A survey and health assessment of cosmetic products for children
7 Exposure assessment
Exposure assessments on Kathon and benzyl alcohol are conducted as an exact determination of the concentration in the analyzed products is available for these two substances. Quantitative analyses of the constituents phenoxyethanol, sodium benzoate or 5-bromo-5-nitro-1,3-dioxane are not undertaken in the same way and therefore no exposure assessment will be conducted for these substances.
The guidelines for the exposure assessment are stated in EU’s Technical Guidance Document (TGD) (European Commission, 2003) and SCNNFP’s guidelines (SCCNFP 0690, 2003).
However, no standard weight for a child is stated in the TGD. Children’s weight for a certain age can be found in official growth curves. Netdoktor.dk has a table of girls’ and boys’ weight (see extract below) dating from an older Scandinavian study. They emphasize that it is an older study and that in general children have become a little taller and heavier since then (Netdoktor, 2006).
Table 7.1: Overview of Scandinavian children’s weight (Netdoktor, 2006).
| The child’s age | Girls’ average weight | Boys’ average weight |
| 3 years | 15.0 kg | 15.4 kg |
| 6 years | 20.8 kg | 21.2 kg |
| 9 years | 27.5 kg | 28.5 kg |
| 12 years | 39.0 kg | 37.5 kg |
| 14 years | 50.0 kg | 48.0 kg |
The weight from 8 years and up must be taken with a reservation as the weight varies much in the puberty.
The target group of this project is children at the age 3-14 years. Worst case is thus a three-year-old girl’s weight, i.e. 15 kg. For a few situations, for instance bath situations where little brother or sister bathes together with the older siblings, it may be considered to apply an even lower weight as worst case.
In all exposure scenarios a child of 15 kilos is applied as a standard equivalent to an age of three years which is the lower age in the target group of the cosmetic products for children being studied in this project.
A standard MoS (Margin of Satety) of 100 is applied for the risk assessment. It is generally accepted that MoS must be at least 100 in order that a substance can be declared as safe to apply. If a credible animal study is available it can be justified to use a factor 10 for differences in kind (animals to humans) and a factor 10 to include sensitive groups in the population – in total a factor 100.
SCCNFP concludes in their guidelines for safety assessment of cosmetics constituents that there is no reason to apply an additional safety factor for children when talking of intact skin (SCCNFP 0690, 2003).
7.1 Kathon
In total 11 products are analyzed for a quantitative content of Kathon. The table below states the intervals of the measured concentrations of Kathon in the different product types being studied. Furthermore, the table is an overview of the product types in which Kathon is found via the study. The maximum permissible concentration of Kathon in cosmetic products is 15 mg/kg.
Table 7.2: Produkts with Kathon. The measured concentrations are stated for the analyzed products.
| Product type | Products analyzed | Products in total with Kathon |
Measured concentrations I mg/kg (ppm) |
| Body shampoo/bath gel | 6 | 9 | < 2 – 7.7 |
| Liquid soap | 1 | 1 | 10 – 12 |
| Bobble bath | 2 | 4 | 3.0 – 11 |
| Body lotion/cream | 1 | 1 | < 2 |
| Shampoo | 1 | 2 | 4.4 – 6.7 |
| Eau de toilette | 0 | 1 | No analyses conducted |
The detection limit is 2 mg/kg
For a cosmetics constituent, the daily exposure quantity – also called the internal exposure (in the EU called SED or Systemic Exposure Dosage) is the quantity of the substance being expected to penetrate into the bloodstream (and thus being systemic disposable) per kg body weight per day (SCCNFP 0690, 2003).
The calculated exposure scenarios for Kathon are worst case scenarios, calculated according to the guidelines and default values in TGD (Appendix II, Table 14) and retention factors from SCCMFP 0690 (2003). The retention factor is introduced by SCCNFP to consider products being diluted when they are used and cleansed off after use, i.e. for shampoo products, body shampoo and similar “rinse-off” products.
No data are available for liquid soap. Here the values from the consumer project “Survey and health and environmental assessment of hand soap”(Larsen JR et al, 2006) are applied. The factors being a part of the calculation are seen in Table 7-3.
