[Front page] [Contents] [Previous] [Next] |
Azocolorants in Textiles and Toys
3 Health and Environment in the Consumer Situation
3.1 General remarks
3.2 Health
3.2.1 Allergy
3.2.2 Carcinogenicity
3.3 Exposure
3.3.1 Levels of aromatic amines found in samples
3.3.2 Duration of exposure
3.4 Risk
3.4.1 Cancer risk
3.4.2 Allergy risk
3.4.3 Discussion of allergy risk
3.5 Conclusion
3.1 General remarks
The following assessment is primarily based on several different reviews. However, original test reports and case reports have been scrutinized whenever deemed necessary. When reviewing the literature in the field of azo-colorants one is greatly hindered by nomenclature complexity. As Burg and Charest (1980) put it: There is a variation in use of old and established names versus modem nomenclature; there are multiple generic terminologies for identical preparations; there exists a multitude of brand names for identical preparations (sometimes even from the same manufacturer); different nomenclatures exist dependent upon the country of origin; many products have very similar names and there is a real probability of error within the literature in reporting results; there are often inadequate descriptions of combination products in which only the major dye ingredient is identified and variations in the salt form, concentration, diluents, surfactants, dedusters, pH modifiers and biocides are not reported; and there exist different materials all described with the same brand name. In some papers, several investigators have tested a particular dye twice under two different trivial names without realizing that they were actually dealing with only one dye.
Names of colorants given in the following text refer to C.I. Generic Name and, where available, C. I. Constitution No. Also where available, CAS number has been added for easier reference.
3.2 Health
The toxicological effects to be dealt with in this chapter are mainly allergy and carcinogenicity. Other effects of some azo-colorants, e.g. irritative and teratogenic effects have been descnibed, but the further investigation into these effects are beyond the scope of this report.
Although some azo dyes have induced teratogenic effects, such effects have not generally been observed across the universe of azo dyes. There is no evidence for teratogenicity associated specifically with the azo or bisazobiphenyl moiety. However, it should be mentioned that the following bisazobiphenyl dyes are teratogenic in animals (Burg and Charest, 1980):
Direct Blue 14
Direct Blue 1
Direct Blue 6
Direct Blue 15
Direct Blue 53
Afridol Blue
Direct Red 28
Direct Blue 25 (embryotoxic)
lt is beyond the scope of this report to assess the risk of these dyes exerting a teratogenic effect on humans, if the dyes are used in textiles and toys.
3.2.1 Allergy
Numerous cases of skin sensitization have been reported in the later years. From 1930 to 1988 the numbers of patients with textile dye dermatitis were small (Feinman and Doyle, 1988). From 1990 and on quite a number of cases have been described. The case descriptions found during our literature search have been summarized in Table 3.1 after checking for repeated description of the same cases. It should be noted, that most cases are due to the use of disperse dyes on synthetic fabrics which do not provide sufficient dye fastness. None of the cases found are caused by pigments, only dyes. All the cases pertain to the final users of the textiles and not textile workers.
Typical clothing mentioned in the cases are tights, panty hoses and leggings, but also wig lining, wrist watch leather straps, bed linen, T-shirts and bodies have caused reactions. Reactions are not limited to atopic individuals, and outbreaks may be severe, requiring emergency treatment and 2-3 weeks of recuperation.
From Table 1 it can be seen, that the most frequent sensitizers are Disperse Black 1, Disperse Blue 106, Disperse Blue 124, Disperse Orange 3, Disperse Orange 76, Disperse Red 1, Disperse Red 17, Disperse Yellow 3, Disperse Yellow 9, p-aminoazobenzene, and p-dimethylaminoazo-benzene. These dyes have all been described as sensitizers by two or more authors independently.
If one has acquired allergy towards a single colorant, one may cross-react to several others within the same category. For instance, people who are sensitized towards Disperse Blue 124 may cross-react to Disperse Blue 106 as they both belong to the azo-azoyl-paraphenylenediamine group. Among the groups within which cross-reactions may take place are the aminoazobenzene group (Disperse Red 1, Disperse Red 17, Disperse Brown 1 etc.), the paraphenylenediamine group (paraphenylenediamine and Disperse Orange 3) and the benzothiazol-azoyl-paraphenylenediamine group (2 dyes in Disperse Red 153) (Nakagawa et al., 1996).
