Health effects of predatory beneficial mites and wasps in greenhouses
Annex 1
1 Methods for analysis of specific IgE
Sera from the 2nd and 3rd year follow-up phase of the BIOGART cohort study, 344 and 338 samples, respectively. Additionally, 59 sera from previous runs have been tested for comparison.
1.1.1 Preparation of allergen extracts
Allergen preparations made by extraction of the following species, purchased from Koppert BV, The Netherlands:
Tetranychus urticae
Phytoseilus persimilis
Hypoaspis miles
Amblyseius cucumeris
Aphidius colemani
The first two were obtained as live animals (n = 25,000 – 200,000; varying per species) largely free of substrate material, while one (*) was supplied with as carrier material corn grid, the second (**) a mixture of sieved wood particles, wheat bran, yeast lyofilisate flakes, grinded buckwheat shells, and vermiculite while the third (***) needs to be kept in enriched fertilized garden soil. In an attempt to separate the carrier material from the animal bodies, these preparations were sieved through a 425 μm sieve, and the crude (>0.425 mm) and fine (<0.425 mm) fractions were extracted separately.
Extraction was performed with 5 % (w/v) dry material suspended in PBS pH 7.00, shaken overnight at room temperature then incubated in an ultrasonic bath for 15 minutes, and vortexed for 30 minutes. The extracts were centrifuged at 25,000 g and the supernatants dialysed against PBS pH 7,40. The dialysed extract was filtered through a 0.45 μm filter and stored in aliquots at –20°C.
Protein yields varied largely, from 1.11 (Hypoaspis miles > 0.425 mm) to 31.6 (Tetranychus urticae) mg protein per g starting material.
Of the pure carrier materials 25 grams were suspended in 400 ml PBS pH 7.00 and extracted as described above. Here protein content varied from 0 mg (Vermiculite) to 38.1 mg (Wheat bran) protein per gram raw material
SDS-PAGE was performed to assess the protein composition: a neat band pattern with many (>20) proteins in the MW range from 10 to 100 kD was found for the Tetranychus urticae and Phytoseiulus persimilis extracts; Amblyseius cucumeris ‘crude’ extract showed a range of ~10-15 proteins at 5-30 kD, while in the other predator extracts hardly any clear protein bands could be distinguished. From the carrier materials Yeast and Wheat showed band patterns in the 4-38 kD and 6-70 kD range respectively.
1.1.2 Screening for IgE
Specific IgE sensitization was tested with an EIA method in which sera are tested in microwells coated overnight with 0.1 mL diluted allergen extract at 20 mg protein per mL, in PBS, as described by Doekes et al (1996), with some modifications:
- Sera incubated for 2 hrs in allergen-coated microwells; all sera tested at 1/10 dilution; dilution medium: PBTG with a 10% diluted pool of negative control plasma (*see below).
- Detection of IgE binding with mouse monoclonal anti-IgE, followed by incubations with biotinylated rabbit anti-mouse Ig, avidin-peroxidase – all diluted in PBTG (PBS-0.05% Tween-20 with 0.2% gelatin -; detection of avidin-peroxidase-binding with the peroxidase-substrate o-phenylenediamine (OPD) in phosphate-citrate buffer with 0.015% H2O2
- Peroxidase reactions stopped after 30 min with 1 M HCl, and optical density (OD) read at 492 nm.
- All sera tested in rows of six micro-wells: five wells coated with the various allergen preparations and one non-coated control well (control 1).
- In all plates ‘no serum/buffer only’ controls for each coated allergen (control 2).
Results expressed as OD values of test sera in coated wells corrected for both OD (control 1) and OD (control 2; reagent blank):
ODc(i, x) = [Crude OD (i, x) - OD (PBTG, x)] – [OD(i, coating = 0) – OD(PBTG, 0)]
After the first complete test series, all sera giving an ODc value >0.025 on at least one of the five test allergens, and a random sample of completely negative sera were retested on all five allergens. In a few cases where a marked discrepancy was noted between the first and the second test results a third test was performed and the average of the two most nearest OD values was used. For all other serum/allergen test combinations, the OD of the first test was used.
