Survey of Chemical Substances in Consumer Products, No. 99, 2008
Survey and environmental/health assessment of fluorinated substances in impregnated consumer products and impregnating agents
Contents
1 Background, goal and target group
2 Producers of fluorinated compounds
3 Fluorinated substances identified
- 3.1 Contact to companies
- 3.2 Internet search
- 3.3 Information obtained from the Danish Product Register
4 Occurrence in consumer products
- 4.1 Occurrence in impregnating agents
- 4.2 Occurrence in impregnated products
- 4.3 Occurrence in other relevant products
5 Consumption of fluorinated substances in Denmark
6 Environmental assessment of polyfluoroalkylated substances
- 6.1 Environmental chemistry, pathways and levels
- 6.2 Environmental fate and levels
- 6.2.1 Environmental release
- 6.2.2 Levels in the ambient air and precipitation
- 6.2.3 Levels indoors
- 6.2.4 Occurrence in remote area and long-range transportation
- 6.2.5 Levels in environmental waters, groundwater and drinking water
- 6.2.6 Levels in wastewater and sludge
- 6.2.7 Levels in soil and sediments
- 6.2.8 Levels in biota and wildlife
- 6.3 Ecotoxicity
- 6.4 Environmental risk assessment
- 7.1 Consumer products
- 7.2 Air exposure
- 7.3 Non-stick cookware
- 7.4 Paper impregnation and migration into food
- 7.5 Food intake
- 7.6 Drinking water
- 7.7 Overall exposure scenarios
8 Levels of polyfluoroalkylated substances in human tissues
10 Animal bioassays and in vitro tests
- 10.1 Toxicokinetics and metabolism
- 10.2 Toxicology
- 10.2.1 Acute toxicity
- 10.2.2 Effects on the liver
- 10.2.3 Toxicological mechanism
- 10.2.4 Toxicogenomics
- 10.2.5 Effects on cell membranes
- 10.2.6 Effect on mitochondrial bioenergetics
- 10.2.7 Effect on intercellular communication
- 10.2.8 Developmental toxicity
- 10.2.9 Endocrine disruption
- 10.2.10 Mutagenicityt
- 10.2.11 Cancer
- 10.2.12 Cocktail effects
- 10.3 Structure-activity relations
12 General discussion, conclusions and recommendations
- 12.1 Discussion, conclusions and recommendations concerning the chemical family
- 12.2 Discussion, conclusions and recommendations concerning the use survey
- 12.3 Discussion, conclusions and recommendations concerning the environmental impacts
- 12.4 Discussion, conclusions and recommendations concerning the human health impacts
Preface
The growing environmental concern of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) derivatives and related substances is due to the fact that these potential harmful compounds now are global environmental pollutants distributed in air, water, soils and biota, including in polar bears living in remote arctic areas. In addition, in many countries PFOS, PFOA and other related substances have been observed in human blood samples of the general population.
The reasons for this widespread occurrence seem to be that perfluorinated substances are increasingly used and are environmentally persistent and bioaccumulative.
A range of polyfluorinated substances are used in numerous industrial products and consumer products because of their special chemical properties, for instance the ability to repel both water and oils.
The most studied and well-known polyfluorinated substances are the banned PFOS and PFOS related substances, which are not produced anymore, and which during the past years have been substituted with other fluorinated compounds – either perfluorinated compounds with shorter chain length or polyfluorinated compounds, such as fluorotelomer alcohols.
PFOA is used as a processing aid in the manufacture of polyfluoroethene polymers e.g. Teflon®. PFOA is a well-documented impurity in such polymers and in fluorotelomers. Furthermore, PFOA and other perfluorinated carboxylic acids (PFCAs) may be formed by degradation of some fluoropolymers and fluorotelomers.
This project was initiated by the Danish Environmental Protection Agency as a part of the work on identifying chemical substances in consumer products. This project report contains a survey of the use of polyfluorinated compounds in consumer products in Denmark, as well as an updated environmental and health assessment of this large group of substances. The report was drafted in the period April 2007 - November 2007, and it contains references to scientific papers being published until November 2007.
Frank Jensen from the Danish EPA supervised the project.
June 2008
List of used acronyms
AcOH Acetic acid
DPPC Dipalmitoylphoshatidyl choline
EtFHpSA N-Ethyl perfluoroheptane sulfonamide
EtFOSE N-Ethyl perfluorooctane sulfonamidoethanol
EtFOSEA N-Ethyl perfluorooctane sulfonamidoethyl acrylate
EtFOSEMA N-Ethyl perfluorooctane sulfonamidoethyl methacrylate
EtFOSA N-Ethyl perfluorooctane sulfonamide
EtFOSAA N-Ethyl perfluorooctane sulfonamidoacetate
8:2 FTMA 1H,1H,2H,2H-Perfluorodecyl methacrylate
8:2 FTA 1H,1H,2H,2H-Perfluorodecyl acrylate
FTCAs Fluorotelomer carboxylates
FTI Fluorotelomer iodide
FTUCAs Fluorotelomer unsaturated carboxylates
FTOHs Fluorotelomer alcohols
4:2 FTOH 1H,1H,2H,2H-Perfluorohexanol
6:2 FTOH 1H,1H,2H,2H-Perfluorooctanol
8:2 FTOH 1H,1H,2H,2H-Perfluorodecanol
10:2 FTOH 1H,1H,2H,2H-Perfluorododecanol
12:2 FTOH 1H,1H,2H,2H-Perfluorotetradecanol
14:2 FTOH 1H,1H,2H,2H-Perfluorohexadecanol
GJIC Gap junction intercellular communication
M570 N-Methyl perfluorooctane sulfonamidoacetate
MeFBSE N-Methyl perfluorobutane sulfonamidoethanol
MeFOSA N-Methyl perfluorooctane sulfonamide
MeFOSAA N-Methyl perfluorooctane sulfonamidoacetate
MeFOSE N-Methyl perfluorooctane sulfonamidoethanol
MeFOSEA N-Methyl perfluorooctane sulfonamidoethyl acrylate
NOAEL No observed adverse effect level
OECD Organization for Economic Co-operation and Development
PFAS Perfluoroalkylated substances or perfluoroalkyl sulfonate
PFBA Perfluorobutanoic acid/perfluorobutanoate
PFBS Perfluorobutane sulfonate/sulfonic acid
PFCs Polyfluorinated compounds/chemicals
PFCAs Perfluorocarboxylic acids/salts
PFDA Perfluorodecanoic acid
PFDoA Perfluorododecanoic acid
PFDS Perfluorodecane sulfonate
PFHpA Perfluoroheptanoic acid
PFHpS Perfluoroheptane sulfonate
PFHxA Perfluorohexanoic acid
PFHxS Perfluorohexane sulfonate
PFNA Perfluorononanoic acid
PFNS Perfluorononane sulfonate
PFOA Perfluorooctanoic acid/perfluorooctanoate
PFOS Perfluorooctane sulfonate/sulfonic acid
(P)FOSA Perfluorooctane sulfonamide
(P)FOSAA Perfluorooctane sulfonamidoacetate
(P)FOSE Perfluorooctane sulfonamidoethanol
PFOSF Perfluorooctane sulfonyl fluoride
PFPeA Perfluoropentanoic acid
PFPeS Perfluoropentane sulfonate
PFTeA Perfluorotetradecanoic acid
PFTrA Perfluorotridecanoic acid
PFUnA Perfluoroundecanoic acid
PNEC Predicted no effect concentration
POPs Persistent organic pollutants
WWTPs Waste water treatment plants
TDI Tolerable daily intake
USEPA U.S. Environmental Protection Agency
Summary and conclusions
Background
During the past years increased awareness has arisen on a new type of persistent organic pollutants, which contains an alkyl chain typically between 4 and 12 carbon atoms, where all or most of the hydrogen atoms have been replaced by fluorine. This makes the chain very stable and practically non-degradable in the environment. The substances also contain a more reactive functional group, which may be an alcohol, a carboxylic acid, a sulfonic acid, a phosphoric acid or their derivatives.
Several hundreds of these per- or polyfluorinated[1] compounds (PFCs) are known today. These substances are all surface active substances with an extreme low surface tension, and they repel water, grease and dirt, and are therefore used as detergents or impregnating agents in numerous industrial products and consumer products under trade names like Scotchgard®, Baygard®, Gore-Tex®, Zonyl® and Stainmaster®.
A couple of years ago the main focus was on PFOS (perfluorooctane sulfonate) and PFOA (perfluorooctanoic acid) and related compounds, as these compounds have been found to be widespread in all environmental compartments in both industrial and remote sites such as the Arctic areas. Today this use has, however, shifted towards either perfluorinated substances with a shorter chain length (C6 or shorter) or other classes of polyfluorinated substances such as fluorotelomer alcohols (FTOH).
The purpose of this project is to estimate the use of polyfluorinated compounds in impregnation and consumer products in Denmark, and to perform an update of the environmental and health assessment of polyfluorinated substances and their degradation products carried out previously for the Danish Environmental Protection Agency (Poulsen et al. 2005).
Estimation of consumption in Denmark
A search was carried out in the Danish Product Register to determine the registered use of fluorinated substances in consumer products in Denmark. The search was based on OECDs Preliminary 2006-list of about thousands PFCs. In total 92 fluorinated substances were identified, of which 48 substances were registered with a use of 0.00 tonnes meaning either a very low consumption or that a use amount had not been registered, as it should have been done. About 7.5 tonnes of the total 16.5 tonnes registered in the Danish Product Register count for substances that have a chain length lower than 8.
The most important use areas were: releasing agents, paint and lacquers, glue, surface active substances and galvano-technical products, which accounted for about 15 tonnes of the 16.5 tonnes in use in 2007. The consumption in the areas: polish and care products, impregnating agents, cleaning agents and surface active substances (non-metal, e.g. for paper and cardboard) accounted for about 0.5 tonnes (of the last 1.5 tonnes). These uses are, however, most likely much greater, because the Danish Product Register does not register all products containing fluorinated compounds on the Danish market but only chemical products for occupational use containing dangerous substances in a concentration of at least 0.1% or 1% (depending on the classification of the substances) . Therefore, the registered amounts will not give an adequate picture of the total sales in Denmark. In addition, contents of fluorinated compounds in imported finished consumer articles, such as all-weather clothes, are not registered at all. The products registered in the Danish Product Register may, however, afterwards be used for production of consumer articles.
Residues of PFOA in finished products, which are typically between 0.1 and 1% of the total content of fluorinated substances, are neither registered.
As a supplement to the search in the Danish Product Register, information was obtained on the content of fluorinated substances in various consumer products from Internet searches, the literature and from the official Danish statistics.
Several companies in Denmark as well as foreign producers/suppliers of fluorinated substances were also contacted in order to obtain information for developing an estimate of the consumption of fluorinated chemicals in consumer products in Denmark. This approach was, however, unsuccessful because most companies either could not provide any available information about these chemicals or would not participate with information to the project.
Based on all information the annual consumption of fluorinated substances in consumer products in Denmark was estimated to between 14 tonnes and more than 38 tonnes.
Environmental fate and levels
Concentrations of various PFCs have been extensively reported for all environmental compartments all over the world. Most studies have been performed on the North American continent, in Europe and in Japan. The number of compounds analyzed has been extended to a large series of perfluorinated carboxylates with carbon number from 7 to 16. Besides PFOS, the list of sulfonates has been extended to compounds with 7, 9 or 10 carbon atoms. The attention has also been focused on the precursor and intermediates of the more persistent PFOS and PFCAs (perfluorocarboxylic acids). The rest of the chemicals may, however, have a potential to degrade to PFOA or other PFCAs in the environment. The exact amount is not known, as this would require detailed knowledge of the fluorinated substances used, as only specific types of fluorinated substances can be degraded to PFOS, PFOA or other PFCAs in the environment.
Several studies performed with the volatile precursor compounds in smog chambers have found that FTOHs and perfluorosulfonamides react in the atmosphere with OH-radicals to yield the acidic compounds. The reaction time is long enough (about 20 days) for these compounds to be transported to remote regions, where deposition and further bioaccumulation in the food chain occurs. The “precursor” theory for explaining the presence of PFCs in remote regions (e.g. the Arctic) has been supported by atmospheric measurements of FTOHs and perfluoroalkylsulfonamides in both industrial and remote regions. Atmospheric concentrations were higher close to sources, but these compounds were also detected in the Tactic atmosphere. Oceanic transport of ionic PFCs has also been proposed as alternative or supplemental transport path to remote regions, with the compounds directly dissolved in water or present as a film on the surface foam. Another theory hypothesizes transport of PFOS and PFCAs directly from the sources bound on atmospheric particulate phase.
Indoor measurements of PFCs concentrations have been performed in both the gaseous and particulate phases and in house dust. Indoor concentrations were up to 100 times higher than outdoor concentrations, and carpets were identified as a major source of PFCs.
Lowering of analytical detection limits has made it possible to measure PFCs in environmental waters, included rainwater and oceanic waters at very low concentrations.
Several studies have been published dealing with concentrations and fate of PFCs in waste water treatment plants (WWTPs), as these systems have been identified as important sources for PFCs to the aquatic and terrestrial environments, as PFOS and PFCAs were not degradable in WWTPs. Both PFOS, PFCAs and their precursors have been found in waste water and sewage sludge from WWTPs.
High PFC concentrations in wildlife have been reported for a wide range of animals globally, including in the Arctic and the Antarctic. Especially PFOS have been more or less found in all samples with concentrations varying from sub-ng/g levels to several µg/g, with birds living close to a fluorochemical plant as the worst case. The bioaccumulation potential of some PFCs has been confirmed by several bioaccumulation studies in different food webs.
Temporal trends studies have been performed on archived biologic materials, in general covering time spans from the 1970s-1980s to the present. The PFCs concentrations, especially those of PFOS, have been found to gradually increase up to the present years. Only in a study from the Canadian Arctic it has been observed a decrease in PFOS concentration after 2000, which was explained as a rapid response after the stop of PFOS production in USA. Such a decline has not been found in Greenland.
Ecotoxicity
The toxicity of PFOS and PFOA has been studied in different aquatic organisms (algae, invertebrates and fish). Generally, PFOA and PFOS are not toxic at the normal environmental concentrations in water. However, effects have been observed for specific cellular functions, such as mechanisms involving the uptake of xenobiotics. Other biological endpoints affected by PFOA and PFOS are survival, growth, and emergence. Some intermediate degradation products of fluorotelomer acids have been found to be more toxic by a factor 10,000 than their end products (PFCAs).
Human exposure
The main exposures to perfluorinated substances seem to be by direct product exposure, through food intake or by inhalation/ingestion of indoor dusts, but exact information is lacking.
Human levels
Polyfluorinated chemicals (PFCs) have in contrary to most other persistent organic pollutants (POPs) a low affinity to lipids in adipose tissues but bind to proteins in cell membranes and accumulate in various body tissues of exposed organisms, including in liver, kidneys, testes and brain. The accumulation in fats and muscles is minimal.
In the blood perfluorinated chemicals are mainly bound to serum proteins, especially albumin. The mean half-lives in human blood were 5.4 years for PFOS, 8.5 years for PFHxS (perfluorohexane sulfonate), and 3.8 years for PFOA in retired fluorochemical workers but the whole body half-life may be even longer, since the elimination of these chemicals from the human body seems to be insignificant.
Blood levels of perfluorinated chemicals have been monitored in many countries but most data has been published from USA. In all countries, besides Korea, PFOS has been determined in far higher concentrations than the other PFCs. Typical average serum levels of PFOS in industrialized countries are 20-30 ng/mL with maximum levels less than 100 ng/mL. The second most abundant PFC is normally PFOA with typical average serum levels of 3-5 ng/mL. Some of the highest PFOS blood levels (2-3 times the typical levels) in the general population were determined in industrial areas of USA and China. Such levels may be 10 times higher than in rural and remote areas.
Recently, some preliminary data from Denmark was published. The average PFOS level in blood serum was 35 ng PFOS/mL with a maximum concentration of 107 ng/mL. That is somewhat higher concentration than in our neighbour countries.
In some studies blood plasma or whole blood were analysed instead of blood serum. Analysis of plasma will give the same results as serum but whole blood levels will be 2-3 times lower than serum levels, because PFCs are attached to the serum proteins. Levels of PFCs in cord blood are about the half of levels in maternal blood, thus some transfer to placenta occurs. Levels of PFCs in human semen were ten times lower than in blood serum, and levels in human milk are 100 times lower than in blood, so these fluids are not quite suitable for biological monitoring of PFCs.
Toxicokinetics
In animal experiments the studied PFCs are readily absorbed in the gastro-intestinal tract, and some compounds penetrate the intact skin. The peak blood levels are seen 1-2 hours after exposure, and the substances clear rapidly from the blood.
PFOA and PFOS are both considered being metabolically inert, and other perfluoroalkyl acids both with shorter and longer alkyl chain do have similar properties. Their precursors and functional derivatives will ultimately be transformed to the basic acids. For example, the fluorotelomer alcohol 8:2 FTOH is rapidly transformed to PFOA, PFNA (perfluorononanoic acid) and other metabolites in mice and rats. In the same way N-Ethyl perfluorooctane sulfonamidoethanol (EtFOSE) is metabolised to perfluorooctane sulfonamidoethanol (PFOSE), perfluorooctane sulfonamide (PFOSA) and finally to perfluorooctane sulfonate (PFOS).
Once absorbed in the body PFOA is eliminated unchanged as the free carboxylic acid and mainly with urine and to a less extent in faeces. Thus the renal elimination is critical for detoxification, and the elimination decreases with increasing chain length among the perfluorocarboxylic acids (PFCAs). The biological half-life of PFOA in male rats is 70 times longer than that in female rats for which it was only 2 hrs. The sex-related clearance of PFOA differs between animal species. In hamsters it is opposite rats. In mice and rabbits there are no sex difference; and mice had a slow excretion as male rats; and rabbits had a fast excretion as female rats. In dogs the plasma half-life of PFOA was about 20 days in males and the half in females. In monkeys the biological half-life was several months. As mentioned above the excretion of PFCs in humans is insignificant, thus animal bioassays may not be a good model for effects in human. The reasons for these large species differences are not known.
Toxicology
In general the knowledge about the toxicology of most perfluorinated compounds is rather sparse, and it will take some years and much effort, before we will have sufficient information for evaluation of the full impact of the present levels in humans. The experience from the work environment has not indicated any important direct adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctane sulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs.
Although the perfluoroalkyl sulfonic acids and -carboxylic acids are closely related structurally, these chemicals elicit different biological responses in vitro and in vivo. The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, and the toxicity of perfluorinated chemicals increases generally with the length of the alkyl chain. PFCAs with a branched alkyl chain seem to be less toxic than linear isomers.
The liver is the primary target organ for PFCs. PFOS and PFCAs cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid and steroid metabolism. Levels of serum cholesterol, thyroid hormones, and testosterone are reduced, and levels of estradiol are increased. PFDA (perfluorodecanoic acid) with a longer alkyl chain seems even accumulative and more active than PFOA. Toxic effects, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain, have been reported.
PFOS, PFOSA, PFHxS and perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner, and with PFDA inhibiting more than PFOA. Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases.
Although the fluorinated chemicals do not seem to be mutagenic, PFOA induces testis tumors, and PFOS and EtFOSE induce liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals.
In experimental animals PFOS causes developmental effects including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams.
Risk assessment
The U.K. Committee on Toxicity (2006) has recommended a provisionally Tolerable Daily Intake (TDI) for PFOA and PFOS of 3 mg/kg bw/d and 0.3 mg/kg bw/d, respectively, using an uncertainty factor of 100. They conclude that for some small children the TDI may already be exceeded.
This assessment was based on results from animal experiments, which may be very arbitrary and unreliable, because the renal clearances of PFOA and PFOS are almost insignificant in humans, contrary to a large active excretion in experimental animals. This means that these chemicals in humans leave the blood mainly by redistribution to internal organs and not by elimination from the body. This may increase the internal exposure time in critical organs considerable.
1 Background, goal and target group
1.1 Background
During the past years an awareness has arisen on a new type of persistent organic pollutants containing an alkyl chain, typically between 4 and 12 carbon atoms, with all or most hydrogen atoms replaced by fluorine. This makes the chain very stable and practically non-degradable in the environment. The substances also contain a more reactive functional group, which may be an alcohol, a carboxylic acid, a sulfonic acid, a phosphoric acid or their derivatives.
Several hundred of these polyfluoroalkyl or perfluoroalkyl[2] compounds are known today. These substances are all surface active substances with an extreme low surface tension, and they repel water, grease and dirt, and are therefore used as detergents or impregnating agents in a number of industrial- and consumer products under trade names like Scotchgard®, Baygard®, Gore-Tex®, Zonyl® and Stainmaster®.
1.1.1 Chemistry
Perfluorooctane sulfonic acid (PFOS) is the best known perfluorinated compound. PFOS has a linear perfluoroalkyl carbon chain of 8 and a sulfonic acid functional group.
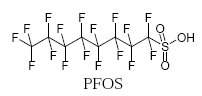
In the year 2000, the American company 3M that was the main producer of the substance, voluntarily ceased the production and sale of PFOS and related compounds, because PFOS surprisingly was found in high concentrations widespread in the environment, included in remote areas such as the Arctic.
PFOS-related compounds were introduced on the List of Undesirable Substances of the Danish EPA in 2004 and are nominated for the Stockholm Convention (2005) List of POP Substances. The European Parliament and the EU Council of Ministers have regulated these substances by the Directive 2006/122/EC of 12 December 2006 on “Restriction on the marketing and use of certain dangerous substances and preparations (perfluorooctane sulfonates)”. According to this directive, PFOS compounds may no longer be marketed or used as a substance or component in preparations in concentrations above 0.005 (w/w) or above, or may no longer be marketed in semi-processed products or articles in a concentration of 0.1% or above by June 27th 2008. However, some exceptions from the limitations exist, for example within the use of photographic coatings for film, paper or printing plates.
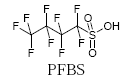
As a substitute for PFOS, 3M is now, for example, producing and trading perfluorobutane sulfonate (PFBS) and its derivatives. The chain length of PFBS is the half of PFOS, and chain lengths of less than 6 carbons are in these types of chemicals considered to be less toxic and less environmentally dangerous.
Another known compound is perfluorooctanoic acid (PFOA), which as the ammonium salt is used for manufacturing fluoropolymers (such as Teflon®).
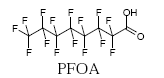
PFOA may occur as impurity in products of fluoropolymers, such as coated non-stick kitchen ware (Washburn et al. 2005). PFOA is also emitted from industries that are manufacturing fluoropolymers. Such type of manufacturing does not take place in Denmark.
PFOA and other perfluorocarboxylic acids (PFCA) with shorter or longer carbon chains can also occur as impurities or as a decomposition product of fluorotelomers, which are used for similar purposes as the formerly used PFOS derivatives (Washburn et al. 2005).
Fluorotelomers are polyfluoroalkyl compounds produced by telomerisation, a process in which a molecule, called telogen, reacts with two or more unsaturated molecules in the ethylene family, called taxogens. The principal reaction is:
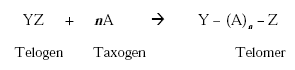
Telomers are thus polymers with a functional group in the end. When trifluoromethyl iodide, as an example, reacts with tetrafluoroethylene, then straight, short-chain telomers are obtained with the general formula: CF3[CF2CF2]nI where n is between 1 and 10. Such telomers have odd number carbon atoms. An even carbon number may be obtained by the reaction of pentafluoroethyl iodide with tetrafluoroethylene, e.g. the so-called C8-chemistry for surfactant production:
![]()
Fluorotelomers are polyfluorinated and precursors of perfluoroalkyl carboxylates. They have non-fluorine substituted hydrogen atoms in between the fluorocarbon chain and the functional group, which often is an alcohol, such as in 1H,1H,2H,2H-perfluorodecanol (8:2 FTOH):
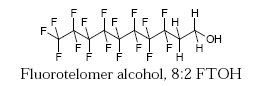
Fluorotelomers are considered to be less environmentally problematic than the PFOS compounds, but they are also persistent in the environment, since they can be degraded to PFOA or other related problematic compounds.
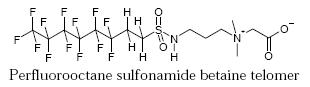
The functional group in a fluorotelomer can also be a substituted sulfonamide as in a perfluorooctane sulfonamide betaine telomer, which is used in fire fighting foams for fighting fires at oil rigs, oil terminals and airports.
1.1.2 Production and use
The world production of polyfluorinated compounds is estimated to be about 10,000 tonnes per year. The world production of fluorotelomers in the years 2000 to 2002 was 5,000 to 6,000 tonnes per year alone (Prevedourous et al., 2006). This production has probably increased considerably since then, which is confirmed by a Swedish and Norwegian study from 2006, which states that the annual global fluorotelomer production is around 10,000 tonnes. The authors also state that about 50% of this production goes to the impregnation of textile consumer products, e.g. in all-weather clothing, carpets and upholstery (Schulze and Norin, 2006)
In earlier surveys impregnation of textiles, carpets, leather, paper and cardboard were the dominating use area for perfluorinated chemicals and amounted to more than half of the total use. Fluorotelomers can be sprayed on the surface of a subject or be integrated permanently into a polymer surface. Impregnated paper and cardboard are used for packaging of food (e.g. popcorn bags for microwave oven) and foodstuff (e.g. paper bags for dry feed for dogs and cats) in order to prevent breakthrough of grease. The use areas for perfluorinated compounds are probably quite stable but the trend is to substitute PFOS related chemicals with fluorotelomers and fluorinated compounds with shorter perfluoroalkyl chains than C8 (Poulsen et al., 2005).
1.1.3 Global pollutants
Polyfluorinated compounds have become a global environmental problem. The substances occur everywhere in indoor air, household dust, outdoor air, earth, sediment, sludge, groundwater, rainwater, surface water, animals and humans, but the occurrence of the individual compounds vary from place to place. The compounds have been found even in remote polar areas, including Greenland (Giesy and Kannan 2002; Bossi et al., 2005ab; Jensen et al., 2006).
The polyfluorinated substances are being upconcentrated through the food chain. As opposed to the most common persistent organic pollutants (POPs) polyfluorinated compounds are not concentrated significantly in fatty tissue. In contrary they are associated to proteins in blood and internal organs, such as spleen, lever and kidney. Polar bears have very high concentrations of several fluoroalkylated compounds, especially PFOS, because of the placement of the polar bear in top of the arctic food chain. Studies from the Danish National Institute for Environmental Research show that the concentrations of fluoroalkylated compounds are growing in seals from Greenland – one of the primary preys of the polar bear. Fluoroalkylated compounds are also wide-spread in the Danish environment, which has been shown in a recent study carried out by DMU (Strand et al., 2007).
1.1.4 Known polyfluorinated substances
OECD has during the past years prepared a list of PFOA, PFOS and PFOS-related as well as polyfluorinated substances. This list has continuously been updated. Today the list contains nearly 1,000 different substances covering PFOS-substances, PFAS, PFOA and other PFCAs, fluorotelomers and other substances that may degrade to PFCAs (OECD, 2007).
The list used in this survey was the OECD 2006-list which was available at the start of the project (OECD, 2006). The OECD 2006-list divides the different fluorinated compounds in different categories as listed below. Some overlap exists between the different categories (e.g. PFOS compounds are also PFAS compounds etc.), why the list contains less than 1,000 unique CAS numbers. The OECD grouping is used in throughout this report. See Table 1.1:
Table 1.1: Categories of perfluorinated and polyfluorinated substances according to OECD 2006-list (OECD, 2006).
| Annex 1 | List of Perfluorooctane Sulfonate (PFOS) and Related Compounds. | |
| Annex 2 | List of Perfluoroalkyl Sulfonate (PFAS) and Related Compounds. | |
| Annex 3 | List of Perfluorooctanoic Acid (PFOA) and Related Compounds. | |
| Annex 4 | List of Perfluoro and Fluoro Chemicals that Potentially Degrade to perfluorocarboxylic Acid (PFCA). | |
| Annex 4 I: List of PFCA & Perfluoro Chemicals that Potentially Degrade to PFCA |
P1: Perfluoro alcohol compounds | |
| P2: Perfluoro amine compounds | ||
| P3: Perfluoro carboxylic compounds (some overlap with annex 3) | ||
| P4: Perfluoro ester compounds | ||
| P5: Perfluoro ether compounds | ||
| P6: Perfluoro iodide compounds | ||
| P7: Perfluoro phosphonic/phosphinic compounds | ||
| P8: Partial perfluoro & miscellaneous perfluoro compounds | ||
| Annex 4 II: List of Fluoro Chemicals that Potentially Degrade to PFCA |
F1: Fluoro alcohol compounds | |
| F2: Fluoro ammonium compounds | ||
| F3: Fluoro amine compounds | ||
| F4: Fluoro carboxylic compounds | ||
| F5: Fluoro ester compounds | ||
| F6: Fluoro ether compounds | ||
| F7: Fluoro iodide compounds | ||
| F8: Fluoro phosphate compounds | ||
| F9: Fluoro sulfate compounds | ||
| F10: Fluoroalkyl silicate compounds | ||
| F11: Fluoro sulfonate/sulfonamide/sulfonyl compounds | ||
| F12: Fluoro siloxane/silicone/silane compounds | ||
| F13: Fluoro thiols compounds | ||
| F14: Fluoro thioether compounds | ||
| F15: Fluoro thioester compounds | ||
| F16: Fluoro urethane compounds | ||
| F17: Partial fluoro & miscellaneous fluoro compounds | ||
1.1.5 Projects on perfluorinated substances carried out previously in Denmark
1.1.5.1 2001/2002: Use of PFOS and related compounds in Denmark
A survey of perfluorooctane sulfonate (PFOS) and related compounds in consumer products carried out by COWI for the Danish EPA in 2001 and 2002 revealed that PFOS-related compounds were used in numerous products in Denmark (Havelund 2001, 2002). The survey showed that 75 of the 175 identified PFOS and PFOS-related compounds on OECD’s list at that time were registered in the Danish Product Register. A total consumption of 8-16 tonnes PFOS-compounds annually was registered. A market survey was carried out in order to quantify the non-registered amount of PFOS-compounds in products. This survey concluded that the total Danish consumption of PFOS-compounds in products probably was between 5 and 50 tonnes per year.
The 2001 search in the Danish Product Register showed that the PFOS-related compounds were mostly present in the following types of products:
- Impregnation agents for textiles, leather and paper
- Wax and other polishes
- Paint, varnish and reprographic agents
- Cleaning products (general cleaning products and cleaning products used for cleaning of metal surfaces or carpets).
1.1.5.2 2002: Use of PFOS and related compounds in impregnating agents, wax and floor polish.
As a follow-up, the Danish EPA initiated another survey of the use of PFOS-related compounds in impregnating agents, wax and floor polish products (Vejrup et al. 2002). In that project PFOS was detected in 3 out of 21 purchased consumer products. Two impregnating agents contained 212 mg perfluorodecane sulfonate/ml and 3.5 mg perfluorooctane sulfonamide/ml, respectively. One of the wax and polish products contained 9 mg N-ethyl-perfluorooctane sulfonamide/ml.
1.1.5.3 2004: Survey of alternatives to PFOS and PFOS-related compounds
In 2004 the Danish EPA initiated a survey with the aim to collect the know-how about the existing technical alternatives to PFOS/PFOS-related compounds and PFOA/PFOA-related compounds. This project also carried out an environmental and health assessment of PFOS/PFOA-related substances and of the possible alternatives to PFOS/PFOA-related compounds (Poulsen et al., 2005).
1.1.5.4 2005: Chemicals used in shoe care products – PFOS discovered
In 2005 the Danish EPA carried out a survey of the use of various chemicals in shoe care products (Engelund and Sørensen, 2005). In 1 out of 4 analysed samples very small amounts (1.1 and 0.36 mg/kg, respectively) of PFOS-related compounds were found. The PFOS-compounds were believed to be present in the product as impurities in a fluoropolymer that was added due to its water and dirt repelling properties.
1.2 Purpose
The former projects carried out for the Danish EPA have mainly covered PFOS and PFOS-related compounds in consumer products; this project is concerned by both perfluorinated and polyfluorinated compounds and includes e.g. fluorotelomers.
The purpose of this project was to
- Estimate the use of fluorotelomers and short-chained perfluorinated compounds in impregnating agents for textiles, carpets, leather, paper and cardboard, cleaning agents for glass, wax, floor polish, paint and other consumer related products in Denmark.
- Update the former environmental and health assessment of the perfluorinated substances and their decomposition products carried out in the previous project for the Danish EPA (Poulsen et al. 2005).
- Assess the need for further investigation and measurements of fluorotelomers in and from consumer products.
Estimation of use of fluorinated compounds within the area of foods is not included in this project, as this product area is not the responsibility of the Danish EPA.
1.3 Methods applied for the survey
Information about the use of fluorinated substances in products in Denmark has been obtained in different ways. A search has been performed in the Danish Product Register of the use of fluorinated compounds in Denmark. Additional information was obtained by contacting stores, suppliers, importers and producers of fluorinated substances and products containing fluorinated substances. However, this approach only gave sparse information, since the companies contacted either did not know about the chemical content of their products or did not want to give away any information about the use of fluorinated substances. Information found on the Internet about the content of fluorinated substances in different products was combined with official statistics of sales of different products in Denmark in order to estimate the use of fluorinated substances in Denmark in different product categories.
2 Producers of fluorinated compounds
Major producers of polyfluorinated compounds are
- DuPont
- 3M
- Clariant
- Bayer
- Ciba Speciality Chemicals
- Daikin
- Arkema
- AGC Chemicals / Asahi Glass
- Solvay Solexis
The three producers DuPont, 3M and Clariant were contacted by phone and e-mail, but only sparse information was received.
Clariant reported that they were producing a range of products and more than 100 products contained either fluorotelomers or fluoropolymers (PFTE). Fluorotelomers are only used in their Nuva series for textile applications, and this company does not have any sale of these products to the Danish market.
When contacting DuPont, they referred to their trade association – Plastics Europe. A meeting was held on October 10th, 2007 with James Franklin and Mike Neal from Plastics Europe and Eric Van Wely from DuPont. They could not provide any specific information about the use areas and consumptions of fluorinated products for the Danish market. The downstream users are not telling them much about the use of their products. However, they noted that USEPA has made an agreement with eight global producers that PFOA emissions and PFOA residue content in fluoropolymers and telomers should be reduced by 95% by 2010 and be eliminated in 2015 (2010/15 PFOA Stewardship Program)[3]. Furthermore, they explained that they are not satisfied with the OECD List of PFAS, PFOS, PFOA and substances that can be degraded to PFCAs, as they believe that too many irrelevant substances are on the list, and that other substances are missing. They had asked for a meeting with OECD about this issue.
A search has been made on the Internet, in order to find out more about the fluorinated chemical products produced by the major world producers of these fluorinated compounds. Trade names and information available for different fluorinated products are listed in Table 2 below. Only some examples are listed, as e. g. DuPont has several more Zonyl and Foraperle Products.[4] Products for food packaging like DuPont’s Zonyl paper products, Clariant’s Cartapack and Solvay Solexis’ Solvera products are intentionally not included in the table as fluorinated compounds, since the food area is not included in this report.
Table 2.1: Examples of fluorinated chemical products from different producers of fluorinated compounds (Information found on the Internet – company webpages).
| Producer | Trade names | Use areas | Concentration in fluorinated product | Concentration in consumer product |
| DuPont | Teflon Advanced[5] | Carpet protector | 3-5 % of fluorinated polyurethane (CAS no. trade secret) | Diluted 4:1 with water, each diluted gallon will treat 200 sq. feet. |
| Zonyl 5180 | Carpet protector | 1-10% of fluorinated polyurethane | Diluted 15:1 with water, each diluted gallon will treat 200 sq. ft. | |
| Zonyl 7950 | Carpet and upholstery shampoo | 15-30% in total of three different fluoroalkyl ethyl phosphates | 0.3-1% in ready to use carpet shampoo. | |
| Zonyl 9361[6] | Paints and coatings | 34% fluorosurfactant | Suggested use rate 0.03-0.2%. | |
| Zonyl FSA | Paints and coatings, adhesives, waxes and polishes | 25% fluorosurfactant | - | |
| Zonyl FSN-100 | Paints and coatings, adhesives, waxes and polishes, polymers, metals and electronics | 100% fluorosurfactant | - | |
| Zonyl FS-610 | Paints and coatings Waxes and polishes Metals and electronics Specialty cleaners |
22% fluorosurfactant | 0.05-0.20% 0.1-0.2% - - |
|
| Zonyl TA-N | Coatings for textiles, leather and non-wowens, cosmetics, fire-fighting formulations | Mixture of perfluorodecyl acrylate (CAS 27905-45-9) and other fluorinated products | - | |
| Zonyl 321 | Building applications (concrete, limestone, bricks, natural stones, terra cotta etc.) | 25% active solids | 5-10% solution. One to two layers of Zonyl 321 is applied of 100-200 g/m² of Zonyl 321 solution. | |
| Zonyl FSO | Graphic arts, paints and coatings, waxes and polishes, polymers, adhesives, metals and electronics | 50% fluorosurfactants | Typically effective at 0.005-0.2% concentration | |
| Foraperle 326[7] | Leather (garments, gloves, accessories, upholstery) | 24-26% solids | - | |
| Foraperle 3116 | Leather (garments, footwear, gloves, accessories, upholstery) | 15-18% solids | - | |
| LX Platform Products | Carpet care; stone, tile and concrete; nonwovens; leather tanning; paper packaging; paints and coatings. | No information, other than the LX Platform products are based on the existing Teflon, Zonyl and Foraperle chemistries, but with a 97% reduction of trace levels of PFOA. | ||
| 3M | Scotchgard | Carpets, apparel and leather, automotive, paint, outdoor | Based on PFBA (perfluorobutanoic acid). No information about levels. | - |
| Novec Fluorosurfactants FC 4432 | Paints and coatings, resins, adhesives, primers | 87% polymeric fluorochemical actives, and 0-0.5% of 2-(2-Propenoic acid)-2-[methyl [(nonafluorobutyl) sulfonyl]-amino] ethyl ester (CAS 67584-55-8 on OECD 2006 list). | 0.05 to 0.3% | |
| Clariant | Licowet F3 liquid | Waxes for cleaners | Fluorinated tensides. No information about content. | - |
| Ceridust 3920 F | Cosmetic waxes (for creams, sticks, powders, and nail enamel) | Polyethylene wax with PTFE. No information about content. | - | |
| Nuva 4200 liq | Impregnation of textiles | Fluoropolymer | - | |
| Nuva N2114 liq | Impregnation of textiles (sportswear, automotive, work wear, medical articles, nonwovens, upholstery, table cloths, umbrellas, sunshades) | Fluorocarbons based on C6 chemistry. | - | |
| Bayer | Baygard | Carpets | Fluorocarbons and organosilicate | No information. |
| Ciba | Oleophobol C | Textiles | No information | Fluorine content is 0.38 % w/w in treated textiles (Hoppenheidt et al., 2007) |
| Oleophobol S | Textiles | No information | Fluorine content is 0.38 % w/w in treated textiles (Hoppenheidt et al., 2007) | |
| Oleophobol 7752 | Textiles | No information | No information | |
| Oleophobol CO | Textiles | No information | No information | |
| Oleophobol SLA | Textiles | No information | No information | |
| Oleophobol 7713 | Textiles (fabric in cars) | No information | No information | |
| Oleophobol SM | Textiles | No information | No information | |
| Oleophobol SL | Awnings, textiles | No information | No information | |
| Oleophobol ZSR[8] | Textiles | Emulsion of a polymer, perfluorinated compound | 30-60 g Oleophobol per litre | |
| Daikin[9] | Unidyne | Textiles, leather, tents, bags, shoes, umbrellas, ski wear, windbreakers, golf wear, caps, medical wear, carpets, furniture, table cloths, shower curtains, wrapping paper, paper cups, car sheet | No information | No information |
| Daifree | Mould releasing agent | No information | No information | |
| Optool DSX | Antifouling coating | 20% solution | No information | |
| Solvay Solexis[10] | Fluorolink® A10 | Natural stone, metal and leather | 10% Fluorolink | 0.5%-1% solution |
3 Fluorinated substances identified
- 3.1 Contact to companies
- 3.2 Internet search
- 3.3 Information obtained from the Danish Product Register
This chapter contains lists of the specific fluorinated substances identified (by CAS number) in this survey. The contact to companies gave only very little information about uses of specific fluorinated substances. The main sources to fluorinated substances identified are the Internet and the search in the Danish Product Register.
The list of fluorinated substances found by the search in the Danish Product Register and the list of substances found by contact to companies (only one substance) are the only substances that for sure are used in chemicals or in products in Denmark. The list of substances found by searching the Internet are of course used globally, and may be used in chemical products and consumer products in Denmark as well, but it may not necessarily be the case.
3.1 Contact to companies
The substances in Table 3.1 have been identified when contacting different companies in Denmark about the use of fluorinated substances. Strangely enough more information about substances not used in Denmark than substances used in Denmark, was received when interviewing companies. However, when one company informs that they know of a use of fluorinated substances in products not sold in Denmark, it does not exclude that other companies may use the same substances in products in Denmark.
