Survey of Chemical Substances in Consumer Products, No. 84, 2007
Survey as well as health assessment of chemical substances in school bags, toy bags, pencil cases and erasers
Contents
3 Analysis results and methods
- 3.1 Screening at Beilstein’s test
- 3.2 FT-IR analyses
- 3.3 Phthalates in PVC
- 3.4 XRF analyses
- 3.5 ICP analysis
- 3.6 UV-VIS screening
- 3.7 GC-MS Analysis
- 3.8 PFOS analysis
- 3.9 Summary of the analysis results
4 Screening for possible harmful effects
- 5.1 Assessment of the health risk when using school bags, toy bags, erasers and pencil cases
- 5.2 Assessment of single substances
- 5.3 Total assessment
Appendix A: List of Internet pages of special interest and products distributed in this way
Appendix B: List of procured products
Appendix C: List of completed analyses
Appendix D: Beilstein, FT-IR and XRF analysis results from screening
Appendix E: XRF analysis results
Appendix F: ICP analysis results
Appendix G: PFOS analysis results
Introduction
The project Survey as well as health assessment of chemical substances in school bags, toy bags, pencil cases and erasers is a part of the total effort of the Danish Environment Protection Agency in connection with mapping of chemical substances in consumer products. The project is divided into three phases.
Phase 1: A market survey of school bags, toy bags, pencil cases and erasers, including a survey of which types being on the market and a survey of which chemical substances being applied in such products found in literature, data sheets and trade contacts. Phase 1 is carried out by FORCE Technology, Department for Plastics and Composites by M.Sc. Nanna Svendsen in the period May – July 2006.
Phase 2: Qualitative and quantitative analyses of constituents as well as analysis of which volatile compounds that can be emitted to the air when handling the products. Furthermore, screening for organic parameters through migration to artificial sweat and artificial saliva. Phase 2 is carried out by FORCE Technology, Department for Chemical Analysis by Ph. D. Erik Bjarnov in the period July – November 2006. In addition, analysis of perfluorinated compounds is carried out. The analyses are performed by Denmark’s National Environmental Research Institute (DMU) by senior researcher Rossana Bossi in the period September – November 2006. Quality responsible is M.Sc.Ole Bundgaard.
Phase 3: Health assessment of relevant chemical substances in school bags, toy bags, pencil cases and erasers. Focus is on the substances being assessed to be a possible risk for the consumers’ health – in this case – primary the children’s health. In addition to this, preparation of exposure scenarios for selected substances is carried out. Phase 3 is carried out by FORCE Technology, Department for Sustainability Management by M.Sc. Pia Brunn Poulsen in November 2006. Quality responsible is senior project manager Anders Christian Schmidt.
Summary and conclusions
As part of the Danish Environmental Protection Agency’s survey of chemical substances in a number of consumer products, knowledge of which substances being a part of and being emitted from school bags, toy bags, pencil cases and erasers is wanted. The project Survey as well as health assessment of chemical substances in school bags, toy bags, pencil cases and erasers is conducted in three phases. The survey includes mapping of the market, qualitative and quantitative analyses as well as a health assessment of possible harmful impacts from substances being emitted from school bags, toy bags, pencil cases and erasers.
Phase 1 includes a study of the types of products which are on the Danish market. Furthermore, a study of which materials they are made of or which materials being a part of them as well as at which age group they are directed at. This information is provided in four ways:
- Search via the Internet
- Purchase of school bags, toy bags, pencil cases and erasers
- Through contact to suppliers and producers whose identity is found on the packaging
- Through contact to a number of relevant associations and organizations
Based on the market analysis 26 different pieces of erasers are bought. These 26 pieces of erasers represent a wide choice of the types of erasers which are on the market today. Also based on the market analysis the most frequently bought school bags and a random choice of toy bags and pencil cases are selected.
Phase 2 includes qualitative and quantitative analyses of constituents in school bags, toy bags, pencil cases and erasers. The following analyses of in total 43 products are carried out:
- Screening by use of FT-IR for identification of polymeric types, phthalates and to some extent inorganic colouring agents. This analysis is conducted on a part of the products to get an indication of which substances they contain.
- Beilstein’s test as verification of the FT-IR analysis with the object of determining whether a polymer is vinyl (PVC). Beilstein’s test is a quick qualitative method for determination of halogens. A little piece of the sample is burnt on a copper wire in a flame. Green colouring of the flame indicates content of chlorine. Beilstein’s test is carried out on all polymers as screening. In the project screening for chlorine on a number of products including a large number of erasers is carried out.
- Quantitative determination of phthalates in a large number of erasers and migration to artificial saliva and sweat.
- Quantitative determination of elements by use of X-ray analysis (XRF). From the analysis it is indicated whether the sample contains chlorinated or brominated flame retardants, chlorinated anti-bacterial means, tin compounds, sulphur or nickel. Emphasis has especially been on single out products containing chromium, arsenic, selenium, antimony, cadmium, barium, mercury and lead as amount of these substances must not exceed a stated maximum amount at extraction according to the Toys Statutory Order.
- ICP-MS analysis of extractions to determine the content of selected metals (chromium, arsenic, selenium, antimony, cadmium, barium, mercury and lead) in the extractions.
- GC-MS headspace analysis. It is discovered that some of the products emit a chemical odour, especially when they are quite new. Therefore, an analysis of volatile compounds being emitted to the air when handling the products is carried out. The analysis is conducted by means of semi-quantitative headspace technique combined with GC-MS.
- Screening for staining colouring agents through UV-VIS analysis. The result of this analysis has been significant information whether more detailed analyses were needed.
- GC-MS for analysis and identification of anti-oxidants and organic colouring agents for assessment of emission of substances from the products to artificial saliva.
- Analysis of perfluorinated compounds.
The result of the Beilstein tests, the FT-IR analyses and the XRF analyses showed that 9 out of 26 erasers are made of PVC with phthalate as plasticizer and that both school bags and toy bags are primarily made of polyester textile with plastic parts of PVC with phthalate.
Compared to the results of the FT-IR analysis where a high content of chalk is identified, a high content of calcium in the XRF analysis is correspondingly measured and in many cases also a high content of magnesium. The occurrence of calcium and/or magnesium in the products originates presumably from the use of chalk or dolomite as fillers. Titanium is measured in some of the products and is probably a white pigment in the form of titanium dioxide. The result of the metal analyses in the extractions showed that these metals have not appreciably migrated to the extractions.
Furthermore, the XRF analysis has shown a high content of Cr, As, Se, Cd, Sb, Ba, Hg and/or Pb in one or more products.
In total, four products exceed the application limitations for lead and cadmium as described in chapter 2, Legislation (no violation for mercury).
In a later GC-MS analysis on extracts after extraction in artificial sweat 25 different compounds of interest for a later health assessment are identified. Especially Isophorone, BHT, Cyclohexanone, Phenol, DIBP, DEHP and 2-Heptanone are emphasized as being of particular interest.
In the headspace analysis for volatile substances 23 different compounds of interests for a later health assessment are identified. Of these, especially Isophorone, BHT, Cyclohexanone, Toluene, tert-Butyl alcohol, Methyl propionate and p-Xylene are emphasized as being of particular interest.
The selection is based on the classification of the substances and description of impacts which may be potentially problematic for the consumer if the emission (the migration) of the substances is too high.
Phase 3
A risk assessment is carried out for the content of the following 11 substances being identified via headspace (i.e. evaporation from the products) and/or via migration to artificial sweat or saliva:
- Isophorone
- BHT
- Cyclohexanone
- Phenol
- Toluene
- DIBP
- DEHP
- 2-Heptanone
- tert-Butyl alcohol
- Methyl propionate
- p-Xylene
Regarding the erasers there is a potential of exposure via the mouth, for instance when the children chew or suck the erasers. At oral exposure the absorption takes place after emission (migration) of the substances from the erasers and mixture in saliva. Absorption is presumed to take place via the mucous membranes in the mouth cavity or in the gastrointestinal tract. As children can suck on the erasers these are presumed to be the most interesting product group among school equipment.
Migration analyses for artificial saliva is carried out only for product no. 22 but the results of the migration analyses for artificial sweat are used as a reasonable approximation for the rest of the products.
In general, the content of the above-mentioned substances in the tested products does not present any health risk at normal use of the products; neither in the individual products nor if children are exposed to several products at once – for instance through use of pencil case, eraser and school bag - at exposure via both inhalation and migration for artificial sweat.
Some of the studied erasers are made of PVC (9 of 26) and four of these erasers have a content of DEHP as plasticizer. Daily intake of a small amount (cube of approx. 4 mm) of eraser with a content of DEHP during a longer period may represent a health risk. Correspondingly, it may represent a health risk if a child daily sucks on an eraser with a high content of DEHP during a longer period.
The calculations are generally based on the analyzed values for a few selected school bags, toy bags, pencil cases and erasers. It cannot be rejected that there may be products with a higher content than found in the tested products in this project. Furthermore, there may be other sources to the same chemical substances in the child’s surroundings which will contribute to the total exposure.
Sammenfatning og konklusioner
Som et led i Miljøstyrelsens kortlægning af kemiske stoffer i en række forbrugerprodukter ønskes viden om, hvilke stoffer der indgår i og afgives fra skoletasker, legetasker, penalhuse og viskelædere. Projektet Kortlægning samt sundhedsmæssig vurdering af kemiske stoffer i skoletasker, legetasker, penalhuse og viskelædere er udført i tre faser. Undersøgelsen omfatter kortlægning af markedet, kvalitative og kvantitative analyser samt sundhedsmæssig vurdering af eventuelle sundhedsskadelige effekter fra stoffer, som afgives fra skoletasker, legetasker, penalhuse og viskelædere.
Fase 1 omhandler en undersøgelse af hvilke typer produkter, der er på markedet i Danmark. Endvidere en undersøgelse af hvilke materialer, de er lavet af, eller som indgår i dem samt hvilken aldersgruppe, disse produkter henvender sig til. Disse oplysninger er fremskaffet ad fire veje:
- Søgning via Internettet
- Indkøb af skoletasker, legetasker, penalhuse og viskelædere
- Kontakt til leverandører og producenter, hvis identitet fremgik af emballagen
- Kontakt til et udvalg af relevante foreninger og organisationer
På baggrund af markedsanalysen er der indkøbt 26 forskellige stykker viskelædere. Disse 26 stykker viskelædere repræsenterer et bredt udvalg af de typer viskelædere, der findes på markedet idag. Der er på baggrund af markedsundersøgelsen udvalgt de mest anvendte skoletasker samt et tilfældigt udvalg af legetasker og penalhuse.
Fase 2 omhandler kvalitative og kvantitative analyser af indholdsstoffer i skoletasker, legetasker, penalhuse og viskelædere. Der er foretaget følgende analyser på ialt 43 produkter:
- Screening vha. FT-IR for identifikation af polymertyper, ftalater og i nogen udstrækning uorganiske farvestoffer. Denne analyse er udført på udsnit af produkterne for at få en indikation af hvilke stoffer, de indeholder.
- Beilsteins test som verifikation af FT-IR analysen med henblik på at konstatere, hvorvidt en polymer er vinyl (PVC). Beilsteins test er en hurtig kvalitativ metode til bestemmelse af halogener, idet lidt prøve afbrændes på en kobbertråd i en flamme. Grønfarvning af flammen indikerer indhold af chlor. Beilsteins test udføres som screening på alle polymerer. Der er i projektet screenet for chlor på en række produkter herunder et større antal viskelædere.
- Kvantitativ bestemmelse af ftalater i en større mængde viskelædere og migration til spyt og sved.
- Kvantitativ grundstofbestemmelse vha. røntgenanalyse (XRF). Ved analysen fås bl.a. indikation af, om prøven indeholder chlorerede eller bromerede flammehæmmere, chlorerede antibakterielle midler, tin-forbindelser, svovl eller nikkel. Der er lagt særligt vægt på at fremhæve produkter, der indeholder krom, arsen, selen, antimon, cadmium, barrium, kviksølv og bly, da disse stoffer i.flg. legetøjsdirektivet ikke må overstige en angivet maksimal mængde ved ekstraktion.
- ICP-MS analyse af ekstrakter for at bestemme indholdet af udvalgte metaller (krom, arsen, selen, antimon, cadmium, barrium, kviksølv og bly) i ekstrakterne.
- GC-MS headspace analyse. Det er konstateret, at nogle af produkterne afgiver en kemisk lugt, specielt når de er helt nye. Der er derfor analyseret for hvilke flygtige forbindelser, der kan afgives til luften ved håndtering af produkterne. Analysen er udført ved semi-kvantitativt headspace teknik kombineret med GC-MS.
- Screening for afsmittende farvestoffer ved UV-VIS analyse. Resultatet af denne analyse har været en væsentlig oplysning for, om der var behov for nærmere analyser.
- GC-MS for analyse og identifikation af antioxidanter og organiske farvestoffer til vurdering af afgivelse af stoffer fra produkterne til kunstigt spyt.
- Analyse af perfluorerede forbindelser.
Resultatet af Beilstein testen, FT-IR anlysen og XRF analysen viste, at 9 ud af 26 af viskelæderne er lavet af PVC med ftalat som blødgører samt, at såvel skoletasker som legetasker primært er fremstillet af polyestertekstil med plastdele af PVC med ftalat.
Sammenholdt med resultaterne fra FT-IR analysen, hvor der er identificeret et højt indhold af kridt, måles tilsvarende et højt indhold af calcium ved XRF analysen foruden i mange tilfælde også et højt indhold af magnesium. Forekomsten af calcium og/eller magnesium i produkterne hidrører antageligt fra anvendelsen af kridt eller dolomit som fyldstoffer. Titan er målt i nogle af produkterne og indgår formentlig som et hvidt pigment i form af titandioxid. Resultatet af metalanlyserne i ekstrakterne viste, at disse metaller ikke i nævneværdig grad er migreret til ekstrakterne.
Der er derudover ved XRF analysen påvist højt indhold af Cr, As, Se, Cd, Sb, Ba, Hg og/eller Pb i et eller flere produkter.
Der er i alt fire produkter, der overskrider anvendelsesbegrænsningerne for bly og cadmium som beskrevet i kapital 2 under Lovgivning (ingen overskridelser for kviksølv).
Ved en efterfølgende GC-MS anlyse på ekstrakter efter ekstraktion i kunstigt sved findes 25 forskellige forbindelser af interesse for en efterfølgende sundhedsvurdering. Af disse fremhæves især Isophoron, BHT, Cyclohexanon, Phenol, DIBP, DEHP og 2-Heptanon som værende af særlig interesse.
Ved headspace analysen for flygtige stoffer findes 23 forskellige forbindelser af interesse for en efterfølgende sundhedsvurdering. Af disse fremhæves især Isophoron, BHT, Cyclohexanon, Toluene, tert-Butylalkohol, Methylpropionat og p-Xylen som værende af særlig interesse.
Udvælgelsen af stoffer til sundhedsvurdering er baseret på stoffernes klassifikation og beskrivelse af effekter, som kan være potentielt problematiske for forbrugeren, hvis afgivelsen (migrationen) af stofferne fra produkterne er for stor.
Fase 3
Der er foretaget risikovurdering for indholdet af de følgende 11 stoffer, der er identificeret via headspace (dvs. afdamper fra produkterne) og/eller via migration til kunstig sved eller spyt:
- Isophoron
- BHT
- Cyclohexanon
- Phenol
- Toluene
- DIBP
- DEHP
- 2-Heptanon
- tert-Butylakohol
- Methylpropionat
- p-Xylen
For viskelæderne gælder, at der er mulighed for en eksponering via munden, eksempelvis ved at børnene tygger eller sutter på viskelæderne. Ved oral eksponering sker absorptionen efter afgivelse (migration) af stofferne fra viskelæderne og opblanding i spyt. Optagelse antages at kunne ske over slimhinder i mundhule eller mave-tarmkanalen. Netop fordi børn kan sutte på dem, antages viskelædere at udgøre den mest interessante produktgruppe blandt skoleudstyr.
Der er kun foretaget migrationsanalyser til kunstigt spyt for produkt 22, men resultaterne fra migrationsanalyserne til kunstig sved anvendes som en rimelig tilnærmelse for resten af produkterne.
Generelt udgør indholdet af ovennævnte stoffer i de undersøgte produkter ikke nogen sundhedsmæssig risiko ved almindelig brug af produkterne. Hverken i de enkelte produkter eller hvis børn udsættes for flere produkter på én gang – eksempelvis via brug af både penalhus, viskelæder og skoletaske og ved eksponering via både indånding og migration til kunstig sved.
Nogle af de undersøgte viskelædere er af PVC (9 af 26), og fire af disse viskelædere har et indhold af DEHP som blødgører. Daglig indtagelse af en lille mængde (kube på ca. 4 mm) viskelæder med et indhold af DEHP over en længere periode kan udgøre en sundhedsmæssig risiko. Tilsvarende kan det udgøre en sundhedsmæssig risiko dagligt at sutte eller tygge på et viskelæder med et højt indhold af DEHP over en længere periode.
Generelt er beregningerne baseret på de analyserede værdier for enkelte udvalgte skoletasker, legetasker, penalhuse og viskelædere. Det kan ikke afvises, at der findes produkter med et større indhold end det, der er fundet i de undersøgte produkter i dette projekt. Der kan desuden være andre kilder til de samme kemiske stoffer i barnets omgivelser, som vil bidrage til den totale eksponering.
Abbreviations
| ADI | Acceptable Daily Intake. Estimated intake which is presumed to have no harmful effect. Might be acute or chronic. Is normally based on ingredients in food (additives) |
| BW | Body Weight |
| DL | Detection Limit |
| EC | Effect Concentration |
| EC50 | Median Effect Concentration, i.e. the concentration where 50% of the test animals show an effect |
| H | Hours |
| LC50 | Median Lethal Concentration, i.e. the concentration where 50% of the test animals are dead |
| LD50 | Median Lethal Dose, i.e. the dose where 50% of the test animals are dead |
| LOAEL | Lowest Observed Adverse Effect Level |
| MAK | Maksimaler Arbeitsplatz Konzentration (Maximum working place concentration): Limits for the working environment defined by the German working environment authorities |
| MOS | Margin of Safety. The relation between the estimated exposure and the concentration which is considered to result in no health risk (e.g. NOAEL) |
| NOAEL | The largest concentration where no harmful effects are observed (No-Adverse-Effect Level) |
| RfC | Inhalation Reference Concentration. A concentration (e.g. µg/m³) which is an estimate of a daily exposure by inhalation which is presumed without any significant harmful effect by inhalation during a human lifetime. It is presumed that a limit of the toxic effect is available from which the limit is derived |
| RfD | Oral Reference Doses is an estimate of a daily intake (e.g. µg/kg lgv/day) which is presumed without any significant harmful effect by intake during a human lifetime. It is presumed that a limit of the toxic effect is available from which the limit is derived |
| TDI | Tolerable Daily Intake. Estimated intake which is presumed to have no harmful effect. May be acute or chronic. Normally, based on chemical pollutants |
| TGD | Technical Guidance Document: EU guidance in risk assessment of chemical substances |
| TLV | Threshold Limit Value which is based on an 8 hours’ time-weighted average exposure in the working environment (one working day) |
| TWA | Time Weighted Average |
1 Introduction
1.1 Preface
School bags and similar bags for children are found in numerous variants and often in very colourful versions. Most often these bags are made of textiles and/or plastics materials. In many cases these plastic materials are PVC with a variable amount of among other things phthalates, colouring agents, perfluorinated compounds, flame retardants, UV stabilizers and volatile compounds.
School bags, toy bags, pencil cases and erasers are used by children at many ages and it is possible that very small children at the age of 0-3 years also get in direct contact with these products. Some products, such as erasers designed as fruits or food with related smell (strawberry smell etc.) may risk ending in the mouth of even the smallest children. To a great extent these products address to children and in many cases they get into direct skin contact during use.
Many of the products emit a chemical smell, especially when they are quite new. The chemical smell might come from a number of solvents such as benzene, styrene and cyclohexanone.
The products are covered by the product safety law and a few substances may be covered by application limitation statutory orders. Products that are toys are also covered by the toys directive.
The soft plastic types may contain one or several kinds of phthalates used as plasticizers. A study published on Greenpeace homepage called Chemikaze Shopping mentions that school accessories such as school bags, drinking bottles and pencil cases, bought in various airports, contain considerable amounts of phthalates. They found DEHP in concentrations up to 23% in a Batman drinking bottle. Therefore, a study of possible content of phthalates is included in this project.
Phase 1: Survey of the market
A survey of the types of products available on the Danish market is carried out. It is also investigated which materials the products are made of or which materials being in the products as well as which age group these products refer to.
Phase 2: Qualitative and quantitative analysis
The following analyses are carried out:
- Screening analysis at FT-IR for determination of the materials which the product is made of as well as for phthalates and to some extent inorganic colouring agents.
- Beilstein test for detection of the presence of Cl (chlorine) for identification of PVC and thus suspicion of phthalates.
- Quantitative determination of phthalates in a large amount of erasers and migration to artificial saliva for a single eraser.
- Quantitative analysis for elements by XRF.
- Quantitative determination of selected metals in extracts by ICP.
- Semi-quantitative analysis of which substances that may be emitted to the air by headspace analysis combined with GC-MS.
- UV-VIS analysis for detection of certain colouring agents.
- Quantitative analysis of which substances being emitted to artificial sweat at GC-MS.
- Analysis for perfluorinated compounds.
Phase 3: Health assessment
Health assessment of relevant chemical substances in school bags, toy bags, pencil cases and erasers as well as preparation of exposure scenarios.
1.2 Purpose
The purpose of the project is to survey the market for school bags, toy bags, pencil cases and erasers including which product types being on the market, the scope of the consumption in Denmark as well as which materials being used for production. The project has focused on whether these products emit chemical substances and if so whether the use of these products can be critical to health.
The project includes a survey of constituents and possible emission of substances such as phthalates, heavy metals, perfluorinated compounds, colouring agents and other problematic substances from product to consumer. A health assessment of the emitted substances has also been carried out.
1.3 Approach
The project is divided into three fazes.
1. A market survey of school bags, toy bags, pencil cases and erasers including a survey of which types being on the market. Based on literature, data sheets and business contacts, a survey of which chemical substances being used in such products is carried out. Emphasis is especially on perfluorinated compounds, volatiles, heavy metals, plasticizers and possibly flame retardants.
2. For selected products, the above-mentioned analyses under phase 2 have been completed.
3. Selection of products in co-operation with the Danish EPA for health assessment and relevant exposure scenarios, including a survey whether the substances are absorbed through the skin.
2 Survey and legislation
2.1 Market surveys
2.1.1 Introduction
A study of the types of products which are on the Danish market has been carried out. Furthermore, a study of which materials they are made of or which materials being a part of them as well as at which age group these products direct.
This information is provided through contact to producers, suppliers and their trade organization, Legetøjsbranchens Fællesråd, which is the trade organization for the whole toy industry in Denmark. Booksellers and their common purchase organization has been contacted as well as large super markets such as Dansk Supermarked importing to Bilka and Føtex and COOP importing to Super-Brugsen and Kvickly. Products from 10 kroner shops such as Tiger, from bag shops and department stores such as Magasin are also included in the study.
For both school bags and erasers Statistics Denmark has calculated the yearly consumption of these products expressed both as quantities and as total value.
2.1.2 Market survey – categories
The Danish Safety Technology Authority has evaluated which products are classified as toys. This evaluation is based in the following documents:
- Toys Statutory Order (TSO)
- The General Product Safety Directive (GPSD)
- Guide no. 4 from the Commission dated 18.02.2003 (Price and size - items mentioned in Guide no. 4) is evaluated as being irrelevant for the purchased products
- Table showing the purchased products delivered to the Danish EPA
- Table 2.3 showing which products being CE labelled
This evaluation is shown in Table 2.3.
We have chosen to divide school bags, toy bags, pencil cases and erasers into the following categories:
School bags
Bags, which from the retail trade are labelled as school bags, may be regarded as and according to the Danish Safety Technology Authority are regarded as being school equipment and not toy as they are used for storing of books, pencil cases, papers etc. and the bag itself is not used for play purposes. There are school bags with appendage where the appendage is toy and not the bag.
Toy bags
Cover a very wide range of bags, for instance:
- Bags for use in the pre-school age for packed lunch and spare clothes in nursery/kindergarten.
- Bags with toy content such as doll change bags, medical bags and tool boxes.
- Bags with another content such as small bags with hair elastics or underwear where the bag only appeals for play purpose.
- Various toy bags such as decoration bags, hand bags, shopping bags and teddy bear bags.
- Bags for games such as V. Smile Pocket carrying case, Gameboy case, PS bag.
Toy bags are estimated as being a product under either the Toys Statutory Order or under the General Product Safety Directive as shown in Table 2.3.
Pencil cases
- For school purpose.
- For play purpose – pencil cases in a size or with content not suitable for use at school. Often these appeal to smaller children. Pencil cases for play purpose are estimated as a product under either the Toys Statutory Order or under the General Product Safety Directive as seen in Table 2.3.
Erasers
- The traditional ones – erasers of a good quality without print, colour or fragrance.
- The popular ones – erasers with popular prints, for instance Batman, Disney figures and Diddl being used for erase purposes. These erasers may contain fragrances.
- For play purposes – erasers in imaginative shapes and colours, perhaps with print and fragrances. Often these erasers are in a quality or size making them most suitable as collector's items or for play purposes. Erasers for play purposes are estimated as being a product under either the Toys Statutory Order or under the General Product Safety Directive as shown in Table 2.3.
2.1.3 Market survey – the Internet
In connection with this project the Internet is used partly to obtain an overview of the market of school bags etc., and partly as basis for continuous contact to relevant companies. A list of Internet pages of special interest and a list of products being sold are shown in Appendix A.
2.1.4 Statistics
According to Statistics Denmark the industry has stated an import and export CN (combined nomenclature) after units, import and export, goods and time in Table 2.1.
Table 2.1 Import and export of goods according to Statistics Denmark
| Product group | Import | Export | ||
| 2004 | 2005 | 2004 | 2005 | |
| Eraser of soft rubber | 2,746,190 | 4,359,845 | 2,027,280 | 2,341,547 |
| Suitcases, brief cases, school bags etc. with an outside of embedded plastic | 19,617,355 | 24,301,813 | 1,222,767 | 535,932 |
Due to a changed practice for the release of the foreign trade figures, the trade figures for the latest month now contain detailed figures for the trade of goods with non-EU countries. The trade figures for the intra-community trade are still available at an aggregated level only. The detailed figures for the trade with countries outside the EU are found under foreign trade – Detailed foreign trade (monthly).
No value is stated for”the Industry’s sale of own goods after unit, product category and time” which indicates that no goods from these product categories are produced in Denmark.
2.1.5 The retail trade
Today, on the Danish market there are two large convenience chains, namely COOP Denmark (among others the convenience store chains Kvickly, Irma, Fakta, Super-Brugsen, Dagli’Brugsen and LokalBrugsen) and Danish Supermarket Group (DSG) (among others the convenience store chains Bilka, Føtex, Netto and A-Z). Both convenience chains control that the toy is delivered with CE labelling. Furthermore, school bags etc. are sold in bookstores, bag shops, department stores and 10 kr. shops.
Information from the retail trade is shown in Appendix A.
2.1.6 Trade organizations
The trade organization, the Toy Business Council (LF), has been contacted to obtain information regarding the market for toy bags etc. The organization works as a consultant for their members and ensures that they are updated with regard to new rules, laws, directives, news and warnings. The organization only works at product level if there are legal matters of dispute. The trade organization does not have its own set of rules but they inform the members about new regulations. Laws and rules are typically provided to the trade organization through the Danish EPA and Euro Commerce.
2.2 Legislation
Safety requirements to toy
For toy”Statutory order on safety requirements to toys and products which due to its appearance can be confused with foods” (Stat. Ord. 1116, 2003) is applicable. Toy is defined as products “which clearly are designed or intended for play purposes for children below 14 years”. If the erasers have a design as a figure (a burger, an ice or similar) the statutory order on safety requirements to toy must also be met.
According to the Toys Statutory Order (Stat. Ord. 1116, 2003) a toy must only be sold if it fulfils the EU legislation on safety requirements to toy or if it is produced in accordance with a technically approved prototype (and approved by a body of the authorities in an EU country). Toy fulfilling these safety requirements must be provided with a CE label before it is sold.
The EU legislation on safety requirements includes the standards which are stated in Appendix 3 of the Toys Statutory Order (Stat. Ord 116, 2003). These are the EN71 series on safety requirements to toy as well as the Executive order on high-voltage power for electric toy. EN71-3 (Part 3: Migration of special substances) includes limits for migration of metals when the toy is put into the mouth. These limits are shown in Table 3.4.
Ban on phthalates in toy for children
According to the Statutory Order no. 151 of 15.3.1999 “Statutory Order on ban on phthalates in toy for children at the age of 0-3 as well as certain infant articles” it is forbidden to produce, sell or import products containing more than 0.05% (w/w) phthalates for the following types of products:
- Toy clearly designed or intended for play purposes for children at the age of 0-3 years.
- Infant articles for children at the age of 0-3 years, i.e. products which are intended to or must be expected to be put into the mouth (dummies, bibs, jewelleries as well as bath equipment etc.).
- Product which is expected to be used as toy by children at the age of 0-3 years as a result of the design of the product including printing.
However, this statutory order is replaced by a new”Statutory Order on ban on phthalates in toy and infant articles” which became effective in the spring of 2007 (Stat. Ord. 786, 2006). The new rules include ban on six phthalates in infant articles and in toy for children up to the age of 14.
According to the new statutory order it is banned to use the phthalates DEHP, DBP and BBP in concentrations above 0.1% as well as to import and sell toy and infant articles containing these phthalates in concentrations above 0.1%. Furthermore, it is banned to use the phthalates DINP, DIDP and DNOP in concentrations above 0.1% in products which children may put into the mouth as well as to import and sell toy and infant articles containing these phthalates in concentrations above 0.1% which children may put in the mouth.
Finally it is banned to use all other phthalates in concentrations above 0.05% in toy for children at the age of 0-3 years as well as in infant articles for children at the age of 0-3 years which are intended to be put into the mouth.
