Environmental Project No. 1256, 2009
Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest
Contents
2 Biology and ecotoxicology of F. fimetaria and F. candida
- 2.1 Introduction to F. fimetaria and F. candida
- 2.2 Comparison of the two species
- 2.3 Genetic variability
- 2.4 Alternative Collembolan test species
- 2.5 Differences in susceptibility of the two species
- 2.6 Variability in Reproduction Rates
3 Testing results obtained at NERI, 1994 to 1999
- 4.1 Test guideline
- 4.2 Participants
- 4.3 Model chemicals
- 4.4 Range finding
- 4.5 Statistical analysis
- 4.6 Experimental design
- 4.7 Test conditions
- 4.8 Control mortality
- 4.9 Control reproduction
- 4.10 Variability of testing results
- 4.11 Conclusion
Annex 3: Bibliometric statistics
Annex 4: Intralaboratory variability
Annex 5: Control mortality and reproduction
Annex 6: OECD Guidelines for Testing Chemicals
1 Preface
Collembolans have been used for ecotoxicological testing for about 4 decades now but they have not yet had the privilege to enter into the OECD test guideline programme. Thus, as a proposal for OECD, two different collembolans, namely Folsomia fimetaria and Folsomia candida are introduced, and the results of a ringtest and a draft test guideline are presented. F. candida is already a well-established testing species and is extensively used for ecotoxicological testing as a representative for soil arthropods, with an ISO standard available since 1999. For F. fimetaria a testing protocol was published in 1998 as an outcome of DK-EPA and EU projects. International guidelines for chemical testing have occasionally included more than one species or included optional species that may be preferred for various reasons, such as representability and target habitat. In the case of alternatives to F. candida we include F. fimetaria due to its sexual mode of reproduction and worldwide distribution in natural and agricultural habitats in contrast to the asexually reproducing F. candida, which is not present in many types of natural and agricultural habitats. Furthermore, additional details needed to perform testing with F. fimetaria are provided.
With F. fimetaria as an optional testing species, the complete biology of the sexual reproduction, lacking for F. candida, will now be included as a potential target for any chemical being tested, including sex hormone disrupting chemicals. As stated in OECD Monograph No. 21 (OECD, 2002), progress in the field of endocrine disrupters is limited by the absence of chemicals accepted as suitable for use as references. Although this is the case too with F. fimetaria, its introduction as a test species nevertheless is needed as no other arthropods, i.e. F. candida, are suitable as a test species in this respect.
2 Biology and ecotoxicology of F. fimetaria and F. candida
- 2.1 Introduction to F. fimetaria and F. candida
- 2.2 Comparison of the two species
- 2.3 Genetic variability
- 2.4 Alternative Collembolan test species
- 2.5 Differences in susceptibility of the two species
- 2.6 Variability in Reproduction Rates
2.1 Introduction to F. fimetaria and F. candida
The use of F. candida and F. fimetaria for ecotoxicological testing purposes has been covered in various publications including: (Riepert and Kula, 1996; Wiles and Krogh, 1998; Fountain and Hopkin, 2005; Scott-Fordsmand and Krogh, 2005; Environment-Canada, 2007). As F. fimetaria is not yet included in internationally approved standards and is less studied than F. candida, it is briefly introduced here. A bibliographic search in Science Citation Index (ISI Web of Knowledge/Web of Science accessed Jan 2008) revealed about 400 papers referring to F. candida and 74 papers referring to F. fimetaria. Of the F. fimetaria papers, about 35 deal with ecotoxicology and some 27 originate from the NERI Soil Fauna laboratory or authors affiliated to this laboratory.
The selection of F. fimetaria for ecotoxicological testing was done by curator, senior researcher, Henning Petersen, Mols Laboratory, Natural History Museum, Aarhus Denmark, in a project supported by the Danish Environmental Protection Agency (DK-EPA) (Petersen and Gjelstrup, 1995), however it was used even earlier in studies to test for DDT effects (Van de Bund, 1965; Scopes and Lichtenstein, 1967). Scopes and Lichtenstein even published a filter paper method on how to use F. fimetaria for general insecticide residue testing (Scopes and Lichtenstein, 1967). Adults of F. fimetaria are 0.8-1.4 mm long (Folker-Hansen et al., 1996), e.g. males 0.9 mm and females 1.3. mm, with a dry weight of 10-40 mg per individual at 20o C. Female F. candida can become 2.0-2.5 mm long (Crouau and Moia, 2006; Widarto et al., 2007), and has a dry weight of 140 mg for adults at the asymptotic maximum size. Adult F. candida males although rarely found are about 1.25 mm long. F. fimetaria reproduces only sexually, and sexual dimorphism is not detectable at low magnification before an age of 20 days after hatching. Males have a more slender body, and they are only half as big as the females (Fig. 2).
Both species are widely distributed (Fig. 1), but maps created particularly from older records cannot be fully trusted due to confusion of the two species (Hopkin, 2008a). F. fimetaria is common in a range of habitats including agricultural soil, and its preference for high organic matter hot spots seems similar to F. candida (Fjellberg, 1980). It occurs less frequently in meadows and in the soils of urban settlements (Chernova et al., 2003). The easiest way to get F. fimetaria is to collect soil samples from agricultural fields, meadows or grassland and make a heat/dry extraction of the soil. In buried lumps of organic hotspots like manure or sludge F. fimetaria can be found in huge numbers (Krogh et al., 1997), and the collection of the lumps is a good source for starting a F. fimetaria culture.
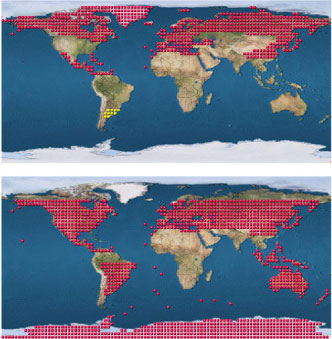
Fig. 1. Biogeographically distribution of F. fimetaria, upper, and F. candida, lower, (Bellinger et al., 1996-2008)[1]. The dotted areas indicate that the species have been found in the corresponding biogeographically region.
F. candida is a cosmopolitan species found almost all over the globe (Fig. 1) and is considered a tramp species (Hopkin, 1997). However, only few outdoor records exists for F. candida who prefers high organic matter like in compost, green-houses, flower pots or manure (Fjellberg, 1980; Chernova et al., 2003; Fjellberg, 2007a), hence the records used to generate the maps of Fig. 1 refers mainly to these domestic habitats. In line with this it is rare in Australian soils (Greenslade and Vaughan, 2003). However, it should be noted that the lack of presence of a standard test species in certain parts of the world may not at all invalidate its general use; it may well have a similar response as other collembolans under the simplified artificial conditions offered in a standard test (see section 2.5).
Discrimination of F. candida and F. fimetaria from species of the same genus is not problematic with the unique position of manubrial setae and other characteristics (Fjellberg, 1980; Potapov, 2000; Potapov and Babenko, 2000; Fjellberg, 2007b).
However, when establishing cultures from field populations, care should be taken to avoid confusion between white and eyeless relatives from the F. fimetaria group such as Folsomia lawrencei, Folsomia kerni and Folsomia litsteri. Using recent keys, e.g. Fjellberg (2007b), should prevent such mistakes. Small F. litsteri was considered to be juvenile F. candida and bigger F. litsteri to be F. lawrencei (Josef Rusek pers. comm.), but later Steve Hopkin considered F. litsteri to be a true species (Hopkin, 2008b) and this is maintained by Fjellberg (2007b).

Fig. 2. Adult F. fimetaria female and male, left, and F. candida female and male, right.
2.2 Comparison of the two species
While the size difference is very obvious for the two species behavioural differences have also been observed, but have rarely been explored scientifically (Chernova et al., 2003). The collembolan family, Sminthuridae, has long been known to display relatively complex mating behaviour (Schaller, 1952), and similarly, the podurids have a sperm transfer requiring male-female interactions as otherwise believed to be non-interactive for the arthropleone collembolans (Schliwa and Schaller, 1963). Although not yet reported for Folsomia the observations by Goloschapova et al. (2006) indicate that isotomids may have more complex mating behaviour than usually assumed.
When being disturbed F. fimetaria will respond by bending down the head and retracting the antenna downwards and inwards to the head, in contrast F. candida will start scattering and jumping. Only few studies have made direct comparisons between the basic biological properties of these two species, however aspects such as fecundity and preference responses to a range of fungi have been demonstrated to be significantly different (Larsen et al., 2008).
At 20°C, the average duration of the five juvenile instars are 3 days for F. candida (Snider, 1973) and maximum 4 days for F. fimetaria (Jensen et al., 2001). Sexual maturity is attained in the 6th instar occurring around age 15-16 days for F. candida and a few days later for F. fimetaria[2] (Snider, 1973; Holmstrup and Krogh, 1996; Widarto et al., 2007).
It is generally assumed that sexually reproducing collembolans need fertilisation for every reproductive instar (Hopkin, 1997). To substantiate this hypothesis specifically reported for only a few non-isotomid species, 24 couples of 25-28 days old, 8th instar, F. fimetaria males and females, and 24 single females were isolated and the oviposition pattern of reproduction was followed for 3 weeks at 20o C (Krogh, 2006). None of the single females produced any eggs and the couples produced averages of 10 and 30 eggs in instars 8 and 10, respectively, with a maximum clutch size of 60 eggs. The same figures for F. candida were 48 and 71 eggs with a maximum clutch of eggs of 114 (Snider, 1973). Egg development for F. fimetaria took 9.5 days, hence similar to 9-11 days observed for F. candida (Snider, 1973). The time between the 8th and 10th reproductive instars were 7 days, with 9 days between the 10th to 12th instars; 1-2 days shorter then the same instars for F. candida. The infertility of isolated females stresses that even if females are coming from a mixed male-female population, as is the case for the reproductive test, this does not enable a female to produce fertile eggs, so the uptake of spermatophores is crucial just at oviposition time shortly after shedding the cuticle.
One of the most interesting differences between the two collembolans is the intracellularly presence of Wolbachia bacteria in F. candida and the absence of it in F. fimetaria[3]. F. candida has always been reported to reproduce parthenogenetically in laboratory cultures and the presence of males in laboratory cultures has never been reported in the literature, since early studies by Goto (1960), Milne (1960), Marshall & Kevan (1962) and Green (1964). Presence of intracellular bacteria in F. candida ovaries has been known since the study by Palévody (1972), and Vandekerckhove et al. (1999) demonstrated the presence of Wolbachia in F. candida ovary cells, fat bodies and institial cells. However, the exact mechanism by which Wolbachia operates in F. candida has not yet been resolved and neither is it yet established if Wolbachia indeed is the reason for parthenogenesis in F. candida (Riparbelli et al., 2006), although it seems plausible (Koivisto and Braig, 2003). When males and females have been found in field populations the population are supposed to reproduce sexually, however as sex rarely has been determined in specimens from field samples, it has never been realized whether naturally occurring F. candida populations reproduce sexually or could have a very low rate of male production. Elin Jørgensen, environmental technician at NERI, discovered few F. candida males in our laboratory cultures in 1993. At that time it was not clear if these males actively took part in sexual reproduction and if a sexually reproducing F. candida population could emerge with these males. A second question arising from the presence of Wolbachia in F. candida was whether the rate of males would change during the life-time of female F. candida. We now know that the males, when reared with females in 10:10 proportion, do not seem to enable establishment of a sexual population with a normal ratio of males and females. Our observations indicate that only about 1 male is produced per 10,000 female offspring during the 8th and 10th reproductive instars, however for older F. candida females, it increases to one for every thousand juveniles.
2.3 Genetic variability
When investigating genetic differences, low variability was found in laboratory populations of F. candida compared to F. fimetaria (Simonsen and Christensen, 2001). Low genetic variability is considered a benefit for a standard test species because it may decrease variability of survival and reproduction between individuals as well as response to toxicants. The variability between clones has been demonstrated to convey minor differences in responses to chemicals and for some chemicals, no differences in sensitivity could be detected at all (Crommentuijn et al., 1995; Chenon et al., 2000). Genetic variability of F. fimetaria has not yet been investigated. To ensure that the species used for testing is well characterised, species cultures would have to be delivered by laboratories with a quality assurance system, such as GLP, who can certify the genetic strain and clone variability.
2.4 Alternative Collembolan test species
Several authors have suggested alternative collembolan species to be used for testing standards because F. candida has limited ecological relevance due to its absence from many natural or agricultural habitats. This has led to suggestions of such species as Paronychiurus kimi (Son et al., 2007), Sinella communis and Proisotoma minuta (Greenslade and Vaughan, 2003) as appropriate test species. Other collembolan species could be selected for testing such as e.g. Isotoma viridis (Wiles and Krogh, 1998), Isotoma anglicana, Orchesella cincta, Sinella curviseta, Orthonychiurus folsomi (Environment-Canada, 2007), and Mesaphorura macrochaeta. The result of a bibliographic search of papers referring to single collembolan species is presented in Annex 3 to give an indication of the present level of scientific knowledge. A number of prerequisites must be fulfilled in advance before using alternative species:
- an unequivocal identification
- a sound rationale for the selection of the species
- ensuring that the reproductive biology is included in the testing phase so it will be a potential target during the exposure
- life-history must be known: age at maturation, duration of egg development and instars subject to exposure
- optimal growth and reproduction conditions are provided with the test substrate and food supply
- variability is sufficiently low for precise and accurate toxicity estimation.
The choice of F. fimetaria as a test species was supported in an evaluation based on practical arguments, acceptability of tests and ecological significance (Van Gestel, 1998).
2.5 Differences in susceptibility of the two species
While Krogh (1995) reported no crucial differences between F. fimetaria and F. candida, Diao et al. (2007) found a difference which proved to be significant for mortality. Pedersen et al. (2000) found that male F. fimetaria differed from females in their copper body burden but reported no statistically significant differences between the growth and reproduction endpoints for the two species.
