Survey of chemical substances in consumer products no. 79, 2006
Survey and health risk assessment of products for treatment of sports injuries and pains
Contents
3 Quantitative chemical analyses
- 3.1 Quantitative determination of volatile and semi volatile compounds (GC/MS screening)
- 3.2 Specific analysis of contents of organic solvents
- 3.3 Prioritisation of substances in products for treatment of sport injuries and pain
Preface
The project “Survey and health risk assessment of products for treatment of sports injuries and pains” has been carried out from April 2005 to December 2005.
The project includes a survey of the products on the Danish market categorised as products for treatment of sports injuries and pains as well as a survey of the chemical substances present according to the product labelling. Subsequently, screening analyses of hazardous substances in selected products have been carried out followed by quantitative analyses of selected substances. Finally, we have made a health risk assessment of a number of hazardous substances.
The project has been carried out by Danish Technological Institute and been headed by laboratory manager Paul Lyck Hansen, who has also been responsible for the laboratory analyses.
Cand.arch. Kathe Tønning has been responsible for the survey, and the health risk assessment has been made by B.Eng. Kirsten Pommer and Lic.techn. Bjørn Malmgren-Hansen. PhD Ole Christian Hansen and PhD Mikael Poulsen have assisted with the quality assurance of the project.
The project has been financed by the Danish EPA.
The reference group on the project consisted of:
Anette Ejersted, EPA (Chairman)
Dorit Skals, EPA
Paul Lyck Hansen, DTI
Kathe Tønning, DTI.
Summary and conclusions
Assessment of products for treatment of sports injuries and pains
Background and purpose
Through recent years the Danish people have become more physically active and are practising some kind of sport regularly. The increase has been significant from year 2000 till today and applies for both men and women.
This increased activity level has, however, also resulted in an increased risk of injuries and sore muscles and joints.
In case of minor injuries most people, professionals as well as exercisers, prefer to self-treat their injuries, often with products which are applied to the skin.
Over generations various minor ailments have been treated by hot and cold therapy products. Recent years have seen an increasing number of therapy products on the market for easing and removing muscle pain. Today the Internet offers a variety of web sites regarding exercise and injuries and more of these recommend use of pain relief creams and gels.
The purpose of this project is to identify the most commonly used products and to asses their popularity. The products selected have been analysed for their content of various substances, especially for active substances. Finally, potential hazardous substances have been identified.
The survey
The project has been carried out by the Danish Technological Institute.
The survey of the sports injury products on the market and their chemical substances has formed the basis of the further assessment of the products.
Due to the very different base formulations of the analysed products, ranging from aqueous to glycerine and paraffin based systems, it was chosen to base the analysis method on headspace measurement.
In order to obtain sufficient sensitivity and lowest possible detection limit it was decided to use Solid Phase Micro Extraction (SPME) combined with GC/MS.
A chemical screening was carried out with the purpose of determining the content of volatile, organic substances in the selected products. The preliminary screening identified more than 30 different organic substances. In consultation with the Danish EPA it was decided to quantify approx. 20 substances and to carry out a subsequent analysis of organic solvents.
Six of the detected substances were selected for further analyses because of their relatively high concentration of substances, which may be hazardous. Further, four solvents were analysed.
Main conclusions
Apart from a health risk assessment of the products it has been investigated whether the products observe the regulations laid down in Statutory Order No. 923, 2005 on Classification, Packaging, Labelling, Sales and Storage of Chemical Substances and Products.
Of the 12 products 2 should have been labelled according to the prevailing labelling requirements.
Additionally, according to Statutory Order Exhibit 2, par. 2.13 the products Nos. 4, 8 and E should be labelled with the text "Contains (substance name). May cause allergic reaction"
Table 0.1 shows the health risks of the 12 selected products and their declaration requirements.
Table 0.1 Potential health impacts of selected sports products and labelling requirements
| Product No. |
Irritation 0: No impact X: Potential impact |
Sensitization 0: No impact X: Potential impact |
Impact by skin absorption 0: No health risk X: Potential impact |
Legislation L: Labelling obligation * A: Labelling requirements ** |
| 1 | X | 0 | 0 | |
| 2 | X | X | X | L |
| 3 | X | X | X | |
| 4 | X | X | 0 | A |
| 5 | 0 | 0 | X | |
| 8 | X | X | X | A |
| 12 | X | O | o | L |
| 13 | o | O | o | |
| 15 | X | X | X | |
| C | o | X | o | |
| E | X | X | X | A |
| H | X | X | X |
* Classification and marking of chemical substances and products acc. to EPA Statutory Order no. 923 of 28 September 2005
** Declaration requirements for allergens acc. to Statutory Order No. 923, 2005, exhibit 2, par. 2.13.
Based on the analysis the following recommendations can be given:
- Substances causing sensitization as e.g. camphor, d-limonene and α-Pinene should be avoided
- If solvents with cooling effect are to be added, ethanol should be preferred as the least poisonous.
Project results
Statistics Denmark has not been able to provide a quantitative survey of the consumption of pain relief products as there is no CN-code[1], for these products. Neither web sites nor the visited shops could provide precise and detailed information about their sales and an estimate of the total sales in Denmark can therefore not be made.
The survey resulted in 39 registered products.
In consultation with the Danish EPA 12 products were selected for further analyses.
The survey disclosed that that the most critical substances would be volatile, organic components, and the analyses were therefore based on this substance group.
Table 0.2 shows the results of the quantitative analyses of the products with the highest content of the mentioned substances.
Table 0.2 Selected results of substances with highest quantified content
| Substance | Contents in weight -% | ||||||||
| 1 | 2 | 3 | 4 | 8 | 13 | 15 | E | H | |
| Camphor | - | 7.7 | 0.51 | 0.10 | - | - | - | - | - |
| Cinnamale | - | 10 | - | - | - | - | - | - | - |
| Dimethyl sulphone | - | - | - | - | - | 8.2 | 0.01 | 0.06 | - |
| Eugenol | - | 2.7 | - | - | - | - | - | - | - |
| Isoeugenol | - | 0.16 | - | - | - | - | - | - | - |
| d-Limonene | - | 1.9 | 0.04 | 0.15 | 0.10 | - | 0.02 | 0.23 | 0.09 |
| Linalool | - | 0.19 | - | 0.10 | 0.01 | - | - | 0.17 | 0.18 |
| Methyl salicylate | 5.1 | 0.53 | - | - | 5.0 | - | 4.5 | 6.7 | 7.6 |
| 2-Phenoxy ethanol | 0.04 | - | 0.90 | - | 0.07 | 0.04 | - | - | - |
| α-Pinene | - | 1.2 | 0.05 | 0.17 | 2.7 | - | 1.9 | 3.3 | 2.2 |
Further, organic solvents were revealed in products Nos. 4, 5, 12 and C. An ethanol content of 54% was detected in product no. 4 and with 2.2% in product No. 5. Product No. 12 holds 27 % 2-propanol and product C contains 14 % ethanol, 0,35 % tert-butanol and 1.9 % acetone.
The analysis has focused on skin irritation and sensitization as well as skin absorption. The results are shown in Table 0.3.
Table 0.3 Possible health impacts of selected substances in sports products
| Substance | CAS no. | Irritation 0: No impact X: Potential impact XX: Major impact /: No data |
Sensitization 0: No impact X: Potential impact XX: Major impact /: No data |
Impact by skin absorption 0: No health risk X: Minor health risk XX: Health risk |
| Acetone | 67-64-1 | O | 0 | 0 |
| t-Butyl alcohol | 75-65-0 | 0 | 0 | 0 |
| Camphor | 76-22-2 | XX | X | X |
| Dimethylsulphon | 67-71-0 | 0 | 0 | 0 |
| Ethanol | 64-17-5 | o | 0 | X |
| d-Limonene | 5989-27-5 | / | XX | X |
| Methylsalicylate | 119-36-8 | XX | / | X |
| 2-Phenoxyethanol | 122-99-6 | o | 0 | X |
| α-Pinene | 80-56-8 | X | X | XX |
| 2-propanol | 67-63-0 | XX | 0 | 0 |
Sammenfatning og konklusioner
Vurdering af produkter til brug ved ømhed og skader efter sport m.m.
Baggrund og formål
En stor del af befolkningen dyrker i dag en eller anden form for motion jævnligt, og dette kan medføre smerter og ømhed i muskler og led. Danskerne er gennem de senere år blevet mere fysisk aktive, og for begge køns vedkommende er der især sket en stigning fra år 2000 til i dag. Et stigende aktivitetsniveau medfører en øget risiko for skader. Såfremt der er tale om mindre skader, vælger mange (både professionelle og motionister) at behandle skaderne selv. Dette sker ofte ved hjælp af produkter, der påsmøres huden.
Småskavanker af forskellig art er igennem flere generationer blevet dulmet med forskellige kulde-/varmeprodukter. Der er imidlertid gennem de senere år udbudt et stigende antal produkter til dette formål, og der findes på markedet i dag således en lang række produkter, der er beregnet til at dulme eller fjerne muskelsmerter. På Internettet er der i dag en lang række sider, der omhandler motion og skader. På flere af disse anbefales brug af smertestillende cremer og geler.
Projektets formål har været at identificere de mest anvendte produkter og forsøge at opgøre, hvor udbredt anvendelsen er af produkter til brug ved ømhed og skader efter sport m.m. Desuden er kortlagt hvilke indholdsstoffer der anvendes i produkterne; herunder specielt hvilke virksomme stoffer der anvendes i produkterne. Endvidere er eventuelle problematiske stoffer identificeret.
Undersøgelsen
Projektet er gennemført af Teknologisk Institut.
Kortlægning af, hvilke produkter inden for kategorien produkter til brug ved ømhed og skader efter sport m.m. der findes på markedet, og hvilke kemiske stoffer der anvendes i disse cremer, har været forudsætningen for den videre vurdering af produkterne.
Pga. den meget varierende basisformulering af de undersøgte produkter, fra vandige systemer til glycerin- og paraffinbaserede, blev det valgt at anvende en analysemetode med udgangspunkt i en headspace-måling.
For at opnå en tilstrækkelig følsomhed med den anvendte analysemetode og derigennem opnå den lavest mulige detektionsgrænse, blev det valgt at benytte Solid Phase Micro Extraction (SPME) kombineret med GC/MS.
Der blev først gennemført en kemisk screening med det formål at konstatere, hvilke flygtige, organiske stoffer der kunne måles i de valgte produkter. Ved den indledende screening blev der konstateret indhold af mere end 30 forskellige organiske stoffer. Det blev i samråd med Miljøstyrelsen valgt at kvantificere omkring 20 stoffer samt foretage en supplerende kvantitativ måling for organiske opløsningsmidler.
Af de fundne stoffer blev seks udvalgt til yderligere undersøgelser, idet der blev fundet relativt høje koncentrationer af stofferne, og fordi de kan være sundhedsskadelige. Herudover er fire opløsningsmidler vurderet.
Hovedkonklusioner
Udover en sundhedsvurdering for produkterne er det vurderet om krav til mærkning og deklaration overholdes i henhold til bekendtgørelse nr. 923, 2005 om klassificering, emballering, mærkning, salg og opbevaring af kemiske stoffer og produkter.
Af de 12 undersøgte produkter skulle 2 have været mærket i overensstemmelse med bekendtgørelsens mærkningsregler.
Herudover skal produkterne 4, 8 og E i henhold til bekendtgørelsens bilag 2, pkt.2.13 mærkes med en sætning med teksten ”Indeholder (stofnavn). Kan udløse allergisk reaktion”.
I Table 0.1 fremgår de sundhedsmæssige risici ved de 12 undersøgte produkter, samt lovgivningsmæssige krav til mærkning.
Tabel 0.1 Mulige sundhedsmæssige påvirkninger for udvalgte sportsprodukter, samt krav til mærkning
| Produkt Nr. |
Irritation 0: Ingen påvirkning X: Mulig påvirkning |
Sensibilisering 0: Ingen påvirkning X: Mulig påvirkning |
Påvirkning ved absorbering gennem huden 0: Ingen sundheds-mæssig risiko X: Mulig sundheds-mæssig risiko |
Lovgivning L: Skal mærkes* A:Krav til mærkning ** |
| 1 | X | 0 | 0 | |
| 2 | X | X | X | L |
| 3 | X | X | X | |
| 4 | X | X | 0 | A |
| 5 | 0 | 0 | X | |
| 8 | X | X | X | A |
| 12 | X | O | o | L |
| 13 | o | O | o | |
| 15 | X | X | X | |
| C | o | X | o | |
| E | X | X | X | A |
| H | X | X | X |
* Klassificering og mærkning af kemiske stoffer og produkter efter Miljøstyrelsens bekendtgørelse nr. 923 af 28. september 2005
** Krav til mærkning med allergisætning i henhold til bek. Nr.923, 2005, bilag 2, pkt.2.13.
På baggrund af undersøgelsen gives følgende anbefalinger:
- Stoffer, der kan forårsage sensibilisering, som fx kamfer, d-Limonen og α-Pinen, bør undgås
- Skal der tilsættes opløsningsmidler, der giver en kølende virkning, bør man primært bruge ethanol, da det er det mindst giftige opløsningsmiddel.
Projektresultater
Det har ikke været muligt at foretage en mængdemæssig kortlægning af forbruget af produkter til brug ved ømhed og skader efter sport mv. via Danmarks Statistik, idet der ikke findes en KN-kode[2], der omhandler disse produkter alene. Oplysninger fra internetbaserede forretninger og besøgte butikker om omfanget af salg af produkter er ikke tilstrækkeligt præcise og detaljerede, til at det er muligt at foretage et estimat over forbruget i Danmark.
Kortlægningen resulterede i registrering af 39 produkter.
I samråd med Miljøstyrelsen blev der udvalgt 12 produkter med henblik på videre undersøgelse i projektet.
Den udførte kortlægning viste, at det var overvejende sandsynligt, at de mest kritiske indholdsstoffer var flygtige, organiske komponenter, hvorfor det blev valgt at fokusere analysedelen på denne stofgruppe.
I Table 0.2 ses resultaterne af de kvantitative analyser for de produkter, hvori der er konstateret det højeste indhold af de i tabellen nævnte stoffer.
Tabel 0.2 Udvalgte resultater for stofferne med de højeste kvantificerede indhold
| Stof | Indhold i vægt-% | ||||||||
| 1 | 2 | 3 | 4 | 8 | 13 | 15 | E | H | |
| Camphor | - | 7,7 | 0,51 | 0,10 | - | - | - | - | - |
| Cinnamal | - | 10 | - | - | - | - | - | - | - |
| Dimethyl sulfon | - | - | - | - | - | 8,2 | 0,01 | 0,06 | - |
| Eugenol | - | 2,7 | - | - | - | - | - | - | - |
| Isoeugenol | - | 0,16 | - | - | - | - | - | - | - |
| d-Limonen | - | 1,9 | 0,04 | 0,15 | 0,10 | - | 0,02 | 0,23 | 0,09 |
| Linalool | - | 0,19 | - | 0,10 | 0,01 | - | - | 0,17 | 0,18 |
| Methyl salicylat | 5,1 | 0,53 | - | - | 5,0 | - | 4,5 | 6,7 | 7,6 |
| 2-Phenoxy ethanol | 0,04 | - | 0,90 | - | 0,07 | 0,04 | - | - | - |
| α-Pinen | - | 1,2 | 0,05 | 0,17 | 2,7 | - | 1,9 | 3,3 | 2,2 |
Derudover er der identificeret indhold af organiske opløsningsmidler i Produkt nr. 4, 5, 12 og C. Der blev målt ethanol i Produkt nr. 4 med et indhold på 54 % og i Produkt nr. 5 med et indhold på 2,2 %. Produkt nr. 12 indeholder 27 % 2-propanol, og Produkt C indeholder 14 % ethanol, 0,35 % tert-butanol og 1,9 % acetone.
Undersøgelsen har været koncentreret om hudirritation og sensibilisering samt absorbering gennem huden. Resultaterne af undersøgelsen for de udvalgte stoffer kan ses i Table 0.3.
