[Front page] [Contents] [Previous] [Next] |
Brominated Flame Retardants
1. Introduction to Brominated Flame Retardants
1.1 Flame retardants
1.2 The Chemistry of Brominated Flame Retardants
1.2.1 Polybrominated Diphenyl Ethers
1.2.2 Polybrominated Bipenyls
1.2.3 Tetrabromobisphenol A and Derivatives
1.2.4 Hexabromocyclododecane
1.2.5 Other Brominated Flame Retardants
1.3 European and Global Consumption of Flame Retardants
1.4 Emission from Products in Service
1.1 Flame retardants
Flame retardants are added to polymeric materials, both natural and synthetic, to enhance the flame-retardancy properties of the polymers.
There are four main families of flame-retardant chemicals:
| Inorganic flame retardants including aluminium trioxide, magnesium hydroxide, ammonium polyphosphate and red phosphorus. This group represents about 50% by volume of the global flame retardant production /9/. | |
| Halogenated flame retardants, primarily based on chlorine and bromine. The brominated flame retardants are included in this group. This group represents about 25% by volume of the global production /9/. | |
| Organophosphorus flame retardants are primarily phosphate esters and represent about 20% by volume of the global production /9/. Organophosphorus flame retardants may contain bromine or chloride. | |
| Nitrogen-based organic flame retardants are used for a limited number of polymers. |
Global production figures and trends in consumption are discussed further in section 1.3.
About 350 different substances used as flame retardants are described in the literature. The index of Flame Retardant /13/, an international guide to more than 1000 products by trade name, chemical, application, and manufacturer, contains more than 200 chemicals used in commercial flame retardants. A comprehensive list of flame retardants is compiled by the Swedish National Chemical Inspectorate /14/.
Mechanisms of action
Depending on their nature, flame retardants can act chemically and/or physically in the solid, liquid or gas phase. They interfere with combustion during a particular stage of this process, e.g. during heating, decomposition, ignition or flame spread.
Substitution of one type of flame retardants with another consequently means a change in the mechanisms of flame retardancy.
Halogen containing flame retardants act primarily by a chemical interfering with the radical chain mechanism taking place in the gas phase during combustion. High-energy OH and H radicals formed during combustion are removed by bromine released from the flame retardant.
Although brominated flame retardants are a highly diverse group of compounds the flame-retardancy mechanism is basically the same for all compounds. However, there are differences in flame-retardancy performance of the brominated compounds, as the presence of the compounds in the polymer will influence the physical properties of the polymer.
In general aliphatic bromine compounds are easier to break down and hence more effective at lower temperatures, but also less temperature resistant than aromatic retardants.
Aluminium hydroxide and other hydroxides act in a combination of various processes. When heated the hydroxides release water vapour that cool the substrate to a temperature below that required for sustaining of the combustion processes. The water vapour liberated has also a diluting effect in the gas phase and forms an oxygen displacing protective layer. Additionally the oxide (e.g. AlO2) forms together with the charring products an insulating protective layer.
Phosphorus compounds mainly influence the reactions taking place in the solid phase. By thermal decomposition the flame retardant are converted to phosphorus acid which in the condensed phase extract water from the pyrolysing substrate, causing it to char. However, some phosphorus compounds may, similar to halogens, act in the gas phase as well by a radical trap mechanism.
Nitrogen based flame retardants as melamine and melamine derivatives act by intumescence. The flame retardants are most often used in combination with other flame retardants. Gasses released from the compounds make the material to swell forming a insulating char on the surface.
A distinction is made between reactive and additive flame retardants.
Reactive flame retardants are built chemically into the polymer molecule, together with the other starting components. This prevents them from bleeding out of the polymer and vaporise and their flame retardancy is thus retained. They have no plasticising effect and do not affect the thermal stability of the polymer. They are used mainly in thermosets, especially polyesters, epoxy resins and polyurethanes (PUR) in which they can be easily incorporated.
The most used reactive brominated flame retardants are tetrabromobisphenol A (TBBPA), tetrabromophthalic anhydride, dibromoneopentylglycol, and brominated styrene.
Additive flame retardants are incorporated in the plastic either prior to, during, or, more frequently, following polymerisation. They are used especially in thermoplastics as ABS, HIPS, PS, PC and thermoplastic polyesters. If they are compatible with the plastic they act as plasticisers, otherwise they are considered as fillers. They are sometimes volatile and can tend to bleed, so their flame retardancy may be gradually lost. High molecular weight products are developed to enable plastics to be made more permanently fire retardant by the additive method.
The most used additive brominated flame retardants are polybrominated diphenyl ethers (PBDEs), Tetrabromobispehol A (mostly used as reactive FR) and hexabromocyclododecane (HBCD).
Synergism
Combinations of flame retardants can produce an additive or synergistic effect. While the additive effect is the sum of the individual actions, the effects of synergism are higher than this sum.
Antimony trioxide
Antimony trioxide, Sb2O3, the main antimony compound used commercially, shows no perceptible flame-retardant action on its own. Together with bromine-containing compounds, however, it produces a marked synergistic effect. Antimony trioxide is widely used in brominated FR formulations.
1.2 The Chemistry of Brominated Flame Retardants
Brominated flame retardants (BFRs) may in accordance with the classification in section 3.1 be defined as non-organophosphorus organic compounds where one or more hydrogen atoms are replaced by bromine. BFRs are usually containing 50-85% of bromine (by weight, the contents of each compound is shown in table 1.1).
Ammonium bromide, which may be used as flame retardants in textiles, and brominated organophosphates are not included under this definition.
Brominated flame retardants can be divided into three classes:
| Aromatic, including tetrabromobisphenol A (TBBPA), polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) | |
| Aliphatic, which are in general used in relatively small quantities | |
| Cycloaliphatic, including hexabromocyclododecane (HBCD) |
Physical/chemical properties of more than 40 brominated flame retardants in commercial use, according to OECD 1994 , IPCS 1997 and product literature are listed in appendix 3.
Until now risk evaluations have mainly focused on the high volume aromatic compounds.
In this report consumption and disposal of flame retardants will be estimated for the following groups of brominated flame retardants:
| Polybrominated diphenyl ethers (penta, octa, and decabromodiphenyl ether) | |
| Tetrabromobisphenol A and derivatives | |
| Polybrominated biphenyls | |
| Hexabromocyclododecane | |
| Other brominated flame retardants |
The first three groups are aromatic compound whereas hexabromocyclododecane is a cycloaliphatic compound.
