Survey of Chemical Substances in Consumer Products, No. 100, 2008
Mapping, emissions and environmental and health assessment of chemical substances in artificial turf
Contents
2 Artificial turf pitches – Materials and chemical substances
5 Procurement of sample material
- 6.1 Analysis methods and analysis programme
- 6.2 Results of screening analyses
- 6.3 Results of leaching tests
- 6.4 Discussion of results
- 6.5 Selection of substances for health and environmental assessment
- 8.1 Zinc and its salts
- 8.2 6PPD
- 8.3 Dicyclohexylamine
- 8.4 Diisobutyl phthalate
- 8.5 Nonylphenol
- 8.6 2,4-di-tert-butylphenol
- 8.7 Diethyl phthalate
- 8.8 Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate
- 8.9 Estimate of effect of discharged substances
9 Abbreviations used in the environmental and health assessments
10 Abbreviations used in the material descriptions and for chemical substances/substance groups
Preface
The “Mapping, emissions and environmental and health assessment of chemical substances in artificial turf” project was carried out between April 2007 and the end of October 2007.
This report describes the results of the project, including mapping of products on the market, materials included in artificial turf pitches, chemical analyses and exposure scenarios as well as health and environmental assessments.
The project was carried out by the Danish Technological Institute with Dr Nils H. Nilsson as project manager and Dr Bjørn Malmgren-Hansen responsible for the health and environmental assessments. Mr Uffe Sognstrup Thomsen contributed with quality assurance of health and environmental assessments.
The monitoring group for the project consisted of:
Frank Jensen Danish Environmental Protection Agency
Dorte Lerche Danish Environmental Protection Agency
Nils H. Nilsson Danish Technological Institute
The project was financed by the Danish Environmental Protection Agency.
Summary and conclusions
In recent years, it has become increasingly popular to use artificial turf as a substitute for natural turf.
Artificial turf is primarily used for football pitches, but it is also becoming increasingly popular for golf greens, school playgrounds and playgrounds in general.
There are a number of advantages in using artificial turf rather than natural turf: The pitches are more hard-wearing and easier to maintain than natural turf pitches, the football season can be extended whatever the weather, and the pitches can be laid in places where it is difficult to get grass to grow. Artificial turf pitches can be used indoors as well as outdoors.
The pitches that have become especially popular are the third-generation pitches. They are characterised by having turf with longer straws (approx. 50-70 mm versus approx. 30 mm previously) and by the pitch infill consisting of a layer of silica sand at the bottom of the loom and a rubber granule layer at the top. The top 20 mm are not filled. Previously, the infill on football pitches was gravel.
The pitches can be laid directly on a smoothed gravel layer, but many pitches are laid with a shock-absorbing pad between the gravel and the artificial turf.
The Danish Football Association, DBU (DBU, 2005) has described the requirements concerning the football functional properties. These requirements were determined by UEFA and FIFA.
Over the past three years, there have been discussions about the possible health and environmental problems associated with the use of artificial turf pitches. This debate arose on the background of Norwegian studies, in particular. A Swedish report published by the Swedish Chemicals Agency (Kemikalieinspektionen) in 2006 supported the concern that the use of artificial turf pitches could have an adverse effect on health and the environment. These concerns relate to both the health risk to the users of artificial turf pitches and to the leaching of chemical substances into the soil and water.
Especially the use of old car tyres in the form of granules has been debated in the Norwegian studies and the Swedish report. It has in several cases been the practice in Denmark, in connection with invitations for tender, to exclude the use of car tyres following from the Norwegian studies and the Swedish report. This has resulted in e.g. grey industrial rubber being used as an alternative.
Against this background, the Danish Environmental Protection Agency has initiated this study of artificial turf pitches, focusing on Danish conditions.
100-120 tonnes of rubber granules are used per pitch as elastic infill in the pitches, unless an elastic pad is used for the base, so the quantities are rather significant.
The study has shown that today (August 2007), 45 artificial turf football pitches have been laid in Denmark. All but one are outdoor pitches. Following 22 interviews with suppliers, municipalities and the Danish Football Association (DBU), there is reason to assume that many more pitches will be laid in the coming years. This is partly due to the excellent technical playing properties of the third-generation pitches, and partly due to the fact that the artificial turf pitches can actually be used for playing sports all 365 days a year.
In order to use the pitches all year, however, it is necessary to salt the pitches during the winter months in order to keep them free of frost and snow. This is one of the aspects that received focus in this study in relation to environmental impacts, as this issue has not previously been investigated in foreign studies, and there is an expectation that using salt impacts the leaching of chemical substances to soil and water, depending on the volume and type of salt (acid or alkaline).
In addition to the Norwegian studies and the Swedish report, a number of results have been published from the Netherlands, Switzerland and France.
The French study was very comprehensive, both as regards the analysis parameters measured and the number of tests. The tests were conducted over a period of one year and covered both the health and environmental aspects of using rubber granules as infill in the pitches.
The conclusion of the French study is that there is no reason for concern in connection with the use of granulated car tyres as infill, neither based on a health nor an environmental assessment. The assessment is based on the low measured values for the emission of substances in relation to the scenarios set up, on which the conclusion is based.
Leaching of chemicals from the artificial turf pitches was studied using a special recovery technique – a so-called lysimeter test. The same applies for a study recently concluded in Switzerland. In lysimeter tests, it is possible to closely approximate “real life” exposure under well-defined test parameters. The lysimeters are constructed in such a way that rainwater or artificial water can be recovered for analysis following passage through the materials and support layers which make up the artificial turf pitches (gravel, elastic pad, artificial turf mat, sand and elastic infill).
The Swiss study included monitoring the sum parameters DOC (dissolved organic carbon), the sum of dissolved organic nitrogen compounds as well as ammonium nitrogen, nitrate nitrogen and nitrite nitrogen. The study furthermore included monitoring the organic substances aniline, alkylated phenylene diamines, benzothiazole, cyclohexylamine, 16 PAHs and the element zinc, as these substances form part of infill based on rubber from car tyres.
After one year of monitoring in Switzerland, the amount of leached substances in almost all tests had dropped to a concentration lower than the detection limit of 0.2 µg/l.
During the study period, no increases of zinc in the drainage water were detected.
One of the conclusions of the Swiss study is that the substances existing on the surface of rubber granules are leached off by rainwater after a short period of time, and there are therefore no reasons for environmental concerns about the use of rubber granules as an infill material.
However, studies on the long-term effects (several years) are lacking.
The Dutch study indicates that there may be increased zinc leaching as a result of ageing of the rubber, which would make zinc more susceptible to leaching. According to one scenario, Dutch threshold values would be exceeded after 11 years (Building Materials Decree (2,100 mg/m²/year)) and after 20 years (Decree on Soil Quality (3,600 mg/m²/year)), respectively. The scenario assumes that the infill is entirely replaced after 10 years of use. If the rubber ages faster than assumed, the threshold values will be exceeded after only 3-4 years. The environmental impact of zinc was assessed at 0.08% of the total zinc impact in the Netherlands, but with an estimated impact of 0.5% if the number of artificial turf pitches is increased from the current 370 to 2,500.
The results from the foreign studies all conclude that on the basis of the measured concentrations of substances that are hazardous to health, there are no health risks in connection with inhalation, neither indoors nor outdoors.
Against this background, and against the background of self-obtained results in connection with preliminary studies under this project in the form of GC/MS headspace analyses conducted on a wide range of infill and artificial turf mat samples, it was decided not to focus on health scenarios in connection with inhalation. In addition, it should be noted that the Danish third-generation pitches are all outdoors, contrary to Norwegian pitches, in particular.
The intention of this study was to supply new information about the health and environmental aspects of the increasing use of artificial turf pitches.
However, as all existing studies show that there are no problems in relation to PAH emissions, this project has not monitored for PAH.
Unlike the foreign studies, however, a larger number of different infill materials and artificial turf mats have been included in this project in order to obtain a better basis of comparison between the different types. The infill materials include granulated tyres, coated tyre granules, thermoplastic elastomers (TPE) with different tinting and, as a new product, coir fibres. All in all, 16 types of infill materials were obtained, eight types of artificial turf mats and two elastic pads. Here, it is important to remember that the term granulated car tyres covers an inhomogeneous mixture, as the infill material could originate from many different types of car tyres.
Furthermore, laboratory studies have been carried out which provide information about leaching of substances from the infill materials, the turf mats and a recycled rubber-based pad as a result of the pitches being salted in winter, as this aspect had not previously been studied. The studies have included road salt in the form of sodium chloride, which is the cheapest de-icing salt, or calcium chloride, which is used in the event of heavy frost. As regards the salt concentration, the leaching tests were based on the recommendations of the Danish Football Association (DBU).
The monitoring included a wide range of organic substances which derive from either the infill or from the artificial turf mats themselves. In particular, there was emphasis on measuring for organic substances which are found in the different tyres as well as their degradation products. Also, particular focus was given to zinc, phthalates and nonylphenols as a consequence of the results from the foreign studies.
Results
A wide range of environmentally harmful substances were found in the contact water (migration medium) from leaching tests on infill, artificial turf and pad, and also a number of substances that are harmful to human health.
For coir infill and “green industrial rubber” infill, substances which have a negative impact on health or the environment were not found in the solvent extract from the materials. However, leaching tests have not been conducted on these to types of infill. It should be emphasised that in one sample of EPDM rubber infill, a content of organic substances was detected (degradation products from peroxide, used in connection with the vulcanisation of the rubber). Therefore, it cannot be excluded that the substances might cause environmental problems, as leaching tests with EPDM rubber infill showed discharge of the substances into the water
Based on the dichloromethane extracts of grey and green industrial rubber, it can be concluded that these were significantly differing types of rubber, even though both infills are labelled industrial rubber by the supplier.
Environmentally harmful substances found in concentrations well above the detection threshold in the contact water are shown in Table 0.1.
Table 0.1 Environmentally harmful substances found in significant concentrations in the contact water in relation to possible health and environmental impacts.
| Substance | Infill | Artificial turf |
| Zinc | X | |
| Diethylphthalate (DEP) | X | X |
| Dibutylphthalate (DBP) | X | X |
| Benzylbutylphthalate (BBP) | X | X |
| Diisobutylphthalate (DIBP) | X | X |
| Dicyclohexylphthalate (DCHP) | X | |
| Diethylhexylphthalate (DEHP) | X | X |
| Nonylphenol | X | |
| Bis-(2,2,6,6-tetramethyl-4-piperidinyl)sebacate | X | |
| Cyclohexanamine, N-cyclohexyl- | X | |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | X | |
| Cyclohexanamine | X |
The zinc leaching measured in this study was of the same magnitude as that found in foreign studies. The leaching of phthalates and nonylphenols, however, was higher in this study.
On the basis of the foreign studies, it was assessed that the leaching conditions from a football pitch differ significantly from the conditions in the leaching tests conducted in the laboratory in this study. Therefore, it cannot be concluded on the basis of the laboratory leaching tests alone whether the substances are an environmental risk under practical conditions. This is due to the fact that during laboratory tests, a better contact is created between the fixed material and the contact liquid than is the case in real life on artificial turf pitches as the result of the weather (e.g. rain). An example is that the sample is placed in the simulated rainwater and shaken for 24 hours before the water is analysed. This should be viewed as an absolutely worst case scenario and would therefore overestimate the actual leaching.
The results from leaching in calcium chloride-based contact liquid (simulated salting) showed a significant drop in the amount of leached phthalates as well as significant changes in the concentration of other substances (both higher and lower values). This is due to the fact that commercially available calcium chloride is highly alkaline due to the calcium hydroxide content. An alkaline environment causes hydrolysis reactions which affect a wide range of organic substances, including phthalates. No significant changes in the zinc leaching were detected as a function of the pH and/or ion strength of the contact liquid, regardless of salt type.
Furthermore, leaching tests have shown the same level of zinc in contact water in new infill as in the same infill after approx. two years of use.
Health assessment
Four representative substances were selected for the health assessment:
benzothiazole, dicyclohexylamine, cyclohexanamine and dibutyl phthalate. These substances are present in high concentrations in contact water from the leaching tests and are representative of the harmful substances emitted from the products.
On the basis of the results from foreign exposure studies and own analyses, it was decided to focus exclusively on exposure via skin contact and oral uptake from particles swallowed.
Microscopy of the dust particles from leaching tests has shown a particle size for the finest particles in the order of 10-50 µm, whereas the majority of the particles are approx. 2 mm. With a high content of fine particles, the leaching of health and environmentally harmful substances may increase due to the larger contact area, and the risk of inhalable flying dust is also increased. However, as mentioned, the amount of fine dust in the products is limited. In the assessments of the results, no health risks were found in connection with exposure to the substances, with the exception of a possible allergenic risk for sensitive individuals to benzothiazole and the amines.
Environmental assessment
The substance concentrations in the contact water from the leaching tests were assessed in relation to possible spillover of drainage water to nearby watercourses. It was assessed that there is an environmental risk from a number of the substances found if the drainage water reaches concentrations on a level with those found in the contact water.
It should be emphasised that – as mentioned above – the actual concentrations of chemical substances in the drainage water from third-generation pitches are probably significantly lower than those measured in the laboratory as a consequence of a less efficient natural leaching, because it was assessed that the contact with water is not as efficient as in the laboratory tests.
For some substances, however, the calculations include very large calculated water concentrations in relation to the estimated no-effect concentration (in excess of 100 times), and thus it cannot be ruled out that there may be an environmental risk with the leaching of these substances.
Furthermore, substances were detected, such as peroxide cross-linking chemicals or degradation products thereof, from EPDM infill. These substances were present in very high concentrations in contact water, but there is no data available on environmental risks. The zinc leaching from EPDM infill, however, is significantly lower than from car tyre infill (SBR rubber).
The results from the laboratory tests in this study show that a number of environmentally harmful substances can be leached from elastic infill as well as from artificial turf with a possible environmental risk in the event of drainage water spilling over into nearby watercourses. However, on the basis of the foreign lysimeter tests and pilot studies showing significantly lower leaching than in laboratory tests, it was assessed that measurements of the substance concentrations under real conditions on football pitches are necessary in order to assess the risk. Therefore, this study found no reason to question the conclusions of the elaborate Swiss, French and Dutch studies that rubber granules from car tyres pose no major environmental risk. Here, it should be noted that the Dutch results regarding zinc leaching are not found in this or the French and Swiss studies. On the other hand, it should also be emphasised that these are only short-term studies, and the effect of ageing of rubber granules etc. is therefore unknown.
However, a more recent Dutch study published in the spring of 2008 concludes that over a period of 10-15 years, zinc is not leached in concentrations that constitute any environmental problem. Continued monitoring for zinc in the Swiss study still shows no sign of increased zinc leaching as a result of rubber ageing (U. Hofstra, 2008, Edwin Müller, 2008).
When setting up a monitoring programme for the drainage water, it is possible, when selecting analysis parameters, to benefit from the results from this study and from the foreign studies mentioned in the report. For instance, measurements for PAHs can be excluded, and measurements for phthalates can be added.
1 Introduction
1.1 Artificial turf pitches are popular
In recent years, it has become increasingly popular to use artificial turf as a substitute for natural turf.
Artificial turf is becoming increasingly popular for golf greens, school playgrounds and playgrounds in general, but it is primarily used for football pitches.
The international football associations UEFA and FIFA thus accept the use of artificial turf pitches for football if the pitches meet the requirements for functional properties in relation to football (FIFA, 2006).

Figure 1.1 Outdoor artificial turf football pitch
There are several advantages in using artificial turf rather than natural turf. Artificial turf pitches are more hard-wearing and easier to maintain than natural turf pitches. The football season can be extended whatever for the weather, and the pitches can be laid in places where it is difficult to get grass to grow.
Artificial turf pitches are used indoors as well as outdoors. In Denmark, however, artificial turf pitches are almost exclusively used outdoors.
According to the Danish Football Association (DBU) (A. Johansen, 2007), there are four indoor pitches in Denmark. However, only two of these are third-generation pitches. One is a three-quarter pitch in Brøndby; the other is a newly installed pitch in Ikast. The infill material in Ikast is polyethylene-coated sand, contrary to the traditional third-generation pitches where the infill is sand plus an elastic granulate.
The sports arenas Valby-hallen and Ballerup-hallen also feature indoor pitches, but according to DBU, these are of the second-generation type (without elastic infill).
According to DBU, there are no immediate plans to establish further indoor pitches in Denmark.
In most cases by far, artificial turf consists of plastic fibres attached to a polypropylene or polyester carpet loom (see Fejl! Henvisningskilde ikke fundet.). The polyester mesh is coated with an adhesive suspension which is cured in an oven. One supplier mentions that a styrene butadiene latex is used for the adhesive.

Figure 1.2 Mesh for artificial turf, front and back
The fibre height is typically 3-6 cm. A mixture of sand and an elastic granulate is used in order to secure the artificial turf pitches. The sand is used because of its weight, and the purpose of the elastic granulate is to provide springiness.
Pitches using an elastic granulate as the springy part of the concept are called third-generation artificial turf pitches. Third-generation artificial turf pitches have been developed from 1990 to the present day.
A fourth-generation artificial turf pitch is also on the way, where an additional foamed elastic sublayer immediately below the turf structure is introduced. This makes it possible to reduce the volume of rubber granules per square metre, thus making it possible to use alternative elastic infill materials, such as ethylene propylene diene rubber (EPDM), at a competitive price (T.V. Pedersen, 2007). However, it does not give better playing properties compared to the other pitch types.
The concept of fourth-generation artificial turf pitches has not yet been defined by DBU.
In Magglingen, Switzerland, an artificial turf pitch has been laid on a pad without the use of elastic infill. The grass (PE) is held upright using polyamide support fibres.

Figure 1.3 PE grass with polyamide support fibres
However, according to the information available, this pitch does not meet the applicable FIFA requirements for playing properties. The requirements were made with a view to the properties of pitches with elastic infill, which was state of the art at that time.
Since then, and practically without exception, rubber granules from obsolete car tyres (styrene butadiene rubber (SBR)) have been used as the springy part of the infill material. This is due to the fact that, so far, tyre granules have been by far the cheapest infill material. Tyre granules are available as a raw material in ample supply, as approx, 60% of all rubber (approx. 20 million tonnes per year) is used to produce tyres which are discarded sooner or later. For a third-generation football pitch, approx. 100-120 tonnes of rubber granules are used. However, this volume can be reduced by installing an elastic pad under the mat on the reverse of the artificial turf.
Coated infill types also exist. The coating is typically polyurethane with an added colourant (e.g. green).
Tyres contain a number of chemical substances which are cause for health and environmental concerns, which means that rubber from discarded car tyres should be used with care.
This aspect, combined with the fact that the use of artificial turf has become increasingly popular, has resulted in several countries launching research projects designed to investigate the health and environmental impacts from artificial turf pitches (T. Källquist, 2005; C. Dye et al., 2006; T.S.W. Plesser, 2004; T. Sanner, 2006; Swedish Chemicals Agency (Kemikalieinspektionen), 2006; H.J. Kolitzus, 2006; Verschoor, 2007; M. van Bruggen et al., 2006; U. Hofstra, 2007; R. Moretto, 2007). The results of these projects will be briefly reviewed in chapter 3. Chapter 2 reviews the chemistry relating to the materials which are used to make artificial turf pitches.
Chapter 4 describes the mapping of the use of artificial turf pitches in Denmark. Chapter 5 follows, containing descriptions of sample materials procured in the form of artificial turf mats, infill materials and pads.
Chapters 6 and 7 deal with analysis methods and analysis programme and the health assessment, respectively. The report concludes with chapter 8 with an environmental assessment.
2 Artificial turf pitches – Materials and chemical substances
2.1 Possible constituents
For artificial turf pitches, plastic fibres made of polyethylene (PE), polypropylene (PP) and polyamide (PA) are used (The Grass Yarn & Tufters Forum, 2006). These are attached to a perforated mesh of PP or polyester. This is coated with a latex-based adhesive which is subsequently cured. Rubber granules or thermoplastic polymers are used for the elastic infill, but there is also a product on the market which is based on natural fibres. Sand is used to weigh down the grass. The sand could be coated with an elastomer or PE. Additionally, an elastic sublayer is usually included under the artificial turf. This could typically be a coarse rubber fraction from tyres glued together using polyurethane, but other types are also available in the market.
Below follows a brief description of the substances which can be expected to leach from artificial turf pitches on the basis of the chemical structure of the plastic or rubber type used as well as the additive chemicals used to stabilise the molecular chains against degradation due to weather.
For rubber, degradation products may be present as a result of vulcanisation. The volume and the chemical structure are often unpredictable, because they depend on the vulcanisation system used, and the time and temperature.
With wear and tear, rubber dust may be produced, which can be a source of exposure via skin or respiratory passages.
2.1.1 Chemical substances in the artificial turf mats
The existing literature (The Grass Yarn & Tufters Forum, 2006) states that the artificial turf fibres are primarily produced from polyethylene (PE) or polypropylene (PP), but that nylon (polyamide) is also an option. The same source indicates that PE is used exclusively for football pitches.
Antioxidants are usually added to PE and PP in order to improve weather resistance, which is otherwise already good due to the saturated carbon chain structure. The antioxidants are typically organic phenolic structures with a relatively high molecular weight in order to prevent evaporation. Organic phosphites are usually added as auxiliaries, as this provides a synergistic protective effect against oxidative degradation.
Furthermore, UV stabilisers are added to protect against light degradation. Typical UV stabilisers are of the HALS type (Hindered Amine Light Stabilisers). Some UV stabilisers are contain zinc, e.g. Tinuvin 494 from Ciba Speciality Chemicals.
The fibres are coloured green. Some of the green colourants can be based on metallic complexes (copper), or they can be of the azo colourant type, of which some, e.g. yellow, are known to be potentially carcinogenic. The green colour is produced by mixing yellow and blue colours.
Previous studies carried out by the Danish Technological Institute show that volatile short-chain hydrocarbons often degas from PE as well as PP. It was thus expected beforehand that the artificial turf mats would degas volatile, short-chain hydrocarbons in a similar manner. It was expected that there might also be softeners in the latex adhesive used, e.g. in the form of phthalates.
2.1.2 Chemical substances in the elastic infill
According to the existing literature (The Grass Yarn & Tufters Forum, 2006), the overwhelming majority of the artificial turf pitches laid are based on recycled, granulated tyres (98%).
Information exists (T.V. Pedersen, 2007) that one of the disadvantages of using the granules is the black colour, and that, especially in wet weather, the rubber easily sticks to clothes, footwear and skin.
Another disadvantage is the smell of rubber, which is most predominant in hot weather, however. The smell can be suppressed by watering.