A serum was considered definitely positive for IgE against a specific allergen if the ODc of the final test result was >0.050, a threshold level at which the chance of false-positive reactions is much lower than at lower cut-off values like 0.025.
All serologic data were provided as data-files with thus corrected OD values (ODc) for each serum on each allergen, and with a 0/1 code indicating specific IgE positivity as defined above.
1.1.3 Modification of IgE test method
In the first IgE test series, serious technical problems were encountered: with several allergen preparations, high background values were observed in the ‘no serum’ control wells, and these high OD values showed high inter-plate and inter-day variation, which further hampered the production of reliable, reproducible test results.
Since it was noted that OD values were often lower in wells in which – apparently negative - serum or plasma had been present, compared to the control wells with coated allergen but PBTG instead of serum (control 2), a panel of plasma samples from a presumably negative population (rubber industry workers) was screened, and a pool of >10 plasma samples with no evidence of any IgE reaction to the five allergen extracts was prepared. Since addition of this pooled plasma (at 10% v/v) to the dilution medium PBTG resulted in much better and more constant background OD values, all subsequent tests were performed with sera diluted 1/10 in PBTG containing 10% of the negative plasma pool. Only IgE serology results obtained in this test system are included in the final data files.
| Serum series | N | No (%) of sera positive on: | |||||
| >1 allergen | Tetranychus urticae | Phytoseiulus persimilis | Hypoaspis miles | Amblyseius cucumeris | Aphidius colemani | ||
| 1-9999 | 59 | 6 (10.1) | 2 (3.3) | 1 (1.7) | 1 (1.7) | 2 (3.3) | 3 (5.1) |
| 20000 (run 2) | 344 | 37 (10.8) | 14 (4.1) | 9 (2.6) | 8 (2.3) | 23 (6.7) | 13 (3.8) |
| 30000 (run 3) | 338 | 35 (10.4) | 10 (3.0) | 8 (2.4) | 7 (2.1) | 15 (4.4) | 10 (3.0) |
Table 1-1 Test sera: numbers and %’s of positive IgE reactions (ODc >0.05):
1.1.3.1 Numbers and associations of positive IgE reactions
Approximately 10 % of all sera showed a positive reaction to at least one allergen. The positive reactions to different allergens showed significant associations:
Thus, of the 6 sera in the 1-9999 series with at least one positive IgE reaction, 1 serum was positive for three allergens (T. urticae, P. persimilis and A. colemani), and one for two allergens (T. urticae and A. colemani). Associations for the 20000 and 30000 series are summarized in the following two-by-two tables.
Associations were most pronounced for IgE to T. urticae and P. persimilis ( c² = 80 to >100), for the combination of IgE to T. urticae and A. colemani ( c2 = 63-73), while IgE to H. miles showed least associations with other positive reactions.
Run 2 |
Phytoseiulus persimilis + | Phytoseiulus persimilis - | Run 3 | Phytoseiulus persimilis + | Phytoseiulus persimilis - | |
| Tetranychus urticae + | 6 | 8 | Tetranychus urticae + | 5 | 5 | |
| Tetranychus urticae - | 3 | 327 | Tetranychus urticae - | 3 | 325 | |
| c² >100 | p < 0.001 | c² = 81 | p < 0.001 | |||
| Run 2 | Hypoaspis miles + | Hypoaspis miles - | Run 3 | Hypoaspis miles + | Hypoaspis miles - | |
| Tetranychus urticae + | 2 | 6 | Tetranychus urticae + | 1 | 9 | |
| Tetranychus urticae - | 6 | 330 | Tetranychus urticae - | 6 | 322 | |