Application limitation of certain heavy metals
For the heavy metals lead, mercury and cadmium legislation regarding limitation of the application of these substances is available. The following legislation is relevant:
- Statutory order on ban on import and sale of products containing lead. Stat. Ord. 1012 of 13.11.2000.
- Statutory order on ban on import, sale and export of mercury and mercurial products. Stat. Ord. 627 of 01.07.2003.
- Statutory order on ban on sale, import and production of products with cadmium content. Stat. Ord. 1199 of 23.12.1992.
According to these statutory orders it is banned to import and sell products containing more than 100 ppm (mg/kg) lead and mercury respectively and more than 75 ppm cadmium in the homogeneous single parts of the product.
2.2.1 Selection criteria
The following selection criteria for products for further analysis have been applied:
- Products from each of the above-mentioned categories have been selected so that both school bags and various categories of toy bags, pencil cases and erasers have been selected for analysis.
- All the above-mentioned contact groups (toy retailers, book sellers, supermarkets, department stores and 10 kroner shops) have been contacted and at visits at representatives for these contact groups all the goods being interesting for this project have been photographed and described. On this basis, products from all the above-mentioned contact groups have been selected.
- All the selected products contain plastic parts or are completely made of plastic.
- The products are selected on the basis of information from contact to various contact groups so that products selling well are selected. (It has not been possible to get a top 3 list of the most sold products within the above-mentioned categories from all contact groups but where possible it has been taken into consideration during the selection of products for analysis).
- Certain products are sold both in the shops and on the Internet and therefore products being sold both on the Internet and in various shops have been selected for further analysis.
- Several types of products are available at several types of contact groups (Spiderman school bag from Marvel is available at supermarkets, book sellers and in bag shops).
2.2.2 Selected products
26 different pieces of erasers are bought based on the above-mentioned selection criteria. These 26 pieces of erasers represent a wide choice of the types of erasers being on the market today. On the basis of the market survey the most frequently sold school bags are selected as well as a random choice of toy bags and pencil cases. In total 43 products are selected for the analyses.
Besides the 26 different erasers, the following products are selected for further analysis:
Table 2.2 Products selected for analyses
| Category | Contact group/purchase place | Description |
| School bags | Toy store | Rucksack |
| Supermarket | School bag | |
| Bag shop | School bag | |
| School bag | ||
| Toy bags | Toy store | Handbag, small |
| 10 kroner shop | Bag with hair rubber band | |
| Decorative bag | ||
| Decorative bag | ||
| Toy bag | ||
| Pencil cases | Toy store | Pencil case |
| Supermarket | Pencil case | |
| Book shop | Pencil case | |
| Pencil case | ||
| Pencil case | ||
| 10 kroner shop | Pencil case |
Information regarding material selection and other relevant information is also printed on the packaging of the individual products being selected for further analysis. This information is listed in Table 2.3 below. The Danish Safety Technology Authority’s assessment is also stated in the table. TSO in the table states that the product must comply with the Toys Statutory Order. GPSD in the table states that the product must comply with the General Product Safety Directive.
Table 2.3 Labelling or remark stated on the packaging of purchased products
| No. | Description | Other labelling or remark | CE labelled [+/-] | The Danish Safety Technology Authority’s assessment of category [TSO/GPSD] |
| 1 | Eraser | - | TSO | |
| 2 | Eraser | - | TSO | |
| 3 | Eraser | Smells pleasantly | - | TSO |
| 4 | Eraser | Smells pleasantly | - | GPSD |
| 5 | Eraser | - | GPSD | |
| 6 | Eraser | Danger labelled for children under 3 years | + | TSO |
| 7 | Eraser | - | GPSD | |
| 8 | Eraser | Conform to ASTM 4236 and EN-71 | - | TSO, EN 71 is stated |
| 9 | Eraser | - | TSO | |
| 10 | Eraser | - | GPSD | |
| 11 | Eraser | Danger labelled for children under 3 years | + | TSO |
| 12 | Eraser | Danger labelled for children under 3 years | - | GPSD |
| 13 | Eraser | Danger labelled for children under 3 years | + | TSO |
| 14 | Eraser | Danger labelled for children under 3 years | + | TSO |
| 15 | Eraser | Danger labelled for children under 3 years | + | TSO |
| 16 | Pencil case | Danger labelled for children under 3 years | + | TSO |
| 17 | Eraser | - | GPSD | |
| 18 | Eraser | - | GPSD | |
| 19 | Eraser | - | GPSD | |
| 20 | Eraser | - | GPSD | |
| 21 | Eraser | - | GPSD | |
| 22 | Eraser | + | TSO | |
| 23 | Eraser | + | TSO | |
| 24 | Eraser | Danger labelled for children under the age of 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard | + | TSO |
| 25 | Eraser | Smells pleasantly | - | Is assessed to be a food imitation |
| 26 | Pencil case | - | TSO | |
| 27 | Eraser | - | TSO | |
| 28 | Eraser | - | GPSD | |
| 29 | Toy bag | - | GPSD | |
| 30 | Toy bag | Danger labelled for children under 3 years | + | TSO |
| 31 | Pencil case | - | TSO | |
| 32 | Toy bag | + | TSO | |
| 33 | Toy bag | - | GPSD | |
| 34 | Pencil case | Danger labelled for children under 3 years | + | TSO |
| 35 | Pencil case | - | TSO | |
| 36 | Pencil case | - | GPSD | |
| 37 | Toy bag | Danger labelled for children under the age of 3 years. This product conforms the safety requirements of ASTM F963 | + | TSO |
| 38 | Toy bag | This bag is not toy keep away from babies | - | TSO |
| 39 | School bag | - | GPSD | |
| 40 | School bag | Not for children under 3 years | + | TSO |
| 41 | School bag | - | TSO | |
| 42 | School bag | Not for children under 3 years | + | TSO |
| 43 | Pencil case | - | GPSD |
3 Analysis methods and results
- 3.1 Screening at Beilstein’s test
- 3.2 FT-IR analyses
- 3.3 Phthalates in PVC
- 3.4 XRF analyses
- 3.5 ICP analysis
- 3.6 UV-VIS screening
- 3.7 GC-MS Analysis
- 3.8 PFOS analysis
- 3.9 Summary of the analysis results
The following qualitative and quantitative analyses are completed as basis for an assessment of the possible health risk when using school bags, toy bags, erasers and pencil cases.
1. Screening analysis by FT-IR for determination of the materials which the products are made of as well as of phthalates and to some extent inorganic colouring agents.
2. Beilstein test for detection of the presence of C1 for identification of PVC and thus suspicion of phthalates.
3. Quantitative determination of phthalates in erasers.
4. Quantitative analysis for elements through XRF for determination of the amount of the individual metals in the product.
5. Quantitative analysis for metals in extracts at ICP-OES for assessment of the quantity of each metals migrated.
6. Quantitative analysis of which substances that may be emitted to the air by headspace analysis combined with GC-MS.
7. UV-VIS analysis for identification of certain colouring agents.
8. Quantitative analysis of which substances being emitted to artificial sweat and in one single case to artificial saliva at GC-MS.
9. Analysis of perfluorinated compounds which may have been applied as preservative on certain products.
3.1 Screening at Beilstein’s test
3.1.1 Used analytical equipment and proofing methods
Beilstein’s test is a quick method for determination of halogens. The principle of the test is that volatile copper salts will colour a flame green because of the copper content. Copper halides (F is accepted) are volatile and only in a few other cases the test will give a positive reaction. If halogens are present in plastic the plastic is most probably a PVC plastic and will typically be softened with a phthalate plasticizer.
A micro-burner and a strong copper wire are needed. The micro-burner must have full air inlet (nearly colourless flame). The copper wire is calcinated and the warm wire is rubbed on the sample so that some of the sample melts upon the wire. The wire is led into the middle of the outer zone of the flame. If the sample is lighted and burns it is burnt out outside the flame. The wire is again led into the flame and shortly before ignition the green colour is seen clearly if the sample contains halogens.
3.1.2 Results of the Beilstein test
The Beilstein test is conducted through a test on all the purchased products of all material types found on the individual products.
The results are shown in Table 3.1 and in Appendix D.
Table 3.1 The result of the Beilstein test
| No. | Description | Remark | Beilstein +/- |
| 1 | Eraser | - | |
| 2 | Eraser | - | |
| 3 | Eraser | Smells pleasantly | Eraser: + |
| Cover: - | |||
| 4 | Eraser | Smells pleasantly | - |
| 5 | Eraser | Eraser: + | |
| Cover: - | |||
| 6 | Eraser | Danger labelled for children under 3 years | Black part: + |
| Rest: - | |||
| 7 | Eraser | - | |
| 8 | Eraser | Conform to ASTM 4266 and EN-71 | - |
| 9 | Eraser | Eraser: + | |
| Cover: - | |||
| 10 | Eraser | - | |
| 11 | Eraser | Danger labelled for children under 3 years | Eraser: - |
| Cover: + | |||
| 12 | Eraser | Danger labelled for children under 3 years | + |
| 13 | Eraser | Danger labelled for children under 3 years | + |
| 14 | Eraser | Danger labelled for children under 3 years | + |
| 15 | Eraser | Danger labelled for children under 3 years | + |
| 16 | Pencil case | Danger labelled for children under 3 years | + |
| 17 | Eraser | - | |
| 18 | Eraser | - | |
| 19 | Eraser | - | |
| 20 | Eraser | - | |
| 21 | Eraser | - | |
| 22 | Eraser | + | |
| 23 | Eraser | + | |
| 24 | Eraser | Danger labelled for children under the age of 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard | - |
| 25 | Eraser | Smells pleasantly | - |
| 26 | Pencil case | + | |
| 27 | Eraser | - | |
| 28 | Eraser | - | |
| 29 | Toy bag | + | |
| 30 | Toy bag | Danger labelled for children under 3 years | Red plastic (A):+ |
| Clear plastic (B): + | |||
| 31 | Pencil case | Canvas (A): + | |
| Plastic front (B): + | |||
| Rest: - | |||
| 32 | Toy bag | - | |
| 33 | Toy bag | - | |
| 34 | Pencil case | Danger labelled for children under 3 years | + |
| 35 | Pencil case | Fabric (A): + | |
| Stickers (B): + | |||
| Gray inside (C): + | |||
| Rest: - | |||
| 36 | Pencil case | + | |
| 37 | Toy bag | Danger labelled for children under the age of 3 years. This product conforms the safety requirements of ASTM F963 | Round plastic plates (A): + |
| Fabric (B): - | |||
| 38 | Toy bag | This bag is not toy keep away from babies | Bag (A): + |
| Lining (B): - | |||
| Rosa plastic (C): | |||
| Red strap (D): - | |||
| 39 | School bag | Lining (A): - | |
| Bag (B): + | |||
| Plastic (C): + | |||
| Coloured strap (D): - | |||
| 40 | School bag | Not for children under 3 years | Rosa canvas (A): + |
| Handle (B): + | |||
| Green/yellow plastic (C): - | |||
| 41 | School bag | Rosa lining (A): + | |
| Black bottom (B): + | |||
| Small bag for accessories (C): + | |||
| Rest (D): - | |||
| 42 | School bag | Not for children under 3 years | Black fabric front (A): + |
| Gym bag (B): + | |||
| Plastic front (C): + | |||
| Straps (D): - | |||
| Lining, back: - | |||
| 43 | Pencil case | + |
From the above Table 3.1 it is seen which products having a positive test. This gives a suspicion of phthalates. The presence of phthalates can be verified through a FT-IR analysis. A comparison of the analysis results for the Beilstein, FT-IR and XRF analyses is shown in Appendix D.
3.2 FT-IR analyses
3.2.1 Applied analytical equipment and preparation methods
The FT-IR analyses are conducted on a Nicolet Impact 400 FT-IR spectrometer.
Initially, only a screening analysis was conducted for an assessment of the material type. If a product consisted of more than one type of material, the part of the product being assessed to be the largest/most important is analyzed.
Different techniques were used depending on the product. Flat, smooth materials were examined using ATR technique. Materials not smooth or flat were investigated by rubbing a silicon carbide sand paper against the sample and absorb the spectrum at DRIFT (diffuse reflectance) with the clean sand paper as reference. Textiles were also examined at DRIFT technique with a steel surface (empty “cup”) as reference.
Both ATR and DRIFT are reflection techniques and the spectra become a little distorted in relation to normal transmission spectra.
For identification of plastic types primarily electronic reference libraries (Hummel-Scholl or Sadtler Know-it-all) were used in combination with FORCE Technology’s general experience.
Plasticizers, such as phthalates, are normally used in large quantity (30%) and can immediately be seen in the spectra. These substances will often camouflage the spectrum of the basis polymer. Normally, a phthalate present in a few per cent of another ester may not be noted. Normally, other additives being used in 0.1% to a few per cent may not be discovered at the screening analysis unless they have absorptions in ranges where basis polymer and perhaps plasticizer for positively do not absorb.
Fillers with characteristic spectra as for example chalk may be detected in levels of 10-30% while other fillers most often cannot be positively identified.
In relation to anti-oxidants which are added in much less amounts than plasticizers, it will only be in the cases where more than about 0.1 weight% is added and the anti-oxidant, or other additives, has strong absorption bands outside the absorptions from the polymer that they may be recognized in the analysis.
3.2.2 Results of the FT-IR screening
The results are shown in Table 3.2 and in Appendix D.
Table 3.2 Results of the FT-IR screening
| No. | Description | Remark | Content |
| 1 | Eraser | ||
| 2 | Eraser | ||
| 3 | Eraser | Smells pleasantly | Eraser: PVC with phthalate and chalk |
| Cover: Not examined | |||
| 4 | Eraser | Smells pleasantly | |
| 5 | Eraser | Eraser: PVC with phthalate | |
| Cover: Not examined | |||
| 6 | Eraser | Danger labelled for children under 3 years | Black part of penguin: Not examined |
| Rest: Not examined | |||
| 7 | Eraser | ||
| 8 | Eraser | Conform to ASTM 4266 and EN-71 | |
| 9 | Eraser | Eraser: PVC with phthalate and chalk | |
| Cover: Not examined | |||
| 10 | Eraser | Liquid paraffin and lots of chalk | |
| 11 | Eraser | Danger labelled for children under 3 years | Eraser: not examined |
| Cover: Not examined | |||
| 12 | Eraser | Danger labelled for children under 3 years | PVC with phthalate and chalk |
| 13 | Eraser | Danger labelled for children under 3 years | Eraser: PVC with phthalate and chalk |
| 14 | Eraser | Danger labelled for children under 3 years | PVC with phthalate and chalk |
| 15 | Eraser | Danger labelled for children under 3 years | Eraser: PVC with phthalate |
| 16 | Pencil case | Danger labelled for children under 3 years | Gray: PVC with phthalate. White: Polyester textile (PET) |
| 17 | Eraser | ||
| 18 | Eraser | ||
| 19 | Eraser | ||
| 20 | Eraser | ||
| 21 | Eraser | ||
| 22 | Eraser | Eraser: PVC with phthalate and chalk. | |
| 23 | Eraser | Eraser: PVC with phthalate and chalk | |
| 24 | Eraser | Danger labelled for children under the age of 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard | Isobutene Isoprene rubber |
| 25 | Eraser | Smells pleasantly | |
| 26 | Pencil case | Polyester Polyurethane (PUR) | |
| 27 | Eraser | ||
| 28 | Eraser | ||
| 29 | Toy bag | ||
| 30 | Toy bag | Danger labelled for children under 3 years | A: |
| B: PVC with phthalate | |||
| 31 | Pencil case | A: Polyester textile with terephthalate (PET) | |
| B: PVC with phthalate | |||
| Rest: Not examined | |||
| 32 | Toy bag | ||
| 33 | Toy bag | ||
| 34 | Pencil case | Danger labelled for children under 3 years | PVC with phthalate |
| 35 | Pencil case | A: Polyester textile with terephthalate (PET) | |
| B: PVC with phthalate | |||
| C: PVC with phthalate and chalk | |||
| Rest: Not examined | |||
| 36 | Pencil case | ||
| 37 | Toy bag | Danger labelled for children under the age of 3 years. This product conforms the safety requirements of ASTM F963 | A: PVC with phthalate |
| B: Polyester textile with terephthalate | |||
| 38 | Toy bag | This bag is not toy keep away from babies | A: Polyester textile with terephthalate |
| B: Polyester textile with terephthalate | |||
| C: Poly Urethane | |||
| D: Not examined | |||
| 39 | School bag | A: Poly Amid textile | |
| B: Polyester textile with terephthalate | |||
| C: PVC with phthalate | |||
| D: Not examined | |||
| 40 | School bag | Not for children under 3 years | A: Polyester textile with terephthalate (PET) |
| B: PVC with phthalate | |||
| C: Polyester textile with terephthalate (PET) | |||
| 41 | School bag | A: PVC with phthalate and chalk | |
| B: Not examined | |||
| C: Not examined | |||
| D: Not examined | |||
| 42 | School bag | Not for children under 3 years | A: PA |
| B: Polyester textile with terephthalate | |||
| C: PVC with phthalate | |||
| D: PP | |||
| Lining, back: Not examined | |||
| 43 | Pencil case | Eraser: PVC with phthalate. |
If the results of the Beilstein test stated in Table 3.1 are compared to the results of FT-IR analyses shown in Table 3.2 it is seen that the erasers showing positive at the Beilstein test mainly consist of PVC with a phthalate plasticizer. It is also seen that the bags and pencil cases showing positive at the Beilstein test mainly consist of polyester textile (PET). A comparison of the analysis results for the Beilstein, FT-IR and XRF analyses is shown in Appendix D.
3.3 Phthalates in PVC
3.3.1 Analysis method
In co-operation with the Danish Environmental Protection Agency a number of erasers have be selected for quantitative analysis for phthalates. 50 mg of the sample is weighed in fragments in 20 ml screw-top glasses. The samples are extracted with CH2Cl2 at room temperature during the night. Dissolved PVC, if any, is precipitated by addition of methanol.
The sample is centrifuged and the extract is analyzed by gas chromatography with mass spectrometric detector (GC-MS). As internal standard butyl-hydroxyl-toluene (BHT) is used.
For the GC-MS analyses Varian Saturn 2000 ion trap system is used.
The detection limit is substantial below the identified levels. The degree of uncertainty of the quantification is approx. 10% relative.
3.3.2 Result of the phthalate analysis
The result of the phthalate analysis is shown in Table 3.3 below.
Table 3.3 The result of the phthalate analysis
| Sample | DEHP w/w% |
DINP w/w% |
| 3 | 0 | 37 |
| 5 | 0 | 54 |
| 9 | 0 | 32 |
| 12 | 35 | Trace |
| 13 | 0 | 50 |
| 14 | 0 | 43 |
| 15 | 0 | 70 |
| 16* | 17 | Trace |
| 22 | 54 | 0 |
| 23 | 22 | 0 |
* Small amounts of dibutyl phthalate
In the analysis, mainly two types of phthalates are identified, DEHP (Bis-(2-ethylhexyl) phthalate) and DINP (Diisononyl phthalate). No attempt to quantify small content of other phthalates has been made. Phthalate is added as a plasticizer to PVC, normally in quantities of about 30% and in some cases up to 50%. It ought to be noted that in sample 15 a phthalate content of 70% is found which is above the normal content.
Subsequently, an analysis of DEHP for sample 22 to an external standard is conducted. Here the result was a content of 44 w/w% DEHP. As it is more precise to apply external standards it is a content of 44 w/w% DEHP for sample 22 which is applied in the health calculations.
3.4 XRF analyses
3.4.1 Analysis method XRF analysis
For the X-ray analyses (energy dispersive X-ray fluorescence) a X-LAB 2000 instrument (Spectro) is used. For quantification of the content the programme TURBO-QUANT is used. Through this technique all elements larger or equal to no. 11, sodium, can be analyzed. The minimum level which can be determined depends on matrix and element but it is <10 ppm for certain elements.
No real sample preparation has been conducted. The sample is either placed directly in the instrument or a piece of approx. 5 cm x 5 cm has been cut. These test samples are analyzed directly in the instrument.
The analysis is a surface analysis, i.e. only elements within a maximum depth of approx. 100µm, dependent on the material, are analysed.
3.4.2 Result of XRF analysis
The procedure of the X-ray analyses is that an analysis of all the material types found on the individual products is conducted on all the purchased products.
The amount of single substances shown in Table 3.4 is the maximum permitted emission at extraction in stomach acid according to the Toys Statutory Order. There is no reason to examine the extraction level to stomach acid if in the X-ray analyses, there is found a lower total value of the content of the metals in the products than the threshold limits of the maximum emission of the substances at extraction in stomach acid.
Table 3.4 Maximum emission of single substances at extraction in stomach acid
| Z | Symbol | Element | Maximum amount [mg/Kg] |
| 24 | Cr | Chromium | 60 |
| 33 | As | Arsenic | 25 |
| 34 | Se | Selenium | 500 |
| 48 | Cd | Cadmium | 75 |
| 51 | Sb | Antimony | 60 |
| 56 | Ba | Barium | 1000 |
| 80 | Hg | Mercury | 60 |
| 82 | Pb | Lead | 90 |
The result of the XRF screening analysis of products is shown in Table 3.5 and in Appendix D. The result of the ICP-OES analysis to quantitative determination of selected metals at extraction in artificial sweat is shown in Table 3.7.
Table 3.5 Results of the XRF screening
| No. | Description | Remark | Content acc. to XRF |
| 1 | Erasers | ||
| 2 | Erasers | ||
| 3 | Eraser | Smells pleasantly | Eraser: Cl (PVC) with Ca (chalk) |
| 4 | Eraser | Smells pleasantly | Ca (Chalk) |
| 5 | Eraser | ||
| 6 | Eraser | Danger labelled for children under 3 years | |
| 7 | Eraser | ||
| 8 | Eraser | Conform to ASTM 4266 and EN-71 | |
| 9 | Eraser | Eraser: Cl (PVC) with Ca (chalk), Silicium | |
| 10 | Eraser | Chalk | |
| 11 | Eraser | Danger labelled for children under 3 years | |
| 12 | Eraser | Danger labelled for children under 3 years | Cl (PVC )with a little Ca (chalk), Cu (pigment) |
| 13 | Eraser | Danger labelled for children under 3 years | |
| 14 | Eraser | Danger labelled for children under 3 years | Cl (PVC) with Ca (chalk), Titan (perhaps titan dioxide (pigment)) |
| 15 | Eraser | Danger labelled for children under 3 years | |
| 16 | Pencil case | Danger labelled for children under 3 years | |
| 17 | Eraser | ||
| 18 | Eraser | ||
| 19 | Eraser | ||
| 20 | Eraser | ||
| 21 | Eraser | ||
| 22 | Eraser | ||
| 23 | Eraser | ||
| 24 | Eraser | Danger labelled for children under the age of 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard | Rubber, Silicium |
| 25 | Eraser | Smells pleasantly | |
| 26 | Pencil case | ||
| 27 | Eraser | Ca (Chalk) | |
| 28 | Eraser | ||
| 29 | Toy bag | ||
| 30 | Toy bag | Danger labelled for children under 3 years | A: Cl (PVC) with Zn (heat stabilizer), ought to be investigated more closely due to Ba content |
| 31 | Pencil case | A: Ca (Chalk), ought to be investigated more closely due to Ce and Pb content (dye) | |
| B: Cl (PVC), ought to be investigated more closely due to Cd and Ba content (stabilizer). Zn content (stabilizer) | |||
| 32 | Toy bag | ||
| 33 | Toy bag | ||
| 34 | Pencil case | Danger labelled for children under 3 years | Cl (PVC), ought to be investigated more closely due to Ce and Ba content (stabilizer). Zn content (stabilizer) |
| 35 | Pencil case | High content of Ti, Ca (chalk), ought to be investigated more closely due to Sb and Ba content | |
| 36 | Pencil case | ||
| 37 | Toy bag | Danger labelled for children under the age of 3 years. This product conforms the safety requirements of ASTM F963 | B: Contains Cl, P and Ni, ought to be investigated more closely due to Sb content |
| 38 | Toy bag | This bag is not toy keep away from babies | A: Chalk, content of CI (could be fire-retardant), Zn. Ought to be investigated more closely due to Sb content (could be fire-retardant) |
| B: Content of Ti, S, Ni, ought to be investigated more closely due to Sb content | |||
| C: Content of S, CI, Ti and Zn (heat stabilizers). Ought to be investigated more closely due to Pb content | |||
| 39 | School bag | A: Contains Ti and Zn | |
| B: Content of S, CI and Zn (heat stabilizers). Ought to be investigated more closely due to Ba content | |||
| C: Cl (PVC), content of Zn. Ought to be investigated more closely due to Ba content | |||
| 40 | School bag | Not for children under 3 years | A: Ought to be investigated more closely due to Ba, Pb and Sb content |
| B: Cl (PVC). Ought to be investigated more closely due to Cd content | |||
| C: Contains Cl and Zn. Ought to be investigated more closely Cd, Sb, Ba and Pb content | |||
| 41 | School bag | ||
| 42 | School bag | Not for children under 3 years | A: Content of Cu, Zn, Br, Sr and Mo. Ought to be investigated more closely due to Sb, Ba and Pb content |
| B: Content of Br. Ought to be investigated more closely due to Sb content | |||
| C: Cl (PVC) with content of Zn and Ba. | |||
| D: Content of S, Ca and Sr | |||
| 43 | Pencil case |
The XRF analysis results confirm the results of the FT-IR analysis and Beilstein test. Most of the erasers consist of PVC with phthalate as plasticizer. As can be seen in Table 3.5 the XRF analysis shows the presence of large amounts of Ca in those products which at the FT-IR analysis showed the presence of chalk.
It shall be noted that the XRF analysis also shows a high content of Cr, As, Se, Cd, Sb, Ba, Hg and/or Pb in one or more products. The amount of single substances stated in Table 3.4 is the maximum permitted emission at extraction in stomach acid according to the Toys Statutory Order while amounts stated in Appendix D being the basis of Table 3.5 state the amount found in the product. The results stated in Table 3.5 and in Appendix D shall exclusively be regarded as an indication of the possibility that amounts exceeding the permitted amounts can be found in a migration analysis. The products where a high content of the above substances is found are thus selected for a more detailed analysis.
Results of heavy metal content
Measurement of the content of metals in the products is conducted through a quantitative element determination by means of X-ray analysis (see the results in Appendix E). These results are compared to the application limitation statutory orders for lead, cadmium and mercury as described in chapter 2 under Legislation.
As it is shown in Table 3.6, in total four products exceed these application limitations.
Table 3.6 Products exceeding the application limitations for Pb, Cd and Hg. (Sample nos. 31, 34, 40 and 42)
| Sample no. | Element | Concentration | Limit acc. to statutory orders |
| 31A | Cd | 389.3 µg/g | 75 ppm (=µg/g) |
| 31A | Pb | 474.3 µg/g | 100 ppm (=µg/g) |
| 31B | Cd | 358.7 µg/g | 75 ppm (=µg/g) |
| 34 | Cd | 256.3 µg/g | 75 ppm (=µg/g) |
| 40A | Pb | 740.4 µg/g | 100 ppm (=µg/g) |
| 40B | Cd | 393.7 µg/g | 75 ppm (=µg/g) |
| 40C | Cd | 375.2 µg/g | 75 ppm (=µg/g) |
| 40C | Pb | 2427 µg/g | 100 ppm (=µg/g) |
| 42A | Pb | 4682 µg/g | 100 ppm (=µg/g) |
3.5 ICP analysis
3.5.1 Analysis method ICP
Barium is analyzed on ICP-OES – inductively coupled plasma optical emission spectrometry – from Jobin Yvon JY 38 S and other metals are analyzed on ICP-MS – inductively coupled plasma mass spectrometry – from Varian according to DS/ISO 17294-2.
3.5.2 Result of the ICP analysis
Migration analyses for metals of selected products are conducted. The results are shown in Table 3.7 below as well as in Appendix F.
Table 3.7 Results of migration analyses for metals of selected products
| Cr | As | Se | Cd | Sb | Ba | Hg | Pb | |
| µg/l | µg/l | µg/l | µg/l | µg/l | mg/l | µg/l | µg/l | |
| 31 A | 7.4 | 1.7 | 49 | 1.4 | 12 | 1.1 | <0.1 | 0.64 |
| 31 B | 4.9 | 0.35 | 57 | 33 | <0.1 | 0.11 | <0.1 | 0.86 |
| 34 | 7.0 | 12 | 62 | 0.19 | 0.1 | 1.6 | <0.1 | 0.62 |
| 35 A | 6.8 | 2.9 | 63 | 1.8 | 1.5 | 1.3 | <0.1 | 0.31 |
| 37 B | 17 | 1.4 | 75 | 2.2 | 43 | 0.087 | <0.1 | 21 |
| 38 A | 9.1 | 0.52 | 63 | 0.030 | 0.82 | 0.75 | <0.1 | <0.1 |
| 38 B | 11 | 1.1 | 60 | 0.13 | 20 | 0.043 | <0.1 | 0.12 |
| 38 C | 19 | 1.0 | 58 | 0.50 | 5.3 | 0.026 | <0.1 | 1.3 |
| 39 B | 7.3 | 0.89 | 56 | 0.064 | 4.5 | 0.45 | <0.1 | 1.5 |
| 39 C | 10 | 2.8 | 56 | 0.18 | <0.1 | 0.075 | <0.1 | 0.48 |
| 40 A | 10 | 1.4 | 59 | 0.16 | 27 | 0.22 | <0.1 | 0.50 |
| 40 B | 6.4 | 0.60 | 63 | 13 | 48 | 0.047 | <0.1 | 0.63 |
| 40 C | 86 | 1.9 | 51 | 39 | <0.1 | 0.80 | <0.1 | 14 |
| 42 A | 41 | 17 | 50 | 1.7 | 5.8 | 0.16 | <0.1 | 88 |
| 42 B | 9.8 | 3.0 | 52 | 0.10 | <0.1 | 0.045 | <0.1 | 18 |
| 42 C | 7.2 | 1.1 | 58 | 1.4 | 3.0 | 0.14 | <0.1 | 6.5 |
| TLV* | 60 mg/kg | 25 mg/kg | 500 mg/kg | 75 mg/kg | 60 mg/kg | 1000 mg/kg | 60 mg/kg | 90 mg/kg |
*TLV = Threshold Limit Value
These values are to be compared with the threshold limits as stated in DS/EN 71-3 – see Table 3.4. For comparison, the threshold limits are repeated in last row in the above Table 3.7.
