|
Environmental Project, 615, 2001 Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent ProductsContents
PrefaceThe present report is the result of a project funded by the Council for recycling and cleaner technology. The project was initiated in January 1998. The report contains a compilation of data describing the environmental and health related properties of substances in detergents, a presentation of new data from ecotoxicological laboratory tests, and an assessment of the potential hazards of the substances to the environment and human health. The report was prepared by CETOX (Centre for Integrated Environment and Toxicology) which is a ‘centre without walls’ between DHI Water & Environment and Danish Toxicology Center. The project was followed by a steering committee, which held six meetings during the project period. The steering committee was composed of the following members:
We thank the members of the steering committee for their contributions and co-operation during the project.
Hørsholm, 18 December, 2000, SummaryThis report reviews the literature describing the inherent environmental and health properties of substances in household detergents and cosmetic detergent products. These products are used in high volumes, and the total annual consumption exceeded 70,000 tons in Denmark (in 1997) and 9,000,000 tons in Europe (in 1998). The review contains an up-to-date presentation of the available data and includes the following groups of substances: Anionic surfactants, nonionic surfactants, cationic surfactants, amphoteric surfactants, complexing agents, preservatives, bleaching agents, acids and bases, solvents, and fragrances. The main emphasis is directed towards the four groups of surface active agents, i.e. anionic, nonionic, cationic, and amphoteric surfactants. The outcome of the study is a compilation of data and information organized in specific chapters for each of the substance groups described above. The report includes a ranking of single substances on the basis of their inherent environmental or health properties. The ranking of substances may be used to indicate cases for a more detailed risk assessment or potentially hazardous chemicals that may be considered for substitution. 1. Introduction
The present study includes an environmental and human health hazard assessment of substances in household detergents and cosmetic detergent products. These products are used in high volumes, and the total annual consumption exceeded 70,000 tons in Denmark (in 1997) and 9,000,000 tons in Europe (in 1998). During the last decade particular attention has been addressed to the substances that are used in consumer products. Some components that were formerly used in these products have now been replaced by substances with better environmental or health properties. For example, the nonionic surfactants alkylphenol ethoxylates are transformed in the environment to recalcitrant metabolites that are more toxic than the original surfactants, and both alkylphenol ethoxylates and the metabolites are suspected to have ‘hormone-mimicking’, estrogenic effects. Today alkylphenol ethoxylates have largely been replaced by other surfactants in household detergents and personal care products by voluntary agreements between the authorities and industry. For other substances (e.g. preservatives), certain limit values define the maximum concentrations that are permitted for the different product types (Cosmetic Directive 2000). The report reviews the literature on the most important groups of substances in household detergents and cosmetic detergent products. The general approach has been to compile and evaluate data from standardized tests in order to direct the review towards the parameters that are included in the European legislation and to facilitate a comparison between the different substances. A few additional ecotoxicological laboratory tests were conducted in order to improve the knowledge on the inherent environmental properties of specific substances. The main emphasis is directed towards the four groups of surface active agents, i.e. anionic, nonionic, cationic, and amphoteric surfactants. The report presents a method for ranking of single substances on the basis of their inherent environmental or health properties. The ranking of substances may be used to indicate cases for a more detailed risk assessment or potentially hazardous chemicals that may be considered for substitution. The term ‘toxicity’ has been used throughout the report to describe either ecotoxicity or the potential effects towards human health. The specific sections for environmental and health assessment will probably solve the potential confusion in most cases. The report includes a large number of taxonomical names that may also lead to confusion as the nomenclature has changed for some species over the years. The approach in the present report is to apply the same names that were used in the original reference and to neglect changes in nomenclature that were decided after its publication. However, an exception was made for the freshwater microalga which was formerly known as Selenastrum capricornutum. Several names were proposed (including Raphidocelis subcapitata and Kirchneria subcapitata), before it was agreed that the correct name for this species is Pseudokirchneriella subcapitata (ISO 1999). For convenience, the name Selenastrum capricornutum is used to describe the studies conducted before 1999, whereas Pseudokirchneriella subcapitata is applied in the few cases where this name is used in the original reference. The following abbreviations have been used throughout the text. 1.1. List of abbreviations
2. Consumption of detergent and cleaning productsMost detergents are formulated products containing surfactants which remove dirt, stain, and soil from surfaces or textiles. Surfactants consist of a hydrophobic and a hydrophilic component and have the ability to change the surface properties of water. In aqueous solutions, surfactants tend to accumulate at air/solution or solid/liquid interfaces, whereby the surface tension of water is reduced. The physico-chemical properties of surfactants are the basis for their numerous applications. A very important effect of surfactants in cleaning products is the wetting effect. Because of the reduced surface tension, the water can be more evenly distributed over the surface and this improves the cleaning process. The emulsifying effect of surfactants is important for both cleansing and washing of textiles. Due to the hydrophobic and hydrophilic parts, surfactants can sorb to non-polar and polar materials at the same time. During cleansing and washing, the non-polar materials are kept in emulsions in the aqueous solution and removed by rinsing. By varying the hydrophobic and hydrophilic part of a surfactant, a number of properties may be adjusted, e.g. wetting effect, emulsifying effect, dispersive effect, foaming ability and foaming control. Surfactants are grouped according to their ionic properties in water:
The two major markets, household detergents and industrial and institutional cleaning products, consume more than 1 million and more than 200 thousand tons surfactants, respectively, in Europe (Morse 1999). The formulations, or products, in which these volumes are used, differ markedly in their contents of surfactants. E.g., a liquid product may contain approximately 50% surfactant compared to less than 25% in powders. The consumption of various household detergent products is estimated below by inclusion of figures from several sources (Table 2.1). Table 2.1
Mildness is an important property that plays a significant role for the use of surfactants in household products. Today, anionic surfactants are used in the largest volume, but the growth of anionic surfactants is expected to be relatively slow in the next few years, as they are gradually replaced by milder nonionic and amphoteric surfactants. The trend towards milder surfactants has already favoured the use of specific surfactant types. Mild components such as the amphoteric surfactants, alkyl betaines and alkylamido betaines, as well as the anionic surfactants, a -olefin sulfonates (AOS), are used in increasing volumes and the consumption of these chemicals is expected to grow (Morse 1999). The consumption of surfactants in household and in industrial and institutional detergents is estimated below by inclusion of figures from several sources (Table 2.2-2.3). Table 2.2
3. Anionic surfactants
3.1 Alkyl sulfatesAlkyl sulfates (AS) are used in laundry detergents, frequently in combination with other anionic surfactants. Besides, AS are used in speciality products, including wool-washing agents, soap bars and liquid bath soaps, hair shampoos, and tooth pastes. Most of the AS used in consumer products are linear primary AS but some linear and branched secondary AS are also used (Painter 1992). Primary AS have the structure:
Secondary AS have the structure:
The hydrophobic alkyl chain (R or R1 + R2) usually contains 12-18 carbon atoms. The sulfate group of secondary AS is found at all positions along the alkyl chain, except at the ends. The most widely used surfactant is the sodium salt, but raw materials with various other cations like, e.g., ammonium, magnesium, mono-, di-, tri-ethanolamine and cyclohexamine, are also produced. 3.1.1 Occurrence in the environmentVery few data on the concentration of AS in the environment could be found. The best basis for predicting the concentrations of AS in the aquatic environment is probably the data obtained in the monitoring program which was executed jointly by the Dutch Soap Association (NVZ) and the Dutch authorities. The monitoring showed that the concentrations of C12-15 AS in the effluent of seven representative municipal sewage treatment plants varied between 0.0012 and 0.012 mg/l with an average value of 0.0057 mg/l (Matthijs et al. 1999). 3.1.2 Environmental fateBiodgradation patjways The biological degradation of AS is initiated by a hydrolytic cleavage of the sulfate ester bond catalysed by alkylsulfatases. The cleavage leaves inorganic sulfate and fatty alcohol which undergo oxidation by dehydrogenases to produce fatty acids via fatty aldehydes. The fatty acids are degraded by b -oxidation and finally totally mineralised or incorporated into biomass (Steber and Berger 1995). The biodegradation pathway for secondary AS differs from that of the primary AS by the formation of a ketone instead of an aldehyde. The ketone undergoes hydroxylation and forms an aldehyde and a carboxylic acid, which are further degraded by the b -oxidation. Biodegradation under anoxic conditions is anticipated to follow the same pathway as for the aerobic degradation (Steber and Berger 1995). Effects of structure on biodegradability of A/S Primary and secondary AS generally undergo complete primary biodegradation within a few days followed by a rapid ultimate biodegradation. Branched AS are also degraded quite rapidly, but multiple branchings of the alkyl chain considerably reduce the rate and extent of primary biodegradation (Swisher 1987; Painter 1992). The effect of branching was illustrated by a study in which the primary biodegradation was examined for a number of C12-15 AS with varying proportions of linear components. Primary biodegradation of anionic surfactants is usually quantified by measurements of methylene blue active substances (MBAS) which indicate a loss of surface-activity. The time required for the removal of 95% MBAS ranged from only 1 day for a coconut oil-AS containing 99% linear material, through 3 days for an oxo-AS containing 50% linear components, to as long as 12 days for an AS derived from tetra propylene containing less than 5% linear material (Painter 1992). The ultimate aerobic biodegradability of AS was more or less unaffected of a 2-alkyl branching, and different structures with 2-alkyl branches of C1 (methyl-), C4 (butyl-) or C6 (hexyl-) were all readily degradable in the closed bottle test. Extensive branching of AS, as in a C13 propylene tetramer (4 internal CH3-groups; 10% quaternary carbons) and a C13 butylene trimer, however, may preclude compliance with the pass criteria for ready biodegradability (Battersby et al. 2000; Table 3.1). Aerobic biodegradability Rapid primary degradation of AS has frequently been reported for OECD tests, model sewage treatment systems, and seawater (Painter 1992; Steber and Berger 1995). There are numerous studies confirming the aerobic biodegradability of AS, and linear primary AS exceeds all other anionic surfactants in the rate of primary and ultimate biodegradation. Also secondary AS are normally readily biodegradable as, e.g., the oxygen uptake from biodegradation of a linear secondary C10-13 AS corresponded to 77% ThOD in 22 days. Some highly branched AS being poorly primary biodegradable may also resist ultimate biodegradation (Painter 1992). The fate of AS in wastewater treatment plants was illustrated in a model system using 14C-labelled C18 AS. At steady state, 60% of the added 14C was mineralized, 30% was associated with the sludge, and 10% was found in the effluent. About 90% of the 14C in the sludge was ascribed to bacterial biomass, and only 0.3% of the 14C found in the effluent was intact AS (Steber and Berger 1995). This indicates that AS are efficiently removed in wastewater treatment plants. Earlier studies have indicated that only 12-55% MBAS of a branched C13 AS was removed in activated sludge simulation tests (Painter 1992). Linear AS are readily biodegradable in the OECD 301 tests, whereas branching of the alkyl chain may lead to a less extensive ultimate biodegradability (Table 3.1). Table 3.1
Anaerobic biodegradation of AS has been investigated in systems using digested sludge. A simple screening method, which was applied by Birch et al. (1989) and in the present study, determines the ultimate anaerobic biodegradability by measuring the gas production (i.e., CO2 and CH4) in sealed vessels containing diluted sludge (ECETOC 1988; ISO 1995). The test substance is added at a high concentration (e.g., 20-50 mg of carbon per litre) in order to measure the total net gas production from mineralization of the test substance. A drawback to the method is that the required concentration of test substance may inhibit the anaerobic bacteria and, hence, provide unfavourable conditions for biodegradation. On the other hand, the bacterial community in the digested sludge may be better adapted to biodegradation of man-made chemicals than the bacteria in natural habitats. The possibilities for predicting the fate in anoxic environments from results obtained in the screening tests have not yet been evaluated. Both linear and 2-alkyl-branched primary AS are degraded to a high extent under anaerobic conditions (Table 3.2). Table 3.2
Bioaccumulation Bioaccumulation of AS in aquatic organisms has been determined in tests with goldfish, rainbow trout, carp and guppy. The majority of these experiments has been performed with radiolabelled compounds, mainly 35S-labelled AS, which do not allow a distinction between parent AS and metabolites. As the AS is metabolised in the organism, the bioconcentration factor for the intact surfactant may be overestimated in experiments using radiolabelling techniques instead of chemical analyses. Whole body BCF values, as well as specific tissue BCF values, have been determined in fish for AS between C12 and C16 (Table 3.3). Table 3.3
3.1.3 Effects on the aquatic environmentThe aquatic toxicity of AS seems to increase with increasing alkyl chain length. This has been shown for daphnids and for some fish species. An overall comparison of the acute toxicity between the primary and secondary AS shows only minor differences in the toxicity, although only a few studies for comparison are available. Algae The available data describing the toxicity of AS towards algae indicate that the lowest EC50 values range between 1 and 10 mg/l for C12 AS (Table 3.4). Table 3.4
1 Test based on assimilation of 14C-NaHCO3.* Effect concentration based on measured concentrations. Invertebrates The toxicity of AS towards invertebrates has mainly been examined in tests with Daphnia magna. Lundahl and Cabridenc (1978) showed that the acute toxicity of AS to Daphnia magna increased with increasing alkyl chain length (Table 3.5). It has been shown that during degradation of C12 AS, the toxicity first increased to a maximum after 30 hours and then fell to almost a negligible value. The increase in toxicity was explained by the formation of the more toxic dodecanoic acid which is rapidly transformed to other and less toxic metabolites (Painter 1992). Table 3.5
* Effect concentration based on measured concentrations. Fish The toxicity of AS to fish has been demonstrated to increase with increasing alkyl chain length as also seen in studies with Daphnia magna. Studies performed by Kikuchi et al. (1976) showed that the 24 h-LC50 values for killifish in distilled water decreased by a factor of about 10 when the alkyl chain was increased by two carbon atoms. C16 was 10 times more toxic than C14, which was about 10 times more toxic than C12 (Table 3.6). Differences between the toxicity values for AS with similar chain lengths may be due to different species, but are probably also a result of different times of exposure and hardness of water (Painter 1992). Table 3.6
Whereas most correlations between AS structure and toxicity show an increasing toxicity with increasing alkyl chain length, the budding in Hydra attenuata was apparently more affected by C10 AS than by C12, C14, and C16 AS (Bode et al. 1978) (Table 3.7). The authors suggested that the decrease in toxicity with increasing alkyl chain length was attributable to reduced solubility in water. Table 3.7
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AS |
Species |
Route |
LD50 (mg/kg body weight) |
Reference |
Various |
Rat |
Oral |
5,000-15,000 |
Kirk-Otmer 1994 |
Various |
Rat |
Oral |
1,000-11,000 |
Falbe 1986; Gloxhuber and Künstler 1992; |
C6-18 |
Mouse |
Oral |
2,200 - < 8,000 |
Gloxhuber and Künstler 1992 |
C12 |
Rat |
Oral |
1,200 |
Gloxhuber and Künstler 1992 |
C12 |
Rat |
Oral |
1,000 – 2,700 |
Singer and Tjeerdema 1993 |
Skin and eye irritation
For a homologous series of AS (C8 to C16), maximum swelling of stratum corneum (the outermost layer of epidermis) of the skin was produced by the C12 homologue. This is in accordance with the fact that the length of the hydrophobic alkyl chain influences the skin irritation potential. Other studies have shown that especially AS of chain lengths C11, C12 and C13 remove most amino acids and soluble proteins from the skin during washing (Prottey and Ferguson 1978; Rhein et al. 1986). Concentrated samples of AS are skin irritants in rabbits and guinea pigs. AS are non-irritant to laboratory animals at a 0.1% concentration (Gloxhuber and Künstler 1992). C12 AS is used in research laboratories as a standard substance to irritate skin and has been shown to induce an irritant eczema (Frankild 1992). AS were found, by many authors, to be the most irritating of the anionic surfactants, although others have judged the alkyl sulfates only as irritant as laurate (fatty acid soap) (Tupker 1990).
A structure/effect relationship with regard to the length of the alkyl chain can also be observed on mucous membranes. The maximum eye irritation occurs at chain lengths of C10 to C14 (Falbe 1986). In acute ocular tests, 10% C12 AS caused corneal damage to the rabbit eyes if not irrigated (Davies et al. 1976). Another study showed that a 1.0% aqueous C12 AS solution only had a slight effect on rabbit eyes, whereas 5% C12 AS caused temporary conjunctivitis, and 25% C12 AS resulted in corneal damage (Singer and Tjeerdema 1993).
Subchronic toxicity
In a 13-week feeding study, rats were fed dietary levels of 0, 40, 200, 1,000 or 5,000 ppm of C12 AS. The only test material related effect observed was an increase in absolute organ weights in the rats fed with the highest concentration which was 5,000 ppm. The organ weights were not further specified and no other abnormalities were found (Walker et al. 1967).
Mutagenicity and carcinogenicity
In a mutagenicity study, rats were fed 1.13 and 0.56% C12 AS in the diet for 90 days. This treatment did not cause chromosomal abberations in the bone marrow cells (Hope 1977). Mutagenicity studies with Salmonella typhimurium strains (Ames test) indicate no mutagenic effects of C12 AS (Mortelmans et al. 1986). The available long-term studies in experimental animals (rats and mice) are inadequate to evaluate the carcinogenic potential of AS. However, in studies in which animals were administered AS in the diet at levels of up to 4% AS, there was no indication of increased risk of cancer after oral ingestion (Falbe 1986; IPCS 1996).
Reproductive toxicity
No specific teratogenic effects were observed in rabbits, rats or mice when pregnant animals were dosed with 0.2, 2.0, 300 and 600 mg C12 AS/kg body weight/day by gavage during the most important period of organogenesis (day 6 to 15 of pregnancy for mice and rats and day 6 to 18 of pregnancy for rabbits). Reduced litter size, high incidence of skeletal abnormalities and foetal loss were observed in mice at 600 mg C12 AS/kg/day, a dose level which also caused severe toxic effects in the parent animals in all three species (Palmer et al. 1975a; Singer and Tjeerdema 1993). An aqueous solution of 2% AS was applied (0.1 ml) once daily to the dorsal skin (2 x 3 cm) of pregnant mice from day 1 to day 17 of gestation. A solution of 20% AS was tested likewise from day 1 to day 10 of gestation. The mice were killed on days 11 and 18, respectively. A significant decrease in the number of implantations was observed when mice were treated with 20% AS compared to a control group which was dosed with water. No evidence of teratogenic effects was noted (Nomura et al. 1980).
When aqueous solutions of 2% and 20% AS (0.1 ml) were applied once per day to the dorsal skin (2 x 3 cm) of pregnant ICR/Jc1 mice from day 12 to day 17 of gestation no effects on pregnancy outcome were detected. Treatment with 20% AS resulted in growth retardation of suckling mice, but this effect disappeared after weaning (Nomura et al. 1980). A 10% AS solution (0.1 ml) was applied twice daily to the dorsal skin (2 x 3 cm) of pregnant lCR/Jc1 mice during the preimplantation period (days 0-3 of gestation). A significant number of embryos collected on day 3 as severely deformed or remained at the morula stage. Nomura et al. (1980) reported that the number of embryos in the oviducts was significantly greater for the mice dosed with AS as compared to the control mice. No pathological changes were detected in the major organs of the dams.
Classification
AS are generally classified according to Comité Européen des Agents de Surface et leurs Intermédiaires Organiques (CESIO) as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes). An exception has been made for C12 AS which is classified as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed) and R38 and R41 (CESIO 2000).
AS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.2 Alkyl ether sulfates
Alkyl ether sulfates (AES), or alkyl ethoxy sulfates, are being used increasingly, frequently in combination with other anionic and nonionic surfactants, in liquid bath soaps, hair shampoos, and mechanical dishwashing agents. Besides, AES are important as ingredients in industrial cleaning agents and as auxiliaries in some industrial process steps (Steber and Berger 1995). AES are primary sulfate esters manufactured from the corresponding alcohol ethoxylates.
AES have the following structure(s):
![]()
Alkyl ether sulfates: R2 = H; R1 = C10-14; n = 1-4
Oxoalkyl ether sulfates: R2 = H, C1, C2; R1 +
R2 = C11-15; n = 1-4
The structures above describe the normal alkyl chain length for AES, but sometimes longer alkyl or ethoxylate chains are seen.
3.2.1 Occurrence in the environment
Very few data on the concentration of AES in the environment have been found. The monitoring conducted in the Netherlands showed that the concentrations of C12-15 AES in the effluent of seven representative municipal sewage treatment plants varied between 0.003 and 0.012 mg/l with an average value of 0.0065 mg/l (Matthijs et al. 1999).
3.2.2 Environmental fateBiodegradation pathways
The most frequent initial step in the biodegradation of AES is the cleavage of an ether bond (Steber and Berger 1995). The cleavage may take place at any ether bond producing a fatty alcohol or an alcohol ethoxylate and ethylene glycol sulfates of various lengths. The alcohol is degraded by w /b -oxidation, whereas the ethylene glycol sulfate is eliminated stepwise by oxidation and cleavage of C2-units along with a desulfation (Steber and Berger 1995). The ether cleavage and the desulfation may also take place in the absence of molecular oxygen, but the anaerobic biodegradation pathway has not yet been verified (Steber and Berger 1995).
Effects of structure on biodegradability of AES
The length of the alkyl chain and the number of EO units apparently do not affect the degree of aerobic biodegradation, but branching of the alkyl chain may hinder the primary biodegradation of AES. E.g., according to studies reported by Painter (1992), the removal of MBAS was 97% for a linear primary AES, 90% for a linear primary oxo-AES, and 50% for a branched tetra-propylene based primary AES during 3 days.
Aerobic biodegradability
AES are degraded readily and completely under aerobic conditions. E.g., for C12-14 AE3S, a rapid primary degradation of 90-100% is reported to take place within a period of 1 to 5 days (Painter 1992). In activated sludge simulation tests 67-99% DOC was removed by degradation of C12-14 AE2S and C12-15 AE3S (Schöberl et al. 1988). The ultimate biodegradation of AES has been confirmed in OECD 301 tests for ready biodegradability (Table 3.9).
Table 3.9
Ultimate aerobic biodegradability of AES.
AES |
Test |
Result |
Reference |
C12-14 AE2S |
Closed bottle test, 28 d |
58-100% ThOD |
Schöberl et al. 1988 |
C12-15 oxo-AE3S |
Modified OECD screening test, 28 d |
96-100% DOC |
Schöberl et al. 1988 |
CO2 evolution test, 28 d |
65-83% ThCO2 |
Schöberl et al. 1988 |
|
C12-18 AE8.5S |
Closed bottle test, 28 d |
100% ThOD |
Steber and Berger 1995 |
Anaerobic biodegradability
The primary of AES has been confirmed in early studies in which a removal of 64% MBAS for C12-14 AE3S (in 28 days) and 70% MBAS for C16 AE1S (in 17 days) were observed (Painter 1992). The ultimate anaerobic biodegradability of C12 AE3S was examined in gas production screening tests using either digested sludge, a marine sediment or material from a freshwater swamp as inoculum. The 20 mg of AES carbon per litre which was applied in these tests proved to be inhibitory to the anaerobic bacteria, and only in the digested sludge a net gas production corresponding to 23% ThGP was observed during 56 days (Madsen et al. 1996a). Experiments using a higher inoculum to test substrate ratio have shown that extensive biodegradation of AES may occur under anoxic conditions. Nuck and Federle (1996) examined the anaerobic degradation of a C14 AE3S which was 14C-labelled in the ethoxylate moiety. By using an inoculum of 24-29 g of digester sludge per litre of medium, the recovery of 14CO2 and 14CH4 equalled 88.4% (1 mg AES/l) and 87.6% (10 mg AES/l) after 17 days of incubation at 35oC.
Bioccumulation
The uptake, distribution and elimination of 35S labelled C12 AE3S and C12 AE5S have been investigated in carp (Cyprinus carpio) without distinction between parent AES and metabolites (Kikuchi et al. 1980). The following BCF values for the two substances, respectively, were determined: Whole body, 18 and 4.7; gall bladder, 3,400 and 940; and hepatopancreas, 46 and 18. Both the uptake and the elimination were reported to be rapid. Due to metabolisation of AES in the organism, the BCF for the intact surfactant may be overestimated in experiments using radiolabelled compounds. For the whole body, as well as for the gall bladder, the steady state was not reached within 72 hours and, hence, the reported BCF values are considered to be invalid. Furthermore, the fish were not fed during the study. The high concentrations found in the gall bladder are thus most probably due to biotransformation of AES in the liver and subsequent excretion of radiolabelled metabolites in the gall bladder (Comotto et al. 1979; Wakabayashi et al. 1987; Goodrich et al. 1991; Toshima et al. 1992). Based on the studies above, AES are not considered to bioconcentrate in aquatic organisms.
3.2.3 Effects on the aquatic environment
The chemical structure of AES highly influences the effect on aquatic organisms. The relations between alkyl chain length, number of EO groups and toxicity are complex and not yet resolved, but in general, changes in EO numbers affects toxicity more than changes in the alkyl chain length. In AES with alkyl chains of less than C16, the toxicity tended to decrease with increasing numbers of EO, but this was reversed for alkyl chain lengths above C16. The toxicity of AES thus seems to peak at alkyl chain lengths of C16. In a study of the acute toxicity of various AES (C8 to C19.6 and 1-3 EO) to bluegill sunfish (Lepomis macrochirus), the LC50 fell from > 250 mg/l for C8 and 375 mg/l for C10 to 24 mg/l for C13, 4-7 mg/l for C14, 2 mg/l for C15 and 0.3 mg/l for C16, and then increased to 10.8 mg/l for C17.9 and 17 mg/l for C19.6 (Little 1981).
Algae
Not very many and mainly quite old data describing the effects of AES towards algae were found in the literature. Besides the effect concentrations presented in Table 3.10, Kutt and Martin (1974) reported very low toxicity values for the marine red tide dinoflagellate, Gymnodium breve, when this species was exposed to coconut ethoxylate sulfate. The authors observed 87%, 63% and 44% inhibition at 0.0025; 0.0125 and 0.05 mg/l, respectively, after 48 hours of exposure. Experiments in which Gymnodium breve was exposed with LAS confirm that this species is highly sensitive to surfactants (Hitchcock and Martin 1977), and occasionally available data for Gymnodium breve should therefore not be used for comparison of the aquatic toxicity between various surfactants. Typical EC50 values describing the toxicity of AES towards algae vary between 4 and 65 mg/l (Table 3.10). In a microcosmos study performed by Belanger et al. (1996), the NOEC values appeared to be above the concentrations tested.
Table 3.10
Effects of AES to algae.
Species |
AES |
EC50 (mg/l) |
Test duration |
Reference |
Selenastrum capricornutum |
C10-15 AE3S |
65 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 AEnS |
20 (97% inhibition of growth) |
21 d |
Nyberg 1988 |
Selenastrum capricornutum |
C10-16 AE2S |
30 (91% inhibition of growth) |
21 d |
Nyberg 1988 |
Selenastrum capricornutum |
AES |
65 |
72 h |
Fendinger et al. 1994 |
Selenastrum capricornutum |
C12-14 AES |
32 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
Cx AE9S |
4-8 |
- |
Painter 1992 |
Nitzschia fonticula |
Cx AE9S |
5-10 |
- |
Painter 1992 |
Microcystis aeruginosa |
Cx AE9S |
10-50 |
- |
Painter 1992 |
MicrocosmosAlgae community |
C14.5 AES |
NOEC: 0.61* |
28 d |
Belanger et al. 1996 |
* Effect concentrations based on measured concentrations.
Invertebrates
Painter (1992) reported ranges for EC50 for the acute toxicity of AES to daphnids between 1 and 50 mg/l. However, an EC50 of 0.37 mg/l was observed in a 21-day reproduction test with Daphnia magna (Maki 1979). Also Belanger et al. (1995) observed very low effect concentrations of AES on invertebrates as both mayfly and bivalve populations were impaired at 0.77 mg/l during an 8-week mesocosmos study (Table 3.11).
Table 3.11
Effects of AES to invertebrates.
Species |
AES |
EC50/LC50 |
Test duration |
Reference |
Daphnia magna |
C13.67 AE2.25S |
1.17* (0.82-1.66)** |
96 h |
Maki 1979 |
Daphnia magna |
C13.67 AE2.25S |
0.74* |
21 d |
Maki 1979 |
Daphnia magna |
C13.67 AE2.25S |
0.37* |
21 d |
Maki 1979 |
Mesocosmos |
C14-15 AE2.17S |
LOEC: 0.77* |
56 d |
Belanger et al. 1995 |
*Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Fish
The LC50 values for fish are in the range between 0.39 to 450 mg/l (Table 3.12). A LOEC value of 0.22 mg/l has been reported for a chronic life cycle test with a duration of 1 year (Maki 1979). The toxicity of AES towards fish seems to increase with increasing alkyl chain length for AES with up to 16 carbons.
Table 3.12
Effects of AES to fish.
Species |
AES |
LC50 (mg/l) |
Test duration |
Reference |
Fathead minnow (Pimephales promelas) |
C11 AE4S |
17.0 |
24 h |
Painter 1992 |
Fathead minnow |
C12 AE2S |
1.5 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE2S |
1.8 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE2S |
1.0 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE2S |
80 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE4S |
4.0 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE4S |
0.9 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE4S |
15 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE6S |
9.3 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE6S |
0.8 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE6S |
2.1 |
24 h |
Painter 1992 |
Rainbow trout |
C9-10 AE2.5S |
400-450 |
96 h |
Painter 1992 |
Rainbow trout |
C12-13 AE2S |
28 |
96 h |
Painter 1992 |
Rainbow trout |
C12-15 AE3S |
8.9 |
96 h |
Painter 1992 |
Brown trout |
C12-15 AE3S |
1.0-2.5 |
96 h |
Reiff et al. 1979 |
Harlequin Fish |
C12-15 AE3S |
3.9 |
48 h |
Reiff et al. 1979 |
Golden orfe |
C12-15 AE3S |
3.95 |
48 h |
Reiff et al. 1979 |
Fathead minnow, fry |
C14-16 AE2,25S |
0.63 |
45 d |
Little 1981 |
Fathead minnow, juvenile |
C14-16 AE2,25S |
0.94 |
45 d |
Little 1981 |
Fathead minnow |
C13.7 AE2.25S |
LOEC: 0.22* |
365 d |
Maki 1979 |
Sheepshead minnow (Cyprinodon variegatus) |
C14-16 AE2.25S |
0.39 |
45 d |
Little 1981 |
* Effect concentrations based on measured concentrations.
Toxicokinetics and acute toxicity
AES are easily absorbed in the intestine in rats and humans after oral administration. Radiolabelled C11 AE3S and C12 AE3S were extensively metabolized in rats and most of the 14C-activity was eliminated via the urine and expired air independently of the route of administration (oral, intraperitoneal or intravenous). The main urinary metabolite from C11 AE3S is propionic acid-3-(3EO)-sulfate. For C12 and C16 AE3S, the main metabolite is acetic acid-2-(3EO)-sulfate. The alkyl chain appears to be oxidized to CO2 which is expired. The EO-chain seems to be resistant to metabolism (McDermott et al. 1975; Taylor et al. 1978). Only small amounts of non-specified AES were absorbed through the skin (Painter 1992). The LD50 values after oral administration of AES range from 1,000 – 5,000 mg/kg body weight for rats (Falbe 1986; Gloxhuber and Künstler 1992; Painter 1992) indicating a low acute toxicity.
Skin and eye irritation
AES are better tolerated on the skin than, e.g., alkyl sulfates and it is generally agreed that the irritancy of AES is lower than that of other anionic surfactants. Alkyl chain lengths of 12 carbon atoms are considered to be more irritating to the skin compared to other chain lengths (Tupker 1990; Gloxhuber and Künstler 1992). The skin irritating properties of AES normally decrease with increasing level of ethoxylation (Falbe 1986; KEMI 1990). Undiluted AES should in general be considered strongly irritating. Even at concentrations of 10% moderate to strong effects can be expected. However, only mild to slight irritation was observed when a non-specified AES was applied at 1% to the skin (SFT 1991).
Subchronic and long term toxicity
A 90-day subchronic feeding study in rats with 1% of AE3S or AE6S with alkyl chain lengths of C12-14 showed only an increased liver/body weight ratio (Scailteur et al. 1986). In a chronic oral study with a duration of 2 years, doses of C12-AE3S of 0.005 – 0.05% in the diet or drinking water had no effects on rats. The concentration of 0.5% sometimes resulted in increased kidney or liver weight (Falbe 1986; Scailteur et al. 1986; Painter 1992).
Carcinogenicity
There is no indication of increased risk of cancer after oral ingestion of AES. Carcinogenic effects were not observed after skin application (Falbe 1986; SFT 1991).
Reproductive toxicity
No evidence of reproductive and teratogenic effects was seen in a two-generation study in rats fed with a mixture (55:45) of AES and linear alkylbenzene sulfonates. Dietary levels of 0.1, 0.5, and 1% were administered to the rats either continuously or during the period of major organogenesis during six pregnancies. No changes in reproductive or embryogenic parametres were observed (Nolen et al. 1975).
Classification
AES are generally classified according to Comité Européen des Agents de Surface et leurs Intermédiaires organiques (CESIO) as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R36 (Irritaing to eyes). An exception has been made for AES (2-3E0) in a concentration of 70-75% where R36 is substituted with R41 (Risk of serious damage to eyes).
AES are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
3.3 Linear alkylbenzene sulfonates
Linear alkylbenzene sulfonates (LAS) are, by volume, the most important group of synthetic anionic surfactant today. LAS are mainly used in laundry detergents and cleaning agents. LAS are frequently used as the sodium salts as the sole surfactant in a formulation or in conjunction with other anionic, nonionic or cationic surfactants. LAS consist of an alkyl chain attached to a benzene ring in the para position to the sulfonate group. Sometimes toluene, xylene and naphthalene are used in place of benzene. The homologue distribution in commercial products covers alkyl chain lengths from C10 to C13 with an average chain length of C11.6. LAS raw materials are derived from linear alkyl benzenes in which the ring is attached to a C-atom which is itself attached to two other C-atoms. The benzene ring may be attached to any of the C atoms from C2 to C6 but not to C1. Structures in which the benzene ring may be attached to different C atoms are described as isomers. E.g., the structure with a C12 alkyl chain and the benzene ring attached at the second alkyl carbon is designated as the C12-2-isomer and abbreviated C12-2.
LAS have the following structure:
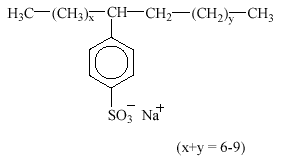
3.3.1 Occurrence in the environment
The concentrations of LAS have been monitored in several environmental compartments. The monitoring conducted in the Netherlands showed that the concentrations of LAS in the effluent of seven representative municipal sewage treatment plants varied between 0.019 and 0.071 mg/l with an average value of 0.039 mg/l (Matthijs et al. 1999). Concentrations of LAS in sewage sludges have been measured in the range of 2 to 12 g/kg for primary and anaerobically digested sludge (most in the range 4-10 g/kg), whereas aerobically digested sludge and activated sludge contained 2.1-4.3 g/kg and 0.09-0.86 g/kg, respectively (Painter 1992). A monitoring of contaminants in sludge samples from municipal sewage treatment plants in Denmark showed that the concentrations of LAS varied between 0.01 and 16 g/kg (Tørsløv et al. 1997). The median concentration of all examined sludge samples (20) was 0.53 g/kg, whereas the medians were 0.02 g/kg for 11 activated sludge samples and 0.94 g/kg for 9 samples consisting of a mixture of activated and anaerobically digested sludge (Madsen et al. 1998). LAS are found in soils that are treated with sewage sludge as a fertilizer. E.g., the concentration of LAS ranged from 2.5 to 40.3 mg/kg (median 25 mg/kg) in 7 soil samples that were collected immediately after dosing of the fields with sludge; these values fell to ‘control’ values within 21 to 122 days (Painter 1992).
Aquatic sediments may also contain LAS at mg/kg levels. E.g., the LAS concentrations were 1.5 to 10 mg/kg in 10 river sediments in Germany, whereas 25 to 174 mg/kg were found at four other sites. A Danish monitoring of contaminants in coastal marine sediments showed LAS concentrations of up to 22 mg/kg (Lillebæltssamarbejdet 1998). The highest concentration of 22 mg/kg was found in a fjord in the vicinity of the discharge of effluent from a municipal wastewater treatment plant.
3.3.2 Environmental fate
Biodegradation pathways
The initial step in the biodegradation of LAS under aerobic conditions is an w -oxidation of the terminal methyl group of the alkyl chain to form a carboxylic acid. Further degradation proceeds by a stepwise shortening of the alkyl chain by b -oxidation leaving a short-chain sulfophenyl carboxylic acid. In the presence of molecular oxygen the aromatic ring structure hydrolyses to form a dihydroxy-benzene structure which is opened before desulfonation of the formed sulfonated dicarboxylic acid. The final degradation steps have not been investigated in details but are likely to occur by general bacterial metabolic routes involving a total mineralisation and assimilation into biomass (Steber and Berger 1995). Both the initial w -oxidation and the hydroxylation of the ring structure of LAS require molecular oxygen, and they are not expected to take place under anoxic conditions (Steber and Berger 1995).
Aerobic biodegradability
Numerous data on primary and ultimate biodegradation of LAS have been reported. Primary degradation of 93-97% was measured as MBAS removal in OECD tests (Schöberl et al. 1988) and removal in wastewater treatment plants are reported to exceed 85% measured as MBAS (Steber and Berger 1995). The removal of LAS in wastewater treatment plants includes sorption to the sludge particles. For different treatment plants the sorbed amount was reported to be 3-15% with a total removal of 95-99% (Painter 1992). The ultimate biodegradation of LAS in aerobic screening tests fulfils the requirements for ready biodegradability in OECD 301 tests (Table 3.13). The degradation of LAS does not lead to an accumulation of metabolites as indicated by a 95% DOC removal in a test for detection of recalcitrant metabolites (Steber and Berger 1995).
Table 3.13
Ultimate aerobic biodegradability of LAS.
LAS |
Test |
Result |
Reference |
C10-13 |
Closed bottle test, 28 d |
55-65% ThOD |
Schöberl et al. 1988 |
Modified OECD screening test, 28 d |
73-84% DOC |
Schöberl et al. 1988 |
|
CO2 evolution test, 28 d |
45-76% ThCO2 |
Schöberl et al. 1988 |
Anaerobic biodegradability
Ultimate biodegradation of LAS under anoxic conditions has not been documented, and the known mechanisms that precede the aerobic mineralization, i.e. the w -oxidation and the hydroxylation of the benzene ring, require molecular oxygen. A primary anaerobic biodegradation of LAS may occur dependent on the environmental conditions. This has, e.g., been shown in continuous stirred tank reactors with anaerobically digested sludge, operated at 37° C, in which the anaerobic transformation of C12 LAS corresponded to between 20 and 25% of the initial concentration (Angelidaki et al. 2000). Another recent study by Denger and Cook (1999) showed that commercial LAS and C12-3 LAS were desulfonated under sulphur-limited anoxic conditions. The two studies show that LAS can be attacked and transformed by bacteria in the absence of molecular oxygen which implies that LAS is possibly not entirely persistent in anoxic environments. However, it is too early to assess the environmental relevance of the observed anaerobic transformation of LAS, and studies of the fate of LAS in aquatic sediments that are adapted via the continuous discharge of treated effluents should be conducted in the future. Sulphur-limited conditions are not expected to exist in anoxic sediments and, especially, marine sediments usually contain high levels of sulfate. E.g., the molar concentrations of SO4-- in coastal Danish sediments during summer have been measured to approximately 16 mM (Randers Fjord and Kysing Fjord) and 25 mM (Limfjorden) at the surface and approximately 5 mM in deeper layers (Jørgensen 1977; Sørensen et al. 1979).
Fate of LAS in sludgeamended soil
Since LAS are generally not degraded under anoxic conditions, levels of LAS in the g/kg range can be found in sludge which is applied to agricultural soil. The LAS in the sludge will normally biodegrade rapidly in well-aerated and aerobic soils. An extensive environmental monitoring of LAS concentrations in agricultural fields following sludge-amendment showed losses of LAS ³ 98% in the majority of the sites with calculated half-lives for LAS in soil between 7 and 22 days (Holt et al. 1989; Waters et al. 1989). The field monitoring data are in agreement with laboratory studies of the mineralization of 14C-labelled C12-LAS in mixtures of sludge and soil. In these studies, more than 68% of the added 14C-LAS was mineralized during 2 months, when aerobic conditions prevailed, while a lower mineralization was seen in mixtures that were partly anoxic (Gejlsbjerg et al., in press). The fate of LAS was recently evaluated for two catchment areas reflecting the eastern and western parts of Denmark (Madsen et al. 1999). The model simulations indicated that worst-case LAS concentrations in the upper 0-15 cm will be between 1 and 10 mg/kg with higher concentrations immediately after sludge application (sludge dosage: 2,000 kg/ha/year; LAS concentration 16 g/kg). The LAS concentrations in sludge are usually much slower than 16 g/kg and, hence, typical LAS concentrations in the upper 0-15 cm were estimated to between 0.1 and 1 mg/kg with higher concentrations immediately after sludge application. A substantial fraction of 98-99% of the sludge-bound LAS was predicted to degrade in the upper soil layer within one year, and the degree of leaching of LAS to depths below 1 m was predicted to be < 1.3% of the LAS applied with sludge (Madsen et al. 1999).
Bioaccumulation
Earlier studies of the bioaccumulation of LAS in aquatic organisms have mainly been performed with 14C- or 35S-radiolabelled LAS. By using radioactivity measurements, whole fish BCF values for C12 LAS have been determined to 108-280 for bluegill sunfish (Lepomis macrochirus; Bishop and Maki 1980), 173-245 for fathead minnow (Pimephales promelas; Kimerle et al. 1975), and 231 for zebra fish (Brachydanio rerio; Coenen 1988). Several studies show that LAS are transformed in fish (Comotto et al. 1979; Kikuchi et al. 1980; Newsome et al. 1995), but the experimental data do not allow a quantitative analysis of intact LAS and biotransformation products. Identification of metabolites suggests that biotransformation of LAS occurs via w -oxidation followed by b -oxidation. These processes lead to the formation of short-chained intermediates that are less toxic because of their lower lipophilicity compared to LAS (Newsome et al. 1995). Bioconcentration experiments using radiolabelled compounds are likely to overestimate the BCF for the intact surfactant because the radiotracer technique does not distinguish between the parent compound and radiolabelled metabolites.
Tolls (1998) examined the uptake and depuration of different LAS homologues by chemical analyses of the parent compound. The BCF tended to increase with increasing alkyl chain length but also the position of the aryl sulfonate moiety was important. A higher BCF was seen for LAS isomers with the aryl sulfonate attached to the second carbon at the alkyl chain, i.e. C11-2, C12-2, and C13-2 (Table 3.14). As it can be seen from the data in Table 3.14, the obtained BCF values differ markedly which indicates that inter-experimental difference exists. The only difference between the experiments with C12-2 LAS is the concentration of the compound in the tested mixture. Apparently, the BCF is inversely related to the concentration of the compound in the mixture, i.e. a higher BCF is obtained with decreasing test concentrations. As the toxicity of LAS is expected to decrease after the primary biotransformation, the BCF studies using chemical analyses of intact surfactant are of higher value than experiments based on radiolabelled compounds. The data in Table 3.14 indicate that the homologues in commercial LAS (i.e., C10-x - C13-x) have a low-to-moderate bioaccumulation potential with the exception of the C13-2 LAS.
Table 3.14
Whole body BCF values in fathead minnow (Pimephales promelas). Data from Tolls (1998).
LAS |
Uptake/depuration period |
BCFss |
C10-2 |
168-192 h/96 h |
6.0 (46) |
C11-2 |
168-192 h/96 h |
31.9 (29) |
C12-2 |
168-192 h/96 h |
99.1 – 211.5 |
C13-2 |
168-192 h/96 h |
987.2 (22) |
C11-5 |
168-192 h/96 h |
6.1 – 9.8 |
C12-5 |
168-192 h/96 h |
10.0 (44) |
C13-5 |
168-192 h/96 h |
34 (34) |
C12-6 |
168-192 h/96 h |
31.9 (48) |
C10-in |
168-192 h/96 h |
3.0 (50) |
C11-in |
168-192 h/96 h |
9.1 (41) |
C12-in |
168-192 h/96 h |
29.9 (27) |
C13-in |
168-192 h/96 h |
112.4 (28) |
Note: Cn-in represents the ‘inner isomers’, i.e.
the sum of the 3-, 4-, 5-, 6-, and 7-sulfophenylalkanes, in contrast to the 2-isomer. The
values in parentheses specify the relative standard variation in %.
3.3.3 Effects on the aquatic environment
Numerous studies have been performed to determine the effects of LAS towards aquatic organisms. The aquatic effect concentrations that were observed in these studies are highly variable. This variation is partly related to the testing of different isomers and homologues, but it may also be due to the specific test conditions and species. The length of the alkyl chain is an important factor determining the aquatic toxicity. In general, the homologues with the highest number of carbons in the alkyl chain are more toxic than are those with shorter alkyl chains. Today, commercial LAS have a homologue distribution between C10 and C13 with a typical average alkyl chain length of C11.6.
Algae
The widest range in the toxicity of LAS towards species belonging to the same group is found for algae (Table 3.15). Approximately 90% of the data found in the literature fall between 0.1 and 100 mg/l. Typical ranges of EC50 values are 1 to 100 mg/l for fresh water species and < 1 to 10 mg/l for marine species (Painter 1992). A very low EC100 value of 0.025 mg/l was determined for Gymnodium breve (Hitchcoch and Martin 1977). Previous studies in which Gymnodium breve was exposed with AES confirm that this species is highly sensitive to surfactants (Kutt and Martin 1974), and occasionally available data for Gymnodium breve should therefore not be used for comparison of the aquatic toxicity between various surfactants.
Table 3.15
Effects of LAS to algae.
Species |
LAS |
EC50 (mg/l) |
Test duration |
Reference |
Selenastrum capricornutum |
C10 |
270 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C11 |
111 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C12 |
48 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C13 |
30 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C14 |
18 |
72 h |
Verge et al. 1996 |
Navicula pelliculosa |
C13 |
1.4 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C13 |
5 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C13 |
116 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C12 |
0.9 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C12 |
29 |
96 h |
Lewis and Hamm 1986 |
Dunaliella sp. |
C12 |
3.3 (3.0-3.7)** |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenoidosa |
C12 |
29 (38-31)** |
96 h |
Utsunomiya et al. 1997 |
Natural periphyton |
C11.9 |
3.3 |
21 d |
Lewis et al. 1993 |
Natural algae populations |
C13 |
1.9* |
3 h |
Lewis and Hamm 1986 |
Natural algae populations |
C12 |
3.4* |
3 h |
Lewis and Hamm 1986 |
* Effect concentrations based on measured concentrations.
** Parentheses indicate 95% confidence intervals.
Invertebrates
LC50 values have been found in the range of 1 to 10 mg/l when Daphnia magna were exposed with LAS homologues between C10 and C13. The acute toxicity of LAS to Daphnia magna generally increases with increasing alkyl chain length. This is illustrated by studies performed by Maki and Bishop (1979) showing that LAS homologues ³ C14 produce EC50 values below 1 mg/l (Table 3.16). Similar results were obtained in a study of LAS homologues between C10 to C14 as the 48 h-LC50 values were 1.2 mg/l for C14 LAS and 53.1 mg/l for C10 LAS (Kimerle and Swisher 1977). A study with the marine crustacean Acartia tonsa indicated that a C10-13 LAS affected the survival at 0.54 mg/l (LC50) and the development rate at 0.51 mg/l (EC50) after 8 days of exposure. The 48 h-LC50 that was obtained in the same study with Acartia tonsa was 2.1 mg/l (Kusk and Petersen 1997). Metabolites from biotransformation of LAS are reported to have a much lower toxicity to invertebrates compared to the toxicity of the intact surfactant (Painter 1992).
Table 3.16
Effects of LAS to Daphnia magna unless otherwise indicated.
LAS |
EC50/LC50 |
Test duration |
Reference |
C18 |
0.12* |
48 h |
Maki and Bishop 1979 |
C16 |
0.11* |
48 h |
Maki and Bishop 1979 |
C14 |
0.68* |
48 h |
Maki and Bishop 1979 |
C13 |
2.6* |
48 h |
Maki and Bishop 1979 |
C13 |
2.19 |
96 h |
Maki 1979 |
C13 |
1.17 |
21 d |
Maki 1979 |
C13 |
1.11 |
21 d |
Maki 1979 |
C12 |
5.9* |
48 h |
Maki and Bishop 1979 |
C11 |
21.2* |
48 h |
Maki and Bishop 1979 |
| C10 | 29.5* (27.9-31.1)** |
48 h | Maki and Bishop 1979 |
| C11.8 | 3.94 (2.87-6.83)** |
96 h | Maki 1979 |
| C11.8 | 1.67 (1.228-2.18)** |
21 d | Maki 1979 |
| C11.8 | 1.50 (0.75-3.33)** |
21 d (reproduction) |
Maki 1979 |
| C10-13 | 0.54 Acartia tonsa |
8 d (survival) |
Kusk and Petersen 1997 |
| C10-13 | 2.1 Acartia tonsa |
48 h (survival) |
Kusk and Petersen 1997 |
* Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Fish
The toxicity of LAS to fish generally increases with increasing alkyl chain length, and approximately a 10-fold difference in toxicity between homologues separated by two carbon atoms has been observed. As also noted for invertebrates, fish are less susceptible to metabolites from biotransformation of LAS (Painter 1992). LC50 values below 1 mg/l were found for C11.9 (0.71 mg/l), C13 and C14 (both 0.4 mg/l) in studies with fathead minnow (Table 3.17) and for C10-15 (0.36 mg/l; 96 h) in a study with rainbow trout (Brown et al. 1978).
Table 3.17
Effects of LAS to fathead minnow (Pimephales promelas) unless otherwise indicated.
LAS |
LC50 (mg/l) |
Test duration |
Reference |
C11.9 |
0.71* (0.49-0.98)** |
7 d |
Fairchild et al. 1993 |
C14 |
0.5 LOEC:0.05-0.10 (estimated) |
96 h |
Macek and Slight 1977 |
C13 |
1.8 LOEC: 0.12-0.28 |
96 h |
Macek and Slight 1977 |
C12 |
6.6 LOEC: 1.08-2.45 |
96 h |
Macek and Slight 1977 |
C11 |
21.9 LOEC: 7.2-14.5 |
96 h |
Macek and Slight 1977 |
C10 |
57.5 LOEC:14-28 (estimated) |
96 h |
Macek and Slight 1977 |
C10-13 |
4.6 LOEC:1.02-2.05 (estimated) |
96 h |
Macek and Slight 1977 |
C10 |
43 |
48 h |
Kimerle and Swisher 1977 |
C11 |
16 |
48 h |
Kimerle and Swisher 1977 |
C12 |
4.7 |
48 h |
Kimerle and Swisher 1977 |
C13 |
0.4 |
48 h |
Kimerle and Swisher 1977 |
C14 |
0.4 |
48 h |
Kimerle and Swisher 1977 |
C10-15 |
0.36* (0.25-0.51)** |
96 h |
Brown et al. 1978 |
C13 |
NOEC:0.15* |
30 d |
Maki 1979 |
C11.8 |
NOEC:0.9* |
30 d |
Maki 1979 |
C11.2 |
LOEC:5.1-8.4* (life cycle) |
- |
Holman and Macek 1980 |
C11.7 |
LOEC:0.48-0.49* (life cycle) |
- |
Holman and Macek 1980 |
C13.3 |
LOEC:0.11-0.25* (life cycle) |
- |
Holman and Macek 1980 |
* Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Sediment organisms
LAS sorb to sediment with partition coefficients of 50 to 1,000. The toxicity of LAS bound to sediment is relatively low compared to LAS in solution. NOEC and LOEC values were as high as 319 and 993 mg LAS/kg, respectively, for the sediment-living Chironomus riparius. The corresponding NOEC for LAS in solution was as low as 2.4 mg/l indicating that only a small fraction of the sorbed LAS was bioavailable (Painter 1992). Bressan et al. (1989) investigated the effects of LAS dissolved in water and found acute effects in the range of 0.25 to 200 mg/l dependent of the species. Copepods and embryos of the sea urchin Paracentrotus lividus were the most sensitive organisms. LAS dissolved in water may also cause chronic effects like reduction of the growth rate of the marine mussel Mytilus galloprovincialis. LAS sorbed to sediments did not have similar effects. No alterations of the treated organisms were observed although the LAS concentrations in the sediment were 3 to 10 times higher than the effect concentrations observed for LAS in water. The 96 h-LC50 values for sediment-bound LAS were 182.5 mg/kg and 200 mg/kg for the bivalve molluscs Unio elongatulus and Anodonata cygnea.
3.3.4 Effects on human healthToxicokinetics and acute toxicity
LAS are readily absorbed by the gastrointestinal tract after oral administration in animals. LAS are not readily absorbed through the skin (IPCS 1996). The bulk is metabolized in the liver to sulfophenylic carboxyl acids. The metabolites are excreted primarily via the urine and faeces. The main urinary metabolites in rats are sulfophenyl butanoic acid and sulfophenyl pentanoic acid. Accumulation of LAS or its main metabolites has not been established in any organ after repeated oral ingestion (SFT 1991).
Dodecylbenzene sulfonate (C12 LAS) was administrated daily in the diet at a dose level of 1.4 mg/kg body weight for 5 weeks. Of the administered dose of C12 LAS, 52.4% was excreted in faeces and 29.4% in urine during the dosing period. A single application of 0.385 mg C12 LAS per rat resulted in a total elimination of 85% within the first 24 hours and 95% within 10 days (Lay et al. 1983). No data on skin absorption were identified, but the skin absorption of anionic surfactants is generally considered to be very low.
Table 3.18
Acute toxicity (LD50) of alkyl benzene sulfonates.
Surfactant |
Species |
Route |
LD50 (mg/kg/ body weight) |
Reference |
Branched alkylbenzene sulfonate |
Rat |
Oral |
700 – 2,480 |
SFT 1991; Gloxhuber and Künstler 1992 |
LAS |
Rat |
Oral |
401 – 1,900 |
IPCS 1996 |
LAS |
Mouse |
Oral |
1,259 – 2,300 |
IPCS 1996 |
LAS |
Rabbit |
Dermal |
> 500 |
CIRP 1993 |
No serious injuries or fatalities in man have been reported following accidental ingestion
of LAS-containing detergent (Painter 1992; IPCS 1996). The main clinical signs observed
after oral administration to rats of doses near or greater than the LD50 values consisted
of reduced voluntary activity, diarrhoea, weakness etc. Death usually occurred within 24
hours of administration. Rats appear to be more sensitive to LAS than mice (IPCS 1996).
Skin and eye irritation
LAS and branched alkylbenzene sulfonates may cause irritation of the eyes, skin and mucous membranes. LAS are relatively more irritating to the skin than the corresponding branched alkylbenzene sulfonates (KEMI 1990). The potential of LAS to irritate the skin depends on the concentration applied. LAS have been classified as irritating to skin at concentrations above 20% according to EU-criteria. Human skin can tolerate contact with solution of up to 1% LAS for 24 hours resulting in only mild irritation (IPCS 1996). Application of > 5% LAS to the eyes of rabbits produced irritation. Concentration of < 0.1% LAS produced mild to no irritation (CIRP 1993).
Sensitization
Skin sensitization was not seen in 2,294 volunteers exposed to LAS or in 17,887 exposed to formulations of LAS (Nusair et al. 1988).
Subchronic and long-term toxicity
A feeding study indicated that LAS, when administered for 2 years at extremely high levels (0.5%) in the diets to rats, produced no adverse effects on growth, health or feed efficiency (Buehler et al. 1971).
Mutagenicity and carcinogenicity
The mutagenic potential of LAS was tested using Salmonella typhimurium strains, using Ames test. In these studies, LAS was not mutagenic (Inoue and Sunakawa 1980). The available long-term studies are inadequate for evaluating the carcinogenic potential of LAS in laboratory animals. The studies available (oral administration to rats and mice) do not show any evidence of carcinogenicity (Gloxhuber and Künstler 1992; IPCS 1996).
Reproductive toxicity
LAS was applied daily from day 0 through day 20 of gestation to the shaved skin of pregnant rats. The applied concentrations of LAS were 0.05-0.5%, and the doses remained on the skin. Furthermore, concentrations of 1%, 5% and 20% LAS were applied to the skin of pregnant rats, and these doses were removed 30 minutes after exposure. The only effects attributed to LAS were reduced body weight in the dams given the highest concentration (20%), and local skin changes in the dams which received the two highest concentrations (5% and 20%). There were no findings indicative of effects of LAS on the foetal parameters evaluated and no indication of teratogenic or embryotoxic effects (Daly and Schroeder 1980).
A 20% LAS solution (0.1 ml) was applied twice daily to the dorsal skin (2 x 3 cm) of pregnant 1CR/Jc1 mice during the preimplantation period (days 0-3 of gestation). A significant number of embryos collected on day 3 were severely deformed or remained at the morula stage. Nomura et al. (1980, 1987) reported that the number of embryos in the oviducts was significantly greater for the mice dosed with LAS as compared to the control mice used in that study. No pathological changes were detected in the major organs of the dams.
In general no specific effect of LAS on reproductive processes has been seen, although dosages causing maternal toxicity may also induce some effects on reproduction. No teratogenic effects attributed to LAS exposure were observed (Gloxhuber and Künstler 1992; IPCS 1996).
Classification
LAS are classified as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes) according to CESIO (CESIO 2000).
LAS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.4 Secondary alkane sulfonates
Alkane sulfonates are used in liquid detergents like, e.g., dishwashing agents, cleaning agents, and hair shampoos, frequently in combination with AES. Commercial products are almost exclusively composed of secondary alkane sulfonates (SAS) with the following structure:
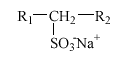
The alkyl chain (R1+R2), normally C11 to C17, is linear and the SO3- group is placed randomly along the alkyl chain. Thus, e.g., C14 alkane sulfonate is a mixture of the six isomers 2-, 3-, 4-, 5-, 6- and 7-sulfotetradecane.
No data were found on the occurrence of SAS in the environment.
3.4.1 Environmental fate
Biodegradation pathways
The of SAS biodegradation has only been scarcely investigated. A pathway similar to that of LAS involving an initial w /b -oxidation is an obvious assumption, but this has not yet been proven. One study suggests that the initial degradation step is a desulfonation requiring molecular oxygen (Painter 1992). This process involves the formation of a hydroxysulfonate which is hydrolysed to inorganic sulfate and a ketone. The ketone is subsequently oxidised to an ester which is cleaved to acetic acid and the corresponding alcohol (Painter 1992).
Aerobic biodegradability
SAS undergo rapid primary biodegradation with MBAS removals higher than 90% within a few days (Swisher 1987). Removals of 96% were seen in the OECD screening test for primary biodegradation (Schöberl et al. 1988). In activated sludge simulation tests, 96% of C10-18 SAS was removed, while the parent C13-18 SAS was removed by 83-96% (Painter 1992). The fate of a 14C-labelled C17 SAS was followed in a continuous activated sludge test to illustrate the ultimate biodegradation. After 3 days, 47% of the added C17 SAS were detected as 14CO2 and 25% were incorporated into sludge biomass (Steber and Berger 1995). The ultimate biodegradability of SAS fulfils the criteria for ready biodegradability in OECD 301 tests (Table 3.19).
Table 3.19
Ultimate aerobic biodegradability of SAS.
Compound |
Test |
Result |
Reference |
C12-18 |
Closed bottle test, 30 d |
93% ThOD |
Painter 1992 |
C13-17 |
Closed bottle test, 28 d |
99% ThOD |
Madsen et al. 1994 |
C13-18 |
Closed bottle test, 28 d |
63-95% ThOD |
Schöberl et al. 1988 |
Modified OECD screening test, 28 d |
88-96% DOC |
Schöberl et al. 1988 |
|
CO2 evolution test, 28 d |
56-91% ThCO2 |
Schöberl et al. 1988 |
Anaerobic biodegradability
Wagener and Schink (1987) investigated the anaerobic biodegradability of SAS in tests incubated with either digested sludge or creek sludge and came to the conclusion that alkyl sulfonates are not degraded under anoxic conditions.
Bioaccumulation
No experimental data describing the bioaccumulation potential of SAS were found in the literature.
3.4.2 Effects on the aquatic environment
Algae
The toxicity of various SAS homologues was determined in tests with Chlamydomonas variabilis. After 24 hours of exposure at 20° C, there was a tendency to an increased toxicity with increasing chain length. The EC50 values were 125 mg/l for C10.3, 74.9 mg/l for C11.2, 32.4 mg/l for C14, 15.8 mg/l for C15, 9.42 mg/l for C16, 3.93 mg/l for C17, 3.71 mg/l for C18.9, and 8.47 mg/l for C20.7 (Lundahl and Carbridenc 1978).
Invertebrates
The same tendency to an increased toxicity of SAS with increasing chain length was seen in tests with Daphnia magna. The tests with Daphnia magna showed 24 h-EC50 values at 319 mg/l for C10.3, 133 mg/l for C11.2, 111 mg/l for C14, 34.2 mg/l for C15, 30.1 mg/l for C16, 12.3 mg/l for C17, 3.31 mg/l for C18.9, and 6.30 mg/l for C20.7 (Lundahl and Carbridenc 1978). Schöberl et al. (1988) reported an EC50 range of 8.7-13.5 mg/l for daphnia in studies with C13-18 SAS, whereas Painter (1992) reported a lower EC50 range of 0.7-6 mg/l for C15-18 SAS.
Fish
Also for fish the longer chain length SAS are more toxic than the shorter chained homologues (Table 3.20). This has been shown both for minnow (Lundahl and Carbridenc 1978) and for bluegill sunfish (Painter 1992). Schöberl et al. (1988) reported a range of LC50 values of 3-24 mg/l for C13-18 SAS in tests with fish species that were not specified.
Table 3.20
Effects of SAS to fish.
Species |
SAS |
LC50 |
Test duration |
Reference |
Minnow (Phoxinus phoxinus) |
C14 |
34.5 |
24 h |
Lundahl and Carbridenc 1978 |
Minnow |
C15 |
8.5 |
24 h |
Lundahl and Carbridenc 1978 |
Minnow |
C16 |
3.11 |
24 h |
Lundahl and Carbridenc 1978 |
Bluegill sunfish (Lepomis macrochirus) |
C13 |
144 |
96 h |
Painter 1992 |
Bluegill sunfish |
C16 |
4.6 |
96 h |
Painter 1992 |
Bluegill sunfish |
C18 |
1.3 |
96 h |
Painter 1992 |
Fish |
C13-18 |
3-24 |
- |
Schöberl et al. 1988 |
3.4.3 Effects on human health
Toxicokinetics and acute toxicity
SAS are readily absorbed from the gastrointestinal tract of rats after oral administration. Following administration of C12 and C16 SAS the main metabolite is butyric acid-4-sulfonate. This metabolite is eliminated in the urine (McDermott et al. 1975; Taylor et al. 1978).
The acute toxicity of a SAS of non-specified chain length in the rat was moderate with LD50 values between 1,000 and 3,000 mg/kg body weight when administrated by the oral route (Falbe 1986; SFT 1991; Gloxhuber and Künstler 1992).
Skin and eye irritation
The irritating potential of SAS to skin is almost the same as that of alkyl sulfates. Concentrations of more than 20% alkane sulfonate are strongly irritating to the skin of rabbits (SFT 1991).
Chronic toxicity, carcinogenicity, mutagenicity
Subchronic studies with rats receiving 300 mg SAS/kg body weight/day orally for 45 and 90 days revealed no adverse effects. Similarly, rats fed 0.5% SAS in their diets for 91 days developed no adverse symptoms (Scailteur et al. 1986; Painter 1992). There was no indication of increased risk of cancer after oral ingestion of SAS in studies that were not further specified (Falbe 1986; SFT 1991).
Classification
The skin irritating potential of SAS is about the same as for alkyl sulfates. SAS may therefore also be classified as Irritant (Xi) with R38 (Irritating to skin) and R41 (Risk of serious damage to eyes).
SAS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.5 a -Olefine sulfonates (AOS)
a -Olefine sulfonates (AOS) are used in laundry powder detergents, liquid dishwashing agents, as well as in hair shampoos, and mainly in Japan and the USA (Steber and Berger 1995). AOS consist of a mixture of alkene sulfonates (about 60-65%) and hydroxyalkane sulfonates (about 30-40%). The normally linear C-chain in alkene 1-sulfonates and hydroxyalkane 1-sulfonates may contain 11 to 20 carbons with 14 to 18 carbons as the usual range (Painter 1992).
The alkene sulfonates have the structure:
H3C-(CH2)m-CH=CH-(CH2)n-SO3-Na+
(m = 1, 2, 3, …; n = 0, 1, 2, …; m + n = 9-15)
The hydroxyalkane sulfonates have the structure:
R-CH2-CH(OH)-(CH2)m-SO3-Na+
(R = C7-13; m = 1,2,3)
The a -olefine sulfonates are expressed as, e.g., C18 AOS or Cx AOS if the number of C atoms is not known. The hydroxyalkane sulfonates are expressed as C18-xOH AOS, where x indicates the C atom at which the –OH group is attached on the carbon chain.
No data were found on the occurrence of AOS in the environment.
3.5.1 Environmental fateBiodegradation pathways
Very little is known about the biodegradation pathways of AOS. Steber and Berger (1995) report a hypothetical pathway involving an initial desulfonation catalyzed by an alkane sulfonate-a -hydroxylase yielding a desulfonated ketene that could be hydrolysed to the corresponding acid.
Aerobic biodegradability
AOS are rapidly primary biodegradable with MBAS removals between 95 and 100% in 2 to 8 days in river water and inoculated media (Painter 1992). The ultimate biodegradability of AOS exceeds the pass requirements in OECD 301 tests for ready biodegradability. Schöberl et al. (1988) report 85% DOC removal in the modified OECD screening test, 85% ThOD in the closed bottle test, and 65-80% ThCO2 in the Sturm test. In activated sludge simulation tests, AOS was removed by 100% MBAS and 88% DOC (Painter 1992). The alkene sulfonates and hydroxyalkane sulfonates in commercial AOS are both ultimately biodegraded as approximately 84% ThCO2 was obtained during degradation of C14, C16, and C18 within 27 days, whereas the corresponding 3-hydroxyalkane sulfonates were degraded by approximately 86% under the same conditions (Painter 1992).
Anaerobic biodegradability
The studies of Wagener and Schink (1987) indicate that AOS are not degraded anaerobically. However, Painter (1992) reports a range of 31% to 43% MBAS removal under anoxic conditions indicating primary biodegradation.
Bioaccumulation
No experimental data describing the bioaccumulation potential of AOS were found in the literature.
3.5.2 Effects on the aquatic environment
Toxicity studies describing the effects of AOS to aquatic organisms have mainly been performed with fish. Only a few data have been found describing the effects towards algae and crustaceans.
Algae
Schöberl et al. (1988) report a range of 10-100 mg/l for C14-18 AOS as being toxic to the growth of algae.
Invertebrates
EC50 values for Daphnia magna have been determined within the range 5-50 mg/l for C14-18 AOS (Schöberl et al. 1988). Another study with Daphnia magna, referred by Painter (1992), showed EC50 values of 16.6 mg/l for C14-16 AOS and 7.7 mg/l for C16-18 AOS.
Fish
The studies performed with fish show that the higher homologues of AOS are more toxic than the lower ones. This has been illustrated for different fish species (see Table 3.21).
Table 3.21
Effects of AOS to fish.
Species |
AOS |
LC50 (mg/l) |
Test duration |
Reference |
Harlequin fish (Rasboa heteromorpha) |
C14-16 C16-18 |
3.3 0.5 |
96 h 96 h |
Reiff et al. 1979 |
Brown trout (Salmo trutta) |
C14-16 C16-18 |
2.5-5 0.5 |
96 h 96 h |
Reiff et al. 1979 |
Golden orfe (Idus idus) |
C14-16 C16-18 |
3.4 0.9 |
96 h 96 h |
Reiff et al. 1979 |
Fathead minnow (Pimephales promelas) |
C14-16 C16-18 |
5.3 1.4 |
24 h 24 h |
Painter 1992 |
Rainbow trout (Salmo gairdneri) |
C14-16 C16-18 |
5.1 0.8 |
24 h 24 h |
Painter 1992 |
3.5.3 Effects on human health
Toxicokinetics and acute toxicity
The absorption of AOS through intact skin is considered to be very low (IPCS 1996). sUnchanged a -olefine sulfonate (AOS) and/or metabolites of AOS are primarily eliminated in the urine and, to a lesser extent, in the faeces within 24 hours of administration. The chemical structures of the metabolites have not yet been identified.
AOS has a moderately low acute oral toxicity as indicated by LD50 values between 1,300 and 2,400 mg/kg body weight for rats and between 2,500 and 4,300 mg/kg body weight for mice (SFT 1991; IPCS 1996). The toxic effects at high oral doses were reduced voluntary activity, diarrhoea and anaemia (IPCS 1996).
Skin and eye irritation
AOS are mildly to moderately irritating to human skin depending on the concentration. In patch tests, human skin can tolerate contact to solutions containing up to 1% AOS for 24 hours resulting in only mild irritation (IPCS 1996). Instillation in the rabbit eye of 0.5% AOS caused no irritation after 24 hours, while 1% AOS caused a weak irritation (Gloxhuber 1974).
Chronic toxicity, carcinogenicity, mutagenicity
The long-term toxicity and potential tumorigenic activity of AOS were assessed in a 2 year feeding study in rats at dietary levels of 0.1, 0.25 and 0.5%. No adverse clinical effects were observed, and survival rates were not affected by treatment with AOS. Histological examination of the tissues did not provide any evidence of toxicity or tumour induction (Hunter and Benson 1976). In the Salmonella/microsome assay (Ames test) AOS were tested as negative showing a negligible potential to cause genetic damage (Yam et al. 1984).
Reproductive toxicity
AOS were studied in rabbits, mice and rats for teratogenic potential. AOS were administered orally once a day by gavage on day 6-15 of pregnancy in mice and rats and on day 6-18 of pregnancy in rabbits. The doses were from 0.2–600 mg/kg body weight. The study showed no evidence of teratogenic potential (Palmer 1975b).