Table 3.1: Fluorinated substances identified by searching the Internet or contacting Danish companies
| CAS No. | Substance name | OECD class | Chain length | Found in |
| 65530-65-6 | Poly(difluoromethylene), a-fluoro-w-[2-[(1-oxooctadecyl)oxy]ethyl]- (TSCA, NDSL) | Fluoro ester compounds (F5) | n | One sealer product for cars (auto polish) in a concentration between 0.085-0.45% |
| 203743-03-7 | Poly(hexadecyl methacrylate/2-hydroxyethyl methacrylate/octadecyl methacrylate)/gamma-omega-perfluoro-C10-C16-alkyl acrylate. | Not on the OECD list | 10-16 | Textile impregnation spray for car interiors in a concentration of about 0.01 mg/kg (uncertain concentration). |
| 9002-84-0 | PFTE-wax | Not on the OECD list | n | Used in printing inks to protect the surface and make the surface smoother |
| 65530-85-0 | Polytetrafluoroethane (Teflon wax) | Not on OECD’s 2006 or 2007 list, but on a former OECD list and identified in the Product Register. | n | Used in paint to form a water repellent film, but not used in products sold in Denmark. Typical content is about 11-18%. |
| 355-49-7 | Perfluorohexadecane (C16F34) | Not on the OECD list | 16 | Used in ski wax (produced in Norway). Questionable if used in Denmark. |
3.2 Internet search
The web-pages of the producers of fluorinated substances have been examined closely in order to learn more about the specific fluorinated substances used. It was only possible to find specific information for DuPont fluorinated products.
When searching the DuPont website it is possible to find MSDS’s of their Zonyl, Foraperle and Forafac products, where some of the fluorinated compounds are listed. However, it is only possible to find the information about the specific substances, if the search is performed on German MSDS’s.
In total, 93 Zonyl, 14 Foraperle and 10 Forafac product MSDS’s exist in German language (September 2007). Table 3.2 below contains a listof the fluorinated compounds used in chemical products from DuPont.
Table 3.2: List of fluorinated substances identified in DuPont fluorinated chemical products. The information is found on the DuPont webpage[11]. Both the chemical name mentioned in the MSDS and used by OECD are listed.
3.3 Information obtained from the Danish Product Register
A search in the Danish Product Register has been performed during the summer of 2007 in order to map the use of fluorinated compounds in products in Denmark. The search represents figures updated in 2007 – the figures thereby represent 2006 volumes.
Only substances and chemical products used occupationally and containing substances classified as dangerous in a concentration of at least 0.1% or 1% (depending on the classification of the substance) have to be registered in the Danish Product Register. As none of the fluorinated compounds are classified as dangerous themselves, the registration will only occur, if they are constituents of products, which are labelled and classified as dangerous of other reasons. In other words, the Danish Product Register does not register all products containing fluorinated compounds on the Danish market, and the registered amounts do not give a complete picture of the total sales in Denmark. The amounts registered are for occupational use, but the chemical products may later on be used for production of consumer articles. Finally, imported finished consumer articles, such as carpets containing fluorinated compounds are not registered in the Product Register. However, the search can be used as a starting point for the following market survey.
In order to be able to compare with the former 2001-search in the Danish Product Register on PFOS and PFOS-related compounds, a search was first made by using the same about 200 substances already used in the search in 2001. This search was supplemented by a search using the OECD preliminary list of PFOS, PFAS, PFOA and related compounds and chemicals that may degrade to PFCA (OECD, 2006) with about 1,000 substances (CAS numbers from the first search was removed).
Most substances on the Danish 2001-list are also on the OECD list, but a few substances was found only on the list used in the 2001 survey. By performing a search using both lists it is possible to compare the new with the former search in the Danish Product Register.
Since the search in the Danish Product Register was carried out for this project, the OECD has updated their list, and now named it: “Lists of PFOS, PFAS, PFOA, PFCA, related compounds and chemicals that may degrade to PFCA” (OECD, 2007). The comparison of these two OECD lists has not been included in this project.
3.3.1 Search in the Danish Product Register
The search in the Danish Product Register resulted in a total of 92 different fluorinated substances. Only 72 of the substances were identified searching the same approximately 200 CAS numbers from the 2001 survey. The other 20 fluorinated substances were identified by using the large OECD list of substances, where the overlaps between the first and the second list were removed in advance.
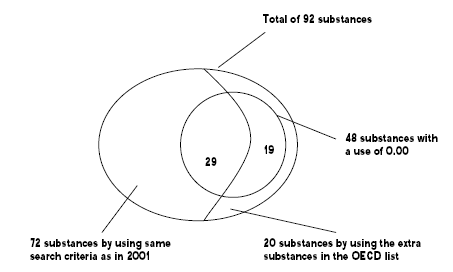
For 48 of the 72 substances an annual use 0.00 tons was registered. That means that the companies have reported a use of the substances, but have failed to report any amount. The result of the search is illustrated in Figure 3.1.
Figure 3.1: Result of the search in the Danish Product Register
3.3.2 Fluorinated substances identified in the Danish Product Register
The fluorinated substances identified by the search in the Danish Product Register are listed in Table 3.3 below. The substances with the highest use are listed first in the table. For confidentiality reasons no exact amounts are given, but the amounts are instead listed as uses over 5 tonnes in total, below 1 tonnes, below 0.1 tonnes and as 0.00/0.000 i.e. indicating that no amount have been reported to the Danish Product Register. In total, the 44 fluorinated substances registered with an amount, account for an import of 16.5 tonnes in 2006.
These data from the Danish Product Register includes all reported uses of the fluorinated substances, including industrial uses, which may not be consumer relevant. However, it is not possible with the received data to perform an exact calculation of how large a part of the amount that is assumed to be used in consumer products only. Since the products are classified as dangerous it is likely that more are for industrial uses. However, the products for industrial uses may end up as components in consumer products.
Table 3.3: Fluorinated substances identified by the search in the Danish Product Register
3.3.3 The identified substances and their uses
The most important use areas (according to the reported totals) are releasing agents, paint and lacquers, glue, surface active substances and galvano-technical products, which accounts for about 15 of the 16.5 tonnes in total.
The use areas polish and care products, impregnating agents, cleaning agents and surface active substances (non-metal, e.g. for paper and cardboard) accounts for about 0.5 tonnes (of the last 1.5 tonnes). The amounts used in these areas are most likely much higher, as only chemical products labelled as dangerous have to be registered in the Danish Product Register.
In other words, the Danish Product Register does not register all products containing fluorinated compounds on the Danish market, and the registered amounts do not give an adequate picture of the total sales in Denmark. Finally, imported finished products such as raincoats containing fluorinated compounds are not registered in the Product Register.
All identified substances in the Danish Product Register are associated to a specific use area. Figure 3.2 below illustrates the total uses and total amounts of all registered fluorinated compounds within the different use areas. As the total imported amounts only have been registered for about half of the substances, the distribution between the different areas is therefore not necessarily correct, as many numbers are missing. However, for every import registration, which is notified to the Product Register, a use area must be associated. The use areas therefore represent the different uses for the registered fluorinated compounds.
It is difficult to say which uses are only consumer related and which are not. However, it is assumed that hydraulic fluids and lubricants are the only uses, which are not consumer related, and these uses constitute a very small part of the total registered use. It can be discussed whether releasing agents will be a part of consumer products. Releasing agents are compounds used e.g. in moulds in order to get the moulded plastic product for example to release easily from the mould. However, releasing agents can also be used on e.g. frying pans to ensure a non-stick surface.
Figure 3.2: Use of fluorinated substances within different use areas
3.3.4 The identified substances and their OECD grouping
We have appointed all the fluorinated substances a grouping corresponding to the grouping used by OECD as presented in the beginning of this chapter. A few substances have a grouping of “none”, which means that these substances are not on the OECD list, but are on the Danish list from 2001. For these substances the grouping used in this project has been added. The grouping of the substances can be found in the table above, but is also presented in the Figure 3.3 below.
Figure 3.3: The fluorinated substances found in the Danish Product Register grouped in the different OECD categories.
Figure 3.3 shows that 10 of 92 (11%) substances are PFOS related, 3 substances (3%) are PFOS/PFAS[12] related, 29 substances (32%) are PFAS substances, 1 substance (1%) is PFOA, and a large part (42 substances or 46%) are substances that potentially can degrade to perfluorocarboxylic acids, PFCA. The rest of the substances (8 or 9%) are substances that are not listed on the OECD preliminary list of PFOS, PFAS, PFOA and related compounds and chemicals that may degrade to PFCA.
The total use in 2006 of these 44 identified fluorinated substances was 16.5 tonnes. The total amount of fluorinated substances could be much higher, if there had been consumption data of the other 48 substances without registered amounts. For the 16.5 tonnes, which have been reported, the amounts are distributed between the different OECD categories as illustrated in the Figure 3.4 below.
Figure 3.4: Use of fluorinated substances distributed between different OECD fluor categories.
The figure shows that about 2 tonnes of the registered fluorinated substances still are PFOS substances. The category “No OECD category” accounts for almost half of the total registered amount of 16.5 tonnes.
3.3.4.1 Chain length of the identified substances
We have looked at the chain length of the identified substances as the chain length is important with respect to the health and environmental effects of the substances.
The variation of the chain length for the 92 different identified fluorinated substances are illustrated in the figure below. Some of the CAS numbers (21) do not specify a specific chain length, but a range in stead – for example a chain length of 4-8 or 6-18. For these substances the middle equal number has been used as a chain length. Some substances are polymers. For these substances the chain length of the monomer has been used for the calculations. Furthermore, some CAS numbers (13) are listed with a chain length of “n” indicating that the chain length could be almost any large number. These substances have been omitted from the calculations. The table can therefore only be used as an indication of the chain length of the substances registered.
Figure 3.5: Illustration of the distribution of chain length for the different fluorinated substances.
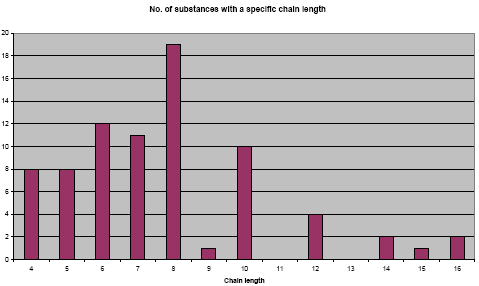
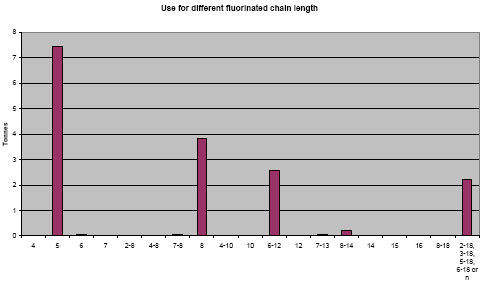
As illustrated in Figure 3.5 above most of the identified fluorinated substances have a chain length between 4 and 10, and the average is just below 8.
When dividing the use of the identified fluorinated substances on their chain length, it can be seen that the main part of the identified fluorinated substances has a chain length of eight or lower (see Figure 3.6).
Figure 3.6: Illustration of the distribution of the use of fluorinated substances for different chain lengths.
3.3.5 Comparison with the former search in 2001
It is not possible directly to compare the 2001-search in the Danish Product Register (Havelund, 2002) directly with the search performed in this project. The 2001-search was only carried out for 175 identified PFOS and PFOS-related compounds on the OECD list at that time. The search in this project has been much broader (about 1,000 PFAS, PFOS, PFOA and substances that can be degraded to PFCAs).
In comparison, 72 fluorinated substances were found in this 2007 search in the Danish Product register, when looking at the same search group from 2001. 20 additional fluorinated compounds have been identified by using the OECD list from 2006 in this 2007-search.
In comparison the amounts of fluorinated compounds used in Denmark seem to have decreased from 2001 to 2007. An amount of 16 tonnes were the result of the both the 2001 search and the 2007 search. However, if the use registered for the 20 “new” substances is subtracted, the registered amount in the 2007-search is 9 tonnes for the same search group of substances as in the 2001-search. This is in line with the decreased use of PFOS precursors since 2001. However, it is important to notice that the amounts in the Product Register most probably only account for a smaller part of the total amount used in Denmark. It is not known whether the use of fluorinated compounds in products not classified as dangerous, and thereby not registered in the Danish Product Register, has increased.
When comparing the substances found in the 2001 search with this search in 2007 in the Product Register, it can be seen that 48 chemicals of the 75 (2001) and 72 (2007), respectively, are exactly the same substances. In other words 27 fluorinated substances identified in the 2001 search are no longer used. Groups of fluorinated compounds (OECD grouping of 2001), where most of the substances are no longer in use are:
- Perfluoroalkylsulfonyl derivates
- Perfluoroalkylsulfonamide aminopropyl derivates
- Perfluoroalkylsulfonamide chrom complex derivates
When comparing the substances found in the 2001 search with this search, it is striking that many (14) of the repeaters – substances registered both in 2001 and in 2007 are used within galvano-technical products.
It is not possible to say whether the use decrease from 2001 until today is the evidence of a total decrease of the use of fluorinated substances or just a shift from PFOS and PFOS-related substances to fluorotelomers. It may, however, be a logical explanation as production of these chemicals has ceased and use is forbidden by summer 2008.
4 Occurrence in consumer products
- 4.1 Occurrence in impregnating agents
- 4.2 Occurrence in impregnated products
- 4.3 Occurrence in other relevant products
The information from the Danish Product Register has been supplemented with information from different companies such as producers, importers, suppliers and stores.
Producers of fluorinated substances have been identified by searching the Internet, and the results were presented in Chapter2. Stores and companies marketing and selling consumer products with a content of fluorinated substances have been identified initially by identifying the different products that contain fluorinated substances, and then secondly identifying the sectors in which the products are sold or produced. A search for companies belonging to these sectors have been carried out by using the Internet-based program Dun & Bradstreet that contains all companies listed in Denmark under different sector codes.
The consumer products and sectors as listed in Table 4.1 below have been identified to be relevant.
When searching for companies in the identified sectors, the result was of course many thousand different companies, and it has not been possible within the budget of this project to contact them all, but a few companies within the most relevant sectors have been contacted.
Suppliers and importers of fluorinated substances or products containing fluorinated substances have mostly been identified indirectly, i.e. different shops or companies selling or producing products containing fluorinated substances were contacted and gave information about their suppliers.
It was decided to use the direct approach, i.e. telephone calls when contacting the different sources, as it was not expected to pay off in the short available time, just by sending questionnaires to the different companies.
In all 59 different companies have been contacted.
However, as this approach only gave sparse information (either they did not know the chemical content of their products or they did not want to give away any information about the use of fluorinated substances), information found on the Internet about the content of fluorinated substances in different products was combined with statistics of sales of different products in Denmark in order to estimate the use of fluorinated substances in Denmark in different product categories.
Statistics from Statistics Denmark have been used in order to collect information about the amounts used of relevant consumer products. Both import/export statistics and production statistics have been used for relevant consumer products in order to calculate the supply of products in Denmark via the equation:
Supply = Import – export + Danish production
Table 4.1: List of consumer products and matching sectors with probable uses of fluorinated compounds
| Product group | Products | Relevant companies/shops | Relevant sectors |
| Impregnating agents for surfaces (monomer and telomer compounds) | |||
| Impregnating agents for coats of textiles and leather | Clothes, sports and scout shops | 524845 Sports shops including hunting/camping equipment, 5242101 Women’s/girls’ clothes shops 52422 Men’s and boys’ clothes shops |
|
| Impregnating agents for footwear | Shoe shops. | 52431 Shoe shops, suppliers | |
| Impregnating agents for carpets and mats | Carpet dealers | 524801 Carpet shops | |
| Impregnating agents for furniture of textiles and leather | Furniture shops | 524410 Furniture shops | |
| Wax for cars and skis etc. | Shops for accessories for cars and skis etc. | 5030 Spare parts/accessories etc. retail/wholesale 524845 Sports shops |
|
| Floor polish agents | Do-it-yourself market, paint shop | 52462 Do-it-yourself markets 52463 Paint and wallpaper shop |
|
| Cleansing agents for glass, hotplates etc. | Supermarkets. White goods shops. | 52113 Supermarkets 51431 White goods, retail |
|
| Paint and lacquers | Do-it-yourself market, paint shop | 52462 Do-it-yourself markets 52463 Paint and wallpaper shop |
|
| Impregnating products with added or integrated polymers and telomers | |||
| Packaging | Greaseproof paper and cardboard for fast food, chips, popcorn bags for microwave oven, paper cups, dry food etc.* | Supermarkets, pet shops | 52113 Supermarkets |
| Clothing | All-weather clothing, raincoats, sports clothes, caps, umbrellas | Clothes shops, sports shops. scout shops, supermarkets |
1822 Coats, dresses, trousers etc. production 524845 Sports shops including hunting/camping equipment 1824901 Sports clothing, production |
| Leather jackets, -coats and -gloves | Leather shops | 52432 Leather goods shops | |
| Footwear | Shoes and boots | Shoe shops | 19301 Shoe factories 51422 Shoes wholesale |
| Furnishings | Carpets and mats | Carpets dealers | 1751 Carpet factories |
| Furniture of textile and leather Nano treatment? |
Furniture dealer | 524410 Furniture shops 3614109 Furniture factories |
|
| Shower curtains and table clothes | Hosiery shops | 17402 Curtains, linen, textiles, production 1754 Textiles other, production 17409 Textile goods, other finished goods, production |
|
| Non-stick kitchen equipment* | Hardware | 5246102 ironmonger's shops | |
| Luggage | Bags and suitcases of leather and textile | Leather shops | 1920 Bags, suitcases etc., production |
| Camping, leisure and sports equipment | Tents and sails Sunshades |
Sports and leisure shops | 524845 Sports shops incl. Hunting/camping equipment 17401 Flag and tent factories, producers of sails |
* Not investigated in this project
In the following text the information regarding products containing fluorinated substances are listed. The following headlines are used:
- Occurrence in impregnating agents (for textiles and leather).
- Occurrence in impregnated products (such as clothes, footwear, furniture, carpets and sunshades).
- Occurrence in other relevant products (such as paints, inks, wax, polish, and cleaning agents).
In this Chapter 4 information from the different areas are listed and calculations are performed with use of statistics where possible, in order to estimate a total use of fluorinated compounds. Chapter 5 is a summary of the information described in this chapter 4.
4.1 Occurrence in impregnating agents
The main brands on the market for impregnating agents are:
- Boston
- Kiwi
- Imprenex
- Nikwax
- Granger
Impregnating agents are used on many different products such as shoes, textiles, carpets, furniture, tents, etc. In the following paragraphs, impregnating agents for footwear, carpets, furniture and textiles are discussed in detail.
Impregnating agents identified for general impregnating purposes (i.e. leather, textiles, furniture, tents etc.) are listed below. Whether the impregnating agents contain fluorinated compounds is, however, uncertain for some products (if the products contain fluorinated substances that are mentioned in brackets).
- TexCare Imprægneringsspray (impregnation spray) (with TexCare® fluor anti-spot system)
- Coxy Super Protector Spray (impregnation with fluor)
- NanoCover Tekstil & Læder (textile and leather). Nanotechnology has been used for this product. This product can be used for clothes, coats, skiwear, ties, cushions, furniture, tents, shoes, etc.
- NikWax impregnating products (at least one of their products contains fluorinated chemicals according to analysis carried out by the Swedish Society for Nature Conservation (Naturskyddsföreningen, 2007)).
Fluorinated levels in products
In a project carried out for the Danish EPA in 2001, perfluorodecane sulfonate was found in a concentration of 212 mg/ml in an impregnating agent for tents, sleeping bags, etc. (Vejrup et al., 2002).
In another project carried out for the Danish EPA in 2004, PFOA was found in an impregnating product used by professional dry cleaners. The impregnating product for textiles was analyzed towards artificial sweat and this resulted in levels of 0.2 µg perfluoroheptanoic acid/dm² textile and 0.44 µg perfluorooctane acid/dm² textiles (Glensvig et al., 2004).
In a third project carried out for the Danish EPA in 2004, perfluoroheptanoic acid and perfluorooctane acid were found in impregnating agents for shoes in levels of 1.1 mg/kg and 0.36 mg/kg respectively. It is stated that this probably is due to impurities in the fluorocarbon polymer, which is added in the impregnating products, i.e. the PFOS-compounds identified are the monomers of the fluorocarbon polymer/telomer used in the impregnation agent (Engelund and Sørensen, 2005).
Levels of PFCA precursors (e.g. fluorotelomers) up to 3.8% have been measured in carpet impregnating agents. (Information from the Government of Canada 2006 cited from Swedish Chemicals Agency, 2006).
Recently, Swedish Society for Nature Conservation published a report about PFAS in impregnation fluids. Thirteen commercial products were analysed for a variety of perfluoroalkylsulfonates, PFCAs, perfluorooctanesulfonamides (FOSA and FOSE), and FTOHs (in all 27 different fluorinated chemicals). Some of these impregnating products have been found on the Danish market as well. Two of the products did not contain any of the fluorinated substances that were analysed for. Six of the products contained PFOS-related chemicals. Three of the products had very high contents of FTOH, between 1 and 9 g/L. Five products had high concentrations of FTOH, between 0.2-0.9 g/L, and contained PFCAs as well in concentrations between 0.2-8.4 mg/L. Seven of the investigated products contained PFOA, varying between 45 and 692 µg/L. Additionally, other PFCAs as well as up to 1000-times higher amounts of 8:2 FTOH were detected (Naturskyddsföreningen, 2007). The results are summarised in Table 4.2 below.
Table 4.2: Amounts of fluorinated substances extracted from 13 Swedish commercial impregnation products for all-weather clothing and shoes (Naturskyddsföreningen, 2007).
| Product name | Sum FTOH (ng/mL) | Sum PFAS (ng/mL) |
Sum PFCA (ng/mL) |
Sum FTS + FTCA (ng/mL) |
Sum FOSA + FOSE (ng/mL) |
Total sum (ng/mL) |
| Ecco Universal waterproofing spray | 224 310 | 0 | 2 783 | 0 | 0 | 227 093 |
| Armour | 3 480 | 0 | 0 | 0 | 0 | 3 480 |
| Nikwax TX Direct wash-in | 760 | 0 | 244 | 0 | 0 | 1 004 |
| Boston Raingard allover | 585 490 | 12.1 | 223 | 0 | 0 | 585 725 |
| Kiwi select all protector | 664 250 | 12.5 | 6 286 | 58.8 | 0 | 670 607 |
| Imprenex plus | 0 | 81.3 | 0 | 0 | 0 | 81 |
| Nikwax nubuck & mocka proof | 0 | 0 | 0 | 0 | 0 | 0 |
| Springyard Waterproofer | 1 144 630 | 22.4 | 91 | 0 | 0 | 1 144 743 |
| XT | 4 649 520 | 17.7 | 298 | 0 | 0 | 4 649 836 |
| Boston protector | 203 300 | 0 | 0 | 0 | 0 | 203 300 |
| Nikwax TX Direct Spray-on | 0 | 0 | 0 | 0 | 0 | 0 |
| Atsko Waterguard | 9 419 980 | 5.1 | 1 075 | 0 | 0 | 9 421 060 |
| Collonil classic waterstop | 882 250 | 0 | 8 372 | 0 | 0 | 890 622 |
| Average content (for the 11 products containing fluorinated substances) |
1 616 179 | 13.7 | 1 761 | 5.3 | 0 | 1 617 959 |
We got access to confidential information from a company producing impregnating agents primarily to the Scandinavian market. Two of their products for textiles had been analysed for PFCAs and contained 2.2 mg/kg total PFCAs and 5.3 mg/kg total PFCAs, respectively. The dominating PFCA was PFOA with contents of 0.73 mg PFOA/kg and 4 mg PFOA/kg in the respective products.
According to the search in the Danish Product Register fluorinated substances in impregnating agents are used in concentrations between 0.003% and 6%. The actual span between the lowest and highest concentrations is not necessarily this large, but these intervals are given in the Product Register as the exact concentration is confidential.
Companies contacted
None of the contacted companies were willing to give any information to the project.
Danish Statistics
A total estimation for impregnating agents for textiles and leather can be calculated using statistics for the larger product group “Preparations for treatment of textiles, leather and hide” (see Table 4.3). This product group are assumed to cover all sorts of impregnating agents, but probably not what is used for furniture. Furthermore, this product group is expected to cover much more than just impregnating agents.
Table 4.3: Supply of preparations for treatment of textiles, leather and hide (tonnes) in Denmark according to Danish Statistics. The product group is most likely covering other products than just impregnating agents.
| Import (tonnes) | Export (tonnes) | Production | Supply (tonnes) | |
| 2004 | 1589,9 | 149,3 | 0 | 1440,6 |
| 2005 | 2232,7 | 145,0 | 0 | 2087,7 |
| 2006 | 2206,6 | 126,1 | 9 | 2089,5 |
It is assumed that a maximum of 10% of the supply of impregnating agents for textiles and leather are based on fluorinated compounds but with an unknown concentration, as no information was received from producers of impregnating agents about the amount of fluorinated substances used in impregnating agents. It is necessary to know the concentration (percentage or in g/kg) of fluorinated substances to be able to calculate the total amount of fluorinated substances used in impregnating agents in Denmark.
According to the Product Register the concentration of fluorinated agents in impregnating agents for shoes is between 0.003 and 6%. This would mean a content of fluorinated compounds between 0.03 g/kg and 60 g/kg. This is intentionally a large span, as the actual concentrations are confidential.
The Swedish analysis of 13 impregnating agents, shown in Table 4.2, reported a large span of the concentrations of fluorinated substances of between 0.00008 g/L and 9.4 g/L with an average of 1.6 g/L (which is assumed to be equal to 1.6 g/kg).,
The Danish supply of preparations for treatment of textiles, leather and hide is 2089.5 tonnes, and if assuming that 10% of that amount is impregnating agents containing fluorinated substances in a concentration of 1,6 g/kg, the total use of fluorinated compounds in impregnating agents in Denmark for 2006 can be calculated to around 338 kg.
As 170 kg of fluorinated substances are reported for use in impregnating agents in Denmark according to the Product Register, this amount is regarded as a minimum use in Denmark and the 338 kg as the maximum use.
These calculations cover the entire area of impregnating agents, as the Danish Statistics is only available on this detail level. Below more detailed information on the specific types of impregnating agents is presented:
- Impregnating agents for footwear
- Impregnating agents for carpets and mats
- Impregnating agents for textiles, leather and furniture
- Impregnating agents for car interiors (textiles)
4.1.1 Impregnating agents for footwear
According to the report from the Danish EPA “Mapping and health assessment of chemical substances in shoe care products” (Engelund and Sørensen, 2005), the sale of impregnation products for shoes in Denmark in 2003 was about 29.5 tonnes. As mentioned earlier small concentrations of PFOS-compounds was found in impregnating agents for shoes on the Danish market.
By searching the Internet or visiting shoe shops it is easy to discover a number of impregnating agents for shoes and boots with a content of fluorinated compounds. The label on many products states directly that this product contains a fluorinated agent. The following impregnating products especially for footwear have been identified, and can be found in many ordinary shoe stores or in Internet trading. All the mentioned impregnating agents have a fluorinated content either according to the products or the information obtained on the Internet:
- WOLY Combo Proper (with fluorine agent)
- WOLY Velour Nubuck (with fluorine agent)
- WOLY Protector 3 x 3 (with fluorine agent)
- WOLY Multi effect (with fluorine agent)
- WOLY Oil Protector (with fluorine agent)
- Collonil Classic Waterstop (with GORE-TEX - contains fluorinated chemicals (PFCAs) according to analysis carried out by the Swedish Society for Nature Conservation (Naturskyddsföreningen, 2007)
- Ecco Universal Waterproofing spray (contains fluorinated chemicals (PFCAs) according to analysis carried out by the Swedish Society for Nature Conservation (Naturskyddsföreningen, 2007))
Companies contacted
Shoecare, which is the Danish distributor for the WOLY products, was contacted. They referred to their German supplier Melvo. Shoecare informed that they have received a declaration from their supplier that the products do not contain PFOS or PFOS-related compounds, and that the spray products contain fluorotelomer alcohols (FTOH) and traces of PFOA.
Melvo was contacted in order to learn more about content and concentration of fluorinated compounds in their products. However, this German company would not participate in the project with information about the content of the fluorinated compounds. They referred to the MSDS of the products, which did not reveal anything about the fluorinated compounds other than the products can decompose to HF (hydrogen fluoride), which means that they do contain fluorinated substances. However, the supplier of the fluorinated compounds is DuPont and therefore the product would probably be based on their “X Platform”, “Zonyl“ or “Foraperle” products.
Danish Statistics
It was impossible to find information about the use of impregnating agents for shoes via Statistics Denmark. The statistics are not available at sufficient detail level. A product code can be found for “Shoe polish and similar products for footwear and leather” and for “Preparations for treatment of textiles, leather and hide”. However, the sale of impregnation products for shoes of 29.5 tonnes for 2003 from the Danish EPA study can be used for the calculations, assuming that the annually use has not changed.
When using the average content of 1.6 g fluorinated substances per kg impregnating agents as found in the impregnating agents on the Swedish market (see Table 4.2), the annual use of fluorinated substances amounts to between 12 and 24 kg fluorinated substances for use in impregnating agents for shoes only, if it is assumed that around 25-50% of the impregnating agents for shoes are based on fluorinated compounds (others may be silicone-based).
4.1.2 Impregnating agents for carpets and mats
By searching the Internet or visiting carpet stores it is easy to discover a number of impregnating agents for carpets. Whether the impregnating agents contain fluorinated compounds is, however, uncertain for most products. The following impregnating products for carpets have been identified:
- Ditog imprægnering (contains fluorocarbons)
- Juhlin’s Textil og Tæppeimprægnering
- Stainshield
- Pure Guard
- TM-Textil og Tæppeimprægnering (textile and carpet impregnation)
- Baygard SF-A 02, Baygard SPT(contains fluorocarbons).
Visits to carpet stores showed that impregnation agents for carpets are easily accessible for consumers, but no information have been received, whether they are based on fluorinated compounds or about the amounts sold. This has not been investigated further. It is, however, assumed that this market is small, as not all consumers have carpets in their homes, and as most carpets today already are impregnated from the factory, when new carpets are bought (see 4.2.5 Carpets and mats). The use in industry (impregnation) and service (cleaning of mats) may be greater.
The contacted carpet factories and –stores informed that carpets made by man-made fabrics (nylon etc.) often are impregnated at the production. Carpets made by wool are seldom imprægnated
It is not possible to find information about the use of impregnating agents for carpets via Statistics Denmark. The statistics are not available at sufficient detail level.
4.1.3 Impregnating agents for textiles, leathers and furniture
By searching the Internet a number of impregnating agents for furniture and textiles have been identified. It is, however, uncertain for most products whether the impregnating agents contain fluorinated compounds. The following impregnating products for furniture and textiles have been identified:
- Point2you textil & læder (textile and leather). Nanotechnology has been used for this product.
- Cetox KWI used as impregnating agent for textiles at dry cleaning services in Denmark[13] (contains a fluoroalkyl polymer).
- Matas stofimprægnering (fabric impregnation)
- Guardian Textil Imprægnering (fluor anti-spot system)
- Granger´s XT Spray (contains fluorinated chemicals (FTOHs, PFAS and Pica’s) according to analysis carried out by the Swedish Society for Nature Conservation (Naturskyddsföreningen, 2007).
Companies contacted
Point2you and Guardian Protection Products were contacted, but no information was received. Furthermore, the firm using the impregnating agent Cetox KWI containing a fluoroalkyl polymer (used before 2003 for impregnating textiles at dry cleaners) was contacted, but it was not possible to get information about any continuous use of impregnating agents with fluorinated substances. The rest was not contacted.
Ecolab has developed a product called Saprit protect plus in order to protect garments from water, dirt and oils[14]. It is a professional impregnation product used for finishing in the textile industry. It is used as the final step in an industrial washing process of textiles, e.g. washing of work-wear[15]. It contains fluorocarbon chemicals according to their website[16]. Ecolab was contacted and informed that there is not content of PFOS, and the fluorocarbon content is below 2 ppm.
Danish Statistics
It is not possible to find information about the use of impregnating agents for furniture and textiles via Statistics Denmark. The statistics are not available at sufficient detail level.
4.1.4 Impregnating agents for car interiors (textiles)
The Danish EPA is carrying out a project with the title “Mapping and health assessment of products for indoor car care” (2007). In this project different products for indoor car care have been examined, among other impregnating agents for textiles. Two products seem to have a content of fluorinated substances in the concentration of 0.01 mg/kg (ppm). The concentration is, however, uncertain, as it was measured by use of a screening technique (headspace analysis), and the identification of the fluorinated substance was also uncertain. Nevertheless, one of the products: “SONAX Textil Imprægnering” is containing a fluorocarbon resin according to the MSDS.
“SONAX Textil Imprægnering” has it primarily use on indoor car upholstery, but is also usable for tents, backpacks and and other textiles with climate membrane.
The German producer of SONAX, Salzenbrodt GmbH, has been contacted, and they have confirmed that their textile impregnation agents are containing fluorinated substances. SONAX themselves do not have information about the content of fluorinated compounds in their product. However, the supplier to Salzenbrodt GmbH informed that the product “SONAX Textil Imprægnering” contains the DuPont product Foraperle 225, which contains the compound 2-Propenoic acid, 2-methyl-, hexadecyl ester, polymers with 2-hydroxyethyl methacrylate, g-w-perfluoro-C10-C16-alkyl acrylate and stearyl methacrylate (CAS 203743-03-7). Salzenbrodt GmbH estimated that a total of 1.8 kg of this fluorinated polymer was used in products sold to Denmark in 2006. Additional questions were asked about their content and sales amount on the Danish market, but no information was received.
4.2 Occurrence in impregnated products
Chapter 4.1 focused on impregnating agents, i.e. chemical products. This chapter focuses on products/articles that are being impregnated by the producer before being sold on the market. It is somewhat easier to find information about the content of fluorinated substances in chemical products, as e.g. Material Safety Data Sheets exist. Information about the content of chemicals in consumer articles/consumer products is not easily accessible.
This survey does not focus on the use of fluorinated compounds within the area of food, as this product area is not the responsibility of the Danish EPA.
4.2.1 Clothes
Three companies trading normal daily clothes have been contacted. None of the contacted companies are using fluorinated compounds in their products to their knowledge. No products are sold with water- or stain proof properties.
4.2.2 Waterproof clothing and all-weather clothes
Fluorinated levels in products
A Swedish and Norwegian study from 2006 states that the annual global fluorotelomer production is around 10000 tonnes, and that about 50% of this production goes to the impregnation of textile consumer products, e.g. in all-weather clothing, carpets and upholstery (Schulze and Norin, 2006; Berger and Herzke, 2006). In the same study six all-weather jackets for children from five different brands (Gore-Tex Paclite, HellyTech, FineTex/DuPont Teflon, TCS Water and Dermizax) bought in the Nordic countries were investigated for the content of fluorinated substances. The study analysed the unbound content of fluorinated compounds in the jackets: unbound content of fluorotelomer alcohols (FTOH) between 25 and 1000 µg/m² of textile, an unbound content of PFCA between <5 and 400 µg/m² of textile and an unbound content of PFOS related compounds between <5 and 100 µg/m² of textile.
Companies contacted
Three different “sportswear” companies were contacted on the matter. One company claimed that their products produced in China did not contain fluorinated compounds. The two other companies informed that many of their products are Gore-Tex products, i.e. they are based on fluorochemistry.
Helly Hansen, Norway, confirmed the use of fluorocarbons in their products, such as e.g. breathable jackets. No information has been received with respect to total amounts used in their products today. Helly Hansen informed that they have been monitoring the discussions around PFOS and PFOA usage in textiles and have concluded that from ultimo 2008 or during summer 2009 the latest, all HH products will be free from fluorocarbons or fluorotelomers.
A supplier of fluorinated impregnating products to the Danish textile market was contacted. They reported an annual sale of around 650-700 kg of their products. These products contain fluoropolymers with an average content of 17%. This results in an annual sale of 111-119 kg fluoropolymers to textile industries in Denmark. The supplier estimated their market share to be about 30%. This will result in an estimation of about 400 kg fluorinated substances being used for impregnation of textiles in Denmark (excluding carpets).
Danish Statistics
In the Danish statistics it is possible to find some product groups that specifically mention impregnated clothing. However, it is not possible to distinguish the type of impregnation, i.e. it can be other types of impregnation than with fluorinated compounds – for example silicones. The product groups are:
- 6113 0090 ”Beklædningsgenstande, trikotage, konfektioneret af imprægneret, belagt eller lamineret tekstilstof, undtagen gummieret” (Clothing, hosiery, ready-made clothing impregnated, covered or laminated textile fabric, but not rubberised) exist.
- 62101010 “Beklædningsgenstande konfektioneret af filt, også imprægneret, overtrukket, belagt eller lamineret” (Ready-made apparel of felt, also impregnated, coated, covered or laminated).
- 62104000 “Konfektioneret tekstilbeklædning, imprægneret, overtrukket, belagt eller lamineret, til mænd/drenge, ej trikotage, i.a.n[17].” (Ready-made textile clothing, impregnated, coated, covered or laminated, for men/boys, not hosiery, not mentioned elsewhere).
- 62105000 “Konfektioneret tekstilbeklædning, imprægneret, overtrukket, belagt el lamineret, til kvinder/piger, ej trikotage, i.a.n.” (Ready-made textile clothing, impregnated, coated, covered or laminated, for women/girls, not hosiery, not mentioned elsewhere).
The import and export are given in both currency and kg whereas the production is given in currency and in pieces, which makes it difficult to estimate the total use of impregnated clothing in Denmark. As the production is close to 15-20% of the imported amount, the production amount is estimated by using the ratio of tonnes per DKK for the imported amount for the different years. These estimations give the following values (see Table 4.4):
Table 4.4: Supply of clothing impregnated (tonnes) in Denmark according to Danish Statistics
| Import (tonnes) | Export (tonnes) | Production (tonnes estimated) | Supply (tonnes) | |
| 2004 | 1624 | 934 | 342 | 1032 |
| 2005 | 2240 | 874 | 422 | 1788 |
| 2006 | 3110 | 1048 | 534 | 2596 |
It has not been possible to identify use concentrations for different fluorinated impregnating agents for clothes. However, some of the Zonyl products can be used for both textiles and carpets. It is therefore assumed in the calculations that the use concentrations are about the same. The unbound measured figures for total content of fluorinated substances in the Norwegian and Swedish study cannot be used, as it is not measured for the total content but for the unbound chemicals, which are probably only about less than 1‰ of the total content.
If it is assumed that between 5 and 50% of the impregnated clothing are impregnated with fluorinated substances, and that the total use of fluorinated substances are between 0.27 and 2.7 g/kg textile as used for carpets (according to use instructions for Zonyl products), then the content of fluorinated compounds in clothing in Denmark would be between 35 kg and 3.5 tonnes (density assumed to be 0.5 kg/m²)[18].
The minimum value is set to 400 kg based on the information from a supplier (as described above). The maximum value used is the 3.5 tonnes, as the estimate based on the market share for the one company giving information is too uncertain.
For comparison, the above-mentioned Swedish and Norwegian study estimates that the total global use of fluorotelomer compounds for impregnated products (textiles, carpets, etc.) is around 5,000 tonnes globally.
To sum up the minimum use of fluorinated compounds used for impregnated clothings is estimated to be 400 kg and the maximum use is estimated to 3.5 tonnes.
4.2.3 Footwear
The footwear category covers a range of different kind of footwear, such as slippers, boots, shoes, footwear for sports, etc. It is assumed that mainly boots are being coated with fluorinated products such as Teflon. Visits to shoe stores in Denmark show that especially boots for children are treated with Teflon.
Companies contacted
Two producers of footwear were contacted. One company informed that they do use an impregnating agent (MI 1113) with a content of a fluorine compound for finishing of their product. The production is however carried out abroad, and it is only for a special type of product for a special customer abroad, i.e. no fluorinated compounds are used for impregnating the products for the Danish market.
The Danish supplier of this MI 1113 impregnating agent was contacted and they could inform that the impregnating agent is not sold to any other companies in Denmark.
The other footwear producing company did not want to participate with information. It is supposed that fluorinated compounds are used.
Danish Statistics
The Danish Statistics on footwear are divided in different categories dependant on the type of footwear, e.g. waterproof (rubber), upper section of textile, leather, etc. These footwear categories cover a range of different kind of footwear, such as slippers, boots, shoes, footwear for sports, etc.
It is, however, impossible to perform a calculation for content of fluorinated substances in footwear, as:
- No information can be found regarding the content of fluorinated substances per pair of shoes/boots.
- The Danish supply of footwear cannot easily be calculated, as the import/export statistics is calculated in kilos, and the Danish production is calculated either in pair of shoes or 1,000 pieces dependant on the product code, i.e. the numbers cannot be added.
To sum up: We know that especially childrens boots are being treated with Teflon, but have not any information about amounts. The statistics in this area cannot be used for any educated guess, i.e. the amount of fluorinated substances used in this area is unknown.
4.2.4 Furniture
Companies contacted
Five companies selling furniture were contacted. Three of the companies were not interested in participating in the project. The two other companies informed that they did not use any kind of fluorinated compounds to impregnate their furniture. IKEA answered that they had been using Scotchgard in some of their furniture fabrics before 2000, but decided to phase out the use before the 3M Company withdrew their products from the market. Instead, IKEA is now using the concept of removable and washable covers for their furniture. The only Teflon-treated products to be found in IKEA are in non-stick cookware.
4.2.5 Carpets and mats
Two carpet stores were visited in order to learn about the sale of impregnated carpets in Denmark. The information provided varied. In one store we were told that almost every carpet sold today are impregnated (99%). Some are impregnated with Teflon, and carpets for sale in both stores were marked Scotchgard™. However, in the other store, we were told that the use of Teflon impregnated carpets is on its way out, as the coating is expensive.
Fluorinated levels in carpets (see also Section 6.2.4.)
