Survey and health risk assessment of products for treatment of sports injuries and pains
5 Health Assessment
5.1 Introduction
In this chapter, potential health effects from identified and selected substances in section 3.3 are assessed. The focus of the assessment is primarily aimed at adults.
For each of the identified and quantified substances, information on the substances’ identity as well as chemical and physical properties are presented. It will include data on material state, melting point, boiling point, vapour pressure and solubility.
A search in the open literature has been performed with focus on possibility of skin absorption and the most important test results, effects and circumstances are presented in this report. The aim was to find data for NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) for the selected substances or other relevant data, if available.
Based on NOAEL or similar data and the amount of the substances of the tested products it can be assessed whether the substances may cause negative health effects.
5.2 Method
It is assumed that the substances can be absorbed in the body by penetration through skin.
None of the product labellings had directions for a recommended amount to use. From the information on the products no special directions regarding the recommended amount to use was given for any of the products. For assessment and comparison purposes the same amount of product should be used.
Based on experiments where the legs of two test persons were exposed to 4-5 gram product, the exposure scenario was determined to be 5 grams of product per day. The exposure scenarios are defined according to the EU's Technical Guidance Document (TGD, 2003).
The uptake is calculated as:
Uptake per day per kg body weight = C [mg/gram] * 5 gram per day * F / body weight [kg]
C: Content of substance in mg per gram sample
F: Fraction of absorbed substance. If no specific values for F is found then the default value is used: F = 100 % if Log KOW < 4 and F = 10% if Log KOW > 4.
The body weight (b.w.) is assumed to be 70 kg (TGD 2003).
The equation can be reduced to:
Uptake per kg b.w. per day = Content of substance × * F * 5 / 70
Uptake [mg/kg b.w.] per day = 0.0714 * F * C [mg/gram] per day
The intake per day then have to be compared with data for absorption through skin, if available, and/or oral intake.
Assessment of risk
In the assessment of health risks the calculated intake has to be compared with the NOAEL or similar values. As NOAEL typically is based on tests on animals a safety factor (MOS: Margin of safety) is introduced by dividing NOEAL in mg/kg b.w by the intake.
If the data for animals is a chronic long term study of high quality the safety factor in the risk assessment is typically MOS=100. This is based on a factor of 10 for extrapolation between species (interspecies) and a factor of 10 meant to protect sensitive individuals like children (intraspecies). If the data is of less quality eg. based on LOAEL or a subchronic study an additional safety factor is applied (typically 10). The total safety factor is the combined product of the individual safety factors.
In the assessment of health effects MOS is not used for sentisizing effects as these effects do not have a lower concentration limit.
5.3 Selected substances
The substances described in the following are selected as the most important substances for the potential heath risks when using these products. The selected substances are:
- Camphor
- Dimethylsulphone
- d-Limonene
- Methylsalicylate
- α-Pinene
- 2-Phenoxyethanol
Also a short description of the solvents ethanol, 2-propanol, acetone, and t-butyl alcohol is included because these substances are found in large quantities in the products.
5.3.1 Camphor
5.3.1.1 Identity
| Name | Camphor (EINICS name:Bornan-2-one) |
| CAS-number | 76-22-2 |
| EINECS number | 200-945-0 |
| Molecular formula | C10H16O |
Molecular structure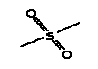 |
|
| Molecular weight | 152.23 |
| Synonyms | Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl- 1,7,7-Trimethylbicyclo(2.2.1)heptan-2-one 2-Bornanone Gum camphor Spirit of camphor |
The substance consists of colourless or white crystals. It has a boiling point of 204° C and a melting point of 179° C (The Merck Index, 1983).
The substance is more soluble in organic solvents than in water. Yalkowsky and Yan (2003) state that 1.6 gram of camphor can be dissolved in 1 litre of water at 25° C. In another reference the following is given: At 25° C one gram dissolves in about 800 ml water, in 1 ml alcohol, 1 ml ether, 0.5 ml chloroform. The substance is freely soluble in carbon disulfide, petroleum, fixed and volatile oils. It is also soluble in concentrated mineral acids, in phenol, in liquid ammonia and in liquid sulfoxide (O'Neil, M.J. 2001).
The partition coefficient Log KOW is determined to be 2.38 (Daylight Chemical Information Systems. 2004).
Vapour pressure is determined to be 0.65 mm Hg at 25° C (Jones AH, 1960).
Some values are given for odour threshold values. The lowest odour value is 0.0026 ppm and the highest is 0.96 ppm. Both odour values are below the threshold limit value :TLV (Haz-map, 2005).
5.3.1.2 Detected quantities
The substance is detected in 7 products. The most important are considered to be the samples with the highest concentrations. In
Sample number 2 is found 77 mg/gram equal to 7.7 w%
Sample number 3 is found 5.1 mg/gram equal to 0.51 w%
Sample number 12 is found 2.5 mg/gram equal to 0.25 w%
Sample number 4 is found 1.0 mg/gram equal to 0.10 w%
The remaining 3 samples contain less than 0.01 mg/gram.
5.3.1.3 Function of substance
The substance is included in the INCI-database. Here is stated that the function of the substance can be denaturants / film formers and as a fragrance. O'Neil, M.J. (2001) states that the substance is normally used as an odorant and flavourant and it can be used as emollient in cosmetics and as a preservative.
Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
The Danish threshold limit value is 2 ppm equal to 12 mg/m³. The same limit is set in USA (ACGIH, 2005).
5.3.1.4 Health Effects
Data regarding health effects are retrieved from TOXNET and the databases related to this host. The substance has not been included in IUCLID.
Acute toxicity
The substance is irritating to the eyes, the skin, and the respiratory tract (IPCS, 2003). Camphor applied on the skin of volunteers as a 20% solution in alcohol produced no significant sensation of irritation or pain at normal skin temperatures. It did appear to have a slight sensitising effect on the perception of temperature change during heating and cooling, and increased the sensation of burning at high temperatures (National Poisons Information Service Center, 1996).
Acute toxicity by ingestion based on test with animals indicates that camphor may be slightly toxic (i.e LD50 rat <2000 mg/kg):
- LD50 Mouse oral 1310 mg/kg (Lewis, R.J. 1996)
- LD50 Rat subcutaneously 70 mg/kg (Lewis, R.J. 1996)
- LD50 Mouse ip 3000 mg/kg (ACGIH, 2001)
Several exposure studies with humans have been reported. In one study 1.5 g camphor has been ingested by an adult, who recovered. In children 0.7 to 1.0 g has proved to be fatal. Urinary retention, albuminuria, and anuria are described in non-fatal cases, but kidney lesions in fatal poisonings are not always prominent. Mild and transient hepatic derangements may occur and widespread hemorrhages are described in one fatal case. Fetal death resulted after camphor ingestion by mother and postmortem exam revealed severe atelectasis (collapse of lung) and central neuronal necrosis (Gosselin et al, 1984).
