Survey as well as health assessment of chemical substances in school bags, toy bags, pencil cases and erasers
5 Health assessment
- 5.1 Assessment of the health risk when using school bags, toy bags, erasers and pencil cases
- 5.2 Assessment of single substances
- 5.3 Total assessment
For assessment of the health risk at daily use of school bags, toy bags, erasers and pencil cases a selection of the effect levels of the found substances are assessed in relation to the relevant exposure time and way of exposure.
The calculations are made with basis in EC’s Technical Guidance Document (TGD) (European Commission, 2003).
In the survey 11 specific substances are selected for an assessment in corporation with the Danish Environmental Protection Agency. The selection is based on an interaction between the classification of the substances, the found concentrations as well as the number of products in which they are found.
At first an assessment of the health properties of the selected substances is carried out. Then exposure calculations are made based on worst case considerations which are used to assess the health risk of the selected substances in the analyzed products.
5.1 Assessment of the health risk when using school bags, toy bags, erasers and pencil cases
Exposure from school bags, toy bags, erasers and pencil cases takes partly place by inhalation of volatiles which the products emit (measured through the “headspace” analyses) and partly through dermal absorption of substances through skin contact with the products (measured through emission to the artificial sweat analyses).
For erasers there is a further exposure possibility through oral intake as it may be expected that some children bite or suck the erasers and even swallow a bit of them. Some erasers in the survey smell good and have a shape which may encourage children to put them into the mouth. Erasers with a design as a lip stick, a burger, grapes or similar are a part in the survey.
No analyses with the purpose to quantify the total amounts of the constituents in erasers are made – with exception of a few phthalates.
As a general rule no migration analyses of the erasers are made in relation to artificial saliva but on the contrary migration analyses in relation to artificial sweat. These analysis results will be used in a risk assessment of oral intake of constituents in the erasers as the difference of the liquids artificial saliva and artificial sweat is not large (the main difference is the temperature, which of course have an impact on the amount that migrates to the two different solutions). However, this means that there may be certain reservations in the conclusions but it is assumed that there are larger uncertainties in the concentrations of the semi-quantitative analyses than assuming that the results from the artificial sweat analyses may be transferred directly as artificial saliva results. However, the phthalate analyses showed relatively high concentrations of certain phthalates (among others DEHP) and the risk assessment showed that the concentrations can be problematic. Based on this, a single migration analysis to artificial saliva for the eraser with the highest content of DEHP is made afterwards.
The basic calculations for the three types of exposures are stated in the following.
5.1.1 Exposure through inhalation
In theory, exposure by inhalation can last from the purchase of the product until it is no longer in use (discarded). The substances which the consumer is exposed to during a possible unpacking of the product and at the beginning of the use period can roughly be assumed to be the substances found via the “headspace” analyses (analyse of substances evaporating from the products).
However, it must be noted that due to problems with the analytical equipment the results from the “headspace” analyses are not exact but only indicative. It is assess med that the error rate is between 10 and 500%.
Exposure by inhalation is expressed as the concentration of the chemical substance in the air in the inhalation zone and the exposure is expressed as an average concentration during a reference period of for instance one day. For exposure by inhalation both a short-term scenario for the acute toxicity and a long-term scenario for the chronic toxicity are calculated according to TGD if the related NOAEL values are found.
For estimation of the exposure by inhalation the concentration in the air, the inhalation rate and the air volume must be known (the inhalation zone or the size of the room).
The inhalation rate for children at moderate activity is 1.2 m³/hour according to TGD.
At short-term exposure air volume is set to 2 m³ according to TGD to represent the air gap which is directly around the person. This value is most probably valid for an adult and therefore 1 m³ is used instead to represent a child’s inhalation zone.
At long-term exposure an air volume of 20 m³ is used as a standard (8 m³ room with 2.5 m height to the ceiling). It may be discussed which value to use. In theory the children are exposed both to the substances evaporating from the products at home (and here only from their own products) and at school but here from many more (similar?) products in a much larger room. This is not taken into consideration in the exposure calculation but as worst case a relatively small room of 20 m³ is used.
The concentration in closed rooms is assumed to be larger than in ventilated rooms. For the calculation of the concentration in the room it is assumed that the substance is evaporated immediately to the whole room and is homogeneously spread out. It is left out of consideration that the evaporation and thus the concentration of the substances become smaller over time.
As even small children may be assumed to be in contact with the products or be in the same room in which the products are used/placed, a long-term scenario with long-term exposure is chosen from a worst case consideration where a respiration rate of 8.3 m³/day for a child at the age of 3-5 years is used (according to TGD).
In TGD no standard weight for a child is stated. For the sake of a realistic “worst case”, children’s weight is used at the youngest age where they are expected to play with toy bags, i.e. 3 years. Children are generally somewhat older (close to the school age) before they use school bags, erasers and pencil cases but they can still be exposed by inhalation by being in the same room as the products. Children’s weight for a certain age can be found via official growth curves. Netdoktor.dk has a table of girls’ and boys’ weight which comes from an old Scandinavian survey. They point out that it is an old survey and that in general the children have become a little higher and heavier since then (Netdoktor, 2006. According to these tables 3-year-old boys weight 12.7 kg (low weight) and 3-year-old girls 12.0 kg (low weight). Therefore, 12 kg is used a child’s weight as worst case.
It is presumed that children can be exposed to the substances which evaporate from the products up to 6 hours during one day. In a school situation children will have school bag, pencil case and eraser close to the body during the whole school day.
For the weight of the products the total weight of the product is used even if some of the substances are only found in the handle or in the outside of a school bag.
According to the Technical Guidance Document on Risk Assessment of the EU the exposure is calculated by inhalation through the following formula (European Commission, 2003):
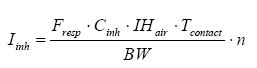
where
| Iinh | The amount of inhaled substance | µg/kg bw/day |
| Fresp | Inhalable fraction of the substance | 1, i.e. 100% |
| Cinh | The concentration in the air | µg/m³ |
| IHair | The inhalation rate of the person | m³/hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
Where the concentration in the air Cinh is calculated according to the following formula (European Commission, 2003):
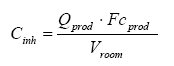
where
| Cinh | The concentration in the air | µg/m³ |
| Qprod | Amount of the product used in the room | g |
| Fcprod | Weight fraction of substance in the product | µg/g |
| Vroom | Volume of the room | m³ |
The equation used for the calculations is thus:
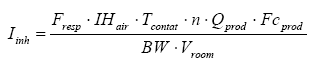
It must be noted that the values of analysis used for the risk calculations for exposure by inhalation are too high – maybe between a factor 100 to 500 too high. This is due to the fact that the samples were set for evaporation – at first during the night at 40 ºC where after the intention was that the samples were to be analyzed. But due to problems with the analysis equipment all the samples have hereafter been placed for about three weeks at room temperature before they were analyzed. This means that the results do not represent the evaporation during the 6 hours which is used as daily exposure but on the contrary the evaporation during three weeks.
Quite clearly, the evaporation will be largest at the beginning and at a certain time a kind of equilibrium will be reached for which reason the evaporation will be smaller. Besides the temperature plays a role. The evaporation is significantly higher at the beginning at the higher temperature than at room temperature. Thus, it is difficult to estimate the factor with which the analysis results shall be divided to illustrate the evaporation per day but if the analyzed values are used as they are the calculated MoS (Margin of Safety) will thus be significantly lower than the real value. When the calculated MoS are above 100 it is certain that the exposure will not present any health risk.
5.1.2 Exposure through the skin
In the scenario for skin exposure it is assumed that the products are used in the hand which will then have the primary exposure. For erasers and pencil cases this is clear while skin exposure for toy bags and school bags may also occur when the bag is on the back. However, it is assumed that the children wear clothes whereby the skin exposure is minimal. Therefore, only skin exposure through the hand is used.
Before skin absorption the chemical substance shall be transferred from the product to the skin. When it is on the skin the substance can be absorbed through the skin and from there to the blood and then spread in the rest of the body.
Migration analyses simulating sweat have been conducted. These analyses show how large amounts of the substances that can migrate (be transferred) when the product is touched by the hand. The substances being found in the extraction liquid are the substances which can potentially be absorbed through the skin at contact with the products.
The potential absorption (the exposure) can be expressed through the following equation (European Commission, 2003):
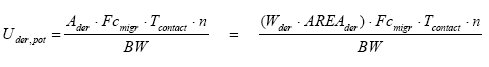
where
| Uder,pot | Potential absorption of the chemical substance | µg/kg bw/day |
| Ader | Total amount of substance which the skin potentially is exposed to | g |
| Wder | The weight of the product on the skin | g/cm² |
| AREAder | Area of the contact between the product and the skin | cm² |
| Fcmigr | Fraction of substance which migrates | µg/g per hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
Starting point is the amount of the substance which migrates per cm² of the product and this value is compared with the area of the product which touches the skin for a certain time.
It is assumed that it is only the area of a child’s palms (on both hands) which are in touch with the product. This value is not found in TGD but a list of men’s and women’s surface area of the hands in relation to the total surface area of the body is available (for women 731 cm² for both front and rear on both hands in relation to a total body area of 16,900 cm²). This ratio is compared with the information from TGD that the total body area of a child body is 6,030 cm² for a child at the age of 2-3 years, i.e. same age as in the definition of the body weight. In this way the result is that a 3-year-old child’s hands have a surface area of 131 cm², when the area is divided by 2 as it is assumed that the products are only touched with the inside of the hands.
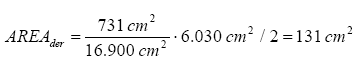
The surface of a child’s hands is only an approximation as it is assumed that the ratio of sizes between an adult woman’s hands and body is the same for a child.
As basis of the calculations is chosen that a child has its school bag/toy bag and its pencil case in the hands for maximum 1 hour a day in total. The migration analyses to artificial sweat are conducted in a way where a piece of the product is extracted in artificial sweat for 4 hours. The migration amounts are divided by 4 as the samples have stayed for 4 hours in artificial sweat and thus represent the migration amount per 4 hours.
For the analyses the products are cut into small pieces (cubes) of 2-3 mm crosswise. This means that the surface becomes significantly larger than the normal surface of the products. Furthermore, touch of the products will normally only take place on the outside of the product. The measured concentration can thus be overestimated by a factor 3 as a minimum.
For a school child the skin exposure for an eraser can be significantly longer as children often use it and also play with it. Therefore, for erasers, exposure time is calculated for 4 hours as worst case. The child’s weight is set to 12 kg in all cases.