The measured migration values are all stated in µg/l except for barium which is stated in mg/l. The threshold limits in DS/EN 71-3 are all stated in mg/kg. The conversion factor is: 1 µg/l = 0.001 mg/kg. So all numbers must be devided with 1,000 (except those for barium) to be able to compare them with the threshold limits.
3.6 UV-VIS screening
3.6.1 Analysis method UV-VIS screening
If the products contain colouring agents which can migrate to artificial sweat this will be important information whether more detailed analyses are needed.
Products for UV-VIS screening are selected on basis of their strong colours. Products from all product categories are selected.
The UV-VIS spectra of the extracts are recorded in the range of 800 – 200 nm on a Perkin Elmer Lambda 2 spectro photometer. Substances having an absorbance of 0.01 at a given wavelength can be detected. Substances absorbing in the range 400 to 700 nm will indicate the presence of colouring agents while substances only absorbing in UV (200-400 nm) indicate other additives like BHT, MBT, phthalates and similar. As colouring agents have different intensity it is not possible to state a general detection limit but as indication it can be informed that strong coloured agents in a concentration of 5 mg/l can have absorbance about 0.2 to 0.5 measured in a 10 mm cuvette. For the analysis a partial sample from the migration test in artificial sweat has been applied.
3.6.2 Result of the UV-VIS screening
The sweat extracts are recorded using a 10 mm cuvette in the wavelength range of 200-800 nm.
Except for sample 24, substances with strong UV absorption are found in all samples. UV absorption can derive from plasticizers (phthalates), solvent residues (isochrones) on various additives.
Only three samples have an absorption in the visible range of the spectra indicating staining. One sample, 42A, has shown an immediately visible yellow colour in the extract. Two samples 4 and 38C have given a weak red tinting of the extract. However, the red tinting was so weak that it is not noted immediately. The result of the UV-VIS screening is stated in Table 3.8.
Sample 41A is not analyzed through UV-VIS but shows a clear pink colouring in the extract.
Table 3.8 The results of the UV-VIS screening:
| Description of spectra | ||||
| Sample no. | 200-300 nm | 300-400 nm | 400-800 nm | Colour |
| 3 | Medium, 0.6 210, shoulder 290 | nothing | nothing | |
| 4 | Very strong >1 220nm, medium 0.5 290 nm | medium 0.4 340 nm | very weak 550 | weak red tinted |
| 9 | Strong 0.95 200+230nm, shoulder 280 nm | nothing | nothing | |
| 10 | Medium 0.6 210 nm, strong =1 240 nm, shoulder 280 | nothing | nothing | |
| 14 | Strong 0.9 210, 0.9 250 nm, | nothing | nothing | |
| 24 | No absorption | nothing | nothing | |
| 30A | Medium 0.6 210 nm, 0.6 250 nm, sv 280 | nothing | nothing | |
| 30B | Weak 0.3 210 nm, 0.2 250 nm, shoulder 281 | nothing | nothing | |
| 31A | Very strong >1, 220-240 nm, shoulder 280 | nothing | nothing | |
| 31B | Weak 0.15 210nm, 0.14 250 nm | nothing | nothing | |
| 34 | Medium 0.5 210 nm, 0.4 250 nm | nothing | nothing | |
| 35A | Strong 1 210 nm, 0.7 250 nm | decreasing (fluff) | (fluff) | |
| 37B | Very strong >1 200-250 nm, shoulder 290 nm, | decreasing | decreasing | |
| 38A | Very strong >1, 220-240 nm | very weak, decreasing | nothing | |
| 38B | Medium 0.7 210 nm, 0.5 250 nm, sv 280 | nothing | nothing | |
| 38C | Very strong >1 200-250 nm shoulder 300 | something | a little max. at 500 | weak red tinted |
| 39A | Strong 0.9 210, 0.6 250 nm, shoulder 290 | decreasing | nothing | |
| 39B | Very strong >1, 200-280 nm | decreasing | nothing | |
| 39C | Very strong >1 250 nm | nothing | nothing | |
| 40A | Very strong >1 200-240 nm shoulder 280 | decreasing | decreasing | |
| 40B | Strong 0.9 210 nm, 0.4 250 nm, shoulder 290 nm | nothing | nothing | |
| 40C | Very strong >1 210-240 nm shoulder 290 | nothing | nothing | |
| 42A | Very strong >1 200-240 nm shoulder 280 | decreasing | 450 nm + little at 600 nm | yellow |
| 42B | Very strong >1 200-280 nm, shoulder 290 nm, | decreasing | decreasing | |
| 42C | Strong 0.7 210, 0.9 250 nm | nothing | nothing | |
| 42D | Strong 0.8 210 nm, shoulder at 280 nm | nothing | nothing | |
3.7 GC-MS Analysis
3.7.1 Analysis method
It is observed that some of the products emit a chemical smell, especially when they are quite new. Therefore, a number of products have been selected for an analysis of which volatiles that can be emitted to the air when handling the products. The analysis is conducted semi-quantitatively by headspace technique combined with GC-MS.
Screening of volatiles, headspace technique
Approx. 1 g of the sample is cut into small pieces. The samples are placed in a closed sample bottle of 10 ml. The samples are placed at 40°C during the night and then left at room temperature for about three weeks. The samples are analyzed by GS-MS by use of the headspace technique. The samples are reheated at 40°C for ten minutes and are shaken at regular time intervals. Hereafter, 1000 µl of the air over the sample (headspace) is injected in the GC. For the GC-MS analyses a Varian Saturn 2000 ion-trap GC-MS system is used. At the headspace technique only substances with a certain steam pressure is observed. It must be noted that due to problems with the analysis equipment, the results from the headspace analyses are not exact but only indicative. It is assessed that the error rate is between 10 and 500%. Furthermore, the problems with the analysis equipment caused that the samples were at evaporation for three weeks and not for six. Therefore, the analysis values represent far more than typical daily values. It is difficult to asses the consequence of the extended period for the analysed values compared to a typical use situation. The evaporation will clearly be largest at the beginning and in time there will be a kind of equilibrium and the evaporation will abate. Furthermore, the temperature will have an influence. The evaporation is significantly larger at the beginning at the higher temperature than at room temperature. As quantization p-xylene is applied as external standard and it is assumed that the total-ion area for a peek proportional with the concentration with same factor as p-xylene. There are differences in the ionization efficiency and the degradation patterns of the different substances so the assumption can only give semi-quantitative values for other substances than xylene but for the same substance in two different samples a higher number will mean a correspondingly higher content.
Migration to artificial sweat
Artificial sweat is produced according to DS/EN 1811:2000.
2 g sample of varying area is placed in 25 ml artificial sweat and left at 40°C for 4 hours where after the water phase is decanted from the sample pieces. The water phase is examined through UV-VIS for staining and GC-MS with solid phase micro extraction (SPME) of substances migrated to the water phase. A Carboxen-DVB or a 30 m PDMS fibre is applied but a few samples are analyzed using both fibres. There is a certain difference in the concentrating efficiency for the two fibres. Furthermore, only calibration for substances in EN71-9 is used as well as four specific phthalates (DIBP, DBP, DEHP DnOP). Of the substances mentioned in EN71-9 it is in fact only isophorone which is found in the samples. Therefore, the values in the table of other substances than isochrones and phthalates are only comparable for differences between releases of the same substance for the different samples as the difference in the concentration efficiency is unknown.
As the migration period is 4 hours the analysis results are divided by 4 in the risk calculations in chapter 5 when the risk of a daily exposure of 1 hour is calculated.
Migration to artificial saliva
According to agreement with the Danish Environmental Protection Agency a migration analysis to artificial saliva is carried out on eraser 22. Artificial saliva contains in 1000 ml 4.5 g NaCl + 0.3 g KCl + 0.3 g Na2SO4 +0.4 g NH4Cl + 3.0 g C3H6O3 + 0.2 g Urea dissolved in demineralised water where after pH is adjusted to 5.0 with 2 N NaOH. The extraction is made of 1 g sample in 20 ml saliva at 37 degrees for 1 hour to imitate a child sucking the eraser for 1 hour daily. Thereafter the saliva is made alkaline, pH 10, and extracted 3 times with dichloromethane. The extract is dried with sodium sulphate and then evaporated to dryness. The residue is dissolved in 1 ml dichloromethane added tetradeutero-bis(2-ethylhexyl)-phthalate as internal standard.
3.7.2 Result of GC-MS screening
The result is shown in Table 3.9A and Table 3.9B
Table 3.9A The Head-space analysis
| Sample | rt | Substance | CAS | ug/sample in the glass |
| Pr 3 B | 4.704 | Toluene | 108-88-3 | 0.01 |
| 7.229 | p-xylene | 108-38-3 | 0.01 | |
| 6.707 | Butanoic acid, 2-methyl-, ethyl ester | 7452-79-1 | 0.01 | |
| 7.792 | 1,3,5,7-Cyclooctatetraene | 629-20-9 | 0.02 | |
| 8.84 | ||||
| 8.946 | Butanoic acid, 2,3-dimethyl-, ethyl ester | 54004-42-1 | 0.02 | |
| 9.993 | Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-me | 18172-67-3 | 0.01 | |
| Pr 3 B | 10.211 | Hexane, 2,2,5,5-tetramethyl- | 1071-81-4 | 0.31 |
| 10.475 | Hexanoic acid, ethyl ester | 123-66-0 | 0.04 | |
| 10.805 | Acetic acid, hexyl ester | 142-92-7 | 0.01 | |
| 11.134 | Heptane, 4-propyl- | 3178-29-8 | 0.03 | |
| 11.243 | D-Limonene | 5989-27-5 | 0.05 | |
| 11.305 | 1-Hexene, 3,5,5-trimethyl- | 4316-65-8 | 0.04 | |
| 11.63 | 1-Hexene, 3,5,5-trimethyl- | 4316-65-8 | 0.02 | |
| 11.697 | Hexane, 2,2,5,5-tetramethyl- | 1071-81-4 | 0.01 | |
| 12.428 | Hexanoic acid, 2-propenyl ester | 123-68-2 | 0.29 | |
| Pr 4 B | 2.43 | Propanoic acid, methyl ester | 554-12-1 | 0.05 |
| 4.527 | Propanoic acid, 2-methyl-, ethyl ester | 97-62-1 | 0.01 | |
| 6.89 | 3-Hexen-1-ol, (Z) - (leaf alcohol)? | 928-96-1 | 0.01 | |
| 8.868 | 1S-à-Pinene | 7785-26-4 | 0.01 | |
| 12.43 | Hexanoic acid, 2-propenyl ester | 123-68-2 | 0.04 | |
| 12.872 | 1,6-Octadien-3-ol, 3,7-dimethyl- | 78-70-6 | 0.10 | |
| 11.775 | Heptadecane, 2,6-dimethyl- | 54105-67-8 | 0.03 | |
| 14.257 | Heptadecane | 629-78-7 | 0.02 | |
| 14.399 | Heneicosane | 0.02 | ||
| 15.036 | Heptadecane | 629-78-7 | 0.03 | |
| 16.098 | Decane, 2,6,7-trimethyl- | 62108-25-2 | 0.02 | |
| 16.135 | 0.02 | |||
| 16.242 | Heneicosane, 11-(1-ethylpropyl)- | 0.02 | ||
| 16.354 | Eicosane | 112-95-8 | 0.03 | |
| 16.487 | Heptadecane, 2,6-dimethyl- | 54105-67-8 | 0.02 | |
| 16.555 | Heptadecane | 629-78-7 | 0.01 | |
| 17.086 | Hydroxylamine, O-decyl- | 29812-79-1 | 0.02 | |
| 18.448 | Triacontane, 11,20-didecyl- | 55256-09-2 | 0.02 | |
| 11-18 | White spirit | 0.26 | ||
| 20.955 | Butylated Hydroxytoluenee | 128-37-0 | 0.14 | |
| Pr 10 B | 1.813 | tert butanol (?) | 75-65-0 | 0.09 |
| 8.958 | 1-Hexene, 3,5,5-trimethyl- | 4316-65-8 | 0.01 | |
| 10-18 | White spirit, high aromat | 0.14 | ||
| 20.951 | Butylated Hydroxytoluene | 128-37-0 | 0.35 | |
| Pr 16 B | 4.699 | Toluene | 108-88-3 | 0.01 |
| Pr 24 B | 4.699 | Toluene | 108-88-3 | 0.02 |
| 9.557 | Benzene, 1-ethyl-2-methyl- | 611-14-3 | 0.03 | |
| 9.637 | Benzene, 1-ethyl-2-methyl- | 611-14-3 | 0.01 | |
| 9.766 | Benzene, 1,3,5-trimethyl- | 108-67-8 | 0.02 | |
| 10.388 | Benzene, 1,2,3-trimethyl- | 526-73-8 | 0.05 | |
| 12.014 | Heptadecane, 2,6-dimethyl- | 29812-79-1 | 0.01 | |
| 12.159 | Docosane | 629-97-0 | 0.01 | |
| 12.843 | Heptadecane, 2,6-dimethyl- | 29812-79-1 | 0.03 | |
| 14.052 | 1-Tetradecene | 1120-36-1 | 0.02 | |
| 14.146 | Heptadecane, 2,6-dimethyl- | 29812-79-1 | 0.02 | |
| 14.258 | Heptadecane | 629-78-7 | 0.04 | |
| 14.401 | Heptadecane | 629-78-7 | 0.03 | |
| Pr 24 B | 14.56 | 2(1H)-Naphthalenone, octahydro-4a,5-dime | 51557-64-3 | 0.05 |
| 15.035 | Heptadecane, 2,6-dimethyl- | 29812-79-1 | 0.05 | |
| 16.098 | Decane, 2,6,7-trimethyl- | 62108-25-2 | 0.03 | |
| 16.139 | 1-Hexadecene | 29812-79-1 | 0.04 | |
| 16.241 | Heneicosane | 29812-79-1 | 0.03 | |
| 16.353 | Heptadecane | 629-78-7 | 0.05 | |
| 16.489 | Heptadecane, 2,6-dimethyl- | 54105-67-8 | 0.04 | |
| 17.087 | 29812-79-1 | 0.04 | ||
| 18.043 | 0.04 | |||
| 18.102 | Docosane | 629-97-0 | 0.03 | |
| 18.203 | Docosane | 29812-79-1 | 0.02 | |
| 18.32 | Eicosane | 112-95-8 | 0.03 | |
| 18.45 | Triacontane, 11,20-didecyl- | 55256-09-2 | 0.03 | |
| 19.007 | Heneicosane | 29812-79-1 | 0.02 | |
| 9-18min | White spirit, high aromat, total | 0.78 | ||
| 20.956 | Butylated Hydroxytoluene | 128-37-0 | 0.02 | |
| Pr 34 B | 7.953 | Cyclohexanone | 108-94-1 | 0.01 |
| 13.457 | Isophorone | 78-59-1 | 0.02 | |
| Pr 42 B | 7.954 | Cyclohexanone | 108-94-1 | 0.01 |
| 13.45 | Isophorone | 78-59-1 | 0.21 | |
| 2.904 | Presumably aldehyde | None | 0.02 |
Table 3.9B GC-MS of the sweat extract analysis – [µg/g]
| Identified name of substance | CAS | 4 | 10 | 24 | 30B | 31A | 31B | 34 | 35A | 35B | 35C | 37A,1 | 37B | 38A | 38B |
| 1,2,3,5- Tetra- methyl- benzene |
527-53-7 | ||||||||||||||
| 1,2,4,5- Tetra- methyl- benzene |
95-93-2 | ||||||||||||||
| 1,2-Diphenyl- ethanedione |
134-81-6 | ||||||||||||||
| 1,4-Methano- azulene, decahydro-4,8,8-trim |
475-20-7 | 100 | |||||||||||||
| 2,6-Di-tert- butyl-p- benzo- quinone |
719-22-2 | 4 | 3 | 2 | 1 | 2 | 1 | 2 | |||||||
| 2-Ethyl-1-hexanol | 104-76-7 | 2 | 10 | 20 | 40 | 20 | 60 | 1 | 8 | 2 | |||||
| 2-Ethyl- hexanal |
123-05-7 | 1 | |||||||||||||
| 2-heptanone | 110-43-0 | ||||||||||||||
| 2-Nonanone | 821-55-6 | ||||||||||||||
| 2-Octanone | 111-13-7 | ||||||||||||||
| 3,5,5-trimethyl-3-Cyclo- hexen-1 -one |
471-01-2 | 1 | |||||||||||||
| alpha- methyl- styrene |
98-83-9 | ||||||||||||||
| Benzoic acid, 2-methyl- propyl ester |
120-50-3 | ||||||||||||||
| BHT | 128-37-0 | 25 | 70 | 3 | 1 | 10 | 1 | ||||||||
| Bicyclo [2.2.1] heptan-2- one, 1,3,3-trimet |
1195-79-5 | 1 | |||||||||||||
| Cedrol | 77-53-2 | trace | 100 | 20 | 100 | trace | |||||||||
| Cyclo- hexanol |
108-93-0 | ||||||||||||||
| Cyclo- hexanone |
108-94-1 | 5 | 2 | 10 | 5 | 3 | |||||||||
| DEHP | 117-81-7 | 4 | 6,0 | 2,4 | |||||||||||
| Diisobutyl Phthalate | 84-69-5 | 1,5 | 2 | 0,7 | 0,1 | 1,3 | 0,3 | 2 | |||||||
| Dibutyl Phthalate | 84-74-2 | 2 | trace | ||||||||||||
| Isophorone | 78-59-1 | 1 | 2 | 5 | 3 | 15 | 10 | 40 | 40 | trace | 3 | 110 | 5 | ||
| Linalool | 78-70-6 | 35 | |||||||||||||
| Nonylphenol example CAS | 25154-52-3 | 2 | |||||||||||||
| Phenol | 108-95-2 | 1 | |||||||||||||
| Phathales not identified | 2 | ||||||||||||||
| Tinuvin - Drometrizole | 2440-22-4 | 20 |
| Identified name of substance | CAS | 38C | 39B | 39C | 40A | 40B | 40C | 41A | 42A | 42B | 42C | 43 | Number | Max |
| 1,2,3,5-Tetra- methyl- benzene |
527-53-7 | 1 | 1 | 2 | 1 | |||||||||
| 1,2,4,5-Tetra- methyl- benzene |
95-93-2 | 1 | 1 | 2 | 1 | |||||||||
| 1,2-Diphenyl- ethan- edione |
134-81-6 | 10 | trace | trace | 3 | 10 | ||||||||
| 1,4- Methan- oazulene, decahydro-4,8,8-trim |
475-20-7 | 1 | 100 | |||||||||||
| 2,6-Di- tert- butyl-p- benzo- quinone |
719-22-2 | 5 | trace | 2 | 7 | 5 | ||||||||
| 2-Ethyl-1-hexanol | 104-76-7 | 1 | 5 | 8 | 5 | 30 | 5 | 5 | 1 | 4 | 25 | 11 | 60 | |
| 2-Ethyl- hexanal |
123-05-7 | 1 | 2 | 3 | 2 | |||||||||
| 2-hepta- none |
110-43-0 | 20 | 1 | 20 | ||||||||||
| 2-Nona- none |
821-55-6 | 10 | 3 | 2 | 10 | |||||||||
| 2-Octa- none |
111-13-7 | 3 | 1 | 3 | ||||||||||
| 3,5,5-trimethyl- 3-Cyclo- hexen- 1-one |
471-01-2 | 3 | 2 | 3 | ||||||||||
| alpha- methyl- styrene |
98-83-9 | 2 | 1 | 2 | ||||||||||
| Benzoic acid, 2-methyl- propyl ester |
120-50-3 | 1 | 1 | 1 | ||||||||||
| BHT | 128-37-0 | 1 | 7 | 70 | ||||||||||
| Bicyclo [2.2.1] heptan-2 -one, 1,3,3- trimet |
1195-79-5 | 1 | 1 | |||||||||||
| Cedrol | 77-53-2 | 1 | 4 | 100 | ||||||||||
| Cyclo- hexanol |
108-93-0 | 0 | 0 | |||||||||||
| Cyclo- hexanone |
108-94-1 | 1 | 4 | 2 | 1 | 2 | 1 | 7 | 10 | |||||
| DEHP | 117-81-7 | 1 | trace | 1 | 5 | 6 | ||||||||
| Diisobutyl Phthalate | 84-69-5 | 15 | 0,1 | 1,5 | 88 | 0,5 | 0,4 | trace | 0,1 | trace | 0,1 | 11 | 88 | |
| Dibutyl Phthalate | 84-74-2 | 0,1 | 3 | 1,5 | ||||||||||
| Isophorone | 78-59-1 | 150 | 250 | 70 | 1 | 1 | 10 | 20 | 1 | 95 | 1 | 12 | 250 | |
| Linalool | 78-70-6 | 1 | 35 | |||||||||||
| Nonyl- phenol examplel CAS |
25154-52-3 | 1 | 2 | |||||||||||
| Phenol | 108-95-2 | 1 | 2 | 1 | 3 | trace | 4 | 3 | ||||||
| Phthalate, not identified | 1 | 2 | ||||||||||||
| Tinuvin - Drome- trizole |
2440-22-4 | 1 | 20 |
Number: Number of products in which the substance is found. If the substance is found in two different examined parts of the product (for instance A and B) it is only counted as one product.
Max: Maximum concentration in which the substance is found.
Migration to artificial saliva
The result of the migration analysis of eraser 22 is that 0.1% (w/w) DEHP or 1 mg/g eraser is emitted to artificial saliva, i.e. that the concentration in the saliva was 0.05 mg/ml. Uncertainty of measurement is 50%, i.e. the real value is between 0.05 and 0.2%.
Screening of volatiles, headspace technique
By screening of volatiles through headspace technique the following 23 substances of particular interest are identified. The selection of substances for health assessment is based on the classification of the substances and description of effects which may be potentially problematic for the consumer if the migration of the substances from the products is too high.
- BHT
- Isophorone
- Toluene
- t-Butyl alcohol
- Methyl propionate
- Cyclohexanone
- p-Xylene
- D-limonene
- (1S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane
- Linalool
- White spirit – high aromat (several CAS numbers)
- 3-Hexen-1-ol, (Z)-
- Hexanoic acid, ethyl ester
- Hexanoic acid, 2-propenyl ester
- Methylisobutyrate
- ethyl 2-methylbutyrate
- 1,3,5,7-Cyclooctatetraene
- Butanoic acid, 2,3-dimethyl-, e
- 2,2,5,5-Tetramethylhexane
- n-Hexyl Acetate
- 4-Propylheptane
- 3,3,5-Trimethyl-1-hexene
Migration to artificial sweat
The following 25 substances of particular interest are identified through migration in artificial sweat and GC-MS analysis. The selection of substances for health assessment is based on the classification of the substances and description of effects which may be potentially problematic for the consumer if the migration of the substances from the products is too high.
- DEHP
- DIBP
- Isophorone
- BHT
- Nonylphenol (typical in CAS – exact isomer identification not possible)
- 2-heptanone
- Cyclohexanone
- Phenol
- DBP
- aa-Methylstyrene
- Linalool
- 1,4-Methanoazulene, decahydro-4,8,8-trim
- 1,2,4,5-Tetramethylbenzene
- 1,2,3,5-Tetramethylbenzene
- Cedrol
- 2-Ethyl-1-hexanol
- 2,6-Di-tert-butyl-p-benzoquinone
- 2-Ethylhexanal
- 2-Nonanone
- 3,5,5-trimethyl-3-Cyclohexen-1-on
- Tinuvin - Drometrizole
- 1,2-Diphenylethanedione
- 2-Octanone
- Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimet
- Benzoic acid, 2-methylpropyl ester
3.8 PFOS analysis
Perfluoroctanesulfonate (PFOS) and a number of associated perfluorinated compounds are applied in many industrial products and consumer products due to their special chemical properties, for instance the ability of repelling water and oil.
An increasing concern over these potentially harmful compounds has arisen as they are now found as globally widespread pollutions in air, water, soil as well as flora and fauna which indicate that these perfluorinated substances are environmentally persistent and accumulate in animals and humans.
PFOS and associated substances are easily absorbed in the body where they can be connected to proteins and especially accumulate in blood and liver but with regard to some compounds also in testicles and brain tissue. The half-life period in the body seems to be several years. The acute toxicity of PFOS and PFOA is moderate and the first-mentioned is most harmful to health. The toxicity of the associated substances increases with the chain length.
The liver is the primary target organ of perfluorinated compounds and they generate peroxisome proliferation in rat liver as well as induction of different enzymes involved in the metabolism. PFOS seems to be more active than PFOA regarding this effect but again PFDA with a longer alkyl chain is even more active. PFOA and PFOS also have an influence on the blood level of various hormones, for instance by decreasing the testosterone concentration and increasing the concentration of estradiol in rats. Therefore, the substances must be regarded as hormone-disrupting (endocrine disruptor).
PFOS may be applied as impregnating agent in certain products, especially bags might contain PFOS. Therefore, a number of bags are selected for analysis for PFOS.
3.8.1 Analysis method PFOS analyses
The samples (2 g textile cut into small pieces) are extracted by use of methanol, diluted with water followed by centrifugation. The extracts are analyzed using LC-MS-MS with electrospray.
3.8.2 Result of the PFOS analysis
Table 3.10 Result of the PFOS analysis
| Concentrations: ng/g textile | ||||||||||
| Substance | 35A | 38A | 39A | 39B | 40A | 41A | 41B | 42A | 42B | 42C |
| PFOS | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 |
| PFOSA | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 |
| PFBS | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 |
| PFHXS | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| PFOA | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| PFNA | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 |
| PFDA | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 |
| PFUnA | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 |
Ten selected products were analyzed for a possible content of perfluorinated compounds. The analyses showed that all measurements were below the detection limit.
3.9 Summary of the analysis results
A wide selection of the school bags, toy bags, pencil cases and erasers being on the market today has been analyzed. The analyses have mainly shown that:
- 10 out of 26 erasers consist of PVC with phthalate as plasticizer.
- At the GC-MS analysis of selected erasers mainly two types of phthalates, DEHP and DINP, are identified.
- Most of the bags and pencil cases consist of polyester textile (PET).
- To a large extent chalk is applied as filler material.
- The XRF analysis shows a high total content of Cr, As, Se, Cd, Sb, Ba, Hg and/or Pb in one or more products. These products are selected for a more detailed analysis (extraction followed by ICP).
- ICP analysis for quantitative content of Cr, As, Se, Cd, Sb, Ba, Hg and Pb in artificial sweat extracts showed very low content of the selected metals which consequently are not released in a large quantity.
- Four products exceed the application limitations of cadmium and/or lead.
- Of organic compounds found after extraction in artificial sweat or by the headspace analysis the following substances are emphasized as being of interest for a health assessment:
- Isophorone
- BHT
- Cyclohexanone
- Phenol
- Toluene
- DIBP
- DEHP
- 2-heptanone
- tert-Butyl alcohol
- Methyl propionate
- p-Xylene
The 10 products selected for analysis of perfluorinated compounds showed that all measurements were below the detection limit.
In total four products exceed the application limitations for lead, cadmium and mercury as described in chapter 2 under Legislation (no violation for mercury).
In the selection for a more detailed health assessment/risk assessment emphasis has been on selection of the substances having harmful properties.
4 Screening for possible harmful effects
Based on the identified compounds in the different analyses in phase 2 of the project a screening for possible harmful substances has been conducted. The screening is based on classifications in the list of dangerous substances (LODS) supplemented by the advisory list for self classification of the Danish Environmental Protection Agency (both available via www.mst.dk).
The identified compounds are summarized in the table below and a more detailed description of selected substances is made in the texts below where their harmful effects also are clarified.
Ten selected products are analyzed for a possible content of perfluorated compounds. The analyses showed that all measurements were below the detection limit and therefore these substances are not included as a part of the screening.
Table 4.1: Summary of the screening for harmful effects of the found constituents
LODS The list of dangerous substances (Danish EPA) (Stat. Ord. 923, 2005).
LOUS The list of undesirable substances (Danish EPA, 2004).
1 The advisory list for self classification of dangerous substances of the Danish EPA (Danish EPA, 2001).
2 In the EU list of substances with documentation of hormone disturbing effects (EC DG Env., 2000).
3 Covered by Statutory Order on ban on phthalates in toys and childcare articles (in products for children at the age of
0-14 years) (Stat. Ord. 786, 2006).
DEHP – 2-ethylhexyl phthalate
The phthalate DEHP is classified as Reproduction toxic category 2 (Rep2[1]) with R60-61 “May impair fertility” and “May cause harm to the unborn child”. DEHP is in the list of undesirable substances of the Danish EPA because this phthalate is in the EU list of substances with documentation of hormone disrupting effects.
DEHP is covered by the Statutory Order on ban on phthalates in toys for children at the age of 0-3 years as well as in some childcare articles (Stat. Ord. 151, 1999). However, this statutory order is replaced by the Statutory Order on ban on phthalates in toys and childcare articles which became effective in the spring of 2007 (Stat. Ord. 786, 2006; Stat. Ord. 1074, 2006). Thus DEHP is banned in concentrations above 0.1% in toys and childcare articles for children at the age of 0-14 years. At the time of writing (2006) DEHP is banned in concentrations above 0.05% in toys and childcare articles for children at the age of 0-3 years (Stat. Ord. 151, 1999). The products selected in this project are bought before the new phthalate regulations and were allowed to contain phthalates as all the products are for children above 3 years.
DEHP is found in four of the examined products in concentrations between 17 and 44% and at the same time it is found at migration to artificial sweat in five products in a maximum concentration of 6 µg/g. Finally, a single eraser is examined through migration to artificial saliva where the emission is measured to 0.1% (i.e. 1 mg/g).
DINP (diisononyl phthalate)
DINP is not classified as a dangerous substance and is not found in the list of undesirable substances of the Danish EPA. However, DINP is covered by the Statutory Order on ban on phthalates in toys and childcare articles which became effective in the spring of 2007 (Stat. Ord. 786, 2006; Stat. Ord. 1074, 2006). Thus DINP is banned in concentrations above 0.1% in toys and childcare articles for children at the age of 0-14 years which children may put into the mouth.
DINP is found in eight of the examined products in concentrations between 3 and 70%.