Camphor remains in over 950 products listed in Poisindex according to the following reference. A review of all camphor ingestions estimated to be 2 mg/kg or greater was made. Seventy-three patients (90%) remained asymptomatic, three (4%) developed minor symptoms, and five (6%), all ingesting over 59 mg/kg, developed major symptoms. No deaths were reported (Geller RJ et al; 1984).
From IPCS, Poisons Information Monograph the following has been retrieved
- Camphor crosses the placenta and has been implicated in fetal and neonatal death. It has been used to induce abortions. Camphor poisoning during pregnancy was reported in four cases and, in each case, camphorated oil was mistaken for castor oil. The topical use of camphorated oil in pregnancy was not associated with teratogenic effects.
- Deafness has been reported in association with camphor. Ulceration of the mucous membranes has been reported following the use of toothache solutions containing camphor (along with menthol, phenol, clove oil and chloroform).
- Camphor administered in doses of 60 mg to 4 g was reported to cause flickering, darkening or veiling of vision along with noises in the ears. Corneal erosions have been reported in association with the use of inhalant capsules containing camphor.
Sub-chronic toxicity
D-Camphor elicited no evidence of teratogenicity when administered orally during the fetal period of organogenesis to pregnant rats at doses up to 1000 mg/kg b.w./day, and to pregnant rabbits at doses up to 681 mg/kg b.w./day. The NOEL for the fetal organism for the rat was above 1000 mg/kg b.w., and for the rabbit above 681 mg/kg b.w. (Leuschner J, 1997).
Chronic toxicity
With chronic dermal exposure, systemic effects and contact dermatitis can occur as well as significant allergic responses. Ocular exposure results primarily in irritation only, although oral intake has been associated with visual problems (Ford MD et al., 2001).
Camphor is classified as “A4; Not classifiable as a human carcinogen” (ACGIH, 2005).
Summary
Only values for NOEL for teratogenicity are given for short-term studies with animals. The lowest value was 680 mg/kg b.w. per day.
Observations on humans showed that ingestion of 2 mg/kg b.w. gave none or minor symptoms.
References above show that camphor may cause irritation by skin contact and may by chronic exposure cause allergies.
5.3.1.5 Exposure scenarios
For camphor Log KOW is less than 4 and therefore, 100% of the substance is assumed absorbed through the skin.
The maximum content in a sample was 77 g/kg.
Intake per day per kg b.w. = 0.0714 * 77 = 5.5 mg/kg b.w./day
For the other 3 samples with a relative high content, intake per day per kg b.w. is between 0.01 and 0.36 mg/kg b.w.
5.3.1.6 Assessment
Camphor is a substance that may cause irritations and allergies by skin contact. It may be toxic if ingested in relative large amounts - more than 1 mg/kg b.w. Camphor may cause teratogenic effects; NOEL based on a subacute test is estimated to 680 mg/kg. Indications for other long-term effects have not been found.
Camphor has been detected in seven samples. Based on dermal contact with 5 gram of product the maximum daily uptake will be 5.5 mg per kg b.w.
Based on the data for teratogenicity a margin of safety (MOS) is only about 123. Compared with the observations on humans with 2 mg/kg b.w. by oral intake, the exposure with this substance is higher for sample no. 2 and lower for the other 3 samples. As the data is based on a subchronic study, the safety factor for risk evaluation is assumed to be at least 1000.
Therefore, when assuming 100% uptake through skin it can be concluded that there is a minor risk of teratogen health effects from dermal exposure to product no. 2. Pregnant should avoid using the product on larger skin areas.
Based on the available data there is a risk that camphor may cause irritations and allergic reactions for products no. 2, 3, 4, 12.
5.3.2 Dimethyl sulphone
5.3.2.1 Identity
| Name | Dimethyl sulphone |
| CAS-number | 67-71-0 |
| EINECS number | 200-665-9 |
| Molecular formula | C2H6O2S |
Molecular structure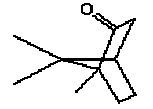 |
|
| Molecular weight | 94.13 |
| Synonyms | Methyl sulfonyl methane Methylsulfonylmethane Dimethylsulfone MSM |
The following data is retrieved from CHEMid:
Dimethyl sulphone has a melting point of 109oC and a boiling point of 238oC.
The substance is completely miscible with water; solubility is 1,000 gram per litre water. Log KOW is -1.41, which means that the substance is much more soluble in water than in organic solvents.
The vapour pressure is estimated to 5.15 mm Hg.
5.3.2.2 Detected quantities
Dimethyl sulphone is determined in three products, - sample number 13, where 8.2 mg/gram was detected and in sample number 15 and C, where very small amounts were found (<0.06 mg/g).
5.3.2.3 Function of substance
The substance is a naturally occurring nutrient found in the human body. Dimethyl sulphone is an important source for organic sulphur.
The substance is included in the INCI-database as a solvent.
Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
No threshold limit values or restrictions for the substance have been found.
5.3.2.4 Health Effects
Very limited information on this substance has been available. Most information retrieved focused on the advantages of using the substance as natural non-prescription drug or nutrient supplement.
Acute toxicity
Food contains dimethyl sulphone at the level of a few ppm; e.g. cow milk contains 3.3ppm and tomatoes 0.86 ppm (Methylsulfonylmethane, 2003).
The substance is believed to be non-toxic. Oral dosage for humans is often in the range of 1 to 3 grams daily (Methylsulfonylmethane, 2003).
The natural level of dimethyl sulphone in the circulatory system of an adult human male is about 0.2 mg/kg (MSM Research Information).
From CHEMid the following data on acute toxicity is found (National Technical Information Service. Vol. OTS0533525):
Rabbit, skin LD50 > 5000 mg/kg
Rat, oral LD50 > 5000 mg/kg
Sub-chronic toxicity
In a 90 day study with rats, the animals were orally exposed to 1.5 grams per kg per day. No adverse effects or increased mortality were observed (Methylsulfonylmethane, 2003).
Chronic toxicity
No data on long-term effects has been retrieved. The only references on long-term effects and dimethyl sulphone are tests trying to verify some positive health effects regarding for instance arthritis.
Summary
Dimethyl sulphone is not acute toxic. No data indicating long-term effects has been found. Potential positive health effects have not been assessed.
