Survey of Chemical Substances in Consumer Products, No. 95, 2008
Survey and safety assessment of Chemical substances in artificial nails and nail hardeners
Contents
- 3.1 Literature search
- 3.2 Delimination of the survey
- 3.3 Survey
- 3.4 Ingredients of the products
- 3.5 Selection for analysis
- 4.1 Quantification of formaldehyde in nail hardeners
- 4.2 Quantification of acrylic compounds in products for manufacture of artificial nails
- 4.3 Screening for ingredients in products for manufacture of artificial nails
- 4.4 Quantification of benzophenon or azobenzen
- 4.5 Selection of substances for safety assessment
- 6.1 Safety assessment of substances in nail hardeners
- 6.2 Safety assessment of substances in artificial nails
7 Exposure and risk assessment
- 7.1 Exposure and risk assessment for consumers using nail hardeners
- 7.2 Exposure and risk assessment for consumers using artificial nails
Preface
The project ”Survey and health assessment of chemical substances in artificial nails and nail hardeners” has been carried out in period April 2007 until November 2007.
The report describes the result of the project including a survey of the products and chemical analyses and health assessment of a number of selected products.
The survey of the project includes the products found on the Danish market within the categories nail hardeners and products for building up artificial nails. Selected products have been analysed for selected ingredients. The potentially allergenic ingredients found in higher concentrations in the products were selected for a health assessment and subsequent risk assessment.
The project has been carried out by DHI, Centre for Environment and Toxicology and is managed by Dorthe Nørgaard Andersen, Head of Projects, who is also responsible for the survey and the health assessment. Jørgen Andersen, Lantmännen Analycen is responsible for the performed analyses. Jette Rud Larsen, Consultant, Hanne Sørensen, Project Assistant have both contributed to the survey, and Karl-Heinz Cohr, Chief Toxicologist, has contributed with sparring during the process and quality assurance of the project.
The project is financed by the Danish Environmental Protection Agency.
The project was followed by a Reference Group consisting of:
Magnus Løfstedt, Danish Environmental Protection Agency (Chairman of the Reference Group)
Elisabeth Paludan, Danish Environmental Protection Agency
Dorthe Nørgaard Andersen, DHI
Summary and conclusions
The objective of this survey was
- To make a survey of allergy causing chemicals in nail hardeners and products for manufacture of artificial nails to be sold to private persons from the retail trade, the internet and beauty shops.
- Analyse selected products for their content of e.g. acrylic compounds
- To perform health risk assessment of selected chemicals found during the analyses
- To suggest a safe concentration of formaldehyde in nail hardeners.
A wide selection of products was purchased. Nail hardeners were purchased from the retail trade, while products for artificial nails could only be purchased from the internet. Except from this was nail tips that can be bought from the materialists etc. From a health perspective point of view, these products were assessed as not interesting and are therefore not included in this survey and instead acrylic nails (liquid and powder) and gel nails were focused on. The glues for fastening of these nail tips were however also included in the project.
The purchased products were examined and their labelling was assessed. Many of the checked products appeared not to be in accordance with the demanded labelling requirements. Most significant was the insufficient ingredient declaration and the lacking directions for use. As shown in this survey, directions for use are imperative especially for artificial nail products to secure a correct application of the products on the nails, which is extremely important to avoid allergy health risks.
Products for building up artificial nails can be divided into different systems. In this project products for building up acrylic nails (liquid and powder) - one of the most used systems - have been examined. Products for building up gel nails have also been analysed during the project. Only selected products of each system were included. They were product types considered to have the biggest content of acrylates, e.g. acrylic powders and belonging acrylic liquids.
Other product types (topcoat, primer, etc.) which are included in parts of the process were not included in the survey.
Product from the different purchased product groups were selected for analysis. Nail hardeners were analysed for free formaldehyde, whereas products for artificial nails were analysed for the acrylates:
- Triethyleneglycol diacrylate (CAS no. 1680-21-3)
- 2-Hydroxypropyl acrylate (CAS no. 999-61-1)
- 2-Hydroxyethyl acrylate (CAS no. 818-61-1)
- Ethyl acrylate (CAS no. 140-88-5)
- Ethylene dimethacrylate (CAS no. 97-90-5)
- 2-Hydroxypropyl methacrylate (CAS no. 27813-02-1)
Toxicological investigations of selected substances (formaldehyde, 2-hydroxyethyl acrylate 2-hydroxypropyl acrylate, 2-hydroxypropyl methacrylate and ethylene dimathacrylate) were performed and the critical effects of the substances were found. Based on the toxicological investigations it is assessed that the critical effects of the main part of the examined substances is allergy. Besides, a review of the literature showed that allergy after use of artificial nails and nail hardeners is described.
A realistic worst-case scenario was set up for nail hardeners and the risk of systemic effects when using nail hardeners was calculated. The calculation showed that there is no risk of systemic effects when using nail hardeners containing the permitted amount of formaldehyde which is 5%. As formaldehyde can evaporate during the process, it is recommended to let in some air after application of nail hardeners to limit the exposure most possible by inhalation and deposition of vapour on the skin.
Formaldehyde may cause allergy and is classified as allergenic. Elicitation of allergy has been observed in formaldehyde sensitive persons in concentrations down to 0.05% formaldehyde, while induction of allergy in animal studies is seen at about 0.4 – 0.96% formaldehyde. The greatest risk of allergy is expected to develop if the nail hardener comes into contact with the skin. Exposure of the skin through application of nail hardener is expected to be limited, but application of nail hardener on the cuticle and the skin around the cuticle is hazardous and may cause allergic reactions. Irritation and allergy may also occur, if nail hardeners are applied on broken nails. In this case, formaldehyde may come into contact with the tissue under the nail and the risk of irritation and allergy will increase.
On the basis of the material generated in this project the permitted concentration of 5% formaldehyde in nail hardeners is assessed to constitute a risk of allergy induction in healthy persons and a high risk of elicitation of allergy in formaldehyde sensitive persons. Based on the literature presented in this report, a maximum concentration of 0.01% formaldehyde in nail hardeners is assessed to be a safe concentration in relation to the risk of allergy when applied both by healthy persons and persons already suffering from allergy. If the objective is to prevent healthy persons from developing formaldehyde allergy, a higher limit of concentration may be argued. However, this is difficult to set as there is no existing clear limit value for induction of formaldehyde allergy for this type of exposure. Formaldehyde is permitted as preservative in cosmetic products in a concentration of 0.2%. It can be argued that the concentration in nail hardeners as a minimum should not exceed this limit to diminish the risk of induction of allergy in healthy persons. A warning to already sensitive persons should appear on the product about the content of formaldehyde in a concentration above 0.05%. The Scientific Committee of Consumer Products (SCCP) in EU has been requested to assess a safe limit value for the content of formaldehyde in nail hardeners. This survey showed that several nail hardeners on the Danish market contains below 1% formaldehyde (4 out of 6 tested). Therefore, there are alternatives to the products containing larger amounts of formaldehyde. One product of the six tested only contains 0.01% formaldehyde.
The health assessment of product for building up artificial nails showed that there is not health risks of systemic effects related to the use. On the other hand, the risk of contact allergy by applying these products is assessed to be significant. This assessment was due to the amount of acrylates found in the products compared with the allergenic potential. Skin contact will result in a risk of contact allergy, as the products contain allergenic acrylate monomers in concentration of up to 15%. This applies specifically for the gel nails that contain high concentrations (up to 8%) of particularly 2-hydroxyethyl acrylate, which is considered to be the ingredient with the highest allergenic potential of the tested ingredients. In patch tests with acrylate sensitized persons elicitation of allergy at concentrations of 0.1% 2-hydroxyethyl acrylate was observed. The acrylic nails (liquid and powder) appeared to constitute a lower risk of contact allergy, as these products contain lower concentrations of the most allergy potent acrylates. However using the products still constitutes a significant risk of allergy.
The conclusion of the survey is that the products contain chemical substances in concentrations that constitute a significant risk of contact allergy. It has also been clarified how important the application of this type of project is. It is suggested that professional and competent personnel build up artificial nails instead of the consumer. This survey project does not comprise the working environment for personnel in beauty clinique’s etc., but it is important to emphasize that these products contain substances that may give rise to concern within the working environment. Many of the purchased products are marketed for professional use, but it is assessed that the private consumer will not have difficulty in obtaining the products for private use. However, the risk of contact allergy by use increases considerably, if the products come into contact with the skin. It is also assessed that the risk of skin contact decreases with the increasing experience in building up artificial nails.
The conclusions of the project are the following recommendations to the consumer:
Recommendations to the consumers
To reduce the amount of formaldehyde that is inhaled and deposited on the skin, the room should be well aired when you use nail hardeners
Be careful when applying nail hardener so that it only covers the nail and not the skin. This decreases the risk of formaldehyde.
Do not use nail hardeners containing formaldehyde on damaged nails. If the nail is broken, the ingredients can come into contact with the tissue under the nail and increase the risk of formaldehyde allergy.
Use qualified nail technicians for building up ”powder + liquid nails”, ”gel nails”, ”wrapping nails” and ”dip on nails”. These products should not be applied by private consumers, because the exposure of allergenic substances increases considerably
1 Introduction
Importers of materials for production of artificial nails report an increasing interest in these products. If we follow the tendencies in USA where there is said to be more nail cliniques than hair dressers, artificial nails may gradually be a commonly used cosmetic product also in Denmark. Already today several nail cliniques and hair dressers sell and fix artificial nails. A simple search for nail cliniques on the internet showed 70 cliniques within an individual chain. Besides, there are several do-it- yourself products on the market.
The extent of the damages caused by using nail hardeners and artificial nails is not known, but The National Allergy Research Centre for Consumer Products assumes that a couple of cases are reported per year. They also assume that mainly the serious allergy cases are reported and that the actual number of cases is higher. Damage on the nails is described in literature, including allergenic reactions, irritation and bacteria attacks.
As a result of the above, a survey of artificial nails and nail hardeners has been carried out on the retail market and the professional market respectively. The project examines and describes the type of products offered on the Danish market and to which chemical substances the user is exposed by using them. Due to the considerable range of products an examination of the complete market was not possible and instead individual selected products were selected.
Products for building up artificial nails often contain acrylic powder in the form of ethyl methyl (metha) acrylate polymers and different types of other methyl (metha) acrylate polymers and small amounts of monomer methyl (metha) acrylate and ethyl (metha) acrylate. Investigations in scientific papers and contact with suppliers show that today there are four methods for building up artificial nails each containing a different chemical substance. There is also a fifth method. Here, the artificial nails are pre-manufactured nail tips to be fastened with cyanoacrylate containing glue. Some of the methods use several components and besides the acrylic part also components for pre- and after treatment, such as for instance primers and top coat. The components with the highest considered concentration of acrylates have been selected for the survey, as these types of substances are considered most relevant to investigate from a health point of view. The methods for production and usage scenarios are described in Chapter 3.
A number of nail hardener products, of which most of them are found in the retail shops, has been purchased. Several nail hardeners contain formaldehyde but also other reinforcing ingredients have been seen. The different products are described in Chapter 3.
The products are comprised by the Cosmetics Directive and must be labelled as cosmetics. The labelling has been reviewed and for several products considered insufficient.
2 Objective
The overall objective of the project is to create e general view of the possible health risk involved using artificial nails and nail hardeners. For this purpose we have composed three intermediate projects:
- A survey of the presence of chemicals with a sensitisation potential in artificial nails, in glue for artificial nails and in nail hardeners sold to private persons in the retail shops, through the internet as well as in products from beauty cliniques.
- A part in which selected products for artificial nails are analysed for their content of for example acrylic compounds and nail hardener products for content of formaldehyde.
- A safety assessment of acrylic compounds, formaldehyde compounds, formaldehyde and other potentially problematic chemicals in the products including – if possible - a suggestion for a concentration which is safe to the user.
3 Survey
- 3.1 Literature search
- 3.2 Delimination of the survey
- 3.3 Survey
- 3.4 Ingredients of the products
- 3.5 Selection for analysis
3.1 Literature search
By literature searches in public databases on the internet ((ToxLine, PubMed, Google) articles describing health problems caused by the use of nail hardeners and artificial nails have been searched. The following search terms were used: ’Acrylates and artificial nails’, ’artificial nails and sensitisation’, ’nail extenders’ nail hardener’ ’powder and liquid’. This search identified literature describing effects such as allergy, irritation and bacteria infections by using artificial nails and nail hardeners. Many of the articles showed that use of artificial nails often causes allergy and irritation, whereas formaldehyde is mentioned in relation to nail hardeners. Based on the result of the literature search, the products that are potentially most harmful to the consumer were pointed out.
3.2 Delimination of the survey
There are a great number of products for manufacture of artificial nails in Denmark, whereas the selection of nail hardener products is more limited.
On a visit to a supplier, the present methods for fixing artificial nails were presented to us. The producer emphasized that the product for building up artificial nails on the natural nail hardeners is only for professional use and is only for sale to and thus to be used by educated, professional nail technicians. Due to this, the consumer will not themselves be able to use these products but can only get in touch with them when visiting nail cliniques and hair dressers. However, DHI assesses that the private consumer on the internet has access to professional products for use at home.
The education of nail technicians comprises not only techniques and skills but also hygiene and health aspects. Today there is no authorised education of nail technicians in Denmark.
There are four difference methods for building up artificial nails:
- Powder + liquid nails (the nails are built up by using a mixture of acrylic powder and acrylic liquid)
- Gel nails (the nails are built up by using one-component product in the form of a thick liquid that hardens in UV light).
- "Wrapping nails” ( the nails are built up by glass fibre and silk nails wetted with glue/resin)
- Dip on nails (acrylic powder and glue/resin are mixed for building up the nail hardeners)
When building up artificial nails, a number of different products are required to obtain the desired result. Besides acrylic powders, gels and liquids for example primers and degreasing products for cleaning and ready making of the natural nail are required before affixing or building up the artificial nail. Also oil or fat for protection of skin and nail cuticle, and top coat used for the last shiny surface, often added UV-filter is used.
