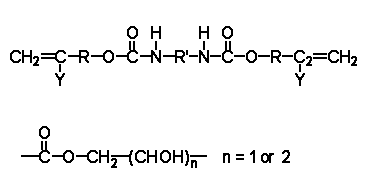|
Environmental Project no. 901, 2004 Evaluation of Alternatives for Compounds under Risk Assessment in the EU, Bisphenol AContents3 Search in the Danish Product Register
4 Bisphenol A in products in contact with food
8 Evaluation of the possible alternatives to bisphenol A PrefaceThe present report is the result of a project funded by the Danish Environmental Protection Agency (Danish EPA), Programme for Cleaner Products etc., 2002, which was initiated in September 2002. The report contains a compilation of data describing the use of bisphenol A and possible alternatives in coated food and beverage containers, polycarbonates, thermographic printing, toners and printing inks and an assessment of the potential hazards of the alternative substances to the environment and human health. CETOX (Centre for Integrated Environment and Toxicology) which is a "centre without walls" between DHI Water & Environment (DHI) and Danish Toxicology Center (DTC) prepared the report. The project was followed by an advisory group, which held two meetings during the project period. The advisory group was composed of the following members:
We thank the members of the steering committee for their contribution and co-operation during the project. Hørsholm, 18 December 2003 Lise Møller, CETOX Summary and conclusionsThis report reviews the use of bisphenol A and its possible alternatives in coated food and beverage containers, polycarbonates, thermografic printing, toners and printing inks. Bisphenol A has shown estrogenic properties (in vitro) and has endocrine modulating activity in both in vitro and in vivo screening assays but the levels causing endocrine disrupting effects in fetuses of animals and humans are widely debated. Bisphenol A is classified with Xi; R36/37/38 R43 (List of dangerous substances, Danish EPA, 2002), which is suggested to be changed to Repr. Cat. 3; R62 (possible risk of impaired fertility); Xi; R37-41, R43 (irritating to respiratory system and risk of serious damage to eyes, may cause sensitisation by skin contact) (EU, 2003). The objective was to identify and evaluate possible alternatives to bisphenol A in certain application areas identified by the Danish EPA on the basis of the draft EU risk assessment for bisphenol A (EU, 2002), i.e. can coatings and lids for glass containers etc., polycarbonate bottles, thermal paper and toners supplemented with printing inks. As bisphenol A may be contained in these products imported to and used in Denmark, the project includes both bisphenol A used as an intended chemical ingredient and bisphenol A occurring as a residual monomer in polymeric materials. Possible alternatives to bisphenol A in can coatings, polycarbonates and printing inks were identified on the basis of the information given by the industry, Danish Product Registry (DPR) and additional information from the internet. The available information on alternatives pointed out by the industry and on the possible alternatives verified on the internet consisted mainly of groups of chemicals. For some applications, e.g. polycarbonates, health and environmental assessments of alternatives should include an overall assessment of the polycarbonate product as a whole and thus not be limited to one or a few substances substituting bisphenol A in a plastic product. The screening of environmental and health properties of the polyester and polyamide alternatives indicates that these groups are possibly less harmful to health and the environment than bisphenol A. On the other hand, the polyacrylate and polymerised rosin alternatives may cause the same effects or more hazardous effects on both environment and health as bisphenol A (Table 8.1). The economical consequences of substituting alternatives for bisphenol A will depend on the specific case. This is exemplified by the use of polyester-based coatings in cans. However, polyester-based coatings cannot always replace the epoxy-based coatings because of failing security (limited chemical resistance and adherence). In some cases, polyester-based coatings are more expensive compared to the epoxy-based coatings, limiting their use to applications in which their particular properties, e.g. higher flexibility in drawn cans, are needed whereas the use of drawn cans is limited to certain can sizes. The economical consequences of substituting an alternative substance for bisphenol A will thus depend on many factors and can be investigated case by case on the basis of detailed information on the specific use of a chemical. Considering the relatively short project period of approx. one year, the coating industry has not been able to commit itself to a co-operation regarding an assessment of specific alternatives. As the development of such alternatives typically takes several years, the industry has estimated the possibilities of achieving usable results within such a short project period to be limited. The industry is, however, open to consider co-operation if the project period is prolonged (CEPE, 2003). The two European associations, APME (the plastic manufacturers) and CEFIC (the European Chemical Industry Council) indicate, however, that the industry does not want to co-operate with the individual national projects but prefers joint European initiatives (Plastindustrien i Danmark, 2003). As a result of this investigation it seems that compared to the focus on the bisphenol A diglycidylether (BADGE)-related can coatings and polycarbonates there is generally less focus on the bisphenol A content and substitution hereof in thermographic printing, toners and printing inks. In order to reduce the hazardous effects on both health and the environment, it is recommended to make careful assessments of the specific environmental and health properties of the alternatives to bisphenol A. Sammenfatning og konklusionerDenne rapport opsumerer anvendelsen af bisphenol A og dets mulige alternativer i overfladebelægninger i fødevareemballage, polycarbonater, termopapir, tonere og trykfarver. Bisphenol A har østrogene egenskaber, men hvilke niveauer, der forårsager hormonforstyrrende effekter i dyre- og menneskefostre, diskuteres. Bisphenol A er klassificeret med Xi; R36/37/38 R43 (Listen over farlige stoffer, Miljøstyrelsen, 2002), der foreslås ændret til Repr. Cat. 3; R62 (mulighed for skade på forplantningsevnen); Xi; R37-41, R43 (irriterer åndedrætsorganerne og forårsager risiko for alvorlig øjenskade; kan give overfølsomhed ved kontakt med huden) (EU, 2003). Formålet var at identificere og vurdere de mulige alternativer til bisphenol A i bestemte anvendelsesområder, identificeret af Miljøstyrelsen, på baggrund af EU's risikovurdering af bisphenol A (EU, 2002), dvs. belægninger i konservesdåser og låg til glasemballage m.m., flasker af polycarbonat, termopapir og tonere suppleret med trykfarver. Da disse produkter, der importeres til og anvendes i Danmark, kan indeholde bisphenol A, omfatter projektet både bisphenol A anvendt som kemisk bestanddel og restmonomerer af bisphenol A i polymere materialer. Bisphenol A's mulige alternativer i belægninger i konservesdåser, i polycarbonater og trykfarver blev identificeret på baggrund af informationer fra industrien, søgning i Produktregisteret og supplerende information fra internettet. Oplysninger om alternativerne fra industrien og internettet bestod hovedsageligt af informationer om kemikaliegrupper. For nogle anvendelser, f.eks. polycarbonater, bør miljø- og sundhedsvurderingerne af alternativerne omfatte en samlet vurdering af polycarbonatproduktet som en helhed og ikke være begrænset til nogle få stoffer, der erstatter bisphenol A i et plasticprodukt. Screeningen af polyester- og polyamidalternativernes miljø- og sundhedsmæssige egenskaber viser, at disse grupper kan forårsage færre skadelige effekter på miljø og sundhed end bisphenol A, mens polyacrylat og de polymeriserede harpiks alternativer på den anden side kan forårsage de samme eller mere skadelige effekter på både miljø og sundhed end bisphenol A (tabel 8.1). De økonomiske omkostninger ved at substituere bisphenol A med alternativer afhænger af den konkrete situation. Dette kan eksemplificeres ved brugen af de polyesterbaserede belægninger i konservesdåser. De polyesterbaserede belægninger kan ikke ubetinget erstatte epoxylakkerne pga svigtende sikkerhed (begrænset kemisk resistens og vedhæftning). De polyesterbaserede belægninger er i nogle tilfælde dyrere sammenlignet med de epoxybaserede, hvilket begrænser deres anvendelse til områder, hvor deres egenskaber er essentielle, f.eks. større fleksibilitet, der er en forudsætning i produktionen af trukne dåser, mens anvendelsen af trukne dåser er begrænset til bestemte dåsestørrelser. De økonomiske omkostninger ved substitution af bisphenol A med alternativer vil derfor afhænge af mange faktorer og skal undersøges fra sag til sag på baggrund af oplysningerne om anvendelsen af det specifikke kemiske stof. Set i lyset af den relativt korte projektperiode på omtrent 1 år har dåseindustrien ikke ønsket at indgå i et samarbejde om vurdering af specifikke alternativer. Da det typisk tager flere år at udvikle sådanne alternativer, har industrien vurderet, at mulighederne for at opnå brugbare resultater vil være begrænset i så kort en projektperiode. Men industrien er åben over for samarbejdsmuligheder ved en eventuel projektforlængelse (CEPE, 2003) De to europæiske sammenslutninger APME (plasticindustrien) og CEFIC (rådet for den europæiske industri) påpeger at industrien ikke ønsker at indgå samarbejde med de enkelte lande, hver især, som f.eks. et nationalt projekt som dette, men foretrækker fælles europæiske initiativer (Plastindustrien i Danmark, 2003). Nærværende undersøgelse, tyder på at der i forhold til den fokus, der er rettet mod de bisphenol A diglycidylether (BADGE)-relaterede dåsebelægninger og bisphenol A holdige polycarbonater, generelt er mindre fokus på bisphenol A indhold og substitution heraf i termopapir, tonere og trykfarver. Med henblik på at reducere de skadelige effekter på både miljø og sundhed anbefales det at foretage en grundig vurdering af de specifikke bisphenol A alternativers miljø- og sundhedsmæssige egenskaber. 1 IntroductionTraces of bisphenol A found in canned food and beverages after contact with epoxy or polycarbonate surfaces have brought bisphenol A into focus. Bisphenol A has shown estrogenic properties in laboratory tests and acts as an endocrine disrupter but the levels causing effects in fetuses of animals and humans are widely debated. The aim of this project was to identify and evaluate alternatives to certain applications of bisphenol A, identified by the Danish EPA on the basis of the draft EU risk assessment for bisphenol A (EU, 2002). The following applications had been identified:
These applications could, however, be extended to cover further focus areas, identified from the EU risk assessment (EU, 2002). The EU risk assessment Only for thermal paper recycling, the use of bisphenol A in PVC production (inhibitor, additive packages) and its use as an antioxidant in the production of PVC plasticizers, it was concluded that there is a need for limiting the risks to the water and sediment environments. Furthermore, it was concluded that there is a need for limiting the risks of eye and respiratory tract irritation, liver effects and reproductive toxicity during the manufacture of bisphenol A (EU, 2002). For the remainder of applications/processes evaluated in the EU risk assessment of bisphenol A, it was concluded that there is either no need for risk reduction beyond what has already been applied or that there is a need for more information/testing. Of the three applications singled out in the EU risk assessment, thermal paper was already a focus area in this project. The use of bisphenol A in PVC is expected to be of less relevance since the industry has agreed to phase out the use of bisphenol A for these purposes (EU, 2002). Furthermore, the manufacture of bisphenol A was not relevant for studies of substitution in this project and thus no other focus areas than those pinned out above were considered in this project. However, based on search in the Danish Product Register in which bisphenol compounds are registered in 72 printing ink products, the focus area on toner was extended to cover printing inks as well. According to the EU risk assessment the applications specified in Table 1.1 are the major applications of bisphenol A in Europe, with a total EU consumption of 685,000 tonnes/year (EU, 2003A). Table 1.1 The major applications of bisphenol A in Europe (EU, 2003A)
2 ObjectiveThe objective of this project was to gather knowledge of alternatives to bisphenol A used in thermal paper, toners, printing inks and in food and beverage packaging. The project included bisphenol A used as an intended chemical ingredient and bisphenol A occurring as a residual monomer in polymeric materials. The use of bisphenol A in Denmark for the production of the above goods is estimated to be very modest. However, bisphenol A occurs in these types of products imported to and used in Denmark. The project included the use of bisphenol A in products in the EU and, therefore, the identification and evaluation of alternatives were made in collaboration with international producers and users. The project included the following phases:
3 Search in the Danish Product RegisterThe Danish Product Register (DPR) is registering hazardous products for professional use according to the Danish legislation. This means that any person starting professional manufacture or importation of products, which may constitute a danger to or in any other way adversely affect safety or health, prior to commencement of these activities must submit notification to the DPR. The notification of a product includes among other things:
A manufacture or importer who has submitted a notification must inform the DPR about any changes in the information submitted. However, experience shows that some manufacturers and importers fail to inform the DPR about such changes. In the following sections, bisphenol A substances (BPA-substances) are defined by derivatives of bisphenol A (BPA) or polymers containing BPA as monomer. The purpose of the search in the DPR was:
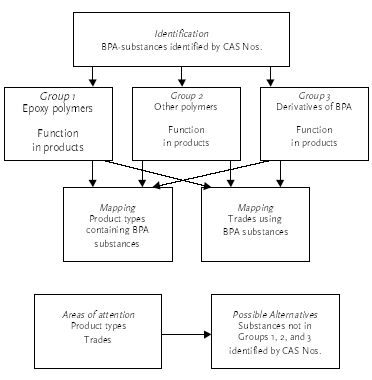
The overview of product types containing BPA-substances and trades using the products was mainly used to verify the chosen focus areas or to state new areas of attention in respect to this project. The information describing the function of BPA-substances in the products was used to identify alternatives to certain applications related to the selected focus areas. The strategy of the DPR search is illustrated in Figure 3.1. The registered BPA-based substances were identified and subsequently grouped in three groups. The function of the substances in the products was mapped out for each group. Furthermore, product types containing and industrial sector using BPA-substances were mapped out for each of the three groups. Potential alternatives to BPA-substances were identified in selected product types and trade categories relevant to the focus areas of this project.
Figure 3.1 The strategy of the D P R search The results of the DPR search related to the strategy are described in the following sections. 3.1 Identification of bisphenol A substancesThe identification of BPA-substances registered in the DPR was based on several searches with different inputs. The CAS No. 80-05-7 for BPA and synonyms/names of BPA were used as search inputs. A list with BPA-substances was generated. The list is divided into three groups: Group 1: Epoxy polymers Group 1 is a list of 347 substances. In this search, epoxy polymers are characterized as polymers containing monomers such as bisphenol A and epichlorhydrin or bisphenol A diglycidylether (BADGE) as a minimum. In addition, the polymers may contain other monomers. It has not been possible to determine the molecular weight or the degree of free epoxy-groups of the polymers from the search. Examples of polymers in group 1 are stated below:
Group 2 is a list of 69 substances. The polymers of this group contain BPA as monomer without having epoxy group functionalities. Examples are:
Group 3 is a list of 57 substances. Examples of derivatives of bisphenol A (not polymers) are stated below:
The function registered in the DPR of BPA-substances in products is stated below according to the three groups:
The function of the substances/polymers is mainly registered as binders in the DPR. The registered function "monomer" in group 3 is probably related to the acrylates represented by this group. Acrylates are monomers used for the manufacture of thermosetting acrylic surface-coating resins. The registered function of substances in products is based on a predetermined list of functions and on the knowledge of the persons working in the DPR. The manufacturer or the supplier of a product is not required to provide this kind of information. The substances mentioned in the three lists were mapped according to product types and trades in which the substances are used. 3.2 Mapping of bisphenol A substancesThe results of mapping the BPA-substances in groups 1, 2 and 3 are stated below in Tables 3.1 and 3.2. Table 3.1 lists the various product types containing BPA-substances from the three groups. Volume (T) is the total quantity (tonnes/year) of all BPA-substances used in products in a certain product type. The number columns give the total of products containing BPA-substances within each product type. Table 3.1 gives a selection of product types. The selection criterion was:
In Table 3.1 the product types relevant to the focus area of this project are typed in italics. Table 3.1 shows, for instance, that there are 72 "printing inks products" containing BPA-substances from Group 1 and that the total quantity of substances from all groups is 28 tonnes/year. Table 3.1 shows a selection of product types containing BPA-based substances. For each of the three groups, the table states the number of products containing BPA-substances within each product type (Number). Furthermore, the table gives the total amount (in tons) of BPA-substances from all three groups forming part of products within each product type (Volume). Product types relevant to the focus areas of this project are typed in italics. The product types
are related to the epoxy-coating of food and beverage containers, which is one of the focus areas of this project. The product types
are related to toners, which are another focus area of this project. The product types
are related to thermographic printing, which is also a focus area of this project. Table 3.1 Mapping of product types containing bisphenol A substances.
Table 3.1 shows that Group 1, epoxy polymers, is the largest group both with regard to members and to quantity in the investigated product types. This is not surprising considering the number of substances in Group 1. The table shows that epoxy polymers are widely used in many products. Table 3.2 lists the various trades using the BPA-substances in the three groups. The volume (T) is the total quantity (tonnes/year) of all BPA-substances used in products within a certain trade category. For each trade category the number columns specify the total products containing BPA-substances. Table 3.2 gives a selection of trades. The selection criterion was:
Table 3.2. shows, for instance, that there are 801 products containing Group 1 BPA-substances, which are used in the trade category "Manufacture of fabricated metal products, except machinery and equipment", and that the total quantity of BPA-substances from all three groups is 6648 tonnes/year. Table 3.2 gives a selection of trades using products containing BPA-based substances. For each of the three groups, the table states the number of products containing BPA-substances within a certain trade (Number). Furthermore, the table gives the total amount (in tons) of BPA-substances from all three groups used by a certain trade (Volume). Trade categories relevant to the focus areas of this project are typed in italics. In Table 3.2 the product types relevant to the focus area of this project are typed in italics. The trade categories
are related to epoxy-coating of food and beverage containers as well as to toners, which are the focus areas of this project. The trade category
is related to thermographic printing, which is another focus area of this project. Table 3.2 Mapping of trade categories using products containing bisphenol A substances.