2.6 Variability in Reproduction Rates
Variability of F. candida reproduction is obvious from different scientific publications. Van Amelsvoort and Usher (1989) observed probably the lowest reproduction rate of F. candida fed Baker’s yeast, with the population already declining after the first clutch appeared; this was in remarkable contrast to the classical findings by Snider (1973)[4] where F. candida produced eggs throughout its lifetime. According to her findings, 10 F. candida females would on the average produce 628 juveniles on plaster-charcoal during the first two reproductive instars, instar 6 and 8; this is probably possible in soil as well. However, if the eggs of the third clutch produced by instar 10, hatched before the 4 week test duration of the F. candida test, a mean of 1342 juveniles would be produced per replicate. This would require that the duration of instars and egg development are faster than the average. For F. fimetaria, which would produce 400 juveniles during the 3 week test suggested here (section 2.2), the variability may be due to similar changes in timing and instar duration. Attempts to clarify the sources of variability was done by Axelsen et al. (1998) in a modelling exercise. They found that a precise sexual differentiation, when individuals for testing are selected from a synchronous culture of F. fimetaria, was important for variability. Crouau and Cazes (2003) demonstrated that the individual age and test duration was important for F. candida testing when performed according to ISO 11267 (ISO, 1999).
[1] Maps reproduced with permission from the authors
[2] Life history data on F. fimetaria are not yet precise enough to give accurate figures.
[3] We have made an analysis of Wolbachia in F. candida and F. fimetaria (Krogh et al in prep.)
[4] This observation led to the conclusion that yeast would affect the life history tactics by F. candida.
3 Testing results obtained at NERI, 1994 to 1999
3.1 Introduction
Since 1992, plenty of toxicity tests and experiments have been conducted with F. fimetaria at Department of Terrestrial Ecology, Danish National Environmental Research Institute (NERI). This section describes a compilation of a subset of these tests to illustrate the intra-laboratory variability of an experienced testing facility. The data has previously been reported to Environment Canada (Krogh, 2004).
The database included the control reproduction observed in 57 tests with F. fimetaria (Annex 4). The procedure followed was a standard test guideline in effect at the laboratory since 1994 (Krogh, 1995; Wiles and Krogh, 1998). The tests were performed during a period of 6 years, potentially representing variability of culture health and performance properties. Different soil types ranging from sandy soils to clay soils were used in the tests.
3.2 Performance
The mean survival of initially 20 adult F. fimetaria, 10 females and 10 males, and their reproduction in the 3 week standard tests were: 17.7 [17.2-18.2] and 430.8 [405-457], respectively; for frequency distributions see Fig. 3; the reproduction was normally distributed, P>15% (Kolmogorov’s D statistic). 5% of the tests would have a reproduction £233 according to the normally distributed reproduction. The average CV was 18.1 [15.4-21.2][5] None of the tests had a mean reproduction less than 100, but 7% had a coefficient of variation CV>30% (Annex 4), which is the validity criteria of the ISO F. candida test (ISO, 1999). Two of the CV’s qualified as outliers, according to the validity criterion and 14% of the tests had an average adult mortality >20%, the validity criteria, and 2% a mortality >30%.
F. fimetaria performed generally well in all soils tested, so when reduced performance was observed this might be the result of other factors, such as health condition, seasonality or feeding condition.
3.3 Influence of soil type
Linking soil properties to collembolan performance, i.e. survival and reproduction, must be done with caution, due to the fact that the NERI data does not originate from experimentally designed studies with soil factors applied as treatments, but from independently assessments of the performance. Thus, the level of performance in the tests may have been caused by the actual condition of the test animals. Uncontrolled microbial factors differing from test to test may exert an influence on performance too.
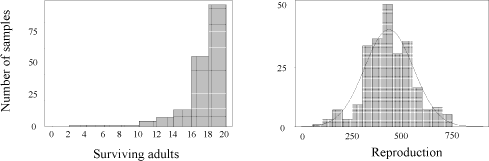
Fig. 3. Frequency distributions of surviving F. fimetaria adults of the initial 20 males and females and their reproduction for each replicate sample analysed, n=243, the normal distribution with mean 433.7 and variance 14,924 is included on the reproduction graph.
To explore the relationships between the two performance measurements and soil characteristics, the correlations are given in Table 1. Adult survival was not correlated with soil constituents but reproductive output, in terms of number of juveniles, was significantly positively correlated with clay and silt but was negatively correlated with sand content of the soil.
3.4 Conclusion
The soil particle fractions clay and silt was positively correlated with the reproduction, while sand was negatively correlated with the reproduction. The performance of F. fimetaria was generally good for all soil types tested, with only less than an average of 200 juveniles per replicate in a control series observed in 2 tests. Survival was on the average 88.5% and was not affected by the soil types. Test performance was equivalent to the requirements for the F. candida test and therefore supports the same validity criteria as stated in ISO 11267: a mean maximum adult mortality of 20%, a mean minimum reproductive output of 100 juveniles in the controls with a maximum coefficient of variation (CV) of 30%.
The tests with F. fimetaria met the validity criteria as defined in the ISO 11267 standard (ISO, 1999) of at least 100 juveniles in the controls and in 91% of the tests the CV<30%
Table 1 Pearson correlation coefficients and the significance of the correlations between the soil characteristics and performance data of F. fimetaria from standard tests. Number of observations, n=243.
| Correlation, r |
Correlation strength, r² |
Significance | ||
| Clay | Organic matter | 7.5% | 0.6% | 24% |
| Silt | Organic matter | 14% | 1.9% | 3% |
| Silt | Clay | 91% | 83% | <0.01% |
| Sand | Organic matter | -22% | 5.0% | 0% |
| Sand | Clay | -95% | 90% | <0.01% |
| Sand | Silt | -99% | 97% | <0.01% |
| Adult | Organic matter | 1.5% | 0.0% | 92% |
| Adult | Clay | 4.2% | 0.2% | 66% |
| Adult | Silt | 4.0% | 0.2% | 65% |
| Adult | Sand | -3.5% | 0.1% | 71% |
| Juveniles | Organic matter | -1.0% | 0.0% | 87% |
| Juveniles | Clay | 17% | 2.9% | 0.75% |
| Juveniles | Silt | 22% | 4.6% | 0.07% |
| Juveniles | Sand | -21% | 4.3% | 0.1% |
| Juveniles | Adult | 34% | 11% | <0.01% |
[5] Confidence intervals of the log-normal distribution: ![]() (Olsson, 2005) obtained by back-transformation.
(Olsson, 2005) obtained by back-transformation.
4 Ringtest results
- 4.1 Test guideline
- 4.2 Participants
- 4.3 Model chemicals
- 4.4 Range finding
- 4.5 Statistical analysis
- 4.6 Experimental design
- 4.7 Test conditions
- 4.8 Control mortality
- 4.9 Control reproduction
- 4.10 Variability of testing results
- 4.11 Conclusion
4.1 Test guideline
A draft OECD test guideline was developed in the prevalidation phase of this project (OECD, 2006b) by Scott-Fordsmand and Krogh (2005) and was changed according to input from ringtest participants and further refined during the final reporting phase (Annex 6). Existing OECD guidelines were used as templates to ensure consistency and to ensure that the content was sufficient to perform the test.
F. candida and F. fimetaria is reared in lab cultures in closed containers with a bottom layer of a mixture of plaster of Paris and activated charcoal in a ratio of 9:1 by weight. The charcoal absorbs waste products that may be harmful to the optimal productivity of the cultures. The black colour of the bottom layer eases the visibility of the white collembolans. Wholes and furrows in the plaster may help stimulating oviposition (Fountain and Hopkin, 2005), although this was not needed for our cultures to thrive. The substrate is kept moist but not waterlogged to ensure saturated air humidity. The collembolans are watered and fed granulated dry Baker’s yeast weekly; during this operation they are aerated.
Breeding of synchronous cultures is induced by transferring adults to fresh containers and collecting the eggs after three days, recommendable over a week-end. Alternatively the adults may be removed from the substrate and the eggs left behind. In the first case eggs are collected, in the second case adults are removed. After approx. 10 days the eggs hatch and at the age of 9-12 days the juveniles of F. candida or the 23-26 days old adults of F. fimetaria are ready for testing. Allowing for an age range span of 3 days in the test has important practical consequences, as it now provides for a working schedule that does not involve working with the test during the weekend spanning over 3 days.
The test exposes the collembolans to chemicals through the test soil, which is the artificial OECD soil based on a recipe originating from the earthworm acute test (OECD, 1984). On the day of preparing the mixture of moist soil and chemical collembolans, 10 F. candida or 10 male and 10 female F. fimetaria are added.
Test and breeding conditions are 20 oC and a light:dark cycle of 12:12 hours and light intensity of 400–800 lux.
At test termination after 3 weeks for F. fimetaria and 4 weeks for F. candida the collembolans are removed from the soil by flotation or heat extraction. While flotation immediately terminates the test, heat extraction runs for 2 days where the collembolans actively have to move out of the soil.
The practicability of performing the tests with the two species is identical with the exception of the need to discriminate the F. fimetaria males from females.
4.2 Participants
Participants spanned a broad range of laboratories from highly experienced professional contract laboratories to research laboratories at universities. This has aided in exposing the guideline procedure to diverse situations exposing weak or yet unresolved issues even for the existing ISO standard test for F. candida. A list of the 14 participating laboratories is presented in Annex 1; they have been given a code to enable linking the data to a certain laboratory in Annex 2. A total of 51 tests were performed in the ringtest exercise (Table 4).
4.3 Model chemicals
The three model compounds chosen for the ringtest are evaluated for use as positive controls and reference chemicals for the guideline. Boric acid is the preferred candidate because it is easily accessible, while dimethoate is less accessible and the commercial production may cease, and CuCl2 is more difficult to handle in the test due to the need to compensate for a pH effect changing with the CuCl2 concentration. While boric acid and copper chloride are generally available, dimethoate was kindly delivered to the participants from Cheminova.
The model chemicals boric (H3BO4), copper chloride (CuCl2), and the insecticide dimethoate, were chosen to cover 3 different modes of action, i.e. effects caused by: acidity, heavy metal inhibition of fecundity and inhibition of choline esterase. The benefit of boric acid is its accessibility and it has been suggested as a positive control for tests with plants, mites and collembolans (Environment-Canada, 2005b, 2007; OECD, 2007). Boric acid was applied in the concentrations corresponding to 0, 25, 50, 100, 200, 400, 800 mg kg-1; anhydrous copper chloride in the concentrations: 0, 200, 400, 800, 1200, 1600, 2000; and dimethoate in the concentrations 0, 0.25, 0.5, 1, 2, 3, 4 mg kg-1.
4.4 Range finding
In many cases range-finding tests were not performed or did not contribute to an appropriate final concentration series. A general problem of range-finding is that it is usually performed as a lethal test, but is used to guide the selection of concentrations for reproduction tests. Obviously this would give faulty guidance for chemicals with sublethal effects.
4.5 Statistical analysis
Statistical analyses for the estimation of control mortality and reproduction and concentrations causing a decrease of 10% and 50% in reproduction or survival (i.e., LC10, LC50, EC50 and EC50) and their 95% confidence limits were performed using SAS/STAT® version 9.1.3 procedures NLIN and NLMIXED (SAS-Institute-Inc., 2004b). Non-linear modelling was used to estimate concentration-response relationships by fitting the binomially distributed mortality data to the mortality rate (m) formula (probit analysis):
m = c + (1 - c) Φ (a+bd)
where c is control mortality rate, Φ (phi) is the cumulative normal probability function, a slides the curve along the x-axis, b determines the slope, and d is the mg kg-1 concentration of the testing compound in soil. Other models were employed when it was more appropriate to fit the actual mortality data: asymptotic growth, c+(1-c)(1-ead), and exponential growth, c×ead. The reproduction data was fit to the sigmoid model:
![]()
and to exponential decay, c×ead, and a convex decrease, (k/(1-b))×(1-b×e(ad)).
Often a concentration-response curve does not contain sufficient information to estimate parameters for a non-linear curve such as the logistic or exponential because the curve is simply linear, the variability is too high[6], or the fitting procedure cannot attain reasonable parameters, i.e., it cannot converge. In such cases, there still may be a clear and significant decrease of the response with increasing concentration, and therefore, a linear section of the data can be selected by choosing a lower and an upper concentration limit within the decreasing section. Responses outside and on these borders were then added together and a new linear dataset created containing the sum of data for the upper and lower limit and the original data between these concentrations.
95% confidence limits are written in brackets [ ] throughout. Tests for normality were performed with the distribution analysis tool of SAS/INSIGHT (SAS-Institute-Inc., 2004a). The Coefficient of Variation (CV) is calculated as ![]() .
.
4.6 Experimental design
A spacing factor of 1.8 has been recommended for other tests such as the H. aculeifer and the enchytraeid test (OECD, 2004a, 2007), while the guideline on plant growth states that “the number and spacing of the concentrations or rates should be sufficient to generate a reliable concentration-response relationship and regression equation and give an estimate of the ECx or ERx.” (OECD, 2006c). The ringtest does not per se support the spacing factor approach as an inspection of the concentration-response figures reveal (Fig. 4 to Fig. 7). As the purpose of using the spacing factor is to evenly cover the whole response curve, the actual result of the factor is to lump together many low concentrations at the expense of covering the higher concentrations.
4.7 Test conditions
The draft guideline (Annex 6), prescribes a soil humidity content of approximately 50% of the soil’s WHC, but it should be ensured that the soil will maintain a crumbled structure. Hence, the water content is not regulated according to the usual 50% of the WHC. Generally the loss of water is controlled during the test and should not impose any stress on the collembolans.
4.8 Control mortality
The highest mortality was observed in tests with F. fimetaria (Table 2).For F. candida control mortality was less than 20% for 79% of the tests and F. fimetaria had a mortality of less than 20% for 44% of the tests (Table 2). These proportions were significantly different. The failure of some tests to meet the mortality validity criterion is indeed expected to happen even for highly experienced laboratories but at a much lower rate as observed here for F. fimetaria.
