Survey of Chemical Substances in Consumer Products, No. 98, 2008
Survey and Health Assessment of Possible Health Hazardous Compounds in Proofing Sprays
Contents
2 Literature retrieval and information search
- 2.1 Introduction
- 2.2 Preliminary searches
- 2.3 Bibliographical database searches
- 2.4 Results
- 2.5 Summary of results and conclusion
- 4.1 Background. Summary of literature retrieval and survey
- 4.2 Selection of products
- 4.3 Analysis programme
5 Results of screening of compounds
6 Results of quantitative analyses and aerosol analyses
7 Discussion of analysis results
- 8.1 Butyl acetate
- 8.2 Butanone
- 8.3 1-Butanol
- 8.4 Cyclohexane
- 8.5 Perfluoroctane-1-ol
- 8.6 Dodecamethylpentasiloxane
- 8.7 Recapitulation on health assessment and information collection
Enclosure 1: Report from the Danish Poison Information Centre
Preface
The project ”Survey and Health Assessment of Possible Health Hazardous Compounds in Proofing Sprays” was carried out from April 2007 till November 2007.
This report describes the project results, comprising a literature retrieval and information search about cases of toxification from proofing agents, survey of products and chemical analyses and a health assessment of a number of selected products.
As a starting point, registered information was collected about toxification of consumers who had used proofing sprays.
In addition, it was examined which products exist on the Danish market within the category textile proofing sprays.
Subsequently, a plan was drawn up for analyses and experimental investigations in co-operation with the Danish Environmental Protection Agency. When the plan had been accepted, chemical and aerosol analyses of selected products as well as a health assessment of selected compounds were carried out.
The project was carried out by Danish Technological Institute with M.Sc., Ph.D. Anders Feilberg as project manager and cand. arch. Kathe Tønning, M.Sc., Ph.D. Anne-Gry Hemmersam and laboratory manager Eva Jacobsen as project co-workers. The health assessment was carried out by graduate in pharmacology Inge Søborg and M.Sc., Karl-Heinz Cohr from DHI.
In addition, the Danish Poison Information Centre at the Bispebjerg Hospital (Danish Clinic for Occupational and Environmental Medicine) contributed with an outline of Danish cases of toxification in connection with textile proofing agents. The outline can be seen in enclosure 1.
The project was followed by a reference group consisting of the following persons:
Anette Ejersted the Danish Environmental Protection Agency (chairman of the reference group)
Magnus Løfstedt the Danish Environmental Protection Agency
Bettina Ørsnæs Andersen the Danish Environmental Protection Agency
Anders Feilberg Danish Technological Institute
The project was financed by the Danish Environmental Protection Agency.
Summary and conclusions
Many different types of proofing sprays are sold directly to the consumers as agents for aftertreatment of different types of textiles especially in order to obtain a water- and dirt-repellant effect.
In recent years, it has been observed internationally and in Denmark that spray products for proofing of textiles in certain cases result in acute respiratory illness and similar acute poisoning symptoms. During the period from 1991 to 2007, 84 cases of varying degrees of poisoning in connection with the use of textile proofing were identified in Denmark. It has not been possible to find any unambiguous reason for the cases of poisoning on the basis of the information about the compounds.
Therefore, this project has been implemented in order to investigate textile proofing sprays on the Danish market.
The starting point of the project was a need for greater knowlege about the compounds in this type of product and the size of the aerosols humans are exposed to.
The following elements form part of the project:
- Literature retrieval and information search
- Survey of products on the market
- Investigation of chemical composition of substances
- Investigation of liberation of small aerosols during use
- Health assessment of the products.
The most important project results will be exmined in the following.
Literature and information search
By means of systematic searches in scientific data bases information has been collected about toxic effects in connection with spray proofing and about the composition of the proofing sprays with regard to proofing agent, solvent and possible propellant.
Many of the cases of poisoning that have been reported for proofing sprays have in common that a previous rewording has taken place of the products in connection with substitution of the solvents used.
Some proofing sprays that have caused acute toxicity in humans have subsequently been tested on animals. No information exists about the toxicological impact mechanism of particular proofing sprays but is must be assumed that the proofing agents influence the surface conditions in the lungs e.g. the surface tension and thus the lung function and might hamper the passage of oxygene across the alveolars.
The spray proofing agents involved in the reported cases of toxification most often contain some type of fluorcarbon polymer (15 out of 17 products). The manufacturers keep the chemical structure secret to avoid product copying. Please note that a few products in addition to fluorine compounds also contain silicone compounds.
In general, it is easier to procure information about which solvents and propellants form part of the product, whereas amount specifications rarely are stated.
Only limited information exists about the size distribution of the aerosols consumers are exposed to when using proofing spray. This project uses the term aerosols about material and substances that are not gaseous and that are suspended in air. As a starting point, liquid aerosols are in question but it cannot be ruled out that these subsequently will assume a solid or amorphous physical structure.[1]
The type of the solvent as well as the appearance of specific fluorcarbon compounds and the aerosol size can be of importance to the observed cases of poisoning but a more precise reason cannot be concluded from literature.
Survey
The survey comprised the following activities:
- Contact to the retail trade. 21 of the procured products were purchased in physical shops.
- Search on the internet. Many homepages with internet shops were visited and 5 of the products were purchased on the internet.
- Contact to distributors/importers. Approaching importers of the products that form part of the survey resulted in information about the substances in the products whereas information on sale of the products in Denmark only has been received from few importers.
Products have been purchased for textile proofing within the product groups:
- Products for proofing of shoes
- Products for proofing of tents and the like
- Products for proofing of furniture
- Products for proofing of clothes for outdoor use, e.g. jackets or the like.
The main selection criteria for purchase of products have been that the products have to be sold to a certain degree. It has especially been possible to use that criterion when visiting physical shops and the staff was asked which products are “best selling”, but it has not been possible to use that criterion in connection with internet trade.
Consumption of sprays for textile proofing
It has not been possible to procure information from any of the contacted importers about their sale on the Danish market and therefore it has not been possible to estimate the extent of products sold for textile proofing.
Selection of products for further investigation
The survey resulted in the registration of 29 products (17 spray products with propellant and 12 spray products with pump) and in co-operation with the Danish Environmental Protection Agency 16 products were selected for further investigation.
Chemical analyses
16 products were chosen for analysis and the principle was that spray as well as pump products should be represented, that fluorine as well as silicone based products should be investigated and that products with known as well as unkown substances should be examined.
Subsequently, the following screening analyses were carried out:
- Element analyses for content of fluorine or silicone in the surface coating of proofed textile by x-ray.
- Screening for content of volatile and semi-volatile organic substances in the aerosol mist that appears when the products are used, by means of gas chromatography with mass spectrometric detection (GC/MS).
The screening analyses showed that nearly all products contained varied amounts of fluorine (0.1-15 %). Fluorine was not detected in 2 out of the 16 analysed products. Silicium was measured in 11 products.
Summarised, the results show that 13 of the 16 products probably are based on a fluorcarbon coating. One single sample contained only a small amount of fluorine and substantially more silicium.
The screening analyses for volatile and semi-volatile organic substances showed content of a wide range of solvents and propellants. However, in two products it was not possible to demonstrate content of volatile or semi-volatile organic substances. 10 of the 14 other products contained large amounts of hydrocarbons in the form of hydrocarbon mixtures that function as organic solvents. Most products contained varying amounts of polar organic solvents. Some products also contained aromatic compounds and one single product contained chlorinated solvents.
In addition, the screening analyses showed the appearance of one fluorine compound and silicone/siloxane compounds. From the chemical analyses, the assumed fluorine substances turned out to be structurally related to the so-called fluortelomers, meaning substances with the structure CF3(CF2)nCH2CH2OH. An example is 1H,1H,2H,2H-perfluoroctanol.
In the light of the screening analyses, 10 products were chosen for quantitative chemical analyses. The quantitative analyses were carried out on 14 substances in the chosen products. For some products, the concentration in the products was below the detection limit but most substances could be analysed in one or several products.
When comparing with an analysis of a standard it could be ruled out that some of the products contained 1H,1H,2H,2H perfluoroctanol. Additional analyses could not uncover the exact chemical structure of the detected fluorine compounds. The concentrations of detected fluorine compounds were low compared with the x-ray analyses and therefore it must be assumed that the main part of the fluorine compounds is polymerised during the analysis and therefore they cannot be detected. That might be because the active ingredient is designed to polymerise on contact with air and in that way create a proofing coating.
Aerosol analyses
All 16 products that were chosen for analyses were analysed for liberation of small aerosols in the size interval of 6-650 nm. As far as it is known, it is the first time systematic measurements were carried out on small aerosols and nanoaerosols which the consumer is exposed to when using spray proofing.
The results unambiguously show that middle-sized aerosols in the interval of 50-200 nm are liberated when propellant based spray products are used. The measured aerosol concentrations are in the area of 105-106 cm-3 at an exposure time of 10 s. When using pump products the amount of liberated small aerosols is very small or insignificant.
The reason for the difference between pump products and propellants is that pump products give larger primary aerosols that are deposited more efficiently on the textile surface than the smaller aerosols from propellants. In the case of non-deposited aerosols a quick evaporation of solvents will take place and then aerosols consisting of non-volotile substances will remian in the air.
As part of the project, a test rig was developed for investigation of proofing products with regard to liberation of small aerosols and determination of the aerosol size distribution.
Health assessment
In the project, health assessments were carried out on 6 substances found either in the semi-quantitative screenings or the quantitative analyses of chemical substances in spray products intended for textile proofing. The assessments of the health conditions were carried out on the basis of worst worst case scenarios. The 6 investigated substances were cyclohexane, butan-2-on, 1-Butanol and butyl acetate which are solvents and perfluoroctan-1-ol and dodecamethylpentasiloxane.
The assessments showed that the procured textile sprays only contained substances that were listed in the Danish Ministry of the Environment’s Regulation on propellants and solvents to be used in aerosol products (the Danish Environmental Protection Agency, 1984). However, the organic solvent butyl acetate must not appear in products for indoor household use. The content of organic solvents is not a health related problem in these spray products assessed in relation to substance limit values of the Danish Working Environment Authority.
In connection with the assessed substances the rule is that margin of safety (MOS) has to be at least 100 compared to the NOAEL value (no observed adverse effect level) in the critical effect in a relevant animal study. A factor 10 is used for extrapolation from animals to humans and an additional factor 10 is used to protect the particularly sensitive groups or individuals. That criterion is normally used to protect users of consumer products.
On the basis of that criterion, the content of a polydimethyl siloxane that was found in one single spray product will not be a health hazardous risk.
Substances that structurally are similar to 1H,1H,2H,2H-perfluoroctanol were estimated to have MOS values of approx. 10, that is 1/10 of the protection level that normally is used for consumer products. In addition, it has only been possible to account for a small part of the total amount of fluorine compounds in the products and it is only that small part that forms part of the health assessment. This type of substance gives another reason for cautiousness as the available literature shows that fluorine compounds exist in most of the cases of poisoning where information about the chemical composition is available.
Aerosols from proofing products consist of small drops of proofing agent dissolved in solvents. The proofing agents are solid or liquid with extremely low vapour pressure. The solvents have a rather high vapour pressure and will evaporate quickly and leave liquid or solid particles of the proofing substances floating in the air – the smaller the aerosol particles the quicker the evaporation. In practice, the aerosols that are inhaled mainly consist of heavy volatile proofing substances. In concentrated form that can influence the surface tension in the lungs and result in changed lung function. No information exists about the combined influence of solvent vapours and aerosols on the respiratory system (possibly with a small solvent content).
Conclusion
Most ascertained cases of poisoning that arise when textile proofing has been used involve products that are based on fluorcarbon compounds.
It has not been possible to determine the exact chemical structure of the fluorcarbon compounds that exist in textile proofing agents and therefore it has not been possible to carry out a final health assessment of the products. However, in the light of the project results that prove the appearance of fluorine in most products it must be assessed as possible that exposure to non-polymerised or partly polymerised fluorcarbon compounds in rather high concentrations is possible.
The use of textile proofing agents sprayed with propellant results in a considerable exposure to fine (< 1 µm) and ultra fine aerosols (nanoaerosols) (< 100 nm). The toxicological effect from inhaling nanoaerosols is not yet known. Existing information in the field cannot document that small aerosols in themselves are harmful. However, many international research activities are being carried out on the toxicology of nanoaerosols and in a couple of years they will hopefully be able to shed more light on this problem. Aerosols can be carriers of (re)active chemical substances, e.g. fluorcarbon monomers but the importance is not known as the chemical structure of the substances could not be detected or procured in this project.
The classic toxicological assessments of the individual substances in a product are apparently insufficient when the product is sprayed by means of propellant. Physical properties, e.g. aerosol size, are determining factors that show if and which toxic effect might arise in the respiratory system. Toxic effects can arise when the solvents in aerosols evaporate after inhalation and result in a high local concentration in lungs/alveolars. When the solvent is evaporated small, solid or liquid aerosols are created. Respiratory symptoms could also be due to possible depositing of insoluble substances, e.g. fluorcarbon compounds on the surfaces of the respiratory passages. In that way, the proofing substances can affect the surface conditions in the lungs and thus the lung function and possibly restrain the passage of oxygen across the alveolars.
Sammenfatning og konklusioner
Der findes en lang række forskellige imprægneringsmidler, der sælges direkte til forbrugerne som midler til efterbehandling af forskellige typer tekstiler for primært at opnå en vand- og smudsafvisende effekt.
Gennem de seneste år er det observeret både internationalt og i Danmark, at sprayprodukter til imprægnering af tekstiler i visse tilfælde medfører akutte luftvejslidelser og lignende akutte forgiftningssymptomer. I Danmark er der således i perioden 1991 til 2007 registreret 84 tilfælde af varierende grad af forgiftning i forbindelse med anvendelse af tekstilimprægnering. Der har ikke ud fra viden om indholdsstoffer kunnet udledes nogen entydig årsag til forgiftningstilfældene.
Nærværende projekt er på den baggrund iværksat med henblik på at undersøge spraymidler til tekstilimprægnering på det danske marked.
Projektets udgangspunkt er, at der er behov for mere viden om denne type produkters indholdsstoffer samt størrelsen af de aerosoler, man eksponeres for.
Følgende elementer indgår i projektet:
- Litteratur- og informationssøgning
- Kortlægning af produkter på markedet
- Undersøgelser af kemisk sammensætning af indholdsstoffer
- Undersøgelse af frigivelse af små aerosoler under anvendelse
- Vurdering af den sundhedsmæssige risiko ved produkterne.
De væsentligste resultater af projektet er gennemgået i det følgende.
Litteratur- og informationssøgning
Ved hjælp af systematiske søgninger i videnskabelige databaser er der indsamlet information om toksiske effekter i forbindelse med sprayimprægnering samt om imprægneringsmidlernes sammensætning med hensyn til imprægneringsmiddel, opløsningsmiddel og eventuel drivgas.
Mange af de forgiftningstilfælde, der er rapporteret for imprægneringsspray, har til fælles, at der forudgående er sket en omformulering af produkterne i forbindelse med substitution af de anvendte opløsningsmidler.
Enkelte imprægneringssprays, der har forårsaget akut toksicitet i mennesker, har efterfølgende været testet i dyremodeller. Der findes ingen oplysninger om den toksikologiske virkningsmekanisme af partikulære imprægneringsstoffer, men det må formodes, at imprægneringsstofferne påvirker overfladeforholdene i lungerne, fx overfladespændingen og dermed lungefunktionen, og eventuelt hæmmer passagen af oxygen over alveolerne.
Sprayimprægneringsmidler, der er involveret i rapporterede forgiftningstilfælde, indeholder oftest en form for fluorcarbon-polymer (15 ud af 17 produkter). De kemiske strukturer hemmeligholdes af producenterne for at undgå kopiering af produkterne. Det skal bemærkes, at enkelte produkter ud over fluorforbindelser også indeholder silikoneforbindelser.
Det er generelt nemmere at få oplysninger om, hvilke opløsningsmidler og drivmidler der indgår i produkterne, men der er sjældent tale om mængdeangivelser.
Der foreligger kun begrænset information om størrelsesfordelingen af de aerosoler, man udsættes for ved anvendelse af tekstilimprægnering. I dette projekt anvendes betegnelsen aerosoler om materialer og stoffer, der ikke er på gasform, og som er suspenderet i luft. I udgangspunktet er der tale om væskeformige aerosoler, men det kan ikke udelukkes, at disse efterfølgende antager en fast eller amorf fysisk struktur.[2]
Såvel typen af opløsningsmiddel som forekomsten af specifikke fluorcarbon-forbindelser og aerosolstørrelsen kan have betydning for de observerede forgiftningstilfælde, men en nærmere årsag kan ikke udledes fra litteraturen.
Kortlægning
I kortlægningen er indgået følgende aktiviteter:
- Kontakt til detailhandel. 21 af de anskaffede produkter er indkøbt i fysiske butikker.
- Søgning på internettet. En lang række hjemmesider med internetbutikker er besøgt, og 5 af de indkøbte produkter er købt i internetbutikker.
- Kontakt til producenter/importører. Henvendelserne til importørerne for de produkter, der indgår i kortlægningen, har resulteret i oplysninger om indholdsstoffer i produkterne, hvorimod oplysninger om omfanget af solgte produkter i Danmark kun er modtaget fra enkelte importører.
Der er indkøbt produkter til tekstilimprægnering inden for produktgrupperne:
- Produkter til imprægnering af fodtøj
- Produkter til imprægnering af telte og lignende
- Produkter til imprægnering af møbler
- Produkter til imprægnering af beklædning til udendørs brug, som fx jakker eller lignende.
Udvælgelseskriterierne for indkøb af produkter har primært været, at det skulle være produkter, der sælges i et vist omfang. Dette kriterium har primært kunnet anvendes, hvor der har været tale om besøg i fysiske butikker, hvor personalet er blevet spurgt om, hvilke af deres produkter der ”går bedst”, mens det ikke har kunnet anvendes ved handel på internettet.
Forbrug af spraymidler til tekstilimprægnering
Det har ikke været muligt at få oplysninger fra samtlige kontaktede importører om omfanget af deres salg på det danske marked, og det har således ikke været muligt at estimere omfanget af solgte produkter til tekstilimprægnering.
Udvælgelse af produkter til videre undersøgelse
Kortlægningen resulterede i registrering af 29 produkter (17 sprayprodukter med drivgas og 12 sprayprodukter med pumpe), og i samråd med Miljøstyrelsen blev der udvalgt 16 produkter til videre undersøgelse.
Kemiske analyser
De 16 produkter til analyser blev valgt ud fra, at både spray- og pumpeprodukter skulle være repræsenteret, at både fluor- og silikonebaserede produkter undersøges, samt at både produkter med kendt og ukendt virkningsstof undersøges.
Der er herefter foretaget følgende screeningsanalyser:
- Grundstofanalyser for indhold af fluor eller silikone i overfladebelægningen på imprægneret tekstil ved røntgen
- Screening for indhold af flygtige og semiflygtige organiske stoffer i aerosoltågen, som fremkommer ved brug af produkterne, ved hjælp af gaschromatografi med massespektrometrisk detektion (GC/MS).
Screeningsanalyserne viste, at næsten samtlige produkter indeholdt varierende mængder af fluor (0,1-15 %). Kun i 2 ud af de 16 analyserede produkter kunne fluor ikke detekteres. Silicium blev målt i 11 produkter.
Resultaterne viser sammenfattende, at 13 af de 16 produkter efter al sandsynlighed er baseret på en fluorcarbon-belægning. En enkelt prøve indeholdt kun en lille mængde fluor og væsentligt mere silicium.
Screeningsanalyserne for flygtige og semiflygtige organiske stoffer viste indhold af en lang række opløsningsmidler og drivgasser. I to produkter kunne der dog ikke konstateres indhold af flygtige og semiflygtige organiske stoffer. 10 af de 14 øvrige produkter indeholdt store mængder af kulbrinter i form af kulbrinteblandinger, der fungerer som organisk opløsningsmiddel. De fleste produkter indeholdt varierende mængder af polære organiske opløsningsmidler. Enkelte produkter indeholdt tillige aromatiske forbindelser, og et enkelt produkt indeholdt klorerede opløsningsmidler.
Screeningsanalyserne viste endvidere forekomst af enkelte fluorforbindelser og silikone/siloxan-forbindelser. De formodet fluorholdige stoffer viste sig ud fra de kemiske analyser at være strukturelt beslægtede med såkaldte fluortelomerer, dvs. stoffer med strukturen CF3(CF2)nCH2CH2OH. Et eksempel herpå er 1H,1H,2H,2H-perfluoroctanol.
På baggrund af screeningsanalyserne blev 10 produkter udvalgt til kvantitative kemiske analyser. De kvantitative analyser blev udført for 14 stoffer i de valgte produkter. For enkelte stoffer var koncentrationen i produkterne under detektionsgrænsen, men de fleste stoffer kunne analyseres i et eller flere produkter.
Ved sammenligning med analyse af en standard kunne det udelukkes, at nogen af produkterne indeholdt 1H,1H,2H,2H-perfluoroctanol. Supplerende analyser kunne ikke afdække de nøjagtige kemiske strukturer af de detekterede fluorforbindelser. Koncentrationerne af detekterede fluorforbindelser var lave sammenlignet med røntgenanalyserne, og det må derfor formodes, at hovedbestanddelen af fluorforbindelser polymeriseres under analysen og derfor ikke kan detekteres. Dette kan skyldes, at den aktive ingrediens er designet til at polymerisere ved kontakt med luft og dermed danne en imprægneringsbelægning.
Aerosolanalyser
Alle 16 produkter, der blev udvalgt til analyser, blev analyseret for afgivelse af små aerosoler i størrelsesintervallet 6-650 nm. Såvidt vides er det første gang der er udført systematiske målinger af de små aerosoler og nanoaerosoler, man udsættes for ved brug af sprayimprægneringsmidler.
Resultaterne viser entydigt, at der ved anvendelse af drivgasbaserede sprayprodukter sker en frigivelse af aerosoler med middelstørrelse i intervallet 50-200 nm. De målte aerosolkoncentrationer er i niveauet 105-106 cm-3 ved en eksponeringstid på 10 sekunder. Ved anvendelse af pumpeprodukter er mængden af frigivne små aerosoler meget lille eller insignifikant.
Forklaringen på forskellen mellem pumpeprodukter og drivgasprodukter er, at pumpeprodukter giver større primære aerosoler, der deponeres mere effektivt på tekstiloverfladen end de mindre aerosoler fra drivgasprodukter. For ikke-deponerede aerosoler vil der ske en hurtig fordampning af opløsningsmidlerne, hvorefter der i luften vil restere aerosoler bestående af ikke-flygtige stoffer.
Som en del af projektet er der udviklet en testopstilling til undersøgelse af imprægneringsprodukter mht. afgivelse af små aerosoler og bestemmelse af aerosolernes størrelsesfordeling.
Sundhedsvurdering
Der er i projektet gennemført sundhedsvurderinger for 6 stoffer, fundet enten ved de semikvantitative screeninger eller ved de kvantitative analyser af kemiske stoffer i sprayprodukter beregnet til tekstilimprægnering. Vurderinger af de sundhedsmæssige forhold er foretaget ud fra opstillede worst case-scenarier. De 6 undersøgte stoffer er cyclohexan, butan-2-on, 1-butanol og butylacetat, der alle er opløsningsmidler, samt perfluoroctan-1-ol og dodecamethylpentasiloxan.
Vurderingerne viste, at de anskaffede tekstilsprays kun indeholdt stoffer, som var listet i Miljøministeriets bekendtgørelse om driv- og opløsningsmidler til brug i aerosolprodukter (Miljøstyrelsen, 1984). Derimod må det organiske opløsningsmiddel butylacetat ikke forekomme i produkter til indendørs husholdningsbrug. Indholdet af organiske opløsningsmidler er imidlertid ikke et sundhedsmæssigt problem i disse sprayprodukter vurderet i forhold til Arbejdstilsynets grænseværdier for stofferne.
For de vurderede stoffer er anvendt den regel, at margin of safety (MOS) skal være mindst 100 i forhold til NOAEL-værdien (no observed adverse effect level) i den kritiske effekt i et relevant dyrestudie. Der anvendes en 10-faktor for ekstrapolation fra dyr til menneske og yderligere en 10-faktor for at beskytte de særligt følsomme grupper eller individer. Dette kriterium er det normalt anvendte for at beskytte brugerne af forbrugerprodukter.
Ud fra dette kriterie vil heller ikke indholdet af en polydimethylsiloxan, der blev fundet i et enkelt sprayprodukt, udgøre en sundhedsmæssig risiko.
