Survey of Chemical Substances in Consumer Products, no. 86, 2007
Survey and risk assessment of chemical substances in deodorants
Contents
2 Market survey and product sampling
- 2.1 Market survey
- 2.2 Legislation
- 2.3 Sampling of products and control of labelling
- 2.4 Selection of products for analysis
- 5.1 Deodorants and contact allergy
- 5.2 Risk assessment – in general
- 5.3 The selected fragrance substances
- 5.4 Farnesol
- 5.5 Comments concerning other fragrance substances
- 5.6 Allergen load of fragrance substances
- 5.7 Triclosan
Sammenfatning
Deodoranter anvendes dagligt af store dele af befolkningen og kan indeholde ingredienser, som visse duftstoffer og konserveringsmidler, der er hyppige årsager til hudallergi. Herudover mistænkes større forbrug af visse antimikrobielle midler, f.eks. triclosan at kunne medføre specifik bakterieresistens.
Hovedformålet med projektet var at kortlægge forekomsten af udvalgte duftstoffer og konserveringsmidler/antibakterielle midler i deodoranter på det danske marked samt vurdere allergirisikoen ved en række udvalgte duftstoffer i deodoranter.
Projektet var opdelt i 3 faser: i) kortlægning af deodoranter på det danske marked, indsamling af de mest solgte produkter og kontrol af mærkning ifølge kosmetikbekendtgørelsen, ii) bestemmelse af indholdet af 26 deklarationspligtige duftstoffer (som fra marts 2005 har skullet deklareres på kosmetiske produkter) og udvalgte konserveringsmidler/antibakterielle midler, samt iii) risikovurdering for udvalgte duftstoffer m.h.p. hudallergi, på baggrund af analyseresultaterne.
Kortlægning og indsamling af de mest solgte deodoranter blev udført på basis af oplysninger modtaget fra producenter/importører og forhandlere af deodoranter samt andre relevante oplysninger fra Internettet, SPT (Brancheforeningen for sæbe parfume og teknisk/kemiske artikler) og Informationscenter for Miljø og Sundhed. Der blev i alt indkøbt 97 deodoranter, således at de fleste mærker på markedet, forskellige typer af deodoranter samt produkter til både mænd og kvinder indgik i undersøgelsen. Af de 97 deodoranter, blev 23 produkter udvalgt til kemisk analyse for indhold af de udvalgte duftstoffer og 15 produkter, blev udvalgt til bestemmelse af triclosanindholdet.
Risikovurdering med hensyn til hudallergi blev foretaget for duftstofferne isoeugenol, hydroxycitronellal, hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC), cinnamal og cinnamyl alcohol samt konserveringsmidlet triclosan. Duftstofferne blev udvalgt således, at der var tale om potente allergener og/eller hyppige årsager til allergi. Desuden blev der lagt vægt på, at der var data fra klinisk undersøgelser af personer med allergi over for deodoranter, som grundlag for risikovurdering. Erfaringen viser, at grænseværdier baseret på data fra personer med erhvervet allergi, er meget effektive til at forebygge nye tilfælde af allergi såvel som at mindske sygdommens konsekvenser for de personer, der har erhvervet allergien.
Undersøgelsen viste at:
- 65,9 % af deodoranterne indeholdt en eller flere af de 26 deklareringspligtige duftstoffer ifølge deklarationsoplysningerne på produkterne.
- Duftstoffer i produkterne var af meget forskellige i potens og allergiforekomst. De mest potente stoffer - cinnamal, methyl heptin carbonat og evernia prunastri extract (oak moss abs.) - var i færrest produkter (1,1 % - 4,6 %).
- Evernia prunastri extract indeholder to meget potente allergifremkaldende stoffer (atranol og chloratranol), som den Videnskabelig Komité, der rådgiver EU-kommissionen vedrørende kosmetik (SCCP), har vurderet ikke bør forekomme i kosmetik på grund af allergirisikoen. Evernia Prunastri extract var i 4,6 % af produkterne.
- Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC), hydroxycitronellal og isoeugenol, der er årsag til mange allergitilfælde, var i henholdsvis 33 %, 27,3 % og 9,1 % af de parfumerede deodoranter.
- Den kemiske analyse af de 23 udvalgte produkter viste, at alle produkter indeholdt de duftstoffer, som fremgik af deklarationsoplysningerne og således overholdt kravene i kosmetikbekendtgørelsen.
- Risikovurderingen viste, at mellem 3,4 % - 6,8 % af produkterne indeholdt duftstofferne, HICC, hydroxycitronellal eller isoeugenol i en mængde, der overskred det maximalt acceptable ud fra den anvendte metode.
- Cinnamal blev kun fundet i et produkt i 5 ppm, hvilket ikke udgør nogen risiko for allergi. Cinnamyl alcohol, som omdannes til cinnamal i huden, var i 12,5 % af produkterne.
- Alle produkter overholdt gældende lovgivningen, som ikke foreskriver maksimum tilladte koncentrationer for de udvalgte duftstoffer.
- Ialt ca. 40 % af deodoranterne indeholdt tilladte konserveringsmidler. Det mest anvendte konserveringsmiddel/antimikrobielle stof i deodoranterne var triclosan (i 15 % af produkterne) efterfulgt af parabener (i 9 % af produkterne) og phenoxyethanol (i 7 % af produkterne).
- Triclosan var kun til stede i deodorantprodukter i den dyre priskategori. Triclosanindholdet i de undersøgte produkter var inden for den maksimalt tilladte koncentration (0,3 %) i kosmetiske produkter.
- Triclosan er et allergen, men hyppigheden af allergi er ikke klarlagt. Der er ikke foretaget dosis-respons undersøgelser af triclosans allergifremkaldende effekter, og det var således ikke muligt at gennemføre en risikovurdering.
Det kan konkluderes at duftstoffer, der både vides at være potente allergener og hyppige årsager til allergi hos forbrugere af kosmetik, hyppigt forekommer i deodoranter. De mest potente er dog også de mest sjældne. Allergenerne forekommer ofte i kombinationer. Allergentrykket er betydeligt i denne produkttype og der forekommer duftstoffer, som den Videnskabelig Komité, der rådgiver EU-kommissionen vedrørende kosmetik, har vurderet ikke bør forekomme i kosmetik. Endvidere forekommer der i et mindretal af produkter niveauer af duftstoffer, som udgør en ikke ubetydelig risiko for allergi. Alle produkter opfyldte gældende lovgivning vedr. deklaration af indholdsstoffer samt indholdet af duftstoffer og triclosan.
Summary
Deodorants are used daily by major parts of the population and they may contain ingredients, such as certain fragrance ingredients and preservatives, which are among the most frequent causes of skin allergy. Furthermore, increased use of antimicrobials, eg. triclosan, has been suspected of causing microbiological resistance.
The aim of this project was to map the presence of selected fragrance ingredients and preservatives/antibacterials in deodorants on the Danish market and to perform risk assessment for a selected group of fragrance ingredients concering allergic reactions in the skin
The project was divided into tree phases: i) mapping of deodorants on the Danish market, collection of the most sold products and control of labelling concerning declarations of contents according to the Cosmetic Directive ii) determination of the contents of 26 fragrance ingredients, which since March 2005 have to be declared, and iii) risk assessment of selected fragrance ingredients concerning contact allergy in view of the results of chemical analyses.
Mapping and collection of the most sold deodorants was based on information received from producers/importers and distributors of deodorants as well as other relevant information from the internet, SPT (Association of Danish Cosmetics Toiletries Soap and Detergent Industries) and Informationcenter for Environment and Health. In total, 97 deodorants were purchased, in such a way that most brands on the market, different types of deodorants and products intended for men and women were included in the investigation. Of the 97 deodorants, 23 products were chosen for chemical analysis for the 26 regulated fragrance ingredients and 15 products were selected for the analysis of triclosan content.
Risk assessment concerning contact allergy was performed for the fragrance ingredients isoeugenol, hydroxycitronellal, hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC), cinnamal and cinnamyl alcohol and the preservative triclosan. The fragrance ingredients were selected to include potent allergens as well as frequent causes of allergic reactions. In addition, data from clinical investigation of individuals with allergic reactions to deodorants was also required as basis for the risk assessment. It is the experience that limit values, derived from data from allergic individuals, are very efficient in preventing new cases of allergy as well as reducing symptoms in those who are already sensitized.
The results of the present investigation are as follows:
- According to ingredients list on the products, one or more of the 26 fragrance ingredients, regulated for labelling, were present in 65.9 % of the deodorants.
- The fragrance ingredients differed in potency and frequency of allergic reactions. The most potent substances cinnamal, methyl heptin carbonat and evernia prunastri extract (oak moss abs.) was in the fewest products (1.1 % - 4.6 %).
- Evernia prunastri extract contains two very potent allergenic substances, which according to an opinion of The Scientific Committee on Consumer Products (SCCP) advisory to the European Commission should not be present in cosmetics, due to the risk of allergic reactions. Evernia Prunastri extract was present in 4.6 % of the products.
- Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC), hydroxycitronellal and isoeugenol, which are the cause of many allergic reactions, were present in 33 %, 27.3 % and 9.1 % of the fragranced deodorants respectively.
- The chemical analysis of the 23 selected products showed that the contents of fragrance ingredients conformed to the labelling on respective products, and thus, they were in accordance with the regulations.
- The risk assessment showed that between 3.4 % - 6.8 % of the produkts, as a minium, contained the fragrance ingredients HICC, hydroxycitronellal or isoeugenol in an amount, which surpassed the maxium acceptable concentration derived from the applied method.
- Cinnamal was found only in one product at a level of 5 ppm, which is of no risk concerning allergy. Cinnamyl alcohol, which is transformed to cinnamal in the skin, was present in 12.5 % of the products.
- All products respected the cosmetic regulation, which provides no limit values for the investigated fragrance ingredients.
- Approximately 40 % of the deodorants contained permitted preservatives. The most used preservative/antimicrobial in the deodorants was triclosan (in 15 % of the products) followed by parabens (in 9 % of the products) and phenoxyethanol (in 7 % of the products).
- Triclosan was only present in deodorants in the expensive product category. The triclosan contents in the investigated products were with- in the maximal allowed concentration (0.3 %) in cosmetic products.
- Triclosan is an allergen, but the frequency of allergy is not known. No dose-response investigations of triclosan’s allergenic effects have been performed, and therefore, it was not possible to perform its risk assessment.
It is concluded that fragrance ingredients, which are both potent allergens and frequent causes of allergy among consumers, are used as ingredients in deodorants. However, the most potent allergens were also the most infrequent ingredients. The allergens are often present in combination and the allergen load is significant. Some fragrance ingredients are found in deodorants, which according to The Scientific Committee on Consumer Products advisory to the European Commission (SCCP) should not be present in cosmetics. Furthermore in a minority of products the concentration levels of fragrance substances constitute a not insignificant risk of allergy. All products conformed to the requirements of present regulations concerning labelling as well as content of fragrance ingredients and triclosan.
1 Introduction
A majority of the population uses deodorants on a daily basis. The active ingredients in the deodorants are fragrances, preservatives/antimicrobials and substances used for the reduction/inhibition of sweating. Fragrances and preservatives are among the most common causes of skin allergy from cosmetic products (1). The content as well as the use pattern of deodorants are responsible for the increased risk of fragrance allergy associated to this product category (2). Moreover, excessive use of certain antimicrobials in deodorants, for example triclosan may lead to development of bacterial resistance against antibiotics.
Contact allergy developes when low molecular weight substances, such as fragrance chemicals in deodorants, penetrate the skin and activate the immune system in such a way that the immune system recognises and reacts to the allergenic substance. The cells that participate in the allergic reaction are T-lymphocytes, which circulate in the whole body. The process of allergy development is called induction and occurs without symptoms. On subsequent exposure to sufficient amounts of the allergenic substance, the immune system will react to the susbstance and eczema will develop.
The symptoms of eczema are itchy redness, papules, swelling and some time blisters on the exposed skin area. Allergy to ingredients of deodorants will appear in the armpits, but can spread to other parts of the body, if the use of the product is continued. The process of developing allergic symptoms is called provocation or elicitation. Once a person has developed allergy it is a livelong condition, where exposure to sufficient amounts of allergen will result in eczema and therefore exposure should be avoided. Otherwise the person may experience recurring or chronic eczema.
A fragrance formula is typically composed of 10-300 fragrance substances among approximately 2500 fragrance chemicals in use. In EU, 26 fragrance substances, reported to cause allergy in humans, have been identified to help the fragrance allergic persons to avoid exposure to the fragrance substance they cannot tolerate. Since March 2005, it is required that these 26 substances should be labelled on cosmetic products, when their content in leave-on cosmetics is =10 ppm, or =100 ppm in rinse-off cosmetics, according to the EU Cosmetic Directive (4). The same regulation has also been introduced for detergent and cleaning products, both for household and industrial use, since October 2005. This regulation is also useful for doctors in the case of suspected fragrance allergy as it provides the tool to test patients with relevant fragrance ingredients, establish the correct diagnosis and advise patients to avoid exposure to specific substances in future in order not to develop eczema.
According to the EU Cosmetic Directive, 55 different chemical substances can be used as preservatives in cosmetic products under the conditions described in the Directive.
In a survey of the ingredient labelling on 31 deodorants, the Information Centre for Environment & Health found that these products contained potential skin allergens including one or more of the 26 fragrance substances, which should now be declared in the ingredient list (3). The news paper Morgenavisen Jyllandsposten has also found allergenic substances in 10 expensive (prestige products of high price category) deodorants. On this background, the Danish Environmental Protection Agency (DEPA) requested an investigation of fragrance substances and preservatives/antimicrobials used in deodorants as well as their concentration levels in these products. In addition an evaluation of health risks of selected substances present in these products was required.
The project was divided in 3 phases: i) survey of deodorants on the Danish market, collection of the most sold products and control of labelling to check the compliance with the Cosmetic Directive (4); ii) in cooperation with DEPA regarding selection of a number of products for the determination of the contents of the 26 fragrance substances and some selected preservatives, which are regulated according to Annex 3 and Annex VI of the Cosmetic Directive, and finally conformity testing with the regulation according to the Cosmetic Directive; and iii) risk assessment of deodorants on the basis of the analytically determined concentrations of the selected fragrance substances and preservatives with regard to skin allergy.
2 Market survey and product sampling
- 2.1 Market survey
- 2.2 Legislation
- 2.3 Sampling of products and control of labelling
- 2.4 Selection of products for analysis
2.1 Market survey
The following strategy was adopted to ascertain a realistic overview of the deodorants sold on the Danish market:
- Contact to The Association of Danish Cosmetics Toiletries Soap and Detergent Industries (SPT) to get an overview of deodorants on the Danish market and the most sold deodorants,
- An overview of deodorants on the Danish Market via the internet,
- An overview of deodorants on the Danish market by visiting big supermarkets and some special perfume shops,
- Written/telephone contact to Danish manufacturers/importers/distributors of deodorants as well as to deodorants responsible persons in big supermarkets to get information on most sold deodorants,
- Contact to Information Centre for Environment and Health to get relevant information on the deodorants, which were included in their survey.
SPT and Information Centre for Environment and Health informed that all information was on their respective home pages. There was no information on the most sold deodorants on the Danish market on these webpages (5). However at the SPT’s homepage (5), information was given on the most sold brands of perfumes (both for men and women) based on turnover in Danish Kroner. SPT recommended a contact to its members to get more information. The Information Centre for Environment and Health suggested that the expensive deodorants (international brands with relatively high price) should also be included in the present survey.
Google search on ”deodorant” revealed over six million pages with deodorants, of which approximately 53.000 pages with Danish addresses. The impression from going through the first 200 pages was that the most deodorant manufacturers, both Danish and international, advertise their products through the internet and also inform about the quality and property of the respective products. In some cases the ingredient list was also given, for example 14 variants of Axe deodorant with different composition. The internet search also revealed that the same products can be bought through several different addresses, but some specific products were available only through specified shops. The addresses of Danish deodorant dealers on the internet are described in Table 1. The list is not exhaustive as only 200 of the 53000 pages were reviewed.
A number of retail shops were visited to get an overview of deodorants on the Danish market. Thus several supermarkets, special perfume shops, clothing- sport articles shops were visited. All of the shops visited were in the greater Copenhagen area and in Roskilde County. Several shop owners/administrators were requested to give information on the most sold products in their respective shops, but in most cases such information was not given.
Table 1: Internet addresses for the shopping of deodorants in Denmark (found in the first 200 of the 53000 web pages in Google search)
Twenty nine manufacturers and importers/distributors of deodorants in Denmark, whose products were estimated to cover a major part of deodorant market in Denmark, and six big supermarkets/magazines were contacted in writing or by phone to get information on the most sold deodorants and the number of units sold per year in Denmark. The administrators in three retail shops provided information about the names of the most sold deodorants in their shops. Five manufactures/importers of deodorants also informed about the most sold brand of their respective products as well as the numbers of individual products sold per year. Furthermore, two manufacturers of deodorants formulated products only for specific dealers. Three cosmetic importers had no deodorants in their assortment. According to the information received, approximately 2000-175000 units/year of individual deodorants of different brands were sold in Denmark in 2006. All information received is treated confidentially at NERI. The information received is used only for the present study and has not been forwarded to anyone else.
