHA has been found in the blood shortly after application to the skin (17). According to Petersen et al. (23) it is therefore possible that DHA may penetrate down through the stratum corneum and affect the DNA and proteins in other cells in the body.
Because of an in vitro study, in which DHA demonstrated genotoxic and mutagenic properties, the substance is suspected of having these properties. These properties have not been confirmed in other test systems. Therefore there is some doubt about the use of DHA in skin treatment over longer periods (23).
The information available about the genotoxicity of DHA can seem contradictory as DHA has demonstrated genotoxic properties in vitro, but it is also an intermediary in the carbohydrate metabolism of higher plants and animals, and therefore a natural phenomenon in the body (7).
Local irritation
Frequent use of self-tanning products containing DHA may make skin more prone to irritation (20).
Laboratory tests of a self-tanning product showed no skin irritation, but moderate eye irritation. However, there is no evidence that the irritation is due to the content of DHA (25).
Allergies
Patients have been tested for allergic reactions to DHA using patch tests, but with negative results. The author has a theory that previously described cases of allergies may be due to exposure to other substances at the same time as DHA (26,27).
Morren et al. report on two cases of allergic reactions to DHA as a result of using self-tanning products on the skin (28). The US FDA mentions that it is possible that other ingredients in self-tanning products such as oils or Juglans regia (walnut) extract may cause discomfort for those with nut allergies (29).
5.1.1.1 Critical effect
The critical effect of DMA is considered to be irritation of the eyes. Allergic reactions rarely occur. Because of an in vitro study, DHA is suspected of being a genotoxic substance with mutagenic properties.
There is no data on inhalation of DHA. The US Magic Tan (5) have completed studies of the effects of exposure to self-tanning agents through inhalation, and on the eyes and mucous membranes, but they do not want to reveal the results.
There is no data on NOAEL, LOAEL, NOEL or LOEL in the literature.
The attitude of the US FDA to DHA
DHA is an ingredient in food and it has been approved for ingestion by the US Food and Drug Administration (FDA). In fact the health industry is the world's largest user of DHA. As DHA is a pyruvite or "fat-burner", it is used in many slimming products.
DHA has also been approved by the FDA for use as a cream and lotion. The FDA assesses that DHA has no known harmful effects on the body when it is applied to the skin, and DHA is the only self-tanner approved for use in self-tanning products. Approval of DHA as a self-tanner for use in cosmetics is limited to external use. The FDA defines external use as "applied only to external parts of the body and not to the lips or any body surface covered by mucous membrane". Furthermore, no colouring additive may be used in cosmetics intended for use around the eyes unless it has special approval (30).
According to the latest document from the FDA on sunscreens, self-tanning products must be labelled with a warning that they do not protect against UV radiation or sunburn (1).
Recommendations from the FDA
- DHA should not be inhaled as in rare cases it can cause an allergic reaction in allergy sufferers, and the long-term effects of the carbohydrate from repeated inhalation have never been examined.
- The eyes should be kept shut for the reasons mentioned above.
- It may be advisable to protect the lips with a lip-salve and the nose with nose filters prior to treatment.
- The mouth should be kept shut during treatment.
5.2 BRIEF HEALTH ASSESSMENT OF ETHOXYDIGLYCOL (CAS NO. 111-90-0)
Ethoxydiglycol is on the INCI list and there are no restrictions on use in cosmetic products under the Statutory Order on Cosmetic Products (Statutory Order no. 422 of 4 May 2006).
Ethoxydiglycol generally has low toxicity. This applies for injection, inhalation, and acute and sub-chronic skin toxicity tests (31).
Ethoxydiglycol provokes minimal to mild skin irritation in rabbits and mild to serious ocular (eye) irritation (31,32).
Ethoxydiglycol does not provoke an allergic reaction on contact with the skin (31).
Use of ethoxydiglycol is deemed to be low risk when used as an ingredient in cosmetics under current practices for use and concentration. (31).
