Survey of nanotcnological consumer products
Attachment B Exposure
Quantification of human exposure to the active substance in four fictive products.
This attachment describes scenarios of human exposure which are based on four fictive products. The products represent a facial lotion, a sun lotion, a fluid product for outdoor surface treatment, and spray product for indoor surface treatment.
The calculations are based on default values and formulas taken from part 1, appendix II – “Consumer Exposure”, in Technical Guidance Document (TGD) on risk assessment for existing substances (European Commission, 2003). If the TGD did not contain any data, the default values were based partly on contact with relevant actors and partly on values estimated by DHI.
With respect to the concentration of the active substance, very few producers/distributors have been able to give information about the content of the substance in the products. Thus, the values indicated in the tables below the individual scenarios, is the best possible estimate that can be acquired from the very sparse information received by DHI (DHI, 2006). In cases when the producer has stated an order of magnitude and not a definite value, the estimate is based on a worst case scenario using the highest value. For the scenario of the facial lotion no information at all could be acquired about the content of active substance. As the content in the facial lotion is expected to be lower than in the sun lotion, where the active substance is intended to act as a sun filter, the value 0.1 % has been used. However, this value must be considered more arbitrary than the values for sun lotion and the product for surface treatment.
Scenario for cosmetics – facial day lotion
The route of exposure for cosmetic products will mainly be dermal contact. Intake of smaller quantities by contact with the area around the mouth is not taken into consideration. As the product is a “leave on” product, which should neither be diluted when used nor washed off, the quantity of active substance on the skin (Ader) can be estimated as follows:
Equation 1: ![]()
Thus, the potential uptake per kilogram body weight per day is derived as:
Equation 2: ![]()
Table 4 Explanation of symbols and default values
| Symbol | Explanation | Default values | Unit | Reference |
| Ader | The quantity of active substance on the skin per application |
mg | (European Commission, 2003) | |
| Qpro | The quantity of product per application for an adult |
800 mg | mg | (European Commission, 2003) |
| Fpro | Concentration of active substance in the product | 0.1 | % | (DHI, 2006) |
| Uder,pot | Potential daily uptake of quantity of active substance | mg/kg bw/day | ||
| n | Number of applications per day | 1 | Number/day | (European Commission, 2003) |
| bw | Body weight | 60 for adult women and 70 for adult men | kg | (European Commission, 2003) |
Based on equation 1, the quantity of active substance on the skin per application for a facial day lotion containing 0.1% of nano material, can be calculated:
Ader = 0.8 mg.
The potential daily uptake of active substance can be calculated:
Uder,pot = 0.013 mg/kg bw/day for women.
Use of both a facial day lotion and night lotion containing 0.1% nano material is equal to application twice a day and consequently Uder,pot will be 0.026 mg/bw/day.
Scenario for cosmetics – Sun lotions
The route of exposure is as for the facial lotion mainly by dermal contact. Intake of smaller quantities by contact with the area around the mouth is not taken into consideration. As the product is a “leave on” product, which should neither be diluted when used nor washed off, the quantity of active substance on the skin (Ader) can be estimated as shown in the above Equation 1 and 2.
The conversion of the value of applied sun lotion in an adult compared to a child is shown in Equation 3.
Equation 3: 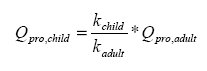
Table 5 Explanation of symbols and default values
| Symbol | Explanation | Default values | Unit | Reference |
| Ader | The quantity of active substance on the skin per application |
kg | (European Commission, 2003) | |
| Qpro,adult | The quantity of product per application for an adult |
8000 | g | (European Commission, 2003) |
| Qpro,adult | The quantity of recommended product per application for an adult | 36000 | mg | (European Commission, Recommendation, 2006) |
| Qpro,child | The quantity of product per application for a child |
2600 | mg | (European Commission, 2003) |
| Qpro,child | The quantity of recommended product per application for a child | 12000 | mg | (European Commission, Recommendation, 2006) |
| Fpro | Concentration of active substance In the product |
2 | % | (DHI, 2006) |
| Uder,pot | Potential daily uptake of active substance | kg/kg bw/day | ||
| n | Number of applications per day | 3 | Number/day | (European Commission, 2003) |
| bw | Body weight | 12.34 for a 2 year old child | kg | (Lentner C.,1981) |
| Kchild | Body area of a 2 year old child, weighing 12,34 kg and 86,8 tall | 0.55 | cm² | (Lentner C.,1981) |
| Kadult | Body area of an adult (woman) | 1.69 | cm² | (European Commission, 2003) |
With reference to the default values indicated in the TGD for the quantity of the actually applied sun lotion the following exposure can be calculated:
The quantity of the active substance on the skin per application of sun lotion containing 2% of nanomaterial for a 2 year old child can be calculated:
Ader = 52 mg.
The potential daily uptake of active substance per day for a two year old child can be calculated as Uder,pot = 12,6 mg/kg bw/day.