After exposure of the skin the chemical substance shall pass the skin before it is a real absorption. The dermal absorption of the substances is generally estimated due to lacking data. If no other information is found a dermal absorption of 100% and a dermal absorption of 10% for substances with a molar weight larger than 500 g/mole which at the same time has a log KOW less than -1 or larger than 4 (as stated in TGD) are used as standard. It is more difficult for large molecules to penetrate the skin like very lipophilic substances.
The factor for the dermal absorption (1 or 0.1) is multiplied by the potential absorption (worst case).
5.1.3 Exposure through intake
Regarding the erasers there is a possibility of an exposure through the mouth, for instance if the children chew or suck the erasers. At oral exposure the absorption takes place after migration of the substances from the erasers and mixture in saliva. Absorption is assumed to take place via the mucous membranes in the oral cavity or the gastrointestinal canal.
As described earlier, as a main rule no migration analyses of the erasers in relation to artificial saliva are conducted but on the contrary migration analyses in relation to artificial sweat. Based on relatively high concentrations of certain phthalates (i.e. DEHF) a single migration analysis for artificial saliva of the eraser with the highest content of DEHP has subsequently been conducted. The results of the migration analyses for artificial sweat are used as a reasonable approximation for the rest of the products.
As basis for the oral intake the equation for migration of substances from a product to foods/beverages has been used. The foods/beverages are then ingested (European Commission, 2003). However, it is not exactly this situation which occurs when a child sucks/chews an eraser and for this reason the equation has been adjusted. The erasers are all so small that a child can suck, lick and chew the whole surface of the eraser. This is assumed as worst case that the measured migration from the whole eraser is ingested – no matter the size of the eraser.
The oral intake can thus be calculated from the formula below:
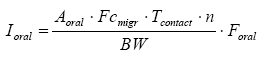
where
| Ioral | Amount of ingested substance | µg/kg bw/day |
| Aoral | Total amount of product which is licked or sucked | g |
| Fcmigr | Fraction of substances which migrates per time unit | µg/g/hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
| Foral | Fraction which is absorbed (bio available part) |
As basis of the calculation is chosen that a child licks, chews or sucks the eraser several times a day. The total exposure is assumed to be maximum 1 hour a day. As previously, the child’s weight is set to 12 kg (however, for a few calculations a weight of 20 kg is used to illustrate a school child’s weight).
The migration analyses for artificial sweat and artificial saliva are conducted in the way that a piece of the product is extracted in artificial sweat/saliva for 4 hours for which reason the result shall be divided by a factor 4 so that the measured values correspond to the exposure time. For the analyses the erasers are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface becomes substantially larger than the surface which an eraser normally has but if a child chews the eraser there is access to a larger surface of the eraser. The measured concentration may be overestimated by a factor 3 or more.
It must be noted that oral intake can also take place through hand-to-mouth, i.e. that hand or fingers having touched the product are put into the mouth afterwards. Thereby a transmission of a substance from fingers to mouth can take place. In literature it is stated that hand-to-mouth as an average lasts 3-10 minutes for which reason this part is assumed included in the chosen exposure time of 1 hour (Bremmer and van Veen, 2002; Green, 2002; Kiss, 2001).
After exposure of the mouth cavity the chemical substance shall pass the mucous membranes before a real absorption can be in question. The oral absorption of the substances is generally estimated due to lacking data. Therefore, an oral absorption of 100% is assumed as a standard.
For the phthalates (especially) where a total determination of the phthalate content is made the Danish Environmental Protection Agency has wanted a calculation of a scenario where it is assumed that the children will swallow a part of the eraser when they chew it. It is assumed that between 0.008 and 0.1 g of eraser is swallowed which corresponds to between approx. 0.01 and 0.08 cm³ for the relevant erasers – i.e. cubes of approx. 1.9 to 4.3 mm in height, width and length – an amount not quite unrealistic to swallow.
The oral intake can be calculated from the formula below:
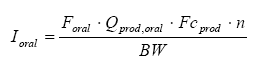
where
| Ioral | Amount of ingested substance | mg/kg bw/day |
| Foral | The oral absorption | |
| Qprod, oral | Amount of product which is ingested | g |
| Fcprod | Fraction of substance in the product | mg/g |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
5.1.4 Margin of Safety
For an assessment of the risk for the individual chemical substances the so-called Margin of Safety (MoS) is calculated. Here the calculated daily exposure (Iinh or Uder or Ioral) is set in relation to the zero effect level (NOAEL – No Observed Adverse Effect Level) according to the following formula:
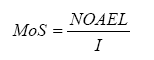
It is generally accepted that MoS must be at least 100 to declare a product as safe in use. In this way a safety factor of 10 for extrapolation of data from animals to humans is taken into account and a safety factor of 10 to take especially sensitive human individuals into account.
5.1.5 Total exposure
If the child is exposed to a substance from the same product through different ways of exposure the total absorption can be added.
Furthermore, other sources for the same chemical substances in the child’s surroundings may contribute to the total exposure.
5.2 Assessment of single substances
5.2.1 Isophorone
Application
Isophorone has a wide application as dissolvent for different artificial resins and polymers as well as for wax, fatty substances and oil. Isophorone is used in some printing inks, paints, varnishes and glues. Furthermore, it is used as chemical intermediate and in some pesticides (ATSDR, 1989; HSDB; IUCLID, 2000d; IPCS, 1995; Jensen AA, 1997a).
Isophorone occurs naturally in cranberries (Jensen AA, 1997a).
Identification
| Chemical name | 3,5,5-Trimethyl-2-cyclohexen-1-on |
| CAS-No. | 78-59-1 |
| EINECS No. | 201-126-0 |
| Gross formula | C9H14O |
| Molecular structure | 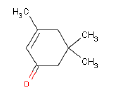 |
| Molecular weight | 138,21 g/mol |
| Synonyms | Isophorone Trimethylcyclohex-2-enon 3,5,5-Trimethyl-2-Cyclohexenon 1,1,3-trimethyl-3-cyclohexen-5-on |
Physical-chemical data
| Physical form | Colourless liquid with a peppermint like fragrance | Chemfinder, HSDB |
| Melting point | -8 °C | Chemfinder |
| Boiling point | 215.2 °C | Chemifinder |
| Steam pressure | 0.438 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Soluble. 12 g/l | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.7 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
XN; R21/22. XI; R36/37. Carc3; R40. |
Harmful. Dangerous in contact with skin and if swallowed. Local irritating. Irritates the eyes and the respiratory system. Possible carcinogenic effect. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Isophorone is easily absorbed in the body through lungs, skin or gastrointestinal tract (Jensen AA, 1997a). 14C labelled isophorone shows that 93% of the ingested oral amount of isophorone is mainly found in the urine and in the exhalation air after 24 hours (IPCS, 1995; HSDB; ATSDR, 1989). 100% absorption is thus assumed in the calculations.
Effects on health
Isophorone is damaging to health through ingestion and in contact with skin. In acute experiments and experiments of 90 days with mice and rats damages on liver and the central nervous system as well as deaths are seen at high doses. In long-term studies with mice and rats kidney damages are demonstrated (IPCS, 1995; ATSDR, 1989).
LD50 values (oral intake for rats) for isophorone are between 1000 and 3450 mg/kg bw (HSDB; IUCLID, 2000d).
Isophorone is irritating for both the eyes and the respiratory organs (HSDB; ATSDR, 1989; IPCS, 1995; IUCLID, 2000d). In the working environment examples of complaints about irritation effects at air levels of isophorone from 0.7 to 14 ppm are seen (Jensen AA, 1997a).
No reports about sensitizing properties of isophorone are made (IUCLID, 2000d; IPCS, 1995; HSDB).
NOAEL for rats fed with isophorone for 90 days was set to 102.5 – 163.8 mg/kg bw. At the test significant reductions of the body weight at high doses were seen. At a test of 90 days with dogs (oral intake) a NOAEL of more than 150 mg/kg bw was observed (highest dose tested and no effects observed) (IUCLID, 2000d).
NOAEL for rats and guinea pigs having inhaled isophorone for six weeks was 0.144 mg/l air based on kidney effects. No statement of how much air and thus how large a dose the animals have ingested per kg body weight (IUCLID, 2000d). NOAEL for rats and rabbits having inhaled isophorone for six months was 250 ppm (250 mg/kg) based on death rates (ATSDR, 1989).
Some weeks’ exposure of isophorone vapours of more than 100 ppm has given serious kidney and lung damages in test animals. At 2-3 times larger exposure effects on the liver are also seen (Jensen AA, 1997a).
Isophorone is classified as carcinogenic category 3 (Carc3) with R40”Possible risk of cancer”. Substances in this carcinogenic group are substances giving cause for reservation as they are possibly carcinogenic for humans but there is not sufficient information to conduct a satisfactory assessment of these (Stat. Ord. 329, 2002). A survey being the basis of this assessment is from a 2-year study of the carcinogenic properties of isophorone in mice and rats. The result of the study is that there was some indication of carcinogenic effect in male rats but no indication of carcinogenic effect neither in female rats nor female mice. In male mice an ambiguous indication of a carcinogenic effect was seen.
Some test animals with mice and rats indicate that isophorone is not reprotoxic (HSDB; ATSDR, 1989). However, there are tests with pregnant rats and mice exposed to a little above 100 ppm indicating the possibility of foetal malformation (Jensen AA, 1997a).
Isophorone does not show mutagenic properties (HSDB; IPCS, 1995).
Threshold limits
The threshold limit in the working environment for isophorone is 5 ppm or 25 mg/m³ with the remarks L and C (cancer), i.e. that the threshold limit is a limit value (L) which is not allowed to be exceeded and that the substance is on the list of substances which are regarded as carcinogenic (The Danish Working Environment Authority, 2005).
