|
| Front page | | Contents | | Previous |
Survey of Dioxin Emission from PCP-treated Wood
Bilag A
Appendix A: Dioxin analysis - method
Dioxin analysis in general
Dioxin is not a single substance, but a family of approximately 200 isomers with different degrees of toxicity. An analysis for
dioxins and furans is therefore not directly equal to a specific value of dioxin. The immediate result is a diagram showing an
isomeric pattern. The isomeric pattern shows the mutual quantitative proportion between the present isomers. The isomeric
pattern therefore functions as a finger print, as the presence and the amount of the different isomers can vary depending on the
dioxin source.
The total toxicity of the dioxin isomers can however be measured in the so-called tox equivalents, where the toxicity of the
present types of isomers is compared to the most toxic isomer 2,3,7,8-TCDD. The conversion to tox equivalents is typically
done by using International Tox Equivalent Factors (I-TEF), which means that the total toxicity is expressed in I-TEQ -
International Tox Equivalents. The International Tox Equivalent Factors are shown in table 4.1, which also includes the Nordic
and WHO Tox Equivalent Factors which were previously quite commonly used. The WHO-TEF system differs from I-TEF,
because this system in contrast to previous systems gives separate toxicity equivalent factors for humans/mammals, fish and
birds. However I-TEF is still the most used system.
In this project it has been chosen to use I-TEQ values as description of the total toxicity for dioxins. The results are also given in
WHO-TEQ values, but only I-TEQ values are used in the text.
Analysis method for PCDD/F and PCP in wood
The method that has been used to analyse the wood samples from both construction wood and disposable pallet boards has
earlier been used for dioxin analyses on sludge and textiles. These methods have earlier been described by the National
Environmental Research Institute.
The analyses and description of methods for this project have been carried out by Senior research associate Jørgen Vikelsøe
and Laboratory technician Elsebeth Johansen from DMU (National Environmental Research Institute, Denmark), Department
of Atmospheric Environment.
Table A.1
Important toxicity equivalency factor systems for dioxins
| Congener |
WHO1998 |
Nordic1988 |
International1989 |
| WHO-TEF 1) |
Nordic-TEF 2) |
I-TEF 2) |
| 2,3,7,8-TCDD |
1 |
1 |
1 |
| Other TCDDs |
0 |
0 |
0 |
| 1,2,3,7,8-PeCDD |
1 |
0.5 |
0.5 |
| Other PeCDDs |
0 |
0 |
0 |
| 1 2,3,4,7,8-HxCDD |
0.1 |
0.1 |
0.1 |
| 1,2,3,6,7,8-HxCDD |
0.1 |
0.1 |
0.1 |
| 1,2,3,7,8,9-HxCDD |
0.1 |
0.1 |
0.1 |
| Other HxCDDs |
0 |
0 |
0 |
| 1,2,3,4,6,7,8-HpCDD |
0.01 |
0.01 |
0.01 |
| Other HpCDDs |
0 |
0 |
0 |
| OCDD |
0.0001 |
0.001 |
0.001 |
| |
|
|
|
| 2,3,7,8-TCDF |
0.1 |
0.1 |
0.1 |
| Other TCDFs |
0 |
0 |
0 |
| 1,2,3,7,8-PeCDF |
0.05 |
0.01 |
0.05 |
| 2,3,4,7,8-PeCDF |
0.5 |
0.5 |
0.5 |
| Other PeCDFs |
0 |
0 |
0 |
| 1 2,3,4,7,8-HxCDF |
0.1 |
0.1 |
0.1 |
| 1,2,3,6,7,8-HxCDF |
0.1 |
0.1 |
0.1 |
| 2,3,4,6,7,8-HxCDF |
0.1 |
0.1 |
0.1 |
| 1,2,3,7,8,9-HxCDF |
0.1 |
0.1 |
0.1 |
| Other HxCDFs |
0 |
0 |
0 |
| 1,2,3,4,6,7,8-HpCDF |
0.01 |
0.01 |
0.01 |
| 1,2,3,4,7,8,9-HpCDF |
0.01 |
0.01 |
0.01 |
| Other HpCDFs |
0 |
0 |
0 |
| OCDF |
0.0001 |
0.001 |
0.001 |
1. The TEF-values stated cover exposure to humans and mammals. Separate and slightly different TEF-values have been stated
for fish and birds /UNEP 1999/.