Cosmetic products including deodorants are also sold via shops in airports, at ferries and also through travel agencies. The customers in the airports and at ferries can be Danes as well as of other nationalities. Therefore, sale of deodorants in these shops has not been considered in the present study, which is focused on the deodorants on the Danish market. One of the travel agencies in Denmark was contacted, in writing, to get information on the most sold deodorant via its shop, but no answer was received.
In the present investigation, information on the most sold deodorants via membership of various cosmetic clubs (post-order cosmetic dealers) is not requested and internet dealers of deodorants are not contacted, as it was considered a minor part of deodorants sold in Denmark.
2.2 Legislation
Cosmetic products including deodorants marketed in Denmark/EU should comply with the EU Cosmetic Directive/Danish Statutory Order on Cosmetic Products (4). The Cosmetic Directive requires labelling of cosmetic products among others full declaration of ingredients. This also means that content of all ingredients in deodorants should be declared on these products, according to § 25 of the Danish Satutory Order concerning labelling of cosmetic products. Furthermore, according to § 33 of the Danish Satutory Order the manufacturer in EU or the responsible for marketing should also be identified on the label, so that Member State authorties, for example DEPA, have easy access to information concerning ingredients and risk assessment of the products.
This project is focussed on fragrance substances and preservatives, which should only be used in deodorant formulation under the restrictions laid down in Annex III and Annex VVI respectively. The requirements concerning contents of other ingredients according to the Cosmetic Directive should also be met, obviously. Deodorants should be labelled with the 26 named fragrance substances if there concentration in the products is over 10 ppm. Furthermore, the products should be labelled “contains perfume (or aroma)”, if it contains other than the 26 named fragrance substances, or less than 10 ppm of these 26 substances. Maximum permitted concentration of triclosan, which is analysed in the selected products in this study, in cosmetic products, and thus, in deodorants is 0.3% (w/w).
2.3 Sampling of products and control of labelling
To control the labelling of the deodorants as well as to get an impression of a reliable pattern of the exposure of Danish population by deodorant ingredients, sampling of deodorants was performed on the basis of:
- information received on the number of sold units of different brands of deodorants,
- the most popular deodorants (number of units sold) according to the salesmen in various shops/supermarkets visited
- the most sold men and women perfumes according to SPT’s homepage
The deodorants purchased for the present survey are described in Annex 1. All products are bought in retail-outlets/magazines in the greater Copenhagen area or in Roskilde in the period 18-22 May 2006. All-in-all 97 deodorants were bought, so that most brands were included in the study, which included all types of deodorants (55 sprays and 42 deo roll-on, cream deo and deostick) both for men and women. Identification of the purchased products, information of manufacturers/importers/dealers and labelling of contents on the products are described in Annex 1.
Labelling of all products was checked for the conformity of the declaration of ingredients with the guidance in the Cosmetic Directive, and it was also checked that the manufacturers/importers of the products were clearly identified. Names of some substances in the ingredient lists (in italics) were not in agreement with the corresponding names in the Danish Statutory Order on Cosmetic Products: anisyl alcohol/anise alcohol, hexyl cinnamicaldehyde/hexyl cinnamal, hydroxymethypentyl cyclohexenecarboxaldehyde/hydroxyisohexyl 3-cyclohexene carboxaldhyde, 3-methyl-4-(2,6,6-2-cyclohexen-1-yl)-3-butene-2-one/alpha-isomethylionone and 2-(4-ter-butylbenzyl)-propionaldehyd/butylphenyl methylpropional. A possible explanation for this is that the names of the respective substances in the Danish Statutory Order are not INCI names. Furthermore, labelling of ”limonene” can be interpreted as the products contain ”d-limonene”, as one of the 26 regulated fragrance substances.
Nine of the sampled products were not declared to contain perfume, but only eight of these were explicitly labelled as perfume free. Seventeen of the sampled products were labelled with “perfume”, without mentioning any specific fragrance substance. This indicates that these products either did not contain any of the 26 regulated fragrance substances, or the concentrations of these 26 fragrance substances in the products were less than the concentration (10 ppm) required for obligatory labelling according to the Cosmetic Directive.
The distribution of the individual 26 fragrance substances/extracts, whose content should be declared according to the Cosmetic Directive, in the 88 perfume containing products, is described in Table 2. The frequency of labeled essential oils, which could be sources of some of the 26 fragrance substances, is not included in the Table 2. Besides the 26 regulated fragrance substances, two other fragrance ingredients were labelled on the sampled deodorants: bisbolol in 4 products and triethyl citrate in 4 products.
Among the 26 fragrance substances, most commonly used in the formulation of perfume containing deodorants was citronellol (65.9%), followed by linalool
(53.4 %), d-limonene (53.4 %), geraniol (48.9 %), butylphenyl methyl propional (48.9 %), alpha isomethylionone (46.6 %), benzyl salicylate (39.8 %), hydroxyisohexyl 3-cyclohexene carboxaldehyd (32.3 %), coumarin (32.3 %), hydroxycitronellal (27.3 %), eugenol (27.3 %), citral (26.1 %) and benzyl benzoate (25 %). The remaining 13 of the 26 fragrance substances were used in less than 25% of the deodorants (Table 2). Comparison of the declared contents of fragrance substances in the sampled deodorants with the content of selected fragrance substances in deodorants on the European market, in an earlier study (6) may indicate that the use of strong fragrance allergens in deodorants is decreasing. The two studies, however, cannot be directly compared, as only a subsample of the deodorants were analysed chemically in the present study.
Table 2. Fragrance substances labelled on the 88 perfume containing deodorants*
| Fragrance substance | No. of products | % of products containing fragrance substances |
| Amyl cinnamal | 9 | 10.2 |
| Amylcinnamyl alcohol | - | - |
| Anise alcohol | 2 | 2.3 |
| Benzyl alcohol | 15 | 17.1 |
| Benzyl benzoate | 22 | 25.0 |
| Benzyl cinnamate | 3 | 3.4 |
| Benzyl salicylate | 35 | 39.8 |
| Butylphenyl methylpropional | 43 | 48.9 |
| Cinnamal | 1 | 1.1 |
| Cinnamyl alcohol | 11 | 12,5 |
| Citral | 23 | 26.1 |
| Citronellol | 58 | 65.9 |
| Coumarin | 29 | 33.0 |
| Eugenol | 24 | 27.3 |
| Farnesol | 13 | 14.8 |
| Geraniol | 43 | 48.9 |
| Hexyl cinnamal | 29 | 33.0 |
| Hydroxycitronellal | 24 | 27.3 |
| Hydroxyisohexyl-3-cyclohexene carboxaldehyde (HICC) | 29 | 33.0 |
| Isoeugenol | 8 | 9.1 |
| alpha-isomethyl ionone | 41 | 46.6 |
| d-Limonene/limonene | 47 | 53.4 |
| Linalool | 47 | 53.4 |
| Methyl 2-octynoate | 1 | 1.1 |
| Evernia Prunastri extract/ oakmoss/egemossekstrakt | 4 | 4.6 |
| Evernia Furfuracea extract/Træmossekstrakt | 2 | 2.3 |
INCI (Inventory of Ingredients, Official Journal of the European Union) is used, except for the substances with no INCI name
*17 products were labelled to contain perfume without mentioning any specific fragrance substance.
The declared content of preservatives/antimicrobials in the deodorants is described in Table 3. Only about 40% of the deodorants contained the permitted preservatives. It is therefore possible that one or more of the other ingredients in the remaining products functions as preservative/antimicrobial. For example, ethanol which is used as solvent in the formulation of deodorants is a well know antimicrobial substance, and the fragrance substance benzyl alcohol can also be used as a preservative according to the Cosmetic Directive. In general, 0-3 preservatives/antimicrobial substances were present in the sampled deodorants, except in the product No. 306, which contained 5 different preservatives. The most commonly used preservative/antimicrobial substance in the deodorants was triclosan (in 15 % of the products), followed by parabens (in 9 % of the products) and phenoxyethanol (in 7 % of the products). Other preservatives (Table 3) were present in 1-2 % of the sampled products. Triclosan was present only in the products of upper price category.
Table 3. Preservative/antimicrobial labelling on the purchased deodorants.
| Fragrance substance | No. of products |
| Benzoic acid/Na-benzoate or other inorganic benzoates | 3 |
| 2-Bromo-2-nitropropane-1,3-diol | 1 |
| DMDH Hydantoin | 1 |
| Parabenes | 9 |
| Imidazolidinyl urea | 2 |
| Iodopropynyl butylcarbamate | 2 |
| Phenoxyethanol | 7 |
| Sorbic acid (and its salts) | 1 |
| Triclosan | 15 |
2.4 Selection of products for analysis
Products for the analyses as well as risk assessment of fragrance substances and preservatives/antimicrobial substances were selected in cooperation with DEPA. Fifteen of the products labelled to contain triclosan (Table 3), were selected for the determination of triclosan. Only few products contained other permitted preservatives/antimicrobial substances, and therefore, risk assessment of additional fragrance substances than planned was prioritised, instead of analysis and risk assessment of an additional preservative.
Fragrance substances were selected in such a way that these were potent allergens and/or frequently involved in skin allergy, and a dose-response study in allergic persons, with the deodorants containing these substances, had been performed earlier. Isoeugenol, hydroxycitronellal and hydroxyisohexyl-3-cyclohexene carboxaldehyde (HICC) were prioritised, because these are among the most frequent allergenic fragrance substances and dose-response studies using deodorants containing these fragrance substances on persons allergic to these fragrance substances have been performed (7-10). Moreover cinnamyl alcohol was selected, because it metabolises in the skin to cinnamal, one of most allergenic fragrance substances, which itself was found only in one of the products in this study. Finally, it was decided to include farnesol to get an overview of the content of farnesol in deodorants, as this substance can also be used as antimicrobial in addition to its function as fragrance substance.
For the analysis of the content of 26 regulated fragrance substances, 23 deodorants were selected in a way, so that these were among the most sold products and at least two of the fragrance substances selected for risk assessment were declared on the respective ingredient lists. Furthermore, all products containing isoeugenol and/or cinnamyl alcohol were selected for the analysis. Determination of the contents of evernia prunastri extract (oakmoss) and evernia furfuracea extract (treemoss) (among the 26 fragrance substances) is omitted, as a suitable method for the determination of these substances is not yet available. Thus, only 24 of the 26 fragrance substances were analysed in the selected samples.
3 Analysis
3.1 Materials
The fragrance substance standards were obtained through various sources as described in an earlier report (11). Triclosan-standard was purchased from Sigma-Aldrich, Denmark. All other chemicals used were of pro analysi or HPLC quality.
3.2 Analysis
3.2.1 Sample preparation
3.2.1.1 Fragrance substances
Aerosol spray-products were opened as described before (11) to remove and measure the amount of propellant. A portion of the samples, without propellant, was transferred into vials for the analysis by gas chromatography-mass spectrometry (GC-MS).
Deostick and roll-on products were treated as follows: To approximately 2 g sample, weighed exactly in a dark bottle, a few boiling chips and 8 ml methanol were added and the flask was closed with a screw cap. The mixture was mixed gently and warmed thereafter at 60°C for 5 min to dissolve the fatty substances (heating of homogenous liquid products was not required). The sample solution/suspension was then cooled to room temperature (20°C). A 20 cm (length) x 1.8 cm (diameter) glass column was packed with wet silica gel (in methanol) to 7 cm. The cooled sample solution was quantitatively transferred on to the silica-gel column and that was eluted with 20 ml methanol. The first 5 ml of the eluate was discarded, and thereafter the eluate was collected in a 25 ml measuring flask. The flask was filled up to the mark with methanol. The fragrance extract thus obtained was transferred into GC vials and analysed within 24 hours. Duplicate analysis was performed on each sample.
3.2.1.2 Triclosan
To approximately 1 g homogenous sample weighed in a 100 ml dark bottle, 0.25 ml sulfuric acid (4M) and 10 ml methanol were added. The mixture was shaken for 15 min at 60°C and then filtered through Whatman No. 2 filter paper and collected in a 25 ml measuring flask. The measuring flask was filled with methanol up to the mark. For each sample, two extracts were made. The sample extracts were transferred into vials and analysed by high performance liquid chromatography (HPLC).
3.2.2 Analysis of fragrance substances
Analysis of fragrance substances in the sample extracts as well as in the undiluted samples was performed by GC-MS, as described before (12). Each sample as well as each calibration standard solution (2-100 ppm) were analysed in duplicate. Identification and determination was performed in selective ion mode (SIM). Repeatability of the analytical method was evaluated for 10 determinations of two mixtures of fragrance substances (10 ppm and 25 ppm of each substance except farnesol). Repeatability test of farnesol (10 ppm and 25 ppm) determination was performed separately, because this in itself is a mixture of three substances. Oakmoss/treemoss in the samples was identified by the presence of evernic acid ethyl ester. Recovery of the fragrance substances, except oakmoss/treemoss, was investigated by the analysis of two products, which were spiked with 10 ppm of each of the target fragrance substance. Only qualitative analysis of oakmoss/treemoss in the deodorants is performed.
The detection limit of each substance was ca. 1 ppm and the limit of quantification of the fragrance substances was ca. 2 ppm. Recovery of all fragrance substances was 80-115% and relative standard deviation (repeatability) of the method for all substances was 8-12%.
3.2.3 Analysis of triclosan
Each sample extract as well as all calibration solutions were analysed in duplicate by HPLC as described before (13). Analyses of several dilutions of triclosan solution (5-300 ppm) were performed to generate calibration curve of the substance. Repeatability of the analytical method was determined by the analysis of two solutions of triclosan (30 ppm and 120 ppm), and the recovery of triclosan in the products was investigated by the analysis of two products spiked to concentration levels 60 ppm and 120 ppm.
Identification of triclosan in the samples was performed by the comparison of HPLC-retention time and UV-spectrum of the HPLC-peak of standard triclosan with those of the samples analysed under the same conditions as the standard triclosan. The content of triclosan was determined by the use of calibration curve of the standard triclosan. The recovery of triclosan from the spiked samples was ca. 98%, and the relative standard deviation of the analytical method was less than 5%.
4 Results
Analysis of 24 regulated fragrance substances in 23 selected deodorants (19 spray products, 2 deostick and 2 roll-on) was performed by GC-MS at selective ion monitoring mode. The content of the fragrance substances in the investigated products is described in Table 4. The concentrations are not corrected for the recovery of the fragrance substances.
None of the investigated products were found to contain amylcinnamyl alcohol or methyl heptin carbonate. The content of the remaining 22 fragrance substances in the deodorants varied from approximately 2 ppm (detection limit) to 11300 ppm (w/w for deostick and roll-on, w/v for other samples). Frequency and concentration range of all of the target fragrance substances in the investigated products are described in Table 5. As the contents of individual fragrance substances in the investigated products were in a broad range, only concentration range (minimum and maximum concentration) for these is reported in Table 5, while mean (± SD) and median values (Table 6) were calculated only for the five fragrance substances selected for the risk assessment. All 23 selected products contained the fragrance substances, which were listed in the ingredient lists of the respective products, and they were labelled correctly with respect to content of fragrance substances.