There are alternative options when choosing materials. You can either use coated granules from tyres in order to reduce the migration of substances from the granules, or you can use EPDM rubber granules and thermoplastic elastomers (TPE) or mixed granules. Coated sand is also mentioned as an option. Infill material based on natural fibres also exists. Finally, an infill material is marketed under the designation industrial rubber. According to the supplier, this is surplus rubber from the production of window piping.
The problem with several of the alternatives to granulated car tyres is that the price level of the infill materials is increasing.
According to (The Grass Yarn & Tufters Forum, 2006), coated tyre granules are four times as expensive as the base raw material.
According to the same reference, if you switch to EPDM or TPE, the price increases further to eight to 14 times, respectively, compared to natural rubber granules (The Grass Yarn & Tufters Forum, 2006). Since the price of an artificial turf football pitch using tyre granules is approx. DKK 5 million, substituting the tyre granules results in a considerably increased installation price. This is assuming that the figures are correct, which is questionable on the basis of information from suppliers to the Danish market.
Information is thus provided that some of the alternative infill materials are only two-to-four times more expensive than pure tyre granules. From one supplier to the Danish market, the following approximate prices were stated for 120 tonnes of infill in the types of infill used for the pitches: Granulated car tyres: DKK 180,000, coated granules from car tyres: DKK 500,000, EPDM, TPE and coir: DKK 850,000. If the density of EPDM compared to granulated car tyres is taken into consideration, the price of EPDM increases to DKK 1,100,000.
Information was provided, however (T.V. Pedersen, 2007), that by using fourth-generation structures with foamed cross-linked polyethylene (PEX) pads it is possible to achieve competitive prices for alternative infill materials made from EPDM rubber.
2.1.2.1 Granulated tyres
In connection with the “Emission and health evaluation of PAHs and aromatic amines in car tyres” project (Danish Environmental Protection Agency, Kortlægning nr. 54, 2005), detailed descriptions were given of the raw rubber types forming part of the recipes for passenger cars tyres as well as for truck tyres.
The raw rubber types are natural rubber (NR), styrene butadiene rubber (SBR) and butadiene rubber (BR). SBR rubber is the main component in tyre treads.
In addition to carbon black (soot), aromatic oils, zinc oxide, stearic acid, antioxidants and antiozonants as well as sulphur and accelerators form part of the recipe. The accelerators contain nitrogen and sulphur and, when heated, can emit carbon disulphide and split off amines, of which several can be nitrosamine-forming. In addition to zinc, rubber granules made from discarded car tyres can emit copper and chromium from the steel cord used to reinforce the tyres. The accelerators used are typically based on benzothiazole, which can be split off during vulcanisation. The antiozonants are predominantly 6-PPD [N(1,3-di-methyl-butyl)N´phenyl-p-phenylene diamine], but other p-phenylene diamine-based antiozonants, e.g. IPPD (N´-isopropyl-N´phenyl-p-phenylene diamine) are also used. The Norwegian studies (C. Dye et al., 2005; C. Dye et al., 2006; T.S.W. Plesser, 2004; T. Sanner, 2006) also observed emission of phthalates as well as long-chain alkylphenols from the rubber granules. The phthalates could originate from adhesives and the alkylphenols from reactive resins used in the vulcanisation of butyl rubber types (J.S. Dick, 2001). Butyl rubber forms part of tyres as an airtight layer.
2.1.2.2 EPDM rubber
EPDM rubber consists of carbon chains constructed from the monomers ethylene and propylene and a diene component, typically norbornene, built in as a side chain. The advantage of this polymeric structure is great weather resistance, making it unnecessary to add antiozonants to EPDM rubber. The volume of antioxidants can also be reduced. EPDM can be subjected to either peroxide or sulphur vulcanisation. In both cases, zinc oxide may be a constituent, but in greater volume in the sulphur vulcanisation. In sulphur vulcanisation, the usual accelerators based on nitrogen and sulphur are used. For the peroxide vulcanised types, organic peroxides are used, typically dicumyl peroxide which splits off acetophenone during vulcanisation. Other types may split off tert-butyl alcohol. The softeners used for EPDM are predominantly naphthenic oils with a relatively low aromatic content. Triallyl cyanurate is used in peroxide vulcanised EPDM rubber as a cross-linking regulator.
2.1.2.3 Industrial rubber
Municipalities and football clubs that do not want tyre granules specify instead that they want infill in the form of industrial rubber.
In principle, industrial rubber is all rubber, as all technical rubber products are produced industrially. One supplier calls industrial rubber a surplus product from the rubber industry, originating from window piping production.
Based on the supplier’s information alone, it is not possible to assess the chemical composition of the granules.
2.1.2.4 TPE
TPE is an abbreviation of thermoplastic elastomers. SEBS is a typical thermoplastic elastomer based on a Styrene Ethylene Butadiene Styrene structure. TPE is distinct from rubber in that it is not vulcanised. Instead, a mesh structure is formed by the styrene segments forming crystalline domains. Since the chain structure is saturated, SEBS is characterised by good weather resistance, as was described for EPDM rubber. The emission of chemical substances from SEBS is predicted to be limited, because no vulcanisation chemicals are used as is the case for rubber.
2.1.2.5 Natural fibre-based infill
A single supplier offers a natural fibre-based infill material. This is based on coir fibres.
3 Foreign studies
Detailed studies of artificial turf pitches have primarily been conducted in Norway (T. Källquist, 2005; C. Dye et al., 2006; T.S.W. Plesser, 2004; T. Sanner, 2006), but ongoing as well as concluded studies with comprehensive monitoring programmes for a number of environmental parameters are also being conducted in Sweden (Swedish Chemicals Agency (Kemikalieinspektionen), 2006), the Netherlands (Verschoor, 2007; M. van Bruggen et al., 2006; U. Hofstra, 2007; R. Moretto, 2007), Switzerland (E. Müller, 2007; H.J. Kolitzus, 2006) and France (R. Moretto, 2007). The Swedish report is primarily based on information from other sources.
3.1.1 Norwegian studies
Four Norwegian reports are available which, in different respects, assess health and environmental risks as a consequence of the laying and use of artificial turf pitches in Norway.
Two of the studies were conducted at the behest of the Norwegian Pollution Control Agency (Statens Forurensningstilsyn (SFT)) (T. Källquist, 2005; C. Dye et al., 2006). One was conducted for the Norwegian football association (T. Sanner, 2006), and the final one was conducted by Byggforsk (T.S.W. Plesser et al., 2004).
SINTEF Building and Infrastructure (Byggforsk) (T.S.W. Plesser et al., 2004) mentioned above has published its final report on potential health and environmental impacts associated with artificial turf pitches for the Norwegian football association in September 2004.
The Byggforsk study included three infill rubber granule types based on recycled rubber (all three presumably from tyres) and an EPDM rubber granule product (presumably not recycled). In addition, two artificial turf fibre types based on PE and PE/PP copolymer were used. One type was split fibre, the other monofibre. Split fibres are produced by slitting PE film. Monofibres are produced by each fibre being extruded separately. This should provide better long-term wear resistance.
The study analysed for total contents of arsenic, lead, cadmium, copper, chromium, mercury, PCB, PAH, phthalates and phenols in the materials. Furthermore, leaching tests and degassing tests were conducted.
The risk assessment was performed in a simplified version, as the total content of environmentally harmful substances in the source materials was compared to the SFT’s normative values for most sensitive area use. In Norway “most sensitive area use” means areas intended for housing, gardens, kindergartens, schools etc.
The contact water from the leaching tests of rubber granules from recycled rubber contained zinc, PAHs, phthalates and phenols. The amount of zinc places the contact water in the SFT class V (very polluted water), but it is lower than the zinc content allowed in the Canadian Environmental Quality Guidelines for drinking water.
The amount of antracene, fluoroanthene, pyrene, phthalates and nonylphenols is higher than that allowed in the Canadian Environmental Quality Guidelines.
According to the authors, it will require a more detailed risk analysis and further tests to determine whether there is a real risk of harm to the environment and health from the substances measured in the contact water.
The rubber granules from the recycled rubber emit a number of alkylated gaseous benzenes. In one of the tests, degassing of trichloromethane was detected, and in another cis-1,2-dichloroethene. The report recommends performing actual measurements on an artificial turf pitch in order to determine whether the emission is problematic.[1]
With the exception of the of chromium and zinc content, the EPDM granules contained smaller amounts of environmentally dangerous substances than the granules from recycled rubber. EPDM also emitted smaller amounts of volatile substances.
In the actual artificial turf fibres, copper and zinc were detected. In both cases, the leaching of zinc from these types of artificial turf to demineralised water are above the SFT’s threshold values for zinc in drinking water (class V).
In one artificial fibre type, contents of octylphenol and nonylphenol were detected. Furthermore, DEHP was detected in both types of artificial fibre. One sample also contained DMP and DINP.
The Norwegian Institute for Water Research (Norsk Institutt for vannforskning) (T. Källquist, 2005) has assessed the environmental risk on the basis of studies of environmentally harmful substances existing in materials used when laying artificial turf pitches, and their potential as regards leaching via rainwater.
The risk was assessed on the basis of the results in the Byggforsk report described above, and by calculating the PEC/PNEC ratio, where:
PEC is “Predicted Environmental Concentration”, and PNEC is “Predicted No Effect Concentration”, i.e. the highest concentration which will not result in harmful impacts on the environment.
The assessment follows the standard procedures used in risk assessment of chemicals in the EU.
The scenario on which the assessment is based is an artificial turf pitch with an area of 7,200 m², annual precipitation of 800 mm and drainage into a nearby stream. Naturally, the flow rate in the stream will have an impact on the dilution ratio. This was set to be a factor of 10.
The amount of artificial fibre turf and rubber granules for a pitch with the area mentioned was set at 5,760 kg and 129,600 kg, respectively.
It is a supposition for the calculations that the concentrations found in the contact water are equilibrium concentrations, i.e. independent of the water/material volume ratio.
The calculations showed that there is a possibility of environmental impacts for small recipients receiving surface water from artificial turf pitches.
The substances contributing the most are zinc and alkylphenols (in particular octylphenol).
The concentration of leached substances is expected to diminish, but over many years.
The amounts leached into the water are modest, and according to the report, any environmental impacts will therefore only be local.
In order to obtain a better basis for evaluating the environmental impacts of the artificial turf pitches, the authors believe that measurements should be conducted directly on drains from laid artificial turf pitches. The measurements should include toxicity tests.
The Norwegian Institute for Air Research (Norsk Institutt for Luftforskning (NILU)) conducted air pollution measurements in three indoor artificial turf sports halls in 2005 (C. Dye et al., 2006).
The measurements were conducted for artificial turf pitches with newly laid rubber granules (SBR tyres), with rubber granules laid one year previously (SBR tyres) and for an artificial turf pitch with thermoplastic elastomer (TPE) infill.
The measuring programme included analysing flying dust PM10 and PM2.5 for the proportion of rubber, and the concentration of vulcanisation compounds, anti-ageing agents, phthalates and tar substances (PAHs). Furthermore, measurements were made for volatile organic compounds (VOC) and polycyclic aromatic hydrocarbons (PAHs) in the air phase.
Measurements were made for the total sum of volatile organic compounds (TVOC) in the three sports halls, as well as for a wide range of specific volatile compounds.
The study lists concentrations for benzothiazole, toluene, 4-methyl-2-pentanone, diethyl phthalate (DEP), diisobutyl phthalate (DIBP) and dibutyl phthalate (DBP) as well as TVOC. TVOC was in the interval 136 µg/m³ to 716 µg/m³. The largest concentrations were found for toluene: 15-85 µg/m³ and benzothiazole: 3-16 µg/m³.
The study concludes that TPE provides a lower TVOC than the granules based on SBR from used tyres.
The content of organic materials in the flying dust was considerable in all three sports halls. Typically, these are PAHs, phthalates, semi-volatile organic compounds, benzothiazoles and aromatic amines.
In the report (T. Sanner, 2006) prepared by the Norwegian Institute of Public Health (Nasjonalt folkehelseinstitutt) and the Radium Hospital (Radiumhospitalet), the health risk for football players was assessed.
The assessment used nine exposure scenarios, including inhalation via the respiratory passages, skin exposure and oral intake.
The inhalation scenarios are based on the highest detected VOC level of approx. 716 µg/m³, even though VOC in the other two sports halls monitored was 2.5-3 times lower.
On the basis of the scenarios, the report concludes that, based on the exposures calculated from the use of indoor halls with artificial turf, and where granulated rubber from discarded car tyres is used, there is no reason to assume that these will lead to an increased health risk. However, certain reservations are made regarding the development of asthma/airway allergies, in which area the existing knowledge is limited. This applies in particular to latex allergenes.
It is an open question whether these latex allergenes can occur in dust from artificial turf pitches with infill based on discarded car tyres.
For several of the detected substances in the sports hall air, the toxicological knowledge is limited. However, the concentrations of the substances are very low, and for this reason they are not expected to pose any health risk.
According to the report, the exposure amounts for benzene and PAHs pose no health risks.
According to the authors, it is deemed necessary to replace the current SBR rubber granule infill in the sports halls based on the existing knowledge on health effects, but it is recommended that when adding infill, recycled SBR from tyres should not be used, as there is a possible risk of inducing latex allergy.
3.1.2 Swedish status report
In 2006, the Swedish Chemicals Agency (Kemikalieinspektionen) published a status report “Artificial turf from a chemical perspective” (Konstgræs ur et kemikalieperspektiv) (Kemikalieinspektionen, 2006) concerning health and environmental problems in connection with the use of artificial turf.
The report focuses exclusively on artificial turf football pitches with an infill made from recycled car tyres.
According to the report from June 2006, Sweden has approx. 150 artificial turf football pitches laid on infill made from rubber granules from recycled tyres.
Most pitches are for outdoor use, but indoor pitches also exist.
In the Stockholm area alone, there are apparently plans for 30 new artificial turf pitches.
It is assessed that approx. 90% of the pitches will be based on infill from recycled tyres.
To a large extent, the report is based on the Norwegian studies, the results of which are reviewed.
There are also references to a few individual measurements in artificial turf halls in Sweden.
Measurements and calculations showed a very low content of benzo(a)pyrene, approx. 10,000 times lower than the threshold set by the Swedish working environment authority.
The report reviews European guidelines, including the German provisional standard DIN 18035-7:2002.
The report finally concludes that granules from recycled rubber originating from car tyres should not be used as infill when laying new artificial turf pitches. The reason for this is an environmental objective set by the Swedish parliament, determining that materials containing particularly dangerous substances should not be used.
The other conclusions are very close to the corresponding Norwegian recommendations and with the very same arguments.
3.1.3 Dutch studies
Three comprehensive Dutch studies are available (A.J. Verschoor, 2007; M. van Bruggen et al., 2006; U. Hofstra, 2007):
- RIVM report 601774001, on the leaching of zinc from rubber infill in artificial turf pitches (A.J. Verschoor, 2007)
- RIVM report 609300001, on nitrosamines in rubber granules (M. van Bruggen)
- INTRON report on health and environmental aspects of infill rubber granules from recycled car tyres (in Dutch, but with English summary) (U. Hofstra, 2007).
The report on zinc leaching concludes that the leaching of zinc from SBR infill will show an increasing trend as a consequence of the ageing of the rubber. It is assessed that the zinc impact from infill will be 800 mg/m²/year. In the Dutch Building Materials Decree, an acceptable zinc impact is set at 2,100 mg/m²/100 years. According to the authors, this value is thus exceeded already after approx. three years. It is stated that the zinc impact locally would be approx. 20 times higher than from agriculture (slurry manure and pesticides).
The report concludes that further studies on zinc leaching could reduce the experimental uncertainty which underlies the risk assessment.
It is suggested that studies be made on the ageing of different types of rubber and on the effect of the ageing on the leaching of zinc and other substances which may migrate from rubber.
Furthermore, field measurements are suggested for artificial turf pitches with infill rubber of different ages and qualities. Bioassays of the drainage water are recommended to determine the impact of the mixture of substances emitted from the infill material. Finally, mini field tests (lysimeter) are recommended – e.g. on 1 m³ samples.
An INTRON report (U. Hofstra, 2007) concludes that leaching of zinc from rubber granules from used car tyres is considered to be the most serious source of environmental pollution from outdoor artificial turf pitches. It states that the Dutch threshold values for zinc emissions are expected to be exceeded within a 3-20 years utilisation phase.
A conference presentation (N. Salzmann, 2006) concludes that the amount of airborne nitrosamines (N-nitrosodimethylamine) measured on a pitch[2] is higher than the health threshold. The assessment is based on a scenario in which the pitch is used for football three times a week for a duration of two hours. According to the paper, the threshold was exceeded after eight years. There were no further details about the experimental parameters and the monitoring.
In another very comprehensive study (P.C.J.M. Janssen, 2006), the concentration of nitrosamines was measured in the air above four artificial turf football pitches in Arnhem at a height of 30-100 cm. It was concluded that nitrosamines could not be detected in the air. Laboratory measurements detected nitrosamines in small concentrations in the actual infill material.
The overall conclusion of this study was that nitrosamines are not a health problem (contrary to the conclusion above).
As regards health risks in the form of skin contact, inhalation or oral intake, it is concluded that there is no significant risk for football players as a consequence of infill in artificial turf pitches based on rubber granules from used car tyres.
3.1.4 Swiss study
In Bern, Switzerland, a project was launched in May 2006 involving at least one year of monitoring of the leaching of selected chemical substances and common parameters (H.J. Kolitzus, 2006).
The study was based on the use of lysimeters, which are used for e.g. studies of the uptake of nutrients by plants. The lysimeters are constructed from reinforced polyester tubes with a diameter of 1 m and a height of 1.5 m. The tubes contain an automatic water collection system. This enables collection of the total amount of rainwater from an artificial turf surface. The collected water is subsequently analysed.

Figure 3.1 Lysimeter test, Bern, Switzerland
Collection takes place in containers connected to the concrete bottoms of the lysimeters.
For the test series, ten different types of surface were selected, which are used in connection with the laying of artificial turf pitches.
EPDM and SBR rubber are included as infill material, as is sand and different types of support layers, e.g. bitumen-based or as recycled SBR rubber granules on top of a free mineral support layer.
A preliminary conclusion has been made on the basis of test results from May-September 2006.
Emissions of aromatic amine complexes and benzothiazoles were detected in the concentration range 10-300 µg/l, but no significant discharge of PAHs to the drainage water was detected, and this was also the case for recycled SBR. The detection limit for the individual PAHs was at approx. 0.02 µg/l.
The preliminary conclusions of the completed study, prepared in German by E. Müller, the professional expert associated with the study, were sent to the Danish Technological Institute by email on 29 August 2007.
In addition, the results were summarised (E. Müller, 2007) in September 2007 at a meeting arranged by the German BASPO (Bundesamt für Sport (Federal Office for Sport) in Magglingen, Switzerland.
Below is the English translation (from Danish) of the most important information and conclusions from the study.

Figure 3.2 Detail from lysimeter tests in Bern, Switzerland
Table 3.1 Studied artificial turf materials in the Swiss lysimeter tests
| Artificial turf pitch | Artificial turf pitch with EPDM-granules (peroxide vulcanised) and silica sand on elastic pad Artificial turf with rubber granules from car tyres and silica sand. Artificial turf pitch with EPDM granules (sulphur vulcanised) and quarts on elastic pad Artificial turf pitch without infill |
| Elastic pad | Water permeability one-layer EPDM pad Water permeability multilayer coated plastic Waterproof sandwich coating |
| 0-Sample (for investigating the natural background contribution for the measured analysis parameters) | Gravel with smoothed gravel layer Gravel with smoothed gravel layer and bitumen layer Elastic pad based on granulated car tyres |
The study included leaching of the following chemical substances and common parameters:
- Aniline
- Alkylated phenylene diamines
- Benzothiazole
- 16 PAHs
- Zinc
- Ammonium nitrogen
- Nitrate nitrogen
- Nitrite nitrogen
- Sum of dissolved organic nitrogen compounds
- Sum of dissolved organic compounds (DOC)
Additional determination of the zinc content of the different rubber granules was made, as well as thermal analyses and leaching tests in order to characterise the different granules.
It was concluded that with modern analytical measuring equipment, it is possible to detect even the smallest traces of organic substances. The substances are washed from the surface by rainwater after a relatively short period of time. At the end of the one-year period, the concentrations of leached substances were in almost all cases smaller than the detection limit of 0.2 µl per litre. In leaching tests in the laboratory, the leaching profile from the artificial turf pitches was confirmed in the lysimeter tests.
It was also established that the substances leached from rubber granule infills and rubber pads are the same substances which are drained from roads as a result of rubber wear particles from the car tyres, and which are discharged from municipal treatment plants.
During the one-year study period, there was no increase in the leached amount of zinc ions to the aquatic environment. All tests showed the same low content of PAHs on a level with the contents of the O samples. No health or environmentally harmful PAHs could be detected in the water from the tests.
The preliminary conclusion is as follows:
- The rubber granules and elastic pads used for artificial turf pitches are insoluble in water. All granules, newly manufactured as well as granules from crushed car tyres, leave traces of chemical substances which can be detected in the rainwater collected.
- The organic substances present on the surface of the rubber granules will be leached by the rainwater within a short period of time.
- With the knowledge we have today, there is no foundation for any claim about environmental problems concerning the leaching of undesirable chemical substances from artificial turf pitches and elastic layers (infill and pad). However, no long-term studies are available (covering several years).
- The preparation of testing methods and norms should be discouraged as the necessary basis for their development is not available.
3.1.5 French study
A French study is available (R. Moretto, 2007) which was prepared for ADEME, Aliapur and Fieldturf Tarkett i 2007. Aliapur is a French company involved in granulating tyres in France. In 2005, the company’s production of rubber granules from tyres corresponded to a volume of 283,000 tonnes of tyres (85% of tyre refuse in the French market). Fieldturf Tarkett is one of the major international players within third-generation artificial turf. Annually, the company lays 650 major sports pitches, of which 100 were laid in France in 2006. ADEME is the French ministry of the environment, which has supported the fact-finding project. The practical implementation took place in a French research network (EEDEMS).
The study was initiated on the basis of, among other things, the information (the Norwegian reports and the Swedish report) which was presented on the possible health and environmental problems in using recycled tyres as infill for artificial turf pitches.
The reports also formed the basis for implementing some of the studies referred to previously, e.g. those from the Netherlands and Switzerland.
The study included environmental impacts on water as well as an assessment of the health risks associated with gaseous emissions.
As regards materials, the studies included artificial fibres from Fieldturf Tarkett combined with granules from used tyres, virgin EPDM and TPE-based infill.