Classification
AOS are classified as Irritant (Xi) with the risk phrases R38 and R41 for concentrations > 80% and R36/38 (Irritating to eyes and skin) for concentrations of 40-80% according to CESIO (CESIO 2000).
AOS are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
3.6 Sulfosuccinates
Sulfosuccinates are used in special detergent formulations and personal care products. Besides, sulfosuccinates are used as emulsifiers in the textile, plastics, photography and leather industries (Hales 1993; Steber and Berger 1995). The structurally related alkyl ether sulfosuccinates are used in personal care products. Sulfosuccinates have the following structure:
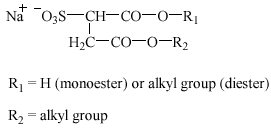
The alkyl chain(s) normally consist of less than nine carbons and can be either linear or branched. Branching increases the water solubility.
No data were found on the occurrence of sulfosuccinates in the environment.
3.6.1 Environmental fate
Biodegradation pathways
Relatively few studies have attempted to elucidate the biodegradation pathway of sulfosuccinates. High removals of carbon but no release of inorganic sulfate suggest that the biodegradation is initiated by hydrolysis of the ester bonds followed by b -oxidation of the alcohols (Steber and Berger 1995). Hales (1993) studied the formation of metabolites during degradation of C6-8 dialkyl sulfosuccinate under aerobic and anoxic conditions. This study confirmed the ester cleavage leaving one of two structural distinct monoalkyl sulfosuccinates, one being readily degraded and the other being less readily degraded. The suggested pathway for the easily degradable metabolite is hydrolytic cleavage leaving the corresponding alcohol and sulfosuccinate, whereas the other compound is sequentially degraded by w - and b -oxidations. In the absence of molecular oxygen, the ester bonds may be cleaved and followed by b -oxidation of the alcohol but the cleavage of the C-S-bond occurs only in the presence of oxygen. Thus a primary biodegradation is possible, whereas ultimate biodegradation is unlikely to occur under anoxic conditions (Hales 1993; Steber and Berger 1995).
Aerobic biodegradability
Data for the aerobic biodegradability have only been found for dialkyl sulfosuccinates and not for the ethoxylated compounds. High degrees of primary biodegradation (97-99%) are reported for C6-8 dialkyl compounds in OECD tests (Schöberl et al. 1988; Hales 1993). The biodegradation is highly affected by the structure of the carbon chain as indicated by a decreased primary biodegradation rate for structures with branched alkyl chains (Steber and Berger 1995). Dialkyl sulfosuccinates are not readily biodegradable according to OECD criteria for ready biodegradability (Table 3.22). Also coupled units tests have shown incomplete biodegradation with 70% DOC removal for C6-8 dialkyl sulfosuccinate (Hales 1993) and 49% for C8 dialkyl sulfosuccinate (Schöberl et al. 1988). A modified semi-continuous activated sludge test for ultimate inherent biodegradability showed 85-94% removal based on measurements of C6-8 dialkyl sulfosuccinate carbon (Hales 1993).
Table 3.22
Ultimate aerobic biodegradability of sulfosuccinates.
Compound |
Test |
Result |
Reference |
C6-8 dialkyl sulfosuccinate |
Modified OECD screening test, 28 d |
51-62% DOC 45-55% ThCO2 |
Hales 1993 |
C8 dialkyl sulfosuccinate |
Closed bottle test, 28 d |
50% ThOD |
Schöberl et al. 1988 |
Anaerobic biodegradability
No data have been found confirming an ultimate biodegradation of sulfosuccinates under anoxic conditions. As described in relation to the biodegradation pathway, only a primary biodegradation is anticipated in the absence of molecular oxygen (Hales 1993; Steber and Berger 1995).
Bioaccumulation
No experimental data describing the bioaccumulation potential of sulfosuccinates were found in the literature.
3.6.2 Effects on the aquatic environment
Very few data describing the aquatic toxicity of sulfosuccinates are available. Schöberl et al. (1988) report EC/LC50 values of 33 mg/l for daphnia and 39 mg/l for fish for C8 dialkyl sulfosuccinate.
3.6.3 Effects on human health
No data are available on the effects on human health. Sulfosuccinates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.7 Fatty acid soaps
Fatty acid soaps are the alkali salts of fatty acids. Soaps are primarily used in toilet soap bars and, also in solid form, as a cleaning agent. Typical raw materials for the production of soap are palm kernel oil (C8-14), coconut oil (C12-16), palm oil (C14-18), and tallow fat (C16-18).
R-CH2-COO-Na+
(R = 10-16)
3.7.1 Occurrence in the environment
Only few data on the concentration of soap in the environment have been found. The monitoring conducted in the Netherlands showed that the concentrations of soap in the effluent of six representative municipal sewage treatment plants varied between 0.091 and 0.365 mg/l with an average value of 0.174 mg/l (Matthijs et al. 1999).
3.7.2 Environmental fate
Biodegradation pathways
The degradation of fatty acids proceeds by b -oxidation in which coenzyme A is involved. Stepwise shortening of the alkyl chain occurs under the formation of acetyl coenzyme A fragments, which are used in living cells for energy production (Steber and Berger 1995). The b -oxidation may proceed in the absence of oxygen as well which implies that the same biodegradation pathway is anticipated in anoxic environments (Steber and Berger 1995).
Aerobic biodegradability
The general method for measuring primary biodegradation of anionics (MBAS analyses) is not applicable for fatty acids and, hence, no concrete data on primary biodegradability of soaps are available (Steber and Berger 1995). However, fatty acid soaps are rapidly and ultimately biodegradable which indicates a rapid primary biodegradation of these compounds. Fatty acids and soaps are ultimately biodegraded in the OECD 301 tests for ready biodegradability as illustrated by the data in Table 3.23.
Table 3.23
Ultimate aerobic biodegradability of fatty acids and soaps.
Compound |
Test |
Result |
Reference |
Na-soap |
Sturm test, 28 d |
80-90% ThCO2 |
Schöberl et al. 1988 |
Ca-stearate |
Sturm test, 28 d |
63% ThCO2 |
Schöberl et al. 1988 |
Na-laurate |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-palm kernelate |
BOD/COD, 10 d |
³ 90% |
Steber and Berger 1995 |
Na-oleate |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-tallow soap |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-stearate |
BOD/COD, 10 d |
> 85% |
Steber and Berger 1995 |
Na-behenate |
BOD/COD, 10 d |
> 75% |
Steber and Berger 1995 |
C8-18 fatty acids |
BOD/COD, 28 d* |
100% |
Steber and Berger 1995 |
C16 fatty acid |
BOD/COD, 28 d* |
100% |
Steber and Berger 1995 |
C18 fatty acid |
BOD/COD, 28 d* |
79% |
Steber and Berger 1995 |
C22 fatty acid |
BOD/COD, 28 d* |
69% |
Steber and Berger 1995 |
*Modified for poorly water-soluble compounds
Anaerobic biodegradability
The anaerobic biodegradability of palmitic acid has been confirmed in a digester model system (Steber and Wierich 1987) and in the more stringent ECETOC/ISO 11734 test (Table 3.24). Gas production measurements in a fermentor, in which the soaps were added in a semi-continuous mode, showed that the anaerobic biodegradability corresponded to 95% degradation of laurate (C12), 70% of oleate and palm kernel-based soap (C18 and C12-18), 60% of tallow-based soap, and only 14% of behenate (C22) (Steber and Berger 1995). Madsen et al. (1996a) examined the anaerobic biodegradability of Na-cocoate (C8-18) in screening tests by using either digested sludge, freshwater swamp material, or marine sediment as inoculum. The biodegradability observed after 28 and 56 days of incubation at 35° C was, respectively, 70 and 93% ThGP in the digested sludge, 60 and 84% ThGP in the freshwater swamp, and 50 and 96% ThGP in the marine sediment.
Table 3.24
Ultimate anaerobic biodegradability of fatty acids and soaps in digested sludge.
Compound |
Type of test and duration |
Result |
Reference |
Palmitic acid, C16 |
Measurement of 14CH4 and 14CO2 evolution, 28 d |
92-97% ThCH4 + ThCO2 |
Steber and Wierich 1987 |
Na-cocoate, |
Measurement of gas production, 35° C, 56 d |
93% ThGP |
Madsen et al. 1996a |
K-cocoate, C12-16 |
Measurement of gas production, 35° C, 56 d |
99% ThGP |
This study (Appendix; Table A8, Figure A8) |
Palmitate, C16 |
Measurement of gas production, 35° C, 28 d |
79-94% ThGP |
Birch et al. 1989 |
Bioaccumulation
No experimental data describing the bioaccumulation potential of fatty acid soaps were found in the literature.
3.7.3 Effects on the aquatic environment
The aquatic toxicity of fatty acid soaps is very variable and seems to be highly dependent on both the species and the specific fatty acid soap tested.
Algae
Schöberl et al. (1988) reported that the growth of algae was inhibited at concentrations of 10-50 mg/l of Ca-soap. Yamane et al. (1984) investigated the effects of C8-18 soap towards three different alga species and obtained EC50 (72 h) values of 10-50 mg/l for Selenastrum capricornutum, 20-50 mg/l for Microcystis aeruginosa, and 10-20 mg/l for Nitzschia fonticula. All of these EC50 were determined by using the growth rate of the algae (Table 3.25).
Table 3.25
Effects of fatty acids and soaps to algae.
Species |
Fatty acid soap |
EC50 |
Test duration |
Reference |
Selenastrum capricornutum |
C8-18 soap |
10-50 |
72 h |
Yamane et al. 1984 |
|
20-50 |
72 h |
|
|
|
10-20 |
72 h |
|
|
Algae |
Ca soap |
10-50 |
Not indicated |
Schöberl et al. 1988 |
Microcystis aeruginosa |
Soap |
18-32 |
96 h |
Canton and Slooff 1982 |
|
180-320 |
96 h |
|
|
Scenedesmus
|
Na-laurate |
53 |
72 h |
BKH |
Na-oleate |
58 |
72 h |
Consulting |
|
Na-palmoil soap |
140 |
72 h |
Engineers 1994 |
|
Na-tallow acid |
190 |
72 h |
|
|
Na-behenate |
230 |
72 h |
|
Invertebrates
The variability in the toxicity of fatty acid soaps towards Daphnia magna is approximately a factor of 20. The effect concentrations reported for Daphnia magna and Gammarus pulex are presented in Table 3.26.
Table 3.26
Effects of fatty acids and soaps to crustaceans.
Species |
Fatty acid soap |
EC50/LC50 |
Test duration |
Reference |
Daphnia magna
|
Soap |
32-56 |
48 h |
Canton and Slooff 1982
|
|
36;NOEC:10 |
21 d (mortality) |
||
|
> 10; NOEC:10 |
21 d (reprod.) |
||
Daphnia magna
|
Na-oleate |
4.2 |
24 h |
BKH Consulting engineers 1994
|
Soap |
10 |
- |
||
Palmoil soap |
25 |
24 h |
||
Talgseife |
40 |
24 h |
||
Haushaltseife |
42.3 |
- |
||
Na-laurate |
48 |
24 h |
||
Lauric acid |
2-5.4 |
48 h |
||
Gammarus |
Hardened tallow soap |
88 |
72 h |
BKH Consulting engineers 1994 |
Fish
Schöberl et al. (1988) reported that the adverse effects of fatty acids to fish depend on the hardness of the water. At a water hardness of 0° dH the LC50 of soap towards golden orfe (Idus idus melanotus) was 6.7 mg/l, while it was 20-150 mg/l at 3-23° dH. The same dependence of water hardness was documented by Kikuchi et al. (1976) who exposed killifish (Oryzias latipes) to Na-soap. In distilled water, the 48 h-LC50 was 5.9 mg/l, while no effects were seen at 84 mg/l, when the water hardness was 25 mg CaCO3/l. A relatively high toxicity has been found for oleic acid as the LC50 was between 0.1-2.1 mg/l for rainbow trout (BKH Consulting Engineers 1994). LC50 values for different fish species are presented in Table 3.27.
Table 3.27
Effects of fatty acids and soaps to fish.
Species |
Fatty acid soap |
LC50 (mg/l) |
Test duration |
Reference |
Golden orfe |
Ca-soap |
6.7 (0° dH) |
Not indicated |
Schöberl et al. 1988 |
Killifish |
Na-soap |
5.9 (distilled water) |
48 h |
Kikuchi et al. 1976 |
Guppy |
Soap |
320-560 (200mgCaCO3/l) |
96 h |
Canton and Sloof 1982 |
Oryzias latipes |
Soap |
1,000-1,800 (200mgCaCO3/l) |
96 h |
Canton and Sloof 1982 |
Rainbow trout |
Oleic acid |
0.1-2.1 |
96 h |
BKH Consulting engineers 1994 |
Rice fish
|
Sodium laurate (C12) |
11 |
96 h |
BKH Consulting engineers 1994 |
Sodium myristate (C14) |
118 |
96 h |
|
|
Sodium stearate (C18) |
125 |
96 h |
|
|
Sodium palmitate (C16) |
150 |
96 h |
|
|
Sodium oleate (C18) |
217 |
96 h |
|
|
Haushaltseife |
1,342 |
96 h |
|
|
Fathead minnow |
Oleic acid |
205 |
96 h |
BKH Consulting engineers 1994 |
Bluegill sunfish |
Lauric acid Oleic acid |
63.3 66.6 |
96 h 96 h |
BKH Consulting engineers 1994 |
Trout (Oncorhynchus kisuth) |
Oleic acid Palmitoleic acid |
12 12 |
33 h 2.5 h |
BKH Consulting engineers 1994 |
Golden orfe (Leuciscus idus melanotus) |
Na-fatty acid soap |
54 |
48 h |
BKH Consulting engineers 1994 |
3.7.4 Effects on human health
Toxicokinetics and acuate toxicity
The rate of percutaneous absorption of sodium laurate is greater than that of most other anionic surfactants. The greatest skin penetration of the human epidermis was found with C10 and C12 soaps (Prottey and Ferguson 1975).
The LD50 –values by oral administration of soaps are more than 10,000 mg/kg body weight for rats. This indicates a very low acute toxicity (Gloxhuber and Künstler 1992).
Skin and eye irritation
The existence of unsaturated carbon chains and carbon chain lengths of C16 to C18 contribute to a low skin irritation effect while soaps based on unsaturated C12-chains may be irritating to the skin (KEMI 1990).
Series of sodium soaps were studied to investigate the effect of the lipophilic chain length upon extraction of amino acids and proteins from the stratum corneum. The soaps, sodium laurate (C12) and sodium myristate (C14) removed most amino acids and proteins from the skin (Prottey and Ferguson 1975).
Soap concentrations of 10% or more may be irritating to skin and concentrations above 30% cause severe local irritation (Gloxhuber and Künsler 1992).
The only soaps that lead to permanent corneal damage are those containing large amounts free alkali and having a pH value of more than 12 (Grant and Schuman 1993). Accidental contact of the human eye with soap or soap powder followed by rapid rinsing of the eyes is not expected to cause severe reactions and reactions observed usually disappear quickly (Gloxhuber and Künsler 1992).
Carcinogenicity
Both oral administration and dermal exposures to soap (potassium soap) gave negative results in carcinogenicity tests with laboratory animals (Gloxhuber and Künsler 1992). Sodium oleate (C18) was given to rats in concentrations of 2.5% and 5.0% in the drinking water for 108 weeks. The soap did not induce tumours in the rats (Hiasa et al. 1985).
4.Nonionic surfactants
Nonionic surfactants are surface-active compounds with hydrophobic and hydrophilic moieties. These surfactants do not ionize in aqueous solutions. Commercial nonionic surfactants are normally a mixture of homologuos structures composed of alkyl chains that differ in the number of carbons and with hydrophilic moieties that differ in the number of ethylene oxide (ethoxylate, EO), propylene oxide (propoxylate, PO) and butylene oxide (butoxylate, BO) units. Nonionic surfactants are widely used in consumer products like, e.g., laundry detergents, cleaning and dishwashing agents, and personal care products. Nonionic surfactants are also widely used in cleaning agents formulated for the industrial and institutional sector. By volume, the most important nonionic surfactants are included in the very versatile group of alcohol ethoxylates and alcohol alkoxylates.
4.1 Alcohol ethoxylates and alcohol alkoxylates
Alcohol ethoxylates (AE) are nonionic surfactants composed of a hydrophobic alkyl chain (fatty alcohol) which is combined with a number of ethoxylate, or ethylene oxide, units via an ether linkage. Alcohol alkoxylates (AA) normally contain both ethylene oxide (EO) and propylene oxide (PO) in their hydrophilic moiety, whereas butylene oxide (BO) is less frequently used. The abbreviation AA has been used to designate nonionic surfactants with a hydrophilic part containing PO (or BO), frequently in combination with EO. AE are used in many types of consumer and industrial products like, e.g., laundry detergents, all-purpose cleaning agents, dishwashing agents, emulsifiers, and wetting agents. AA are used as weakly foaming and foam-mitigating surfactants in household cleaning agents, dishwashing agents and cleaning agents designed for the food industry (Bertleff et al. 1997). Other applications of AA include textile lubricants, agricultural chemicals, and rinse aid formulations.
The nonionic surfactants described in this section include several chemical structures of which a few representative structures are given below.
Lineary primary AE, C13 EO7:
CH3-(CH2)12-O-(CH2-CH2-O-)7H
Iso-C13 branched primary AE, EO7:
![]()
Linear primary AE, C13 EO10, end-capped with n-butylether
CH3-(CH2)12-O-(CH2-CH2-O-)10-CH2-CH2-CH2-CH2-OH
Linear primary AA, EO5, PO4:
![]()
The hydrophobic fatty alcohol usually contains 12-15 carbon atoms, but chain lengths of C9-11 are also used.
4.1.1 Occurrence in the environment
During the 1980s non-quantitative methods for detection of AE were used together with analyses for nonionic surfactants (bismuth iodide active substances, BiAS) to determine the presence of AE in e.g. effluents from wastewater treatment plants. Today, the efforts are directed towards the development of new methods for specific determination of AE at low concentrations in environmental samples. Effluent concentrations of AE in wastewater treatment plants were in the order of 0.006-0.02 mg/l for AE concentrations of 0.19-0.91 mg/l in the influent. A higher AE concentration of 3.4 mg/l in the influent resulted in an effluent concentration of 0.04 mg/l (Holt et al. 1992). A recent environmental monitoring showed that the effluent concentrations of AE from municipal sewage treatment plants in the Netherlands varied between 0.0022 and 0.013 mg/l with an average value of 0.0062 mg/l (Matthijs et al. 1999).
The presence of AE in the aquatic environment has been reported for a Japanese river. The concentration of AE in the river water was below the detection limit of 0.005 mg/l, whereas the concentration in the sediment ranged from 0 to 1.0 mg/kg. A concentration of 0.004 mg/l for C14-15 AE was observed in Ohio River, USA (Holt et al. 1992).
4.1.2 Environmental fate
Biodegradation pathways
Three different mechanisms have been proposed for the biological degradation of AE under aerobic conditions (Marcomini et al. 2000a, 2000b).
| 1. | The first mechanism is a central scission, or ether cleavage, which leads to the formation of fatty alcohols and polyethylene glycols (PEG). The fatty alcohols are first transformed to fatty acids by w -oxidation of the terminal carbon, whereafter the fatty acids are degraded by b -oxidation. The b -oxidation of the fatty acid releases pairs of C-atoms from the carbon chain which are mineralized to CO2. The PEG are degraded via a non-oxidative shortening which releases one glycol unit at a time from the terminus of the PEG, and/or via an oxidative hydrolysis forming monocarboxylated PEG. |
| 2. | The second mechanism is a microbial attack at the terminal carbon of the alkyl chain, via an w -oxidation, followed by a series of b -oxidations. By this mechanism the AE is first transformed to a carboxylated AE (with the carboxylic group at the alkyl chain) which is further degraded via the formation of monocarboxylated and dicarboxylated PEG. |
| 3. | The third mechanism is an w -oxidation of the terminal carbon of the polyethoxylic chain. This mechanism proceeds via the formation of a carboxylated AE (with the carboxylic group at the polyethoxylic chain), which is further degraded via dicarboxylated AE (with carboxylic groups at both alkyl and polyethoxylic ends) and dicarboxylated PEG. |
Recent studies have elucidated the relations between the biodegradation mechanisms and the structure of the AE (Marcomini et al. 2000a, 2000b). The formation of PEG was observed only for a linear AE and an oxo-AE (composed of linear AE and monobranched AE with short 2-alkyl chains, i.e. 2-methyl-, 2-ethyl-, 2-propyl-, and 2-butyl-), whereas only carboxylated AE (with the carboxylic group at the polyethoxylic chain) were detected during biodegradation of a multibranched AE. The absence of carboxylated AE in the experiments with the linear and the monobranched (2-alkyl branched) oxo-AE indicates that the central scission (mechanism 1) was the primary mechanism for the biodegradation of linear and most monobranched AE in the examined commercial mixtures, whereas the multibranched AE was degraded via w -oxidation of the polyethoxylic chain (mechanism 3) (Marcomini et al. 2000a). Biodegradation of an oxo-2-butyl-substituted AE only resulted in carboxylated AE (mainly metabolites with the carboxylic group at the alkyl chain) suggesting that w -oxidation of the alkyl chain was the primary mechanism (mechanism 2). The results obtained with the 2-butyl-substituted AE show that a shift from the central scission to the w ,b -oxidation is introduced when the length of the 2-alkyl branch exceeds three carbon atoms (Marcomini et al. 2000b).
Far less is known about the biodegradation of AA and of end-capped AE. AA containing butoxylate (BO) or propoxylate (PO) groups in their hydrophilic moiety are degraded via cleavages of the hydrophilic chain, which may be either non-oxidative or oxidative like the degradation of PEG. A secondary carbon atom in the hydrophilic moiety, e.g. in PO groups, inhibits the oxidative route (Balson and Felix 1995). End-capped AE are degraded by a combination of w -oxidation of the hydrophilic chain and central hydrophobe-hydrophile scission. The w -oxidation is inhibited by the presence of PO in the hydrophilic chain, whereas the extent of central scission is determined by the degree of 2-alkyl branching (Balson and Felix 1995). The findings in the above-mentioned studies with 2-butyl-substituted AE (Marcomini et al. 2000b) further illustrate the effect of the length of the 2-alkyl substituent.
The anaerobic biodegradation of linear AE is apparently initiated by a stepwise release of C2 units as acetaldehyde to form the corresponding shortened AE and, eventually, a fatty acid (Wagener and Schink 1988). This pathway was recently confirmed in anaerobic assays with a linear pure C12 AE (with 8 EO) and a linear technical C12 AE (with an average of 9 EO), as the first identifiable metabolites were shortened AE and subsequent metabolites included dodecanoic acid and acetic acid. No PEG was observed during the degradation of linear AE, which indicates that central scission of the AE molecule was not the biodegradation mechanism under the applied anaerobic conditions (Huber et al. 2000).
Effects of structure of AE on biodegradability
The biodegradability of the AE is relatively unaffected by the alkyl carbon chain length and the number of EO units but highly affected by the molecular structure of the hydrophobic chain (Dorn et al. 1993). The linear AE are normally easily degraded under aerobic conditions. Only small differences are seen in the time needed for ultimate degradation of linear AE with different alkyl chain lengths. AE with a typical alkyl chain (e.g., C12 to C15) will normally reach more than 60% degradation in standardized tests for ready biodegradability. The length of the EO chain determines the hydrophobicity of the AE and, hence, influences the biodegradability in terms of the bioavailability. Longer EO chains decrease the bioavailability of the AE due to increased hydrophilicity and molecular size, which limits the transport of the molecule through the cell wall (Balson and Felix 1995). For AE containing more than 20 EO units, a reduced rate of biodegradation has been observed (Scharer et al. 1979; Holt et al. 1992).
The biodegradation of branched AE tends to be slower than biodegradation of linear AE. One important observation that may explain this fact is that the b -oxidation is hindered by the branching of the alkyl chain (Holt et al. 1992; Balson and Felix 1995). Furthermore, branching at the C-atom forming the internal ether linkage may hinder the hydrophobe-hydrophile scission (Balson and Felix 1995). The biodegradability of AE depends on degree and structure of the branching. The general trend is that the biodegradation decreases considerably with an increasing branching of the carbon chain (Kaluza and Taeger 1996). A highly branched C13 AE, prepared from 2-propyl-C10 and 2-pentyl-C8 with 46% branching, was not readily biodegradable in the DOC die-away screening test as only 50% of the initial DOC was removed during 28 days (Kaluza and Taeger 1996). The structure of the backbone in the carbon chain also affects the biodegradability. Swisher (1987) found that one single internal methyl group had no effect on the biodegradation compared to the entirely linear AE, whereas two methyl groups decreased the degradation rate markedly, especially if the methyl groups were located at the same carbon resulting in a quaternary structure. The rate of biodegradation of monobranched AE is strongly influenced by the length of the side chain. Although the 60% pass level was fulfilled in the CO2 evolution test (but not the 10-day window), the degradation of an oxo-2-butyl-substituted AE occurred more slowly than the degradation of an oxo-AE blend containing 2-methyl-, 2-ethyl-, 2-propyl-, and 2-butyl side chains. The degradation of the 2-butyl-substituted AE showed a time profile similar to that of a multibranched AE (Marcomini et al. 2000b). Kaluza and Taeger (1996) compared the biodegradability of branched AE based on different carbon chains (all with 7-8 EO units). They found that an iso-C13 AE based on propylene tetramer (four internal methyl groups) did not pass a test for ready biodegradability, whereas an iso-C13 based on butylene trimer (three internal methyl groups) did. The ultimate degradation of iso-C10 based on propylene trimer (three internal methyl groups) also complied with the criteria for ready biodegradability. Kravetz et al. (1991) studied the degradation of a C11-15 AE based on propylene and containing four internal methyl groups as well as a C10-14 AE containing three internal groups (both with 7 EO units). The structures of the two substances were complex as they both contained a quaternary carbon. None of the branched AE passed the criteria for ready biodegradability and no difference in the degradation rates for the two substances was observed.
Aerobic biodegradability
Linear C12-18 AE, containing 5-14 EO units, are ultimately degraded under aerobic conditions. The degradation rate of AE containing more than 20 EO units is slower, although an extensive primary degradation may take place for AE containing up to 50 EO units (Birch 1984). Only a few studies report the fate of AE in wastewater treatment plants. Average concentrations of 0.33 mg/l (0.19-0.47 mg/l) in the influent and 0.009 mg/l (0.006-0.012 mg/l) in the effluent of C14-15 EO7 indicate a removal of 97-98% of the AE during wastewater treatment (Holt et al. 1992). Data on the ultimate aerobic biodegradability of linear AE are shown in Table 4.1. As described previously, the aerobic biodegradation of branched AE depends on the structure of the hydrophobic carbon chain. In general the biodegradability decreases with increasing branching of the alkyl chain, but also the number of internal methyl groups and the presence of quaternary carbon atoms affect the biodegradability of AE. Normally, AE containing a quaternary carbon atom are not readily biodegradable (Table 4.2).
Table 4.1
Ultimate aerobic biodegradability of linear AE.
Compound |
Test |
Result |
Reference |
C9-11 EO8 |
Closed bottle test, 28 d |
80% ThOD |
Madsen et al. 1994 |
C12-14 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C12-15 EO7 |
BOD, 30 d |
92% ThOD |
Kravetz et al. 1991 |
|
CO2 evolution test, 28 d |
82% ThCO2 |
Madsen et al. 1996b |
C12-15 EO9 |
CO2 evolution test, 28 d |
64-79% ThCO2 |
Kravetz et al. 1991 |
C12-18 EO10-14 |
Closed bottle test, 28 d |
69-86% ThOD |
Schöberl et al. 1988 |
C13 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C13-15 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C14-15 EO7 |
BOD, 30 d |
83% ThOD |
Kravetz et al. 1991 |
C15 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C16-18 EO5 |
Closed bottle test, 28 d |
65-75% ThOD |
Schöberl et al. 1988 |
C16-18 EO30 |
Closed bottle test, 28 d |
27% ThOD |
Schöberl et al. 1988 |
Table 4.2
Ultimate aerobic biodegradability of branched AE.
Compound |
Comments |
Test |
Result |
Reference |
Iso-C10 EO7-8 |
3 internal CH3-groups, highly branched |
Die away screening test, 28 d |
90% DOC |
Kaluza and Taeger 1996 |
Oxo-C11 EO7-8 |
10% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C10-14 EO7 |
2.9 internal CH3-groups, quaternary C-atom |
BOD, 30 d |
40% ThOD |
Kravetz et al. 1991 |
Oxo-C12 EO5 |
2-butyl-substituted |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Marcomini et al. 2000b |
C12-15 EO7 C12-15 EO18 C12-15 EO30 |
75% primary alcohol |
CO2 evolution test, 28 d |
> 80% ThCO2 |
Scharer et al. 1979 |
Iso-C13 EO7-8 |
3 internal CH3-groups, highly branched |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
4 internal CH3-groups, highly branched |
Die away screening test, 28 d |
62% DOC |
Kaluza and Taeger 1996 |
C11-15 EO7 |
4 internal CH3-groups, quaternary C-atom |
CO2 evolution test, 28 d |
40-50% ThOD |
Kravetz et al. 1991 |
C13 EO7-8 |
< 1 internal CH3-group, 10% branching |
Die away screening test, 28 d |
95% DOC |
Kaluza and Taeger 1996 |
C13 EO7-8 |
1 internal CH3-group, 25% branching |
Die away screening test, 28 d |
95% DOC |
Kaluza and Taeger 1996 |
C13 EO7-8 |
» 1 internal CH3-group, 46% branching |
Die away screening test, 28 d |
50% DOC |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO7-8 |
10% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO3-12 |
|
Modified OECD screening test, 28 d |
75% DOC |
Schöberl et al. 1988 |
Oxo-C14-15 EO9-20 |
|
Die away screening test, 28 d |
65-75% DOC |
Schöberl et al. 1988 |
C15 EO7-8 |
1 internal CH3-group, 25% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
The biodegradability of AA generally decreases with an increasing number of PO units in
the hydrophilic part. The results summarized in Table 4.3 confirm this trend as, e.g., the
C12-18 AA containing 6 PO units did not pass the level required for ready
biodegradability whereas the same alcohol containing 2 PO units attained 83% ThOD in the
closed bottle test (Schöberl et al. 1988). The general trend for linear AA is
that, apparently, there is a limit of 6-7 PO units in order to qualify for primary
biodegradation (Balson and Felix 1995). However, increased branching of the carbon chain
determines the construction of the hydrophilic part of the surfactant, as fewer PO units
can be tolerated in branched AA in order to comply with the requirement for primary
biodegradability (Naylor et al. 1988). Naylor et al. (1988) showed that a
primary biodegradation > 80% was achieved for the most linear AA (20% branching)
containing up to 3.5 PO units, an AA with 1 internal methyl group containing up to 2.0 PO
units, and an AA with 2 internal methyl groups containing up to 0.4 PO units.
The removal of alcohol propoxylates in German wastewater treatment plants has been reported to be in the range of 73-81% (Holt et al. 1992). If the AA is terminated with PO units the degradation is highly influenced by the branching because the w -hydrophile oxidation is inhibited by the presence of PO. In this case, the level of branching determines the biodegradation which proceeds by the hydrophobe-hydrophile scission (Balson and Felix 1995). This was confirmed by a study showing that the primary biodegradability of an AA containing 6 EO units and 6.5 PO units was 97% for 20% 2-alkyl branching and 10% for 100% 2-alkyl branching (Balson and Felix 1995). Balson and Felix (1995) showed a primary degradation of 83-97% for a C9-11-AE capped with an alkyl group and 80-99% primary degradation of a C9-11-AE capped with an aryl group. Data describing the ultimate biodegradability of end-capped AE are sparse. A C12-14 EO9 and a C12-18 EO10, both end-capped with n-butylether, were confirmed to be readily biodegradable (Table 4.3).
Table 4.3
Ultimate aerobic biodegradability of end-capped AE and AA.
Compound |
Test |
Result |
Reference |
C12-14 EO9, n-butyl-ether (end-capped) |
Closed bottle test, 28 d |
80% ThOD |
Schöberl et al. 1988 |
C12-18 EO10, n-butyl-ether (end-capped) |
Manometric respirometry test, 28 d |
98% ThOD |
This study (Appendix; Table A1, Figure A1) |
C8-10 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C9-11 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C10-12 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
Iso-C13 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C13-15 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C12-18 EO2.5, PO 6 |
Modified OECD screening test, 28 d |
43% DOC |
Schöberl et al. 1988 |
C12-18 EO2.5, PO 6 |
Closed bottle test, 28 d |
36% ThOD |
Schöberl et al. 1988 |
C12-18 EO6, PO 2 |
Modified OECD screening test, 28 d |
69% DOC |
Schöberl et al. 1988 |
C12-18 EO6, PO 2 |
Closed bottle test, 28 d |
83% ThOD |
Schöberl et al. 1988 |
Anaerobic biodegradability
Most of the relatively few studies of the anaerobic biodegradability of AE have been performed with linear AE. Anaerobic biodegradation tests have been performed with various inocula like, e.g., anaerobically digested sludge (Steber and Wierich 1987; Salanitro and Diaz 1995; Madsen et al. 1995; 1996a) and anoxic sediments (Wagener and Schink 1987; Madsen et al. 1995, 1996a; Federle and Schwab 1992). Anaerobic biodegradability tests with diluted digested sludge have either been performed by use of screening methods (e.g., ECETOC 1988; ISO 1995) or by use of 14C-labelled model compounds (e.g., Steber and Wierich 1987). Since the concentration of surfactant in the screening test may inhibit its degradation by anaerobic bacteria, the results from studies using 14C-labelled compounds are generally considered to be of higher value. The results indicate that linear AE are normally mineralized in anaerobically digested sludge. The mineralization observed in experiments with 14C-labelled surfactants suggests that almost complete degradation of linear AE may be expected in anaerobic digesters and that the lower mineralization observed in the screening test was caused by inhibition (Table 4.4). AE end-capped with butylether were either partially mineralized or not degraded in the ISO 11734 screening test (Table 4.4; Appendix). Linear AE were also degraded in anoxic sediments, where a lower mineralization was observed at 22° C compared to the mineralization at higher temperatures (Table 4.5).
Table 4.4
Ultimate anaerobic biodegradability of AE in digested sludge.
Compound |
Type of test and duration |
Result |
Reference |
C9-11 EO8 |
Measurement of gas production, 35° C, 40-50 d |
60-83% ThCH4 |
Salanitro and Diaz 1995 |
C9-11 EO8 |
Measurement of gas production, 35° C, 56 d |
79% ThGP |
Madsen et al. 1996a |
C12-15 EO7 |
Measurement of gas production, 35° C, 56 d/84d |
38%; 35% ThGP |
Madsen et al. 1996b This study (Appendix; Table A9, Figure A9) |
C18 EO7 |
Measurement of 14CH4 and 14CO2 evolution, 35° C, 28 d |
84% ThCH4 + ThCO2 |
Steber and Wierich 1987 |
C8 EO5, n-butylether (end-capped) |
Measurement of gas production, 35° C, 84 d, ISO 11734 |
Inhibition |
This study (Appendix; Table A10, Figure A10) |
C12-18 EO10, n-butyl-ether (end-capped) |
Measurement of gas production, 35° C, 84 d, ISO 11734 |
54% ThGP |
This study (Appendix; Table A11, Figure A11) |
Table 4.5
Ultimate anaerobic biodegradability of AE in sediments.
Compound |
Type of test and duration |
Result |
Reference |
C9-11 EO8 |
Measurement of gas production in freshwater swamp material, 35° C, 56 d |
77% ThGP |
Madsen et al. 1996a |
C9-11 EO8 |
Measurement of gas production in marine sediment, 35° C, 56 d |
66% ThGP |
Madsen et al. 1996a |
C10-12 EO7.5 |
Measurement of CH4-production in polluted creek mud, 28° C, 37 d |
70% ThCH4 |
Wagener and Schink 1987 |
C12 EO8-9 |
Measurement of 14CH4 and 14CO2 evolution in wastewater pond sediment, 22° C, 87 d |
24-40% ThCH4 + ThCO2 |
Federle and Schwab 1992 |
C12 EO8-9 |
Measurement of 14CH4 and 14CO2 evolution in pond sediment, 22° C, 87 d |
13% ThCH4 + ThCO2 |
Federle and Schwab 1992 |
C12 EO23 |
Measurements of CH4-production in polluted creek mud, 28° C, 37 d |
80% ThCH4 |
Wagener and Schink 1987 |
Bioaccumulation
Bioaccumulation of AE in aquatic organisms has been determined only for fish. The majority of the very few data is based on studies with 14C-labelled compounds that do not allow the distinction between the parent compound and metabolites. Because AE are metabolized in aquatic organisms, the bioconcentration factor for the parent compound may well be overestimated in experiments in which 14C-labelled model surfactants are used. By use of 14C-labelled surfactants, whole body concentration ratios have been estimated for four different AE in fish (Table 4.6).
Table 4.6
Whole body BCF values of AE in fish.
Compound/species |
Uptake/ depuration |
BCF |
Reference |
C14 EO7 Bluegill sunfish (Lepomis macrochirus) |
28 d/? |
799* |
Bishop and Maki 1980 |
C12 EO4 Carp (Cyrinus carpio) |
72 h/168 h |
309* |
Wakabayashi et al. 1987 |
C12 EO8 Carp |
72 h/168 h |
222* |
Wakabayashi et al. 1987 |
C12 EO16 Carp |
72 h/168 h |
4.3* |
Wakabayashi et al. 1987 |
C12 EO8 Fathead minnow (Pimephales promelas) |
54-72 h/- |
12.7 |
Tolls 1998 |
C13 EO4 Fathead minnow |
54-72 h/- |
232.5 |
Tolls 1998 |
C13 EO8 Fathead minnow |
54-72 h/- |
29.5-55.0 |
Tolls 1998 |
C14 EO4 Fathead minnow |
54-72 h/24 h |
237.0 |
Tolls 1998 |
C14 EO8 Fathead minnow |
54-72 h/24 h |
56.7-135.2 |
Tolls 1998 |
C14 EO11 Fathead minnow |
54-72 h/24 h |
15.8 |
Tolls 1998 |
C14 EO14 Fathead minnow |
54-72 h/24 h |
< 5 |
Tolls 1998 |
C16 EO8 Fathead minnow |
54-72 h/24 h |
387.5 |
Tolls 1998 |
* BCF values based on radioactivity measurements.
Tolls (1998) combined 14C-techniques and chemical analysis and showed that the parent AE (C13 EO8) was rapidly eliminated by transformation into metabolites, which were eliminated at a slower rate. The bioconcentration factors for C12 EO8 and C13 EO8 were 12.7 and 29.5-55.0, respectively, when the AE were monitored by chemical analysis. The BCF values for C13 EO4 and C14 EO4 were 232.5 and 237.0, respectively. The influence of the hydrophobe chain length was illustrated by BCF values of 56.7 to 135.2 for C14 EO8 and 387.5 for C16 EO8. AE with a relatively high number of EO units, i.e. C14 EO11 and C14 EO14 did not bioaccumulate in fish as indicated by the BCF values of < 5 and 15.8 (Tolls 1998; Table 4.6). The data in Table 4.6 indicate that the more hydrophobic AE (e.g. C13 EO4, C14 EO4, and C16 EO8) have a moderate bioaccumulation potential.
In the study by Tolls (1998) the BCF values ranged from < 5 to 387.5, whereas the uptake rates (k1) varied from 330 to 1660 (l x kg x d-1) and the elimination rates (k2) varied from 3.3 to 59 (d-1). According to the guideline on bioaccumulation studies in fish (OECD 305) the time to 95% steady state conditions can be estimated by the equation t95 = 3.0/k2. Using this equation, the t95 for the AE investigated by Tolls (1998) range from 1.2 to 22 hours. The results obtained by Tolls (1998) indicate that the time to steady state and the BCF for AE increase with decreasing length of the ethoxylate chain (e.g., t95 for C13 EO8 = 2.4 h and BCF = 30-55, and t95 for C13 EO4 = 17.1 h and BCF = 233).
The achievement of steady state conditions for AE (C9-11 EO6, C12-13 EO6.5, and C14-15 EO7) after a relatively short exposure period has also been illustrated by Lizotte et al. (1999) who observed that ‘steady state’ mortality occurred within 240 hours of exposure in the higher exposure concentrations. At the lower exposure concentrations with C9-11 EO6 and C14-15 EO7, the mortality continued, however, throughout the treatment period. For an illustration of the time needed for achievement of maximum toxicity a comparison of toxicity data for AE obtained in short-term and long-term studies is presented in Table 4.7.
Table 4.7
Effects of different exposure periods on the toxicity of AE to fish.
Species |
AE |
LC50 (mg/l) |
Test duration |
Reference |
Fathead minnow (Pimephales promelas) |
C9-11 EO6 |
2.7 |
240 h |
Dorn et al. 1997 |
Fathead minnow, larvae |
C9-11EO6 |
4.87 (4.47-5.26)* |
672 h |
Lizotte et al. 1999 |
Fathead minnow |
C12-13 EO6.5 |
1.3 (0.72-2.7)* |
96 h |
Wong et al. 1997 |
Fathead minnow, larvae |
C12-13 EO6.5 |
2.39 (2.26-2.52)* |
672 h |
Lizotte et al. 1999 |
Fathead minnow |
C14-15 EO7 |
0.63-1.65 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow, larvae |
C14-15 EO7 |
1.02 (0.94-1.11)* |
672 h |
Lizotte et al. 1999 |
* Parentheses indicate 95% confidence limits.
The data in Table 4.7 indicate that an increase of the exposure period did not lead to lower effect concentrations (LC50) and that maximum toxicity of the AE was achieved after a relatively short exposure period. However, the AE examined by Lizotte et al. (1999) did not include relatively hydrophobic types like, e.g., C13 EO4, C14 EO4, and C16 EO8, for which BCF values above 100 have been determined (Table 4.6).
4.1.3 Effects on the aquatic environment
Many studies have been performed to determine the toxic effects of AE towards aquatic organisms. Extrapolation from laboratory toxicity tests to the environment is obviously not easy for readily biodegradable surfactants, because biodegradation of the compounds in the sewers and in wastewater treatment plants is expected to alter the composition of isomers and homologues. The toxicity of a linear C12-15 EO9 and a branched C11-15 EO7 was investigated after treatment in a continuously activated sludge reactor (Kravetz et al. 1991). Both AE were degraded to products that were not acutely toxic. A higher chronic toxicity was observed for the effluent from the branched AE than from the linear AE. The degradation products were not identified but it was believed that the EO-chain was shortened and, hence, more toxic AE metabolites would have been produced. Garcia et al. (1996) investigated whether the toxicity of AE (C12) was affected by a broad-range or a narrow-range EO distribution. The AE with the narrow-range distribution were less toxic than were the AE with the broad-range EO distribution when the surfactants contained more than 8-10 EO, whereas no differences were observed for a lower degree of ethoxylation. The AE with narrow-range and broad-range EO distribution differed by the presence of a lower amount of free fatty alcohols in the AE with the narrow-range EO distribution. The following paragraphs describe the toxicity of AE and AA towards algae, invertebrates, and fish.
Algae
Algae constitute the group of aquatic organisms which appears to be the most sensitive to AE. The acute toxicity of linear and branched AE to algae is in the same range with EC50 values from 0.05 to 50 mg/l. Besides the differences in chemical structure, the reason for the variation may be due to different test conditions and different test species. For the linear types, the toxicity increases with increasing hydrophobe chain length (comparison of C13 EO7-8 and C15 EO7-8, Table 4.8) and decreasing EO chain length (comparison of C12-14 with 4-13 EO, Table 4.8). The toxicity of AE to algae tends to decrease with increasing degree of branching (Table 4.9). Based on the low EC50 values (£ 1 mg/l), the linear AE of C12-15 EO6-8 are considered as very toxic to algae. When the degree of branching is low (£ 25%), the branched types are also considered very toxic to algae. A C12-14 EO9 end-capped with an n-butyl-group was very toxic to a non-specified alga as the EC50 was 0.3 mg/l (Schöberl et al. 1988).
The effect of the carbon chain length and structure on the toxicity to algae was examined for two AA containing 6 EO and 3 PO-groups (Bertleff et al. 1997). It was observed that the toxicity increased with an increasing carbon chain length and that branching of the carbon chain reduced the toxicity (Table 4.10).
Table 4.8
Effects of linear AE to algae.
Species |
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Selenastrum capricornutum |
C12-14 EO4 |
2-4 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 EO9 |
4-8 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 EO13 |
10 |
48 h |
Yamane et al. 1984 |
Nitzschia fonticula |
C12-14 EO9 |
5-10 |
48 h |
Yamane et al. 1984 |
Microcystis aeruginosa |
C12-14 EO9 |
10-50 |
72 h |
Yamane et al. 1984 |
Scenedesmus subspicatus |
C12-14 EO7 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C12-15 EO7 |
0.85 (0.84-0.85)* NOEC:0.50 |
72 h |
Madsen et al. 1996b |
Scenedesmus subspicatus |
C13 EO7-8 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13-15 EO7-8 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C14-15 EO6 |
0.09 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C14-15 EO6 |
0.60 |
96 h |
Lewis and Hamm 1986 |
Navicula pelliculosa |
C14-15 EO6 |
0.28 |
96 h |
Lewis and Hamm 1986 |
Scenedesmus subspicatus |
C15 EO7-8 |
0.05 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C12-15 EO9 |
0.7 |
96 h |
Dorn et al. 1993 |
* Parenthesis indicate 95% confidence interval.
Table 4.9
Effects of branched AE to algae.
Species |
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Not indicated |
Oxo-C9-15 EO2-10 |
4-50 |
- |
Schöberl et al. 1988 |
Scenedesmus subspicatus |
Iso-C10 EO7-8 (3 internal CH3-groups, highly branched) |
50 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Iso-C13 EO7-8 (3 internal CH3-groups, highly branched) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Iso-C13 EO7-8 (4 internal CH3-groups, highly branched) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (< 1 internal CH3-group, 10% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (1 internal CH3-group, 25% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (» 1 internal CH3-group, 46% branching) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Oxo-C13-15 EO7-8 (10% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C15 EO7-8 (1 internal CH3-group, 25% branching) |
0.05 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C13 EO7 (2 internal CH3-groups, quaternary C-atom) |
7.5 NOEC: 10.0 |
96 h |
Dorn et al. 1993 |
Selenastrum capricornutum |
C11-15 EO7 (4 internal CH3-groups, quaternary C-atom) |
10.0 NOEC: 4.0 |
96 h |
Dorn et al. 1993 |
Table 4.10
Effects of AA and end-capped AE to algae.
Species |
AA |
EC50 (mg/l) |
Test Duration |
Reference |
Algae |
C8-10 EO6, PO3 |
10-100 |
- |
Bertleff et al. 1997 |
Algae |
C9-11 EO6, PO3 |
1-10 |
- |
Bertleff et al. 1997 |
Algae |
C10-13 EO6, PO3 |
1-10 |
- |
Bertleff et al 1997 |
Algae |
Iso-C13 EO6, PO3 |
10-100 |
- |
Bertleff et al. 1997 |
Algae |
C13-15 EO6, PO3 |
0.1-1 |
- |
Bertleff et al. 1997 |
Algae |
C12-14 EO9, butylether (end-capped) |
0.3 |
- |
Schöberl et al. 1988 |
Invertebrates
The acute toxicity of AE to invertebrates varies with EC50 values from 0.1 mg/l to more than 100 mg/l for the linear types and from 0.5 mg/l to 50 mg/l for the branched types. The toxicity is species specific and may vary between 0.29 mg/l to 270 mg/l for the same linear AE (Lewis and Suprenant 1983). The most commonly used invertebrates for testing are Daphnia magna and Daphnia pulex, and they are also among the most sensitive invertebrates to AE. Apparently, the toxicity of AE to invertebrates was not related to hydrophobicity as it is the case for algae. Some AE are very toxic to invertebrates, i.e., linear AE of C12-15 EO1-8 and branched AE with a low degree of branching, i.e. < 10-25%. Branching of the alkyl chain reduces the toxicity of AE to invertebrates as also observed for algae. This effect of branching is evident by comparison of the toxicity of the linear C13 AE and C13 AE containing more or less branched alkyl chains (Tables 4.11-4.12). The toxicity of commercial AE was recently determined by using a sperm cell toxicity test with the sea urchin Paracentrotus lividus. The EC50 obtained in this test have proven to be closer to chronic data for all the tested AE. Whereas a fully linear C12 AE exhibited an EC50 of 0.96 mg/l in the sperm cell toxicity test, a fully monobranched C12 AE exhibited an EC50 of 4.0 mg/l. In this case, the alkyl side chain reduced the toxicity of the C12 AE by approximately a factor of 4 (Marcomini et al. 2000c). A C12-14 EO9 end-capped with an n-butyl-group was toxic to daphnids as the acute and chronic EC50 values were 1-2 mg/l and 0.3 mg/l, respectively (Schöberl et al. 1988). Schöberl et al. (1988) report that an AA with 2-5 EO and 4 PO was toxic to daphnids as the EC50 ranged between 2.4 and 6.0 mg/l.
Table 4.11
Effects of linear AE to invertebrates.
Species |
AE |
EC/LC50 (mg/l) |
Test Duration |
Reference |
Hyalella azteca |
C9-11 EO6 |
14 |
10 d |
Dorn et al. 1997 |
Chironomus tentans |
C9-11 EO6 |
5.7 |
10 d |
Dorn et al. 1997 |
Mysidopsis bahia |
C10 EO4 |
5.6 |
48 h |
Hall et al. 1989 |
Daphnia magna |
C12-13 EO5 C12-13 EO4.5-6 C12-13 EO6.5 |
0.46 (0.39-0.56)* 0.59 (0.42-0.83)* 0.74 (0.63-0.86)* |
48 h |
Wong et al. 1997 |
Daphnia magna |
C12-14 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia pulex |
C12-15 EO7 |
0.76 |
48 h |
Salanitro et al. 1988 |
Daphnia magna |
C12-15 EO7 |
1.0-2.0 |
48 h |
Madsen et al. 1996b |
Daphnia magna |
C12-15 EO9 |
1.3 (1.1-1.4)* NOEC: 1.0 |
48 h |
Dorn et al. 1993; Kravetz et al. 1991 |
Daphnia magna |
C13 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Mysidopsis bahia |
C13 EO10 |
2.2 |
48 h |
Hall et al. 1989 |
Daphnia magna |
C13-15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia magna |
C14 EO1 |
0.83 |
48 h |
Maki and Bishop 1979 |
Daphnia pulex |
C14 EO1 |
0.10 |
48 h |
Maki and Bishop 1979 |
Daphnia magna |
C14-15 EO7 |
0.29-0.4 |
48 h |
Lewis and Perry 1981 |
Paratanytarus parthenogenica (midge) |
C14-15 EO7 |
23 |
48 h |
Lewis and Suprenant 1983 |
Gammarus sp. (amphipod) |
C14-15 EO7 |
3.3 |
48 h |
Lewis and Suprenant 1983 |
Asellus sp. (isopod) |
C14-15 EO7 |
270 |
48 h |
Lewis and Suprenant 1983 |
Dugesia sp. (flatworm) |
C14-15 EO7 |
1.8 |
48 h |
Lewis and Suprenant 1983 |
Dero sp. (oligochaete) |
C14-15 EO7 |
1.7 |
48 h |
Lewis and Suprenant 1983 |
Rhabditis sp. (nematode) |
C14-15 EO7 |
16 |
48 h |
Lewis and Suprenant 1983 |
Daphnia magna |
C14-15 EO13 |
1.2 (0.65-1.9)* |
48 h |
Wong et al. 1997 |
Daphnia magna |
C15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia |
C16-18 EO2-4 |
20-100 |
- |
Schöberl et al. 1988 |
Daphnia |
C16-18 EO5-7 |
5-200 |
- |
Schöberl et al. 1988 |
Daphnia |
C16-18 EO10-14 |
40-60 |
- |
Schöberl et al. 1988 |
Daphnia magna |
C16-18 EO18 |
20 |
48 h |
Talmage 1994 |
Daphnia magna |
C16-18 EO30 |
18 |
48 h |
Talmage 1994 |
* Parentheses indicate 95% confidence intervals.
Table 4.12
Effects of branched AE to Daphnia magna.
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Oxo-C9-15 EO2-10 |
2-10 |
- |
Schöberl et al. 1988 |
Oxo-C9-15 EO> 10 |
4-20 |
- |
Schöberl et al. 1988 |
Iso-C10 EO7-8 |
50 |
48 h |
Kaluza and Taeger 1996 |
Oxo-C11 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7 |
9.8 |
48 h |
Dorn et al. 1993 |
C11-15 EO7 |
11.6 |
48 h |
Kravetz et al. 1991 Dorn et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Fish
The acute toxicity of AE to fish varies with LC50 values from 0.4 mg/l to more than 100 mg/l for the linear types and from 0.25 mg/l to 40 mg/l for the branched AE (Tables 4.13-4.14). For linear AE the toxicity increases with decreasing EO units (comparison within C12-15 EO7-9 and within C14-15 EO 7-11). C12-15 AE containing 7-11 EO groups are considered to be very toxic to fish (EC/LC50 £ 1 mg/l). There are only few data on the toxicity of branched AE to fish and only oxo-C9-15 EO2-10 is considered very toxic. A C12-14 EO9 end-capped with an n-butyl-group was toxic to fish (species not specified) as the EC50 was 0.5-4.6 mg/l (Schöberl et al. 1988). Schöberl et al. (1988) report that an AA with 2-5 EO and 4 PO was toxic to fish as the LC50 ranged between 0.7 and 5.7 mg/l.
Table 4.13
Effects of linear AE to fish.
Species |
AE |
LC50 (mg/l) |
Test Duration |
Reference |
Bluegill sunfish |
C10-12 EO6 |
6.4 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow |
C12-13 EO5 |
1.0 (0.84-1.3)A |
96 h |
Wong et al. 1997 |
Brown trout |
C12-14 EO8 |
0.8 |
96 h |
Reiff et al. 1979 |
Golden orfe |
C12-14 EO8 |
1.8 |
96 h |
Reiff et al. 1979 |
Harlequin fish |
C12-14 EO10-11 |
1.6-2.8 |
96 h |
Reiff et al. 1979 |
Zebra fish |
C12-15 EO7 |
1.0-2.0 |
96 h |
Madsen et al. 1996 |
Bluegill sunfish |
C12-15 EO3 |
1.5 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow |
C12-15 EO7 |
0.48 |
96 h |
Salanitro et al. 1988 |
Fathead minnow |
C12-15 EO9 |
1.6 (1.3-1.8) A |
96 h |
Dorn et al. 1993; Kravetz et al. 1991 |
Bluegill sunfish |
C12-15 EO9 |
2.1 |
96 h |
Macek and Krzeminski 1975 |
Atlantic salmon |
C12 EO4 |
1.5 25.0 |
96 h |
Wildish 1972 |
Bluegill sunfish |
C13 EO9 |
7.5 |
96 h |
Macek and Krzeminski 1975 |
Rainbow trout |
C14-15 EO7 |
0.78 |
96 h |
Turner et al. 1985 |
Rainbow trout |
C14-15 EO11 |
1.08 |
96 h |
Turner et al. 1985 |
Rainbow trout |
C14-15 EO18 |
5.0-6.3 |
96 h |
Talmage 1994 |
Bluegill sunfish |
C14-15 EO7 |
0.66 |
96 h |
Lewis and Perry 1981 |
Bluegill sunfish |
C14-15 EO7 |
0.7-1.12 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow |
C14-15 EO7 |
0.63-1.65 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow |
C14-15 EO13 |
1.0 (0.62-1.9)A |
96 h |
Wong et al. 1997 |
Not indicated |
C16-18 EO2-4 |
> 100 |
- |
Schöberl et al. 1988 |
Not indicated |
C16-18 EO5-7 |
3-30 |
- |
Schöberl et al. 1988 |
Not indicated |
C16-18 EO10-14 |
1.7-3 |
- |
Schöberl et al. 1988 |
Brown trout |
Tallow EO14 |
0.4 |
96 h |
Reiff et al. 1979 |
Golden orfe |
Tallow EO14 |
2.3 |
96 h |
Reiff et al. 1979 |
Harlequin fish |
Tallow EO14 |
0.7 |
96 h |
Reiff et al. 1979 |
A
Parentheses indicate 95% confidence intervals.Table 4.14
Effects of branched AE to fish.
Species |
AE |
LC50 (mg/l) |
Test Duration |
Reference |
Not indicated |
Oxo-C9-15 EO2-10 |
0.25-4 |
- |
Schöberl et al. 1988 |
Not indicated |
Oxo-C9-15 EO> 10 |
1-40 |
- |
Schöberl et al. 1988 |
Bluegill sunfish |
C11-15 EO9 |
4.6 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow (Pimephales promelas) |
C13 EO7 (2 internal CH3-groups, quaternary C-atom) |
4.5 |
96 h |
Dorn et al. 1993 |
Fathead minnow |
C11-15 EO7 |
6.1 (5.8-6.3)A |
96 h |
Kravetz et al. 1991 |
A
Parentheses indicate 95% confidence intervals.4.1.4 Effects on human health
Toxicokinetics and acute toxicity
In general, AE are readily absorbed through the skin of guinea pigs and rats and through the gastrointestinal mucosa of rats. AE are quickly eliminated from the body through the urine, faeces, and expired air (CO2) (CIRP 1983; SFT 1991).
Orally dosed AE was absorbed rapidly and extensively in rats, and more than 75% of the dose was absorbed. When applied to the skin of humans, the doses were absorbed slowly and incompletely (50% absorbed in 72 hours). Half of the absorbed surfactant was excreted promptly in the urine and smaller amounts of AE appeared in the faeces and expired air (CO2) (Drotman 1980). The metabolism of C12 AE yields PEG, carboxylic acids, and CO2 as metabolites (SFT 1991). Data describing the acute toxicity of various AE, as indicated by LD50, are presented in Table 4.15. The LD50values after oral administration to rats range from about 1-15 g/kg body weight indicating a low to moderate acute toxicity.
Table 4.15
Acute toxicity (LD50) of AE.
Type of surfactant |
Species |
Route of administration |
LD50 (g/kg body weight) |
Reference |
AE |
Rat |
Oral |
1.6 - > 25 |
Kirk-Otmer 1994 |
AE |
Rat |
Oral |
0.87 - > 25 |
SFT 1991 |
C9-11 EO6 |
Rat |
Oral |
1.4 |
Gingell and Lu 1991 |
C9-11 EO6 |
Rabbit |
Dermal |
< 2 |
Gingell and Lu 1991 |
C12 EO23 |
Rat |
Oral |
8.6 |
CIRP 1983 |
C12 EO23 |
Mouse |
Oral |
3.5 |
CIRP 1983 |
C12 EO4 |
Rat, mouse |
Oral |
5-10 |
CIRP 1983 |
C13 EO6 |
Rat |
Oral |
2.1 |
Benke and Brown 1977 |
C13 EO6 |
Rat |
Dermal |
< 2.0 ml |
Benke and Brown 1977 |
C14 EO7 |
Rat |
Oral |
3.3 |
Benke and Brown 1977 |
C18 EO10 |
Rat |
Oral |
2.91 |
CIRP 1988 |
C18 EO20 |
Rat |
Oral |
1.92 |
CIRP 1988 |
C18 EO2 |
Rat |
Oral |
> 25.1 |
CIRP 1988 |
Oxo-AE |
Rat |
Oral |
< 10 |
Hüls 1993 |
Skin and eye irritation
The ability of nonionic surfactants to cause a swelling of the stratum corneum of guinea pig skin has been studied. C12 AE containing 23 EO groups caused little or no swelling. It was concluded that swelling is due to a reversible conformation change, resulting from coorporative binding of the surfactant (Putterman et al. 1977). The swelling mechanism of the skin involves a combination of ionic binding of the hydrophilic group as well as hydrofobic interactions of the alkyl chain with the substrate. One of the mechanisms of skin irritation caused by surfactants is considered to be denaturation of the proteins of skin. It has also been established that there is a connection between the potential of surfactants to denaturate protein in vitro and their effect on the skin. Nonionic surfactants do not carry any net charge and, therefore, they can only form hydrophobic bonds with proteins. For this reason, proteins are not deactivated by nonionic surfactants, and proteins with poor solubility are not solubilized by nonionic surfactants.
Undiluted C9-11 EO6 was found severely irritant to the rabbit skin. The exposure site was evaluated for erythema and edema using the Draize method of scoring. The Primary Irritation Index (PII) was determined to be 5.3 of a possible 8.0. Less than 2 is mildly irritating, 2 – 5 is moderately irritating and > 5 is severely irritating. According to this system, the undiluted C9-11 EO6 is classified as moderately irritating to rabbit skin (Gingell and Lu 1991). Undiluted C12 EO23 caused no primary irritation when applied to the rabbit skin. No primary cutaneous irritation was observed in clinical studies using 60% C12 EO23 or 100% C12 EO4 (CIRP 1983). C18 AE with either 2, 10 or 20 EO were not irritants when applied to the skin of 200 humans at a concentration of 60% in water (CIRP 1988).
A 1% solution of C13 EO6 and a 10% solution of C14 EO7 were tested for skin irritation using a rabbit closed-patch test. The C13 AE was mildly irritating under these conditions as indicated by a PII score of 1.6, whereas the C14 AE was only moderately irritating with a PII score of 4.2 (Benke and Brown 1977). Undiluted C12 EO23 only caused a slight conjunctival reaction in a Draize eye test with rabbits and no corneal and iridial effects were recorded, both in washed and unwashed eyes, for up to 72 hours (CIRP 1983).
The Draize system for evaluation of eye irritation consists of 8 descriptive ratings with increasing intensity of irritation. The maximum values for scoring are 80 for the cornea, 20 for the conjunctiva and 10 for the iris. The higher the score the more severe the damage. The maximum total score is 110. In Draize eye irritation studies with rabbits, undiluted C12 EO4 was moderately and minimally irritating in the unrinsed and rinsed eye, respectively. Ten and twenty percent solutions were both classified as either slightly or non-irritating to unrinsed and rinsed eyes (CIRP 1983). Undiluted C13 EO6 and C14 EO7 produced severe eye irritation in rabbits. The maximum average scores calculated according to Draize were 59.1 for unrinsed eyes. When a 10% solution was used, or when the eyes were rinsed after application of undeluted AE, a moderate irritation was produced as indicated by a maximum average Draize score of 10 to 35 (Benke and Brown 1977).
Aqueous concentrations of up to 60% of C18 AE with either 2EO or 10EO were mildly and minimally irritating to the rabbit eye, respectively. In rabbits C18 EO10 was practically non-irritating to the eye, whereas C18 EO2 and C18 EO20 were minimally irritating to the eye with no water rinse. All of the three C18 AE were non-irritating to the eyes when the eyes were rinsed with water. No irritation of the cornea and iris was observed in rinsed eyes (CIRP 1988).
Sensitization
A 1% w/v aqueous dilution of a C9-11 EO6 was not a skin sensitizer in a guinea pig skin sensitization assay according to the Buehler method. It is an EEC accepted allergy test method and is mentioned in the OECD test guideline No. 406, "Skin Sensitization" (Gingell and Lu 1991). No evidence of sensitization was reported when a 25% solution of C12 EO23 was used in a repeated insult patch test on 168 subjects. The surfactant was applied at 48 hours intervals three times per week for 3 weeks. Then a 3 week non-treatment period followed before the subjects were challenged using the same procedure. A C12 EO4 did not produce sensitization when applied at 100% to 50 subjects in an other patch test. No reactions were observed after the induction or the challenge application (CIRP 1983).
Subchronic and chronic toxicity
A diet containing 1% C14 EO7 or C13 EO6 produced increased liver–to-body weight ratios after administration to rats for 91 days, although, histologically, these livers appeared normal (Brown and Benke 1977). Systemic toxicity of C12 EO4 was not observed in subchronic (21 days) and chronic (3 months, twice daily) dermal tests with diluted formulations (6% in 52% aqueous ethanol solution) on rabbits (CIRP 1983). No observable systemic toxicity was produced in 4 or 13 week subchronic percutaneous toxicity studies after repeated dermal doses (up to 50 mg/day) of C13 EO6 and C14 EO7 in rabbits (Brown and Benke 1977; Talmage 1994).
Reproductive toxicity
The possible adverse effects of dermally applied C9-11 EO6 on the reproductive performance of rats and their offspring over two generations were evaluated by monitoring fertility, gestation, lactation, pup growth and survival. The rats were exposed unoccluded, three days per week, to 0.1 ml/kg body weight of concentrations of 1, 10 and 25% AE. No effects on the reproductive performance or on the growth and development of the offspring were detected (Gingell and Lu 1991). No teratogenic or embryotoxic effects were seen when rats were treated topically with 6% C12 EO4 in 52% ethanol on day 6 to day 15 of gestation (CIRP 1983).
Mutagenicity
There was no evidence of mutagenicity of C9-11 EO6 when tested in the Ames test (gene mutation test). The mutagenic response was investigated in Salmonella typhimurium strains by evaluation of their ability to induce base-pair substitution and frame-shift mutations (Gingell and Lu 1991). Data on genotoxicity were collected in a survey of nine short-term genotoxicity testing for many different types of nonionic surfactants. None of these data indicated any mutagenic potential of AE (Yam et al. 1984; Dean 1985; Zeiger and Anderson 1988).
Classification
Alcohol ethoxylates are according to CESIO (2000) classified as Irritant or Harmful depending on the number of EO-units:
- EO < 5 gives Irritant (Xi) with R38 (Irritating to skin) and R41 (Risk of serious damage to eyes)
- EO > 5-15 gives Harmful (Xn) with R22 (Harmful if swallowed) – R38/41
- EO > 15-20 gives Harmful (Xn) with R22-41
- > 20 EO is not classified (CESIO 2000)
- Oxo-AE, C13 EO10 and C13 EO15, are Irritating (Xi) with R36/38 (Irritating to eyes and skin) (Hüls 1993).
AE are not included in Annex 1 of the list of dangerous substances of the Council Directive 67/548/EEC.
4.2 Block copolymers
Block copolymers are weakly foaming substances that have found applications within areas as detergents (foam-mitigating agents), wetting agents, emulsifiers, textile lubricants, and agricultural chemicals. Block copolymers are now being replaced in many household detergents by alcohol alkoxylates (AA) that comply better with the current requirements for biodegradability. The block copolymers consist of long chains of ethylene oxide (EO) and propylene oxide (PO) units. Contrary to other nonionic surfactants, the block copolymers do not contain a hydrophobic moiety based on a fatty alcohol. Instead, the PO units function as the hydrophobic part which establish surface active properties in combination with the more hydrophilic EO units.
4.2.1 Environmental fate
Aerobic biodegradability
The block copolymers fail to meet the requirements for ready biodegradability and also their primary biodegradability may be limited. The biodegradation mechanisms are supposed to be similar to the mechanisms responsible for the degradation of the hydrophilic part of AA: The EO/PO chain is degraded from the terminus by sequential cleavage of individual glycol units. Inclusion of PO units may reduce the biodegradability due to the possible presence of a secondary C-atom which is known to inhibit the degradation (Balson and Felix 1995). The high molecular weight of the copolymers increases the time needed for biodegradation, as the degradation proceeds by terminal attack only. Furthermore, the molecular weight also limits the transport through the bacterial cell wall and thus limits the intracellular degradation. Primary biodegradability of copolymers varies between 5 and 58%, the higher values representing polymers with a high content of EO (Balson and Felix 1995). Removal of EO/PO block polymers was found to be 7% in a confirmatory test and 2-4% in a coupled unit’s test (Holt et al. 1992).
4.2.2 Effects on the aquatic environment
Block copolymers are some of the least toxic types of nonionic surfactants. Aquatic toxicity of block copolymers is reported with EC/LC50 values of more than 100 mg/l for fish and invertebrates (Schöberl et al. 1988). In spite of the limited biodegradability, the block copolymers are generally not considered to cause adverse effects in aquatic environments at concentrations below 100 mg/l.
4.3 Alkyl glycosides and glucose amides
Alkyl polyglycosides (APG) and fatty acid glucose amides (FAGA) are used in household products like cleaning agents, liquid dishwashing agents and laundry detergents. APG are composed of a linear fatty alcohol which is bound to the C-1 carbon of the glucose molecule by a glycosidic bond. Commercial APG mixtures usually have an average degree of polymerization (DP) of approximately 1.4 moles of glucose per mole of fatty alcohol.
APG have the following structure:
The alkyl chain usually contains either 8-10 or 12-14 carbons (Steber et al. 1995).
FAGA have the following structure:
R-CH2-CO-N-(CH3)-CH2-(CHOH)4-CH2-OH
No data were found on the occurrence of APG or FAGA in the environment.
4.3.1 Environmental fate
Effects of structure of APG on biodegradability
The effects of the APG structure on the aerobic degradation pathway have not been described and no metabolites have been identified. Under strictly anoxic conditions, a branched C8 APG was only partially degraded in contrast to the extensive anaerobic degradation of linear APG (Madsen et al. 1996b). Similarly, the pathways by which FAGA biodegrades are not yet known.
Aerobic biodegradability
According to the results obtained in OECD tests for ready biodegradability, APG with alkyl chain lengths from C8 to C16 are readily biodegradable (Table 4.16). With the exception of the C8 APG, all APG in Table 4.16 are based on linear alkyl chains. Ultimate aerobic biodegradability of C12-14 APG was also tested in an OECD confirmatory test showing 96-100% removal of DOC (Schöberl 1997). A similar high biodegradability of C12-14 APG was seen in a coupled units test in which a 89% DOC removal was achieved (Steber et al. 1995). The primary biodegradation of APG was also rapid in the OECD confirmatory test as indicated by a specific analysis of APG (Steber et al. 1995). Ultimate biodegradation without formation of stable metabolites was confirmed in a modified coupled units test. In this test, the effluent from the treatment unit was circulated to detect any possible accumulation of non-readily degradable substances. The C12-14 APG reached 100% of DOC removal indicating that the APG was completely mineralized without any accumulation of metabolites (Steber et al. 1995).