Table 4: Content of selected fragrance substances in the 23 investigated deodorants.
| Fragrance substance | Content in ppm (µg/ml in spray, µg/g in deostick/roll-on) | |||||||
| spray 1 | spray 2 | spray 3 | spray 4 | spray 5 | spray 6 | spray 7 | spray 8 | |
| alpha-Amylcinnamic aldehyde |
2.3 | - | - | - | - | - | - | - |
| alpha-Amylcinnamic alcohol |
- | - | - | - | - | - | - | - |
| Anise alcohol | - | - | - | - | - | - | - | - |
| Benzyl alkohol | 31.6 | - | 166.2 | - | - | - | 52.1 | - |
| Benzyl benzoate | 878.0 | - | 4054.2 | - | 53.9 | - | - | - |
| Benzyl cinnamate | - | - | - | 143.2 | - | - | - | - |
| Benzyl salicylate | 900.0 | 145.4 | 2472.8 | - | - | 2085.7 | - | 1646.8 |
| Butylphenyl methyl-propional | 112.0 | 716.0 | 5455.0 | 9.4 | - | 15.0 | 597.5 | 1092.9 |
| Cinnamal | 5.0 | - | - | - | - | - | - | - |
| Cinnamyl alkohol | - | - | - | 296.7 | 1.7 | 37.9 | - | - |
| Citral | 45.3 | - | - | - | - | - | 110.7 | - |
| Citronellol | 350.0 | 1069.6 | 783.3 | 280.0 | 93.7 | 41.5 | - | 289.2 |
| Coumarin | 7.2 | - | - | 29.9 | 3.8 | - | 100.1 | - |
| Eugenol | 195.3 | - | - | - | 99.1 | - | - | - |
| Farnesol | - | 595.0 | 660.9 | - | - | 978.9 | 1791.0 | 1402.5 |
| Geraniol | 39.5 | 61.6 | 399.0 | - | 45.1 | - | 80.3 | 140.9 |
| a-Hexylcinnamic aldehyde | 369.4 | 1019.0 | - | - | - | 71.3 | - | 2502.9 |
| Hydroxycitronellal | dl | 499,8 | 310,8 | 23,9 | - | - | 114,5 | 354,6 |
| HICC | 274.0 | 143.6 | 73.1 | 18.3 | 12.2 | 28.9 | 892.1 | - |
| Isoeugenol | 20.7 | - | - | - | 6.9 | - | - | 28.9 |
| alpha-isomethylionone | - | 245.0 | 2588.0 | - | - | - | 548.8 | 1035.4 |
| Limonene | 3778.1 | 1977.0 | 1619.3 | 1083.3 | 5760.6 | - | 3794.6 | 4002.6 |
| Linalool | 110.2 | 302.3 | 73.9 | 42.2 | 115.6 | 1102.0 | 597.0 | 525.1 |
| Methyl-2-octynoate | - | - | - | - | - | - | - | - |
| Oakmoos/Treemoss | - | - | - | - | X | - | - | - |
HICC: Hydroxyisohexyl 3-cyclohexene carboxaldehyde; dl: detection limit ca. 1 ppm; X: contains oakmoss/treemoss
Table 4: continued
| Fragrance substance | Content in ppm (µg/ml in spray, µg/g in deostick/roll-on) | |||||||
| Deostick 1 | spray 9 | spray 10 | spray 11 | spray 12 | spray 13 | deostick 2 | spray 14 | |
| alpha-Amylcinnamic aldehyde |
- | - | - | - | 66.3 | - | - | - |
| alpha-Amylcinnamic alcohol |
- | - | - | - | - | - | - | - |
| Anise alcohol | - | - | dl | - | - | - | - | - |
| Benzyl alcohol | - | 129.9 | - | - | - | - | - | - |
| Benzyl benzoate | - | 20.2 | 11.2 | 183.5 | 65.3 | - | - | - |
| Benzyl cinnamate | - | - | - | - | - | - | - | - |
| Benzyl salicylate | - | 2078.1 | - | - | 474.0 | - | - | - |
| Butylphenyl methyl-propional | 114.2 | - | 23.6 | - | - | 2405.0 | dl | - |
| Cinnamal | - | - | - | - | - | - | - | - |
| Cinnamyl alkohol | - | 9.5 | - | 406.3 | 503.0 | - | - | 35.5 |
| Citral | 202.4 | - | - | - | 38.8 | 99.9 | - | - |
| Citronellol | 197.6 | - | 63.7 | 35.2 | 851.7 | 5847.5 | dl | 52.4 |
| Coumarin | 116.4 | 84.9 | - | 55.0 | 258.0 | - | - | 49.5 |
| Eugenol | 125.4 | - | 0.8 | - | 131.6 | - | - | - |
| Farnesol | - | - | - | - | - | 649.1 | 9.0 | - |
| Geraniol | 103.1 | 30.3 | 206.9 | 23.9 | 141.2 | 242.4 | dl | 24.4 |
| a-Hexylcinnamic aldehyde | - | - | - | - | 136.3 | - | dl | 4434.0 |
| Hydroxycitronellal | - | 89.2 | dl | 37.8 | 1746.5 | - | - | 377.2 |
| HICC | 184.8 | 323.7 | 19.4 | - | - | 168.5 | - | 4431.0 |
| Isoeugenol | 123.0 | 34.7 | - | - | 138.4 | - | dl | - |
| alpha-isomethylionone | - | - | 5.8 | - | 477.0 | - | 14.8 | 269.1 |
| Limonene | - | - | - | 1194.3 | 11386.5 | 1226.7 | 1022.8 | - |
| Linalool | 111.1 | 133.6 | 121.3 | 46.6 | 1164.9 | 998.5 | 86.0 | 32.1 |
| Methyl-2-octynoate | - | - | - | - | - | - | - | - |
| Oakmoos/Treemoss | - | - | - | - | X | - | - | - |
HICC: Hydroxyisohexyl 3-cyclohexene carboxaldehyde; dl: detection limit ca. 1 ppm; X: contains oakmoss/treemoss
Table 4: continued
| Fragrance substance | Content in ppm (µg/ml in spray, µg/g in deostick/roll-on) | ||||||
| spray 15 | spray 16 | spray 17 | spray 18 | roll-on 1 | roll-on 2 | spray 19 | |
| alpha-Amylcinnamic aldehyde |
42.3 | - | - | 164.7 | - | - | - |
| alpha-Amylcinnamic alcohol |
- | - | - | - | - | - | - |
| Anise alcohol | - | - | - | - | - | 50.5 | - |
| Benzyl alcohol | 83.2 | 98.4 | - | ||||
| Benzyl benzoate | 107.6 | - | 38.1 | - | - | 3.2 | 201.6 |
| Benzyl cinnamate | 74.1 | - | - | - | - | - | - |
| Benzyl salicylate | 136.3 | - | 5279.0 | 828.7 | - | - | 211.7 |
| Butylphenyl methyl-propional | - | - | 3788.0 | dl | 2.4 | dl | 172.5 |
| Cinnamal | - | - | - | - | - | - | - |
| Cinnamyl alkohol | 240.1 | 39.6 | 36.1 | - | - | 223.1 | |
| Citral | 553.9 | 249.9 | 119.7 | - | 44.0 | - | 296.2 |
| Citronellol | 344.3 | 363.8 | 3161.0 | 107.9 | 16.5 | 89.2 | 289.9 |
| Coumarin | 1254.9 | - | - | - | 127.4 | - | 170.3 |
| Eugenol | 514.0 | - | - | - | - | dl | - |
| Farnesol | - | 269.4 | - | - | 969.2 | - | - |
| Geraniol | 124.7 | 184.8 | 101.2 | dl | 48.6 | dl | - |
| a-Hexylcinnamic aldehyde | - | - | 2962.9 | 374.2 | 5.2 | - | 186.1 |
| Hydroxycitronellal | 292.6 | 337.5 | 1092.5 | dl | - | 46.3 | - |
| HICC | - | 28.3 | 350.5 | - | dl | 28.5 | 270.6 |
| Isoeugenol | 67.5 | - | - | - | - | - | - |
| alpha-isomethylionone | 1674.2 | 46.4 | 1788.0 | 292.1 | 128.2 | 250.0 | 120.9 |
| Limonene | 11229.0 | 2662.2 | 4507.1 | - | 5489.4 | - | 4538.3 |
| Linalool | 3447.1 | 1308.0 | 350.4 | 8.2 | 377.3 | - | 230.5 |
| Methyl-2-octynoate | - | - | - | - | - | - | - |
| Oakmoos/Treemoss | X | - | - | - | - | - | - |
HICC: Hydroxyisohexyl 3-cyclohexene carboxaldehyde; dl: detection limit ca. 1 ppm; X: contains oakmoss/treemoss
Table 5: Frequency and content of selected fragrance substances in the 23 deodorants investigated.
| Fragrance substance | Products containing the fragrance substance No. (%) |
Concentration range (ppm) | |
| alpha-Amylcinnamic aldehyde? |
4 | 17 | 2.3 - 164.7 |
| alpha-Amylcinnamic alcohol |
- | - | - |
| Anise alcohol | 2 | 9 | dl*, 50.5 |
| Benzyl alcohol | 6 | 26 | 31.6 - 166.2 |
| Benzyl benzoate | 11 | 48 | 3.2 - 4054.2 |
| Benzyl cinnamate | 2 | 9 | 74.1, 143.2 |
| Benzyl salicylate | 11 | 48 | 136.3 – 5279.0 |
| Butylphenyl methylpropional | 16 | 70 | dl* - 5.455.0 |
| Cinnamal ? | 1 | 4 | 5.0 |
| Cinnamyl alcohol ? | 11 | 48 | 1.7 - 503.0 |
| Citral? | 10 | 44 | 38.8 - 553.9 |
| Citronellol? | 21 | 91 | dl* - 5847.5 |
| Coumarin? | 12 | 52 | 3.8 - 1254.9 |
| Eugenol? | 7 | 30 | dl*- 514.0 |
| Farnesol? | 9 | 39 | 9.0 -1791.0 |
| Geraniol? | 20 | 87 | dl* - 399.0 |
| Hexyl cinnamal ? | 11 | 48 | dl* - 4434.0 |
| Hydroxycitronellal? | 16 | 70 | dl* - 1746.5 |
| HICC? | 17 | 74 | dl* - 4431.0 |
| Isoeugenol? | 8 | 35 | dl* - 138.4 |
| Alpha-isomethylionone | 15 | 65 | 5.8 - 2588.0 |
| d-Limonene? | 16 | 70 | 1022.8 - 11386.5 |
| Linalool? | 22 | 96 | 8.2 - 3447.1 |
| Methyl-2-octynoate? | - | - | - |
| Evernia prunastri?/ furfuracea extract (Oakmoos/Treemoss) |
3 | 13 | X |
*detection limit of the respective substances: about 1 ppm
The most common fragrance allergens are contained in the two mixtures, which is used for diagnosing fragrance allergy, called fragrance mix I (?) and fragrance mix II (?), besides oxidation product of terpens (?), and tree moss extract are commen allergens. Methyl-2-octynoate is an extreme, but rare allergen. X: contains oakmoss/treemoss
Table 6: Mean and median concentrations of the 5 selected fragrance substances in 23 deodorants selected for the analysis.
| Fragrance substance | Concentration i ppm | |
| Mean±SD | Median | |
| Cinnamyl alcohol | 166±177 | 40 |
| Farnesol | 814±547 | 661 |
| Hydroxycitronellal | 333±470 | 203 |
| HICC | 426±1055 | 144 |
| Isoeugenol | 53±52 | 32 |
The contents of triclosan in all of the investigated products are described in Table 7. The content of triclosan could not be determined in one of the samples (2006-316) due to presence of a large interfering peak just adjacent to triclosan peak in the HPLC chromatogram of the sample. The investigated products contained 0.05-0.24% (w/v) triclosan, and thus, the triclosan contents in the investigated products were within the maximum allowed concentration (0.3%) in cosmetic products.
Table 7: Content of triclosan in the investigated products
| Sample | Triclosan content % (w/w) |
| Spray 20 | 0.0797 |
| Spray 21 | 0.0841 |
| Deostick 3 | 0.0480 |
| Spray 22 | 0.1853 |
| Deostick 23 | 0.1158 |
| Spray 24 | 0.0814 |
| Spray 25 | 0.0516 |
| Deostick 4 | 0.0536 |
| Spray 26 | 0.0842 |
| Spray 27 | 0.0781 |
| Spray 15 | 0.2403 |
| Spray 28 | Interference |
| Spray 29 | 0.0538 |
| Spray 30 | 0.1248 |
| Spray 31 | 0.0666 |
5 Risk assessment
- 5.1 Deodorants and contact allergy
- 5.2 Risk assessment – in general
- 5.3 The selected fragrance substances
- 5.4 Farnesol
- 5.5 Comments concerning other fragrance substances
- 5.6 Allergen load of fragrance substances
- 5.7 Triclosan
5.1 Deodorants and contact allergy
The use of perfumed deodorants is associated with an increased risk of perfume allergy. In a study of 925 eczema patients and a control group of 806 persons, randomly selected from the population, a statistically significant correlation was found between skin rash to a perfumed deodorant as first time symptom (odds ratio: 2,3-2,9) and later diagnosis of perfume allergy (14). In a German study, eczema patients were patch tested with their own deodorants (15). All-in-all 1069 deodorants were tested, of these 6.7% produced allergic reactions. There was a statistically significant correlation between an allergic reaction to ones own deodorant and perfume allergy, among these allergy to HICC and cinnamal (15).
The environment in the armpit is moist and occluded, which like the presence of hair follicles can increase penetration of certain allergens (16-18). Shaving also increases penetration and thus the risk for skin allergy (19). In a case study, 14 perfume allergic patients were asked to use one of their own deodorants in the armpit and as well as on the upper arm for one week. Twenty deodorants were tested, and 12 of these (60%) produced eczema in the armpit, while only 4 (20%) produced eczema on the upper arm (20). The deodorants, which gave a positive test, contained 1.3-8.6 times higher concentration of allergenic fragrance substances than those products which were negative in the study (20)
Deodorants are available in different formulations, such as aerosol sprays, roll-ons, and sticks. There may be differences in bioavailability of allergenic fragrance substances in different formulations of deodorants. In a small study, a deodorant spray and a deostick with the same concentrations of allergenic fragrance substances were tested in the cubital fossa of 7 perfume allergic patients. Five of these persons reacted to deospray, while only one reacted to deostick. (21). The influence of deodorant matrix on allergy has not been investigated systematically. Other products are also important for perfume allergy, especially perfumes, colognes, aftershave lotion and creams/lotions (14-15).
5.2 Risk assessment – in general
Exposure in the form of dose/cm² of an allergen is a decisive determinant for the development of allergy (22, 23). Thus, the concentration of allergen in a given product is important. The EU Commission has published guidelines for risk assessment. The standard dose of deodorant is 0.5 g/day on a total surface area of 100 cm², i.e. 5 mg/ cm² (24). The Research Institute for Fragrance Materials (RIFM), which is financed by the perfume producers association called International Fragrance Association (IFRA) use, in a new model for risk assessment, 9.1mg/cm²/day. In several risk assessments, different doses and surface areas have been used (18, 24, 25), for example the total skin area exposed to deodorants has been estimated to be 100 cm² -240 cm².
In a recent study, the use of 6 different cosmetic products by American women of 19-65 year of age was measured during a 14 days period (26), including use of
deostick. The mean dose per application of deostick was 0.61 g (median 0.45 g), which corresponds to standard dose, and the average application frequency was 1.3 per day (0-4). The 10% of the participants, who were the most frequent users, used deodorant as a minimum twice a day (26). Other deodorant formulations were not investigated in this study. Other factors, than allergen dose, are of importance for development of allergy, e.g. the skin area of the body, vehicle, simultaneous ocurrence of skin irritants in the formulation and preexisting eczema (27).
Since 1973, the perfume industry’s’ association IFRA has published recommendations for the use of allergenic fragrance substances. (28), which are based on tests conducted in healthy voluenteers. In their earlier used model, which is still in force for the majority of the substances, the scientific rationale for the recommendations was seldom given. The problems of fragrance allergy are a consequence of these recommendations being insufficient (29, 30).
Toxicologists from cosmetic industry have worked several years on the development of a model for risk assessment. This is based on the results of a predictive test in mice, the so called Local Lymph Node Assay (LLNA), in some cases supplemented with tests performed in healthy voluenteers (18, 23, 31). The potential of a substance to induce skin allergy is predicted from the data obtained through these tests (31, 33). The basis for the calculation includes exposure estimate and uncertainty factors (18, 32).
The risk assessment model developed by the scientists in the cosmetic industry includes scientific elements and it establishes safe limits, but it is not validated with respect to whether these limits will be able to prevent allergy. Besides, the model aims exclusively at prevention of new cases of allergy and it does not take into account the existing allergy problem in the population. It has been demonstrated that limit values, which are based on data from tests performed in persons with allergy, are very effective in preventing new cases of allergy as well as in minimizing the consequences of the disease in persons, who have already developed allergy (27). The results of such dose-response investigations employing allergic persons have been shown to be highly reproducible, even when these are performed in different clinics and in different European countries (34).
The risk assessment presented here, is based on the clinical data derived from tests employing patients with allergy. For comparison, an example of risk assessment based on animal experiments and an overview of actual limit values, which are in force or are proposed as official limit values as well as those recommended by perfume industry’s’ organisations (RIFM/IFRA), are presented.
5.3 The selected fragrance substances
5.3.1 Hydoxyisohexyl 3-cyclohexene carboxaldehyde (HICC)
Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC) with the trade name Lyral®, is one of the most frequent causes of perfume allergy. In studies performed in European Dermatology clinics, 1.6%-2.7% of eczema patients have been found to be allergic to HICC (35, 36). In a multicenter study in Germany, 1.9% of eczema patients were found to be allergic to HICC (37) without any significant sex difference. Similar results have also been found in the Danish monitoring network of dermatologists. Here, 2.3% of men and 2.6% of female eczema patients were found to be allergic to HICC in 2005; and in 2006, 1.3% of men and 2.7% of female eczema patients were allergic to HICC (38). The frequency of HICC allergy among the tested eczema patients in a dermatology department in Copenhagen is shown in Figure 1. There is a tendency that patients with HICC allergy have eczema in the armpits more frequently than other eczema patients, p = 0.065 (37).
Figure 1. Frequency of eczema patients with allergic reaction to selected fragrance substances among all eczema patients (n = 3179) allergy tested at the Dermatology Department of Gentofte Hospital in the period 2002-2005 (unpublished results)
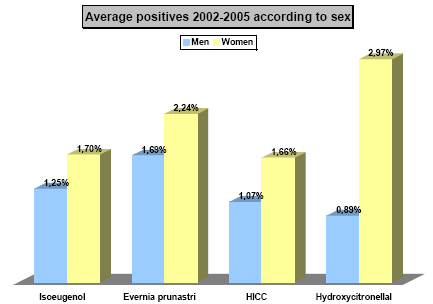
HICC: Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC). Isoeugenol and Evernia Prunastri extract (oak moss abs.) are tested at 1 %, while hydroxycitronellal and HICC are testet at 5 % in petrolatum.