3.1.5.1 Discharge of substances into water
In the studies involving the discharge of substances into water, infill sand was included as well as the joinings and the polyurethane adhesive used to join the artificial turf pitches.
17.5 kg of sand and 15 kg of elastic infill were used in the experiments.
The experiments were performed as in situ experiments and pilot scenario experiments.
For the in situ experiments, a lysimeter system was applied. The system was based on a stainless steel tray with a surface area of 2 m² and a height of 10 cm at one end and 5 cm at the other. The tray was placed in an artificial turf football pitch. The rainwater percolate was collected in the bottom of the tray and pumped into analysis receptacles.
The monitoring period was 11 months.
For the pilot studies, rectangular aluminium tanks with a length of 2.5 m and a width of 1 m were used. Bottoms and sides were based on waterproof geomembranes. The percolate was collected via drainage pipes at the bottom of the structure.
The test setup was indoors, and rain simulation was carried out by using nozzles which dosed an amount of water corresponding to the average precipitation in a number of European capitals (800 mm of rain annually).
As with the in situ experiments, the monitoring period was 11 months.
The analysis programme in connection with the two types of test setups was extensive. For instance, 42 chemical/physical parameters were monitored:
Total cyanide, phenolic index, total hydrocarbons (THC), 16 PAHs, TOC, Al, As, Ba, Cd, Co, Cr, Cu, Hg, Mo, Ni, Pb, Sb, Se, Sn, Zn, fluorides, nitrates, ammonium, chlorides and sulphates as well as pH and conductivity.
Furthermore, ecotoxicological characterisation of the percolates was carried out through inhibition tests with Daphnia magna in accordance with EN ISO 6341 and with Pseudokirchneriella subcapitata in accordance with EN ISO 28692 (inhibition of algae).
The volume of water dosed in the four pilot studies was 800 l/m²/15 kg of granulate, and the percolate collected was 580 l/m². This is taken to indicate that approx. 27-30% of the water evaporates into the atmosphere.
The conclusion from the two types of experiments is that the concentrations of the substances and common parameters measured are compatible with French and European guidelines, but the authors have not provided detailed specification of the guidelines to which reference is made.
The conclusion from the ecotoxicity tests is that the percolate from third-generation artificial turf pitches, regardless of the type of infill, does not demonstrate a negative impact on the environment.
3.1.5.2 Emission of volatile substances
In the studies looking at the emission of volatile substances, the following materials were tested: artificial turf with green artificial fibres including a band of white fibres, polyurethane adhesive, sand and three different infill granulates. EPDM, SBR from used car tyres and TPE were used as granulates.
As in the studies for discharge into water, 17.5 kg of sand and 15 kg of elastic infill were used in the experiments.
For a sample of 0.15 m², which was actually tested, this corresponded to 2.625 kg of sand and 2.25 kg of granulate.
The test was performed on sample material placed in steel trays measuring 0.78 m x 0.19 m at 23°C and a relative humidity of 50% ± 5%. The test was conducted in accordance with prEn ISO 16000-9: Indoor air – Part 9: Determination of the emission of volatile organic compounds from building products and furnishing – Emission test chamber method.
A specific ventilation speed of q = 1.25 m3/m²/h was used.
The tests measured for VOC and aldehydes.
The conclusion from the studies were:
- The emission from artificial turf alone is very low (TVOC = 8.3 µg/m³ over a period of 28 days) compared to parquet flooring.
- The emission from artificial turf with granulate from used tyres as infill is low (TVOC = 134 µg/m³ over a period of 28 days).
- The emission from artificial turf with TPE granulate as infill is also low (TVOC = 118 µg/m³ over a period of 28 days).
- The emission from artificial turf with EPDM as infill is higher (TVOC =490 µg/m³ over a period of 28 days).
On the basis of a worst case scenario (for indoor use, the starting point is a hall with an artificial turf surface of 1,800 m² and a volume of 20,000 m³), it was concluded that:
- On the basis of the concentration of VOC and aldehyde emitted to the indoor air, there should be no concerns in connection with outdoor use of artificial turf pitches seen from a health perspective.
- On the same background of measurements, neither should there be any concerns in connection with indoor use, seen from a health perspective. This applies to professional athletes, amateur athletes, children and adults as well as the people installing the pitches.
There exists, however, a risk for the people installing the indoor artificial turf pitches if this takes place in badly ventilated halls over a period of more than five years. In such cases, air replacement of two volumes per hour is recommended.
It should be added that the conclusions regarding the impact as a result of the discharge of substances into water were made on a short-term and medium-term basis.
Furthermore, it was emphasised that there is a comparable impact from the infill material, regardless whether this is based on virgin TPE and EPDM or on granulate from used car tyres.
The conclusion is interesting when compared to the conclusions of the Norwegian, Swedish and Dutch studies. On the basis of the French study, there are thus no grounds for phasing out the use of granulated car tyres (SBR), and in this study, EPDM emits more VOC than SBR. It should be noted, however, that there are many different recipes for EPDM, so a particularly oily type may have been chosen for the comparison.
3.2 Overall conclusion of the foreign studies and strategy for this study
Overall, it can be concluded that more or less all foreign studies conclude that there are no health problems for users of artificial turf pitches, neither indoors or outdoors.
On the other hand, the foreign studies do not reach a common conclusion as regards recommendations/preferences regarding the use of different infill materials for artificial turf pitches and their environmental impact. Here, the results of lysimeter studies carried out in France and Switzerland indicate that, in practice, there should be no problems in using granulated car tyres as infill in the pitches, compared to other infill types. A Dutch study concludes that increased zinc leaching may occur from granulated car tyres as a result of ageing of the rubber. This is the only report making this hypothesis. In Norway, the risk of an environmental impact as a result of leaching of chemical substances from granulated tyres is assessed to be small, with the exception of particularly sensitive areas. The Swedish status report discourages the use of granulated car tyres with reference to restrictions in Swedish law. No lysimeter tests have been carried out in Sweden unlike in France and Switzerland, but the conclusion was largely based on the Norwegian studies.
The foreign studies include a limited number of artificial turf mats and infill types. For instance, coir infill and grey industrial rubber have not been reviewed. This Danish study has therefore emphasised collecting as many different types of artificial turf mats and infills as possible in order to perform a mutual comparison of the materials used on the market as well as the chemistry associated therewith.
In the assessment of the environmental impact on soil and groundwater from the pitches, the Danish Environmental Protection Agency has also been interested in mapping and reviewing the significance of using salt on the artificial turf pitches, as well as the use of any herbicides. This interest is related to the weather in Denmark as well as the fact that all Danish artificial turf pitches, with the exception of two, are outdoors. One Danish municipality has used large quantities of salt, and a hypothesis was therefore made that larger volumes of salt in the form of sodium chloride, as a result of the change in ion strength, could increase the leaching of certain substances to the water and soil. Calcium chloride or magnesium chloride are also used for salting, and it is therefore also possible that divalent positive ions (Ca++ and Mg++) could have an impact on the leaching of zinc from the artificial turf pitches containing zinc.
The strategy of this project has therefore been to provide comparisons between the artificial turf mats and infill materials available in Denmark, with the main emphasis being on the environmental impact on the soil and water from outdoor artificial turf pitches. At the same time, the focus on the health assessment of the impact on users in connection with contact and inhalation was reduced during the project period as a consequence of the results which were published during the course of the project, and which, more or less without exception, conclude that there are no health problems associated with the use of artificial turf pitches, regardless of the infill type.
4 Mapping
4.1 Method
Manufacturers and suppliers of rubber granules and artificial turf fields have been contacted to identify the market for third-generation artificial turf pitches and the expectations for new pitches in the coming years.
The knowledge gathering has included information about the actual structure of artificial turf pitches and the materials used for establishing the pitches.
At the request of the Danish Environmental Protection Agency, information has been gathered about any use of chemicals in connection with winter salting of the pitches and any use of herbicides as part of pitch maintenance.
The mapping of conditions specific for Denmark has been based on interviews conducted via questionnaires (see Appendix 1).
The mapping has also included a review and summary of publicly available reports or lectures on environmental studies. The results summary of this literature study is reviewed in chapter 3.
The mapping in relation to Danish conditions has furthermore been based on inquiries made to and interviews with Dansk Boldspil-Union (DBU), football clubs and municipalities with a view to obtaining an overview of artificial turf pitches already laid and expectations for new turf pitches being laid in the coming years.
4.2 Interview
As mentioned above, a questionnaire has been used (Appendix 1). The questionnaire has been forwarded to a couple of large players within manufacture, supply and establishment of artificial turf pitches in Denmark in June 2007. Subsequently, the questionnaire has been used as a basis for telephone interviews, among other things for registering information acquired in connection with questions about local conditions for artificial turf pitches such as maintenance, salting etc. A total of 14 municipalities, football clubs and sports associations have been asked questions and suppliers of artificial turf pitches approved by DBU and suppliers of elastic infill materials have been contacted.
The information acquired in connection with the mapping, including the literature study of international experiences, form the basis of the conclusions drawn with respect to:
- The extent of artificial turf pitches in Denmark
- Materials used
- Chemical constituents significant for the survey
- Exposure scenarios
4.2.1 Artificial turf pitches in Denmark
4.2.1.1 Number of artificial turf pitches and forecast for new pitches
According to information obtained from a seminar held at the Faculty of Life Sciences, Forest & Landscape, University of Copenhagen, on 28 March 2007, 45 full-scale third-generation artificial turf pitches are currently established in Denmark.
This corresponds with information obtained from suppliers of artificial turf pitches approved by DBU on the basis of a FIFA certificate.
This also corresponds with DBU’s website (visited on 15 August 2007) which lists 45 third-generation pitches.
However, the indoor pitch in Ikast mentioned in the introduction is not included in DBU’s list. The reason for this could be that it is not a third-generation artificial turf pitch according to DBU’s definition as the infill is polyethylene-coated (PE) sand. PE is not an elastic material in itself, but it is flexible even at minus degrees due to the extremely low glass transition temperature (÷120°C).
It has been stated that 25 new artificial turf pitches have been put out for tender this year (2007), which means that the municipal mergers in Denmark apparently have not temporarily postponed the propensity to invest in the area due to a restructuring of tasks in a transition period which had been expected in some supplier circles.
In addition, several suppliers of artificial turf pitches have stated that more pitches, approx. ten, are already being established.
4.2.1.2 Pitch maintenance experiences (salting etc.)
In relation to the maintenance of artificial turf pitches, experience is are limited as most third-generation pitches have been established in recent years (2002 and later).
All football clubs and municipalities contacted, except from one respondent, have stated that they winter salt the pitches as required. The volume of salt used has not been stated for most of the pitches.
If stated, the volume is between one to 16 tonnes per season. The figure of 16 tonnes was stated by a municipality in the Greater Copenhagen Area which has found it difficult to use snow removal machines. The figure is from the winter 2005/2006, which saw periods with a lot of snow.
Both dry salting and salt solutions have been used. One respondent states that 150 kg of dry salt is used per application. Another states 200-400 kg salt is used per application in conditions with light frost and little snow.
Both sodium chloride and potassium chloride are used in connection with the salting.
One of the respondents states that magnesium chloride is used under conditions with severe frost and a lot of snow.
None of the respondents stated that they use urea for defrosting pitches, but one artificial turf supplier recommends urea.
One municipality provided detailed information about salting.
During the 2004/2005 season, a total of 136 tonnes of road salt was used for two pitches (second-generation pitches), during the 2005–2006 season, 150 tonnes of salt was used for the pitches, while only 16 tonnes of road salt was used during the 2007 season which saw very little snow. The reduced consumption is, among other things, a result of an upgrade to third-generation pitches with rubber infill. Rubber maintains its elasticity even at frost temperatures and the salting requirement is thus expected to be heavily reduced.
In snowy conditions, salting is still expected. The municipality has based its calculation on six snowy days a year. As two tonnes of salt per application is expected, the estimated consumption will be six tonnes for three pitches, i.e. a total of 36 tonnes per season.
However, one of the large suppliers of artificial turf pitches states that several pitches are not salted, but only cleared of snow using snow removal machines.
With respect to any use of herbicides or biocides, only one of the clubs contacted states that they have used Round Up once. All other clubs contacted stated that they had not used herbicides.
Most of the pitches are still so new that they have yet to be filled with new infill material. One respondent (a high school for physical education) states that eight tonnes of a new infill is expected to be used over a period of five years.
4.2.1.3 DBU’s guides on establishing and maintaining artificial turf pitches
DBU’s website contains a guide on and a description of how to establish artificial turf football pitches (DBU, 2005) as well as a leaflet with good advice on maintaining third-generation artificial football pitches (DBU, 2004).
A distinction is made between DBU, category 1 certificate pitches and DBU, category 2 certificate pitches. The category 2 pitches are only filled with silica sand while category 1 comprises FIFA STAR** and FIFA STAR* pitches with rubber granule and silica sand fillings. DBU, category 1 also covers pitches with a UEFA certificate established before 31 December 2005.
In the DBU, category 1, the sublayer must be in the form of a laid-out rubber pad or a polyurethane-bound ET base course. In connection with the rubber pad, an additional bitumen-bound single-layer cast drainage asphalt base course can be used. For artificial turf surfaces with leaves >/= 60 mm, a sublayer can be deselected. However, for FIFA STAR** for stadiums, there is a requirement for underlying padding.
For FIFA STAR**, artificial turf straight leaves of 50-70 mm in length must be used, and FIFA STAR* requires the leaves to be >/= 40 mm. Mono or split-fibres can be used for both types.
In connection with the selection of a surfacing system, the following must be considered:
- Sports functional properties
- Material technological properties
- Warranty
- Maintenance
- Price
Alternative surfacing options are available (selection and thickness of material), but the owner will ultimately make the final decision in this matter.
If a polyurethane casting is used, the contractor must document compliance with statutory requirements in connection with the handling of the polyurethane and that the required permits have been given.
The maintenance guide offers a number of practical tips on pitch maintenance. An important element is compliance with the guide prepared by the supplier. The tips include regular maintenance in the form of cleaning, marking, deep-cleaning, surface loosening, filling up and watering. Watering can be relevant during the summer months with respect to cooling down and reduced friction.
There is also a guide on winter maintenance, including salting. Salting must be approved by both the turf supplier and the rubber granule supplier. It is stated that the use of salt solution (e.g. in a mixing ratio of 1:6) seems more effective that spreading dry salt. Presalting should only be performed if frost is expected or during a change from frost to thaw. It is recommended that sodium chloride be used for the salting, but according to the guide, potassium chloride can also be used. There is a comment that large salt volumes in the long term can impede water permeability in the gravel bed.
DBU[3] estimate on the annual consumption of materials in connection with maintenance:
- Salt in the form of sodium chloride or calcium chloride, two tonnes at DKK 2,000
- Filling up with rubber granules, three tonnes at DKK 5,000
According to DBU, there should be a minimum warranty period of five (5) years and the pitch depreciation is estimated at ten (10) years. This only applies to the pitch surface, the artificial turf and the removal of old artificial turf.
The pitch supplier must be able to document compliance with the materials quality and local environment and fire regulations.
The third-generation artificial turf pitches differ from the second-generation pitches in having leaves that are significantly longer (approx. 50-70 mm compared to 30 mm previously), in having infill which consists of a layer of silica sand at the bottom of the sward and a rubber granule layer at the top (or a mixture of the rubber granule and sand) and having approx. 20 mm of the artificial turf not being filled.
It is stated that the new pitches abroad are laid directly on a flattened gravel layer (frost-proof) without a bound base course, but some pitches are laid with a shock-absorbing pad between the gravel and the artificial turf. All second-generation pitches in Denmark have so far been laid on a bound base course.
DBU has prepared an estimate of the establishment costs for second- and third-generation artificial turf pitches. The estimated establishment costs are DKK 4.9 million and DKK 5.5-6 million, respectively.
4.2.1.4 Other comments on artificial turf pitches
A few respondents state that odour problems may occur in hot weather (rubber smell). The smell of rubber originates from a number of volatile sulphur compounds, lower organic acids (butyric acid and valeric acid) and terpenes, among others.
Generally, the municipalities and football clubs surveyed indicate a high degree of satisfaction among users as regards the playing properties of the artificial turf pitches.
However, one respondent states that professional football players prefer natural turf pitches.
The Danish football players’ association website www.spillerforeningen.dk (in Danish only) hosts an ongoing debate on artificial turf pitches. The association conducted a questionnaire survey on this topic. Players prefer playing on natural turf, but they recognise the advantage of being able to also train in winter on artificial turf pitches. There is a debate as to whether tournament matches can be played on artificial turf as well as natural turf.
The survey indicates that many people are interested in the environmental debate in connection with artificial turf.
4.2.2 Monitoring parameters
Based on the knowledge gathered (see chapter 3) about the materials used in artificial turf pitches in the form of the artificial turf mat, elastic infill and possibly an elastic sublayer in the form of a pad, the following substances, based on the available material knowledge about the chemical substances which are part of the recipes or which are present as degradation products, could be relevant to monitor:
- Heavy metals
Zinc, copper, lead, cadmium, tin, mercury, nickel and chromium
- Volatile organic compounds
Amines (dimethylamine, diethylamine, dicyclohexylamine), nitrosamines, hydrocarbons, degradation products from accelerators (carbon disulfide), styrene
- Flying dust from the elastic infill
- Semi-volatile organic compounds
Antiozonants in the form of p-phenylene diamines (primarily 6 PPD) and possibly degradation products (for SBR rubber types)
Mercaptobenzothiazole (degradation product from the accelerator CBS)
PAHs
Alkylphenols
Phthalates
- Other parameters
For instance TVOC (total volatile organic carbon), DOC (dissolved organic carbon), EOX (extractable organic halogenes), total nitrogen, smell, impact on organisms (daphnia, algae etc.)
The parameters which are relevant to monitor depend on the infill used for the pitches (rubber type, TPE, any PU coating, dyeing, natural fibres), the substances constituting the actual mat in the form of leaves, mesh, adhesive and joinings, and whether a pad is used (foamed PE, rubber granulate with PUR adhesive). For TPE and natural fibres, a number of the substances mentioned above are not relevant to monitor, as no vulcanisation is applied to these infill types. On the other hand, degradation of the natural fibre material may occur over time as a result of microbial activity.
The parameters to be monitored depend on whether the health impacts are to be assessed or whether the assessment relates to the environmental impact through seeping as a result of rain and salting. Therefore, the scenarios have been divided into two main groups.
The health scenarios cover the uptake of chemical substances through inhalation, via skin exposure or through oral intake. In case of inhalation, the volatile substances must be monitored, whereas uptake via skin and oral intake also include heavy substances or non-volatile substances.
As regards monitoring in relation to environmental impacts, it is especially relevant to measure for the leaching of heavy substances or heavy metals in the scenarios.
As many outdoor pitches are salted during periods of snow and frost, this is an important parameter to take into account in the leaching tests. In this connection, the impact of this salting on the leaching of undesirable substances into the soil and water could be of particular interest, not only in Denmark, but also in countries with climatic conditions resembling those in Denmark.
There have been discussions on the relevance of leaching tests with water saturated with carbon dioxide, which is included in some of the leaching tests listed in the German preliminary standard DIN V 18035-7, because acid pH is claimed to be unrealistic as rainwater has no buffer capacity worth mentioning. It may therefore be interesting to observe leaching from the materials constituting artificial turf pitches in the presence of calcium chloride, which is mentioned as one of the substances that can be used for salting according to DBU. Calcium chloride results in an acid reaction in solutions, contrary to sodium chloride and potassium chloride, and may thus increase the leaching of heavy metals, primarily zinc.
4.2.3 Various standards
Germany has prepared a preliminary standard DIN V 18 035-7 “Sportzplätze Teil 7: Kunststoffrasenflächen” (Sports areas – Part 7: Artificial turf pitches). Among other things, this standard established threshold values for the migration of heavy metals and dissolved organic compounds through specified analysis methods at laboratory scale in relation to limiting the possibility of soil and water pollution.
It has been known to use analysis results from tests carried out in accordance with this preliminary standard as documentation for the environmental properties of infill materials for third-generation artificial turf.
In Switzerland, there is criticism about the relevance of some of the tests of the German standard, as it is believed that it does not reflect reality in relation to established full-scale artificial turf pitches laid. One element of the critique has concerned acid leaching of heavy metals which has been thought to be unrealistic (H.J. Kolitzus, 2006). This reference also criticises the toxicity test. Switzerland (H.J. Kolitzus, 2006; E.Müller, 2007) has therefore initiated its own field measurements for leaching from artificial turf. As mentioned previously, this project was completed in 2007, but the report has not yet been published.
Drafts also exists for German standards for the evaluation of dust properties, which can be used to assess infill for artificial turf pitches. These are DIM 33897-1, “Arbeitsplatzatmosphäre – Routineverfarhren zur bestimmung des Staubungsverhaltens von Schüttgütern – Teil 1: Grundlagen and Teil 3: Verstaubung in ruhender Luft” (Workplace atmosphere – Routine procedures for the determination of dust properties in infill – Part 1: Basics and Part 3: Dust properties in still air”.
In prEN 15330-1, “Surfaces for sports areas – Synthetic turf and needle-punched surfaces primarily designed for synthetic turf”, a number of functional requirements for playing properties have been listed for artificial turf pitches, including football pitches. The standard also includes requirements for ageing resistance and water permeability as well as requirements that supplier list maintenance requirements.
Both the ageing resistance and water permeability requirements can have an impact on the leaching rate and the leaching profile. The ageing of the artificial turf can have an impact on the availability of substances which can be leached, and the water permeability can impact the contact time between the materials of the artificial turf pitch and the rainwater.
For measuring emissions from artificial turf pitches, several studies use the standard DS/EN ISO 16000 – 10 (2006): “Indendørsluft – del 10. Bestemmelse af emissionen af flygtige organiske substances fra byggematerialer og møbler - Emissionsprøvningsmetoden” (Indoor air - Part 10: Determination of the emission of volatile organic compounds from construction materials and furniture – Emission testing method). This study has used a simpler measurement of the headspace composition, corresponding to the screening method used in Norway.
4.2.4 Description of health exposure scenarios
On the basis of the chemical substances which are used for the structure of the artificial turf pitches, and the published results from the many foreign studies, the exposure scenarios have attempted to take the already existing knowledge and the particular Danish climate conditions into consideration.
Furthermore, the scenarios include an assessment of the differences existing in connection with exposure through the respiratory passages with outdoor use, as the Norwegian studies are related to indoor use of the artificial turf pitches, because these are more common due to the colder climate.
The principles of the assessments are based on the EU Technological Guidance Document (TGD) for risk assessments. The exposed users could be adults as well as children.
