Survey of Chemical Substances in Consumer Products, No. 82, 2007
Survey and health assessment of selected respiratory sensitizers in consumer products
Contents
2 Inventory of consumer products, which may contain any of the five R42 substances
- 2.1 Glutaraldehyde, CAS 111-30-8
- 2.2 Cyclohexane-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
- 2.3 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
- 2.4 Methyltetrahydrophthalic anhydride, CAS 11070-44-3
- 2.5 Methylene diphenyl diisocyanate, CAS 26447-40-5; 5873-54-1; 101-68-8
- 2.6 Results of inquiries
- 2.7 Results of search for products with likely emission of the five substances
3 Results of emission analysis from selected consumer products
- 3.1 Analysis of MDI emission from various consumer products
- 3.1.1 Sample treatment and collection
- Polyurethane one component sealant and adhesive
- Floor adhesive
- Hair conditioner
- Hair spray with polyurethane as declared ingredient
- Polyurethane rain coat
- Foam mattress and spring mattress
- 3.1.2 Method of analysis
- Extraction
- Chromatography
- 3.1.3 Calculation of detection limit
- 3.1.4 Results
- 3.2 Analysis of phthalic anhydride derivatives in consumer products
- 3.3 Analysis of glutaraldehyde in consumer products
4 Exposure and risk assessment
- 4.1 Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
- 4.2 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
- 4.3 Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
- 4.4 Methylenediphenyldiisocyanate, CAS 26447-40-5, 5873-54-1 and 101-68-8
- 4.5 Glutaraldehyde, CAS 111-30-8
Foreword
This project was carried out with DTC Health and Environment, a business unit of
DHI Water and Environment, as project leader, and NERI (National Environmental Research Institute) as partner.
DTC carried out the initial search for relevant products containing respiratory sensitizers.
Project assistant Hanne Sørensen, DTC, made the tracking and purchases of most of the products analyzed.
Toxicologist Inge Søborg reviewed the first phase report, and Chief toxicologist Karl-Heinz Cohr carried out the quality control of the final report.
Secretary Vibeke Salmon carried out the text editing and translation.
At the NERI, Senior Scientist Betty Bügel Mogensen, planned, executed and reported the chemical emission analysis with the aid of laboratory technicians Inga Jensen and Kitty Petersen. Quality control on the performed analyses was carried out by Betty Bügel Mogensen.
The project was carried out for the Danish Environmental Protection Agency (DEPA) on contract no. 7041-0513 under the programme for surveys on chemical substances in consumer products.
The primary contact person in DEPA for this project was Annette Ejersted, who was initially seconded by Lea Frimann Hansen, and later Frank Jensen.
The project was initiated in March 2006 with final delivery in November 2006.
Summary
In 2004, the Danish EPA issued a revised list of unwanted substances (Orientering fra Miljøstyrelsen, nr. 8, 2004). The criteria for inclusion in this list were among others the classification of the substances. One of the criteria was classification R42: “May cause sensitization by inhalation”. Five substances are on the list exclusively because of this classification:
Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
Methylendiphenyldiisocyanate (MDI), CAS 26447-40-5, 5873-54-1 and 101-68-8
Glutaraldehyde, CAS 111-30-8
The purpose of the present project was to
- Make an inventory of consumer product types that may contain any of the five R42-substances.
- Examine, if consumers actually are exposed to the substances when using such products.
- Assess, if the exposure is large enough to cause health effects in the consumers
The inventory below was created by searching SPIN – database on substances in preparations in Nordic countries, the INCI list, and data from earlier projects on consumer products. In addition, five trade associations and retail stores were asked about their knowledge on the matter, and the literature was searched for cases of respiratory sensitization due to any of the five substances in consumer products.
All in all, 19 products were sampled for inclusion in an emission analysis. The number of products within each group is given in parenthesis.
The following consumer products may contain phthalic anhydride derivatives:
- Nail Lacquer (6)
- Two-component epoxy adhesive (2)
The following consumer products may contain glutaraldehyde:
- Cosmetic products (mouth washes and creams) (0)
- Disinfectants (0)
- Film developers for consumers (0)
- Paper handkerchiefs and toilet paper (2)
The following consumer products may contain monomeric MDI:
- PU-foam articles, such as mattresses (2)
- One component spray foam or adhesive (2)
- Boat and car repair kits (1)
- Two-component adhesives and putties (0)
- Liquid roof coating (0)
- Hot melt adhesives (0)
- Floor adhesives (1)
- Polyurethane materials in clothing (1)
- Spray hair fixatives and conditioner (2)
When searching the market it was not possible to obtain cosmetic products, disinfectants, nor film developers with glutaraldehyde. Neither was it possible to obtain two-component adhesives and putties, liquid roof coating, nor hot melt adhesives with a content of monomeric MDI.
All purchases were carried out while pretending to be ordinary consumers, not professionals. Although some of the outlets visited are also used by professionals, we were not offered products intended only for professionals. This indicates a fairly good separation between the markets for consumers and the markets for professionals, at least pertaining to these kinds of products.
Emissions from the 19 products were analyzed under realistic and, in some cases also extreme use conditions. No emissions of the respiratory sensitizers from the 19 products obtained could be detected by chemical analysis. Hence, no risk of sensitisation to phthalic anhydrides, MDI or glutaraldehyde can be attributed to any of the products analyzed.
Introduction
In 2004, the Danish EPA issued a revised list of unwanted substances (Orientering fra Miljøstyrelsen, nr. 8, 2004). The criteria for inclusion in this list were among others the classification of the substances. One of the criteria was classification R42: “May cause sensitization by inhalation”. Five substances are on the list exclusively because of this classification:
Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
Methylendiphenyldiisocyanate (MDI), CAS 26447-40-5, 5873-54-1 and 101-68-8
Glutaraldehyde, CAS 111-30-8
Exposure to these five substances through consumer products is virtually unknown, and the Danish EPA needs more knowledge of the use of these substances in consumer products, and the emission of the five substances from products containing the substances.
The purpose of the present project was to
- Make an inventory of what kind of consumer products may contain any of the five R42-substances.
- Examine, if consumers are actually exposed to the substances when using such products.
- Assess, if the exposure is large enough to cause health effects in the consumers
Products, which are exclusively used in the occupational setting and lead only to occupational exposure, are outside the scope of this project.
The final goal of the project is to present an assessment of the health risk of the consumers from the five R42 substances.
The project was divided into two phases:
- Inventory of the kind of consumer products, which may contain any of the five R42 substances
- Exposure assessment and assessment of health risks
1 Methods
For the phase 1 survey the occurrence of the five substances has been searched in the sources given below.
Data on the use of the five respiratory sensitizers have been collected in SPIN – database on substances in preparations in Nordic countries.
The INCI list (International Nomenclature of Cosmetic Ingredients) has been searched for occurrence of the five substances.
In addition, data have been retrieved from earlier projects on consumer products published on the web page of the Danish Environmental Protection Agency.
The following trade associations and retail stores have been requested to provide available data on the use of some or all of the five substances:
Plastindustrien i Danmark
Danmark Farve- og Lakindustri
COOP Danmark
Dansk Supermarked
Association of Danish Cosmetic, Toiletries, Soap and Detergent Industries (SPT)
Glutaraldehyde has been searched on the Internet via Google using the search words ”disinfection” and ”glutaraldehyde”.
In the database PubMed, additional searches have been made for cases of respiratory sensitization due to any of the five substances in consumer products.
After having established the type of consumer products most likely containing the five substances, products have been searched and sampled by personal purchase in retail outlets, both physically and on the Internet.
The purchased products were delivered to the National Environmental Research Institute for emission analysis.
2 Inventory of consumer products, which may contain any of the five R42 substances
- 2.1 Glutaraldehyde, CAS 111-30-8
- 2.2 Cyclohexane-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
- 2.3 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
- 2.4 Methyltetrahydrophthalic anhydride, CAS 11070-44-3
- 2.5 Methylene diphenyl diisocyanate, CAS 26447-40-5; 5873-54-1; 101-68-8
- 2.6 Results of inquiries
- 2.7 Results of search for products with likely emission of the five substances
2.1 Glutaraldehyde, CAS 111-30-8
Synonyms: Glutaral; 1,5 pentanedial
The SPIN database has registered 428 preparations with glutaraldehyde in Finland, Norway, Sweden and Denmark in 2003. Of these, 139 preparations are registered in Denmark. Some of the 428 preparations are registered as consumer preparations in Norway and Sweden. The industrial use for which the preparations are registered in Denmark is:
- Farming of animals
- Hospital activities
- Human and health services
- Manufacture of paints, varnishes and similar coating, printing inks and mastics
- Manufacture of food and beverages
- Dentists
- Painting
- Manufacture of machinery and equipment
- Other motor vehicle services
- “All kinds of activities”
- Private households with employed persons
The use category for which the preparations have been registered in Denmark is:
- Non-agricultural pesticides and preservatives
- Biocides – pesticides for non-agricultural uses
- Reprographic agents
- Photochemicals
- Developers – for developing pictures, but not photographic film
- Paints, lacquers and varnishes
- Colouring agents
- Cleaning/washing agents
- Disinfecting agents
Glutaraldehyde is permitted in cosmetics as a preservative (no. 26, VI, 1,48). The maximum permitted concentration is 0.1% and it is banned in aerosol and spray products. Labelling with “Contains glutaraldehyde“ is mandatory, if the concentration in the final products exceeds 0.05%.