Tabel 0.3 Mulige sundhedsmæssige påvirkninger af udvalgte stoffer i sportsprodukter
| Stof | CAS nr. | Irritation 0: Ingen påvirkning X: Mulig påvirkning XX: Risiko for væsentlig påvirkning /: Ingen data |
Sensibilisering 0: Ingen påvirkning X: Mulig påvirkning XX: Risiko for væsentlig påvirkning /: Ingen data |
Påvirkning ved absorbering gennem huden 0: Ingen sundhedsmæssig risiko X: Mindre sundhedsmæssig risiko XX: Sundhedsmæssig risiko |
| Acetone | 67-64-1 | o | 0 | 0 |
| t-Butylalkohol | 75-65-0 | 0 | 0 | 0 |
| Kamfer | 76-22-2 | XX | X | X |
| Dimethylsulfon | 67-71-0 | 0 | 0 | 0 |
| Ethanol | 64-17-5 | o | 0 | X |
| d-Limonen | 5989-27-5 | / | XX | X |
| Methylsalicylat | 119-36-8 | XX | / | X |
| 2-Phenoxyethanol | 122-99-6 | o | 0 | X |
| α-Pinen | 80-56-8 | X | X | XX |
| 2-propanol | 67-63-0 | XX | 0 | 0 |
1 Survey
1.1 Introduction
A large part of the population is today doing some sort of regular exercise often resulting in sore muscles and joints. Through recent years the Danish people are becoming more physically active and the increase is significant for both men and women from year 2000 till today[3]. An increasing activity level means increased risk of injuries. In case of minor injuries most people (professionals as well as exercisers) prefer to self-treat their injuries, this often means by products which are applied to the skin.
Over generations various minor ailments have been treated by hot and cold therapy products. Recent years have seen an increasing number of therapy products for easing and removing muscle pain.
The Internet contains a variety of web sites on exercise and injuries and more of these recommend use of pain relief creams and gels.
1.1.1 Purpose
The purpose of the survey was to identify the most commonly used products and try to assess how extensively products for sports pain and injuries are used.
A survey of the products in the market within the category products for sports pains and injuries and their content of chemical substances has formed the basis of the subsequent assessment of the products.
1.1.2 Definition
The project includes only products which are promoted as suitable for relief of joint and muscle pain and only products which are not prescription drugs and not comprised by the Medicines Act.
1.1.3 Method
The following activities are comprised by the survey:
- Contact to retailers
- Internet searching including contact to manufacturers/suppliers
- Contact to professional users and sports clubs including contact to physiotherapists/sports massagers.
Inquiries have been made about the products through the internet and by shop visits.
1.1.4 Product selection
The criteria for selecting the products for the analyses, was that it should be products which were selling well.
Thus the distributors in the shops have been asked which products were best-selling and which products he would recommend to customers without any specific product wish. Further, we have inquired about exact sales figures of the individual products.
The same inquiries were made to the Internet distributors; however, here we did not always receive response from the supplier of the relevant product. Further, it has not been possible to get data on the exact number of units sold. The information given about sales figures was usually rather vague, such as "this product sells fairly well"...
1.2 Implementation
The survey comprises the following three main elements:
- Retail trade
- Internet sale
- Professional therapists and sports clubs.
In the following each product is specified by a number (purchased products) or by a letter (only product information, either through the Internet or through professional therapists and sports clubs).
1.2.1 Retail trade
For the interview, questionnaires were handed out with the following questions:
- Products included in the shop assortment?
- Distribution of sale of the individual products?
- Estimated total sales of the category?
- Age and sex distribution of the customers?
- Are product instructions given in connection with sale?
- Which products are recommended?
8 shops were visited, comprising:
- 3 sports shops
- 2 drug stores
- 2 health food shops
- 1 pharmacy.
1.2.1.1 Sports shops
Two of the visited sports shops had no sale of products for treatment of sports injuries. One shop stated that they refer any customers of such products to drug stores.
The 3rd sports shop (a chain store) had two products[4] for treatment of sports injuries, product 15 and product F.
According to the shop personnel they sell 4 units a month of which product 15 is the most popular. Product 15 comes in 3 concentrations, whereof 1 and 2 sell evenly, whereas 3, being the strongest, has low sales figures.
The customers of these products are mainly young sports males at the age of 19 to 30 years. The majority of the customers to such creams already knows the product and therefore requires no product instructions.
When instructions are given the customer is questioned about the cause of the injury. In case of genuine injuries Product no. 15 is recommended, whereas e.g. muscle stiffness can be treated by a heat therapy product prior to sports practising. (Product F).
1.2.1.2 Drug stores
2 drug stores were contacted. Drug store 1 sells 17 products (Products 2, 3, 4, 5, 6, 7, 12, 13, 14, C, L, M, N, O, R, S and T), and drug store 2 is selling 11 products (Product no. 1, 2, 3, 4, 6, 7, C, L, P, Q and T). Altogether they sell 20 different products for treatment of sore muscles and joints.
On a monthly basis drug store 1 estimates their sale to be approx. 100 units of sports injury skin creams, drug store 2 estimated their sales to be approx. 20 units.
The major part of the sold products in the drug store chain is of their own brand.
Both drug stores report that the customers to these products are mainly sports people and elderly people.
Drug store 1 estimates their sale to be evenly distributed between men and women, whereas drug store 2 has a majority of male customers. Both stores estimate the age group to be 19-40 years.
Within the elderly buyers both stores estimate the majority of the sale of joint pain relief products to go to women.
If the customer does not ask for a specific product, but just something for pain relief, it is always recommended to buy one of the chain's own brands.
The shops always give advice in connection with sale of this product type, but both shops state that their advice is limited to the informative labelling on the individual product.
1.2.1.3 Health food
Visits were paid to two health food shops, selling Product 13, Product K and Product M.
Besides, health food shop 2 sells Product No. R.
Both health food shops sell very little of these products and are therefore not able to inform about the general sales pattern, but both are aware of the media's influence on sales, as they have registered increased sales following a product advertisement.
Health food shop 1 estimates the total, monthly sale for the category to be approx. 6 units, half being Product no. M. Further, this health food shop has also registered seasonal interest as sales have a small increase during summer half.
The customers are primarily men aged 19-40 years.
The estimated sale of health food shop 2 is approx. 4 units. Here they see two distinct customer groups, namely sports people and elderly people at the age of 19-30 years and 60 years plus. The younger groups are primarily men and the older group mainly women.
Practically no instructions and advice are given, if so, it is limited to the information on the product labelling. The health food shops state that as the legislation stipulate that only medicine is allowed to "make promises" the health food shop personnel is subject to restrictions as to how they are allowed to advise on "alternative" products.
1.2.1.4 Pharmacy
A statement from one pharmacy totals 51 units sold during the first half of 2005 distributed on 4 products categorised as products for sports pains and injuries.
The pharmacy's assortment includes 4 products (Product no. 4, Product no. 6, Product no. C and Product no. V).
Of the total sales, Product no. C represents approx. half of the sale (23 units), and Product no. 4 (17 units) one third of the sale.
In the same period the pharmacy has sold 235 units distributed on two products which are covered by the Medicines Act.
The pharmacy's customers of this type of products are aged 30 and upwards with an even distribution between men and women.
Further they informed that generally they do not give advice in connection with sale of these products.
If customers do not ask for a specific product but only for a pain relief cream they are usually recommended to buy products which are covered by the Medicines Act (over-the-counter-drugs).
1.2.1.5 Summary - Retail Trade
Visits have been paid to 8 shops comprising 3 sports shops, 2 drug stores, 2 health food shops and 1 pharmacy.
Summary of pain relief products sold in the selected shops appear from Table 1.1.
Table 1.1 Survey of products and number of sold units
| Shop | Product | No. of sold units/month |
| Sports shop 1 | Do not sell sports injury products | 0 |
| Sports shop 2 | Do not sell sports injury products | 0 |
| Sports shop 3 | Product no. 15 (concentrations 1, 2, 3) and F | Approx. 4 units |
| Drug store 1 | Product nos. 2, 3, 4, 5, 6, 7, 12, 13, 14, C, L, M, N, O, R, S and T | Approx. 100 units |
| Drug store 2 | Product nos. 1, 2, 3, 4, 6, 7, C, L, P, Q and T | Approx. 20 units |
| Health food shop 1 | Product no. 13, K and M | Approx. 6 units |
| Health food shop 2 | Product no. 13, K, M and R | Approx. 4 units |
| Pharmacy | Product no. 4, 6, C and V | Approx. 9 units |
As it appears products no. 4, 6, 13 and M are sold in three of the six shops dealing in products for treatment of sports pains and injuries.
The customer groups of the 6 shops are almost identical. The age structure is approx. 19-40 years or approx. 60 year plus. The general rule is that the customers are sports practising people or elderly people. Two of the six shops claim to have primarily male customers, whereas three of the six shops maintain to have an even distribution between men and women.
Common to all the shops is that they give very little advice in connection with sale of these products types, if so, the advice given is limited to the information from the product labelling and manuals.
1.2.2 Internet search
Different words and word combinations regarding sports pains and injuries have been searched on www.google.dk.
Table 1.2 Survey of searched words and word combinations
| Words and combinations | Hits |
| Treatment of sports injuries | 3,680 |
| Pains after sports | 697 |
| Sports injuries | 7,020 |
| Sports creams | 66 |
| Sports gels | 55 |
Through the Internet searching we found a number of Danish web sites offering partly products partly instructions in connection with treatment of sports pains and injuries.
In total 18 web sites have been reviewed. All advertisements and articles concerning sports products from Internet shops numbered 1-12 and web fora 1-6 have been examined.
Based on the searchings contact has been made to a number of companies behind the web sites, where we have enquired about declaration of contents, e.g. data sheets, and further information about sales figures.
Some of the companies have responded by sending data sheets and information about their most popular products, others have answered that they lack knowledge of the product contents, and some did not respond at all.
1.2.3 Professional therapists and sports clubs
Contact has been made to a number of football and handball clubs, physiotherapists, sports therapists, and similar in order to obtain information about which products are being used in professional connections.
17 professional therapists and sports clubs have been contacted distributed on
- 7 physiotherapists
- 1 chiropodist
- 1 acupuncturist
- 1 combined reflexologist/ acupuncturist
- 3 sports clubs
- 3 sports massager college
- 1 self-employed sports massager.
One of the physiotherapists uses creams for treatment of sports injuries, another uses product no. G. The other 6 physiotherapists are not using this product type. The chiropodist and the reflexologist/acupuncturist both use Product no. 12.
One of the three contacted sports clubs uses Product no. 2 and Product no. T, another uses Product no. E. Sports club 3 is using Product E and F.
One of the three interviewed educational establishments for sports massage and therapy uses product No. W and heat/cold products of brand B. The products are used only by trained therapists and they do not recommend the products for private use.
Another educational establishment uses only products of one special brand – Product C. For heating massage Product no. X is used.
Besides we have contacted one self-employed sports therapist, who did not want to use sports creams because of lack of documented effect.
The sports therapist was attached to a football team, and he confirmed that the players were using heat/cold products mainly of brand B.
1.2.3.1 Summary of professionals’ and sports clubs’ use of sports creams
Table 1.3 gives an overview of sports products used by professional users as well as their estimated consumption.
Table 1.3 Survey of products used by professional users and sports clubs
| Professional users | Product | Quantity applied per month |
| Physiotherapist 1 | No use of sports products | |
| Physiotherapist 2 | Product no. G | Approx. 25o ml |
| Physiotherapist 3 | No use of sports products | |
| Physiotherapist 4 | No use of sports products | |
| Physiotherapist 5 | No use of sports products | |
| Physiotherapist 6 | No use of sports products | |
| Physiotherapist 7 | No use of sports products | |
| Chiropodist | Product no. 12 | <100 ml |
| Acupuncturist | No use of sports products | |
| Combined reflexologist/acupuncturist | Product no. 12 | Approx. 150 ml |
| Sports club 1 | Product no. E | <100 ml |
| Sports club 2 | Product no. 2 and T | <100 ml |
| Sports club 3 | Product E and F | <100 ml |
| Sports massage school 1 | Product W and Brand B | Not reported |
| Sports massage school 2 | Product X and brand C | Not reported |
| Sports massage school 3 | No use of sports products | Not reported |
| Self-employed sports therapist | No use of sports products | Not reported |
1.3 Purchased products
In total 39 products were registered.
Part of the products has been found on the Internet and only few of the web sites offer information about the contents of the products.
15 products were purchased (Product no. 1-15) and another 11 products (Product no. A, C, D, E, F, G, H, I, J and K) were registered only with the information available on the web sites.
Finally, we became acquainted with further 13 products (Product no. L, M, N, O, P, Q, R, S, T, U, V, W and X) through professional users, sports clubs, shops, etc.
All registered products are sold in shops or through the Internet.
1.4 Consumption
Statistics Denmark has not been able to provide data for a quantitative analysis of the consumption of products for treatment of sports injuries, as there is no CN-code[5] solely for these products acc. to the Central Customs and Tax Administration.
Information about product sale from both Internet-based shops and visited shops is inaccurate and undetailed therefore it is not possible to elaborate a proper estimate of the consumption in Denmark.
Table 1.4 Survey of total sales of the contacted companies within the category stated in approx. number per month
| Shop | Sold units per month |
| Sports shop 1 | No sale of sports injury products |
| Sports shop 2 | No sale of sports injury products |
| Sports shop 3 | Approx. 4 units |
| Drug store 1 | Approx. 100 units |
| Drug store 2 | Approx. 20 units |
| Health food shop 1 | Approx. 6 units |
| Health food shop 2 | Approx. 4 units |
| Pharmacy | Approx. 9 units |
1.5 Products
Through the survey we have become acquainted with 39 products for treatment of sports injuries and pains.
Part of the products has been found on the Internet, where only some of the web sites include contain information about the product contents.
1.5.1 Product survey
15 products (Product no. 1-15) have been purchased and Table 1.5 shows the disclosed constituents of the products. The information is a direct copy of the product labelling.
Additionally, further 24 products (Product no. A-X) have been registered. The information about the product contents has either been given by the retailer or obtained from the Internet.