Assessment
Through analysis, isophorone is identified in the following 12 products. Isophorone is primarily identified through migration to artificial sweat but also through evaporation from two products. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g |
|
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 1 | ||
| Toy bag | 30B | 2 | ||
| Toy bag | 37B | 3 | ||
| Toy bag | 38C | 150 | ||
| School bag | 39B | 250 | ||
| School bag | 40B | Handle | 1 | |
| School bag | 41A | Inside | 10 | |
| School bag | 42C | 95 | 0.21 | |
| Pencil case | 31 A | 5 | ||
| Pencil case | 34 | 15 | 0.02 | |
| Pencil case | 35B | 40 | ||
| Pencil case | 43 | 1 | ||
| Product type | Product ID | Maxi- mum meas- ured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the pro- duct |
Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| School bag | 42C | 0.21 | 1 | 0.35 | 900 | 6 | 12 | 20 | 1.63 | 150 | 91,796 |
| Pencil case | 34 | 0.02 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.01 | 150 | 18,456, 806 |
The following exposure to isophorone is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.25 | 1 | 0.299 | 131 | 4 | 12 | 3.27 | 150.0 | 45., 3 |
| Toy bag | 30B | 0.5 | 1 | 0.020 | 131 | 1 | 12 | 0.11 | 150.0 | 1,394,931 |
| Toy bag | 37B | 0.75 | 1 | 0.009 | 131 | 1 | 12 | 0.08 | 150.0 | 1,965,069 |
| Toy bag | 38C | 37.5 | 1 | 0.066 | 131 | 1 | 12 | 26.99 | 150.0 | 5,558 |
| School bag | 39B | 62.5 | 1 | 0.058 | 131 | 1 | 12 | 39.60 | 150.0 | 3,788 |
| School bag | 40B | 0.25 | 1 | 0.136 | 131 | 1 | 12 | 0.37 | 150.0 | 405,618 |
| School bag | 41A | 2.5 | 1 | 0.042 | 131 | 1 | 12 | 1.14 | 150.0 | 131,744 |
| School bag | 42C | 23.75 | 1 | 0.119 | 131 | 1 | 12 | 30.76 | 150.0 | 4,877 |
| Pencil case | 31 A | 1.25 | 1 | 0.036 | 131 | 1 | 12 | 0.49 | 150.0 | 308,446 |
| Pencil case | 34 | 3.75 | 1 | 0.040 | 131 | 1 | 12 | 1.66 | 150.0 | 90,577 |
| Pencil case | 35B | 10 | 1 | 0.087 | 131 | 1 | 12 | 9.48 | 150.0 | 15,829 |
| Pencil case | 43 | 0.25 | 1 | 0.054 | 131 | 1 | 12 | 0.15 | 150.0 | 1,014,595 |
The following exposure to isophorone is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.25 | 1 | 21.1 | 1 | 12 | 0.44 | 150.0 | 341,232 |
As worst case the highest of the above exposure values can be added as a child might be exposed to isophorone during a long time both by inhalation from a school bag and a pencil case at the same time as an exposure through the skin from a school bag, a toy bag, a pencil case and a eraser takes place as well as oral exposure when the child sucks or chews a eraser.
This scenario gives a total exposure of 81.42 µg/kg bw/day and when this value is compared with a NOAEL of 150 mg/kg bw/day the result is a Margin of Safety of 1842.
It is generally accepted that MoS must be at least 100 before a substance can be declared as safe in use. All the calculated MoS of the individual products are significantly above 100 and the assessment is thus that they do not represent any health risk with regard to isophorone. Exposure of isophorone through both inhalation and skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.2 BHT
Application
BHT is used as an anti-oxidant in foods (E321), animal feed, petroleum products, synthetic rubber, plastics material as well as vegetable oils and soaps. BHT is also widely used in cosmetic products. Furthermore, it functions as anti-skinning agent in paints and inks (Merck, 1983; OECD SIDS, 2002).
Identification
| Chemical name | 2,6-Di-tert-butyl-p-cresol |
| CAS-No. | 128-37-0 |
| EINECS No. | 204-881-4 |
| Gross formula | C15-H24-O |
| Molecular structure | 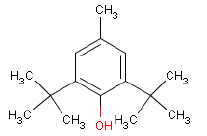 |
| Molecular weight | 220.35 g/mol |
| Synonyms | Butylenes hydroxytoluen (BHT) 2,6-Bis(1,1-dimethylethyl)-4-methylphenol |
Physical-chemical data
| Physical form | Colourless solid substance. | OECD SIDS, 2002 |
| Melting point | 71 °C | TOXNET ChemIDplus |
| Boiling point | 265 °C | TOXNET ChemIDplus |
| Steam pressure | 0.015 mm Hg at 20 °C | Dutch Institute for the Working Environment, 1991. |
| Water solubility | Weakly soluble: 0.0006 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 5.1 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
No | |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish Self classification (The Danish Environmental Protection Agency, 2001) |
Yes Xn;R22 N;R50/53 |
Harmful. Dangerous if swallowed. Dangerous for environment. Very toxic for aquatic organisms; may cause long-term adverse effects in the aquatic environment. |
Bioavailability
BHT is easily absorbed through the gastrointestinal tract and to a certain degree also through intact skin. Rats fed with a single dose BHT separated 80-90% of the dose via the urine after four days, most of it during 24 hours. For humans 66% is separated during 11 days via the urine (OECD SIDS, 2002).
In a test, rat skin was applied with 14C labelled BHT. Here the skin absorption was 13% of the applied dose (Nordic Council of Ministers, 1997). This value is therefore used in the calculations with dermal absorption.
Effects on health
BHT has a low acute toxicity. Tests with rats which orally consumed BHT resulted in a LD50 value of more than 2930 mg/kg bw (OECD SIDS, 2002).
BHT is slightly irritating for both skin and eyes (based on tests with rabbits) but does not show signs of sensitizing properties in animal tests (OECD SIDS, 2002; IUCLID, 2000e). Few cases of allergic responses towards BHT at humans are reported and this result despite the wide application of BHT as anti-oxidant in both foods and cosmetic products (OECD SIDS, 2002).
In animal tests, prolonged exposure to BHT has shown effects on lungs, liver, kidneys and thyroid gland. High sub-chronic doses of BHT can result in deaths at mice and rats either due to serious lung damages or massive bleedings. At chronic oral exposure, effects are first and foremost on liver and thyroid gland. Doses of above 25 mg BHT/kg bw/day result in hyperactivity of the thyroid gland and magnification of the liver from daily exposure of 7 days. Therefore NOAEL is 25 mg/kg bw/day (OECD SIDS, 2002).
IARC assesses that BHT is not classifiable in relation to the carcinogenic properties of the substance in humans. There are limited indications of the carcinogenic properties of BHT in animals and therefore an assessment for humans cannot be made (IARC, 1986).
BHT does not show mutagenic properties – Ames test is negative (OECD SIDS, 2002; IUCLID, 2000e).
Reproduction studies with mice and rats showed an effect (fewer pups per litter) at doses above 100 g/kg bw/day. NOAEL for this study was 25 mg/kg bw/day for rats (OECD SIDS, 2002).
Threshold limits
The threshold limit in the working environment for BHT is 10 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
BHT is identified in the following 6 products through analyses. BHT is primarily identified through migration to artificial sweat but also through evaporation from three erasers. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). More parts from same product have been analyzed. In the table below the highest measured value is stated when several values from same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 25 | 0.14 | |
| Eraser | 10 | 70 | 0.35 | |
| Eraser | 24 | 3 | 0.02 | |
| Pencil case | 35C | Inside | 10 | |
| Toy bag | 37B | 1 | ||
| Toy bag | 38C | 1 | ||
The found NOAEL value for BHT is for chronic effects for which reason a long-term scenario for exposure to BHT by inhalation from erasers, pencil cases and toy bags is solely calculated. In this way the following exposure to BHT is found:
| Product type | Product ID | Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
M³/hour | g | hours/- day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.14 | 1 | 0.35 | 900 | 6 | 12 | 20 | 1.09 | 25 | 22,949 |
| Eraser | 10 | 0.35 | 1 | 0.35 | 105 | 6 | 12 | 20 | 0.32 | 25 | 78,682 |
| Eraser | 24 | 0.02 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.01 | 25 | 3,076, 134 |
The following exposure to BHT is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 6.25 | 0.13 | 0.299 | 131 | 4 | 12 | 10.62 | 25 | 2,353 |
| Eraser | 10 | 17.5 | 0.13 | 0.671 | 131 | 4 | 12 | 66.67 | 25 | 375 |
| Eraser | 24 | 0.75 | 0.13 | 0.093 | 131 | 4 | 12 | 0.40 | 25 | 63,183 |
| Pencil case | 35C | 2.5 | 0.13 | 0.026 | 131 | 1 | 12 | 0.09 | 25 | 273,400 |
| Toy bag | 37B | 0.25 | 0.13 | 0.009 | 131 | 1 | 12 | 0.00 | 25 | 7.557,957 |
| Toy bag | 38C | 0.25 | 0.13 | 0.066 | 131 | 1 | 12 | 0.02 | 25 | 1,068,937 |
The following exposure to BHT is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | Kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 6.25 | 1 | 21.1 | 1 | 12 | 10.99 | 25 | 2,275 |
| Eraser | 10 | 17.5 | 1 | 105 | 1 | 12 | 153.13 | 25 | 163 |
| Eraser | 24 | 0.75 | 1 | 21.1 | 1 | 12 | 1.32 | 25 | 18,957 |
As worst case the highest of the above exposure values can be added as a child might be exposed to BHT both by inhalation from an eraser at the same time as an exposure through the skin from a toy bag, a pencil case and an eraser as well as oral exposure when the child sucks or chews an eraser.
This scenario gives a total exposure of 221 µg/kg bw/day and when this value is compared with a NOAEL of 25 mg/kg bw/day the result is a Margin of Safety of 113.
All the calculated MoS of the individual products are above 100 and therefore they are not assessed to represent any health risk with regard to their content of BHT. Exposure to BHT by inhalation, through skin absorption and oral intake from several products at the same time is neither assessed to represent any health risk for the examined products – but MoS is close to 100.
However, it must be noted that the most critical of the above exposure levels (lowest MoS) is for oral intake of an eraser (Product ID 10). In these calculations the values from artificial sweat are assumed to be the same as for artificial saliva (as analyses of artificial saliva are not conducted with analysis for BHT). Furthermore, it is assumed that a child sucks and chews the whole eraser which in this case is a rather big eraser of 4 x 1.3 x 11 cm giving most likely a too high estimate.
Furthermore, the erasers for the analyses are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface becomes significantly larger that the normal surface of an eraser.
To illustrate this surface of the eraser is here calculated if the whole eraser is cut into cubes of 0.3 x 0.3 x 0.3 mm. Thus it gives approx. 13 x 4 x 36 = 1872 pieces of eraser, each with a surface of 0.3 x 0.3 x 6 = 0.54 cm², i.e. a total surface of 1010 cm². For purposes of comparison, the eraser in uncut condition has a surface of ((4 x 1.3) + (4 x 11) + (1.3 x 11)) x 2 = 127 cm², i.e. nearly a factor 8 in difference. Therefore, the measured concentrations of BHT are most probably overestimated by a factor 8 whereby MoS is then approx. 1,300 for Product no. 10 at oral intake. MoS for total exposure from several products through several ways of exposure are thus approx. 287 instead.