The INCI name for glutaraldehyde is Glutaral.
The search on the internet by Google for disinfectants containing glutaraldehyde gave only products intended for use in food industry and agriculture. No sale to private consumers could be identified.
A search for glutaral on the internet by Google turned up one body lotion containing Glutaral. This body lotion was sold from a French internet page, claiming that it helps against staphylococci adherence to the skin.
A search among the previous surveys of chemical substances in consumer products revealed that glutaraldehyde residues may be present in paper handkerchiefs and toilet paper in concentrations of up to 0.08 kg/t (1).
An additional search for cases of respiratory allergy resulting from exposure to glutaraldehyde emission from consumer products was carried out in the database PubMed. Several cases of asthma resulting from occupational exposure to glutaraldehyde containing disinfectants were found, but none were related to private use. Cases of exposure to glutaraldehyde in the private setting, were only found in relation to contact dermatitis, but not to respiratory hypersensitisation.
2.1.1 Conclusion
With regard to glutaraldehyde containing consumer products, we should look for:
- Cosmetic products (mouth washes and creams)
- Disinfectants
- Film and picture developers for consumers
- Paper handkerchiefs and toilet paper
2.2 Cyclohexane-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
Synonyms: hexahydro-1,3-isobenzofurandione
hexahydrophthalic anhydride
The SPIN database has registered 57 preparations with cyclohexane-1,2-dicarboxylicanhydride (CDA) in the Nordic countries in 2003. Of these, 38 preparations are registered in Denmark. None of the 57 preparations are registered as consumer preparations. The industrial use for which the preparations are registered in Denmark is:
- Manufacture of other transport equipment
- Sale, maintenance and repair of motor vehicles and motor cycles; retail sale of automotive fuel
- Manufacture of aircraft and spacecraft (includes repair of air planes (2))
- Motor vehicle painters
The use category for which the preparations have been registered in Denmark is:
- Paints, lacquers and varnishes
In Sweden, CDA is also used for curing agents in plastic.
Hence, CDA may occur as a residual monomer in consumer products.
In cosmetics, CDA may occur as a monomer in different film forming polymers used in e.g. nail lacquer. CDA as such is not found on the INCI list. In a declaration, it might be listed as phthalic anhydride in the kind of polymer used, e.g. Phthalic Anhydride/Benzoic Acid/Trimethylolpropane Copolymer.
We do not know if CDA or only phthalic anhydride is actually employed in the phthalic anhydride copolymers.
In 2002, the information centre for Environment and Health published a list of products containing unwanted substances. Among these were nine nail lacquers containing phthalic anhydride according to the product declaration (3).
An additional search in PubMed for cases of respiratory sensitization gave several hits indicating that occupational exposure to epoxy resins and their hardeners is a common reason for respiratory sensitization to CDA (4,5,6).
No cases of CDA allergy from private use of epoxy products were found in the PubMed database. However, as two-component epoxy products are available for private use, some of these were sampled for CDA emission testing.
2.2.1 Conclusion
With regard to CDA containing or –emitting products, two-component epoxy adhesives and nail lacquer were considered relevant for emission analysis.
2.3 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
Synonyms: Hexahydro-4-methylphthalsyreanhydrid,
Hexahydro-5-methyl-1,3-isobenzofurandione
In 2003, the SPIN database has registered 3 preparations with hexahydro-4-methylphthalic anhydride (HMPA) in the Nordic countries. None of these were registered in Denmark. In fact, all three were registered in Sweden, and none of these were consumer preparations.
Industrial use categories are:
- Manufacture of chemicals and chemical products
- Manufacture of rubber and plastic products
- Manufacture of electrical machinery and apparatus
- Heat transferring agents
- Adhesives, binding agents
Hence, HPMA may occur as a residual monomer in consumer products.
In cosmetics, HPMA may occur as a monomer in different film forming polymers used in e.g. nail lacquer. HPMA as such is not found on the INCI list. On a declaration, it might be listed as Phthalic anhydride in the kind of polymer used, e.g. Phthalic Anhydride/Benzoic Acid/Trimethylolpropane Copolymer.
We do not know if HPMA or only phthalic anhydride is actually employed in the phthalic anhydride copolymers.
In 2002, the information centre for Environment and Health published a list of products containing unwanted substances. Among these were nine nail lacquers, which contained phthalic anhydride according to the product declaration (3).
An additional search in PubMed for cases of respiratory sensitization gave a few hits indicating that occupational exposure to epoxy resins, their hardeners, and unsaturated polyester resins (UP resins) is a common reason for respiratory sensitization to HPMA (5,7,8). No consumer product related cases were found during this search.
2.3.1 Conclusion
With regard to HPMA containing or –emitting products, we decided to sample
two-component epoxy adhesives and nail lacquer.
2.4 Methyltetrahydrophthalic anhydride, CAS 11070-44-3
Synonyms: MTHPA
tetrahydromethyl-1,3-isobenzofurandione
In 2003, the SPIN database has registered 5 preparations with methyltetrahydrophthalic anhydride (MTHPA) in 2003. None of these were registered in Denmark. In fact, all five were registered in Sweden, and none of these were consumer preparations.
Industrial use categories are:
- Manufacture of chemicals and chemical products
- Manufacture of rubber and plastic products
- Manufacture of electrical machinery and apparatus
- Process regulators
- Heat transferring agents
- Curing agents for plastic
- Others not mentioned specifically
Hence, MTHPA may occur as a residual monomer in consumer products.
In cosmetics, MTHPA may occur as a monomer in different film forming polymers used in e.g. nail lacquer. MTHPA as such is not found on the INCI list. On a declaration, it might be listed as phthalic anhydride in the kind of polymer used, e.g. Phthalic Anhydride/Benzoic Acid/Trimethylolpropane Copolymer.
We do not know if MTHPA or only phthalic anhydride is actually employed in the phthalic anhydride copolymers.
In 2002, the information centre for Environment and Health published a list of products containing unwanted substances. Among these were nine nail lacquers containing phthalic anhydride according to the product declaration (3).
An additional search in PubMed for cases of respiratory sensitization gave a few hits indicating that occupational exposure to epoxy resins, their hardeners, and unsaturated polyester (UP) resin is a common reason for respiratory sensitization to MTHPA (5,7,8). No consumer product related cases were found during this search.
2.4.1 Conclusion
In conclusion, with regard to MTHPA containing or –emitting products, we decided to sample two-component epoxy adhesives and nail lacquer.
2.5 Methylene diphenyl diisocyanate, CAS 26447-40-5; 5873-54-1; 101-68-8
Synonyms: 1,1’-methylenebis(isocyanato)benzene,
MDI
In 2003 The SPIN database has registered 308 preparations with MDI in The Nordic countries. Of these, 148 preparations are registered in Denmark. Some of the 308 preparations are registered as consumer preparations in Norway and Sweden. The industrial use for which the preparations are registered is:
- Construction
- Manufacture of motor vehicles, trailers and semi-trailers
- Manufacture of basic metals
- Manufacture of furniture
- Manufacture of rubber and plastic products
- Manufacture of wood and products of wood and cork, except furniture; manufacture of articles of straw and plating materials
- Wholesale trade and commission trade, except of motor vehicles and motorcycles
- Private households with employed persons
- Sale, maintenance and repair of motor vehicles and motorcycles; repair of personal and household goods
- Manufacture of electrical machinery and apparatus
- Manufacture of pulp, paper and paper products
- Manufacture of chemicals and chemical products
- Manufacture of other transport equipment
- Floor and wall covering except floor planning
- Joinery installation
- Painting
The use categories for preparations registered in Denmark are:
- Paints, lacquers and varnishes
- Adhesives, binding agents
- Curing agents
- Construction materials
- Process regulators
- Fillers
- Flooring materials (joint-less floors)
Deeming from this, MDI or residues of MDI might be emitted from consumer products, such as boat and car repair kits, furniture (mattresses), two-component adhesives and putties, flooring and wall paper. Polyurethane materials in clothing may also emit MDI.
Secondary exposure to MDI may occur when polyurethane materials are processed by grinding or heating (9).
MDI has been subject to an EU risk assessment, published in 2005 (10). One of the conclusions for consumers was that there is a need for limiting risks, since health risks due to combined occupational and consumer exposure could not be excluded with regard to sensitization (dermal contact and inhalation exposure).