1.2.1 Polybrominated Diphenyl Ethers
Brominated diphenyl ethers are a group of aromatic brominated compounds in which one to ten hydrogens in the diphenyl oxide structure are replaced by bromine.
The polybrominated diphenyl ethers (PBDEs) with three to ten bromine atoms are used in commercial flame retardants. The compounds are designated tri (3), tetra (4), penta (5), hexa (6), hepta (7), octa (8), nona (9) and decabromodiphenyl ether.
Commercial products are not pure substances. Three different flame retardants are commercially available. They are referred to as penta-, octa- and decabromodiphenyl ether, but each product is a mixture of brominated diphenyl ethers.
Synonyms
Various synonyms and abbreviations of polybrominated diphenyl ethers are used in the literature. In this report - in accordance with the monograph from IPCS /7/ - the chemical name polybrominated diphenyl ethers is used. To indicate that it is a group of compounds the abbreviation PBDEs is used instead of the more widespread PBDE. The same group may as well be named polybrominated biphenyl ethers (PBBEs), polybrominated biphenyl oxides (PBBOs), or polybrominated diphenyl oxides (PBDOs).
Global consumption
The annual global consumption of all polybrominated diphenyl ethers was in 1992 estimated at 40,000 tonnes, which was broken down as 30,000 tonnes (75%) of decabromodiphenyl ether, 6,000 tonnes (15%) of octabromodiphenyl ether and 4,000 tonnes (10%) of pentabromodiphenyl ether /7/. The 40,000 tonnes corresponded to about 30% of the world market.
Data on the Western European market of flame retardants shown in table 1.5 indicate that the consumption of PBDEs until 1996 did not show a significant decrease, and PBDEs accounted for about 26% of the European market for brominated flame retardants in 1996 /19/. A market analysis from 1999 shows that the market share of the PBDEs has deceased to about 11% in 1998. The decrease in the consumption of PBDEs is especially pronounced in Germany, The Netherlands and the Nordic countries /18/.
In 1986 members of the German Association of Chemical Industries voluntarily stopped the production of PBDEs and PBBs /15/. In the recent year leading European companies in the electric and electronic industry have proclaimed an official policy of avoiding PBDEs and PBBs in their products.
Environmental Health Criteria monograph has been prepared for polybrominated diphenyl ethers in 1994 /7/.
Decabromodiphenyl ether
Decabromodiphenyl ether (DeBDE) is a fine, white to off white crystalline powder. IPCS reported that a typical composition for modern products would be 97-98% decaBDE with 0.3-3.0% of other brominated diphenyl ethers, mainly nonaBDE /7/.
Decabromodiphenyl ether is mostly used for applications in plastic and textiles. It is an additive flame retardant, i.e. it is physically combined with the material being treated rather than chemically combined.
As for the PBDEs as a group, various names and abbreviations are used for decabromodiphenyl ether. Box 2.1 gives the chemical names and synonyms of the compound. Physical and chemical properties can be found in appendix 3.
Box 1.1
Decabromodiphenyl ether
| Chemical names | Decabromodiphenyl ether (DeBDE) Decabromodiphenyl oxide (DeBDO) |
| CAS no. | 1163-19-5 |
| CAS name | 1,1´-oxybis[2,3,4,5,6-pentabromo]-benzene |
| IUPAC name | Bis(pentabromophenyl) ether |
| Synomyms | Decabromobiphenyl ether (DBBE) Decabromobiphenyl oxide (DBBO) Decabromo phenoxybenzene Benzene 1,1' oxybis-, decabromo derivative |
| Bromine content | 81-83% |
Figure 1.1
Chemical structure of decabromodiphenyl ether
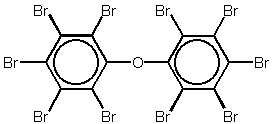
Industry information indicates that decabromodiphenyl ether is used at loadings of 10-15% weight in polymers and is always used in conjunction with antimony trioxide /3/. Traditionally the major application for decabromodiphenyl ether has been in high impact polystyrene (HIPS) used for TV-set backplates. In the beginning of the 1990'ies the total global consumption of DeBDE was broken down as follows /11/:
30% Polystyrene (HIPS) [moulding parts, panels, housing ]
20% Terephthalates (PBT, PET) [moulding products, connectors, switchgears, electrical equipment]
15% Polyamides (PA) [injection moulding, contactors, bobbins, electrical elements]
10% Styrenic rubbers (SBR) [latex, carpet backing, furniture]
5% Polycarbonates (PC) [moulding parts, panels, housing, computers, aircraft]
5% Polypropylene (PP) [injection moulding, capacitors, TV, electronics]
15% Other polymer applications and end uses, notably: Acetate copolymer (EVA) [extrusion, coating, wire, cables, electrical distribution] and unsaturated polyester resins (UPE) [moulding compounds, panels, boxes, electrical equipment]
It should be noted that consumption of PBDEs has changed significantly in Europe during the last years as discussed in the following chapters. For many uses PBDEs have, however, been substituted by other brominated flame retardants and the brake down of the consumption shown above still gives some indication of the use of additives BFRs by plastic raw material.
Octabromodiphenyl ether
The commercially supplied octabromodiphenyl ether (OcBDE) is an off-white mixture of brominated diphenyl ethers typically consisting of 31-35% octaBDE. The other main components are hexaBDE (10.5-12%), heptaBDE (around 44%), nonaBDE (9.5-11.3%) and decaBDE (0-0.7%) /7/. The product is a solid of low water solubility and vapour pressure.
Chemical/physical properties are listed in appendix 3.
The chemical structure of octabromodiphenyl ether is shown in figure 1.2. On the basis of the chemical structure there are 12 possible isomers of octaBDE.
Figure 1.2
Chemical structure of OcBDE
General structure
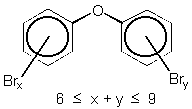
Specific isomer
Information provided by industry to the EU risk assessment /4/ indicates that octabromodiphenyl ether is always used in combination with antimony trioxide. In Europe it is primarily used in acrylonitrile butadiene styrene (ABS) polymers at 12-18% weight loadings. Around 95% of the total octabromodiphenyl ether supplied in the EU (around 1990) is used in ABS. Other minor uses, accounting for the remaining 5%, include high impact polystyrene (HIPS), polybutylene terephthalate (PBT) and polyamide polymers, at typical loading of 12-15% weight. The flame retarded polymer products have typically been used for the housings of office equipment and business machines.