Table 2 Summary of control performance evaluation criteria for the two collembolan tests for all tests, including tests not fulfilling the validity criteria, and detection of number of outliers for the boric acid tests. Percentages are the % of tests fulfilling the criteria. CV: Coefficient of Variation for the reproduction. Juv.: Reproductive output of the test in number of juveniles. Raw data presented in Annex 4.
| F. fimetaria | F. candida | |
| Mean reproduction | 132 [67-197] | 399 [310-488] |
| Mean mortality | 35% [22-48] | 14% [8.7-20] |
| Mean control mortality <20% | 44% | 79% |
| Mean control reproduction > 100 juv. | 50% | 97% |
| CV < 30% | 44% | 76% |
| Mean CV | 59.5 | 25.5 |
| Mean CV when reproduction>100 juv. | 28.8 | 24.8 |
| Outliers: Inter-laboratory variability of LC50, h (P<1%) | 0 | 1 |
| Outliers: Inter-laboratory variability of EC50, h (P<1%) | 0 | 0 |
4.9 Control reproduction
The validity criterion for the F. candida, F. fimetaria and O. folsomi control reproduction is an average minimum of 100 juveniles (ISO, 1999; Environment-Canada, 2007). The coefficient of variability (CV) of the reproduction has been set to a maximum of 30% (ISO, 1999), identical to the earthworm and draft mite reproduction tests (ISO, 1998; OECD, 2004b, 2007). For the ringtest, it was suggested to adopt the validity criteria of 100 juveniles and a CV of <30% for both species; therefore, these values are used for the evaluation of the ringtest results (Table 2). For comparison it should be noted that experience from the ringtest paving the way for the F. candida ISO 11267 standard has shown that variability in terms of the CV was greater than 30% for 30% of the tests (BBA, 1995) and 10% of the tests had a mean number of juveniles in the controls less than 100. Intrinsically F. candida has a reproduction rate twice the reproduction rate of F. fimetaria.
One of the F. candida tests had a reproductive output below the suggested validity criteria of 100 juveniles and 24% produced less than 200 juveniles. F. fimetaria produced less than 100 juveniles in 43% of the tests. The mean reproductive CV for F. fimetaria was significantly larger than the CV for F. candida (ANOVA F-test P<0.1%) (Table 2). But when excluding the data sets not meeting the mean minimum 100 juvenile reproduction criterion, the mean CV of the F. candida control reproduction was 23.6 [19-28] (n=33) and 28.7 [17-40] (n=8) for F. fimetaria, which did not differ significantly from each other (one-way ANOVA, P>5%) for the mean or the variance. Thus, this demonstrates that if a sufficient reproduction is obtained, a F. fimetaria test would have a normally accepted CV. In other words it can be concluded that the precision of the control reproduction is potentially identical for the two species. The reproduction was particularly high in three F. candida tests (ref. no. 4, 48, 49), and this may be explained by the appearance of a third clutch (see section 2.6).
4.10 Variability of testing results
The inter-laboratory variability is evaluated by calculating h, the standardized difference of a toxicity test result observed for one laboratory from the mean toxicity values as given in Table 2 (Weyers et al., 2002). The test statistic (x-m)/STD is t-distributed and if x, the individual toxicity estimate from one laboratory, deviates considerably from the mean, it is considered an outlier. The criterion for outliers consists of toxicity estimates that differ from the mean at the 1% level of significance (Weyers et al., 2002). For mortality, only the LC50 of 815 mg kg-1 for boric acid (ref. no. 43) qualified as an outlier, which was the outcome of an otherwise fully valid F. candida test. For the boric acid reproduction tests, none of the EC50 values were detected as outliers.
Graphical presentations of the EC50’s and the LC50’s are presented in Fig. 8 and Fig. 9 for all three testing compounds. However, as boric acid testing results were most numerous only those have been used for evaluation of the endpoint variability.
Boric acid has a pronounced sublethal effect for both species (Table 3). The variances of the two identical mean EC50‘s for F. candida and F. fimetaria with boric acid were not significantly different (P>10%) and both proved to conform to a normal distribution (P>15% for Kolmogorov’s D). The precision of the EC50 estimates in terms of width of the 95% confidence limits (Table 4), were roughly spread up to ±50% around the EC50 for both species, and they were statistically identical. This precision depends on a proper model choice and it would not reflect the true precision if the model has a poor fit.
Table 3 Mean LC50 and EC50 for the two species and the three model compounds. Numbers in brackets: 95% confidence limits.
| Species | Compound | N | LC50 | EC50 | ||
| F. candida | Boric acid | 16 | 259 | [154-364] | 90.8 | [61.8-120] |
| Copper | 11 | 1541 | [442-2639] | 1251 | [423-2080] | |
| Dimethoate | 8 | 2.1 | [0.74-3.4] | 1.65 | [0.4-2.9] | |
| F. fimetaria | Boric acid | 9 | 560 | [271-849] | 107 | [67.9-146] |
| Copper | 2 | 1260 | [-3551-6070] | |||
| Dimethoate | 3 | 1.0 | [-1.7-3.7] | 0.81 | [-1.9-3.5] |
The LC50 for F. fimetaria were significantly higher than the LC50 of F. candida (ANOVA, F-test) (Table 3), but the apparent higher variability of F. fimetaria LC50’ies is related to the mean and vanishes by transformation to obtain variance-homogeneity. The precision in terms of the width of the 95% confidence limits of the LC50-estimates were seemingly better for F. candida ranging up to ±50% of the LC50, but the wider range of F. fimetaria, ±100%, did not differ significantly (ANOVA, P>5%) (Table 4). When excluding the two F. fimetaria boric acid tests with a control mortality >50%, the LC50‘ies of F. fimetaria tests still varied within a factor of 2.6 (n=7, CV=38%) and the F. candida tests varied within a factor of 3.1 (n=16, CV=70%) in relation to the mean LC50. This alternative to the outlier analysis way of describing variability (Table 2) gives the same result, and it is concluded that variability of the LC50 and EC50 toxicity outcome does not differ for the two species.
4.11 Conclusion
The reliability and performance of the test with the new standard species F. fimetaria were assessed by comparing its performance with the F. candida test, which then acted as a reference test method currently accepted by regulatory agencies, while still being a candidate species of the new draft guideline. The range of criteria used for this assessment was largely fulfilled, but the control reproduction and survival performed badly in some tests. In spite of this the toxicity was accurately and precisely estimated, in particular when invalid test results were omitted.
Table 4 Control mortality and reproduction and toxicity endpoints of the ringtest in terms of LC10, LC50, EC10 and EC50 estimated from the complete concentration-response data of each test. Missing cells is due to no effects detected or 50% effect levels outside the concentration range. C.V.: Coefficicient of variability of the control reproduction.
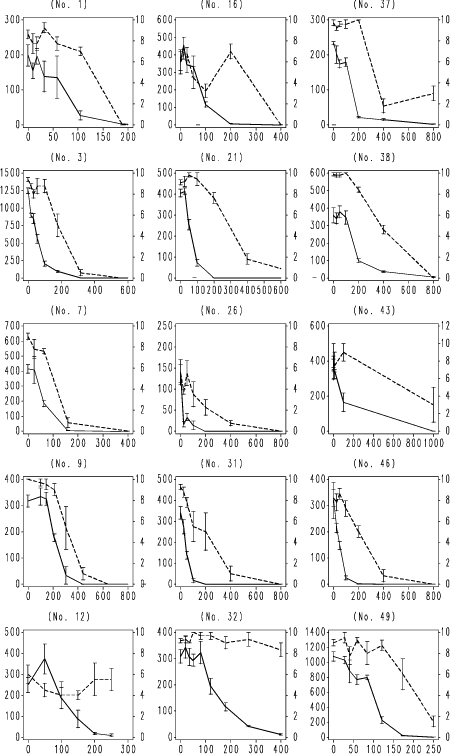
Fig. 4. F. candida testing results with boric acid. Horizontal axis: Nominal concentration of boric acid, mg kg-1 soil; Left vertical axis: number of juveniles produced per replicate; Right vertical axis surviving adults per replicate. Vertical bars: standard error of the mean. Numbers in brackets: ringtest ref. no. used to anonymize the laboratory. Broken line: adult survival per replicate; unbroken line reproduction, number of juveniles per replicate produced by initially 10 adults.
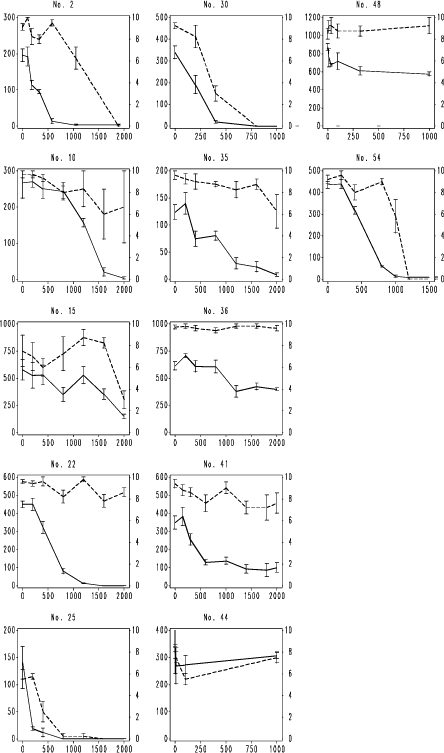
Fig. 5. F. candida testing results with nominal CuCl2 concentration. Legend as Fig. 4.
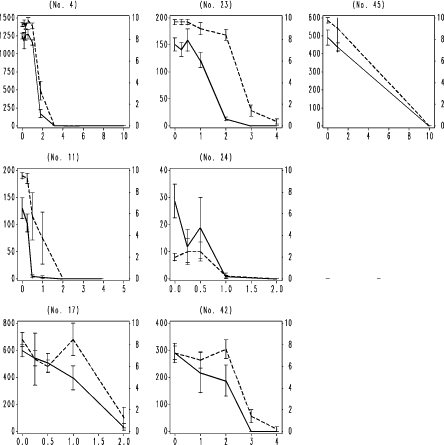
Fig. 6. F. candida testing results with nominal dimethoate concentration. Legend as Fig. 4
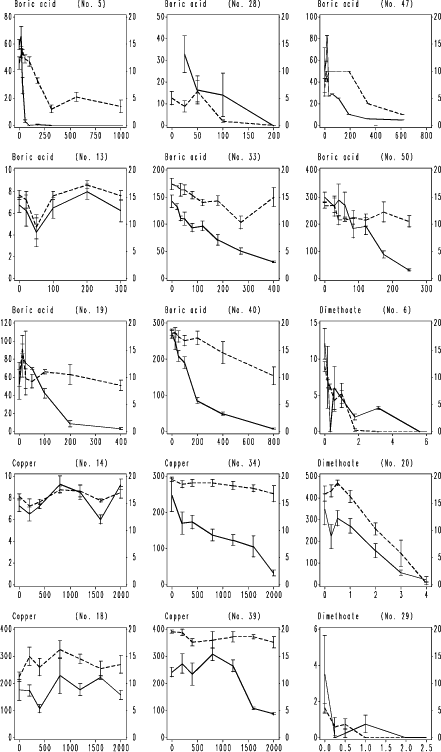
Fig. 7. F. fimetaria testing results with the three model compounds. Legend as Fig. 4, except unbroken line is the reproduction of juveniles per replicate produced by initially 20 adults.
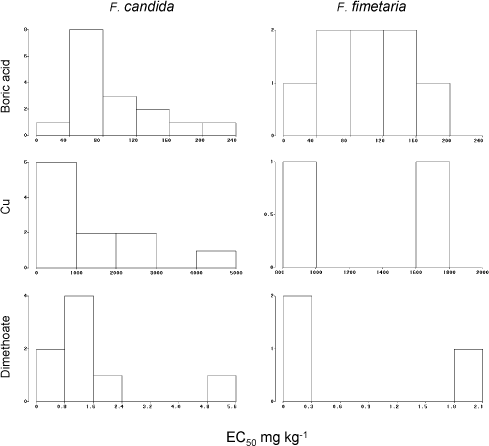
Fig. 8. Frequency distribution of chronic reproduction EC50‘ies from the ringtest. Y-axis number of occurrences of EC50.
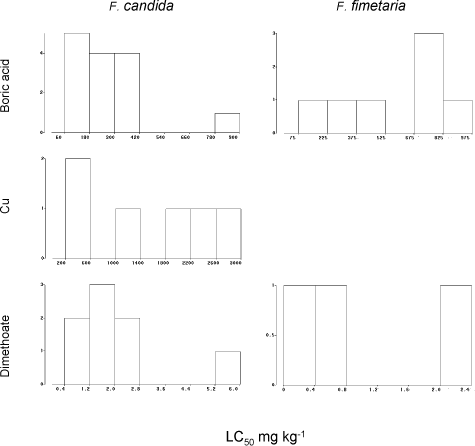
Fig. 9. Frequency distribution of chronic LC50‘ies from the ringtest. Y-axis number of occurrences of LC50.
[6] Presently no OECD guideline has validity criteria for the power of a test and the confidence limits of ECX-estimates, and high variability will lead to lower power and undesirable wide confidence intervals. A maximum of 50% width of the confidence interval would be a reasonable validity criterion. To implement such a criterion guidance should be followed concerning modelling as provided by Environment Canada and OECD (Environment-Canada, 2005a; OECD, 2006a).
5 Summary and conclusions
The collembolans Folsomia fimetaria L. and F. candida Willem are proposed for inclusion in the OECD guidelines for testing of chemicals programme. The present ISO guideline 11267 for the collembolan F. candida has been applied successfully for toxicity testing in soil since its release, but due to the restricted parthenogenetic reproductive biology of F. candida, a collembolan species with a sexual mode of reproduction is needed. The relative F. fimetaria reproduces sexually and fulfils basic requirements for performance and feasibility of laboratory test animals. Each female reproductive instar of F. fimetaria requires the presence of males, of which three instars are usually completed during the standard reproductive tests with F. candida and F. fimetaria.
Extensive experience of the ringtest coordinating laboratory with the F. fimetaria test demonstrates intra-laboratory repeatability during the years 1994 to 1999. Fifty-seven control reproduction data sets tests with F. fimetaria all fulfilled the validity criterion for reproduction while the survival and the CV criteria were not met in 14% and 7% of the tests, respectively.