Table 3.1
Cases of allergy observed for different colorants 1990-1996.
|
Name of colorant |
Number of cases |
References |
|
Acid Black 48
|
3 |
Balato et al., 1990 |
|
Acid Red 118 |
1 |
Seidenari et al., 1995 |
|
Acid Red 359 |
2 |
Seidenari et al., 1995 |
|
Acid Yellow 61 |
5 |
Seidenari et al., 1995 |
|
Basic Brown 1 |
2 |
Balato et al., 1990 |
|
Bismark Brown |
1 |
Lisboa et al., 1994 |
|
Black Acid 48 |
4 |
Seidenari et al., 1991 |
|
Black Base 1 |
9 |
Seidenari et al., 1991 |
|
Direct Orange 34 |
8 |
Seidenari et al., 1995 |
|
Disperse Black 1
|
17 |
Seidenari et al., |
|
Disperse Black 2 |
5 |
Lisboa et al., 1994, |
|
Disperse Blue 1 |
3 |
Hausen, 1993 |
|
Disperse Blue 106 |
15 |
Lisboa et al., 1994, |
|
Disperse Blue 124 |
59 |
Balato et al., 1990, |
|
Disperse Blue 3 |
4 |
Seidenari et al., 1991 |
|
Disperse Blue 35 |
9 |
Balato et al., 1990, |
|
Disperse Blue 85 |
1 |
Brown, 1990 |
|
Disperse Brown l |
2 |
Brown, 1990, Nakagawa |
|
Disperse Orange 1 |
1 |
Shehade and Beck, |
|
Disperse Orange 13 |
2 |
Brown, 1990, Nakagawa |
|
Disperse Orange 3
|
39 |
Balato et al., 1990, |
|
Disperse Orange 76 |
14 |
Balato et al., 1990, |
|
Disperse Red 1
|
46 |
Brown, 1990, Balato |
|
Disperse Red 17 |
28 |
Balato et al., 1990, |
|
Disperse Red 7 |
1 |
Sousa-Basto and Azenha,1994 |
|
Disperse Yellow 3 |
41 |
Balato et al., 1990, |
|
Disperse Yellow 54 |
3 |
Seidenari et al., 1991 |
|
Disperse Yellow 9 |
12 |
Brown, 1990, Seidenari et al., 1991 |
|
p-aminoazobenzene |
28 |
Seidenari et al.,1991, |
|
p-aminophenol |
9 |
Seidenari et al., 1991 |
|
p-dimethylamino |
19 |
Balato et al., 1990,
|
3.2.2 Carcinogenicity
There are two aspects of carcinogenicity of azo-colorants:
- The carcinogenicity of time colorant as is, and
- The carcinogenicity of the aromatic amines which may occur as degradation products upon reductive cleavage of the azo group.
Far from all azo-colorants have been tested sufficiently to determine whether they should be regarded as carcinogens or not. In Table 3.2 below is a list of those azo-colorants which should be regarded as carcinogens. In Table 3.3 is a list of those azo-colorants which are probably not carcinogenic to humans, corresponding to classification in group 4 in the IARC (International Agency for Research on Cancer, World Health Organization) evaluation system. Group 4 is used for agents or mixtures for which there is evidence suggesting lack of carcinogenicity in humans and in experimental animals. In some instances, agents or mixtures for which there is inadequate evidence of carcinogenicity in humans but evidence suggesting lack of carcinogenicity in experimental animals, consistently and strongly supported by a broad range of other relevant data may be classified in this group.
If a colorant is not mentioned on either Table 3.2 or Table 3.3 there has not been found sufficient data to allow for a classification as carcinogen or non-carcinogen. Of course, this leaves us with a large amount of azo-colorants of which we can not determine the carcinogenicity with a reasonable degree of certainty.
The data summarized in the Table 3.2 and 3.3 have been taken from review articles, since time has not allowed to review the large amount of original test reports for all these azo-colorants. Doing so, we are relying on the reviewers ability to distinguish between satisfactory and inadequate methods used in the studies.