For stoffer, der strukturelt minder om 1H,1H,2H,2H-perfluoroctanol, blev estimeret MOS-værdier på ca. 10, altså 1/10 af det beskyttelsesniveau, som normalt anvendes ved forbrugerprodukter. Hertil kommer, at det kun har været muligt at redegøre for en mindre del af den totale mængde af fluorforbindelser i produkterne, og at det kun er denne mindre del, der indgår i sundhedsvurderingen. For denne type stoffer er der yderligere grund til forsigtighed, idet den foreliggende litteratur viser, at fluorforbindelser optræder i langt de fleste forgiftningstilfælde, hvor der foreligger information om den kemiske sammensætning af produktet.
Aerosoler af imprægneringsprodukter består af små dråber af imprægneringsstof opløst i opløsningsmidler. Imprægneringsstofferne er faste eller flydende stoffer med ekstremt lave damptryk. Opløsningsmidler har forholdssvis høje damptryk og vil hurtigt fordampe og efterlade flydende eller faste partikler af imprægneringsstofferne svævende i luften - jo mindre aerosolpartiklerne er, desto hurtigere fordampning. I praksis vil de aerosoler, der indåndes, hovedsaglig bestå af det tungtflygtige imprægneringsstoffer. Dette vil i koncentreret form kunne påvirke overfladespændingsforholdene i lungerne og derved medføre en forandring af lungefunktionen. Der findes ingen viden om den kombinerede virkning på luftvejene af opløsningsmiddeldampe og aerosoler (eventuelt med et lille indhold af opløsningsmiddel).
Konklusion
De fleste konstaterede forgiftningstilfælde efter anvendelse af tekstilimprægnering involverer produkter, der er baserede på fluorcarbon-forbindelser.
De nøjagtige kemiske strukturer af de fluorcarbon-forbindelser, der indgår i tekstilimprægneringsmidler, har ikke kunnet fastlægges, og en endegyldig sundhedsvurdering af produkterne kan derfor ikke foretages. Ud fra projektets resultater, der påviser forekomst af fluor i de fleste produkter, må det dog vurderes som sandsynligt, at eksponering for ikke-polymeriserede eller delvist polymeriserede fluorcarbon-forbindelser i relativt høje koncentrationer kan forekomme.
Anvendelse af tekstilimprægneringsmidler, der sprayes med drivgas, medfører en betydelig udsættelse for fine (< 1 µm) og ultrafine aerosoler (nanoaerosoler) (< 100 nm). Den toksikologiske betydning af indånding af nanoaerosoler er endnu ikke kendt. Den eksisterende viden på området kan ikke dokumentere, at små aerosoler i sig selv er skadelige. Aerosolerne kan være bærere af (re)aktive kemiske stoffer, fx fluorcarbon-monomerer, men betydningen heraf er ikke kendt, da stoffernes kemiske strukturer ikke har kunnet detekteres eller været tilgængelige i dette projekt.
Klassiske toksikologiske vurderinger af de enkelte indholdsstoffer i et produkt er tilsyneladende utilstrækkelige, når produktet sprayes ved hjælp af et drivgas. Fysiske karakteristika, fx aerosolens størrelse er en bestemmende faktor for om og hvilken toksisk effekt, der vil kunne opstå i luftvejene. Toksiske effekter kan opstå ved, at opløsningsmidlerne i aerosoler fordamper efter indånding og medfører en høj lokal koncentration i lunger/alveoler. Ved fordampningen af opløsningsmidlet dannes små, faste eller væskeformige aerosoler. Luftvejssymptomer kan også skyldes eventuel deponering af uopløselige stoffer, fx fluorcarbon-forbindelser på overfladerne i luftvejene. Derved kan imprægneringsstofferne påvirke overfladeforholdene i lungerne, og dermed lungefunktionen, og eventuelt hæmme passagen af oxygen over alveolerne.
Det tilbagestår endnu at blive vist om toksiciteten af stoffer på aerosolform stiger yderligere, når aerosolstørrelsen i tågerne aftager til nanostørrelser (< 100 nm). Der foregår dog adskillige internationale forskningsaktiviteter vedrørende nanoaerosolers toksikologi, som i løbet af nogle år forhåbentlig kan kaste mere lys over denne problemstilling.
1 Introduction
1.1 Background
A wide range of different proofing agents exist and they are sold directly to the consumers as agents for restorative treatment of different types of textiles most often to obtain water and stain repellency. Frequently, cases of toxification in connection with the use of these products have been reported. In a case from 2005, 10 people for instance became ill within two months as a result of using a certain product.
The main part of the products is sold as sprays. During use, consumers will therefore be exposed to aerosols from the chemical substances. That is why, it is relevant to assess if there might be a health hazard involved when inhaling the substances.
The chemical composition of the proofing agents differs. The products can e.g. be based on emulsions of wax or paraffin, on polysiloxanes or fluorine compounds. In addition, the products contain various solvents and aerosol propellants that in themselves can be problematic. In recent years, more so-called nanotechnological proofing agents have entered the market. Neither the chemical composition nor the nanotechnical characters of the products have been stated.
A possible health hazard from using the products is expected to depend on the chemical substances as well as on the size of the aerosols that are created in the spray products. Products using a pump mechanism typically result in aerosols with a size of approx. 100 µm whereas propellant sprays also result in aerosols below 10 µm. Ultra fine aerosols (< 100 nm) potentially pose a particular health hazard due to their extremely small size. In scientific literature, examples exist of ultra fine particles that are not hazardous in the same way as larger units, but they have toxic effects merely because of their size. However, it is unclear if that goes for all types of ultra fine particles. At the same time, ultra fine particles have a large capacity with regard to sorption of other substances due to the rather large specific area of surface (area of surface per volume or mass unit). In addition, the size of the particles might influence the exposure/bio accessibility as very small particles hypothetically can penetrate further into the finely branched alveolars.
As the number of aerosols and perhaps the specific area of surface of the aerosols can be of importance to the health effect it is important to know the size distribution and the aerosol concentration (amount per volume) rather than merely the mass per volume concentration when the potential health effect is to be assessed.
In general, it should be emphasized that there still is some uncertainty as to which extent ultra fine particles always pose health hazards or if the toxicity presupposes certain physical and/or chemical properties including the ability to sorb toxic substances.
In the light of the above, the Danish Environmental Protection Agency implemented the project Survey and Health Assessment of Possible Health Hazardous Compounds in Proofing Sprays.
1.2 Objective
Objectives of the project:
1. On the basis of existing knowledge (i.a. scientific literature) as far as possible to investigate if health hazards exist either due to the chemical substances of the products or due to the size of the aerosols created during use.
2. To identify possible problematic substances in such products.
3. To investigate the size distribution of the aerosols the consumer is exposed to when using spray products for textile proofing.
4. To determine which textile proofing sprays exist on the Danish market, to investigate them and determine if they have a content of problematic substances and to investigate the type of aerosol creating mechanism.
2 Literature retrieval and information search
- 2.1 Introduction
- 2.2 Preliminary searches
- 2.3 Bibliographical database searches
- 2.4 Results
- 2.5 Summary of results and conclusion
2.1 Introduction
The first phase of the project comprised the collection of literature data with regard to known registered information about toxification of consumers during the use of proofing spray.
In addition to human cases of toxification this report also includes a number of studies on animals during the use of as a rule commercial proofing sprays with a more or less well-known composition. There are two reasons for that: 1) to procure more information about ingredients in spraying products that have been involved in human cases of toxification, and 2) to get the opportunity to explain certain effects through pathological investigations.
A wide range of different proofing agents exist and they are sold directly to the consumers as agents for restorative treatment of different types of textiles, most often to obtain water and stain repellency. The main part of the products is sold as sprays.
Cases of poisoning have often been reported in connection with the use of these types of products. In the newest, larger case from Denmark, 10 people became ill within two months in 2005 as a result of using a certain product.
The first phase of the project has the following objective:
- To clarify the reasons for the registered cases of poisoning when using this type of products, including specifically if they mainly are due to certain chemical substances or if the size of the aerosols created during use have a decisive influence.
2.2 Preliminary searches
In order to create an outline of which substances and/or substance groups it would be relevant to target the payment database literature survey against, a number of preliminary internet searches on relevant Danish and foreign homepages were carried out.
The internet searches were carried out by means of the search machine Google and in one particular case it was subsequently chosen to use the same search word in Google Scholar which focuses on scientific references.
2.2.1 Google searches
A number of Danish cases of toxification have been registered in connection with using different types of proofing sprays and therefore a Danish keyword was initially used for the searches.
For this preliminary screening the following Danish words were used: spray forgiftning imprægnering, as the word tekstilspray combined with forgiftning did not give any search results.
That only resulted in few interesting results.
The English search combination was: fluor resin textile spray pulmonary poison, and the advanced search strategy - that all words had to be found - was employed. That combined with results in English gave a total of 14.100 results.
2.2.1.1 Selected search results in Google
The Danish search localised a scientific article from the Danish magazine Ugeskrift for Læger from 1999 (Jacobsen et al., 1999). That reference contains a summary of the chemical composition of a number of proofing products. The examination of the compositions was instituted by Giftinformationen (Danish Poison Information Centre at Bispebjerg Hospital: Clinic for Occupational and Environmental Medicine) and it was carried out by the Danish Emergency Management Agency (Beredskabsstyrelsen).
It is appropriate to divide the substances found in the investigated products into 3 main groups:
- Propellants (however, not in sprays with a pump)
- Proofing agents
- Solvents.
The propellants consist of low molecular hydrocarbons such as propane, butane and isobutane. Earlier, the so-called CFC gases (fluorine and chlorine containing hydrocarbons) were used. The proofing agents can be siloxane compounds, fluorcarbons, urethanes, esters/wax or phthalates.
The solvents are typically mixtures of aliphatic hydrocarbons (e.g. heptane isomers) and cyclic hydrocarbons (e.g. cyclohexane) and chlorinated hydrocarbons (e.g. 1,1,1-trichloroethane) and esters (e.g. butylated acetate). Butylated acetate is forbidden in products for indoor household use and 1,1,1-trichloroethane is forbidden in spray cans as it is ozone layer decomposing. Therefore, these two solvents are forbidden in spray cans intended for indoor household use.
The searches in English gave two usable results. One Japanese article (Jinn et al., 1998) reports the following content of spray cans: 1,1,1-trichloroethane, liquid petroleum gas (low molecular alkanes) and fluorine based polymers (fluoride resin). In this case, it is a proofing spray that has caused lung injury.
The other article (Lazor-Blanchet et al., 2004) does not mention a textile spray but an agent to treat floors (tiles) so discoloration is prevented. The proofing substance in this agent is stated to be: < 1 % acrylic ester fluorpolymers dissolved in a > 90 % mixture of isoalkanes (C9-C12). This product does not exist in a spray can with propellant but is intended for application with a brush. However, the professional tiling company that was mentioned in the article had chosen to fill the liquid into a container with pump spray and apply the agent in that way resulting in toxification. The same acrylate fluorpolymer as in this product (and from the same manufacturer) is in article[3] stated to have caused a number of respiratory problems in connection with household proofing of leather and textiles.
2.3 Bibliographical database searches
In order to involve knowledge about international experience, a goal-oriented search was carried out in a suited cluster of literature databases (TOXCENTER) and at the same time in a couple of the large databases EMBASE and SCISEARCH that do not form part of this cluster at the database host STN (see description under 2.3.1.2).
The search was carried out on the combination of textile proofing and/or the identified chemical substances in relation to the registered symptoms including the word toxification.
2.3.1 Goal-oriented literature searches
2.3.1.1 Preparation of search profile
On the basis of keywords from Vernez et al. (2006) and literature references in that article, it was chosen to search for cases of toxification where the following words and word combinations appeared:
- Acute Respiratory Syndrome
- Lung Injury
- Pulmonary Toxicity
- Pulmonary Collapse
- Pneumonia
- Respiratory Disease.
The search terms for these parameters are: Acute Respiratory Syndrome OR Lung Injury OR Pulmonary Toxicity OR Pulmonary collapse OR Pneumonia OR Respiratory disease.
The mentioned cases of toxification can occur by exposure to the following:
- Proofing spray
- Waterproofing spray
- Spray impregnation
- Fluor resin
- (Airborne particle)
as it was established that the term "textile" had a limiting effect on the number of search results:
Search term: Proofing Spray OR Waterproofing spray OR Spray Impregnation.
Other conditions that might manifest themselves are:
- Particle size
- Orifice spraying pressure.
2.3.1.2 Payment database searches
The above search profile was used to search in the below databases.
TOXCENTER (Toxicology Center) is a bibliographical database that covers the pharmacological, biochemical, physiological and toxicological effects from medicines and drugs and other chemicals.
EMBASE (Excerpta Medica) is a bibliographical database covering literature within the biomedical and pharmaceutical field.
Science Citation Index (SciSearch®) contains all recordings published in Science Citation Index ExpandedTM.
The search resulted in 9 references, see chapter 8.
2.3.1.3 No cost bibliographical database searches
The complete search term:
((Acute AND Respiratory AND Syndrome) OR (Lung AND Injury) OR (Pulmonary AND Toxicity) OR (Pulmonary AND collapse) OR Pneumonia OR (Respiratory AND disease)) AND ((Proofing AND Spray) OR (Waterproofing AND spray) OR (Spray AND Impregnation))
was also used in PubMed and on Scirus.com.
This very specific search gave 8 search results in PubMed and 11 results in Scirus related to lung effects arising after having used proofing spray. There was a certain overlapping between the references from the payment databases and the no cost databases. The entire reference list can be seen in chapter 8.
2.3.2 Articles and references referred to
Vernez et al. (2006) and several of the procured articles contain a number of references to additional literature. The complete bibliography of the project comprises a score of references to scientific investigations; see the reference list (chapter 9). However, several of the references relate to proofing products to be used on other materials than textiles.
The identified relevant literature was purchased with a couple of exceptions where repeated attempts to place an order gave no result. In addition, three of the identified articles were commented on from the English abstract as the original article was in Japanese.
The literature has been investigated in order to identify possible cause and effect relationships between toxifications/symptoms and exposure to chemical substances (isolated substances or combinations) and/or the physical characteristics of aerosols, also including the special conditions for nanoaerosols. Only one of the scientific articles dealt with measurements of the aerosol diameter. In addition, Vernez et al., (2006) have a rather rough measurement of the size distribution on mass basis. Most of the articles discuss the creation of very fine aerosols when spraying liquids from propellant cans and mention that this condition can contribute to the registered lung effects.
2.4 Results
2.4.1 Data from referred to articles
2.4.1.1 Product composition
The collected information is presented in Table 2.1.
Table 2.1 Complete outline of accessible composition data from procured literature.
| Reported toxicity | Proofing agent/ active substance | Solvent | Propellant | Reference |
| Coughing, respiratory distress, headache, fever, shivers (Does not specifically refer to one single substance mixture.) | Fluorcarbons, silicone compounds, urethanes, esters/wax, phthalics | Aliphatic hydrocarbons (heptane, methylhexane) - also cyclohexane. Possibly e.g. butyl acetate | Propane, butane and/or isobutane | (1) |
| Immediate lung injuries | Fluorpolymers | 1,1,1-Trichlorethane | Propane and butane | (3) |
| Lung reactions | Mixture of fluor-acrylate polymer and isoparaffin hydrocarbons | Changed – not stated from what to what | Not informed | (5) |
| Lung reactions | Fluor resin (fluorcarbon resins) | Petroleum hydrocarbons | Butane/propane | (6) |
| Immediate respiratory symptoms. Fever |
Fluorpolymer resin and a co-polymer, 1 % silicone resin and 1 % polymerised C10-alkenes | 95 % Soltrol-10, consisting of 70 % 2,2,4-trimethyl-pentane and 30 % C7- and other C8-isoparaffines. The 5 % have not been informed |
Pump spray | (7) |
| Serious respiratory problems | Nanospray with very fine atomisation - has later turned out not to be nanoaerosols, combination otherwise not stated | Propellant is used, but the combination is not informed | (8)/(9) | |
| Respiratory problems | Aliphatic fluorine compounds | n-Heptane; ethyl acetate | Isobutane | (10) |
| Immediate lung injuries in test animals | Perfluoralkyethylacrylate/n-alkyl acrylate copolymer 1 % | Naphta 95 % heptane 3 % ethyl acetate 1 % |
Carbon dioxide | (11) |
| Leather spray. Quick breathing, pulmonary edema and haemorrhage from the lungs and some deaths. Examined in rats and guinea pigs. |
Fluoralkenes, fluorphenyl and/or fluor alcohol | C7-C8-alkanes and traces of ethyl acetate and 2-butoxy ethanol, dipropylenglycol methyl ether and C10-C12-alkanes | Propane | (12) |
| Not textile spray Acute lung toxicity |
Acrylate-fluorpolymer | C9 - C12-isoalkanes | Atomised with pumping device | (4) |
| Serious lung change | Fluorine resin and silicone | Liquid petroleum gas | (13) | |
| Respiratory problems | Fluorcarbon component (fluorpolymer) | (14) | ||
| Morphological changes in lung tissue in test animals | Fluorine resin with/without silicone | Ethyl acetate, mineral turpentine, n-heptane |
Propane | (15) |
| Lung collapse at aerosol diameter of up to 90 µm (mice) | Fluorine resin | n-Heptane, ethyl acetate | Liquid petroleum gas | (16) |
| Serious lung toxicity - very old article |
Melamine resin, Organic methyl soap. |
Petroleum, petrol, Methylene chloride, freon (trichlorofluor-methane; dichlorofluor-methane) |
Propane Butane |
(17) |
| Coughing for a long time, short of breath, chest pains as during pleurisy | 1.2 % fluoralkylpolymer (FC-3537) | Isooctane | Propane | (17) |
| Short of breath, coughing and weight on the chest | Fluorpolymer (FS-4565) |
Hexane | Isobutane | (18) |
Liquid petroleum gas is a mixture of low molecular hydrocarbons – presumably most propane, butane and isobutane.
Some of the studies that were found do not contain information about the proofing liquid composition and therefore they have not been included in the table.
2.4.2 Assessment of reported cases of toxification
All the cases of toxification, reported for proofing spray in the found references, have in common that the products previously have been used without reported lung injuries, often for several years. It is also a common trait that a formulation change of the product has taken place immediately before the observed cases of toxification. That has often taken place with reference to the solvents or propellants being harmful to the environment, and therefore they had to be replaced.
The solvents less harmful to the environment and subsequently allowed have often not been able to dissolve a sufficient amount of the originally used water repelling proofing agents and therefore they have been replaced with other substances. That has i.a. been reported in investigations from Switzerland, France, Denmark and the USA (Vermez et al., 2006; BfR, 2006a; Gregersen et al., 2006; Smilkstein et al., 1992; Kulig et al., 1993).
On the whole, respiratory injuries connected with the use of proofing spray were observed in a number of cases (Burkardt et al., 1996; Tagawa et al., 2003). Many of the other references describe individual cases (Tanino et al., 1999; Kobayashi et al., 2006). Several of the references stress that tobacco was smoked at the same time as spraying took place or that cigarettes were held between the fingers which still had surplus proofing liquid on them. This is with reference to Teflon compounds (being fluorcarbon polymers) are known to cause ”polymer fume fever” when heated and cases of pulmonary edema owing to pyrolytic products from these polymers (Jinn et al., 1998) have been reported.
In two issues of Morbidity and Mortality Weekly Report from 1993, there is reference to poisoning with leather proofing spray in Oregon (Smilkstein et al., 1992) and an "epidemiological note" from Colorado concerning three cases (Kulig et al., 1993). However, in the editorial comment after the actual reports at least 157 cases of consultations to doctors were registered concerning toxification with the same product in the USA. In both cases, the editorial states that a formulation change of the product had taken place shortly before, as the use of 1,1,1-trichloroethane was to be phased-out before 1994 according to the change in the Clean Air Act in 1990. The composition of the leather spray liquids involved in the cases of toxification appears from Table 2.1. The reported composition of spray liquid corresponds to the combination of several textile spray liquids and has therefore been included here.
Through questionnaires used as follow-up on a Swiss collection of reported cases of toxification (approx. 200 cases), Vernez et al. (2006) retrospectively investigated to which degree the exposure concentration had influenced various parameters e.g. with regard to consultations at doctors/casualty wards. In the cases where the hospital had been visited the results of the clinical investigations and analyses carried out at the hospital have been further investigated.
On the basis of the questionnaires, individual exposure data was generated from a classic 2 zone model for aerosol dispersing in the community and in the distance during use. The resulting evaluated dosage and exposure data were spread over several sizes. A connection was not found between exposure and indicators of health effects (own perception of the seriousness and clinical indicators). A minor connection was found between unspecified inflammation indicators e.g. leucocytes and C reactive protein (a test that measures the blood’s content of a protein that indicates an immediate inflammation) and the maximum exposure concentration.
The found results demonstrated that there was considerable individual variation indicating that one or more indirect mechanisms determine the development of the respiratory problems. No threshold value was found for safe exposure. That indicates that increased requirements to the surroundings (ventilation, through draught, room size) during use are not enough to prevent future outbreaks of toxification with proofing spray. The authors conclude that additional precautions have to be taken when marketing new spray products.
2.4.3 Other information from procured literature
Yamashita and Tanaka (1995) investigated the administration of aerosols in a number of female mice from the CD-1 strand. They found that prescriptions containing fluor resin caused immediate respiratory disease but none of the other ingredients worked that way. They refer to recent preceding cases of toxification and discuss that changes in solvents ease the creation of aerosols and give a smaller drop size. That could explain the increased toxicity of the reworded spray liquid.
A couple of years later, Yamashita et al. (1997) in CD-1 female mice again investigated the toxicity of a spray liquid that had been made water-repellent with fluorcarbon resin. This time different average aerosol diameters in the spray mist were tested. The article demonstrates that the aerosol size is of great importance. When the aerosol diameter increased to 89.1 µm with 0.2 % of particles with a diameter less than or equal to 10 µm, there was no toxicity of the fluorcarbon resin. When the average aerosol diameter in the spray mist was 62.0 µm with 1.6 % of aerosols with a diameter less than or equal to 10 µm there were on the contrary many toxic lung changes in the mice.
Tashiro et al., (1997) investigated the effect of a textile spray containing perfluoralkylethylacrylate/n-alkylacrylat copolymer as proofing agent on rats. A sample was taken of the severally damaged surface mucus in the lungs of the rats. Then the group investigated if it was possible to administrate new surface mucus.
The objective of the test was to investigate if it was possible to treat damaged lungs through inhalation of an aerosol of lung surface mucus (from pigs). At the same time, the test demonstrates that a commercially available textile spray is very damaging to rat lungs.
Hubbs et al. (1997) partly investigated the product composition and partly investigated how a proofing agent (for leather) effects guinea pig and rat lungs. After rewording, the product had been the cause of many respiratory diseases in humans. The previous product caused no toxic changes in guinea pig or rat lungs. The new spray product caused quick breathing, pulmonary edema, haemorrhage from the lung and one death in the exposed guinea pigs and rats. The electro microscopic samples showed direct cytotoxicity in the lungs with alveolar necrosis in type 1 cells and interstitial edemata certain places in the lungs and no effects in other samples. The test demonstrated that the old product with fluoralkenes did not show lung toxicity, but the new product that also contained fluoralkenes demonstrated toxicity in guinea pigs as well as in rats. The change in the composition of the product took place in connection with the phasing-out of 1,1,1-trichloroethane (Clean Air Act amendment from 1990).
2.4.4 Nanoaerosols
Here, nanoaerosols are used as the term for small (<100 nm) units of substances or material that are suspended in air and that are not gaseous. Liquid or solid materials can be in question, including amorphous structures.
As already mentioned, it was not possible to find information about possible health effects from spray with nanoaerosols – apart from the press release mentioned below.
At an expert meeting on 7 April 2006, the German federal agency for Risk Assessment (BfR) discussed if they could find the reason for 97 cases of toxification, of which some were serious, caused by two new sealing spray products that contained nanoparticles (BfR, 2006a). The expert meeting analysed to which extent respiratory problems and pulmonary edema could have been caused by the nanoparticles in the 2 products or if other dangerous substances from traditional proofing agents could be responsible. As the suppliers of the 2 products were unable to supply complete product declarations, it was not possible to carry out a discussion on a sufficiently scientific basis. However, it was agreed that a classic toxiological weighing-out of the individual compound in a mixture is not enough when the product is applied from an aerosol spray with propellant. Here, physical factors such as e.g. drop size play a decisive role for toxic effects in the respiratory passages.
The health effects of products from a propellant spray can only be determined with a test strategy that imitates the actual indoor application conditions.
Subsequently, (26 May 2006) BfR sent a press release (BfR, 2006b) stating that the two sealing sprays did not contain nanoaerosols (aerosols < 100 nanometer). The reference to "nano" in the marketing of the products was supposed to underline the very thin layer of sealing that was necessary. The cause of the 110 cases of health injuries - of which some were serious - has not yet been established.