To reach the protection level indicated by the sun protection factor, the quantity of applied sun protection lotion must be equal to the quantity used in the test, which is approx. 36 g for an average adult. This amount exceeds the quantity which the consumers normally use (Commission Recommendation,2006).This quantity is equal to 4.5 times more sun lotion than what TGD stipulates is the actual use. For consumers that apply the recommended quantity, the quantity of active substance on the skin per application of sun lotion containing 2% of nanomaterials can be calculated as follows: Ader = 234 mg for a 2 year old child.
Daily uptake of active substance can be calculated as:
Uder,pot = 56.7 mg/kg bw/day for a 2 year old child.
Scenario of liquid products for outdoor surface treatment
As an example a product for surface treatment of car glasses is chosen.
In this scenario it is assumed that all glasses of the car will be treated. Based on personal communication, the estimated total glass area of a standard passenger car is 5.4 m² (Carglass, 2006). It is assumed that the product is applied with a cloth and that it is not diluted before use and thus a dilution factor is not required in the scenario. It is also assumed that the nanomaterial is not volatile and therefore exposure by inhalation can be excluded. The route of exposure will mainly be dermal contact. The scenario is based on many assumptions, as it has been impossible to collect more precise product data. The below is an example on how a scenario can be established. The estimated values below cannot be used as basis for a risk assessment of this type of products without more precise product information
The total quantity of active substance for treatment of all car glasses of a standard passenger car can be estimated using equation 4 and 5, and the below default values.
Equation 4: ![]()
Equation 5 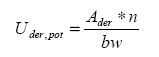
Table 6 Explanation of symbols and default values
| Symbol | Explanation | Default values | Unit | Reference |
| Ader | The quantity of active substance on the skin per application |
ml | (European Commission, 2003) | |
| Qpro | The quantity of product per application for an adult |
10 ml/ m² * 5.4 m² = 54 ml |
ml | (DHI, 2006),(Carglass, 2006) |
| Fpro | Concentration of active substance In the product |
0.1 | % | (DHI, 2006) |
| f | Allocation factor for the part of the applied quantity of the product with dermal contact | 1 | % | (DHI, 2006) |
| Uder,pot | Potential Uptake of active substance per year | ml/kg bw/year | ||
| n | Number of application per year | 2 | Number/year | (DHI, 2006) |
| bw | Body weight | 60 for adult women and 70 for adult men | kg | (European Commission, 2003) |
The quantity of active substance on the skin per application of a surface treatment product containing 0.1% of nanomaterial can be calculated as: Ader = 5.4 * 10-4 ml using an allocation factor of 1%.
The potential uptake of active substance per application thus can be calculated as 8 * 10-6 ml/kg bw/application for men.
The potential uptake of the active substance per year provided two yearly applications are Uder,pot = 1.5 * 10-5 ml/kg bw/year.
Based on an assumed mass density of 1 g/ml, the potential uptake of active substance in a person per application corresponds to 8* 10-3 mg/kg bw/application and per year to 1.5 * 10-2 mg/kg bw/year (based on two applications per year).
Scenario for a spray product for indoor surface treatment.
In this scenario, a spray product is used for surface treatment of tiles in a bathroom. Liquid aerosols are formed when liquid vapours condense or through mechanical liquefaction, e.g. by atomization with a nozzle and when a liquid jet hits an obstacle (The Danish Working Environment Service, 1983). Thus, it is not expected that a certain formation of aerosols can be avoided and a scenario should account for both exposure by inhalation and dermal contact when the product is applied with a cloth. The product is applied undiluted and consequently a dilution factor is not required in the scenario.
Based on personal communication with the Danish Building Research Institute (SBI) there are many small ‘wet rooms’ in Denmark with a floor area of approximately 3 m². Therefore, an estimated room volume of 7.5 m² corresponding to a floor area of 3 m² and 2.5 m’s distance to the ceiling is assumed to be a reasonable scenario (SBI, 2006). The total surface area of this room will be approximately 20.5 m². Assuming that the floor and 2 of 4 walls are tiled, it is equivalent with an area of 11.75 m².
As is the case with the example of outdoor surface treatment, the scenario of indoor surface is based on a number of assumptions, because of lack of precise product data. Above is shown an example of how to establish a scenario. Therefore, the estimated values above cannot be used for a risk assessment of this type of products, unless more precise product information is available.
The total applied quantity of the active substance with dermal contact and the potential uptake during treatment of tiles in a bathroom can be estimated by applying equation 4 and 5 and the default values in table 7:
Table 7 Symbol explanation and default values
| Symbol | Explanation | Default values | Unit | Reference |
| Ader | The quantity of active substance on the skin per application | ml | (European Commission, 2003) | |
| Qpro | The application of product per application | 10 ml/ m² * 11.75 m² = 117.5 ml |
ml | (DHI, 2006), (SBI, 2006) |
| Fpro | Concentration of the active substance in the product | 0.1 | % | (DHI, 2006) |
| f | Allocation factor to calculate the part of the applied quantity with dermal contact | 1 | % | (DHI, 2006) |
| Uder,pot | Potential Uptake of active substance per year | ml/kg bw/year | ||
| n | Number of applications per year | 2 | Number/year | (DHI, 2006) |
| bw | Body weight | 60 for adult women and 70 for adult men | kg | (European Commission, 2003) |
The amount of active substance on the skin per application of a surface treatment product containing 0.1% of nanomaterial can be calculated:
Ader = 1.18 * 10-3 ml using an allocation factor of 1%.