Table 1.5 Survey of registered creams for treatments of sports pains and injuries
| Product no. |
Contents acc. to manufacturer (disclosed substances) | Comments/Information from web sites | Category | Type | Application |
| 1 | Petrolatum, Methyl Salicylate, Polyglyceryl-2 Dipolyhydroxystearate, Zingiber Officinalis | No comments. | Ointment | Heat | After injury |
| 2 | Petrolatum, Camphor, Menthol, Paraffin, Melaleuca Leucadendron Cajaputi, Metha Peperita, Eugenia Caryophyllus Cinnamomum Cassia, Cinnamale, Eugenol, Limonene, Benzylalcohol, Linalool | No comments. | Balm | Heat | General use |
| 3 | Aqua, Ethylhexyl Stearate, Paraffinum Liquidum, Isohexadecane, Sorbitane Isostearate, PEG-2-Hydrogenated Castor Oil, Ozokerite, Hydrogenated Castor Oil, Sorbeth-30, Cyclomethicone, PPG-15 Stearyl Ether, Mentha Piperita, Menthol, Camphor, PEG-40 Sorbitan Peroleate, Magnesium Sulfate, Lactic Acid, Phenoxyethanol, Sodium Benzoate, BHT | No comments. | Lotion | Heat | General use |
| 4 | Alcohol denat., Aqua, Carbomer, Camphor, Menthol, Abies alba, Citrus limonum, Juniperus communis, Myristica fragans, Thymus vulgaris, PEG-60 Hydrogenated Castor Oil, PEG 12, Triethanolamine | No comments. | Gel | Not reported | After injury |
| 5 | Aqua, Prunus armeniaca (apricot seed oil), Arnica Montana (Mountain tobacco), Paullinia Cupana (Guarana), Capsicum frutescens (Chili), Laureth-7, Polyacrylamice, Citrus limonium, Phenoxyethanol, Methyldibromo, Glutaronitrile, Yucca Vera, Citric acid | No comments. | Stick | Heat | General use |
| 6 | Menthol, Eucalyptus oil, Glycerine, Ethanol, Emulsifier, Carbomer, Food colours (E131) | No comments. | Gel | Cold | After injury |
| 7 | Aqua, Mentha Arvensis, Cetearyl Alcohol, Sorbitan Oleate, Glycerin, Isopropyl Palmitate, Polysorbate 80, Stearic Acid, Alcohol, Carbomer, Aminomethyl, Propanol, Lactic Acid, Methylparaben, Cl 75810 (Chlorophyll) | No comments. | Balm | Cold | After injury |
| 8 | Petrolatum, Lanolin, Turpentine, Methyl Salieylate, Cetearyl Alcohol, Peanut Glycerides, Isopropyl Myristate, Capsicum Frutescens, Aluminum Atearates | ”100 g contains: 4 g Methylsalicylate, 2 g pepper extract, 5 g cleaned French turpentine, 89 g ointment (ordered twice)” |
Balm | Heat | After injury |
| 9 | Aqua, isopropyl Alcohol, Arnica Montana, Chamomilla Recutita, Mentha Arvensis Menthol, Triethanolamine, Carbomer, Hamamelis, Virginiane, Echinacea Purpurea. | ”Menthol, corn mint oil, arnica, camomile, echinacea, hamamelis. No preservatives” | Gel | Cold | After injury |
| 10 | Aqua, Peanut Glycerides, Beheneth -10, Camphor, Alcohol, Triethanolamine, Mentha Arvensis Carbomer Menthol Benzyl Alcohol, Methylchloroisothiazolinone, Methylsothiazolinone. | ”Water, vegetable oils, camphor, menthol, JHP-Oil (Japanese peppermint oil), euxyl” | Cream | Not reported | Before sport |
| 11 | Aqua, Triethanolamine, Diethylamine Salicylate, Mentha Arvensis, Carbomer, Sodium Benzoate | ”100 g contains 3 g peppermint oil, 2 g diethylaminsalicylate, 1 g natriumbenzoate, 2 g triethanolamine, 92 g resingel.” | Gel | Not reported | After injury |
| 12 | Active substances: isopropyl alcohol; inactive substances: water, herbal (ilex paraguariensis), carbornes, triethanolamine, menthol, camphor, siliciumdioxide, methylparabene, E 102 yellow, E 133 blue | Instructions in Danish | Gel | Cold | After injury |
| 13 | Aloe Barbadensis, Hydrolyzed Glycosaminoglycans, Emu Oil, Dimethyl Sulfone (MSM), PEG-6 Stearate, Glyceryl Stearate, Ceteth-20, Steareth-20, Heduchium Coronarium, Arnica Montana, Juniperus Communis, Panax Gensing, Hypericum Perforatum, Urtica, Dioica, Rosmarin Officinalis, Sage Officinalis, Methyl Glyceth-10, Bromelain, Phenoxyethanol, Ethylhexyglycerin, Cananga Odorata, Sclerotium Gum, Capsicum Frutescens, Sodium Benzoate, Leptospernum Scoparium, Yucca Schidigera | ”Replacing water are Aloe Vera Gel and two aliphatic acids Omega 3 and Omega 6, Awapui (white ginger from Hawai), Arnica, juniper, Ginseng, carob herbs, Urtica Urens, Rosmarin, Sage, Capsicum and Ylang Ylang.” | Gel | Heat | General use |
| 14 | Petrolatum, viburnum prunifolium, camphor, menthol, eucalyptus globulus, rosmarinus officinalis, lavendula angustifolia, silica, dichlorobenzyl alcohol | ”Product no. 14 is a combination of a semiocclusive plaster and a muscle ointment, based on following natural extracts: Viburnum prunifolium camphor, menthol, eucalyptus globulus, rosmarinus officinalis and lavendula angustifolium.” | Plaster and ointment | Not reported | After injury |
| 15 | Winter green oil, Cleaned French turpentine, , Extract Capsici, Juniper oil | Ointment | Heat | After injury | |
| A | ”Contains a.o. peppermint, rosmarin, eucalyptus, camomile, menthol and arnicaoil” | Gel | Cold | After injury | |
| B | Aqua, Paraffinum, Liquidum, Cetearyl alcohol, PEG-20 Stearate, Ethanol, Camphor, Oleoresus capsicum | No comments. | Gel | Heat | Before sports |
| C | Ethanol, Menthol, Eucalyptus oil, Dimethylsulphon, Ginger-based biocomplex containing a.o. Zingiber Officinalis Rosco extract and Alpinia galanga extract, carbones, E 131 | No comments. | Gel | Cold | After injury |
| D | Petrolatum, Camphor, Menthol, Methyl Salicylate, Eucalyptus Globulis, Pinus Punsilic, Titanium Dioxide | No comments. | Balm | Cold | General use |
| E | ” Contents: 100 g contains 4 g methylsalicylate, 2 g pepper extract, 5 g. cleaned turpentine oil, 89 g. ointment base. Special precautions: Not to be used for children below 5 years. Most popular trauma heat balm in our league clubs” |
Ointment | Heat | After injury | |
| F | ”Heat cream made of pH balances, vegetable and mineral raw materials” No information about contents on the web | Ointment | Heat | Not reported | |
| G | No | Ointment | Heat | General use | |
| H | No information about contents on the web | Balm | Heat | Before sports | |
| I | No information about contents on the web | Gel | Not reported | After injury | |
| J | ”Combination of ingredients, incl. Dead Sea salt, menthol, mistletoe and arnica extract” | Gel | Cold | After injury | |
| K | ”Contains alcohol. Menthol fragrance.” | Spray | Cold | After injury | |
| L | ”Product no. L is a 100 % pure Danish nature product. The product is approved as naturopath medicine and consists of essential oil, natural vitamins and minerals.” | Not reported | Not reported | General use | |
| M | ” Ingredients (INCI): Alcohol, Arnica montana, Aqua, Glycerin, Hydroxypropyl methylcellulose.” |
Gel | Not reported | General use | |
| N | ”Product no. N is a mineralized ointment, to be applied to the affected skin and the surrounding area.” | Ointment | Not reported | General use | |
| O | ”Product no. O is the genuine Japanese peppermint oil of the plant Mentha arvensis L. var. piperascens Holmes ex Christy. The Mentha arvensis oil may contains up to 90 % menthol, against only 50 % from Mentha piperita. The content of i.e. menthol gives the peppermint oil its unique effect.” | Ointment | Not reported | After injury | |
| P | ”Contents: Essential juniper oil, wheat germ oil, coconut oil, vaseline.” | Ointment | Not reported | General use | |
| Q | No web information about contents | Ointment | Heat | After injury | |
| R | ” Ingredients: Aqua, Dimethyl Sulfone (MSM), Prunus Armeniaca (apricot seed oil), Squalene, Glyceryl Stearate se, Cetyl Alcohol, Hydrolyzed Glucosaminglycans, Capsicum Frutescens (Spanish pepper), Cocoimino Dipropionate, Hedychium Coronarium (hvid, Hawaiian ginger), Origanum Vulgare (oregano), Xanthan Gum, Citrus spp., Mentha Viridis (garden mint), Sodium Benzoate, Sodium ethyl-paraben, Sodium methyl-parabene.” | Cream | Not reported | General use | |
| S | ”Declaration: Rhus Tox D6, Gift eg, Lachesis D6, snake poison, Arnica D6, Mountain Tobacco, Zinc D6, Zink, Graphite D6, Graphite, Ointment base” | Ointment | Not reported | General use | |
| T | No information about contents on the web | Ointment | Heat | General use | |
| U | No information about contents on the web | Cream | Heat | General use | |
| V | No information about contents on the web | Not found | Not found | Not found | |
| W | ”Product no. W contains camphor, menthol, peppermint oil, sage extract and eucalyptus plant extract” | Ointment | Heat | General use | |
| X | ” Innehåll: mandelolja, tistelolja, ricinolja, eterisk olja pepparmint, eukalyptus, camphor.” | Oil | Heat | General use |
1.5.2 Selection criteria
In cooperation with the Danish EPA 12 products were selected for further analyses in the project.
The selection was made on basis of information about the product contents, i.e. substances which have been declared by the manufacturer as well as estimated consumption.
The selection was based on the following criteria:
- Where two or more products contain the same substances, only one has been selected.
- Products assessed according to the complexity of the substance content
- Different manufacturers should be represented
- Popular products
1.5.3 Selected products
The following 12 products have been selected for analysis: Product nos. 1, 2, 3, 4, 5, 8, 12, 13, 15, C, E and H.
Product numbers and letters from the survey are maintained in the following.
2 Screening of substances
Based on the selection criteria described in paragraph 1.5.2 screening analyses were made of the content of organic substances in the selected products with the main purpose of detecting and identifying substances with negative health effects.
2.1 Chemical screening analysis
As the tested products had rather different base formulations, see Table 1.5, it has been chosen to use a headspace-based analysis method.
The applied sampling method based on sampling on ATD-tubes measures organic compounds from the volatile, organic solvents (ethanol, acetone, etc.) to the semi-volatile compounds (parabenes, phthalates etc.).
The examination of the product declarations did not discover any substances which are not covered by the applied measuring method. Therefore, it was not considered necessary to have the products analysed for contents of inorganic substances.
2.1.1 Method of GC/MS headspace-analysis
A weighed out sample amount was put in a 100 ml glass jar.
A glass tube with adsorbent (tenax TA) was placed next to the sample and the Tenax filters were passively exposed for different time periods (10 and 15 minutes resp.).
Subsequently, the Tenax filters were analysed by thermal desorption combined with gas chromatography - mass spectrometry. (ATD/GC-MS in scan mode). The following parameters were applied.
Table 2.1 Analysis parameters
| GC/MS-Instrument | Perkin Elmer Turbomass and ATD 400 |
| MS-parameters | Scan mode 29-500 m/z, solvent delay: 0,1 min. |
| GC-parameters | Furnace prog: 35° C for 3 min., 10° C/min. to 260° C hold for 5 min. Carrier gas: Helium Column:, CP Sil 8CB Low bleed MS 30 m x 0,25 mm, film: 0.5 ∝m |
| ATD-parameters | ATD-pipes: Tenax TA Desorption temp: 290° C |
Detected substances are solely identified by using the NIST-library of mass spectres.
In connection with the qualitative screening no quantitative assessment of the contents of the identified organic compounds was carried out.
2.1.2 Substances
Table 2.2 shows the total screening result of the 12 tested products. Detected substances are marked with "X".
Table 2.2 Screening results - 12 products
| Substance name | CAS no. | Product no. | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 8 | 12 | 13 | 15 | C | E | H | ||
| 1,2-Propandiol | 57-55-6 | x | |||||||||||
| 2-Methyl-1,3-dioxane | 626-68-6 | x | |||||||||||
| 2-Methyl-2-propanol | 75-65-0 | x | |||||||||||
| 2-Phenoxy-ethanol | 122-99-6 | x | |||||||||||
| 2-Propanol | 67-63-0 | x | x | x | |||||||||
| 3-Carene | 13466-78-9 | x | |||||||||||
| Acetaldehyde | 75-07-0 | x | |||||||||||
| Acetone | 67-64-1 | x | |||||||||||
| Benzaldehyde | 100-52-7 | x | |||||||||||
| Benzyl acetate | 140-11-4 | x | |||||||||||
| Borneol acetate | 76-49-3 | x | |||||||||||
| Camphene | 79-92-5 | x | x | x | x | x | x | x | |||||
| Caryophyllen | 87-44-5 | x | |||||||||||
| Camphor | 76-22-2 | x | x | x | x | x | x | ||||||
| Cinnamaldehyde | 104-55-2 | x | x | ||||||||||
| Dimethyl sulphone | 67-71-0 | x | |||||||||||
| Ethanol | 64-17-5 | x | x | x | |||||||||
| Ethyl acetate | 147-78-6 | x | x | ||||||||||
| Eucalyptol | 470-82-6 | x | x | x | |||||||||
| Eugenol | 97-53-0 | x | |||||||||||
| Geranyl benzoate | 94-48-4 | x | |||||||||||
| d-Limonene | 5989-27-5 | x | x | x | x | x | x | x | |||||
| Menthol | 1490-04-6 | x | x | x | x | x | x | ||||||
| Methyl salicylate | 119-36-8 | x | x | x | x | x | |||||||
| b-Myrcene | 123-35-3 | x | x | x | x | x | x | x | |||||
| b-Phellandrene | 555-10-2 | x | |||||||||||
| α-Pinene | 80-56-8 | x | x | x | x | x | x | x | x | x | x | ||
| β-Pinene | 127-91-3 | x | x | x | x | x | x | x | x | x | |||
| p-Cresol methyl ether | 104-93-8 | x | |||||||||||
2.2 Health Risk screening
The preliminary screening revealed a number of substances and substance groups, e.g. aliphatic hydrocarbons. The substances which were identified with an unambiguous name were subjected to a screening based on
- Chemical structure
- Physical/chemical properties
- Classification etc.
Table 2.3 shows selected physical/chemical data of these substances.
The physical/chemical data of the selected substances have been found through searchings in ChemID and HSDB under TOXNET.
Table 2.3 Physical/chemical data of substances revealed by the screening
| Name | CAS no. | Melting point °C |
Boiling point °C |
Vapour pressure mmHg |
Water solubility mg/l |
Log KOW |
| 1,2-Propandiol | 57-55-6 | -60 | 187.6 | 0.129 | ∝ | -0.92 |
| 2-Methyl-1,3-dioxane | 626-68-6 | - | ||||
| 2-Methyl-2-propanol | 75-65-0 | 25.4 | 82.4 | 40.7 | ∝ | 0.35 |
| 2-Propanol | 67-63-0 | -89.5 | 82.3 | 45.4 | ∝ | 0.05 |
| 3-Carene | 13466-78-9 | <25 | 170 | 3.72 | - | 4.38 |
| Cinnamaldehyde | 104-55-2 | -7.5 | 246 | 0.0289 | 1420 | 1.90 |
| Acetaldehyde | 75-07-0 | -123 | 20.1 | 902 | ∝ | -0.34 |
| Acetone | 67-64-1 | -94.8 | 56 | 232 | ∝ | -0.24 |
| α-Pinene | 80-56-8 | -62.5 | 156 | 4.75 | 2.5 | 4.83 |
| Benzaldehyde | 100-52-7 | -26 | 179 | 0.127 | 6570 | 1.48 |
| Benzyl acetate | 140-11-4 | -51.3 | 213 | 0.177 | 3100 | 1.96 |
| Borneol acetate | 76-49-3 | 29 | 221 | 0.228 | 3.86 | |
| b-Phellandrene | 555-10-2 | - | 171.5 | 1.59 | 4.7 | |
| β-Pinene | 127-91-3 | -64.5 | 166 | 2.93 | - | 4.16 |
| b-Myrcene | 123-35-3 | <-10 | 167 | 2.01 | 5.6 | 4.17 |
| Camphene | 79-92-5 | 51.2 | 160 | 2.51 | 4.2 | 4.22 |
| Eucalyptol | 470-82-6 | 1.5 | 176.4 | 1.9 | 3500 | 2.74 |
| Caryophyllen | 87-44-5 | <25 | - | - | - | 6.3 |
| Camphor | 76-22-2 | 180 | 204 | 0.072 | 1600 | 2.38 |
| Dimethyl sulphone | 67-71-0 | 109 | 238 | 5.15 | ∝ | -1.41 |
| D-limonene | 5989-27-5 | -74.3 | 176 | 1.98 | 13.8 | 4.57 |
| Ethanol | 64-17-5 | -114 | 78.2 | 59.3 | ∝ | -0.31 |
| Ethanol, 2-phenoxy | 122-99-6 | 14 | 245 | 0.007 | 26700 | 1.16 |
| Ethyl acetate | 141-78-6 | -83.6 | 77.1 | 93.2 | 80000 | 0.73 |
| Eugenol | 97-53-0 | -7.5 | 253.2 | 0.0226 | 2460 | 2.27 |
| Menthol | 1490-04-6 | 43 | 212 | 0.0637 | 456 | 3.4 |
| Methyl salicylate | 119-36-8 | -8.0 | 222.9 | 0.034 | 700 | 2.55 |
| p-Cresol methyl ether | 104-93-8 | -32.0 | 175.5 | 1.14 | - | 2.66 |
∝: Miscible with water
2.2.1.1 State of matter
From Table 2.3 it appears that the majority of the substances are liquid at room temperature. Camphene, Camphor, Menthol and Dimethylsulphone, however, have high melting points and are solid at room temperature.
Most substances have boiling points above 150 °C. The six substances: 2-methyl-2-propanol, 2-propanol, acetaldehyde, acetone ethanol and ethyl acetate have boiling points below 100 °C and vapour pressures above 40 mm Hg (highest for the low-boiling substances).
Several substances have very low vapour pressures and do not evaporate. This is the case for 1,2-propandiol, Benzaldehyde, benzyl acetate, borneol acetate, camphor and methyl salicylate, all having a vapour pressure below 1 mm Hg.
