Toxicological Evaluation and Limit Values for 2-Ethylhexyl acrylate, Propylene carbonate, Quaternary ammonium compounds, Triglycidyl isocyanurate, and Tripropyleneglycol diacrylate
Contents
Principles for setting of limit values for chemical substances
Preface
This series of reports constitutes a part of the work related to the setting of health based limit values for chemical substances in air, soil and drinking water.
In this report, the toxicological documentation for the setting of limit values for 2-ethylhexyl acrylate, propylene carbonate, quaternary ammonium compounds, triglycidyl isocyanurate, and tripropyleneglycol diacrylate are presented.
For every substance, the following items are considered:
| part 1, | physicochemical properties, production and uses, environmental occurrence and fate, and human exposure |
| part 2, | toxicokinetic properties and toxicological mechanisms |
| part 3, | human toxicity |
| part 4, | animal toxicity |
| part 5, | regulations and limit values in different media |
| part 6, | summary of sections 1 to 5 |
| part 7, | evaluation of toxicity and identification of critical effects |
| part 8, | estimation of tolerable daily intake (TDI) and health based limit values |
| part 9, | implementation of health based limit values to quality criteria |
The work has been carried out by the Institute of Food Safety and Toxicology, Danish Veterinary and Food Administration as a contract work for the Danish Environmental Protection Agency.
The work has been followed by a Steering Committee who has contributed to the work with professional expertise, proposals and criticism:
Linda Bagge, Chairman, Danish Environmental Protection Agency
Poul Bo Larsen, Danish Environmental Protection Agency
Erik Thomsen, Danish Environmental Protection Agency
Hans Chr. Ellehauge, Danish Environmental Protection Agency
Anders Carlsen, Medical Health Office for Viborg County
Elle Laursen, National Board of Health
Ole Ladefoged, Institute of Food Safety and Toxicology
Elsa Nielsen, Institute of Food Safety and Toxicology
Principles for setting of health based limit values for chemical substances
In the following, the principles upon which the Danish Environmental Protection Agency bases the health based limit values, in the following referred to as limit values, for chemical substances are briefly outlined. For further and more specific information, the reader is referred to the references mentioned below.
Purpose
The purpose of setting limit values for chemical substances is to prevent health hazards in the human population caused by chemicals as pollutants. The scientific method for setting of limit values comprises a hazard identification and hazard assessment which together with an exposure assessment constitute the risk assessment part in the proces of setting limit values.
Selection of data
Data concerning exposure and harmful effects of a chemical substance are collected from national and international criteria documents, monographs and original scientific literature. During the review of the data, the quality and reliability of the studies and research work are critically assessed. This is an important step since conflicting viewpoints regarding the hazards may be present. Unpublished data from industry or other sources are only seldom used, as such data have not been published in scientific journals and have not been subjected to critical review by other scientists.
If adequate human data are available these are preferred as the basis for the assessment. For most substances however, human data are not adequate or available. In these cases, limit values are based upon data from experimental animal studies.
When all the relevant data have been evaluated, the hazard considered most important - "the critical effect" - for setting the limit value, is identified. In this step it is assessed whether an effect should be considered as adverse and of relevance to humans.
A substance may have different effects at different concentrations or doses. Generally, the effects are of more concern the lower the concentration or dose at which they occur, and the effect observed at the lowest concentration or dose often forms the basis for setting the limit value.
Threshold chemicals, NOAEL or LOAEL
The next step for assessment of a limit value is to identify the "no observed adverse effect level" (NOAEL) which is the highest dose at which the critical effect was not observed or, in cases where a NOAEL cannot be identified, the "lowest observed adverse effect level" (LOAEL) which is the lowest dose at which the critical effect was observed.
TDI / safety factors
Having identified a NOAEL or a LOAEL, three "safety factors" (SF) are used to extrapolate from NOAEL or LOAEL to the tolerable daily intake, TDI (expressed in mg/kg b.w. per day) or the limit value for air, LVair, (expressed in mg/m3). The purpose of the safety factors is to take into account the fact that:
| SF1: | The toxicological effect of a chemical substance on animals need not reflect the toxicological effect on "normal" humans, this factor is historically set at 10. | |
| SF2: | The toxicological effect of a chemical substance may vary considerably between different persons, and that i.e. children, elderly or sick people may be much more sensitive to exposure than "normal" people, this factor is often set at 10. | |
| SF3: | The data may be of varying quality and relevance to the actual problem, this factor is set at a value from 1 to 1000 depending on a concrete evaluation. |
Thus in cases where a threshold value for the toxic effect is assumed and a NOAEL or a LOAEL can be identified, the TDI or the LVair are obtained by the following calculation:
Exposure routes
In general, limit values for air are based upon data from inhalation studies and limit values for soil (LVsoil) and drinking water (LVdw) are based upon data from oral studies. However, if data for the relevant exposure route are not available, data from alternative exposure routes may be used as well, although it is realized that the degree of uncertainty may increase. This will then influence the value of the SF3.
Analogy
In cases where no data on harmful effects are available, an evaluation may be made upon the basis of data for related substances and a consequent increase in the value of the SF3.
Non threshold chemicals
For chemical substances where a threshold value for the toxic effect cannot be assumed (i.e. genotoxic carcinogenic substances), the concept of lifetime risk is applied. Thus, for these potential carcinogenic substances, the TDI corresponding to a specific lifetime risk, is calculated upon the basis of animal studies by means of the "One Hit" model. A lifetime risk of 10-6 (life-time exposure to the dose that may lead to cancer for one in a million) is considered as tolerable.
Exposure air, water, soil
Having obtained the tolerable daily intake for a chemical substance, the limit values for drinking water and soil are calculated taking into account the daily exposure from the various media. The following exposure standard estimates for the various media are used in the calculation of limit values:
| Soil* oral intake |
Soil* dermal contact |
Air inhalation |
Water oral intake |
|
| Child, 10 kg average/maximum |
0.2 / 10 g | 1 / 10 g | 10 / 12 m3 | 1 / 2 liter |
| Adult, 70 kg average/maximum |
0.025 / 0.1 g | 0.1 / 1 g | 20 / 30 m3 | 2 / 4 liter |
*For the soil exposure estimates, it has to be emphasized that these are based upon exposure scenarios which cover the most sensitive applications, e.g. domestic gardens, play grounds or kindergartens.
To ensure that the total daily intake of a chemical substance from the various media does not exceed the tolerable daily intake, a certain percentage of the tolerable intake to the various media may be assigned (allocation).
Limit values
The limit value for soil and drinking water are obtained by the following calculations:
*TDI or a percentage of the TDI (allocation)
w: body weight for a child (10 kg) or an adult person (70 kg)
C-value, quality criteria
Finally, the limit values are used as the basis for the setting of quality criteria for soil, drinking water, and air (C-values). In this step, other than health based viewpoints may be taken into account. This may include aesthetical factors such as odour (all media), discoloration (soil, drinking water), taste and microbial growth (drinking water). Furthermore, economic or political administrative factors may be taken into account.
It has to be stressed that no ecotoxicological considerations are taken into account in the process of setting health based limit values.
References
Industrial Air Pollution Control Guidelines. Vejledning fra Miljøstyrelsen Nr. 9 1992. Ministry of the Environment, Denmark, Danish Environmental Protection Agency.
Health Based Evaluations of Chemical Substances in Drinking Water. Vejledning fra Miljøstyrelsen Nr. 1 1992. Ministry of the Environment, Denmark, Danish Environmental Protection Agency. In Danish.
Risk Evaluation of Contaminated Sites. Miljøprojekt Nr. 123 1990. Ministry of the Environment, Denmark, Danish Environmental Protection Agency. In Danish.
2-Ethylhexyl acrylateand estimation of a limit value in air.
Pia Berthelsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
| Molecular formula: | C11H20O2 |
Structural formula:
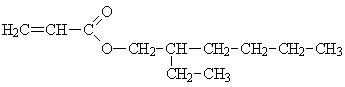
| Molecular weight: | 184.3 |
| CAS-no.: | 103-11-7 |
| Synonyms | Acrylic acid, 2-ethylhexyl ester 1-Acryloyloxy-2-ethyl-hexan 3-Acryloyloxymethyl-heptan 2-Ethylhexyl 2-propenoate 1-Hexanol, 2-ethyl-, acrylate Octyl-acrylate 2-Propenoic acid, 2-ethylhexyl ester 2-Propenoic acid, octyl ester |
1.2 Physical / chemical properties
| Description: | Colourless liquid with a sharp and musty odour. |
| Purity: | 99.5% |
| Melting point: | -90° C |
| Boiling point: | 213-218° C |
| Density: | 0.887 g/ml (at 20° C) |
| Vapour pressure: | 0.14 mmHg (19 Pa) at 20° C |
| Concentration of saturated vapours: | 184 ppm (calculated) at 20° C and 760 mmHg. |
| Vapour density: | 6.35 (air = 1) |
| Conversion factor: | 1 ppm = 7.66 mg/m3 20°
C 1 mg/m3 = 0.130 ppm 1 atm |
| Flash point: | 82-92° C (open cup), 86° C (closed cup) |
| Flammable limits: | 0.8-6.4 (v/v% in air) |
| Autoignition temp.: | 252° C |
| Solubility: | Water 0.1g/l (at 20° C). Soluble in alcohols, ethers, and many organic solvents (acetone, benzene, ethyl ether, heptane, methanol, carbon tetrachloride). |
| logPoctanol/water: | 3.67 - 4.32 |
| Henry’s constant: | 3.54 x 10-4 (atm x m3)/mole at 20° C. |
| pKa-value: | - |
| Stability: | Polymerises readily unless inhibited. Rapid, uncontrolled polymerisation can cause explosion. Reacts readily with electrophilic, free-radical, and nucleophilic agents. |
| Incompatibilities: | - |
| Odour threshold, air: | 0.55-1.36 mg/m3 |
| References: | BUA (1994), HSDB (1999), IARC (1994), ICSC (1993), IUCLID (1996), Ruth (1986) |
Direct, acid-catalysed esterification of acrylic acid with 2-ethylhexanol is the principal method for the manufacture of 2-ethylhexyl acrylate. A polymerisation inhibitor is added. (IARC 1994).
The major current use of 2-ethylhexyl acrylate is in acrylic pressure-sensitive adhesives. An adhesive for general purpose tape typically contains about 75% 2-ethylhexyl acrylate. Other uses of 2-ethylhexyl acrylate is in the production of plastics, latex, paints, textile and leather finishes, coatings for paper and industrial metal finishing. (HSDB 1999, IARC 1994).
In Denmark, the principal use of 2-ethylhexyl acrylate is in UV curable inks, lacquers and varnishes. Emission occurs in the form of aerosols.
2-Ethylhexyl acrylate is not known to occur as a natural compound. It may be released into the environment in fugitive and stack emissions or in wastewater during its production and use. (HSDB 1999, IARC 1994).
Air
2-Ethylhexyl acrylate is expected to exist almost entirely in the vapour phase based on its vapour pressure. It may photolyse in sunlight. It will react with photochemically produced hydroxyl radicals and ozone with an estimated half-life of 10.3 hours. (HSDB 1999).
Water
2-Ethylhexyl acrylate is not expected to adsorb to sediment or suspended particulate matter. It may hydrolyse, especially in alkaline waters based upon hydrolysis data for the structurally similar ethyl acrylate. It may photolyse in sunlight. It may biodegrade based upon the biodegradability of butyl acrylate and ethyl acrylate. It will significantly volatise from water with an estimated half-life of between 7.3 hours and 2.7 days. (HSDB 1999).
Soil
2-Ethylhexyl acrylate is expected to exhibit moderate mobility in soil and, therefore, it may leach to groundwater. It may hydrolyse, especially in alkaline soils based upon hydrolysis data for the structurally similar ethyl acrylate. It may biodegrade based upon the biodegradability of butyl acrylate. It may volatilise from near surface soil and other surfaces. (HSDB 1999).
Bioaccumulation
According to HSDB (1999) 2-Ethylhexyl acrylate is not expected to bioconcentrate in aquatic organisms. However BUA (1994) is stating that considerable bioaccumulation is to be expected.
The most probable route of human exposure of 2-ethylhexyl acrylate is by inhalation of contaminated air especially at plants where it is manufactured and used. Workers also may be exposed dermally during spills or leaks. (Samimi & Falbo 1982).
Inhalation
Urinary excretion of metabolites following inhalational exposure indicates absorption occurring by this route (Vodicka et al. 1990).
Oral intake
Excretion of a radioactive marked dose following oral exposure indicate absorption occurring by this route (Sapota 1988).
Dermal contact
No data were found.
Intraperitoneal application
In one study male Wistar rats were administrated an intraperitoneal dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons (Gut et al. 1988). In another study male Wistar albino rats were administrated an intraperitoneal dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate (Sapota 1988). In both studies plasma radioactivity concentration reached a peak level at about 2-3 hours after administration indicating easy absorption through this route. In tissues the highest concentrations of radioactivity was found in kidney, liver, spleen and the lungs. In the study with a dose of 100 mg/kg bw 6.5% of the dose was found in tissues at 3 hours after administration. The radioactivity in the tissues decreased slowly with time. At 72 hours after administration 1% of the dose was still found in the examined tissues. The radioactivity in adipose tissue and sciatic nerve was still relatively high.
Intravenous application
In one study male Wistar rats were administrated an intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons (Gut et al. 1988). In another study male Wistar rats were administrated an intravenous dose of 10 mg/kg bw or 50 mg/kg bw of (14C)-2-ethylhexyl acrylate (Cikrt et al. 1986). The highest concentrations of radioactivity in tissues was found in kidney, liver, brain, thymus and spleen.
Metabolism
2-Ethylhexyl acrylate is believed to undergo carboxylesterase-catalysed hydrolysis to 2-ethylhexanol and acrylic acid, like other acrylate esters (Cikrt et al. 1986, Miller et al. 1981 - quoted from IARC 1994).
2-Ethylhexyl acrylate to a minor extent reacts with non-protein SH groups in for instance glutathione causing depletion of the non-protein SH groups and excretion of thioethers in urine as described in the following studies:
Male Wistar rats exposed by 6 hours inhalation to 2-ethylhexyl acrylate in concentrations from 250 to 4800 mg/m3 over 24 hours excreted thioethers in urine in a dose dependent manner decreasing from 8.0 to 3.0% (at 1000 mg/m3) of the dose of 2-ethylhexyl acrylate indicating saturable metabolism along this pathway. Dose related depletion of non-protein SH groups in blood, liver and brain was seen at concentrations of and above 2400 mg/m3. (Vodicka et al. 1990).
When male Wistar rats were administrated an intraperitoneal dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons 2% of the dose was found as thioethers in the urine (Gut et al. 1988).
The principal eliminated metabolite in expired air was carbon dioxide in two studies in male Wistar rats given an intraperitoneal or intravenous dose of (14C)-2-ethylhexyl acrylate (Gut et al. 1988, Sapota 1988).
When 2-ethylhexyl acrylate and its metabolite acrylic acid reacts with the reduced form of glutathion (GSH), mercapturic acids can be formed (Cikrt et al. 1986).
Excretion
Two mercapturic acids N-acetyl-S-(2-carboxyethyl) cysteine and N-acetyl-S-2-(2-ethylhexyloxycarbonyl)ethyl-cysteine is excreted in the bile and urine (Cikrt et al. 1986, Kopecký et al. 1985 - quoted from IARC 1994). Besides some unidentified metabolites have been detected in the bile of rats (Cikrt et al. 1986).
Male Wistar rats were administrated an intravenous dose of 10 mg/kg bw or 50 mg/kg bw of (14C)-2-ethylhexyl acrylate. Biliary excretion of radioactivity was followed in 1-3 hour intervals within the first 24 hours after administration. A significant increase in bile flow (243%) was observed. In the 24-hours 2.2% of the dose was eliminated via bile at both doses, most of it (83%) during the first 3 hours. (Cikrt et al. 1986).
Male Wistar rats were administrated an intraperitoneal or intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons. Within the first 24 hours about 50% of the dose had been excreted in expired air, 7-13% in urine and less than 0.01% in faeces. (Gut et al. 1988).
Male Wistar albino rats were administrated an intraperitoneal or oral dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate. Within the first 72 hours more than 90% of the dose had been excreted (78% in expired air, 10% in urine, and 3% in faeces for intraperitoneal application and 50% in expired air, 41% in urine, and 1% in faeces for oral application). (Sapota 1988).
Half-life
Male Wistar rats were administrated an intraperitoneal or intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons. Elimination of radioactivity from blood was bi-exponential. The plasma half-life for the distribution phase was 60 minutes (i.p.) and 30 minutes (i.v.) for 4 months old and 130 minutes (i.p.) and 115 minutes (i.v.) for 7 months old. For the elimination phase, the half-life was 6 hours (i.p.) and 5 hours (i.v.) for the youngest rats and 14 hours (i.p. and i.v.) for the oldest. (Gut et al. 1988).
Male Wistar albino rats were administrated an intraperitoneal dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate. Elimination of radioactivity from blood had a monophasic character. The plasma half-life was about 22 hours. The half-life for excretion was calculated to be about 1½ hour. (Sapota 1988).