| c² = 21.8 | p < 0.001 | c² = 0.44 | p = NS | |||
| Run 2 | Amblyseius cucumeris + | Amblyseius cucumeris - | Run 3 | Amblyseius cucumeris + | Amblyseius cucumeris - | |
| Tetranychus urticae + | 9 | 5 | Tetranychus urticae + | 4 | 6 | |
| Tetranychus urticae - | 14 | 316 | Tetranychus urticae - | 11 | 317 | |
| c² = 62.3 | p < 0.001 | c² = 22.7 | p < 0.001 | |||
| Run 2 | Aphidius colemani + | Aphidius colemani - | Run 3 | Aphidius colemani + | Aphidius colemani - | |
| Tetranychus urticae + | 7 | 7 | Tetranychus urticae + | 5 | 5 | |
| Tetranychus urticae - | 6 | 324 | Tetranychus urticae - | 5 | 323 | |
| c² = 73.0 | p < 0.001 | c² = 63.4 | p < 0.001 | |||
| Run 2 | Hypoaspis miles + | Hypoaspis miles - | Run 3 | Hypoaspis miles + | Hypoaspis miles - | |
| Phytoseiulus persimilis + | 2 | 7 | Phytoseiulus Persimilis + |
1 | 7 | |
| Phytoseiulus persimilis - |
6 | 329 | Phytoseiulus persimilis - |
6 | 324 | |
| c² = 8.37 | p = 0.004 | c² = 0.71 | p = NS | |||
| Run 2 | Amblyseius cucumeris + | Amblyseius cucumeris - | Run 3 | Amblyseius cucumeris + | Amblyseius cucumeris - | |
| Phytoseiulus persimilis + |
6 | 3 | Phytoseiulus persimilis + |
3 | 5 | |
| Phytoseiulus persimilis - |
17 | 318 | Phytoseiulus persimilis - |
12 | 318 | |
| c² = 43.9 | p < 0.001 | c² = 13.9 | p < 0.001 | |||
| Run 2 | Aphidius colemani + | Aphidius colemani - | Run 3 | Aphidius colemani + | Aphidius colemani - | |
| Phytoseiulus persimilis + |
6 | 3 | Phytoseiulus persimilis + |
4 | 4 | |
| Phytoseiulus persimilis - |
7 | 328 | Phytoseiulus persimilis - |
6 | 324 | |
| c² = 83.5 | p < 0.001 | c² = 47.5 | p < 0.001 | |||
| Run 2 | Amblyseius cucumeris + | Amblyseius cucumeris - | Run 3 | Amblyseius cucumeris + | Amblyseius cucumeris - | |
| Hypoaspis miles + | 4 | 4 | Hypoaspis miles + | 1 | 6 | |
| Hypoaspis miles - | 19 | 317 | Hypoaspis miles - | 14 | 317 | |
| c² = 18.0 | p < 0.001 | c² = 0.12 | p = NS | |||
| Run 2 | Aphidius colemani + | Aphidius colemani - | Run 3 | Aphidius colemani + | Aphidius colemani - | |
| Hypoaspis miles + | 3 | 5 | Hypoaspis miles + | 1 | 6 | |
| Hypoaspis miles - | 10 | 326 | Hypoaspis miles - | 9 | 322 | |
| c² = 17.0 | p < 0.001 | c² = 0.44 | p = NS | |||
| Run 2 | Aphidius colemani + | Aphidius colemani - | Run 3 | Aphidius colemani + | Aphidius colemani - | |
| Amblyseius cucumeris + | 8 | 15 | Amblyseius cucumeris + | 2 | 13 | |
| Amblyseius cucumeris - | 5 | 316 | Amblyseius cucumeris - | 8 | 315 | |
| c² = 56.3 | p < 0.001 | c² = 2.71 | p = 0.10 |
Table 1-2 The association of positive IgE reactions to different allergens.
1.1.3.2 Correlation between year 2 and year 3 results
For 310 subjects sera from both year 2 and year 3 were tested, and the results showed a strong correlation. Table …gives the associations as two-by-two tables for each of the five allergens:
| Tetranychus urticae | Year 3+ | Year 3 - | Phytoseiulus persimilis | Year 3+ | Year 3 - | |
| Year 2 + | 8 | 6 | Year 2 + | 5 | 4 | |
| Year 2 - | 2 | 294 | Year 2 - | 3 | 298 | |
| c² > 100 | p < 0.001 | c² = 82.9 | p < 0.001 | |||
| Hypoaspis miles | Year 3+ | Year 3 - | Amblyseius cucumeris | Year 3+ | Year 3 - | |
| Year 2 + | 2 | 6 | Year 2 + | 7 | 16 | |
| Year 2 - | 4 | 298 | Year 2 - | 7 | 280 | |
| c² = 12.2 | p < 0.001 | c² = 32.5 | p < 0.001 | |||
| Aphidius colemani | Year 3+ | Year 3 - | ||||
| Year 2 + | 8 | 5 | ||||
| Year 2 - | 2 | 295 | ||||
| c² > 100 | p < 0.001 | |||||
Table 1-3 The correlation between the IgE in run 2 and in run 3.