DBP – dibutyl phthalate
The phthalate DBP is classified as Reproduction toxic category 2 with R61 “May cause harm to the unborn child” and Reproduction toxic category 3 with R62 “Possible risk of impaired fertility”. Furthermore, DBP is classified as dangerous for environment with R50 “Very toxic for aquatic organisms”. The Danish EPA has classified DBP as dangerous for environment with R51/53 “Toxic for aquatic organisms; may cause long-term adverse effects in the aquatic environment” in their self classification list. DBP is in the list of undesirable substances of the Danish EPA because this phthalate is in the EU list of substances with documentation of hormone disrupting effects.
DBP is covered by the Statutory Order on ban on phthalates in toys for children at the age of 0-3 years as well as in certain childcare articles (Stat. Ord. 151, 1999). However, this statutory order is replaced by the Statutory Order on ban on phthalates in toys and childcare articles which became effective in the spring of 2007 (Stat. Ord. 786, 2006; Stat. Ord. 1074, 2006). Thus DBP is banned in concentrations above 0.1% in toys and childcare articles for children at the age of 0-14 years. At the time of writing (2006) DBP is banned in concentrations above 0.05% in toys and childcare articles for children at the age of 0-3 years (Stat. Ord. 151, 1999).
DBP is only found in the sweat extract in a single of the examined products in low concentrations.
DIBP – Di-isobutyl phthalate
DIBP is not classified according the List of dangerous substances but the Danish EPA classifies the substance as dangerous for environment with R50/53: “Very toxic for aquatic organisms; may cause long-term adverse effects in the aquatic environment” according to their advisory list for self classification (Danish EPA, 2001).
Furthermore, the Danish EPA informs that the classification of DIBP is about to be changed to Rep2 on development and Rep3 on fertility with the risk phrases R61 “May cause harm to the unborn child” and R62 “Possible risk of impaired fertility”.
DIBP is found at migration to artificial sweat in 11 products in a maximum concentration of 88 µg/g.
Zinc oxide
Zinc oxide is classified as dangerous for environment with R50/53:”Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”. Zinc oxide has a LD50 value for rats of more than 5,000 mg/kg (bw); this means that zinc oxide shall not be classified as harmful. According to IUCLID a zinc oxide is not irritating to skin and is not sensitizing (IUCLID, 2000a). No information is found indicating that zinc oxide represents a health problem.
Copper
In its pure form copper is not classified according to the list of dangerous substances. Compounds of copper are classified differently depending on the compound but most copper compounds are classified as harmful if swallowed as copper may give liver damage (Danish EPA, 2003). The critical effect for copper is only relevant at considerable oral intake (Danish EPA, 2001).
Copper and copper compounds are in the list of undesirable substances of the Danish EPA.
Barium
In its pure form barium is not classified according to the list of dangerous substances. However, barium salts are classified as harmful and dangerous if inhaled and if swallowed.
Cadmium
As a pure substance (unstabilized) Cadmium is classified as carcinogenic category 2 (Carc2[2]) with R45 “May cause cancer”, as highly flammable with R17 “Spontaneously flammable in air”, as very toxic with R26 “Very toxic by inhalation”, as toxic with R48/23/25 “Toxic; danger of serious damage to health by prolonged exposure through inhalation and if swallowed”, as reprotoxic (Rep3) with R62 “Possible risk of impaired fertility” and R63 “Possible risk of harm to the unborn child”, as mutagenic (Mut3) with R68 “Possible risks of irreversible effects” and finally as dangerous for environment with R50/53 “Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
Cadmium is a toxic substance but one of the main problems is that the substance accumulates in the body and especially in the kidneys. This accumulation already starts at birth. Therefore, exposure to cadmium will contribute to the accumulation of cadmium in the body (Danish EPA, 2003).
Cadmium is in the list of undesirable substances of the Danish EPA.
Statutory Order on ban on sales, import and production of products with cadmium content (Stat. Ord. 1199 of 23.12.1992) sets application limitations on products containing cadmium. According to the Statutory Order it is banned to import and sell products containing more than 75 ppm (mg/kg) cadmium in the homogeneous single parts of the product.
Lead
Lead compounds are generally classified as harmful (harmful by inhalation and if swallowed), as reprotoxic and as dangerous for environment. A few special lead compounds are classified as toxic/very toxic, reprotoxic and as potentially carcinogenic.
Lead and lead compounds are in the list of undesirable substances of the Danish EPA.
Statutory Order on ban on import and sale of products containing lead (Stat. Ord. 1012 of 13.11.2000) sets application limitations on products containing lead. According to the Statutory Order it is banned to import and sell products containing more than 100 ppm (mg/kg) lead in the homogeneous single parts of the product.
Zinc
As a pure substance (zinc dust) zinc is classified as extremely flammable with the risk phrases R15 “Contact with water liberates extremely flammable gases” and R17 “Spontaneously flammable in air”. Furthermore, zinc is classified as dangerous for environment with the risk phrase R50/53 “Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
Zinc is used in skin drugs and metabolism disorders due to zinc are only relevant at significant and regular oral intake (Danish EPA, 2000).
Antimony
According to the list of dangerous substances antimony compounds are classified as harmful with R20/22 “Harmful by inhalation and if swallowed” and as dangerous for environment with R51/53 “Toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
Antimony and its compounds are known for causing dermatitis (Danish EPA, 2003).
Nickel
In its pure form nickel is classified as carcinogenic category 3 (Carc3[3]) with R40 “Possible risk of cancer” and is allergenic R43 “May cause sensitization by skin contact”. Certain nickel compounds are in the list of undesirable substances of the Danish EPA.
Most nickel compounds are classified as allergenic with R43 and as dangerous for environment with R50/53. Furthermore, a number of nickel compounds are either recognized for or suspected of being carcinogenic (Danish EPA, 2003).
Isophorone
According to the List of dangerous substances isophorone is classified as harmful with R21/22 “Harmful in contact with skin and if swallowed” as well as irritant with R36/37 “Irritating to eyes and respiratory system”. Furthermore, isophorone is classified as carcinogenic category 3 (Carc3[4]) with R40 ”Possible risk of cancer”.
Isophorone is found partly through migration to artificial sweat in 12 products (maximum concentration of 250 µg/g) and partly through headspace analyses in 2 products (maximum contrition of 0.21 µg/g).
BHT
According to the List of dangerous substances BHT is not classified but the Danish EPA gives the substance the following recommended classification: Harmful with R22 “Harmful if swallowed” and dangerous to environment with R50/53 “Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
Furthermore, BHT is in the List of undesirable substances on basis of the self classification from the Danish EPA.
BHT is found partly through migration to artificial sweat in 7 products (maximum concentration of 70 µg/g) and partly through headspace analyses in 3 products (maximum concentration of 0.35 µg/g).
2-heptanone
According to the List of dangerous substances 2-heptanone is classified as R10 “Flammable” and as harmful with R20/22 “Harmful by inhalation and if swallowed”.
2-heptanone is found through migration to artificial sweat in a single product in a maximum concentration of 20 µg/g.
Cyclohexanone
According to the List of dangerous substances cyclohexanone is classified as harmful with R20 “Harmful by inhalation”. According to IUCLID cyclohexanone is not irritating to skin and is not sensitizing (IUCLID, 2000c).
Cyclohexanone is found partly through migration to artificial sweat in 7 products (maximum concentration of 10 µg/g) and partly through headspace analyses in two products (maximum concentration of 0.01 µg/g).
Phenol
According to the List of dangerous substances phenol is classified as being toxic with R23/24/25 “Toxic by inhalation, in contact with skin and if swallowed”, corrosive with R34 “Causes burns” and harmful with R48/20/21/22 “Harmful: danger of serious damage to health by prolonged exposure through inhalation, in contact with skin and if swallowed”. Additionally, phenol is classified as mutagenic category 3 (Mut3) with R68 “Possible risks of irreversible effects”.
In 2000, phenol was in the list of undesirable substances of the Danish EPA but has been removed from the list in 2004 as the substance does not fulfil the new criteria for undesirable properties (based on the classification) (Danish EPA, 2004).
Phenol is found through migration to artificial sweat in 4 products in a maximum concentration of 3 µg/g.
a-Methyl styrene
According to the List of dangerous substances, a-methyl styrene is classified as R10 “Flammable”, irritant with R36/37 “Irritating to eyes and respiratory system”, and dangerous for environment with R51/53 “Toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
a-Methyl styrene is found through migration to artificial sweat in a single product in a maximum concentration of 2 µg/g.
Linalool
According to the List of dangerous substances, Linalool is not classified and is not found in the self classification list of the Danish EPA. However, Linalool is in the List of undesirable substances as it is one of the 26 fragrances which the Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP) assesses to be allergenic through skin contact. Therefore, the fragrance has to be declared separately on cosmetics products.
Linalool is found partly through migration to artificial sweat in a single product (maximum concentration of 35 µg/g) and partly through headspace analyses in a single product (maximum concentration of 0.1 µg/g).
1,2,4,5-Tetramethyl benzene
According to the list of dangerous substances 1,2,4,5-tetramethyl benzene is not classified but the Danish EPA classifies the substance as dangerous for environment with R51/53 “Toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment” according to their advisory list for self classification (Danish EPA, 2001).
The substance is found through migration to artificial sweat in two products in a maximum concentration of 1 µg/g.
1,2,3,5-Tetramethyl benzene
According to the list of dangerous substances 1,2,3,5-tetramethyl benzene is not classified but the Danish EPA classifies the substance as dangerous for environment with R51/53 “Toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment” according to their advisory list for self classification (Danish EPA, 2001).
The substance is found through migration to artificial sweat in two products in a maximum concentration of 1 µg/g.
Toluene
According to the List of dangerous substances toluene is classified as R11 “Highly flammable”, irritant with R38 “Irritating to skin” and harmful with R48/20 “Harmful: danger of serious damage to health by prolonged exposure through inhalation”, R65 “Harmful: may cause lung damage if swallowed” and R67 “Vapours may cause drowsiness and dizziness”. Additionally, toluene is classified as reprotoxic category 3 with R63 “Possible risk of harm to the unborn child”.
Toluene is found in 3 products through headspace analyses in a maximum concentration of 0.02 µg/g.
t-Butyl alcohol
According to the List of dangerous substances t-butyl alcohol is classified as being highly flammable (R11) and harmful with R20 “Harmful by inhalation”.
t-Butyl alcohol is found in a single product via headspace analyses in a concentration of 0.09 µg/g.
Methyl propionate
According to the List of dangerous substances methyl propionate is classified as being highly flammable (R11) and harmful with R20”Harmful by inhalation”.
Methyl propionate is found in a single product through headspace analyses in a concentration of 0.05 µg/g.
D-limonene
According to the List of dangerous substances D-limonene is classified as flammable (R10), irritant with R38 “Irritating to skin” and R43 “May cause sensitization by skin contract”. Furthermore, the substance is classified as dangerous for environment with R50/53 “Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
D-limonene is in the list of undesirable substances as it is one of the 26 allergenic fragrances which must be declared separately in cosmetics.
D-limonene is found in a single product through headspace analyses in a concentration of 0.05 µg/g.
(1S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane
According to the List of dangerous substances the substance is not classified but the Danish EPA gives the substance the following advisory classification: Dangerous for environment with R50/53 “Very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment”.
The substance is found in a single product through headspace in a concentration of 0.01 µg/g.
Other substances
Additionally, an aromatic turpentine compound is identified in a single product through headspace in a concentration of 0.78 µg/g and a kind of white spirit in a single product through headspace in a concentration of 0.26 µg/g. For both substances applies that certain variants of the substances are in the list of undesirable substances of the Danish EPA. However, it has been impossible to find a definitive identification. It should be noted that both compounds are found in a relatively high concentration through headspace (compared with the other measured concentrations).
The substance 2-ethyl-1-hexanol is found in many of the analyzed products – 11 in total – and in a maximum concentration of 60 µg/g. additionally, the substance cedrol is found in four products in a relatively high maximum concentration of 100 µg/g. These substances are not commented further as they have no classification according to the List of dangerous substances or via the self classification list of the Danish EPA.
In addition to this, a number of other compounds are identified – many of them only in one of the examined products. These are not commented further as they have no classification according to the List of dangerous substances or via the self classification list of the Danish EPA.
4.1 Selection of chemicals for health risk assessment
Via an X-ray analysis metals have been identified for a number of products. These have a higher total than the recommended threshold limits for migration to stomach acid according to the Toys Statutory Order. A migration analysis for stomach acid must be carried out to assess whether the recommended threshold limits may be exceeded in these cases. Based on the selected analysis programme these products are not analyzed further through migration analyses and therefore these are not selected for a more detailed health assessment.
With the exception of BHT and some of the phthalates, substances with an environmental hazard classification are only found in one product. For this reason no environmental assessment of the individual substances was made.
In the selection for a more detailed health assessment/risk assessment it has been emphasized to select some of the above substances having properties harmful to health. However, a risk assessment of substances can only be carried out where quantitative measurements through analyses are available. Therefore, focus is on substances analyzed through the analyses for either artificial sweat or headspace (emission to air).
The 11 substances below which in co-operation with the Danish EPA have been selected for a more detailed health assessment are selected with basis in their classification and because they are the most frequent substances being found via either the migration analysis or headspace.
- Isophorone
- BHT
- Cyclohexanone
- Phenol
- Toluene
- DIBP
- DEHP
- 2-heptanone
- tert-Butyl alcohol
- Methyl propionate
- p-Xylene
5 Health assessment
- 5.1 Assessment of the health risk when using school bags, toy bags, erasers and pencil cases
- 5.2 Assessment of single substances
- 5.3 Total assessment
For assessment of the health risk at daily use of school bags, toy bags, erasers and pencil cases a selection of the effect levels of the found substances are assessed in relation to the relevant exposure time and way of exposure.
The calculations are made with basis in EC’s Technical Guidance Document (TGD) (European Commission, 2003).
In the survey 11 specific substances are selected for an assessment in corporation with the Danish Environmental Protection Agency. The selection is based on an interaction between the classification of the substances, the found concentrations as well as the number of products in which they are found.
At first an assessment of the health properties of the selected substances is carried out. Then exposure calculations are made based on worst case considerations which are used to assess the health risk of the selected substances in the analyzed products.
5.1 Assessment of the health risk when using school bags, toy bags, erasers and pencil cases
Exposure from school bags, toy bags, erasers and pencil cases takes partly place by inhalation of volatiles which the products emit (measured through the “headspace” analyses) and partly through dermal absorption of substances through skin contact with the products (measured through emission to the artificial sweat analyses).
For erasers there is a further exposure possibility through oral intake as it may be expected that some children bite or suck the erasers and even swallow a bit of them. Some erasers in the survey smell good and have a shape which may encourage children to put them into the mouth. Erasers with a design as a lip stick, a burger, grapes or similar are a part in the survey.
No analyses with the purpose to quantify the total amounts of the constituents in erasers are made – with exception of a few phthalates.
As a general rule no migration analyses of the erasers are made in relation to artificial saliva but on the contrary migration analyses in relation to artificial sweat. These analysis results will be used in a risk assessment of oral intake of constituents in the erasers as the difference of the liquids artificial saliva and artificial sweat is not large (the main difference is the temperature, which of course have an impact on the amount that migrates to the two different solutions). However, this means that there may be certain reservations in the conclusions but it is assumed that there are larger uncertainties in the concentrations of the semi-quantitative analyses than assuming that the results from the artificial sweat analyses may be transferred directly as artificial saliva results. However, the phthalate analyses showed relatively high concentrations of certain phthalates (among others DEHP) and the risk assessment showed that the concentrations can be problematic. Based on this, a single migration analysis to artificial saliva for the eraser with the highest content of DEHP is made afterwards.
The basic calculations for the three types of exposures are stated in the following.
5.1.1 Exposure through inhalation
In theory, exposure by inhalation can last from the purchase of the product until it is no longer in use (discarded). The substances which the consumer is exposed to during a possible unpacking of the product and at the beginning of the use period can roughly be assumed to be the substances found via the “headspace” analyses (analyse of substances evaporating from the products).
However, it must be noted that due to problems with the analytical equipment the results from the “headspace” analyses are not exact but only indicative. It is assess med that the error rate is between 10 and 500%.
Exposure by inhalation is expressed as the concentration of the chemical substance in the air in the inhalation zone and the exposure is expressed as an average concentration during a reference period of for instance one day. For exposure by inhalation both a short-term scenario for the acute toxicity and a long-term scenario for the chronic toxicity are calculated according to TGD if the related NOAEL values are found.
For estimation of the exposure by inhalation the concentration in the air, the inhalation rate and the air volume must be known (the inhalation zone or the size of the room).
The inhalation rate for children at moderate activity is 1.2 m³/hour according to TGD.
At short-term exposure air volume is set to 2 m³ according to TGD to represent the air gap which is directly around the person. This value is most probably valid for an adult and therefore 1 m³ is used instead to represent a child’s inhalation zone.
At long-term exposure an air volume of 20 m³ is used as a standard (8 m³ room with 2.5 m height to the ceiling). It may be discussed which value to use. In theory the children are exposed both to the substances evaporating from the products at home (and here only from their own products) and at school but here from many more (similar?) products in a much larger room. This is not taken into consideration in the exposure calculation but as worst case a relatively small room of 20 m³ is used.
The concentration in closed rooms is assumed to be larger than in ventilated rooms. For the calculation of the concentration in the room it is assumed that the substance is evaporated immediately to the whole room and is homogeneously spread out. It is left out of consideration that the evaporation and thus the concentration of the substances become smaller over time.
As even small children may be assumed to be in contact with the products or be in the same room in which the products are used/placed, a long-term scenario with long-term exposure is chosen from a worst case consideration where a respiration rate of 8.3 m³/day for a child at the age of 3-5 years is used (according to TGD).
In TGD no standard weight for a child is stated. For the sake of a realistic “worst case”, children’s weight is used at the youngest age where they are expected to play with toy bags, i.e. 3 years. Children are generally somewhat older (close to the school age) before they use school bags, erasers and pencil cases but they can still be exposed by inhalation by being in the same room as the products. Children’s weight for a certain age can be found via official growth curves. Netdoktor.dk has a table of girls’ and boys’ weight which comes from an old Scandinavian survey. They point out that it is an old survey and that in general the children have become a little higher and heavier since then (Netdoktor, 2006. According to these tables 3-year-old boys weight 12.7 kg (low weight) and 3-year-old girls 12.0 kg (low weight). Therefore, 12 kg is used a child’s weight as worst case.
It is presumed that children can be exposed to the substances which evaporate from the products up to 6 hours during one day. In a school situation children will have school bag, pencil case and eraser close to the body during the whole school day.
For the weight of the products the total weight of the product is used even if some of the substances are only found in the handle or in the outside of a school bag.
According to the Technical Guidance Document on Risk Assessment of the EU the exposure is calculated by inhalation through the following formula (European Commission, 2003):
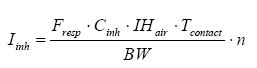
where
| Iinh | The amount of inhaled substance | µg/kg bw/day |
| Fresp | Inhalable fraction of the substance | 1, i.e. 100% |
| Cinh | The concentration in the air | µg/m³ |
| IHair | The inhalation rate of the person | m³/hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
Where the concentration in the air Cinh is calculated according to the following formula (European Commission, 2003):
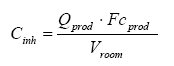
where
| Cinh | The concentration in the air | µg/m³ |
| Qprod | Amount of the product used in the room | g |
| Fcprod | Weight fraction of substance in the product | µg/g |
| Vroom | Volume of the room | m³ |
The equation used for the calculations is thus:
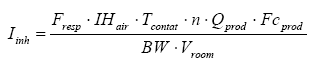
It must be noted that the values of analysis used for the risk calculations for exposure by inhalation are too high – maybe between a factor 100 to 500 too high. This is due to the fact that the samples were set for evaporation – at first during the night at 40 ºC where after the intention was that the samples were to be analyzed. But due to problems with the analysis equipment all the samples have hereafter been placed for about three weeks at room temperature before they were analyzed. This means that the results do not represent the evaporation during the 6 hours which is used as daily exposure but on the contrary the evaporation during three weeks.
Quite clearly, the evaporation will be largest at the beginning and at a certain time a kind of equilibrium will be reached for which reason the evaporation will be smaller. Besides the temperature plays a role. The evaporation is significantly higher at the beginning at the higher temperature than at room temperature. Thus, it is difficult to estimate the factor with which the analysis results shall be divided to illustrate the evaporation per day but if the analyzed values are used as they are the calculated MoS (Margin of Safety) will thus be significantly lower than the real value. When the calculated MoS are above 100 it is certain that the exposure will not present any health risk.
5.1.2 Exposure through the skin
In the scenario for skin exposure it is assumed that the products are used in the hand which will then have the primary exposure. For erasers and pencil cases this is clear while skin exposure for toy bags and school bags may also occur when the bag is on the back. However, it is assumed that the children wear clothes whereby the skin exposure is minimal. Therefore, only skin exposure through the hand is used.
Before skin absorption the chemical substance shall be transferred from the product to the skin. When it is on the skin the substance can be absorbed through the skin and from there to the blood and then spread in the rest of the body.
Migration analyses simulating sweat have been conducted. These analyses show how large amounts of the substances that can migrate (be transferred) when the product is touched by the hand. The substances being found in the extraction liquid are the substances which can potentially be absorbed through the skin at contact with the products.
The potential absorption (the exposure) can be expressed through the following equation (European Commission, 2003):
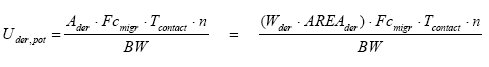
where
| Uder,pot | Potential absorption of the chemical substance | µg/kg bw/day |
| Ader | Total amount of substance which the skin potentially is exposed to | g |
| Wder | The weight of the product on the skin | g/cm² |
| AREAder | Area of the contact between the product and the skin | cm² |
| Fcmigr | Fraction of substance which migrates | µg/g per hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
Starting point is the amount of the substance which migrates per cm² of the product and this value is compared with the area of the product which touches the skin for a certain time.
It is assumed that it is only the area of a child’s palms (on both hands) which are in touch with the product. This value is not found in TGD but a list of men’s and women’s surface area of the hands in relation to the total surface area of the body is available (for women 731 cm² for both front and rear on both hands in relation to a total body area of 16,900 cm²). This ratio is compared with the information from TGD that the total body area of a child body is 6,030 cm² for a child at the age of 2-3 years, i.e. same age as in the definition of the body weight. In this way the result is that a 3-year-old child’s hands have a surface area of 131 cm², when the area is divided by 2 as it is assumed that the products are only touched with the inside of the hands.
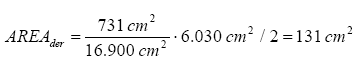
The surface of a child’s hands is only an approximation as it is assumed that the ratio of sizes between an adult woman’s hands and body is the same for a child.
As basis of the calculations is chosen that a child has its school bag/toy bag and its pencil case in the hands for maximum 1 hour a day in total. The migration analyses to artificial sweat are conducted in a way where a piece of the product is extracted in artificial sweat for 4 hours. The migration amounts are divided by 4 as the samples have stayed for 4 hours in artificial sweat and thus represent the migration amount per 4 hours.
For the analyses the products are cut into small pieces (cubes) of 2-3 mm crosswise. This means that the surface becomes significantly larger than the normal surface of the products. Furthermore, touch of the products will normally only take place on the outside of the product. The measured concentration can thus be overestimated by a factor 3 as a minimum.
For a school child the skin exposure for an eraser can be significantly longer as children often use it and also play with it. Therefore, for erasers, exposure time is calculated for 4 hours as worst case. The child’s weight is set to 12 kg in all cases.
After exposure of the skin the chemical substance shall pass the skin before it is a real absorption. The dermal absorption of the substances is generally estimated due to lacking data. If no other information is found a dermal absorption of 100% and a dermal absorption of 10% for substances with a molar weight larger than 500 g/mole which at the same time has a log KOW less than -1 or larger than 4 (as stated in TGD) are used as standard. It is more difficult for large molecules to penetrate the skin like very lipophilic substances.
The factor for the dermal absorption (1 or 0.1) is multiplied by the potential absorption (worst case).
5.1.3 Exposure through intake
Regarding the erasers there is a possibility of an exposure through the mouth, for instance if the children chew or suck the erasers. At oral exposure the absorption takes place after migration of the substances from the erasers and mixture in saliva. Absorption is assumed to take place via the mucous membranes in the oral cavity or the gastrointestinal canal.
As described earlier, as a main rule no migration analyses of the erasers in relation to artificial saliva are conducted but on the contrary migration analyses in relation to artificial sweat. Based on relatively high concentrations of certain phthalates (i.e. DEHF) a single migration analysis for artificial saliva of the eraser with the highest content of DEHP has subsequently been conducted. The results of the migration analyses for artificial sweat are used as a reasonable approximation for the rest of the products.
As basis for the oral intake the equation for migration of substances from a product to foods/beverages has been used. The foods/beverages are then ingested (European Commission, 2003). However, it is not exactly this situation which occurs when a child sucks/chews an eraser and for this reason the equation has been adjusted. The erasers are all so small that a child can suck, lick and chew the whole surface of the eraser. This is assumed as worst case that the measured migration from the whole eraser is ingested – no matter the size of the eraser.
The oral intake can thus be calculated from the formula below:
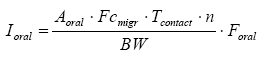
where
| Ioral | Amount of ingested substance | µg/kg bw/day |
| Aoral | Total amount of product which is licked or sucked | g |
| Fcmigr | Fraction of substances which migrates per time unit | µg/g/hour |
| Tcontact | The duration of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
| Foral | Fraction which is absorbed (bio available part) |
As basis of the calculation is chosen that a child licks, chews or sucks the eraser several times a day. The total exposure is assumed to be maximum 1 hour a day. As previously, the child’s weight is set to 12 kg (however, for a few calculations a weight of 20 kg is used to illustrate a school child’s weight).
The migration analyses for artificial sweat and artificial saliva are conducted in the way that a piece of the product is extracted in artificial sweat/saliva for 4 hours for which reason the result shall be divided by a factor 4 so that the measured values correspond to the exposure time. For the analyses the erasers are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface becomes substantially larger than the surface which an eraser normally has but if a child chews the eraser there is access to a larger surface of the eraser. The measured concentration may be overestimated by a factor 3 or more.
It must be noted that oral intake can also take place through hand-to-mouth, i.e. that hand or fingers having touched the product are put into the mouth afterwards. Thereby a transmission of a substance from fingers to mouth can take place. In literature it is stated that hand-to-mouth as an average lasts 3-10 minutes for which reason this part is assumed included in the chosen exposure time of 1 hour (Bremmer and van Veen, 2002; Green, 2002; Kiss, 2001).
After exposure of the mouth cavity the chemical substance shall pass the mucous membranes before a real absorption can be in question. The oral absorption of the substances is generally estimated due to lacking data. Therefore, an oral absorption of 100% is assumed as a standard.
For the phthalates (especially) where a total determination of the phthalate content is made the Danish Environmental Protection Agency has wanted a calculation of a scenario where it is assumed that the children will swallow a part of the eraser when they chew it. It is assumed that between 0.008 and 0.1 g of eraser is swallowed which corresponds to between approx. 0.01 and 0.08 cm³ for the relevant erasers – i.e. cubes of approx. 1.9 to 4.3 mm in height, width and length – an amount not quite unrealistic to swallow.
The oral intake can be calculated from the formula below:
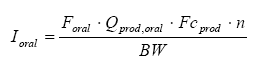
where
| Ioral | Amount of ingested substance | mg/kg bw/day |
| Foral | The oral absorption | |
| Qprod, oral | Amount of product which is ingested | g |
| Fcprod | Fraction of substance in the product | mg/g |
| N | The number of occurrences per day | per day |
| BW | Body weight | kg |
5.1.4 Margin of Safety
For an assessment of the risk for the individual chemical substances the so-called Margin of Safety (MoS) is calculated. Here the calculated daily exposure (Iinh or Uder or Ioral) is set in relation to the zero effect level (NOAEL – No Observed Adverse Effect Level) according to the following formula:
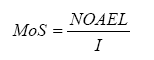
It is generally accepted that MoS must be at least 100 to declare a product as safe in use. In this way a safety factor of 10 for extrapolation of data from animals to humans is taken into account and a safety factor of 10 to take especially sensitive human individuals into account.
5.1.5 Total exposure
If the child is exposed to a substance from the same product through different ways of exposure the total absorption can be added.
Furthermore, other sources for the same chemical substances in the child’s surroundings may contribute to the total exposure.
5.2 Assessment of single substances
5.2.1 Isophorone
Application
Isophorone has a wide application as dissolvent for different artificial resins and polymers as well as for wax, fatty substances and oil. Isophorone is used in some printing inks, paints, varnishes and glues. Furthermore, it is used as chemical intermediate and in some pesticides (ATSDR, 1989; HSDB; IUCLID, 2000d; IPCS, 1995; Jensen AA, 1997a).
Isophorone occurs naturally in cranberries (Jensen AA, 1997a).
Identification
| Chemical name | 3,5,5-Trimethyl-2-cyclohexen-1-on |
| CAS-No. | 78-59-1 |
| EINECS No. | 201-126-0 |
| Gross formula | C9H14O |
| Molecular structure | 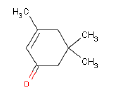 |
| Molecular weight | 138,21 g/mol |
| Synonyms | Isophorone Trimethylcyclohex-2-enon 3,5,5-Trimethyl-2-Cyclohexenon 1,1,3-trimethyl-3-cyclohexen-5-on |
Physical-chemical data
| Physical form | Colourless liquid with a peppermint like fragrance | Chemfinder, HSDB |
| Melting point | -8 °C | Chemfinder |
| Boiling point | 215.2 °C | Chemifinder |
| Steam pressure | 0.438 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Soluble. 12 g/l | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.7 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
XN; R21/22. XI; R36/37. Carc3; R40. |
Harmful. Dangerous in contact with skin and if swallowed. Local irritating. Irritates the eyes and the respiratory system. Possible carcinogenic effect. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Isophorone is easily absorbed in the body through lungs, skin or gastrointestinal tract (Jensen AA, 1997a). 14C labelled isophorone shows that 93% of the ingested oral amount of isophorone is mainly found in the urine and in the exhalation air after 24 hours (IPCS, 1995; HSDB; ATSDR, 1989). 100% absorption is thus assumed in the calculations.
