[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for 2-Ethylhexyl acrylate, Propylene carbonate, Quaternary ammonium compounds, Triglycidyl isocyanurate, and Tripropyleneglycol diacrylate
Evaluation of health hazards by exposure to
2-Ethylhexyl acrylateand estimation of a limit value in air.
Pia Berthelsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
| Molecular formula: | C11H20O2 |
Structural formula:
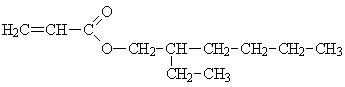
| Molecular weight: | 184.3 |
| CAS-no.: | 103-11-7 |
| Synonyms | Acrylic acid, 2-ethylhexyl ester 1-Acryloyloxy-2-ethyl-hexan 3-Acryloyloxymethyl-heptan 2-Ethylhexyl 2-propenoate 1-Hexanol, 2-ethyl-, acrylate Octyl-acrylate 2-Propenoic acid, 2-ethylhexyl ester 2-Propenoic acid, octyl ester |
1.2 Physical / chemical properties
| Description: | Colourless liquid with a sharp and musty odour. |
| Purity: | 99.5% |
| Melting point: | -90° C |
| Boiling point: | 213-218° C |
| Density: | 0.887 g/ml (at 20° C) |
| Vapour pressure: | 0.14 mmHg (19 Pa) at 20° C |
| Concentration of saturated vapours: | 184 ppm (calculated) at 20° C and 760 mmHg. |
| Vapour density: | 6.35 (air = 1) |
| Conversion factor: | 1 ppm = 7.66 mg/m3 20°
C 1 mg/m3 = 0.130 ppm 1 atm |
| Flash point: | 82-92° C (open cup), 86° C (closed cup) |
| Flammable limits: | 0.8-6.4 (v/v% in air) |
| Autoignition temp.: | 252° C |
| Solubility: | Water 0.1g/l (at 20° C). Soluble in alcohols, ethers, and many organic solvents (acetone, benzene, ethyl ether, heptane, methanol, carbon tetrachloride). |
| logPoctanol/water: | 3.67 - 4.32 |
| Henry’s constant: | 3.54 x 10-4 (atm x m3)/mole at 20° C. |
| pKa-value: | - |
| Stability: | Polymerises readily unless inhibited. Rapid, uncontrolled polymerisation can cause explosion. Reacts readily with electrophilic, free-radical, and nucleophilic agents. |
| Incompatibilities: | - |
| Odour threshold, air: | 0.55-1.36 mg/m3 |
| References: | BUA (1994), HSDB (1999), IARC (1994), ICSC (1993), IUCLID (1996), Ruth (1986) |
Direct, acid-catalysed esterification of acrylic acid with 2-ethylhexanol is the principal method for the manufacture of 2-ethylhexyl acrylate. A polymerisation inhibitor is added. (IARC 1994).
The major current use of 2-ethylhexyl acrylate is in acrylic pressure-sensitive adhesives. An adhesive for general purpose tape typically contains about 75% 2-ethylhexyl acrylate. Other uses of 2-ethylhexyl acrylate is in the production of plastics, latex, paints, textile and leather finishes, coatings for paper and industrial metal finishing. (HSDB 1999, IARC 1994).
In Denmark, the principal use of 2-ethylhexyl acrylate is in UV curable inks, lacquers and varnishes. Emission occurs in the form of aerosols.
2-Ethylhexyl acrylate is not known to occur as a natural compound. It may be released into the environment in fugitive and stack emissions or in wastewater during its production and use. (HSDB 1999, IARC 1994).
Air
2-Ethylhexyl acrylate is expected to exist almost entirely in the vapour phase based on its vapour pressure. It may photolyse in sunlight. It will react with photochemically produced hydroxyl radicals and ozone with an estimated half-life of 10.3 hours. (HSDB 1999).
Water
2-Ethylhexyl acrylate is not expected to adsorb to sediment or suspended particulate matter. It may hydrolyse, especially in alkaline waters based upon hydrolysis data for the structurally similar ethyl acrylate. It may photolyse in sunlight. It may biodegrade based upon the biodegradability of butyl acrylate and ethyl acrylate. It will significantly volatise from water with an estimated half-life of between 7.3 hours and 2.7 days. (HSDB 1999).
Soil
2-Ethylhexyl acrylate is expected to exhibit moderate mobility in soil and, therefore, it may leach to groundwater. It may hydrolyse, especially in alkaline soils based upon hydrolysis data for the structurally similar ethyl acrylate. It may biodegrade based upon the biodegradability of butyl acrylate. It may volatilise from near surface soil and other surfaces. (HSDB 1999).
Bioaccumulation
According to HSDB (1999) 2-Ethylhexyl acrylate is not expected to bioconcentrate in aquatic organisms. However BUA (1994) is stating that considerable bioaccumulation is to be expected.