To provide sufficient information for the adoption of these collembolans for the OECD test guideline programme an international ringtest was initiated in 2005. For both species, test validity is achieved with a mean maximum adult mortality of 20%, a mean minimum reproductive output of 100 juveniles in the controls with a maximum coefficient of variation (CV) of 30%. Due to less experience of the participants in performing the F. fimetaria test, the control reproduction validity criteria were not met in half of the test, while the F. candida test generally was successful.
As the proposed test for F. fimetaria is mechanistically and functionally similar to the previously validated ISO F. candida test method with established performance criteria, the reliabilities of the test methods were compared and the overall test performance was evaluated against a range of criteria: control survival and reproduction and their variability, variability of toxicity endpoints for the model chemicals, the precision of the LC50 and EC50 and intra- and interlaboratory variability. Most data was produced for boric acid, so it was selected for this analysis. Survival was successful for 44% of the F. fimetaria tests and 79% of the F. candida tests. F. fimetaria tests with a valid reproduction had a CV similar to F. candida. A mean control reproduction of 130 and 400 for F. fimetaria and F. candida, respectively, resulted in 43% F. fimetaria tests not meeting the validity criteria, while the F. candida tests were practically all valid. The precision of the EC50 and LC50 estimates were identical for the two species. Stable EC50 and LC50 estimates with low inter-laboratory differences were produced by both tests with no outliers, except for one F. candida LC50 figure, and identical mean and variance for EC50 values with boric acid. Thus, based on the overall inter- and intralaboratory validation results the following validity criteria are proposed for both species in the draft test guideline and should be met in the untreated controls for a test to be considered valid:
- Adult mortality should not exceed a mean of 20 % at the end of the tests
- An average minimum of 100 juveniles per vessel should be produced during the test
- The coefficient of variation of the number of the juveniles per vessel should be less than 30%
- The reference compound, boric acid, should cause a 50% decrease in reproduction at 100 mg kg-1 in an OECD artificial soil substrate with 5% organic matter.
The proposed draft guideline includes comprehensive information and details to successfully perform toxicity testing with either of the two collembolan species. It is especially recommended to employ F. fimetaria for chemicals that are suspected to interfere with any parts of the reproductive biology of sexually reproducing species, while F. candida may be used more generally to assess less specific toxicity.
6 Acknowledgements
The ringtest participants are truly acknowledged for contributing their results for free and Cheminova for providing dimethoate. Janeck Scott-Fordsmand initiated the introduction of springtails into the OECD TG programme and OECD national coordinator Henrik Tyle, DK-EPA, proposed the project for an OECD TG. The technical staff at the Soil Fauna laboratory, Karen Kjær Jacobsen, Zdenek Gavor and Elin Jørgensen performed most of the reported tests from NERI, I am grateful for their careful work with the tests and their culture handling since the very first test were done in 1991. Dr. Esko Martikainen, Finland, is acknowledged for his improvement of the F. fimetaria test protocol during his stay as a visiting scientist in 1994. The Danish EPA, Ministry of the Environment, and NordUTTE provided the financial support. Hans Løkke introduced collembolan laboratory testing to Danish ecotoxicology. Dr. Rick Scroggins, Environment Canada, is acknowledged for providing support for a previous review on F. fimetaria performance. Henrik Tyle, DK-EPA, and Jukka Ahtiainen, SYKE, provided valuable comments to the report.
References
Amundsen, C.E., Andersen, S., Nordal, O., Linjordet, R., Hartnik, T., Warner, B., Krogh, P.H., 1999. Forurensninger i veistøv. Miljøgifter og biologiske virkninger. Jordforsk, Senter for jordfaglig miljøforskning.
Axelsen, J.A., Holmstrup, M., Krogh, P.H., 1998. Simulation of development and reproduction of Collembola sampled from synchronized cultures. Pedobiologia 42, 1-9.
BBA, 1995. 1st report of the second international ring test on a method for determining the effects of chemicals or soil contaminants on the reproduction of collembola. Biologische Bundesanstalt für Land. und Forstwirtschaft, Institut fur Ùkotoxikologie im Pflanzenschutz, Kùnigin-Luise-Str 19, 14195 Berlin, Berlin.
Bellinger, P.F., Christiansen, K.A., Janssens, F., 1996-2008. Checklist of the Collembola of the World.
Chenon, P., Rousset, A., Crouau, Y., 2000. Genetic polymorphism in nine clones of a parthenogenetic collembolan used in ecotoxicological testing. Applied Soil Ecology 14, 103-110.
Chernova, N.M., Goloshchapova, N.P., Potapov, M.B., 2003. Behavior of closely related collembolan species of Isotomidae family. Zoologicheskii Zhurnal 82, 1191-1200.
Crommentuijn, T., Stäb, J.A., Doornekamp, A., Estoppey, O., Van Gestel, C.A.M., 1995. Comparative ecotoxicity of cadmium, chlorpyrifos and triphenyltin hydroxide for four clones of the parthenogenetic collembolan Folsomia candida in an artificial soil. Functional Ecology 9, 734-742.
Crouau, Y., Cazes, L., 2003. What causes variability in the Folsomia candida reproduction test? Applied Soil Ecology 22, 175-180.
Crouau, Y., Moia, C., 2006. The relative sensitivity of growth and reproduction in the springtail, Folsomia candida, exposed to xenobiotics in the laboratory: An indicator of soil toxicity. Ecotoxicology and Environmental Safety 64, 115-121.
Diao, X.P., Jensen, J., Hansen, A.D., 2007. Toxicity of the anthelmintic abarnectin to four species of soil invertebrates. Environmental Pollution 148, 514-519.
Environment-Canada, 2005a. Guidance Document on Statistical Methods for Environmental Toxicity Tests (Including June 2007 Amendments). In: Scroggins, R.P. (Ed.). Environmental Protection Series, EPS 1/RM/46, p. 241. Available from: www.etc-cte.ec.gc.ca/organization/bmd/bmd_publist_e.html.
Environment-Canada, 2005b. Test for measuring emergence and growth of terrestrial plants exposed to contaminants in soil. In: Scroggins, R.P. (Ed.). Environmental Protection Series. Environment Canada, Environmental Technology Centre, Ottawa, ON, Canada Ottawa, Report EPS 1/RM/45, p. 131. Available from: http://www.etc-cte.ec.gc.ca/organization/bmd/pubs/pubs_en/1RM45EnglishFinal.pdf.
Environment-Canada, 2007. Test for measuring survival and reproduction of springtails exposed to contaminants in soil. In: Scroggins, R.P. (Ed.). Environmental Protection Series. Environment Canada, Environmental Technology Centre, Ottawa, ON, Canada Ottawa, Report EPS 1/RM/47, p. 146. Available from: http://www.etc-cte.ec.gc.ca/organization/bmd/bmd_publist_e.html.
Fjellberg, A., 1980. Identification keys to Norwegian Collembola. Norsk Entomologisk Forening, Ås, Norway.
Fjellberg, A., 2007a. Checklist of Nordic Collembola. With notes on habitat preferences and presence/absence in individual countries. Accessed: January 15, 2008, Available from: http://www.geocities.com/~fransjanssens/publicat/collnord.pdf.
Fjellberg, A., 2007b. The Collembola of Fennoscandia and Denmark. Part II: Entomobryomorpha and Symphypleona. Fauna Entomologica Scandinavica 42, 264.
Folker-Hansen, P., Krogh, P.H., Holmstrup, M., 1996. Effect of dimethoate on body growth of representatives of the soil living mesofauna. Ecotoxicology and Environmental Safety 33, 207-216.
Fountain, M.T., Hopkin, S.P., 2005. Folsomia candida (Collembola): A “Standard” Soil Arthropod. Annual Review of Entomology 50, 201-222. Available from: http://arjournals.annualreviews.org/loi/ento.
Goloschapova, N.P., Potapov, M.B., Chernova, N.M., 2006. Sexual behaviour in Isotomidae (Collembola). Pedobiologia 50, 111-116.
Goto, H.E., 1960. Facultative parthenogenesis in Collembola (Insecta). Nature 10, 958-959.
Greenslade, P., Vaughan, G.T., 2003. A comparison of Collembola species for toxicity testing of Australian soils. Pedobiologia 47, 171-179. Available from: http://dx.doi.org/10.1078/0031-4056-00180.
Holmstrup, M., Krogh, P.H., 1996. Effects of an anionic surfactant, linear alkylbenzene sulfonate, on survival, reproduction and growth of the soil-living collembolan Folsomia fimetaria. Environmental Toxicology and Chemistry 15, 1745-1748.
Holmstrup, M., Krogh, P.H., 2001. Effects and risk assessment of linear alkylbenzene sulphonates (LAS) in agricultural soil. 3. Sub-lethal effects on soil invertebrates. Environmental Toxicology and Chemistry 20, 1673–1679.
Holmstrup, M., Krogh, P.H., Løkke, H., de Wolf, W., Marshall, S., Fox, K., 2001. Effects and risk assessment of linear alkylbenzene sulphonates (LAS) in agricultural soil. 4. The influence of salt speciation, soil type, and sewage sludge on toxicity using the collembolan Folsomia fimetaria and the earthworm Aporrectodea caliginosa as test organisms. Environmental Toxicology and Chemistry. 20, 1680–1689.
Hopkin, S.P., 1997. Biology of springtails. Oxford University Press, Oxford.
Hopkin, S.P., 2008a. Folsomia fimetaria. Accessed: 8-1-2008, Available from: http://www.stevehopkin.co.uk/collembolamaps/
Entomobryomorpha/264FOfim/.
Hopkin, S.P., 2008b. Folsomia litsteri. Accessed: 21-1-2008, Available from: http://www.stevehopkin.co.uk/collembolamaps/
Entomobryomorpha/264FOfim/.
ISO, 1998. ISO 11268-2:1998 (E) Soil quality - Effects of pollutants on earthworms (Eisenia fetida) - Part 2: Determination of effects on reproduction.
ISO, 1999. ISO 11267:1999 Soil quality - Inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. ISO, Geneve, Switzerland. Available from: www.iso.org.
Jensen, J., Van Langevelde, J., Pritzl, G., Krogh, P.H., 2001. Effects of the phthalates di-(2-ethyl-hexylphthalate) (DEHP) and di-butylphthalate (DBP) on the Collembolan Folsomia fimetaria. Environmental Toxicology and Chemistry 20, 1085–1091.
Koivisto, R.K.K., Braig, H.R., 2003. Intraclonal genetic variation: ecological and evolutionary aspects. Edited by H. D. Loxdale fls, fres and G. Lushai fres. Microorganisms and parthenogenesis. Biological Journal of the Linnean Society 79, 43-58. Available from: http://www.blackwell-synergy.com/doi/abs/10.1046/j.1095-8312.2003.00185.x
Krogh, P.H., 1995. Does a heterogeneous distribution of food or pesticide affect the outcome of toxicity tests with Collembola? Ecotoxicology and Environmental Safety 30, 158-163. Available from: http://dx.doi.org/10.1006/eesa.1995.1020.
Krogh, P.H., 2004. Compilation and Review of Existing Control Performance Data for the Collembolan species, Folsomia fimetaria L., in Artificial and Natural Soils. Report prepared by NERI, Silkebiorg, Denmark, for the Biological Methods Division, Environment Canada, Ottawa, Ontario, 24.
Krogh, P.H., 2006. Recordings of the reproduction of Folsomia fimetaria couples. Available from: http://krogh.ph/shared/fimetaria_repro.
Krogh, P.H., Holmstrup, M., Petersen, S.O., Jensen, J., 1997. Ecotoxicological assessment of sewage sludge in agricultural soil. Danish Ministry for the Environment, Environmental Protection Agency. Working Report No. 69. Available from: http://www.mst.dk/udgiv/Publikationer/1997/87-7810-865-9/pdf/87-7810-865-9.PDF.
Larsen, J., Johansen, A., Larsen, S.E., Heckmann, L.H., Jakobsen, I., Heckmann, L.H., Krogh, P.H., 2008. Population performance of collembolans feeding on soil fungi from different ecological niches. Soil Biology and Biochemistry 40, 360-369. Available from: http://dx.doi.org/10.1016/j.soilbio.2007.08.016.
Martikainen, E.A.T., Krogh, P.H., 1999. Effects of soil organic matter content and temperature on toxicity of dimethoate to Folsomia fimetaria (Collembola : Isotomidae). Environmental Toxicology & Chemistry 18, 865-872.
OECD, 1984. OECD Guidelines for testing of chemicals no. 207. Earthworm acute toxicity tests. Adopted 4.
OECD, 2002. OECD's Appraisal of Test Methods for Sex Hormone Disrupting Chemicals (OECD Monograph No. 21). ENV/JM/MONO(2002)8 OECD Series on Testing and Assement No. 21. Available from: http://appli1.oecd.org/olis/2002doc.nsf/
43bb6130e5e86e5fc12569fa005d004c/
f84d6ed7aacd2fc8c1256b750052d744/
$FILE/JT00122123.PDF.
OECD, 2004a. Test No. 220: Enchytraeid reproduction test. OECD guidelines for testing chemicals. OECD. Available from: http://titania.sourceoecd.org/vl=2481688/cl=61/nw=1/
rpsv/ij/oecdjournals/1607310x/v1n2/s20/p1.
OECD, 2004b. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Esienia andrei ). Available from: http://lysander.sourceoecd.org/vl=2006461/cl=12/
nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s21/p1.
OECD, 2006a. Current approaches in the statistical analysis of ecotoxicity data: a guidance to application. OECD series on testing and assessment Number 54. Available from: http://appli1.oecd.org/olis/2006doc.nsf/linkto/env-jm-mono(2006)18.
OECD, 2006b. Guidance document for the development of OECD guidelines for the testing of chemicals. OECD Series on Testing and Assessment 1, 1-33. Available from: http://appli1.oecd.org/olis/2006doc.nsf/linkto/env-jm-mono(2006)20.
OECD, 2006c. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. OECD guidelines for the testing of chemicals ENV/JM/TG(2005)4. Available from: http://puck.sourceoecd.org/vl=8550520/cl=43/nw=1/rpsv/ij/
oecdjournals/1607310x/v1n2/s9/p1.
OECD, 2007. Proposal for a new guideline. Predatory mite reproduction test in soil (Hypoaspis (Geolaelaps) aculeifer). Accessed: September, Available from: http://www.oecd.org/dataoecd/54/61/38972970.pdf.