Quantitative relations between levels of IgE to each allergen as measured in the two consecutive years were assessed as the (Pearson) correlations for the ODc values; thus calculated correlation coefficients (for non-transformed values) were 0.915, 0.666, 0.784, 0.906 and 0.781 for IgE to T. urticae, P. persimilis, H. miles, A. cucumeris and A. colemani, respectively.
These relations are further illustrated in the following graphs, which clearly show that the stronger reactions (OD’s >0.1-0.2) in year 2 were practically always reproduced in year 3; that there were apparently no examples of a markedly present ‘new’ incident sensitization during follow-up; and that the large majority of ‘discordant’ results was found among sera with relatively weak to borderline reactions.
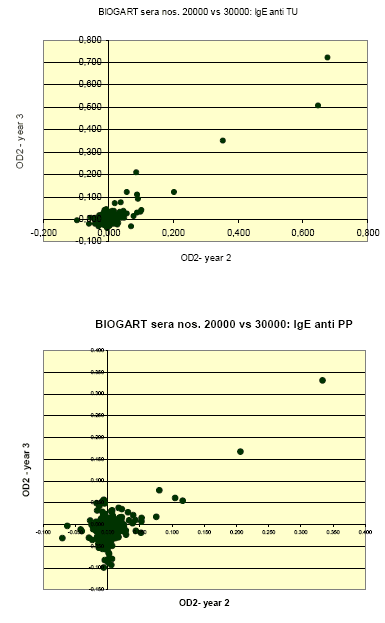
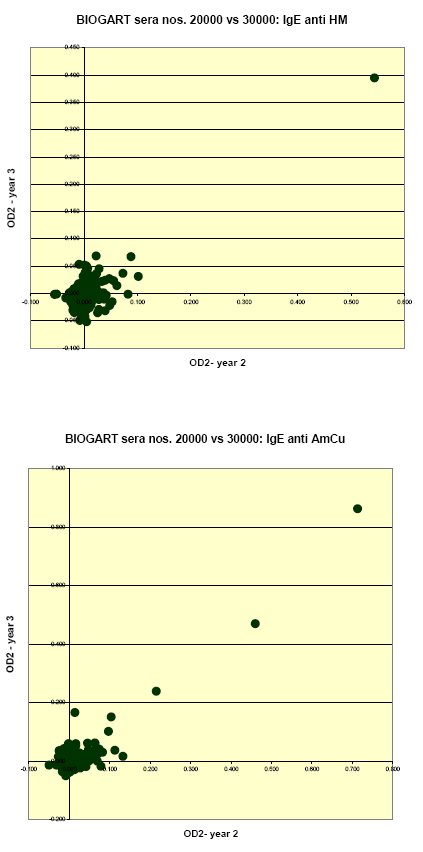
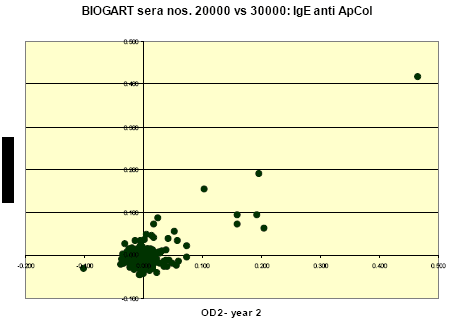
1.1.4 IgE inhibition experiments and immunoblotting
The strong or at least significant correlations between reactions to IgE different allergens suggest that the T. urticae and P. persimilis preparations might be partially cross-reactive. Therefore IgE inhibition EIAs were performed in which the reactions of moderately to strongly positive sera with a coating of T. urticae or P. persimilis allergens respectively were blocked by adding the same or a possibly cross-reacting allergen extract to the fluid phase.