No data were found.
3 Human toxicity
Inhalation
No data were found.
Oral intake
No data were found.
Dermal contact
Seven persons have developed allergic contact dermatitis due to an acrylic based adhesive tape. Patch-testing revealed that all persons reacted to 2-ethylhexyl acrylate. Five of the persons were further tested for cross-sensitisation patterns. They all reacted to 2-ethylbutylacrylate and some of them reacted to other acrylates as well. (Jordan 1975).
No cases of respiratory sensitisation have been reported.
In Finland, 5 cases of occupational contact urticaria caused by 2-ethylhexyl acrylate has been reported from 1990 to 1994 (Kanerva et al. 1996 - quoted from Toxline pre1981-1999).
Generally acrylates are potent contact allergens with polyfunctional acrylates and epoxyacrylates being the strongest and polyfunctional methacrylates and cyanoacrylates being the weakest. A lot of the acrylates cross react. The Danish National Institute of Occupational Health (AMI) has made a list of allergens. It contains about 65 acrylates all causing contact sensitisation. Two of the acrylates (2,3-epoxypropyl-acrylate and methylmethacrylate) also cause respiratory sensitisation. Several epoxy compounds cause respiratory sensitisation so the epoxy-group in 2,3-epoxypropyl-acrylate might be the cause of its allergic effect on the respiratory system. (AMI 1990). In the Nordic countries 23 acrylates were classified for the ability to cause sensitisation by skin contact and were labelled with R43. (Nordisk Ministerråd 1991).
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
No data were found.
4 Toxicity, animal data
Inhalation
The LC50-value for mice is greater than 7700 mg/m3 (BASF 1967 - quoted from IUCLID 1996).
When rats were exposed to a saturated atmosphere (about 1400 mg/m3) of 2-ethylhexyl acrylate for 8 hours no mortality occurred (BASF 1958 - quoted from IUCLID 1996).
Alderley Park rats (2 animals of each sex per group) were exposed to 2-ethylhexyl acrylate in ethanol at 375 and 1000 mg/m3 6 hours a day, 5 days per week for 2½ week. A reduced body weight gain, lethargy, and dyspnoea were observed in high-dose animals. No changes in blood, urine and pathology were observed. Low-dose animals showed no toxic signs. (Gage 1970 - quoted from IUCLID 1996).
Oral administration
The reported oral LD50-values for 2-ethylhexyl acrylate ranged from 4.4 to 12.8 g/kg for rats (5 values reported), and from 4.4 to greater than 5.0 g/kg for mice (2 values reported). Rabbits have an oral LD50-value greater than 3.5 g/kg and the value for cats is greater than 1.8 g/kg (1 value reported for each species). (Studies quoted in IUCLID 1996, Clayton & Clayton 1994, DPIMR 1981, BUA 1994).
Rabbits (1 animal per sex) were fed 2-ethylhexyl acrylate as a 10% emulsion through a tube at a dose of 1774 mg/kg for 6 days (male) or 8 days (female). Four female rabbits were fed a dose of 887 mg/kg for 10 days. The high-dose animals died after 6 or 8 days. A reduced body weight gain, lack of desire to eat, and weak muscle tonus were observed and the gastric mucosa and kidneys were damaged. Low-dose animals only showed momentary lack of desire to eat and a slightly reduced body weight gain. (BASF 1960 - quoted from BUA 1994).
Dermal contact
The dermal LD50-value for rats is greater than 12 g/kg (1 value reported). For rabbits the value is between 7.5 and 16 g/kg (8 values reported), and for the guinea pig it is greater than 8.8 g/kg (2 values reported). (Studies quoted in IUCLID 1996, Clayton and Clayton 1994, DPIMR 1981, BUA 1994).
Skin sensitisation
Female Dunkin Hartley outbred guinea pigs were induced with intradermal injections of 2-ethylhexyl acrylate in concentrations of 0.5 M or 0.17 M in Freund´s complete adjuvant three times during 9 days. Sensitisation was observed when the animals were challenged at day 21, 35 and 49 with 1 M 2-ethylhexyl acrylate applied epicutaneously. At an induction concentration of 0.5 M 6-11 out of 16 animals were sensitised. At an induction concentration of 0.17 M 11-13 out of 16 animals were sensitised. Four control animals were sensitised (3 at day 35 and 1 at day 49). Cross reaction was seen with ethyl acrylate, n-butyl acrylate and hexyl acrylate. (Waegemaekers & van der Walle 1983).
Guinea pigs were induced with 0.1% (w/v) 2-ethylhexyl acrylate applied epicutaneous or intracutaneous 3 times a week for 3 weeks. Sensitisation was observed when the animals were challenged at day 11 after the induction with the same concentration of 2-ethylhexyl acrylate as used for the induction. For the epicutaneous test, 10 out of 10 animals were sensitised. For the intracutaneous test, 7 out of 10 animals were sensitised. (Hunter et al. 1966 - quoted from Nordisk Ministerråd 1991).
In the Polak method, 6 Hartley outbred guinea pigs of either sex were induced with 1 mg 2-ethylhexyl acrylate in Freund´s complete adjuvant applied as injections in the footpads and the neck. Sensitisation was observed when the animals were challenged at day 7 after the induction with 1% or 5% 2-ethylhexyl acrylate. (Parker & Turk 1983).
Eye contact
2-Ethylhexyl acrylate was non-irritating to rabbit eyes in a study done by the Swedish military in accordance to OECD Guidelines 405 (Koch et al. 1985 - quoted from Toxline pre 1981-1999).
2-Ethylhexyl acrylate was irritating to rabbit eyes in a study done by BASF (BASF - quoted from IUCLID 1996).
Inhalation
Wistar rats (10 animals of each sex per group) were whole-body exposed (OECD-guideline 413) to 2-ethylhexyl acrylate vapours at 75, 225 and 750 mg/m3 6 hours a day, 5 days per week for 90 days. No animals died in any dose-group. In mid and high-dose animals, a reduced body weight gain, lethargy and reduced levels for albumin were observed; the olfactory epithelium of the nasal mucosa was degenerated; and female rats had reduced levels of total protein and glucose. Besides high-dose female rats had a higher level than normal of liver enzymes. Low-dose animals showed no toxic signs. (BASF 1989 - quoted from IUCLID 1996 and BUA 1994).
Mice were exposed to 2-ethylhexyl acrylate at 103 mg/m3 for 4.5 months. Exposure time and interval is not stated in this Russian study. Respiratory tract irritation, dyspnoea, increased level of liver enzymes and reduced diuresis were observed. The study states a limit value of 10 mg/m3 for inhalation and a limit value of 46 mg/m3 for the effect on CNS. (Lomonova 1982 - quoted from IUCLID 1996).
Oral administration
No data were found.
Dermal contact
There was an apparent increase in the frequency of chronic nephritis in C3H/HeJ mice (68%) treated three times a week for their lifetime with 20 mg 75% (v/v) 2-ethylhexyl acrylate in acetone applied to clipped dorsal skin compared to the negative control (15%). Survival was not affected by the treatment with 2-ethylhexyl acrylate. (DePass et al. 1985).
100 ml of 86.5% 2-ethylhexyl acrylate in acetone applied 3 or 5 times a week for 16 or 68 days to female NMRI mice caused no skin changes in the short term experiment but skin irritation in the 68 day experiment. (BASF 1981 - quoted from IUCLID 1996).
Male NMRI and C3H/HeJ mice (10 animals per group) exposed to doses of 25ml 21% or 86.5% 2-ethylhexyl acrylate in acetone 3 times a week for 3 months exhibited skin irritation. The NMRI mice were less sensitive than the C3H/HeJ mice. No skin irritation were observed for the NMRI mice given 21% 2-ethylhexyl acrylate. (BASF 1985 - quoted from IUCLID 1996).
In long term (2 years or for life) carcinogenicity studies with 2-ethylhexyl acrylate applied to NMRI or C3H/HeJ mice (80 animals per group) 3 times a week, skin irritation (scaling, scabbing, hyperkeratosis, hyperplasia, crust formation and ulceration) was observed. Survival were not affected by the treatment with 2-ethylhexyl acrylate and no systemic effects were seen. (Mellert et al. 1994, Wenzel-Hartung et al. 1989). The lowest dose administered to the C3H/HeJ mice was 25 ml of a 2.5% (w/w) solution of 2-ethylhexyl acrylate in acetone. Skin irritation was observed at this dose, however, after the 11th week of treatment, these lesions were reversible. One group of C3H/HeJ mice was treated with a 43% solution for 24 weeks and thereafter observed for lifetime. Skin lesions were reversible in the 43% group immediately after treatment was stopped. For the higher doses (21% and 86.5%) further skin lesions developed. (Wenzel-Hartung et al. 1989).
Male and female New Zealand white rabbits (2 or 1 animals of each sex) exposed to 1 ml per day of 2-ethylhexyl acrylate for 3 or 12 days developed skin inflammation. After 12 days necroses and ulcerations were also observed. (Hunter et al. 1981 - quoted from IUCLID 1996).
Male and female guinea pigs (5 animals of each sex) exposed to 0.5 ml per day of 2-ethylhexyl acrylate for 12 days developed skin inflammation, necroses and ulcerations. The lesions were worse than the lesions seen in rabbits exposed to the double dose of 2-ethylhexyl acrylate. (Hunter et al. 1981 - quoted from IUCLID 1996).
4.3 Reproductive and developmental effects
2-Ethylhexanol is a metabolite of 2-ethylhexyl acrylate. 2-Ethylhexanol in high doses (above 800 mg/kg b.w.) has caused developmental effects in rats. (Ritter et al. 1987).
4.4 Mutagenic and genotoxic effects
2-Ethylhexyl acrylate was not mutagenic in 4 strains (TA98, TA100, TA1535, and TA1537) of Salmonella typhimurium in an Ames test with or without metabolic activation systems (Zeiger et al. 1985).
2-Ethylhexyl acrylate tested in cultured L5178Y mouse lymphoma cells without exogenous activation produced an equivocal result for an increased mutant frequency as well as for induced aberrations. No increase in the number of micronuclei was seen. (Dearfield et al. 1989).
In another experiment the mutation frequency was up to 4.6 times greater than in controls for the highest dose levels of 2-ethylhexyl acrylate added to cultured L5178Y mouse lymphoma cells with metabolic activation. No reproducible increase in mutation frequency was seen without the metabolic activation. (Litton Bionetics 1984 - quoted from HSDB 1999).
2-Ethylhexyl acrylate did not induce a dose-related increase in the hgprt mutant frequency in either the suspension or monolayer assay in Chinese hamster ovary cells (Moore et al. 1991).
A cell transformation assay in C3H-10T1/2 cells tested negative with 2-ethylhexyl acrylate (BASF 1982 - quoted from IUCLID 1996).
The sister chromatid exchange assay in CHO cells with and without metabolic activation was slightly positive when tested with 2-ethylhexyl acrylate with metabolic activation (ambiguous result) (BASF 1980 - quoted from IUCLID 1996).
Unscheduled DNA synthesis in primary rat hepatocytes was slightly increased when tested with 2-ethylhexyl acrylate (ambiguous result) (BASF 1980 - quoted from IUCLID 1996).
No chromosome aberrations were observed when mice were given an oral dose of 2.5 g/kg once a day for 1 or 5 days in an in vivo cytogenetic assay (BASF- quoted in IUCLID 1996).
In a 2-year carcinogenicity study 25 ml of a 21.5, 43 or 85% (w/w) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of male NMRI mice (80 per group) three times a week. After about 7 months half of each group was rested for treatment for 2 months and then treated with a promoter for 20 weeks. None of the mice treated with 2-ethylhexyl acrylate alone developed a skin tumour at the application site. One squamous cell papilloma occurred in each of the groups treated with 2-ethylhexyl acrylate and the promoter. Squamous cell carcinomas were observed only in the positive control groups (exposed to 0.015 % benzo[a]pyrene alone or in combination with promoter). (Mellert et al. 1994).
In a lifetime carcinogenicity study 25 ml of a 2.5, 21 or 86.5% (w/w) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of male C3H/HeJ mice (80 per group) three times a week. Another group was treated with a 43% solution for 24 weeks and thereafter observed for lifetime. Only in the 86.5% and 21% test groups showing chronic irritative skin damage there was a high incidence of neoplastic skin lesions (total of 15 papillomas, 36 carcinomas, and 16 melanomas) with no dose dependency. In contrast, no skin tumours were found in the negative control groups, in the group treated with 2.5% 2-ethylhexyl acrylate for lifetime or in the group treated with 43% 2-ethylhexyl acrylate for about 6 months and then observed for lifetime. (Wenzel-Hartung et al. 1989).
In a lifetime carcinogenicity study 20 mg of a 75% (v/v) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of 40 male C3H/HeJ mice three times a week. The concentration was chosen as the highest concentration that caused neither grossly observable irritation nor reduced weight gain in a preliminary 2-week study. Two animals had squamous cell carcinomas and four additional animals had squamous cell papillomas all on the treated skin. (DePass et al. 1985).
5 Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
2-Ethylhexyl acrylate is classified for irritative effects (Xi;R37/38 - irritating to respiratory system and skin) and for sensitising effects (R43 - may cause sensitisation by skin contact) (MM 1997).
EU
-
IARC/WHO
Ethylhexyl acrylate is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 1994).
US-EPA
-
RD50
-
6 Summary
Description
2-Ethylhexyl acrylate is a colourless liquid with a sharp, musty odour. Its solubility in water is low (0.1g/l). Its vapour pressure is 0.14 mmHg. The odour threshold in air is 0.55-1.36 mg/m3.
In Denmark, the principal use of 2-ethylhexyl acrylate is in UV curable inks, lacquers and varnishes.
Environment
2-Ethylhexyl acrylate is not known to occur as a natural compound. It may be released into the environment in fugitive and stack emissions or in wastewater during its production and use.
If released to the atmosphere, 2-ethylhexyl acrylate will react with photochemically produced hydroxyl radicals and ozone with an estimated half-life of 10.3 hours. It may also photolyse in sunlight.
If released to soil and water, 2-ethylhexyl acrylate may biodegrade and it may hydrolyse, especially in an alkaline environment. 2-Ethylhexyl acrylate is not expected to adsorb to sediment or suspended particulate matter in water. It is expected to exhibit moderate mobility in soil. 2-Ethylhexyl acrylate will significantly volatise from water and near surface soil with an estimated half-life of between 7.3 hours and 2.7 days.
Human exposure
The most probable route of human exposure of 2-ethylhexyl acrylate is by inhalation of contaminated air especially at plants where it is manufactured and used.
Toxicokinetics
In the rat 2-ethylhexyl acrylate is readily absorbed through the gastrointestinal tract and after intraperitoneal application. The plasma radioactivity concentration peaked at 2-3 hours. Following absorption 2-ethylhexyl acrylate is distributed to various organs with the highest concentration occurring in the liver and kidney. 2-Ethylhexyl acrylate is rapidly metabolised and excreted. At high doses (100 mg/kg bw) the decline in concentration in the tissues was slower with levels remaining almost constant for 72 hours in adipose tissue. 2-Ethylhexyl acrylate is believed to undergo carboxylesterase-catalysed hydrolysis to alcohol and acrylic acid, like other acrylate esters. 2-Ethylhexyl acrylate to a minor extent reacts with non-protein SH groups resulting in thioethers and mercapturic acids being excreted in the urine and/or bile. The major route of excretion is the lungs (50-75%) followed by urine (7-41%) and faeces (0.01-3 %). The metabolite in the lungs is CO2.
Human toxicity
Seven cases of allergic contact dermatitis due to an acrylic based adhesive tape have been reported. Patch-testing revealed that all persons reacted to 2-ethylhexyl acrylate. No cases have been reported of respiratory sensitisation. In Finland 5 people have got contact urticaria due to 2-ethylhexyl acrylate.
Animal toxicity
Single peroral, dermal or inhalatory administration of 2-ethylhexyl acrylate each proved to be only slightly toxic (LC50-value for mice is greater than 7700 mg/m3; oral LD50-values ranged from 1.8 to 12.8 g/kg for different species; and dermal LD50-values ranged from 7.5 to 16 g/kg for different species).
When rats were inhaling about 1400 mg/m3 of 2-ethylhexyl acrylate for 8 hours no mortality occurred.
A reduced body weight gain, lethargy, and dyspnoea were observed in Alderley Park rats inhaling 2-ethylhexyl acrylate at 1000 mg/m3 6 hours a day, 5 days per week for 2½ week. Animals inhaling 375 mg/m3 for the same exposure period showed no toxic signs.
Skin sensitisation was observed in challenged guinea pigs that had been induced with intradermal injections of 2-ethylhexyl acrylate in concentrations of 0.5 M or 0.17 M in Freund´s complete adjuvant three times during 9 days; that had been induced with epicutaneous or intracutaneous application of 2-ethylhexyl acrylate in concentrations of 0.1% (w/v) 3 times a week for 3 weeks; or that in the Polak method had been induced with 1 mg 2-ethylhexyl acrylate in Freund´s complete adjuvant applied as injections in the footpads and the neck.