The most probable route of human exposure of 2-ethylhexyl acrylate is by inhalation of contaminated air especially at plants where it is manufactured and used. Workers also may be exposed dermally during spills or leaks. (Samimi & Falbo 1982).
Inhalation
Urinary excretion of metabolites following inhalational exposure indicates absorption occurring by this route (Vodicka et al. 1990).
Oral intake
Excretion of a radioactive marked dose following oral exposure indicate absorption occurring by this route (Sapota 1988).
Dermal contact
No data were found.
Intraperitoneal application
In one study male Wistar rats were administrated an intraperitoneal dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons (Gut et al. 1988). In another study male Wistar albino rats were administrated an intraperitoneal dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate (Sapota 1988). In both studies plasma radioactivity concentration reached a peak level at about 2-3 hours after administration indicating easy absorption through this route. In tissues the highest concentrations of radioactivity was found in kidney, liver, spleen and the lungs. In the study with a dose of 100 mg/kg bw 6.5% of the dose was found in tissues at 3 hours after administration. The radioactivity in the tissues decreased slowly with time. At 72 hours after administration 1% of the dose was still found in the examined tissues. The radioactivity in adipose tissue and sciatic nerve was still relatively high.
Intravenous application
In one study male Wistar rats were administrated an intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons (Gut et al. 1988). In another study male Wistar rats were administrated an intravenous dose of 10 mg/kg bw or 50 mg/kg bw of (14C)-2-ethylhexyl acrylate (Cikrt et al. 1986). The highest concentrations of radioactivity in tissues was found in kidney, liver, brain, thymus and spleen.
Metabolism
2-Ethylhexyl acrylate is believed to undergo carboxylesterase-catalysed hydrolysis to 2-ethylhexanol and acrylic acid, like other acrylate esters (Cikrt et al. 1986, Miller et al. 1981 - quoted from IARC 1994).
2-Ethylhexyl acrylate to a minor extent reacts with non-protein SH groups in for instance glutathione causing depletion of the non-protein SH groups and excretion of thioethers in urine as described in the following studies:
Male Wistar rats exposed by 6 hours inhalation to 2-ethylhexyl acrylate in concentrations from 250 to 4800 mg/m3 over 24 hours excreted thioethers in urine in a dose dependent manner decreasing from 8.0 to 3.0% (at 1000 mg/m3) of the dose of 2-ethylhexyl acrylate indicating saturable metabolism along this pathway. Dose related depletion of non-protein SH groups in blood, liver and brain was seen at concentrations of and above 2400 mg/m3. (Vodicka et al. 1990).
When male Wistar rats were administrated an intraperitoneal dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons 2% of the dose was found as thioethers in the urine (Gut et al. 1988).
The principal eliminated metabolite in expired air was carbon dioxide in two studies in male Wistar rats given an intraperitoneal or intravenous dose of (14C)-2-ethylhexyl acrylate (Gut et al. 1988, Sapota 1988).
When 2-ethylhexyl acrylate and its metabolite acrylic acid reacts with the reduced form of glutathion (GSH), mercapturic acids can be formed (Cikrt et al. 1986).
Excretion
Two mercapturic acids N-acetyl-S-(2-carboxyethyl) cysteine and N-acetyl-S-2-(2-ethylhexyloxycarbonyl)ethyl-cysteine is excreted in the bile and urine (Cikrt et al. 1986, Kopeckı et al. 1985 - quoted from IARC 1994). Besides some unidentified metabolites have been detected in the bile of rats (Cikrt et al. 1986).
Male Wistar rats were administrated an intravenous dose of 10 mg/kg bw or 50 mg/kg bw of (14C)-2-ethylhexyl acrylate. Biliary excretion of radioactivity was followed in 1-3 hour intervals within the first 24 hours after administration. A significant increase in bile flow (243%) was observed. In the 24-hours 2.2% of the dose was eliminated via bile at both doses, most of it (83%) during the first 3 hours. (Cikrt et al. 1986).
Male Wistar rats were administrated an intraperitoneal or intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons. Within the first 24 hours about 50% of the dose had been excreted in expired air, 7-13% in urine and less than 0.01% in faeces. (Gut et al. 1988).
Male Wistar albino rats were administrated an intraperitoneal or oral dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate. Within the first 72 hours more than 90% of the dose had been excreted (78% in expired air, 10% in urine, and 3% in faeces for intraperitoneal application and 50% in expired air, 41% in urine, and 1% in faeces for oral application). (Sapota 1988).
Half-life
Male Wistar rats were administrated an intraperitoneal or intravenous dose of 10 mg/kg bw of (14C)-2-ethylhexyl acrylate labelled on the vinyl carbons. Elimination of radioactivity from blood was bi-exponential. The plasma half-life for the distribution phase was 60 minutes (i.p.) and 30 minutes (i.v.) for 4 months old and 130 minutes (i.p.) and 115 minutes (i.v.) for 7 months old. For the elimination phase, the half-life was 6 hours (i.p.) and 5 hours (i.v.) for the youngest rats and 14 hours (i.p. and i.v.) for the oldest. (Gut et al. 1988).