Effects on health
Isophorone is damaging to health through ingestion and in contact with skin. In acute experiments and experiments of 90 days with mice and rats damages on liver and the central nervous system as well as deaths are seen at high doses. In long-term studies with mice and rats kidney damages are demonstrated (IPCS, 1995; ATSDR, 1989).
LD50 values (oral intake for rats) for isophorone are between 1000 and 3450 mg/kg bw (HSDB; IUCLID, 2000d).
Isophorone is irritating for both the eyes and the respiratory organs (HSDB; ATSDR, 1989; IPCS, 1995; IUCLID, 2000d). In the working environment examples of complaints about irritation effects at air levels of isophorone from 0.7 to 14 ppm are seen (Jensen AA, 1997a).
No reports about sensitizing properties of isophorone are made (IUCLID, 2000d; IPCS, 1995; HSDB).
NOAEL for rats fed with isophorone for 90 days was set to 102.5 – 163.8 mg/kg bw. At the test significant reductions of the body weight at high doses were seen. At a test of 90 days with dogs (oral intake) a NOAEL of more than 150 mg/kg bw was observed (highest dose tested and no effects observed) (IUCLID, 2000d).
NOAEL for rats and guinea pigs having inhaled isophorone for six weeks was 0.144 mg/l air based on kidney effects. No statement of how much air and thus how large a dose the animals have ingested per kg body weight (IUCLID, 2000d). NOAEL for rats and rabbits having inhaled isophorone for six months was 250 ppm (250 mg/kg) based on death rates (ATSDR, 1989).
Some weeks’ exposure of isophorone vapours of more than 100 ppm has given serious kidney and lung damages in test animals. At 2-3 times larger exposure effects on the liver are also seen (Jensen AA, 1997a).
Isophorone is classified as carcinogenic category 3 (Carc3) with R40”Possible risk of cancer”. Substances in this carcinogenic group are substances giving cause for reservation as they are possibly carcinogenic for humans but there is not sufficient information to conduct a satisfactory assessment of these (Stat. Ord. 329, 2002). A survey being the basis of this assessment is from a 2-year study of the carcinogenic properties of isophorone in mice and rats. The result of the study is that there was some indication of carcinogenic effect in male rats but no indication of carcinogenic effect neither in female rats nor female mice. In male mice an ambiguous indication of a carcinogenic effect was seen.
Some test animals with mice and rats indicate that isophorone is not reprotoxic (HSDB; ATSDR, 1989). However, there are tests with pregnant rats and mice exposed to a little above 100 ppm indicating the possibility of foetal malformation (Jensen AA, 1997a).
Isophorone does not show mutagenic properties (HSDB; IPCS, 1995).
Threshold limits
The threshold limit in the working environment for isophorone is 5 ppm or 25 mg/m³ with the remarks L and C (cancer), i.e. that the threshold limit is a limit value (L) which is not allowed to be exceeded and that the substance is on the list of substances which are regarded as carcinogenic (The Danish Working Environment Authority, 2005).
Assessment
Through analysis, isophorone is identified in the following 12 products. Isophorone is primarily identified through migration to artificial sweat but also through evaporation from two products. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g |
|
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 1 | ||
| Toy bag | 30B | 2 | ||
| Toy bag | 37B | 3 | ||
| Toy bag | 38C | 150 | ||
| School bag | 39B | 250 | ||
| School bag | 40B | Handle | 1 | |
| School bag | 41A | Inside | 10 | |
| School bag | 42C | 95 | 0.21 | |
| Pencil case | 31 A | 5 | ||
| Pencil case | 34 | 15 | 0.02 | |
| Pencil case | 35B | 40 | ||
| Pencil case | 43 | 1 | ||
| Product type | Product ID | Maxi- mum meas- ured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the pro- duct |
Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| School bag | 42C | 0.21 | 1 | 0.35 | 900 | 6 | 12 | 20 | 1.63 | 150 | 91,796 |
| Pencil case | 34 | 0.02 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.01 | 150 | 18,456, 806 |
The following exposure to isophorone is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.25 | 1 | 0.299 | 131 | 4 | 12 | 3.27 | 150.0 | 45., 3 |
| Toy bag | 30B | 0.5 | 1 | 0.020 | 131 | 1 | 12 | 0.11 | 150.0 | 1,394,931 |
| Toy bag | 37B | 0.75 | 1 | 0.009 | 131 | 1 | 12 | 0.08 | 150.0 | 1,965,069 |
| Toy bag | 38C | 37.5 | 1 | 0.066 | 131 | 1 | 12 | 26.99 | 150.0 | 5,558 |
| School bag | 39B | 62.5 | 1 | 0.058 | 131 | 1 | 12 | 39.60 | 150.0 | 3,788 |
| School bag | 40B | 0.25 | 1 | 0.136 | 131 | 1 | 12 | 0.37 | 150.0 | 405,618 |
| School bag | 41A | 2.5 | 1 | 0.042 | 131 | 1 | 12 | 1.14 | 150.0 | 131,744 |
| School bag | 42C | 23.75 | 1 | 0.119 | 131 | 1 | 12 | 30.76 | 150.0 | 4,877 |
| Pencil case | 31 A | 1.25 | 1 | 0.036 | 131 | 1 | 12 | 0.49 | 150.0 | 308,446 |
| Pencil case | 34 | 3.75 | 1 | 0.040 | 131 | 1 | 12 | 1.66 | 150.0 | 90,577 |
| Pencil case | 35B | 10 | 1 | 0.087 | 131 | 1 | 12 | 9.48 | 150.0 | 15,829 |
| Pencil case | 43 | 0.25 | 1 | 0.054 | 131 | 1 | 12 | 0.15 | 150.0 | 1,014,595 |
The following exposure to isophorone is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.25 | 1 | 21.1 | 1 | 12 | 0.44 | 150.0 | 341,232 |
As worst case the highest of the above exposure values can be added as a child might be exposed to isophorone during a long time both by inhalation from a school bag and a pencil case at the same time as an exposure through the skin from a school bag, a toy bag, a pencil case and a eraser takes place as well as oral exposure when the child sucks or chews a eraser.
This scenario gives a total exposure of 81.42 µg/kg bw/day and when this value is compared with a NOAEL of 150 mg/kg bw/day the result is a Margin of Safety of 1842.
It is generally accepted that MoS must be at least 100 before a substance can be declared as safe in use. All the calculated MoS of the individual products are significantly above 100 and the assessment is thus that they do not represent any health risk with regard to isophorone. Exposure of isophorone through both inhalation and skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.2 BHT
Application
BHT is used as an anti-oxidant in foods (E321), animal feed, petroleum products, synthetic rubber, plastics material as well as vegetable oils and soaps. BHT is also widely used in cosmetic products. Furthermore, it functions as anti-skinning agent in paints and inks (Merck, 1983; OECD SIDS, 2002).
Identification
| Chemical name | 2,6-Di-tert-butyl-p-cresol |
| CAS-No. | 128-37-0 |
| EINECS No. | 204-881-4 |
| Gross formula | C15-H24-O |
| Molecular structure | 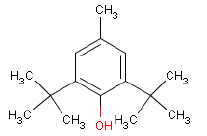 |
| Molecular weight | 220.35 g/mol |
| Synonyms | Butylenes hydroxytoluen (BHT) 2,6-Bis(1,1-dimethylethyl)-4-methylphenol |
Physical-chemical data
| Physical form | Colourless solid substance. | OECD SIDS, 2002 |
| Melting point | 71 °C | TOXNET ChemIDplus |
| Boiling point | 265 °C | TOXNET ChemIDplus |
| Steam pressure | 0.015 mm Hg at 20 °C | Dutch Institute for the Working Environment, 1991. |
| Water solubility | Weakly soluble: 0.0006 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 5.1 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
No | |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish Self classification (The Danish Environmental Protection Agency, 2001) |
Yes Xn;R22 N;R50/53 |
Harmful. Dangerous if swallowed. Dangerous for environment. Very toxic for aquatic organisms; may cause long-term adverse effects in the aquatic environment. |
Bioavailability
BHT is easily absorbed through the gastrointestinal tract and to a certain degree also through intact skin. Rats fed with a single dose BHT separated 80-90% of the dose via the urine after four days, most of it during 24 hours. For humans 66% is separated during 11 days via the urine (OECD SIDS, 2002).
In a test, rat skin was applied with 14C labelled BHT. Here the skin absorption was 13% of the applied dose (Nordic Council of Ministers, 1997). This value is therefore used in the calculations with dermal absorption.
Effects on health
BHT has a low acute toxicity. Tests with rats which orally consumed BHT resulted in a LD50 value of more than 2930 mg/kg bw (OECD SIDS, 2002).
BHT is slightly irritating for both skin and eyes (based on tests with rabbits) but does not show signs of sensitizing properties in animal tests (OECD SIDS, 2002; IUCLID, 2000e). Few cases of allergic responses towards BHT at humans are reported and this result despite the wide application of BHT as anti-oxidant in both foods and cosmetic products (OECD SIDS, 2002).
In animal tests, prolonged exposure to BHT has shown effects on lungs, liver, kidneys and thyroid gland. High sub-chronic doses of BHT can result in deaths at mice and rats either due to serious lung damages or massive bleedings. At chronic oral exposure, effects are first and foremost on liver and thyroid gland. Doses of above 25 mg BHT/kg bw/day result in hyperactivity of the thyroid gland and magnification of the liver from daily exposure of 7 days. Therefore NOAEL is 25 mg/kg bw/day (OECD SIDS, 2002).
IARC assesses that BHT is not classifiable in relation to the carcinogenic properties of the substance in humans. There are limited indications of the carcinogenic properties of BHT in animals and therefore an assessment for humans cannot be made (IARC, 1986).
BHT does not show mutagenic properties – Ames test is negative (OECD SIDS, 2002; IUCLID, 2000e).
Reproduction studies with mice and rats showed an effect (fewer pups per litter) at doses above 100 g/kg bw/day. NOAEL for this study was 25 mg/kg bw/day for rats (OECD SIDS, 2002).
Threshold limits
The threshold limit in the working environment for BHT is 10 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
BHT is identified in the following 6 products through analyses. BHT is primarily identified through migration to artificial sweat but also through evaporation from three erasers. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). More parts from same product have been analyzed. In the table below the highest measured value is stated when several values from same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 25 | 0.14 | |
| Eraser | 10 | 70 | 0.35 | |
| Eraser | 24 | 3 | 0.02 | |
| Pencil case | 35C | Inside | 10 | |
| Toy bag | 37B | 1 | ||
| Toy bag | 38C | 1 | ||
The found NOAEL value for BHT is for chronic effects for which reason a long-term scenario for exposure to BHT by inhalation from erasers, pencil cases and toy bags is solely calculated. In this way the following exposure to BHT is found:
| Product type | Product ID | Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
M³/hour | g | hours/- day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.14 | 1 | 0.35 | 900 | 6 | 12 | 20 | 1.09 | 25 | 22,949 |
| Eraser | 10 | 0.35 | 1 | 0.35 | 105 | 6 | 12 | 20 | 0.32 | 25 | 78,682 |
| Eraser | 24 | 0.02 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.01 | 25 | 3,076, 134 |
The following exposure to BHT is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 6.25 | 0.13 | 0.299 | 131 | 4 | 12 | 10.62 | 25 | 2,353 |
| Eraser | 10 | 17.5 | 0.13 | 0.671 | 131 | 4 | 12 | 66.67 | 25 | 375 |
| Eraser | 24 | 0.75 | 0.13 | 0.093 | 131 | 4 | 12 | 0.40 | 25 | 63,183 |
| Pencil case | 35C | 2.5 | 0.13 | 0.026 | 131 | 1 | 12 | 0.09 | 25 | 273,400 |
| Toy bag | 37B | 0.25 | 0.13 | 0.009 | 131 | 1 | 12 | 0.00 | 25 | 7.557,957 |
| Toy bag | 38C | 0.25 | 0.13 | 0.066 | 131 | 1 | 12 | 0.02 | 25 | 1,068,937 |
The following exposure to BHT is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | Kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 6.25 | 1 | 21.1 | 1 | 12 | 10.99 | 25 | 2,275 |
| Eraser | 10 | 17.5 | 1 | 105 | 1 | 12 | 153.13 | 25 | 163 |
| Eraser | 24 | 0.75 | 1 | 21.1 | 1 | 12 | 1.32 | 25 | 18,957 |
As worst case the highest of the above exposure values can be added as a child might be exposed to BHT both by inhalation from an eraser at the same time as an exposure through the skin from a toy bag, a pencil case and an eraser as well as oral exposure when the child sucks or chews an eraser.
This scenario gives a total exposure of 221 µg/kg bw/day and when this value is compared with a NOAEL of 25 mg/kg bw/day the result is a Margin of Safety of 113.
All the calculated MoS of the individual products are above 100 and therefore they are not assessed to represent any health risk with regard to their content of BHT. Exposure to BHT by inhalation, through skin absorption and oral intake from several products at the same time is neither assessed to represent any health risk for the examined products – but MoS is close to 100.
However, it must be noted that the most critical of the above exposure levels (lowest MoS) is for oral intake of an eraser (Product ID 10). In these calculations the values from artificial sweat are assumed to be the same as for artificial saliva (as analyses of artificial saliva are not conducted with analysis for BHT). Furthermore, it is assumed that a child sucks and chews the whole eraser which in this case is a rather big eraser of 4 x 1.3 x 11 cm giving most likely a too high estimate.
Furthermore, the erasers for the analyses are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface becomes significantly larger that the normal surface of an eraser.
To illustrate this surface of the eraser is here calculated if the whole eraser is cut into cubes of 0.3 x 0.3 x 0.3 mm. Thus it gives approx. 13 x 4 x 36 = 1872 pieces of eraser, each with a surface of 0.3 x 0.3 x 6 = 0.54 cm², i.e. a total surface of 1010 cm². For purposes of comparison, the eraser in uncut condition has a surface of ((4 x 1.3) + (4 x 11) + (1.3 x 11)) x 2 = 127 cm², i.e. nearly a factor 8 in difference. Therefore, the measured concentrations of BHT are most probably overestimated by a factor 8 whereby MoS is then approx. 1,300 for Product no. 10 at oral intake. MoS for total exposure from several products through several ways of exposure are thus approx. 287 instead.
All in all, these circumstances mean that exposure to BHT from several products at the same time and through several ways of exposure will most probably not constitute any risk for the examined products. However, it is unknown if other products may have a higher content of BHT and thus constitute a health problem if a child is exposed to several products with a high content of BHT. As BHT is much used as an anti-oxidant in foods there is a possibility of exposure through other sources. The total exposure is not assessed in this project.
5.2.3 Cyclohexanone
Application
Cyclohexanone is an artificial organic liquid which is primarily used as intermediate in the production of nylon. Additionally, it is also used as intermediate, additive agent and solvent in a number of products (IARC, 1989).
Identification
| Chemical name | Cyclohexanone |
| CAS-Nr. | 108-94-1 |
| EINECS Nr. | 203-631-1 |
| Gross formula | C6H10O |
| Molecular structure | 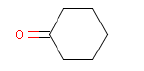 |
| Molecular weight | 98.14 g/mol |
| Synonyms | Cyclohexyl ketone Hexanon Ketohexamethylene Pimelic keton |
Physical-chemical data
| Physical form | White to weak yellowish oily liquid with a peppermint like fragrance | Chemfinder |
| Melting point | -47 °C (-31°C at TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 155.6 °C (155.4 °C at TOXNET ChemIDplus) | Chemfinder |
| Steam pressure | 4.33 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble: 25 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.81 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
R10 XN;R20 |
Flammable. Harmful. Dangerous by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about bioavailability of cyclohexanone is found but according to the threshold limit list the substance can be absorbed through the skin for which reason 100% absorption is assumed in the calculations.
Effects on health
Test of the acute toxicity of cyclohexanone on animals has shown a low acute oral toxicity. Oral LD50 values for rats are between 1296 and 3460 mg/kg bw/day and LD50 values (inhalation, 4 hours) for rats are between 10.7 and 32.5 mg/L (IUCLID, 2000h).
Tests with rabbits show that cyclohexanone is irritating for the skin and for the eyes. Cyclohexanone vapours can irritate mucous membranes and contact with the liquid can cause dermatitis in sensitive individuals (IUCLID, 2000h; HSDB). Exposure to vapours of 25 ppm of a few minutes’ duration seems to be unpleasant whereas at 75 ppm a severe irritation of nose, throat and eyes is observed (Jensen AA, 2003c).
Cyclohexanone does not seem to be sensitizing according to several animal tests whereas patch tests on humans have shown that cyclohexanone resins give an allergic contact dermatitis (IUCLID, 2000h; HSDB).
Exposure to 3,000 ppm cyclohexanone for a few hours is mortal for test animals. Exposure to 200-500 ppm affects the nervous system as it can give a prolonged response time (Jensen AA, 2003c).
IARC assesses that cyclohexanone is not classifiable in relation to the carcinogenic properties of the substance in humans. Indication of the carcinogenic properties of cyclohexanone in animals is insufficient (IARC, 1989).
The majority of the experimental data indicates that cyclohexanone is not genotoxic. Long-term tests with mice and rats indicate that cyclohexanone is not carcinogenic (OECD SIDS).
In a two generation study with rats, effects on the fertility at 1400 ppm were demonstrated but not at 500 ppm. However, it turned out that the effect was reversible during a subsequent recovery period after completion of exposure (OECD SIDS).
NOAEL for the chronic effects (weight increase) of cyclohexanone is calculated to 462 mg/kg bw/day for rats (Nilsson et al, 2006).
Threshold limits
The threshold limit in the working environment for cyclohexanone is 10 ppm or 40 mg/ m³ with the remark H, i.e. the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Cyclohexanone is identified in the following 7 products through analyses. Cyclohexanone is primarily identified through migration to artificial sweat but also through evaporation from two products. There are more values of analysis than those stated in the table below (see table 3.9A and 3.0B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Pencil case | 34 | 5 | 0.01 | |
| Pencil case | 35B | 10 | ||
| Toy bag | 38A | 3 | ||
| School bag | 39B | 4 | ||
| School bag | 40C | 1 | ||
| School bag | 41A | Inside | 2 | |
| School bag | 42A | 1 | 0.01 | |
No acute NOAEL for cyclohexanone is identified for which reason a long-term scenario for exposure to cyclohexanone through pencil cases, toy bags and school bags by inhalation is carried out.
At long-term exposure the following exposure to cyclohexanone through inhalation is found.
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/- day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 34 | 0.01 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.00 | 462 | 113,693, 925 |
| School bag | 42B | 0.01 | 1 | 0.35 | 900 | 6 | 12 | 20 | 0.08 | 462 | 5,937, 349 |
The following exposure to cyclohexanone is absorbed through skin contact. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 34 | 1.25 | 1 | 0.040 | 131 | 1 | 12 | 0.55 | 462 | 836,932 |
| Pencil case | 35B | 2.5 | 1 | 0.087 | 131 | 1 | 12 | 2.37 | 462 | 195,013 |
| Toy bag | 38A | 0.75 | 1 | 0.041 | 131 | 1 | 12 | 0.33 | 462 | 1,389,245 |
| School bag | 39B | 1 | 1 | 0.058 | 131 | 1 | 12 | 0.63 | 462 | 729,184 |
| School bag | 40C | 0.25 | 1 | 0.036 | 131 | 1 | 12 | 0.10 | 462 | 4,750,065 |
| School bag | 41A | 0.5 | 1 | 0.042 | 131 | 1 | 12 | 0.23 | 462 | 2,028,850 |
| School bag | 42A | 0.25 | 1 | 0.037 | 131 | 1 | 12 | 0.10 | 462 | 4,70,626 |
As worst case the highest of the above exposure values can be added as a child might be exposed to cyclohexanone during a long time both by inhalation from a school bag and a pencil case at the same time as an exposure through the skin from a school bag, a toy bag, a pencil case and a eraser takes place.
This scenario gives a total exposure of 3.41 µg/kg bw/day and when this value is compared with a NOAEL of 462 mg/kg bw/day the result is a Margin of Safety of 135.484.
All the calculated MoS of the individual products are significantly above 100 and therefore they are not assessed to represent any health risk with regard to cyclohexanone. Exposure to cyclohexanone both by inhalation and through skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.4 Phenol
Application
Phenol is primarily applied as an intermediate in organic syntheses and is a raw material in the production of bisphenol A, alkylphenols, caprolactam, salicylic acid, nitrophenols, diphenyl ether and halogeneous phenols. Beyond this a small amount is applied as component in cosmetic, medical drugs, binding agents, impregnating agents, paints, varnishes and solvents (EU ECB, 2006a).
Identification
| Chemical name | Phenol |
| CAS-Nr. | 108-95-2 |
| EINECS Nr. | 203-632-7 |
| Gross formula | C6H6O |
| Molecular structure | 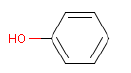 |
| Molecular weight | 94.11 g/mol |
| Synonyms | Benzenol Hydroxybenzene Oxybenzene |
Physical-chemical data
| Physical form | Light sensitive solid/thick liquid with sweet tarry fragrance. The colour varies from colourless to pink. | Chemfinder |
| Melting point | 40.5 °C | Chemfinder |
| Boiling point | 181.7 °C | Chemfinder |
| Steam pressure | 0.35 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Soluble: 82.8 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.46 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
T;R23/24/25 C;R34 XN;R48/20/21/22 Mut3;R68 |
Toxic by inhalation, in contact with skin and if swallowed. Risk of corrosion. Harmful to health. Harmful: Danger of serious damage to health by prolonged exposure by inhalation, in contact with skin and if swallowed. Mutagenic cat. 3. Possible risks of irreversible effects. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Was earlier in the list but does not fulfil the new criteria for undesirable properties (classifications). |
Bioavailability
Phenol is quickly absorbed and nearly completely through lungs, gastrointestinal tract and skin. The absorption through skin is so large that a few hours’ contact with 2% phenol solution can result in acute intoxication with shock, cramps, coma and death. Of the absorbed amount 90% is separated within 24 hours (Jensen AA, 1997b).
In the EU Risk Assessment Report for phenol the absorption through oral intake and by inhalation is calculated to 100% but only to 80% through dermal exposure (EU ECB, 2006a). Same values are used in the calculations in this project.
Effects on health
Indication of acute toxicity at humans and animals are similar no matter the way of exposure. Absorption of phenol is quick as indications of toxicity are already seen after a few minutes at exposure to phenol. Deaths for humans are reported after exposure to phenol concentrations of 140-290 mg/kg bw (EU ECB, 2006a).
LD50 values for rats through oral intake are stated to 340 mg/kg bw (EU ECB, 2006a). However, humans seem to be more sensitive to the acute toxicity of phenol than animals as ingestion of 1 g of phenol may be mortal for an adult human whereas the mortal concentration in animals only corresponds to the same as applicable for a substance hazardous to health (Jensen AA, 1997b).
Both at acute and chronic intoxications at large amounts of phenol by inhalation or through ingestion serious damages on lungs, heart, liver and kidneys are seen. Furthermore, phenol is toxic to the white blood corpuscles (Jensen AA, 1997b).
Phenol can cause serious skin damages in contact with skin and is thus classified as corrosive (EU ECB, 2006a; Stat. Ord. 923, 2005). No studies indicate that phenol is allergenic (Jensen AA, 1997b; EU ECB, 2006a).
Human data indicate that phenol has a serious effect on the nervous system after prolonged exposure whether it is oral, dermal or through inhalation. At oral intake LOAEL is stated to 1.8 mg/kg bw/day (no NOAEL). In contact with skin NOAEL is stated to 130 mg/kg bw/day (EU ECB, 2006a).
Phenol does not seem to harm the unborn child or to be carcinogenic. In a two generation study with rats a NOAEL of 93 mg/kg bw/day is found. The effects at higher concentrations were reduced body weight (EU ECB, 2006a).
Both positive and negative results in different tests of the mutagenic properties of phenol are found for which reason the EU classifies phenol as mutagenic in category 3, i.e. possible risk of irreversible effects (EU ECB, 2006a; Stat. Ord. 923, 2005).
Threshold limits
The threshold limit in the working environment for phenol is 1 ppm or 4 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Phenol is identified in the following four products through analyses and solely through migration to artificial sweat.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Toy bag | 38A/C | 1 | ||
| School bag | 40B | Handle | 2 | |
| School bag | 41A | Inside | 1 | |
| School bag | 42A | 3 | ||
Data indicate that the effect of phenol depends on the way of exposure. A higher NOAEL value is stated for exposure to skin than for oral intake. The primary exposure to phenol will be by inhalation and in contact with skin through phenol vapours. Using the NOAEL value for contact with skin (based on test animals) the following dermal exposure of phenol in contact with skin is found. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours. Furthermore, an absorption factor of 100% is used as it is assumed that the listed NOAEL for dermal exposure considers the dermal absorption.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Toy bag | 38A/C | 0.25 | 1 | 0.066 | 131 | 1 | 12 | 0.18 | 130 | 722,602 |
| School bag | 40B | 0,5 | 1 | 0.136 | 131 | 1 | 12 | 0.74 | 130 | 175,768 |
| School bag | 41A | 0.25 | 1 | 0.042 | 131 | 1 | 12 | 0.11 | 130 | 1.141777 |
| School bag | 42A | 0.75 | 1 | 0.037 | 131 | 1 | 12 | 0.30 | 130 | 428,702 |
All the calculated MoS of the individual products are significantly above 100 and therefore it is assessed that the examined products do not constitute any health risk with regard to migration of phenol even if tests indicate that humans are more sensitive towards phenol than animals.
5.2.5 Toluene
Application
Toluene is applied in the production of petrol as well as in certain types of paints, diluting agents, ink, binders, medical drugs and cosmetics products. Furthermore, it is applied in some kinds of varnishes, nail varnishes, rubber products and leather colouring processes (IPCS, 1985; ATSDR, 2000).
Identification
| Chemical name | Methylbenzene |
| CAS-Nr. | 108-88-3 |
| EINECS Nr. | 203-625-9 |
| Gross formula | C7H8 |
| Molecular structure |  |
| Molecular weight | 92,14 g/mol |
| Synonyms | Toluene Methylbenzol Monomethyl benzene Phenyl methane |
Physical-chemical data
| Physical form | Colourless liquid with benzene-like smell | Chemfinder |
| Melting point | -93 °C (-94.9 at TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 110.6 °C | Chemfinder |
| Steam pressure | 28.4 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble: 0.526 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 2.73 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XI;R38 XN;R48/20-65 Rep3;R63 R67 |
Highly flammable. Local irritating. Irritating to skin. Harmful to health. Harmful: Danger of serious damage to health by prolonged exposure by inhalation. Harmful: May cause lung damage if swallowed. Toxic to reproduction cat. 3. Possible risk of harm to the unborn child. Vapours may cause drowsiness and dizziness. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Toluene is easily absorbed in the body. Toluene can be absorbed through the skin and about half the amount of toluene being inhaled is absorbed in the body. Toluene is accumulated after absorption in the body in fatty tissue (nerve system and fat deposits). The half life of toluene in humans can be up to three days. Toluene is easily transferred to the womb and about 75% of the toluene concentration being found in the mother’s blood can be found in the unborn child (Jensen AA, 1997c; EU ECB, 2003).
Effects on health
The acute toxicity of toluene is low. LD50 values for rats are between 5,500 and 7,500 mg/kg bw. Indications of acute toxicities are headache, dizziness, feeling of intoxication and at high concentrations also unconsciousness. Toluene is also classified with R67 “Vapours may cause drowsiness and dizziness” (EU ECB, 2003; Jensen AA, 1997c).
Toluene is classified as skin irritating and has a degreasing effect on the skin. Furthermore, toluene irritates the eyes and the airways. No indication of toluene being allergenic in contact with skin or by inhalation but only limited data is available (EU ECB, 2003).
In a two-year’ inhalation study with rats a NOAEC value of 1,125 mg/ m³ (corresponding to approx. 300 ppm) is found. No clear indications of intoxication at the highest doses were found. A 13-weeks’ study with both rats and mice showed a NOAEL of 625 mg/kg lw. In the rats nerve damages were found in the brain at doses above NOAEL (1,250 mg/kg bw) and there was one single death at the mice at the same dose (1,250 mg/kg bw) (EU ECB, 2003). Prolonged inhalation of toluene in high concentrations can thus give nerve and brain damages (Jensen AA, 1997c) and toluene is also classified with “danger of serious damage to health by prolonged exposure through inhalation”.
Based on experiences with work-related exposures it is assessed that it takes more than 10 years’ exposure to toluene at low concentrations before damages on the brain such as the painter’s syndrome are a reality EU ECB, 2003).
Toluene is neither mutagenic nor carcinogenic (EU ECB, 2003). IARC assesses that toluene is not classifiable in relation to the carcinogenic properties of the substance in humans and indications of lack of carcinogenic effect in animals (IARC, 1999).
Toluene is considered as possibly harmful to the unborn child and is also classified as possibly harmful to the unborn child category 3. Limited human data indicate that there is an increased risk of spontaneous abortion at doses of approx. 88 ppm (Jensen AA, 1997c; EU ECB, 2003).
Threshold limits
The threshold limit in the working environment is 25 ppm or 94 mg/ m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
Toluene is identified in the following three products through analyses and solely through evaporation.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 3 | 0.01 | ||
| Eraser | 24 | 0.02 | ||
| Pencil case | 16 | 0.01 | ||
No acute NOAEL of toluene is identified for which reason a long-term scenario for exposure to toluene through erasers and pencil cases by inhalation is conducted.
At long-term exposure the following exposure to toluene by inhalation is found:
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day | kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 3 | 0.01 | 1 | 0.35 | 900 | 6 | 12 | 20 | 0.08 | 625 | 8,032, 129 |
| Eraser | 24 | 0.02 | 1 | 0.35 | 21 | 6 | 12 | 20 | 0.00 | 625 | 171,301, 319 |
| Pencil case | 16 | 0.01 | 1 | 0.35 | 47 | 6 | 12 | 20 | 0.00 | 625 | 153,806, 716 |
All the calculated MoS of the individual products are far above 100 and therefore it is assessed that the toluene evaporation from the examined products does not constitute any health risk. This also applies if the 88 ppm (increased risk of spontaneous abortion) is used in the calculations.