Therefore, there are for the time being no examples of directly proven toxic lung injuries due to nanoaerosols in spray products.
2.5 Summary of results and conclusion
A number of articles were found with information partly about toxic effects in connection with spray proofing and partly about the proofing agents composition with regard to proofing agent, solvent and possible propellant.
Some few proofing sprays that caused toxicity in humans were subsequently tested in animals.
Many cases of reported toxification from proofing spray have in common that products with the same name previously were used without reported cases of lung injuries. Immediately before the observed cases of toxification a rewording of the product had taken place often in connection with more rigorous environmental laws where the original solvent or propellant was regarded as dangerous to the environment and therefore had to be replaced.
The more environmentally friendly solvents that subsequently were used have not been able to dissolve a sufficient amount of the originally used proofing agents which therefore have been replaced by other chemical compounds.
In Vernez et al., 2006 there is a rough measurement of the aerosol size distribution on mass basis. In several products that have caused respiratory problems among the users the size of 90% of the aerosol drops was approx. 2-10 µm (Vernex et al, 2006). Most articles that are referred to, discuss why it is important that propellants in aerosols lower the average aerosol diameter in the spray mist.
Spray proofing agents involved in reported cases of toxification often contain one or other type of fluorcarbon polymers. There is no actual description of the compounds e.g. in the form of a CAS no. However, in some few American reports there are some chemical describing letter/number references so it should be possible to find detailed descriptions of the chemical structures. On the other hand, solvents and propellants are in general unambiguously described, but amount specifications are rarely in question.
Generally speaking and after having gone through the many references, it is still not clear if the registered lung injuries are caused by a kind of immediate chemical pneumonia or if it could be the reaction of the lungs to a fine vaporized hydrophobic mist that penetrates down to the finest bronchioles. Literature lacks data about the aerosol size as well as the chemical composition. When such data has been procured experimental toxicological investigations of the demonstrated substances, including the substances on aerosol basis and the importance of the aerosol size will be necessary.
3 Survey
3.1 Introduction
3.1.1 Objective
The objective of the survey was to:
- Identify which products within the category of textile proofing sprays have been used the most.
- Procure products for chemical analyses.
- Try to procure information about the material (including substances) in the products in question.
The investigation of which products within the category exist in the market has been the condition for the further assessment of the products.
3.1.2 Delimitation
As described in chapter 1, a wide range of proofing agents are sold directly to the consumers as agents for restorative treatment of various types of textiles primarily to achieve water and stain repellency.
The Danish Environment Protection Agency chose to focus on the product category spray agents[4] for textile proofing. That means, that textile proofing agents to be used when washing textiles or intended for application on textiles have not been included in the project.
3.1.3 Procedure
The following activities form part of the survey (including purchase of products):
- Internet search
- Contact to the retail trade
- Contact to manufacturers and suppliers.
The survey has aimed at including expensive as well as inexpensive products.
It has not been possible through Statistics Denmark to carry out a quantitative survey of the consumption of textile proofing sprays. Skat (Danish Tax and Customs Administration) has informed that there is no KN code[5] that solely deals with these products.
3.2 Purchase
The part of the survey dealing with purchase of products comprised:
- Internet search – purchase in internet shops and contact to distributors
- Shop visits – purchase in physical shops
Products were purchased for textile proofing within the product groups:
- Products for shoe proofing
- Products for proofing of tents and the like
- Products for furniture proofing
- Products for proofing of clothes for outdoor use such as jackets or the like.
The main selection criteria for purchase of products have been that the products have to be sold to a certain degree. It has especially been possible to use that criterion when visiting physical shops and the staff was asked which products are “best selling” but it has not been possible to use that criterion in connection with internet trade.
3.2.1 Internet search and trade
Searches mainly took place through Google.dk with the word combination textile proofing and spray. In addition, homepages registered in catalogues, daily papers and magazines have been visited.
3.2.1.1 Contact to distributors
In the light of the internet searches, a number of the companies behind the internet shops, selling spray agents for textile proofing, have been contacted.
Contact to distributors/importers concerned information about substances (safety data sheets) in each product and enquiries about amounts sold.
The far majority of the distributors/importors have sent information about the substances in the form of safety data sheets whereas the request for information about amounts sold has been met to a very limited degree and therefore it is not possible to estimate total sales/consumption in Denmark of the product type ”Spray products for textile proofing”.
3.2.2 Shop visits
A wide range of shops have been visited, including:
- Furniture dealers
- Chemist’s shops
- Shoe shops
- Sports goods shops/”outdoor” shops
- Supermarkets
- Department stores
- DIY markets
- Auto detailing shops.
When visiting shops, it was asked which products are sold the most and also to which degree the customers ask for directions when purchasing the products. Only few of the visited shops said that the consumers ask for directions when purchasing the products and those few cases the requests for directions mainly concerned which product was the ”best”.
3.3 Products
All products were purchased through national chain of shops or on the internet.
The survey resulted in the registration of 26 products, of them 5 on the internet.
Some of the specialist shops (e.g. the furniture dealers and shoe shops) typically only sell one product, while other shops (e.g. chemist’s shops, sports goods shops, DIY centres etc.) in some cases sell several products. In the latter case, it was asked which products are best selling and mainly those products were the ones that were purchased.
Regarding contact to importers, several importers have said that ”private labelling” is used to a certain degree within the product category, meaning that the dealers import (or purchase from an importer) identical products and then give the products different names.
3.3.1 Product outline
Table 3.1 states the declared substances in each product of the registered spray agents for textile proofing. The information partly originates from the packaging and partly from the safety data sheets (MSDS) of the individual product.
Table 3.1 Outline of products. Information originates from packaging and safety data sheets, respectively.
| Lab no. | Spray/ pump |
Danger symbols | Substances | CAS no. | R- and S- sentences |
| 1 | Spray | E.g. | No safety data sheet. The product has been deleted from the product range. Dimethylether Heptane Ethyl acetate Sec-butylacetate Fluor polymer |
115-10-6 142-82-5 141-78-6 105-46-4 |
- |
| 2 | Pump -spray is available | - | No R- or S-sentences | ||
| 3 | Spray | Fx, Xi, N (MSDS) |
Low boiling hydrogenated nafta | 64742-49-0 | R11, R38, R51/53, R67 S2, S23, S24, S51, S61 |
| Butyl acetate | 123-86-4 | ||||
| 4 | Pump | Modified organo functional siloxane polymer | Not informed | No R- or S-sentences | |
| 5 | Spray | Fx, Xi | Propan-1-ol | 71-23-8 | R11, R41, R67 S(2), S7, S16, S26, S24/25 |
| Silicone | |||||
| 2-propanol | 67-63-0 | ||||
| 6 | Spray | Fx, Xi, N (MSDS) |
3M Fluortensid | - | R12, R38, R51/53, R67 S23, S51, S61 |
| Butane (content < 0.1 % 1.3 Butadien) | 106-97-8 | ||||
| Propane | 74-98-6 | ||||
| Low boiling hydrogenated nafta, naphta (crude oil), hydrotreated light (<0.1 % benzene) | 64742-49-0 | ||||
| Propan-2-ol; isopropylalcohol | 67-63-0 | ||||
| Isopropyl acetate | 203-561-1 | ||||
| 7 | Pump | Propan-2-ol | 67-63-0 | S26, S61 | |
| 8 | Spray | Fx, Xn, N | Low boiling hydrogenated nafta, naphta (crude oil), hydrotreated light (<0.1% benzene) | 64742-49-0 | R11, R36/38, R51/53, R67 S-sentences only as text. |
| Isopropyl alcohol, propan-2-ol | 67-63-0 | ||||
| Isopropyl acetate | 108-21-4 | ||||
| 9 | Spray | Fx, Xi, N | Naphta (crude oil), hydrotreated light | 64742-49-0 | R12, R38, R51/53, R67 S2, S3, S9, S16, S51, S56 |
| 2-Propanol | 67-63-0 | ||||
| Naphta (crude oil), hydrotreated heavy | 64742-48-9 | ||||
| Propane as liquid | 74-98-6 | ||||
| Butane, chemically clean | 106-97-8 | ||||
| 10 | Pump | Propan-2-ol | 67-63-0 | None | |
| Paraffines | |||||
| Wax | |||||
| 11 | Pump | Fluorcarbon resin | Not informed | R52/53 S7, S16, S24/25, S26, S61 |
|
| Cationic tensides | Not informed | ||||
| Non-ionic tensides | Not informed | ||||
| Propan-2-ol | 67-63-0 | ||||
| 12 | Spray | Fx, Xi, N | Naphta (crude oil), hydro treated light | 64742-49-0 | R12, R38, R51/53, R67 S2, S3, S9, S16, S51, S56 |
| 2-Propanol | 67-63-0 | ||||
| Naphta (crude oil), hydrotreated heavy | 64742-48-9 | ||||
| Propane as liquid | 74-98-6 | ||||
| Butane, chemically clean | 106-97-8 | ||||
| 13 | Spray | Fx | No safety data sheet. The product has been discontinued. | - | |
| Dimethylether | 115-10-6 | ||||
| Heptane | 142-82-5 | ||||
| Ethyl acetate | 141-78-6 | ||||
| Sec-butyl acetate | 105-46-4 | ||||
| Fluor polymer | |||||
| 14 | Spray | Fx, Xi, N (MSDS) |
Naphta (petroleum), hydrotreated light | 64742-49-0 | R12, R38, R51/53, R67 S2, S16, S23, S29, S51 |
| Isobutane | 75-28-5 | ||||
| Propane | 74-98-6 | ||||
| Butane | 106-97-8 | ||||
| n-Butyl acetate | 123-86-4 | ||||
| 15 | Pump | F* Xn (MSDS) | Mixture of organic solvents with special additives | - | R10, R65, R67 S2, S7, S16, S24/25, S33, S62 |
| Iso-Alkane | 90622-57-4 | ||||
| n-Butyl acetate | 123-86-4 | ||||
| Isopropyl acetate | 108-21-4 | ||||
| 16 | Pump | Perfluoralkylacrylcopolymerised | Not informed | No R-sentences. S2, S23, S24/25, S26, S36/37/39, S46 |
|
| 17 | Pump | Aquous mixture of potassium salts | - | No R-sentences. S2, S25 |
|
| Acetic acid | 64-19-7 | ||||
| 18 | Spray | Fx | Propane | 74-98-6 | R12, R66 S2, S46 |
| Butane | 106-97-8 | ||||
| Hydrocarbons, C4, 1,3-butadiene-free, polymerised | Not informed | ||||
| 19 | Spray | Fx, Xi | Isopropanol | 67-63-0 | R11, R36, R67 S2, S16, S26, S51 |
| 20 | Spray | No safety data sheet | |||
| Contains petroleum distillates | |||||
| Contains CO2 as propellant | 124-38-9 | ||||
| 21 | Spray | No safety data sheet | |||
| Contains petroleum distillates | |||||
| Contains CO2 as propellant | 124-38-9 | ||||
| 22 | Spray | Xi, N, Fx | Mixture of heptane-isomers 2-Propanol Non-aromatic gas propane as liquid Butane chemically clean |
67-63-0 74-98-6 106-97-8 |
R12, R38, R51/53 |
| 23 | Pump | Formic acid Methanol |
64-18-6 67-56-1 |
||
| 24 | Pump | Methanol | S2 | ||
| 25 | Spray | Fx, Xi | Dimethyl ether Ethyl acetate Ethanol Propan-2-ol |
115-10-6 141-78-6 64-17-5 67-63-0 |
R12, R36, R67 S2, S23-a, S26, S46, S51 |
| 26 | Spray | Fx | Naphta (crude oil), hydrodesulphurized heavy (<0.1 % benzene) | Not informed | R10, R12, R65 S2, S16, S23, S24, S46, S51 |
| Crude oil gases, liquefied (<0.1 % 1.3 butadien) | Not informed | ||||
| Not purchased | Spray | Heptane | 142-82-5 | R38, R67, R50/53 S2, S29, S51, S61 |
|
| 2-Propanol | 67-63-0 | ||||
| Isopropyl acetate | 108-21-4 | ||||
| Butane (propellant) | 106-97-8 | ||||
| Not purchased | Pump | No safety data sheet the product is water based. | |||
| Not purchased | Pump | No safety data sheet the product is water based. |
* Error in the supplier manual, the right marking is stated.
3.3.2 Legislative conditions
Consumer products intended for textile proofing have to follow the ordinary rules in accordance with Regulation no. 329 dated 16 May 2002 of the Danish Ministry of the Environment concerning classification, packaging, marking, sale and storage of chemical substances and products (Danish Environmental Protection Agency).
If the products contain substances that are included in the list of hazardous substances in Regulation no. 923 dated 28 September 2005 of the Danish Ministry of the Environment they have to be marked in accordance with the classifications in the regulation (Danish Environmental Protection Agency, 2007). In addition, there might be a prohibition against use in aerosol cans (AE marking).
Furthermore, new obligations have been imposed on companies that produce, import, use or distribute chemical substances and products in connection with the implementation of the EU chemical regulation REACH ((EC) No. 1907/2006). REACH came into force on 1 June 2007, but will be implemented gradually in the course of 15 years. REACH i.a. imposes producers and importers to register chemical substances and in that connection to report data about the properties of the substances to a central chemical agency. In addition, producers and importers of substances requiring a safety data sheet have to give detailed information to their customers about how the substances can be handled properly.
Finally, propellants and solvents in products intended for textile proofing and sold in aerosol cans have to be in accordance with regulation no. 571 dated 29 November 1984 concerning the use of propellants and solvents in aerosol cans (Danish Environmental Protection Agency). Aerosol cans are defined as cans with a volume of max. 1.0 litre, containing a liquid or nebulized gas intended for discharge through a device so the content is emptied in the form of solid or liquid aerosols or as foam.
Only propellants and solvents stated in enclosure 1 of the regulation are allowed in concentrations exceeding 1%, unless they are comprised by other legislation. In this connection, all chemical substances contained in aerosol cans with a boiling point below 168ºC (Danish Environmental Protection Agency) are characterised as propellants or solvents. As this regulation is old, the positive list in enclosure 1 does contain substances that no longer are allowed in spray cans due to other legislation. That goes for substances with AE marking in the list of hazardous substances and substances that are controlled via the regulation on certain ozone layer destroying substances.
In special cases, where neither health related nor environmental conditions speak against it, the Danish Environmental Protection Agency can allow the rules of the regulation to be departed from. The Danish Environmental Protection Agency has to deal with requests concerning deviations from the rules in the course of 45 days.
4 Experimental investigations
- 4.1 Background. Summary of literature retrieval and survey
- 4.2 Selection of products
- 4.3 Analysis programme
4.1 Background. Summary of literature retrieval and survey
As it appears from Table 3.1, 29 products were registered of which 26 were purchased. To a certain degree, we succeeded in obtaining information about the solvent and propellant content especially from the procured safety data sheets. That information also appears from Table 3.1. For some products it also appears which type of coating (fluorpolymer, silicone based or the like) was used.
The information has been collected in Table 4.1.
Table 4.1 Outline of advance knowledge about substances distributed on spray and pump products.
| No. of spray products | No. of pump products | Total | |
| Total | 17 | 12 | 29 |
| Active substance: | |||
| Fluorpolymer based | 4 | 2 | 6 |
| Silicone based | 3 | 1 | 4 |
| Wax | 0 | 1 | 1 |
| Not informed | 10 | 8 | 18 |
| Solvent: | |||
| Organic solvent1 | 16 | 6 | 22 |
| Water | 0 | 3 | 3 |
| Not informed | 1 | 3 | 4 |
1Alcohols, ketones, esters, oil fractions (”nafta”, petroleum distillates). In some cases, mixtures of water and solvents are in question.
In connection with products where the active substance (coating type) was informed, either fluorine based products or silicone/siloxane based products are in question. In addition, one single product is based on wax. The exact chemical structure of the applied coating has not been informed in any of the cases.
The vast majority of the products contain organic solvents and they either constitute the main part in the product or are found in a mixture with water. A few pump products are declared as purely water based and it is not clear which type of coating is in question in connection with these products.
The literature searches show that products that have caused health effects mainly contain fluorine containing polymers (15 out of 17 products). In connection with 1 product the content is not stated and the last product that is described in an older article contains melamine. It should be noted that some products in addition to fluorine compounds also contain silicone compounds.
It is most likely that products with fluorpolymers in certain cases can give unwanted health effects in the respiratory passages. In principle, the health effects can be due to the following effects or a combination:
- The applied fluorine compounds are directly toxic to respiratory passages/ lung tissue.
- During use small particles are created that can penetrate into the lung tissue and e.g. give rise to harmful inflammatory conditions.
- The solvents that are used for fluorine compounds are injurious in the resulting concentration.
In literature (Yamahita et al, 1995), there are indications that aerosol size plays an important part. It is known from other connections that certain materials are non-toxic when they exist as larger aerosols, but they can be toxic when they exist as nanoaerosols (diameter < 100 nm). It has not been possible to find accessible information that indicates that solid or non-aerosol fluorpolymers in general are toxic.
Proofing agents that are used as spray (under pressure and with propellant) or by means of a pump are applied to the exposed material as aerosols in varying sizes that deposit on the material. Spray agents give rise to smaller aerosols (~10 µm) than pumped agents (~100 µm). However, the main drops will nevertheless mainly consist of very volatile organic solvents or water that quickly evaporate and therefore can leave substantially smaller aerosols consisting of non-volatile material behind.
In the light of the above, the following experimental investigations were carried out:
- Determination of size distribution of liberated aerosols during use and for a well-defined period of time after use.
- Screening for possible content of fluorine and/or silicium (as the proofing agent in many cases is unknown).
- Investigation of the compostion of the applied solvent, including content of other organic additives and microcompounds and the content of monomers or oligomers in the used proofing material.
4.2 Selection of products
16 products were chosen for analysis and the principle was that spray as well as pump products should be represented, that fluorine as well as silicone based products should be investigated and that products with known as well as unkown substances should be examined. The selected products appear from Table 4.2.
Table 4.2 Products selected for analysis.
| No. | Active substance | Aerosol mechanism |
| 1 | Fluorpolymer | Spray |
| 3 | Unknown | Spray |
| 4 | Siloxane polymer | Pump |
| 6 | Fluorpolymer | Spray |
| 8 | Unknown | Spray |
| 9 | Unknown | Spray |
| 11 | Fluorpolymer | Pump |
| 14 | Unknown | Spray |
| 15 | Unknown | Pump |
| 16 | Unknown | Pump |
| 18 | Silicone | Spray |
| 20 | Silicone | Spray |
| 21 | Fluorpolymer | Spray |
| 24 | Unknown | Pump |
| 25 | Unknown | Spray |
| 26 | Unknown | Spray |
4.3 Analysis programme
4.3.1 Screening analyses
X-ray
Screening was carried out to determine the content of the elements fluorine and silicium by means of wavelength dispersive x-ray spectroscopy. That is a quick method used to determine if the proofing agent is based on fluorine compounds, silicone compounds or others.
Undyed cotton fabric was spray proofed with the product for 10 s. The samples were analysed directly with the proofed side turned towards the x-ray pipe after evaporation of the solvent. The result gives a quantitative measurement of the fluorine and silicium content. Elements with atom numbers larger than fluorine are also detected by this method if they are present in significant amounts.
Table 4.3 Parameters for x-ray measurements
| X-ray equipment | Wavelength dispersive x-ray equipment with model Philips PW 2400 with UNIQUANT calculation programme ver 5.49 |
| Counting time | 6-20 sec. per element |
| Power pipe | 2400 W |
The achieved knowledge about content of elements has created the basis for applying subsequent GC/MS analyses so the greatest possible amount of information about substances and coating type has been obtained.
Aerosol measurements
In connection with the aerosol analyses it has not been necessary to carry out a separation of qualitative and quantitative measurements as the measurements always are quantitative and as a result state the amount of aerosols per volume unit. Please also see 4.3.2.6.
Semi-quantitative GC/MS screening
A subsample, approx. 2-3 g, was weighed and a known amount of dichloromethane containing internal standards was added. Internal standard was added in order to obtain semi-quantitative results. The product was sprayed directly into a calibrated flask and dissolved in dichloromethane. The extracts were subsequently analysed by means of gas chromatography (GC/MS).
The results from this analysis cover the semi-volatile compounds and not propellants or the most volatile organic solvents. In connection with the screening that was carried out, the detected compounds were merely identified by comparison with the NIST MS library (NIST02 Version 2.0).
The detection limit of the analysis method is estimated to be 0.01 mg/g and the measuring uncertainty is estimated to be ± 20 %, however, it is higher for some compounds as semi-quantification only has been carried out against an internal standard, bromobenzene. In some cases, another internal standard was used, o-terphenyl, due to interference in relation to bromobenzene.
Table 4.4 GC/MS analysis parameters
| GC/MS instrument | Agilent HP 5973 ALS |
| GC parameters | Column: Zebron ZB-1, 20 m x 0,18 mm id., 0.18 µm film thickness Carrier gas: Helium, constant flow at 0.8 ml/min. Oven programme: 40ºC for 2 min., 15ºC/min. up to 300ºC Injection: 275ºC, split 1:10. |
| MS parameters | Scan mode: 35-550 m/z Solvent delay: 2 min |
In addition, the samples were screened for volatile compounds by means of fixed-time microextraction (SPME) which makes it possible to detect very volatile substances as no solvents are used that can interfer with these substances.
A subsample, approx. 0,2 g, was weighed directly in headspace glass. The gas phase was subsequently analysed by means of SPME-GC/MS.
The results of this analysis mainly cover the content of propellants and organic solvents. In connection with the screening that was carried out, the detected compounds were merely identified by comparison with the NIST MS library (NIST02 Version 2.0).
The detection limit of the analysis method is estimated to be 0.001-0.1 mg/g, but will depend on the vapour pressure and affinity against the applied SPME fibre of the individual component. No assessment of the amount of the identified substances in the product was carried out, as the results only are qualitative and therefore no analysis uncertainty is stated.
Table 4.5 SPME-GC/MS analysis parameters
| GC/MS instrument | Finnigan Focus GC-DSQ |
| GC parameters | Column: Zebron ZB-1, 30 m x 0.25 mm id., 1.0 µm film thickness Carrier gas: Helium, constant flow at 0.8 ml/min. Oven programme: 40ºC for 1 min., 10ºC/min. up to 275ºC, 275ºC for 10 min. Injection: 275ºC, split 20 ml/min. |
| SPME parameters | Fiber: 85 µm Carboxen/PDMS Absorption: 35ºC, 15 min. Desorption: 3 min. |
| MS parameters | Scan mode: 35-450 m/z Ion source 225ºC |
The combination of x-ray and GC/MS analyses can give information about which type of proofing can be obtained from the different products and which chemical compounds form part of the structure. However, it should be stressed that the finished surface/proofing typically will be a polymerized material and it can be difficult to finally determine the exact structure of that material.
4.3.2 Quantitative analyses
In the light of the screening analyses, 10 products were selected for quantitative analyses in co-operation with the Danish Environmental Protection Agency. However, aerosol analyses were carried out on all 16 products from the screening phase.
With a starting point in which substances were identified during the screening analyses and an evaluation of the relevance of the different substances to a health assessment, a number of organic compounds in the 10 products were selected for quantification. In order to quantify the selected organic compounds it was necessary to use three different analysis methods due to the difference of the substances with regard to volatility. From the screening analyses it was assessed that it is not relevant to analyse all products with all methods and for all compounds. External standards were used to identify and quantify the organic compounds. Analyses in duplicate were carried out. The detection limits are estimated from analysis of external standards and they appear from the result tables. The analysis uncertainty of the analysis methods was estimated to be 10 % while uncertainty on analyses in duplicate appears from the result tables.
4.3.2.1 GC/MS analysis of dichloromethane solution
A sub sample (2-3 gram) was weighed and a known amount of dichloromethane (50 ml) containing internal standards was added. The samples were subsequently analysed by means of GC/MS, see Table 4.6.
By using that method, it was possible to quantify the following compounds: n-butyl acetate, n-propylacetat, 2-butoxyethyl acetate, d-limonene, toluene, ethylbenzene, xylene, dodecamethylpentasiloxane, 1-perfluoroctan-1-ol and other fluorine compounds. The following products were analysed: 1, 3, 4, 8, 14, 16, 18, 21 and 25.
Table 4.6 GC/MS analysis parameters
| GC/MS instrument | Agilent HP 5973 ALS |
| GC parameters | Column: Zebron ZB-1, 20 m x 0.18 mm id., 0.18 µm film thickness Carrier gas: Helium, constant flow at 0.8 ml/min. Oven programme: 40ºC for 2 min., 15ºC/min. up to 300ºC Injection: 275ºC, split 1:10. |
| MS parameters | Scan mode: 35-550 m/z Solvent delay: 2 min |
4.3.2.2 GC/MS analysis of carbon disulphide solutions
A sub sample (approx. 1 g) was weighed and a known amount of carbon disulphide (25 ml) containing an internal standard was added. The samples were subsequently analysed by means of GC/MS.