Table 3.2
Azo-colorants which should be regarded as carcinogens.
|
Name of colorant |
Degree of |
Remarks |
References |
|
(4-Dimethylamino)- benzeneazo-1- |
2B |
Three different studies on male rats gave liver tumors, squamous carcinoma of the forestomach, and mammary tumors. |
Longstaff, |
|
(4-Dimethylamino)- |
2A |
Topically induced tumors in mice, orally induced liver tumors in rats. |
Longstaff, 1983 |
|
Acid Dye |
2B |
Rats produced metastatic liver tumors in dose-related manner. Liver tumors reported in mice. |
Longstaff, 1983 |
|
Acid Red 114 |
2B |
Probably linked to formation of the metabolite 3,3’- |
IARC, |
|
Acid Red 26 |
2B |
Carcinogenic in mouse by oral dosing causing liver tumors. No NOEL** found. |
Longstaff, 1983, Burg and Charest, 1980 |
|
Direct Black 38 |
2A |
large - benzidine based |
IARC, |
|
Direct Blue 15 |
2B |
Probably linked to formation of the metabolite 3, 3’- |
IARC, |
|
Direct Blue 6 |
2A |
large - benzidine based |
IARC, |
|
Direct Brown 95 |
2A |
large - benzidine based |
IARC, |
|
Solvent Yellow 1 |
2A |
production of skin tumors in rats following topical application |
Longstaff, 1983 |
|
Solvent Yellow 2 |
2A |
production of skin tumors in rats following topical application |
Longstaff, 1983 |
|
Solvent Yellow 3 |
1 |
Skin application in mice produces liver tumors, and topical application to pregnant mice produces tumors in F1 generation |
Longstaff, 1983 |
*IARC categories: Group 1 - The agent is carcinogenic to humans. The exposure circumstance entails exposures that are carcinogenic to humans.
Group 2A- The agent is probably carcinogenic to humans. The exposure circumstance entails exposures that are probably carcinogenic to humans.
Group 2B - The agent is possibly carcinogenic to humans. The exposure circumstance entails exposures that are possibly carcinogenic to humans.
** NOEL= No Observed Effect Level or Maximum No-Effect Dose
Table 3.3
link to tabel
Azo-colorants which should not be regarded as human carcinogens.
Azo-colorants may form aromatic amines upon reductive cleavage of one or more azo groups. Most often the aromatic amine degradation products are the same as the ones from which the azo-colorant was manufactured, but there are important exceptions. If an aromatic amine is used as the »diazo component« in the manufacturing process the degradation product will be the same aromatic amine, but not necessarily if it is used as the coupling component. Examples to illustrate this distinction are:
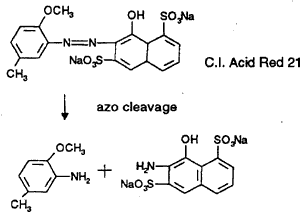
a) CI Acid Red 21, formed under coupling diazotised 6-methoxy-mtoluidine to 1 -naphthol-3,8-disulphonic acid is cleaved to 6-rnethoxy-mtoluidine, which falls under the German ordinance, and 2-amino- 1 naphthol-3,8-disulphonic acid, sodium salt:
b) CI Disperse Red 31, formed by coupling diazotised p-nitroaniline to 6-methoxy-m-toluidine is cleaved to p-nitroaniline and 4-amino-6-methioxy-m-toluidine
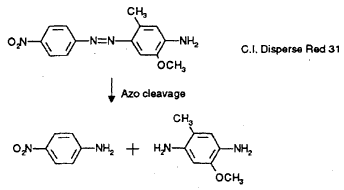
(ETAD, 1995)
The formation of carcinogenic amines is regarded to be the major causative factor of the carcinogenicity of a given dye. Typical examples are benzidine dyes, which are metabolized to the known human carcinogen benzidine (LKRC group 1). Benzidine-based dyes have Malso been placed in group 2A (probably carcinogenic to humans) by IARC. Aromatic amines which may be considered carcinogenic are listed in Table 3.4 below. The list is not exhaustive, since there is a multitude of aromatic amines, which may be formed during break-down of azo-colorants, and not all aromatic amines have been sufficiently tested to allow for an evaluation of carcinogenicity.
Table 3.4
link to tabel
Aromatic amines which may be considered carcinogenic.
All of the aromatic amines in Table 3.4 are also on the German prohibition list, except o-anisidine and p-aminoazobenzene.
5-Nitro-o-toluidine, CAS 99-55-8, and 2,4,5-Trimethyl-aniline, CAS 137-17-7, are both on the German prohibition list, but they are only classified in group 3 (not classifiable) by IARC.