HICC is included in the new perfume mixture, fragrance mix II, which is routinely used for the diagnosis of perfume allergy. Furthermore, it is recommended to test separately with HICC, because it is a very frequent allergen (36).
In a European experimental study, 18 HICC allergic persons were patch-tested with a serial dilution of HICC (6%-0.0006%), i.e. tested under occlusion for 48 hours (39). It was possible from the dose response curve to calculate the dose, which will cause an allergic reaction in 10% of allergic patients. This dose was found to be 29 ppm HICC (CI: 7-69) (39). In a later investigation employing a group of Danish eczema patients, an identical dose-response curve was obtained (7), i.e. 10% reacted to 25 ppm HICC (CI: 0.8-120).
HICC allergy is less frequent in North America, where this allergy has been demonstrated in approximately 0.4 % of eczema patients. The possible explanation is that HICC is less frequenly used in deodorants in North America, and when used, it is in lower concentrations (40), while HICC is shown to be very common on the European market. In a study in 1996, HICC was found in 53% of deodorants (6), and in the present study HICC was present in 33% of the deodorants (Table 2). On the basis of the high frequency of allergy to HICC, EU’s Scientific Committee on Consumer Products (SCCP) has recommended 200 ppm as maximum amount of HICC in cosmetic products (41) (Table 8).
Table 8. Recommendations for limit values of selected fragrance substances by the EU Scientific Committee (SCCP) and perfume industry’s organisation IFRA.
| Fragrance substance | SCCP* | Year | IFRA** | Year |
| HICC | 200 ppm*** | 2003 | 15.000 ppm | 2004 |
| Hydroxycitronellal | 10.000 ppm | 2001 | 10.000 ppm | 1992 |
| Isoeugenol | 200 ppm | 2001 | 2000 ppm 200 ppm |
before 1998 1998 |
| Cinnamal | 1000 ppm | 2001 | None 500 ppm |
before 2007 2007 |
| Cinnamyl alcohol | 8000 ppm | 2001 | 8000 ppm 4000 ppm |
before 2004 2004 |
* EU Scientific Committee on Consumer Products.
** International Fragrance Association publishes guidelines and establishes limit values for the use of fragrance substances with regard to health effects. Limit values in Table 8 are established on the basis of allergenic effects (28).
*** Recommended through SCCP Opinion on HICC (41), but not yet implemented.
The limits of other substances are taken from the public hearing, prior to implementation in Cosmetic Directive (46).
In the present investigation, 6 deodorants were found to contain more than 200 ppm HICC. This means that approximately 1/3 of the analysed deodorants and at least 6.8% of the 88 perfumed deodorants, within this study, contained more HICC than recommended due to the risk of allergy (41). There was one deodorant, which contained 4431 ppm HICC, i.e. more than 20 times the limit recommended by the SCCP (41), and more than twice the maximum concentration (1874 ppm) which was found in a similar study 10 years back (6). Use of deodorants implies a special kind of exposure as described above, and the mentioned 200 ppm limit value of HICC is based on a model calculation. This limit value has been tested later in a study employing 14 HICC allergic persons and 10 controls (7). They were supplied with a deodorant containing 200 ppm HICC for the use two times daily for 14 days in one armpit, and a similar deodorant without HICC to be used in the contralateral armpit. Within 14 days, 9/14 (69%) of the HICC allergic persons developed an allergic eczema soley in the armpit where HICC containing deodorants had been applied. A control group of healthy voluenteers used deodorants in the same way, but none developed eczema (Table 9). Thus, there was a statistically significant correlation between the exposure to 200 ppm HICC in a deodorant and development of eczema (p=0.02). When the exposure dose was increased from 200 ppm HICC to 600 ppm and 800 ppm, all persons in the test group developed eczema, but none in the control group (Table 9). The amount of the deodorant used per day was estimated as realistic (7). It was concluded that 200 ppm is not a safe limit value for HICC in deodorants with regard to elicitation of the allergy, and it should be considered whether HICC should be used at all in this type of product (7). HICC was present in 33% of the perfumed deodorants in the present study.
Table 9. Overview of results of deodorant provocation investigations with different allergens. Frequency in % of test groups, which reacted at different doses of allergen applied in a roll-on antiperspirant, is given.
| Isoeugenol | Cinnamal(1) | Cinnamal(2) | Hcitron | HICC | |
| Dose in ppm | |||||
| 0 | 0 | 0 | 0 | 0 | 0 |
| 63 | 23 | ||||
| 100 | 11 | ||||
| 200 | 69 | 64 | |||
| 320 | 75 | 55 | 57 | ||
| 600 | 85 | ||||
| 630 | 76 | 100 | |||
| 1000 | 99 | 71 | |||
| 1800 | 100 | ||||
| 3200 | 100 | ||||
| No. test persons | 13 | 8 | 9 | 7 | 14 |
| No. of control persons | 10 | 20 | 7 | 10 | |
| % control persons, who reacted | 0 | 0 | 0 | 0 | |
| Exposure should be: | < 63 ppm | <100 ppm | <320 ppm | < 200 ppm | |
| Reference | (10) | (9) | (8) | (7) | |
Hcitron: Hydroxycitronellal; HICC: hydroxyisohexyl cyclohexenecarboxaldehyde (Lyral®).
As a major part of the test persons reacted to deodorants with the lowest concentration of HICC, it was attempted to statistically evaluate the data to determine a concentration level, which can elicit allergic eczema in 10% of the allergic persons, the so called ED 10% (Table 10). Although the data permits such a calculation, i.e. the logistic dose-response model is statistically acceptable; the relative low number of test persons influences the strength and give a low precision of the estimates as well as large confidence intervals. For HICC, it was estimated that 10% of HICC allergic patients will react to the use of a deodorant with 29 ppm HICC in the product (Table 10). Only four of the analysed deodorants in the present study contained less than 29 ppm HICC. The estimates for the other selected fragrance substances have also a low presicion and therefore, these data are not used further in the risk assessment.
Table 10. The allergen concentration in deodorant (roll-on), which provokes allergic eczema in persons with allergy to the substance. Estimates on the basis of fitted dose-response curves with corresponding confidence intervals (58).
| Substance | ED10 % (95 %CI) | ED25 % (95 % CI) | ED50 % (95 % CI) | Ref. |
| HICC | 29 ppm (<5ppb-89) | 62 ppm (0,05-148) | 136 ppm (4,3-296) | 7 |
| Hydroxycitronellal | 38 ppm (*) | 103 ppm (*) | 276 ppm (*) | 8 |
| Isoeugenol | 20 ppm (0,4-56) | 53 ppm (5,8-130) | 146 ppm (47-478) | 10 |
| Cinnamal (1+2) | 109 ppm (39-169) | 169 ppm (86-245) | 263 ppm (170-397) | 9 |
ED: Elicitation dose (concentration) for a defined part of the test population, for example 10 %.
CI: Confidence interval.
*: can not be calculated. Group size in some studies is small (see Table 9), thus there is a considerable uncertainty of the estimates.
For comparison, the levels of allergenic fragrance substances, which will be acceptable according to the risk assessment model used by the cosmetic/perfume industry, are presented in Table 11. Characteristic for this model is that as a starting point it uses the amount of the allergenic substance which can induce allergy in experimental animals (mice) or in healthy voluenteers. The model, which can be used in different ways, is based on several assumptions. Furthermore, the model is not validated with regard to the levels of the allergens which in fact are effective in prevention of development of new cases of allergy, and it does not consider the existing allergy problems in the population. Generally, the levels which will be considered acceptable according to this model will be higher than those based on clinical data. This is also seen for HICC, where the safe limit value is calculated to 2000 ppm. (Table 11).
Table 11. Example of risk estimates for induction. Dose per application (concentration in deodorant), which is theoretically acceptable and calculated on the basis of induction experiments using animals/healthy voluenteers (59).
| Fragrance substance | Potency (31,59) | Reference dose* | Acceptable conc. |
| HICC | Weak | 1 µg/cm² | < 2000 ppm |
| Hydroxycitronellal | Weak | 1 µg/cm² | < 2000 ppm |
| Isoeugenol | Moderate | 0,1 µg/cm² | < 200 ppm |
| Cinnamal | Moderate | 0,1 µg/cm² | < 200 ppm |
*The reference dose for sensitisation is based on the potency of the substances (derived from experiments) as default values and uncertainty factors. In the calculation, the maximum possible uncertainty factors have been used: a factor 10 for inter-individual variation, a factor 10 for difference in matrix between the experiment and the real product, a factor 10 for experimental conditions (armpit), all together 1000. The acceptable concentration is calculated from exposure (0.5 g deodorant/day on a total of 100 cm² skin and the reference dose, so that the ratio of the reference dose and exposure in µg/cm² is below 1.
The perfume industry (IFRA) has since April 2004 recommended that maximum 1.5 % (15.000 ppm) HICC should be used in cosmetics (28), a limit, which is associated with risk of allergy, independently of the model used for the risk assessment (Tabel 9-11).
5.3.2 Hydroxycitronellal
Hydroxycitronellal is included in fragrance mix I, a perfume mixture containing eight fragrance substances, which is used for the diagnosis of perfume allergy. It is the 3rd most frequent cause of allergy among the 8 substances in the mixture (42). It is responsible for allergic reactions on an average in 13% of the test persons, who show a positive reaction to fragrance mix I or who are suspected of fragrance allergy. In a European study from the beginning of 1990’s, 0.75% of 1072 eczema patients reacted to hydroxycitronellal (43). Danish data reveals that among eczema patients tested at a dermatology department in Copenhagen, 3% among women and 0.9% of men show an allergic reaction to hydroxycitronellal (Figure 1).
The perfume industry’s association IFRA has for several years recommended the use of maximum 1% hydroxycitronellal in the products, because of its allergenic properties (28). This limit is based on experiments employing healthy voluenteers (44). In the present investigation, hydroxycitronellal was found in 27% of the products with up to 0.17%; chemical analysis of the 23 selected products revealed a median concentration of 203 ppm hydroxycitronellal.
In a case study, it was found that persons with the diagnosis of hydroxycitronellal allergy had used products containing an average of 0.18% hydroxycitronellal compared to products containing 0.032 % used by the persons, who did not have hydroxycitronellal allergy (45). In a provocation study with 14 eczema patients, among these seven with allergy to hydroxycitronellal, the patients were supplied with two deodorants in a blind and randomized way, one with hydroxycitronellal and the other without hydroxycitronellal (8). Start concentration of hydroxycitronellal was 320 ppm (0.032%), which was applied, in an armpit, two times daily up to 14 days; and the unperfumed deodorant was applied in the contralateral armpit as control. None in the control group reacted, while all who were allergic to hydroxycitronellal reacted to deodorants containing hydroxycitronellal: four persons to the deodorant with 320 ppm (0.032%) hydroxycitronellal, one to 1000 ppm (0.1%) hydroxycitronellal and yet two to the deodorant containing 3200 ppm (0.32%) hydroxycitronellal (Table 9). The amount of deodorants used was estimated as realistic (8). This means that approximately half of the patients will react to concentrations, which are found in 10% of the deodorants (8/88) on the market. This is a pattern similar to HICC, which is structurally related. The study employing allergic patients indicated that deodorants should not contain more than 320 ppm hydroxycitronellal (8). Concentrations at this level or higher were found in six products, corresponding to approximately 1/3 of the products declared to contain hydroxycitronellal and 6.8% of the of the 88 perfumed products included in the study.
5.3.3 Isoeugenol
Isoeugenol is a moderate/strong allergen. It is included in fragrance mix I, a perfume mixture containing eight fragrance substances, which is used for the diagnosis of perfume allergy. This is the second most frequent allergen among the eight substances in the mixture (42). It is responsible for allergic reactions on an average in 18.9% of the tested persons, who show a positive reaction to fragrance mix I or who are suspected of having fragrance allergy (42). Danish data show that among eczema patients tested at a dermatology department in Copenhagen, 1.5% show an allergic reaction to isoeugenol (Figure 1). In an earlier investigation, 20/73 (29%) of the deodorants on the European market were found to contain 1-458 ppm isoeugenol (6). In the present investigation eight products (9%) contained a median concentration of isoeugenol at 32 ppm and a maximum concentration of 138.4 ppm. The perfume industry has since 1992 recommended a maximum isoeugenol concentration in cosmetic products at 0.2%, and from May 1998, a maximum concentration of 0.02%. This limit is in public consultation within EU with regard to implementation in the Cosmetic Directive (46). Deodorants in the present investigation conform to this limit.
In a provocation study with 23 eczema patients, among these 13 with allergy to isoeugenol, these were supplied with two deodorants, one with and another without isoeugenol (10). The start concentration was 63 ppm (0.0063%) isoeugenol in the deodorant, which was applied in an armpit two times daily up to 14 days, and the unperfumed deodorant was applied in the other armpit as control. If no reaction was observed, a deodorant with higher concentration of isoeugenol, 200 ppm (0.02%), was applied for additional 14 days, and if still no reaction was observed then a deodorant with higher isoeugenol concentration, 630 ppm (0.063%), was applied for addition 14 days (Table 9). None in the control group reacted, while 10 of the 13 patients with isoeugenol allergy reacted to deodorants with isoeugenol. Of these, three patients reacted to a deodorant with 63 ppm isoeugenol, 4 patients to a deodorant with 200 ppm isoeugenol and 3 more reacted to the use of a deodorant with 630 ppm isoeugenol. None of the control persons reacted to the deodorants. This means that seven of the 13 (53%) allergic person got allergic reactions from the use of deodorants containing isoeugenol at 200 ppm concentration level, the maximum concentration recommended by the industry. On the basis of this study the isoeugenol level in products should be kept below 63 ppm. This was the case in 5/8 isoeugenol containing deodorants in the present study. This means that isoeugenol concentration in at least 3.4% of the 88 perfumed deodorants in the present study was higher than recommended.
Although the perfume industry’s organisation IFRA in 1998 reduced the recommended concentration of isoeugenol from 2000 ppm to 200 ppm, the incidence of isoeugenol allergy has not reduced (47). This is possibly due to the use of structurally related substances (for example isoeugenol acetate) instead of isoeugenol (48). It will be important to make a survey of the use of substances structurally related to isoeugenol.
5.3.4 Cinnamal/cinnamyl alcohol
Cinnamal is a moderate/strong allergen, which has been in focus for several years due to its allergenic properties (1, 49). In the earlier mentioned investigation cinnamal was found in 17% of the deodorants (6). In the present investigation, cinnamal was present in only one deodorant (1.1%) at a concentration level of 5 ppm. A limit of 0.1% cinnamal is in public consulation for the implementation in the Cosmetic Directive (46). The risk estimate, performed by the cosmetic industry, indicates that there is a significant risk of sensitisation by exposure to 0.1% cinnamal (31). Perfume industry has recently recommended use of maximum 0.05% (500 ppm) in cosmetic products, fully implemented in 2007 (28). Earlier, perfume industry did not give any recommendation to a maximum limit for cinnamal. Both in a Danish and a British investigation, the frequency of allergy to cinnamal and cinnamyl alcohol was found to be reduced over a long period (50, 51), possibly related to reduction in use of these substances. In a study of eczema patients allergic to cinnamal, allergic eczema was developed in one of nine patients (11%) by the use of a 100 ppm cinnamal containing deodorant, and in 99% of the patients by the use of deodorant containing 1000 ppm cinnamal (9). An unperfumed control deodorant did not give any reaction. In the paper (9), it is recommended to keep the cinnamal concentration in the products below 100 ppm. The products in the present investigation conform to this recommendation.
In the analysis of deodorants cinnamyl alcohol, which can transform to cinnamal in the skin (52-55) was included. Animals which were sensitised to one of these substances also reacted to the other substance (52). Simultaneous reaction of the two substances is also often seen in humans (56). Cinnamal is a more potent allergen and induces allergy in animals at 15 times lower concentration than cinnamyl alcohol (52). In humans, 80% of the persons allergic to cinnamal will also react to cinnamyl alcohol in identical concentrations at patch testning (56). In the present investigation, cinnamyl alcohol was found in 11 deodorants (12 %) in concentrations from 1.7 ppm to 503 ppm, median 40 ppm. A limit of 0.8 % cinnamyl alcohol is in public consultation for implementation in the Cosmetic Directive (46). The Perfume industry’s organisation IFRA has, since 2002 with final implementation in 2004, recommended its members not to use cinnamyl alcohol in concentrations above 0.4% (28). This is based on a number of old studies, where the concentration which did not produced allergy in healthy persons was 4 % cinnamyl alcohol (no-effect level) (55, 57). In perfume industry’s earlier risk assessments, this level was divided by an uncertainty factor of 10, to define the safe limits (58). Although the data has been available for many years, the limit has not previously been in accordance to this principle.