By using that method it was possible to quantify the following compounds: cyclohexane, heptane and 1-butanol. The following products were analysed: 1, 3, 4, 8, 18, 21, 25 and 26.
Table 4.7 GC/MS analysis parameters
| GC/MS instrument | Finnigan Focus GC-DSQ |
| GC parameters | Column: Zebron ZB-1, 30 m x 0.25 mm id., 1.0 µm film thickness Carrier gas: Helium, constant pressure, 0.8 psi. Oven programme: 40ºC for 2 min., 10ºC/min. up to 130ºC, 120ºC/min. up to 270ºC, 270ºC for 10 min. Injection: 275ºC |
| MS parameters | Scan mode: 40-400 m/z Solvent delay: 3.8 min |
4.3.2.3 GC/MS analysis of xylene solutions
A sub sample (approx. 1 g) was weighed and a known amount of xylene (25 ml) containing an internal standard was added. The samples were subsequently analysed by means of GC/MS.
By using that method it was possible to quantify the following compounds: 1,1-dichloroethane, methylene chloride, 1,2-dichloroethene and 2-butanon. The following products were analysed: 8 and 4.
Table 4.8 GC/MS analysis parameters
| GC/MS instrument | Finnigan Focus GC-DSQ |
| GC parameters | Column: Zebron ZB-1, 30 m x 0.25 mm id., 1.0 µm film thickness Carrier gas: Helium, constant pressure, 0.8 psi. Oven programme: 100ºC for 2,5 min., 30ºC/min. up to 250ºC, 250ºC/min. for 1 min. Injection: 225ºC |
| MS parameters | Scan mode: 40-400 m/z |
4.3.2.4 Analysis of fluorinated organic compounds
Samples that in the screening analyses turned out to contain substantial amounts of fluorine were analysed by means of GC/MS with negative chemical ionisation (NCI), see Table 4.9. This method is specific for organic substances that contain halogen atoms, including fluorine. In addition, this method can give information about the melocular mass of the substances which with advantage can be combined with the knowledge about the structure of the substances obtained by normal GC/MS. A subsample was weighed and acetone was added.
The following products were analysed: 4, 8, 14, 21 and 25.
Table 4.9 GC/MS analysis parameters
| GC/MS instrument | Agilent HP 5973 ALS |
| GC parameters | Column: Zebron ZB-1, 20 m x 0.18 mm id., 0,18 µm film thickness Carrier gas: Helium, constant flow at 0.8 ml/min. Oven programme: 40ºC, 10ºC/min. up to 300ºC, 300ºC for 5 min. Injection: 280ºC |
| MS parameters | Scan mode: 50-650 m/z |
4.3.2.5 Analyses of product no. 4
No organic substances were detected during the screening analyses of product no. 4. Therefore, it was attempted to dissolve product no. 4 in various solvents and they were analysed by means of GC/MS to determine if the solvent had any influence on this. The following solvents were tested: dichloromethane, acetone, carbon disulphide and xylene.
The content and concentration of the solvents were analysed by means of GC/MS. Similar to the screening analyses the products were injected directly into a calibrated flask and diluted.
4.3.2.6 Aerosols
All 16 products were analysed for liberation of aerosols of up to 1 µm in aerodynamic diameter during use on a piece of textile. Exposure took place in a purpose-built pipe system where it is possible to carry out dynamic measurements during use and measurement after maintaining the air for a shorter period of time. Measuring took place 1 min. and 7 min. , respectively, after application.
The objective of the analysis carried out after 7 min. was to investigate if the size distribution changed in the period immediately after use due to solvent evaporation.
The suggested times are not necessarily representative of typical application patterns but the results can from the knowledge of the applied product amount be immediately scaled to more or less comprehensive use. This procedure was chosen because it is not possible to state a standardised application pattern as the products are used for small and large items where exposure time and amount vary substantially.
By determining the applied product amount gravimetrically, a measure for the aerosol concentration per mass unit was obtained.
Undyed cotton fabric with a pore size of 200-300 µm (Figure 4.1) covered the purpose-built semi-closed experimental chamber (Figure 4.2 and Figure 4.3) and the distance from the spraying can to the fabric was 24 cm.
Figure 4.1 Optical microskope image of the undyed cotton fabric.
Fabric proofing was carried out by applying the product through a small cylinder at the top of the experimental chamber. Spraying took 10 s as it appeared that enough proofing liquid was liberated in that amount of time to carry out particle measurements. If proofing takes longer time e.g. 1-2 min. very large amounts of proofing liquid is liberated compared to the volume of the experimental chamber (7.5 litres). All products with propellant for spraying of the content were kept horizontal during proofing, and all produts with a pump mechanism for spraying of the content were kept vertical during proofing. After proofing the experimental chamber was closed with a plug so the time-related development in size and number of aerosols could be determined. There was no sign of condensation in the chamber.
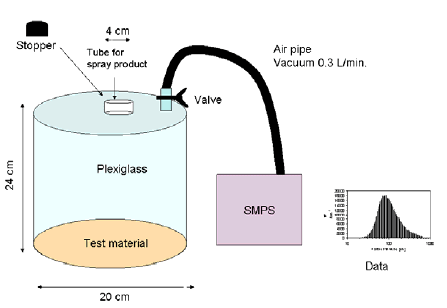
Figure 4.2 Schematic drawing of the experimental setup.

Figure 4.3 Experimental setup in plexiglass which is shown schematically in figure 4.2.
Aerosols created by the spray products were measured behind the product corresponding to the ordinary application situation where the user directs the spraying away from the body. That means that spraying does not take place directly into the measuring device, but measuring takes place on aerosols liberated to the air during use of the products.
Aerosol size distribution of the aerosols was measured with a Scanning Mobility Particle Sizer (TSI SMPS 3934 equipped with Differential Mobility Analyzer (DMA model 3081) and ultra fine Condensation Particle Counter (CPC model 3776)). Aerosols are drawn into the device and pass a radioactive source by means of which the aerosols obtain a known charge distribution. Then the aerosols are led to a laminar air flow through an electric field that separates the aerosols according to size. The aerosols are counted by a condensation particle counter.
Depending on the configuration, the instrument can measure particles in the interval of 2.5-1000 nm. In this project, measuring initially took place in the interval of 6-650 nm. In certain products, larger aerosols were suspected and in those cases the products were analysed for particles of between 650 nm and 1000 nm. The aerosols were sucked into a measuring instrument with a flow of 0.3 L/min. or 1.5 L/min. through a purpose-built silicone tube. Significant amounts of aerosols were not deposited in this tube. Each measurement of size distribution lasted 60 s.
Figure 4.4 gives a schematic presentation of the experimental course. After 10 s of proofing, the experimental setup was closed with a plug. After 60 s, aerosols were measured in the order of magnitude of 20-650 nm. This measurement took 60 s. Then another 5 min. passed and the measurement was repeated. The aerosol flow was then increased to 1.5 L/min. in order to measure particles down to 6 nm. Measurements took place at two different points of time to see if there was a time-related change in the size and the amount of the liberated aerosols.
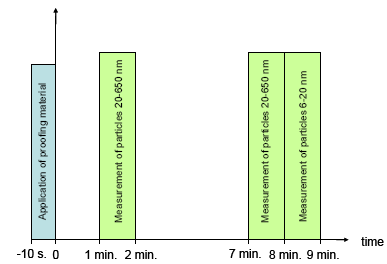
Figure 4.4 Schematic presentation of the experimental course.
Before each measurement, a measurement of the background level of aerosols was carried out and it showed that the number of background aerosols varied from 500-4000 aerosols/cm³ per minute, corresponding to the expected level of background aerosols in an indoor environment.
5 Results of screening of compounds
The selected products were examined to find out which substances they contain in order to assess if they consist of health hazardous substances that require closer investigation through quantitative analyses. All products were analysed quantitatively with regard to aerosol liberation and size distribution and the results are shown in chapter 6: Results of quantitative analyses and aerosol analyses.
5.1 Results of the chemical screening
The substances that were identified in connection with the effectuated screenings are summed up in the following tables. They are divided according to analysis method.
5.1.1 Results before x-ray measurements
Table 5.1 states the results of the fluorine and silicium analysis. The content of silicium in product no. 6, 8, 9, 14, 15 and 16 might be an expression of a background value from the used fabric on which the proofing product was applied. The results are based on the condition that the samples are homogeneous, meaning that the product was applied in a uniform layer on the fabric.
Table 5.1 Results of x-ray measurements, weight%
| Product no. | F , % | Si , % | Information from declaration or data sheet |
| Textile (Blind) | < 0.05 | 0.011 | |
| 1 | 0.43 | 0.081 | F |
| 3 | 0.61 | 0.092 | Unknown |
| 4 | 15 | 0.76 | Si |
| 6 | 0.59 | 0.013 | F |
| 8 | 3.1 | 0.0069 | Unknown |
| 9 | 0.90 | 0.0057 | Unknown |
| 11 | 7.3 | 0.020 | F |
| 14 | 2.0 | 0.0083 | Unknown |
| 15 | 1.4 | 0.012 | Unknown |
| 16 | 3.1 | 0.0075 | Unknown |
| 18 | 0.069 | 0.19 | Si |
| 20 | < 0.05 | 1.6 | Si |
| 21 | 4.2 | 0.23 | F |
| 24 | 5.4 | 0.26 | Unknown |
| 25 | 0.67 | 0.024 | Unknown |
| 26 | < 0.05 | 0.26 | Unknown |
Results of the semi-quantitative GC/MS screening
Table 5.2 shows the results of the GC/MS screening and the calculated estimated content (mg/g). All identified substances are stated with a CAS no.
Organic compounds were not detected in product no. 4 and 24 during extraction with dichloromethane.
Table 5.2 Results of the semi-quantitative GC/MS screening, mg/g
| Product no. | ||||||||
| Name | CAS no. | 1 | 3 | 6 | 8 | 9 | 11 | 14 |
| Hydrocarbons * | - | 360 | 360 | 310 | 320 | 160 | - | 250 |
| Norbonane ** (Bicyclo[2.2.1]heptane) | 279-23-2 | - | 2.2 | - | - | - | - | - |
| Butyl acetate | 123-86-4 | 50 | 21 | - | - | 23 | - | 63 |
| D-Limonene | 5989-27-5 | 1.7 | - | - | - | - | - | - |
| Decahydronaphthalene ** | 493-02-7 | - | - | - | - | 1,0 | - | - |
| Sum of fluorine compounds ** | - | - | - | 0.05 | 0.10 | - | 0.04 | 0.06 |
| 1H,1H,2H2H-perfluoroctan-1-ol ** | 647-42-7 | - | - | 0.17 | 0.29 | - | - | 0.03 |
| Diisooctyl 1,2-benzendicarboxyl acid ** | 27554-26-3 | - | - | 0.25 | - | - | - | - |
* This result covers a sum of several hydrocarbons
** It has not been possible to identify these compounds with reasonable probability by means of the NIST library. The component can be a similar compound.
Table 5.2 Results of the semi-quantitative GC/MS screening, mg/g continued
| Product no. | ||||||||
| Name | CAS no. | 15 | 16 | 18 | 20 | 21 | 25 | 26 |
| Hydrocarbons * | - | 500 | - | 500 | 640 | - | - | 780 |
| Alcohol | - | - | - | - | - | - | 0.44 | - |
| n-propyl acetate ** | 109-60-4 | - | - | - | - | - | 0.08 | - |
| Bromnitromethane ** | - | - | 0.03 | - | - | - | - | - |
| Butoxytrimethylsilan | 1825-65-6 | - | - | - | 0.42 | - | - | - |
| Butyl acetate | 123-86-4 | 39 | 0.04 | - | - | - | 0.05 | - |
| Toluene | 108-88-3 | - | - | 0.80 | 0.10 | - | 0.06 | - |
| Ethyl benzene | 100-41-4 | - | - | 1.4 | - | - | - | - |
| Xylenes | 95-47-6, 108-38-3, 106-42-3 | - | - | 5.5 | - | - | 0.05 | - |
| 3,5-dimethyl-1H-pyrazole ** | - | - | 0.12 | - | - | - | - | - |
| 1-(2-methoxy-1-methylethoxy)-2-propanol, 2-(2-hydroxyproxy)-1-propanol, 1-(2-methoxypropoxy)-2-propanol and similar compounds | 20324-32-7, 106-62-7, 13429-07-7 etc. | - | - | - | - | 4.8 | - | - |
| Decahydro-naphthalen e** | 493-02-7 | - | - | - | 1.7 | - | - | - |
| decahydro-naphthalene ** | 91-17-8 | - | - | - | - | - | - | 21 |
| 2-Methyl-trans-decalin or decahydro-2-methyl-naphthalene ** | 1000152-47-3, 2958-76-1 | - | - | - | 1.2 | - | - | 36 |
| Sum of fluorine compounds ** | - | - | - | - | - | 0.03 | 0.17 | - |
| Dodecamethylpentasiloxane | 141-63-9 | - | - | 1.1 | - | - | - | - |
| 2,5,8,11,14-Pentaoxapentadecan ** | 143-24-8 | - | 1.6 | - | - | - | - | - |
| Siloxane compounds ** | - | - | - | 0.53 | 1.8 | - | - | 0.37 |
| Octadecan acid ** | - | - | - | - | - | - | 0.02 | - |
| Piperonylbutoxid | 51-03-6 | - | - | - | - | - | 0.05 | - |
* This result covers a sum of several hydrocarbons
** It has not been possible to identify these compounds with reasonable probability by means of the NIST library. The component can be a similar compound.
”-”: not detected
5.1.2 Results of the SPME-GC/MS screening
Table 5.3 shows the results of the SPME-GC/MS screening that was carried out. The identified substances are marked with ”X”. The substances are shown according to their retention time which is an expression for their boiling point and thus their ability to evaporate. Therefore, substances with the lowest boiling point are stated first in the table. All identified substances are stated with a CAS no.
Volatile organic compounds were not identified in product no. 4 and 24 of this analysis method.
Table 5.3 Results of SPME-GC/MS screening
| Identification | CAS no. | Product no. | |||||||||||||
| 1 | 3 | 6 | 8 | 9 | 11 | 14 | 15 | 16 | 18 | 20 | 21 | 25 | 26 | ||
| Propane | 74-98-6 | x | x | ||||||||||||
| Isobutane | 75-28-5 | x | x | x | |||||||||||
| Dimethyl ether | 115-10-6 | x | x | ||||||||||||
| Butane | 106-97-8 | x | x | x | x | ||||||||||
| Acetone | 67-64-1 | x | x | x | X | ||||||||||
| Isopropyl alcohol | 67-63-0 | x | x | x | x | x | x | x | x | ||||||
| 1,1-Dichlorethane | 75-34-3 | x | |||||||||||||
| Methylenchloride | 75-09-2 | x | |||||||||||||
| 1-Propanol | 71,23-8 | x | |||||||||||||
| 1,2-Dichlorethene | 156-60-5 | x | |||||||||||||
| Acetic acid | 64-19-7 | x | |||||||||||||
| 2-Butanone | 78-93-3 | x | X | ||||||||||||
| 2-Butanol | 78-92-2 | x | |||||||||||||
| Ethyl acetate | 141-78-6 | x | x | x | x | x | |||||||||
| Isopropyl acetate | 108-21-41 | x | x | x | x | x | |||||||||
| 1-Butanol | 71-36-3 | x | x | x | x | ||||||||||
| 3,3-Dimethylpentane, 2,3-dimethylpentane | 562-49-2, 565-59-3 | x | x | x | x | x | |||||||||
| Cyclohexane | 110-82-7 | x | x | x | x | x | |||||||||
| 2-Methylhexane, 3-methylhexane | 591-76-4, 589-34-4 | x | x | x | x | x | x | ||||||||
| Alcohol | x | ||||||||||||||
| Dimethylcyclopentane | 1638-26-2 | x | x | x | x | x | x | ||||||||
| Heptane | 142-82-5 | x | x | x | x | x | x | ||||||||
| Methyl-cyclohexane | 108-87-2 | x | x | x | x | x | |||||||||
| Ethyl cyclopentane | 1678-91-7 | x | x | x | x | ||||||||||
| Pentane, 3-ethyl- | 617-78-7 | x | |||||||||||||
| Trimethylcyclopentane | 15890-40-1 | x | x | x | |||||||||||
| 2-Methylheptane, 3-methylheptane | 592-27-8 | x | |||||||||||||
| Butyl acetate | 123-86-4 | x | x | x | x | x | x | ||||||||
| Octane | 111-65-9 | x | x | x | |||||||||||
| Dimethyl cyclohexane | 638-04-0 | x | |||||||||||||
| Ethyl benzene | 100-41-4 | x | |||||||||||||
| Hydrocarbons* | x | x | x | x | x | x | x | x | x | X | x | ||||
| Alcohols or cyclic alkanes | X | ||||||||||||||
| Xylene | 95-47-6, 108-38-3, 106-42-3 | x | x | x | |||||||||||
| Trimethylcyclohexane | 1839-63-0 | x | x | ||||||||||||
| Trimethylcycloheptane | x | ||||||||||||||
| Hexylen glycol | 107-41-5 | x | |||||||||||||
| Nonane | 111-84-2 | x | x | X | |||||||||||
| 3-Methylnonane, 2-methylnonane | 06-04-5911 | x | |||||||||||||
| Dipropylene glycol monomethyl ether ** | 20324-32-7 | x | |||||||||||||
| Decan | 124-18-5 | x | x | x | X | ||||||||||
| 5-Ethyl-2-methyl-heptane | 13475-78-0 | x | |||||||||||||
| D-limonene ** | 5989-27-5 | x | |||||||||||||
| 2-Butoxyethyl acetate | 112-07-2 | x | |||||||||||||
| Undecane | 1120-21-4 | x | x | x | x | X | |||||||||
| Dodecane | 112-40-3 | X | |||||||||||||
* This result covers a sum of several hydrocarbons
** It has not been possible to identify these compounds with reasonable probability by means of the NIST library. The component can be a similar compound.
”-”: not detected
6 Results of quantitative analyses and aerosol analyses
In co-operation with the Danish Environmental Protection Agency, 10 products were selected for quantitative analyses and investigation for content of possible perfluoralcohols. The selection took a starting point in the screening analyses results by x-ray and GC/MS.
The 10 selected products appear from Table 6.1.
Table 6.1 Outline of selected products
| Product no. | 1 | 3 | 4 | 8 | 14 | 16 | 18 | 21 | 25 | 26 |
| Type | Spray | Spray | Pump | Spray | Spray | Pump | Spray | Spray | Spray | Spray |
9 of the products (no. 1, 3, 8, 14, 16, 18, 21, 25, 26) were analysed to quantify selected organic compounds that might constitute a health risk.
Analyses were carried out on the selected products (no. 4, 8, 14, 21, 25) where x-ray measurements either detected a content of fluorine or where GC/MS screening analyses detected content of fluorinated alcohols related to 1H,1H,2H,2H-perfluoroctane-1-ol, in order to examine if it was possible to identify these compounds and quantify them against 1H,1H,2H,2H-perfluoroctan-1-ol.
In connection with the initial analyses, product no. 4 showed a high content of fluorine but it was not possible by means of GC/MS analysis to detect content of organic compounds. In agreement with the Danish Environmental Protection Agency it was therefore decided to investigate if it was possible to find another analysis method for determination of the substances in this product.
6.1 RESULTS OF ANALYSES
In the following, the results of the quantitative analyses, investigations of fluorinated compounds and analyses of product no. 4 will be presented. The results are discussed closer in chapter 7, Discussion of analysis results.
6.1.1 Results of quantitative analyses of organic compounds
The tables below show the results of the quantitative GC/MS analyses of the selected products and the selected organic compounds that are considered to be relevant in relation to the health assessment. The results are the averages of the analyses in duplicate and the standard deviation is stated.
Table 6.2 Results of quantitative analyses, mg/g
| Identification | CAS no. | Sample no. | |||||
| 1 | 3 | 4 | 8 | 14 | Det.limit | ||
| 1,1-Dichlorethane | 75-34-3 | i.a. | i.a. | - | 0.06 ± 0.01 | i.a. | 0.02 |
| 1,2-Dichlorethene | 156-60-5 | i.a. | i.a. | - | - | i.a. | 0.02 |
| Methylene chloride | 75-09-2 | i.a. | i.a. | - | - | i.a. | 0.02 |
| 2-Butanone | 78-93-3 | i.a. | i.a. | - | - | i.a. | 0.02 |
| 1-Butanol | 78-92-2 | - | - | - | - | i.a. | 0.2 |
| Cyclohexane | 110-82-7 | 6.5 ± 0.3 | 0.29 ± 0.02 | - | 6.0 ± 0,2 | i.a. | 0.01 |
| Heptane | 142-82-5 | 105 ± 4 | 267 ± 11 | - | 210 ± 24 | i.a. | 0.01 |
| Toluene | 108-88-3 | - | - | - | - | - | 0.02 |
| Ethyl benzene | 100-41-4 | - | - | - | - | - | 0.02 |
| p-Xylene | 95-47-6 | - | - | - | - | - | 0.02 |
| m- and o-xylene | 108-38-3, 106-42-3 | - | - | - | - | - | 0.02 |
| n-Butyl acetate | 123-86-4 | 98 ± 3 | 20 ± 2 | - | - | 80 ± 3 | 0.03 |
| d-Limonene | 5989-27-5 | 0.50 ± 0.01 | - | - | - | - | 0.02 |
| 2-Butoxyethyl acetate | 112-07-2 | - | - | - | - | - | 0.02 |
| Dodecamethylpenta siloxane | 141-63-9 | - | - | - | - | - | 0.03 |
| 1H,1H,2H2H-perfluoroctane-1-ol | 647-42-7 | i.a. | i.a. | - | - | - | 0.06 |
| Other fluorine containing compounds* | i.a. | i.a. | - | 0.61 ± 0.04 | 0.68 ± 0.01 | 0.1 | |
”-” Means that the component was not identified or below the detection limit
”i.a.” Means that analysis has not been carried out for this component
* Calculated against 1H,1H,2H2H-perfluoroctane-1-ol
Det. limit: Detection limit
Table 6.2 Results of quantitative analyses, mg/g, continued…
| Identification | CAS no. | Sample no. | |||||
| 16 | 18 | 21 | 25 | 26 | Det.limit | ||
| 1-Butanol | 78-92-2 | - | 3.8 ± 0.5 | - | - | 0.44 ± 0.01 | 0.2 |
| Cyclohexane | 110-82-7 | - | - | - | - | - | 0.01 |
| Heptane | 142-82-5 | - | 0.048 ± 0.002 | - | - | - | 0.01 |
| Toluene | 108-88-3 | - | 0.78 ± 0.01 | - | 0.065 ± 0.003 | 0.020 ± 0.001 | 0.02 |
| Ethyl benzene | 100-41-4 | - | 0.97 ± 0.01 | - | 0.027 ± 0.001 | - | 0.02 |
| p-Xylene | 95-47-6 | - | 2.4 ± 0.1 | - | 0.046 ± 0.001 | - | 0.02 |
| m- g o-xylene | 108-38-3, 106-42-3 | - | 0.84 ± 0.01 | - | 0.032 ± 0.001 | - | 0.02 |
| n-Butyl acetate | 123-86-4 | 0.058 ± 0.009 | - | - | 0.065 ± 0.03 | - | 0.03 |
| d-Limonene | 5989-27-5 | - | - | - | - | - | 0.02 |
| 2-Butoxyethyl acetate | 112-07-2 | 0.037 ± 0.002 | - | - | - | - | 0.02 |
| Dodecamethylpenta siloxane | 141-63-9 | - | 0.66 ± 0.01 | - | - | - | 0.03 |
| 1H,1H,2H2H-Perfluoroctane-1-ol | 647-42-7 | i.a. | i.a. | - | - | i.a. | 0.06 |
| Other fluorine containing compounds* | i.a. | i.a. | 0.33 ± 0.01 | - | i.a. | 0.1 | |
”-” Means that the component was not identified or below the detection limit
”i.a.” Means that analysis has not been carried out for this component.
* Calculated against 1H,1H,2H2H-perfluoroctane-1-ol
Det. limit: Detection limit
6.1.2 Results of analyses of fluorine containing compounds
The analysis of product 4, 8, 14, 21 and 25 together with the analysis of an external standard showed that none of the products contain 1H,1H,2H2H-perooctane-1-ol. In product 8, 14, 21 and 25 a number of compounds were detected that cannot be identified. From the mass spectrum of the substances it was assessed that they are related to 1H,1H,2H2H-perfluoroctane-1-ol.
Through analysis by means of NCI-GC/MS it was also confirmed that fluorine compounds are in question, please also see chapter 7.