2.2.1.2 Fat/water-ratio
As to solubility, the lower alcohols and ketones/aldehydes have high water solubility, which is also the case for the sulphur compound dimethyl sulphone.
Most of the compounds containing an oxygen atom will have solubility between 0.1 and 10 g/litre.
Among the selected substances, 8 have water solubilities below 25 mg/litre.
The octanol/water partition coefficient is expressed by the value log KOW. With a negative log KOW the substance will primarily be at the aqueous phase and when the value is positive in the fat phase. It is assumed that substances with log KOW below 4 will be 100 % absorbed through the skin, while substances with higher values are absorbed only by 10 % (TGD, 2003).
Five of the selected substances have a negative log KOW and are therefore primarily water-soluble. This is seen via a very high water solubility of these substances.
3-carene, α-Pinene, b-Phellandrene, β-Pinene, b-mycene, camphene and d-limonene have a log KOW above 4. Common to these substances is the sum formula C10H16 and their cyclic structure.
2.2.2 Classification etc. for selected substances
Information has been retrieved about the classification and limit values of the selected substances and further whether they are listed in "List of Cosmetic Ingredients" (INCI, 2005).
Information about classification has been procured from "List of Hazardous Substances (liste, 2005) and from the Danish EPA's Advisory List for Self-classification (Advisory List 2001). Data from the list is marked by ”*”.
Information about limit values has been taken from Threshold limit
values for substances and materials (C.0.1, 2005) from the Danish Working Environment Service.
Abbreviations:
L: Threshold value
H: Skin penetration
K: Carcinogenic
Searchings for all substances have been made in the INCI database. Table 2.4 gives information about the substances in the list and their functions and limitations.
Table 2.4 Classification for substances revealed by screening
| Nr. | Name | CAS no. | Classification | GV mg/m³ | INCI, function | Cosmetics Regulations and other |
| 1 | 1,2-Propanediol | 57-55-6 | Solvent | |||
| 2 | 2-Methyl-1,3-dioxane | 626-68-6 | No info | |||
| 3 | 2-Methyl-2-propanol | 75-65-0 | F;R11 Xn; R20 |
150 LH | Fragrance | |
| 4 | 2-Propanol | 67-63-0 | F;R11 Xi;R36 R67 |
490 | Fragrance Antifoaming Solvent |
|
| 5 | 3-Carene | 13466-78-9 | N;R51/53 * | Fragrance | ||
| 6 | Cinnamaldehyde | 104-55-2 | R43 * N;R50 * |
Denaturants | EU List of contact allergens. III/23, 76, when concentrations exceed 0.001 % in products which are not cleaned off, 0.01 % in products to be cleaned off. |
|
| 7 | Acetaldehyde | 75-07-0 | F+; R12 Carc3; R40 Xi; R36/37 |
25 LK | Additives | CMR substances not permitted in cosmetics |
| 8 | Acetone | 67-64-1 | F; R11 Xi; R36 R66 R67 |
600 | Denaturants/ solvent | |
| 9 | α-Pinene | 80-56-8 | N;R50/53 * | Fragrance | ||
| 10 | Benzaldehyde | 100-52-7 | Xn; R22 | Solvent | ||
| 11 | Benzyl acetate | 140-11-4 | - | 61 | Additive | |
| 12 | Borneol acetate | 76-49-3 | - | Not in the INCI list | ||
| 13 | β-Phellandrene | 555-10-2 | Xn;R22 N;R51/53 * | Fragrance | ||
| 14 | β-Pinene | 127-91-3 | N;R50/53 * | Fragrance | ||
| 15 | β-Myrcene | 123-35-3 | - | Fragrance | ||
| 16 | Camphene | 79-92-5 | N;R50/53 * | Fragrance | ||
| 17 | Eucalyptol | 470-82-6 | - | Fragrance | ||
| 18 | Caryophyllen | 87-44-5 | - | Fragrance | ||
| 19 | Camphor | 76-22-2 | - | 12 | Fragrance | |
| 20 | Dimethyl sulphone | 67-71-0 | - | Solvent | ||
| 21 | d-Limonene | 5989-27-5 | R10 Xi; R38 R43 N; R50-53 |
D VI/1,29 Max 1 % fragrance |
EU's list of contact allergens III/27,88. To be declared when the concentration exceed 0.001 % in products which are not cleaned off, and 0.01 % in products to be cleaned off. |
|
| 22 | Ethanol | 64-17-5 | F; R11 | 1900 | Solvent | |
| 23 | Ethanol, 2-phenoxy | 122-99-6 | Xn; R22 Xi; R36 |
Fragrance Preservative | 38, VI/1,29 Max 1 % |
|
| 24 | Ethyl acetate | 141-78-6 | F; R11 Xi; R36, R66, R67 |
540 | Solvent | |
| 25 | Eugenol | 97-53-0 | Xn; R22 Mut3;R40 R43 * |
Fragrance | EU List of contact allergens. III/23, 76, when concentrations exceed 0.001 % in products which are not cleaned off, 0.01 % in products to be cleaned off. |
|
| 26 | Menthol | 1490-04-6 | - | Fragrance | ||
| 27 | Methyl salicylate | 119-36-8 | - | Denaturant | ||
| 28 | p-Cresol methyl ether | 104-93-8 | - | Fragrance |
As can be seen a good deal of the substances are classified - 8 substances are on the List of Hazardous substances and 7 on the Advisory List. The remaining substances are not listed.
Preliminarily it is estimated that 1,2-propandiol (no. 1) is not covered by the labelling requirements. The other 11 substances may not be self-classified due to lack of information.
The substances 3-carene (no. 5), α-Pinene (no. 9), β-Pinene (no. 14) and camphene (no. 16) are entered on the Advisory List for classification with (N, R50/53) Environmental Hazard, however, no classification for health hazard.
Of the classified substances, two substances are suspected of causing chronic damages - Acetaldehyde (no. 7), suspected of being carcinogenic (Carc3, R40) and Eugenol (no. 25), suspected of changing the genes (Mut3, R40).
Three substances are classified as contact allergens (R43) and are on the EU List of Major contact allergens. (nos. 6, 21, 25).
The vast majority of the detected substances are odorants. According to data INCI, there are 17 odorants and 7 are solvents. Of the selected substances there is only one preservative and two of the substances are not included in the INCI list.
Four of the detected substances are mentioned in the Statutory Order for Cosmetic Products. The preservative 2-phenoxy ethanol is permitted in concentrations up to max. 1 %. Cinnamale, d-Limonene and Eugenol are to be declared when the concentration exceeds 0.001 % in products which are not cleaned off, and 0.01 % in products which are cleaned off.
3 Quantitative chemical analyses
- 3.1 Quantitative determination of volatile and semi volatile compounds (GC/MS screening)
- 3.2 Specific analysis of contents of organic solvents
- 3.3 Prioritisation of substances in products for treatment of sport injuries and pain
3.1 Quantitative determination of volatile and semi volatile compounds (GC/MS screening)
In consultation with the Danish EPA a number of substances were selected for quantification. As the method used in connection with the screening was not a suitable analysis method for quantification of contents of organic substances, it was decided to use Solid Phase Micro Extraction (SPME) as sampling principle combined with GC/MS.
3.1.1 Method for quantitative SPME-GC/MS analysis
The sample was weighed out in a 20 ml membrane glass. 3 different isotope- marked, internal standards (Toluene-d8, phenol-d6 and naphthalene-d8) were added to the sample. Headspace was sampled for 15 min. at SPME and subsequently analysed by gas chromatography/mass spectrometry (GC/MS). The parameters were as follows:
Table 3.1 Parameters for SPME-GC/MS analysis
| GC/MS-Instrument | Thermo Finnigan, DSQ |
| MS-parameters | Autotune, 35-300 m/z, solvent delay: 4,0 min. |
| GC-parameters | Furnace prog: 40 ° C for 1 min., 10 ° C/min. To 260 ° C hold for 2 min. Injector: 290 ° C, splitless time: 0.5 min. Carrier gas: Helium, constant flow: 1,0 ml/min. Column: Valcobond VB-1, 30 m x 0.25 mm, film: 1.5 μm |
| SPME-parameters | Fibre: 100 μm PDMS Headspace-temperature: 35 ° C |
Calibration of the method has been carried out at 6 different concentration levels from 0.1 to 50 μg per headspace glass.
The detection limit of the method was determined to 1 mg/kg and the uncertainty is between 10-25 % RSD, depending on the substance.
3.1.2 Results of SPME-GC/MS-analysis in mg/kg
The quantified contents of the GC/MS analysis are listed in below tables.
Table 3.2 SPME-GC/MS analysis results in mg/kg
| Substance | CAS no. | Sample marking | |||||
| 1 | 2 | 3 | 4 | 5 | 8 | ||
| Toluene | 89-83-8 | <1 | 3.0 | <1 | <1 | <1 | 2.3 |
| Dimethyl sulphone | 67-71-0 | <1 | <1 | <1 | <1 | <1 | <1 |
| Benzaldehyde | 100-52-7 | <1 | 4,200 | <1 | <1 | <1 | <1 |
| α-Pinene | 7785-70-8 | <1 | 12.000 | 550 | 1.700 | <1 | 27.000 |
| Phenol | 108-95-2 | <1 | <1 | <1 | 2.4 | 5.6 | 11 |
| Camphene | 79-92-5 | <1 | 360 | 3.4 | 300 | <1 | 130 |
| Benzyl alcohol | 100-51-6 | <1 | 9.2 | <1 | <1 | <1 | <1 |
| p-Methylanisol | 104-93-8 | <1 | <1 | <1 | <1 | <1 | <1 |
| 3-Carene | 13466-78-9 | <1 | 240 | 1.7 | 290 | <1 | <1 |
| d-Limonene | 5989-27-5 | 9.8 | 19.000 | 400 | 1.500 | <1 | 1.000 |
| Linalool | 78-70-6 | 2,2 | 1.900 | 93 | 1.000 | <1 | 100 |
| Camphor | 21368-68-3 | 5,3 | 77.000 | 5.100 | 980 | <1 | 8,2 |
| Methyl salicylate | 119-36-8 | 51.000 | 5300 | 6,4 | <1 | <1 | 50.000 |
| Estragole | 140-67-0 | <1 | <1 | <1 | <1 | <1 | 11 |
| 2-Phenoxy ethanol | 122-99-6 | 390 | <1 | 9.000 | <1 | 4.900 | 680 |
| Cinnamale | 104-55-2 | <1 | 10.000 | <1 | <1 | <1 | <1 |
| Eugenol | 97-53-0 | <1 | 27.000 | <1 | <1 | <1 | <1 |
| Isoeugenol | 97-54-1 | <1 | 1.600 | <1 | <1 | <1 | <1 |
| Substance | CAS no. | Sample marking | |||||
| 12 | 13 | 15 | C | E | H | ||
| Toluene | 89-83-8 | <1 | <1 | 1,7 | <1 | 2,6 | 4,4 |
| Dimethyl sulphone | 67-71-0 | <1 | 8.200 | 8.0 | 57 | <1 | <1 |
| Benzaldehyde | 100-52-7 | <1 | <1 | <1 | 2.0 | <1 | <1 |
| α-Pinene | 7785-70-8 | <1 | 16 | 19.000 | <1 | 33.000 | 22.000 |
| Phenol | 108-95-2 | <1 | 16 | 4.4 | <1 | 6.5 | 2.0 |
| Camphene | 79-92-5 | <1 | <1 | 48 | <1 | 47 | 150 |
| Benzyl alcohol | 100-51-6 | <1 | 7.3 | <1 | <1 | <1 | <1 |
| p-Methylanisol | 104-93-8 | <1 | 9.5 | <1 | <1 | <1 | <1 |
| 3-Carene | 13466-78-9 | <1 | <1 | <1 | <1 | <1 | <1 |
| d-Limonene | 5989-27-5 | 1.1 | 1.1 | 170 | 44 | 2.300 | 870 |
| Linalool | 78-70-6 | <1 | 43 | 30 | 6.3 | 1.700 | 1.800 |
| Camphor | 21368-68-3 | 2.500 | <1 | 9.0 | <1 | <1 | <1 |
| Methyl salicylate | 119-36-8 | <1 | <1 | 45.000 | <1 | 67.000 | 76.000 |
| Estragole | 140-67-0 | <1 | <1 | 8.0 | <1 | 10 | 14 |
| 2-Phenoxy ethanol | 122-99-6 | <1 | 410 | <1 | <1 | <1 | <1 |
| Cinnamale | 104-55-2 | <1 | <1 | <1 | <1 | <1 | <1 |
| Eugenol | 97-53-0 | <1 | <1 | <1 | <1 | <1 | <1 |
| Isoeugenol | 97-54-1 | <1 | <1 | <1 | <1 | <1 | <1 |
”<1” means that the content is lower than the detection limit for the applied analysis method. (1mg/kg)
3.2 Specific analysis of contents of organic solvents
With the applied SPME-GC/MS analysis it was not possible to carry out a quantification of the contents of the identified organic solvents and a GC/FID-based method was therefore developed for the purpose.
3.2.1 Method for quantitative analysis of organic solvents
Following parameters were applied for the specific analysis of organic solvents.
Table 3.3 Analysis parameters for GC/FID based method
| GC-Instrument | HP 5890 |
| GC-parameters | Furnace progr: isotherm at 80° C. Injector: 225° C, FID-detector: 225° C Carrier gas: Nitrogen, constant flow: 25 ml/min. Column: 4 m, 10 % carbowax 1500 |
| Solvent | Dimethylformamide (DMF) |
3.2.2 Results of the specific analysis of organic solvents
Table 3.4 lists the identified contents
Table 3.4 Results of specific analysis of organic solvents
| Substance | CAS no. | Sample marking | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 8 | 12 | 13 | 15 | C | E | H | ||
| Toluene* | 89-83-8 | 3 ppm | 2.3 ppm | 1.7 ppm | 2.6 ppm | 4.4 ppm | |||||||
| Ethanol | 64-17-5 | 54% | 2.2% | 14% | |||||||||
| 2-Propanol | 67-63-0 | 27% | |||||||||||
| Tert-butanol | 75-65-0 | 0.35% | |||||||||||
| Acetone | 67-64-1 | 1.9% | |||||||||||
Empty fields = The solvent was not found in the sample
* The content of toluene is determined by SPME-GC/MS see paragraph SPME-GC/MS 3.1.1.
3.3 Prioritisation of substances in products for treatment of sport injuries and pain
Based on the screening of health effects and the qualitative analysis a number of results has been selected for health assessment.
The most interesting substance has been selected in co-operation with the danish environmental protection agency.
- Camphor
- Dimethyl sulphone
- D-limonen
- Methyl salicylat
- 2-phenoxyethanole
- α-Pinene
Furthermore the following solvents has been selected
- Etanol
- 2-Propanol
- Acetone
- T-Butyl alcohol
4 Legislation
4.1 Introduction
Due to the way these sport products are used there might be some doubts regarding which regulations are covering the sport products. The products are regulated by the law on chemical substances and products (LBK nr. 21 af 16/01/1996 om kemiske stoffer og produkter). Due this the sport products are also regulated by the general Statutory Order on classification and labelling of chemical substances and products (Klassificering, 2005). Some of the substances in the products are required to have a warning label and therefore, all the products are assessed with respect also to classification and labelling.
The Statutory Order on cosmetics (Cosmetics, 2006) does not regulate sports products, although the exposure route seems a like. Sports products are not covered by the definition of cosmetic products, which states:
§2 The statutory order is not applicable for products which have to be applied to humans or animals to prevent, realise, ease, treat or cure sickness, sickness symptoms or pain or to affect the functions of the body.
§3 Cosmetic products means any substance or preparation intended for placing in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with the view exclusively or principally to cleaning them, perfuming them or protecting them in order to keep them in good condition, change their appearance or correct body odours.
A number of perfume substances have to be declared on for products which are not meant to be cleaned off the skin. Substances in sports products are assumed not to be cleaned off the skin.
The 26 perfume substances analysed here must be declared on stay-on cosmetic products, if the content is more than 0.001% (10 mg/kg). 9 of the 12 analysed products contains more than 0.001% of one or more of the 26 perfume substances but since the sport products as described above are not covered by these declaration demand, the users of the products do not have access to this information about the content of the allergic perfume substances.