2. From /Jensen 1997/.
Principle of the analysis method
The wood is divided into fine particles, and a mixture of 13C-marked PCDD/F standards (extraction spikes) is added. The
sample is soxhlet extracted in toluene; the extract is evaporated and parted in two part-extracts for PCP and PCDD/F
respectively. For PCDD/F the decontamination is made by a four-step liquid-chromatographical method. For PCP the
decontamination is made by an acid and alkaline shaking followed by methylation. Quantification by high-dissolving GC/MS
using the isotop dilusion method. Decontamination, detection and quantification for PCDD/F are based on an adaptation of
European standard for analysis of dioxin in flue gas, DS/EN 1948 2-3.
Apparatus
Soxhlet extraction equipment 250 ml
Rotary vacuum evaporator
Nitrogen evaporator
Automatic pipettes: 20-100 l, 100 l, 500l
Columns for liquid chromatography:
2.5x12 cm with reservoir 150 ml, Teflon tap, NS12 bottom cone ground joint
1x17 cm med Teflon tap, NS12 upper mantle ground joint 1x10 cm
Gas chromatography: Hewlett-Packard HP 5890 series II, connected to mass spectrometer
Pre-column: Chrompack Retention Gap, capillary quartz glass 2.5 m x 0.3 mm in diameter.
Column: J&W Scientific DB-5ms, capillary quartz glass 60 m x 0.25 mm in diameter, 25 μm film thickness
Autosampler: LEAP Technologies CTC A200S
Mass spectrometer: Kratos Concept 1S, high-dissolving sector instrument
Chemicals
| Toluene |
Rathburn, glass distilled |
| n-hexane |
Rathburn, glass distilled |
| Dichloromethane |
Rathburn, glass distilled |
| Methanol |
Merck, LiChrosolv |
| Na2SO4 |
Merck, anhydrous for analysis |
| Silica gel |
Merck, silica gel 60 0.063-0.20 mm |
| H2SO4 |
Merck for analysis |
| NaOH |
Merck for analysis |
| HCl |
Merck for analysis |
| Al2O3 |
ICN Biomedicals, Alumina A |
| n-dodecan |
BDH, Purity > 99% (GC area) |
| Active C |
Supelco, CarbopackC 80/100 mesh |
| Celite |
Fluka, Celite 545, 20-45μ |
| PFK |
Fluka, Perfluorokerosine, high boiling, for mass spectroscopy |
Internal and external standards
The applied marked and unmarked standards are all produced by CIL, Andover, Massachusetts, USA. Original dissolution 1
or 2 μg/ml in toluene, to be kept at 4°C.
Table A.2
Extraction spike dissolution
| Substance |
ng/ml |
Marking |
2378-TCDD
12378-PeCDD
123678-HxCDD
|
4 |
13C12 |
1234678-HpCDD
OCDD
|
8 |
13C12 |
2378-TCDF
12378-PeCDF
23478-PeCDF
123789-HxCDF
123678-HxCDF
234678-HxCDF
|
4 |
13C12 |
1234678-HpCDF
1234789-HxCDF
OCDF
|
8 |
13C12 |
| Toluene |
Dissolvent |
Table A.3
Injection spike dissolution
| Substance |
ng/ml |
Marking |
| 1234-TCDD
123789-HxCDD
|
16 |
13C12 |
| n-dodecan |
Dissolvent |
Table A.4
External standard dissolutions
| Substance |
Unmarked |
13C12 |
| |
ng/ml |
ng/ml |
ng/ml |
ng/ml |
ng/ml |
ng/ml |
| 1234-TCDD |
- |
- |
- |
- |
- |
4 |
| 2378-TCDD |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 12378-PeCDD |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 123478-HxCDD |
0.4 |
1 |
4 |
10 |
40 |
- |
| 123678-HxCDD |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 123789-HxCDD |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 1234678-HpCDD |
0.8 |
2 |
8 |
20 |
80 |
8 |
| OCDD |
0.8 |
2 |
8 |
20 |
80 |
8 |
| 2378-TCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 12378-PeCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 23478-PeCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 123478-HxCDF |
0.4 |
1 |
4 |
10 |
40 |
- |
| 123678-HxCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 123789-HxCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 234678-HxCDF |
0.4 |
1 |
4 |
10 |
40 |
4 |
| 1234678-HpCDF |
0.8 |
2 |
8 |
20 |
80 |
8 |
| 1234789-HpCDF |
0.8 |
2 |
8 |
20 |
80 |
8 |
| OCDF |
0.8 |
2 |
8 |
20 |
80 |
8 |
| n-dodecan |
Dissolvent |
All dissolutions in the dilution row contain the same concentration of 13C12 marked standards (spikes), stated in the last
column.
