Survey, emission and health assessment of chemical substances in baby products
3 Health Assessment
- 3.1 Introduction
- 3.2 Method
- 3.3 Selected substances
- 3.3.1 Hexabromocyclododecane (HBCD)
- 3.3.2 Toluene 2,4-diisocyanate (TDI)
- 3.3.3 2-Ethylhexanoic acid (2-EHA)
- 3.3.4 Acetophenone
- 3.3.5 1,1,2,2-Tetrachloroethane
- 3.3.6 Formaldehyde
- 3.3.7 Styrene
- 3.3.8 2-bromo-4,6-dinitroaniline (BDNA)
- 3.3.9 Hexaethylene glycol dimethyl ether
- 3.3.10 Tetrapropylene glycol monomethyl ether
- 3.3.11 DEHP
- 3.3.12 DINP
- 3.3.13 Xylene
- 3.4 Overall Assessment
3.1 Introduction
In this section, potential health effects from identified and selected substances are assessed. The focus of the assessment is primarily aimed towards children aged 0 to 1 year.
For each of the identified and quantified substances, information of the substances identity as well as chemical and physical properties is presented. This will include data on material state, melting point, boiling point, vapour pressure and solubility.
A search in the open literature and in recently published research papers has been performed. Focus has been on the ability of absorption of the substance through skin, lung and the gastrointestinal tract. The most important test results, the effects and circumstances are presented. The aim was to find data for NOAEL/LOAEL (No or low observed adverse effect levels) for the selected substances or other relevant data if available.
Based on NOAEL or similar data and the amount of the substances the margin of safety (MOS) can be calculated, and it can be assessed whether the substance may cause a negative health effect for the tested products. However, it is very important to note that for most compounds it is impossible to calculate a true margin of safety because essential information on toxicokinetics, biotransformation, and molecular interaction with biomolecules are lacking. Therefore, much care must be taken when MOS values are used for decision making on xenobiotic chemicals, especially when it is a question of protecting children and young adults.
Further it is assessed from the gathered data whether the substances may cause a negative environment effect for the tested products.
3.2 Method
It is assumed that the substances can be absorbed in the body or may act negatively on external and internal surfaces of an organism.
Regarding exposure the following scenarios are assessed.
- Products with uptake through skin
As worst case, the baby is assumed to have a naked body with skin contact with the product in an area of 15×30 = 450 cm². This area roughly corresponds to the back of a 8 months old baby (measured out) and has been estimated as a realistic worst case exposure area when using nursing pillows, mattresses, etc. If no values for uptake through skin is available 100% uptake is assumed if Log KOW < 4 and 10% uptake if Log KOW > 4 of the substance. The body weight of a baby is set at 5 kg based on the range 3-10 kg the first year. The time of use is dependent on product and shown in Table 3.1.
- Products with oral uptake
As worst case, the baby is assumed to lick on an area of contact with the product in an area of 5 cm × 5 cm =25 cm² from which the migrated substance is transferred. As the uptake is orally 100% uptake is assumed.
The body weight of a baby is set at 5 kg based on the range 3-10 kg the first year. The time of use is dependent on product and shown in Table 3.1.
- Products with uptake through air
For some products with contents of polystyrene pellets, specific volatile components may be emitted and inhaled. For these products it is assumed that the chemical substance is emitted from the total weight of the product to a volume of approximately 1 m³ as the baby will be positioned in close connection with the product. The analysis of emitted volatile components is performed in a laboratory climate chamber of 5 litres with a 3 gram sample. The total weight of PS pellets in the product is approximately 400 gram which gives the same concentration of emitted components from a climate chamber of 0.7 m³.
Table 3.1 Time of use
| Product no. | Type | Time of use, skin contact (h/d) ¹ | Time of use, oral contact (h/d) ¹ |
| 1 | Disposable foam wash cloth | 0.2 | 0.2 |
| 2 | Disposable foam wash cloth | 0.2 | 0.2 |
| 3 | Disposable foam wash cloth | 0.2 | 0.2 |
| 4 | Pillow for feeding baby | 3 | 0.2 |
| 5 | Pillow for feeding baby | 3 | 0.2 |
| 6 | Baby mattress | 3 | 3 |
| 7 | Nursing pad | 1 | 0.2 |
| 8 | Nursing pad | 1 | 0.2 |
| 9 | Baby carrier | 1 (summer) | 1 |
| 10 | Baby carrier | 1 (summer) | 1 |
| 11 | Apron to perambulator | None | 3 |
| 12 | Apron to perambulator | None | 3 |
| 13 | Baby mattress | 3 | 3 |
1 The time of use is a worst case estimate based on interviews with a number of parents
The exposure scenarios are defined according to the EU's Technical Guidance Document (TGD, 2003).
The exposure from scenario 1 is calculated by:
Uptake per day per kg b.w. = [ M × A × H × F] / b.w. {1}
| M: | Migrated amount of substance (mg/cm²×h) |
| A: | Exposed skin area (cm²) |
| H: | Time of exposure per day (hours) |
| F: | Fraction absorbed |
| b.w.: | Body weight (kg) |
F: Fraction of absorbed substance. If no specific values for F is found then the default values is used: F = 1, i.e. 100 % if Log KOW < 4 and F = 0.1, i.e.10% if Log KOW > 4.
By inserting the bodyweight of 5 kg, the equation can then be reduced to:
Uptake per day per kg b.w. = 0.2 × M × A × H × F
In some cases the migrated amount is not available, but only analysis of content by extraction with solvent.
The dependence between migration and concentration is dependent on characteristics of the product, the chemical substance and the simulant contact medium (e.g. artificial sweat) and the exact dependence can only be found from experiments.
In some cases the migration of substances from materials may be explained by using Ficks law J=-D ×dc/dx where
D is diffusion coefficient of the substance
J is the flux (mole of substance per time unit)
dc/dx is the concentration difference of the substance over the diffusion distance
From Ficks law a linear relation between concentration and flux can be expected for some products.
Therefore, in order to obtain an indication of the migration for products where only the content has been measured, it is assumed that there is a linear dependency between migration and concentration.
In case the migration is known for a comparable product M(2), an indicative migration can be estimated for the product M(1) as
M(1)=M(2) × C(2)/C(1) × T(2)/T(1) × A(2)/A(1)
where
M: Migrated amount of substance (mg/cm²/h)
C: Content (mg/g)
A: Exposure area (cm²)
T: Time of use (h)
There will be a considerable uncertainty in the estimate especially as the material characteristics can be different and therefore the estimate must only be used as a crude estimate of the migration.
Evaluation of risk
In the evaluation of health risks the calculated intake has to be compared with the NOAEL or similar values.
Because NOAEL typically is based on studies with animals and different durations uncertainty factors are used to make the value comparable. The uncertainty factors are based on an uncertainty factor of 10 for extrapolation between species (interspecies) and a factor of 10 meant to protect sensitive individuals like children (intraspecies). If the data is of less quality or based on LOAEL an additional uncertainty factor may be applied (typically 10).
In the evaluation of health risks, NOAEL is compared with the calculated uptake. The ratio between the NOAEL and the exposure (substance uptake) is defined as the margin of safety (MOS: Margin of safety). If the data for animals is of sufficient valid the margin of safety factor of 100 may be considered sufficient. But are data inadequate further addition of uncertainty factors may be necessary.
3.3 Selected substances
The substances described in the following are selected as the most important substances for the potential health risks of using these products. The selected substances for health and environmental assessment are:
- Hexabromocyclododecane (HBCD or HBCDD)
- Toluene 2,4-diisocyanate (TDI)
- 2-ethylhexanoic acid (2-EHA)
- Acetophenone
- Formaldehyde
- 1,1,2,2-Tetrachloroethane
- Styrene
- 2-Bromo-4,6-dinitroaniline (BDNA)
- Hexaethylene glycol dimethyl ether,
- Tetrapropylene glycol, monomethyl ether
Apart from the selected substances, the health risk from DEHP, DINP and xylene has been assessed.
3.3.1 Hexabromocyclododecane (HBCD)
3.3.1.1 Identity
| Name | 1,2,5,6,9,10-Hexabromocyclododecane |
| CAS-number | 3194-55-6, 25637-99-4 |
| EINECS number | 221-695-9, 247-148-4 |
| Molecular formula | C12H18Br6 |
Molecular structure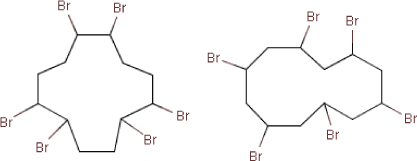 |
|
| Molecular weight | 641.70 |
| Synonyms | Cyclododecane, 1,2,5,6,9,10-hexabromo- Hexabromocyclododecane HBCD |
Hexabromocyclododecane are presented by two different CAS numbers. CAS no. 25637-99-4 (EINECS no. 247-148-4) describes a mixture of mainly three stereoisomers. CAS no. 3194-55-6 (EINECS no. 221-695-9) is 1,2,5,6,9,10-hexabromocyclododecane. The properties of the substances are assumed to be comparable if no other data are available.
The substance consists of a white odourless solid. It has documented melting points between 170 and 190ºC depending on the relative composition of the three stereo-isomers. A boiling point has not been documented since the compound starts to decompose just above the melting point (EC Draft RAR 2003).
The substance has a very low solubility in water. Several values have been reported and evaluated in the EC risk assessment that concluded on a water solubility of 0.0034 mg/L in pure water at 25°C (EC Draft RAR 2003). HBCD has a high solubility in ketone, chlorinated and aromatic solvents (HSDB 6110).
The partition coefficient Log Kow has been estimated to be 7.74 and the Henry’s Law Constant 4.60 E-5 atm-m³/mole at 25°C (Syracuse Research Corporation 2005).
The vapour pressure of HBCD is low. Thus the vapour pressure is stated to 6.3x10-5 Pa in the EU risk assessment and 4.7 x E-7 mm Hg at 21°C (HSDB 6110).
This means that HBCD will evaporate only slowly from aquatic surfaces. However, since HBCD can be predicted to adsorb to suspended matter and in the aquatic environment eventually end up in sediment, evaporation of HBCD is not a probable exposure route (EC Draft RAR 2003).
There are however some uncertainties in the reported physico-chemical properties of HBCD, since the commercial HBCD is composed of three isomers and also some impurities, this, should, have no influence on the assessment.
3.3.1.2 Function of substance
HBCD is as an additive-type flame retardant and is thus added to the plastics materials without reacting with the plastic polymer. HBCD is used in extruded and expanded polystyrene foam. Other applications include crystal and high-impact polystyrene, SAN (Styrene-AcryloNitrile) resins, adhesives, and coatings (HSDB 6110).
3.3.1.3 Classification and TLV
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
It is a high production volume (HPV) chemical and it is a prioritised substance within the Programme of Existing Substances in the EU. It is currently undergoing a comprehensive EU risk assessment with Sweden as rapporteur country (EC Draft RAR 2003).
Threshold limit value (TLV) was not found.
3.3.1.4 Detected quantities
The substance is detected in 2 products.
In sample number 4C is found 457 µg/gram
In sample number 5C is found 433 µg/gram
Note: The chemical is thermal instable and therefore it is likely that decomposition occurs in both the injector inlet of the chromatographic device as well as in the detection chamber (EC Draft RAR 2003). The determinations may therefore be underestimated.