4.2 Classification of products
In the assessment of which products should be classified only substances detected in more than 0.01 percent (100 mg/kg) have been considered.
A table for each of the 12 samples is shown below. For substances included in the EU-list on allergic fragrances (SCCNFP, 1999) are stated “potential sensitizer” and the substance and its effect are stated in the classification for the product. Substances included on the Danish advisory list for self-classification (Vejledende liste) are marked with an *.
Table 4.1 Classification for sports product number 1
| Sample number: 1 | ||
| Substance | Amount (w%) | Classification |
| Methyl salicylate | 5.10 | - |
| 2-phenoxyethanol | 0.04 | Xn;R22 Xi;R36 |
| Product classification | None | |
Table 4.2 Classification for sports product number 2
| Sample number: 2 | ||
| Substance | Amount (w%) | Classification |
| Benzaldehyde | 0.42 | Xn;R 22 |
| α-Pinene | 1.2 | N;R50/53* |
| d-Limonene | 1.9 | R10 Xi;R38 R43 N;R50/53 |
| Linalool | 0.19 | Potential R43 |
| Camphor | 7.7 | None |
| Methyl salicylate | 0.53 | None |
| Cinnamal | 10.00 | R43 * |
| Eugenol | 2.7 | Xn;R22 Mut3;R68 R43* |
| Isoeugenol | 0.16 | Xn;R22 R43* |
| Product classification | Xi R43 | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
Table 4.3 Classification for sports product number 3
| Sample number: 3 | ||
| Substance | Amount (w%) | Classification |
| α-Pinene | 0.06 | N;R50/53* |
| d-Limonene | 0.04 | R10 Xi;R38 R43 N;R50/53 |
| Camphor | 0.51 | None |
| 2-Phenoxyethanol | 0.90 | Xn;R22 Xi;R36 |
| Product classification | None | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
Table 4.4 Classification for sports product number 4
| Sample number: 4 | ||
| Substance | Amount (w%) | Classification |
| Ethanol | 52.00 | F;R11 |
| α-Pinene | 0.17 | N;R50/53* |
| Camphene | 0.03 | N;R50/53* |
| d-Limonene | 0.15 | R10 Xi;R38 R43 N;R50/53 |
| Linalool | 0.10 | Potential R43 |
| Camphor | 0.10 | None |
| Product classification | None | |
| To be labelled for allergens | Contains d-limonene. May cause allergic reactions. | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
The flashpoint of product number 4 was measured to below 45°C meaning that the product should be labelled with R10 (21-55°C) or R11 (flashpoint<21°C). However, a product having a flash point equal to or greater than 21° C and less than or equal to 55° C need not be classified as flammable if the product could not in any way support combustion and only so long as there is no reason to fear risks to those handling these products or to other persons. Due to this it is assessed that product 4 do not have to labelled for flammability.
Table 4.5 Classification for sports product number 5
| Sample number: 5 | ||
| Substance | Amount (w%) | Classification |
| Ethanol | 2.20 | F;R11 |
| 2-Phenoxyethanol | 0.49 | Xn;R22 Xi;R36 |
| Product classification | None | |
Table 4.6 Classification for sports product number 8
| Sample number: 8 | ||
| Substance | Amount (w%) | Classification |
| α-Pinene | 2.70 | N;R50/53* |
| d-Limonene | 0.10 | R10 Xi;R38 R43 N;R50/53 |
| Linalool | 0.01 | Potential R43 |
| Methyl salicylate | 5.00 | None |
| 2-Phenoxyethanol | 0.07 | Xn;R22 Xi;R36 |
| Product classification | None | |
| To be labelled for allergens | Contains d-limonene. May cause allergic reactions. | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
Table 4.7 Classification for sports product number 12
| Sample number: 12 | ||
| Substance | Amount (w%) | Classification |
| 2-propanol | 27.00 | F; R11 Xi; R36 R67 |
| Camphor | 0.25 | None |
| Product classification | Xi; R36 R67 | |
As shown in Table 4.7 product number 12 should be labelled irritant.
The flashpoint of product 12 was measured to be between 45-50°C. This means that the product should be labelled with R10. However, a product having a flash point equal to or greater than 21° C and less than or equal to 55° C need not be classified as flammable if the product could not in any way support combustion and only so long as there is no reason to fear risks to those handling these products or to other persons. Due to this it is assessed that product 12 do not have to labelled with R10.
Table 4.8 Classification for sports product number 13
| Sample number: 13 | ||
| Substance | Amount (w%) | Classification |
| Dimethyl sulphone | 0.82 | None |
| 2-Phenoxyethanol | 0.04 | Xn;R22 Xi;R36 |
| Product classification | None | |
Table 4.9 Classification for sports product number 15
| Sample number: 15 | ||
| Substance | Amount (w%) | Classification |
| α-Pinene | 1.90 | N;R50/53* |
| d-Limonene | 0.02 | R10 Xi;R38 R43 N;R50/53 |
| Methyl salicylate | 4.500 | None |
| Product classification | None | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
Table 4.10 Classification for sports product number C
| Sample number: C | ||
| Substance | Amount (w%) | Classification |
| Acetone | 1.90 | F; R11 Xi; R36 R66 R67 |
| t-Butyl alcohol | 0.35 | F; R11 Xn; R20 |
| Ethanol | 14.00 | F; R11 |
| Product classification | None | |
The flashpoint of product C was measured to be between 45-50°C. This means that the product should be labelled with R10. However, a product having a flash point equal to or greater than 21° C and less than or equal to 55° C need not be classified as flammable if the product could not in any way support combustion and only so long as there is no reason to fear risks to those handling these products or to other persons. Due to this it is assessed that product C do not have to labelled with R10.
Table 4.11 Classification for sports product number E
| Sample number: E | ||
| Substance | Amount (w%) | Classification |
| α-Pinene | 3.30 | N;R50/53* |
| d-Limonene | 0.23 | R10 Xi;R38 R43 N;R50/53 |
| Linalool | 0.17 | Potential R43 |
| Methyl salicylate | 6.70 | None |
| Product classification | None | |
| To be labelled for allergens | Contains d-limonene. May cause allergic reactions. | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
Table 4.12 Classification for sports product number H
| Sample number: 2 | ||
| Substance | Amount (w%) | Classification |
| α-Pinene | 2.20 | N;R50/53* |
| d-Limonene | 0.09 | R10 Xi;R38 R43 N;R50/53 |
| Linalool | 0.18 | Potential R43 |
| Methyl salicylate | 7.60 | None |
| Product classification | None | |
The product contains substances which may be hazardous to the environment. This aspect is not covered by the project.
According to the Statutory Order on classification of chemicals the above tables show that products nos. 2 and 12 should have been labelled. The products no. 4, 8 and E have to be labelled for allergens and with warning of allergic reactions.
5 Health Assessment
5.1 Introduction
In this chapter, potential health effects from identified and selected substances in section 3.3 are assessed. The focus of the assessment is primarily aimed at adults.
For each of the identified and quantified substances, information on the substances’ identity as well as chemical and physical properties are presented. It will include data on material state, melting point, boiling point, vapour pressure and solubility.
A search in the open literature has been performed with focus on possibility of skin absorption and the most important test results, effects and circumstances are presented in this report. The aim was to find data for NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) for the selected substances or other relevant data, if available.
Based on NOAEL or similar data and the amount of the substances of the tested products it can be assessed whether the substances may cause negative health effects.
5.2 Method
It is assumed that the substances can be absorbed in the body by penetration through skin.
None of the product labellings had directions for a recommended amount to use. From the information on the products no special directions regarding the recommended amount to use was given for any of the products. For assessment and comparison purposes the same amount of product should be used.
Based on experiments where the legs of two test persons were exposed to 4-5 gram product, the exposure scenario was determined to be 5 grams of product per day. The exposure scenarios are defined according to the EU's Technical Guidance Document (TGD, 2003).
The uptake is calculated as:
Uptake per day per kg body weight = C [mg/gram] * 5 gram per day * F / body weight [kg]
C: Content of substance in mg per gram sample
F: Fraction of absorbed substance. If no specific values for F is found then the default value is used: F = 100 % if Log KOW < 4 and F = 10% if Log KOW > 4.
The body weight (b.w.) is assumed to be 70 kg (TGD 2003).
The equation can be reduced to:
Uptake per kg b.w. per day = Content of substance × * F * 5 / 70
Uptake [mg/kg b.w.] per day = 0.0714 * F * C [mg/gram] per day
The intake per day then have to be compared with data for absorption through skin, if available, and/or oral intake.
Assessment of risk
In the assessment of health risks the calculated intake has to be compared with the NOAEL or similar values. As NOAEL typically is based on tests on animals a safety factor (MOS: Margin of safety) is introduced by dividing NOEAL in mg/kg b.w by the intake.
If the data for animals is a chronic long term study of high quality the safety factor in the risk assessment is typically MOS=100. This is based on a factor of 10 for extrapolation between species (interspecies) and a factor of 10 meant to protect sensitive individuals like children (intraspecies). If the data is of less quality eg. based on LOAEL or a subchronic study an additional safety factor is applied (typically 10). The total safety factor is the combined product of the individual safety factors.
In the assessment of health effects MOS is not used for sentisizing effects as these effects do not have a lower concentration limit.
5.3 Selected substances
The substances described in the following are selected as the most important substances for the potential heath risks when using these products. The selected substances are:
- Camphor
- Dimethylsulphone
- d-Limonene
- Methylsalicylate
- α-Pinene
- 2-Phenoxyethanol
Also a short description of the solvents ethanol, 2-propanol, acetone, and t-butyl alcohol is included because these substances are found in large quantities in the products.
5.3.1 Camphor
5.3.1.1 Identity
| Name | Camphor (EINICS name:Bornan-2-one) |
| CAS-number | 76-22-2 |
| EINECS number | 200-945-0 |
| Molecular formula | C10H16O |
Molecular structure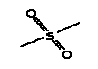 |
|
| Molecular weight | 152.23 |
| Synonyms | Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl- 1,7,7-Trimethylbicyclo(2.2.1)heptan-2-one 2-Bornanone Gum camphor Spirit of camphor |
The substance consists of colourless or white crystals. It has a boiling point of 204° C and a melting point of 179° C (The Merck Index, 1983).
The substance is more soluble in organic solvents than in water. Yalkowsky and Yan (2003) state that 1.6 gram of camphor can be dissolved in 1 litre of water at 25° C. In another reference the following is given: At 25° C one gram dissolves in about 800 ml water, in 1 ml alcohol, 1 ml ether, 0.5 ml chloroform. The substance is freely soluble in carbon disulfide, petroleum, fixed and volatile oils. It is also soluble in concentrated mineral acids, in phenol, in liquid ammonia and in liquid sulfoxide (O'Neil, M.J. 2001).
The partition coefficient Log KOW is determined to be 2.38 (Daylight Chemical Information Systems. 2004).
Vapour pressure is determined to be 0.65 mm Hg at 25° C (Jones AH, 1960).
Some values are given for odour threshold values. The lowest odour value is 0.0026 ppm and the highest is 0.96 ppm. Both odour values are below the threshold limit value :TLV (Haz-map, 2005).
5.3.1.2 Detected quantities
The substance is detected in 7 products. The most important are considered to be the samples with the highest concentrations. In
Sample number 2 is found 77 mg/gram equal to 7.7 w%
Sample number 3 is found 5.1 mg/gram equal to 0.51 w%
Sample number 12 is found 2.5 mg/gram equal to 0.25 w%
Sample number 4 is found 1.0 mg/gram equal to 0.10 w%
The remaining 3 samples contain less than 0.01 mg/gram.
5.3.1.3 Function of substance
The substance is included in the INCI-database. Here is stated that the function of the substance can be denaturants / film formers and as a fragrance. O'Neil, M.J. (2001) states that the substance is normally used as an odorant and flavourant and it can be used as emollient in cosmetics and as a preservative.
Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
The Danish threshold limit value is 2 ppm equal to 12 mg/m³. The same limit is set in USA (ACGIH, 2005).
5.3.1.4 Health Effects
Data regarding health effects are retrieved from TOXNET and the databases related to this host. The substance has not been included in IUCLID.
Acute toxicity
The substance is irritating to the eyes, the skin, and the respiratory tract (IPCS, 2003). Camphor applied on the skin of volunteers as a 20% solution in alcohol produced no significant sensation of irritation or pain at normal skin temperatures. It did appear to have a slight sensitising effect on the perception of temperature change during heating and cooling, and increased the sensation of burning at high temperatures (National Poisons Information Service Center, 1996).
Acute toxicity by ingestion based on test with animals indicates that camphor may be slightly toxic (i.e LD50 rat <2000 mg/kg):
- LD50 Mouse oral 1310 mg/kg (Lewis, R.J. 1996)
- LD50 Rat subcutaneously 70 mg/kg (Lewis, R.J. 1996)
- LD50 Mouse ip 3000 mg/kg (ACGIH, 2001)
Several exposure studies with humans have been reported. In one study 1.5 g camphor has been ingested by an adult, who recovered. In children 0.7 to 1.0 g has proved to be fatal. Urinary retention, albuminuria, and anuria are described in non-fatal cases, but kidney lesions in fatal poisonings are not always prominent. Mild and transient hepatic derangements may occur and widespread hemorrhages are described in one fatal case. Fetal death resulted after camphor ingestion by mother and postmortem exam revealed severe atelectasis (collapse of lung) and central neuronal necrosis (Gosselin et al, 1984).
Camphor remains in over 950 products listed in Poisindex according to the following reference. A review of all camphor ingestions estimated to be 2 mg/kg or greater was made. Seventy-three patients (90%) remained asymptomatic, three (4%) developed minor symptoms, and five (6%), all ingesting over 59 mg/kg, developed major symptoms. No deaths were reported (Geller RJ et al; 1984).
From IPCS, Poisons Information Monograph the following has been retrieved
- Camphor crosses the placenta and has been implicated in fetal and neonatal death. It has been used to induce abortions. Camphor poisoning during pregnancy was reported in four cases and, in each case, camphorated oil was mistaken for castor oil. The topical use of camphorated oil in pregnancy was not associated with teratogenic effects.
- Deafness has been reported in association with camphor. Ulceration of the mucous membranes has been reported following the use of toothache solutions containing camphor (along with menthol, phenol, clove oil and chloroform).
- Camphor administered in doses of 60 mg to 4 g was reported to cause flickering, darkening or veiling of vision along with noises in the ears. Corneal erosions have been reported in association with the use of inhalant capsules containing camphor.
Sub-chronic toxicity
D-Camphor elicited no evidence of teratogenicity when administered orally during the fetal period of organogenesis to pregnant rats at doses up to 1000 mg/kg b.w./day, and to pregnant rabbits at doses up to 681 mg/kg b.w./day. The NOEL for the fetal organism for the rat was above 1000 mg/kg b.w., and for the rabbit above 681 mg/kg b.w. (Leuschner J, 1997).
Chronic toxicity
With chronic dermal exposure, systemic effects and contact dermatitis can occur as well as significant allergic responses. Ocular exposure results primarily in irritation only, although oral intake has been associated with visual problems (Ford MD et al., 2001).
Camphor is classified as “A4; Not classifiable as a human carcinogen” (ACGIH, 2005).
Summary
Only values for NOEL for teratogenicity are given for short-term studies with animals. The lowest value was 680 mg/kg b.w. per day.
Observations on humans showed that ingestion of 2 mg/kg b.w. gave none or minor symptoms.
References above show that camphor may cause irritation by skin contact and may by chronic exposure cause allergies.
5.3.1.5 Exposure scenarios
For camphor Log KOW is less than 4 and therefore, 100% of the substance is assumed absorbed through the skin.
The maximum content in a sample was 77 g/kg.
Intake per day per kg b.w. = 0.0714 * 77 = 5.5 mg/kg b.w./day
For the other 3 samples with a relative high content, intake per day per kg b.w. is between 0.01 and 0.36 mg/kg b.w.
5.3.1.6 Assessment
Camphor is a substance that may cause irritations and allergies by skin contact. It may be toxic if ingested in relative large amounts - more than 1 mg/kg b.w. Camphor may cause teratogenic effects; NOEL based on a subacute test is estimated to 680 mg/kg. Indications for other long-term effects have not been found.
Camphor has been detected in seven samples. Based on dermal contact with 5 gram of product the maximum daily uptake will be 5.5 mg per kg b.w.