Substitution alternatives
The altemative to using the aromatic amines in the manufacture of colorants could be to use the sulphonated aromatic amines instead. Jung et al. (1992) carried out a comparison of the genotoxicity and carcinogenicity data on sulphonated aromatic amines and their unsulphonated analogues. The comparison showed that the sulphonated aromatic amines generally have no or very low genotoxic effects.
3.3 Exposure
Exposure to either azo-colorants or their degradation products such as aromatic amines may take place via ingestion, e.g. by infants mouthing their toys, and by skin contact,. e.g. by rubbing or extraction via sweat.
Stomach acid
When analyzing textile samples for content of aromatic amines it makes sense to try to mimic the actual conditions of exposure. It is generally known that the environment in the human bowel is acidic, and therefore the analysis can be made on hydrochloric acid solution at pH 1.5.
Sweat
Sweat is secreted by two types of sweat glands, the small, eccrine (minores) and the larger, apocrine (majores) ones. The eccrine sweat glands are far greater in number and are found mostly in hairless skin areas, while the apócrine ones are found more in hairy regions (particularly in the armpits). Eccrine sweat is clear, watery and odorless. Apocrine sweat is cloudy, viscous, often slightly yellow and fluorescent, at times bluish or nearly black. Sterile apocrine sweat is odorless but quickly takes on a characteristic odor due to the action of bacteria.
pH of sweat
At a low rate of sweating, eccrine sweat is acid as a result of the high rate of lactic acid secretion: with increasing flow it turns alkaline owing to bicarbonate secretion. pH at low rates is 5-7, while pH at high sweat flow is 7- 8. Apocrine sweat, because of its higher ammonia content, is somewhat less acidic than eccrine sweat. Sweat from children is generally less acidic than sweat from adults and has pH 6-8 (Lentner, 1981).
These conditions can be mimicked by subjecting the textile sarnples to two different simulants: a »sour sweat« solution at pH 5.5, and an »alkaline sweat« solution at pH 8.
3.3.1 Levels of aromatic amines found in samples
A detailed account of what has been found in samples of textile goods on the Danish market is given in chapter 5.
In the Danish survey some samples have been subjected to treatment with »stomach acid« simulant, some with »sour sweat« simulant, and all of the samples have been subjected to treatment with »alkaline sweat« simulant. The latter treatment produced the largest proportion of positive findings, indicating that clothes being worn while sweating profusely for a long time give time largest exposure to aromatic amines.
Aniline was found in 13 out of 59 samples in total. Levels found were 0.4-160 mg/kg textile. The sample with the largest content was a pink cotton sheet.
Aromatic amines listed in Table 4 was found in 17 out of the 59 samples. Lowest positive finding was 0. l mg o-toluldine/kg textile and the highest finding was 70 mg o-toluldine in a child cotton sweater of dark olive color. The same sweater also had a content of a variety of the other amines listed in Table 4 and aniline and p-phenylenediamine (which is allergenic).
Benzidine or isomers hereof were found at a level of 300 mg/kg in two samples, one was a burgundy lady silk pajamas, and the other was a pair of brown cotton boxer shorts.
A recent Swedish survey of 11 pieces of children’s clothes found a content of »hazardous« azo-colorants in two out the 11 samples. The report does not state the identity and quantity of the azo-colorants, but it says that certain hazardous aromatic amines were found, which may be formed from certain azo-colorants. In addition to this a content of 27 mg of chromium was found in a pair of baby pants, and up to 0.22 mg of lead/kg and 37 mg of copper/kg was found. Also, rather high pH values, above 8 in 5 out of the 11 samples. The highest pH measured was 9.4 (Meisner, 1997). With high pH values in the clothes it is not necessary to sweat alkaline sweat in order to extract aromatic amines. The exposure can come about just by to spilling water, food or the like on the clothes, as children often do.
3.3.2 Duration of exposure
Textiles for clothing will normally provide a peak exposure the first time they are worn, if they are not laundered by the consumer before wearing. Shoes will, however, provide a constant exposure, since shoes are rarely laundered. Since feet can get very sweaty, and provide an alkaline environment there is a good chance of extracting aromatic amines from any azo colorants used in the shoe material. Unfortunately, we have not had access to results of test for amines carried out on shoes, be it leather ot other material.