Repeated application to the skin of mice, rabbits and guinea pigs caused skin irritation and subsequent degeneration of the treated areas. C3H mice were more sensitive than NMRI mice. In a lifetime study with 2-ethylhexyl acrylate applied to mice 3 times a week skin irritation was seen. The lowest dose administered was 25 ml of a 2.5% (w/w) solution of 2-ethylhexyl acrylate in acetone. Skin irritation was observed at this dose, however, after the 11th week of treatment, these lesions were reversible. For the higher doses (21% and 86.5%) further skin lesions developed.
In one study 2-ethylhexyl acrylate was irritant to the rabbit eye but in another one it was non-irritating.
The olfactory epithelium of the nasal mucosa was degenerated when Wistar rats inhaled 2-ethylhexyl acrylate at 225 and 750 mg/m3 6 hours a day, 5 days per week for 90 days. A reduced body weight gain, lethargy and reduced levels for albumin were also observed at these doses. Animals inhaling 75 mg/m3 for the same exposure period showed no toxic signs.
An apparent increase in the frequency of chronic nephritis was seen in male C3H/HeJ mice treated three times a week for their lifetime with 20 mg 75% (v/v) 2-ethylhexyl acrylate in acetone applied to clipped dorsal skin.
Reproductive and developmental effects
2-Ethylhexanol is a metabolite of 2-ethylhexyl acrylate. 2-Ethylhexanol in high doses has caused developmental effects in rodents.
Mutagenic and genotoxic effects
2-Ethylhexyl acrylate was negative in Ames test with and without metabolic activation, in assays in Chinese hamster ovary cells and in a cell transformation assay in C3H-10T1/2 cells. In various in vitro studies in cultured L5178Y mouse lymphoma cells, CHO cells and primary rat hepatocytes, 2-ethylhexyl acrylate produced an equivocal result. An in vivo cytogenetic assay in mice was negative.
Carcinogenicity
In two dermal carcinogenicity studies performed on C3H/HeJ mice for 2 years or the duration of their natural lives, 2-ethylhexyl acrylate proved to be locally tumourigenic at doses above 21% 2-ethylhexyl acrylate in acetone applied epicutaneously 3 times a week. Skin irritation was seen at the site of application. When the treatment in one group was discontinued after 6 months and observations were kept up for as long as these animals lived, the local skin damage receded almost completely and no skin tumours were observed. In a group receiving 2.5 % 2-ethylhexyl acrylate, skin lesions were reversible after the 11th week of treatment and no tumours developed in this group. NMRI mice did not develop skin tumours in a 2-year study equivalent to the study in C3H/HeJ mice.
7 Evaluation
The critical effects in humans following exposure to 2-ethylhexyl acrylate are considered to be the skin sensitising and irritative/damaging effects on respiratory tract, skin and eyes. This is based on the following:
In humans, the available data on health effects are limited to seven cases of allergic contact dermatitis after exposure to 2-ethylhexyl acrylate and to some cases of contact urticaria. No cases have been reported of respiratory sensitisation.
In laboratory animals, the main effects observed is skin sensitisation, irritation in the respiratory tract, skin and eye and carcinogenicity.
Long term (90 days) inhalation of 2-ethylhexyl acrylate (225 and 750 mg/m3) by Wistar rats degenerated the olfactory epithelium of the nasal mucosa, reduced body weight gain, caused lethargy and induced changes in some biochemical substances. Skin sensitisation and irritation has been observed in several animal experiments as described earlier in this paper. Those concentrations administered in long-term animal trials which no longer led to any local irritative effects were around 2.5% for dermal application and 75 mg/m3 for inhalation. The two references to eye irritating properties is not mentioning the doses causing the effect/ lack of effect on the eye. The different results are therefore likely to be a result of different doses. Since 2-ethylhexyl acrylate is irritating to the skin, it is most likely that it is also irritating to the eye in high enough doses or concentrations.
The carcinogenic effect of 2-ethylhexyl acrylate is considered to be related to its skin irritating effect and not to a genotoxic mechanism. This is based on that 2-ethylhexyl acrylate is negative or has showed equivocal results in different in vitro mutagenic assays and no chromosome aberrations were observed in mice in an in vivo cytogenetic assay. 2-Ethylhexyl acrylate is not carcinogenic in NMRI mice and the carcinogenic activity of 2-ethylhexyl acrylate in the skin of male C3H/HeJ mice could be detected only in association with a chronic skin irritation. As long as human dermal exposure remains far below levels causing skin irritation or does not persist chronically it is very unlikely that tumours will develop.
With the available studies, a NOAEL cannot be set for skin sensitisation. However, a subchronic inhalation study of good quality exist. This study will be used as the basis for estimating a limit value in air. A level of 75 mg/m3 is considered as a NOAEL for degeneration of the olfactory epithelium in rats inhaling 2-ethylhexyl acrylate for 6 hours a day, 5 days a week for 90 days.
8. Limit value in air
The limit value is calculated based on a NOAEL of 75 mg/m3 for degeneration of the olfactory epithelium in rats inhaling 2-ethylhexyl acrylate 6 hours a day, 5 days a week for 90 days.
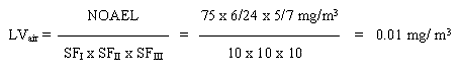 |
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 because: a) the study on which the NOAEL is based is not a chronic study and concern for the development of cancer in the respiratory system exist; b) no data on reproductive toxicity are available; c) 2-ethylhexyl acrylate is known to cause skin sensitisation and can therefore possibly cause respiratory sensitisation as well.
9. C-value
A limit value of 0.01 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990). A C-value of 0.01 mg/m3 and placing in Main Group 2, is proposed.
2-Ethylhexyl acrylate has a low odour threshold in air (0.55-1.36 mg/m3). However, the proposed limit value of 0.01 mg/m3 is considered to take into account the discomfort from odour.
C-value
0.01 mg/m3, Main Group 2.
10. References
AMI (1990). Allergi- og overfølsomhedsfremkaldende stoffer i arbejdsmiljøet. AMI-rapport 33.
BUA (1994). 2-Ethylhexyl acrylate (December 1991). Beatergremium für umweltrelevante Alstoffe (BUA) 88, Geschellschaft Deutscher Chemiker, p. 1-55.
Cikrt M, Vodicka P, Sapota A, Gut I, Stiborová A and Kopecký J (1986). Biliary excretion and organ distribution of 14C radioactivity after 14C-2-2-ethylhexyl acrylate administration in rats. J Hyg Epidemiol Microbiol Immunol 30, 365-370.
Clayton GD and Clayton FE (1994). Acrylates. In: Patty´s Industrial Hygiene and Toxicology, 4th ed. New York, John Wiley Sons, vol. 2D, 2999-3007.
DPIMR (1981). 2-Ethylhexyl acrylate. In: Dangerous Properties of Industrial Materials Report, vol. 1, 57-59.
Dearfield KL, Millis CS, Harrington-Brock K, Doerr CL and Moore MM (1989). Analysis of the genotoxicity of nine acrylate/methacrylate compounds in L5178Y mouse lymphoma cells. Mutagenesis 4, 381-393.
DePass LR, Maronpot RR and Weil CS (1985). Dermal oncogenicity bioassays of monofunctional and multifunctional acrylates and acrylate-based oligomers. J Toxicol Environ Health 16, 55-60.
Gut I, Vodicka P, Cikrt M, Sapota A and Kavan I (1988). Distribution and elimination of (14C)-2-ethylhexyl acrylate radioactivity in rats. Arch Toxicol 62, 346-350.
HSDB (1999). 2-Ethylhexyl acrylate. In: Hazardous Substances Data Base.
IARC (1994). 2-Ethylhexyl acrylate. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 60, 475-487.
ICSC (1993). 2-Ethylhexyl acrylate. In: International Chemical Safety cards (WHO/IPCS/ILO), ICSC number: 0478, http//www.cdc.gov/niosh/ipcneng/neng0478.html.
IUCLID (1996). 2-Ethylhexyl acrylate. In: International Uniform Chemical Information Database. Existing Chemicals 1996. ECB, JRC, Ispra.
Jordan WP (1975). Cross-sensitization patterns in acrylate allergies. Cont Derm 1, 13-15.
Mellert W, Kühborth B, Gembardt C and Munk R (1994). 2-year carcinogenicity study in the male NMRI mouse with 2-ethylhexyl acrylate by epicutaneous administration. Fd Chem Toxic 32, 233-237.
MM (1997). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
Moore MM, Parker L, Huston J, Harrington-Brock K and Dearfield KL (1991). Comparison of mutagenicity results for nine compounds evaluated at the hgprt locus in the standard and suspension CHO assays. Mutagenesis 6, 77-85.
Nordisk Ministerråd (1991). Review over 2-ethylhexylacrylate´s (EHA) allergene effekter. In: Kriteriedokumenter fra et nordisk allergiprojekt 51, 135-137.
Parker D and Turk JL (1983). Contact sensitivity to acrylate compounds in guinea pigs. Cont Derm 9, 55-60.
Ritter EJ, Scott WJ, Randall JL and Ritter JM (1987). Teratogenicity of di(2-ethylhexyl)phthalate, 2-ethylhexanol, 2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine. Teratology 35, 41-46.
RTECS (1999). Acrylic acid, 2-ethylhexyl ester. In: Registry of Toxic Effects of Chemical Substances.
Ruth JH (1986). Odor thresholds and irritation levels of several chemical substances: a review. Am Ind Hyg Assoc J 47, A142-A151.
Samimi B and Falbo L (1982). Monitoring of workers exposure to low levels of airborne monomers in a polystyrene production plant. Am Ind Hyg Assoc J 43, 858-862.
Sapota A (1988). The disposition of [2,3-14C]-methyl and [2,3-14C]-2-ethylhexyl acrylate in male Wistar albino rats. Arch Toxicol 62, 181-184.
Toxline (pre 1981-1999). 2-Ethylhexyl acrylate. In: Toxline database.
Vodicka P, Gut I and Frantík E (1990). Effects of inhaled acrylic acid
derivatives in rats. Toxicology 65, 209-221.
Waegemaekers TH and van der Walle HB (1983). The sensitizing potential of 2-ethylhexyl acrylate in the guinea pig. Cont Derm 9, 372-376.
Wenzel-Hartung RP, Brune H and Klimisch HJ (1989). Dermal oncogenicity study of 2-ethylhexyl acrylate by epicutaneous application in male C3H/HeJ mice. J Cancer Res Clin Oncol 115, 543-549.
Zeiger E, Haworth S, Mortelmans K and Speck W (1985). Mutagenicity testing of di(2-ethylhexyl)phthalate and related chemicals in Salmonella. Environ Mutag 7, 213-232.
Propylene carbonateand estimation of a limit value in air.
Vibe Beltoft
Elsa Nielsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
1. General description
| Molecular formula: | C4H6O3 |
| Structural formula: | 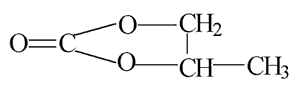 |
| Molecular weight: | 102.09 |
| CAS-no.: | 108-32-7 |
| Synonyms: | 4-methyl-1,3-dioxolan-2-one; dipropylene carbonate; 1,2-propanediolcarbonate; 1,2-PDC; cyclic methylethylene carbonate; cyclic propylene carbonate; cyclic 1,2-propylene carbonate; 1,2-propylene carbonate; propylene glycol cyclic carbonate; 4-methyl-2-oxo-1,3-dioxolane; carbonic acid, cyclic propylene ester. |
1.2 Physical / chemical properties
| Description: | Propylene carbonate is a colourless and odourless non-viscous liquid. |
| Purity: | - |
| Melting point: | -48.8 - -49.2 °C |
| Boiling point: | 241.7 - 243.4 °C |
| Density: | 1.189; 1.2069 g/ml (at 20°C) |
| Vapour pressure: | 0.03 mmHg (4 Pa) at 20°C |
| Concentration of saturated vapours: | 40 ppm (165 mg/m3) (calculated) |
| Vapour density: | - |
| Conversion factor: | 1 ppm = 4.17 mg/m3 20° C 1 mg/m3 = 0.236 ppm 1 atm |
| Flash point: | 135° C (open cup); 130-132° C |
| Flammable limits: | - |
| Autoignition temp.: | 510° C |
| Solubility: | Water: 83 g/l (at 20°C). Soluble in alcohol, ether, acetone, benzene, chloroform, ethyl acetate, carbon tetrachloride. |
| logPoctanol/water: | - |
| Henry’s constant: | - |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | Incompatible with strong oxidising agents, acid, bases, and reducing agents. |
| Odour threshold, air: | - |
| References: | CIR (1987), EPA (1998), HSDB (1998). |
Propylene carbonate is produced through the thermal cracking of saturated hydrocarbons. It can also be produced by the reaction of propylene oxide with carbon dioxide over a tetraethyl/ammonium bromide catalyst. (EPA 1998).
Propylene carbonate is used in CO2 recovery, in lithium batteries, in solvent extraction, plasticiser, organic synthesis, natural gas purification, synthetic fibre spinning solvent, and for the detection of N-containing drugs and their salts. (HSDB 1998).
Propylene carbonate is also used in painting and paint-stripping, to extraction of metals, and as a component of cooling agents and brake fluids. (EPA 1998).
It is also used in cosmetic products as a polar solvent at concentrations ranging from < 0.1 to 5%. In some products, up to 20% is used. (CIR 1987).
No data were found.
Air
No data were found.
Water
Propylene carbonate may decompose in aqueous solutions with the transient formation of propylene oxide. Based on the available physical and chemical properties, evaporation from water would be slow. (EPA 1998).
Soil
Leaching to water may occur, however, no data were available on soil to air or water to air mobility. Based on the available physical and chemical properties, evaporation from soil would be slow. (EPA 1998).
Bioaccumulation
No data were found.
Humans are exposed to propylene carbonate as cosmetic ingredient (up to 5%), and during work as a e.g. paint-stripping agent. (CIR 1987, EPA 1998).
2 Toxicokinetics
Inhalation
No data were found
Oral intake
No data were found
Dermal contact
A procedure to measure the steady-state rate of permeation of commercial solvents through living human skin was used on human female skin. The skin was removed from healthy females during plastic surgery of the breast. The samples were thinned by removing the dermal tissue from the epidermis and then stretched to a thickness of 300 to 600 m m. The permeability rate of propylene carbonate was reported to be 0.7 g/m2hour compared to a permeability rate for water of 24 g/m2h indicating that propylene carbonate is not readily absorbed through the skin. (Ursin et al. 1995 - quoted from EPA 1998).
No data were found.
No data were found.
3. Human toxicity
No data on short term toxicity are available except for studies on irritative effects.
In clinical studies, subjects were exposed to a wide range of cosmetic products containing from 0.5 to 10% propylene carbonate. In most of the studies, no skin irritation or sensitisation was noted. However, in one study (Hill Top Research 1977 - quoted from CIR 1987) of an antiperspirant (containing 2% propylene carbonate) using a modified Draize procedure, four subjects out of 51 developed skin erythema on intact sites and another four subjects developed erythema on abraded sites during the induction phase. In one study, moderate skin irritation was reported in subjects exposed to 20% solution of propylene carbonate. Occasionally other symptoms, e.g. hyperpigmentation, dryness, oedema and vesicles of the skin have been reported. (CIR 1987).
When five subjects were exposed to undiluted propylene carbonate applied to scarified skin once daily for three days, moderate skin irritation was observed; no evidence of phototoxicity was noted (CIR 1987).
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
No data were found.
4. Toxicity, animal data
Inhalation
"Concentrated vapours" (concentration not stated) for 8 hours was not lethal to six rats during a 14 days observation period. (Smyth et al. 1954 - quoted from CIR 1987).
Dogs, guinea pigs, and rats were exposed to an aerosol of propylene carbonate at a concentration of 2800 mg/m3 6 hours/day for 5 days/week for 21 days. The rats developed rhinorrhea and diarrhoea; no other toxicological effects were reported. (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Oral administration
Propylene carbonate was given by oral intubation in logarithmic doses to groups of five rats. Animals were observed for 14 days following the single oral dose. The LD50-value was reported to be 29.1 g/kg. (CIR 1987).
In mice, the oral LD50-value was reported to be 20.7 g/kg; no other data was reported (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Five male and female rats were administered undiluted propylene carbonate at a dose of 5 g/kg by oral gavage. They were observed for 14 days. Salivation was noted immediately after the single dose. No deaths and no lesions were observed at terminal necropsy. (Pharmakon Research International 1985 - quoted from CIR 1987).
Dermal contact irritation
Undiluted propylene carbonate was applied to the intact and abraded, clipped skin of rabbits (three males and three females). Skin responses were assessed at 24 and 72 hours after treatment. Very slight to well defined erythema and very slight oedema were noted at the 24 hours evaluation. All treated sites were normal at the 72 hours evaluation. The results indicated slight skin irritation. (Pharmakon Research International 1985 - quoted from CIR 1987).
Eye irritation
Undiluted propylene carbonate (0.1 ml, pH 8.82) was instilled into the right eye of three male and three female albino rabbits. Ocular irritation was reported to be only minimally; five had irritation of the conjunctivae only and one had irritation of the cornea, iris, and conjunctiva. (Pharmakon Research International 1985 - quoted from CIR 1987).