3.3.1.5 Health Effects
Very few official data on health effects on humans exist. Skin irritation and sensitisation test on guinea pigs have been performed resulting in conclusion of HBCD being a mild skin allergen (HSDB 6110).
Consumers are exposed to HBCD from products containing the chemical, e.g. polymers. The flame retardant is physically bound within the polymer matrix, however it is not chemically bound and may therefore migrate out of the matrix. Therefore, release of HBCD from the surface of the product and to atmosphere from plastic products may be a potential way of exposure.
In the EU report, no measured data on consumers’ exposure have been submitted by the industry. The available mathematical models for consumer exposure are not applicable for the calculation of consumer exposure to HBCD. Products, e.g. textiles, may contain up to 25% HBCD (EC Draft RAR 2003).
Acute toxicity
Two acute dermal toxicity studies have been reported, both with negative results (no animals died) (EC Draft RAR 2003).
Acute oral toxicity studies also showed no toxic or gross pathological changes after high dosing (10 g/kg).
Some acute studies have reported different kinds of sublethal effects; diarrhoea, body weight reduction, depressed activity, increasing apathy and trembling (EC Draft RAR 2003).
Inhalation studies have also confirmed the low acute toxicity of HBCD.
Sub-chronic toxicity
Two 28-day studies both demonstrate a rather low order of toxicity of HBCD upon repeated administration. The increase in liver weights was not accompanied by any pathological findings, and it might reflect a reversible adaptive change characterised by induction of the microsomal enzymes and proliferation of the endoplasmic reticulum. When the exposure is for a limited period of time, like in this case, the hypertrophy is reversible but if it continues for a longer period of time, exposure may exceed the metabolic capacity of the liver and necrosis occurs. (Newberne, 1982).
Two 90-days and one lifetime (18 month) studies on rats and mice support the theory that the liver and the thyroid hormone system are the target for HBCD toxicity in mammals. LOAEL based on these studies of 100 mg/kg/day have been deduced (EC Draft RAR 2003).
Two ordinary developmental toxicity studies have failed to demonstrate any fetotoxicity, teratogenic potential, or adverse effects from HBCD on development of rats.
Few studies on mutagenicy and carcinogenity have been performed and none have reported positive results for HBCD.
Recent studies has indicated that HBCD may cause developmental neurotoxic effects as illustrated by statistically significant changes in spontaneous behaviour, learning and memory defects, and reduced number of nicotine receptors. An indicative LOAEL of 0.9 mg/kg b.w/day can be deduced from these studies (Darnerud 2003, Eriksson et al 2002). The neurotoxicological potential of HBCD has also been confirmed in a study where it was demonstrated that HBCD inhibit plasma membrane uptake of the neurotransmitters dopamine, glutamate and GABA. The response occurred at concentration levels similar to what previously have been found for PCBs and Ecstasy (Mariussen and Fonnum 2003).
Summary
HBCD shows similar chemical and physical properties as well-known persistent organic pollutants and at the same time at a first glance seems to lack toxic action, as were the case with PCBs back in the 1950-ties.
Relevant toxicity studies are lacking for the substance. However, the few studies that have been published so far are enough for being the basis for an immediate strict regulation of the compound. Effects on hormone systems and behaviour at low concentration levels indicate that infants definitely should be protected from ever possible risk of exposure.
3.3.1.6 Exposure scenarios
Human exposure of different populations and subpopulations by multiple exposure routes is possible. These are workers, consumers, and humans exposed to HBCD via the environment (via food, drinking water and air). Worker and consumer exposure are mainly via the dermal and inhalation routes, whereas exposure via the environment occurs via the oral route.
HBCD was not found in the migration experiments.
3.3.1.7 Assessment
HBCD has a high log KOW value and a low indicative LOAEL of 0.9 mg/kg/day. The substance has only been analysed in PS pellets covered by outer layers in product 4, and5 (pillows for feeding the baby). The substance is solid at the temperatures of use and has a low solubility in water. Therefore, it is rather unlikely that a high diffusion through the outer cover layers will occur even if the pillow gets wet accidentally.
As no data was found in the migration analysis for HCBD a margin of safety can not be calculated.
3.3.2 Toluene 2,4-diisocyanate (TDI)
3.3.2.1 Identity
| Name | Toluene 2,4-diisocyanate |
| CAS-number | 584-84-9 |
| EINECS number | 209-544-5 |
| Molecular formula | C9H6N2O2 |
| Molecular structure | 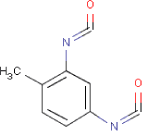 |
| Molecular weight | 174.16 |
| Melting point | 20.5ºC at 760 mmHg |
| Boiling point | 251ºC at 760 mmHg |
| Log Kow (octanol/water) | 3.74 (estimated, esc.syrres.com) |
| Synonyms | 2,4-Diisocyanato-1-methylbenzene (9CI) 2,4-Toluene diisocyanate 4-Methyl-m-phenylene diisocyanate Benzene, 2,4-diisocyanato-1-methyl- Isocyanic acid, 4-methyl-m-phenylene ester (8CI) |
Technical toluene diisocyanate is a mixture of 2.4- and 2.6-isomers (80:20). The pure substance is a colourless to light yellow clear liquid or crystals. It is a quite reactive compound used in the manufacture of polyurethane foams and other elastomers.
The compound has a sharp and pungent odour (Budavari 1996). It is miscible with alcohol, ether, acetone, benzene, carbon tetrachloride, chlorobenzene, diglycol monomethyl ether, kerosene, olive oil, as well as soluble in ethyl acetate (Budavari 1996). The vapour pressure is stated to 1.07 Pa (0.008 mm Hg) at 20°C (Boublik et al 1984).
Along with the estimated octanol-water partitioning coefficient log Kow 3.74, an estimate of water solubility of 37.6 mg/L, as well as a Henry’s law constant of 1.11 E-5 atm-m³/mole, is also reported (esc.syrres.com). However, as TDI is highly reactive and reacts with water with evolution of carbon dioxide (Budavari 1996), these estimates should not be regarded as particularly valid.
3.3.2.2 Function of substance
The substance is a chemical intermediate used in the preparation of polyurethane foams, elastomers and coatings.
3.3.2.3 Classification and TLV
TDI is classified in the List of dangerous substances (Miljøministeriet 2005):
| Tx;R26 | Very toxic by inhalation |
| Xi;R36/37/38 | Irritating to eyes, respiratory system and skin |
| Carc.cat.3;R40 | Limited evidence of carcinogenic effects |
| R42/43 | May cause sensitization by inhalation and skin contact |
| R52/53, | Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
The classification is depending on the concentration in the product as:
Conc.>=20%: Tx;R26 Xi;R36/37/38 Carc3;R40 R42/43
7%<=conc.<20%: Tx;R26 Carc3;R40 R42/43
1%<=conc.<7%: T;R23 Carc3;R40 R42/43
0.1%<=conc.<1%: Xn;R20 R42. (MSDS 584-84-9)
The threshold limit value (Occupational exposure limits, OEL) in Denmark is 0.035 mg/m³ corresponding to 0.005 ppm. The substance is marked with K, which means that the substance is included in the list of substances considered carcinogenic (Arbejdstilsynet 2005).
TDI is a high production volume chemical.
3.3.2.4 Detected quantities
Foam based polyurethane products may in principle emit small amounts isocyanates or the corresponding diamines (Kortlægning nr.28, 2003). TDI was quantitatively analysed by the GC/MS screening of the products 1,2,3,6B,7B,8B,11E,13B but this results is regarded as reaction products from the heating in the GC column. By using HPLC for analysis of foam wash cloths the content was below the detection limit of 5 µg/g.
3.3.2.5 Health effects
In the safety data sheet MSDS the substance is described as a potent skin irritant and allergen.
Potential Health Effects stated are:
Eye: Causes severe eye irritation. Lachrymator (substance which increases the flow of tears).
Skin: Causes severe skin irritation and eczema. May be harmful if absorbed through the skin. May cause sensitization by skin contact.
Ingestion: May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation: Causes respiratory tract irritation. May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung oedema. Toxic if inhaled.
Chronic: Repeated exposure may cause allergic respiratory reaction (asthma).
May cause allergic skin reaction in some individuals. Limited evidence of a carcinogenic effect.
Acute toxicity
Exposure to levels as low as 0.014 mg/m³ (0.002 ppm) can result in chronic loss of pulmonary function. A more acute, asthmatic type of bronchitis is not uncommon (IARC 1979).
Acute skin absorption tests on rabbits produced severe irritation but failed to kill, even with very high doses (16 g/kg b.w) (IARC 1979). Another reported acute value is LD50 for rat oral at 3060 mg/kg (EHC 75). For mouse with oral intake LD50 1950 mg/kg was reported (Lewis 1996).
Men complained of neurological symptoms after single exposure to TDI. Effects were euphoria, ataxia, and loss of consciousness; headache, difficulty in concentration, poor memory and confusion. Four year after the exposure incident personality changes, irritability and depression was still noted (HSDB 874).
Sub-chronic toxicity
There is inadequate evidence for the carcinogenicity of TDI in humans. There is sufficient evidence for the carcinogenicity of TDI in experimental animals. Overall evaluation: Toluene diisocyanates are possibly carcinogenic to humans (Group 2B) (IARC 1999, HSDB 874).
Groups of mice and rats in a sub-chronic study were feed corn oil spiked with TDI in different concentrations. The mice in the experiment were administrated 120 or 240 mg/kg. No treatment related tumour was seen in male mice. However, the female mice got two different tumour types; 0% in control, 10% in low-dose, and high dose animals gave a positive trend (p=0.01) (IARC 1986). In the rat test the males were administrated 30 or 60 mg/kg b.w and females 60 or 120 mg/kg b.w of TDI. Treatment related effects were seen in males at low-dose (IARC 1986).
Summary
A broad range of toxic effects is reported for TDIs in the literature. The majority of reported endpoints and observed harm are directly related to TDIs inherent property of being an aggressive and highly reactive compound. Therefore, it is also natural that populations subjected to occupational exposure are at highest risk. However, because of the compounds high toxicity and possibility to induce damage to epithelium, small residues of free compound in/on products that can come in contact with infants should not be ignored.
The long-term effects on brain functions mentioned above must naturally be taken into account when evaluating potential health effects in children.
3.3.2.6 Exposure scenarios
There is no available data for migration of TDI. The content in the foam wash cloths was below 5µg/g. If a worst case assumption is made where the extractable amount of isocyanate corresponds to the detection limit, the maximum extractable amount can be calculated to 5µg*2,34 g /351 cm² =0,033 µg/cm² as a foam washing cloth weighs 2,34 g and has an area of approx. 18,5*19=351 cm².
Based on an extractable content of 0,033 µg/cm², 6 times daily use, an area of exposure of 450 cm² and 100 % absorption, the uptake in a worst case scenario can be calculated to
Uptake = 6*0,033*450*1*0,2 = 17,8 µg/kg/day.
3.3.2.7 Assessment
Based on a LOAEL of 30 mg/kg/day for carcinogenic effects in rats and the highest calculated uptake, the margin of safety (MOS) is 1700 for foam wash cloths
Table 3.2 Estimated margin of safety for products
| Product no. | MOS |
| 1,2,3 | >1700 |
As the data is based on a subchronic study and LOAEL, the uncertainty factor for risk evaluation is assumed to be at least 1000.