ETAD (1997) has carried out a study on extractability of dyestuffs from textiles over a normal lifetime of use, which has been set at 50 wearing-washing cycles for any piece of textile. Three disperse dyes, Disperse Yellow 3, Disperse Blue 3 and Acid Red 114 were chosen to dye polyamide for the experimental study. This is to be regarded as a worst case study since deep shade dyeing with disperse dyes on polyamide gives poor fastness. The study showed that measured migration was much lower than what could be predicted when applying the following default model proposed by the German BgVV:
External exposure (predicted) in µg/kg/day =
1 m2 (textile) x D x 50 x 0.001 x 106 µg/kg bw./day
70 kg (person)
where
| D 50 0.001 |
dyeload (g/m2) number of wearings default migration value (0.1%/day) |
The study also showed that:
| for a typical reference dyeing strength, a fastness of approximately 4 results in an average exposure of ca. 1 µg /kg bw./day, | |
| the predicted average exposure calculated on the basis of 0.1% migration of the dyestuff can be as high as 2500 µg/kg bw./day, and | |
| that the measured amount of migrating dyestuffs declines over the normal lifetime of use. |
One of the reasons for the high discrepancy between measured average exposure and predicted average exposure could be that due to alkalinity of laundering agents the free aromatic amines and surplus dyes are extracted fairly rapidly.
3.4 Risk
3.4.1 Cancer risk
In connection with the findings of aromatic amines in toy animals the risk of cancer was calculated by the National Food Agency of Denmark (1996). Under the preconditions that a child is exposed to a dose of 0.1 mg of o-toluidine, corresponding to the maximum finding in 1 kg of toy animal, the calculated risk is 1-2 x
Due to limitations in time and data, risk calculations for other relevant amines have not been carried out. However, it should be borne in mind that the carcinogenic potency of o-toluidine is relatively small, compared to some of the other aromatic amines as e.g. benzidine. Furthermore, there is an estimated uncertainty on the risk calculations for o-toluidine of a factor 10, compared to other linear models.
The US-EPA (IRIS, 1997) has calculated the risk of cancer from oral exposure to benzidine expressed as a slope factor of 2.3 x
Therefore, applying the principle of caution, a general limit of 0.1 mg aromatic amines/kg textile and a limit specifically for benzidine as »not detectable« by the most sensitive analytical method available is hereby recommended.
3.4.2 Allergy risk
The risk of getting allergic reactions to certain azo dyes and amines from textiles must be regarded as substantial, deeming from the number of cases recently reported by several independent authors in different countries in Europe and Japan.
The most frequent sensitizers reported are Disperse Black 1, Disperse Blue 106, Disperse Blue 124, Disperse Orange 3, Disperse Orange 76, Disperse Red 1, Disperse Red 17, Disperse Yellow 3, Disperse Yellow 9, p-aminoazobenzene, and p-dimethylaminoazobenzene. Contents of Phenylendiamines, such as those found in our survey of textiles on the Danish market, may also cause allergic reactions, either directly or as cross-reactions owing to allergy to Disperse Orange 3 for instance.
3.4.3 Discussion of allergy risk
When characterizing risk, both the probability of the effect and the severity of time effect should be considered.
Probability
A quantitative risk assessment would require that we have some knowledge of the number of people exposed to textiles with the dyes, which have resulted in cases of allergic reactions, namely contact dermatitis.
The risk would then be expressed as:
percentage of people expected to react after exposure to azo-dye in textile.
However, we do not have access to data on the number of people exposed. Information on e.g. »number of panty hoses produced with the azo-dyes in question« is not obtainable. Hence, we are not able to express the risk assessment numerically.
On the other hand, judging from our experience with numerous literature searches for allergic cases resulting from exposure to other chemicals than azo-dyes the number of cases found in this study is relatively large. This indicates that we are dealing with a substantial risk, i.e. a risk, which is real, and which is not negligible. If we had found only a few cases of reactions per dye substance, we would have called the risk negligible. We do not know if the risk is »small«, »considerable« or »large«, since we have no idea of the number of people actually exposed nor the actual number of people reacting allergic after exposure. We only know that the people who had reactions have been exposed at least twice, unless we are dealing with cross reactions. It should be noted that there is a clear causal relationship. All cases have been confirmed as far as it is possible with the identification of azo-dyes.