Based on the subchronic test with rats NOAEL is assessed to be at least 1,500 mg/kg b.w. per day.
5.3.2.5 Exposure scenarios
For dimethyl sulphone Log KOW is less than 4 and therefore, 100% of the substance is assumed absorbed through the skin.
The maximum content in a sample was 8.2 mg/kg.
Intake per kg b.w. per day = 0.0714 * 8.2 = 0.6 mg/kg b.w. per day
5.3.2.6 Assessment
Dimethyl sulphone does not cause allergy or irritations by skin contact. Data does not indicate any negative long-term effects. Based on the NOAEL the Marginal of Safety is more than 2,500 for the sample with the highest content of the substance.
Several articles indicate that there might be a positive health effect from dimethyl sulphone, but this is not assessed in this study.
It can be concluded that dimethyl sulphone in the amounts detected does not cause any negative health effect.
5.3.3 d-Limonene
5.3.3.1 Identity
| Name | d-Limonene |
| CAS-number | 5989-27-5 |
| EINECS number | 227-813-5 |
| Molecular formula | C10H16 |
Molecular structure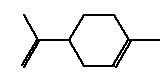 |
|
| Molecular weight | 136.23 |
| Synonyms | (+)-(4R)-Limonene (+)-4-Isopropenyl-1-methylcyclohexene (+)-Dipentene (+)-Limonene Citrene (+)-alpha-Limonene (+)-p-Mentha-1,8-diene |
d-Limonene is a liquid with a fresh citrus odour. The substance has a boiling point of 176°C (Budavari, 1989) and a melting point of -74.35°C (Lide, 1992).
The vapour pressure is 1.44 mmHg (Hansen and Eggert, 2003). Solubility in water is 13.8 mg/litre at 25°C. The partition coefficient Log KOW is measured to 4.57.
5.3.3.2 Detected quantities
d-Limonene was found in 9 products, sample 2 representing the highest amount of 19 mg/gram.
In sample E, 2.3 mg/gram was detected and samples 4, 8 and H held about 1 mg/gram. In samples nos. 1, 3, 15 and C the amount was less than 0.4 mg/gram.
5.3.3.3 Function
d-Limonene is used as a fragrance in cosmetics and as a flavouring agent in food and beverage.
d-Limonene is included in INCI as a fragrance.
5.3.3.4 Classifications and TLV’s
d-Limonene is included in the List of dangerous substances and classified as:
R10 Flammable
Xi; R38 Irritant; Irritating to skin.
R43 May cause sensitization by skin contact.
N; R50/53 Hazardous to the environment; Very toxic to aquatic organisms, may cause long-term adverse effects in aquatic environments.
A general TLV is given for terpenes, 25 ppm equivalent to 140 mg/m³ (C.0.1, 2005). No specific values are found for d-limonene.
The substance is included in the EU-list of allergenic perfume substances (SCCNFP, 1999).
5.3.3.5 Health Effects
d-Limonene is included in IUCLID, but the data sheet comprises relatively few data. The following is based on the data sheet, data bases in TOXNET and two previous survey reports, - one on printed matter (Hansen OC and Eggert T, 2003) and one on stain removers (Engelund et al, 2004).
Acute toxicity
Data for acute toxicity by ingestion is determined by LD50 to more than 4,000 mg/kg. This indicates no major potential health risk (Hansen and Eggert, 2003).
Oxidisation products of d-Limonene are strong allergens. A number of cases of contact allergy from occupational exposures to d-Limonene are reported. The frequency of contacts allergy to oxidised limonene is 1-2 % in several groups of eczema patients. The relationship between contact allergy to oxidised d-Limonene and fragrances in cosmetic products need to be further examined (SCCNFP, 1999).
Chronic toxicity
There is inadequate evidence for carcinogenicity in humans. There is evidence for carcinogenicity in animals, but the mechanism is not relevant for humans. Therefore d-Limonene is not classifiable as to its carcinogenicity to humans (Group 3) (IARC, 1999).
Data for NOAEL and LOAEL is included in the report on stain removers (Engelund et al, 2004). Data are given for ingestion with liver damage as the critical effect.
NOAEL : 250 mg/kg b.w. per day
LOAEL: 500 mg/kg b.w. per day
The type of test that the data are based upon (chronic or subchonic experiment) is not described in the report.
IUCLID does not provide data for estimating NOAEL or similar threshold limits for ingestion or dermal uptake. The same applies for the TOXNET data bases.
Summary
d-Limonene is a substance that by skin contact may cause allergy. It is not harmful by ingestion and no indications for long term effects have been found. NOAEL is 250 mg/kg (liver damages) and LOAEL is 500 mg/kg (liver damages).
5.3.3.6 Exposure scenarios
The maximum content in sample no. 2 was 19 mg per gram.
Intake per kg b.w. per day= 0.0714 * 19 = 1.4 mg/kg b.w.per day
For the other samples with a relative high content the daily intake will be from 0.07 to 0.18 mg/kg b.w.
Because of the relative high value of Log KOW (>4) it seems reasonable to expect that not 100% of the substance will be absorbed by skin contact.
5.3.3.7 Assessment
After oxidation of d-Limonene the substances formed are allergens. The content of d-Limonene may cause allergy.
NOAEL for liver damages is 250 mg/kg b.w. which gives a margin of safety of 175 for sample 2 and about 1500 and more for the other samples. As it is not identified whether the data is based on a subchonic or chronic experiment the safety factor must be set at 1000.
It is therefore assumed that there is a minor non sensitizing health risk for sample number 2 and a negligible health risk for the remaining samples.
It can be concluded that d-Limonene may cause allergies by skin contact at least in the samples containing more than 1 mg/gram corresponding to the limit in the Statutory Order on Classification and labeling, appendix 2, point 2.13 (sample no. 2, 4, 8, E).
5.3.4 Methyl salicylate
5.3.4.1 Identity
| Name | Methyl salicylate |
| CAS-number | 119-36-8 |
| EINECS number | 204-317-7 |
| Molecular formula | C8H8O3 |
Molecular structure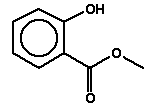 |
|
| Molecular weight | 152.14 |
| Synonyms | Hydroxybenzoic acid, methyl ester Benzoic acid, 2-hydroxy-, methyl ester 2-(Methoxycarbonyl)phenol 2-Carbomethoxyphenol 2-Hydroxybenzoic acid methyl ester Birch oil, sweet Methyl 2-hydroxybenzoate Oil of wintergreen Sweet birch oil |
Methyl salicylate is a colourless, yellowish or reddish oily liquid having a characteristic odour of wintergreen. The substance has a boiling point of 220-224° C and a melting point of -8.6° C (Budavari, 1989).