In the EU risk assessment report, possible exposure to free MDI from the following products has been identified:
- Spray (PU-foam)
- Putty/filler in cartridge
- Liquid glue for wood
- Paints
Spray foam or One Component Foam (OCF): MDI-based OCF is offered in the consumer market and to professional tradesmen for use as a filler in small gaps in buildings (e.g. around window frames, between floor boards etc). In this context, the word ‘spraying’ is not entirely appropriate. The OCF is supplied in pressurised cans and is applied through a pre-expansion tube (always part of the package). The product is released from the nozzle as viscous foam, rather than as a sprayed aerosol. Curing starts immediately and moves from the outside inwards. Therefore, emission and hence potential exposure virtually ceases once the outer coat has been cured. However, Sweden has a warning when using the one-component frothed foam.
PU wood adhesives are used for waterproof bonding and on moist wood. Flooring adhesives are used for wood-parquet.
PU paint is used as a primer for liquid roof coating, with a long in-service life (10 to 15 years), and for decorative painting. It is confirmed by industry that hot melt adhesives are currently offered to the D.I.Y. (do-it-yourself) market. According to industry, even 2-component products are offered to the D.I.Y. market. One company stated:
“Moreover, other products normally offered only to the craftsmen can reach end-consumers by self-service at craftsmen retailers”. However, it is very unlikely that the consumer’s working conditions are ever appropriate for the use of 2-component products containing free MDI (10).
A search in the INCI list for the three CAS numbers connected with MDI showed no result. A second search on “MDI” gave three copolymers, in which MDI may have been used, and may therefore emit residual monomer:
PEG-8/SMDI copolymer, CAS 39444-87-6
PPG-12/SMDI copolymer, CAS 9042-82-4
PPG-51/SMDI copolymer, CAS 9042-82-4
These substances are copolymers of the respective PEG’s and saturated MDI monomer. They are used as hair conditioning agents, hair fixative, plasticizer, skin-conditioning agent (emollient and miscellaneous), in eye shadows, foundations, miscellaneous makeup preparations , and moisturizing preparations (11).
Since the present project deals with the substances as respiratory sensitizers we considered it prudent to test the emission of MDI from aerosol hair fixatives with the above mentioned polymers on the ingredient list.
2.5.1 Conclusion
With regard to MDI containing and –emitting products, we decided to look for samples of the following products:
- PU-foam articles, such as mattresses
- One component spray foam
- Boat and car repair kits
- Two-component adhesives and putties
- Liquid roof coating
- Hot melt adhesives
- Floor adhesives
- Polyurethane materials in clothing
- Spray hair fixatives
2.6 Results of inquiries
Answers to general inquiries about the knowledge of inclusion of any of the five substances in consumer products were given from:
COOP
Dansk Supermarked A/S
The Association of Plastic Industries in Denmark.
Danmarks Farve- og Lakindustri
Association of Danish Cosmetic, Toiletries, Soap and Detergent Industries (SPT)
None of these respondents were aware of the presence of any such products on the Danish market.
2.7 Results of search for products with likely emission of the five substances
Glutaraldehyde
The search for products containing glutaraldehyde was very difficult. The search for film and picture developers with content of glutaraldehyde, and not exclusively for occupational use, was negative after several telephone inquiries. One inquiry led us to a company who thought they might provide a developer with glutaraldehyde for youth schools with classes in photography. However, upon further investigation, this company was not able to deliver any such developer. Upon this, we concluded that the use of glutaraldehyde in developers for hobby use seems to have been phased out.
Next, we thought glutaraldehyde might be present in disinfectants for home use or mouth washes. Telephone inquiries and store searches revealed no such thing.
Finally, since glutaraldehyde according to our investigation may be found in paper towels and paper handkerchiefs we purchased some toilet paper and some kitchen rolls made of recycled paper.
Phthalic anhydride derivatives
Two component epoxy adhesives were readily available from do-it-yourself-markets, so samples of these products were purchased and sent for analysis for emission phthalic anhydride derivatives.
Nail lacquers containing polymers based on phthalic anhydride derivatives were identified in stores by scrutiny of the INCI declaration. The nail lacquers were chosen according to place of the polymers in the INCI declaration, since ingredients are supposed to be mentioned in order of falling concentration. Hence, if the polymer is mentioned as one of the first ingredients the nail lacquer was preferred for sampling rather than the one where the polymer was mentioned later. This was thought to give the best chance of measuring the emission, if any would be present.
MDI
Consumer products made with polyurethane, MDI-copolymer or MDI was easier to find, although some discrepancy in information regarding the content of residual MDI-monomer in adhesives and joint fillers were found. Cosmetic products were sampled if the ingredient list contained MDI based polymers or copolymers.
We purchased samples of car window adhesive, floor adhesive, joint filler, PU-rainjacket, hairspray and conditioner, and mattresses.
3 Results of emission analysis from selected consumer products
- 3.1 Analysis of MDI emission from various consumer products
- 3.1.1 Sample treatment and collection
- Polyurethane one component sealant and adhesive
- Floor adhesive
- Hair conditioner
- Hair spray with polyurethane as declared ingredient
- Polyurethane rain coat
- Foam mattress and spring mattress
- 3.1.2 Method of analysis
- Extraction
- Chromatography
- 3.1.3 Calculation of detection limit
- 3.1.4 Results
- 3.2 Analysis of phthalic anhydride derivatives in consumer products
- 3.3 Analysis of glutaraldehyde in consumer products
The products that were purchased and submitted for analysis are listed in annex 2.
3.1 Analysis of MDI emission from various consumer products
Collection and analysis of air samples were carried out according to OSHA (Occupational Safety and Health Administration, U.S. Department of Labour), method 47 for analysis of MDI in the occupational environment.
Air samples are collected by sucking a known amount of air through a glass fibre filter, which is coated with 1-(2-pyridyl)piperazin (1-2PP). Together with MDI 1-2PP forms a complex, which absorbs UV-light. The filter is extracted with acetonitril:dimethylsuphoxide (90:10) and is analysed by HPLC with UV detector (254 nm).
3.1.1 Sample treatment and collection
The purpose of the collection was to treat the sample in a way as close to the user situation as possible.
Car window adhesive
A car window was placed on a table (see figure 1). The sample was opened, and the adhesive was squeezed out of the container with a joint gun while distributing it along the edge of the window pane. After this, the adhesive was covered with the moulding that protects the window pane during transport, mimicking the placement of the window in the car frame (realistic work scenario).
During the out-squeezing and distribution of the adhesive, air was sampled from a height equal to the position of the nose of the user. Application of the adhesive lasted 12 minutes (realistic work scenario).
After this, the filter was exchanged with an un-exposed filter, and air was sampled right over the adhesive in nose height for two hours.
The remainder of the adhesive was squeezed out of the container, and air was sampled over the adhesive for six minutes (maximum exposure).

Figure 1. Application of adhesive to car window
Polyurethane one component sealant and adhesive
Sealant (160-180 g) was squeezed out in stripes on a non-absorbing surface placed in a plastic tray (see figure 2 below). Collection of sample was carried out 25 cm above the sealant. The collection of air and expression of sealant was started simultaneously and continued for 15 minutes.
Floor adhesive
Approximately 200 g of the thin fluid adhesive was spread over an area of approximately 25x40 cm². (see figure 2 below). Suction took place 25 cm above the surface for 15 minutes counting from the opening of the bottle.
Hair conditioner
A large handful of conditioner (app. 25 g) was spread over an area of app. 25x30 cm² (see figure 2 below). Air was collected 25 cm above the area for 15 minutes counting from the opening of the container.

Figure 2. Hair conditioner. The plastic tray with non-absorbing material was used for sealant, adhesive, hair conditioner, and hair spray.
Hair spray with polyurethane as declared ingredient
Hair spray was sprayed out over a non-absorbing surface of 25x30 cm² placed in a plastic tray (see figure 2 above). Spray for 10 seconds, pause for 10 seconds, and spray for 10 seconds. Air was collected 25 cm above the area for 15 minutes.
Polyurethane rain coat
Collection of air was started as soon as the plastic bag around the rain coat was opened. The rain coat was spread out and turned over and around several times during the air collection. Air was collected 25 cm above the rain coat for 15 minutes.
Foam mattress and spring mattress
Collection of air was started when the plastic cover was removed. The mattress was placed on the floor, and air was collected 25 cm above the surface for 7 hours (see figure 3 below). During this time the mattress was sat on and walked on every half hour.
The sampling air flow velocity was 1 L/min in all samplings.

Figure 3. Collection of air sample over mattress.
3.1.2 Method of analysis
Extraction
The filters were extracted with 4 mL acetonitril:dimethyl sulfoxide (90:10).
Chromatography
The extract was analyzed on HPLC (Agilent 1100)
Method 1:
Column: Zorbax XDB 5μ C8 from Agilent, length 150 mm, diameter 4,6 mm. Mobile phase: 50% acetonitril and 50% 0,05M ammoniumacetate in MilliQ water (pH 6.07), isocratic. Flow velocity 1 mL/min. Temperature 25° C. Injection volume 25 μl.