The health assessments in this study and fact-finding project are based on the Norwegian exposure scenarios according to the worst case principle, but related to outdoor sports. The scenarios for inhalation, skin contact and oral intake are reviewed summarily below.
It should be noted that, according to the French study, which is also based on indoor exposure as regards exposure through inhalation, no models exist for outdoor exposure within the relevant distance (the models use distances of 100 m up to 1 km radius from the area where the VOC emission takes place).
This means that it comes down to pure estimation how much less the exposure is outdoors.
According to the Danish Football Association (DBU), there is no major difference between the number of training and actual playing hours in Denmark and Norway. Therefore, as mentioned above, it is possible to use the Norwegian scenarios as a starting point. These are:
4.2.4.1 Inhalation
- Scenario 1: Adults (>= 20 years) training and playing matches indoors
Body weight = 70 kg
Inhalation volume during training/match = 6 m³/hour
Duration per week = 20 hours
Duration in months = 6 months
In addition to this should be added six hours of match play per week for six months.
This results in a weekly exposure volume of 156 m³/week for six months or 0.32 m³/kg body weight per day.
- Scenario 2: Juniors (16-19 years) training and playing matches indoors
Body weight = 65 kg
Inhalation volume during training/match = 4.8 m³/hour
Per-exposure duration = 2 hours
No. of exposure episodes per week = 7
Duration in months = 5.5 months
In addition to this should be added two two-hour matches per month for three months.
This results in a weekly exposure volume of 75 m³/week for 16 weeks or 0.16 m³/kg body weight per day.
- Scenario 3: Older children (12-15 years) training and playing matches indoors
Body weight = 50 kg
Inhalation volume during training/match = 3.6 m³/hour
Duration per week = 10 hours
Duration in months = 6 months
In addition to this should be added two hours of match play per week for six months.
This results in a weekly exposure volume of 43.2 m³/week for six months or 0.12 m3/kg body weight per day.
- Scenario 4: Children (7-12 years) training and playing matches indoors
Body weight = 30 kg
Inhalation volume during training/match = 1.8 m³/hour
Duration per week = 10 hours
Duration in months = 6 months
In addition to this should be added two hours of match play per week for six months.
This results in a weekly exposure volume of 21.6 m³/week for six months or 0.10 m3/kg body weight per day.
Health assessments were performed for the highest exposure and for the most sensitive user group (scenarios 1 and 4).
4.2.4.2 Skin contact
- Scenario 5: Adults (>= 20 years) training and playing matches indoors
Body weight = 70 kg
Skin surface exposed = 7,100 cm²
Per-exposure duration = 4 hours
No. of exposure episodes per week = 5
Duration in months = 6 months
In addition to this should be added six hours of match play per week for six months. It is assumed that the matches take place in the same period as the training.
This results in a weekly skin exposure of 42,600 mg of rubber granules per week or 87 mg of rubber granules per kg of body weight per day.
- Scenario 6: Juniors (16-19 years) training and playing matches indoors
Body weight = 65 kg
Skin surface exposed = 6,600 cm²
Per-exposure duration = 2 hours
No. of exposure episodes per week = 7
Duration in months = 5.5 months
In addition to this should be added two two-hour matches in three months. It is assumed that the matches take place in the same period as the training.
This results in a weekly skin exposure of 49,500 mg of rubber granules per week or 109 mg of rubber granules per kg of body weight per day.
- Scenario 7: Big children (12-15 years) training and playing matches indoors
Body weight = 50 kg
Skin surface exposed = 5,100 cm²
Per-exposure duration = 2.5 hours
No. of exposure episodes per week = 4
Duration in months = 5.5 months
In addition to this should be added two hours of match play per week for six months. It is assumed that the matches take place in the same period as the training.
This results in a weekly skin exposure of 25,500 mg of rubber granules per week or 73 mg of rubber granules per kg of body weight per day.
- Scenario 8: Children (7-12 years) training and playing matches indoors
Body weight = 30 kg
Skin surface exposed = 3,000 cm²
Per-exposure duration = 2.5 hours
No. of exposure episodes per week = 4
Duration in months = 6 months
In addition to this should be added two hours of match play per week for six months. It is assumed that the matches take place in the same period as the training.
This results in a weekly skin exposure of 15,000 mg of rubber granules per week or 71 mg of rubber granules per kg of body weight per day.
Health assessments were performed for the highest exposure (scenario 6). If the project results indicate a health risk, some of the other scenarios are added.
4.2.4.3 Oral intake
There is no concrete knowledge on the extent to which children playing football on an artificial turf pitch will accidentally swallow some of the granulate.
In the Norwegian study, the absolute worst case scenario was assumed to be somewhere between 23.7 mg per kg of body weight per day and 93.4 mg of rubber granules per kg of body weight per day for a duration of six months.
The scenarios on which the estimated intake are based take an oral intake of 1.0 g of rubber dust per match as their starting point. It seems unrealistically high because of the unpleasant taste of rubber, but nonetheless serves as the worst case scenario.
4.2.5 Suggestions for exposure scenarios for migration to soil/water
In this project, it was not possible to complete comprehensive tests including collection of drainage from lysimeters, neither based on a financial or a temporal evaluation. The foreign studies were typically conducted over a period of one year with monitoring of up to 42 analysis parameters.
Therefore, this project has conducted relatively simple leaching tests, corresponding to those conducted in Norway (in accordance with prEN 12457-4 at L/S=10) or in accordance with the German DIN 18035-7. If possible, a comparative test was made in accordance with guidelines for leaching of heavy metals and salts from soil and cinders in Danish Executive Order no. 1635, 2006 on recycling of residual products and soil for construction works. The executive order for testing of soil (prEN 12457-3) uses a lower liquid content (L/S=2), which may be difficult to handle when testing rubber particles (prEN 12547-3). If this is taken into consideration, the method is otherwise deemed to be suitable for rubber.
The leaching tests include leaching to demineralised water, a sodium chloride solution and a calcium chloride solution (alkaline pH). The starting point is the guideline volumes of salt which, according to DBU, are estimated to be used per year (2 tonnes), and the information gathered through interviews with football clubs, municipalities and artificial turf suppliers. The starting point is watering with a solution of salt in water at a ratio of 1:6.
The samples (primarily infill) selected for migration tests would be the samples for which the risk was expected to be biggest for high migration of zinc, phthalates and octylphenols/nonylphenols or other substances which are harmful to human health.
Based on the leaching tests, a simple assessment was made of the impact of substances in drainage water on drinking water quality by comparing the concentration of substances in the contact medium to drinking water requirements. Furthermore, the impact of any leaching of drainage water from football pitches into nearby watercourses was assessed, e.g. in case of heavy rainfall, and considerations were made on the significance of leached substances in relation to wastewater discharge.
Comparative worst case reviews or simplified model observations were applied, as the financial framework of the project did not allow for comprehensive modelling which could take the actual soil conditions in connection with water recovery at the football pitches tested into consideration.
5 Procurement of sample material
Samples of infill, artificial turf mats and any pads have been procured by contacting suppliers approved by DBU as well as by contacting manufacturers or importers of infill for artificial turf.
The following samples have been procured:
Table 5.1 List of infill materials
| Infill no. | Sample type | Supplier no. |
| 1 | Granulated car tyres, black | 1 |
| 2 | Granulated car tyres, black | 2 |
| 3 | PUR-coated granulated car tyres, dyed green | 3 |
| 4 | EPDM granules | 3 |
| 5 | SBR rubber granules | 4 |
| 6 | SBR granules, dyed brown | 4 |
| 7 | Grey industrial rubber | 4 |
| 8 | TPE | 5 |
| 9 | Coir fibre based | 5 |
| 10 | TPE in 3 colours, brown, light brown, green | 6 |
| 11 | TPE, dark brown (Terra XPS) | 7 |
| 12 | Green (unknown type) | 4 |
| 13 | Industrial rubber, dyed green | 4 |
| 14 | Industrial rubber from artificial turf pitch 1 | 4 |
| 15 | Industrial rubber, used, from artificial turf pitch 1 | 4 |
| 16 | Granulated car tyres from artificial turf pitch 2 | 4 |
Table 5.2 List of artificial turf mats
| Artificial turf no. | Sample type | Supplier no. |
| 1 | Artificial turf mats | 5 |
| 2 | Artificial turf mats | 8 |
| 3 | Artificial turf mats | 9 |
| 4 | Artificial turf mats | 9 |
| 5 | Artificial turf mats | 10 |
| 6 | Artificial turf mats | 10 |
| 7 | Artificial turf mats | 11 |
| 8 | Artificial turf mats | 4 |
Table 5.3 List of pads
| Elastic pad no. | Sample type | Supplier no. |
| 1 | Foamed reuse-PEX | 10 |
| 2 | PUR-bound granulated rubber (not tyres) | 11 |
Both used and new infills from artificial turf pitch no. 1, where the infill is grey industrial rubber, and used infill from artificial turf pitch no. 2, where the infill is granulated car tyres, have been collected.
Table 5.4 List of artificial turf pitches
| Artificial turf pitch no. | Sample type | Supplier no. |
| 1 | Infill | 4 |
| 2 | Infill | 4 |
The expectation is that an indication can be obtained, based on the used infills from the two pitches, of whether the zinc leaching is reduced, unchanged or increased, given the pitches were installed two years ago.
Among the many samples collected, it has been prioritised which products should be selected for analysis and which analysis parameters should be included for the selected samples. This has been done based on a knowledge of the chemical structure of the various materials used, auxiliaries added and known degradation products.
It is, for example, not relevant to analyse for zinc in TPE, which is not vulcanised rubber.
6 Analyses
- 6.1 Analysis methods and analysis programme
- 6.2 Results of screening analyses
- 6.3 Results of leaching tests
- 6.4 Discussion of results
- 6.5 Selection of substances for health and environmental assessment
6.1 Analysis methods and analysis programme
6.1.1 Chemical screening analyses
Chemical screening analyses have been carried out for organic substances on the majority of the products collected in the form of elastic infills, artificial turf mats and pads.
The analyses for the organic components have comprised headspace analyses for emissions of volatile substances and analysis of dichloromethane extracts (DCM) for constituents in the actual products.
For a small selection of the artificial turf mats, a supplementary screening for elements has been carried out using X-ray analysis.
6.1.1.1 Semi-quantitative GC/MS headspace screening for emission of volatile organic substances
0.5 g of sample is placed in a 60 ml glass with screw cap for one hour at 70°C, and the headspace is analysed for volatile substances. The analyses are carried out as single determinations. Blind samples were included in the form of headspace analyses of empty glasses. The equipment was flushed with nitrogen to prevent cross-sample contamination.
An HP 5890 gas chromatograph with an HP 5972 mass spectrometer was used.
6.1.1.2 Semi-quantitative analysis for content of organic component in the products
A sample quantity weighed out (0.5-1 g) was transferred to an annealed 100-ml red cap glass.
Extraction was carried out with DCM (10 ml – sample labelled 33617-1, however 20 ml) to which was added deuterium-labelled internal standards (benzene-d6, toluene-d8, p-xylene-d10, naphthalene-d8 and DEHP-d4) using ultrasound and mechanical shaking.
Double determinations were carried out.
Analysis of extracts using GC-MS in scan mode.
Standards for BTEX, n-alkanes (C10-C36) and phthalates as well as other selected analytes were co-analysed.
The listed components were identified by comparing the current mass spectres with mass spectres in NIST library (Nist02 Version 2.0).
The quantities specified are calculated against the internal standard of naphthalene-d8 (5.69 µg/ml extraction liquid).
6.1.1.3 Screening for content of elements in artificial turf mats
The screening was carried out on artificial tuft mats nos. 1, 2, 4, 6, 7 and 8. The analysis was carried out on an Eagle III EDXRF instrument. The method is non-destructive to the material, as the sample is placed in the instrument’s test chamber which is evacuated, and the sample is then irradiated using X-rays. 300 µm spot was used at a voltage of 40 kV. Single determinations were carried out.
6.1.1.4 Quantitative screening analyses for zinc
Zinc analyses were made on selected samples of elastic infill and artificial turf mats in order to determine the concentration in the materials before the leaching tests.
The analysis conditions were as follows:
0.5 g of sample – carefully weighed – were prepared with 10 ml of concentrated nitric acid (subboiling quality) in an PFA autoclave through microwave-induced heating. The destruate was diluted with demineralised water (MilliQ plus) to 50 ml and filtered.
Blind samples were produced in a similar manner.
Double determinations were carried out.
The destruates were analysed for zinc content through inductively coupled plasma-atomic emission spectrometry (ICP-AES).
Quantification was made against standards made in nitric acid.
The calibration was verified against traceable control samples.
6.1.2 Leaching tests
Laboratory-scale leaching tests were carried out on the products selected for quantitative analysis, either to test for emissions of zinc or organic substances, based on the results from the preliminary screening analyses and quantitative zinc analyses.
Three aqueous contact media were subjected to leaching:
- Pure water (MilliQ quality)
Pure water to which was added 70 g technical sodium chloride road salt in a concentration of 70 g per litre - Pure water to which was added 70 g technical calcium chloride road salt in a concentration of 70 g per litre
The sodium chloride used was labelled Pioner Stensalt, Brøste, food quality, no additives, production date 22/02/07.
The calcium chloride was labelled calcium chloride, Flakes 77% CC tech, KOCC210198, Tetra 52 854 401, made in Finland by Tetra Chemicals.
Contact with pure water was selected for the scenario where the leaching from the artificial turf pitches is caused by rain.
Contact with sodium chloride and calcium chloride was selected for the two scenarios where winter salting is carried out. Sodium chloride, which is the cheapest type of thawing salt, is usually used, but during very cold periods either calcium chloride or magnesium are used instead.
The concentration of salt in the contact water was selected on the basis of DBU’s recommended salt solution concentration of 142 g/litre for the removal of ice and snow on the pitches. It was decided to halve this concentration as melting dilutes the solution. This has been estimated to be a factor of 2.
The pH of the sodium chloride solution was measured to be 4.7 using a pH meter, and the calcium chloride had a pH of 11.5, i.e. strongly alkaline. Following leaching, the calcium chloride liquids were still strongly alkaline (approx. pH = 11 measured using sticks). The strongly alkaline pH of the calcium chloride solution is deemed to be caused by calcium hydroxide residues.
The test conditions were as follows. 80.0 g of product was weighed out and to this was added 800.0 g of the contact medium. The artificial turf samples were divided up before contact with the water. The same applied to the tested pad.
For the zinc emission tests, 1 l polyethylene (PE) containers with PE screw-on caps were used. Prior to the test, the containers were flushed with diluted nitric acid of analysis quality and post-flushed with the contact medium. PE containers with the pure contact media were used as blank samples.
Annealed 1 l glass bottles with PE screw-on caps were used for the organic substance emission tests. As blind samples, glass bottles to which were added the contact media were also used.
The leaching occurred over a 24-hour period on a Gerhard Laboshake shaker. The containers and bottles were placed in horizontal position in the shaker.
Good liquid contact with all material was observed during the shaking process at the shaking rate applied of 120 directional changes per minute.
Upon completion of the tests, the contact water was filtered through a Büchner funnel using a Whatmann filter paper no. 42.
During filtration of the calcium chloride-based contact liquids, in a number of instances a significantly lower pressure drop was observed during filtration as well as foaming, indicating the conversion of organic substance and possibly saponification reactions as a result of the high pH of the contact liquid.
The filtrates were stored in a fridge until analysis.
The filtrates to be analysed for zinc content were made 0.14 M nitrate and analysed for the element using inductively coupled plasma atomic emission spectrometry (ICP-AES).
As mentioned above, the quantification was carried out against standards made in nitric acid and verified against traceable control samples.
The filtrates to be analysed for emissions of organic substances to the contact water were extracted using the following procedure:
500 ml of filtrate (possibly a small quantity) is made alkaline (pH = 10 using sodium hydroxide). Internal standards (bromobenzene and o-tert phenyl) were added together with 20 ml of DCM. The mixture was shaken for 30 min. The DCM phase was separated off and transferred to a glass bottle with anhydrous sodium sulphate as drying agent. The extraction was repeated with 10 ml of DCM which was transferred to the bottle.
The water phase was then made acidic (pH = 2) using hydrochloric acid.
The extraction procedure was the same as for the alkaline extraction to 20 ml of DCM. After having separated off the DCM phase, it was transferred to the bottle with the alkaline extract. The extraction was repeated with 5 ml of DCM, which was combined with the previous three extracts in the bottle.
Following the addition of internal standards, the extracts were analysed using GC/MS.
6.2 Results of screening analyses
6.2.1 Results of quantitative analysis for zinc in products
Table 6.1 Quantitative zinc content in selected products
| Product | Zn (mg/kg) |
| Infill no. 1 | 16,200 |
| Infill no. 2 | 18,500 |
| Infill no. 3 | 16,800 |
| Infill no. 4 | 16 |
| Infill no. 7 | 10,000 |
| Infill no. 13 | 15 |
| Infill no. 14 | 8,500 |
| Infill no. 15 | 8,300 |
| Infill no. 16 | 21,000 (stddev = 8,500) |
| Artificial turf no. 2 | 6.5 |
| Artificial turf no. 4 | < 3 |
| Artificial turf no. 6 | 1,100 (stddev = 1,240) |
| Artificial turf no. 8 | 150 |
| Pad no. 2 | 10,100 |
6.2.2 Results of X-ray screening for elements in selected turf mats
The analysis showed calcium as the only element in the turf base of the all turf mats tested.
Iron was observed in all grass leaves. In four out of seven turf mats tested, zinc in varying quantities was detected. In two of the samples, titanium was also detected. The surface of the sample (turf no. 6) with the highest zinc content was washed using 96% ethyl alcohol without causing changes in the zinc concentration. One of the samples also showed calcium. It is known that a supplier of UV stabilisers for plastics has a product containing calcium and zinc. This may explain why no leaching of zinc occurs with alcohol as would be expected if there was zinc stearate on the surface of the turf. Salts of stearic acid (Ca, Zn) act as release agents for plastic materials.
6.2.3 Headspace analysis
Headspace analyses were performed on infill nos. 1-5, nos. 7-9 as well as no. 11 and on artificial turf nos. 1, 2, 4 and nos. 6-8, as well as on both pads. The background for this prioritisation is to screen a representative selection of infill materials, artificial turf mats and pads within the limited framework of the project.
Table 6.2 Results of headspace analysis of selected infills and artificial turf products (µg/g)
| Component | CAS-no. | Pad no. 2 | Infill no. 1 | Infill no. 2 | Infill no. 3 | Infill no. 4 | Infill no. 7 | Infill no. 8 | Infill no. 9 | Artificial turf no. 2 |
| 2-Pyrrolidinone. 1-methyl- | 872-50-4 | 3.5 | ||||||||
| Benzene. 1.3-bis(1-methylethenyl)- | 3748-13-8 | 0.8 | ||||||||
| Benzene. 1.4-bis(1-methylethenyl)- | 1605-18-1 | traces | ||||||||
| Benzenemethanol. à.à-dimethyl- cumylalcohol | 617-94-7 | 0.7 | ||||||||
| Benzothiazole | 95-16-9 | 0.1 | ||||||||
| Butoxyethoxyethanol | 112-34-5 | 0.5 | 0.5 | |||||||
| Butylated hydroxytoluene (BHT) | 128-37-0 | 1.4 | ||||||||
| Cyclohexanamine | 108-91-8 | 2.7 | ||||||||
| Cyclohexanone | 108-94-1 | 0.9 | 0.7 | |||||||
| Ethanone. 1-[4-(1-hydroxy-1-methylethyl)phenyl]- * | 54549-72-3 | 1.3 | ||||||||
| Ethanone. 1-[4-(1-methylethenyl)phenyl]- (isopropenylacetophenone) | 5359-04-6 | 1.8 | ||||||||
| Heptanonitrile | 629-08-3 | 4.7 | ||||||||
| MIBK/2 hexanone | 108-10-1/ 591-78-6 |
0.5 | 5 | 12 | 3.4 | |||||
| N-cyclohexyl-formamide | 766-93-8 | |||||||||
| Nonanale | 124-19-6 | 0.3 | ||||||||
| Tertbutylacetophenone | 943-27-1 | 1.1 |
Results are only shown for products where substances above the detection limit were identified.
Table 6.3 shows substances identified in headspace analysis with potentially health or environmentally harmful effects based on the reference list of hazardous substances, the guiding list for self-classification or other data.
Table 6.3 Substances found with potential health and environmental effects
| Component | CAS-no. | Classification |
| 2-Pyrrolidinone, 1-methyl- | 872-50-4 | XI;R36/38 |
| Benzene, 1,3-bis(1-methylethenyl)- | 3748-13-8 | * R43 N;R50/53 |
| Benzene, 1,4-bis(1-methylethenyl)- | 1605-18-1 | * N;R50/53 |
| Benzothiazole | 95-16-9 | * Xn;R22 R43 |
| Butoxyethoxyethanol | 112-34-5 | XI;R36 |
| Butylated hydroxytoluene (BHT) | 128-37-0 | * Xn;R22 N;R50/53 |
| Cyclohexanamine | 108-91-8 | R10 XN;R21/22 C;R34 |
| Cyclohexanone | 108-94-1 | R10 XN;R20 |
| MIBK/2 hexanone | 108-10-1/591-78-6 | F;R11 XN;R20 XI;R36/37 R66 |
| Nonanale | 124-19-6 | * N;R50 |
| Tertbutylacetophenone | 943-27-1 | * R52/53 |
* Danish Environmental Protection Agency, 2001
6.2.4 Results of analysis for content of organic substances by extraction in dichloromethane
Table 6.4 Results of extraction in DCM of pads and infills (µg/g)
Table 6.5 Results of extraction in DCM of artificial turf (µg/g)
| Component | CAS-no. | Artificial turf no. 1 |
Artificial turf no. 2 |
Artificial turf no. 4 |
Artificial turf no. 6 |
Artificial turf no. 7 |
Artificial turf no. 8 |
| 4,4'-((p-Phenylene) diisopropylidene)di-phenol |
2167-51-3 | 51 | |||||
| Erucylamide | 112-84-5 | 88 | 146 | 177 | |||
| Bis(2,2,6,6-tetramethyl-4-piperidinyl) sebacate | 52829-07-9 | 173 | |||||
| Butoxyethoxy- ethylacetate |
124-17-4 | 22 | |||||
| Cyclohexanamine | 108-91-8 | ||||||
| DEHP | 117-81-7 | 80 | 104 | 43 | |||
| Nonylphenol | 25154-52-3 | 16 | |||||
| Octylphenols/ nonylphenols |
56 | 57 |
Table 6.6 shows substances found by extraction in DCM having potentially health or environmentally harmful effects based on the reference list of hazardous substances, the guiding list for self-classification or other data.