5.3 BRIEF HEALTH ASSESSMENT OF PHENOXYETHANOL (CAS NO.122-99-6)
Phenoxyethanol is on the INCI list and it is an aromatic ether permitted for use as a preservative in cosmetic products at concentrations of up to 1 per cent. According to the Cosmetic Ingredient Review Expert Panel, phenoxyethanol has low toxicity in rats from ingestion and application to the skin. There is no information on the concentrations tested. On the other hand a subchronic test with phenoxyethanol showed reduced body weight in rats in a 90-day study (33).
It was not possible to demonstrate harmful effects on rats exposed to single exposures of saturated vapours of phenoxyethanol (saturated at 100 degrees C and then cooled to room temperature) for a period of seven hours (Hazardous Substance Databank, HSDB, Phenoxyethanol).
The concentrated phenoxyethanol was extremely irritant on the eyes. This irritation disappeared at concentrations of < 2.2 per cent. Exposure to phenoxyethanol in a concentration of 2 per cent was slightly irritant on the skin in a test on rabbits. The same test on guinea pigs provoked neither irritation nor allergic reactions. There was no sign of teratogenicity, embryotoxicity or foetotoxicity at doses which were toxic for the mother animal in tests on mice where phenoxyethanol was applied to the skin. Phenoxyethanol was not mutagenic in Ames' test, with or without metabolic activation or in micronucleus tests (mice). In clinical studies phenoxyethanol provoked no primary irritation or allergic reaction. Phenoxyethanol did not demonstrate phototoxicity in clinical studies (33).
5.4 BRIEF HEALTH ASSESSMENT OF GLYCERINE (CAS NO. 56-81-5)
Glycerine is on the INCI list and there are no restrictions on use in cosmetic products under the Statutory Order on Cosmetic Products.
When administered on the skin and in the rectum, glycerine can cause irritation. Application of glycerine to the eyes can damage the cornea (34,35).
5.5 BRIEF HEALTH ASSESSMENT OF POLYSORBATES AND SORBITAN ESTERS
Polysorbates and sorbitan esters are on the INCI and there are no restrictions on use in cosmetic products under the Statutory Order on Cosmetic Products. They are used as emulsification agents/surface-active substances in self-tanners.
There is an LD50, oral at >15g/kg (36). The FAO/WHO Expert Committee on Food Additives states the acceptable daily intake of polysorbate esters at 25 mg/kg body weight (34,36).
Polysorbates can increase the absorption of fat-soluble substances. There are instances of allergic reactions after skin contact (34).
Undiluted polysorbates provoked no or slight skin irritation in tests on rabbits. Dilution of 15 per cent gave no skin irritation. Draize’ test of undiluted polysorbates in the eyes of rabbits provoked minimal eye irritation. Dilution to 75 or 30 per cent in water or 10 per cent in mineral oil provoked no irritation (36).
Tests on the mucous membranes in the mouth on hamsters with Polysorbate 20 (unspecified volume and concentration) and 10 per cent Polysorbate 40 showed no inflammatory reaction (36).
A lotion containing 4 per cent Polysorbate 40 and a cream formulation containing 1 per cent Polysorbate 85 gave no irritation after application to penile and vaginal mucous membranes on rabbits. Three beagle dogs exposed in the vaginal area to a foam-bath product containing 6 per cent Polysorbate 20 once a day, five days a week for three weeks suffered serious irritation. Exposure to the same foam-bath product diluted to 5 per cent in water provoked no visible effects (36).
Polysorbate 60 and 80 are not deemed to have any harmful effects from ingestion of amounts of up to 25 mg/kg BW (ADI total polysorbate tests) (36).
Polysorbate 20 provoked no harmful effects on the eyes at concentrations of up to 40 per cent. Undiluted Polysorbate 20 applied to the eyes of rabbits gave slight irritation. There is no documentation regarding skin irritation (36).
Polysorbates are not regulated by the Statutory Order on Cosmetic Products, but use in cosmetic products has been assessed by a panel of experts. On the basis of available toxicological data, the panel of experts assessed that Polysorbates (20, 21, 40, 60, 65, 80 and 85) do not pose a risk for the consumer when they are used in cosmetics under the current practice for use and concentration (36).