On the basis of the results of the literature search we chose to concentrate the survey of the artificial nails on the components mainly containing acrylates. From collected safety data sheets for some of the acrylic powder-, acrylic liquid- and acrylic gel products it could be established that all these products contain from 60% to more than 90% acrylates. Acrylic powder and gels are sold in many different colours with the possibility of inserting tinsel and jewels into the nails. In the survey we have chosen only to use the colourless variants of the selected powders and gels.
There is also a fifth method besides the above mentioned: The artificial nails can be pre-manufactures nail tips which are glued on the nails. These nails and the glue are sold in many retail shops. The pre-manufactured nails are not included in the survey, as they are not assumed to pose risks to the users. Glues for fixing nail tips are however included.
Searches on the internet showed many suppliers of the products. We have endeavoured to select products from big as well as smaller suppliers and we have recognized that it is not possible to cover the complete market.
3.3 Survey
In May and June 2007 we carried out a survey of nail hardeners and products for manufacture of artificial nails sold on the Danish retail market and via the internet from nail cliniques and importers of nail products.
3.3.1 Products on the retail market
On the retail market nail hardeners and glues for fixing artificial nails were identified in the following types of stores:
- Super market/chains
- Department stores
- Drugstores
- Internet shops
No products for building up artificial nails were found in the retail trade during our survey. Subsequently, however, complete start sets have been introduced to the market, e.g. on the internet directly to the consumers. Typically these start sets contain whatever is needed to build up artificial nails and an instruction.
During the survey, most nail hardeners were found in the retail market, and only one single product was purchased directly from an importer. Just as nail lacquer, all nail hardeners are sold in small bottles with a brush in the lid. The package and the label of the hardener mainly cater for women. That the product is a nail hardener and not a nail lacquer is indicated on the text of the packing.
In addition, several of the products had a colour, which is normally not used for nail lacquers, and most products had a thin, spirit like consistency which is not found in nail lacquers.
We have purchased seven nail hardeners for the project, one of these from an agent for professional users.
Table 3.1 Nail hardeners, purchased in type of store, volume and price
| Nail hardener | Volume | Type of store | Price DKK incl. VAT |
Price DKK incl. VAT pr. ml |
| 1 | 8 ml | Drugstores | 89.95 | 11.24 |
| 2 | 8 ml | Drugstores | 89.95 | 11.24 |
| 3 | 10 ml | Department stores | 84.00 | 8.40 |
| 4 | 8 ml | Super markets/chains | 39.50 | 4.94 |
| 5 | 5 ml | Drugstores | 94.95 | 19.00 |
| 9 | 12 ml | Drugstores | 59.95 | 5.00 |
| 20 | 15 ml | Internet shop | 42.50 | 2.84 |
The usage scenario for nail hardeners can be described as being identical with the scenario for nail lacquer.
The products contain volatile inhalable solvents. The products must be painted on the nail and nail band and skin may come into contact with the products.
According to the directions for use of some of the nail hardeners, it is only permitted to use them for a limited period of time, for instance once or twice a week, or in other cases as a two-, three- or four weeks cure with application for two or three days and lotion day 4 etc. The product is intended to remain on the nail until next day, when it is cleaned and renewed. According to the directions for use, the treatment with several products should be stopped or reduced to for instance 1-2 times a week or less when the nail has hardened.
Five glues for fixing the nail tips have been purchased.
Table 3.2 Glues for nails, purchased in type of shop, volume and price
| Glue | Volume | Type of shop | Price DKK incl. VAT |
Price DKK incl. VAT pr. g or ml |
| 6 | 3 g | Supermarkets/chains | 39.50 | 13.16 |
| 7 | 3 g | Supermarkets/chains | 25.00 | 8.33 |
| 8 | 3 g | Drugstores | 29.95 | 14.98 |
| 19 | 2 g | Internet shops | 32.50 | 16.25 |
| 24 | 15 ml | Internet shops | 61.25 | 4.08 |
Glues for artificial nails are all sold in small packing’s of 2 g until 15 ml, and three of the five purchased glues could be purchased in the retail stores. The glues for professional use are also called “resin”. The products contain cyanoacrylates that stick in few seconds.
The usage scenario for the glues can be described as follows:
The glues are applied on the nail surface, involving inhalation of fumes and risk of contact with nail bands and skin. The glues are applied for fixing pre-manufactured nail tips or repair of splitted nails. Warnings are given in all five products that the glues stick within seconds.
3.3.2 Products for professional use
By nail products for professional use is meant products only used for manufacture of artificial nails in nail cliniques or hair dressers. Contact to suppliers of products considered to have an important market share of artificial nails for professional use in the nail cliniques is endeavoured.
The selection of the purchased products is based on the components containing most acrylates. Based on product data sheets from the nail producers and literature, the following products have been selected and purchased from the suppliers:
Acrylic powder
Acrylic liquid
Acrylic gel
Glue (resin)
7 acrylic powders and 7 acrylic liquids were purchased for the survey and all the products were purchased from the internet shops.
Table 3.3 Acrylic powder for nails, volume and price
| Acrylic powder product | Volume | Price DKK incl. VAT | Price DKK incl. VAT per. g |
| 10 | 20 g | 59.00 | 2.95 |
| 13 | 28 g | 137.50 | 4.91 |
| 16 | 22.68 g | 148.75 | 6.56 |
| 21 | 30 g | 86.25 | 2.88 |
| 25 | Not informed | 61.00 | - |
| 28 | 28 g | 234.00 | 8.36 |
| 31 | 30 g | 137.50 | 4.58 |
The greater part of the acrylic liquids were packed in plastic cans. Only one of the seven products was packed in a glass bottle, and this product was the only that did not emit a strong smell. It will be necessary to store the plastic cans with the acrylic liquids in a well ventilated room.
Table 3.4 Acrylic liquids for nails, volume and price
| Acrylic liquid product | Volume | Price DKK incl. VAT | Price DKK incl. VAT per. g |
| 12 | 120 ml | 69.00 | 0.58 |
| 15 | 118 ml | 280.00 | 2.37 |
| 18 | 118 ml | 325.00 | 2.75 |
| 23 | 30 ml | 98.75 | 3.29 |
| 27 | 30 ml | 49.50 | 1.65 |
| 30 | 118 ml | 324.00 | 2.75 |
| 33 | 100 ml | 236.25 | 2.36 |
Also the gel products are found in different colours. Seven different copies of the variant were purchased for the survey.
Table 3.5 Acrylic gel for nails, volume and price
| Acrylic gel product | Volume | Price DKK incl. VAT | Price DKK incl. VAT per. g |
| 11 | 0.5 oz = 14.2 g | 69.00 | 4.86 |
| 14 | 14 g | 248.75 | 17.77 |
| 17 | 15 g | 248.75 | 16.58 |
| 22 | 30 ml | 248.75 | 8.29 |
| 26 | Not indicated | 189.00 | - |
| 29 | 15 ml | 250.00 | 16.67 |
| 32 | 30 g | 323.75 | 10.79 |
Usage scenarios and ways of application for Powder and Liquid nails:
Acrylic powder and acrylic liquid are mixed and placed on the centre of the natural nail leaving few millimetres to cuticle and skin. The nail tips must often be extended during the operation and special forms or templates are used. The nails are built up in several turns and the process may last many hours. Cuticle and skin may come into contact with the products. Volatile acrylic fumes may be inhaled. During the process, the nails are cut and filed into the intended shape, and this may cause inhalation and contact with fine acrylic dust.
Usage scenarios and ways of application for Gel nails:
Acrylate-containing gel is placed on the centre of the natural nail leaving few millimetres to cuticle and skin. The nail tips must often be extended during the operation and for this purpose are used special forms or templates. The nails are hardened using UV-light. The nails are built up in several turns (up to 10 times) and this process may last many hours. Cuticle and skin may come into contact with the products. Volatile acrylic fumes may be inhaled. During the process, the nails are cut and filed into the intended shape and this may cause inhalation and contact with fine acrylic dust.
Usage scenarios and ways of application for wrapping nails:
Pre-manufactured nail tips are affixed to the natural nails, and a piece of silk or glass fibre is placed on the nails. The glass fibre or the silk must be fitted to the surface of the nails. After this, a layer of glue is placed on the nails to fasten the glass fibre/silk nails pieces. The nails are built up with the glue so that the glass fibre/silk nail pieces are totally embedded in the glue. Cuticle and skin may come into contact with the products. Inhalation of acrylic fumes may occur while the glue dries. Surfaces and sides are grinded in, and inhalation and of and contact with dust may occur.
Usage scenarios for Dip on nails:
These nails are produced by cutting the nail tips to the desired shape and by gluing them on. After that, a layer of glue is placed on the nail whereupon it is dipped in acrylic powder. The remaining acrylic powder is brushed away, and the process is repeated. The process is terminated by a layer of glue. Cuticle and skin may come into contact with the products. Inhalation of acrylic fumes may occur while the glue dries, and inhalation of and contact with dust may occur.
3.4 Ingredients of the products
3.4.1 Ingredients in nail hardeners
Nail hardeners can consist of several principles. In the first place, formaldehyde can act as intensifier as the substance can polymerise keratin in the nail plate. Other intensifying components, such as for instance different types of polymers may also be contained.
Table 3.6 Identified hardening components in nail hardeners indicated on the labels
| Ingredients | CAS No. | Products in which the ingredients is contained Product number |
| Formaldehyde | 50-00-0 | 1, 2, 3, 4, 5, |
| Nitrocellulose | 9004-70-0 | 1, 2, 3, 4, 9, 20 |
| Tosylamide/Formaldehyde resin | 25035-71-6 | 4, 20 |
| Acrylate copolymer | 25035-69-2 | 3, 4, 9 |
| Tosylamide/epoxy resin | Not indicated | 1, 2, 9, |
| Adipic Acid/Isophthalic Acid/ Neopentyl Glycol/ Trimethylopropane copolymer | 25950-34-9 | 1, 2 |
| Styrene/Acrylate copolymer | 9010-92-8 | 1, 2 |
| Adipic Acid/ Neopentyl Glycol/ Trimetllitic Anhydride copolymer | 28407-73-0 | 9 |
| Phthalic Anhydride/ Trimellitic Anhydride/ Glycols copolymer | Not informed | 3 |
| Polyvinyl Butyral | 63148-65-2 | 20 |
| Phthalic Anhydride/ Glycerin/ Glycidyl Decanoate copolymer | Not indicated | 9 |
| Trifluoropropyl Dimethicone | Not indicated | 9 |
Besides, the following substances have been identified in the survey of nail hardeners, cf. the INCI-declarations:
Butyl Acetate, Ethyl acetate, Heptane, Isopropyl Alcohol, Acetyl Tributyl citrate, Stearalkonium Hectorite, Dromethizole, Camphor.
Formaldehyde which can act as intensifier and/or preservative can be added directly and indirectly. Indirectly it is added in the form of a substance that can liberate formaldehyde after addition. Formaldehyde liberating substances may be benzyl hemiformal, 2-bromo-2-nitropropane-1,3-diol, 5-bromo-5-nitro-1,3-dioxane, diazolidinyl urea, DMDM hydantoin, imidazolidinyl urea, methenamine, paraformaldehyde, sodium hydroxymethylglycinate and quaternium-15.
3.4.2 Ingredients in nail hardeners
The declaration list on the products or material safety data sheets from the suppliers have been reviewed to identify which acrylates are contained in acrylic powders, –liquids, - gels and glues. The substances have been identified and listed in table 3.7 below.
Table 3.7 Identified acrylates in the products
| Ingredients | CAS No. | Products in which the product is contained Product number |
| Cyanoacrylate | Not indicated | 6 |
| Ethyl cyanoacrylate | 7085-85-0 | 7, 8, 19, 24 |
| Polymethyl Methacrylate | 9011-14-7 | 7, 19, 21 |
| Polyethylene Glycol Dimethacrylate | 109-17-1 | 7 |
| Ethyl Methacrylate | 97-63-2 | 15, 18, 23 |
| Acrylate copolymer | 25685-29-4 | 13 |
| Glycol HEMA-Methacrylate (= Ethylene Glycol Dimethacrylate esters) |
97-90-5 | 15 |
| HEMA (=2-Hydroxyethyl Methacrylate) | 868-77-9 | 15 |
| Polyethylmethacrylate | 9003-42-3 | 16, 21 |
| Methacrylate copolymer | Not indicated | 16 |
| Urethane Methacrylate Oligomers | Not indicated | 17 |
| Methacrylate Monomers | Not indicated | 17, 18 |
| Polyurethane acrylate oligomer | Not indicated | 26 |
| Trimethylopropane Trimethacrylate | Not indicated | 32, 33 |
| 2-EthoxyethylMethacrylate | 2370-63-0 | 33 |
| Triethyleneglycol Dimethacrylate | 109-16-0 | 33 |
| 2-Hydroxypropyl Methacrylate | 27813-02-1 | 33 |
Besides the acrylates mentioned in table 3.7, it appears from safety data sheets or declaration lists available to us that the following substances are contained in acrylic powders:
Benzoylperoxide (polymerisation initiator). The powders may also contain silica (filler) and titanium dioxide (pigment).
In acrylic liquids:
Benzophenone (UV-filter), dimenthyltolylamine, dimethylparatoluidine (catalysator), MeHQ (stabilisator).
In acrylic gels:
Hydroxycyclohexylphenylketone, benzophenone (UV-filter), benzoyl isopropanol (polymerisation initiator), calcium pantothenate (vitamin), silica (filler). Pigments in the form of blue or violet colours may be added.
Glues:
Boron trifluoride (strength), BHA (antioxidant), citric acid (pH-regulator), 2-bromo-2-nitropropane-1,3-diol (preservative), hydroquinone (stabilisator), silica (filler), alcohol (solvent), sulfur dioxide.
The substances are added in amounts below 1% and act as catalysators and stabilisators in the polymerisation process. The remainder in the artificial nail is therefore expected to be extremely limited.
3.5 Selection for analysis
Based on the survey, products both within nail hardeners and artificial nail products (liquid and powder and gel nails) were selected for analysis, and it was determined to include both product systems (liquid powder and gel nails). In cooperation with the Danish Environmental Protection Agency, it was determined which substances to analyse for in the products concerned (See Chapter 4).