5.2.6 DIBP
Application
DIBP is applied among others in paint, varnish, paper and cardboard. Furthermore, it is applied as softeners and binding agents in especially plastic products as well as for regulation of the viscosity in certain products (IUCLID, 2000f).
Identification
| Chemical name | Diisobutyl phthalate |
| CAS-Nr. | 84-69-5 |
| EINECS Nr. | 201-553-2 |
| Gross formula | C16H22O4 |
| Molecular structure |  |
| Molecular weight | 278.35 g/mol |
| Synonyms | 1,2-Benzendi carboxylic acid, bis(2-methylpropyl) ester Phthalic acid, diisobutyl ester Isobutyl phthalate Palatinol IC |
Physical-chemical data
| Physical form | Clear viscous liquid. | Chemfinder |
| Melting point | -64 °C | HSDB |
| Boiling point | 296 °C | Chemfinder |
| Steam pressure | 6.65E-03 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Non-soluble. 0.0062 g/L at 24 °C |
Chemfinder TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 4.11 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
No | According to the Danish Environmental Protection Agency the classification will be changed to Rep2 on development and Rep3 on fertility. I.e. Rep2; R61-62 (May cause harm to the unborn child. Possible risk of impaired fertility). |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No N;R50/53 |
Harmful to environment. Very toxic to aquatic organisms may cause long-term adverse effects in the aquatic environment. |
Bioavailability
Phthalates in general – and thus DIBP – are easily absorbed in the body either through lungs, gastrointestinal tract or skin (Jensen AA, 1997d). In the calculations the absorption is thus calculated to 100%.
Effects on health
The acute toxicity of phthalates is generally low. LD50 values for rats for BIPB are between 10,400 and 15,000 mg/kg bw (IUCLID, 2000f).
Tests with rabbits show that DIBP is not irritating for neither skin nor eyes (IUCLID, 2000f). Only very limited data on the sensitizing properties of the substance are available. HSDB reports that several examples of allergenic reactions are seen at contact with plastic products containing DIBP.
A test with rats being fed orally with DIBP for 14 days gave a NOAEL of 50 mg/kg bw/day. The highest dose (2000 mg/kg) resulted in an increased liver weight as well as an increase in triglyceride and cholesterol levels. In the intermediate doses (of 100 and 200 mg/kg) only small effects were seen such as an increase in the triglyceride level. DEHP was given to a positive control group and the effects were the same at high dose of DIBP as of DEHP (IUCLID, 2000f).
Generally phthalates are seldom active in genetic short-term tests. Some phthalates are not mutagenic (Ames test) and this also applies DIBP (IUCLID, 2000f; Jensen AA, 1997d).
No data on the carcinogenic properties of DIBP are available but generally phthalates are not assessed to constitute a high cancer risk (Jensen AA, 1997d).
In general there are limited data on the properties of DIBP being toxic to reproduction but a single test with rats getting doses of DIBP (390, 780 and 1300 mg/kg bw respectively) on the 5th, 10th and 15th day of pregnancy respectively shows that DIBP has effects being toxic to reproduction. For all doses the average fetal weight was strongly reduced and abnormalities were found on the skeletons of the foetuses (dose-dependent). At the intermediate dose two dead foetuses without eyes were found (IUCLID, 2000f).
Threshold limits
The threshold limit in the working environment for DIBP is 3 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
DIBP is identified in the following 10 products through analyses. DIBP is solely identified through migration to artificial sweat. There are more values of analysis than those stated in the table below (see table 3.9B). More parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 1.5 | ||
| Pencil case | 31 A | 2 | ||
| Pencil case | 34 | 0.1 | ||
| Pencil case | 43 | 0.1 | ||
| Toy bag | 37B | 1.3 | ||
| Toy bag | 38C | 15 | ||
| School bag | 39B | 0.1 | ||
| School bag | 40B | Handle | 88 | |
| School bag | 41A | Inside | 0.4 | |
| School bag | 42B | Gym bag | 0.1 | |
The following exposure to DIBP is absorbed in contact with skin. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.375 | 1 | 0.299 | 131 | 4 | 12 | 4.90 | 50 | 10,198 |
| Pencil case | 31 A | 0.5 | 1 | 0.036 | 131 | 1 | 12 | 0.19 | 50 | 257,038 |
| Pencil case | 34 | 0.025 | 1 | 0.040 | 131 | 1 | 12 | 0.01 | 50 | 4,528,855 |
| Pencil case | 43 | 0.025 | 1 | 0.054 | 131 | 1 | 12 | 0.01 | 50 | 3,381,985 |
| Toy bag | 37B | 0.325 | 1 | 0.009 | 131 | 1 | 12 | 0.03 | 50 | 1,511,591 |
| Toy bag | 38C | 3.75 | 1 | 0.066 | 131 | 1 | 12 | 2.70 | 50 | 18,528 |
| School bag | 39B | 0.025 | 1 | 0.058 | 131 | 1 | 12 | 0.02 | 50 | 3,156,641 |
| School bag | 40B | 22 | 1 | 0.136 | 131 | 1 | 12 | 32.54 | 50 | 1,536 |
| School bag | 41A | 0.1 | 1 | 0.042 | 131 | 1 | 12 | 0.05 | 50 | 1,097,863 |
| School bag | 42B | 0.025 | 1 | 0.011 | 131 | 1 | 12 | 0.00 | 50 | 16,695,573 |
The following exposure to DIBP is absorbed through oral intake when a child sucks or chews an eraser. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours. As mentioned earlier it is assumed that the results from migration to artificial sweat can be transferred directly to artificial saliva.
| Product type | Product ID | Maximum measured concentration in µg/g | Foral | Weight Aoral |
Tcontact | BW | Ioral | NOAEL | MoS |
| Migration artificial sweat | g | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 4 | 0.375 | 1 | 21.1 | 1 | 12 | 0.66 | 50 | 75,829 |
As absolute worst case the highest of the above exposure values can be added as a child might be exposed to DIBP both through the skin from a school bag, a toy bag, a pencil case and an eraser as well as oral exposure when the child sucks or chews an eraser.
This scenario gives a total exposure of 40.99 µg/kg bw/day and when this value is compared with a NOAEL of 50 mg/kg bw/day the result is a Margin of Safety of 1219.
All the calculated MoS of the individual products are significantly above 100 and this assessment is thus that they do not represent any health risk with regard to DIBP. Exposure to DIBP both by inhalation and through skin absorption from several products at the same time is neither assessed to represent any health risk for the examined products.
5.2.7 DEHP
Application
DEHP is primarily applied as plasticizer as it has an ability of plasticizing plastic without reacting chemically with it. DEHP is especially used in PVC products like tubes, hoses and parts for medical equipment. Furthermore, it is used as plasticizer in plastics materials of cellulose ester and synthetic elastomer (IPCS, 1992).
Identification
| Chemical name | 1,2-Benzene dicarboxylic acid, bis(2-ethylhexyl)ester |
| CAS-No. | 117-81-7 |
| EINECS No. | 204-211-0 |
| Gross formula | C24H38O4 |
| Molecular structure |  |
| Molecular weight | 390.56 g/mol |
| Synonyms | Bis(2-Ethylhexyl)Phthalate (DEHP) Ethylhexyl phthalate Dioctyl phthalate Bisoflex 81 |
Physical-chemical data
| Physical form | Colourless oily liquid with nearly no smell. | Chemfinder |
| Melting point | -50 °C (-55 °C according to TOXNET ChemIDplus) | Chemfinder |
| Boiling point | 386.9 °C (384 °C according to TOXNET ChemIDplus) | Chemfinder |
| Steam pressure | 1.42E-07 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Insoluble: 0.00027 g/Lat 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 7.6 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
Rep2;R60-61 | Toxic to reproduction cat.2. May impair fertility. May cause harm to the unborn child. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
Yes No |
Bioavailability
DEHP is easily absorbed in the body through either lungs or the gastrointestinal tract. The skin permeability of DEHP is not large and is measured to 6.5 and 26% depending on the animal species. DEHP is one of the long-chained phthalates where the permeability is smallest. The EU Draft Risk Assessment Report on DEHP uses the following relevant bioavailability percentages which are also used in the calculations. Oral exposure – 50%, but 100% for children. Dermal – 5% for both adults and children (EU ECB, 2006b).
Effects on health
The acute toxicity of DEHF is very low. The LD50 value for rats is above 20,000 mg/kg bw – in some tests even above 30,000 mg/kg bw (IUCLID, 2000g). A single survey of the acute toxicity of DEHP in humans is available. Here oral intake of 5 g led to no symptoms and intake of 10 g only led to mild symptoms such as stomach disorders. It was only men who ingested DEHP as single dose (EU ECB, 2000b; Jensen AA, 1997d).
DEHP is slightly irritating for both skin and eyes. Animal tests indicate that DEHP has no sensitizing properties (EU ECB, 2006b).
In animal tests with rats a NOAEL for the acute effects of DEHP on the heart rate is determined to 28.5 mg/kg bw/day (EU ECB, 2006b).
The mutagenic properties of DEHP (gene mutations, DNA damages and chromosome effects) are tested in several studies and the results are predominantly negative (EU ECB, 2006b; IUCLID, 2000g).
IARC assesses DEHP as being non-classifiable in relation to the carcinogenic properties of the substance in humans. There is insufficient information about the carcinogenic properties of DEHP in humans and there are sufficient indications of the carcinogenic properties of DEHP in animals (IARC, 2000). But the mechanism behind the carcinogenic effect of DEHP in rodents is very special and does not seem to be relevant for humans (Jensen AA, 1997d; EU ECB, 2000b).
A two generation study with rats sets a NOAEL value of 8 mg/kg bw/day for testicular toxicity. The effects were a reduction in testicular weight. The same test sets a NOEAL value of 77 mg/kg bw/day for damages to reproduction. In another two generation study with rats a NOAEL value of 4.8 mg/kg bw/day is set for testicular toxicity and a NOAEL of 46 mg/kg for damages to reproduction. DEHP is therefore regarded as being toxic to reproduction and is also classified as toxic to reproduction category 2, i.e. that it can damage the fertility and can cause harm to the unborn child (Stat. Ord. 923, 2005).
Threshold limits
The threshold limit in the working environment for DEHP is 3 mg/m³ (The Danish Working Environment Authority, 2005).
Assessment
DEHP is identified in four products through migration to artificial sweat. There are more values of analysis than those stated in the table below (see table 3.9A and 3.9B). Several parts from the same product have been analyzed. In the table below the highest measured value is stated when several values from the same product are found.
Additionally DEHP is identified through total determination in four erasers in a maximum concentration of 44%. Based on these numbers a scenario is calculated where it is assumed that a child will swallow between 0.008 and 0.1 g of eraser corresponding to approx. 0.01 and 0.08 cm³ for the relevant erasers – i.e. cubes of approx. 1.9 to 4.3 mm in height, width and length – an amount not unrealistic to swallow. The value of 0.008 g eraser is chosen as it is the upper limit of intake of toy material which is used in DS/EN 71-3”Toys. Safety requirements. Part 3: Migration of special substances”.
Finally a migration analyse to artificial saliva for a single eraser is conducted – the eraser with the highest DEHP. Based on this value a scenario is calculated for a child who sucks this eraser 1 hour a day.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | mg/g |
| Migration artificial sweat | Total content | |||
| Eraser | 12 | 350 | ||
| Eraser | 16 | 170 | ||
| Eraser | 22 | 440 | ||
| Eraser | 23 | 220 | ||
| Pencil case | 35C | Inside | 6 | |
| Toy bag | 38A | 2.4 | ||
| School bag | 39C | 1 | ||
| School bag | 41A | Inside | 1 |
The following exposure to DEHP is absorbed in contact with skin. The measured concentrations are corrected by a factor 0.25 (divided by 4) as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concen- tration in µg/g |
Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/ day |
kg | ug/kg bw/day | mg/kg bw/day | ||||
| Pencil case | 35C | 1.5 | 0.05 | 0.026 | 131 | 1 | 12 | 0.02 | 4.8 | 227,469 |
| Toy bag | 38A | 0.6 | 0.05 | 0.041 | 131 | 1 | 12 | 0.01 | 4.8 | 360,843 |
| School bag | 39C | 0.25 | 0.05 | 0.039 | 131 | 1 | 12 | 0.01 | 4.8 | 901,198 |
| School bag | 41A | 0.25 | 0.05 | 0.042 | 131 | 1 | 12 | 0.01 | 4.8 | 843,158 |
As worst case the highest of the above exposure values can be added as a child might be exposed to DEHP through the skin from a school bag, a toy bag and a pencil case.
This scenario gives a total exposure of 0.04 µg/kg bw/day and when this value is compared with a NOAEL of 4.8 mg/kg bw/day the result is a Margin of Safety of 120.000.
All the calculated MoS of the individual products are significantly above 100 and this assessment is thus that they do not represent any health risk with regard to skin absorption of DEHP. Exposure to DEHP through skin absorption from several products at the same time is neither assessed to represent any health risk.
Intake of small amounts of eraser
Through intake of small pieces of eraser of 0.008, 0.5 and 0.1 g respectively, corresponding to cubes of 1.9 mm, 3.5 mm and 4.3 mm respectively, the result is the following exposure to DEHP. Absorption from the gastrointestinal tract is calculated to 100% for children. The calculations are based on a body weight of both 12 and 20 kg. The 20 kg is applied to illustrate children’s weight during the first school year.
| Product type | Product ID | Measured concentration in mg/g | Foral | Qoral | BW | Ioral | NOAEL | MoS |
| Total content | g | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 12 | 350 | 1 | 0.1 | 12 | 2.92 | 4.8 | 1.6 |
| Eraser | 12 | 350 | 1 | 0.1 | 20 | 1.75 | 4.8 | 2.7 |
| Eraser | 12 | 350 | 1 | 0.05 | 12 | 1.46 | 4.8 | 3.3 |
| Eraser | 12 | 350 | 1 | 0.05 | 20 | 0.88 | 4.8 | 5.5 |
| Eraser | 12 | 350 | 1 | 0.008 | 12 | 0.23 | 4.8 | 20.6 |
| Eraser | 12 | 350 | 1 | 0.008 | 20 | 0.14 | 4.8 | 34.3 |
| Eraser | 16 | 170 | 1 | 0.1 | 12 | 1.42 | 4.8 | 3.4 |
| Eraser | 16 | 170 | 1 | 0.1 | 20 | 0.85 | 4.8 | 5.6 |
| Eraser | 16 | 170 | 1 | 0.05 | 12 | 0.71 | 4.8 | 6.8 |
| Eraser | 16 | 170 | 1 | 0.05 | 20 | 0.43 | 4.8 | 11.3 |
| Eraser | 16 | 170 | 1 | 0.008 | 12 | 0.11 | 4.8 | 42.4 |
| Eraser | 16 | 170 | 1 | 0.008 | 20 | 0.07 | 4.8 | 70.6 |
| Eraser | 22 | 440 | 1 | 0.1 | 12 | 3.67 | 4.8 | 1.3 |
| Eraser | 22 | 440 | 1 | 0.1 | 20 | 2.20 | 4.8 | 2.2 |
| Eraser | 22 | 440 | 1 | 0.05 | 12 | 1.83 | 4.8 | 2.6 |
| Eraser | 22 | 440 | 1 | 0.05 | 20 | 1.10 | 4.8 | 4.4 |
| Eraser | 22 | 440 | 1 | 0.008 | 12 | 0.29 | 4.8 | 16.4 |
| Eraser | 22 | 440 | 1 | 0.008 | 20 | 0.18 | 4.8 | 27.3 |
| Eraser | 23 | 220 | 1 | 0.1 | 12 | 1.83 | 4.8 | 2.6 |
| Eraser | 23 | 220 | 1 | 0.1 | 20 | 1.10 | 4.8 | 4.4 |
| Eraser | 23 | 220 | 1 | 0.05 | 12 | 0.92 | 4.8 | 5.2 |
| Eraser | 23 | 220 | 1 | 0.05 | 20 | 0.55 | 4.8 | 8.7 |
| Eraser | 23 | 220 | 1 | 0.008 | 12 | 0.15 | 4.8 | 32.7 |
| Eraser | 23 | 220 | 1 | 0.008 | 20 | 0.09 | 4.8 | 54.5 |
In the calculations here a NOAEL for effects being toxic to reproduction is used – i.e. long-term effects. This means that it clearly constitutes a health risk to eat eraser daily, even in small amounts. However, it must be assumed that in general it is a one-time occurrence to swallow a piece of eraser.
Suck on an eraser
For eraser 22 a migration analysis to artificial saliva is carried out as this eraser has the highest content of DEHP. The analysis is conducted at 37 degrees for 1 hour to imitate a child who sucks an eraser for 1 hour daily. The result of the migration analysis is that 0.1% (w/w) DEHP is released, i.e. 1 mg/g of eraser to artificial saliva. Uncertainty is 50%. The calculations are based on a body weight of 20 kg to illustrate children’s weight during the first school year.
The following exposure to DEHP is absorbed through oral intake when a child sucks or chews eraser 22. The duration of the exposure is assumed to be one hour daily. As the migration analysis is completed with a duration of one hour for one gram of eraser the amount of DEHP being ingested may be calculated like the migration (1 mg/g eraser) multiplied by the amount of eraser being sucked (here 14.4 g).
| Product type | Product ID | Maximum measured concentration in mg/g | F oral | Weight A product oral |
Tcontact | BW | I oral | NOAEL | MOS |
| Migration | g | Hours/ day | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 22 | 1 | 1 | 14.4 | 1 | 20 | 0.72 | 4.8 | 6.7 |
MoS are significantly below 100 and thus it constitutes a health problem if a child of 20 kg sucks this eraser one hour daily for a longer period (NOAEL is for effects being toxic to reproduction – long-term effects).
In the calculations it is assumed that the child sucks and chews the whole eraser which in this case is an eraser of 3.8 x 3.1 x 1 cm, i.e. a large eraser. Most probably, the calculation gives a too high estimate as it is unrealistic that a child sucks or chews the whole eraser at one time.
If it is assumed that a child will only have one end of the eraser in the mouth, i.e. the first cm of the eraser, then it is only 1 x 3.1 x 1 cm = 3.1 cm³ of the total area of 11.78 cm³ of the eraser which the child sucks. Thus the child sucks 3.79 g of the total weight of 14.4 g of the eraser. MoS can then be calculated to 25 instead. However, this does not change the fact that MoS is still significantly below 100.
| Product type | Product ID | Maximum measured concentration in mg/g | F oral | Weight A product oral |
Tcontact | BW | I oral | NOAEL | MOS |
| Migration | g | Hours/day | kg | mg/kg bw/day | mg/kg bw/day | ||||
| Eraser | 22 | 20 | 1 | 3.79 | 1 | 20 | 0.19 | 4.8 | 25.3 |
At the migration analysis to artificial saliva the eraser was cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface will be significantly larger than the surface which an eraser normally has. To illustrate the surface of the eraser is here calculated if the whole eraser is cut into cubes of 0.3 x 0.3 x 0.3 mm. This gives thus approx. 13 x 10 x 3 = 390 pieces of eraser, each with at surface of 0.3 x 0.3 x 6 = 0.54 cm², i.e. a total surface of 221 cm². For purposes of comparison, the eraser in uncut condition has a surface of ((3.8 x 3.1) + (3.1 x 1) + (3.8 x 1)) x 2 = 37.4 cm², i.e. nearly a factor 6 in difference. Therefore, the measured concentrations of DEHP are probably overestimated by a factor 6. If this is considered MoS will be 150, i.e. above 100 and without health risk but this may only apply if the child solely sucks the first cm of the eraser. If the child also chews the eraser or sucks a larger area the surface area from which DEHP can migrate will become larger and thus MoS can get under 100.
If the analysis error is taken into account – i.e. that the measured value in fact can be halved – this does not change much on MoS. A doubling of MoS will take place at a 50% reduction of the exposure.
All in all these conditions mean that it may be assumed to be hazardous to health for a child of 20 kg to suck or chew this eraser one hour daily for a longer period.
5.2.8 2-Heptanone
Application
2-heptanone is applied as indutrial solution, among others for resins and varnishes and is also applied as flavouring agent in foods (HSDB).
Identification
| Chemical name | 2-heptanone |
| CAS-No. | 110-43-0 |
| EINECS Nr. | 203-767-1 |
| Gross formula | C7H14O |
| Molecular structure |  |
| Molecular weight | 114.19 g/mol |
| Synonyms | Amyl methylketone Butylacetone Methyl amyl ketone Pentyl methyl ketone |
Physical-chemical data
| Physical form | Clear, colourless liquid with a mild fragrance of banana. | Chemfinder |
| Melting point | -35 °C (-31 °C according to Chemfinder) | TOXNET ChemIDplus |
| Boiling point | 151 °C (150 °C according to Chemfinder) | TOXNET ChemIDplus |
| Steam pressure | 3.86 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Weakly soluble 43 g/L | Chemfinder |
| Octanol water distribution coefficient (log KOW) | 1.98 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
R10 XN;R20/22 |
Inflammable. Harmful to health. Harmful by inhalation and if swallowed. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about the bioavailability of 2-heptanone is found but according to the Danish threshold list the substance can be absorbed through the skin for which reason 100% absorption is assumed in the calculations.
Effects on health
Only few information about the health effects of 2-heptanone is found. If nothing else is stated data are found through TOXNET’s HSDB database.
2-heptanone is classified as harmful by inhalation and if swallowed. LD50 (oral, rat) is 1,670 mg/kg (HSDB, TOXNET ChemIDplus).
2-heptanone is irritating for both skin and eyes and evaporations of the substance are also irritating for the mucous membranes.
Skin sensitization of 2-heptanone on humans has been examined on 26 voluntary persons. At a concentration of 4% in Vaseline no positive reactions were found.
During 13 weeks rats were fed with 0 (control), 20, 100 and 500 mg 2-heptanone/kg bw. At a dose of 500 mg an increase of the weight of liver and kidney was seen. At 100 mg similar effects were seen, just to a lesser extent. No serious effects were seen at 20 mg/kg bw.
In a reproduction study rats were exposed to up to 1000 ppm 2-heptanone. The effects were such as reductions in the food intake and changes in the body weight but no reproductive and development toxic effects.
2-heptanone has shown negative result in Ames test and other tests for mutagenic properties have also been negative.
Very few data are found on the carcinogenic properties of 2-heptanone but the few results indicate that 2-heptanone is not carcinogenic.
Threshold limits
The threshold limit in the working environment for 2-heptanone is 50 ppm or 238 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
2-heptanone is identified in one product through migration to artificial sweat.
| Product type | Product ID | Remark | Maximum measured concentrating in µg/g | |
| Migration artificial sweat | Headspace | |||
| School bag | 39C | 20 | ||
Assuming a NOAEL value based on the few data available for 2-heptanone on 20 mg/kg bw/day the following exposure for 2-heptanone in contact with skin is found where Tcontact (the duration of the exposure) is divided by a factor 4 as the measured migration concentrations are for 4 hours.
| Product type | Product ID | Maximum measured concentration in µg/g | Fabs | Wder | AREAder | Tcontact | BW | Uder | NOAEL | MoS |
| Migration artificial sweat | g/cm² | cm² | hours/day | kg | ug/kg bw/day | mg/kg bw/day | ||||
| School bag | 39C | 5 | 1.00 | 0.039 | 131 | 1 | 12 | 2.13 | 20.0 | 9,387 |
The calculated MoS for the examined product is far above 100 and therefore it is assessed that the exposure through contact with skin with 2-heptanone from the examined product does not constitute any health risk.
5.2.9 tert-Butyl alcohol
Application
tert-Butyl alcohol is especially important due to its properties as a solvent. It is applied to remove water from substances in the production of perfume (especially artificial musk), for recrystallization of chemicals and as denaturant in spirit. Furthermore, tert-butyl alcohol is applied as an intermediate in the production of other chemicals such as MTBE (methyl-tert-butyl ether) (IPCS, 1987; Jensen AA, 2003a).
Identification
| Chemical name | tert-Butanol |
| CAS-Nr. | 75-65-0 |
| EINECS Nr. | 200-889-7 |
| Gross formula | C4H10O |
| Molecular structure | 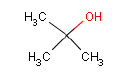 |
| Molecular weight | 74.12 g/mol |
| Synonyms | 1,1-Dimethylethanol 2-Methyl-2-propanol 2-methylpropan-2-ol t-Butylhydroxid t-Butylalkohol |
Physical-chemical data
| Physical form | Solid substance at normal room temperature. Has a strong smell. | Jensen AA, 2003a |
| Melting point | 25.4 °C (25.5 °C at Chemfinder) | TOXNET ChemIDplus |
| Boiling point | 82.4 °C | Chemfinder |
| Steam pressure | 40.7 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Easily soluble: 1000 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.35 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XN;R20 |
Highly flammable. Harmful to health. Harmful by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
tert-Butyl alcohol is easily absorbed through skin, lungs and gastrointestinal tract. It is easily decomposed in the body to carbon dioxide and water which thereafter are exhaled within 24 hours (Jensen AA, 2003a).
Effects on health
The acute toxicity of tert-butyl alcohol is low. Oral LD50 values for rats are found between 2733 and 3500 mg/kg bw (IUCLID, 2000i; IPCS, 1987). tert-Butyl alcohol has a narcotic effect (about 1½ times higher compared to ethanol). Toxic symptoms are headache, dizziness, nausea, sleepiness and drowsiness (Jensen AA, 2003a; IPCS, 2003).
tert-Butyl alcohol is degreasing on the skin and can cause contact eczema. Skin drugs based in tert-butyl alcohol has caused contact allergy (Jensen AA, 2003a).
Not much information about effective values for tert-butyl alcohol is found in the literature. IUCLID refers to a test with mice getting doses of tert-butyl alcohol through the drinking water for 90 days. The effects at the high doses were among others ataxia (loss of full control of bodily movements), loss of weight and hyperactivity). NOEL for the direct chemical effects was set to 1566 mg/kg bw/day for male mice and 4363 mg/kg bw/day for female mice (IUCLID, 2000i).
tert-Butyl alcohol is found inactive in Ames test as well as in many other short-term tests and thus it has no mutagenic properties (Jensen AA, 2003a; IUCLID, 2000i).
Only a few studies regarding the properties of the substance being toxic to reproduction are available. Inhalation of high concentrations being toxic for the female animal resulted in damages to foetus (Jensen AA, 2003a).
The carcinogenic properties are investigated in a long-term study with mice and rats exposed to tert-butyl alcohol through the drinking water for 2 years. Some indications of carcinogenic effect in male rats at 420 mg/kg bw and female mice at 2110 mg/kg bw were found but no indications in female rats. In male mice an ambiguous indication of carcinogenic effect was found (NTP, 1995).
Threshold limits
The threshold limit in the working environment of tert-butyl alcohol is 50 ppm or 150 mg/m³ with the remarks L and H, i.e. that the threshold limit is a limit value (L) which is not allowed to be exceeded and that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
tert-Butyl alcohol is identified in one product exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 10 | 0.09 | ||
No acute NOAEL of tert-butyl alcohol is identified for which reason a long-term scenario for exposure to the substance by inhalation through school bags and pencil cases are solely conducted. A NOAEL value of 1566 mg/kg bw/day is found based on a study of 90 days but at the same time indications of carcinogenic effect in male rats at 420 mg/kg bw are found in a two-years’ study. However, no NOAEL based on this long-term study is specified. In the calculations the 420 mg/kg bw/day is applied knowing that no NOAEL value is specified.
| Pro- duct type |
Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hour/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 10 | 0.09 | 1 | 0.35 | 105 | 6 | 12 | 20 | 0.08 | 420 | 5,140,562 |
The calculated MoS of the examined product is far above 100 and therefore it is assessed that the exposure through contact with skin with med tert-butyl alcohol of the examined product does not constitute any health risk even if it is not a real NOAEL value being used for the calculation of MoS.
5.2.10 Methyl propionate
Application
Propionates are permitted as additive in foods and is applied as aromatic compound (for instance in pastry, sweets and ice cream). The substance is naturally found in some fruits such as kiwi fruits and some strawberries as well as in shellfish (mussels). Furthermore, methyl propionate is applied as solvent in for instance paints and varnishes (HSDB).
Identification
| Chemical name | Propane acid, methyl ester |
| CAS-Nr. | 554-12-1 |
| EINECS Nr. | 209-060-4 |
| Gross formula | C4H8O2 |
| Molecular structure | 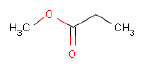 |
| Molecular weight | 88.11 g/mol |
| Synonyms | Methyl propionate Methyl propylate |
Physical-chemical data
| Physical form | Flammable colourless liquid | Chemfinder. HSDB. |
| Melting point | -87.5 °C | TOXNET ChemIDplus |
| Boiling point | 79.8 °C | TOXNET ChemIDplus |
| Steam pressure | 84 mmHg at 25 °C | TOXNET ChemIDplus |
| Water solubility | 62.4 g/L at 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 0.84 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord. 923, 2005) |
F;R11 XN;R20 |
Highly flammable. Harmful to health. Harmful by inhalation. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
No information about the bioavailability of methyl propionate is found.
Effects on health
Only few information about the health effects of methyl propionate is found. If nothing else is stated data are found through the HSDB database of TOXNET.
Methyl propionate has a low toxicity at intake. LD50 value for rats through oral intake is 5,000 mg/kg bw. Effects such as ataxia (loss of full control of bodily movements), gasping breathing and hypothermia (cooling of the body) are seen at mortal levels. The dermal toxicity of the substance is low. The substance is harmful to health by inhalation.
No reports on any toxic effects of methyl propionate at humans are found.
Methyl propionate is a highly flammable liquid which is skin irritating.
No information about possible carcinogenic, reproduction or mutagenic effects of the substance is found.
Threshold limits
The Danish Working Environment Authority has set no threshold limit for the substance (The Danish Working Environment Authority, 2005).
Assessment
Methyl propionate is identified in one product, exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 4 | 0.05 | ||
No information is found about tests having identified a NOAEL value of methyl propionate for which reason no exposure calculations of the substance are conducted.
5.2.11 p-xylene
Application
p-Xylene (a mixture of ortho-, meta- and para-xylene) is one of the most important solvents. p-Xylene is used in the production of among others dimethyl etherphthalate (IPCS, 1997). In Denmark xylene is primarily applied as solvent and diluents in paint, varnish, remover, stain remover, printing inks, ink, drain cleaner, nail polish etc. (Jensen AA, 2003b).