No ocular irritation was noted in six rabbits exposed to 10.5 or 17.5% propylene carbonate. (Kuramoto et al. 1972 - quoted from CIR 1987).
Instillation of 0.5 ml propylene carbonate into the conjunctival sac of the eyes of rabbits produced marked erythema of the conjunctivae, vascularisation of the sclera, and oedema of the lids and nictitating membrane within 24 hours; all eyes appeared normal by the 7th day. (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Inhalation
Rats (15/sex/group) were exposed to 100, 500, or 1000 mg/m3 propylene carbonate as aerosol for 90 days. No significant signs of toxicity were noted, except for some periocular swelling in the high and mid-dose groups. No systemic toxicity was reported.
An additional 20 rats per group were studied to investigate acute and subchronic neurotoxicity. For the acute study, rats received a single 6-hour exposure to propylene carbonate aerosol; no behavioural alterations were noted in any exposure groups at 1 hour and 24 hours after exposure. Standard neurobehavioral tests and motor activity were examined after 6 and 13 weeks; no behavioural alterations were noted in any of these exposure groups.
(Huntsman Corporation - quoted in EPA 1998).
Oral administration
Rats were given 1000, 3000, 5000 mg/kg/day propylene carbonate by gavage for 90 days. A recovery group was followed further from day 90 to day 118. No consistent dose-related findings were reported following necropsy or histopathological examination. (Huntsman Corporation - quoted in EPA 1998).
Dermal contact
To investigate dermal carcinogenicity, 50 ml propylene carbonate was applied twice a week to the shaved backs of 50 male mice for 104 weeks. A total of 10.4 ml was applied to each animal during the study. No deaths were observed, nor were any consistent body weight changes or significant dermal effects noted during the course of the study. (Huntsman Corporation - quoted in EPA 1998).
4.3 Reproductive and developmental effects
Twenty-seven dams (Sprague-Dawley rats) per group were orally exposed by gavage to 1000, 3000, and 5000 mg/kg/day propylene carbonate on days 6 through 15 of gestation. No developmental toxicity was observed at any dose. Maternal toxicity (decreased body weight gain and food consumption) was observed in high-dose dams. (Huntsman Corporation - quoted in EPA 1998).
4.4 Mutagenic and genotoxic effects
Propylene carbonate was evaluated for mutagenicity in Salmonella typhimurium, strains TA1535, TA1537, TA1538, TA98, TA100 with and without metabolic activation by liver homogenate. Doses of 50 to 5000 m g/plate were used. In the case of TA100, propylene carbonate showed some minor activity with and without activation at five doses, however, a dose-response relationship was not observed. (CIR 1987).
Propylene carbonate at five doses up to 4000 mg/plate was negative for genotoxicity in rat hepatocyte primary culture (CIR 1987).
To investigate dermal carcinogenicity, 50 ml propylene carbonate was applied twice a week to the shaved backs of 50 male mice for 104 weeks. A total of 10.4 ml was applied to each animal during the study. No tumours were noted during the course of the study. (Huntsman Corporation - quoted in EPA 1998).
5. Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
Propylene carbonate is classified for irritative effects (Xi;R36 - irritating to eyes) (MM 1997).
Cosmetics
As propylene carbonate is widely used in cosmetic products at concentrations from < 0.1% to 5%, it was concluded (based on clinical studies and animal studies) by the CIR panel (CIR 1987) that propylene carbonate is safe as a cosmetic ingredient in the present practices of use and concentrations.
EU
-
IARC/WHO
-
US-EPA
-
RD50
-
6. Summary
Description
Propylene carbonate is an odourless, clear liquid with a low vapour pressure (0.03 mmHg) and a high water solubility (83 g/l) at room temperature.
Environment
Only limited data are available. Based on the physical and chemical properties, evaporation from soil to water and air is considered to be slow.
Toxicokinetics
One study has reported that propylene carbonate is not readily absorbed through the skin. No other data have been found.
Human toxicity
No data on systemic toxicity have been found. In clinical studies, a 20% solution of propylene carbonate and undiluted propylene carbonate caused moderate skin irritation, whereas 5 and 10% aqueous solutions produced no skin irritation or sensitisation. However, in one study, subjects (4/51) developed skin erythema on intact sites and other four subjects developed erythema on abraded sites during the induction phase (2% solutions).
Animal toxicity
No deaths were observed in rats exposed to "concentrated vapours" for 8 hours. Dogs, guinea pigs, and rats exposed to propylene carbonate (aerosol) at 2800 mg/m3 6 hours/day for 5 days/week for 21 days developed rhinorrhea and diarrhoea; no other toxicological effects were reported. LD50-values was reported to be around 29 g/kg for rats and 20 g/kg for mice. Slight skin irritation were indicated in rabbits after undiluted propylene carbonate was applied to the intact skin, whereas marked irritation was observed following instillation in the eyes of rabbits.
No systemic effects were observed in rats exposed at concentrations of 100, 500 and
1000 mg/m3 propylene carbonate (aerosol) for 90 days; however, some periocular
swelling was noted at 500 and 1000 mg/m3; thus, a NOAEL of 100 mg/m3
is considered in this study.
Likewise, no effects were seen in rats following oral administration at doses up to 5000
mg/kg/day for 90 days or in mice following dermal application for 104 weeks.
Reproductive and developmental effects
No human data have been found.
No developmental toxicity was observed in rats following oral doses of 1000, 3000 and 5000 mg/kg/day during gestation days 6 through15; maternal toxicity (decreased body weight gain and food consumption) was noted at 5000 mg/kg.
Mutagenic and genotoxic effects
Propylene carbonate was negative when tested in Salmonella typhimurium (strains TA1535, TA1537, TA1538, TA98) with and without metabolic activation whereas strain TA100 showed minor activity although not in a dose related manner. Propylene carbonate was negative in rat hepatocyte primary culture.
Carcinogenicity
No human data have found.
In a 2-year dermal mice study, no tumours were observed.
7. Evaluation
The available data on health effects of propylene carbonate in humans are limited to data on irritative effects. Following application to the skin of undiluted propylene carbonate (one study) or of a 20% solution (one study), a moderate skin irritation was observed; however, no irritative effects were observed following application of solutions containing up to 10% propylene carbonate, One study using a modified Draize procedure has reported that 4/51 subjects developed skin erythema on intact sites and other four subjects developed erythema on abraded sites during the induction phase (2% propylene carbonate).
Propylene carbonate is of low acute toxicity in experimental animal following a single oral administration; no data have been found for inhalation exposure.
Following repeated inhalation exposure to propylene carbonate as aerosol, no systemic effects were observed following 21 days (dogs, guinea pigs, and rats - 2800 mg/m3) or 90 days (rats - up 1000 mg/m3); local effects in form of rhinorrhea and diarrhoea (21-day study) and some periocular swelling (90-day study) were reported. Likewise, no effects were seen in rats following oral administration (up to 5000 mg/kg/day) for 90 days or in mice following dermal application for 104 weeks. As diarrhoea was not reported in the oral study, the relevance of this finding is uncertain.
No reproductive toxicity studies have been found. In the only developmental study, no developmental toxicity including teratogenicity was observed in rats even when tested at dose levels giving rise to maternal toxicity.
The mutagenicity and genotoxicity tests available do not indicate that propylene carbonate is a genotoxic substance. No tumours were observed in the dermal carcinogenicity study on mice, the only study available.
The available studies indicate that exposure to propylene carbonate does not result in systemic toxicity even when tested at high dose levels by inhalation or oral administration. Thus, the critical effects following exposure to propylene carbonate are considered to be the local effects observed in the inhalation studies, effects which are probably related to the irritative properties of propylene carbonate. For the estimation of a limit value in air, a NOAEL of 100 mg/m3 is considered for local effects (periocular swelling) observed in the 90-day rat study.
8. Limit value in air
The limit value is calculated based on a NOAEL of 100 mg/m3 for local effects (periocular swelling) in rats exposed to propylene carbonate for 90 days.
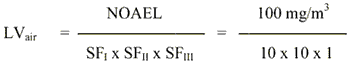 |
= 1 mg/m3
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 1 because of using a NOAEL for local effects and as no systemic effects have been observed even following administration of high doses.
9. C-value
A limit value of 1 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990). A C-value of 1 mg/m3 and placing in Main Group 2 is proposed.
C-value
1 mg/m3, Main Group 2.
10. References
EPA (1998). Environmental profile for propylene carbonate. EPA/600/R-98/068. National risk management research laboratory office of research and development. U.S. environmental protection agency. Cincinnati.
CIR (1987). Final report on the safety assessment of propylene carbonate (1987). J Amer Coll Toxicol 6, 23-51.
HSDB (1998). Propylene carbonate. In: Hazardous Substances Data Base.
MM (1997). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
MST (1990). Begrænsning af luftforurening fra virksomheder. Vejledning fra Miljøstyrelsen nr. 6 1990.
Triglycidyl isocyanurate
(Cationic surfactants) and estimation of a limit value in air.
Inger Thorup
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
1 General description
Quaternary ammonium compounds (QACs) are cationic surfactants. They are synthetic organically tetrasubstituted ammonium compounds, where the R substituents are alkyl or heterocyclic radicals. A common characteristic of these synthetic compounds is that they have one long-chain hydrophobic alkyl group. The products used in the technical field are normally not distinct individual compounds, but mixtures of homologues, in which the average chain length and the distribution of chain lengths in the lipophilic parts of the molecules may vary according to the starting materials used. The most well investigated compound is benzalkonium chloride.
In 1988, EPA suggested the QACs clustered into four groups, so that the toxicity studies would be facilitated by selecting one representative from each group for testing (Merianos 1991).
Below is given the structural formula for one representative of each of the four groups:
Group I: Straight-chain alkyl or hydroxyalkyl QACs
(e.g. CAS no.124-03-8, Hexadecyl ethyl dimethyl ammonium bromide; CAS no. 1119-97-7 , Tetradecyl trimethyl ammonium bromide; CAS no. 57-09-0, Hexadecyl trimethyl ammonium bromide; CAS no. 112-03-8, Octadecyl trimethyl ammonium chloride; CAS no. 1120-02-1, Octadecyl trimethyl ammonium bromide); CAS no. 1119-94-4, Dodecyl trimethyl ammonium bromide.)
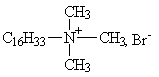 |
|
| Hexadecyl trimethyl ammonium bromide (CTAB) |
Group II: Alkyl dimethyl benzyl ammonium compounds
(e.g. CAS no. 139-08-2, Tetradecyl dimethyl benzyl ammonium chloride (Benzalkonium chloride); CAS no. 122-18-9, Hexadecyl dimethyl benzyl ammonium chloride.)
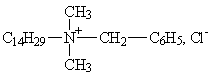 |
|
| Tetradecyl dimethyl benzyl ammonium chloride (benzalkonium chloride) |
Group III: Alkyl [di- and tri- chlorobenzyl] dimethyl ammonium compounds
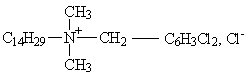 |
|
| Tetradecyl dimethyl dichlorobenzyl ammonium chloride |
Group IV: Heterocyclic ammonium compounds
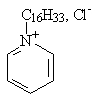 |
|
| 1-Hexadecylpyridinium chloride (cetylpyridinium chloride) |
1.2 Physical / chemical properties
QACs are white, crystalline powders. Low molecular weight QACs are very soluble in water, but slightly or not at all soluble in solvents such as ether, petrol and benzene. As the molecular weight and chain lengths increases, the solubility in polar solvents (e.g. water) decreases and the solubility in non-polar solvents increases.
References: Gloxhuber (1974), Gosselin (1984), Kirk-Othmer (1985), Merianos (1991).
QACs are synthesised industrially by alkylation of tertiary amines with alkyl halides or other alkylating species.
QACs are used as antiseptics, bactericides, fungicides, sanitisers, and softeners, but are also used in deodorants and as conditioning agents in hair cosmetics. The compounds are normally applied in concentrations between 0.01 and 1%. Concentrations in the low range are used in pharmaceutical products as topical antiseptics (skin, conjunctivae and mucous membranes). Benzalkonium chloride is a common used preservative in ophthalmic and nasal solutions.
In general, QACs within the field of antiseptics etc. contain alkyl chain lengths in the range C8 to C16 as these show good antimicrobial activities. For the use as softeners and hair conditioning agents chain lengths between C16 to C18 are used. The QACs are most effective against micro-organisms at neutral or slightly alkaline pH and become virtually inactive below pH 3.5. They are incompatible with anionic detergents such as soap, and demonstrate a high degree of binding to non-ionic surfactants.
References: Gosselin (1984), Kirk-Othmer (1985), Merianos (1991), MST (1991).
QACs are synthetic compounds and therefore not naturally occurring substances.
Levels of 1-5 mg QACs/litre in the water influx at sewage plants have been measured in Europe and USA (MST 1991).
A major part of the QACs is discharged into wastewater and removed in the biological processes of sewage treatment plant. A 90% reduction of the QACs in the water phase of sludge has been reported and alkyl di-/ trimethyl ammonium and alkyl dimethyl benzyl ammonium compounds seem almost completely degraded in sewage sludge.
However, the aerobic and anaerobic biodegradability of QACs is not well investigated. Only sparse data are available concerning stability, solubility and biodegradability. In general, it seems that the biodegradability decreases with increasing numbers of alkyl chains: R(CH3)3N+ > R2(CH3)2N+ > R3(CH3)N+ . Within each category the biodegradability seems inversely proportional to the alkyl chain length. Heterocyclic QACs are less degradable than the non-cyclic.
Investigations have shown that bioaccumulation of considerable dimensions will probably not take place. (MST 1991).
The general population are exposed to QACs directly through their use in disinfectants, hair conditioning agents and fabric softening agents, and indirectly through food stuffs due to the use to clean food contact surfaces.
2 Toxicokinetics
2.1 Absorption, distribution and elimination
Oral intake
Rats received orally 14C-labeled hexadecyl trimethyl ammonium bromide (CTAB, group I). About 80% of the dose of radioactivity was found in the gastrointestinal tract 8 hours after the administration, only small amounts were found in the blood plasma and about 2% of the administrated radioactivity was excreted in the bile during the first 12 hours after treatment. The low levels of radioactivity in the plasma and bile, together with the large amount of radioactivity found in the gastrointestinal tract indicated poor intestinal absorption of CTAB. Only small amounts of radioactivity were found in the liver, kidneys, spleen, heart, lung and skeletal muscles. Within three days of ingestion 92% of the radioactivity was excreted via the faeces and 1% via urine. (Isomaa 1975a).
Intraperitoneal application
Intraperitoneal injections of 14C-labeled CTAB to bile-duct cannulated rats showed that after 24 hours 36% of the radioactivity was excreted in the bile and 1% in the urine. The study indicated that CTAB was subjected to metabolic transformation, but the metabolites were not identified. (Isomaa 1975a).
After i.p. injections to pregnant rats small amounts of the compound could be detected in placenta and foetus (Anon. 1976).
Dermal, mucosal and eye application
Benzalkonium chloride was not detected in either venous blood or breast milk from woman using vaginal tampons containing 60 mg benzalkonium chloride (Bleau 1983 - quoted from Anon. 1989).
Following the instillation of a C14 benzalkonium chloride solution onto the corneal surface of rabbits, radioactivity was detected in the corneal epithelium, endothelium and stroma, and in conjunctivae. No radioactive material was found in the aqueous humour or any other tissues, including the blood (Green 1986 - quoted from Anon. 1989).
Although the absorption of QACs through normal skin probably is of less importance (Cutler & Drobeck 1970, Gosselin 1984), studies with excised guinea pig skin have shown that the permeability constants strongly depends on the exposure time and type of skin (Gloxhuber 1974) .
The cationic surface active compounds are in general more toxic than the anionic and non-ionic surfactants. The positively-charged cationic portion is the functional part of the molecule and the local irritation effects of QACs appear to result from the quaternary ammonium cation.
Due to their relative ability to solubilise phospholipids and cholesterol in lipid membranes, QACs affect cell permeability which may lead to cell death. Further QACs denature proteins as cationic materials precipitate protein and are accompanied by generalised tissue irritation.
It has been suggested that the shown decrease in acute toxicity of QACs with chain lengths above C16 is due to decreased water solubility (Cutler & Drobeck 1970, Gloxhuber 1974, Gosselin 1984, Effendy 1995).
In general it appears that QACs with a single long-chain alkyl groups are more toxic and irritating than those with two such substitutions. Only the first mentioned are useful as germicides/detergents (Gosselin 1984).
The straight chain aliphatic QACs have been shown to release histamine from minced guinea pig lung tissue (Cutler & Drobeck 1970). However, studies with benzalkonium chloride have shown that the effect on histamine release depends on the concentration of the solution. When cell suspensions (11% mast cells) from rats were exposed to low concentrations, a decrease in histamine release was seen. When exposed to high concentrations the opposite result was obtained (Anon. 1989).
In addition, QACs may show curare-like properties, a muscular paralysis with no involvement of the central nervous system. This is most often associated with lethal doses (Cutler & Drobeck 1970, Merianos 1991).