4 Chemical Analyses
- 4.1 Quantification of formaldehyde in nail hardeners
- 4.2 Quantification of acrylic compounds in products for manufacture of artificial nails
- 4.3 Screening for ingredients in products for manufacture of artificial nails
- 4.4 Quantification of benzophenon or azobenzen
- 4.5 Selection of substances for safety assessment
4.1 Quantification of formaldehyde in nail hardeners
In cooperation with the Danish Environmental Protection Agency, a number of nail hardeners were selected to be analysed for content of formaldehyde. Six of the seven purchased nail hardeners were analysed. As two of the products were of the same brand, only one of them was selected. Thus, all six different brands were represented in the analysis.
4.1.1 Quantitative GC-MS method of analysis
Before breaking the sealing of the sample, the sample is heavily shaken. The next step is to weigh out quickly approx. 1 g to a measuring flask half filled with dichlormethane. The measuring flask is filled up to the marking dicloromethane.
The samples are dissolved/extracted during a period of approx. 2 hours. After extraction/dissolution, part of the dichlormethane phase is transferred to GC-vial, whereupon it is ready for analysis. In connection with very viscuous extracts, the sample is appropriately diluted before analysis (typical 10*). The dichloromethane used for the analyses is also used to dilute the sample.
Also analyses of blind samples of the dichloromethane used for the analyses are carried out.
The extract is analysed on GC-MS-SIM. The following method parameters are used:
Oven: Starting temperature 40o C to be kept for 3 minutes, then a slope of 30 o C/minute until 300 o C has been reached. The final temperature is kept for 2 minutes.
Injector parameters: 280 o C, split less injection. 2µl injected. Starting pressure 6.0 psi.
The method is an internal method used by Analycen. The reference number for internal method is KG.18.
Calculation of the content is carried out based on internal standards. The detection limit of the analysis method is determined to be 10 mg/kg, and the analyses uncertainty is 10%.
By this method both the content of free formaldehyde and the content of formaldehyde donors were determined.
4.1.2 Results in % of the GC-MS analyse
The quantified content obtained from the performed GC-MS analysis is illustrated in table 4.1.
The analyses show that 2 of the analysed 6 products contain more than 2% formaldehyde, whereas the remaining 4 products all contain between 0.01 and 0.8% formaldehyde. The formaldehyde of the two products with the lowest content of formaldehyde (product no. 9 and 20) has not been declared.
Table 4.1 Content of formaldehyde (%) in selected nail hardeners analysed in double determination. The indicated content is the average of the double determination. The difference between the two determinations varies less than 3% for all samples except for product identification 20 that varies 9%. Besides it is indicated for each product if formaldehyde is declared (√) or not (-). The detection limit is 0.001%.
| Product identification | Formaldehyde (%) | Declared product |
| 1 | 2.37 | √ |
| 3 | 0.80 | √ |
| 4 | 0.69 | √ |
| 5 | 4.05 | √ |
| 9 | 0.01 | - |
| 20 | 0.19 | - |
4.2 Quantification of acrylic compounds in products for manufacture of artificial nails
In cooperation with the Environmental Protection Agency, a number of nail products were selected for analysis of content of different acrylic compounds. The products were selected within the categories gel nails, acrylic powders for building up acrylic nails and liquids that are also applied for formation of acrylic nails (see Chapter 3). All three types of products were selected in order to be able to compare and evaluate the degree of health risk of the different systems.
During the analyses, the monomers are focused on. In accordance with the safety data sheets, the artificial nail products contain different forms of polymers. Polymers are not particularly interesting with respect to safety, as their toxicity is considered low due to their big size, and very often the data found are very few. On the other hand, polymers may contain small amounts of monomers (the starting material of the polymers) that may have a high toxicity. Therefore the focus in the analyses were the monomers in order to illustrate the levels of monomers in the different products.
In cooperation with the Environmental Protection Agency it was determined to test for the following acrylate monomers in the selected products:
- triethyleneglycol diacrylate (CAS nr. 1680-21-3)
- 2-hydroxypropyl acrylate (CAS nr. 999-61-1)
- 2-hydroxyethyl acrylate (CAS nr. 818-61-1)
- ethyl acrylate (CAS nr. 140-88-5)
- ethylene dimethacrylate (CAS nr. 97-90-5)
- 2-hydroxypropyl methacrylate (CAS nr. 27813-02-1)
4.2.1 Method of quantitative GC-MS analysis
For description of methods, please see Chapter 4.1.1.
The content was calculated based on internal standards. The detection limit is 10 mg/kg and the uncertainty margin 10%. All analyses were performed in double determination.
4.2.2 Results in % of the GC-MS analysis
The content of acrylic compounds quantified in the GC-MS analysis is shown in table 4.2 – 4.3.
Tabel 4.2 Content of acrylic compounds (%) in selected products for gel nails. The indicated content is the average of a double determination. (-) indicates that the substance is below the detection limit of 0.001%.
| Product identifi- cation |
Acrylic compound | |||||
| Triethylene- glycol diacrylate |
2-Hydroxy- propyl acrylate |
2-Hydroxy- ethyl acrylate |
Ethyl acrylate | Ethylen- glycol dimetha- crylate |
2-Hydroxy- propyl methacrylate |
|
| 11 | - | 0.041 | 5.35 | - | 0.020 | - |
| 14 | - | - | 3.05 | - | - | - |
| 17 | - | - | - | - | 0.007 | 1.45 |
| 22 | 0.004 | 0.062 | 6.70 | - | 0.041 | - |
| 26 | 0.024 | 0.063 | 8.25 | - | - | - |
| 29 | 0.011 | 0.865 | 5.60 | - | 0.011 | - |
| 32 | - | - | 4.45 | - | - | - |
The analyses for acrylic compounds show that the amount of tested compounds contained in the products for production of artificial nails (acrylic nails and gel nails) is limited. Some products contain 10% of one of the 6 analysed substances, but in all cases only one of the substances is contained in the product in such a great magnitude.
Generally, it can be seen that the products for formation of gel nails contain several of the tested acrylic compounds. The analysis shows that the products for manufacture of gel nails generally contain the substance 2-hydroxyethyl acrylate in the highest concentrations (3-8%) and only one product out of seven tested does not contain the substance (table 4.2). Unlike the other products, this product (product number 17) contains mainly 2-hydroxypropyl methacrylate. The other tested acrylic compounds are only found in small amounts (< 0.8%) in the tested products.
Except for a small amount of ethylene dimethacrylate found in product no. 25 (0.015%) (table 4.3), none of the tested acrylate compounds were found in the powders for manufacture of acrylic nails. This is somewhat surprising but the reason may be that the content of this type of products are mainly polymers, whereas the content of monomers in the products are low. Based on safety data sheets on the individual products, the ingredients have been identified as different types of polymers such as polyethyl methacrylate, polymethyl methacrylate orcopolymerer of ethyl and methyl methacrylate. By screening the ingredients in the powders, butyl methacrylate has most probably been found in 6 of the seven analysed products (Chapter 4.3.2 – table 4.5). However, no methyl methacrylate and ethyl methacrylate have been found in the powders. This indicates that there is only a very small amount or none of these monomers in the product.
In the liquids which together with the powders are used for manufacture of acrylic nails, the amount and type of acrylic compounds vary to some extent between the products. For two of the products, the content of 2-hydroxypropyl methacrylate is found to be approx. 10%, while another product contains 15% ethylene dimethacrylate. From the Safety Data Sheets for a couple of the products it appears that this type of product often contains quite a considerable amount of ethyl methacrylate (up to 80%). An assumed content of ethyl methacrylate was also identified during the screening (Chapter 4.3.2 – table 4.5).
Table 4.3 Content of acrylic compounds (%) in selected product for building up acrylic nails divided into powders and liquids. The indicated content is the average of a double determination. (-) indicates that the substance is below the detection limit of 0.001%.
| Product identifi- cation |
Acrylic compound | |||||
| Triethylene- glycol diacrylate |
2-Hydroxy- propyl acrylate |
2-Hydroxy- ethyl acrylate |
Ethyl acrylate | Ethylene dimeth- acrylate |
2-Hydroxy- propyl methacrylate |
|
| Acrylic powders | ||||||
| 10 | - | - | - | - | - | - |
| 13 | - | - | - | - | - | - |
| 16 | - | - | - | - | - | - |
| 21 | - | - | - | - | - | - |
| 25 | - | - | - | - | 0.015 | - |
| 28 | - | - | - | - | - | - |
| 31 | - | - | - | - | - | - |
| Acrylic liquids | ||||||
| 12 | - | - | - | - | - | - |
| 15 | - | - | - | - | 6.45 | - |
| 18 | - | - | - | 0.002 | - | 10.25 |
| 23 | - | - | - | - | 0.89 | - |
| 27 | - | - | - | 0.007 | 15 | - |
| 30 | - | - | - | 0.004 | - | - |
| 33 | - | - | 0.03 | - | - | 10 |
4.3 Screening for ingredients in products for manufacture of artificial nails
The quantitative analyses for acrylic compounds showed that it is very obvious that the nail products contain other ingredients than the analysed 6 acrylic compounds due to the fact that the GC-MS analysis showed many other peaks in the mass spectres than the 6 acrylic compounds. Consequently it was decided to perform a screening (GC-MS-SCAN) of the ingredients that appeared with the highest peasks in GC-MS based on the quantitative analyses. With a reasonable probability it will be possible by this method to assume the ingredients of the highest peaks.
4.3.1 Principle of analyses
A well-known amount of sample is diluted/extracted with dichlormethane. The extract is analysed using the GC-MS-SCAN. During the analysis the mass- spectres of the substances contained in the sample are absorbed. Different substances have different mass-spectres; however isomers (for instance xylenes) may have (almost) identical mass-spectres.
The absorbed mass-spectres can be compared with the mass-spectres in a library containing more than 100,000 mass-spectres. Based on the congruity between the absorbed spectres and the spectres of the library, the possible substance is suggested. Also the library shows a quality assessment of the suggested substance. This is indicated as a number between 0 and 100. The closer to 100 the better is the congruity, and the greater the probability that the suggestion is correct. The laboratory has experienced that suggestions of a quality above 80 are often correct. However it should be emphasized that it cannot be finally verified, until a standard containing the suggested substance has been analysed.
Table 4.4 and 4.5 show the components that have been suggested using GC-MS-SCAN through library search. The number of suggested components varies from 1 to 6. The tables do NOT reflect the complete number of components in the products, on the contrary it is estimated that by far the most products contain between 50 and 100 components.
4.3.2 Screening results
21 products to be applied for artificial nails (acrylic nails and gel nails) were screened for ingredients. Each product has been screened for the ingredients showing the highest peaks during the quantitative GC-MS analyses for acrylic compounds.
Table 4.4 Screening of ingredients in selected products for gel nails.
| Ingredients | CAS no. | Quality | Product identification | ||||||
| 11 | 14 | 17 | 22[1] | 26 | 29 | 32 | |||
| Azobenzene or Benzophenone | 119-61-9 / 103-33-3 | 90 | x | ||||||
| Benzaldehyde | 100-52-7 | 96 | x | ||||||
| 2-Butenoic acid, ethyl ester | 623-70-1 | 78 | x | x | |||||
| 2-butenoic acid, methyl ester | 4358-59-2 | 72 - 87 | x | x | |||||
| Butylated hydroxytoluene | 128-37-0 | 94 | x | ||||||
| Mequinol | 150-76-5 | 95 | x | ||||||
| 2-phenoxyethanol | 122-99-6 | 95 | x | ||||||
| 2-henyl-1,2-propandiol | 4217-66-7 | 90 | x | ||||||
| 2-propenoicacid | 79-10-7 | 91 | x | ||||||
| Butyl methacrylate | 97-88-1 | 80 | x | x | |||||
Table 4.5 Screening of ingredients in selected products for acrylic nails (powders)
| Ingredients | CAS no. | Quality | Product identification | ||||||
| 10 | 13 | 16 | 21 | 25 | 28 | 31 | |||
| Benzoic acid phenyl ester | 93-99-2 | 83 - 91 | x | x | x | x | x | ||
| Biphenyl | 92-52-4 | 93 | x | x | x | x | x | x | |
| 2-Butenoic acid, ethyl ester | 623-70-1 | 78 - 87 | x | x | x | x | x | x | x |
| 2-Butenoic acid, methyl ester | 4358-59-2 | 72 - 87 | x | x | x | x | x | x | x |
| Diethylphthalat | 84-66-2 | 96 | x | ||||||
| Propanenitrile 2,2-azobis-2-methyl | 78-67-1 | x | |||||||
| Butyl methacrylate | 97-88-1 | 80 | x | x | x | x | x | x | |
Table 4.6 Screening of ingredients in selected products for acrylic nails (liquids)
| Ingredients | CAS no. | Quality | Product identification | ||||||
| 12 | 15 | 18 | 23 | 27 | 30 | 33 | |||
| Benzaldehyde | 100-52-7 | 96 | x | ||||||
| Benzenamin-N,N,4-trimethyl | 99-97-8 | 94 | x | x | |||||
| Benzenamin-N,N,2-trimethyl | 609-72-3 | 72 - 87 | x | x | x | ||||
| Benzyl benzoat | 120-51-4 | 90 | x | ||||||
| 2-butenoic acid, ethyl ester | 623-70-1 | 87 | x | x | |||||
| 2-butenoic acid, methyl ester | 4358-59-2 | 72 - 87 | x | x | x | ||||
| Butylated hydroxytoluene |
128-37-0 | 94 | x | ||||||
| 2-ethoxymethyl methacrylate |
2370-63-0 | 83 | x | ||||||
| d-limonen | 5989-27-5 | 94 | x | ||||||
| Mequinol | 150-76-5 | 95 | x | ||||||
| Oxybenzone | 131-57-7 | 94 | x | ||||||
| 1,1-oxybis-2-propanol | 110-98-5 | 91 | x | ||||||
| Phenyl- ethylalkohol |
60-12-8 | 97 | x | ||||||
| 1-propanol, 2-(-hydroxy- propoxy) |
106-62-7 | 90 | x | ||||||
| 2-ethylhexyl-p-methoxycin-namate | 5466-77-3 | 97 | x | ||||||
| Ethyl methacrylate | 97-63-2 | 93 | x | x | x | ||||
| Butyl methacrylate | 97-88-1 | 80 | x | ||||||
4.4 Quantification of benzophenon or azobenzen
In connection with screening for ingredients, a product was assumed to possibly contain azobenzene, which is classified as mutagenic and carcinogenic (see table 5.1). Therefore there was a need to state if the product contained the substance, or if it was benzophenon.