The severity of the effect
Concerning the nature of allergic contact dermatitis it should be noted that:
| Reaction to allergens do usually not occur unless the person has had previous exposure to the specific allergen. The first - and maybe several - exposures is called the sensitization phase during which the immune system is primed for recognition of the allergen. Patients are often surprised to learn that substances to which they have been exposed for years suddenly are identified as the cause of an allergic reaction. | |
| Reactions during first-time exposure may occur as a result of cross-sensitizations due to close resemblance of the allergens in question. If, for instance, one is sensitized to p-phenylendiamine in hair dyes, a cross reaction to Disperse Orange 3 may occur. | |
| When a person is sensitized it usually requires a smaller amount of allergen to elicit the reaction than it took to induce the sensitization. This agrees nicely with the following scenario: a person wears a dyed garment a few times before washing it for the first time. During this period the peak exposure takes place. After wash there is only little surplus dye left in the garment, but maybe enough to elicit a reaction, or a reaction may not be elicited until the person is exposed to another garment providing a peak exposure. | |
| Sensitization lasts for a life time. Desensitization cures (vaccinations) are only feasible for proteinaceous allergens such as pollen or animal hair, and in such cases only with a success rate of about 50%. However, the symptom, i. e. the contact dermatitis, may be relieved by medical treatment and/or avoidance of exposure to the allergen, if at all possible. |
Azo-dye allergy in perspective
It seems peculiar that the literature search came up within many cases world wide whereas such cases are rarely reported in the Nordic countries. This may be due to a number of reasons of which at least five should be mentioned: First, azo-dyes are not part of the standard test kit used in dermatology clinics. Second, azo-dye contact dermatitis might be subject to publication bias. Some clinicians find cases worthwhile reporting, while others do not. However, the lack of published cases from the Nordic countries does not disqualify the findings in other countries. Third, many cases of contact dermatitis are never resolved, and the causal agent remains unknown. If the medical treatment works, and the patient does not return with further eruptions, there is no cause for costly and uncomfortable further investigations. Fourth the use of hair dye containing p-phenylendiamine may be more prevalent in Southern Europe giving rise to cross-sensitization, which may account for some of the cases. Fifth, the textile quality available and the pattern of use in other countries may be different.
According to Menné (1998) maybe less than five cases a year are seen in one clinic in Denmark. For a clinician who sees a lot more cases caused by other chemicals, such as perfume or nickel, the few cases caused by azo-dyed textiles looks like a relatively small incidence.
When considering the incidence, it should be borne in mind, that although we have established that there is a substantial risk, the risk will not show up as cases if exposure ceases. The offending dyes are not reported as those commonly sold by the European Dye Manufacturers. Of course, textiles dyed with the offending dyes may still be imported from outside Europe. We know that in the period up to 1988 textile dye dermatitis was not considered a problem, and the numbers of patients with textile dye dermatitis was denoted as »small«.
For the period of 1990 - 1996 we have found a relatively high number of cases resulting from exposure to azo dyes via textile contact. We do not know if the number of cases in the future is going to increase, stagnate or decrease. Administratively, there has not been set any acceptability limit for allergic risk, as there has been for cancer risk.
3.5 Conclusion
Judging from contents of aromatic amines found in the present Danish survey the risk of getting cancer from exposure to azo-dyed textiles is small, but existing. However, the majority of azo-colorants and amines have not been adequately tested.
The risk of getting allergies against some of the azo dyes may be substantial, especially if the consumer does not launder the textiles before wearing them, since it is the first wearing which provides the peak exposure that usually causes sensitization.
The most frequent sensitizers reported are Disperse Black 1, Disperse Blue 106, Disperse Blue 124, Disperse Orange 3, Disperse Orange 76, Disperse Red 1, Disperse Red 17, Disperse Yellow 3, Disperse Yellow 9, p-aminoazobenzene, and p-dimethylaminoazobenzene. Contents of Phenylendiamines, such as those found in our survey of textiles on the Danish market, may also cause allergic reactions, either directly or as cross-reactions owing to allergy to Disperse Orange 3 for instance.
Alternatives to the azo-dyes giving off the offending aromatic amines could be sulphonated azo dyes (with a few exceptions), since the sulphonated analogues to the aromatic amines do not seem to possess the same carcinogenic potential.
[Front page] [Contents] [Previous] [Next] [Top] |