Pentabromodiphenyl ether
The commercially supplied pentabromodiphenyl ether (PeBDE) is a mixture of brominated diphenyl ethers. It contains typically 50-60% pentaBDE and 24-38% tetraBDE and 4-8% hexaBDE /11/.
The chemical structure of the pure pentabromodiphenyl ether is similar to the structure of octabromodiphenyl ether, but with only five bromine atoms. Chemical names and structure, abbreviations, synonyms, physical properties, etc. are listed in appendix 3.
PeBDE has traditionally been used as an additive flame retardant in epoxy resins, polyesters, polyurethanes and textiles /7/.
1.2.2 Polybrominated Bipenyls
Polybrominated biphenyls (PBBs) are a group of halogenated hydrocarbons which are formed by substituting bromine for hydrogen in biphenyl. The bromine content can vary between two and ten.
According to OECD, decabromobiphenyl (DeBB) is the only brominated biphenyl that has been identified in commercial use /11/. The technical product contains about 97% DeBB, the rest being nona and octabromobiphenyls. The demand for decabromobiphenyl in 1992 was limited to the Benelux, France and the South European countries at a level of less than 2000 tonnes per year. The W. European market of DeBB was in 1998 about 600 tonnes (see table 1.5).
The chemical structure of decabromobiphenyl is shown in figure 1.3.
Figure 1.3
Decabromobiphenyl
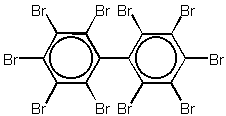
DeBB has traditionally been used as additive flame retardant for styrenic polymers and engineering plastics /11/. It has also been considered a general purpose FR additive for other polymers such as unsaturated polyester (UPE) resins.
Environmental Health Criteria monograph has been prepared for polybrominated biphenyls in 1994 /6/.
1.2.3 Tetrabromobisphenol A and Derivatives
Tetrabromobisphenol A (TBBPA) and derivatives are a group of aromatic brominated flame retardants in which four hydrogens in the bisphenol structure are replaced by bromine. In all tables in the report TBBPA represent the whole group.
TBBPA and derivatives are globally speaking the most important group of brominated flame retardants in terms of actual production and demand, which in 1992 was more than 60,000 tonnes per annum cooresponding to 40% of the market. In Western Europe TBBPA and derivatives accounted for about 26% of the total market in 1998 (see table 1.5).
The group includes tetrabromobisphenol A as well as its dimethylether, dibromopropylether, bis(allylether), bis(2-hydroxyethyl oxide), carbonates and epoxy oligomer derivatives.
Chemical names and structures, abbreviations, synonyms, physical properties, etc. are listed in appendix 3.
The chemical structure of TBBPA and the dimetylether derivative are shown in figure 1.4.
Figure 1.4
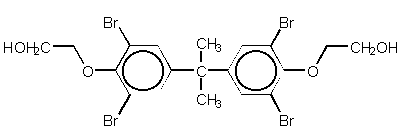
Chemical structures of TBBPA and TBBPA- bis-(2-hydroxyethylether)
TBBPA
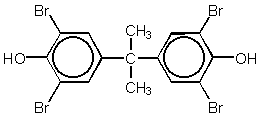
TBBPA bis-(2-hydroxyethylether)
TBBPA is used as reactive flame retardant in the production of epoxy resins, replacing bisphenol A, partially or totally, in the reaction with epichlorhydrin. Commercial epoxy FR resins containing 20% bromine (48% if bisphenol A is totally replaced by TBBPA) are widely used in the manufacturing of rigid epoxy laminated printed circuit boards. Other epoxy based TBBPA end uses are glass reinforced panels for construction, motor housings and terminal boards.
When TBBPA is used as a reactive flame retardant, the chemical identity of the compound is lost in the process of polymerisation. This means that TBBPA per se is not present in the final product. However, in this report the content of flame retardants in products will be indicated by the quantity of TBBPA used for production of the product.
TBBPA can be used as an additive flame retardant in acrylonitrile-butadiene-styrene (ABS), polystyrene (PS), thermoplastic polyesters (PET/PBT) and phenolic resin. In the beginning of the nineties additive use accounted for approximately 10% of the global TBBPA consumption /8/. The additive use may account for a larger part of the consumption today, but updated information have not been available.
Other TBBPA derivatives included in this group are TBBPA bis(2-hydroxyethyloxide), TBBPA bis(2,3-dibromopropyl) oxide and TBBPA bis(allyloxide), used for polyolefins, in particular polypropylene (PP) extrusion grade, surface coatings and polystyrene (PS) foams, respectively.
Environmental Health Criteria monograph has been prepared for tetrabromobisphenol A and derivatives in 1995 /8/. TBBPA is not covered by EU risk assessments.
1.2.4 Hexabromocyclododecane
Hexabromocyclododecane is a cycloaliphatic compound with six bromine atoms. The chemical structure is shown in figure 1.5.
Chemical names and structures, abbreviations, synonyms, physical properties, etc. are listed in appendix 3.
Figure 3.5
Chemical structure of hexabromocyclododecane
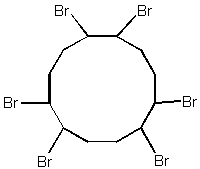
Hexabromocyclododecane has traditionally been used as an additive flame retardants for textiles coatings and production of flame retarded expanded polystyrene used for insulation in the building industry.
In Western Europe HBCD in 1998 accounted for approximately 14% of the total consumption of BFRs (see table 1.5).
Environmental Health Criteria monograph has not been prepared for hexabromocyclododecane, but the compound is under risk assessments within the EU. A draft of the risk assessment has been finished by March 1999.
1.2.5 Other Brominated Flame Retardants
The above mentioned brominated flame retardants represented about 76% of the 1992 global production of BFRs. The remaining is covered by a number of other retardants.
More details on the consumption of the flame retardants in Western Europe is given in the following chapter. The mentioned flame retardants only represented about 52% of the W. European market in 1998.
In table 1.1 the globally speaking most widely used BFRs are listed.
CAS number, application and properties of the flame retardants are listed in appendix 3.