Retention time 4.333 min.
Method 2:
Column: Prodigy, 5μ from Phenomenex, length 250 mm, diameter 4,6 mm. Mobile phase 50% acetonitril, 50% ammoniumacetate in MilliQ water (pH 6.07), isocratic. Flow velocity 1 mL/min. Temperature 25° C. Injection volume 25 μl.
Retention time 10.98 min.
Detection
Detection was carried out at 254 nm on a G1314A VWD variable wavelength detector from Agilent.
The extracted samples were analyzed against a row of standard solutions of MDI derivatized with 1-2PP in the concentrations 10, 25, 50, 100, 250, and 500 ng/ml. The standard curve was linear within the entire concentration span.
The lowest concentration of standard solution which gave a signal significantly different from the base line was 25 ng/ml.
3.1.3 Calculation of detection limit
The detection limit depends on the collected amount of air. Collection in 12 minutes corresponds to 12 litres of collected air.
The filter was extracted with 4 ml of solvent. 25 ng/ml in 4 ml corresponds to 100 ng in 12 l of air, corresponding to 8 ng/ l air or 8 μg/m³ of air.
The detection limits for the different analyses appear from table 1 below.
3.1.4 Results
In some of the chromatogrammes, which were obtained by the HPLC method 1, one peak was situated very close to the MDI peak. All samples were therefore reanalyzed with HPLC method 2, which showed that there was no MDI in any of the samples. The analytical result for the collected air samples are shown in table 1 below.
Table 1 Concentration of MDI in the collected air samples
| Sample | DMU no. | Collected amount of air, L | Concentration of MDI, μg/m³ | Limit of detection μg/m³ |
| 1 Car window adhesive Realistic work scenario |
ATMI 2006-460 |
12 | n.d. | 8 |
| 1 2 hours following work process |
- | 120 | n.d. | 0.8 |
| 1 Maximum exposure |
- | 6 | n.d. | 17 |
| 11 Sealant |
ATMI 2006-918 |
15 | n.d. | 7 |
| 17 Adhesive /sealant |
ATMI 2006-924 |
15 | n.d. | 7 |
| 6 Floor adhesive |
ATMI 2006-913 |
15 | n.d. | 7 |
| 5 Hair conditioner |
ATMI 2006-912 |
15 | n.d. | 7 |
| 4 Hair spray |
ATMI 2006-911 |
15 | n.d. | 7 |
| 9 Rain coat |
ATMI 2006-916 |
15 | n.d. | 7 |
| 19 Foam mattress |
ATMI 2006-1037 |
420 | n.d. | 0.2 |
| 18 Spring mattress |
ATMI 2006-1038 |
420 | n.d. | 0.2 |
3.2 Analysis of phthalic anhydride derivatives in consumer products
The following phthalic anhydride derivatives are comprised by the analytical method:
Cyclohexane-1,2-dicarboxylic anhydride (unspec.) (HHPA), CAS 85-42-7
Hexahydro-4-methylphtalic anhydride (HHMPA), CAS 19438-60-9
Methyltetrahydrophtalic anhydride (unspec.) (MTHPA), CAS 11070-44-3
Collection and analysis of phthalic anhydride derivatives was carried out after a method described by Welinder and Gustavsson in 1992 (12). The method was described for MTHPA, but it is also applicable to other phthalic anhydride derivatives.
Air samples were collected by sucking a known amount of air through a little glass column packed with glass wool and two layers of XAD-2, which adsorbs the anhydrides. XAD-2 is extracted with toluene which is analyzed for phthalic anhydride derivatives by gas chromatography with mass spectrometry detection (GC-MS).
3.2.1 Sample treatment and collection
The purpose of the sample collection was to treat the samples in a way, which as close as possible to the normal way of application.
Nail Lacquer
A glass plate was placed on top of a drawing of ten rectangles the size of fingernails (1.2 cm x 2 cm). Nail Lacquer was applied in an even, thick layer with the supplied brush, which was dipped in the bottle. Air was collected 25-32 cm above the glass plate for 10 min., and the pump was started when the bottle was opened and the application started (see figure 4 below).
In addition, 10 drops of nail lacquer was put on a watch glass, and air was sucked from right above the watch glass for 10 min. to simulate maximum exposure.
Figure 4: Application of nail lacquer on glass plate. Air is sucked through XAD-2 column, which is placed in nose height over the nail lacquer.
Epoxy adhesive
Epoxy adhesive is a two-component adhesive. Half of each tube was squeezed out on a glass plate, and the two parts were mixed with the supplied spatula or a cotton swab. As long as the adhesive was liquid, it was used for gluing various surfaces (app. 8 min.). The air sample was taken in nose height above the work surface for 10 minutes with start of the pump when the adhesive was first squeezed out (see figure 5 below)

Figure 5: Application of epoxy adhesive. Air is sucked through an XAD-2 column, which is placed in nose height above the work surface.
The sampling air flow velocity was 1 l/min during all collections.
Control of method
A standard solution of the three phthalic anhydride derivatives in toluene was aerated with nitrogen at 37° C. Air was collected just above the surface for 10 min. During this a part of the anhydrides were liberated, and it was made possible to perform a positive qualitative control of the method of collection and extraction.
Analytical method
Extraction: The tips of the XAD-2 column were removed with a pair of pliers. 500 μL toluene was added and the content of the column (XAD and glass wool) was pushed out into a 4 mL capped vial and the column was rinsed with an additional 500 μL of toluene. The extraction was performed for 20 minutes in ultrasound bath. The toluene extract was aspirated with a 1 mL plastic syringe and filtered through a 0.2 μm filter.
Chromatography: The extracts were analyzed by GC-MS (Turbomass from Perkin Elmer)
Column: Rtx 200 MS from Retstek, length 30 m, diameter 0.25 mm, film thickness 0.25μm. Carrier gas: helium with a flow velocity of 2 mL/min. Injection volume 1μL. Injection temperature 300° C. Temperature program of the oven: 100° C for 5 minutes increasing to 230° C with 5° C a minute, hold for 10 minutes, then increasing to 280° C with 25° C a minute, hold for 10 minutes. The column was changed to a new one of same type after analysis of the two first samples.
Detection: The substances were detected by mass spectrometry after electro impact (EI+) ionisation. Collection of masses appears from table 2 below.
Table 2: Analysis parameters for phthalic anhydrides on GC-MS
| Substance | CAS nr | Collection time [Minutes] |
Retention time [Minutes] |
Mass m/z |
| Cyclohexane-1,2-dicarboxylic anhydride (unspec.) (HHPA) | 85-42-7 | 7-12 | 11.43 | 54 67 82 |
| Hexahydro-4-methylphthalic anhydride (HHMPA) | 19438-60-9 | 12-18 | 12.72 12.79 |
54 81 96 |
| Methyltetrahydrophthalic anhydride (unspec.) (MTHPA), | 11070-44-3 | 12-18 | 12.94 13.01 13.36 |
79 93 94 |
The samples were analyzed against a series of standard solutions of 1, 5, 10, 50, and 100 ng/mL. The calibration curve was linear in the entire area of measurement. The lowest concentration of standard solution, which gave a signal significantly different from the noise on the baseline was 5 ng/mL.
Calculation of detection limit
The detection limit depends on the collected amount of air. In the realistic work scenarios the exposure time was 8-10 minutes corresponding to 8-10 litres of air.
The column was extracted with 1 mL solvent. 5 ng/mL in 1 mL corresponds to 5 ng in 8-10 L of air corresponding to 0.5-0.6 μg/m³ of air.
3.2.2 Results of phthalic anhydride measurements
The chromatogrammes for a standard solution and the method control run before change of GC column is seen in figure 6 and 7 below.
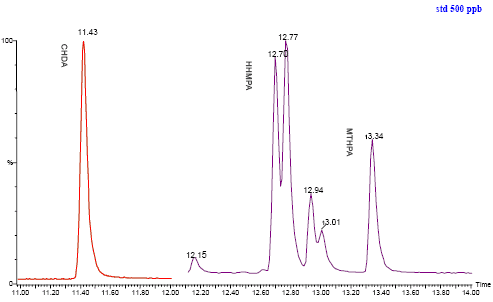
Figure 6: Chromatogram of standard solution of the three phthalic anhydride derivatives.
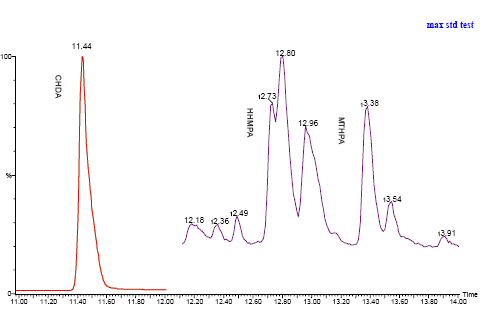
Figure 7: Chromatogram for control of method for collection and extraction of three phthalic anhydride derivatives.