Standard dissolutions level 1.4 and 10 ng/ml (2,3,7,8-TCDD) are used for quantification
Standard dissolution level 40 ng/ml is used for linearity sample
Standard dissolution level 0.4 ng/ml is used for check of sensibility
Pre-treatment of sample
The wood samples are bored up by means of a 10 mm spiral drill. The blocks are bored all through, the holes placed as close
as technically possible. The drill is to be rinsed by means of n-hexane and acetone between each sample. The bore dust from
each sample is mixed carefully, and approx. 1 g dry weight is weighed out. 100 μl extraction spike dissolution (Table 4.2,
internal and external standards) is added.
Extraction
The spiked sample soxhlet is extracted for 20 hours in 700 ml toluene. The extract is added 0.5 ml n-dodecan as keeper and
evaporated to approx. 0.5 ml under vacuum in rotary evaporator at 35°C, 25 torr. The samples to be separated (i.e. pallet
wood that is to be analysed for both PCDD/F and PCP) are transferred to a 100 ml calibrated flask and redissolved in
n-hexane. Thereafter part samples of 10 ml are taken with full pipette.
Dioxin analysis
Decontamination by means of liquid chromatography
The evaporated extract or part-extract is placed on the upper one of two columns connected in series, containing (from the top)
Column 1: 2.5 x 12 cm m/ reservoir 250 ml
1 g anhydrous Na2SO4.
1 g silica gel (activated at 105°C),
4 g silica gel modified by 33% NaOH
1 g silica gel
4 g silica gel modified by 44% H2SO4
2 g silica gel
Column 2: 1 x 17 cm
1 g anhydrous Na2SO4.
6 g acid Al2O3 (activated at 250C).
Elution with 90 ml n-hexane through both columns connected in series is made. The columns are separated, after which column
2 only is eluted with 20 ml n-hexane. Both eluates containing various impurities are to be discarded. The PCDD/F fraction,
which is adsorbed on Al2O3 in column 2, is thereafter eluted with 20 ml dichloromethane/n-hexane 20/80. The eluate is placed
on a column containing
Column 3: 1 x 10 cm
1g Carbopack C/Celite 40/60 (activated at 15°C).
The column is fastened with the clean end upwards. Before placement of the sample the column is to be rinsed by 8 ml toluene,
16 ml dichloromethane/methanol 75/20 and 8 ml n-hexane.
After placement of the sample the column is eluted by 8 ml n-hexane followed by 16 ml dichloromethane/methanol 75/20. The
eluates are discarded. The column is placed with the clean end downwards and is finally eluted by 120 ml toluene.
The last eluate containing the decontaminated PCCD/F fraction is evaporated under vacuum in the rotary evaporator to some
ml and thereafter under N2 to almost dryness. The evaporation residue is redissolved in 25 μl internal standard mixture (Table
4.3) and 25 μl n-dodecan. After this the sample is ready for analysis by gas chromatography/mass spectrometry (GC/MS).
Gas chromatography
| Injection |
Automatic split/splitless, 2 μl + 1 μl n-dodecan, purge closed 40 sec. |
| Injector |
290°C, gooseneck insert 4 mm |
| Pre-column |
Chrompack Retention Gap, capillary quartz glass, 2.5 m x 0.3 mm in diameter |
| Column |
J&W Scientific DB-5ms, capillary quartz glass, 60 m x 0.25 mm in diameter
0.25 μm film thickness |
| Carrier gas |
He, pressure 150 kPa |
| Temperature program |
40 sec. at 200°C, 20°C/min. to 230°C, 3°C/min. to 290°C, 28 min. at 290°C |
| Transfer line |
290°C |
Mass spectrometry
| Dissolving power |
6000-10000 |
| Ionization |
Electron impact (EI) |
| Ionization energy |
35-45 eV depending on tuning |
| Ionization current |
5 μA |
| Ion source temperature |
290°C |
| Acceleration voltage |
8 kV |
| Electron multiplier voltage |
2.5-3 kV |
| Noise filter |
300 Hz digital |
| Magnet stabilization |
Current intensity |
| Solvent filament disconnect |
10 min |
| Cooling water temperature |
20-21°C |
| Calibration gas |
PFK |
| Scan parameter |
Cycle time 1 sec
Lock mass sweep 500 ppm, dwell 100 msec
ESA sweep 20 ppm
Dwell per mass 90-100 msec
Dwell for check mass 20 msec
Skew (between masses) 10 msec
Return time (between scan) 20 msec |
| Detection mode |
Selected Ion Monitoring (SIM). 5 windows, each with its mass combination (Table 4.4), are used |
2 masses for each substance, corresponding to the most intense lines in the molecular-ion group for both unmarked and marked
substances, are monitored. Furthermore, in all windows a lock mass and a check mass, which are standing out in the spectrum
of PFK, are used.