Table 3.2 also shows that Group 1 is the largest group both with regard to numbers and to quantity in the investigated trade categories. There is not direct connection between Tables 3.1 and 3.2 as regards the figures and amounts stated. The individual product may be registered under more product types and especially under more trade categories and subcategories. Tables 3.1 and 3.2 must thus be considered as independent tables and amounts and numbers in the two tables must be considered as relative figures. The two tables show that the BPA-substances are widely used in most product types and trade categories investigated and that the DPR thus covers the focus areas of this project. However, some of the product types and trade categories cover more than just the focus areas. For instance, products, such as "paint and lacquers/varnishes" are not only related to the application "epoxy coated food and beverage containers". Products as "paint and lacquers/varnishes" have other applications, such as floor varnishing. The product type "toner" is not listed as containing BPA-substances. This may be due to the fact that toners do not have to be registered in the DPR or that they do not contain BPA-substances at all. 3.3 Information on alternativesThe search for information on substances/alternatives to BPA-substances from the DPR was divided into the following areas:
The generated lists of non-BPA-based substances in the above mentioned product types are only indications of possible alternatives to BPA-substances for the specified applications. The proposals for alternative substances are based on polymers which do not contain the monomer bisphenol A. Tables 3.3 – 3.7 state the alternatives found with respect to the above areas 1-5. Table 3.3 Substances/polymers in products used by manufactures of other fabricated metal products, e.g. manufacturing of light metal packaging
Table 3.4 Substances/polymers in products of the product type "Surface treatment of metal (not paints etc.)"
Table 3.5 Substances/polymers in products with product type "Paint and lacquers/varnishes"
Table 3.6 Substances/polymers in products with product type "Toners and Writing materials"
Table 3.7 Substances/polymers in products with product type. "Printing inks"
3.4 The DPR and search for bisphenol A alternativesThe Danish Product Register (DPR) registers hazardous products for professional use according to Danish legislation. This means that non-hazardous product like paper, bottles and other articles as well as products for private use are not covered by the DPR. The mapping showed that BPA-substances are widely used in several product types. The DPR registered function of BPA-substances in products is not designed for identifying alternative substances. The registration of function is not based on information from the manufacturer or supplier but is an assessment made by the case officer at the DPR. In addition, the DPR has not registered the function of substances for the last two years. Instead, the listed tables with possible alternatives are based on polymers relating to the focus areas of this project. Polymers are normally considered to have binding properties in the majority of products. However, the binding properties depend on molecular weight and functionality groups. The listed tables with alternative substances are only a supplement to the information from the industry. It has not been possible to obtain information from the DPR on alternatives to BPA-substances in thermal paper. The function of pure BPA as developing agent in thermal paper has not been considered in the DPR search. 4 Bisphenol A in products in contact with foodBisphenol A (BPA) containing products in contact with food mainly consist of coated food and beverage cans and vats (wine), coated lids for glass containers, besides some mineral water bottles and food and beverage containers, such as jugs made of polycarbonate (PC). 4.1 Coated food and beverage containersDue to the corrosive nature of much food and beverages, containers and lids made of metal (aluminium; iron with or without tin) are coated to ensure the integrity of the container and to avoid metal migration into the food, attack on the metal substrate by the food or deleterious organoleptic effects. Some food is sterilised in the container by thermal processing (e.g. 120°C for 1 hour) and the coating thus needs to retain its resistance properties at these elevated temperatures and throughout the shelf-life of the canned food. The required properties of can coatings can be summarised as:
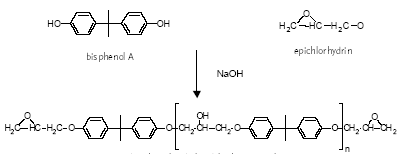
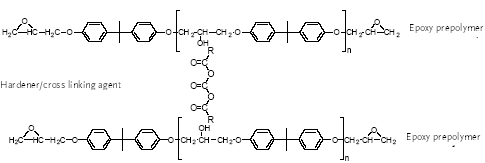
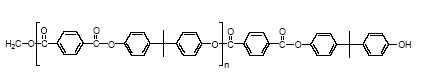
Several materials can be used for coating food and beverage containers (epoxy-phenolics, polyester, PVC, epoxy-anhydrides and epoxy-aminos or epoxy-acrylic aminos) but the choice of suitable coatings is limited for containers intended for very corrosive food. 4.1.1 Bisphenol A based epoxy coatingEpoxy-based coatings can withstand highly corrosive food and beverages (Glud & Marstrand, 2003; CEPE, 2003). Epoxy coatings are mainly thermoset, being based upon high molecular weight epoxy resins. A thermoset polymeric material cannot be melted or dissolved in solvents. The final dried film of the epoxy coating is made by curing/hardening an epoxy resin (pre-polymer) in the coating on the container or metal before can formation (Glud & Marstrand, 2003; CEPE, 2003). Epoxy resins are characterised by molecules with more than one epoxy group. In the curing process, the epoxy resin molecules are linked by a curing compound, which establishes itself as a permanent bridge (covalent bonds) between the epoxy and hydroxyl groups of the epoxy resin molecules (Figure 4.1). The curing renders the epoxy chemically resistant. The most common epoxy resins used in coatings are the bisphenol A glydicylether epoxy resins, of which bisphenol A is a major constituent. Bisphenol A glycidylether resins or prepolymers (Figure 4.1) are made from bisphenol A and epichlorhydrin of which the epichlorhydrin forms the glycidyl groups.
bisphenol A diglycidyl ether pre-polymer CURING:
READY POLYMER:
Figure 4.1 The process and structure of a bisphenol A glycidylether resin For most purposes, n ranges from 0.2 to 13 (average numbers) and for can coatings, prepolymers with n = 2 to 30 are used (surface coatings). According to the EU risk assessment, high molecular weight epoxy resins are used for can coating. If n is 0 or 1, the product is a viscous liquid while it is a brittle solid if n is larger than 1 (Polycondensation, 2003). Low molecular weight epoxy resins are liquid while higher molecular weight epoxies are solid at room temperature (Ullmann, 2002). For can coatings and other uses, for which high molecular weight prepolymers are required, short chain bisphenol A glydicylether epoxy resins (e.g. n = 0) are extended with additional bisphenol A an advancement process (EU, 2002 and Polycondensation, 2003). The most widely used epoxy resins for can coating are based on bisphenol A diglycidylether (BADGE). BADGE is an intermediate for preparing all bisphenol A/epichlorhydrin-based liquid epoxy resins (SPI, 2002B). The amount of `free' (residual) BADGE in the resin is dependent on many factors, of which molecular weight is one. Common curing compounds (cross-linking compounds) are polyamines (ethylene diamine, diethylene triamine, triethylene tetraamine), polyamides, acid anhydrides, polymercaptans, isocyanates, amino resins and phenolic resins. Among these, the polyamine and polyamide curing agents are used for heavy duty coatings (storage tanks, holds of ships, pipes etc.) and normally not for can coatings (CEPE, 2003). Epoxy resins reacted with an excess of polyamine yields an adduct, a polymer with amine functionality, which has certain advantages over aliphatic polyamines. Adducts can be made from both solid and liquid epoxy resins. These adducts are used in heavy-duty coatings as curing agents for epoxy resin-based coatings. For can coatings, high temperature cured coatings are used. As high molecular weight epoxy resin prepolymers are used, the hydroxyl groups become as important or even more important than the epoxy groups for cross-linking and thus curing agents able to cross-link between hydroxyl groups are used. They are phenol formaldehyde resins (Figure 4.2), melamine formaldehyde resins, urea formaldehyde resins and polyisocyanates (outside only).
Figure 4.2 Phenol formaldehyde resin Epoxy-anhydride coatings The epoxy-anhydride coatings are high molecular weight epoxy resins cross-linked with anhydride hardeners. The epoxy-anhydrides are thermoset systems, which are normally pigmented with titanium dioxide. With a good flexibility and a very good packing resistance, the BPA-containing epoxy-anhydride coatings are used for e.g. some drawn cans as the white internal lacquer in some maize (sweet corn) cans (CEPE, 2003). Epoxy-amino and epoxy-acrylic amino coatings The epoxy-amino and epoxy-acrylic amino coatings are high molecular weight epoxy cross-linked with amino resins. With a good flexibility but a limited packing resistance, the BPA-containing lacquers are used for e.g. beer and beverage cans (CEPE, 2003). 4.1.2 BPA-free alternatives in can liningsThere are only few BPA-free alternatives available on the market today and these alternatives are limited for use in specific products. Non-BPA containing epoxies are epoxy novolac resins and cycloaliphatic epoxy resins. Besides these, PVC is not containing epoxy, but most coatings using PVC contain molecules/resins with some epoxy functionality (CEPE, 2003). Phenolic resins Phenolic resins (Figure 4.3) are reaction products of phenols and an aldehyde, usually formaldehyde (HCHO), in acid solution (surface coatings). Commercially used phenols are bisphenols, phenol, cresols, xylenols, p-t-butylphenol, p-phenylphenol and resorcinol (SPI, 2002A). Many of the phenolic resins are made from mixed feedstock.
