Pesticides Research No. 116, 2008
Sensor-based graduation of fungicide application in winter wheat
Contents
- 1.1 Fungicide use in winter wheat
- 1.2 Spatial variation in diseases
- 1.3 Spatially varying fungicide application
- 1.4 Objectives
- 2.1 Field experiments
- 2.2 Measurements of soil and topography
- 2.3 Yield measurements
- 2.4 Disease assessments
- 2.5 Sensor measurements of plants
- 2.6 Fungicide deposition on leaves and soil
- 2.7 Overview of measurement dates
- 2.8 Experiment with fungicide deposition at different driving speed
- 2.9 Statistical analyses
- 3.1 Characterisation of the sites
- 3.2 Yields
- 3.3 Diseases
- 3.4 Sensor measurements of crop parameters
- 3.5 Fungicide deposition
- 3.6 Fungicide deposition at different driving speed
- 4.1 Regression model
- 4.2 Causal model
- 4.3 Yield and economic benefits of applying the models
- 4.4 Spatial variation in soil and plant properties
- 4.5 Quality of sensor measurements
- 4.6 Fungicide treatment effects
- 4.7 Models for estimating yield response to fungicide
- 4.8 Evaluation of estimated spatial variation in fungicide rate
Preface
The project has been conducted in collaboration between University of Aarhus, Faculty of Agricultural Sciences (DJF) and the Danish Agricultural Advisory Service (DAAS). The basic experimental treatments and the yield determination were performed by DAAS, whereas all disease assessments, measurements of fungicide deposition and sensor measurements were conducted by DJF, who also was responsible for the model development.
The project leader was Jørgen E. Olesen (DJF), and the following researchers from DIAS contributed to the different research topics: Lise Nistrup Jørgensen, Peter Kryger Jensen and Anton G. Thomsen. The following advisors from DAAS were involved in designing and conducting the field experiments. Jens Erik Jensen, Bo J.M. Secher and Hanne Schønning. Ole Møller Hansen from DAAS was involved in the planing process. The following technicians from DIAS and DAAS contributed to the project: Finn Christensen, Uffe Pilegård Larsen, Carsten Sparholt, Søren H. Sørensen and Henning Nissen.
The project was funded under the crop protection research programme of the Danish Environment Agency. Additional funding was provided from "Gluds Legat" for a more detailed analysis of the laser sensors measurements in the project.
The input of the scientific sparring group under the crop protection research programme is gratefully acknowledged. This sparring group had the following members: Tommy Dalgaard, Peter Esbjerg, Lene Gravesen, Mette Jensen, Pernille Kaltoft, Jørn Kirkegaard, Kristian Kristensen, Niels Lindemark, Peter Odderskær, Hanne L. Pedersen, Bo Svenning Petersen, Jesper Rasmussen, Peter Sandøe, Anne Mette Langvad, Bent J. Nielsen, Chris Topping, Egon Noe, Jens Erik Ørum, Per Rydahl, Poul Henning Petersen, Jacob Weiner, Kim Martin Lind and Lise Samsøe-Petersen. A special acknowledgement is given to the reviewers of the report, which were Niels Lindemark and Lise Samsøe-Petersen.
Foulum, November 2007
Jørgen E. Olesen
Summary
The control of leaf diseases in cereals is usually performed using the same dose in the entire field based on an assessment of disease incidence in the field as a whole. However, there may be large differences in the need for disease control in different parts of a field. A site-specific fungicide application therefore potentially offers several advantages, including a better overall yield response to applied fungicide, a smaller amount of fungicide deposited on the soil surface and a reduction in fungicide use.
The objectives of the project were to clarify which factors influence the need to spatially vary fungicide treatments in winter wheat, and how these factors can be monitored using available tractor mounted sensors. The following specific objectives were considered:
- To study how the spatial variation in diseases in winter wheat (particularly mildew and septoria) depends on the variation in canopy structure (leaf area), nitrogen (N) concentration in leaves and variation in topography and soil type.
- To study the effects of canopy structure for yield and deposition of fungicides on wheat, including the distribution of fungicides on the top three leaves and the soil surface.
- To study how the yield increase from fungicide application depends on canopy structure, N-uptake, soil conditions, yield level and disease occurrence.
- To describe and parameterise a model to be used for optimised spatially varying fungicide doses, and to assess the effect of application of this model on yield and fungicide use.
Field experiments were carried out in winter wheat in 2005 and 2006 at two sites in Denmark ín each year. The sites were Nissumgård and Schackenborg in 2005 and Nissumgård and Dybvad in 2006. All sites had high spatial variation in soil and terrain. Each experiment was conducted in a two-factorial design with fungicide dose (4 levels) and N strategy (4 levels). The fungicide treatments consisted of increasing doses of Opus (0 to 0.8 L ha-1) applied at GS 39. The N strategies consisted of three different rates of mineral fertiliser N applied in a split treatment and a normal rate of N applied in a single treatment. The experimental factors were laid out in a randomised split-plot design with N-strategy as whole-plot factor and fungicide dose as sub-plot factor. These blocks were repeated 10 times across the field in an attempt to cover most of the soil variation between replicate blocks.
The soil and terrain variation were characterised in spring by measurements with RTK-GPS (for elevation, slope and aspect), EM38 (geoelectric measurement of soil texture) and MobilTDR (soil water capacity and soil impedance). There was a good relationship between measurements with EM38 and MobilTDR at all sites, except Schackenborg, most likely due to large organic matter contents in parts of the field at Schackenborg.
There was a consistent grain yield response to N strategy in all experiments, although this response was weak at Nissumgård in 2006 due to a very large spatial variation in crop establishment. However, the N strategies were primarily included to increase the variation in crop density and N status. There was only a small, but significant, yield response to fungicide rate in 2005, but a larger yield response to fungicide application in 2006, in particular at Dybvad. The yield gains of fungicide application became zero or negative in 2005, when the costs of applying fungicides were subtracted.
Measurements of crop status were taken by hand held sensors (SPAD, LAI2000 and VIScan) and using tractor-mounted sensors (MobilLas) at GS39. These measurements were taken in all plots. For comparison destructive plant sampling was performed in selected plots. This comparison showed that the SPAD measurements give good estimates of leaf N concentration and LAI2000 good estimates of crop leaf area index (LAI). The measurements of crop spectral reflectance with VIScan were expressed as the ratio vegetation index (RVI), which was found to correlate well with wheat LAI throughout the growing season, except for the measurements at Schackenborg at GS39, probably due to a high stand of weeds (mainly Poa annua) at this site and measurement time.
The diseases were assessed four times during the growing season. The diseases were dominated by Septoria tritici, and the coverage of septoria on the 2nd leaf at GS65 and the flag leaf at GS75 were taken as indicators for disease occurrence. This disease index decreased with increasing fungicide rate. The occurrence of septoria varied spatially, in particular depending on leaf N concentration and possibly also related to LAI.
The deposition of fungicides on each of the top three leaves and on the soil surface was measured using a tracer mixed in the fungicide solution used for spraying the crop at GS39. The fungicide deposition in terms of tracer concentration per leaf area varied strongly between the top three leaves with the highest concentration on the upper leaves. There was a tendency for the highest concentrations at high LAI, which may be related to leaf inclination, as there was a significant correlation to mean leaf angle. However, this effect was strongest for leaf 3 and there were only weak relationships for leaf 1 and 2. As it is the disease control on the upper two leaves that provides the main effect on yield gain from disease control, the effect of varying leaf fungicide concentrations with varying canopy density was ignored in the development of the algorithms for sensor based fungicide application.
The low driving speed (4 km h-1) used in the experiment may be supposed to give a deeper penetration into the canopy and thus less deposition on the upper part of the canopy compared to the deposition pattern obtained using a driving speed (8 km h-1) closer to the situation in practical agriculture. A supplementary experiment showed such a deposition pattern. At 4 km h-1 deposition of spray liquid per area unit leaf was only slightly reduced at the 2nd leaf and the value on the 3rd leaf was close to 75% of the deposit on the flag leaf. At 8 km h-1 a much steeper gradient in deposit was found, with a value on the 3rd leaf close to 40% of the per area unit deposit on the flag leaf.
The grain yield without disease (disease free yield) was found to be better correlated to crop sensor measurements (LAI, leaf N concentration and RVI) at GS39 than to soil measurements taken in spring. However, the usefulness of the RVI measurements at Schackenborg was reduced due to a large weed population.
The generally low disease pressure in 2005 and 2006 gave a poor relationship between yield response to fungicide application and crop characteristics. Analysis of yield gain from fungicide application showed significant positive correlations between yield gain and leaf N concentration or RVI at GS39, in particular at Dybvad with the highest septoria attack and the smallest error variation in grain yields. There were also significant positive relationships between normalised disease response to fungicide application and the measurements of leaf N concentration and RVI, which indicates that fungicides may be less effective in controlling disease in dense and nutritious crops.
The relevant crop characteristics could be measured using handheld sensors. Analyses of the tractor-mounted measurements using the MobilLas measurements indicate that it is possible also to perform these measurements in practice for the RVI measurements, whereas it is considerably more difficult to measure leaf area index (LAI) with sufficient precision using tractor mounted sensors. This is due to the saturation of the laser sensor measurements at LAI above 2.5-3.0.
Two different algorithms (an empirical model and a causal model) for spatially varying fungicide application were developed. Both models make use of RVI and EM38 measurements. EM38 describes the soil characteristics, in particular the soil clay content. This measurement can be made only once and used subsequently, whereas the RVI measurement needs to be taken at GS39 at the time of fungicide application. Both types of measurements are operationally available today.
Both algorithms were estimated to give a higher need for fungicide application for crops having a higher RVI. This was an indirect effect for the empirical model, whereas both higher grain yield and a higher septoria occurrence and high RVI contributed to this effect for the causal model.
The applicability of the sensor-based models for spatially varying fungicide application was evaluated using data from the four experiments. However, only plots that had received the normal N fertiliser rate were used for this purpose.
The evaluation of the model showed that the estimated variation in fungicide rate within the field varied between experimental sites, but the standard deviation was generally in the order of 0.1 to 0.2 L ha-1 Opus. There were only small yield gains from applying the spatially variable fungicide rate using data on variation in soil and crop conditions at the normal N rate in the field experiments conducted in this project, when comparing with a uniform fungicide rate of 0.4 L ha-1 Opus. There was mostly a reduction in fungicide deposition on the soil from applying the sensor-based fungicide rates compared with the uniform fungicide rate.
The evaluation showed that the largest yield gains from sensor-based fungicide application were obtained in situations with either very low or high disease infestation. There may thus be some scope for applying sensor-based fungicide rates in winter wheat. However, this will require further testing under a wider range of soil and climatic conditions, and this should also include a larger variation in the selection of varieties.
Sammenfatning
Bekæmpelse af sygdomme i kornafgrøder foretages normalt med samme dosering i hele marken ud fra en vurdering af sygdomsforekomst i marken som helhed. I praksis kan der dog være store forskelle i behovet for bekæmpelse i forskellige dele af marken. En sted-specifik tildeling af fungicider giver derfor mulighed for at opnå flere fordele, herunder en bedre udbytte-respons på tildelt fungicid, en mindre mængde fungicid afsat på jorden og potentielt en reduktion i fungicidanvendelsen.
Formålet med projektet er at undersøge hvilke parametre, der har betydning for arealgraduering af fungicidtildelingen i vinterhvede, og hvorledes disse parametre kan registreres med tilgængelige traktormonterede sensorer. Projektet har følgende delmål:
- At undersøge hvordan arealvariationen i forekomst af sygdomme i vinterhvede (især meldug og septoria) afhænger af variationen i afgrødestruktur (bladareal), N-indhold i bladene samt variation i topografi og jordtype.
- At undersøge betydningen af afgrødestrukturen for udbytte og afsætning af fungicid i vinterhvede, herunder fordeling af fungicid på de tre øverste blade i afgrøden og jorden.
- At undersøge hvordan merudbyttet ved svampebekæmpelse afhænger af afgrødestruktur, N-optagelse, jordbundsforhold, udbytteniveau samt forekomst af sygdommene.
- At beskrive en model for graduering af fungicidanvendelse i vinterhvede og vurdere effekten af gradueret fungicidtildeling på udbytte og fungicidforbrug.
Der blev i 2005 og 2006 gennemført markforsøg i vinterhvede på to lokaliteter hvert år. Stederne var Nissumgård og Schackenborg i 2005 og Nissumgård og Dybvad i 2006. Alle lokaliteter havde høj rumlig variation i jordbund og terrænforhold. Hvert forsøg blev gennemført i et to-faktorielt design med fungiciddosering (4 niveauer) og N-strategi (4 niveauer). Fungicidbehandlingerne bestod af stigende dosis af Opus tildelt på VS39 (0 to 0.8 L ha-1). N-strategierne bestod af tre forskellige N-mængder i en to-delt strategi suppleret med en normal N-mængde som engangstildeling. Forsøgsfaktorerne blev udlagt i et randomiseret split-plot design med N-strategi som helplotfaktor og fungicid som delplotfaktor. Blokkene blev gentaget 10 gange på tværs af marken i et forsøg på at dække jordvariationen mellem blokkene.
Jord- og terrænvariationen blev karakteriseret i foråret ved målinger med RTK-GPS (højde, hældning og hældningsretning), EM38 (geoelektrisk måling af jordtekstur) og MobilTDR (vandkapacitet og impedans i jorden). Der var en god sammenhæng mellem målinger af EM38 og MobilTDR på alle lokaliteter, undtagen Schackenborg, formentlig på grund af et højt indhold af organisk stof i dele af marken på Schackenborg.
Der var en god og konsistent respons af udbytte på N-strategi i alle forsøg. Dog var denne respons svag ved Nissumgård i 2006 på grund af en meget stor rumlig variation i afgrødeetableringen. N-strategierne blev dog hovedsageligt medtaget i forsøget for at øge variationen i afgrødetæthed og N-indhold i bladene. Der var kun en lille, men dog signifikant, udbytterespons på fungicidtildelingen i 2005, men en større udbytterespons på fungicid i 2006, især ved Dybvad. Efter korrektion for omkostninger ved fungicidtildelingen var der ingen signifikant økonomisk fordel ved fungicidanvendelse i 2005.
Målinger af afgrødestatus blev taget med håndbårne sensorer (SPAD, LAI2000 og VIScan) og med traktormonterede sensorer (MobilLas) ved VS39. Disse målinger blev foretaget i alle parceller. Til sammenligning blev der taget destruktive planteprøver af blad N koncentration og bladarealindeks (LAI) i udvalgte parceller. Denne sammenligning viste, at SPAD målinger kan tages som et godt udtryk for bladenes N koncentration og LAI2000 giver et godt estimat for LAI . Målinger af afgrødens spektrale reflektans med VIScan blev udtrykt ved et vegetationsíndeks (RVI), som korrelerede godt med hvedens LAI i hele vækstsæsonen, dog med undtagelse af målingerne ved Schackenborg ved VS39, formentlig på grund af en stor bestand af enårig rapgræs på dette sted ved dette måletidspunkt.
Sygdomsforekomsten blev bedømt fire gange i vækstsæsonen. Sygdommene var domineret af Septoria tritici, og dækningsgraden af septoria på andet blad ved VS65 og på flagbladet ved VS75 blev benyttet som indikator for sygdomsforekomst. Dette sygdomsindeks faldt med stigende fungiciddosering. Forekomsten af septoria varierede rumligt, især afhængig af N koncentration i bladene og muligvis også afhængig af LAI.
Afsætningen af fungicid på de tre øverste blade og på jordoverfladen blev målt ved anvendelse af en tracer, som blev opbladet i sprøjtevæsken og tildelt på VS39. Fungicidafsætningen i form af tracer per bladareal varierede stærkt mellem de tre øverste blade, og med de højeste koncentrationer på det øverste blad. Der var en tendens til højere tracerkoncentrationer ved højt LAI, hvilket formentlig er relateret til bladvinkler, idet der var en signifikant sammenhæng mellem tracerkoncentration på flagbladet og afgrødens gennemsnitlige bladvinkel. Denne effekt var dog stærkest for tredje blad, og der var kun svage sammenhænge for blad 1 og 2. Da det er sygdomskontrollen på de to øverste blade, der er afgørende for merudbyttet ved sygdomsbekæmpelsen, blev effekten af varierende fungicidkoncentrationer på bladene ignoreret ved udviklingen af algoritmer til sensorbaseret fungicidtildeling.
Ved den lave kørehastighed (4 km time-1), der blev anvendt i forsøgene, opnås formentlig en dybere nedtrængning af fungicid i afgrøden og derfor mindre afsætning på de øvre blade sammenlignet med afsætningsprofilen ved de højere kørehastigheder (ca. 8 km time-1), der anvendes i praksis. Dette blev eftervist i et supplerende forsøg i vinterhvede. Ved en kørehastighed på 4 km time-1 var afsætningen på andet blad kun lidt reduceret i forhold til fanebladet, og afsætningen på tredje blad var tæt på 75% af afsætningen på fanebladet. Ved 8 km time-1 var der en meget stejlere gradient i afsætningen, med en værdi for tredje blad på ca. 40% af afsætningen på fanebladet.
Udbyttet i en afgrøde uden sygdom (sygdomsfrit udbytte) var bedre korreleret med sensormålinger af afgrøden (LAI, blad N koncentration og RVI) ved VS39 end med jordmålinger taget i foråret. Værdien af RVI målingerne ved Schackenborg var dog reduceret på grund af en tæt ukrudtsbestand.
Det generelt lave sygdomstryk i både 2005 og 2006 gjorde det vanskeligt at kvantificere en sammenhæng mellem merudbytte ved fungicidtildeling og afgrødetæthed. Analyser af merudbytte ved fungicidtildeling viste signifikante positive korrelationer mellem merudbytte for fungicidtildeling og blad N koncentration eller RVI ved VS39. Dette var især tilfældet ved Dybvad, som havde det højeste septoriaangreb og den laveste forsøgsfejl i kerneudbytte. Der var også signifikante positive sammenhænge mellem normaliseret sygdomsrespons på fungicidtildeling og målingerne af blad N koncentration og RVI, hvilket indikerer at fungiciderne kan være mindre effektive i tætte og velnærede afgrøder.
De relevante afgrødekarakteristika kunne måles med håndholdte sensorer. Analyser af målingerne med den traktormonterede MobilLas viste at det også er muligt at gennemføre disse målinger i praksis når det drejer sig om RVI, hvorimod det er betydeligt vanskeligere at måle bladarealindekset (LAI) med tilstrækkelig præcision med traktormonterede sensorer. Dette skyldes, at sensormålingerne mætter ved LAI over 2,5-3,0.
Der blev udviklet to forskellige algoritmer (en empirisk og en kausal model) for rumlig varierende fungicidtildeling. Begge modeller udnytter RVI og EM38 målinger. EM38 beskriver variationen i jordbundsforhold, især i relation til jordens lerindhold og dermed den vandholdende evne. Denne måling behøver kun at blive foretaget én gang for alle, hvorimod RVI målingerne skal foretages på tidspunktet for fungicidtildeling. Begge typer målinger er kommercielt tilgængelige.
Begge algoritmer vil estimere et større behov for fungicidtildeling for afgrøder med et højt RVI. Dette er en indirekte effekt i den empiriske model, hvorimod det i den kausale model er forårsaget af et estimeret højere kerneudbytte og en højere forekomst af septoria ved højt RVI.
Effekterne af anvendelse af de sensor-baserede modeller på økonomisk udbytte og fungicidafsætning på jorden blev estimeret ved anvendelse af data fra de fire forsøg. Dog blev kun parceller med normal N-niveau anvendt til dette formål.
Evalueringen af algoritmerne viste at den estimerede variation i fungicidtildeling inden for marken varierede mellem forsøgsstederne, men spredningen i fungicidtildeling inden for marken var generelt på 0,1 til 0,2 L ha-1 Opus. Der var kun meget små merudbytter for anvendelse af en rumligt varierende fungicidtildeling sammenlignet med en ensartet behandling med 0,4 L ha-1 Opus. Der var i de fleste tilfælde en reduktion i fungicidafsætningen på jorden ved anvendelse af sensor-baserede fungicidtildeling sammenlignet med den ensartede behandling.
Evalueringen viste de største merudbytter for sensor-baseret fungicidtildeling i situationer med enten meget lave eller meget høje angrebsgrader. Der kan således være muligheder for praktisk anvendelse af sensor-baseret fungicidtildeling i praksis. Dette vil dog kræve yderligere afprøvning med en større variation i jord og klimaforhold, og her bør også indgå en større variation i sortsvalget.
1 Introduction
- 1.1 Fungicide use in winter wheat
- 1.2 Spatial variation in diseases
- 1.3 Spatially varying fungicide application
- 1.4 Objectives
1.1 Fungicide use in winter wheat
Fungicides are commonly used for controlling leaf diseases in winter wheat. On average the number of treatments today is two, and the average dose of fungicides per treatment varies between 20 and 50% of the full dose (Farmstat and Kleffmann, 2002). The main targets for disease control in winter wheat are powdery mildew (Blumeria graminis) and septoria leaf blotch (Septoria tritici). Septoria tritici has during the last 20 years been the most yield reducing disease and been the major factor influencing the fungicide input. Input of pesticides in Denmark is today generally measured as Treatment Frequency Index (TFI), which quantifies the number of full dosages applied in the field. The target figure for 2003 in Pesticide Action Plan II for fungicide input in winter wheat was recommended to 0.75. In the new action plan extending to 2009 the recommended TFI is 0.65. The target of 0.75 was reached in general in 2002 (Anonymous, 2003). A further reduction requires increased use of resistant cultivars and new techniques to optimise the input. More than a 50% reduction in fungicide input in winter wheat has already taken place during the last 20 years mainly due to use of appropriate and reduced dosages.
1.2 Spatial variation in diseases
Mildew often occurs near the borders of fields, where local shelter effects may give favourable conditions for infection and spreading of mildew spores (Koch, 1980; Secher et al., 1995; Bjerre et al., 1998). This is probably caused by more favourable temperature and humidity conditions near shelterbelts, and topographic variation may give rise to similar variation in occurrence of mildew. Mildew has also been found to increase at higher crop densities during early crop development (Bødker et al., 1994). The occurrence of mildew on the upper leaves (including the flag leaf) on the other hand seems to be more related to nitrogen (N) application rates and N concentration in the leaves around growth stage 39 (Olesen et al., 2000a, 2003b).