In a study PFOS and PFOA were found in all 16 tested vacuum cleaner dust samples from different Japanese households and offices. The levels found varied between 11-2500 ng/g dust for PFOS and 69-3700 ng/g dust for PFOA. These findings could be an indication of use of fluorinated compounds in carpets, but other products in the homes are of course also possible sources. (Moriwaki et al., 2003).
Dinglasan-Panlilio and Mabury (2006) have investigated seven different commercially available and industrial applied polymeric and surfactant materials, i.e. the chemical products that are applied to impregnated consumer products. Two investigated chemical products were carpet protectors. They identified the presence of between 0.04 and 3.8% residual unbound fluorotelomer alcohols in varying chain lengths.
Companies contacted
One of the major Danish carpet producers was contacted. They are according to their environmental report 2005/2006 producing around 6 million m² carpets. They are using fluorinated compounds for impregnation, but not PFOS or PFOS-related compounds. They are using a DuPont product for their carpets. They are impregnating 100% of the carpets they are producing. It was not possible to get more detailed information. The company referred to the trade union Federation of Danish Textile and Clothing Industry (Dansk Tekstil og Beklædning), but they could not give any information about the use of fluorinated compounds in carpets (did not have that kind of information).
Another large producer of carpets was contacted. They are producing around 7.5 million m² carpets yearly. Only about 20% of these carpets are being impregnated with a Zonyl product from DuPont. Around 50% of the carpets in total were exported; the rest was for the Danish market. It is only specific fibres that are being impregnated. Nylon fibres are always impregnated, whereas carpets based on wool fibres are never impregnated. Primarily carpets for industrial uses (offices etc.) are impregnated. In average for these two contacted carpet producers around 56% of the carpets produced in Denmark are impregnated with fluorinated compounds.
Danish Statistics
Data of the Danish supply of carpets from Danish Statistics can be found in the Table 4.5 below. The supply is calculated for the entire product group no. 57 called carpets and other textile floor coverings, which are covering carpets made of both natural fibres such as wool and coconut, and synthetic fibres.
Table 4.5: Supply of carpets (m²) in Denmark according to Danish Statistics
| Import (m²) | Export (m²) | Production (m²) | Supply (m²) | |
| 2004 | 7,814,221 | 10,081,361 | 11,558,000 | 9,290,860 |
| 2005 | 7,325,222 | 9,856,891 | 12,226,000 | 9,694,331 |
| 2006 | 7,918,408 | 10,893,686 | 13,387,000 | 10,411,722 |
A calculation of the Danish supply of carpets that are not based on natural fibres such as wool and coconut (which most likely are not impregnated), shows that 90% (2006) of the total Danish supply of carpets are based on synthetic fibres. As the two largest carpet producers in Denmark (producing in total 13.5 million m² of carpets) in average are impregnating 56% of the carpets, this value is used as the lower value of the fraction of carpets being impregnated. 90% is used as the upper value (equals the percentage of carpets based on synthetic fibres).
According to MSDS of Scotchgard Carpet Protectors the content of fluorochemicals in the concentrated products is 1-5% or 2-10% for different examples of carpet protectors. The types of fluorinated chemicals are a trade secret. However, no information was found on, how the product should be used (in which concentration and how much per square meters).
According to MSDS of DuPont’s Teflon products for carpets (wool), the concentration of fluorochemicals in the concentrated products is 3-4%. This example product should be used in a 1:8 dilution, and be applied to carpets at the rate of 1.0 gallon of dilution for every 100 square feet area carpet. As one gallon is 3.785 litre and one square foot is 0.0929 m² each m² carpet impregnated will contain between 1.5 and 2.0 g fluorinated compounds[19].
When using another of DuPont’s impregnation agents for carpets, e.g. Zonyl 5180 Carpet Protector, the fluorinated content per m² carpet will be between 0.1 g and 1.4 g[20]. The MSDS and technical data sheet of this product state that the content of fluorinated compounds is between 1 and 10%, and that a 1:15 dilution should be used. One diluted litre will treat 200 square feet of carpet. It is uncertain, whether this product is used for pre- or after treatment of carpets.
If assumed that between 56 and 90% of the carpets in Denmark are impregnated with fluorinated compounds, and the content of fluorinated compounds is between 0.1 and 2 g per m² of carpet, this will amount to a yearly consumption of fluorinated compounds between 745 kg and 18.0 tonnes.
4.2.6 Sunshades/awnings, tents, umbrellas and parasols
Sunshades/awnings, tents, umbrellas and parasols are all products that can be impregnated with fluorinated compounds. As mentioned earlier some impregnating agents, e.g. the SONAX Tekstil Imprægnering (Textile Impregnation) spray may also be used for tents, backpacks and textiles with climate membrane (like e.g. umbrellas and parasols).
Companies contacted
A Danish firm selling awnings was contacted. It turns out they are getting the fabric for the awnings from a company in Sweden – they were also contacted. The Swedish company supplies impregnated awnings to 10 different companies in Denmark. The fabric for the awnings is impregnated with Oleophobol SL-A01, which contains a dispersion of fluoropolymers. There is no information about the percentage content of fluoropolymers. According to the Swedish company, they supply 30,000 m² of impregnated fabric for awnings to Danish companies. It has not been possible, neither to estimate the total use of fluorinated substances for impregnation of awnings sold in Denmark, nor to uncover how widespread the use of fluorinated substances for impregnation of awnings is in Denmark.
Another company gave the information that outside the Danish market, Teflon wax (polytetrafluoroethane, CAS 65530-85-0) is used in products that are applied to sunshades in order to make them water-repellent.
4.3 Occurrence in other relevant products
4.3.1 Paints
Fluorinated levels in products
According to DuPont the fluorotelomer-based surfactants are added to paints to improve flow, wetting and levelling, and for reducing the surface defects and craters. The fluorotelomer-based products are added in an amount between 300 and 500 mg of product/kg of paints (Washburn et al., 2005).
Dinglasan-Panlilio and Mabury (2006) have investigated seven different commercially available and industrial applied polymeric and surfactant materials, i.e. the chemical products that are applied to impregnated consumer products. Two investigated chemical products were fluorosurfactants for paint, polish and other coatings. They identified the presence of between 0.04 and 3.8% residual unbound fluorotelomer alcohols in varying chain lengths.
Companies contacted
Four paint and lacquers producing companies in Denmark have been contacted, as well as the trade organisation for paint and lacquers in Denmark (Foreningen for Danmarks Farve- og Lakindustri).
They were asked about their use of fluorinated compounds in paints and lacquers. We were told that some paint producers use a so-called Teflon wax (polytetrafluoroethane, CAS 65530-85-0). The typical content will be about 11-18% dry matter in water. The contacted paint producers do not use Teflon wax in products for the Danish market, but is certain that it is used in paints sold in Denmark. Teflon wax is added to leave the paint with a water repellent film, when the paint dries. An alternative to this is to use a polyethylene wax, however, that will not result in a water repellent film, but a scratch resistant surface. Teflon wax is, according to the contacted paint producing companies, not used for products sold in Denmark, but one of the paint producers uses Teflon wax in products sold outside of Denmark.
This general impression of the interviews with the four companies is in contrast to the information from the search in the Danish Product Register, where more than 10 substances in a total of about 3.5 tonnes are registered as used within the Danish paint & lacquer industry. The companies and the trade association were confronted with this, and the trade association made an inquiry at its members, and got the following response:
- Fluorinated compounds are only used by a few of the companies within the paint and lacquer industry.
- The members of the trade association were confronted with the CAS numbers found by the Product Register search and the members who responded could in general not recognisee the fluorinated substances.
- One company uses about 250 kg per year of Zonyl FSN. Zonyl is used for aqueous paints for indoor use. According to DuPont data sheet on Zonyl FSN[21], the content of fluorosurfactants is 40%. This amounts to a total confirmed use of fluorinated substances of 100 kg. Zonyl FSN is according to the MSDS containing a fluorinated telomer (CAS 65545-80-4) – a compound that is also registered for use in paints by the Danish Product Register.
According to the trade organisation about two thirds of their members responded to the inquiry. It is not possible to say, what causes the difference in amounts between the information from the trade organisation and the Danish Product Register but it may, according to the trade organisation, be any of the following explanations:
- The amounts registered in the Danish Product Register have not been updated (the companies have not informed about that the substances are no longer used).
- The members that have not replied are using the substances.
- Companies outside the trade organisation are using the substances.
- The substances are used in raw materials that are imported by the paint & lacquer companies, but the amounts of the substances are reported to the Danish Product Register by the producers outside of Denmark, because of confidentiality. This means that the companies in Denmark do not know the total content of their paints.
Therefore, it is concluded that the use of fluorinated substances within the paint area is minimum 100 kg and maximum 3.5 tonnes (as registered in the Product Register). The contents of fluorochemicals in paint products not classified dangerous are not included. The total consumption may be somewhat higher, however, since information from an industru association showed a consumption of only 100 kg, it was decided to use the information in the Product Register at upper limit.
4.3.2 Printing inks
Companies contacted
The trade association for paint and lacquers in Denmark (Foreningen for Danmarks Farve- og Lakindustri) was contacted. The trade association made an inquiry at its members, and got the response that a fluorinated wax (PFTE-wax; CAS 9002-84-0) is used in printing inks. The wax is used to protect the surface and make the surface smoother. No information about amounts was received.
According to the search in the Danish Product Register, the use of fluorinated substances in printing inks is low (15 kg in total).
4.3.3 Auto polish and -wax
Fluorinated levels in products
In a project for the Danish EPA auto polish and wax products were investigated. In one sealing product a polyfluorinated compound not on the OECD list (CAS no. 65530-65-6: 2-Propenoic acid, 2-methyl-, dodecyl ester, polymer with a-fluoro-?-[2-[(2-methyl-1-oxo-2-propenyl)oxy]
ethyl]poly(difluoromethylene) and N-(hydroxymethyl)-2-propenamide) was found in a concentration of 0.085 to 0.45%. No products were analysed specifically for the content of fluorinated substances. This content of a polyfluorinated compound was based on Material Safety Data Sheets. Other of the examined products could therefore also contain fluorinated substances (Ferdinand et al., 2004).
Contact to companies
Contact to a German producer of car care products has given the information that the auto polish and waxes they are producing do not have a content of fluorinated substances. However, the market share of these products on the Danish market is not known.
Danish Statistics
Data of the Danish supply of wax and polish from Danish Statistics can be found in Table 4.6. In the statistics auto polish and wax has its own product group. The supply is calculated for the entire product group no. 3405 30 called “Polermidler o. l. præparater til karrosserier/automobilpolermidler” (polishes and similar preparations for coachwork, other than metal polishes/polishes for automobiles).
Table 4.6: Supply of auto polish and wax (tonnes) in Denmark according to Danish Statistics
| Import (tonnes) | Export (tonnes) | Production (tonnes) | Supply (tonnes) | |
| 2004 | 636 | 321 | 17 | 332 |
| 2005 | 706 | 9 | 3 | 700 |
| 2006 | 982 | 32 | 4 | 954 |
It looks like the market for auto polish and waxes is rising, especially as the similar numbers for 2000-2002 was 297, 414 and 373 tonnes, respectively (Ferdinand et al., 2004).
In the former project from the Danish EPA, the market for the consumers was judged to be about one third of the total market. It is uncertain whether this distribution is still valid for 2006, where the use of auto polish and waxes nearly has tripled. If assuming that between 33 and 50% of the used amount of auto polish and waxes are used by consumers, and that auto polish contains between 0.0025 and 0.1% fluorosurfactants (Zonyl FSO used as an example[22]), then the content of fluorinated compounds in auto polish and wax used in Denmark in 2006 will be between 8 kg and 477 kg.
However, fluorinated substances may not necessarily be used for every polish or wax product. As limited information has been found on the prevalence of fluorinated substances in polish and wax, a minimum value of 1% and maximum value of 75% of the products on the market containing fluorinated substances are assumed. This results in a content of fluorinated substances in auto polish and wax used in Denmark in 2006 for the consumer market between 79 g and 358 kg.
4.3.4 Floor polish
Fluorinated levels in products
Moriwaki et al found PFOS and PFOA in all 16 tested vacuum cleaner dust samples from different Japanese households and offices. The levels found varied between 11-2500 ng/g dust for PFOS and 69-3700 ng/g dust for PFOA. These findings could be an indication of use of fluorinated compounds in carpets, but one of the samples was taken from a house with no carpets. The wooden floor in this home was treated 6 months before the sampling day with a floor wax coating. In this house the level of PFOA and PFOS in the vacuum cleaner dust was 250 ng/g dust and 94 ng/g dust, respectively. It was concluded that the wax-coating may be one of the sources to the PFOA and PFOS levels found, but no analysis of the wax was carried out. Other sources may be impregnated products, such as clothes/all-weather clothes and impregnating agents (Moriwaki et al. (2003).
Dinglasan-Panlilio and Mabury (2006) have investigated seven different commercially available and industrial applied polymeric and surfactant materials, i.e. the chemical products that are applied to impregnated consumer products. Two investigated chemical products were DuPont Zonyl fluorosurfactants for paint, polish and other coatings. They identified the presence of between 1.0 and 3.8% of dry weight residual unbound fluorotelomer alcohols in varying chain lengths for the Zonyl products.
According to DuPont website[23] “almost every acrylic/wax floor polish formulation on the market contains a fluorosurfactant (100-500 ppm)”. This equals 0.01% to 0.05% of the product is a fluorosurfactant. This range of contents on wet weight basis seems to be significantly lower than the results published by Dinglasan-Panlilio and Mabury (2006), however, their results were reported on dry weight.
Danish Statistics
The supply is calculated for the entire product group no. 3405 9090 called “Pudse- og polermidler, undtagen til sko, læder, træværk, biler og metalvarer samt skurepræparater” (polishes except for shoes, leather, woodwork, cars and metal goods, and scrub agents). The supply of polish products is listed in Table 4.7.
Table 4.7: Supply of polish for other polish products (floor polish etc.) in Denmark according to Danish Statistics
| Import (tonnes) | Export (tonnes) | Production (tonnes) | Supply (tonnes) | |
| 2004 | 662 | 481 | 7 | 188 |
| 2005 | 692 | 499 | 6 | 199 |
| 2006 | 567 | 333 | 6 | 240 |
When using the percentages of content of fluorinated compounds taken from the DuPont website and the supply of 240 tonnes of “other polish products” in Denmark in 2006, the content of fluorinated compounds in “other polish products” used in Denmark in 2006 is between 24 kg and 120 kg. However, as this group of “other polish products” in the Danish Statistics may cover other products than floor polish products, and as fluorinated substances may not necessarily be used for every polish product, a minimum value of 1% and a maximum value of 50% of the products on the market containing fluorinated substances are assumed. This results in a content of fluorinated substances in floor polish used in Denmark in 2006 between 0.2 kg and 60 kg.
4.3.5 Ski wax
The market for ski wax was investigated by contact to a single Norwegian producer of ski wax. Only one special type of ski wax is containing a perfluorinated alkane (CAS No. 355-49-7, perfluorohexadecane, C16F34). The ski wax is very expensive (around 1000 NOK per 30 g of wax) and is hence only used by top-professional skiers. The Norwegian production is just below 1 tonne of this wax that contains 80-100% of the perfluorinated compound. 50% is being exported. It is assumed that the use of this kind of product in Denmark is negligible.
4.3.6 Cleaning agents for glass
By searching the Internet a number of cleaning agents for glass have been identified. It is however uncertain for most products whether the cleaning agents contain fluorinated compounds. The following cleaning agents have been identified:
- “Percenta Glas- & Keramik Rengøring”. Nanotechnology has been used.
- “Point2you Glas & Keramikforsegling”. Nanotechnology has been used.
- “Point2you Nano-glasforsegling”. Nanotechnology has been used.
- “NanoCover basis klargøring”
- Aro, Glas universal spray.
- Ajax, Glas universal, Colgate-Palmolive.
- “Mr. Muscle Glasrengøring Professionel”, JohnsonDiversey.
- Cif Windows Spray Regular, Unilever (no content of fluorinated substances according to the ingredients on their website – fluorinated substances are not mentioned specific, but all ingredients are listed, so it is assumed that no fluorinated substances are in the products).
- Froggy Sprit Glasspray (no content of fluorinated substances according to the ingredients on their website – fluorinated substances are not mentioned specific, but all ingredients are listed, so it is assumed that no fluorinated substances are in the products).
Companies contacted
Several of the companies were contacted, but only few were interesting in giving information. JohnsonDiversey is only selling window cleaning products on the professional market, and they are not using any kind of fluorinated compounds in their products. Presently, Unilever has not any glass cleaning products on the Danish market. About two years ago they sold the product (Cif Windows Spray), which did not contain any fluorinated substances.
It seems like the “ordinary” glass cleaning products found in every day stores do not contain fluorinated compounds. It is however, uncertain whether the glass cleaning products that are based on nanotechnology contain fluorinated compounds.
5 Consumption of fluorinated substances in Denmark
5.1 Experiences
In all 59 different companies were contacted.
In general, it has been very difficult to get any useable information out of the companies. The companies are divided in two groups:
- A large group that has no or almost no knowledge about fluorinated substances and therefore also their uses.
- A smaller group that has a high knowledge about the fluorinated compounds.
The survey has shown that the second group, in most cases are unwilling to participate in the study or are referring to the trade union within their sector.
Some of the problems in mapping the use of fluorinated compounds are:
- Importers and end users may not be aware that the products contain fluorinated compounds because it is confidential information, and the content is not necessarily mentioned in material safety data sheets.
- The supply chain may be long, meaning that companies are giving up, when trying to retrieve the information from their suppliers, as it often is the supplier of the supplier that has the necessary information.
- Not all companies are willing to tell the identity of their suppliers, and in most cases the companies preferred to contact their suppliers themselves.
- The producers do not necessarily know which specific types of products their products are used for. They do not get feedback from their customers.
- Short chain fluorinated compounds may appear as contaminants or impurities in products containing fluoropolymers. For example, in products for impregnation fluorinated compounds may appear as impurities at the level of parts per million.
5.2 Results
Based on the above descriptions and calculations in chapter 4 the total estimated consumption of fluorinated substances in Denmark is somewhere between 14 and more than 38 tonnes annually (see Table 5.1). Table 5.1 is a summary of the calulations and estimates described in chapter 4. It is, however, an uncertain estimate because some figures are calculated with a use of educated guesses based on information from industry and literature. The amounts found by the search in the Product Register must for most used be regarded as minimum values as only chemicals that contain substances classified as dangerous in a concentration of at least 0.1% or 1% (depending on the classification of the substance) are to be registered in the Danish Product Register, i.e. there may be a use of chemicals not classified as dangerous with a content of fluorinated substances. Furthermore a use of articles/products with a content of fluorinated substances is not registered in the Danish Product Register. If no other information was available the amounts from the Danishg Product Register were used as both minimum and maximum amounts.
Table 5.1: Total calculated estimated amount of fluorinated substances used or contained in products in Denmark
| Use area | Min. estimated amount of fluorinated substances in products (kg) | Max. estimated amount of fluorinated substances in products (kg) |
| Releasing agents | 7200 | > 7200 |
| Paint and lacquers | 100 | 3500 |
| Printing inks | 15 | > 15 |
| Glue | 2500 | > 2500 |
| Surface active substances | 1100 | > 1100 |
| Cleaning agents | 100 | > 100 |
| Polish and care products | 170 | 590 |
| Auto polish and wax | 0.08 | 360 |
| Floor polish | 0.2 | 60 |
| Carpets | 745 | 18000 |
| Sunshades/awnings, tents, umbrellas, parasols etc. | not estimated | not estimated |
| Impregnated clothing | 400 | 3,500 |
| Footwear | not estimated | not estimated |
| Impregnating agents | 170 | 340 |
| Impregnation agents for footwear only | 12 | 24 |
| Impregnation agents for car textiles only | 1.8 | 1.8 |
| Galvano-technical products | 760 | > 760 |
| Inhibitors | 400 | > 400 |
| Pesticides | 180 | > 180 |
| Soldering agents | 280 | > 280 |
| Total | 14120 @ 14.tonnes | > 38465 = > 38 tonnes |
One thing is the use of fluorinated substances, another thing is, however, the type of fluorinated substances used, and the possibility of the substances to be degraded to PFOS, PFOA or other PFCAs in the environment, as these substances are the most critical in the environment.
DuPont has published a scientific paper, in which they calculate the PFO (Perfluorooctanoate) content in selected consumer articles (Washburn et al., 2005). This content covers the impurities of PFOA, but not compounds being degraded to PFCAs, as for example the possibility of fluorotelomer alcohols to degrade to PFCA. By using the data given in the DuPont paper on the above figures, it can be seen that the impurities of PFOA typically is somewhere between 0.1 and 1% of the total content of fluorinated substances.
It must be noted that about 7.5 tonnes of the total 16.5 tonnes registered in the Danish Product Register counts for substances that have a chain length lower than 8, and that are furthermore substances that are not on the OECD list of PFAS, PFOS, PFOA and substances that can be degraded to PFCA.
The rest of the chemicals may, however, have a potential to degrade to PFOA or other PFCAs in the environment. The exact amount is not known, as this would require detailed knowledge of the fluorinated substances used, as only specific types of fluorinated substances can be degraded to PFOS, PFOA or other PFCAs in the environment.
At the meeting with DuPont and the trade association Plastics Europe, they commented on the list of substances from the Danish Product Register, and informed that in their opinion, not all of the substances could be degraded to PFAS and only in small amounts.
This survey of fluorinated substances used in consumer products in Denmark has unfortunately not given any detailed information (as CAS numbers) about the specific substances that are used in the products. For information about the specific substances we must rely on the list of substances registered in the Danish Product Register and the list of substances that can be found by going into detail with the Material Safety Data Sheets on different products containing fluorinated substances.
6 Environmental assessment of polyfluoroalkylated substances
- 6.1 Environmental chemistry, pathways and levels
- 6.2 Environmental fate and levels
- 6.2.1 Environmental release
- 6.2.2 Levels in the ambient air and precipitation
- 6.2.3 Levels indoors
- 6.2.4 Occurrence in remote area and long-range transportation
- 6.2.5 Levels in environmental waters, groundwater and drinking water
- 6.2.6 Levels in wastewater and sludge
- 6.2.7 Levels in soil and sediments
- 6.2.8 Levels in biota and wildlife
- 6.3 Ecotoxicity
- 6.4 Environmental risk assessment
6.1 Environmental chemistry, pathways and levels
6.1.1 Background
The family of about 1000 polyfluorinated chemical substances (PFCs) is very diverse with different chemical structures and properties determining their environmental pathways and fate in the biosphere. Some substances are simple perfluoroalkyl carboxylic acids (PFCAs like e.g. PFOA) or sulfonic acids (like e.g. PFOS) and their salts with various lengths of the perfluorinated alkyl groups and eventual branched chains. Other polyfluorinated substances - the substances mostly used in consumer products - are complex, high-molecular derivatives of the acids, e.g. bulky substituted sulfonamides), fluorotelomers (e.g. polyfluorinated alcohols (FTOH) and perfluorinated phosphates (PAPS) or polymers). These substances are mostly non-polar and more volatile but less persistent than the parent perfluoroalkanoic acids (PFAA), they are precursors of.
Polyfluorinated substances are used because of their surfactant properties. They have both hydrophobic and oleophobic properties and are chemically and thermally inert; especially the fluorocarbon chain is extremely resistant to heat and chemical attack, e.g. by acids and bases, and reducing and oxidizing agents.
The C-F binding is very strong. Thus the perfluoro chain is a stable identity, which in practice is non-degradable in nature. On the other hand a bulky functional end group will be more readily transformed in the environment and in organisms, and therefore the compounds will be degraded to the persistent sulfonates (e.g. PFOS) and carboxylates (PFOA) in the end. The sulfonates and carboxylates are polar species, which will not accumulate in fatty compartments but mainly in blood and liver, and these substances will often interact with polar sites in sediments.
The low biodegradability of PFCs is, together with their tendency to bioaccumulate, characteristics typical of persistent organic pollutants (POPs). After PFCs have been found to be distributed globally in all environmental compartments, the use and production of several perfluorinated compounds have been regulated by national and international agencies such as U.S. EPA (2006), Environment Canada (Renner, 2005) and European Union (2006). PFOS has recently been added to the OSPAR list of chemicals for priority action (OSPAR, 2003) and nominated for inclusion in the Stockholm Convention (2005) as a persistent organic pollutant.
6.1.2 Physical-chemical properties
Large differences exist between the water solubility and vapour pressure for the individual PFOS- and PFOA-related substances. Some data are shown in Table 6.1 and Table 6.2. It should be noted that commercial products are not pure substances but may be mixtures and can contain several percentages of branched isomers and traces of other PFCs.
The acids and their free salts are solids that are soluble and dissociated in water and insoluble in lipids and do not evaporate from the water phase. The aqueous solubility will decrease with increased chain length. In absence of being in solution 8:2 FTOH will rapidly sublime at ambient temperature (Kaiser et al. 2006).
Liu & Lee (2007) discovered that the fluorocarbon chain length was the dominant structural feature influencing water solubility and sorption to soil of fluorotelomer alcohols (polyfluorinated alcohols). The water solubility of fluorotelomer alcohols is low and decreases with increasing chain length. Each CF2 moiety decreased the aqueous solubility by about 0.78 log units and increased the sorption by surface soils with 0.87 log units.
Fluorotelomer alcohols have higher calculated vapour pressures than the parent alcohol; for example 10:2 FTOH is 1000 times more volatile than dodecanol, possibly because of the unique molecular geometry with possibility of formation of intramolecular hydrogen bonding (Stock et al. 2004b).
Table 6.1: Water solubility and vapour pressure for some perfluorinated sulfonates (Heckster et al. 2003; Shoeib et al. 2004a; Lei et al. 2004b).
| Substance name | Acronym | CAS no. | Solubility in water (g/L) | Vapour pressure (Pa) | Octanol-air partition coefficient (Log Koa) |
| Perfluorooctane sulfonyl fluoride | PFOSF | 307-35-7 | |||
| Perfluorooctane sulfonic acid | PFOS | 1763-23-1 | |||
| Potassium perfluorooctane sulfonate | PFOS-, K+ | 2795-39-3 | 0.519 0.57 |
3.31 x 10-4 | |
| Perfluorooctane sulfonamide | PFOSA; FOSA | 754-91-6 | |||
| N-Methyl perfluorooctane sulfonamide | MeFOSA | 31506-32-8 | |||
| N-Ethyl perfluorooctane sulfonamide | EtFOSA | 4151-50-2 | 6.9 (25°C) | ||
| N-Methyl perfluorooctane sulfonamidoethanol | MeFOSE | 24448-09-7 | 0.002 | 7.7 (20°C) 7.05 (25°C) |
|
| N-Ethyl perfluorooctane sulfonamidoethanol | EtFOSE | 1691-99-2 | 1.51 x 10-4 | 0.504 | 7.78 (20°C) 7.30 (25°C) |
| N-Methyl perfluorooctane sulfonamidoethyl acrylate | MeFOSEA | 25268-77-3 | |||
| N-Ethyl perfluorooctane sulfonamidoethyl acrylate | EtFOSEA | 423-82-5 | 8.9 x 10-4 | 0.002 | 7.87 (20°C) |
| Perfluorobutane sulfonic acid | PFBS | 29420-49-3 | 0.51 | ||
| Potassium perfluorobutane sulfonate | PFBS-, K+ | 29420-43-3 | |||
| Perfluorohexane sulfonic acid | PFHxS | 432-50-7 | |||
| Perfluorononane sulfonic acid | PFNS | 474511-07-4 | |||
| Perfluorodecane sulfonic acid/sulfonate | PFDS | 335-77-3 |
Table 6.2: Water solubility and vapour pressure for some perfluorinated carboxylates and fluorotelomer alcohols (Heckster et al. 2003; Shoeib et al. 2004a; Toxnet/HSDB; Liu & Lee 2007; Thuens et al. 2007; Kaiser et al. 2006; Stock et al. 2004b).
| Substance name | Acronym | CAS no. | Solubility in water (g/L) | Vapour pressure (Pa) | Octanol-air partition coefficient (Log Koa) |
| Perfluorobutanoic acid | PFBA | 375-22-4 | |||
| Perfluoropentanoic acid | PFPeA | 2706-90-3 | |||
| Perfluorohexanoic acid | PFHxA | 307-24-4 | |||
| Perfluoroheptanoic acid | PFHpA | 375-85-9 | 118 (24°C) | 128 | |
| Pentadecafluorooctanoic acid; Perfluorooctanoic acid | PFOA | 335-67-1 | 9.5 3.4 4.3 (24oC) |
70 20 (25 ºC) |
|
| Branched perfluorooctanoic acid | 90480-55-0 | ||||
| Ammonium perfluorooctanoate | PFOA-, NH4+ APFO | 3825-26-1 | >500 374 (24°C) |
<1.3-9.2 x 10-3 | |
| Sodium perfluorooctanoate | PFOA-, Na+ | 335-95-5 | 169 (24°C) | ||
| Perfluorononanoic acid | PFNA | 375-95-1 | |||
| Perfluorodecanoic acid | PFDA | 335-76-2 | 0.26 (24°C) | ||
| Perfluoroundecanoic acid | PFUnA | 2058-94-8 | 0.092 (24°C) | ||
| Perfluorododecanoic acid | PFDoA | 307-55-1 | |||
| Perfluorotridecanoic acid | PFTrA | 72629-94-8 | |||
| Perfluorotetradecanoic acid | PFTeA | 376-06-7 | |||
| 1H,1H,2H,2H-Perfluorohexanol | 4:2 FTOH | 2043-47-2 | 0.97 (22oC) | 992 | 3.3-4.8 (25oC) |
| 1H,1H,2H,2H-Perfluorooctanol | 6:2 FTOH | 647-42-7 | <1.2-1.7 x 10-2 1.9 x 10-2 (22oC) |
713 | 3.6 -5.3 (25oC) |
| 1H,1H,2H,2H-Perfluorodecanol | 8:2 FTOH | 865-86-1 | 1.40 x 10-4 1.1 x 10-5 (22oC) |
254 | 4.2-5.6 (25oC) |
| 1H,1H,2H,2H-Perfluorododecanol | 10:2 FTOH | 678-39-7 | 6,0 x 10-6 – 8,9 x 10-4 (22oC) |
144 | 4.8 -5-5 (25oC) |
| 1H,1H,2H,2H-Perfluorotetradecanol | 12:2 FTOH | 39239-77-5 | 5.3-5.6 (25oC) | ||
| 1H,1H,2H,2H-Perfluorohexadecanol | 14:2 FTOH | 60699-51-6 | 6.2 (25oC) |
For PFOA the octanol-water partition coefficient (Log Pow) is 5, but the special solubility profiles and surface-active properties of PFOA (and other perfluorinated acids) make environmental fate predictions based on octanol-water partition coefficients irrelevant for these chemicals. Water is believed to be the target compartment for perfluorinated acids in the abiotic environment.
Perfluorinated carboxylic acids (PFCA) are stronger acids than their non-fluorinated counterparts and have the corresponding lower pKa. For PFOA the pKa is 2.80 (Kissa 2001).
6.1.3 Degradation
Because of the very strong C-F binding, the perfluoroalkyl chain is extremely resistant to heat, UV-radiation, chemical attacks by acids and bases, or reducing and oxidizing agents. The functional groups (sulfonamides, esters, alcohol etc.) at the other end of the molecule will be more readily transformed in the environment and in organisms, and the more complex molecules will gradually be degraded to the ultimate, perfluorinated sulfonates and carboxylates, which under normal circumstances seem to persist in the environment for foreseeable time.
The 175 polyfluorinated substances on a list developed by Canadian authorities were studied with a computer program simulating microbial degradation. The prediction was that 109 substances might be precursors and be degraded to PFOS and 61 to PFOA (Dimitrov et al. 2004).
6.1.3.1 Abiotic degradation
Fluorinated organic polymers are very stable to hydrolysis resulting in half-lives from 1-5 years to 500 years. However, heating of fluoropolymers such as poly[tetrafluoroethylene] (PTFE) to more than 350oC, degradation in smaller molecules occurs, including a small (0.01%) formation of PFOA (Ellis et al. 2001).
Yamada et al. (2005) investigated the thermal degradation of a small polyester/cellulose fabric substrate treated with a fluorotelomer-based acrylic polymer under laboratory conditions conservatively representing typical combustion conditions of time, temperature, and excess air level in a municipal incinerator, with an average temperature of at least 1000 oC and 2 seconds residence time. The fabric was destroyed by this treatment, and no PFOA was detected, only SiF4. The authors concluded that under typical municipal waste incineration conditions no significant quantity of PFOA would be formed from the incineration of a textile or paper substrate treated with a fluorotelomer based acrylic polymer, even without consideration of post-combustion pollution control equipment for acid gas scrubbing in place at municipal incinerators. This conclusion is however questionable. Actual waste incineration is performed in another and larger scale and is inhomogeneous and less controlled.
Photodegradation of PFOS by UV irradiation in air is not yet confirmed experimentally but in both water and alkaline 2-propanol slow photodegradation occurred in the laboratory tests; mostly (ten times more) in the alkaline solvent (rate constant: 0.93 days-1). The resulting compounds are mixtures of shorter chain compounds by stepwise removal of CF2 (Yamamoto et al. 2007).
Perfluorocarboxylic acids including PFOA were effectively photodegraded after 4 hrs in water containing persulfate (S2O82-) (Hori et al. 2005).
PFOS and related fluorochemicals are almost completely decomposed in the laboratory using zerovalent iron in subcritical water for six hours forming a. o. CHF3 and F- (Hori et al. 2006).
A few minutes ultrasonic irradiation of PFOS and PFOA in aqueous solution in the laboratory degraded both chemicals to perfluoroalkyl substances with a shorter chain length (Moriwaki et al. 2005).
6.1.3.2 Degradation in air
A prevailing hypothesis on the origin of non-volatile perfluorinated compounds such as PFOS and PFCAs in remote places is that volatile precursors, among others, substituted sulfonamides for PFOS and fluorotelomer alcohols (FTOH) for PFCAs, undergo long-range transport and hereby reach remote areas (Ellis et al. 2004).
The volatile compounds are transport vehicle for the non-volatile fluorinated acids. In the air the precursors will by time degrade to the stable and water-soluble PFCAs, which are washed out of the air or deposited to the surface. Atmospheric lifetime of short chain FTOHs, as determined with its reaction with OH-radicals, was approximately 20 days making the molecule able to travel about 7000 km. A possible chemical reaction (many steps) in the atmosphere is:
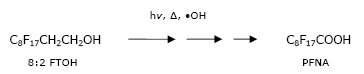
Smoke chamber experiments with fluorotelomer alcohols (4:2, 6:2 and 8:2 FTOH) exposed to chlorine atoms, as a surrogate for OH-radicals, indicated that these chemicals in the atmosphere can oxidise/degrade to series of perfluorinated carboxylic acids (Ellis et al. 2004). The yields from 8:2 FTOH were mainly PFNA (1.6%) and PFOA (1.5%) and to a lesser extend shorter chain acids.
The atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol (MeFBSE), a short-chain alternative to PFOS derivatives, was studied by reaction with OH radicals (Martin et al. 2006; D’Eon et al. 2006). The atmospheric life time of this basic chemical was estimated to 2 days; however, it was only degraded to the sulfonamide MeFBSA, which again had an atmospheric life time of >20 days. The ultimate degradation products were perfluorobutane sulfonic acid and perfluorobutanoic acid, short-chain acids which are not considered very bioaccumulative.
6.1.3.3 Biodegradation
Dinglasan et al. (2004) examined the aerobic biodegradation of the 8:2 telomer alcohol (8:2 FTOH) using a mixed microbial system. The initial measured half-life of the 8:2 FTOH (purity 97%) was 0.2 days/mg of initial biomass protein. Volatile and non-volatile metabolites were identified and quantified. The telomer acids: CF3(CF2)7CH2COOH (8:2 FTCA) and CF3(CF2)6CF=CHCOOH (8:2 FTUCA) and PFOA were identified as major metabolites. The telomer acids may further degrade to PFNA and PFOA.
In a laboratory test using a microbial enrichment culture aerobic degradation of 8:2 FTOH was observed (Schröder 2003). Telomer acids and PFOA were identified as metabolites. 85% of the telomer alcohol was degraded after a week. The half-life was about one day. No aerobic degradation of either PFOS or PFOA derivatives was observed. However, microbiological degradation of PFOS and PFOA in contaminated sludge occurred under anaerobic conditions. PFOS was biodegraded more easily than PFOA. The former compound disappeared within 2 days and the later in 25 days. The degradation products were not identified (Schröder 2003).
In another test activated sludge from a domestic sewage plant was used as degradation medium for 8:2 FTOH (Wang et al. 2005a). After 28 days 35% was transformed to unsaturated (5%) and saturated C10 acids (27%) and PFOA (2.1%). This study was followed by a more comprehensive biodegradation study in mixed bacterial culture and activated sludge medium conducted up to 4 months (Wang et al., 2005b). The authors reported the presence of 3 new metabolites along with other five metabolites previously reported. It was found that strong adsorption to the activated sludge greatly reduced partitioning of 8:2 FTOH or any transformation product to air. It was also shown that replenishment of organic carbon enhanced microbial mineralization of multiple –CF2- groups. After 90 days a 12% total mineralization of 8:2 FTOH was observed. Fiebig et al. (2007) found that 8:2 fluorotelomer alcohol also was biodegraded under anaerobic conditions in digested sludge. The main degradation product was, however, an 8:2 fluorotelomer acid.
Researchers at the Clariant Company have found that 8:2 FTOH undergoes biotransformation in soil to oxidation products, including PFOA. The half-life was calculated to 28 days. Bound to a commercial acrylate type polymer this telomer was not released or degraded in an aerobic soil test for one year. Traces of PFOA detected originated from degradation of fluorotelomer residuals in the polymer (Koch et al. 2007).
Biodegradation of 8:2 FTOH in soil by two strains of Pseudomonas has been recently investigated by Liu et al. (2007). These authors observed that transformation of 8:2 FTOH to PFOA was highly dependent on the presence of other easily metabolized carbon sources. However, they concluded that other microbes directly able to utilize 8:2 FTOH as carbon source may exist in the environment.
6.1.3.4 Conclusion
PFOS, PFOA and other perfluoroalkylated acids/salts are very stable chemicals; however, under extreme laboratory conditions with addition of potent chemicals, high-energy radiation and high temperatures some degradation products are formed. However, these chemicals are not biodegradable and will persist in the environment.
The case is different with functional derivatives (e.g. substituted sulfonamides and esters) of these perfluorinated acids and with other polyfluorinated substances not fully fluorinated such as fluorotelomer alcohols. These polyfluorinated substances are somewhat degradable in the environment and will slowly but finally be transformed to the basic perfluorinated acids, which will persist.
A prevailing hypothesis on the origin of non-volatile perfluorinated compounds such as PFOS and PFCAs in remote places is that volatile precursors undergo long-range air transportation and hereby reach remote areas.
6.2 Environmental fate and levels
6.2.1 Environmental release
Emissions of polyfluorinated chemicals to the environment may happen either directly from production and processing plants, or during product use. PFOA is mainly emitted from production of fluoropolymers, such as PTFE (e.g. Teflon®), and from use of products containing PFOA as an impurity.
Production processes and use of fluorotelomers (impregnation, fire-fighting etc.) may emit polyfluorinated substances, which degrade into the persistent perfluorinated acids in the environment. Therefore, polyfluorinated substances are determined in the environment primarily in the form of the final stable degradation products PFOS, PFOA and higher PFCAs. Se also section 6.1 above.
Fluorinated polymeric materials used for textiles, carpets etc. may also release residual amounts of the telomer that failed to be covalently linked to the polymer during production, or the polymeric material may decomposes itself by heat or release polyfluorinated substances by wearing.
Armitage et al. (2006) estimated that between 2700 and 5900 tons of polyfluorinated compounds were emitted to the environment between the years 1950 and 2004. The largest single source contribution (~72% of the total) originated from fluoropolymer manufacturing, followed by ammonium perfluorooctanoate manufacturing (~12% of total) and fluoropolymer dispersion processing (~7% contribution). Direct sources were in this study estimated to be approximately an order of magnitude larger than indirect sources (emissions from product application of telomers etc.).
The historical industry-wide emissions of total PFCAs from direct- and indirect sources were estimated by Prevedouros et al. (2006) to be 3200-7300 tonnes in the period from 1950 to 2004.
The overall emissions from the global fluorotelomer industry have been estimated to contribute approx. 1-2% of the PFCAs in North American rainfall, considered consistent with previous global emissions estimates (Yarwood et al. 2007).
6.2.2 Levels in the ambient air and precipitation
6.2.2.1 Levels in North America
Six different polyfluorinated substances (EtFOSE, MeFOSE, EtFOSA, 4:2 FTOH, 6:2 FTOH, 8:2 FTOH and 10:2 FTOH) have been detected in the air at a highly urbanized site of Toronto, Canada, in 2001. Tropospheric concentrations typically range from 7 to 106 pg/m³ and from 14 to 393 pg/m³. The mean concentrations ranged from 14 pg/m³ for EtFOSA to 205 pg/m³ for EtFOSE, and totally 260 pg/m³. At a rural site (Long Point) in Canada the levels were 2-3 fold less (74 pg/m³) and only one substance (EtFOSE) was found (Martin et al. 2002). In samples from warm summer periods more PFAS was in the gas phase than in the colder seasons.