It is concluded that there is no risk to the health for consumers from toluene 2,4-diisocyanate by using foam wash cloths based on an assumption that all TDI present at a concentration corresponding to the detection limed can be extracted and absorbed.
It must be mentioned that no quantitative measurements has been made for the other foam containing products. A content of extractable isocyanates will be a source for allergic reactions.
3.3.3 2-Ethylhexanoic acid (2-EHA)
3.3.3.1 Identity
| Name | 2-ethylhexanoic acid |
| CAS-number | 149-57-5 |
| EINECS number | 205-743-6 |
| Molecular formula | C18H16O2 |
| Molecular structure | 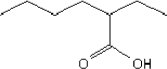 |
| Molecular weight | 144.22 |
| Synonyms | 2-Ethylhexanoic acid 2-Ethylhexoic acid Ethyl hexanoic acid, 2- Hexanoic acid, 2-ethyl- |
The substance is a clear liquid. It has a boiling point of 228ºC (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. It is soluble in ethyl ether, carbon tetrachloride and slightly soluble in ethanol. The solubility in water according to (Ashford, 1994) is 1.4 g/l at 25ºC.
The octanol/water partition coefficient Log KOW is determined to be 2.64 (Hansch, 1995).
Vapour pressure is determined to be 4Pa (0.03 mmHg) at 20ºC (Flick, 1991).
The substance has a mild odour (Flick, 1991).
3.3.3.2 Function of substance
The function of the substance is as stabiliser for PVC products. The substance may also be regarded as a residue from PU production since the salt of 2-EHA and tin, stannous octoate, is the most popular catalyst in PU production. The fact that the substance in this study is found in PU samples and that the presence of tin also is verified in these samples indicate an origin from the catalyst.
3.3.3.3 Classification and TLV
2-Ethylhexanoic acid is included in the List of dangerous substances (corresponding to Annex I of Directive 57/548/EC) and classified as:
Repr.cat.3;R63 Possible risk of harm to unborn child
No Danish threshold limit value for the substance has been found.
3.3.3.4 Detected quantities
The substance is detected in 8 of the samples when extracted with dichloromethane and in 1 in migration tests.
Table 3.3 Detected quantities
| Product no. | Type | Content, µg/g |
Area migration cm² |
Migrated amount µg/cm² |
| 1 | Disposable foam wash cloth | 241 | - | - |
| 2 | Disposable foam wash cloth | 55 | - | - |
| 3 | Disposable foam wash cloth | 424 | - | - |
| 6B | Baby mattress | 30 | - | - |
| 7B | Nursing pad | 81 | - | - |
| 8A | Nursing pad | 21 | - | - |
| 8B | Nursing pad | 495 | - | - |
| 13B | Baby mattress | 66 | 260 | 0.25 |
”-” No analysis
3.3.3.5 Health effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Tests for acute toxicity on animals show that 2-ethylhexanoic acid has a low acute toxicity by ingestion.
- LD50 Rat oral 1,600-3,000 mg/kg (Clayton and Clayton 1993-1994)
- LD50 Rabbit oral 1,300 mg/kg (Clayton and Clayton 1993-1994)
The pure substance is harmful if swallowed, inhaled or absorbed through the skin and is extremely destructive to tissues of mucous membranes and upper respiratory tract, eyes, and skin (Prager, 1996).
Some results on rabbits in the IUCLID data set show the component is irritating, other not.
Subchronic toxicity
Data in HSDB and IUCLID report teratogenic effects of 2-ethylhexanoic acid.
Results with continuous administration in drinking water for Wistar rats up to day 20 of gestation shows skeletal malfunctions in offspring like clubfoot, absence of fibula etc. for doses from 100 mg/kg/day and above. The number of affected foetuses was control: 2.4%,100 mg/kg/day: 4.9%, 300 mg/kg/day: 8.9% and 600 mg/kg/day: 15.3%. The NOAEL for teratogenic effects was set to 100 mg/kg/day.
The developmental toxicity of 2-ethylhexanoic acid was studied in animals treated by gavage with doses 0, 100, 250, 500 mg/kg b.w/day on gestation day 6-15 for rats and with doses 0, 25, 125, 250 mg/kg b.w/day on gestation day 6-18 for rabbits. The results suggest that 2-ethylhexanoic acid induces developmental toxicity in rats only at doses that cause maternal toxicity. 2-Ethylhexanoic acid causes maternal toxicity in rabbits without affecting foetal development. The no observable effect levels for maternal and developmental toxicity in rats are 250 and 100 mg/kg, respectively. The no observable effect levels for maternal and developmental toxicity in rabbits are 25 mg/kg and 250 mg/kg or more. (Hendrickx, 1993)
Data is also reported in IUCLID for fertility effects for rats with 100, 300 or 600 mg/kg/day added in drinking water with a premating exposure of 10 weeks for male and 2 weeks for female. The result was a value of NOAEL parental =300 mg/kg/day and NOAEL offspring =100 mg/kg/day.
No data was found for carcinogenic or sensitizing effects.
Summary
2-Ethylhexanoic acid is a substance that may cause reproduction toxic effects including fertility or teratogenic effects in humans. Indications for other long term effects have not been found.
Values for teratogenic effects in rats gave NOAEL=100 mg/kg b.w/day.
Values for fertility effects in rats gave NOAEL=100 mg/kg/day whereas values for developmental toxicity in rabbits was NOAEL=25 mg/kg b.w. per day.
3.3.3.6 Exposure scenarios
From the highest value of the migration results for product 13B (foam in mattress) of 0.25 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.25*450*(3/4)*1*0.2=16.6 µg/kg/day.
This will require that the mattress is wetted somewhat in order to make contact between skin, outer layer of the mattress and the foam containing the substance. This is most likely when the baby is sweating (during summer).
For product 3 (wash cloth), the content is 6 times as high as in product 13, but the time of use is 15 times less indicating a 2.5 times lower uptake:
Uptake=(424/66)*0.25*450*(0.2/4)*1*0.2=7 µg/kg/day. Similar estimates are shown for product no.1,2 and the other products in Table 3.4.
Table 3.4 Calculated and estimated uptake for products
| Product no | Content (mg/g) | Migration (µg/cm²) | Uptake worst case (µg/kg b.w.) |
| 1 | 241 | Not analysed | 4² |
| 2 | 55 | Not analysed | 0.9 2, |
| 3 | 424 | Not analysed | 7 2,, |
| 6B | 30 | Not analysed | 7.5 ² |
| 7B | 81 | Not analysed | 6.8 ² |
| 8A | 21 | Not analysed | 1.8 ² |
| 8B | 495 | Not analysed | 0 ¹ |
| 13B | 66 | 0.25 | 16.6 |
1: Insignificant uptake as the foam has plastic cover
2: Estimate based on assuming migration of product 13B
3.3.3.7 Assessment
Based on a NOAEL of 25 mg/kg/day for maternal and developmental toxicity in rabbits and the highest calculated uptake, the margin of safety (MOS) is 1504 for product 13.
In the estimate it is assumed that the observed effects will also have an effect on a baby.
Table 3.5 Estimates margin of safety for products
| Product no. | MOS |
| 1 | 6200 |
| 2 | 27000 |
| 3 | 3513 |
| 6B,7B | 3300-3700 |
| 8A | >14000 |
| 13B | 1504 |
As the data are based on a subchronic study, the uncertainty factor for risk evaluation is assumed to be at least 1000 for product 13B. For the products 1,2,3,6,7,8 there is an uncertainty regarding the estimated migration, as the materials may not be of the same physical characteristic and thereby the diffusion coefficient of the substance may be different. It is likely that the uncertainty in the estimation is less than a factor 10.
It is concluded that there is no health risk for the consumer from 2-ethylhexanoic acid for the product no. 13. Based on analysis results from extraction in dichloro methane and assuming similar migrations as for product 13 which introduces an additional uncertainty factor (<10), it is concluded that there is a possible minor risk for products nos.1, 3, 6 and 7. A real assessment of the risk will require a migration analysis.
For product 8A there is no risk of health effects.
3.3.4 Acetophenone
3.3.4.1 Identity
| Name | Acetophenone |
| CAS-number | 98-86-2 |
| EINECS number | 202-708-7 |
| Molecular formula | C8H8O |
| Molecular structure | 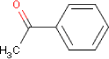 |
| Molecular weight | 120.15 |
| Synonyms | 1-phenylethanone Acetophenon Benzoyl methide Ethanone, 1-phenyl- Ketone, methyl phenyl- Methyl phenyl ketone Phenyl methyl ketone 1-Phenyl-1-ethanone |
Liquid. Forms laminar crystals at low temperature.
Orange blossom or jasmine-like odour. Bitter, aromatic flavour.
Boiling point: 202°C and melting point 20.5°C. Vapour Pressure: 53 Pa (0.397 mmHg) at 25° C. Henry's Law constant= 1.04 E-5 atm-cu m/mole.
Log Kow is 1.58. Slightly soluble in concentrated sulphuric acid; freely soluble in alcohol, chloroform, ether, acetone, benzene, fatty oils, and glycerol. The water solubility is 6.13 mg/L at 25°C.
3.3.4.2 Function of substance
Acetophenone has several different uses: In perfumery to impart an orange-blossom-like odour; catalyst for the polymerization of olefins; in organic syntheses, esp. as photosensitizer; flavouring agent in non-alcoholic beverages, ice cream, candy, baked goods, gelatines and puddings, chewing gum; fragrance ingredient in soaps, detergents, creams, lotions, perfumes.
Solvent for synthesis of pharmaceuticals, rubber chemicals, dyestuffs and corrosion inhibitors (HSDB 969).
3.3.4.3 Classification and TLV
This chemical substance is classified in the Annex I of Directive 67/548/EEC and in the Danish List of hazardous compounds as:
Xn;R22 Harmful if swallowed.
Xi;R36 Irritating to eyes.
Occupational exposure to acetophenone may occur through inhalation and dermal contact with this compound at workplaces where acetophenone is produced or used. The European regulation recommends (as well as the Danish Working Environment Authorities) a TLV-TWA of 49 mg/m³ (10 ppm, 8-hr) (Arbejdstilsynet 2005).
3.3.4.4 Detected quantities
In the screening with dichloromethane the substance was detected in two products.
Table 3.6 Detected quantities
| Product no. | Type | Content in µg/g |
Area migration (cm²) |
Migrated amount µg/cm² |
Migrated amount µg/g |
VOC µg/m³ |
| 4A | Pillow for feeding baby | 200 | 0.005 | - | - | |
| 5A | Pillow for feeding baby | 210 | 0.01 | - | - | |
| 7A | Pillow for nursing | 196 | 0.003 | - | - | |
| 4C | Pillow for feeding baby | 1010 | - | - | 1.6 | - |
| 5C | Pillow for feeding baby | 1572 | - | - | 5.9 | 0.41 |
“-“ No analysis
3.3.4.5 Health effects
Acute toxicity
Acetophenone had a moderate to low acute oral toxicity in laboratory animals and a low dermal toxicity in guinea-pigs. Central nervous system depression occurred in laboratory animals exposed orally and by injection.