Table 1.1
Most widely used brominated flame retardants (based on /11/)
| Chemical | Bromine content (%) |
Reactive/ Additive |
Main applications (globally) |
| Tetrabromobisphenol A (TBBPA) | 59 |
R,A |
epoxy, PC, UPE |
| TBBPA carbonate oligomer | 55 |
A | ABS, engin. thermoplastics |
| TBBPA-bis(2,3-dibromopropyl ether) | 68 |
A | polyolefins |
| TBBPA-bis(allyl ether) | 51 |
R |
1) |
| TBBPA epoxy oligomer | 52 |
A | styrenics, PBT |
| TBBPA-bis(2-hydroxyethyl ether) | 51 |
A | engin. plastics, coatings |
| Decabromodiphenyl ether (DeBDE) | 83 |
A | general purpose |
| Octabromodiphenyl ether (OcBDE) | 79 |
A | ABS, PC, thermosets |
| Pentabromodiphenyl ether (PeBDE) | 71 |
A | textile, PUR |
| Decabromobiphenyl (DeBB) | 84 |
A | general purpose |
| Tetrabromophthalic anhydride (TBPA) | 69 |
R |
UPE, coating |
| TBPA diester/ether diol | 45 |
R |
rigid PUR foams |
| Ethylene bis(tetrabromophthalimide) (EBTBP) | 67 |
A | similar to DeBDE |
| Tetrabromophthalimide | 69 |
A | engin. thermoplast. |
| Disodium salt of tetrabromophthalate | 15 |
A | textile, coatings |
| Hexabromocyclododecane (HBCD) | 75 |
A | EPS and XPS |
| Dibromoethyldibromocyclohexane | 75 |
A | PS, PUR |
| Ethylene bis(dibromonorbornanedicarboximide) | 48 |
A | nylons and polyolefins |
| Dibromoneopentyl glycol (DBNPG) | 61 |
R |
UPE, PUR |
| Tribromoneopentyl alcohol (TBNPA) | 72 |
R |
PUR |
| Vinyl bromide (VBr) | 75 |
R |
modacrylic fibers |
| 2,4,6-Tribromophenol (TBP) | 73 |
R |
phenolics, epoxy |
| Bis(tribromophenoxy)ethane (HBPE) | 70 |
A | ABS, PC, thermosets |
| Tribromophenyl allyl ether (TBP-AE) | 65 |
R |
1) |
| Poly(dibromophenylene oxide) (PDBPO) | 62 |
A | similar to DeBDE |
| Pentabromoethylbenzene (5BEB) | 82 |
A | UPE, SBR, textile |
| Tetradecabromodiphenoxybenzene (TDBDPB) | 82 |
A | similar to DeBDE |
| Poly(pentabromobenzyl acrylate) (PBB-PA) | 70 |
A | polymeric, PBT |
| Polydibromostyrene (PDBS) | 59 |
A | PA, PBT, styrenics |
| Brominated polystyrene (BrPS) | 60 |
A | PBT, PS |
| 1) | The OECD report /11/ says PS foam, but this is presumably not the case. The table in the report include also ammonium bromide. |
1.3 European and Global Consumption of Flame Retardants
As it will be shown in chapter 6 imported products account for about 90% of the consumption of brominated flame retardants with end products in Denmark. As background information for the assessments of the content of brominated flame retardants in imported products, analyses of the World and Western European market for flame retardants will be discussed in the following section.
Use of bromine
World production of bromine in 1996 is estimated at 440,000 tonnes /16/. Brominated flame retardants accounted in 1997 for approximately 30% of global bromine consumption /17/.
In 1992 brominated the flame retardants accounted for approximately 20% of the global bromine consumption. Other uses were agrochemicals and sanitary (15%), gasoline additives (14%), drilling fluids (10%), dyes (8%), water chemicals (6%), photographic chemicals, pharmaceuticals, synthetic rubbers, minerals separation and electrolytes /11/.
Production of brominated flame retardants
Brominated flame retardants are produced by a few major manufacturers. The world's major BFR manufacturers are Great Lakes Chemical (USA), Albemarle Corporation (USA) and Dead Sea Bromine Group (Israel). More than 70% of the market in the USA and Western Europe are held by these companies /17/.
World consumption of brominated flame retardants increased from 1992 to 1995 from around 150,000 tonnes/y to 200,000 tonnes/y representing 22% of the world consumption of flame retardants (see table 1.2). According to an analysis from Roskill Information Services, the consumption is expected to have grown to around 254,000 tonnes/y by the year 2000. The growth is expected to be fastest in Asian countries other than Japan.
Table 1.2
Global consumption of flame retardants according to base element content 1992
/11/ and 1997 /17 /
| Base element | Market volume 1992 tonnes |
Market volume 1996 1) tonnes |
| Bromine | 150,000 |
202,000 |
| Chlorine | 60,000 |
50,000 |
| Phosphorus | 100,000 |
137,000 |
| Antimony | 50,000 |
70,000 |
| Nitrogen | 30,000 |
2) |
| Aluminium | 170,000 |
410,000 |
| Other | 50,000 |
55,000 |
| Total | 610,000 |
924,000 |
| 1) | Includes only USA, W. Europe and Asia. |
| 2) | Included in "Other". |
The global consumption of 150,000 tonnes in 1992 can, according to OECD 1994 /11/, be broken down to approx. 40% TBBPA, 20% DeBDE, 4% OcBDE, 3% PeBDE, <1,5% PBBs, and finally 34% other flame retardants. This consumption pattern has presumably changed during recent years as will be shown for the Western European market in the following.
Brominated flame retardants are primarily used in plastics. Of the world consumption, applications other than for plastics account for less than 25% /17/. Other applications are for textiles, adhesives, rubbers, paints, wood treatment and paper. Although textiles represent a growing market because of more stringent fire safety regulations, BFRs have become less popular in this sector of the market than some of the other flame retardants /17/.
Market volume figures for Western Europe according to two different market analyses are shown in table 1.3. The two analyses refer to 1996 and 1998, respectively, and estimate that brominated flame retardants accounted for about 15% and 18% of the total market volume.
Table 1.3
Western European market for flame retardants 1996 and 1998 according to two
different market analyses
| Base element | Frost & Sullivan, 1997 /19/ | IAL, 1999 /18/ | ||
Market volume 1996 tonnes |
% of total volume |
Market volume 1998 tonnes |
% of total volume |
|
| Bromine | 44,000 |
15 |
62,500 |
18 |
| Chlorine | 8,000 |
9 |
29,100 |
9 |
| Phosphorus | 75,000 |
21 |
58,000 1) |
17 |
| Antimony | 23,000 |
7 |
23,650 |
7 |
| Aluminium | 121,000 |
41 |
136,000 |
40 |
| Other | 20,000 |
7 |
31,400 |
9 |
| Total | 291,000 |
100 |
340,000 |
100 |
| 1) | Includes only .only organophosphorus compounds. Inorganic phosphorus compounds are included in 'other' |
A first estimate on the consumption with finished products in Denmark can be obtained by assuming that the Danish consumption pattern equals a W. European average. This first estimate will later in the report be compared to the estimates obtained by the more detailed assessment. The comparison is done to secure that all the main applications are covered by the detailed assessment.