None of the phthalic anhydride derivatives were detected in any of the air samples (see table 3 below)
Table 3: Concentration of phthalic anhydride in selected products
| DMU no. | Collected amount of air [L] | Concen-tration | Detection limit [μg/m³] |
|
| 2 Nail lacquer |
ATMI 2006-461 |
10 | n.d. | 0.5 |
| 3 Two-component adhesive |
ATMI 2006-462 |
10 | n.d. | 0.5 |
| 10 Two-component adhesive |
ATMI 2006-917 |
10 | n.d. | 0.5 |
| 12 Nail lacquer |
ATMI 2006-919 |
10 | n.d. | 0.5 |
| 13 Nail lacquer |
ATMI 2006-920 |
10 | n.d. | 0.5 |
| 14 Nail lacquer |
ATMI 2006-921 |
10 | n.d. | 0.5 |
| 15 Nail lacquer |
ATMI 2006-922 |
10 | n.d. | 0.5 |
| 16 Nail lacquer |
ATMI 2006-923 |
10 | n.d. | 0.5 |
3.3 Analysis of glutaraldehyde in consumer products
Collection and analysis of air samples was carried out according to OSHA method 64 (13) for analysis of glutaraldehyde in the occupational environment.
Air samples were collected by sucking a known amount of air through a silica column (Sep-Pak) coated with 2,4-dinitrophenylhydrazine (DNPH). Together with DNPH glutaraldehyde forms a derivative, which can be analyzed by HPLC and UV-detection.
Sample treatment and collection
The intention with the air collection was to treat the sample in a way that closely resembles the use scenario.
Kitchen roll
Collection of air was begun when the package of 4 kitchen rolls was opened. One roll was rolled out and crumpled up as if it was going to be used for wiping, while the three other rolls were left standing on the table. The collection time was 10 minutes.
Toilet paper
Collection of was begun when the package of 8 toilet rolls was opened. All 8 rolls were put out on the table. One roll was unrolled partly (approx. 2 m). The other rolls were squeezed and turned somewhat during the first 5 minutes. The collection time was 10 minutes.
The flow velocity of the air sampling was 1 L/min. at all collection sessions. The collection was performed with Sep-Pak DNHP-Silica cartridge from Waters (14).
Analytical method
Elution: the Sep-Pak column was eluted with 5 ml of acetonitril.
Chromatography: The eluate was analyzed on HPLC (Agilent 1100)
Column: Nova-pak, 4μ C18 from Waters, length 150 mm, diameter 3,9 mm.
Pre column C18 (Waters).
Method 1
Mobile phase A: 100% acetonitril, B: 0,1% phosphoric acid in Milli-Q water. Flow velocity 1,0 ml/min. Gradient: 55% A increasing to 100% A at 8 minutes. Falling to 55% A at 9 minutes, hold until 20 min.
Method 2
Mobile phase A: 100 % acetonitril, B: 50% metanol +50% MilliQ water. Flow velocity 1 mL/min. Gradient: 40% A increasing to 100% A at 40 min. Falling to 40% A at 41 min, hold until 55 min.
Temperature 25° C. Injection volume: 20 μl.
Detection: The samples were detected at 360 nm on a G1314A VWD variable wavelength detector from Agilent. Retention time: 5.66 minutes.
The samples were analyzed with elution method 1 against standard solutions of 10, 25, 50, 100, 250, and 500 ng/mL. The calibration curve was linear in the entire measuring range. The lowest concentration of standard solution, which gave a signal significantly different from the noise on the base line, was 10 ng/mL.
With elution method 1, the samples and the blank gave a signal in the chromatograms close to glutaraldehyde. The samples were therefore analyzed again with elution method 2, and all samples were analyzed with and without spiking with 500 ng/ml glutaraldehyde. Elution method 2 could separate glutaraldehyde from the peak in the samples. Hence, this peak does not come from glutaraldehyde. The applied method of analysis detects a host of aldehydes and ketones, and one of these may have been present in the ambient air.
Calculation of detection limit
The detection limit depends on the collected amount of air. At the realistic use scenarios the exposure time was 10 minutes corresponding to 10 L of air.
The column was extracted with 5 mL of solvent. 10 ng/mL in 5 mL corresponds to 50 ng in 10 L air corresponding to 5 μg/m³ air.
3.3.1 Results of glutaraldehyde measurements
Table 4. Concentration of glutaraldehyde in selected consumer products
| Product | DMU no. | Collected volume of air [L] | Concen-tration | Limit of detection [μg/m³] |
| 7 Kitchen roll made from recycled paper |
ATMI 2006-914 |
10 | n.d. | 5 |
| 8 Toilet paper made from recycled paper |
ATMI 2006-915 |
10 | n.d. | 5 |
4 Exposure and risk assessment
- 4.1 Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
- 4.2 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
- 4.3 Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
- 4.4 Methylenediphenyldiisocyanate, CAS 26447-40-5, 5873-54-1 and 101-68-8
- 4.5 Glutaraldehyde, CAS 111-30-8
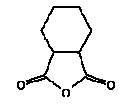
4.1 Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
Synonyms: hexahydrophthalic anhydride, HHPA
hexahydro-1,3-isobenzofurandione
Molecular formula: C8H10O3
Molecular weight: 154.17
Structural formula:
Cyclohexan-1,2-dicarboxylic anhydride (HHPA) is a solid substance at room temperature. (15).
Melting point: 34° C (15)
Vapour pressure: 0.01 hPa at 20° C (15)
Boiling point: 296° C at 1013 hPa (15)
Vapour density: not found
Water solubility: very low solubility in water, and is slowly reacting with water (15).
Odour threshold, air: not found
Conversion factor at 20° C, 1 atm.:1 ppm = 6.293 mg/m³
1 mg/m³ = 0.159 ppm (16)
4.1.1 Hazards
Cyclohexan-1,2-dicarboxylic anhydride (HHPA) has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of HHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, HHPA is an irritant because of formation of corresponding acids in wet surroundings.
HHPA rarely induces contact allergy of the skin (delayed type hypersensitivity), but more easily induce IgE-mediated contact urticaria. This only comes about after initial respiratory sensitization, and subsequent skin contact.
The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
HHPA has caused both sensitization and work-related symptoms at exposure levels as low as 10-50 μg/m³. The level of exposure needed to cause specific IgE antibody production and work-related symptoms in mucous membranes and respiratory organs may be less than 10 μg/m³ (16). No information on the duration needed to induce respiratory sensitization was found.
There is cross sensitivity to MHHPA (see below).
The critical effect is sensitization.
4.1.2 Limit values
No health based limit values for HHPA have been found, but a limit value should be below 10 μg/m³ to ensure absence of sensitizing effect.
4.2 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
Synonyms: MHHPA, 5-methyl- hexahydro-1,3-isobenzofurandione
Molecular formula: C9H12O3
Molecular weight: 168.19
Structural formula:
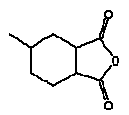
MHHPA is an oily liquid at room temperature.
Melting point: - 30° C- -29° C (16,17)
Boiling point: 120° C at 130 Pa (16)
Vapour density: not found
Vapour pressure: not found
Water solubility: 36 g/l at 20° C (17)
Odour threshold, air: not found
Conversion factor at 20° C, 1 atm.: 1 ppm = 6.865 mg/m³
1mg/m³ = 0.146 ppm
4.2.1 Hazards
MHHPA has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MHHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, MHHPA is an irritant because of formation of corresponding acids in wet surroundings. It rarely induces contact allergy of the skin but more easily induces IgE-mediated contact urticaria. The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
The critical effect is sensitization.
There is cross-sensitivity to HHPA.
MHHPA has caused both sensitization and work-related symptoms at exposure levels as low as 10-50 μg/m³. The level of exposure needed to cause specific IgE antibody production and work-related symptoms in mucous membranes and respiratory organs may be less than 10 μg/m³ (16).
4.2.2 Limit values
No health based limit values for MHHPA have been found, but a limit value should be below 10 μg/m³ to ensure absence of sensitizing effect.
4.3 Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
Synonyms: Tetrahydromethylphthalic anhydride, 1,2,3,6-tetrahydro-4-methylphthalic anhydride, MTHPA.