Male Wistar albino rats were administrated an intraperitoneal dose of 100 mg/kg bw of 2-ethylhexyl [2,3-14C]-acrylate. Elimination of radioactivity from blood had a monophasic character. The plasma half-life was about 22 hours. The half-life for excretion was calculated to be about 1½ hour. (Sapota 1988).
No data were found.
3 Human toxicity
Inhalation
No data were found.
Oral intake
No data were found.
Dermal contact
Seven persons have developed allergic contact dermatitis due to an acrylic based adhesive tape. Patch-testing revealed that all persons reacted to 2-ethylhexyl acrylate. Five of the persons were further tested for cross-sensitisation patterns. They all reacted to 2-ethylbutylacrylate and some of them reacted to other acrylates as well. (Jordan 1975).
No cases of respiratory sensitisation have been reported.
In Finland, 5 cases of occupational contact urticaria caused by 2-ethylhexyl acrylate has been reported from 1990 to 1994 (Kanerva et al. 1996 - quoted from Toxline pre1981-1999).
Generally acrylates are potent contact allergens with polyfunctional acrylates and epoxyacrylates being the strongest and polyfunctional methacrylates and cyanoacrylates being the weakest. A lot of the acrylates cross react. The Danish National Institute of Occupational Health (AMI) has made a list of allergens. It contains about 65 acrylates all causing contact sensitisation. Two of the acrylates (2,3-epoxypropyl-acrylate and methylmethacrylate) also cause respiratory sensitisation. Several epoxy compounds cause respiratory sensitisation so the epoxy-group in 2,3-epoxypropyl-acrylate might be the cause of its allergic effect on the respiratory system. (AMI 1990). In the Nordic countries 23 acrylates were classified for the ability to cause sensitisation by skin contact and were labelled with R43. (Nordisk Ministerråd 1991).
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
No data were found.
4 Toxicity, animal data
Inhalation
The LC50-value for mice is greater than 7700 mg/m3 (BASF 1967 - quoted from IUCLID 1996).
When rats were exposed to a saturated atmosphere (about 1400 mg/m3) of 2-ethylhexyl acrylate for 8 hours no mortality occurred (BASF 1958 - quoted from IUCLID 1996).
Alderley Park rats (2 animals of each sex per group) were exposed to 2-ethylhexyl acrylate in ethanol at 375 and 1000 mg/m3 6 hours a day, 5 days per week for 2½ week. A reduced body weight gain, lethargy, and dyspnoea were observed in high-dose animals. No changes in blood, urine and pathology were observed. Low-dose animals showed no toxic signs. (Gage 1970 - quoted from IUCLID 1996).
Oral administration
The reported oral LD50-values for 2-ethylhexyl acrylate ranged from 4.4 to 12.8 g/kg for rats (5 values reported), and from 4.4 to greater than 5.0 g/kg for mice (2 values reported). Rabbits have an oral LD50-value greater than 3.5 g/kg and the value for cats is greater than 1.8 g/kg (1 value reported for each species). (Studies quoted in IUCLID 1996, Clayton & Clayton 1994, DPIMR 1981, BUA 1994).
Rabbits (1 animal per sex) were fed 2-ethylhexyl acrylate as a 10% emulsion through a tube at a dose of 1774 mg/kg for 6 days (male) or 8 days (female). Four female rabbits were fed a dose of 887 mg/kg for 10 days. The high-dose animals died after 6 or 8 days. A reduced body weight gain, lack of desire to eat, and weak muscle tonus were observed and the gastric mucosa and kidneys were damaged. Low-dose animals only showed momentary lack of desire to eat and a slightly reduced body weight gain. (BASF 1960 - quoted from BUA 1994).
Dermal contact
The dermal LD50-value for rats is greater than 12 g/kg (1 value reported). For rabbits the value is between 7.5 and 16 g/kg (8 values reported), and for the guinea pig it is greater than 8.8 g/kg (2 values reported). (Studies quoted in IUCLID 1996, Clayton and Clayton 1994, DPIMR 1981, BUA 1994).
Skin sensitisation
Female Dunkin Hartley outbred guinea pigs were induced with intradermal injections of 2-ethylhexyl acrylate in concentrations of 0.5 M or 0.17 M in Freund´s complete adjuvant three times during 9 days. Sensitisation was observed when the animals were challenged at day 21, 35 and 49 with 1 M 2-ethylhexyl acrylate applied epicutaneously. At an induction concentration of 0.5 M 6-11 out of 16 animals were sensitised. At an induction concentration of 0.17 M 11-13 out of 16 animals were sensitised. Four control animals were sensitised (3 at day 35 and 1 at day 49). Cross reaction was seen with ethyl acrylate, n-butyl acrylate and hexyl acrylate. (Waegemaekers & van der Walle 1983).