Table 6.6 Substances found with potential health and environmental effects
| Component | CAS no. | Classification |
| 1,4-Benzendiamine, N-(1,3-dimethylbutyl)-N'-phenyl- (6PPD) | 793-24-8 | * R43 N;R50/53 |
| 1-Dodecanamine, N,N-dimethyl- | 112-18-5 | * R43 N;R50/53 |
| 1-Tetradecanamine, N,N-dimethyl- | 112-75-4 | * Xn;R22 R43 N;R51/53 |
| 2-(5-Chloro-2-benzotriazolyl)-6-tert-butyl-p-cresol | 3896-11-5 | * Xn;R22 |
| 2-Pyrrolidinone, 1-methyl- | 872-50-4 | XI;R36/38 |
| Erucylamide | 112-84-5 | |
| Aniline | 62-53-3 | T;R23/24/25-48/23/24/25 CARC3;R40 XI;R41 R43 MUT3;R68 N;R50 |
| Benzophenone, 2-hydroxy-4-(octyloxy)- (Octabenzone) | 1843-05-6 | A fraction in hsdb benzophenone: 119-61-9 (hormone effects) |
| Benzothiazole | 95-16-9 | * Xn;R22 R43 |
| Bis(2,2,6,6-tetramethyl-4-piperidinyl) sebacate | 52829-07-9 | * N;R51/53 |
| Butoxyethoxyethylacetate | 124-17-4 | * R52/53 |
| Butylated hydroxytoluene (BHT) | 128-37-0 | * Xn;R22 N;R50/53 |
| Cyclohexanamine | 108-91-8 | R10 XN;R21/22 C;R34 |
| DEHP | 117-81-7 | REP2;R60-61 |
| Dibutylphthalate | 84-74-2 | * N;R51/53 |
| Diisobutylphthalate | 84-69-5 | * N;R50/53 |
| D-limonene | 5989-27-5 | R10 XI;R38 R43 N;R50/53 |
| Nonylphenol | 25154-52-3 | XN;R22 C;R34 REP3; R62-63 N;R50/53 |
| N-phenyl-1-naphtaleneamine | 90-30-2 | * Xn;R22 R43 |
| Octylphenols/nonylphenols | See nonylphenol octylphenol:27193-28-8: *Xn;R22 R43 N;R50/53 | |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 96-76-4 | * N;R51/53 |
| Phenol, 2-(5-chloro-2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylethyl)- or similar | 3864-99-1 | * N;R50/53 |
| Hexylbenzene | 1077-16-3 | * N;R50/53 |
* Danish Environmental Protection Agency, 2001
Based on the results of extraction in DCM, a number of representative products were selected for subsequent tests of leaching into aqueous contact liquids. The products for the leaching tests were selected so that substances having health and environmentally harmful properties and high concentration were weighted most. In addition, it has also been taken into account whether the substances occur in many or few products.
6.3 Results of leaching tests
Table 6.7 Leaching of zinc
| Sample marked | Zn mg/l |
| Infill no. 1 MilliQ 1 | 0.59 |
| Infill no. 1 NaCl | 7.4 ² |
| Infill no. 1 CaCl2 | 0.36 |
| Infill no. 3 MilliQ | 1.4 |
| Infill no. 3 NaCl | 0.38 |
| Infill no. 3 CaCl2 | < 0.05 |
| Infill no. 7 MilliQ | < 0.05 |
| Infill no. 14 MilliQ | 0.06 |
| Infill no. 14 NaCl | 0.11 |
| Infill no. 14 CaCl2 | 0.94 |
| Infill no. 15 MilliQ | 2.3 |
| Infill no. 15 NaCl | 11 ² |
| Infill no. 15 CaCl2 | 1.5 |
| Infill no. 16 MilliQ | 0.80 |
| Blank NaCl | < 0.05 |
| Blank CaCl2 | < 0.05 |
| Pad 2 MilliQ | 0.27 |
| Pad 2 NaCl | 0.41 |
1 MilliQ is extremely pure ion-exchanged, membrane-filtered water
2 The values are considered to be contaminated analyses as results in MilliQ and CaCl2 in the same series give much lower results. These high analysis values are not included in the subsequent environmental assessment.
Table 6.8 Leaching of organic substances from elastic infills (µg/l)
| Component | CAS-no. | Infill no. 1 | Infill no. 1 | Infill no. 2 | Infill no. 3 | Infill no. 3 | Infill no. 4 |
| MilliQ | CaCl2 | MilliQ | MilliQ | CaCl2 | MilliQ | ||
| 5-Methyl-2-hexanone | 110-12-3 | 7 | 6 | 6 | |||
| Cyclohexanone | 108-94-1 | 80 | 95 | 107 | 112 | 123 | |
| Ethanol, 2-butoxy- | 111-76-2 | 13 | 19 | ||||
| Benzaldehyde | 100-52-7 | ||||||
| Benzene, isocyanato- | 103-71-9 | 17 | 9 | 8 | |||
| Cyclohexane, isocyanato- | 3173-53-3 | 27 | 46 | 31 | 37 | 31 | |
| 2-Pyrrolidinone, 1-methyl- | 872-50-4 | 613 | 847 | ||||
| Benzaldehyde, 2-hydroxy- | 90-02-8 | 6 | |||||
| Acetophenone | 98-86-2 | 6 | 5 | 6 | 6 | 108 | |
| Hexanoic acid, 2-ethyl- | 149-57-5 | 38 | 64 | 31 | 35 | 23 | |
| Ethanol, 1-(2-butoxyethoxy)- | 54446-78-5 | 7 | 171 | 334 | |||
| Cyclohexane, isothiocyanato- | 1122-82-3 | 10 | 17 | 16 | |||
| N-cyclohexyl-formamide | 766-93-8 | 161 | 214 | 139 | 172 | 233 | |
| Acetamide, N-cyclohexyl- | 1124-53-4 | 9 | 21 | 56 | |||
| Phenol, m-tert-butyl- | 585-34-2 | 365 | 207 | ||||
| Cyclohexanamine, N-cyclohexyl- | 101-83-7 | 1167 | 12 | 573 | 255 | ||
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 96-76-4 | ||||||
| Cyclohexanamine, N-cyclohexyl-N-methyl- | 7560-83-0 | 126 | 138 | 76 | |||
| Cycloheptasiloxane, tetradecamethyl- | 107-50-6 | ||||||
| Diethylphthalate | 84-66-2 | 61 | 32 | 73 | |||
| Benzothiazole, 2-(methylthio)- | 615-22-5 | 6 | 29 | 11 | 13 | 24 | |
| Dodecanoic acid | 143-07-7 | 14 | 28 | 19 | |||
| Benzothiazolone | 934-34-9 | 239 | 754 | 277 | 231 | 802 | |
| 3,5-Di-tert-Butyl-4-hydroxybenzaldehyde | 1620-98-0 | 132 | 86 | ||||
| Diisobutylphthalate | 84-69-5 | 83 | 21 | 98 | 94 | 93 | |
| Dibutylphthalate | 84-74-2 | 72 | 170 | 69 | 178 | ||
| 1,3-Dicyclohexylurea | 2387-23-7 | 30 | 19 | 38 | 28 | 18 | |
| 1,4-Benzendiamin, N-(1-methylethyl)-N’-phenyl-, (IPPD) | 101-72-4 | 73 | |||||
| Benzylbutylphthalate | 85-68-7 | ||||||
| DEHP | 117-81-7 | 14 | |||||
| Hexa(methoxymethyl)melamine | 68002-20-0 | 1380 | 1320 | 1200 | 1860 | 1760 | |
| Aniline | 62-53-3 | 16 | |||||
| Benzothiazole | 95-16-9 | 528 | 385 | 293 | 578 | 437 | |
| Cyclohexanamine | 108-91-8 | ||||||
| Ethanone, 1,1'-(1,3-phenylene)bis- | 6781-42-6 | 23747 | |||||
| Ethanone, 1,1'-(1,4-phenylene)bis- | 1009-61-6 | 6965 | |||||
| Ethanone, 1-[4-(1-methylethenyl)phenyl]- | 5359-04-6 | 13378 | |||||
| Ethanone, 1-[4-(1-methylethenyl)phenyl]- | 5359-04-6 | 4949 | |||||
| Drometrizol | 2440-22-4 | ||||||
| 2-(1-phenylethyl)-phenol | 4237-44-9 | 11 | |||||
| Degradations products of 1,4-Benzenediamine, N-(1,3-dimethylbutyl)-N'-phenyl- (6PPD) | 793-24-8 | 687 | 391 | 641 | 324 | 266 |
Table 6.8 Leaching of organic substances from elastic infills (µg/l), contd.
| Component | CAS-no. | Infill no. 10 | Infill no. 11 | Infill no. 11 | Infill no. 14 | Infill no. 14 |
| MilliQ | MilliQ | CaCl2 | MilliQ | CaCl2 | ||
| 5-ethyl-2-hexanone | 110-12-3 | |||||
| Cyclohexanone | 108-94-1 | 219 | 99 | |||
| Ethanol, 2-butoxy- | 111-76-2 | |||||
| Benzaldehyde | 100-52-7 | 2 | 2 | |||
| Benzene, isocyanato- | 103-71-9 | 8 | ||||
| Cyclohexane, isocyanato- | 3173-53-3 | 22 | 26 | |||
| 2-Pyrrolidinone, 1-methyl- | 872-50-4 | |||||
| Benzaldehyde, 2-hydroxy- | 90-02-8 | |||||
| Acetophenone | 98-86-2 | 3 | 22 | 11 | ||
| Hexanoic acid, 2-ethyl- | 149-57-5 | |||||
| Ethanol, 1-(2-butoxyethoxy)- | 54446-78-5 | 26 | 42 | |||
| Cyclohexane, isothiocyanato- | 1122-82-3 | |||||
| N-cyclohexyl-formamide | 766-93-8 | 561 | 321 | |||
| Acetamide, N-cyclohexyl- | 1124-53-4 | 62 | 35 | |||
| Phenol, m-tert-butyl- | 585-34-2 | 14 | 16 | |||
| Cyclohexanamine, N-cyclohexyl- | 101-83-7 | 99 | 12 | |||
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 96-76-4 | 250 | 78 | 54 | 56 | 20 |
| Cyclohexanamine, N-cyclohexyl-N-methyl- | 7560-83-0 | |||||
| Cycloheptasiloxane, tetradecamethyl- | 107-50-6 | 56 | ||||
| Diethylphthalate | 84-66-2 | 71 | 100 | 146 | ||
| Benzothiazole, 2-(methylthio)- | 615-22-5 | 23 | ||||
| Dodecanoic acid | 143-07-7 | 306 | 28 | 56 | 58 | |
| Benzothiazolone | 934-34-9 | 118 | 449 | |||
| 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | 1620-98-0 | 6 | 10 | |||
| Diisobutylphthalate | 84-69-5 | 30 | 76 | 81 | 24 | |
| Dibutylphthalate | 84-74-2 | 61 | 65 | 158 | 7 | |
| 1,3-Dicyclohexylurea | 2387-23-7 | 73 | 68 | |||
| 1,4-Benzendiamine, N-(1-methylethyl)-N’-phenyl-, (IPPD) | 101-72-4 | |||||
| Benzylbutylphthalate | 85-68-7 | 43 | ||||
| DEHP | 117-81-7 | 114 | 83 | |||
| Hexa(methoxymethyl)melamine | 68002-20-0 | |||||
| Aniline | 62-53-3 | 6 | ||||
| Benzothiazole | 95-16-9 | 18 | 245 | 574 | ||
| Cyclohexanamine | 108-91-8 | 1610 | 533 | |||
| Ethanone, 1,1'-(1,3-phenylen)bis- | 6781-42-6 | |||||
| Ethanone, 1,1'-(1,4-phenylen)bis- | 1009-61-6 | |||||
| Ethanone, 1-[4-(1-methylethenyl)phenyl]- | 5359-04-6 | |||||
| Ethanone, 1-[4-(1-methylethenyl)phenyl]- | 5359-04-6 | |||||
| Octylphenols/Nonylphenols | 0 | |||||
| Drometrizol | 2440-22-4 | 955 | 5794 | 494 | ||
| 2-(1-Phenylethyl)-phenol | 4237-44-9 | |||||
| 2-Ethyl-1-hexanol | 104-76-7 | 3 |
Table 6.9 Leaching of organic substances from artificial turf (µg/l)
| CAS-nr. | Artificial turf no. 2 |
Artificial turf no. 4 |
Artificial turf no. 4 |
Artificial turf no. 7 |
Blank | Blank | |
| MilliQ | MilliQ | CaCl2 | MilliQ | MilliQ | CaCl2 | ||
| Diethylphthalate (DEP) | 84-66-2 | 359 | 302 | < LOD | 335 | < LOD | < 0,2 |
| Diisobutylphthalate(DIBP) | 84-69-5 | 118 | 112 | 10 | 144 | 5 | 8 |
| Dibutylphthalate (DBP) | 84-74-2 | 137 | 155 | 5 | 183 | 3 | 2 |
| Benzylbutylphthalate (BBP) | 85-68-7 | < LOD | 42 | < LOD | 47 | < LOD | < LOD |
| Bis(2-ethylhexyl)phthalate(DEHP) | 117-81-7 | 14 | 183 | 5 | 87 | 2 | 2 |
| Dicyclohexylphthalate (DCHP) | 84-61-7 | < LOD | 82 | 6 | 88 | < LOD | < LOD |
| Nonylphenol | 84852-15-3 | 143 | 384 | 175 | 150 | < LOD | < LOD |
| Bis-(2,2,6,6-tetramethyl-4-piperidinyl)sebacate | 52829-07-9 | 137 | 5 | 183 | 353000 1 | < LOD | < LOD |
| Erucylamide | 112-84-5 | 644 | 1771 | 4 | < LOD | < LOD | < LOD |
1 The concentration of the substance was extremely high in this sample, and the figure is based on a series of dilutions. The analysis result correlates with a very high content of the substance found in the DCM extraction.
6.3.1 Microscopy
As the particle size of rubber dust is significant regarding any impact on a potential inhalation health risk, microscopy was performed of filtered-off fine dust particles from two of the leaching tests for zinc analysis with infill 14 and infill 15. In these leaching tests, high filter resistance was observed, indicating the presence of fine particulate material.
The sample from infill 14 contained black and white/transparent particles (10-50 µm) – the majority being transparent.
The sample from infill 15 almost only contained black particles, and there were no white particles on the one rubber fragment in the dish.
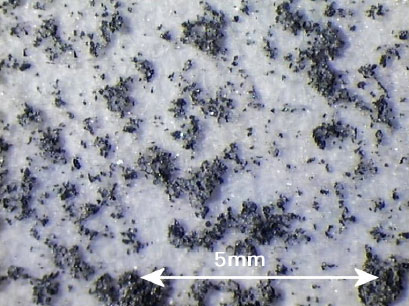
Figure 6.1 Picture of filtered-off dust particles on filter paper from leaching test with infill 14, large-scale
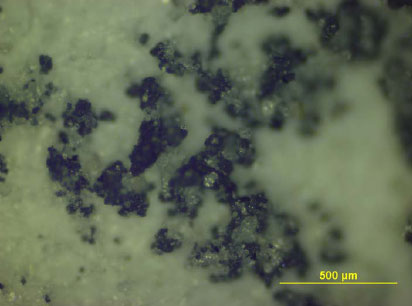
Figure 6.2 Picture of filtered-off particles on filter paper from leaching test with infill 14, enlarged
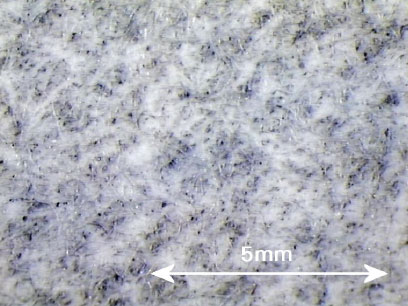
Figure 6.3 Picture of filtered-off particles on filter paper from leaching test with infill 15, large-scale
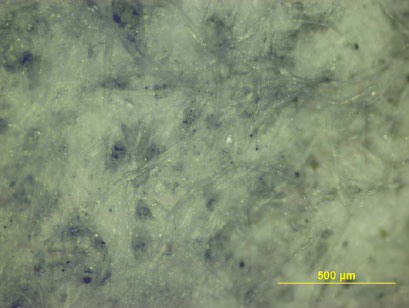
Figure 6.4 Picture of filtered-off particles on filter paper from leaching test with infill 15, enlarged
It is assessed that the fine dust is the probable cause of the increased filter resistance in the pores during filtration.
There is no simple pattern for filter clogging from the contact tests. The filtration problems were thus observed both for SBR rubber infill and for EPDM rubber infill. The filtration problems were observed on both new and used infills. This indicates that the fine particulate substance with a clogging effect is also present in the unused infill material from the start and not as a result of wear. The most probable explanation for the fine dust is that it is fine particulate rubber in the actual infill material, which is also supported by the microscopy pictures.
As mentioned above, the contact tests using a calcium chloride solution were generally found to provide the best filtration.
6.4 Discussion of results
Significantly higher amounts of leached phthalates were found from elastic infills of SBR-based infills in this study than in the results from the Norwegian study (T. S.W. Plesser, 2004). The reason for this may, e.g., be a higher content of the substances, that the substances are found in higher amounts on the surface of particles, that the substances are bound to finer particles with a shorter diffusion path, or that a more efficient leaching process was used with better liquid-solid contact in the present studies.
Table 6.10 shows, for some substances, how much the leached amount corresponds to in relation to the content in elastic infills and artificial turf
Table 6.10 Amount of leached substance in relation to content as determined by DCM extraction
| Substance | Elastic infill % leached, 24 hours |
Artificial turf % leached, 24 hours |
| Dibutylphthalate (DBP) | 3.6 | |
| Diisobutylphthalate (DIBP) | 0.6 | |
| Diethylhexylphthalate (DEHP) | 1.9 | 1.8 |
| Nonylphenol | 6.7 |
As can be seen, a limited amount of phthalate and nonylphenol leached out after a 24-hour extraction period compared to the total sample content.
Bis-(2,2,6,6-tetramethyl-4-piperidinyl)sebacate was found in very high concentrations in leaching tests of artificial turf no. 7. The amount indicates that the substance is present in a weight quantity of 0.35 percent by weight, where the measurement result of the DCM extraction was 0.0173 percent by weight. In this context, it should be noted that the substance is very water-soluble with log Kow = 0.35, for which reason only a small quantity is expected to be extracted to DCM. The high concentration observed in the leaching test is thus not unrealistically high if it is assumed that most of the substance added has leached out. The substance is a so-called HALS (hindered amine stabiliser), which is a group of chemical substances that stabilise plastics against photochemically induced degradation. The water solubility of the substance makes it an inappropriate choice.
Table 6.12 shows results of leaching of infills from the present Danish study as well as the Norwegian study (T.S.W. Plesser, 2004).
Table 6.11 Leached substances in Norwegian and Danish infill studies
| Substance | Liquid from leaching test. Elastic infill Norway µg/l |
Liquid from leaching test. Elastic infill Denmark µg/l |
| Zn | 3300 | 600-2300 |
| DEHP | 5.5 | 14-114 |
| Diethylphthalate (DEP) | 6.6-8.3 | 32-146 |
| Dibutylphthalate (DBP) | 2.1-3.3 | 61-178 |
| Sum of phthalates (not DEHP) | 6-16 | 162-428 |
| Sum of octylphenol and nonylphenol | 4.5 | - |
Table 6.12 shows the content of phthalates and nonylphenol observed in infills and artificial turf in the Norwegian and Danish studies.
Table 6.12 Content of some substances in infills, Danish and Norwegian studies
| Substance | Elastic infill, Norway µg/g | Elastic infill, Denmark µg/g | Elastic artificial turf, Norway µg/g | Elastic artificial turf, Denmark µg/g |
| Diethylphthalate (DEP) | 1.5 | ? | ? | |
| Dibutylphthalate (DBP) | 1.6-3.9 | 50 | 1 | ? |
| Diisobutylphthalate (DIBP) | Not detected | 77-175 | ||
| Diethylhexylphthalate (DEHP) | 3.9-29 | 52-62 | 1.7-8 | 43-104 |
| Diisononylphthalate (DINP) | 57-78 | Not found | 5.5 | |
| Nonylphenol | 21 | 0.2 | 16-57 |
As shown in the table, there is a significantly higher concentration of DBP, DIBP and DEHP in infills in the Danish study compared to the Norwegian study. The Norwegian study has, on the other hand, found DINP in concentrations comparable to the phthalate (DBP, DIBP, DEHP) concentrations in the Danish study. This may go some way to explaining the higher leaching of phthalates observed from infills in the Danish study, as shown in Table 6.11. The content of both phthalates and nonylphenols in artificial turf is far higher in the Danish study than in the Norwegian, resulting in a high leaching level.
6.5 Selection of substances for health and environmental assessment
Foreign studies of the emissions of volatile chemical substances unanimously conclude that the health effects from inhalation are insignificant. Headspace analyses of evaporation from elastic infills, artificial turf mats and pads in the present study have confirmed that the emission of volatile substances is insignificant. It has thus been decided to exclusively focus on oral intake and dermal uptake in the health assessment.
Based on the concentrations of chemical substances found in leaching tests as well as the hazardous properties of the substances, it has been decided to assess the effects of:
- Benzothiazole
- Dicyclohexylamine
- Cyclohexanamine
- Dibutyl phthalate
In the leaching tests, a number of potentially environmentally harmful substances were also found, and it has been decided to assess a representative selection of these:
- Zinc and its salts
- 6PPD
- Dicyclohexylamine
- Diisobutyl phthalate
- Nonylphenol
- 2,4-Di-tert-butylphenol
The substances selected are representative for the substance groups which were expected to be relevant to assess before carrying out the analyses (see Section 4.2.2).
7 Health assessment
7.1 Introduction
This chapter assesses the potential health effects of the identified substances. The assessment is based on worst case scenarios for football players using artificial turf pitches.
For each of the identified and quantified substances, there is information available on the identity and on the chemical and physical properties of the substances. The information concerns state, melting point, boiling point, specific gravity, vapour pressure and solubility.
A review was carried out of the literature via international databases and supplementary searches in scientific literature. As foreign studies have shown that effects caused by inhalation of vapour are insignificant, the focus of the present study was on effects caused by dermal uptake or oral intake. Oral intake may occur by inhalation of, e.g., rubber particles. The most important test results and effects in the literature found are presented. The overall objective has been to find data for NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) on the selected substances or other relevant data available which have been able to contribute further to the assessment.