All in all, these circumstances mean that exposure to BHT from several products at the same time and through several ways of exposure will most probably not constitute any risk for the examined products. However, it is unknown if other products may have a higher content of BHT and thus constitute a health problem if a child is exposed to several products with a high content of BHT. As BHT is much used as an anti-oxidant in foods there is a possibility of exposure through other sources. The total exposure is not assessed in this project.
5.2.3 Cyclohexanone
Application
Cyclohexanone is an artificial organic liquid which is primarily used as intermediate in the production of nylon. Additionally, it is also used as intermediate, additive agent and solvent in a number of products (IARC, 1989).
Identification
| Chemical name | Cyclohexanone |
| CAS-Nr. | 108-94-1 |
| EINECS Nr. | 203-631-1 |
| Gross formula | C6H10O |
| Molecular structure | 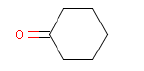 |
| Molecular weight | 98.14 g/mol |
| Synonyms | Cyclohexyl ketone Hexanon Ketohexamethylene Pimelic keton |
Physical-chemical data
| Physical form | White to weak yellowish oily liquid with a peppermint like fragrance | Chemfinder |
| Melting point | -47 °C (-31°C at TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 155.6 °C (155.4 °C at TOXNET ChemIDplus) | Chemfinder |
| Steam pressure | 4.33 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble: 25 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.81 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
R10 XN;R20 |
Flammable. Harmful. Dangerous by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about bioavailability of cyclohexanone is found but according to the threshold limit list the substance can be absorbed through the skin for which reason 100% absorption is assumed in the calculations.
Effects on health
Test of the acute toxicity of cyclohexanone on animals has shown a low acute oral toxicity. Oral LD50 values for rats are between 1296 and 3460 mg/kg bw/day and LD50 values (inhalation, 4 hours) for rats are between 10.7 and 32.5 mg/L (IUCLID, 2000h).
Tests with rabbits show that cyclohexanone is irritating for the skin and for the eyes. Cyclohexanone vapours can irritate mucous membranes and contact with the liquid can cause dermatitis in sensitive individuals (IUCLID, 2000h; HSDB). Exposure to vapours of 25 ppm of a few minutes’ duration seems to be unpleasant whereas at 75 ppm a severe irritation of nose, throat and eyes is observed (Jensen AA, 2003c).
Cyclohexanone does not seem to be sensitizing according to several animal tests whereas patch tests on humans have shown that cyclohexanone resins give an allergic contact dermatitis (IUCLID, 2000h; HSDB).
Exposure to 3,000 ppm cyclohexanone for a few hours is mortal for test animals. Exposure to 200-500 ppm affects the nervous system as it can give a prolonged response time (Jensen AA, 2003c).
IARC assesses that cyclohexanone is not classifiable in relation to the carcinogenic properties of the substance in humans. Indication of the carcinogenic properties of cyclohexanone in animals is insufficient (IARC, 1989).
The majority of the experimental data indicates that cyclohexanone is not genotoxic. Long-term tests with mice and rats indicate that cyclohexanone is not carcinogenic (OECD SIDS).
In a two generation study with rats, effects on the fertility at 1400 ppm were demonstrated but not at 500 ppm. However, it turned out that the effect was reversible during a subsequent recovery period after completion of exposure (OECD SIDS).
NOAEL for the chronic effects (weight increase) of cyclohexanone is calculated to 462 mg/kg bw/day for rats (Nilsson et al, 2006).
Threshold limits
The threshold limit in the working environment for cyclohexanone is 10 ppm or 40 mg/ m³ with the remark H, i.e. the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Cyclohexanone is identified in the following 7 products through analyses. Cyclohexanone is primarily identified through migration to artificial sweat but also through evaporation from two products. There are more values of analysis than those stated in the table below (see table 3.9A and 3.0B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Pencil case | 34 | 5 | 0.01 | |
| Pencil case | 35B | 10 | ||
| Toy bag | 38A | 3 | ||
| School bag | 39B | 4 | ||
| School bag | 40C | 1 | ||
| School bag | 41A | Inside | 2 | |
| School bag | 42A | 1 | 0.01 | |
No acute NOAEL for cyclohexanone is identified for which reason a long-term scenario for exposure to cyclohexanone through pencil cases, toy bags and school bags by inhalation is carried out.
At long-term exposure the following exposure to cyclohexanone through inhalation is found.
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/- day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 34 | 0.01 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.00 | 462 | 113,693, 925 |
| School bag | 42B | 0.01 | 1 | 0.35 | 900 | 6 | 12 | 20 | 0.08 | 462 | 5,937, 349 |
The following exposure to cyclohexanone is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 34 | 1.25 | 1 | 0.040 | 131 | 1 | 12 | 0.55 | 462 | 836,932 |
| Pencil case | 35B | 2.5 | 1 | 0.087 | 131 | 1 | 12 | 2.37 | 462 | 195,013 |
| Toy bag | 38A | 0.75 | 1 | 0.041 | 131 | 1 | 12 | 0.33 | 462 | 1,389,245 |
| School bag | 39B | 1 | 1 | 0.058 | 131 | 1 | 12 | 0.63 | 462 | 729,184 |
| School bag | 40C | 0.25 | 1 | 0.036 | 131 | 1 | 12 | 0.10 | 462 | 4,750,065 |
| School bag | 41A | 0.5 | 1 | 0.042 | 131 | 1 | 12 | 0.23 | 462 | 2,028,850 |
| School bag | 42A | 0.25 | 1 | 0.037 | 131 | 1 | 12 | 0.10 | 462 | 4,70,626 |
As worst case the highest of the above exposure values can be added as a child might be exposed to cyclohexanone during a long time both by inhalation from a school bag and a pencil case at the same time as an exposure through the skin from a school bag, a toy bag, a pencil case and a eraser takes place.
This scenario gives a total exposure of 3.41 µg/kg bw/day and when this value is compared with a NOAEL of 462 mg/kg bw/day the result is a Margin of Safety of 135.484.
All the calculated MoS of the individual products are significantly above 100 and therefore they are not assessed to represent any health risk with regard to cyclohexanone. Exposure to cyclohexanone both by inhalation and through skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.4 Phenol
Application
Phenol is primarily applied as an intermediate in organic syntheses and is a raw material in the production of bisphenol A, alkylphenols, caprolactam, salicylic acid, nitrophenols, diphenyl ether and halogeneous phenols. Beyond this a small amount is applied as component in cosmetic, medical drugs, binding agents, impregnating agents, paints, varnishes and solvents (EU ECB, 2006a).
Identification
| Chemical name | Phenol |
| CAS-Nr. | 108-95-2 |
| EINECS Nr. | 203-632-7 |
| Gross formula | C6H6O |
| Molecular structure | 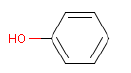 |
| Molecular weight | 94.11 g/mol |
| Synonyms | Benzenol Hydroxybenzene Oxybenzene |
Physical-chemical data
| Physical form | Light sensitive solid/thick liquid with sweet tarry fragrance. The colour varies from colourless to pink. | Chemfinder |
| Melting point | 40.5 °C | Chemfinder |
| Boiling point | 181.7 °C | Chemfinder |
| Steam pressure | 0.35 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Soluble: 82.8 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.46 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
T;R23/24/25 C;R34 XN;R48/20/21/22 Mut3;R68 |
Toxic by inhalation, in contact with skin and if swallowed. Risk of corrosion. Harmful to health. Harmful: Danger of serious damage to health by prolonged exposure by inhalation, in contact with skin and if swallowed. Mutagenic cat. 3. Possible risks of irreversible effects. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Was earlier in the list but does not fulfil the new criteria for undesirable properties (classifications). |
Bioavailability
Phenol is quickly absorbed and nearly completely through lungs, gastrointestinal tract and skin. The absorption through skin is so large that a few hours’ contact with 2% phenol solution can result in acute intoxication with shock, cramps, coma and death. Of the absorbed amount 90% is separated within 24 hours (Jensen AA, 1997b).
In the EU Risk Assessment Report for phenol the absorption through oral intake and by inhalation is calculated to 100% but only to 80% through dermal exposure (EU ECB, 2006a). Same values are used in the calculations in this project.
Effects on health
Indication of acute toxicity at humans and animals are similar no matter the way of exposure. Absorption of phenol is quick as indications of toxicity are already seen after a few minutes at exposure to phenol. Deaths for humans are reported after exposure to phenol concentrations of 140-290 mg/kg bw (EU ECB, 2006a).
LD50 values for rats through oral intake are stated to 340 mg/kg bw (EU ECB, 2006a). However, humans seem to be more sensitive to the acute toxicity of phenol than animals as ingestion of 1 g of phenol may be mortal for an adult human whereas the mortal concentration in animals only corresponds to the same as applicable for a substance hazardous to health (Jensen AA, 1997b).
Both at acute and chronic intoxications at large amounts of phenol by inhalation or through ingestion serious damages on lungs, heart, liver and kidneys are seen. Furthermore, phenol is toxic to the white blood corpuscles (Jensen AA, 1997b).
Phenol can cause serious skin damages in contact with skin and is thus classified as corrosive (EU ECB, 2006a; Stat. Ord. 923, 2005). No studies indicate that phenol is allergenic (Jensen AA, 1997b; EU ECB, 2006a).
Human data indicate that phenol has a serious effect on the nervous system after prolonged exposure whether it is oral, dermal or through inhalation. At oral intake LOAEL is stated to 1.8 mg/kg bw/day (no NOAEL). In contact with skin NOAEL is stated to 130 mg/kg bw/day (EU ECB, 2006a).
Phenol does not seem to harm the unborn child or to be carcinogenic. In a two generation study with rats a NOAEL of 93 mg/kg bw/day is found. The effects at higher concentrations were reduced body weight (EU ECB, 2006a).
Both positive and negative results in different tests of the mutagenic properties of phenol are found for which reason the EU classifies phenol as mutagenic in category 3, i.e. possible risk of irreversible effects (EU ECB, 2006a; Stat. Ord. 923, 2005).
Threshold limits
The threshold limit in the working environment for phenol is 1 ppm or 4 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Phenol is identified in the following four products through analyses and solely through migration to artificial sweat.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Toy bag | 38A/C | 1 | ||
| School bag | 40B | Handle | 2 | |
| School bag | 41A | Inside | 1 | |
| School bag | 42A | 3 | ||
Data indicate that the effect of phenol depends on the way of exposure. A higher NOAEL value is stated for exposure to skin than for oral intake. The primary exposure to phenol will be by inhalation and in contact with skin through phenol vapours. Using the NOAEL value for contact with skin (based on test animals) the following dermal exposure of phenol in contact with skin is found. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours. Furthermore, an absorption factor of 100% is used as it is assumed that the listed NOAEL for dermal exposure considers the dermal absorption.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Toy bag | 38A/C | 0.25 | 1 | 0.066 | 131 | 1 | 12 | 0.18 | 130 | 722,602 |
| School bag | 40B | 0,5 | 1 | 0.136 | 131 | 1 | 12 | 0.74 | 130 | 175,768 |
| School bag | 41A | 0.25 | 1 | 0.042 | 131 | 1 | 12 | 0.11 | 130 | 1.141777 |
| School bag | 42A | 0.75 | 1 | 0.037 | 131 | 1 | 12 | 0.30 | 130 | 428,702 |
All the calculated MoS of the individual products are significantly above 100 and therefore it is assessed that the examined products do not constitute any health risk with regard to migration of phenol even if tests indicate that humans are more sensitive towards phenol than animals.