Several investigations have demonstrated a relationship between incidence of septoria and crop density (Jørgensen, 1997; Bjerre et al., 1998; Olesen et al., 2000a), showing that septoria increases at low crop densities, where the fungal disease spores more readily spreads upwards in the crop canopy. However, other investigation have found higher disease incidence at high crop densities (Broscious et al., 1985; Tompkins et al., 1985). The different results obtained may be an effect of a correlation between crop density and leaf nitrogen (N) concentrations, since a large crop N supply often leads to dense crops. However, if a low crop density is determined by a low plant density, then high leaf N concentrations may also occur at low densities. High leaf N concentrations at growth stage 39 have in several investigations been found to promote Septoria tritici (Leitch and Jenkins, 1995; Olesen et al., 2003b; Simon et al., 2003).
1.3 Spatially varying fungicide application
Control of leaf diseases in cereals is normally performed with the same fungicide dosages throughout the field from an assessment of the disease occurrence in the field as a whole. However, there may be large differences in the need for disease control in different parts of a field (Bjerre et al., 1998). A site specific fungicide application may improve disease control under the following conditions (Bjerre et al., 2006): (a) if the disease varies spatially, (b) if the crop sensitivity to disease varies, and/or (c) if the effect of disease control varies spatially.
Analyses of yield gains from fungicide control have shown a tendency for higher yield increases at sites with high yield potentials (Paveley et al., 1996; Oerke and Dehne, 1997; Dansk Landbrugsrådgivning, 2003). The causes of this have not been fully clarified, but it may be at least partly related to low yielding crops primarily occurring on sandy soils, where the full potential of disease control cannot be exploited, since the crop yield is often reduced by early senescence. The crop density and the leaf area also affects the amount of fungicide deposited on the individual leaves, and thus also the effect obtained from a fungicide treatment (Secher, 1998).
The qualitative deposition in the crop canopy with conventional spraying technology is primarily varied through changes in driving speed and drop size and nozzle choice. A low driving speed and a large drop size gives the largest penetration in the canopy, whereas a high speed and fine drops lead to a deposition in the upper part of the canopy. The deposition is often characterised by a capture efficiency, which is correlated with the crop leaf area index (Jagers op Akkerhuis et al., 1998; Gyldenkærne et al., 1999). A constant capture efficiency will lead to a steeper decline of fungicide deposition in a dense compared with an open crop canopy. However, Secher (1998) found the same deposition profiles at different crop densities, but generally a lower deposition per unit leaf area in the more dense canopies. If the fungicide treatments within a field are varied based on crop density and the same amount of fungicide is used, then a spatially varied application may lead to less fungicide being deposited on the soil surface. Experiments have shown that there is a small, but significant yield gain from adjusting the fungicide rate (+/- 20%) according to the crop density (Secher, 1998).
A spatially varied fungicide rate requires that the relevant influencing factors can be measured using tractor-mounted soil and plant sensors for mapping the spatial variation in soil and plant characteristics. The soil characteristics may be measured using time domain reflectrometry (MobilTDR) (Thomsen and Schelde, 2006) or electrical conductivity (EM38) (Greve et al., 2002). These soil characteristics mostly reflect the soil water retention capacity, which is related to yield potential and therefore possibly also to yield gain from fungicide application. The plant characteristics may be measured by using spectral reflectance combined with laser measurements to characterise crop canopy geometry (MobilLas) (Thomsen and Schelde, 2007). These plant characteristics mostly reflect leaf area index and plant nitrogen content, which are related yield potential, fungicide deposition and disease susceptibility.
There thus appears to be a potential for spatially optimising the fungicide application in winter wheat. This may increase the overall efficacy of fungicide applications and at the same time reduce the amount of fungicide lost to the soil surface. However, there is a need to assess a number of factors, which thus have been the focus of this project:
- How much does disease occurrence vary depending on plant density, crop canopy structure and N supply, and how does this influence the need for fungicide application?
- How does the yield potential depend on canopy density, and will dense canopy give higher yield gains for fungicide applications?
- Can the new sensor technologies be used to measure the relevant soil and plant characteristics, and can they be used in practice for spatially optimising the fungicide rate?
1.4 Objectives
The objectives were to clarify which factors influence the need to spatially vary fungicide treatments in winter wheat, and how these factors can be monitored using available tractor mounted sensors. This requires clarification of the relationships between the influencing crop parameters and a range of processes including disease occurrence, fungicide deposition on leaves and disease control effects of fungicides. The investigations were performed with the objective of developing algorithms for spatially varying the fungicide dose when spraying at heading in winter wheat.
The following specific objectives were considered:
- To study how the spatial variation in diseases in winter wheat (particularly mildew and septoria) depends on the variation in canopy structure (leaf area), N concentration in leaves and variation in topography and soil type.
- To study the effects of canopy structure for yield and deposition of fungicides on wheat, including the distribution of fungicides on the top three leaves and the soil surface.
- To study how the yield increase from fungicide application depends on canopy structure, N-uptake, soil conditions, yield level and disease occurrence.
- To describe and parameterise a model to be used for optimised spatially varying fungicide doses, and to assess the effect of application of this model on yield and fungicide use.
2 Materials and methods
- 2.1 Field experiments
- 2.2 Measurements of soil and topography
- 2.3 Yield measurements
- 2.4 Disease assessments
- 2.5 Sensor measurements of plants
- 2.6 Fungicide deposition on leaves and soil
- 2.7 Overview of measurement dates
- 2.8 Experiment with fungicide deposition at different driving speed
- 2.9 Statistical analyses
2.1 Field experiments
Field experiments were carried out in winter wheat at four sites in Denmark with high spatial variation in soil and terrain; two sites in each of the years 2005 (Schackenborg and Nissumgaard) and 2006 (Nissumgaard and Dybvad). The site at Schackenborg is located close to the village Møgeltønder and west of the town Tønder in southern Jutland on a sandy soil overlaying clayey deposits from the marshes. The site at Nissumgård is located close to the village Gjesing between Skanderborg and Odder in eastern Jutland on moraine deposits varying in soil type from sandy to sandy loam. The site at Dybvad is also located in eastern Jutland on moraine deposits varying in soil type from loamy sand to sandy loam.
All locations were chosen to be relatively inhomogeneous fields with respect to soil type, and at Nissumgård and Dybvad also with variable topography, in order to favour different responses to nitrogen and fungicide treatments.
Each experiment was conducted in a two-factorial design with fungicide dose and N strategy (Table 1). The fungicide treatments consisted of increasing doses of Opus (125 g L-1 epoxiconazol) applied at GS 39. The N strategies consisted of three different rates of mineral fertiliser N applied in a split treatment and a normal rate of N applied in a single treatment. In all split treatments 50 kg N ha-1 was applied in early April. The remaining N and the full rate of the single N treatment were applied at stem elongation (late April).
Table 1. Factors and treatments in the experiment.
| Factor | Treatment |
| 1. Fungicide | 1. No fungicide control |
| 2. 0.2 L ha-1 of Opus applied at GS 39 | |
| 3. 0.4 L ha-1 of Opus applied at GS 39 | |
| 4. 0.8 L ha-1 of Opus applied at GS 39 | |
| 2. N-strategy | 1. 160 kg N ha-1 (50 + 110 kg N ha-1) |
| 2. 80 kg N ha-1 (50 + 30 kg N ha-1) | |
| 3. 240 kg N ha-1 (50+190 kg N ha-1) | |
| 4. 160 kg N ha-1 (single application) |
The experimental factors were laid out in a randomised split-plot design with N-strategy as whole-plot factor and fungicide dose as sub-plot factor. These blocks were repeated 10 times across the field in an attempt to cover most of the soil variation between replicate blocks, giving a total of 160 plots in each experiment. Net plot sizes were 27.6 m² at Nissumgård and 30 m² at Schackenborg in 2005, and 16 m² at Nissumgård and 17.7 m² at Dybvad in 2006.
The crop management details are outlined in Table 2. The previous crop was winter wheat at both sites in 2005 and winter oilseed rape at both sites in 2006. The wheat was sown in mid September in both years. The site at Nissumgård in 2005 was sprayed with manganesesulphate in April. However, despite of this, manganese deficiency could be observed in some of the plots in May.
There were three plots at Schackenborg in 2005 with errors in N fertiliser application. In about a third of plots 2108 and 2208 too little fertiliser had been applied, and plot 2811 had received too much fertiliser.
Table 2. Crop management at the two experimental sites in 2005 and 2006.
| 2005 | ||
| Nissumgård | Schackenborg | |
| Previous crop | winter wheat | winter wheat |
| Variety | Deben | Grommit |
| Sowing | 21-09 | 18-09 |
| Harvest | 01-09 | 18-08 |
| N fertiliser | 11-04 (1st rate) | 06-04 (1st rate) |
| 26-04 (2nd rate) | 26-04 (2nd rate) | |
| Fungicide | 08-06 (Opus) | 30-05 (Opus) |
| Herbicide | 28-10 (9 g ha-1 Lexus 50 WG) | 22-04 (100g ha-1 Hussar) |
| 28-10 (1.5 L ha-1 Boxer EC) | ||
| 30-04 (100 g ha-1 Hussar) | ||
| 30-04 (0.04 L ha-1 DFF) | ||
| 18-05 (12.5 g ha-1 Monitor) | ||
| Insecticide | 18-08 (0.1 kg ha-1 Pirimor) | |
| Micronutrient | 30-04 (2 kg ha-1 MnSO4) | |
| 2006 | ||
| Nissumgård | Dybvad | |
| Previous crop | winter oilseed rape | winter oilseed rape |
| Variety | Deben | Deben |
| Sowing | 13-09 | 15-09 |
| Harvest | 30-08 | 29-08 |
| N fertiliser | 19-04 (1st rate) | 19-04 (1st rate) |
| 05-05 (2nd rate) | 05-05 (2nd rate) | |
| Fungicide | 06-06 (Opus) | 02-06 (Opus) |
| Herbicide | 06-10 (1 L ha-1 Stomp) | 12-10 (1.25 L ha-1 Boxer EC) |
| 06-10 (0.025 L ha-1 DFF) | 12-10 (0.4 L ha-1 Stomp) | |
| 06-10 (0.15 L ha-1 Boxer EC) | 12-10 (0.03 L ha-1 DFF) | |
| 10-05 (18 g ha-1 Monitor) | 12-10 (0.1 L ha-1 Oxitril CM) | |
| Insecticide | 24-10 (0.05 L ha-1 Karate EW) | 20-08 (0.1 L ha-1 Cyperb) |
| Micronutrient | 24-10 (2 kg ha-1 MnSO4) | 20-10 (2 kg ha-1 MnSO4) |
| 10-05 (2.5 kg ha-1 MnSO4) |
The fungicide treatments including the tracer treatment were applied using an experimental plot sprayer equipped with a 2.5 m wide boom. The spray boom was equipped with conventional hydraulic flat fan nozzles with a mutual distance of 50 cm. The nozzle used was a Hardi S 4110-14 flat fan nozzle delivering 0.7 l min-1 corresponding to 230 l ha-1 at a driving speed of 3.6 km h-1.
There was a spraying error at Dybvad in 2006, where plot 4312 did not get any fungicide application.
2.2 Measurements of soil and topography
Measurements of soil texture, water content, topography etc. were made with three sensors:
- MobilTDR (time domain reflectometry) instrument developed at Research Centre Foulum (Thomsen et al., 2005; Thomsen and Schelde, 2006). The TDR instrument is mounted on farm tractor. The MobilTDR instrument was used for making precise measurements of water content and electrical conductivity of the top 50 cm of the soil. Early season measurements of water content is closely related to the water holding capacity of the topsoil and hence the yield potential. In combination, measurements of water content and electrical conductivity are closely related to the sum of the clay and silt fractions of a given soil type. The MobilTDR instrument is only available as a single prototype. A maximum of 15-30 ha can be mapped in a single day. These measurements were included for comparison with EM38 measurements and generally to provide data of reference quality.
- EM38 instrument mounted on a low sledge made from non-metallic materials (plastics) (Greve et al., 2002; Korsaeth, 2006). The instrument is pulled by a light ATV vehicle. The EM38 instrument is used for measuring soil electrical conductivity of the topsoil. The measuring depth varies with especially the clay and water content of the soil. The measurements are related to relative rather than absolute differences in soil texture. The commercially available EM38 instrument is widely used today and large areas can be mapped in a single day.
- RTK (GPS) instrument. The RTK instrument is used for manual measurements of the exact position and elevation of plot corners. The data is used in the extrapolation of mobile measurements made outside plots and in the calculation of plot orientation.
Both mobile instruments (MobilTDR and EM38) were equipped with GPS (global positioning satellite) receivers for geo-referencing measurements. The mobile measurements related especially to soil texture were included in order to map differences in growth conditions for the individual plots. The mobile measurements were made outside the research plots in order to avoid disturbing the wheat crop. Consequently interpolation is needed in order to extend measurements inside the plots.
Additionally samples of the topsoil (0-20 cm) were taken across each of the fields for comparison with the sensor measurements. Ten samples were taken at each site and analysed for soil texture.
2.3 Yield measurements
The plots were harvested using a plot combiner. Crude grain yield was determined immediately after combining by weighing the amount of grain harvested in each plot. Samples were taken for subsequent analyses. The first step of the analysis was separation of grain and impurities, resulting in an estimate of percentage of impurities. The pure grain samples were analysed for content of water, crude protein and starch and for specific weight (100 litre weight) using NIT (Buchmann et al., 2001).
Based on content of impurities and water the grain yields were transformed to dry matter grain yields, which were used in subsequent analyses. Only yield was analysed further.
Due to errors at harvest, two plots at Nissumgård and one plot at Schackenborg were lost in 2005, and consequently any yield related value for these plots was set to missing in the subsequent analyses. At Nissumgård yields from 21 plots in 2006 were discarded due to errors in marking the location of the field plots. There were 6 missing yield observations at Dybvad in 2006. This number of missing data should be compared to the total of 160 plots at each site.
2.4 Disease assessments
Disease assessments were carried out 4 times in the trials with focus on Septoria tritici (septoria leaf blotch) and Blumeria graminis (powdery mildew). Cultivars susceptible to Septoria tritici were chosen to increase the possibilities of investigating the impact on this disease.
- GS 32-33; Assessments were carried on whole plots with the 4 different N strategies. The disease severity was assessed as percentage of green area of the canopy with visible symptoms of the diseases.
- GS 37-39; Severity was assessed in all plots. The disease severity was assessed as percentage of green area of the canopy with visible symptoms of the diseases.
- GS 65; approximately 3-4 weeks after fungicide application. All plots were assessed. Severity of individual diseases was assessed on individual leaf levels (2nd leaf and flag leaf).
- GS 75 approximately 30-40 days after application. All plots were assessed. Severity of individual diseases was assessed on individual leaf levels (2nd leaf and flag leaf).
Apart from plant pathogenic spots, physiological spots developed significantly in the cultivar Grommit grown at Schackenborg in 2005. These spots are cultivar specific as well as being influenced by various stress factors in the crop. The physiological spots complicated the assessment of septoria leaf blotch at GS 39 and 65 as the different symptoms can be difficult to separate. In 2006 only Sepotoria blotch developed significantly in the trials. In the report Septoria leaf blotch will generally just be called Septoria.
2.5 Sensor measurements of plants
Measurements of canopy structure (leaf area index (LAI), leaf mean tip angle (MTA) and height) and N-status were all made during two major campaigns (growth stages BBCH_32 and BBCH_39) using four instruments:
- LAI-2000 canopy analyser from LI-COR Biosciences was used for measuring LAI and MTA (Stroppiana et al., 2006). A limited number of destructive samples were collected and analysed in the laboratory with respect to leaf area in order to check the sensor measurements. Both the total crop area index (CAI) including both green and senescent canopy material and the mean tip angle (related to canopy N-status) output by the instrument. For the early measurements included here the CAI values are sufficiently close to green LAI.
- ViScan radiometer developed at research Centre Foulum (Thomsen et al. 2002). The hand carried ViScan radiometer is used for measuring the spectral reflectance of crop canopies. Spectral reflectance especially in the form of a spectral index calculated from two or more spectral bands (Broge and Leblanc, 2001) is closely related to e.g. green leaf area index and canopy chlorophyll content (Broge and Mortensen, 2002). The simple ratio vegetation index, RVI (Broge and Thomsen, 2002), calculated as the ratio of near infrared (780 nm) and visible red (660 nm) reflectance values, was used here as indicator of canopy green leaf area and chlorophyll content (N-status).
- SPAD-502 chlorophyll meter from Konica Minolta. SPAD measurements are made manually by placing a leaf in a clip. Meter readings are related to the chlorophyll concentration in the leaf (Broge and Mortensen, 2002). 30 individual readings were made in each plot on the upper fully extended leaves in the canopy and averaged to a mean value by the instrument. Instrument readings were used as index values and not calibrated into absolute chlorophyll content.
- The MobilLas sensor includes the following major hardware components (Thomsen and Schelde, 2007): a) Near-infrared laser range finder, AccuRange 4000, with high-speed interface produced by Acuity Research Inc., CA, USA. Range finder configured for close range, narrow beam (0.5 mm) and daylight operation. b) Two four-band radiometers, SKR 1850, produced by Skye Instrument Ltd., UK. Radiometers filtered (650, 710, 730 and 780 nm) for the measurement of common spectral indexes (RVI, NDVI etc.) and red edge position (Broge and Leblanc, 2001; Thomsen et al., 2002; Broge et al., 2002). c) Global positioning sensor, GPS 16, produced by GARMIN International Inc., KS, USA.
Due to errors in delineation of some of the plots at Nissumgård in 2006, measurements of ViScan and MobilLas were not performed in 22 plots on any of the measurement dates in 2006. Due to data storage problems measurements of crop canopy reflectance using ViScan from 31 May 2006 at Nissumgård were only available from 11 plots. The missing ViScan data were substituted by similar data measured using MobilLas.
2.6 Fungicide deposition on leaves and soil
Deposition of spray liquid was measured on the crop and at the soil surface. A tracer, brillantsulfoflavin, was used to quantify the amount of spray liquid and hence fungicide deposited on the crop and at soil level. Brillantsulfoflavin is a stable product at 5 °C and storage for several months did not cause loss of activity (Jensen and Spliid, 2003). The tracer at a concentration of 100 g ha-1 brillantsulfoflavin was added to the spray solution in treatments with the lowest fungicide dose. This means that deposition was measured in the combinations N strategy ´ replicates giving a total of 40 plots per location. Before application of tracer and fungicide mixture in the field experiment, fluorescence interactions between fungicide and tracer was tested without revealing any problems.
Shortly before the experimental applications were carried out, objects were placed at soil level in order to collect the spray used for quantification of soil deposits. Rectangular filter paper objects with a size of 1.8 ´ 12 cm were used. This size allows a representative sampling, as the objects could reach from the middle of one plant row to the middle of the next row. The paper objects were placed on metal rods in order to avoid contamination with soil and in order to achieve a horizontal placement. Eight paper objects were placed in each plot. After the fungicide treatment was carried out, the paper objects were collected with two samples each consisting of four paper objects per plot. The filter papers were stored in 100 ml amber glass bottles under dark conditions at 5 oC until the samples were analysed. The tracer was solved in 50 ml demineralised water and the bottles were shaken thoroughly and a small proportion of the liquid was used for the analysis. Samples of the spray liquid were taken and stored the same way.
After the fungicide treatment, 9 crop plants from each plot was taken and divided into three sections: 1) 1st leaf (flag leaf), 2) 2nd leaf and 3) 3rd leaf. These plant samples were collected in plastic bags and after transportation stored at 5 °C. The tracer was solved using 100 ml demineralised water and the samples were shaken gently in order to avoid fluorescent material from the leaves. The liquid with tracer and fungicide was collected and stored dark at 5 °C until the analysis of tracer. The leaf area of each section was determined using a Licor Area Meter (model 3100). Following this the dry weight of the leaf sections were determined by drying at 80 °C for 24 hours.
The concentration of tracer in the liquid samples was determined using a Perkin Elmer model LS50B luminescence spectrometer. The bottles were shaken and a sample of 6 ml was used in the fluorescence detector. The sample was excited at a wavelength of 420 nm and after excitation emission was measured at 518 nm. The content of the sample was quantified using a number of standard concentrations ranging from 10 to 2000 mg l-1. From the concentration of brillantsulfoflavin in the sample the actual amount of tracer on the paper objects and on the leaf sections were calculated. For control purposes tracer concentration was also determined in the remaining spray solution from the experimental sprayer. Paper objects and leaf samples from untreated control were also washed with demineralised water and tested.
2.7 Overview of measurement dates
The measurements of crop characteristics were coordinated, so that the different measurement could be compared. However, due to weather conditions and time required for taking measurements, not all measurements could be performed on the intended dates (Table 3).