4.4.1 Method of analysis for quantitative GC-MS method
Please refer to Chapter 4.1.1 for description of the GC-MS analysis method.
The content was calculated based on internal standards. The detection limit is 10 mg/kg and the uncertainty 10%. All analyses were performed in double determination.
4.4.2 Results in % of the GC-MS analysis
The quantified content obtained from the GC-MS analysis is illustrated in table 4.7. The analyses show that the gel product contained benzophenon and not the cacinogenic azobenzene.
Table 4.7 Content of benzophenon (%) and azobenzene in selected gel nail products. The indicated content is the average of a double determination. The detection limit of the analysis amounted to 0.01%. (-) indicates that the substance is below the detection limit of 0.001%.
| Product identification | Benzophenon (%) | Azobenzene (%) |
| 29 | 0.69 | - |
4.5 Selection of substances for safety assessment
Based on the analyses performed on nail hardeners and products for manufacture of artificial nails, the substances to be toxicologically assessed were selected in cooperation with the Danish Environmental Protection Agency.
The following substances were selected:
- Formaldehyde (in nail hardeners)
- 2-Hydroxyethyl acrylate (in gel nails)
- 2-Hydroxypropyl acrylate (in gel nails)
- 2-Hydroxypropyl methacrylate (in liquid and gel nails)
- Ethylene dimethacrylate (in liquid and gel nails)
5 Legislation
Artificial nails and nail hardeners are comprised by the Statutory Order on Cosmetic Products (BEK no 422 of 4/05/2006) (1). With respect to marketing of cosmetic products, the marketing responsible person in the EU is responsible for labelling of the products in accordance with the regulations for cosmetic products. The products must not contain ingredients which are not allowed in cosmetic products; the final cosmetic product should not cause a risk to the consumers by normal use or use that can be reasonably anticipated.
5.1 Demands on labelling of products
The products must be labelled as follows on box and packing.
- Name of company and address or head office of the company responsible for marketing the product.
- Weight/volume of the product
- Expiry date, if validity of the product unopened is less than 30 months.
- If the validity is more than 30 months, validity after opening with indication of symbol for open box is indicated.
- Safety declarations for correct use of the cosmetic product – including special considerations with respect to cosmetic products used professionally.
- The manufacturing number (batch number), possibly only on box.
- Function of the cosmetic product
- List of ingredients. The ingredients must be stated with INCI (International Nomenclature of Cosmetic Ingredients) name and listed by decreasing weight in the product.
- Scenting and aromatic compounds and their raw materials must be named “perfume” or “aroma”.
5.1.1 Evaluation of labelling
The purchased products have been reviewed for possible lack of labelling. Generally, the labelling showed many discrepancies, especially the acrylic artificial nail products. The following discrepancies were recurred on many of the purchased products.
- Missing information of durability
- Directions for use
- The labelling is often in other languages than Danish (English, German, French)
- Missing batch number
- Wrong or no INCI list
The functioning of most of the products was unclear, and the list of ingredients did not comply with the rules in the Statutory Order on Cosmetic Products. In the labelling of the nail hardeners were observed fewer discrepancies than in that of artificial nails.
5.2 Classification of ingredients
The classification of ingredients in the purchased and analysed nail products has been investigated in accordance with the chemicals legislation and the demands stipulated in the cosmetics legislation. It is important to emphasize that this classification applies to products that are marketed in accordance with the chemicals legislation and not the cosmetics legislation. However, the classification may indicate if the products contain hazardous substances. Glues for fixing artificial nails belong under a so-called “borderline” area meaning that they are either classified as cosmetic products or as chemical products depending on the purpose, the application, and how the product is presented to the consumer. The Danish Environmental Protection Agency has assessed the glues for fixing the nail tips (see Chapter 3.3.1) to be chemicals. Therefore, they must be labelled in accordance with the chemical legislation.
5.2.1 Classification of formaldehyde
The content of formaldehyde in the nail hardeners is shown in the analyses of 6 different nail hardeners on the Danish market. The classification of formaldehyde is shown in table 5.1.
Table 5.1 Classification of formaldehyde in nail hardeners
| Chemical name | CAS no. | Number of productsa | Classification | Statutory Order on Cosmetic Products |
| Formaldehyde | 50-00-0 | 6 | T;R23/24/25, C;R34 CARC3;R40 R43 | Permitted in nail hardeners up to 5% |
In accordance with the Statutory Order on Cosmetic Products (1), the allowed content of formaldehyde in nail hardeners is up to 5% total formaldehyde. In addition, all final products containing formaldehyde or substances from which formaldehyde can be decomposed (see Chapter 3.4.2) can be labelled with the text “contains formaldehyde”, if the concentration of formaldehyde in the final product exceeds 0.05%. The analyses of the 6 nail hardeners products illustrate that none of the products exceeds this limit of 5%, whereas a product (product number 20) does not declare formaldehyde, even the concentration exceeds the limit of declaration of 0.005%.
If formaldehyde has been added as a preservative, the maximum permitted concentration is 0.2%.
5.2.2 Classification of acrylic compounds
Acrylic compounds are found in a selection of products for manufacture of artificial nails purchased on the Danish market. In order to find indications of the toxic effects of the substances, information on the classification of the acrylic compounds has been collected from the list of hazardous compounds. Furthermore, information on the regulation of the ingredients in cosmetic products was found.
Table 5.2 Classification of the analysed acrylates
| Chemical name | CAS no. | Number of productsa | Classification | Statutory Order on Cosmetic Products |
| Ethyl acrylate | 140-88-5 | 3 | F;R11 Xn;R20/21/22 Xi;R36/37/38 R43 | Not permitted when the product is used as a fragrant |
| Ethylene dimethacrylate | 97-90-5 | 8 | Xi;R37 R43 | - |
| 2-Hydroxyethyl acrylate | 818-61-1 | 7 | T;R24 Xi;R36/38 R43 (conc < 10%) | - |
| 2-Hydroxypropyl acrylate | 999-61-1 | 4 | T;R23/24/25 C;R34 R43 |
- |
| 2-Hydroxypropyl methacrylate | 27813-02-1 | 3 | - | - |
| Triethyleneglycol diacrylate | 1680-21-3 | 3 | R43 (conc < 20% og > 1 %) |
- |
a Number of products containing the acrylic compound
As shown in table 5.2, 5 out of 6 of the analysed acrylic compounds are classified. The 5 substances have been classified for ´sensitisation by skin contact (R43). This classification must appear in the labelling of the product, if the products are labelled in accordance with the chemicals legislation, but not if the product is covered by the cosmetics legislation. However, all ingredients except perfume and aromatic substances must be included in the list of ingredients of cosmetic products.
5.2.3 Classification of screened ingredients in products for manufacture of artificial nails
Information of the classification of the identified ingredients has been searched. This information has been searched in the List of Hazardous Substances and in the consultative list for self classification of the Danish Environmental Protection Agency. The data collected from the consultative list are marked with ‘*’. Also, data on the permitted use of the ingredients in cosmetic products have been identified.
Table 5.3 Classification of the ingredients identified through GC-MS SCAN of 21 nail products. Data are collected from the consultative list of the Danish Environmental Protection Agency marked with ‘*’. Also, data on the permitted use of the ingredients in cosmetic products have been identified
| Chemical name | CAS no. | Number of productsa | Classification | Statutory Order on Cosmetic Products |
| Benzophenone | 119-61-9 | 1 | - | - |
| Benzaldehyde | 100-52-7 | 2 | Xn; R22 | - |
| Benzenamin-N,N,4-trimethyl | 99-97-8 | 2 | T;R23/24/25 R33 R52/53 (conc > 25%) |
- |
| Benzenamin-N,N,2-trimethyl | 609-72-3 | 3 | T;R23/24/25 R33 R52/53 (conc > 25%) |
- |
| Benzoic acid phenyl ester | 93-99-2 | 5 | R43 N;R51/53* | - |
| Benzyl benzoate | 120-51-4 | 1 | Xn;R22 | Must be indicated when the conc. exceeds 0.001% for products that are not cleaned |
| Biphenyl | 92-52-4 | 6 | Xi;R36/37/38 N;R50/53 | - |
| 2-Butenoic acid, ethyl ester | 623-70-1 | 11 | - | - |
| 2-Butenoic acid, methyl ester | 4358-59-2 | 12 | - | - |
| Butyleret hydroxytoluen | 128-37-0 | 2 | Xn;R22 N;R50/53* | - |
| Diethylphthalat | 84-66-2 | 1 | - | - |
| 2-Ethoxy-methylmethacrylate | 2370-63-0 | 1 | R43 R52/53* | - |
| d-Limonene | 5989-27-5 | 1 | R10 Xi;R38 R43 N;R50/53 | Must be indicated when the conc. exceeds 0.001% for products that are not washed off. |
| Mequinole | 150-76-5 | 2 | Xn;R22 Xi;R36 R43 | Max. conc.: 0.02% (after mixing for use) Only for professional use The directions for use must contain the following: Only for professional use Avoid skin contact The directions for use should be read carefully. |
| Oxybenzone | 131-57-7 | 1 | R43* | On the list of approved UV-filters: Max 10%. |
| 1,1-oxybis-2-propanol | 110-98-5 | 1 | - | - |
| 2-Phenoxyethanol | 122-99-6 | 1 | Xn;R22 Xi;R36 | Highest permitted concentration: 1.0% Only to be used in products that is rinsed off. |
| Phenylethylalkohole | 60-12-8 | 1 | - | - |
| 2-Phenyl-1,2-propandiol | 4217-66-7 | 1 | - | - |
| Propanenitrile 2,2-azobis-2-metyl | 78-67-1 | 1 | E;R2 F;R11 Xn;R20/22 R52/53 | - |
| 1-propanol, 2- (-hydroxypropoxy) |
106-62-7 | 1 | - | - |
| 2-Propenoic acid | 79-10-7 | 1 | R10 Xn;R20/21/22 C;R35 N;R50 (conc. > 25%) |
- |
| 2-Ethylhexyl-p-methoxycinnamate | 5466-77-3 | 1 | N;R50/53* | On the list of approved UV-filters. Max 10%. |
| Butyl methacrylate | 97-88-1 | 9 | R10 Xi;R36/37/38 R43 | - |
| Ethyl methacrylate | 97-63-2 | 3 | F;R11 Xi;R36/37/38 R43 | - |
a Number of products with the GC-MS SCAN ingredient identification.
* Data from guide for self classification
As it appears from table 5.3, many of the screened ingredients of the products for manufacture of artificial nails are classified in accordance with the chemicals legislation. Several substances are also regulated by the cosmetics legislation – for instance with respect to declaration. For instance, d-limonene is not declared on product no. 30, benzyl benzoate not on product no. 12
More of the substances are probably found in the products as impurities. With cosmetic products is distinguished between ingredients and impurities. Ingredients are actively added substances that must be declared on the packing. The substances to be added legally, and in which the concentrations are regulated in accordance with the Statutory Order on Cosmetic Products (1). Contrary to ingredients, impurities are not actively added. Impurities are present in low concentrations in the used raw materials, or they can be introduced during the industrial manufacturing of the product. Such impurities are not to be declared, however they should be avoided, and it is always the responsibility of the producer to ensure that the final product is not hazardous to human health.
6 Safety assessment
- 6.1 Safety assessment of substances in nail hardeners
- 6.2 Safety assessment of substances in artificial nails
Safety assessments were performed on formaldehyde found in nail hardeners, and 2-hydroxyethyl acrylate, 2-hydroxypropyl acrylate, 2-hydroxypropyl dimethacrylate and ethylene dimethacrylate found in artificial nail products. The toxicological effects of the substances are described, including the ability of the substances to induce allergy. This effect is considered to be the most critical effect due to the amount of literature describing contact allergy after use of nail hardeners and/or artificial nails.
When data were available, a NO(A)EL[2] for the critical effect of the substances has been established. The NO(A)EL value is applied in Chapter 7 on exposure scenarios and risk assessments of the products.
6.1 Safety assessment of substances in nail hardeners
6.1.1 Formaldehyde
Identification
Formaldehyde is a colourless, flammable, reactive and readily polymerising gas at normal temperature and pressure. It is a gas with a pungent, irritating odour (2).
Formaldehyde is a normal metabolite in mammalian organisms. It can be generated by the metabolism of certain xenobiotics or endogenous compounds, such as amino acids (3).
| Chemical name | Formaldehyde |
| Synonyms | Formic aldehyde, methanal, methylene oxide, methylaldehyde, oxymethylene, oxomethane |
| CAS-No. | 50-00-0 |
| EINECS No. | 200-001-8 |
| Molecular formula | CH2O |
| Molecular structure | 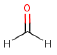 |
| Legislation: Classification in accordance with the list of hazardous substances (4) Cosmetics (1) |
T; R23/24/25: Toxic by inhalation, in contact with skin and if swallowed. C; R34: Causes burns. CARC3; R40: Limited evidence of a carcinogenic effect R43: May cause sensitization by skin contact. A maximum amount of 5% in nail hardeners allowed |
Physical-Chemical properties
| Physical state | Gas |
| Molecular weight (g/mol) | 30.03 |
| Melting point | -92° C (5) |
| Boiling point | -19.5° C (5) |
| Vapour pressure | 3890 mm Hg at 25° C (5) |
| Octanol/water partition coefficient (log Kow) | 0.35 (5) |
Acute toxicity
Systemic effects
The toxic effect observed after exposure of formaldehyde (by inhalation) may be, depending on dose, weakness, headache, abdominal pain, anaesthesia, anxiety, burning sensation in the nose and throat, thirst, clammy skin, central nervous system depression, cyanosis, diarrhoea, dizziness, irritation and necrosis of mucous membranes and gastrointestinal tract, vomiting, nausea, coma, and shock (6).
Ingestion of aqueous solutions of formaldehyde may cause renal injury and lead to an increase in formate levels in the urine (6).