Acetophenone was a skin irritant in rabbits and guinea-pigs. It was a severe eye irritant in rabbits. No skin- sensitizing potential was demonstrated when solutions of acetophenone were tested on guinea-pigs.
The highest toxicity value reported in HSDB (2005) is 200 mg/kg (LD50, mouse).
Only effects on human beings have been examined as result of its use as hypnotic or sedative, and with fairly high dosage there appears to be a slightly depressant action on pulse and slight but continuous decrease of haemoglobin.
Among healthy subjects no effects of any kind were perceptible following ingestion of 0.1-0.3 g, but with 0.45-0.6 g micturition (urination frequency) was increased, pulse weakened and slowed after 5-6 hr, and there was slight but continuous decrease of haemoglobin, returning to normal when dosage ceased.
Sub-chronic toxicity
Acetophenone levels ranging from 1-102 mg/kg/day failed to cause any reduction in body weight or any histopathologic abnormalities in the liver, kidney, spleen, or testis when incorporated in the diet of Sherman rats for 30 days. Acetophenone in the diet of male and female Osborne-Mendel rats at levels 1,000, 2,500, or 10,000 ppm (0.1, 0.25, 1.0%) for 17 weeks found no toxic effects on body weight, haematological indices (red and white blood cell counts, haemoglobin, and hematocrit), nor histopathological abnormalities of the liver, kidney, spleen, heart, testis, muscle, or bone marrow.
Application of 480 mg/kg of acetophenone to the skin of pregnant rats on days 10-15 of pregnancy did not cause any change in the gestation period, size of litter, weight of the offspring, time for appearance of teeth or hair, opening of the eyes, or appearance of reflexes.
There was no evidence of mutagenicity in Ames bacterial tests.
In USEPAs integrated information system (IRIS 2005) oral reference dose (RfD) have been calculated to 0.14 mg/kg bw/day, including an uncertainty factor of 3000. NOAEL was 10,000 ppm or 423 mg/kg/day in a supporting study (Hagan, 1967)
Single dose oral LD50 values for rats range from 0.9-3.2 g/kg bw indicating that the subchronic NOAEL defined by (Hagan,1967) may be close to the threshold for toxicity.
Summary
Thresholds for toxic responses to acetophenone seem in general to be high. If any risk to exposure other than occupational should be regarded, it should be the one that may occur in the scenarios depicted in this investigation. Some references indicate that acetophenone might have irritating properties on both skin and lungs. Therefore, there may be a potential risk for the establishment of chronically diseases as asthma and allergies in sensitive individuals.
3.3.4.6 Exposure scenarios
From the highest value of the migration results for product 5A of 0.01 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.01*450*(3/4)*1*0.2=0.65 µg/kg/day.
The internal PS pellets contain a considerable amount of acetophenone, but the amount can only be extracted if the outer and inner cover layers are penetrable by water. Assuming that this would be possible and that 450 cm² was wetted in a depth of 1 cm this corresponds to 450 cm³ which is approximately 6 g of PS pellets or 36 µg/450 cm² =0.08 µg/cm² (80 cm³ of PS pellets weighs 1 g)
In this case the uptake would be
Uptake=0.08*450*(3/4)*1*0.2=5.4 µg/kg/day.
From the area of exposure and exposure time, the oral uptake will be a factor 270 less.
Table 3.7 Calculated and estimated uptake for products
| Product no | Migrated amount (µg/cm²) |
Migration (µg/g) |
Uptake worst case (µg/kg b.w.) |
| 4A | 0.005 | Not analysed | 0.3 |
| 5A | 0.01 | Not analysed | 0.65 |
| 7A | 0.003 | Not analysed | 0.2 |
| 4C | Not analysed | 1.6 | 1.5 ¹ |
| 5C | Not analysed | 5.9 | 5.4 ¹ |
1 Assuming wetting of in 1 cm depth of 450 cm² of PS pellets
3.3.4.7 Assessment
Based on a NOAEL of 423 mg/kg/day and the highest calculated uptake by skin contact, the margin of safety (MOS) is 658500 for product 5A and MOS =78000 for product 5C.
The corresponding ratio between RfD and uptake is 217 for product 5A and 26 for product 5C. The last is a very unlikely scenario and it is assessed that there is no health effects from oral uptake or skin contact from acetophenone for the tested products.
Table 3.8 Estimates margin of safety for products
| Product no. | MOS |
| 4A, 5A, 7A | >658000 |
| 4C,5C | >78000 |
Regarding uptake by air the ratio between the TLV of 49000 µg/m³ and the concentration of acetophenone measured in climate chamber of 0.41 µg/m³ is 119500. Based on this it is assessed that there is no health effects from intake by air for acetophenone for the tested products.
3.3.5 1,1,2,2-Tetrachloroethane
Identification
| Name | 1,1,2,2-Tetrachlorethane |
| CAS no. | 79-34-5, 25322-20-7 (tetrachloro-ethane) |
| EINECS no. | 201-197-8 |
| Molecular formula | C2H2Cl4 |
| Molecular structure | 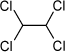 |
| Molecular weight | 167.85 g/mol |
| Synonyms | Sym-tetrachloroethane |
| acetylene tetrachloride |
The melting point of the substance is –43.8ºC. The boiling point is 146ºC (Budavari 1989). The vapour pressure is 800 Pa at 25ºC (Howard 1990), 1200 Pa at 30ºC (9 mmHg) (Flick 1985: HSDB). The water solubility is 2860 mg/l at 25ºC (1 g/350 ml, Budavari 1989). The partitioning coefficient log Kow has experimentally been determined to 2.39 (Hansch et al. 1995).
3.3.5.1 Function of substance
1,1,2,2-Tetrachloroethane is produced by chlorination of ethylene, ethane or 1,2-dichlorethane. 1,1,2,2-Tetrachloroethane is used as solvent for a wide range of substances, but the use is decreasing due to the high toxicity of the substance and the emergence of suitable alternatives.
The source of the recoveries might be the use in the production of polymers or the use as solvent in adhesives. Thus, 1,1,2,2-tetrachloroethane can be a residue from the use in the production process, but it can also be an accidental by-product from the production of another substance, which is used in the production.
3.3.5.2 Classification and TLV
1,1,2,2-Tetrachloroethane, CAS no. 79-34-5, is classified (Miljøministeriet 2005):
| Tx;R26/27 | Toxic. Very toxic by inhalation and in contact with skin |
| N;R51/53 | Dangerous for the environment. Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
Classification Tx;R26/27 N;R51/53 Tx;R26/27 N;R52/53 T;R23/24 N;R52/53 T;R23/24 Xn;R20/21 |
Concentration Conc.>=25% 7% <= conc.<25% 2.5%<= conc.<7% 1%<= conc.<2.5% 0.1%<= conc.<1% |
The threshold limit value is 7 mg/m³ and the substance is marked H which means that the substance can be absorbed through the skin (Arbejdstilsynet, 2005).
3.3.5.3 Detected quantities
In the screening with dichloromethane 1,1,2,2-tetrachloroethane was detected in 2 products (278 µg/g in 4C and 493 µg/g in 5C). The content of 1,1,2,2-tetrachloroethane is analysed in migration studies by sweat test of pillows for feeding the baby (4A, 5A) and a nursing pillow (7A) and in sweat and saliva tests of PS pellets in pillows for feeding the baby (4C, 5C), and by emission measurements of PS pellets in pillows for feeding the baby (4C, 5C). No concentrations of 1,1,2,2-tetrachloroethane were determined above the detection limit in the analysed products. The detection limit is 0.5 µg corresponding to 0.0025 µg/cm² for the products 4A, 5A and 7A.
3.3.5.4 Health effects
The acute toxicity of 1,1,2,2-tetrachloroethane is slight to moderate.
The data below is partly base don Survey report no. 42 (Miljøstyrelsen 2004).
Acute toxicity:
| Acute oral, rat | LD50 | 800 mg/kg | NIOSH 1997 |
| Acute oral, rat | LD50 | 200 mg/kg | HSDB 2003 |
| Acute oral, human | TDLO* | 30 mg/kg | NIOSH |
*: Lowest observed dose with toxic effect
Based on the results of principally limited short-term and subchronic studies, the liver appears to be the most sensitive target organ.
1,1,2,2-Tetrachloroethane is found to be hepathotoxic and nephrotoxic. In a rat study with short-time oral exposure of 1,1,2,2-tetrachloroethane, hepathotoxicity, nephrotoxicity, effects on testes etc. were observed at the lowest dose level. Thus, LOAEL was 8 mg/kg b.w/day (Hassauer et al. 1993).
The acceptable daily intake (ADI) for short-time oral absorption of 8 µg/kg b.w/day is based on a LOAEL for rats of 8 mg/kg b.w/day and a safety factor of 1000 (Hassauer et al. 1993).
In a rat inhalation study, based on immunotoxicity a NOAEL of 2 mg/m³ was observed. The reference has recalculated the exposure to a NOAEL of 60 µg/kg/day assuming an absorption from inhalation of 50% (Hassauer et al. 1993).
ADI: 0.6 µg/kg bw/day is based on absorption via inhalation (NOAEL 60 µg/kg/day from 2 mg/m³, and a safety factor of 100) (Hassauer et al. 1993).
Chronic toxicity
In a long-term oral exposure of 1,1,2,2-tetrachloroethane to rats, hepathotoxicity, nephrotoxicity, effects on testes etc. were observed. NOAEL was 3.2 mg/kg bw/day (Hassauer et al. 1993).
ADI for long-time oral absorption of 0.3 µg/kg bw/day (based on a NOAEL for rats of 3.2 mg/kg bw/day and a safety factor of 10000).
Long-time oral intake of tetrachloroetane resulted in an increased number of liver-tumours in mice. It has not been possible to repeat the results in other species of animal. The exposure for 78 weeks for 0, 142 or 284 mg/kg bw/day is used in an American model (Multistage model) for the evaluation of its carcinogenic potency. The potency, which resulted in 5% increase of liver-tumours (TD0.05), was between 5.8 and 28 mg/kg bw/day (CICAD 1998).
3.3.5.5 Bioavailability
1,1,2,2-Tetrachloroethane is readily absorbed via the skin (MSDS, HSDB).
References suggest an absorption between 70 and 100% after oral exposure. In an experiment with 1.5 mg/kg for rats and mice, 41% was recovered in the exhaled air, 23% in urine and 4% in faeces for rats, for mice the figures were 51%, 22% and 6%, respectively (ATSDS 1996). An absorption of 100% is used in the evaluation.
3.3.5.6 Exposure
In the worst case is assumed that the migration corresponds to the detection limit or 0.0025 µg/cm² (4 hour experiment) for the products 4A, 5A, 7A. Based on this a maximum uptake is calculated to:
Uptake=0.0025*450*(3/4)*1*0.2=0.17 µg/kg/day by skin adsorption
From exposure area and time is found that maximum oral uptake is 270 times less.
3.3.5.7 Assessment
Based on NOAEL 3.2 mg/kg/day and the maximum uptake using the detection level is determined a margin of safety (MOS) of 19000 by absorption through skin while the ratio between ADI and maximum uptake is 1.9.
From this it is concluded that no health risk by absorption through the skin exists for the products 4, 5, 7.
Oral uptake is assessed not to pose any health risk as MOS is 5200000.