Cinnamal was found at 5 ppm in one product in this investigation, which does not pose any risk. The level of cinnamyl alcohol is difficult to assess as there is no quantitative clinical data. However, the data illustrates that persons allergic to cinnamal can be exposed to substances in the products, which may produce ezema, even though cinnamal is not present in the products. For this reason, cinnamyl alcohol should be used under the same conditions as cinnamal, i.e. below 100 ppm (Table 9), which was the case for about half of the products containing cinnamyl alcohol in the present investigation.
5.4 Farnesol
Farnesol can be used in deodorants both as an antimicrobial substance and as a fragrance substance. It is considered to be an important allergen (6). Farnesol is included in the new perfume mixture, fragrance mix II, which is used routinely for the diagnosis of perfume allergy. Farnesol allergy was detected in 0.4%-0.5% of eczema patients tested in two different European multicenter studies (35, 36).
In a German multicenter study, 2021 eczema patients were tested and 1.1% was found to be allergic to farnesol. On this basis, it was estimated that 10.000 persons in Germany were allergic to farnesol (61). In the present investigation 13 products (14.8%) were found to contain farnesol, and in the subgroup of 23 deodorants, subjected to chemical analysis, 9 of the products contained farnesol. The median concentration of farnesol in these products was 661 ppm (range 9-1771 ppm, Table 3 and Table 7).
Perfume industry’s organisation IFRA has adopted a new model for quantitative risk assessment, which is based on same principles as in Table 11, except that the concrete reference value is calculated specifically on the basis of experimental results employing humans and not on the basis of normal values as in Table 12 (28). Until now, no limit values for farnesol have been adopted, but for new formulations limit values are introduced from June 2007 and for old formulation from June 2008. The limit values are laid down because of allergenic properties and introduced for 11 different product categories. The limit value for deodorants is 0.11% (1100 ppm). In the present investigation, only two deodorants were found to contain more than 1100 ppm farnesol. Thus in the present situation, this limit will probably not affect the Danish market or incidence of allergy. A safe limit is, however, required to prevent the use of higher concentrations in future. The proposed limit value can be validated by dose-response study employing persons with farnesol allergy.
5.5 Comments concerning other fragrance substances
The 26 fragrance substances, which should be declared when used in cosmetics, are very different with regard to both allergenic potency as well as incidence of allergy. The most allergenic and frequently occurring allergens are the 14 fragrance substances, which are routinely used for the screening of perfume allergy, i.e. the ingredients of fragrance mix I and fragrance mix II (indicated in Table 5). However, there are differences in allergenic properties of the individual substances even within this group (62). Besides, methyl 2-octynoate is a very potent allergen (28), and oxidation products of d-limonene and linalool are frequent allergens (63, 64).
Evernia Prunastri extract (oak moss) is a natural extract, which is included in fragrance mix I and this is also an ingredient that frequently gives positive reaction (42). It contains the most potent known allergens, atranol and chloroatranol (65, 66). On the basis of allergy risk, the SCCP has recommended that these two substances should not be present in cosmetic products (67). Four of the products in the present investigation contained evernia prunastri extract. Even though the content is not quantified, this is inappropriate.
Methyl 2-octynoate has been shown to be an extreme allergen in experiments using healthy voluenteers (28). It was declared on one deodorant (Table 4). On the basis its of sensitisation properties the recommended limits of use by IFRA is 0.01% and this limit is proposed for adoption in the Cosmetic Directive (46). Methyl 2-octynoate allergy is not frequent, because it is seldom used and has had a low limit value compared to other fragrance substances.
5.6 Allergen load of fragrance substances
The present investigation included 88 perfumed deodorants from the Danish market. According to ingredients listing on the products, up to 65.9 % of these contained one or more of the fragrance substances which were selected for the study. Among these cinnamal was present in 1% of the deodorants, isoeugenol in 9%, hydroxycitronellal in 27.3% and HICC in 33 % of the deodorants. Approximately one fourth of the deodorants (n=23), which was selected for the quantitative analysis of fragrance substances, contained 5-17 of the 26 target fragrance substances in the products, median eight fragrance substances per product. Thus, there is a considerable allergen load in a considerable part of deodorants on the Danish market, further the same allergenic substances are commonly used. There are approximately 2500 fragrance substances used in the formulation of perfumes. Of these, about 10% have been described as allergenic in humans (68).The products in the present investigation concerned 1% of available fragrance ingredients, even so it was the same substances which were used in many of the deodorants.
Although, there is a great difference in allergenic potency and use concentration of individual substances, as described above, the more potent allergens were found in a number of products and in combination with other allergens. This may be of concern, as several substances are structurally alike and thus they will contribute to additional allergy risk when they are used together. How the simultaneous allergen exposure affects the risk of allergy is not known, but simultaneous exposure to several allergens in allergic patients can produce synergistic reactions (69)
5.7 Triclosan
Triclosan is an antibacterial substance, which is used in deodorants and other cosmetics (70) and also in other types of products, such as cutting oils (71). The Swiss Contact Dermatitis Group tested 2295 eczema patients with tricolsan in the years 1989/90 and found a positive reaction in 0.8% of these. In the same study, 5.7% patients gave a positive reaction to the preservative formaldehyde and 5.5% reacted positive to methylchloroisothiazolinone/methylisothiazolinone (72). In a study from 1970’s, no positive reaction was recorded when 902 eczema patients were tested with 0.5% and 1% triclosan in a 16 months period, but two cases of positive reaction were recorded when tests were performed with 2% triclosan in another period of 17 month (73). Both of these patients were sensitised by tricosan containing deodorants, and one of these also had used a soap containing triclosan. In another study from the same period, 292 patients tested negative to triclosan while positive reaction was observed in two cases. One was sensitized by the use of triclosan containing foot powder deodorant and the other by the use of a deostick containing 0.12% triclosan for a couple of years. This last patient later performed a use-test with a soap containing 0.5% triclosan and developed eczema on one arm, where the soap was used, while the other arm, where a trriclosan free soap was applied, was eczema free. Also in the same time period a case from England was reported, where a woman developed allergic eczema after using a deodorant spray containing triclosan for 3 months (74).
In a Norwegian study, three persons were found to be sensitised to triclosan among 103 tested patients, in two of the persons from the use of a room disinfection product containing 3% triclosan, and in the third case for unknown reason (75). Three cases of allergy to triclosan as result of the use of the same room disinfection product were also reported from Italy (76). A nurse trainee with allergy to triclosan as a result of the use of a hand disinfection product is reported from England (77) and a patient with allergy to triclosan and other preservatives from a soap (78). In a recent report from Finland, 0.2% of the 5376 tested patients with suspected cosmetic allergy were shown to be allergic to triclosan in the period 1995-1996 and 0.1% of the 6598 patients tested in the period 2000-2002 (79).
Allergy to triclosan is not tested routinely, and thus, the cases of allergy to triclosan are possibly overseen. In the present investigation, triclosan was found in 15 products (17%) in concentrations from 0.05%-0.24%, which is within the maximum permitted limit in cosmetic products. In USA, triclosan is typically used in the concentration range 0.15-0.30% (80), which also corresponds to that found in the present investigation. Triclosan is an allergen, the frequency of allergy to this substance has not been mapped, but it is possibly less frequent compared to that due to other preservatives, for example methylchloroisothiazolinone/ methylisothiazolinone. A dose-response study of triclosan’s allergenic effects has not been performed, and therefore, it is not possible to perform a risk assessment.
6 Discussion
Deodorants are associated with an increased risk of skin allergy as a consequence of their contents and use pattern related to especially, allergenic fragrance substances but also in some cases preservatives. In the present investigation labelling of the most sold deodorants on the Danish market was checked, followed by the analysis of selected products and a risk assessment of selected substances.
Ninety seven deodorants, considered to be the most sold on the Danish market, were purchased, of these, nine products were not declared to contain perfume, while ”perfume” was declared on 17 products but without naming any individual fragrance substance.
On the basis of the labelling of the contents on the purchased deodorants, 23 products were selected for the analysis of the content of 26 fragrance substances, and 15 deodorants were selected for the determination of triclosan content. The products for the analyses were selected such that exposure to the fragrance substances cinnamyl alcohol, farnesol, hydroxycitronellal, isoeugenol, HICC and isoeugenol could be estimated. The fragrance substances to be included in the risk assessment were selected on the basis that these were frequent/potent allergens and a dose-response study of persons allergic to the respective substance was available. This was the case for cinnemal, hydroxycitronellal, HICC and isoeugenol (7-10). Cinnamyl alcohol can transform in the skin to cinnamal and was also chosen for the quantitative analysis to illustrate potential cinnamal exposure. Farnesol has been identified as an important allergen in deodorants (61). It was therefore selected to get an overview of the exposure from this substance.
According to the labelling of the products, 65.9% of the deodorants contained one or more of the fragrance allergens, which were selected for the study. The 26 fragrance substances, which should be declared when used in cosmetics, include allergens which differes in potency and incidence of allergy. The most allergenic and frequent allergens are 14 fragrance substances, which are used for the screening of perfume allergy, i.e. the ingredients of fragrance mix I and fragrance mix II. However even with in this group there is a difference in allergenic properties of the individual substances. In addition the substance methyl heptin carbonate is very potent allergen (28), and oxidation products of d-limonene and linalool are frequent allergens (63, 64). Evernia furfuracea extract (tree moss), which is among the 26 fragrance substance, has been shown to be a very common allergen (62). These important allergens were present in 1.1%-65.9% of the products (Table 4). The most potent allergens, cinnamal, methyl 2-octynoate and evernia prunastri extract were present in realtively few products. Evernia Prunastri extract contains some of the most potent allergens ever identified: chloroatranol and atranol (65, 66). The EU Commission’s Scientific Committee SCCP, has recommended that these substances should not be present in cosmetic products due to their allergenic properties (67). In the present investigation, evernia prunastri extract was present in four products, and although their content in the products is not quantified, it is not appropriate.
Although there is a great difference in the allergenic potency and use concentration of the individual fragrance substances, even potent allergens are used in several products and in combination with other allergens. Several substances are structurally similar and they will contribute additionally to the risk of allergy, when they are used simultaneously. The influence of a high allergen load on the risk of allergy is not known, but simultaneous exposure to several allergens in allergic individuals can give synergistic reactions (69).
Among the fragrance substances selected for risk assessment, hydroxyisohexyl 3-cyclohexene carboxaldehyd (HICC) was declared on 33 % of the products, hydroxycitronellal on 27.3 %, isoeugenol on 9.1 %, and cinnamal on 1.1 % of the deodorants. In an investigation from 1996, HICC was found in 53 % of 73 deodorants, hydroxycitronellal in 50 %, isoeugenol in 29 %, and cinnamal in 17 % (6). Obviously, there appears to be a decline in the use of these substances. However, both local and international products from five European countries were included in the first investigation. Furthermore, in the previous investigation, the content of the fragrance allergens was determined by chemical analysis, while in the present investigation the frequency was based on the information from the labelling on the products. In addition, there is an administrative limit of 10 ppm, under which the regulated fragrance substances need not be labelled. Thus, alone on this basis, the results of the two investigations may come out differently and cannot be compared. Thus it is not possible to conclude that use pattern of these fragrance allergens has changed over time.
The risk assessment was based on investigations employing persons with allergy to the respective substances, as it is known that limit values derived from such data are very effective in prevention of new cases of allergy as well as in limiting the consequences of the disease in persons who have aquired allergy (27). From the studies on HICC-allergic persons, the acceptable concentration of this substance in deodorant will be under 200 ppm (7); the exact limit could not be established in the study. Among the 23 products selected for chemical analysis, 17 of which were declared to contain HICC, six products were found to contain more than 200 ppm HICC. This means that 1/3 of the products containing HICC, corresponding to 6.8% of the 88 perfumed deodorants included in the present study, did not comply with the recommendation made by SCCP. The HICC content in one deodorant was 4431 ppm, i.e. more than 20 fold the above mentioned limit and more than twice the maximum concentration found in the earlier deodorant investigation performed 10 years back (6).
Perfume industry’s organisation IFRA has decided that 15.000 ppm is the limit value for HICC to limit the risk of allergy. The scientific basis for the limit value is not available in the open scientific literature. It has been proposed that HICC should not be present in deodorants because of allergy risk (7). HICC was present in 33% of the deodorants on the Danish market.
From the studies on hydrocycitronellal allergic persons, the acceptable limit of hydroxycitronellal in deodorants would be under 320 ppm (8); the precise limit could not be established in the study. The chemical analysis of 23 selected deodorants revealed that six products contained 320 ppm or more hydroxycitronellal. This means that hydroxycitronellal in at least 6.8% of the 88 perfumed deodorants in the present study was 320 ppm or more, maximum 1746 ppm. This level of hydroxycitronellal is higher than the the maximum hydroxycitronellal, i.e. 1023 ppm found in deodorants in the previous study, 10 years back (6). Perfume industry’s organisation IFRA has set 10.000 ppm as limit value for hydroxycitronellal to limit the risk of allergy. The scientific basis for the limit value is not available in the open scientific literature.
From the studies on isoeugenol-allergic persons, the acceptable concentration of isoeugenol in deodorants will be under 63 ppm (10); the precise limit could not be set in the study. The chemical analysis of 23 selected deodorants revealed that the concentration of isoeugenol in three of the products was 63 ppm or more. This means that at least 3.4% of the 88 deodorants in the present study contained 63 ppm or more isoeugenol, maximum 138 ppm, which is lower than the maximum concentration (458 ppm), found in deodorants 10 years back (6).
Perfume industry’s organisation IFRA has set 200 ppm as acceptable limit for isoeugenol to limit the risk of allergy. The scientific basis for the limit value is not available in the open scientific literature, but this limit value is not far from the above mention 63 ppm. In the mean time, the lower limit (200 ppm) has not resulted in lower incidence of isoeugenol allergy, which is possibly due to the use of substances structurally related to isoeugenol (47, 48).
From the studies on cinnamal allergic persons, the acceptable concentration of cinnamal in deodorants will be under 100 ppm (9); the precise limit could not be established in the study. The chemical analysis of the 23 selected products revealed that one product contained 5 ppm cinnamal, which is not considered to be of any risk. Perfume industry’s organisation IFRA has set 500 ppm as acceptable limit for cinnamal to limit the risk of allergy. The scientific basis for the limit value is not available in the open scientific literature. Cinnamyl alcohol can transform to cinnamal in the skin (52-55), so that animals sensitised to one of these substances reacted to the other substance (52). Simultaneous reactions of these two substances in humans have also often been observed (56). Cinnamy alcohol should be included in the risk assessment of cinnamal. Cinnamy alcohol was determined in 23 selected deodorants in the present study.
Farnesol was present in 14.8 % of the investigated products. The chemical analysis of 23 selected products revealed 9-1791 ppm farnesol in these products. A dose-response study on farnesol allergic persons has not yet been performed; therefore, it is not possible to perform risk assessment of farnesol exposure.
Perfume industry has from summer 2007 introduced an 1100 ppm limit for the use of farnesol. In the present study, only two deodorants contained farnesol over this limit. Thus, in present situation, the proposed limit will not affect the Danish market or incidence of farnesol allergy. This limit should be validated in the studies employing farnesol allergic persons.
The preservative triclosan was found in 15 products in concentrations from 480 ppm to 2400 ppm and was present mainly in expensive deodorants. Triclosan is an allergen, the frequency of allergy is not yet mapped, but is considered to be lower than for several other preservatives, for example methylchloroisothiazolinone/methylisothiazolinone (80). A dose-response study of allergenic effect of triclosan has not yet been performed, and therefore, it is not possible to perform a risk assessment.
Analyses of fragrance substances in the 23 selected deodorants revealed that labelling of the products with respect to the content of these substances complied with the guidelines in the Cosmetic Directive. None of the limits set by the perfume industry’s organisation IFRA were violated. There are no adopted/ implemented limits in EU for permitted concentrations of the selected fragrance substances, and thus neither in Danish Statutory Order on Cosmetics.
It can be concluded that fragrance substances, which are both potent allergens and frequent causes of allergy in cosmetic users, are present in deodorants. The most potent fragrance allergens are also used the least. The allergens are often present in combination, and the allergen load in these products is considerable. The deodorants contain fragrance substances, which SCCP has evaluated and recommended should not be present in cosmetics. In some products the concentrations of certain fragrance substances were at levels, which are considered to cause a not insignificant risk of allergy. All products complied with the existing legislation.
Fragrance substances are among the most frequent causes of allergy. Deodorants are associated with an increased risk of development of skin allergy because of the exposure conditions in the armpit. The use of allergenic fragrance substances in deodorants is an area, where an effort to prevent fragrance allergy will be advantageous.
7 References
1. Uter W et al. Patch testing with patients’ own cosmetics and toiletries- results of the IVDK 1998-2002. Contact Dermatitis 2005: 53(4):226-33
2. Johansen JD. Contact allergy to fragrances: clinical and experimental investigations of the fragrance mix and its ingredients. Contact Dermatitis 2002: suppl 3:46:1-31
3. http://www.miljoeogsundhed.dk/default.aspx?node=5233
4. Miljøministeriets bekendtgørelse om kosmetiske produkter. Bekendtgørelse Nr. 74 af 14. januar 2005.
5. http://www.spt.dk/media//dk/Statistik/
Branchestatistik%201%20halvår05.pdf
6. Rastogi SC, et al. Deodorants on the European Market: quantitative chemical analysis of 21 fragrances. Contact Dermatitis 1998: 38: 29-35.