Based on NOAEL or similar data as well as the amount of substance to which the person is exposed, a margin of safety (MOS) is calculated. Once the margin has been established, it is possible to assess whether the substance has a potentially adverse effect on health when using football pitches installed with the artificial turf mats, infills and pads tested in the study.
7.2 Method
7.2.1 Routes of exposure
The preliminary health screenings identified hazardous substances in both rubber granules and artificial turf material.
The assessment exclusively focuses on the uptake of substances from rubber particles.
The model assumes that the substances can be absorbed (taken up) in the body by oral intake of stirred-up rubber particles or similar inhalation of larger particles which do not reach the lower respiratory passages as well as via the migration of substances from collected rubber particles to the skin via secreted perspiration. It was thus decided not to investigate the inhalation of gaseous substances as the foreign studies have shown that this does not pose a health problem.
7.2.2 Exposure scenarios
There is no information in (TGD 2003) on the amount of rubber particles which are transferred.
Instead, exposure scenarios from foreign studies were used, as described in Section 4.2.4.
It was decided to use worst case scenarios, which means that the maximum exposure levels for rubber particles were used for the calculation of substance exposure.
Skin contact
- Scenario 6: Junior players (16-19 years)
Body weight = 65 kg
Skin surface exposed = 6,600 cm²
Per-exposure duration = 2 hours
No. of exposure episodes per week = 7
Duration in months = 5.5 months
To the above calculation of playing time should be added two two-hour matches every three months. It is assumed that they take place in the same period as the training.
It results in a weekly skin exposure of 49,500 mg of rubber granules or 109 mg of rubber granules per kg of body weight/day (109 mg/kg/day= 49,500 mg per week/7 days/65 kg).
Oral intake
In the Norwegian study (Tore Sander, 2006), the absolute worst case scenario is assumed to be somewhere between 23.7 mg per kg of body weight/day and 93.4 mg of rubber granules per kg of body weight/day for a duration of six months.
The scenarios on which the estimated intake are based take an oral intake of 1.0 g of rubber dust per match as their starting point. It seems unrealistically high because of the unpleasant taste of rubber, but nonetheless serves as a basis for the scenarios.
As worst case scenario, it was decided to assess for 93.4 mg per kg of body weight/day.
Calculation
The exposure scenarios are defined in accordance with EU’s Technical Guidance Document (TGD, 2003).
The skin uptake/oral intake of a substance is calculated as:
I = Q * M * F/BW
Where:
I Intake/uptake per day per kg of body weight
Q Concentration of substance (mg substance/g of sample)
M Amount of substance (g per day) taken in/up
F Fraction of substance absorbed
BW Body weight (kg)
Thus, I = Q * F * 109 mg/kg for skin with the exposure level applied for rubber particles.
Analysis data is only available for leaching of substance into water (µg of leached substance/l of water) over a 24-hour period. These data are used to calculate the substance concentration, Q, as 100 g of rubber granules are used per litre of water. It is assumed that the leached amount of substance per g of granules into water can similarly leach into perspiration and from there through skin.
Q (mg of substance/g) = concentration in leaching tests (mg/l)/100 g.
If there is no data available for dermal uptake, 100% uptake is assumed (F = 1) if the substance log KOW < 4, and 10% uptake (F = 0.1), if log KOW < -1 and log KOW > 4.
If there is no data available for oral intake, 100% uptake is assumed, i.e. F = 1.
Risk assessment
In the assessment of health risks, the calculated exposure, i.e. the uptake, must be compared with NOAEL or similar values. As NOAEL is typically based on animal tests, a margin of safety (MOS) is calculated by dividing NOAEL in mg per kg of body weight with the exposure/uptake level.
If the animal data are based on a chronic long-term high-quality study, the safety factor in the risk assessment is typically 10. The safety factors used to derive a NOAEL for humans are often based on animal tests with, e.g., mice or rats. A factor of 10 is, e.g., used for extrapolation between species (different species) and a factor of 10 to protect sensitive individuals of the species, such as children. If the data are based on LOAEL or a subchronic study, an additional safety factor is added (typically 10). The total safety factor is the total product of the individual safety factors.
In assessing health effects, MOS is not used for sensitising effects, as the effects do not have a lower concentration limit.
7.3 Selected substances
The substances described below have been singled out as being the most significant in terms of health risk when using the products. The substances in question are as follows:
- Benzothiazole
- Dicyclohexylamine
- Cyclohexanamine
- Dibutyl phthalate
The health assessment of the substances is as follows:
7.3.1 Benzothiazole
7.3.1.1 Identity
| Name | Benzothiazole |
| CAS-no. | 95-16-9 |
| EINECS-no. | 202-396-2 |
| Molecular formula | CtH5NS |
Molecular structure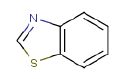 |
|
| Molecular weight (g/mol) | 135.19 |
| Synonyms | |
| Description | Yellow liquid |
| Boiling temperature | 227 ºC (Budavari, 1996) |
| Melting point | 2 ºC (Weast, 1976) |
| Solubility | 3000 mg/l, 25 ºC, water (Iuclid dataset benzothiazole) The substance is slightly soluble in water, alcohol and carbon disulphide (Lide, 1996) |
| Distribution coefficient Log Kow | 2 (Iuclid dataset benzothiazole) |
| Vapour pressure | 0.13 hPa at 20 ºC (Iuclid dataset benzothiazole) |
| Smell | Unpleasant smell (Lewis, 1993) |
7.3.1.2 Amounts found
The substance is found in leaching tests of infill nos. 1, 2, 3 and 14 in concentrations from 245-578 µg/l. There is also a small amount in no. 10 (10 µg/l). The substance is also found by leaching into calcium chloride comparable concentration levels.
7.3.1.3 Function of the substance
The substance originates presumably from degradation products of added mercaptobenzothiazole-based accelerators.
7.3.1.4 Classification and threshold values
The substance is not included in the list of hazardous substances (Danish Ministry of the Environment, 2005), but is included in the guiding list for self-classification (Danish Environmental Protection Agency, 2001) with the specification:
Xn;R22 Harmful if swallowed
R43 May cause sensitisation by inhalation
There is no Danish occupational hygiene threshold value for the substance.
7.3.1.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 177-479 mg/kg (IUCLID data set benzothiazole, 2003)
- LD50 rat, dermal 500 mg/kg (IUCLID data set benzothiazole, 2003)
- LD50 rabbit, dermal 126-400 mg/kg (IUCLID data set benzothiazole, 2003)
Subchronic toxicity
Some benzothiazoles of the type mercaptobenzothiazoles are sensitising, but no data was found from animal tests for benzothiazole. Data were, however, found which show an allergenic effect on a 10-year-old girl (Contact dermatitis 2007, v57, p 56).
The substance has not exhibited genetic toxicity in Ames test or in other bacterial mutation tests (IUCLID data set benzothiazole, 2003).
Chronic toxicity
No relevant data were found (IUCLID data set benzothiazole, 2003).
7.3.1.6 Exposure scenarios
The maximum benzothiazole content in liquid from leaching is 578 µg of benzothiazole per litre of liquid containing 100 g of rubber granules. Of this is calculated an expected maximum leached amount of substance of 5.78 µg of substance/g of rubber particles per day.
With a log KOW = 2, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g/kg * 1 * 5.78 µg/g = 0.63 µg/kg of body weight/day.
Oral intake is at the same level in worst case scenario:
Intake, oral = 0.093 g /kg * 1 * 5.78 µg/g = 0.54 µg/kg of body weight/day.
7.3.1.7 Assessment
Health data for assessment
The substance may be allergenic to some individuals, but there are no data for the frequency.
There are no data for long-term effects in animals, for which reason the lowest acute toxicity is used for the assessment (LD50 rabbit, dermal 126 mg/kg/day).
Health risk
Based on LD50 rabbit, dermal 126 mg/kg/day a safety margin of MOS = 126/0.00063 = 200,000 is obtained. MOS for oral intake is at the same level. As the health effect is acute, an uncertainty factor of at least 10,000 should be used in risk assessment. MOS is approx. 20 times above the uncertainty factor, and it is thus assessed that there is no risk of toxic effects. The data basis is, however, very limited.
It is assessed that there may be an allergy risk for sensitive individuals.
7.3.2 Cyclohexanamine
7.3.2.1 Identity
| Name | Cyclohexanamine |
| CAS-no. | 108-91-8 |
| EINECS-no. | 203-629-2 |
| Molecular formula | C6H13N |
Molecular structure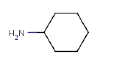 |
|
| Molecular weight (g/mol) | 99.18 |
| Synonyms | Cyclohexylamine |
| Description | Colourless or yellow liquid |
| Boiling temperature | 134.5 ºC (Budovari,1996) |
| Melting point | -17.7 ºC (Budovari,1996) |
| Solubility | The substance is mixable with water 20 ºC (Iuclid dataset cyclohexylamine). Is highly soluble in ethanol and mixable in ether, acetone (Lide, 1996) |
| Distribution coefficient Log Kow | 1.49 (Hansch, 1995) |
| Vapour pressure | 14 hPa at 20 ºC (Iuclid dataset cyclohexylamine) |
| Smell | Strong fishy amine smell (Budovari, 1996) |
7.3.2.2 Amounts found
The substance is found in leaching tests of infill no. 14 in a concentration of 1610 µg/l. In calcium chloride, the concentration is lower with 533 µg/l.
7.3.2.3 Function of the substance
The substance is presumed to be a dissociation product from an added accelerator.
7.3.2.4 Classification and threshold values
The substance is included in the list of hazardous substances (Danish Ministry of the Environment, 2005) and is classified as:
R10 Flammable
Xn;R21/22 Harmful in contact with skin and if swallowed
C;R34 Causes burns
The threshold value for working environment is 10 ppm or 40 mg/m³ (Danish Working Environment Authority (WEA) guide, 2007).
7.3.2.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 136-496 mg/kg (IUCLID data set cyclohexylamine)
- LD50 mouse, oral 224 mg/kg (IUCLID data set cyclohexylamine)
- LD50 rabbit, dermal 208-372 mg/kg (IUCLID data set cyclohexylamine)
It is a weak methemoglobin-forming substance (American Conference, 1991).
Subchronic toxicity
The substance is specified as a moderately sensitising substance (American Conference, 1991). It is also specified (Lewis, 1996) that the substance may cause dermatitis.
The substance has a neurotoxic effect. Tests with oral doses to mice thus showed increased movement activity after one hour at 37, 74 and 148 mg/kg with an increase of 41% at 148 mg/kg (IR PRODS & CHEM CO 7/2/87).
Chronic toxicity
A 90-day feeding study in 2 * 25 rats in doses (start/end): 75/30, 227/100 and 525/296 mg/kg showed weight loss and reduced food intake in the two highest dose groups as well as a 80% reduction in sperm production in the highest dose group (reprotoxic effect) (IUCLID data set cyclohexylamine).
Another 90-day feeding study in 100 rats in each group with 50, 100, 200 and 300 mg/kg showed weight loss and reduced sperm production in the two highest dose groups.
The NOAEL determined for this study was 100 mg/kg (IUCLID data set cyclohexylamine).
A five-generation study in rats shows an effect on the reproductive ability from 100 mg/kg, but no mutagenic or teratogenic effects up to the highest dose of 150 mg/kg/day for the F0 generation. No effects were observed at 50 mg/kg/day (IUCLID data set cyclohexylamine).
A study with six dogs per group was carried out over 9.5 years. The dose during the first four years was 0.15, 1.5 and 15 mg/kg/day, respectively. In the remaining period, the dose was 50, 100 and 150 mg/kg/day, respectively. No health effects were found during the first four years, but a weight loss was observed when changing dose. The weight was, however, slowly regained, and no other effects were observed during the study period (IUCLID data set cyclohexylamine).
Two-year feeding studies in 2 * 25 rats with oral doses, 0.15, 1.5 and 15 mg/kg, respectively, as well as 30-month feeding studies in 2 * 52 rats with 200 mg/kg show no significant carcinogenic effects, the level of effects in test animals being the same as in the control group (IUCLID data set cyclohexylamine).
No teratogenic effects were observed in feeding studies in rats on days 7-13 of the pregnancy at doses up to 36 mg/kg/day and no teratogenic effect in studies in pregnant rhesus monkeys on days 20-45 of the pregnancy at doses up to 75 mg/kg (IUCLID data set cyclohexylamine).
A reference dose exists for the substance in IRIS (TOXNET database) based on a two-year study in 2 * 48 rats as well as a six-generation feeding study in rats where NOAEL determined for testicular damage in rats was 18 mg/kg/day with a LOAEL of 60 mg/kg/day.
With an uncertainty factor of 100, a reference dose of RfD = 0.2 mg/kg/day is obtained.
7.3.2.6 Exposure scenarios
The maximum content in liquid from leaching is 1,610 µg cyclohexanamine per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 16.1 µg of substance/g of rubber particles per day.
With a log KOW = 1.49, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g /kg * 1 * 16.10 µg/g = 1.75 µg/kg of body weight/day.
At an exposure of a maximum of 93 mg/kg/day, the uptake by oral intake will be at a level comparable to that of skin uptake.
7.3.2.7 Assessment
Health data for assessment
Data show that the substance is not carcinogenic, harmful to reproduction or mutagenic.
The substance is moderately sensitising.
NOAEL for rats is 18 mg/kg/day, and Rfd = 0.2 mg/kg/day.
Health risk
Based on the NOAEL for rats of 18 mg/kg/day, a safety margin of MOS = 18/0.00175 = 10,200 for skin uptake is obtained, which is approx. 100 times above the uncertainty factor of 100 specified in the calculation of reference dose. MOS for oral intake is at the same level.
Exposure to the substance is not assessed to be associated with health effects with the exception of particularly sensitive individuals who may develop allergic reactions.
7.3.3 Dicyclohexylamine
7.3.3.1 Identity
| Name | Dicyclohexylamine |
| CAS-no. | 101-83-7 |
| EINECS-no. | 202-980-7 |
| Molecular formula | C12H23N |
Molecular structure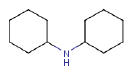 |
|
| Molecular weight (g/mol) | 181.31 |
| Synonyms | Cyclohexanamine, N-cyclohexyl- |
| Description | Colourless liquid |
| Boiling temperature | 255.8 ºC (Budovar, 1996i) |
| Melting point | -0.1 ºC (Budovari, 1996) |
| Solubility | 800 mg/l, 25 ºC, water (Iuclid dataset dicyclohexylamine). The substance is soluble in ethanol, ether, benzene (Lide, 1996) |
| Distribution coefficient Log Kow | 3.5 (Iuclid dataset dicyclohexylamine) |
| Vapour pressure | 1 hPa ved 65 ºC (Iuclid dataset dicyclohexylamine) |
| Smell | Aminelike smell (Ashford, 1994) |
7.3.3.2 Amounts found
The substance is found in leaching tests of infill no. 1, 2, 3 and 14 in concentrations from 99 to 1,167 µg/l. The substance appears to be converted in the alkaline calcium chloride, e.g. by oxidation, which means that the concentration drops to 1/10-1/100 as compared to leaching with neutral water.
7.3.3.3 Function of the substance
The substance is presumed to be a dissociation product from an added accelerator.
7.3.3.4 Classification and threshold values
The substance is included in the list of hazardous substances (Danish Ministry of the Environment, 2005) and is classified as:
Xn, R22 Harmful if swallowed
C;R34 Causes burns
N; R50/53 Environmentally hazardous substance; Very toxic to aquatic organisms/
May cause long-term adverse effects in the aquatic environment
There is no occupational hygiene threshold value for the substance.
7.3.3.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 200-373 mg/kg (IUCLID data set dicyclohexylamine)
- LD50 mouse, oral 500 mg/kg (IUCLID data set dicyclohexylamine)
- LD50 rabbit, dermal 200-316 mg/kg (MONSANTO CO, 03/18/77)
Tests in rabbits show that the substance causes burns at 45 mg/animal for 8 hours and severely irritating at 0.75 mg/animal for 24 hours (IUCLID data set dicyclohexylamine).
(E. Goettinger, 1971) states that 40% of dicyclohexylamine is converted into cyclohexanamine.
Subchronic toxicity
No test data showing a sensitising effect on animals have been found. In (Budovari, 1996), the substance is, however, listed as a potentially sensitising substance.
Chronic toxicity
No long-term test data have been found, but data for acute toxicity show that the acute toxicity is comparable with that of cyclohexylamine within a factor of 2-3.
As 40% of dicyclohexylamine is converted into cyclohexanamine, it is presumed that NOAEL for dicyclohexylamine is at the same level as for cyclohexanamine, corresponding to approx. 20 mg/kg/day. In the assessment, data for acute toxicity are, however, used.
7.3.3.6 Exposure scenarios
The maximum content in liquid from leaching is 1,167 µg cyclohexanamine per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 11.67 µg of substance/g of rubber particles per day.
With a log KOW = 3.5, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g /kg * 1 * 11.67 µg/g = 1.27 µg/kg of body weight/day.
At an exposure of a maximum of 93 mg/kg/day, the uptake by oral intake will be at a level comparable to that of skin uptake.
7.3.3.7 Assessment
Health data for assessment
The data for the substance only comprises acute toxicity with the lowest acute toxicity LD50 rat, oral = 200 mg/kg/day.
The substance may be sensitising.
Health risk
A calculation based on the lowest acute toxicity LD50 rat, oral = 200 mg/kg/day gives MOS = 200/0.00127 = 157,000 for skin uptake, which is 16 times above an uncertainty factor of 10,000, which should be used in the assessment of acute toxicity. MOS for oral intake is at the same level.
Exposure to the substance is not assessed to be associated with health effects with the exception of particularly sensitive individuals who may develop allergic reactions.
7.3.4 Dibutyl phthalate
The substance has been described in (Mapping no. 43, 2003). The following data have been updated.
7.3.4.1 Identity
| Navn | Dibutylphtalate |
| CAS-no. | 84-74-2 |
| EINECS-no. | 201-557-4 |
| Molecular formula | C16H22O4 |
Molecular structure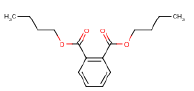 |
|
| Molecular weight (g/mol) | 278.34 |
| Synonyms | |
| Description | Oily liquid (Rar dibutylphtalate) |
| Boiling temperature | 340 ºC (Rar dibutylphtalate) |
| Melting point | -69 ºC (Rar dibutylphtalate) |
| Solubility | 10 mg/l, 20 ºC, water (Rar dibutylphtalate) |
| Distribution coefficient Log Kow | 4.57 (Rar dibutylphtalate) |
| Vapour pressure | 9.7 hPa ved 55 ºC (Rar dibutylphtalate) |
| Smell | - |
7.3.4.2 Amounts found
The substance is found in leaching tests of all studied infill nos. 1, 2, 3, 4, 10 and 11 in concentrations from 61-178 µg/l. The substance is degraded (hydrolysis of ester binding) to < 5% of the concentration in neutral water in all the alkaline calcium chloride-based contact liquids (nos. 1, 3, 11 and 14).
7.3.4.3 Function of the substance
The substance is a softener which may be a contaminant, or which may originate from an adhesive.
7.3.4.4 Classification and threshold values
The substance is included in the guiding list for self-classification (Danish Ministry of the Environment, 2005) and is classified as:
N;R51/R53 Environmentally hazardous substance; Toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
In (Rar dibutyl phthalate), the substance is classified as:
T;R61,R62 Toxic; May cause harm to the unborn child/Possible risk of impaired fertility
N; R50 Environmentally hazardous substance; Very toxic to aquatic organisms
The working environment threshold value for the substance is 3 mg/m³ (Danish Working Environment Authority (WEA) guide, 2007).
7.3.4.5 Health effects
The data are based on the risk assessment for the substance (Rar dibutyl phthalate).
Acute toxicity
The acute toxicity is very low:
- LD50 rat, oral 6,300-8,000 mg/kg (Rar dibutyl phthalate)
- LD50 mouse, oral 4,840 mg/kg (Rar dibutyl phthalate)
- LD50 rabbit, dermal 20,000 mg/kg (Rar dibutyl phthalate)
Subchronic toxicity
The substance has not been found to be sensitising in animal tests (Rar dibutyl phthalate).
In a three-month feeding study in rats, a NOAEL of 152 mg/kg/day for hepatic effects (peroxisomal-based hepatic changes) was obtained.
Chronic toxicity
According to (Rar dibutyl phthalate), there are no data to decide whether the substance has carcinogenic effects.
As for reprotoxic effects, a one-generation study in rats gave a NOAEL of 50 mg/kg/day for teratogenic effects, whereas a two-generation study gave a LOAEL of 52 mg/kg/day for teratogenic effects for male rats and 80 mg/kg/day for female rats, with a maternal toxicity of 375 mg/kg/day.
In (Rar dibutyl phthalate), the LOAEL determined for teratogenic effects was thus 52 mg/kg/day based on the two-generation study, whereas a NOAEL of 152 mg/kg/day is used for non-pregnant adult rats.
7.3.4.6 Exposure scenarios
The maximum content in liquid from leaching is 178 µg dibutyl phthalate per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 1.78 µg of substance/g of rubber particles per day.
With a log KOW = 4.57, the skin absorption is assumed to be 10%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g/kg * 0.1 * 1.78 µg/g = 0.019 µg/kg of body weight/day.
For oral intake, 100% uptake is assumed.
Intake, oral = 0.093 g/kg * 1 * 1.78 µg/g = 0.16 µg/kg of body weight/day.
7.3.4.7 Assessment
Health data for assessment
The substance causes hepatic damage in rats at a NOAEL of 152 mg/kg/day and has teratogenic effects at a LOAEL of 52 mg/kg/day.
Health risk
A LOAEL for rats of 52 mg/kg/day gives a safety margin of MOS = 52/0.00016 = 314,000 for oral intake. The MOS for skin uptake is approx. ten times higher. With an uncertainty factor of 1,000 based on the LOAEL, it is thus deemed that there are no health effects associated with exposure to the substance.
7.3.5 Conclusion
The overall assessment is that there are no health effects associated with exposure to the four substances tested, with the exception of a potential risk for developing allergy in particularly sensitive individuals (benzothiazole and the two amines).
8 Environmental assessment
- 8.1 Zinc and its salts
- 8.2 6PPD
- 8.3 Dicyclohexylamine
- 8.4 Diisobutyl phthalate
- 8.5 Nonylphenol
- 8.6 2,4-di-tert-butylphenol
- 8.7 Diethyl phthalate
- 8.8 Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate
- 8.9 Estimate of effect of discharged substances
A number of environmentally harmful substances were identified in the analyses of the liquids from the leaching tests.