The assessment of intake via inhalation can be estimated from the ratio between the occupational threshold limit value (7000 µg/m³) and the concentration from the measurement in climate chamber (<0.1 µg/m³). From this the ratio is >70000. Based on this is assessed that no health effects is expected by inhalation of tetrachloroethane from the tested products.
3.3.6 Formaldehyde
3.3.6.1 Identity
| Name | Formaldehyde |
| CAS-number | 50-00-0 |
| EINECS number | 200-001-8 |
| Molecular formula | CH2O |
| Molecular structure | 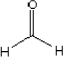 |
| Molecular weight | 30.03 |
| Synonyms | Formalin Methanal |
The substance is a gas. It has a melting point of -92ºC and a boiling point of -19°C (Budavari 1996)
The substance has a high water solubility of 40,000 mg/l at 20 °C
(Pickrell,1983). At high concentrations the substance polymerises.
The partition coefficient log KOW is determined to be 0.35 (Hansch,1995).
Vapour pressure is determined to be 518 kPa (3890 mmHg) at 25ºC (Boublik, 1984).
The substance has a pungent, suffocating odour (NIOSH, 1997).
3.3.6.2 Function of substance
The presence of formaldehyde is probably a reaction by-product which arises from used additives during the production process of textiles
3.3.6.3 Classification and TLV
Formaldehyde is included in the List of dangerous substances and classified as:
| Carc. Cat. 3;R40 | Limited evidence of a carcinogenic effect |
| T;R23/24/25 | Toxic by inhalation, in contact with skin and if swallowed |
| C;R34 | Causes burns |
| R43 | May cause sensitisation by skin contact |
At concentrations in the interval 1-5 % formaldehyde is classified Carc. Cat.3;R40 and R43 and at concentrations 0.2-1% the classification is R43.
The Danish threshold limit value for the working environment is for the substance 0.4 mg/m³ and marked with H for penetrable through skin and K for being considered carcinogenic. For indoor climate there is a Danish norm value of 0.15 mg/m³ (Arbejdstilsynet, 2005) which is close to WHOs guidance value of 0.1 mg/m³. Sensitive persons react with mucous membrane and eye irritation from 0.06 mg/m³.
3.3.6.4 Detected quantities
Formaldehyde was detected in the following products
Table 3.9 Detected quantities
| Product no. | Type | Content µg/g |
Area migration cm² |
Migrated amount µg/cm² |
| 4A | Pillow for feeding baby | 26 | 200 | 0.37 |
| 5A | Pillow for feeding baby | 65 | 219 | 1.7 |
| 7A | Pillow for nursing | 100 | 196 | 0.36 |
3.3.6.5 Health effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET and Survey no. 39 (Eggert and Hansen 2004)..
Acute toxicity
Tests for acute toxicity on animals shows:
- LD50 Rat oral 600 mg/kg (IUCLID)
- LD50 Rat oral 100 mg/kg (Lewis,1996)
- LD50 mouse oral 42 mg/kg (IUCLID)
- LC50 Rat inhalation 480 mg/m³ (Tomlin, 1994)
- Formaldehyde is irritating to skin, eyes and mucous membranes and causes allergic sensitisation. (Thomsen,1990) and (Tomlin, 1994)
Chronic toxicity
Formaldehyde is a probably human carcinogen with limited evidence in humans and sufficient evidence in animals. The evidence include an increased amount of nasal squamous cell carcinomas in long term inhalation studies on rats and mice supported with in vitro genotoxicity data. Formaldehyde is placed in group 2A by IARC (IARC, 1995)
Formaldehyde is revaluated and in the draft (IARC, 2005) it has been concluded that there is sufficient evidence for nasopharyngeal carcinoma in humans for placing formaldehyde in group 1.
The reference dose for chronic oral exposure, RfD, is 0.2 mg/kg/day. The value is based on a 2-year experiment with Wistar rats where formaldehyde was administered daily with drinking water. The LOAEL for weight gain and histopathy was 82 mg/kg/day whereas NOAEL was 15 mg/kg/day. By using an uncertainty factor of 100 for inter- and intraspecies differences, a RfD value of 0.2 mg/kg/day was obtained.
Summary
Formaldehyde causes allergic sensitisation and is a probable human carcinogen. The NOAEL value is 15 mg/kg/day.
3.3.6.6 Exposure scenarios
From the highest value of the migration results for product 5A of 1.7 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=1.7*450*(3/4)*1*0.2 = 115 µg/kg/day. Uptake for 5A and 7A is shown in Table 3.10.
Table 3.10 Calculated and estimated uptake for products
| Product no | Migrated amount (µg/cm²) |
Uptake worst case (µg/kg b.w.) |
| 4A | 0.37 | 25 |
| 5A | 1.7 | 115 |
| 7A | 0.36 | 24 |
3.3.6.7 Assessment
Based on a NOAEL of 15 mg/kg/day for effects on weight gain and histopathy on rats and the highest calculated uptake, the margin of safety (MOS) is 128 for product 5.
The ratio between RfD and the calculated uptake is 1.3 for product 5 and 6 for product 4, 7.
Table 3.11 Estimates margin of safety for products
| Product no. | MOS |
| 4A | 584 |
| 5A | 128 |
| 7A | 604 |
It is concluded that there is a no potential risk to the health of the consumer from products no. 4, 5, and 7. However, it must be noted that the formaldehyde exposure is close to the acceptable daily intake and that the uptake therefore may contribute significantly to the other sources for formaldehyde found in homes (wood sources, electronics etc.). It is assumed that formaldehyde has an absorbtion of 100% and this is not very likely, but it can not be rejected that formaldehyde in the pillow could have a sentisising effect.
3.3.7 Styrene
3.3.7.1 Identity
| Name | Styrene |
| CAS-number | 100-42-5 |
| EINECS number | 202-851-5 |
| Molecular formula | C8H8 |
| Molecular structure | 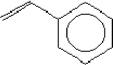 |
| Molecular weight | 104.15 |
| Synonyms | Ethenylbenzene Vinyl benzene |
The substance is a colourless to yellowish oily liquid. It has a melting point of -31ºC and a boiling point of 145°C (Lide, 2000).
The substance is less soluble in water than organic solvents with a water solubility of 310 mg/l at 25°C (Yalkowsky, 1990). At high concentrations the substance polymerises.
The partition coefficient Log KOW is determined to be 2.95 (Hansch, 1995).
Vapour pressure is determined to be 850 Pa (6.4 mmHg) at 25ºC (Chao, 1983).
The substance has a sweet and pleasant odour if pure, but usually contains aldehydes that have a typical penetrating smell, sharp, sweet, and unpleasant odour (Verschueren, 2001).
3.3.7.2 Function of substance
The presence of styrene is not intended but is a monomer residue from the production process.
3.3.7.3 Classification and TLV
Styrene is included in the List of dangerous substances and classified as:
| R10 | Flammable |
| Xn;R20; | Harmful by inhalation |
| Xi;R36/R38 | Irritating to eyes and skin |
The threshold limit value for the working environment is 105 mg/m³ (25 ppm) with notation LHK. L means that the threshold limit value is a ceiling value which at no time must be exceeded. H means that the substance is penetrable to the skin. K means that the substance is adopted on the list of substances that may be carcinogenic (Arbejdstilsynet 2005).
3.3.7.4 Detected quantities
In the screening with dichloromethane the styrene was detected in 4 products
Table 3.12 Detected quantities
| Product no. | Type | Content in µg/g |
Area migration (cm²) |
Migrated amount µg/cm² |
Migrated amount µg/g |
VOC µg/m³ |
| 4A | Pillow for feeding baby | 200 | <0.0025 | <0.5 | - | |
| 5A | Pillow for feeding baby | 9 | 210 | <0.0024 | <0.5 | - |
| 7A | Pillow for nursing | 14 | 196 | <0.0025 | <0.5 | - |
| 4C | Pillow for feeding baby | 315 | 200 | - | <0.5 | 0,3 (max) |
| 5C | Pillow for feeding baby | 679 | 210 | - | <0.5 | 0,3 (max) |
| 6A | Cover for mattress | 20 | - | - | - | - |
| 7B | Pillow for nursing | 79 | - | - | - | - |
| 8B | Pillow for nursing | 28 | - | - | - | - |
“-“ No analysis
3.3.7.5 Health effects
The substance is under evaluation in the EU Risk Assessment Programme for Existing Substances but the assessment is not yet finalised.
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Tests for acute toxicity on animals shows:
- LD50 Rat oral 1000 mg/kg (Verschueren, 1983)
- LD50 Mouse oral 316 mg/kg (Lewis, 1996)
- LD50 Rat inhalation 24000 mg/m³ (Lewis, 1996)
Acute exposure to high concentrations of styrene may produce irritation of the mucous membranes of the upper respiratory tract, nose and mouth
(Environment Canada, 1981).
Chronic toxicity
There is limited evidence in animals and limited evidence in humans for carcinogenity of styrene.
Data from both laboratory (in vitro and in vivo) and human studies indicate that styrene exposure can result in DNA damage in individuals who possess the capacity to activate styrene metabolically to styrene-7,8-oxide.
The lung tumours in mice probably develop as a result of in-situ formation of styrene 7, 8-oxide which causes cytotoxicity and increased cell proliferation, but the roles of circulating styrene 7,8-oxide and of DNA adducts cannot be discounted. Based on metabolic considerations, it is likely that the proposed mechanism involving metabolism of styrene to styrene 7,8-oxide in mouse Clara cells is not operative in human lungs to a biologically significant extent. However, based on the observations in human workers regarding blood styrene 7,8-oxide, DNA adducts and chromosomal damage, it cannot be excluded that this and other mechanisms are important for other organs (IARC, 2002).
Studies of reproductive effects on rats where styrene was administered on day 6-15 of gestation showed effects like decreased maternal and foetal body weight and increased foetal resorption at 400 mg/kg/day but not at 250 mg/kg/day (Srivastava, 1990).
An embryotoxic study showed a toxicity of the metabolite styrene oxide of 0.038 umol/ml, 1 umol/ml of styrene and 1.56 umol/ml of benzene (Brown-Woodman, 1994).
The reference dose for chronic oral exposure RfD is 0.2 mg/kg/day.
The value is based on a subchronic study where beagle dogs were gavaged with 0, 200, 400, 600 mg/kg/day of styrene in peanut oil for 560 days. Effects where found on red blood cells and liver from at 400 mg/kg/day but not at 200 mg/kg/day. From this NOAEL = 200 mg/kg/day. An uncertainty factor of 1000 was used reflecting a factor of 10 for intraspecies, 10 for interspecies and a factor of 10 for extrapolation of subchronic to chronic effects (IRIS).
Summary
There is limited evidence for carcinogenity of styrene.
A study showed reproductive effects on rats.
The NOAEL value=200 mg/kg/day based on effects on red blood cells and liver on beagle dogs.
3.3.7.6 Exposure scenarios
Styrene was not detected in the migration measurements but from the highest value based on the detection limit for product 5A a maximum migration of 0.0025 µg/cm² (4 hour experiment)can be calculated. Using 3 hours of exposure time, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.0025*450*(3/4)*1*0.2 = 0.17 µg/kg/day.