The ready biodegradability of a special type of glycoside surfactant, an ethyl glycoside fatty acid 6-O monoester (C12) (EGE), was examined in the CO2 evolution test and the closed bottle test (Table 4.16). The C12 EGE was degraded more rapidly than the other examined surfactants (C12-14 APG, C8 branched APG, and C12-15 AE), and, for C12 EGE, 65% of ThOD was reached after only 5 days in the closed bottle test (Madsen 1996b). The C12 EGE has previously been succesfully applied in pilot-scale laundry detergents (Andresen et al. 1995), but, to our knowledge, no commercial household products containing this type of surfactant are available. A C12-14 glucose amide (C12-14 FAGA) reached 89 and 86% of ThCO2, respectively, for substrate concentrations of 10 and 20 mg/l (Stalmans et al. 1993; Table 4.16). In an activated sludge mineralization experiment with a 14C-labelled C12 FAGA, 89% of the added 14C was recovered as 14CO2 after 28 days and the mineralization half-life was calculated to 1.26 days (Stalmans et al. 1993).
Table 4.16
Ultimate aerobic biodegradability of alkyl glycosides and glucose amides.
Compound |
Test |
Result |
Reference |
C8 branched APG |
CO2 evolution test, 28 d |
78% ThCO2 |
Madsen et al. 1996b |
C8 branched APG |
Closed bottle test, 28 d |
68% ThOD |
Madsen et al. 1996b |
C8-10 APG |
Modified OECD screening test, 28 d |
94% DOC |
Steber et al. 1995 |
C8-10 APG |
Closed bottle test, 28 d |
81-82% ThOD |
Steber et al. 1995 |
C8-16 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C8-16 APG |
Closed bottle test, 30 d |
80% ThOD |
Garcia et al. 1997 |
C9-11 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C9-11 APG |
Closed bottle test, 30 d |
94% ThOD |
Garcia et al. 1997 |
C12-14 APG |
Die away screening test, 28 d |
95-96% DOC |
Steber et al. 1995 |
C12-14 APG |
Modified OECD screening test, 28 d |
90-93% DOC |
Steber et al. 1995 |
C12-14 APG |
Closed bottle test, 28 d |
73-88% ThOD |
Steber et al. 1995 |
C12-14 APG |
Closed bottle test, 28 d |
67% ThOD |
Madsen et al. 1996b |
C12-14 APG |
CO2 evolution test, 28 d |
81% ThCO2 |
Madsen et al. 1996b |
C12-16 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C12-16 APG |
Closed bottle test, 30 d |
78% ThOD |
Garcia et al. 1997 |
C12 EGE |
CO2 evolution test, 28 d |
78% ThCO2 |
Madsen et al. 1996b |
C12 EGE |
Closed bottle test, 28 d |
80% ThOD |
Madsen et al. 1996b |
C12-14 FAGA |
CO2 evolution test, 35 d |
86; 89% ThCO2 |
Stalmans et al. 1993 |
Anaerobic biodegradability
Several studies have shown that APG with a linear alkyl chain are ultimately biodegradable in the absence of molecular oxygen (Table 4.17). The anaerobic biodegradation of these surfactants is normally rapid and may exceed 60% of ThGP within 28 days (Madsen et al. 1995). Also the glycoside monoesters, C10 and C12 EGE, were extensively biodegraded in an anaerobic screening test with digested sludge (Table 4.17). The biodegradability of alkyl glycosides has also been determined in screening tests with anoxic sediments. By using material from a freshwater swamp as the inoculum, the ultimate biodegradability during 56 days at 35° C reached 76% of ThGP for C12-14 APG, 83% of ThGP for C10 EGE, and 89% of ThGP for C12 EGE. In a similar test with an inoculum obtained from a marine sediment, the biodegradability during 56 days at 35° C attained 79% of ThGP for C10 EGE (Madsen 1996a). Branching of the alkyl chain may limit the anaerobic mineralization as indicated by the low biodegradability of a branched C8 APG (Table 4.17).
Table 4.17
Ultimate anaerobic biodegradability of alkyl glycosides in digested sludge.
Compound |
Test |
Result |
Source |
C8 branched APG |
Measurement of gas production, 35° C, 56 d |
22% ThGP |
Madsen et al. 1996b |
C8-10 APG |
Measurement of gas production, 35° C, 56 d ECETOC test |
95% ThGP |
Steber et al. 1995 |
C12-14 APG |
Measurement of gas production, 35° C, 56 d ECETOC test |
84% ThGP |
Steber et al. 1995 |
C12-14 APG |
Measurement of gas production, 35° C, 56 d |
72; 92% ThGP |
Madsen et al. 1996b; Madsen et al. 1996a |
C10 EGE |
Measurement of gas production, 35° C, 56 d |
96% ThGP |
Madsen et al. 1996a |
C12 EGE |
Measurement of gas production, 35° C, 56 d |
82% ThGP |
Madsen et al. 1996a |
Bioaccumulation
No experimental data describing the bioaccumulation potential of APG or FAGA were found in the literature.
4.3.2 Effects on the aquatic environment
The aquatic toxicity of alkyl glycosides and glucose amides is characterized by EC/LC50 values in the range from 2.5 to more than 100 mg/l with the lowest toxicity for the short-chained APG. With EC/LC50 values of 2.5-12 mg/l, C12-14 APG are considered toxic to aquatic organisms, whereas C8-10 APG have a lower toxicity with EC/LC50 ³ 20 mg/l. The EC/LC50 values for algae, crustaceans and fish were between 11 and 38 mg/l for C12 EGE and between 2.9 and 57 mg/l for FAGA with C12 to C14 alkyl chains (Table 4.18-4.20).
Table 4.18
Effects of alkyl glycosides and glucose amides to algae.
Compound |
Species |
EC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
Selenastrum capricornutum |
1,543 (1,474-1,621)* |
72 h |
Madsen et al. 1996b |
C8 branched APG |
Selenastrum capricornutum |
NOEC: 100 |
72 h |
Madsen et al. 1996b |
C8-10 APG |
Scenedesmus subspicatus |
21 |
72 h |
Steber et al. 1995 |
C8-10 APG |
Scenedesmus subspicatus |
NOEC: 5.7 |
72 h |
Steber et al. 1995 |
C8-16 APG |
Scenedesmus subspicatus |
14.8 |
96 h |
Henkel KgaA |
C8-16 APG |
Scenedesmus subspicatus |
NOEC: 5.0 |
96 h |
Henkel KgaA |
C12-14 APG |
Scenedesmus subspicatus |
6.0 |
72 h |
Steber et al. 1995 |
C12-14 APG |
Scenedesmus subspicatus |
NOEC: 2.0 |
72 h |
Steber et al. 1995 |
C12-14 APG |
Selenastrum capricornutum |
11 (10-13)* |
72 h |
Madsen et al. 1996b |
C12-14 APG |
Selenastrum capricornutum |
NOEC: 3.1 |
72 h |
Madsen et al. 1996b |
C12 EGE |
Selenastrum capricornutum |
38 (37-38)* |
72 h |
Madsen et al. 1996b |
C12 EGE |
Selenastrum capricornutum |
NOEC: 11 |
72 h |
Madsen et al. 1996b |
C12 FAGA |
Selenastrum capricornutum |
57 (50-64)* |
96 h |
Stalmans et al. 1993 |
C12 FAGA |
Selenastrum capricornutum |
NOEC: 21 |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Selenastrum capricornutum |
13 (12-14)* |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Selenastrum capricornutum |
NOEC: 5.6 |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Selenastrum capricornutum |
3.9 (2.5-6.4)* |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Selenastrum capricornutum |
NOEC: 2.9 |
96 h |
Stalmans et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Table 4.19
Effects of APG to Daphnia magna.
Compound |
EC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
557 (465-717)* |
48 h |
Madsen et al. 1996b |
C8-10 APG |
20 |
48 h |
Steber et al. 1995 |
C8-16 APG |
85 |
48 h |
Henkel KgaA |
C12-14 APG |
12 (10-14)* |
48 h |
Madsen et al. 1996b |
C12-14 APG |
7.0 |
48 h |
Steber et al. 1995 |
C12-14 APG |
NOEC: 1.0 |
21 d (reprod.) |
Steber et al. 1995 |
C12 EGE |
23 (21-25)* |
48 h |
Madsen et al. 1996b |
C12 FAGA |
44 (38-53)* |
48 h |
Stalmans et al. 1993 |
C12-14 FAGA |
18 (16-21)* |
48 h |
Stalmans et al. 1993 |
C12-14 FAGA |
NOEC: 4.3 (survival) |
21 d |
Stalmans et al. 1993 |
C14 FAGA |
5.0 (3.3-9.2)* |
48 h |
Stalmans et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Table 4.20
Effects of APG to fish.
Compound |
Species |
LC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
Zebra fish (Brachydanio rerio) |
558 |
96 h |
Madsen et al. 1996b |
C8-10 APG |
Zebra fish |
101 |
96 h |
Steber et al. 1995 |
C8-16 APG |
Zebra fish |
7.8 |
96 h |
Henkel KgaA |
C12-14 APG |
Zebra fish |
2.5-5.0 |
96 h |
Madsen et al. 1996b |
C12-14 APG |
Zebra fish |
3.0 |
96 h |
Steber et al. 1995 |
C12-14 APG |
Zebra fish |
NOEC: 1.8 |
28 d |
Steber et al. 1995 |
C12 EGE |
Zebra fish |
11-17 |
96 h |
Madsen et al. 1996b |
C12 FAGA |
Fathead minnow (Pimephales promelas) |
39 (31-51) |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Zebra fish |
7.5 |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Fathead minnow |
2.9 (2.4-3.7) |
96 h |
Stalmans et al. 1993 |
4.3.3 Effects on human health
Acute toxicity
The toxicity of APG by oral and dermal administration is low (Table 4.21).
Table 4.21
Acute toxicity (LD50) of APG.
Compound |
Species |
Application |
LD50 (g/kg body weight) |
Reference |
C10 APG |
Rat |
Oral |
> 10 |
Hughes and Lew 1970 |
C8 alkyl glycoside |
Rat |
Oral |
> 2 |
Akzo Nobel 1998 |
C8 alkyl glycoside |
Rabbit |
Dermal |
> 2 |
Akzo Nobel 1998 |
n-Octadecyl-9.0-glycoside |
Rat |
Oral |
> 35.5 |
Hughes and Lew 1970 |
Skin and eye irritation
Patch test carried out on 10 volunteers at concentrations up to 10% active matter of a C10 APG showed no skin irritation (Hughes and Lew 1970).
Classification
Alkyl glycosides are considered non-irritating to skin, but irritating to eyes at very high concentrations. A general classification of a 65% C8 alkyl glycoside solution according to the Substance Directive 67/548/EEC is Irritating (Xi) with the risk phrase R41 (Risk of serious damage to the eyes) or R36 (Irritating to the eyes) (Akzo Nobel 1998).
Alkyl glycosides are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
4.4 Fatty acid amides
Fatty acid amides (FAA) are used in hair shampoo, liquid soaps, shaving creams and other personal care products. FAA consist of a fatty acid, usually derived from coconut oil, which is linked to an amide group by a C-N bond. The amide may either be monoethanolamide (MEA), diethanolamide (DEA), or monoisopropanolamide (MIPA). Representative structures of FAA are indicated below.
The alkyl chain usually contains 12 to 18 carbon atoms.
4.4.1 Environmental fate
Aerobic biodegradability
Most fatty acid amides (FAA), like e.g. the widely used cocodiethanolamide (cocoamide DEA) and cocomonoethanolamide (cocoamide MEA), are ultimately degraded in the OECD tests for ready biodegradability. The available data describing the biodegradability of the ethoxylated FAA are contradictory. Data cited by Schöberl et al. (1988) indicate that these surfactants do not pass the criteria for ready biodegradability, whereas the opposite is the case for data obtained from Akzo Nobel (1999a, 1999b) (Table 4.22).
Table 4.22
Ultimate aerobic biodegradability of FAA.
FAA |
Test |
Result |
Reference |
Cocoamide MEA |
Closed bottle test, 30 d |
82% ThOD |
IUCLID 2000 |
Cocoamide DEA |
Closed bottle test, 30 d |
71% ThOD |
IUCLID 2000 |
C12-18 amide DEA |
Modified OECD screening test, 28 d |
74% DOC |
Schöberl et al. 1988 |
C18 amide DEA |
Coupled units test |
87% DOC |
Schöberl et al. 1988 |
C12-14 amide MEA EO 4 |
Closed bottle test, 28 d |
47% ThOD |
Schöberl et al. 1988 |
C12-14 amide MEA EO 10 |
Closed bottle test, 28 d |
35% ThOD |
Schöberl et al. 1988 |
C12-14 amide MEA E05 |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Akzo Nobel 1999a |
C12-14 amide MEA E012 |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Akzo Nobel 1999b |
The primary biodegradability of FAA during 19 days attained 91-100% for C12
amide MEA, 90-99% for C12 amide DEA, and 90-98% for the ethoxylated C12
amide DEA EO5 (Swisher 1987). Primary biodegradation of C18 amide MEA EO6
attained 97-98% removal in an OECD-confirmatory test (Schöberl 1997).
Anaerobic biodegradability
The anaerobic biodegradability of FAA has been examined for cocoamide MEA by using the ECETOC screening test (ECETOC 1988). Ultimate anaerobic biodegradability of cocoamide MEA reached 79% of the theoretical gas production, ThGP, during incubation of diluted digested sludge for 42 days at 35° C (IUCLID 2000). By use of the ISO 11734 screening test, which corresponds to the ECETOC method, the ultimate anaerobic biodegradability of cocoamide MEA attained 81% during 56 days (Appendix; Table A12, Figure A12).
Bioaccumulation
No experimental data describing the bioaccumulation potential of fatty acid amides were found in the literature.
4.4.2 Effects on the aquatic environment
The aquatic toxicity of FAA has been determined for species representing the three trophic levels algae, invertebrates, and fish. Cocoamide DEA appears to be more toxic to aquatic organism than cocoamide MEA.
An exceptionally high toxicity of cocoamide MEA was reported for two tests with the green alga Scenedesmus subspicatus as the 96 h-EC50 were 1.0 and 1.1 mg/l (IUCLID 2000). More recent tests with a pure cocoamide MEA (purity ³ 95.5% C12-18, personal communication with Jørgen Hyldgaard, Plum Hudsikkerhed) gave EC50 values of 16.6 mg/l for Scenedesmus subspicatus and 17.8 mg/l for Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum) (Plum Hudsikkerhed 2000a; 2000b). The latter data indicate that the toxicity of cocoamide MEA to algae are not markedly higher than the toxicity to daphnids and fish, and EC50 values above 10 mg/l are probably more representative for the toxicity towards algae. The ethoxylated FAA show the same level of aquatic toxicity as the non-ethoxylated FAA (Table 4.23-4.24).
Table 4.23
Aquatic toxicity of FAA to algae.
FAA |
Species |
EC/LC50(mg/l) |
Duration |
Reference |
Cocoamide MEA |
Scenedesmus subspicatus |
1.0; 1.1 |
96 h |
IUCLID 2000 |
Cocoamide MEA |
Scenedesmus subspicatus |
Biomass |
72 h |
Plum Hudsikkerhed 2000a |
Cocoamide MEA |
Pseudokirchneriella subcapitata |
Biomass |
72 h |
Plum Hudsikkerhed 2000b |
Cocoamide DEA |
Scenedesmus subspicatus |
2.2; 2.3 |
96 h |
IUCLID 2000 |
C12-14 amide MEA EO5 |
Scenedesmus subspicatus |
20 |
96 h |
Akzo Nobel 1999a |
C12-14 amide MEA EO4 |
Scenedesmus subspicatus |
14 |
72 h |
Akzo Nobel 1999c |
A
Parentheses indicate 95% confidence intervals.Table 4.24
Aquatic toxicity of FAA to crustaceans and fish.
FAA |
Species |
EC/LC50 (mg/l) |
Duration |
Reference |
Cocoamide MEA |
Daphnia magna |
24.8; 37.5 NOEC: 10.1; 11 |
24 h |
IUCLID 2000 |
Cocoamide MEA |
Zebra fish (Brachydanio rerio) |
28.5; 31 NOEC: 10.1; 11 |
96 h |
IUCLID 2000 |
Cocoamide DEA |
Daphnia magna |
4.2; 5.4 NOEC: 2.5; 2.8 |
24 h |
IUCLID 2000 |
Cocoamide DEA |
Daphnia magna |
2.4 |
48 h |
IUCLID 2000 |
Cocoamide DEA |
Zebra fish |
3.6; 4.0 NOEC: 2.5; 2.8 |
96 h |
IUCLID 2000 |
Cocoamide DEA |
Rice fish (Oryzias latipes) |
10.8-13.8 |
24 h |
IUCLID 2000 |
C12-14 amide MEA EO4 |
Daphnia sp. |
10-100 |
- |
Schöberl et al. 1988 |
C12-14 amide MEA EO4 |
Fish |
4-20 |
- |
Schöberl et al. 1988 |
C12-14 amide DEA EO4 |
Daphnia sp. |
2-3 |
- |
Schöberl et al. 1988 |
4.4.3 Effects on human health
Acute toxicity
The fatty acid diethanolamides all have a low oral toxicity (Table 4.25).
Table 4.25
Acute toxicity (LD50) of FAA.
FAA |
Species |
Application |
LD50 (g/kg body weight) |
Reference |
Cocoamide DEA |
Rat |
Oral |
12.2 |
CIRP 1996 |
Lauramide DEA |
Rat |
Oral |
2.7 |
CIRP 1986 |
Linoleamide DEA |
Rat |
Oral |
> 5 |
CIRP 1986 |
Oleamide DEA |
Rat |
Oral |
> 10 |
CIRP 1986 |
Skin and eye irritation
A 30% cocoamide DEA solution was a moderate skin irritant in rabbits. Test sites were scored for irritation according to Draize, and the Primary Irritation Index (PII) was 3.1 (maximum irritation is indicated by the score of 8). In products intended for prolonged contact with the skin, the concentration of cocoamide DEA should not exceed 5% (CIRP 1996). Low concentrations (0.6%) of cocoamide DEA are severely irritating to the eyes of rabbits. The substance was tested according to a modified Draize eye irritaton test (CIRP 1996).
Sensitization
Several studies of the sensitization potential of cocoamide DEA indicate that this FAA induces occupational allergic contact dermatitis and a number of reports on skin allergy patch testing of cocoamide DEA have been published. These tests indicate that allergy to cocoamide DEA is becoming more common (Hindson and Lawlor 1983; DeGroot et al. 1987; Wall and Gebauer 1991; Pinola et al. 1993; Fowler 1998).
Carcinogenicity
Alkanolamides are manufactured by condensation of diethanolamine and the methylester of long chain fatty acids. The alkanolamides are susceptible to nitrosamine formation which constitutes a potential health problem. Nitrosamine contamination is possible either from pre-existing contamination of the diethanolamine used to manufacture cocoamide DEA, or from nitrosamine formation by nitrosating agents in formulations containing cocoamide DEA (Pinola et al. 1993). According to the Cosmetic Directive (2000) cocoamide DEA must not be used in products with nitrosating agents because of the risk of formation of N-nitrosamines. The maximum content allowed in cosmetics is 5% fatty acid dialkanolamides, and the maximum content of N-nitrosodialkanolamines is 50 µg/kg. The preservative 2-bromo-2-nitropropane-1,3-diol is a known nitrosating agent for secondary and tertiary amines or amides. Model assays have indicated that 2-bromo-2-nitropropane-1,3-diol may lead to the N-nitrosation of diethanolamine forming the carcinogenic compound, N-nitrosodiethanolamine which is a potent liver carcinogen in rats (IARC 1978).
Mutagenicity
Several FAA have been tested in short-term genotoxicity assays. No indication of any potential to cause genetic damage was seen (Yam et al. 1984). Lauramide DEA was tested in mutagenicity assays and did not show mutagenic activity in Salmonella typhimurium strains or in hamster embryo cells (Inoue and Sunakawa 1980). Cocoamide DEA was not mutagenic in strains of Salmonella typhimurium when tested with or without metabolic activation (Zeiger and Anderson 1988).
Classification
Cocoamide DEA is a possible occupational allergen. Nitrosamine contamination is possible when fatty acid diethanolamides are used together with nitrosating agents.
Fatty acid diethanolamides (C8-C½8) are classified by CESIO as Irritating (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes). Fatty acid monoethanolamides are classified as Irritant (Xi) with the risk phrases R41 (CESIO 2000).
Fatty acid amides are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
5. Cationic surfactants
Cationic surfactants are surface-active compounds with at least one hydrophobic alkyl
chain and a hydrophilic group carrying a positive charge. Cationic surfactants are
positively charged in aqueous solutions. Of the cationic surfactants especially the
quaternary ammonium compounds are used in commercial products. The quaternary ammonium
compounds are characterized by a positively charged quaternary nitrogen atom. Commercial
raw materials are normally derived from natural oils which implies that homologous
mixtures of surfactants with different alkyl chain lengths are used in the products. In
household products, the cationic surfactants are primarily applied in fabric softeners,
hair conditioners, and other hair preparations. Other applications of cationic surfactants
include disinfectants and biocides, emulsifiers, wetting agents, and processing additives.
By volume, the most important cationic surfactants in household products are the alkyl
ester ammonium salts that are used in fabric softeners.
This Chapter focuses entirely on quaternary ammonium compounds. As the surfactants in this group may have long and complicated names, a number of abbreviations are used in the present Chapter.
| ATMAC: | Alkyltrimethylammonium chlorides |
| ATMAB: | Alkyltrimethylammonium bromides |
| DADMAC: | Dialkyldimethylammonium chlorides |
| DADMAMS: | Dialkyldimethylammonium methyl sulfates |
| DSDMAC: | Distearyldimethylammonium chlorides |
| DTDMAC: | Ditallowdimethylammonium chlorides |
| ADMBAB: | Alkyldimethylbenzylammonium bromides |
| ADMBAC: | Alkyldimethylbenzylammonium chlorides |
| EQ: | Esterquats |
| DEQ: | Diesterquats |
| DEEDMAC: | Diethyl ester dimethylammonium chlorides |
Occurrence in the environment
Because of their positive charge, the cationic surfactants sorb strongly to the negatively charged surfaces of sludge, soil and sediments. The widespread use and sorption behaviour of cationic surfactants implies that these substances are expected to be present in many environmental compartments. Particular attention was paid to the presence of ditallowdimethylammonium chloride (DTDMAC) in surface waters of major rivers in the Netherlands, where DTDMAC was found at 2 to 34 µg/l (Leeuwen et al. 1992). On the basis of an environmental risk evaluation of DTDMAC, the authorities and the detergent industry in several countries agreed on a voluntary substitution of DTDMAC and the structurally related distearyldimethylammonium chloride (DSDMAC) with readily biodegradable alternatives. During the ninetees quaternary ammonium salts containing ester groups have replaced traditional cationic surfactants in fabric softeners.
5.1 Alkyltrimethylammonium salts
Alkyltrimethylammonium chlorides (ATMAC) and, to a minor extent, alkyltrimethylammonium bromides (ATMAB) are primarily used in cosmetic products including hair conditioners, hair dyes and colors, and other hair and personal care preparations. The hydrophobic alkyl chains of ATMAC and ATMAB are normally linear. These surfactants have the structure:
The alkyl chain, R, usually contains 12-18 carbon atoms, and the counter-ion, X-, may be either Cl- or Br-.
5.1.1 Environmental fate
Biodegradation
Very little is known about biodegradation pathways of alkyltrimethylammonium salts. Two potential points of attack were proposed by Macrell and Walker (1978, cited in Ginkel 1995): The degradation may either be initiated by a fission of the C-N bond in which the alkyl chain or a methyl group is cleaved from a tertiary amine, or by an w -oxidation in which the far end of the alkyl chain is first oxidized to a carboxylic acid. Biodegradation can then proceed via b -oxidation. Studies with a Xanthomonas sp. capable of biodegrading C10 ATMAC support both degradation mechanisms as 9-carboxynonyl- and 7-carboxyheptyltrimethyl-ammonium chloride were detected during the growth of the organism on this quaternary ammonium compound (Dean-Raymond and Alexander 1977, cited in Ginkel 1995).
Aerobic biodegradability
Test methods in which the biodegradability is determined by analyses of parent substrate concentration (primary biodegradation) or dissolved organic carbon are less applicable for cationic surfactants because of the strong sorption of these substances. However, the ultimate biodegradability of ATMAC has been examined in several standard biodegradation tests by measuring the oxygen uptake or the evolution of carbon dioxide. The review of Ginkel (1995) cites a number of studies indicating that the recalcitrance of ATMAC in screening tests increases with increasing alkyl chain length. E.g., the studies of Masuda et al. (1976; cited in Ginkel 1995) using the MITI test showed that the biodegradability of various ATMAC during 10 days was 73% of ThOD for C8, 63% for C10, 59% for C12, 35% for C14, and 0% for C16 and C18. These data show that ATMAC can be ultimately degraded in aerobic screening tests. However, information on the inoculum used in the tests is lacking, and, therefore, it is difficult to verify whether or not the OECD criteria for ready biodegradability were fulfilled. During the present study a ready biodegradability test was conducted with C16 ATMAC which was added at 10 mg/l. The results of this test showed that 40% of ThOD was reached during 28 days without acclimation of the inoculum (Table 5.1; Appendix). The bacterial toxicity of especially the longer chained ATMAC may be mitigated in the presence of equimolar amounts of anionic surfactants. Several studies have shown that ATMAC may be extensively mineralized when complexated with the anionic surfactant LAS. E.g., Games et al. (1982) showed that C18 ATMAC at 20 mg/l inhibited the endogenous CO2 production in a SCAS test, and thereby biodegradation was precluded, whereas a mineralization corresponding to 81% of ThCO2 was attained during 25 days in a mixture of C18 ATMAC and LAS (both added at 20 mg/l). Due to the bacterial toxicity and sorptive properties of cationic surfactants, results from screening tests may underestimate the biodegradation potential in the aquatic environment. Rapid and extensive mineralization was observed when 14C-labelled C18 ATMAC was added to the SCAS system at initial levels of 0.1 and 1.0 mg/l (Games et al. 1982; Table 5.1). Another study with 14C-labelled C18 ATMAC (10 µg/l) has demonstrated an extensive mineralization in river water as indicated by the evolution of 14CO2 which corresponded to more than 60% and 75% of the added 14C after 7 and 21 days, respectively (Boethling 1984; Table 5.1). The rapid transformation, which may occur in the environment, can also be illustrated by the half-life of C18 ATMAC which was calculated to 2.2 days in acclimated river water (Larson 1983, cited in Ginkel 1995). The biodegradation routes of alkyltrimethylammonium salts which were outlined above do not indicate that recalcitrant metabolites are formed. This is in accordance with the study of the fate of radiolabelled C18 ATMAC by Games et al. (1982). Using mass balance calculations these authors suggested that no metabolites with appreciable half-lives were formed from the degradation of C18 ATMAC.
Table 5.1
Ultimate aerobic biodegradability of alkyltrimethylammonium chlorides.
ATMAC |
Test |
Result |
Reference |
C16 |
Manometric respirometry test, 10 mg/l; 28 d |
40% ThOD |
This study (Appendix; Table A2; Figure A2) |
C18 |
CO2 evolution screening test, 20 mg/l; 25 d |
Inhibition |
Games et al. 1982 |
C18 |
CO2 evolution screening test, ATMAC + LAS (both 20 mg/l); 25 d |
81% ThCO2 |
Games et al. 1982 |
C18 |
14CO2 test, river water, 10 µg/l; 7 d/21 d |
> 60/75% 14CO2 |
Boethling 1984 |
C18 (14CH3 labelled) |
Unacclimated SCAS system, 1,000 mg SS/l, 0.1 mg/l; 172 h |
88% 14CO2 |
Games et al. 1982 |
C18 (14C1 labelled) |
Unacclimated SCAS system, 1,000 mg SS/l, 0.1 mg/l; 172 h |
67% 14CO2 |
Games et al. 1982 |
Anaerobic biodegradability
Although cationic surfactants will sorb onto sludge particles and eventually reach the digester during the treatment of wastewater sludge, there is very limited information about the biodegradability of these compounds under anoxic conditions. It has been demonstrated, however, that the concentration of quaternary ammonium salts does not decrease, or only slightly decrease, in an anaerobic digester (Janicke and Hilge 1979, cited in Ginkel 1995). The anaerobic biodegradability of C16 ATMAC was examined in the present study by using the ISO 11734 screening test, but the applied test concentration of 14.0 mg C/l was toxic to the anaerobic bacteria as seen from the negative net biogas production throughout the test period of 56 days (Appendix; Table A13, Figure A13).
Bioaccumulation
Bioaccumulation studies with ATMAC have been performed with fathead minnow (Pimephales promelas) by using 14C-labelled model compounds (Tolls et al. 1994). The radiolabelling technique does not allow a distinction between the parent compound and their metabolites formed and, hence, the term concentration ratio (CR) was used instead of BCF which normally refers to the intact parent compound. The relatively few data indicate that the bioconcentration of ATMAC are hydrophobicity dependent as the CRs were 2.4 for C8, 35 for C12, and 1,962 for C16-18 (Versteeg and Shorter 1992, cited in Tolls et al. 1994). The high CR of 1,962 for C16-18 ATMAC may represent both the intact surfactant and its metabolites, and the CR may at least partially be due to inter-experimental variation. Although more experiments are needed to understand the bioconcentration of cationic surfactants, the possibility of variation between experiments is indicated by the fact that the CR for a C(18)2 dialkyldimethylammonium chloride was determined to 104 (Versteeg and Shorter 1992, cited in Tolls et al. 1994).
5.1.2 Effects on the aquatic environment
Algae
Algae constitute a group of organisms which appears to be very sensitive to cationic surfactants. The toxicity of ATMAB and ATMAC to algae is characterized by EC50 values below 1 mg/l (Table 5.2).
Table 5.2
Effects of alkyltrimethylammonium salts to algae.
Species |
Surfactant |
EC50 (mg/l) |
Duration |
Reference |
Selenastrum capricornutum |
C16 ATMAB |
0.09 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C16 ATMAB |
« 2.5A |
21 d |
Nyberg 1988 |
Microcystis aeruginosa |
C16 ATMAB |
0.03 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C12 ATMAC |
0.19 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C12 ATMAC |
0.12 |
96 h |
Lewis and Hamm 1986 |
Navicula pelliculosa |
C12 ATMAC |
0.20 |
96 h |
Lewis and Hamm 1986 |
Dunaliella sp. |
C16-18 ATMAC |
0.38 (0.33-0.45)B |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenidosa |
C16-18 ATMAC |
0.28 (0.22-0.26)B |
96 h |
Utsunomiya et al. 1997 |
A
No living cells were observed in the cultures receiving 2.5 mg/l.B 95% confidence limits.
Invertebrates and fish
ATMAC are acutely toxic to aquatic invertebrates as indicated by EC/LC50 values below 1 mg/l for alkyl chain lengths of C16 (Table 5.3). Belanger et al. (1993) exposed artificial stream mesocosms housing the freshwater clam Corbicula fluminea with C12 ATMAC. Minor and transient effects on length gain were observed at 43 m g/l during weeks 2-4 and 6-7, but these effects were not evident at the end of the experiment after 8 weeks. One study with the species Idus melatonus indicates that some ATMAC are also toxic to fish (Boethling and Lynch 1992; Table 5.3).
Table 5.3
Effects of alkyltrimethylammonium chlorides to invertebrates and fish.
Species |
Surfactant |
EC50/LC50 (mg/l) |
Duration |
Reference |
Crustacean |
ATMAC C |
1.2-5.8 |
- |
Boethling and Lynch 1992 |
Crustacean |
C16 ATMAC |
0.1 |
48 h |
Lewis and Suprenant 1983 |
Flatworm |
C16 ATMAC |
0.68 |
48 h |
Lewis and Suprenant 1983 |
Oligochaete |
C16 ATMAC |
0.22 |
48 h |
Lewis and Suprenant 1983 |
Bivalve |
C12 ATMAC |
LOEC:0.18-0.24 A |
56 d |
Belanger et al. 1993 |
Water snail |
ATMAC C |
0.73-23 |
- |
Boethling and Lynch 1992 |
Fish, golden orfe |
ATMAC C |
0.36-8.6 |
- |
Boethling and Lynch 1992 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
C The ranges include tests with C12, C14, C16, C18, and C20-22.
5.1.3 Effects on human health
Toxicokinetics and acute toxicity
The few available absorption studies conducted with cationic surfactants indicate that absorption occurs in small amounts through the skin (Bartnik and Wingen 1979; SFT 1991). Percutaneous absorption of radiolabelled C12 ATMAB in 3% aqueous solution (applied to an 8 cm2 area with occlusion) in the rat was low and corresponded to 0.6% of the applied 14C activity in 72 hours. Most of the absorbed surfactant was excreted in the urine, i.e. 0.35% of the applied 14C activity within the first 24 hours, whereas 13.2% remained on the skin after rinsing. Cutaneous application of the surfactant without rinsing resulted in a greater degree of percutaneous absorption (3.15%) in 48 hours. In the rat elimination after parenteral administration was rapid and was effected primarily via the urine, - more than 80% of the radioactivity was eliminated within 24 hours of application (Bartnik and Wingen 1979).
About 80% of the 14C activity was found in the gastrointestinal tract 8 hours after oral administration of 14C-labelled C16 ATMAB . Only small amounts of the applied radioactivity were found in the urine and in the blood plasma. This indicates poor intestinal absorption. Similar small amounts of 14C were found in the liver, kidneys, spleen, heart, lungs and skeletal muscles. Within 3 days of ingestion, 92% of the administrated radioactivity had been excreted in the faeces and 1% in the urine. No appreciable enterohepatic circulation of the radioactivity was found (Isomaa 1975).
The acute oral toxicity of alkyltrimethylammonium salts (Table 5.4) is somewhat higher than the toxicity of anionic and nonionic surfactants. This may be due to the strongly irritating effect which cationic surfactants exhibit on the mucous membrane of the gastrointestinal tract (SFT 1991). Cationic surfactants are generally about 10 times more toxic when administrated by the intravenous route compared to oral administration (Falbe 1986; SFT 1991).
Table 5.4
Acute toxicity (LD50) after oral administration of alkyl trimethylammonium salts.
Surfactant |
Species |
LD50 (mg/kg body weight) |
Reference |
C16 ATMAB |
Rat |
1,000 |
Richardson 1992-1994 |
C16 ATMAC |
Rat |
410 |
Richardson 1992-1994 |
C12 ATMAC |
Rat |
250-300 |
Kirk-Otmer 1994 |
C18 ATMAC |
Rat |
1,000 |
Kirk-Otmer 1994 |
C18 ATMAC |
Mouse |
633 |
CIRP 1997 |
C16-18 ATMAC |
Rat |
> 500 |
Kirk-Otmer 1994 |
Skin and eye irritation
Skin irritation depends on surfactant concentration. Regardless of the structure, cationic surfactants lead to serious destruction of the skin at high concentrations. Solutions of approximately 0.1% are rarely irritating, whereas irritation is usually pronounced at concentrations between 1.0 and 10.0% surfactant (CIRP 1997). C16 ATMAC was severely irritating to rabbit skin in a concentration of 2.5%. The surfactant was applied to intact and abraded sites and scored after 34 hours. Then the skin was rinsed and then scored again after 48 hours. The erythema and Eschar Index was 3.75 (maximum 4) and the edema Index was 2.0 (maximum 4) (CIRP 1997).
With regard to eye irritation, cationic surfactants are the most irritating of the surfactants (Bartnik and Wingen 1979; SFT 1991). The longer chained alkyltrimethylammonium salts are less irritating to the rabbit eye than the shorter alkyl chain homologues (CIRP 1997). C10 ATMAB, C12 ATMAB, and C16 ATMAC were tested in concentrations between 0.1 and 1.0% in water and were found to be significantly irritating or injurious to the rabbit eye. A 5% solution of C18 ATMAC was instilled into the eyes of guinea pigs, and this concentration was very irritating with a total PII (The Primary Irritation Index) score of 96 (maximum 110) (Bracher et al. 1987).
A homologous series of ATMAB produced very little swelling of the stratum corneum and some homologues produced a shrinkage of the stratum corneum after prolonged exposure (Jungerman 1970; Putterman 1977; Tupker 1990).
Many proteins in the skin are considerably more resistant to the denaturating effects of cationic surfactants compared to those of anionic surfactants. As cationic surfactants frequently have a lower critical micelle concentration than the anionic surfactants, a saturation of the surfactant/protein complex is prevented by the formation of micelles (SFT 1991). Compared to a representative anionic surfactant, the cooperative binding with subsequent protein denaturation requires about a tenfold higher concentration of a cationic surfactant. Contrary to the irreversible denaturating effect of sodium dodecyl sulfate (C12 AS), the adverse effects of some cationic surfactants on proteins may be reversible (Falbe 1986). Cationic surfactants can interact with proteins or peptides by polar and hydrophobic binding. Polar interactions result in electrostatic bonds between the negatively charged groups of the protein molecule and the positively charged surfactant molecule. For example, the enzyme, glucose oxidase, is deactivated by C16 ATMAB through the formation of an ion pair between the cationic surfactant and the anionic amino acid side-chain of the enzyme molecule (Falbe 1986).
Sensitization
A repeated insult patch test of C16 ATMAC was conducted with 114 volunteers. Seventeen days after the last induction of 0.25% surfactant, a challenge patch of 0.25% was applied. No sensitization was observed (CIRP 1997).
Subchronic/Chronic toxicity
C16 ATMAB was administered at concentrations of 10, 20, and 45 mg/kg/day via the drinking water to rats for one year. The only effect observed was a decrease in body weight gain in the 45 mg/day dose group (Isomaa et al. 1976).
Reproductuive toxicology
No embryo toxic effects were seen, when C18 ATMAC was applied dermally to pregnant rats during the period of major organogenesis (day 6-15 of gestation). The concentrations of C18 ATMAC were 0.9, 1.5 and 2.5%. There was no increase in the incidence of fetal malformations (Palmer et al. 1983). C16 ATMAB was not teratogenic in rats after oral doses. Mild embryonic effects were observed with 50 mg/kg/day, but these effects were attributed to maternal toxicity rather than to a primary embryonic effect. Lower doses of C16 ATMAB showed no embryo toxic or teratogenic effects (CIRP 1997).
Mutagenicity
C16 ATMAC was studied in in vitro short-term tests to detect potential mutagenic effects. Cultures of Syrian golden hamster embryo cells were used for an in vitro bioassay. No in vitro transformation of hamster embryo cells was induced, and C16 ATMAC was not mutagenic in Salmonella typhimurium (Inoue and Sunakawa 1980). No mutagenic effects or genetic damages were indicated in a survey of nine short-term genotoxicity tests with C16 and C18 ATMAC (Yam et al. 1984).
Classification
Most undiluted cationic surfactants satisfy the criteria for classification as Harmful (Xn) with R22 and as Irritant (Xi) for skin and eyes with R38 and R41. In addition, certain surfactants will satisfy the criteria for classification as Corrosive with R34 in addition to the acute toxicity (SFT 1991).
According to CESIO, C8-18 ATMAC (i.e., lauryl, coco, soya, and tallow) are classified as Corrosive (C ) with the risk phrases R22 (Harmful if swallowed) and R34 (Causes burns). C16 ATMAC is classified as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed), R38 (Irritating to skin), and R41 (Risk of serious damage to eyes). C20-22 ATMAC are classified as Irritant (Xi) with R36/38 (Irritating to eyes and skin) (CESIO 2000).
The maximum allowed concentration of C12-22 alkyltrimethylammonium salts (bromide or chloride) in cosmetics is 0.1% (Cosmetic Directive 2000).
5.2 Dialkyldimethylammonium salts
Dialkyldimethylammonium chlorides (DADMAC) are used as antistatic agents in cosmetic products including hair conditioners and hair coloring preparations. Furthermore, DADMAC are used as biocides in industrial cleaning agents and, to a minor extent, all purpose household cleaning agents. The alkyl chains of DADMAC are normally linear, although DADMAC containing at least one branched alkyl chain are also used. The general structure of DADMAC is indicated below.
The alkyl chain, R, usually contains 10-16 carbon atoms. The length of the alkyl chains of specific structures is indicated by, e.g., C(12)2 for a DADMAC with two C12 alkyl chains.
5.2.1 Environmental fate
Aerobic biodegradability
The ultimate biodegradability of DADMAC has been examined in several standard biodegradation tests. As for ATMAC, the recalcitrance of DADMAC in screening tests increases with increasing alkyl chain length. This is particularly evident from the studies of Masuda et al. (1976; cited in Ginkel 1995) which indicated that the biodegradability in the MITI test of various DADMAC was 50% of ThOD for C(10)2 and 0% for alkyl chain lengths in the range of C(12)2 to C(18)2. The duration of these tests was 10 days. As also noted for ATMAC, the description of the studies of Masuda et al. does not include information on the inoculum used in the tests. DADMAC with branched alkyl chain(s) like, e.g., decylisononyldimethylammonium chloride are expected to degrade more slowly than similar homologous with linear alkyl chains. Studies by Ginkel et al. (2000) show that DADMAC were transformed in laboratory column experiments in which a slow release of the test compounds were ensured by pre-sorption of the quaternary ammonium salts onto a silica gel. Complete removal of C(10)2 DADMAC, as indicated by HPLC analyses of column effluents, were obtained within 4 days after inoculation of the columns with a pure culture of a bacterium which was able to utilize C(10)2 DADMAC for growth (Table 5.5). In a similar experiment, the same pure culture transformed C(18)2 DADMAC completely after approximately 8 days. A C(16-18)2 DADMAC (ditallow hydrogenated) was transformed in columns inoculated with river water which indicates that microorganisms capable of a primary degradation of DADMAC are common (Ginkel et al. 2000). These results indicate that the poor biodegradability in standard screening tests is not necessarily due to an inherent recalcitrance of DADMAC as other factors like, e.g., toxicity and a slow desorption of the cationic surfactant from surfaces may limit biodegradation. Studies in which 14C-labelled C(16-18)2 DADMAC (ditallow) was added to semi-batch reactors at 2.1 mg/l as a complex with LAS confirm that the entire DADMAC molecule can be ultimately biodegraded. In the reactors, the 14CO2 recovered from mineralization of three radiolabelled forms of C(16-18)2 DADMAC, i.e. [14C]methyl-, [14C]C1-alkyl-, and [14C]uniform-C-labelled, corresponded to between 22 and 53% of the added 14C after 39 days, whereas the primary biodegradation in the same period was somewhat higher, i.e. 59-81% of the initial level (Sullivan 1983). The data in Table 5.5 show that the methyl groups bound to the quaternary nitrogen were more susceptible to biodegradation than the carbons in the alkyl chains. A comparison between the biodegradation of DADMAC (Sullivan 1983) with the C18 ATMAC degradation in the studies of Games et al. (1982) indicates that DADMAC are degraded at a considerably slower rate than ATMAC.
Table 5.5
Ultimate and primary biodegradability of dialkyldimethylammonium chlorides under aerobic
conditions.
DADMAC |
Test |
Result |
Reference |
Ditallow |
Closed bottle test, 283 d |
68% ThOD |
Ginkel 1995 |
Dioctadecyl |
Sturm test, 33 d |
4% ThCO2 |
Ginkel 1995 |
Ditallow, C(16-18)2 [14C]methyl |
Semi-batch reactor, 39 d |
40; 53% 14CO2 |
Sullivan 1983 |
Ditallow, C(16-18)2 |
Semi-batch reactor, 39 d |
31% 14CO2 |
Sullivan 1983 |
Ditallow, C(16-18)2 |
Semi-batch reactor, 39 d |
22; 31% 14CO2 |
Sullivan 1983 |
Didecyl |
Silica gel column, pure culture; 4 d |
100% removal |
Ginkel et al. 2000 |
Dioctadecyl |
Silica gel column, pure culture; 8 d |
100% removal |
Ginkel et al. 2000 |
Ditallow hydrogenated |
Silica gel column, river water; 14 d |
Removal of parent substrate; extent not stated in reference |
Ginkel et al. 2000 |
A short-chained C(8)2 DADMAC was ultimately biodegraded at a concentration of
0.5 mg/l in acclimated river water. The half-lives calculated from the carbon dioxide
produced were 4.9 days in the presence of sediment and 13.8 days without sediment (Larson
1983; Larson and Vashon 1983; both cited in Ginkel 1995).
Anaerobic biodegradability
The information on the biodegradability of cationic surfactants under anoxic conditions is scarce. One study has demonstrated that the concentration of quaternary ammonium salts did not decrease, or only slightly decreased, in an anaerobic digester (Janicke and Hilge 1979, cited in Ginkel 1995).
Bioaccumulation
The bioconcentration of DADMAC has been investigated in studies with bluegill sunfish (Lepomis macrochirus) and fathead minnow (Pimephales promelas). As described for ATMAC in Section 5.1.1 the term CR was used to indicate the bioconcentration which was determined by use of 14C-labelled model compounds. The CR was determined to 32 for C(16-18)2 DADMAC (Lepomis macrochirus) and 104 for C(18)2 DADMAC (Pimephales promelas) (Tolls et al. 1994).
5.2.2 Effects on the aquatic environment
Algae
Algae are very sensitive to dialkyldimethylammonium salts as also noted for the alkyltrimethylammonium salts. The toxicity of DADMAC and DADMAMS to algae is characterized by EC50 values below 1 mg/l (Table 5.6).
Table 5.6
Effects of dialkyldimethylammonium salts to algae.
Species |
Surfactant |
EC50 (mg/l) |
Duration |
Reference |
Dunaliella sp. |
DADMAC, ditallow C(16-18)2 |
18 (13-24)A |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenidosa |
DADMAC, ditallow C(16-18)2 |
6.0 (5.5-6.5)A |
96 h |
Utsunomiya et al. 1997 |
Selenastrum capricornutum |
DADMAC, ditallow C(16-18)2 |
0.06 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
DADMAC, ditallow C(16-18)2 |
0.23 B (0.16-0.32)A |
120 h |
Lewis and Wee 1983 |
Selenastrum capricornutum |
DADMAMS, ditallow C(16-18)2 |
0.1-0.5 B |
120 h |
Lewis and Wee 1983 |
Microcystis aeruginosa |
DADMAC, ditallow C(16-18)2 |
0.05 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
DADMAMS, ditallow C(16-18)2 |
0.1 B |
120 h |
Lewis and Wee 1983 |
Navicula pelliculosa |
DADMAC, ditallow C(16-18)2 |
0.07 |
96 h |
Lewis and Hamm 1986 |
A
95% confidence limits.B Algistatic concentration, i.e. the concentration that inhibits growth, but logarithmic growth will resume, when the algae are resuspended in fresh medium without test substance.
Invertebrates and fish
DADMAC with alkyl chains consisting of 16 carbons or more are acutely toxic to aquatic invertebrates and fish as the lowest EC/LC50 values are below 1 mg/l (Tables 5.7-5.8).
Table 5.7
Effects of DADMAC to invertebrates.
Species |
Surfactant |
EC50/LC50(mg/l) |
Duration |
Reference |
Daphnia magna |
Ditallow C(16-18)2 |
0.19 A |
48 h |
Lewis and Wee 1983 |
Daphnia magna |
Ditallow C(16-18)2 |
0.16-1.06 |
48 h |
Kappeler 1982 |
Daphnia magna |
Dioctadecyl C(18)2 |
0.16 A |
48 h |
Lewis and Wee 1983 |
Ceriodaphnia dubia |
Ditallow C(16-18)2 |
0.54 A |
48 h |
Taylor 1984 |
Mysidopsis bahia |
Ditallow C(16-18)2 |
0.22 A |
96 h |
Lewis and Wee 1983 |
Chironomus riparius |
Ditallow C(16-18)2 |
9.2 (8.1-11)B NOEC: 1.34 |
96 h |
Roghair et al. 1992 |
Lymnaea stagnalis |
Ditallow |
18 |
96 h |
Roghair et al. 1992 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
Table 5.8
Effects of dialkyldimethyl ammonium salts to fish.
Species |
Surfactant |
LC50 (mg/l) |
Duration |
Reference |
Bluegill sunfish (Lepomis macrochirus) |
DADMAC, ditallow |
0.62 A |
96 h |
Lewis and Wee 1983 |
Stickleback |
DADMAC, ditallow |
4.5 |
96 h |
Roghair et al. 1992 |
Bluegill sunfish |
DADMAMS, ditallow C(16-18)2 |
1.23 A |
96 h |
Lewis and Wee 1983 |
Bluegill sunfish |
DADMAC, dioctadecyl |
1.04 A |
96 h |
Lewis and Wee 1983 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
5.2.3 Effects on human health
No specific data describing the health effects of dialkyldimethylammonium salts were obtained. However, many of the properties described for alkyltrimethylammonium salts also apply to dialkyldimethylammonium salts, although these are generally less irritating than the corresponding alkyltrimethylammonium salts (CIRP 1997).
5.3 Alkyldimethylbenzylammonium salts
Alkyldimethylbenzylammonium chlorides (ADMBAC) and bromides (ADMBAB) are used in cosmetic products including hair conditioners and hair coloring preparations. Besides being surfactants and antistatic agents, the alkyldimethylbenzylammonium compounds function as biocides in various cosmetic and detergent products. The biocidal properties are utilized, when ADMBAC are added to all-purpose or specialized cleaning agents.
The linear alkyl chain, R, usually contains 8 to 18 carbons, and the counter-ion, X-, may be either Cl- or Br-.
5.3.1 Environmental fate
Biodegradation patyways
The knowledge about the biodegradation pathways of alkyldimethylbenzylammonium salts is very scarce. A qualitative analysis of the metabolites that were formed in pilot activated sludge plants showed that benzoate, acetate, and tetradecyldimethyl amine were formed during degradation of C14 ADMBAC (Fenger et al. 1973). The average degradation of C14 ADMBAC in this study was 73% of the initial concentration during 36 days (Table 5.9). The identified metabolites indicate that ADMBAC is degraded via a cleavage of the bond linking the benzene group to the alkyldimethylammonium.
Aerobic biodegradability
The aerobic biodegradability of ADMBAC has been examined in various standard screening tests. These tests suffer from methodological problems with toxicity and sorption related to the behaviour of cationic surfactants. As for ATMAC and DADMAC, the recalcitrance of ADMBAC in screening tests generally increases with increasing alkyl chain length. The studies of Masuda et al. (1976; cited in Ginkel 1995) indicated that the biodegradability in the MITI test of various ADMBAC was 79% of ThOD for C8, 95% for C10, 89% for C12, 83% for C14, 5% for C16 and 0% for C18 during 10 days of incubation. However, information on the inoculum used by Masuda et al., which is important to evaluate these results, was not reported by Ginkel (1995). A closed bottle test with C12-14 ADMBAC, using a secondary effluent inoculum and a test substance concentration of 1.5 mg/l, showed that only 8% of ThOD was attained during 28 days. Parallel vessels with C12-14 ADMBAC and sodium benzoate revealed that the applied concentration of the test substance inhibited the inoculum by only 16% which indicates that toxicity alone does not explain the poor biodegradability of C12-14 ADMBAC (Madsen et al. 1994). Gerike and Gode (1990) reported 83% ultimate degradation of C12 ADMBAC, as indicated by DOC removal, in a coupled units test (Table 5.9). However, as noted previously, DOC analyses are less applicable for cationic surfactants and results relying on this parameter should therefore be evaluated with caution. Alkyldimethylbenzylammonium salts are clearly better degradable than DADMAC which is particularly evident when comparing the results of the MITI tests by Masuda et al. (1976; cited in Ginkel 1995). The results of Masuda et al. indicate that extensive ultimate biodegradation of ADMBAC (C8 to C14) may occur, and that these surfactants will probably biodegrade as rapidly as ATMAC (see Table 5.1) when present at environmentally realistic concentrations. However, studies with low concentrations of 14C-labelled ADMBAC would improve the basis for evaluating the biodegradability of these substances.
Table 5.9
Ultimate and primary aerobic biodegradability of ADMBAC.
ADMBAC |
Test |
Result |
Reference |
C12 |
CAS test |
83% DOC A |
Gerike and Gode 1990 |
C12 |
CAS test |
96% loss of disulfine blue active substances (primary degradation) 54% DOC A |
Swisher 1987 |
C12-14 |
Closed bottle test, OECD 301D, 1.5 mg/l, 28 d |
8% ThOD |
Madsen et al. 1994 |
C14 |
Activated sludge pilot plants, 20 mg/l, 36 d |
63-72% loss of parent |
Fenger et al. 1973 |
A
Sorbed DOC, if any, could probably not be differentiated from the sludge itself.Anarobic biodegradability
Only limited information exists on the biodegradability of cationic surfactants under anoxic conditions. A study by Janicke and Hilge (1979, cited in Ginkel 1995) has demonstrated that the concentration of quaternary ammonium salts did not decrease, or only decreased slightly, in an anaerobic digester.
5.3.2 Effects on the aquatic environment
ADMBAC are very toxic to aquatic organisms as also noted for the alkyltrimethyl ammonium and dialkyldimethylammonium salts. Some of the available data on the acute aquatic toxicity (EC/LC50) are below 1 mg/l (e.g. for the green algae Chlorella pyrenidosa), but EC/LC50 values between 1 and 10 mg/l are also observed (Table 5.10).
Table 5.10
Aquatic toxicity of ADMBAC.
Species |
Surfactant |
EC50/LC50 |
Duration |
Reference |
Green alga |
C12-14 |
1.8 |
24 h |
Utsunomiya et al. 1997 |
Green alga |
C12-14 |
0.67 |
96 h |
Utsunomiya et al. 1997 |
Golden orfe |
C12 |
LC0: 3.5 |
- |
Boethling and Lynch 1992 |
Bluegill sunfish |
Hyamine 3500 |
0.5 |
- |
Boethling and Lynch 1992 |
Goldfish |
Hyamine 3500 |
2.0 |
- |
Boethling and Lynch 1992 |
5.3.3 Effects on human health
Toxicokinetics and acute toxicity
No specific toxicokinetic studies were identified for ADMBAC, but the absorption of these surfactants through the skin is anticipated to be low as observed for the alkyltrimethylammonium salts (Section 5.1.3). Different homologues of ADMBAC showed a moderate acute toxicity in experiments with rats and mice (Table 5.11).
Table 5.11
Acute toxicity (LD50) of ADMBAC.
Surfactant |
Species |
Application |
LD50 (mg/ kg body weight) |
Reference |
ADMBAC |
Rat |
Oral |
300 |
Lewis 1996 |
ADMBAC |
Rat |
Oral |
280-445 |
BIBRA 1989 |
C12-18 ADMBAC |
Rat |
Oral |
525 |
CIRP 1989 |
C14-18 ADMBAC |
Mouse |
Oral |
150-340 |
BIBRA 1989 |
C14-18 ADMBAC |
Rat |
Dermal |
1,420 |
Lewis 1996 |
The relationship between alkyl chain length and the acute toxicity of various ADMBAC
homologues (C8 to C19) has been studied in mice. The studies
indicated that chain lengths above C16 had a markedly lower acute toxicity and
that even-numbered alkyl chain homologues appeared to be less toxic than odd-numbered
carbon chains. It was suggested that the decrease in toxicity above C16 was due
to a decreased water-solubility (Zeiger and Anderson 1987; CIRP 1989).
Dermal and eye irritation
ADMBAC is a skin irritant in animals at concentrations above 0.1% (CIRP 1989). A non-specified ADMBAC caused skin irritation and minor to moderate eye irritation at 0.625 and 1.25% concentrations (Skydsgaard and Dideriksen 1991). Inflammation of the eye and deterioration of vision occurred 3 days after change of soaking solution for a soft contact lens to a solution containing C8-18 ADMBAC (Richardson 1992-1994).
Sensitization
The sensitization potential of ADMBAC has been examined in an experiment including 2,295 patients with suspected allergic contact dermatitis. Some of the patients (5.5%) showed positive reactions after exposure to 0.1% ADMBAC. These results were surprising as ADMBAC was not suspected to be a sensitizer. The high irritating potential of ADMBAC, even at low concentrations, could be an explanation of the observed results as the patch test reactions may have been false positives (Perrenoud et al. 1994). However, another group of 2,806 patients with eczema was patch tested with 0.1% ADMBAC, and 2.13% of these patients appeared to be sensitized (Camarasa 1979). Skin sensitization was noted in patients patch tested with ADMBAC in aqueous solutions at 0.07 to 0.1% surfactant. However, there was no incidence of skin sensitization in a population of normal individuals tested with 0.1% ADMBAC. This indicates that individuals with diseased skin may be at risk for sensitization to ADMBAC (Afzelius and Thulin 1979; Lovell and Staniforth 1981).
Mutagenicity
C16 ADMBAC did not induce transformation of the cells in an in vitro bioassay for carcinogenesis by using cultures of Syrian golden hamster embryo cells. The mutagenic potential of this surfactant was also examined by using Salmonella typhimurium strains - no mutagenic effects were seen (Inoue and Sunakawa 1980). In other short-term genotoxicity assays (Salmonella/microsome assay) and rec-assay (bacterial DNA repair test) C16 ADMBAC was tested for ability to cause DNA damage in bacteria. None of the data indicated any mutagenic effects (Yam et al. 1984).
Carcinogenicity
Lifetime studies of ADMBAC were conducted in mice and rabbits that were treated with 8.5 to 17% surfactant dissolved in acetone or methanol. ADMBAC was applied repeatedly to the skin and ADMBAC caused ulceration, inflammations and scars in many animals, but no tumours (Steinbäck 1977).
Reproductivity toxocoty
No embryotoxic activity was detected when C18 ADMBAC was applied topically to pregnant rats during the period of major organogenesis (day 6-15) at doses up to 6.6%, which was sufficient to cause adverse maternal reactions (Palmer et al. 1983). Intravaginal instillation of ADMBAC (single doses up to 200 mg/kg) to pregnant rats on day one of the gestation caused abnormal foetal development and embryotoxicity (Buttar 1985).
Classification
ADMBAC are included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC with the following classification:
C8-18 ADMBAC are classified as Harmful ( Xn) with the risk phrases R21/22 (Harmful in contact with skin and if swallowed) and Corrosive (C) with R34 (Causes burns) and (N) with R50 (Very toxic to aquatic organisms).
5.4 Alkyl ester ammonium salts
During the last decade alkyl ester ammonium salts have largely replaced the dialkyldimethylammonium salts (e.g. DTDMAC and DSDMAC) in fabric softeners for household use. Alkyl ester ammonium salts are quaternary ammonium compounds containing one, or more often two, weak ester linkages in the molecular structure. This group of cationic surfactants consists of at least three different types of esters: (I) the esterquat (EQ), N-methyl-N,N-bis[2-(C16-18–acyloxy) ethyl]-N-(2-hydroxyethyl) ammonium methosulfate, (II) the diesterquat (DEQ), N,N,N-trimethyl-N-[1,2-di-(C16-18–acyloxy) propyl] ammonium, and (III) the diethyl ester dimethylammonium chloride (DEEDMAC), di-(tallow fatty acid) ester of di-2-hydroxyethyl dimethylammonium chloride.
The structures of alkyl ester ammonium salts are given below.
5.4.1 Environmental fate
Effects of structure on biodegradability
The presence of ester linkages implies that a rapid biodegradation is expected for all alkyl ester ammonium salts described above. The ester linkages are readily attacked by microorganisms, and the cleavage of these linkages results in smaller molecules that are easily biodegraded.
Aerobic biodegradability
The aerobic biodegradability of the poorly water-soluble EQ has been examined under simulated sewage treatment plant conditions in the coupled units test in which more than 90% degradation was found (Puchta et al. 1993). The main metabolite formed from the degradation of the EQ was a tris-(hydroxyethyl) methylammonium methosulfate (MTEA), and since this metabolite is a water-soluble substance with a quaternary structure, further tests for ready biodegradability were carried out with MTEA (Table 5.11). The parent molecules of DEQ and DEEDMAC have been examined in standard OECD screening tests for ready biodegradability. Although these compounds also have a low water-solubility (e.g. 2.8 µg/l for DEQ), both DEQ and DEEDMAC have proven to be readily biodegradable under screening test conditions (Table 5.12).
Table 5.12
Ultimate aerobic biodegradability of alkyl ester ammonium salts.
Compound |
Test |
Result |
Reference |
MTEA |
CO2 evolution test, 28 d |
76-94% ThCO2 |
Puchta et al. 1993 |
DEQ |
CO2 evolution test, 10/20 mg/l, 28 d |
85%; 87% ThCO2 |
Waters et al. 1991 |
DEEDMAC |
CO2 evolution test, 10/20 mg/l, 28 d |
80% ThCO2 |
Giolando et al. 1995 |
The mineralization of 14C-stearyl-, 14C-methyl-, and 14C-dihydroxypropyl-labelled
DEQ in river water attained 94.1, 88.4, and 94.6% of the 14C added during 22
days. The associated mineralization half-lives of DEQ were determined to 0.65-0.70 days,
7.1-7.7 days, and 6.1-6.7 days, respectively, for the various positions of the 14C
(Waters et al. 1991). The mineralization of 14C-labelled DEEDMAC was
examined in activated sludge and river water with sediment (Giolando et al. 1995).
The total accumulated 14CO2 from the mineralization of DEEDMAC
attained 76% and 82% of the added 14C for the batch activated sludge and the
river water die-away test, respectively. The estimated half-lives for the mineralization
of DEEDMAC were 1.0 days in activated sludge and 1.1 days in river water with sediment
(Giolando et al. 1995). The findings in the studies with 14C-labelled
DEQ and DEEDMAC indicate that these compounds will be rapidly and completely biodegraded
in a variety of environmental compartments.
Anaerobic biodegradability
The ultimate anaerobic biodegradability of DEEDMAC has been examined in the ECETOC test (ECETOC 1988). The total gas production from mineralization of DEEDMAC reached 90% of ThGP during 60 days under the methanogenic test conditions (Giolando et al. 1995). No data were found on the anaerobic biodegradation of EQ and DEQ, but due to the structural similarity with DEEDMAC (primarily the ester linkages) EQ and DEQ are assumed to be degraded under anoxic conditions as well.
5.4.2 Effects on the aquatic environment
Alkyl ester ammonium salts generally have an acute aquatic toxicity characterized by EC/LC50 values between 2 and 10 mg/l (Table 5.13). The aquatic toxicity of alkyl ester ammonium salts is markedly lower as compared with other cationic surfactants. A comparison with the EC/LC50 values for ATMAC, DADMAC, and ADMBAC shows that the acute aquatic toxicity of alkyl ester ammonium salts is at least one order of magnitude lower (i.e., EC/LC50 are higher) than the toxicity of the ‘traditional’ quaternary ammonium compounds.
Table 5.13
Effects of alkyl ester ammonium salts to aquatic organisms.
Species |
Surfactant |
EC/LC50 (mg/l) |
Duration |
NOEC(mg/l) |
Reference |
Algae |
Esterquat (EQ) |
|
- |
0.3 |
Puchta et al. 1993 |
Algae |
DEQ |
|
72 h |
1.8 |
Waters et al. 1991 |
Algae |
DEEDMAC |
2.9 |
96 h |
|
Giolando et al. 1995 |
Daphnia |
Esterquat (EQ) |
78 |
21 d-NOEC |
3.0 |
Puchta et al. 1993 |
Daphnia magna |
Diesterquat (DEQ) |
7.7 |
48 h-EC50 |
1.0 |
Waters et al. 1991 |
Daphnia magna |
DEEDMAC |
14.8 |
24 h-EC50 |
1.0 |
Giolando et al. 1995 |
Fish |
Esterquat (EQ) |
3.0 |
14 d-NOEC |
4.0 |
Puchta et al. 1993 |
Rainbow trout |
Diesterquat (DEQ) |
7.0 |
96 h-LC50 |
³ 3.5 |
Waters et al. 1991 |
Zebra fish |
DEEDMAC |
5.2 |
96 h |
|
Giolando et al. 1995 |
Fathead minnow |
DEEDMAC |
|
35 d |
0.68 |
Giolando et al. 1995 |
5.4.3 Effects on human health
Acute toxicity
Rats and mice given oral doses of 5,000 mg of EQ/kg body weight exhibited no symptoms of toxic reactions (Puchta et al. 1993). The LD50 values by oral administration and dermal application of DEQ were more than 5,000 mg/kg body weight in rats and more than 2,000 mg/kg body weight for rabbits, respectively (Waters et al. 1991). These results indicate a very low acute toxicity of alkyl ester ammonium salts.
Skin and eye irritation
Concentrated EQ was found to be irritating to the skin of rabbits after 4 hours of semi-occlusive exposure, but the irritation is reversible (Puchta et al. 1993). DEQ was found to be non-irritant to the skin and eye of rabbits (Waters et al.1991).
Skin sensitization
No sensitization potential of EQ was detected in guinea pigs by use of the maximization method (Puchta et al. 1993). Also DEQ was not sensitizing in a modified Buehler test using guinea pigs (Waters et al. 1991).
Subchronic toxicity
A 90-days feeding study in rats showed no systemic toxic effects after administration of doses of up to 300 mg of EQ/kg body weight and even when the dose was increased to 1,000 mg/kg body weight (Puchta et al. 1993). A 28-day subchronic toxicity test with DEQ showed no apperant adverse effects on rats fed a diet containing up to 1% DEQ (Waters et al. 1991).
Mutagenicity
EQ showed no gene mutation effects in the Ames test and no chromosome mutations in the Micronucleus test (Puchta et al. 1993). No genetic damage after exposure to DEQ was indicated in tests for gene mutation and chromosomal aberration (Waters et al. 1991).
6. Amphoteric surfactants
Surface-active compounds with both acidic and alkaline properties are known as amphoteric
surfactants. Amphoteric surfactants include two main groups, i.e. betaines and real
amphoteric surfactants based on fatty alkyl imidazolines. The key functional groups in the
chemical structures are the more or less quaternized nitrogen and the carboxylic group.
Betaines are characterized by a fully quaternized nitrogen atom and do not exhibit anionic
properties in alkaline solutions, which means that betaines are present only as
‘zwitterions’. Another group of amphoterics is designated imidazoline
derivatives because of the formation of an intermediate imidazoline structure during the
synthesis of some of these surfactants. This group contains the real amphoteric
surfactants that form cations in acidic solutions, anions in alkaline solutions, and
‘zwitterions’ in mid-pH range solutions. The mid-pH range (isoelectric range) in
which the surfactant has a neutral charge is compound specific and depends on the
alkalinity of the nitrogen atom and the acidity of the carboxylic group (Domsch 1995).
Amphoteric surfactants are used in personal care products (e.g. hair shampoos and
conditioners, liquid soaps, and cleansing lotions) and in all-purpose and industrial
cleaning agents. The total volume of amphoteric surfactants consumed in commercial
products today is relatively small (see Chapter 2), but the consumption of these chemicals
is expected to increase in the future because of the request for milder surfactants.
Besides acting as mild surfactants, the amphoterics may improve the mildness of especially
anionic surfactants. By volume, the most important groups of amphoteric surfactants today
consist of alkylamido betaines and alkyl betaines. The use of alkylamphoacetates in
personal care products is expected to grow in coming years.
6.1 Betaines
Betaines are primarily used in personal care products like, e.g. hair shampoos, liquid soaps, and cleansing lotions. Other applications include all-purpose cleaning agents, hand dishwashing agents, and special textile detergents. All betaines are characterized by a fully quaternized nitrogen. In alkyl betaines, one of the methyl groups in the ‘betaine’ structure (N,N,N-trimethylglycine) is replaced by a linear alkyl chain. A special type of betaines is the hydroxysulfobetaines in which the carboxylic group of alkyl betaine is replaced by sulfonate and a hydroxy-group is inserted in the hydrophilic part of the molecule. In alkylamido betaines, an amide group is inserted as a link between the hydrophobic alkyl chain and the hydrophilic moiety. The most commonly used alkylamido betaine is alkylamidopropyl betaine (e.g., cocoamidopropyl betaine), whereas alkylamidoethyl betaines are used in smaller amounts.
Representative structures of betaines are shown below.
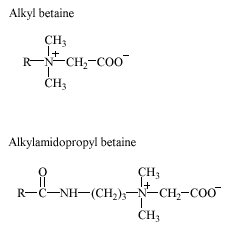
No data were found on the occurrence of betaines in the environment.
6.1.1 Environmental fate
Aerobic biodegradability
The primary biodegradability of betaines approaches 100% as, e.g., the loss of surface-activity attained 100% for C12 alkyl betaine, 98% for cocoamidopropyl betaine, and 96 and 100% for C14-15 hydroxysulfobetaine (Domsch 1995, and references therein). The results from ultimate biodegradability tests of alkyl betaines show some variation with degradation percentages below and above the pass level for ready biodegradability, especially if older data of Fernlay (1978, cited in Domsch 1995) are taken into account. However, both alkyl betaines and cocoalkylamido betaines can be regarded as readily biodegradable on the basis of the data in Table 6.1. The hydroxysulfobetaines are probably not readily biodegradable in standard screening tests as indicated by a biodegradability of 40 and 47% of ThOD in the closed bottle test (Table 6.1).
Table 6.1
Ultimate aerobic biodegradability of betaines.