6.1.3 Results of analyses of product no. 4
It was not possible to find a suited solvent for product no. 4 that makes a screening analysis for volatile and semi-volatile organic compounds by GC/MS possible.
6.1.4 Results of aerosol analyses
The measured aerosol concentrations and average sizes have been summed up in Table 6.4 and are also shown in Figure 6.1. The aerosol size distribution of the 16 selected products appears from table 6.4. The largest uncertainty on the measurement results is found in the reproducibility of the amount and the way the proofing liquid leaves the product at 10 s of continuous use. For products with a pump, continuous use means that pumping takes place continuously for 10 s at a frequency that is as high as possible in order to obtain maximum liberation of a product. The variation in the amount of liberated aerosols is ± 40 %. Please also refer to table 6.3 for liberated substances from pump and spray products. The variation in the mean value of the aerosol diameter is ± 20%. Figure 6.2 shows the product amount that was liberated during 10 s of proofing and it was measured by weighing the can before and after proofing.
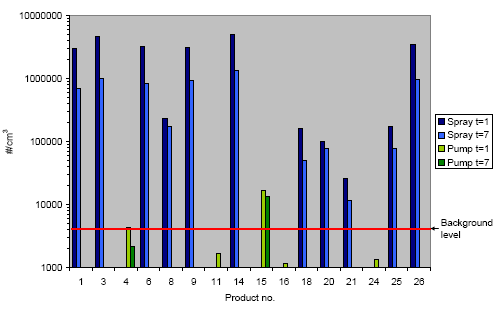
Figure 6.1 Total number of aerosoles (#) liberated during 10 s of proofing with spray or pump products measured at two different times after proofing.
| Aerosol measurement after 1 minute | Aerosol measurement after 7 min. | |||||||||
| Product no. | Libe- rated amount of proofing spray per 10 s. (g) |
Aero- sols 20-650 nm (103 per cm³) |
Aero- sols 20-100 nm (103 per cm³) |
Aero- sols 100-650 nm (103 pee cm³) |
Mean aerosol size (nm) |
Aero- sols 20-650 nm (103 per cm³) |
Aero- sols 6-20 nm (103 per cm³) |
Aero- sols 20-100 nm (103 per cm³) |
Aero- sols 100-650 nm (103 per cm³) |
Aero- sol size Mean value (nm) |
| 1 | 5.0 | 3000 | 2000 | 1000 | 104 | 680 | 0 | 370 | 320 | 124 |
| 3 | 4.3 | 4500 | 1500 | 3000 | 171 | 1000 | 0 | 150 | 860 | 209 |
| 4 | 5.0 | 4 | 2 | |||||||
| 6 | 6.7 | 3200 | 2300 | 930 | 93 | 840 | 0 | 510 | 330 | 110 |
| 8 | 14.6 | 230 | 230 | 8 | 38 | 170 | 67 | 170 | 4 | 36 |
| 9 | 7.3 | 3100 | 2200 | 890 | 98 | 930 | 0 | 470 | 460 | 125 |
| 11 | 17.0 | 2 | 1 | |||||||
| 14 | 7.0 | 5000 | 3200 | 1800 | 105 | 1300 | 0 | 570 | 770 | 129 |
| 15 | 4.3 | 17 | 13 | 3.4 | 79 | 13 | 4 | 11 | 2 | 70 |
| 16 | 16.7 | 1 | 1 | |||||||
| 18 | 1.0 | 2500 | 1500 | 1000 | 114 | 670 | 0 | 300 | 380 | 148 |
| 20 | 9.3 | 100 | 78 | 23 | 83 | 77 | 3 | 60 | 17 | 82 |
| 21 | 5.9 | 26 | 14 | 12 | 136 | 12 | 7 | 8 | 4 | 120 |
| 24 | 2.6 | 1 | 1 | |||||||
| 25 | 12.0 | 170 | 120 | 57 | 99 | 76 | 21 | 63 | 13 | 68 |
| 26 | 12.7 | 3400 | 2800 | 620 | 74 | 950 | 0 | 720 | 230 | 89 |
Table 6,3. Measured aerosol concentrations and average sizes.
Table 6.4. Number (#) of measured aerosols per cm³ as function of aerosol diameter in the interval of 10-1000 nm after 7 min.
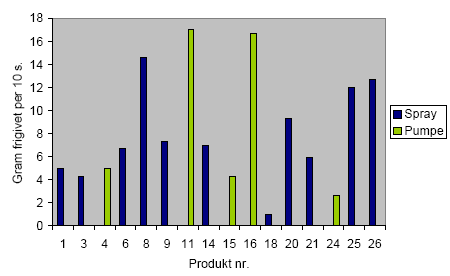
Figure 6.2 Product liberated (g) after 10 s of proofing.
7 Discussion of analysis results
7.1 Chemical analyses
The chemical analyses that were carried out demonstrated and quantified a number of organic chemical compounds that either were on the List of Hazardous Substances or comprised by Regulation no. 571 concerning the use of propellants and solvents in aerosol cans. The importance of these results is also mentioned in Chapter 8.
7.2 Fluorinated compounds
As expected, a content of the element fluorine was demonstrated in a number of the investigated products. However, it has only been possible to detect a limited number of fluorine compounds and on the basis of the estimated concentrations it must be ascertained that the main part of the fluorine compounds contained in the fluorine based products cannot be detected. Most likely because the substances are developed or intended to polymerize rather quickly and create a water-repellent surface. The substances are expected to consist of short fluorinated carbon strings of the type (-CF2-)n or similar, terminated with an active component which leads to polymerisation. The main component is presumably designed to polymerize easily when in contact with air (oxygen and/or water vapour) which therefore also will happen in connection with the analytical procedure. That makes it very difficult to isolate and analyse monomers.
The detected fluorine compounds appear in concentrations of less than 1 mg/g in products where the fluorine content determined by x-ray analysis is more than 20 mg/g. That means that the main part of the fluorine amount exists as a substance that cannot immediately be analysed by means of GC/MS. The reason is most probably that the non-detectable fluorine compounds exist in complete or partly polymerized form. Analysis methods taking this problem into account have not been published and a more complete fluorine mass balance therefore requires the development of completely new analysis methods.
The mass spectra of the detected fluorine compounds are related to known mass spectra for substances of the type CF3(CF2)nCH2CH2OH, for instance 1H,1H,2H,2H-perfluoroctane-1-ol (FTOH 6:2). Such substances are called fluortelomer alcohols (FTOH). However, none of the detected substances have the same chromatographic retention time or a mass spectrum identical to FTOH 6:2. In addition, the mass spectra do not indicate that the nucleus in the substances is fluortelomer aldehydes, fluortelomer acids or unsaturated fluortelomer alcohols. Several of the detected substances have mass spectra that are very similar to FTOH 6:2, however, with the decisive difference that the mass fragment m/z 95 has been replaced by m/z 77. M/z 95 corresponds to –CF2CH2CH2OH and is the same for all fluortelomer alcohols. M/z 77 can correspond to -CFHCH2CH2OH where one single fluorine atom has been replaced with a hydrogen atom. Therefore, it must tentatively be said that several products contain substances of the type CF3-(CF2)n- CFHCH2CH2OH.
On the basis of the chromatographic retention times on the chosen non-polar column it is possible to estimate the boiling point intervals of the detected substances by comparing with hydrocarbon standards.
Product no. 8 contains 3 fluorine compounds with estimated boiling points in the interval of 450-520K.
Product no. 14 contains 6 fluorine compounds with estimated boiling points in the interval of 430-480K.
Product no. 21 contains 4 compounds with estimated boiling points in the interval of 390-470K.
In comparison, FTOH 6:2 has a boiling point of 368K. With the above method a boiling point of 407K is estimated which indicates that this simple method has a tendency to overestimate the boiling point.
As it appears, substances much less volatile than FTOH 6:2 were detected in most cases and therefore they must be assumed to have a longer string length.
7.3 Aerosol analyses
The aerosol analyses that were carried out show that the consumer can be exposed to rather large amounts of small aerosols (6-650 nm) when using textile proofing agents. The concentration of aerosols (propellant products) in the can that was used was in several cases larger than 106 per cm³ at 10 s of exposure. In comparison, the exhaust from diesel vehicles contains 107-108 per cm³ (at the exhaust pipe without dilution from the surroundings). In polluted town air the aerosol concentration is in the order of magnitued of 105 per cm³.
In general, spray products with propellant liberated more aerosols in the interval 20-650 nm compared to other products without propellant. The amount of liberated aerosols from products without propellants was in most cases comparable to the background level. In connection with spray products, there was a great difference between the number of aerosols and aerosols per weight unit that the different products create. No clear connection can be made between the chemical composition and number or aerosol size. Aerosol exposure is first and foremost determined by whether spray or pump products are in question.
The reason why pump based products do not cause exposure to aerosols to an appreciable extent is probably that the pump mechanism gives larger primary aerosols which are deposited much more efficiently on textiles than the smaller aerosols generated from propellant products.
In several of the investigated products, the word ”nano” appears in the product name. However, all those products have a pump mechanism and do not give rise to the liberation of small aerosols as mentioned above.
The obtained results do not indicate that the products contain actual nanoaerosols and the “nano” description presumably refers to the coating that is obtained. In principle, it cannot be ruled out that the products contain nanoparticles in solid form suspended in liquid, but neither the chemical analyses nor the aerosol analyses indicate that. In any case, it can be ascertained that products with pump mechanisms do not expose the user to small aerosols.
Whether or not a nanostructured coating, a coating with nanothickness or added nanoparticles (in solid form) actually are in question has not been investigated in this project.
These results are in agreement with German investigations that concluded that a certain ”nanoproduct” did not contain nanomaterials (BfR, 2006b).
Figure 7.1 shows how fine and ultra fine aerosols are created when a propellant based product is used.
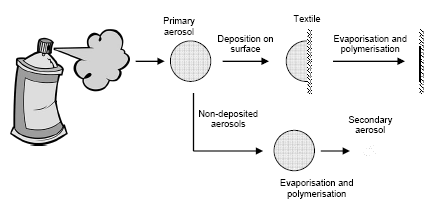
Figure 7.1 Flow diagramme of the creation of fine and ultra fine (nano) aerosols after evaporation of solvent from the fraction of the primary aerosols that are not deposited on the textile surface.
The measurement of aerosols in the interval of 6-650 nm seems to cover the entire relevant measuring area for all products, however, the aerosol distribution of product 3 has a smaller fraction the exceeds 650 nm and for product 8 it seems that few aerosols are liberated with a diameter under 6 nm. The aerosol distribution of product 3 was measured up to 1000 nm. The amount of aerosols in the interval of 650-1000 nm declined evenly. From the obtained size distributions it can be ruled out that there will be significant amounts of aerosols > 1000 nm because then a gradual increase in the number of aerosols in the high end of the size interval would have been observed.
That demonstrates that the solvents (all volatile) evaporate very quickly (within 1 minute) and leave small aerosols. Otherwise, a pronounced change in size would have been observed in the period after 1 minute.
The mean size of the liberated aerosols from the products is shown in Figure 7.2. In connection with spray products, the aerosol size general increased with time after proofing except for product 8 and 20 where the aerosol size was constant and product 21 and 25 where the aerosol size declined. After liberation of spray products, the aerosol size increased as a function of time and that might be because the concentration of aerosols is very high and therefore the collision rate between the aerosols is high. During collision, the aerosols can aggregate and in that way create larger aerosols in time. A decline in aerosol size can be due to additional evaporation of small volatile compounds.
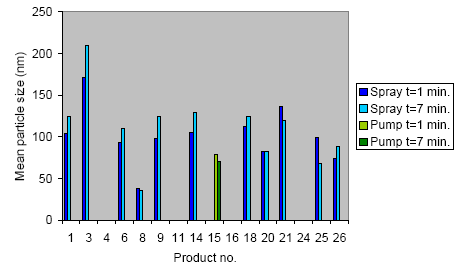
Figure 7.2 Mean particle size (nm), measured 1 minute and 7 min. after proofing.
8 Health Assessment
- 8.1 Butyl acetate
- 8.2 Butanone
- 8.3 1-Butanol
- 8.4 Cyclohexane
- 8.5 Perfluoroctane-1-ol
- 8.6 Dodecamethylpentasiloxane
- 8.7 Recapitulation on health assessment and information collection
In consultation with the Danish Environmental Protection Agency the following substances were selected for health assessment: cyclohexane, butan-2-on, 1-butanol, butyl acetate, perfluoroctane-1-ol and dodecamethylpentasiloxane. In this chapter, the toxicological profiles of the 6 chemical substances have been set up. The four first mentioned substances are assumed to be used in spray products in their capacity of propellants and solvents and therefore they are subject to special control in Regulation 571 dated 29/11/1984 (the Danish Environmental Protection Agency). All four substances are included on the list of permitted propellants and solvents in enclosure 1 of the Regulation, but all four substances are forbidden (in concentrations exceeding 1 %) in products intended for indoor use (all tested spray products) with propellant. The two last-mentioned are the two actual proofing substances where the occurrence has been best documented in the spray products selected for analysis.
All the substances were found in products that are sprayed from aerosol cans with propellant. None of the below assessments deal with the fact that the substances also appear as very fine aerosol mists. That is because it has not been possible to find experimental toxicological data for these substances in the form of aerosols. The end of this chapter examines the importance of the very fine aerosol mists that have been measured for all aerosol products with propellants in this investigation.
8.1 Butyl acetate
8.1.1 Application
Butyl acetate is mainly used as solvent in varnish, artificial leather, photographic film and plastics. To a minor degree, butyl acetate is used in the perfume industry and for the production of artificial aromatic compounds (HSDB, 2007).
8.1.2 Identification
At room temperature, butyl acetate is a clear, colourless liquid with a pleasant smell that often is described as banana-like. It is not easily soluble in water, but it is miscible with most hydrocarbons and very easily soluble in ethanol and ether and soluble in acetone (HSDB, 2007). The odour limit in water is 0.066 mg/m³ (HSDB, 2007). Butyl acetate is included on the list of organic solvents of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Butyl acetate |
| Synonyms: | 1-Butyl acetate; n-Butyl acetate; 1-Butyl acetate; Acetic acid, butyl ester (ECB, 2007) Butyl ethanoate (HSDB, 2007) |
| CAS no.: | 123-86-4 |
| EINECS No.: | 204-658-4 |
| Molecule formula | C6H16O2 |
| Molecule structure | 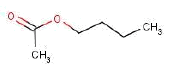 |
| Legislation: Classification according to the list of hazardous substances (Danish Environment Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value of the Danish Working Environment Authority (ppm, mg/m³) |
R10; R66; R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 150 ppm; (710 mg/m³) for all butyl acetates |
8.1.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid (HSDB, 2007) |
| Molar weight | 116.16 (HSDB, 2007) |
| Density | 0.8826 g/cm³ at 25°C (HSDB, 2007) |
| Melting point | -78°C (HSDB, 2007) |
| Boiling point | 126.1°C (HSDB, 2007) |
| Vapour pressure at 25 ?C | 11.5 mm Hg (HADB, 2007) |
| Octanol water (logPow) | 1.78 (HSDB, 2007) |
| Solubility in water | 14 g/L at 20°C; 5 g/L at 25°C (HSDB, 2007) |
| Odour limit in water | 0.066 mg/m³ (HSDB, 2007) |
8.1.4 Toxicological data
8.1.4.1 Absorption
Butyl acetate is quickly absorbed in the blood by inhalation. No measurements exist of gastrointestinal or dermal absorption, but effectuated oral and dermal LD50 studies indicate that the substance also is absorbed through these routes.
8.1.4.2 Acute effects, humans
Butyl acetate has a low systemic effect (HSDB, 2007). The lowest toxic concentration on inhalation was found to be 200 ppm (920 mg/m³), and changes were found on the sensory organs and especially the olfactory sense, on eyes (irritation) and on lungs, on chest and respiration (other changes) (ChemIDPlus, 2007).
Possible toxic symptoms are central nervous system (CNS) effects: headache, muscular weakness, dizziness, stiffness, confusion, delirium and coma. Gastrointestinal tract effects are: nausea, vomiting, and diarrhea (with smell of the alcohol from the faeces); irritation in eyes and neck from vapour as well as liquid, coughing and dyspnoea; ictus disturbance; death due to respiratory failure. (HSDB, 2007).
Butyl acetate is described as a mildly irritating substance, but more irritating than ethyl acetate, and as a CNS depressor. These effects are considered to originate from the physical properties of the substance (HSDB, 2007)).
Skin exposure: prolonged or frequently repeated exposure can lead to drying of the skin.
Butyl acetate vapours lead to eye irritation and inhalation irritates the respiratory passages.
Occupational inhalation has led to effects on the liver (HSDB, 2007).
8.1.4.3 Acute effects, animals
In connection with oral administration, the LD50 values are between 3200 mg/kg bw (rabbit) and >10.000 mg/kg bw (rat). Dermal LD50 17.600 mg/kg bw (rabbit); LD50 values by direct administration in the abdominal cavity was 1230 and 1500 mg/kg bw in guinea pigs and mice, respectively. LC50 was 6000 mg/m³ after 2 hours of inhalation in mice and 390 ppm corresponding to 1850 mg/m³ after 4 hours of inhalation in rats (ChemIDPlus, 2007).
8.1.4.4 Subchronic effects
No studies have been found with repeated dosage in animals apart from one single study in cats where no local changes were found in the cornea or the conjunctival sac of cats dosed either with 500 ppm for 20 days or with 1000 ppm for 4 days. However, according to ACGIH, animals (species of animal not informed) exposed 6 hours a day for 6 days to 3100 ppm showed blood changes (HSDB, 2007).
8.1.4.5 Mutagenicity
Butyl acetate showed no mutagenic properties in Ames' test (Salmonella typhimurium strands TA98, TA100, TA1535, TA1537, TA1538 and Escherichia coli (WP2uvrA strand)) during testing with and without activation with rat microsomal fraction.
8.1.4.6 Chronic effects
No long-term tests with butyl acetate have been carried out in any species of animal. IARC has not considered the carcinogenic properties of butyl acetates. On the other hand, ACGIH in the USA has decided that within a two-year period the substance shall be transferred to an approval list: Cannot be classified as a human carcinogen (HSDB, 2007).
8.1.4.7 Summary
Butyl acetate is not acute toxic on intake or inhalation or during exposure of the skin. Due to the physical/chemical properties – solvent with large vapour pressure – the substance has irritating effects on skin and mucous membrane (eyes and upper respiratory passages) and a number of effects on the CNS after inhalation. No information has been found stating that butyl acetate should be sensitizing.
It is assessed that people working in the chemical industry who have skin diseases, nephropathy, chronic respiratory diseases or hepatic diseases can have increased risk in connection with exposure to butyl acetate.
Toxicological data (animals) |
|
| LD50, mg/kg, oral, rat | 10768 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, guinea pig | 4700 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, mouse | 6000 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, rabbit | 3200 (ChemIDPlus, 2007) |
| LD50, mg/kg, dermal, rabbit | >17600 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 2 hours, mouse | 6000 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 4 timer, rat | 1846 (corresponding to 390 ppm) (ChemIDPlus, 2007) |
| Toxicological data (humans) | |
| LCLo mg/m³, inhalation (time not informed) | 947 (corresponding to 200 ppm) (ChemIDPlus, 2007) |
8.1.5 Health assessment of butyl acetate
Occurrence in investigated spray products:
| Butyl acetate measured in analysed products | Product no. | ||||||
| 1 | 3 | 9 | 14 | 15 | 16 | 25 | |
| Semi-quantitative g/kg (%) | 23 (2.3) |
39 (3.9) |
|||||
| Quantitative g/kg (%) | 98 (9.8) |
20 (2.0) | 80 (8.0) |
0.058 (0.58) |
0.065 (0.65) | ||
| Butyl acetate declared (other remarks) | No (discontinued product) |
Yes | No | Yes | Yes | No | No |
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The aerosol product with the highest concentration of butyl acetate is product no. 1 that true enough has been discontinued, but product no. 14 contains almost as much. The calculation was most logically carried out in product no. 14 which still is marketed.
Product no. 14 is sold in Denmark in 200 ml spray cans but in other countries it is marketed in 400 ml cans.
The density of the spray liquid is not known but a conservative estimate is a density of 1 g/cm3 which means that 200 ml weighs 200 g.
Therefore, a spray can will contain 16 g of butyl acetate and distributed in a 20 m³ large room that will give a concentration of 800 mg/m³.
That is 12 % above the limit value of the Danish Working Environment Authority which is 710 mg/m³ (150 ppm).
The limit value is a “time weighted average” determined according to extensive toxicological estimates as the value to which a worker may be exposed 8 hours daily in an entire working life.
As the product is a consumer product where exposure only will take place now and then, the calculated value can instead be compared with the ceiling value of the Danish Working Environment Authority, at the double of the ordinary limit value.
Therefore, it must be assessed that the use of spray no. 14 is not injurious to health even during the absolute worst case scenario. Presumably, passing and acute sickness can arise (irritation of the eyes and respiratory passages).
8.1.6 Conclusion on butyl acetate (n-butyl acetate)
The content of butyl acetate in the examined spray products for textile proofing is not in itself a health hazard to the consumers.
8.2 Butanone
8.2.1 Application
Is mainly used as solvent in surface coating industries, paint and varnish industries, for polymer and glue production and as an intermediate for chemical syntheses in the chemical and pharmaceutical industry. In addition, it is used to some extent in the aromatics industry.
The Food and Drug Administration (FDA) in the USA has set an acceptable daily intake value (ADI) of 3.2 mg/day (oral intake) (HSDB, 2007).
8.2.2 Identification
Butanone is a clear liquid with a sweet, pleasant, lightly pricking, acetone-like odour. Butanone is easily soluble in water at low temperatures, but the solubility declines with increasing temperatures. The substance is soluble in alcohol, ether, acetone and benzene. (HSDB, 2007).
The substance is included on the list of organic substances of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Butanone |
| Synonyms: | Methyl ethyl ketone; butan-2-on; 2- butanone; methyl ethyl ketone |
| CAS no.: | 78-93-3 |
| EINECS No.: | 210-159-0 |
| Molecule formula | C4H8O |
| Molecule structure | 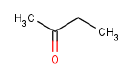 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value (ppm, mg/m³) (The Danish Working Environment Authority, 2007) |
F;R11 XI;R36 R66 R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 145 mg/m³ (H, can be absorbed through the skin) |
8.2.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid (HSDB, 2007) |
| Molar weight | 77.11 (HSDB, 2007) |
| Density | 0.805 g/cm³ at 20°C (HSDB, 2007) |
| Melting point | -86°C (HSDB, 2007) |
| Boiling point | 79.6°C (HSDB, 2007) |
| Vapour pressure at 25°C | 90 mm Hg (HSDB, 2007) |
| Octanol water (logPow) | 0.29 (HSDB, 2007) |
| Solubility in water | 353 g/L at 10°C (HSDB, 2007); 27.1 g/L at 20°C (IUCLID)(IPCS, 1992) |
| Odour limit | low: 0.7375 mg/m³; high = 147.5 mg/m³ (HSDB, 2007) |
8.2.4 Toxicological data
8.2.4.1 Absorption
Butanone is absorbed quickly in the body no matter if oral or dermal exposure is in question or if absorption takes place on inhalation. Butanone seems to be distributed on all tissue. Butanone and its metabolites are eliminated completely in the course of 24 hours. Elimination especially takes place with the expiratory air even though small amounts are eliminated in transformed form via the kidneys (IPCS, 1992).
8.2.4.2 Acute effects, humans
Exposure to 590 mg/m³ (200 ppm) did not cause changes in different behaviour or psychological tests. Nor did experimental exposure to 794 mg/m³ (270 ppm) 4 hours/day have greater effect on behaviour and 5 min. contact with liquid butanone only caused passing bleaching of the skin (IPCS, 1992).
8.2.4.3 Acute effects, animals
Very low acute toxicity was present in the tested species of animals for all routes of administration. The LD50 values for oral studies are 2700 and 5520 mg/kg bw in rats and 34140 mg/kg bw in mice. The inhalation studies carried out on mice and rats are all very old and were not carried out in accordance with the current guidelines but the lethal concentration for 50 % of the animals (LC50) in mice after 45 min. of exposure can be calculated to 205025 mg/m³ (69500 ppm) and in rats after 4 hours of exposure to 23600 mg/m³ (8000 ppm). A dermal LD50 value was found in rabbits at 8000 mg/kg bw with 24 hours of exposure (IPCS, 1992).
Minor to moderate irritation of the skin and moderate to serious irritation of rabbit eyes were observed. Other skin studies did not show irritation (IPCS, 1992).