The internal PS pellets contain a considerable amount of styrene, but the amount can only be extracted for dermal contact if the outer and inner cover layers are penetrable by water. Assuming that this would be possible and that 450 cm² was wetted in a depth of 1 cm this corresponds to 450 cm³ which is approximately 6 g of PS pellets or 3 µg/450 cm² =0.0067 µg/cm². In this case the uptake would be
Uptake=0.0067*450*(3/4)*1*0.2 = 0.45 µg/kg/day.
From the area of exposure and exposure time, the oral uptake will be a factor 270 less.
3.3.7.7 Assessment
Based on a NOAEL of 200 mg/kg/day for effects on red blood cells and liver of beagle dogs and the highest calculated uptake which is based on the detection limit, the margin of safety (MOS) is approximately 1200000 for product 4A, 5A, 7A
For product 4C, 5C and 7C MOS =452000.
Table 3.13 Estimates margin of safety for products
| Product no. | MOS |
| 4A,5A,7A | 1,200,000 |
| 4C,5C,7C | 452,000 |
It is concluded that there is no health effect from styrene by skin contact and oral uptake.
Regarding uptake by air via inhalation the ratio between the TLV of 105000 µg/m³ and the concentration of styrene measured in climate chamber of 0.77 µg/m³ is 136000. Based on this it is assessed that there is no health risk from intake by air of styrene for the tested products.
3.3.8 2-bromo-4,6-dinitroaniline (BDNA)
3.3.8.1 Identity
| Name | 2-bromo-4,6-dinitroaniline |
| CAS-number | 1817-73-8 |
| EINECS number | 217-329-2 |
| Molecular formula | C6H4BrN3O4 |
| Molecular structure | 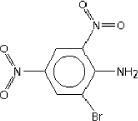 |
| Molecular weight | 262.03 |
| Melting point | 153.5 (C°) |
| Boiling point | Sublimes before reaching BP (C°) |
| Log Kow (octanol-water) | 2.73 (estimate) |
| Synonyms | 2-Bromo-4,6-dinitroaniline Aniline, 2-bromo-4,6-dinitro- Benzenamine, 2-bromo-4,6-dinitro |
It is in its commercial formulation a yellow-orange powder (HSDB 5453).
The substance is very soluble in hot alcohol and acetone, soluble in hot acetic acid.
The water solubility is estimated to 92 mg/l.
3.3.8.2 Function of the substance
The substance is used as a chemical intermediate for Azo derivatives and Disperse Violet 7 and 24.
3.3.8.3 Classification and TLV
This chemical substance is not classified in the Annex I of Directive 67/548/EEC, and not listed in any priority list of existing chemicals (Council Regulation (EEC) No 793/93).
The substance is classified in the Danish Advisory List for self-classification of hazardous substances as:
| N;R51/53 | Dangerous for the environment. Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
No other classification or TLVs are reported except for those in MSDS (cf. Health effects).
It is a high production volume chemical according to US-EPA based on that it is a major intermediate for disperse azo colorants.
3.3.8.4 Detected quantities
Results of extraction with dichloromethane.
Table 3.14 Detected quantities
| Product no. | Type | Content in µg/g | Area migration (cm²) c | Migrated amount µg/cm² |
| 11A | Apron to perambulator | 31 | 200 | 0.0025 |
| 12A | Apron to perambulator | 108 | 200 | 0.0025 |
3.3.8.5 Health effects
In the MSDS for the compound some warnings for occupational handling of the product is described: It may be harmful by inhalation and causes mild eye irritation. It may cause irritation of the digestive tract and cause headache.
It is, however also stated that the toxicological properties of the compound have not been fully investigated. The MSDS state the Hazard symbol: Xn (Harmful) and Risk Phrases R20/21 (Harmful by inhalation and in contact with skin).
Acute toxicity
Oral, rat LD50= 4100 mg/kg (MSDS 1817-73-8).
Acute oral toxicity was evaluated in single male or female rats administered 6-bromo-2,4-dinitroaniline (the less common isomer in the technical grade formulation) at levels of 0.28, 0.62, 1.4, 3.2, 7.1, or 10.7 g/kg body weight. Mortality after 12 days was induced at 7.1 g/kg (female) and 10.7 g/kg (male). However, relevant sublethal endpoints were discovered at lower dosing (3.2 g/kg) and before death. Observations included central depression and lowered response to painful stimuli, brownish urine during the first 24 hours post-dosing and marked loss of body weight. Necropsy of decedents revealed yellowish-brown discoloration of all internal organs, oedema of the liver, hyperaemia of the lungs and contraction of the ventricles of the heart (EPA/OTS 1983).
The disposition and metabolism of BDNA were investigated in rats after a single intravenous dose of either 1, 10, or 100 micromoles per kilogram (micromole/kg) BDNA. Animals were sampled for blood, liver, muscle, skin, kidney, and adipose tissue after different times of metabolism. No signs of toxicity were noted at any dose. About 46 to 62 percent of the BDNA was excreted in the urine and from 33 to 43 percent in the faeces. Relative amounts did not depend on dose. BDNA was found in all major tissues and was more or less evenly distributed except for the organs involved in clearance, metabolism, and excretion. After 72 hours the amount of BDNA remaining in the body was negligible. The authors conclude that BDNA is readily absorbed from the gastrointestinal tract and that rapid metabolism and excretion prevent its accumulation (Chopade and Matthews 1986).
Chronic toxicity
The mutagenicity of 2-bromo-4,6-dinitroaniline (BDNA) has been evaluated in some Salmonella test strains TA98, TA100, TA1535, TA1537 and TA1538 (Ames Test). BDNA was tested for mutagenicity at concentrations ranging from 10-1000 ug/plate using the plate incorporation method. BDNA caused a positive response in all of the bacterial test strains except TA1535, both in the presence and absence of metabolic activation.
In vivo studies have also confirmed evidence of cytotoxicity at each tested concentration (HSDB 5453).
Summary
Because no available chronic studies on the compound were found it is difficult to define a NOAEL value for further risk assessment. The only values found is the LD50 value of 4.1 mg/kg and the value for sublethal endpoint of 3.2 mg/kg bw as there were pronounced detrimental responses at this level.
3.3.8.6 Exposure scenarios
In the migration experiments the substance was below the detection limit of 0.5 µg for product 11A, 12A. This corresponds to 0.5/200=0.0025 µg/cm² (4 hour experiment).
Assuming oral intake with an area of 25 cm² the maximum uptake can be calculated to:
Uptake=0.002*25*(3/4)*1*0.2=0.009375 µg/kg/day.
3.3.8.7 Assessment
Based on the value for sublethal endpoint of 3.2 g/kg and assuming a safety factor of 10000 gives a value of NOAEL of 0.3 mg/kg/day.
Based on this estimate of NOAEL a margin of safety (MOS) of 32000 can be calculated. Thus there is no indication of health effects. But data are seriously missing to perform an actual and reasonable assessment.
3.3.9 Hexaethylene glycol dimethyl ether
3.3.9.1 Identity
| Name | Hexaethylene glycol dimethyl ether |
| CAS-number | 1072-40-8 |
| EINECS number | 214-006-8 |
| Molecular formula | C14H30O7 |
| Molecular structure | |
| Molecular weight | 310.4 |
| Synonyms | 2,5,8,11,14,17,20-heptaoxahenicosane (EINECS name) |
3.3.9.2 Use and function of substance
The substance is assumed to be part of non-reacted poly glycol ether isomers from the main polyurethane foam material in the mattress, in where the breathable structure of flexible polyether foam allows good air circulation (Bayer, 2005).
Other hexaethylene glycols (CAS no. 2615-15-8) are reported to be ingredients in personal care products such as lip liner and toothpastes (National Institute of Health, United States, 2005).
3.3.9.3 Classification and TLV
Hexaethylene glycol dimethyl ether is not included in the List of dangerous substances or the Advisory list of self-classification and there is no Danish threshold limit value.
The Risk phrases for few other different Ethylene glycol ether substances were stated.
Hexaethylene glycol monodecyl ether (CAS no. 3055-96-7, C24H50O7):
R36 R37 R38 R41
Pentaethylene glycol monodecyl ether (CAS no. 3055-95-6, C22H46O6):
R36 R37 R38 R41
Triethylene glycol monodecyl ether (CAS no. 3055-94-5, C18H38O4):
R36 R37 R38 R41
These ethylene glycol ethers are irritating to eyes, the respiratory system and to skin and cause risk of serious damage to the eye (Oxford University, 2005).
3.3.9.4 Detected quantities
The substance is extracted with dichloromethane in the product 13C and measured in a semi quantitative concentration of 2582 µg/g.
3.3.9.5 Health Effects
No data regarding health effects has been found for the substance.
Summary
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
3.3.9.6 Exposure scenarios
Hexaethylene glycol dimethyl ether was not found in the migration experiments.
3.3.9.7 Assessment
Due to the limited data available no assessment is suggested for hexaethylene glycol dimethyl ether.
3.3.10 Tetrapropylene glycol monomethyl ether
3.3.10.1 Identity
| Name | Tetrapropylene glycol monomethyl ether |
| CAS-number | 20324-34-9 |
| EINECS number | |
| Molecular formula | C13H28O5 |
| Molecular structure | 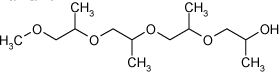 |
| Molecular weight | 264.37 |
| Synonyms | 4,7,10-Trimethyl-2,5,8,11-tetraoxatetradecan-13-ol |
3.3.10.2 Use and function of substance
The substance is assumed to be part of non-reacted poly glycol ether isomers from the main polyurethane foam material in the mattress, in where the breathable structure of flexible polyether foam allows good air circulation (Bayer, 2005).
3.3.10.3 Classification and TLV
Tetrapropylene glycol monomethyl ether is not included in the list of dangerous substances or self classification and there is no Danish threshold limit value.
3.3.10.4 Detected quantities
The substance is extracted with dichloromethane in the product 13C and measured in a semi quantitative concentration of 715 µg/g g divided in two peaks of 622 µg/g and 93 µg/g. Tetrapropylene glycol monomethyl ether is suggested by the NIST chemical identification program as the best match of these peaks.
3.3.10.5 Health effects
No data regarding health effects has been found for the substance.
Summary
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
3.3.10.6 Exposure scenarios
Tetrapropylene glycol monomethyl ether was not found in the migration experiments.
3.3.10.7 Assessment
Due to the limited data available no assessment is suggested for tetrapropylene glycol monomethyl ether.
3.3.11 DEHP
DEHP is not among the substances selected for assessment, but based on the measured concentrations the health effect is evaluated in the following.
The data physico -chemical and toxicological properties for DEHP in this section is based on data in the project (Kortlægning nr. 77, 2006).
3.3.11.1 Identity
| Name | Bis (2-ethylhexyl)phthalate |
| CAS-number | 117-81-7 |
| EINECS number | 204-211-0 |
| Molecular formula | C24H38O4 |
| Molecular structure | 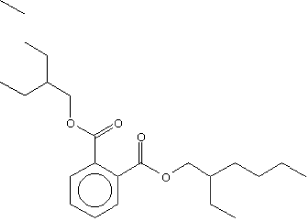 |
| Molecular weight | 390.56 |
| Synonyms | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester Bis(2-ethylhexyl) phthalate DEHP Octyl phthalate Phthalic acid, bis(2-ethylhexyl) ester |
The substance is a colourless, oily liquid. It has a boiling point of 230ºC (Clayton, 1981-1982) and a melting point of -55ºC (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. The solubility in water according to (Yalkowsky, 1992) is 0.285 mg/l at 24ºC.