The results indicated that
- reactions of anti-T. urticae positive sera with T. urticae allergens could be dose-dependently inhibited with T. urticae extract, such that 50% inhibition was achieved at approximately 4 mg/mL T. urticae extract (protein concentration);
- IgE anti-T. urticae could also be inhibited with the P. persimilis extract, with 50% inhibition reached at approx. 45 mg/mL;
- partial inhibition was seen after incubation with A. cucumeris extract: approx. 30% at the highest concentrations (45 mg/mL).
- other extracts: H. miles, A. colemani, and a house dust mite extract (HDM) did not show any significant inhibition.
The results of the plate with P. persimilis coating in which anti-P. persimilis positive serum was tested confirmed the cross-reactivity between T. urticae and P. persimilis extracts:
- P. persimilis, T. urticae and A. cucumeris showed dose-dependent inhibition, with C (inh, 50%) values of approx. 10, 5, and 20 mg/mL, respectively. Thus, T. urticae was a more effective inhibitor of IgE anti-P. persimilis than P. persimilis itself, suggesting that the observed IgE anti-P. persimilis reactions – at least of the sera used for these inhibition experiments - may be largely or completely due to an initial sensitization to Tetranychus allergens and cross-reactivity with P. persimilis.
- The other extracts, H. miles, A. colemani and HDM did not inhibit the IgE anti-P. persimilis binding.
For the H. miles inhibition experiment only serum from one subject with sufficiently high titre was available. Curiously enough, the reaction could not be inhibited with H. miles extract (and neither with the other extracts), which suggest that even this relatively strong reaction might in fact be non-specific.
In the IgE anti-A. cucumeris inhibition EIA only A. cucumeris itself showed sufficient potency to reach close to 50% inhibition – at ~60 mg/mL. None of the other extracts showed inhibition except HDM, which showed a dose-response inhibition curve levelling off at ~30% inhibition – which suggested partial cross-reactivity by allergens shared by A. cucumeris and HDM.
The IgE anti-A. colemani reaction was only inhibited by A. colemani itself, with a C(inh, 50%) of approximately 1 mg/mL , whereas none of the other preparations showed any inhibition.
SDS-PAGE and immunoblotting were performed with the same sera. Results confirmed
- the similarity of T. urticae and P. persimilis preparations, with similar staining patterns for proteins after SDS-PAGE, and after incubation of blot strips;
- the relatively weak, but clearly demonstrable reactions of IgE with one or several proteins in each of tested allergen preparations.
1.2 Analysis of follow-up
1.2.1 Selection of material
A remarkable finding of the IgE serology in the material from run 2 and 3 was the strong correlation between the levels of IgE sensitization for each specific allergen – expressed as the blank-adjusted OD values in EIAs with 1/10 diluted sera - in the two consecutive follow-up years (see Appendix 1, paragraph 1.2.3, Table 3, and the graphs). This strongly suggested that the incidence of new sensitization during the study would have been low – at least during the later follow-up years. To assess whether this was indeed the case, and also true (or not) for the earlier years in the study, all still available sera from workers with one or more positive reactions in run 2 and /or 3 in the thus far reported test series were simultaneously retested on the same allergen(s). Thus, from each greenhouse worker in this group the corresponding serum samples from run 0 and run 1 were recovered and tested in allergen-coated EIA plates, together with his/her serum samples from run 2 and/or 3 of which at least one had been found positive.
1.2.2 Analytical methods
The test method for specific IgE was essentially the same as described in Appendix I for the primary test series, with sera tested at a 1/10 dilution in PBTG plus negative control serum in microwells coated with the various allergen preparations, and with appropriate control microwells in each plate:
- for each allergen controls without serum (reagent blank or no serum control);
- for each serum a non-coated control well to correct for non-specific sticking of IgE to the plate (Appendix 1.1.2).
The general design per test plate was however different, and always such that for each subject all available serum samples (from 2 to 4) were tested on a specific allergen in adjacent wells of the same plate, to allow an optimal comparison of longitudinal changes in allergen-specific IgE reactivity for each individual worker.