Stock et al. (2004a) measured in 2001 perfluoroalkyl sulfonamides and fluorotelomer alcohols in the ambient air in six North American cities (Reno (NV), Griffin (GA), Cleves (OH), Winnipeg (MB), Long Point (ON) and Toronto (ON)). Mean concentrations of total perfluoroalkyl sulfonamides (EtFOSA, MeFOSE and EtFOSE) ranged from 22 pg/m³ in Winnipeg to 403 pg/m³ in Griffin. Mean concentrations of total FTOHs (6:2, 8:2 and 10:2) ranged from 11 pg/m³ in Winnipeg to 165 pg/m³ in Toronto. Surface treatment products, previously manufactured by 3M Company for soil, stain and water protection of home furnishings – including carpets, were primarily MeFOSE-based polymers. It corresponded with that the highest mean and single levels of MeFOSE in the air (359 pg/m³ and 1549 pg/m³) were measured in Griffin, a location of carpet production.
A study of atmospheric levels of perfluoroalkyl substances in the Canadian Arctic (Cornwallis Island) in the summer of 2004 was published by Stock et al. (2007). Mean values of gas plus particle phase concentrations of FTOHs (observed in 20-50% of samples) ranged from 2.8 pg/m³ for 10:2 FTOH to 14 pg/m³ for 8:2 FTOH. Levels of sulfonamide derivatives were higher with a total mean concentration of 112 pg/m³. Mean concentrations of PFOSA and MeFOSE were 20 pg/m³ and 29 pg/m³, respectively. Mean concentration of other perfluorinated compounds (EtFOSE, EtFOSA, MeFBSE and EtFBSE) ranged from 11-23 pg/m³. The high concentration of the two PFBS precursors may be caused by the increasing use of perfluorobutane sulfonamide derivatives as substitutes for perfluorooctane sulfonamide derivatives. In the air particulates the non-volatile perfluorinated acids were also detected in this study. The highest observed concentration was that of PFOS (mean 5.9 pg/m³) followed by PFOA (mean 1.4 pg/m³); PFNA and PFDA had both mean concentrations 0.2 pg/m³.
Samples from the Global Atmospheric Passive Sampling (GAPS) study from 40 sites collected in December 2004 to March 2005 were screened for four PFAS (MeFOSE, EtFOSA, EtFOSE and MeFOSEA) (Lee et al. 2007). MeFOSEA was not detected in any sample. For 42% of the sites the other three substances were not detected. In 70% of the sites EtFOSE and EtFOSA were not detected. Most abundant was MeFOSE with the highest concentration of 280 pg/m³ in Athens, Georgia, a region, where carpet production occurred.
6.2.2.2 Levels in Japan
The content of PFOS in airborne dust collected along Japanese roads was up to 427 ng/g. The air concentrations in cities ranged 0.1-2.1 pg/m³ in a rural town and 2.3 -22 pg/m³ in an urban city (Sasaki et al. 2003). Higher concentrations were observed in summer than in winter.
PFOA and PFOS were measured in atmosphere particulate matter at two stations in the Kyoto area, Japan (Harada et al. 2005b). FOA concentrations were significantly higher than PFOS concentrations and ranged between 72 and 919 pg/m³. PFOS concentrations ranged between 2.5 and 9.8 pg/m³.
Shoeib et al. (2004ab) measured three perfluoroalkyl sulfonamides (MeFOSE, EtFOSE and MeFOSEA) used in surface treatment formulation for textile and paper products to impact oil and water resistance in outdoor air. Levels of MeFOSE and EtFOSE in outdoor air were 16-32 pg/m³ and 8.5-10 pg/m³, respectively. MeFOSEA was not detected.
6.2.2.3 Levels in Germany
Jahnke et al. (2007a) carried out a study on the occurrence of airborne PFAS in the gaseous and particulate phase of air samples taken in spring 2005 at a metropolitan site in Germany (Hamburg) as well as in a rural area (Waldhof). A wide distribution of fluorotelomer alcohols (4:2 FTOH, 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH), fluorinated sulfonamides (MeFOSA and EtFOSA), and -sulfonamidoethanols (MeFOSE and EtFOSE) in air was found, and all of the detected compounds belonged to the group of volatile precursor compounds of PFOS and PFOA. The ?FTOHs concentrations ranged from 64-311 pg/m³ (mean 181 pg/m³) in the rural area to the higher levels 150-546 pg/m³ (mean: 288 pg/m³) in Hamburg. The most abundant compound was 8:2 FTOH. The sum of PFOS precursors was in the range 8-68 pg/m³ (mean 34 pg/m³) in Waldhof and in the range 13-171 pg/m³ (mean 68 pg/m³) in Hamburg. A significant correlation was found with the ambient temperature for the partitioning of airborne FOSEs between the gaseous and particulate phase, whereas FTOHs and FOSAs were almost exclusively found in the gaseous phase.
6.2.2.4 Measurements during ship cruises
In 2005 an icebreaker ship cruising in the Arctic from the North Atlantic to the Canadian Archipelago was equipped with a high volume air sampler, and each day during 6-27 July an air sample (particles and gas phase) was collected from the different places during the expedition and later analysed for 3 fluorotelomers and 3 perfluorooctane sulfonamide derivatives (Shoeib et al. 2006). In the twenty 24-h air samples one of the chemicals (MeFOSEA) was not detected at all (<0.001 pg/m³), and 6:2 FTOH was only found in the gas phase with levels ranging <1.1-5.98 pg/m3 and with an arithmetic mean of 2.65 pg/m³. Levels of the two fluorotelomers alcohols (8:2 FTOH and 10:2 FTOH) in the gas phase ranged 4.16-22.7 pg/m³ (mean 11.4 pg/m³) and 1.45-16.4 pg/m³ (mean 6.27 pg/m³), respectively, and in the particle phase levels were in the range 1.07-8.37 pg/m³ (mean 3.50 pg/m³) and 0.29-1.27 pg/m³ (mean 0.80 pg/m³), respectively; thus the levels were much higher in the gas phase. Levels of the two sulfonamides present (MeFOSE and EtFOSE) were also higher in the gas phase and were in the range <1.9-23.6 pg/m³ (mean 8.30 pg/m³) and 1.0-5.17 pg/m³ (mean 1.87 pg/m³), respectively. In the particle phase the levels were <1.7-15.0 pg/m³ (mean 3.53 pg/m³) and <0.001-5.5 pg/m³ (only detected in 25% of samples; mean 1.05 pg/m³), respectively. The results from the remote regions were compared to the results of three samples from Toronto. The results from Toronto samples for 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, MeFOSE and EtFOSE in gas phase ranged between 12.4 and 7.2 pg/m³ (mean 17.7 pg/m³), 25.1-59.6 pg/m³ (mean 40.2 pg/m³), 12.0-36.1 pg/m³ (mean 21.1 pg/m³), 5.38-11.8 pg/m³ (mean 8.0 pg/m³) and 1.04-3.01 pg/m³ (mean 2.33 pg/m³), respectively. In the particle phase the levels were lower, and the results for 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, MeFOSE and EtFOSE were in the range 0.20-0.42 pg/m³ (mean 0.31 pg/m³), 0.30-1.31 pg/m³ (mean 0.71 pg/m³), 0.42-1.82 pg/m³ (mean 1.09 pg/m³), 2.67-6.51 pg/m³ (mean 4.20 pg/m³) and 0.40-1.68 pg/m³ (mean 0.96 pg/m³), respectively. These findings confirmed model predictions of atmospheric transport of perfluorinated chemicals to the Arctic.
Polyfluorinated alkyl substances (6:2 FTOH, 8:2 FTOH, 10:2 FTOH, EtFOSA, MeFOSA, EtFOSE, and MeFOSE) in 8 air samples have been measured on board a German research ship during a cruise from Bremerhaven to Cape Town in South Africa (Jahnke et al. 2007b). The study showed that airborne PFAS are mainly restricted to the Northern Hemisphere with a maximum concentration of 190 pg/m³ of 8:2 FTOH in the first sample collected in the Channel. That was ten times higher than south of Equator. Ionic PFOA and PFOS were determined in the particulate phase of the first sample but at almost two orders of magnitude lower levels; the maximum observed levels were 2.0 and 2.5 pg/m³ for PFOA and PFOS, respectively.
Barber et al. (2007) analysed PFAS in air samples from 4 field sites in Europe (rural, semi-rural and urban). They found that the prevailing substances found were the fluorotelomers 8:2 FTOH and 6:2 FTOH. These volatile PFAS were ubiquitous in air samples, in the gas phase at 5–243 pg/m³ and 5–189 pg/m³, respectively. The concentrations were several orders of magnitude higher in indoor air than outdoor air, making homes a likely important diffuse source of PFAS to the atmosphere.
6.2.2.5 Levels in wet precipitation
Six fluorotelomer carboxylic acids, which are transformation/oxidation products of telomer alcohols, were analysed in rainwater collected in July 2004 in the city of Winnipeg, Canada (Loewen et al. 2005). Low ppt levels of C10- and C12-FTCA and –FTUCAs (oxidation products of 8:2 and 10:2 FTOH) were detected indicating that FTOH may be removed from the atmosphere by oxidation and wet deposition. The concentration of PFOS in rainwater was 0.59 ng/L but none PFCAs was detected.
Perfluoroalkyl carboxylates (PFCAs), fluorotelomer carboxylates (FTCAs) and fluorotelomer unsaturated carboxylates (FTUCAs) were determined in wet only precipitation samples from nine sites in North America (Scott et al. 2006). Significantly higher concentrations of PFOA were found at 4 northeastern United States and 2 southern urban Canadian sites (range: 0.6-89 ng/L). 8:2- and 10:2-FTUCA were detected at all 4 U.S. sites (<0.07-8.6 ng/L).
Barton et al. (2007) have shown that PFOA exists primarily in the particulate phase and is efficiently scavenged by rain droplets, making wet deposition an important removal mechanism from the atmosphere.
6.2.2.6 Conclusion
The polyfluorinated substances identified in air have mainly been the volatile PFOS precursors MeFOSE and EtFOSE, and fluorotelomer alcohols (FTOH) precursors of PFCAs. In urban areas levels were often 10 times higher than in rural areas and remote background levels further 10 times lower. In cities with carpet production the levels were highest (up to 1500 pg/m³). Concentrations observed in the summer were higher than in the winter. PFOS and PFCAs are not volatile and were only found in low concentrations in particulate matters.
6.2.3 Levels indoors
Indoor air levels of perfluorinated chemicals are especially high and may be up to 100 times higher than outdoors, and since the modern individual spends much time indoor, this exposure will be most important for the human body burden of PFCs (Renner 2004). Indoor air may also act as a key source to these chemicals to the outside environment (Shoeib et al. 2007). It is important to note that PFOS, PFOA and other acids and salts have a low volatility and will concentrate in the dust, whereas the volatile polyfluorinated chemicals such as sulfonamides and telomers will dominate in the particle phase in air. Some indoor levels determined in various parts of the World are presented in the following sections.
6.2.3.1 Indoor air levels
Shoeib et al. (2004a) measured in indoor air with passive sampling three perfluoroalkyl sulfonamides (MeFOSE, EtFOSE and MeFOSEA) used in surface treatment formulation for textile and paper products. Levels in indoor air from 4 houses and an old laboratory ranged 667-8315 pg for MeFOSE/m³ (geom. mean 2590 pg/m³) and 289-1917 pg for EtFOSE/m³ (geom. mean 770 pg/m³). Indoor levels of MeFOSEA in three of the houses were 5-283 pg/m³. Indoor air levels were about 25 times higher than outdoor values.
The high indoors air levels of perfluoroalkyl sulfonamides compared to outdoors levels were also confirmed in another study by the same research group. Shoeib et al. (2004b) found by using passive air samplers 25 times higher levels indoors of MeFOSE (geometric mean 1968 pg/m³) and 13 times higher levels of EtFOSE (geometric mean 1033 pg/m³). Results for EtFOSA (geometric mean 54 pg/m³) and MeFOSEA (geometric mean 38 pg/m³) were a magnitude lower. The mean levels indoors were 20-100 times higher than outdoor levels.
Updates of these studies of PFOS derivatives were published by Shoeib et al. (2005a, 2007). The results of 59 air samples are summarized in Table 6.3.
Table 6.3: PFOS derivatives in indoor air (Shoeib et al. 2005a, 2007).
| Indoor air (pg/m³) passive sampling | ||||
| Substance | No. samples with detectable levels | Range | Mean | Median |
| MeFOSE | 59 | 366 - 8190 | 1970 | 1490 |
| EtFOSE | 59 | 227 - 7740 | 1100 | 744 |
| EtFOSA | 52 | 6- 646 | 59 | 40 |
| MeFOSEA | 10 | 12 - 109 | 35 | 29 |
| 8:2 FTOH | 59 | 261-28600 | - | 2070 |
| 10:2 FTOH | 59 | 104-9210 | - | 891 |
Barber et al. (2007) analysed air samples from indoor locations in Norway. Perfluorooctanoate (PFOA) was often the predominant compound found in the particulate phase at concentrations ranging from 1–818 pg/m³.
6.2.3.2 Levels in window film
One of the major sources to fluorinated chemicals indoors is newly impregnated carpets. In Canada levels of perfluoroalkyl contaminants have been measured in inner window film before and after installation of a carpet. The total concentration of contaminants in the carpeted room increased 2.4-fold from 5.62 pg/cm² window before to 13.4 pg/cm² two months after. The major compounds were PFOA followed by PFOS, PFDS and PFTeA (Gewurtz et al. 2007).
6.2.3.3 Levels in house dust
Vacuum cleaner dust from Japanese homes contained between 11 and 2,500 ng PFOS/g dust (mean: 200 ng/g; median: 24.5 ng/g) and between 69 and 3,700 ng PFOA/g dust (mean: 380 ng/g; median: 165 ng/g), respectively. PFOA levels were in general higher than PFOS levels. The highest concentrations of both were in the same sample, and there was an association between PFOS and PFOA in all samples, which indicates a common source (Moriwaki et al. 2003).
Shoeib et al. (2005ab & 2007) analysed both PFOS derivatives and PFOA precursors (fluorotelomer alcohols) in house dust from homes in Ottawa, Canada, sampled in the winter 2002-2003. The results of 66 dust samples are summarized in Table 6.4.
Table 6.4: PFCs in indoor dust (Shoeib et al. 2005ab, 2007).
| House dust (ng/g) | ||||
| Substance | Samples with detectable levels | Range | Mean | Median |
| PFOS | 66 | <1 - 5063 | 443 | 59 |
| MeFOSE | 66 | 3 - 8860 | 412 | 113 |
| EtFOSE | 66 | 1.4 - 75440 | 2200 | 138 |
| EtFOSA | 0 | - | - | - |
| MeFOSEA | 16 | 0.7 - 44 | 14 | 8 |
| PFHxS | 66 | <1 - 4305 | 391 | 46 |
| PFOA | 66 | <1 - 1234 | 106 | 18 |
| 6:2 FTOH | 66 | 2-2500 | 156 | 33 |
| 8:2 FTOH | 66 | 3-16315 | 410 | 55 |
| 10:2 FTOH | 66 | 2-8176 | 233 | 35 |
Levels in paired air and dust samples from the same homes correlated indicating same source for air and dust contamination. Surprisingly EtFOSA was not detected in dust although it has been found in indoor air.
Older homes had lower levels of PFOA and PFOS in the dust, and houses with more carpeting on the floors had higher levels (Zhu et al. 2005).
A series of ionic PFCs was measured in dust from 67 Canadian homes (Kubwabo et al. 2005). The data revealed a correlation between the concentrations of PFCs and the percentage of carpeting in the house.
6.2.3.4 Conclusion
PFOS, PFOA and other acids and salts have a low volatility and will concentrate in the house dust, whereas the volatile polyfluorinated chemicals, such as sulfonamides and telomers, will concentrate in the indoor air, mainly in the particle phase.Indoor air levels of perfluorinated chemicals may be up to 100 times higher than outdoors, and PFC levels in house dust are also high. Levels up to 75 ppm have been determined. A main source seems to be impregnated carpets. Indoor exposure may sometimes be the most important human source of exposure and body burden of PFCs. Indoor air may also act as a key source to these chemicals to the outside environment.
6.2.4 Occurrence in remote area and long-range transportation
The transport of PFOS, PFOA, other perfluorinated acids and their precursors to remote regions, such as the Arctic, is a puzzle for the scientific community (Simcik 2005). Local use in remote regions of fluorosurfactants in fire-fighting foams (airports, fuel storage) and other products may constitute a source of PFOS and PFOA but cannot explain the high level of contamination. Neither can movement of contaminated wildlife. The binding to water and the low volatility make it less likely that PFOS and PFOA themselves will be transported through long distances by the "grass-hopping" and cold condensation mechanisms as persistent organic pollutants (POPs). Nevertheless, the occurence in the Arctic wildlife indicate that polyfluorinated chemicals are distributed widely in the environment and may be transported over very long distances.
6.2.4.1 Contribution from transport with ocean water
Perfluorinated compounds in waters seem to be most concentrated in the surface foam; whether alone in an aqueous solution or with another co-surfactant it is not known. That suggests that marine aerosol (foam) transport should be considered an important long-range transport mechanism (Kaiser et al. 2006).
PFOA directly emitted from polymerization processes, waste water plants, etc. is expected to be present in the environment almost entirely as the dissociated acid/salt. With its negligible vapor pressure, high water solubility and surfactant properties, accumulations in surface waters and to particulate matter herein are likely.
Based on actual concentrations measured in open ocean water, the annually flux of perfluorooctanoic acid (PFOA) to the Arctic was calculated to be between 2 and 12 tons (Prevedouros et al. 2006a; Stock et al. 2007). However, this flux was not considered by the authors to contribute significantly to the observed biota contamination, as evidence suggests that transport from the Atlantic into the Arctic Ocean will be at a depth greater than 200 m.
Using another model a net flux of approx. 8-23 tons per year of PFOA was estimated to flow into the Northern Polar zone in 2005, and that was 20-60 times greater than the amount estimated to be deposited from the global emission, distribution, and degradation of 8:2 FTOH (Armitage et al. 2006).
Transport of PFOA to the Arctic via the ocean takes on the order of decades (20-30 year) as a result of the time required for extremely persistent chemicals to redistribute throughout the oceans (Armitage et al. 2006). In addition, oceanic rates of exchange between northern temperate oceans and the Arctic are likely to be different for the Atlantic and Pacific Oceans which could lead to varying time lags depending on the major oceanic transport routes for PFOA to the Arctic. This means that a delayed response in the Arctic to emission reductions in industry countries will be observed. If concentrations in the primary exposure media (i.e., surface water) continue to increase due to direct sources, it follows that concentrations in wildlife will also continue to increase long after emissions have been drastically reduced or even eliminated.
6.2.4.2 Contribution from transport with air
It was estimated by Ellis et al. (2004), based on prevailing air concentrations, that atmospheric oxidation of FTOHs gives rise to a (C8-C10) PFCA flux in the range 1-100 t/yr in North America or a deposition of 40-4100 ng/m².
Newer results for the calculation of the globally distribution of PFOA using the IMPACT 3-D chemistry/transport model analysis, suggest that telomers and their degradation products are ubiquitous in the Northern Hemisphere (Wallington et al. 2006; Nielsen and Andersen 2007). The concentrations of 8:2 FTOH and its degradation products are typically a factor of five lower in remote oceans and Arctic locations in the Northern Hemisphere, and it is consistent with an atmospheric lifetime of 20-40 days for the group as a whole, which makes them able to travel more than 7000 km. Telomer species in the remote Northern Hemisphere in the model are one-third primary 8:2 FTOH, one-third long-lived fluorine-containing aldehydes, and one-third terminal reaction products. PFOA is also ubiquitous in the Northern Hemisphere. Using estimated emissions of FTOHs based on air concentrations and the three-dimensional model, 0.4 t/yr of perfluorooctanoic acid was calculated to be deposited at latitudes north of 65°N via atmospheric oxidation. The modeling results do not prove that the atmospheric oxidation of 8:2 fluorotelomer alcohol is the source of PFCAs observed in remote locations. However, the results show that with current estimates of chemistry and flux, the atmospheric oxidation of 8:2 FTOH can provide a quantitative explanation for the presence of PFCAs in remote regions.
Young et al. (2007) described the investigation of high Arctic ice caps to determine seasonal cycles, temporal trends, and atmospheric fluxes in order to illuminate the source of PFOA to the Arctic. They found high fluxes for total PFCAs (PFOA and PFNA dominated) averaging 313 kg in 2004 and 651 kg in 2005. These fluxes agree with those determined through modeling of FTOH degradation by Wallington et al. (2006). The flux for PFOS was a little above 30 kg/year in 2005. PFOS levels were 10 times higher, when they peaked in 1999. That is opposite to the levels of PFCAs, which increase at a constant rate.
6.2.4.3 Comparisons of atmospheric and aquatic input
Wania (2007) carried out a model simulation with the zonally averaged global fate and transport model Globo-POP, in combination with historical emission estimates for FTOHs and PFOA, in order to evaluate the relative efficiency and importance of the two transport pathways (air and sea) of direct and indirect PFOA sources. The author used a global model that considers and contrasts both transport hypotheses with the objective to evaluate their relative efficiency and importance, building on the model used by Armitage et al. (2006). It also needed to be confirmed that reductions of the emissions of volatile precursor compounds would indeed result in fast declines in Arctic seawater concentrations. Estimates of the emission-independent Arctic Contamination Potential reveal that the oceanic transport of directly emitted PFCAs is more than 10-fold more efficient than the atmospheric degradation of FTOHs in delivering PFCAs to the Arctic, mostly because of the low yield of the reaction. The cumulative historic emissions of FTOHs are lower than those estimated for PFOA alone by a factor of 2-3, further limiting the contribution from precursor oxidation.
A focused investigation of environmental media (water and soil) was performed by Davisa et al. (2007) in order to understand the pathways for transport of ammonium perfluorooctanoate (APFO) from a manufacturing plant. These results led to a conceptual model that indicates deposition of airborne material as a primary contributor to aquatic and terrestrial systems near the facility.
6.2.4.4 Conclusion
Simcik and Dorweiler (2005) proposed pathways for environmental distribution of perfluorochemicals and compared atmospheric and non-atmospheric sources to surface waters (see Figure 6.1).
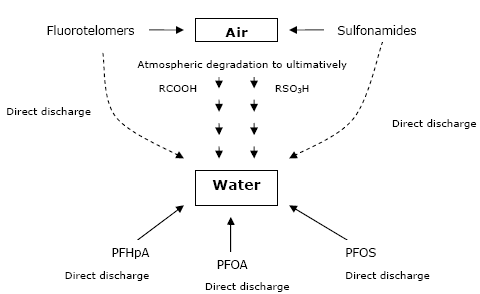
Figure 6.1: Pathways to environmental distribution of perfluorochemicals (modified after Simcik and Dorweiler 2005)
They concluded that “urban” waters, such as Lake Michigan, received most input from direct discharge from waste water treatment effluents, while remote surface waters did receive most perfluorinated chemicals from atmospheric deposition of degradation products. The ratio of PFHpA to PFOA increased with increasing distance from non-atmospheric sources and could be used as an indicator.
According to Ellis et al. (2004) and Wallington et al. (2006), atmospheric transport and degradation of 8:2 FTOH is the most plausible explanation for the fast response to changes in production and changing concentrations of PFOA in ice cores. The findings of the non-commercial PFDA and PFUnA with similar concentrations argue against the deposition of direct transported PFOA but for contamination by atmospheric oxidation. Additionally, these studies state that PFOA levels in the Arctic are too high to be explained by oceanic transport. This is, however, in disagreement with the studies by Armitage et al. (2006) and Wania (2007). Thus, presently no consensus exists in the scientific community regarding the most important transport pathways and environmental fate of PFOA.
6.2.5 Levels in environmental waters, groundwater and drinking water
Contamination sources of aquatic ecosystems from PFCs can mainly be identified as direct discharge from production of fluorochemicals, effluents from wastewater treatsment plants (WWTPs) and accidental discharge of e.g. fire-fightings foams containing fluorochemicals. Atmospheric transport also contributes to contamination of the aquatic environment. In the case of accidental discharge relatively large concentrations of PFCs may occur in surface water or groundwater (concentration level: µg/L). Otherwise, PFCs concentrations in oceanic water or surface waters are in the concentration range ng-pg/L. At these concentrations no toxicity of these compounds is observed. However, these data are important to identify the sources of PFCs in the environment and to understand the global circulation of these compounds in the aquatic environment.
A very sensitive analytical method allowed the detection of PFCs at sub-ppt levels in oceanic water collected during several international research cruises undertaken during 2002-2004 in the central to eastern Pacific Ocean (19 locations), north and mid Atlantic Ocean (12 locations), south China and Sulu Seas (5 locations) and the Labrador Sea (20 locations) (Yamashita et al. 2005). An additional 50 samples of coastal seawater from several Asian countries were analyzed. PFOA was the major contaminant detected in oceanic waters, followed by PFOS. The concentrations of PFOS and PFOA in coastal waters were about a factor 1000 higher than those from oceanic waters.
In a general screening of polar compounds in surface and drinking water from northern Italy, Loos et al. (2007) found PFCs concentrations between 0.2 and 8.1 ng/L. Concentrations in tap water were very close to those found in lake water. Since the tap water was obtained from processing of lake water, the contamination of drinking water from PFCs originates directly from the contamination of lake water. This indicates that perfluorinated surfactants are at present not successfully removed by water treatment steps. Much higher concentrations of PFCs were found in drinking water obtained by surface water treatment in the Rhine-Ruhr region in Germany (Skutlarek et al. 2006). These authors found up to 598 ng/L total PFCs concentration in drinking water and up to 4385 ng/L total PFCs in surface water.
Surface water contamination by PFCs was also found in the Cape Fear drainage basin in North Carolina, USA (Nakayama et al. 2007). One hundred samples from 80 different locations were collected and analysed for 10 target PFCs. Detectable levels of the target compounds were found in all samples with maximum concentration of perfluoroheptanoic acid (C7) of 329 ng/L.
A total of 14 PFCs were quantified in river water samples collected in the Pear River basin (south China) and the Yangtze River basin (central China) (So et al. 2007). PFOS was the dominant compound found in samples from the Pear River basin, while PFOA was the predominant compound found in the Yangtze River basin. Considerable amounts of perfluorobutane sulfonate (PFBS) or 22.9-26.1% of total PFCs analysed, were found at a specific location, indicating local sources of this particular compound. Different PFC profiles were observed in the two basins, indicating the presence of dissimilar sources in the two regions.
The discharge of C6-C9 perfluorinated carboxylates (PFCAs) was studied and employed to assess European emissions of these compounds (McLachlan et al. 2007). PFCAs were determined in water collected to the mouth of 14 major European rivers, including the Rhine, Elbe, Danube, Oder, Seine, Loire and Po. The highest PFOA discharge was found for river Po (Italy) and the authors suggested that these concentrations were due to industrial sources. PFOA concentrations in the lower ng/L range were found for the other rivers, indicating widely distributed sources as significant contributors to the PFCs emissions to waters in Europe.
In conclusion, the contamination of surface water from PFCs may pose an important intake source of PFCs for the consumer when the surface water is used for production of drinking water. Direct contamination of groundwater from accidental spill has not been reported recently. Particularly contaminated surface waters (e.g. some rivers in central Europe) significantly contribute to discharge of PFCs in seas and oceans.
6.2.6 Levels in wastewater and sludge
In the past two years there has been more focus on investigation of mass flow of PFCs in municipal and industrial wastewater plants, since these have been recognized as important point source to the diffuse pollution of the aquatic environment from PFCs.
The origin and amount of PFCs in six wastewater treatment plants (WWTP) and their transformation during treatment have been evaluated by measuring these compounds in influent, effluent, and river water at the point of WWTP discharge in Iowa City (USA) (Boulanger et al. 2005). PFOS and PFOA were quantified in effluent (26±2.0 and 22±2.1 ng/L, respectively) and river water (23±1.5 and 8.7±0.8 ng/L, respectively). The biotransformation of EtFOSE was also investigated in order to determine whether EtFOSE could be the precursor compound for the presence of PFOS-related compounds after treatment. The results suggested that transformation of precursors is not an important source for PFOS and PFOA compared to direct use and disposal of products containing the end products as residual amounts.
Higgins et al. (2005) developed an analytical method for the analysis of perfluoroalkyl sulfonates, perfluoroalkyl carboxylates, and PFOS precursors (MeFOSAA and EtFOSAA). The method was applied to a limited survey of these compounds in domestic sludge and sediments from the San Francisco Bay Area. The concentration of PFCs in domestic sludge ranged from 5 to 153 ng/g for total PFCAs and from 55 to 3370 ng/g for perfluoroalkyl sulfonates. PFOS precursors were also found in both sediment and domestic sludge at levels often exceeding those of PFOS.
A different method was developed by Schultz et al. (2006a) for the analysis of PFCs in municipal wastewater influents and effluents. The method included perfluoroalkyl sulfonates, fluorotelomer sulfonates, perfluorocarboxylates, and selected perfluoroalkylsulfonamides. The method was applied to analysis of raw influent and final effluent of ten wastewater treatment plants located nationwide in USA. PFCs were observed in wastewater at all treatment plants and each plant exhibited unique distributions of PFCs despite similar treatment processes. In nine out of ten plants, at least one class of PFCs showed increased concentrations in the effluent water. In some plants, decreased concentrations in effluent water were attributed to sorption to sludge.
In a following work, Schultz et al. (2006b) investigated the fate of PFCs in a municipal wastewater treatment facility in USA by analysing raw influent, primary and secondary effluent, and sewage sludge. Precursor of the acidic compounds, such as perfluoroalkyl sulfonamides and 6:2 fluorotelomer sulfonate were included in the analytical program. Mass flows of 6:2 fluorotelomer sulfonate and PFOA were unchanged after wastewater treatment, while a net increase in the mass flow of PFOS and perfluorodecane sulfonate (PFDS) occurred after treatment. Mass flows of perfluoroalkyl sulfonamides and perfluorononanoic acid (PFNA) also increase after activated sludge treatment. The authors concluded that conventional treatment in wastewater plants are ineffective in removing most of PFCs and more data are needed on the precursors such as perfluoroalkyl sulfonamides, perfluoroalkylethanols or fluorotelomer olefins, which could account for the observed increases in fluorochemical mass flow.
In a similar study Sinclair and Kannan (2006) observed an increase in mass flow of several PFCs measured in six wastewater treatment plants from New York State. The authors hypothesize that the observed increase may have resulted from degradation of precursor compounds such as fluorotelomer alcohols, which was supported by a significant correlation in the mass flow of PFOA/PFNA and PFDA/PFUnA. Dominance of the longer carbon chained PFCAs were found in sludge samples, suggesting a preferential partitioning of these compounds to sludge.
On the basis of the results of the studies performed on PFCs in municipal and industrial wastewater plants, it can be concluded that these plant are important point sources for discharge of PFCs into the aquatic environment. While domestic waste appears to be a consistent source of PFOS, commercial waste introduces higher and more variable levels of PFOA. Precursor, such as PFOS-based chemicals, fluorotelomer alcohols and sulfonamides are transformed to PFOS and PFCAs after activated sludge treatment. Several studies provide evidence that PFCs are not removed from wastewater by conventional treatment and therefore their dischare into receiving waters is of particular concern.
6.2.7 Levels in soil and sediments
There are few studies reporting the levels of PFCs in soil and sediment and investigations about the potential for bioaccumulation of PFCs from sediment or soil. Complicating the study of PFC fate in soil or sediment-water systems is the presence of precursors. It is not yet clear whether these precursors can contribute to PFOS bioaccumulation or they are a less bioavailable form of PFOS.
A newly developed analytical method for determination of perfluorocarboxylic acids was applied to a screening of these compounds in harbour sediments from Barcelona, Spain (Alzaga et al. 2005). PFCAs with carbon chain from C8 to C10 were detected in the concentration range 10.4-12.4 ng/g in 50% of the samples taken from the commercial harbour. Lower concentrations (<1.3-2.6 ng/g) were detected in sediments from the marina.
The sorption of three classes of PFCs (perfluorosulfonates, perfluorocarboxylates and perfluorooctyl sulfonamide acetic acids) has been reported by Higgins et al. (2006). Sediment content of organic carbon was the dominant parameter affecting sorption, indicating the importance of hydrophobic interactions. Both the length of the perfluorocarbon tail and the functional group were important to determine sorption. Each CF2 moiety contributed by approximately 0.5-0.6 log unit to the distribution coefficients. The sorption of perfluorosulfonates was on average 1.7 times stronger than the perfluorocarboxylate analogues.
In a follow up study Higgins et al. (2007) assessed the bioaccumulation of the same classes of PFAS from contaminated sediments using the freshwater oligochaete Lumbriculus variegatus. The results suggested that PFCs in sediments are readily bioavailable and that bioaccumulation from sediments does not increase with increasing perfluorocarbon chain length. PFOS and PFBA were the most bioaccumulative PFCs. EtFOSAA accumulated in the worm tissues and appeared to undergo biotransformation to PFOS and other PFOS precursors.
6.2.8 Levels in biota and wildlife
Most of the studies published until 2004 have been focused on concentration of PFCs in selected biota from a specific area/ecotype or on bioaccumulation through the food chain of these compounds. In the recent years several studies have been published on temporal trends of PFAS concentrations from 25-30 years ago to the present year. Several studies have extended their analysis program to new compounds such as fluorotelomer sulfonates and fluorotelomer saturated and unsaturated carboxylates (FTCAs, FTUCAs). The last ones are degradation products of fluorotelomer alcohols (FTOH).
6.2.8.1 Temporal trend studies
One of the first published temporal trend study of PFCs was performed using archived guillemot eggs from the Baltic Sea (Holmström et al. 2005). Samples were collected from Stora Karlsö (Sweden) between 1968 and 2003. PFOA was not detected in any sample (MDL 3 ng/g ww). A 30-fold increase was observed for PFOS concentrations, from 25 ng/g ww in 1968 to 614 ng/g ww in 2003, which corresponded to a 7-11% average increase per year (see Figure 6.1).
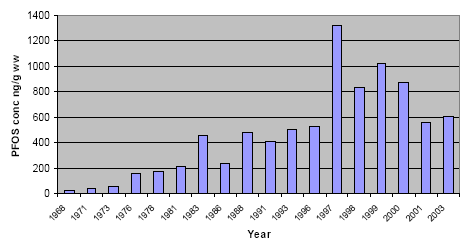
Figure 6.1: Trends of PFOS concentration in Guillemot eggs from Stora Karlsö at Gotland in Sweden (Holmström et al. 2005).
A time trend study of PFCs concentrations was performed in beluga (Delphinapterus leucas) from Baffin Island in the Canadian Arctic in the period 1982-2002 (Tomy et al. 2005). PFOS, PFOSA and PFCAs (C8-C12) were analyzed together with FTCAs, FTUCAs and EtFOSA. The last three groups of compounds were not detected in any sample. Exponential increase in both PFOA and PFDA concentrations were observed with respective doubling times of 6 and 11 years. The concentration profile for both PFOSA and PFOS were similar. PFOS and PFOSA concentrations were found to be increasing up to 2002 although there was a small decrease in concentration in 1995 for both compounds.
Spatial and temporal trends in the concentrations of selected PFCs were measured using archived liver samples of ringed seal (Phoca hispida) from East and West Greenland (Bossi et al. 2005a). The samples were collected in four different years at each location, between 1986 and 2003 in East Greenland and between 1982 and 2003 in West Greenland. PFOS was the major contributor to the burden of PFCs in samples, followed by perfluoroundecanoic acid (PFUnA). Perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) were also detected in most samples. Regression analysis of logarithmic transformed PFOS, PFDA and PFUnA median concentrations indicated a significant temporal trend with increasing concentrations at both locations. A spatial trend in PFOS concentrations was observed between the two sampling locations, with significantly higher concentrations in seals from East Greenland.
Archived polar bears (Ursus maritimus) liver tissue samples from two locations in the North American Arctic were analyzed for PFCs (Smithwick et al. 2006). The study covered the period 1972-2002. The samples originated from an eastern location (Baffin Island, Canada) and a western location (Barrow, Alaska). Concentrations of PFOS and PFCAs with carbon chain length between C9 to C11 showed an exponential increase between 1072 and 2002 at both locations. The doubling time for PFOS was similar to the doubling time of production of perfluorooctylsulfonyl-fluoride-based products in the 1990s. The degradation products of FTOH, 8:2 and 10:2 fluorotelomer acids and their unsaturated acid counterparts, were also analyzed but they were not detected in any sample.
PFOS concentrations in eggs from two colonies of herring gulls (Larus argentatus) from Northern Norway showed a nearly 2-fold increase in the period 1983-1993, followed by a levelling off between 1993 and 2003 (Verreault et al. 2006). The SPFCAs (8 to 15 C) also showed a marked increase in 1983-2003 and only a weak decrease in the period 1993-2003. The accumulation profile of PFCAs was characterized by a higher proportion of odd carbon chain length compounds.
Temporal trends in PFCs concentrations were investigated in liver samples from two ringed seals (Phoca hispida) populations in the Canadian Arctic in the period 1992-2005 (Arviat, West Hudson Bay) and in the period 1972-2005 (Resolute bay, Lancaster Sound) (Butt et al. 2007a). PFAS analysed included C7-C15 perfluorinated carboxylates (PFCAs) and their suspected precursors, the 8:2 and 10:2 fluorotelomer saturated and unsaturated carboxylates (FTCAs, FTUCAs), C4, C6, C8, C10 sulfonates, and perfluorooctane sulfonamide (PFOSA). Perfluoroheptanoate (PFHpA), perfluorohexane sulfonate (PFHxS), perfluorodecane sulfonate (PFDS) were not detected in any sample. Fluorotelomer saturated and unsaturated acids were detected in some individuals, most of the time at concentrations close to the detection limit. The detection of fluorinated telomer acids supports the hypothesis of FTOH as a source of PFCs in the environment. Statistically significant decreasing PFOS concentrations were observed in both populations from 2000 to 2005. According to the authors, these results indicate that the ringed seals and their food web are rapidly responding to the phase out of perfluorooctane sulfonyl fluoride based compounds by 3M in 2001.
Another temporal trend study investigated the concentrations of PFAS in liver samples from two seabird species, thick-billed murre (Uria lomvia) and northern fulmars (Fulmaris glacialis) from the Canadian Arctic (Butt et al. 2007b). In contrast to most other wildlife samples, PFCs profiles were dominated by long-chained perfluorinated carboxylates (PFCAs), mainly in the range C11-C15 PFCAs. PFCs concentrations were found to increase significantly from 1975 to 2003/2004. Again, detection of the 8:2 and 10:2 FTUCAs suggested FTOH as a source of some PFCAs in arctic birds.
Many temporal trend studies on PFOS have shown increasing concentrations over time. Temporal trends studies are particularly useful not only to follow the historical use of PFCs, but also to follow in the future the effect of ceased production and use of PFOS-based products in Europe and North America. In the Arctic regions a different picture is obtained when looking at PFOS concentrations in ringed seals from the Canadian Arctic and Greenland. PFOS is still increasing in ringed seals from Greenland, while a statistically significant decrease was observed in ringed seals from the Canadian Arctic after 2000. This will suggest different sources and transport path to the Arctic regions.
6.2.8.2 Biomagnification studies
Several biomonitoring studies have been conducted in which simultaneous concentrations of PFCs in organisms, water and sediments have been measured, thus enabling to estimate field-based bioaccumulation factors (BAFs). The trophic magnification factor (TMF) has also been determined in several studies performed in different food webs.
Studies involving the biomagnification of PFAS through the food chain involving the analysis not only of biota but also of abiotic matrices from the same environment are reported in this paragraph.
A food web from Lake Ontario was investigated for the presence of PFCs (Martin et al. 2004). The investigation included a top predator fish, forage fish species, and two invertebrates. The highest mean concentration of each PFC was detected in the benthic macroinvertebrate Diporeia hoyi. The observed concentrations were often 10-fold higher than those found in Mysis relicta, a predominantly pelagic feeder. This suggested that the sediment and not the water was the major source for PFCs contamination. Bioaccumulation of PFCs was observed at the top of the food web, with the exception of PFOA.
Trophic transfer of PFCs was examined in Great Lakes benthic food web; in addition, PFCs were analyzed in predatory fish (Chinook salmon, carp and lake whitefish) and in eggs from brown trout from Michigan (Kannan et al., 2005a). PFCs were also analyzed in green frog livers, snapping turtle plasma, mink livers and bald eagle tissue. The calculated bioconcentration factor (BCF) for PFOS concentration in biota/concentration in water was 1000 in benthic invertebrates. Concentrations of PFOS in mink and bald eagles were 5- to 10-fold greater than those in salmon, carp or snapping turtle. Eggs of fish contained notable concentrations of PFOS, suggesting oviparous transfer of this compound. The calculated biomagnification potential of PFOA was lower than that of PFOS.
A preliminary screening of PFOS and related compounds has been performed in liver samples of fish, birds and marine mammals from Greenland and the Faeroe Islands in order to assess the presence and distribution of PFCs in the food chain from these two remote areas (Bossi et al. 2005b). PFOS was found at concentrations above LOQ (10 ng/g wet weight) in 13 out of 16 samples from Greenland and in all samples from the Faeroe Islands. The results from Greenland showed a biomagnification of PFOS along the marine food chain (shorthorn sculpin<ringed seal<polar bear). The greatest concentration of PFOS was found in liver of polar bear from East Greenland (mean: 1285 ng/g wet weight, n = 2).
Distribution of PFCs through the food chain has been investigated in biota from the Southern Ocean and the Antarctic (Tao et al. 2006). In this study, livers from albatrosses, blood from elephant seals, and blood and eggs from penguins and polar skua were analyzed for PFCs. The samples were collected in the period 1995-2005. PFOS was found in all liver samples from albatrosses from the Southern Ocean, while PFOA was found in 30% of the samples. PFOS was also found in the blood of elephant seals from Antarctica at concentrations ranging from <0.08 to 3.52 ng/mL. This study documents the global scale distribution of PFCs.