5.2.5 Toluene
Application
Toluene is applied in the production of petrol as well as in certain types of paints, diluting agents, ink, binders, medical drugs and cosmetics products. Furthermore, it is applied in some kinds of varnishes, nail varnishes, rubber products and leather colouring processes (IPCS, 1985; ATSDR, 2000).
Identification
| Chemical name | Methylbenzene |
| CAS-Nr. | 108-88-3 |
| EINECS Nr. | 203-625-9 |
| Gross formula | C7H8 |
| Molecular structure | 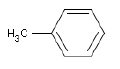 |
| Molecular weight | 92,14 g/mol |
| Synonyms | Toluene Methylbenzol Monomethyl benzene Phenyl methane |
Physical-chemical data
| Physical form | Colourless liquid with benzene-like smell | Chemfinder |
| Melting point | -93 °C (-94.9 at TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 110.6 °C | Chemfinder |
| Steam pressure | 28.4 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble: 0.526 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 2.73 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XI;R38 XN;R48/20-65 Rep3;R63 R67 |
Highly flammable. Local irritating. Irritating to skin. Harmful to health. Harmful: Danger of serious damage to health by prolonged exposure by inhalation. Harmful: May cause lung damage if swallowed. Toxic to reproduction cat. 3. Possible risk of harm to the unborn child. Vapours may cause drowsiness and dizziness. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Toluene is easily absorbed in the body. Toluene can be absorbed through the skin and about half the amount of toluene being inhaled is absorbed in the body. Toluene is accumulated after absorption in the body in fatty tissue (nerve system and fat deposits). The half life of toluene in humans can be up to three days. Toluene is easily transferred to the womb and about 75% of the toluene concentration being found in the mother’s blood can be found in the unborn child (Jensen AA, 1997c; EU ECB, 2003).
Effects on health
The acute toxicity of toluene is low. LD50 values for rats are between 5,500 and 7,500 mg/kg bw. Indications of acute toxicities are headache, dizziness, feeling of intoxication and at high concentrations also unconsciousness. Toluene is also classified with R67 “Vapours may cause drowsiness and dizziness” (EU ECB, 2003; Jensen AA, 1997c).
Toluene is classified as skin irritating and has a degreasing effect on the skin. Furthermore, toluene irritates the eyes and the airways. No indication of toluene being allergenic in contact with skin or by inhalation but only limited data is available (EU ECB, 2003).
In a two-year’ inhalation study with rats a NOAEC value of 1,125 mg/ m³ (corresponding to approx. 300 ppm) is found. No clear indications of intoxication at the highest doses were found. A 13-weeks’ study with both rats and mice showed a NOAEL of 625 mg/kg lw. In the rats nerve damages were found in the brain at doses above NOAEL (1,250 mg/kg bw) and there was one single death at the mice at the same dose (1,250 mg/kg bw) (EU ECB, 2003). Prolonged inhalation of toluene in high concentrations can thus give nerve and brain damages (Jensen AA, 1997c) and toluene is also classified with “danger of serious damage to health by prolonged exposure through inhalation”.
Based on experiences with work-related exposures it is assessed that it takes more than 10 years’ exposure to toluene at low concentrations before damages on the brain such as the painter’s syndrome are a reality EU ECB, 2003).
Toluene is neither mutagenic nor carcinogenic (EU ECB, 2003). IARC assesses that toluene is not classifiable in relation to the carcinogenic properties of the substance in humans and indications of lack of carcinogenic effect in animals (IARC, 1999).
Toluene is considered as possibly harmful to the unborn child and is also classified as possibly harmful to the unborn child category 3. Limited human data indicate that there is an increased risk of spontaneous abortion at doses of approx. 88 ppm (Jensen AA, 1997c; EU ECB, 2003).
Threshold limits
The threshold limit in the working environment is 25 ppm or 94 mg/ m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Toluene is identified in the following three products through analyses and solely through evaporation.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 3 | 0.01 | ||
| Eraser | 24 | 0.02 | ||
| Pencil case | 16 | 0.01 | ||
No acute NOAEL of toluene is identified for which reason a long-term scenario for exposure to toluene through erasers and pencil cases by inhalation is conducted.
At long-term exposure the following exposure to toluene by inhalation is found:
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day | kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 3 | 0.01 | 1 | 0.35 | 900 | 6 | 12 | 20 | 0.08 | 625 | 8,032, 129 |
| Eraser | 24 | 0.02 | 1 | 0.35 | 21 | 6 | 12 | 20 | 0.00 | 625 | 171,301, 319 |
| Pencil case | 16 | 0.01 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.00 | 625 | 153,806, 716 |
All the calculated MoS of the individual products are far above 100 and therefore it is assessed that the toluene evaporation from the examined products does not constitute any health risk. This also applies if the 88 ppm (increased risk of spontaneous abortion) is used in the calculations.
5.2.6 DIBP
Application
DIBP is applied among others in paint, varnish, paper and cardboard. Furthermore, it is applied as softeners and binding agents in especially plastic products as well as for regulation of the viscosity in certain products (IUCLID, 2000f).
Identification
| Chemical name | Diisobutyl phthalate |
| CAS-Nr. | 84-69-5 |
| EINECS Nr. | 201-553-2 |
| Gross formula | C16H22O4 |
| Molecular structure | 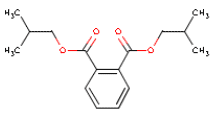 |
| Molecular weight | 278.35 g/mol |
| Synonyms | 1,2-Benzendi carboxylic acid, bis(2-methylpropyl) ester Phthalic acid, diisobutyl ester Isobutyl phthalate Palatinol IC |
Physical-chemical data
| Physical form | Clear viscous liquid. | Chemfinder |
| Melting point | -64 °C | HSDB |
| Boiling point | 296 °C | Chemfinder |
| Steam pressure | 6.65E-03 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Non-soluble. 0.0062 g/L at 24 °C |
Chemfinder TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 4.11 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
No | According to the Danish Environmental Protection Agency the classification will be changed to Rep2 on development and Rep3 on fertility. I.e. Rep2; R61-62 (May cause harm to the unborn child. Possible risk of impaired fertility). |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No N;R50/53 |
Harmful to environment. Very toxic to aquatic organisms may cause long-term adverse effects in the aquatic environment. |
Bioavailability
Phthalates in general – and thus DIBP – are easily absorbed in the body either through lungs, gastrointestinal tract or skin (Jensen AA, 1997d). In the calculations the absorption is thus calculated to 100%.
Effects on health
The acute toxicity of phthalates is generally low. LD50 values for rats for BIPB are between 10,400 and 15,000 mg/kg bw (IUCLID, 2000f).
Tests with rabbits show that DIBP is not irritating for neither skin nor eyes (IUCLID, 2000f). Only very limited data on the sensitizing properties of the substance are available. HSDB reports that several examples of allergenic reactions are seen at contact with plastic products containing DIBP.
A test with rats being fed orally with DIBP for 14 days gave a NOAEL of 50 mg/kg bw/day. The highest dose (2000 mg/kg) resulted in an increased liver weight as well as an increase in triglyceride and cholesterol levels. In the intermediate doses (of 100 and 200 mg/kg) only small effects were seen such as an increase in the triglyceride level. DEHP was given to a positive control group and the effects were the same at high dose of DIBP as of DEHP (IUCLID, 2000f).
Generally phthalates are seldom active in genetic short-term tests. Some phthalates are not mutagenic (Ames test) and this also applies DIBP (IUCLID, 2000f; Jensen AA, 1997d).
No data on the carcinogenic properties of DIBP are available but generally phthalates are not assessed to constitute a high cancer risk (Jensen AA, 1997d).
In general there are limited data on the properties of DIBP being toxic to reproduction but a single test with rats getting doses of DIBP (390, 780 and 1300 mg/kg bw respectively) on the 5th, 10th and 15th day of pregnancy respectively shows that DIBP has effects being toxic to reproduction. For all doses the average fetal weight was strongly reduced and abnormalities were found on the skeletons of the foetuses (dose-dependent). At the intermediate dose two dead foetuses without eyes were found (IUCLID, 2000f).
Threshold limits
The threshold limit in the working environment for DIBP is 3 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
DIBP is identified in the following 10 products through analyses. DIBP is solely identified through migration to artificial sweat. There are more values of analysis than those stated in the table below (see table 3.9B). More parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 1.5 | ||
| Pencil case | 31 A | 2 | ||
| Pencil case | 34 | 0.1 | ||
| Pencil case | 43 | 0.1 | ||
| Toy bag | 37B | 1.3 | ||
| Toy bag | 38C | 15 | ||
| School bag | 39B | 0.1 | ||
| School bag | 40B | Handle | 88 | |
| School bag | 41A | Inside | 0.4 | |
| School bag | 42B | Gym bag | 0.1 | |
The following exposure to DIBP is absorbed in contact with skin. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.375 | 1 | 0.299 | 131 | 4 | 12 | 4.90 | 50 | 10,198 |
| Pencil case | 31 A | 0.5 | 1 | 0.036 | 131 | 1 | 12 | 0.19 | 50 | 257,038 |
| Pencil case | 34 | 0.025 | 1 | 0.040 | 131 | 1 | 12 | 0.01 | 50 | 4,528,855 |
| Pencil case | 43 | 0.025 | 1 | 0.054 | 131 | 1 | 12 | 0.01 | 50 | 3,381,985 |
| Toy bag | 37B | 0.325 | 1 | 0.009 | 131 | 1 | 12 | 0.03 | 50 | 1,511,591 |
| Toy bag | 38C | 3.75 | 1 | 0.066 | 131 | 1 | 12 | 2.70 | 50 | 18,528 |
| School bag | 39B | 0.025 | 1 | 0.058 | 131 | 1 | 12 | 0.02 | 50 | 3,156,641 |
| School bag | 40B | 22 | 1 | 0.136 | 131 | 1 | 12 | 32.54 | 50 | 1,536 |
| School bag | 41A | 0.1 | 1 | 0.042 | 131 | 1 | 12 | 0.05 | 50 | 1,097,863 |
| School bag | 42B | 0.025 | 1 | 0.011 | 131 | 1 | 12 | 0.00 | 50 | 16,695,573 |
The following exposure to DIBP is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours. As mentioned earlier it is assumed that the results from migration to artificial sweat can be transferred directly to artificial saliva.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.375 | 1 | 21.1 | 1 | 12 | 0.66 | 50 | 75,829 |
As absolute worst case the highest of the above exposure values can be added as a child might be exposed to DIBP both through the skin from a school bag, a toy bag, a pencil case and an eraser as well as oral exposure when the child sucks or chews an eraser.