Table 3. Overview of dates of crop measurements.
| Measurement | GS32 | GS39 | GS65 | GS75 |
| Nissumgård (2005) | ||||
| Disease | 10-05 | 24-05 | 28-06 | 11-07 |
| Plant sample | 02-06 | |||
| Canopy height | 12-05 | 06-06 | ||
| SPAD | 12-05 | 06-06 | ||
| VIScan | 12-05 | 01-06 | 22-06 | 04-07 |
| LAI2000 | 12-05 | 01-06 | 22-06 | 04-07 |
| MobilLas | 12-05 | 01-06 | ||
| Schackenborg (2005) | ||||
| Disease | 10-05 | 24-05 | 24-06 | 07-07 |
| Plant sample | 30-05 | |||
| Canopy height | 27-05 | |||
| SPAD | 11-05 | 26-05 | ||
| VIScan | 11-05 | 27-05 | 20-06 | 30-06 |
| LAI2000 | 11-05 | 26-05 | 20-06 | 30-06 |
| MobilLas | 11-05 | 27-05 | ||
| Nissumgård (2006) | ||||
| Disease | 17-05 | 31-05 | 22-06 | 11-07 |
| Plant sample | 31-05 | |||
| SPAD | 23-05 | 31-05 | ||
| VIScan | 23-05 | 31-05 | 20-06 | 04-07 |
| LAI2000 | 23-05 | 01-06 | ||
| MobilLas | 23-05 | 31-05 | ||
| Nissumgård (2006) | ||||
| Disease | 18-05 | 31-05 | 22-06 | 11-07 |
| Plant sample | 31-05 | |||
| SPAD | 17-05 | 31-05 | ||
| VIScan | 17-05 | 31-05 | 20-06 | 04-07 |
| LAI2000 | 17-05 | 01-06 | ||
| MobilLas | 17-05 | 01-06 |
2.8 Experiment with fungicide deposition at different driving speed
In 2006 a supplementary experiment examining the influence of speed of the sprayer on deposition on leaves and soil was carried out. The experiment was carried out in winter wheat crop at Research Centre Flakkebjerg. The variety was Robigus and a two-factorial field experiment was conducted with the following factors:
Factor 1. Driving speed and application technique:
1. 4 km h-1, Low drift LD-02 nozzle at 240 L ha-1
2. 8 km h-1, Low drift LD-02 nozzle at 120 L ha-1
Factor 2. Nitrogen application rate:
1. 80 kg N ha-1
2. 160 kg N ha-1
Nitrogen was applied as a single application on 18 April at both N rates. A randomised block design with 4 replicates and a plot size of 2.5 ´ 10 m was used. At 4 km/h the spray liquid consisted of tap water with addition of the tracer Rhodamin B (100 g ha-1) and a non-ionic surfactant at a concentration of 0.1%. Another tracer, Brillantsulfoflavin (100 g ha-1) was used at 8 km/h and a non-ionic surfactant was again used at a concentration of 0.1%. The surfactant used was a linear alcohol polyethoxylate (Lissapol Bio, Syngenta). The purpose of the surfactant was to give the spray liquid properties that are comparable to a spray liquid with a fungicide. The experimental treatment was carried out at growth stage BBCH 53 when the winter wheat was 75 cm high. Deposition of spray liquid was measured on 1) 1st leaf (flag leaf), 2) 2nd leaf and 3) 3rd leaf and at soil level using the same methodology as described in section 2.6. The concentration of Rhodamin B was also measured on the Perkin Elmer luminescence spectrometer. Rhodamin B was chosen as this tracer was found to interact limited with brillantsulfoflavin and has its fluorescence peak at another wavelength. Measuring Rhodamin B concentration, the sample was excited at a wavelength of 553 nm and after excitation emission was measured at 578 nm.
2.9 Statistical analyses
The plot level data were analysed using the ANOVA, GLM and MIXED procedures in the SAS system (SAS, 1996). In the general linear model, the interaction term block ´ nitrogen was used in the denominator of the F-test corresponding to the factor nitrogen, while the residual error was used for F-tests for differences between fungicide treatments and the interaction term nitrogen ´ fungicide. In the mixed models, the whole plot term (block ´ nitrogen) and blocks were having random effects, and the other factors had fixed effects. Satterthwaites method was used for calculating denominator degrees of freedom in the mixed models.
Correlation and regression analyses on the sensor measurements were performed using the CORR and REG procedures of the SAS system (SAS, 1996).
Regression models were compared in terms of the multiple correlation coefficient (R²) and the root means squared error (RMSE) of the model residuals.
3 Results
- 3.1 Characterisation of the sites
- 3.2 Yields
- 3.3 Diseases
- 3.4 Sensor measurements of crop parameters
- 3.5 Fungicide deposition
- 3.6 Fungicide deposition at different driving speed
3.1 Characterisation of the sites
The soils at the experimental sites were characterised by measurements with RTK-GPS, EM38 and MobilTDR during spring of 2005 and 2006.
The elevation at the corners of each plot was measured using the RTK-GPS, and this was used to derive mean elevation, mean slope and mean aspect for each plot. The elevation at the Nissumgård site in 2005 varied 10 m across the field with a depression at the centre of the field (Fig. 1). At Schackenborg the field sloped towards northwest with a 6 m difference in elevation across the field (Fig. 2). There were little elevation differences for the Nissumgård site in 2006 (Fig. 3), whereas there was a small depression in the north-eastern part of the experimental area at Dybvad (Fig. 4).
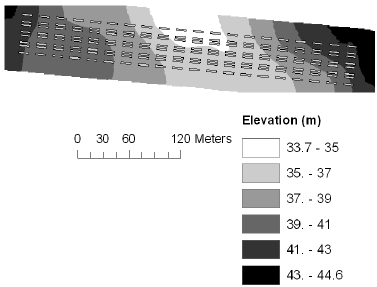
Figure 1. Mean elevation at Nissumgård in 2005.
The soil electrical conductance in two depth intervals (based on measurement with the EM38 sensor in vertical and horizontal position) were measured and interpolated to derive the mean conductance for each plot. The electrical conductance largely reflects the variation in soil texture with low conductance corresponding to sandy soil. There was a large variation in electrical conductance at the Nissumgård site in 2005 (Fig. 5), which only partly corresponds to the topography (compare Figs. 1 and 5). The electrical conductance at Schackenborg showed a large area with sandy soil in the centre of the field, and an area of clayey soil towards the north of the field (Fig. 6). The Nissumgård site in 2006 varied from sandy soil at the southern end to sandy loam at the north-western end (Fig. 7). A similar variation from west to east was seen at Dybvad (Fig. 7) with high electrical conductivity in the depression.
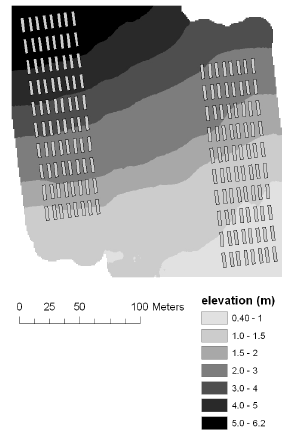
Figure 2. Mean elevation at Schackenborg in 2005.
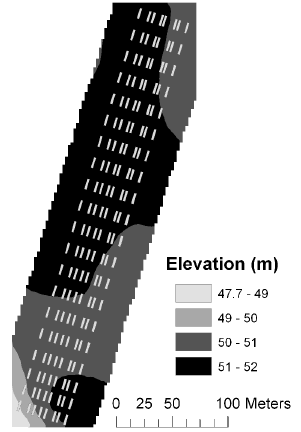
Figure 3. Mean elevation at Nissumgård in 2006.
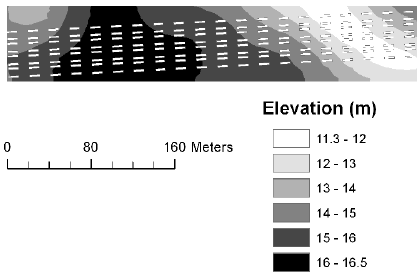
Figure 4. Mean elevation at Dybvad in 2006.
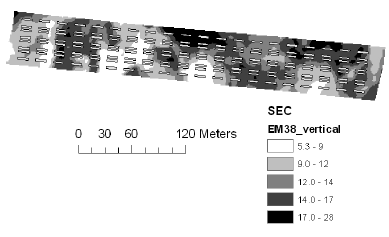
Figure 5. Soil electrical conductance measured with EM38 using vertical polarisation at Nissumgård in 2005.
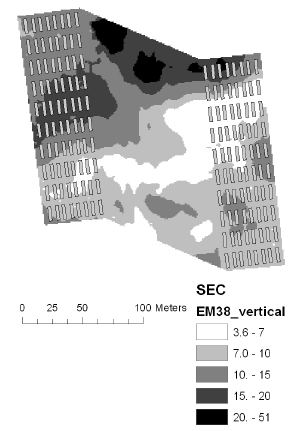
Figure 6. Soil electrical conductance measured with EM38 using vertical polarisation at Schackenborg in 2005.
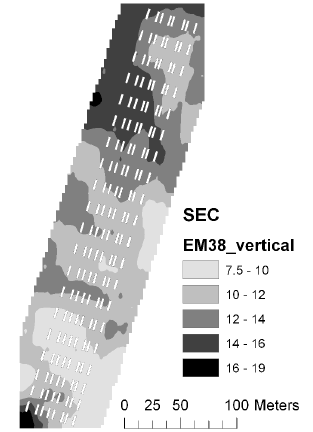
Figure 7. Soil electrical conductance measured with EM38 using vertical polarisation at Nissumgård in 2006.
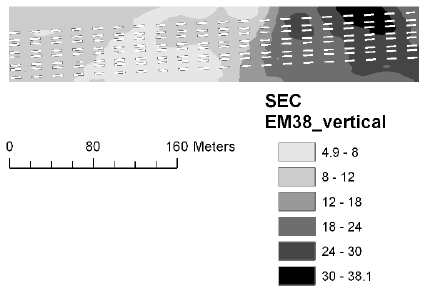
Figure 8. Soil electrical conductance measured with EM38 using vertical polarisation at Dybvad in 2006.
The soil water content was measured with TDR in early April 2005 and early May 2006 and reflects the soil water holding capacity to 50 cm depth. These measurements were interpolated for each plot. The soil water content correlated with the EM38 measurements, although there were separate relationships at the four sites and there was some disparity in the relationship at Schackenborg (Fig. 9ab). There was an even closer relationship between impedance measured using the TDR sensor and conductance measured using EM38 (Fig. 9cd).
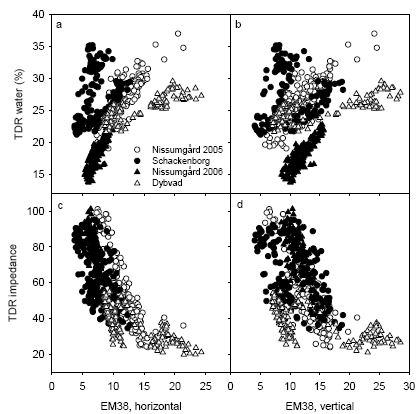
Figure 9. Relationship between TDR measurements of soil water capacity and impedance to 50 cm depth and EM38 measurements of conductance measured using either horizontal or vertical polarisation.
Soil samples were taken at 10 positions at each site for characterisation of soil texture. These positions were chosen based on the TDR and EM38 map to maximise the variation in soil conditions. A comparison of the soil texture measurements and the TDR and EM38 measurements is shown in Fig. 10, where soil clay and soil organic matter contents have been chosen as indicators of soil texture. There is close relationship between soil clay content and soil water content measured using TDR (Fig 10a). However, the measurements of soil water content at Dybvad appears to be lower than for the other sites, which may be related to the fact that these measurements were taken on 9 May and some evapotranspiration may have reduced soil water content at this point in time. There was also a close relationship between soil clay content and EM38 measurements at most sites (Fig. 10c). However, the scatter was much larger at Schackenborg due to the large variation soil organic matter content at this site (Fig. 10d).
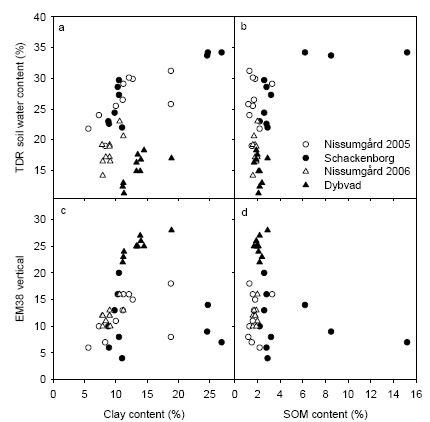
Figure 10. Soil water content at field capacity (TDR) and soil electrical conductivity (EM38) related to soil clay content and soil organic matter in the top soil.
All plots in 2005 were assessed for presence of weeds and for homogeneity of the plant cover on 12 May 2005 at Nissumgård and 11 May 2005 at Schackenborg. The most inhomogeneous plots at Nissumgård occurred in the low area with the highest soil electrical conductance (Fig. 11a). The weed cover was particularly large and rather uniform at Schackenborg (Fig. 11d), and the weed flora here was dominated by Poa annua. The weeds occurred patchier at Nissumgård and the weed flora was also more diverse here.
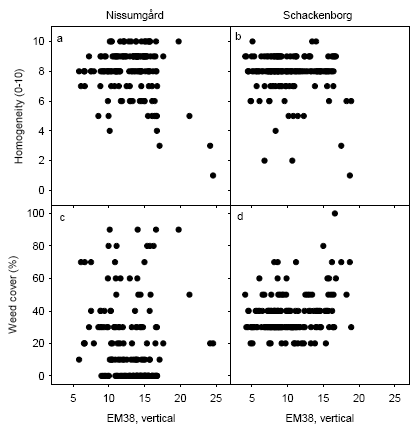
Figure 11. Homogeneity of the crop stand and weed cover of the plots in May 2005 depending on soil electrical conductance measured with EM38 using vertical polarisation at Nissumgård and Schackenborg.
The experiments in 2006 were also assessed for weeds and homogeneity. The site at Nissumgård had several plots with poor establishment and winter damage due to molehills. There were a few plots at Dybvad with a substantial population of grass weeds.
3.2 Yields
The average dry matter yields at Nissumgård and Schackenborg in 2005 were 5.71 and 5.78 t ha-1, respectively. The average dry matter yields at Nissumgård and Dybvad in 2006 were 6.19 and 7.29 t ha-1, respectively. An ANOVA model including the two treatments, their interactions and block showed considerable variation between experimental sites in the variation explained by the model and in the C.V. (Table 4). The C.V. was particularly high at Nissumgård in 2006. However, a comparison with other sensor measurements did not indicate that any of these should be discarded due to errors in yield determination. The large variation at Nissumgård in 2006 may therefore be attributed to variation in crop establishment.
Table 4. Results of ANOVA for grain dry matter yield, either uncorrected or adjusted for fungicide costs (net yield). The R² of the model, the significance levels of the experimental treatments and the coefficient of variation (C.V.) are shown. The net yield was calculated by subtracting fungicide costs (373 DKK L-1 Opus) and application costs (65 DKK ha-1 per application) from the gross yields by assuming a grain dry matter price of 1000 DKK t-1.
| Location | R² | P (N) | P (fung) | P(N´fung) | C.V. (%) | |||||
| Gross grain yield | ||||||||||
| Nissumgård, 2005 | 0.885 | <0.0001 | <0.0001 | 0.5531 | 8.5 | |||||
| Schackenborg | 0.889 | <0.0001 | 0.0007 | 0.6288 | 7.6 | |||||
| Nissumgård, 2006 | 0.513 | 0.0132 | 0.0245 | 0.4666 | 13.0 | |||||
| Dybvad | 0.837 | <0.0001 | 0.0001 | 0.0071 | 4.5 | |||||
| Net grain yield | ||||||||||
| Nissumgård, 2005 | 0.882 | <0.0001 | 0.1472 | 0.5531 | 9.0 | |||||
| Schackenborg | 0.888 | <0.0001 | 0.6029 | 0.6288 | 8.1 | |||||
| Nissumgård, 2006 | 0.497 | 0.0132 | 0.4572 | 0.4666 | 13.7 | |||||
| Dybvad | 0.812 | <0.0001 | <0.0001 | 0.0071 | 4.7 | |||||
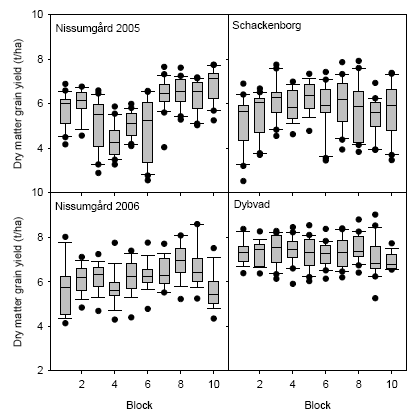
Figure 12. Box-plots showing means and variation in grain dry matter yield for the blocks at the four experimental sites. The box marks the 25th and 75th percentiles, and the whiskers indicate the 10th and 90th percentiles. The line within the box shows the median. The dots mark outlying observations.
The variation in yields across the experimental fields is illustrated in Fig. 12, which shows box-plots of dry matter grain yield for each block at the four experimental sites. There were large variations between blocks at Nissumgård and Schackenborg, whereas there was relatively little variation at Dybvad. At Schackenborg blocks 1 through 5 were located in one rectangular area of the field, and blocks 6 through 10 in an adjacent area. At the other sites the blocks were located in a row along the field.
The grain yields increased with increasing N application from the low N rate to the normal N rate (160 kg N ha-1). However, there was only at Schackenborg a further yield increase from increasing the N rate to 240 kg N ha-1 (Table 5). There was little yield difference between a single and a split N application.
Table 5. Mean grain yield responses (t DM ha'1) to N treatments at the four experimental sites. The LSD for 95% confidence is shown.
| N-strategy | Nissumgård 2005 |
Schackenborg 2005 |
Nissumgård 2006 |
Dybvad 2006 |
| 2. 50+30 N | 4.66 | 4.36 | 5.94 | 6.59 |
| 1. 50+110 N | 6.14 | 5.96 | 6.28 | 7.47 |
| 3. 50+190 N | 6.03 | 6.85 | 5.99 | 7.48 |
| 4. 160 N | 6.03 | 5.99 | 6.50 | 7.68 |
| LSD95 | 0.22 | 0.20 | 0.38 | 0.15 |
Table 6. Mean grain yield responses (t DM ha-1) to fungicide treatments at the four experimental sites. The LSD for 95% confidence is shown.
| Fungicide | Nissumgård 2005 |
Schackenborg 2005 |
Nissumgård 2006 |
Dybvad 2006 |
| 1. None | 5.47 | 5.57 | 5.90 | 6.78 |
| 2. 0.2 L ha-1 | 5.61 | 5.77 | 6.04 | 7.27 |
| 3. 0.4 L ha-1 | 5.78 | 5.83 | 6.30 | 7.44 |
| 4. 0.8 L ha-1 | 6.00 | 5.99 | 6.46 | 7.72 |
| LSD95 | 0.22 | 0.20 | 0.38 | 0.15 |
Table 7. Net yield responses (t DM ha-1) to fungicide treatments at the four experimental sites. Responses are presented as least squares means with standard errors in parentheses. The net yield was calculated by subtracting fungicide costs (373 DKK L-1 Opus) and application costs (65 DKK ha-1 per application) from the gross yields by assuming a grain dry matter price of 1000 DKK t-1. The LSD for 95% confidence is shown.
| Fungicide | Nissumgård 2005 |
Schackenborg 2005 |
Nissumgård 2006 |
Dybvad 2006 |
| 1. None | 5.41 | 5.51 | 5.76 | 6.71 |
| 2. 0.2 L ha-1 | 5.47 | 5.61 | 5.89 | 7.12 |
| 3. 0.4 L ha-1 | 5.57 | 5.61 | 6.08 | 7.23 |
| 4. 0.8 L ha-1 | 5.63 | 5.63 | 6.13 | 7.36 |
| LSD95 | 0.22 | 0.20 | 0.38 | 0.15 |
The yield responses to fungicide treatments are summarized in Table 6. The yield gain from the highest dose was about 1.0 t ha-1 at Dybvad, but only about 0.5 t ha-1 at the other locations. When net yields (obtained by subtracting fungicide and application costs from the gross yields) were analysed, the fungicide responses was low or negative for the experiments in 2005, whereas there was still a considerable yield gain at Dybvad (Table 7).
The analyses of variance showed that the interaction between N strategy and fungicide was significant at Dybvad only (Table 4). This interaction was seen as a considerably higher yield increase with increasing N rate in the fungicide treated plots compared with no fungicide application (Fig. 13). A similar, but non-significant, tendency was seen for the other experimental sites.
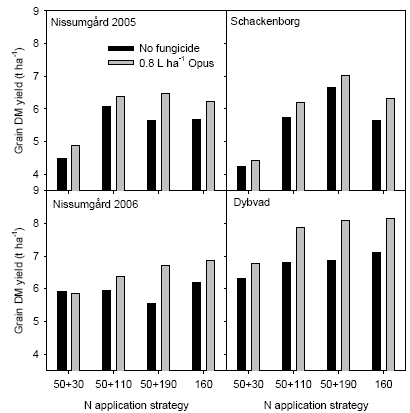
Figure 13. Interaction between N strategy and fungicide rate on dry matter grain yield for the four experimental sites.
3.3 Diseases
Generally the level of Septoria tritici in 2005 was low to moderate compared to other years, and the attack level in 2006 was moderate. The attack level still made it possible to differentiate between treatments. However, the impacts on yields were considerable lower compared with other years.
At the two first assessment dates in 2005 only minor differences were seen in the disease severity. However, differences between N strategies were observed. Also later lower disease levels were seen following the low N strategy at both localities (Fig. 14). Measured on 2nd leaf at GS 65 the N strategy with the single application of 160 kg N ha-1 increased the level of disease most. Increasing the N rate from 160 to 240 kg N ha-1 gave a clear increase in disease level at Schackenborg.
At the 2 first assessments in 2006 quite clear differences were seen in the disease severity between different N strategies. Generally lower levels of diseases were seen following the low N rate at both locations (Fig 14). Measured on 2nd leaf the highest N rate increased the level of disease most. At the later assessment only the low N strategy was clearly found to be different from the 3 other strategies.
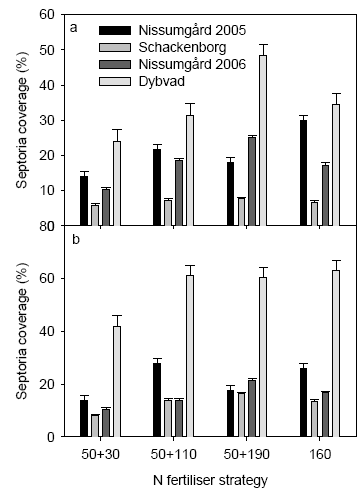
Figure 14. Mean septoria coverage in untreated plots from the different N strategies assessed on 2nd leaf at GS 65 (a) and on flag leaf at GS 75 (b) for the four experimental sites. The whiskers above the bars show the standard error.
At Nissumgård in 2005 low levels of powdery mildew (Blumeria graminis) attack was also assessed. The level of powdery mildew was, however, insignificant and in the range of 0.0 to 0.5%. Still the assessments indicated that the low N rate gave the lowest stimulation of mildew attack as the level of attack stayed at 0% in all treatments.