Occurrence of PFCs was found to be ubiquitous in a study from New York State (Sinclair et al. 2006). This study included the analysis of surface water from the main water bodies of NYS, liver from two species of popular sport fish, smallmouth (Micropterus dolomieu) and largemouth brass (Micropterus salmoides) and liver from both migratory and resident bird species, the totally piscivorous common merganser (Margus merganser) and the highly herbivorous mallard (Anas platyrhyncos). PFOS was the most abundant compound found in all biota, whereas high concentrations of PFOA were found in Hudson River. Significantly higher concentrations of PFOS were found in piscivorous birds than in non-piscivorous birds. An average BCF of 8850 was estimated for PFOS accumulation in fish relatively to ambient water. An average BMF of 8.9 was estimated for the accumulation of PFOS in the fish common merganser.
The fate of PFCs in shallow water marine organisms and a food web represented by sediment-associated tidal flat organisms was investigated in Ariake Sea, Japan (Nakata et al. 2006). In shallow water species, PFOS was the dominant compound, while PFOA was the most abundant compound in tidal flat organisms and sediments, indicating differences in exposure between the two ecosystems.
The environmental distribution and the biomagnification of a series of PFCs were investigated in the food web of the bottlenose dolphin (Tursiops truncatus) near Florida (USA) by Houde et al. (2006). PFCs were analyzed in WWTP effluents, seawater, sediment, zooplankton, whole fish, and plasma from dolphins. The fact that PFCs were found at high concentrations in WWTP effluents suggested that these facilities were sources for PFCs contamination of the food web. Biomagnification factors (BMFs) ranged from <1 to 156. Trophic magnification factors (TMFs) of PFOS were in the range of those predicted for Arctic food webs, indicating that the rate of accumulation of PFOS is not dependent on differences in the food web and climatic conditions.
Concentrations and biomagnification potential of PFCs was investigated in species from the Barents Sea food web, including amphibians, fish and birds (Haukås et al. 2007). PFOS displayed the highest concentrations, with values up to 225 ng/g in glaucous gull liver. Liver based magnification factors displayed values >1 for PFHxS, PFOS, PFNA and SPFCs in the majority of predator-pray relationship. The authors found that the degree of trophic transfer of PFCs was similar to that of PCB, DDT and PBDE.
Based on the empirical laboratory and field data, PFCs with seven or fewer fluorinated carbons are not bioaccumulative and do not possess significant biomagnification potential in food webs. In studies measuring the accumulation of PFCs in aquatic-based food webs, biomagnification of perfluorinated acids has been noted to be several orders of magnitude lower than that of most persistent lipophilic compounds, with PFOS being identified as the only perfluorinated acid exhibiting potential for biomagnification. More research is needed to charachterize the bioaccumulation potential of PFCAs with longer fluorinated carbon chains (>8).
6.2.8.3 Levels in fish and mussels
A study performed in ten north-central Portuguese estuaries evaluated the tissue burden of PFOS in mussel (Mytilus galloprovincialis) and the potential relationship between PFOS content and age/size and sex of the organisms (Cunha et al. 2005). PFOS was observed in all mussels collected with whole body burden concentrations in the range 36.8 – 129 ng/g. Significant different PFOS concentrations were observed in the different body tissues with higher concentrations in haemolimph and digestive gland tissues. Significant differences were also found between mature and non-mature animals, the latter showing significantly lower PFOS levels.
The PFOS concentration in fish liver from freshwater locations in Flanders (Belgium) was correlated to biological endpoints (serum ALT enzyme activity, serum protein content, hematocrit value, serum electrolyte levels, condition and growth rate) (Hoff et al. 2005a). Although some biological endpoints were suggested to be altered by PFOS exposure, no indications were obtained for a decrease in fish condition or reduce growth capacity.
A limited number of mussels and oyster samples from South China and Japan were analyzed in order to test a new developed analytical method for PFCs determination in biota (So et al. 2006). Concentrations ranged from 0.114 to 0.586 ng/g for PFOS, 0.063 to 0.512 ng/g for PFHxS, <0.012 to 0.030 ng/g for PFBS, and 0.038 to 2.957 ng/g for PFOSA. Oyster samples from Tokyo Bay had the greatest concentrations of PFOS and PFOSA.
Perfluorinated carboxylates (PFCAs), perfluorinated sulfonates and unsaturated fluorotelomer carboxylates (8:2 and 10:2 FTUCAs) were measured in lake trout (Salvelinus namaycush) samples from the Great Lakes in Canada (Furdui et al. 2007). The major perfluoroalkyl contaminant observed was PFOS with the highest concentration found in samples from Lake Erie (121±14 ng/g). Perfluorodecane sulfonate (PFDS) was detected in 89% of the samples. The 8:2 FTUCA was detected at concentrations ranging between 0.1 and 0.2 ng/g; with the highest level in samples showing also elevated concentrations of PFOA. The presence of 8:2 FTUCA and PFOA may originate from metabolization of 8:2 fluorotelomer alcohol (8:2 FTOH) as observed in rat metabolism by Martin et al. (2005). The 10:2 FTUCA was detected only in 9% of the samples.
6.2.8.4 Levels in birds
PFCs have been detected worldwide in seabirds and terrestrial birds. The highest PFCs concentrations are found in birds from the industrialized areas. PFCs concentrations in fish-eating birds are usually lower than concentrations measured in fish-eating mammals. This could be due toa different trophic position in the respective foodweb or a shorter elimination half-life in birds.
PFCs levels were investigated in plasma, liver, brain and eggs from adult glaucous gull (Larus hyperboreus) from the Norwegian Arctic (Verreault et al. 2005). PFOS was highest in plasma (48.1-349 ng/g wet weight), followed by liver tissue and egg. PFCAs with 8-15 carbon atoms were found, with the highest concentrations in plasma (sum PFCAs: 41.8-262 ng/g ww). PFBS, PFOSA and the unsaturated fluorotelomer carboxylic acids (FTUCA) were not detected in any sample. The accumulation profiles of PFCAs were characterized by high proportions of the long and odd-numbered carbon-chained-length compounds (C11 and C13).
PFOS was determined in liver and blood of a small song bird, the great tit (Parus major) in the vicinity of a large fluorochemical plant in Belgium (Dauwe et al. 2007). The concentrations of PFOS ranged from 553 to 11,359 ng/g in liver and from 24 to 1625 ng/g in blood. The hepatic concentrations exceeded in most birds the hepatic benchmark concentrations for the protection of avian species (Beach et al. 2006). Near the same fluorochemical plant, Hoff et al. (2005b) found that PFOS significantly affected alanine aminotranferase, triglycerides and cholesterol in plasma of nestling great tits.
6.2.8.5 Levels in aquatic mammals
A greater number of studies have been conducted on marine mammals compared to terrestrial mammals. The studies are often conducted on naturally dead animals (e.g. dolphins and whales), where the concentrations may not be representative for free-ranging animals.
Occurrence and trends of PFCs levels have been investigated in harbour porpoises (Phocoena phocoena) from coastal waters around Iceland, Norway and Denmark, and in the German Baltic Sea (Van de Vijver et al. 2004). Liver samples were collected for a total of 49 individuals. PFOS concentrations ranged from 26 to 1149 ng/g ww. A geographical difference was observed with a decreasing trend in PFCs contamination from south to north, with the highest concentrations in porpoises from the Baltic Sea.
PFCs levels in polar bears (Ursus maritimus) from East Greenland were reported for the first time by Smithwick et al. (2005a). Liver tissue from a total of 29 individuals collected from 1999 to 2001 was analyzed. The mean PFOS concentration (2470 ± 1320 ng/g ww) was similar to Hudson Bay, Canada, and both populations had significantly greater concentrations than those reported for polar bears from Alaska, indicating a spatial trend.
A circumpolar study of perfluoroalkyl contaminants was carried out by analyzing these compounds in liver tissue and blood from polar bears (Ursus maritimus) from five locations in the North American Arctic and two locations in the European Arctic (Smithwick et al. 2005b). PFOS concentrations were significantly correlated with age at four of seven sampling locations, while gender was not correlated to concentration for any compound. Populations in South Hudson Bay (2000-2730 ng/g ww), East Greenland (911-2140 ng/g ww), and Svalbard (756-1290 ng/g ww) had significantly higher PFOS concentrations than western populations (435-729 ng/g ww). Common sources for PFCAs at the same location were suggested by correlating PFCAs with adjacent chain length.
The concentrations of PFCs were compared to those of chlorinated and brominated organic pollutants in two subpopulations of polar bears (Ursus maritimus) from Alaska, the Beaufort Sea and the Chukchi Sea populations (Kannan et al. 2005b). The results of the study indicated differences in concentrations and profiles of organohalogen compounds between the two subpopulations. It was hypothesized that the difference could be due to different exposures from different transport paths of contaminants (from Japan and Southeast Asia or from Russia and Atlantic Ocean). The source of difference could also be due to different feeding habits of the two subpopulations.
The isomer profile of PFCAs was identified in liver tissue from 15 polar bears (Ursus maritimus) from Canadian Arctic and Eastern Greenland (De Silva et al. 2004). The PFOA isomer pattern in Greenland showed a prevalence of branched isomers, while only the linear isomers were found in bears from Canada. The presence of branched isomers suggested some contribution from compounds obtained with electrofluorination (EF), while prevalence of linear isomers suggested the contribution from compounds obtained with telomerisation.
The tissue distribution of PFCs was investigated in harbour seals (Phoca vitulina) from the Dutch Wadden Sea (Van de Vijver et al. 2005). PFCs were analyzed in liver, kidney, blubber, muscle, and spleen tissue. PFOS was the predominant compound with concentrations ranging from 89 to 2724 ng/g wet weight. Perfluorobutane sulfonate (PFBS) was found at concentrations of 2.7 ± 0.7 ng/g ww and only in spleen tissue. Increasing PFOS concentration was observed in the order kidney> liver> blubber> skeletal muscle.
Concentrations of perfluorinated carboxylic acids (PFCAs) and sulfonic acids were determined in plasma of bottlenosed dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean (Houde et al. 2005). PFOS was the predominant compound found at concentrations between 49 ng/g wet weight (dolphins from Bermuda) and 1171 ng/g wet weight (dolphins from Charleston, SC). Fluorotelomer 8:2 and 10:2 unsaturated acids (FTUCAs) were detected for the first time in the concentrations range 0.5-1.4 ng/g.
PFCs were determined in plasma, milk, and urine of free-ranging bottlenosed dolphins (Tursiops truncatus) from Sarasota Bay, Florida (USA) (Houde et al. 2006ab). Samples were taken in the period 2002-2005. Mean seasonal sum of PFCs detected in plasma ranged from 530 to 927 ng/g ww. No significant differences were observed between seasons, indicating a constant exposure to PFCs. Sexually immature calves (age, <10 years) had significantly higher PFC concentrations than their mothers. Analysis of PFCs in milk confirmed the hypothesis that PFCs are transferred through lactation in dolphins. PFCs were also detected in urine samples, indicating that the urinary system is an important path of depuration from PFCs in dolphins.
PFOS and PFOA were measured in liver tissue from 80 female sea otters collected from the California coast during 1992-2002 (Kannan et al. 2006). Concentrations of PFOS and PFOA were in the range <1-884 and <5-147 ng/g wet weight, respectively. PFCs concentrations were compared among the otters that died from infectious diseases, non-infectious causes, and from apparent emaciation. Concentrations of PFOS and PFOA were significantly higher in sea otters with the infectious disease category than in the non-infectious category. Moreover, PFOA concentrations were found to increase from 1992 to 2002, whereas PFOS concentrations increased from 1992 to 1998 and then decreased after 2000.
In a recent paper Van de Vijver et al. (2007) investigated the distribution of PFCs in liver, kidney, muscle, brain, and bubble in harbour porpoises (Phocoena phocoena) from the Black Sea. PFOS concentrations were highest in liver (327 ± 351 ng/g ww) and kidney (147 ± 262 ng/g ww) tissues. Concentrations in muscle, brain and blubber were about a factor 10 lower. Shorter carbon-chained perfluorinated carboxylic acids, such as perfluorobutanoic acid (PFBA) and PFOS were not detected. PFOS concentrations were comparable to those found in porpoises from the German Baltic Sea.
Polar bears and bottlenose dolphins from the United States have the highest PFCs concentrations. Generally, the concentrations of PFCs in top predators can reach a level which may be close to some measurable effects. Moreover, these species are already exposed to other persistent compounds (e.g. PCBs). The presence of PFCs at high concentrations may constitute an additional treathen to these species through additive and synergistic effects with the other contaminants.
6.2.8.6 Levels in terrestrial animals
Few monitoring studies have been conducted on terrestrial mammals. Those species living close to contaminated areas can reach extremely high PFCs concentrations, as observed by Hoff et al. (2004). In this study a concentration of 180 µg/g ww of PFOS was measured in mice inhabiting the area close to a perfluorochemical plant.
PFCs were measured in blood of captive red panda and giant panda from eight cities from six different provinces in China (Dai et al. 2006). PFOS was the predominant compound in all samples with concentrations ranging from 0.80 to 73.80 ng/ml for red panda and from 0.76 to 8.2 ng/ml for giant panda. Concentrations of PFOS and PFOA were positively correlated. No age- or sex-related differences were observed in PFC concentrations. The serum of those individual living close to urbanized areas had the highest PFC concentrations.
With the exception of those species living close to fluorochemicals production plant, the levels of PFCs observed in terrestrial mammals are in the same range as those measured in marine mammals.
6.3 Ecotoxicity
Most ecotoxicity studies with PFCs have used aquatic organisms and have published only in internal reports from the laboratories of 3M and DuPont. Based hereupon it was concluded in the previous assessment that PFOS was moderate acute toxic and slightly chronic toxic to aquatic organisms, and that PFOA was practically non-toxic (Poulsen et al. 2005).
A fish species, fatheaded minnow (Pimephales promelas) was exposed for 39 days to varying concentrations of perfluorooctanoic acid (PFOA) under microcosm conditions (Oakes et al. 2004). The PFOA concentrations used in this study were much higher than those normally found in the aquatic environment. However, no mortality was associated to exposure to these concentrations. Significant declines in circulating plasma steroids were observed, but these were accompanied by only limited increases in time to first oviposition and decreases in overall egg production. Effects such as peroxisome proliferation and oxidative stress were also observed but at low levels. The authors concluded that PFOA is relatively non toxic at environmentally relevant concentrations, but that biochemical and reproductive endpoints might be affected.
The toxicity of PFOS and PFOA was evaluated for the aquatic midge, Chironomus tentans (MacDonald et al. 2004). The median lethal concentration (LC50) for PFOS calculated during a 10 days laboratory test was 45.2 µg/l, while the median effective concentration (EC50) was 27.4 µg/l. A parallel test performed with PFOA in the same concentration range (0.1 to 100,000 µg/l) did not produce any effect. A chronic life test was performed for PFOS in the concentration range 1-100 µg/l. Three of the four endpoints measured - survival, growth, and emergence - were significantly affected with EC50 values of 92.2, 93.8, and 94.5 µg/l, respectively. Reproduction was not affected.
The marine mussel Mytilus californianus was used as a test organism to determine the toxicity of PFCs with respect to the multixenobiotic resistance (MXR) mechanism (Stevenson et al. 2006). This mechanism acts as a first cellular defence against different xenobiotics. The most studied transporter involved in the MXR mechanism is the p-glycoprotein. The function of the MXR mechanism can be compromised by the presence of some xenobiotics. This effect is called chemosensitization. Four of the nine PFCs studied showed chemosensitizing behaviour, with PFNA and PFDA having the highest values.
Recently, the toxicity of precursor compounds of PFCAs (saturated and unsaturated fluorotelomer carboxylic acids, FTCA and FTUCA) has been tested to Daphnia magna, Chironomus tentans, and Lemna Gibba (Phillips et al. 2007). It was observed that the toxicity increased with increasing chain length. In addition FTCA were more toxic than FTUCA. The acute E50 ranged from 0.025 mg/l for D. magna to 63 mg/l for C. tentans. The toxicity threshold found for these compounds is a factor 10.000 lower than those calculated for PFCAs.
One of the few avian toxicology studies revealed for bobwhites a reproductive lowest observable adverse effect level (LOAEL) of 10 ppm in the diet or 0.77 mg PFOS/kg bw/day for male and female birds (Giesy and Jones 2004). This corresponded to 8.7 mg PFOS/mL and 4.9 mg PFOS/g in female serum and liver, respectively, and to 141 mg PFOS/mL and 86 mg PFOS/g in male serum and liver. In mallards the LOAEL was the same (10 ppm) for males but 50 ppm for females.
At the concentrations usually found in the environment, PFCs will not induce acute toxic effects. Generally, the toxicity of PFCs is species dependent and sometimes gender-dependent for the same species. It is therefore difficult to perform risk assessment for these compounds on the basis of the few published studies. The toxicity of the precursors to the perfluorinated acids has been found to be much higher than the toxicity of the acids selves. Further research is needed in order to clarify this aspect, as well as the occurrence of the precursor compounds in the aquatic environment.
6.4 Environmental risk assessment
An environmental risk assessment for PFOS has been performed for the Environmental Protection Agency in England (Brooke et al. 2004). The aim of the study was to review the risks arising from current uses of PFOS-related substances. Risk evaluation was performed for the following compartments: aquatic (surface water and sediments), terrestrial compartment, atmospheric compartment, and marine environment. Moreover, non compartment-specific effects relevant to the food chain (secondary poisoning) were examined. The predicted no-effect-concentration (PNEC) for the aquatic and terrestrial environments is 25 µg/l. The measured levels of PFOS in surface waters and the water concentrations calculated after discharge from a wastewater treatment plant (WWTP) were below the calculated PNEC. The calculated emission to waste water which would give rise to a risk for the terrestrial compartment is 7.4 g/day. This is for applications of sludge over 10 years; the emission which would give rise to a risk following a single application is 0.67 kg/day. No data were available for risk assessment for the atmospheric compartment. For non- compartment-specific effects relevant to the food chain (secondary poisoning) the PNEC for secondary poisoning was 0.0167 mg/kg ww in food. An alternative PNEC of 0.067 mg/kg was also used. These values were applied for risk assessment of both aquatic and terrestrial food chains. A comparison of the PNEC value with measured concentrations for freshwater fish showed that the highest measured concentrations reported exceed the PNEC of 0.0167 mg/kg. A number of values also exceed the alternative PNEC value of 0.067 mg/kg. The emissions to waste water giving rise to a risk for the terrestrial food chain were estimated to be in the range 1.65 – 3.30 g/day. These are for 10 years of application; the emissions giving a risk from a single application of sludge were estimated to be 15 – 30 g/day. The authors concluded that the major area of concern was the secondary poisoning in the aquatic environment, for both freshwater and marine environments.
7 Human exposure
- 7.1 Consumer products
- 7.2 Air exposure
- 7.3 Non-stick cookware
- 7.4 Paper impregnation and migration into food
- 7.5 Food intake
- 7.6 Drinking water
- 7.7 Overall exposure scenarios
The pathways leading to general human exposure to polyfluorinated chemicals, including PFOS, PFOA and telomers are not well-known (Butenhoff et al. 2006).
7.1 Consumer products
The contribution from consumer products containing these chemicals may be significant. PFOA and other polyfluorinated chemicals can be part of products either
- because the product is treated with fluorinated compounds due to intentional application, or
- in form of an unintended impurity, or
- due to degradation of precursor compounds such as FTOHs.
It is not always possible to distinguish between these cases, since recipes of technical applications are mostly confidential or the actual composition of the used mixture of active compounds confidential.
Impregnation of all-weather-clothes, textiles, tents, footwear, furniture and carpets are among the most important uses of polyfluorinated chemicals. Direct skin exposure from product use or inhalation of aerosols from impregnation spray cans may occasionally be an important route of exposure but it is difficult to quantify, however, high concentrations of perfluorinated compounds have been measured in indoor air and dust.
A study performed by Dinglasan-Panlilio and Mabury (2006), indicates the potential for a significant amount of fluorinated alcohols to be released as residuals from a suite of fluorinated materials that are industrially applied and commercially available and hence contribute substantially to the atmospheric burden of FTOHs. It was determined that the examined fluorinated materials contained 0.04-3.8% residual polyfluorinated telomer alcohol (dry mass basis). The seven materials were:
- Polyfox-L-diol (not commercial available) in which the residuals were about 65% 8:2 FTOH and 35% 10:2 FTOH.
- Teflon Advance (a carpet stain repellent for home use), in which the residuals were about 55% 8:2 FTOH and 30% 10:2 FTOH and 15% 6:2 FTOH.
- Zonyl FSO-100 (industrial fluorosurfactant for paint, polish and other coatings), in which the residuals were about 55% 6:2 FTOH, 25% 8:2 FTOH and 15% 10:2 FTOH.
- Zonyl FSE (industrial fluorosurfactant for paint, polish and other coatings), in which the residuals were about 50% 6:2 FTOH, 45% 8:2 FTOH and 5% 10:2 FTOH.
- Motomaster Windshield Washer fluid with Teflon, in which the residuals were about 50% 6:2 FTOH and 40% 8:2 FTOH, and 5% 10:2 FTOH.
- 8:2 Methacrylate (monomer), in which the residual was about 100% 8:2 FTOH.
- 3M Scotchgard Rug and Carpet Protector, in which the residual was about 100% MeFOSE, and therefore must be an old product.
This study also suggests that direct exposure of the general population to these compounds is plausible, if these materials are applied in homes and are out gassing after treatment of surfaces, including carpet, textiles, or paper products. Metabolism of these volatile precursors could then lead to the perfluorinated acids detected in human blood samples worldwide.
7.2 Air exposure
The daily intake of PFOS from air exposure in Japan was calculated to 10-100 pg/day, which would result in 1.2-12 ng/L excess of plasma PFOS levels (Sasaki et al. 2003).
The potential exposure of humans from indoor air of these chemicals was calculated in the study of Shoeib et al. (2005b); see Table 7:
Table 7: Calculated intake (ng/d) of PFOS derivatives via inhalation and intake of dust (Shoeib et al. 2005b).
| Exposure route | Rate | 10 Percentile | Median | 90 Percentile |
| Inhalation (male) | 20 L/min | 17 | 41 | 127 |
| Inhalation (female) | 19 L/min | 16 | 39 | 119 |
| Inhalation (child) | 13 L/min | 12 | 27 | 82 |
| Intake of dust (adult) | 100 mg/d | 5 | 20 | 412 |
| Intake of dust (child) | 200 mg/d | 10 | 44 | 825 |
The results show that adults will inhale more PFOS, and children had a higher intake with dust. Based on body weight, children had a 5-10 times higher PFOS intake than adults. Concerning telomer alcohols the child intakes were estimated to 4.9, 8.0 and 4.6 ng/d for 6:2, 8:2 and 10:2 FTOH, respectively.
7.3 Non-stick cookware
The chemical resistance, non-stick properties and thermal stability of fluoropolymers (polytetrafluoroethylene, PTFE, Teflon) have lead to several applications in dental practice and consumer products. The most publicly well-known occurrence of perfluorinated chemicals is probably PFOA as impurity in the non-stick surface layer of fluoropolymer treated cookware, such as frying pans. DuPont, the producer of the polymer, has detected PFOA content of 4–75 µg/kg in PTFE cookware (Begley et al. 2005). However, another study by DuPont of fluoropolymer treated cookware could, however, not at all detect PFOA under simulated cooking conditions (Powley et al. 2005).
In a recent study by Sinclair et al. (2007) gas-phase release of PFOA, 6:2 FTOH, and 8:2 FTOH were measured from heating new nonstick frying pans. PFOA was reported to vaporize at 189 °C and decompose at >234 °C. PFOA, 6:2 FTOH and 8:2 FTOH were released into the gas phase at 7-337 ng (11-503 pg/cm²), <10-97 ng (<15-204 pg/cm²) and 40-298 ng (42-625 pg/cm²), respectively, per pan from four brands of nonstick frying pans during first use. This suggests that residual PFAS is released from the PTFE coating to the gas phase under the normal cooking temperatures. Gas-phase concentration of PFAS varied depending on the frying pan brand, which suggests that the sintering conditions (temperature, pressure, and duration) used in the coating of fluoropolymers may have an influence on the release of PFAS. In addition, PFOA was detected in water boiled for 10 minutes in nonstick pan brands. A pan in stainless steel did not release any PFAS at even higher temperature.
Larsen et al. (2005) detected small amounts of PFOA (up to 140 ppb) in extracts of PTFE, obtained after applying pressure and increased temperatures to the material. A later study by The Norwegian Institute of Public Health (2007) goes in further detail about these findings. In a worst case scenario, the new study showed that an adult human would be exposed to 66 ng PFOA/kg bw, when drinking 100 ml water cooked in a Teflon coated pan. It was, however, surprisingly concluded that even at an assumption of 100% uptake of PFOA that being no essential intake route for humans.
7.4 Paper impregnation and migration into food
Perfluorochemicals are used to treat paper to improve its moisture and oil barrier properties. In particular, papers used in contact with high-fat content foods and feeds tend to be treated with fluorotelomer or fluorotelomer-based paper additives/coatings to prevent oil stains or oil soak through the paper. Food wrappers may be an important source of perfluorinated chemicals in humans (Renner 2007b).
Typically, these fluorotelomer paper coatings/additives are either very low molecular weight fluorotelomers, which are mixtures of C6-, C8-, C10- and C12-perfluorinated chemicals, or high molecular weight polymers with fluorotelomer-based side chains (Begley et al. 2005; D’Eon and Mabury 2007).
Fluorotelomer-based paper coating/additive formulations may before application onto paper have very high PFOA content (88-160 mg/kg), but during normal application rates this amount of PFOA will be diluted by about 300 times on the final paper product (Begley et al. 2005).
Before 2000 for example, a Canadian study of fast food composites revealed that more than 55% of the composites contained N-ethyl perfluorooctane sulfonamide (EtFOSA). The highest level measured (23.5 mg/kg) was in a pizza. The degradation product or impurity PFOS was also detected in three samples. Most samples after 2000 were free of these contaminants, because fluorotelomers have since substituted PFOS (Tittlemier et al. 2003, 2006).
A well-known occurrence of perfluorinated chemicals and telomers is as greaseproof additive (mg quantities) in microwave popcorn bags, from which the chemicals can be released gradually during microwave heating and leak into the food or found in the vapors (Sinclair et al. 2007). One of the three brands investigated did release 16 ng PFOA, 223 ng 6:2 FTOH and 258 ng 8:2 FTOH. Another did not release anything. In the highly contaminated brand the packaging paper contained seven PFCAs (PFPeA, PFHpA, PFOA, PFNA, PFDA, PFUnA and PFDoA) and the two telomers in concentrations from 0.4-4.7 ng/cm² before cooking.
7.5 Food intake
Food may also be polluted by PFCs via the environment. A study from Poland (Falandysz et al. 2006) reports high level of perfluorinated chemicals in cod and eider duck from the Baltic, and people from Gdansk, who eat much fish from the Baltic Sea had about three times higher levels of perfluorinated chemicals in their blood than a reference population.
7.6 Drinking water
A Japanese study has shown that consumption of drinking water obtained from a polluted river may lead to a significantly increased daily intake of 0.2-1 mg PFOS/day and may contribute 8-16 µg PFOS/L to blood serum levels and result in a 25-50% rise in normal levels (Harada et al. 2003). The New Jersey Department of Environmental Protection has recently recommended a drinking water guidance level of 0.04 µg/L (Renner 2007a).
7.7 Overall exposure scenarios
In a recent PhD thesis the total average internal exposures to PFOS and PFOA in humans were assessed by using a Scenario-Based Risk Assessment (SceBRA) (Horowitz 2007). The modelled average total internal exposure to PFOS was in the range of 17 ng/kg bw/day (adults) to 66 ng/kg bw/day (infants). The pathway contributing most to the internal exposure to PFOS in adults was ingestion of food (>98%). The modelled internal exposure to PFOA is in the range of 1 ng/kg bw/day (adults) to 4 ng/kg bw/day (infants). For PFOA the pathway ingestion of food is also the main contributor (>75%) to the total internal exposure. Oral exposure from hand-to-mouth contact with carpets and incidental ingestion of dust contribute to some extent to the exposure of infants, toddlers, and children. The study concludes that the exposures modelled with SceBRA and exposures deduced from measured blood serum levels correspond well for PFOS, but for PFOA the modelled exposures are one order of magnitude lower than the exposures deduced from the measured blood levels. Possible explanations are either overlooked exposure pathways or contribution from precursor compounds, which have not been included in the Horowitz study.
8 Levels of polyfluoroalkylated substances in human tissues
The physical-chemical properties of perfluorinated chemicals cover a large spectrum. The most abundant in the environment and in Man are the ultimate degradation products of sulfonamides and telomers: Perfluoroalkyl sulfonates and perfluoroalkyl carboxylates. These chemicals have in contrary to most other persistent organic pollutants (POP) a low affinity to lipids in adipose tissues but bind to proteins in cell membranes. The accumulation in the body will therefore mainly be in protein rich parts such as blood and internal organs such as liver, kidney and spleen. A comprehensive review discussing monitoring and toxicological findings for perfluoroalkyl acids has recently been published (Lau et al. 2007).
The quantitative determination of perfluorinated chemicals in environmental and human samples is not an easy task. It includes various extraction and cleanup steps before instrumental analysis. An interlaboratory survey showed great variations in results from various sample types between laboratories (van Leeuwen et al. 2006).
8.1 Levels in human milk
Although human milk mainly concentrates lipophilic pollutants, perfluorinated chemicals have also been found in human milk, however, in much lower levels than in blood. Development of solid phase extraction methods made it recently possible to analyse these low levels in milk. In the first pilot study of two human milk samples from USA only PFPA (1.56 ng/mL) in one sample and PFHxA (0.82 ng/mL) were detected (Kuklenyik et al. 2004).
In a later study from China of 19 individual human milk samples extremely low concentrations of 6 fluorinated chemicals were found in all samples and three others in a few samples (So et al. 2006b). A summary of the data is in Table 8.1.
The relatively high concentrations of PFHxS and PFUnA are remarkable. The first should as a C6-compound not undergo bioaccumulation, and the latter may be a degradation product from 10:2 fluorotelomers. Intakes of fish were positively correlated with the levels of perfluorinated chemicals in human milk. At the greatest exposure the daily intake of PFOS+PFOA in a breastfed infant was calculated to 47 ng/kg bw and evaluated as a potential health risk to the infant.
Table 8.1: Perfluoroalkyl compounds in human milk in China (So et al. 2006b).
| No. of samples with detectable content | Average concentration (pg/mL) | Range (ng/L) | |
| PFBS | 2/19 | 2.0 | <1.0-2.5 |
| PFHxS | 19/19 | 20.5 | 4.0-100 |
| PFOS | 19/19 | 121 | 45-360 |
| PFHxA | 1/19 | 7.6 | <1.0-7.6 |
| PFHpA | 7/19 | 4.9 | 2.6-6.7 |
| PFOA | 19/19 | 106 | 47-210 |
| PFNA | 19/19 | 18.1 | 6.3-62 |
| PFDA | 19/19 | 7.2 | 3.8-15 |
| PFUnA | 19/19 | 19.0 | 7.6-56 |
The newest study is from Sweden (Kärrman et al. 2007). Only PFOS and PFHxS were present in all human milk samples. In nine composite samples (pooled milk samples from many individual mothers in various cities, mainly Uppsala) from the years 1996-2004 showed levels in the period of PFOS levels were from 0.123 to 0.258 ng/mL and levels of PFHxS were from 0.016 to 0.051 ng/mL. PFOA was not detected in these samples but PFNA was detected in three samples. The study also included a matched comparison between levels in milk and blood serum from 12 mothers. The mean levels of PFOS in serum and milk were 20.7 and 0.201 ng/mL, respectively, thus 100 times lower levels in human milk than in blood. The highest PFOS level in human milk in this study was 0.465 ng/mL; if an infant has a daily intake of 150 mL milk/kg bw that will correspond to a daily intake of 70 ng perfluorinated chemicals/kg bw – somewhat above a preliminary reference dose of 25 ng/kg bw/day.
A pilot study in Germany included 19 fresh human milk samples from Munich and 38 archived samples from Leipzig (Völkel et al. 2007). The levels for PFOS ranged between 28 and 309 pg/mL with a median of 119 pg/mL. In addition, 13 frozen milk samples from Gyor, Hungary, were analysed. The PFOS levels were higher and ranged 96-639 pg/mL with a median of 330 pg/mL. PFOA was only detected in 11 out of 70 samples in which the levels ranged 201-460 pg/mL (detection limit: 200 pg/mL).
In another small German study of 12 pooled milk samples from the Hannover area collected in 2000-2004 neither PFOS, PFOSA nor PFHxS were detected (limit 0.1-0.4 ng/mL). On the other hand, surprisingly high levels of PFHxA (range 7.5-22.7 ng/mL) and PFOA (range 4.1-12.7 ng/mL) were found (Suchenwirth et al. 2006).
Although the levels of PFCs in human milk are relatively low the exposure of these chemicals to the breast fed infant may be significant, however, the low levels in milk compared to blood make blood samples better for monitoring use.
8.2 Levels in human blood
In the blood perfluorinated chemicals are mainly bound to serum proteins, especially albumin (Jones et al. 2003b). In most studies blood serum is analysed but other studies analyse whole blood or blood plasma. When comparing such studies it is important to take into account that results will depend on what medium is analysed. In a Japanese study concentrations of PFOS were measured in whole blood and serum from the same persons. It showed that levels in serum were two to three times higher (range: 19-41 ng/mL; mean: 27 ng/mL) than in whole blood (range: 5-14 ng/mL; mean 11 ng/mL) (Taniyasu et al. 2003). Another study by Ehresman et al. (2007) showed that the plasma to serum ratios for PFHxS, PFOS and PFOA were 1:1 independent of concentration level. The serum/plasma to whole blood ratio, regardless the anticoagulant used, was approximately 2:1. The difference between plasma, serum and whole blood corresponds to volume displacement by red blood cells, which are not attracting these chemicals. Red blood cells contribute in general to 41-45% of the weight of blood.
8.2.1 Occupational exposure
Perfluorinated chemicals in human blood have for decades been detected in occupationally exposed individuals and occupational levels may be 100-1000 times higher than levels in the general population. For example in a worker handling the ammonium salt of PFOA the blood contained up to 71 µg organic fluorine/ml blood (Ubel et al. 1980). Later it was found that serum PFOS concentrations among production workers in PFOS-related processes averaged 0.5-2 ppm (mg/L) and ranged <0.1-12 ppm (Olsen et al. 2003ab).
The initial mean blood serum concentrations of PFOS, PFHxS and PFOA in a group of 24 retired fluorochemical production workers and sampled in 1998 were 799 ng/mL for PFOS, 290 ng/mL for PFHxS, and 691 ng/mL for PFOA (Olsen et al. 2007a).
8.2.2 General population exposure
There are two production methods to synthesise PFCAs. The electrochemical fluorination process result in a mixture of linear and branched isomers but the telomerisation process will only result in linear isomers. The isomer distribution of perfluorocarboxylates (PFCAs) in human blood may be used to explain the exposure source.
PFCs are attached to serum proteins, and serum has higher levels of contamination than whole blood. Therefore, it is best to monitor these chemicals using blood serum or plasma. Never the less in some studies whole blood were analysed instead of blood serum. Such results are not directly comparable, as whole blood levels will be 2-3 times lower than serum levels.
8.2.2.1 USA levels
In the first study of 65 commercial available human serum samples the average level of PFOS was 28.4 ng/mL, with a range of 6.7-81.5 ng/ml; PFOA and PFHxS were quantified in half of the samples and PFOSA only in one sample and at much lower levels (Hansen et al. 2001).
Blood serum from 31 American (age range: 5-74 years) were analysed for fluorochemicals by Olsen et al. (2003a). PFOS was determined in all but one serum sample and concentrations ranged between <6.1 to 58.3 ng/mL. The mean of the positive samples for PFOS was 18.3 ng/mL, and the mean serum to liver ratio was 1.3. In blood serum the mean levels for PFOA, FOSA and PFHxS were respectively 3.1, 4.5 and 2.4 ng/mL. Only a few samples had levels of FOSA and PFHxS above the quantification limits.
A larger study characterised PFOS and six other related fluorochemicals in serum samples taken in 2000-2001 from 645 adult American blood donors from cities in six states (Olsen et al. 2003b). The levels ranged from the lower limit of determination of 4.1 to 1,656 ng/mL (ppb) with a geometric mean of 34.9 ng/mL. The mean was a little higher among males than females but no substantial difference was observed by age. The levels of the other fluorochemicals were one magnitude lower. The means of PFOA, PFHxS, PFOSAA (N-ethyl perfluorooctane sulfonamidoacetate, oxidation product of EtFOSE) and M570 (oxidation product of perfluorooctane sulfonamidoethanol) in the six cities were 4.3-5.3, 1.8-2.2, 1.6-2.1 and 1.3-1.4 ng/mL, respectively. PFOSAA and M570 were considered markers of consumer-related exposure.
In a study of an elderly population (238 persons; ages 65-96) in Seattle, the range of PFOS in the blood was <3.4-175 ng/mL with a geometric mean of 31 ng/mL (Olsen et al. 2004). This was considered inside the normal range (30-40 ng/mL) for non-occupationally exposed individuals. The oldest individuals had slightly lower concentrations but no differences between sexes were noted. In the study levels of PFOA, PFHxS, PFOSAA and M570 ranged <1.4-16.7 ng/mL (mean: 4.2 ng/mL), <1.4-40.3 ng/mL (mean: 2.2 ng/mL), <1.6-21.1 ng/mL (mean: 1.5 ng/mL) and <1.0-6.6 ng/mL (mean: 1.2 ng/mL), respectively.
Serum samples collected in July 2003 from 20 adults from Atlanta, GA, all contained PFOS (mean: 56 ng/ml; range: 3.6-164 ng/mL), PFOA (mean: 4.9 ng/mL; range: 0.2-10.4 ng/mL), PFHxS (mean: 3.9 ng/mL; range: 0.4-11.2 ng/mL), PFNA (range: 1.3-4.4 ng/mL) and MeFOSA-AcOH (range: 0.4-5.2 ng/mL). PFOSA, PFDA, PFUnA and EtFOSA-AcOH were determined in even lower levels in most samples but not in all (Kuklenyik et al. 2004).
The levels of PFOS and PFOA in 100 serum samples from year 2000 and 40 plasma samples collected in 2005 from blood donors in Minneapolis-St. Paul were compared in a new study of Olsen et al. (2007b) in order to check, if the production stop of PFOS had any effect on blood levels. It showed that the geometric mean for PFOS of 33.1 ng/mL in 2000 decreased to 15.1 ng/mL or the half in 2005; the same did happen for PFOA with a mean of 4.5 ng/mL in 2000 and 2.2 ng/mL in 2005.
Calafat et al. (2006a) have measured 11 perfluorinated chemicals in 23 archived, pooled serum samples collected from 1990-2002 in various places in the country. The concentrations of PFOS ranged 13.8-56.5 ng/mL (median 31.1 ng/mL), of PFOA 2.8-23.7 ng/mL (median 11.6 ng/mL) and of PFHxS <0.3-3.1 ng/mL (median 2.0 ng/mL).
In another study of 54 pooled serum samples collected from 1832 participants of the 2001-2002 National Health and Nutrition Examination Survey (NHANES) mean concentrations of PFOS among non-Hispanic white male and females were 40 ng/mL and 24 ng/mL, respectively, and higher than for both non-Hispanic males (18 ng/mL) and females (18 ng/mL) or Mexican American males (14 ng/mL) and females (11 ng/mL) (Calafat et al. 2006b). A similar difference was seen for PFOA but at lower levels; among non-Hispanic white male and females 7 ng/mL and 4 ng/mL, respectively, and higher than for both non-Hispanic males (3.6 ng/mL) and females (2.85 ng/mL) or Mexican American males (3 ng/mL) and females (2 ng/mL). In a later study the same research group investigated 1562 NHANES serum samples from the earlier years 1999-2000 for other variables than sex and ethnicity as determined earlier (Calafat et al. 2007a). The conclusion was that higher education was associated with higher PFAS levels but age had no influence.
In a follow-up study 2094 serum samples from the NHANES 2003-2004 were analysed, and PFOS, PFOA, PFHxS and PFNA were detected in >98% of the samples (Calafat et al. 2007b). Geometric mean concentrations in 2003-2004 were significant lower than in 1999-2000 for PFOS (32%), PFOA (25%) and PFHxS (10%) but average level of PFNA had increased 100%.
8.2.2.2 Levels in Canada
A pilot study analysed PFOS, PFOSA and PFOA in 56 Canadian serum samples (Kubwabo et al. 2004). The total PFOS content ranged from 3.7 to 65.1 ng/mL with a mean of 28.8 ng/mL. The concentrations of PFOA (range: <1.2-7.2 ng/mL) were one order of magnitude lower than that of PFOS and only found in 29% of the samples.
A study of 16 Canadian blood serum samples showed that the linear isomers dominated with 98% thus most PFCAs must come from telomerisation (De Silva and Mabury 2006). Even numbered PFCAs had higher concentrations than odd numbered in contrary to the arctic mammals; the highest concentration of PFCAs was of PFOA (mean 4.4 ng/g).
8.2.2.3 Levels in Japan
Concentrations of PFOS in whole blood from 10 Japanese volunteers sampled in June 2002 ranged 2-14 ng/mL with a mean of about 8 ng/mL (Taniyasu et al., 2003).