The partition coefficient Log KOW is measured to 2.55 (Hansch and Leo, 1987).
The solubility in water is 0.74 gram/litre at 30° C (Riddick et al, 1985). Methyl salicylate is soluble in most common organic solvents.
The vapour pressure is 0.0343 mmHg at 25° C (Daubert and Danner, 1989).
5.3.4.2 Detected quantities
Methyl salicylate is determined at very high quantities in 5 products:
Number 1: 51 mg/gram equivalent to 5.1 w%
Number 8: 50 mg/gram equivalent to 5.0 w%
Number 15: 45 mg/gram equivalent to 4.5 w%
Number E: 67 mg/gram equivalent to 6.7 w%
Number H: 76 mg/gram equivalent to 7.6 w%
In product number no. 2, 5.3 mg/gram equivalent to 0.5 w% is found and nothing in the remaining 6 samples.
5.3.4.3 Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
No TLV’s were found.
5.3.4.4 Health Effects
Methyl salicylate is included in the IUCLID database as well as in the TOXNET databases.
Acute toxicity
Data for acute oral toxicity LD50 is determined to be from 880 to 2,100 mg/kg for various types of animals. For dermal toxicity LD50 is determined to be from 700 to more than 5,000 mg/kg (IUCLID, 2000).
Symptoms of poisoning by methyl salicylate differ little from those described for aspirin. Central excitation, intense hyperpnoea, and hyperpyrexia are prominent features. The odour of the drug can easily be detected on the breath and in the urine and vomits. In children as little as 4 ml of methyl salicylate may be fatal (Gilman et al., 1990).
The following data on humans are given (IUCLID, 2000):
Oral LD50, adult 500 mg/kg child 170 mg/kg
Oral LDLO, adult 101-800 mg/kg child 228-700 mg/kg
Generally, ingestion of salicylates at doses larger than 150 mg/kg can produce toxic symptoms such as tinnitus, nausea, and vomiting. Serious toxicity can be seen with ingestions larger than 400 mg/kg, with severe vomiting, hyperventilation, hyperthermia, confusion, coma, convulsions, hyper- or hypoglycaemia, and acid-base disturbances such as respiratory alkalosis or metabolic acidosis. In severe cases, the clinical course may progress to pulmonary oedema, haemorrhage, acute renal failure, or death. It is important to note that the salicylate-overdose patient can progress to a more serious condition over time as additional drug is absorbed from the gastrointestinal tract. Chronic salicylism presents clinically in a similar fashion to the acute situation, although it is often associated with a higher morbidity and mortality as well as more pronounced hyperventilation, dehydration, coma seizures, and acidosis (Amadur et al, 1991).
With respect to pregnancy and effects on the fetus, it is shown that salicylates cross the placental barrier. A 33 week old fetus died in utero 20 hours after 3 gram salicylate was ingested by the mother. The salicylate level of the mother was 568 mg/l at admission and 212 mg/l at the time fetal heart tones stopped. The concentration in autopsy blood from the fetus, which aborted 8 days later, was 243 mg/l (Ellenhorn and Barceloux, 1988).
Tests have showed that methyl salicylate is irritating to skin and eyes when tested on animals (IUCLID, 2000). Humans’ ornaments or liniments should not be applied to burned or damaged skin (American Hospital Formulary Service, 1984). Absorption can occur through the skin. Death has resulted from systemic poisoning from local misapplication of the drug (Gilman et al, 1990).
Chronic toxicity
There is no evidence that moderate therapeutic doses of salicylates cause fetal damage in human beings; however, babies born by women who ingest salicylates for long periods may have significantly reduced weights at birth. In addition, there is an increase in prenatal mortality, anaemia, ante partum and postpartum haemorrhage, prolonged gestation, and complicated deliveries (Gilman et al, 1990).
Methyl salicylate given orally by capsules to dogs at a rate of 500-800 mg/kg per day was fatal in a month. Doses of 350 mg/kg per day could be fed for 2 years; loss in weight and enlargement of the liver were observed (Humpreys, 1988).
Methyl salicylate was found to be negative when tested for mutagenicity using the Salmonella/microsome preincubation assay, using the standard protocol approved by the National Toxicology Program (NTP). Methyl salicylate was tested in as many as 5 Salmonella typhimurium strains (TA1535, TA1537, TA97, TA98, and TA100) in the presence and absence of rat and hamster liver S-9, at doses of 1,000, 3,300, 10,000, 33,300, 100,000, and 333,300 ug/plate. The highest ineffective dose tested in any Salmonella typhimurium strain was 333,000 ug/plate (Mortelmans K et al. 1986).
Methyl salicylate is teratogenic in animals and can be absorbed in toxic quantities by the dermal route. Consequently, the dermal absorption and teratogenic potential of a petroleum-based grease (PBG) manufactured using methyl salicylate (3%) was assessed. The test material (petroleum based grease/methyl salicylate) was dermally applied at doses of either 0, 1, 3, or 6 g/kg/day to groups of pregnant rats on gestational days 6-15. The maternal and developmental No-Observable-Adverse-Effect-Level for petroleum based grease/methyl salicylate was greater than 6 g/kg/day (Infurna R et al; Teratology 41 (5): 566, 1990).
Prenatal exposure to methyl salicylate on kidney function in rats were studied. Pregnant female Sprague Dawley rats were treated with methyl salicylate by intraperitoneal injections between gestational days 10 and 14. Methyl salicylate exposure was teratogenic and embryotoxic. Prenatal exposure decreased fetal weight and increased the number of resorptions, fetal mortality, and the incidence of fetal malformations including ectopic kidneys. The primary postnatal renal defect associated with prenatal methyl salicylate treatment was a decreased urine concentrating ability in weanlings.
(Daston,1988)
Data on other long-term effects were not found.
Summary
Lowest dose causing lethality for humans is 100 mg/kg for adults. Methyl salicylate is irritating by skin and eye contact.
Methyl salicylate may be teratogenic by dermal absorption. The substance was teratogenic and embryotoxic by injection in rats. The maternal and developmental NOAEL for petroleum based grease/methyl salicylate was greater than 6 g/kg/day. Indications of other long-term effects were not found.
5.3.4.5 Exposure scenarios
The maximum content in sample no. H was 76 mg per gram.
Intake per kg b.w. per day = 0.0714 * 76 = 5.4 mg/kg b.w/day
For the other samples with a relative high content the daily intake will be from 3 to 5 mg/kg b.w.