For practical reasons these sera were clustered in a number of groups:
a) sera tested (runs 0 and 1) or retested (run 2 and 3) on four allergens: T. urticae, P. persimilis, A. cucumeris and A. colemani; n = 9
b) sera (re)tested on T. urticae and P. persimilis; n = 12
c) sera (re)tested on A. cucumeris and A. colemani; n = 35
Most of the run 2 and run 3 series included in set a) had been positive on only 2 or 3 of the four test allergens, and similarly many of the run 2 and/or run 3 sera of groups b) and c) had been positive on only one of the allergens. Therefore this design also implicated retesting of a substantial number of previously negative IgE tests.
Given the low number of clearly positive IgE anti-H. miles reactions, and their apparent lack of specificity shown by the EIA inhibition experiments (Appendix 1.2.4), no longitudinal analyses of IgE anti-H. miles reactions were performed.
1.2.3 Results
A relatively high proportion of the positive IgE reactions found in the previous test series were very weak – just above the pre-set cut-off OD values of 0.05. As a consequence, retest results for several of these previously positively tested samples were in a number of cases now just below the limit of detection, and positivity could thus not be confirmed. This was particularly the case for sera from workers who had shown a positive reaction in only one of the two test runs.
In the following tables the results are summarized semi-quantitatively for all tested workers involved in the follow-up serology, with the strength of the IgE reaction (adjusted OD value) given in categories as follows:
below 0.05: - / neg.
0.05 – 0.10 +/-
0.10 - 0.20 +
0.20 – 0.50 ++
>0.50 +++
Not done (serum not available): ND.
| Amblyseius cucumeris | IgE in follow-up study | IgE response vs. time | |||
| participant | year 0 | year 1 | year 2 | year 3 | |
| 20x (3-4 years)* | neg | neg | neg | neg | |
| 2x (2 years)* | neg | neg | neg | neg | |
| A | neg | +/- | + | + | new sensitization in year 1 |
| B | +/- | +/- | + | + | slight increase year 1 -> 2 |
| C | neg | neg | +/- | +/- | new sensitization in year 2 |
| D | +/- | +/- | +/- | +/- | constant, low |
| E | neg | + | neg | neg | transient |
| F | ++ | ++ | ++ | ++ | constant positive |
| G | neg | neg | +/- | nd | new, low in year 2 |
| H | +/- | +/- | +/- | nd | constant, low |
| I | + | +/- | +/- | nd | constant, low |
| J | + | neg | neg | nd | transient |
| K | + | + | + | nd | constant positive |
| L | + | +/- | +/- | nd | constant, low |
| M | +/- | +/- | +/- | nd | constant, low |
*) For 20 subjects, sera from all 3 or 4 years were negative; for 2 workers, sera from 2 years were available and both negative.
Table 1.3.1: IgE response to Amblyseius cucumeris – development in time
Thus, 7 subjects showed a constant (weakly to moderately) positive IgE response to A. cucumeris, 3 showed newly developed sensitization, in one the data suggested a slight increase in an already positive response, and in two there had been an early response that seemed to decrease in later years.
| Aphidius colemani | IgE in follow-up study | IgE response vs. time | |||
| participant | year 0 | year 1 | year 2 | year 3 | |
| 22x (3-4 years)* | neg | neg | neg | neg | |
| 1x (2 years)* | neg | neg | neg | neg | |
| N | +/- | + | + | + | slight increase year 0 -> 1 |
| O | neg | + | neg | neg | transient |
| C | neg | neg | ++ | +/- | new sensitization in year 2, thereafter decrease |
| P | neg | neg | +/- | +/- | new, low in year 2 |
| Q | +/- | ++ | neg | neg | transient |
| R | ++ | ++ | + | ++ | constant positive |
| F | neg | neg | + | neg | transient |
| S | +/- | +/- | +/- | +/- | constant, low |
| H | ++ | + | +/- | nd | constant, gradual decline |
| I | ++ | ++ | + | nd | constant, gradual decline |
| T | +/- | nd | + | nd | constant, (low) positive |
| L | ++ | ++ | ++ | nd | constant positive |
* For 22 subjects, sera from all 3 or 4 years were negative; for 2 workers, sera from 2 years were available and both negative.
Table 1.3.2: IgE response to Aphidius colemani– development in time.