Figure 4.3 Phenolic resins Epoxy resin esters are reaction products of epoxy resins and vegetable oil fatty acids. Polyester-based coatings Polyester resins are used for the interior coating of cans (Ullmann, 2002). The polyester systems can be based on either high molecular weight thermoplastic polyesters or on low molecular weight thermoset polyesters. The ready-made polyester-based can coatings are delivered to the can producers as powders or liquids (CEPE, 2003). Polyester-based coatings are not resistant to corrosive foods, i.e the packing resistance is limited. The polyester bonds tend eventually to hydrolyze, which results in coatings, that can loose their resistance and performance properties, with can perforation being the worst case. However, the polyester-based coatings are generally more flexible compared to epoxy coatings and their use is limited mainly to non-aggressive food (Glud & Marstrand, 2003). Newer polyester-based products are, however, suitable for a wider product range (CEPE, 2003). Bisphenol A-based epoxy resins may also be used as a performance enhancer of polyester-based internal can coatings (Lyons, 2000). However, CEPE explains that the industry does not use BPA for this purpose but may use an BPA-based epoxy resin to improve the performance of the polyester coating. Polyester resins are cross-linked with amino or phenolic resins and may contain lower molecular weight epoxy resins to improve the resistance properties (CEPE, 2003). PVC-based coatings The PVC vinyl-based systems are thermoplastic (non-cross-linked) dispersed in a varnish. With a very good flexibility and packing resistance, the PVC systems are often used on top of the epoxy-phenolic basecoat in e.g. drawn cans. As PVC can thermally degrade during stoving generating hydrochloric acid (HCl), epoxy or oxirane functional substances or resins are often added as HCl scavengers (CEPE, 2003). 4.1.3 Coating properties influencing use and choice of coatingThe can producers (e.g. Glud & Marstrand) receive ready-made can coatings from the coating producers (e.g. Valspar, PPG, ICI and Grace) whereas the coating producers receive the epoxy resins from the resin producers (e.g. Dow, Resolution and Huntsman). The European epoxy resin producers are members of the Association of Plastics Manufacturers in Europe (APME), about 95% of the European coating producers are members of the European Confederation of Paint, Printing Ink and Artists Colours Manufacturers (CEPE) and many of the European can producers are members of the European Secretariat of Manufacturers of Light Metal Packaging (SEFEL). The food and beverage packers and fillers (food industry) are members of the Confederation of the food and drink Industries of the EU (CIAA). APME, CEPE, SEFEL and CIAA are all members of the Joint Industry Group (JIG). The BPA-based epoxy phenolic coatings, often called Phenolic Gold Lacquers due to their characteristic golden/yellowish colour, are used for the majority of applications including highly corrosive foods such as fruit, vegetables, meat products etc. While the adhesion of epoxy-based coatings is good for all materials, the polyester-based coatings may have varying adhesive properties depending on the type of substrate (e.g. tinplate or aluminium) (Glud & Marstrand, 2003). The polyester-based coatings are generally more expensive compared to the epoxy-based coatings, limiting their use mostly to cans or can components, in which their particular properties (e.g. higher flexibility in drawn cans) are needed. Polyester-based coatings are used for non-corrosive food only, e.g. meat such as liver paste etc., in which the fat content of the food protects the can coating against the aggressive food ingredients (Glud & Marstrand, 2003). The packing resistance (shelf-life) of the polyester-based coatings will often be lower (e.g. 2-3 years) compared to the epoxy-based coatings, putting the integrity of the packaging at risk and requiring extensive tests in order to establish the shelf-life of the various types of canned food. When using epoxy-based coatings, a shelf-life of up to 5 years can be assured for a wide range of foods (Glud & Marstrand). The same specification of cans with a given coating is often used for various end uses (e.g. different types of vegetables) under the can producers' guarantee. As a consequence of the requirements to heat and corrosive resistance, durability and price, the can producers select coatings capable of withstanding the most severe requirements, i.e. epoxy coatings (Glud & Marstrand, 2003). In 2002, approx. 90% of the total use of coatings were based on epoxy (including PVC which frequently contains epoxy or oxirane functional species) while polyester-based coatings only constitute one of the alternatives, which also include PVC (non-epoxy, non-oxirane containing) and to a lesser extent the acrylic resins. For external coatings (non-food contact), epoxy resins may or may not be used. In many cases, a small amount of epoxy resin may be present even for acrylic and polyester resins in order to improve performance. For internal can coatings (food contact), about 90% are based on BPA-based epoxy resins (CEPE, 2003). 4.1.4 Development of alternativesThe well-known bisphenol A-containing epoxy, i.e. BADGE-based coatings, has been used by the can coating industry for the past approx. 40 years (SPI, 2002B). Due to the focus on bisphenol A, the reduction of release of free bisphenol A monomers from epoxy coatings and the development of bisphenol A-free can coating alternatives have progressed for the last approx. 5 years (Glud & Marstrand, 2003; CEPE, 2003). For the past 5 years, the migration of BPA monomers from cured can coatings has been in focus and the industry strives for continuous improvement attempting to reduce the BPA migration as well as other migrants (CEPE, 2003; Glud & Marstrand, 2003). Earlier, there existed some coating products with BPA monomers added as plasticizer but nowadays the so-called "Clean Epoxy Technology" is used in which the polymerisation process is completed in order to reduce the content of free BPA monomers to a minimum (Grace, 2003). Based on the measurements of BPA migration from epoxy resins into foodstuff, the concentrations of BPA from food contact applications are approx. 650 μg/l for wine and 100 μg/kg from canned food (EU, 2003A). For adults the estimated daily ingestion of BPA is 500 μg/day from wine and 100 μg/day from canned food. The figures are used in the EU risk characterisation (EU, 2003A). The polyester-based coatings were developed in 1987 in order to replace the PVC organosols and were later considered as alternatives for BADGE-based coatings and later the BPA containing coatings (CEPE, 2003). Several can users, e.g. the military, demand long-lasting packing resistance with a durability of at least 5 years. During recent years, the can coating industry has intensified the development of BPA-free alternatives. The development of a new can coating system takes approx. 10 years from the testing and evaluation of the properties to the successful commercialisation including pack testing, which can last up to at least 5 years. During the development period, all information of the new can coating system is kept strictly confidential. Considering this 10-year time scale for developing and testing of new coatings, the contacted industries decided not to use the offer for "free" health and environmental evaluation of BPA-free alternatives as the project period was considered to be unrealistically short for this purpose. 4.1.5 Other uses of coatingsTo a lesser extent compared to the total use of bisphenol A in coatings, BPA-epoxy based systems are used for lid and cap coatings and for side seam stripes (CEPE, 2003; Metropak, 2003; Pelliconi, 2003). Epoxy-based coatings are used as inside lid coatings in bottle lids, glass container lids, etc. It is estimated that all closing systems for glass jars today and most side seam stripes contain at least one epoxy-based coating system (CEPE, 2003). Based on the experience from Pelliconi and their cooperation with coating suppliers, there are currently no alternatives to epoxy-based coatings for lids (Pelliconi, 2003). To a large extent (but not 100%) coatings for caps contain BPA-based resins, which are especially used when good substrate adhesion and protection against corrosion are needed (Metropak, 2003). The economical difference of the products depends not only on the price of the coating but also on its properties, etc. (e.g. what is the cost of a can with a shelf-life reduced from 1 year compared to 5 years?). Therefore, no price examples comparing caps with BPA-based resins and BPA-free resins were given (Metropak, 2003). 4.1.6 Environmental and health screening of alternatives to bisphenol A in coatings for food and beverage purposesDuring the search in the DPR, several polyester resins and polyacrylates were mentioned as possible alternatives to bisphenol A in coated food and beverage containers (Tables 3.3, 3.4 and 3.5). An internet search was performed in order to investigate the use of these substances. Some of the mentioned polyacrylates could be identified in coatings for floor, electrical insulation (e.g. CAS No. 26376-86-3) etc. but not as ingredients in coatings for food or beverage purposes. Among the polyester resins, mentioned on the internet one of the possible high molecular weight thermoplastic polyesters is based on polybutylene terephthalate (PBT). PBT is used in heat resistant coatings on paper and board (e.g. for packaging of frozen food and oven-ready meals) as in the BASF product Ultradur B2550, where it might be in direct food contact. No specific alternatives were pointed out by the industry (CEPE, 2003). Very little information about the phenolic resins was available but many of the phenols are made from mixed feedstock of bisphenols, phenols etc. and are therefore not evaluated as alternatives to the epoxy-based coatings. The PVC-based coatings are often used in a combination with the epoxy-phenolic coatings or resins and thus not evaluated as alternatives to the epoxy-based coatings. The screening of the environmental and health properties of coatings for food or beverage purposes was thus based on the polyacrylate and polyester properties in general. Properties of polyacrylatesThe properties of polyacrylates differ dependent on the specific substance. However, to some extent, acrylate monomers are likely to be released from the polyacrylate products. Some of the acrylate monomers are classified with N; R51/53, i.e. toxic to aquatic organisms and may cause long-term effects in the aquatic environment (Danish EPA, 2002). Table 4.1 Properties of polyacrylates