Because of the relative low value of Log KOW (<4) it seems reasonable to expect that 100% of the substance will be absorbed by skin contact.
5.3.4.6 Assessment
In five of the products the content of methyl salicylate is so high that 3 to 5 mg per kg b.w. will be absorbed per day. Comparing this with the data on the lowest dose causing lethality for humans the Margin of Safety is only 20.
Methyl salicylate in pure form will cause irritation to skin and eyes (IUCLID, 2000). A product containing about 5 % (no. 1, 8, 15, E, H) may cause irritations.
Methyl salicylate may cause teratogenic effects. Comparing with a NOAEL of more than 6 g/kg b.w. the margin of safety is more than 1200 and therefore the risk for teratogenic effects for humans is negligible.
Based on LD50 =500 mg/kg for humans by oral intake there is a safety factor of 87. Based on this and assuming 100 % uptake by skin, there might be a risk of absorbing larger quantities through skin causing acute poisoning. Symptoms of poisoning by methyl salicylate include central excitation, intense hyperpnoea, and hyperpyrexia.
5.3.5 2-Phenoxyethanol
5.3.5.1 Identity
| Name | 2-Phenoxyethanol |
| CAS-number | 122-99-6 |
| EINECS number | 204-589-7 |
| Molecular formula | C8H10O2 |
Molecular structure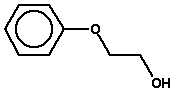 |
|
| Molecular weight | 138.16 |
| Synonyms | Hydroxy-2-phenoxyethane 2-Fenoxyethanol 2-Hydroxyethyl phenyl ether 2-Phenoxyethanol 2-Phenoxyethyl alcohol Ethylene glycol phenyl ether Arosol Dowanol EP Dowanol EPH EGMPE |
The substance 2-phenoxyethanol is a colourless liquid with a faint aromatic odour. Boiling point is 245.2°C and melting point is 14°C (Budavari, 1989).
The partition coefficient Log KOW is measured to 1.16 (Leo, 1985). 2-Phenoxyethanol is freely soluble in alcohol, ether and sodium hydroxide. The solubility in water is 26.7 gram per litre (Budavari, 1989).
The vapour pressure for 2-phenoxyethanol is measured to be 0.07 mm Hg at 25°C (Dow Chem Co, 1990).
5.3.5.2 Detected quantities
2-Phenoxyethanol was found in 9 products. The largest amount was found in sample no. 3, where 9 mg/g equal to 0.9 w% was detected. Other relevant findings were:
Sample no. 5 4.9 mg/g equal to 0.5 w%
Sample no. 8 0.68 mg/g equal to 0.07 w%
5.3.5.3 Function
2-Phenoxyethanol is used for a number of purposes. It is common as fixative for perfumes, as solvent for inks, textile dye carrier as, preservative and as bactericide.
The substance is included in INCI as a preservative and can be used in concentrations less than 1 percent in cosmetics.
5.3.5.4 Classifications and TLV’s
2-Phenoxyethanol is included in the List of dangerous substances and classified as:
Xn;R22 Harmful; Harmful if swallowed.
Xi;R36 Irritating; Irritating to eyes.
With respect to the Statutory Order on cosmetic products (Cosmetics, 2005) is can be used in up to 1 percent.
5.3.5.5 Health Effects
For 2-phenoxyethanol an IUCLID data-set is found, data in TOXNET and a description of the substance in Screening for health effects from chemical substances in textile colorants (Hansen OC, 2005).
Acute toxicity
In IUCLID a number of tests with rats where LD50 by oral exposure was determined are reported. The data range is between 1,200 mg/kg and 5,500 mg/kg. By dermal exposure LD50 was determined to 2,300 mg/kg and up to more than 10,000 mg/kg.
Several negative tests for skin irritation on animals are reported (IUCLID, 2000). Also a 3 week patch-test on humans did not cause irritations. Test on rabbits showed eye irritation. Several tests for sensitizing were reported - all with a negative result.
Sub-chronic toxicity
Several sub-chronic studies are reported in IUCLID. Some of these are briefly referred in the following:
- In a 13 week study with rats orally exposed NOAEL was determined to 200 mg/kg b.w. per day based on changes in blood parameters and weight loss.
- In another 13 week study with rats orally exposed NOAEL was determined to 80 mg/kg b.w. per day based on kidney damages.
- A 13 week study with dermal exposure to rabbits showed no adverse effects at the doses 50, 150 and 500 mg/kg per day. NOAEL was determined to be 500 mg per kg b.w. per day.
Chronic toxicity
Pregnant New Zealand white rabbits were treated dermally with 300, 600, or 1,000 mg/kg/day of undiluted 2-phenoxyethanol on days 6 through 18 of gestation (25 animals per dose group). 2-Phenoxyethanol was toxic to the dams (maternal death) at the 600 and 1,000 mg/kg doses. No adverse effects on pregnancy rate, resorptions, or fetal body measurements were observed at any dose. 2-Phenoxyethanol did not cause malformations in the fetuses as compared with controls (Scortichini et al, 1987).
2-Phenoxyethanol was tested for reproductive toxicity in Swiss CD-1 mice in a 2 generation test. The dose levels were 0.0, 0.25, 1.25, 2.5% in feed equal to 375, 1,875 and 3,700 mg/kg/day. 2-Phenoxyethanol produced significant reproductive and developmental toxicity. Liver weight increased in treated F0 mice. The substance caused significant toxicity in growing animals, as evidenced by the reduced body weight in neonates and the large increase in post-natal lethality as the F1 animals grew to the age of mating (Department of Health & Human Services, 1984).
Teratogenicity was evaluated in pregnant New Zealand White rabbits. They were (25/group) dermally exposed to 2-phenoxyethanol at treatment levels of 0, 300, 600, and 1,000 mg/kg/day on gestation days (GD) 6-18. Surviving animals were sacrificed on GD 28. Significant differences were observed between treated and control animals in the following: slight to moderate reddening of the skin at the application site (all treated animals), maternal mortality with dead animals exhibiting kidney damages, evidence of anorexia, changes in the gastric mucosa, decreased feed and fecal material in the intestines as well as changes in the blood parameters (high- and mid-dose groups). No significant differences were observed between treated and control animals in the following (mid- and low-dose groups unless otherwise noted. No statistical evaluations were performed on the five high-dose group rabbits which survived until GD 28. (Dow Chemicals, 1984).
Summary
The substance may cause irritation by eye contact and may be harmful by ingestion. NOAEL based on oral intake was determined to be 80 mg per kg b.w. per day based on kidney damages. Tests showed reproductive and developmental effects in long term studies with dermal exposure.