Guinea pigs were induced with 0.1% (w/v) 2-ethylhexyl acrylate applied epicutaneous or intracutaneous 3 times a week for 3 weeks. Sensitisation was observed when the animals were challenged at day 11 after the induction with the same concentration of 2-ethylhexyl acrylate as used for the induction. For the epicutaneous test, 10 out of 10 animals were sensitised. For the intracutaneous test, 7 out of 10 animals were sensitised. (Hunter et al. 1966 - quoted from Nordisk Ministerråd 1991).
In the Polak method, 6 Hartley outbred guinea pigs of either sex were induced with 1 mg 2-ethylhexyl acrylate in Freund´s complete adjuvant applied as injections in the footpads and the neck. Sensitisation was observed when the animals were challenged at day 7 after the induction with 1% or 5% 2-ethylhexyl acrylate. (Parker & Turk 1983).
Eye contact
2-Ethylhexyl acrylate was non-irritating to rabbit eyes in a study done by the Swedish military in accordance to OECD Guidelines 405 (Koch et al. 1985 - quoted from Toxline pre 1981-1999).
2-Ethylhexyl acrylate was irritating to rabbit eyes in a study done by BASF (BASF - quoted from IUCLID 1996).
Inhalation
Wistar rats (10 animals of each sex per group) were whole-body exposed (OECD-guideline 413) to 2-ethylhexyl acrylate vapours at 75, 225 and 750 mg/m3 6 hours a day, 5 days per week for 90 days. No animals died in any dose-group. In mid and high-dose animals, a reduced body weight gain, lethargy and reduced levels for albumin were observed; the olfactory epithelium of the nasal mucosa was degenerated; and female rats had reduced levels of total protein and glucose. Besides high-dose female rats had a higher level than normal of liver enzymes. Low-dose animals showed no toxic signs. (BASF 1989 - quoted from IUCLID 1996 and BUA 1994).
Mice were exposed to 2-ethylhexyl acrylate at 103 mg/m3 for 4.5 months. Exposure time and interval is not stated in this Russian study. Respiratory tract irritation, dyspnoea, increased level of liver enzymes and reduced diuresis were observed. The study states a limit value of 10 mg/m3 for inhalation and a limit value of 46 mg/m3 for the effect on CNS. (Lomonova 1982 - quoted from IUCLID 1996).
Oral administration
No data were found.
Dermal contact
There was an apparent increase in the frequency of chronic nephritis in C3H/HeJ mice (68%) treated three times a week for their lifetime with 20 mg 75% (v/v) 2-ethylhexyl acrylate in acetone applied to clipped dorsal skin compared to the negative control (15%). Survival was not affected by the treatment with 2-ethylhexyl acrylate. (DePass et al. 1985).
100 ml of 86.5% 2-ethylhexyl acrylate in acetone applied 3 or 5 times a week for 16 or 68 days to female NMRI mice caused no skin changes in the short term experiment but skin irritation in the 68 day experiment. (BASF 1981 - quoted from IUCLID 1996).
Male NMRI and C3H/HeJ mice (10 animals per group) exposed to doses of 25ml 21% or 86.5% 2-ethylhexyl acrylate in acetone 3 times a week for 3 months exhibited skin irritation. The NMRI mice were less sensitive than the C3H/HeJ mice. No skin irritation were observed for the NMRI mice given 21% 2-ethylhexyl acrylate. (BASF 1985 - quoted from IUCLID 1996).
In long term (2 years or for life) carcinogenicity studies with 2-ethylhexyl acrylate applied to NMRI or C3H/HeJ mice (80 animals per group) 3 times a week, skin irritation (scaling, scabbing, hyperkeratosis, hyperplasia, crust formation and ulceration) was observed. Survival were not affected by the treatment with 2-ethylhexyl acrylate and no systemic effects were seen. (Mellert et al. 1994, Wenzel-Hartung et al. 1989). The lowest dose administered to the C3H/HeJ mice was 25 ml of a 2.5% (w/w) solution of 2-ethylhexyl acrylate in acetone. Skin irritation was observed at this dose, however, after the 11th week of treatment, these lesions were reversible. One group of C3H/HeJ mice was treated with a 43% solution for 24 weeks and thereafter observed for lifetime. Skin lesions were reversible in the 43% group immediately after treatment was stopped. For the higher doses (21% and 86.5%) further skin lesions developed. (Wenzel-Hartung et al. 1989).
Male and female New Zealand white rabbits (2 or 1 animals of each sex) exposed to 1 ml per day of 2-ethylhexyl acrylate for 3 or 12 days developed skin inflammation. After 12 days necroses and ulcerations were also observed. (Hunter et al. 1981 - quoted from IUCLID 1996).