8.2.4.4 Subchronic effects
Most studies with repeated dosage were carried out on rats with exposure on inhalation. Only doses of 5000 ppm (14750 mg/m³) in the one and 5041 ppm (14870 mg/m³) in the other given 6 hours/day 5 days a week for 90 days had effects. Reduced body weight, brain and spleen weight and increased liver weight and changed blood parameters were found and females were more sensitive than males. No histopathological changes or influence on the reproductive organs or morphological changes in CNS or peripheral nervous systems (PNS) were found (IPCS, 1992).
In a test, mice were exposed to increasing concentrations of butanone from 300 to 10000 ppm (total of 5 levels). The dosage time of each concentration was 30 min. and the number of mice that did not react to visual stimuli was counted. The dose at which 50 % of the animals no longer reacted could be calculated to 8528 mg/m³ corresponding to 2891 ppm (IPCS, 1992).
In a teratogenic test in mice, a no observed adverse effect concentration (NOAEC) of 2980 mg/m³ (1010 ppm) given on day 6-15 of the gestation period, 7 hours/day could be determined. No significant toxicity signs were found in the dam, but there was a minor increase in the relative liver weight in the highest dosed group. In the same group, lower foetal body weight was observed and it was significant for the males. Lowest observed adverse effect concentration (LOAEC) was set to 3000 ppm (IPCS, 1992) due to the developmental effects.
8.2.4.5 Mutagenicity
Butanone was not found mutagenic in a number of Ames' tests that were carried out and in vivo micronucleus studies in mice or Chinese guinea pigs showed no positive effects. However, some studies showed that butanone and a number of similar substances induce aneuplodi in yeast cells; an effect that was significantly strengthened by simultaneous exposure to ethyl acetate (IPCS, 1992).
In the light of this, butanone cannot be assessed to be genotoxic in short-term tests in vitro and in vivo.
8.2.4.6 Chronic effects
The only longer study that has been carried out is a one-year dermal study in male mice with application twice weekly of 8 mg (50 mg of a 17 % solution). No papilloma were found after 1 year (7).
Butanone cannot be classified with regard to carcinogenic effect in humans as no information exists about the substance concerning cancer in humans and sufficient data from experiments on animals does not exist.
8.2.4.7 Summary
Butanone is easily absorbed in the body after exposure via the gastrointestinal tract, the skin or the lungs. Absorbed butanone is eliminated in the course of 24 hours. Butanone has a very low acute toxicity in humans as well as in animals.
The results from testing butanone for irritation of skin and mucous membrane conflict a bit, but some irritation was found in most studies. Exposure of human skin to undiluted butanone results in passing bleaching of the skin. Butanone is classified with regard to eye irritation (R36) but not with regard to irritation of skin although repeated exposure can give dry or cracked skin (R66), In addition, a product should be marked, stating that vapours can cause lethargy and dizziness (R67) if it contains 15% or more butanone plus possibly other chemical substances with the same effect.
The critical effect is found to be lower foetal body weight in a teratogenic test with mice with a NOAEC of approx. 3000 mg/m³ corresponding to a bit more than 1000 ppm, treatment time was 7 hours/day on gestation day 6-15.
| Toxicological data (animals) | |
| LD50, mg/kg bw, oral, rat | 2737 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, oral, mouse | 4050 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | 6480 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | 8000 (IPCS, 1992) |
| LC50, mg/m³, inhalation, 8 hours, rat | 23500 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 4 hours, mouse | 32000 (ChemIDPlus, 2007) |
| NOAEC, mg/m³, inhalation, day 6-15 of gestation period, 7 hours/day, mouse | 2980 |
| Toxicological data (humans) | |
| NOAEC, mg/m³, inhalation, (time not stated) | 590 (IPCS, 1992) |
| NOAEC, mg/m³, inhalation, 4 hours | 794(IPCS, 1992) |
8.2.5 Health assessment of butanone
The semi-quantitative screening of all products for textile proofing showed no results stating the content of butanone.
The more sensitive SPME-GC/MS screening of all products registered the occurrence of butanone in product no. 8 and 21, but without measured concentrations.
In connection with the quantitative analyses, butanone was not found in amounts exceeding the detection limit in any of the products – and not in product no. 8 and 21.
8.2.6 Conclusion on butanone in textile proofing sprays
Butanone has been identified in product no. 8 and 21. However, in the quantitative analyses of spray products, butanone was not found in amounts exceeding the detection limit of 0.02 mg/g.
Therefore, butanone is not in itself hazardous to health for consumers in the investigated spray products for textile proofing.
8.3 1-Butanol
8.3.1 Application
1-Butanol is used as solvent in the dyestuff and the varnish industry when making natural and synthetic resins, vegetable oils, dyes and alkaloids. It is used as an intermediate when making medicine and chemicals and is used in industries that make artificial leather, textiles, rubber adhesives, photographic film and perfume (HSDB, 2007). 1-Butanol is included on the list of organic solvents of the Danish Working Environment Authority.
8.3.2 Identification
1-Butanol is a clear colourless liquid with a very characteristic (rancid and sweet) faint smell of alcohol. The substance is rather soluble is water, miscible with ethanol and ether and very easily soluble in acetone. The solubility in benzene exceeds 10 % (HSDB, 2007).
| Identification: | |
| Substance name: | 1-Butanol |
| Synonyms: | 1-Butanol; n-butanol |
| CAS no.: | 71-36-3 |
| EINECS No.: | 200-751-6 |
| Molecule formula | C4H10O |
| Molecule structure | |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value (ppm/mg/m³) (Danish Working Environment Authority, 2007) |
R10 XN;R22 XI;R37/38-41 R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 150 mg/m³ for all butanol-isomers (L, ceiling value; H, absorbed through the skin) |
8.3.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid |
| Molar weight | 74.1(HSDB, 2007) |
| Density | 0.8098 at 20°C (HSDB, 2007) |
| Melting point | -89°C(HSDB, 2007) |
| Boiling point | 117.7°C (HSDB, 2007) |
| Vapour pressure at 25 ?C | 7.0 mmHg (HSDB, 2007) |
| Octanol water (logPow) | 0.88 (HSDB, 2007) |
| Solubility in water | 63.2 g/L (HSDB, 2007); 74 g/L (IUCLID (ECB, 2007)) both at 25°C |
| Odour limit | In water 7.1 mg/L; in air 0.83 ppm (HSDB, 2007) |
8.3.4 Toxicological data
8.3.4.1 Absorption
1-Butanol is absorbed in the body via the lungs, the gastrointestinal tract and the skin. Absorbed substance is quickly distributed to the tissue where the substance is transformed considerably. The main part of absorbed substance is eliminated as CO2 via the lungs; but only a minor part is eliminated via the kidneys (HSDB, 2007).
8.3.4.2 Acute effects, humans
High concentrations in the air cause inhibition of CNS (tiredness, headache, muscular weakness, dizziness, stiffness, confusion, delirium, coma) (HSDB, 2007; IPCS, 1992). In addition, there might be gastrointestinal effects such as nausea, vomiting and diarrhea. Possible lethal toxification would be due to respiratory failure (HSDB, 2007).
1-Butanol is very irritating on the mucous membrane. Irritation of skin, eyes and neck has been observed during exposure to the liquid and vapours. In addition, coughing and difficulty in breathing have been observed.
8.3.4.3 Acute effects, animals
The oral LD50 values of 1-Butanol in rats vary between 700 mg and 2100 mg/kg bw.
The main effects from exposure to the vapour for a shorter time consist of different degrees of irritation of the mucous membrane and inhibition of CNS. Several sources state that it is believed to be approx. 6 times as toxic as ethanol (IPCS, 1987).
The substance seems distinctly irritating during testing with liquid in the eyes and moderately irritating on the skin (HSDB, 2007).
The skin sensitizing potential of 1-Butanol (IUCLID (ECB, 2007)) has not been tested.
8.3.4.4 Subchronic effects, animals
The effect of repeated inhalation comprises pathological changes in lung tissue and degenerative injuries in liver and kidneys (IPCS, 1987).
That was found in a number of inhalation studies carried out on rodents with different dosages (from 0.03 to approx. 40 ppm) and set ups varied from dosage in measured hours/day in a certain number of days to continuous exposure for 30 days, 4 months or 92 days (IUCLID).
The available animal studies are not suited for determining a no observed adverse effect level (NOAEL) to be used in risk assessments.
An inhalation study with exposure of pregnant female rats from day 1 to day 19 during gestation periods 7 hours a day with 3500, 6000 or 8000 ppm, revealed a NOAEC in the dam of 3500, but there was a minor increase in the number of rudimentary cervical vertebra in the offspring in the highest dosed group, and therefore NOAEC for development/teratogenecity was 6000 ppm (corresponding to 18000 mg/m³) (IUCLID from (ECB, 2007)).
No other reproduction toxicity studies are suited for determination of NOAEL.
8.3.4.5 Chronic effects
A wide range of short-term studies especially in vitro, showed no signs of mutagenic or genotoxic properties in 1-Butanol.
Environmental Health Criteria no. 65: Butanols - four isomers, 1987, as well as IUCLID from (ECB, 2007) refer to the fact that 2 long-term studies of very poor quality are supposed to exist, but it has not been possible to find further reference to these studies.
No studies exist with a route of administration that makes it possible to evaluate the chronic effects – not to mention the carcinogenic potential of 1-Butanol in humans.
IARC has not assessed 1-Butanol with regard to carcinogenicity in animals or humans.
8.3.4.6 Summary
1-Butanol is an ignitable colourless liquid that is used as organic solvent in many industrial connections. It has a low acute toxicity regardless of the exposure method. The substance is easily absorbed with the inhaled air, after intake or via the skin and it is distributed very quickly and evenly to all tissue.
High concentrations with the inhaled air induce signs of inhibition of CNS such as drowsiness, headache (in humans) and dizziness in animals as well as in humans.
Pathological changes in the lung tissue and degenerative changes in liver and kidneys appear in animals after repeated dosage via inhalation and anaesthesia is constantly developed.
Another predominating effect of 1-Butanol is skin and especially mucous membrane irritation, so irritation of eyes, nose and throat are effects that are registered at low exposures.
Sensitizing potential tests have not been carried out.
No trustworthy long-term studies have been found in any species of animal but the substance has proved to be non-mutagenic after substantial testing in vitro.
A minor occurrence of developmental disturbance was found at doses where toxic effect on the dam also was observed in a development/teratogenic test.
One of the very sensitive effects is eye irritation on exposure to vapour from 1-Butanol. In that connection, the effect level is 153.9 mg/m³ corresponding to 50 ppm in humans.
| Toxicological data (animals) | |
| LC50, ppm, inhalation, rat, 4 hours | 8000 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, oral, rat | 700 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rat | 800-2000 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rat | 2100 (IPCS, 1987) |
| LD50, mg/kg bw, oral, mouse | 2680 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rabbit | 3500 (IPCS, 1987) |
| LD50, mg/kg bw, dermal, rabbit | 4200 (IPCS, 1987) |
| LD50, mg/kg bw, dermal, rabbit | 5300 (IPCS, 1987) |
| NOAEC1,ppm, 7 hours/day, gd 1-19, female rat | 3500 (IPCS, 1987) |
| NOAEC²,ppm, 7 hours/day, gd 1-19, female rat | 8000 (IPCS, 1987) |
| Toxicological data (humans) | |
| NOAEC³, ppm inhalation – time not stated | 50 (IPCS, 1987) |
gd = gestation day
1General toxic effects
²Developmental toxic effects
³Eye irritation
8.3.5 Health assessment of 1-Butanol
No results were found for content of 1-Butanol during the semi-quantitative screening of all products for textile proofing.
The more sensitive SPME-GC/MS screening of all products registered the occurrence of 1-Butanol in product no. 18, 20, 25 and 26.
The quantitative analyses did not show 1-Butanol in amounts exceeding the detection limit in any product – and not in product no.18, 25 and 26 that were analysed quantitatively.
8.3.6 Conclusion on 1-Butanol in textile proofing spray
1-Butanol was not found in amounts exceeding the detection limit (0.2 mg/g) in the quantitative analyses in any spray.
1-Butanol in the investigated spray products for textile proofing is therefore in itself not a health hazard to consumers.
8.4 Cyclohexane
8.4.1 Application
The main application is as solvent for varnishes and resins, as paint and varnish remover, for extraction of "essential oils" in the analytic chemistry for determination of molar weight, for making adipic acid, benzene, cyclohexanon, cyclohexanol, cyclohexyl chloride, nitrocyclohexane, solid fuel, for industrial re-crystallization of steroids and in fungicides (HDSB, 2007).
8.4.2 Identification
Cyclohexane is a colourless, easily flowing liquid with a mild, sweet petroleum or chloroform-like odour. It is very flammable. Cyclohexane is practically insoluble in water but is soluble in ethanol, ether and acetone and is miscible with olive oil (HDSB, 2007). The odour limit is approx. 25 ppm in air. Cyclohexane is included on the list of organic solvents of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Cyclohexane |
| Synonyms: | Cyclohexane (IUPAC) from (8) Hexahydrobenzene, hexamethylene, |
| CAS no.: | 110-82-7 |
| EINECS No.: | 203-806-2 |
| Molecule formula | C6H6 |
| Molecule structure | 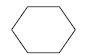 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value of the Danish Working Environment Authority (ppm, mg/m³) (Danish Working Environment Authority, 2007) |
F;R11 Xi;R38 Xn;R65 R67 N;R50/53 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 172 mg/m³ |
8.4.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Clear liquid |
| Molar weight | 84.16 (ECB, 2004) |
| Density | 0.778 g/cm³ at 20°C (HSDB, 2007) |
| Melting point | 6.47°C (HSDB, 2007) |
| Boiling point | 80.7°C (ECB, 2004) |
| Vapour pressure at 25 ?C | 96.9 mm Hg (HSDB, 2007) (103 hPa at 20°C (ECB, 2004) |
| Octanol water (logPow) | 3.44 |
| Solubility in water | 58 mg/L at 25°C (ECB, 2004) |
| Odour limit | Approx. 25 ppm (HSDB, 2007) |
8.4.4 Toxicological data
8.4.4.1 Absorption
Cyclohexane is almost completely absorbed via the gastrointestinal tract and after inhalation. Approx. 50% absorption via the skin has been measured of small doses in the form of vapour, but substantially lower absorption has to be expected from liquid cyclohexane placed directly on undamaged skin (ECB, 2004).
Cyclohexane is distributed in the body with highest concentrations in fatty tissue. Elimination mainly takes place via the lungs either unchanged or as CO2 (ECB, 2004).
8.4.4.2 Acute toxic effects, humans
In a recent study, human volunteers were exposed to 25 or 250 ppm cyclohexane for a 4-hour period. No neuro behaviour effects were found in connection with any of the doses. The 250 ppm (corresponding to 860 mg/m³) is therefore assessed to be a no observed adverse effect concentration (NOAEC) for neuro behaviour toxicity (ECB, 2004).
Skin irritation appears after repeated dermal exposure. That is because cyclohexane has degreasing properties.
Skin sensitizing properties are not expected (ECB, 2004).
8.4.4.3 Acute toxic effects, animals
Oral LD50 values of more than 5000 mg/kg, 29800 mg/kg and 8000-39000 mg/kg were found for cyclohexane in rats. The lowest lethal oral dose in rabbits is 6000 mg/kg; the study showed that toxicity involved the CNS (narcotic effect and cramps).
The dermal LD50 in rabbits is larger than 2000 mg/kg which is the highest dose that has been tested (ECB, 2004).
Exposure of rabbits to cyclohexane vapour for 1 hour gave CNS effects (cramps, shaking, quick respiration, cyanosis and diarrhea). All animals exposed to 26000 ppm (89600 mg/m³) died. LC50 for exposure of rats for 4 hours exceeded 9500 ppm (32800 mg/m³) as no death occurred (ECB, 2004).
NOAEC was 2000 ppm (6880 mg/m³) for neuro toxicity in rats after 6 hours of wholebody exposure (ECB, 2004). A NOAEC of 400 ppm (1400 mg/m³) was found for neuro toxic effects in a sub-acute rat study with 8 hours of exposure daily for 6 days (ECB, 2004).
8.4.4.4 Subchronic effects
After repeated dosage on inhalation, the systematic effects in both mice and rats in the course of the 28 and 90 day studies were limited to effects on the liver: increase in absolute and relative liver weight, increase in mitotic index figures and centrolobular hypertrophy. The study lead to a no observed adverse effect concentration (NOAEC) of 2000 ppm (6880 mg/m³) (ECB, 2004).
It is true that an older study showed a NOAEC of 425 ppm, but the study is very insufficient and therefore this value cannot be used for health assessments (ECB, 2004).
No studies of subchronic effects from oral exposure exist.
An old study exists for rabbits of subcronic effects from dermal exposure but it was not possible to derive a NOAEL value (ECB, 2004).
In a 2-generation rat study (inhalation) no effects were found on fertility and only small weight reductions were found in the newly born offspring at 7000 ppm and toxicity in the dam also appeared. In the study, there was NOAEC of 500 ppm (1720 mg/m³) for systematic toxicity (sedation) and of 2000 ppm (6880 mg/m³) for reproduction.
2 inhalation studies were carried out for toxicity on the development (teratogenecity studies) – one in rats and one in rabbits. Concentrations of up to 7000 ppm, 6 hours a day on gestation day 7-16 (in rats) or on gestation day 7-19 (in rabbits) were used. In rats, there was systematic toxicity in the form of reduced number of implantations and the dam showed reduced body weight and feed consumption at 2000 and 7000 ppm, but no effects were seen in the development of the foetuses. In rabbits, no toxicity was seen in the dam or in the foetuses. Therefore, there is a NOAEC of 500 ppm (1.720 mg/m³) for systematic effects in rats, but with regard to the development of the foetuses there is a NOAEC of 7000 ppm (24.080 mg/m³). In the rabbit study, both NOAEC values are 7000 ppm (24.080 mg/m³) (ECB, 2004).
8.4.4.5 Mutagenicity
Cyclohexane neither appeared genotoxic in short-term in vitro nor in vivo studies (ECB, 2004).
8.4.4.6 Chronic effects
In a doubtful study it appeared that cyclohexane might have a weak cancer promoter potential (ECB, 2004). However, no conventional 2-year carcinogenic study exists, but the EU believes it is unlikely that the substance should be carcinogenic.
IARC has not assessed cyclohexane with regard to carcinogenic potential.
8.4.4.7 Summary
Cyclohexane is absorbed easily via the gastrointestinal tract and on inhalation and it to some degree it is also absorbed via the skin.
There is low acute toxicity from all routes of exposure. The acute effects as well as the effects after repeated dosage are mainly effects from the CNS. In addition, liver effects appear as increased weight and growth of central cells in the liver in subchronic studies in rodents. Cyclohexane has no toxic effects on reproduction.
The critical study is an acute human study with 4-hour exposure to 250 ppm corresponding to 860 mg/m³ for neuro behaviour effect. No effects were seen with this concentration. The critical effect is general toxicity in the dam in the rat teratogenic test. Effects are seen at 500 ppm.
Cyclohexane is not mutagenic and even though no regular carcinogenic study exists it is assessed to be unlikely that cyclohexane should have carcinogenic potential. The substance has not been assessed by IARC.
| Toxicological data (animals) | |
| LC50, ppm, inhalation, rat, 4 hours | >9500 (ECB, 2004) |
| LD50, mg/kg bw, oral, rat | 29820 (HSDB, 2007) |
| LD50, mg/kg bw, oral, rat | 8000 (HSDB, 2007) |
| LD50, mg/kg bw, oral, rat | 12705 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, oral, mouse | 1300 (HSDB, 2007) |
| LD50, mg/kg bw, oral, mouse | 813 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, oral, rabbit | 6000 (ECB, 2004) |
| LD50, mg/kg bw, dermal, rabbit | 18000 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | >2000 (ECB, 2004 |
| NOAEC1,ppm, 6 hours/day, gd 7-16, rat | 500 |
| NOAEC²,ppm, 6 hours/day, gd 7-16, rat | 7000 |
| NOAEC³,ppm, 6 hours/day, gd 7-19, rabbit | 7000 |
| Toxicological data (humans) | |
| NOAEC, ppm, inhalation, 4 hours | 250 |
gd = gestation day
1General systematic toxic effects
²Developmental toxic effects
³Both general and developmental toxic effects
8.4.5 Health assessment of cyclohexane
8.4.5.1 Exposure and health assessment
Occurrence in investigated sprays:
| Cyclohexane measured in analysed products | Product no. | ||||
| 1 | 3 | 6 | 8 | 9 | |
| Identified in SPME-GC/MS screening | X | X | X | X | X |
| Quantitative g/kg (%) | 6.5 (0.65) |
0.29 0.029 |
Not analysed | 6.0 (0.60) |
Not analysed |
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The aerosol product with the highest concentration is product no. 1 that true enough has been discontinued but product no. 8 contains almost as much. The calculation was most logically carried out in product no. 8 that still is marketed.
An aerosol can filled with product no. 8 can hold 500 ml. If the density of the product is fixed at 1 g/cm³, then the spray container can liberate 3.0 g cyclohexane at the most, which distributed in the 20 m³ gives a maximum concentration of 150mg/m³.
Cyclohexane has a limit value determined by the Danish Working Environment Authority of 172 mg/ m³. The obtained concentration in the absolute worst case scenario amounts to approx. 87% of the limit value of the Danish Working Environment Authority.
The limit value is a ”time weighted average” that has been determined according to extensive toxicological estimates as the value to which a worker may be exposed 8 hours daily in an entire working life.
As the product is a consumer product where exposure only will take place now and then, the calculated value can instead be compared with the ceiling value of the Danish Working Environment Authority, at the double of the “ordinary” limit value.
Therefore, it must be assessed that the use of spray no. 8 is not injurious to health compared to exposure to cyclohexane. Even the absolute worst case scenario where 500 ml aerosol liquid is sprayed into a room of only 20 m³ will not lead to passing, acute sickness.
8.4.6 Conclusion on cyclohexane in aerosol products for textile proofing
The content of cyclohexane in the investigated spray products for textile proofing on the Danish market is in itself not a health hazard to the consumers.
8.5 Perfluoroctane-1-ol
8.5.1 Application
Perfluoroctane-1-ol forms part of several goods marked with "Fluortelomer Intermediate”, of which perfluoroctane-1-ol amounts to 27 - 34 %. The rest is formed by homologous substances of which approx. 1 % has fewer -CF2 – and the rest has more -CF2 links (always an equal number C atoms in the substances). These so-called fluortelomer alcohols are used in the production of products that require protective surface properties within the surface coating, pressure, textile and chemical industry.
8.5.2 Identification
telomer alcohols consist of an equal number of fluoridised carbon atoms connected to an ethanol part. Perfluoroctane-1-ol is a wax-like solid substance with a light to yellowish brown colour. The substance has a wax-like smell. It is almost insoluble in water, but soluble in acetone, butanone and isobutanol. The melting point is between 55 and 65°C.
| Identification: | |
| Substance name: | Perfluoroctane-1-ol |
| Synonyms: | 1,1,2,2-Tetrahydroperfluor-1-octanol; 1H,1H,2H,2H-perfluoroctanol; 1-Octanol, 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluor- (systematic name) (ChemlDPlus): 6:2 FTOH or fluortelomer alcohol 6-2 |
| CAS no.: | 647-42-1 |
| EINECS No.: | 211-477-1 |
| Molecule formula | C8H4F13O |
| Molecule structure | 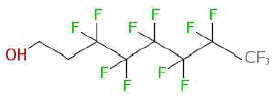 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Limit value of the Danish Working Environment Authority (ppm, mg/m³) (Danish Working Environment Authority) |
Not on the list Not on the list |
8.5.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Solid wax-like yellowish brown substance |
| Molar weight | Approx. 370 |
| Density | Approx. 1.7 g/cm² |
| Melting point | 55-65°C |
| Boiling point | 145 - 245°C |
| Vapour pressure at 25 ?C | - |
| Octanol water (logPow) | - |
| Solubility in water | Insignificant |
| Odour limit | No accessible information |
8.5.4 Toxicological data
As (hardly) any information was found for the substance perfluoroctane-1-ol itself, most data originates from investigations on the immediately higher homolog – the substance with 8 perfluoridised carbon atoms in addition to the 2 surrounded by hydrogen atoms. The terminology in English is often fluortelomer alcohol 6-2 (octanol compound), while the compound on which much data was found is called fluortelomer alcohol 8-2 (possibly 8:2) (decanol compound). These substances are generally written as 6:2 FTOH or 8:2 FTOH, respectively, in scientific literature.
It has been chosen to generalise in the light of the specific substance 8:2 FTOH and the term fluortelomer alcohols will also be used.
8.5.4.1 Absorption
Fluortelomer alcohols (8:2 FTOH) are absorbed quickly after oral intake, but the systematic concentration after 6 hours of skin exposure is insignificant. After oral intake, the plasma concentration is maximal when 1 hour has passed. The half-life period in the blood is 5 hours. The largest part of 8-2 FTOH is eliminated with faeces; the main part in unchanged form. Less than 4 % of an administered dose is eliminated with the urine. Of this, a small amount is oxidized to perfluoroctanoate (PFOA). Absorption is the same in male and female rats (Fasano et al, 2006).