More than 200 serum samples, collected in 2002-2003 from the cities of Kyoto, Yokote and Taiwa, were investigated by Harada et al. (2004). The PFOS and PFOA mean levels in serum ranged from 3.5 to 28.1 ng/mL and from 2.8-12.4 ng/mL, respectively. Highest levels of PFOS and PFOA were measured in an area with known contamination of surface water.
Harada et al. (2004, 2007) have reported the results of analysing 100 historic blood serum samples from Kyoto, Japan, from the period 1983-1999, which showed that PFOA concentrations did increase more than 4 times in that period and continued to increase, but levels of PFOS reached a plateau in late 1980s. The production of fluoropolymers in Japan increased in the same period 3.5 fold. Levels were influenced by several factors. Sex and residential area are the most influential factors, while age and smoking status are not so important. In general males had 50-100% higher concentrations than females.
Other 100 serum samples collected in 2003-2004 from 10 cities showed also large geographical differences (Harada et al. 2007). Regards PFOS the geometric mean levels in males were between 10.7 ng/mL (range: 3.7-17.6 ng/mL) in Sendai to 29.3 ng/mL (range: 22.2-37.7) in Osaka. The highest concentration was 92.2 ng/mL in a sample from Akita. Levels of PFOA were much lower with mean levels in males from 3.0 ng/mL (range: 0.4-7.8 ng/mL) in Sendai to 14.5 ng/mL (range: 10.7-19.8 ng/mL) in Osaka.
8.2.2.4 Levels in China
In China the levels differ with time and place. In one study (Yeung et al. 2006) 85 samples of whole blood collected from nine cities were analysed. The mean concentration of PFOS was greatest in samples collected from Shenyang (79.2 ng/mL) in the north and least in samples from Jintan (3.72 ng/mL) by the Yangtze River. PFHxS was the second most abundant perfluorochemical. There were large differences in the concentration profiles of the various perfluorochemicals among the nine cities. In a later study by the same research group analysed 5 blood samples from each of four cities for 10 perfluorochemicals (Yeung et al. 2007). PFOS was far the most abundant compound - around 90% of content in the three high-level cities. Jintan had again lowest levels of PFOS (mean: 5.04 ng/mL; range 1.5-12.8 ng/mL) compared with Beijing (mean: 13.8 ng/mL; range 4.04-21.2 ng/mL), Guizhou (mean: 20.7 ng/mL; range 7.78-46.2 ng/mL) and Shenyang (mean: 56.3 ng/mL; range 35.7-12.8 ng/mL).
8.2.2.5 Levels in Sweden
In a study from Sweden 66 whole blood samples collected 1997-2000 from the general population were analysed for 12 perfluorinated compounds with chain length 4 to 14 carbon atoms (Kärmann et al. 2004). PFOS, PFOA, PFOSA, PFHxS and PFNA were found in 92-100% of the samples, while traces of PFDA and PFDoA were found in 65% of the samples. Besides this, PFDS, PFHxA, PFDoA and PFTeA were detected in 3-8% of the samples. Levels of PFOS dominated with a range of 1.7-37 ng/mL and a mean of 18.2 ng/mL. The mean sum of all perfluorinated compounds was 24.6 ng/ml.
A later study included blood serum from 12 mothers collected in 2004 in Uppsala (Kärrman et al. 2007). Six out of seven perfluorinated chemicals were present in all serum samples. The compounds were PFOS (range: 8.0-48 ng/mL; mean: 20.7 ng/mL), PFHxS (range: 1.8-11.8 ng/mL; mean: 4.7 ng/mL), PFOA (range: 2.4-5.3 ng/mL; mean: 3.8 ng/mL), PFNA (range: 0.43-2.5 ng/mL; mean: 0.80 ng/mL), PFDA (range: 0.27-1.8 ng/mL; mean: 0.53 ng/mL) and PFUnA (range: 0.2-1.5 ng/mL; mean: 0.40 ng/mL). In addition, traces of PFOSA were present in 9 samples. The medians were generally a little lower than the means.
8.2.2.6 Levels in Norway
In the blood serum from 35 consumers of fish from a polluted lake in Norway the levels of 9 perfluorinated substances were measured (Thomsen et al. 2006). Males had higher levels than females. The overall results were PFOS (range: 11-62 ng/mL; mean: 30 ng/mL), PFHxS (range: 0.32-2.2 ng/mL; mean: 1.2 ng/mL), PFOA (range: 1.3-7.4 ng/mL; mean: 3.4 ng/mL), PFNA (range: 0.56-4.6 ng/mL; mean: 1.5 ng/mL), PFDA (range: 0.23-3.1 ng/mL; mean: 0.67 ng/mL) and PFUnA (range: 0.2-7.7 ng/mL; mean: 1.5 ng/mL). PFDoA, PFOSA and Me-PFOSA-AcOH were found some of the samples in levels close to the detection limit.
8.2.2.7 Levels in Denmark
A recent study by Fei et al. (2007) reports the results of PFOS and PFOA in 1399 blood plasma samples collected during March 1996 - November 2002 among a cohort of pregnant women in Denmark. The average levels of PFOS and PFOA were 35.3 ng/mL (range: 6.4-106.7 ng/mL) and 5.6 ng/mL (range: <1.0-41.5 ng/mL), respectively.
Serum concentrations of 9 perfluorinated compounds (PFOS, PFOA, PFOSA, MeFOSA-AcOH, EtFOSA-AcOH, PFHxS, PFNA, PFDA and PFDoA) have been measured in two groups of Faroe Island residents (Kato et al. 2007). The first group included 12 mothers sampled in 2000 and their 5 year old children sampled in 2005. The median concentrations were for PFOS respectively 23.7 and 16.3 ng/mL; thus the children had lower levels. Levels of the other contaminants were much lower: 2.4 and 4.5 ng/mL, respectively, for PFOA. The second group consisted of 103 children 7 years of age and samples collected in 1993-1994. The median concentration for PFOS was 29 ng/mL and for PFOA 5.5 ng/mL.
8.2.2.8 Levels in Germany
In 2005 in Germany (Bavaria) 356 samples of blood plasma from blood banks were analysed for PFOS and PFOA (Fromme et al. 2007). For males the levels of PFOS ranged from 2.1-55 ng/mL with a median of 13.7 ng/mL, and PFOA levels ranged from 0.5-19.1 ng/mL with a median of 5.7 ng/mL. For females the levels were a little lower with PFOS ranging 2.5-30.7 ng/mL with a median of 10.9 ng/mL, and PFOA ranging 1.5-16.2 ng/mL with a median of 4.8 ng/mL. For females the levels increased significantly with age.
8.2.2.9 Levels in Poland
Whole blood from a reference group of 15 donors from Gdansk in Poland in 2003 was analysed for 11 fluorinated chemicals (PFBS, PFHxS, PFOS, PFOSA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA and PFDoA) (Falandysz et al. 2006). All chemicals, besides PFBS, were quantified in all samples. The concentrations of PFOS and PFOA were highest. The results are shown in Table 8.2:
Table 8.2: Levels of fluorinated chemicals in Polish blood (Falandysz et al. 2006)
| PFHxS | PFOS | PFOSA | PFHxA | PFHpA | PFOA | PFNA | PFDA | PFUnA | PFDoA | |
| Mean ng/mL | 0.51 | 16 | 0.44 | 0.03 | 0.17 | 3 | 0.61 | 0.2 | 0.11 | 0.016 |
| Range | 0.17-1.0 | 6.7-46 | 0.05-0.9 | 0.004-0.065 | 0.017-0.47 | 1.3-5.2 | 0.03-1.5 | 0.09-0.51 | 0.04-0.3 | 0.006-0.038 |
8.2.2.10 Levels in Italy
Fifty serum samples from Siena, Italy, were analysed for PFOS, PFOSA, PFHxS and PFOA (Corsolini and Kannan 2004). Most samples contained PFOS (mean: 4.3 ng/mL), a third contained PFHxS (mean 1.7 ng/mL), and 10% contained PFOSA (mean: 1.8 ng/mL). PFOA was not detected but the limit of detection was also rather high (3 ng/mL).
8.2.2.11 Levels in Sri Lanka
Rather high levels of perfluorinated chemicals were found in blood from Sri Lanka (Guruge et al. 2004). The levels in the capital Colombo were a little higher than in a rural area. Maximum serum concentrations were 18 ng PFOS/mL and 23 ng PFOA/mL.
Concentrations of 12 perfluorinated substances (PFOS, PFOSA, PFBS, PFHxS, PFOA, PFPeA, PFHxA, PFHpA, PFNA, PFDA, PFUnA and PFDoA) were analysed in 30 human sera and in seminal plasma from Sri Lanka (Guruge et al. 2005). PFBS, PFOSA and PFPeA were below the detection limits. Six of these chemicals (PFOS, PFHxS, PFOA, PFNA, PFDA and PFUnA) were found in all serum samples but only PFOS and PFHxS were present in seminal plasma. There were no differences between levels in people living in the capital Colombo (PFOS mean: 7.8 ng/mL; range: 1.5-18.2 ng/mL and PFOA mean: 9.54 ng/mL; range: 0.6-22.8 ng/mL) and in conventional tea workers from a rural area (PFOS mean: 6.3 ng/mL; range: 1.8-17.5 ng/mL and PFOA mean: 9.06 ng/mL; range: 1.9-23.5 ng/mL). However, rural organic tea workers had lower levels (PFOS mean: 0.96 ng/mL; range: 0.3-1.33 and PFOA mean: 0.53 ng/mL; range: 0.2-0.89 ng/mL). The overall mean levels were 5.0 ng/mL for PFOS, 0.57 ng/mL for PFHxS, 6.4 ng/mL for PFOA, 0.17 ng/mL for PFNA, 0.09 ng/mL for PFDA and 0.11 ng/mL for PFUnA. Thus PFOA levels in Sri Lanka are higher than that of PFOS.
8.2.2.12 Levels in Korea
In Korea 50 whole blood samples from Daegu, the fourth largest city in Korea were analysed (Yang et al. 2004; Kannan et al. 2004). The mean concentration of PFOA (88 ng/mL) was higher than that of PFOS (15 ng/mL). There were no sex or age dependencies. Curiously, PFOA was only determined in 19-25% of these samples. This may be explained by individual exposure to a local source.
8.2.2.13 Levels in Peru
Calafat et al. (2006a) measured 11 perfluorinated chemicals in serum samples collected in 2003 from 44 residents from Trujillo in Peru. Perfluorinated chemicals were only determined in less than 25% of the samples. The 95th percentile serum concentrations of PFOS was 1.0 ng/mL, of PFOA was 0.3 ng/mL and of PFHxS 0.4 ng/mL.
8.2.2.14 Levels in the arctic
In the Arctic the human blood levels are generally lower than in industrialised areas. In twelve plasma samples from women giving birth from Bodø in Norway, Naryan Mar in North Russia and Taimyr in Siberia low levels of sixteen perfluorinated compounds were determined (Odland 2006). Highest levels (median 16 ng/mL; max. 49 ng/mL) were found for PFOS in North Russia followed by Norway (median 16 ng/mL; max. 28 ng/mL); lowest levels in Siberia (median 9 ng/mL; max. 14 ng/mL). For PFOA the levels were much lower and about similar the three places (median 2 ng/mL; Max. 6 ng/mL). For PFNA the levels in North Russia (median 6 ng/mL; max. 16 ng/mL) were about five times higher than the other places. PFHxS, PFHpS, PFOSA, PFHpA, PFDA, PFUnA, PFDoA, and PFTrA had median levels of 0.1-1.5 ng/mL. The rest of the substances (PFPS, PFNS, PFDS, EtFOSA, and PFHxA) had median levels of 0.002-0.04 ng/mL. The levels of PFOS were much higher than levels of the more well-known chlorinated and brominated contaminants.
The presence of perfluorinated compounds in 23 archived pooled samples of blood plasma from females of the northern population in Canada was studied by Tittlemier et al. (2004). Average levels were 36.9 ng PFOS/mL and 2.2 ng PFOA/mL. All samples also contained PFNA (0.11-1.98 ng/mL), while 70% of the samples contained detectable levels of PFHpA and only one sample contained PFHxA. There was no difference between levels in Inuit and Caucasian people as there are for PCB and dioxin, thus the source of exposure may not be food.
8.2.2.15 International comparisons
OECD has made an overview of PFOS and PFOA levels in human blood sampled in various countries from 1998-2000. The average PFOS levels ranged 17-53 ng/mL for PFOS and 3-17 ng/mL for PFOA (OECD 2002).
PFOS and other perfluorinated chemicals were determined in blood serum from the general population of the USA, India and Italy by Kannan et al. (2003). PFOS levels (mean: 32 ng/mL) in Americans are 7-8 times higher than in Italians who again have twice the levels of Indians. In the majority of samples from the USA PFHxS, PFOA and PFOSA were also detected (means: 3.9, 5.1, and 3.4 ng/mL, respectively). On the contrary, one third of the samples from Italy and India had detectable levels of PFHxS while PFOA and PFOSA only were detectable in a few or zero samples. The concentration of PFNA and PFDA was between 0.5 and 1 ng/mL. No variation in sex or age was seen.
A later study of Kannan et al. (2004) reported concentrations of perfluorinated compounds (PFOS, PFHxS, PFOA, PFOSA) in 473 blood/serum/plasma samples from 9 countries (USA, Columbia, Brazil, Belgium, Italy, Poland, India, Malaysia and Korea). In all countries, besides Korea, PFOS was far the dominating contaminant with means values ranging from 66 ng/mL in Kentucky, USA, and to 2.3 ng/mL in India.
Figure 8.1 and Figure 8.2 show data from various countries of PFOS and PFOA in blood serum/plasma and whole blood, respectively. The separation is due to that whole blood levels will be 2-3 times lower than serum/plasma levels, because PFCs are attached to the serum proteins.
Figure 8.1: Average concentrations of PFOS and PFOA in human blood serum/plasma from various countries.
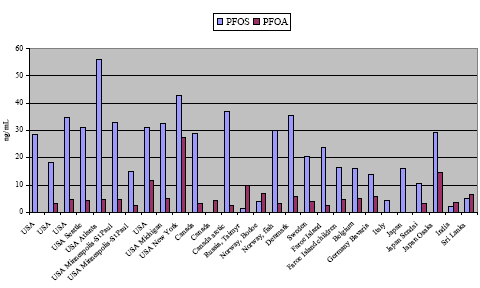
Figure 8.2: Average concentrations of PFOS and PFOA in whole human blood from various countries.
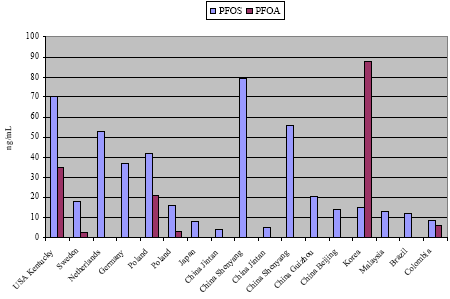
8.2.2.16 Summary and conclusion
Blood levels of perfluorinated chemicals have been monitored in many countries but most data has been published from USA. In all countries, besides Korea, PFOS has been determined in far higher concentrations than the other PFCs. Typical average serum levels of PFOS in industrialized countries are 20-30 ng/mL with maximum levels less than 100 ng/mL. Rural areas show serum levels less than 10 ng PFOS/ml The second most abundant PFC is normally PFOA with typical average serum levels of 3-5 ng/mL. Some of the highest PFOS blood levels (2-3 times the typical levels) in the general population were determined in industrial areas of USA and China. Such levels may be 10 times higher than in rural and remote areas. Preliminary data from Denmark show somewhat higher concentrations than in our neighbour countries with an average PFOS level in blood serum of 35 ng PFOS/mL and with a maximum concentration of 107 ng/mL.
8.2.3 Cord blood
Perfluorinated compounds were investigated in maternal blood and in cord blood in 15 pregnant Japanese women. PFOS concentrations in maternal samples ranged from 4.9 to 17.6 µg/L, whereas those in foetal samples ranged from 1.6 to 5.3 µg/L, or one-third of maternal levels. PFOA was only determined in 3 out of 15 maternal samples with concentrations ranging 0.5-2.3 µg/L. PFOSA was not detected in any samples (Inoue et al. 2004).
Levels of perfluorinated compounds in cord blood plasma (archive pooled samples) from females of the northern population in Canada were about the half of maternal blood or a little higher than in Japan (Tittlemier et al. 2004).
Midasch et al. (2007) determined PFOS and PFOA in maternal blood and cord blood plasma from 11 German mothers. Concentrations of PFOS in maternal blood ranged from 7.8-16.4 ng/mL with a median of 13.0 ng/mL. In cord blood the PFOS concentrations ranged 3.3-9.5 ng/mL with a median of 7.3 ng/mL; thus almost the half of maternal blood as measured in Canada. Regards PFOA the levels in cord blood were much lower and ranged from 1.5-4.0 ng/mL with a median of 2.6 ng/mL, and surprisingly the levels in cord blood were a little higher with a range of 1.5-4.6 ng/mL and a median of 3.4 ng/mL.
Apelberg et al. (2007a) analysed cord serum from 299 newborns delivered in Baltimore, USA, 2004-2005, for 10 polyfluorinated compounds. PFOS and PFOA were detected in all samples with geometric mean concentrations of 4.9 ng/mL and 1.6 ng/mL, respectively. A comparison of PFOS and PFOA in 200 maternal (second sampling) and 50 cord blood plasma samples from Denmark 1996-2002 showed that the average levels in maternal blood of PFOS (29.9 ng/mL) were 2½ times the levels in cord blood (11.0 ng/mL). The difference was much smaller for PFOA having the lower average levels of 4.5 ng/mL in maternal blood and 3.7 ng/mL in cord blood (Fei et al. 2007).
In conclusion: Levels of PFCs in cord blood are about the half of levels in maternal blood, thus some transfer to placenta occurs.
8.3 Levels in semen
Rather high levels of perfluorinated chemicals were found in blood and semen from Sri Lanka (Guruge et al. 2004). The levels in the capital Colombo were a little higher than in a rural area. Levels in semen were ten times lower than in blood serum. Maximum blood serum concentrations were 18 ng PFOS/mL and 23 ng PFOA/mL.
8.4 Levels in the liver
Livers from 31 American deceased donors were analysed for fluorochemicals by Olsen et al. (2003a). Liver PFOS concentrations ranged between <4.5 to 57 ng/mL but in about half of the liver samples levels were below the limit of quantification. The means of the positive samples were 28.0 ng/g ww for liver. The mean serum to liver ratio was 1.3. Only one liver had measurable contents of PFOA.
9 Toxicity to humans
An old study of chemical plant workers exposed to fluorochemicals in the air (up to 3.9 mg/m³) for years showed that these workers had about 100 times higher levels of organic fluorine in their blood (1-71 mg/L serum) than people from the general population. However, no ill effects were attributable to exposure to these fluorochemicals. The half-life of PFOA in these workers was estimated to be 18-24 months (Ubel et al. 1980).
A recent investigation of 24 retired fluorochemical workers showed that the mean half-lives in human blood were 5.4 years for PFOS, 8.5 years for PFHxS, and 3.8 years for PFOA (Olsen et al. 2007a). The half-lives of the fluorinated chemicals in the general population, who is 10-100 times less contaminated than the retired workers, may be even longer.
The renal clearances of PFOA and PFOS are almost neglible in humans (Harada et al. 2005), which means that when these chemicals leave the blood a redistribution probably to internal organs such as the liver and kidneys will happen. The residence times in these organs are not known but must be very much longer.
9.1 Biochemical effects
Findings in animal studies suggest that PFOA affects hormonal states and metabolism of lipids in humans. However, no significant correlation has been observed between serum levels of PFOA (mean 5-7 mg/L) and any biological parameter in ammonium perfluorooctanoate plant workers (Olsen et al. 1998; 1999; 2000).
9.2 Cancer
A retrospective cohort mortality study of a perfluorooctane sulfonyl fluoride (PFOSF) production workforce reported an excess of bladder cancer based on 3 deaths (SMR 12.77) at high-exposure jobs (Alexander et al. 2003).
Workers at a PFOA production plant in the USA whose serum levels averaged 5 ppm had no excess of cancer mortality related to PFOA (Olsen et al. 2004). Olsen et al. (1998) concluded there was reasonable assurance of no substantial hormonal changes associated with PFOA at serum levels measured among these male production employees.
9.3 Developmental toxicity
In a study by Apelberg et al. (2007b) the relationship between PFOS and PFOA concentrations and gestational age, birth weight, and birth size was investigated, and the conclusions were that reductions of birth weight, ponderal index and of head circumference were associated with PFOS and PFOA concentrations among vaginal deliveries. A similar inverse association concerning maternal PFOA concentration and birth weight was found by Fei et al. (2007).
10 Animal bioassays and in vitro tests
- 10.1 Toxicokinetics and metabolism
- 10.2 Toxicology
- 10.2.1 Acute toxicity
- 10.2.2 Effects on the liver
- 10.2.3 Toxicological mechanism
- 10.2.4 Toxicogenomics
- 10.2.5 Effects on cell membranes
- 10.2.6 Effect on mitochondrial bioenergetics
- 10.2.7 Effect on intercellular communication
- 10.2.8 Developmental toxicity
- 10.2.9 Endocrine disruption
- 10.2.10 Mutagenicityt
- 10.2.11 Cancer
- 10.2.12 Cocktail effects
- 10.3 Structure-activity relations
10.1 Toxicokinetics and metabolism
10.1.1 Absorption
PFOS and PFOA (as ammonium salt) are readily and rapidly absorbed in the gastro-intestinal tract and the lungs of rats. Toxic concentrations of PFOA are absorbed through the skin of rabbits (Lau et al 2004; Kennedy et al 2004; Hinderliter et al. 2006). About 93% of an oral dose of PFOA given to rats was absorbed, and peak blood levels were attained 1-2 h after dosing (Hundley et al. 2006). Also an oral dose of 8:2 FTOH is readily absorbed in rats but the skin absorption was neglible. The maximum concentration in the blood occurred by 1 hour after oral dosing, and it cleared rapidly with a half-life of less than 5 hours (Fasano et al. 2006).
10.1.2 Biotransformation
PFOA and PFOS are both considered being metabolically inert (Clark et al. 1973). Other perfluoroalkyl acids with shorter or longer alkyl chain do have similar properties. Their precursors and functional derivatives will ultimately be transformed to their basic acids. For example, the fluorotelomer alcohol 8:2 FTOH and its phosphates are rapidly transformed to PFOA, PFNA and other metabolites in mice and rats (Hagen et al. 1981; Kudo et al. 2005; Fasano et al. 2006; Henderson and Smith 2007; D’Eon and Mabury 2007). The transformation in rat hepatocytes is catalyzed by cytochrom P-450 (Martin et al. 2005).
About 20% of an oral dose of 100 ppm EtFOSE is in male rat metabolised to PFOS (Thomford et al. 2002). Rat liver microsomal fractions also degrade EtFOSE by de-alkylation to FOSE and further to FOSA and finally to PFOS (Xu et al. 2004).
10.1.3 Accumulation
PFOA and PFOS bind to proteins and accumulate - more or less depending on animal species and sex - in various body tissues, mainly in blood, liver, and kidneys of exposed organisms (in total 88% in male rats) but also in lungs, heart, skin, testes (3% in male rats) and brain (Vanden Heuvel et al 1992; Austin et al. 2003; Hundley et al. 2006). The accumulation in fat tissues and muscles is, however, minimal.
Kudo and co-workers (2001) studied PFCAs with different chain length (C7 - C10) in male liver. The result showed that the longer the chain the more of the compound was accumulated in the liver.
10.1.4 Elimination
Once absorbed in the body PFOA is eliminated as the free carboxylic acid mainly with urine and to a less extent in faeces. Thus the renal elimination is critical for detoxification (Vanden Heuvel et al. 1991).
The biological half-life of PFOA in male rats after i.v. administration was 5.6 days or 70 times longer than that in female rats for which it was 2 hrs. The difference was mainly due to the difference in renal clearance, which was significantly reduced by probenezid, suggesting that PFOA is excreted by organic anion transporters. Castration of male rats caused a 14-fold increase in renal clearance, comparable with female rats. The increase was reduced again by treatment with testosterone. Treatment with estradiol also increased the renal clearance, and in female rats ovariectomy increased the renal clearance (Kudo et al. 2002). Elimination of PFOA increases with age in female rats but not in male rats (Hinderliter et al. 2006).
Perfluorocarboxylic acids (PFCA) with a shorter carbon chain length than PFOA are more rapidly eliminated in urine and PFHpA (0.10/0.05) > PFOA (5.6/0.08) >PFNA (30/2.4) >PFDA (40/59 days) for male/female rats (Kudo et al. 2001; Ohmori et al. 2003). The sex differences were most marked for PFOA.
The sex-related clearance of PFOA differs between animal species (Hundley et al. 2006). In hamsters it is opposite of in rats. Male and female hamsters excrete respectively 99% and 58% of a dose in 5 days. In mice and rabbits there was no sex difference, and mice had a slow excretion as male rats, and rabbits had a fast excretion as female rats. In dogs the plasma half-life of PFOA was about 20 days in males and the half in females.
The elimination half-lives in male and female Cynomolgus monkeys for PFOA were 33 days and 21 days, respectively, and the urine was the major excretion route (Butenhoff et al. 2004a). PFOS has a slower elimination rate, and the half-life of PFOS in the Cynomolgus monkey was about 200 days (Seacat et al. 2002a; Andersen et al. 2006).
After an oral dosing of 8:2 FTOH, the majority of the 14C-labelled substance was excreted with feces and 37-55% occurred there as 8:2 FTOH. A small part (1%) was excreted as PFOA in urine (Fasano et al. 2006).
10.2 Toxicology
10.2.1 Acute toxicity
The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, The oral rat LD50 for PFOS is 250 mg/kg bw (3 M 1999), and for PFOA the oral rat LD50 is between 430 and 1800 mg/kg (Kennedy et al. 2004). For PFOA and PFDA the rat intraperitoneal LD50’s are 189 and 41 mg/kg, respectively. Thus PFDA is much more toxic and have delayed effects (Olson and Anderson 1983). Generally, the toxicity of perfluorinated chemicals tested increases with the length of the alkyl chain.
10.2.2 Effects on the liver
Toxicological studies have demonstrated that the liver is the primary target organ for PFOS and PFOA, and body weight loss, increased liver weight, liver cell hypertrophy and changed lipid metabolism with reduction in serum cholesterol are early responses in experimental animals (Kennedy et al. 2004; Seacat et al. 2003).
The no-observed-adverse effect level (NOAEL) for liver effects in male rats exposed for PFOS during 14 weeks feeding was 5 mg/kg/d (Seacat et al. 2003). NOAEL and LOEAL for liver effects by PFOA in male rats exposed by feeding in 13 weeks were 0.06 and 0.64 mg/kg/d, respectively (Perkins et al. 2004). Another repeated-dose study found a LOAEL of 0.3-1 mg/kg for PFOA in male rats, and branched forms of PFOA had lower toxicity than linear forms (Loveless et al. 2006).
PFDA having a longer chain than PFOA was more toxic in rat, hamster, mouse and Guinea pig (van Rafelgheim et al. 1987; Kawashima et al. 1995).
Rats seem to tolerate somewhat higher liver concentrations of PFOS than monkeys, because the NOAEL in a 6-month monkey study was 0.15 mg/kg/d (Seacat et al. 2002a). PFOA with a LOAEL of 3 to 10 mg/kg/d is considerably less liver toxic in monkeys than PFOS, and the target is different (Butenhoff, 2002a). The only site of change in the monkey was liver enlargement at the doses cited.
10.2.3 Toxicological mechanism
PFOA and PFOS induce hepatomegaly characterized by the subcellular proliferation of organelles such as smooth endoplasmic reticulum, mitochondria, but most notably peroxisomes. The changes observed in the liver could be the result of peroxisome proliferation, a well-known toxicological mechanism in rodents. It may cause lipid accumulation in the liver, uncoupling of the mitochondrial oxidative phosphorylation, and reduction of thyroid hormone in circulation. Induction of peroxisome proliferation and benign liver tumors are associated with activation of the nuclear hormone receptor - peroxisome proliferator-activated receptor-a (PPARa). This is one of the three isoforms of PPAR encoded by separate genes and differentially expressed in various tissues, and are found in all mammalian species examined to date. Ligands for PPARs have been widely developed for the treatment of various diseases including dyslipidemias and diabetes. Some hypolipidemic drugs, solvents and environmental chemicals are ligands for PPARa and can induce peroxisome proliferation, for example, clofibrate, phthalates, chloroform, perchloroethylene, trichloroethylene, HFC-123, and MTBE. Humans do not exhibit the liver toxicities by these chemicals found in rodent models (Peraza et al. 2006).
This PPAR receptor is the most likely target of PFOA and PFOS both in rodents and humans (Vanden Heuvel et al. 2006). Polyfluorinated acids are analogue ligands to natural long-chain fatty acids and may displace them in biochemical processes and at receptors such as PPARa. The activation by PFOA and PFOS is more selective but less potent. PFOA is more capable than PFOS in activating PPARa, and mouse is more responsive than human in test systems (Takacs and Abbott 2007).
PFOA is an immunosuppressant through induction of PPARs and enhance the IgE-mediated hypersensitivity response to ovalbumin, and in this way it may provoke asthma (Fairley et al. 2007).
Perfluorocarboxylates (PFCA), particularly PFOA, PFNA and PFDA, are highly potent peroxisome proliferators in rodent livers and affect mitochondrial, microsomal, and cytosolic enzymes and proteins involved in lipid metabolism (Ikeda et al. 1985; Vanden Heuvel 1996; Upham et. al. 1998; Kudo et al. 2000).
PFOS is less reactive as peroxisome proliferator in rodents, and EtFOSE has no effect (Berthiaume and Wallace 2002). PFBA has a slighter effect on indicators of peroxisome proliferation (Ikeda et al. 1985).
The differences between animal species are large for PFDA. Peroxisome proliferation was greatest in mice and almost absent in Guinea pigs. However, accumulation of lipid droplets in liver cells was more pronounced in hamsters and guinea pigs than in rats and mice exposed to PFDA (van Rafelgheim et al. 1987).
Increase in hepatic fatty acid b-oxidation activity (acyl-CoA oxidase) is a biochemical measure of peroxisome proliferation. Kudo and co-workers (2001) studied PFCAs with different chain length (C7 - C10) in male liver. The result indicated that the liver concentration and not the chain length was decisive, but the longer the chain the more of the compound was accumulated in the liver. In vitamin A deficient mice, PFOA had a stronger effect and caused a 3-6 times increase in the ß-oxidation of fatty acids (Sohlenius et al. 1995).
In an in vitro test various perfluorinated chemicals were tested for interference with the liver-fatty acid binding protein (L-FABP). The most potent chemical was PFOS followed by EtFOSA, EtFOSE, and PFOA (Luebker et al. 2002). This interference may contribute to the toxicity of these chemicals.
10.2.4 Toxicogenomics
Toxicogenomic analysis is able to predict toxicity and pathological responses, categorize chemicals, and elucidate mechanism of toxicity. Such analysis showed that PFOA and PFOS exhibited peroxisome proliferator-activated receptor a (PPARa) agonist-like effects on genes (enzymes etc.) associated with lipid metabolism and fatty acid homeostasis (Shipley et al. 2004; Martin et al. 2007). It results e.g. in
- down-regulation of cholesterol biosynthesis genes, matching an in vivo decrease in serum cholesterol, and
- perturbation of thyroid hormone metabolism genes matched by serum thyroid hormone depletion in vivo.
Guruge et al. (2006) used a microarray technique to study gene regulation in livers from male rats treated with hyper doses of PFOA. Over 500 gene expressions were significantly altered. The largest categories of induced up-regulated genes were those involved in transport and metabolism of lipids, particularly fatty acids. Other induced genes were involved in cell communication, adhesion, growth, apoptosis, hormone regulatory pathways, proteolysis and peptidolysis and signal transduction. The gene expressions suppressed (down-regulated) were related to transport of lipids, inflammation and immunity and especially cell adhesion. Genes involved in apoptosis, regulation of hormones, metabolism were also suppressed.
In the lung and liver of PFOA-exposed mouse fetuses the expressions of genes related to fatty acid catabolism were changed (Rosen et al. 2007). That was especially robust in the fetal liver and also genes associated with lipid transport, ketogenesis, glucose metabolism, lipoprotein metabolism, cholesterol biosynthesis, steroid metabolism, bile acid synthesis, phospholipid metabolism, retinol metabolism, proteosome activation and inflammation. Most changes were associated with the PPARa receptor.
10.2.5 Effects on cell membranes
Despite of its lipophobic character PFOS and to a lesser extend PFOA can partition into model bilayers and cell membranes, where it causes changes in membrane structure and function and increased fluidity. Interaction with pulmonary surfactants such as dipalmitoylphoshatidyl choline (DPPC) may be a mechanism by which PFOS causes perinatal mortality in animal studies (Xie et al. 2007). The orientation in the membrane of the PFAS molecules is with the functional group end combined to membrane proteins with the fluorocarbon tails sticking out like hairs (Roon et al. 2006).
Some of the observed effects of perfluorinated compounds may be due to alterations in cell membrane fluidity, which is a measure of the relative mobility of the phospholipid bilayer of the cell membrane. This selectively permeable cell membrane forms the first barrier that separates the cell from exogenous exposures. Effects on the permeability status of the cell membrane could play an important role in mediating the adverse effects of a number of environmental contaminants. In some in vitro assay systems PFOS - but not the shorter chain PFBS and PFHxS – significantly increased in a dose-dependent manner membrane fluidity of fish leukocytes, and decreased mitochondrial membrane potential determined by flow cytometry (Hu et al. 2003).
PFOA, PFOS and EtFOSE caused a slight increase in the intrinsic proton leak of the mitochondrial inner membrane, which resembled a surfactant-like change in membrane fluidity (Starkov and Wallace 2002).
10.2.6 Effect on mitochondrial bioenergetics
PFOS, PFOA, FOSA, FOSAA, EtFOSA, EtFOSE and EtFOSAA had the capacity in vitro to interfere with mitochondrial respiration. FOSA was the most potent uncoupler of the oxidative phosphorylation (Schnellmann and Manning 1990; Starkov and Wallace 2002).
In laboratory tests N-acetyl perfluorooctane sulfonamides (FOSAA, EtFOSAA) disrupt mitochondrial bioenergetics by inducing “the mitochondrial permeability transition”. PFOA had a slight effect but PFOS and EtFOSE had no effect (O’Brien and Wallace 2004).
10.2.7 Effect on intercellular communication
Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases (Trosko et al. 1998).
Upham and co-workers (1998) showed that perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner in vitro and with PFDA inhibiting more than PFOA.
In various test systems (in vitro and in vivo) both PFOS, PFOSA, and PFHxS – but not PFBS - inhibit gap junction intercellular communication in a dose-dependent fashion, and this inhibition occurred rapidly and was reversible (Hu et al. 2002).
Cellular effects such as cell membrane fragility and gap junction communication are two of the hypothetical explanations for the effects of these molecules. These are not considered the hallmark effects, such as interference with liver function or lipid metabolism.
10.2.8 Developmental toxicity
PFOA and PFOS can cross the placenta barrier in rodents and be found in placenta, amniotic fluid, embryo and fetus. The concentration of PFOA in fetal plasma at day 21 was approximately half of the concentration in maternal plasma. Lactational transfer is also occurring but the concentration in milk is ten times lower than in blood (Hinderliter et al. 2005).
PFOA and PFOS in rats and mice have showed developmental toxicity and other adverse effects in vivo. The developmental toxicity of PFOS is higher than that of PFOA. These effects included reduction of fetal weight, cleft palate, anasarca, delayed ossification of bones and cardiac abnormalities, as well as decreased neonatal survival following in utero exposure, reduction in mean post natal body weight, altered mammary gland development, decrease in gestation length, not fully matured lungs with laboured breathing, and significant delay in sexual maturation. The structural abnormalities were only found in teratological studies at the highest dose groups (³ 30 mg/kg/d), where significant reductions of weight gain and food consumption and increase of mortality in the pups were also observed in the pregnant dams (Case et al. 2001; Butenhoff et al. 2004b; Kennedy et al. 2004; Grasty et al. 2005; Luebker et al. 2005b; Lau et al. 2003, 2004, 2006; Thibodeaux et al. 2003; White at al. 2007). Concurrent exposure to PFOS and restraint stress enhance effects (Fuentes et al. 2007).
In a two-generation reproduction study the NOAEL value in rats for PFOS was 0.1 mg/kg/d (Luebker et al. 2005a). However, at this low maternal dose level the longer chain PFDA significantly reduced fetal body weight in mice (Harris and Birnbaum 1989).
High doses of EtFOSE also caused reduced maternal body weight and foetal weight in rodents and had effects quite similar to its metabolite PFOS. Both PFBS and PFHxS have been assessed for developmental and reproductive effects. Maternal exposure to PFBS potassium salt did not produce any adverse effect on embryo/foetal development, and no significant alterations were noted in a two-generation study in rats at doses as high as 1 g/kg. PFHxS is only examined in a screening system at lower doses without any effect observed (Lau et al. 2004).
Similar effects, to what is mentioned for rodents, happen in rabbits exposed to PFOS and EtFOSE during gestation. The no-observed-effect-level (NOEL) for PFOS in rabbits was 0.1 mg/kg/d (Case et al. 2001).
Interaction with pulmonary surfactants, such as dipalmitoylphoshatidyl choline (DPPC), may be a mechanism by which pfos causes perinatal mortality in animal studies (Xie et al. 2007).
10.2.9 Endocrine disruption
Some polyfluorinated chemicals may act as endocrine disruptors. Exposure of adult rats to PFOA (25 mg/kg bw by gavage) decreased the testosterone level in testicular interstitial fluid, increasing the serum estradiol level and decreased relative accessory sex organ weights (Cook et al. 1992; Biegel et al. 1995; Biegel 1997; Shi et al. 2007). It could be explained by induction of a hepatic aromatise by PFOA, which converts testosterone to estradiol. Some other studies show no such effects, for instance in a 6-months oral study in monkeys, daily doses of PFOA up to 20 mg/kg/day did not produce any changes in hormone levels, including oestrogen (Butenhoff 2002a).
Some polyfluorinated chemicals have estrogenic effects in cell cultures (”E-screen assay”; Soto et al. 1995). For example, the fluorotelomer alcohols 6:2 FTOH and 8:2 FTOH induce MCF-7 breast cancer cell proliferation and up-regulates the estrogen receptor, but PFOS, PFOA and PFNA have no estrogenic effect in that test (Maras et al. 2006; Vanparys et al. 2006).
The effects on hormone levels in rodents are reflected in changes in the testis where exposure to perfluorooctanoate results in Leydig cell hyperplasia and eventually development of Leydig cell adenomas (Biegel et al. 1995). A study of testis effects in adult rats exposed to perfluorododecanoic acid (PFDoA) also showed a reduced gene expression of many genes involved in cholesterol transport and steroidogenesis and a reduced serum testosterone level (Shi et al. 2007). Thus, it seems that exposure to some PFCs can severely affect proliferation and function of Leydig cells in the adult rat. Leydig cells in the testis are the main sites for testosterone biosynthesis.
This is of considerable concern, because Leydig cell hyperplasia is common among infertile men (Holm et al. 2003) who, as a group, also shows lower testosterone levels than comparable normal controls (Andersson et al. 2004). Reduced testis function has been linked to the testicular dysgenesis syndrome (TDS) (Skakkebaek et al. 2001). The TDS hypothesis states that in utero exposure to endocrine disruptors can damage testis development and lead to reduced testis function in the adult, with symptoms ranging from a moderately reduced semen quality to testis cancer. The best animal model for TDS consists of rats exposed to long-chain phthalates in a critical time window during development, which results in testis dysgenesis with Leydig cell hyperplasia and clustering of the Leydig cells in the centre of the testis, resulting in reduced testosterone levels and compromised fertility in the adults (Sharpe 2006; Hallmark et al. 2007).
The compromised Leydig cell function is reflected in a reduced expression of genes involved in cholesterol transport and steroidogenesis (Liu et al. 2005). This has striking similarities to the reported effect of PFAS exposure; however, it seems as if PFAS compounds, in contrast to phthalates, can induce the effects in the adult.
PFOS did affect the neuroendocrine system in rats, when female rats were injected intraperitoneal with 0, 1 and 10 mg PFOS/kg bw for two weeks (Austin et al. 2003). The oestrous cyclicity was affected, serum corticosterone level was increased, and serum leptin concentration and norepinephrine concentration in the paraventricular nucleus of the hypothalamus were decreased.
10.2.10 Mutagenicity
PFOA was non-mutagenic in the Ames test using five strains of Salmonella typhimurium and in a single strain of Saccharomyces cerevisiae (Griffith and Long 1980). Several other mutagenicity studies of PFOA published by contract laboratories support the inactivity of PFOA (Kennedy et al. 2004). PFDA is also negative in the Ames-Test and various other test systems. However, PFDA was active in a chromosome aberration assay in the presence of S-9 mix and in S-phase DNA synthesis assay (Godin et al. 1992).
Polyfluorinated chemicals may increase the carcinogenicity of other chemicals since the genotoxicity of cyclophosphamide in the micronucleus assay with hamster lung V79 cells increased many fold by simultaneous exposure to PFOS (Jernbro et al. 2007). See Figure 10.1:
Figure 10.1: Genotoxicity of cyclophosphamide (CPP) + PFOS in micronucleus test (After Jernbro et al. 2007)
No results of mutagenicity testing with other polyfluorinated chemicals were found in the open literature.