The total population in W. Europe is approximately 390 million of which Denmark accounts for 5.3 million corresponding to 1.4%. The GNP per capita is a little higher in Denmark than the W. European average. If it is assumed that the consumption in Denmark accounted for 1.5% of the consumption in W. Europe, the total consumption of brominated flame retardants in Denmark 1997 should be approximately 600-800 tonnes. In the summary in chapter 6 this quantity will be compared to the result of the present analysis.
Consumption of BFRs in Western Europe
In table 1.4 the consumption by type of brominated flame retardant in Japan and Western Europe is shown.
The total consumption of brominated flame retardants in Japan 1994 was 51,450 tonnes - higher than the total consumption in Europe. The flame retardants may be used for production of compounds exported to other countries in the area.
It is noteworthy that the PBDEs take up a significant higher share of the European market in 1996 than of the Japanese in 1994. TBBPA and derivatives accounted for 65% of the Japanese market presumably reflecting the consumption of brominated flame retardants for electronics.
The market analysis will be encumbered with uncertainty and the differences between the 1996 and 1998 analyses may partly be due to the uncertainty of the analyses partly be due to changes in the consumption pattern during the period.
Table 1.4
Market volume for brominated flame retardants in Japan and Western Europe
| Flame retardant | Japan 1994 1) | Benelux, France, UK, and Germany 1996 2) | W. Europe 1998 3) | |||
Volume tonnes |
% |
Volume tonnes |
% |
Volume tonnes |
% |
|
| TBBPA | 24,000 |
47 |
9,700 |
31 |
13,150 |
21 |
| TBBPA deriv. | 9,500 |
18 |
- |
- |
3,650 |
6 |
| PBDEs | 6,000 |
12 |
8,300 |
26 |
7,050 |
11 |
| HBCD | 1,600 |
3 |
2,200 |
7 |
8,950 |
14 |
| EBTBP 4) | 2,500 |
5 |
5) |
5,250 |
8 |
|
| TBPA 4) | 5) |
1,900 |
6 |
5) |
||
| Other | 7,850 |
15 |
9,200 |
29 |
24,450 |
39 |
| Total | 51,450 |
100 |
31,300 |
100 |
62,500 |
100 |
| 1) | Based on IPCS 1997 /9/. |
| 2) | Based on Frost and Sullivan 1997 /19/. The six countries represent 76% of the total European market of halogenated flame retardants. The amount of other brominated flame retardants is derived from figures on the total halogen flame retardants market, assuming that the brominated flame retardants represented 80% of the halogens (European average). TBBPA derivatives are assumed to be included in 'Other'. No specific figures on PBBs are given in the market analysis. |
| 3) | Based on /18/. |
| 4) | EBTBP: Ethylene bis(tetrabromophthalimide. TBPA: Bromophthalic anhydride. |
| 5) | May be included in 'other'. |
According to a market analysis of Frost and Sullivan 1997, no pronounced changes in the consumption pattern are forecast for the period until 2003 /19/. The analysis of IAL consultants forecast a general increase in the market for brominated flame retardants with a minor decrease in the market for PBDEs (-1% growth p.a.) and brominated polyols (-2% growth p.a.).
A more detailed consumption estimate of the W. European market for brominated flame retardants is shown in table 1.5.
Table 1.5
Western European market for brominated flame retardants, 1998 (based on IAL
Consultants /18/)
| Flame retardant | Market volume tonnes |
% |
| Reactive 1) | ||
| TBBPA | 13,150 |
21 |
| TBBPA polycarbonate oligomer | 2,150 |
3 |
| TBBPA bis(2,3-dibromopropyl ether) | 1,500 |
2 |
| Brominated polyols 2) | 8,400 |
13 |
| Brominated epoxy oligomers 3) | 1,250 |
2 |
| Dibromoneopentyl glycol | 1,150 |
2 |
| Other reactive | 250 |
0.4 |
| Subtotal, reactive | 28,800 |
45 |
| Additive | ||
| PBDEs | 7,050 |
11 |
| PBBs | 600 |
1 |
| HBCD | 8,950 |
14 |
| Ethylene bis(tetrabromophtalimide) | 5,250 |
8 |
| Polybrominated polystyrenes 4) | 4,175 |
7 |
| Polydibromophenylene oxide | 3,250 |
5 |
| Saytex 8010 proprietary product | 2,500 |
4 |
| Polybrominated imides 5) | 850 |
1 |
| Brominated phenyl indane | 750 |
1 |
| Poly(pentabromobenzyl) acrylate | 500 |
0.8 |
| Other additive | 775 |
1 |
| Subtotal, additive | 34,700 |
55 |
| Total | 62,500 |
100 |
| 1) | Some of these flame retardants may actually be used as additives. The TBBPA derivatives are cf. OECD 1994 used as additives (see table 1.1). |
| 2) | Includes TBPA diester/ether diol and brominated polyetherpolyol in appendix 3. |
| 3) | Presumably identical to TBBPA epoxy oligomer in table 1.1 and appendix 3. |
| 4) | Include polydibromostyrene and brominated polystyrene in table 1.1 and appendix 3. |
| 5) | The market analysis says by mistake amides. |
Regional differences
According to the market analysis by IAL Consultants there are significant regional differences in the use of PBDEs. In France and the UK PBDEs accounted in 1998 for 19% and 22%, respectively, of the total market of BFRs, whereas the PBDEs only accounted for 4% of the total in Germany. On the Nordic market the consumption of PBDEs is indicated as 'small unquantifiable consumption'. In the analysis of Frost and Sullivan (1996 data), there was no significant differences in the use of PBDEs in Germany and the other European countries. This may be due to a mistake in the analysis or reflect a significant shift away from the use of PBDEs in Germany during the period 1996 to 1998.
Market volumes by base material
For about half of the market volume there is a nearly 100% connection between a single flame retardant, a polymer base material and an application. This is the fact for TBBPA (epoxy for printed circuit boards and electronic component encapsulates), brominated polyols (rigid PUR foam for insulation) and HBCD (EPS/XPS for insulation panels).
The other flame retardants may be used for a number of base materials and a number of flame retardants may be used for the same base material.