Polymers have little specific toxicological activity. The biological effects of polymers may be attributed to residual monomers, oligomers or low-molecular-weight by-products that are incorporated into the polymer, additives or molecular changes during the curing process. Polyacrylates are a large group of resins produced from a large number of feedstock and to which a large number of additives are added. It is thus not possible to make any general assessments regarding toxicological properties, although the polyacrylates themselves are not dangerous to health. Regarding the effects on health, the possible content of the residual monomers in polymers, monoalkyl, monoaryl or monoalkylaryl esters of acrylic acid are included in the Annex 1 of the EU directive on classification and labelling of dangerous substances with a classification as irritating to eyes, skin and respiratory organs. The influence from these monomers should be considered regarding releases from the finished product and regarding the environment and working environment related to the production and manufacture. Properties of polyester Polyester consists of long-chain polymers chemically composed of at least 85% by weight of an ester of a dihydric alcohol and a terephthalic acid. Esters are formed when alcohol reacts with a carboxylic acid. Polyesters can consist of any hydrocarbon chemical group. The properties of the polyester compound will depend on the influence of the specific hydrocarbon chemical group. As the polyester compounds used in coatings for food or beverage purposes could not be specified, the general properties of the polyester are included in the Table 4.2. Table 4.2 Properties of polyester
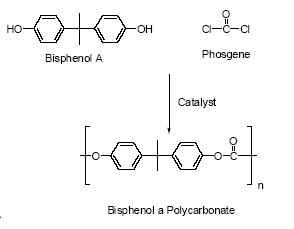
Based on the available data, polyester cannot be classified as dangerous to the environment. However, as the properties depend on the specific substance, it is not possible to make a general assessment of the environmental hazard of polyester-based alternatives. The biological effects of polymers may be attributable to residual monomers; oligomers or low-molecular-weight by-products that become incorporated into the polymer, additives or molecular changes form the curing process. Polyesters are a large group of resins produced from a large number of feedstock and added a large number of additives. It is thus not possible to make any general assessments regarding toxicological properties. However, the polyesters themselves are not classified as dangerous to health. 4.1.7 Legislation related to coating of cansWithin the EU, the European Scientific Committee for Food (SCF) is the responsible for assessing materials intended to come into contact with food (SPI, 2002B). The European Commission (Directorate General of Health and Consumer Protection) has published legislation (2002/16/EC) on the use of BADGE, bisphenol-F diglydicyl ether (BFDGE) and the wider family of novolac glycidyl ethers (NOGE). The use of BADGE as monomer and as reaction intermediate or additive in food cans coatings is permitted until 1 January, 2005. Until then, the sum of the migration levels of BADGE and BADGE derivatives must not exceed 1 mg/kg foodstuff. The EU is currently processing the first amendment to 2002/16/EC. This amendment will extend the deadline until 31/12/2005 in order to enable the European Food Safety Authority (EFSA) to give their opinion on a toxicological dossier for BADGE due to be submitted at the end of the first quarter of 2004 (CEPE, 2003). The same goes for BFDGE (also the sum of BFDGE + BFDGE derivatives + BADGE + BADGE derivatives < 1 mg/kg). NOGE was allowed only until 1 December 2002. It is therefore currently being phased out (Times food, 2003). BPA is permitted under the Directive 2002/72/EC and due to a recent opinion from the SCF, the Specific Migration Limit (SML) is expected to be 0.6 mg/kg. According to CEPE, the typical BPA migration levels in foodstuffs are up to about 0.05 mg/kg (CEPE, 2003). BADGE is included in current danish legislation regarding materials and subjects intended for foodcontact (Retsinfo, 2003). BADGE may only be released to the food from plastic materials, surface coatings and adhesives. BADGE must not exceed 1 mg/kg in the food or 1 mg/dm2 for cans etc. (Retsinfo, 2003). 4.2 Polycarbonate food and beverage containersPolycarbonate products are used in food contact materials such as mineral water bottles, infant feeding bottles, returnable beverage bottles (e.g. used in water cooler machines), tableware (plates and mugs), jugs, beakers, microwave ovenware and storage containers (EC, 2002; EU 2003A). Polycarbonate is a hard clear lightweight thermoplast, which is often based on bisphenol A. Approximately 487,000 tonnes bisphenol A is used in the production of polycarbonates per year within the EU (EU, 2003A). It can be made in three different ways:
Figure 4.4 Interfacial polycondensation of bisphenol A and phosgene Polycarbonate is a very stable hard heat-tolerant material with high electrical insulating properties, impact resistance, ductility and optical clarity (Bisphenol A org., 2003; EU, 2003A). Polycarbonate resins readily undergo hydrolysis under alkaline conditions leading to the release of bisphenol A, e.g. in paper recycling plants (Fukazawa et al., 2002). It is a well-known fact that polycarbonate hydrolyses after prolonged contact with hot water (>60°C) hydrolyses (Pedersen, 2001). However, short sterilisation with water at 120°C is not considered to lead to hydrolysis of polycarbonate (Leisewitz & Schwarz, 1997). The maximum residual content of bisphenol A in polycarbonates is reported to be 50 mg/kg but the typical residual content is < 10 mg/kg (EU, 2003A). The amount of unreacted monomers in polycarbonate is less than 100-150 mg/kg and, in general, it is <25 mg/kg according to the polycarbonate producers, Bayer and Dow (Leisewitz & Schwarz, 1997). In a European-wide survey, migration of BPA from the bottles into food simulants was not detectable at <3 μg/kg (EC, 2002). For used bottles BPA was detected in water and/or acetic acid samples from five of twelve bottles, the levels measured ranging from 20-50 μg/l, i.e.20-50 ppb. 50 μg/l is considered to represent the realistic worst-case exposure conditions of BPA migration from polycarbonates into food (EU, 2003A). The estimated daily ingestion of BPA for infants is 35-50 μg/day from polycarbonate feeding bottles, which is used in the EU risk characterisation (EU, 2003A). 4.2.1 Alternatives to polycarbonateThere are different types of non-bisphenol A plastic products on the market. Compared to the properties of polycarbonate, the bisphenol A-free nylon products e.g. Grilamid TR, are considered to have similar properties and may be seen as an alternative to polycarbonate. A few companies produce nylon (polyamide) that can be used for baby bottles as a replacement for polycarbonate bottles. The companies are EMS-Grivory in Germany and Sumter in the USA. Grilamid TR grades are transparent thermoplastic polyamides based on aliphatic and cycloaliphatic blocks. Grilamid TR is characterised by its UV resistance, high chemical and stress crack resistance etc. (Grivory, 2003). Polyamide (nylon) The high molecular weight polyamide, hexamethylene adipamide, is formed from adipic acid and hexamethylene diamine in a polymerisation process, and is also known as nylon 66. Polyamide has puncture resistance and thermoformability with very good barrier properties and is therefore used for food packaging for e.g. meat and cheese products (BASF, 2003). BASF is one of the major resin suppliers to the manufacturers of polyamide. The manufactured quantity of nylon 66 in the EU by e.g. BASF, Rhone-Poulenc and Du Pont is >1,000,000 tonnes/year (Iuclid, 2003). Polyamide was thus considered as a possible alternative to polycarbonates and the environmental and health assessments were based on data available for polyamide. 4.2.2 Environmental and health screening of alternatives to polycarbonatesThe environmental and health screening of polyamide are based on the properties listed in Table 4.3. Table 4.3 Properties of the polyamide, nylon 66
The degree of degradability will depend on the aliphatic and cycloaliphatic blocks in the polyamide. Polyamide is degradable, and with a BOD/COD ratio above 0.5 it seems to fulfil the criteria for ready biodegradability. The acute toxicity to aquatic organisms is about 100 mg/l for daphnia, which is considered to be the most sensitive group. There are no data describing the bioaccumulation of polyamide. Based on the available data it is, however likely that polyamide should not be classified as dangerous to the environment. Polymers have as such little specific toxicological activity. The biological effects of polymers may be attributable to residual monomers, oligomers or low-molecular-weight by-products that are incorporated into the polymer, additives or molecular changes of the polymer during the curing process. It is not possible to make any general assessments regarding toxicological properties of polyamides. However, the polymer itself is not classified as dangerous to health. 4.2.3 Legislation related to plastic materialsThe limit to migration of bisphenol A from plastic materials used for food contact materials is 3 mg/kg (Retsinfo, 2001). At present, there are no legal restrictions on the amount of bisphenol A that can be present in the final plastic product (EU, 2003A). 5 Thermographic printingIn thermographic printing, heat is used to develop an image on a substrate, usually paper. Either an image forming material (thermoplastic ink) is heated and transferred to the substrate, the "thermal ink transfer printing", or the substrate is coated with an image forming material, which can be developed by applying heat during printing, the "direct thermal transfer printing" (Ullmann, 2002). Thermal ribbons and resistive ribbons can be used for "thermal ink transfer printing" but neither of these ribbons contain bisphenol A. In "direct thermal transfer printing", heat generated in a thermal head causes a heat sensitive material on the paper to react, forming a coloured image. This paper is referred to as thermal paper and may contain bisphenol A. In the EU, e.g. in Austria, the main source of bisphenol A released to the environment is believed to be the recycling of thermal paper (Leisewitz & Schwartz, 1997; EU 2002). In the process of recycling, the waste paper is often bleached with sodium hypochlorite and chlorinated derivatives may be formed. In a study of waste water from twenty Japanese paper recycling plants, 3,3'-dichlorinated bisphenol A derivatives were detected. They were found to be 28 times more estrogenic than the non-chlorinated bisphenol A (Fukazawa et al., 2002). Thermal paper consists of a smooth base paper with a 5-10 m coating. The coating contains a leuco dye and a phenol developer (e.g. bisphenol A) as well as a binder (e.g. styrene, maleic anhydride copolymer) and other substances. The developer and the leuco dye react to the heat application causing the visible colour to be formed (Figure 5.1).