5.3.5.6 Exposure scenarios
The maximum content in sample no. 3 was 9 mg per gram.
Intake per kg b.w. per day = 0.0714 * 9 = 0.64 mg/kg b.w. per day
For sample no. 5 with a content of 4.9 mg/gram the intake per day will be 0.35 mg/kg b.w. per day whereas the intake per day for sample no. 8 will be 0.05 mg/kg b.w.
Log KOW is relatively low and it is therefore assumed that most of the substance will be absorbed through the skin.
5.3.5.7 Assessment
Sample no. 3 contains 0.9 percent 2-Phenoxyethanol.
Compared with NOAEL of 80 mg/kg b.w. per day for sample 3 it results in a margin of safety of MOS=125 and for sample 5 MOS is 229 and for sample no. 8 MOS is 1650.
As the data is based on a subchronic study, the safety factor for risk assessment is assumed to be at least 1000.
The products 3 and 5 may cause health problems within the margin of safety.
The content of 2-Phenoxyethanol is not expected to cause irritation by skin contact, but it may cause severe irritation upon contact with eyes.
5.3.6 α-Pinene
5.3.6.1 Identity
| Name | α-Pinene |
| CAS-number | 80-56-8 |
| EINECS number | 201-291-9 |
| Molecular formula | C10H16 |
Molecular structure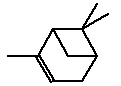 |
|
| Molecular weight | 136.24 |
| Synonyms | 2-Pinene 2,6,6-Trimethylbicyclo(3.1.1)-2-hept-2-ene 2,6,6-Trimethylbicyclo(3.1.1)hept-2-ene 4,6,6-Trimethylbicyklo(3,1,1)hept-3-en Bicyclo(3.1.1)hept-2-ene, 2,6,6-trimethyl Acintene A Monoterpenes |
α-Pinene is a colourless liquid with a characteristic odour of pine. The boiling point is 156°C and melting point is -62.5°C (Fenaroli, 1975).
Log KOW for α-Pinene is determined to 4.83 (Li and Perdue, 1995).
The solubility of the substance in water is 2.49 mg/l at 25°C. α-Pinene is soluble in alcohol, chloroform, ether and concentrated acetic acid. It is almost insoluble in propylene glycol and glycerine ( Fenaroli, 1975).
The vapour pressure of α-Pinene is 4.75 mm Hg at 25°C (Daubert and Danner, 1989).
5.3.6.2 Detected quantities
α-Pinene was detected in 8 products. The important findings were:
Sample 2: 12 mg/g equal to 1.2 w%
Sample 4: 1.7 mg/g equal to 0.2 w%
Sample 8: 27 mg/g equal to 2.7 w%
Sample 15: 19 mg/g equal to 1.9 w%
Sample E: 33 mg/g equal to 3.3 w%
Sample H: 22 mg/g equal to 2.2 w%
5.3.6.3 Function
α-Pinene has many functions and is widely used. Common uses are as solvent, emollient, in pesticides as base for synthetic oils and perfumes
The substance is included in INCI as a fragrance.
5.3.6.4 Classifications and TLV’s
α-Pinene is not classified in the Annex I of Directive 67/548/EEC. The substance is included in the Danish advisory list for selfclassification (Vejledende liste) with the classification:
R43 May cause sensitization by skin contact.
N;R50/53 .Hazardous to the environment; Very toxic to aquatic organisms, may cause long-term adverse effects in aquatic environments..
No TLV are given for α-Pinene. For terpenes in general like turpentine TLV in Denmark is 140 mg/m³ or 25 ppm (C.0.1, 2005). In USA, TLV as an 8 hour Time Weighted Average (TWA) is set to 20 ppm (ACGIH, 2003).
5.3.6.5 Health Effects
Data for α-Pinene is relatively limited. The following is based on a data set from IUCLID, the databases in TOXNET and a general search at the internet. A general search of terpenes is also included.
Acute toxicity
Acute oral toxicity is tested in studies with rats, which showed LD50 of 2,100 mg/kg and up to 5,100 mg/kg. Dermal toxicity based on test with rabbits showed results of LD50 of more than 5,000 mg/kg (IUCLID).
α-Pinene has essentially the same toxicity as turpentine (Gosselin et al, 1984).
Fatal dose for humans is about 180 gram orally as turpentine, which contains 58-65% α-Pinene (The Merck Index, 1976).
It is referred that α-Pinene irritates skin and mucous membranes and causes skin eruption and irritation of the respiratory system (Budavari, 1989). In IUCLID there are references to some tests on rabbits, mice and rats, where some were positive and some negative. A patch test on 5 humans tested with 10% α-Pinene in petrolatum for 48 hours showed no effects.
In IUCLID a test of eye irritation with the result moderately irritation is described. Another source states that α-Pinene is an eye, mucous membrane, and severe human skin irritant (Lewis, 1996).
Several tests on sensitizing on humans are reported (IUCLID, 2000). Most of the patch test showed that several people reacted positive. Turpentine oil, which normally has a high content of α-Pinene is labelled R43: May cause sensitization by skin contact.
Sub-chronic toxicity
In a 14 day test rats were orally exposed daily with 0, 250 and 500 mg/kg. In the group exposed to 500 mg per kg, reduced body weight and increased weight of liver was observed.
No relevant data for turpentine oil was found.
Chronic toxicity
No data on chronic toxicity for α-Pinene were found.
From OSHA’s Health Guidelines (2005) the following information has been retrieved for turpentine and it is assumed to be valid for α-Pinene as well.
- In one study, dermal application of turpentine produced skin tumours in rabbits but not in mice ; in another experiment, however, painting the skin of mice with 240 g/kg turpentine did cause tumours.
- Turpentine is a skin, eye, mucous membrane, and upper respiratory tract irritant in humans. It may also cause skin sensitization and central nervous system, gastrointestinal, and urinary tract effects. The lowest estimated oral dose reported to be lethal in humans is 441 mg/kg.
- A case-control study of workers in particle-board, plywood, sawmills, and formaldehyde glue factories demonstrated a statistically significant association between chronic exposure (longer than 5 years) to terpenes (the principal component of turpentine) and the development of respiratory tract cancers.
Summary
α-Pinene causes irritation by skin and eye contact and may cause allergies by skin contact.
Data on long-term effects are very limited and risk of cancer and other long-term effects from skin contact and ingestion are very uncertain.
Based on the one sub-chronic test NOAEL is determined to be 250 mg/kg with increased liver weight as the critical effect.
5.3.6.6 Exposure scenarios
The maximum content in sample no. E was 33 mg/gram.