10.2.11 Cancer
In animal studies with CD rats, a strain that has a low spontaneous incidence of these tumours, PFOA produced a dose-dependent increase in Leydig cell adenomas (Biegel et al. 1995; Liu et al. 1996). The tumours may be a result of endocrine changes, because a reduced aromatase activity and a sustained increase in serum estradiol were observed (Biegel et al. 2001). Nevertheless, US Environmental Protection Agency classifies nevertheless PFOA as a carcinogen in animals (US EPA 2002).
A finished two-years rat feeding study with PFOS has only been briefly reported (Seacat et al. 2002b). A modest liver tumour response was observed in the high dose group of 20 ppm PFOS as potassium salt.
A dietary dose of 100 ppm EtFOSE caused an increase of hepatocellular adenomas in females and thyroid follicular cell adenomas in males rats (Thomford et al. 2002). About 20% of an oral dose of EtFOSE is metabolised to PFOS.
10.2.12 Cocktail effects
Since exposure to polyfluorinated compounds is ubiquitous to humans this exposure is added to all other exposures humans may experience. This raises the question of possible mixture effects, which could increase effects from other exposures.
Mixture effects have already been shown in vitro and in vivo for estrogenic compounds (Silva et al. 2002; Tinwell and Ashby 2004) and for anti-androgens (Birkhoj et al. 2004; Metzdorff et al. 2007), but there are very few studies of mixture effects of compounds with different modes of action.
Kudo and Kawashima (1997) found that fish oil-feeding prevents PFOA induced fatty liver in mice.
For polyfluorinated substances there are more reports of such effects. It was just mentioned that the genotoxicity of cyclophosphamide in the micronucleus assay with hamster lung V79 cells increased many fold by simultaneous exposure to PFOS (Jernbro et al. 2007). Therefore, polyfluorinated chemicals may increase the carcinogenicity of other chemicals.
Co-administration of PFOS and dioxin (TCDD) resulted in an increased p450 A1A expression as compared to TCDD alone (Hu et al. 2003).
10.3 Structure-activity relations
The major pile of toxicological information is about PFOS and PFOA, thus the knowledge about other polyfluorinated chemicals is scarce. Qualitatively we know that PFOS and PFOS derivates seem to be more toxic than PFOA and derivatives. Furthermore, the persistence and toxicity of perfluorinated acids increases in general with the chain length and substances with branched chain are less toxic than linear substances. There seems presently to be insufficient background data to make quantitative structure-activity relationships (QSAR) for these homologues but it may be possible at a later stage.
It is even more difficult making theoretical predictions of the properties of the more complex telomers and other complex polyfluorinated compounds used in consumer products. They belong to various chemical classes and may ultimately be degraded or metabolised to their parent perfluorinated acids. Qualitatively, it will be possible with some certainty to predict, which perfluorinated acid the chemical compound is precursor for, and OECD has made an attempt in their substances lists to indicate the potential lengths (or range of lengths) of the fluorinated carbon chain as a result of degradation.
11 Human risk assessment
In general the knowledge about the toxicology of the perfluorinated compounds is rather sparse, and it will take some time and efforts, before we will have sufficient information for evaluation of the full impact of the present levels in humans.
11.1 Toxicological summary
PFOA and PFOS may bind to proteins in the blood and accumulate in various body tissues of exposed organisms, including in liver, kidneys, testes and brain. The half-lives of PFOS and PFOA in human blood have been estimated to about 4 years but the whole body half-life will be even longer, since the elimination of these chemicals in humans is neglible.
Although the perfluoroalkyl sulfonic acids and carboxylic acids are closely related structurally, these chemicals elicit different biological responses in vitro and in vivo. The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, and the toxicity of perfluorinated chemicals increases generally with the length of the alkyl chain.
The liver is the primary target organ for perfluorinated compounds. PFOS and PFCAs cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFDA with a longer alkyl chain seems even more active than PFOA. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain.
Subchronic exposure of animals to PFOS may lead to significant weight loss accompanied by hepatotoxicity and reduction of serum cholesterol and thyroid hormones. PFOA and PFOS also interact with serum levels of various sex hormons, e.g. reduction of testosterone and increase of oestradiol in rats. Thus it is an endocrine disruptor.
PFOS, PFOSA, PFHxS and perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner, and with PFDA inhibiting more than PFOA. Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases.
PFOA and PFOS also affect the serum levels of various hormones, i.e. reducing testosterone, and increasing estradiol in rats. Thus it acts as an endocrine disruptor.
Although the fluorinated chemicals do not seem to be mutagenic, PFOA induces testis tumors and PFOS and EtFOSE induce liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals.
PFOS causes developmental effects including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance of these effects may be questioned. PFOA causes reduction of foetal weight. Other PFAS (PFBS and PFHxS) have neither a significant effect on reproduction nor on development, even at high doses.
The experience from the working environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctane sulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs.
The risk characterisation for the general population shows according to Butenhoff et al. (2004) a sufficient margin of exposure and safety. This assessment was, however, based on animal data regarding liver weight increase and Leydig cell adenoma, which may be irrelevant for humans.
Two recent human studies link prenatal exposure to perfluorinated compounds to lower birth weight (Apelberg et al. 2007b; Fei et al. 2007)
11.2 Tolerable daily intake
The Committee on Toxicity (2006ab) in the U.K. has recently performed a risk assessment of PFOA and PFOS:
- The lowest NOAEL for PFOA found was 0.06 mg/kg bw/d for increased liver weight in rats in a 13-week study. However, a five times higher level of 0.3 mg/kg bw/d and a uncertainty factor of 100 to allow for inter- and intra-species variation was used for deriving the TDI for PFOA of 3 mg/kg bw/d. That was compared with an estimated food intake of up to 0.1 mg/kg bw/d for adults and 0.3 mg/kg bw/d for toddlers.
- The lowest NOAEL for PFOS found was 0.03 mg/kg bw/d for decreased T3 levels in a 26-week monkey study. It was used to propose a provisionally TDI for PFOS of 0.3 mg/kg bw/d using an uncertainty factor of 100 to allow for inter- and intra-species variations. The food intake of PFOS in UK was estimated to max. 0.2 mg/kg bw/d in adults and 0.5 mg/kg bw/d in 1.5-2.5 year olds, thus for some small children the TDI is exceeded.
There are several aspects in these risk assessments which may be questioned:
- The lowest NOAEL for PFOA was not used.
- The durations of the animal experiments, from which data used were collected, were 3-6 months. Life-time bioassays are likely to generate lower NOAELs. Normally, a further uncertainty factor of 10 is used when using sub-chronic instead of chronic toxicity studies. This has not been done in the UK risk assessment.
- The conservative threshold approach is used for PFOA, although PFOA is considered to be an animal carcinogen by US EPA.
- The uncertainty factor of 100 applied in the estimations above seems to be very conservative, because the half-lives of these chemicals in human blood are 20-100 times longer than half-lives in rodents, and there are other differences to take into account. The fact that the renal clearances of PFOA and PFOS are insignificant in humans, contrary to a large active excretion in experimental animals (Harada et al. 2005a) means that these chemicals in humans leaves the blood by redistribution to internal organs and not by elimination from the body. This increases the internal exposure time in critical organs considerable and makes risk assessment of perfluorinated chemicals based on animal experiments very arbitrary and unreliable.
- The referred maximum food intake in the U.K. may not be typically for other countries and the time trend is not known.
- People are exposed to these chemicals and their precursors from other sources than food. Some studies have shown that direct product exposure, indoor air and dust may be more important.
12 General discussion, conclusions and recommendations
- 12.1 Discussion, conclusions and recommendations concerning the chemical family
- 12.2 Discussion, conclusions and recommendations concerning the use survey
- 12.3 Discussion, conclusions and recommendations concerning the environmental impacts
- 12.4 Discussion, conclusions and recommendations concerning the human health impacts
12.1 Discussion, conclusions and recommendations concerning the chemical family
During the past years an awareness has arisen on a new type of persistent organic pollutants, which contains an alkyl chain typically between 4 and 12 carbon atoms, where all or most of the hydrogen atoms have been replaced by fluorine. This makes the chain very stable and practically non-degradable in the environment. The substances also contain a more reactive functional group, which may be an alcohol, a carboxylic acid, a sulfonic acid, a phosphoric acid or their derivatives.
Today more than thousand polyfluorinated substances are known. These substances are surface active substances with an extreme low surface tension, and they repel water, grease and dirt, and are therefore used as surfactants or impregnating agents in numerous industrial products and consumer products under trade names such as Scotchgard®, Baygard®, Gore-Tex®, Zonyl® and Stainmaster®.
Until the start of this century, the most used polyfluorinated compounds were PFOS (perfluorooctane sulfonate) and PFOS-related compounds. When it became clear that these persistent chemicals were global pollutants, and high levels were found in polar bears from remote arctic areas, the production and use of these compounds stopped, and a formal ban has recently been introduced in the European Union. Today PFOS has been substituted in products by either perfluorinated substances with a shorter chain length (C6 or shorter) or other classes of more complex polyfluorinated substances such as fluorotelomer alcohols (FTOH) and their derivatives.
These more complex compounds may be precursors of and be degraded to the ultimate perfluorinated acids. The fluorocarbon tail is, however, rather stable.
12.2 Discussion, conclusions and recommendations concerning the use survey
The purpose of this project has been to estimate the use of polyfluorinated compounds (PFCs) in impregnation and consumer products in Denmark, and to perform an update of the environmental and health assessment of polyfluorinated substances and their degradation products carried out previously for the Danish EPA (Poulsen et al. 2005).
In order to estimate the use of fluorinated compounds used in consumer products in Denmark, the following approach has been used. Initially, a search was carried out in the Danish Product Register in order to determine the registered use of these substances in Denmark. Secondly, several companies in Denmark as well as foreign producers/suppliers of these fluorinated substances were contacted in order to obtain information for developing an estimate of the consumption of fluorinated chemicals in consumer products in Denmark. Searches on the Internet were used as an extra source of information, and information found about the level of fluorinated substances in products was combined with Danish statistical information in order to estimate an amount of fluorinated substances in consumer products within specific use areas in Denmark.
Results of the survey
The search in the Danish Product Register showed a total use of fluorinated substances of 16.5 tonnes. As a basis for the search OECDs Preliminary lists of PFOS, PFAS, PFOA and related compounds and chemicals that may degrade to PFCA (OECD, 2006) were used. In total 92 fluorinated substances were identified in the Danish Product Register, of which 48 substances were registered with a use of 0.00 tonnes (meaning either a very low consumption or that a use amount had not been registered, as it should have been done).
The most important use areas (according to the reported totals) are releasing agents, paint and lacquers, glue, surface active substances and galvano-technical products, which accounts for about 15 of the 16.5 tonnes in total. Releasing agents are compounds used e.g. in moulds in order to get the moulded plastic product for example to release easily from the mould. However, releasing agents can also be used on e.g. frying pans to ensure a non-stick surface.
The use in areas polish and care products, impregnating agents, cleaning agents and surface active substances (non-metal, e.g. for paper and cardboard) accounts for about 0.5 tonnes (of the last 1.5 tonnes). These uses are most likely much greater, as only chemical products labelled as dangerous have to be registered in the Danish Product Register.
The Danish Product Register does not register all products containing fluorinated compounds on the Danish market, and the registered amounts do not give an adequate picture of the total sales in Denmark. In addition, imported finished products, such as raincoats containing fluorinated compounds, are not registered in the Product Register.
As a supplement to the search in the Danish Product Register information was obtained from Danish Statistics on the content of fluorinated substances in different consumer products. The estimated total consumption of fluorinated substances in consumer products in Denmark is shown in Table 12.1.
Table 12.1: Total estimated amount of fluorinated substances used or contained in products in Denmark
| Use area | Min. estimated amount of fluorinated substances in products (kg) | Max. estimated amount of fluorinated substances in products (kg) |
| Releasing agents | 7200 | > 7200 |
| Paint and lacquers | 100 | 3500 |
| Printing inks | 15 | > 15 |
| Glue | 2500 | > 2500 |
| Surface active substances | 1100 | > 1100 |
| Cleaning agents | 100 | > 100 |
| Polish and care products | 170 | 590 |
| Carpets | 745 | 18000 |
| Sunshades/awnings, tents, umbrellas, parasols etc. | not estimated | not estimated |
| Impregnated clothing | 400 | 3,500 |
| Footwear | not estimated | not estimated |
| Impregnating agents | 170 | 340 |
| Galvano-technical products | 760 | > 760 |
| Inhibitors | 400 | > 400 |
| Pesticides | 180 | > 180 |
| Soldering agents | 280 | > 280 |
| Total | 14120 kg @ 14.tonnes | > 38465 kg @ > 38 tonnes |
One thing is the use of fluorinated substances, another thing is, however, the type of fluorinated substances used, and the possibility of the substances to be degraded to PFOS, PFOA or other PFCAs in the environment, as these substances are the most critical in the environment. According to documentation from DuPont impurities of PFOA in products containing fluorinated substances are typically between 0.1 and 1% of the total content of fluorinated substances. Besides this potential content of PFOA as impurities, products may contain precursor compounds, such as fluorotelomer alcohols, being able to degrade to PFCAs.
It must be noted that about 7.5 tonnes of the total 16.5 tonnes registered in the Danish Product Register count for substances that have a chain length lower than 8, and there are furthermore substances that are not on the OECD list of PFAS, PFOS, PFOA and other substances that can be degraded to PFCA.
The rest of the chemicals may, however, have a potential to degrade to PFOA or other PFCAs in the environment. The exact amount is not known, as this would require detailed knowledge of the fluorinated substances used, as only specific types of fluorinated substances can be degraded to PFOS, PFOA or other PFCAs in the environment.
Conclusions and recommendations to survey
During this project several companies in Denmark as well as foreign producers and suppliers of fluorinated substances have been contacted in order to obtain information about the consumption of fluorinated substances in Denmark. This approach was, however, unsuccessful because either the companies had no knowledge about these fluorinated chemicals or they simply did not want to participate with information to the project.
It is, therefore, difficult or impossible to obtain a more precise estimate of the consumption of these substances in Denmark than by using the information in the Danish Product Register.
This is however, also problematic, as the data in the Danish Product Register is not complete. First of all, the Product Register data only covers chemical products (and only classified chemical products) and not consumer products/articles. Secondly, the information in the Product Register does not seem to be completely up to date – a lot of uses are registered with a consumption of 0.00 tonnes, indicating information is lacking.
The complexity of the area is further demonstrated by the fact that the chemicals are not only found in chemical products (more easy to track and measure), but also as content or impurity in consumer products/articles. It is almost impossible to track e.g., which impregnating agents that have been used to produce all-weather clothes in China, and to find out, which amounts are sold in Denmark.
The last resort has been to use Danish Statistics to estimate the consumption of fluorinated chemicals in consumer products in Denmark. These estimates have a high uncertainty as the statistics on supply on certain consumer products in Denmark, not necessarily is very precise – the product groups are too large for the purpose of this project. Furthermore, these statistical data have been multiplied with a concentration (range) of fluorinated substances in the products, in order to estimate a total amount used in consumer products in Denmark. This concentration range has been based on information from published chemical analysis of products and on information found on impregnating chemicals and use amounts on the Internet.
In order to learn more about the content and concentration of fluorinated substances in consumer products in Denmark, more chemical analysis have to be carried out in order to learn more about the substances used and the range of concentrations that can be found.
This survey has shown that chemical analysis have been carried out mainly for impregnating agents for e.g. footwear and all-weather clothes. In all other consumer product areas the information about the content of fluorinated substances is limited: carpets, footwear, sunshades etc., paints, printing inks, auto polish and waxes, floor polish and cleaning agents.
According to the estimations carried out in this project, carpets seem to be the largest use areas. A use could not be estimated for sunshades, awnings, parasols etc., but this area also seems to be of relevance for further investigation. It is therefore suggested that these products are in focus if chemical analysis of the content of fluorinated substances are carried out.
12.3 Discussion, conclusions and recommendations concerning the environmental impacts
Environmental fate and levels
Concentrations of PFCs have been extensively reported for all environmental compartments all over the world. Most studies have been performed in the North American continent, Europe and Japan. The number of compounds analyzed has been extended to a large series of perfluorinated carboxylates with carbon number from 7 to 16. Besides PFOS, the list of sulfonates has been extended to compounds with 7, 9 or 10 carbon atoms. The attention has also been focused on the precursor and intermediates of the more persistent PFOS and PFCAs.
Biodegradation studies of precursor compounds such as fluorotelomer alcohols (FTOHs) and perfluorosulfonamides demonstrated that these compounds are degraded to the more persistent PFOS and PFCAs.
Several studies performed with the volatile precursor compounds in smog chambers have found that FTOHs and sulfonamides react in the atmosphere with OH radicals to yield the acidic compounds. The reaction time is long enough (about 20 days) for these compounds to be transported to remote regions, where deposition and further bioaccumulation in the food chain occurs. The “precursor” theory for explaining the presence of PFCs in remote regions (e.g. the Arctic) has been supported by atmospheric measurements of FTOHs and perfluorosulfonamides in both industrial and remote regions. Atmospheric concentrations were higher close to sources, but these compounds were also detected in the Arctic atmosphere. Oceanic transport of ionic PFCs has also been proposed as alternative or supplemental transport path to remote regions, with the compounds directly dissolved in water or present as a film on the surface foam. Another theory hypothesizes direct transport of PFOS and PFCAs directly from the sources directly bound on atmospheric particulate phase.
Indoor measurements of PFCs concentrations have been performed in both gaseous and particulate phase. Indoor concentrations resulted up to 100 times higher than outdoor concentrations and carpets were identified as one of the major sources of PFCs on the basis of measurements of household vacuum cleaner dust; no actual analysis of carpets have been carried out.
Improvements in analytical detection limits have made possible measurements of PFCs levels at ppt levels in environmental waters, included rainwater and oceanic waters, where PFCs have been detected at very low concentrations.
Several studies have been published on concentrations and fate of PFCs in wastewater treatment plants (WWTPs), as these systems have been identified as important sources for PFCs to the aquatic and terrestrial environments. Precursors of PFOS and PFCAs have also been analyzed. These compounds have been found in wastewater together with PFOS and PFCAs. PFCs were also found in sludge from WWTPs. PFOS and PFCAs were found more or less not degradable in WWTPs.
PFCs concentrations in wildlife have been reported for a wide range of animals at all latitudes, including the Arctic and the Antarctic. PFCs and especially PFOS have been more or less found in all samples with concentrations varying from sub-ng/g levels to several µg/g, with birds living close to a fluorochemical plant as the worst case. The bioaccumulation potential of PFCs has been confirmed by several bioaccumulation studies in different food webs.
Temporal trends studies have been performed on archived biologic materials, in general covering time spans from the 1970s-1980s to the present. The PFCs concentrations, especially those of PFOS, have been found to gradually increase up to the present years. Only in a study from the Canadian Arctic it has been observed a decrease in PFOS concentration after 2000, which was explained as a rapid response after the stop of PFOS production in USA.
Ecotoxicity
PNEC (Predicted No Effect Levels) values of 0.0167 and 0.067 mg/kg food have been reported for secondary poisoning in the food chain for the aquatic environment for PFOS. The comparison of PNEC values with measured environmental PFOS concentrations raised concern about secondary poisoning in the aquatic environment. The toxicity of PFOS and PFOA has been tested for different aquatic organisms (algae, invertebrates and fish).
Generally, PFOA and PFOS are not found to be toxic at the normal environmental concentrations in water. However, different effects have been observed for specific cellular functions such as mechanisms involving the uptake of xenobiotics. Other biological endpoints affected by PFOA and PFOS are survival, growth, and emergence. The degradation products of FTOH (saturated and unsaturated fluorotelomer acids) have been found to be more toxic than their end products (PFCAs) by a factor of 10,000.
12.4 Discussion, conclusions and recommendations concerning the human health impacts
Human levels
Perfluorinated chemicals (PFC) have in contrary to most other persistent organic pollutants (POP) a low affinity to lipids in adipose tissues but bind to proteins in cell membranes and accumulate in various body tissues of exposed organisms, including in liver, kidneys, testes and brain. The accumulation in fats and muscles is minimal.
In the blood perfluorinated chemicals are mainly bound to serum proteins, especially albumin. The mean half-lives in human blood were 5.4 years for PFOS, 8.5 years for PFHxS, and 3.8 years for PFOA in retired fluorochemical workers but the whole body half-life may be even longer, since the elimination of these chemicals from the human body is insignificant. Further, the half life for the lower steady state concentrations in the general population is probably much longer.
Blood levels of perfluorinated chemicals have been monitored in many countries but most data has been developed in USA. In all countries, besides Korea, PFOS has been determined in far higher concentrations than the other PFCs. Typical average serum levels of PFOS in industrialized countries are 20-30 ng/mL with maximum levels less than 100 ng/mL. The second most abundant PFC is normally PFOA with typical average serum levels of 3-5 ng/mL. Some of the highest PFC blood levels (2-3 times the typical levels) in the general population were determined in industrial areas of USA and China. Such levels may be 10 times higher than in rural and remote area.
Recently data from Denmark was published. The average PFOS level in blood serum was 35 ng PFOS/mL with a maximum concentration of 107 ng/mL. That is somewhat higher concentration than in our neighbour countries. It may at least partly be explained by that the Danish samples are old (1996-2002); thus there is a need for further studies.
Some studies have analysed blood plasma or whole blood instead of serum. Analysis of plasma will give the same results as serum but whole blood levels will be 2-3 times lower than serum levels. Levels of PFCs in cord blood are about the half of levels in maternal blood thus some transfer to placenta occurs. Levels of PFCs in human semen are ten times lower than in blood serum and levels in human milk are 100 times lower than in blood, so these fluids are not suitable for biological monitoring of PFCs.
Toxicokinetics
In animal experiments the studied PFCs are readily absorbed in the gastro-intestinal tract and some compounds penetrate the intact skin. The peak blood levels are seen 1-2 hours after exposure and the substances clear rapidly from the blood.
PFOA and PFOS are both considered being metabolically inert, and other perfluoroalkyl acids with shorter or longer alkyl chain do have similar properties. Their precursors and functional derivatives will ultimately be transformed to their basic acids. For example, the fluorotelomer alcohol 8:2 FTOH is rapidly transformed to PFOA, PFNA and other metabolites in mice and rats. In the same way EtFOSE is metabolised to FOSE, FOSA and finally to PFOS.
Once absorbed in the body PFOA is eliminated as the free carboxylic acid mainly with urine and to a less extent in faeces. Thus the renal elimination is critical for detoxification, and the lamination decreases with increasing chain length among the perfluorocarboxylic acids (PFCAs). The biological half-life of PFOA in male rats is 70 times longer than that in female rats for which it was only 2 hrs. The sex-related clearance of PFOA differs between animal species. In hamsters it is opposite rats. In mice and rabbits there are no sex difference; and mice had a slow excretion as male rats; and rabbits had a fast excretion as female rats. In dogs the plasma half-life of PFOA was about 20 days in males and the half in females. In monkeys the biological half-life is several months. As mentioned above the excretion of PFCs in humans are insignificant, thus animals may not be a good model.
Toxicology
Although the perfluoroalkyl sulfonic acids and carboxylic acids are closely related structurally, these chemicals elicit different biological responses in vitro and in vivo. The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, and the toxicity of perfluorinated chemicals increases generally with the length of the alkyl chain. PFCAs with a branched alkyl chain seem to be less toxic than linear isomers.
The liver is the primary target organ for perfluorinated compounds. PFOS and PFCAs cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFDA with a longer alkyl chain seems even accumulative and more active than PFOA. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain.
Subchronic exposure of animals to PFOS may lead to significant weight loss accompanied by hepatotoxicity and reduction of serum cholesterol and thyroid hormones. The lowest NOAEL for PFOA found was 0.06 mg/kg bw/d for increased liver weight in rats in a 13-week study. The lowest NOAEL for PFOS found was 0.03 mg/kg bw/d for decreased T3 levels in a 26-week monkey study.
Inhibits cellular communication
PFOS, PFOSA, PFHxS and perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner, and with PFDA inhibiting more than PFOA. Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases.
Endocrine disruptors
Both PFOA and PFOS affect the serum levels of various hormones, i.e. reducing testosterone, and increasing estradiol in rats. Thus these substances may act as endocrine disruptors.
Cancer
Although the fluorinated chemicals do not seem to be mutagenic, PFOA induces testis tumors and PFOS and EtFOSE induce liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals.
The experience from the work environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctane sulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs.
Developmental toxicity
PFOS causes developmental effects including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance may be questioned. PFOA causes reduction of foetal weight. Other PFAS (PFBS and PFHxS) have neither a significant effect on reproduction or development even at high doses.
Risk assessment
The U.K. Committee on Toxicity (2006) has recommended a provisionally TDI for PFOA and PFOS of 3 mg/kg bw/d and 0.3 mg/kg bw/d, respectively, using an uncertainty factor of 100. They conclude that for some small children the TDI may already be exceeded. This assessment was based on results from animal experiments, which may be very arbitrary and unreliable, because the renal clearances of PFOA and PFOS are insignificant in humans, contrary to a large active excretion in experimental animals (Harada et al. 2005). This means that these chemicals in humans leave the blood by redistribution to internal organs and not by elimination from the body. This increases the internal exposure time in critical organs considerable
Conclusions and recommendations to human toxicology
The main exposures to perfluorinated substances seem to be by direct product exposure, through food intake or by inhalation/ingestion of indoor dusts but it is not sufficient explained.
Perfluorinated chemicals are readily absorbed in the organism and concentrate in the blood and internal organs associated with proteins. The half-lives in the blood are several years in humans compared to hours or days in experimental animals, in which the renal clearance is fast contrary to in humans, where it is insignificant.
The levels of perfluorinated chemicals in human blood differ between countries and areas. The levels are higher in industrial area than in rural areas. PFOS is still the most abundant followed by PFOA but here seems to be a time trend downwards for these chemicals and upward trend for PFNA. The blood levels of PFOS in Denmark are in the upper end of the global average, and new studies are warranted.
The toxicology of PFOS and PFOA are rather well-studied in animals but the available information about the health-related properties of other polyfluorinated substances is limited.
The great difference in residence time in the body between humans and animals makes it questionable using the animal data for risk assessment as it is presently done.
The recent independent studies showing that exposure to perfluorinated chemicals at the present levels may affect pregnancy and decrease birth weight in humans is worrying but presently, we have insufficient information for evaluation of the full health impact of the present exposure levels in humans.
13 References
3M. Perfluorooctane sulfonate: Current summary of human sera, health and toxicology data. 3M, January 21, 1999.
http://www.chemicalindustryarchives.org/
dirtysecrets/scotchgard/pdfs/226-0548.pdf
Alzaga R, Salgado-Petinal C, Jover E, Bayona JM. Development of a procedure for the determination of perfluorocarboxylic acids in sediment by pressurized fluid extraction, headspace solid-phase microextraction followed by gas chromatographic-mass spectrometric detection. J Chromat A 2005; 1083: 1-6.
Alexander BJ, Olsen GW, Burris JM, Mandel JH, Mandel JS. Mortality of employees of a perfluorooctane sulfonyl fluoride manufacturing facility. Occup Environ Med 2003; 60: 722-729.
Andersen ME, Clewell HJ, Tan Y-M, Butenhoff JL, Olsen GW. Pharmacokinetic modelling of saturable, renal resorption of perfluoroalkylacids in monkeys – probing the determinants of long plasma half-lives. Toxicology 2006; 227: 156-164.
Andersson AM, Jorgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab 2004; 89: 3161-3167.
Apelberg BJ, Goldman LR, Calafat AM, Herbstman JB, Kuklenyik Z, Heidler J, Needham LL, Halden RU, Witter FR. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol 2007a; 41: 3891-3897.
Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Heidler J, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspec 2007b; 115: 1670-1676.
Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M, Korzeniowski SH Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ Sci Technol 2006; 40: 6969-6975.
Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SMJ. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ. Health Perspec 2003; 111: 1485-1489.
Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones K. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ Monit 2007; 9: 530-541.
Barton CA, Kaiser MA, Russel MH. Partitioning and removal of perfluorooctanoate during rain events: the importance of physical-chemical properties. J Environ Monit 2007; 9: 839-846.
Beach SA, Newsted JL, Coady K, Giesy JP. Ecotoxicological evaluation of perfluorooctanesulfonate (PFOS). Rev Environ Contam Toxicol 2006; 186: 133-174.
Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit Contam 2005; 22: 1023-1031.
Berger U, Järnberg U, Kallenborn R. Perfluorinated alkylated substances (PFAS) in the European Nordic environment. Organohalogen Compounds 2004; 66: 4046-4052.
Berger U, Herzke D. Per- and polyfluorinated alkyl substances (PFAS) extracted from textile samples. Organohalogen Compounds 2006; 68: 2023-2026.
Berthiaume J, Wallace KB. Perfluorooctanoate, Perfluorooctane sulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondria biogenesis. Toxicol Lett 2002; 129: 23-32.
Betts KS. Perfluoroalkyl acids. What is the evidence telling us? Environ Health Perspec 2007; 115: A250-A357.
Biegel LB, Hazard characterization for human health C8 exposure CAS registry no. 3825-26-1. DuPont, 1997.
Biegel LB, Liu RCM, Hurtt ME, Cook JC. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo and ex vivo studies. Toxicol Appl Pharmacol 1995; 134: 18-25.
Biegel LB, Hurtt ME, Frame SR, O'Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 2001; 60: 44-55.
Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, Vinggaard AM. The combined antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol 2004; 201: 10-20.
Bossi R, Riget FF, Dietz R. Temporal and spatial trends of perfluorinated compounds in ringed seal (Phoca hispida) from Greenland. Environ Sci Technol 2005a; 39: 7416-7422.
Bossi R, Riget FF, Dietz R, Sonne C, Fauser P, Dam M, Vorkamp K. Preliminary screening of perfluooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faeroe Islands. Environ Poll 2005b; 136: 323-329.
Boulanger B, Vargo J, Schnoor JL, Hornbuckle KC. Evaluation of perfluorooctane surfactants in a wastewater treatment system and in a commercial surface protection product. Environ Sci Technol 2005; 39:5524-5530.
Brooke D, Foottit A, Nwaogu TA. Environmental risk evaluation report: perfluorooctane sulfonate (PFOS). Environment Agency, 2004. http://www.environment-agency.gov.uk/
Butenhoff JL, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, Jung R, Kennedy GL Jr, Lieder P, Olsen G, Thomford P. Toxicity of ammonium perfluorooctanoate in male Cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci 2002a; 69: 244-257.
Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, Zobel LR. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol 2004b; 39: 363-380.
Butenhoff JL, Kennedy GL Jr, Hinderliter PM, Lieder P, Jung R, , Hansen KJ, Gorman GS, Noker PE, Thomford P. Pharmacokinetics of perfluorooctanoate in male Cynomolgus monkeys. Toxicol Sci 2004a; 82: 394-406.
Butenhoff JL, Olsen GW, Pfahles-Hutchens A. The applicability of biomonitoring data for perfluorooctane sulfonate to the environmental public health continuum. Environ. Health Perspec 2006; 114: 1776-1782.
Butenhoff J, York R, Seacat A, Luebker D. Perfluorooctane sulfonate-induced perinatal mortality in rat pups is associated with a steep dose-response. Toxicologist 2002b; 66(1-S): 25 (abstract).
Butt CM, Muir DCG, Kwan M, Mabury SA. Rapid response of arctic ringed seals to changes in perfluoroalkyl production. Environ Sci Technol 2007a; 41: 42-49.
Butt CM, Mabury SA, Muir DCG, Braune BM. Prevalence of long-chained perfluorinated carboxylates in seabirds from the Canadian Arctic between 1975 and 2004. Environ Sci Technol 2007b; 3521-3528.
Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ Sci Technol 2006b; 40: 2128-2134.
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Sci Technol 2007a; 41: 2237-2242.
Calafat AM, Needham LL, Kuklenyik Z, Reidy JA, Tully JS, Aguilar-Villalobos M, Naeher LP. Perfluorinated chemicals in selected residents of the American continent. Chemiosphere 2006a; 63: 490-496.
Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspec 2007b; 115: 1596-1602.
Case MT, York RG, Christian MS. Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. Int J Toxicol 2001; 20: 101-109.
Clark LC, Becattini F, Kaplan S, Obrock V, Cohen D, Becker C. Perfluorocarbons having a short dwell time in the liver. Science 1973; 181: 680-682
Committee on Toxicity. COT statement on the tolerable daily intake for perfluorooctanoic acid. UK Food Standards Agency, 8 Nov 2006a. http://www.food.gov.uk/multimedia/pdfs/cotstatementpfoa200610.pdf
Committee on Toxicity. COT statement on the tolerable daily intake for perfluorooctane sulfonate. UK Food Standards Agency, 8 Nov 2006b. http://www.food.gov.uk/multimedia/pdfs/cotstatementpfos200609.pdf
Cook JC Murray SM, Frame RS, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol 1992; 1134: 209-217.
Corsolini S, Kannan K. Perfluorooctane sulfonate and related fluorochemicals in several organisms including humans from Italy. Organohalogen Compounds 2004; 66: 4079-4085.
Cunha I, Hoff P, Van de Vijver K, Guilhermino L, Esmans E, De Coen W. Baseline study of perfluorooctane sulfonate occurrence in mussels, Mytilus galloprovincialis, from north-central Portuguese estuaries. Mar Poll Bull 2005; 50: 1121-1145.
Dai J, Li M, Saito N, Xu M, Wei F. Perfluorooctanesulfonate and perfluorooctanoate in red panda and giant panda from China. Environ Sci Technol 2006; 40: 5647-5652.
Dauwe T, Van de Vijver C, De Coen W, Eens M. PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant. Environment International 2007; 33: 357-361.
Davisa KL, Barbara S, Larsen BS, Kaiser MA, Andrew S. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 2007; 67: 2011-2019.
D'Eon JC, Hurley MD, Wallington TJ, Mabury SA. Atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and mechanism of reaction with OH. Environ Sci Technol 2006; 40:1862-1868.
D'Eon JC, Mabury SA. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): Exploring routes of human contamination. Environ Sci Technol 2007; 41: 4799-4805.
De Silva AO, Mabury SA. Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations. Environ Sci Technol 2004; 38: 6538-3545.
De Silva AO, Mabury SA. Isomer distribution of perfluorocarboxylates in human blood: Potential correlation to source. Environ Sci Technol 2006; 40: 2903-2909.
Dimitrov S, Kamenska V, Walker JD, Windle W, Purdy R, Lewis M, Mekenyan O. Predicting the biodegradation products of perfluorinated chemicals using catabol. SAK QSAR Environ Res 2004; 15: 69-82.
Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol 2004; 38: 2857-2864.
Dinglasan-Panlilio MJA, Mabury SA. Significant residual fluorinated alcohols present in various fluorinated materials. Environ Sci Technol 2006; 40: 1447-1453.
Directive 2006/122/EC of the European Parliament and of the Council of 12 December 2006 amending for the 30th time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (perfluorooctane sulfonates). http://eur-lex.europa.eu/LexUriServ/site/en/oj/2006/
l_372/l_37220061227en00320034.pdf
Ellis DA, Mabury SA, Martin JW, Muir DCG. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001; 412: 321-324.
Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Andersen MPS, Wallington TJ. Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environ Sci Technol 2004; 38: 3316-3321.
Ehresman DJ, Froelich JW, Olsen GW, Chang S-C, Butenhoff JL. Comparison of whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 2007; 103: 176-184.
Engelund B, Sørensen H. Mapping and health assessment of chemical substances in shoe care products. Survey of chemical substances in consumer products no. 52. Copenhagen: Danish EPA, 2005.
Fairly KJ, Purdy R, Kearns S, Anderson SE, Meade BJ. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol Sci 2007; 97: 375-383.
Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U. Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic Coast? Environ Sci Technol 2006; 40: 748-751.
Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, Buck RC, Korzeniowski SH, Hinderliter PM, Kemper RA. Absorption, distribution, metabolism, and elimination of 8-2 fluorotelomer alcohol in the rat. Toxicol Sci 2006; 91: 341-355.
Fei C, McLaugghlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within Danish national birth cohort. Environ Health Perspec 2007; 115: 1677-1682.
Ferdinand J, Kaysen O, Petersen C. Chemical substances in auto polish and wax. Survey of chemical substances in consumer products no. 41. Copenhagen: Danish EPA, 2004.
Fiebig S, Geffke T, Schulze D, et al. Anaerobic biodegradation of 8:2 fluorotelomer alcohol in digested sludge. Poster, SETAC-Europe’07, Porto.
Fromme H, Midasch O, Twardella D, Angerer J, Boehmer S, Liebl B. Occurrence of perfluorinated substances in an adult German population in Southern Bavaria. Int Ach Occup Environ Health 2007; 80: 313-319.
Fuentes S, Colomina MT, Vicens P, Franco-Pons N, Domingo JL. Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: effects on postnatal development and behavior of the offspring. Toxicol Sci 2007; 98: 589–598.
Furdui VI, Stock NL, Ellis DA, Butt CM, Whittle DM, Crozier PW, Reiner EJ, Muir DCG, Mabury SA. Spatial distribution of perfluoroalkyl contaminants in lake trout from the Great Lakes. Environ Sci Technol 2007; 41:1554-1559.
Gauthier SA, Mabury SA. Aqueous photolysis of 8:2 fluorotelomer alcohol. Environ Toxicol Chem 2005; 24: 1837-1846.
Gewurtz SB, Crozier P, Bhavsar SP, Diamond ML, Helm PA, Marvin C, Reiner E. Perfluoroalkyl contaminants in indoor window film before and after a carpet installation. Organohalogen Compounds 2007; 69: 1005-1008.
Giesy JP, Jones PD. Toxicological perspectives on perfluorinated compounds in avian species. Organohalogen Compounds 2004; 66: 4086-4089.
Giesy JP, Kannan K. Perfluorochemical surfactants in the environment. Environ Sci Technol 2002; 36: 147A-152A.
Glensvig D, Buck C, Abildgaard A, Stuer-Lauridsen F. Chemical substances in selected impregnating agents. Survey of chemical substances in consumer products No. 50. Copenhagen: Danish EPA, 2004.
Godin CS, Myhr BC, Lawlor TE, Young RR, Murli H, Cifone MA. Assessment of the potential genotoxicity of perfluorodecanoic acid and chlorotrifluoroethylene trimer and tetramer acids. Toxicol Sci 1992; 18:557-569.
Grasty RC, Bjork JA, Wallace KB, Lau CS, Rogers JM. Effects of prenatal perfluorooctane sulfonater (PFOS) exposure on lung maturation in the perinatal rat. Birth Defects Res (Part B) 2005; 74: 405-416.
Griffith FD, Long JE. Animal toxicity studies with ammonium perfluorooctanoate. Am Ind Hyg Assoc J 1980; 41: 576-583.
Guruge K, Taniyasu S, Yamashita N, Miyazaki S, Yamanaka N, Wijeratna S, Seneviratne H. Perfluorinated compounds in human serum and seminal plasma from an urban and rural population in Sri Lanka. Organohalogen Compounds 2004; 66: 4004-4008.
Guruge KS, Taniyasu S, Yamashita N, Wijeratna S, Mohotti KM, Seneviratne HR, Kannan K, Yamanaka N, Miyazaki S. Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka. J Environ Monit 2005: 7: 371-377.
Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PKS, Giesy JR, Jones PD, Yamashita N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol Sci 2006; 89: 93-107.
Hagen DF, Belisle J, Johnson JD, Venkateswarlu P. Characterization of fluorinated metabolites by a gas chromatographic-helium microwave plasma detector – the biotransformation of 1H, 1H, 2H, 2H-perfluorodecanol to perfluorooctanoate. Anal Biochem 1981; 118: 336-343.
Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe R M. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspec 2007; 115: 390-396.
Hansen KJ, Clemen LA, Ellefson ME, Johnson HO. Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol 2001; 35: 766-770.
Harada K, Saito N, Sasaki K, Inoue K, Koizumi A. Perfluorooctane sulfonate contamination of drinking water in the Tama River, Japan: Estimated effects of resident serum levels. Bull Environ Contam Toxicol 2003; 71: 31-36.
Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, Kamiyama S, Koizumi A. The influence of time, sex and geographical factors on levels of perfluorooctane sulfonate in human serum over the last 25 years. J Occup Health 2004; 46: 141-147.
Harada K, Nakanishi S, Saito N, Tsutsui T, Koizumi A. Airborne perfluorooctanoate may be a substantial source contamination in Kyoto area, Japan. Bull Environ Contam Toxicol 2005; 74: 64-69.
Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 2005; 99: 253-161.
Harada K, Koizumi A, Saito N, Inoue K, Yoshinaga T, Date C, Fujii S, Hachiya N, Hirosawa I, Koda S, Kusaka Y, Murata K, Omae K, Shimbo S, Takenaka K, Takeshita T, Todoriki H, Wada Y, Watanabe T, Ikeda M. Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan. Chemosphere 2007; 66: 293-301.
Harris MW, Birnbaum LS. Developmental toxicity of perfluorodecanoic acid in C57BL/6N mice. Toxicol Sci 1989: 12: 442-448.
Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barent Sea food web. Environ Poll 2007; 148: 360-371.
Havelund S. Kortlægning af perfluorooktanylsulfonat og lignende stoffer i forbrugerprodukter – fase 1. Miljøprojekt Nr. 605. København: Miljøstyrelsen, 2001 (in Danish). Title in English: Survey of perfluorooctanylsulfonate and similar substances in consumer products – phase 1.
Havelund S., Kortlægning af perfluorooktanylsulfonat og lignende stoffer i forbrugerprodukter – fase 2. Miljøprojekt Nr. 691. København: Miljøstyrelsen, 2002 (in Danish). Title in English: Survey of perfluorooctanylsulfonate and similar substances in consumer products – phase 2.