Table 7-3. Applied factors in the exposure assessment.
| Product type | Number of appplications | Applied quantity per application | Concentra-tion of Kathon | Uptake through the skin | Retention factor | The child’s weight |
| Body shampoo/ Bath gel |
1-2 per day | 5.0 g | 7.7 mg/kg | 100% | 0.01 | 15 kg |
| Fluid soap | 6 per day | 1.0 g | 12 mg/kg | 0.01 | ||
| Bobble bath | 1-2 per week | 17.0 g | 11 mg/kg | 0.01 | ||
| Body lotion/cream | 1-2 per day | 7.5 g | 2 mg/kg | 1 | ||
| Shampoo | 2-7 per week | 12.0 g | 6.7 mg/kg | 0.01 | ||
| Eau de toilette | 1-2 per day1 | 3.0 g² | 15 mg/kg³ | 1 |
1 – assumption as no data in TGD or SCCNFP guidelines.
2 – the value for deodorant spray is applied. This value will be significantly higher than for eau de toilette (but there is no available value for eau de toilette in TGD).
3 – no eau de toilette products have been analyzed. Therefore, the maximum permissible concentration of 15 mg/kg is applied.
As seen in the table no data for eau de toilette are available. A density of 1 g/litre is assumed as eau de toilette mainly consists of water and about 8% fragrances according to the analyse of an eau de toilette in this project. It is assumed that eau de toilette is applied once per day. Typically, adults will have a use of 1-2 applications per day whereas very small children may be expected to apply significantly less. It may be assumed that the girls have a certain age before they start using eau de toilette. The older children can be expected to imitate the mother’s behaviour, i.e. 1-2 applications per day.
Regarding application quantity, no quantity is stated in TGD for eau de toilette. Instead the value for deodorant spray of 3.0 g per application is used. This value is probably significantly higher than the real value as experience shows that less eau de toilette than deodorant spray is used.
Correspondingly, no standard weight for a child is stated in TGD. As described in the beginning of the chapter, a worst-case value of 15 kg for a child’s weight is applied.
The daily exposure is calculated by use of the formula below where SED (Systemic Exposure Dosage) is the daily exposure, A is the quantity being applied daily, C is the concentration of the substance in the cosmetics product, DA (Dermal Absorption) is the absorption through the skin indicated in %, Rf is the retention factor (introduced by SCCNFP to consider “rinse-off” products) and bw (body weight) is the body weight of the child.
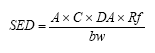
The daily exposure of Kathon when using body lotion on a three-year-old child can be calculated to:
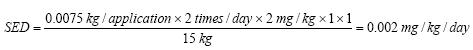
In the table below the daily exposure (SED) for Kathon is stated for the different product types.
Table 7.4: Daily exposure (SED) for Kathon for the different product types
| Applied quantity per application (in kg) | Number of daily applications | Concentration (mg/kg) |
Rf | SED (mg/kg/day) |
|
| Body shampoo/bath gel | 0.005 | 2 | 7.7 | 0.01 | 0.000051 |
| Liquid soap | 0.001 | 6 | 12 | 0.01 | 0.000048 |
| Bobble bath | 0.017 | 0.33 | 11 | 0.01 | 0.000041 |
| Body lotion/cream | 0.0075 | 2 | 2 | 1 | 0.0020 |
| Shampoo | 0.012 | 1 | 6.7 | 0.01 | 0.000054 |
| Eau de toilette | 0.003 | 2 | 15 | 1 | 0.0060 |
It is seen that the highest daily exposures occur when using eau de toilette and body lotion. It must be noted that the apply of eau de toilette is assessed to be much overestimated as the same exposure values as for normal deodorant are applied.
7.1.1 Risk assessment for Kathon
The risk assessment is conducted for the critical effect of Kathon which at acute toxicity is ataxia and serious stomach irritation. It is presumed that 100% of the Kathon to which the child is exposed will also be absorbed in the body. The applied NOAEL value is identified for Kathon in the health assessment in section 6.3.2, i.e. for the mixture MI/CMI. This value is 8 mg/kg bw/day CIRP, 1992).