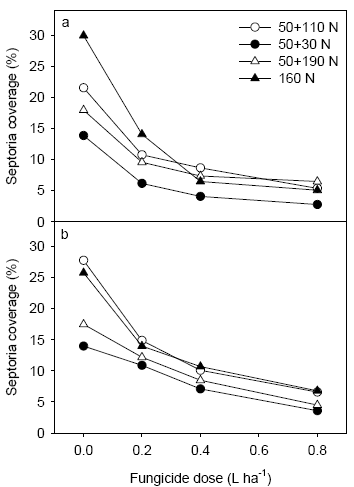
Figure 15. Level of septoria following application of different fungicide dosages at Nissumgård in 2005 assessed on 2nd leaf at GS 65 (a) and on flag leaf at GS 75 (b).
Fungicide treatments reduced the disease level significantly and a clear dose response could be measured at both 2nd leaf and flag leaf. At Nissumgård in 2005 septoria was controlled between 80 and 50% going from 80% to 20% of the full rate (1 L ha-1) (Fig. 15). At Schackenborg control levels varied from 60% to 20% at the same interval of fungicide dosages (Fig. 16).
At Schackenborg low levels of tan spot (Drechslera tritici repentis) attack were also assessed. However, the level of tan spot was insignificant in the range of 0.1 to 0.4% and showed no effect of N strategy. Physiological spotting was quite severe on lower leaves at GS 39 and 65. There was a clear tendency for more severe attack of physiological spots in the N strategy with the low N rate.
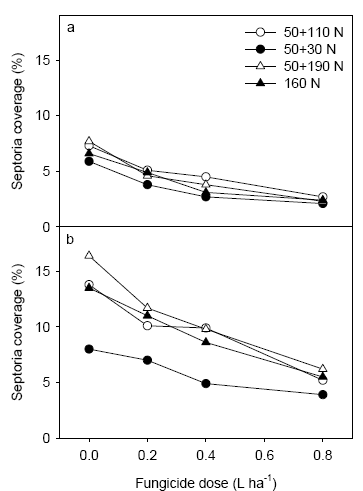
Figure 16. Level of septoria following application of 3 fungicide dosages at Schackenborg assessed on 2nd leaf at GS 65 (a) and on flag leaf at GS 75 (b).
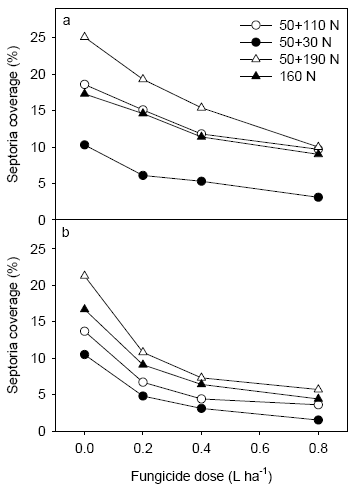
Figure 17. Level of septoria following application of 3 fungicide dosages at Nissumgård in 2006 assessed on 2nd leaf at GS 65 (a) and on flag leaf at GS 75 (b).
Fungicide treatments reduced the disease level significantly and a clear dose response could be measured at both 2nd leaf and flag leaf at both locations in 2006 (Figs. 17 and 18). At Dybvad septoria was controlled between 97 and 56% going from 80% to 20% of the full rate. In the low N rate strategy a higher level of control was generally found for 20 and 40% of the full dose. At Nissumgård control levels varied from 73% to 46% at the same interval of fungicide dosages. Also here a tendency to high levels of control was seen for the low N strategy, although the differences were smaller.
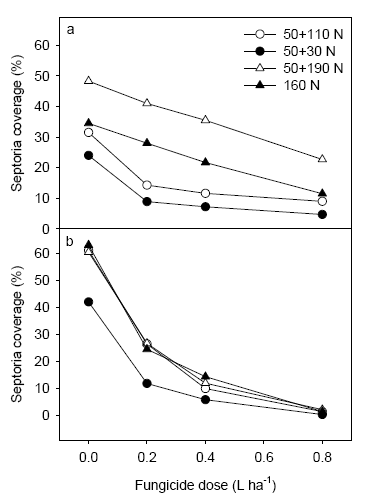
Figure 18. Level of septoria following application of 3 fungicide dosages at Dybvad assessed on 2nd leaf at GS 65 (a) and on flag leaf at GS 75 (b) at both locations.
3.4 Sensor measurements of crop parameters
Canopy measurements of spectral reflectance using VIScan were taken four times during the season at all sites. Measurements of leaf area (LAI-2000), leaf N concentration and of crop structure using the MobilLas were only taken on the two first measurement dates at GS32 and GS39. Samples of above-ground biomass for measurements of leaf area and crop N were only taken in selected plots at GS39.
3.4.1 Manual measurements
The measurements of leaf area index (LAI) using the LAI2000 sensor is compared with the destructive plant samples in Fig. 19. The LAI2000 underestimated total LAI from the destructive samples, which included both leaves and stems (Fig. 19a). There was a better agreement when LAI2000 measurements were compared with destructive samples of LAI for leaves only (Fig. 19b). There was in both cases a tendency for a larger underestimation at Schackenborg and Dybvad compared with Nissumgård in both years. The underestimation of LAI using the LAI2000 sensor at Schackenborg may partly be due to the fact that the measurements were taken at a height of 10-20 cm above the ground to avoid weeds interfering with the measurements.
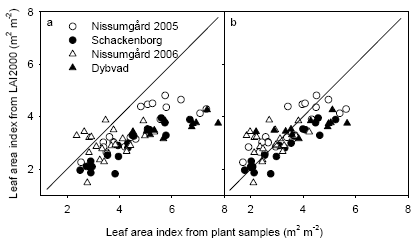
Figure 19. Comparison of leaf area index at GS39 measured using LAI2000 and with destructive plant samples including all plant components (a) or leaves only (b). The 1:1 line is shown.
There was a good relationship between SPAD readings and the leaf N concentrations in the destructive plant samples (Fig. 20). However, slightly lower SPAD readings were obtained at Schackenborg compared with other sites at similar N concentrations. This may be related to the fact that a different variety (Grommit) was used at Schackenborg compared with the other sites (Deben).
There was in general a good relationship between LAI and RVI with similar ratios of RVI to LAI at all sites for given dates (Fig. 21). The exception was the measurements taken at GS39, where there was considerably higher RVI at Schackenborg compared with the other sites at similar levels of LAI.
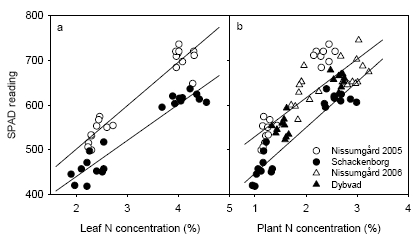
Figure 20. Relation between SPAD reading and leaf N concentration (a) and above-ground plant N concentration (b) in the destructive plant samples at GS39. Regression lines are shown separately for Schackenborg and the other three sites jointly.
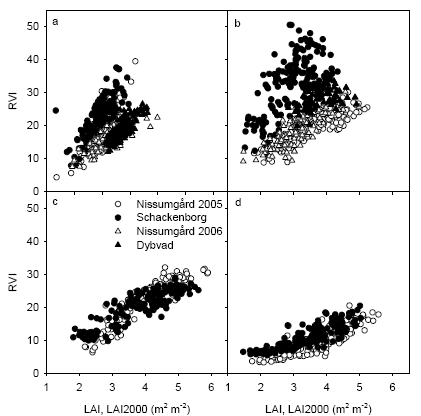
Figure 21. Relation between LAI measurements with LAI2000 and ratio vegetation index (RVI) measured with VIScan on four different dates: GS32 (a), GS39 (b), GS65 (c) and GS75 at Nissumgård and Schackenborg in 2005.
3.4.2 MobilLas measurements
The MobilLas software first makes 50 % of the laser range measurements, then the spectral measurements, and finally the remaining laser measurements. Spectral measurements are averaged to a single value. The accumulated height-of-hit distribution is calculated from the laser range measurements and stored. The GPS receiver is transmitting positions at a rate of 1 Hz. The most recently received position is stored together with sensor measurements. At a driving speed of 2 km h-1 a complete dataset is generated for every approximately 1 m travelled.
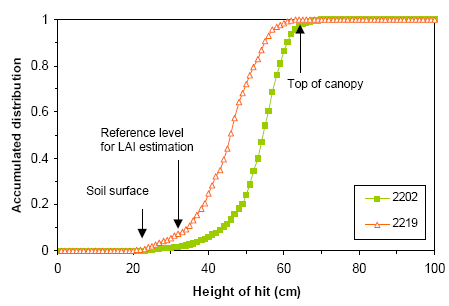
Figure 22. Two examples of accumulated height-of-hit distributions measured by the laser range finder at Dybvad on 17 May 2006. The accumulated distribution or canopy gap fraction is shown as a function of the relative height inside the canopy. Both distributions calculated as the mean of 8 individual distributions measured inside the plots. The approximate locations of soil surface, reference level for total LAI estimation and top of canopy are indicated. Calculated total LAI values for plots 2202 and 2219 were 3.0 and 2.6, respectively. The estimated crop heights for plots 2202 and 2219 were 42 and 39 cm, respectively.
Figure 22 shows two examples of a laser measured accumulated ‘height-of-hit’ distributions. The distributions are calculated as the mean of 8 individual distributions measured within each plot. It was found that LAI estimates based on a mean distribution for each plot were more robust than estimates based on LAI values calculated from the individual distributions.
The height-of-hit distributions are calculated from 1000 range measurements made at a 53-degree zenith angle. Based on distance to hit and look angle the height of the hit above an arbitrary reference plane is calculated. Based on the accumulated distribution the crop height is calculated as the difference between the 99.5% (top of canopy) and 0.5% (soil surface) percentiles. The laser 53-degree zenith angle was selected in order to calculate LAI using
LAI = -1.2 •• ln(GF) (1)
where GF is the canopy gap fraction (Jones, 1992). Canopy gap fraction at any height within the canopy can be obtained from the height-of-hit distribution. Thus vertical profiles of LAI or single values can be calculated from the same data. Usually the leaf area of the entire canopy is desired and the gap fraction is read directly from the accumulated distribution slightly above the apparent soil surface. For the distributions shown in Figure 22, gap fraction values of 0.032 (plot 2202) and 0.072 (plot 2219) were obtained at a relative height of 10 cm.
Gap fractions are measured at a 53-degree zenith angle because at this angle gap fraction is largely insensitive to leaf angle distribution (Jones, 1992) and only at this angle is there a simple relationship between gap fraction and leaf area.
Because of the non-infinite diameter (0.5 mm) of the laser beam, canopy gap fractions are systematically underestimated especially for dense canopies (Wilson, 1963). In order to correct for this effect, the gap fraction values were corrected before LAI was calculated using
GF = GFl + CF •• (1 – GFl ) (2)
where GFl is the gap fraction estimated from laser measurements as discussed above, and CF is a correction factor depending on the gap size distribution of the crop. For the winter wheat crop a CF value of 0.05 was empirically found to yield estimates of LAI comparable to manual measurements. After correction, the initial gap fraction values of 0.032 and 0.072 were transformed to 0.080 and 0.118 respectively, and the LAI values shown in Figure 23 were calculated using eq. (1).
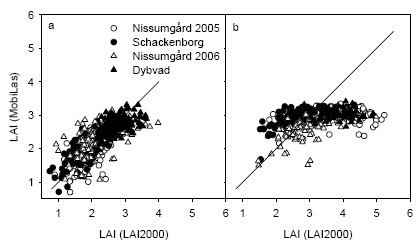
Figure 23. Comparison of leaf area index (LAI) estimated from the MobilLas instrument compared with the LAI-2000 instrument at GS32 (a) or GS39 (b). The 1:1 line is shown.
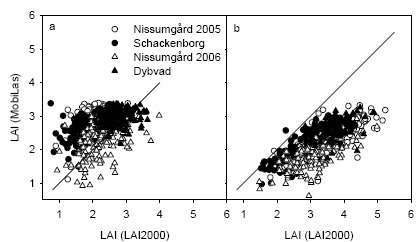
Figure 24. Comparison of leaf area index (LAI) estimated from the MobilLas instrument to 30 cm depth in the canopy compared with the LAI-2000 instrument at GS32 (a) or GS39 (b). The 1:1 line is shown.
The LAI estimated using MobilLas correlated well with LAI2000 measurements for measurements taken at GS32 (Fig. 23). However the MobilLas could not estimate LAI above about 3.5, which becomes a limitation for the measurements taken at GS39. Instead the MobilLas measurements of LAI at GS39 to a depth of 30 cm in the canopy correlated better with LAI measurements taken with the LAI2000 instruments (Fig. 24). Similar results were obtained when the MobilLas estimates were compared with destructive LAI samples (Fig. 25). The MobilLas measurements of LAI to 30 cm depth at GS39 was could be corrected to the level of LAI of the destructive samples by multiplying by 1.55 and this relation explained 49% of the variation in the destructive LAI measurements.
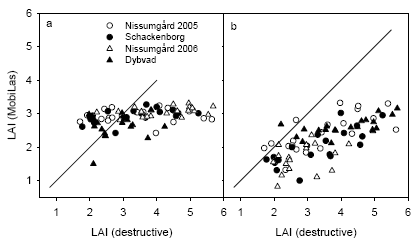
Figure 25. Comparison of leaf area index at GS39 measured using MobilLas to either ground level (a) or to 30 cm depth in the canopy (b) compared with destructive plant samples of leaf area only. The 1:1 line is shown.
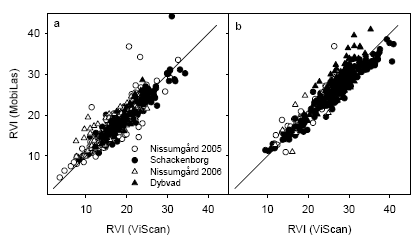
Figure 26. Comparison of ratio vegetation index (RVI) estimated from the MobilLas instrument compared with the ViScan instrument at GS32 (a) or GS39 (b). The 1:1 line is shown.
The spectral reflectance measurements using the MobilLas instrument was intercalibrated with the ViScan instrument in each year, and good relationships were therefore obtained between the two sets of observations of RVI (Fig. 26).
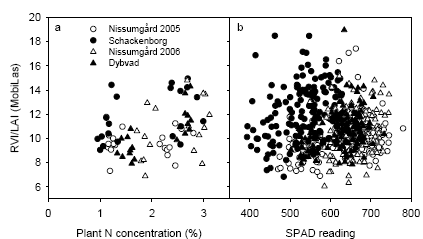
Figure 27. The relationship between RVI/LAI from the MobilLas sensor against total plant N concentration (a) or readings from the Yara SPAD sensor (b). The measurements were taken at GS39 and the LAI was taken at the LAI estimated to a depth of 30 cm.
The RVI spectral index is closely related to the amount of chlorophyll present in the canopy on an area basis. Hence by calculating the RVI/LAI ratio a new index related to the amount of chlorophyll per leaf area unit - or concentration - is obtained. This variable should ideally be correlated to plant N concentration or to the Yara SPAD readings, which are a measure of chlorophyll content. However, there was only a weak correlation between the RVI/LAI indicator and the plant N concentration or SPAD readings (Fig. 27). The correlation coefficients between RVI/LAI and SPAD were 0.24 (P=0.0015), 0.20 (P=0.0126), 0.26 (P=0.0017) and 0.35 (P=0.0001) for Nissumgård 2005, Schackenborg, Nissumgård 2006 and Dybvad, respectively. The weak correlation may partly be due to the problems in accurately estimating LAI at GS39 with the MobilLas sensor and for Schackenborg possibly due to interference of weeds with the RVI measurements.
3.5 Fungicide deposition
3.5.1 Leaf size
The examination of leaf deposition of fungicides in the two experiments involved the measurement of leaf area and leaf weight of the three leaf sections. The leaf area measurements in Table 8 show responses to the nitrogen strategies used in the four experiments. In 2005 differences were significant concerning the flag leaf and the 2nd leaf at Nissumgård, whereas significant differences were seen for all three leaf sections at Schackenborg. It is not surprising that the largest differences found were between the treatments with the lowest and the highest nitrogen supply. Fungicide was applied at an earlier growth stage in 2006 and as a consequence the flag leaf was generally less developed and had a smaller area than the preceding year. Significant differences in leaf area were found at both Nissumgård and at Dybvad and for all three leaf sections in 2006. However, leaf area in plots with the single N application was the largest at both places in 2006. Leaf area in the low N treatment had the smallest leaf area at both locations in 2006.
Table 8. Leaf area (cm²/leaf) of three leaf levels at the four experimental sites.
| Fertiliser treatment | Flag leaf | 2nd leaf | 3rd leaf |
| Nissumgård 2005 | |||
| 1. 160 N split | 28.07 | 29.41 | 19.08 |
| 2. 80 N split | 20.95 | 24.59 | 18.24 |
| 3. 240 N split | 30.81 | 30.78 | 19.81 |
| 4. 160 N once | 26.76 | 29.34 | 20.17 |
| LSD95 | 2.66 | 2.17 | 1.77 |
| Schackenborg 2005 | |||
| 1. 160 N split | 24.27 | 24.56 | 15.79 |
| 2. 80 N split | 18.93 | 21.95 | 15.16 |
| 3. 240 N split | 19.15 | 26.19 | 17.46 |
| 4. 160 N once | 24.03 | 24.96 | 19.58 |
| LSD95 | 1.58 | 2.43 | 1.59 |
| Nissumgård 2006 | |||
| 1. 160 N split | 15.80 | 19.14 | 19.34 |
| 2. 80 N split | 13.92 | 17.89 | 18.36 |
| 3. 240 N split | 16.09 | 18.71 | 18.58 |
| 4. 160 N once | 18.02 | 23.00 | 21.32 |
| LSD95 | 1.75 | 1.86 | 1.53 |
| Dybvad 2006 | |||
| 1. 160 N split | 16.62 | 22.29 | 18.01 |
| 2. 80 N split | 15.42 | 22.06 | 18.20 |
| 3. 240 N split | 16.87 | 22.62 | 18.12 |
| 4. 160 N once | 18.70 | 26.83 | 20.34 |
| LSD95 | 1.56 | 1.27 | 1.15 |
The dry weight of the leaf sections (Table 9) shows a corresponding influence of N supply, with the primary differences for the flag leaf and at the 2nd leaf. However apart from the influence of total N supply, an effect of N timing is seen especially in the 2005 experiments. In both years the two treatments with 160 kg N ha-1 showed a greater dry weight of the 3rd leaf, when N was supplied once. On the other hand it seems that the N supply in these plants was limiting the development of the flag leaf as this leaf section in 2005 was significantly lighter than the flag leaf in the treatment, where 160 kg N ha-1 was applied in a split strategy. In 2006 there were the same difference between the two treatments as in 2005 looking at the 3rd leaf. However, leaf weight of the flag section was not reduced in 2006 in the treatment with a single N application. The difference could be due to the earlier fungicide treatment and leaf area measurement in 2006.
Table 9. Leaf weight (g dry matter/leaf) of three leaf levels at the four experimental sites.
| Fertiliser treatment | Flag leaf | 2nd leaf | 3rd leaf |
| Nissumgård 2005 | |||
| 1. 160 N split | 0.141 | 0.136 | 0.0742 |
| 2. 80 N split | 0.108 | 0.125 | 0.0779 |
| 3. 240 N split | 0.155 | 0.142 | 0.0778 |
| 4. 160 N once | 0.124 | 0.132 | 0.0809 |
| LSD95 | 0.013 | 0.004 | 0.0072 |
| Schackenborg2005 | |||
| 1. 160 N split | 0.137 | 0.143 | 0.0841 |
| 2. 80 N split | 0.115 | 0.145 | 0.0911 |
| 3. 240 N split | 0.163 | 0.156 | 0.0852 |
| 4. 160 N once | 0.132 | 0.148 | 0.0957 |
| LSD95 | 0.013 | 0.011 | 0.009 |
| Nissumgård 2006 | |||
| 1. 160 N split | 0.0877 | 0.103 | 0.0912 |
| 2. 80 N split | 0.0760 | 0.091 | 0.0830 |
| 3. 240 N split | 0.0898 | 0.099 | 0.0879 |
| 4. 160 N once | 0.0961 | 0.116 | 0.0963 |
| LSD95 | 0.0108 | 0.0104 | 0.0094 |
| Dybvad 2006 | |||
| 1. 160 N split | 0.0851 | 0.114 | 0.0852 |
| 2. 80 N split | 0.0777 | 0.111 | 0.0837 |
| 3. 240 N split | 0.0891 | 0.120 | 0.0872 |
| 4. 160 N once | 0.0938 | 0.133 | 0.0901 |
| LSD95 | 0.0072 | 0.007 | (0.0060) |
The specific leaf weight results are shown in Table 10. Nitrogen strategies had significant influence on the specific leaf weight on some of the leaf sections at the two experimental sites in 2005. In 2006 on the other hand, no significant differences in specific leaf weight were seen at any combination of leaf section and location.