Over-all, 4 subjects showed a constant (weakly to moderately) positive IgE response to A colemani, and two a positive response that seemed to become gradually weaker in time. In 2 workers there appeared to be new sensitization during the study, and in one the data suggested a slight increase in an already positive response. In three workers a transient response was observed.
| Phytoseiulus persimilis | IgE in follow-up study | IgE response vs. time | |||
| participant | year 0 | year 1 | year 2 | year 3 | |
| 15x (3-4 years)* | neg | neg | neg | neg | |
| U | +/- | + | + | + | increase year 0 -> 1 |
| P | neg | neg | + | neg | transient |
| R | + | + | + | + | constant positive |
| V | neg | neg | neg | + | new in year 3 |
| H | + | +/- | +/- | nd | constant, with decline |
| I | + | + | +/- | nd | constant, with decline |
* For 15 subjects, sera from all 3 or 4 years were negative; for 2 workers, sera from 2 years were available and both negative.
Table 1.3.3: IgE response to Phytoseiulus persimilis – development in time.
Over-all, 1 worker showed a constant positive IgE response to P. persimilis, and two a positive response that seemed to become gradually weaker in time. In 1 worker there appeared to be new sensitization during the study, and in one the data suggested a slight increase in an already positive response. In one worker a transient response was observed.
| Tetranychus urticae | IgE in follow-up study | IgE response vs. time | |||
| participant | year 0 | year 1 | year 2 | year 3 | |
| 9x (3-4 years)* | neg | neg | neg | neg | |
| X | +/- | +/- | neg | neg | transient (weak) |
| Y | +/- | neg | neg | neg | transient (weak) |
| U | ++ | +++ | ++ | +++ | constant positive, with increase year 0 -> 1 |
| Z | ++ | + | + | + | constant positive |
| aa | neg | neg | neg | + | new in year 3 |
| R | ++ | + | + | ++ | constant positive |
| ab | +/- | +/- | +/- | neg | weak, gradual decline |
| ac | neg | nd | neg | +/- | new (weak) sensitization in yr 3 |
| V | +/- | +/- | +/- | +/- | constant, weak |
| S | +/- | +/- | +/- | +/- | constant, weak |
| I | + | + | +/- | nd | constant positive, with decline (?) |
| ad | nd | ++ | +++ | nd | strong, increase in time |
* For 15 subjects, sera from all 3 or 4 years were negative; for 2 workers, sera from 2 years were available and both negative.
Table 1.3.4: IgE response to Tetranychus urticae – development in time
Over-all, 7 workers showed a constantly positive IgE response to T. urticae, and one a positive response that seemed to become gradually weaker in time. In 2 workers there appeared to be new sensitization during the study, and in 2 the data suggested a slight increase in an already positive response. In 2 workers a transient response was observed.
1.3 Discussion
No data are available in the literature on the development in time of these IgE responses, thus no comparison with previously published data is possible. The results however further underline that the IgE responses detected with these EIA methods were very weak and therefore in many cases difficult to reproduce upon re-testing. Nevertheless, definitely positive IgE reactions (eg. OD values >0.15-0.20) could in general be easily reproduced, and for a number of the workers clearly positive IgE responses could be noted over all the years. On the other hand, sera from these subjects did - with a few exceptions - not show an obvious increase in IgE levels in time. Instead it appeared that the most pronounced IgE responses had been present from the start of the study. This may be in line with the fact that the study is no real cohort study but essentially a large cross-sectional survey with an extensive follow-up component. Thus the relatively strong IgE reactions of some sera from the first study periods (runs 0 and 1) could be due to work-related sensitization in the years of employment preceding the study. Alternatively, the reactions may reflect cross-reactivity with other insects or mites encountered in teh general environment, in which case the IgE responses might have been much less relevant.
1.4 Conclusions
The rather low frequency of specific IgE responses to the beneficial predatory mites and insects, and to the pest mite Tetranychus urticae was confirmed in this follow-up serology study. In fact, where positive, the reactions of most sera were rather weak. There was very little evidence of incident sensitization during the follow-up; in those few individuals where serology data suggested an increase in the response, or newly emerging sensitization, the levels remained usually relatively low, at least much lower than those found in sera from subjects with more or less constant positive responses.
Version 1.0 August 2007, © Danish Environmental Protection Agency