Molecular formula:C9H10O3
Molecular weight: 166.19
Structural formula (1,2,3,6-tetrahydro-4-methylphthalic anhydride, CAS 26590-20-5, shown):
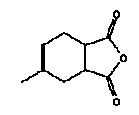
Melting point: -38° C (18)
Boiling point: 150° C at 13.5 hPa, 210° C at 136 hPa (18)
Vapour density: none found
Vapour pressure: not found
Water solubility: 176.4 g/l at 20° C (18)
Odour threshold, air: none found
Conversion factor at 20° C, 1 atm.: 1 ppm = 6.783 mg/m3
1 mg/m³ = 0.147 ppm (16)
4.3.1 Hazards
MTHPA has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MTHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, MTHPA is an irritant because of formation of corresponding acids in wet surroundings. It rarely induces contact allergy of the skin but more easily induces IgE-mediated contact urticaria. The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
Among MTHPA-exposed workers, even at low levels of exposure (5-20 μg/m³) 56% had allergy symptoms of the eyes and upper airways, 9% had asthma, and 16% had MTHPA specific IgE antibodies. The corresponding numbers were 65%, 11%, and 22% in the more heavily (20-150 μg/m³) exposed groups (16).
The critical effects for MTHPA are irritation of mucous membranes of the eyes and airways and sensitization-induced work-related diseases. Sensitization, work-related rhinoconjunctivitis, and asthma have been verified for workers exposed to MTHPA levels of 5-20 μg/m³ (16).
4.3.2 Limit values
No health based limit values for MTHPA have been found, but a limit value should be below 5 μg/m³ to ensure absence of sensitizing effect.
4.3.3 Exposure and risk assessment for consumers for all the above phthalic anhydride derivatives
The emission of the three phthalic anhydride derivatives was measured during realistic use scenarios for 6 nail lacquers and 2 two-component epoxy adhesives.
The results of these emission measurements were all below the detection limit of 0.5 μg/m³. This is at least 10 times below the lowest level of 5 μg/m³, which has been found to induce respiratory sensitization.
Nail lacquers were chosen for emission measurements, because allergic contact dermatitis cases have been found in the literature. These cases were caused by phthalic anhydride used in the copolymer base of the nail lacquers (19). The nail lacquers chosen were selected among those with phthalic anhydride copolymers listed among the very first on the ingredients list, meaning that the highest concentrations of phthalic anhydride derivatives would be expected in these particular nail lacquers.
Epoxy adhesives were chosen because phthalic anhydrides are known to be part of these products, and occupational exposure via epoxy resins has been reported to cause allergic rhinitis and conjunctivitis (20).
Since no measurable emission from the selected nail lacquers and epoxy adhesives could be found, the risk of respiratory sensitization must be considered to be low.
There is a slight possibility that people who have acquired respiratory allergy from other sources, e.g. occupationally, may react to very minute amounts in consumer products. However, no such reactions toward consumer products have been found in the literature.
4.4 Methylenediphenyldiisocyanate, CAS 26447-40-5, 5873-54-1 and 101-68-8
Synonyms: MDI
The possible isomeric forms of MDI are:
4,4’-methylenediphenyl diisocyanate, CAS 101-68-8
2,4’-methylenediphenyl diisocyanate, CAS 5873-54-1
2,2’-methylenediphenyl diisocyanate, CAS 2536-05-2
Molecular formula:C15H10N2O2
Molecular weight: 250.26
Structural formula (4,4’-MDI, CAS no. 101-68-8 shown):
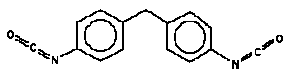
Polymeric MDI is a dark amber viscous liquid while the pure 4,4’ MDI is a white waxy solid. The odour of MDI is slightly musty (10).
Melting point: 34 - 43° C (10)
Boiling point: 314- 364° C (10)
Vapour density: not found
Vapour pressure: <0.014 Pa (2,4’-MDI)
<0.002 Pa (4,4’-MDI)
<0.005 Pa (polymeric MDI), all at 20° C (10)
Water solubility: Determination of the MDI solubility in water is difficult because of the high reactivity of the NCO groups towards OH groups, e.g. in water giving rise to aromatic amines. Consequently it is not possible to measure the solubility of MDI in water using the EC standard methods.
Odour threshold, air: none found
Conversion factor at 20° C, 1 atm.: 1 ppm = 10.22 mg/m3
1 mg/m³ = 0.098 ppm (21)
4.4.1 Hazards
MDI has the following classification:
Xn: Harmful
Xi: Irritant
Xn: Sensitising
R20: Harmful by inhalation
R36/37/38: Irritating to eyes, respiratory system, and skin
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MDI in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%. However, even below 0.1% the product should be labelled. “Contains isocyanates. See information provided by the manufacturer”. If the product is an article there is no such requirement for labelling.
In the EU risk assessment report (10) a different classification was proposed:
Xn: Carcinogenic, category 3
Xn: Harmful
Xi: Irritant
Xn: Sensitising
R20: Harmful by inhalation
R36/37/38: Irritating to eyes, respiratory system, and skin
R42/43: May cause sensitization by inhalation and skin contact
R 40: Limited evidence of a carcinogenic effect
R 48/20: Harmful: danger of serious damage to health by prolonged exposure through inhalation
Strictly speaking MDI should be classified as toxic by inhalation on the basis of a 4-hour LC50 of 490 mg/m³. However, a consensus was reached among European experts (Directive 67/548/EEC; 25th ATP, i.e. Dir. 98/8/EC, O.J.30.12.1998) to
consider this value as irrelevant in terms of real-life exposure, because such high values are said not to be achievable except under experimental testing conditions. This pragmatic reasoning is acceptable provided that such high concentrations are indeed never achieved, even through misuse or (further) technological changes in work processes. Consequently it is proposed to classify MDI as harmful by inhalation. Taken together, in terms of pure hazard characterisation MDI is toxic by inhalation. However, if one considers the exposure assessment, it is reasonable to consider MDI as harmful only and to apply the risk management phrase ‘harmful by inhalation’(10).
It should be noted that since the vapour pressure of MDI (4,4’) is only 0.002 Pa at room temperature it is only possible to reach a saturated air concentration of 0.0197 ppm or 0.2 mg/m³. In order to reach a concentration of as much as 490 mg/m³ it is necessary to heat the MDI, which in turn will condensate and form particles, not vapour, in the indicated concentration.
For irritant effects a NOAEL of 0.5 mg/m³ was found (10).
Animal data as well as studies in humans provide clear evidence of possible skin sensitization due to MDI. Animal studies indicate that MDI is a strong allergen. Human case reports describe the occurrence of allergic contact dermatitis due to MDI skin exposure (10).
MDI is a potential respiratory sensitizer in animals and humans. Animal studies have shown that respiratory sensitization can be induced by skin contact with MDI. The quantitative relationships between exposures (concentration, duration, rate of exposure, route of exposure) have not been established. At the present time it is not possible to define reliable exposure-response relationships with regard to the risk of sensitization for MDI. The current knowledge/state of the art in this field does not yet allow deciding a threshold level for sensitization. Because animal data support the hypothesis that respiratory hypersensitivity may be induced by skin contact and because such possibility has not been excluded in studies involving humans, it is reasonable to consider that it is not only important to reduce inhalation exposure but also to avoid skin contact (10).
The mechanism behind isocyanate-related hypersensitivity is still obscure. Several publications indicate that complex immunological reactions are involved in the sensitization process to MDI. Immediate allergic, late allergic and dual-phase responses can occur. Humoral as well as cellular immunity may be involved in the pathogenesis of hypersensitivity due to isocyanates. The specific humoral response can be IgE as well as IgG mediated. Cross-reactivity with other isocyanates has been described in several publications (10).
There is inadequate evidence of carcinogenicity in humans and limited evidence in experimental animals (10), which is the reason the EU risk assessment report proposed as classification as carcinogenic in category 3.
In conclusion, dermal and respiratory sensitization seems to be the most critical effects.
4.4.2 Limit values
The Danish occupational limit value is 0.005 ppm or 0.05 mg/m³ (22). This is a time weighted average (TWA) over 8 hours. In practise, the Danish ceiling limit (for a random 15 minute measuring period) is twice the TWA, i.e. 0.01 ppm or 0.1 mg/m³.
The American Conference of Governmental Industrial Hygienists (ACGIH) has recommended the same limit as a time weighted average (21). The ACGIH notes that the recommended limit may not necessarily protect susceptible workers from possible sensitization or an allergic reaction in previously sensitized individuals.
The Occupational Safety and Health Administration (OSHA) of the US department of Labor has set a permissible exposure limit of 0.02 ppm or 0.2 mg/m³ as a ceiling limit (23).
4.4.3 Exposure and risk characterisation for consumers
The EU risk assessment report finds that as respiratory hypersensitivity may be induced by skin contact, respiratory and skin sensitisation due to MDI cannot be excluded during spray painting, the use of one component foam, during gluing or using a putty/filler cartridge or during the use of a hot melt adhesive. However, there is already sufficient information available upon which to base a conclusion (iii) for this endpoint for all scenarios: there is a need for limiting the risks; risk reduction measures which are already being applied shall be taken into account. (Specific attention should be paid to the situation where a subject has an occupationally acquired sensitisation to MDI) (10).
The EU risk assessment report considers chronic toxicity from the use of consumer products containing MDI of less concern as consumer exposure of the identified products is expected to occur on occasional events of short duration. For chronic toxicity and carcinogenicity, conclusion (ii) is reached for all scenarios: there is at present no need for further information or testing or risk reduction measures beyond those which are being applied already (10).