Male and female guinea pigs (5 animals of each sex) exposed to 0.5 ml per day of 2-ethylhexyl acrylate for 12 days developed skin inflammation, necroses and ulcerations. The lesions were worse than the lesions seen in rabbits exposed to the double dose of 2-ethylhexyl acrylate. (Hunter et al. 1981 - quoted from IUCLID 1996).
4.3 Reproductive and developmental effects
2-Ethylhexanol is a metabolite of 2-ethylhexyl acrylate. 2-Ethylhexanol in high doses (above 800 mg/kg b.w.) has caused developmental effects in rats. (Ritter et al. 1987).
4.4 Mutagenic and genotoxic effects
2-Ethylhexyl acrylate was not mutagenic in 4 strains (TA98, TA100, TA1535, and TA1537) of Salmonella typhimurium in an Ames test with or without metabolic activation systems (Zeiger et al. 1985).
2-Ethylhexyl acrylate tested in cultured L5178Y mouse lymphoma cells without exogenous activation produced an equivocal result for an increased mutant frequency as well as for induced aberrations. No increase in the number of micronuclei was seen. (Dearfield et al. 1989).
In another experiment the mutation frequency was up to 4.6 times greater than in controls for the highest dose levels of 2-ethylhexyl acrylate added to cultured L5178Y mouse lymphoma cells with metabolic activation. No reproducible increase in mutation frequency was seen without the metabolic activation. (Litton Bionetics 1984 - quoted from HSDB 1999).
2-Ethylhexyl acrylate did not induce a dose-related increase in the hgprt mutant frequency in either the suspension or monolayer assay in Chinese hamster ovary cells (Moore et al. 1991).
A cell transformation assay in C3H-10T1/2 cells tested negative with 2-ethylhexyl acrylate (BASF 1982 - quoted from IUCLID 1996).
The sister chromatid exchange assay in CHO cells with and without metabolic activation was slightly positive when tested with 2-ethylhexyl acrylate with metabolic activation (ambiguous result) (BASF 1980 - quoted from IUCLID 1996).
Unscheduled DNA synthesis in primary rat hepatocytes was slightly increased when tested with 2-ethylhexyl acrylate (ambiguous result) (BASF 1980 - quoted from IUCLID 1996).
No chromosome aberrations were observed when mice were given an oral dose of 2.5 g/kg once a day for 1 or 5 days in an in vivo cytogenetic assay (BASF- quoted in IUCLID 1996).
In a 2-year carcinogenicity study 25 ml of a 21.5, 43 or 85% (w/w) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of male NMRI mice (80 per group) three times a week. After about 7 months half of each group was rested for treatment for 2 months and then treated with a promoter for 20 weeks. None of the mice treated with 2-ethylhexyl acrylate alone developed a skin tumour at the application site. One squamous cell papilloma occurred in each of the groups treated with 2-ethylhexyl acrylate and the promoter. Squamous cell carcinomas were observed only in the positive control groups (exposed to 0.015 % benzo[a]pyrene alone or in combination with promoter). (Mellert et al. 1994).
In a lifetime carcinogenicity study 25 ml of a 2.5, 21 or 86.5% (w/w) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of male C3H/HeJ mice (80 per group) three times a week. Another group was treated with a 43% solution for 24 weeks and thereafter observed for lifetime. Only in the 86.5% and 21% test groups showing chronic irritative skin damage there was a high incidence of neoplastic skin lesions (total of 15 papillomas, 36 carcinomas, and 16 melanomas) with no dose dependency. In contrast, no skin tumours were found in the negative control groups, in the group treated with 2.5% 2-ethylhexyl acrylate for lifetime or in the group treated with 43% 2-ethylhexyl acrylate for about 6 months and then observed for lifetime. (Wenzel-Hartung et al. 1989).
In a lifetime carcinogenicity study 20 mg of a 75% (v/v) solution of 2-ethylhexyl acrylate in acetone was applied epicutaneously to the clipped dorsal skin of 40 male C3H/HeJ mice three times a week. The concentration was chosen as the highest concentration that caused neither grossly observable irritation nor reduced weight gain in a preliminary 2-week study. Two animals had squamous cell carcinomas and four additional animals had squamous cell papillomas all on the treated skin. (DePass et al. 1985).
5 Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
2-Ethylhexyl acrylate is classified for irritative effects (Xi;R37/38 - irritating to respiratory system and skin) and for sensitising effects (R43 - may cause sensitisation by skin contact) (MM 1997).
EU
-
IARC/WHO
Ethylhexyl acrylate is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 1994).
US-EPA
-
RD50
-
6 Summary
Description
2-Ethylhexyl acrylate is a colourless liquid with a sharp, musty odour. Its solubility in water is low (0.1g/l). Its vapour pressure is 0.14 mmHg. The odour threshold in air is 0.55-1.36 mg/m3.