8.5.4.2 Acute toxic effects, humans
No information has been found with regard to the acute effects of fluortelomer alcohols on humans.
An estrogenic effect of fluortelomer alcohols appeared on some human estrogenic receptor isoforms (in a test carried out on yeast cells) (Ishibashi et al, 2007) but neither perfluoroctanoate (PFOA) or perfluoroctane sulphonate (PFOS) had that effect. It is uncertain, what the specific biological importance of this is.
8.5.4.3 Acute toxic effects, animals
No information was found with regard to the acute effects of fluortelomer alcohols on animals (Herzke et al., 2007).
8.5.4.4 Subchronic effects
In a 90-day oral rat study, with 8-2 FTOH with daily doses of 1, 5, 25 and 125 mg/kg bw a no observed adverse effect level (NOAEL) was found on 5 mg/kg bw for male rates and 25 mg/kg bw for female rats. The effects at higher doses were liver necrosis and kidney injuries. There were signs of peroxisom proliferation in females at 25 mg/kg bw/day and in both sexes at 125 mg/kg bw/day (Fasano et al., 2006).
In a test concerning the toxic effects on development/teratogenecity it appeared that 8-2 FTOH does not effect the foetus development selectively (Fasano et al., 2006).
8.5.4.5 Mutagenicity
No information was found that could shed light on the mutagenic potential of fluortelomer alcohols.
8.5.4.6 Chronic effects
No studies of longer duration that could illustrate the chronic effects or carcinogenic potential of fluortelomer alcohols were found.
8.5.4.7 Summary
Nearly all accessible data on fluortelomer alcohols with 8 or with 10 carbon atoms was found as short background information in a larger investigation about absorption, distribution, metabolism and elimination (ADME study) of perfluordecan-1-ol. The background information originates from non-publicised studies.
Fluortelomer alcohols are absorbed in rats after oral administration but not after dermal exposure to the substance. ADME is the same in male and female rats.
No information was found about acute human effects.
After 90 days of oral administration effects were found in rodents on liver and kidneys. No observed adverse effect level (NOAEL) was found at 5 mg/kg bw in male rats and 25 mg/kg bw in female rats. That is in accordance with the demonstration that the substances give peroxisom proliferation in rodents.
| Toxicological data (animals) | |
| NOEL, mg/kg bw/day, oral, 90 days, female rat | 25 |
| NOEL, mg/kg bw/day, oral, 90 days, male rat | 5 |
8.5.5 Health assessment of perfluoroctane-1-ol
In the semi-quantitative analyses a substance that is expected to be perfluoroctane-1-ol (called 1H,1H,2H,2H–perfluoroctane-1-ol) (6:2 FTOH) was found in 3 products. On analysis of the procured standard it appeared that another substance was in question that is closely related to perfluoroctane-1-ol. In 2 additional products a sum of fluorine compounds was measured.
The quantitative analyses showed no 6:2 FTOH in the analysed products, but substances similar to this were measured in 3 products.
| Different fluorine compounds measured in analysed products | Product no. | ||||
| 6 | 8 | 14 | 21 | 25 | |
| Semi-quantitative screening results | |||||
| 6:2 FTOH g/kg | 0.17 | 0.29 | 0.03 | ||
| Sum of fluorine compounds (g/kg) | 0.03 | 0.17 | |||
| Quantitative analysis results | |||||
| Other fluorine compounds (g/kg) | Not analysed | 0.61 | 0.68 | 0.33 | Not found |
As the other quantitatively determined fluorine containing substances are very similar to 6:2 FTOH it was chosen to assess the content in these products as if fluortelomer alcohols were in question.
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The highest concentration is found in product no. 14 where 1 kg spray liquid contains 680 mg. Product no. 14 is sold in Denmark in spray cans with a content of 200 ml, but in other European countries it is sold in 400 ml spray cans.
If a spray can of 200 ml (200 g) is emptied completely into the 20 m³, an average concentration of fluortelomer alcohol of (680 x 0.2/20 mg/m³) = 6.8 mg/m3 per m³ air is obtained.
In Technical Guidance Document on Risk Assessment (TGD, part 1), European Chemicals Bureau (European Commission, 2003), the inhalation rate for adults has been determined to an average of 0.83 m³/hour. And if we anticipate that the person remains in the small room non-stop (and without ventilation) for 8 hours, then the inhaled amount is 6.8 x 0.83 x 8 mg = 45 mg.
We have no data of how much of the substance will be absorbed in the body from the inhalation air. Therefore, absorption must be set to 100 %.
In TGD, part 1, the standard average weight is 60 kg for females and 70 kg for males.
Exposure can be calculated to 0.75 mg/kg bw for a female and 0.64 mg/kg bw for a male.
In connection with an aerosol for household use it can be anticipated that spray treatment corresponding to worst case scenario only happens at long intervals between treatments and therefore it would be relevant to compare the actual exposure with a no effect level from an acute study. However, that is not possible as only few data exist for flurotelomer alcohols.
In the toxicological data there is no observable effect level (NOEL) for male rats of 5 mg/kg bw in a 90-day test.
If that value is compared with the calculated exposure for a female, then there is a margin of safety (MOS) of 5/0.75 = 6.7.
MOS becomes a bit higher for a male: 5/0.64 = 7.8.
For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
8.5.5.1 Discussion
The calculated margin of safety (MOS) that is less than 10 does not give sufficient safety in connection with use of spray product no. 14 in accordance with the scenario set up for spray proofing.
The analysis results of the fluorine compounds in product no. 8 are only approx. 10 % lower than for product no. 14. For this product, the margin of safety is also below 10.
It should also be considered that neither of the two products state a content of fluorine compounds on the label (product no. 8) or in the safety data sheet (product no. 14), respectively. The low content and the fact that these fluorine compounds are not included on the list of hazardous substances (the Danish Environmental Protection Agency, 2005) of the Danish Environmental Protection Agency result in no absolute demand for declaration, but the impression easily arises from the given declarations that they are exhaustive.
The consumer might get the impression that the proofing agent itself in both cases is low-boiling, hydrogenated naphta-fractions.
In connection with the screening investigations a high content of fluorine was found in more products than in which fluortelomer alcohols were analysed. Therefore, only a small part of this fluorine has been accounted for. A polymerisation might have taken place in connection with the analysis. However, it is possible that the consumer could be exposed to non-polymerised fluorine compounds in rather high concentrations. The problem is especially that we do not know the identity of the substances but if it is assumed that they can be compared to FTOH 6:2 then they might constitute a substantial problem that we cannot include in our conclusion because it only considers the substances found through analyses.
8.5.6 Conclusion on fluortelomer alcohol-like substances in proofing spray
Based on the very small amount of data material for the industrially very widespread fluortelomer alcohols a no observed effect level (NOEL) can be determined on male rats of 5 mg/kg bw/day from a 90-day study.
A quantitative analysis showed a content of similar substances of 0.61 g/kg (product no. 8), 0.68 g/kg (product no. 14) and 0.33 g/kg (product no. 21), respectively.
By calculating the margin of safety (MOS) of the two products with the highest concentrations, values arise that are less than 10. For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent (fluortelomer alcohols). In concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.6 Dodecamethylpentasiloxane
8.6.1 Application
Dodecamethylpentasiloxane is one of several linear polydimethylsiloxanes that when mixed often creates a group of artificial polymers that are among the most produced silicone substances. They are very widespread because of their physical-chemical properties and are used in many connections for production of cosmetics and foodstuffs, for surface treatment and many other things including the production of breast implants. In addition, they are often used in the textile industry and for the production of proofing liquids.
8.6.2 Identification
It has not been possible to find very many physical-chemical data for precisely dodecamethylpentasiloxane, but the substance is one of many linear polydimethylsiloxanes that are very similar to each other. Dodecamethylpentasiloxane is a viscous liquid with a low vapour pressure.
As other polydimethylsiloxanes, the substance is almost insoluble in water but is soluble in methylene chloride, ether, xylene and methyl ethyl ketone (butanone).
No data was found with regard to specific appearance or odour.
| Identification: | |
| Substance name: | Dodecamethylpentasiloxane |
| Synonyms: | |
| CAS no.: | 141-63-9 |
| EINECS No.: | 205-492-2 |
| Molecule formula | C12H36O4Si5 |
| Molecule structure | 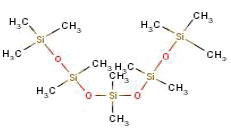 |
| Legislation: Classification according to the list of hazardous substances Limit value of the Danish Working Environment Authority (ppm, mg/m³) |
Not on the list Not on the list |
8.6.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Liquid |
| Molar weight | |
| Density | 0.940 g/cm³ at 25°C |
| Melting point | |
| Boiling point | 232°C (ChemlDPlus, 2007) |
| Vapour pressure at 25 ?C | |
| Octanol water (logPow) | 6 |
| Solubility in water | Almost insoluble |
| Odour limit | Not found |
8.6.4 Toxicological data
8.6.4.1 Absorption
The absorption, distribution and elimination of dodecamethylpentasiloxane after one single oral dose were measured in rats. It was calculated that approx. 25 % of an oral dose is absorbed from the gastrointestinal tract. In the course of the first day, approx. 65 % of the administered dose is eliminated; most of it through faeces. In the course of the next 24 hours, an additional 34 % is eliminated. Around 23 % is eliminated with the expiratory air and approx. 2 % with the urine (TOXNET, 1984).
8.6.4.2 Acute toxic effects, humans
The descriptions of effects in humans is to a high degree limited to the use of polydimethylsiloxanes in implants of different kinds or the use of the substances for direct injection in the vitreuos body of the eye in connection with treatment of glaucoma (HSDB, 2007). These are not relevant in this connection.
No reports were found on allergy in connection with polydimethylsiloxanes in cosmetic products (Fischer, 1986).
8.6.4.3 Acute toxic effects, animals
One single oral dose of 600 mg/kg bw has not provoked systematic effects in rats (TOXNET, 1984).
Polydimethylsiloxanes cause irritation in rabbit eyes but do not damage cornea (HSDB, 2007).
8.6.4.4 Subchronic effects
Injected doses of up to 20 mg/kg bw did not give developmental toxicity in rats (HSDB, 2007).
8.6.4.5 Mutagenicity
No genotoxic or mutagenic properties were found of linear polydimethylsiloxanes (HSDB, 2007).
8.6.4.6 Chronic effects
In a two-year investigation on rats with polydimethylsiloxane concentrations in the feed of up to 0.28 % there were no signs of unwanted effects (HSDB, 2007). There is a no observed adverse effect level (NOAEL) of 0.28 % in the feed, corresponding to 140 mg/kg bw/day, as a rat according to OECD eats 20 g feed a day and in average weighs 0.4 kg.
In another test, mice were dosed with polydimethylsiloxane in a concentration of 2.35 % in the feed for 80 weeks. That did not give rise to any significant increase in deaths or significant increase in the number of benign or malignant tumours (HSDB, 2007). As mice according to OECD eat 3 g feed a day and weigh 0.020 kg, 2.35 % in the feed corresponds to a NOAEL of 3525 mg/kg bw/day.
8.6.4.7 Summary
Polydimethylsiloxanes are often referred to as practically inert (biologically and chemically inactive) substances.
Despite the widespread use of linear polydimethylsiloxanes, including dodecamethylpentasiloxane in many industrial connections and consumer products, these substances seem to be very poorly investigated in experiments on animals.
In the light of 2 long-term feed tests, NOAEL values of 140 mg/kg bw in rats and 3525 mg/kg bw in mice, respectively, were found calculated on the basis of the highest tested concentrations in feed.
| Toxicological data (animals) | |
| NOEL, mg/kg bw, oral, rat, acute | >600 |
| NOAEL, mg/kg bw/day, oral, rat, 2 years | >140 |
| NOAEL, mg/kg bw/day, oral, mouse, 18 months | >3525 |
| Toxicological data (humans) | |
| No relevant data found |
8.6.5 Health assessment of dodecamethylpentasiloxane
The content of dodecamethylpentasiloxane could only be determined quantitatively for product no. 18. The product contains 0.66 g/kg.
The absolute worst case scenario is that 1 spray can is emptied in a 20 m³ room and that the person stays in the same room for 8 hours without airing.
During spraying of up to 1 kg of proofing liquid, corresponding to the content in the largest aerosol can that is allowed for non-industrial use, an average concentration of 33 mg/m³ is obtained.
If a human remains in the room for 8 hours 33 mg/m³ x 0.83 m³/hour x 8 hours = 219 mg (European Commission, 2003) is inhaled.
No data states to which high degree polydimethylsiloxanes are absorbed on inhalation, so here it is anticipated that 100 % is absorbed.
A male will therefore be exposed to 219/70 mg/kg bw = 3.13 mg/kg bw and a female correspondingly to 219/60 mg/kg bw = 3.65 mg/kg bw.
The margin of safety (MOS) is calculated in the light of it having been informed that no systematic effects were seen of the individual dose of 600 mg/kg bw in connection with the investigation of absorption from the gastrointestinal tract.
Therefore, MOS amounts to: 192 for males and 164 for females which are acceptable rates. For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
8.6.6 Conclusion on the appearance of dodecamethylpentasiloxane in proofing spray
A study with oral absorption, distribution, metabolism and elimination (ADME) of polydimethylsiloxanes in rats has been reported and therefore an acute no observed effect level of 600 mg/kg bw could be determined.
Compared with the worst case scenario, margins of safety (MOS) could be calculated of at least 192 for males and 164 for females for the only spray liquid in which dodecamethylpentasiloxane was measured. These safety margins are acceptable.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent (polydimethylsiloxanes). In concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.7 Recapitulation on health assessment and information collection
8.7.1 Chemical substances
In this chapter, health assessments were carried out on 6 substances found either through semi-quantitative screenings or through quantitative analyses of chemical substances in spray products intended for textile proofing. Assessments of the health related conditions were carried out in the light of the worst case scenarios that had been set up.
The assessments demonstrated that the content of organic solvent in these spray products in itself does not compose a health hazardous problem.
The content of polydimethylsiloxane found in one single spray product based on the calculations that were carried out cannot constitute a health hazardous risk.
Based on measurements of substance concentrations that look like a certain fluortelomer alcohol and compared with the small amount of toxicological data that is available for this and similar substances only a very low safety margin was found compared to the worst case scenario that was set up. On the basis of the analysis data, the products should not during use in the present form in themselves compose a health hazardous risk, but for chemical substances in consumer products a margin of safety (MOS) of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account. It is believed that several of the products do not fulfil that requirement.
In connection with these substances there are additional reasons to recommend cautiousness and to use a large safety margin. The literature study that was carried out by using available information about cases of poisoning caused by textile proofing agents demonstrated that the main part of all registered cases of poisoning precisely have occurred when using spray liquids containing organic perfluorinated polymers.
In addition, the same obersvation was reported by Lyngenbo et al. (2007). That investigation specifies the cases of poisoning that were reported to the Danish Poison Information Centre from 1991 to 2007 and that have involved sprays for surface treatment of many different materials. In 84 of the cases, the majority of the sprays reported for cases of poisoning contained a fluorine compound. However, it is concluded: the cause and mechanism of the lung diseases is not known and prevention of the problem is not straightforward.
Finally, the problem might be greater and more confusing than the analysis results in this project disclose. In connection with the screeing investigations a high content of fluorine was found in more products than in which substances similar to fluortelomer alcohol were analysed. Therefore, an account has only been given for a small part of that fluorine.
However, it is possible that the consumer can be exposed to non-polymerized fluorine compounds in rather high concentrations. The exact identities of the substances are not known but if it is assumed that they can be compared to FTOH 6:2, then they can form a substantial problem which it has not been possible to include in the health assessment that was carried out.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent that in concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.7.2 Products
Spray cans are only allowed to contain the propellants and solvents stated in the enclosure to Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans from the Danish Environmental Protection Agency. In addition, it appears from that enclosure that a number of allowed propellants or solvents must not be used in cosmetics or in products for indoor household use. That means that they must not appear in concentrations of more than 1 % unless the Danish Environmental Protection Agency has given their permission (§8 in the Regulation).
Most of the surveyed spray products are marketed principally for indoor use as none of the products are marked and it has not been stated in any other way that the product must only be used outdoors, e.g ”only for outdoor use”. On other products it is stated that they have to be used in the open or only in places with good ventilation. Directions for use often recommend ventilation at the place of treatment.
8.7.2.1 Butyl acetate in the investigated products
In the enclosure of the previously mentioned Regulation the amount of butyl acetate is stated comprising 1-butyl acetate (n-butyl acetate), 2-butyl acetate and tert-butyl acetate. The 2 last mentioned were not found in any product by semi-quantitative screening. Therefore, butyl acetates must not be used as solvents in spray cans for indoor household use unless the Danish Environmental Protection Agency has given dispensation.
In connection with product no. 3, 14 and 15 the content of butyl acetate has been declared on the safety data sheet. They contain 2, 8 and 3.9 %, respectively. On the safety data sheet of product no. 14 the content of n-butyl acetate has been declared to 1-5 %.
In connection with product no. 1 and 9 the content of butylacetate has not been declared, but they contain 9.8 and 2.3 %, respectively.
In connection with product no. 16 and 25 the content of butylacetate has not been declared. Analyses have shown 0.0058 and 0.0065 %, respectively. The content is very low and therefore it does not have to be declared.
Compared to the rules in Regulation no. 571 dated 29/11/1984 concerning the use of propellants and solvents in aerosol cans product no. 1, 3, 9, 14 and 15 exceed the allowed concentration of butyl acetate in aerosols intended for indoor household use.
8.7.2.2 Butanone in the investigated products
In the enclosure of the previously mentioned Regulation butanone is stated under the description methyl ethyl ketone.
Butanone was identified in product no. 8 and 21 by SPME-GC/MS analysis. However, in the quantitative analyses of spray products butanone was not found in amounts exceeding the detection limit of 0.02 mg/g.
8.7.2.3 1-Butanol in the investigated products
In the enclosure of the previously mentioned Regulation, the amount of butanol is stated comprising 1-Butanol (n-Butanol), 2-Butanol and tert-butanol. The 2 latter were not found in any product by semi-quantitative screening.
1-Butanol was identified in product no. 18, 20, 25 and 26 by SPME-GC/MS screening of all products. In connection with the quantitative analyses 1-Butanol was not found in amounts exceeding the detection limit in analysed products (no. 18, 25 and 26).
8.7.2.4 Cyclohexane in the investigated products
Cyclohexane is stated in the enclosure of the previously mentioned Regulation.
The three analysed products no. 1, 3 and 8 contain cyclohexane in concentrations of 0.65, 0.029 and 0.60 %, respectively. The content is very low and therefore it does not have to be declared.
8.7.2.5 Perfluoroctane-1-ol in the investigated products
Perfluoroctane-1-ol is not stated in the enclosure of the previously mentioned Regulation as it solely deals with propellants and solvents.
Perfluorctane-1-ol was not found in the products. However, screening identified fluortelomer alcohols that are closely related to perfluoroctane-1-ol in product no. 6, 8, 14, 21 and 25 and quantitatively determined in product no. 8, 14 and 21 at 0.61, 0.68 and 0.33 mg/kg, respectively. Based on a worst case scenario, MOS was calculated for product no. 14 to 7.8 for males and 6.7 for females which is less than 1/10 of the MOS of 100 that is required for consumer products. The same goes for product no. 8 and 21.
None of the analysed products declare the content of fluorine compounds as there is no requirement. The consumer could get the impression that the proofing agent itself is low boiling, hydrogenated naftafractions.
8.7.2.6 Dodecamethylpentasiloxane in the investigated products
Dodecamethylpentasiloxane has not been stated in the enclosure of the previously mentioned Regulation.
Dodecamethylpentasiloxane is only identified in product no. 18 and determined quantitavely to 0.66 g/kg. Based on a worst case scenario, MOS is calcualted to 192 for males and 164 for females which is acceptable as a MOS of at least 100 is required for chemical substances in consumer products.
8.7.3 Effects of propellants in spray cans
Cases of toxification when using marketed proofing sprays in Germany, the Netherlands and Switzerland have not led to serious health problems such as respiratory diseases or pulmonary edema if the aerosol mists cannot reach the alveolar tissue in the lungs. In order to reach those parts of the lungs (respirable) the drop sizes have to be less than approx. 4 µm. That drop size is easily obtained when the product is applied when using a propellant and a correspondingly small nozzle in the spray head - as demonstrated in this investigation. When the same liquids are used when using a pump mechanism the drops do not become smaller than approx. 100 µm and therefore they cannot reach the alveolars. A new investigation shows that the registered cases of toxification in Denmark apparently all have comprised products with a propellant (see enclosure 1).
This project has demonstrated that the consumer can be exposed to high local concentrations of aerosol mists with respirable aerosols. In connection with using textile proofing agents considerable concentrations of fine (<1 µm) and ultra fine aerosols (nanoaerosols) (<100 nm) can be created and they must be regarded as being 100 % respirable.
The toxicological effect from inhaling nanoaerosols is not yet known. Existing knowledge in the field cannot document that small aerosols in themselves are harmful. Aerosols can be carriers of (re)active chemical substances, e.g. fluorcarbon monomers, but the effect is not know either, as the chemical structure of the (re)active substances is not known and it has not been possible to determine it on the basis of the chemical analyses that were carried out.
Cases of toxification in Germany with claimed nanoaerosol containing spray liquids have been discussed by a number of German experts (BfR, 2006 a). They could not agree on a final toxicological assessment of the effect on the lungs. The experts pointed out that the classic toxicological assessments of the individual substances in a product are not sufficient when the product is sprayed by means of a propellant. Physical properties, e.g. aerosol size are determining factors for if and which toxicological effect could arise in the respiratory passages. Therefore, it was not possible to disregard the possibility that the observed toxic effects could have arisen solely as a result of the aerosol use, meaning not an effect from inhalation of nanoaerosols.
The experts agreed that the health effects of spray products with propellant only can be determined by means of a test streategy that copies the actual conditions of use indoors. Toxic effects are only seen when the product itself, meaning the complete mixture of substances in the consumer product, is inhaled as a fine aerosol with the corresponding small drop size. That goes for products with as well as without nanoaerosols.
As mentioned, the toxicological effect from inhaling nanoaerosols in not yet known. Several international research activities are taking place concerning the toxicity of nanoaerosols and in a couple of years they will hopefully shed more light on the problem.
8.7.4 Proposals for further investigations
In order to carry out a more complete health assessment and clarify the reasons for the cases of illness that have been observed in Denmark and abroad it is necessary to have:
- an improved experimental basis to describe the toxicity of fluorcarbon compounds.
- an understanding of whether or not the toxicity of substances in aerosol form, including fluorcarbon compounds increases additionally when the aerosol size in the aerosol mists declines to nanosizes (< 0.1 µm).
- develop completely new analysis methods that take the reactivity of the components to be analysed into account.
8.7.5 Good advice to consumers when using textile proofing spray
- As far as possible use textile proofing sprays outdoors. Avoid standing in the wind direction.
- If the product has to be used indoors it is important to provide good ventilation in the room during and after use.
- Only use small amounts indoors.
- Spray for a short period of time and avoid inhaling aerosol mists.
- Keep the spray can as far away from your face as possible.
- Read possible user instructions on the product and follow them carefully.
- Max. use the amount recommended on the product.
- Use pump spray rather than spray with propellants.
- Do not use spray products when children are around.
- Do not let children use spray products.
- If possible, use a dust filter mask and rubber gloves to reduce inhalation and skin contact.
9 Reference list
Arbejdstilsynet. AT-Vejledning C.O.1 "Grænseværdier for stoffer og materialer". http://www.at.dk/graphics/at/04-Regler/05
-At-vejledninger/C-vejledninger/
C-0-1-Graensevaerdilisten/C-0-1-Graensevaerdilisten-2007.pdf 2007.
(The Danish Working Environment Authority, AT Guideline C.O.1 “Limit values for substances and materials”)
BfR (2006b) - Nanoparticles were not the cause of health problems triggered by sealing sprays! Press release 12/2006. 1-2. 26-5-2006. Berlin, Germany, Federal Institute for Risk Assessment. Lukassowitz, I.
BfR (2006a) - Cause of intoxication with nanospray not yet fully elucidated, Press release 10/2006. 1-2. 12-4-2006. Berlin, Germany, Federal Institute for Risk Assessment. Lukassowitz, I.
Bonte F, Rudolphus A, Tan KY, Aerts JGJV. Ernstige respiratoire verschijnselen na het gebruik van impregneersprays. Ned Tijdschr Geneeskd. 2003;147(24):1185-8.