Based on the data for teratogenicity a margin of safety (MOS) is only about 123. Compared with the observations on humans with 2 mg/kg b.w. by oral intake, the exposure with this substance is higher for sample no. 2 and lower for the other 3 samples. As the data is based on a subchronic study, the safety factor for risk evaluation is assumed to be at least 1000.
Therefore, when assuming 100% uptake through skin it can be concluded that there is a minor risk of teratogen health effects from dermal exposure to product no. 2. Pregnant should avoid using the product on larger skin areas.
Based on the available data there is a risk that camphor may cause irritations and allergic reactions for products no. 2, 3, 4, 12.
5.3.2 Dimethyl sulphone
5.3.2.1 Identity
| Name | Dimethyl sulphone |
| CAS-number | 67-71-0 |
| EINECS number | 200-665-9 |
| Molecular formula | C2H6O2S |
Molecular structure |
|
| Molecular weight | 94.13 |
| Synonyms | Methyl sulfonyl methane Methylsulfonylmethane Dimethylsulfone MSM |
The following data is retrieved from CHEMid:
Dimethyl sulphone has a melting point of 109oC and a boiling point of 238oC.
The substance is completely miscible with water; solubility is 1,000 gram per litre water. Log KOW is -1.41, which means that the substance is much more soluble in water than in organic solvents.
The vapour pressure is estimated to 5.15 mm Hg.
5.3.2.2 Detected quantities
Dimethyl sulphone is determined in three products, - sample number 13, where 8.2 mg/gram was detected and in sample number 15 and C, where very small amounts were found (<0.06 mg/g).
5.3.2.3 Function of substance
The substance is a naturally occurring nutrient found in the human body. Dimethyl sulphone is an important source for organic sulphur.
The substance is included in the INCI-database as a solvent.
Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
No threshold limit values or restrictions for the substance have been found.
5.3.2.4 Health Effects
Very limited information on this substance has been available. Most information retrieved focused on the advantages of using the substance as natural non-prescription drug or nutrient supplement.
Acute toxicity
Food contains dimethyl sulphone at the level of a few ppm; e.g. cow milk contains 3.3ppm and tomatoes 0.86 ppm (Methylsulfonylmethane, 2003).
The substance is believed to be non-toxic. Oral dosage for humans is often in the range of 1 to 3 grams daily (Methylsulfonylmethane, 2003).
The natural level of dimethyl sulphone in the circulatory system of an adult human male is about 0.2 mg/kg (MSM Research Information).
From CHEMid the following data on acute toxicity is found (National Technical Information Service. Vol. OTS0533525):
Rabbit, skin LD50 > 5000 mg/kg
Rat, oral LD50 > 5000 mg/kg
Sub-chronic toxicity
In a 90 day study with rats, the animals were orally exposed to 1.5 grams per kg per day. No adverse effects or increased mortality were observed (Methylsulfonylmethane, 2003).
Chronic toxicity
No data on long-term effects has been retrieved. The only references on long-term effects and dimethyl sulphone are tests trying to verify some positive health effects regarding for instance arthritis.
Summary
Dimethyl sulphone is not acute toxic. No data indicating long-term effects has been found. Potential positive health effects have not been assessed.
Based on the subchronic test with rats NOAEL is assessed to be at least 1,500 mg/kg b.w. per day.
5.3.2.5 Exposure scenarios
For dimethyl sulphone Log KOW is less than 4 and therefore, 100% of the substance is assumed absorbed through the skin.
The maximum content in a sample was 8.2 mg/kg.
Intake per kg b.w. per day = 0.0714 * 8.2 = 0.6 mg/kg b.w. per day
5.3.2.6 Assessment
Dimethyl sulphone does not cause allergy or irritations by skin contact. Data does not indicate any negative long-term effects. Based on the NOAEL the Marginal of Safety is more than 2,500 for the sample with the highest content of the substance.
Several articles indicate that there might be a positive health effect from dimethyl sulphone, but this is not assessed in this study.
It can be concluded that dimethyl sulphone in the amounts detected does not cause any negative health effect.
5.3.3 d-Limonene
5.3.3.1 Identity
| Name | d-Limonene |
| CAS-number | 5989-27-5 |
| EINECS number | 227-813-5 |
| Molecular formula | C10H16 |
Molecular structure |
|
| Molecular weight | 136.23 |
| Synonyms | (+)-(4R)-Limonene (+)-4-Isopropenyl-1-methylcyclohexene (+)-Dipentene (+)-Limonene Citrene (+)-alpha-Limonene (+)-p-Mentha-1,8-diene |
d-Limonene is a liquid with a fresh citrus odour. The substance has a boiling point of 176°C (Budavari, 1989) and a melting point of -74.35°C (Lide, 1992).
The vapour pressure is 1.44 mmHg (Hansen and Eggert, 2003). Solubility in water is 13.8 mg/litre at 25°C. The partition coefficient Log KOW is measured to 4.57.
5.3.3.2 Detected quantities
d-Limonene was found in 9 products, sample 2 representing the highest amount of 19 mg/gram.
In sample E, 2.3 mg/gram was detected and samples 4, 8 and H held about 1 mg/gram. In samples nos. 1, 3, 15 and C the amount was less than 0.4 mg/gram.
5.3.3.3 Function
d-Limonene is used as a fragrance in cosmetics and as a flavouring agent in food and beverage.
d-Limonene is included in INCI as a fragrance.
5.3.3.4 Classifications and TLV’s
d-Limonene is included in the List of dangerous substances and classified as:
R10 Flammable
Xi; R38 Irritant; Irritating to skin.
R43 May cause sensitization by skin contact.
N; R50/53 Hazardous to the environment; Very toxic to aquatic organisms, may cause long-term adverse effects in aquatic environments.
A general TLV is given for terpenes, 25 ppm equivalent to 140 mg/m³ (C.0.1, 2005). No specific values are found for d-limonene.
The substance is included in the EU-list of allergenic perfume substances (SCCNFP, 1999).
5.3.3.5 Health Effects
d-Limonene is included in IUCLID, but the data sheet comprises relatively few data. The following is based on the data sheet, data bases in TOXNET and two previous survey reports, - one on printed matter (Hansen OC and Eggert T, 2003) and one on stain removers (Engelund et al, 2004).
Acute toxicity
Data for acute toxicity by ingestion is determined by LD50 to more than 4,000 mg/kg. This indicates no major potential health risk (Hansen and Eggert, 2003).
Oxidisation products of d-Limonene are strong allergens. A number of cases of contact allergy from occupational exposures to d-Limonene are reported. The frequency of contacts allergy to oxidised limonene is 1-2 % in several groups of eczema patients. The relationship between contact allergy to oxidised d-Limonene and fragrances in cosmetic products need to be further examined (SCCNFP, 1999).
Chronic toxicity
There is inadequate evidence for carcinogenicity in humans. There is evidence for carcinogenicity in animals, but the mechanism is not relevant for humans. Therefore d-Limonene is not classifiable as to its carcinogenicity to humans (Group 3) (IARC, 1999).
Data for NOAEL and LOAEL is included in the report on stain removers (Engelund et al, 2004). Data are given for ingestion with liver damage as the critical effect.
NOAEL : 250 mg/kg b.w. per day
LOAEL: 500 mg/kg b.w. per day
The type of test that the data are based upon (chronic or subchonic experiment) is not described in the report.
IUCLID does not provide data for estimating NOAEL or similar threshold limits for ingestion or dermal uptake. The same applies for the TOXNET data bases.
Summary
d-Limonene is a substance that by skin contact may cause allergy. It is not harmful by ingestion and no indications for long term effects have been found. NOAEL is 250 mg/kg (liver damages) and LOAEL is 500 mg/kg (liver damages).
5.3.3.6 Exposure scenarios
The maximum content in sample no. 2 was 19 mg per gram.
Intake per kg b.w. per day= 0.0714 * 19 = 1.4 mg/kg b.w.per day
For the other samples with a relative high content the daily intake will be from 0.07 to 0.18 mg/kg b.w.
Because of the relative high value of Log KOW (>4) it seems reasonable to expect that not 100% of the substance will be absorbed by skin contact.
5.3.3.7 Assessment
After oxidation of d-Limonene the substances formed are allergens. The content of d-Limonene may cause allergy.
NOAEL for liver damages is 250 mg/kg b.w. which gives a margin of safety of 175 for sample 2 and about 1500 and more for the other samples. As it is not identified whether the data is based on a subchonic or chronic experiment the safety factor must be set at 1000.
It is therefore assumed that there is a minor non sensitizing health risk for sample number 2 and a negligible health risk for the remaining samples.
It can be concluded that d-Limonene may cause allergies by skin contact at least in the samples containing more than 1 mg/gram corresponding to the limit in the Statutory Order on Classification and labeling, appendix 2, point 2.13 (sample no. 2, 4, 8, E).
5.3.4 Methyl salicylate
5.3.4.1 Identity
| Name | Methyl salicylate |
| CAS-number | 119-36-8 |
| EINECS number | 204-317-7 |
| Molecular formula | C8H8O3 |
Molecular structure |
|
| Molecular weight | 152.14 |
| Synonyms | Hydroxybenzoic acid, methyl ester Benzoic acid, 2-hydroxy-, methyl ester 2-(Methoxycarbonyl)phenol 2-Carbomethoxyphenol 2-Hydroxybenzoic acid methyl ester Birch oil, sweet Methyl 2-hydroxybenzoate Oil of wintergreen Sweet birch oil |
Methyl salicylate is a colourless, yellowish or reddish oily liquid having a characteristic odour of wintergreen. The substance has a boiling point of 220-224° C and a melting point of -8.6° C (Budavari, 1989).
The partition coefficient Log KOW is measured to 2.55 (Hansch and Leo, 1987).
The solubility in water is 0.74 gram/litre at 30° C (Riddick et al, 1985). Methyl salicylate is soluble in most common organic solvents.
The vapour pressure is 0.0343 mmHg at 25° C (Daubert and Danner, 1989).
5.3.4.2 Detected quantities
Methyl salicylate is determined at very high quantities in 5 products:
Number 1: 51 mg/gram equivalent to 5.1 w%
Number 8: 50 mg/gram equivalent to 5.0 w%
Number 15: 45 mg/gram equivalent to 4.5 w%
Number E: 67 mg/gram equivalent to 6.7 w%
Number H: 76 mg/gram equivalent to 7.6 w%
In product number no. 2, 5.3 mg/gram equivalent to 0.5 w% is found and nothing in the remaining 6 samples.
5.3.4.3 Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
No TLV’s were found.
5.3.4.4 Health Effects
Methyl salicylate is included in the IUCLID database as well as in the TOXNET databases.
Acute toxicity
Data for acute oral toxicity LD50 is determined to be from 880 to 2,100 mg/kg for various types of animals. For dermal toxicity LD50 is determined to be from 700 to more than 5,000 mg/kg (IUCLID, 2000).
Symptoms of poisoning by methyl salicylate differ little from those described for aspirin. Central excitation, intense hyperpnoea, and hyperpyrexia are prominent features. The odour of the drug can easily be detected on the breath and in the urine and vomits. In children as little as 4 ml of methyl salicylate may be fatal (Gilman et al., 1990).
The following data on humans are given (IUCLID, 2000):
Oral LD50, adult 500 mg/kg child 170 mg/kg
Oral LDLO, adult 101-800 mg/kg child 228-700 mg/kg
Generally, ingestion of salicylates at doses larger than 150 mg/kg can produce toxic symptoms such as tinnitus, nausea, and vomiting. Serious toxicity can be seen with ingestions larger than 400 mg/kg, with severe vomiting, hyperventilation, hyperthermia, confusion, coma, convulsions, hyper- or hypoglycaemia, and acid-base disturbances such as respiratory alkalosis or metabolic acidosis. In severe cases, the clinical course may progress to pulmonary oedema, haemorrhage, acute renal failure, or death. It is important to note that the salicylate-overdose patient can progress to a more serious condition over time as additional drug is absorbed from the gastrointestinal tract. Chronic salicylism presents clinically in a similar fashion to the acute situation, although it is often associated with a higher morbidity and mortality as well as more pronounced hyperventilation, dehydration, coma seizures, and acidosis (Amadur et al, 1991).
With respect to pregnancy and effects on the fetus, it is shown that salicylates cross the placental barrier. A 33 week old fetus died in utero 20 hours after 3 gram salicylate was ingested by the mother. The salicylate level of the mother was 568 mg/l at admission and 212 mg/l at the time fetal heart tones stopped. The concentration in autopsy blood from the fetus, which aborted 8 days later, was 243 mg/l (Ellenhorn and Barceloux, 1988).
Tests have showed that methyl salicylate is irritating to skin and eyes when tested on animals (IUCLID, 2000). Humans’ ornaments or liniments should not be applied to burned or damaged skin (American Hospital Formulary Service, 1984). Absorption can occur through the skin. Death has resulted from systemic poisoning from local misapplication of the drug (Gilman et al, 1990).
Chronic toxicity
There is no evidence that moderate therapeutic doses of salicylates cause fetal damage in human beings; however, babies born by women who ingest salicylates for long periods may have significantly reduced weights at birth. In addition, there is an increase in prenatal mortality, anaemia, ante partum and postpartum haemorrhage, prolonged gestation, and complicated deliveries (Gilman et al, 1990).
Methyl salicylate given orally by capsules to dogs at a rate of 500-800 mg/kg per day was fatal in a month. Doses of 350 mg/kg per day could be fed for 2 years; loss in weight and enlargement of the liver were observed (Humpreys, 1988).
Methyl salicylate was found to be negative when tested for mutagenicity using the Salmonella/microsome preincubation assay, using the standard protocol approved by the National Toxicology Program (NTP). Methyl salicylate was tested in as many as 5 Salmonella typhimurium strains (TA1535, TA1537, TA97, TA98, and TA100) in the presence and absence of rat and hamster liver S-9, at doses of 1,000, 3,300, 10,000, 33,300, 100,000, and 333,300 ug/plate. The highest ineffective dose tested in any Salmonella typhimurium strain was 333,000 ug/plate (Mortelmans K et al. 1986).
Methyl salicylate is teratogenic in animals and can be absorbed in toxic quantities by the dermal route. Consequently, the dermal absorption and teratogenic potential of a petroleum-based grease (PBG) manufactured using methyl salicylate (3%) was assessed. The test material (petroleum based grease/methyl salicylate) was dermally applied at doses of either 0, 1, 3, or 6 g/kg/day to groups of pregnant rats on gestational days 6-15. The maternal and developmental No-Observable-Adverse-Effect-Level for petroleum based grease/methyl salicylate was greater than 6 g/kg/day (Infurna R et al; Teratology 41 (5): 566, 1990).
Prenatal exposure to methyl salicylate on kidney function in rats were studied. Pregnant female Sprague Dawley rats were treated with methyl salicylate by intraperitoneal injections between gestational days 10 and 14. Methyl salicylate exposure was teratogenic and embryotoxic. Prenatal exposure decreased fetal weight and increased the number of resorptions, fetal mortality, and the incidence of fetal malformations including ectopic kidneys. The primary postnatal renal defect associated with prenatal methyl salicylate treatment was a decreased urine concentrating ability in weanlings.
(Daston,1988)
Data on other long-term effects were not found.
Summary
Lowest dose causing lethality for humans is 100 mg/kg for adults. Methyl salicylate is irritating by skin and eye contact.
Methyl salicylate may be teratogenic by dermal absorption. The substance was teratogenic and embryotoxic by injection in rats. The maternal and developmental NOAEL for petroleum based grease/methyl salicylate was greater than 6 g/kg/day. Indications of other long-term effects were not found.
5.3.4.5 Exposure scenarios
The maximum content in sample no. H was 76 mg per gram.
Intake per kg b.w. per day = 0.0714 * 76 = 5.4 mg/kg b.w/day
For the other samples with a relative high content the daily intake will be from 3 to 5 mg/kg b.w.
Because of the relative low value of Log KOW (<4) it seems reasonable to expect that 100% of the substance will be absorbed by skin contact.
5.3.4.6 Assessment
In five of the products the content of methyl salicylate is so high that 3 to 5 mg per kg b.w. will be absorbed per day. Comparing this with the data on the lowest dose causing lethality for humans the Margin of Safety is only 20.
Methyl salicylate in pure form will cause irritation to skin and eyes (IUCLID, 2000). A product containing about 5 % (no. 1, 8, 15, E, H) may cause irritations.