Intake per kg b.w. per day= 0.0714 * 33 = 2.4 mg/kg b.w. per day
Intake for the other products is between 0.1 to 1.9 mg/kg b.w.
5.3.6.7 Assessment
Six samples (2, 4, 8, 15, E and H) contain more than 0.1w% α-Pinene. α-Pinene is a potential skin sensitizer and therefore there is a substantial risk of allergic reaction when contact with these products.
NOAEL is estimated to 250 mg/kg based on limited data.
As the data is based on a subchronic study, the safety factor for risk assessment is assumed to be at least 1,000.
The margin of safety is from 100-300 for products 2, 8, 15, E, and H and above 1,000 for the rest. Taken the limited data and the MOS into account it can be concluded that there is a possible risk by uptake through skin of the substance.
5.3.7 Solvents
A special focus has been directed towards the content of organic solvents, because it is expected that the evaporation of these substances causes the cooling effects in some of products.
5.3.7.1 Substances and amounts
All the selected products have been tested for content of organic solvents. In the analysis the detection limit was 0.1 %. The results are shown in Table 5.1. It shows that solvents were found only in 4 products.
Table 5.1Content of solvents in sports products
| Solvent 1 | CAS-number | Product 4 | Product 5 | Product 12 | Product C |
| Ethanol | 64-17-5 | 54 % | 2,2 % | 14 % | |
| 2-Propanol | 67-63-0 | 27 % | |||
| Acetone | 67-64-1 | 1.9 % | |||
| tert-Butyl alcohol | 75-65-0 | 0.35 % |
1 Toluene was also found but in ppm level (<0,0005%)
5.3.7.2 Physical properties
Relevant data for assessing the solvents with respect to evaporation and cooling effects is shown in Table 5.2.
Table 5.2 Physical properties for solvents in sports products
| Solvent | CAS-number | Vapour pressure mmHg | Heat of Vaporization J/ gram |
| Ethanol | 64-17-5 | 59.3 | 878 |
| 2-Propanol | 67-63-0 | 45.4 | 757 |
| Acetone | 67-64-1 | 232 | 510 |
| tert-Butyl alcohol | 75-65-0 | 40.7 | 528 |
As shown in Table 5.2, the vapour pressure is about the same for ethanol, 2-propanol and t-butyl alcohol. For acetone, the vapour pressure is about 4 or 5 times higher, which means that this substance will have the fastest rate of evaporation.
The amount of energy (heat) necessary for evaporation is expressed by the term “Heat of evaporation”. When 1 gram of ethanol is evaporated 878 J is removed and this is felt as the cooling effect. The total cooling effect is the amount of solvent that evaporates times the heat of evaporation. The evaporation per time unit is not known and therefore the cooling effect per time unit cannot be determined.
The total cooling effect based on the use of 5 grams of product is shown in Table 5.3.
Table 5.3: Potential cooling effect based on content of solvents in sports products
| Solvent | CAS-number | Content in 5 gram | |||
| Product 4 | Product 5 | Product 12 | Product C | ||
| Ethanol | 64-17-5 | 2.6 g | 0.11 g | 0.70 g | |
| 2-Propanol | 67-63-0 | 1.35 g | |||
| Acetone | 67-64-1 | 0.10 g | |||
| tert-Butyl alcohol | 75-65-0 | 0.02 g | |||
| Energy per 5 gram product | 2282 J | 97 J | 1022 J | 676 J | |
As shown in Table 5.3 the potential cooling effect is very different for the 4 products.
5.3.7.3 Classification and TLVs
The classification and the TLV’s for the four solvents are shown in Table 5.4.
Table 5.4 Classification for solvents in sports products
| Solvent | CAS-number | Classification | TLV mg/m³ | INCI function |
| Ethanol | 64-17-5 | F;R11 | 1900 | Solvent |
| 2-Propanol | 67-63-0 | F;R11 Xi;R36 R67 |
490 | Fragrance Anti foam agent Solvent |
| Acetone | 67-64-1 | F;R11 Xi R36 R66 R67 |
600 | Denaturants / Solvent |
| tert-Butyl alcohol | 75-65-0 | F;R11 Xn;R20 |
150 | Fragrance |
Table 5.4 shows that all the solvents are flammable, and that two substances are irritating and one is harmful by ingestion. Two substances are marked with R67 and the substances may therefore cause dizzyness by direct inhalation.
The threshold limit values are relatively high for all for solvents when compared to substances with greater health risks like chloroform (10 mg/m³) and formaldehyde (0.4 mg/m³)
5.3.7.4 Health effects
The solvents will be absorbed into the body either by absorption through the skin or by inhalation. No data indicate that the solvents cannot be absorbed and therefore it is assumed that 100 % may be by either route.
Data on acute toxicity for the four solvents are retrieved from Chemid:
Inhalation
| Ethanol | LC50, rat | 20,000 mg/kg | |
| 2-Propanol | LC50, rat | 16,000 mg/kg | |
| Acetone | TCLo, man | 12,000 mg/kg, | nausea or vomiting, muscle weakness |
| LC50, rat | 5,500 mg/kg | ||
| t-Butyl alcohol | LC50, rat | 10,000 mg/kg |
Skin
| Ethanol | LDLo, rabbit | 20,000 mg/kg |
| 2-Propanol | LD50, rabbit | 12,800 mg/kg |
| Acetone | LD50, guinea pig | 9,400 mg/kg |
| LDLo, rabbit | 20,000 mg/kg | |
| t-Butyl alcohol | LD50, rabbit | >2,000 mg/kg |
| LD50, guinea pig | >10,000 mg/kg |
From the data given on inhalation and skin absorption it can be seen that there is no potential acute health risk when comparing the criteria for classification of chemical substances.
The classification for the four solvents does not include any long-term effects, although, the four solvents are well known for causing CNS-damages (Central nervous System) when exposed repeatedly to low concentrations and/or for a long time.
No data on concentrations or uptake has been found for showing when long term health effects occur.
5.3.7.5 Exposure assessment
The assessment of the potential health risk by inhalation is based on the Danish TLV’s and the total amount that may evaporate.
- One product contains 54 % ethanol. If 5 grams of product is used per person, it means that 2600 mg ethanol may evaporate.
If all ethanol evaporates momentarily in more than 1.5 m³ of air, the threshold limits will be observed.
Therefore, although more people are gathered and use the product at the same time, ethanol will not cause any short-term health risks.
- Tert-butyl alcohol is found in product no. C in the amount of 0.35 % equivalent to 17 mg in 5 gram.