The tests thus identified a number of environmentally harmful substances either classified with R50, R51 or R52 and possibly in combination with R53 in leaching tests of either elastic infills, artificial turf or pads.
Six substances were selected which occur in significant concentrations in the contact liquids from the leaching tests. For these, ecotoxicological data have been found for the assessment of the lowest effect level (predicted no-effect concentration (PNEC)) in the aquatic environment.
The substances are as follows:
- Zinc and its salts
- 6PPD
- Dicyclohexylamine
- Diisobutyl phthalate
- Nonylphenol
- 2,4-Di-tert-butylphenol
In this assessment, a reassessment was also made of data for calculating the PNEC for diethyl phthalate used in the Norwegian study, and it was also decided to add an environmental assessment of bis(2,2,6,6-tetramethyl-4-piperidinyl)sebacate.
In the model scenario used, it is assessed that drainage water from artificial turf football pitches may leach in three different ways:
- The drainage water can seep through the underlying soil and potentially contaminate the groundwater.
- The drainage water can be drained via a sewer system with potentially increased impact from environmentally harmful substances on inlets to sewage treatment plants.
- During heavy showers, the drainage water may also be drained to adjacent watercourses. Surplus water from the sewer may during heavy showers also be discharged to the aquatic environment.
It is not possible to make a complete environmental assessment of all three scenarios within the framework of the present project. It is thus decided to assess the drainage of surplus drainage water to a watercourse nearby in accordance with TGD’s standard models, which were also used in a Norwegian environmental assessment (T. Källquist, 2005).
At the same time, the potential contribution to drinking water contamination is assessed based on the concentrations of environmentally harmful and hazardous substances, and it is estimated whether there may be an increased impact from environmentally harmful substances on drainage water drained to a sewer.
8.1 Zinc and its salts
Zinc, CAS no. 7440-66-6, is classified with:
N;R50/53: Very toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
The leaching of zinc will be in the form of zinc ions – probably as a zinc chloride solution, especially in the winter season.
As the ecotoxicological effect of zinc is caused by the dissolved zinc ions, such data are not found in the IUCLID data set for the metal zinc (IUCLID data set zinc), and it was thus decided to find ecotoxicological data for zinc chloride, CAS no. 646-85-7.
8.1.1 Ecotoxicological data
Table 8.1 shows ecotoxicological data for fish, invertebrates and algae.
Table 8.1 Ecotoxicological data for dissolved zinc
| Organism | Value (concentration as zinc) | References |
| Fish (Brachydanio rerio) |
LC50, 96 hours = 18.2 mg/l | (IUCLID dataset zincchloride) |
| Invertebrates (Daphnia magna) |
EC50, 72 hours = 0.073-0.39 mg/l | (IUCLID dataset zincchloride) |
| Alga (Selenastrum apricornutum) |
NOEC (EC20) 96 hours = 0.05 mg/l | (IUCLID dataset zincchloride) |
| Alga (Navicula incerta) |
EC50, 96 hours = 10 mg/l , EC10, 96 timer = 1 mg/l | IUCLID dataset zincchloride) |
The solubility of zinc chloride is very high, the solubility being 4,320 g/l at 25°C.
The bioaccumulation factor for dissolved zinc in algae is specified to be up to approx. BCF = 10,000, whereas the accumulation in most molluscs is up to 500, apart from molluscs living in sediment, e.g. crabs, with a BCF of up to 10,000 and oysters of up to 15,000 (IUCLID data set zinc).
For fish, the bioaccumulation of dissolved zinc is < 500 (IUCLID data set zinc).
8.1.2 Estimate of no-effect concentration
With an assessment factor of 100, the NOEC value for algae gives a no-effect concentration of PNEC = 0.5 µg/l.
In the Norwegian environmental study (T. Källquist, 2005), PNECwater = 3.1 µg/l was used based on a better data basis in a draft EU risk assessment of environmental effects for zinc, and this value is thus used in the environmental assessment.
8.2 6PPD
6PPD 1,4-Benzenediamine, N-(1,3-dimethylbutyl)-N'-phenyl-, CAS no. 793-24-8, with the advisory classification:
N;R50/53: Very toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
8.2.1 Ecotoxicological data
Table 8.2 shows ecotoxicological data for fish, invertebrates and algae.
Table 8.2 Ecotoxicological data for 6PPD
| Organism | Value | References |
| Fish (Lepomis macrochirus) | LC50, 96 hours = 0.4 mg/l | (IUCLID dataset 6PPD) |
| Fish (Salmo gairdneri) | LC50, 96 hours = 0.14 mg/l | (IUCLID dataset 6PPD) |
| Fish (Primephales promelas) | LC50, 96 hours = 0.15 mg/l | (IUCLID dataset 6PPD) |
| Invertebrates (Daphnia magna) | EC50, 48 hours = 0.51-0.82 mg/l NOEC = 0,25 mg/l |
(IUCLID dataset 6PPD) |
| Alga (Selenastrum capricornutum) | EC50, 96 hours = 0.6 mg/l | (IUCLID dataset 6PPD) |
The distribution coefficient between octanol and water is log Kow = 5.4 (IUCLID data set 6PPD), for which reason bioaccumulation can be expected (risk at log Kow = 3).
Biodegradation tests show that the substance is non-degradable. An aerobic biodegradation test with activated sludge thus resulted in 7.2% degradation after 32 days and an aerobic test with household sludge resulted in 13-40% after 28 days.
In connection with the biodegradation tests, the EC50 specified for activated sludge is 450 mg/l for three hours’ exposure (IUCLID data set 6PPD).
Chronic toxicity
No data have been found for long-term tests.
8.2.2 Estimate of no-effect concentration
Data for short-time exposure show that the substance is more toxic to fish with LC50 = 0.14 against 0.5-0.6 mg/l for invertebrates and algae. As data have been found for short-term tests for the three trophic levels, but not for long-term tests, an assessment factor of 1,000 and LC50 = 0.14 mg/l is used in accordance with TGD. It can thus be estimated that PNEC = 0.14 µg/l.
8.3 Dicyclohexylamine
Dicyclohexylamine, CAS no. 101-83-7, with the advisory classification:
N;R50/53: Very toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
8.3.1 Ecotoxicological data
Table 8.3 shows ecotoxicological data for fish and algae.
Table 8.3 Ecotoxicological data for dicyclohexylamine
| Organism | Value | References |
| Fish (Brachydanio rerio) |
LC50, 96 hours = 62 mg/l | (IUCLID dataset dicyclohexylamine) |
| Alga (Scenedesmus subspicatus) |
EbC 50 72 hours = 0.38 mg/l EbC 10 72 hours = 0.02 mg/l (alga inhibition test for biomass production) |
(IUCLID dataset dicyclohexylamine) |
| Alga (Scenedesmus subspicatus) |
ErC 10 72 hours > 0.063 og < 0.125 mg/l (growth rate test) NOEC = 0.016 mg/l |
(IUCLID dataset dicyclohexylamine) |
The distribution coefficient between octanol and water is log Kow = 3.5 (IUCLID data set dicyclohexylamine).
The water solubility is 800 mg/l, 25°C.
Biodegradation tests show that the substance is easily degradable. Aerobic biodegradation tests with household sludge thus resulted in > 90% degradation after 20 days at a concentration of 0.8 mg/l, and tests in activated sludge resulted in 76.9% degradation after 14 days at a substance concentration of 100 mg/l (IUCLID data set dicyclohexylamine).
Chronic toxicity
Data are only available for algal growth tests.
8.3.2 Estimate of no-effect concentration
The data for ecotoxicity are inadequate as there are no data for invertebrates.
In the growth test of Scenedesmus subspicatus algae shown in the table, an NOEC of 0.016 mg/l was specified with an LOEC of 0.031 mg/l, which may be used in the assessment of chronic effect on algae in accordance with TGD.
As no data are available for invertebrates, a PNEC cannot immediately be estimated.
To supplement the data basis, the related substance cyclohexylamine is reviewed.
Table 8.4 Ecotoxicological data for cyclohexylamine
| Organism | Value | References |
| Fish (Leucismus idus) |
LC50, 48 hours = 58 mg/l | (IUCLID dataset cyclohexylamine) |
| Invertebrates (Daphnia magna) |
EC50, 24 hours = 49-80 mg/l | |
| Alga (Microcystis aeruginosa) |
Toxicity threshold = 0.02 mg/l (8 days growth rate test) |
(IUCLID dataset cyclohexylamine) |
| Alga (Scenedesmus quadricauda) |
Toxicity threshold = 0.51 mg/l (8 days growth rate test) |
(IUCLID dataset cyclohexylamine) |
| Alga (Selenastrum apricornutum) |
EC50, 96 hours = 20 mg/l | (IUCLID dataset cyclohexylamine) |
The substance shows short-term effects with LC50 for fish being at the same level as dicyclohexylamine. The short-term effects for invertebrates are at the same level as the data for fish, and the data for algae suggest that the toxicity of the two substance is more or less the same.
As an estimate of the no-effect concentration PNEC for both substances, the lowest value for the algal growth tests is used and an assessment factor of 100, corresponding to PNEC = 0.2 ug/l.
As the short-term effects for fish and invertebrates are approx. 50 mg/l, the expected factor for the no-effect concentration for these trophic levels will be 1,000 under LC/EC50, corresponding to 50 ug/l. The estimated PNEC thus provides adequate protection for fish and algae.
8.4 Diisobutyl phthalate
Diisobutyl phthalate, CAS no. 84-69-5, with the advisory classification:
N;R50/53: Very toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
8.4.1 Ecotoxicological data
Table 8.5 shows ecotoxicological data for fish, invertebrates and algae.
Table 8.5 Ecotoxicological data for diisobutyl phthalate
| Organism | Value | References |
| Fish (Primaphales promelas) |
LC50, 96 hours = 0.73 mg/l | (IUCLID dataset DIBP) |
| Invertebrates (Daphnia magma) |
EC50, 24 hours = 7.4 mg/l | (IUCLID dataset DIBP) |
| Invertebrates (Daphnia magma) |
21 days test: LOEC = 3 mg/l, NOEC = 1 mg/l | (IUCLID dataset DIBP) |
| Invertebrates (Nitogra spinipes) |
EC50, 48 hours = 3 mg/l | IUCLID dataset DIBP) |
| Alga (Scenedesmus subspicatus) |
EC50, 72 hours = 1 mg/l NOEC = 0.19 mg/l |
(IUCLID dataset DIBP) |
The distribution coefficient between octanol and water is log Kow= 4.11 (IUCLID data set DIBP), for which reason bioaccumulation can be expected (risk at log Kow = 3). The bioaccumulation factors for cyprinus carpio are specified to be 780 based on model calculations.
Biodegradation tests show that the substance is easily degradable. Two aerobic biodegradation tests with household sludge resulted in 79 and 94% degradation, respectively, after 28 days (IUCLID data set DIBP).
Chronic toxicity
For Daphnia magna, an NOEC of 1 mg/l was found in tests carried out over a 21-day period with LOEC = 3 mg/l (IUCLID data set DIBP).
8.4.2 Estimate of no-effect concentration
Algae are almost 7.4 times more sensitive than invertebrates as EC50, 72-hour algae = 1 mg/l against EC50, 24-hour daphnia magma, for which reason PNECv, in accordance with TGD’s standard assessment factors, is estimated from the lowest short-time value for algae to be PNEC = 1µg/l by using an assessment factor of 1,000.
8.5 Nonylphenol
Nonylphenol, CAS no. 25154-52-3, classified as:
N;R50/53: Very toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
8.5.1 Ecotoxicological data
Table 8.6 shows ecotoxicological data for fish, invertebrates and algae.
Table 8.6 Ecotoxicological data for nonylphenol
| Organism | Value | References |
| Fish (Primaphales promelas) |
LC50, 96 hours = 0.128 mg/l | (Rar nonylphenol) |
| Invertebrates (Daphnia Magma) |
EC50, 48 hours = 0.085 mg/l | (Rar nonylphenol) |
| Alga (Scenedesmus subspicatus) |
EC50, 72 hours = 0.0653 mg/l | (Rar nonylphenol) |
The distribution coefficient between octanol and water is log Kow= 4.48 (Rar nonylphenol), for which reason bioaccumulation can be expected (risk at values above log Kow = 3). In the reference, the bioaccumulation factor is specified to be 1280.
Biodegradation tests show that the substance is not easily degradable. Two aerobic biodegradation tests with household sludge resulted in 10 and 19% degradation, respectively, after 10 days, as well as 53 and 62% degradation after 28 days (Rar nonylphenol).
Chronic toxicity
For fish and invertebrates, the NOEC from long-term tests is specified to be between 1 and 10 µg/l, whereas the NOEC value for algae is based on an EC10 value and put at 3.3 µg/l (Rar nonylphenol).
8.5.2 Estimate of no-effect concentration
The estimated no-effect concentration for aquatic organisms, PNECv, is calculated to be 0.33 µg/l by using an assessment factor of 10 (Rar nonylphenol).
8.6 2,4-di-tert-butylphenol
2,4-di-tert-butylphenol, CAS no. 96-76-4, with the advisory classification:
N;R51/53: Toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
8.6.1 Ecotoxicological data
Table 8.7 shows ecotoxicological data for fish, invertebrates and algae.
Table 8.7 Ecotoxicological data for 2,4-di-tert-butylphenol
| Organism | Value | References |
| Fish (Leuciscus idus) | LC50, 48 hours = 1.8 mg/l | (IUCLID dataset 2,4-di-tert-butylphenol) |
The distribution coefficient between octanol and water is log Kow= 5.13 (IUCLID data set 2,4-di-tert-butylphenol), for which reason bioaccumulation can be expected.
The water solubility is 12 mg/l, 20°C.
Biodegradation tests show that the substance is not easily degradable. An aerobic biodegradation test with activated sludge and a substance concentration of 34.5 mg/l exhibited 2% degradation after 28 days (IUCLID data set 2,4-di-tert-butylphenol).
Chronic toxicity
No data have been found for long-term tests.
8.6.2 Estimate of no-effect concentration
As data have only been found for fish, a PNEC cannot immediately be estimated.
A search for data on related substances has thus been carried out.
Table 8.8 shows data for the related substance 2,6-di-tert-butylphenol, CAS no. 128-39-2.
Table 8.8 Ecotoxicological data for 2,6-di-tert-butylphenol
| Organism | Value | References |
| Invertebrates (Daphnia magma) |
EC50, 48 hours = 0.45 mg/l | (IUCLID dataset 2,6-di-tert-butylphenol) |
| Invertebrates (Gammarus fasciatus) |
EC50, 48 hours = 0.6 mg/l | (IUCLID dataset 2,6-di-tert-butylphenol) |
Table 8.9 shows data for phenol, CAS no. 108-95-2.
Table 8.9 Ecotoxicological data for phenol
| Organism | Value | References |
| Fish (Leuciscus idus) |
LC50, 48 hours = 14 mg/l | (rar phenol) |
| Fish (larver cirrhina mrigala) |
NOEC = 77-94 µg/l | (rar phenol) |
| Invertebrates (Daphnia magma) |
EC50, 48 hours = 4.2-13 mg/l | (rar phenol) |
| Alga (Selenastrum apricornitum) |
EC50, 96 hours = 37-84 mg/l | (rar phenol) |
For phenol, a PNEC of 7.7 ug/l has been specified based on long-term effects for fry.
As can be seen, in short-term tests 2,4-di-tert-butylphenol and the related substance 2,6-di-tert-butylphenol have LC50 values almost ten times lower than phenol for fish of the same species (leuciscus idus) and EC50 for invertebrates of the same species (daphnia magna). This may indicate a no-effect concentration which may be lower than for phenol. As is the case for phenol, it is assumed that algae are the least sensitive organisms to the substance 2,4-di-tert-butylphenol and the related substance 2,6-di-tert-butylphenol. In this case, the no-effect concentration can be estimated to be PNEC = 0.45/1,000 = 0.45 ug/l based on the lowest short-time effect and an assessment factor of 1000.
8.7 Diethyl phthalate
The data for PNEC for diethyl phthalate, CAS no. 884-66-2, have in the following been reassessed in relation to the assessment made in a Norwegian study of environmental effects from artificial turf pitches (T. Källquist, 2005).
8.7.1 Ecotoxicological data
The following ecotoxicological data have been found:
| Organism | Value | References |
| Fish (Primaphales promelas) | LC50, 96 hours = 17 mg/l | (IUCLID dataset DEP) |
| Fish (Salmo gairdneri) | LC50, 96 hours = 12 mg/l | (IUCLID dataset DEP) |
| Invertebrates (Daphnia magma) |
EC50, 48 hours = 36-54 mg/l NOEC= 10 mg/l (shortterm) 21 days test: NOEC = 13 mg/l |
IUCLID dataset DEP) |
| Invertebrates (Musidopsis bahia) |
EC50, 24 hours = 5.3 mg/l NOEC =5.3 mg/l |
IUCLID dataset DEP) |
| Alga (Scenedesmus subspicatus) |
EC50, 72 hours = 23 mg/l EC10, 72 hours = 9 mg/l |
(IUCLID dataset DEP) |
8.7.2 Estimate of no-effect concentration
The data suggest that fish in short-term tests are three to four times more sensitive than invertebrates and a factor of two more sensitive than algae. The NOEC from the long-term test can therefore not be used for invertebrates, you should use the lowest short-term effect LC50 for fish = 12 mg/l, thus resulting in an estimate of no-effect concentration PNEC = 12 µg/l
(In the Norwegian study, a PNEC = 900 ug/l was estimated based on NOEC = EC10 for algae of 9 mg/l and an assessment factor of 10.)
8.8 Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate
The data for PNEC for Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate, CAS no. 52829-07-9, are in the following assessed on the basis of information in the supplier’s instructions for use (Instructions for use, Lowilite 77). The substance is a polymer stabiliser found in very high concentrations in liquid from leaching tests of an artificial turf product.
8.8.1 Ecotoxicological data
The following ecotoxicological data have been found:
| Organism | Value | References |
| Fish | LC50, 96 hours = 4.4 mg/l | (Instructions Lowilite 77) |
| Invertebrates (Daphnia magma) |
EC50, 24 hours = 17 mg/l | (Instructions Lowilite 77) |
| Alga | EC50, 72 hours = 1.9 mg/l | (Instructions Lowilite 77) |
Distribution coefficient between octanol and water is log Kow= 0.35 (Instructions for use, Lowilite 77).
8.8.2 Estimate of no-effect concentration
Only short-term data are available, showing that algae are the most sensitive organisms. Based on this, a no-effect concentration PNEC = 1.9/1000 = 1.9 µg/l can be estimated with an assessment factor of 1,000.
8.9 Estimate of effect of discharged substances
8.9.1 Discharged substances in drainage water
Below is a calculation of the amount of substances from rubber granules and artificial turf mat in drainage water for a one-year period, based on the assumption that the concentrations found from leaching tests are equilibrium concentrations which do not decrease over time.
The calculation uses an area of a pitch of 8,000 m² and precipitation of 702 mm/year based on the Danish Meteorological Institute statistics 1966-1990 for East Jutland.
The amount of artificial turf mat is calculated to be 24 tonnes based on an average weight of the samples received of 3 kg/m², whereas the weight of the rubber granules is put at 100 tonnes per pitch based on mapping information.
The precipitation corresponds to a liquid-infill ratio of 0.15:1 for 24 hours as well as a ratio for artificial turf of 0.64:1 for 24 hours. This is much less per 24 hours than for the leaching test.
Table 8.10 Worst case estimate of discharged amounts of substances from a football pitch with elastic infill per year
| Substance | Content in drainage water g/year | Max. content in product from DCM- extraction and ICP (µg/g) | Content in product per pitch (g) | % substance in drainage water compared to content |
| Zn | 12,917 | 17,000 | 1,700,000 | 0.76 |
| Dibutylphtalate (DBP) | 999.6 | 50 | 5,000 | 20 |
| Diisobutylphtalate (DIBP) | 550.4 | 175 | 17,500 | 3 |
| Diethylhexylphthalate (DEHP) | 640 | 60 | 6,000 | 10.7 |
The table shows that less than 1% of the zinc content and up to 20% of the phthalate content leach during a year, assuming that the leaching occurs with the same efficiency during a one-year period as during a 24-hour period. As mentioned in the analysis section, it is assessed that the substance concentrations in the contact water are maximum concentrations which are probably somewhat higher than the concentrations that will be obtained in drainage water from a football pitch. In addition, the drainage water concentrations of phthalates will probably fall over time provided that the infills remain intact over time. Further studies are, however, required to confirm this.
Table 8.11 Worst case estimate of discharged amounts from artificial turf from a football pitch per year
| Substance | Substance in drainage water g/year | Max. content in products from DCM extraction (µg/g) |
Content in product per pitch (g) | % substance in drainage water compared to content |
| Diethylhexylphthalate (DEHP) | 1028 | 104 | 2496 | 41 |
| Nonylphenol | 2157 | 57 | 1368 | 158 |
The table shows that a large part of the phthalate content and 158% of the nonylphenol leach during a one-year period if it is assumed that the leaching occurs with the same efficiency during a one-year period as during a 24-hour period. As mentioned in the analysis section, it is assessed that the substance concentrations in the contact water are maximum concentrations which are probably somewhat higher than the concentrations that will be obtained in drainage water from a football pitch. In addition, the drainage water concentrations of phthalates and nonylphenols will probably fall over time provided that the infills remain intact over time. Further studies are, however, required to confirm this.
8.9.2 Discharge of drainage water to watercourses
The following model calculation investigates the effect of the discharge of selected substances to any watercourses near football pitches (local effects). This is particularly during heavy showers where the pitch’s drainage system cannot take all the rainwater. The calculation is made under the same assumptions as the Norwegian study (T. Källquist, 2005).
It is thus assumed that the concentration in water from leaching at a water-sample ratio of 10:1 corresponds to the concentration in the drainage water. The concentration of the drainage water Ceff is assumed to be diluted 10 times (f) when drained to a catchwater drain/small watercourse as specified as default in TGD (Technical Guidance Document, 2003).
The concentration in the water phase can be calculated to be:
PECwater = Ceff /((1+Kpsusp x SUSPwater x 10-6)*f)
where Kpsusp is the distribution coefficient between solid and water calculated from KOC with a content of organic substance in suspended material put at the default value c org,susp= 10%.
This means that Kpsusp= 0.1* KOC.
SUSPwater is the concentration of suspended material (mg/l) – the value is put at the default value of 15 mg/l.
The dilution factor f = 10.
Koc is calculated on the basis of Kow from the QSAR model for non-hydrophobic organic substances in TGD.
Thus, log KOC = 0.81*log KOW + 0.1.