Compound |
Test |
Result |
Reference |
C12-14 alkyl betaine |
Closed bottle test, 28 d |
63% ThOD |
Madsen et al. 1994 |
C12-18 alkyl betaine |
Closed bottle test, 28 d |
> 60% ThOD |
Brøste 1998 |
Cocoalkyl betaine |
Closed bottle test, 30 d |
> 60% ThOD |
Domsch 1995 |
Cocoalkyl betaine |
Closed bottle test, 30 d |
57% ThOD |
Domsch 1995 |
Cocoalkyl amidopropyl betaine |
Closed bottle test, 30 d |
84% ThOD |
IUCLID 2000 |
Cocoalkyl amidopropyl betaine |
Modified OECD screening test, 28 d |
100% DOC |
IUCLID 2000 |
Cocoalkyl amidopropyl betaine |
Modified OECD screening test |
90-94% DOC |
Domsch 1995 |
C14-15 hydroxysulfo betaine |
Closed bottle test |
40% ThOD |
Domsch 1995 |
Cocoalkyl hydroxysulfo betaine |
Closed bottle test |
47% ThOD |
Domsch 1995 |
Anaerobic biodegradability
The knowledge about the biodegradability of betaines under anoxic conditions is relatively scarce. A search in the literature by Goldschmidt (1993-1994) indicates that sulfate-reducing marine bacteria belonging to the genus Desulfobacterium are able to grow on betaine with the stoichiometric formation of N,N-dimethylglycine (Heijthuijsen and Hansen 1989, cited in Goldschmidt 1993-1994). Another study indicated that betaine was anaerobically degraded to methylamine in sewage sludge at a betaine concentration of 2 g/l and a solids concentration of 3.3 g/l (Gwardys and Nowakowska-Waszczuk 1981, cited in Goldschmidt 1993-1994). The anaerobic biodegradability of cocoamidopropyl betaine was examined in the present study by using the ISO 11734 screening test. Under the methanogenic test conditions, the ultimate biodegradability of cocoamidopropyl betaine attained 45 and 75% of ThGP after 28 and 56 days, respectively, at the applied test concentration of 14.4 mg C/l (Appendix; Table A14, Figure A14).
Bioaccumulation
No experimental data describing the bioaccumulation potential of betaines were found in the literature.
6.1.2 Effects on the aquatic environment
The aquatic toxicity of betaines varies considerably, even within the same species, which is particularly evident by evaluating the EC50 values determined for the green alga Scenedesmus subspicatus. For this species, the EC50 obtained in tests with cocoamidopropyl betaine are between 0.55 and 48 mg/l. The geometric mean of the EC50 obtained for S. subspicatus is 3.1 mg/l, when the values 0.55, 1.84, and 30 mg/l are used (Table 6.2). The EC/LC50 of alkyl and cocoamidopropyl betaines towards crustaceans and fish are between 1 and 100 mg/l.
Table 6.2
Effects of alkyl and alkylamidopropyl betaines to aquatic organisms.
Species |
Surfactant |
EC/LC50 (mg/l) |
Duration |
Reference |
Algae |
C12-14 alkyl betaine |
2.5 |
72 h |
Berol Nobel 1993 |
Algae |
Cocoamidopropyl betaine |
1.84 |
72 h |
IUCLID 2000 |
Algae |
Cocoamidopropyl betaine |
Growth rate: |
96 h |
IUCLID 2000 |
Algae |
Cocoamidopropyl betaine |
Biomass: |
72 h |
Goldschmidt 1993-1994 |
Daphnia magna |
Cocoamidopropyl betaine |
6.5 |
48 h |
IUCLID 2000 |
Daphnia magna |
Cocoamidopropyl betaine |
21.7 |
48 h |
IUCLID 2000 |
Zebra fish |
C12-14 alkyl betaine |
21.9 |
96 h |
Berol Nobel 1993 |
Fish |
C12-18 alkyl betaine |
10-100 |
- |
Brøste 1998 |
Zebra fish |
Cocoamidopropyl betaine |
2.0 |
96 h |
IUCLID 2000 |
6.1.3 Effects on human health
Toxicokinetics and acute toxicity
Amphoteric surfactants are easily absorbed in the intestine and are excreted partly unchanged via the faeces. Metabolization to CO2 and short-chained fatty acids also occur. No tendency to accumulation in the organism or storage of betaines in certain organs has been detected (SFT 1991). Betaines generally have a low acute toxicity. E.g., LD50 values for cocoamidopropylbetain (30% solution) by oral administration have been determined to 4,910 mg/kg body weight in rats (CIRP 1991a).
Skin and eye irritation
Betaines do not carry any net charge, and, therefore, they can only form hydrophobic bonds with proteins in the skin. This may be the explanation for the low protein denaturation potential of betaines as the ion-binding of other surfactants contributes to denaturation. In combination with anionic surfactants a positive synergistic effect with regard to skin compatibility is often found. Compared to a 20% solution of C12 alkyl sulfate (AS; sodium lauryl sulfate) alone, decreased erythema was observed for the combination of 20% C12 AS and 10% cocoamidopropyl betaine one hour after the removal of patches (Dillarstone and Paye 1993). The combination of cocoamidopropyl betaine and C12 AS also reduced swelling of the skin, and generally interactions between amphoterics and AS produce less swelling and result in milder skin reactions (Rhein et al. 1986).
Concentrated betaines are expected to be irritant to skin and eyes. Diluted solutions (3-10%) are not irritant to skin, but they are mildly irritant to the eyes (4.5%) (KEMI 1994).
Solutions containing 7.5% and 10% cocoamidopropyl betaine were not irritating to intact or abraded rabbit skin in a single insult occlusive patch test. The PII (Primary Irritation Index) for the solution was < 0.3 (maximum score is 8). When a 15% solution was tested under occlusive patches for 24 hours by using the same procedure, a PII of 3.5 was achieved and well-defined erythema and edema were observed (CIRP 1991a).
In a Draize test for ocular irritation a concentration of 4.5% cocoamidopropyl betaine produced a slight conjunctival irritation (erythema and swelling of conjunctiva) in unrinsed eyes and a very slight conjunctival irritation in rinsed eyes of rabbits. The surfactant was instilled into the conjunctival sac of the eye. No corneal involvement or iris congestion was seen (CIRP 1991a). The maximum mean irritation scores for eyes of rabbits treated with 30% cocoamidopropyl betaine and left unrinsed were in the range between 26 and 42 (maximum score is 110) (CIRP 1991a).
Sensitization
No evidence of delayed contact hypersensitivity was found in guinea pigs after topically administrated solutions of 10% cocoamidopropyl betaine by using the Magnusson-Kligman maximization test (CIRP 1991a). Various instances of contact allergy to cocoamidopropyl betaine have been reported. In all of the reports it was concluded that the observed skin reactions were due to the presence of 3-dimethylaminopropylamine which is an impurity in cocoamidopropyl betaine. This impurity is an intermediate in the synthesis of alkylamidopropyldimethylamines that are intermediates in the synthesis of the corresponding alkylamido betaines (Angelini et al. 1995, 1996a, 1996b; Armstrong et al. 1999).
Mutagenicity
Cocoamidopropyl betaine was proven to be non-mutagenic to Salmonella typhimurium in the Ames Salmonella/microsome reverse mutation assay (CIRP 1991a). Short-term genotoxicity tests have shown negative results of mutagenicity for lauryl betaine in various strains of Salmonella typhimurium (Yam et al. 1984).
No tests on reproductive toxicity and carcinogenicity were available.
Classification
Betaines are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
6.2 Imidazoline derivatives
The amphoteric surfactants in this group include structures designated as alkylamphoacetates, alkylamphopropionates, and alkyliminopropionates. These surfactants are usually produced by the reaction of fatty acids or their esters with amines (e.g. aminoethylethanol amine). Alkylamphopropionates may be obtained by the addition of acrylic acid, methyl acrylate, or ethyl acrylate to the reaction product of fatty acids and amines. During the synthesis of most of the surfactants an intermediate imidazoline ring structure may be formed (hence the common name ‘imidazoline derivatives’). The imidazoline ring is probably opened by the influence of hydrolysing conditions and does not appear in the final products (Domsch 1995). Alkylamphoacetates, alkylamphopropionates, and alkyliminopropionates are used in products like hair shampoos, liquid soaps, and shower gels. Other major applications of alkylamphopropionates and alkyliminopropionates include highly acidic and alkaline household cleaning agents. Commercial products may contain complex mixtures of the amphoteric surfactants described in this section. Representative structures are given below.
No data were found on the occurrence of these amphoteric surfactants in the environment.
6.2.1 Environmental fate
Aerbic biodegradability
The ultimate aerobic biodegradability of alkylamphodiacetates, alkylamphopropionate, and alkyliminodipropionate complies with the pass levels for ready biodegradability in OECD 301 screening tests (Table 6.3).
Table 6.3
Ultimate aerobic biodegradability of amphoteric imidazoline derivatives.
Compound |
Test |
Result |
Reference |
Cocoamphodiacetate |
Closed bottle test, 30 d |
> 60%; 66% ThOD |
Domsch 1995 |
Cocoamphodiacetate |
Modified OECD screening test |
> 70% DOC |
Domsch 1995 |
C12-18 alkylampho-propionate |
Modified OECD screening test |
79% ThOD |
Domsch 1995 |
C12 alkylimino-dipropionate |
Manometric respirometry test, 28 d |
99% ThOD |
This study (Appendix; Table A3, Figure A3) |
Anaerobic biodegradability
Information on the ultimate anaerobic biodegradability of imidazoline derivatives has not been found in the literature. The anaerobic biodegradability of C12 alkyliminodipropinate (16.4 mg C/l) reached only 2.5% of ThGP during 56 days in the ISO 11734 screening test which was performed in the present study. However, the test substrate concentration inhibited the anaerobic bacteria, and inhibitory effects may have precluded biodegradation (Appendix; Table A15, Figure A15).
Bioaccumulation
No experimental data describing the bioaccumulation potential of alkylamphoacetates, alkylamphopropionates, or alkyliminopropionates were found in the literature.
6.2.2 Effects on the aquatic environment
No data describing the aquatic toxicity of the amphoteric surfactants in this group were found in the literature. Because of the variability in the effect concentrations observed for betaines (see Table 6.2), it is not tempting to base the assessment upon structural analogy and betaine aquatic toxicity. Testing of the aquatic toxicity and the subsequent release of data to the open literature should be encouraged as the consumption of these surfactants is expected to increase.
6.2.3 Effects on human health
Alkylamphoacetates and akylamphopropionates have a low acute toxicity after oral administration to rats (Table 6.4).
Table 6.4
Acute toxicity (LD50) of amphoteric surfactants by oral administration.
Surfactant |
Species |
LD50 (g/kg body weight) |
Reference |
Cocoamphoacetate |
Rat |
15.9 – 28 ml |
CIRP 1990 |
Cocoamphodiacetate |
Rat |
> 5.0 – 16.6 |
CIRP 1990 |
Cocoamphopropionate |
Rat |
20.0 ml* |
CIRP 1990 |
Cocoamphodipropionate |
Rat |
> 5.0 – 16.3 |
CIRP 1990 |
* Commercial solution in water, probably 40-50%.
Skin and eye irritation
Generally these amphoteric surfactants do not seem to be irritant to the skin and only to a small extent irritating to the eye (SFT 1991). Some variation in test results have been reported.
Cocoamphodipropionate was found to be non-irritating as a concentration of 7.5-70% (PII = 0), whereas cocoamphopropionate was slightly irritating to rabbit skin at a concentration of 15–16%. Cocoamphodiacetate was non-irritating to slightly irritating at a concentration of 10-12% (CIRP 1990).
A Draize test has shown that cocoamphodipropionate was practically non-irritating to the eye at a concentration of 7.5%, whereas cocoamphopropionate was non-irritating to slightly irritating at 5% and 16%. Cocoamphodiacetate was moderately to severely irritating to the eye at a concentration of 10-12%. Cocoamphoacetate was slightly to severely irritating at 16 to 50% (CIRP 1990).
Sensitization
Cocoamphoacetate and cocoamphopropionate were non-irritating and non-sensitizing in a repeated insult patch test (non-occlusive) involving 141 subjects. The concentration of the surfactants was 10% in distilled water. During induction, each chemical was applied to the back three times per week for three weeks. The challenge phase was initiated 10 to 15 days after application of the final induction patch. Cocoamphoacetate and cocoamphopropionate did not induce sensitization in any of the subjects (CIRP 1990). Cocoamphoacetate was non-sensitizing in guinea pigs when tested in the Magnusson-Kligman maximization test. The tested concentrations for induction and challenge were 25, 50 and 100% (CIRP 1990).
Mutagenicity
Cocoamphodiacetate, cocoamphopropionate, and cocoamphodipropionate were non-mutagenic, when evaluated in the Ames Salmonella/microsome assay using different strains of Salmonella typhimurium (CIRP 1990).
No tests on reproductive toxicity and carcinogenicity were available.
Classification
The amphoteric surfactants described in this section are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7. Complexing agents
Complexing agents, or builders, are used in laundry detergent powders and liquids as well
as in all-purpose cleaning agents. Commonly used complexing agents are phosphates,
phosphonates, polycarboxylates, and zeolites. Complexing agents improve cleaning
efficiency by inactivating water hardness. They keep calcium and magnesium ions in
solution and prevent them from interfering with the surfactants, and they prevent
redeposition of soil on the washed fabric or surface. Zeolites are used in combination
with other builders in phosphate-free detergents.
Eutrophication and associated problems have received considerable attention during many years, particularly with respect to the effects on freshwater lakes tending to be phosphorus limited (Lee et al. 1978). In order to reduce the phosphorus content of municipal sewage, voluntary and statutory restrictions have been introduced to limit the use of the detergent builder sodium tripolyphosphate (TPP). The release of complexing agents into the environment may affect the distribution and partitioning of metals in soils, sediments and sludge. Complexing agents may potentially cause active desorption of trace metals from particulate matter or interfere with natural sorption processes. Remobilization of metals has expecially been examined for EDTA and NTA (see Sections 7.6 and 7.7) although this is probably a general effect of complexing agents.
7.1 Phosphates
7.1.1 Environmental fate and effects
From 1947 until the late eighties sodium tripolyphosphate (TPP) was used almost exclusively as the complexing agent in detergents due to its multifunctional contribution to washing and cleaning processes. Other complexing agents like phosphonates, polycarboxylates and zeolite have now partially replaced phosphates in household detergents. However, in Denmark the strategy is to establish phosphorus removal processes at the major wastewater treatment plants –a goal which generally speaking has been fulfilled – and TTP is still used in many household detergents. Phosphates serve many functions in detergent products. It removes hardness, reduces surfactant use, improves emulsification and dispersion, prevents re-deposition, and controls alkalinity.
Detergents containing phosphorus contribute together with other sources of phosphorus to the eutrophication of many fresh waters. Algae are the first step in the food chain and a number of factors are needed to promote their growth. These factors are sunlight for photosynthesis, temperature, certain water conditions (turbulence) and nutrients like carbon, nitrogen and phosphorus. Typical plant organic matter of aquatic algae and macrophytes contain phosphorus, nitrogen and carbon in approximately the ratios:
1P : 7N: 40C per 100 g dry weight or
1P : 7N: 40C per 500 g wet weight
Thus broadly speaking, if one of the above mentioned elements is limiting and all other elements are present in excess of physical needs, phosphorus can theoretically generate its weight 500 times in algae, nitrogen 71 times and carbon 12 times in algae (Wetzel 1983).
Whereas the primary production in marine waters is mainly nitrogen limited, freshwaters are considered to be phosphorus limited. A large part of the sewage effluents in many countries is released untreated into freshwater recipients, and here the use of phosphorus as complexing agents is still an environmental concern.
7.1.2 Effects on human health
Toxicokinetics and acute toxicity
Polyphosphates are hydrolyzed into smaller units (orthophosphates) in the gut before absorption, which may induce a metabolic acidosis (Gosselin et al. 1984). The orthophosphates are excreted in the urine (HSDB 1998). Ingested diphosphate is readily converted to monophosphate. No diphosphate was found in faeces or urine of rats treated with diets containing up to 5% tetrasodium diphosphate. Diphosphate was almost completely absorbed by the gut and excreted as monophosphate in the urine (IPCS 1982). The acute toxicity of polyphosphonates is low as the lowest LD50 after oral administration is > 1,000 mg/kg body weight (IPCS 1982; ACGIH 1991).
Skin and eye irritation
The most important human health effect, which may be caused by the use of phosphates in household detergents, is the potential irritation to skin and eyes. Polyphosphates are moderately irritating to skin and mucous membrane (Merck 1989). Polyphosphates can be irritating because of their alkalinity. A 1% aqueous solution of TTP has a pH of 9.8 and the pH of concentrated solutions is about 10.5 (Gosselin et al. 1984). Acute studies with tetrasodium diphosphate show that direct contact causes severe irritation and corneal injury in the rabbit eyes and that it may be irritating to skin (ACGIH 1991).
Mutagenicity and carcinogenecity
No mutagenic potential was observed when TTP was tested in a Salmonella/microsome assay (Ames test) and in a chromosomal aberration assay in vitro using a Chinese hamster fibroblast cell line (Ishidate et al. 1984). Tetrasodium pyrophosphate was not mutagenic in an in vitro assay using S. cerevisiae strains and S. typhimurium strains with and without the addition of mammalian metabolic activation preparations (IPCS 1982).
Reproductive toxicity
Sodium tripolyphosphate showed no maternal toxicity or teratogenic effects at dose levels up to 238 mg/kg body weight in mice and 40 mg/kg in rats (IPCS 1982). Reproduction studies in three generations of rats on diets with 0.5% TTP were performed. TTP had no effects on fertility or litter size, or on growth or survival on offspring (Hodge 1964). Tetrasodium diphosphate showed no maternal toxicity or teratogenic effects at dose levels up to 130 mg/kg body weight in mice and 238 mg/kg in rats (IPCS 1982).
Classification
Polyphosphates are of low toxicity. No tests on sensitization and carcinogenicity were available. Polyphosphates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7.2 Phosphonates
Phosphonate compounds containing more than one phosphonate group are effective sequestrants and possess other useful properties such as high water solubility, chemical stability, bleach stabilizing effects, and the ability to prevent precipitation of calcium salts at substoichiometric concentrations.
Phosphonates are characterized by the presence of one or more –C-PO3-H2 groups. Most phosphonates are synthesized from phosphorous acid by reaction with formaldehyde and either ammonia or amines.
An example of a phosphonate synthesized by reaction with ammonia is:
Amino tris methylenephosphonic acid (ATMP; CAS No. 6419-19-8).
Examples of phosphonates synthesized by reaction with amines are:
Ethylenediamine tetra methylenephosphonic acid (EDTMP;
CAS No. 1429-50-1), Hexamethylenediamine tetra methylenephosphonic
acid (HDTMP; CAS No. 23605-74-5), Diethylenetriamine penta methylenephosphonic
acid (DTPMP; CAS No. 15827-60-8).
1-Hydroxy ethane diphosphonic acid (HEDP; CAS No. 2809-21-4) is formed from PCl3 and acetic acid (Gledhill and Feijtel 1992).
7.2.1 Occurrence in the environment
A large percentage of European phosphonate consumption occurs in detergents and, thus, phosphonates are continuously released to the environment in Europe. At present sufficiently sensitive analytical methods for measuring phosphonates are unavailable and environmental concentrations are predicted from models. According to the model simulations the maximum phosphonate levels in aquatic environments are expected to be < 30 m g/l. If partitioning to sediments (100:1) and limited photo- and biodegradation are assumed, the average phosphonate concentrations in European streams are predicted to be in the order of 0.25 m g/l (Gledhill and Feijtel 1992).
7.2.2 Environmental fate
A variety of natural and synthetic chemicals contain a C-P bond. The C-P bond provides the molecule stability and a relatively high resistance to chemical, photolytic and thermal decomposition. Phosphonates seem to be recognized by bacteria only as a possible P-source, which may explain poor results in standard biodegradation tests. However, several laboratory studies report phosphonate degradation by pure microbial cultures when supplied as the sole source of phosphorus (Gledhill and Feijtel 1992). Orthophosphate has been found to suppress phosphonate utilisation in many microrganisms. Thus organisms preferentially use inorganic phosphate, which may explain the low biodegradability of phosphonates in synthetic test media and natural sewage systems.
Aerobic biodegradability
Numerous studies have shown that little, if any, primary or ultimate biodegradation occurs for any phosphonate product in standard biodegradation tests such as the OECD screening test, BOD20 test, sapromat test and closed bottle test. Phosphonates may serve as a carbon source when present at very high concentrations and, e.g., DOC removals of 23-33% of HEDP and ATMP have been observed in a Zahn-Wellens test (Gledhill and Feijtel 1992).
Anaerobic biodegradability
Reports of anaerobic biodegradability are sparse. For HEDP and ATMP less than 4% of the 14C-labelled phosphonate carbon was converted to 14CO2 and 14CH4 in a model digestor (Gledhill and Feijtel 1992).
Bioaccumulation
As expected for highly water-soluble substances, the log Kow values for phosphonates are low (ATMP: -3.53; HEDP: –3.49; EDTMP: –4.10; HDTMP: –4.43; DTMP: -3.40). The potential for bioaccumulation of phosphonates in aquatic organisms is therefore expected to be low as well. Experimental bioconcentration studies with zebra fish have been conducted with radiolabelled ATMP and HEDP. For both substances, the BCF values determined after 4-6 weeks of exposure were less than 24.
7.2.3 Effects on the aquatic environment
Algae
Investigation of the effects of phosphonates in alga bioassays is quite complex as the alga medium contains a precise level of micro nutrients which are held in solution by a chelator, EDTA. The introduction of an additional chelator, such as a phosphonate, may indirectly either inhibit or stimulate alga growth. The phosphonate may bind essential metals (indirect inhibition) or it may release additional phosphorus via photodegradation (indirect stimulation). Cell counts were performed at day 4 and day 14 during a toxicity study with Selenastrum capricornutum. The day 4 results indicate EC50 values for the examined phosphonates between 0.4 and 30 mg/l, with EDTMP being the most toxic (Table 7.1). For HEDP, EDTMP and DTPMP the EC50 values measured on day 14 were lower than the values measured on day 4. Initial concentrations of phosphonates may have chelated some essential micronutrients for alga growth, thus resulting in the low EC50 values observed on day 4. In the time period from day 4 to day 14, HEDP, EDTMP and DTPMP may have photodegraded to release these nutrients plus additional phosphorus, which resulted in observed growth stimulation and thus the lower EC50 value observed on day 14. For the reasons described above, the apparent toxicity of phosphonates to algae cannot be regarded as a reliable indication of the toxicity of phosphonates in the aquatic environment.
Table 7.1
Effects of phosphonates to the green algae Selenastrum capricornutum (data from Gledhill
and Feijtel 1992).
Substance |
EC50 (mg/l) |
NOEC (mg/l) |
Test duration |
ATMP |
19.6 19.6 |
7.4 7.4 |
96 h 14 d |
HEDP |
3.0 39.1 |
1.3 13.2 |
96 h 14 d |
EDTMP |
0.42 27.1 |
0.09 9.3 |
96 h 14 d |
HDTMP |
28 27 |
10.2 10.2 |
96 h 14 d |
DTPMP |
1.9 8.7 |
5.2 5.2 |
96 h 14 d |
Invertebrates
Generally, the acute EC/LC50 values for phosphonates towards invertebrates are well above 100 mg/l. One exception is the Eastern oyster for which acute LC50 values below 100 mg/l are found (Table 7.2).
Table 7.2
Effects of phosphonates to invertebrates (data from Gledhill and Feijtel 1992).
Species |
Substance |
EC50/LC50(mg/l) |
NOEC(mg/l) |
Test duration |
Chironomus sp. |
ATMP |
11,000 |
7,040 |
48 h |
Grass Shrimp |
ATMP |
7,870 |
4,575 |
96 h |
Eastern Oyster |
ATMP |
201 |
95 |
96 h |
Daphnia magna |
ATMP |
297 < 54 |
125 > 25 |
48 h 28 d |
Chironomus sp. |
HEDP |
8,910 |
3,925 |
48 h |
Grass Shrimp |
HEDP |
1,770 |
104 |
96 h |
Eastern Oyster |
HEDP |
89 |
< 52 |
96 h |
Daphnia magna |
HEDP |
527 < 25 |
400 > 12 |
48 h 28 d |
Chironomus sp. |
EDTMP |
7,320 |
1,956 |
48 h |
Grass Shrimp |
EDTMP |
1,436 |
605 |
96 h |
Eastern Oyster |
EDTMP |
67 |
55 |
96 h |
Daphnia magna |
EDTMP |
510 |
250 |
48 h |
Chironomus sp. |
HDTMP |
4,660 |
1,803 |
48 h |
Grass Shrimp |
HDTMP |
942 |
537 |
96 h |
Eastern Oyster |
HDTMP |
212 |
< 161 |
96 h |
Daphnia magna |
HDTMP |
574 |
125 |
48 h |
Chironomus sp. |
DTPMP |
9,910 |
7,589 |
48 h |
Grass Shrimp |
DTPMP |
4,849 |
2,125 |
96 h |
Eastern Oyster |
DTPMP |
156 |
56 |
96 h |
Daphnia magna |
DTPMP |
242 |
125 |
48 h |
Fish
As also noted for invertebrates, the LC50 values for phosphonates are well above 100 mg/l. The aquatic toxicity data obtained in long-term studies with fish are not markedly different from the data from short-term studies (96 hours). This indicates that phosphonates do not accumulate and that the maximum toxicity is obtained in short term tests (Table 7.3).
Table 7.3
Effects of phosphonates to fish (data from Gledhill and Feijtel 1992).
Species |
Substance |
LC50 (mg/l) |
NOEC (mg/l) |
Test duration |
Bluegill sunfish |
ATMP |
> 330 |
330 |
96 h |
Channel catfish |
ATMP |
1,212 |
924 |
96 h |
Sheepshead minnow |
ATMP |
8,132 |
4,831 |
96 h |
Rainbow trout |
ATMP |
160 |
- |
96 h |
Bluegill sunfish |
HEDP |
868 |
529 |
96 h |
Channel catfish |
HEDP |
695 |
529 |
96 h |
Sheepshead minnow |
HEDP |
2,180 |
104 |
96 h |
Rainbow trout |
HEDP |
200 |
- |
96 h |
Bluegill sunfish |
EDTMP |
> 164 |
164 |
96 h |
Channel catfish |
EDTMP |
967 |
522 |
96 h |
Sheepshead minnow |
EDTMP |
1,513 |
605 |
96 h |
Rainbow trout |
EDTMP |
> 164 250 |
164 - 35 |
96 h |
Bluegill sunfish |
HDTMP |
> 273 |
273 |
96 h |
Channel catfish |
HDTMP |
> 2,400 |
2,400 |
96 h |
Sheepshead minnow |
HDTMP |
> 954 |
< 954 |
96 h |
Rainbow trout |
HDTMP |
> 273 |
273 |
96 h |
Bluegill sunfish |
DTPMP |
758 |
576 |
96 h |
Channel catfish |
DTPMP |
657 |
432 |
96 h |
Sheepshead minnow |
DTPMP |
5,377 |
2,125 |
96 h |
Rainbow trout |
DTPMP |
>180-252 |
180 |
96 h |
7.2.4 Effects on human health
Toxicokinetics and acute toxicity
The intestinal absorption and kinetics of 32P–labelled HEDP have been studied in man. After oral administration 70-90% of the administered dose was found in faeces after 6 days. HEDP was poorly absorbed (Caniggia and Gennari 1977). When 32P-labelled HEDP was given intravenously 35-50% of the administered dose was excreted in the urine after 6 days. No metabolism occurred (Caniggia and Gennari 1977). 14C-labelled EDTMP was poorly absorbed from the gastrointestinal tract and most of the absorbed dose was rapidly excreted by the kidneys or sequestered in bone. EDTMP is not metabolized as the entire radioactivity in the urine was identified as EDTMP (Calvin et al. 1988). No data on percutaneous absorption was available.
Phosphonates show low oral and dermal toxicity (Table 7.4).
Table 7.4
Acute toxicity (LD50) of phosphonates.
Type |
Species |
Route of administration |
LD50 |
Reference |
ATMP |
Rat |
Oral |
2,910 |
SFT 1991 |
ATMP |
Rat |
Oral |
2,100 |
RTECS 1997 |
ATMP |
Rat |
Dermal |
> 6,310 |
SFT 1991 |
ATMP, pentasodium salt |
Rat |
Oral |
17,800 |
RTECS 1997 |
ATMP, pentasodium salt |
Rabbit |
Dermal |
15,800 |
RTECS 1997 |
HEDP |
Rat |
Oral |
2,400 |
SFT 1991 |
HEDP |
Rat |
Dermal |
> 7,940 |
SFT 1991 |
EDTMP |
Rat |
Oral |
6,900 |
SFT 1991 |
EDTMP |
Rat |
Dermal |
> 5,010 |
SFT 1991 |
1,2,4-Butantricarboxylic acid, 2-phosphono |
Rat |
Oral |
> 6,500 |
IUCLID 2000 |
1,2,4-Butantricarboxylic acid, 2-phosphono, tetrasodium salt |
Rat |
Dermal |
> 4,000 |
IUCLID 2000 |
DTPMP, sodium salt |
Rat |
Oral |
> 5,000 |
RTECS 1997 |
DTPMP, sodium salt |
Rabbit |
Dermal |
> 5,000 |
RTECS 1997 |
Skin and eye irritation
Concentrated solutions of ATMP and HEDP have pH values of about 2.1. Only moderately skin and eye irritation have been seen (SFT 1991). In Guinea pig maximization test 1,2,4-butantricarboxylic acid, 2-phosphono, tetrasodium salt in a 32% solution was not sensitizing (IUCLID 2000). ATMP, HEDP and EDTMP did not show sensitizing effects (SFT 1991).
Mutagenicity and carcinogenecity
EDTMP was tested for genotoxicity in the Ames, mouse lymphoma, unscheduled DNA synthesis and in vivo cytogenetics assays. No mutagenic activity was seen in any of the assays (Calvin et al. 1988). HEDP showed no mutagenicity in microsome test with Salmonella typhimurium and mouse lymphoma assays (SFT 1991). A 50% solution of 1,2,4-butantricarboxylic acid, 2-phosphono was tested according to Guideline 474 "Genetic toxicology: Micronucleus Test " as a single oral administration in mice. No mutagenic effects were seen (IUCLID 2000). Rats were fed EDTMP in the diet over a 2 year period. The dose was up to 100 mg/kg/day. No carcinogenic potential was seen (Calvin et al. 1988).
Reproductive toxicity
Rabbits were given HEDP by gavage in the doses 25, 50 and 100 mg/kg/day from day 2 to 16 of gestation. No differences between the controls and the treated animals were seen with respect to teratogenicity and maternal toxicity (Nolen and Buehler 1971). A 49% solution of 1,2,4-butantricarboxylic acid, 2-phosphono was given orally to rats. The doses were 0, 100, 300 or 1,000 mg/kg and they were given from day 6 to 15 of gestation according to Guideline 414 "teratogenicity". No teratogenicity, embryotoxicity or maternal toxicity were seen (IUCLID 2000).
Classification
Phosphonates show no sensitizing, mutagenic or reproductive effects. Low acute oral and dermal toxicity is seen. Phosphonates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7.3 Polycarboxylates
Polycarboxylates used in washing powders and detergents are homopolymers of acrylic acid or copolymers of acrylic acid and maleic anhydride, generally as sodium salts. Relevant CAS Nos. are: Sodium polyacrylate (9003-04-7), polyacrylic acid (9003-01-4), and acrylic acid polymers with maleic anhydride, sodium salt (52255-49-9). The various polycarboxylates are distinguished by the monomers used for their preparation, acrylic acid (AA) and maleic anhydride (MA), and their mass-average molar mass or molecular weight (MW). The polymers are designated by codes of the corresponding abbreviations, P(AA) for polyacrylic acid, and P(AA-MA) for the copolymer of acrylic acid and maleic anhydride, to which the numerical value of MW is suffixed.
As a consequence of the reduction of phosphate content in detergents, the concentrations of free calcium and magnesium rise in the washing water. The metal ions tend to form precipitates with hard water and some detergent components. Polycarboxylates inhibit the crystal growth of inorganic precipitates so that these salts remain in suspension and do not precipitate onto textile fabrics
7.3.1 Environmental fate
Due to their major use in detergents, the main route for the emission of polycarboxylates to the environment is via domestic sewage treatment plants to surface waters receiving the treated effluents.
Polycarboxylates are removed from sewage water by physico-chemical processes such as sorption onto particulate matter and precipitation, which implies that the polycarboxylates will partition into the sludge. Sewage sludge is frequently stabilised by anaerobic digestion and subsequently used as fertilizer in agriculture. Therefore, degradation and elimination processes in sewage treatment plants, surface waters and soils are of main interest.
Aerobic biodegradabilty
Polycarboxylates are generally not rapidly biodegradable, and no evidence for short-term biodegradation has been obtained when P(AA)3,000-4,000 was evaluated for BOD5, BOD10 and DOC removal in test systems inoculated with effluent from a municipal sewage treatment plant (ECETOC 1993). A respirometric screening test with P(AA-MA)70,000 showed a biodegradability corresponding to < 14% biodegradation. A number of 14C-labelled P(AA)1,000; 2,000; 4,500; 10,000 and P(AA-MA)12,000; 70,000 were tested in flasks fitted with CO2 absorbers. The polycarboxylates (test concentration: 0.1 and 1 mg/l) were incubated for up to 19 weeks in river water, pre-adapted river water or a mixture of river water and sediment. Mineralisation in river water was < 20% for all polymers tested. The P(AA) were mineralised to a higher degree in pre-adapted river water and river water plus sediments than in river water alone: 63% and 58% for P(AA)1,000 and 15% and 12% for P(AA)10,000). The results for P(AA-MA) were not significantly different in the three test waters and indicate that their degradation is slow (< 20%) under discontinuous test conditions (ECETOC 1993).
A partial biodegradation of polycarboxylates with a molecular weight of 1,000-70,000 has been indicated in tests with activated sludge inoculum. P(AA)1,000 was mineralised to an average extent of 43%, whereas P(AA)2,000 and P(AA-MA)70,000 were mineralized 19% and 15%, respectively (ECETOC 1993).
The fate of radiolabelled P(AA-MA)70,000 has been examined in a sewage-treatment plant model system using pre-adapted sewage sludge. Both by continuous and pulse loading more than 90% of the 14C was recovered in the sludge, while 2-3% remained in the supernatant (ECETOC 1993).
Several studies have shown that the biodegradation of polycarboxylates in soils is poor. E.g., the 14CO2 production was followed for 1 year in a standard soil which was treated with 14C-labelled P(AA-MA)70,000. The total formation of 14CO2 was 4-7% of the added 14C, and it occurred mainly within the first month (ECETOC 1993).
Anaerobic biodegradability
No evidence exists for the biodegradation of high molecular weight polycarboxylates under anoxic conditions. Anaerobic incubation of 14C-labelled P(AA-MA)70,000 in a model digester containing domestic sewage sludge showed that the substance was not mineralized under the applied conditions as 94-95% of the added 14C was sorbed to the sludge particles (ECETOC 1993).
Bioaccumulation
No experimental data are available describing the bioaccumulation potential of polycar-boxylates. However, the molecular weight of polycarboxylates used in laundry detergents is normally between approx. 1,000 and 100,000 and, hence, the bioaccumulation potential of typical commodity chemicals is assumed to be low. Uptake through biological membranes is only anticipated for substances with a molecular weight < 1,000 (OECD 2000).
7.3.2 Effects on the aquatic environment
Algae
Inhibitory effects on the growth rate of algae have been observed with Scenedesmus subspicatus, where the 96 h-EC10 was 180 mg/l for P(AA)4,500. The 96 h-EC10 values were 32 mg/l and ³ 200 mg/l for P(AA-MA)70,000 in tests with Scenedesmus subspicatus. A similar low toxicity was seen for P(AA)78,000 as the EC10 (4-14 days) were 82 mg/l for Scenedesmus subspicatus and 30 to more than 1,000 mg/l for Chlorella kessleri (ECETOC 1993).
Crustaceans and fish
Data describing the acute toxicity for daphnids and fish are available for a number of polycarboxylates with different molecular weights. A consistently low toxicity has been observed with LC50 above the highest concentration tested (LC50 > 100 – 1,000 mg/l) (ECETOC 1993).
Sediment and fish
The acute toxicity of P(AA)4,500 to chironomid larvae was tested in a sediment batch system. After 96 hours, no effects were observed at the highest concentration tested (4,500 mg/kg dry matter) (ECETOC 1993). The acute toxicity of polycarboxylates to earth worms (Eisenia foetida) is also low. For P(AA)4,500 the LC50 was > 1,000 mg/kg soil. The LC0 values reported for P(AA)78,000 and for P(AA-MA)70,000 were 1,000 mg/kg soil and 1,600 mg/kg soil, respectively.
7.3.3 Effects on human health
Toxicokinetics and acute toxicity
14
C-labelled P(AA-P)2,500 (50% aqueous solution of phosphonated P(AA)) was given to rats by gavage in the concentrations of 25 mg/kg body weight. After 4 days 0.35% of the administered dose was recovered in expired air, 0.47% in the urine and 82-94% was recovered in the faeces. This result indicates a very little absorption from the intestinal tract (ECETOC 1993). In a study of skin penetration of P(AA-P)2,500, only 0.3% was recovered after 2 days in expired air, urine and faeces combined. In general components with a molecular weight > 1,000 have difficulties in penetrating the skin (ECETOC 1993).The LD50 values by oral administration for rats and mice are over 5 g/kg body weight and by dermal administration for rabbits over 5 g/kg body weight (ECETOC 1993). These values indicate a low acute toxicity by oral and dermal administration.
Skin and eye irritation
Irritation of polycarboxylates has not been observed in man. A 40% active solution of P(AA)7,000 and a 45% solution of P(AA)8,000 were not irritant to the skin of rabbits (ECETOC 1993). When P(AA)1,000 or P(AA)1,200 were applied to the eyes no damage to the cornea or iris was observed. A slight conjunctivae irritation was observed but this cleared within 24 hours after administration. The concentrations were no further specified (ECETOC 1993). P(AA) with different molecular weights were not found to be sensitising (ECETOC 1993).
Subchronic toxicity
No serious adverse effects were observed by oral, dermal or pulmonal administration (ECETOC 1993).
Mutagenecity and carcinogenicity
No evidence of mutagenic potential for polycarboxylates P(AA) and P(AA-MA) tested in a variety of genetic tests, such as Ames test, gene mutation in mammalian cells (mouse lymphoma), UDS (unscheduled DNA synthesis) assay and micronucleus test (Thompson et al. 1989). The International Agency for Research on Cancer (IARC) has evaluated polyacrylic acid and the data available to the working group did not permit an evaluation of the carcinogenicity to humans of polyacrylic acid (IARC 1979).
Reproductive toxicity
P(AA)90,000 and 4,500 and P(AA-MA)12,000 have been tested. The compounds were administrated by gavage to rats during major organogenesis (on day 6 to 15 of gestation) at dose levels of 500 – 7,000 mg/kg bw/day. No treatment related adverse effects on foetal development (skeletal abnormalities and soft tissue) were seen (Nolen et al. 1989).
Classification
In general P(AA) and P(AA-MA) have a low acute toxicity after oral and dermal administration. No data on carcinogenicity were available. No evidence of a mutagenic and a teratogenic potential has been reported. Some P(AA) were slightly irritating to rabbit eyes. No sensitizing potential has been identified. Polycarboxylates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7.4 Sodium citrate
Sodium citrates (disodium citrate (CAS No. 144-33-2) and trisodium citrate (CAS No. 68-04-2)) are salts of citric acid. Sodium citrates are widely used in phosphate-free detergents and cleaners. Sodium citrate solutions will exhibit a pH of about 8.5 and are subject to microbial growth. Citrate is a chelating agent for di- and trivalent metal ions.
7.4.1 Environmental fate
Biodegradability
Sodium citrates are rapidly and ultimately biodegradable under aerobic and anoxic conditions. E.g., sodium citrate attained 90% ThOD in a closed bottle test for ready biodegradability during 30 days (IUCLID 2000).
Bioaccumulation
Due to a low log Kow value of –1.72, sodium citrate is not expected to accumulate in aquatic organisms.
7.4.2 Effects on the aquatic environment
Sodium citrate has a low toxicity towards aquatic organisms (Table 7.5).
Table 7.5
Effects of sodium citrate to algae, crustaceans and fish.
Species |
Effect concn. |
Test |
Reference |
Scenedesmus quadricauda |
NOEC: 640 |
7 d |
Verschueren 1997 |
Daphnia magna |
EC50: 825 |
48 h 21 d |
Verschueren 1997 |
Chinook salmon |
LOEC: 10 |
96 h |
Bringmann and Kuhn 1977 |
7.4.3 Effects on human health
Toxicokinetics and acute toxicity
Sodium citrate is oxidized to bicarbonate and excreted in the urine (HSDB 1998). Sodium citrate is a normal human metabolite of carbohydrates in the Krebs cycle (citric acid cycle). It is the glycolytic pathway in which glucose is converted into pyruvate. An LD50-value was found to be 7.1 g/kg body weight after oral administration to mice (Hoyt and Gewanter 1992). This value indicates a low acute toxicity by oral administration.
Skin and eye irritation
Sodium citrates are not irritating to rabbit skin in a test performed according to OECD Guideline 404 (IUCLID 2000).
Sodium citrate is well tolerated by the eye and has proven effective in experimental treatment of cornea injuries caused by alkalines. Sodium citrate reduced the incidence of ulceration and perforation (Grant and Schuman 1993).
Mutagenicity and carcinogenicity
Citrates have shown no mutagenic effects, and no potential carcinogenicity is suspected for citric acid and its salts (Hoyt and Gewanter 1992). Sodium citrate was tested in Salmonella/microsome assay (Ames test) and chromosomal aberrations assay in vitro using a Chinese hamster fibroblast cell line. No mutagenic potential was observed in either test (Ishidate et al. 1984).
Reproductive toxicity Classification
Sodium citrate was negative in teratogenicity studies (Schardein 1993). Based on available information, sodium citrates are considered safe when used in detergents and cleaners. Sodium citrates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7.5 Zeolites
Zeolite (CAS No. 1318-02-1) is an inert, insoluble aluminium silicate, which softens water by ion exchange (Henning et al. 1977).
7.5.1 Environmental fate
Removal during wastewater treatment
The removal of zeolite during wastewater treatment is mainly due to sorption to sludge. The removal of zeolite A in primary treatment has been investigated by Carrondo et al. 1981 in static column tests using raw waste water. Removals of 55% and 69% were observed for retention times of one and two hours, respectively. Carrondo et al. 1980 investigated zeolite A removal in the activated sludge process at pilot scale. Assuming a 50% removal during primary treatment, the plant was dosed with zeolite at 15 and 30 mg/l. Average removal rates were 88%, and the removal rate was always greater than 80%.
7.5.2 Effects on the aquatic environment
The toxicity of zeolite towards aquatic organisms is low (Table 7.6).
Table 7.6
Effects of zeolite A to algae, crustaceans and fish.
Species |
EC/LC50 |
Test duration |
Reference |
Selenastrum capricornutum |
100-1,000 |
8 h |
Morse et al. 1994 |
Chlorella vulgaris |
NOEC: 70 |
8 h |
Morse et al. 1994 |
Daphnia magna |
>70211-1,000NOEC: 129-1,000 |
48 h |
Morse et al. 1994 |
Fathead minnow |
> 680 NOEC: > 87 |
96 h |
Morse et al. 1994 |
Fathead minnow |
NOEC: 175 |
30 d |
Maki 1979 |
7.5.3 Effects on human health
Toxicokinetics and acute toxicity
The gastrointestinal absorption of silicic acids depends on the degree of polymerisation. The lower the degree of polymerisation, the higher the absorption. Silicic acid monomers were absorbed at a very high rate (Yokoi and Enomoto 1979; IUCLID 2000). In a human stomach model it was found that zeolite Na-A is hydrolyzed to silicates and aluminates. Studies with rats indicate that the silicate is excreted by the urinary system and the aluminate in the faeces (Christophiemk et al. 1992). The LD50-value of zeolites by oral administration for rats is > 5 g/kg body weight (Gloxhuber et al. 1983; IARC 1997).
Skin and eye irritation
Zeolite A is not irritating to rabbit skin according to OECD –guideline No. 404 "Acute Dermal Irritation/Corrosion" (IUCLID 2000). In a patch test, a 1% suspension of Zeolite A was exposed to human skin for 24 hours and no irritation was observed (Gloxhuber et al. 1983). Zeolite A is slightly to non-irritating to the eyes (IUCLID 2000). No sensitization potential of zeolites was observed (Gloxhuber et al. 1983; Christophiemk et al. 1992).
Subchronic toxicty
No indications of any chronic toxicity have been found.
Mutagenicity and carcinogenicity
Synthetic zeolite A was tested for carcinogenicity in rats by oral administration of 0.6, 6.0 or 60 mg/kg/day via the diet for two years. No increase in tumour incidence was found. No human data are available (Gloxhuber et al. 1993). No indications of any chronic toxic or tumorigenic effects in rats given 1,000 ppm zeolite A (about 50 mg/kg/day) orally in 2 years (Christophiemk et al. 1992). In Salmonella typhimurium strains (Ames test) no mutagenic potential of zeolite A was seen (IUCLID 2000).
Reproductive toxicity
Zeolite A was tested for its teratogenic potential in rats and rabbits. The zeolite was given in destilled water by gavage on day 6-15 of gestation for rats and day 6-18 to rabbits. No adverse effects were observed on the dams, the embryo or the fetuses at the doses tested. The highest dose tested was 1,600 mg/kg body weight (Nolen and Dierckman 1983).
Classification
Zeolite A is considered non-sensitizing and non-irritating to the skin, but may be slightly eye irritating. No carcinogenic and teratogenic potential has been observed. Very low acute toxicity of zeolites by oral administration is observed. Zeolites are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
7.6 EDTA and EDTA tetrasodium salt
Ethylenediamine tetraacetate, EDTA, (CAS No. 60-00-4) and EDTA tetrasodium salt (CAS No. 64-02-8) are used at low levels in fabric washing powders as a bleach stabiliser. They are also used in soaps as stabilisers and in some liquid products to enhance the action of preservatives. Besides, EDTA is used in detergents for the industrial and institutional market like, e.g., machine dishwashing agents.
7.6.1 Environmental fate
EDTA is a hexadentate chelator capable of combining stoichiometrically with metals. The EDTA-metal interactions depend on metal concentrations, pH, nature of the sediment, concentrations of organics etc. Therefore, it is not possible to give a single value for an EDTA concentration at which no effects on metal remobilization occur. The most preferred EDTA-metal complex is Ni followed by Cu, Zn or Pb, but the formation of these complexes is very dependent on the water-specific conditions. At low EDTA concentrations, nearly all of the EDTA is bound to Ni. With increasing EDTA concentrations other metal ions are complexed successively (EU, Risk Assessment 2000). The metal which forms the most stable EDTA complex is Fe(III), and iron is the most frequent heavy metal in river water. However, studies on the EDTA speciation in surface waters have shown that no major amounts of FeEDTA are present as insoluble Fe(OH)3 and Fe(O)OH are formed. When EDTA is discharged to aquatic environments it has been shown that it will always occur as a metal complex. In German rivers, heavy metal concentrations of approximately 0.5 m mol/l (sum Cd, Cu, Hg, Ni, Pb, Zn) are detected. The stoichiometric EDTA equivalent is about 150 m g/l. In most rivers, the EDTA concentration is lower. Therefore, all EDTA is bound onto actually emitted heavy metal, and there is no free EDTA available to remobilize metals from sediments. Remobilization from the deeper layers is limited by formation of nearly insoluble metal sulfides. Only if the sediments are whirled up during high water flows, a significant increase of heavy metal abundance in the water phase may occur (EU, Risk Assessment 2000).
Aerobic biodegradability
EDTA is not readily biodegradable in standardized OECD tests, but several lines of evidence suggest that the compound is inherently ultimately biodegradable under aerobic conditions. By use of a pre-adapted inoculum, 10% carbon dioxide evolution and 22% DOC removal were observed in the Sturm test, whereas a higher DOC removal (37%) was attained in the Zahn-Wellens test (Wolf and Gilbert 1992). Other data indicate an interesting relation between pH and the biodegradation of CaNa2EDTA. By using samples from a river, a ditch and a lake as inocula in the closed bottle test, a biodegradation between 60 and 83% was obtained after 49 days at pH 6.5, whereas between 53 and 72% were obtained after 28 days at pH 8.0 (EU Risk Assessment 2000).
Anaerobic biodegradability
No biodegradation of EDTA has been observed under anoxic conditions.
Bioaccumulation
A highly polar, water-soluble compound such as EDTA is not expected to bioaccumulate by partitioning into the lipid component of aquatic organisms. A whole body bioconcentration factor of 1, with a half-life for depuration of 128-242 hours, was observed for bluegill sunfish exposed for 28 days to radiolabelled EDTA (Bishop and Maki 1980). Much information about the influence of EDTA on the accumulation on heavy metals is available. E.g., studies of the influence on Cd accumulation on rainbow trout (Oncorhynchus mykiss) have indicated that EDTA decreases the accumulation of Cd (Pärt and Wikmark 1984).
7.6.2 Effects on the aquatic environment
The toxicity of EDTA to aquatic organisms is dependent on the hardness of the test medium and the pH. This has been shown in experiments with bluegill sunfish (Lepomis macrochirus): The LC50 of EDTA was 61.2 mg/l in very soft water, 401.7 mg/l in medium hard water and 807.3 mg/l in very hard water. At a pH of 3.7 the LC50 was 159 mg/l, 486 mg/l at a pH of 8.9 and 2,340 mg/l at a pH of 7.4 (Wolf and Gilbert 1992).
Information on the chronic toxicity of EDTA towards aquatic organisms is lacking, although it is reported that the NOEC is usually higher than one tenth and almost always higher than one hundredth of the corresponding LC50 (ECETOC 1984).
7.6.3 Effects on human health
Toxicokinetics and acute toxicity
Calcium disodium EDTA is poorly absorbed from the gastrointestinal tract in humans with only 2.5% of an oral dose of 3.0 gram being excreted in the urine (Richardson 1992-1994). Studies in rats also indicated that calcium disodium EDTA was poorly absorbed from the gastrointestinal tract. About 80–95% of the dose appeared in the faeces after 24 hours. The amount absorbed in 24 hours, determined from the quantity found in the tissues and urine ranged from 2–18% with most of the values between 2 and 4% (WHO 1998). Low acute toxicity by oral administration is observed. Conversion from the tetrasodium salt to the calcium disodium salt greatly reduced toxicity. The acute toxicity of EDTA is given in Table 7.7.
Table 7.7
Acute toxicity (LD50) of EDTA and salts.
Type |
Species |
Route of administration |
LD50 (mg/kg body weight) |
References |
EDTA |
Rat |
Oral |
4,000 |
Richardson 1992-1994 |
Tetrasodium EDTA (powder) |
Rat |
Oral |
1,000-2,000 |
IUCLID 2000 |
Tetrasodium EDTA |
Rat |
Oral |
2,400 |
Wolf and Gilbert 1992 |
Calcium-disodium EDTA |
Rat |
Oral |
1,0000 |
Wolf and Gilbert 1992 |
Disodium EDTA |
Mice |
Oral |
1,000 |
Gosselin et al. 1984 |
Disodium EDTA |
Rat |
Oral |
2,000-2,200 |
WHO 1998 |
Skin and irritation
In a test performed according to OECD Guideline 404 "Acute Dermal Irritation/Corrosion" Tetrasodium EDTA is found non-irritating to the rabbit skin (IUCLID 2000). A 1% aqueous solution of tetrasodium EDTA has a pH of 11.8. Unless first neutralized, EDTA should not be applied to the eyes, because the solutions are alkaline enough to be injurious to the eye (Grant and Schuman 1993). Disodium EDTA has been used therapeutically on the cornea for decontaminating the eye after alkaline splashes and removal of superficial calcific opacities that occur in band keratopathy. EDTA is a common component in many eye drops and contact lens wetting and cleansing solutions (WHO 1998). In normal human eyes, a near neutral 0.1% solution of disodium EDTA applied as eyedrops or as an eye bath causes only mild stinging sensation (Grant and Schuman 1993).
Sensitization
EDTA is not found sensitizing in guinea pig maximization tests (Fisher 1986; IUCLID 2000).
Mutagenicity and carcinogenicity
Trisodium EDTA was tested for its mutagenic potential in Salmonella typhimurium strains and Escherichia coli in laboratories, and no mutagenicity was observed either with or without the S9 metabolic activation system (Dunkel et al. 1985). Trisodium EDTA was tested for its mutagenic potential in the mouse lymphoma cell forward mutation assay, with and without S9 metabolic activation system. No mutagenicity was observed (McGregor et al. 1988).
EDTA induced an increased mutant frequency in a mouse lymphoma assay without metabolic activation at concentrations of 25 and 30 mmol/l. Whether the mutagenic activity of EDTA was due to pH effects which has been suggested earlier is unclear (Wangenheim and Bolcfoldi 1988).
EDTA disodium salt administered 186 mg/kg body weight in mice showed no effects of inducing chromosomal aberrations in mouse spermatogonia, but induced micronuclei in the mouse after treatment of germ cells at the late stages of meiosis (Russo and Lewis 1992).
Zordan et al. (1990) investigated the genetic effects of EDTA disodium salt in the germ cells and the somatic cells in Drosophila melanogaster and mouse. The dosages were 93 and 186 mg/kg body weight. No increase in aneuploidy incidence was seen in bone marrow cells of the mouse and EDTA did not induce increased aneuploidy in spermatocytes of mouse either. EDTA induced aneuploidy in the germ cells of Drosophila but was negative in the somatic cells of Drosophila.
In mouse lymphoma cells DNA-strand breaks were measured in vitro without metabolic activation. There was a clear evidence of DNA-damaging activity in high concentrations from 40 mmol/l (Garberg et al. 1998).
In the alkaline elution assay EDTA disodium salt in a concentration of 30 mmol/l, with and without metabolic activation, showed no mutagenic activity (Swenberg et al. 1976).
EDTA disodium salt was studied in mice for mutagenic activity in a bone marrow micronucleus assay, a dominent lethal assay and in the incidence of spermhead abnormalities. The doses ((5-20 mg/kg body weight) were given orally. EDTA disodium salt induced a dose dependent increase in the incidence of micronucleated polychromatic erythrocytes, but no mutations in the dominant lethat assay and no increase in the spermhead abnormalities were seen (Muralidhara and Narasimhamurthy 1991).
Contrasting results are thus obtained concerning the mutagenicity of EDTA. Additional evaluation may be considered.
Reproductive toxicity
EDTA and its salt were studied for teratogenic potential in rats. The equimolar dose of 1,000 mg/kg body weight was given by gastric intubation twice daily on day 7 to 14 of gestation. No teratogenic effects occurred with any of the compounds even at maternally toxic doses (Shardein et al. 1981). Disodium EDTA was given to pregnant rats on day 7 to 14 of gestation by gavage (954 mg/kg body weight/day) and by subcutanous injection (375 mg/kg body weight/day). Disodium EDTA in the diet resulted in severe maternal toxicity and malformations in 71% of the offspring. Disodium EDTA given by gastric intubation (1,250 mg/kg/day or 1,500 mg/kg/day) was much more toxic to the dams. 87.5% maternal deaths but fewer malformed offspring. Disodium EDTA given subcutaneously was lethal to 24% of the dams at a much lower dose than given by either oral route, but did not produce a significant number of malformations in the offspring. For subcutaneous absorption the compound might not reach the embryo in concentrations high enough to produce malformations. A greater absorption of dietary EDTA into the circulation would correlate with the large increase in malformations following this route of administration. The route of administration had significant effect on the toxicity and teratogenicity (Kimmel 1977). It was suggested that the teratogenic effects of EDTA given to rats at very high levels were due to zinc deficiency. The binding of EDTA to zinc may be the most important interaction during pregnancy in that the developing embryo is extremely sensitive to zinc deficiency. Teratogenicity could be prevented by zinc diet supplement (Swenerton and Hurley 1971; Wolf and Gilbert 1992; WHO 1998).
EDTA salts are considered, by the Danish Labour Inspection Service, as a suspected reproductive toxicant at medium dose, meaning 20 to 200 mg/kg body weight (Arbejdsmiljøinstituttet 1990b).
Classification
EDTA is irritating to the eyes. The teratogenic potential of EDTA and its salts has been investigated but with variable results. EDTA and salts have been shown to be teratogenic after oral administration in rats. EDTA is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC. BASF classify EDTA, tetrasodium salt as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed) and R36 (Irritating to the eyes) (BASF 1999). No data showed ability to induce sensitisation to human skin, but EDTA disodium salt is listed as a potential contact allergen by the Danish Labour Inspection Service (Arbejdsmiljøinstituttet 1990a).
7.7 Trisodiumnitrilotriacetate (NTA)
Nitrilotriacetate, NTA, (CAS No. 139-13-9) is an organic compound belonging to the group of amino carboxylic acids, which have strong chelating capacity. Chelating agents react with polyvalent metal ions to form one or more ring structures. NTA acts by sequestering metal ions and is very effective in removing both calcium and magnesium from wash waters. In terms of washing performance NTA can largely replace phosphates (Perry 1981). However, NTA has received considerable attention primarily due to its demonstrated carcinogenicity and heavy metal chelating properties.
7.7.1 Environmental fate
The strong complexing capacity of NTA is expected to have adverse effects upon heavy metal removal during sewage treatment and upon mobilisation of metals from sediments in receiving waters. Several investigations have shown that the presence of NTA in water/sediment systems increases the concentration of heavy metals in the water phase (Perry et al. 1984; Garnett et al. 1986; Dehnad and Radeke 1993). However, these experiments have usually been performed with a sediment water suspension. The fact that the mobilisation of metals from stable sediments into the water phase depends on the diffusion rate has not been taken into consideration. The diffusion from stable sediments is slow and several days are normally required before a steady state is achieved (Källqvist). An experiment with stable artificial sediment (kaolinite) indicated that high concentrations of NTA remobilized Zn and it was concluded that NTA concentrations above 200 m g/l might mobilise heavy metals from stable sediments (Bernhardt 1991, cited in Källqvist). A continuous exposure with NTA may enhance the risk of metal remobilization although the low diffusion of metals from the sediment reduces the transport of metals (Källqvist).
Aerobic biodegradabilty
NTA is known to be aerobically biodegradable by acclimated microorganisms. Biodegradability tests with NTA have been inconsistent; 90% degradation has been reported after 9 and 13 days in tests with activated sludge, while degradation attained only 20% in a CO2 evolution test after 28 days and did not occur in shake flask and BOD tests (Perry et al. 1984). Following a period of acclimatisation, almost complete biodegradation has been reported for the activated sludge process when operated under optimum conditions. The efficiency of NTA removal during biological sewage treatment and the period of acclimatisation prior to NTA biodegradation has shown to be affected by factors like, e.g., the concentration of heavy metals, treatment temperature, NTA concentration and water hardness (Perry et al. 1984).
Anaerobic biodegradability
The removal of NTA during anaerobic sludge digestion has been found to be variable and affected by operational characteristics. E.g., studies indicating no removal and up to 29-45% removal in digesters receiving co-settled primary and activated sludge over a period of 120 days have been reported (Perry et al. 1984).
7.7.2 Effects on the aquatic environment
The toxicity of NTA towards algae, crustaceans and fish is low with EC/LC50 values well above 100 mg/l (Table 7.8).
Table 7.8
Effects of NTA towards aquatic organisms.
Species |
EC50/LC50 (mg/l) |
Test duration |
Reference |
Microcystis aeruginosa |
180-320 |
96 h |
Canton and Sloof 1982 |
Daphnia magna |
560-1,000 150; NOEC:100 |
48 h |
Canton and Sloof 1982 |
Fathead minnow (Pimephales promelas) |
NOEC: 53.9 |
Full life cycle |
Arthur et al. 1974 |
Guppy (Poecilia reticulata) |
560-1,000 |
96 h |
Canton and Sloof 1982 |
7.7.3 Effects on human health
Toxicokinetics and acute toxicity
Na3NTA is poorly absorbed from the gastrointestinal tract in humans. When absorbed the compound is rapidly excreted in the urine. About 87% of the absorbed dose were excreted within the first 24 h post dosing. NTA is not biotransformed and is excreted almost entirely unchanged in urine (Budny and Arnold 1973).
14C-labelled NTA was given intravenously and by stomach intubation to mice and the distribution was studied with autoradiography. Up to 48 hours after dosing a high concentration of radioactivity in the skeleton was seen. NTA has a preference for bone where it forms complexes with divalent cations such as calcium. In addition to the skeleton, a high concentration of radioactivity was seen in the kidney and the urinary bladder up to 8 hours after injection (Tjälve 1972).
The absorption, distribution and metabolic excretion of NTA in mice were determined by oral administration. Excretion of 14C-labelled NTA after a single oral dose showed that 99% of the dose was eliminated within 24 h. About 96% in the urine and the rest in faeces. NTA was readily absorbed from the gastrointestinal tract of the mice and was rapidly distributed into all tissues with highest concentrations in the bladder, kidney and bone. Elimination of NTA from the skeletal tissue was also rapid – after 8 hours no detectable radioactivity was left. This indicates no serious accumulation in the bone (Chu et al. 1978). NTA is poorly absorbed in humans compared with experimental animals. The absorption through skin is minimal. Less than 0.1% of dermal doses are absorbed (Anderson and Alden 1989).
The acute toxicity of NTA and its salts in animals are relatively low. The acute toxicity (LD50 values) of NTA are given in Table 7.9.
Table 7.9
Acute Toxicity (LD50) of NTA and its salts.
Type |
Species |
Route of admini- |
LD50 |
Reference |
NTA |
Rat |
Oral |
1,470 |
Richardson 1992-1994 |
NTA |
Mice |
Oral |
3,160 |
Richardson 1992-1994 |
NTA, trisodium salt |
Rat |
Oral |
1,900 |
Anderson et al. 1985 |
NTA, trisodium salt (40%) |
Rat |
Oral |
2,330 |
Nixon 1971 |
NTA, disodium salt (30%) |
Rat |
Oral |
1,460 |
Nixon 1971 |
Skin and eye irritation
NTA is a skin irritant. The degree depends on the neutralization (Richardson 1992-1994). A 20% solution of Na3NTA was not skin irritating in a patch test on 66 persons (Nixon 1971). NTA is a mild eye irritant (Grant and Schuman 1993).
Dermal exposure to NTA does not cause sensitization (Anderson and Alden 1989). A 20% solution of Na3NTA was not allergenic in a patch test on 66 persons (Nixon 1971).
Subchronic toxicity
Rats fed for 90 days with diets containing 2,000 ppm (0.2 g/kg bw/day) Na3NTA and no effects were observed. Rats fed a diet containing 20,000 ppm (2 g/kg bw/day) had abnormal kidneys and a significant decrease in weight gain with a corresponding increase in organ/body weight ratios (liver and kidney) (Nixon 1971).
Mutagenicity and Carcinogenicity
NTA induces tumours only after prolonged exposure to higher doses than those producing kidney toxicity. The reported induction of tumours in rodents is considered to be due to cytotoxicity resulting from the chelation of divalent cationics such as zinc and calcium in the urinary tract (WHO 1996). Dosages of NTA that do not alter Zn or Ca distribution do not produce any urinary tract toxicity even after chronic exposure. When toxic doses are supplied chronically some of the severely damaged tissues may develop tumours (Anderson et al. 1985). Rats were given 0.1% NTA trisodium salt in drinking water for 2 years. The exposed animals showed an increase in hyperplasia and tumourigenesis in the kidney (Goyer et al. 1981). Nitrilotriacetic acid and nitrilotriacetic acid, trisodium salt were tested for carcinogenicity in mice and rats by oral administration and induced tumours of the urinary system (kidney, ureter and bladder). The monohydrate administered in the diet induced malignant tumours of the urinary system. When administered in drinking water to rats, it induced renal adenomas and adenocarcinomas (IARC 1990).
The International Agency for Research on Cancer (IARC) has evaluated that there is sufficient evidence for the carcinogenicity of NTA and its sodium salts in experimental animals and the overall evaluation is that nitriloacetic acid and its salt are possibly carcinogenic to humans. IARC has placed NTA in Group 2B (IARC 1990).
The potential of NTA to cause chromosome abnormalities was investigated in cell culturs (human lymphocytes and Chinese hamster ovary cells) and in vivo in mice (micronucleus test). NTA was not found mutagenic in any of the three test assays (Monaldi et al. 1988; Loveday et al. 1989).
Reproductive toxicity
The effect on reproduction and development of Na3NTA in the diet was studied in rats for two generations and in rabbits during a single pregnancy. Na3NTA was fed to rats either continuously or only during organogenesis (from day 6 to 15) in each pregnancy at one or two dietary levels, 0.1 and 0.5%. For the rabbits doses of 2.5, 25, 100 and 250 mg Na3NTA/kg body weight were given by stomach tube during organogenesis (on day 7 to 16 of pregnancy). Na3NTA caused no effects on reproduction or embryonic development in either rats or rabbits. The only effects of Na3NTA on the rats were some growth depression in both adults and wealing animals fed 0.5% (Nolen et al. 1971). Pregnant mice were given 0.2% NTA in the drinking water from day 6 to 18 of pregnancy. The fetuses were examined for malformations. Skeletal or visceral examination did not reveal any teratogenic effects, although NTA also accumulated in the foetal skeleton (Tjälve 1972).
NTA was not found teratogenic in the frog embryo teratogenicity assay (Dawson et al. 1989).
Exposure to nitrilotriacetic acid, and presumably also to its water-soluble metal complexes, occurs as a result of its presence in household detergents and in drinking water. Little information on the toxicity of NTA in humans is available. The kidney is the primary target for NTA toxicity in animals. There is a clear evidence of carcinogenicity in rats and mice, causing kidney, bladder and urinary tract tumours in high doses and after long-term exposure. No human carcinogenic data are available. There is no evidence of teratogenicity and mutagenicity. The mechanism of the toxicity can be partly explained by chelation of essential divalent metal ions such as Ca++, Mg++ and Zn++.
Classification
Nitrilotriacetic acid with sodium salts is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC. Sodium salts of nitrilotriacetic acid are included in the list of carcinogenic components of the Executive Order on precautions to prevent cancer risk issued by the National Working Environment Authority (Executive Order 1999).
BASF classify NTA as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed) and R36 (Irritating to the eyes) (BASF 1999).
8. Preservatives
Preservatives are chemical agents that prevent growth of microorganisms in the product,
thereby rendering it safe in use and increasing its shelf life. Microorganisms may cause
several problems as, e.g., undesired visible growth or chemical changes of the product.
Contamination by microorganisms may also be associated with health hazards. Preservatives
are typically used in liquid products that do not have extreme pH values or a high
concentration of surfactants. E.g., products with a pH between 3 and 10 generally require
preservative(s) to avoid growth. As a group, preservatives consist of many structurally
different substances.
8.1 Isothiazolinones
Isothizolinones are used in household detergents and cosmetic detergent products. The most frequently used are 2-methyl-4-isothiazolin-3-one (MI) with the CAS No. 2682-20-4 and 5-chloro-2-methyl-4-isothiazolin-3-one (CMI) with the CAS No. 26172-55-4. These two substances are used as a mixture in the preservative product with the commercial name Kathon.
8.1.1 Kathon
Kathon (CAS No. 55965-84-9) is a commercial mixture of MI and CMI in the ratio 1:3. In cosmetic products the maximum allowed concentration is 15 ppm of the mixture of MI and CMI (Directive 97/18/EC and Directive 98/16/EC). The products may include water at levels more than 75% and various kinds of salts, e.g. magnesium salts. Examples of commercial products are Kathon CG (cosmetic grade): 0.35% MI and 1.15% CMI = 1.5% active ingredients + magnesium salts, and Kathon 886: 3.8% MI and 10.1% CMI = 13.9% active ingredients.
Ecotoxicology
The primary aerobic biodegradability of MI has been examined in a river sediment-water system by use of 14C-labelled model compound. During the 7-day experiment 14C-labelled
Primary aerobic biodegradability
MI (1 m g/g) was rapidly transformed as only 12.6% of the initial MI was present after 24 hours of incubation at 25° C. The calculated half-life for the parent compound was 9.1 hours (Reynolds 1994a). MI was transformed to several unidentified metabolites. One of the major metabolites reached a level corresponding to 18.2% of the 14C added after 24 hours of incubation. This metabolite decreased to 6.8% after 7 days which indicates further transformation. Other metabolites tended to increase during the 7-day experiment. At the end of the experiment metabolites that were bound in the sediment corresponded to 55%, whereas 14CO2 attained 9% of the added 14C. Most of the formed metabolites have shorter chromatographic retention times than MI which indicates that they are polar compunds. On the basis of the identification of metabolites from transformation of 4,5-dichloro-2-(n-octyl)-4-isothiazolin-3-one, it has been proposed that MI is transformed via N-methyl malonamic acid, N-methyl acetamide, and malonic acid (Madsen 2000).
The primary biodegradability of CMI has been examined with the same type of sediment and water as described for MI. The 14C-labelled CMI (1 m g/g) was rapidly transformed as only 30% of the initial CMI remained after 24 hours of incubation at 25° C. The calculated half-life for the intact CMI was 17.3 hours (Reynolds 1994b). At the end of the 7-day experiment, the sediment bound metabolites corresponded to 57.1% of the added 14C whereas 2.8% of the added 14C was released as 14CO2. Due to the structural similarities of MI and CMI, it is suggested that the major metabolites for the transformation of CMI are the same as described for MI.
Ultimate aerobic biodegradability
The ultimate aerobic biodegradability of MI has been examined in a CO2 evolution test (OECD 301B) which was modified for low concentrations of 14C-labelled compounds (Bashir 1998a). MI was added at initial concentrations of 0.1, 0.03, and 0.01 mg/l. The duration of the test was 29 days and the test was performed at 22° C. At the end of the test the accumulated 14CO2 attained 54.1%, 55.8%, and 47.6% in the respective concentrations (0.1, 0.03 and 0.01 mg/l). During the 10-day window 37%, 30% and 30%, respectively, of the initial MI was mineralized to 14CO2.
The ultimate biodegradability of CMI was examined in the CO2 evolution test (OECD 301B) under the same conditions as described for MI (Bashir 1998b). CMI was added at initial concentrations of 0.3, 0.1, and 0.03 mg/l. The 14CO2 formed from the mineralization of CMI during 29 days reached 38.8%, 55.3%, and 62% of the added 14C in the respective concentrations (0.3, 0.1, and 0.03 mg/l). The percentages of 14CO2 attained within the 10-day window were 25%, 40%, and 48% of the added 14C-activity. The ultimate biodegradability of CMI exceeded the 60%-pass level for ready biodegradability at the lowest test concentration of 0.03 mg/l, but the pass level was not reached within the 10-day window.