Identification
| Chemical name | p-xylene |
| CAS-Nr. | 106-42-3 |
| EINECS Nr. | 203-396-5 |
| Gross formula | C8H10 |
| Molecular structure |  |
| Molecular weight | 106.12 |
| Synonyms | 1,4-Dimethylbenzene 1,4-Xylene Benzene, 1,4-dimethyl- Chromar |
Physical-chemical data
| Physical form | Clear liquid, with characteristic sweet aromatic smell. | Chemfinder, Jensen AA, 2003b |
| Melting point | 13.2 °C | TOXNET ChemIDplus |
| Boiling point | 138.3 °C | TOXNET ChemIDplus |
| Steam pressure | 8.84 mm Hg at 25 °C | TOXNET ChemIDplus |
| Water solubility | Slightly soluble: 0.162 g/Lat 25 °C | TOXNET ChemIDplus |
| Octanol water distribution coefficient (log KOW) | 3.15 | TOXNET ChemIDplus |
Classification
| The list of dangerous substances (Stat. Ord 923, 2005) |
R10 XN;R20/21 XI;R38 |
Flammable. Harmful to health. Harmful by inhalation and in contact with skin. Local irritating. Irritates the skin. |
| The list of undesirable substances (The Danish Environmental Protection Agency, 2004) The Danish EPA Self classification (The Danish Environmental Protection Agency, 2001) |
No No |
Bioavailability
Evaporations of p-xylene are largely absorbed (60-70%) through the lungs. In fluid form the substance is quickly absorbed from the gastrointestinal tract and it penetrates easily through the skin (ATSDR, 1995; Jensen AA, 2003b).
The absorbed p-xylene is quickly spread in the body with the blood, especially to bone marrow, brain, spleen and fatty tissue. The main part of the absorbed p-xylene is separated during a few hours with the urine. A smaller part, approx. 5%, is eliminated unchanged with the air inhaled. The part of the absorbed xylene being spread to the fat deposits is eliminated more slowly (half-life 2-4 days) (Jensen AA, 2003b).
Effects on health
The acute toxicity of p-xylene is low. LD50 values for rats at oral intake are between > 3400 and 4779 mg/kg bw (IUCLID, 2000j). Examples of serious acute intoxications with deaths after exposure to very high concentration (10,000 ppm) in the air at work with cleaning of tanks with leftovers of p-xylene are seen (Jensen AA, 2003b).
Symptoms of an acute intoxication are fatigue, foam at the mouth, visual disturbances, uncoordinated movements, muscular spasms, paralysis, unconsciousness and coma. Damages on heart, liver and kidneys may occur. Alcohol strengthens the toxicity of p-xylene (Jensen AA, 2003b).
At direct contact with the skin p-xylene is degreasing and irritating (IUCLID, 2000j). At direct exposures to the eyes severe burns on the cornea are seen. A few minutes’ exposure to p-xylene in a concentration of 200 ppm results in irritation of eyes, nose and throat (Jensen AA, 2003b).
Exposure during a long period to p-xylene may result in the so-called painter’s syndrome where the effects are unnatural fatigue during the day, sleep problems during the night, headache, amnesia, irritability and other personality changes (Jensen AA, 2003b).
The National Research Centre for the Working Environment assesses that p-xylene leads to a high risk of permanent and severe damages on the nervous system even at normal work with the substances. A number of animal tests indicate that p-xylene and its isomers have a neurotoxic effect at exposure by inhalation. Effects observed at the animals are among others trembling, muscular spasms, strenuous breathing, hearing loss etc. after inhalation of p-xylene (ATSDR, 2005).
No specific knowledge about the reproduction effects of p-xylene in humans is available but a number of population studies indicate that exposure to solvents can cause damages to foetus and an increased number of spontaneous abortions. p-Xylene is easily transported with the blood from mother to foetus through the placenta. Exposure to p-xylene in a concentration which affected the female animal generated an increased foetus mortality, an impaired growth and development of the foetus as well as foetus malformations in tests with pregnant mice and rats (ATSDR, 2005; Jensen AA, 2003b).
p-Xylene is tested negative in Ames test as well as in a number of other short-term tests for mutagenic effects (IUCLID, 2000j; Jensen AA, 2003b).
IARC assesses that p-xylenes are non-classifiable in relation to the carcinogenic properties of the substance in humans. There are insufficient indications of the carcinogenic properties of p-xylenes in both humans and animals (IARC, 1999). Long-term tests with mice and rats, where the animals got technical p-xylene in doses up to 1 mg/kg bw/day orally for two years, have not shown any carcinogenic effects (NTP, 1986b; Jensen AA, 2003b).
A NOAEL value of 500 mg/kg bw/day is found for p-xylenes for reproductive, neurological and other systemic effects in rats in a test of two-year’ duration (ATSDR, 2005; NTP, 1986b).
Threshold limits
The threshold limit in the working environment for p-xylene is 25 ppm or 109 mg/m³ with the remark H, i.e. that the substance can be absorbed through the skin (The Danish Working Environment Authority, 2005).
Assessment
p-Xylene is identified in one product exclusively through headspace.
| Product type | Product ID | Remark | Maximum measured concentration in µg/g | |
| Migration artificial sweat | Headspace | |||
| Eraser | 3 | 0.01 | ||
The identified NOAEL value is based on long-term effects for which reason a long-term scenario for exposure to the substance by inhalation through the eraser is conducted. The following exposure for p-xylene by inhalation is found:
| Product type | Pro- duct ID |
Maxi- mum mea- sured concen- tration in µg/g |
Fresp | IHair, long | Q weight of the product | Tcontact | BW | Vroom, long | Iinh, long | NOAEL | MoSlong |
| Head- space |
m³/hour | g | hours/ day |
kg | m³ | ug/kg bw/day | mg/kg bw/day | ||||
| Eraser | 3 | 0.01 | 0.7 | 0.35 | 900 | 6 | 12 | 20 | 0.27 | 625 | 11,474, 469 |
The calculated MoS of the examined product are far above 100 and therefore it is assessed that the exposure by inhalation of p-xylene from the examined product does not constitute any health risk.
5.3 Total assessment
A risk assessment of content of the following 11 substances has been conducted. These substances are identified through headspace (i.e. evaporate from the products) and/or through migration to artificial sweat or artificial saliva (for one single eraser):
- Isophorone
- BHT
- Cyclohexanone
- Phenol
- Toluene
- DIBP
- DEHP
- 2-Heptanone
- tert-Butyl alcohol
- Methyl propionate
- p-Xylene
In general, the content of the above-mentioned in the examined products does not constitute any health risk; neither in the individual products nor if children are exposed to several products at one time – for instance through use of pencil case, eraser and school bag and at exposure both by inhalation and through migration to artificial sweat.
However, for BGT applies that Margin of Safety for a single scenario and a single product – an eraser – is fairly close to 100 (is 163). In this case it is a rather large eraser of 4 x 1.3 x 11 cm and in the calculations it is assumed that the child sucks and chews the whole eraser. Furthermore, the erasers for the analyses are cut into small pieces (cubes) with a width of 2-3 mm. This means that the surface is significantly larger than the surface that an eraser normally has. The measured concentrations can thus be overestimated by a factor 8.
All in all these conditions mean that exposure to BHT from several products at the same time and through several ways of exposure may not constitute any risk for the examined products. However, it is unknown whether other products can have a larger content of BHT and thus constitute a health problem if a child is exposed to several products with a high content of BHT. As BHT is much applied as an anti-oxidant in foods there is a possibility of exposure through other sources. The total exposure is not assessed in this project.
The total amounts in selected erasers are analyzed for DEHP. Based on these results a scenario is calculated where it is assumed that a little piece of an eraser of between 0.008 and 0.1 g is swallowed. For the calculations it is assumed that a little piece of eraser of between approx. 1.9 x 1.9 x 1.9 mm and 4.3 x 4.3 x 4.3 mm is ingested. In this scenario Margin of Safety is significantly below 100 (based on NOAEL value for effects being toxic to reproduction). Thus it is clear that repeated eating of eraser may cause serious health effects.
Furthermore, a scenario is assessed where a school child sucks an eraser for 1 hour daily. The calculations are conducted for the eraser with the highest content of DEHP. The calculations show that it can constitute a health risk daily to suck an eraser with a high content of DEHP during a long period.
In general, the calculation is based on the analyzed values for a few selected school bags, toy bags, pencil cases and erasers. It cannot be rejected that there are products with a higher content than the content found in the products examined in this project. Furthermore, there may be other sources to the same chemical substances in the child’s surroundings which will contribute to the total exposure.
6 References
ATSDR, 1989. ”Toxicological profile for Isophorone”. Agency for Toxic Substances and Disease Registry (ATSDR). US Public Health Service in collaboration with US Environmental Protection Agency (EPA), December 1989. http://www.atsdr.cdc.gov/toxprofiles/tp138.pdf
ATSDR, 2000. “Toxicological profile for Toluene”. Agency for Toxic for Toxic Substances and Disease Registry (ATSDR). U.S. Department of Health and Human Services. September 2000. http://www.atsdr.cdc.gov/toxprofiles/tp56.pdf.
ATSDR, 2005. “Draft toxicological profile for Xylene”. Agency for Toxic for Toxic Substances and Disease Registry (ATSDR). U.S. Department of Health and Human Services. September 2005. http://www.atsdr.cdc.gov/toxprofiles/tp71.pdf
Borch J. et al, 2005. ”Diisobutyl ftalate has comparable anti-drogenic effects to di-n-butyl ftalate in feral rat testis”, Borch J, Axelstad M, Vinggaard AM, Dalgaard M, Danish Institute for Food and Veterinary Research. Toxicology Letters (2006). Article in press. Accepted 20 October 2005.
Bremmer and van Veen, 2002. “Children's toys fact sheet. To assess the risks for the consumer”. Bremmer HJ, van Veen MP. RIVM report 612810012/2002. National Institute of Public Health and the Environment, Bilthoven, The Netherlands. As described in (Svendsen et al, 2006).
Chemfinder. Chemfinder.com – Database & Internet searching. http://chemfinder.cambridgesoft.com
The Danish Environmental Protection Agency, 2000. ”Kemikalier i tekstiler”. Larsen HF et al, DHI – Institut for Vand og Miljø, Dansk Toksikologi Center, Teknologisk Institut. Miljøprojekt nr. 534, 2000. The Danish Environmental Protection Agency.
The Danish Environmental Protection Agency, 2001. ”Rapport om vejledende liste til selvklassificering af farlige stoffer”. Miljøprojekt nr. 635, 2001. The Danish Environmental Protection Agency.
The Danish Environmental Protection Agency, 2003. “Kortlægning af kemiske stoffer i tekstilmetervarer”. Laursen SE et al, Teknologisk Institut. Kortlægning af kemiske stoffer i forbrugerprodukter nr. 23, 2003. The Danish Environmental Protection Agency.
The Danish Environmental Protection Agency, 2004. ”Listen over uønskede stoffer 2004”. Orientering fra Miljøstyrelsen nr. 8, 2004. The Danish Environmental Protection Agency.
The Danish Environmental Protection Agency, 2005. “Kortlægning af læbeplejeprodukter med duft, smag m.v.”. Jette Rud Larsen & Rikke D. Holmberg. Kortlægning af kemiske stoffer i forbrugerprodukter. Nr. 55, 2005. The Danish Environmental Protection Agency.
The Danish Working Environment Authority, 2005. “Grænseværdier for stoffer og materialer”. At-vejledning, Stoffer og materialer – C.0.1. April 2005 – replaces October 2002. The Danish Working Environment Authority.
Dutch Institute for the Working Environment, 1991. Dutch Institute for the Working Environment; Dutch Chemical Industry Association; Chemical safety sheets: Working safely with hazardous chemicals. Dordrecht: Samson Chemical Publishers; 1991. As described in (Pors og Fuhlendorff, 2002).
EC DG Env., 2000. ”Towards the establishment of a priority list of substances for further evaluation in their role in endocrine disruption” – preparation of a candidate list of substances as a basis for priority setting”. Final report (Incorporating corrigenda to final report dated 21 June 2000). European Commission DG Environment. BKH Consulting Engineers, Delft, The Netherlands in association with TNO Nutrition and Food Research, Zeist, the Netherlands. Delft, 10 November 2000. http://www.edenresearch.info/public/bkh_main.pdf
EU ECB, 2003. “European Union Risk Assessment Report. Toluene.“ European Commission. Joint Research Centre. European Chemicals Bureau. Institute for Health and Consumer Protection. 2nd. Priority List. Vol. 30. 2003.
EU ECB, 2006a. “European Union Risk Assessment Report. Phenol.“ European Commission. Joint Research Centre. European Chemicals Bureau. Institute for Health and Consumer Protection. 1st. Priority List. Vol. 64. 2006.
EU ECB, 2006b. “European Union Risk Assessment Report. Bis(2-ethylhexyl) ftalate”. Draft of March 2006. European Commission. Joint Research Centre. European Chemicals Bureau. Institute for Health and Consumer Protection.
European Commission, 2003. “Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances. Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances. Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market”. Part I. European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau, 2003.
Green, 2002. “Mouthing times among young children from observational data”, Green MA, 2002. Division of Hazard Analysis, US Consumer Product Safety Commission (www.cpsc.gov). Washington DC, USA. As described in (Svendsen et al, 2006).
HSDB. Hazardous Substance Data Bank. TOXNET. Extraction from HSDB for the different substances on CAS-no. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
IARC, 1986. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. “Some Naturally Occuring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation”. Volume 40 (p. 161). 1986. World Health Organization. International Agency for Research on Cancer.
IARC, 1989. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. “Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting.” Volume 47 (p. 157). 1989. World Health Organization. International Agency for Research on Cancer.
IARC, 1999. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. “Re-Evaluation of Some Organic Chemicals, Hydrozine and Hydrogen Peroxide”. Volume 71 (p. 829 + 1189). 1999. World Health Organization. International Agency for Research on Cancer.
IARC, 2000. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. “Some Industrial Chemicals.” Volume 77 (p. 41). 2000. World Health Organization. International Agency for Research on Cancer.
IPCS, 1985. “Toluene”. Environmental Health Criteria 52. IPCS Inchem, International programme on chemical safety. WHO. Geneva, 1985.
IPCS, 1987. “t-Butanol. Health and safety guide”. Health and Safety guide No. 7. IPCS International Programme on Chemical Safety. WHO, Geneva, 1987.
IPCS, 1992. ”Diethylhexyl ftalate”. Environmental Health Criteria 131. IPCS Inchem, International programme on chemical safety. WHO, Geneva, 1992.
IPCS, 1995. ”Isophorone”. Environmental Health Criteria 174. IPCS Inchem, International programme on chemical safety. WHO, Geneva, 1995.
IPCS, 1997. ”Xylenes”. Environmental Health Criteria 190. IPCS Inchem, International programme on chemical safety. WHO, Geneva, 1997.
IUCLID, 2000a. “IUCLID Datasheet. Substance ID: 1314-13-2, Zink oxide”, European Commission – European Chemicals Bureau. Creation data 18.2.2000.
IUCLID, 2000b. “IUCLID Datasheet. Substance ID: 13463-67-7, Titanium dioxide”, European Commission – European Chemicals Bureau. Creation data 18.2.2000.
IUCLID, 2000c. “IUCLID Datasheet. Substance ID: 108-94-1, Cyclohexanone”, European Commission – European Chemicals Bureau. Creation data 18.2.2000.
IUCLID, 2000d. ”IUCLID Datasheet, Substance ID: 78-59-1, 3,5,5-trimethylcyclohex-2-enone”. Creation date 19.2.2000. European Commission, European Chemicals Bureau.
IUCLID, 2000e. ”IUCLID Datasheet, Substance ID: 1289-37-0, 2,6-di-tert-butyl-p-cresol”. Creation date 18.2.2000. European Commission, European Chemicals Bureau.
IUCLID, 2000f. ”IUCLID Datasheet, Substance ID: 84-69-5, Diisobutyl ftalate”. Creation date 19.2.2000. European Commission. European Chemicals Bureau.
IUCLID, 2000g. ”IUCLID Datasheet, Substance ID: 117-81-7, bis(2-ethylhexyl) ftalate”. Creation date 18.2.2000. European Commission. European Chemicals Bureau.
IUCLID, 2000h. ”IUCLID Datasheet, Substance ID: 108-94-1, cyclohexanone”. Creation date 18.2.2000. European Commission. European Chemicals Bureau.
IUCLID, 2000i. ”IUCLID Datasheet, Substance ID: 75-65-0, 2-methylpropan-2-ol”. Creation date 19.2.2000. European Commission. European Chemicals Bureau.
IUCLID, 2000j. ”IUCLID Datasheet, Substance ID: 106-42-3, p-xylene”. Creation date 18.2.2000. European Commission. European Chemicals Bureau.
Jensen AA, 1997a. ”Fokus på farlige stoffer i arbejdsmiljøet nr. 43. Isophoron”, Jensen AA. Updated 08/96. The Working Environment Fund 1997.
Jensen AA, 1997b. ”Fokus på farlige stoffer i arbejdsmiljøet nr. 30. Phenol”, Jensen AA. Updated 08/96. The Working Environment Fund 1997.
Jensen AA, 1997c. ”Fokus på farlige stoffer i arbejdsmiljøet nr. 16. Toluen”, Jensen AA. Updated 08/96. The Working Environment Fund 1997.
Jensen AA, 1997d. ”Fokus på farlige stoffer i arbejdsmiljøet nr. 32. Ftalater”, Jensen AA. Updated 08/96. The Working Environment Fund 1997.
Jensen AA, 2003a. ”Fokus på farlige stoffer nr. 53. Butylalkoholer”, Jensen AA. Complete and partly updated/revised 5 January 2003. ”Det ny ABF”.
Jensen AA, 2003b. ”Fokus på farlige stoffer nr. 28. Xylener”, Jensen AA. Complete and partly updated/revised 5 January 2003. ”Det ny ABF”.
Jensen AA, 2003c. ”Fokus på farlige stoffer nr. 58. Cyclohexanoner”, Jensen AA. Complete and partly updated/revised 5 January 2003. ”Det ny ABF”.
Kiss, 2001. “A mouthing observation study of children under 6 years of age”, Kiss CT, 2001. Division of human factors. US Consumers Product Safety Commission (www.cpsc.gov). As described in (Svendsen et al, 2006).
Merck, 1983. The Merck Index, Whitehouse Station, NJ, USA: Windholz M. Merck and Co., Inc(ed) 1983. 215-216, No. 1521: Butylated Hydroxytoluene. As described in (SIDS, 2002).
Netdoktor, 2006. ”Drenges højde og vægt i forhold til alder” and ”Pigers højde og vægt i forhold til alder”. Found on the homepage of Netdoktor in October, 2006. http://www.netdoktor.dk/boern/fakta/drengevaeksttabel.htm and http://www.netdoktor.dk/Sunderaad/fakta/pigevaeksttabel.htm.
Nilsson NH et al, 2006. ”Kortlægning og sundhedsmæssig vurdering af kemiske stoffer i sexlegetøj”. Kortlægning af kemiske stoffer i forbrugerprodukter nr. 77. The Danish Environmental Protection Agency. 2006.
Nordic Council of Ministers, 1997. ”Health effects of selected chemicals to the environment – 4: Summaries and classification proposals”. Copenhagen: Nordisk Ministerråd, 1997. Vol TemaNord 1997:605CP – the environment. As described in (Pors og Fuhlendorff, 2002).
NTP, 1986a. ”Toxicology and carcinogenesis studies of Isophorone (CAS No. 78-59-1) in F344/N Rats and B6C3F1 mice (gavage studies)”. National Toxicology Program, Technical Reports Series No. 291. US Department of Health and Human Services, Public Health Services, National Institutes of Health.
NTP, 1986b. “Toxicology and Carcinogenesis Studies of Xylenes (Mixed) (60% m-Xylene, 14% p-Xylene, 9% o-Xylene, and 17% Ethylbenzene) (CAS No. 1330-20-7) in F344/N Rats and B6C3F1 Mice (Gavage Studies)”. National Toxicology Program, Technical Report No. 327. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health.
NTP, 1995. “Toxicology and Carcinogenesis Studies of t-Butyl Alcohol (CAS No. 75-65-0) in F344/N Rats and B6C3F1 Mice”. National Toxicology Program, Technical Report No. 436. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health.
OECD SIDS, 2002. ”SIDS Initial Assessment Report For SIAM 14”. 2,6-di-tert-butyl-p-cresol (BHT) CAS No. 128-37-0. UNEP Publication. Paris, France, 2002.
OECD SIDS. ”Cyclohexanone CAS No. 108-94-1. UNEP Publication.
Pors og Fuhlendorff, 2002. Pors, J., Fuhlendorff, R. “Kortlægning af kemiske stoffer i hygiejnebind”. Kortlægning nr. 13, 2002. The Danish Environmental Protection Agency, 2002.
Stat. Ord. 1012, 2000. ”Bekendtgørelse om forbud mod import og salg af produkter, der indeholder bly”. BEK 1012 af 13.11.2000. The Danish Ministry of the Environment.
Stat. Ord. 1074, 2006. ”Bekendtgørelse om ændring af bekendtgørelse om forbud mod ftalater i legetøj og småbørnsartikler”. BEK nr. 1074 af 3.11.2006. The Danish Ministry of the Environment.
Stat. Ord. 1199, 1992. ”Bekendtgørelse om forbud mod salg, import og fremstilling af cadmiumholdige produkter”. BEK 1199 af 23.12.1992. The Danish Ministry of the Environment.
Stat. Ord. 151, 1999. ”Bekendtgørelse om forbud mod ftalater i legetøj til børn i alderen 0-3 år samt i visse småbørnsartikler m.v.”. BEK nr. 151 af 15.3.1999. The Danish Ministry of the Environment and Energy.
Stat. Ord. 329, 2002. ”Bekendtgørelse om klassificering, emballering, mærkning, salg og opbevaring af kemiske stoffer og produkter”. BEK nr. 329 af 16.5.2002. The Danish Ministry of the Environment.
Stat. Ord. 627, 2003. ”Bekendtgørelse om forbud mod import, salg og eksport af kviksølv og kviksølvholdige produkter”. BEK nr. 627 af 01.07.2003. The Danish Ministry of the Environment.
Stat. Ord. 786, 2006. ”Bekendtgørelse om forbud mod ftalater i legetøj og småbørnsartikler”. BEK nr. 786 af 11.7.2006. The Danish Ministry of the Environment.
Stat. Ord. 923, 2005. ”Bekendtgørelse om listen over farlige stoffer”. BEK nr. 923 af 28.09.2005. The Danish Ministry of the Environment.
Stat. Ord. 975, 2002. ”Bekendtgørelse om ændring af bekendtgørelse om forbud mod ftalater i legetøj til børn i alderen 0-3 år samt i visse småbørnsartikler m.v.”. BEK nr. 975 af 27.11.2002. The Danish Ministry of the Environment.
Svendsen et al, 2006. “Kortlægning og afgivelse af kemiske stoffer I “slimet” legetøj”. Svendsen N, Pedersen SF, Berth N, Pedersen E, Hansen OC, Teknologisk Institut, Kortlægning af kemiske stoffer i forbrugerprodukter, nr. 67, The Danish Environmental Protection Agency, 2006.
TOXNET ChemIDplus. Udtræk fra TOXNETs ChemIDplus database for de forskellige stoffer på CAS-nr. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CHEM
Appendix A List of Internet pages of special interest and products distributed in this way
The following Internet pages are of special interest:
http://www.mst.dk/udgiv/Publications/2005/87-7614-668-5/html/default_eng.htm
http://www.motto.dk/diddl-dk/Produkter/produktliste_tasker.htm
Sale on the Internet
From www.leg-hoppy.dk:
School bags: JEVA skoletasker, Spiderman Rygsæk (for school, sport and leisure), Witch Rygsæk (or school, sport and leisure), Manchester United Rygsæk,
Pencil cases: Spiderman Penal Rund, Penal Peter Plys, Penal Peter Plys med hoved.
Toy pencil cases: Sparkle Unicorn Skrivesæt med Penal.
Toy bags: BabyBorn Pusletaske Rygsæk, Baby Annabell Taske med musik, Sparkle Unicorn Perletaske, Lægetaske Little Doktor (from 3 years), Postmand Per cykeltaske med albue-/knæbeskyttere og drikkedunk (from 3 years)
From www.disney.dk: http://www.disneystore.co.uk :
Toy bags: Sleeping Beauty Mini Doll Crown Handbag Set ( 3+ years), Minnie Nurses Set (not suitable for children below 3 years due to small parts), Marie Small Vet Kit, Power Rangers Skateboard, Pads & Bag Set, Mickey Builders Box Set, Minnie 'Fresh' Stamp Set, Cinderella Girls Blue Handbag, Little Mermaid 'Summer Time' Backpack, W.I.T.C.H Denim & Flower Girls Magazine Bag, Buzz with Canteen Swim Bag, Dash Swim Bag, Mickey Nightmare Tote Bag, Mickey Messenger Bag, Mickey Surfer Boys Backpack, Stitch Backpack, Power Rangers Swim Backpack, W.I.T.C.H Colourful Girls Shoulder Bag, Fairies Flower Girls Backpack, Fairies Girls Straw Bag, Minnie Fruit Girls Straw Bag.
Pencil cases: Narnia Pencil Case, Little Mermaid Stationery Set (penal, viskelæder mm.), Fairies Stationery Set (tin penal, viskelæder mm.), Stitch Triple Filled Pen Case (3+ years), Nemo Turquoise Triple Filled Pen Case (3+ years) (is available with several motives): Power Rangers, Fairies, Cinderella, Marie), Little Mermaid Small Filled Pencil case, Eeyore Double Filled Pen Case.
Toy pencil case: Fairies Mini Stationery Set (“penal” viskelæder mm.).
From www.diddl.dk
Here a large product programme is presented with many school bags and pencil cases in polyester as well as pencil cases in metal and plush. Furthermore, toy bags in the form of cosmetics bag and cosmetics purse as well as a set of suitcases.
Diddl erase pen in different types and Diddl erasers in different types.
The following information comes from inquiry to the retail trade:
NEYE:
School bags: JEVA, Panino, EASTPAK, Disney, Lego, Ticket to Heaven and JanSport.
Bog&Ide:
School bags: Bratz og Justic League, Diddl (producted of water-repellent polyester and all materials are “AZO-free”). The Lego bags are produced without use of nickel and harmful AZO colouring agents.
Pencil cases: Diddl handbag pencil case, Diddl nylon pencil case, Diddl pencil case with content. Lego pencil cases with content (several types).
Producers
JEVA:
All the JEVA products are produced of PVC-free nylon and polyester.
JEVA produces the series Tessa for kindergarten and leisure. In the range Shopper bag with handle, Accesorize-penal, Posh handbag, Niña-bagpack, Chica-bagpack are available.
A range of miniature bagpacks for use in the pre-school age is produced. Furthermore, a school bag programme for different levels of ages divided into a programme for boys and a programme for girls is available. For each programme there is a pencil case.
JEVA A/S is a Danish company with proud traditions which have produced, marketed and sold school bags, bagpacks, travel bags and accessories since the early seventies.
JEVA A/S,Langgade 2, DK-8350 Hundslund, Tel.: +45 8655 0100
Fax: + 45 8655 0462, E-mail: info@jeva.dk
Lego:
“LEGO® BAGS is a school and leisure time bag program for active girls and boys between 3 and 10 years. The bags are designed to meet all expectations regarding durability, function, comfort, ergonomics and safety”.
There are 7 series. All with 2 matching pencil cases (except for the series directing at the smallest children, it has no penal).
Eastpack:
The home page gave no information about materials.
Disney:
The home page gave no information about materials at first.
Ticket to Heaven:
The home page does not mention bags.
JanSport:
The home page informs that the material is ribstop with a ballistic bottom.
Diddl:
The home page informs that products are of water-repellent polyester and that all materials are ”AZO-free”.
Bratz:
The home page does not mention the bags.