7. Haslund Jørgensen P, Devantier Jensen C, Rastogi S, Andersen K, Johansen JD. Experimental elicitaion with hydroxyisohexyl-3-cyclohexene carboxaldehyde-containing deodorants. Contact Dermatitis 2007: 56: 146-150.
8. Svedman C, Bruze M, Johansen JD, Goossens A, Frosch P, Lepoittevin J-P, Rastogi, SC, White I, Menné T. Deodorants: an experimental provocation study with hydroxycitronellal. Contact Dermatitis 2003: 48:217-223.
9. Bruze M, Johansen JD, Andersen KE et al. Deodorants: An experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol 2003:48:194-200
10. Bruze M, Johansen JD, Andersen KE et al. Deodorants: An experimental provocation study with isoeugenol 2005:52:260-267
11. Rastogi S.C. (1993) Sample preparation for gas chromatographic analysis of organic solvents in aerosol cans. Chromatographia 36: 201-203
12. Miljøstyrelsen, Kortlægning nr. 8 – 2002: Indholdet af udvalgte duftstoffer i rengøringsprodukter og andre forbrugerprodukter
13. Miljøstyrelsen, Kortlægning nr. 24 – 2003: Antibakterielle midler i beklædningsgenstande
14. Johansen JD, Andersen TF, Kjøller M, Veien N et al. Identification of risk
products for fragrance contact allergy: a case-referent study based on patients’ histories. Amercian Journal of Contact Dermatitis 1998:9:80-87.
15. Uter W, Balzer C, Geier J, Schnuch A, Frosch p. Ergebnisse der epikutantestung mit patienteneigenen Parfüms, Deos und Rasierwassern. Dermatologie in Beruf und Umwelt 2005:53:25-36.
16. Hotchkiss SAM. Absorption of fragrance ingredients using in vitro models. In Fragrances. Beneficial and adverse effects. Eds. Frosch PJ, Johansen JD, White IR. Springerverlag 1998:125-135
17. Feldmann RJ, Maibach HI. Absorption of some organic compounds trough the skin in man. J Invest Derm 1969:54:339-404
18. Felter S, Ryan CA, Basketter DA, Gilmour NJ, Gerberick FG. Application of the risk assessment paradigm to the induction of allergic contact dermatitis. Regulatory Toxicology and Pharmacology 2003:37:1-10.
19. Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis 1994:31:291-292
20. Johansen JD, Rastogi SC, Bruze M, Andersen KE, Frosch P et al. Deodorants: a clinical provocation study in fragrance-sensitive individuals. Contact Dermatitis 1998:39:161-165
21. von Peter C, Hoting E. Anwendungstest mit parfümierten Kosmetika bei Patienten mit positivem Epikutantest auf Duftstoff-Mischung. Dermatosen 1993:41:237-241.
22. Friedmann PS. The immunology of allergic contact dermatitis: the DNCB story. Adv Dermatol 1990:5:175-9
23. Robinson MK, Gerberick GF, Ryan C, McNamee P, White, Basketter DA. The importance of exposure estimation in the assessment of skin sensitization risk. Contact Dermatitis 2000:42:251-259.
24. The Scientific Committee on Cosmetic Products and non-Food Products Intended for Consumers. The SCCNFP’s notes of guidance for testing of cosmetic ingredients and their safety evaluation. 5th revision. Adopted by the SCCNFP during the 25th plenary meeting of 20 October 2003.
25. Cadby PA, Troy WR, Vey MGH. Consumer exposure to fragrance ingredients: providing estimates for safety evaluation. Regulatory Toxicology and Pharmacology 2002:246-252
26. Loretz L, Api AM, Barraj L et al. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food and Chemical Toxicology 2006:44:2008-2018.
27. Johansen JD, Frosch PJ, Menné T. Allergic contact dermatitis in Humans - experimental and quantative aspects. In Contact Dermatitis eds. Frosch PJ, Menné T, Lepoittevin JP. Springer 4. ed 2006: kap 13: 189-200.
28. IFRA. IFRA code and standards. http://www.ifraorg.org/GuideLines.asp
29. White IR. Fragrances – future aspects. Beneficial and adverse effects. Eds. Frosch PJ, Johansen JD, White IR. Springerverlag 1998:216-225
30. Menne T, Wahlberg JE; European
Environmental and Contact Dermatitis Research Group. Risk assessment failures of chemicals commonly used in consumer products. Contact Dermatitis 2002:46(4):189-90
31. Gerberick FG, Robinson MK, Felter S, White IR, Basketter D. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis 2001:45:333-340
32. Kimber I, Basketter DA, Butler M, Gamer A, Garrigue JL, Gerberick GF
Newsome C, Steiling W, Vohr HW. Classification of contact allergens according to potency: proposals. Food Chem Toxicol 2003:41:1799-809.
33. Basketter DA, Andersen KE, Liden
C, Van Loveren H, Boman A, Kimber I, Alanko K, Berggren E. Evaluation of the skin sensitizing potency of chemicals by using the existing methods and considerations of relevance for elicitation.
Contact Dermatitis 2005:52:39-43.
34. Fischer LA, Menné T, Johansen JD. Experimental nickel elicitation thresholds – a review focusing on occluded nickel exposure. Contact Dermatitis 2005: 52: 57-64.
35. Frosch PJ, Johansen JD, Menné T, Pirker C, Rastogi SC, Andersen KE, Bruze M, Goossens A, Lepoittevin JP, White IR (2002). Further important sensitizers in patients sensitive to fragrances. I. Reactivity to 14 frequently used chemicals. Contact Dermatitis 47:78-85
36. Frosch PJ, Rastogi SC, Pirker C, Brinkmeier T, Andersen KE, Bruze M, Svedman C, Goossens A, White IR, Uter W, Giménez Arnau E, Lepoittevin JP, Johansen JD, Menné T. Patch testing with a new fragrance mix – reactivity to the single constituents and chemical detection in relevant cosmetic products. Contact Dermatitis 2005: 52:216-25
37. Geier J, Brasch J, Schnuch A et al (2002). LyralÒ has been included in the patch test standard series in Germany. Contact Dermatitis 46:295-297
38. www.videncenterforallergi.Nationale allergital for 2005/2006
39. Johansen JD, Frosch PJ, Svedman C, Andersen KE, Bruze M, Pirker C, Menné T. Hydroxyisohexyl 3-cyclohexene carboxaldehyde – known as Lyral. Quantitative aspects and risk assessment of an important fragrance allergen. Contact Dermatitis 2003:48:310-16.
40. Belsito DV, Fowler JF Jr, Sasseville D, Marks JG Jr, De Leo VA, Storrs FJ. Delayed-type hypersensitivity to fragrance materials in a select North American population. Dermatitis 2006:17:23-8.
41. SCCNFP. Opinion of The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning hydroxyisohexyl 3-cyclohexene carboxaldehyde. Adopted 9. December 2003
42. Schnuch A, Lessmann H, Geier J, Frosch PJ, Uter W; IVDK. Contact allergy to fragrances: frequencies of sensitization from 1996 to 2002. Results of the IVDK.Contact Dermatiti. 2004 Feb;50(2):65-76
43. Frosch PJ, Pilz B, Andersen KE, Burrows D,
Camarasa JG, Dooms-Goossens A, Ducombs G,
Fuchs T, Hannuksela M, Lachapelle JM, et al. Patch testing with fragrances: results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes.Contact Dermatitis 1995:33:333-342.
44. Johansen JD, Rastogi SC, Menné T. Exposure to selected fragrance materials. Contact Dermatitis 1996:34:106-110.
45. Suskind RR. The hydroxycitronellal story: what can we learn from it? In Fragrances. Beneficial and adverse effects. Eds. Frosch PJ, Johansen JD, White IR. Springerverlag 1998:159-165
46. EU-commission. Public consultation on perfumery materials in the framework of council directive 76/768/EEC relative to cosmetic products.
http://ec.europa.eu/health/ph_risk/
committees/sccp/documents/out93_en.pdf
47. White JML, Fleming J, Buckley D, White IR, McFadden JP. Frequency of allergic contact dermatitis to isoeugenol is not decreasing: a review of 3636 patients tested from 2001 to 2005. Contact Dermatitis 2006:55:27
48. Tanaka S, Royds C, Buckley D, Basketter
DA, Goossens A, Bruze M, Svedman C, Menne
T, Johansen JD, White IR, McFadden JP. Contact allergy to isoeugenol and its derivatives: problems with allergen substitution.
Contact Dermatitis. 2004:51:288-91
49. Cocchiara J, Letizia CS, Lalko J, Lapczynski A, Api AM. Fragrance material review on cinnamaldehyde. Food Chem Toxicol. 2005;43:867-923.
50. Buckley DA, Wakelin SH, Seed PT, Holloway D, Rycroft RJ, White IR, McFadden JP. The frequency of fragrance allergy in a patch-test population over a 17-year period. Br J Dermatol. 2000;142(2):279-83.
51. Johansen JD, Menné T. The fragrance mix and its constituents- a 14 year material. Contact Dermatitis 1995:32:18-23.
52. Weibel H, Hansen J, Andersen KE. Cross-sensitization patterns in guinea pigs. Acta Derm Venereol 1989:69:302-7
53. Elahi EN, Wright Z, Hinselwood D et al. Protein binding and metabolism influence the relative skin sensitization potential of cinnamic compounds. Chem Res Toxicol 2004:17:301-10
54. Smith CK, Moore CA, Elahi EN, Smart AT, Hotchkiss SA. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde and cinnamic alcohol. Toxicol Appl Pharmacol 2000:168:189-99
55. Letizia CS, Cocchiara J, Lalko J, Lapczynski A, Api AM. Fragrance material review on cinnamyl alcohol. Food Chem Toxicol 2005:43:837-66.
56. Buckley DA, Basketter DA,
Smith Pease CK, Rycroft RJ, White IR, McFadden JP. Simultaneous sensitivity to fragrances. Br J Dermatol 2006:154:885-8.
57. Bickers D, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH, Sipes IG, Smith RL, Tagami H; The RIFM expert panel. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem Toxico. 2005:43:799-836
58. Vølund A. Potency evaluation of contact allergens. Statistical appendix. Nordiske Seminar og Arbejdsrapporter 1993:570:83-107
59. Basketter D, Clapp, C, Jefferies D. Predictive identification of human skin sensitization thresholds. Contact Dermatitis 2005:53:260-267
60. Felter S, Robinson, Basketter D, et al. A review of the scientific basis for uncertainty factors for use in quantitative risk assessment for the induction og allergic contact dermatitis. Contact Dermatitis 2002:47:257-266
61. Schnuch A, Uter W, Geier J, Lessmann H, Frosch P. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis 2004:50:117-121
62. Matura M, Skold M, Borje A, Andersen KE, Bruze M, Frosch P, Goossens A, Johansen JD, Svedman C, White IR, Karlberg AT. Not only oxidized R-(+)- but also S-(-)-limonene is a common cause of contact allergy in dermatitis patients in Europe. Contact Dermatitis 2006 55:274-279.
62. Schnuch A, Uter W, Geier J, Lessmann H, Frosch PJ. Sensitization to 26 fragrances to be labelled according to current European regulation. Contact Dermatitis 2007:57:1-10
63 Matura M, Skold M, Borje A, Andersen KE, Bruze M, Frosch P, Goossens A.
Johansen JD, Svedman C, White IR, Karlberg AT. Selected oxidized fragrance
terpenes are common contact allergens. Contact Dermatitis 2005:52:320-328.
64. Bernard G, Gimenez-Arnau E, Rastogi SC, Heydorn S, Johansen JD, Menne T, Goossens A, Andersen K, Lepoittevin JP. Contact allergy to oak moss: search for sensitizing molecules using combined bioassay-guided chemical fractionation, GC-MS, and structure-activity relationship analysis. Arch Dermatol Res. 2003;295:229-235. Epub 2003 Sep 16.
65. Johansen JD, Andersen KE, Svedman C, Bruze M, Bernard G, Gimenez-Arnau E, Rastogi SC, Lepoittevin JP, Menne T. Chloroatranol, an extremely potent allergen hidden in perfumes: a dose-response elicitation study. Contact Dermatitis 2003 49:180-184.
66. Scientific Committee on Cosmetic Products (SCCP). Opinion on Atranol and Chloroatranol present in natural extracts (e.g. oak moss and tree moss extract). Adopted by the SCCP during the 2nd plenary meeting of 7 december 2004.
67. de Groot AC, Frosch PJ. Adverse reactions to fragrances. A clinical review.
Contact Dermatitis 1997:36(2):57-86
68. Johansen JD, Skov L, Volund A, Andersen K, Menne T. Allergens in combination have a synergistic effect on the elicitation response: a study of fragrance-sensitized individuals. Br J Dermatol. 1998;139(2):264-270.
69. Roed-Petersen J, Auken G, Hjorth N. Contact sensitivity to Irgasan DP 300. Contact Dermatitis 1975:1:293-294
70. Geier J, Lessmann H, Becker D et al. Patch testing with components of water-based metalworking fluids: results of a multicentre study with a second series. Contact Dermatitis 2006:55:322-329.
71. Perrenoud D, Bircher A, Hunziker T et al. Frequency of sensitization to 13 common preservatives in Switzerland. Swiss Contact Dermatitis Group. Contact Dermatitis 1994;30:276-279
72. Wahlberg JE. Routine patch testing with Irgasan DP 300. Contact Dermatitis 1976:2:292
73. Hindson T. Irgasan DP 300 in a deodorant. Contact Dermatitis 1975:1:328
74. Steinkjer B, Braaten LR. Contact dermatitis from triclosan (Irgasan DP 300). Contact Dermatitis 1988:18:243-244
75. Veronesi S, Padova MP, Vanni D, Melino M. Contact Dermatitis to triclosan. Contact Dermatitis 1986:15:257-58
76. Wong CSM, Beck MH. Allergic contact dermatitis from triclosan in antibacterial handwashes. Contact Dermatitis 2001:45:307.76.
77. Zaugg T, Hunziker T. Germall II and triclosan. Contact Dermatitis 1987:17:262
78. Hasan T, Rantanen T, Alanko K, Harvima RJ, Jolanki R, Kalimo K, et al. Patch test reactions to cosmetic allergens in 1995-1997 and 2000-2002 in Finland – a multicentre study. Contact Dermatitis 2005:53:40-45.
79. Campell L, Zirwas MJ. Triclosan. Dermatitis 2006:17:204-7.
80. Wilkinson JD, Shaw S, Andersen KE, Brandao FM, Bruynzeel DP, Bruze M, Camarasa JM, Diepgen TL, Ducombs G, Frosch PJ, Goossens A, Lachappelle JM, Lahti A, Menne T, Seidenari S, Tosti A, Wahlberg JE. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991-2000). Contact Dermatitis 2002 :46:207-10.