In the present investigation we were not able to find hot melt adhesive containing MDI nor MDI containing spray paint for private consumers on the Danish market.
The products that were tested for emission of MDI were: car window adhesive, mattresses, one component adhesives and sealers, polyurethane rain-coat, floor adhesive, and a hair conditioner.
No emission of MDI could be detected under the testing conditions.
We have not examined exposure scenarios involving the grinding or thermal removal of MDI containing material. Such secondary exposure is known to be a hazard in the occupational setting (9), but only normal, predictable consumer exposure has been the scope of this project.
With limits of detection varying from 0.2-17 μg/m³ and no emission found in the different exposure scenarios, the exposure is well below the occupational exposure limit of 50 μg/m³. Hence, the risk of inducing hypersensitivity during the use of the available consumer products made with MDI seems very low.
It is important that consumers follow instructions to avoid skin contamination, since dermal sensitization can lead to general sensitization, thus causing risk of respiratory allergy with asthma-like symptoms upon later exposure by inhalation.
In the case of already acquired hypersensitivity towards MDI or other isocyanates, occupationally or accidentally, even small exposures by inhalation from consumer products like window sealing foams, may cause an outbreak of respiratory allergy with asthma-like symptoms (10).
4.5 Glutaraldehyde, CAS 111-30-8
Synonyms: Glutaral; 1,5-pentanedial
A colourless oily liquid. Commercial solutions often have an amber tint and an odour similar to spoiled fruit.
Molecular formula: C5H8O2
Molecular weight: 100.12
Structural formula:
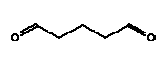
Boiling point: 187-189° C (with decomposition)
Vapour density: 3.4 (air=1)
Vapour pressure: 16.5 mmHg (2.2 kPa) at 20° C. There is some confusion
about the purity of the test substance used: IUCLID (1996) gives the same value for pure glutaraldehyde and for a 50% aqueous solution, ACGIH (1991) gives a vapour pressure of 0.0152 mmHg (2.0 Pa at 20° C) for a 50% solution. In
SUBFAC (a computer model), vapour pressures (20° C) has been calculated to 24.9 mmHg for a 100% solution, 19.4 mmHg for a 50% solution, and 2.7 mmHg for a 2% solution (24). In 2001, ACGIH gave a vapour pressure of 0.102 mmgHg for a 50% solution, and 0.003 mmHg for a 2% solution (21).
Odour threshold, air: 0.14 ppm (0.17 mg/m³)
Conversion factor at 20° C, 1 atm.: 1 ppm = 4.2 mg/m³
1 mg/m³ = 0.240 ppm
Data taken from (24).
4.5.1 Hazards
Glutaraldehyde has the following classification:
R23/25: Toxic by inhalation and if swallowed.
R34: Causes burns.
R42/43: May cause sensitization by inhalation and skin contact.
R50: Very toxic to aquatic organisms.
with the following classification limits in products:
| Concentration | Classification |
| C = 50 % | T, N; R23/25-34-42/43-50 |
| 25 % = C < 50 % | T; R22-23-34-42/43 |
| 10 % = C < 25 % | C; R20/22-34-42/43 |
| 2 % = C < 10 % | Xn; R20/22-37/38-41-42/43 |
| 1 % = C < 2 % | Xn; R36/37/38-42/43 |
| 0,5 % = C < 1 % | Xi; R36/37/38-43 |
The occurrence in chemical consumer products, other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
Inhalation studies in mice and rats showed no carcinogenic activity when exposed to air concentrations of 62.5 – 250 ppb (0.0625 – 0.250 ppm) 6 hours a day, 5 days a week for 104 weeks (25).
Inhalation allergy in the occupational setting has occurred in several cases, particularly during cold sterilization of hospital equipment and use of glutaraldehyde containing chemicals during radiographic processing. Symptoms vary from watering of eyes, rhinitis, respiratory difficulty, nausea to headache. The vapours from glutaraldehyde may act as an irritant to bronchial and laryngeal mucous membranes, and prolonged exposure could produce localized edema and other symptoms of allergic response, including asthma. In these cases, the air concentrations in the breathing zone varied from 0.05 ppm to 0.12 ppm. In the cases where information on air concentration was not given, the workers had worked with preparations containing 2 – 3.6 % glutaraldehyde (25).
Numerous symptoms have been found in individuals with exposure to less than 0.05 ppm glutaraldehyde, which is the recommended peak exposure limit in many countries (26). One such case was described in a 61-year-old nurse, an ex-smoker, who began working in a renal dialysis unit in 1976 (22 years before).
Ten years later, she experienced sporadic and mild episodes of chest tightness and shortness of breath related to exposure to formalin, which was used to sterilize artificial kidney machines. In 1994, formalin was replaced with 2% glutaraldehyde, which she handled daily in an open environment. She was symptomless until February 1998, when she developed symptoms of irritation of the eyes and upper respiratory tract, dyspnea on exertion, dry cough, and episodic attacks of wheezing, which she associated which glutaraldehyde exposure.
Her symptoms were progressively severe, and she had an acute asthma attack requiring hospitalization. She then took sick leave, during which she slowly recovered. After 3 months with medical treatment, she was symptom free, and she was clinically evaluated.
She underwent a the specific bronchial challenge test with activated 2% glutaraldehyde aqueous solution painted on a cardboard in a 7 m³ challenge chamber for 10 min. No changes FEV1 (forced expiratory volume in one second) were observed in a 24 hour monitoring period. However, at the end of this period, the methacholine inhalation test became positive (PC20 0.74 mg/ml, PC20: precise concentration of methacoline where FEV1 falls by 20%). One week later, the challenge test with 2% glutaraldehyde was repeated, and it elicited an early asthmatic response. Although no late reaction was observed, a recurrent nocturnal asthmatic reaction occurred in the following days.
Similar cases of rhinitis and asthma have been diagnosed by specific bronchial challenge with glutaraldehyde concentrations in the range of 0.064-0.081 mg/m³, which is well below the ceiling limit of 0.8 mg/m³ (0.2 ppm) in Denmark or even the more stringent 0.05 ppm ceiling limit in many other countries. Cross reactivity between formaldehyde and glutaraldehyde has been suggested.
The type of mechanism responsible for glutaraldehyde induced asthma is not known because IgE specific antibodies have not been demonstrated in affected subjects, or can be detected in only a small percentage of workers with symptoms related to work with glutaraldehyde (27).
According to the American Conference of Industrial Hygienists (ACGIH) no clear dose-response relationships in humans have been established for airborne glutaraldehyde exposure. Reported industry experience indicates an absence of glutaraldehyde-induced skin or respiratory sensitizations for workers routinely exposed to airborne glutaraldehyde concentrations ranging from 0.01 to 0.34 ppm (21,26).
4.5.2 Limit values
In Denmark, the occupational exposure limit is 0.2 ppm (0.8 mg/m³), and this a ceiling limit (22).
The American Conference of Industrial Hygienists (ACGIH) recommends a threshold limit value – ceiling of 0.05 ppm (21).
In Denmark, a general limit (a so-called C-value) of 0.001 mg/m³ for ambient air has been calculated (24). The C-value is a limit value for how much an installation may contribute to air pollution.
4.5.3 Exposure and risk characterisation for consumers
In general, it was very difficult to obtain consumer products with a content of glutaraldehyde. No cosmetic products were found, even though glutaraldehyde is a permitted ingredient with restrictions.
Glutaraldehyde may sometimes be used as a disinfectant during the manufacture of paper towels, toilet paper and the like. The two samples taken did not emit any detectable amounts of glutaraldehyde, i.e. the emission was below 5 μg/m³. This is well below the Danish occupational exposure limit, and at the level of general limit for ambient air.
Hence no risk of respiratory sensitization to glutaraldehyde can be attributed to these paper samples.
No cases of sensitization to glutaraldehyde in consumer products have been found in the literature.
The risk of respiratory sensitization to glutaraldehyde via consumer products must be considered very low, both because of low availability, non-detectable emissions, and absence of cases in the literature.
It is possible that consumers with already acquired sensitization towards glutaraldehyde or formaldehyde from other sources may react to even small residual concentrations of glutaraldehyde in consumer products. However, since no such cases were found in the literature to confirm this, the risk must be considered very low.
5 Conclusion
The purpose of the present project was to
- Make an inventory of consumer product types that may contain any of the five R42-substances.
- Examine, if consumers actually are exposed to the substances when using such products.
- Assess, if the exposure is large enough to cause health effects in the consumers
All in all, 19 products were sampled for inclusion in an emission analysis. The number of sampled products within each group is given in parenthesis.