In Denmark, the principal use of 2-ethylhexyl acrylate is in UV curable inks, lacquers and varnishes.
Environment
2-Ethylhexyl acrylate is not known to occur as a natural compound. It may be released into the environment in fugitive and stack emissions or in wastewater during its production and use.
If released to the atmosphere, 2-ethylhexyl acrylate will react with photochemically produced hydroxyl radicals and ozone with an estimated half-life of 10.3 hours. It may also photolyse in sunlight.
If released to soil and water, 2-ethylhexyl acrylate may biodegrade and it may hydrolyse, especially in an alkaline environment. 2-Ethylhexyl acrylate is not expected to adsorb to sediment or suspended particulate matter in water. It is expected to exhibit moderate mobility in soil. 2-Ethylhexyl acrylate will significantly volatise from water and near surface soil with an estimated half-life of between 7.3 hours and 2.7 days.
Human exposure
The most probable route of human exposure of 2-ethylhexyl acrylate is by inhalation of contaminated air especially at plants where it is manufactured and used.
Toxicokinetics
In the rat 2-ethylhexyl acrylate is readily absorbed through the gastrointestinal tract and after intraperitoneal application. The plasma radioactivity concentration peaked at 2-3 hours. Following absorption 2-ethylhexyl acrylate is distributed to various organs with the highest concentration occurring in the liver and kidney. 2-Ethylhexyl acrylate is rapidly metabolised and excreted. At high doses (100 mg/kg bw) the decline in concentration in the tissues was slower with levels remaining almost constant for 72 hours in adipose tissue. 2-Ethylhexyl acrylate is believed to undergo carboxylesterase-catalysed hydrolysis to alcohol and acrylic acid, like other acrylate esters. 2-Ethylhexyl acrylate to a minor extent reacts with non-protein SH groups resulting in thioethers and mercapturic acids being excreted in the urine and/or bile. The major route of excretion is the lungs (50-75%) followed by urine (7-41%) and faeces (0.01-3 %). The metabolite in the lungs is CO2.
Human toxicity
Seven cases of allergic contact dermatitis due to an acrylic based adhesive tape have been reported. Patch-testing revealed that all persons reacted to 2-ethylhexyl acrylate. No cases have been reported of respiratory sensitisation. In Finland 5 people have got contact urticaria due to 2-ethylhexyl acrylate.
Animal toxicity
Single peroral, dermal or inhalatory administration of 2-ethylhexyl acrylate each proved to be only slightly toxic (LC50-value for mice is greater than 7700 mg/m3; oral LD50-values ranged from 1.8 to 12.8 g/kg for different species; and dermal LD50-values ranged from 7.5 to 16 g/kg for different species).
When rats were inhaling about 1400 mg/m3 of 2-ethylhexyl acrylate for 8 hours no mortality occurred.
A reduced body weight gain, lethargy, and dyspnoea were observed in Alderley Park rats inhaling 2-ethylhexyl acrylate at 1000 mg/m3 6 hours a day, 5 days per week for 2½ week. Animals inhaling 375 mg/m3 for the same exposure period showed no toxic signs.
Skin sensitisation was observed in challenged guinea pigs that had been induced with intradermal injections of 2-ethylhexyl acrylate in concentrations of 0.5 M or 0.17 M in Freund´s complete adjuvant three times during 9 days; that had been induced with epicutaneous or intracutaneous application of 2-ethylhexyl acrylate in concentrations of 0.1% (w/v) 3 times a week for 3 weeks; or that in the Polak method had been induced with 1 mg 2-ethylhexyl acrylate in Freund´s complete adjuvant applied as injections in the footpads and the neck.
Repeated application to the skin of mice, rabbits and guinea pigs caused skin irritation and subsequent degeneration of the treated areas. C3H mice were more sensitive than NMRI mice. In a lifetime study with 2-ethylhexyl acrylate applied to mice 3 times a week skin irritation was seen. The lowest dose administered was 25 ml of a 2.5% (w/w) solution of 2-ethylhexyl acrylate in acetone. Skin irritation was observed at this dose, however, after the 11th week of treatment, these lesions were reversible. For the higher doses (21% and 86.5%) further skin lesions developed.
In one study 2-ethylhexyl acrylate was irritant to the rabbit eye but in another one it was non-irritating.
The olfactory epithelium of the nasal mucosa was degenerated when Wistar rats inhaled 2-ethylhexyl acrylate at 225 and 750 mg/m3 6 hours a day, 5 days per week for 90 days. A reduced body weight gain, lethargy and reduced levels for albumin were also observed at these doses. Animals inhaling 75 mg/m3 for the same exposure period showed no toxic signs.
An apparent increase in the frequency of chronic nephritis was seen in male C3H/HeJ mice treated three times a week for their lifetime with 20 mg 75% (v/v) 2-ethylhexyl acrylate in acetone applied to clipped dorsal skin.