3 Human toxicity
The toxicity of QACs in general is not well established, although several human fatalities have been ascribed to them. Far from all of the compounds have been put through toxicological investigation and specific investigations are used to characterise the toxicological properties of the QACs. As mentioned before, the QACs has been clustered into four groups (Merianos 1991), so that the toxicity would be facilitated by selecting one member from each group for testing.
The major part of the present data refer to investigation on benzalkonium chloride/alkyl dimethyl benzyl ammonium chloride which belong to group II. In the literature the generic term alkyl dimethyl benzyl ammonium chloride is often used as a general term for benzalkonium chloride.
3.1 Short term/single exposure
At least 10 human fatalities (9 adults and one child) implicating QACs are medically recorded as resulting from alkyl dimethyl benzyl ammonium chloride (C8-C18) solutions of 10 to 15% that were introduced into the victims via oral ingestion, intramuscular, intravenous or intrauterine instillation (Gleason 1969 - quoted from Merianos 1991).
Inhalation
Five deep breaths of benzalkonium chloride (4 mg/ml in 0.9% sodium chloride, nebulised) caused constrictions of the airways in asthmatic persons. The mechanism of this effect is unclear, but it was not considered by the investigators to be an allergic response. (Miszkiel 1988).
Oral intake
Ingestion of 100-400 mg/kg b.w. of alkyl dimethyl benzyl ammonium chloride (10-15% solutions) caused rapid death within a few minutes to three hours in five persons. Superficial necrosis of mucous membranes was seen in the upper alimentary tract and erosion, ulceration and petecchial haemorrhages were seen throughout the small intestine. Severe changes were seen in the liver, kidneys and heart. Even in the case of prompt death lesions were seen in these organs. In addition glottic and pulmonary oedema was reported. (Cutler & Drobeck 1970, Gosselin 1984).
In humans poisoning paralysis is not a well established phenomenon. However, curare-like paralysis was reported in three persons poisoned with dimethyl benzyl ammonium chloride (benzalkonium chloride). (Gosselin 1984).
Dermal application skin irritation
From human testing of different QACs the generalised conclusion is obtained that all the compounds investigated to date exhibit similar toxicological properties.
It has been concluded that the maximum concentration that did not produce irritating effect on intact skin is 0.1%. Irritation became manifest in the 1-10% range. Concentrations below 0.1% have caused irritation in persons with contact dermatitis or broken skin. (Anon. 1989, BIBRA 1989, Cutler & Drobeck 1970, Merianos 1991).
Sensitisation
Topical mucosal application of QACs may produce sensitisation. Reports on case stories and patch test have shown that compounds such as benzalkonium chloride (group II), cetalkonium chloride (group II) and cetrimide (group I) may possibly act as sensitisers (Anon. 1989, BIBRA 1989, Cronin 1980, Cutler & Drobeck 1970, Merianos 1991). However, in general it is suggested that QACs have a low potential for sensitising man (Cronin 1980).
In several studies patients from dermatological clinics have been patch tested with 0.1% benzalkonium chloride (according to standard international procedures). It was shown that the compound was able to induce skin sensitisation in about 0.5-5.5 % of the patients. (Camarasa 1979 - quoted from Anon. 1989, Fuchs et al. 1993, Perrenoud et al. 1994, Schnuch et al.1998).
In patch studies carried out in the general population and in healthy volunteers, no sensitivity to 0,1 % benzalkonium chloride was detected (BIBRA 1989, Lovell 1992 – quoted from Anon. 1989).
It is difficult to distinguish between an allergic and an irritative skin reaction due to the inherent skin irritating effect of QACs.
Mucous membranes and eye
A 0.1% benzalkonium chloride instilled into the eye produced burning and stinging reactions. In general, a 0.02% solution seems without irritating effect. A few cases of unpleasant reactions have been reported at this concentration, however only conjunctival redness and not corneal damage has been described. 0.01% did not cause any damage. (BIBRA 1989, Anon. 1989).
Intrauterine instillation of alkyl dimethyl benzyl ammonium chloride in the range of 5-15 mg /kg/b.w. (10-15% solutions) has lead to death (Gosselin 1984).
Intramuscular or intravenous administration
Intramuscular or intravenous administration of 5-15 mg alkyl dimethyl benzyl ammonium chloride /kg/b.w. (10-15% solutions) caused death.
In total five deaths are reported due to intramuscular, intravenous or intrauterine administration. Three of the persons who received intravenous injections died within 21-46 hours. Another person survived for 15 days (Gosselin 1984).
3.2 Long term/repeated exposure
Inhalation
A group of 196 farmers (with or without respiratory symptoms) were evaluated for the relationship between exposure to QACs (unspecified, exposure levels not given) and respiratory disorders by testing for lung function and bronchial responsiveness to histamine. After histamine provocation statistically significant associations were found between the prevalence of mild bronchial responsiveness (including asthma-like symptoms) and the use of QACs as disinfectant. The association seems even stronger in people without respiratory symptoms. (Vogelzang et al. 1997).
Oral intake
No data have been found.
Dermal application
No data have been found.
3.3 Reproductive / developmental effects
No data have been found.
No data have been found.
No data have been found.
4 Toxicity, animal data
The toxicological data available for most of the QACs are limited (see 3 - Human toxicity). However, studies have been performed on some of the widely used compounds. The most investigated QACs belong to group II as particularly benzalkonium chlorides have been studied.
4.1 Short term/single exposure
The acute toxicity of QACs varies with the compound and, especially, the route of administration. For some substances the LD50 value is several hundreds times lower by the i.p. or i.v. than the oral route, whereas toxicities between the congeners only differ in the range of two to five times.
At least some QACs are significantly more toxic in 50% dimethyl sulfoxide than in plain water when given orally (Gloxhuber 1974, Merianos 1991, Gosselin 1984).
Probably all common QAC derivatives produce similar toxic reactions, but as tested in laboratory animals the oral mean lethal dose varies with the compound between the approximate limits given below (Merianos 1991, Gosselin 1984).
Inhalation
Wistar rats were exposed to an alkyl dimethyl ethyl benzyl ammonium compound at a concentration of 5.4 mg/litre (maximum attainable) for one hour. This concentration lead to 100% death. (Levenson 1965- quoted from Cutler & Drobeck 1970).
Recently, a whole-body inhalation study on cetylpyridinium chloride has been reported. This is a heterocyclic QAC belonging to group IV. Groups of five rats per sex were exposed to air containing 0, 0.05, 0.07, 0.13 and 0.29 mg cetylpyridinium chloride dust/l for four hours (equal to 50, 70, 130 and 290 mg dust/m3). The particle size was less than 5 µm. The LC50 was 0.09 mg/l (90 mg/m3) with upper and lower 95% confidence limits at 0.13 and 0.07 mg/l respectively. Deaths occurred in all treated groups (2/10, 1/10, 8/10 and 10/10). No deaths were seen among controls and all the deaths occurred within 4 days of exposure. Nasal discharge and chromodacryorrhoea (red discoloration around the nares) was found in all exposed groups and during the first week transient laboured breathing/respiratory difficulty (most pronounced at the higher exposure levels) was seen. The remaining animals were killed after 14 days. Besides lesions in the eyes (see below), no gross lesions attributed to the treatment were seen in these animals. Histopathological examination of lungs and other major organs were not carried out. (Lin 1991). The author has calculated that the total cetylpyrimidinium chloride exposure at the LC50 level (0.09 mg/l) was about 4-8 mg/kg b.w. and based upon this it was inferred that cetylpyrimidinium chloride could be more toxic by inhalation exposure than by oral or dermal exposure.
Oral administration LD50
LD50 values for QACs have been reported within the range of 250-1000 mg/kg for rats, 150-1000 mg/kg for mice, 150-300 mg/kg for guinea pigs and about 500 mg/kg b.w. for rabbits and dogs (Cutler & Drobeck 1970, Gloxhuber 1974, Anon. 1976). The ranges observed reflect differences in the study designs of these rather old experiments as well as differences between the various QACs.
The oral route of administration was characterised by delayed deaths, gastrointestinal lesions and respiratory and central nervous system depression. It was also found that given into a full stomach, the QACs lead to lower mortality and fewer gastrointestinal symptoms. This support the suggestion of an irritating effect. (Cutler & Drobeck 1970, Gloxhuber 1974, Merianos 1991, Gosselin 1984, BIBRA 1989).
In an attempt to elucidate the relationship between structure and toxicity of QACs, various homologues alkyl dimethyl benzyl ammonium chloride (C8-C19) were investigated with respect to LD50 in mice. The results indicated that increasing chain length beyond C16 decreased the acute toxicity markedly and that even numbered members appeared to be more toxic than those with odd numbered carbon chains. It was suggested that the decrease in toxicity above C16 was due to decreased water solubility. (Cutler & Drobeck 1970).
Dermal application LD50
Only a very few LD50 data are available. For benzalkonium unspecified (group II) a LD50 at about 1500 mg/kg b.w. for rats has been reported. In mice a LD50 value at 1600 mg/kg b.w. for octadecyl trimethyl ammonium chloride (group I) and in rabbits a LD50 at 7700 mg/kg b.w. for cetylpyridinium chloride (group IV) have been obtained.
CTAB (group I) given subcutaneously to rabbits and guinea pigs lead to a LD50 at about 100 mg/kg. Unspecified alkyl dimethyl benzyl ammonium chloride (group II) applicated subcutaneously gave rise to values in the range of 60 (mice) to 400 (rats). (BIBRA 1989, RTECS 1998).
0.1, 1.5, 6.5 and 50% solutions of benzalkonium chloride were applied on the fur (0.05 ml and then rubbed in) of two strains of mice. Each dilution was applied to 8 mice. 29 of 96 mice receiving 6.5 and 50% solutions (approximately 160 and 1250 mg/kg b.w./day) died within 72 hours after the application. Weight reduction was seen in the 6.5 and 50% groups, but not at lower levels. Necropsy of animals which died revealed discoloration of the subcutis on application site and absence of content in the gastrointestinal tract. The cause of death was not apparent. (Serrano 1972 - quoted from Anon. 1989).
skin irritation
From animal testing (rabbits, guinea pigs, rats and mice) of different QACs within groups I and II the generalised conclusion is obtained that all the QACs investigated to date exhibit similar skin irritating properties. In general, the maximum concentration that did not produce effect on intact skin is 0.1%. Solutions of 0.3-5% induces reactions ranging from skin irritation (erythema) to necrosis. (Gosselin 1984, BIBRA 1989, Merianos 1991).
Sensitisation
Various protocols involving repeated dermal or intradermal applications of benzalkonium chloride and challenge with 0.01-0.3% solutions have shown that benzalkonium chloride is able to induce sensitisation in guinea pigs and mice (Anon. 1989, BIBRA 1989). Older studies performed on other QACs did not reveal any signs of sensitising effect (Cutler & Drobeck 1970).
Mucous membranes and eye
Various studies concerning toxic effect of benzalkonium chloride to the eye have been performed. Instillation of different concentrations of benzalkonium chloride solutions in the rabbit eye have revealed that microscopic changes in the corneal epithelium can be induced at levels 0.01% or more. 0.001% is without damaging effect. (Anon. 1989).
Examination of five groups I or II QACs showed that 0.063-0.125% was the "threshold irritant concentration" range. (Cutler & Drobeck 1970).
Investigation of two QACs -alkyl dimethyl benzyl ammonium chloride and cetylpyridinium chloride- showed that instillation of a 330 ppm (0.033%) solution was the maximum concentration which did not produce irritation in rabbit eyes (Whitehall 1945 - quoted from Merianos 1991).
Eye irritation due to airborne cetylpyridinium chloride (group IV) has been reported once (see 4.1, inhalation above). Groups of five rats per sex were exposed to air containing 0, 0.05, 0.07, 0.13 and 0.29 mg cetylpyridinium chloride dust/l for four hours (equal to 50, 70, 130 and 290 mg dust/m3). Eye irritation was found in one or more animals per sex in all groups, except the controls. Lesions of the cornea, iris and/or conjunctiva were found in 4/10, 4/10, 6/10 and 6/10, respectively. All the ocular lesions were reversible (Lin 1991). In general, the longer chain alkyl trimethyl ammonium compounds are less irritating to the eye than the shorter chain homologues (C18<C12) and the dialkyl dimethyl ammonium compounds are less irritating than the corresponding mono alkyl trimethyl ammonium compounds.
Other tests for mucous membrane irritation occasionally applied to study the QACs include a penile irritation test. Seven group I QACs were tested (alkyl trimethyl ammonium compounds) in such assay. Irritating effect were seen after application of a 1-10% solutions. (Cutler & Drobeck 1970).
Others
Benzalkonium chloride or benzethonium chloride (group II) was instilled in the middle ear of guinea pigs. A single application of a 0.1% the respective solutions were placed in the tympanic cavity for 10-60 minutes. After two or 9 weeks the animals were killed. For both compounds severe lesions were seen in both the middle and inner ear. The extent of the damages were related to both the duration of exposure and the length of survival after the exposure. (Aursnes 1982).
A curare-like paralysis of skeletal muscles have been ascribed to QACs, specifically benzalkonium chloride and cetyl pyrimidinium chloride. Parenteral injections in rats, rabbits and dogs have resulted in prompt but transient limb paralysis and sometimes fatal paresis of the respiratory muscles. This effect seems to be transient. (Gosselin 1984).
4.2 Long term/repeated exposure
Inhalation
An inhalation toxicity study of an aerosolised hair conditioner containing an effective benzalkonium chloride concentration at 0.1% has been carried out in rats and hamsters. 12 CD rats and 12 golden hamsters were exposed to 9.9 mg conditioner/m3 five days a week, four hours/day, for 14 weeks (9.9 mg conditioner corresponds to 9.9 µ g benzalkonium chloride/m3). Body weights, haematological and biochemistry data were recorded, and gross and histopathological examination were conducted. No changes related to inhalation of the benzalkonium chloride conditioner were seen in any of the species. (CFTA 1979 - quoted from Anon. 1989).
Oral administration rat
The most widely investigated group is the alkyl dimethyl benzyl ammonium chlorides, particularly benzalkonium chloride. Many long term studies have been carried out, however, they are all of a very old date and do not meet the requirements of today’s quality guidelines.
Osborn-Mendel rats were fed 0.063, 0.125, 0.25 and 0.5% alkyl dimethyl benzyl ammonium chloride (group II) in the diet for two years. The measured toxicity parameters were growth rate, food consumption, mortality, and gross and microscopic (at least ten tissues) pathological examination. Suppression of growth occurred even at the lowest concentration (about 63 mg/kg b.w./day). For the remaining parameters toxic effects were seen at the 0.25% level. At about this level (250 mg/kg b.w./ day) pathologic changes were reported including diarrhoea and bloating of the abdomen, brown syrupy material in the intestine, distension of the coecum and foci of haemorrhagic necrosis in the gastro-intestinal tract. All rats at the 0.5% level died within 10 weeks. (Fitzhugh 1948 – quoted from Cutler & Drobeck 1970 and BIBRA 1989).
In another two years study, however, using a larger number of animals (12/sex), levels of 0.015, 0.031, 0.062, 0.125, 0.25 and 0.5% alkyl dimethyl benzyl ammonium chloride in the diet were tested. This study revealed that alkyl dimethyl benzyl ammonium chloride at 0.125% (125 mg/kg b.w./day) in the diet did not affect the growth, food consumption, blood picture or histopathology of the treated animals. At the 0.5% level only 50% of the animals survived 50 days. The pathological findings at this level were in agreement with Fitzhugh (1948) in that diarrhoea, brown viscid contents in the upper intestinal tract and acute gastritis were observed. Histopathological investigation revealed mucosal necrosis of the gastrointestinal tract. (Alfredson 1951 - quoted from Cutler & Drobeck 1970 ).
In these long-term studies the alkyl dimethyl benzyl ammonium chloride were fed in the diet. To obviate the difficulties concerning calculation of the exact doses administered to the animals, studies with benzalkonium chloride given by gavage were carried out. Rats were given the compound at 50 and 100 mg/kg b.w./day for 12 weeks with water or milk as vehicle. The compound was well tolerated at 50 mg/kg b.w./day, but depression of weight gain was seen at 100 mg/kg b.w./day when water was used as vehicle. (Coulston 1961 - quoted from Cutler & Drobeck 1970). It is not clear if tissue examination was performed in this study.
In a rat study doses of 5, 12.5 and 25 mg benzalkonium chloride /kg b.w./day given by gavage for two years lead to decrease in body weight at the highest dose level and increased cell growth in the gastric mucosa (probably at all dose levels) (Shelanski 1949 - quoted from Cutler & Drobeck 1970 and BIBRA 1989).