Figure 5.1 The reaction process between the leuco dye and the phenol developer Both the developed areas and the non-developed areas of the thermal paper will contain bisphenol A, which may ultimately be released if e.g. the paper is recycled. The total amount of BPA used for thermal paper production within the EU is estimated to of 1,400 tonnes/year. The total amount of BPA-containing thermal paper manufactured in the EU is 105,000 tonnes/year (EU, 2003A). "Direct thermal transfer printing" is used when low resolution and relatively low permanence are acceptable. Popular uses are airline, event and cinema tickets, online lottery and gaming tickets, labels, point of sale applications, computer printers and charting devices such as portable typewriters, diagnostic printers and portable data collectors; laptop computers, desktop calculators and handheld calculators; medical, industrial and testing charting devices and facsimile (Appleton, 2003; Ullmann, 2002). 5.1 Alternatives to BPA in thermal paperThe large producers of thermal paper are Nashua and Appleton in the USA, Kanzaki in Japan and Kohler in Germany (MeadWestvaco, 2003). Examples of the phenol developers are Nippon Soda and SongWon Industrial. The manufacturer, Nippon Soda, produces bisphenol S to be used as a developer in thermal paper (Nippon Soda, 2003) but Bisphenol F can be used as a developer in thermal paper as well (SongWon Industrial, 2003). There are indications that, in Japan, bisphenol A has recently been substituted in thermal paper (MeadWestvaco, 2003). In order to verify this, Kanzaki was contacted but no response was received from the company (Kanzaki, 2003). 5.1.1 Environmental and health screening of alternatives to bisphenol A in thermal paperBoth bisphenols, S and F are used as phenol developers in thermal paper. Bisphenols S and F Only very few data were available on bisphenol F and no data were found on bisphenol S. Table 5.1 Properties of bisphenol F
Both bisphenols F and S have different structures compared with bisphenol A. However, bisphenol F, which was earlier used as an additive to PVC, has been phased out due to its toxicological properties, which was considered to be very similar to the properties of bisphenol A (Glud & Marstrand, 2003). In order to concentrate on one substance, the use of bisphenol F as an additive in PVC was thus phased out. A comparison of the QSAR estimations of bisphenol F and bisphenol A also shows little difference between them. The calculated acute EC/LC50 values for fish, crustaceans and algae are between 2.6 and 4.0 for bisphenol A and between 3.9 and 11.4 for bisphenol F. On the basis of the available data, their properties are, however, considered to be similar to those of bisphenol A, and they are thus not considered as alternatives. 6 TonersToners are used in copying and non-impact printing processes, i.e. in office copiers, plain paper fax machines, digital printers and copiers. These machines print by a method called electrophotography. In electrophotography, a charge pattern replicating the light image is formed on a photoconductive film. Charged pigmented thermoplastic particles (toner) are attracted to the charged areas of the photoconductive film from where it is transferred to the paper onto which the toner is fixed by softening and fusing the toner to the paper (Ullmann, 2002). Toners are thermoplastic particles, typically 5-25 m in size with 5-10% of pigment to give the desired colour. The function of the thermoplastic is to fix and fuse the image onto the paper, which is done by heat and/or pressure (Ullmann, 2002). Typical thermoplastics used in toners are random copolymers of styrene with methacylates or acrylates. Some toners contain a small percentage of very large thermoplastic particles (100-700 m) called beads, which carry many small toner particles (Ullmann, 2002). Xerox is one of the toner suppliers, from which the products contain bisphenol A. E.g. in the Xerox Cyan developer (i.e. DocuColor 12, Document Centre colorSeries 50, DocuColor 2045, DocuColor 2060, DocuColor 6060), there is <7% wt. toner of which 80-90% wt. is a bisphenol A polyester resin (CAS No. 122970-65-4) (Xerox, 2003B). An example of the structure of a bisphenol A polyester resin is shown in Figure 6.1. Whereas, in the Xerox Dry ink document centre 240DC/STx, a bisphenol A propylene oxide fumarate polymer (CAS No. 39382-25-7) constituting 60-75% (wt/wt). is used and the same polymer is used in other dry inks and magnetic toners produced by Xerox (Xerox, 2003A). Also Lexmark uses bisphenol A polyester resins in their toners (Lexmark, 2002).
Figure 6.1 Bisphenol A dimethyl terephthalate polyester resin Polyesters are macromolecules prepared from different diabasic acids (e.g. isophthalic, orthophthalic, terephthalic acids, fumaric/maleic acids) and diols (dihydric alcohol) such as ethylene glycol, propylene glycol, neopentyl glycol, bisphenol A (BPA) etc. Polyester resins are generally divided into ortho resins, iso-resins, BPA fumarates and vinyl ester resins. Due to differences in their structures different combinations of these di-acids & diols impart variation in mechanical properties like flexural strength, tensile strength and compressive strength. Bisphenol A seems only to appear in toners as part of a polymer/resin. There were no alternatives to the bisphenol A polymer/resins related to the production or use of toners, and thus no industrial input to the list of possible alternative substances available from the DPR (Tables 3.6 and 3.7). 7 Printing inksPrinting inks are applied in thin films on many substrates such as paper, paper board, metal sheets and metallic foil, plastic films and molded plastic articles, textiles and glass (Kirk-Othmer, 1996). Printing inks can be coloured or non-coloured and are used on e.g. labels and ice lollies, which are in contact with food (The Danish Veterinary and Food Administration, 2003). Printing inks typically contain three main components: Pigments (the coloured or solid ingredient), vehicles (the fluid ingredients) and additives (such as driers and extenders). Printing ink is thus a mixture of colouring matter dispersed or dissolved in a vehicle or carrier, forming a fluid or paste, which can be printed on a substrate and dried (Kirk-Othmer, 1996). The vehicles consist of a combination of resin, oil and solvent. The solvent is absorbed by the paper, leaving a thin ink film of resin and oil that binds the pigment to the paper. This film hardens by oxidation (Kirk-Othmer, 1996). Printing inks are produced by e.g. Resino Trykfarver A/S (Resino, 2003), BASF (2003B), Akzo Nobel (2003) and Sun Chemical (2003B). The bisphenol A resin is used as e.g. an ingredient in rosin-modified phenolics for printing inks, where rosin-modified phenolics possess good oil solubility, which may be used for water-washable inks and adhesives (Shanghai Nanda, 2003). Another phenolic resin is used in printing ink products from the Japanese Arakawa Chemical Industries (2003) in Japan. Raw materials, e.g. bisphenol A, are supplied for the printing ink industry by e.g. the Eastern Chemical Corporation (2003). 7.1 Alternatives to BPA in printing inksPrinting inks produced by BASF have no content of bisphenol A (BASF, 2003B). According to Akzo Nobel Inks, binders based on bisphenol A have only very limited use in traditional printing inks whereas UV-printing ink types more often use binders based on bisphenol A (Akzo Nobel, 2003). Resino Trykfarver mainly uses nitrocellulose, polyvinyl butyral, polyamide, blocked polyurethane as binder and ketones, maleinate, polyester, etc. as auxillary binders. The main binder in their cationic UV-printing inks is, however, based on bisphenol A containing epoxy (Resino Trykfarver, 2003). Also urethane resins are used in food packaging gravure inks. They are used as binders for gravure inks for printing on polyolefin films for food packaging, e.g. polypropylene, polyester and nylon, which has good adhesion and flexibility properties (Arakawa Chemical Industries, 2003). The urethane acrylates are among the popular monomers and prepolymers acrylate resins used as drier (UV-curing inks) in the UV printing inks (HDM, 2003). Furthermore, one company offers polymerised rosin in hectograph ink to replace bisphenol resin, for the printing ink industry (Pinechem, 2003). 7.1.1 Environmental and health screening of alternatives to bisphenol A in printing inksUrethane acrylates and polymerised rosin were considered as possible alternatives to bisphenol A in UV-printing inks. Comparing this with the information available from the DPR (Tables 3.6 and 3.7), one polymerised rosin substance can be identified, but none of the polyacrylates were identified as an urethane acrylate. Urethane acrylates Urethane acrylates are acrylates, which contain diisocyanates (Figure 7.1). Where R is CH3,; Y is H and R' is an isocyanate. The isocates can be:
Figure 7.1 Urethane acrylates (HDM, 2003) Urethane acrylates are various substances such as urethane diacrylate, urethane dimethacrylate or diurethane dimethacrylate (CAS No. 103597-45-1), which is a 4-tert-octylphenol compound (Chemfinder, 2003). The environmental and health properties of a urethane acrylate will depend on the specific substance. Octylphenols, for instance, is included on the list of substances considered having endocrine disrupting properties. However, in general, some acrylate monomers are classified with N; R51/53, i.e. toxic to aquatic organisms and may cause long-term effects in the aquatic environment (Danish EPA, 2002). Monoalkyl, monoaryl or monoalkylaryl esters of acrylic acid are included in the Annex 1 of the EU directive on classification and labelling of dangerous substances with a classification as irritating to eyes, skin and respiratory organs (HDM, 2003). The influence from these monomers should be considered as regards releases from the finished product and as regards the environment and working environment related to the production and manufacture. The polyurethanes are as such relatively inert and harmless materials, having little specific toxicological activity. However, feedstock for polyurethanes, the isocyanates, are a group of very reactive substances, which may cause disabling respiratory diseases and skin sensitization by repeated exposure. In many countries, isocyanates are recognized as the most frequent cause of occupational asthma (Engelund & Pratt, 2001). Isocyanates (MDI, IPDI; HMDI and TDI) are included in the Annex 1 to the EU directive on classification and labelling of dangerous substances with classifications as very toxic, toxic or harmful by inhalation, irritating to eyes, skin and respiratory organs and sensitizing by inhalation and skin contact. Isocyanates may be formed in connection with heating of polyurethanes, e.g. during welding. Polymerised rosin Polymerised rosin is based on natural organic compounds such as gum and pine rosin. In printing inks, rosin is mainly used as a colour carrier of the ink and to increase its adhesion to the paper (Pinechem, 2003). In EU the produced quantity of rosin (also named colophony), which is registered as CAS No. 8050-09-7, is 100,000 – 500,000 tonnes/year from e.g. Akzo Nobel, Helm AG and Henkel (Iuclid, 2003). This registered colophony is used as e.g. binder in printing inks (KemI, 2003). The rosin specified in Table 3.7 as CAS No. 68333-69-7 (maleated polymer with pentaerythritol) is not included in the Iuclid database of High Production Volume Chemicals and it was not verified by the industry. The maleated rosin, which was indicated as a gum rosin in a datasheet, could not be identified as an ink ingredient in a search on the internet but was indicated as ingredient in pesticides. The environmental and health essessments were thus based on available data for colophony, the registered polymerised rosins. Colophony is produced by fractionated distillation of raw tall oil, a by-product from the paper and pulp industry. The main components (approx. 90%) are diterpenenes of the abietic acid (CAS No. 514-10-3) and pimaric acid types (KemI, 2003). Table 7.1 Properties of polymerised rosin
Colophony not pass the level for ready biodegradability (Iuclid, 2003). However, according to the N-Class database (KemI, 2003), colophony is considered to be readily degradable. Colophony is very toxic to fish. Based on the aquatic toxicity and the uncertainty of the biodegradability, colophony is presently considered to fulfil the criteria for the N;R50/53 classification. Rosin may cause sensitisation at repeated skin contact. Thermal decomposition products of rosin may be irritating to eyes, nose and throat in acute exposure and can cause allergic reactions including asthma, contact dermatitis and eczema at repeated exposure. 7.2 Legislation on printing inks for food packaging materialsThe description from the Danish Food Ministry regarding printing inks in food contact materials refers to the present work in the EU, in which it is stated that food contact materials shall be safe and not transfer their components into the foodstuff in unacceptable quantities (EU, 2003B). Therefore, printing inks are not covered by EU legislation other than the general Food Safety Framework Directive (89/109/EEC) and additional legislation which covers plastic packaging materials (90/128/EEC), (Coates Lorilleux International, 2003). Bisphenol A is, however among the substances, that are not to be used in printing inks for food packaging materials in Japan as stated in the Voluntary Regulation concerning Printing Inks for Food Packaging Materials, 1999, from the Japan Printing Ink Makers Association (Sun Chemical , 2003A). Both bisphenol A type epoxy liquid resins and methylene bisphenol type epoxy liquid resins are included in this list. 8 Evaluation of the possible alternatives to bisphenol ADuring the project period no specific substance was pointed out by the industry as an alternative to bisphenol A in coated food or beverage containers, polycarbonates, thermographic printing, toners or printing inks. However, several possible alternatives or groups of substances were mentioned and investigated. The evaluation of the possible alternatives was based on the internationally accepted principles for classification of single substances, i.e. risk phrases (R-phrases). A large number of chemicals have been officially classified on the basis of their potential toxicity to human health whereas only very few of these chemicals have been classified for their potential hazardous environmental effects. But due to the implementation of Directive 1999/45/EC of the European Parliament and of the Council, preparations of chemicals must now be evaluated and classified with respect to both human health and to the environment. The environmental and health properties of a substance are dependent on the inherent properties of the specific substance. Therefore, the environmental and health properties can vary within a group of substances, i.e. a detailed risk assessment of e.g. a polyacrylate will require specific data on that specific substance. An environmental and health screening based on the general properties of a chemical group or based on a single substance of a chemical group may, however, be used to indicate the potential hazards of the alternative substance to the environment and human health. A summary of the results of the environmental and health hazard assessment of possible alternatives to bisphenol A in coatings, polycarbonates and printing inks is given in Table 8.1. Furthermore, the EU environmental and health assessment of BPA is given for comparison (EU, 2003A). Bisphenol A is considered to be readily biodegradable and not bioaccumulative but toxic to aquatic organisms. It does thus not fulfil the criteria for classification as regards the environment but further discussion is needed as the observed effects at low concentrations of bisphenol A in long-term toxicity studies justify the application of suitable risk and safety phrases (EU, 2003A). From an environmental point of view the alternative polyester and polyamide, depending on the specific substances, may turn out to cause less harmful effects than BPA whereas polymerised rosin and monomers from polyacrylates may cause the same or more hazardous effects on the environment as bisphenol A. From the health point of view the possible alternatives, polyesters and polyamides depending on the specific substances, may turn out to cause less harmful effects than BPA, whereas some polyacrylates may be irritating to eyes, respiratory system and skin and polymerised rosins may cause sensitisation by skin contact. Bisphenol A is known to be irritating to respiratory system, cause risk of serious damage to eyes and sensitisation by skin contact. Furthermore, bisphenol A is on the list of substances, which are suspected as endocrine disrupters, and it has been suggested to classify it with R62 (possible risk of impaired fertility) (EU, 2003A). None of the mentioned alternatives are on this list but it should be mentioned that potential endocrine disrupting effects have most likely not been examined for the alternatives. The environmental and health effect of the alternative will, however, totally depend on the specific chemical compound in focus including possible migration of monomers etc. and the assessment should be revised on the basis of the properties of the specific chemical compound. Table 8.1 Screening of environmental and health properties of the possible alternatives to bisphenol A
1) List of substances, which are or are potential endocrine disrupting (Danish EPA, 2003) 9 BibliographiesArakawa Chemical Industries (2003). Online. http://www.arakawachem.co.jp/e/ & http://www.arakawa-usa.com/tech_Resin_for_ink.htm. BASF (2003A). Online. http://www.basf.com/static/OpenMarket/Xcelerate/ Bisphenol A Org (2003). http://www.bisphenol-a.org/human/polyplastics.html Chemfinder (2003). Online. http://chemfinder.cambridgesoft.com/ Coates Lorilleux International (2003). Online. http://www.coateslorilleux.com/thd/she/Documents/Foodinkslett5.pdf Danish EPA (2002). List of dangerous substances. Online. http://www.mst.dk. (in Danish). Listen over farlige stoffer. Danish EPA (2003). EU's list of 118 substances, considered to be endocrine disrupting or potential endocrine disrupting. Online. http://www.mst.dk. Miljøstyrelsen EU's liste over 118 stoffer, der anses for at være hormonforstyrrende eller potentielt hormonforstyrrende. (in Danish). DermNet (2003). Online. http://www.dermnetnz.org/index.html Dow (2003). Online. http://www.styrofoameurope.com/products_services/industry/pack.htm Eastern Chemical Corporation (2003). Online. http://www.easternchemcorp.com/products.htm EC (2002). Opinion of the Scientific Committee on Food on Bisphenol A. SCF/CS/PM/3936 Final 3 May 2002. http://europa.eu.int/comm/food/fs/sc/scf/out128_en.pdf Epiwin (2003). Online. http://esc-plaza.syrres.com/interkow/kowdemo.htm Engelund, B. & C. Pratt, (2001). Secondary exposure to isocyanates, Danish Toxicology Centre. EU (2002). Risk assessment of bisphenol A, Draft. EU (2003A). Risk assessment of bisphenol A, Final Report, October 2003. EU (2003B). Food Contact Materials. EU legislation. Online . http://europa.eu.int/comm/food/ Fukazawa, H., M. Watanabe, F. Shiraishi, H. Shiraishi, T. Shizawa, H. Matsushita & Y. Terao (2002). Formation of chlorinated derivatives of bisphenol A in waste paper recycling plants and their estrogenic activities. Journal of Health Science. Vol. 48, 3, pp. 242-249. GESTIS-Stoffdatenbank (2003). Berufsgenossenschaftliches Institut für Arbeitsicherheit. Online.