This scenario gives a total exposure of 40.99 µg/kg bw/day and when this value is compared with a NOAEL of 50 mg/kg bw/day the result is a Margin of Safety of 1219.
All the calculated MoS of the individual products are significantly above 100 and this assessment is thus that they do not represent any health risk with regard to DIBP. Exposure to DIBP both by inhalation and through skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.7 DEHP
Application
DEHP is primarily applied as plasticizer as it has an ability of plasticizing plastic without reacting chemically with it. DEHP is especially used in PVC products like tubes, hoses and parts for medical equipment. Furthermore, it is used as plasticizer in plastics materials of cellulose ester and synthetic elastomer (IPCS, 1992).
Identification
| Chemical name | 1,2-Benzene dicarboxylic acid, bis(2-ethylhexyl)ester |
| CAS-No. | 117-81-7 |
| EINECS No. | 204-211-0 |
| Gross formula | C24H38O4 |
| Molecular structure | 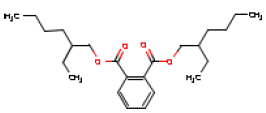 |
| Molecular weight | 390.56 g/mol |
| Synonyms | Bis(2-Ethylhexyl)Phthalate (DEHP) Ethylhexyl phthalate Dioctyl phthalate Bisoflex 81 |
Physical-chemical data
| Physical form | Colourless oily liquid with nearly no smell. | Chemfinder |
| Melting point | -50 °C (-55 °C according to TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 386.9 °C (384 °C according to TOXNET ChemIDplus) | Chemfinder |
| Steam pressure | 1.42E-07 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Insoluble: 0.00027 g/Lat 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 7.6 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
Rep2;R60-61 | Toxic to reproduction cat.2. May impair fertility. May cause harm to the unborn child. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
Yes No |
Bioavailability
DEHP is easily absorbed in the body through either lungs or the gastrointestinal tract. The skin permeability of DEHP is not large and is measured to 6.5 and 26% depending on the animal species. DEHP is one of the long-chained phthalates where the permeability is smallest. The EU Draft Risk Assessment Report on DEHP uses the following relevant bioavailability percentages which are also used in the calculations. Oral exposure – 50%, but 100% for children. Dermal – 5% for both adults and children (EU ECB, 2006b).
Effects on health
The acute toxicity of DEHF is very low. The LD50 value for rats is above 20,000 mg/kg bw – in some tests even above 30,000 mg/kg bw (IUCLID, 2000g). A single survey of the acute toxicity of DEHP in humans is available. Here oral intake of 5 g led to no symptoms and intake of 10 g only led to mild symptoms such as stomach disorders. It was only men who ingested DEHP as single dose (EU ECB, 2000b; Jensen AA, 1997d).
DEHP is slightly irritating for both skin and eyes. Animal tests indicate that DEHP has no sensitizing properties (EU ECB, 2006b).
In animal tests with rats a NOAEL for the acute effects of DEHP on the heart rate is determined to 28.5 mg/kg bw/day (EU ECB, 2006b).
The mutagenic properties of DEHP (gene mutations, DNA damages and chromosome effects) are tested in several studies and the results are predominantly negative (EU ECB, 2006b; IUCLID, 2000g).
IARC assesses DEHP as being non-classifiable in relation to the carcinogenic properties of the substance in humans. There is insufficient information about the carcinogenic properties of DEHP in humans and there are sufficient indications of the carcinogenic properties of DEHP in animals (IARC, 2000). But the mechanism behind the carcinogenic effect of DEHP in rodents is very special and does not seem to be relevant for humans (Jensen AA, 1997d; EU ECB, 2000b).
A two generation study with rats sets a NOAEL value of 8 mg/kg bw/day for testicular toxicity. The effects were a reduction in testicular weight. The same test sets a NOEAL value of 77 mg/kg bw/day for damages to reproduction. In another two generation study with rats a NOAEL value of 4.8 mg/kg bw/day is set for testicular toxicity and a NOAEL of 46 mg/kg for damages to reproduction. DEHP is therefore regarded as being toxic to reproduction and is also classified as toxic to reproduction category 2, i.e. that it can damage the fertility and can cause harm to the unborn child (Stat. Ord. 923, 2005).
Threshold limits
The threshold limit in the working environment for DEHP is 3 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
DEHP is identified in four products through migration to artificial sweat. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
Additionally DEHP is identified through total determination in four erasers in a maximum concentration of 44%. Based on these numbers a scenario is calculated where it is assumed that a child will swallow between 0.008 and 0.1 g of eraser corresponding to approx. 0.01 and 0.08 cm³ for the relevant erasers – i.e. cubes of approx. 1.9 to 4.3 mm in height, width and length – an amount not unrealistic to swallow. The value of 0.008 g eraser is chosen as it is the upper limit of intake of toy material which is used in DS/EN 71-3”Toys. Safety requirements. Part 3: Migration of special substances”.
Finally a migration analyse to artificial saliva for a single eraser is conducted – the eraser with the highest DEHP. Based on this value a scenario is calculated for a child who sucks this eraser 1 hour a day.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | mg/g |
| Migration artificial sweat | Total content | |||
| Eraser | 12 | 350 | ||
| Eraser | 16 | 170 | ||
| Eraser | 22 | 440 | ||
| Eraser | 23 | 220 | ||
| Pencil case | 35C | Inside | 6 | |
| Toy bag | 38A | 2.4 | ||
| School bag | 39C | 1 | ||
| School bag | 41A | Inside | 1 |
The following exposure to DEHP is absorbed in contact with skin. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 35C | 1.5 | 0.05 | 0.026 | 131 | 1 | 12 | 0.02 | 4.8 | 227,469 |
| Toy bag | 38A | 0.6 | 0.05 | 0.041 | 131 | 1 | 12 | 0.01 | 4.8 | 360,843 |
| School bag | 39C | 0.25 | 0.05 | 0.039 | 131 | 1 | 12 | 0.01 | 4.8 | 901,198 |
| School bag | 41A | 0.25 | 0.05 | 0.042 | 131 | 1 | 12 | 0.01 | 4.8 | 843,158 |
As worst case the highest of the above exposure values can be added as a child might be exposed to DEHP through the skin from a school bag, a toy bag and a pencil case.
This scenario gives a total exposure of 0.04 µg/kg bw/day and when this value is compared with a NOAEL of 4.8 mg/kg bw/day the result is a Margin of Safety of 120.000.
All the calculated MoS of the individual products are significantly above 100 and this assessment is thus that they do not represent any health risk with regard to skin absorption of DEHP. Exposure to DEHP through skin absorption from several products at the same time is neither assessed to represent any health risk.
Intake of small amounts of eraser
Through intake of small pieces of eraser of 0.008, 0.5 and 0.1 g respectively, corresponding to cubes of 1.9 mm, 3.5 mm and 4.3 mm respectively, the result is the following exposure to DEHP. Absorption from the gastrointestinal tract is calculated to 100% for children. The calculations are based on a body weight of both 12 and 20 kg. The 20 kg is applied to illustrate children’s weight during the first school year.
| Product type | Product ID | Measured concentration in mg/g | Foral | Qoral | BW | Ioral | NOAEL | MoS |
| Total content | g | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 12 | 350 | 1 | 0.1 | 12 | 2.92 | 4.8 | 1.6 |
| Eraser | 12 | 350 | 1 | 0.1 | 20 | 1.75 | 4.8 | 2.7 |
| Eraser | 12 | 350 | 1 | 0.05 | 12 | 1.46 | 4.8 | 3.3 |
| Eraser | 12 | 350 | 1 | 0.05 | 20 | 0.88 | 4.8 | 5.5 |
| Eraser | 12 | 350 | 1 | 0.008 | 12 | 0.23 | 4.8 | 20.6 |
| Eraser | 12 | 350 | 1 | 0.008 | 20 | 0.14 | 4.8 | 34.3 |
| Eraser | 16 | 170 | 1 | 0.1 | 12 | 1.42 | 4.8 | 3.4 |
| Eraser | 16 | 170 | 1 | 0.1 | 20 | 0.85 | 4.8 | 5.6 |
| Eraser | 16 | 170 | 1 | 0.05 | 12 | 0.71 | 4.8 | 6.8 |
| Eraser | 16 | 170 | 1 | 0.05 | 20 | 0.43 | 4.8 | 11.3 |
| Eraser | 16 | 170 | 1 | 0.008 | 12 | 0.11 | 4.8 | 42.4 |
| Eraser | 16 | 170 | 1 | 0.008 | 20 | 0.07 | 4.8 | 70.6 |
| Eraser | 22 | 440 | 1 | 0.1 | 12 | 3.67 | 4.8 | 1.3 |
| Eraser | 22 | 440 | 1 | 0.1 | 20 | 2.20 | 4.8 | 2.2 |
| Eraser | 22 | 440 | 1 | 0.05 | 12 | 1.83 | 4.8 | 2.6 |
| Eraser | 22 | 440 | 1 | 0.05 | 20 | 1.10 | 4.8 | 4.4 |
| Eraser | 22 | 440 | 1 | 0.008 | 12 | 0.29 | 4.8 | 16.4 |
| Eraser | 22 | 440 | 1 | 0.008 | 20 | 0.18 | 4.8 | 27.3 |
| Eraser | 23 | 220 | 1 | 0.1 | 12 | 1.83 | 4.8 | 2.6 |
| Eraser | 23 | 220 | 1 | 0.1 | 20 | 1.10 | 4.8 | 4.4 |
| Eraser | 23 | 220 | 1 | 0.05 | 12 | 0.92 | 4.8 | 5.2 |
| Eraser | 23 | 220 | 1 | 0.05 | 20 | 0.55 | 4.8 | 8.7 |
| Eraser | 23 | 220 | 1 | 0.008 | 12 | 0.15 | 4.8 | 32.7 |
| Eraser | 23 | 220 | 1 | 0.008 | 20 | 0.09 | 4.8 | 54.5 |
In the calculations here a NOAEL for effects being toxic to reproduction is used – i.e. long-term effects. This means that it clearly constitutes a health risk to eat eraser daily, even in small amounts. However, it must be assumed that in general it is a one-time occurrence to swallow a piece of eraser.