CTAB (group I) was offered to 10 SD rats of each sexes in concentrations of 0.007, 0.014 and 0.032% in drinking water for one year. These concentrations were calculated to deliver doses of approximately 10, 20 and 45 mg/kg b.w./day. The compound was well tolerated at the two lowest dose levels. At the highest dose level reduction in body weight, wetting and discoloration of the fur in the ventral region, decreased relative liver weight and increased relative coecum weight were seen. No compound related haematological or gross pathologic changes were seen and no microscopic alterations were found in the wall of stomach and small intestine of treated rats. No other tissues were histopathological examined. (Isomaa 1976).
dog
Dogs fed alkyl dimethyl benzyl ammonium chloride in the diet for 15 weeks at levels of 0.031, 0.062, 0.125, 0.25, 0.5 and 1.0% showed that 0.125% (approximately 30 mg/kg b.w./day) was the level without toxic effect. At the 0.25% level decreased body weight and food consumption were seen. Dogs fed the 0.5 and 1% levels died. As in the rats, the pathological changes were restricted to the gastrointestinal tract and included haemorrhage and necrosis in the gastrointestinal mucosa. (Alfredson 1951 - quoted from Cutler & Drobeck 1970).
In another study dogs (6 animals/dose) were given doses of 12.5, 25 and 50 mg benzalkonium chloride/kg b.w./day by gavage for 52 weeks with water or milk as vehicle. The benzalkonium chloride was given as a 10% solution. Deaths occurred among dogs at the two highest dose levels, but only when water was used as vehicle. The toxic effects seen at these levels - salivation, emesis and enteritis - were most intense in the dogs given the compound in water. When water was used as vehicle, intestinal congestion and inflammation was seen even in the dogs receiving 12.5 mg/kg b.w./day. These observations were, however, regarded as minor changes. (Coulston 1961 - quoted from Anon. 1989).
guinea pig
Groups of 20 guinea pigs were given 5, 12.5 or 25 mg alkyl dimethyl benzyl ammonium chloride by gavage for one year. No overt adverse effects or cellular changes in the major organs (not further specified) were seen. (Shelanski 1949 - quoted from Anon. 1989).
The above mentioned repeated toxicity studies do not cover all the studies carried out on group I and II QACs, but include the data which seems most pertinent. For the remaining studies not described above, the reported non-toxic (unspecified) levels are within the same range -or even higher- than those stated above. (Cutler & Drobeck 1970).
Dermal application
Application (probably uncovered) of benzalkonium chloride at 10 mg/kg b.w./day or more five times per week for three months to rats caused changes in the blood picture, liver and kidney damage and changes in certain organ weights (Berezovskaya 1978 - quoted from BIBRA 1989).
In a dermal study involving 100 female Swiss mice and ten New Zealand rabbits (both males and females), half of the mice and rabbits were treated with 8.5% benzalkonium chloride and the remaining half with 17% for about 80 weeks. An untreated group consisting of 100 mice and 19 rabbits served as controls. The solutions were applied uncovered twice a week (0.02 ml) on shaved dorsal skin (mice) or ear (rabbit). The highest dose level corresponds to approximately 85 mg/kg b.w./day for mice and 0.85 mg/kg/day for rabbits. Complete necropsy was performed on each animal. Skin samples and lesions in the lung, liver, kidneys were examined microscopically. Benzalkonium chloride caused ulceration, inflammation and scarring at the application site at both dose levels. No effects were seen on survival. The study indicated lack of systemic toxicity. (Stenbäck 1977).
4.3 Reproductive / developmental effects
The toxicological data available are primarily related to benzalkonium salts. Only a few studies on other relevant QACs have been found.
Oral intake
There were no overt adverse effects on reproduction in groups of 15 rats and 10 guinea pigs given up to 25 mg benzalkonium chloride/kg b.w./ day by gavage for two generations (Shelanski 1948 - quoted from BIBRA 1989).
Pregnant rats given up to 50 mg benzalkonium chloride/kg/day by gavage from days 6-15 of pregnancy showed no evidence of foetal malformations or decrease in litter size (FDRL 1977 - quoted from BIBRA 1989).
A brief review reported maternal and embryo toxicity (unspecified) when pregnant rabbits were fed 30 mg/kg b.w./day or more of an unspecified benzalkonium salt by gavage on days 7-19 of pregnancy. No malformations were seen. (CEC 1987 - quoted from BIBRA 1989).
Dermal application
Benzalkonium chloride (0.5 ml) in concentrations up to 6.6% was applied (uncovered) to the shaved skin of rats on days 6-15 of pregnancy (6.6% corresponds to approximately. 150 mg /kg/day). The doses induced local adverse maternal reaction (skin reactions), but not systemic toxicity. No effects on litter size, post-implantation loss, litter and mean foetal weights were seen. No signs of embryotoxicity or foetal abnormalities. (Palmer et al. 1983).
Dermal exposure to up to 120 mg /kg/day of an unspecified benzalkonium salt on days 6-15 of pregnancy apparently caused no adverse effects on the foetus in rats. No further details available. (CEC 1987 - quoted from BIBRA 1989).
Dimethyl distearyl ammonium chloride (group I) (0.5 ml) in concentrations up to 9.9% or 0.5 ml trimethyl stearyl ammonium chloride in concentrations up to 2.5% was applied (uncovered) to the shaved skin of rats on days 6-15 of pregnancy (2.5 and 9.9% corresponds to approximately 60 and 250 mg /kg/day, respectively). The doses induced local adverse maternal reaction (skin reactions), but not systemic toxicity. No effects on litter size, post-implantation loss, litter and mean foetal weights were seen. No signs of embryotoxicity or foetal abnormalities. (Palmer et al. 1983).
Mucous membranes
Single doses of 0, 25, 50, 100 and 200 mg benzalkonium chloride/kg b.w. were instilled into the vagina of pregnant rats. No adverse effects on pregnancy outcome at the lowest dose level. At 50 mg/kg b.w. and above, there were decreases in the number of live pups per litter and in litter size and weight. No visceral anomalies were seen, however abnormal bone development (sternal defects), increases in early embryo/foetal death (resorptions), reduced foetal growth and slight decreases in pregnancy rate were seen at 100 mg/kg b.w. In all rats given 100 mg/kg b.w. or more vaginal inflammation was seen at necropsy. (Buttar 1985 - quoted from BIBRA 1989).
Intraperitoneal application
I.p. administration of 10.5 or 35 mg CTAB /kg b.w. to pregnant mice as a single dose increased the incidence of dead implantations and malformations, principally cleft palate and minor skeletal defects in the skull and sternum. At the high dose CTAB increased foetal mortality. As QACs are able to alter the cell permeability it was suggested by the authors, that the embryotoxic and teratogenic effects of CTAB was due to a disturbance of the functional integrity of the placenta. (Isomaa & Ekman 1975b).
Primarily benzalkonium chloride, but also other QACs have been investigated for mutagenicity in microbial test systems.
In Ames tests using Salmonella typhimurium with and without metabolic activation no signs of mutagenicity has been observed. Negative results were also obtained in E. coli reversion and B. subtilis rec assays. However, for benzalkonium chloride also positive and equivocal results were seen in the B. subtilis rec assays. In an E. coli DNA polymerase assay benzalkonium chloride induced repairable DNA damage, which points towards a genetic damage. (Yam 1984, BIBRA 1989, Anon. 1989).
QACs have been tested in hamster and mouse cell-transformation tests with negative results (Yam 1984, BIBRA 1989, Anon. 1989).
In vivo (i.p., micronucleus test) and/or in vitro tests (mouse and hamster cell cultures) carried out with unspecified benzalkonium salts did not lead to sister chromatid exchanges or any chromosomal aberrations (BIBRA 1989, NTP – quoted from Toxline, 1995-1998).
A few oral and dermal carcinogenicity studies on representative QACs (from groups I and II) are available. However, they are of an earlier date and do not meet the requirements of today’s quality guidelines.
Oral intake
In a two years study rats (12-24/group) were given an alkyl dimethyl benzyl ammonium chloride in dietary levels of 0.015 to 0.5%. Only the highest level showed signs of toxic effect. The incidence of neoplasms among the treated groups was not significantly different from that observed in the control group. Only a limited number of organs were examined. (Alfredson 1951 – quoted from Cutler & Drobeck 1970 and BIBRA 1989).
Dermal contact
The tumorigenicity of benzalkonium chloride was evaluated in a dermal study involving 100 female Swiss mice and ten New Zealand rabbits (both males and females). Half of the mice and rabbits were treated with 8.5% benzalkonium chloride and the remaining half with 17% for about 80 weeks. An untreated group consisting of 100 mice and 19 rabbits served as controls. The solutions were applied uncovered twice a week (0.02 ml) on shaved dorsal skin (mice) or ear (rabbit). Complete necropsy was performed on each animal. Skin samples, grossly observed tumours, and other lesions in the lung, liver, kidneys were examined microscopically. Neither local skin tumours or systemic tumours were induced. (Stenbäck 1977).
In a well performed NTP study (1995) benzethonium chloride (group II) was investigated in rats and mice. Groups of 60 animals of each sex and species were topically administered up to 1.5 mg benzethonium chloride/kg b.w. 5 days/week for 103 weeks. The doses were administered in ethanol. There were no evidence of carcinogenic activity of benzethonium chloride in neither rats nor mice. (NTP - quoted from Toxline, 1995-1998).
5 Regulations, limit values
Ambient air
-
Drinking water
-
Soil
-
OELs
-
Classification
QACs in the category benzyl-C8-18-alkyldimethyl chloride are classified for acute toxicity (Xn;R21/22 - harmful in contact with skin or if swallowed), for corrosive properties (C;R34 - causes burns); and for environmental toxicity (N;R50 - dangerous for the environment; very toxic to aquatic organisms) (MM 1999).
Cosmetics
Denmark: The content of alkyl (C8-C18) dimethyl benzyl ammonium chloride and alkyl trimethyl ammonium chloride/bromide in cosmetics must not exceed 0.1% (MEM 1998).
IARC/WHO
-
USA
FDA has concluded for some QACs, that the food additive regulations should be amended to provide for the safe of use on food equipment and food contact surfaces, if the solution does not exceed 200 ppm. In this instance, safe use does not require potable water rinse (Federal Register 1969 & 1974 - quoted from Merianos 1991).
6 Summary
Description and use
Quaternary ammonium compounds (QACs) are cationic surfactants. They are synthetic organically tetrasubstituted ammonium compounds, where the R substituents are alkyl or heterocyclic radicals.
A common characteristic of these synthetic compounds is that one of the R’s is a long-chain hydrophobic aliphatic residue. The most well investigated compound is benzalkonium chloride.
QACs are used as antiseptics, bactericides, fungicides, sanitisers, and softeners, but are also used in deodorants and as conditioning agents in hair cosmetics. Benzalkonium chloride is a common used preservative in ophthalmic and nasal solutions. The compounds are normally applied in concentrations between 0.01 and 1%.
Environment
QACs are synthetic compounds and therefore not naturally occurring substances. A major part of the QACs is discharged into wastewater and removed in the biological processes of sewage treatment plant. A 90% reduction of the QACs in the water phase of sludge has been reported and alkyl di/trimethyl ammonium and alkyl dimethyl benzyl ammonium compounds seem almost completely degraded in sewage sludge.
Investigations indicate that bioaccumulation of considerable dimensions will not take place.
The general population are exposed to QACs directly through their use in disinfectants, hair conditioning agents and fabric softening agents, and indirectly through food stuffs due to the use as sanitising food contact surfaces.
Toxicokinetics
Studies in rats have indicated poor intestinal absorption of QACs. For CTAB 92% of the radioactivity was excreted via the faeces and 1% via urine within three days of ingestion.
Toxicity
The mammalian toxicity of QACs in general is not well established and far from all of the compounds have been put through toxicological investigations.
The major part of the present data refer to investigations on benzalkonium chloride/alkyl dimethyl benzyl ammonium chloride.
Human toxicity
A few deep breaths benzalkonium chloride (4 mg/ml in 0.9% sodium chloride, nebulised) has caused constrictions of the airways in asthmatic persons.
A group of 196 farmers were evaluated for the relationship between exposure to QACs and respiratory disorders. Associations were found between the prevalence of mild bronchial responsiveness and the use of QACs as disinfectant.
Human fatalities implicating QACs are recorded as resulting from alkyl dimethyl benzyl ammonium chloride (C8-C18) solutions of 10 to 15% (100-400 mg/kg b.w.). Erosion, ulceration and necrosis of mucous membranes was seen in the alimentary tract. Severe changes were seen in the liver, kidneys and heart.
From human dermal testing of different QACs it is concluded that all the compounds investigated to date exhibit similar toxicological properties. The maximum concentration that did not produce irritating effect on intact skin is 0.1%. Irritation became manifest in the 1-10% range. Concentrations below 0.1% have caused irritation in persons with contact dermatitis or broken skin. Concerning eye and mucosa, the level which did not cause any damage is 0.01%.
It cannot be excluded that topical mucosal application of certain QACs may produce sensitisation. 0.1 % benzalkonium chloride has been shown to lead to positive skin reaction, but in general it is suggested that QACs have a low potential for sensitising man.
Animal toxicity
Exposure to a dimethyl ethyl benzyl ammonium compound for one hour in concentrations of 5.4 mg/l air lead to 100% dead. In a study rats were exposed to air containing 0, 0.05, 0.07, 0.13 and 0.29 mg cetylpyridinium chloride dust/l for four hours (equal to 50, 70, 130 and 290 mg dust/m3) and the LC50 was estimated to 0.09 mg/l (90 mg/m3). Deaths within 4 days of exposure occurred in all treated groups. Eye irritation, nasal discharge and discoloration around the nares and transient laboured breathing/respiratory difficulty was seen in all exposed groups.
The oral LD50 values for QACs have been reported within the ranges of 250-1000 mg/kg for rats, 150-1000 mg/kg for mice, 150-300 mg/kg for guinea pigs and about 500 mg/kg b.w. for rabbits and dogs. The administration lead to deaths, gastrointestinal lesions and respiratory and central nervous system depression. Only a very few dermal LD50 data are available and the reported values are in the ranges of 1500-7700 mg/kg b.w. for mice, rats and rabbits.
From animal testing (rabbits, guinea pigs, rats and mice) of different QACs within group I and II it can be concluded that all the QACs investigated to date exhibit similar skin irritating properties. The maximum concentration that does not produce effect on intact skin is 0.1%. Solutions of 0.3-5% induces reactions ranging from skin irritation to necrosis. Studies have shown that benzalkonium chloride is able to induce sensitisation in guinea pigs and mice.
Many studies concerning toxic effect of benzalkonium chloride to the eye have been performed. Instillation of different concentrations of benzalkonium chloride solutions in the rabbit eye have revealed that microscopic changes in the corneal epithelium can be induced at levels 0.01% or more. 0.001% is without damaging effect. Eye irritation may also be induced via exposure to QACs in the air.
A 14 weeks inhalation study with an aerosolised hair conditioner containing 0.1% benzalkonium chloride (corresponding to 9.9 m g benzalkonium chloride/m3) has been carried out in rats and hamsters. No changes related to inhalation of the benzalkonium chloride conditioner were seen in any of the species.
Long term studies in rats (one and two years duration) showed that QACs in doses as low as 0.032% in drinking water (45 mg/kg b.w./day) or 0.063% in the diet (63 mg/kg b.w./day) may affect the growth of rats and cause slight gastro-intestinal disturbances. In dogs severe gastro-intestinal lesions were seen after administration of 0.25% QAC in the diet (60 mg/kg b.w./day) for 15 weeks. Concentrations at 0.5% lead to deaths among both rats and dogs. The effects observed is primarily of local nature due to irritation of surface tissues. Based upon the available studies a NOAEL at about 0.01% in drinking water/diet can be established (roughly corresponding to 15 mg/kg b.w.). Long term dermal studies in rats showed that application of a 8.5% benzalkonium chloride solution twice a week lead to local skin inflammation and ulceration. No systemic toxic effect was observed.
Reproductive and developmental effects
No developmental toxicity was observed in oral studies causing local, but not systemic, maternal toxicity. Toxic effect was observed when QACs have been applied locally near the developing foetus (i.p. application or instillation into vagina). This could be due to a disturbance of the functional integrity of the placenta.
Genotoxicity
The results of the genotoxicity tests were in the vast majority of the cases negative, indicating that QACs have negligible potential to cause genetic damage.
Carcinogenicity
The results of the carcinogenicity tests were negative, indicating that QACs have no carcinogenic potential.
7 Evaluation
As the QACs are solid substances (which are used in form of solutions) the exposure in form of vapour is irrelevant whereas dust as well as aerosol exposure is relevant. No data with respect to dust exposure is available and for aerosol exposure, only one relevant animal study has been carried out.
The toxicity profile of QACs is primarily based upon data from studies with benzalkonium chloride. Other QACs have only been more or less sparsely investigated. However, it seems that the various QACs exhibit similar toxicological properties.
Oral, dermal and few inhalation toxicity studies on representative QACs are available. However, they are of an earlier date and do not meet the requirements of today’s quality guidelines.
The studies have shown that QACs may induce adverse effect, including death, in humans as well as animals. From the available studies it can be concluded that the critical toxic effect of QACs apparently can be ascribed to the local irritating effects on surface tissues (skin, gastro-intestinal mucosa, eye, and respiratory system). These effects of QACs can be induced at levels not causing systemic effect. Therefore, the threshold for induction of oral toxic effect seems more related to the concentration of the solution than the daily amount of compound ingested.
Studies have shown that 0.001% solutions are without adverse effect even on the most sensitive membranes (eye).