The partition coefficient Log KOW is determined to be 7.6 (Debruijin, 1989).
Vapour pressure is determined to be 9.6´10-6 Pa (7.23 X10-8 mmHg) at 25ºC (Daubert, 1989).
The substance has a slight odour (NIOSH, 1994).
3.3.11.2 Function of substance
The function of the substance is as plasticizer.
3.3.11.3 Classification and TLV
Bis(2-ethylhexyl)phthalate is included in the List of dangerous substances and classified as:
| Repr.Cat. 2;R60-61 | May impair fertility and may cause harm to the unborn child |
The Danish occupational threshold limit value is 3 mg/m³ (Arbejdstilsynet, 2005).
3.3.11.4 Detected quantities
DEHP has been detected in analysis for phthalates in one of the products, which is 11A a front cover (apron) to a perambulator. DEHP has been detected in semi quantitative analysis of 0.04 mg/g and in migration tests with artificial sweat the concentration was between 0.48 µg/g (in 3.45 g product) and 0.49 µg/g (in 3.80 g product).
3.3.11.5 Health Effects
DEHP is in the process of being evaluated by EU in the Programme on existing chemical substances. Germany is the rapporteur country. The risk assessment report is not yet finalised, but a draft can be found at the ECB homepage (ecb.jrc.it).
Data regarding health effects is included in IUCLID. The following is based on the data sheet, databases in TOXNET and the EU risk assessment above.
Acute toxicity
Tests for acute toxicity on animals show that DEHP is not acute toxic.
LD50 Mouse oral >30,000 mg/kg (WHO, 1992 )
LD50 Rat oral ca. 25,000 mg/kg (WHO, 1992 )
Sub-chronic toxicity
DEHP has been shown to be a weak irritant to mammalian skin when administered topically or intradermally (0.2 mL of an emulsion of 100 g/L) (WHO, 1992).
Chronic toxicity
DEHP is classified as A3 Confirmed animal carcinogen with unknown relevance to humans (ACGIH, 2005).
Studies for carcinogenity in mouse and rats have been found in the dataset for DEHP (IUCLID) with values for effects at approximately 400 mg/kg/day.
The Reference Dose for Chronic oral exposure RfD = 0.02 mg/kg/day. (IRIS)
In the risk assessment on bis(2-ethylhexyl) phthalate (Risk assessment, 2003), a 3 generation rat guideline study is reported. Testicular as well as developmental toxicity was found with increased incidences of small testes, epididymes, and seminal vesicles, as well as cases of minimal testes atrophy. The toxicity was aggravated by exposure during the gestational/pup-period. LOAEL was estimated to 14 mg/kg/day and NOAEL 4.8 mg/kg/day. (Wolfe, 2003)
Summary
Values for carcinogenity for mouse and rats showed effects at approximately 400 mg/kg/day.
In the new draft for risk assessment on DEHP the value of NOAEL is 4.8 mg/kg/day for testicular and developmental effects.
3.3.11.6 Exposure scenarios
There has been no migration experiment for DEHP in the present product. However, in (Kortlægning nr. 77, 2006) was found a migration of 0.01µg/cm² (1 hour experiment) for a product with a concentration of 730 µg/g. As a worst case it is assumed that the migration is the same from product no.11 which have a lower concentration of DEHP=40µg/g.
From this the following maximum oral uptake can be estimated:
Uptake=0.01*25*3*1*0.2=0.15 µg/kg/day
3.3.11.7 Assessment
Based on a NOAEL of 4.8 mg/kg/day and the estimated maximum uptake, the margin of safety (MOS) is 32000 for product 13
The ratio between the RfD value and the uptake is a factor 133.
From these data it is concluded that there seems to be no health risk for DEHP by oral uptake for product 11 (apron for perambulator). However from (Kortlægning nr. 77, 2006), it must be mentioned that the uptake is very dependent on the actual migration conditions, as DEHP has a high solubility in organic solvents but not in water. Therefore the migration may be somewhat different in saliva than under the conditions in (Kortlægnin nr. 77, 2006).
3.3.12 DINP
DINP is not among the substances selected for assessment, but based on the measured concentrations the health risk is evaluated in the following.
3.3.12.1 Identity
| Name | Diisononylphthalate |
| CAS-number | 28553-12-0 |
| EINECS number | 249-079-5 |
| Molecular formula | C26H42O4 |
| Molecular structure | 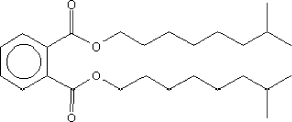 |
| Log KOW | 8.8 (EU risk assessment on DINP, 2003). |
3.3.12.2 Detected quantities
DINP has been found in analysis for product 8A with 144 mg/g (14.4%), in product 8C with 220 mg/g (incl. DIDeP) and in migration tests for product 8A with 0.033 µg/cm² as the highest value.
3.3.12.3 Health effects
A NOAEL of 88 mg/kg/day is found for effects on the liver and kidneys in rats based on a chronic/carcinogenic study. For reproductive organs NOAEL =276 mg/kg/day based on a mouse study (EU risk assessment of DINP, ECB 2003). A lower value of NOAEL 15 mg/kg/day has, however, been suggested in the EU by the scientific committee CSTEE, which is used here.
3.3.12.4 Exposure scenarios
From the highest value of the migration results for product 8A of 0.033 µg/cm² (4 hour experiment), a use of 1 hour, an area of 450 cm² of exposure and 10% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.033*450*(1/4)*0.1*0.2=0.0743µg/kg/day.
The oral uptake will be 9 times less if 100% absorption is assumed by oral contact.
3.3.12.5 Assessment
Based on a NOAEL of 15 mg/kg/day and the estimated maximum uptake, the margin of safety (MOS) is 202800 for product 8A.
From these data it is concluded that there is no health risk for DINP by skin contact or oral uptake for product 8A. However, from (Kortlægning nr. 77, 2006), it must be mentioned that the uptake is very dependent on the actual migration conditions, as phthalates has a high solubility in organic solvents but not in water. In (Kortlægning nr. 77, 2006) the migration for DEHP was increased a factor 8 in water based cream and a factor 1000 in oil based cream. Therefore the concentration on the skin of DINP released from the product may be somewhat higher if creams, moisturizers etc. is used on the baby.
Assuming worst case and a factor 1000 increase of migration in oil based media the uptake will be 74 µg/kg which gives a margin of safety (MOS) of 203. As the estimate is based on data for another product and another phthalate an additional uncertainty factor of a factor of 10 for uptake is applied leading to a combined uncertainty of 10000.
It is concluded that there is a possible minor risk from DINP exposure for product 8A if the baby is in direct contact between the plastic layer and skin and oil based cream or moisturizers is used.
It must also be mentioned that the content of DINP phthalate of 14.4 % is a factor 288 above the allowed limit value of 0.05 wt% for use of phthalates in products for children.
3.3.13 Xylene
Xylene has been measured for product 4C, 5C in climate chamber.
The highest concentration was for product 5C with 0.4 µg/m³.
Regarding uptake by air the ratio between the TLV (109 mg/m³) and the concentration of xylene measured in climate chamber is 272500. Based on this it is assessed that there is no health effects from intake by air for xylene for the tested products.
3.4 Overall Assessment
The health assessment focuses on:
1) What kind of chemical exposure risks that may be found in certain consumer products aimed for use with babies
2) Risks for potential health effects on infants during their critical stages of development
The following conclusions could be drawn from the study:
- All studied baby products contain measurable quantities of more than one compounds classified as hazardous to health and/or environment
- Disposable foam washing cloths contain 2 substances with carcinogenic, sensitizing and reproduction toxic effects. There may be a minor risk of presence of 2-ethylhexanoic acid but a real assessment will require a migration analysis.
- Pillows for baby feeding emit formaldehyde which is carcinogenic and sensitizing. The assessment shows that the worst case migration to skin may contribute significantly to the acceptable daily intake
- Nursing pads contain substances with carcinogenic, sensitizing and reproduction toxic effects which may have a possible health risk in some cases.
- Baby mattresses contain substances with a possible reproduction toxic effect in some cases.
- Some products contain compounds (phthalates) forbidden by Danish and EU-legislation to occur in baby products.
- Some of the products contain several substances with chronic effects which are also found in a number of other products in the society. The effect of such multi-source exposure must be of major concern as babies are a sensitive part of the population and should be protected from chemical exposure.
In the following is shown the results of evaluation of the substances.
Table 3.15 and Table 3.16 show results of health assessments for the selected substances based on the highest uptake found either by uptake by skin or by oral uptake.
Table 3.15 Results of health assessment for selected substances
| Substance | CAS no. | Measured concentrations (sample: µg/g) |
Calculated and estimated uptake for products (sample: µg/kg b.w. per day) |
NOAEL (mg/kg b.w. per day) |
Health effects |
| Hexabromocyclo- dodecane (HBCD) |
25637-99-4 | 4C: 457 5C: 433 |
Not possible (no migration data) | LOAEL approx 0.9 | Developmental, neurotoxic effects |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | 1,2,3: <5 | 1,2,3<18 | LOAEL =30 | Carc3, R40 (carcinogen) R42/R43 (sensitization) |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | 1: 241 2: 55 3: 424 6B: 30 7B: 81 8A: 21 13B: 66 |
1: 4 ¹, 2: 0.9 ¹ 3: 7 ¹ 6B: 7.5 ¹ 7B: 6.8 ¹ 8A: 1.8 ¹ 13B: 16.6 |
25 | R63 possible harm to unborn child |
| Acetophenone | 98-86-2 | 4C :1010 5C: 1572 |
4A :0.3 5A :0.65 7A: 0.2 4C: 1.5 ² 5C: 5.4 ² |
423 | Irritating |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 4C: 278 5C: 493 |
4A,5A,7A<0.17 | 3.2 | Very toxic by skin contact |
| Formaldehyde | 50-00-0 | 4A: 26 5A: 65 7A: 100 |
4A: 25 5A: 115 7A: 24 |
15 | Carc3, R40 (carcinogen) R43 (sensitization) |
| Styrene | 100-42-5 | 4A: none 5A: 9 7A: 14 5C: 679 6A: 20 7B: 79 8B: 28 |
4A,5A,7A <0.17 4C,5C,7C <0.45 |
200 | Irritating |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | 11A : 31 12A: 108 |
11A, 12A< 0.009375 |
0.3 ³ | Few data |
| Hexaethylene glycol Dimethyl ether | 1072-40-8 | 13C:2582 | Not possible: Not found in migration experiments |
No data found | Few data |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | 13C:715 | Not possible: Not found in migration experiments |
No data found | Few data |
1: The estimate has a considerable uncertainty as it is based on migration data for product 13
2: Based on wetting of PS pellets in 1cm depth in an area of 450 cm²
3: Estimate
Table 3.16 Results of health assessment for selected substances continued
| Substance | CAS nr. | MOS ² | Uncertainty factor |
| Hexabromocyclododecane (HBCD) | 25637-99-4 | Not possible | |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | 1,2,3: >1700 | 1,2,3 1000 |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | 1: 6200 2: 27000 3: 3513 6B,7B 3300-3700 8A:>14000 13B :1500 |
1,2,3,6,7,8: 10000 13: 1000 |
| Acetophenone | 98-86-2 | 4A,5A,7A>658000 4C,5C >78000 |
4,5,7 : 3000 |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 4A,5A,7A>19000 | 10000 |
| Formaldehyde | 50-00-0 | 4A: 584 5A: 128 7A: 604 |
100 |
| Styrene | 100-42-5 | 4A,5A,7A >1200000 4C,5C,7C>452000 |
1000 |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | 11A,12A>32000 | 10000 |
| Hexaethylene glycol Dimethyl ether | 1072-40-8 | Not possible | |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | Not possible |
1: The uncertainty is a combination of uncertainty on the NOAEL value and an additional uncertainty on uptake as described in chapter 3.2.