Burkardt KK, Britt A, Petrini G, O'Donnell S, Donovan W. Pulmonary toxicity following exposure to an aerosolized leather protector. Clinical Toxicology. 1996;34(1):21.
ChemIDPlus Lite database. National Library of Medicine. http://chem.sis.nlm.nih.gov/chemidplus 2007.
ECB. European Chemical Bureau. European Union Risk Assessment Report, Phenol, CAS No. 108-95-2, 203-632-7. http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/
RISK_ASSESSMENT/REPORT/phenolreport060.pdf. Publication Office; Publications.eu.int: European Commission Directorate General Joint Research Centre; 2007.
ECB. European Chemicals Information System. ECB, JRC, EEC, Ispra, Italy [updated 2007]. Available from: http://ecb.jrc.it/esis/.
ECB. European Chemical Bureau. European Union Risk Assessment Report:Cyclohexan, CAS no.110-82-7, EINECS No. 203-806-2. http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/cyclohexanereport031.pdf European Commission Directorate General Joint Research Centre; 2004. (41).
European Commission, 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
http://eur-lex.europa.eu/LexUriServ/site/da/oj/2007/
l_136/l_13620070529da00030280.pdf
European Commission. Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC (part 1). 1-311. 2003. European Commission, Joint Research Centre.
Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, et al. Absorption, distribution, metabolism, and elimination of 8-2 fluortelomer alcohol in the rat. Toxicol Sci. 2006;91(HSDB, 2007):341-55.
Fisher AA. Contact Dermatitis. 3 ed. Philadelphia, Pa. 1986.
Gregersen PA, Klixbüll U, Skanning PG, et al. Outbreak of respiratory distress after exposure to textile proofing spray with fluorpolymer - effective toxicovigilance through a poison center. [Abstract]. Clinical Toxicology. 2006;44:569.
Herzke D, Schlabach M, Mariussen E, Uggerud H, Heimstad E. A literature survey on selected chemical compounds - Literature survey of polyfluorinated organic compounds, phosphor containing flame retardants, 3-nitrobenzanthrone, organic tin compounds, platinum and silver. http://www.sft.no/publikasjoner/2238/ta2238.pdf Sft - Nilu; 2007.
HSDB. National Library of Medicine U. Hazardous Substance Data Bank . http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB Bethesda, Maryland; 2007.
Hubbs AF, Castranova V, Ma JY, Frazer DG, Siegel PD, Ducatman BS, et al. Acute lung injury induced by a commercial leather conditioner. Toxicol Appl Pharmacol. 1997;143(1):37-46.
IPCS. Methyl Ethyl Ketone (Environmental Health Criteria 143, 1992).IPCS Inchem; 1992. (Environmental Health Criteria; vol. 143).
IPCS. Butanols - four isomers (ehc, 1987).IPCS Inchem; 1987. (Environmental Health Criteria; vol. 65).
Ishibashi H, Ishida N, Matsuoka M, Tominaga N, Arizono K. Estrogenic effects of fluortelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro. Biol Pharm Bull. 2007;30(IPCS, 1992):1358-9.
Jacobsen P, Klixbüll U, Jensen K. [Lung damage after use of conditioner sprays for leather and textiles]. Ugeskrift for Læger. 1999;161(26):4030-1.
Jinn Y, Akizuki N, Ohkouchi M, Inase N, Ichioka M, Marumo F. Acute lung injury after inhalation of water-proofing spray while smoking a cigarette. Respiration. 1998;65(6):486-8.
Kobayashi K, Tachikawa S, Horiguchi T, et al. [A couple suffering acute respiratory illness due to waterproofing spray exposure]. [Abstract]. Nihon Kokyuki Gakkai Zasshi. 2006;44:(9)647-52.
Kulig K, Brent J, Phillips S, Messenger T, Hoffmann RE, Burkhart K, Tavris DR, and Miller GB. Severe acute respiratory illness linked to use of shoe sprays - Colorado, November 1993. Morbidity and Mortality Weekly Report (MMWR) 42(46), 885-887. 26-11-1993. Washington, DC, Centers for Disease Control and Prevention.
Laliberté M, Sanfacon G, Blais R. Acute pulmonary toxicity linked to use of a leather protector. Annals of Emergency Medicine. 1995;25(6).
Lazor-Blanchet C, Rusca S, Vernez D, Berry R, Albrecht E, Droz PO, et al. Acute pulmonary toxicity following occupational exposure to a floor stain protector in the building industry in Switzerland. Int Arch Occup Environ Health. 2004;77(4):244-8.
Miljøstyrelsen. Bekendtgørelse om anvendelse af driv- og opløsningsmidler i aerosolbeholdere. Miljøministeriets bekendtgørelse nr. 571 af 29. november 1984. Danmark.
(The Danish Environmental Protection Agency: Regulation on use of propellants and solvents in aerosol cans. Regulation no. 571 of 29 November 1984, the Danish Ministry of the Environment.)
Miljøstyrelsen, 2002. Bekendtgørelse nr. 329 af 16/05/2002 om klassificering, emballering, mærkning, salg og opbevaring af kemiske stoffer og produkter. https://www.retsinformation.dk/Forms/R0710.aspx?id=12387&exp=1
(The Danish Environmental Protection Agency, 2002. Regulation no. 329 of 16/05/2002 on classification, packaging, marking, sale and storage of chemical substances and products).
Miljøstyrelsen, 2007. bekendtgørelsen nr. 923 af 28. september 2005 om Listen over farlige stoffer. Online adgang til søgning i listen på: http://www.mst.dk/Kemikalier/Stoflister+og+databaser
/Listen+over+farlige+stoffer/Søgning+i+farlige+stoffer.htm
(The Danish Environmental Protection Agency, 2007. Regulation no. 923 of 28 September 2005 on the list of hazardous substances).
Smilkstein MJ, Burton BT, Keene W, Barnett M, Hedberg K, Flemin D, and Jacobson CM. Acute respiratory illness linked to use of aerosol leather conditioner -- Oregon, December 1992. Morbidity and Mortality Weekly Report (MMWR) 41(52), 965-967. 8-1-1993. Atlanta, GA, Centers for Disease Control and Prevention.
Tagawa A, Ikehara K, Tsuburai T, et al. [Acute lung injury caused by inhalation of waterproofing spray]. [Abstract]. Nihon Kokyuki Gakkai Zasshi. 2003;41:(2)123-6.
Tanino M, Kamishima K, Miyamoto H, et al. [Acute respiratory failure caused by inhalation of waterproofing spray fumes]. [Abstract]. Nihon Kokyuki Gakkai Zasshi. 1999;37:(12)983-6.
Tashiro K, Matsumoto Y, Nishizuka K, Shibata K, Yamamoto K, Yamashita M, et al. Mechanism of acute lung injury caused by inhalation of fabric protector and the effect of surfactant replacement. Intensive Care Med. 1998;24(1):55-60.
Testud F, Gabrielle L, Paquin ML, Descotes J. Alvéolite aiguë après utilisation d'un aérosol imperméabilisant: à propos de deux observations [Acute alveolitis after using a waterproofing aerosol: apropos of 2 cases]. Rev Med Interne. 1998;19(4):262-4.
Thibaut G, Wylomanski JL, Laroche D. [Pulmonary intoxication by accidental inhalation of a household aerosol water-repellent]. Toxicol Eur Res. 1983;5(2):81-4.
TOXNET. Pharmacokinetic profile of dodecamethylpentylsiloxane in rats following oral administration, with cover letter dated 4/20/94. EPA/OTS; Doc #86940001447. TOXNET EPA OTC; 1984. Available from: http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~rM7x5s:2.
Vernez D, Bruzzi R, Kupferschmidt H, De-Batz A, Droz P, Lazor R. Acute respiratory syndrome after inhalation of waterproofing sprays: A posteriori exposure-response assessment in 102 cases. Journal of Occupational and Environmental Hygiene. 2006;3:250-61.
Yamashita M, Tanaka J. Pulmonary collapse and pneumonia due to inhalation of a waterproofing aerosol in female CD-1 mice. Clinical Toxicology. 1995;33(6):631-7.
Yamashita M, Yamashita M, Tanaka J, Hirai H, Suzuki M, Kajigaya H. Toxicity of waterproofing spray is influenced by the mist particle size. Vet Hum Toxicol. 1997;39(6):332-4.
Enclosure 1: Report from the Danish Poison Information Centre
Lung injuries from proofing sprays
Ole Lyngenbo, John Bang, Peter Jacobsen
Bispebjerg Hospital, Clinic for Occupational and Environmental Medicine and Danish Poison Information Centre.
Summary
Sprays for proofing of textile, ceramics and other surfaces can involve respiratory disease, ranging from slight irritation to diffuse pulmonary involvement with infiltrates on x-ray and reduced oxygenation. General malaises, non-specific symptoms from the central nervous system and gastro-intestinal tract are other common features.
84 cases were identified retrospectively through the Danish Poison Centres databases from the period January 1. 1991 till May 31. 2007. Analyses were largely descriptive and included frequencies, time trends and association between product types and severity.
Respiratory effect was present in most patients (92%). The majority of these also had general symptoms including fever, general malaise, gastrointestinal upset and symptoms from the central nervous system. In a large proportion of the patients symptoms did not start until some time after cessation of exposure, typically min. up to one hour.
Reduced oxygen saturation was present in 19 out of 47 cases with available data. Pulmonary changes on x-ray were reported in 13 of 30 patients. The severity was estimated as moderate/severe for 58% of the cases, mild for 37% and as no poisoning for 4%. One case could not be classified. Severity was significantly associated with spraying of furniture (p=0,001). Follow up through hospital records was successful for 33 patients (39%), of these 20 were graded with moderate/severe and 13 with mild poisoning.
Conclusions: Aerosol sprays for surface coating have a potential for causing lung disease including severe morbidity. The cause and mechanism of this effect is not known and prevention of the problem is not straightforward. Future analytical and experimental studies should both consider the chemical composition and aerosol properties.
Introduction
Recommended use of ordinary consumer product does rarely cause serious harm. One exception is sprays for proofing of textile, ceramics and other surfaces, which for some decades regularly have been involved in outbreaks of acute pulmonary illness (1,2,3,4). Both small series and outbreaks with more than 100 victims associated to a single product have been reported (4,5,6). From Denmark information on 3 outbreaks with limited numbers of victims have been published (2,7,8).
Respiratory disease, ranging from slight irritation to diffuse pulmonary involvement with infiltrates on x-ray and reduced oxygenation has been the most common manifestation. General malaises, non-specific symptoms from the central nervous system and gastro-intestinal tract are other common features. Two cases with fatal course due to complicated respiratory illness have been reported (9,10).
Fluorcarbon polymers, silicone compounds, solvents and other components have been suggested to cause the pulmonary effect (7,11,12,13,14). However, none of these components have been present in all instances and usually the sprays do not induce harm. Thus, the cause and mechanisms of the diseases remains unknown and its also unknown why small changes in the composition of a product may change the associated risk (7,11).
The latest Danish outbreak involved 16 cases associated to use of a product based on Fluoracrylates and Cyclosiloxanes as active ingredients. The product had been sold for several years without apparent problems, and chemical analyses detected dodecyl acrylate (CAS: 2156-97-0) in high concentration. A component that could not be demonstrated in previous production series but on the other hand not has a strong potential for respiratory toxicity.
In order to obtain more information on the risks associated with proofing products the Danish EPA has initiated of studies on chemical composition and toxicology of the products and of disease associated with them. The present study represents the clinical epidemiology of pulmonary injuries associated with the use of proofing agents sold on the Danish market. It is based on data from the Danish Poisons Information Centre, which has poisoning surveillance as one of its aims.
Methods and data
Cases were identified retrospectively through the Danish Poison Centres databases from the period January 1. 1991 till May 31. 2007. After case identification the original records were retrieved and information was extracted from these. Additional information on clinical course and outcome of the poisoning was obtained through hospital discharge records when possible.
For the last five months in 2005 and the first five months in 2007 the retrospective case identification was substituted by active surveillance and expanded data collection through the poison centres ongoing activities. The background for this was an outbreak of lung injuries associated with aerosol sprays in 2005 and an effort to get better data for the present study.
Cases were defined as individuals presented to the poison centre with acute exposure to a product for surface proofing in an aerosol spray.
The databases were searched with phrases expected to identify this kind of products and substrings of the phrases in order to catch different spelling. Additional searches were performed using commercial names of identified brands and also using substrings of these names.
Information on product, exposure, demographic characteristics and clinical condition of the patient was extracted from the original record. Exposure was assessed using several parameters: Volume, number of containers used for proofing, object sprayed, time spraying, indoor/outdoor and ventilation.
However, this information had not been systematically collected, why an additional and simple exposure measure was constructed. In this exposure was classified as small when the treated object was small like shoes and when larger objects had been treated for short time (< 2 min. ) in good ventilation. All other exposures were classified as moderate/large or unknown.
The severity was classified as no poisoning when there was no indication of an effect, mild when symptoms were expected to disappear without treatment and moderate/severe when treatment was judged necessary. The basis for this classification was the original assessment and available clinical and Para clinical data. When follow up in hospital records with facts about the actual course was available, outcome was classified in the same groups.
Analyses were largely descriptive and included frequencies, time trends and association between product types and severity. As statistical test chi square test was applied with a 5% level for statistical significance.
Results
The search identified 126 potential cases. After exclusion of 42 cases with exposure to products not fulfilling the definition and cases that only had eye exposure, 84 cases remained for analyses.
Characteristics of the cases are shown in table 1. The majority were middle aged and young adults who had been exposed by their own spraying at home. Only one case had been exposed during professional work. Two puppets – the only non-human exposures - 4 children below 10 years and one adult had been exposed from other peoples work (passive exposure). All cases were accidentally exposed, i.e. not by sniffing or other intended exposures.
Table 1. Main attributes of 84 cases with accidental poisoning from proofing sprays. Number of cases with available data in ( )
| Characteristic | Statistics |
| Mean age ± SD (77) | 34,6 ± 14,0 years |
| Male sex (81) | 51% |
| Animal exposure (81) | 2,5% |
| Brand name known (64) | 76% |
| Ingredients known (42) | 50% |
| Indoor exposure (57) | 93% |
| Limited exposure (60) | 18,3% |
Information on the intended use of the products was available for 78 cases. Sprays for furniture proofing were by far most prevalent, table 2. Of these products 9 were meant for leather, 37 for textile surfaces and 7 were unclassifiable in this respect. Some information on composition was available for half of the products. Fluorinated carbon compounds were the most common active ingredients, but also silicone compounds and in some products both ingredients were used.
Table 2. Intended use of proofing sprays involved in accidental poisoning.
| Purpose | Number |
| Furniture proofing | 54 |
| Clothes | 9 |
| Shoes | 4 |
| Ceramic surfaces | 4 |
| Carpet | 2 |
| Tent | 2 |
| Riding equipment | 1 |
| Car seat | 1 |
| Sealing foundation for paint | 1 |
| Unknown | 6 |
Brand names were available for 64 products. Three brands for furniture proofing included 47 of these products, appendix 1.
The available information on quantitative exposure is presented in, table 3. Only for type of object and indoor/outdoor exposure was information available in more than 50% of cases.
Table 3. Information on quantitative exposure to proofing sprays.
| Variable | Information | Missing data |
| Volume | 75 – 2200 ml | 77% |
| Number of cans | 0,33 – 5,5 | 73% |
| Time spraying | 2-120 min | 71% |
| Indoor/outdoor | 53/4 | 32% |
| Ventilation present/absent | 15/17 | 62% |
| Object size | 93% | 7% |
Following the constructed measure exposure was small for 10 cases, typically for proofing of shoes and clothes and moderate for 50 cases. Data were insufficient for a realistic exposure assessment for 24 cases.
Clinical effects
The clinical information is summarized in table 4. Respiratory effect was present in most patients (92%). The majority of these also had general symptoms including fever, general malaise, gastrointestinal upset and symptoms from the central nervous system. In a large proportion of the patients symptoms did not start until some time after cessation of exposure, typically min. up to one hour.
Reduced oxygen saturation was present in 19 out of 47 cases with available data. Pulmonary changes on x-ray were reported in 13 of 30 patients, table 4.
Table 4. Clinical data on 84 cases accidentally exposed to proofing sprays.
| Parameter | Number of cases | Percent |
| Airway effects only (N=84) | 27 | 32% |
| Airways + general (N=84) | 50 | 60% |
| General effects only (N=84) | 3 | 4% |
| No symptoms (N=84) | 3 | 4% |
| Latency till effects (N=49) | 31 | 63% |
| Reduced oxygenation (N=47) | 19 | 40% |
| Pulmonary infiltrates (N=30) | 13 | 43,4% |
The severity was estimated as moderate/severe for 58% of the cases, mild for 37% and as no poisoning for 4%. One case could not be classified. Severity was significantly associated with spraying of furniture (p=0,001). Follow up through hospital records was successful for 33 patients (39%), of these 20 were graded with moderate/severe and 13 with mild poisoning.
Time trends
Figure 1 shows a non-regular distribution over the period with clustering in 2005 – 2007. A smaller cluster around 1995 is also indicated. Figure 2 shows that the clusters largely are explained by cases associated with 3 brands. Also the group of other and unknown brands seems to increase in 2006 and 2007.
Figure 1. Time trend for poisoning with proofing sprays: Jan 1991 - May 2007. (Note: Only 5 months in 2007)
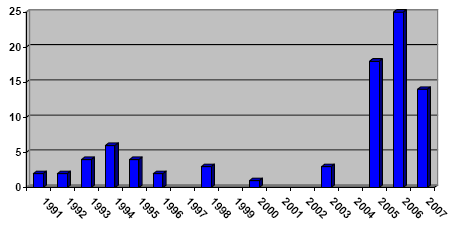
Figure 2. Time trend for poisoning with proofing sprays, distributed on brand and year: Jan. 1991- May 2007.
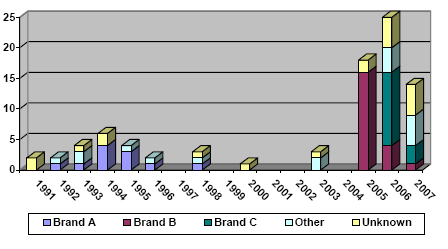
Discussion
The present study demonstrates that a wide range of spray products for surface proofing can cause lung injury and other health effects with ordinary use. Products for furniture dominate but this may have several interpretations: A greater exposure when treating such object or differences in chemical composition or physical properties of the products.
A toxicological interpretation is not possible with the lacking information on composition of the majority of products. A Fluorinated compound as active ingredient was present in most products for which information was available, but also products based on silicones alone was implicated.
Several different changes in chemical composition of spray products have increased the associated risk (7,11,12,13,14). This could be interpreted in favour of a significant role for the products physical (aerosol) properties; interaction between chemical and physical properties of the sprays might be responsible for the increased risk.
The epidemiological characteristic of outbreaks was confirmed in this study ranging more than 16 years. Three different brands for furniture proofing were responsible for 45 of sixty-four cases for which the brand name was known. One of the brands was associated with a small increase in incidence in the mid nineties. The two other brands were involved in an outbreak that started abruptly in 2005 and seems still to be going on.
The outbreaks are related to sprays for furniture. However there may be a general increase in pulmonary injuries from sprays since 14 of twenty-four cases associated with product for other use than furniture proofing occurred within 2006 and 2007. Four cases caused by products for ceramic surfaces occurred in 2007.
The total number of inquires per year to the Danish Poison Centre has increased through the period under study from approximately 1500 in the early 1990ies to a little more than 2500 in 2005. In 2006 the number doubled by the change of the centre from a doctors only to a centre open to the general public in mid August 2006.
However adjusting for increase in contacts will not smooth the outbreaks out, especially not if particular brands are considered. The relative severity of the cases and their close association to the use of a consumer product makes contact to the poison centre likely both from the public and from physicians. Thus we find it reasonable to believe that the variations in poison centre cases represents true variations in incidence.
Although the occurrence in outbreaks indicates a significant role for the product as such other factors might also influence the incidence. Fore instance increase in use of the products or change in the purposes for which they are used. We have no information about these parameters, but statistics on sale of the products and information about recommended or suggested use from producers and dealers might help.
In this study more than one fifth of all cases had reduced oxygen saturation and one in six had pulmonary infiltrates or other changes on x-ray. Although the majority of cases only had a moderate or less severe course this indicates potential for more severe diseases in concordance with reports of ARDS and even deaths from other countries (14,15,16).
If prevention measures are not succeeded in short time, a shift to alternative forms of administration (others than spraying) must be considered and discussed. The non-professional use of aerosol sprays for surface coating of furniture must - because of the risk for severe lung disease - be avoided.
Conclusions
- Aerosol sprays for surface coating have a potential for causing lung disease including severe morbidity.
- The cause and mechanism of this effect is not known and prevention of the problem is not straightforward.
- Future analytical and experimental studies should both consider the chemical composition and aerosol properties.
References
1. Müller-Esch G, Brunk E, Djonlagic H, Hoffman J, Wiesmann K-J. Inhalationsintoxikation durch Lederimprägnationsmittel. DMW 1982; 107: 692-5.
2. Jacobsen P, Klixbüll U Jensen K. Lungeskade efter anvendelse af spraymidler til imprægnering. Ugeskr Læger 1999; 161: 4030-1.
3. Wallace GMF, Brown PH. Horse rug lung: toxic pneumonitis due to fluorcarbon inhalation. Occup Environ Med 2005; 62: 414-16.
4. Vernez D, Bruzzi R, Kupferschmidt H, De-Batz A, Droz P, Lazor R. Acute respiratory syndrome after inhalation of waterproofing sprays: A posteriori exposure-response assessment in 102 cases. J Occup Environ Hyg. 2006; 3: 250-61.
5. Centres for Disease Control. Brief report: Respiratory illness associated with boot sealant products. Five states 2005-2006. MMWR 2006; 55: 488-90.
6. Ebbecke M, Schaper A, Kotseronis N, Desel H. Toxicovigilance of German poisons Centers. An epidemic of serious intoxications caused by new sealing sprays based on Nanotechnology. Clin Toxicol 2007; 45: 337-8 (abstract).
7. Gregersen P, Jensen MS, Klixbüll U, Jacobsen P. Respiratory illness after exposure to a textile-proofing agent – toxicovigilance through a poison centre (abstract 279, Eapcct congress, Prague 2006).
8. Christensen KJS, Olsen JE, Fogh A. Akut forgiftning forårsaget af indånding af spray-middel anvendt til imprægnering. Ugeskr Læger 1984; 146: 274-5.
9. Testud F. Toxicité des imperméabilisants pour le cuir et les tissues. Vigitox 2004; 24:1-2. http://www.centres-antipoison.net/lyon/index.html.
10. Tizzard Z Edwards J. Acute respiratory effects following use of
waterproofing sprays: Some UK experience. Thorax (online)
http://thorax.bmj.com/cgi/eletters/59/6/541-a.
11. Groot R de, Vries I de, Meulenbelt J. Sudden increase of acute respiratory illness after using a spray product to waterproff clothing and shoes. (Abstract 45, Eapcct congress Strasbourg 2004).
12. Woo OF, Healey KM. Chest pain and hypoxemia from inhalation of a trichloroethane aerosol product. J Toxicol Clin Toxicol 1983; 20: 333-41.
13. Yamashita M, Yamashita M, Tanaka J, Hirai H Suzuki M, Kajigaya H. Toxicity of waterproofing spray is influenced by the mist particle size. Vet Hum Toxicol 1997; 39: 332-4.
14. Laliberté M, Sanfacon G, Blais R. Acute pulmonary toxicity linked to use of a leather protector. Ann Emerg Med 1995; 25: 841-4.
Footnotes
[1] In literature, the terms aerosol and particle are often used without an unambiguous definition of the difference between the terms. In some cases, aerosol is used as term for liquid materials and particle as term for solid materials.
[2] I litteraturen anvendes begreberne aerosoler og partikler ofte uden en entydig definition af forskellen mellem begreberne. I nogle tilfælde anvendes aerosoler som betegnelse for væskeformige materialer og partikler som betegnelse for faste materialer.
[3] Quotation: Interestingly, during the winter 2002-2003, the Swiss Toxicological Information Centre had also recorded an unusual increase in respiratory troubles following household exposure to proofing sprays for conditioning of leather and textiles. After the occurrence of more than 150 such cases, three incriminated aerosols were removed from stores and distribution channels. Investigations by Public Health authorities showed that this outbreak of domestic cases also occurred after a formulation change of the proofing agent. The same new acrylate fluorpolymer produced by the same manufacturer was found as the common component in both our occupational cases and the domestic cases (4). It has not been possible to procure the reference: Office Fédéral de la Santé Publique from 2003.
[4] With and without propellant, respectively
[5] The KN code is an 8 digit product code number (KN ~ combined nomenclature)
Version 1.0 October 2008, © Danish Environmental Protection Agency