Table 10. Specific leaf weight (g dry matter/cm² leaf) of three leaf sections at the four experimental sites.
| Fertiliser treatment | Flag leaf | 2nd leaf | 3rd leaf |
| Nissumgård 2005 | |||
| 1. 160 N split | 0.00503 | 0.00462 | 0.00388 |
| 2. 80 N split | 0.00517 | 0.00514 | 0.00429 |
| 3. 240 N split | 0.00505 | 0.00462 | 0.00392 |
| 4. 160 N once | 0.00465 | 0.00452 | 0.00414 |
| LSD95 | 0.00036 | 0.00013 | 0.00049 |
| Schackenborg | |||
| 1. 160 N split | 0.00564 | 0.00582 | 0.00538 |
| 2. 80 N split | 0.00607 | 0.00662 | 0.00605 |
| 3. 240 N split | 0.00560 | 0.00613 | 0.00489 |
| 4. 160 N once | 0.00551 | 0.00609 | 0.00491 |
| LSD95 | 0.00036 | 0.00097 | 0.00049 |
| Nissumgård 2006 | |||
| 1. 160 N split | 0.00555 | 0.00538 | 0.00472 |
| 2. 80 N split | 0.00547 | 0.00511 | 0.00451 |
| 3. 240 N split | 0.00559 | 0.00527 | 0.00473 |
| 4. 160 N once | 0.00534 | 0.00508 | 0.00453 |
| LSD95 | (0.00028) | (0.00031) | (0.00037) |
| Dybvad | |||
| 1. 160 N split | 0.00513 | 0.00510 | 0.00473 |
| 2. 80 N split | 0.00505 | 0.00503 | 0.00459 |
| 3. 240 N split | 0.00523 | 0.00529 | 0.00482 |
| 4. 160 N once | 0.00504 | 0.00496 | 0.00449 |
| LSD95 | (0.00027) | 0.00016 | 0.00025 |
3.5.2 Leaf deposition
The deposition of tracer on the three leaf sections is shown as deposition per leaf area unit (Table 11). The tracer was applied at an area dose corresponding to 1 mg per cm² horizontal surface. Leaf orientation, however, is not horizontal, but the figure gives an indication that the values in the table are within the expected interval.
There was a large difference in the overall deposition level between the two experimental sites in 2005, and this was primarily caused by differences in deposition values on the flag leaf. The flag leaf is the first “filtering unit” of the spray cloud. The differences between the treatments in deposition per area unit leaf on the flag leaf are supposed to be caused mainly by differences in leaf orientation. In 2005 the two treatments, 80 kg N ha-1 and 160 kg N ha-1 in one application had more erect leaves compared to the two other treatments with split application of 160 and 240 kg N ha-1 and the deposition per area leaf unit on the flag leaf is correspondingly lower on these more erect leaves.
Deposition values on the 2nd leaf and especially on the 3rd leaf at Nissumgård in 2005 show a greater penetration and deposition in the more open crops with a reduced deposition on the flag leaf. The deposition values at Schackenborg were at a much lower level. This is probably caused by the fact that the crop generally was more open and erect. Differences between treatments were smaller at Schackenborg. However, the same influence of crop density on penetration is seen, because deposition on the 3rd leaf is much higher in the open crop fertilized with 80 kg N ha-1 compared to the 240 kg N ha-1 treatment with a much denser crop structure.
Deposition values in 2006 were much more even within the three leaf sections for the four nitrogen treatments at both Nissumgård and Dybvad and no significant differences between N treatments were found. Compared to 2005, differences in leaf orientation between the four nitrogen treatments were much smaller at the time of fungicide/tracer in 2006 at both locations. This is supposed to be the primary reason for the small differences in deposition per leaf area unit between nitrogen treatments in 2006 compared to the preceding year. In all experiments, deposition per unit area decreased from the flag leaf to the 3rd leaf. The decrease seems to be related to crop density.
Table 11. Deposition of tracer (mg BSF/cm² leaf) on the three leaf sections at the four experimental sites.
| Fertiliser treatment | Flag leaf | 2nd leaf | 3rd leaf |
| Nissumgård 2005 | |||
| 1. 160 N split | 0.306 | 0.155 | 0.091 |
| 2. 80 N split | 0.225 | 0.177 | 0.124 |
| 3. 240 N split | 0.288 | 0.154 | 0.077 |
| 4. 160 N once | 0.222 | 0.160 | 0.105 |
| LSD95 | 0.038 | 0.027 | 0.024 |
| Schackenborg | |||
| 1. 160 N split | 0.143 | 0.132 | 0.084 |
| 2. 80 N split | 0.126 | 0.151 | 0.112 |
| 3. 240 N split | 0.165 | 0.157 | 0.070 |
| 4. 160 N once | 0.136 | 0.154 | 0.093 |
| LSD95 | 0.032 | 0.038 | 0.019 |
| Nissumgård 2006 | |||
| 1. 160 N split | 0.238 | 0.245 | 0.157 |
| 2. 80 N split | 0.218 | 0.227 | 0.151 |
| 3. 240 N split | 0.272 | 0.242 | 0.157 |
| 4. 160 N once | 0.237 | 0.212 | 0.135 |
| LSD95 | (0.043) | (0.031) | (0.021) |
| Dybvad | |||
| 1. 160 N split | 0.195 | 0.220 | 0.100 |
| 2. 80 N split | 0.197 | 0.225 | 0.113 |
| 3. 240 N split | 0.209 | 0.195 | 0.102 |
| 4. 160 N once | 0.207 | 0.212 | 0.094 |
| LSD95 | (0.036) | (0.032) | (0.021) |
Table 12 show the deposition of tracer per weight unit of the leaf sections. These results correspond with the values in Table 11.
Table 12. Deposition of tracer (mg BSF/g DM) on the three leaf sections at the four experimental sites.
| Fertiliser treatment | Flag leaf | 2nd leaf | 3rd leaf |
| Nissumgård 2005 | |||
| 1. 160 N split | 60.95 | 33.61 | 23.33 |
| 2. 80 N split | 43.97 | 34.94 | 28.83 |
| 3. 240 N split | 57.04 | 33.05 | 19.16 |
| 4. 160 N once | 47.23 | 35.28 | 25.53 |
| LSD95 | 7.34 | (5.26) | 4.73 |
| Schackenborg | |||
| 1. 160 N split | 25.54 | 22.78 | 15.55 |
| 2. 80 N split | 20.79 | 22.95 | 18.51 |
| 3. 240 N split | 29.49 | 25.34 | 14.20 |
| 4. 160 N once | 24.67 | 25.31 | 18.84 |
| LSD95 | 5.72 | (4.02) | (3.14) |
| Nissumgård 2006 | |||
| 1. 160 N split | 42.96 | 45.69 | 33.42 |
| 2. 80 N split | 40.01 | 44.64 | 33.97 |
| 3. 240 N split | 48.81 | 46.12 | 33.48 |
| 4. 160 N once | 44.60 | 41.76 | 29.50 |
| LSD95 | (8.02) | (6.46) | (4.38) |
| Dybvad | |||
| 1. 160 N split | 38.11 | 43.22 | 21.23 |
| 2. 80 N split | 38.92 | 44.83 | 24.69 |
| 3. 240 N split | 39.64 | 36.06 | 20.57 |
| 4. 160 N once | 41.29 | 42.96 | 20.81 |
| LSD95 | (6.64) | (6.40) | (4.65) |
3.5.3 Soil deposition
Deposition of tracer on the soil surface is shown in Table 13 The values at Schackenborg was influenced by a large population of Poa annua that is supposed to be the primary reason to the difference in absolute deposition level between the two experimental sites in 2005. Although the level is different, the actual order of treatments is the same. Generally the highest soil deposition in 2005 was found in the 80 kg N ha-1 whereas there were small and non-significant differences between the other treatments. In 2006 soil deposit values were again highest at the 80 kg N ha-1 treatment at both locations. However, soil deposition values was lower at both locations, where the normal N rate was applied once compared to the other 3 nitrogen treatments.
Table 13. Deposition of tracer (% of applied) on objects at soil level at the two experimental sites.
| Treatment | Nissumgård 2005 |
Schackenborg | Nissumgård 2006 |
Dybvad |
| 1. 160 N split | 19.0 | 12.7 | 24.7 | 19.3 |
| 2. 80 N split | 33.6 | 20.9 | 28.2 | 22.2 |
| 3. 240 N split | 16.3 | 11.2 | 25.6 | 18.4 |
| 4. 160 N once | 19.2 | 11.9 | 20.6 | 14.1 |
| LSD95 | 5.5 | 3.0 | 3.8 | 4.3 |
3.6 Fungicide deposition at different driving speed
The experiment with application of tracer at different driving speeds showed no significant influence of nitrogen level on deposition of spray liquid on any of the three leaf sections. The primary purpose of the experiment was to investigate whether the application speed influenced the profile of deposition in the crop canopy. The deposition values are given as relative values as there were some problems with absolute values due to different recovery of the two tracers from the plant material. Deposition values on the flag leaf were set equal to 100 and the deposition per area unit on the second and on the third leaf was calculated relative to this value.
Table 14. Relative deposition of spray liquid on three leaf sections at two sprayer speeds, using LD-02 low drift nozzle.
| Speed of application and water volume | ||
| 4 km h-1, 240 l ha-1 | 8 km h-1 120 l ha-1 | |
| Flag leaf | 100 | 100 |
| 2nd leaf | 94 | 58 |
| 3rd leaf | 75 | 39 |
| LSD0.95 | 15 | 11 |
The results in Table 14 show a decreasing deposition of spray liquid per area unit as the spray penetrates the canopy. At 4 km h-1 deposition per area unit on the 2nd leaf was slightly reduced and there was a significantly reduced deposition on the 3rd leaf. However at 4 km h-1 deposition per area unit on the 3rd leaf was still approximately 75% of the value on the flag leaf. At 8 km h-1 deposition per area unit decreased much faster, and there was a significant difference between deposition values on all three leaf sections.
The measurements of deposition of spray liquid on the soil surface in the experiment are shown in Table 15. The results show the deposition values per area unit at the soil surface as a percentage of the applied area dose.
Table 15. Deposition of spray liquid at soil level in winter wheat at GS53 BBCH at two different sprayer speeds and at two nitrogen levels.
| Speed of application and water volume | ||
| Nitrogen level | 4 km h-1, 240 l ha-1 | 8 km h-1 120 l ha-1 |
| 80 kg N ha-1 | 18.0 | 22.7 |
| 160 kg N ha-1 | 13.5 | 15.6 |
| LSD0.95 | (5.1) | 6.5 |
There was no significant influence of speed on soil deposition values. This is in accordance with earlier results by Jensen and Spliid (2005) comparing soil deposition values in winter wheat at the same sprayer speeds as in this investigation. At 4 km h-1 there is a tendency towards a lower soil deposition value at the high N rate, and at 8 km h-1 there is a significant difference.
4 Model development
- 4.1 Regression model
- 4.2 Causal model
- 4.3 Yield and economic benefits of applying the models
- 4.4 Spatial variation in soil and plant properties
- 4.5 Quality of sensor measurements
- 4.6 Fungicide treatment effects
- 4.7 Models for estimating yield response to fungicide
- 4.8 Evaluation of estimated spatial variation in fungicide rate
Two types of models were tested in the project; a regression model and a causal model. The regression model attempts to use the experimental results directly for establishing a link between yield increase from fungicide control and the sensor readings. The causal model establishes the underlying causal relations between the fungicide application and their effect on disease and thus on wheat grain yield, and how these processes relate to sensor measurements.
4.1 Regression model
The split-plot design allowed the response of yield and disease to fungicide application to be determined within each of the 40 sub-blocks in each experiment. The crop density within each subplot varied considerably less than between sub-blocks, since the N strategy was identical for all plots within a su-block and since most of the soil variation was partitioned between blocks and sub-blocks. This then allowed the responses to fungicide dose to be compared with crop characteristics measured at GS39. For spatially varied fungicide applications to be of any value, there must be a relationship between either the yield or fungicide response to fungicide dose and some of the measurable crop characteristics at time of fungicide spraying.
Table 16. Correlation coefficients between yield and disease response to fungicide and sensor measurements of leaf N concentration (SPAD), LAI (LAI2000) and RVI (VIScan) at GS39. The yield response was taken as the slope of a regression of grain yield (t DM ha-1) on fungicide dose. The disease response was taken as either the slope of a regression of septoria coverage (%) on fungicide dose or the normalised disease response, which was calculated by the slope divided by the intercept of the regression of septoria on fungicide dose.
| Sensor measurement | Yield | Disease | Normalised disease | |||
| Nissumgård 2005 | ||||||
| Leaf N (SPAD) | 0.06 | -0.21 | 0.24 | |||
| LAI (LAI2000) | 0.19 | -0.29 | 0.21 | |||
| RVI (VIScan) | 0.17 | -0.40 | * | 0.16 | ||
| Schackenborg | ||||||
| Leaf N (SPAD) | 0.06 | -0.69 | *** | -0.14 | ||
| LAI (LAI2000) | 0.17 | -0.74 | *** | -0.23 | ||
| RVI (VIScan) | 0.09 | -0.50 | *** | 0.11 | ||
| Nissumgård 2006 | ||||||
| Leaf N (SPAD) | 0.38 | -0.53 | *** | 0.47 | ** | |
| LAI (LAI2000) | -0.23 | 0.01 | 0.36 | * | ||
| RVI (VIScan) | -0.10 | -0.01 | 0.45 | * | ||
| Dybvad | ||||||
| Leaf N (SPAD) | 0.55 | *** | -0.59 | *** | 0.68 | *** |
| LAI (LAI2000) | 0.17 | -0.35 | * | 0.51 | ** | |
| RVI (VIScan) | 0.59 | *** | -0.52 | ** | 0.53 | *** |
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
The yield and disease responses to fungicide dose were estimated for each sub-block by linear regression of the grain yield and the septoria coverage against fungicide dose. However, only sub-blocks with no missing data for yield or septoria coverage were used. The septoria coverage was estimated as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75. The responses were then taken as the slope of these regressions. As the disease response will to some extent depend on the disease level, a normalised disease index was calculated as the ratio of the slope to the intercept in the regression.
Figure 28. Relationship between yield and normalised disease response to fungicide rate and three selected crop characteristics at GS39; leaf N concentration measured with SPAD (a,b), leaf area index measured with LAI2000 (c,d) and ratio vegetation index measured with ViScan (f, g).
There were significant relationships between yield response to fungicide and the measurements of leaf N and RVI at GS39 for Dybvad only (Table 16). At this location higher leaf N concentrations or high RVI gave a higher yield response to fungicide application. The relationship between the yield response to fungicide and leaf N was also positive for the other locations, but not significant. Across locations there was a lot of scatter in the responses (Fig. 28).
There were significant negative correlations between disease response to fungicide and all sensor measurements, in particular at Schackenborg and Dybvad. Much of these relationships between disease response to fungicide and the sensor measurements are caused by differences in disease levels depending on crop density and N supply, since the significant relationships disappeared at most locations, when the disease responses were normalised for the disease level without fungicide application (Table 16).
However, there was a consistent relationship between normalised disease response to fungicide and leaf N for Nissumgård and Dybvad (Fig. 28b). Excluding the data from Schackenborg, which had consistently lower SPAD readings than the other sites, gave an overall correlation coefficient of 0.49 (P<0.0001) between normalised disease response to fungicide and the SPAD readings. This indicates that higher leaf N concentrations give a relatively flatter dose response curve thus reducing the efficacy of the fungicide application.
Table 17. Regression of yield response to fungicide (t DM L-1) against Yara SPAD sensor readings (SPAD) and EM38 measurements with vertical polarisation (EM38v). The yield response was taken as the slope of a regression of grain yield (t DM ha-1) on fungicide dose.
| Model | Location | Intercept | SPAD | EM38v | R² | ||
| All sites | All | -1.42 | 0.0029 | *** | 0.034 | * | 0.10 |
| Individual | -1.39 | 0.15 | |||||
| sites | 1 | 0.0023 | 0.048 | ||||
| 2 | 0.0025 | 0.047 | |||||
| 3 | 0.0043 | * | -0.037 | ||||
| 4 | 0.0036 | * | 0.023 | ||||
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
Table 18. Regression of yield response to fungicide (t DM L-1) against ViScan reflectance measurements of ratio vegetation index (RVI) and EM38 measurements with vertical polarisation (EM38v). The yield response was taken as the slope of a regression of grain yield (t DM ha-1) on fungicide dose.
| Model | Location | Intercept | RVI | EM38v | R² | ||
| All sites | All | -0.17 | 0.018 | 0.040 | * | 0.06 | |
| Individual | -0.40 | 0.13 | |||||
| sites | 1 | 0.032 | 0.023 | ||||
| 2 | 0.017 | 0.039 | |||||
| 3 | 0.006 | 0.102 | |||||
| 4 | 0.045 | * | 0.022 | ||||
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
The yield response to fungicide across all sites was significantly influenced by plant N concentration (SPAD readings) and by soil type as reflected by the EM38 value (Table 17). The yield response to fungicide was increased for crops with high N concentration and also for areas with higher clay content (higher EM38 values). Similar effects were seen for RVI, but RVI was not as good a predictor of yield response to fungicide as SPAD readings (compare Tables 17 and 18). This difference may partly be related the fact that RVI values were higher for Schackenborg in 2005 at GS39 due to infestation with grass weeds. These results show that SPAD, RVI and EM38 measurements significantly influence yield response to fungicide, and that therefore higher fungicide doses should be applied to areas in the field with higher leaf N concentrations, higher RVI or higher clay content.
An alternative method for estimating the possibilities for varying fungicide rate is to estimate the yield response to sensor measurements and to fungicide dose. Several regression models of grain yield on sensor measurements and fungicide dose are shown in Table 19. A sensor-based variation in fungicide dose will be possible, if the there is a significant interaction between one of the sensor measurements and fungicide dose. This was the case for the interaction between RVI and square root of fungicide dose (Table 19). The regression coefficient for this interaction was consistent at 0.023 t DM L-1 for all regression equations involving several predictors. This value is close to the value of 0.018 t DM L-1 estimated from the regression of the yield response on fungicide dose on RVI (Table 18).
Table 19. Regression of dry matter grain yield (t DM ha-1) on RVI measured with ViScan (RVI), Yara SPAD sensor readings (SPAD), EM38 measurements with vertical polarisation (EM38v) and the interaction of RVI and square root of fungicide dose. The coefficients of the regression equations and the R² and RMSE of these equations are given.
| Intercept | RVI | RVI² | SPAD | EM38v | RVI*vdose | R² | RMSE |
| 3.53 | 0.110 | 0.32 | 0.977 | ||||
| 2.28 | 0.0064 | 0.17 | 1.069 | ||||
| 5.59 | 0.050 | 0.05 | 1.141 | ||||
| 5.68 | 0.047 | 0.13 | 1.108 | ||||
| 1.38 | 0.307 | -0.0042 | 0.34 | 0.958 | |||
| 0.40 | 0.099 | 0.0056 | 0.43 | 0.892 | |||
| 2.99 | 0.108 | 0.046 | 0.35 | 0.950 | |||
| 3.55 | 0.098 | 0.022 | 0.34 | 0.960 | |||
| -0.72 | 0.220 | -0.0026 | 0.0052 | 0.44 | 0.885 | ||
| 1.19 | 0.279 | -0.0036 | 0.041 | 0.37 | 0.935 | ||
| -0.74 | 0.197 | -0.0021 | 0.0050 | 0.034 | 0.46 | 0.870 | |
| 1.15 | 0.273 | -0.0038 | 0.041 | 0.021 | 0.40 | 0.920 | |
| 0.39 | 0.086 | 0.0056 | 0.024 | 0.46 | 0.870 | ||
| -0.78 | 0.210 | -0.0027 | 0.0053 | 0.025 | 0.47 | 0.862 | |
| -0.80 | 0.190 | -0.0022 | 0.0050 | 0.033 | 0.023 | 0.49 | 0.850 |
4.2 Causal model
The causal model links the different responses of crop and disease to environmental conditions and fungicide application (e.g. Olesen et al., 2003a). The following relationship were initially assumed:
- A relationship between disease free yield (Yp) and sensor measurements.
- A relationship between yield reduction and disease occurrence, possibly depending on yield level or sensor measurements.
- A relationship between disease and sensor measurements without fungicide application.
- A relationship between disease and fungicide concentration on the leaves.
- A relationship between fungicide dose, canopy structure and fungicide concentration on the leaves.
These relationships can, if quantified, be combined into a system of equations, which then allows the response function of yield and disease to fungicide application to be estimated at any given site in the field, provided sensor measurements are available.
4.2.1 Yield responses
The grain yield obtained for a non-diseased crop (disease free yield) was estimated for each sub-block as the intercept of a regression of yield on septoria coverage. However, only sub-blocks with no missing data for yield or septoria coverage were used. The septoria coverage was estimated as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75.
The disease free yield correlated well with crop canopy measurements at GS39, in particular LAI and possibly RVI seem to be good predictors of disease free yield (Table 20 and Fig. 29). The soil measurements showed a correlation with disease free yield at Nissumgård in 2005, but not at the other sites, which indicates that it may be more difficult to derive a generally applicable relationship between soil sensor measurements and disease free yield than between crop sensor measurements at GS39 and disease free yield.
Table 20. Correlation coefficients between disease free yield and yield response to septoria disease and sensor measurements of leaf N concentration (SPAD), LAI (LAI2000), RVI (VIScan), soil electrical conductance (EM38 with horizontal or vertical polarisation) and TDR measurements of soil water capacity or impedance. The crop sensor measurements were taken at GS39. The disease free yield was taken as the intercept of a regression of yield (t DM ha-1) on septoria coverage (%), and the yield response was taken as the slope of the same regression.
| Sensor measurement | Nissumgård 2005 |
Schackenborg 2005 |
Nissumgård 2006 |
Dybvad 2006 |
||||
| Disease free yield | ||||||||
| Leaf N (SPAD) | 0.53 | *** | 0.74 | *** | 0.32 | 0.88 | *** | |
| LAI (LAI2000) | 0.62 | *** | 0.78 | *** | 0.07 | 0.64 | *** | |
| RVI (VIScan) | 0.61 | *** | 0.70 | *** | 0.21 | 0.83 | *** | |
| EM38, horizontal | 0.57 | *** | -0.03 | 0.26 | 0.10 | |||
| EM38, vertical | 0.57 | *** | -0.06 | 0.22 | 0.12 | |||
| TDR water | 0.60 | *** | 0.16 | 0.13 | 0.02 | |||
| TDR impedance | -0.72 | *** | -0.14 | -0.20 | -0.08 | |||
| Yield response to disease | ||||||||
| Leaf N (SPAD) | -0.05 | 0.01 | -0.28 | -0.35 | * | |||
| LAI (LAI2000) | -0.17 | -0.01 | 0.15 | -0.08 | ||||
| RVI (VIScan) | -0.13 | -0.03 | 0.05 | -0.30 | ||||
| EM38, horizontal | -0.20 | -0.08 | -0.13 | -0.48 | ** | |||
| EM38, vertical | -0.22 | -0.07 | -0.11 | -0.48 | ** | |||
| TDR water | -0.23 | -0.07 | 0.05 | -0.35 | * | |||
| TDR impedance | 0.21 | 0.17 | 0.01 | 0.23 | ||||
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
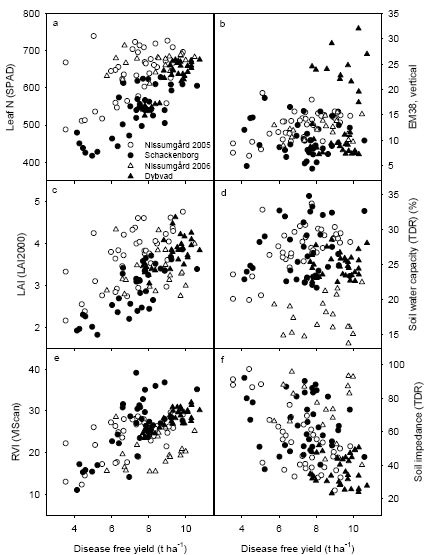
Figure 29. Relationship between disease free yield and three selected crop characteristics at GS39; leaf N concentration measured with SPAD (a), leaf area index measured with LAI2000 (c) and ratio vegetation index measured with VIScan (e), and with soil characteristics; soil electrical conductivity (EM38) (b), soil water capacity (TDR) (d) and soil impedance (TDR) (f).