Of special interest for the estimate of BFRs with imported products in the next chapter is the distribution between PBDEs, PBBs, TBBPA derivatives and other BFRs for polyolefins and engineering plastics. Of the additive BFRs (except HBCD used for XPS and EPS) PBDEs accounted for about 24%, PBBs for 2%, TBBPA derivatives for 11% whereas other flame retardants accounted for the remaining 62%. Few years ago the PBDEs made up a significant higher part.
A detailed estimate on the market of brominated flame retardants by base materials is shown in table 1.6. For each base material the percentage of the total consumption of flame retardants that is accounted for by BFRs and antimony trioxide is additionally shown. Antimony trioxide is often used in combination with BFRs, but may for instance in PVC be used in other combinations or solely.
BFRs (in combination with antimony trioxide) are the sole flame retardants used for PBT/PET, PC and EPS/XPS. For ABS, HIPS, PA and epoxies BFRs (and antimony trioxide) account for about half or more of the consumption of flame retardants.
The consumption of BFRs for phenolics, PVC, rubbers, coatings, functional fluids (for paints) and timber is in the market analysis indicated as small and unquantifiable.
The flame retardants are predominantly used reactively in epoxies, PUR and UP. The consumption for these base materials was about 36% of the total BFR consumption.
Table 1.6
Western European market for brominated flame retardants by base material 1998
(based on IAL Consultants 1999 /18/)
| Base material 1) | Market volume tonnes |
% of total BFR volume 2) |
BFR/antimony % of total FR volume 4) |
Major applications where BFRs may be used 4) |
| PE | 3,500 |
6 |
12 |
Cable covering, pipes, sheets for transportation and construction |
| PP | 5,000 |
8 |
27 |
Pipes, sheets for transportation and construction, appliances, switchgear, video tape, film, IT housing, flooring, |
| ABS | 6,000 |
10 |
74 |
Automotive components, IT housing, electric and electronic appliances |
| HIPS | 2,500 |
4 |
48 |
Electronic appliances, switchgear, sheet, lighting, telephones, IT housing |
| UP | 1,000 |
2 |
6 |
Transportation, roof sheets, sanitary ware, switchgear, electronics. |
| PET/PBT | 6,500 |
10 |
100 |
Relays, motors, switchgear, electronics |
| PA | 3,000 |
5 |
49 |
Switchgear, fuse boxes, terminal blocks, print connectors, etc. |
| PC | 3,000 |
5 |
100 |
Electric and electronic equipment |
| EPS/XPS | 8,500 |
14 |
100 |
Insulation panels |
| Epoxies | 12,000 |
19 |
54 |
Printed circuit boards (major), encapsulation, specialist flooring on oil rigs |
| PUR | 9,500 |
15 |
19 |
Insulation panels (major) |
| Textiles | 1,500 |
2 |
5 |
|
| Other 5) | 500 |
1 |
29 |
| 1) | Appendix 2 gives a list of abbreviations. |
| 2) | Indicates the percentage of the total consumption of BFRs that are used for each base material. |
| 3) | Indicates for each base material the percentage of the total consumption of flame retardants for the base material that is made up by BFRs and antimony trioxide (antimony trioxide is mostly used as synergist in combination with BFRs). |
| 4) | The analysis gives joint information on all flame retarded grades of the plastics/textiles. Only selected applications where BFRs may be used are mentioned here (selected by the authors of this report). For some of the mentioned applications BFRs may not be used. |
| 5) | The consumption of BFRs for phenolics, PVC, rubbers, coatings, functional fluids (for paints) and timber is indicated as small, unquantifiable. |
Consumption by end products
The use of flame retardants for the production of electric and electronic equipment in Europe in 1995 has been reported in a booklet from the Association of Plastics Manufacturers in Europe /20/. The consumption of flame retarded plastics and the percentage of flame retarded plastics containing BFRs are shown in table 1.7
The assessment shows that nearly half of the brominated flame retardants used by the EEE industry in 1995 were used for consumer electronics (brown products); particularly external parts of TV sets. Of the plastics used for consumer electronics 55% was flame retarded; of this 83% with brominated flame retardants. As it will be discussed in section 2.2.2 TV sets and other consumer electronics are not estimated to account for such high share of today consumption. This is in accordance with the market volumes shown above where ABS and HIPS (traditionally used for consumer electronics external parts) only account for 14% of the total W. European consumption of BFRs.
The data on consumption of flame retardants for the production of 'electrical equipment materials', as well as small and large domestic appliances will be included in the estimate of BFR consumption with these products in Denmark in chapter 4.
Table 1.7
Consumption of plastics treated with flame retardants for production of electric
and electronic equipment in Western Europe, 1995 (after /20 /)
| Sector | Plastic parts treated with FR | Percentage of plastics parts treated with FR |
Weight of FR treated plastics (tonnes) |
% of FR plastics containing BFR |
| Brown products | For TV sets the majority of external parts are treated. Extremely low percentage for internal parts. Some epoxy and PA are treated with phosphorus. |
55% |
128,000 |
83% |
| Data processing | Apart from keyboards, the majority of monitors' external
parts are treated. Epoxy internal parts are treated. Phenolic internal parts are not treated. PBT supporting electronic circuits are treated. |
63% |
71,000 |
83% |
| Electrical equipment materials | Circuit breakers and carry fusible | 20% |
35,000 |
54% |
| Office equipment | As for 'Data processing' | 63% |
25,000 |
- |
| Small domestic appliances | Inner parts | 2% |
3,000 |
- |
| Large domestic appliances | Inner parts | 1% |
11,000 |
- |
| Medical equipment | - | - |
- |
- |
| Telecommunications | - | 0% |
0 |
- |
1.4 Emission from Products in Service
The emission of flame retardants to the in-door environment from products in service has during the last years been a focal point in the debate about the use of brominated flame retardants.
The available data do not allow detailed estimates on the emission from single product groups, but in the following the total emission from products in use in Denmark will be estimated. The estimate will only give the order of magnitude of the emission.
Emission to the air and waste water from industrial processes is included in section 2.1.5.
Significance of the emission
The significance of the emission of brominated flame retardants from office machines has be demonstrated by the detection of the compounds in the in-door atmosphere of office rooms, computer halls /22 / and control rooms /22/.
The most obvious sources of emissions to the air would be from products where the flame retardants are used as additive. Phenol-paper laminates used for printed circuit boards in consumer electronics or thermoplastic components that heat up during operation, e.g. computer monitors, could be good candidates. Unreacted flame retardants from reactive use in for instance printed circuit boards may also be emitted.