Annex 1
Products purchased for the survey of deodorants
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-242-MIMI | The Body Shop, UK |
Alcohol Denat, Aqua, Parfum, Benzyl Benzoate, Benzyl Salicylate, Dipropylene Glycol, 1-Butyl Alcohol, Hexyl Cinnamal, Limonene, Eugenol, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Citronellol, Linalool, Buthylphenyl methylpropional, Geraniol, Isoeugenol, Citral, Denatonium Benzoate, Amyl Cinnamal, Hydroxycitronellal, Coumarin, Benzyl Alcohol. |
| 2006-243-MIMI | The Body Shop, UK | Alcohol Denat, Aluminium Chlorohydrate, parfum, Hydroxyethylcellulose, 1-Butyl Alcohol; Denatonium Benzoate. |
| 2006-244-MIMI | P & G Prestige Beaute, UK |
Alcohol Denat. Water, Butane, Octyldodecanol, Parfum/Fragrance, Triethyl citrate, Farnesol, Limonene, Linalool, Butylphenyl methylpropional, Citral, Geraniol |
| 2006-245-MIMI | P & G Prestige Beaute, UK |
Aluminium chlorohydrate, PPG-15 stearyl ether, Steareth-2, Parfum, Steareth-21, Benzyl alcohol, Phenoxyethanol, Disodium EDTA, BHT |
| 2006-246-MIMI | COTY SA Isabella Rossellini parfums, UK |
Alcohol Denat., Butane, Isobutane, Propane, Parfum/Fragrance, Propylene glycol, Triethyl citrate, Butylphenyl methylpropional, Hexyl cinnamal, Farnesol, Linalool, Citronellol, Limonene, Hydroxycitronellal, Alpha-isomethyl ionone, Hydroxyisohexyl 3-cyclohexene carboxaldehyde, Benzylsalicylate, Geraniol |
| 2006-247-MIMI | Elisabeth Arden, UK |
Alcohol Denat., Water, Parfum/Fragrance, Glycerin, BHT, Alpha-isomethyl ionone, Benzyl alcohol, Benzyl benzoate, Benzyl salicylate, Butylphenyl methylpropional, Citronellol, Farnesol, Geraniol, Hydroxycitronellal, Hydroxyisohexyl 3-cyclohexene carboxaldehyde, Limonene, Linalool |
| 2006-249-MIMI | Valmistusmaa Tanska for COOP Norden AB | Aqua, Aluminium chlorohydrate, PPG-6 Stearate, Glyceryl Sterate, Ceteth-20, Steareth-20, Octyldodecanol, PPG-15 stearyl ether, Aloe barbadensis (Aloe Vera), Allantoin, Citric Acid, Parfum/Fragrance, Methylparaben, Propylparaben |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-249-MIMI | Valmistusmaa Tanska for COOP Norden AB | Aqua, Aluminium chlorohydrate, PPG-6 Stearate, Glyceryl Sterate, Ceteth-20, Steareth-20, Octyldodecanol, PPG-15 stearyl ether, Aloe barbadensis (Aloe Vera), Allantoin, Citric Acid, Parfum/Fragrance, Methylparaben, Propylparaben |
| 2006-250-MIMI | Cederroth DK-Lynge |
Alcohol Denat., Propane, Butane, Parfum/Fragrance, Dipropylene glycol, Alpha-isomethyl ionone, Limonene, Hexyl cinnamal, Linalool, Hydroxyisohexyl 3-cyclohexene carboxyaldehyde, Benzyl salicylate, Butylphenyl methylpropional |
| 2006-251-MIMI | Derma Pharm A/S for COOP danmark | Aqua, Aluminium chlorohydrate, Steareth-2, Caprylic/Capric triglyceride, Steareth-20 |
| 2006-252-MIMI | Hunca France www.hunca.com |
Butane, Alcohol Denat., Propane, Parfum/Fragrance, Alpha-isomethyl ionone, Benzyl benzoate, Butylphenyl methylpropional, Citral, Citronellol, Eugenol, Geraniol, Hexyl cinnamal, Hydroxycitronellal, Hydroxyisohexyl 3-cyclohexene carboxaldehyde, Isoeuganol, Limonene, Linalool, Propylene glycol, Ethylhexylglycerin |
| 2006-253-MIMI | DOETSCH GRETHER AG, Germany www. Fenjal.com |
Alcohol Denat., Butane, Propane, Isobutane, Triethy lcitrate, Parfum/Fragrance, BHT, Isopropyl myristate, Propylene glycol, Cinnamyl alcohol, Hydroxycitronellal, Coumarin, Hydroxyisohexyl 3-cyclohexene carboxaldehyde, Butylphenyl methylpropional, Linalool, Benzyl benzoate, Citronellol, Limonene |
| 2006-254-MIMI | Colgate-Palmolive | Aqua, Aluminium sesquichlorohydrate, Steareth-2, PPG-15 stearyl ether, Steareth-20, Cyclopentasiloxane, Parfum/Fragrance, Tetrasodium EDTA, DMDMHydantoin, BHT, Aloe barbadensis, Iodopropynyl Butylcarbamate, Amyl cinnamal, Citronellol, Hexyl cinnamal, Hydroxyisohexyl 3-cyclohexene carboxaldehyde, Linalool |
| 2006-255-MIMI | www.ray-saxx.com | Alcohol Denat, Butane, Propane, Isobutane, Triethyl Citrate, Parfum, aqua |
| 2006-256-MIMI | MATAS DK-3450 Allerød |
Butane, Propane, isobutane, cyclomethicone, almuminium chlorohydrate, parfum, quaternium-18 Hectorite |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-257-MIMI | Biotherm, France |
Aluminium Chlorohydrate, PPG-15 Stearyl ether, Cetearyl Alcohol, Ceteareth-33, Dimethicone, Bisabolol, Methylparaben, Propylparaben, C12-13 Alkyl Lactate, Silica, Parfum/Fragrance. |
| 2006-258-MIMI | MATAS DK-3450 Allerød |
Alcohol (denatureret med Denatonium Benzoate), Ricinus Communis, Stearic Acid, Cetearyl Alcohol, Ethylhexylglycerin, Parfum, Denatonium Benzoate, Phenoxyethanol, Sodium Hydroxide. |
| 2006-259-MIMI | MATAS DK-3450 Allerød |
Aqua, Aluminium Chlorohydrate, Octyldodecanol, Cetearyl Alcohol, Ceteareth-20, Ceteareth-12, Zinc Ricinoleate, Parfum, Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben. |
| 2006-260-MIMI | Plaisir M-cosmetics, Allerød |
Aqua, almuminium chlorohydrate, alcohol(denatureret med denatonium benzoate), PEG-40, Hydrogenated Cator Oil, Ethylhexyl glycerine, Creatine, Fucus vesiculosus, denatonium benzoate, maltodextrin |
| 2006-261-MIMI | Parfums Vanderbilt, France |
Isobutane, Alcohol denat., Parfum/Fragrance, Eugenol, Triclosan, Limonene, Linalool, Benzyl Salicylate, Benzyl Benzoate, Alpha-Isomethyl Ionone, Benzyl Salicylate, Benzyl Benzoate, Alpha-Isomethyl, Ionone, Geraniol, Citronellol, Hexyl Cinnamal, Amyl Cinnamal. |
| 2006-262-MIMI | MATAS DK-3450 Allerød |
Aqua, Aluminium chlorohydrate, Cyclopentasiloxane, Alcohol (Denatureret med Denatonium Benzoate), Glyceryl Stearate, Cetyl Alcohol, PEG-100 Stearate, Dicaprylyl, Carbonate, PEG-75 Strarate, Ceteth-20, Steareth-20, Tocopherol, Zinc Ricinoleate, Polyglyceryl-3 caprylate, Denatonium Benzoate. |
| 2006-263-MIMI | A/s Blumøller, DK-Odense | Aqua, Aluminium chlorohydrate, Glycerin, PPG-15 stearyl Ether, Steareth-2, Cyclopentasiloxane, Steareth-21, Parfum, Talc, Allantoin, Dimethicone, BHT. |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-264-MIMI | Unilever www.unilever.dk |
Butane, Isobutane, Propane, Aluminium Chlorohydrate, Cyclomethicone, PPG-14 Butyl Ether, Parfum, Disteardimonium Hectorite, Helianthus Annuus, Dimethiconol, Octyldodecanol, BHT, Alpha-Isomethyl, Ionone, Benzyl Alcohol, Benzyl Salicylate, Butylphenyl methylpropional, Citronellol, Coumarin, Eugenol, Geraniol, Hexyl Cinnamal, Linalool. |
| 2006-265-MIMI | Procter & Gamble, UK | Butane, Dipropylene glycol, isobutane, propane, isopropyl myristate, zinc phenolsulfonate, hydroxyisohexyl 3-cyclohexene, carboxaldehyde, limonene, linalool, coumarin, isoeugenol, eugenol, citronellol, geraniol, benzyl benzoate, cinnamyl alcohol, evernia prunastri |
| 2006-266-MIMI | Parfumeurs Createurs, France | Aqua, Alcohol denat., Isobutane, linalool, geraniol, triclosan, parfum, alpha isomethyl ionone, coumarin, limonene, hydroxyisohexyl 3-cyclohexene carboxaldehyde, citronellol, citral, butylphenyl-methylpropional, benzyl salicylate |
| 2006-267-MIMI | Licence by Puma Metropolitan cosmetics, Germany |
Alcohol denat., isobutane, propane, butane, triethyl citrate, isopropyl myristate, farnesol, linalool, benzyl salicylate, benzophenone-2, hexyl cinnamal, citronellol, hydroxyisohexyl 3-cyclohexene carboxaldehyde, butylphenyl methylpropional, cinnamyl alkohol |
| 2006-268-MIMI | Unilever DK www.unilever.com |
Isobutane, propane, butane, cyclomethicone, aluminium chlorohydrate, C12-15 alkyl benzoate, disteardimonium hectorite, propylene carbonate, alpha-isomethyl ionone, benzyl salicylate, butylphenyl methylpropional, citronellol, hexyl cinnamal, hydroxycitronellal, hydroxyisohexyl 3-cyclohexene carboxaldehyde, limonene, linalool |
| 2006-269-MIMI | COTY SA, France |
Aqua, Alcohol denat., PEG-40, hydrogenated castor oil, trideceth-9, PEG-5, ethylhexanoate, triethyl citrate, bisabolol, farnesol, linalool, butylphenyl, methylpropional, hydroxyisohexyl 3-cyclohexene carboxaldehyde, alpha-isomethyl ionone, limonene, hydroxycitronellal, coumarin, citronellol, geraniol, benzyl alcohol |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-270-MIMI | COTY SA, France |
Aqua, PEG-40, hydrogenated castor oil, trideceth-9, peg-5, ethylhexanoate, hexyl cinnamal, benzyl salicylate, triethyl citrate, butylphenyl methylpropional, bisabolol, farnesol, alpha-isomethyl ionone, linalool, hydrocitronellal, citronellol, limonene, geraniol, isoeugenol |
| 2006-271-MIMI | Cindy Crawford, Star Parfume, Germany www.cindy.com |
Isobutane, propane, propylene glycol, butane, triethyl citrate, isopropyl, myristate, farnesol, BHT |
| 2006-272-MIMI | www.hummel.dk | Alcohol denat., isobutane, propane, butane, cyclopentasiloxane, aluminium, chlorohydrate, quaternium-18 hectorite, parfum |
| 2006-273-MIMI | COTY SA, UK |
Alcohol denat., isobutane, propane, butane, propylene glycol, triethyl citrate, limonene, hexyl cinnamal, butylphenyl methylpropional, farnesol, linalool |
| 2006-274-MIMI | Gillette Espoo-Espo |
Cyclopentasiloxane, stearyl alcohol, aluminium zirconium pentachloro-hydrex, GLY, PEG-14 butyl ether, hydrogenated castor oil, myristyl, myristate, zea mays, silica dimethyl-silylate, silica, butylphenyl methyl-propional, citral, citronellol, coumarin, eugenol, geraniol, hydroxyisohexyl 3-cyclohexene carboxaldehyde, isoeugenol, linalool, CI 42090 |
| 2006-275-MIMI | E. Tjellesen A/S www.goshcosmetics.com |
Alcohol denat., Aqua, isobutane, propane, butane, triethyl citrate, parfum, benzyl salicylate, hydroxyisohexyl 3-cyclohexene carboxaldehyde, linalool, hydroxycitronellal, coumarin, isoeugenol, benzyl alcohol, geraniol, benzyl benzoate, cinnamyl alcohol |
| 2006-276-MIMI | Unilever DK www.unilever.com |
Aqua, Aluminium chlorohydrate, helianthus annuus, steareth-2, steareth-20, cholesterol, lecithin, tocopherol, alpha-isomethyl ionone, benzyl alcohol, benzyl salicylate, citronellol, geraniol, limonene, linalool |
| 2006- 277-MIMI | Schwarzkopf & henkel, Germany | Alcohol denat., isobutane, propane, butane, isopropyl myristate, phenoxyethanol, ethyl- hexylglycerin, tocopheryl acetate, benzyl salicylate, linalool, limonene, coumarin, butylphenyl methylpropional, citral, eugenol, geraniol, benzyl benzoate citronellol |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-278-MIMI | Unilever DK www.unilever.com |
Isobutane, propane, butane, aluminium chlorohydrate, cyclomethicone, disteardimonium hectorite, corn starch modified, mannitol, BHT citric acid, sodium ascorbate, calcium disodium EDTA, propylene carbonate, parfum, benzyl benzoate, benzyl salicylate, citronellol, coumarin, eugenol, geraniol, limonene, linalool |
| 2006-279-MIMI | A/s bluemøller DK-Odense |
Aqua, aluminium chlorohydrate, glycerin, PEG-15, stearyl ether, steareth-2, cyclopentasiloxane, steareth-21, talc, dimethicone, allantoin, BHT |
| 2006-280-MIMI | A/s bluemøller DK-Odense |
Aluminium chlorohydrate, glycerin, PEG-15, stearyl ether, steareth-2, cyclopentasiloxane, steareth-21, dimethicone, allantoin, BHT |
| 2006-281-MIMI | Unilever DK www.unilever.com |
Alcohol denat., isobutane, propane, butane, pentetic acid, isopropyl myristate, aminomethyl propanol, BHT, parfum, alpha-isomethyl ionone, benzyl alcohol, benzyl benzoate, benzyl salicylate, butylphenyl methylpropional, citral, citronellol, coumarin, eugenol, evernia furfurscea, geraniol, hexyl cinnamal, hydroxycitronellal, limonene, linalool |
| 2006-282-MIMI | Consiva Non-Food A/S, DK | Alcohol denat., isobutane, propane, isopropyl myristate, parfum, anise alkohol, benzyl benzoate, citronellol, eugenol, geraniol, hydroxycitronellal, hydroxyisohexyl 3-cyclohexene carboxaldehyde, linalool, alpha-isomethyl ionone, butylphenyl methylpropional |
| 2006-283-MIMI | E. Tjellesen A/S www.goshcosmetics.com |
Aqua, isobutane, propane, butane, triethyl citrate, isopropyl myristate, propylene glycol, parfum, benzyl benzoate, limonene, coumarine, hydroxycitronellal, linalool, citronellol, cinnamyl alkohol, geraniol |
| 2006-284-MIMI | Beiersdorf (BDF), Germany | Aqua, Aluminium chlorohydrate, PEG-15, stearyl ether, steareth-2, steareth-21, chitosan, hydrolyzed pearl, persea gratissima, trisodium EDTA, parfum |
| 2006-285-MIMI | A/s bluemøller DK-Odense www.sanex.net |
isobutane, propane, butane, cyclopentasiloxane, aluminium sesquichlorohydrate, dimethicone, talc disteardimonium hectorite, propylene carbonate, dimethiconol, parfum |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-286-MIMI | COTY AS, Germany |
Water, Aluminium chlorohydrate, PEG-15, stearyl ether, steareth-2, steareth-20, allantion, coumarin, cyclomethcone, alpha-isomethyl ionone, linalool, silica dimethyl silylate, ethylhexylglycerin, acacia Senegal gum |
| 2006-287-MIMI | Unilever DK www.unilever.com |
Aqua, Aluminium chlorohydrate, glycerin, helianthus annuus, steareth-2, steareth-20, alpha-isomethyl ionone, benzyl alcohol, benzyl benzoate, benzyl salicylate, butylphenyl methylpropional, citronellol, geraniol, hexyl cinnamal, hydroxycitronellal, linalool |
| 2006-288-MIMI | A/s bluemøller DK-Odense www.sanex.net |
Aqua, Isobutan, Aluminium chlorohydrate, propane, isododecane, butane, isopropyl, palmitate, glycerin, isohexadecane, lauryl PEG/PPG 18/18, methicone, BHT |
| 2006-289-MIMI | E. Tjellesen A/S www.goshcosmetics.com |
Alcohol denat., propane, butane, isobutane, triethyl citrate, parfum, aqua |
| 2006-290-MIMI | Mäurer + wirtz www.tabac-original.de |
Alcohol denat., Butane, dipropylene glycol, ethylhexylglycerin, tocopheryl acetate, fragrance, limonene, linalool, hydroxycitronellal, coumarin, citro-nellol, alpha-isomethyl ionone, benzyl salicylate, evernia prunastri (oak moss) extract, cinnamyl alcohol, citral, euge-nol, hexyl cinnamal, isoeugenol, evernia furfuracea (treemoss) extract, geraniol, amyl cinnamal, benzyl benzoate |
| 2006-291-MIMI | E. Tjellesen A/S www.goshcosmetics.com |
Aqua, Ethylhexyl stearate, Aluminium chlorohydrate, steareth-6, propylene glycol, stearyl alcohol, cyclomethicone, methylparabene, allantoin, hexyl cinnamal, benzyl salicylate, eugenol, linalool. geraniol, hydroxycitronellal, citronellol |
| 2006-292-MIMI | Parfums Vanderbilt, France | Aqua, Aluminium chlorohydrate, PPG-15, stearyl ether, cetearyl alcohol, ceteareth-33, iodopropynyl butyl-carbamate, PEG-4 dilaurate, PEG-4 laurate, eugenol, dimethicone, limo-nene, benzyl salicylate, linalool, benzyl benzoate, alpha-isomethyl ionone, geraniol, citronellol, citral, hexyl cinnamal, amyl cinnamal |
| 2006-293-MIMI | Calvin Klein Cosmetics Dist. By Unilever |