Anaerobic biodegradability
The biodegradability of 14C-labelled CMI has been examined under anoxic conditions in a system containing river sediment and water (Liu and Reynolds 1994). During the incubation at 25° C the evolved 14CO2 increased to 16.6% and 55.7% of the added 14C after 30 and 365 days, respectively. The half-life of the parent CMI was calculated to 4.6 h. The level of accumulated 14CO2 from the mineralization of CMI demonstrates that the isothiazolone ring was cleaved and that the metabolites were further oxidized. On the basis of the observed mineralization of CMI and the fate of 14C residuals it has been proposed that the anaerobic degradation of CMI leads to the same type of metabolites as proposed for aerobic degradation of MI and CMI (Liu and Reynolds 1994).
Bioaccumulation
The high water-solubility and the low log Kow values determined for MI and CMI (0.4 and -0.5, respectively) indicate a low potential for bioaccumulation of both substances. Studies of the bioaccumulation of CMI in bluegill sunfish (Lepomis macrochirus) showed BCF values of 102, 114, and 67 at nominal concentrations of 0.02, 0.12, and 0.8 mg/l (Erikson et al. 1995). These BCF values are based on total accumulated 14C and include both the parent compound and metabolites. The BCF for MI has been determined to 2.3 at a nominal concentration of 0.12 mg/l (Erikson et al. 1995).
Aquatic toxicity
The toxicity of the formulated product (Kathon) has been investigated towards different aquatic organisms and for all species investigated EC/LC50 values were well below 1 mg/l (Table 8.1).
Table 8.1
Effects of Kathon (MI: CMI, 1:3) to aquatic organisms. Data are obtained from From (1996).
Species |
EC50/LC50 (mg/l) |
Test duration |
Selenastrum capricornutum |
0.003 |
Not indicated |
Daphnia magna |
0.16 |
48 h |
Eastern oyster (Crassostrea virginica) |
0.028 |
48 h |
Bay mussel (embryo/larvae) |
0.014 |
48 h |
Rainbow trout (Salmo gairdneri) |
0.19 |
96 h |
Sheepshead minnow (Cyprinodon variegatus) |
0.3 |
96 h |
Bluegill sunfish (Lepomis macrochirus) |
0.28 |
96 h |
The aquatic toxicity of the proposed metabolites N-methyl malonamic acid, malonamic acid,
and N-methyl acetamide for the transformation of both MI and CMI has not been determined.
However, QSAR estimates of the baseline toxicity (based on the lipophilicity of the
substances) have shown that the EC/LC50 values are well above 100 mg/l for all the
proposed metabolites (Madsen 2000). N-methyl malonamic acid and malonamic acid are
structually related to the more lipophilic N-(n-octyl) malonamic acid for which the
aquatic toxicity has been examined. The 96 h-LC50 of N-(n-octyl) malonamic acid to rainbow
trout (Oncorhynchus mykiss) was determined to 250 mg/l and the NOEC to 160 mg/l. A
similar toxicity was seen for Daphnia magna, as the 48 h-EC50 was reported to be in
the range of 90-160 mg/l (Madsen et al. 2000).
Interpretation of biodegradability and toxicity
Both MI and CMI inhibit the inoculum in biodegradability screening tests which implies that the conditions are very unfavourable in tests aiming at determing the ready biodegradability, even when low contrations are used. MI and CMI may thus be regarded as candidates for an assessment of other available "convincing scientific evidence" to demonstrate that the substances can be degraded (biotically and/or abiotically) in the aquatic environment to a level of > 70% within a 28-day period". Primary biodegradation of MI and CMI occurred with half-lives of less than 24 hours in aerobic and anoxic sediments, and within a period of less than one week the parent compounds were depleted to very low levels that could not be clearly distinguished from analytical artefacts. The ultimate aerobic biodegradability of both MI and CMI attained levels of > 55% within 29 days. Furthermore, the proposed metabolites of MI and CMI are considered to have a low aquatic toxicity on the basis of QSAR estimates and the measured toxicity of the structurally related N-(n-octyl) malonamic acid.
Human health
As it is Kathon in the MI/CMI ratio of 1:3 which is used in cleaning agents and cosmetics it is this mixture which is assessed in the human health and hazard assessment. Most studies have been carried out with the commercial mixture and not with the pure isothiazolones.
Toxicokinetics and acute toxicity
After oral administration of Kathon 886 to rats, the majority of MI and CMI was readily excreted in the urine or faeces while storage in the tissues was minimal. Up to 62% of a single percutaneous dose was bound to the site of application 24 hours after exposure (CIRP 1992). N-methyl malonamic acid was detected as the main metabolite in the urine of rats given oral doses of either of the two isothiazolones. Malonamic acid and malonic acid were also identified as metabolites (DFG 1993). Kathon 886 was rapidly distributed to the blood, liver, kidneys, and testes after an intravenous dose (0.8 mg/kg body weight). The chlorinated compound was 14C-labelled and after 24 hours more than 50% of the administered radioactivity had been excreted in the faeces and urine, after 96 hours about 70% (faeces 35%, urine 31%, and CO2 4%) was excreted (Debethizy et al. 1986).
The half-life of dermally absorbed compounds was found to be 13.1 day. This suggests an increased potential for accumulation on the skin with repeated application or use (Connor et al. 1996).
Isothiazolinones are moderately to highly toxic by oral administration. The major signs of toxicity were severe gastric irritation, lethargy, and ataxia (CIRP 1992) (Table 8.2).
Table 8.2
Acute toxicity (LD50) of isothiazolinones.
Types |
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
CMI |
Rat/mouse |
Oral |
53 – 60 |
DFG 1993 |
Kathon CG |
Rat |
Oral |
3350 |
CIRP 1992 |
CMI |
Rabbit |
Dermal |
80 |
DFG 1993 |
Skin and eye irritation
Aqueous dilutions of Kathon 886 were tested for skin irritation in rabbits. A concentration of 0.056% a.i. was non-irritating, and 5.65% a.i. was corrosive. Kathon CG with an a.i. concentration of 1.5% was severely irritating (CIRP 1992). Solutions which contain more than 0.5% (5000 ppm/active isothiazolones) produce severe irritation of human skin and can cause corrosion of mucous membranes and the cornea. Solutions containing > 100 ppm active isothiazolones can irritate the skin (DFG 1993). Kathon 886 with concentrations of 0.056% a.i. was non-irritating to the eye. Conc of 2.8% and 5.65% a.i. were severely irritating (corrosive) to the eye. Kathon CG with a 1.5% a.i. concentration were corrosive to the eye (CIRP 1992). Instillation of 0.1ml of an aqueous solution containing 560 ppm isothiazolones into the rabbit eye did not produce irritation. Higher concentration caused dose-dependent mild to severe irritation. After instillation into the rabbit eye of a single dose of undiluted Kathon 886 containing 13.9% active ingredients, clouding of the cornea, chemosis, and swelling of the eyelids were observed (DFG 1993).
Sensitization
The sensitization potential of Kathon CG and Kathon 886 in humans has been studied extensively. There is general agreement among investigators that Kathon CG is a sensitizer (Björkner et al. 1986; Bruze 1987a; Gruvberger 1997). It is primarily CMI which is the sensitizing substance (strong sensitizer) in the product but 2-methyl-4-isothiazolin-3-one also has sensitizing properties (weak sensitizer – moderate allergen) (Bruze 1987a; Gruvberger 1997). Kathon CG is a part of the standard test series at skin clinics. The risk of sensitization depends on how contact with the product occurs. The risk is greater when the skin barrier has been damaged and smaller when the skin is healthy. The sensitizing capacity of the preservatives Kathon CG has been established in both humans and guinea pigs (Bruze 1987b).
Several reports on occupational allergic contact dermatitis from MI and CMI have been published (Gruvberger 1997). A large number of patients (8,521) were tested from 1985-1997 for contact allergy to antimicrobials. The MI/CMI mixture was the most common antimicrobial allergen (Goossens et al. 1997). A high frequency (17.6%) of contact allergy to MI and CMI was demonstrated in 51 production workers in a factory handling preservatives with high concentrations of MI and CMI. Four of the workers sensitized to Kathon CG suffered from chemical burns caused by preservatives with high corrosive concentration of MI and CMI (Gruvberger 1997). Dermatological studies have demonstrated that isothiazolone concentrations below 20 ppm may cause sensitization and that allergic reactions can be provoked in sensitized persons even with concentrations in the range of 7-15 ppm active isothiazolones (DFG 1993). Sodium bisulfite and glutathione, (Gruvberger1997), can chemically inactivate MI and CMI. A review of studies of MI/CMI allergic contact potential indicate that the actual sensitization rate observed is extremely dependent on dose and type of exposure. This review of data leads to the conclusion that, under normal use conditions, within the current permitted/recommended use concentration for MI/CMI (up to 15 ppm), the risk of primary sensitization is negligible (Fewings and Menné 1999).
Subchronic toxicity
Kathon 886 administrated in the drinking water to rats for three months produced slight gastric irritation at a dose of 20 mg/kg/day; the no effects level (NOEL) was 8 mg/kg/day. Dermal application of Kathon 886 at doses up to 0.4 mg/kg/day for three months produced no systemic toxicity in rabbits (CIRP 1992).
Mutagenicity and carcinogenicity
Kathon CG and Kathon 886 have been evaluated in a number of mutagenicity assays. Although there have been conflicting reports in the literature, it has been reported by several investigators that these biocides are mutagenic in Salmonella typhimurium strains (Ames test) (Monte et al. 1983; Wright et al. 1983; Connor et al. 1996). Negative results were obtained in studies of the DNA-damaging potential of Kathon in mammalian cells in vitro and of cytogenetic effects and DNA-binding in vivo (DFG 1993). The addition of rat liver S-9 (metabolic activation) reduced toxicity but did not eliminate mutagenicity. The compounds bind to the proteins in the S-9. At higher concentrations of Kathon the increase in mutagenicity may be due to an excess of unbound active compounds (Connor et al. 1996).
A study of cutaneous application of Kathon CG in 30 months, three times per week at a concentration of 400 ppm (0.04%) a.i. had no local or systemic tumorigenic effect in male mice. No dermal or systemic carcinogenic potential was observed (Scribner et al. 1983; CIRP 1992; DFG 1993).
Reprooductive toxicity
No adverse effects on fertility, reproduction, fetal survival, or fetal health were observed in rats administrated > 20 mg/kg/day Kathon 886 in the drinking water for 15 weeks prior to mating (CIRP 1992). Reproduction and teratogenicity studies with rats, given isothiazolone doses of 1.4-14 mg/kg/day orally from day 6 to day 15 of gestation, showed no treatment related effects in either the dams or in the foetuses (DFG 1993).
Classification
MI and CMI are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
Table 8.3
Classification of the MI: CMI mixture in the ratio 1:3 (Rohm and Hass 1998).
Concentration of the mixture (a.i) |
Classification symbols |
Risk phrases |
> 25% |
T,C,N |
23/24/25,34,43,50 |
3 – 25% |
C,Xn |
34,20/21/22,43 |
0.6 – 3% |
C |
34,43 |
0.06 – 0.6% |
Xi |
36/38,43 |
50ppm – 0.06% In DK and S: > 15ppm |
Xi Xi |
43 43 |
R23/24/25: Toxic by inhalation, in contact with skin and if swallowed.
R34: Causes burns
R43: May cause sensitisation by skin contact.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
R36/38: Irritating to eyes and skin.
The highest allowed concentration of Kathon in cosmetics is 15 ppm according to the cosmetic directive (Cosmetic Directive 2000).
8.1.2 1,2-Benzisothiazolin-3-one
Ecotoxicology
1,2-Benzisothiazolin-3-one (CAS No. 2634-33-5) is used in specialized cleaning agents, although it is used less frequently than Kathon.
There are no experimental data available regarding the biodegradability and bioaccumulation of 1,2-benzisothiazol-3-one. However, QSAR calculations indicate a high probability of aerobic biodegradation and a low potential for bioaccumulation in aquatic organisms (log Kow = 0.64) (EPIWIN 1994).
Data describing the acute toxicity of 2-benzisothiazolin-3-one towards algae, crustaceans and fish are given in Table 8.4.
Table 8.4
Effects of 2-benzisothiazolin-3-one to aquatic organisms (data obtained from Biochema
Schwaben 2000).
Species |
EC50/LC50 |
Test duration |
Green algae (species not indicated) |
0.15 |
72 h |
Daphnia magna |
1.35 |
48 h |
Rainbow trout (Salmo gairdneri) |
1.6 |
96 h |
Bluegill sunfish (Lepomis macrochirus) |
5.9 |
96 h |
Human health
1,2-Benzisothiazolin-3-one is rapidly and totally metabolized in animals. Neither the substance itself nor the metabolites accumulate in the liver or adipose tissue. Excretion is mostly via the kidneys and almost completely within 24 hours. The main metabolites are o-methylsulphonylbenzamide and o-methylsulphinylbenzamide. Rats excreted 96% of an oral dose of 1,2-benzisothiazolin-3-one within 5 days (DFG 1989).
Toxicokinetics and acute toxicity
1,2-Benzisothiazolin-3-one has a relatively low toxicity by oral administration (Table 8.5).
Table 8.5
Acute toxicity (LD50) of 1,2-benzisothiazolin-3-one.
Species |
Route |
LD50 (mg/kg body weight) |
Reference |
Rat |
Oral |
1,020 |
Bertaccini et al. 1971 |
Mice |
Oral |
1,150 |
Bertaccini et al. 1971 |
Rat |
Oral (73.1% solution)* |
670 – 784 |
DFG 1989 |
Rat |
Dermal (73.1% solution)* |
> 2,000 |
DFG 1989 |
Rat |
Oral(100% solution)* |
900 – 1,200 |
DFG 1989 |
* A Proxel product.
Skin and eye irritation
1,2-Benzisothiazolin-3-one has strong irritating or corrosive properties in animals. These properties are related to its alkaline reaction in water solutions. Solutions of 1,2-benzisothiazolin-3-one (> 5%) in water have a pH of 10-12 (ICI 1990). A solution of 1% 1,2-benzisothiazolin-3-one has been reported to cause strong irritation of the guinea pig skin (Alomar et al. 1985). In routine patch testing 1% 1,2-benzisothiazolin-3-one in alcohol gave weak irritant reaction in 30% of a total of 404 patients tested (Andersen and Hamann 1984). Concentrations from 0.1% 1,2-benzisothiazolin-3-one have been found irritating to the skin in clinical studies of 56 subjects (Chew and Maibach 1997). 1,2-Benzisothiazolin-3-one in 0.08 and 0.16% aqueous solutions produced some irritant responses when patch tested on a group of 25 healthy volunteers (Damstra et al. 1992). In the rabbit eye 12.5% 1,2-benzisothiazolin-3-one was a strong and severe irritant (DFG 1989).
Sensitization
The allergenic potential of 1,2-benzisothiazolin-3-one has been assessed in very few animal studies, but there are numerous reports about humans being sensitized due to handling products containing small amounts of 1,2-benzisothiazolin-3-one. The sensitizing potential of 1,2-benzisothiazolin-3-one was evaluated using the guinea pig maximization test of Magnusson and Kligman and was found to be a week sensitizer. Three of 20 guinea pigs exhibited sensitizaton with 0.2% 1,2-benzisothiazolin-3-one in aqueous propylene glycol (Andersen and Hamann 1984). Using the murine local lymph node assay the lowest concentration at which 1,2-benzisothiazolin-3-one were able to induce a significant proliferative response was at 10% 1,2-benzisothiazolin-3-one. The murine local lymph node assay assesses contact sensitization potential by measuring T cell activation and, in particular, T lymphocyte proliferation in the lymph nodes (Botham et al. 1991). In several published case reports 1,2-benzisothiazolin-3-one has induced allergic dermatitis. The allergic effects appear from 0.01% 1,2-benzisothiazolin-3-one and have been confirmed in a series of patch test studies. The majority of cases are occupational exposure to 1,2-benzisothiazolin-3-one in cutting oils, paper, gum arabic, air fresheners, water softeners and paints (Freeman 1984; Alomar et al. 1985; DeBoer et al. 1989; Damstra et al. 1992; Diaz et al. 1992; Sanz-Gallen et al. 1992; Cooper and Shaw 1999). According to Hopkins (1994) 1,2-benzisothiazolin-3-one possesses a fairly high sensitizing potential in man and it is significantly more active in the workplace than in the test laboratory in guinea pigs or mice. One case story from a detergent formulation factory has been reported on occupational astma or rhinitis after exposure to 1,2-benzisothiazolin-3-one (Moscato et al. 1997).
Mutagenicity and carcinogenicity
1,2-Benzisothiazolin-3-one (94% solution) was nonmutagenic in an Ames test when tested in Salmonella strain TA98 (Riggin et al. 1983). In the in vivo micronucleus test, where a solution of 73.4% 1,2-benzisothiazolin-3-one was given orally to mice, no evidence of mutagenicity was observed. This method involves the use of polychromatic erythrocyte stem cells of mice. The bone marrow is collected and an increase in micronucleated cells over the controls is considered as a positive mutagenic effect (DGF 1989).
An UDS test (unscheduled DNA synthesis) in cultures of rat hepatocytes also gave no mutagenic effects at a concentration of 73.4% 1,2-benzisothiazolin-3-one. This test is a measure of DNA repair capability after direct damage to DNA (DGF 1989). Finally, the mouse lymphoma cell mutation test showed no mutagenic potential of 1,2-benzisothiazolin-3-one at a concentration of 73.1% 1,2-benzisothiazolin-3-one. This mutation assay is used to determine the ability of chemicals to cause gene mutations in cultured mamalian cells (DGF 1989).
Reproductive toxicity
Female rats were given a product containing 73.4% 1,2-benzisothiazolin-3-one by gavage from day 7 to 16 after mating in doses of 10, 40, or 1,009 mg product/kg/day. The rats were sacrificed shortly before expected day of delivery. The dose 40 mg/kg/day were neither embryotoxic, fetotoxic nor teratogenic. The dose 100 mg/kg/day was considered to be slightly fetotoxic because the fetuses were on average 4% lighter and that ossification was sometimes slightly delayed. No teratogenic effect at this concentration was seen but it caused moderate maternal toxicity (DFG 1989).
Classification
1,2-Benzisothiazolin-3-one is included in Annex 1 of list of dangerous substances of
Council Directive 67/548/EEC and classified as follows:
Harmful (Xn) with R22 (Harmful if swalloved) and Irritant (Xi) with R38 (Irritating to the
skin), R41 (Risk of serious damage to eyes), R43 (May cause sensitization by skin
contact), N; R50 (Very toxic to aquatic organisms).
C > 25%: Xn; R22-38-41-43
20% < C < 25%: Xi; R38-41-43
10% < C < 20%: Xi; R41-43
5% < C < 10%: Xi; R36 (Irritating to eyes)-43
0.05% < C < 5%: R43
1,2-Benzisothiazolin-3-one is not allowed as preservative in cosmetics according to the cosmetic directive (Cosmetic Directive 2000).
8.2 Parabens
The parabens are all esters of 4-hydroxybenzoic acid, only differing in the ester group, which may be a methyl-, an ethyl-, a propyl- or a butyl group giving methylparaben (CAS No. 99-76-3), ethylparaben (CAS No. 120-47-8), propylparaben (CAS No. 94-13-3), or butylparaben (CAS No. 94-26-8). The most frequently used parabens are methylparaben and propylparaben. Methylparaben is used as a preservative in foods, beverages and cosmetics. Propylparaben is used as a preservative in food and antifungal agents. In shampoos/conditioners methyl paraben is preferred, frequently in combination with propyl paraben and/or ethyl paraben. The concentration used is below 0.2% (Rastogi and Johansen 1993). Parabens are stable in acidic solutions. Hydrolysis occurs above pH 7. In strong alkaline solutions parabens hydrolyze to the corresponding carboxylic acid. As the carbon number of the alkyl chain increases, anti-microbial activity increases but water solubility decreases. The individual esters differ in their relative anti-microbial activities. For this reason, optimum effectiveness is usually obtained with combinations of two or more paraben esters (of different chain lengths).
Ecotoxicology
The tests that were conducted in the present study showed that methyl-, ethyl-, and propylparaben are readily biodegradable under aerobic conditions. The parabens were only partially degraded in anaerobic screening tests (ISO 11734) as illustrated by an ultimate biodegradability in the range of 18 to 40% of the theoretical gas production (Table 8.6). Of the three parabens examined, methylparaben attained the highest biodegradability in the anaerobic screening test. It is possible that the parabens inhibit the anaerobic bacteria at the applied test concentration (20 mg of C/l) and that ethyl- and propylparaben were more toxic than methylparaben. The potential for bioaccumulation is low to moderate as judged from the QSAR estimated log Kow values that range between 1.96 and 3.57 (Table 8.6).
Table 8.6
Biodegradability and potential bioaccumulation of methyl-, ethyl-, propyl-, and
butylparaben.
Parameter |
Method |
Results |
Data source |
Methylparaben |
|
||
Aerobic biodegradability |
Manometric respirometry test, 28 d |
92% ThOD |
This study (Appendix; Table A4, Figure A4) |
Anaerobic biodegradability |
Measurement of gas production, 35o, 56 d ISO 11734 |
40% ThGP |
This study (Appendix; Table A16, Figure A16) |
Potential bioaccumulation |
QSAR log Kow |
1.96 |
EPIWIN 1994 |
Ethylparaben |
|
||
Aerobic biodegradability |
Manometric respirometry test, 28 d |
89% ThOD |
This study (Appendix; Table A5, Figure A5) |
Anaerobic biodegradability |
Measurement of gas production, 35o, 90 d ISO 11734 |
33% ThGP |
This study (Appendix; table A17, Figure A17) |
Potential bioaccumulation |
QSAR log Kow |
2.47 |
EPIWIN 1994 |
Propylparaben |
|
||
Aerobic biodegradability |
Manometric respirometry test, 28 d |
92% ThOD |
This study (Appendix; Table A6, Figure A6) |
Anaerobic biodegradability |
Measurement of gas production, 35o, 90 d ISO 11734 |
18% ThGP |
This study (Appendix; Table A18, Figure A18) |
Potential bioaccumulation |
QSAR log Kow |
3.04 |
EPIWIN 1994 |
Butylparaben |
|
||
Potential bioaccumulation |
QSAR log Kow |
3.57 |
EPIWIN 1994 |
The data in Table 8.7 indicate that the toxicity of parabens towards aquatic organisms is
low.
Table 8.7
Effects of methyl-, ethyl-, and propylparaben to aquatic organisms.
Species |
EC50/LC50 (mg/l) |
Test duration |
Reference |
Methylparaben |
|||
Green alga (Pseudokirchneriella subcapitata) |
91 (90-93) |
72 h |
This study (Appendix; Table A20) |
Daphnia magna |
11.2 (5.7-22.0) |
48 h |
This study (Appendix; Table A20) |
Fish Golden orfe (Leuciscus idus) |
NOEC: 50 |
48 h |
Nipa 1991 |
Ethylparaben |
|||
Pseudokirchneriella subcapitata |
18 (17-19) |
72 h |
This study (Appendix; Table A20) |
Daphnia magna |
20-50 |
48 h |
This study (Appendix; Table A20) |
Golden orfe |
LC0: 20 |
96 h |
Nipa 1997 |
Propylparaben |
|||
Pseudokirchneriella subcapitata |
15 (15-16) |
72 h |
This study (Appendix; Table A20) |
| Daphnia magna | 15.4 (8.0-32.3) | 48 h | This study (Appendix; Table A20) |
| Golden orfe | NOEC: 5 | 96 h | Nipa 1992 |
Phenoxyethanol and benzyl alcohol
Phenoxyethanol (CAS No. 122-99-6) and benzyl alcohol (CAS No. 100-51-6) have some structural similarities with parabens. These preservatives are readily biodegradable and the few data indicate a low aquatic toxicity. Phenoxyethanol attained > 90% ThOD in a BOD test and the log Kow of 1.16 indicates that the substance is not expected to bioconcentrate in aquatic organisms. The toxicity of phenoxyethanol to fish has been determined in studies with fathead minnow (Pimephales promelas; LC50: 344 mg/l) and golden orfe (Idus idus melanotus; NOEC: 200 mg/l (Bayer 1997). Benzyl alcohol reached a level of more than 70% ThOD in a BOD test and has a log Kow of 1.1. The anaerobic biodegradability of a mixed product containing 55-80% benzyl alcohol, 15-30% methylparaben, and 5-15% propylparaben attained 66% of ThGP in the ISO 11734 screening test after 56 days (Appendix; Table A19, Figure A19). The aquatic toxicity of benzyl alcohol has been determined in test with Daphnia magna (24 h-EC50: 55 mg/l) and fish (Idus idus melanotus; LC50: 646 mg/l) (CETOX 2000).
Endocrine disrupting effects
The estrogenic effects of parabens have been investigated in juvenile rainbow trout (Oncorhynchus mykiss) where the induction of yolk protein (vitellogenin) was used as an estrogen-specific endpoint after repeated injections of the parabens (ethyl-, propyl-, and butylparaben (Petersen et al., in press). All of the tested parabens showed estrogenic activity in doses between 100 and 300 mg/kg with propyl- and butylparaben being the most active. The major metabolite of the parabens, p-hydroxybenzoic acid, was tested as well but showed no estrogenic activity.
In a receptor-binding assay, it was shown that butylparaben was able to compete with 3H-estradiol for binding to the rat estrogen receptor with an affinity approximately 5 orders of magnitude lower than that of diethylstilbestrol (DES) and between 1 and 2 orders of magnitude less than nonylphenol (Routledge et al. 1998). In an in vitro yeast-based estrogen assay, methyl-, ethyl-, propyl-, and butylparaben were all found to be weakly estrogenic with butylparaben as the most potent with an estrogenic activity which was 10,000 fold less than that of 17b -estradiol. Oral administration of parabens to immature rats showed no activity, however, subcutaneous administration of butylparaben produced a positive uterotrophic response in vivo 100,000 times less potent than 17b -estradiol. When parabens are applied to skin they are known to be metabolised by four carboxyl esterases capable of hydrolysing, the different parabens to p-hydroxybenzoic acid (Lobemeier et al. 1996). However, in vitro studies on penetration of rat skin by butylparaben and propylparaben have indicated that 4% of butylparaben and 30% of propylparaben were not hydrolysed (Bando et al. 1997).
If parabens in the concentrations used in household products and cosmetics will have endocrine disrupting effects in the environment or in humans is unknown. For such an evaluation the rapid biodegradation and metabolization of the parabens should be taken into account.
Human health
After oral administration parabens are quickly absorbed from the gastrointestinal tract. They are hydrolyzed to p-hydroxybenzoic acid, conjugated, and the conjugate is excreted in the urine. Parabens do not accumulate in the body. Most of an administered dose can be recovered within 5 to 72 hours as p-hydroxybenzoic acid or its conjugates (CIRP 1984; Rastogi and Johansen 1993). Propylparaben was readily hydrolysed when administered orally to dogs, with peak tissue concentration 6 hrs after administration. After 48 hrs the compound was completely eliminated. The hydrolyses occurs in the liver, kidney and muscle, but not in other tissues. The metabolites excreted were 4-hydroxybenzoic acid, 4-hydroxyhippuric acid, ester glucuronides and ester sulphates (Richardson 1992-1994). Parabens are rapidly absorbed through intact skin (CIRP 1984).
Toxicokinetiics and acute toxicity
The lower paraben homologues have minimal acute and chronic toxicity and are therefore cleared as human diet additives (WHO 1974; Clayton and Clayton 1993; Positivlisten 1998) (Table 8.8).
Table 8.8
Acute toxicity (LD50) of parabens.
Type |
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
Methylparaben |
Rat |
Oral |
> 5,600 |
CIRP 1984 |
Methylparaben |
Dog |
Oral |
3,000 |
Richardson 1992-1994 |
Methylparaben, sodium salt |
Mouse |
Oral |
2,000 |
WHO 1974 |
Methylparaben, sodium salt |
Mouse |
Subcutaneous |
1,200 |
Lewis 1996 |
Propylparaben |
Mouse |
Oral |
6,300 |
Lewis 1996 |
Propylparaben |
Dog, rabbit |
Oral |
6,000 |
Richardson 1992-1994 |
Propylparaben, sodium salt |
Mouse |
Oral |
3,700 |
WHO 1974 |
Propylparaben, sodium salt |
Mouse |
Subcutaneous |
1,650 |
Lewis 1996 |
Butylparaben |
Mouse |
Oral |
13,200 |
Lewis 1996 |
Ethylparaben |
Mouse |
Oral |
6,000 |
CIRP 1984 |
Ethylparaben |
Rat |
Oral |
4,300 |
CIRP 1984 |
Skin and eye irritation
The parabens have a low irritant potential (Clayton and Clayton 1993). The sodium salt, however, may be strongly alkaline and lead to severe irritation and corrosion damage. Undiluted methylparaben was tested with the Draize skin irritation technique using rabbits. Mild skin irritation was observed (CIRP 1984). A 5% concentration of butylparaben caused mild irritation in guinea pigs (Richardson 1992-1994). Pure methylparaben was slightly irritating when instilled into the eyes of rabbits (CIRP 1984).
Sensitization
Parabens are not strong sensitizers. The incidence of sensitivity induced primarily by parabens is extremely small (Cronin 1980). The skin allergenic qualities of parabens appear to be apparent primarily if they come into contact with damaged skin by e.g. eczema. Normal skin is affected to a lesser degree (Fisher 1986; Rastogi and Johansen 1993). Particularly medicinal liniments and creams preserved with parabens cause a certain frequency of contact eczema. This is due to the fact that the products are applied to damaged skin which is more vulnerable to sensitizing substances. However, the number of cases of contact allergic eczema in relation to the widespread exposure is low. Many paraben-sensitive individuals tolerate paraben-containing cosmetics provided the product is applied to normal skin not subjected to a dermatitis in the past. This is called the "paraben paradox" (Fisher 1979). Paraben hypersensitivity has been reported in a number of cases (Schamberg 1967; Henry et al. 1979; De Groot et al. 1986; Cooper and Shaw 1998; Carradorri et al. 1990).
In a period from 1985-1997, a total of 8,521 patients were tested in a contact allergy clinic. Anti-microbials were tested for allergic contact dermatitis and sensitivity to parabens had a frequency 0.8% and was thus the seventh most frequent anti-microbial allergen in this study (Goossens et al. 1997). In another study a paraben mixture in 5% petrolatum was used in a comparison between the frequency of sensitization in healthy subjects and in patients with dermatitis. In 2,150 patients, 1.01% were sensitized with the paraben-mix, and 0.67% of 593 healthy volunteers were sensitized (Seidenari et al. 1990).
Chronic toxicity
The chronic toxicity of methylparaben and propylparaben was tested in white rats. The rats were fed diets containing 2 and 8% each of methylparaben or propylparaben for 96 weeks. Only mild growth retardation was observed at 8% levels (Furia 1972). Dogs fed 700 mg propylparaben /kg body weight/day for 90 days suffered no ill effects. Growth retardation occurred when rats were fed 1,600 mg propylparaben/kg/day (Richardson 1992-1994).
Mutagenicity and carcinogenicity
Numerous in vitro mutagenicity studies indicate that parabens are non-mutagenic (CIRP 1984). Butyl paraben and ethylparaben were tested in Salmonella/microsome assay (Ames test) and chromosomal aberrations assay in vitro using a Chinese hamster fibroblast cell line. No mutagenic potential was observed in either of the tests (Ishidate et al. 1984). Methylparaben was tested for mutagenic activity in Salmonella typhimurium strains and was found negative (Prival et al. 1982).
No evidence of tumorigenic effects were seen in a 2 year study at doses up to 0.06% of butylparaben by oral administraton to mice (Inai et al. 1985).
Reproductive toxicity
Teratogenic studies on methylparaben were negative (CIRP 1984).
Classification
Parabens are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC. The highest allowed concentration of parabens in cosmetics is 0.4% for one type of paraben and 0.8% for paraben mixtures (Cosmetic Directive 2000).
8.3 Nitrosubstituted compounds
Two nitrosubstituted substances were included in this review: 2-bromo-2-nitropropane-1,3-diol (BNPD) with the CAS No. 52-51-7 and 5-bromo-5-nitro-1,3-dioxane (CAS No. 30007-47-7). Both preservatives are used in cosmetic products, liquid soaps and cleaning agents.
8.3.1 2-Bromo-2-nitropropane-1,3-diol (Bronopol)
The highest concentration allowed in cosmetics is 0.1%. Formation of nitrosamines in the presence of amines should be avoided (Cosmetic Directive 2000).
BNPD reacts with iron and aluminium with some loss of microbial activity. It is quite stable about pH 5.5 and can be used with good effect at low pH values. BNPD is one of the most frequently used preservatives in cosmetics and cleaning agents in concentrations of about 0.1% or less. It is a broad spectrum preservative with a wide range of antimicrobial properties. It is active against gram positive bacteria, gram negative bacteria, fungi and yeast, and has a special effect on Pseudomonas aeruginosa. The antibacterial activity of BNPD relates to its interaction with essential thiols within the cell. In the presence of air, BNPD acts as a catalyst for the oxidation of thiol-groups to disulfides, with the rapid consumption of oxygen (DFG 1989).
BNPD is a formaldehyde-releasing compound, also called a formaldehyde donor. In alkaline solution and at increasing temperature, it dissociates to form formaldehyde, bromide and nitrite. BNPD acts as an antibacterial and antifungal agent because of its intrinsic properties and not through release of formaldehyde. 0.02% BNPD in an emulsion has been reported to release up to 15 ppm formaldehyd (Storrs and Bell 1983; Ford and Beck 1986).
Ecotoxicology
Biodegradability
According to the OECD criteria BNPD is not readily biodegradable. BNDP was not readily biodegradable in a closed bottle test (OECD 301D) at concentrations of 3 and 6 mg/l (Knoll MicroCheck 1996). The low biodegradability is not unexpected as BNPD inhibits the inoculum at the applied concentration in standard biodegradability tests. 14C-Labelled BNPD at 1 mg/l was partially mineralized by an inoculum, which was probably a mixture of activated sludge and soil, as indicated by a 14CO2 evolution of approximately 40% during 17 days. At day 21 over 80% of the 14C was present either as CO2 or in the biomass. BNPD was completely transformed by day 3 and one major metabolite (probably trishydroxynitromethane or 2-nitropropane-1,3-diol) was formed. However, this substance was a transient metabolite as its concentration had decreased to negligible levels by day 17 (Knoll MicroCheck 1996). No evidence confirming an ultimate biodegradation of BNPD under anoxic conditions was found in the literature.
Bioaccumulation
No experimental data describing the bioaccumulation potential of BNPD were found in the literature. However, due to the low log Kow value of 0.18 (Knoll MicroCheck 1996), BNPD is unlikely to accumulate in aquatic organisms.
Aguatic toxicity
BNPD is very toxic to aquatic organism with effect concentrations below 1 mg/l for algae and crustaceans. BNPD was not particularly toxic towards the examined fish as indicated by LC50 values between 20 and 59 mg/l (Table 8.9).
Table 8.9
Effects of BNPD to aquatic organisms.
Species |
EC50/LC50 (mg/l) |
Test duration |
Reference |
Selenastrum capricornutum |
0.37 |
72 h |
Knoll MicroCheck 1996 |
Scenedesmus subspicatus |
> 1.0 |
72 h |
Knoll MicroCheck 1996 |
Chlorella vulgaris |
1.87 |
72 h |
Knoll MicroCheck 1996 |
Daphnia magna |
1.4 |
48 h |
Knoll MicroCheck 1996 |
Pacific oyster (Crassostrea gigas) |
0.78 |
48 h |
Office of Pesticide Programs 1995 |
Mysid (Mysidopsis bahia) |
0.59 |
96 h |
Office of Pesticide Programs 1995 |
Fish |
20-59 |
96 h |
Office of Pesticide Programs 1995 |
Rainbow trout (Salmo gairdneri) |
41.2 |
96 h |
Knoll MicroCheck 1996 |
Sheepshead minnow (Cyprinodon variegatus) |
58 |
96 h |
Knoll MicroCheck 1996 |
Bluegill sunfish (Lepomis macrochirus) |
35.7 |
96 h |
Knoll MicroCheck 1996 |
Human health
Toxicokinetics and acute toxicity
BNDP and its breakdown products administrated intravenously to rats and rabbits were excreted in the urine and expired air. BNPD did not accumulate in the organism. Metabolic breakdown products included 2-nitropropane-1,3-diol, which may be further metabolized to glycerol and CO2 (CIRP 1984a). When 14C –labelled BNPD was administered either orally or intravenously to rats a rapid elimination of radioactivity occurred from the body. 70-80% was excreted in urine and 6-10% in expired air during 24 hours. The highest concentration of radioactivity, 24 hours after the percutaneous application, was found in kidneys, liver and lung. No unchanged BNPD was detected in the urine samples examined. Within 24 hours approx 40% of topically applied dose of 14C-labelled BNPD was absorbed through the skin of rats. About 19% of the applied radioactivity were excreted in the urine, faeces and expired air at the end of 24 hours. The 24 hour recoveries of 14C were about 15% in the urine and about 2% in expired air of the dose applied to the skin (Buttar and Downie 1980). When BNPD was applied orally the maximum body burden was reached after 60 min. The muscle, liver and blood had the highest levels. About 86% of the applied dose is excreted during 24 hours, about 75% in the urine and about 9% as CO2 (Kujawa et al. 1987).
BNPD causes gastrointestinal lesions after oral administration to rodents. BNPD is moderately toxic by oral administration (Table 8.10).
Table 8.10
Acute toxicity (LD50) of BNPD.
|
Species |
Route of administration |
LD50 (mg/kg body weight) |
|
BNPD |
Rat |
Oral |
180-400 |
DFG 1989 |
BNPD |
Mouse |
Oral |
250-500 |
Richardson 1992-1994 |
BNPD |
Mouse |
Oral |
374 |
Kujawa et al. 1987 |
BNPD |
Dogs |
Oral |
250 |
CIRP 1984a |
BNPD (in water) |
Rat |
Percutaneous |
1,600 |
DFG 1989 |
BNPD (in acetone) |
Rat |
Subcutaneous |
200 |
CIRP 1984a |
Skin and eye irritation
23 patients of 129 showed irritant reactions in patch test to 1% BNPD. 3 patients showed irritant reaction to 0.5% and 2 patients to 0.25% BNPD (Peters et al. 1983). A study of 149 eczematous patients determined that 0.25% BNPD in soft yellow paraffin caused mild irritation (Richardson 1992-1994). A 20% aqueous solution was moderately to severely irritating to abraded and nonabraded rabbit skin. Primary irritation score was 6.75 of 8.0 (maximum possible score). A 0.5% emulsion and a 0.5% solution of BNPD were not irritating after four daily applications. The irritation to nonabraded rabbit skin depends to some extent on the vehicle (CIRP 1984a). Solid BNPD and 10% and 20% aqueous solutions of BNPD placed in the eye of rabbits produced severe ocular damage, washing after application either did not reduce the reaction, or reduced it only slightly. 2% BNPD in solution and in emulsion was irritating to the rabbit eye. 4 daily applications of a 0.5% solution and emulsion or a 0.5% solution in saline was nonirritating to the eyes (CIRP 1984a; DFG 1989).
Skin Sensitization
After repeated intradermal injection of a 0.02% solution of BNPD followed by an application of a 15% aqueous solution of BNPD, no sensitization was observed in the guinea pig maximization test (DFG 1989). Contact sensitization was not demonstrated in any of 93 normal subjects on whose skin 5% BNPD in yellow paraffin was applied 10 times in 3 weeks (induction phase) followed by a 2 week rest period prior to challenge with 0.25% BNPD (Maibach 1977).
Acute allergic contact dermatitis was reported in patients using Eucerin cream preserved with BNPD in concentration above 0.05%. The patients were BNPD patch test-positive. Eucerin is a cream used by many dermatologist in USA to patients with abnormal skin (Storrs and Bell 1983). Patients with suspected allergic contact dermatitis were tested with 13 preservatives. 2,295 patients were included. BNPD was one of the preservatives with the lowest sensitization rate of 1.2% (Perrenoud et al. 1994). 8,149 patients were patch tested with BNPD (0.5%). Reactivity was quite low, with 38 allergic reactions, corresponding to 0.47% (Frosch et al. 1990). In some cases there were indications of cross-sensitization between BNPD and formaldehyde and in others no cross-reactions were observed (Storrs and Bell 1983).
Subchronic toxicity
Rats tolerated oral doses (by intubation) of 20 mg BNPD/kg /day for 90 days. No other symtoms than occasional vomiting were seen. A dose of 160 mg/kg/day for six weeks in the drinking water caused reduced water intake by rats and slightly enlarged kidneys. Some deaths (2 of 80) occurred at a dose level of 300 mg/kg/day (CIRP 1984a). In 72 day feeding trial, rats receiving up to 100 mg/kg diet (corresponding to 5-10 mg/kg body weight/day) showed no ill-effects (DFG 1989).
Mutagenicity and carcinogencity
BNPD was not considered mutagenic in the Ames test with Salmonella typhimurium with and without metabolic activation (Bryce et al. 1978; DFG 1989).
Oral administration of BNPD to rats in drinking water at doses 160 mg/kg/day for 2 years did not affect the incidence of tumors (Bryce et al. 1978). No carcinogenic effect was observed in concentration of up to 0.5% applied topically to mice 3 times pr week for 80 weeks (CIRP 1984a). BNPD is a known nitrosating agent for secondary and tertiary amines or amides. Model assays have indicated that, in the presense of secondary and tertiary amines and amides, nitrite is released during the breakdown of BNPD. This may lead to N-nitrosation of diethanolamin and formation of the carcinogenic compound N-nitrosodiethanolamine (Scmeltz and Wenger 1979; Ong and Rutherford 1980).
Reproductive toxicity
No effects on reproduction were observed when male rats were given 40 mg/kg body weight BNPD orally for 63 days prior to mating, or when female rats were given the same dose level 14 days prior to mating (CIRP 1984a). Dermal application of up to 2% BNPD to rats from day 6 –15 of pregnancy had no adverse effect other than local skin reactions (Bryce et al. 1978).
Classification
BNPD is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
Harmful (Xn) with R21/22 (Harmful by inhalation and if swalloved) and Irritant (Xi) with R37/38 (Irritating to respiratory system and skin)- R41(Risk of serious damage to eyes), N; R50/53 (Very toxic to aquatic organisms, may cause long-term adverse effects).
The highest allowed concentration of BNPD in cosmetics is 0.1% according to cosmetic directive (Cosmetic Directive 2000).
8.3.2 5-Bromo-5-nitro-1,3-dioxane
Ecotoxicology
Information regarding degradation, bioaccumulative potential and aquatic toxicity is not available for 5-bromo-5-nitro-1,3-dioxane (CAS No. 30007-47-7). However, due to the structural similarity to 2-bromo-2-nitropropane-1,3-diol, the ecotoxicological properties of 5-bromo-5-nitro-1,3-dioxane are expected to be similar to those of 2-bromo-2-nitropropane-1,3-diol (Section 8.3).
Human health
5-Bromo-5nitro-1,3-dioxane is moderately toxic for rats and mice. Significant skin and eye irritation was observed in animal studies at 0.5%, but not at 0.1% (Table 8.11).
Table 8.11
Toxicological data for 5-bromo-5-nitro-1,3-dioxane (data obtained from CIR 1990).
Study type |
End point |
Results Mg/kg bw or %1 |
Ingestion/inhalation |
LD50 |
455-590 |
Skin contact |
LD50 |
No data |
|
Irritation |
Skin irritation |
|
NOEC Irritation |
0.1 |
Eye contact |
Irritation |
Eye irritation |
|
NOEC |
0.05 |
Allergy |
Sensitisation |
Sensitisation by skin contact |
|
LOEC Sensitisation |
0.1 |
Chronic effects |
Carcinogenicity |
No data |
1: Numbers marked with asterisk (*) are mg/kg body weight (bw). Other numbers are %. NOEC: No Observed Effect Concentration. LOEC: Lowest Observed Effect Concentration.
5-Bromo-5-nitro-1,3-dioxane was neither a sensitiser nor a photosensitiser in guinea pig studies. This ingredient was neither mutagenic nor teratogenic. Sensitisation was observed in clinical patients at 0.1 and 0.5%, but not in a study on nonclicical volunteers.
5-Bromo-5-nitro-1,3-dioxane may react with amines and amides to form nitrosamines or nitrosamides, which are considered as carcinogenic substances. Concerning cosmetic products, there are special conditions laid down for the use of this preservativ, stating that formation of nitrosamines must be avoided. As a consequence, 5-bromo-5-nitro-1,3-dioxane must not be mixed with amines and amides in cosmetic products. Further this preservative must only be used in rinse-off products, which are products intended not to remain on skin.
The highest allowed concentration of 5-bromo-5-nitro-1,3-dioxane in cosmetics is 0.1%, and it is only allowed in cosmetic products which are rinsed away after use (Cosmetic Directive 2000).
8.4 Halogenated compounds
8.4.1 Chloroacetamide
Chloroacetamide (CAS No. 79-07-2) is used as a preservative in cosmetics, pharmaceutical products, paints, glues, emulsions and as a wood preservative. It is used in concentrations of less than 1% and most often 0.2 – 0.5%.
Ecotoxicology
The environmental properties of chloroacetamide are scarcely described. There are no data available on the biodegradability and the potential for bioaccumulation. The log Kow was calculated to – 0.582 (EPWIN 1994) which indicates that chloroacetamide will not bioconcentrate in aquatic organisms. The 48 h-EC50 of chloroacetamide has been determined to 55.6 mg/l for Daphnia magna (CETOX 2000).
Human health
There are no data available on the toxicokinetics of chloroacetamide. The data concerning acute oral toxicity indicate high acute toxicity (Table 8.12).
Toxicokinetics and acute toxicity
Table 8.12
Acute Toxicity (LD50) of chloroacetamide.
Species |
Route Of administration |
LD50 (mg/ kg body weight) |
Reference |
Mouse |
Oral |
155 |
Lewis 1996 |
Rat |
Oral |
70 |
Richardson 1992-1994 |
Rat |
Oral |
138 |
CIRP 1991b |
Dog |
Oral |
31 |
CIRP 1991b |
Rabbit |
Oral |
122 |
CIRP 1991b |
Skin and eye irritatin
The skin irritancy response of a 0.2% solution of chloroacetamide in water was tested in 25 patients. The solution did not cause any reaction (Damgård Nielsen 1983). No irritation was observed when a 9% solution of chloroacetamide was applied to guinea pig as part of a sensibilization study (CIRP 1991b). Instillation of 0.1 ml of a 5% solution of chloroacetamide into the eyes of albino rabbits caused no irritation (CIRP 1991b).
Skin sensitization
Using a test to determine the potential to induce a sensitization reaction in humans (the Draize test) with 1.25% chloroacetamide, 35 of 205 (17%) human volunters were sensitized (Nord 1991). Several case reports have been published where sensitization to chloroacetamide is described. These reports show a strong sensitizing potential (Bang Pedersen and Fregert 1976; Wahlberg et al. 1978; Doom-Goossens 1981; DeGroot and Weyland 1986; Lama et al. 1986; Detmar and Agathos 1988; Jones and Kennedy 1988; Jelen et al. 1989; Wantke et al. 1993). Several animal studies with Guinea pigs were performed, and no sensitization was observed. The concentration range was 0.07 – 9% (CIRP 1991b).
Subchronic toxicity
Four groups of rats were exposed to 0, 20, 100 or 500 ppm chloroacetamide in the diet for 90 days. Effects were observed at the highest dose. Increase in leucocytes, decrease in female liver weight and decrease in testicular weight were seen (CIRP 1991b).
Mutagenecity and carcinogenecity
Chloroacetamide in solution (70% and 30% sodium benzoate) was nonmutagenic in gene mutation and chromosomal aberration assays (CIRP 1991b).
Reproductive toxicity
Pregnant rats were tested at dose levels of 20 mg/kg chloroacetamide on single days (7th, 11th or 12th ) and no effects on litter size or fetuses and no effect on dams were observed (Thiersch 1971; Shepard 1995). A subacute study indicate that chloroacetamide with a dose level of 50 mg/kg body weight has an effect on the male reproductive function, when dosed orally repeatedly in a 90 days study (CIRP 1991b). Dosing 50 mg/kg chloroacetamide to rats on day 13 and 14 of gestation resulted in the postnatal death of approx. half of the embryos. Surviving offspring developed normally (Kreybig et al. 1969).
Classification
Chloroacetamide is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
Reprotoxic category 3 (Rep 3) with R62 (Possible risk of impaired fertility), Toxic (T) with R25 (Toxic if swallowed) and Irritant with R43 (May cause sensitization by skin contact).
0.1% < C < 3%: Xi; R43
3% < C < 5%: Xn; R22 - 43
5% < C < 25%: Xn; R22-43-62
C > 25%: T; R25-43-62
The highest allowed concentration of chloroacetamide in cosmetics is 0.3% and the mandatory warning text on the label is "contains chloroacetamide" (Cosmetic Directive 2000).
8.4.2 5-Chloro-2-(2,4-dichlorophenoxy) phenol (Triclosan)
5-Chloro-2-(2,4-dichlorophenoxy) phenol (Triclosan) with the CAS No. 3380-34-5 is used in surgical scrub preparations, medicated cosmetics, deodorants, body, and hand preparations, moisturing preparations, cleansing products, bath soaps, detergents, skin care preparations, powders, eye makeup, aftershave etc. (Wenninger and McEwen 1997)
Ecotoxicology
Residues of methyl triclosan (4-Chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene) have been reported in rivers, industrial wastewater, and aquatic biota. The concentrations of methyl triclosan ranged from 1-38 m g/kg body weight in the freshwater fish topmouth gudgeon in Tama River, whereas 1-2 m g/kg body weight was found in the goby fish (A. flavimanus) and 3-20 m g/kg body weight was found in clam, oyster, and mussels in Tokyo Bay. The highest levels reported in Tokyo Bay (20 ppb) were measured in the blue mussel Mytilus edulis (Miyazaki et al. 1984).
Triclosan was not biodegraded (0% ThOD) after 4 weeks in a standard test for ready biodegradability at concentrations of 30 and 100 mg/l (MITI 1992).
Triclosan would be expected to bioaccumulate in aquatic organisms on the basis of its log Kow of 4.76. However, a bioaccumulation study over 8 weeks with fish has shown relatively low BCF values between 2.7 and 90 (MITI 1992).
The acute aquatic toxicity of Triclosan has been determined to 0.39 mg/l for Daphnia magna (48 h-EC50) and to 0.25 mg/l for fathead minnow (Pimephales promelas) (96 h-LC50) (Office of Pesticide Programs 1995).
Human health
Triclosan has shown not to be toxic by oral administration, and has not acted as a carcinogen, mutagen or teratogen (Table 8.13). Direct contact with the material under exaggerated exposure conditions has been reported to cause dermal irritation in laboratory animals. Triclosan has rarely been associated with skin irritation or sensitisation in humans in formulated products (Bhargava and Leonard 1996).
Table 8.13
Toxicological data for Triclosan
Study type |
End point |
Results mg/kg bw or %1 |
Reference |
Ingestion/inhalation |
LD50 |
3700* |
RTECS 2000 |
Skin contact |
LD50 |
9300* |
RTECS 2000 |
|
Irritation |
Mild irritation |
RTECS 2000 |
Eye contact |
Irritation |
No data |
|
Allergy |
Sensitisation |
Sensitisation by skin contact may occur |
Fisher 1986 |
Chronic effects |
Carcinogenicity Reprotoxicity/Teratogennicity Mutagenicity |
No evidence of effects |
Bhargava and Leonard 1996 |
1: Numbers marked with * are mg/kg body weight (bw). Other numbers are %. NOEC: No Observed Effect Concentration, LOEC: Lowest Observed Effect Concentration
A few investigations of allergic contact dermatitis to Triclosan have been reported when used in cosmetic products (e.g., deodorants). Daize testing showed a low sensitising potential to Triclosan (Fisher 1986).
The highest allowed concentration of Triclosan in cosmetics is 0.3% according to the cosmetic directive (Cosmetic Directive 2000).
8.4.3 Methyldibromoglutaronitrile
Methyldibromoglutaronitrile (CAS No. 35691-65-7) is used in hair shampoos, hair conditioners, hair preparations, bubble baths, indoor tanning preparations, face and neck preparations, permanent waves and all types of blushers.
Ecotoxicology
Methyldibromoglutaronitrile has been shown to be readily biodegradable in a standard OECD screening test (CTFA 1997). The log Kow was determined to 1.022 and the potential for accumulation of methyldibromoglutaronitrile in aquatic organisms is thus regarded as low.
The toxicity of methyldibromoglutaronitrile has been determined towards algae, crustaceans, and fish with the following effect values determined: Fish (96 h-LC50), 1.75-8.3 mg/l; daphnia (48 h-EC50), 2.2 mg/l; and algae (72 h-EC50), 0.15 (CTFA 1997).
The highest allowed concentration of methyldibromoglutaronitrile in cosmetics is 0.1% according to the cosmetic directive (Cosmetic Directive 2000).
8.5 Other preservatives
8.5.1 1,3,5-Triazine - 1,3,5 (2H,4H,6H)-triethanol (THT) (Grotan)
1,3,5-Triazine - 1,3,5 (2H,4H,6H)-triethanol (THT) with the CAS No. 4719-04-4 is a formaldehyde-releasing preservative which is primarily used for industrial applications, e.g. as a bacteriocide in cooling oils. The triazine-group releases formaldehyde.
Ecotoxicology
The environmental fate and effect of THT are only sparsely described. There were no experimental data that describe the biodegradation and bioaccumulation potential of THT. However, on the basis of a log Kow value of –4.67 which was calculated by use of QSAR estimation (EPWIN 1994), the potential for bioaccumulation in aquatic organism is considered to be low. The toxicity of THT towards aquatic organisms has been described for crustaceans and fish where the following LC50 values have been reported: Bluegill sunfish (Lepomis macrochirus) adults, 44.8 mg/l and fingerlings, 27.0 mg/l; rainbow trout (Oncorhynchus mykiss); 67.3 mg/l; mud crab; 72.6 mg/l; and grass shrimp (Palaemonidae sp.), 147.0 mg/l. The duration of the exposure periods was not indicated (RTECS 1997).
Human health
There were no data on the toxicokinetics available. By oral administration THT is of moderate acute toxicity (Table 8.14).
Toxicokinetics and acute tixicity
Table 8.14
Acute Toxicity (LD50) of THT.
Species |
Route of administration |
LD50 (mg/kg body weight) |
|
Rat |
Oral |
763 |
Lewis 1996 |
Rat |
Oral |
580 |
Rossmoore 1981 |
Rat |
Oral |
928 |
Schülke and Mayr 1998 |
Rat |
Dermal |
> 2000 |
Lewis 1996 |
Rabbit |
Dermal |
> 3500 |
Rossmoore 1981 |
Skin and eye irritation
Higher concentration of THT can cause irritation, as is demonstrated by the results of Danish dermatologists. Of 694 patients who underwent a skin test with a 2% or 5% aqueous solution of THT, 13% developed skin irritation (Roed Pedersen 1977). Skin irritation or other skin changes are generally not observed during occupational exposure with THT. Irritation may develop at higher concentrations (> 1%), especially in persons with sensitive skin or eczema. Prolonged and frequent skin contact can result in skin damage and even eczema. The severity of the reaction seems to depend on the concentration, and the high pH seems to be important in this context (MAK 1995). THT caused transient irritation in rabbit eye, but eyes recovered after 96 hours (Rossmoore 1981).
Skin sensitization
There is disagreement as to the sensitizing potential of THT, if it is a strong or weak sensitizer. Numerous studies with concentrations up to 1% and more yielded negative results, but some revealed positive reactions, mostly on persons with eczema - at concentrations below 0.5% (Rycroft 1978; Ketel and Kirch 1983; De Groot et al. 1986; Fisher 1986; Veronesi et al. 1987). 4 of 19 men with occupationally derived soluble oil dermatitis reacted positively to patch testing with 0.2% Grotan BK. However, when re-patch tested about a year later, with no contact with the allergen occurred, only 1 reacted to 0.2%, 1 reacted to 1% and the last 2 men did not react to 0.1-5% (Keczkes and Brown 1976). 230 metal workers with occupational dermatitis were patch tested with 1% THT. 16 subjects (6.9%) were sensitized (Alomar et al. 1985). In studies on sensitization with THT using the guinea pig maximization test it has been observed that the sensitization frequency increases with increasing concentration. THT was applied in 3 concentrations: 1.0, 0.5 and 0.1%. Four of twenty animals were sensitized at 1% THT (Andersen et al. 1984).
Mutagenicity and carcinogenicity
THT was tested for mutagenicity (chromosome abnormalities) in micronucleus test. THT was administered by intragastric intubation, dermal application or subcutaneous injection. Bone marrow preparations were screened for the presence of micronucleated cells in polychromatic erythrocytes. Doses administered were 15, 60, 240 or 960 mg/kg body weight. THT did not show any detectable mutagenic activity in the micronucleus test (Urwin et al. 1976). As part of a testing programme this component was tested in the Ames test with S. typhimurium strains. A positive response was observed in some of the strains (Mortelmans et al. 1986). No carcinogenicity studies were available.
Classification
THT is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
Harmful (Xn) with R22 (Harmful if swalloved) and Irritant (Xi) with R43 (May cause
sensitization by skin contact).
0.1% < C < 25% Xi; R43.
C > 25% Xn R22-43.
THT is not allowed in cosmetics (Cosmetic Directive 2000).
8.5.2 Formaldehyde
Formaldehyde (CAS No. 50-00-0) is a colourless gas and mostly marketed as aqueous solution with typical content of 37-50%, stabilized with 10-15% methanol to prevent polymerisation (Flyvholm 1997). Formaldehyde is frequently used as preservative in concentrations of about 0.1% in cosmetics and cleaning agents. Formaldehyde is added to the product or generated in the product from formaldehyde releasers. BNPD is an example of a formaldehyde releaser, with concentrations in cosmetics of 0.01-0.1%, and it can release up to 75 ppm formaldehyde in the product. Quaternium 15 (methanamine –3-chloroallylochloride) is another formaldehyde releaser. The concentrations of Quaternium-15 in products are between 0.02-0.3% and it can release up to 300 ppm formaldehyde in the product (Flyvholm and Menné 1992). The use of formaldehyde as a preservative is small in amounts compared with other applications of formaldehyde (Flyvholm 1997).
Ecotoxicology
The biodegradability of formaldehyde has been determined according to BOD5 methods (DIN 38409) by which a degradation of 97.4% and > 60% was determined (IUCLID 2000).
The log Kow of formaldehyde has been reported to -0.78 (IUCLID 2000), and hence, the potential for accumulation in aquatic organism is considered to be low.
A number of studies have been performed for determination of the toxicity of formaldehyde towards aquatic organisms. Some of the effect concentrations are given in Table 8.15.
Table 8.15
Effects of formaldehyde to aquatic organisms (data from IUCLID 2000).
Species |
EC/LC50 (mg/l) |
Test duration |
Scenedesmus quadricauda |
74 |
8 d |
Daphnia magna |
2 |
48 h |
Rainbow trout (Oncorhynchus mykiss) |
47.2 |
96 h |
Golden orfe (Leuciscus idus) |
22 |
48 h |
Largemouth bass (Micropterus salmoides) |
57.2 |
96 h |
Zebra fish (Danio rerio) |
41 |
96 h |
Human health
Toxicokinetics and acute toxicity
Formaldehyde can enter the body through skin and by ocular contact, inhalation and ingestion. It does not accumulate in the body. Formaldehyde disappears rapidly in the bloodstream because of condensation reactions with DNA, protein, amino acids, as well as by oxidation to CO2. The liver and erythrocytes appear to be primary sites of rapid oxidation of formaldehyde to the nontoxic chemical formate, which is excreted in the urine, and to CO2, which is exhaled. Almost every tissue in the body has the ability to break down formaldehyde. Numerous enzymes (e.g. formaldehyde dehydrogenase) can catalyze conversion to formate, which is further metabolized to CO2 and water. Formate is a normal metabolite in mammalian systems (CIRP 1984b; Richardson 1992-1994).
Formaldehyde is characterized by a high acute toxicity by oral administration (Table 8.16).
Table 8.16
Acute toxicity (LD50) of formaldehyde.
Type |
Species |
Route of administration |
LD50 |
Reference |
Formaldehyde, 2% solution |
Rat |
Oral |
800 |
WHO 1996 |
Formaldehyde |
Guinea pig |
Oral |
260 |
CIRP 1984b |
Formaldehyde |
Rabbit |
Dermal |
270 |
CIRP 1984b |
Skin and eye irritation
No significant irritant effects on the skin were noted following exposure to a 1% aqueous solution of formaldehyde. Liquid formaldehyde may irritate the skin, causing a rash or burning feeling on contact. It can also cause severe burns, leading to permanent damage, depending on the concentration (CIRP 1984b). Formaldehyde may in some individuals be mildly irritating to the eyes in airborne concentrations down to 0.01 ppm (Arbejdstilsynet 1991). Aqueous solutions of formaldehyde accidentally splashed into the eyes have caused severe injuries. Ocular irritation is observed in animals exposed to formaldehyde vapour at concentrations of 15 ppm (CIRP 1984b). The most important exposure of formaldehyde is through inhalation. Upper airway irritation to formaldehyde vapour occurs at 0.1-25 ppm. Lower airway irritation is reported at 5-30 ppm (CIRP 1984b).
Sensitization
Formaldehyde may cause allergic asthma. Formaldehyde is a relatively strong contact allergen and contact allergy may develop after contact with products, which contain less than 1% formaldehyde (Arbejdstilsynet 1991).
Subchronic and chronic toxicity
Chronic studies with rats given formaldehyde in drinking water showed adverse effects in the animals receiving the highest dose (about 100 mg/kg of body weight). The effects were a.o. low body weight and pathological changes in the stomach (Til et al. 1989; Tobe et al. 1989).
Reproductive toxicity
No teratogenic effects were seen in mice given formaldehyde orally, in an aqueous solution containing about 0.2% formaldehyde, on day 6-15 of gestation. The oral doses were 74, 148, 185 mg/kg body weight. No effects on fetus size and no skeletal or visceral abnormalities were observed. Neither was any teratogenic effect of formaldehyde observed in mice in inhalation studies (Marks et al. 1980). No effects on reproductive performance or on the health of the offspring were observed in beagle dogs exposed to formaldehyde via the diet on day 4-56 after mating. The concentration administered was 125 or 375 ppm formaldehyde (Hurni and Ohder 1977). Sperm abnormalities and inhibition of spermatogenesis has been observed in rat studies with doses administrated 100-200 mg/kg body weight (WHO 1996). Pregnant hamsters were treated with a 37% aqueous formaldehyde solution to evaluate the embryotoxic effects of topical exposure on day 8, 9,10, and 11 of gestation. No treatment related malformation or significant effects on fetal weight and length were seen (Overman 1985).
Mutagenicity and carcinogenicity
Formaldehyde increased the number of micronuclei and nuclear anomalies in epithelial cells in rats by oral administration (Migliore et al. 1989). There is little evidence that formaldehyde is carcinogenic by oral route. Though exposure to formaldehyde by inhalation gives an increased incidence of carcinomas of the nasal cavity in rats and mice at doses that caused irritation of the nasal epithelium (WHO 1996; Kerns et al. 1983). The International Agency for Research on Cancer (IARC) concluded that there is limited evidence for the carcinogenicity to humans and sufficient evidence for carcinogenicity in experimental animals. IARC has placed formaldehyde in group 2A (probably carcinogenic to humans) (IARC 1995). Epidemiologic studies of cancer risk and formaldehyde have shown no convincing evidence of a relationship (ECETOC 1995). Formaldehyde is included in the list of carcinogenic components of the Executive Order on precautions to prevent cancer risk issued by the National Working Environment Authority (Executive Order 1999).
Classification
Formaldehyde is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
Toxic (T) with R23/24/25 (Toxic by inhalation, in contact with skin, and if swallowed), Corrosive (C) with R34 (Causes burns) and Carc3, R40 (Possible risk of irreversible effects) and R43 (May cause sensitization by skin contact).
0.2% < C < 1% Xi; R43
1% < C < 5% Xn; R40 R43
5% < C < 25% Xn; R20/21/22 Xi; R36/37/38 Carc3; R40 R43
C > 25% T; R23/24/25 C; R34 Carc3; R40 R43
The highest allowed concentration of formaldehyde in cosmetics is 0.2%, except for products for dental hygiene where the concentration allowed is 0.1%. The concentration is expressed as free formaldehyde. The mandatory warning text "contains formaldehyde" must be placed on the label if the content of formaldehyde is more than 0.05% in the product (Cosmetic Directive 2000). The Danish occupational threshold limit value is 0.4 mg/m3 (Arbejdstilsynet 2000).
8.5.3 Diazolidinylurea
Ecotoxicology
The environmental fate and effect of diazolidinylurea (CAS No. 78491-02-8) has only been scarcely examined. Diazolidinylurea is not readily biodegradable as only 24% ThCO2 was attained in a standard laboratory test, OECD 301B (CETOX 2000). According to a QSAR estimation (EPIWIN 1994) the log Kow of diazolidinyl urea is –7.49 which implies that the potential bioaccumulation in aquatic organisms is expected to be low. The toxicity of diazolidinylurea has been examined in test with fish (species not indicated) and Daphnia magna where LC50 and EC50 (48-h) were determined to > 100 mg/l and 35 mg/l, respectively (CETOX 2000).
The highest allowed concentration of diazolidinglurea in cosmetics is 0.5% according to cosmetic directive (Cosmetic Directive 2000).
8.5.4 Sodium hydroxymethylglycinate
Ecotoxicology
Only very few data were found describing the fate and effects of sodium hydroxymethylglycinate (CAS No. 70161-44-3). There are no data available describing the biodegradability of sodium hydroxymethylglycinate. According to a QSAR estimation (EPIWIN 1994) log Kow is –3.41. The potential of bioaccumulation in aquatic organisms is thus regarded as being low. The toxicity of sodium hydroxymethylglycinate has been examined in tests with fish (species not indicated) and Daphnia magna where LC50 and EC50 (48-h) were determined to 94-100 mg/l and 26.5 mg/l, respectively (CETOX 2000).
The highest allowed concentration of sodium hydroxymethylglycinate in cosmetics is 0.5% according to cosmetic directive (Cosmetic Directive 2000).
9. Bleaching Agents
Bleaching agents of either the peroxygen type (perborates and percarbonates) or the
chlorine type (cyanurates and hypochlorite) are used in laundry detergents, dishwashing
agents and cleaning agents. The bleaching agents oxidize and decolorize stains originating
from natural substances (e.g. protein, tea, red wine, and fruit juice). The peroxygen type
bleaching agents are especially efficient at high temperatures, and an activator is
usually added to enhance the bleaching effect at lower temperatures. The most common
bleaching activator in European product is tetraacetylethylenediamine (TAED).
9.1 Tetraacetyl ethylenediamine
Tetraacetyl ethylenediamine (TAED; CAS No. 10543-57-4) is a bleach activator in products containing perborates and percarbonates. The concentration used typically ranges from 1 to 3%.
9.1.1 Environmental fate and effects
TAED has been shown to be readily biodegradable according to OECD criteria, and, e.g., a typical biodegradability of TAED is 95% DOC removal during 28 days (OECD 301E; IUCLID 2000). Highly water-soluble materials are unlikely to bioaccumulate to any significant degree. The octanol/water partition coefficient (log Kow) is 1.8 for TAED which indicates a low bioaccumulation potential for this substance.
Aguatic toxicity
The toxicity of TAED towards algae is scarcely investigated. A NOEC > 500 mg/l was found in a test over 14 days with Chlorella vulgaris (IUCLID 2000).
TAED has a low toxicity towards crustaceans as indicated by the effect concentrations determined for Daphnia magna (LC50 > 500 mg/l) and Gammarus pulex (LC50 > 800 mg/l) (IUCLID 2000).
TAED has a low toxicity towards fish as indicated by the reported LC50 values that are all above 250 mg/l (IUCLID 2000).
9.1.2 Effects on human health
Toxicokinetics and acute toxicity
TAED is rapidly absorbed from intestinal tract and metabolized by hydroxylation and deacetylation to N,N-diacetyl N glycolyl ethylenediamine, TriAED, N acetyl N glycolyl ethylene diamine and DAED, which are excreted via the urine (Gilbert 1992). Test with radioactively labelled TAED applicated on the skin of rats showed minimal absorption through the skin (SFT 1991). TAED has a low acute toxicty (Table 9.1).
Table 9.1
Acute toxicity (LD50) of TAED.
|
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
TAED |
Rat |
Oral |
10,000 |
Gilbert 1992 |
TAED |
Rat |
Oral |
> 2,000 |
IUCLID 2000 |
TAED |
Mouse |
Oral |
5,900 |
Gilbert 1992 |
Skin and ryr irritation
TAED has a low irritation potential (Gilbert 1992).
Sensitization
TAED was not a sensitizer in guinea pigs using the Magnusson Kligman maximization test (Gilbert 1992).
Mutagenicity
TAED was non-mutagenic in Ames test using Salmonella typhimurium strains, with and without activation (rat liver enzymes, S9 mix) (Gilbert 1992).
Reproductive toxicity
TAED administered orally to rats daily from day 6 to 15 of gestation at doses of 0, 40, 200 and 1,000 mg/kg body weight/day showed no embryotoxic effects and no significant increase in malformations (IUCLID 2000).
Classification
TAED is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
9.2 Perborates and percarbonates
Sodium perborate tetrahydrate (Cas No. 10486-00-7) and sodium percarbonates (Cas. No. 15630-89-4) are used primarily as bleaching agents in detergent powders and in bleaching powders. They are also to a smaller extent used as mild disinfectants in cosmetics and pharmaceutical preparations. Sodium perborate monohydrate (Cas No. 10332-33-9) is primarily used as a bleaching agent in detergent powders (IPCS 1998).
9.2.1 Environmental fate and effects
Sodium perborate is rapidly hydrolysed to boron, peracetic acid and acetic acid in the aquatic environment, whereas sodium percarbonate is rapidly hydrolysed to sodium carbonate, hydrogen peroxide, peracetic acid and acetic acid. Boron is a naturally occurring element which is found in the form of borates in the oceans, sedimentary rocks, coal, shale, and some soils. The boron content of environmental samples in inland surface waters is generally in the range 0.001-0.5 mg/l and up to 5 mg/l in seawater or in concentrated sewage (IPCS 1998). The octanol/water partition coefficients (log Kow) are 0.175 for boric acid, -1.25 for peracetic acid and –0.17 for acetic acid, which indicate a low bioaccumulation potential for these substances.