Palévody, C., 1972. XIntracellular Microorganisms in Ovary of Collembolae-Isotomid Folsomia candida. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Série D: Sciences naturelles 275, 401-404.
Pedersen, M.B., van Gestel, C.A.M., Elmegaard, N., 2000. Effects of copper on reproduction of two collembolan species exposed through soil, food, and water. Environmental Toxicology & Chemistry 19, 2579-2588.
Petersen, H., Gjelstrup, P. (Eds.), 1995. Development of a semi-field method for evaluation of laboratory tests as compared to field conditions. Danish EPA (Download from http://www.mst.dk/udgiv/Publications/1995/87-7810-305-3/pdf/87-7810-305-3.PDF), Copenhagen.
Potapov, M.B., 2000. The use of macrochaetotaxy in taxonomy of palaearctic Folsomia (Collembola, Isotomidae). Pedobiologia 44, 234-239.
Potapov, M.B., Babenko, A.B., 2000. Species of the genus Folsomia (Collembola : Isotomidae) of northern Asia. European Journal of Entomology 97, 51-74.
Riepert, F., Kula, C., 1996. Entwicklung von Labormethoden zur Prüfung der Wirkung von chemischen Stoffen und Pflanzenschutzmitteln auf Collembolen und Regenwürmer [Development of laboratory methods for testing effects of chemicals and pesticides on Collembola and earthworms]. Mitteilungen aus der Biologischen Bundesanstalt für Land und Forstwirtschaft. Berlin Dahlem. Heft 320, 82. Available from: http://www.bba.de/veroeff/mitt/pdfs/mitt320.pdf.
Riparbelli, M.G., Giordano, R., Callaini, G., 2006. Centrosome inheritance in the parthenogenetic egg of the collembolan Folsomia candida. Cell and Tissue Research 326, 861-872. Available from: http://dx.doi.org/10.1007/s00441-006-0253-x
SAS-Institute-Inc., 2004a. SAS/INSIGHT® 9.1 User's Guide, Volumes 1 and 2. Cary, NC: SAS Institute Inc. Available from: http://support.sas.com/documentation/onlinedoc/
91pdf/sasdoc_91/insight_ug_9984.pdf.
SAS-Institute-Inc., 2004b. SAS/STAT® 9.1 User's Guide. SAS Institute Inc., Cary, NC. Available from: http://support.sas.com/documentation/onlinedoc/
91pdf/sasdoc_91/stat_ug_7313.pdf.
Schaller, F., 1952. Die Copula der Collembolen (Springschwanze). Naturwissenschaften 39, 48-49.
Schliwa, W., Schaller, F., 1963. Die Paarbildung des Springschwanzes Podura aquatica (Apterygota [Urinsekten], Collembola). Naturwissenschaften 50, 698.
Scopes, N.E.A., Lichtenstein, E.P., 1967. The use of Folsomia fimetaria and Drosophila melanogaster as test insects for the detection of insecticide residues. J. Econ. Entomology 60, 1539-1541.
Scott-Fordsmand, J.J., Krogh, P.H., 2005. Background report on pre-validation of an OECD springtail test guideline. In: EPA, D. (Ed.). Environmental Project No. 986. Available from: http://www2.mst.dk/Udgiv/publications/2005/87-7614-535-2/pdf/87-7614-536-0.pdf.
Simonsen, V., Christensen, P.G., 2001. Clonal and genetic variation in three collembolan species revealed by isozymes and randomly amplified polymorphic DNA. Pedobiologia 45, 161-173.
Snider, R., 1973. Laboratory observations on the biology of Folsomia candida (Willem) (Collembola: Isotomidae). Revue D'Écologie et Biologie du Sol 10, 103-124.
Son, J., Ryoo, M.I., Jung, J., Cho, K., 2007. Effects of cadmium, mercury and lead on the survival and instantaneous rate of increase of Paronychiurus kimi (Lee) (Collembola). Applied Soil Ecology 35, 404-411.
Sverdrup, L.E., Jensen, J., Krogh, P.H., Stenersen, J., 2002. Studies on the effect of soil aging on the toxicity of pyrene and phenanthrene to a soil-dwelling springtail. Environmental Toxicology and Chemistry 21, 489-492.
Sverdrup, L.E., Kelley, A.E., Krogh, P.H., Nielsen, T., Jensen, J., Scott-Fordsmand, J.J., Stenersen, J., 2001. Effects of eight polycyclic aromatic compounds on the survival and reproduction of the springtail Folsomia fimetaria L. (Collembola, Isotomidae). Environmental Toxicology and Chemistry 20, 1332-1338.
Van Amelsvoort, P.A.M., Usher, M.B., 1989. Egg Production related to food quality in Folsomia candida (Collembola: Isotomidae): effects on life history strategies. Pedobiologia 33, 61-66.
Van de Bund, C.F., 1965. Changes in the soil fauna caused by application of insecticides. Boll. Zool. agr. Bachic. 7, 185-212.
Van Gestel, C.A.M., 1998. The developmental status of ecotoxicity tests. In: Løkke, H., Van Gestel, C.A.M. (Eds.). Handbook of soil invertebrate toxicity tests. John Wiley & Sons, Ltd., Chichester, pp. 57-67.
Vandekerckhove, T.T.M., Watteyne, S., Willems, A., Swing, J.G., Mertens, J., Gillis, M., 1999. Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. Fems Microbiology Letters 180, 279-286.
Weyers, A., Rùmbke, J., Moser, T., Ratte, H.T., 2002. Statistical results and implications of the enchytraeid reproduction ringtest. Environmental Science & Technology 36, 2116-2121.
Widarto, T.H., Krogh, P.H., Forbes, V., 2007. Nonylphenol stimulates fecundity but not population growth rate (l) of Folsomia candida. Ecotoxicology and Environmental Safety 67, 369-377. Available from: http://dx.doi.org/10.1016/j.ecoenv.2006.11.005.
Wiles, J.A., Krogh, P.H., 1998. Testing with the collembolans I. viridis, F. candida and F. fimetaria. In: Løkke, H., Van Gestel, C.A.M. (Eds.). Handbook of soil invertebrate toxicity tests. John Wiley & Sons, Ltd., Chichester, pp. 131-156.
Annex 1
Participants
The list include contact person of laboratories delivering testing results to the ringtest.
Study director Ir. Ine Geujin
NOTOX B.V.
Hambakenwetering 7
PO Box 3476
5203 DL's - Hertogenbosch
The Netherlands
Prof. Gabor Bakonyi
SZIE Department of Zoology and Ecology
2100 Godollo, Hungary
Pater K. u. 1.
Prof. Berndt-Michael Wilke
Berlin University of Technology
Institute of Ecology
Franklinstr. 29
D-10587 Berlin, Germany
Dr. Hege Stubberud
Jordforsk
Frederik A. Dahls vei 20
N-1432 Ås, Norway
Dr. Jan Lana
RECETOX, Faculty of Science, Masaryk University
TOCOEN, Ltd.
Kamenice 126/3
625 00 Brno, Czech Republic
Dr. Maike Schaefer
University of Bremen, UFT
Department of General and Theoretical Ecology
Leobener Str.
D - 28359 Bremen,
Germany
Dr. Kristin Becker van Slooten
Laboratory for Environmental Chemistry and Ecotoxicology (CECOTOX)
ENAC-ISTE
Ecole polytechnique fédérale de Lausanne (EPFL)
Station 2
1015 Lausanne, Switzerland
Dr. Janeck J. Scott-Fordsmand
Dept. Terrestrial Ecology
National Environmental Research Institute
Vejlsoevej 25, PO Box 314
8600-Silkeborg, Denmark
Dr. Mónica João de Barros Amorim
Departamento de Biologia
Universidade de Aveiro
3810-193 Aveiro, Portugal
Prof. Nobuhiro Kaneko
Soil Ecology, Graduate School of Environment &
Information Sciences, Yokohama National University
79-7 Tokiwadai, Yokohama 240-8501
Japan
Silvio Knäbe
GAB Technologie
Gmbh Eutinger Strasse 24
75223 Niefern Ùschelbronn Germany
Dr. Pilar Andrés and Dr. Xavier Domene
Centre de Recerca i Aplicacions Forestals (CREAF)
Universitat Autònoma de Barcelona
Spain
Vladimír Kocí
Department of Environmental Chemistry
ICT Prague Ústav chemie ochrany prostredí
VŠCHT Praha Technická 5, 166 28 Praha 6
Czech Republic
Dr. Thomas Moser
ECT Oekotoxikologie GmbH
Bùttgerstrasse 2-14
D-65439 Flùrsheim
Germany
Juliska Princz
Biological Methods Division, Environmental Technology Centre
Science & Technology Branch, Environment Canada
335 River Road South
Ottawa, ON K1A 0H3
Annex 2
Laboratory code
All the datasets were coded by a reference no. in Fejl! Henvisningskilde ikke fundet., and in Fejl! Henvisningskilde ikke fundet. to Fejl! Henvisningskilde ikke fundet. to conceal the identity of the participating laboratories. However, the tests performed by each coded laboratory is presented below. B: Boric acid; C: copper chloride; D: Dimethoate.
| Laboratory no. | Testing data set reference no. (Ref. no.) |
F. candida | F. fimetaria |
| 1 | 21, 22, 23 | BCD | |
| 2 | 1, 2 | BC | |
| 3 | 52, 53 | B | B |
| 4 | 12, 13, 14, 15, 16, 17, 18, 19, 20 | BCD | BCD |
| 5 | 55, 56 | BD | |
| 6 | 47, 48 | CB | |
| 7 | 36, 37, 38, 39, 40 | BC | BC |
| 8 | 9, 10, 11 | BCD | |
| 9 | 3, 4, 5, 6 | BD | |
| 10 | 49, 50 | B | B |
| 11 | 24, 25, 26, 27, 28, 29, 30, 31 | BCD | BCD |
| 12 | 7, 8 | B | |
| 13 | 32, 33, 34, 35 | BC | BC |
| 14 | 41, 42, 43, 44, 45, 46 | BCD |
Annex 3
Bibliometric statistics
An impression of the accumulated knowledge available on collembolans was obtained by searching for common collembolan species names in Science Citation Index (ISI Web of Knowledge/Web of Science accessed Jan 2008). Thus, whenever a species name occurred in a title, abstract or keyword of papers it was counted as a citation. To indicate the attention papers on a certain species have received, the average number of citations was included as a “citation rate” (auto-citations excluded).
| Species | ISI indexed papers | Citations | Citation rate |
| F. candida | 398 | 2082 | 5 |
| Orchesella cincta | 109 | 1025 | 9 |
| Onychiurus armatus/Protaphorura armata[1] | 85 | 919 | 11 |
| F. fimetaria | 63 | 401 | 6 |
| Parisotoma notabilis and Isotoma notabilis | 42 | 467 | 11 |
| Proisotoma minuta | 38 | 155 | 4 |
| Onychiurus arcticus/Megaphorura arctica | 37 | 381 | 10 |
| Isotoma viridis/anglicana | 36 | 250 | 7 |
| Sminthurus viridis | 36 | 115 | 3 |
| Folsomia quadrioculata | 27 | 225 | 8 |
| Isotomiella minor | 20 | 138 | 7 |
| Isotomurus palustris / I. prasinus | 18 | 134 | 7 |
| Sinella curviseta | 13 | 50 | 4 |
| Tullbergia macrochaeta, Mesaphorura macrochaeta, or T. krausbaueri | 14 | 91 | 7 |
| Paronychiurus kimi² | 5 | 5 | 1 |
| Onychiurus folsomi / Orthonychiurus folsomi[2] | 2 | 9 | 5 |
Some species is present under more than one name either because the genus name has been changed or because a species complex has been illuminated and a new species name has been given to a member of a species complex.
[1] The similar onychiurid pair Onychiurus fimatus/Protaphorura fimata was mentioned 26 times, and cited 219 times, so the species group are similarly “popular” than O. cincta.
[2] Due to its association with Onychiurinae it should be considered together with the other members of this subfamily, which will make the subfamily Onychiurinae the second most commonly published species group.
Annex 4
Intralaboratory variability
Survival and reproduction of control replicate results from 57 F. fimetaria standard tests performed over a period of 6 years (n=243) at NERI. Mean and 95% C.L., are given for adult survival and reproduction. Adults: Average number of surviving adults of the initial 10 males and 10 females at test termination; Reproduction: Average number of juvenile F. fimetaria at test termination; 95% C.L.: Confidence Limits.