The present studies of BFRs in the in-door atmosphere cannot, however, be used for quantitative estimates of emission rates. Emission rates can be estimated from chamber experiments or may be estimated based on volatilisation models and physico-chemical properties of the compounds.
The most straight way to estimate long-term emissions of bromine compounds from the plastics would be to analyse the total bromine content of the same plastics with e.g. a 10 years intervening period. Such analyses are unfortunately not available.
Chamber experiments
Only a few chamber experiments have been performed.
Ball et al. (1991) analysed the emission of PBDEs, dibenzofurans and dibenzodioxins from three printers, two TV sets and two computer monitors /22/. The units were flushed with 85-100 m3 of air over a period of 3 days. The temperatures within the TV sets and monitors were 36-39°C and 46-48°C, respectively. Very different results were obtained from the products. Total PBDE emission from each of the two TV sets was 192 and 4 ng PBDE/unit, respectively, whereas the emission from each of the monitors was 9 and 889 ng PBDE/unit, respectively. Only small amounts of PBDEs were emitted from the printers. The TV sets and monitors emitted predominantly tetra-BDE and penta-BDE. Both compounds are present in commercial PeBDE and OcBDE. Analysis of TBBPA was not performed in the experiment. The content of flame retardants in the products was not determined, and the explanation for the low values in one of the TV sets and one monitor could be that other BFRs were used as flame retardants in these units. This is supported by the fact that no correlation between the concentration of PBDEs and dibenzofurans and dibenzodioxins was found. The highest values of dibenzofurans and dibenzodioxins were found in the monitor with an emission of only 9 ng PBDEs.
If the highest emission values of the TV set are assumed to represent a unit where PBDEs are used in the back-plate, the total content of the unit can be roughly estimated at 180 g (12% of 1.5 kg plastic in the back-plate /32/). If the emission rate of 192 ng/unit/3days is extrapolated to a total service-life of 10 years, around 0.2 g PBDEs will be emitted during the service-life. This corresponds to 0.1% of the total content.
In the PC-monitor with high PBDE emission, PBDEs are assumed to be present in the casing of the monitor. On average a PC monitor contains about 340 g PBDEs (20% of 1.7 kg plastic in the housing /32/). If the emission rate of 889 ng PBDE/unit/3days is extrapolated to a total service-life of 10 years, some 1.4 g PBDEs will be emitted during the service-life. This corresponds to 0.4% of the total content.
From a study on the formation of polybrominated dibenzofurans and dibenzodioxins sponsored by the bromine industry /23 / a few unpublished analyses of PBDE emission from TV sets are available /24/. From two TV sets on average 35 ng tetra-BDE and 27 ng penta-BDE were emitted during 24 hours of operation with 18m3 of air passing. The cabinets of the TV sets were flame retarded with DeBDE, but no data on this compound are reported.
Unreacted TBBPA in epoxy laminates
TBBPA and other BFRs when used as reactive flame retardants will be incorporated in the polymer structure and not be present as a chemical entity in the product. Unreacted TBBPA from epoxy laminates may be emitted, but analyses performed on pulverised epoxy laminates have shown that only around 4 mg unreacted TBBPA could be extracted per g of TBBPA in the laminate. This corresponds to 0.0004%. The values are - according to the authors - probably underestimated due to incomplete extraction, but the result indicates that emission of unreacted TBBPA from epoxy laminates may be insignificant in comparison to emission from phenol-paper laminates and other plastics in which the flame retardants are used as additive.
Model estimates
The experimental data shows that significant amounts of PBDEs are emitted from the appliances. Considering the few available experimental data, the emission estimate will be based on theoretical considerations.
The emission of brominated flame retardants from products in service will depend on two factors:
| Volatility of the flame retardants from the surface | |
| Migration of the flame retardants in the polymer |
Volatility
The possible emission of PBDEs per year from products in service is in the ongoing EU risk assessments estimated from the following equation:
Emission by volatilisation = 1.1 · 106 · P%
where P = vapour pressure of flame retardant (mmHg at 20°C)
The equation is derived for the loss of plasticiser additives in various plastics films, but is used in the assessments in the absence of other information.
Table 1.8 shows the vapour pressure of the compounds and the calculated annual emissions.
The emission of DecaBDE is calculated to be 0.4% over a ten year period. Compared to the above estimates from the chamber experiments of 0.1 and 0.4% per ten year, respectively, the model estimate of DeBDE seems not to be unreasonable.
Table 1.8
Vapour pressure and estimated emission of PBDEs from plastics
| Compound | Vapour pressure 1) mm Hg at 21 ° C |
Emission percentage/year |
| DecaBDE | 3.47·10-8 |
0.038% |
| OctaBDE | 4.9·10-8 |
0.054% |
| PentaBDE | 3.5·10-7 |
0.39% |
| 1) | Vapour pressure values for PBDEs are derived from the EU risk assessments. |
The above mentioned equation is derived from 'Use category document. Plastic additives'. The revised draft version of the document from 1998 /25 /, uses the following worst case emission factors for organic flame retardants.
Indoor service, volatility to the atmosphere : 0.05%
Indoor service, leaching to liquid waste: 0.05%
Outdoor service, volatility to atmosphere: 0.05%
Outdoor service, leaching to environment: 0.7%
The emission factor for organic flame retardants is taken to be similar to that of the least volatile of plasticisers and antioxidant groups.
The relatively high factor for leaching to liquid waste from indoor service seems to be a heritage from the plasticisers (in flooring) that do not apply on flame retardants. The factor for leaching to the environment from outdoor service will later be discussed in relation to flame retardants in roofing.
TBBPA
The vapour pressure of TBBPA is by Perenius 1995 stated to be 4.15·10-5 mm Hg (no temperature specification) /26/. It has not been possible to confirm the vapour pressure values from other sources. If a value of 4.15·10-5 mm Hg is put into the equation above, 46% of the content should be emitted per year. Although the calculated emission factor seems to be unreasonably high, the calculation calls for analyses of the actual emission rates of TBBPA used as additive.
Migration in the polymer
The long-term emission of flame retardants from the plastics will also be dependent on the migration of the flame retardants in the polymer. Reactive flame retardants used in thermosets are assumed to be totally bound in the polymer structure, but additive flame retardants may be considered plasticisers and will be able to migrate through the polymer structure. Migration of flame retardants to the surface of the plastics, designated "blooming" cause problem to the application of DeBDE in some polymers /27/.