Algae
The effects of borate towards algae have been reviewed by Guhl (1992) who found that low concentrations generally promoted the growth of algae, whereas higher concentrations inhibited algal growth. In a growth inhibition test with Scenedesmus subspicatus an EC50 value of 34 mg B/l was determined (Steber 1992). The toxicity of peracetic acid has been reported in the range of 0.7-16 mg/l (IUCLID 2000).
Invertebrates
In a study of the acute toxicity of boric acid to daphnia the static 48 h-LC50 was found to be 95 mg B/l (Bringman and Kuhn 1977). In a study by Steber (1992) it was concluded that chronic effects of boron to daphnia may occur at a concentration of > 10 mg/l. The toxicity peracetic acid towards crustaceans has been reported in the range of 2.2-3.3 mg/l (IUCLID 2000).
Fish
The toxicity of boron is often higher in soft water than in hard water. The acute toxicity of boron towards Danio rerio (96 h-LC50) has been determined to 14.2 mg B/l (Guhl 1992). In a fish early life stage test with rainbow trout NOEC levels of boron have been determined in the range between 0.009 and 0.103 mg B/l, whereas the EC50 ranged from 27 to 100 mg B/l dependent on the water hardness (Birge and Black 1977). For peracetic acid the toxicity towards fish is reported in the range of 13-89 mg/l (IUCLID 2000).
Hydrogen peroxide
Besides being a product from the hydrolysis of percarbonate, hydrogen peroxide (Cas No. 7722-84-1) is used as a bleaching agent and disinfectant. Hydrogen peroxide is a very reactive chemical and will decompose to water under release of oxygen. The half-life of hydrogen peroxide in fresh water has been determined to be between 8 and 31 hours. The half-life in waste water is between minutes and hours and in sludge only a few seconds. Hydrogen peroxide which is used in cleaning agents is decomposed to water before it is released to the environment. Hydrogen peroxide is thus not expected to cause adverse effects in the environent.
9.2.2 Effects on human health
Toxicokinetics and acute toxicity
Sodium perborate hydrolyses to give hydrogen peroxide plus metaborate (WHO 1998). Sodium perborates are hydrolytically unstable salts because they contain boron-oxygen-oxygen bonds that react with water to form hydrogen peroxide and stable sodium metaborate (ECETOC 1995). Borate excretion occurs mainly through the kidneys in which about half is excreted within the first 12 hours and the remainder is eliminated over a period of 5-7 days (HSDB 1998). Ingested borates are readily absorbed and do not appear to be metabolised via the liver. Borates are excreted primarily in the urine regardless of the route of administration (ECETOC 1995). Both sodium perborate and percarbonates have a low acute toxicty (Table 9.2).
Table 9.2
Acute toxicity (LD50) of sodium perborates.
|
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
Sodium perborate tetrahydrate |
Rat |
Oral |
1,200 |
Lewis 1996 |
Sodium perborate tetrahydrate |
Rat |
Oral |
> 1,000 |
Kirk-Otmer 1994 |
Sodium perborate tetrahydrate |
Rat |
Oral |
2,243 |
ECETOC 1995 |
Sodium perborate monohydrate |
Rat |
Oral |
1,120 |
ECETOC 1995 |
Sodium perborate monohydrate |
Rat |
Oral |
1,600-2,100 |
ECETOC 1995 |
Sodium perborate monohydrate |
Rabbit |
Dermal |
> 2,000 |
ECETOC 1995 |
Skin and eye irritation
In the OECD Guideline test No. 404 for irritation/corrosion on the skin of rabbits, sodium perborate monohydrate (solid) was found to be slightly irritating (ECETOC 1995). The substance appears to have little effect on the skin in normal handling operations. However some drying and minor irritation have been observed and prolonged or continuous contact should be avoided (Kirk-Otmer 1994). Perborate powders were tested for eye irritation and found severely irritating to the rabbit eye (100 mg in one eye). A 1% solution of sodium perborate tetrahydrate was non-irritating to the rabbit eye (ECETOC 1995). Sodium perborate (conc. about 1.5%) will provide a local environment with a pH of around 10, which may be partially responsible for some of the acute inflammatory and tissue reactions (ECETOC 1995). Sodium peroxyborate tetrahydrate is irritating to the eyes and mucous membranes, which should be washed promptly with water in the event of contact (Kirk-Otmer 1994).
Sensitization
Sodium perborate monohydrate did not cause skin sensitization in guinea pigs (ECETOC 1995).
Subchronic and chronic toxicity
Oral administration of sodium perborate tetrahydrate in a 28-day study gave no specific toxic effects. The observed findings were considered to be of secondary nature, due to local effects on the gastric mucosa (ECETOC 1995).
Mutagenicity
Sodium perborate induced a weak mutagenic effect in some strains of Salmonella typhimurium (Ames test) (Seiler 1989).
Reproductive toxicity
In a study performed according to OECD Guideline No. 414 (teratogenicity), sodium perborate tetrahydrate was given dose levels of 0, 100, 300 and 1,000 mg/kg body weight/day by gavage on day 6 to15 of gestation. A statistically significant dose related to lower mean body weight gain and mean daily food consumption were observed in the 300 and 1,000 mg/kg/day groups. These doses were maternally toxic doses. An increase of malformations (mainly related to the skeletal and to the cardiovascular system) was present at 1,000 mg/kg/day. On the basis of these results perborates do not seem to be toxic to development (Bussi et al. 1996).
9.3 Sodium hypochlorite
Sodium hypochlorite (CAS No. 7681-52-9) with the chemical structure of NaOCl is used for cleaning, desinfection, and bleaching. Hypochlorite is widely used in the food processing industry. Household applications of hypochlorite include cleaning of toilet bowls, removing stains from hard surfaces and bleaching of textiles in connection with washing. Sodium hypochlorite is always found dissolved in water as the pure substance is very unstable. The sodium hypochlorite solution is strongly alkaline and the strength of a solution is stated in % active chlorine. Solutions contain up to 15% active chlorine with a pH of up to 11. In cleaning products containing bleach the concentration of sodium hypochlorite is 0.5-2%. All hypochlorite salts in aqueous solutions produce equilibrium mixtures of hypochlorous acid, hypochlorite ion and chlorine (IARC 1991).
9.3.1 Environmental fate and effects
Hypochlorite is a strong oxidant which oxidizes other substances and thereby reduces itself to chloride ions. Halogenated organic compounds may be formed by the reactions of hypochlorite with organic substances. The possible reaction products include trihalomethanes (e.g. chloroform), haloacetic acids, haloacetonitriles, and chloronitromethanes. Some of these halogenated compounds may be toxic and slowly degradable in the aquatic environment. Several studies have examined the halogenation of organic compounds by reactions with hypochlorite. When hypochlorite is used in the household the typical degree of NaOCl-to-halogenated organic compound conversion has been shown to vary within the interval of 0.5 to 3% of the chlorine added as hypochlorite of which up to 15% is represented by chloroform (Rasmussen 1998 and references therein).
Aquatic toxicity
Most of the consumed amounts of hypochlorite end in the sewer, and a large proportion of the hypochlorite will be converted to chloride ions before entering the wastewater treatment plant. Possible effects of hypochlorite on operational parameters in wastewater treatment plants have been examined by frequent additions of NaOCl to activated sludge (up to 25 mg/l) which did not affect the removal of BOD, COD, NH3-N and suspended solids (AISE 1997). Due to the rapid reactions with other substances, the inherent toxicity of hypochlorite, with EC/LC50 values below 1 mg/l, is of little, if any, relevance for aquatic environments. Inherent environmental properties of possible hypochlorite reaction products are shown in Table 9.3.
Table 9.3
Acute aquatic toxicity and aerobic biodegradability of possible products formed by the
reactions between sodium hypochlorite and organic substances (data from IUCLID 2000).
Reaction product |
Aquatic toxicity (EC/LC50, mg/l) |
Biodegradability |
||
|
Algae |
Daphnia |
Fish |
|
Chloroform |
560-950 |
29-350 |
18-100 |
98% in 5 days |
Chloroacetic acid |
0.028 |
77-500 |
100-500 |
100% in 28 days |
2-Chlorophenol |
- |
2.6-23 |
2.6-20 |
68% in 40 days |
2,4-Dichlorophenol |
21 |
1.4-5.1 |
1.7-8.6 |
74% in 10 days |
Toxicokinetics and acute toxicity
HO36Cl was readily absorbed into the blodstream after oral administration. The highest 36Cl activity was in the plasma and whole blood, whereas the lowest activity was measured in the liver, ileum and adipose tissue. Hypochlorite is converted and eliminated in the chloride form and the excretion was found to be mainly through the urinary route (Abdel-Rahman et al. 1983).
Ingestion causes irritation and corrosion of mucous membranes, pain, vomiting, and oedema of the pharynx and larynx; reduced blood pressure, delerium and coma may occur (Richardson 1992-1994). Inhalation of hypochlorous fumes causes coughing, respiratory tract irritation and pulmonary oedema (Richardson 1992-1994). When hypochlorite preparations come into contact with acidic substances or dirt particles, chlorine gas may be formed. Acute toxicity values of sodium hypochlorite is given in Table 9.4.
Table 9.4
Acute toxicity (LD50) of sodium hypochlorite.
Type |
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
Sodium hypochlorite, pentahydrate |
Rat |
Oral |
8,910 |
Richardson 1992-1994 |
Sodium hypochlorite |
Mouse |
Oral |
5,800 |
RTECS 1998 |
Sodium hypochlorite, 12.5% active chlorine |
Rat |
Oral |
8,200 |
IUCLID 2000 |
Sodium hypochlorite, pentahydrate |
Rabbit |
Dermal |
> 10,000 |
IUCLID 2000 |
Skin and eye irritation
More concentrated solutions (15%) would naturally be expected to cause more serious injury from splash in the eye. In tests on rabbit eyes, 5% solutions (approx. pH 11) caused immediate pain. If washed off immediately, only slight edema was seen for about one day. If not washed with water, the reactions were more severe (Grant and Schuman 1993). A solution of 0.5% hypochlorite, applied to the cornea and conjunctiva of rabbit eyes for 3 to 5 minutes, caused considerable superficial disturbance, but the eyes returned to normal within two weeks (Delft et al. 1983).
Sensitization
Sodium hypochlorite is not found to be a sensitizing agent in animals (ICSC 1998). Positive patch tests with sodium hypochlorite have been reported (Eun et al. 1984; Joost et al. 1987; Ng and Goh 1989).
Mutagenicity and carcinogenicity
Sodium hypochlorite did not induce chromosome aberrations in the micronucleus test in mice (Hayashi et al. 1988). Sodium hypochlorite was tested in Salmonella/microsome test and chromosomal aberration test in vitro using a Chinese hamster fibroblast cell line. The Salmonella/microsome test is a reverse mutation test in which the number of induced revertant colonies (his+) is countered. Sodium hypochlorite was positive in both tests (Ishidate et al. 1984). Oral administration of hypochlorite to mice at doses of 1.6, 4.0 or 8.0 mg chlorine/kg body weight per day resulted in dose-related increases in the number of sperm-head abnormalities. The mouse sperm head assay was used to test the ability of the desinfectant to disrupt normal sperm morphology as a measure of mutagenic potential to a germ cell line (Meier et al. 1985).
The carcinogenic potential of sodium hypochlorite was examined in rats. Sodium hypochlorite in concentrations of 0.1 and 0.5 % was dosed to drinking water for 104 weeks. No dose related change in the incidence of tumors was observed for any organ or tissue (Hasegawa et al. 1986). The International Agency for Research on Cancer (IARC) has concluded that there is inadequate evidence for the carcinogenicity of sodium hypochlorite in animals, and sodium hypochlorite is not classifiable as to its carcinogenicity in humans (Group 3).
Classification
Sodium hypochlorite is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows: Corrosive (C) with R34 (Causes burns) and R31 (Contact with acids liberates very toxic gas):
C > 10% active chlorine: R31 C; R34
5% < C < 10% active chlorine: R31, Xi; R36/38
The threshold limit value for chlorine in Denmark is 0.5 ppm (1.5 mg/m3) (Arbejdstilsynet 2000).
9.4 Dichloroisocyanurates
Besides the use as a bleaching agent, dichloroisocyanurates (e.g., sodium dichloroisocyanurate (CAS No. 2893-78-9); potassium dichloroisocyanurate (CAS No. 2244-21-5)) are used in the leather processing, and textile industry. Furthermore dichloroisocyanurates are used as disinfectants and as cleaning agents.
9.4.1 Environmental fate and effects
Chlorinated salts of isocyanuric acid hydrolyze in water to form cyanurate and hypochlorous acid (Hammond et al. 1986). Dichloroisocyanurates are inorganic compounds which implies that assessment of their biodegradation is not relevant. Dichloroisocyanurates are highly water soluble and practically insoluble in octanol (IUCLID 2000). The potential for bioaccumulation of dichloroisocyanurates in aquatic organisms is therefore considered to be low.
Dichloroisocyanurates are used as algaecides in swimming pools and are thus expected to be toxic towards algae at a level below 1 mg/l. Toxicity tests with Daphnia magna have shown EC50 values of 0.19 and 0.28 mg/l which correspond to the acute toxicity found in tests with fish (Table 9.5).
Table 9.5
Effects of dichloroisocyanurates to crustaceans and fish.
Species |
EC50/LC50 (mg/l) |
Test duration |
Reference |
Daphnia magna |
0.19 |
48 h |
Office of Pesticide Programs 1995 |
Daphnia magna |
0.28 NOEC: < 0.062 |
48 h |
IUCLID 2000 |
Bluegill sunfish (Lepomis macrochirus) |
0.46 NOEC: 0.25 |
96 h |
IUCLID 2000 |
Bluegill sunfish |
0.28 |
96 h |
IUCLID 2000 |
Rainbow trout (Oncorhynchus mykiss) |
0.36 |
96 h |
Office of Pesticide Programs 1995 |
Rainbow trout |
0.13 |
96 h |
IUCLID 2000 |
9.4.2 Effects on human health
Toxicokinetics and acute toxicity
Dichlorocyanurates are readily absorbed in the gastrointestinal tract and excreted primarily in the urine as unchanged compounds in rats. No evidence of bioaccumulation in tissues is observed (Clayton and Clayton 1993). The acute toxicity of chloroisocyanurates are shown in Table 9.6.
Table 9.6
Acute toxicity (LD50) of chloroisocyanurates.
Type |
Species |
Route of administration |
LD50 (mg/kg body weight) |
Reference |
Sodium dichloroisocyanurates (10%) |
Rat |
Oral |
1,670 |
Clayton and Clayton 1984 |
Trichloroisocyanuric acid |
Rat |
Oral |
750 |
Gosselin et al. 1984 |
Sodium cyanurate |
Rat |
Oral |
7,500 |
Clayton and Clayton 1993 |
Sodium dichloroisocyanurates (10%) |
Rabbit |
Dermal |
> 2,000 |
IUCLID 2000 |
Trichloroisocyanuric acid |
Rabbit |
Dermal |
> 5,000 |
Hammond et al. 1986 |
Sodium dichloroisocyanurates (10%) are moderately toxic to animals by ingestion, and they
are practically non-toxic when applied as a single dose to rabbit skin. The toxicity is
apparently due to corrosive action on stomach lining rather than to any systemic effects
(Gosselin et al. 1984).
Skin and eye irritation
Exposure to high levels of chlorinated isocyanurates may cause tissue irritation because of their ability to release hypochlorite (Hammond et al. 1986). The chlorinated isocyanurates are generally corrosive to rabbit eye and skin applied under occluded conditions with 24 hour contact (Clayton and Clayton 1993). In a study dichlorocyanuric acid, sodium salt has been evaluated for primary dermal irritation. The test substance was applied to the clipped, intact skin of rabbits and the abraded skin of rabbits for 24 hours. The test substance was determined to be non-irritant to intact skin and a moderate to moderately severe irritant to abraded skin (HSDB 1998). Dichlorocyanuric acid, sodium salt was evaluated for eye irritation. The test substance was placed in the eye of rabbits at a dose level of 10 mg of finely ground powder. The test substance was determined to be a moderately severe eye irritant (HSDB 1998).
Sensitization
Chlorinated isocyanurates are not known to be dermal sensitizers (IUCLID 2000; Clayton and Clayton 1993).
Mutagenicity and carcinogenecity
Dichloroisocyanuric acid, sodium salt, was evaluated for mutagenicity in the Salmonella/microsome preincubation assay (Ames test). In the presence and absence of rat or hamster metabolising enzymes, the test results did not induce gene mutations (Zeiger et al. 1987). In an in vivo test, rats were administered sodium cyanurate by gavage at single doses up to 5000 mg/kg and sacrificed 24 and 48 hours after dosing. Bone marrow cells were collected and examined. There was no evidence of cyanurate-induced chromosomal aberrations in rat bone marrow cells (Hammond et al. 1986).
Reproductive toxicity
Dichloroisocyanurate was administered by gavage to pregnant mice on day 6 to 15 of gestation. The dose levels were 0, 25, 100 and 400 mg/kg body weight. Maternal mortality occurred in about 50% of the high dose group, owing to gastrointestinal tract irritation. There was no evidence that dichloroisocyanurate was fetotoxic or teratogenic in mice (Hammond et al. 1986).
Classification
Sodium/potassium dichloroisocyanurate is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
O with R8 (Contact with combustible material may cause fire), Harmful (Xn) with R22 (Harmful if swallowed) and R31 (Contact with acids liberates toxic gas) and Irritant (Xi) with R36/37 (Irritating to eyes and respiratory system).
C > 10%: Xn; R22 R31, Xi; R36/37.
Sodium dichloroisocyanurate, dihydrate is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as follows:
Harmful (Xn) with R22 (Harmful if swallowed) and R31 (Contact with acids liberates toxic gas) and Irritant (Xi) with R36/37 (Irritating to eyes and respiratory system).
10. Acids and bases
| 10.1 | Acetic acid |
| 10.2 | Citric acid |
| 10.3 | Sulfamic acid |
| 10.4 | Phosphoric acid |
| 10.5 | Sulfuric acid |
| 10.6 | Potassium hydroxide |
| 10.7 | Sodium carbonate |
| 10.8 | Sodium hydrogen carbonate |
| 10.9 | Sodium hydroxide |
Acids and bases are added to liquid laundry detergents, hard surface cleaning agents, hair
shampoos, and liquid soaps in order to obtain a certain pH. The acids in household
detergents may either be organic (e.g. acetic acid, citric acid, hydroxyethane
diphosphonic acid, and sulfamic acid) or inorganic (e.g. phosphoric acid and sulfuric
acid). Commonly used sources of alkalinity are potassium and sodium hydroxide, sodium
carbonate, sodium hydrogen carbonate, and ammonia.
10.1 Acetic acid
Ecotoxicology
Acetic acid (CAS No. 64-19-7) is a weak acid. Acetic acid is ultimately biodegradable under aerobic and anoxic conditions. E.g., 95% of the substance was degraded during 5 days in a Zahn-Wellens test (OECD 302B) with non-adapted activated sludge.
The toxicity of acetic acid is generally low, with EC50 values towards algae and crustaceans around 100 mg/l, and LC50 (96 h) values towards fish in the range 75-88 mg/l (IUCLID 2000).
Human health
Most acidic cleaning agents will give burns on the skin, the eyes and mucous membranes in concentrated form. In diluted solutions, these agents will be strongly irritant. The skin will generally tolerate acids better than bases. When burns occur on the skin, pain is immediately sensed, and the skin becomes red and swollen. Long-term and repeated inhalation of aerosolized acidic cleaning agents may damage the lungs and give rise to chronical bronchitis.
Classification
Acetic acid is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Corrosive (C) wih the risk phrase R35 (Causes severe burns).
> 90%: Cx; R35
25% < C < 90%: C; R34
10% < C < 25%: Xi; R36/38
10.2 Citric acid
Ecotoxicology
Citric acid (CAS No. 77-92-9) is easily oxidized by a variety of oxidizing agents such as peroxides or hypochlorites. Citric acid is readily biodegradable with 98% biodegraded in 48 hours (OECD 302B, Zahn-Wellens test). Citric acid is found in almost all living systems. It is an intermediate in the tricarboxylic acid (TCA) cycle. The toxicity of citric acid towards aquatic organisms is very low with EC/LC50 values far beyond 100 mg/l (IUCLID 2000). See also sodium citrate (Section 7.4).
Human health
Citric acid tested on rabbit eyes as a single drop of a 2-5% solution in water caused little or no injury. Irrigation for 30 minutes with 0.5-2% solutions caused severe injury; the 0.5% solution caused permanent cloudiness of the cornea, and the 2% solution causes severe dense opacification. In one patient a splash of a large quantity of saturated solution of citric acid in the eyes caused severe conjunctival reaction and ulceration of the cornea (Grant and Schuman 1993). In conclusion, citric acid must be considered hazardous to the eyes if accidentally hit by strong solutions (> 5%). Citric acid is only mildly irritating to the skin (RTECS 1998). Inhalation of citric acid aerosols will induce cough. Long-term and repeated inhalation of aerosolized acidic cleaning agents may damage the lungs and give rise to chronical bronchitis.
Classification
Citric acid is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
10.3 Sulfamic acid
Ecotoxicology
Sulfamic acid (CAS No. 5329-14-6) is a strong acid. Sulfamic acid hydrolyses slowly to ammonium hydrogen sulfate in the aquatic environment. The toxicity of sulfamic acid to aquatic organisms is related to the effects on the pH of the aquatic medium. LC50 (96 h) towards fish (Pimephales promelas) has been determined to 70.3 mg/l (IUCLID 2000). No data was found on the toxicity of sulfamic acid towards algae and crustaceans.
Human health
Mild irritation was observed when human skin was exposed with a 4% solution for five days, whereas 500 mg on rabbit skin for 24 hours gave a severe irritation. Instillation of sulfamic acid in the eyes of rabbits gave moderate to severe irritation. The substance has a low acute toxicity as measured by its oral rat LD50 of 3,160 mg/kg (RTECS 1998).
Classification
Sulfamic acid is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Irritant (Xi) with the risk phrases R36/38 (Irritating to skin and eyes).
10.4 Phosphoric acid
Ecotoxicology
Phosphoric acid (CAS No. 7664-38-2) undergoes ionic dissociation as any strong acid. Phosphates are normal body or skeleton components of practically all life forms. In the aquatic environment, the acid may affect the pH of the water body and the phosphate entity may persist indefinitely. The presence of phosphate in surface waters can produce a fertiliser action and produce algae blooms. No exact LC50 values have been determined, however, for both crustaceans and fish LC50 is reached when pH decreases 3,5 (IUCLID 2000).
Human health
Phosphoric acid is a moderately toxic substance as measured by the oral rat LD50: 1,530 mg/kg. The dermal LD50 on rabbit skin is 2,740 mg/kg (HSDB 1998; RTECS 1998).
Most acidic cleaning agents will give burns on the skin, the eyes and mucous membranes in concentrated form. In diluted solutions, these agents will be strongly irritant. The skin will generally tolerate acids better than bases. When burns occur on the skin, pain is immediately sensed, and the skin becomes red and swollen. Long-term and repeated inhalation of aerosolized acidic cleaning agents may damage the lungs and give rise to chronical bronchitis.
As phosphoric acid is a hygroscopic substance inhalation of such aerosols will lead to formation of hygroscopic growth in the airways when the aerosol is deposited in the humid airways. This means that when evaluating occupational exposure with phosphoric acid, not only the respirable fraction and aerodynamic diameter must be taken into consideration, but also hygroscopic growth. Otherwise the total deliverable dose to the lungs may be underestimated by 600-700% (HSDB 1999).
The US Environmental Protection Agency has calculated a so-called reference concentration (RfC) below which it is considered safe to inhale phosphoric acid for a lifetime: 0.01 mg/m3. The Danish occupational threshold limit value is 1 mg/m3 (Arbejdstilsynet 2000).
Classification
Phosphoric acid is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Corrosive (C) with the risk phrase R34 (Causes burns).
> 25%: C; R34
10% < C. < 25%: Xi; R36/38
10.5 Sulfuric acid
Ecotoxicology
Sulfuric acid (CAS No. 7664-93-9) is a strong acid. Sulfuric acid will ultimately react with calcium and magnesium in water to form sulfate salts. Sulfate-reducing bacteria are known to utilize sulfuric acid (IUCLID 2000).
The toxicity of sulfuric acid to aquatic organisms is related to the effects on the pH of the medium. EC50 (24-48 h) values towards different species of crustaceans (Daphnia magna and Crangon crangon) have been determined in the range from 43 to 80 mg/l, whereas LC50 (24-96 h) values towards fish (Gambusia affinis, Lepomis machrochirus, Danio rerio) are in the range from 42 to 82 mg/l. No data were found on the toxicity of sulfuric acid towards algae. For crustaceans and fish the LC50 is reached when pH decreases to 3,5 (IUCLID 2000).
Human health
Most acidic cleaning agents will give burns on the skin, the eyes and mucous membranes in concentrated form. In diluted solutions, these agents will be strongly irritant. The skin will generally tolerate acids better than bases. When burns occur on the skin, pain is immediately sensed, and the skin becomes red and swollen. Long-term and repeated inhalation of aerosolized acidic cleaning agents may damage the lungs and give rise to chronical bronchitis.
Classification
Sulfuric acid is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Corrosive (C) with the risk phrase R35 (Causes severe burns).
> 15%: C; R35
5% < C < 15%: Xi; R36/38
10.6 Potassium hydroxide
Ecotoxicology
Potassium hydroxide (CAS No. 1310-58-3) is a strong base, which is harmful to aquatic organisms due to alkalinity. However, following neutralization to pH 5.5 to 8.5 the substance is not harmful. The aquatic toxicity of potassium hydroxide has been investigated in tests with fish (Gambusia affinis), and a 96 h-LC50 of 80 mg/l has been determined (IUCLID 2000).
Human health
Burns caused by bases may be deep and destructive and give slowly healing wounds. Contact with corrosive cleaning agents do not always give immediate pain, but just a greasy feeling on the skin. When pain and redness finally occurs, damage has already occurred . Bases have a degreasing effect on the skin, facilitating penetration of the skin with irritant and allergenic substances.
Inhalation of basic aerosols may give burns in the airways, giving rise to cough and respiratory distress. Long-term exposure to basic aerosols may give irreversible lung damage.
Classification
Pottasium hydroxide is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Harmful (Xn) with the risk phrase R 22 (Harmful if swallowed) and Corrosive (C) with the risk phrase R35 (Causes severe burns).
< 25%: Xn; R 22 C; R35
5% < C < 25%: C; R35
2% < C < 5%: C; R34
0.5% < 2%; Xi; R36/38
10.7 Sodium carbonate
Ecotoxicology
Aqueous solutions of sodium carbonate (CAS No. 497-19-8) are strongly alkaline. Sodium carbonate will hydrolyse as a function of pH. The carbon system in water is important because of the ubiquity of carbon dioxide and carbonate bearing minerals in the environment. Initial species of carbonates in the aqueous environment are H2CO3 and (CO3)2- which are in equilibrium with other forms depending on environmental conditions: closed or open system, pH, temperature, etc. The toxicity of sodium carbonate towards algae (Nitzschia sp.) is characterized by EC50 values in the range 137-242 mg/l. EC50 (48 h) values towards Daphnia magna have been determined in the range from 265 to 565 mg/l, whereas LC50 (96 h) values towards fish (Gambusia affinis and Lepomis machrochirus) have been found in the range of 300 to 740 mg/l (IUCLID 2000).
Human health
Sodium carbonate has low acute toxicity as measured by its oral rat LD50 of 4,090 mg/kg (RTECS 1998). Teratology tests on mice, rats and rabbits have all been negative (HSDB 1998).
Classification
Sodium carbonate is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Irritant (Xi) with the risk phrase R36 (Irritating to eyes).
10.8 Sodium hydrogen carbonate
Ecotoxicology
In freshwater, only a minor part of the carbonate and bicarbonate form complexes with metals. It has been calculated that 99% of the total carbonate carbon exists as free ions (IUCLID 2000). In seawater, much larger proportions of CO3- and HCO3- exist as complexes. NaHCO3 (CAS No. 144-55-8) and more specifically HCO3- is a naturally occurring intermediate molecule or ion. Due to its weak base properties it is easily transformed to CO2, metal carbonate, or remaining in aqueous solutions at different concentrations depending on the environmental compartment, its alkalinity, composition, temperature, etc. The toxicity of sodium hydrogen carbonate towards aquatic organisms (algae, crustaceans and fish) is very low with EC/LC50 values far beyond 100 mg/l.
Human health
The acute toxicity of sodium hydrogen carbonate is low as measured by the oral rat LD50 of 4220 mg/kg (RTECS 1998). Teratology tests on mice, rats and rabbits have all been negative (HSDB 1998).
Classification
Sodium hydrogen carbonate is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
10.9 Sodium hydroxideEcotoxicology
Sodium hydroxide (CAS No. 1310-73-2) is a strong base. It is highly soluble in water and dissociates to sodium and hydroxide ions, with the effect of increasing pH and alkalinity. Na+ and OH- persist indefinitely in the environment with equilibrium between various forms of complexes and precipitates. The aquatic toxicity of sodium hydroxide has been investigated towards crustaceans and fish. EC50 values in the range 30-180 mg/l have been determined for crustaceans, while LC50 (96 h) values for fish have been found in the range 45-125 mg/l, with rainbow trout (Oncorhynchus mykiss) being the most sensitive species (IUCLID 2000).
Human health
Burns caused by bases may be deep and destructive and give slowly healing wounds. Contact with corrosive cleaning agents do not always give immediate pain, but just a greasy feeling on the skin. When pain and redness finally occurs, damage has already occurred. Bases have a degreasing effect on the skin, facilitating penetration of the skin with irritant and allergenic substances.
Inhalation of basic aerosols may give burns in the airways, giving rise to cough and respiratory distress. Long-term exposure to basic aerosols may give irreversible lung damage.
Classification
Sodium hydroxide is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Corrosive (C) with the risk phrase R35 (Causes severe burns).
>5%: C; R35
2% < C < 5%: C; R34
0.5% < C < 2%: Xi; R36/38
11. Solvents
| 11.1 | Ethanol |
| 11.2 | Isopropanol (propan-2-ol) |
| 11.3 | 2-Butoxy ethanol (butyl glycol) |
| 11.4 | 1-Decanol |
| 11.5 | Butoxy diglycol |
| 11.6 | Propylene glycol |
| 11.7 | Glycerol |
| 11.8 | 2-Amino ethanol |
| 11.9 | Dipropylene glycol |
The most common groups of solvents in household detergents are alcohols, glycols, and
glycol ethers. Solvents are used in all-purpose cleaners and in so called in situ cleaners
that are "self-working" without manual scrubbing. Short chain alcohols are used
in liquid laundry detergents and liquid dishwashing agents in order to ensure solubility
and stability of the products.
11.1 Ethanol
Ecotoxicology
Ethanol (CAS No. 64-17-5) is rapidly biodegraded in aerobic and anoxic environments like activated sludge, waste water, sediments, and soil. BOD5 values range from 37 to 86% ThOD. The biodegradability of ethanol has been determined to 74 and 84% removal of DOC during 5 and 20 days, respectively (IUCLID 2000). The low log Kow value (-0.32) indicates that ethanol will not bioconcentrate in aquatic organisms. The toxicity of ethanol towards aquatic organisms is very low with EC/LC50 values > 1,000 mg/l (IUCLID 2000).
Human health
Occupational exposure includes inhalation and dermal exposure. Inhalational exposure at the occupational limit value of 1,900 mg/m3 will not produce significant blood alcohol concentrations. The maximal concentration of ethanol in the blood for a 70 kg hard working person is 20 mg ethanol/l blood, when the air concentration is 1,900 mg/m3. In comparison, ingestion of a single drink will after 20-30 minutes give rise to a maximal concentration of 150-200 mg/l blood (Campbell and Wilson 1986).
Ethanol is only absorbed in negligible amounts through skin. Ethanol precipitates protein. Briefly applied to the skin ethanol does no damage, but it is irritating if left on for long periods of time. Applied to wounds or raw surfaces it not only increases the injury but also forms a coagulum under which bacteria may subsequently thrive. It is thus not used to disinfect open wounds (Goodman et al. 1980).
Classification
Ethanol is not classifiable as pertains to health hazards. Ethanol is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Highly flammable (F) with the risk phrase R11 (Highly flammable).
11.2 Isopropanol (propan-2-ol)
Ecotoxicology
Isopropanol (CAS No. 67-63-0) is rapidly biodegraded in ready biodegradability tests (OECD 301E) with 95% DOC removal during 21 days and in a coupled unit test (OECD 303A) with 99.9% degradation during 3 hours. Under anoxic conditions isopropanol is first oxidized to acetone and hydrogen, after which acetone is fermented to methane and CO2. The removal of isopropanol was in the range of 69-74% in 20-40 days in a test using concentrated anaerobic waste as inoculum (IUCLID 2000). The low log Kow value (0.05) indicates that isopropanol will not bioconcentrate in aquatic organisms. The toxicity of isopropanol towards aquatic organisms is very low with EC/LC50 values > 1,000 mg/l (IUCLID 2000).
Human health
Occupational exposure includes inhalation and dermal exposure. Isopropanol is about twice as toxic as ethanol, although it generally has a low acute toxicity as measured by its oral rat LD50 of 5,045 mg/kg. It increases the toxicity of chlorinated solvents if exposure occurs simultaneously (HSDB 1999).
Inhalation of high concentrations may give central nervous system depression, however reversible upon cessation of exposure. It does not produce adverse effects on reproduction; is neither a teratogen, a selective developmental toxicant, nor a developmental neurotoxicant; and it is not genotoxic or an animal carcinogen. The metabolism of isopropanol appears equivalent across species with rapid conversion to acetone and carbon dioxide (Kapp et al. 1996).
Isopropanol can be absorbed through skin. Since isopropanol has greater fat-solvent effects than ethanol, repeated use has a drying effect on skin. Isopropanol is not an irritant. Severe cases of allergic contact dermatitis have been reported, but they are rare (HSDB 1999).
Classifcation
Isopropanol is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Highly flammable (F) with the risk phrase R11 (Highly flammable) and Irritant (Xi) with the risk phrases R36 (Irritating to eyes) and R67 (Vapours may cause drowsiness and dizziness).
The Danish occupational exposure limit is 490 mg/m3 (Arbejdstilsynet 2000).
11.3 2-Butoxy ethanol (butyl glycol)
Ecotoxicology
2-Butoxy ethanol (CAS No. 111-76-2) is rapidly biodegraded in ready biodegradability tests (OECD 301E) with 95% DOC removal during 28 days (IUCLID 2000). The low log Kow value (0.74) indicates that 2-butoxy ethanol will not bioconcentrate in aquatic organisms. The toxicity of 2-butoxy ethanol towards aquatic organisms is very low with EC/LC50 values > 500 mg/l (IUCLID 2000).
Human health
The lethal dose of ethylene glycols in humans is approximately 1.4 ml/kg, which would be equivalent to approximately 100 ml for a 70 kg person. The oral rat LD50 is 1.48 g/kg (HSDB 1999). Exposure to high concentrations of vapors, probably in the range of 300-600 ppm for several hours, would be expected to cause respiratory and eye irritation, central nervous system depression and damage to kidney and liver (HSDB 1999). The Danish occupational threshold limit value is 20 ppm (Arbejdstilsynet 2000).
2-Butoxyethanol penetrates the skin readily and the toxic action from excessive skin exposure may be more likely than from vapor inhalation. The rate of absorption through human skin is about 0.2 mg/cm2/h (HSDB 1999).
Classification
Butyl glycol is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Harmful (Xn) with the risk phrase R20/21/22 (Harmful by inhalation, in contact with skin and if swallowed) and Irritant (Xi) with the risk phrase R37 (Irritating to respiratory system).
11.4 1-Decanol
Ecotoxicoligy
The aerobic biodegradability of 1-decanol (CAS No. 112-30-1) is reported to 86% ThOD during 30 days in a closed bottle test (OECD 301D). The relatively high log Kow value (4.23) indicates that 1-decanol has the potential to bioconcentrate in aquatic organisms. The toxicity of 1-decanol has been determined towards crustaceans and fish (Table 11.1).
Table 11.1
Effects of 1-decanol to crustaceans and fish. Data from IUCLID (1996).
Species |
EC50/LC50 |
Test duration |
Daphnia magna |
3; 11 |
48 h; 24 h |
Nitocra spinipes |
3.1-5.2 |
96 h |
Fathead minnow (Pimephales promelas) |
2.3 |
96 h (flow through) |
Bleak (Alburnus alburnus) |
6-8.6 |
96 h |
Golden orfe (Leuciscus idus) |
0.6; 3.2 |
48 h |
Human health
ubstance has a low vapor pressure, 0.00851 mm Hg at 25° C, meaning that under normal conditions hazardous vapor concentrations will not build up. The substance has been tested for developmental toxicity by inhalation in rats with negative results (Nelson et al. 1990).
Classification
1-Decanol is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
11.5 Butoxy diglycol
Ecotoxicology
Butoxy diglycol (CAS No. 112-34-5) is readily biodegradable as more than 60% ThOD was attained during 28 days in the MITI (I) test (OECD 301C) (IUCLID 2000). The low log Kow value (0.15-0.91) indicates that butoxy diglycol will not bioconcentrate in aquatic organisms. The toxicity of butoxy diglycol towards aquatic organisms is very low with EC/LC50 values > 1,000 mg/l (IUCLID 2000).
Human health
The substance is of low acute toxicity as measured by its oral rat LD50 of app. 6 g/kg (HSDB 1999; RTECS 1999). It is of low acute toxicity by inhalation, but repeated dosage may cause lesions of the kidney. Though slightly irritating to the skin with prolonged contact it is only toxic by this route in large amounts and with continuous and prolonged contact.
Butoxy diglycol has been tested by the dermal route for subchronic and reproductive toxicity in rats with negative results in doses as high as 2 g/kg/day. The substance produced dermal irritation which was dependent on concentration in incidence, severity, and time of onset, and more severe in females than in males (Auletta et al. 1991).
Classification
Butoxy diglycol is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
11.6 Propylene glycol
Ecotoxicology
Propylene glycol (CAS No. 57-55-6) is rapidly degraded by microorganisms and 100% biodegradability during 24 hours has been observed in an aerobic biodegradability test with activated sludge. Several studies have shown that propylene glycol is also rapidly degraded under anoxic conditions in sludge as, e.g., 100% biodegradation was observed during 9 days (IUCLID 2000). The low log Kow value ( –0.92) indicates that propylene glycol will not bioconcentrate in aquatic organisms. The toxicity of propylene glycol towards aquatic organisms is very low with EC/LC50 values > 1,000 mg/l (IUCLID 2000).
Human health
The toxicity of the substance is low, both in experimental animals and in man. Propylene glycol is metabolized to lactic acid, a substance which is normally occurring in the body. No indications on mutagenicity or carcinogenicity have been found. Subcutaneous injections in mice led to a small increase in fetal malformations, but experiments with oral exposure of mice over several generations did not show any effects of toxicity to reproduction.
Propylene glycol is mildly to moderately irritating to skin in concentrations above 10%. No irritation was seen in rabbit eyes. Several cases of allergy have been described, and concentrations above 10%, particularly if occluded, may give rise to allergic skin reactions. With skin affected by disease or damage the risk of irritation and allergic reaction is increased. Reactions have been described by 2% on eczematous skin. As propylene glycol is widely used, allergy cases are considered unusual. Propylene glycol may be absorbed through skin and increase the absorption of other substances (Roberts and Walters 1998; LaKind et al. 1999).
CIR (1994) estimates that propylene glycol may be safely used in cosmetic preparations in concentrations up to 50%.
Classification
Propylene glycol is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
11.7 Glycerol
Ecotoxicology
Glycerol (CAS No. 56-81-5) is readily biodegradable as 63% ThOD was attained during 14 days in the MITI test (OECD 301C), whereas 93% ThOD was reached during 30 days in the closed bottle test (OECD 301D). Inherent anaerobic biodegradability of glycerol was confirmed in an acetate-enriched bacterial culture from digested domestic sludge as 90% degradation was observed during 8 days (IUCLID 2000). The low log Kow value (-2.56) indicates that glycerol will not bioconcentrate in aquatic organisms. The toxicity of glycerol towards aquatic algae, invertebrates and fish is very low, with EC50 values > 1,000 mg/l (IUCLID 2000).
Human health
The adverse effects of glycerol are due to the dehydrating effects of the substance. Pure glycerol may irritate the skin. Contact with the eyes will give strong irritation and pain. Glycerol may damage the endothelial cell of the cornea of the eye because of the osmotic effect – the eye is dried out, so to speak (Grant and Schuman 1993). Sensitization is very rare (Fisher 1986).
Classification
Glycerol is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
11.8 2-Amino ethanol
Ecotoxicology
2-Amino ethanol (CAS No. 141-43-5) is rapidly biodegraded in ready biodegradability tests as > 95% DOC removal was seen after 4 days in the DOC die away test (OECD 301A), whereas > 80 ThCO2 was reached during 19 days in the CO2 evolution test (OECD 301 B) (IUCLID 2000). The low log Kow value (-1.91) indicates that 2-amino ethanol will not bioconcentrate in aquatic organisms. The toxicity of 2-amino ethanol towards aquatic invertebrates and fish is low with EC50 values > 100 mg/l (IUCLID 2000).
Human health
The substance reacts as a base in aqueous solution, and the pH of 0.1N 2-amino ethanol is approximately 12. The nitrosation of the ethanol amines may result in the formation of N-nitrosodiethanolamine (NDELA) which is carcinogenic in laboratory animals. 2-amino ethanol can react with an aldehyde to form DEA, and then can be nitrosated to form NDELA. The optimum pH for nitrosamine formation is variously reported to be between 1 and 6. However, in the presence of catalysts such as chloral or an aldehyde, nitrosation reactions may occur up to a pH of 11 (CIR 1994).
Weeks et al. 1960 reported that the dominant effects of continuous exposure of dogs, guinea pigs, and rats to 5-6 ppm 2-amino ethanol vapor were skin irritating and lethargy. The inhalation of 2-amino ethanol vapor at concentrations of 12-26 ppm for 90 days did not result in any mortality in dogs or rodents. Some deaths did occur after 25 days in dogs exposed to 102 ppm 2-amino ethanol vapor, and after 24-28 days in rodents exposed to 66-75 ppm 2-amino ethanol vapor. Exposure to 66-102 ppm 2-amino ethanol vapor caused behavioral changes and produced pulmonary and hepatic inflammation, hepatic and renal damage, and hematologic changes in dogs and rodents. Inhalation by humans has been reported to cause immediate allergic responses of dyspnea and asthma and clinical symptoms of acute liver damage and chronic hepatitis (CIR 1994).
CIR (1994) recommends that in products intended for prolonged contact with the skin, the concentration of ethanolamines should not exceed 5%. 2-Amino ethanol should be used only in rinse-off products.
Classification
2-Amino ethanol is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as Harmful (Xn) with the risk phrase R20 (Harmful by inhalation) and Irritant (Xi) with the risk phrases R36/37/38 (Irritating to eyes, respiratory system and skin). The Danish occupational exposure limit is 2.5 mg/m3 (Arbejdstilsynet 2000).
11.9 Dipropylene glycol
Ecotoxicology
Dipropylene glycol (CAS No. 25265-71-8) is inherently biodegradable as 100% degradation was observed in a Zahn-Wellens tests (OECD 302B). In a closed bottle test (OECD 301D), only 16% ThOD was attained during 28 days (IUCLID 2000). Dipropylene glycol does not bioconcentrate in aquatic organisms as BCF values in the range 0.3-4.6 were observed in a 42 day bioaccumulation study with carp (Cyprinus carpio) (IUCLID 2000). A low bioconcentration potential is also indicated by the log Kow of -1.49. The toxicity of dipropylene glycol towards fish is very low with EC50 values > 1,000 mg/l (IUCLID 2000). There was no data found on the effects of dipropylene glycol towards algae and crustaceans.
Human health
Dipropylene glycol is of low acute oral and dermal toxicity in laboratory animals. Oral rat LD50 is 14,850 mg/kg (RTECS 1999). Ingestion of large amounts may give effects on the central nervous system and kidney, liver, lung and spleen damage. Dipropylene glycol is a mild irritant. Repeated applications of dilute solutions have not produced sensitization in volunteers (BIBRA 1991). No developmental toxicity was seen after oral exposure of pregnant rats and rabbits (NTIS 1992a; NTIS 1992b).
Classification
Dipropylene glycol is not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
12. Fragrances
| 12.1 | Potential hazard to health |
| 12.2 | Polycyclic musks |
| 12.3 | Camphene |
| 12.4 | 2-Pinene |
| 12.5 | D-Limonene |
| 12.6 | Camphor |
| 12.7 | Coumarin |
| 12.8 | Terpineol |
| 12.9 | a -hexylcinnamaldehyde |
| 12.10 | Eucalyptus oils |
Perfume may be made up by hundreds of constituents. Single chemical substances or simple herbal extracts may also be used to impart fragrance to the product. The purpose of the perfume may be to mask unpleasant odours from other constituents, or to leave a fragrance trace where the product has been used. Perfumes have frequently received attention because of their potential hazard to health, and, consequently, the health hazard assessment constitutes the main part of this Chapter. The environmental hazard assessment has received far less attention and, hence, available data describing the environmental fate and effects of some fragrance constituents are included.
12.1 Potential hazard to health
Contact allergy
The main hazard to health of perfume is hypersensitivity, i.e. contact allergy or intolerance. Contact allergy to perfume occurs with a relatively high incidence. Thus, in an assessment of an unselected population of 567 Danes, 1.1% were found to be allergic to Balsam of Peru and 1.1% were allergic to fragrance mix (Nielsen and Menné 1992). Both Balsam of Peru and fragrance mix are markers for perfume allergy. The incidence of perfume allergy is only surpassed by nickel allergy, which had an incidence of 6.7% in the same population. There is no cure for perfume allergy. Once a person is sensitized exposure to even minute amounts give rise to eruptions and eczema. Eruptions and eczema may be alleviated with steroid creams, although this treatment is not without side effects if used extensively and frequently. The best prophylaxis is avoidance of perfumes, which is not easy as the use of perfume in various household products is widespread. Research is being carried out in order to establish safe concentration below which reactions cannot be elicited.
Most manufacturers who use perfume in their formulations refer to the IFRA Code of Practice when considering type and concentration of perfume in the products. The recommendations in IFRA Code of Practice should be used with caution and evaluated critically. Many of the references are given as "private communication to IFRA" with neither date nor source.
Intolerance by inhalation
Intolerance to perfumes by inhalation is another debated hazard. Symptoms may vary from feeling ill, over coughing, phlegm, wheeze, chest tightness, headache, exertional dyspnea, acute respiratory illnesses, hay fever, child respiratory trouble, to physician confirmed asthma. Symptoms of hyperreactivity of the respiratory tract and asthma without IgE-mediated allergy or demonstrable bronchial obstruction can be induced by perfume. This was shown by placebo-controlled challenges of nine patients with perfume. The same patients were also subjected to perfume provocation with or without a carbon filter mask to ascertain whether breathing through a filter with active carbon would prevent the symptoms. The patients breathed through the mouth during the provocations, as they used a nasal clamp to prevent any smell of perfume. The patient’s earlier symptoms could be verified by the perfume provocation. Breathing through the carbon filter had no protective effect. The symptoms were not transmitted via the olfactory nerve, since the patients could not smell the perfume, but they may have been induced by a trigeminal reflex via the respiratory tract or by the eyes (Millqvist and Lowhagen 1996). Cases of occupational asthma induced by perfume substances such as isoamyl acetate, limonene, cinnamaldehyde and benzaldehyde tend to give persisting symptoms even though the exposure is below the occupational exposure limits (Jensen and Petersen 1991).
Inhalation intolerance has also been reproduced in animals. The emissions of five fragrance products for 1 hour produced various combinations of sensory irritation, pulmonary irritation, decreases in expiratory airflow velocity as well as alterations of the functional observational battery indicative of neurotoxicity in mice. Neurotoxicity was found to be more severe after mice were repeatedly exposed to the fragrance products, being four brands of cologne and one brand of toilet water (Anderson and Anderson 1998).
Potency ranking for sensitization
According to a Japanese study (Nakayama 1998) perfume constituents may be classified in four classes, A, B, C and D, where A is common and primary sensitizers, B is rare sensitizers, C is virtually non-sensitizing fragrances and D is considered as non-sensitizers. The classification was the result of patch test trials on cosmetic dermatitis patients and controls. During the trials a number of fragrances were discovered to produce no positive reactions on either the patients or the controls even at high concentrations of 5-10%. In Japan, the recommendation of using class C and D fragrances rather than A and B fragrances in cosmetic products has produced significantly lower reaction rates in Japan than in the United States and Europe.
Table 12.1
Potency ranking for sensitization of fragrances
Class A fragrances (common cosmetic sensitizers and primary sensitizers) |
|
Hydroxycitronellal |
Geranium oil |
Jasmine absolute |
Sandalwood oil |
Ylang-ylang oil |
Artificial sandalwood |
Cananga oil |
(Bornyl methoxy cyclohexanol containing artificial sandalwood) |
Cinnamic alcohol |
|
Cinnamic aldehyde |
Hay green |
Eugenol |
Musk ambrette |
Isoeugenol |
Armoise oil |
Benzyl salicylate |
Narcissus absolute |
Balsam of Peru |
Lavender oil |
d-Carvone |
Bay oil |
l-Carvone |
Violet leaves absolute |
Costus root oil* |
Methylheptine carbonate |
a -Damascone |
Methyloctine carbonate |
Geraniol |
b -Damascone |
Class B fragrances (reare sensitizers |
|
Amylis oil |
Fir balsam absolute |
Citral diethyl acetate |
Nutmeg oil |
l-Hydroxycittonellal |
d-Methoxycitronellal |
Vetiver oil |
cis-3-Hexenyl acetate |
a -Ionone |
Acetivenol |
b -Ionone |
Allyl cyclohexyl propionate |
Methylisoeugenol |
Bourgeons de cassis absolute |
Clove buds oil |
b -Damacenone |
Cedarwood oil |
5-Cyclohexadecenone |
Basil oil |
Rose de May absolute |
Cedramber |
g -Methylionone |
Oakmoss absolute |
a -Methylionone |
Petitgrain citronnier |
Others |
Iso E super |
|
Class C fragrances (virtually nonsensitizing fragrances) |
|
Isoamyl salicylate |
Gerany nitril |
g -Dodecalactone |
Lyral |
Guaiacyl acetate |
Musk tibetene |
6-Isobutyl quinoline |
Ligustral |
g -Undecalactone |
e -Nonalactone |
Neroli oil |
Rosemary oil |
Bergamot oil FL (furocoumanrinfree) |
p-tert-Butyl cyclohexyl acetatee |
Tetrahydrogeraniol |
Allyl amyl glycolatee |
e -Decalactone |
Allyl ionone |
cis-3-Hexenol |
Ambrette seed oil |
Musk ketone |
Bois de rose oil |
Citral hexylene glycol acetal |
Linalool |
Caraway oil |
Mentyl acetate |
Citronellyl acetate |
Petigrain oil |
Cumin oil |
l-Nonanal |
l-Methoxycitronellal |
l-Decanal |
Isobutyl salicylate |
Bacdanol |
Phenyl propyl alcohol |
Others |
Lavandin oil |
|
Class D fragrances (Considered as nonsensitizers) |
|
Linalool oxide |
e -Dodecalactone |
Dihydro linalool |
Phenylethyl isoamyl ether |
Dihydro myrcenol |
Mandarin oil |
Myrcenyl acetate |
Octyl dodecanol |
Pentalide |
Almond oil |
Phenyl ethyl salicylate |
Phenyl acetaldehyde dimethylacetal |
Tonka absolute |
Jasmal |
Tetrahydro linalool |
d -Nonalacetone |
Tetrahydromuguol |
d -Undecalactone |
Tetrahydromyrcenol |
g -Nonalacetone |
Isopropyl myristate |
g -Decalactone |
Hedione |
d -Dodecalactone |
Citronellyl nitrile |
Isobuthyl angelate |
Lemon FL (furocoumarinfree) |
cis-3-Hexenyl salicylate |
Lime Oil FL (furocoumarinfree) |
Others |
The following sections describe the potential hazards to the environment and health of
some of the most frequently used fragrances in detergent and cleaning products.
12.2 Polycyclic musks
AHTN (7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene) (CAS No. 1506-02-1; 21145-77-7) and HHCB (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-[gamma]-2-benzopyran) (CAS No. 1222-05-5) are used as fragrances in cosmetics and detergents, fabric softeners, household cleaning products, air fresheners, etc. Both substances are high volume chemicals with a use volume of 585 and 1,482 tonnes in Europe in 1995, respectively. AHTN and HHCB represent about 95% of the market for polycyclic musks in EU (Plassche and Balk 1997). The following survey of the environmental properties of AHTN and HHCB is based entirely on the risk assessment by Plassche and Balk (1997).
Occurrence in the environment
Both AHTN and HHCB have been found in the environment, e.g., in river water and fish and in samples of human fat and milk. Emissions of AHTN and HHCB take place by private use, and the total volume of these substances is expected to be discharged via wastewater treatment plants. A part of the AHTN and HHCB is released into the aquatic environment when the effluent is discharged into the recipient. Another part will enter the terrestrial environment after sorption to wastewater sludge and application of the sludge on agricultural land. The highest measured influent concentrations in different wastewater treatment plants were 0.0044 and 0.0029 mg/l for AHTN and HHCB, respectively, whereas the highest measured effluent concentrations were 0.0031 an 0.0025 mg/l, respectively. The presence of polycyclic musks in the aquatic environment has been reported for rivers in Germany, the Netherlands, Sweden, and Japan. E.g., concentrations of up to 0.4 and 0.26 m g/l were reported for German rivers. Concentrations in suspended organic matter have been found to be in the range of 0.06-1.2 mg/kg dry weight for AHTN and 0.05-0.58 mg/kg dry weight for HHCB.
Aerobic biodegradability
The ultimate aerobic biodegradability of AHTN and HHCB has been determined in various standard screening tests. All the available results indicate a low level of ultimate biodegradation of both AHTN and HHCB under screening test conditions (Table 12.2). However, it has been shown that primary biodegradation of AHTN and HHCB may occur by different soil-born fungi.
Bioaccumulation
Both AHTN and HHCB have high log Kow values (>> 3.0) and have the potential to bioconcentrate in aquatic organisms.
Table 12.2
Ultimate aerobic biodegradability of polycyclic musks.
Compound |
Test |
Result |
AHTN |
Modified MITI (II) test; 28 d |
0% ThOD |
AHTN |
Modified OECD 301B, sealed vessel TIC test |
0% ThIC |
AHTN |
Two-phase closed bottle test; 7 weeks |
12-21% ThOD |
AHTN |
CO2 evolution test, OECD 301B; 28 d |
0% ThCO2 |
HHCB |
Modified OECD 301B, sealed vessel TIC test; 28 d |
0% ThIC |
HHCB |
CO2 evolution test, OECD 301B; 28 d |
0% ThCO2 |
Aguatic toxicity. Algae
The toxicity towards algae has been determined for both AHTN and HHCB according to OECD Test Guideline 201 with the green alga Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum). The 72 h-EC50 for the growth rate were > 0.80 mg/l for AHTN (NOEC, 0.44 mg/l) and > 0.85 mg/l for HHCB (NOEC, 0.47 mg/l).
Invertebrates
The chronic toxicity of AHTN and HHCB has been determined in a Daphnia magna 21-day test according to OECD Test Guideline 202. For AHTN the 21 d-EC50 for the immobilisation of the parent generation was 0.34 mg/l (95% confidence interval, 0.24-0.43 mg/l). The 21 d-EC50 on reproduction was 0.24 mg AHTN per litre (95% confidence interval, 0.24-0.25 mg/l), the NOEC for reproduction was 0.20 mg/l, and reproduction was almost completely inhibited at 0.40 mg/l. For HHCB 21 d-EC50 for immobilisation was 0.29 mg/l (95% confidence interval, 0.20-0.42 mg/l). The 21 d-EC50 on reproduction was 0.28 mg HHCB per litre (95% confidence interval, 0.24-0.25 mg/l), and the NOEC for reproduction was 0.11 mg/l.
Fish
A 21-d prolonged toxicity test has been carried out with bluegill sunfish (Lepomis macrochirus) according to the OECD Test Guideline 204. Concentrations of AHTN up to 0.18 mg/l did not affect the survival of the fish. The 21 d-LC50 for AHTN was determined to 0.31 mg/l and fish growth was significantly reduced at 0.18 mg/l. For HHCB the 21 d-LC50 was 0.45 mg/l and fish growth was significantly reduced at 0.39 mg/l. The overall NOEC of the test was 0.093 mg/l as determined by the onset of clinical signs (Table 12.3).
The toxicity to early life stages of fathead minnow (Pimephales promelas) was examined according to the OECD Test Guideline 210. The hatchability of eggs was not significantly affected by AHTN in any of the test concentrations. Larvae survival after 32 days of exposure was not affected at 0.067 mg/l and below, while larvae growth was not affected at 0.035 mg/l. For HHCB hatchability was not significantly affected in any of the test concentrations. Larvae survival and larvae growth was not affected at 0.68 mg/l and below, after 32 days of exposure.
Table 12.3
Effects of AHTN and HHCB to fish.
Species |
Substance |
Effect concn. |
Test duration |
Bluegill sunfish (Lepomis macrochirus) |
AHTN |
LC50: 0.341 (0.226-0.448) LOEC: 0.184 NOEC: 0.089 (growth) |
21 d |
Fathead minnow (Pimephales promelas) |
AHTN |
LC50: 0.100 (0.097-0.100) |
32 d (early life stage test) |
Bluegill sunfish |
HHCB |
LC50: 0.452 (0.316-0.911) |
21 d |
Fathead minnow |
HHCB |
LC50: > 0.140 |
32 d (early life stage test) |
Soil organisms
Toxicity tests with earthworms (ISO 11268) showed no mortality or growth inhibition of adult earthworms after 4 weeks of exposure with AHTN at 250 mg/kg, whereas reproduction was not affected at 105 mg/kg. For HHCB, survival of adult earthworms was not affected at 250 mg/kg, whereas the growth rate and reproduction were inhibited at 250 mg/kg and 105 mg/kg, respectively.
Effects on human health
AHTN and HHCB have been under evaluation by The EU Scientific Committee on Cosmetic and Non-Food Products according to the record of their 3rd plenary meeting in Brussels, 20 May 1998. AHTN and HHCB have been tested in a rat two-generation study. The oral doses producing levels of AHTN and HHCB in milk of the lactating rat being approximately 1,000 times higher than the levels reported in human milk were determined. Groups of 28 time-mated rats were then dosed at that level and multiples of that level starting in the third week of pregnancy. This dosing was then continued post-partum until weaning. From the litters, randomly selected off-spring (24 males and females/group) were retained to maturity and assessed for general health and development as well as for behavioural effects and reproductive capability. The F2 generation was maintained until 21 days post-partum at which time all F1 and F2 animals were sacrificed. No effects were seen even at the highest doses. This study was performed by the Research Institute for Fragrance Materials (Ford and Bottomley 1997).
HHCB was negative in two genotoxicity (mutagenicity) tests: the micronucleus test with human lymfocytes and with the human hepatoma cell line Hep G2, in doses up to cytotoxicity (Kevekorde et al. 1997), and in the SOS chromotest (Mersch-Sundermann 1998). HHCB acts as a moderate irritant on rabbit skin (RTECS 2000). The acute toxicity for both AHTN and HHCB is relatively low as the lowest toxic doses exceed 4,500 mg/kg/day administered over a few days (RTECS 2000). No data on allergenicity were found.
12.3 CampheneEcotoxicology
Camphene (CAS No. 79-92-5) is a natural component in essential oils and a terpene found in camphor. In a modified MITI (I) test (OECD 301C) only 1-4% of camphene was degraded in 28 days (IUCLID 2000). Camphene is thus not readily biodegradable. The log Kow is 4.1 and camphene is therefore potentially bioaccumulative in aquatic organisms. BCF values of 432-922 and 606-1290 were determined at exposure concentrations of 15 and 1.5 m g/l, respectively, in a 56-day bioaccumulation test with carp (Cyprino carpio).
The toxicity of camphene towards algae is low with EC50 values > 1,000 mg/l. For Daphnia magna an EC50 value of 22 mg/l has been determined (IUCLID 2000). The highest toxicity of camphene has been found in tests with fish. E.g., the LC50 were 0.72 mg/l (96 hours, flow-through) for zebra fish (Danio rerio), 1.9 mg/l (96 hours, static) for sheepshead minnow (Cyprinodon variegatus), and 2.0 mg/l (48 hours, static) for ricefish (Oryzias latipes) (IUCLID 2000).
Effects on humman health
When tested at 4% in petrolatum, camphene produced no irritation in a 48-hour closed patch test on human subjects. In a study of the sensitizing properties of 17 terpenes and related compounds found in essential oils, camphene was found not to be a sensitizer for human skin. Camphene is absorbed through the skin (HSDB 1998).
12.4 2-Pinene
Ecotoxicology
2-Pinene (CAS No. 80-56-8) is a main component of turpentine. Biotransformation has been examined in experiments confirming that the bacterium Pseudomonas maltophilia is able to grow on alpha-pinene with formation of the following metabolites: Limonen, borneol, campher, 2-(4-methyl-3-cyclohexeneyliden) propionic acid, and perill-acid. The log Kow of 4.83 indicates that 2-pinene has the potential to bioaccumulate in aquatic organisms. The aquatic toxicity of 2-pinene to crustaceans has been examined in tests with two different species. The 48 h-EC50 was 41 mg/l towards Daphnia magna, whereas the LC50 ranged between 1 and 1.5 mg/l for Chaetogammarus marinus (48-96 hours) (IUCLID 2000).
The highest acute toxicity to aquatic organisms has been found in tests with fish, as an 96 h-LC50 of 0.28 mg/l was determined in a static test with fathead minnow (Pimephales promelas) (IUCLID 2000).
Human health
Contact sensitization is uncommon (De Groot et al. 1994). Application of pure pinene on human skin gives severe irritation. The oral rat LD50 is 3,700 mg/kg. Pinene is absorbed through the skin and lungs (HSDB 1998). The Danish occupational exposure limit is 25 ppm (Arbejdstilsynet 2000).
12.5 d-Limonene
Human health
d-Limonene (CAS No. 5989-27-5) itself has a low sensitizing capacity. However, it is easily oxidized at air exposure and the oxidation products formed are strong sensitizers. The frequency of allergic reactions to d-limonene containing oxidation products is comparable to that of common allergens such as formaldehyde (Karlberg 1998). The oxidation of d-limonene may be counteracted by addition of antioxidants. The effects of such antioxidants, however, wear off with time, whereupon formation of oxidation products starts. Furthermore, the antioxidant BHT, which is commonly used in hand soaps, may constitute a health hazard, since it has been shown to promote skin cancer in mice after induction with benzo[a]pyrene (Taffe and Kensler 1988; Danish Toxicology Centre 1995). The Danish occupational exposure limit is 75 ppm (tentative, dipentene).
Classification
d-Limonene is included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC and classified as flammable with the risk phrase R10 (Flammable) and Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R43 (May cause sensitisation by skin contact).
12.6 Camphor
Human health
Camphor (CAS No. 76-22-2) is moderately toxic with an LD50 of 1.31 g/kg. A fatal dose for a 1-year old child is 1 g of camphor. Cases of collapse have been reported after local application of camphor in the nostrils. The substance can be transferred to the fetus through placenta. Dust and vapors are very irritating to skin and mucous membranes. Camphor is quickly absorbed through skin. It is irritating to skin and eyes. Sensitization to camphor is rare.
12.7 Coumarin
Human health
Contact sensitization due to exposure with coumarin (CAS No. 91-64-5) may occur (De Groot et al. 1994). Oral rat LD50 is 293 mg/kg. Recent experiments have shown clear evidence of carcinogenic activity of coumarin in female B6C3F1 mice by oral administration, while there is some evidence in male F344/N rats and male B6C3F1 mice.
12.8 Terpineol
Human health
Contact sensitization due to exposure with terpineol is uncommon (De Groot et al. 1994). Oral rat LD50 is 4,300 mg/kg (RTECS 1998).
12.9 a -hexylcinnamaldehyde
Human health
Contact sensitization due to exposure with a -hexylcinnamaldehyde (CAS No. 101-86-0) is rare (De Groot et al. 1994). Oral rat LD50 is 3,100 mg/kg (RTECS 1998).
12.10 Eucalyptus oils
Human health
Contact sensitization is rare, but has been seen at concentrations as low as 2%. Oral rat LD50 is 2,480 mg/kg (RTECS 1998). Eucalyptus oils (CAS No. 8000-48-4) are moderately irritating for skin and eyes.
13. Methods for ranking of substances and ingredients
| 13.1 | Environmental hazard assessment |
| 13.2 | Human health hazard assessment |
Comparison of the inherent environmental and toxicological properties of substances and
ingredients in detergents is frequently conducted in relation to formulation of new
products and environmental labelling. The methods presented in this Chapter are based on
internationally accepted principles for classification of single substances. A large
number of chemicals that are used in household detergents have been officially classified
on the basis of their potential toxicity to human health, whereas only very few of these
chemicals have been classified for their potential environmental hazards. The
implementation of the Directive 99/45/EC of the European Parliament and of the Council
implies that chemical preparations may be classified as dangerous for both human health
and for the environment. The methods for ranking of single substances are derived from the
criteria for the risk phrases (R-phrases) also forming the basis of the classification of
preparations in the Directive 99/45/EC. The relation between this Directive and the
ranking criteria implies that the ranking methods may contribute to the production and
request of products that will not be classified as dangerous for health or the
environment. However, the ranking is entirely based on the data evaluation by the authors
of this review, and the purpose has not been to recommend a classification of specific
substances. The ranking presented in this Chapter should be interpreted as a screening of
the potential hazards of the substances to health and the environment. Substitution of a
potentially hazardous substance identified on the basis of inherent properties is not
always the only solution, and substances may exist, where substitution is not attractive
for technical, economical, and even environmental and health based reasons. In such cases,
the ranking may be seen as a tool to identify substances for which a more thorough risk
assessment should be performed.
13.1 Environmental hazard assessment
The environmental hazard assessment is performed on the basis of the inherent properties of the substances. The method for environmental ranking of single substances is based on the weighting of R-phrases in the Directive 99/45/EC and follows commonly accepted criteria and data interpretation that are used for classification (Directive 67/548/EEC) and effects assessment for the aquatic compartment (EC 1996).
The inherent environmental properties are used for ranking of single substances in four main groups, i.e. environmental hazard classes 1-4, where class 4 contains the substances considered to have the lowest potential for environmental hazard (and vice versa). The environmental ranking system operates with a class 3A and a class 3. Class 3A contains substances with an acute aquatic toxicity corresponding to EC/LC50 £ 1 mg/l and for which other additional evidence suggests that the substances are environmentally less acceptable (than the substances in class 3) or that further investigations are necessary. Substances with an acute aquatic toxicity characterized by EC/LC50 £ 1 mg/l are placed in class 3A, if one or more of the following criteria apply:
| 1. | Limited ultimate biodegradability in aerobic tests for ready biodegradability (i.e. below pass level) due to sorption, toxicity or other inherent properties that preclude sufficient biodegradability under standard test conditions, provided that other convincing scientific evidence is available to demonstrate that the substance can be degraded to harmless products in the aquatic environment (> 70% within 28 days). The expert judgement of the scientific evidence may always be an issue of debate as long as no criteria have been defined for biodegradation simulation tests, their environmental relevance, and their technical quality. The substances with the above mentioned characteristics are, therefore, placed in class 3A, and a more thorough evaluation of the evidence proving a rapid degradation is recommended on a case by case basis. |
| 2. | Limited ultimate biodegradability under anoxic conditions as indicated by < 60% ThGP during 60 days in screening tests (e.g. ISO 11734), unless > 60% mineralization or complete primary degradation to harmless products is confirmed under relevant anoxic conditions. Recalcitrance under anoxic conditions may lead to an accumulation of the substance in aquatic sediments. Anaerobic biodegradability of surfactants is included in the criteria for environmental labelling (e.g., the Nordic Swan). |
| 3. | Indications for potential bioaccumulation in aquatic organisms as indicated by an experimentally determined BCF > 100 or a log Kow ³ 3.0. This additional criterion recognizes that indications for potential bioaccumulation may exist for some substances, although the evidence is not sufficient to fulfil the criteria for class 1. E.g., there are difficulties in the extrapolation from experimental data for defined model chemicals to more complex raw materials like, e.g., surfactants composed of several homologues and/or isomers. Such substances are placed in class 3A, because the potential bioaccumulation may imply a higher impact on the aquatic environment, e.g., when sub-lethal effects are considered. |
The criteria for environmental ranking of substances in the environmental hazard classes
1, 2, 3A, 3, and 4 are given below. Class 0 is used for substances for which the available
data are considered insufficient (Table 13.1).
Table 13.1
Criteria for environmental hazard assessment.