Methyl salicylate may cause teratogenic effects. Comparing with a NOAEL of more than 6 g/kg b.w. the margin of safety is more than 1200 and therefore the risk for teratogenic effects for humans is negligible.
Based on LD50 =500 mg/kg for humans by oral intake there is a safety factor of 87. Based on this and assuming 100 % uptake by skin, there might be a risk of absorbing larger quantities through skin causing acute poisoning. Symptoms of poisoning by methyl salicylate include central excitation, intense hyperpnoea, and hyperpyrexia.
5.3.5 2-Phenoxyethanol
5.3.5.1 Identity
| Name | 2-Phenoxyethanol |
| CAS-number | 122-99-6 |
| EINECS number | 204-589-7 |
| Molecular formula | C8H10O2 |
Molecular structure |
|
| Molecular weight | 138.16 |
| Synonyms | Hydroxy-2-phenoxyethane 2-Fenoxyethanol 2-Hydroxyethyl phenyl ether 2-Phenoxyethanol 2-Phenoxyethyl alcohol Ethylene glycol phenyl ether Arosol Dowanol EP Dowanol EPH EGMPE |
The substance 2-phenoxyethanol is a colourless liquid with a faint aromatic odour. Boiling point is 245.2°C and melting point is 14°C (Budavari, 1989).
The partition coefficient Log KOW is measured to 1.16 (Leo, 1985). 2-Phenoxyethanol is freely soluble in alcohol, ether and sodium hydroxide. The solubility in water is 26.7 gram per litre (Budavari, 1989).
The vapour pressure for 2-phenoxyethanol is measured to be 0.07 mm Hg at 25°C (Dow Chem Co, 1990).
5.3.5.2 Detected quantities
2-Phenoxyethanol was found in 9 products. The largest amount was found in sample no. 3, where 9 mg/g equal to 0.9 w% was detected. Other relevant findings were:
Sample no. 5 4.9 mg/g equal to 0.5 w%
Sample no. 8 0.68 mg/g equal to 0.07 w%
5.3.5.3 Function
2-Phenoxyethanol is used for a number of purposes. It is common as fixative for perfumes, as solvent for inks, textile dye carrier as, preservative and as bactericide.
The substance is included in INCI as a preservative and can be used in concentrations less than 1 percent in cosmetics.
5.3.5.4 Classifications and TLV’s
2-Phenoxyethanol is included in the List of dangerous substances and classified as:
Xn;R22 Harmful; Harmful if swallowed.
Xi;R36 Irritating; Irritating to eyes.
With respect to the Statutory Order on cosmetic products (Cosmetics, 2005) is can be used in up to 1 percent.
5.3.5.5 Health Effects
For 2-phenoxyethanol an IUCLID data-set is found, data in TOXNET and a description of the substance in Screening for health effects from chemical substances in textile colorants (Hansen OC, 2005).
Acute toxicity
In IUCLID a number of tests with rats where LD50 by oral exposure was determined are reported. The data range is between 1,200 mg/kg and 5,500 mg/kg. By dermal exposure LD50 was determined to 2,300 mg/kg and up to more than 10,000 mg/kg.
Several negative tests for skin irritation on animals are reported (IUCLID, 2000). Also a 3 week patch-test on humans did not cause irritations. Test on rabbits showed eye irritation. Several tests for sensitizing were reported - all with a negative result.
Sub-chronic toxicity
Several sub-chronic studies are reported in IUCLID. Some of these are briefly referred in the following:
- In a 13 week study with rats orally exposed NOAEL was determined to 200 mg/kg b.w. per day based on changes in blood parameters and weight loss.
- In another 13 week study with rats orally exposed NOAEL was determined to 80 mg/kg b.w. per day based on kidney damages.
- A 13 week study with dermal exposure to rabbits showed no adverse effects at the doses 50, 150 and 500 mg/kg per day. NOAEL was determined to be 500 mg per kg b.w. per day.
Chronic toxicity
Pregnant New Zealand white rabbits were treated dermally with 300, 600, or 1,000 mg/kg/day of undiluted 2-phenoxyethanol on days 6 through 18 of gestation (25 animals per dose group). 2-Phenoxyethanol was toxic to the dams (maternal death) at the 600 and 1,000 mg/kg doses. No adverse effects on pregnancy rate, resorptions, or fetal body measurements were observed at any dose. 2-Phenoxyethanol did not cause malformations in the fetuses as compared with controls (Scortichini et al, 1987).
2-Phenoxyethanol was tested for reproductive toxicity in Swiss CD-1 mice in a 2 generation test. The dose levels were 0.0, 0.25, 1.25, 2.5% in feed equal to 375, 1,875 and 3,700 mg/kg/day. 2-Phenoxyethanol produced significant reproductive and developmental toxicity. Liver weight increased in treated F0 mice. The substance caused significant toxicity in growing animals, as evidenced by the reduced body weight in neonates and the large increase in post-natal lethality as the F1 animals grew to the age of mating (Department of Health & Human Services, 1984).
Teratogenicity was evaluated in pregnant New Zealand White rabbits. They were (25/group) dermally exposed to 2-phenoxyethanol at treatment levels of 0, 300, 600, and 1,000 mg/kg/day on gestation days (GD) 6-18. Surviving animals were sacrificed on GD 28. Significant differences were observed between treated and control animals in the following: slight to moderate reddening of the skin at the application site (all treated animals), maternal mortality with dead animals exhibiting kidney damages, evidence of anorexia, changes in the gastric mucosa, decreased feed and fecal material in the intestines as well as changes in the blood parameters (high- and mid-dose groups). No significant differences were observed between treated and control animals in the following (mid- and low-dose groups unless otherwise noted. No statistical evaluations were performed on the five high-dose group rabbits which survived until GD 28. (Dow Chemicals, 1984).
Summary
The substance may cause irritation by eye contact and may be harmful by ingestion. NOAEL based on oral intake was determined to be 80 mg per kg b.w. per day based on kidney damages. Tests showed reproductive and developmental effects in long term studies with dermal exposure.
5.3.5.6 Exposure scenarios
The maximum content in sample no. 3 was 9 mg per gram.
Intake per kg b.w. per day = 0.0714 * 9 = 0.64 mg/kg b.w. per day
For sample no. 5 with a content of 4.9 mg/gram the intake per day will be 0.35 mg/kg b.w. per day whereas the intake per day for sample no. 8 will be 0.05 mg/kg b.w.
Log KOW is relatively low and it is therefore assumed that most of the substance will be absorbed through the skin.
5.3.5.7 Assessment
Sample no. 3 contains 0.9 percent 2-Phenoxyethanol.
Compared with NOAEL of 80 mg/kg b.w. per day for sample 3 it results in a margin of safety of MOS=125 and for sample 5 MOS is 229 and for sample no. 8 MOS is 1650.
As the data is based on a subchronic study, the safety factor for risk assessment is assumed to be at least 1000.
The products 3 and 5 may cause health problems within the margin of safety.
The content of 2-Phenoxyethanol is not expected to cause irritation by skin contact, but it may cause severe irritation upon contact with eyes.
5.3.6 α-Pinene
5.3.6.1 Identity
| Name | α-Pinene |
| CAS-number | 80-56-8 |
| EINECS number | 201-291-9 |
| Molecular formula | C10H16 |
Molecular structure |
|
| Molecular weight | 136.24 |
| Synonyms | 2-Pinene 2,6,6-Trimethylbicyclo(3.1.1)-2-hept-2-ene 2,6,6-Trimethylbicyclo(3.1.1)hept-2-ene 4,6,6-Trimethylbicyklo(3,1,1)hept-3-en Bicyclo(3.1.1)hept-2-ene, 2,6,6-trimethyl Acintene A Monoterpenes |
α-Pinene is a colourless liquid with a characteristic odour of pine. The boiling point is 156°C and melting point is -62.5°C (Fenaroli, 1975).
Log KOW for α-Pinene is determined to 4.83 (Li and Perdue, 1995).
The solubility of the substance in water is 2.49 mg/l at 25°C. α-Pinene is soluble in alcohol, chloroform, ether and concentrated acetic acid. It is almost insoluble in propylene glycol and glycerine ( Fenaroli, 1975).
The vapour pressure of α-Pinene is 4.75 mm Hg at 25°C (Daubert and Danner, 1989).
5.3.6.2 Detected quantities
α-Pinene was detected in 8 products. The important findings were:
Sample 2: 12 mg/g equal to 1.2 w%
Sample 4: 1.7 mg/g equal to 0.2 w%
Sample 8: 27 mg/g equal to 2.7 w%
Sample 15: 19 mg/g equal to 1.9 w%
Sample E: 33 mg/g equal to 3.3 w%
Sample H: 22 mg/g equal to 2.2 w%
5.3.6.3 Function
α-Pinene has many functions and is widely used. Common uses are as solvent, emollient, in pesticides as base for synthetic oils and perfumes
The substance is included in INCI as a fragrance.
5.3.6.4 Classifications and TLV’s
α-Pinene is not classified in the Annex I of Directive 67/548/EEC. The substance is included in the Danish advisory list for selfclassification (Vejledende liste) with the classification:
R43 May cause sensitization by skin contact.
N;R50/53 .Hazardous to the environment; Very toxic to aquatic organisms, may cause long-term adverse effects in aquatic environments..
No TLV are given for α-Pinene. For terpenes in general like turpentine TLV in Denmark is 140 mg/m³ or 25 ppm (C.0.1, 2005). In USA, TLV as an 8 hour Time Weighted Average (TWA) is set to 20 ppm (ACGIH, 2003).
5.3.6.5 Health Effects
Data for α-Pinene is relatively limited. The following is based on a data set from IUCLID, the databases in TOXNET and a general search at the internet. A general search of terpenes is also included.
Acute toxicity
Acute oral toxicity is tested in studies with rats, which showed LD50 of 2,100 mg/kg and up to 5,100 mg/kg. Dermal toxicity based on test with rabbits showed results of LD50 of more than 5,000 mg/kg (IUCLID).
α-Pinene has essentially the same toxicity as turpentine (Gosselin et al, 1984).
Fatal dose for humans is about 180 gram orally as turpentine, which contains 58-65% α-Pinene (The Merck Index, 1976).
It is referred that α-Pinene irritates skin and mucous membranes and causes skin eruption and irritation of the respiratory system (Budavari, 1989). In IUCLID there are references to some tests on rabbits, mice and rats, where some were positive and some negative. A patch test on 5 humans tested with 10% α-Pinene in petrolatum for 48 hours showed no effects.
In IUCLID a test of eye irritation with the result moderately irritation is described. Another source states that α-Pinene is an eye, mucous membrane, and severe human skin irritant (Lewis, 1996).
Several tests on sensitizing on humans are reported (IUCLID, 2000). Most of the patch test showed that several people reacted positive. Turpentine oil, which normally has a high content of α-Pinene is labelled R43: May cause sensitization by skin contact.
Sub-chronic toxicity
In a 14 day test rats were orally exposed daily with 0, 250 and 500 mg/kg. In the group exposed to 500 mg per kg, reduced body weight and increased weight of liver was observed.
No relevant data for turpentine oil was found.
Chronic toxicity
No data on chronic toxicity for α-Pinene were found.
From OSHA’s Health Guidelines (2005) the following information has been retrieved for turpentine and it is assumed to be valid for α-Pinene as well.
- In one study, dermal application of turpentine produced skin tumours in rabbits but not in mice ; in another experiment, however, painting the skin of mice with 240 g/kg turpentine did cause tumours.
- Turpentine is a skin, eye, mucous membrane, and upper respiratory tract irritant in humans. It may also cause skin sensitization and central nervous system, gastrointestinal, and urinary tract effects. The lowest estimated oral dose reported to be lethal in humans is 441 mg/kg.
- A case-control study of workers in particle-board, plywood, sawmills, and formaldehyde glue factories demonstrated a statistically significant association between chronic exposure (longer than 5 years) to terpenes (the principal component of turpentine) and the development of respiratory tract cancers.
Summary
α-Pinene causes irritation by skin and eye contact and may cause allergies by skin contact.
Data on long-term effects are very limited and risk of cancer and other long-term effects from skin contact and ingestion are very uncertain.
Based on the one sub-chronic test NOAEL is determined to be 250 mg/kg with increased liver weight as the critical effect.
5.3.6.6 Exposure scenarios
The maximum content in sample no. E was 33 mg/gram.
Intake per kg b.w. per day= 0.0714 * 33 = 2.4 mg/kg b.w. per day
Intake for the other products is between 0.1 to 1.9 mg/kg b.w.
5.3.6.7 Assessment
Six samples (2, 4, 8, 15, E and H) contain more than 0.1w% α-Pinene. α-Pinene is a potential skin sensitizer and therefore there is a substantial risk of allergic reaction when contact with these products.
NOAEL is estimated to 250 mg/kg based on limited data.
As the data is based on a subchronic study, the safety factor for risk assessment is assumed to be at least 1,000.
The margin of safety is from 100-300 for products 2, 8, 15, E, and H and above 1,000 for the rest. Taken the limited data and the MOS into account it can be concluded that there is a possible risk by uptake through skin of the substance.
5.3.7 Solvents
A special focus has been directed towards the content of organic solvents, because it is expected that the evaporation of these substances causes the cooling effects in some of products.
5.3.7.1 Substances and amounts
All the selected products have been tested for content of organic solvents. In the analysis the detection limit was 0.1 %. The results are shown in Table 5.1. It shows that solvents were found only in 4 products.
Table 5.1Content of solvents in sports products
| Solvent 1 | CAS-number | Product 4 | Product 5 | Product 12 | Product C |
| Ethanol | 64-17-5 | 54 % | 2,2 % | 14 % | |
| 2-Propanol | 67-63-0 | 27 % | |||
| Acetone | 67-64-1 | 1.9 % | |||
| tert-Butyl alcohol | 75-65-0 | 0.35 % |
1 Toluene was also found but in ppm level (<0,0005%)
5.3.7.2 Physical properties
Relevant data for assessing the solvents with respect to evaporation and cooling effects is shown in Table 5.2.
Table 5.2 Physical properties for solvents in sports products
| Solvent | CAS-number | Vapour pressure mmHg | Heat of Vaporization J/ gram |
| Ethanol | 64-17-5 | 59.3 | 878 |
| 2-Propanol | 67-63-0 | 45.4 | 757 |
| Acetone | 67-64-1 | 232 | 510 |
| tert-Butyl alcohol | 75-65-0 | 40.7 | 528 |
As shown in Table 5.2, the vapour pressure is about the same for ethanol, 2-propanol and t-butyl alcohol. For acetone, the vapour pressure is about 4 or 5 times higher, which means that this substance will have the fastest rate of evaporation.
The amount of energy (heat) necessary for evaporation is expressed by the term “Heat of evaporation”. When 1 gram of ethanol is evaporated 878 J is removed and this is felt as the cooling effect. The total cooling effect is the amount of solvent that evaporates times the heat of evaporation. The evaporation per time unit is not known and therefore the cooling effect per time unit cannot be determined.
The total cooling effect based on the use of 5 grams of product is shown in Table 5.3.
Table 5.3: Potential cooling effect based on content of solvents in sports products
| Solvent | CAS-number | Content in 5 gram | |||
| Product 4 | Product 5 | Product 12 | Product C | ||
| Ethanol | 64-17-5 | 2.6 g | 0.11 g | 0.70 g | |
| 2-Propanol | 67-63-0 | 1.35 g | |||
| Acetone | 67-64-1 | 0.10 g | |||
| tert-Butyl alcohol | 75-65-0 | 0.02 g | |||
| Energy per 5 gram product | 2282 J | 97 J | 1022 J | 676 J | |
As shown in Table 5.3 the potential cooling effect is very different for the 4 products.
5.3.7.3 Classification and TLVs
The classification and the TLV’s for the four solvents are shown in Table 5.4.
Table 5.4 Classification for solvents in sports products
| Solvent | CAS-number | Classification | TLV mg/m³ | INCI function |
| Ethanol | 64-17-5 | F;R11 | 1900 | Solvent |
| 2-Propanol | 67-63-0 | F;R11 Xi;R36 R67 |
490 | Fragrance Anti foam agent Solvent |
| Acetone | 67-64-1 | F;R11 Xi R36 R66 R67 |
600 | Denaturants / Solvent |
| tert-Butyl alcohol | 75-65-0 | F;R11 Xn;R20 |
150 | Fragrance |
Table 5.4 shows that all the solvents are flammable, and that two substances are irritating and one is harmful by ingestion. Two substances are marked with R67 and the substances may therefore cause dizzyness by direct inhalation.