5.6 BRIEF HEALTH ASSESSMENT OF ERYTHRULOSE (CAS NO. 40031-31-0)
Erythrulose is not on the INCI list. Erythrulose is a sugar molecule formed through aerobic fermentation of Gluconobacter. Erythrulose reacts with free primary and secondary amino groups (Maillard reaction) in the outer layer of the skin (stratum corneum). Acute toxicity tests have revealed LD50 >2000 mg/kg (rats, oral) for 16 per cent erythrulose (43).
Erythrulose is neither irritating nor sensitising on contact with the skin.
Erythrulose was not mutagenic in Ames' test (43).
5.7 BRIEF HEALTH ASSESSMENT OF PARABENS (METHYLPARABEN CAS NO. 99-76-3, ETHYLPARABEN, CAS NO. 120-47-8 AND PROPYLPARABEN CAS NO. 94-13-3)
Methyl, ethyl and propylparabens are present in several self-tanning products.
Parabens are used as a preservative and are regulated by the Statutory Order on Cosmetic Products (Statutory Order no. 422 of 4 May 2006). This Statutory Order sets limits on the content of parabens in cosmetics at 0.4 per cent (as acid) for an individual ester, and 0.8 per cent for ester mixtures.
Parabens are effective preservatives in the pH interval 4-8, but they are best at lower pHs. Effectiveness increases and water solubility decreases for longer alkyl chains. The effectiveness of parabens can be influenced by other additives or other ingredients, such as polysorbates (34,35)
Methyl, ethyl and propyl parabens can be absorbed through the skin. Parabens are hydrolysed and conjugated and released through the urine. Data from life-time studies indicate that parabens do not accumulate in the body. Studies with different administration routes on animals indicate that parabens have low acute toxicity and that toxicity seems to fall with increasing lengths of alkyl chain (37)
Parabens can provoke allergic reactions on contact with the skin. The sensitising effect is more limited on normal skin, however. Hypersensitivity to methylparaben will often imply hypersensitivity to ethylparaben and propylparaben. Ingesting food and drugs containing parabens does not seem to lead to rashes on people allergic to parabens, if the mucous membrane is intact (37).
A large number of mutagenic tests, including Ames' test, have been carried out, and none of these indicate the parabens have a mutagenic effect (37).
A teratogenic effect has been demonstrated in rats from administration of a 10 per cent solution of ethylparaben (corresponding to 9,000 mg/kg BW/day). There were no clear teratogenic effects at lower concentrations. Methyl, ethyl and propylparabens have demonstrated potent spermatocidal effects. In rats there were clear oestrogen-like effects at oral doses of 600 mg/kg BW/day (37).
No carcinogenic effects have been demonstrated in studies of propylparaben and methylparaben (37).
Trials lasting 96 weeks with 2 per cent and 8 per cent methyl and propylester showed no significant pathological results. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) has estimated that 2 per cent parabens in food corresponds to 1,000 mg/kg BW (37).
The estimated acceptable daily intake is < 10 mg/kg BW. (34).
5.8 BRIEF HEALTH ASSESSMENT OF PCA (CAS NO. 98-79-3 and 149-87-1) AND SODIUM PCA (CAS NO. 28874-51-3 AND 54571-67-4)
Use of PCA is not restricted in cosmetic products and it is used as a moisturiser.
PCA is polyglutamic acid. Sodium PCA is the sodium salt of polyglutamic acid. It is used in both skin and hair lotions. Recommended concentrations in these substances are 0.2-4 per cent. Sodium PCA has been tested as non-irritant for the eyes and skin at concentrations of up to 50 per cent. There are no indications that Sodium PCA and PCA are photo-toxic, allergenic, or genotoxic (38).
On the basis of available data, it can be concluded that Sodium PCA and PCA are safe ingredients in cosmetic formulas as they are used today (38).
Version 1.0 September 2006, © Danish Environmental Protection Agency