Environmental hazard class |
Explanations |
Class 0 |
The available data are insufficient |
Class 1 |
Acute aquatic toxicity: EC/LC50 £ 1 mg/l. The substance is not readily degradable, or the log Kow ³ 3.0 unless the experimentally determined bioconcentration factor (BCF) £ 100. |
Class 2 |
Acute aquatic toxicity: 1 mg/l < EC/LC50 £ 10 mg/l. The substance is not readily degradable, or the log Kow ³ 3.0 unless the experimentally determined bioconcentration factor (BCF) £ 100. |
Class 3A |
Acute aquatic toxicity: EC/LC50 £ 1 mg/l. The substances in this class have inherent properties that either make them less acceptable compared to the substances in class 3 or imply a need for further investigations. Substances with EC/LC50 £ 1 mg/l are placed in class 3A, if one or more of the following criteria apply: Ultimate biodegradability in ready biodegradability tests below the specified pass level provided that other convincing scientific evidence demonstrates rapid degradation to harmless products in the aquatic environment. Limited ultimate biodegradability under anoxic conditions as indicated
by Indications for potential bioaccumulation in aquatic organisms, but the data are considered insufficient to fulfil the criteria for class 1 (e.g. technical surfactants composed of several homologues and/or isomers). |
Class 3 |
Acute aquatic toxicity: EC/LC50 £ 1 mg/l. or: Acute aquatic toxicity: 10 mg/l < EC/LC50 £ 100 mg/l. The substance is not readily degradable. or: The substance is not readily degradable. The log Kow ³ 3.0 unless the experimentally determined bioconcentration factor (BCF) £ 100. or: The substance does not comply with the criteria mentioned above, but it is considered potentially hazardous to the aquatic environment on the basis of available evidence. |
Class 4 |
The substance does not comply with the criteria mentioned above, and it is not con-sidered hazardous to the aquatic environment on the basis of available evidence. |
The EU Technical Guidance Document recommends that the lowest of the relevant available
toxicity data (EC50 or LC50) is used for risk assessment, and that the effect
concentration is expressed as the arithmetic mean if more than one EC/LC50 value is
available for the same species (EC 1996). In order to obtain a high level of compliance
with internationally accepted criteria, the following approach was applied in the
environmental ranking presented in Table 13.5.
The EC/LC50 were derived by an evaluation of the available data describing the acute aquatic toxicity towards a number of commonly accepted species.
Algae
Selenastrum capricornutum (Pseudokirchneriella subcapitata)
Scenedesmus subspicatus
Chlorella sp.
Skeletonema costatum
Phaeodactylum tricornutum
Microcystis aeruginosa
Crustaceans
Daphnia magna
Daphnia pulex
Ceriodaphnia
Acartia tonsa
Brine shrimp (Artemia salina)
Scud (Gammarus pulex)
Nitocra spinipes
Mysid shrimp (Mysidopsis bahia)
Fish
Rainbow trout (Oncorhynchus mykiss)
Fathead minnow (Pimephales promelas)
Zebra fish (Danio rerio)
Bluegill sunfish (Lepomis macrochirus)
Medaka, Rice fish, Red killifish (Oryzias latipes)
Carp (Cyprinus carpio)
Golden orfe (Leuciscus idus melanotus)
Guppy (Poecilia reticulata)
Turbot (Scophthalmus maximus)
The geometric mean value was calculated if more than one EC/LC50 value was available for the same species. The geometric mean was preferred instead of the arithmetic mean, because the geometric mean implies that extreme toxicity values (‘outliers’) have less influence on the calculated mean value.
The effect concentrations (EC50 or LC50) that were obtained for the most sensitive species within one of the three trophic levels, algae, crustaceans, and fish, were used for the environmental ranking.
13.2 Human health hazard assessment
The ranking of human health effects are based on the EU-classification system. In this system classification of chemical substances and the assignment of R-phrases are performed on the basis of the inherent properties of the substances. The following different effect types are taken into consideration:
- Acute toxicity
- Corrosive/irritative effect
- Organ toxicity
- Allergy/sensibilization
- Genotoxicity
- Carcinogenicity
- Reproductive toxicity
- Neurotoxicity
For substances classified in accordance with the EU-classification criteria (Annex 1), the official classification is used as guidance for the human health ranking. For substances, that are not officially classified, the human health ranking in this Chapter is based on the inherent toxicological properties of the substances.
The inherent toxicological properties are used for ranking of single substances in five "acute" health hazard classes (1-5) and five "subchronic/chronic" health hazard classes (A-E), where the classes 5 and E contain substances being considered to have the lowest potential for human health hazards (and vice versa).
The health hazard classes 1-5 describe the acute toxicological properties which cover acute toxicity, corrosive/irritative effects and organ toxicity. These toxicological effects are termed "acute effects". The health hazard classes A-E decribe chronic toxicological properties which cover allergy, neurotoxicity, carcinogenicity, genotoxicity and reproductive toxicity. These toxicological effects are termed "subchronic/chronic effects".
Class 1-3 and A-C are assigned to substances classified in accordance with the EU classification system. Class 4 and D are assigned to substances classified in accordance with the EU classification system, but also substances for which the evidence is too weak for classification, even though some evidence does exist. Class 5 and E include substances for which no evidence exists regarding human health or no concern exists based on existing evidence. Class 0 (acute effects) and 0 (chronic and sub-chronic effects) are assigned to substances for which there are no available data or the available data are not applicable in relation to the classification criteria.
The criteria for ranking the substances in the human health classes 1-5, A-E, and 0 are described in Table 13.2 and 13.3, respectively.
The health hazard class is stated for 100% pure substance, as well as for the substance diluted with a non-hazardous substance like water by use of the conventional method for product classification. However, it should be noted that the ranking of a substance in a health hazard class is a characterization of the health effect of a single substance and not a hazard assessment of a product containing the substances.
Table 13.2
Criteria for human health hazard assessment on acute, corrosive/irritative and organ
toxicity (acute effects)
Health hazard class |
Effect type |
Human health hazard criteria for acute effects |
Class 0 |
All |
No data or available data not applicable to assessment of the human health hazard |
Class 1 |
Acute toxicity |
Very high acute toxicity
|
|
Corrosive/ Irritative effect |
Severely corrosive
|
|
Organ toxicity |
Irreversible effects after a single exposure to low doses
|
Class 2 |
Acute toxicity |
High acute toxicity
|
|
Corrosive/ |
Corrosive
|
|
|
Irritating to respiratory tract
|
|
|
Severely damaging to eyes
|
|
Organ toxicity |
Irreversible effects after a single exposure to medium doses
|
Class 3 |
Acute toxicity |
Moderate acute toxicity
|
|
Corrosion/ |
Irritating to skin and/or eyes
|
|
Organ toxicity |
Irreversible effects after a single exposure to high doses
|
Class 4 |
Acute toxicity |
Low acute toxicity
|
|
Corrosive/ |
Mildly irritating to skin and/or eyes
|
|
Organ toxicity |
No observed effect |
Class 5 |
All |
No reason for concern with regard to health hazardous effects |
Table 13.3
Criteria for human health hazard assessment on allergy, neurotoxicity, carcinogenicity,
genotoxicity and reproductive toxicity (subchronic/chronic effects).
Health hazard Class |
Effect type |
Human health hazard criteria for subchronic/chronic effects |
Class 0 |
All |
No data or available data not applicable to assessment of the human health hazard |
Class A |
Allergy/ Sensibilisation |
Sensitising
|
|
Neurotoxicity |
Severe effects after repeated or prolonged exposure of low doses
|
|
Carcinogenicity |
Known carcinogenic to humans
|
|
Genotoxicity |
Known mutagenic
|
|
Reproductive Toxicity |
Known toxic to reproduction
|
Class B |
Allergy/ Sensibilisation |
Sensitising
|
|
Neurotoxicity |
Severe effects after repeated or prolonged exposure to medium doses
|
| Carcinogenicity | Probably carcinogenic to humans
|
|
| Genotoxicity | Probably mutagenic
|
|
| Reproductive toxicity | Probably toxic to reproduction
|
|
| Class C | Neurotoxicity | Severe effects after repeated or prolonged
exposure to high doses
|
| Carcinogenicity | Possibly carcinogenic to humans
|
|
| Genotoxicity | Possibly mutagenic
|
|
| Reproductive toxicity | Possibly toxic to reproduction
|
|
| Class D | Allergy/ Sensibilisation |
Sensitising Allergy has been found in a few isolated cases |
| Neurotoxicity | No observed effect | |
| Carcinogenicity | Probably not carcinogenic to humans
|
|
| Genotoxicity | No observed effect | |
| Reproductive toxicity | No observed effect | |
| Class E | All subchronic/ chronic effects | No reason for concern with regard to health hazardous effects |
Table 13.4
Examples on human health ranking of substances.
Substance |
CAS No. |
Human health hazard classes |
|
pH regulators |
|
Acetic acid > 90% |
P64-19-7 |
1E |
Acetic acid, 25-90% |
|
2E |
Acetic acid, 10-25% |
|
3E |
Acetic acid < 10% |
|
4E |
Formic acid > 90% |
64-18-6 |
1E |
Formic acid, 10-90% |
|
2E |
Formic acid, 2-10% |
|
3E |
Formic acid, < 2% |
|
4E |
Table 13.5 Look here!
Ranking of substances in household detergents (gmv.: geometric mean value).
14. References
Abdel-Rahman, M.S., D.M. Waldron and R.J. Bull. 1983. A comparative kinetics study of monochloramine and hypochlorous acid in rat. Journal of Applied Toxicology, 3, 175-179.
ACGIH. 1991. Documentation of the threshold limit values and biological exposure indices. Vol.3. 6.ed. American Conference of Governmental Industrial Hygienists, Inc., Cincinnati, Ohio, United States.
Afzelius, H. and H. Thulin. 1979. Allergic reactions to benzalkonium chloride. Contact Dermatitis, 5, 60.
AISE. 1997. Benefits and safety aspects of hypochlorite formulated in domestic product. Scientific dosier.
Akzo Nobel. 1998. Safety Data Sheets. Alkylpolyglycosides. Akzo Nobel Surface Chemistry, Stenungsund, Sweden.
Akzo Nobel. 1999a-c. Product Information. Bermodol SPS 2525 (1999a), Bermodol SPS 2532 (1999b), Bermodel SPS 2543 (1999c). Akzo Nobel Surface Chemistry, Stenungsund, Sweden.
Alomar, A., L. Conde-Salazar and C. Romaguera. 1985. Occupational dermatoses from cutting oils. Contact Dermatitis, 12, 129-138.
Andersen, K.E., A. Boman, K. Hamann and J.E. Wahlberg. 1984. Guinea pig maximization tests with formaldehyde releasers. Contact Dermatitis, 10, 257-266.
Andersen, K.E. and K. Hamann. 1984. The sensitizing potential of metalworking fluid biocides (phenolic and thiazole compounds) in the guinea-pig maximization test in relation to patch-test reactivity in eczema patients. Fd. Chem. Toxicol., 22 (8), 655-660.
Anderson, R.C. and J.H. Anderson. 1998. Acute toxic effects of fragrance products. Archives of Environmental Health, 53 (21), 138-146.
Anderson, R.L. and C.L. Alden. 1989. Risk assessment for nitrilotriacetic Acid (NTA). In D. Paustenbach (ed.), The Risk Assessment of Environmental and Human Health Hazards: A Textbook of Case Studies. John Wiley & Sons. New York, United States.
Anderson, R.L., W.E. Bishop and R.L. Campell. 1985. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. CRC. Crit. Rev. Toxicol., 15, 1-102.
Andresen, O., S.A. Christensen and T. Madsen. 1995. Substitution of surface active substances in detergents with glycolipids. Working Report No. 38, Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark, 77 p.
Angelidaki, I., F. Haagensen and B.K. Ahring. 2000. Anaerobic transformation of LAS in continuous stirred tank reactors treating sewage sludge. 5th World Surfactants Congress, May 29 – June 2, 2000, Florence. Proceedings, Vol. 2, p. 1551-1557.
Angelini, G., C. Foti, L. Rigano and G. A. Vena. 1995. 3-Dimethylaminopropylamine: A key substance in contact allergy to cocaamidopropylbetaine? Contact Dermatitis, 32, 96-99.
Angelini, G., L. Rigano, C. Foti, P. Rossi and G.A. Vena. 1996a. Pure cocamidopropylbetaine is not the allergen in patients with positive reactions to commercial cocamidopropylbetaine. Contact Dermatitis, 35, 252.
Angelini, G., L. Rigano, C. Foti, G.A. Vena and M. Grandolfo. 1996b. Contact allergy to impurities in surfactants: Amount, chemical structure and carrier effect in reactions to 3-dimethylaminopropylamine. Contact Dermatitis, 34, 248-252.
Arbejdsmiljøinstituttet. 1990a. Allergi- og overfølsomhedsfremkaldende stoffer i arbejdsmiljøet (in Danish). [Allergenic chemicals in the working environment]. Report No. 33, Danish Labour Inspection Service, Copenhagen, Denmark.
Arbejdsmiljøinstituttet. 1990b. Reproduktionsskadende kemiske stoffer i arbejdsmiljøet (in Danish). [Reproductively toxic chemicals in the working environment]. Report No. 35, Danish Labour Inspection Service, Copenhagen, Denmark.
Arbejdstilsynet. 1991. Hud og luftveje (in Danish). [Skin and respiratory passages]. Compendium 2, National Working Environment Authority, Copenhagen, Denmark.
Arbejdstilsynet. 2000. Grænseværdier for stoffer og materialer (in Danish). [Exposure limit values for substances and materials]. At-vejledning No. 6.0.1 oktober 2000, National Working Environment Authority, Copenhagen, Denmark.
Armstrong, D.K., H.R. Smith, J.R. Ross and J.R. White. 1999. Sensitization to cocamidopropylbetaine: An 8-year review. Contact Dermatitis, 40 (6), 335-336.
Arthur, J.W., A.E. Lemke, U.R. Mattson, and B.S. Halligan. 1974. Toxicity of sodium nitrilotriacetate (NTA) to the fathead minnow and an amphipod in soft water. Water Res., 8, 187-193.
ASTM. 2000. Annual Book of ASTM Standards. Water and environmental technology; pesticides; resource recovery; hazardous substances and oil spill responses; waste management; biological effects. American Society for Testing and Materials, Philadelphia, PA, United States.
Auletta, C.S., R.E. Schroeder, W.J. Krasavage and C. Stack. 1991. Toxicology of diethylene glycol butyl ether: 3. Dermal subchronic toxicity/fertility study in rats. Toxicologist, 11 (1), 316.
Balson, T. and M.S.B. Felix. 1995. Biodegradability of non-ionic surfactants, p. 204-230. In D.R. Karsa and M.R. Porter (eds.), Biodegradability of Surfactants. Blackie Academic & Professional, Glasgow, United Kingdom (review).
Bando, H., S. Mohri, F. Yamashita, Y. Takakura and M. Hashida. 1997. Effects of skin metabolism on percutaneous penetration of lipophilic drugs. J. Pharmaceut. Sci., 86, 759-761.
Bang Pedersen, N. and S. Fregert. 1976. Occupational allergic contact dermatitis from chloroacetamide in glue. Contact Dermatitis, 2, 122-123.
Bartnik, F.K. and F. Wingen. 1979. Percutaneous absorption of dodecyltrimethylammonium bromide, a cationic surfactant, in the rat. Fd. Cosmet. Toxicol., 17, 633-637.
BASF. 1999. Safety Data Sheet. Trilon B Flüssig. BASF A/S, Copenhagen, Denmark.
Bashir, M. 1998a. Ready biodegradability of 14C-RH-573: Modified Sturm Test . Covance Laboratories Inc., Madison, WI. Rohm and Haas Technical Report No. TR-97-076.
Bashir, M. 1998b. Ready biodegradability of 14C-RH-651: Modified Sturm Test . Covance Laboratories Inc., Madison, WI. Rohm and Haas Technical Report No. TR-97-15.
Battersby, N.S., L. Kravetz and J.P. Salanitro. 2000. Effect of branching on the biodegradability of alcohol-based surfactants. 5th World Surfactants Congress, May 29 – June 2, 2000, Florence. Proceedings, Vol. 2, p. 1397-1407.
Bayer. 1997. Materials safety datasheet, 2-phenoxyethanol (Phenoxyethanol rein), Bayer A/S, Lyngby, Denmark.
Belanger, S.E., D.H. Davidson, J.L. Farris, D. Reed and D.S. Cherry. 1993. Effects of cationic surfactant exposure to a bivalve mollusc in stream mesococms. Environ. Toxicol. Chem., 12, 1989-1802.
Belanger, S.E., E.M. Meiers and R.G. Bausch. 1995. Direct and indirect ecotoxicological effects of alkyl sulfate and alkyl ethoxysulfate on macroinvertebrates in stream mesocosms. Aquatic Toxicology, 33, 65-87.
Belanger, S.E. and K.L. Rupe. 1996. A flow-through laboratory microcosm suitable for assessing effects of surfactants on natural periphyton. Environmental Toxicology and Water Quality: An International Journal, 11, 65-76.
Benke, G.M. and M.J. Brown. 1977. Safety testning of alkyl polyethoxylate. Nonionic surfactants. I. Acute effects. Fd. Cosmet. Toxicol., 15, 309-318.
Berol Nobel. 1993. Amphoteen 24. Produktinformation (in Swedish). [Amphoteen 24. Product information].
Bertleff, W., R. Baur, H. Gümbel and M. Welch. 1997. Schaumarme Tenside (in German). [Weakly foaming surfactants]. SÖFW-Journal, 123, 222-233.
Bhargava, H.N. and P.A. Leonard. 1996. Triclosan: Applications and safty. American Journal of Infection Control, 24 (3).
BIBRA. 1989. Benzalkonium chloride. Toxicity profile. BIBRA Toxicology International, British Industrial Biological Research Association, Carshalton, United Kingdom.
BIBRA. 1994. Dipropylene glycol. Toxicity profile. BIBRA Toxicology International, Surrey, United Kingdom, 4 p.
Biochema Schwaben. 2000. Rodurol BIT. Technisches Spezial-konservierungsmittel gemäss BgVV –Empfehlung XIV. Biochema Schwaben – Dr. Lehmann GMBH & Co. KG, Memmingen, Germany.
Birch, R.R. 1984. Biodegradation of nonionic surfactants. J. Am. Oil Chem. Soc., 62, 340-343.
Birch, R.R., C. Biver, R. Campagna, W.E. Gledhill, U. Pagga, J. Steber, H. Reust and W.J. Bontinck. 1989. Screening of chemicals for anaerobic biodegradability. Chemosphere, 19, 1527-1550.
Birge, W.J. and J.A. Black. 1977. Sensitivity of vertebrate embryos to boron compounds. Washington D.C., US Environmental Protection agency, Office of Toxic Substances (EPA-560/1-76-008).
Bishop, W.E. and R.I. Perry. 1979. Development and evaluation of a flow-through growth inhibition test with duckweed (Lemna minor), p. 421-435. In D.R. Branson and K.L. Dickson (eds.), Aquatic Toxicology and Hazard Assessment: 4th Conference. STP 737, ASTM, Philadelphia, PA, United States.
Bishop, W.E. & A.W. Maki. 1980. A critical comparison of bioconcentration test methods, p. 116-129. In J.G. Eaton, P.R. Parrish and A.C. Hendricks (eds.), Aquatic Toxicology. American Society for Testing and Materials, ASTM STP 707.
Björkner, B., M. Brize, I Dahlquist, S. Fregert, B. Gruvberger and K. Persson. 1986. Contact allergy to the preservative Kathon CG. Contact Dermatitis, 14, 85-90.
BKH Consulting Engineers. 1994. Environmental data review of soaps. Nederlandse Vereniging van Zeepfabrikanten NVZ, Delft, The Netherlands, 31 pp (review).
Bode, H., R. Ernst and J. Arditti. 1978. Biological effects of surfactants. III Hydra as a highly sensitive assay animal. Environ. Pollut., 17, 175-185.
Boer, E.M. de, W.G. van Ketel and D.P. Bruynzeel. 1989. Dermatoses in metal workers. Contact Dermatitis, 20, 280-286.
Boethling, R.S. 1984. Environmental fate and toxicity in wastewater treatment of quaternary ammonium surfactants. Water Research, 18, 1061-1076 (review).
Boethling, R.S. and D.G. Lynch. 1992. Quaternary ammonium surfactants, p. 145-177. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany (review).
Botham, P.A., J. Hilton, C.D. Evans, D. Lees and T.J. Hall. 1991. Assessment of the relative skin sensitizing potency of 3 biocides using the murine local lymph node assay. Contact Dermatitis, 25, 172-177.
Bracher, M., C. Faller, J. Spengler and C. A. Remhart. 1987. Comparison of in vitro cell toxicity with in vivo eye irritation. Mol. Toxicol., 1 (4), 561-570.
Bressan, M., R. Brunetti, S. Casellato, G.C. Fara, P. Giro, M. Marin, P. Negrisolo, L. Tallandini, S. Thomann, L. Tosoni and M. Turchetto. 1989. Effects of linear alkylbenzene sulfonate (LAS) on benthic organisms. Tenside Surfactants Detergents, 26, 148-158.
Bringmann, G. and R. Kuhn. 1977. The effects of water pollutants on Daphnia magna. Z. Wasser-Abwasser-Forsch. 10(5), 161-166.
Brown, N.M. and G.M. Benke. 1977. Safety testing of alkyl polyethoxylate. Nonionic surfactants. II. Subchronic studies. Fd. Cosmet. Toxicol., 15, 319-324.
Brown, V.M., F.S.H. Abram and L.J. Collins. 1978. The acute lethal toxicity to rainbow trout of an LAS surfactant and of its residues and degradation products. Tenside Detergents, 2, 57-59.
Bruze, M., I. Dahlquist, S. Fregert, B. Gruvberger and K. Persson. 1987a. Contact allergy to the active ingredients of Kathon CG. Contact Dermatitis, 16, 183-188.
Bruze, M., S. Fregert, B. Gruvberger and K. Persson.1987b. Contact allergy to the active ingredients of Kathon CG in the guinea pig. Acta Derm Venereol (Stockholm) 67, 315-320.
Bryce, D.M., B. Croshaw, J.E. Hall, V.R. Holland and B. Lessel. 1978. The activity and safety of the antimicrobial agent Bronopol (2-bromo-2 nitropropane 1,3- diol). J. Soc. Cosmet. Chem., 29, 3-24.
Brøste. 1998. Brugsanvisning for Dehyton AB 30 (in Danish). [Materials safety datasheet for Dehyton AB 39]. P. Brøste A/S, Lyngby, Denmark.
Budny, J.A. and J.D. Arnold. 1973. Nitrilotriacetate (NTA): Human metabolism and its importance in the total safety evaluation program. Toxicology and Applied Pharmacology, 25, 48-53.
Burke, B., A.H. Olavesen, C.G. Curtis and G.M. Powell. 1975. The biodegradation of some anionic detergents in the rat. A common metabolic pathway. Xenobiotica, 5 (9), 573-584.
Bussi, R., G. Chierico, N. Drouot, V. Garny, S. Hubbart, G. Malinverno and W. Mayr. 1996. Rat embryo-fetal development study on sodium perborate tetrahydrate. Teratology, 53 (5), 26A.
Buttar, H.S. and R.H. Downie. 1980. The biotransformation and disposition of bronopol following topical and intravenous administration to rats. Toxicology Letters, 6, 101-107.
Buttar, S. 1985. Embryotoxicity of benzalkonium chloride in vaginally treated rats. Journal of Applied Toxicology, 5 (6), 398-401.
Calvin, G., P.H. Long, K.A Stitzel, R.L. Anderson, R.R. Balmbra and R.D. Bruce. 1988. Ethylenediaminetetra (methylenephosphonic acid): Genotoxicity, biodistribution, and subchronic and chronic toxicity in rats. Food and Chemical Toxicology, 26, 601-610.
Camarasa, J.M. 1979. First epidemiological study of contact dermatitis in Spain: 1977. Acta Derm. Venereol. (Suppl)(Stoch.), 59 (85), 33-37.
Campbell, L. and H.K. Wilson. 1986. Blood alcohol concentrations following the inhaltion of ethanol vapour under controlled conditions. J. Forensic Science Soc., 26 (2), 129-35.
Caniggia, A. and C. Gennari. 1977. Kinetics and intestinal absorption of 32P-EHDP in man. Calc. Tiss. Res., 22, 428-429.
Canton, J.H. and W. Sloof. 1982. Substitutes for phosphate containing washing products: their toxicity and biodegradability in the aquatic environment. Chemosphere, 11 (9), 891-907.
Cardwell, R.D., C.E. Woelke, M.I. Carr and E. Sanborn. 1977. Appraisal of a reference toxicant for estimating the quality of oyster larvae. Bull. Environ. Contam. Toxicol., 18, 719-725.
Cardwell, R.D., C.E. Woelke, M.I.Carr and E.Sanborn. 1978. Variation in toxcity tests of bivalve mollusc larvae as a function of termination technique. Springer-Verlag New York, United States, 128-134.
Carradorri, S., A.M. Peluso and M. Faccioli. 1990. Systemic contact dermatitis due to parabens. Contact Dermatitis, 22, 238-239.
Carrondo, M.J.T, R. Perry and J.N. Lester. 1980. Behaviour of zeolite type a in the activated sludge process-I. Influence on treatment parameters. J. Wat. Pollut. Control Fed., 52, 2796-2800.
Carrondo, M.J.T., R. Perry and J.N. Lester. 1981. Sedimentation of zeolite type A in water and waste water. Can. J. Civ. Engng., 8, 206-217.
CESIO. 2000. Comité European des Agents de Surface et leurs Intermediaires Organiques. Classification and labelling of surfactants for human health hazards according to the Dangerous Substances Directive. CESIO recommendations for anionic and non-ionic surfactants. january 2000. CESIO Toxicology Advisory Group (CTAG).
CETOX. 2000. Preservatives 2000. Report prepared by CETOX for the Swedish Society for Nature Conservation. Foundation concerning criteria for Bra Miljöval, Göteborg, Sweden (review).
Chew, A.L. and H.I. Maibach. 1997. 1,2-Benzisothiazolin-3-one (Proxel R): Irritant or allergen? Contact Dermatitis, 36, 131-136.
Christophiemk, P., P. Gerike and M. Potokar. 1992. Zeolites, p. 205- 228. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany.
Chu, I., G.C. Becking, D.C. Villeneuve and A. Viau. 1978. Metabolism of Nitrilotriacetic Acid (NTA) in the mouse. Bull. Environ. Contam. Toxicol., 19, 417-422.
CIR. 1990. Final report on the safety assessment of 5-bromo-5-nitro-1,3-dioxane. Cosmetic Ingredient Review, Vol. 9, No. 2.
CIR. 1994. Propylene glycol, 2-aminoethanol. Cosmetic Ingredient Review, Vol. 13, No. 6.
CIRP. 1983. Final report on the safety assessment of Laureths –4 and –23. Cosmetic Ingredient Review Program, Vol. 2, No 7.
CIRP. 1984a. Addendum to the final report on the safety assessment of 2-bromo-2-nitropropane-1,3-diol. Cosmetic Ingredient Review Program, Vol. 3, No. 3.
CIRP. 1984b. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. Cosmetic Ingredient Review Program, Vol. 3, No. 5.
CIRP. 1986. Final report on the safety assessment of Cocamide DEA, Lauramide DEA, Linoleamide DEA, and Oleamide DEA. Cosmetic Ingredient Review Program, Vol. 5, No. 5.
CIRP. 1988. Final report on the safety assessment of Steareth-2,-4,-6,-7,-10,-11,-13,-15,-20. 7 (6). Cosmetic Ingredient Review Program, Vol. 7; No. 6.
CIRP. 1989. Final report on the safety assessment of benzalkonium chloride. Cosmetic Ingredients Review Program, Vol. 8, No. 4.
CIRP. 1990. Final report on the safety assessment of cocoamphoacetate, cocoamphopropionate, cocoamphodiacetate, and cocoamphodipropionate. Cosmetic Ingredient Review Program, Vol. 9, No. 2.
CIRP. 1991a. Final report on the safety assessment of cocamidopropyl betaine. Cosmetic Ingredient Review Program, Vol. 10, No. 1.
CIRP. 1991b. Final report on the safety assessment of chloroacetamide. Cosmetic Ingredient Review Program. Vol. 10, No. 1.
CIRP. 1992. Final report on the safety assessment of methylisothiazolinone and methylchloroisothiazolinone. Cosmetic Ingredient Review Program, Vol. 11, No. 1.
CIRP. 1993. Final report on the safety assessment of sodium dodecylbenzene sulfonate/TEA- dodecylbenzene sulfonate/sodiumdecylbenzene sulfonate. Cosmetic Ingredients Review Program, Vol. 12, No. 3.
CIRP. 1996. Amended final report on the safety assessment of Cocamide DEA. Cosmetic Ingredient Review Program, Vol. 15, No. 6.
CIRP. 1997. Final report on the safety assessment of cetrimonium chloride, cetrimonium bromide, and steartrimonium chloride. Cosmetic Ingredients Review Program, Vol 16, No. 3.
Clayton, GD. and F.E. Clayton. 1993. Pattys Industrial Hygiene and Toxicology. 4th ed. Vol.II, Part A-F. John Wiley & Sons Inc. New York, United States.
Coenen, W. 1988. Einfluss des linearen Alkylbenzolsulfonates (LAS) auf die Kinetik von Lindan, 4-Nitrophenol und DDT beim Zebrabärbling (in German). [Effect of linear alkylbenzene sulfonates (LAS) on the kinetics of Lindan, 4-nitrophenol, and DDT in zebra fish]. Diploma Thesis, University of Mainz, Germany.
Comotto, R.M., R.A. Kimerle and R.D. Swisher. 1979. Bioconcentration and metabolism of linear alkylbenzenesulfonate by daphnids and fathead minnows, p. 232-250. In L.L. Marking and R.A. Kimerle (eds.), Aquatic Toxicology , ASTM, 1979, Vol. ASTM STP 667.
Connor, T.H., P.G. Tee, M. Afshar and K.M. Connor. 1996. Mutagenicity of cosmetic products containing Kathon. Environmental and Molecular Mutagenesis, 28, 127-132.
Cooper, S.M. and S. Shaw. 1998. Allergic contact dermatitis from parabens in a tar shampoo. Contact Dermatitis, 39, 140-141.
Cooper, S.M. and S. Shaw. 1999. Occupational hand dermatitis due to 1,2-benzisothiazolin-3-one in the water-softener manufacturing industry. Contact Dermatitis, 40, 221.
Cosmetic Directive. 2000. Commission Directive 2000/6/EC of 29 February 2000 and Commission Directive 2000/11/EC of 10 March 2000 adapting to technical progress Annexes II, III, VI and VII of Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products.
Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances.
Cronin, E. 1980. Contact Dermatitis. Churchill Livingstone Inc., Edinburgh, United Kingdom.
CTFA. 1997. Data sheet on methyldibromo glutaronitrile.
Daly, I.W. and R.E. Schroeder. 1980. A teratology study of topically applied linear alkylbenzene sulphonate in rats. Fd. Cosmet. Toxicol., 18, 55–58.
Damgård Nielsen, G. 1983. The toxicity of chloroacetamide. A survey. Arch. Pharm. Chem., Sci. Ed., 11, 115-123.
Damstra, R.J., W.A. van Vloten and C.J.W. van Ginkel. 1992. Allergic contact dermatitis from the preservative 1,2-benzisothiazolin-3-one (1,2-BIT, Proxel): A case report, its prevalence in those occupationally at risk and in the general dermatological population, and its relationship to allergy to its analogue Kathon CG. Contact Dermatitis, 27, 105-109.
Danish Toxicology Centre. 1995. Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl- (Butylhydroxytoluene, BHT). In Nordic Council of Ministers. Health Effects of Selected Chemicals Hazardous to the Environment – Volume 4. TemaNord 1997:605.
Davies, R.E., S.R. Kynoch and M.P. Liggett. 1976. Eye irritation tests assessment of the maximum delay time for remedial irrigation. J. Soc. Cosmet. Chem., 27 (7), 301-306.
Dawson, D.A., D.J. Fort, D.L. Newell and A. Bantle. 1989. Developmental toxicity testing with Fetax: Evaluation of five compounds. Drug Chem. Toxicol., 12, 67-75.
Dean, B.J. 1985. Genetic toxicology testing of 41 chemicals. Mutation Research, 153, 57-77.
Debethizy, J.D., S.L. Longacre, R.B. Steigerwalt, F.W. Deckert, J.N. Moss, A.W. Hayes, J.M. Smith and H.E. Scribner. 1986. Absorption and disposition of 14C-labelled Kathon biocide, a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one, following intravenous or dermal administration to male sprague-Dawley rats. Fd. Chem. Toxicol., 24 ( 1), 43-49.
De Boer, E.M., W.G. van Ketel and D.B. Bruynzell. 1989. Dermatoses in metal workers. (II) Allergic contact dermatitis. Contact Dermatitis, 20, 208-286.
De Groot, A.C., J.D. Bos, B.A. Jagtman, D.P. Bruynzell, T. van Joost and J.W. Weyland. 1986. Contact allergy to preservatives – II. Contact Dermatitis, 15, 218-222.
De Groot, A.C. and J.W. Weyland. 1986. Contact allergy to chloroacetamide in an "anti-wrinkle serum". Contact Dermatitis, 15, 97-98.
De Groot, A.C., F.S. DeWit, J.D. Bos and J.W. Weyland 1987. Contact allergy to Cocamide DEA and Lauramide DEA in shampoo. Contact Dermatitis, 16, 117-118.
De Groot, A.C., J.W. Weyland and J.P. Nater. 1994. Unwanted Effects of Cosmetics and Drugs Used in Dermatology, 3rd. ed., Elsevier, Amsterdam, The Netherlands.
Dehnad, F. and K.-H. Radeke. 1993. Beschreibung der Desorption von Zink aus Fluss-sedimenten mittels Freundlich- und Langmuir-Isothermen. Acta Hydrochim. Hydrobiol. 21(4), 221-225.
Delft, J.L. van, D. de Wolff-Rovendaal and J.A. Oosterhuis. 1983. Irrigation with mercury chloride and sodium hypochlorite to prevent local recurrence after excision of conjunctival melanoma. An experimental study. Doc. Ophthalmol., 56, 61-67.
Denger, K. and A.M. Cook. 1999. Note: Linear alkylbenzenesulphonate (LAS) bioavailable to anaerobic bacteria as a source of sulphur. Journal of Applied Microbiology, 86, 165-168.
Detmar, U. and M. Agathos. 1988. Contact allergy to chloroacetamide. Contact Dermatitis, 19, 66-67.
DFG. 1989. Critical data evaluation for MAK values and classification of carcinogens. Vol. 2, VCH Verlagsgesellschaft mbH, Weinheim, Germany.
DFG. 1993. Deutsche Forschungsgemeinschaft. Occupational Toxicants. Critical data evaluation for MAK values and classification of carcinogens. Vol 5, VCH Verlagsgesellschaft mbH, Weinheim, Germany.
Diaz, M.P. Lamarao and T. Vale. 1992. Occupational contact allergy to 1,2-benzisothiazolin-3-one in the manufacture of air fresheners. Contact Dermatitis, 27, 205-207.
Dillarstone, A. and M. Paye. 1993. Antagonism in concentrated surfactant systems. Contact Dermatitis, 28, 198.
Domsch, A. 1995. Biodegradability of amphoteric surfactants, p. 231-254. In D.R. Karsa and M.R. Porter (eds.), Biodegradability of surfactants. Blackie Academic & Professional, Glasgow, United Kingdom.
Doom-Goossens, A., H. Degreef, J. Vanhee, L. Kerkhofs and M.T. Chrispeels. 1981. Chlorocresol and chloroacetamide: Allergens in medications, glues, and cosmetics. Contact Dermatitis, 7, 51-52.
Dorn, P.B., J.P. Salanitro, S.H. Evans and L. Kravetz. 1993. Assessing the aquatic hazard of some branched and linear non-ionic surfactants by biodegradation and toxicity. Environ. Toxicol. Chem., 12, 1751-1762.
Dorn, P.B., J.H. Rodgers, Jr., S.T. Dubey, W.B. Gillespie, Jr. and R.E. Lizotte, Jr. 1997. An assessment of the ecological effects of a C9-11 linear alcohol ethoxylate surfactant in stream mesocosm experiments. Ecotoxicology, 6, 275-292.
Drotman, R.B. 1980. The absorption, distribution, and excretion of alkylpolyethoxylates by rats and humans. Toxicology and Applied Pharmacology, 52, 38-44.
Dunkel, V.C., E. Zeiger, D. Brusick, E. McCoy, D. McGregor, K. Mortelmans, H.S. Rosenkranz and V.F. Simmon. 1985. Reproducibility of Microbial Mutagenicity Assays: II. Testing of Carcinogens and Noncarcinogens in Salmonella typhimurium and Escherichia coli. Environmental Mutagenesis, 7(5), 1-248.
EC. 1996. Technical guidance document in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No. 1488/94 on risk assessment for existing substances. Part II. ISBN 92-827-8012-0. ECSC-EC-EAEC, Luxembourg.
ECETOC. 1984. The EEC sixth amendment. A guide to risk evaluation for effects in the environment. Technical report No. 13. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium.
ECETOC. 1988. Evaluation of anaerobic biodegradation. Technical report No. 28. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium.
ECETOC. 1993. Polycarboxylate polymers as used in detergents. Joint Assessment of Commodity Chemicals. No.23. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium.
ECETOC. 1995. Reproductive and general toxicology of some inorganic borates and risk assessment for human beings. Technical Report No.63. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium.
EDTA, Risk Assessment. 2000. Draft report by Bundesanstalt für Arbeitsschutz und Arbeitsmedizin. Anmeldstelle Chemickaliengesetz, Dortmund, Germany.
EPIWIN. 1994. Estimation of log octanol/water partition coefficient. Version 1.35a, August 1994. Syracuse Research Corp., New York, United States.
Erikson, U., A. Johnson and M. Törnlund. 1995. Risk Assessment of Slimicides. KEMI Report No. 9/95. The Swedish National Chemicals Inspectorate, Solna, Sweden.
Eun, H. C., A. Y. Lee and Y. S. Lee. 1984. Sodium hypochlorite dermatitis. Contact Dermatitis, 11 (1), 45.
Executive Order. 1999. Executive Order No. 1001 of 15 December 1999 on precautions to prevent cancer risk when working with substances and materials issued by the National Working Environment Authority.
Falbe, J. (ed). 1986. Surfactants in Consumer Products. Theory, Technology and Application. Springer-Verlag, Berlin, Germany.
Fairchild, J.F., F.J. Dwyer. T.W. La Point, S.A. Burch and C.G. Ingersoll. 1993. Evaluation of a laboratory-generated NOEC for linear alkylbenzene sulfonate in outdoor experimental streams. Environ. Toxicol. Chem., 12, 1763-1775.
Federle, T.W. and B.S. Schwab. 1992. Mineralization of surfactants in anaerobic sediments of a laundromat wastewater pond. Water Res., 26, 123-127.
Fendinger, N.J., D.J. Versteeg, E. Weeg, S. Dyer and R.A. Rapaport. 1994. Environmental behaviour and fate of anionic surfactants, p. 528-557. In L.A. Baker (ed), Environmental Chemistry of Lakes and Reservoirs. ACS Advances in Chemistry Series No. 237. American Chemical Society, Washington DC, United States.
Fenger, B.M. Mandrup, G. Rohde and J.C.K. Sørensen. 1973. Degradation of a cationic surfactant in activated sludge pilot plants. Water Research, 7, 1195-1208.
Fewings, J. and T. Menné. 1999. An update of the risk assessment for ethylchloroisothiazolinone/methylisothiazoinone (MCI/MI) with focus on rinse-off products. Contact Dermatitis, 41, 1-13.
Fisher, A.A. 1979. Paraben dermatitis due to a new medicated bandage: The" paraben paradox". Contact Dermatitis, 5, 273-274.
Fisher, A.A. 1986. Contact Dermatitis. 3rd ed. Lea & Fibiger, Philadelphia, United States.
Flyvholm, M.-A. and T. Menné. 1992. Allergic contact dermatitis from formaldehyde. Contact Dermatitis, 27, 27-36.
Flyvholm, M.-A. 1997. Formaldehyde exposure at the workplace and in the environment. Allergologie, 20 (5), 225-231.
Ford, G.P. and M.H. Beck. 1986. Reactions to Quaternium 15, Bronopol and Germall 115 in a standard series. Contact Dermatitis, 14, 271-274.
Ford, R.A. and A.A. Bottomley. 1997. Method for evaluation of the potential toxicity to the neonate from exposure to xenobiotics to mother’s milk – application to three fragrance materials. Toxicologist, Mar. (1 Pt. 2), 367 (reviewed from abstract).
Fowler, J.F. 1998. Allergy to Cocamide DEA. American Journal of Contact Dermatitis, 9, 40-41.
Frankild, S. 1992. Dosis-effekt undersøgelse af natrium lauryl sulfats (SLS) indflydelse på 4 modelstoffers hudpenetration in vitro (in Danish). [Dosis-effect study of the effect of sodium lauryl sulfate on the in vitro penetration of the skin of 4 model chemicals]. Dermato-venerologiske Afdeling 1, Odense Sygehus, miljømedicin ISH, Odense University, Denmark.
Freeman, S. 1984. Allergic contact dermatitis due to 1,2-benzisothiazolin-3-one in gum arabic. Contact Dermatitis, 11, 146-149.
Frohm, U. 1996. Leverandørbrugsanvisning. Helse-, miljø- og sikkerhedsdatablad for Kathon CG (in Danish). [Materials safety datasheet for Kathon CG]. Intern nr. 1900. Vendico Chemicals AB/Rohm and Haas.
Frosch, P.J., I.R. White, R.J.G. Rycroft, A. Lahti, D. Burrows, J.G. Camarasa, G. Ducombs and J.D. Wilkinson. 1990. Contact allergy to Bronopol. Contact Dermatitis, 22, 24—26.
Furia, T.E. ed. 1972. CRC Handbook of Food Additives. 2nd ed. Cleveland.
Games, L.M., J.E. King and R.J. Larson. 1982. Fate and distribution of a quaternary ammonium surfactant, octadecyltrimethylammonium chloride (OTAC), in wastewater treatment. Environ. Sci. Technol., 16, 483-488.
Garberg, P., E. L. Åkerblom and G. Bolcsfoldi. 1988. Evaluation of a genotoxicity test measuring DNA-strand breaks in mouse lymphoma cells by alkaline unwinding and hydrozyapalitete elution. Mutat. Res., 203, 155-176.
Garcia, M.T., I. Ribosa, J. Sanchez Leal and W. Hrezcuch. 1996. Effect of homolog distribution on the toxicity of alcohol ethoxylates. J. Am. Oil Chem. Soc., 73, 903-906.
Garcia, M.T., I. Ribosa, E. Campos and J. Sanchez Leal. 1997. Ecological properties of alkylglycosides. Chemosphere, 35, 545-556.
Garnett, K., P.W.W. Kirk, N. Lester and R. Perry. 1986. Assessment of the interactions of metals and nitrilotriacetic acid in soil/sludge mixtures. Wat. Air Soil Pollut., 34, 175-184.
Gejlsbjerg, B., C. Klinge and T. Madsen. Mineralization of organic contaminants in sludge-soil mixtures. Environ. Toxicol. Chem. In press.
Gerike, P. and P. Gode. 1990. The biodegradability and inhibitory threshold concentration of some disinfectants. Chemosphere, 21, 799-812.
Gilbert, P.A. 1992. TAED - Tetraacetylethylenediamine, p. 319- 328. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany (review).
Gingell, R. and C.C. Lu. 1991. Acute, subchronic, and reproductive toxicity of a linear alcohol ethoxylate surfactant in the rat. J. Am. Coll. Toxicol.,10, 477-86.
Ginkel, C.G. van. 1995. Biodegradation of cationic surfactants, p.183-203. In D.R. Karsa and M.R. Porter (eds.), Biodegradability of Surfactants. Blackie Academic & Professional, Glasgow, United Kingdom (review).
Ginkel, C.G. van, M.A. Venema and M.G.J. Geurts. 2000. Rapid biodegradation of dissolved long-chain dialkyldimethylammonium salts. 5th World Surfactants Congress, May 29 – June 2, 2000, Florence. Proceedings, Vol. 2, p. 1408-1413.
Giolando, S.T. R.A. Rapaport, R.J. Larson and T.W. Federle. 1995. Environmental fate and effects of DEEDMAC: a new rapidly biodegradable cationic surfactant for use in fabric softeners. Chemosphere, 30 (6), 1067-1083.
Gledhill, W.E. and T.C.J. Feijtel. 1992. Environmental properties and safety assessment of organic phosphonates used for detergent and water treatment applications, p. 261-285. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds. Springer-Verlag, Berlin Heidelberg, Germany (review).
Gloxhuber, C. 1974. Toxicological Properties of Surfactants. Arch. Toxicol., 32, 245-270.
Gloxhuber, C.H., M. Potokar, W. Pittermann, S. Wallat, F. Bartnik, H. Reuter and S. Braig. 1983. Zeolithe A – A phosphate substitute for detergents: toxicological investigation. Fd. Chem. Toxicol., 21 (2), 209-220.
Gloxhuber, C., and K. Künstler. 1992. Anionic surfactants: Biochemistry, Toxicology, Dermatology. 2.ed., Vol. 43. Marcel Dekker, Inc., New York, United States.
Goldschmidt. 1993-1994. Literature search on anaerobic biodegradability of betaines and test reports on toxicity to the green alga S. subspicatus. Th. Goldschmidt AG, Essen, Germany.
Goodman, L.S., A.G. Gilman and A. Gilman. 1980. Goodman and Gilman’s the pharmacological basis of therapeutics. 6th ed., McMillan, New York, United States.
Goodrich, M.S., M.J. Melancon, R.A. Davis and J.J. Lech. 1991. The toxicity, bioaccumulation, metabolism and elimination of dioctyl sodium sulfosuccinate DSS in rainbow trout (Oncorhynchus mykiss). Water Res., 25, 119-124.
Goossens, A., L. Claes, J. Drieghe and E. Put. 1997. Antimicrobials: preservatives, antiseptics and disinfectants. Contact Dermatitis, 39, 133-134.
Gosselin, R.E., R.P. Smith and H.C. Hodge. 1984. Clinical toxicology of commercial products. 5th ed. Williams & Wilkins, Baltimore, United States.
Goyer, R.A., H.L. Falk, H. Hogan, D.D. Feldman and W. Richter. 1981. Renal tumors in rats given trisodium nitrilotriacetate acid in drinking water for 2 years. J. Natl. Cancer Inst., 66, 869-880.
Grant, W.M. and J.S. Schuman. 1993. Toxicology of the Eye. 4. ed. Charles C. Thomas Publisher, Springfield, Illinois, United States.
Gruvberger, B. 1997. Methylisothiazolinones. Diagnosis and prevention of allergic contact dermatitis. Doctoral dissertation. Department of Occupational and environmental dermatology, University Hospital, Malmö, Sweden.
Guhl, W. 1992. Ökologische Aspekte von Bor. Vertrag gehalten anlässlich der 39. Sepawa-Jahrestagung 1992 in Bad Dürkheim. SÖFW-Journal, 18, 3-10.
Hales, S.G. 1993. Biodegradation of the anionic surfactant dialkyl sulphosuccinate. Environ. Toxicol. Chem., 12, 1821-1828.
Hall, W.S., J.B. Patoczka, R.J. Mirenda, B.A. Porter and E. Miller. 1989. Acute toxicity of industrial surfactants to Mysidopsis bahia. Arch. Environ. Contam. Toxicol., 18, 765-772.
Hammond, B.G, S.J. Barbee, T. Inoue, N. Ishida, G.J. Levinskas, M.W. Stevens, A.G. Wheeler and T. Cascieri. 1986. A review of Toxicology Studies on Cyanurate and its Chlorinated Derivatives. Environmental Health Perspectives, 69, 287-292.
Hasegawa, R., M. Takahashu, T. Kokubo, F. Furukawa, K. Toyoda, H. Sato, Y. Kurokawa and Y. Hayashi. 1986. Carcinogenicity study of sodium hypochlorite in F344 rats. Chem. Toxic., 24 (12), 1295-1302.
Hayashi, M., M. Kishi, T. Sofuni and M. Ishidate Jr. 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Fd. Chem. Toxicol., 26 (6), 487-500.
Henkel KGaA. Department of Ecology. Ecological certificate with respect to the Nordic Environmental Labelling Programme of textile detergents. Henkel, Düsseldorf, Germany.
Henning, K., J. Kandler, H.D Nielsen. 1977. Actuelle Fragen zur Substitution von Phosphaten in Waschmitteln. Seife-Öle-Fette-Wachse, 103, 571-578.
Henry, J.C., E.H. Tschen and L.E. Becker. 1979. Contact urticaria to parabens. Archives of Dermatology, 115, 1231-1232.
Hiasa, Y., N. Konishi, Y. Kitahori and T. Shimoyama. 1985. Carcinogenicity study of a commercial sodium oleate in fisher rats. Fd. Chem. Toxicol., 23 (6), 619-623.
Hindson, C. and F. Lawlor. 1983. Coconut diethanolamine in cosmetic products: An overview. Cosmet. Toilet., 109, 53-62.
Hitchcock, W.S. and D.F. Martin. 1977. Effects and fate of a surfactant in cultures of the red tide organisms, Gymnodium breve. Bull. Environ. Contam. Toxicol., 18, 291-296.
Hodge, H.C. 1964. Toxicity studies on phosphates. Food Cosmet. Toxicol., 2, 147-154.
Holman, W.F. and K.J. Macek. 1980. An aquatic safety assessment of linear alkylbenzene sulfonate (LAS): Chronic effects on fathead minnows. Trans. Am. Fish Soc., 109, 122-130.
Holt, M.S., E. Matthijs and J. Waters. 1989. The concentrations and fate of linear alkylbenzene sulphonate in sludge amended soils. Water Res., 23, 749-759.
Holt, M.S., G.C. Mitchell and R.J. Watkinson. 1992. The environmental chemistry, fate and effects of nonionic surfactants, p. 89-144. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany (review).
Hope, J. 1977. Absence of chromosome damage in the bone marrow of rats fed detergent actives for 90 days. Mutat. Res., 56, 47-50.
Hopkins, J. 1994. Missed opportunities inclinical dermatology – the case of 1,2-benzisothiazolin-3-one. Fd. Chem. Toxicol., 32 (2), 189-191.
Hoyt, H.L. and H.L. Gewanter. 1992. Citrate, p 229-242. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany.
HSDB. 1998. Hazardous Substances Data Bank. Produced by the National Library of Medicine (NLM), United States. CHEM-BANKTM, Compact disc SP-018-047, SilverPlatter International N.V., November 1998. HSDB No. 164, last update: October 1998.
HSDB. 1999. Hazardous Substances Data Bank. Produced by the National Library of Medicine (NLM), United States. CHEM-BANKTM , Silver Platter International NV.
HSDB. 2000. Hazardous Substances Data Bank. Produced by the National Library of Medicine (NLM), United States. CHEM-BANKTM , Silver Platter International NV.
Huber, M., U. Meyer and P. Rys. 2000. Biodegradation mechanisms of linear alcohol ethoxylates under anaerobic conditions. Environ. Sci. Technol., 34, 1737-1741.
Hughes, F.A. and B.W. Lew. 1970. Physical and functional properties of some higher alkyl polyglucosides. J. Am. Oil Chem Soc, 47, 162-167.
Hüls. 1993. Safety Data Sheets. Marlipal 0 13/150, Marlipal 0 13/100. Hüls AG, Marl., Germany.
Hunter, B. and H.G. Benson. 1976. Long-term toxicity of the surfactant alpha-olefin sulphonate (AOS) in the rat. Toxicology, 5, 359-370.
Hurni, H, and H. Ohder. 1977. Reproduction study with formaldehyde and hexamethylenetetramine in beagle dogs. Food and Cosmetic Toxicology, 11, 459-462.
IARC. 1978. The International Agency for Research on Cancer. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Volume 17. Some N-nitroso compounds. IARC, World Health Organization, Lyon, France.
IARC. 1979. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Some monomers, plastics and synthetic elastomers and acrolein. Vol. 19. The International Agency for Research on Cancer, World Health Organization, Lyon, France.
IARC. 1990. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol. 48. The International Agency for Research on Cancer. World Health Organization, Lyon, France.
IARC. 1991. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol. 52. The International Agency for Research on Cancer, World Health Organization, Lyon, France.
IARC. 1995. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Wood dust and formaldehyde. Vol. 62. The International Agency for Research on Cancer, World Health Organization, Lyon, France.
IARC. 1997. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Silica, some silicates, coal dust and para-aramid fibrils. Vol. 68. The International Agency for Research on Cancer, World Health Organization, Lyon, France.
ICI. 1990. Safety data sheet of Proxels. ICI Speciality Chemicals.
ICSC. 1998. International Chemical Safety Cards. WHO.1119. NIOSH.
Inai, K., Y. Aoki, H. Akamizu, R. Eto, T. Nishida and S. Tokuoka. 1985. Tumorigenicity study of butyl and isobutyl p-hydroxybenzoates administered orally to mice. Fd. Chem. Toxicol., 23 (6), 575-578.
Inoue, K. and T. Sunakawa. 1980. Studies of in vitro cell transformation and mutagenicity by surfactants and other compounds. Fd. Cosmet. Toxicol., 18, 289-296.
IPCS. 1982. Toxicological evaluation of certain food additives. FAO/WHO Expert Committee on Food Additives, International Programme on Chemical Safety, World Health Organization, Geneva, Schwitzerland.
IPCS. 1996. Linear alkylbenzene sulfonates and related compounds. Environmental Health Criteria 169. World Health Organization, Geneva, Schwitzerland.
IPCS. 1998. Environmental Health Criteria 204, Boron. International Programme on Chemical Safety, World Health Organization, Geneva, Schwitzerland.
Ishidate, M., T. Sofuni, K. Yoshikawa, M. Hayashi, T. Nohmi, M. Sawada and A. Matsuoka. 1984. Primary mutagenicity screening of food additives currently used in Japan. Fd. Chem.Toxic., 22 (8), 623-636.
ISO. 1989. Fresh water algal growth inhibition test with Scenedesmus subspicatus and Selenastrum capricornutum. International Standard 8692. International Organization for Standardization, Geneva, Schwitzerland.
ISO. 1995. Water quality – evaluation of the "ultimate" anaerobic biodegradability of organic compounds in digested sludge – method by measurement of the biogas production. ISO 11734. International Organization for Standardization, Geneva, Schwitzerland.
ISO. 1996. Water quality – determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea). International Standard 6341. International Organization for Standardization, Geneva, Schwitzerland.
ISO. 1998. ISO international standards. International Organization for Standardization, Geneva, Switzerland.
ISO. 1999. ISO/TC 147/SC 5/WG N 182. Nomenclature Selenastrum capricornutum. International Organization for Standardization, Geneva, Schwitzerland.
Isomaa, B. 1975. Absorption, distribution and excretion of (14C)CTAB, a quaternary ammonium surfactant in the rat. Fd. Cosmet. Toxicol., 13, 231-237.
Isomaa, B., J. Reuter and B.M. Djupsund. 1976. The subacute and chronic toxicity of cetyltrimethylammonium broimide (CTAB), a cationic surfactant, in the rat. Archives of Toxicology, 35, 91-96.
IUCLID. 2000. CD-ROM. Public data on high volume chemicals. Year 2000 edition, Joint Research Centre, European Chemicals Bureau. Ispra, Italy.
Jelen, G., C. Cavelier, J.P. Protois and J. Foussereau. 1989. A new allergen responsible to shoe allergy: Chloroacetamide. Contact Dermatitis, 21, 110-111.
Jensen, O.C. and I. Petersen. 1991. Erhvervsasthma fremkaldt af duftstoffer I kattegrus (in Danish). [Occupational asthma precipitated by perfume added to gravel in cat toilets]. Ugeskrift for Læger, 153, 13, 25, 939-40.
Jones, S.K. and C.T.C. Kennedy. 1988. Chloroacetamide as an allergen in the paint industry. Contact Dermatitis, 18, 304-305.
Joost, Th. van, J. M. W. Habets, E. Stolz and A.M. Geursen-Reitsma. 1987. Sodium hypochlorite sensitization. Contact Dermatitis, 16 (2), 114-115.
Jungermann, E. (ed.). 1970. Cationic Surfactants. Surfactant Science Series, Vol. 4. Marcel Dekker, Inc., New York, United States.
Jørgensen, B.B. 1977. The sulfur cycle of a coastal marine sediment (Limfjorden, Denmark). Limnology and Oceanography, 22, 814-832.
Källqvist, T. NTA Vurdering av økotoksikologiske egenskaper. Norwegian Institute for Water Research, NIVA, Oslo, Norway, 10 p.
Kaluza, U. and K. Taeger. 1996. Einfluss der chemischen Struktur auf ökotoxikologische Eigenschaften von Alkanol-Ethoxylaten (in German). [Effect of chemical structure on the ecotoxicological properties of alcanol ethoxylates]. Tenside Surfactants Detergents, 33, 46-51.
Kapp, R.W., C. Bevan, T.H. Gardiner, M.I. Banton, T.R. Tyler and G.A. Wright. 1996. Isopropanol: Summary of TSCA test rule studies and relevance to hazard identification. Reg. Tox. Pharm., 23, 183-192.
Kappeler, T.U. 1982. The aquatic toxicology of DTDMAC and its ecological significance. Tenside Detergents, 19, 169-176.
Karlberg, A.T. 1998. D-Limonene – an old perfume ingredient introduced as a "natural" solvent in industry: Is there a risk of sensitization? p. 106-112. In P.J. Frosch, J.D. Johansen and I.R White (ed.), Fragrances. Beneficial and Adverse Effects, Springer-Verlag, Berlin Heidelberg, Germany.
Keczkes, K. and P.M. Brown. 1976. Hexahydro, 1,3,5, tris (2-hydroxyethyl) triazine, a new bacteriocidal agent as a cause of allergic contact dermatitis. Contact Dermatitis, 2, 92-98.
KEMI. 1990. Tvätt- och rengöringsmedel för hushållsbruk (in Swedish). [Laundry detergents and cleaning agents for consumer use]. Report No. 7/1990, Swedish National Chemicals Inspectorate, Solna, Sweden.
KEMI. 1994. Tvätt-disk- och rengöringsmedel (in Swedish). [Laundry detergents and cleaning agents]. Report No. 5/1994, Swedish National Chemicals Inspectorate, Solna, Sweden.
Kerns, W.D., K.L. Pavkov and D.J. Donofrio. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Research, 43, 4382-4392.
Ketel, W.G and L.S. Kirch. 1983. The problem of the sensitizing capacity of some grotans used as bacteriocides in cooling oils. Dermatosen, 31 (4), 118-121.
Kevekorde, S., V. Mersch-Sundermann, M. Diez and H. Dunkelberg. 1997. In vitro genotoxicity of polycyclic musk fragrances in the micronucleus test. Muta. Res., 395 (2-3), 145-150.
Kikuchi, M., M. Wakabayashi, T. Nakamura, W. Inoune, K. Takahashi, T. Kawana, H. Kawahara and Y.Koido. 1976. A study of detergents II. Acute toxicity of anionic surfactants on aquatic organisms. Ann. Rep. Tokyo. Metrop. Res. Inst. Environ. Prot., p. 57-69.
Kikuchi, M., M. Wakabayashi, H. Kojima and T. Yoshida. 1980. Bioaccumulation profiles of 35S-labelled sodium linear alkylpoly(oxythylene) sulfates in carp (Cyprinus carpio). Water Res., 14, 1541-1548.
Kimerle, R.A., R.D. Swisher and R.M. Schroeder-Comotto. 1975. Surfactant structure and aquatic toxicity. Proc. IJC Symposium on structure activity correlations in studies on toxicity and bioconcentration with aquatic organisms. Burlington, Ontario, Canada, p. 22-35.
Kimerle, R.A. and R.D. Swisher. 1977. Reduction of aquatic toxicity of linear alkylbenzene sulfonate (LAS) by biodegradation. Water Res., 11, 31-37.
Kimmel, C.A. 1977. Effect of route of administration on the toxicity and teratogenicity of EDTA in the rat. Toxicology and Applied Pharmacology, 40, 299-306.
Kirk-Otmer. 1994. Encyclopedia of Chemical Technology. Dialog on Disc. Third Edition. John Wiley & Sons, Inc. Dialog Information Services, Inc. Palo Alto, United States.
Knoll MicroCheck. 1996. Product Information. Bronopol Environmental Safety. Knoll MicroCheck, Nottingham, United Kingdom.
Kravetz, L., J.P. Salanitro, P.B. Dorn and K.F. Guin. 1991. Influence of hydrophobe type and extent of branching on environmental response factors of nonionic surfactants. J. Am. Oil Chem. Soc., 68, 610-618.
Kreybig, T. von, R. Preussmann and I. von Kreybig. 1969. Chemische Konstitution und teratogene Wirkung bei der Ratte. Arzneim Forsch., 19, 1073-76.
Kujawa, M., R. Macholz, H. Seidler, M. Härtig, H.J. Lewerenz, W. Schnaak and G. Zydek. 1987. Verteilung und Metabolismus von 2-Brom-2-nitropropan-1,3-diol (Bronopol). Z fur die gesamte Hygiene und ihre Grenzgebiete, 33, 27-29.
Kusk, O. and S. Petersen. 1997. Acute and chronic toxicity of tributyltin and linear alkylbenzene sulfonate to the marine copepod Acartia tonsa. Environ. Toxicol. Chem., 11, 1629-1633.
Kutt, E.C. and D.F Martin. 1974 Effect of selected surfactants on the growth characteristics of Gymnodium breve. Marine Biology, 28, 253-259.
LaKind, J.S., E.A. McKenna, R.P. Hubner and R.G. Tardiff. 1999. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Critical Reviews in Toxicology, 29 (4), 331-365.
Lama, L., D. Vanni, M. Barone, P. Patrone and C. Antonelli. 1986. Occupational dermatitis to chloroacetamide. Contact Dermatitis, 15 (4), 243.
Lay, J.P., W. Klein and F. Korte. 1983. Elimination and biodistribution studies of (14C)dodecylbenzene sulfonate in rats, following low dosing in the daily diet and a single i.p. administration. Toxicology Letters, 17, 186-192.
Lee, G.F., W. Rast and R.A. Jones. 1978. Eutrophication of water bodies: Insights for an age-old problem. Environ. Sci. Technol., 12, 900-908.
Leuwen, K. van, C. Roghair, T. de Nijs and J. de Greef. 1992. Ecotoxicological risk evaluation of the cationic fabric softener DTDMAC. III. Risk assessment. Chemosphere, 24, 629-639.
Lewis, R.J. 1996. Sax´s Dangerous Properties of Industrial Materials.9th ed. Van Nostrand Reinhold, New York, United States.
Lewis, M.A. and R.L. Perry. 1981. Acute toxicities of equimolar and ecotoxic surfactant mixtures to Daphnia magna and Lepomis macrochirus, p. 401-418. In D.R. Branson and K.L. Dickson (eds.), Aquatic Toxicology and Hazard Assessment, Fourth Conference, ASTM STP 737, American Society for Testing and Materials, United States.
Lewis, M.A. and D. Suprenant. 1983. Comparative acute toxicity of surfactants to aquatic invertebrates. Ecotoxicology and Environmental Safety, 7, 313-322.
Lewis, M.A. and V.T. Wee. 1983. Aquatic safety assessment for cationic surfactants. Environ. Toxicol. Chem., 2, 105-108.
Lewis, M.A. and B.G. Hamm. 1986. Environmental modification of the photosynthetic response of lake plankton to surfatants and significance to a laboratory – field comparison. Water Res., 20, 1575-1582.
Lewis, P.A. and W.B. Horning. 1991. Differences in acute toxicity test results of three reference toxicants on Daphnia at two temperatures. Environ. Toxicol. Chem., 10, 1351-1357.
Lewis, M.A., C.A. Pittinger, D.H. Davidson and C.J. Ritchie. 1993. In situ response of natural periphyton to an anionic surfactant and an environmental risk assessment for phytotoxic effects. Environ. Toxicol. Chem., 12, 1803-1812.
Lillebæltssamarbejdet. 1998. Miljøfremmede stoffer i havbunden (in Danish). [Xenobiotic substances in marine sediments]. Fyns, Sønderjyllands, and Vejle Counties, 70 p.
Little, A.D. 1981. Human safety and environmental aspects of major surfactants (supplement). A report to the Soap and Detergent Association, ADL. Reference 84048.
Liu, P. and J.L. Reynolds. 1994. Anaerobic aquatic metabolism of 14C-RH-651. Rohm and Haas Technical Report No. 34-94-63. XenoBiotic Laboratories, Inc., Plainsboro, NJ, United States.
Lizotte Jr., R.E., D.C.L. Wong, P.B. Dorn and J.H. Rogers, Jr. 1999. Effects of a homologous series of linear alcohol ethoxylate surfactants on fathead minnow early life stages. Arch. Environ. Contam. Toxicol., 37, 536-541.
Lobemeier, C., C. Tschoetschel, S. Westie and E. Heymann. 1996. Hydrolysis of parabens by extracts from differing layers of human skin. Biol. Chem., 377, 647-651.
Loveday, K.S., M.H. Lugo, M.A. Resnick, B.E. Anderson and E. Zeiger. 1989. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro: II. Results with 20 chemicals. Environ. Mol. Mutagen. 13 (1), 60-94.
Lovell, C.R. and P. Staniforth. 1981. Contact allergy to benzalkonium chloride in plaster of Paris. Contact Dermatitis, 7 (2), 343-344.
Lundahl, P. and R. Cabridenc. 1978. Molecular structure – biological properties relationships in anionic surface-active agents. Water Res., 12, 25-30.
Macek, K.J. and S.F. Krzeminski. 1975. Susceptibility of Bluegill Sunfish (Lepomis macrochirus) to noninonic surfactants. Bull. Environ. Contam. Toxicol., 13, 377-384.
Macek, K.J. and B.H. Slight. 1977. Utility of toxicity tests with embryos and fry of fish in evaluating hazards associated with the chronic toxicity of chemicals to fishes, p. 137-146. In F.I. Mayer and G.L. Hamelink (eds), Aquatic Toxicity and Hazard Assessment, ASTM STP 634, United States.
Madsen, T., A. Damborg, H.B. Rasmussen and C. Seierø. 1994. Evaluation of methods for screening surfactants. Ultimate aerobic and anaerobic biodegradability. Working Report No. 38, Danish Environmental Protection Agency, Ministry of Environment, Copenhagen, Denmark, 69 p.
Madsen, T., H.B. Rasmussen and L. Nilsson. 1995. Anaerobic biodegradation potentials in digested sludge, a freshwater swamp and a marine sediment. Chemosphere, 31, 4243-4258.
Madsen, T., H.B. Rasmussen and L. Nilsson. 1996a. Methods for screening anaerobic biodegradability and toxicity of organic chemicals. Environmental Project No. 336, Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark, 58 p.
Madsen, T., G. Petersen, C. Seierø and J. Tørsløv. 1996b. Biodegradability and aquatic toxicity of glycoside surfactants and a nonionic alcohol ethoxylate. J. Am. Oil Chem. Soc., 73, 929-933.
Madsen, T., M. Winther-Nielsen and L. Samsøe-Petersen. 1998. Effects of organic chemicals in sludge applied to soil. Degradation and toxicity to organisms living in soil. Environmental Project No. 432, Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark, 36 p.
Madsen, T., M. Winther-Nielsen and D. Rasmussen. 1999. Studies on the fate of linear alkylbenzene sulfonates (LAS) in sludge and sludge-amended soil. The CLER Review, 5, 14-19.
Madsen, T. 2000. Evaluation of the biodegradability of RH-573 and RH-651. Report to Rohm and Haas. Study No. 12113. March 2000. DHI – Water and Environment, Hørsholm, Denmark.
Madsen, T., L. Samsøe-Petersen, K. Gustavson and D. Rasmussen. 2000. Ecotoxicological assessment of antifouling biocides and non-biocidal antifouling paints. Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark. http://www.mst.dk/200006pubs/87-7944-084-3/default_eng.htm.
Maibach, H.I. 1977. Dermal sensitization potential of 2-bromo-2-nitropropane-1,3-diol (Bronopol). Contact Dermatitis, 3, 99-108.
MAK. 1995. Gesundheitschädliche Arbeitsstoffe. Toxikologisch-arbeitsmedizinische Begründung von MAK-Werten. VCH Verlagsgesellschaft, Weinheim, Germany.
Maki, A.W. 1979. Correlations between Daphnia magna and Fathead minnow (Pimephales promelas) chronic toxicity values for several classes of test substances. J. Fish Res. Board Can., 36, 411-421.
Maki, A.W. and W.E. Bishop. 1979. Acute toxicity studies of surfactants to Daphnia magna and Daphnia pulex. Arch. Environ. Contam. Toxicol., 8, 599-612.
Marcomini, A., M. Zanette, G. Pojana and M.J.-F. Suter. 2000a. Behaviour of aliphatic alcohol polyethoxylates and their metabolites under standardized aerobic biodegradation conditions. Environ. Toxicol. Chem., 19, 549-554.
Marcomini, A., G. Pojana, C. Carrer, L. Cavalli, G. Cassani and M. Lazzarin. 2000b. Aerobic biodegradation of monobranched aliphatic alcohol polyethoxylates. Environ. Toxicol. Chem., 19, 555-560.
Marcomini, A., G. Pojana, C. Carrer, A. Giacometti, L. Cavalli and G. Cassani. 2000c. Biodegradation behavior of alcohol polyethoxylates (AE): An up to date. 5th World Surfactants Congress, May 29 – June 2, 2000, Florence. Proceedings, Vol. 2, p. 1380-1386.
Marks, T.A., W.C. Worthy and R.E. Staples. 1980. Influence of formaldehyde and Sonacide (potentiated acid glutaraldehyde) on embryo and fetal development in mice. Teratology, 22, 51-58.
Matthijs, E., M.S. Holt, A. Kiewiet and G.B.J. Rijs. 1999. Environmental monitoring for linear alkylbenzene sulfonate, alcohol ethoxylate, alcohol ethoxy sulfate, and soap. Environ. Toxicol. Chem., 18, 2634-2644.
McDermott, J.A., D.H. Hughes and P.M. Quinlin. 1975. Alkyl ethoxy sulphates, absorption, distribution, excretion and metabolite identification studies in rats and man. Toxicol. Appl. Pharmacol., 33, 145.
McGregor, D.B., A. Brown, P. Cattanach, I. Edwards, B. McBride, C. Riach and W.J. Caspary. 1988. Responses of the L51784tk+/tk- mouse lymphoma cell forward mutation assay:III. 72 coded chemicals. Environmental and Molecular Mutagenesis, 12, 85-154.
Meier, J.R, R.J. Bull, J.A. Stober and M.C. Cimino. 1985. Evaluation of chemicals used for drinking water disinfection for production of chromosomal damage and sperm-head abnormalities in mice. Environmental mutagenesis, 7, 201-211.