Table A.5
SIM masses for PCDD/F analysis
| Substance |
m/z 1 |
m/z 2 |
m/z 313C12- |
m/z 413C12- |
% I
mz1/mz2
|
| Group 1, tetra |
10-18 min |
Lock/check
TCDF
TCDD
|
292.9824
303.9016
319.8965
|
304.9824
305.8987
321.8936
|
315.9419
331.9368
|
317.9389
333.9339
|
77.3/100
77.2/100
|
| Group 2, penta |
18-24 min |
Lock/check
PeCDF
PeCDD
|
330.9792
339.8597
355.8546
|
342.9792
341.8567
357.8517
|
351.9005
367.8954
|
353.8976
369.8925
|
154.3/100
154.3/100
|
| Group 3, hexa |
22-28 min |
Lock/check
HxCDF
HxCDD
|
392.9760
373.8207
389.8156
|
392.9760
375.8178
391.8127
|
385.8610
401.8559
|
387.8579
403.8530
|
123.5/100
123.5/100
|
| Group 4, hepta |
28-34 min |
Lock/check
HpCDF
HpCDD
|
442.9729
407.7818
423.7767
|
442.9729
409.7788
425.7737
|
419.8220
435.8169
|
421.8189
437.8140
|
102.9/100
102.9/100
|
| Group 5, octa |
34-45 min |
Lock/check
OCDF
OCDD
|
442.9729
441.7428
457.7377
|
442.9729
443.7398
459.7348
|
453.7860
469.7780
|
455.7830
471.7750
|
88.2/100
88.2/100
|
Quantification, determination and identification of isomers
A chromatogram showing the signal of each mass (so-called mass traces) according to the SIM tables is recorded. For
determination of a dioxin or furan isomer in the unknown sample, there must be a well-defined peak with signal/noise conditions
on at least 3 on both the belonging mass traces. The retention times of the peaks must correspond to a 2,3,7,8-substituted
dioxin isomer or furan isomer. This can be determined by comparing with the retention times of the corresponding
isotope-marked standards, as there might be 1-2 sec. delay on the unmarked substances. The peaks fulfilling the above
conditions are quantified on both mass traces. The relation between the areas of the peaks of the two mass traces must
correspond to the natural chlorine isotope relation stated in table 4.5, with an error range of 25%. For the following calculations
the sum of both areas is to be used.
Quantification, calculation of response factors from standard
For each peak of an unmarked isomer on the chromatograms of external standard a response factor is calculated according to
the formula:
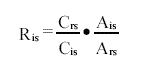
in which
Ris = Response factor for the i'th iso.mer
Cis = Concentration of the i'th isomer in external standard (table c)
Crs = Conc. of the belonging r'th laboratory spike isomer in external standard (table 4.4)
Ars = Area for the belonging r'th spike isomer in external standard 1)*)
Ais = Area for the i'th isomer *)
*) sum of peaks on both mass traces
1) in cases with an identical spike this spike is to be used in the calculation; otherwise the closest related spike is to be used for
the calculation as follows:
13C12-1,2,3,6,7,8-HxCDF til 1,2,3,4,7,8-HxCDF
13C12-1,2,3,6,7,8-HxCDD til 1,2,3,4,7,8-HxCDD
In this way there are 17 response factors for each GC/MS analysis of the external standard, one for each congener.
In order to obtain further precision the average of the various standard levels of response factors is used - and normally 2-4
repetitions (average response factor method).