Reproductive and developmental effects
2-Ethylhexanol is a metabolite of 2-ethylhexyl acrylate. 2-Ethylhexanol in high doses has caused developmental effects in rodents.
Mutagenic and genotoxic effects
2-Ethylhexyl acrylate was negative in Ames test with and without metabolic activation, in assays in Chinese hamster ovary cells and in a cell transformation assay in C3H-10T1/2 cells. In various in vitro studies in cultured L5178Y mouse lymphoma cells, CHO cells and primary rat hepatocytes, 2-ethylhexyl acrylate produced an equivocal result. An in vivo cytogenetic assay in mice was negative.
Carcinogenicity
In two dermal carcinogenicity studies performed on C3H/HeJ mice for 2 years or the duration of their natural lives, 2-ethylhexyl acrylate proved to be locally tumourigenic at doses above 21% 2-ethylhexyl acrylate in acetone applied epicutaneously 3 times a week. Skin irritation was seen at the site of application. When the treatment in one group was discontinued after 6 months and observations were kept up for as long as these animals lived, the local skin damage receded almost completely and no skin tumours were observed. In a group receiving 2.5 % 2-ethylhexyl acrylate, skin lesions were reversible after the 11th week of treatment and no tumours developed in this group. NMRI mice did not develop skin tumours in a 2-year study equivalent to the study in C3H/HeJ mice.
7 Evaluation
The critical effects in humans following exposure to 2-ethylhexyl acrylate are considered to be the skin sensitising and irritative/damaging effects on respiratory tract, skin and eyes. This is based on the following:
In humans, the available data on health effects are limited to seven cases of allergic contact dermatitis after exposure to 2-ethylhexyl acrylate and to some cases of contact urticaria. No cases have been reported of respiratory sensitisation.
In laboratory animals, the main effects observed is skin sensitisation, irritation in the respiratory tract, skin and eye and carcinogenicity.
Long term (90 days) inhalation of 2-ethylhexyl acrylate (225 and 750 mg/m3) by Wistar rats degenerated the olfactory epithelium of the nasal mucosa, reduced body weight gain, caused lethargy and induced changes in some biochemical substances. Skin sensitisation and irritation has been observed in several animal experiments as described earlier in this paper. Those concentrations administered in long-term animal trials which no longer led to any local irritative effects were around 2.5% for dermal application and 75 mg/m3 for inhalation. The two references to eye irritating properties is not mentioning the doses causing the effect/ lack of effect on the eye. The different results are therefore likely to be a result of different doses. Since 2-ethylhexyl acrylate is irritating to the skin, it is most likely that it is also irritating to the eye in high enough doses or concentrations.
The carcinogenic effect of 2-ethylhexyl acrylate is considered to be related to its skin irritating effect and not to a genotoxic mechanism. This is based on that 2-ethylhexyl acrylate is negative or has showed equivocal results in different in vitro mutagenic assays and no chromosome aberrations were observed in mice in an in vivo cytogenetic assay. 2-Ethylhexyl acrylate is not carcinogenic in NMRI mice and the carcinogenic activity of 2-ethylhexyl acrylate in the skin of male C3H/HeJ mice could be detected only in association with a chronic skin irritation. As long as human dermal exposure remains far below levels causing skin irritation or does not persist chronically it is very unlikely that tumours will develop.
With the available studies, a NOAEL cannot be set for skin sensitisation. However, a subchronic inhalation study of good quality exist. This study will be used as the basis for estimating a limit value in air. A level of 75 mg/m3 is considered as a NOAEL for degeneration of the olfactory epithelium in rats inhaling 2-ethylhexyl acrylate for 6 hours a day, 5 days a week for 90 days.
8. Limit value in air
The limit value is calculated based on a NOAEL of 75 mg/m3 for degeneration of the olfactory epithelium in rats inhaling 2-ethylhexyl acrylate 6 hours a day, 5 days a week for 90 days.
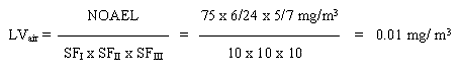 |
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 because: a) the study on which the NOAEL is based is not a chronic study and concern for the development of cancer in the respiratory system exist; b) no data on reproductive toxicity are available; c) 2-ethylhexyl acrylate is known to cause skin sensitisation and can therefore possibly cause respiratory sensitisation as well.
9. C-value
A limit value of 0.01 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990). A C-value of 0.01 mg/m3 and placing in Main Group 2, is proposed.
2-Ethylhexyl acrylate has a low odour threshold in air (0.55-1.36 mg/m3). However, the proposed limit value of 0.01 mg/m3 is considered to take into account the discomfort from odour.
C-value
0.01 mg/m3, Main Group 2.
10. References
AMI (1990). Allergi- og overfølsomhedsfremkaldende stoffer i arbejdsmiljøet. AMI-rapport 33.