4: Italic type means that MOS <Uncertainty factor which means that there is a possible risk of health effect
Table 3.17 states the results of additional health assessments based on the highest uptake found either by uptake via skin or by oral uptake.
Table 3.17 Results of health assessment for additional substances
| Substance | CAS no. | Measured concentrations (sample: µg/g) |
Calculated and estimated uptake for products (sample: µg/kg b.w. per day) | NOAEL (mg/kg b.w. per day) |
MOS 4 | Uncertainty factor |
| DEHP | 117-81-7 | 11A: 40 | 0.15 1, 2 | 4.8 | 32000 | 10000 |
| DINP | 584-84-9 | 8A: 144000 8C 220000 |
8A: 0.0743 ² 8A:74.3 1,3 |
15 | 8A: 203000 ² 8A:203 ³ |
10000 |
1: Based on a worst case estimate of the migration from another product with higher content of DEHP
2: In artificial sweat. The MOS value is expected to decrease significantly (1-3 decades) if moisturizers and creams are used on baby
3: Estimate for oil like creams and moisturizers
4: Bold means that MOS <Uncertainty factor which means that there is a possible risk of health effect
The main results for each assessed substance is:
- Hexabromocyclododecane (HBCD)
HBCD shows similar chemical and physical properties as well-known persistent organic pollutants and at the same time at a first glance seems to lack toxic action, as were the case with PCBs back in the 1950-ties.
Relevant toxicity studies are lacking for the substance. However, the few studies that have been published so far are enough for being the basis for an immediate strict regulation of the compound. Effects on hormone systems and behaviour at low concentration levels indicate that infants definitely should be protected from ever possible risk of exposure.
HCBD was detected in PS pellets inside product 4,5 but not found in migration tests.
It is rather unlikely that the pillow’s content of the substance will migrate to the surface even if the pillow for feeding babies gets wet.
- Toluene 2,4-diisocyanate (TDI)
TDI was not found quantitatively in foam wash cloths (products no. 1,2,3)
The evaluation is based on NOAEL of 30 mg/kg/day for carcinogenic effects in rats in a subchronic study and an uncertainty factor for NOAEL of 1000, and a worst case assumption where a content corresponding to the detection limit is absorbed through the skin.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects by skin contact:
- There is no risk for products no. 1,2 and 3.
- Data for other foam based products is insufficient for a risk evaluation.
- 2-Ethylhexanoic acid (2-EHA)
2-EHA was found in migration tests or estimated for product 1,2,3,6,7,8,13.
The evaluation is based on a NOAEL of 25 mg/kg/day for maternal and developmental toxicity in a subchronic study on rabbits and an uncertainty factor for NOAEL of 1000.
There is an additional uncertainty factor of 10 on uptake for products nos. 1,2,3,6,7,8.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects by skin contact:
- There is no risk the product no. 13
- There is a possible minor risk for products nos. 1,3, 6 and 7 within the uncertainty in estimating the amount of uptake
In the estimate it is assumed that the observed effects will also have an effect on a baby.
- Acetophenone
Acetophenone was found in migration tests for product 4,5,7.
The evaluation is based on a NOAEL of 423 mg/kg/day and an uncertainty factor for NOAEL of 3000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for acetophenone for the tested products by oral uptake or skin contact.
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of acetophenone measured in climate chamber is 119500. Based on this it is assessed that
- there is no health effects from intake by air for acetophenone for the tested products.
- 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloromethane was found in analysis for product 4,5,7, but in migration tests the concentrations was below detection limits. This limit is used in a worst-case estimate.
The evaluation is based on a NOAEL of 3.2 mg/kg/day and an uncertainty factor for NOAEL of 10000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for acetophenone for the tested products by oral uptake or skin contact
Regarding uptake by air the ratio between the occupational threshold limit value and the concentration of tetrachloroethane measured in climate chamber is 70000. Based on this it is assessed that
- there is no health effect from uptake by air for tetrachloroethane for the tested products.
- Formaldehyde
Formaldehyde was found in migration tests for product 4,5,7.
The evaluation is based on a NOAEL of 15 mg/kg/day for effects on weight gain and histopathy on rats and an uncertainty factor of 100.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for formaldehyde for the tested products by oral uptake or skin contact
- The estimated formaldehyde exposure is very close to the acceptable daily intake for product 5 and 1/6 of the uptake for product 4 and 7 and therefore formaldehyde may contribute significantly to other sources of formaldehyde in the home, when it is assumed that 100% of the formaldehyde is absorbed via the skin.
- Styrene
Styrene was found in analysis for product 4,5,7, but in migration tests the concentrations was below detection limits. This limit is used in a worst-case estimate.
The evaluation is based on a NOAEL of 200 mg/kg/day for effects on red blood cells and liver of beagle dogs and an uncertainty factor of 1000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for styrene for the tested products by oral uptake or skin contact
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of styrene measured in climate chamber is 136000. Based on this it is assessed that
- there are no health effects from intake by air for styrene for the tested products.
- 2-Bromo-4,6-dinitroaniline (BDNA)
BDNA was found in analysis for product 11,12 but in migration tests the concentrations was below detection limits. This limit is used in a worst case estimate.
Because no available chronic studies on the compound was found, an estimate of NOAEL was made based on a value for sublethal end point of 3.2 mg/kg bw and an uncertainty factor of 10000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for BDNA for the tested products by oral uptake or skin contact
- Hexaethylene glycol dimethyl ether
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
Hexaethylene glycol dimethyl ether was not found in the migration experiments.
Due to the limited data available no assessment is suggested for hexaethylene glycol dimethyl ether.
- Tetrapropylene glycol monomethyl ether
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
Tetrapropylene glycol monomethyl ether was not found in the migration experiments.
Due to the limited data available no assessment is suggested for tetrapropylene glycol monomethyl ether.
- DEHP
DEHP was detected in product 11.
The evaluation is based on a NOAEL of 4.8 mg/kg/day and a combined uncertainty factor for NOAEL and the uncertainties in estimating the uptake of 10000. The assessment is based on migration data for another product.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for DEHP for the tested products by oral uptake or skin contact
There is a considerable uncertainty in the estimate and the migration conditions may be somewhat different by oral uptake for a baby than in the in the migration conditions used for the assessment.
- DINP
DINP was found in migration tests for product 8.
The evaluation is based on a NOAEL of 15 mg/kg/day for effects on liver and kidneys in rats in a chronic/carcinogenic study and a combined uncertainty factor for NOAEL and the uncertainties in estimating the uptake of 10000. The assessment is based on migration data for another product and another phthalate.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is a possible risk for DINP for the tested product by skin contact if oil based cream or moisturizer is used on the baby’s skin
Apart from this it must be mentioned that
- the content of DINP in product 8 is above the allowed value for childcare articles for children aged 0-3 years as mentioned later.
- Xylene
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of xylene measured in climate chamber is 272500. Based on this it is assessed that
- there are no health effects from intake by air for xylene for the tested products.
3.4.1 Products
In the following are shown products and the components with identified risk for health effects.
- Product no.1, 2, 3 Disposable foam washing cloths
For the products 1 and 3 there may be a minor risk by skin exposure to 2-ethylhexanoic acid within the uncertainty in estimating the uptake amount.
The risk can be minimised by washing the baby with fresh water and drying with a towel after each use of the foam washing cloths.
- Product no 4, 5 Pillow for feeding baby
For these products the estimated formaldehyde exposure is very close to the acceptable daily intake for product 5 and 1/6 of the uptake for product 4
Therefore formaldehyde may contribute significantly to other sources of formaldehyde exposure in the home. The risk assessment assumes that 100% of the formaldehyde is absorbed via the skin.
- Product no.7, 8 Nursing pads
For product no.7
- the calculated uptake of formaldehyde is 1/6 of the acceptable daily intake and therefore the product is a significant source for formaldehyde exposure.
- Within the uncerntainties there is a possible risk for 2-ethyl-hexanoic acid.
For product no.8
- the content of the phthalate DINP above the allowed limit value of 0.05 wt%
- There is a possible risk for DINP for the tested product by skin contact if oil based cream or moisturizers are used on the baby’s skin.
- Product no. 6 and 13 Baby mattress
For product 6 there may be a minor risk by skin exposure to 2-ethylhexanoic acid within the uncertainty in estimating the uptake amount.
For product no.13 it was not possible to evaluate the health effects from the found content of hexaethylene glycol dimethyl ether and tetrapropylene monomethyl ether.
The content of phthalates was above the allowed limit value of 0.05 wt% in the foam part of product 13.
- Product no. 9, 10 Baby carriers
No risk of health effects was found in the assessment.
- Product no.11, 12 Apron to perambulator
No risk of health effects was found for product 12.
In product 11, the phthalate DEHP was found and although the assessment showed no risk there is a considerable uncertainty in the estimate as the migration is based on an estimate and not the actual migration conditions for oral uptake.
In summation, possible health risks were found for product no.1, 2,3,6,7,8.
Product no. 4 and 5 contributes with a significant part of the acceptable daily intake of formaldehyde
DEHP was found in product 11 with no risk found in the assessment, but with a considerable uncertainty in the estimate regarding migration conditions.
In product no.8,10,13 the content of phthalates in the product or parts of the product was above the allowed limit of 0.05 wt%.
For the products 9,12 , no possible risk of health effects was found and no phthalates with content above allowed limits was present.
3.4.1.1 Alert report to the Danish Environmental Protection Agency during investigation
As it is forbidden to produce, import and sell toys and childcare articles for children aged 0-3 if the products contain more than 0,05 weight percent phthalates, the measured content above this level has been reported to the Danish EPA - referring to "Statutory order no. 151 of March 15. 1999. Banning phthalates in toys for children aged 0-3 and in certain childcare articles etc."
The following has been reported:
The content of DINP:
144 mg/g (14,4%) in the product 8A (pillow for nursing).
3.8 mg/g (0,38%) in the product 8B (foam part of pillow for nursing).
The content of DIBP:
0.76 mg/g (0,076%) in product 10E (Mark on a carrying sling).
The content of DINP+DIDeP:
220 mg/g (22%) in product 8C (Plastic underlayer of a pillow for nursing).
The content of Diundecylphthalat:
4.4 mg/g (0,44%) in product 13C (white foam part of a mattress).
Only the pillow for nursing was assessed as an infringement. The Danish EPA has handled the case and the nursing pillow is no longer on the Danish marked.
Version 1.0 March 2008, © Danish Environmental Protection Agency