Water, Propylene glycol, butylene glycol, PEG-8, sodium stearate, poloxamer 407, oleth-20, sodium hydroxide, tetrasodium EDTA, triclosan, parfum |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-294-MIMI | Chanel, Paris | Aqua, Propylene glycol, triethyl citrate, triclosan, PEG-40 hydrogenated castor oil, linalool, butylphenyl methylpropional, BHT, limonene, citric acid, benzyl salicylate, alpha-isomethyl ionone, citronellol, citral, coumarin |
| 2006-295-MIMI | www.lemale-jpg.com | Aqua, Propylene glycol, butylene glycol, sodium stearate, laureth-4, sorbitol, phenoxyethanol, butylphenyl methylpropional, coumarin, hydroxyisohexyl 3-cyclohexene carboxaldehyde, alpha-isomethyl ionone, limonene, linalool, citral, cinnamal, eugenol, CI 60730, CI14700, parfum |
| 2006-296-MIMI | Parfums Issey Miyake, France |
Alcohol denat. Dipropylene glycol, phenoxyethanol, farnesol, butylphenyl methylpropional, linalool, limonene, glyceryl laurate, menthoxy propanediol, hydroxyisohexyl 3-cyclohexene carboxaldehyde, citronellol, geraniol, citral, PEG hydrogenated castor oil, propylene glycol, salvia officinalis (sage water), methylparaben, butylparaben, ethylparaben, propylparaben, isobutylparaben |
| 2006-297-MIMI | Parfums ralph lauren, France | Aqua, Propylene glycol, dipropylene glycol, sodium stearate, triclosan, parfum, limonene, linalool, butylphenyl methylpropional, alpha-isomethyl ionone, hexyl cinnamal, triethanolamine, panthenol, eugenol, tocopheryl acetate, citronellol, geraniol, isoeugenol |
| 2006-298-MIMI | Giorgio armani parfums Dist. Disigner fragrances, Montreal, canada |
Alcohol denat, aqua, Isobutane, triclosan, linalool, limonene, butylphenyl methylpropional, alpha-isomethyl ionone, benzyl salicylate, geraniol, citral, citronellol, eugenol |
| 2006-299-MIMI | Clinique labs., UK |
Alcohol denat. parfum, Limonene, linalool, butylphenyl methylpropional, benzyl salicylate, citronellol, citral, triclosan |
| 2006-300-MIMI | Tommy hilfinger, UK |
Alcohol denat., water, Propylene glycol, sodium stearate, capryloyl glycine, parfum, alpha-isomethyl ionone, limonene, linalool, hydroxyisohexyl 3-cyclohexene carboxaldehyde, citral |
| 2006-301-MIMI | Giorgio armani parfums Dist. Disigner fragrances, Montreal, canada |
Aqua, Propylene glycol, glycerin, sodium stesrate, steareth-100, behenic acid, EDTA, sodium hydroxide, triclosan, parfum, limonene, butylphenyl methylpropional, linalool, alpha-isomethyl ionone, hydroxycitronellal, coumarin, hexyl cinnamal, geraniol, citronellol, citral |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-302-MIMI | P&G prestige beaute, UK |
Alcohol denat., water, Propylene glycol, butylene glycol, stearic acid, Palmitic acid, octyldodecanol, sodium hydroxide, farnesol, limonene, linalool, butylphenyl methylpropional, citral, parfum |
| 2006-303-MIMI | Clinique labs., UK |
Purified water, aluminium chlorohydrate, ceteth-2, PPG-11 stearyl ether, steareth-20, myristalkonium chloride, quaternium-14, trisodium EDTA, sorbic acid, methylparaben, propylparaben, butylparaben |
| 2006-304-MIMI | Dr. Hauschka skin care Manufactured by WALA Heilmittel GmbH |
Aqua, Alcohol, triethyl citrate, glycerin, ricinus communis, zinc ricinoleate, bentonite, salvia offcinalis, hemamelis virginiana, limonene, linalool, geraniol, citral, benzyl benzoate, farnesol, coumarine, citronellol, eugenol, benzyl salicylate, buxus chinensis, sucrose laurate, cetearyl alcohol, lecithin, sodium magnesium silicate, xanthan gum, citric acid, components of natural essential oils |
| 2006-305-MIMI | Allison A/S www.allison.dk |
Water, Propylene glycol, sodium stearate, PEG-7 glyceryl cocoate, PPG-3 myristyl ether, polyceryl-3 caprylate, sodium gluconate, sodium hydroxide, parfum |
| 2006-306-MIMI | Allison A/S www.allisoncosmetics.com |
Aqua, Aluminium chlorohydrate, cetearyl alcohol, octyldodecanol, ceteareth-12, ceteareth-20, glycerin, oryza sativa, butylene glycol, Propylene glycol, ethylhexylglycerin, ginkgo biloba, zingriber officinale, tocophenol, sodium benzoate, potassium sorbate, sorbic acid, phenoxyethanol, methylparaben, ethylparaben, butylparaben, isobutylparaben, propylparaben, imidazolidinyl urea, Aroma |
| 2006-307-MIMI | Naomi Campbell metropolitan cosmetics, Germany | Alcohol denat., Propane, butane, isobutane, propylene glycol, ethylhexylglycerin, isopropyl myristate, bisabolol, limonene, linalool, cirtonellol, BHT |
| 2006-308-MIMI | Parfums Cacharel Paris | Alcohol denat., aqua, Isobutane, triclosan, hydroxyisohexyl 3-cyclohexene carboxaldehyde, hydroxycitronellal, alpha-isomethyl ionone, coumarin, geraniol, citronellol, cinnamyl alcohol, linalool |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-309-MIMI | Sprite Antonio Banderas | Propane, isobutane, dipropylene glycol, triethylcitrate triclosan, hydroxyisohexyl 3-cyclohexene carboxaldehyde, limonene, alpha-isomethyl ionone, linalool, coumarine, benzyl benzoate, parfum |
| 2006-310-MIMI | Jackpot fragrances, NL |
Propane, butane, isobutene, cyclomethicone, dimethicone, isopropyl myristate, octoxyglycerin, parfum |
| 2006-310-MIMI | Jackpot fragrances, NL |
Propane, butane, isobutene, cyclomethicone, dimethicone, isopropyl myristate, octoxyglycerin, parfum |
| 2006-311-MIMI | GUCCI Scannon S.A., Paris |
Aqua, Propylene glycol, butylene glycol, glycerin, sodium stearate, sorbeth-30, PPG-3 myristyl ether, PPG-1, PPG-9 lauryl glycol ether, bis-PEG-18 methyl ether dimethyl silane, methylpropanediol, hexyl cinnamal, butylphenyl methylpropional, hydroxypropyl cyclodextrin, ethylhexylglycerin, hydroxyisohexyl 3-cyclohexene carboxaldehyde, dipotassium glycyrrhizate, sodium thiosulfate, BHT, methyl lactate, alpha-isomethyl ionone, linalool, citronellol, amyl cinnamal, geraniol, CI 17200, CI 47005 |
| 2006-312-MIMI | Kenzo parfums, France |
Aqua, Propylene glycol, sodium stearate, nylon-12, laureth-23, ethylhexylglycerin, CI 77891, nylon-6, citronellol, geraniol, butylphenyl methylpropional, benzyl benzoate, sodium hydroxide, limonene, alpha-isomethyl ionone, hexyl cinnamal, benzylcinnamal, linalool, methyl-2-octynoate, linaool, benzyl alcohol, hydroxycitronellal, eugenol |
| 2006-313-MIMI | Chanel, France | Aqua, Alcohol denat., Propylene glycol, triethyl citrate, PEG-40 hydrogenared castor oil, triclosan, BHT, citric acid, parfum, amyl cinnamal, benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, cinnamyl alcohol, citral, citronellol, coumarin, eugenol, geraniol, hydroxycitronellal, isoeugenol, limonene, linalool, alpha-isomethyl ionone, evernia prunastri (oakmoss) extract |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-314-MIMI | Orgins Nat. Res., UK | butylene glycol, hamamekis virginiana (winterbloom), sodium stesrate, pentadoxynol-200, PEG-12 dimethicone, essential oils, menthe arvensis (field mint), litsea cubeba, cinnamomum camphora (shiu), ocimum basilicum (basil), ribes nigrum (black currant), rosa damascene (rose), jasmine grandiflorum (jasmine), cananga odorata (ylang ylang), citrus aurantium (neroli), vetiveria zizanoides (vetiver), myrocarpus fastgiatus (cabreuva), pimento acris (bay), geraniol, linalool, farnesol, benzyl salicylate, eugenol, limonene, gaultheria fragrantissima, isosteareth-20, glyceryl laurate, glycerin, sodium chloride, benzoic acid |
| 2006-315-MIMI | Lancome, France |
Aqua, Aluminium chlorohydrate, cetearyl alcohol, isopropyl myristate, cetyl esters, Propylene glycol, oleth-12, CI 15510, CI 14700, hydroxyisohexyl 3-cyclohexene carboxaldehyde, evernia furfuracea, limonene, linalool, benzyl salicylate, benzyl alcohol, benzyl benzoate, cinamyl alcohol, propylparaben, alpha-isomethyl ionone, geraniol, methylparaben, citronellol, coumarin, hexyl cinnamal, amyl cinnamal, parfum |
| 2006-316-MIMI | ESTEE LAUDER.com | Propane, butane, isobutane, cyclopentasiloxane, aluminium chlorohydrate, isopropyl myristate, diethylhexyl malate, disteardimonium hectorite, propylene carbonate, triclosan, parfum |
| 2006-317-MIMI | Cosmea ACO A/S Hørsholm |
Aqua, Aluminium chlorohydrate, propylene glycol, glyceryl stearate, PEG-100 stearate, cetyl alkohol, octyldodecanol, cyclomethcone, hydroxyethylcellulose, allantoin, parfum |
| 2006-318-MIMI | Cosmea ACO A/S Hørsholm |
Aqua, Aluminium chlorohydrate, propylene glycol, glyceryl stearate, PEG-100 stearate, cetyl alkohol, octyldodecanol, cyclomethcone, hydroxyethylcellulose, allantoin |
| 2006-319-MIMI | Lóreal Danmark A/S | Aqua, Aluminium sesquichlorohydrate, PPG-15 stearyl ether, cetearyl alcohol, ceteareth-33, C12-13 alkyl lactate, dimethicone, iodopropylnyl butylcarbamate, PEG4- dialurate, PEG-4 dilaurate, PEG-4 laurate, parfum |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-320-MIMI | Lóreal Danmark A/S | Isobutane, Aluminium chlorohydrate, cyclopentasiloxane, triethyl citrate, isopropyl palmitate, stearalkonium bentonite, parfum |
| 2006-321-MIMI | Van Gils parfume www.vangilsparfume.com |
Isobutane, propane, octyl stearate, butane, talc, octylglycerin, PEG-10 rapeseed sterol, persea gratissima, parfum |
| 2006-322-MIMI | Parfums Davidoff, paris Lancaster Group |
Alcohol denat., Cetearyl ethylhexanoate, balm mint (Melissa officinalis) extract, bisabolol, limonene, linalool, triethyl citrate, alpha isomethyl ionone, hydroxyisohexyl 3-cyclohexene carboxaldehyde, farnesol, hydroxyl-citronellal, citral, citronellol, geraniol |
| 2006-323-MIMI | Calvin Klein Cosmetics Dist. By Unilever cosmetics Intl. |
Alcohol denat., water, Pentylene glycol, alpha-isomethyl ionone, benzyl salicylate, butylphenyl methylpropional, citronellol, coumarin, diethylhexyl sebacate, hexyl cinnamal, hydroxycitronellal, limonene, linalool |
| 2006-324-MIMI | Gorgio Armani parfumes, italy | Alcohol, butylphenyl methylpropional, linalool, triclosan, benzylsalicylate, alpha isomethyl ionone, limonene, geraniol, citronellol, coumarin |
| 2006-325-MIMI | Dolce & Gabbana dealers | Aqua, Alcohol denat., Triclosan, benzophenone-2, parfum |
| 2006-326-MIMI | Parfums Christian Dior www.dior.com |
Alcohol, Dipropylene glycol, triethyl citrate, ethylhexylglycerin, parfum, butylphenyl methylpropional, benzyl salicylate, hexyl cinnamal, hydroxycitronellal, benzophenone-2, citronellol, alpha isomethyl ionone, hydroxyisohexyl 3-cyclohexene carboxaldehyde, limonene, linalool, BHT, geraniol, benzyl benzoate, cinnamyl alcohol, citral, CI 14700 |
| 2006-327-MIMI | Cosmeurop parfums www. Bettybarclayfragrance.de |
Butane, propane, dipropylene glycol, ethylhexylglycerin, tocopheryl acetat, hydroxyisohexyl 3-cyclohexene carboxaldehyde, butylphenyl methylpropional, linalool, alpha isomethyl ionone, coumarin, limonene, parfum |
| 2006-328-MIMI | Unilever DK www.unilever.com |
Aqua, Aluminium chlorohydrate, Glycerine helianthus annuus, stearath-2, stearath-20, hydrolysed silk, parfum, alpha isomethyl ionone, amyl cinnamal, benzyl alcohol, benzyl salicylate, butylphenyl methylpropional, cinnamyl alkohol, citronellol, geraniol, hexyl cinnamal, hydroxycitronellal, linalool |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-329-MIMI | Beiersdorf www. Nivea.com |
Butane, isobutane, propane, cyclomethicone, Aluminium chlorohydrate, disteardimonium hectorite, dimethcone, octyldodecanol, persea gratissima, butyloctanoic acid, tocopheryl acetate, benzyl salicylate, hydroxyisohexyl 3-cyclohexene carboxaldehyde, citronellol, geraniol, coumarin, butylphenyl methylpropional, alpha isomethyl ionone, limonene, eugenol, parfum |
| 2006-330-MIMI | A/s bluemøller DK-Odense |
Alcohol denat., isobutane, propane, octyldodecanol, parfum, alpha isomethyl ionone, butylphenyl methylpropional, citral, citronellol, coumarin, geraniol, farnesol, hydroxyisohexyl 3-cyclohexene carboxaldehyde, limonene, linalool |
| 2006-331-MIMI | Beiersdorf www. Nivea.com |
Aqua, Butane, isobutane, propane, Aluminium chlorohydrate, cyclomethicone, C12-15 alkyl benzoate, dicaprylyl carbonate, dicaprylyl ether, cetyl PEG/PPG-10/1 dimerhicone, polyglycetyl-2 dipolyhydroxylstearate, polysorbate 65, phenoxyethanol, butyloctanoic acid, methyl paraben |
| 2006-332-MIMI | Consiva non-food | Aluminium chlorohydrate, PPG-10 cetyl ether, stearate-2, stearate-20, silk amino acid, methylmethacrylate, crosspolymer, imidazolidinyl urea, sodium benzoate, anise alcohol, benzyl benzoate, citronellol, eugenol, geraniol, hydroxycitronellal, hydroxyisohexyl 3-cyclohexene carboxaldehyde, alpha isomethyl ionone, butylphenyl methylpropional |
| 2006-333-MIMI | A/s bluemøller DK-Odense |
Aqua, Aluminium chlorohydrate, cetearyl alcohol, octyldodecanol, ceteareth-12, ceteareth-20, 2-bromo-2-nitropropane-1,3-diol |
| 2006-334-MIMI | Beiersdorf www. Nivea.com |
Butane, isobutane, propane, cyclomethicone, Aluminium chlorohydrate, dimethicone, disteardimonium Hectorite, octyldodecanol, persea gratissima, butyl octanoic acid, tocopheryl acetate, hydroxyisohexyl 3-cyclohexene carboxaldehyde, limonene, alpha isomethyl ionone, citronellol, butylphenyl methylpropional. linalool, benzyl salicylate, hexyl cinnamal, cinnmyl alkohol, coumarin, citral, benzyl benzoate, parfum |
| NERI No. | Manufacturer/ Importer |
Ingredients labelled |
| 2006-335-MIMI | Parfumeurs Createurs, Belgium | Isobutane, linalool, geraniol, triclosan, coumarin, citronellol, butylphenyl methylpropional, hexyl cinnamal, benzyl alcohol, parfum |
| 2006-336-MIMI | Unilever DK www.unilever.com |
Alcohol denat., aqua, Aluminium chlorohydrate, hydroxypropylcellulose, benzyl alcohol, benzyl salicylate, butylphenyl methylpropional, coumarin, limonene, silica, parfum |
| 2006-337-MIMI | Teraline DK-skødstrup Wwwteraline.dk |
Ammonium alum |
| 2006-338-MIMI | Beiersdorf www. Nivea.com |
Aqua, Aluminium chlorohydrate, PPG-15 stearyl ether, stearate-2, stearate-21, chitosan, persea gratissima, trisodium EDTA, limonene, hydroxyisohexyl 3-cyclohexene carboxaldehyde, linalool, alpha isomethyl ionone, citronellol, geraniol, benzyl salicylate, hexyl cinnamal, butylphenyl methylpropional |
| 2006-338-MIMI | Beiersdorf www. Nivea.com |
Aqua, Aluminium chlorohydrate, PPG-15 stearyl ether, stearate-2, stearate-21, chitosan, persea gratissima, trisodium EDTA, limonene, hydroxyisohexyl 3-cyclohexene carboxaldehyde, linalool, alpha isomethyl ionone, citronellol, geraniol, benzyl salicylate, hexyl cinnamal, butylphenyl methylpropional |
| 2006-339-MIMI | Unilever DK www.unilever.com |
Butane, isobutane, propane, Aluminium chlorohydrate, cyclomethicone, PPG-14 butyl eyher, disteardimonium hectorite, helianthus annuus, dimethiconol octyldodecanol, BHT, alpha isomethyl ionone, benzyl cinnamate, benzyl salicylate, butylphenyl methylpropional, citronellol, eugenol, hexyl cinnamal, hydroxycitronellal |
Version 1.0 October 2007, © Danish Environmental Protection Agency