Suck on an eraser
For eraser 22 a migration analysis to artificial saliva is carried out as this eraser has the highest content of DEHP. The analysis is conducted at 37 degrees for 1 hour to imitate a child who sucks an eraser for 1 hour daily. The result of the migration analysis is that 0.1% (w/w) DEHP is released, i.e. 1 mg/g of eraser to artificial saliva. Uncertainty is 50%. The calculations are based on a body weight of 20 kg to illustrate children’s weight during the first school year.
The following exposure to DEHP is absorbed through oral intake when a child sucks or chews eraser 22. The duration of the exposure is assumed to be one hour daily. As the migration analysis is completed with a duration of one hour for one gram of eraser the amount of DEHP being ingested may be calculated like the migration (1 mg/g eraser) multiplied by the amount of eraser being sucked (here 14.4 g).
| Product type | Product ID | Maximum measured concentration in mg/g | F oral | Weight A product oral |
Tcontact | BW | I oral | NOAEL | MOS |
| Migration | g | Hours/ day | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 22 | 1 | 1 | 14.4 | 1 | 20 | 0.72 | 4.8 | 6.7 |
MoS are significantly below 100 and thus it constitutes a health problem if a child of 20 kg sucks this eraser one hour daily for a longer period (NOAEL is for effects being toxic to reproduction – long-term effects).
In the calculations it is assumed that the child sucks and chews the whole eraser which in this case is an eraser of 3.8 x 3.1 x 1 cm, i.e. a large eraser. Most probably, the calculation gives a too high estimate as it is unrealistic that a child sucks or chews the whole eraser at one time.
If it is assumed that a child will only have one end of the eraser in the mouth, i.e. the first cm of the eraser, then it is only 1 x 3.1 x 1 cm = 3.1 cm³ of the total area of 11.78 cm³ of the eraser which the child sucks. Thus the child sucks 3.79 g of the total weight of 14.4 g of the eraser. MoS can then be calculated to 25 instead. However, this does not change the fact that MoS is still significantly below 100.
| Product type | Product ID | Maximum measured concentration in mg/g | F oral | Weight A product oral |
Tcontact | BW | I oral | NOAEL | MOS |
| Migration | g | Hours/day | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 22 | 20 | 1 | 3.79 | 1 | 20 | 0.19 | 4.8 | 25.3 |
At the migration analysis to artificial saliva the eraser was cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface will be significantly larger than the surface which an eraser normally has. To illustrate the surface of the eraser is here calculated if the whole eraser is cut into cubes of 0.3 x 0.3 x 0.3 mm. This gives thus approx. 13 x 10 x 3 = 390 pieces of eraser, each with at surface of 0.3 x 0.3 x 6 = 0.54 cm², i.e. a total surface of 221 cm². For purposes of comparison, the eraser in uncut condition has a surface of ((3.8 x 3.1) + (3.1 x 1) + (3.8 x 1)) x 2 = 37.4 cm², i.e. nearly a factor 6 in difference. Therefore, the measured concentrations of DEHP are probably overestimated by a factor 6. If this is considered MoS will be 150, i.e. above 100 and without health risk but this may only apply if the child solely sucks the first cm of the eraser. If the child also chews the eraser or sucks a larger area the surface area from which DEHP can migrate will become larger and thus MoS can get under 100.
If the analysis error is taken into account – i.e. that the measured value in fact can be halved – this does not change much on MoS. A doubling of MoS will take place at a 50% reduction of the exposure.
All in all these conditions mean that it may be assumed to be hazardous to health for a child of 20 kg to suck or chew this eraser one hour daily for a longer period.
5.2.8 2-Heptanone
Application
2-heptanone is applied as indutrial solution, among others for resins and varnishes and is also applied as flavouring agent in foods (HSDB).
Identification
| Chemical name | 2-heptanone |
| CAS-No. | 110-43-0 |
| EINECS Nr. | 203-767-1 |
| Gross formula | C7H14O |
| Molecular structure |  |
| Molecular weight | 114.19 g/mol |
| Synonyms | Amyl methylketone Butylacetone Methyl amyl ketone Pentyl methyl ketone |
Physical-chemical data
| Physical form | Clear, colourless liquid with a mild fragrance of banana. | Chemfinder |
| Melting point | -35 °C (-31 °C according to Chemfinder) | TOXNET ChemIDplus |
| Boiling point | 151 °C (150 °C according to Chemfinder) | TOXNET ChemIDplus |
| Steam pressure | 3.86 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble 43 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.98 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
R10 XN;R20/22 |
Inflammable. Harmful to health. Harmful by inhalation and if swallowed. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about the bioavailability of 2-heptanone is found but according to the Danish threshold list the substance can be absorbed through the skin for which reason 100% absorption is assumed in the calculations.
Effects on health
Only few information about the health effects of 2-heptanone is found. If nothing else is stated data are found through TOXNET’s HSDB database.
2-heptanone is classified as harmful by inhalation and if swallowed. LD50 (oral, rat) is 1,670 mg/kg (HSDB, TOXNET ChemIDplus).
2-heptanone is irritating for both skin and eyes and evaporations of the substance are also irritating for the mucous membranes.
Skin sensitization of 2-heptanone on humans has been examined on 26 voluntary persons. At a concentration of 4% in Vaseline no positive reactions were found.
During 13 weeks rats were fed with 0 (control), 20, 100 and 500 mg 2-heptanone/kg bw. At a dose of 500 mg an increase of the weight of liver and kidney was seen. At 100 mg similar effects were seen, just to a lesser extent. No serious effects were seen at 20 mg/kg bw.
In a reproduction study rats were exposed to up to 1000 ppm 2-heptanone. The effects were such as reductions in the food intake and changes in the body weight but no reproductive and development toxic effects.
2-heptanone has shown negative result in Ames test and other tests for mutagenic properties have also been negative.
Very few data are found on the carcinogenic properties of 2-heptanone but the few results indicate that 2-heptanone is not carcinogenic.
Threshold limits
The threshold limit in the working environment for 2-heptanone is 50 ppm or 238 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
2-heptanone is identified in one product through migration to artificial sweat.
| Product type | Product ID | Remark | Maximum measured concentrating in µg/g | |
| Migration artificial sweat | Headspace | |||
| School bag | 39C | 20 | ||
Assuming a NOAEL value based on the few data available for 2-heptanone on 20 mg/kg bw/day the following exposure for 2-heptanone in contact with skin is found where Tcontact (the duration of the exposure) is divided by a factor 4 as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| School bag | 39C | 5 | 1.00 | 0.039 | 131 | 1 | 12 | 2.13 | 20.0 | 9,387 |
The calculated MoS for the examined product is far above 100 and therefore it is assessed that the exposure through contact with skin with 2-heptanone from the examined product does not constitute any health risk.
5.2.9 tert-Butyl alcohol
Application
tert-Butyl alcohol is especially important due to its properties as a solvent. It is applied to remove water from substances in the production of perfume (especially artificial musk), for recrystallization of chemicals and as denaturant in spirit. Furthermore, tert-butyl alcohol is applied as an intermediate in the production of other chemicals such as MTBE (methyl-tert-butyl ether) (IPCS, 1987; Jensen AA, 2003a).
Identification
| Chemical name | tert-Butanol |
| CAS-Nr. | 75-65-0 |
| EINECS Nr. | 200-889-7 |
| Gross formula | C4H10O |
| Molecular structure |  |
| Molecular weight | 74.12 g/mol |
| Synonyms | 1,1-Dimethylethanol 2-Methyl-2-propanol 2-methylpropan-2-ol t-Butylhydroxid t-Butylalkohol |
Physical-chemical data
| Physical form | Solid substance at normal room temperature. Has a strong smell. | Jensen AA, 2003a |
| Melting point | 25.4 °C (25.5 °C at Chemfinder) | TOXNET ChemIDplus |
| Boiling point | 82.4 °C | Chemfinder |
| Steam pressure | 40.7 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Easily soluble: 1000 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.35 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XN;R20 |
Highly flammable. Harmful to health. Harmful by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
tert-Butyl alcohol is easily absorbed through skin, lungs and gastrointestinal tract. It is easily decomposed in the body to carbon dioxide and water which thereafter are exhaled within 24 hours (Jensen AA, 2003a).
Effects on health
The acute toxicity of tert-butyl alcohol is low. Oral LD50 values for rats are found between 2733 and 3500 mg/kg bw (IUCLID, 2000i; IPCS, 1987). tert-Butyl alcohol has a narcotic effect (about 1½ times higher compared to ethanol). Toxic symptoms are headache, dizziness, nausea, sleepiness and drowsiness (Jensen AA, 2003a; IPCS, 2003).
tert-Butyl alcohol is degreasing on the skin and can cause contact eczema. Skin drugs based in tert-butyl alcohol has caused contact allergy (Jensen AA, 2003a).
Not much information about effective values for tert-butyl alcohol is found in the literature. IUCLID refers to a test with mice getting doses of tert-butyl alcohol through the drinking water for 90 days. The effects at the high doses were among others ataxia (loss of full control of bodily movements), loss of weight and hyperactivity). NOEL for the direct chemical effects was set to 1566 mg/kg bw/day for male mice and 4363 mg/kg bw/day for female mice (IUCLID, 2000i).
tert-Butyl alcohol is found inactive in Ames test as well as in many other short-term tests and thus it has no mutagenic properties (Jensen AA, 2003a; IUCLID, 2000i).
Only a few studies regarding the properties of the substance being toxic to reproduction are available. Inhalation of high concentrations being toxic for the female animal resulted in damages to foetus (Jensen AA, 2003a).
The carcinogenic properties are investigated in a long-term study with mice and rats exposed to tert-butyl alcohol through the drinking water for 2 years. Some indications of carcinogenic effect in male rats at 420 mg/kg bw and female mice at 2110 mg/kg bw were found but no indications in female rats. In male mice an ambiguous indication of carcinogenic effect was found (NTP, 1995).
Threshold limits
The threshold limit in the working environment of tert-butyl alcohol is 50 ppm or 150 mg/m³ with the remarks L and H, i.e. that the threshold limit is a limit value (L) which is not allowed to be exceeded and that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
tert-Butyl alcohol is identified in one product exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 10 | 0.09 | ||
No acute NOAEL of tert-butyl alcohol is identified for which reason a long-term scenario for exposure to the substance by inhalation through school bags and pencil cases are solely conducted. A NOAEL value of 1566 mg/kg bw/day is found based on a study of 90 days but at the same time indications of carcinogenic effect in male rats at 420 mg/kg bw are found in a two-years’ study. However, no NOAEL based on this long-term study is specified. In the calculations the 420 mg/kg bw/day is applied knowing that no NOAEL value is specified.