It has been shown that benzalkonium chloride is able to induce sensitisation in guinea pigs and mice at concentrations not leading to skin irritation. In humans, it has been difficult to distinguish between an allergic and an irritative skin reaction due to the inherent skin irritating effect of QACs. However, it is suggested that QACs have a low potential for sensitising man (Andersen 1999).
Only a few studies reflect of QACs after inhalation. In a human study, a group of farmers were investigated for a possible relationship between exposure to QACs and respiratory disorders. Associations were found between the prevalence of mild bronchial responsiveness and the use of QACs as disinfectant. The design of the study does not allow the use for setting a N/LOAEL.
The most pertinent study for setting a N/LOAEL seems to be the four hours rat study on cetylpyrimidinuim chloride in dust form. A NOAEL cannot be estimated from this experiment as effects (death as well as irritating effect) were seen even at the lowest dose level at 0.05 mg/l. This is only about 6 times lower than the dose level, which caused 100% death. Cetylpyrimidinuim chloride seems to cause adverse effects at dose levels similar to other QACs after application on surfaces and after repeated oral intake. Based upon calculations from the cetylpyrimidinium chloride inhalation study it could be inferred that the compound is more toxic by inhalation exposure than by oral or dermal exposure. This may be the case for other QACs too.
A repeated animal inhalation study performed with a hair conditioner containing a very low concentration of benzalkonium chloride (9.9 mg/m3) was without adverse effect.
The results from the reprotoxicity studies do not indicate developmental toxicity. Effects have only been observed when QACs have been applied locally near the developing foetus (i.p. or instillation into vagina). This could be due to a disturbance of the functional integrity of the placenta.
The results of the genotoxicity tests were in the majority of the cases negative, indicating that QACs have negligible potential to cause genetic damage. This is in accordance with the results from the carcinogenicity studies, although these do not meet today’s quality guidelines.
Based on the available data, the critical effect in humans following exposure to QACs is considered to be the irritative effect on skin, mucosal membranes and eye and respiratory system. The effect may be induced following exposure to QACs in solutions or as aerosols. For the purpose of estimating a limit value in air, an exposure level of 50 mg cetylpyrimidinuim chloride/m3 (as aerosols, particle size less than 5 µm) is considered a LOAEL for death and irritative effects in rats.
8 Limit value in air
Limit value in air
The limit value is calculated based on an acute inhalation study in rats (cetylpyrimidinuim chloride as aerosols, particle size less than 5 µm). A NOAEL could not be established as effect was seen even at the lowest dose level of 50 mg/m3. At this concentration irritative effect and even death was seen.
LOAEL 50 mg/m3
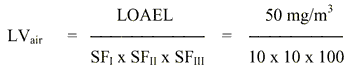 |
= 0.005 mg/m3
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 100 since deaths were recorded even at the lowest dose level and the calculations were based upon a four hour exposure study.
9 C-value
A limit value of 0.005 mg/m3 has been calculated for the sum of QACs. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value. A C-value of 0.005 mg/m3 and placing in Main Group 2 is proposed (MST 1992).
C-value
0.005 mg/m3 (sum of QACs), Main Group 2.
10 References
Andersen KE. Personal communication, May 1999.
Anon. (1989). Final Report on the Safety Assessment of Benzalkonium Chloride. J Am Coll Toxicol 8, 589-625.
Anon. (1976). Raad van Europa Werkgroep Cosmetica: Alkyltrimethylammoniumbromide; Alkyltrimethylammoniumchloride;. Dialkyldimethylammoniumchloride; Alkyldimethyl benzylammoniumchloride.
Aursnes J (1982). Ototoxic effect of quaternary ammonium compounds. Acta Otolarylgol 93, 421-433.
BIBRA Working Group (1989). Benzalkonium chloride. Toxicity profile. The British Industrial Biological Research Association.
Cronin E (1980). Cont Derm, 692-695. Churchill Livingstone.
Cutler RA and Drobeck HP (1970). Toxicology of Cationic Surfactants. In: Cationic Surfactants. Vol. 4 (Chap. 15). Jungermann E (Ed.) Marcel Dekker, Inc., New York.
Effendy I and Maibach HI (1995). Surfactants and experimental irritant contact dermatitis. Cont derm 33, 217-225.
Fuchs T, Meinert A, Aberer W, Bahmer FA, Peters KP, Lischka GG, Schulze Dirks A, Enders F and Frosch PJ (1993). [Benzalkonium chloride- a relevant contact allergen or irritant? Results of a multicenter study of the German Contact Allergy Group] In German. Hautarzt 44, 699-702.
Gosselin RE, Smith RP and Hodge HC (1984). Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins.
Gloxhuber C (1974). Review articles. Toxicological Properties of Surfactants. Arch Toxicol 32, 245-270.
HSDB (through 1998). Tetradecylbenzyl-dimethyl-ammonium-chloride. Alkyl-dimethyl-benzyl-ammonium-chloride In: Hazardous Substances Database.
Isomaa B, Reuter J and Djupsund BM (1976). The Subacute and Chronic Toxicity of Cetyltrimethylammonium Bromide (CTAB), a Cationic Surfactant, in the Rat. Arch Toxicol 35, 91-96.
Isomaa B (1975a). Absorption, distribution and excretion of [14C]CTAB, a quaternary ammonium surfactant, in the rat. Fd Cosmet Toxicol 13, 231-237.
Isomaa B and Ekman K (1975b). Embryotoxic and teratogenic effects of CTAB, a cationic surfactant, in the mouse. Fd Cosmet Toxicol 13, 331-334
Kirk-Othmer (1985). Quaternary ammonium compounds. In: Concise Encyclopedia of Chemical Technology. John Wiley & Sons. A Wiley-Interscience Publication, 162-63.
Lin GHY (1991). Acute inhalation toxicity of Cetylpyridinium chloride. Fd Chem Toxic 29, 851-854.
Lægemiddelkataloget. København 1996. Danmarks Apotekerforening, Foreningen af danske Medicinfabrikker og Medicinindustriforeningen. ISSN 0105-287X.
MEM (1998). Bekendtgørelse om kosmetiske produkter. Miljø- og Energiministeriets bekendtgørelse nr. 303 af 18. maj 1998.
Merianos JJ (1991). Quaternary Ammonium Antimicrobial Compounds. In: Disinfection, Sterilisation, and Preservation (Chap. 13). Block S. (Ed.) Fourth edition. Lea & Febiger, USA.
Miskiel KA, Beasly R, Rafferty P and Holgate ST (1988). The contribution of histamine release to bronchoconstriction provoked by inhaled benzalkonium chloride in asthma. Br J Clin Pharmacol 25, 157-163.
MM (1999). Danish Environment and Energy Ministry Standing Order No. 510 af 18th.June, 1999.
MST (1992). Industrial Air Pollution Control Guidelines. Vejledning fra Miljøstyrelsen nr. 9 1992.
MST (1991). Overfladeaktive stoffer - spredning og effekter i miljøet. Miljøprojekt nr. 166. Miljøstyrelsen.
Palmer AK, Bottomley AM, Edwards JA and Clark R (1983). Absence of embryotoxic effects in rats with three quarternary ammonium compounds (cationic surfactants). Toxicology 26, 313-315.
Perrenoud D, Bircher A, Hunziker T, Suter H, Bruckner Tuderman L, Stager J, Thurlimann W, Schmid P Suard A and Hunziker N (1994). Frequency of sensitization to 13 common preservatives in Switzerland. Swiss Contact Dermatitis Research Group. Cont Derm 30, 276-9.
Preller L, Doekes G, Heederik D, Vermeulen R, Vogelzang PF and Boleij JS (1996). Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Resp J. 9, 1407-13.
RTECS (1998). Hexadecyl trimethyl ammonium bromide. In the data base: Registry of Toxic Effects of Chemical Substances.
Schnuch A, Geier J, Uter W and Frosch PJ (1998). Patch testing with preservatives, antimicrobials and industrial biocides. Results from a multicentre study. Br J Dermatol 138, 467-76.
Stenbäck F (1977). Local and Systemic Effects of Commonly Used Cutaneous Agents: Lifetime Studies of 16 Compounds in Mice and Rabbits. Acta Pharmacol Toxicol 41, 417-431.
Toxline data base:1995-1998/09.
Vogelzang PFJ, van der Gulden JWJ, Preller L, Tielen MJM, van Schayck CP and Folgering H (1997). Bronchial hyperresponsiveness and exposure in pig farmers. Int Arch Occup Environ Health 70, 327-333.
Yam J, Booman KA, Broddle W, Geiger L, Heinze JE, Lin YJ, McCarthy K, Reiss S, Sawin V, Sedlak RI, Slesinski RS and Wright GA (1984). Surfactants: A survey of short-term genotoxicity testing. Fd Chem Toxicol 22, 761-769.
Tripropyleneglycol diacrylate
1 General description
1.1 Identity
| Molecular formula: | C15H24O6 |
| Structural formula: | 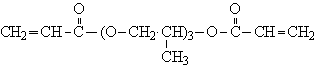 |
| Molecular weight: | 300.39 |
| CAS-no.: | 42978-66-5 |
| Synonyms: | Acrylic acid, propylenebis(oxypropylene) ester; 2-Propenoic acid, (1-methyl-1,2-diethanediyl)- bis(oxy(methyl-2,1-ethanediyl)) ester; Tripropyleneglycol diacrylate; TPGDA; TRPGDA. |
1.2 Physical / chemical properties
| Description: | Pale, yellow tinted liquid with a mild odour. |
| Purity: | Technical product: 80% pure monomer with > 18% oligomer (Nylander-French & French 1998). |
| Melting point | - |
| Boiling point: | - |
| Density: | 1.030 g/ml (at 20°C) |
| Vapour pressure: | < 0.01 mmHg (< 1.33 Pa) at 20°C 0.0106 mmHg (1.41 Pa) at 25°C |
| Concentration saturated vapours: | of 14 ppm (175 mg/m3 (calculated) at 20°C and 760 mmHg |
| Vapour density: | - |
| Conversion factor: | 1 ppm = 12.5 mg/m3 20°C 1 mg/m3 = 0.080 ppm 1 atm |
| Flash point: | > 110° C, closed cup |
| Flammable limits: | - |
| Autoignition temp.: | - |
| Solubility: | Insoluble in water. Soluble in many organic solvents. |
| logPoctanol/water: | - |
| Henry’s constant: | - |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | - |
| References: | RTECS (1999), Nylander-French & French (1998), Mortensen (1991). |
1.3 Production and use
No data were found on production.
Tripropyleneglycol diacrylate is used in a variety of UV curable inks, lacquers and varnishes (surface coatings). A lacquer contains 56.4% TPGDA monomer (Tice et al. 1997). Surface coating materials in Sweden normally contain 10 - 30% TPGDA (Nylander-French et al. 1994).
1.4 Environmental occurrence
No data were found
1.5 Environmental fate
No data were found
1.6 Human exposure
No data on human exposure have been found. However, the general population may be exposed to tripropyleneglycol diacrylate by inhalation of contaminated air and through contact with TPGDA containing products.
2 Toxicokinetics
2.1 Absorption, distribution
Inhalation
No data were found.
Oral intake
No data were found.
Dermal contact
No data were found, but systemic effects have been observed following dermal application indicating that absorption through the skin takes place (Celanese Corporation - quoted from Mortensen 1991)
2.2 Elimination
Metabolism
The major route of detoxification of acrylates is their conjugation with glutathione via the Michaëlis addition reaction or glutathione-S-transferase. Conjugation of acrylates by glutathione is expected to be proportional to the number of functional acrylate groups. The available data suggest that the acrylates most likely act on the site of contact, conjugate available glutathione, and are hydrolysed by carboxylesterases. (Tice et al. 1997).
Excretion
No data have been found.
Half-life
No data have been found.
2.3 Toxicological mechanisms
Based on the chemical structure and molecular reactivity, acrylates have the potential to interact with biomolecules, including nucleic acids and nucleoproteins and thus to induce DNA damage, being limited in the biological activity only by the physico-chemical properties and access to biological systems (Tice et al. 1997).
3 Human toxicity
3.1 Short term toxicity
Inhalation
No data were found
Oral intake
No data were found
Dermal contact
Acrylates are generally potent contact allergens. Polyfunctional acrylates and epoxyacrylates are the most potent ones, whereas the polyfunctional methacrylates and cyanoacrylates are much weaker. Many of the acrylates cross-react.
The list of allergens contain some 60 acrylates which all cause contact sensitisation (National Institute of Occupational Health 1990). In the Nordic countries, 23 acrylates are considered contact allergens and a classification with R43 (may cause sensitisation by skin contact) has been agreed (Nordic Council of Ministers 1991).
One American woman working with silk screening of computer discs with UV curable inks developed acute allergic contact dermatitis on hands and forearms. Patch testing revealed a number of strong reactions to epoxy resins and many multifunctional epoxy resins. The only material listed on the safety data sheet to which she reacted was tripropyleneglycol diacrylate. The other positive reactions likely represent cross-reactions. (Skotnicki & Pratt 1998 - quoted from TOXLINE PLUS 1998).
Occupational allergic contact dermatitis due to tripropyleneglycol diacrylate exposure was observed in a male silk screen maker in Belgium (Goossens et al. 1998).
Fingertip paraesthesia and occupational allergic contact dermatitis caused by acrylics (one of which was tripropyleneglycol diacrylate) occurred in a dental nurse in Finland. (Kanerva et al. 1998 – quoted from TOXLINE PLUS 1998).
Three out of 59 workers exposed to polyfunctional acrylic monomers (including tripropyleneglycol diacrylate) in 5 Swedish furniture companies developed severe acute toxic skin reactions due to insufficient product knowledge and lack of introduction routines, handling directions and protective equipment. A follow-up period of three years upon implementation of new routines revealed no further cases of acute toxic eczema in the five companies. (Voog & Jansson 1992).
Based on 10 years of patch testing with the (meth)acrylate series, Kanerva et al. (1997) put a low score (ranked 22 of 24) on tripropyleneglycol diacrylate with respect to its sensitising capacity.
3.2 Long term toxicity
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
3.5 Carcinogenic effects
No data were found.
4 Toxicity, animal data
4.1 Short term toxicity
Inhalation
No data were found.
Oral administration
An LD50-value after oral administration of a single dose of tripropyleneglycol diacrylate (TPGDA) to rats of 6200 mg/kg has been reported (NTIS - quoted from RTECS 1999).
Dermal contact
An LD50-value after single dermal application of TPGDA to rabbit skin of > 2000 mg/kg has been reported (Celanese Corporation - quoted from RTECS 1999).
A study on repeated dermal exposure was performed. Rabbits were painted with 500 mg/kg b.w. TPGDA 5 days a week for 2 weeks. The observation period was 4 week after the last application. This repeated exposure led to systemic toxicity, including convulsions, tremors and ataxia. The test methods and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
Irritation skin
TPGDA was tested for skin irritation after a single application to rabbits. The rabbits were examined 24 hours, 72 hours and 7 days after the application. The irritation was scored as moderately irritating both after 24 and 72 hours. At day 7, a delayed skin effect was observed. For most of the animals, skin necrosis and escar formation were noted. The delayed effects were assigned to category I (refers to corrosion). The dose was not stated. The test method is not described further and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
eye
When TPGDA was tested for eye irritation in rabbits, it was scored as category IV (slight irritation) after 72 hours. The test method is not described further and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
Sensitisation
TPGDA (purity not specified) was tested for sensitising capacity in guinea pigs using the Guinea Pig Maximization Test (GPMT). Groups of exposed and control animals consisted of 15 albino Dunkin-Hartley strain guinea pigs. For intradermal induction was used 1% TPGDA in olive oil:acetone (9:1), and for the topical induction was used a solution of 25% TPGDA in petrolatum. The challenge concentration was 0.2% TPGDA in petrolatum. The optimal test concentration was determined in a preliminary test. Of the guinea pigs treated with TPGDA, 11 of 15 (73%) became sensitised, which indicates that TPGDA is a strong sensitiser. (Björkner 1984).
The same study shows that other multifunctional acrylates such as 1,4-butanediol diacrylate, diethyleneglycol diacrylate, tetraethyleneglycol diacrylate and neopentylglycol diacrylate cross-react with TPGDA. (Björkner 1984).
The same test performed with acetone as vehicle show that the vehicle influences the allergic response to a very large extent. Of 15 animals tested, none was positive if acetone was used (11 of 15 were positive when petrolatum was used). The discrepancy between the results is due to a polymerisation process of the acrylates in acetone. Petrolatum prevents polymerisation of acrylic monomers. (Björkner & Niklasson 1984).
TPGDA stabilised with 500 ppm hydroquinone methylether was tested for sensitising properties in the Guinea Pigs Maximization Test (GPMT). The group of treated animals consisted of 10 male and 10 female and the control group of 5 male and 5 female Dunkin Hartley guinea pigs. On day 1, 0.1 ml of the test substance, in the presence of Freund’s adjuvant, was administered by intradermal route at a concentration of 0.5% in paraffin oil. On day 9, 0.5 ml of the test substance was applied by cutaneous route and kept on the application site for 48 hours. After a period of 15 days without treatment, a 0.5 ml challenge cutaneous application of the vehicle (left flank) and 0.5 ml of the test substance (right flank) at a concentration of 25% in paraffin oil was performed in all animals. The substances were held in place by means of an occlusive dressing for 24 hours. The cutaneous reactions were evaluated at the challenge application site, 24 and 48 hours after removal of the dressing. After the last scoring, the animals were sacrificed. No behavioural abnormalities, or cutaneous reactions were observed in any animal from the control or treated groups.