The yield response to septoria disease did not depend on crop characteristics at GS39 at most of the sites (Table 20 and Fig. 30). However, this may in part be due to large uncertainties in the estimated responses. Some of the very large yield responses (both positive and negative) at Schackenborg thus seem highly unlikely (Fig. 30). Dybvad was the only location, where significant relationships between sensor measurements and yield responses to disease were obtained, and even these responses were rather weak. Also there was very little variation in yield response to disease at Dybvad. Overall the yield response to disease can probably be considered to be constant across fields.
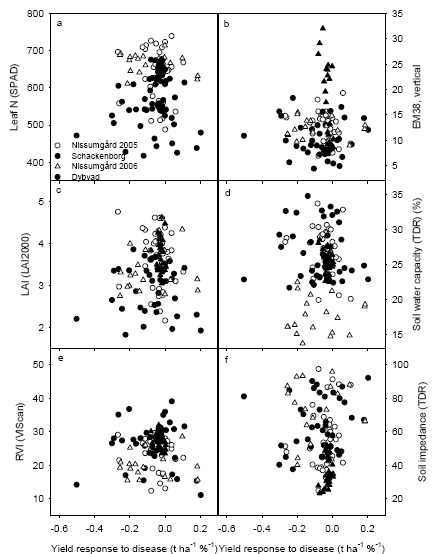
Figure 30. Relationship between yield response to disease and three selected crop characteristics at GS39; leaf N concentration measured with SPAD (a), leaf area index measured with LAI2000 (c) and ratio vegetation index measured with VIScan (f), and with soil characteristics; soil electrical conductivity (EM38) (b), soil water capacity (TDR) (d) and soil impedance (TDR) (e).
4.2.2 Disease responses
The septoria coverage was estimated as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75. This disease attack depended on fungicide application and analyses of the relationship with sensor measurements at GS39 were therefore performed separately for each fungicide rate. The highest correlation coefficients were obtained between septoria coverage and measurements of either leaf N or RVI (Table 21).
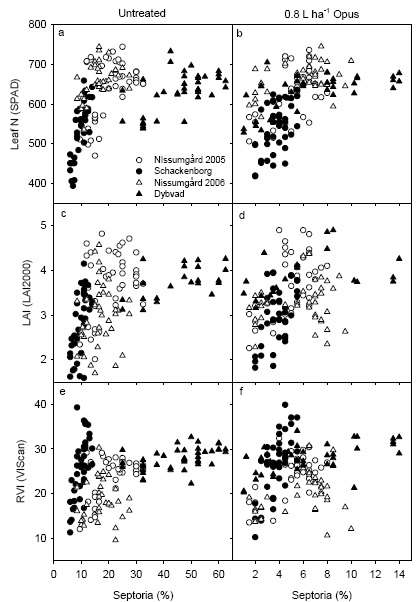
Figure 31. Relationship between septoria attack and three selected crop characteristics at GS39; leaf N concentration measured with SPAD (a, b), leaf area index measured with LAI2000 (c, d) and ratio vegetation index measured with VIScan (e, f) for untreated (a, c, e) and full fungicide treatments (b, d, f).
The highest correlation coefficients were obtained at Schackenborg and Dybvad across fungicide treatments. The weakest correlations were obtained for Nissumgård in 2006, which may reflect the large inhomogeneity of the crop stand at this particular location. The data from the different locations could not be fitted onto a joint relationship between septoria attack and sensor measurements, although there seems to be some relationship, in particular between septoria attack and leaf N (Fig. 31).
Table 21. Correlation coefficients between septoria coverage on the top two leaves and sensor measurements of leaf N concentration (SPAD), LAI (LAI2000), RVI (VIScan).
| Sensor measurement | Untreated | 0.2 L ha-1 | 0.4 L ha-1 | 0.8 L ha-1 | ||||
| Nissumgård 2005 | ||||||||
| Leaf N (SPAD) | 0.45 | ** | 0.37 | * | 0.51 | *** | 0.54 | *** |
| LAI (LAI2000) | 0.34 | * | 0.34 | * | 0.36 | * | 0.52 | *** |
| RVI (VIScan) | 0.46 | ** | 0.57 | *** | 0.42 | ** | 0.61 | *** |
| Schackenborg | ||||||||
| Leaf N (SPAD) | 0.79 | *** | 0.54 | *** | 0.61 | *** | 0.51 | *** |
| LAI (LAI2000) | 0.63 | *** | 0.58 | *** | 0.50 | ** | 0.48 | ** |
| RVI (VIScan) | 0.67 | *** | 0.61 | *** | 0.61 | *** | 0.69 | *** |
| Nissumgård 2006 | ||||||||
| Leaf N (SPAD) | 0.45 | ** | 0.46 | ** | 0.53 | *** | 0.45 | ** |
| LAI (LAI2000) | 0.14 | 0.37 | * | 0.16 | -0.01 | |||
| RVI (VIScan) | 0.06 | 0.42 | * | 0.14 | 0.31 | |||
| Dybvad | ||||||||
| Leaf N (SPAD) | 0.58 | *** | 0.82 | *** | 0.56 | *** | 0.64 | *** |
| LAI (LAI2000) | 0.57 | ** | 0.70 | ** | 0.41 | * | 0.42 | |
| RVI (VIScan) | 0.50 | *** | 0.56 | *** | 0.55 | *** | 0.55 | *** |
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
The slopes of the relationship between septoria coverage and the sensor measurements were estimated for the different fungicide rates using a common intercept. The slope declined with increasing fungicide rate (Fig. 32).
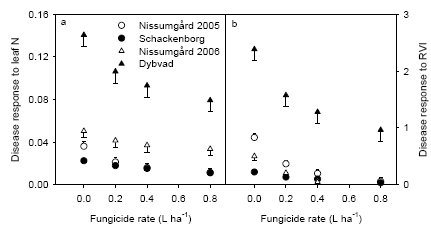
Figure 32. Response of disease to sensor measurements estimated as the slope of a regression of septoria coverage on either leaf N (SPAD) (a) or RVI (VIScan) (b) at GS39 for different fungicide rates. The vertical lines show the standard error.
4.2.3 Fungicide deposition
The deposition of fungicide was measured by addition of a tracer to the fungicide spray. The amount of tracer that was deposited on the soil surface depended on the leaf area index as expected (Fig. 33a). However, different relationships were obtained at the Schackenborg and the other sites, most likely because of the dense stand of Poa annua at Schackenborg. This large weed population is probably also the reason why the deposition of fungicide on the soil surface fits better to the measured RVI (Fig. 33b). The relationship between soil deposition of the fungicide and LAI or RVI fits an inverse exponential relationship, suggesting that the deposition efficiency is constant.
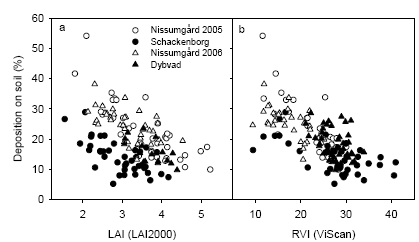
Figure 33. Deposition of tracer on the soil surface (% of applied tracer) in relation to measured LAI (a) and RVI (b).
The deposition on the leaves was measured as concentration of the tracer per unit leaf area. These measurements were compared with measurements of LAI and mean leaf angle derived from the LAI2000 and with RVI from the VIScan. There was a tendency for slightly higher concentrations on the upper leaves at higher LAI and RVI (Table 22 and Fig. 34). However, this was probably caused by the correlation between LAI and leaf angle. More horizontal leaves, which are associated with a higher leaf area, were thus found to give a higher concentration on the upper leaves. The opposite trends were found for the lower leaves (Table 22).
Table 22. Correlation coefficients between concentration of tracer (mg cm-2) on each of the top three leaves and measured LAI (LAI2000), leaf angle (LAI2000) and RVI (VIScan).
| Sensor measurement | Leaf 1 | Leaf 2 | Leaf 3 | |||
| Nissumgård 2005 | ||||||
| LAI (LAI2000) | 0.21 | -0.43 | ** | -0.62 | *** | |
| Leaf angle (LAI2000) | -0.34 | * | 0.29 | 0.43 | ** | |
| RVI (VIScan) | 0.26 | -0.32 | * | -0.56 | *** | |
| Schackenborg | ||||||
| LAI (LAI2000) | 0.11 | -0.10 | -0.54 | *** | ||
| Leaf angle (LAI2000) | -0.16 | 0.07 | 0.52 | *** | ||
| RVI (VIScan) | -0.07 | 0.07 | -0.42 | ** | ||
| Nissumgård 2006 | ||||||
| LAI (LAI2000) | 0.16 | -0.26 | -0.35 | * | ||
| Leaf angle (LAI2000) | -0.08 | -0.06 | 0.06 | |||
| RVI (VIScan) | 0.19 | -0.10 | -0.24 | |||
| Dybvad | ||||||
| LAI (LAI2000) | 0.22 | -0.05 | -0.69 | ** | ||
| Leaf angle (LAI2000) | -0.48 | * | -0.20 | -0.01 | ||
| RVI (VIScan) | 0.14 | 0.03 | -0.04 | |||
Significance levels: *: 0.05<P<0.01, ** 0.01<P<0.001, *** 0.001<P.
Figure 34. Deposition of tracer on leaf 1 (a, b), 2 (c, d), 3 (e, f) in relation to measured LAI (a, c, e) and RVI (b, d, f).
4.2.4 Parameterisation of the causal model
The causal model attempts to estimate the effect of disease and fungicide application on grain yield based on sensor measurements. It was originally anticipated that this estimation would be based on the following assumed equations:
Yield = Pot.Yield x f1(Disease) (3)
Pot.Yield = f2(Sensor) (4)
Disease = f3(LAI,N-conc,Variety) x f4(FungicideConc) (5)
FungicideConc = FungicidDose x f5(LAI) (6)
LAI = f6(Sensor) (7)
N-Conc = f7(Sensor) (8)
The study of fungicide deposition on leaves has not shown any major effects of leaf area index or canopy structure on fungicide concentration on the top two leaves (section 4.2.3), whereas there were clear effects of canopy structure on the fungicide concentration on the third leaf level. However, the protection of disease on the third leaf is not considered as important as for the top two leaves. The effects of fungicide concentration in eqns (5) and (6) were therefore ignored, when developing the causal model.
The potential yield (Pot.Yield) in eqn (4) can be estimated using the measurements of RVI, SPAD and EM38 as shown for the regression equations in Table 20. RVI appears to be just as good a predictor of yield as the other measurements, and as RVI is the simplest measurement, this measure is used here for estimating potential yield. Data from all plots in the experiments with highest fungicide rate (0.8 L ha-1 Opus) were used for this estimation to avoid effects of large disease attacks on the potential yield estimation.
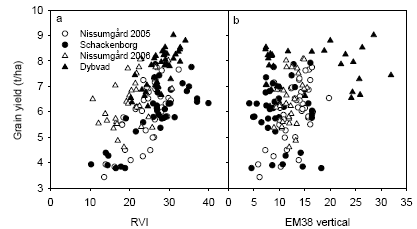
Figure 35. Relationship between dry matter grain yield and (a) RVI at GS39 or (b) EM38 with vertical polarisation for all plots with the highest dose of fungicide (0.8 L ha-1 Opus).
There was clear relationship between RVI at GS39 and grain yield (Fig. 35), resulting in the following estimated regression equation (R² = 0.34, RMSE = 1.01):
Ymax = 3.38 + 0.124 RVI (9)
where Ymax is a surrogate for potential yield and taken as the observed yield at the highest fungicide rate (t DM ha-1), and RVI is the ratio vegetation index at GS39.
The regression equation was slightly improved by also introducing the EM38 measurements as an explanatory variable (R² = 0.39, RMSE = 0.97):
Ymax = 2.74 + 0.119 RVI + 0.059 EM38v (10)
where EM38v is the EM38 measurement with vertical polarisation.
Here only effects of septoria are considered, since this disease was the primary disease in the experiments and generally the major disease affecting yields in winter wheat. The septoria coverage was estimated as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75.
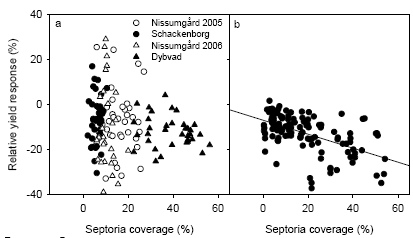
Figure 36. Relative yield response taken as the change in dry matter yield between fungicide treated and non-treated plots in percent of yield of treated plots versus the difference in septoria coverage of the two sets of treatments. Data from the treatments with full fungicide rate in the experiments described in this report (a) and fungicide trials in winter wheat from 2000 to 2006 (b). The line shows a linear regression of yield response on septoria coverage.
The response of grain yield to septoria disease was considered using two different data sources (Fig. 36). Firstly the data from the four field experiments conducted in this project were used (Fig. 36a). These data showed only a moderate yield response to septoria, which may be related to the climatic conditions (relatively dry summers) during these years. In fact only the data from Dybvad indicated a lower yields at higher septoria coverage (Fig. 36a). Therefore additional data were included from fungicide trials in winter wheat carried out at different locations during 2000 to 2006 (Fig. 36b). A total of 29 different experiments were included. The relative yield response was taken from the experiments as the grain dry matter yield difference between fungicide treated and non-treated plots. This relative yield response was regressed on the difference between septoria coverage between fungicide treated and non-treated plots.
The regression equation included a response to septoris coverage (%) (Sep) and a response to fungicide dose (L ha-1 Opus) (Dose). The estimation had to consider that the septoria coverage was based on uncertain assessments, which potentially influences the parameter estimation. Therefore a structural regression approach was used for the parameter estimation using the CALIS procedure of SAS. The parameter estimation was based on the observation that the standard deviation of the septoria coverage was 1.5 times the standard error of the relative yield. The following regression equation was obtained for the data from the fungicide trials carried out during 2000 to 2006 (R² = 0.31, RMSE = 6.9):
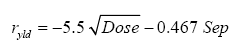 (11)
(11)
All parameters were significant at the 99.9% confidence level.
The regression equation and Fig. 36 show that average response of grain yield to septoria disease is generally considerably larger than what was observed in the experiments conducted in this experiment. Also there appears to be a 7% yield increase from fungicide application, which is unrelated to control of the septoria disease.
Regression and correlation analyses showed that the major determinants of septoria coverage as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75 was septoria coverage at GS39, fungicide rate and RVI at GS39. A multiple linear regression was made on the product of septoria and RVI at GS39 with separate regression coefficients for each fungicide rate (R² = 0.81, RMSE = 5.1). These regressions coefficients were subsequently fitted to an exponential equation, resulting in the following equation for estimation of septoria coverage:
Sep = 3.05 + [0.0105 + 0.166 exp(-Dose / 0.291)] Sep39 RVI (12)where Sep is the septoria coverage (%) taken as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75, Sep39 is the septoria coverage at GS39, RVI is the ratio vegetation index at GS39, and Dose is the dose of fungicide applied at GS39 (L ha-1 Opus). The predicted versus the observed septoria coverage is shown in Fig. 37.
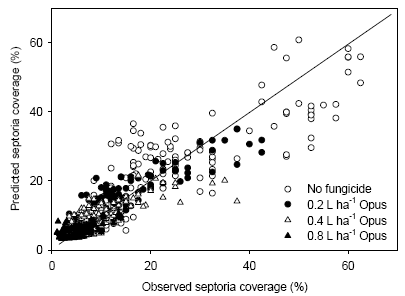
Figure 37. Predicted versus observed septoria coverage taken as the mean cover of septoria on leaf 2 at GS65 and septoria on leaf 1 at GS75. The predictions were made using eqn (12). The 1:1 line is shown.
4.3 Yield and economic benefits of applying the models
The effect of spatially varying the fungicide rate was estimated using two types of models, either an empirical regression model (section 4.1) or a causal model (section 4.2). Both types of models estimate the yield response to sensor measurements and fungicide application.
The empirical regression model was selected from Table 19 as the model including responses to RVI, EM38v and fungicide dose:
![]() (13)
(13)
where Y is grain dry matter yield (t ha-1), RVI is ratio vegetation index, EM38v is EM38 measurement with vertical polarisation, and Dose is fungicide dose (L ha-1 Opus).
The causal model was based on eqns (10), (11) and (12).
All plot based data with normal N fertiliser rate (N treatments 1 and 4) were selected from the four experiments. The data obtained from these plots were used as input to the yield prediction models, which were applied to fungicide doses varying from 0 to 1 L ha-1 Opus in steps of 0.01 L ha-1. The net grain yield was calculated under the assumption of a grain price of 1000 DKK per ton DM and a fungicide price of 373 DKK L-1. The optimal fungicide rate was calculated by finding the fungicide dose giving the highest net grain yield. These calculations did not include application costs, and the fungicide dose was thus calculated under the assumption that at least some part of the field would need to be sprayed and that driving would be needed also to obtain the sensor measurements.
Table 23. Mean and standard deviation (in brackets) of estimated fungicide dose for each site estimated using either the empirical or causal model. The causal model was applied with either observed septoria at GS39 for each plot (causal 1) or for the location as an average (causal 2).
| Location | N treatment | Empirical | Causal 1 | Causal 2 |
| Nissumgård 2005 | 1 (split) | 0.51 (0.09) | 0.23 (0.18) | 0.12 (0.16) |
| 4 (single) | 0.55 (0.09) | 0.28 (0.18) | 0.23 (0.15) | |
| Schackenborg | 1 (split) | 0.64 (0.20) | 0.00 (0.00) | 0.00 (0.00) |
| 4 (single) | 0.69 (0.21) | 0.00 (0.00) | 0.00 (0.00) | |
| Nissumgård 2006 | 1 (split) | 0.27 (0.18) | 0.16(0.18) | 0.20 (0.19) |
| 4 (single) | 0.33 (0.23) | 0.26 (0.22) | 0.27 (0.20) | |
| Dybvad | 1 (split) | 0.65 (0.15) | 0.46 (0.09) | 0.51(0.09) |
| 4 (single) | 0.60 (0.11) | 0.52 (0.09) | 0.50 (0.09) |
The average estimated fungicide rates were generally higher for the empirical compared with the causal model (Table 23), except for Nissumgård in 2006, where similar results were obtained (Fig. 38). This difference may partly be explained by the fact that the causal model also requires information on septoria coverage at GS39, which in the model regulates for different disease pressures between sites and years. There was little difference between using measured septoria coverage at plot or location level (Table 23), primarily because most of the variation in septoria at GS39 was site and year dependent.
The estimated effect of using a spatially variable fungicide rate compared with a fixed fungicide rate was estimated by calculating the net grain yield (grain yield minus fungicide costs) for the spatially variable fungicide rates and for a standard fungicide rate of 0.4 L ha-1 Opus. The causal model was used for estimating the grain yield response. The effect on soil deposition was also estimated by deriving the following relationship between soil deposition and RVI using the data shown in Figure 33b:
Dep = 49 exp(-RVI / 26) (14)
where Dep is soil deposition of the fungicide (%), and RVI is the ratio vegetation index at time of fungicide application.
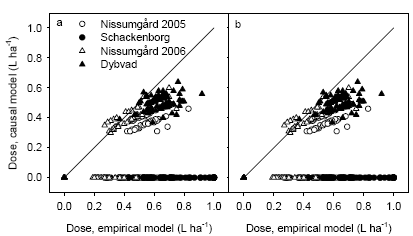
Figure 38. Estimated fungicide dose calculated using the causal model versus fungicide dose calculated using the empirical model. The causal model was applied with either observed septoria at GS39 for each plot (a) or for the location as an average (b). The 1:1 line is shown.
Table 24. Net dry matter grain yield (estimated grain yield using the empirical model minus fungicide costs) and soil fungicide deposition for either variable fungicide rates using the empirical model or using as standard fungicide rate of 0.4 L ha-1.
| Net yield (t ha-1) | Soil deposition (L ha-1) | ||||
| Location | N treat. | Variable | Standard | Variable | Standard |
| Nissumgård 2005 | 1 | 5.81 | 5.84 | 0.093 | 0.075 |
| 4 | 5.87 | 5.91 | 0.097 | 0.072 | |
| Schackenborg | 1 | 5.98 | 6.11 | 0.102 | 0.068 |
| 4 | 6.09 | 6.25 | 0.104 | 0.064 | |
| Nissumgård 2006 | 1 | 5.15 | 5.13 | 0.093 | 0.092 |
| 4 | 5.44 | 5.44 | 0.073 | 0.083 | |
| Dybvad | 1 | 6.13 | 6.13 | 0.065 | 0.065 |
| 4 | 6.09 | 6.09 | 0.100 | 0.067 | |
Table 25. Net dry matter grain yield (estimated grain yield using the causal model minus fungicide costs) and soil fungicide deposition for either variable fungicide rates using the causal model (Causal 2 in Table 23) or using as standard fungicide rate of 0.4 L ha-1.
| Net yield (t ha-1) | Soil deposition (L ha-1) | ||||
| Location | N treat. | Variable | Standard | Variable | Standard |
| Nissumgård 2005 | 1 | 5.83 | 5.84 | 0.021 | 0.075 |
| 4 | 5.91 | 5.91 | 0.040 | 0.072 | |
| Schackenborg | 1 | 6.38 | 6.11 | 0.000 | 0.068 |
| 4 | 6.51 | 6.25 | 0.000 | 0.064 | |
| Nissumgård 2006 | 1 | 5.14 | 5.13 | 0.049 | 0.092 |
| 4 | 5.44 | 5.44 | 0.060 | 0.083 | |
| Dybvad | 1 | 6.14 | 6.13 | 0.083 | 0.065 |
| 4 | 6.10 | 6.09 | 0.084 | 0.067 | |
The application of the empirical model for estimating the spatial variation in fungicide rate resulted in very small yield differences compared with a fixed fungicide rate (Table 24), except for Schackenborg where there was a net yield reduction of about 0.1 t DM ha-1 for varying fungicide rate spatially. On average grain yields were 1% lower for the spatially varied fungicide rate compared with fixed fungicide rate. There was in most cases an increase in fungicide deposition on the soil for the spatially variable compared with the fixed fungicide rate. On average the fungicide deposition increased by 24%.