When calculating Margin of Safety (MoS) for Kathon the NOAEL value and the calculated daily exposure (= SED) are placed in the following formula:
![]()
Margin of Safety for Kathon when using body lotion on a three-year-old child will be:
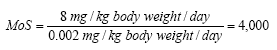
Table 7.5: Margin of safety for Kathon for the different product types
| SED (mg/kg/day) |
MoS | |
| Body shampoo/bath gel | 0.000051 | 155,844 |
| Liquid soap | 0.000048 | 166,667 |
| Bobble bath | 0.000041 | 194,458 |
| Body lotion/cream | 0.0020 | 4,000 |
| Shampoo | 0.000054 | 149,254 |
| Eau de toilette | 0.0060 | 1,333 |
Margin of Safety ought to be above 100 to take account of a safety factor of 10 for extrapolation of data from animals to humans and a safety factor of 10 to take account of especially sensitive human individuals.
The calculation shows that the margins of safety are substantially above 100 even if the calculation is made as a worst case calculation, i.e. for a three-year-old child with a high daily use of body lotion. However, it must be emphazied that MoS is not calculated for allergy but for another critical effect, cf. the health assessments in chapter 6.
7.2 Benzyl alcohol
In total 17 products are analyzed for a quantitative content of the 26 fragrances, including benzyl alcohol. Benzyl alcohol was found in 12 of the 17 analyzed products. The table below states the intervals of the measured concentrations of benzyl alcohol in the different product types being studied. Furthermore, the table is an overview of the product types in which benzyl alcohol is found via the study. The maximum permissible concentration of a preservative in cosmetic products is 1% according to the Cosmetics Statutory Order. As a fragrance there is no upper limit.
Through the analyses, more products with a content of benzyl alcohol are identified than found via the survey (applies to the product types body shampoo/bath gel and shampoo). This reflects the fact that there is benzyl alcohol in the products but in a low concentration not mandatory to declare on the product.
Table 7.6: Products with benzyl alcohol. The measured concentrations are stated for the analyzed products.
| Product type | Products with an analyzed content of benzyl alcohol | Products in total with benzyl alcohol | Measured concentrations in mg/kg (ppm) |
| Body shampoo/bath gel | 6 | 1 | 4 – 120 |
| Shampoo | 3 | 1 | 1 – 8 |
| Body lotion/cream | 1 | 1 | 780 – 790 |
| Eau de toilette | 1 | 1 | 5 |
| Bath confetti/caviar/fizzle salt | 1 | 6 | 2 |
| Bobble bath | - | 1 | No analyses conducted |
| Tooth paste | - | 1 | No analyses conducted |
| Hair dye (rinsing colour) | - | 7 | No analyses conducted |
| Others (body splash) | - | 1 | No analyses conducted |
The detection limit is 1 mg/kg
The calculated exposure scenarios for benzyl alcohol are worst case scenarios. The daily exposure quantity, Systemic Exposure Dosage, SED, is calculated on basis of the factors below which appear from TGD and SCCNFP 0690 (2003) (Table 7-7).
Table 7-7. Factors in the exposure assessment of benzyl alcohol.
| Product type | Number of applications | Applied quantity per application | Concentration of benzyl alcohol (worst case) |
Absorption through the skin (worst case) |
Retention factor | The child’s weight (worst case) |
| Body shampoo/ Bath gel |
1-2 per day | 5.0 g | 120 mg/kg | 100% | 0.01 | 15 kg |
| Body lotion/cream | 1-2 per day | 7.5 g | 790 mg/kg | 1 | ||
| Shampoo | 2-7 per week | 12.0 g | 8 mg/kg | 0.01 | ||
| Eau de toilette | 1-2 per day1 | 3.0 g² | 5 mg/kg | 1 | ||
| Bath caviar | 1 per week³ | 25.0 g4 | 2 mg/kg | 0.01 |
1 – assumption as no data in TGD or SCCNFP guidelines.
2 – the value for deodorant spray is applied. This value will be significantly higher than for eau de toilette (but there is no available value for eau de toilette in TGD).
3 – assumption as no data in TGD or SCCNFP guidelines.
4 – assumption as no data in TGD or SCCNFP guidelines.