Heckster FM, Laane RWPM, Voogt P de. Environmental and toxicity effects of perfluoroalkylated substances. Rev Environ Contam Toxicol 2003; 179: 99-121.
Henderson W, Smith MA. Perfluorooctanoic acid and perfluorononanoic acid in fetal and neonatal mice following In utero exposure to 8-2 fluorotelomer alcohol. Toxicol Sci 2007; 95: 452–461.
Higgins CP, Field JA, Criddle CS, Luthy RG. Quantitative determination of perfluorochemicals in sediments and domestic sludge. Environ Sci Technol 2005; 39:3946-3956.
Higgins CP, McLeod PB, MacManus-Spencer LA, Luthy RG. Bioaccumulation of perfluorochemicals in sediments by the aquatic oligochaete Lumbriculus variegatus. . Environ Sci Technol 2007; 41: 4600-4606.
Higgins CP, Luthy RG. Sorption of perfluorinated surfactants on sediments. Environ Sci Technol 2006; 40: 7251-7256.
Hoff PT, Van Campenhout K, Van de Vijver K, Covaci A, Bervoets L, Moens L, Huyskens G, Goemans G, Belpaire C, Blust R, De Coen W. Perflurooctane sulfonic acid and organohalogen pollutants in liver of three freshwater fish species in Flanders (Belgium): relationship with biochemical and organismal effects. Environ Pollut 2005a; 137: 324-333.
Hoff PT, Van de Vijver K, Dauwe T, Covaci A, Maervoet J, Eens M. Evaluation of biochemical effects related to perflurooctane sulfonic acid exposure in organohalogen-contaminated great tit (Parus major) and blue tit (Parus caeruleus) nestlings. Chemosphere 2005b; 61: 1558-1569.
Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy GJ. Perfluorooctanoate: placental and Lactational transport pharmacokinetics in rats. Toxicology 2005; 211: 139-148.
Hinderliter PM, Han X, Kennedy GJ, Butenhoff JL. Age effect on perfluorooctanoate (PFOA) plasma concentration in post-weaning rats following oral gavage with ammonium perfluorooctanoate (APFO). Toxicology 2006; 225: 195-203.
Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased te-stosterone/LH ratio. J Pathol 2003; 199: 378-86.
Holmström KE, Järnberg U, Bignert A. Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea, 1968-2003. Environ Sci Technol 2005; 39: 80-84.
Hoppenheidt K, Kottmair A, Mücke W. Influence of Organofluorine-treated Textiles on Biowaste Composting. Textile Res J 2000; 1: 84-90. Found at http://www.bifa.de/download/influence.pdf
Hori H, Yamamoto A, Hayakawa E et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ Sci Technol 2005; 39: 2383-2388.
Hori H, Nagaoka Y, Yamamoto A et al. Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water. Environ Sci Technol 2006; 40: 1049-1054.
Horowitz L. Assessment of consumer exposure to perfluorinated compounds using the SceBRA-method. Diploma Thesis, supervised by Trudel D, Wormuth M, Scheringer M, Hungerbühler K. ETH – Swiss Federal Institute of Technology Zurich. Zürich, 31 January, 2007.
Houde M, Wells RS, Fair PA, Bossart GD, Hohn AA, Rowles TK, Sweeney JC, Solomon KR, Muir DCG. Perfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ Sci Technol 2005; 39: 6591-6598.
Houde M, Balmer BC, Brandsma S, Wells RS, Rowles TK, Solomon KR, Muir DCG. Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay , Florida, USA. Environm Toxicol Chem 2006a; 25: 2405-2412.
Houde M, Bujas TAD, Small J, Wells RS, Fair PA, Bossart GD, Solomon KR, Muir DCG. Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ Sci Technol 2006b; 40: 4138-4144.
Hu W, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 2002; 68: 429-436.
Hu W, Jones PD, De Coen W, King L, Fraker P, Newsted J, Giesy JP. Alterations in cell membrane properties caused by perfluorinated compounds. Comp Biochem Physiol C 2003; 135: 77-88.
Hundley SG, Sarrif AM, Kennedy GL Jr. Absorption, distribution and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem Toxicol 2006; 29: 137-145.
Ikeda T, Aiba K, Fukuda K, Tanaka M. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabolically inert derivatives of fatty acids. J Biochem 1985; 98: 475-482
Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspec 2004; 112: 1204-1207.
Jahnke A, Ahrens L, Ebinghaus R, Temme C. Urban versus remote air concentrations of fluorotelomer alcohols and other polyfluorinated alkyl substances in Germany. Environ Sci Technol 2007a; 41: 745-752.
Jahnke A, Berger U, Ebinghaus R, Temme C. Latitudinal gradient of airborne polyfluorinated alkyl substances in the marine atmosphere between Germany and South Africa. Environ Sci Technol 2007a; 41: 3055-3061.
Jernbro S, Rocha P S, Keiter S, Skutlarek D, Färber H, Jones PD, Giesy JP, Hollert H, Engwall M. Perfluorooctane sulfonate increases the genotoxicity of cyclophosphamide in the micronucleus assay with V79 cells. Env Sci Pollut Res 2007; 14: 85-87.
Jensen AA, Poulsen PB, Bossi R. Kemi, anvendelse, forekomst og effekter af perfluoralkylsyrer (PFOS, PFOA etc.) - en ny type miljøgifte. Miljø og Sundhed 2006: 12(30); 20-30.
Jones PD, Hu W, De Coen W, Newsted J, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 2003b; 22: 2639-2649.
Kaiser MA, Barton CA, Botelho M, Buck RC, Buxton LW, Gannon J, Kao C-PC, Larsen BS, Russell MH, Wang N, and Waterland, RL. Understanding the Transport of Anthropogenic Fluorinated Compounds in the Environment. Organohalogen Compounds 2006; 68: 675-678.
Kallenborn R, Berger U, Järnberg U, Dam M, Glesne O, Hedlund B, Hirvi J-P, Lundgren A, Mogensen BB, Sigurdsson AS. Perfluorinated alkylated substances (PFAS) in the Nordic environment. Nordic Council of Ministers, September 2004.
Kannan K, Perrotta E, Thomas NJ. Association between perfluorinated compounds and pathological conditions in southern sea otters. Environ Sci Technol 2006; 40: 4943-4948.
Kannan K, Tao L., Sinclair E, Pastva SD, Jude DJ, Giesy JP. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 2005a; 48: 559-566.
Kannan K, Yun SH, Evans TJ. Chlorinated, brominated and perfluorinated contaminants in livers of polar bears from Alaska. Environ Sci Technol 2005b; 39: 9057-9063.
Kannan K, Corsolini S, Falandysz J, Fillmann, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldous KM. Perfluorooctane sulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 2004; 38: 4489-4495.
Kannan K, Kumar KS, Corsolini S, Aldous KM. Perfluoroalkylated compounds in human blood. Organohalogen Compounds 2003; 64: 29-32.
Kato K, Calafat AM, Reidy JA et al. Serum concentrations of polyfluoroalkyl compounds in Faroe Island residents. Organohalogen Compounds 2007; 69: 161-164.
Kawashima Y, Kobayashi H, Miura H, Kozuka H. Characterisation of hepatic responses of rat to administration of perfluorooctanoic and perfluorodecanoic acids at low levels. Toxicology 1995; 99: 169-178.
Kennedy GL, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol 2004; 34: 351-384.
Kissa, E., 2001. Fluorinated surfactants and repellents, 2nd edition, Marcel Dekker Inc., New York, USA.
Koch V, Knaup W, Fiebig S, Geffke T, Schulze D. Biodegradation kinetic of a Clariant fluorotelomer-based acrylate polymer - Results from a test on aerobic transformation in soil with prolonged exposure. Poster, SETAC- Europe’07, Porto.
Kubwabo C, Stewart B, Zhu J, Marro L. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J Environ Monit 2005; 7: 1074-1078.
Kubwabo C, Vais N, Benoit FM. A pilot study on the determination of Perfluorooctane sulfonate and other perfluorinated compound in blood of Canadians. J Environ Monit 2004: 6: 540-545.
Kudo N, Bandai N, Suzuki E, Katakura M, Kawashima Y. Induction by perfluorinated fatty acids with different carbon length of peroxisomal oxidation in the liver of rats. Chem-Biol Interact 2000; 124: 119-132.
Kudo N, Iwase Y, Okayachi H, Yamakawa Y, Kawashima Y. Induction of hepatic peroxisome proliferation by 8-2 telomer alcohol feeding in mice: formation of perfluorooctanoic acid in the liver. Toxicol Sci 2005; 86: 231-238.
Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem-Biol Interact 2002; 139: 301-316.
Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem-Biol Interact 2002; 139: 301-316.
Kudo N, Kawashima Y. Fish oil-feeding prevents perfluorooctanoic acid-induced fatty liver in mice. Toxicol Appl Pharmacol 1997; 145: 285-293.
Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem-Biol Interact 2001; 134: 203-216.
Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol 2004; 38: 3698-3704.
Kärrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. Levels of perfluoroalkylated compounds in whole blood from Sweden. Organohalogen Compounds 2004; 66: 4058-4062.
Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindström G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996-2004, in Sweden. Environ Health Perspec 2007; 115: 226-230.
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007; 99: 366-394.
Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 2006; 90: 510-518.
Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol 2004; 198: 231-241.
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse II: Postnatal evaluation. Toxicol Sci 2003; 74: 382-392.
Lee SC, Harner T, Pozo K, Shoeib M, Wania F, Muir DCG, Jones KC, Barrie LA. Screening for perfluoroalkyl sulfonamides (PFASs) in the global atmospheric Passive Sampling (GAPS) Study. Poster, SETAC Europe Congress, May 20-24, 2007, Porto, Portugal.
Lei YD, Wania F, Mathers D, Mabury SA. Determination of vapor pressures, octanol-air, and octanol-air partition coefficients for polyfluorinated sulfonamide, sulfonamidoethanols, and telomer alcohols. J Chem Eng Data 2004; 49: 1013-1022.
Liu RCM, Hurtt ME, Cook JC, Biegel LB. Effect of the peroxisome proliferators, ammonium perfluorooctanoate, on hepatic aromatise activity in adult male Crl:CD BR (CD) rats. Fundam Appl Toxicol 1996; 30: 220-228.
Liu J, Lee LS, Nies LF, Nakatsu CH, Turco RF. Biotransformation of 8:2 fluorotelomer alcohol in soil by soil bacteria isolates. Environ Sci Technol 2007 (in press).
Liu J, Lee LS. Effect of fluorotelomer alcohol chain length on aqueous solubility and sorption by soils. Environ Sci Technol 2007; 41: 5357-5362
Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod 2005; 73: 180-192.
Loewen M, Halldorson T, Wang F, Tomy G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ Sci Technol 2005; 39: 2944-2951.
Loewen M, Halldorson T, Wang F, Tomy G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ Sci Technol 2005; 39: 2944-2951.
Loos R, Woolgast J, Huber T, Hanke G. Polar herbicides, pharmaceutical products, perfluorooctanesulfonte (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates end ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal Bioanal Chem 2007; 387: 1469-1478.
Loveless SE, Finlay C, Everds NE, Frame SR, Gillies PJ, O’Connor JC, Powley CR, Kennedy GL. Comparative response of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 2006; 220: 203-217.
Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 2005a; 215: 126–148.
Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL, Seacat AM. Interactions of fluorochemicals with rat liver fatty-acid binding protein. Toxicology 2002; 176: 175-185.
Luebker DJ, York RG, Case MT, Hansen KJ, Moore JA, Butenhoff JL. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharmacokinetic parameters. Toxicology 2005b; 215: 149–169.
MacDonald MM, Warne AL, Stock NL, Mabury SA, Solomon KR, Sibley PK. Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid to Chironomus tentans. Environ Tox Chem 2004; 23: 2116-2123.
Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W. Estrogen-like properties of fluorotelomer alcohols as revealed by MCF-7 breast cancer cell proliferation. Environ Health Perspec 2006; 114: 100-105.
Martin JW, Ellis DA, Mabury SA, Hurley MD, Wallington TJ Atmospheric chemistry of perfluoroalkanesulfonamides: Kinetic and product studies of the OH radical and Cl atom initiated oxidation of N-ethyl
perfluorobutanesulfonamide. Environ Sci Technol 2006; 40: 864-872.
Martin JW, Mabury SA, O’Brien PJ. Metabolic pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chem-Biol Interact 2005; 155: 165-180.
Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, Mabury SA. Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal Chem 2002; 74: 584-590.
Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, Wood CR, Corton JC, Kavlock RJ, Dix DJ. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 2007; 97: 595-613.
Martin JW, Whittle DM, Muir DCG, Mabury SA. Perfluoroalkyl contaminants in a food web from Lake Ontario. Environ Sci Technol 2004; 38: 5379-5385.
McLachlan MS, Holmstrom KE, Reth M, Berger U. Riverine discharge of perfluorinated carboxylates from the European Continent. Environ Sci Technol 2007; 41:7260-7265.
Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, Scholze M, Kortenkamp A,Vinggaard AM. Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol Sci 2007; 98: 87-98.
Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health 2007; 80: 643-648.
Moriwaki H, Takagi Y, Tanaka M, et al. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ Sci Technol 2005; 39: 3388-3392.
Moriwaki H, Takata Y, Arakawa R. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in vacuum cleaner dust collected in Japanese homes. J Environ Monit 2003; 5: 753-757.
Nakata H, Kannan K., Nasu T, Cho H, Sinclair E, Takemura A. Perfluorinated contaminants in sediment and aquatic organisms collected from shallow water and tidal flat areas of the Ariake Sea, Japan: environmental fate of perfluorooctane sulfonate in aquatic ecosystems. Environ Sci Technol 2006; 40: 4916-4921.
Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, Lindstrom AB. Perfluorinated compounds in the Cape Fear drainage basin in North Carolina. Environ Sci Technol 2007; 41: 5271-5276.
Naturskyddsföreningen (Swedish Society for Nature Conservation). Fluorerade miljögifter i impregneringsmedel. Rapport 901909-2. 2007. Found at http://www.snf.se/pdf/rap-hmv-impreg.pdf
Nielsen OJ, Andersen MPS. Atmosfærisk nedbrydning af FTOH – en kilde til perfluorerede carboxylsyrer. Dansk Kemi 2007; 88: 28-30.
Oakes KD, Sibley PK, Solomon KR, Mabury SA, and Van Der Kraak GJ. Impact of perfluorooctanoic acids on fathead minnow (Pimephales promeas) fatty acyl-coa oxidase activity, circulating steroids, and reproduction in outdoor microcosmos. Environ Toxicol Chem 2004; 23: 1912-1919.
O’Brien TM, Wallace KB. Mitochondrial permeability transition as the critical target of N-acetyl perfluorooctane sulfonamide toxicity in vitro. Toxicol Sci 2004; 82: 333-340.
Odland JØ. Kartlegging av “nye” miljøgifter I humane blodprøver fra Nord-Norge, Nord-Vest Russland og Sibir. SFT Rapport: 963/2006. Oslo: Statens Forurensningstilsyn, 2006. http://www.sft.no/publikasjoner/overvaking/2184/ta2184.pdf
OECD, Hazard Assessment of perfluorooctane sulfonate (PFOS) and its salts, Co-operation on existing chemicals, Organisation for Economic Co-operation and Development (OECD), ENV/JM/RD (2002)17/FINAL, November 21 2002.
OECD. Preliminary lists of PFOS, PFAS, PFOA and related compounds and chemicals that may degrade to PFCA. Environment Directorate, Joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology, ENV/JM/MONO(2006)15, 12-Apr-2006.
OECD. Lists of PFOS, PFAS, PFOA, PFCA, related compounds and chemicals that may degrade to PFCA. Environment Directorate, Joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology, ENV/JM/MONO(2007)15, 21-Aug-2007. Found at http://www.oecd.org/LongAbstract/0,3425,en
_2649_34375_39160347_119666_1_1_1,00.html
Ohmori K, Kudo N, Katayama K, Kawashima Y. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 2003; 184: 135-140.
Olson CT, Andersen ME. The acute toxicity of perfluorooctanoic and perfluorodecanoic acids in male rats and effect on tissue fatty acids. Toxicol Appl Pharmacol 1983; 70: 362-372.
Olsen GW, Burris JH, Burlew MM. Plasma cholecystokinin and hepatic lipoproteins in ammonium perfluorooctanoate production workers. Drug Chem Toxicol 2000; 23: 603-620.
Olsen GW, Burris JM, Ehresman DJ, Froelich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctane sulfonate, perfluorohexane sulfonate and perfluorooctanoate in retired fluorochemicals production workers. Environ Health Perspec 2007a; 115: 1298-1305.
Olsen GW, Burris JH, Mandel JH, Zobel LR. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees J Occup Environ Med 1999; 41: 799-806.
Olsen GW, Church TR, Larson EB, van Belle G, Lundberg JK, Hansen KJ, Burris JM, Mandel JH, Zobel LR. Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere 2004; 54: 1599-1611.
Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, O'Neill EM, Mandel JH, Zobel LR. Perfluorooctane sulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspec 2003b; 111: 1892-1901.
Olsen GW, Gilliland FD, Burlew MM, Burris JH, Mandel JS, Mandel JH. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 1998; 40: 614-622.
Olsen GW, Hansen KJ, Stevenson LA, Burris JM, Mandel JH. Human donor liver and serum concentrations of perfluorooctane sulfonate and other perfluorochemicals. Environ Sci Technol 2003a; 37: 888-891.
Olsen GW, Muir DC, Reagan WK, Ellefson ME, Ehresman DJ, Butenhoff JL, Zobel LR. Preliminary evidence of a decline in perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations in American Red Cross blood donors. Chemosphere 2007b; 68: 105-111.
Olson CT, Andersen ME. The acute toxicity of perfluorooctanoic and perfluorodecanoic acids in male rats and effect on tissue fatty acids. Toxicol Appl Pharmacol 1983; 70: 362-372.
OSPAR Commission. Summary record of the meeting of the hazardous substances committee (HSC), The Hague, 7-11 April 2003. http://www.ospar.org/eng/html/ welcome.html
Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicol Sci 2006; 90: 269-295.
Perkins RG, Butenhoff JL, Kennedy GL, Palazzolo MJ. 13-week dietary toxicity study of ammonium perfluorooctanoate (APFO) in male rats. Drug Chem Toxicol 2004; 27: 361-378.
Phillips MM, Dinglasan-Panlilio MJA, Mabury SA, Solomon KR, Sibley P. Fluorotelomer acids are more toxic than perfluorinated acids. Environ Sci Technol 2007; 41:7159-7153.
Poulsen PB, Jensen AA, Wallström E. More environmentally friendly alternatives to PFOS-compounds and PFOA. Environmental project no. 1013. Copenhagen: Danish EPA, 2005.
Powley CR, Michalczyk MJ, Kaiser MA, Buxton LW Determination of perfluorooctanoic acid (PFOA) extractable from the surface of commercial cookware under simulated cooking conditions by LC/MS/MS. Analyst 2005; 130: 1299-1302.
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 2006; 40: 32-44.
Renner R. Perfluorinated sources outside and inside. Environ Sci Technol 2004: 38: 80A.
Renner, R. Canada bans fluoropolymer stain repellents. Environ Sci Technol 2005; 39: 56A-57A.
Renner R. New Jersey dives into PFOA water guidance. Environ Sci Technol 2007a; 41: 3395-3396.
Renner R. PFOA in people. Environ Sci Technol 2007b; 41: 4497-4500.
Roon A van, den Besten C, de Voogt P. A theoretical study on the partitioning of perfluorinated alkylated substances (PFAS) between water and a bi-layer membrane. Organohalogen Compounds 2006; 68: 1565-1568.
Rosen MB, Thibodeaux JR, Wood CR, Zehr RD, Schmid JE, Lau C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicology 2007; 239: 15-33.
Ruch RJ. Intercellular communication, homeostasis and toxicology. Toxicol Sci 2002; 68: 265-266.
Sasaki K, Harada K, Saito N, Tsutsui T, Nakanishi S, Koizumi A. Impact of airborne perfluorooctane sulfonate on the human body burden and the ecological system. Bull Environ Contam Toxicol 2003; 71: 408-413.
Schnellmann RG, Manning RO. Perfluorooctane sulfonamide: a structural novel uncoupler of oxidative phosphorylation. Biochim Biophys Acta 1990; 1016: 344-348.
Schröder HF. Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurised liquid extraction and separation on fluorine-modified reverse-phase sorbents. J Chromatography 2003; 1020: 131-151.
Schultz MM, Barofsky DF, Field GA. Quantitative determination of fluorinated alkyl substances by large-volume-injection liquid chromatography tandem mass spectrometry – Characterization of municipal wastewaters. Environ Sci Technol 2006a; 40: 289-295.
Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field GA. Fluorochemical mass flows in a municipal wastewater treatment plant. . Environ Sci Technol 2006b; 40:7350-7357.
Schultze P-E, Norin H. Fluorinated pollutants in all-weather clothing, Friends of the Earth Norway – Report 2/2006, Swedish Society for Nature Conservation. http://www.snf.se/pdf/rap-hmv-allvadersklader-eng.pdf
Scott BF, Spencer C, Mabury SA, Muir DCG. Poly- and perfluorinated carboxylates in north American precipitation. Environ Sci Technol 2006; 40: 7167-7174.
Seacat AM, Thomford PJ, Butenhoff JL. Terminal observations in Sprague-Dawley rats after lifetime dietary exposure to potassium perfluorooctane sulfonate Toxicologist 2002b; 66: 185 (abstract).
Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldrigde SR, Elcombe CR, Butenhoff JL. Sub-chronic dietary toxicity of potassium perfluorooctane sulfonate in rats. Toxicology 2003; 183: 117-131.
Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL. Subchronic toxicity studies on perfluorooctane sulfonate potassium salt in Cynomolgus monkeys. Toxicol Sci 2002a; 68: 249-264.
Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab 2006; 20: 91-110.
Shi Z, Zhang H, Liu Y, Xu M, Dai J. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to perfluorododecanoic acid. Toxicol Sci 2007; 98, 206-215.
Shipley JM, Hurst CH, Tanaka SS, DeRoss FL, Butenhoff JL, Seacat AM, Waxman DJ. Trans-Activation of PPARa and induction of PPARa target genes by Perfluorooctane-based chemicals. Toxicol Sci 1994; 80: 151-160.
Shoeib M, Harner T, Ikonomou M, Kannan K. Indoor and outdoor air concentrations and phase partitioning of Perfluoroalkyl sulfonamides and polybrominated diphenyl ethers. Environ Sci Technol 2004a; 38: 1313-1320.
Shoeib M, Harner T, Vlahos P. Perfluorinated chemicals in the arctic atmosphere. Environ Sci Technol 2006; 40: 7577-7583.
Shoeib M, Harner T, Wilford B, Jones K, Zhu J. A survey of perfluoroalkyl sulfonamides in indoor and outdoor air using passive air samplers. Organohalogen Compounds 2004b; 66: 3999-4003.
Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: Occurrence, partitioning, and human exposure. Environ Sci Technol 2005a; 39: 6599-6606.
Shoeib M, Harner T, Wilford B, Zhu J. Polyfluorinated telomer alcohols (FTOHs) in indoor dust. Organohalogen Compounds 2005b; 67: 801-804.
Shoeib M, Harner T, Zhu J. Indoor air & dust concentration of fluorotelomer alcohols. Organohalogen Compounds 2007; 69: 146-149.
Silva E, Rajapakse N, Kortenkamp A. Something from "nothing"--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol 2002; 36: 1751-1756.
Simcik MF. Global transport and fate of perfluorochemicals. J Environ Monit 2005; 7: 759-763.
Simcik MF, Doweiler KJ. Ratio of perfluorochemicals concentrations as a tracer of atmospheric deposition to surface waters. Environ Sci Technol 2005; 39: 8678-8683.
Sinclair E, Kim SK, Akinleye HB, Kannan K. Quantitation of gas-phase perfluoroalkyl surfactants and fluoroteleomer alcohols released form non-stick cookware and microwave popcorn bags. Environ Sci Technol 2007; 41: 1180-1185.
Sinclair E, Mayack DT, Roblee K, Yamashita N, Kannan K. Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Arch Environm Contam Toxicol 2006; 500: 398-410.
Sinclair E, Kannan K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ Sci Technol 2006; 40: 1408-1414.
Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16: 972-978.
Skutlarek D, Exner M, Färber H. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res 2006; 13: 299-307.
Smithwick M, Muir DCG, Mabury SA, Solomon K, Martin JW, Sonne C, Born EW, Letcher RJ, Dietz R. Perfluoroalkyl contaminants in liver tissue from East Greenland polar bears (Ursus maritimus). Environ Toxicol Chem 2005a; 24:981-986.
Smithwick M, Mabury SA, Solomon K, Sonne C, Martin JW, Born EW, Dietz R, Derocher AE, Letcher RJ, Evans TJ, Gabrielsen GW, Nagy J, Stirling I, Taylor MK, Muir DCG. Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus). Environ Sci Technol 2005b; 39:5517-5523.
Smithwick M, Norstrom RJ, Mabury SA, Solomon K, Evans TJ, Stirling I, Taylor MK, Muir DCG. Temporal trends of perfluoroalkyl contaminants in polar bears (Ursus maritimus) from two locations in the North American Arctic, 1972-2002. Environ Sci Technol 2006; 40: 1139-1143.
So MK, Miyake Y, Yeung WY, Ho YM, Taniyasu S, Rostkowski P, Yamashita N, Zhou BS, Shi XJ, Wang JX, Giesy JP, Yu H, Lam PKS. Perfluorinated compounds in the Pearl River and Yangtze River of China. Chemosphere 2007; 68: 2085-2095.
So MK, Taniyasu S, Lam PKS, Zheng J, Giesy JP, Yamashita N. Alkaline digestion and solid phase extraction method fro perfluorinated compounds in mussel and oysters from South China and Japan. Arch Environ Contam Toxicol 2006a; 50: 240-248.
So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PKS. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol 2006b; 40: 2924-2929.
Sohlenius A-K, Andersson K, Olsson J, DePierre JW. Peroxisome proliferation and associated effects caused by perfluorooctanoic acid in vitamin A-deficient mice. Chem-Biol Interact 1995; 98: 45-50.
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspec (1995; 103 (Suppl 7): 113-122.
Starkov AA, Wallace KB. Structural determinants of fluorochemicals-induced mitochondrial dysfunction. Toxicol Sci 2002; 66: 244-252.
Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, Mabury SA. Polyfluorinated telomer alcohols and sulfonamides in the North American troposphere. Environ Sci Technol 2004a; 38: 991-996.
Stock NL, Ellis DA, Deleebeck L, Muir DCG, Mabury SA. Vapor pressures of the fluorinated telomer alcohols – limitation of estimation methods. Environ Sci Technol 2004b; 38: 1693-1699.
Stevenson CN, MacManus-Spencer LA, Luckenbach T, Luthy RG, Epel D. New perspectives on perfluorochemical ecotoxicology: inhibition and induction of an efflux transporter in the marine mussel, Mytilus californianus. Environ Sci Technol 2006; 40: 5580-5585.
Stock NL, Furdui VI, Muir DCG, Mabury SA. Perfluoroalkyl contaminants in the Canadian arctic: Evidence of atmospheric transport and local contamination. Environ Sci Technol 2007; 41: 3529-3536.
Stockholm Convention 2005. Decision POPRC-1/7: Perfluorooctane sulfonate. http://www.pops.int/documents/meetings/poprc/docs/chem_review.htm
Strand J, Bossi R, Sortkjær O, Landkildehus F, Larsen MM. PFAS og organotin forbindelser i punktkilder og det akvatiske miljø. DMU Faglig Rapport nr. 608, 2007. http://www.dmu.dk/
Suchenwirth RHR, Jürling H, Huppmann R, Bücking M. Perfluorierte Alkyl-Substanzen (PFAS) in der Muttermilch. Bericht des Niedersächsischen Landesgesundheitsamtes, Hannover, 2006. http://www.nlga.niedersachsen.de
Swedish Chemicals Agency. Perfluorinated substances and their uses in Sweden. Report nr. 7, 2006. Swedish Chemicals Agency, Stockholm, 2006.
Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (a,b/d,g) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 2007; 95: 108-117.
Taniyasu S, Kannan K, Horii Y, Hanari N, Yamashita N. A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ Sci Technol 2003; 37: 2634-2639.
Taniyasu S, Yamashita N, Petrick G, Gamo T, Lam PKS, Kannan K. The long range transportation of PFOS and PFOA by global scale water current. Organohalogen Compounds 2007; 69: 150-152.
Tao L., Kannan K, Kajiwara N, Costa M, Fillmann G, Takahashi S, Tanabe S. Perfluorooctanesulfonate and related fluorochemicals in albatrosses, elephant seals, penguins, and polar skuas from the Southern Ocean. Environ Sci Technol 2006; 40: 7642-7648.
Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. I: Maternal and Prenatal Evaluations. Toxicol Sci 2003; 74: 369-381.
Thomford PJ, Seacat AM, Butenhoff JL. Terminal observations in Sprague-Dawley rats after lifetime dietary exposure to N-ethyl perfluorooctane sulfonamide ethanol. Toxicologist 2002; 66: 185 (abstract).
Thomsen C, Kuklenyik Z, Frøshaug M, Calafat AM. The ranges of polyfluorinated compounds in serum from a group of high consumers of fish in from a contaminated lake in Norway. Organohalogen Compounds 2006; 68: 1701-1704.
Thuens S, Dreyer A, Sturm R, Temme C, Ebinghaus R. Determination of octanol-air partition coefficients of several fluorotelomer alcohols. Poster: SETAC’07, Porto.
Tinwell H, Ashby J. Sensitivity of the immature rat uterotrophic assay to mixtures of estrogens. Environ Health Perspec 2004; 112: 575-82.
Tittlemier SA, Edwards L, Pepper K. Concentrations and temporal trends of two perfluorooctyl sulfonamides in fast food composites collected during the Canadian total diet study. Organohalogen Compounds 2003; 62: 315-319.
Tittlemier SA, Pepper K, Edwards L. Concentrations of perfluorooctane sulfonamides in Canadian Total Diet Study composite food samples collected between 1992 and 2004. J Agric Food Chem 2006; 54: 8385-8389.
Tittlemier S, Ryan JJ, Van Oostdam J. Presence of anionic perfluorinated organic compounds in serum collected from northern Canadian populations. Organohalogen Compounds 2004; 66: 4009-4014.
Tomy G, Halldorson T, Stern G. Time trend studies of perfluorinated compounds in beluga (Delphinapterus leucas) from SE Baffin Island. Organohalogen Compounds 2005; 67: 1001-1004.
Trosko JE, Chang C-C, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular communication. Toxicol Lett 1998; 102-103: 71-78.
Ubel FA, Sorenson SD, Roach DE. Health status of plant workers exposed to fluorochemicals – a preliminary report. Am Ind Hyg Assoc J 1980; 41: 584-589.
Upham BL, Deocampo ND, Wurl B, Trosko JE. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int J Cancer 1998; 78: 491-95.
US EPA (2002), Revised draft – Hazard assessment of Perfluorooctanoic Acid and Its Salts, Office of Pollution Prevention and Toxics, Risk Assessment Division, November 4, 2002.
U.S. Environmental Protection Agency. 2010/15 PFOA Stewardship Program 2006. http://www.epa.gov/opptintr/pfoa/pubs/pfoastewardship.htm.
Van de Vijver KI, Hoff P, Das K, Brasseur S, Van Dongen W, Esmans E, Siebert U, Bouquegneau JM, Blust R, De Coen W. Baseline study of perfluorochemicals in harbour porpoises (Phocoena phocoena) from Northern Europe. Mar Pollut Bull 2004; 48: 986-1008.
Van de Vijver KI, Hoff P, Das K, Brasseur S, Van Dongen W, Esmans E, Reijnders P, Blust R, De Coen W. Tissue distribution of perfluorinated chemicals in harbour seals (Phoca vitulina) from the Dutch Wadden Sea. Environ Sci Technol 2005; 39: 6978-6984.
Van de Vijver KI, Holsbeek L, Das K, Blust R, Joiris C, De Coen W. Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ Sci Technol 2007; 41: 315-320.
Vanden Heuvel JP. Perfluorodecanoic acid as a useful pharmacologic tool for the study of peroxisome proliferation. Gen Pharmacol 1996; 27: 1123-1129.
Vanden Heuvel JP, Kuslikis BI, Peterson RE. Covalent binding of perfluorinated fatty acids to proteins in the plasma liver and testes of rats. Chem-Biol Interact 1992; 82: 317-328.
Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol 1991; 6: 83-92.
Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: comparison of human, mouse, and rat peroxisome proliferator-activated receptor-a,-b, and -g, liver X receptor-b, and retinoid X receptor-a. Toxicol Sci 2006; 92: 476-489.
Van Leeuwen SPJ, Kärrman A, van Bavel B, de Boer J, Lindström G. Struggle for quality in determination of perfluorinated contaminants in environmental and human samples. Environ Sci Technol 2006; 40: 7854-7860.
Vanparys C, Maras M, Lenjou M, Robbens J, Van Bockstaele D, Blust R, De Coen W. Flow cytometric cell cycle analysis allows for rapid screening of estrogenicity in MCF-7 breast cancer cells. Toxicology in Vitro 2006; 20: 1238-1248.
Van Rafelgheim MJ, Mattie DR, Bruner RH, Anderson ME. Pathological and hepatic ultrastructural effects of a single dose of perfluoro-n-decanoic acid in the rat, hamster, mouse, and guinea pigs. Toxicol Sci 198; 9: 522-540.
Vejrup KV, Lindblom B. Analysis of perfluorooctanesulfonate compounds in impregnating agents, wax and floor polish products. Survey of Chemical Substances in Consumer Products No. 17. Danish EPA, 2002.
Verreault J, Houde M, Gabrielsen GW, Berger U, Haukås M, Letcher RJ, Muir DCG. Perfluorinated alkyl substances in plasma, liver, brain and eggs of glaucous gull (Larus hyperboreus) from the Norwegian Arctic. Environ Sci Technol 2005; 39: 7439-7445.
Verreault J, Gabrielsen GW, Berger U. Perfluorinated alkyl substances in eggs from herring gulls from Northern Norway: spatial and temporal trends. SPFO Report 971/2006; TA-2208; ISBN 82-7655-502-0.
Völkel W, Genzel-BoroviczenyO, Demmelmair H et al. Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human milk: results of a pilot study. Int J Hyg Environ Health 2007: online Sept 14.
Wallington TJ, Hurley MD, Xia J, Wuebbles DJ, Sillman S, Ito A, Penner JE, Ellis DA, Martin J, Mabury SA, Nielsen OJ, Andersen MPS. Formation of C7F15COOH (PFOA) and other perfluorocarboxylic acids during the atmospheric oxidation of 8:2 fluorotelomer alcohol. Environ Sci Technol 2006; 40: 924-930.
Wang N, Szostek B, Folsom PW, Sulecki LM, Capka V, Buck RC, Berti WR, Gannon JT. Aerobic biotransformation of 14C-labelled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ Sci Technol 2005a; 39: 531-538.
Wang N, Szostek B, Buck RC, Folsom PW, Sulecki LM, Capka V, Berti WR, Gannon JT. Flurorotelomer alcohol biodegradation-Direct evidence that perfluorinated carbon chains break down. Environ Sci Technol 2005b; 39: 7516-7528.
Wania F. A global mass balance analysis of the source of perfluorocarboxylic acids in the Arctic Ocean. Environ Sci Technol 2007; 41: 4529-4535.
Washburn ST, Bingmann TS, Braithwaite SK, Buck RC, Buxton LW, Clewell HJ, Haroun LA, Kester JE, Rickard RW, Shipp AM. Exposure Assessment and Risk Characterization for Perfluorooctanoate (PFO) in Selected Consumer Articles. ENVIRON International Corporation and DuPont, 2005.
White SS, Calafat AM, Kuklenyik Z, Villanueva LT, Zehr RD, Helfant L, Strynat MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 2007; 96: 133-144.
Xie W, Kania-Korwel I, Bummer PM, Lehmler H-J. effect of potassium perfluorooctanesulfonate, perfluorooctanoate and octanesulfonate on the phase transition of dipalmitoylphosphatidylcholine (DPPC) bilayers. Biochem Biophys Acta 2007; 1768: 1299-1308.
Xu L, Krenitsky DM, Seacat AM, Butenhoff JL, Anders MW. Biotransformation of N-ethyl-N-(2-hydroxyethyl)perfluorooctanesulfonamide by rat liver microsomes, cytosol, and slices and by expressed rat and human cytochrom P450. Chem Res Toxicol 2004; 17; 767-775.
Yamada T, Taylor PH, Buck RC, Kaiser MA, Giraud RH. Thermal degradation of fluorotelomer treated articles and related materials. Chemosphere 2005; 61: 974-984.
Yamamoto T, Noma Y, Sakai S-I, Shibata Y. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol. Environ Sci Technol 2007; 41: 5660-5665.
Yamashita N, Kannan K, Taniyasu S, Horii Y, Okazawa T, Petrick G, Gamo T. Anaysis of perfluorinated acids at part-per-quadrillion levels in seawater using liquid-chromatography-tandem mass spectrometry. Environ Sci Technol 2004; 38:5522-5528.
Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T. A global survey of perfluorinated acids in oceans. Mar Poll Bull 2005; 51:658-668.
Yang JH, Kannan K, Kim S-Y, Shin I-H. Levels of Perfluorooctane sulfonate and related fluorochemicals in human blood from the general population of Korea. Organohalogen Compounds 2004; 66: 4041-4045.
Yarwood G, Kemball-Cook S, Keinath M, Waterland RL, Korzeniowski SH, Buck RC, Russell MH, Washburn ST. High-resolution atmospheric modelling of fluorotelomer alcohols and perfluorocarboxylic acids in the North American troposphere. Environ Sci Technol 2007; 41:5756-5762.
Yeung LWY, So MK, Jiang G, Taniyasu S, Yamashita N, Song M, Wu Y, Li J, Giesy JP, Guruge KS, Lam PKS. Perfluorooctane sulfonate and related fluorochemicals in human blood samples from China. Environ Sci Technol 2006; 40: 715-720.
Yeung LWY, Myyake Y, Taniyasu S, Wang Y, Zhan JB, So MK, Yu H, Jiang GB, Wu YN, Li JG, Giesy JP, Yamashita N, Lam PKS. Extractable organic fluorine and perfluorinated compounds in Chinese blood samples. Organohalogen Compounds 2007; 69: 2816-2819.
Young CJ, Furdui VI, Franklin J, Koerner RM, Muir DC, Mabury SA. Perfluorinated acids in Arctic snow: new evidence for atmospheric formation. Environ Sci Technol 2007; 41: 3455-3461.
Zhu J, Marro L, Shoeib M, Kubawabo C, Harner T. Comparison of perfluorinated sulfonamides and their degradation products in indoor dusts. Organohalogen Compounds 2005; 67: 797-800.
Fodnoter
[1] ”Poly” means that many of the hydrogen atoms have been replaced with fluor; ”per” means that all hydrogen in the alkyl chain have been replaced with fluor.
[2] ”Poly” means that many of the hydrogen atoms have been replaced with fluor; ”per” means that all hydrogen atoms in the alkyl chain have been replaced with fluor.
[3] http://www.epa.gov/oppt/pfoa/pubs/pfoastewardship.htm
[4] http://www2.dupont.com/Zonyl_Foraperle/en_US/products/products.html
[5] http://www.dupont.com/teflon/carpetprotector/tech_info.html
[6]http://www2.dupont.com/Zonyl_Foraperle/en_US/products/zonyl_pgs/surfactants.html
[7]http://www2.dupont.com/Zonyl_Foraperle/en_US/
assets/downloads/Hydrology_Overview_me03.pdf
[8] http://www.chemtexindia.com/oleophobolzsr.pdf
[9] http://www.daikin.com/product/chm.html
[10]http://www.solvaysolexis.com/products/byproductline/
productline/productgroup/0,,18527-2-0,00.htm
[11] http://msds.dupont.com/NASApp/msds/Mediator
[12] In the OECD report PFAS is defined as perfluoroalkyl sulfonates, i.e. sulfonic acids with a chain length other than 8 for PFOS (OECD, 2006).
[13] According to the project for the Danish EPA ”Mapping of chemicals in dry-cleaned textiles from Rynex and hydrocarbon dry-cleaning shops”, Survey no. 21, 2003.
[14] http://www.ecolab.dk/html_sider/produkter/Tekster/Arbejdsbeklaedning.pdf
[15] http://www.ecolab.dk/drift/servlet/se.ibs.ns.is.ItemDetailServlet?KEY_ITEM=101664
[16] http://www.ecolab.at/website/hygiene/home/
divisions/textile-care/waschmittelsysteme/finish-produkte/
[17] i.a.n = ikke andetsteds nævnt (not mentioned elsewhere)
[18] http://www2.mst.dk/common/Udgivramme/Frame.asp?
pg=http://www2.mst.dk/udgiv/publikationer/2000/87-7944-156-4/html/kap04.htm
[19] Information found at http://www.dupont.com/teflon/carpetprotector/tech_info.html.
[20] Information found at http://www.dupont.com/teflon/carpetprotector/tech_info.html.
[21] http://www2.dupont.com/Zonyl_Foraperle/en_US/assets/downloads/Zonyl_FSN.pdf
[22] According to information on Zonyl FSO on DuPont website, Zonyl FSO contains 50% fluorosurfactants, and should be used in a solution between 0.005-0.2%.
[23] http://www2.dupont.com/Zonyl_Foraperle/en_US/uses_apps/industrial.html
Version 1.0 October 2008, © Danish Environmental Protection Agency