Application of the causal model for spatial variation in fungicide rate gave small increases in mean net grain yield of -0.01 to 0.27 t DM ha-1 compared with the fixed fungicide rate (Table 25). On average grain yields were 1% lower for the spatially varied fungicide rate compared with fixed fungicide rate. There was on average a reduction of 43% in the deposition of fungicide on the soil surface with the use of spatially varying compared with the fixed fungicide rate.
Discussion
4.4 Spatial variation in soil and plant properties
There was a considerable spatial variation in soil properties at the four experimental sites, which also resulted in a large yield variation, in particular at Nissumgård in both years. The use of four different N-strategies gave additional variation in crop density and N-status. There was also some unintended variation in plant cover, caused by weeds, plant stand, molehills and manganese deficiency. Most of this unintended variation does not appear to have influenced the applicability of the data for analyses of the relationships between sensor measurements and crop performance.
The large stand of weeds (Poa annua) at Schackenborg was most likely the cause of the very high RVI measured at Schackenborg at GS39. Because of this large weed stand, measurements of LAI using the LAI2000 sensor were underestimated because the measurements were not taken at the soil surface but above the weed stand. The large weed stand also influenced the measurements of soil fungicide deposition on the soil surface, since much of the tracer that was not deposited on the wheat was deposited on the weeds.
There were large intra-block variations in soil and plant properties and therefore also a large coefficient of variation for grain yield measurements at all sites, except Dybvad (Table 4). This made it difficult to directly determine the relationship between the yield response to fungicide and sensor measurements (Table 16), and these relationships were only significant at Dybvad.
There was generally a good correlation between the different types of soil measurements (TDR and EM38), so that a high conductance was linked with a high water holding capacity. However, parts of the field at Schackenborg did not follow this general trend. In general a high conductance is linked with a high clay content (Greve et al., 2002), which also has a large water holding capacity. However, this relationship often breaks down where the soil has a large soil organic matter content, and measurements of soil texture showed that a high variation in soil organic matter content at Schackenborg may indeed be the reason the discrepancies between EM38 measurements and soil water content (compare Figs. 9 and 10).
4.5 Quality of sensor measurements
The comparisons of manual sensor measurements and destructive plant samplings showed that the LAI2000 measurements could be used as good estimates of canopy leaf area index (LAI) (Fig. 19), although there was a tendency for underestimation at high LAI. Similar results have been found in other studies (e.g. Stroppiana et al., 2006). There was a small underestimation of LAI using LAI2000 at Schackenborg, which most likely was caused by the need to raise the sensor above the grass weeds in the lower crop canopy at this location.
There was in general a good relationship between SPAD measurements and leaf and plant N concentration (Fig. 20), so that these sensor measurements can be used as reasonably accurate indicators of leaf N concentration. However, two different varieties were used in the experiments. The variety was Grommit at Schackenborg and Deben at all other locations. There appear to have been variety differences in some of the plant characteristics. The specific leaf weight was higher at Schackenborg compared with the other locations (Table 10), and the leaf N concentrations were also slightly lower at Schackenborg compared to the other locations (Fig. 20). However, the SPAD measurements of leaf N generally underestimated leaf and plant N concentrations at Schackenborg compared with the other location (Fig. 20), which may be caused by the higher specific leaf weight at Schackenborg reducing light transmission. It is therefore likely that accurate measurements of leaf N using the SPAD method will require variety specific calibrations.
The manual sensors are, however, much too laborious to use operationally for spatially varying fungicide applications. The recordings of the manual sensors were therefore compared with results of the tractor-mounted MobilLas sensor system, which both measures spectral reflectance (RVI) and uses an infrared laser to estimate LAI. There was good agreement between the tractor-mounted measurements of RVI and the measurements of RVI based on the manual VIScan measurements (Fig. 26).
It is technically very demanding to measure leaf area index (LAI) reliably from a mobile platform, and the MobilLas instrument is the only known remote sensing instrument capable of doing this (Thomsen and Schelde, 2007). It was known from a previous study comparing different approaches that dense canopies with LAI values exceeding 2.5-3.0 is a challenge for any indirect measurement of LAI. As shown in Fig. 22, the MobilLas laser range measurements are used to first calculate the accumulated height of hit distribution. From this distribution, the canopy gap fraction can be directly obtained for any height within the canopy. From the canopy gap fraction LAI is then calculated using eqn. (1). Because of the non-infinite diameter (0.5 mm) of the laser beam and the resulting underestimation of gap fractions, the initial estimates of canopy gap fractions have to be corrected using eqn. (2). The denser the canopy, the greater is the underestimation of gap fraction. For canopies with LAI values exceeding 2.5-3.0, the ground surface can no longer be consistently detected and LAI values become seriously underestimated. Fig. 23 shows the considerable scatter observed in both manual and mobile measurements of LAI. Fig. 23 also shows a generally good relationship between mobile and reference measurements for LAI values below approximately 2.7 and the underestimation of higher LAI values by the mobile instrument.
The software calculating total canopy LAI also calculates profiles of LAI starting at the top of the canopy. Information on the LAI profiles may potentially be useful for optimising crop protection, but this aspect has not been investigated in the present project. The LAI profiles are expected to be reliably estimated for accumulated LAI values not exceeding 2.5-3.0 as for total canopy measurements. Mobile measurements of LAI to a depth of 30 cm are compared in Fig. 24 with reference measurements of total canopy LAI at GS 32 and 39. At GS32 the plant height is typically less than the 40 cm required for calculating LAI at a depth of 30 cm with a ground reference distance of 10 cm (Fig. 22), and the data are thus not comparable. At GS39, where the height is typically more than 40 cm, there is a fair relationship between mobile and reference measurements. The mobile measurements are approximately 1.5 LAI units lower than the reference measurements because only the top 30 cm of the canopy is included. The challenge is thus to develop better algorithms for estimating LAI in dense canopy stands such as for winter wheat at GS39. There may be some prospects for looking at profiles of gap fractions and empirically relate this to observed LAI. However, such approaches are likely to be sensitive to variety and management effects.
The RVI/LAI ratio is in principle closely related to the nitrogen status of the entire canopy, and the MobilLas sensor was primarily developed for precision nitrogen management making use of this ratio. The RVI/LAI ratio is only applicable to green developing canopies for growth stages less than GS32. Thomsen and Schelde (2007) showed that mobile and manual measurements of the RVI/LAI ratio compared favourably to each other and to SPAD readings. Mobile measurements of the RVI/LAI ratio were compared to plant N concentration (Fig. 27a) and to SPAD readings (Fig. 27b). The poor correlations obtained in Fig. 27 are in part due to the late development stage (GS39) and the problems associated with estimating high LAI values. However, some of the scatter in Fig. 27 is also due to observations from Schackenborg differing from the response obtained from the other sites. This is caused both by deviations in RVI and SPAD measurements at Schackenborg compared with the other sites due to effects of both weeds and differences in variety. Therefore, in practice the applicability of the RVI/LAI ratio as a measure of plant N status, will also depend on how sensitive this index is to such influences, and this needs to be further investigated.
4.6 Fungicide treatment effects
4.6.1 Disease and yield responses
The underlying assumption in the project is that there is a yield gain for fungicide application in winter wheat and that this yield gain can be related to one or more sensor measurements that can be made before fungicide application. Septoria was the major disease occurring in both experimental years, and this disease is generally known to cause considerable yield reductions (Shaw and Royle, 1993; Jørgensen and Nielsen, 2003; Olesen et al., 2003a). However, only small yield gains from fungicide application were obtained in the experiments reported here (0.1 to 0.9 t DM ha-1), possibly because of the dry weather conditions during much of the summer in both years. Under conditions more conducive for disease development, yield responses of 1 to 2 t DM ha-1 can be expected (Ørum et al., 2006; Jørgensen et al., 2007).
The small yield increases from fungicide application in this project could not cover the costs of fungicide application in 2005, and it is therefore not surprising that no significant relationship between yield increases from fungicide application and sensor measurements could be found in this year (Table 16). The disease pressure was considerably higher at Dybvad in 2006, and this did indeed result in significant interactions between N strategy and fungicide rate on grain yield (Fig. 13) and a significant relationship between yield response to fungicide application and sensor measurements (Table 16). There was, however, a tendency in all experiments for the response of grain yield to fungicide application to be larger at the highest compared with the lowest N rate.
The results clearly confirm previous findings that increasing N fertiliser rates increases attack of Septoria tritici (Olesen et al., 2003ab; Simon et al., 2003). This pattern was present at both GS65 and GS75 (Fig. 14). There was no clear effect of single or split N application on septoria attack. Olesen et al. (2003a) also found little difference in septoria attack between single and split N application strategies.
4.6.2 Fungicide deposition
The fungicide treatments were applied with conventional hydraulic flat fan nozzles at a driving speed of 3.6 km h-1. This is a typical speed used with manually driven experimental plot sprayers. The nozzle type used and the volume rate of 230 l ha-1 is within the normally recommended and used by farmers. However the driving speed used is much lower than the normal practice where the typical driving speed lies in the interval from 6 to 9 km h-1. The driving speed influences the distribution of the droplets in the canopy in combination with other spray technical factors. Reducing the driving speed increases penetration into the canopy (Göhlich et al., 1976). The driving speed used in the experiment is therefore supposed to give a deeper penetration into the canopy and thus less deposition on the upper part of the canopy compared to the deposition pattern obtained using a driving speed closer to the situation in practical agriculture.
The supplementary experiment (section 3.6) showed that increasing driving speed caused a steeper deposition gradient in the crop canopy. At 4 km h-1 deposition of spray liquid per area unit leaf was only slightly reduced for the 2nd leaf and the value for the 3rd leaf was close to 75% of the deposition on the flag leaf. At 8 km h-1 a much steeper gradient in deposit was found, with a value for the 3rd leaf close to 40% of the per area unit deposit on the flag leaf. What influence such a difference in deposition pattern in the canopy has on disease control and crop yield probably depends on mobility of the fungicides used and interval between fungicide applications. No published investigations on this topic can be found in literature.
The effect of driving speed on fungicide deposition was greatest for the third leaf, which will result in poorer control of septoria on the lower leaves in the crop canopy at realistic driving speeds compared with the driving speed used in the experiment. However, the control of septoria on the lower leaves probably has little effect on grain yield, because the upper leaves are the primary sources of assimilates for grain growth (Dimmock and Gooding, 2002).
Loss of spray liquid to the soil was not significantly affected by the sprayer speed used and this is in accordance with a previous study by Jensen and Spliid (2005).
4.7 Models for estimating yield response to fungicide
The response of grain yield and septoria disease to fungicide application was estimated for each sub-block using regression analysis. For spatially variable fungicide application to be valuable, there must be a relationship between grain yield response to fungicide and one or more of the sensor measurements. This was only the case at Dybvad (Table 16), where the coefficient of variation in yield was small and where there was a substantial septoria infestation.
There was for all experiments significant negative relationships between disease response to fungicide and sensor measurements, in particular for SPAD and RVI (Table 16). This could be attributed to the relationship between disease level and the sensor measurements of crop characteristics at GS39. However, when a correction was made for this effect there were significant positive relationships between disease response to fungicide and sensor measurements for the sites in 2006, which points to higher disease prevalence with higher leaf N concentrations, higher leaf area index and RVI. It also indicates that higher leaf N concentrations give a relatively flatter dose response curve thus reducing the efficacy of the fungicide application. There is little literature on the effect of crop N supply on fungicide efficacy (Jørgensen et al., 1997; Olesen et al., 2003a), and these results indicate that a deeper understanding of the causes of such responses is called for.
There are several indicators from the experiments pointing towards the need for increasing fungicide application rate with increasing crop canopy size and N concentration. These two variables are in practice closely correlated, since increasing N supply will cause winter wheat to increase its leaf area index (Olesen et al., 2002). Firstly, yield gains from fungicide application increased with increasing N fertiliser rate (Fig. 13). Secondly, grain yield and disease response to fungicide rate were at least for some sites significantly positively correlated with sensor measurements of canopy size and leaf N content (Table 16). This suggests that it should be possible to use sensor measurements for determining the optimal spatial variation in fungicide rate.
The results of the correlation analyses in Table 16 and the regressions in Table 17 and 18 indicate that leaf N and RVI measurements are the best candidates for sensor measurements to determine yield response to fungicide rate. This is in line with previous experience indicating that septoria incidence increases with increasing leaf N (Olesen et al., 2003b), and leaf N is generally positively correlated with RVI thus making both these sensor measurements suitable for estimating crop susceptibility to septoria disease.
The results of regression of yield response to fungicide application on SPAD, RVI and EM38v in Tables 17 and 18 could not be used directly for determining the spatially optimal fungicide rate. The reason is that this analysis did not include the diminishing yield response with increasing fungicide rate, which is fundamental for determining the optimal fungicide rate. Instead an empirical regression equation that included the interaction between RVI and the square root of fungicide rate was used (Table 19). The selected empirical regression equation (eqn. 13) included RVI and EM38v measurements, which are operationally available measurements. The EM38v measurement describes the soil characteristics, in particular the soil clay content. Increasing soil clay content means higher soil water availability and therefore a higher yield potential as also reflected in a positive effect of EM38v on disease free yield (Fig. 29). The EM38 measurement can be made only once and used subsequently, whereas the RVI measurement needs to be taken at GS39 at the time of fungicide application.
The empirical regression equation (eqn. 13) implies a higher yield response of fungicide application with increasing RVI. Since high RVI is associated with high yields, this should lead to a higher profitability of applying fungicide for high yielding crops, as also indicated in the data for the varying fertiliser rates (Fig. 13).
As an alternative to the simple regression model, a causal model was developed, which is based on a number of verified relationships between crop yield, disease occurrence and sensor measurements. There were initially four basic assumptions for these relationships:
1. The maximum (potential) yield can be determined from sensor measurements taken before or at GS39.
2. The yield effect of fungicide application is related to the control effect of septoria, and thus yield reduction from disease is directly related to septoria severity.
3. The septoria disease without fungicide can be predicted from sensor measurements.
4. The disease control effect of depends on leaf fungicide concentration and this concentration declines with increasing leaf area index.
The first three assumptions were verified in the experiments, and the analyses of leaf fungicide concentration based on tracer measurements also showed a relationship to leaf area index. However, this effect was strongest for leaf 3 and there were only weak relationships for leaf 1 and 2 (Table 22). It is the disease control on the upper two leaves that provides the main effect on yield gain from disease control (Gooding et al., 2000). This relationship between fungicide concentration and leaf area index was therefore ignored in the development of the causal model.
The actual yield is described as a product of a potential (disease free) yield and an effect of disease. The potential yield was related to both RVI and EM38v measurements (eqn. 10). Similar relationships have previously been reported for Danish winter wheat crops and used for determining the spatial variation in N fertiliser (Berntsen et al., 2002, 2006). The positive relationship between yield and RVI shows that a well-established crop with a high RVI and thus a high biomass and leaf area index at GS39 will generally result in high grain yields. However, the likelihood of high yields is improved under good soil conditions, which is indicated by the positive effect of EM38v on grain yield. A high EM38v measurement is generally associated with high soil clay content and high soil water availability (Fig. 10) (Greve et al., 2002).
Septoria was assumed to influence the ratio of actual to potential yield. The data from the four field experiments gave only a small effect of disease on grain yield (Fig. 36). This may be related to the relatively low infestation level with septoria in the experiments and the warm and dry conditions during both summer seasons, which reduced the effects of septoria on grain yield. Data from fungicide trials in Denmark from 2000 to 2006 were therefore used to develop this relationship, resulting in a stronger grain yield response to disease compared with the response from the present experiments only (Fig. 36). The regression analyses showed a significant additional yield increase from fungicide application, which was unrelated to control of the septoria disease. This effect was estimated to be related to fungicide dose. This is a substantial effect, which should be further investigated. The effect may be related to control of other diseases or to uncertainties in determining the proper effects of disease control on septoria disease levels.
There was no consistent relationship between the yield response to disease and any of the sensor measurements (Table 20), and this was also not hypothesised. The disease free yield was best related to crop characteristics at GS39, whereas soil characteristics were less good predictors of yield (Table 20). The inadequacy of the soil sensor measurements for estimating crop yield may be related to the fact that more factors than soil water supply affect crop establishment and growth and these effects are integrated in the sensor measurements of crop characteristics at GS39. However, soil water availability will affect crop growth and production after this measurement resulting in positive effects of EM38 measurements on disease free yield in addition to the effect of the RVI measurements (eqn. 10).
There was a clear relationship between both RVI and leaf N concentration at GS39 and septoria attack during the following two months (Table 21 and Fig. 31). These relationships were significant at all locations and for all fungicide dosages for leaf N and in most situations for RVI (Table 21). This confirms previous studies of Leitch and Jenkins (1995) and Olesen et al. (2003b), but it contradicts the usual concepts of septoria primarily being influenced by the effects of spore dispersal on disease prevalence. Several studies have thus found that septoria is most dominating in open stands (Jørgensen et al., 1997; Bjerre et al., 2006). However, the good relationship obtained strongly indicates new opportunities for predicting occurrence of septoria in winter wheat.
The analyses of septoria response to sensor measurements showed large variations between years (Fig. 31). This could effectively be accounted for by taking the observed septoria coverage at GS39 as a covariate in the analyses. The best predictor of septoria severity using this approach was the product of RVI and septoria coverage at GS39 (eqn. 12). The fungicide response was accounted for by assuming an exponential dose response curve. In effect this gave a very good description of the observed septoria severity (Fig. 37). However, this is partly based on the assumption that septoria coverage at GS39 can be used as a reliable predictor of conditions for septoria proliferation in the given crop. In practice, more factors are likely to influence septoria, including variety and weather conditions, and there is therefore a need to further improve the basis for predicting septoria under different climatic and management conditions to supplement sensor measurements.
The models developed here only make use of EM38 measurements of soil conditions and of RVI at GS39. In fact these measurements proved to be the most reliable predictors of yield and disease occurrence. However, they are also the measurements, which can most readily be obtained on an operational basis. Analyses of the individual experiments indicated that in particular the leaf N concentrations would be good candidate for a sensor measurement to predict disease development. However, reliable measurements of leaf N concentrations can currently not be obtained on an operational scale at GS39.
4.8 Evaluation of estimated spatial variation in fungicide rate
The applicability of the sensor based models for spatially varying fungicide application was evaluated using data from the four experiments. However, only plots that had received the normal N fertiliser rate were used for this purpose. This included N treatments 1 and 4, where treatment 1 had received the N fertiliser in a split application, whereas the N fertiliser was applied in a single treatment in treatment 4. In practice both fertiliser application strategies may be used, and it is therefore relevant to evaluate the sensor-based models for both N treatments, whereas the two other N treatments would be outside the N fertiliser rate normally applied in practice.
Two types of sensor-based models were used in the evaluation, the empirical regression model (eqn. 13) and the causal model (eqns. 10, 11 and 12). Both models were developed on the data from the same four experiments as used for deriving the spatial variation in input variables for the evaluation. However, the causal model assumed a stronger yield response to disease than was obtained in the experiments (Fig. 36). This higher yield response is in better agreement with other experimental results (Olesen et al., 2003a; Jørgensen et al., 2007).
Since the causal model also includes septoria infestation at GS39 as an input variable this value was either taken for each plot individually or as the average for the experimental site. In practice it will not be possible to obtain spatially varying estimates of this input variable, and maybe not even field level estimates. There was, however, little difference in estimated fungicide rates depending on whether whole field or plot level septoria infestation was used as input to the causal model (Table 23, Fig. 38). It may therefore also be possible to substitute observations of this input variable with proxy estimates using variety information, cropping history and weather data to get an indication of general infection level at a given site in a given year.
The estimated variation in fungicide rate within the field varied between experimental sites, but the standard deviation was generally in the order of 0.1 to 0.2 L ha-1 Opus. This is a higher variation than used in previous experiments with spatial variation in fungicide rate, where Secher et al. (1998) varied fungicide rate +/- 20% depending on RVI measurements.
There was a large discrepancy between the fungicide rates predicted by the two types of models for Schackenborg. The causal model in most cases did not estimate a need for fungicide application. This was primarily due to the low level of septoria disease observed at GS39. On the other hand high fungicide rates were estimated with the empirical model, which relies heavily on RVI measurements for estimating fungicide rate. The RVI values were particular and unrealistically high at Schackenborg due to a large infestation with grass weeds, and this indicates that these models, which rely heavily on one type of sensor measurement, may be vulnerable to other factors affecting these measurements.
The profitability of using the algorithms outlined in the two models were analysed by comparing net grain yield from application of the algorithms with grain yield from a fixed uniform application of 0.4 L ha-1 Opus. This fixed rate application can be considered a typical fungicide treatment in winter wheat in Denmark. The yield results of using the fixed rate application were evaluated by using the yield predictions of the causal model, since no observed data could be provided in this respect that would compare with the other modelled results.