Local effects
Skin irritation and sensitisation
Formaldehyde is irritating to eyes, skin and mucosal membranes (3). With standard patch test protocols, formaldehyde concentrations of 2% and higher is observed to produce skin irritation in non-sensitised individuals (7).
No significant pulmonary function decrements have been observed in adults with or without asthma after three hours of exposure to 0.5 to 3 ppm (0.6 - 3.6 mg/m³) formaldehyde. Most studies show no effect on lung function after formaldehyde exposure (3,8).
Induction of dermal sensitisation to formaldehyde has been observed after dermal exposure. A threshold for induction has not been clearly established, but it is estimated to be less than 5 % aqueous solution (3). Different studies has classified formaldehyde as a moderate to strong sensitizer (9,10,11).
In an guinea pig maximization test sensitisation was observed in 100% of the animals at a formaldehyde concentration of 2% (7).
An LLNA (Local Lymph Node Assay) test has been performed on formaldehyde showing that formaldehyde has a potential to induce sensitisation. Induction of sensitisation was observed at a concentration of 0.96% formaldehyde (12). In another LLNA test a formaldehyde concentration of 0.4 % induced sensitisation (9).
Over the last 30 years, a large number of human patch tests have been performed with aqueous formaldehyde solutions in concentrations up to 2% (7). Allergic reactions to formaldehyde concentrations of 1% has been observed in patients with skin problems (7).
In a serial dilution test 8/35 formaldehyde-sensitised subjects developed allergy to the lowest concentration tested (0.1%) (7). In another test, a few positive reactions were observed at concentrations below 0.1% formaldehyde. The following response frequencies were observed: 9/20 at 0.5%, 3/20 at 0.1%, 2/20 at 0.05%, and 1/20 at 0.025% (7).
Studies of concentration-response relationships for skin allergic reactions caused by occluded dermal exposures of formaldehyde in formaldehyde-sensitised subjects suggest that a elicitation of sensitisation to formaldehyde concentrations below about 0.025 – 0.05% is rare (7).
In conclusion, LLNA tests has shown that formaldehyde is able to induce sensitisation and indicated that formaldehyde concentrations as low as 0.4 – 0.96% may induce sensitisation in non-sensitised subjects. For formaldehyde-sensitised subjects, a large number of human patch tests have been performed. It is concluded that formaldehyde concentrations below 0.025 – 0.05% rarely elicitate sensitisation.
Furthermore, it has been reported that with standard patch test protocols, formaldehyde concentrations of 2% and higher may produce skin irritation in non-sensitised individuals demonstrating that concentrations that evoke a skin irritation response is similar to those evoking allergic skin responses.
Toxicity from repeated or prolonged exposure
Repeated formaldehyde exposure causes toxic effects only in the tissues of direct contact after inhalation, oral or dermal exposure characterized by local tissue destruction and subsequent repair of the damage. The typical locations of lesions in experimental animals are the nose after inhalation, the stomach after oral administration and the skin after dermal application. The nature of the lesions depends on the inherent abilities of the tissues involved to respond to the noxious event and on the local concentration of the substance (3).
In a 2 years chronic feeding study in rats a NOAEL of 15 mg/kg/day was obtained. The NOAEL was based on development of lesions of the gastric mucosa and histopathological changes in the kidneys after administration of formaldehyde via the drinking water (13).
An oral RfD[3] of 0.2 mg/kg bw/day is established based on this study on chronic toxicity in rats (13).
Genotoxicity
Formaldehyde is genotoxic in different in vitro systems (14,15,16).
Several in vivo studies are available on the mutagenic potential of formaldehyde. The conclusion of the studies is insufficient and there is conflicting evidence for genotoxic potential of formaldehyde in humans exposed to occupational levels (6).
Carcinogenicity
Formaldehyde is carcinogenic at the site of contact as a consequence of epithelial cell regenerative proliferation resulting from tissue destruction and mutation, based on studies in both animals and humans (14).
A TD50[4] has been reported for rats and mice. The lowest TD50 was found for rats at 1.35 mg/kg bw/day (17). Based on this value, an acceptable dose may be estimated to 2.7 × 10-6 mg/kg bw/day[5]. After exposure to this dose, there is a risk of cancer in 1 out of 1 million exposed persons. This level of risk is generally accepted.
Reproductive toxicity
There is no evidence that formaldehyde may induce teratogenicity or may affect reproduction by inhalation exposure (6).
Critical effect
The induction and elicitation of dermal sensitisation are considered as critical effect from dermal formaldehyde exposure. A threshold for induction has not been clearly established for humans. However, a concentration level of 0.4 – 0.96% formaldehyde has been established as the lower concentration for induction of skin sensitisation in LLNA tests. Furthermore, a large number of human patch tests have been performed. From these it is concluded that formaldehyde concentrations below 0.025 – 0.05% rarely elicitate sensitisation in formaldehyde-sensitised subject.
Therefore, a concentration of 0.01% formaldehyde may be considered as a safe concentration including both formaldehyde-sensitised subjects and the induction of non-sensitised subjects. A safety factor of 100 is included for the data from LLNA tests for extrapolation of data from animals to humans and including of particularly sensitive human individuals.
An oral Reference Dose (RfD) of 0.2 mg/kg bw/day is established for formaldehyde based on a NOAEL of 15 mg/kg bw/day in rats administrated formaldehyde by drinking water. The effects were reduced body weight gain and effects in the gastro-intestinal tract.
Formaldehyde has a carcinogenic potential. An acceptable dose for lifetime risk of cancer has been estimated to 2.7 × 10-6 mg/kg bw/day. After exposure to this dose, there is a risk of cancer in 1 out of 1 million exposed persons. This risk level is generally accepted.
Table 6.1 Summary of data for formaldehyde
| Toxicological data (animals) | |
| NOAEL, intake, rat | 15 mg/kg bw/day |
| Acceptable dose - lifetime risk, cancer | 2.7 × 10-6 mg/kg bw/day |
| Oral Reference Dose (RfD) | 0.2 mg/kg bw/day |
6.2 Safety assessment of substances in artificial nails
6.2.1 2-Hydroxyethyl acrylate
Identification
2-Hydroxyethyl acrylate is colourless liquid with a sweet pleasant odour (5).
2-Hydroxyethyl acrylate is included in the positive list of monomers and other starting substances for plastics and coatings intended to come into contact with foodstuffs. While there are no recommendations for a specific migration limit or residual level, the European Commission has suggested a group maximum total daily intake of 0.1 mg/kg body weight acrylates (measured as acrylic acid). (18).
| Chemical name | 2-Hydroxyethyl acrylate |
| Synonyms | Ethylene glycol, monoacrylate, hydroxyethyl acrylate, 2-propenoic acid, 2-hydroxyethyl ester |
| CAS-No. | 818-61-1 |
| EINECS No. | 212-454-9 |
| Molecular formula | C5H8O3 |
| Molecular structure | 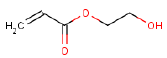 |
| Legislation: Classification in accordance with the list of hazardous substances (4) |
T; R24: Toxic in contact with skin C; R34: Causes burns Xi; R36/38: Irritating to eyes and skin R43: May cause sensitisation by skin contact N; R50: Very toxic to aquatic organisms |
Physical-Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 116.13 |
| Melting point | No information |
| Boiling point | 191° C (19) |
| Vapour pressure | 0.0523 mmHg at 25° C (19) |
| Octanol/water partition coefficient (log Kow) | -0.21 (5) |
Acute toxicity
Systemic effects
Studies on the acute toxicity of 2-hydroxyethyl acrylate indicate oral LD50 values of 540 – 1070 mg/kg bw. Clinical signs (following administration of a 10% aqueous solution) included hypoactivity, rough fur, muscle weakness, gastro-intestinal tract haemorrhage in animals that died. Neat material may have caused chemically burns in the tissues of the mouth, throat, and gastro-intestinal tract (18).
Acute dermal toxicity studies showed LD50 values of 154 (rabbits, undiluted material) and >1000 mg/kg bw (rats, vehicle olive oil). At high concentrations decreased eyelid tone, decreased corneal reflex, loss of righting reflex and muscle coordination were noted (18).
Acute inhalation data indicate that exposures of rats to 333 to 394 ppm (» 1580 - 1870 mg/m³) 2-hydroxyethyl acrylate for 4 or 8 hours caused irritation and were in the threshold area for lethality. Nearly 100% lethality was observed for rats at exposures of 500 ppm (2500 mg/m³) and above (18).
These data indicate that 2-hydroxyethyl acrylate is moderately acute toxic.
Local effects
Skin irritation and sensitisation
2-Hydroxyethyl acrylate is severely irritating to the skin (5,18). Several studies have shown that undiluted 2-hydroxyethyl acrylate is severely irritating to the skin if left in contact with the skin for a sufficient period of time (18). Upon eye contact, 2-hydroxyethyl acrylate may cause severe irritation with irreversible corneal injury (18).
Skin sensitization studies in animals and humans indicate that 2-hydroxyethyl acrylate is a sensitizer and may cross-react with other acrylates in some exposed individuals. 2-Hydroxyethyl acrylate has been found to be a sensitizer in both local Lymph Node Assays (LLNA), Buehler tests and maximization tests in guinea pigs; however some of the studies being of older date (1970 – 1995) (18). Twelve acrylate-sensitised patients were tested with various substances including 2-hydroxyethyl acrylate in a patch test. Ten out of twelve patients had a positive reaction to 2-hydroxyethyl acrylate when tested at a concentration of 0.1% (20). In another human patch test with acrylate-sensitised subjects 8% of the test persons reacted positive to 2-hydroxyethyl acrylate in a 0.1% concentration (21).
Based on these studies 2-hydroxyethyl acrylate is evaluated to have a moderate potential to elicitate sensitisation in acrylate-sensitised subjects.
Toxicity from repeated or prolonged exposure
Repeated exposures to vapours of 2-hydroxyethyl acrylate (0, 5, 10, 25 ppm » 0, 24, 48, 119 mg/m³) to rats via inhalation (7 hr/day, 5 days/week for four weeks) caused severe nasal irritation, resulting in death due to respiratory failure at higher concentrations. The LOAEC was estimated to 5 ppm (25 mg/m³) for 2-hydroxyethyl acrylate based on corneal irritation (18). In another study the effects observed following 18 months exposure of laboratory rats to 5 ppm of 2-hydroxyethyl acrylate were related to irritation of the respiratory tract, without significant evidence of systemic toxicity (18).
Dietary studies with rats and dogs have shown that 2-hydroxyethyl acrylate has a low toxicity after repeated dosing via this route. No effects were observed in the highest tested dose up to 131 mg/kg bw/day. A NOAEL of 131 mg/kg bw/day was established in dogs fed up to 131 mg/kg bw in their diet (18).
Genotoxicity
2-Hydroxyethyl acrylate was not mutagenic to S. typhimurium (bacterial reverse mutation assay) in vitro with or without metabolic activation but was positive with metabolic activation when tested with two E. coli strains. No evidence of chromosomal damage was seen in an 18-month inhalation study. Four rats pr. sex pr. group were killed after 12-months exposure and the bone marrow cells examined for chromosomal damage. No effects were observed (18). Overall, 2-hydroxyethyl acrylate did not show evidence of mutagenic potential in vivo by the inhalation route of exposure (18).
Carcinogenicity
No evidence of a carcinogenic effect was observed in a chronic toxicity/oncogenicity study conducted by the inhalation route of exposure (18).
Reproductive toxicity
Histopathological examination of the reproductive organs of rats from an 18-month inhalation study revealed an increase in age-related lesion (fibrinoid degeneration in the vascular channels of the testes) and uterine inflammation (without any other associated histopathological effects). Neither effects were considered treatment-related or adverse to reproduction (18).
Dietary administration of 2-hydroxyethyl acrylate to rats or dogs did not result in treatment-related effects on testicular weight or histopathology of the testes or uterus (18).
Critical effect
The critical effect of 2-hydroxyethyl acrylate is its potential to induce and elicitate irritation and sensitisation. No lower dose has been established for sensitisation, but elicitation of allergy was observed at a concentration of 0.1%. Based on the available studies on 2-hydroxyethyl acrylate it is evaluated that the substance has a moderate potential to induce sensitisation.
Table 6.2 Summary of data for 2-hydroxethyl acrylate
| Toxicological data (animals) | |
| NOEL, (mg/kg bw), oral, dog | 131 mg/kg bw/day |
6.2.2 2-Hydroxypropyl acrylate
Identification
2-Hydroxypropyl acrylate (HPA) is a clear to light yellow liquid with a sweet odour (22).
Only limited data has been identified on 2-hydroxypropyl acrylate. However, data was found on hydroxypropyl acrylate (CAS no. 25584-83-2). A typical commercial product of hydroxypropyl acrylate contains approximately 75-80% 2-hydroxypropyl acrylate and 20-25% 1-methyl-2-hydroxyethyl acrylate. Below, data on toxicology for this commercial product is also used to describe the toxicity of 2-hydroxypropyl acrylate.
| Chemical name | 2-hydroxypropyl acrylate |
| Synonyms | 1,2-Propanediol, 1-acrylate, propylene glycol monoacrylate, beta-hydroxypropyl acrylate |
| CAS-No. | 999-61-1 |
| EINECS No. | 213-663-8 |
| Molecular formula | C6H10O3 |
| Molecular structure | 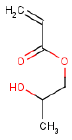 |
| Legislation: Classification in accordance with the list of hazardous substances (4) |
T; R23/24/25: Toxic by inhalation, in contact with skin and if swallowed. C; R34: Causes burns. R43: May cause sensitisation by skin contact |
Physical-Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 130.14 (22) |
| Melting point | No information |
| Boiling point | 77° C (22) |
| Vapour pressure | 0.174 mmHg at 25° C (19) |
| Octanol/water partition coefficient (log Kow) | 0.35 (19) |
Acute toxicity
Systemic effects
2-Hydroxypropyl acrylate is of moderate to low toxicity after oral exposure. The rat oral LD50 was 250 to 500 mg/kg bw with reports of values as high as 590 to 1300 mg/kg bw. The mouse oral LD50 value was 1060 mg/kg bw (22).