Quantification, calculation of concentration in sample
The concentration of the i'th isomer in the unknown sample at the sample-taking, which is the final analysis result, is calculated
according to the formula:
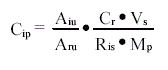
in which
Cip = Concentration of the i'th isomer in sample at the sample-taking
corrected for loss
Aiu = Area of the i'th isomer in unknown *)
Aru = Area of belonging laboratory spike isomer in unknown 1)*)
Cr = Concentration of belonging spike in laboratory spike mixture
(here 10 ng/ml) 1)
Vs = Volume of laboratory spike mixture added before extraction
(here 0.1 ml)
Ris = Average response factor for the i'th isomer calculated from external
standard
Mp = Quantity of sample at the sample-taking
*) sum af peaks on both mass traces
1) see under calculation of standard
Calculation of toxic equivalents (TEQ)
Toxic equivalent (TEQ) is a measurement expressing the total toxicity of the sample, normally used for an environmental
assessment. These TEQs express the toxicity of the individual isomers related to the toxicity of 2,3,7,8-TCDD, which is the
most toxic one (the so-called Seweso dioxin) by means of toxic equivalent factors (TEF).
TEQ in the sample is calculated according to the formular:
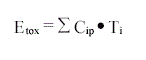
in which
Etox = Tox-equivalent concentration in the sample at sample-taking
Cip = Concentration of i'th isomer in the sample at sample-taking
Ti = Tox-equivalent factor for the i'th isomer according to table 4.1.
Blank specimens
In each analysis series a blank laboratory specimen is included, produced as follows: The extraction and decontamination
procedure is carried out simultaneously with the unknown samples as described above, an empty spiked soxhlet case being
analysed.
Analysis sequence
Each analysis series is analysed in the following sequence: a) Diluted-solution series of external standard, b) a sample with solely
n-dodecan for check of carry-over, c) blank specimen, d) the unknown samples, e) diluted-solution series of external standard.
In connection with long analysis series one or more rows of external standard are included among the unknown samples.
Standards in concentrations above the concentration area of the samples are not to be included. This rule was introduced to
avoid spill-over effects from very strong standards on the unknown samples. The unknown samples are analysed in individual
determinations.
Linearity
The linearity is checked at the deviation of response factors for each level of external standard. The deviation is calculated in the
form of a standard deviation between the various levels. No significant deviations from the linearity for any congener in the
concentration area (0.4 - 40 ng/ml) have been found.
Retrievals
The retrieval varies from 60 - 140%. It is calculated for each of the added extraction standards (extraction spikes) for each
sample and appears from previously (in the paragraph of quantification of sample) mentioned Concentration Report and from
the final result tables printed from the Quattro Pro spreadsheet (Appendix A). Retrievals below 50% or above 150% are not
acceptable. However retrieval for 2,3,7,8-TCDF down to 20% might be found and must be accepted.
Detection limits
The detection limits range from around 0.6 ng/kg for 2,3,7,8-TCDD to 10 ng/kg for OCDD.
Detection limits are calculated on the basis of the signal/noise relation in the software of the mass spectrometer. The relevant
detection limit is calculated as the sum of the blank-specimen value and the average of the detection limits of the analysis series
at nominal sample quantity.
PCP-screening
Pre-treatment and shaking
The 10 ml part-extract taken out for PCP analysis is to be evaporated, added 25 ml CH2Cl2 and 50 ml H2O, after which pH
is adjusted to 10. The H2O phase is to be shaken with 3 x 25 ml CH2Cl2, which is to be discarded. pH is adjusted to 2, after
which the H2O phase is shaken with 3 x 25 ml CH2Cl2. These CH2Cl2 phases are collected, dried by means of anhydrous
Na2SO4 and filtered through glass wool. The sample is to be subjected to methylation with diazomethan and evaporated.
Injection spike
The sample is redissolved in injection spike, containing 10 ng/ml D6-33'44'-PCB dissolved in n-hexane.
External standard
An external standard containing 10 ng/ml PCP (like methyl ether) and 10ng/ml D6-33'44'-PCB dissolved in n-hexane.
Gas chromatography
As with the dioxin analysis.
Mass spectrometry
As with dioxin analysis - however the following SIM-descriptor is to be used.
Table A.6
SIM masses for PCP analysis
| Substance |
m/z 1 |
m/z 2 |
| PCP-methyl ether |
279.8597 |
281.8568 |
| D6-33'44'-PCB |
295.9600 |
297.9571 |
| PFK lock/check |
292.9824 |
292.9824 |
1 scan per sec.
Quantification
Takes place under the same procedure as the one used for dioxin analysis.
| Front page | | Contents | | Previous | | Top |
Version 1.0 Juli 2004, © Danish Environmental Protection Agency
|