The results for the empirical model mostly showed no differences in net yield between the uniform and the variable fungicide rate. However, net negative yields were obtained at Schackenborg from using the variable fungicide rate, which primarily was due to much larger yield gains from fungicide application predicted by the empirical compared with the causal model due to the reasons described above. There was also a general increase in fungicide deposition on the soil due to generally higher fungicide rates applied with the variable fungicide rate compared with the uniform application.
For the causal model there was either no difference or a small increase in net yield from using the variable compared with the uniform fungicide rate. The variable fungicide rate gave in most cases a reduction in fungicide deposition on the soil, indicating possible positive environmental effects of using sensor-based variable fungicide rates in winter wheat.
The primary reasons for the small yield gains obtained from applying the sensor-based algorithms for varying fungicide rate is the generally rather small yield gains from disease control obtained in the experiment conducted in this project (Table 7). The largest predicted yield gains from spatially variable fungicide application was obtained at Schackenborg (Table 25), and this is only because the model predicted that no fungicide application would be needed here, largely in accordance with observed results (Table 7). The second largest predicted yield gains from spatially variable fungicide application were obtained at Dybvad, which had the highest septoria infestation observed in this project. There may therefore be scope for using these algorithms for improving farm economy and optimising (and reducing) fungicide use in winter wheat. However, this will require further testing of the algorithms under field conditions.
The analyses do not provide definite experimental conclusions on which of the empirical or the causal model is best suited for application in practice. However, the causal model is based on a stronger theoretical foundation of the effects of crop and soil characteristics on disease and the resulting effects on grain yield. The causal model should therefore be preferred. However, this model requires information on the general septoria infestation level at GS39, which may be estimated either by visual assessments or from general surveys and information on variety susceptibility.
5 Conclusions
The project has confirmed that there is a spatial variation in septoria disease and that this variation is to a large extent related to crop canopy structure and leaf N concentration, for which proxy estimates are available from tractor mounted sensor measurements. In particular the use of ratio vegetation index (RVI) from measurements of canopy spectral reflectance at GS39 seems to be a good indicator of crop susceptibility to septoria disease. A high RVI thus gives a high risk of septoria attack.
Analysis of yield gain from fungicide application showed significant positive correlations between yield gain and leaf N concentration or RVI at GS39, in particular at the site with the highest septoria attack and the smallest error variation in grain yields. There were also significant positive relationships between normalised disease response to fungicide application and the measurements of leaf N concentration and RVI, which indicates that fungicides may be less effective in controlling disease in dense and nutritious crops.
The disease free yield was found to be better correlated to crop sensor measurements (LAI, leaf N concentration and RVI) at GS39 than to soil measurements taken in spring. However, the usefulness of the RVI measurements at Schackenborg was reduced due to a large weed population. The best prediction of disease free yield was obtained using a combination of RVI and EM38 measurements.
The relevant crop characteristics could all be measured using handheld sensors. Analyses of the tractor-mounted measurements using the MobilLas measurements indicate that it is possible to perform these measurements in practice for the RVI measurements, whereas it is considerably more difficult to measure leaf area index (LAI) with sufficient precision using tractor mounted sensors. This is due to the saturation of the laser sensor measurements at LAI above 2.5-3.0.
The fungicide deposition in terms of tracer concentration per leaf area varied strongly between the top three leaves with the highest concentration on the upper leaves. There was a tendency for the highest concentrations at high LAI, which may be related to leaf inclination, as there was a significant correlation to mean leaf angle. However, this effect was strongest for leaf 3 and there were only weak relationships for leaf 1 and 2. As it is the disease control on the upper two leaves that provides the main effect on yield gain from disease control, the effect of varying leaf fungicide concentrations with varying canopy density could be ignored in the models.
Two different algorithms (an empirical model and a causal model) for spatially varying fungicide application were developed. Both models make use of RVI and EM38 measurements. EM38 describes the soil characteristics, in particular the soil clay content; and this measurement should be made only once and used subsequently, whereas the RVI measurement needs to be taken at GS39 at the time of fungicide application. Both types of measurements are operationally available today.
Both algorithms were estimated to give a higher need for fungicide application for crops having a higher RVI. This was an indirect effect for the empirical model, whereas both higher grain yield and a higher septoria occurrence and high RVI contributed to this for the causal model.
The evaluation of the model showed that the estimated variation in fungicide rate within the field varied between experimental sites, but the standard deviation was generally in the order of 0.1 to 0.2 L ha-1 Opus. There were only small yield gains from applying the spatially variable fungicide rate using data on variation in soil and crop conditions at the normal N rate in the field experiments conducted in this project when comparing with a uniform fungicide rate of 0.4 L ha-1 Opus. There was mostly a reduction in fungicide deposition on the soil from applying the sensor-based fungicide rates compared with the uniform fungicide rate.
The evaluation showed that the largest yield gains from sensor-based fungicide application were obtained in situations with either very low or high disease infestation. There may thus be some scope for applying sensor-based fungicide rates in winter wheat. However, this will require further testing under a wider range of soil and climatic conditions.
The evaluation was performed for wheat grain price of 1000 DKK per ton DM, and the price has during 2007 increased considerably. This will result in increases in optimal fungicide dose, but not necessarily in increased variation in fungicide dose across a wheat field.
6 Perspectives
Sensor-based graduation of fungicide application in crops can be seen as an integral part of precision agriculture, where the combination of data collection, data communication, biological models and decision support systems enable more precise determination of need for crop management inputs in a spatial context (Srinivasan, 2006). This development was initially driven by the availability of georeferenced technologies, in particular the GPS system, and by new sensor systems to monitor crops and soils. However, more recently there has been an increasing recognition that there is a substantial need to improve the decision support models and algorithms, which converts the sensor readings to decisions and variable application rates (Stafford, 2006).
Precision agriculture has in Denmark mostly been used for patch spraying of weeds and for varying N fertiliser rates. However, experiments and modelling studies have shown that the economic benefit for spatially varying N fertiliser rates in Denmark is very small (Berntsen et al., 2006). There may be some environmental benefits of varying N fertiliser rates, but this still needs to be better quantified. The results presented here for sensor-based graduation of fungicide in winter wheat seems to resemble the situation for spatially varying N fertiliser rates. There is very little economic gain, whereas there is probably an environmental benefit, in this case in the form of reduced fungicide deposition on the soil surface.
The reasons for the small economic gains from sensor-based graduation of either fertilisers or fungicides in Denmark is probably related to the fact, that there has already been a large focus on reducing and targeting N fertiliser rates and fungicides to the need of the crop at field scale. A high economic gain from spatially varying fertiliser or fungicide inputs is only obtained if there is a strong non-linear response between input rate and grain yield within the field, or if there is a general over-application in the reference situation. Neither of this is the general picture in Danish agriculture. However, there may certainly be situations (particular locations or climatic conditions), where such conditions may arise, and the use of this technology should therefore not be discarded.
One of the main reasons for investing in technologies for spatially varying fertiliser or pesticide application rates is the ever-increasing farm and field sizes in Danish agriculture. This trend is caused by the economy of scale with increasing profitability caused by reductions in operational costs per area. This results in larger intra-field spatial variation in soils and crops, giving rise to spatial variation in need for inputs, including fungicides. The use of automated detection of need for fungicide application may increase the willingness by farmers to accept reduced dosages and thereby contribute to the political targets for reduced pesticide use in Danish agriculture.
6.1 Application of sensor-based algorithms
The results have shown that it is possible to derive algorithms, which based on commercially available sensor measurements can be used for varying fungicide application to winter wheat, where septoria is the main disease problem. For practical applications to be developed, these algorithms need to be implemented in commercial systems such as the N-sensor system developed by Yara, which is primarily used for adjusting N fertiliser rates, but which has also been applied for varying fungicide dosages.
However, before the algorithms can be effectively integrated in commercial systems, some remaining issues need to be dealt with, and the algorithms as well as the total system would require some additional test. These issues can be summarised in the following points:
- The models should account for variety differences in susceptibility to septoria. This may be difficult to obtain for the empirical model, where it probably will be the coefficient of the dose response in eqn (13) that needs changing. For the causal model, it will also be the coefficient of the dose response in eqn (12) that needs to be changed. However, available data from fungicide experiments in winter wheat may be used for estimating these parameters.
- The causal model needs information on the septoria disease pressure at GS39. It may be difficult for farmers to visually assess septoria at this stage. Therefore it is suggested that such information is made available on the web (e.g. PlanteInfo). This information could be based on recordings in dedicated fields across the country, e.g. from the existing Danish network for disease monitoring. However, the applicability of such information needs to be tested.
- There is a need to explore whether additional available field maps, e.g. of crop yield distribution, can be used for improving the precision on the algorithms.
- There is a need to test and document the algorithms in field experiments over several (2-3) years to ensure that algorithms function properly and to estimate the economic and environmental benefits of applying the system.
Given that steps are taken to resolve these needs for further model refinement and implementation, it should be realistic to implement sensor-based graduation of fungicide application to winter wheat within 3-4 years.
6.2 Research issues
We verified one of the initial hypotheses in the project that the crop conduciveness to septoria is related to leaf N concentrations. However, it is not currently possible to measure leaf N concentration using tractor-mounted sensors. In theory the leaf N concentration should be related to the ratio of RVI to LAI. However, it is very difficult to measure LAI values of above 2.5 to 3 using sensors that measure from above the crop canopy. This is due to a saturation response of the sensors at higher LAI. There seems to be some scope for estimating LAI in dense canopies by only measuring gap fraction profile in the upper part of the canopy. However, such approximate methods are likely to be influenced by variety and management effects affecting leaf angle distribution and canopy height. Such methods therefore need to be developed and tested for a range of conditions. Another problem with the estimation of leaf N concentration from indirect measurements is the influence of weeds and other factors affecting either RVI or LAI measurements. The sensitivity of the leaf N concentration measurements to such factors needs to be studied and ways of dealing with this should be researched.
The results indicated that it was more difficult to obtain good fungicide control of septoria in crops with high leaf N concentrations (Table 16) and/or at high N fertiliser rates (Figs. 15 to 18). The dose-response functions evidently varied between the experiments and in some cases also between N fertiliser treatments, and a further analysis of this may point towards possibilities of better predicting the dose-response under practical conditions. This is needed for better evaluating situations, where fungicide use may be avoided or of particular benefit. This may be possible since there seems to be a relationship to the crop N nutrition.
There was a rather poor relationship between yield gain from fungicide application and the level of septoria control, despite the fact that septoria was the main yield reducing disease. A better relationships was obtained by Olesen et al. (2003b), who however used a different indicator for septoria disease based on several observations during the growing season, whereas the index used in this study is based on observations at two times only (GS65 and GS75). However, this is possibly not the only cause for the lack of response of grain yield to septoria, and other reasons should be investigated, including the effects of other diseases and uncertainties in disease assessments.
The results have shown that crop measurements of leaf N concentration relate to crop susceptibility to septoria disease. However, there are also considerable variety differences in susceptibility to septoria. Only two different varieties were used in this project. However, the results indicate that there may be variety differences in leaf N concentration and possibly in other crop physiological traits that may relate to crop susceptibility to disease. If such differences can be revealed using sensor based measurements, this also opens opportunities for breeding for more resistant varieties.
References
Anonymous, 2005. Bekæmpelsesmiddelstatistik 2004. Orientering nr. 6 2005. Miljøstyrelsen. Miljøministeriet.
Berntsen, J., Thomsen, A., Schelde, K., Hansen, O.M., 2002. Ny strategi for GPS-gødskning med sensor. Agrologisk 3-02, 26-27.
Berntsen, J., Thomsen, A., Schelde, K., Broge, N., Hansen, O.M., Hougaard, H., Hørfarter, R., Knudsen, L., 2006. Algorithms for sensor based redistribution of nitrogen. Precision Agriculture 7, 65-83.
Bjerre, K.D., Jørgensen, L.N., Secher, B.J.M., 1998. Sygdomskort og positionsbestemt fungicidanvendelse. 15. Danske Planteværnskonference – Sygdomme og skadedyr. DJF rapport no 3 133-144.
Bjerre, K.D., Jørgensen, L.N., Olesen, J.E.., 2006. Site-specific management of crop disease. In: A. Shrinivasan (Ed.) Handbook of precision agriculture - principles and applications. The Hayworth Press, USA. p. 207-251.
Broge, N.H., Leblanc, E., 2001. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sensing of Environment 76, 156-172.
Broge, N.H., Mortensen, J.V., 2002. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sensing of Environment 81, 45-57.
Broge, N., Thomsen, A., 2002. Comparison of selected vegetation indices as indicators of crop status – The red edge inflection point (REIP), the radio vegetation index (RVI) and the second soil adjusted vegetation index (SAVI2). Proceedings of 22.nd. EARSel Symposium – Geoinformation for European-wide integration, Prague, Czech Republic, 4-6 June 2002.
Broscious, S. C., Frank, J. A., Frederick, J. R., 1985. Influence of winter wheat management practices on the severity of powdery mildew and septoria blotch in Pennsylvania. Phytopathology 75, 538-542.
Buchmann, N.B., Josefsson, H., Cowe, I.A., 2001. Performance of European artificial neutral network (ANN) calibration for moisture and protein in cereals using the Danish near-infrared transmission (NIT) network. Cereal Chemistry 78, 572-577.
Bødker, L., Jørgensen, L.N., Secher, B.J.M., 1994. Meldugudvikling i vinterhvede ved forskellige dyrkningsstrategier. SP rapport 1994-7, 29-46.
Dansk Landbrugsrådgivning, 2003. Sammenhæng mellem udbytteniveau og merudbytte for svampebekæmpelse i korn. Planteavlsorientering nr. 09-624.
Dimmock, J.P.R.E., Gooding, M.J., 2002. The influence of foliar diseases, and their control by fungicides, on the protein concentration in wheat grain: a review. Crop Protection 138, 349-366.
Farmstat, Kleffmann, 2002. Statistical data on pesticide use in DK (Company data).
Gooding, M.J., Dimmock, J.P.R.E., France, J., Jones, S.A., 2000. Green leaf area decline of wheat flag leaves: the influence of fungicides and relationships with mean grain weight and grain yield. Annals of Applied Biology 136, 77-84.
Greve, M.H., Nehmdahl, H., Krogh, L., 2002. Soil mapping on the basis of soil electric conductivity measurements with EM38. NJF Seminar 336 on Implementation of Precision Farming in Practical Agriculture, Skara, Sweeden, 10-12 June, 2002. DIAS Report 100, 26-34.
Gyldenkærne, S., Secher, B.J.M., Nordbo, E., 1999. Ground deposit of pesticides in relation to the cereal canopy density. Pesticide Science 55, 1210-1216.
Göhlich, H., Jegatheeswaran, P., Heidt, H., 1976. Zur ablagerung von Pflanzenschutzmitteln in höheren Beständen. Landtechnik 31, 148-150.
Jagers op Akkerhuis, G.A.J.M., Axelsen, J.A., Kjær, C., 1998. Towards predicting pesticide deposition from plant phenology; a study in spring barley. Pesticide Science 53, 252-262.
Jensen, P.K., Spliid, N.H., 2003. Deposition of spray liquid on the soil below cereal crops after applications during the growing season. Weed Research 43, 362-370.
Jensen, P. K., Spliid, N. H., 2005. Loss of spray liquid to the soil below cereal crops as related to formulation, drop size, spray angling, travel speed and boom height. Annual Review of Agricultural Engineering 4, 323-331.
Jones, H.G., 1992. Plants and Microclimate. A quantitative approach to environmental plant physiology (Cambridge University Press, New York), 411 pp.
Jørgensen, L.N., Nielsen, G.C., 2003. Septoria control using threshold-based systems and fungicides. In Kema, G.H.J., van Ginkel, M., Harrabi, M. (eds.) Proceedings on of the 6th International Symposium on Septoria and Stagnospora Diseases of Cereals. 8-12 December 2003, Tunis, p. 49-61.
Jørgensen, L.N.., Secher, B.J.M., Olesen, J.E., Mortensen, J., 1997. Need for fungicide treatments when varying agricultural parameters. Aspects of Applied Biology 50, 285-292.
Jørgensen, L.N., Rydahl, P., Noe, E., Langvad, A.M., Ørum, J.E., Pinnschmidt, H., Bøjer, O.Q., 2007. Vurdering af Planteværn Onlines økonomiske og miljømæssige effekt. Bekæmpelsesmiddelforskning fra Miljøstyrelsen.
Koch, H., 1980. Räumliche Befallsmunster beim echten Mehltau der Gerste. Zeitschift für Pflanzenkrankheiten und Pflazenschutz 87, 731-737.
Korsaeth, A., 2006. Height above ground corrections of EM38 readings of soil apparent electrical conductivity. Acta Agriculturae Scandinavica Section B - Soil and Plant Science 56, 333-336.
Leitch, M.H., Jenkins, P.D., 1995. Influence of nitrogen on the development of Septoria epidemics in winter wheat. Journal of Agricultural Science, Cambridge 124, 361-368.
Oerke, E.-C., Dehne, H.-W., 1997. Global crop production and the efficacy of crop protection - current situation and future trends. European Journal of Crop Protection 103, 203-215.
Olesen, J.E., Mortensen, J.V., Jørgensen, L.N., Andersen, M.N., 2000a. Nitrogen application, irrigation strategy and fungicide control in winter wheat on a sandy soil. I. Yield, yield components and nitrogen uptake. Journal of Agricultural Science, Cambridge 134, 1-11.
Olesen, J.E., Jørgensen, L.N., Mortensen, J.V., 2000b. Irrigation strategy, nitrogen application and fungicide control in winter wheat on a sandy soil. II. Radiation interception and conversion. Journal of Agricultural Science, Cambridge 134, 13-23.
Olesen, J.E., Berntsen, J., Hansen, E.M., Petersen, B.M., Petersen, J., 2002. Crop nitrogen demand and canopy area expansion in winter wheat during vegetative growth. European Journal of Agronomy 16, 279-296.
Olesen, J.E., Jørgensen, L.N., Petersen, J., Mortensen, J.V., 2003a. Effects of rates and timing of nitrogen fertiliser on disease control by fungicides in winter wheat. 1. Crop yield and nitrogen uptake. Journal of Agricultural Science, Cambridge 140, 1-13.
Olesen, J.E., Jørgensen, L.N., Petersen, J., Mortensen, J.V., 2003b. Effects of rates and timing of nitrogen fertiliser on disease control by fungicides in winter wheat. 2. Crop growth and disease development. Journal of Agricultural Science, Cambridge 140, 15-29.
Paveley, N.D., Clark, W.S., Sylvester-Bradley, R., Bryson, R.J., Dampney, P., 1996. Responding to intere- and intra-field variation to optimise foliar disease management in wheat. BCPC Pest and diseases 1227-1234.
SAS, 1996. SAS Institute SAS/STAT Software. Changes and enhancements through release 6.11. SAS Institute, Cary, NC.
Secher, B.J.M., Murali, N.S., Gadegård, K.E., 1995. Site specific control of plant diseases - a great potential. In Olesen, S.E. (red.): Seminar on site-specific farming. SP-report no. 26, 165-169.
Secher, B.J.M., 1998. Justering af dosering i forhold til afgrødetæthed – et nyt koncept for dosering af svampe- og insektmidler. 15. Danske Planteværnskonference, Sygdomme og skadedyr. DJF Rapport Markbrug 3, 145-150.
Shaw, M.W., Royle, D.J., 2003. Factors determining the severity of epidemics of Mycosphaerella graminicola (Septoria tritici) on winter wheat in the UK. Plant Pathology 42,882-889.
Simon, M.R., Cordo, C.A., Perello, A.E., Struik, P.C., 2003. Influence of nitrogen supply on the susceptibility of wheat to Septoria tritici. J. Phytopathology 151, 283-289.
Srinivasan, A., 2006. Precision agriculture: An overview. In: A. Shrinivasan (Ed.) Handbook of precision agriculture - principles and applications. The Hayworth Press, USA. p. 3-18.
Stafford, J.V., 2006. The role of technology in the emergence and current status of precision agriculture. In: A. Shrinivasan (Ed.) Handbook of precision agriculture - principles and applications. The Hayworth Press, USA. p. 19-56.
Stroppiana, D., Boschetti, M., Confalonieri, R., Bocchi, S., Brivio, P.A., 2006. Evaluation of LAI-2000 for leaf area index monitoring in paddy rice. Field Crops Research 99, 167-170.
Thomsen, A., Broge, N., 2002. Principles of crop growth measurements by reflectance sensors. Proceedings of NJF Seminar no. 336 – Implementation of Precision Farming in Practical Agriculture, Skara, Sweden, June 2002.
Thomsen, A., Schelde, K., 2006. Mobile TDR unit for mapping soil water content and electrical conduktivity. 8th Conference on Precision Agriculture, Minneapolis, USA, 23-26 July, 2006. Book of Abstracts, 141-141.
Thomsen, A., Schelde, K. 2007. Double sensor approach to crop monitoring. NJF 23rd Congress 2007, Copenhagen, Denmark, 26-29 June, 2007. NJF Report 3, 63-64.
Thomsen, A., Steffensen, F.S.F., Broge, N.H., 2002. Four band radiometer for portable and mobile measurements of canopy reflectance and red edge posititon. In Proceedings of 22nd EARSel Symposium - Geoinformation for European-wide integration edited by T. Benes (Millpress, Rotterdam), (Prague, Czech Republic), June 4-6, 2002, p. 597-600.
Thomsen, A., Drøscher, P., Steffensen, F., 2005. Mobile TDR for georeferenced measurement of soil water content and electrical conductivity. The 5th European Conference on Precision Agriculture, Uppsala, Sweden, 9-12 June 2005. Proceedings, p. 481-486. Wageningen Academic Publishers, NL.
Tompkins, D. K., Wright, A. T., Fowler, D. B., 1992. Foliar disease development in no-till winter wheat: influence of agronomic practices on powdery mildew development. Canadian Journal of Plant Science 72, 965-972.
Wilson, J.W., 1963. Errors resulting from thickness of point quadrats. Australian Journal of Botany 11, 178-188.
Ørum, J.E., Pinnschmidt, H., Jørgensen, L.N., 2006. A model for fungicide application in winter wheat. 3. Danske Plantekongres. p. 490-492.
Version 1.0 January 2008, © Danish Environmental Protection Agency