LD50 values in rabbits after topical application range from 170 mg/kg bw to 250 mg/kg bw. The animals that survived developed moderate oedema, moderate to severe necrosis, and local evidence of irritation at the application site (22).
Local effects
Skin irritation and sensitisation
2-Hydroxypropyl acrylate is irritating to the eyes, nasal and respiratory organs (22). Hydroxypropyl acrylate is highly irritating to the skin (23).
Skin sensitization studies in animals and humans indicate that hydroxypropyl acrylate is likely to be a sensitizer and will cross-react with other acrylates in some exposed individuals. 2-Hydroxypropyl acrylate is among the more potent sensitisers in guinea pigs (22). Nine acrylate-sensitised patients were tested with various substances including 2-hydroxypropyl acrylate in a patch test. Seven out of nine patients had a positive reaction to 2-hydroxypropyl acrylate tested in a concentration of 0.1% indicating a sensitisation potential (20).
It is evaluated based on human and animal data that 2-hydroxypropyl acrylate has a moderate potential to elicitate and induce allergy.
Toxicity from repeated or prolonged exposure
Repeated exposure to vapours of hydroxypropyl acrylate (6 hr/day, 5 days/week for 21 or 20 exposures to rats and mice and rabbits and dogs, respectively) did result in severe irritation of the upper respiratory tract, resulting in death due to respiratory failure at higher concentrations and concentration-related local irritation at sub-lethal exposures. The LOAEC, based on irritation, was 5 ppm (27 mg/m³) for hydroxypropyl acrylate. No systemic toxicity was observed (23).
Genotoxicity
2-Hydroxypropyl acrylate was not mutagenic in an in vivo mouse micronucleus study (23).
Hydroxypropyl acrylate was not mutagenic to S. typhimurium (bacterial reverse mutation assay) in vitro with or without metabolic activation but was positive with metabolic activation when tested with two E. coli strains. In mammalian cells in vitro, hydroxypropyl acrylate was negative in a gene mutation assay but had clastogenic activity in cytogenetic and chromosomal aberration assays. In these mammalian cell assays, positive results occurred only at concentrations that resulted in significant cell death. Thus, the positive results are considered equivocal. Hydroxypropyl acrylate was not mutagenic in an in vivo mouse micronucleus study (23).
Overall, hydroxypropyl acrylate is considered not to have mutagenic potential in vivo based on available data.
Carcinogenicity
No data found
Reproductive toxicity
Based on results from animal studies evaluating exposure to hydroxypropyl acrylate and 2-hydroxyethyl acrylate vapours, no reproductive toxicity is anticipated for 2-hydroxypropyl acrylate. Hydroxypropyl acrylate is not selectively toxic to the embryo or foetus and is not teratogenic via inhalation exposure (23).
Critical effect
The critical effect of 2-hydroxypropyl acrylate is its potential to elicitate and induce allergic reactions and to irritate the skin. No clearly threshold dose has been established for this moderate potential for sensitisation effects, but elicitation of allergic reactions was observed at a concentration of 0.1% 2-hydroxypropyl acrylate in humans.
Table 6.3 Summary of data for 2-hydroxpropyll acrylate
| Toxicological data (animals) | |
| LOAEC, (mg/m³), inhalation, rat | 27 mg/m³ » 7 mg/kg bw/day |
6.2.3 2-Hydroxypropyl methacrylate
Identification
2-Hydroxypropyl methacrylate is a dear liquid with a slightly acrylic odour (5).
| Chemical name | 2-hydroxypropyl methacrylate |
| Synonyms | 1,2-Propanediol, 2-methyl, monomethacrylate, Hydroxypropyl methacrylate, Propylene glycol monomethacrylate |
| CAS-No. | 27813-02-1 |
| EINECS No. | 248-666-3 |
| Molecular formula | C7H12O3 |
| Molecular structure |  |
| Legislation: Classification in accordance with the list of hazardous substances (4) |
Not classified |
Physical-Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 144.18 (5) |
| Melting point | -89° C (19) |
| Boiling point | 87° C (24) |
| Vapour pressure | 0.022 mmHg at 25° C (24) |
| Octanol/water partition coefficient (log Kow) | 0.48 (24) |
Acute toxicity
Systemic effects
2-Hydroxypropyl methacrylate has a low oral acute toxicity. LD50 in rats is 11200 mg/kg bw after oral exposure (19,24).
Local effects
Skin irritation and sensitisation
2-Hydroxypropyl methacrylate vapour is irritating to eyes and respiratory system. The liquid is irritating to eyes (5).
In guinea pigs 2-hydroxypropyl methacrylate had a weak potential to induce sensitisation in a maximisation test (24).
2-Hydroxypropyl methacrylate is observed to elicitate allergic reactions in patch tests. 17.5% of the tested acrylate-sensitised patients were reacting to 2-hydroxypropyl methacrylate in a concentration of 2% (21). In another patch test eleven acrylate-sensitised patients were tested with various substances including 2-hydroxypropyl methacrylate. Six out of eleven patients had a positive reaction to 2-hydroxypropyl methacrylate tested in a concentration of 2% indicating a weak potential for elicitation of allergic reactions (20).
Toxicity from repeated or prolonged exposure
No data found
Genotoxicity
2-Hydroxypropyl methacrylate induced clastogenicity at 0.35 mg/ml with and without activation in an in vitro assay (24). No other studies have been found on the mutagenic effects of 2-hydroxypropyl methacrylate.
Carcinogenicity
No data found
Reproductive toxicity
In a rat study a NOAEL of 1000 mg/kg bw/day was established for reproductive and developmental effects indicating that 2-hydroxypropyl methacrylate has a low potential to cause reproductive toxicity (24).
Critical effect
The critical effects of 2-hydroxypropyl methacrylate are its potential to elicitate and induce allergic reactions. No clearly threshold dose has been established for this effect, but elicitation of allergic reactions was observed at a concentration of 2% 2-hydroxypropyl methacrylate in humans.
A NOAEL was established in a reproductive toxicity study in rats.
Table 6.4 Summary of data for 2-hydroxypropyl methacrylate
| Toxicological data (animals) | |
| NOAEL, (mg/kg bw/day), reprotoxicity, rat | 1000 |
6.2.4 Ethylene dimethacrylate
Identification
Biologically, methacylates resemble acrylates, except for lower reactivity and thus decreased toxicity. This is probably due to steric hindrance by its methyl group (5).
| Chemical name | Ethylene dimethacrylate |
| Synonyms | Ethylene glycol dimethacrylate, ethyldiol metacrylate, glycol dimethacrylate |
| CAS-No. | 97-90-5 |
| EINECS No. | 202-617-2 |
| Molecular formula | C10H14O4 |
| Molecular structure | 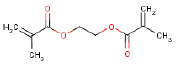 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 923 of 2005) (4) |
Xi;R37: Irritating to respiratory system R43: May cause sensitisation by skin contact |
Physical-Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 198.22 (5) |
| Melting point | -40° C (5) |
| Boiling point | 80.7° C (19) |
| Vapour pressure | 0.188 mmHg at 25° C (19) |
| Octanol/water partition coefficient (log Kow) | 2.2 (5) |
Acute toxicity
Systemic effects
Ethylene dimethacrylate has a low acute toxicity. LD50 orally in mice is 2,000 mg/kg bw, while in rats the value is 3,300 mg/kg bw (19).
Local effects
Skin irritation and sensitisation
Ethylene dimethacrylate is irritating to eyes and respiratory tract (5).
Twenty six acrylate-sensitised patients were tested with various substances including ethylene dimethacrylate in a patch test. Twenty out of twenty six patients had a positive reaction to ethylene dimethacrylate tested in a concentration of 2% indicating a potential for eliciting allergic reactions (20). In another human patch test 13% of the acrylate-sensitised subjects reacted positive to ethylene dimethacrylate in a 2% concentration (21). Based on a Local Lymph Node Assay (LLNA) ethylene dimethacrylate was classified with an extremely weak potency for sensitisation. The concentration inducing sensitisation was 35% ethylene dimethacrylate (25).
Based on these animal and human studies it is evaluated that ethylene dimetacrylate have a weak potential as a sensitiser.
Toxicity from repeated or prolonged exposure
Repeated exposure to ethylene dimethacrylate (6 hr/day, for 13 exposures to rats) in a concentration of 1 mg/m³ produced slightly lethargy. Body weights and urine parameters were unchanged (26).
Carcinogenicity
No data found.
Genotoxicity
Ethylene dimethacrylate was not mutagenic in Ames test (S. typhimurium) with or without activation (26). The substance did induce mutations at the TK gene locus in L5178Y mouse lymphoma cells with metabolic activation in concentrations from 400 to 700 nl/ml. Without metabolic activation, concentrations up to 800 nl/ml caused high cytotoxicity without increasing mutation frequency (5).
Based on these studies the mutagenic potential of ethylene dimethacrylate is not fully verified.
Reproductive toxicity
No data found.
Critical effect
The critical effects of ethylene dimethacrylate are its potential to elicitate and induce allergic reactions. No clearly threshold dose has been established for this effect but elicitation of allergic reactions was observed at a concentration of 2% ethylene dimethacrylate in humans.
No NOAEL for systemic effects were found.
Table 6.5 Summary of data for ethylene dimethacrylate
| Toxicological data (animals) | |
| - | - |
7 Exposure and risk assessment
- 7.1 Exposure and risk assessment for consumers using nail hardeners
- 7.2 Exposure and risk assessment for consumers using artificial nails
The assessment of exposure to substances in nail hardeners and artificial nails is based on the analysed content of substances in selected products on the Danish market (see part 3) and is performed in accordance with the principles in the EU Technical guidance Document (TGD) (27) and The Scientific Committee for Consumer Products (SCCP) guidelines (28). The internal body dose (Systemic Exposure Dose, SED) is estimated in a worst case scenario for a model person by applying standard parameters from TGD and SCCP. The health risks of exposure to selected substances in nail hardeners and artificial nails are assessed by calculating the margin of safety (MoS). The calculation is based on NOAEL, possibly LOAEL established in the toxicological profiles prepared in this survey and the estimated systemic exposure dose (SED).
The safety assessment (part 6) showed that sensitisation is the critical effect of most of the selected components in the nail products. However, sensitisation of contact allergens are dependent not only on the inherent sensitisation capacity of the chemical, but also on the dose and type of exposure, the amount of allergen per unit area surface of skin, the vehicle and the condition of the skin (29). Establishment of a lower safe threshold concentration for the induction and elicitation of sensitisation may be difficult, particularly for use in regulatory toxicology. A validated method for risk assessment for the sensitisation effect has not yet been established. Therefore the below risk assessment is split in a risk assessment of the systemic effects and a part describing the potential risk of sensitisation.
7.1 Exposure and risk assessment for consumers using nail hardeners
In this survey it was decided to focus on free formaldehyde in the nail hardeners. Other substances could also be interesting from a health perspective. However, it was out of range of this project to cover additional ingredients.
Formaldehyde in nail hardeners may react with proteins on the surface of the nail and thereby be immobilized. Depending on the applied concentration some of the formaldehyde may, however, penetrate the nail plate and reach the tissue under the nail. This may especially be the case if the nail is thin, soft or damaged. Formaldehyde may also reach the skin surrounding the nail if the product is not very carefully smeared on the nail plate. As formaldehyde is volatile at room- and skin temperature and may be inhaled after evaporation from the nail and areas of the skin, it is also relevant to include calculation of inhalation in the systemic dose estimation.
The daily exposure of formaldehyde in nail hardeners has been calculated for a model person of 40 kg. This body weight is based on a conservative approach also including younger girls from 15 years and up in the risk assessment.
The daily exposure was calculated for the highest allowed content of formaldehyde in nail hardeners; 5%. The maximum amount of formaldehyde found in the analysed products was 4.05%.
The following assumptions were used as realistic worst-case scenario:
Weight of person, adult: 40 kg
Number of applications per day: 1/day[6]
Applied amount per application (10 nails): 0.25 g (28)
Maximum amount of formaldehyde in nail hardeners: 5 %
7.1.1 Risk assessment for systemic effects
It is assessed that the amount of formaldehyde in nail hardeners will either react with proteins on the surface of the nail, reach the skin surrounding the nail or evaporate during the drying process of the nail hardener. It is evaluated that around 25% of the total content of formaldehyde in the product will evaporate during the drying process. The remaining 75% will cover the nail plate and the skin surrounding the nail. The nails approximately amount to maximum 40 cm². The skin surrounding the nails will amount to about 4 cm² (28), corresponding to about 9% of the total area of nail and skin. Therefore it is assumed that 9% of the applied nail hardeners will cover the skin surrounding the nail, and thereby may contribute to the systemic dose.
It is assumed that none of the formaldehyde reacting on the nail plates will contribute to the systemic dose as no specific data for this possible contribution to a systemic dose is known. The possible effects of formaldehyde penetrating the nail are discussed later in the chapter.
Twenty-five percent formaldehyde will evaporate during the drying process. It is assumed that it is released in the close area around the person (1 m³), and that half of it is absorbed via inhalation or dermal absorption.
Therefore, calculating the systemic exposure dose (SED) only includes the part constituting the skin surrounding the nail (9%) and half the part evaporating during the drying process; 12.5% of the total amount contained in the product. In total 21.5% of the total content of formaldehyde will contribute to a systemic dose.
Daily exposureinhalation+dermal, formaldehyde:
![]()
The daily exposure dose (SED) of formaldehyde in nail hardeners is calculated as mg per kg bodyweight per day (mg/kg bw/day) for the model person.
Normally, a cosmetic product with a margin of safety (MoS) of more than 100 is considered to be a product exposing the user to an acceptable (minimum) health risk (28). When calculating the margin of safety, a safety factor of 10 for extrapolation of data from animals to humans and a safety factor of 10 for particularly sensitive human individuals are taken into account.
![]()
Based on the demonstrated NOAEL values for systemic effects of formaldehyde (15 mg/kg bw/day), a margin of safety is calculated for the maximum allowed concentrations of formaldehyde in nail hardeners; 5% (table 7-1).