Click here to see Annex 4 table
Annex 5
Control mortality and reproduction
Control values estimated from the control data only, in contrast to values in Fejl! Henvisningskilde ikke fundet., that were based on the complete concentration-response dataset.
| Ref.no. | Species | Compound | n | Adults | CV | Mortality, % | Reproduction | CV | |||
| 1 | F. candida | Boric acid | 8 | 8.6 | [7.6-9.6] | 14 | 13.8 | [3.8-23.7] | 199 | [126-271] | 44 |
| 31 | F. candida | Boric acid | 8 | 9.4 | [8.9-9.8] | 5.5 | 6.3 | [1.9-10.6] | 1244 | [1152-1336] | 8.8 |
| 7 | F. candida | Boric acid | 5 | 9.0 | [8.1-9.9] | 7.9 | 10.0 | [1.2-18.8] | 414 | [334-494] | 16 |
| 9 | F. candida | Boric acid | 3 | 10.0 | 0.0 | 0.0 | 317 | [216-417] | 13 | ||
| 12 | F. candida | Boric acid | 8 | 6.0 | [3.9-8.1] | 42 | 40.0 | [19.0-61.0] | 249 | [163-334] | 41 |
| 16 | F. candida | Boric acid | 4 | 6.0 | [2.3-9.7] | 38 | 40.0 | [3.3-76.7] | 365 | [196-534] | 29 |
| 21 | F. candida | Boric acid | 10 | 9.1 | [8.6-9.6] | 8.1 | 9.0 | [3.7-14.3] | 405 | [362-447] | 15 |
| 261 | F. candida | Boric acid | 8 | 5.5 | [3.5-7.5] | 45 | 45.0 | [24.5-65.5] | 140 | [69-212] | 61 |
| 311 | F. candida | Boric acid | 8 | 9.3 | [8.7-9.8] | 7.6 | 7.5 | [1.6-13.4] | 339 | [269-409] | 25 |
| 32 | F. candida | Boric acid | 5 | 9.2 | [8.6-9.8] | 4.9 | 8.0 | [2.4-13.6] | 308 | [236-381] | 19 |
| 37 | F. candida | Boric acid | 7 | 9.7 | [9.0-10.4] | 7.8 | 2.9 | [-4.1-9.8] | 233 | [221-245] | 5.5 |
| 38 | F. candida | Boric acid | 7 | 9.9 | [9.5-10.2] | 3.8 | 1.4 | [-2.1-4.9] | 358 | [253-462] | 32 |
| 43 | F. candida | Boric acid | 4 | 7.8 | [6.2-9.3] | 12 | 22.5 | [7.3-37.7] | 386 | [249-523] | 22 |
| 46 | F. candida | Boric acid | 5 | 8.2 | [6.0-10.4] | 22 | 18.0 | [-4.2-40.2] | 319 | [128-510] | 48 |
| 49 | F. candida | Boric acid | 7 | 9.0 | [8.2-9.8] | 9.1 | 10.0 | [2.4-17.6] | 1076 | [918-1234] | 16 |
| 51 | F. candida | Boric acid | 6 | 9.0 | [8.3-9.7] | 7.0 | 10.0 | [3.4-16.6] | 349 | [277-421] | 20 |
| 53 | F. candida | Boric acid | 6 | 9.3 | [8.5-10.2] | 8.7 | 6.7 | [-1.9-15.2] | 537 | [465-610] | 13 |
| 55 | F. candida | Boric acid | 6 | 8.0 | [7.1-8.9] | 10 | 20.0 | [11.2-28.8] | 345 | [251-440] | 26 |
| 2 | F. candida | Copper | 8 | 9.1 | [8.4-9.8] | 9.1 | 8.8 | [1.8-15.7] | 196 | [155-237] | 25 |
| 10 | F. candida | Copper | 3 | 9.7 | [8.2-11.1] | 6.0 | 3.3 | [-11.0-17.7] | 267 | [84-450] | 28 |
| 15 | F. candida | Copper | 4 | 7.5 | [2.9-12.1] | 38 | 25.0 | [-20.9-70.9] | 578 | [278-878] | 33 |
| 22 | F. candida | Copper | 10 | 9.6 | [9.2-10.0] | 5.4 | 4.0 | [0.3-7.7] | 449 | [410-489] | 12 |
| 251 | F. candida | Copper | 8 | 5.5 | [3.5-7.5] | 45 | 45.0 | [24.5-65.5] | 140 | [69-212] | 61 |
| 301 | F. candida | Copper | 8 | 9.3 | [8.7-9.8] | 7.6 | 7.5 | [1.6-13.4] | 339 | [269-409] | 25 |
| 35 | F. candida | Copper | 5 | 9.6 | [8.5-10.7] | 9.3 | 4.0 | [-7.1-15.1] | 124 | [86-161] | 24 |
| 36 | F. candida | Copper | 7 | 9.7 | [9.3-10.2] | 5.0 | 2.9 | [-1.7-7.4] | 614 | [521-707] | 16 |
| 41 | F. candida | Copper | 5 | 9.4 | [8.3-10.5] | 10 | 6.0 | [-5.1-17.1] | 349 | [246-452] | 24 |
| 44 | F. candida | Copper | 4 | 8.3 | [7.5-9.0] | 6.1 | 17.5 | [9.5-25.5] | 415 | [164-666] | 38 |
| 48 | F. candida | Copper | 4 | 8.5 | [6.9-10.1] | 12 | 15.0 | [-0.9-30.9] | 875 | [753-997] | 8.8 |
| 54 | F. candida | Copper | 5 | 9.2 | [8.2-10.2] | 9.1 | 8.0 | [-2.4-18.4] | 435 | [390-480] | 8.3 |
| 4[1] | F. candida | Dimethoate | 8 | 9.4 | [8.9-9.8] | 5.5 | 6.3 | [1.9-10.6] | 1244 | [1152-1336] | 8.8 |
| 11 | F. candida | Dimethoate | 4 | 9.5 | [8.6-10.4] | 6.1 | 5.0 | [-4.2-14.2] | 130 | [69-191] | 29 |
| 17 | F. candida | Dimethoate | 4 | 8.5 | [6.4-10.6] | 15 | 15.0 | [-5.5-35.5] | 596 | [458-734] | 15 |
| 23 | F. candida | Dimethoate | 10 | 9.6 | [9.1-10.1] | 7.3 | 4.0 | [-1.0-9.0] | 150 | [123-178] | 25 |
| 24 | F. candida | Dimethoate | 16 | 2.0 | [1.2-2.8] | 71 | 80.0 | [72.5-87.5] | 29 | [15-42] | 86 |
| 42 | F. candida | Dimethoate | 4 | 7.3 | [5.2-9.3] | 17 | 27.5 | [7.5-47.5] | 290 | [178-402] | 24 |
| 45 | F. candida | Dimethoate | 4 | 9.8 | [9.0-10.5] | 5.1 | 2.5 | [-5.5-10.5] | 490 | [357-623] | 17 |
| 5 | F. fimetaria | Boric acid | 8 | 11.5 | [8.8-14.2] | 28 | 42.5 | [28.9-56.1] | 59 | [51-68] | 17 |
| 13 | F. fimetaria | Boric acid | 8 | 15.4 | [13.4-17.4] | 16 | 23.1 | [13.1-33.1] | 6.8 | [5.2-8.3] | 28 |
| 19 | F. fimetaria | Boric acid | 4 | 10.5 | [3.3-17.7] | 43 | 47.5 | [11.6-83.4] | 52.3 | [-0.4-105] | 63 |
| 28 | F. fimetaria | Boric acid | 8 | 5.0 | [2.1-7.9] | 70 | 75.0 | [60.3-89.7] | |||
| 33 | F. fimetaria | Boric acid | 5 | 17.4 | [14.5-20.3] | 13 | 13.0 | [-1.3-27.3] | 142 | [110-174] | 18 |
| 40 | F. fimetaria | Boric acid | 7 | 18.1 | [16.9-19.4] | 7.4 | 9.3 | [3.1-15.5] | 270 | [240-301] | 12 |
| 47 | F. fimetaria | Boric acid | 6 | 7.0 | [2.8-11.2] | 57 | 65.0 | [44.0-86.0] | 50.8 | [-1.9-104] | 99 |
| 50 | F. fimetaria | Boric acid | 7 | 13.4 | [12.3-14.6] | 9.5 | 32.9 | [27.0-38.7] | 300 | [256-343] | 16 |
| 52 | F. fimetaria | Boric acid | 2 | 17.5 | [11.1-23.9] | 4.0 | 12.5 | [-19.3-44.3] | 197 | [-476-870] | 38 |
| 14 | F. fimetaria | Copper | 8 | 16.3 | [15.1-17.4] | 8.5 | 18.8 | [12.9-24.6] | 7.3 | [6.0-8.5] | 21 |
| 18 | F. fimetaria | Copper | 4 | 11.3 | [8.5-14.0] | 15 | 43.8 | [30.2-57.3] | 176 | [53-299] | 44 |
| 27 | F. fimetaria | Copper | 8 | 5.0 | [2.1-7.9] | 70 | 75.0 | [60.3-89.7] | 0.4 | [-0.2-1.0] | 198 |
| 34 | F. fimetaria | Copper | 5 | 19.4 | [18.3-20.5] | 4.6 | 3.0 | [-2.6-8.6] | 248 | [119-376] | 42 |
| 39 | F. fimetaria | Copper | 7 | 19.6 | [18.8-20.3] | 4.0 | 2.1 | [-1.5-5.8] | 242 | [200-284] | 19 |
| 6 | F. fimetaria | Dimethoate | 8 | 12.0 | [9.8-14.2] | 22 | 40.0 | [29.1-50.9] | 12.1 | [7.1-17.2] | 50 |
| 20 | F. fimetaria | Dimethoate | 4 | 16.8 | [12.4-21.1] | 16 | 16.3 | [-5.7-38.2] | 347 | [121-573] | 41 |
| 29 | F. fimetaria | Dimethoate | 16 | 5.5 | [3.8-7.2] | 58 | 72.5 | [64.0-81.0] | 3.5 | [-1.1-8.1] | 246 |
[1] These control data are duplicated as two tests shared the same controls, so for summaries and statistics only one figure is used.
Annex 6
Version 3.4, September 2008
PROPOSAL FOR A NEW GUIDELINE
OECD GUIDELINES FOR TESTING CHEMICALS
Collembolan reproduction test
INTRODUCTION
1. This Test Guideline is designed for assessing the effects of chemicals on the reproductive output of the collembolans, Folsomia fimetaria L. and Folsomia candida Willem in soil. It is based on existing procedures [1, 2]. The two species are some of the most accessible species of Collembola and are culturable and commercially available. When specific habitats not covered by the two species need to be assessed the procedure is extensible to other species of Collembola.
2. Soil-dwelling Collembola are ecologically relevant species for ecotoxicological testing. Collembolans are hexapods with a thin exoskeleton highly permeable to air and water, and represent arthropod species with a different route and a different rate of exposure compared to earthworms and enchytraeids.
3. Population densities of Collembola commonly reach 105 m-2 in soil and leaf litter layers in many terrestrial ecosystems [3, 4]. Adults typically measure 0.5 - 5 mm, their contribution to total soil animal biomass and respiration is low, estimated between 1% and 5% [5]. Their most important role may therefore be as potential regulators of processes through microbivory and microfauna predation. Springtails are prey animals for a wide variety of endogeic and epigeic invertebrates, such as mites, centipedes, spiders, Carabidae and rove beetles. Collembola contribute to decomposition processes in acidic soils where they may be the most important soil invertebrates besides enchytraeids, since earthworms and diplopods are typically absent.
4. F. fimetaria has a worldwide distribution and is common in several soil types ranging from sandy to loamy soils and from mull to mor soils. It is an eyeless, unpigmented collembolan. It has been recorded in agricultural soils all over Europe [6]. It has an omnivorous feeding habit, including fungal hyphae, bacteria, protozoa and detritus in its food. It interacts through grazing with infections of plant pathogenic fungi [7] and may influence mycorrhiza, as is known to be the case for F. candida. As most collembolan species it reproduces sexually requiring the permanent presence of males for egg fertilization.
5. F. candida is also distributed worldwide. Although it is not common in most natural soils, it often occurs in very high numbers in humus rich sites. It is an eyeless, unpigmented collembolan. It has a well-developed furca (jumping organ) and an active running movement and jumps readily if disturbed. The ecological role is similar to the role of F. fimetaria, but the habitats are more organic rich soils. It reproduces parthenogenetically. Males may occur at less than 1 per mille.
PRINCIPLE OF THE TEST
6. Synchronous adult (F. fimetaria) or juvenile (F. candida) Collembola are exposed to a range of concentrations of the test substance mixed into an artificial soil according to OECD 207 using a 5% organic matter content (or an alternative soil) [8]. The test scenario can be divided into two steps:
- A range-finding test, in case no sufficient information on toxicity is available, in which mortality and reproduction are the main endpoints assessed after two weeks for F. fimetaria and 3 weeks for F. candida.
- A definitive reproduction test in which the total number of juveniles produced by parent animals and the survival of parent animals are assessed. The duration of this definitive test is 21 days for F. fimetaria or 28 for F. candida.
The toxic effect of the test substance on adult mortality and reproductive output is expressed as LCx and ECx by fitting the data to an appropriate model by non-linear regression to estimate the concentration that would cause x % mortality or reduction in reproductive output, respectively, or alternatively as the NOEC value [9].
INFORMATION ON THE TEST SUBSTANCE
7. The physical properties, water solubility, the log Kow, the soil water partition coefficient and the vapour pressure of the test substance should preferably be known. Additional information on the fate of the test substance in soil, such as the rates of photolysis and hydrolysis and biotic degradation, is desirable. Chemical identification of the test substance according to IUPAC nomenclature, CAS-number, batch, lot, structural formula and purity should be documented when available.
8. This Guideline can be used for water soluble or insoluble substances. However, the mode of application of the test substance will differ accordingly. The Guideline is not applicable to volatile substances, i.e. substances for which the Henry's constant or the air/water partition coefficient is greater than one, or substances for which the vapour pressure exceeds 0.0133 Pa at 25 °C.
VALIDITY OF THE TEST
9. The following criteria should be satisfied in the untreated controls for a test result to be considered valid:
- Mean adult mortality should not exceed 20% at the end of the test;
- The mean number of juveniles per vessel should be at least 100 at the end of the test;
- The coefficient of variation calculated for the number of juveniles per replicate should be less than 30% at the end of the definitive test.
REFERENCE SUBSTANCE
10. A reference substance must be tested at its EC50 concentration for the chosen test soil type either at regular intervals or possibly included in each test run to verify that the response of the test organisms in the test system are responding within the normal level. A suitable reference substance is boric acid, which should reduce reproduction by 50% [10, 11] at about 100 mg/kg.
DESCRIPTION OF THE TEST
Test vessels and equipment
11. Containers capable of holding 30 g of fresh soil are suitable test vessels. The material should either be glass or inert plastic (non-toxic). The test vessels should have a cross-sectional area allowing the actual soil depth within the test vessel to be 2-4 cm. The vessels should have lids (e.g. glass or polyethylene) that are designed to reduce water evaporation whilst allowing gas exchange between the soil and the atmosphere. The container should be at least partly transparent to allow light transmission.
12. Normal laboratory equipment is required, specifically the following:
- drying cabinet;
- stereo microscope;
- pH-meter and luxmeter;
- suitable accurate balances;
- adequate equipment for temperature control;
- adequate equipment for air humidity control (not essential if exposure vessels are covered by lids);
- temperature-controlled incubator or small room;
- forceps or a low-suction air flow device.