It was concluded that under the experimental conditions and according to the maximization method of Magnusson and Kligman, no cutaneous reactions related to a sensitisation potential of TPGDA were observed in the guinea pig.
(C.I.T. unpublished study 1989).
An identical study was performed by the same laboratory using exactly the same substance (TPGDA with 500 ppm hydroquinone methylether as stabiliser), same number of animals and same procedure, only was the concentration used for intradermal induction by day 1, 1% TPGDA in paraffin oil and the concentration used for the cutaneous induction on day 9, was 10% in paraffin oil. In this study, no behavioural abnormalities were observed in the animals throughout the study. Minimal to barely perceptible cutaneous reactions (very slight erythema) were noted 24 hours after removal of the dressing from the cutaneous challenge application, on the right flank (test substance) in 4 out of 10 males and in 2 out of 10 females in the treated group.
It was again concluded that under the experimental conditions and according to the maximization method of Magnusson and Kligman, no cutaneous reactions related to a sensitisation potential of TPGDA were observed in the guinea pig.
(C.I.T. unpublished study 1989).
Photomer 4061 (TPGDA with unspecified purity) was tested in the GMPT for sensitising properties using 10 and 5 female Dunkin Hartley guinea pigs as treated and control group, respectively. Six additional animals were used for the preliminary (dose finding) investigations. Alembicol D - a product of coconut oil was used as solvent. Based on the preliminary investigations, the following concentrations of Photomer 4061 were chosen: for the induction intradermal injection, 0.5% v/v in Alembicol D; for the induction topical application, - 25% v/v in Alembicol D; and for the topical challenge, 10% (anterior) or 5% (posterior) v/v in Alembicol D. Intradermal induction was performed on day 1 with 0.1 ml of each of 50% Freund’s complete adjuvant, 0.5% v/v Photomer 4061 in Alembic D and 0.5% v/v Photomer 4061 in 50:50 Freund’s complete adjuvant and Alembic D (control animals received the same but without Photomer 4061). One week later, the treated animals were inducted topically with 0.4 ml 25% v/v Photomer 4061 in Alembic D (controls with Alembic D alone) held on place for 48 hours with an occlusive dressing. Two weeks after this, all animals were challenged with 0.2 ml 10% and 5% v/v Photomer 4061 in Alembic D for 24 hours and the challenge sites were evaluated 24, 48 and 72 hours after removal of the patches. In this study, Photomer 4061 produced evidence of skin sensitisation (delayed contact hypersensitivity) in 8 of the 10 tested animals (2 were inconclusive). (Huntingdon Research Centre unpublished study 1993).
TPGDA was shown to induce contact sensitivity in Guinea pigs using the Polak method. In the test, 6 outbred Hartley guinea pigs were used. For intradermal induction, the guinea pigs received 1 mg TPGDA dissolved in ethanol:saline (1:4) and Freund’s complete adjuvant (FCA). Seven days later, the skin tests were performed with 0.2 ml of a solution (0.5% or 1% TPGDA in acetone:olive oil (4:1)) being applied to the skin. The test concentrations were 5% of the maximum concentration, which gave no irritation. The skin tests were repeated weekly up to 12 weeks. The time for the first positive skin reaction was at day 28. The number of sensitised animals was not mentioned. (Parker & Turk 1983).
4.2 Long term toxicity
Inhalation
No data were found.
Oral administration
No data were found.
Dermal contact
Rats were tested in a 90-day dermal test with TPGDA at dose levels of 20, 67, or 200 mg/kg b.w. for 5 days a week. Doses up to and including 67 mg/kg b.w. did not give rise to any systemic effects and is thus a NOAEL for systemic effects. All 3 doses led to skin irritation early in the study. The skin appeared to acclimatise and after 3 weeks, the severity of the irritation declined. There was no sign of irritation during the last few weeks of the study in rats treated with 20 mg/kg. The test methods and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
4.3 Reproductive and developmental effects
TPGDA was screened for teratogenic potential by repeated dermal exposure. Twenty pregnant rats were given a daily dermal dose of 250 mg/kg b.w. during day 6 to 15 of pregnancy. The dose level was based on results from a preliminary dermal maternal toxicity screening. TPGDA was not found to be teratogenic or foetotoxic based on maternal observations, including number of implantations, number of live and dead foetuses, number of early and late resorptions, and number of corpora lutea, as well as external, skeletal and visceral evaluation of foetuses for malformations. No further details of the test methods are described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
4.4 Mutagenic and genotoxic effects
TPGDA has been tested for mutagenicity in bacteria (Ames test) with and without metabolic activation; no mutagenic potential was found. The test method and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
In another test with mammalian cells in vitro, the mouse lymphoma cell mutagenicity assay, a positive mutagenic response was found both with and without metabolic activation. The test method and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
TPGDA or Lacquer A (an ultraviolet radiation curable lacquer containing 56.4% TPGDA as the active ingredient) were applied dermally to Tg.AC mice - 3 times a week for 20 weeks. Peripheral blood leukocytes were evaluated for DNA damage (single-strand breaks, alkali labile sites, and DNA crosslinking) at week 4, 8, 12, 16, and 20 by using the alkaline (pH:13) single cell gel (SCG) assay. Peripheral blood polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) were evaluated for the presence of micronuclei at week 20. The extent of DNA migration in leukocytes and the frequency of micronucleated erythrocytes were not significantly altered by treatment with TPGDA when administered alone or in Lacquer A, at doses that induced cell proliferation in keratinocytes. The absence of genotoxicity in these two cell populations suggests that these acrylates are not genotoxic or that they are not absorbed when applied dermally. However, a significant dose-dependent increase in the percentage of PCE relative to the vehicle control was present in mice treated with TPGDA, while a dose-dependent, but non-significant, increase in the percentage of PCE was observed in mice treated with Lacquer A. This observed rate of erythropoiesis may reflect bone marrow/blood toxicity. (Tice et al. 1997).
4.5 Carcinogenic effects
The carcinogenic potential of TPGDA was investigated in mice by dermal administration. The test group consisted of 50 male C3H/HeJ mice that were treated dermally with approximately 100 mg/kg TPGDA (2.5 mg/mouse) twice a week for 80 weeks or until tumours were diagnosed or animals died. The dose used in the study was selected in a preliminary pilot study, where male C3H/HeJ mice were treated with different dilutions of TPGDA. The concentration that only produced minimal skin irritation was selected for the study. Two negative control groups (one untreated and one treated with mineral oil) and one positive control group (treated with benzo(a)pyrene) were included. No obvious skin irritation was seen although fibrosis and changes in pigmentation occurred. At necropsy, skin and body cavities were examined and tissues were taken for histopathological analysis, which did not reveal any increased incidence of skin or visceral tumours. Ten of 50 mice died in the study. In the positive control group, 42 of 50 mice developed carcinomas and in the negative control groups, 1 of 50 mice developed skin papilloma among non-treated and mineral oil treated mice. The test method is not further specified and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
According to Mortensen (1991), the study is not in accordance with OECD guidelines and is inadequate for an evaluation of the carcinogenic potential of TPGDA.
Insertion of the zeta-globin promoted v-Ha-ras transgene into the FVB mouse genome (Tg.AC) produce a defined lesion, which is critical but insufficient by itself to induce benign or malignant tumours in the skin unless activated. Groups of 10 female Tg.AC (v-Ha-ras) mice (12 weeks old) were administered dermal doses of 1, 5 or 10 µmoles/mouse of TPGDA either alone or mixed with acetone (totally 200 µl per application) or as an equimolar amount of a lacquer (lacquer A), three times per week for 20 weeks to the shaved dorsal skin (8 cm2). Negative controls were vehicle treated animals and positive controls were 12-O-tetradecanonylphorbol-13-acetate (TPA) treated mice. The treatment with 5 and 10 µmoles of TPGDA either as the substance itself or as lacquer A induced a dose related increase in papillomas between 6 and 12 weeks of treatment that reached a maximum number of papillomas per mouse between 19 and 20 weeks of treatment. These result indicate that TPGDA may be predicted to be carcinogenic at the site of contact in a long-term cancer bioassay. More studies are advised to clarify the significance of the role of the TPGDA-induced cellular proliferation in the induction of papillomas. (Nylander-French & French 1998).
5 Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
TPGDA is classified for irritative effects (Xi;R36/37/38 - irritating to eyes, respiratory system and skin), for sensitising effects (R43 - may cause sensitisation by skin contact), and for environmental effects (N;R51-53 - toxic to aquatic organism, may cause long-term adverse effects in the aquatic environment) (EU 2000).
EU
-
IARC/WHO
-
US-EPA
-
RD50
-
6 Summary
Description
Tripropyleneglycol diacrylate (TPGDA) is a pale, yellow tinted liquid with a mild odour. It is insoluble in water but soluble in many organic solvents. It has a very low vapour pressure (0.0106 mmHg at 25° C).
Environment
No data were found.
Human exposure
No data on human exposure have been found. However, the general population may be exposed to tripropyleneglycol diacrylate by inhalation of contaminated air and through contact with TPGDA containing products.
Toxicokinetics
No data have been found with respect to absorption and distribution after inhalation or oral intake. Absorption after dermal contact has not been examined but systemic effects have been observed in rabbits after repeated topical application indicating that absorption through the skin takes place.
Detoxification of TPGDA would most likely be by conjugation with glutathione. Acrylates most likely react on the site of contact.
Human toxicity
A number of individuals using TPGDA occupationally have developed allergic contact dermatitis.
Animal toxicity
single dose toxicity
An LD50-value of 6200 mg/kg b.w. has been reported for oral administration to rats and of > 2000 mg/kg b.w. for dermal application to rabbits.
irritation
TPGDA was at first only moderately irritating to rabbit skin but had a delayed effect corresponding to a corrosiveness. It was only slightly irritating to the rabbit eye.
sensitisation
TPGDA has shown equivocal results in the Guinea Pig Maximisation Tests (GPMT) with both positive and negative results being observed. However, the vehicle strongly influences the sensitising response. Cross reactions with other multifunctional acrylates have been observed.
repeated dose toxicity
Systemic toxicity, including convulsions, tremors and ataxia was observed in rabbits following application to the skin of 500 mg TPGDA for 5 times a week for 2 weeks. Systemic toxicity was observed in rats receiving topically doses of 200 mg/kg b.w. for 5 days a week for 90 days; the NOAEL in this study was 67 mg/kg b.w.
Reproductive and developmental effects
TPGDA was not foetotoxic or teratogenic in female rats when 250 mg/kg b.w./day of TPGDA was applied to the skin during day 6 to 15 of gestation.
Mutagenic and genotoxic effects
Tripropyleneglycol diacrylate was not mutagenic in Ames’ test with or without metabolic activation, but gave a positive response in the mouse lymphoma cell mutagenicity assay both with and without metabolic activation.
Following dermal application (3 times a week for 20 weeks) to transgenic mice (Tg.AC (v-Ha-ras)), no DNA damage (single-strand breaks, alkali labile sites, DNA crosslinking) was observed in peripheral blood leukocytes using the alkaline single cell gel assay and the frequency of micronucleated erythrocytes (polychromatic and normochromatic) was not altered.
Carcinogenicity
No signs of carcinogenicity were found in male CH3/HeJ mice treated topically twice every week for 80 weeks with 2.5 mg TPGDA (100 mg/kg b.w.).
An increased number of skin tumours were observed in TPGDA-treated female Tg.AC (v-Ha-ras) mice in a twenty week short-term tumourigenesis study. TPGDA was applied topically 3 times a week for 20 weeks in doses of 1, 5 or 10 µmoles/mouse either in form of technical quality TPGDA or as a lacquer intended for UV cured coatings. Negative controls were vehicle treated animals and positive controls were 12-O-tetradecanoylphorbol-13-acetate treated mice (10 animals per dose group). Number of papillomas was increased in mice treated with 5 and 10 µmoles and all doses of the lacquer and likewise the latency periods (time to occurrence) were shorter than for the negative controls.
7 Evaluation
The available data on human health effects are limited to some cases of allergic contact dermatitis. No data on respiratory sensitisation have been found.
The critical effects in humans following exposure to tripropyleneglycol diacrylate (TPGDA) is considered to be the irritative effects on the respiratory system as also observed following exposure to other acrylates, the skin damaging effects observed in animal studies upon repeated dermal exposure, and the sensitising potential (delayed contact hypersensitivity) observed among workers as well as in animal studies.
Systemic effects (not further specified in the publication) have been observed in one study of rats following dermal application. Based on the available data, it cannot be excluded that systemic effects may occur in humans following inhalation of TPGDA.
The available data on TPGDA are considered to be inadequate for the purpose of estimating a health based limit value in air. As TPGDA, in analogy to other acrylates as e.g., 2-ethylhexyl acrylate, is irritative to the respiratory system and a skin sensitiser, the C-value of 0.01 mg/m3 proposed for 2-ethylhexyl acrylate is proposed for TPGDA as well.
8 C-value
The available data on TPGDA are considered to be inadequate for the purpose of estimating a health based limit value in air. As TPGDA, in analogy to other acrylates, is irritative to the respiratory system and a skin sensitiser, the C-value of 0.01 mg/m3 proposed for 2-ethylhexyl acrylate and placing in Main Group 2 is proposed for TPGDA as well.
C-value
0.01 mg/m3, Main Group 2.
9 References
Andrews LS and Clary JJ (1986). Review of the toxicity of multifunctional acrylates. J Toxicol Environ Health 19, 149-164.
Anonymous (1999). Vurdering af UV-hærdende trykfarver og lakker i et samlet miljøperspektiv. Miljø og Energiministeriet, Miljøstyrelsen.
Björkner B (1984). The sensitising capacity of multifunctional acrylates in the Guinea pig. Cont Derm 11, 236-346.
Björkner B and Niklasson B (1984). Influence of the vehicle on contact allergic reactions to acrylic compounds in the Guinea pig. Cont Derm 11, 268-278.
Clouzeau J (1989). TPGDA sensitisation test in the guinea-pig (study No. 4680 TSG). Centre International de Toxicologie (C.I.T.), France. Unpublished report.
Clouzeau J (1989). TPGDA sensitisation test in the guinea-pig (study No. 4683 TSG). Centre International de Toxicologie (C.I.T.), France. Unpublished report.
Denton SM (1993). Photomer 4061 Bx.92315 skin sensitisation in the guinea-pig. HCR Report. Huntingdon Research Centre. Unpublished report.
EU (2000). Commission directive 2000/32/EC of 19 May 2000 adapting to technical progress for the 26th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Official Journal of the European Communities L 136.
Golden R (1997). Safety and handling of UV/EB curing materials. J Coat Technol 69, 83-89.
Goossens A, Connix D, Rommens K and Verhamme B (1998). Occupational dermatitis in a silk-screen maker. Cont Derm 39, 40-42.
Kanerva L, Jolanki R and Estlander T (1997). 10 years of patch testing with the (meth)acrylate series. Cont Derm 37, 255-258.
Kanerva L, Mikola H, Henriks-Eckerman ML, Jolanki R and Estlander T (1998). Fingertip paresthesia and occupational allergic contact dermatitis caused by acrylics in a dental nurse. Cont Derm 38, 114-116.
Mortensen B (1991). Tripropylene glycol diacrylate. In: Health effects of selected chemicals. Nordic Chemicals Group Vol. 1, 123-132.
Nylander-French LA, Fischer T, Hultengren M, Lewné M and Rosén G (1994). Exponering vid ytbehandling med ultravioletthärdande akrylatlacker i träindustrin. Arbete och Hälsa 13, 1-33.
Nylander-French LA and French JE (1998): Tripropyleneglycol diacrylate but not ethyl acrylate induces skin tumours in a twenty-week short-term tumourigenesis study in Tg.AC (v-Ha-ras) mice. Toxicol Pathol 26, 476-483.
Nylander-French LA, Priha E, Berglund GB and Rosén G (1994). A method for monitoring worker exposure to airborne multifunctional acrylates. Appl Occup Environ Hyg 9, 977-983.
Parker D and Turk JL (1983). Contact sensitivity to acrylate compounds in guinea pigs. Cont Derm 9, 55-60.
RTECS (through April 1999).
Roberts DW (1987). Structure-activity relationships for skin sensitisation potential of diacrylates and dimethacrylates. Cont Derm 17, 281-289.
Tice RR, Nylander-French LA and French JE (1997). Absence of systemic in vivo genotoxicity after dermal exposure to ethyl acrylate and tripropylene glycol diacrylate in Tg.AC (v-Ha-ras) mice. Environ Mol Mutagen 29, 240-249.
Voog L and Jansson B (1992). Identification and control of contact dermatitis from polyfunctional acrylic monomers in five Swedish furniture companies. J Environ Sci Health 27, 1925-1938.
|
[Front page] [Top] |