Table 7.1 The systemic exposure dose (SED) and NOAELs for formaldehyde are used to calculate the margin of safety.
| Substance | NO(A)EL (mg/kg bw/d) |
Daily dose, adult, 40 kg (SED) (mg/kg bw/d) |
MoS |
| Formaldehyde | 15a | 0,067 | 224 |
a Based on systemic effects other than cancer
The calculated margin of safety for formaldehyde in nail hardeners is larger than 100, which indicates an acceptable health risk in relation to systemic effects and the scenario described above.
A critical effect of formaldehyde is its possible potential as carcinogen. The carcinogenic potential of formaldehyde is observed in humans after inhalation of large concentrations in the working environment (2).
The threshold limiting value (TLV) of formaldehyde is 0.4 mg/m³. The threshold limiting value of 0.4 mg/m³ is based on the concentration in the working environment for a working day of 8 hours corresponding to a total concentration of 3.2 mg/m³.
It is assumed that 25 percent of the formaldehyde contained in the nail hardener will evaporate during the drying process. It is further assumed that it is released in the close area around the person (» 1 m³). This corresponds to a concentration in the close area of the person of 3.125 mg/m³ in a short period of time each time the nail hardener is used. The use of nail hardeners’ during one year is maximum 124 times (see appendix 10.1) giving a daily mean exposure of 1.06 mg/m³ (3 times lower than the total threshold value) indicating that the life-time risk of cancer by using nail hardener is negligible. However sensitive persons may experience irritation of the nasal cavity and the upper respiratory tract during the short time of use (½ hour) of nail hardeners as the short-term concentration during use is higher than the threshold limiting value.
7.1.2 Risk assessment for sensitisation
Based on published literature the most critical effect of nail hardeners are sensitisation. However, it is difficult to establish a lower limit for this effect. Thus the above exposure scenario did not include this effect. One of the effects observed after use of nail hardeners are dermatitis at sites distant from the fingers, commonly eyelids, around the mouth and chins, sides of the neck, and on the genitalia (30),(31). However, effects directly at or around the finger nails are also observed (30). Depending on the concentration of applied formaldehyde and the physiological condition of the nail, some formaldehyde may penetrate the nail plate and come into contact with the underlying tissue. So far there have not been any scientific studies that have identified the amount of formaldehyde penetrating the nail under different conditions. Therefore, there are no quantitative estimates on how much formaldehyde there will be present at the underlying tissue after application of nail hardener. The possible effects may be irritation of the tissue under the nail and possible allergic reactions.
Formaldehyde has both a irritating and sensitisation potential. With standard patch tests protocols it has been observed that formaldehyde concentrations of 2% and higher may produce skin irritation and sensitisation in non-sensitised individuals (7). Allergic reactions to formaldehyde concentrations of 1% has also been observed in patients with skin problems (7). Based on Local Lymph Node assays (LLNA) induction of sensitisation was observed at formaldehyde concentration from 0.4 to 0.96% (9),(12).
A large range of human patch tests has been performed with formaldehyde during the last 30 years. Based on the large amount of data it was suggested that a dermal allergic response to formaldehyde concentrations below 0.025 – 0.05% is rare in formaldehyde-sensitised subjects (7).
This indicates that the legal amount of 5% formaldehyde in nail hardeners adds a risk of sensitisation to the users. Based on the data presented in the safety assessment a maximum allowable amount of formaldehyde of 0.01% in nail hardeners would be considered safe for both non-sensitive and formaldehyde-sensitised subjects, while a higher concentration may be considered safe for non-sensitized individuals. The scientific committee on consumer products (SCCP) has recently been requested for an opinion on formaldehyde in cosmetic products including the use in nail hardener products. This opinion may help establish a safe concentration for induction of formaldehyde allergy under this specific exposure.
As described before, the sensitisation of contact allergens are dependent not only on the inherent sensitisation capacity of the chemical, but also on the dose and type of exposure, the amount of allergen per unit area surface of skin, the vehicle and the condition of the skin. Such data has not been evaluated in this project. However, it may be argued that a higher concentration of formaldehyde than 0.01% in nail hardeners may be safe based on the limited exposure of the skin during normal use of the products. The legal amount of formaldehyde as a preservative in cosmetics is 0.2%. The opinion from SCCP may clarify if a higher concentration than this can be considered safe in nail hardener products. Furthermore, a warning on the products may help protecting formaldehyde-sensitised individuals. The products could be labelled with the warning ‘contains more than 0.05% formaldehyde’.
7.1.3 Overall discussion on nail hardeners’
The risk assessment of formaldehyde in nail hardeners shows that the allowable concentration of free formaldehyde in nail hardeners gives no health concerns regarding systemic effects of formaldehyde. However, the legal allowable amount of formaldehyde seems high regarding the sensitisation potential of the substance. To avoid unacceptable numbers of induction of allergic reactions and elicitation in already sensitised individuals to this kind of products a lower concentration may be argued. Based on the data presented in the safety assessment a maximum concentration of 0.01% formaldehyde in nail hardeners would be regarded as safe for all kind of users. A higher concentration limit can be argued if the purpose is to protect non-sensitised individuals.
Several products were found to contain much lower levels of formaldehyde (< 1 %) than the allowed 5 %. This shows that it is possible to produce nail hardeners with lower levels of formaldehyde. Other kinds of substances with a hardening effect in the products are, however, not covered by his safety assessment. It can not be excluded that they also may pose a risk for the users.
During use of the product it is further recommend that it should take place in rooms with well airing minimising the exposure via inhalation and thereby the risk of irritation of the air ways. Furthermore, the users should be very carefully when they apply nail hardeners at the nail plate to avoid skin contact.
7.2 Exposure and risk assessment for consumers using artificial nails
The safety assessment of the selected 4 acrylates showed that the critical effect of the substances is their potential to cause sensitisation or/and irritation. Limited data was found describing systemic effects.
Substances in artificial nails may be absorbed through the skin surrounding the nail when/if the material is in contact with the skin. Some of the substances in the products are volatile at room- and skin temperature and may be inhaled after evaporation from the nail and areas of the skin. Therefore, it may be relevant to include calculation of inhalation in the dose estimation. During the preparation of the nail, one should file the nail into shape. By that procedure, fine dust from the nail may expose the user further for different chemicals. This exposure is not included in the scenarios as it is evaluated to be most important for the safety of the nail technician, which is not included in the project.
The user may also bite or suck their fingers and thereby expose them self via the oral route. However, in this risk assessment the focus was the dermal exposure as this exposure route is evaluated to constitute the largest contribution to possible risks for the users.
The exposure of the substances in artificial nails depends on which system is used. Exposure has been calculated for a model person of 40 kg to include younger girls. The model person uses two different artificial nail systems; acrylic artificial nails (liquid and powder) or gel nails. Artificial nails are added on the nails maximum every second month. But as the largest exposure happens the day of fixing the artificial nails an acute scenario is described for that day; independent of the long-term exposure.
7.2.1 Risk assessment for systemic effects – artificial acrylic nails
Acrylates
2-Hydroxyethyl acrylate, 2-hydroxypropyl methacrylate and ethylene dimethacrylate were found in artificial acrylic nails products.
The nails approximately amount to maximum 40 cm². The skin surrounding the nails will amount to about 4 cm² (28), corresponding to about 9% of the total area of nail and skin. Therefore, it is assumed that 9% of the applied nail product will cover the skin surrounding the nail, and thereby may contribute to the systemic dose.
The following assumptions were used as realistic worst-case scenario:
Weight of person, adult: 40 kg
Applied amount per application (liquid): 2 ml » 2 g
Content in product (maximum): 15% » 150 g/kg product
Percent of product reaching the skin: 9%
Absorption: 100%
It is further assumed that a negligible amount of acrylate penetrate the nail.
Using the described assumptions a daily exposure would be:
Daily exposuredermal, ethylene dimethacrylate:
![]()
NOAELS for systemic effects were only found for 2 of the 3 acrylates (table 7.2).
Table 7.2 The systemic exposure dose (SED) and NOAELs for acrylates found in artificial acrylic nails are used to calculate the margin of safety (MoS).
| Substance | NO(A)EL (mg/kg bw/d) |
Maximum amount in product (%) |
Daily dose, adult, 40 kg (SED) (mg/kg bw/d) |
MoS |
| 2-Hydroxyethyl acrylate | 131 | 0.03 | 0.001 | 131,000 |
| 2-Hydroxypropyl methacrylate | 1000 | 10.25 | 0.46 | 2,174 |
| Ethylene dimethacrylate | - | 15 | 0.675 | - |
Based on this scenario and the NOAELS found for the acrylates it clearly shows that the substances will pose no health risk for the users of artificial acrylic nails concerning systemic effects. For ethylene dimethacrylate no NOAEL was identified. However, with the calculated exposure, it is evaluated that the risk of systemic effects is negligible.
7.2.2 Risk assessment for systemic effects – artificial gel nails
The realistic worst-case exposure scenario for a model person using the gel nail system was based on the following assumptions:
Weight of person, adult: 40 kg
Applied amount per application (gel) 3 g
Content in product (Gel): 8.25% » 82.5 g/kg product
Percent of product reaching the skin: 9%
Absorption: 100%
It is further assumed that a negligible amount of acrylate penetrate the nail.
As for the artificial acrylic nail system it is assumed that 9% of the applied nail product will cover the skin surrounding the nail, and thereby may contribute to the systemic dose.
Daily exposuredermal, 2-hydroxyethyl acrylate:
![]()
Table 7.3 The systemic exposure dose (SED) and NOAELs for acrylates found in gel nails products are used to calculate the margin of safety (MoS).
| Substance | NO(A)EL (mg/kg bw/d) |
Maximum amount in product (%) |
Daily dose, adult, 40 kg (SED) (mg/kg bw/d) |
MoS |
| 2-Hydroxyethyl acrylate | 131 | 8.25 | 0.56 | 234 |
| 2-Hydroxypropyl acrylate | 7A | 0.865 | 0.06 | 116 |
| 2-Hydroxypropyl methacrylate | 1000 | 1.45 | 0.1 | 10,000 |
| Ethylene dimethacrylate | - | 0.041 | 0.003 | - |
A LOAEC
As for the acrylic nail system, the gel nails pose no health risks for the users concerning systemic effects of the substances.
7.2.3 Risk assessment for sensitisation – artificial nails
This survey of artificial nails has shown that the products contain a large range of substances; ex. different acrylates (chapter 4). Thus, a comprehensive risk assessment of the products is difficult to perform as not all the ingredients are known. The critical effect of many acrylates is their sensitisation potential, for which a lower safe exposure dose is complex to establish. Furthermore, literature shows that many acrylates cross-react with other acrylates making the safety assessment more complex (21,32).
Literature shows that sensitisation is a health problem for the users of artificial nails (20,21,33,34,35). The data from the literature is based on human patch tests performed with already sensitised persons, and not test of the induction of allergic reaction in healthy persons. The literature shows that the allergic lesions due to artificial nails are classically around the nails, but are also seen spread to the face and eyelids (31). Allergic reactions may occur 2 to 4 months, and even as long as 16 months after the application. The first indication is an itch in the nail bed. Paronychia, which is usually present in allergic reactions, is associated with excruciating pain in the nail area, and sometimes with paresthesia. The nail bed, thickened, and there is usually onycholysis. The natural nail plate becomes thinner, split and sometimes discoloured. It takes several months for the nail to return to normal. Permanent nail loss is rare (30).
Artificial acrylic nails
The acrylic artificial nail system consists of a liquid and a powder. 2-Hydroxypropyl methacrylate and ethylene dimethacrylate was found in the liquid used to develop the acrylic artificial nail. Five different liquids contained the two acrylates in concentrations from 0.89 to 15% (table 4.3). The maximum amount found was 10.25% for 2-hydroxypropyl methacrylate and 15% for ethylene dimethacrylate. The powder contained none of the analysed acrylates.
Based on human patch tests and animal studies the two acrylates are evaluated as weak sensitisers in a concentration of 2%. However, in these products up to 15% of one acrylate has been observed. The substances potential to elicitate sensitisation in minimum 13% of the tested persons, when tested in a concentration of 2% indicates that concentrations of 10-15% may pose a significant risk of sensitisation.
It is evaluated that the risk can be minimised by avoiding skin contact. The very central point is not by accident to smear the product outside the nail, and thereby avoid contact with the surrounding skin. By avoiding skin contact it is evaluated that the risk of sensitisation is considerably lowered.
Artificial gel nails
The gel nail system is based on one product (a gel) that is smeared on the nail like nail polish about ten times after each other. 2-Hydroxyethyl acrylate was found in 6 out of 7 analysed gel nail products in concentrations from 3.05 to 8.25 %. 2-Hydroxypropyl acrylate was found in 4 out of 7 products, but in lower concentrations; from 0.041 to 0.865. Ethylene dimethacrylate was found in very low concentrations in 4 products, while 2-hydroypropyl methacrylate only was found in 1 product in a concentration of 1.45% (table 4.2). 2-Hydroxyethyl acrylate is clearly the most frequent substance of the analysed acrylates in gel nails.
Based on human patch tests and animal studies 2-hydroxyethyl acrylate and 2-hydroxypropyl acrylate are evaluated as moderate sensitisers. The two acrylates is found to elicitate allergic reactions in a considerable number of humans (> 8% of acrylate-sensitised subjects) in a concentration of 0.1%. The acrylates are evaluated to have a higher potential as sensitisers than the methacrylates found in acrylic nails. This indicates that the use of gel nails may pose a higher risk of sensitisation than acrylic nails.
7.2.4 Overall discussion on artificial nails
It was shown in the risk assessment that use of artificial nails will pose no risk of systemic effects. However, the assessments showed that the amounts of acrylates in the products are important for the health risks of the user regarding the sensitisation potential. This survey only handled a few acrylates. It is evaluated that the products may contain a large spectrum of other acrylates as well (see table 4.4 – 4.5) with the same or even larger potential of contact allergy. The survey further showed that the potential risk of contact allergy seems higher for the gel nail system than for the acrylic artificial nails (liquid and powder) based on the content of acrylates in the products.
8 Conclusion
The objective of this survey was
- To make a survey of allergy causing chemicals in nail hardeners and products for manufacture of artificial nails to be sold to private persons from the retail trade, the internet and beauty shops.
- Analyse selected products for their content of e.g. acrylic compounds
- To perform health risk assessment of selected chemicals found during the analyses
- To suggest a safe concentration of formaldehyde in nail hardeners.