Preparation of the test soil
13. The artificial soil used for the test is prepared according to OECD guideline 207 [8], but with an organic matter content of 5%. Alternatively a natural standard soil could be used, as the artificial soil does not resemble natural soils. The recommended composition of the artificial soil is as follows (based on dry weights, dried to a constant weight at 105 °C):
- 5% sphagnum peat, air-dried and finely ground (a particle size of 2 +/- 1 mm is acceptable);
- 20% kaolin clay (kaolinite content preferably above 30%);
- approximately 74% air-dried industrial sand (depending on the amount of CaCO3 needed), predominantly fine sand with more than 50% of the particles between 50 and 200 microns. The exact amount of sand depends on the amount of CaCO3 (see below), together they should add up to 75 %.
- < 1.0% calcium carbonate (CaCO3, pulverised, analytical grade) to obtain a pH of 6.0 ± 0.5; the amount of calcium carbonate to be added may depend principally on the quality/nature of the peat (see Note 1).
Note 1: The amount of CaCO3 required will depend on the components of the soil substrate and should be determined by measuring the pH of soil sub-samples immediately before the test.
Note 2: It is recommended to measure the pH and optionally the C/N ratio and CEC of the soil in order to enable a normalisation at a later stage and to better interpret the results.
Note 3: If required, e.g. for specific testing purposes, natural soils from unpolluted sites may also serve as test and/or culture substrate. However, if natural soil is used, it should be characterised at least by origin (collection site), pH, texture (particle size distribution) and organic matter content and it should be free from any contamination. For natural soil it is advisable to demonstrate its suitability of in the test and for achieving the test validity criteria before using the soil in a definitive test.
14. The dry constituents of the soil are mixed thoroughly (e.g. in a large-scale laboratory mixer). The maximum water holding capacity (WHC) of the artificial soil is determined in accordance with procedures described in Annex 4. The moisture content of the testing soil should be optimised to attain a loose porous soil structure allowing collembolans to enter into the pores. This is usually between 40-60% of the maximum WHC.
15. The dry artificial soil is pre-moistened by adding enough de-ionised water to obtain approximately half of the final water content 2-7 days before the test start, in order to equilibrate/stabilise the acidity. For the determination of pH a mixture of soil and 1 M potassium chloride (KCl) or 0.01 M calcium chloride (CaCl2) solution in a 1:5 ratio is used (according to Annex 5). If the soil is more acidic than the required range, it can be adjusted by addition of an appropriate amount of CaCO3. If the soil is too alkaline it can be adjusted by the addition of a harmless inorganic acid.
16. The pre-moistened soil is divided into portions corresponding to the number of test concentrations (and reference substance where appropriate) and controls used for the test. The test compounds are added and the water content is regulated according to the paragraph 24.
Selection and preparation of test animals
17. At the start of the test the animals must be well fed and the age between 23-26 days for F. fimetaria and 9-12 days for F. candida. For each replicate, the number of F. fimetaria must be 10 males and 10 females, and for F. candida 10 females must be added (see ANNEX 1 and 2). The synchronous animals are selected randomly from the dishes and their health and physical condition is checked for each batch added to a replicate. Each group of 10/20 individuals is added to a randomly selected test container and the biggest females of F. fimetaria are selected to ensure a proper distinction from the F. fimetaria males.
Preparation of test concentrations
18. Four methods of application of the test substance can be used: 1) mixing the test substance into the soil with water as a carrier, 2) mixing the test substance into the soil with an organic solvent as a carrier, 3) mixing the test substance into the soil with sand as a carrier, or 4) application of the test substance onto the soil surface. The selection of the appropriate method depends on the characteristic of the compound and the purpose of the test. In general, mixing of the test substance into the soil is recommended. However, application procedures that are consistent with the practical use of the test substance may be required (e.g. spraying of liquid formulation or use of special pesticide formulations such as granules or seed dressings). The soil is treated before the collembolans are added, except when the test compound is added to the soil surface.
Test substance soluble in water
19. A solution of the test substance is prepared in deionised water in a quantity sufficient for all replicates of one test concentration. Each solution of test substance is mixed thoroughly with one batch of pre-moistened soil before being introduced into the test vessel.
Test substance insoluble in water
20. For chemicals insoluble in water, but soluble in organic solvents, the test substance can be dissolved in the smallest possible volume of a suitable solvent (e.g. acetone) still ensuring proper mixing of the chemical in the soil and mixing it with a portion of the quartz sand required. Only volatile solvents should be used. When an organic solvent is used, all test concentrations and an additional solvent negative control should contain the same minimum amount of the solvent. Application containers should be left uncovered for a certain period to allow the solvent associated with the application of the test substance to evaporate, ensuring no dissipation of the toxic compound during this time.
Test substance poorly soluble in water and organic solvents
21. For substances that are poorly soluble in water and organic solvents, the equivalent of 2.5 g of quartz sand per test vessel (this must be a part of the total sand added to the soil) is mixed with the quantity of test substance to obtain the desired test concentration. This mixture of quartz sand and test substance is added to the pre-moistened soil and thoroughly mixed after adding an appropriate amount of deionised water to obtain the required moisture content. The final mixture is divided between the test vessels. The procedure is repeated for each test concentration and an appropriate control is also prepared.
Application of the test substance onto the soil surface
22. When the test substance is a pesticide, it may be appropriate to apply it onto the soil surface by spraying. The soil is treated after the collembolans are added. The test containers are first filled with the moistened soil substrate, and the animals added and then weighed. In order to avoid any direct exposure of the animals with the test substance by direct contact, the test substance is applied at least half an hour after introducing the Collembola. The test substance should be applied to the surface of the soil as evenly as possible using a suitable laboratory-scale spraying device to simulate spray application in the field. Before application, the cover of the test container should be removed and replaced by a liner, which protects the exterior side walls of the container from spray. The liner can be made from a test container with the base removed. The application should take place at a temperature within ± 2 °C of variation and for aqueous solutions, emulsions or dispersions at a water application rate according to the risk assessment recommendations and the test soil WHC requirements. The rate should be verified using an appropriate calibration technique. Special formulations like granules or seed dressings could be applied in a manner consistent with agricultural use.
Test containers should be left uncovered for a period of one hour or overnight to allow any volatile solvent associated with the application of the test substance to evaporate.
PROCEDURE
Test conditions
23. The test mean temperature must be 20 ± 1 °C with a temperature range of 20 ± 2 °C. To discourage collembolans from escaping from the soil, the test is carried out under controlled light-dark cycles (preferably 12 hours light and 12 hours dark) with illumination of 400 to 800 lux in the area of the test vessels.
24. In order to check the soil humidity the vessels are weighed at the beginning, in the middle and at the end of the test. Weight loss > 2% is replenished by the addition of de-ionised water. It should be noted that loss of water can be reduced by maintaining a high air-humidity (> 80%) in the test incubator.
25. The pH must be measured at the beginning and the end of both the range-finding test and the definitive test. Measurements should be made in one extra control sample and one extra sample of the treated (all concentrations) soil samples prepared and maintained in the same way as the test cultures, but without addition of the collembolans.
Test procedure and measurements
26. For each test concentration, an amount of test soil corresponding to 30 g fresh weight is placed into the test vessel. Controls, without the test substance, are also prepared. If a vehicle is used for application of the test substance, one control series containing the vehicle alone should be run in addition to the test series. The solvent or dispersant concentration should be the same as that used in the test vessels containing the test substance.
27. The individual springtails are carefully transferred into each test vessel (allocated randomly to the test vessels) and placed onto the surface of the soil. For efficient transfer of the animals a low-suction air flow device can be used. The number of replicates for test concentrations and for controls depends on the test design used. The test vessels are positioned randomly in the test incubator and these positions are re-randomised weekly.
28. For the F. fimetaria test twenty adults, 10 males and 10 females, should be used per test-vessel. Food is added to the test vessels at the beginning of the test and then after 14 days. On day 21 the soil samples should be extracted and counted. For F. fimetaria the gender are discriminated by size in the synchronised animal batch used for the test. Females are distinctively larger than the males (See Annex 2)
29. For the F. candida test 10 juveniles per test vessel should be used. Food is added to the test vessels at the beginning of the test and then after about 2 weeks. On day 28 the soil samples should be extracted and counted.
30. As a suitable food source a sufficient amount, e.g. 2-10 mg, of granulated dried baker's yeast, commercially available for household use, is added to each container at the beginning of the test and after about 2 weeks.
31. At the end of the test, mortality and reproduction are assessed. After 21 (F. fimetaria) and 28 (F. candida) days, they are extracted from the test soil (see Annex 3) and counted [12]. A collembolan is recorded as dead if not present in the extraction. The extraction and counting method should be validated. The validity includes extraction efficiency of juveniles greater than 95%.
32. Practical summary and timetable of the test procedure are described in ANNEX 1.
Test design
Range-finding test
33. When necessary, a range-finding test is conducted with, for example, five test substance concentrations of 0.1, 1.0, 10, 100, and 1000 mg/kg (dry weight of soil). Two replicates for each treatment and control are sufficient.
34. The duration of the range-finding test is two weeks for F. fimetaria and 3 weeks for F. candida. At the end of the test, mortality and reproduction of the Collembola are assessed. The number of adults, and the occurrence of juveniles should be recorded. Additional information, from tests with similar compounds or from literature, on mortality or reproduction of Collembola may also be useful in deciding on the range of concentrations to be used in the range-finding test.
Definitive test
35. For determination of the ECx (e.g. EC10, EC50), twelve concentrations should be tested. At least two replicates for each test concentration treatment and six control replicates are recommended. The spacing factor may vary, i.e. less than or equal to 1.8 in the expected effect range and above 1.8 at the higher and lower concentrations.
36. For determination of the NOEC, at least five concentrations in a geometric series should be tested. Four replicates for each test concentration treatment plus eight controls are recommended. The concentrations should be spaced by a factor not exceeding 1.8.
37. A combined approach allows for determination of both the NOEC and ECx. For the two approaches, eight treatment concentrations in a geometric series should be used. Four replicates for each treatment plus eight controls are recommended. The concentrations should be spaced by a factor not exceeding 1.8.
38. If no effects are observed at the highest concentration in the range-finding test (i.e. 1000 mg/kg) the reproduction test can be performed as a limit test, using a test concentration of 1000 mg/kg and the control. A limit test will provide the opportunity to demonstrate that there is no statistically significant effect at the limit concentration. Eight replicates should be used for both the treated soil and the control.
DATA AND REPORTING
Treatment of results
39. Test main endpoint is the reproductive output (e.g. the number of juveniles produced per test vessel). The statistical analysis requires the arithmetic mean (X) and the variance (s²) for the reproductive output to be calculated per treatment and per control. X and s² are used for ANOVA procedures such as the Student t test, Dunnett test, or Williams’ test as well as for the computation of 95% confidence intervals.
40. The number of surviving females in the untreated controls is a major validity criterion and has to be documented. As in the range-finding test, all other harmful signs should be recorded in the final report as well.
ECx
41. ECx-values including their associated lower and upper 95% confidence limits for the parameter are calculated using appropriate statistical methods (e.g. probit analysis, logistic or Weibull function, trimmed Spearman-Karber method, or simple interpolation). An ECx is obtained by inserting a value corresponding to x% of the control mean into the equation found. To compute the EC50 or any other ECx, the per treatment means (X) should be subjected to regression analysis.
NOEC/LOEC
42. If a statistical analysis is intended to determine the NOEC/LOEC, per-vessel statistics (individual vessels are considered replicates) are necessary. Appropriate statistical methods should be used according to OECD Document 54 on the Current Approaches in the Statistical Analysis of Ecotoxicity Data: a Guidance to Application [9]. In general, adverse effects of the test item compared to the control are investigated using one-tailed (smaller) hypothesis testing at p = 0.05.
43. Normal distribution of data can be tested e.g. with the Kolmogorov-Smirnov goodness-of-fit test, the Range-to-standard-deviation ratio test (R/s-test) or the Shapiro-Wilk test (two-sided, p = 0.05). Cochran's test, Levene test or Bartlett's test, (two-sided, p = 0.05) may be used to test variance homogeneity. If the prerequisites of parametric test procedures (normality, variance homogeneity) are fulfilled, One-way Analysis of Variance (ANOVA) and subsequent multi-comparison tests can be performed. Multiple comparisons (e.g. Dunnett's t-test) or step-down trend tests (e.g. Williams' test in case of a monotonous dose-response relationship) can be used to calculate whether there are significant differences (p = 0.05) between the controls and the various test item concentrations (selection of the recommended test according to OECD Document 54 on the Current Approaches in the Statistical Analysis of Ecotoxicity Data: a Guidance to Application [9]). Otherwise, non-parametric methods (e.g. Bonferroni-U-test according to Holm or Jonckheere-Terpstra trend test) should be used to determine the NOEC and the LOEC.
Limit test
44. If a limit test (comparison of control and one treatment only) has been performed and the prerequisites of parametric test procedures (normality, homogeneity) are fulfilled, metric responses can be evaluated by the Student test (t-test). The unequal-variance t-test (Welch t-test) or a non parametric test, such as the Mann-Whitney-U-test may be used, if these requirements are not fulfilled.
45. To determine significant differences between the controls (control and solvent control), the replicates of each control can be tested as described for the limit test. If these tests do not detect significant differences, all control and solvent control replicates may be pooled. Otherwise all treatments should be compared with the solvent control.
Test report
46. The test report should at least include the following information:
Test substance
- the identity of the test substance, batch, lot and CAS-number, purity;
- physico-chemical properties of the test substance (e.g. log Kow, water solubility, vapour pressure, Henry’s constant (H) and preferably information on the fate of the test substance in soil).
Test organisms
- identification of species and supplier of the test organisms, description of the breeding conditions and age range of test organisms.
Test conditions
- description of the experimental design and procedure;
- preparation details for the test soil; detailed specification if natural soil is used (origin, history, particle size distribution, pH, organic matter content)
- water holding capacity of the soil;
- a description of the technique used to apply the test substance to the soil;
- test conditions: light intensity, duration of light-dark cycles, temperature;
- a description of the feeding regime, the type and amount of food used in the test, feeding dates;
- pH and water content of the soil at the start and end of the test (control and each treatment)
- detailed description of the extraction method and extraction efficiency.