BUA (1994). 2-Ethylhexyl acrylate (December 1991). Beatergremium für umweltrelevante Alstoffe (BUA) 88, Geschellschaft Deutscher Chemiker, p. 1-55.
Cikrt M, Vodicka P, Sapota A, Gut I, Stiborová A and Kopeckı J (1986). Biliary excretion and organ distribution of 14C radioactivity after 14C-2-2-ethylhexyl acrylate administration in rats. J Hyg Epidemiol Microbiol Immunol 30, 365-370.
Clayton GD and Clayton FE (1994). Acrylates. In: Patty´s Industrial Hygiene and Toxicology, 4th ed. New York, John Wiley Sons, vol. 2D, 2999-3007.
DPIMR (1981). 2-Ethylhexyl acrylate. In: Dangerous Properties of Industrial Materials Report, vol. 1, 57-59.
Dearfield KL, Millis CS, Harrington-Brock K, Doerr CL and Moore MM (1989). Analysis of the genotoxicity of nine acrylate/methacrylate compounds in L5178Y mouse lymphoma cells. Mutagenesis 4, 381-393.
DePass LR, Maronpot RR and Weil CS (1985). Dermal oncogenicity bioassays of monofunctional and multifunctional acrylates and acrylate-based oligomers. J Toxicol Environ Health 16, 55-60.
Gut I, Vodicka P, Cikrt M, Sapota A and Kavan I (1988). Distribution and elimination of (14C)-2-ethylhexyl acrylate radioactivity in rats. Arch Toxicol 62, 346-350.
HSDB (1999). 2-Ethylhexyl acrylate. In: Hazardous Substances Data Base.
IARC (1994). 2-Ethylhexyl acrylate. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 60, 475-487.
ICSC (1993). 2-Ethylhexyl acrylate. In: International Chemical Safety cards (WHO/IPCS/ILO), ICSC number: 0478, http//www.cdc.gov/niosh/ipcneng/neng0478.html.
IUCLID (1996). 2-Ethylhexyl acrylate. In: International Uniform Chemical Information Database. Existing Chemicals 1996. ECB, JRC, Ispra.
Jordan WP (1975). Cross-sensitization patterns in acrylate allergies. Cont Derm 1, 13-15.
Mellert W, Kühborth B, Gembardt C and Munk R (1994). 2-year carcinogenicity study in the male NMRI mouse with 2-ethylhexyl acrylate by epicutaneous administration. Fd Chem Toxic 32, 233-237.
MM (1997). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
Moore MM, Parker L, Huston J, Harrington-Brock K and Dearfield KL (1991). Comparison of mutagenicity results for nine compounds evaluated at the hgprt locus in the standard and suspension CHO assays. Mutagenesis 6, 77-85.
Nordisk Ministerråd (1991). Review over 2-ethylhexylacrylate´s (EHA) allergene effekter. In: Kriteriedokumenter fra et nordisk allergiprojekt 51, 135-137.
Parker D and Turk JL (1983). Contact sensitivity to acrylate compounds in guinea pigs. Cont Derm 9, 55-60.
Ritter EJ, Scott WJ, Randall JL and Ritter JM (1987). Teratogenicity of di(2-ethylhexyl)phthalate, 2-ethylhexanol, 2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine. Teratology 35, 41-46.
RTECS (1999). Acrylic acid, 2-ethylhexyl ester. In: Registry of Toxic Effects of Chemical Substances.
Ruth JH (1986). Odor thresholds and irritation levels of several chemical substances: a review. Am Ind Hyg Assoc J 47, A142-A151.
Samimi B and Falbo L (1982). Monitoring of workers exposure to low levels of airborne monomers in a polystyrene production plant. Am Ind Hyg Assoc J 43, 858-862.
Sapota A (1988). The disposition of [2,3-14C]-methyl and [2,3-14C]-2-ethylhexyl acrylate in male Wistar albino rats. Arch Toxicol 62, 181-184.
Toxline (pre 1981-1999). 2-Ethylhexyl acrylate. In: Toxline database.
Vodicka P, Gut I and Frantík E (1990). Effects of inhaled acrylic acid
derivatives in rats. Toxicology 65, 209-221.
Waegemaekers TH and van der Walle HB (1983). The sensitizing potential of 2-ethylhexyl acrylate in the guinea pig. Cont Derm 9, 372-376.
Wenzel-Hartung RP, Brune H and Klimisch HJ (1989). Dermal oncogenicity study of 2-ethylhexyl acrylate by epicutaneous application in male C3H/HeJ mice. J Cancer Res Clin Oncol 115, 543-549.
Zeiger E, Haworth S, Mortelmans K and Speck W (1985). Mutagenicity testing of di(2-ethylhexyl)phthalate and related chemicals in Salmonella. Environ Mutag 7, 213-232.
[Front page] [Contents] [Previous] [Next] [Top] |