PECsediment =Ksusp-water/RHOsuspxPECwater*1000 where
the volume-based distribution coefficient of suspended material in relation to water Ksusp-water (m³/m³) is calculated using the model tool EUSES 2.0 with input of KOC and the selected QSAR model (EUSES 2.0).
The no-effect value for sediment is calculated from the no-effect value for water with the following expression:
PNECsediment = Ksusp-water/RHOsuspxPNECwater*1000.
Finally, the following ratios are calculated: PECwater/PNECwater as well as PECsediment/PNECsediment
(margins of safety) to assess whether there is a potential local environmental effect from discharge of the substances investigated.
Table 8.12 shows a worst case calculation of the environmental effect of discharging drainage water from elastic infills to an adjacent watercourse, under the assumption that the drainage water concentrations of substances correspond to those found in contact water.
Table 8.12 Calculation of discharge of leached substances from infills to watercourses
| Substance | CAS-no. | C eff | log KOW | KOC | c org, susp % | Kpsusp | PEC water |
| Max conc. µg/l | µg/l | ||||||
| Zn | 2300 | 110000 | 86.8 | ||||
| Diethylphthalate (DEP) | 84-66-2 | 146 | 2.65 | 176 | 10 | 18 | 14.6 |
| Dibutylphtalate (DBP) | 84-74-2 | 178 | 4.57 | 6334 | 10 | 633 | 17.6 |
| Benzylbutylphthalate (BBP) | 0 | 43 | 4.84 | 10481 | 10 | 1048 | 4.2 |
| Diisobutylphtalate (DIBP) | 84-69-5 | 98 | 4.11 | 2686 | 10 | 269 | 9.8 |
| Diethylhexylphthalate (DEHP) | 117-81-7 | 114 | 7.6 | 1803018 | 10 | 180302 | 3.1 |
| Cyclohexanamine, N-cyclohexyl- | 101-83-7 | 1167 | 3.5 | 861 | 10 | 86 | 116.5 |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 96-76-4 | 250 | 5.13 | 18001 | 10 | 1800 | 24.3 |
| Cyclohexanamine | 108-91-8 | 1610 | 1.49 | 20 | 10 | 2 | 161.0 |
| 6PPD (based on degradation products) | 793-24-8 | 687 | 5.4 | 29785 | 10 | 2979 | 66.0 |
Table 8.12 Calculation of discharge of leached substances from elastic infills to watercourses, contd.
As described in 8.9.1, the concentrations found in contact water are worst case scenarios as the concentration of leached substances must be expected to decrease over time just as the leaching method used is deemed to be more efficient with more liquid-solid contact than the actual situation on a football pitch.
In this connection, it should be noted that a lysimeter test probably underestimates the leaching level as the physical impact from the players on a wet pitch (during rain) should increase the liquid-solid contact compared to a lysimeter where there is no mechanical impact on the pitch from the football players.
Table 8.12 shows – based on the assumption that the leaching from elastic infills during use of a football pitch corresponds to the results from the leaching test – an effect on adjacent watercourses from zinc at a level corresponding to the result in the Norwegian study. Phthalates may also cause environmental effects with concentration levels in watercourses in the order of 10 times above the no-effect concentration. Similar concentration levels are observed for phenol, 2,4-bis(1,1-dimethylethyl)-.
The amine compounds seem to be capable of causing significant effects with concentrations in the aquatic environment of around 1,000 times above the no-effect concentration.
The 6PPD concentration is based on an uncertain determination of degradation products, but the calculation indicates that the concentration in the aquatic environment may be above the no-effect concentration.
Table 8.13 shows a worst case calculation of the environmental effect of discharge of drainage water from artificial turf into an adjacent watercourse.
Table 8.13 Calculation of discharge of leached substances from artificial turf to watercourses.
| Substance | CAS-no. | C eff | log KOW | KOC | c org, susp % | Kpsusp | PEC water |
| µg/l | µg/l | ||||||
| Diethylphthalate (DEP) | 84-66-2 | 302 | 2.65 | 176 | 10 | 18 | 30.2 |
| Dibutylphtalate (DBP) | 84-74-2 | 155 | 4.57 | 6334 | 10 | 633 | 15.4 |
| Benzylbutylphthalate (BBP) | 85-68-7 | 42 | 4.84 | 10481 | 10 | 1048 | 4.1 |
| Diisobutylphtalate (DIBP) | 84-69-5 | 112 | 4.11 | 2686 | 10 | 269 | 11.2 |
| Dicyclohexylphthalate (DCHP) | 84-61-7 | 82 | 5.6 | 43251 | 10 | 4325 | 7.7 |
| Sum other phthalates (not DEHP) | |||||||
| Diethylhexylphthalate (DEHP) | 117-81-7 | 183 | 7.6 | 1803018 | 10 | 180302 | 4.9 |
| Nonylphenol | 84852-15-3 | 384 | 4.48 | 5355 | 10 | 536 | 38.1 |
| (bis-(2,2,6,6-tetramethyl-4-piperidinyl)sebacate (artificial turf no. 7) |
52829-07-9 | 353000 | 0.35 | 2 | 10 | 0 | 35300 |
| (bis-(2,2,6,6-tetramethyl-4-piperidinyl)sebacate (artificial turf no. 4) |
52829-07-9 | 183 | 0.35 | 2 | 10 | 0 | 18.3 |
The concentrations specified in drainage water C eff from artificial turf are based on concentrations from the leaching tests on artificial turf no. 4
Table 8.13 Calculation of discharge of leached substances from artificial turf to watercourses, contd.
As described in 8.9.1, the concentrations found are worst case scenarios as the concentration of leached substances must be expected to decrease over time just as the leaching method used, as mentioned above, is deemed to be more efficient with more liquid-solid contact than the actual situation on a football pitch.
In this connection, it should be noted once again that a lysimeter test probably underestimates the leaching level as the physical impact from the players on a wet pitch (during rain), all other things being equal, should increase the liquid-solid contact compared to a lysimeter where there is no mechanical impact on the pitch from the football players.
Table 8.13 shows – based on the assumption that the leaching from artificial turf during use of a football pitch corresponds to the results from the leaching test on artificial turf mats – that there may be a very large effect on adjacent watercourses from nonylphenol, where the concentration in water is 115 times above the no-effect concentration. Phthalates may also cause environmental effects as the sum of the phthalate concentrations in the water phase is approx. 16 times above the no-effect concentration. The substance bis(2,2,6,6-tetramethyl-4-piperidinyl)sebacate may also cause environmental effects for all types of artificial turf with a concentration in the water phase of approx. 10 times above the no-effect concentration for artificial turf no. 4, and seven times above the no-effect concentration for artificial turf mat no. 2 (calculated values).
Artificial turf mat no. 7 does, however, cause a very intense effect, suggesting that the artificial turf mat is stabilised using an inappropriate water-soluble chemical. The leaching of the stabiliser is significant due to its solubility in water, resulting in a concentration in the water phase that exceeds the no-effect concentration 18,000 times.
The results show that a number of environmentally harmful substances can be leached from both elastic infills and artificial turf having a potential environmental risk in the case of any spillover of drainage water to adjacent watercourses, but it is assessed that measurements are required of the substance concentrations under real conditions on the football pitches to be able to assess the risk. This is because significantly lower leaching values from football pitches were expected. The expectation of lower leaching values is based on the previously cited Swiss and French studies mentioned above.
In such measurements, it is possible to benefit from the substances found and assessed in this study.
8.9.3 Risks for drinking water
The substance concentrations in contact water have been compared to the requirements set out in the Executive Order on Drinking Water.
Table 8.14 Drinking water requirements and concentrations found in contact water
| Substance | Threshold limit drinking water Denmark µg/l | Liquid from leaching tests Elastic infill Norway µg/l |
Measured in drainage water for sewage from Sjællandsk football pitch µg/l |
Liquid from leaching test Elastic infill Denmark µg/l |
Liquid from leaching test Artificial turf Denmark µg/l |
| Zn | 100 | 3300 | 59 | 600-2300 | |
| DEHP | 1 | 5.5 | 7 | 14-114 | 14-183 |
| Sum other phthalates (not DEHP) | 1 | 6-16 | 162-428 | 614-797 | |
| Nonylphenol | 20 | 4.5 | 1.5 | - | 384 |
As can be seen, concentrations of phthalates have been measured in contact water which are potentially significantly higher than those allowed in drinking water. One single measurement of the drainage water from a football pitch on Zealand also shows that the concentration in drainage water may exceed the drinking water levels set out and thus poses a potential risk in connection with the seeping of drainage water. The present leaching tests were carried out on new infills and artificial turf, and no comparable measurements of drainage water from newly-installed pitches are available. Analyses of drainage water from artificial turf pitches are required to be able to assess the real risk of groundwater contamination including an investigation of how the leaching of substances changes over time. It has not been possible to carry out analyses of drainage water within the financial framework of this project.
8.9.4 Significance of drainage of water to sewer
The leaching tests showed high concentrations of phthalates in the contact water, which may contribute to an impact from phthalates on sewage treatment plants.
It is assessed that the leaching values will be highest at the beginning when a pitch is installed, and that the concentration in the drainage water from the pitch is probably somewhat lower than in the liquid from the contact tests due to a poorer liquid-solid contact.
In (Status, 2003), the concentration of phthalates discharged to Danish sewage treatment plants is estimated to be approx. 25 µg/l (see Table 8.15). The results from the leaching of infills and artificial turf show that there may be a not insignificant contribution from drainage water from football pitches. The significance of the leached substances for the impact on sewage treatment plants is expected to be very dependent on local conditions.
Table 8.15 Phthalates in waste water to sewage treatment plants
| mg/l (mg/m³) in |
mg/l (mg/m³) out | |
| BBP | 0.99 | |
| DEHP | 17.7 | 1.9 |
| DBP | 1.19 | |
| DEP | 4.6 | |
| DINP | 0.22 | |
| DNOP | 0.13 | |
| SUM | 24.83 |
It is assessed that an analysis is required for phthalates in drainage water directly from football pitches to be able to assess the contribution of phthalates discharged to sewage treatment plants. An aspect of the monitoring should be to observe how quickly the concentration decreases following the installation of a new pitch.
8.9.5 Conclusion
A number of environmentally harmful substances were found in the contact water from leaching tests on infills and artificial turf mats. Based on a comparison with foreign lysimeter tests, the leaching tests are expected to have exhibit significantly better liquid-solid contact. A leaching velocity higher than that obtained in lysimeter tests should thus be expected. The lysimeter tests are assessed to be more representative for the conditions on a football pitch.
A worst case assessment has been made on any discharge of drainage water to watercourses based on the Danish leaching tests.
Here, it is assessed that there may be environmental effects associated with infill tests for:
- Zinc PEC/PNEC water = 28
- Phthalates PEC/PNEC water = approx. 10
- Cyclohexanamine and cyclo-
hexanamine, N-cyclohexyl- PEC/PNEC water = approx. 1,000 - Phenol 2,4-bis (1.1-dimethyl-
ethyl)- PEC/PNEC water = approx. 10 - Possibly 6PPD PEC/PNEC water = approx. 470
It should be noted that the ratio between the concentration and the no-effect concentration for the amines and 6PPD is a factor of almost 1,000.
It is assessed that leaching from artificial turf mats may result in potential environmental effects for:
- Phthalates PEC/PNEC water = approx. 10
- Nonylphenol PEC/PNEC water = 115
- And in one case the substance Bis-(2,2,6,6- tetramethyl-4-piperidinyl)
sebacate PEC/PNEC water = approx. 20,000
It should be noted that the ratio between the concentration and the no-effect concentration for nonylphenol is above 100 and for Bis-(2,2,6,6- tetramethyl-4-piperidinyl)sebacate almost 20,000.
It is not known how much the leaching for the substances tested decreases under more realistic leaching conditions, e.g. lysimeter tests, but it is assessed that it cannot be ruled out that there may be an environmental effect for the substances for which a high value was observed in the ratio between the concentration and the no-effect concentration. To determine whether it is a real environmental effect requires lysimeter tests or measurements on drainage water from football pitches over a longer period of time (e.g. one year). The fact is that foreign results suggest a decreasing concentration over time (e.g. one year). It cannot, however, be ruled out that degradation of rubber may occur over a long period of time, which again increases the leaching values (e.g. a time-frame of 10-20 years).
Such lysimeter tests or measurements on drainage water from football pitches over time are also required to be able to assess whether there is any risk to the drinking water. The substances which may pose a potential risk based on the requirements set out in the Executive Order on Drinking Water are assessed to be:
- Zinc
- Phthalates and
- Nonylphenols
as the concentrations in the contact water from leaching tests are in the order of 20-800 times above the threshold values for drinking water. Here, it should be noted that the substances, to a varying degree, will be absorbed by the sand/clay layers which the drainage water passes. Zinc concentrations in percolate of significance for the drinking water quality have thus not been observed in foreign lysimeter tests. No data are, however, available for phthalates and nonylphenols under such realistic conditions.
The assessment of the impact on waste water systems also requires more realistic lysimeter tests or measurements on drainage water from football pitches over time.
9 Abbreviations used in the environmental and health assessments
| Carc | Carcinogenic |
| HSDB | Hazardous Substance Data Bank |
| IRIS | Integrated Risk Information System |
| IUCLID | International Uniform Chemical Information Database |
| EC50 | Effective concentration 50 % |
| ErC50 | Concentration with 50 % impact on growth rate |
| EbC50 | Concentration med 50 % impact on biomass production |
| LC50 | Lethal concentration 50 percent |
| LD50 | Lethal dosage 50 percent |
| LOAEL | Low observed adverse effect level |
| LOEL | Low observed effect level |
| MOS | Margin of safety at lowest observed adverse effect level |
| Mut | Mutagenic, harmful to the genes |
| NOEC | No observed effect concentration |
| NOAEL | No observed adverse effect level, o-effect level for permanent damages |
| NOEL | 0-effect level |
| PNEC | Predicted 0-effect concentration |
| Rep | Reproductive, harmful to foetus and/or reproduction |
| RfD | Reference dosage |
| QSAR | Quantitative Structure Activity Relationship |
10 Abbreviations used in the material descriptions and for chemical substances/substance groups
| EPDM | Ethylene Propylene Diene Monomer (rubber with saturated main chain ) |
| NR | Natural Rubber. Rubber with unsaturated main chain |
| SBR | Styrene Butadiene Rubber, used as tyres tread |
| SEBS | TPE type with saturated main chain |
| TPE | ThermoPlastic Elastomer, rubberlike unvulcanised material |
| PE | PolyEthylene, plastic material with very low glass transition temperature |
| PEX | Crosslinked polyethylene with chemical linkage between the PE chains |
| PP | Polypropylene, plastic materials with glass transitions temperature at approx. 0 °C |
| PU, PUR | Polyurethane, Polyurethane Rubber |
| BTEX | Benzene, toluene, ethylbenzene and xylenes |
| DOC | Dissolved Organic Compounds |
| VOC | Volatile Organic Compounds |
| TOC | Total Organic Compounds |
| TVOC | Total Volatile Organic Compounds |
| THC | Total HydroCarbons |
| EOX | Extractable Organic Halogen (e.g. chlorine) |
| DCM | DiChloroMethane |
| PAH | Polycyclic Aromatic Hydrocarbons |
| 6 PPD | 1,4-Benzendiamine, N-(1,3-dimethylbutyl)-N´-phenyl- |
| Zn | Zinc |
11 References
| A.J. Verschoor, Leaching of zinc from rubber infill on artificial turf (Football pitches), RIVM rapport 61774001/2007 |
| Afgivelse og sundhedsmæssig vurdering af PAH’er i bildæk, Miljøstyrelsen, Kortlægning nr. 54 |
| American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991 |
| Anders Johansen, DBU, telefoninterview 31. januar 2007 |
| Anlæg af artificial turfbaner til fodbold, Artificial turfbelægningen, vejledning og beskrivelse, DBU December 2005 |
| Ashford, R.D. Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd., 1994. |
| At-vejledning Grænseværdier for stoffer og materialer C.0.1, august 2007 |
| Brugsanvisning Lowilite 77, 2004 |
| Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996 |
| C. Dye, A. Bjerke, N. Schmidbauer, S. Manø, Måling av luftforurensning i innendørs kunstgresshaller, NILU OR 03/2006. TA-2148/2006. |
| E.Müller, BAFU,Bern, Umweltvertraäglichkeit von Kunststoffrasen: Resultate eines Feldversuchs, foredrag BASPO (Bundesamt für Sport), Magglingen, Schweiz, 13. september 2007 |
| E. Müller, BAFU, Bern, 2008, Investigations into the Behaviour of Synthetic Surfaces and Artificial Turf Exposed to Natural Weather Conditions. Federal Office of Sport FOSPO, samt personlig kommunikation med Teknologisk Institut |
| Environmental Compability of Sports Surfaces an ISSS project, H.J. Kolitzus, www.isss.de/ist-ch |
| EUSES 2.0 modelværktøj fra EU http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/EUSES/EUSES_2.0/ |
| FIFA Quality Concept – Handbook of test Methods for Football Turf, Marts 2006 |
| FIFA Quality Concept for Artificial Turf Guide |
| Gode råd om vedligehold af 3. generations artificial turf-fodbolbaner, DBU juli 2004. |
| GOETTINGER E; WIEN MED WOCHENSCHR 121 (25-26): 515-516, 1971 |
| Hans J. Kolitzus, Investigations and Assessment of Synthetic Sports Surfaces in Switzerland Including Athletic and Soccer facilities, 2006 |
| Hans J. Kolitzus, IST, Artificial Turf surfaces for Soccer, Study KR6943, 2007 |
| Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995 |
| IR PRODS & CHEM CO; Cyclohexylamine: Acute Toxicity and Irritation Studies and Mutagenicity; 7/2/87; EPA Doc No. 88-920004473; Fiche No. OTS0540890 |
| IUCLID dataset 2,4-di-tert-butylphenol, 2000 |
| IUCLID dataset 2,6-di-tert-butylphenol, 2000 |
| IUCLID dataset 6PPD: N-1,3-dimethylbutyl-N’-phenyl-p-phenyleneamine, 2000 |
| Iuclid dataset benzothiazole 201-14953B1 16.6.2003 |
| Iuclid dataset cyclohexylamin, 2000 |
| Iuclid dataset DEP: diethyl ftalate, 2000 |
| Iuclid dataset DIBP: diisobutylftalate, 2000 |
| Iuclid dataset dicyclohexylamin, 2000 |
| IUCLID dataset zink, 2000 |
| IUCLID dataset zinkklorid, 2000 |
| J.S.Dick, Rubber Technology, Hanser, 2001, side 443 |
| Kemikalieinspektionen PM 2/06: Kunstgräs ur ett kemikalieperspektiv – en lägesrapport |
| Kortlægning af kemiske stoffer i forbrugerprodukter, Kortlægning af pletfjernere nr. 43, 2003, Miljøstyrelsen |
| Kunstgressbaner - Vurdering av helserisiko for fotballspillere. Nasjonalt folkehelseinstitutt og Radiumhospitalet, Oslo, januar 2006 |
| Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996 |
| Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 12th ed. New York, NY: Van Nostrand Rheinhold Co., 1993 |
| Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 76th ed. Boca Raton, FL: CRC Press Inc., 1995-1996. |
| M. van Bruggen, E. M. van Putten, P.C.J.M. Janssen, Nitrosaminen uit rubbergranulaat. Centrum voor Inspectie-onderzoek. Milieucalamiteiten en Drinkwater, RIVM rapport 609 300 001, 2006 |
| Miljøstyrelsen, 2001: Vejledende liste til selvklassificering af farlige stoffer. http://www.mst.dk/Kemikalier/Kemikalier/Stoflister/02040000.htm |
| Miljøministeriet, 2005: Bekendtgørelse om listen over farlige stoffer. Bekendtgørelse nr. 923 af 28. september 2005. Miljøministeriet, København |
| MONSANTO CO; Toxicological Investigation of Dicyclohexylamine; 03/18/77; EPA Doc No. 88-920007594; Fiche No. OTS0545785 |
| Nicole Salzmann, Foredrag ”Environmental and Health Study, SBR Rubber Granulates”, ISA sport, 2006 |
| Nilsson, N.H, Afgivelse og sundhedsmæssig vurdering af PAH’er i bildæk, Miljøstyrelsen, Kortlægning nr. 54, 2005 |
| Rar dibutylftalat: European Union Risk Assessment report dibutyl ftalate, 2004 |
| Rar nonylphenol: European Union Risk Assessment report 4-nonylphenol (branched) and nonylphenol, 2002 |
| Rar phenol: European Union Risk Assessment report phenol, revised edition, 2006 |
| Robert Moretto, Environmental and health assessment of the use of elastomer granulates (virgin and from used tyres) as filling in third-generation artificial turf, EEDEMS (Ademe, Aliapur, Fieldturf Tarkett, 2007 |
| Rubber Technology, J.S. Dick, Hanser,2001, side 443 |
| Status for ftalater, 2003, Miljøministeriet |
| T. Källquist, NIVA Rapport LNR 5111-2005: Miljørisikovurdering av kunstgressystemer |
| Technical guidance document on risk assessment, European commission, 2003 |
| Thale S.W. Plesser, Ole J. Lund, Potensielle helse- og miljøeffekter tilknyttet kunstgressystemer-slutrapport, Byggforsk 10.09.04 |
| The Grass Yarn & Tufters Forum, 2006, Applied Mearket Information Ltd., Düsseldorf 11.-13. December 2006 |
| The Grass Yarn & Tufters Forum, 2006, Applied Mearket Information Ltd., Düsseldorf 11.-13. December 2006 |
| Torben V. Pedersen, Virklund Sport, telefoninterview februar 2007. |
| Tore Sanner, Artificial turf pitches- an assessment of the health risks for football players. Nasjonalt folkehelseinstitutt og Radiumhospitalet, Oslo, januar 2006 |
| TOXNET database : http://toxnet.nlm.nih.gov/ |
| U. Hofstra ,INTRON “Environmental and Health Risks of Rubber Infill, rubber crumb from car tyres as infill on artificial turf, 2007 |
| U. Hofstra, INTRON: Follow-Up Study of the Environmental Aspects of Rubber Infill, 31st March 2008 |
| Weast, R.C. (ed.). Handbook of Chemistry and Physics. 57th ed. Cleveland: CRC Press Inc., 1976 |
Footnotes
[1] It is not indicated whether the pitch is indoors or outdoors.
[2] It is not indicated whether the pitch is indoors or outdoors.
[3] Based on detailed information from one municipality, DBU’s figures in the maintenance guide must be expected to be somewhat unreliable in relation to the actual consumption of salt on some of the pitches.
Version 1.0 October 2008, © Danish Environmental Protection Agency