Test results
- the number of juveniles determined in each test vessel at the end of the test;
- number of adult females and adult mortality (%) in each test vessel at the end of the test
- a description of obvious physiological or pathological symptoms or distinct changes in behaviour;
- the results obtained with the reference test substance;
- the LC50, the NOEC and/or ECx (e.g. EC50, EC10) for reproduction with 95% confidence intervals, and a graph of the fitted model used for its calculation, the slope of the dose-response curve and its standard error (See [9]).
- all information and observations helpful for the interpretation of the results
- power of the actual test if hypothesis testing is done [9].
- deviations from procedures described in this guideline and any unusual occurrences during the test.
LITERATURE
(1) Wiles JA and Krogh PH (1998) Testing with the collembolans I. viridis, F. candida and F. fimetaria. In Handbook of soil invertebrate toxicity tests (ed. H Løkke and CAM Van Gestel), pp. 131-156. John Wiley & Sons, Ltd., Chichester
(2) ISO (1999) Soil Quality - Effects of soil pollutants on Collembola (Folsomia candida): Method for determination of effects on reproduction. No. 11267. International Organisation for Standardisation, Geneve
(3) Burges A and Raw F (Eds) (1967) Soil Biology. Academic Press. London
(4) Petersen H and Luxton M (1982) A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39: 287-388
(5) Petersen H (1994) A review of collembolan ecology in ecosystem context. Acta Zoologica Fennica 195: 111-118
(6) Hopkin SP (1997). Biology of the Springtails (Insecta : Collembola). Oxford University Press. 330pp (ISBN 0-19-854084-1)
(7) Ulber B (1983) Einfluss von Onychirurus fimatus Gisin (Collembola, Onychiuridae) und Folsomia fimetaria L. (Collembola, Isotomidae) auf Pythium ultimum Trow. einen Erreger des Wurzelbrandes der Zuckerrübe. In New trends in soil Biology (Lebrun Ph, André HM, De Medts A, Grégoire-Wibo, Wauthy G (Eds), Proceedings of the VI. international colloquium on soil zoology, Louvain-la-neuve (Belgium), 30 August-2 September 1982, I Dieu-Brichart, Ottignies-Louvain-la-Neuve, pp. 261-268
(8) OECD (Organisation for Economic Cooperation and Development) (1984) Earthworm, Acute Toxicity Tests, Guideline No. 207. OECD, Paris
(9) OECD, (2006) Current approaches in the statistical analysis of ecotoxicity data: a guidance to application. OECD series on testing and assessment Number 54. OECD Paris ENV/JM/MONO(2006)18
(10) Scott-Fordsmand JJ and Krogh PH (2005) Background report on prevalidation of an OECD springtail test guideline. Environmental Project Nr. 986. Miljøstyrelsen 61 pp. Danish Ministry for the Environment.
(11) Krogh, P.H., 2008. Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest. Danish EPA Report Series, In press.
(12) Krogh PH, Johansen K and Holmstrup M (1998) Automatic counting of collembolans for laboratory experiments. Appl. Soil Ecol. 7, 201-205
(13) Fjellberg A (1980) Identification keys to Norwegian collembolans. Norsk Entomologisk Forening.
(14) Edwards C.A. (1955) Simple techniques for rearing Collembola, Symphyla and other small soil inhabiting arthropods. In Soil Zoology (Kevan D.K. McE., Ed). Butterworths, London, pp. 412-416
(15) Goto HE (1960) Simple techniques for the rearing of Collembola and a not on the use of a fungistatic substance in the cultures. Entomologists' Monthly Magazine 96:138-140.
ANNEX 1
Main actions and timetable for performing a collembolan test
The steps of the test can be summarised as follows:
| Time (day) |
Action |
| -33 to -36 | Preparation of synchronous F. fimetaria culture |
| -14 | Prepare artificial soil (mixing of dry constituents) Check pH of artificial soil and adjust accordingly Measure max WHC of soil Pre-moist soil |
| -9 to -12 | Preparation of synchronous F. candida culture |
| -2 to -1 | Sorting Collembola for testing |
| -1 | Distribute into batches Prepare stock solutions and apply test substance if solvent required |
| 0 | Prepare stock solutions and apply test substance if solid compound or surface application is required. Measure soil pH and weigh the containers. Add food. Introduce collembolans. |
| 14 | Range-finding test: Terminate test, extract animals, measure soil pH and loss of water (weight) Definitive tests: Measure moisture content and replenish water and add 2-10 mg yeast |
| 21 | Definitive F. fimetaria test: Terminate test, extract animals, measure soil pH and loss of water (weight) |
| 28 | Definitive F. candida test: Terminate test, extract animals, measure soil pH and loss of water (weight) |
ANNEX 2
GUIDANCE on REARING AND SYNCHRONISATION OF F. fimetaria and F. candida
The time and durations given in this guidance should be checked for each specific collembolan strain to ensure that timing will allow for sufficient synchronized juveniles. Basically incidence of oviposition after the adults are transferred to fresh substrate and egg hatching determines the appropriate day for egg collection and collection of synchronous juveniles.
It is recommended to have a permanent stock culture consisting of e.g. 50 containers/Petri dishes with F. fimetaria. The stock culture should be kept in a good feeding condition by weekly feeding, watering and removal of old food and carcasses. Too few collembolans on the substrate may result in inhibition by more fungal growth. If the stock culture is used for egg production too often, the culture may get fatigue. Signs of fatigue are dead adults and mould on the substrate. The remaining eggs from the production of synchronous animals can be used to rejuvenate the culture.
In a synchronous culture of F. fimetaria, males are distinguished from females primarily by size. Males are clearly smaller than females, and the walking speed of the males is faster than for females. Correct selection of the gender requires little practice and can be confirmed by microscopic inspection of the genital area [13].
1. Rearing
1.a. Preparation of culturing substrate
The culturing substrate is plaster of Paris (calcium sulphate) with activated charcoal. This provides a moist substrate, with the function of the charcoal being to absorb waste gases and excreta [14, 15]. Different forms of charcoal may be used to facilitate observations of the Collembola. For example, powdered charcoal is used for F. candida and F. fimetaria (producing a black/grey plaster of Paris).
Substrate constituents:
- 20 ml of activated charcoal
- 200 ml of distilled water
- 200 ml of plaster of Paris
or
- 50 g of activated pulverized charcoal
- 260-300 ml of distilled water
- 400 g plaster of Paris.
1.b. Breeding
Collembolans are held in containers such as Petri dishes (90 mm x 13 mm), with the bottom covered by a 0.5 cm layer of plaster /charcoal substrate. They are cultured at 20 ±? 1 °C at a light:dark cycle of 12:12 hours (400-800 Lux). Containers are kept moist at all times ensuring that the relative humidity of the air within the containers is 100%. This can be guaranteed by presence of free water within the porous plaster, but avoiding generating a water film on the plaster surface. Any dead individuals should be removed from the containers, as should any mouldy food. To stimulate production of eggs it is necessary to transfer the adult animals to Petri dishes with newly prepared plaster of Paris/charcoal substrate.
1.c. Food source
Granulated dried baker's yeast is used as the sole food supply for both F. candida and F fimetaria. Fresh food is provided either once or twice a week, to avoid moulding. It is placed directly on the plaster of Paris in a small heap. The mass of baker's yeast added should be adjusted to the size of the collembolan population, but as a general rule 2-15 mg is sufficient.
2. Synchronisation.
The test must be performed with synchronized animals to obtain homogeneous test animals of the same instar and size. Furthermore, the synchronisation enables discrimination of F. fimetaria males and females from the age of 3 weeks and onwards based on sexual dimorphism, i.e. size differences. The procedure below is a suggestion on how to obtain synchronized animals (the practical steps are optional).
2.a. Synchronisation.
- Prepare containers with a 0.5 cm layer of plaster of Paris/charcoal substrate.
- For egg laying transfer 150-200 adult F. fimetaria and 50-100 F. candida from the best 15-20 containers of the stock culture with 4-8 weeks old substrate to the containers and feed them 15 mg baker's yeast. Avoid bringing juveniles together with adults as presence of juveniles may inhibit egg production.
- Keep the culture at 20 ± 1 °C (the mean should be 20 °C) and a light:dark cycle of 12:12 hours (400-800 Lux). Ensure that fresh food is available and the air is water saturated. Lack of food may lead the animals to defecate on the eggs resulting in fungal growth on the eggs or F. candida may cannibalize its own eggs.
- After 10 days the eggs are carefully collected with a needle and spatula and moved to “egg-paper” (small pieces of filter paper dipped in plaster of Paris/charcoal slurry) which is placed in a container with fresh plaster/charcoal substrate. A few grains of yeast are added to attract the juveniles and make them leave the egg-paper. It is important that the egg-paper and substrate are humid, or the eggs will dehydrate.
- After three days most of the eggs on the egg-paper will have hatched, and some juveniles may be found under the egg-paper.
- To have evenly aged juveniles, the egg-paper with un-hatched eggs is removed from the Petri dish with forceps. The juveniles, now 0-3 days, stay in the dish and are fed baker's yeast. Un-hatched eggs are discharged.
- Eggs and hatched juveniles are cultured in the same manner as the adults. In particular for F. fimetaria the following measures should be taken: ensuring sufficient fresh food, old moulding food is removed, after 1 week the juveniles are divided into new Petri dishes provided that the density is above 200.
2.b. Handling collembolans at test initiation
- 9-12 days old F. candida or the 23-26 days old F. fimetaria are collected, e.g. by suction, and released into a small container with moist plaster/charcoal substrate and their physical condition is checked under the binocular (injured and damaged animals are disposed). All steps should be done while keeping the collembolans in a moist atmosphere to avoid drought stress, e.g. by using wetted surfaces etc.
- Turn the container up-side down and knock on it to transfer the collembolans to the soil. Static electricity should be neutralized, otherwise the animals may just fly into the air, or stick to the side of the test container and dry out. An ionizer or a moist cloth below the container may be used for neutralisation.
- The food should be spread all over the soil surface and not just in one lump.
- During transportation and during the testing period it should be avoided to knock or otherwise physically disturb the test containers, as this may increase the compaction of the soil, and hamper the interaction between the collembolans.
3. Alternative Collembolan species
Other collembolan species may be selected for testing according to this guideline such as Proisotoma minuta, Isotoma viridis, Isotoma anglicana, Orchesella cincta, Sinella curviseta, Paronychiurus kimi, Orthonychiurus folsomi, Mesaphorura macrochaeta. A number of prerequisites must be fulfilled in advance before using alternative species:
- they must be unequivocally identified;
- the rationale for the selection of the species should be given;
- it must be ensured that the reproductive biology is included in the testing phase so it will be a potential target during the exposure;
- the life-history must be known: age at maturation, duration of egg development, and instars subject to exposure;
- optimal conditions for growth and reproduction must be provided by the test substrate and food supply;
- variability must be sufficiently low for precise and accurate toxicity estimation.
ANNEX 3
Extraction and counting of animals
1. Two methods of extraction can be performed.
1.a. First method: A controlled temperature gradient extractor based on principles by MacFayden can be used [1]. The heat coming from a heating element at the top of the extraction box (regulated through a thermistor placed on the surface of the soil sample). The temperature in the cooled liquid surrounding the collecting vessel is regulated through a thermistor situated at the surface of the collection box (placed below the soil core). The thermistors are connected to a programmable controlling unit which raises the temperature according to a pre-programmed schedule. Animals are collected in the cooled collecting box (2 °C) with a bottom layer of plaster of Paris/charcoal. Extraction is started at 25 °C and the temperature is increased automatically every 12 h by 5 °C. After 12 h at 40 °C the extraction is finished.
1.b. Second method: After the experimental incubation period the number of juvenile Collembola present is assessed by flotation. This involves emptying the tube of soil into a 250 ml vessel and adding approx. 200 ml of distilled water. The soil is gently agitated with a fine paintbrush to allow Collembola to float to the water surface. A small amount, approx. 0.5 ml, of black Kentmere photographic dye may be added to the water to aid counting by increasing the contrast between the water and the white Collembola. The dye is not toxic to Collembola.
2. Counting: Counts of numbers may be carried out by eye or under a light microscope using a grid placed over the floatation vessel or by photographing the surface of each vessel and later counting the Collembola on enlarged prints or projected slides. Counts may also be performed using digital image processing techniques [12]. All techniques should be validated.
ANNEX 4
DETERMINATION OF THE MAXIMUM WHC OF THE SOIL
The following method for determining the maximum water holding capacity (WHC) of the soil has been found to be appropriate. It is described in Annex C of the ISO DIS 11268-2 (Soil Quality - Effects of pollutants on earthworms (Eisenia fetida). Part 2: Determination of effects on reproduction (3)).
Collect a defined quantity (e.g. 5 g) of the test soil substrate using a suitable sampling device (auger tube etc.). Cover the bottom of the tube with a wet piece of filter paper and then place it on a rack in a water bath. The tube should be gradually submerged until the water level is above to the top of the soil. It should then be left in the water for about three hours. Since not all water absorbed by the soil capillaries can be retained, the soil sample should be allowed to drain for a period of two hours by placing the tube onto a bed of very wet finely ground quartz sand contained within a covered vessel (to prevent drying). The sample should then be weighed, dried to constant mass at 105 °C. The water holding capacity (WHC) must be calculated as follows:
WHC (in % of dry mass)
Where:
S = water-saturated substrate + mass of tube + mass of filter paper
T = tare (mass of tube + mass of filter paper)
D = dry mass of substrate
ANNEX 5
DETERMINATION OF SOIL pH
The following method for determining the pH of a soil is based on the description given in ISO DIS 10390: Soil Quality – Determination of pH (15).
A defined quantity of soil is dried at room temperature for at least 12 h. A suspension of the soil (containing at least 5 grams of soil) is then made up in five times its volume of either a 1 M solution of analytical grade potassium chloride (KCl) or a 0.01 M solution of analytical grade calcium chloride (CaCl2). The suspension is then shaken thoroughly for five minutes and then left to settle for at least 2 hours but not for longer than 24 hours. The pH of the liquid phase is then measured using a pH-meter that has been calibrated before each measurement using an appropriate series of buffer solutions (e.g. pH 4.0 and 7.0).
Version 1.0 December 2008, © Danish Environmental Protection Agency