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hour/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 10 | 0.09 | 1 | 0.35 | 105 | 6 | 12 | 20 | 0.08 | 420 | 5,140,562 |
The calculated MoS of the examined product is far above 100 and therefore it is assessed that the exposure through contact with skin with med tert-butyl alcohol of the examined product does not constitute any health risk even if it is not a real NOAEL value being used for the calculation of MoS.
5.2.10 Methyl propionate
Application
Propionates are permitted as additive in foods and is applied as aromatic compound (for instance in pastry, sweets and ice cream). The substance is naturally found in some fruits such as kiwi fruits and some strawberries as well as in shellfish (mussels). Furthermore, methyl propionate is applied as solvent in for instance paints and varnishes (HSDB).
Identification
| Chemical name | Propane acid, methyl ester |
| CAS-Nr. | 554-12-1 |
| EINECS Nr. | 209-060-4 |
| Gross formula | C4H8O2 |
| Molecular structure |  |
| Molecular weight | 88.11 g/mol |
| Synonyms | Methyl propionate Methyl propylate |
Physical-chemical data
| Physical form | Flammable colourless liquid | Chemfinder. HSDB. |
| Melting point | -87.5 °C | TOXNET ChemIDplus |
| Boiling point | 79.8 °C | TOXNET ChemIDplus |
| Steam pressure | 84 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | 62.4 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.84 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XN;R20 |
Highly flammable. Harmful to health. Harmful by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about the bioavailability of methyl propionate is found.
Effects on health
Only few information about the health effects of methyl propionate is found. If nothing else is stated data are found through the HSDB database of TOXNET.
Methyl propionate has a low toxicity at intake. LD50 value for rats through oral intake is 5,000 mg/kg bw. Effects such as ataxia (loss of full control of bodily movements), gasping breathing and hypothermia (cooling of the body) are seen at mortal levels. The dermal toxicity of the substance is low. The substance is harmful to health by inhalation.
No reports on any toxic effects of methyl propionate at humans are found.
Methyl propionate is a highly flammable liquid which is skin irritating.
No information about possible carcinogenic, reproduction or mutagenic effects of the substance is found.
Threshold limits
The Danish Working Environment Authority has set no threshold limit for the substance (The Danish Working Environment Authority, 2005).
Assessment
Methyl propionate is identified in one product, exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 0.05 | ||
No information is found about tests having identified a NOAEL value of methyl propionate for which reason no exposure calculations of the substance are conducted.
5.2.11 p-xylene
Application
p-Xylene (a mixture of ortho-, meta- and para-xylene) is one of the most important solvents. p-Xylene is used in the production of among others dimethyl etherphthalate (IPCS, 1997). In Denmark xylene is primarily applied as solvent and diluents in paint, varnish, remover, stain remover, printing inks, ink, drain cleaner, nail polish etc. (Jensen AA, 2003b).
Identification
| Chemical name | p-xylene |
| CAS-Nr. | 106-42-3 |
| EINECS Nr. | 203-396-5 |
| Gross formula | C8H10 |
| Molecular structure |  |
| Molecular weight | 106.12 |
| Synonyms | 1,4-Dimethylbenzene 1,4-Xylene Benzene, 1,4-dimethyl- Chromar |
Physical-chemical data
| Physical form | Clear liquid, with characteristic sweet aromatic smell. | Chemfinder, Jensen AA, 2003b |
| Melting point | 13.2 °C | TOXNET ChemIDplus |
| Boiling point | 138.3 °C | TOXNET ChemIDplus |
| Steam pressure | 8.84 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Slightly soluble: 0.162 g/Lat 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 3.15 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord 923, 2005) |
R10 XN;R20/21 XI;R38 |
Flammable. Harmful to health. Harmful by inhalation and in contact with skin. Local irritating. Irritates the skin. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Evaporations of p-xylene are largely absorbed (60-70%) through the lungs. In fluid form the substance is quickly absorbed from the gastrointestinal tract and it penetrates easily through the skin (ATSDR, 1995; Jensen AA, 2003b).
The absorbed p-xylene is quickly spread in the body with the blood, especially to bone marrow, brain, spleen and fatty tissue. The main part of the absorbed p-xylene is separated during a few hours with the urine. A smaller part, approx. 5%, is eliminated unchanged with the air inhaled. The part of the absorbed xylene being spread to the fat deposits is eliminated more slowly (half-life 2-4 days) (Jensen AA, 2003b).
Effects on health
The acute toxicity of p-xylene is low. LD50 values for rats at oral intake are between > 3400 and 4779 mg/kg bw (IUCLID, 2000j). Examples of serious acute intoxications with deaths after exposure to very high concentration (10,000 ppm) in the air at work with cleaning of tanks with leftovers of p-xylene are seen (Jensen AA, 2003b).
Symptoms of an acute intoxication are fatigue, foam at the mouth, visual disturbances, uncoordinated movements, muscular spasms, paralysis, unconsciousness and coma. Damages on heart, liver and kidneys may occur. Alcohol strengthens the toxicity of p-xylene (Jensen AA, 2003b).
At direct contact with the skin p-xylene is degreasing and irritating (IUCLID, 2000j). At direct exposures to the eyes severe burns on the cornea are seen. A few minutes’ exposure to p-xylene in a concentration of 200 ppm results in irritation of eyes, nose and throat (Jensen AA, 2003b).
Exposure during a long period to p-xylene may result in the so-called painter’s syndrome where the effects are unnatural fatigue during the day, sleep problems during the night, headache, amnesia, irritability and other personality changes (Jensen AA, 2003b).
The National Research Centre for the Working Environment assesses that p-xylene leads to a high risk of permanent and severe damages on the nervous system even at normal work with the substances. A number of animal tests indicate that p-xylene and its isomers have a neurotoxic effect at exposure by inhalation. Effects observed at the animals are among others trembling, muscular spasms, strenuous breathing, hearing loss etc. after inhalation of p-xylene (ATSDR, 2005).
No specific knowledge about the reproduction effects of p-xylene in humans is available but a number of population studies indicate that exposure to solvents can cause damages to foetus and an increased number of spontaneous abortions. p-Xylene is easily transported with the blood from mother to foetus through the placenta. Exposure to p-xylene in a concentration which affected the female animal generated an increased foetus mortality, an impaired growth and development of the foetus as well as foetus malformations in tests with pregnant mice and rats (ATSDR, 2005; Jensen AA, 2003b).
p-Xylene is tested negative in Ames test as well as in a number of other short-term tests for mutagenic effects (IUCLID, 2000j; Jensen AA, 2003b).
IARC assesses that p-xylenes are non-classifiable in relation to the carcinogenic properties of the substance in humans. There are insufficient indications of the carcinogenic properties of p-xylenes in both humans and animals (IARC, 1999). Long-term tests with mice and rats, where the animals got technical p-xylene in doses up to 1 mg/kg bw/day orally for two years, have not shown any carcinogenic effects (NTP, 1986b; Jensen AA, 2003b).
A NOAEL value of 500 mg/kg bw/day is found for p-xylenes for reproductive, neurological and other systemic effects in rats in a test of two-year’ duration (ATSDR, 2005; NTP, 1986b).
Threshold limits
The threshold limit in the working environment for p-xylene is 25 ppm or 109 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
p-Xylene is identified in one product exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 3 | 0.01 | ||
The identified NOAEL value is based on long-term effects for which reason a long-term scenario for exposure to the substance by inhalation through the eraser is conducted. The following exposure for p-xylene by inhalation is found:
| Product type | Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 3 | 0.01 | 0.7 | 0.35 | 900 | 6 | 12 | 20 | 0.27 | 625 | 11,474, 469 |
The calculated MoS of the examined product are far above 100 and therefore it is assessed that the exposure by inhalation of p-xylene from the examined product does not constitute any health risk.
5.3 Total assessment
A risk assessment of content of the following 11 substances has been conducted. These substances are identified through headspace (i.e. evaporate from the products) and/or through migration to artificial sweat or artificial saliva (for one single eraser):
- Isophorone
- BHT
- Cyclohexanone
- Phenol
- Toluene
- DIBP
- DEHP
- 2-Heptanone
- tert-Butyl alcohol
- Methyl propionate
- p-Xylene
In general, the content of the above-mentioned in the examined products does not constitute any health risk; neither in the individual products nor if children are exposed to several products at one time – for instance through use of pencil case, eraser and school bag and at exposure both by inhalation and through migration to artificial sweat.
However, for BGT applies that Margin of Safety for a single scenario and a single product – an eraser – is fairly close to 100 (is 163). In this case it is a rather large eraser of 4 x 1.3 x 11 cm and in the calculations it is assumed that the child sucks and chews the whole eraser. Furthermore, the erasers for the analyses are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface is significantly larger than the surface that an eraser normally has. The measured concentrations can thus be overestimated by a factor 8.
All in all these conditions mean that exposure to BHT from several products at the same time and through several ways of exposure may not constitute any risk for the examined products. However, it is unknown whether other products can have a larger content of BHT and thus constitute a health problem if a child is exposed to several products with a high content of BHT. As BHT is much applied as an anti-oxidant in foods there is a possibility of exposure through other sources. The total exposure is not assessed in this project.
The total amounts in selected erasers are analyzed for DEHP. Based on these results a scenario is calculated where it is assumed that a little piece of an eraser of between 0.008 and 0.1 g is swallowed. For the calculations it is assumed that a little piece of eraser of between approx. 1.9 x 1.9 x 1.9 mm and 4.3 x 4.3 x 4.3 mm is ingested. In this scenario Margin of Safety is significantly below 100 (based on NOAEL value for effects being toxic to reproduction). Thus it is clear that repeated eating of eraser may cause serious health effects.
Furthermore, a scenario is assessed where a school child sucks an eraser for 1 hour daily. The calculations are conducted for the eraser with the highest content of DEHP. The calculations show that it can constitute a health risk daily to suck an eraser with a high content of DEHP during a long period.
In general, the calculation is based on the analyzed values for a few selected school bags, toy bags, pencil cases and erasers. It cannot be rejected that there are products with a higher content than the content found in the products examined in this project. Furthermore, there may be other sources to the same chemical substances in the child’s surroundings which will contribute to the total exposure.
Version 1.0 August 2007, © Danish Environmental Protection Agency