There are no data for eau de toilette in TGD. A density of 1 g/litre is assumed as eau de toilette mainly consists of water and about 8% fragrances according to the analyse of an eau de toilette in this project. It is assumed that eau de toilette is applied once per day. Regarding application quantity, no quantity is stated in TGD for eau de toilette. Instead the value for deodorant spray of 3.0 g per application is used. This value is probably significantly higher than the real value as experience shows that less eau de toilette than deodorant spray is used.
Nor any data are found for bath caviar in TGD. Therefore, an educated guess of an applied frequency of 1 time per week is made and for the applied quantity per application a value of 25 g is used. In the purchased bath caviar products, the content varies between 25 and 100 g. In the smallest packages the content corresponds to a small handful. As worst case it is assumed that the whole package of 25 g is used.
Correspondingly, no standard weight for a child is stated in TGD. As described in the beginning of this chapter, a value of 15 kg for a child’s weight is applied.
The daily exposure (SED) for benzyl alcohol is calculated as described for Kathon and gives for instance the following daily exposure when using body lotion on a three-year-old child:
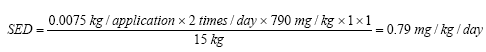
In the table below the daily exposure (SED) for benzyl alcohol is stated for the different product types.
Table 7.8: Daily exposure (SED) for benzyl alcohol for the different product types
| Applied quantity per application (in kg) | Number of daily applications | Concentration (mg/kg) |
Rf | SED (mg/kg/day) | |
| Body shampoo/ Bath gel |
0.005 | 2 | 120 | 0.01 | 0.00080 |
| Body lotion/cream | 0.0075 | 2 | 790 | 1 | 0.79 |
| Shampoo | 0.012 | 1 | 8 | 0.01 | 0.000064 |
| Eau de toilette | 0.003 | 2 | 5 | 1 | 0.0020 |
| Bath caviar | 0.025 | 0.14 | 2 | 0.01 | 0.0000048 |
It is seen that the highest daily exposure occurs when using body lotion.
7.2.1 Risk assessment for benzyl alcohol
The risk assessment for benzyl alcohol is conducted for the critical effect of benzyl alcohol which is lethargy at acute toxicity. It is presumed that 100% of the benzyl alcohol to which the child is exposed will also be absorbed in the body.
Several NOAEL values for benzyl alcohol from different studies are stated in section 6.2.1 in the health assessment of benzyl alcohol. For these worst case calculations, the lowest NOAEL value found in the literature for benzyl alcohol is applied, i.e. the value 188 mg/kg bw/day found in tests with acute toxicity with mice (OECD SIDS, 2001).
When calculating Margin of Safety (MoS) for benzyl alcohol the calculated daily exposure (= SED) is placed in the following formula:
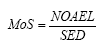
Margin of Safety for benzyl alcohol when using body lotion on a three-year-old child will be:
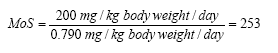
Tabel 7.9: Margin of safety for benzyl alcohol for the different product types
| SED (mg/kg/day) |
MoS | |
| Body shampoo/bath gel | 0.00080 | 235,000 |
| Body lotion/cream | 0.79 | 238 |
| Shampoo | 0.000064 | 2,937,000 |
| Eau de toilette | 0.0020 | 94,000 |
| Bath caviar | 0.0000048 | 39,480,000 |
Margin of Safety ought to be above 100 to take account of a safety factor of 10 for extrapolation of data from animals to humans and a safety factor of 10 to take account of especially sensitive human individuals. The calculation above shows that Margin of Safety is not exceeded in any of the concrete examples. However, for the body lotion product Margin of Safety is relatively close to 100 whereas for the other products there is an even very large margin of safety.
However, it must be emphazied that MoS is not calculated for allergy but for another critical effect, cf. the health assessments in chapter 6.
It must also be emphazied that the mapped body lotion/cream is close to the limit to represent a health risk on basis of the content of benzyl alcohol. Therefore, it cannot be denied that other non-analyzed products may have a higher content of benzyl alcohol and thus form a potential health risk. It may be stay-on products such as body lotion/cream which may represent a potential health risk in the worst-case scenario. However, the calculated Margin of Safety shows that there is no health risk in using the products in relation to the content of benzyl alcohol.
Version 1.0 October 2007, © Danish Environmental Protection Agency