The following consumer products may contain phthalic anhydride derivatives:
- Nail Lacquer (6)
- Two-component epoxy adhesive (2)
The following consumer products may contain glutaraldehyde:
- Cosmetic products (mouth washes and creams) (0)
- Disinfectants (0)
- Film developers for consumers (0)
- Paper handkerchiefs, kitchen roll and toilet paper (2)
The following consumer products may contain monomeric MDI:
- PU-foam articles, such as mattresses (2)
- One component spray foam or adhesive (2)
- Boat and car repair kits (1)
- Two-component adhesives and putties (0)
- Liquid roof coating (0)
- Hot melt adhesives (0)
- Floor adhesives (1)
- Polyurethane materials in clothing (1)
- Spray hair fixatives and conditioner (2)
When searching the Danish market it was not possible to obtain cosmetic products, disinfectants, nor film developers with glutaraldehyde. Neither was it possible to obtain two-component adhesives and putties, liquid roof coating, nor hot melt adhesives with a content of monomeric MDI.
All purchases were carried out while pretending to be ordinary consumers, not professionals. Although some of the outlets visited are also used by professionals, we were not offered products intended only for professionals. This indicates a fairly good separation between the markets for consumers and the markets for professionals, at least pertaining to these kinds of products.
No emissions of the respiratory sensitizers from the 19 products obtained could be detected by chemical analysis. Hence, no risk of sensitisation to phthalic anhydride derivatives, MDI or glutaraldehyde can be attributed to any of the products analyzed.
Phthalic anhydride derivatives
For the phthalic anhydride derivatives the risk of respiratory sensitization from consumer products must be considered very low.
There is a slight possibility that people, who have acquired respiratory allergy to phthalic anhydride derivatives from other sources, e.g. occupationally, may react to very minute amounts in consumer products, such as nail lacquers and epoxy adhesives. However, in the literature no information was found to confirm such reactions toward consumer products.
MDI
The products that were tested for emission of MDI were: car window adhesive, mattresses, one component adhesives and sealers, polyurethane rain-coat, floor adhesive, and a hair conditioner.
No emission of MDI could be detected under the testing conditions.
We have not examined exposure scenarios involving the grinding or thermal removal of MDI containing material. Such secondary exposure is known to be a hazard in the occupational setting, but only normal, predictable consumer exposure has been the scope of this project.
With limits of detection varying from 0.3-17 μg/m³ the exposure is well below the occupational exposure limit of 50 μg/m³. Hence, the risk of inducing hypersensitivity during the use of the available consumer products made with MDI seems very low.
It is important that consumers follow instructions to avoid skin contamination, since dermal sensitization can lead to general sensitization, thus causing risk of allergic asthma upon later exposure by inhalation.
In the case of already acquired hypersensitivity towards MDI or other isocyanates, occupationally or accidentally, even small exposures by inhalation from consumer products like window sealing foams, can cause an outbreak of allergic asthma (10).
Glutaraldehyde
The products that were tested for glutaraldehyde emission was toilet paper and kitchen roll made of recycled paper. No other consumer products with a probable emission of glutaraldehyde could be obtained.
No emission of glutaraldehyde could be detected in the analyzed products.
The risk of respiratory sensitization to glutaraldehyde via consumer products must be considered very low, both because of low availability, non-detectable emissions, and absence of cases in the literature.
6 Reference List
1. Abildgård A, Mikkelsen SH, Lauridsen FS. Survey of chemcial substances in paper handkerchiefs and toilet paper. Copenhagen: Danish Environmental Protection Agency; 2003. 34. Survey of chemcial Substances in Consumer Products)
2. Cohr,K.-H. Epoxy products which may contain phthalic anhydride derivatives are not only used in aircraft manufacture, but also aircraft repair. 2006.
3. Grøn Information. Produkter med uønskede kemiske stoffer 2002.
4. Drexler H, Schaller KH, Nielsen J, Weber A, Weihrauch M, Welinder H, et al. Efficacy of measures of hygiene in workers sensitised to acid anhydrides and the influence of selection bias on the results. Occup Environ Med. 1999;56(3):202-5.
5. Nielsen J, Welinder H, Jonsson B, Axmon A, Rylander L, Skerfving S. Exposure to hexahydrophthalic and methylhexahydrophthalic anhydrides--dose-response for sensitization and airway effects. Scand J Work Environ Health. 2001;27(5):327-34.
6. Nielsen J, Welinder H, Ottosson H, Bensryd I, Venge P, Skerfving S. Nasal challenge shows pathogenetic relevance of specific IgE serum antibodies for nasal symptoms caused by hexahydrophthalic anhydride. Clin Exp Allergy. 1994;24(5):440-9.
7. Kanerva L, Hyry H, Jolanki R, Hytonen M, Estlander T. Delayed and immediate allergy caused by methylhexahydrophthalic anhydride. Contact Dermatitis. 1997;36(1):34-8.
8. Tarvainen K, Jolanki R, Estlander T, Tupasela O, Pfaffli P, Kanerva L. Immunologic contact urticaria due to airborne methylhexahydrophthalic and methyltetrahydrophthalic anhydrides. Contact Dermatitis. 1995;32(4):204-9.
9. Pratt C, Engelund B, Dansk Toksikologi Center. Sekundær eksponering for isocyanater. Rekvireret arbejde for BAR Industri 2001.
10. European Commission - European Chemicals Bureau. European Union Risk Assessment Report. CAS no. 26447-40-5. Methylenediphenyl diisocyanate (MDI). 2005.; vol. 59).
11. Pepe RC, Wenninger JA, and McEwen jr GN, editors. International cosmetic ingredient dictionary and handbook. Vol.1-4 [Medium (Data File): Compact disc]. Cosmetic, Toiletry, and Fragrance Association, Washington, D.C. [updated 2001].
12. Welinder H and Gustavsson C. Determination of methyltetrahydrophtalic anhydride in air. Sampling, analysis and occupational exposure levels. The Annals of Occupational Hygiene. 1992;36:189-97.
13. U.S.Department of Labor.Occupational Safety & Health Administration. Method 64. Glutaraldehyde. http://www.osha.gov/dts/sltc/methods/organic/org064/org064.html 2006.
14. Waters. Vejledning i brug af Sep-Pak DNPH-silica cartridge. 2006.
15. European Commission - European Chemicals Bureau. IUCLID Dataset. Cyclohexane-1,2-dicarboxylic anhydride. http://ecb.jrc.it/IUCLID-DataSheets/85427.pdf 2000.
16. Keskinen H. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards. - 136: Cyclic acid anhydrides. Solna: Arbetslivsinstitutet; 2004. (Arbete och hälsa; 2004:15
17. European Commission - European Chemicals Bureau. IUCLID dataset. 19438-60-9 hexahydro-4-methylphthalic anhydride. http://ecb.jrc.it/IUCLID-DataSheets/19438609.pdf 2000.
18. European Commission - European Chemicals Bureau. IUCLID Dataset. 11070-44-3 tetrahydromethylphthalic anhydride. http://ecb.jrc.it/IUCLID-DataSheets/11070443.pdf 2000.
19. Gach JE, Stone NM, Finch TM. A series of four cases of allergic contact dermatitis to phthalic anhydride/trimellitic anhydride/glycols copolymer in nail varnish. Contact Dermatitis. 2005;53(1):63-4.
20. Yokota K, Johyajama Y, Yamaguchi K, Takeshita T, Morimoto K. Exposure-response relationships in rhinitis and conjunctivitis caused by methyltetrahydrophthalic anhydride. Int Arch Occup Environ Health. 2006;72:14-8.
21. ACGIH. Documentation of the Threshold Limit Values and Biological Exposure Indices. - [2]: E-O. 7 ed. Cincinnati, Ohio: ACGIH (American Conference of Governmental Industrial Hygenists) Worldwide; 2001.
22. Arbejdstilsynet. At-vejledning. Stoffer og materialer - C.0.1 Grænseværdier for stoffer og materialer. 2005.
23. U.S.Department of Labor OS&HA. Regulations (Standards - 29 CFR). TABLE Z-1 Limits for Air Contaminants. - 1910.1000 TABLE Z-1. http://www.osha.gov/pls/oshaweb/owadisp.show_
document?p_table=STANDARDS&p_id=9992 2006.
24. Jelnes JE, Nielsen E. Toxicological Evaluation and Limit Values for Methyl Tertiary-butyl ether (MTBE), Formaldehyde, Glutaraldehyde and Furfural. http://www.mst.dk/udgiv/Publications/1999/87-7909-563-1/pdf/87-7909-562-3.PDF Miljøstyrelsen, Denmark; 1999.[70]
25. HSDB. Glutaraldehyde. RN: 111-30-8. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB 2005.
26. Takigawa T, Endo Y. Effects of glutaraldehyde exposure on human health. J Occup Health. 2006;48(2):75-87.
27. Quirce S, Gomez M, Bombin C, Sastre J. Glutaraldehyde-induced asthma. Allergy. 1999;54(10):1121-2.
Version 1.0 July 2007, © Danish Environmental Protection Agency