This amount cannot cause the concentration to rise above the threshold limit value.
Based on the two examples it can be concluded that the amount of vapours from the products will not exceed the threshold limits at the workplace (8 hours daily). Therefore it is assessed that exposure by inhalation will cause a negligible health risk.
Assessing the potential health by skin contact it is assumed that the weight of an adult is 70 kg and that 5 gram of product is used per day. The assessment is based upon acute toxicity data and shown in Table 5.5.
Table 5.5 Assessing skin absorption of solvents in sports products
| Solvent | CAS-number | Potential amount absorbed by skin contact 70 kg adult using 5 gram [mg/kgb.w.] |
LD50, rabbit mg/kg |
MOS Margin of safety |
|||
| Product 4 | Product 5 | Product 12 | Product C | ||||
| Ethanol | 64-17-5 | 37 | 2 | 10 | >20,000 | > 500 | |
| 2-Propanol | 67-63-0 | 19 | 12,800 | 670 | |||
| Acetone | 67-64-1 | 1,4 | >20,000 | >14,000 | |||
| tert-Butyl alcohol | 75-65-0 | 0.25 | >2,000 | >8000 | |||
Based on the data in Table 5.5 it can be concluded that the MOS for ethanol and 2-propanol in product no. 4 and 12 are relatively low taken into account that acute toxicity-data are used.
The solvents in product 4 and 12 may cause a minor potential health risk, whereas in product 5 and C the amounts are so limited that they will cause no health risk.
Conclusion
Intake of solvents by inhalation will not cause any health risk. Uptake by skin absorption may cause a minor potential heath risk for product 4 and 12.
2-Propanol in product 12 may cause irritation.
5.4 Overall Assessment
5.4.1 Substances
In the following section an overview of the assessment of the substances in section 5.3 is given. Data in the tables are given for the samples with the highest concentration of the actual substance.
Table 5.6 Irritation and allergy effects for selected substances in sports products
| Substance | Max. Conc. W% | Irritation to skin | Sensitization by skin contact | Remarks |
| Camphor | 7.7 | Potential effect | Potential effect | With the content there is a risk of both irritation and sensitization in products no. 2, 3, 4 and 12. |
| Dimethyl sulphone | 0.82 | No effect | No effect | No risk |
| d-Limonene | 1.90 | No data | Potential effect | Risk of allergies in the samples containing more than 1 mg/gram (product no. 2, 4, 8, E) |
| Methylsalicylate | 7.60 | Potential effect | No data | Products containing about 5 % (no. 1, 8, 15, E, H) may cause irritations |
| 2-Phenoxyethanol | 0.9 | No effect | No effect | No risk |
| α-Pinene | 3.30 | Potential effect | Potential effect | Risk of irritation and sensitization. Six products (2, 4, 8, 15, E and H) contain more than 0.1w% |
The solvents, 2-propanol and acetone may cause irritations. This will mostly be relevant for product no. 12. None of the solvents are known for causing allergies.
Table 5.7 Toxic effects for selected substances in sports products
| Substance | Max uptake mg. per kg b.w. | NOAEL mg/kg b.w. per day | MOS | Remarks |
| Acetone | 1 | >20000 | >20000 | No risk of health effects. |
| t-Butyl alcohol | 0.3 | >2000 | >6667 | No risk of health effects. |
| Camphor | 5.5 | 680 | 123 | There is a minor risk of health teratogenic effects from dermal exposure for 5 g of product 2. |
| Dimethyl sulphone | 0.6 | >1500 | >2500 | The amounts detected do not cause any negative health effect. |
| Ethanol | 37 | >20000 | >500 | No risk of health effects because most will evaporate. |
| d-Limonene | 1.4 | 250 | 175 | Minor health risk for product no. 2 (effects on liver) |
| Methylsalicylate | 5 | >6000 | >1200 | Minor risks of health effects. |
| 2-Phenoxyethanol | 0,64 | 80 | 125-229 | Health risk for the products 3 and 5. |
| α-Pinene | 2.4 | 250 | 100 | Based on the limited data it can be concluded than the health risk is relatively high for product no.2,8,15,E,H (increased liver weight) |
| 2-Propanol | 19 | 12800 | 670 | No risk of health effects because most will evaporate. |
From Table 5.7 it can be seen that the most problematic substances with respect to health effects caused by skin absorption are Camphor, 2-Phenoxyethanol, α-Pinene and d-Limonene.
5.4.2 Products
Among the 12 tested products two products, number 2 and 12 shall be classified and labelled according to the Statutory Order on classification of chemicals. The products 4, 8 and E shall also be labelled with a sentence specifying the content of allergens and that an allergic reaction may arise
Irritation effected are found in Camphor, Methylsalicylate, 2-Phenoxyethanol and 2-Propanol.
- Camphor causes a risk of irritation, product no. 2.
- Methylsalicylate may cause irritation, products nos. 1, 8, 15, E and H
- 2-Propanol may cause irritation, product no. 12
Regarding allergies and sensitization the substances d-Limonene, Camphor and α-Pinene are potential allergens.
- d-Limonene may cause sensitization and are found in more than 0.1% in product no. 2, 4, 8 and E.
- Camphor may cause sensitization and are found in more than 0.1% in no. 2, 3, 4 and 12.
- α-Pinene may cause sensitization and are found in more than 0.1% in no. 2, 4, 8, 15, E and H.
The products 1, 5 and 13 contain no potential allergens.
Some of the substances may cause potential health risk by skin absorption. The most problematic substances are Camphor, 2-Phenoxyethanol, α-Pinene and d-Limonene.
- Camphor was found in relatively large amounts in product 2.
- α-Pinene was found in amounts of 1-3% in products nos. 2, 8, 15, E and H.
- d-Limonene was found in 1-2% in product no. 2.
- Ethanol and 2-Propanol was found in products nos. 4 and 12, but it is assumed that the health effect is minimal because most of the substances evaporate.
The overall conclusion of the health assessment is:
- Product nos. 2, 8, , 12, 15, E and H contain potential irritating and sensitizing substances as well as substances with the potential of causing health risks by dermal uptake.
- Product nos. 1, 2, 3, 4, 8, 12, 15, E and H contain potential irritating substances.
- Product nos. 2, 3, 4, 8, 13, 15, C, E, and H contain potential sensitizing substances in concentrations larger than 0.001 wt%
- Two products (nos. 2 and 12) have to be classified and labelled for health effects.
- Product nos. 4, 8 and E have to be labelled: Contains "substance name(s)". May cause allergic reactions.
Version 1.0 Decmeber 2006, © Danish Environmental Protection Agency