Brominated flame retardants are large molecules, and the migration must be expected to be slow. No actual long-term migration rates of flame retardants in plastics have been found. In the absence of data it will be assumed that the migration is fast enough to support the evaporation of flame retardants from the surface.
Total emission
For a calculation of the total emission of BFRs from products in service in Denmark it is necessary to know the total amount of flame retardants in products in service in the society. As the consumption of the flame retardants has changed over the years, an account of the brominated flame retardants accumulated in the Danish society cannot be extrapolated from the current consumption figures.
It seems more reasonable to extrapolate the accumulated amount from European consumption figures under the assumption that the Danish consumption with end products a few years back did not differ that much from the average W. European consumption.
For the calculation it will be assumed that the accumulated amount of BFRs in products in service in 1997 corresponded to 10 years consumption. The average annual consumption is estimated at 1.5% of the W. Europe consumption in 1992 corresponding to 500 tonnes BFRs. The distribution of the flame retardants is estimated from the global distribution according to the OECD 1994. Emission factors for PBDEs are derived from table 1.8. It is in the calculation assumed that the vapour pressure of the main congener of each commercial flame retardants is representative for the commercial flame retardant, i.e. the vapour pressure of pentaBDE is used for PeBDE. The general emission factor from /25/ is used for TBBPA and other flame retardants used as additives. The emission from TBBPA and other flame retardants used as reactives is assumed to be 0. The share of TBBPA used as additive is derived from IPCS /8/, whereas the distribution for "other" BFRs are roughly estimated by the authors.
Under these assumptions the total emission is estimated at 1.5 tonnes as shown in table 1.9.
If the general value of 0.05% is applied for all flame retardants used as additives, the total amount to 1.2 tonnes.
The estimate is very uncertain. The used emission factors are considered worst case factors, and the real emission may be significantly lower. It will roughly be estimated that the right value probably will be within a factor of 10 of the calculated worst case value. The total emission is consequently estimated to be 0.2-1.5 tonnes per year.
The worst case estimates place a flag on the issue and call for more data on the emission of additive flame retardants from products in use.
Table 1.9
Estimated worst case emission of brominated flame retardants from products in
service in Denmark 1997
| Group | % of |
Accumulated in Denmark |
Emission factor |
Emission |
| TBBPA, reactive | 36 |
1,800 |
0 |
0 |
| TBBPA, additive | 4 |
200 |
0.05 |
0.1 |
| DeBDE | 20 |
1000 |
0.038 |
0.4 |
| OcBDE | 4 |
200 |
0.054 |
0.1 |
| PeBDE | 2.7 |
130 |
0.39 |
0.5 |
| Other, reactive | 17 |
830 |
0 |
0 |
| Other, additive | 17 |
830 |
0.05 |
0.4 |
| Total | 5,000 |
1.5 |
| 1) | Gives the distribution of BFRs on the world market 1992 /11/. |
| 2) | The total amount of BFRs accumulated in Denmark is assumed to correspond to 10 years consumption of 500 tonnes/year with a distribution corresponding to the world market distribution shown in column 2. |
Fate of the BFRs after emission
The major part of BFR containing products is used indoors and the emission will initially be to the indoor environment. When emitted the flame retardants are likely to adsorb to particles. The particles (dust) may adhere to surfaces within appliances, on other surfaces in the indoor environment or may be spread to the outdoor environment by airing of the rooms.
PBDEs in air samples and on atmospheric particles of rooms with a very high content of electronics (control rooms) have been analysed by Ball et al. (1992) /22/. The concentration in the dust of four analysed rooms ranged from 0.5 to 3 mg PBDEs per g dust (blank control was 0.0003 mg/g). All PBDEs from tetraBDE to decaBDE were present in significant amounts in the dust. The total PBDE concentration in the air from the four rooms ranged from 96 to 969 pg/Nm3 (blank: 0.06 pg/Nm3).
Bergman et al. has demonstrated the presence of TBBPA and PBDEs on particles in offices and a computer hall. The concentrations of the compounds were not determined. The results are the only demonstration of TBBPA release from products in use.
BFRs in dust adhered to surfaces within a TV set have been analysed by de Boer et al. (1998) /28/. The object of the study was to quantify the possible exposure of a boy, who had been watching TV and played computer games for several hours a day in a small room. Wipe from the back wall of the TV set was reported to contain 15 and 43 mg/m2 of two nonaBDE isomers and 40 mg/m2 of one DeBDE isomer. The content of the three isomers in the side wall wipe was lower. The side wall wipe was additionally analysed for heptaBDE at a content of 3 mg/m2. Circuit boards wipe was analysed for two isomers of hexaBDE at <0.4 and 0.3 mg/m2. Analyses for other PBDEs and the concentration of the compounds in the dust were not reported.
The results demonstrate that significant amounts of brominated flame retardants may be present in the dust within electronic appliances. When the appliances are dismantled for reprocessing some of the dust will be released to the workplace air. Compared to the office environment the exposure by dismantling of the appliances may be several orders of magnitude higher. The presence of brominated flame retardants in the workplace air of a recycling company is at present studied in Sweden.
Dust released from electronic appliances when dismantled is known to cause working environmental problems. In some of the Danish recycling plants the dust of TV sets is removed in a blow chamber before dismantling.
In the absence of detailed information it will here be assumed that the brominated flame retardants emitted to the air sooner or later are released to the environment, although a significant part may be disposed of to solid waste with dust in vacuum cleaner bags, etc.
Atmospheric transport
PBDEs adsorbed onto atmospheric particles will be removed from the atmosphere by wet or dry deposition. The available monitoring data indicate that long range transport via the atmosphere may be occurring for the main components of commercial PeBDE /5/.
Photolysis
Photolytic reductive debromination of DeBDE, forming lower congeners of PBDEs, has been demonstrated in experiments. In the EU risk assessment it is concluded that this reaction in the environment is likely to be small. The atmospheric half-life of DeBDE is estimated at 94 days. Removal from the atmosphere by deposition is thus assumed to be of much higher significance than photodegradation in the atmosphere.
Consequently it will in the estimate of PBDEs in rain water in section 3.4 be assumed that emitted PBDEs will be deposited without degradation.
The fate of TBBPA in the atmosphere seems to be different from the fate of the PBDEs. A half-life value of 0.12 day has been obtained for TBBPA from photodegradation experiments (Ref. in /26/ and /8/). In the absence of other information it will in the estimate on BFRs in rain water in section 3.4 be assumed that TBBPA and derivatives emitted to the air, will be degraded before they are deposited.
[Front page] [Contents] [Previous] [Next] [Top] |