The threshold limit values are relatively high for all for solvents when compared to substances with greater health risks like chloroform (10 mg/m³) and formaldehyde (0.4 mg/m³)
5.3.7.4 Health effects
The solvents will be absorbed into the body either by absorption through the skin or by inhalation. No data indicate that the solvents cannot be absorbed and therefore it is assumed that 100 % may be by either route.
Data on acute toxicity for the four solvents are retrieved from Chemid:
Inhalation
| Ethanol | LC50, rat | 20,000 mg/kg | |
| 2-Propanol | LC50, rat | 16,000 mg/kg | |
| Acetone | TCLo, man | 12,000 mg/kg, | nausea or vomiting, muscle weakness |
| LC50, rat | 5,500 mg/kg | ||
| t-Butyl alcohol | LC50, rat | 10,000 mg/kg |
Skin
| Ethanol | LDLo, rabbit | 20,000 mg/kg |
| 2-Propanol | LD50, rabbit | 12,800 mg/kg |
| Acetone | LD50, guinea pig | 9,400 mg/kg |
| LDLo, rabbit | 20,000 mg/kg | |
| t-Butyl alcohol | LD50, rabbit | >2,000 mg/kg |
| LD50, guinea pig | >10,000 mg/kg |
From the data given on inhalation and skin absorption it can be seen that there is no potential acute health risk when comparing the criteria for classification of chemical substances.
The classification for the four solvents does not include any long-term effects, although, the four solvents are well known for causing CNS-damages (Central nervous System) when exposed repeatedly to low concentrations and/or for a long time.
No data on concentrations or uptake has been found for showing when long term health effects occur.
5.3.7.5 Exposure assessment
The assessment of the potential health risk by inhalation is based on the Danish TLV’s and the total amount that may evaporate.
- One product contains 54 % ethanol. If 5 grams of product is used per person, it means that 2600 mg ethanol may evaporate.
If all ethanol evaporates momentarily in more than 1.5 m³ of air, the threshold limits will be observed.
Therefore, although more people are gathered and use the product at the same time, ethanol will not cause any short-term health risks.
- Tert-butyl alcohol is found in product no. C in the amount of 0.35 % equivalent to 17 mg in 5 gram.
This amount cannot cause the concentration to rise above the threshold limit value.
Based on the two examples it can be concluded that the amount of vapours from the products will not exceed the threshold limits at the workplace (8 hours daily). Therefore it is assessed that exposure by inhalation will cause a negligible health risk.
Assessing the potential health by skin contact it is assumed that the weight of an adult is 70 kg and that 5 gram of product is used per day. The assessment is based upon acute toxicity data and shown in Table 5.5.
Table 5.5 Assessing skin absorption of solvents in sports products
| Solvent | CAS-number | Potential amount absorbed by skin contact 70 kg adult using 5 gram [mg/kgb.w.] |
LD50, rabbit mg/kg |
MOS Margin of safety |
|||
| Product 4 | Product 5 | Product 12 | Product C | ||||
| Ethanol | 64-17-5 | 37 | 2 | 10 | >20,000 | > 500 | |
| 2-Propanol | 67-63-0 | 19 | 12,800 | 670 | |||
| Acetone | 67-64-1 | 1,4 | >20,000 | >14,000 | |||
| tert-Butyl alcohol | 75-65-0 | 0.25 | >2,000 | >8000 | |||
Based on the data in Table 5.5 it can be concluded that the MOS for ethanol and 2-propanol in product no. 4 and 12 are relatively low taken into account that acute toxicity-data are used.
The solvents in product 4 and 12 may cause a minor potential health risk, whereas in product 5 and C the amounts are so limited that they will cause no health risk.
Conclusion
Intake of solvents by inhalation will not cause any health risk. Uptake by skin absorption may cause a minor potential heath risk for product 4 and 12.
2-Propanol in product 12 may cause irritation.
5.4 Overall Assessment
5.4.1 Substances
In the following section an overview of the assessment of the substances in section 5.3 is given. Data in the tables are given for the samples with the highest concentration of the actual substance.
Table 5.6 Irritation and allergy effects for selected substances in sports products
| Substance | Max. Conc. W% | Irritation to skin | Sensitization by skin contact | Remarks |
| Camphor | 7.7 | Potential effect | Potential effect | With the content there is a risk of both irritation and sensitization in products no. 2, 3, 4 and 12. |
| Dimethyl sulphone | 0.82 | No effect | No effect | No risk |
| d-Limonene | 1.90 | No data | Potential effect | Risk of allergies in the samples containing more than 1 mg/gram (product no. 2, 4, 8, E) |
| Methylsalicylate | 7.60 | Potential effect | No data | Products containing about 5 % (no. 1, 8, 15, E, H) may cause irritations |
| 2-Phenoxyethanol | 0.9 | No effect | No effect | No risk |
| α-Pinene | 3.30 | Potential effect | Potential effect | Risk of irritation and sensitization. Six products (2, 4, 8, 15, E and H) contain more than 0.1w% |
The solvents, 2-propanol and acetone may cause irritations. This will mostly be relevant for product no. 12. None of the solvents are known for causing allergies.
Table 5.7 Toxic effects for selected substances in sports products
| Substance | Max uptake mg. per kg b.w. | NOAEL mg/kg b.w. per day | MOS | Remarks |
| Acetone | 1 | >20000 | >20000 | No risk of health effects. |
| t-Butyl alcohol | 0.3 | >2000 | >6667 | No risk of health effects. |
| Camphor | 5.5 | 680 | 123 | There is a minor risk of health teratogenic effects from dermal exposure for 5 g of product 2. |
| Dimethyl sulphone | 0.6 | >1500 | >2500 | The amounts detected do not cause any negative health effect. |
| Ethanol | 37 | >20000 | >500 | No risk of health effects because most will evaporate. |
| d-Limonene | 1.4 | 250 | 175 | Minor health risk for product no. 2 (effects on liver) |
| Methylsalicylate | 5 | >6000 | >1200 | Minor risks of health effects. |
| 2-Phenoxyethanol | 0,64 | 80 | 125-229 | Health risk for the products 3 and 5. |
| α-Pinene | 2.4 | 250 | 100 | Based on the limited data it can be concluded than the health risk is relatively high for product no.2,8,15,E,H (increased liver weight) |
| 2-Propanol | 19 | 12800 | 670 | No risk of health effects because most will evaporate. |
From Table 5.7 it can be seen that the most problematic substances with respect to health effects caused by skin absorption are Camphor, 2-Phenoxyethanol, α-Pinene and d-Limonene.
5.4.2 Products
Among the 12 tested products two products, number 2 and 12 shall be classified and labelled according to the Statutory Order on classification of chemicals. The products 4, 8 and E shall also be labelled with a sentence specifying the content of allergens and that an allergic reaction may arise
Irritation effected are found in Camphor, Methylsalicylate, 2-Phenoxyethanol and 2-Propanol.
- Camphor causes a risk of irritation, product no. 2.
- Methylsalicylate may cause irritation, products nos. 1, 8, 15, E and H
- 2-Propanol may cause irritation, product no. 12
Regarding allergies and sensitization the substances d-Limonene, Camphor and α-Pinene are potential allergens.
- d-Limonene may cause sensitization and are found in more than 0.1% in product no. 2, 4, 8 and E.
- Camphor may cause sensitization and are found in more than 0.1% in no. 2, 3, 4 and 12.
- α-Pinene may cause sensitization and are found in more than 0.1% in no. 2, 4, 8, 15, E and H.
The products 1, 5 and 13 contain no potential allergens.
Some of the substances may cause potential health risk by skin absorption. The most problematic substances are Camphor, 2-Phenoxyethanol, α-Pinene and d-Limonene.
- Camphor was found in relatively large amounts in product 2.
- α-Pinene was found in amounts of 1-3% in products nos. 2, 8, 15, E and H.
- d-Limonene was found in 1-2% in product no. 2.
- Ethanol and 2-Propanol was found in products nos. 4 and 12, but it is assumed that the health effect is minimal because most of the substances evaporate.
The overall conclusion of the health assessment is:
- Product nos. 2, 8, , 12, 15, E and H contain potential irritating and sensitizing substances as well as substances with the potential of causing health risks by dermal uptake.
- Product nos. 1, 2, 3, 4, 8, 12, 15, E and H contain potential irritating substances.
- Product nos. 2, 3, 4, 8, 13, 15, C, E, and H contain potential sensitizing substances in concentrations larger than 0.001 wt%
- Two products (nos. 2 and 12) have to be classified and labelled for health effects.
- Product nos. 4, 8 and E have to be labelled: Contains "substance name(s)". May cause allergic reactions.
6 References
| ACGIH, 2001, American Conference of Governmental Industrial Hygienists. Documentation of Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2001. Cincinnati, OH. 2001 |
| ACGIH, 2003, American Conference of Govenmental Industrial Hygiensts. TLV's BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2003. Cincinnati, OH. 2003 |
| ACGIH, 2005 American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinatti, OH, 2005, p. 17 |
| Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991 |
| American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984 |
| Bekendtgørelse om kosmetiske produkter. Bekg. Nr. 74 af 14. jan. 2005 |
| Bekendtgørelse nr.923 af 28. september 2005 om klassificering, emballering, mærkning, salg og opbevaring af kemiske stoffer og produkter No. 923, 2005 on Classification, Packaging, Labelling, Sales and Storage of Chemical Substances and Products |
| Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989 |
| C.0.1 (2005) At-vejledning: Grænseværdier for stoffer og materialer. Arbejdstilsynet |
| Cosmetics, 2006. Statutory Order on cosmetic products No 422 of 4th May 2006. Ministry of the Environment |
| Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989 |
| Daylight Chemical Information Systems. CLOGP and CMR Models. Camphor (SMILES: O=C(C(C(C1C2)(C)C)(C2)C)C1). Available from the Database Query page at . HYPERLINK "http://www.daylight.com/cgi-bin/contrib/pcmodels.cgi" \t "new" .http://www.daylight.com/cgi-bin/contrib/pcmodels.cgi. as of Feb 24, 2004 |
| Daston GP et al; Fundam and Appl Toxicol 11 (3): 381-400 (1988) |
| Department of Health & Human Services/National Institute of Environmental Health Sciences, National Toxicology Program; Reproduction and Fertility Assessment of Ethylene Glycol Monophenyl Ether (CAS #122-99-6) in CD-1 Mice When Administered in Feed, NTP Study No. RACB83101 (November 1984) available at http://ntp.niehs.nih.gov/ index.cfm?objectid=0847F35A-0850-D1E7-B02ED4DDD150F990 as of August 14, 2002 |
| Dow Chemical Corporation; 2-Phenoxyethanol: Dermal Teratology Study in Rabbits, Final Report. (1984), EPA Document No. 88-8500760, Fiche No. OTS0509695 |
| Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988 |
| Engelund B, Hùglund L, Skjødt D (2004) Mapping of stain removers. Survey of Chemical Substances in Consumer Products no. 43, 2004. Danish Environmental Agency |
| Ford MD, Delaney KA, Ling LJ, Erickson T; Clinical Toxicology. W.B. Saunders Company., Philadelphia, PA. 2001, p. 339 |
| Geller RJ, Spyker DA, Garrettson LK & Rogol AD (1984) Camphor toxicity. Development of a triage system. Vet Hum Toxicol, (Suppl 2): 8-10 |
| Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990 |
| Gosselin R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984 |
| Hansch, C. and A. Leo. The Log P Database. Claremont, CA: Pomona College, 1987 |
| Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society, 1995., p. 53 |
| Hansen OC (2005) Screening for health effects from chemical substances in textile colorants. Survey of Chemical Substances in Consumer Products, 57. Danish Environmental Protection Agency |
| Hansen OC and Eggert T (2003). Survey, emissions and evaluation of volatile organic chemicals in printed matter. Survey of Chemical Substances in Consumer Products no. 36, 2003. Danish Environmental Agency |
| Haz-map can be found at: http://hazmap.nlm.nih.gov/ cgi-bin/hazmap_search?queryx=76-22-2&tbl=TblAgents |
| Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988 |
| IARC (1999). Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. 73 322, 1999 |
| INCI, Fortegnelse over ingredieser i kosmetiske produkter (International Nomenclature of Cosmetic Ingreedients, http://pharmacos.eudra.org/F3/cosmetic/cosm_inci_index.htm) |
| Infurna R et al; Teratology 41 (5): 566 (1990) |
| IPCS,2003 CEC; International Chemical Safety Card on Camphor. (Date of review: May 2003). Available from http://www.inchem.org/documents/icsc/icsc/eics1021.htm as of February 5, 2004 |
| IUCLID, 2000, European Communities, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. http://ecb.jrc.it/esis |
| Jones AH; J Chem Eng Data 5: 196-200 (1960) |
| Klassificering, 2005: Miljøministeriets bekendtgørelse nr. 970 af 16. oktober 2005 om klassificering og mærkning. (Classification 2005. Statutory Order on classification, packaging, labelling, sale and marketing of chemical substances and products, no. 970 of 16 October, 2005. Ministry of the Environment) |
| Leuschner J; Arzneimittelforschung 47 (2): 124-8 (1997) |
| Lewis, R.J. 1996: Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996 |
| Li J, Perdue EM (1995); Physicochemical Properties of Selected Monoterpines. Preprints of Papers Presented at the 209th ACS National Meeting; Anaheim, CA; April 2-7 (1995) 35:134-7 (1995) |
| Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 71st ed. Boca Raton, FL: CRC Press Inc., 1990-1991., p. 3-180 |
| Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 73rd ed. Boca Raton, FL: CRC Press Inc., 1992-1993., p. 3-308 |
| Liste over farlige stoffer. Miljøministeriets bekendtgørelse nr. 923 af 28. september 2005 (List of dangerous substances. Statutory Order on the list of dangerous substances. No. 923 of 28 September 2005. Ministry of Environment.) Methylsulfonylmethane, 2003 |
| Mortelmans K et al; Environ Mutagen 8: 1-119 (1986) |
| National Poisons Information Service Center, (1996) United Kingdom; Poisons Information Monograph: Camphor. (March 1996). Available from: http://www.intox.org/databank/documents/ pharm/camphor/ukpid19.htm as of February 5, 2004 |
| O'Neil, M.J. (ed.) 2001. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001 |
| OSHA’s Health Guidelines (2005) retrieved at http://www.osha.gov/SLTC/healthguidelines/ turpentine/recognition.html |
| Produktsikkerhedsloven, 1994: Lov om produktsikkerhed, LOV nr. 364 af 18/05/1994 med senere ændringer. (Law on Product Safety, Act no. 364 of 18 May, 2005.) |
| Riddick, J.A., W.B. Bunger, Sakano T.K. Techniques of Chemistry 4th ed., Volume II. Organic Solvents. New York, NY: John Wiley and Sons., 1985 |
| SCCNFP, 1999. Fragrance allergy in consumer products. The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers |
| Scortichini BH et al; Fundam Appl Toxicol 8: 272-79 (1987) IUCLID |
| TGD, 2003. Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk assessment for new notified substances, Commission Regulation (EC) 1488/94 on Risk assessment for existing substances and Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Parts 1-4. European Commission (EC), Joint Research Centre, EUR 20418 EN, Office for Official Publications of the EC, Luxembourg |
| The Merck Index (1983). 10th ed. Rahway, New Jersey: Merck Co., Inc., 1983., p. 238 |
| TOXNET:Databaser, bl.a. HSDB (Hasardous Substance DataBase) og ChemID. http://toxnet.nlm.nih.gov/ |
| Vejledende liste til selvklassificering. Miljøstyrelsen, 2001 (Advisory list on self-classification. Danish Environmental Protection Agency 2001) |
Footnotes
[1] CN-code is a 8-digit product code number (CN ~ combined nomenclature)
[2] KN-kode er et 8-cifret varekodenummer (KN ~ kombineret nomenklatur)
[3] Politiken, 4th Februar 2005
[4] According to the sports shop is it the same products that are sold in all the the chain store shops
[5] CN-code is an 8-digit product code no. (CN ~ combined nomenclature)
Version 1.0 December 2006, © Danish Environmental Protection Agency