The potential uptake of active substance per application can be calculated as 2 * 10-5 ml/kg bw/application for women.
The potential uptake of the active substance per year, based on 2 yearly applications, will then be:
Uder,pot = 4 * 10-5 ml/kg bw/year.
To estimate the inhaled quantity of the substance the below equations should be used:
Equation 6 estimates the concentration in the air after application of a product (Qprod).
Equation 6: 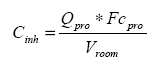
Equation 7 estimates the inhaled quantity of the active substance based on the estimated concentration in air calculated in equation 6.
Equation 7: 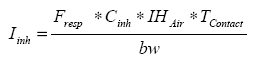
Table 8 Explanation of symbols and default values
| Symbol | Explanation | Default values | Unit | Reference |
| Qpro | Quantity of product per application. | 117.5 ml | ml | (DHI, 2006), (SBI, 2006) |
| Fcpro | Concentration of active substance in the product | 0.1 | % | (DHI, 2006) |
| Vroom | Room area | 7.5 | m³ | (SBI, 2006) |
| Fresp | Respirable part of inhaled substance | 1 | % | (DHI, 2006) |
| IHAir | Respiratory rate | 26 | m³/day – light activity and short time exposure | (European Commission, 2003) |
| TContact | Duration of contact per application | 40 | Min. | Based on the maximum value of the TGD (European Commission, 2003): 3 g spray is applied in 1 min. Assuming that the density is 1 g/ml, the application time of 117.5 will be 40 min. |
| bw | Body weight | 60 for adult women and 70 for adult men | kg | (European Commission, 2003) |
| n | Number of applications per year | 2 | Number/year | (European Commission, 2003) |
| Iinh | Inhaled quantity of substance per application | ml/kg bw/application | ||
| Cinh | Concentration of substance in the room | ml/m³ | (European Commission, 2003) |
The concentration in the air after application of a surface treatment product containing 0.1 % of nano material in a bathroom with the above assumptions can be calculated as Cinh = 0.016 ml/m³.
The estimated inhaled quantity of the substance per application can be calculated as: 1.9 * 10-6 ml/kg bw/application for women.
The quantity of an active substance to be inhaled per year based on 2 applications per year can be calculated as 3.8 * 10-6 ml/kg bw/year.
The total exposure by dermal contact and inhalation, per application of a spray product, can be calculated as follows: (2 * 10-5 ml/kg bw/application + 1.9 * 10-6 ml/kg bw/application) = 2.19 * 10 -5 ml/kg bw/application for a woman.
The total exposure per year for a woman based on exposure by dermal contact and inhalation by application of a spray product twice a year can be calculated as follows: (4 * 10-5 ml/kg bw/application + 3.8 * 10-6 ml/kg bw/application) = 4.38 * 10 -5 ml/kg bw/application.
Based on an assumed density of 1 g/ml, the total quantity of potential uptake of active substance in a woman per application corresponds to 2.19 * 10 -2 mg/kg bw/application and per year to 4.38 * 10 -2 mg/kg bw/application (based on 2 applications per year).
References
European Commission JRC. Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) 1488/94 on risk assessment for existing substances. http://ecb.jrc.it 2003. Available from: http://ecb.jrc.it/existing-chemicals/.
DHI. Estimeret af DHI I samarbejde med aktører på det danske marked. November 2006.
Carglass. Personlig kommunikation med Carglass. Estimat af samlet rudeareal for en standard personbil. 16. November 2006.
Arbejdstilsynet, Arbejdsmiljøinstituttet. Basisbog i arbejdsmiljø. Del II. Risikofaktorer i arbejdsmiljøet. 2. oplag. 1983 Nov.
SBI. Personlig kommunikation med Statens Byggeforskningsinstitut. Estimat af badeværelsevolume efter danske forhold. 16 November 2006.
Lentner C, editor. Geigy Scientific Tables: - 1: Units of Measurement, Body Fluids, Composition of the Body, Nutrition. 8 ed. Basle: CIBA-GEIGY; 1981.
Kommissionens henstilling af 22. September 2006 om effektiviteten af solbeskyttelsesmidler og om angivelser i forbindelse hermed (meddelt under nummer K(2006) 4089) (EØS-relevant tekst) 26.9.2006. Den Europæiske Unions Tidende L 265/39 (2006/647/EF).
Version 1.0 August 2007, © Danish Environmental Protection Agency