http://www.cdromverlag.de/CGI-sd4/om_isapi.dll?client Grivory (2003): Online. http://www.ems-grivory.ch/en/02produkte/01marken/01grilamid/main_grilamid_tr.asp. HDM (2003). Online. Huntsman (2003). Online. IARC (1999). International Agency for Research on Cancer (1979). IARC Monograph on acrylic acid and acrylates (CAS No. 79-10-7, 96-33-3, 140-88-5, 9003-01-4). Vol. 19 (1979) Summary of Data reported and Evaluation. Last update 1999. Iuclid (2003). Online. http://ecb.jrc.it/ KemI (2003). Online. http://www.kemi.se. Kirk-Othmer (1996). Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. On-line. Article Online Posting Date: December 4, 2000. Leisewitz, A. & W. Schwarz (1997). Stroffströme wichtiger endokrin wirksamer industriechemikalien (Bisphenol A). Umweltbundesamt Berlin. Lexmark (2002). Personal communication with Dave Caldwell, Lexmark. Lyons, G. (2000). Bisphenol A, a known endocrine disrupter. WWF European toxics programme report. N-Class (2003). Online. http://www.kemi.se/ Nippon Soda (2003). http://www.nippon-soda.co.jp Pedersen, L.B. (2001). [Plastic and the environment]. Plast og miljø. 2. Udgave. Ingeniøren bøger. Ingeniøren A/S. (in Danish). Pinechem (2003). Chinese Gum Rosin. Online. Polycondensation (2003). http://www.psrc.usm.edu/macrog/lab/epoxy.htm. Reprotext System (2003). Reproductive Toxicology Centre 1991-2003. Resolution (2003). Online. http://www.resins.com/resins/eu/ http://www.nanda-chem.com/product2.htm Retsinfo (2001). Vejledning om materialer og genstande bestemt til at komme i berøring med fødevarer. VEJ nr 12114 af 2001 (Gældende). Retsinfo (2003). The valid legislation regarding materials and subjects to foodcontact. Statutory Order No. 111 of 20/02/2003. In Danish: Den gældende bekendtgørelse om materialer og genstande bestemt til kontakt med fødevarer. RTECS (2003). Registry of Toxic Effects of Chemical Substances. Styren. Last update: 2003. Online. http://csi.micromedex.com(Available from: http://csi.micromedex.com/DATA/RT/RTWL3675000D.htm) Sheftel VO (2000). Indirect food additives and polymers : migration and toxicology. Boca Raton, Fla. Lewis. SongWon Industrial (2003). Online. http://www.songwonind.com. SPI (2002A). The Society of the Plastics Industry. www.socplas.org. SPI (2002B). BADGE safety in can coatings. The Society of the Plastics Industry, Inc. (SPI). September 2002. http://www.plasticsindustry.org/about/epoxy/BADGEcans.pdf Sun Chemical (2003A). Voluntary Regulation Concerning Printing Inks For Food Packaging Materials, 1999, from the Japan Printing Ink Makers Association. Online. Sun Chemical (2003B). Homepage. Online. The Danish Veterinary and Food Administration (2003). Fødevaredirektoratet. Materialer og genstande, trykfarver. Online.
http://www.foedevaredirektoratet.dk/Foedevare/ Times food (2003). Times food processing journal Internet Jan 2003, http://www.etfoodprocessing.com/dec-jan2002/safety1.html). Ullmann (2002). Ulmann's encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH Weinheim, Germany. Verschueren, K.(1997). Handbook of Environmental Data on Organic Chemicals, 3rd Edition. CD-ROM. Xerox (2003A). Material Safety Data Sheet p-05. Xerox (2003B). Material Safety Data Sheet B-0312. 10 Contacted Partners10.1 AuthorityDanish EPA (2002- 2003). Personal communication with Lise Emmy Møller & Shima Dobel, D-EPA Aug. 2002- Dec. 2003. Danish EPA, DK. 10.2 Organizations"Danmarks Farve- og lakindustri", DK. Personal communication with Anette Harbo, Jul. – Dec. 2003. "Grafisk Arbejdsgiverforening", DK. Personal communication with Anette Møller, Jul. - Nov. 2003. "Plastindustrien i DK". Personal communication with Lars Blom, Febr. - Nov. 2003. CEPE (2003). CEPE, EU. Personal communication with Peter Oldring, Jul. – Dec. 2003. 10.3 Industries10.3.1 Polycarbonate relatedBayer (2003). Bayer AB, Gothenburg, S. Personal communication with Inger Petterson, Sept. – Nov. 2003. Cambro (2003). Cambro, US. Personal information via email to Jim Ingram, Febr. – Aug. 2003. Cretex (2003). Cretex A/S, Brøndby DK. Personal communication with Frank Duckert and Niels Skov Hansen, Mar. – Nov. 2003. DMI Plast (2003). DMI Plast Øst, Greve DK. Personal information via email and letter to Christian Koue, Mar. – Nov. 2003. Efo-Plast (2003). Efo-Plast A/S, Kolding, DK. Personal communication with Ebbe Fogh Hansen, Mar – Nov. 2003. Expladan (2003). Expladan A/S, Hårlev, DK. Personal communication with Steen Birkdal, Mar – Nov. 2003. Kaiserplast (2003). Kaiserplast A/S, Karise, DK. Personal communication with Kenny Rosendahl, Mar – Nov. 2003. Teknoplast (2003). Teknoplast A/S, Vejen, DK. Personal communication with Freddy Blaaberg Mar. – Nov. 2003. 10.3.2 Coating relatedEmballator (2003). Emballator Thy Plast A/S, DK. Personal communication with Preben Hansen, Jul. 2003. Flint-Schmidt (2003). Flint-Schmidt (Earlier named Flint Ink), DK. Personal communication with Eigil Reinholdt, Jul. - Nov. 2003. Grace (2003). W.R. Grace, D. Personal communication with Thue Thuesen Jul. – Nov. 2003. Hartmann (2003). Hartmann, DK. Personal communication with Lene Andersen, Jul. 2003. ICI (2003). ICI, DK. Personal communication Mar. – Jul. 2003. Glud & Marstrand (2003). Glud & Marstrand, Odense DK, Personal communication with Rolando Mazzone. Febr. – Dec. 2003. GN Industri (2003). GN Industri, DK. Personal communication with Jan Håkansson, Jul. 2003. Lind Plast (2003). Lind Plast, DK. Personal communication Jul. 2003. Metal Agencies (2003). Metal Agencies, GB. Personal communication with John Koufapanos, Jul. 2003. Metropak (2003), Hedehusene, DK. Personal communication with Annelise H. Boye, Aug. - Oct. 2003. Pelliconi (2003). Pelliconi Scandinavia, Taastrup, DK. Personal communication with Mikkel Fälling, Aug. – Oct. 2003. PPG (2003). PPG Packaging Coatings, DK. Personal communication with Sten Ove Kollén, Jul. – Aug. 2003. Superfos (2003). Superfos, DK. Personal communication Jul. 2003. Valspar (2003A). Valspar, DK. Personal communication with Bill Currie Jul. – Nov. 2003. Valspar (2003B). Valspar, UK. Personal communication with Peter Oldring, Jul. – Nov. 2003. 10.3.3 Toner, printing ink and thermal paper relatedAkzo Nobel (2003). Akzo Nobel Inks, DK. Personal communication with Frank Pedersen, Michael Mandrup and Henning Svendsen, Jun. - Oct. 2003. Appleton (2003). Appleton papers, US. Personal communication with Gregg Ublacker, Aug. 2002 – Jul. 2003 BASF (2003B). BASF-Tryksystemer, DK,. Personal communication with Gitte Just Pedersen and Majbrit Petersen, Juli – Oct. 2003. Kanzaki (2003). Kanzaki; US. Information via email to info@kanzakiusa.com, Febr. - Jul. 2003. MeadWestvaco (2003). Personal communication with W.H. Wooden. http://www.westvaco.com/products/chemicals/poly/polychem.htm Nashua (2003). Nashua, US. Information via email to productinfo@nashua.com, Mar.- Jul. 2003. Resino (2003). Resino Trykfarver, DK. Personal communication with Poul Erik Stenfelt, Jul – Oct. 2003. Ricoh (2003). Ricoh Industrie France, F. Personal communication with Franck Revillion, Febr.- July 2003. Skjern Papirfabrik (2003). Skjern Papirfabrik A/S, DK. Personal communication with Lene Andersen, July 2003. Sun Chemical (2003). DK. Personal communication with Ralph Juul Sørensen. Oct. - Nov. 2003.
|