Merck. 1989. The Merck Index. An Encyclopedia of Chemicals, Drugs and Biologicals. 11th ed. Merck and Co. Inc Rahway, New Jersey, United States.
Mersch-Sundermann, V., S. Kevekordes and C. Jenter. 1998. Testing of SOS induction of artificial polycyclic musk fragrances in E. coli PQ37 (SOS chromotest). Toxicology Letters, 95 (2), 147-54.
Migliore, L., L. Ventura, R. Barale, N. Loprieno, S. Castellino and R. Pulci. 1989. Micronuclei and nuclear anomalies induced in the gastro-intestinal epithelium of rats treated with formaldehyde. Mutagenesis, 4(5), 327-334.
Millqvist, E. and O. Lowhagen. 1996. Placebo-controlled challenges with perfume in patients with asthma-like symptoms. Allergy, 51 (6), 434-439.
MITI. 1992. Data of existing chemicals based on the CSCL Japan. Ministry of International Trade and Industry, Japan. ISBN 4-89074-101-1.
Miyazaki, O., T. Yamagishi and M. Matsumoto. 1984. Residues of 4-chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene (triclosan methyl) in aquatic biota. Bull. Environ. Contam. Toxicol., 32 (2), 227-232.
Monaldi, A., R. Mariot, M. Zordan, M. Paleologo and A.G. Levis. 1988. Nitrilotriacetic acid (NTA) does not induce chromosomal damage in mamalian cells either in vitro or in vivo. Mutation Research, 208, 95-100.
Monte, W.C., S.H. Ashoor and B. J. Lewis. 1983. Mutagenicity of two non-formaldehyde-forming antimicrobial agents. Fd. Chem. Toxicol., 21 (5), 695-697.
Morse, G.G., J.N. Lester and R. Perry. 1994. The environmental and economic impact of key detergent builder systems in the European union. Environmental Engineering Laboratory Imperial College of Science, Technology and Medicine. London SW7 2BU. ISBN 0 948411 09 0, United Kingdom.
Morse, P.M. 1999. Soaps and detergents. Chemical and Engineering News, February 1, 1999.
Mortelmans, K., S. Haworth, T. Lawlor, W. Speck, B. Tainer and E. Zeiger. 1986. Salmonella mutagenicity tests II. Results from the testing of 270 chemicals. Environmental Mutagenesis, 8 (7), 1-119.
Moscato, G., P. Omodeo, A. Dellabianca, M.C. Colli, F. Pugliese, C. Locatelli and J. Scibilia. 1977. Occupational asthma and rhinitis caused by 1,2-benzisothiazolin-3-one in a chemical worker. Occup. Med., 47 (4), 249-251.
Muralidhara and K. Narasimhamurthy. 1991. Assessment of in vivo mutagenic potency of ethylendiamine tetraacetic acid in albino mice. Fd. Chem. Toxicol., 29(12), 845-849.
Nakayama, H. 1998. Fragrance hypersensitivity and its control, p. 83-91. In P.J. Frosch, J.D. Johansen and I.R. White (eds.), Fragrances. Beneficial and Adverse Effects. Springer-Verlag, Berlin Heidelberg, Germany.
Naylor, C.G., F.J. Castaldi and B. J. Hayes. 1988. Biodegradation of nonionic surfactants containing propylene oxide. J. Am. Oil Chem. Soc., 65, 1669-1676.
Nelson, B.K., W.S. Brightwell, A. Khan, E.F. Krieg and A.M. Hoberman. 1990. Developmental toxicology assessment of 1-ocatanol, 1-nonanol, and 1-decanol administered by inhalation to rats. J. Am. Coll. Toxicol., 9 (1), 93-97.
Newsome, C.S., D. Howes, S.J. Marshall and R.A. van Egmond. 1995. Fate of some anionic and alcohol ethoxylate surfactants in Carassius auratus. Tenside Surfactants Detergents, 32, 498-503.
Ng, S.K. and C.L. Goh. 1989. Contact allergy to sodium hypochlorite in Eusol. Contact Dermatitis, 21, 281.
Nielsen, N.H. and M.T. Menné. 1992. Allergic contact dermatitis in an unselected Danish population. Acta Derm. Venereol., 72, 456-60.
Nipa. 1991. Nipa Laboratories Ltd. Finished product specification on Nipagin M. Reference No. P-M1., Nipa Laboratories Ltd., Mid Glamorgan, United Kingdom.
Nipa. 1992. Nipa Laboratories Ltd. Finished product specification on Nipasol M. Reference No. P-P1. Nipa Laboratories Ltd., Mid Glamorgan, United Kingdom.
Nipa. 1997. Nipa Laboratories Ltd. Finished product specification on Nipagin A. Reference No. FP 1020. Nipa Laboratories Ltd., Mid Glamorgan, United Kingdom.
Nixon, G.A. 1971. Toxicology evaluation of trisodium nitrilotriacetate. Toxicol. Appl. Pharmacol., 18, 398-406.
Nolen, G.A. and E.V. Buehler. 1971. The effects of disodium etidronate on the reproductive functions and embryogeny of albino rats and New Zealand rabbits. Toxicol. Appl. Pharmacol., 18, 548-561.
Nolen, G.A., L.W. Klusman, D.L. Back and E.V. Buehler. 1971. Reproduction and teratology studies of trisodium nitrilotriacetate in rats and rabbits. Fd. Cosmet. Toxicol., 9, 509-518.
Nolen, G.A., L.W. Klusman, L.F. Patrick and R.G. Geil. 1975. Teratology studies of a mixture of tallow alkyl ethoxylate and linear alkylbenzene sulfonate in rats and rabbits. Toxicology, 4, 231-243.
Nolen, G.A. and T.A. Dierckman. 1983. Test for aluminosilicate teratogenicity in rats. Food and Chemical Toxicology, 21 (5), 697.
Nolen, G.A., A. Monroe, C.D. Hassall, J. Iavicoli, R.A. Jamieson and G.P. Daston. 1989. Studies of the developemental toxicity of polycarboxylate dispersing agents. Drug and Chem. Tox., 12, 95-110.
Nomura, T., S. Kimura, S. Hata, T. Kanzaki and H. Tanaka. 1980. The synthetic surfactants AS and LAS interrupt pregnancy in mice. Life Sciences, 26, 49-54.
Nomura, T., S. Hata, K. Shibata and T. Kusfuka. 1987. Killing of preimplantation mouse embryos by main ingredients of cleaners AS and LAS. Mutation Research, 190 (1), 25-29.
Nord. 1991. Kriteriedokumenter fra et nordisk allergiprojekt. Review over 2-chloracetamids allergene effekter (in Danish). [Review of the allergenic effects of 2-chloroacetamide]. Nord 1991:51. Nordisk Ministerråd, Copenhagen, Denmark.
NTIS. 1992a. Final report on the developmental toxicity of dipropylene glycol (CAS#25265-71-8) in New Zealand WhiteQ rabbits. NTIS Technical report (NTIS/PB92-238294) (NTP/TER-90-14), 128 p.
NTIS. 1992b. Final report on the developmental toxicity of dipropylene glycol (CAS#25265-71-8) in Sprague-Dawley (CDQ) rats. NTIS Technical report (NTIS/PB92-191679), 166 p.
Nuck, B.A. and T.W. Federle. 1996. Batch test for assessing the mineralization of 14C-radiolabeled compounds under realistic anaerobic conditions. Environ. Sci. Technol., 30, 3597-3603.
Nusair, T.L., P.J. Danneman, J. Stotte and P.H.S. Bay. 1988. Consumer products: Risk assessment process for contact sensitization. Toxicologist, 8, 258.
Nyberg, H. 1988. Growth of Selenastrum capricornutum in the presence of synthetic surfactants. Water Res., 22, 217-223.
OECD. 1998. OECD guidelines for the testing of chemicals. Ninth addendum to the OECD guidelines for the testing of chemicals, Organisation for Economic Co-operation and Development, Paris, France.
OECD. 2000. Guidance document on global harmonisation of classification criteria. Chapter 5. Bioaccumulation (draft).
Office of Pesticide Programs. 1995. Environmental effects database (EEDB). Environmental Fate and Effects Division, U.S. EPA, Washington, D.C., United States.
Ong, J.H. and B.S. Rutherford. 1980. Some factors affecting the rate of N-nitrosodiethanolamine formation from 2-bromo-2-nitropropane-1,3-diol and ethanolamines. J. Soc. Cosmet. Chem., 31, 153-159.
Overman, D. O. 1985. Absence of embryotoxic effects of formaldehyde after percutaneous exposure in hamsters. Toxicology Letters, 24, 107-110.
Painter, H.A. 1992. Anionic surfactants, p. 1-88. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds. Springer-Verlag, Berlin Heidelberg, Germany (review).
Palmer, A.K., M.A. Readshaw and A.M. Neuff. 1975a. Assessment of the teratogenic potential of surfactants. Part I: LAS, AS and CLD. Toxicology, 3, 91-106.
Palmer, A.K., M.A. Readshaw and A.M. Neuff. 1975b. Assessment of the teratogenic potential of surfactants. Part II: AOS. Toxicology, 3, 107-113.
Palmer, A.K., A.M. Bottomley, J.A. Edwards and R. Clark. 1983. Absence of embryotoxic effects in rats with three quarternary ammonium compounds (cationic surfactants). Toxicology, 26, 313-315.
PARCOM. 1995. Protocol on methods for the testing of chemicals used in the offshore industry. Oslo and Paris Commissions, London, United Kingdom.
Pärt, P. and G. Wikmark. 1984. The influence of some complexing agents (EDTA and citrate) on the uptake of cadmium in perfused rainbow trout gills. Aquatic Tox., 5, 277-289.
Perrenoud, D., A. Bircher, T. Hunziker, H. Suter, L. Bruckner-Tuderman, J. Stäger, W. Thürlimann, P. Schmid, A. Svand and N. Hunziker. 1994. Frequency of sensitization to 13 common preservatives in Switzerland. Contact Dermatitis, 30, 276-279.
Perry, R. 1981. Detergent builders and water-quality –a changing scene. Effl. Water Treat. J., 21, 446-449.
Perry, R., P.W.W. Kirk, T. Stephenson and J.N. Lester. 1984. Environmental aspects of the use of NTA as a detergent builder. Water Res., 18 (3), 255-276.
Peters, M.S., S.M. Connolly and A.L. Schroeter 1983. Bronopol allergic contact dermatitis. Contact Dermatitis, 9, 397-401.
Petersen, K.L., S.N. Pedersen, L.B. Christiansen, B. Korsgaard, P. Bjerregaard. The preservatives ethyl-, propyl-, and butylparaben are oestrogenic in an in vivo fish assay. Pharmacology & Toxicology, 86. In press.
Pinola, A., T. Estlander, R. Jolanski, K. Tarvainen and L. Kanerva 1993. Occupational allergic contact dermatitis due to coconut diethanolamide (Cocamide DEA). Contact Dermatitis, 29, 262-265.
Plassche, E.J. van de, and F. Balk. 1997. Environmental risk assessment of the polycyclic musks AHTN and HHCB according to the EU-TGD. Natonal Institute of Public Health and the Environment Bilthoven, the Netherlands. Report No. 601503008.
Plum Hudsikkerhed. 2000a. Algal growth inhibition test of Coco-MEA 20% with the micro alga Scenedesmus subspicatus. Plum Hudsikkerhed A/S, Assens, Denmark.
Plum Hudsikkerhed. 2000b. Algal growth inhibition test of Coco-MEA 20% with the micro alga Pseudokircheriella subcapitata. Plum Hudsikkerhed A/S, Assens, Denmark.
Positivlisten. 1998. Fortegnelse over tilsætningsstoffer til levnedsmidler (in Danish). [The positive list - list of food additives]. Veterinær- og Fødevaredirektoratet, Denmark.
Prival, M.J., A.T. Sheldon, Jr and D. Popkin. 1982. Evaluation, using Salmonellatyphimurium, of the mutagenicity of seven chemicals found in cosmetics. Fd. Chem. Toxicol., 20, 427-432.
Prottey, C. and T. Ferguson. 1975. Factors which determine the skin irritation potential of soaps and detergents. J. Soc. Cosmet. Chem., 26, 29-46.
Puchta, R., P. Krings and P. Sandkühler. 1993. A new generation of softeners. Tenside Surf. Det., 30, 186-191.
Putterman, G.J, N.F. Wolejsza, M.A. Wolfram and K. Laden. 1977. The effect of detergents on swelling of stratum corneum. J. Soc. Cosmetic. Chem, 28, 521-532 .
Rasmussen, D. 1998. Evaluation of literature on the effects of hypochlorite in the environment. Report to the Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark, May 1998, 22 p.
Rastogi, S.C. and E. Johansen. 1993. Indhold af parabener i kosmetik. Analytisk-kemisk kontrol af kemiske stoffer og produkter (In Danish). [Content of parabens in cosmetics]. DMU report No. 66, National Environmental Research Institute, Ministry of Environment, Roskilde, Denmark.
Reiff, B., R. Lloyd, M.J. How, D. Brown and J.S. Alabaster. 1979. The acute toxicity of eleven detergents to fish: Results of an interlaboratory exercise. Water Res., 13, 207-210.
Reynolds, J.L. 1994a. Aerobic aquatic metabolism of 14C-RH-573. Rohm and Haas Technical Report No. 34-94-122. XenoBiotic Laboratories, Inc., Plainsboro, NJ. United States.
Reynolds, J.L. 1994b. Aerobic aquatic metabolism of 14C-RH-651. Rohm and Haas Technical Report No. 34-94-64. XenoBiotic Laboratories, Inc., Plainsboro, NJ. United States.
Rhein, L.D., C.R. Robbins, K. Fernee and R. Cantore. 1986. Surfactant structure effects on swelling of isolated human stratum corneum. J. Soc. Cosmet. Chem., 37, 125-139.
Richardson, M.L. 1992-94. The Dictionary of Substances and Their Effects. Volume 1-7. Royal Society of Chemistry, London, United Kingdom.
Riggin, R. M., W.L. Margard and G.W. Kinzer. 1983. Characterization of impurities in commercial lots of sodium saccharin produced by the Sherwin-Williams process II. Mutagenicity. Fd. Chem. Toxicol., 21 (1), 11-17.
Roberts, M.H., J.E. Warinner, C.F. Tsai, D. Wreight and L.E. Cronin. 1982. Comparison of estuarine species sensitivity to three toxicants. Arch. Environ. Contam. Toxicol., 11, 681-692.
Roberts, M.S. and K.A. Walters. 1998. Dermal absorption and toxicity assessment, p. 728-729. Drugs and the Pharmaceutical Sciences, Vol. 91. Marcel Dekker Inc., New York, United States.
Roed Pedersen, J. 1977. Frequency of sensitivity to Grotan BK in Denmark. Contact Dermatitis, 3, 212-213.
Roghair, C.J., A. Buijze and H.N.P. Schoon. 1992. Ecotoxicological risk evaluation of the cationic fabric softener DTDMAC. I. Ecotoxicological effects. Chemosphere, 24 (5), 599-609.
Rohm and Haas Company. 1998. Safety Data Sheet for Kathon LXE Biocide. Rohm and Haas Nordiska AB, Solna, Sweden.
Rossmoore, H.W. 1981. Antimicrobial agents for water-based metalworking fluids. Journal of Occupational Medicine, 23 (4), 247-254.
Routledge, E.J., J. Parker, J. Odum, J. Ashby and J.P. Sumpter. 1998. Some alkyl hydroxy benzoate preservatives (parabens) are oestrogenic. Toxicol. Appl. Pharmacol., 153, 12-19.
RTECS. 1997. Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, United States.
RTECS. 1998. Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, United States. CHEM-BANKTM, Compact disc SP-018-031, SilverPlatter Internationel N.V., November, 1998. Last update: October 1998.
RTECS. 1999. Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, United States. CHEM-BANKTM, CD-ROM, SilverPlatter Internationel N.V.
RTECS. 2000. Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, United States. Latest version CD-ROM, CHEM-BANKTM or online.
Russo, A. and A.G. Levis. 1992. Further evidence for the aneuploidogenic properties of chelating agents: Induction of micronuclei in mouse male germ cells by EDTA. Environ. Mol. Mutagen, 19, 125-131.
Rycroft, R.1978. Is Grotan BK a contact sensitizer? British Journal of Dermatology, 99, 346-347.
Salanitro, J.P., G.C. Langston, P.B. Dorn and L. Kravetz. 1988. Activated sludge treatment of ethoxylate surfactants at high industrial use concentrations. Water Res., 20, 125-130.
Salanitro, J.P. and L.A. Diaz. 1995. Anaerobic biodegradability testing of surfactants. Chemosphere, 30, 813-830.
Sanz-Gallen, P. , J. Planas, P. Martinez and J.M. Giménez-Arnau. 1992. Allergic contact dermatitis due to 1,2-benzisothiazolin-3-one in paint manufacture. Contact Dermatitis, 4, 271-272.
Scailteur, V., J.K. Maurer, A.P. Walker and G. Calvin. 1986. Subchronic oral toxicity testing in rats with a liquid hand-washing detergent containing anionic surfactant. Fd. Chem. Toxicol., 24 (2), 175-181.
Schamberg, I.L. 1967. Allergic contact dermatitis to methyl and propyl paraben. Archives of Dermatology, 95, 626-628.
Schardein, J.L., R. Sakowski, J. Petrere and R.R. Humphrey. 1981. Teratogenesis studies with EDTA and its salts in rats. Toxicol. Appl. Pharmacol., 61,423-428.
Schardein, J.L. 1993. Chemically Induced Birth Defects, 2nd ed., Marcel Dekker Inc., New York, United States.
Scharer, D.H., L. Kravetz and J. B. Carr. 1979. Biodegradation of nonionic surfactants. Tappi, 62, 10, 75-78.
Schöberl, P., K.J. Bock and L. Huber. 1988. Ökologisch relevanten Daten von Tensiden in Wasch- und Reinigungsmitteln (in German). [Ecologically relevant data for surfactants in laundry detergents and cleaning agents]. Tenside Surfactants Detergents, 25, 86-98 (review).
Schöberl, P. 1997. Ökologishe Bewertung von Tensiden (in German). [Ecological assessment of surfactants]. Tenside Surfactants Detergents, 34, 28-36 (review).
Schülke and Mayr. 1998. Safety data sheet for Grotan BK. Schülke & Mayr, Norderstedt, Germany.
Scribner, H.E., K.L. McCarthy, J.N. Moss, A.W. Hayes, J.M. Smith, M.A. Cifone, G.S. Probst and R. Valencia. 1983. The genetic toxicology of Kathon biocide, a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one. Mutation Research, 118, 129-152.
Seidenari, S., B.M. Manzini, P. Danese and A. Motolese. 1990. Patch and prick test study of 593 healthy subjects. Contact Dermatitis, 23, 162-167.
Seiler, J.P. 1989. The mutagenic activity of sodium perborate. Mutat. Res., 224 (2), 219-227.
SFT. 1991. Tensider i vaske- og rengjøringsmidler – helsefarevurdering (in Norwegian). [Surfactants in laundry detergents and cleaning agents - health assessment]. Report No. 91:06B, Statens Forureningstilsyn, Oslo, Norway.
Shepard, T.H. 1995. Catalog of Teratogenic Agents. 8th ed. The John Hopkins University Press, Baltimore, United States.
Singer, M.M. and R.S. Tjeerdema. 1993. Fate and effects of the surfactant sodium dodecyl sulfate. Reviews of Environmental Contamination and Toxicology, 133, 95-148 (review).
Skydsgaard, K. and L.H. Dideriksen. 1991. Primary skin and eye irritation. Experimental investigation with hydrochloric acid, calcium hydroxide, benzalkonium chloride and calcium hypochlorite. Lab.No. 12100, Scantox A/S, Denmark.
Stalmans, M., E. Matthijs, E. Weeg and S. Morris. 1993. The environmental properties of glucose amide – a new nonionic surfactant. SÖFW-Journal, 13, 795-806.
Statens Naturvårdsverk. 1992. Probit analysis, Ver. 2.3. National Swedish Environmental Protection Board; The data section, Sweden.
Steber, J and P. Wierich. 1987. The anaerobic degradation of detergent range fatty alcohol ethoxylates. Studies with 14C-labelled model surfactants. Water Res., 21, 661-667.
Steber, J. 1992. Henkel KgaA, International Report, Ökologische Information zu Bor (Natriumtetraborat), October 1991. W. Guhl, Henkel laboratory for Ecotoxicology in Düsseldorf, "Ökologische Aspekte von Bor", SÖFW-Journal, 118 (18), 1159-1168.
Steber, J. and H. Berger. 1995. Biodegradability of anionic surfactants, p. 134-182. In D.R. Karsa and M.R. Porter (eds.), Biodegradability of Surfactants. Blackie Academic & Professional, Glasgow, United Kingdom (review).
Steber, J., W. Guhl, N. Stelter and F.R. Schröder. 1995. Alkyl polyglycocides – ecological evaluation of a new generation of nonionic surfactants. Tenside Surfactants Detergents, 32, 515-521.
Steinbäck, F. 1977. Local and systemic effects of commonly used cutaneous agents: Lifetime studies od 16 compounds in mice and rabbits. Acta Pharmacol. et Toxicol., 41, 417-431.
Storrs, F.J. and E.D. Bell. 1983. Allergic contact dermatitis to 2-bromo-2-nitropropane-1,3-diol in a hydrophilic environment. Journal of the American Academy of Dermatology, 8, 157-170.
Sullivan, D.E. 1983. Biodegradation of a cationic surfactant in activated sludge. Water Research, 17, 1145-1151.
Swenberg, J.A., G.L. Petzold and P.R. Harbach. In vitro DNA damage/alkaline elution assay for predicting carcinogenic potential. Biochem. Biophys. Comm., 72, 732-738.
Swenerton, H. and L.S. Hurley. 1971. Teratogenic effects of a chelating agent and their prevention by zinc. Science, 173, 62-64.
Swisher, R.D. 1987. Surfactant Biodegradation. Surfactant Science Series, Vol. 18. Marcel Dekker, Inc., New York, United States, 1083 p (review).
Sørensen, J., B.B. Jørgensen and N.P. Revsbech. 1979. A comparison of oxygen, nitrate, and sulfate respiration in coastal marine sediments. Microbial Ecology, 5, 105-115.
Taffe, B.G. and T.W. Kensler. 1998. Tumor promotion by a hydroperoxide metabolite of butylated hydroxytoluene, 2,6-di-tert-butyl-4-hydroxyperoxy-4-methyl-2,5-cyclohexadienone, in mouse skin. Res. Comm Chem. Pathol. Pharmacol., 61, 291-303.
Talmage, S.S. 1994. Environmental and human safety of major surfactants. Alcohol ethoxylates and alkylphenol ethoxylates. The Soap and Detergent Association. Lewis publishers, Boca Raton, Florida, United States, 374 p (review).
Taylor, A.J., G.M. Powell, D. Howes, J.G. Black and A.H. Olavsen. 1978. Metabolism of the surfactant sodium undecyltriethoxy sulphate and sodium dodecyltriethoxy sulphate in the rat. Biochem. J., 174, 405-412.
Taylor, M.J. 1984. Comparative sensitivity of Ceriodaphnia sp. and Daphnia magna to selected surfactants. The Procter and Gamble Company, Research and Development Report, TDR-84002.
Thiersch, J.B. 1971. Investigations into the differental effect of compounds on the rat litter and mother. Malform. Congenitales Mammiferes, 95-113.
Thompson, E.D., M.J. Aardema and R.A. LeBoeuf. 1989. Lack of genotoxicity with acrylate polymers in five short-term mutagenicity assays. Environ. Mol. Mutag., 14, 98-106.
Til, H.P., V.J. Wountersen and V.J. Feron. 1989. Two-year drinking-water study of formaldehyde in rats. Food Chemistry and Toxicology, 27, 77-87.
Tjälve, H. 1972. A study of the Distribution and Teratogenicity of Nitrilotriacetic Acid (NTA) in Mice. Toxicol. Appl. Pharmacol., 23, 216-221.
Tobe, M., K. Natio and Y. Kurokawa. 1989. Chronic toxicity study on formaldehyde administered orally to rats. Toxicology, 56, 79-86.
Tolls, J., P. Kloepper-Sams and D.T.H.M. Sijm. 1994. Surfactant bioconcentration – a critical review. Chemosphere, 29, 693-717 (review).
Tolls, J. 1998. Bioconcentration of Surfactants. Ph.D. Thesis. Utrecht University, Utrecht, The Nederlands, 208 p.
Topcuoglu, S. and E. Birol. 1982. Bioaccumulation of sodium alkyl sulfate, zinc chloride and their mixture in young goby, Proterorhinus marmoratus. Pall. Turk. J. Nucl. Sci., 9, 100-107.
Toshima, S., T. Moriya and K. Yoshimura. 1992. Effects of polyoxyethylene (20) sorbitan monooleate on the acute toxicity of linear alkylbenzensulfonate (C12-LAS) to fish. Ecotoxicology and Environmental Safety, 24, 26-36.
Tovell, P.W.A., D. Howes and C.S. Newsome. 1975. Absorption, metabolism and excretion by goldfish of the anionic detergent sodium lauryl sulphate. Toxicology, 4, 17-29.
Tupker, R. 1990. The influence of detergents on the human skin. A study on factors determining the individual susceptibility assessed by transepidermal water loss. Thesis, University Groningen, The Netherlands.
Turner, A.H., F.S. Abram, V.M. Brown and H.A. Painter. 1985. The biodegradability of two primary alcohol ethoxylate nonionic surfactants under practical conditions, and the toxicity of the biodegradation products to rainbow trout. Water Res., 19, 45-51.
Tørsløv, J., L. Samsøe-Petersen, J.O. Rasmussen and P. Kristensen. 1997. Use of waste products in agriculture. Environmental Project No. 366, Danish Environmental Protection Agency, Ministry of Environment and Energy, Copenhagen, Denmark, 216 p.
Urwin, C., J.C. Richardson and A.K. Palmer. 1976. An evaluation of the mutagenicity of the cutting oil preservative Grotan BK. Mutation Research, 40, 43-46.
US-EPA. 1989. Dunnett’s procedure in the analysis of data from short-term toxicity tests with aquatic organisms, version 1.1. US-EPA, Cincinatti, United States.
Utsunomiya, A., T. Watanuki, K. Matsushita and I. Tomita. 1997. Toxic effects of linear alkylbenzene sulfonate, quaternary alkylammonium chloride and their complexes on Dunaliella sp. and Chlorella pyrenoidosa. Environ. Toxicol. Chem., 16, 1247-1254.
Verge, C., A. Moreno and S. Roque. 1996. Toxicity of anionic surfactants to green microalgae "Scenedesmus subspicatus" and "Selenastrum capricornutum". Tenside Surfantants Detergents, 33, 166-169.
Veronesi, S., L. Guerra, F. Valeri and F. Toni. 1987. 3 cases of contact dermatitis sensitive to Grotan BK. Contact Dermatitis, 17 (4), 255.
Verschueren, K. 1997. Handbook of environmental data on organic chemicals. 3rd Edition. Van Nostrand Reinhold Company, New York, United States.
Versteeg, D.J. and S.J. Shorter. 1992. Effect of organic carbon on the uptake and toxicity of quaternary ammonium compounds to the fathead minnow, Pimephales promelas. Environ. Toxicol. Chem., 11, 571-580.
VKI. 1992. TOXEDO Ver. 1.2. Program for statistical estimation of EC-values, based on experimentsl data from ecotoxicological assays. DHI – Water and Environment, Hørsholm, Denmark.
Wagener, S. and B. Schink. 1987. Anaerobic degradation of nonionic and anionic surfactants in enrichment cultures and fixed-bed reactors. Water Res., 21, 615-622.
Wagener, S. and B. Schink. 1988. Fermentative degradation of nonionic surfactants and polyethylene glycol by enrichment cultures and by pure cultures of propionate-forming bacteria. Appl. Env. Microbiol., 54, 561-565.
Wahlberg, J.E., M. Högberg and L. Skare. 1978. Chloroacetamide allergy in house painters. Contact Dermatitis, 4, 116-117.
Wakabayashi, M., M. Kikuchi, H. Kojima and T. Yoshida. 1980. Effect of alkyl chain on the uptake, distribution, and excretion of 35S-labelled alkyl sulfates in carp. Ecotoxicology and Environmental Safety, 4, 195-206.
Wakabayashi, M., M. Kikuchi, A. Sato and T. Yoshida. 1987. Bioconcentration of alcohol ethoxylates in carp (Cyprinus carpio). Ecotoxicology and Environmental Safety, 13, 148-163.
Walker, A.I.T., V.K.H. Brown, L.W. Ferrigan, R.G. Pickering and D.A. Williams. 1967. Toxicology of sodium lauryl sulphate, sodium lauryl ethoxy sulphate, and corresponding surfactants derived from synthetic alcohols. Food Cosmet. Toxicol., 5(6), 763-769.
Wall, L.M. and K.A. Gebauer. 1991. Occupational skin desease in Western Australia. Contact Dermatitis, 24, 101-109.
Wangenheim, J. and J. Bolcsfoldi. 1988. Mouse lymphoma L51784 thymidine kinase locus assay of 50 componds. Mutagen. 3, 193-205.
Wantke, F., C.M. Demmer, M. Götz and R. Jarisch. 1993. Sensitization to chloroacetamide. Contact Dermatitis, 29 (4), 213-214.
Waters, J., M.S. Holt and E. Matthijs. 1989. Fate of LAS in sludge amended soils. Tenside Surfactants Detergents, 26, 129-135.
Waters, J., H.H. Kleiser, M.J. How, M.D. Barratt, R.R. Birch, R.J. Fletcher, S.D. Haigh, S.G. Hales, S.J. Marshall and T.C. Pestell. 1991. A new rinse conditioner active with improved environmental properties. Tenside Surf. Det., 28, 460-468.
Weeks, M.H., T.O. Downing, N.P. Musselman, T.R. Carson and W.A. Groff. 1960. The effects of continuous exposure of animals to ethanolamine vapor. Am. Indus. Hyg. Assoc. J., 21, 374-381.
Wenninger, J.A. and G.N. McEwen. 1997. International Cosmetic Ingredients Dictionary and Handbook, Seventh Edition, The Cosmetic, Toiletry, and Fragrance Association, Washington DC, United States.
Wetzel, R.G. 1983. Limnology. 2. Ed. Saunders College Publishing, United States, ISBN 0-03-057913-9.
WHO. 1974. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. WHO Food Additives Series, No. 5, World Health Organization, Geneva, Schwitzerland.
WHO. 1996. Guidelines for drinking water quality. Health criteria and other supporting information. Vol 2nd ed., World Health Organization, Geneva, Schwitzerland.
WHO. 1998. Guidelines for drinking-water quality. Edetic acid (EDTA). Vol. 2, World Health Organization, Geneva, Schwitzerland.
Wildish, D.J. 1972. Acute toxicity of polyoxyethylene esters and polyoxyethylene ethers to S. salar and G. oceanicus. Water Res., 6, 759-762.
Wolf, K. and P.A. Gilbert. 1992. EDTA – Ethylenediaminetetraacetic acid, p. 243-259. In N.T. de Oude (ed.), Detergents, The Handbook of Environmental Chemistry, Volume 3. Part F. Anthropogenic Compounds.Springer-Verlag, Berlin Heidelberg, Germany (review).
Wong, D.C.L., P.B. Dorn, E.Y. Chai. 1997. Acute toxicity and structure-activity relationships of nine alcohol ethoxylate surfactants to fathead minnow and Daphnia magna. Environ. Toxicol. Chem., 16, 1970-1976.
Wright, C., E. Gingold, S. Venitt and C. Crofton-Sleigh. 1983. Mutagenic activity of Kathon, an indusrial biocide and cosmetics preservative containing 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one. Mutation Research, 119, 35-43.
Yam, J., K.A. Booman, W. Broddle, L. Geiger, J.E. Heinze, Y.J. Lin, K. McCarthy, S. Reiss, V. Savin, R.I. Sedlak, R.S. Slesinski and G.A. Wright. 1984. Surfactants: A survey of short-term genotoxicity testing. Fd. Chem. Toxicol., 22, 761-769.
Yamane, A.N., M. Okada and R. Sudo. 1984. The growth inhibition of planktonic algae due to surfactants used in washing agents. Water Res., 18, 1101-1105.
Yokoi, H. and S. Enomoto. 1979. Effect of degree of polymerization of silicic acid on the gastrointestinal absorption of silicate in rats. Chem. Pharm. Bull., 27 (8), 1733-1739.
Zeiger, E., B. Andersson, S. Haworth, T. Lawlor, K. Mortelmans and W. Speck. 1987. Salmonella mutagenicity tests. III. Results from the testing of 255 chemicals. Environmental Mutagenesis, 9 (9), 1-110.
Zeiger, E and B. Anderson. 1988. Salmonella mutagenicity tests: IV. Results from the testning of 300 chemicals. Environmental Mutagenesis, 11 Suppl 12, 1-158.
Zordan, M. A. Russo, R. Costa, N. Bianco, C. Beltrame, and A. G. Leuis. 1990. A concerted approach to the study of the aneuploidogenic properties of two chelating agents (EDTA and NTA) in the germ and somtic cell lines of drosophila and the mouse. Environ. Mol. Mutagen., 15, 205-213.
Appendix Part 1 - Biodegradability tests
Ready biodegradability tests
The ready biodegradability tests were performed according to the OECD 301F guideline (manometric respirometry test) with the sole deviation that the concentration of the test substances was 20 mg/l. One substance, C16 alkyltrimethylammonium chloride, was tested at 10 mg/l. The tests were performed in a an automatic respirometer which was set to monitor the biochemical oxygen demand (BOD) three times per day. The biodegradability of the reference substance, sodium benzoate, was > 60% during 14 days.
Table A1
Ultimate aerobic biodegradability of C12-18 alcohol ethoxylate, EO10
(end-capped with n-butylether) in the manometric respirometry test OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
2,7 |
0,1 |
2 |
20,8 |
0,2 |
3 |
26,1 |
0,6 |
4 |
33,2 |
0,1 |
5 |
39,9 |
1,0 |
6 |
44,1 |
0,8 |
7 |
48,4 |
2,2 |
8 |
53,9 |
4,3 |
9 |
60,1 |
4,7 |
10 |
65,4 |
4,5 |
11 |
70,0 |
4,6 |
12 |
74,4 |
5,4 |
13 |
77,6 |
6,0 |
14 |
80,8 |
6,1 |
15 |
83,4 |
5,6 |
16 |
85,5 |
5,2 |
17 |
87,2 |
5,0 |
18 |
88,7 |
4,9 |
19 |
89,9 |
4,6 |
20 |
91,0 |
4,4 |
21 |
91,9 |
4,3 |
22 |
92,8 |
4,3 |
23 |
93,6 |
4,3 |
24 |
94,5 |
4,5 |
25 |
95,5 |
4,6 |
26 |
96,3 |
4,6 |
27 |
97,0 |
4,4 |
28 |
97,7 |
4,1 |
*SD, standard deviation of 3 replicates.
Figure A1 Look here!
Ultimate aerobic biodegradability of C12-18 alcohol ethoxylate, EO10
(end-capped with n-butylether) in the manometric respirometry test OECD 301F.
Table A2
Ultimate aerobic biodegradability of C16 alkyltrimethylammonium chloride (10
mg/l) in the manometric respirometry test OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
-17,0 |
0,8 |
2 |
-26,8 |
2,1 |
3 |
25,7 |
4,2 |
4 |
31,4 |
3,8 |
5 |
30,0 |
3,7 |
6 |
28,4 |
4,5 |
7 |
34,9 |
6,9 |
8 |
38,7 |
4,4 |
9 |
39,6 |
4,1 |
10 |
37,5 |
4,3 |
11 |
34,8 |
5,1 |
12 |
32,7 |
5,4 |
13 |
31,4 |
7,3 |
14 |
30,4 |
8,5 |
15 |
31,2 |
8,4 |
16 |
34,9 |
5,2 |
17 |
35,3 |
5,7 |
18 |
36,0 |
6,3 |
19 |
36,7 |
7,1 |
20 |
37,7 |
7,9 |
21 |
38,6 |
8,7 |
22 |
39,0 |
9,9 |
23 |
39,6 |
10,4 |
24 |
39,3 |
11,1 |
25 |
39,2 |
11,7 |
26 |
39,3 |
12,6 |
27 |
39,7 |
13,6 |
28 |
39,7 |
14,2 |
*SD, standard deviation of 4 replicates.
Figure A2 Look here!
Ultimate aerobic biodegradability of C16 alkyltrimethylammonium chloride (10
mg/l) in the manometric respirometry test OECD 301F.
Table A3
Ultimate aerobic biodegradability of C12 alkyliminodipropionate in the
manometric respirometry test OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
-1,9 |
0,2 |
2 |
1,3 |
1,0 |
3 |
12,8 |
1,9 |
4 |
27,3 |
1,4 |
5 |
33,0 |
1,7 |
6 |
41,1 |
2,1 |
7 |
49,7 |
1,7 |
8 |
55,2 |
1,9 |
9 |
60,2 |
2,0 |
10 |
64,3 |
2,5 |
11 |
68,7 |
2,2 |
12 |
72,7 |
1,9 |
13 |
76,1 |
1,8 |
14 |
79,1 |
2,4 |
15 |
81,5 |
2,9 |
16 |
83,5 |
3,5 |
17 |
85,3 |
4,2 |
18 |
87,0 |
4,8 |
19 |
88,4 |
5,4 |
20 |
89,8 |
5,8 |
21 |
91,1 |
6,1 |
22 |
92,3 |
6,4 |
23 |
93,7 |
6,5 |
24 |
94,9 |
6,7 |
25 |
96,1 |
6,8 |
26 |
97,2 |
6,9 |
27 |
98,0 |
7,0 |
28 |
98,6 |
7,1 |
*SD, standard deviation of 4 replicates.
Figure A3 Look here!
Ultimate aerobic biodegradability of C12 alkyliminodipropionate in the
manometric respirometry test OECD 301F.
Table A4
Ultimate aerobic biodegradability of methylparaben in the manometric respirometry test
OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
-5,1 |
0,2 |
2 |
-8,2 |
1,1 |
3 |
35,5 |
1,2 |
4 |
49,1 |
0,6 |
5 |
57,0 |
0,3 |
6 |
63,0 |
0,8 |
7 |
67,2 |
0,7 |
8 |
70,1 |
1,4 |
9 |
72,7 |
2,3 |
10 |
75,2 |
3,2 |
11 |
77,8 |
3,4 |
12 |
80,0 |
3,4 |
13 |
81,9 |
3,4 |
14 |
83,6 |
3,5 |
15 |
85,2 |
3,7 |
16 |
86,6 |
3,7 |
17 |
87,9 |
3,7 |
18 |
89,1 |
3,6 |
19 |
89,8 |
3,5 |
20 |
90,4 |
3,4 |
21 |
90,8 |
3,3 |
22 |
91,1 |
3,4 |
23 |
91,4 |
3,4 |
24 |
91,6 |
3,5 |
25 |
91,7 |
3,5 |
26 |
91,9 |
3,6 |
27 |
92,1 |
3,6 |
28 |
92,2 |
3,7 |
*SD, standard deviation of 4 replicates.
Figure A4 Look here!
Ultimate aerobic biodegradability of methylparaben in the manometric respirometry test
OECD 301F.
Table A5
Ultimate aerobic biodegradability of ethylparaben in the manometric respirometry test
OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
0,4 |
0,5 |
2 |
-0,2 |
0,7 |
3 |
17,5 |
4,0 |
4 |
51,8 |
9,4 |
5 |
57,0 |
10,7 |
6 |
61,2 |
10,9 |
7 |
64,4 |
10,9 |
8 |
67,1 |
11,1 |
9 |
69,8 |
11,6 |
10 |
72,2 |
12,7 |
11 |
74,4 |
13,1 |
12 |
76,4 |
13,6 |
13 |
78,4 |
13,7 |
14 |
80,0 |
13,9 |
15 |
81,4 |
13,9 |
16 |
82,3 |
14,1 |
17 |
83,4 |
13,8 |
18 |
84,3 |
13,6 |
19 |
85,0 |
13,5 |
20 |
85,5 |
13,5 |
21 |
86,2 |
13,4 |
22 |
86,6 |
13,5 |
23 |
87,1 |
13,6 |
24 |
87,5 |
13,6 |
25 |
87,8 |
13,7 |
26 |
88,1 |
13,9 |
27 |
88,3 |
14,0 |
28 |
88,4 |
14,1 |
*SD, standard deviation of 4 replicates.
Figure A5 Look here!
Ultimate aerobic biodegradability of ethylparaben in the manometric respirometry test OECD
301F.
Table A6
Ultimate aerobic biodegradability of propylparaben in the manometric respirometry test
OECD 301F.
Time |
Average biodegradability |
SD |
0 |
0,0 |
0,0 |
1 |
-0,5 |
0,2 |
2 |
-2,5 |
0,7 |
3 |
20,1 |
13,2 |
4 |
52,4 |
1,7 |
5 |
56,3 |
2,1 |
6 |
61,1 |
2,0 |
7 |
64,5 |
2,0 |
8 |
67,4 |
3,1 |
9 |
70,3 |
4,5 |
10 |
73,0 |
6,0 |
11 |
75,3 |
6,1 |
12 |
77,4 |
6,2 |
13 |
79,2 |
6,1 |
14 |
80,9 |
5,8 |
15 |
82,5 |
5,5 |
16 |
83,9 |
5,1 |
17 |
85,2 |
4,7 |
18 |
86,4 |
4,5 |
19 |
87,4 |
4,3 |
20 |
88,2 |
4,3 |
21 |
88,8 |
4,3 |
22 |
89,4 |
4,4 |
23 |
89,9 |
4,5 |
24 |
90,3 |
4,5 |
25 |
90,7 |
4,6 |
26 |
91,0 |
4,7 |
27 |
91,3 |
4,8 |
28 |
91,5 |
4,9 |
*SD, standard deviation of 4 replicates.
Figure A6 Look here!
Ultimate aerobic biodegradability of propylparaben in the manometric respirometry test
OECD 301F.
Anaerobic biodegradability tests
The anaerobic biodegradability tests were performed as described in the ISO 11734 guideline. The inoculum concentration was 1.0 g digested sludge dry weight per litre of final test medium, and the concentration of the test substances was typically 15-20 mg C/l (actual concentrations are stated for each test run). The incubation temperature was 35° C during a test period of normally 56 days. The test period was prolonged to 84 or 90 days for some of the substances. The materials and methods used have been described previously (Madsen et al. 1995; 1996a). The anaerobic biodegradability of the reference substance, sodium benzoate, attained 66 and 71% ThGP during 56 days in two test runs.
Table A7
Ultimate anaerobic biodegradability of C12-14 alkyl sulfate (13.0 mg C/l) in
the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-48.9 |
2.5 |
14 |
-91.1 |
9.8 |
21 |
-56.5 |
20.6 |
28 |
20.3 |
11.1 |
35 |
60.7 |
10.8 |
42 |
75.3 |
4.1 |
49 |
78.1 |
1.6 |
56 |
81.3 |
2.5 |
56 (after acidification) |
84.8 |
1.9 |
*SD, standard deviation of 5 replicates.
Figure A7
Ultimate anaerobic biodegradability of C12-14 alkyl sulfate (13.0 mg C/l) in
the biogas production screening test ISO 11734.
Table A8
Ultimate anaerobic biodegradability of coconut fatty acid, potassium salt (20.0 mg C/l) in
the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-47.7 |
4.5 |
14 |
22.1 |
8.9 |
21 |
71.3 |
8.1 |
28 |
82.2 |
4.3 |
35 |
90.5 |
2.3 |
42 |
96.3 |
1.9 |
49 |
97.9 |
7.0 |
56 |
98.8 |
1.0 |
56 (after acidification) |
99.2 |
2.5 |
*SD, standard deviation of 5 replicates.
Figure A8
Ultimate anaerobic biodegradability of coconut fatty acid, potassium salt (20.0 mg C/l) in
the biogas production screening test ISO 11734.
Table A9
Ultimate anaerobic biodegradability of C12-15 alcohol ethoxylate, EO7 (15.7 mg
C/l) in the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-47.3 |
0.1 |
14 |
-115.1 |
< 0.05 |
21 |
-146.4 |
< 0.05 |
28 |
-161.6 |
< 0.05 |
35 |
-131.2 |
0.1 |
42 |
-44.9 |
0.1 |
49 |
-11.3 |
0.1 |
56 |
13.1 |
< 0.05 |
63 |
30.5 |
< 0.05 |
70 |
35.5 |
< 0.05 |
84 |
34.2 |
0.1 |
84 (after acidification) |
35.2 |
0.1 |
*SD, standard deviation of 5 replicates.
Figure A9
Ultimate anaerobic biodegradability of C12-15 alcohol ethoxylate, EO7 (15.7 mg
C/l) in the biogas production screening test ISO 11734.
Table A10
Ultimate anaerobic biodegradability of C8 alcohol ethoxylate, EO5 (end-capped
with n-butylether; 13.5 mg C/l) in the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-68.0 |
1.2 |
14 |
-153.1 |
2.1 |
21 |
-194.2 |
1.2 |
28 |
-217.4 |
1.2 |
35 |
-205.8 |
15.0 |
42 |
-137.5 |
9.5 |
49 |
-85.7 |
30.9 |
56 |
-62.8 |
9.2 |
63 |
-45.6 |
4.6 |
70 |
-38.9 |
2.4 |
84 |
-40.1 |
1.5 |
84 (after acidification) |
-35.2 |
7.0 |
*SD, standard deviation of 5 replicates.
Figure A10
Ultimate anaerobic biodegradability of C8 alcohol ethoxylate, EO5 (end-capped
with n-butylether; 13.5 mg C/l) in the biogas production screening test ISO 11734.
Table A11
Ultimate anaerobic biodegradability of C12-18 alcohol ethoxylate, EO10
(end-capped with n-butylether; 16.3 mg C/l) in the biogas production screening test ISO
11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-41.6 |
16.5 |
14 |
-106.6 |
4.6 |
21 |
-138.1 |
1.0 |
28 |
-157.4 |
1.0 |
35 |
-150.3 |
10.9 |
42 |
-93.7 |
29.4 |
49 |
-15.2 |
63.2 |
56 |
12.9 |
22.3 |
63 |
26.7 |
2.8 |
70 |
35.8 |
2.8 |
84 |
43.4 |
8.4 |
84 (after acidification) |
53.6 |
14.2 |
*SD, standard deviation of 5 replicates.
Figure A11 Look here!
Ultimate anaerobic biodegradability of C12-18 alcohol ethoxylate, EO10
(end-capped with n-butylether; 16.3 mg C/l) in the biogas production screening test ISO
11734.
Table A12
Ultimate anaerobic biodegradability of cocoamide MEA (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Time |
Average
biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-37.2 |
4.7 |
14 |
35.6 |
7.0 |
21 |
59.5 |
7.4 |
28 |
65.8 |
3.7 |
35 |
72.4 |
2.5 |
42 |
75.7 |
1.2 |
49 |
78.6 |
< 0.05 |
56 |
78.6 |
1.4 |
56 (after acidification) |
80.7 |
2.3 |
*SD, standard deviation of 5 replicates.
Figure A12
Ultimate anaerobic biodegradability of cocoamide MEA (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Table A13
Ultimate anaerobic biodegradability of C16 alkyltrimethylammonium chloride
(14.0 mg C/l) in the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-78.0 |
6.8 |
14 |
-157.7 |
1.2 |
21 |
-195.5 |
2.4 |
28 |
-216.7 |
3.2 |
35 |
-227.4 |
3.0 |
42 |
-232.4 |
< 0.05 |
49 |
-238.0 |
< 0.05 |
56 |
-238.0 |
< 0.05 |
56 (after acidification) |
-268.1 |
2.1 |
*SD, standard deviation of 5 replicates.
Figure A13
Ultimate anaerobic biodegradability of C16 alkyltrimethylammonium chloride
(14.0 mg C/l) in the biogas production screening test ISO 11734.
Table A14
Ultimate anaerobic biodegradability of cocomidopropyl betaine (14.4 mg C/l) in the biogas
production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-6.9 |
7.5 |
14 |
19.5 |
5.2 |
21 |
36.4 |
5.4 |
28 |
45.0 |
2.9 |
35 |
63.3 |
3.4 |
42 |
69.4 |
2.9 |
49 |
71.1 |
1.7 |
56 |
73.7 |
1.4 |
56 (after acidification) |
75.4 |
0.9 |
*SD, standard deviation of 5 replicates.
Figure A14
Ultimate anaerobic biodegradability of cocoamidopropyl betaine (14.4 mg C/l) in the biogas
production screening test ISO 11734.
Table A15
Ultimate anaerobic biodegradability of C12 alkyliminodipropionate (16.4 mg C/l)
in the biogas production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-11.1 |
5.0 |
14 |
-9.1 |
2.8 |
21 |
-12.1 |
2.3 |
28 |
-17.1 |
2.0 |
35 |
-17.6 |
3.8 |
42 |
-9.8 |
2.8 |
49 |
0.0 |
3.8 |
56 |
2.5 |
< 0.05 |
56 (after acidification) |
2.5 |
1.0 |
*SD, standard deviation of 5 replicates.
Figure A15
Ultimate anaerobic biodegradability of C12 alkyliminodipropionate (16.4 mg C/l)
in the biogas production screening test ISO 11734.
Table A16
Ultimate anaerobic biodegradability of methylparaben (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
2.3 |
0.1 |
14 |
9.7 |
0.1 |
21 |
29.9 |
0.1 |
28 |
37.6 |
< 0.05 |
35 |
38.0 |
< 0.05 |
42 |
39.2 |
< 0.05 |
49 |
38.4 |
< 0.05 |
56 |
40.0 |
< 0.05 |
63 |
39.2 |
< 0.05 |
77 |
36.3 |
< 0.05 |
90 |
36.3 |
< 0.05 |
90 (after acidification) |
37.1 |
< 0.05 |
*SD, standard deviation of 5 replicates.
Figure A16
Ultimate anaerobic biodegradability of methylparaben (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Table A17
Ultimate anaerobic biodegradability of ethylparaben (20.0 mg C/l) in the biogas production
screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
0.2 |
< 0.05 |
14 |
1.4 |
< 0.05 |
21 |
-2.1 |
< 0.05 |
28 |
1.0 |
< 0.05 |
35 |
5.2 |
< 0.05 |
42 |
8.7 |
< 0.05 |
49 |
12.6 |
< 0.05 |
56 |
14.2 |
< 0.05 |
63 |
17.7 |
< 0.05 |
77 |
24.1 |
< 0.05 |
90 |
28.3 |
< 0.05 |
90 (after acidification) |
33.0 |
< 0.05 |
*SD, standard deviation of 5 replicates.
Figure A17
Ultimate anaerobic biodegradability of ethylparaben (20.0 mg C/l) in the biogas production
screening test ISO 11734.
Table A18
Ultimate anaerobic biodegradability of propylparaben (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
-6.0 |
< 0.05 |
14 |
-1.0 |
< 0.05 |
21 |
-4.1 |
< 0.05 |
28 |
-3.9 |
< 0.05 |
35 |
0.6 |
< 0.05 |
42 |
3.9 |
< 0.05 |
49 |
7.2 |
< 0.05 |
56 |
10.5 |
< 0.05 |
63 |
11.4 |
< 0.05 |
77 |
14.2 |
< 0.05 |
90 |
18.0 |
< 0.05 |
90 (after acidification) |
17.6 |
< 0.05 |
*SD, standard deviation of 5 replicates.
Figure A18
Ultimate anaerobic biodegradability of propylparaben (20.0 mg C/l) in the biogas
production screening test ISO 11734.
Table A19
Ultimate anaerobic biodegradability of Nipaguard (55-80% benzyl alcohol, 15-30%
methylparaben, 5-15% propylparaben; 20.0 mg C/l) in the biogas production screening test
ISO 11734.
Time |
Average biodegradability |
SD |
0 |
0.0 |
0.0 |
7 |
11.6 |
0.1 |
14 |
36.1 |
< 0.05 |
21 |
59.0 |
< 0.05 |
28 |
62.5 |
< 0.05 |
35 |
62.5 |
< 0.05 |
42 |
63.6 |
< 0.05 |
49 |
66.1 |
< 0.05 |
56 |
65.6 |
< 0.05 |
63 |
66.7 |
< 0.05 |
77 |
65.8 |
< 0.05 |
90 |
65.2 |
< 0.05 |
90 (after acidification) |
66.9 |
< 0.05 |
*SD, standard deviation of 5 replicates.
Figure A19
Ultimate anaerobic biodegradability of Nipaguard (55-80% benzyl alcohol, 15-30%
methylparaben, 5-15% propylparaben; 20.0 mg C/l) in the biogas production screening test
ISO 11734.
Appendix Part 2 Aquatic toxicity tests
Algae growth inhibition test.
The toxicity of the test substances to the growth of the micro algae (Pseudokirchneriella subcapitata formerly Selenastrum capricornutum) was determined in a 72 hour test according to ISO 8692 guideline (ISO 1989). Exponentially growing batch cultures of algae were exposed to a serial dilution of the test substance. The test was performed with 3 replicate batches at each concentration and 6 control batches. The test was incubated on a shaking table under constant temperature and light. The cell densities were determined by fluoremetric measurement after 24, 48, and 72 hours of exposure. The inhibition of growth at each test concentration was determined as the average growth rate in per cent of the control growth rate. The concentrations, which inhibited the growth rate 10 and 50%, were calculated by use of a computer program ‘Toxedo’ (VKI 1992). The highest concentration at which no significant effect was observed (No Observed Effect Concentration, NOEC), was determined by Dunnett’s procedure (US-EPA 1989).
Daphnia, acute immobilization test.
The toxicity of the test substances was tested in a 48-hour immobilization test with Daphnia magna according to ISO 6341 guideline (ISO 1996). Groups of daphnids (4 replicates of 5 animals) were exposed to a serial dilution of the substance. The number of immobile animals was registered after 24 and 48 hours. Oxygen level, pH and temperature were determined at test start and termination of the test. On the basis of the test results, the concentrations, at which 10, 50 and 90% of the animals were immobilized (EC10, EC50 and EC90) were calculated by Probit analysis (Statens Naturvårdsverk 1992).
The results of the tests are summarized below:
Table A20
Effects of parabens to Pseudokirchneriella subcapitata and Daphnia magna. Detailed data
are given in the following sections.
Test substance/species |
EC50 |
NOEC |
Test duration |
Methylparaben |
|||
Pseudokirchneriella subcapitata |
91 (90-93)A |
20 |
72 h |
Daphnia magna |
11.2(5.7-22.0) |
2.0 |
48 h |
Ethylparaben |
|||
Pseudokirchneriella subcapitata |
18 (17-19) |
5.0 |
72 h |
Daphnia magna |
20-50 |
10 |
48 h |
Propylparaben |
|||
Pseudokirchneriella subcapitata |
15 (15-16) |
5.0 |
72 h |
Daphnia magna |
15.4(8.0-32.3) |
1.0 |
48 h |
A
Parentheses indicate 95% confidence intervals.Inhibition of the growth of Pseudokirchneriella subcapitata with " Ethylparaben".
Statistical parameters calculated from continuous responses based on continuous mean.
Testtype : Growth Inhibition Test.
6 doses and 16 responses have been used during calculations.
Control values.
Concentration |
Growth |
Inhibition |
Control 1 |
1.956 |
- |
Control 2 |
1.998 |
- |
Control 3 |
1.970 |
- |
Control 4 |
2.017 |
- |
Control 5 |
2.054 |
- |
Control 6 |
2.004 |
- |
Control mean |
2.000 |
0 |
Experimental Data.
Concentration |
Growth |
Inhibition |
1 |
2.009 |
0.0 |
1 |
1.991 |
0.4 |
1 |
2.018 |
0.0 |
2 |
2.016 |
0.0 |
2 |
2.027 |
0.0 |
2 |
2.018 |
0.0 |
5 |
1.985 |
0.7 |
5 |
1.982 |
0.9 |
5 |
1.973 |
1.3 |
10 |
1.698 |
15.1 |
10 |
1.677 |
16.1 |
10 |
1.719 |
14.0 |
20 |
0.667 |
66.6 |
20 |
0.776 |
61.2 |
20 |
0.661 |
66.9 |
50 |
0.330 |
83.5 |
50 |
0.202 |
89.9 |
50 |
0.336 |
83.2 |
100 |
0.072 |
96.4 |
100 |
0.048 |
97.6 |
100 |
0.029 |
98.5 |
Dunnett’s procedure:
NOEC: 5 mg/l
LOEC: 10 mg/l
EC-values and limits of the 95% confidence interval.
y(EC) |
LCL |
EC(yo) |
UCL |
10 |
6 |
6 |
7 |
50 |
17 |
18 |
19 |
90 |
45 |
49 |
53 |
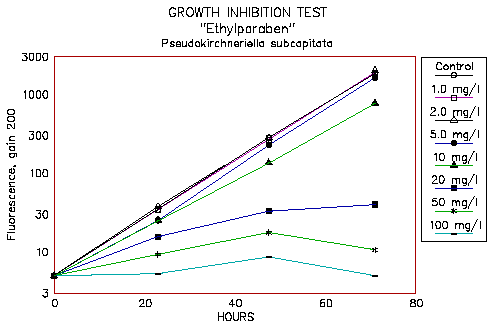
Inhibition of the growth of Pseudikirchneriella subcapitata with "Methylparaben".
Statistical parameters calculated from continuous responses based on continuous mean.
Testtype : Growth Inhibition Test.
6 doses and 13 responses have been used during calculations.
Control values.
Concentration |
Growth |
Inhibition |
Control 1 |
2.102 |
- |
Control 2 |
2.094 |
- |
Control 3 |
2.101 |
- |
Control 4 |
2.123 |
- |
Control 5 |
2.118 |
- |
Control 6 |
2.102 |
- |
Control mean |
2.107 |
0 |
Experimental Data.
Concentration |
Growth |
Inhibition |
2 |
2.046 |
2.9 |
2 |
2.129 |
0.0 |
2 |
2.086 |
1.0 |
5 |
2.129 |
0.0 |
5 |
2.109 |
0.0 |
5 |
2.093 |
0.6 |
10 |
2.151 |
0.0 |
10 |
2.132 |
0.0 |
10 |
2.029 |
3.7 |
20 |
2.108 |
0.0 |
20 |
2.114 |
0.0 |
20 |
2.137 |
0.0 |
50 |
1.836 |
12.8 |
50 |
1.820 |
13.6 |
50 |
1.779 |
15.6 |
100 |
0.917 |
56.5 |
100 |
0.908 |
56.9 |
100 |
0.908 |
56.9 |
200 |
0.337 |
84.0 |
200 |
0.337 |
84.0 |
200 |
0.341 |
83.8 |
Dunnett’s procedure:
NOEC: 20 mg/l
LOEC: 50 mg/l
EC-values and limits of the 95% confidence interval.
y(EC) |
LCL |
EC(yo) |
UCL |
10 |
29 |
31 |
32 |
50 |
90 |
91 |
93 |
90 |
> 200 |
||
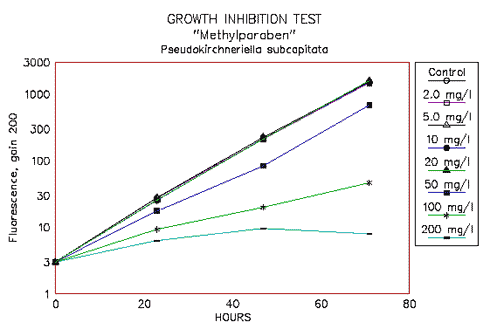
Inhibition of the growth of Pseudokirchneriella subcapitata with " Propylparaben ".
Statistical parameters calculated from continuous responses based on continuous mean.
Testtype : Growth Inhibition Test.
6 doses and 14 responses have been used during calculations.
Control values.
Concentration |
Growth |
Inhibition |
Control 1 |
2.070 |
- |
Control 2 |
2.066 |
- |
Control 3 |
1.972 |
- |
Control 4 |
2.043 |
- |
Control 5 |
2.046 |
- |
Control 6 |
2.060 |
- |
Control mean |
2.043 |
0 |
Experimental Data.
Concentration |
Growth |
Inhibition |
1 |
2.062 |
0.0 |
1 |
2.039 |
0.2 |
1 |
2.056 |
0.0 |
1 |
2.090 |
0.0 |
1 |
2.072 |
0.0 |
1 |
2.058 |
0.0 |
2 |
2.033 |
0.5 |
2 |
2.063 |
0.0 |
2 |
2.055 |
0.0 |
5 |
1.992 |
2.5 |
5 |
1.976 |
3.3 |
5 |
1.966 |
3.8 |
10 |
1.675 |
18.0 |
10 |
1.625 |
20.5 |
10 |
1.661 |
18.7 |
20 |
0.529 |
74.1 |
20 |
0.596 |
70.8 |
20 |
0.522 |
74.4 |
50 |
0.070 |
96.6 |
50 |
0.085 |
95.8 |
50 |
0.283 |
86.1 |
Dunnett’s procedure:
NOEC: 5 mg/l
LOEC: 10 mg/l
EC-values and limits of the 95% confidence interval.
y(EC) |
LCL |
EC(yo) |
UCL |
10 |
7 |
7 |
8 |
50 |
15 |
15 |
16 |
90 |
30 |
32 |
35 |
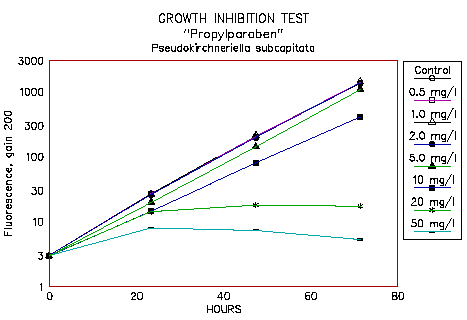
Primary data for acute test |
|||||
TEST ORGANISM: |
Daphnia Magna |
LAB. NO.: |
8119/365 |
||
TEST COMPOUND: |
Ethylparaben |
FILE NO.: |
11166 |
||
START DATE: |
1999.09.07 |
OBSERVERS: |
HeE |
||
|
pH |
O2-content (% saturation) |
|||
|
START |
END |
START |
END |
|
Control |
7.8 |
7.8 |
99 |
97 |
|
Highest concentration |
7.9 |
7.9 |
> 100 |
95 |
|
Concentration |
Total |
No. of anim. |
NUMBER OF IMMOBILE |
|||||||||
mg/l |
number of |
per vessel |
24 HOURS |
48 HOURS |
||||||||
|
animals per |
A |
C |
E |
A |
C |
E |
Total |
A |
C |
E |
Total |
|
conc. |
B |
D |
F |
B |
D |
F |
|
B |
D |
F |
|
Control |
30
|
5 |
5 |
5 |
0 |
0 |
0 |
0
|
0 |
0 |
0 |
0
|
|
5 |
5 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
|||
1 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
2 |
21
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
6 |
|
0 |
0 |
|
0 |
0 |
|
|||
5 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
10 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
20 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
2 |
4 |
|
14
|
|
5 |
5 |
|
0 |
0 |
|
4 |
4 |
|
|||
50 |
20
|
5 |
5 |
|
5 |
5 |
|
20
|
5 |
5 |
|
20
|
|
5 |
5 |
|
5 |
5 |
|
5 |
5 |
|
|||
100 |
20
|
5 |
5 |
|
5 |
5 |
|
20
|
5 |
5 |
|
20
|
|
5 |
5 |
|
5 |
5 |
|
5 |
5 |
|
|||
Ethylparaben |
||||||
mg/l |
0 HOURS |
24 HOURS |
48 HOURS |
|||
Conc. |
O2 |
pH |
O2 |
pH |
O2 |
pH |
Control |
99 |
7.8 |
- |
- |
97 |
7,8 |
20 |
> 100 |
7.9 |
- |
- |
95 |
7,9 |
50 |
> 100 |
7.9 |
98 |
7,8 |
- |
- |
100 |
> 100 |
7.9 |
97 |
7,8 |
|
|
O2-contents are given in % of saturation
|
24 hours |
48 hours |
E10 |
between 20 - 50 |
between 10-20 |
E50 |
20 - 50 |
20-50 |
E90 |
20 - 50 |
20-50 |
Unit: mg/l
Primary data for acute test |
|||||
TEST ORGANISM: |
Daphnia magna |
LAB. NO.: |
81119/366 |
||
TEST COMPOUND: |
Methylparaben |
FILE NO.: |
11166 |
||
START DATE: |
1999.09.14 |
OBSERVERS: |
CS |
||
|
pH |
O2-content (% saturation) |
|||
|
START |
END |
START |
END |
|
Control |
7.7 |
7.9 |
100 |
100 |
|
Highest concentration |
7.6 |
7.7 |
100 |
100 |
|
Concentration |
Total |
No. of anim. |
NUMBER OF IMMOBILE |
|||||||||
mg/l |
number of |
per vessel |
24 HOURS |
48 HOURS |
||||||||
|
animals per |
A |
C |
E |
A |
C |
E |
Total |
A |
C |
E |
Total |
|
conc. |
B |
D |
F |
B |
D |
F |
|
B |
D |
F |
|
Control |
30 |
5 |
5 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
5 |
5 |
5 |
0 |
0 |
0 |
|
0 |
0 |
0 |
|
2.0 |
20 |
5 |
5 |
|
0 |
0 |
|
0 |
0 |
0 |
|
0 |
|
|
5 |
5 |
|
0 |
0 |
|
|
0 |
0 |
|
|
5.0 |
20 |
5 |
5 |
|
0 |
0 |
|
0 |
0 |
0 |
|
1 |
|
|
5 |
5 |
|
0 |
0 |
|
|
0 |
1 |
|
|
10 |
20 |
5 |
5 |
|
2 |
2 |
|
7 |
3 |
2 |
|
10 |
|
|
5 |
5 |
|
1 |
2 |
|
|
2 |
3 |
|
|
20 |
20 |
5 |
5 |
|
3 |
1 |
|
11 |
5 |
4 |
|
19 |
|
|
5 |
5 |
|
5 |
2 |
|
|
5 |
5 |
|
|
50 |
20 |
5 |
5 |
|
3 |
3 |
|
11 |
5 |
5 |
|
18 |
|
|
5 |
5 |
|
3 |
2 |
|
|
5 |
3 |
|
|
100 |
20 |
5 |
5 |
|
5 |
5 |
|
20 |
5 |
5 |
|
20 |
|
|
5 |
5 |
|
5 |
5 |
|
|
5 |
5 |
|
|
200 |
20 |
5 |
5 |
* |
5 |
5 |
|
20 |
5 |
5 |
|
20 |
|
|
5 |
5 |
|
5 |
5 |
|
|
5 |
5 |
|
|
* Dead after 10 minutes
Methylparaben |
||||||
mg/l |
0 HOURS |
24 HOURS |
48 HOURS |
|||
Conc. |
O2 |
pH |
O2 |
pH |
O2 |
pH |
Control |
100 |
7.7 |
- |
- |
100 |
7.9 |
50 |
100 |
7.6 |
- |
- |
100 |
8.0 |
100 |
100 |
7.6 |
100 |
7.6 |
- |
- |
200 |
100 |
7.6 |
100 |
7.7 |
- |
- |
O2-contents are given in % of saturation
LOG |
24 hours |
48 hours |
EC10 |
6.24 |
4.50 |
EC50 |
22.4 |
11.2 |
EC90 |
80.7 |
27.8 |
Unit: mg/l
Primary data for acute test |
|||||
TEST ORGANISM: |
Daphnia Magna |
LAB. NO.: |
8119/367 |
||
TEST COMPOUND: |
Propylparaben |
FILE NO.: |
11166 |
||
START DATE: |
1999.09.15 |
OBSERVERS: |
CS |
||
|
pH |
O2-content (% saturation) |
|||
|
START |
END |
START |
END |
|
Control |
7.7 |
7.6 |
100 |
100 |
|
Highest concentration |
7.7 |
7.6 |
100 |
100 |
|
Concentration |
Total |
No. of anim. |
NUMBER OF IMMOBILE |
|||||||||
mg/l |
number of |
per vessel |
24 HOURS |
48 HOURS |
||||||||
|
animals per |
A |
C |
E |
A |
C |
E |
Total |
A |
C |
E |
Total |
|
conc. |
B |
D |
F |
B |
D |
F |
|
B |
D |
F |
|
Control |
30
|
5 |
5 |
5 |
0 |
0 |
0 |
0
|
0 |
0 |
0 |
0
|
|
5 |
5 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
|||
0.5 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
1 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
2 |
20
|
5 |
5 |
|
0 |
1 |
|
1
|
0 |
1 |
|
1
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
5 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
10 |
20
|
5 |
5 |
|
0 |
0 |
|
0
|
0 |
0 |
|
0
|
|
5 |
5 |
|
0 |
0 |
|
0 |
0 |
|
|||
20 |
20
|
5 |
5 |
|
1 |
1 |
|
4
|
4 |
4 |
|
18
|
|
5 |
5 |
|
0 |
2 |
|
5 |
5 |
|
|||
50 |
20
|
5 |
5 |
|
5 |
5 |
|
20
|
5 |
5 |
|
20
|
|
5 |
5 |
|
5 |
5 |
|
5 |
5 |
|
|||
100 |
20
|
5 |
5 |
|
5 |
5 |
|
20
|
5 |
5 |
|
20
|
|
5 |
5 |
|
5 |
5 |
|
5 |
5 |
|
|||
Propylparaben |
||||||
mg/l |
0 HOURS |
24 HOURS |
48 HOURS |
|||
Conc. |
O2 |
pH |
O2 |
pH |
O2 |
pH |
Control |
100 |
7.7 |
- |
- |
100 |
7.6 |
2 |
100 |
7.7 |
- |
- |
100 |
7.6 |
50 |
100 |
7.7 |
100 |
7.6 |
- |
- |
100 |
100 |
7.7 |
100 |
7.6 |
- |
- |
O2-contents are given in % of saturation
LIN |
24 hours |
48 hours |
EC10 |
15.2 |
9.57 |
EC50 |
27.5 |
15.4 |
EC90 |
39.8 |
21.3 |
Unit: mg/l