A wide selection of products was purchased. Nail hardeners were purchased from the retail trade, while products for artificial nails could only be purchased from the internet.
The purchased products were examined for ingredients and their labelling was assessed. Many of the examined products appeared not to be in accordance with the demanded labelling requirements. Most significant was the insuffiently prepared ingredient declaration and the lacking directions for use. As shown in this survey, directions for use are imperative especially for artificial nail products to secure a correct application of the products on the nails, which is extremely important to avoid allergy health risks.
Nail hardeners were examined for free formaldehyde while the artificial nails were examined for different acrylic compounds.
None of the examined nail hardeners contained more than the permitted 5% formaldehyde. For only one product, formaldehyde was not on the list of ingredients, even if the content was greater than the demanded labelling requirement of 0.05%.
The investigations showed that the content of acrylates is very different from product to product. Within the framework of this project it was not possible to identify the complete composition of the products.
The health assessment of the products showed that especially contact allergy is the critical effect of the examined ingredients of the products.
A realistic worst-case scenario was set up for nail hardeners and the risk of systemic effects when using nail hardeners was calculated. The calculation showed that there is no risk of systemic effects when using nail hardeners containing the permitted amount of formaldehyde which is 5%. As formaldehyde can evaporate during the process, it is recommended to let in some air after application of nail hardeners to limit the exposure most possible by inhalation and deposition of vapour on the skin.
Formaldehyde may cause allergy and is classified as allergenic. Elicitation of allergy has been observed in formaldehyde sensitive persons in concentrations down to 0.05% formaldehyde, while induction of allergy in animal studies is seen at about 0.4 – 0.96% formaldehyde. The greatest risk of allergy is expected to develop if the nail hardener comes into contact with the skin. Exposure of the skin through application of nail hardener is expected to be limited, but application of nail hardener on the cuticle and the skin around the cuticle is hazardous and may cause allergic reactions. Irritation and allergy may also occur, if nail hardeners are applied on broken nails. In this case, formaldehyde may come into contact with the tissue under the nail and the risk of irritation and allergy will increase.
On the basis of the material generated in this project the permitted concentration of 5% is assessed to constitute a risk of allergy induction in healthy persons and a high risk of elicitation of allergy in formaldehyde sensitive persons. Based on the literature presented in this report, a maximum concentration of 0.01% formaldehyde in nail hardeners is assessed to be a safe concentration compared with the risk of allergy when applied both by healthy persons and persons already suffering from allergy. If the objective is to prevent healthy persons from developing formaldehyde allergy, a higher limit of concentration may be argued. However, this is difficult to set as there is no existing clear limit value for induction of formaldehyde allergy for this type of exposure. Formaldehyde is permitted as preservative in cosmetic products in a concentration of 0.2%. It can be argued that the concentration in nail hardeners as a minimum should not exceed this limit to diminish the risk of induction of allergy in healthy persons. A warning to already sensitive persons should appear on the product about the content of formaldehyde in a concentration above 0.05%. The Scientific Committee of Consumer Products (SCCP) of EU has been requested to assess a safe limit value of the content of formaldehyde in nail hardeners. This survey showed that several nail hardeners on the Danish market contains below 1% formaldehyde (4 out of 6 tested). Therefore, there are alternatives to the products containing larger amounts of formaldehyde. One of the six tested products only contains 0.01% formaldehyde.
The health assessment of products for building up artificial nails showed that there is no health risks of systemic effects related to the use. On the other hand, the risk of contact allergy by applying these products is assessed to be significant. This assessment was due to the amount of acrylates found in the products compared with the allergenic potential. Skin contact will result in a risk of contact allergy, if the products contain allergenic acrylate monomers in concentrations of up to 15%. This applies specifically for the gel nails that contain high concentrations (up to 8%) of particularly 2-hydroxyethyl acrylate, which is considered to be the ingredient with the highest allergenic potential of the tested ingredients. In patch tests with acrylate sensitized persons elicitation of allergy at concentrations of 0.1% 2-hydroxyethyl acrylate was observed. The acrylic nails (liquid and powder) appeared to produce a lower risk of contact allergy, as these products contain lower concentrations of the most allergy potent acrylates in these products. However using the products still constitutes a significant risk of allergy.
The conclusion of this survey project is that the products contain chemical substances in concentrations so high that they constitute a significant risk of contact allergy. The survey also demonstrates how important the application of this type of product is. It is suggested that professional and competent personnel builds up artificial nails instead of the consumer. This survey project does not comprise the working environment for personnel in beauty clinique’s etc., but it is important to emphasize that these products contain substances that may give rise to concern within the working environment. Many of the purchased products are marketed for professional use, but the private consumer is assessed not to have difficulty in obtaining the products for private use. However, the risk of contact allergy increases considerably by use, if the products come into contact with the skin. It is also assessed that the risk of skin contact decreases with the increasing experience in building up artificial nails.
8.1 Recommendations to the consumers
Be careful when applying nail hardener so that it only covers the nail and not the skin. This decreases the risk of formaldehyde.
To reduce the amount of formaldehyde that is inhaled and deposited on the skin, the room should be well aired when using nail hardeners
Do not use nail hardeners containing formaldehyde on broken nails. If the nail is broken, the ingredients can come into contact with the tissue under the nail and increase the risk of allergy.
Use qualified nail technicians for building up ”powder + liquid nails”, ”gel nails”, ”wrapping nails” and ”dip on nails”. These products should not be applied by private consumers, due to the increasing risk of exposure of allergenic substances when misused.
9 References
1. Miljø- og Energiministeriets bek. nr. 422 af 4. maj 2006 (Bekendtgørelse om kosmetiske produkter), (2006)
2. Formaldehyde. Geneva: World Health Organization; 2002. (Concise international chemical assessment document; 40
3. SIDS (Screening Information DataSet). Screening Information Data Set for High Production Volume Chemicals; Formaldehydel; CAS No. 50-00-0. Paris, France: UNEP Publications; 2002. SIDS initial Assessment Reports )
4. European Chemicals Bureau (ECB), editor. ESIS. [updated 2007]. Available from: http://ecb.jrc.it/esis/.
5. Hazardous Substances Data Bank (HSDB). National Library of Medicine, Bethesda, Maryland. Thomson MICROMEDEX®, Greenwood Village, Colorado, USA [updated 2007]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
6. Wibowo A. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committe on Occupational Standards. - 132: Formaldehyde. Solna: Arbetslivsinstitutet; 2003. (Arbete och hälsa; 2003:11
7. Wilbur S, et al. Toxicological Profile for Formaldehyde. Atlanta, Ga: United States Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1999.
8. Hilton J, Dearman RJ, Basketter DA, Scholes EW, Kimber I. Experimental assessment of the sensitizing properties of formaldehyde. Food Chem Toxicol. 1996;34(6):571-8.
9. Basketter DA, Kimber I. Predictive testing in contact allergy: facts and future. Allergy. 2001;56(10):937-43.
10. Hilton J, Dearman RJ, Harvey P, Evans P, Basketter DA, Kimber I. Estimation of relative skin sensitizing potency using the local lymph node assay: a comparison of formaldehyde with glutaraldehyde. Am J Contact Dermat. 1998;9(1):29-33.
11. Andersen KE, Boman A, Volund A, Wahlberg JE. Induction of formaldehyde contact sensitivity: dose response relationship in the guinea pig maximization test. Acta Derm Venereol. 1985;65(6):472-8.
12. de Jong WH, ter Beek M, Veenman C, de Klerk, A, van Loveren H. Effects of repeated and prolonged exposure of Low Molecular Weight chemicals on local lymph node responses RIVM; 2005. RIVM report 340300001)
13. IRIS: Integrated Risk Information System. U.S. Environmental Protection Agency, Washington, D.C. Toxicology Data Network (TOXNET®) [updated 2007]. Available from: http://toxnet.nlm.nih.gov/.
14. Naya M, Nakanishi J. Risk assessment of formaldehyde for the general population in Japan. Regul Toxicol Pharmacol. 2005;43(3):232-48.
15. Hagiwara M, Watanabe E, Barrett JC, Tsutsui T. Assessment of genotoxicity of 14 chemical agents used in dental practice: ability to induce chromosome aberrations in Syrian hamster embryo cells. Mutat Res. 2006;603(2):111-20.
16. Emri G, Schaefer D, Held B, Herbst C, Zieger W, Horkay I, et al. Low concentrations of formaldehyde induce DNA damage and delay DNA repair after UV irradiation in human skin cells. Exp Dermatol. 2004;13(5):305-15.
17. The Carcinogenic Potency Database (CPDB). Berkely Lab, [updated 2007]. Available from: http://potency.berkeley.edu/cpdb.html.
18. SIDS (Screening Information DataSet). Screening Information Data Set for High Production Volume Chemicals; 2-Hydroxyethyl acrylate; CAS No. 818-61-1. Paris, France: UNEP Publications; 2005. SIDS initial Assessment Reports )
19. ChemIdplus Lite Full Record. ChemIdplus Lite 2007. Available from: http://chem.sis.nlm.nih.gov/chemidplus.
20. Constandt L, Hecke EV, Naeyaert JM, Goossens A. Screening for contact allergy to artificial nails. Contact Dermatitis. 2005;52(2):73-7.
21. Lazarov A. Sensitization to acrylates is a common adverse reaction to artificial fingernails. J Eur Acad Dermatol Venereol. 2007;21(2):169-74.
22. Documentation of the Threshold Limit Values and Biological Exposure Indices. - [2]: E-O. 7 ed. Cincinnati, Ohio: ACGIH (American Conference of Governmental Industrial Hygenists) Worldwide; 2001.
23. SIDS (Screening Information DataSet). Screening Information Data Set for High Production Volume Chemicals; Hydroxypropyl acrylate; CAS No. 25584-83-2. Paris, France: UNEP Publications; 2002. SIDS initial Assessment Reports )
24. Andersen A.F. Safety assessment of cosmetic ingredients; Final Report of the safety Assessment of Methylacrylate ester monomers used in Nail Enhancement Products. International Journal of Toxicology. 2005;24(Suppl. 5):53-100.
25. Basketter DA, Evans P, Gerberick GF, Kimber IA. Factors affecting thresholds in allergic contact dermatitis: safety and regulatory considerations. Contact Dermatitis. 2002;47(1):1-6.
26. Toxicity Profile: Ethylene Glycol Dimethacrylate. Carshalton: BIBRA International, Information and Advisory Service; 1988.
27. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part I. 2nd ed. Luxembourg: Office for Official Publications of the European Communities: European Commission, Joint Research Centre, European Chemicals Bureau, Institute for Health and Consumer Protection; 2003.
28. SCCP. The SCCP's notes of guidance for the testing of cosmetic ingredients and their safety evaluation Scientific Committee on Consumer Products; European Commission; 2006.
29. Wilkinson JD, Shaw S, Andersen KE, Brandao FM, Bruynzeel DP, Bruze M, et al. Monitoring levels of preservative sensitivity in Europe. A 10-year overview (1991-2000). Contact Dermatitis. 2002;46(4):207-10.
30. Baran R. Nail cosmetics: allergies and irritations. Am J Clin Dermatol. 2002;3(8):547-55.
31. Kohl L, Blondeel A, Song M. Allergic contact dermatitis from cosmetics. Retrospective analysis of 819 patch-tested patients. Dermatology. 2002;204(4):334-7.
32. Rietschel RL, Fowler jr JF, editors. Fisher's Contact Dermatitis. 5 ed. Philadelphia, Pa : Lippincott, Williams & Wilkins: 2001.
33. Hemmer W, Focke M, Wantke F, Gotz M, Jarisch R. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3 Pt 1):377-80.
34. Mowad CM, Ferringer T. Allergic contact dermatitis from acrylates in artificial nails. Dermatitis. 2004;15(1):51-3.
35. Kanerva L, Lauerma A, Estlander T, Alanko K, Henriks-Eckerman ML, Jolanki R. Occupational allergic contact dermatitis caused by photobonded sculptured nails and a review of (meth) acrylates in nail cosmetics. Am J Contact Dermat. 1996;7(2):109-15.
10 Annex
10.1 Lifetime exposure to formaldehyde
As one of the critical effects of formaldehyde is cancer a scenario to calculate the life-time inhalation exposure and risk of formaldehyde by using nail hardeners has been performed.
The following use of nail hardeners has been stated in different product descriptions:
1. Apply one layer on the first day and a second layer on the second day.
2. On the third day remove both layers.
3. Repeat the steps for 14 days.
4. After this 14-days procedure the product should only be used 1-2 times peer week.
5. The 14-day treatment should not be repeated more than 1-2 times a year.
Estimating a life-time exposure based on these instructions of use will result in the following exposure:
2 times x 14 days = 28 times
Rest of the year 2 times peer week: 48 weeks x 2 times/week = 96 times
Each year; maximum of use is: 124 times
As the carcinogenic potential of formaldehyde is expected to be only after inhalation exposure only the assumed percentage of inhalation after use of nail hardeners are used to calculate the total life-time exposure.
Twenty-five percent formaldehyde is expected to evaporate during the drying process. It is assumed that it is released in the close area around the person corresponding to 1 m³.
Exposure of formaldehyde was calculated to be:
Daily exposureinhalation, formaldehyde:
![]()
The mean exposure during a year: 3.125 mg/m³ of apply x (124 times/365 days) = 1.06 mg/m³
Footnotes
[1] No reliable hits appear, but the sample is assumed to contain up to 100 components.
[2] No Observed (Adverse) Effect Level
[3] RfD: Reference Dose; calculated from NOAEL and an safety factor of normally 100
[4] TD50: The lifetime dose rate (mg/kg bw/day) to induce tumours in half of test animals that would have remained tumour free at zero dose.
[5] Acceptable dose: (TD50 x 2) x 10-6
[6] From the instruction of use the following use of product is recommended: Apply one layer on the first day and a second layer on the second day. On the third day remove both layers. Repeat the steps for 14 days. After this 14-day treatment the product should only be used 1-2 times peer week. The 14-day treatment should not be repeated more than 1-2 times a year.
Version 1.0 July 2008, © Danish Environmental Protection Agency