Appendix B List of procured products
| No | Description | Remark |
| 1 | Erasers | |
| 2 | Erasers | |
| 3 | Eraser | Smells pleasantly |
| 4 | Eraser | Smells pleasantly |
| 5 | Eraser | |
| 6 | Eraser | Danger labelled for children under 3 years |
| 7 | Eraser | |
| 8 | Eraser | Conform to ASTM 4266 and EN-71 |
| 9 | Eraser | |
| 10 | Eraser | |
| 11 | Eraser | Danger labelled for children under 3 years |
| 12 | Eraser | Danger labelled for children under 3 years |
| 13 | Eraser | Danger labelled for children under 3 years |
| 14 | Eraser | Danger labelled for children under 3 years |
| 15 | Eraser | Danger labelled for children under 3 years |
| 16 | Pencil case | Danger labelled for children under 3 years |
| )17 | Eraser | |
| 18 | Eraser | |
| 19 | Eraser | |
| 20 | Eraser | |
| 21 | Eraser | |
| 22 | Eraser | |
| 23 | Eraser | |
| 24 | Eraser | Danger labelled for children under 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard |
| 25 | Eraser | Smells pleasantly |
| 26 | Pencil case | |
| 27 | Eraser | |
| 28 | Eraser | |
| 29 | Toy bag | |
| 30 | Toy bag | Danger labelled for children under 3 years |
| 31 | Pencil case | |
| 32 | Toy bag | |
| 33 | Toy bag | |
| 34 | Pencil case | Danger labelled for children under 3 years |
| 35 | Pencil case | |
| 36 | Pencil case | |
| 37 | Toy bag | Danger labelled for children under 3 years. This product conforms the safety reqiurements of ASTM F963 |
| 38 | Toy bag | This bag is not toy keep away from babies |
| 39 | School bag | |
| 40 | School bag | Not for children under 3 years |
| 41 | School bag | |
| 42 | School bag | Not for children under 3 years |
| 43 | Pencil case |
Appendix C List of completed analyses
Appendix D Beilstein, FT-IR and XRF analysis results from screening
| No | Description | Remark | Beilstein +/- | FT-IR analysis results | Content acc. to XRF |
| 1 | Erasers | - | |||
| 2 | Erasers | - | |||
| 3 | Eraser | Smells pleasantly | Eraser: + | Eraser: PVC with phthalate and chalk | Eraser: Cl (PVC) with Ca (chalk) |
| Case: - | Case: not examined | ||||
| 4 | Eraser | Smells pleasantly | - | Ca (chalk) | |
| 5 | Eraser | Eraser: + | Eraser: PVC with phthalate | ||
| Case: - | Case: not examined | ||||
| 6 | Eraser | Danger labelled for childen under 3 years | Black part: + | Black part of penguin: Not examined | |
| The rest: - | The rest: not examined | ||||
| 7 | Eraser | - | |||
| 8 | Eraser | Conform to ASTM 4266 and EN-71 | - | ||
| 9 | Eraser | Eraser: + | Eraser: PVC with phthalate and chalk | Eraser: Cl (PVC) with Ca (chalk), Silicium | |
| Case: - | Case: not examined | ||||
| 10 | Eraser | - | Liquid paraffin and lots of chalk | Chalk | |
| 11 | Eraser | Danger labelled for childen under 3 years | Eraser: - | Eraser: not examined | |
| Case: + | Case: not examined | ||||
| 12 | Eraser | Danger labelled for childen under 3 years | - | PVC with phthalate and chalk | Cl (PVC )wit a little Ca (chalk), Cu (pigment) |
| 13 | Eraser | Danger labelled for childen under 3 years | + | Eraser: PVC with phthalate and chalk | |
| 14 | Eraser | Danger labelled for childen under 3 years | + | PVC with phthalate and chalk | Cl (PVC) with Ca (chalk), Titanium(perhaps titaniumdioxide (pigment)) |
| 15 | Eraser | Danger labelled for childen under 3 years | + | Eraser: PVC with phthalate | |
| 16 | Pencil case | Danger labelled for childen under 3 years | + | Gray: PVC with phthalate. White: Polyester textile (PET) |
|
| 17 | Eraser | - | |||
| 18 | Eraser | - | |||
| 19 | Eraser | - | |||
| 20 | Eraser | - | |||
| 21 | Eraser | - | |||
| 22 | Eraser | + | Eraser: PVC with phthalate and chalk. | ||
| 23 | Eraser | + | Eraser: PVC with phthalate and chalk. | ||
| 24 | Eraser | Danger labelled for children under 3 and 5 years. Non toxic. Do not swallow. Warning: Chocking Hazard | - | Isobuten Isoprene rubber | Rubber, Silicium |
| 25 | Eraser | Smells pleasantly | - | ||
| 26 | Pencil case | + | Polyester Polyurethane (PUR) | ||
| 27 | Eraser | - | Ca (chalk) | ||
| 28 | Eraser | - | |||
| 29 | Toy bag | + | |||
| 30 | Toy bag | Danger labelled for childen under 3 years | Red plastic (A):+ | A: | A: Cl (PVC) with Zn (heat stabilizer), too high Ba content |
| Clear plastic (B): + | B: PVC with phthalate | ||||
| 31 | Pencil case | Canvas (A): + | A: Polyester textile with phthalate (bound) (PET) | A: Ca (chalk), Cd and Pb conent too high (dye) | |
| Plastic front (B): + | B: PVC with phthalate | B: Cl (PVC), too high Cd and Ba content (stabilizer). High Zn content (stabilizer) | |||
| The rest: - | The rest: not examined | ||||
| 32 | Toy bag | - | |||
| 33 | Toy bag | - | |||
| 34 | Pencil case | Danger labelled for childen under 3 years | + | PVC with phthalate | Cl (PVC), too high Cd and Ba content (stabilizer). High Zn content (stabilizer) |
| 35 | Pencil case | Textile (A): + | A: Polyester textile with phthalate (bound) (PET) | High content of Ti, Ca (chalk). Too high content of Sb and Ba | |
| Labels (B): + | B: PVC with phthalate | ||||
| Gray inside (C): + | C: PVC with phthalate and chalk | ||||
| The rest: - | The rest: not examined | ||||
| 36 | Pencil case | + | |||
| 37 | Toy bag | Danger labelled for childen under 3 years | Round plastic plates (A): + | A: PVC with phthalate | |
| Textile (B): - | B: Polyester textil with terephthalate | B: Contains Cl, P and Ni. Sb content too high | |||
| 38 | Toy bag | This bag is not toy keep away from babies | The bag (A): + | A: Polyester textile with terephthalate | A: Chalk, high content of Cl (could be fire retardant), Zn. Too high content of Sb (could be fire retardant) |
| Lining (B): - | B: Polyester textile with terephthalate | B: High content of Ti, S, Ni and too high content of Sb | |||
| Pink plastic (C): | C: Poly Urethane | C: High content of S, Cl, Ti and Zn (heat stabilizers). Too high content of Pb | |||
| Red strap (D): - | D: not examined | ||||
| 39 | School bag | Lining (A): - | A: Poly Amide textile | A: Contains Ti and Zn | |
| The bag (B): + | B: Polyester textile with terephthalate | B: High content of S, Cl and Zn (heat stabilizers). Too high content of Ba | |||
| Plastic (C): + | C: PVC with phthalate | C: Cl (PVC), high content of Zn. Too high content of Ba | |||
| Coloured strap (D): - | D: not examined | ||||
| 40 | School bag | Not for children under 3 years | Pink linen (A): + | A: Polyester textile (polyethylen terephthalate) | A: Too high content of Ba and Pb. High content of Sbndhold af Sb |
| Handle (B): + | B: PVC with phthalate | B: Cl (PVC). Too high contet of Cd | |||
| Green/yellow plastic (C): - | C: Polyester textile with terephthalate | C: Contains Cl and Zn. Too hih content of Cd, Sb, Ba and Pb | |||
| 41 | School bag | Pink lining (A): + | A: PVC with phthalate and chalk. | ||
| Black bottom (B): + | B: Not examined | ||||
| Little accessory bag (C): + | C: Not examined | ||||
| The rest (D): - | D: Not examined | ||||
| 42 | School bag | Not for children under 3 years | Black textile front (A): + | A: PA | A: High content of Cu, Zn, Br, Sr and Mo. Too high content of Sb, Ba and Pb |
| Gymnastic bag (B): + | B: Polyester textile with terephthalate | B: High content of Br. Too high content of Sb | |||
| Plastic front (C): + | C: PVC with phthalate | C: Cl (PVC) with high content of Zn and Ba | |||
| Straps (D): - | D: PP | D: High content of S, Ca and Sr | |||
| Lining, back: - | Lining, back: Not examined | ||||
| 43 | Pencil case | + | Eraser: PVC with phthalate. |
Appendix E XRF analysis results
Sample 3
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 5.82 | % |
| 12 | Mg | Magnesium | 0.242 | % |
| 13 | Al | Aluminum | < 0.022 | % |
| 14 | Si | Silicon | < 0.0098 | % |
| 15 | P | Phosphorus | 0.1194 | % |
| 16 | S | Sulfur | < 0.0070 | % |
| 17 | Cl | Chlorine | 25.95 | % |
| 19 | K | Potassium | < 0.017 | % |
| 20 | Ca | Calcium | 6.104 | % |
| 22 | Ti | Titanium | 0.1986 | % |
| 23 | V | Vanadium | 0.0102 | % |
| 24 | Cr | Chromium | 0.00085 | % |
| 25 | Mn | Manganese | 0.00164 | % |
| 26 | Fe | Iron | < 0.00024 | % |
| 27 | Co | Cobalt | 1.5 | µg/g |
| 28 | Ni | Nickel | 5 | µg/g |
| 29 | Cu | Copper | < 0.7 | µg/g |
| 30 | Zn | Zinc | 129.1 | µg/g |
| 31 | Ga | Gallium | 1.4 | µg/g |
| 32 | Ge | Germanium | 0.4 | µg/g |
| 33 | As | Arsenic | < 0.4 | µg/g |
| 34 | Se | Selenium | 1.1 | µg/g |
| 35 | Br | Bromine | 0.5 | µg/g |
| 37 | Rb | Rubidium | 0.8 | µg/g |
| 38 | Sr | Strontium | 31.5 | µg/g |
| 39 | Y | Yttrium | < 0.4 | µg/g |
| 40 | Zr | Zirconium | < 1.9 | µg/g |
| 41 | Nb | Niobium | < 1.2 | µg/g |
| 42 | Mo | Molybdenum | < 1.0 | µg/g |
| 47 | Ag | Silver | < 0.6 | µg/g |
| 48 | Cd | Cadmium | < 0.6 | µg/g |
| 49 | In | Indium | < 0.6 | µg/g |
| 50 | Sn | Tin | < 0.8 | µg/g |
| 51 | Sb | Antimony | < 0.8 | µg/g |
| 52 | Te | Tellurium | < 1.3 | µg/g |
| 53 | I | Iodine | < 2.3 | µg/g |
| 55 | Cs | Cesium | < 3.4 | µg/g |
| 56 | Ba | Barium | < 5.2 | µg/g |
| 57 | La | Lanthanum | < 7.1 | µg/g |
| 58 | Ce | Cerium | < 10 | µg/g |
| 74 | W | Tungsten | < 2.7 | µg/g |
| 80 | Hg | Mercury | 0.6 | µg/g |
| 81 | Tl | Thallium | < 0.7 | µg/g |
| 82 | Pb | Lead | 2.6 | µg/g |
| 83 | Bi | Bismuth | 0.7 | µg/g |
| 90 | Th | Thorium | 0.8 | µg/g |
| 92 | U | Uranium | < 3.0 | µg/g |
Sample 4
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.265 | % |
| 12 | Mg | Magnesium | 0.323 | % |
| 13 | Al | Aluminum | 0.091 | % |
| 14 | Si | Silicon | 1.346 | % |
| 15 | P | Phosphorus | 0.00603 | % |
| 16 | S | Sulfur | 0.03513 | % |
| 17 | Cl | Chlorine | 0.04719 | % |
| 19 | K | Potassium | < 0.0100 | % |
| 20 | Ca | Calcium | 22.2 | % |
| 22 | Ti | Titanium | 0.02524 | % |
| 23 | V | Vanadium | < 0.00072 | % |
| 24 | Cr | Chromium | 0.00147 | % |
| 25 | Mn | Manganese | 0.00541 | % |
| 26 | Fe | Iron | 0.01758 | % |
| 27 | Co | Cobalt | < 2.1 | µg/g |
| 28 | Ni | Nickel | 11 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 31.1 | µg/g |
| 31 | Ga | Gallium | 1.9 | µg/g |
| 32 | Ge | Germanium | < 0.4 | µg/g |
| 33 | As | Arsenic | 1.2 | µg/g |
| 34 | Se | Selenium | 1 | µg/g |
| 35 | Br | Bromine | 0.7 | µg/g |
| 37 | Rb | Rubidium | 0.6 | µg/g |
| 38 | Sr | Strontium | 109.4 | µg/g |
| 39 | Y | Yttrium | < 0.4 | µg/g |
| 40 | Zr | Zirconium | < 5.3 | µg/g |
| 41 | Nb | Niobium | < 1.9 | µg/g |
| 42 | Mo | Molybdenum | < 1.7 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | 0.4 | µg/g |
| 49 | In | Indium | < 0.9 | µg/g |
| 50 | Sn | Tin | < 1.2 | µg/g |
| 51 | Sb | Antimony | < 1.3 | µg/g |
| 52 | Te | Tellurium | < 2.0 | µg/g |
| 53 | I | Iodine | < 3.8 | µg/g |
| 55 | Cs | Cesium | < 5.8 | µg/g |
| 56 | Ba | Barium | 316.8 | µg/g |
| 57 | La | Lanthanum | < 12 | µg/g |
| 58 | Ce | Cerium | < 18 | µg/g |
| 74 | W | Tungsten | < 2.2 | µg/g |
| 80 | Hg | Mercury | 0.7 | µg/g |
| 81 | Tl | Thallium | 1.8 | µg/g |
| 82 | Pb | Lead | 3.4 | µg/g |
| 83 | Bi | Bismuth | 1.4 | µg/g |
| 90 | Th | Thorium | 0.7 | µg/g |
| 92 | U | Uranium | < 3.2 | µg/g |
Sample 9
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 5.07 | % |
| 12 | Mg | Magnesium | 0.312 | % |
| 13 | Al | Aluminum | 0.0244 | % |
| 14 | Si | Silicon | < 0.0089 | % |
| 15 | P | Phosphorus | 0.0709 | % |
| 16 | S | Sulfur | 0.0257 | % |
| 17 | Cl | Chlorine | 16.89 | % |
| 19 | K | Potassium | < 0.018 | % |
| 20 | Ca | Calcium | 15.73 | % |
| 22 | Ti | Titanium | 0.0817 | % |
| 23 | V | Vanadium | < 0.0030 | % |
| 24 | Cr | Chromium | 0.00095 | % |
| 25 | Mn | Manganese | 0.00686 | % |
| 26 | Fe | Iron | < 0.00021 | % |
| 27 | Co | Cobalt | 3.6 | µg/g |
| 28 | Ni | Nickel | 5.9 | µg/g |
| 29 | Cu | Copper | < 0.7 | µg/g |
| 30 | Zn | Zinc | 53.3 | µg/g |
| 31 | Ga | Gallium | 1.8 | µg/g |
| 32 | Ge | Germanium | 0.3 | µg/g |
| 33 | As | Arsenic | < 0.4 | µg/g |
| 34 | Se | Selenium | 1.1 | µg/g |
| 35 | Br | Bromine | 1 | µg/g |
| 37 | Rb | Rubidium | 0.9 | µg/g |
| 38 | Sr | Strontium | 95.1 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 3.3 | µg/g |
| 41 | Nb | Niobium | < 1.5 | µg/g |
| 42 | Mo | Molybdenum | < 1.2 | µg/g |
| 47 | Ag | Silver | < 0.7 | µg/g |
| 48 | Cd | Cadmium | 1 | µg/g |
| 49 | In | Indium | < 0.7 | µg/g |
| 50 | Sn | Tin | < 1.0 | µg/g |
| 51 | Sb | Antimony | < 1.0 | µg/g |
| 52 | Te | Tellurium | < 1.5 | µg/g |
| 53 | I | Iodine | < 2.6 | µg/g |
| 55 | Cs | Cesium | < 3.9 | µg/g |
| 56 | Ba | Barium | 235.4 | µg/g |
| 57 | La | Lanthanum | < 8.1 | µg/g |
| 58 | Ce | Cerium | < 11 | µg/g |
| 74 | W | Tungsten | < 2.1 | µg/g |
| 80 | Hg | Mercury | < 0.8 | µg/g |
| 81 | Tl | Thallium | 2 | µg/g |
| 82 | Pb | Lead | 4 | µg/g |
| 83 | Bi | Bismuth | 1.6 | µg/g |
| 90 | Th | Thorium | 0.8 | µg/g |
| 92 | U | Uranium | < 3.7 | µg/g |
Sample 10
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.6 | % |
| 12 | Mg | Magnesium | 0.216 | % |
| 13 | Al | Aluminum | 0.061 | % |
| 14 | Si | Silicon | 0.3151 | % |
| 15 | P | Phosphorus | 0.0138 | % |
| 16 | S | Sulfur | 0.1639 | % |
| 17 | Cl | Chlorine | 1.245 | % |
| 19 | K | Potassium | < 0.013 | % |
| 20 | Ca | Calcium | 36.24 | % |
| 22 | Ti | Titanium | < 0.0020 | % |
| 23 | V | Vanadium | < 0.0012 | % |
| 24 | Cr | Chromium | 0.00176 | % |
| 25 | Mn | Manganese | 0.00182 | % |
| 26 | Fe | Iron | < 0.00031 | % |
| 27 | Co | Cobalt | < 2.7 | µg/g |
| 28 | Ni | Nickel | 6.4 | µg/g |
| 29 | Cu | Copper | < 1.2 | µg/g |
| 30 | Zn | Zinc | 622.5 | µg/g |
| 31 | Ga | Gallium | < 0.9 | µg/g |
| 32 | Ge | Germanium | 0.7 | µg/g |
| 33 | As | Arsenic | < 0.6 | µg/g |
| 34 | Se | Selenium | 0.7 | µg/g |
| 35 | Br | Bromine | 1 | µg/g |
| 37 | Rb | Rubidium | 0.6 | µg/g |
| 38 | Sr | Strontium | 272.2 | µg/g |
| 39 | Y | Yttrium | < 0.6 | µg/g |
| 40 | Zr | Zirconium | < 6.1 | µg/g |
| 41 | Nb | Niobium | < 1.8 | µg/g |
| 42 | Mo | Molybdenum | < 1.7 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | < 0.9 | µg/g |
| 49 | In | Indium | < 0.8 | µg/g |
| 50 | Sn | Tin | < 1.1 | µg/g |
| 51 | Sb | Antimony | 1.6 | µg/g |
| 52 | Te | Tellurium | < 1.6 | µg/g |
| 53 | I | Iodine | < 3.0 | µg/g |
| 55 | Cs | Cesium | < 4.3 | µg/g |
| 56 | Ba | Barium | < 6.9 | µg/g |
| 57 | La | Lanthanum | < 9.2 | µg/g |
| 58 | Ce | Cerium | < 13 | µg/g |
| 74 | W | Tungsten | < 6.3 | µg/g |
| 80 | Hg | Mercury | < 1.1 | µg/g |
| 81 | Tl | Thallium | < 1.0 | µg/g |
| 82 | Pb | Lead | 6.5 | µg/g |
| 83 | Bi | Bismuth | 0.8 | µg/g |
| 90 | Th | Thorium | < 0.8 | µg/g |
| 92 | U | Uranium | < 4.2 | µg/g |
Sample 12
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 4.64 | % |
| 12 | Mg | Magnesium | 0.226 | % |
| 13 | Al | Aluminum | < 0.018 | % |
| 14 | Si | Silicon | 0.851 | % |
| 15 | P | Phosphorus | 0.0757 | % |
| 16 | S | Sulfur | 0.01607 | % |
| 17 | Cl | Chlorine | 25.08 | % |
| 19 | K | Potassium | < 0.015 | % |
| 20 | Ca | Calcium | 3.34 | % |
| 22 | Ti | Titanium | 0.3545 | % |
| 23 | V | Vanadium | 0.0111 | % |
| 24 | Cr | Chromium | 0.00616 | % |
| 25 | Mn | Manganese | < 0.00042 | % |
| 26 | Fe | Iron | < 0.00019 | % |
| 27 | Co | Cobalt | < 1.6 | µg/g |
| 28 | Ni | Nickel | 4.7 | µg/g |
| 29 | Cu | Copper | 101.8 | µg/g |
| 30 | Zn | Zinc | 48 | µg/g |
| 31 | Ga | Gallium | 2.1 | µg/g |
| 32 | Ge | Germanium | < 0.5 | µg/g |
| 33 | As | Arsenic | 10.6 | µg/g |
| 34 | Se | Selenium | 0.4 | µg/g |
| 35 | Br | Bromine | 5.7 | µg/g |
| 37 | Rb | Rubidium | 0.6 | µg/g |
| 38 | Sr | Strontium | 18.7 | µg/g |
| 39 | Y | Yttrium | < 0.4 | µg/g |
| 40 | Zr | Zirconium | 18.4 | µg/g |
| 41 | Nb | Niobium | 7.2 | µg/g |
| 42 | Mo | Molybdenum | < 1.1 | µg/g |
| 47 | Ag | Silver | < 0.5 | µg/g |
| 48 | Cd | Cadmium | < 0.4 | µg/g |
| 49 | In | Indium | < 0.5 | µg/g |
| 50 | Sn | Tin | 0.6 | µg/g |
| 51 | Sb | Antimony | < 0.8 | µg/g |
| 52 | Te | Tellurium | < 1.2 | µg/g |
| 53 | I | Iodine | 1.6 | µg/g |
| 55 | Cs | Cesium | < 3.1 | µg/g |
| 56 | Ba | Barium | < 4.6 | µg/g |
| 57 | La | Lanthanum | < 6.3 | µg/g |
| 58 | Ce | Cerium | < 8.5 | µg/g |
| 74 | W | Tungsten | < 2.1 | µg/g |
| 80 | Hg | Mercury | < 0.8 | µg/g |
| 81 | Tl | Thallium | 1.5 | µg/g |
| 82 | Pb | Lead | 58.4 | µg/g |
| 83 | Bi | Bismuth | 1.1 | µg/g |
| 90 | Th | Thorium | 0.8 | µg/g |
| 92 | U | Uranium | < 2.6 | µg/g |
Sample 14
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 2.79 | % |
| 12 | Mg | Magnesium | 0.303 | % |
| 13 | Al | Aluminum | 2.887 | % |
| 14 | Si | Silicon | 3.54 | % |
| 15 | P | Phosphorus | 0.0862 | % |
| 16 | S | Sulfur | 0.1241 | % |
| 17 | Cl | Chlorine | 8.559 | % |
| 19 | K | Potassium | 0.0286 | % |
| 20 | Ca | Calcium | 8.237 | % |
| 22 | Ti | Titanium | 10.64 | % |
| 23 | V | Vanadium | < 0.015 | % |
| 24 | Cr | Chromium | < 0.00049 | % |
| 25 | Mn | Manganese | 0.00264 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | 2.2 | µg/g |
| 28 | Ni | Nickel | 7.5 | µg/g |
| 29 | Cu | Copper | 26.9 | µg/g |
| 30 | Zn | Zinc | 44.7 | µg/g |
| 31 | Ga | Gallium | 2.2 | µg/g |
| 32 | Ge | Germanium | 0.6 | µg/g |
| 33 | As | Arsenic | 1.1 | µg/g |
| 34 | Se | Selenium | 0.8 | µg/g |
| 35 | Br | Bromine | 1.2 | µg/g |
| 37 | Rb | Rubidium | 1.1 | µg/g |
| 38 | Sr | Strontium | 89.9 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 3.8 | µg/g |
| 41 | Nb | Niobium | 6.2 | µg/g |
| 42 | Mo | Molybdenum | < 1.9 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | < 0.9 | µg/g |
| 49 | In | Indium | < 0.8 | µg/g |
| 50 | Sn | Tin | < 1.2 | µg/g |
| 51 | Sb | Antimony | 4.2 | µg/g |
| 52 | Te | Tellurium | < 2.0 | µg/g |
| 53 | I | Iodine | < 3.9 | µg/g |
| 55 | Cs | Cesium | 6.2 | µg/g |
| 56 | Ba | Barium | 174.8 | µg/g |
| 57 | La | Lanthanum | < 14 | µg/g |
| 58 | Ce | Cerium | < 20 | µg/g |
| 74 | W | Tungsten | < 2.5 | µg/g |
| 80 | Hg | Mercury | < 0.9 | µg/g |
| 81 | Tl | Thallium | 2 | µg/g |
| 82 | Pb | Lead | 3.9 | µg/g |
| 83 | Bi | Bismuth | < 0.7 | µg/g |
| 90 | Th | Thorium | 1.7 | µg/g |
| 92 | U | Uranium | < 4.3 | µg/g |
Sample 24
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | < 0.059 | % |
| 12 | Mg | Magnesium | 0.154 | % |
| 13 | Al | Aluminum | < 0.012 | % |
| 14 | Si | Silicon | 22.2 | % |
| 15 | P | Phosphorus | < 0.0037 | % |
| 16 | S | Sulfur | 4.099 | % |
| 17 | Cl | Chlorine | 0.06242 | % |
| 19 | K | Potassium | 0.0269 | % |
| 20 | Ca | Calcium | 0.02221 | % |
| 22 | Ti | Titanium | 0.0076 | % |
| 23 | V | Vanadium | < 0.00043 | % |
| 24 | Cr | Chromium | < 0.00060 | % |
| 25 | Mn | Manganese | 0.0005 | % |
| 26 | Fe | Iron | 0.00559 | % |
| 27 | Co | Cobalt | < 1.6 | µg/g |
| 28 | Ni | Nickel | 4.6 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 57.9 | µg/g |
| 31 | Ga | Gallium | 0.5 | µg/g |
| 32 | Ge | Germanium | 0.4 | µg/g |
| 33 | As | Arsenic | < 0.3 | µg/g |
| 34 | Se | Selenium | 0.3 | µg/g |
| 35 | Br | Bromine | 0.8 | µg/g |
| 37 | Rb | Rubidium | 0.5 | µg/g |
| 38 | Sr | Strontium | 0.7 | µg/g |
| 39 | Y | Yttrium | 1.2 | µg/g |
| 40 | Zr | Zirconium | 37.9 | µg/g |
| 41 | Nb | Niobium | 0.4 | µg/g |
| 42 | Mo | Molybdenum | < 1.0 | µg/g |
| 47 | Ag | Silver | < 0.4 | µg/g |
| 48 | Cd | Cadmium | < 0.4 | µg/g |
| 49 | In | Indium | < 0.5 | µg/g |
| 50 | Sn | Tin | < 0.8 | µg/g |
| 51 | Sb | Antimony | < 0.8 | µg/g |
| 52 | Te | Tellurium | < 1.3 | µg/g |
| 53 | I | Iodine | < 2.4 | µg/g |
| 55 | Cs | Cesium | 3.8 | µg/g |
| 56 | Ba | Barium | < 6.0 | µg/g |
| 57 | La | Lanthanum | < 8.1 | µg/g |
| 58 | Ce | Cerium | < 11 | µg/g |
| 74 | W | Tungsten | < 2.1 | µg/g |
| 80 | Hg | Mercury | 0.8 | µg/g |
| 81 | Tl | Thallium | 0.9 | µg/g |
| 82 | Pb | Lead | 1.5 | µg/g |
| 83 | Bi | Bismuth | 0.9 | µg/g |
| 90 | Th | Thorium | < 0.5 | µg/g |
| 92 | U | Uranium | < 1.8 | µg/g |
Sample 27
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | < 0.064 | % |
| 12 | Mg | Magnesium | 0.158 | % |
| 13 | Al | Aluminum | 0.0752 | % |
| 14 | Si | Silicon | 0.1041 | % |
| 15 | P | Phosphorus | < 0.0016 | % |
| 16 | S | Sulfur | 0.01425 | % |
| 17 | Cl | Chlorine | 0.01998 | % |
| 19 | K | Potassium | < 0.0095 | % |
| 20 | Ca | Calcium | 32.91 | % |
| 22 | Ti | Titanium | 0.00662 | % |
| 23 | V | Vanadium | < 0.00096 | % |
| 24 | Cr | Chromium | 0.00126 | % |
| 25 | Mn | Manganese | 0.00188 | % |
| 26 | Fe | Iron | < 0.00020 | % |
| 27 | Co | Cobalt | 2.6 | µg/g |
| 28 | Ni | Nickel | 5.5 | µg/g |
| 29 | Cu | Copper | < 0.7 | µg/g |
| 30 | Zn | Zinc | 8.3 | µg/g |
| 31 | Ga | Gallium | 2.4 | µg/g |
| 32 | Ge | Germanium | 0.5 | µg/g |
| 33 | As | Arsenic | 0.3 | µg/g |
| 34 | Se | Selenium | 0.8 | µg/g |
| 35 | Br | Bromine | 5 | µg/g |
| 37 | Rb | Rubidium | 0.7 | µg/g |
| 38 | Sr | Strontium | 123.6 | µg/g |
| 39 | Y | Yttrium | 2 | µg/g |
| 40 | Zr | Zirconium | < 3.8 | µg/g |
| 41 | Nb | Niobium | < 1.8 | µg/g |
| 42 | Mo | Molybdenum | < 1.5 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | < 0.6 | µg/g |
| 49 | In | Indium | < 0.7 | µg/g |
| 50 | Sn | Tin | 10.9 | µg/g |
| 51 | Sb | Antimony | < 0.9 | µg/g |
| 52 | Te | Tellurium | 1.9 | µg/g |
| 53 | I | Iodine | < 2.5 | µg/g |
| 55 | Cs | Cesium | < 3.6 | µg/g |
| 56 | Ba | Barium | < 5.7 | µg/g |
| 57 | La | Lanthanum | < 7.6 | µg/g |
| 58 | Ce | Cerium | < 10 | µg/g |
| 74 | W | Tungsten | < 2.2 | µg/g |
| 80 | Hg | Mercury | 1.1 | µg/g |
| 81 | Tl | Thallium | 2.1 | µg/g |
| 82 | Pb | Lead | 3.6 | µg/g |
| 83 | Bi | Bismuth | 1.9 | µg/g |
| 90 | Th | Thorium | 1.3 | µg/g |
| 92 | U | Uranium | < 4.5 | µg/g |
Sample 30A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 9.7 | % |
| 12 | Mg | Magnesium | < 0.12 | % |
| 13 | Al | Aluminum | < 0.039 | % |
| 14 | Si | Silicon | < 0.016 | % |
| 15 | P | Phosphorus | 0.2323 | % |
| 16 | S | Sulfur | < 0.013 | % |
| 17 | Cl | Chlorine | 49.91 | % |
| 19 | K | Potassium | < 0.020 | % |
| 20 | Ca | Calcium | < 0.0072 | % |
| 22 | Ti | Titanium | 0.2018 | % |
| 23 | V | Vanadium | < 0.0056 | % |
| 24 | Cr | Chromium | 0.00181 | % |
| 25 | Mn | Manganese | 0.00274 | % |
| 26 | Fe | Iron | < 0.00023 | % |
| 27 | Co | Cobalt | 2.1 | µg/g |
| 28 | Ni | Nickel | 8.1 | µg/g |
| 29 | Cu | Copper | < 0.8 | µg/g |
| 30 | Zn | Zinc | 523 | µg/g |
| 31 | Ga | Gallium | 0.8 | µg/g |
| 32 | Ge | Germanium | < 0.5 | µg/g |
| 33 | As | Arsenic | < 0.5 | µg/g |
| 34 | Se | Selenium | < 0.4 | µg/g |
| 35 | Br | Bromine | 3.6 | µg/g |
| 37 | Rb | Rubidium | 3.4 | µg/g |
| 38 | Sr | Strontium | 40.5 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 2.8 | µg/g |
| 41 | Nb | Niobium | < 2.0 | µg/g |
| 42 | Mo | Molybdenum | < 2.7 | µg/g |
| 47 | Ag | Silver | 21 | µg/g |
| 48 | Cd | Cadmium | 13 | µg/g |
| 49 | In | Indium | < 1.6 | µg/g |
| 50 | Sn | Tin | 8.7 | µg/g |
| 51 | Sb | Antimony | < 2.5 | µg/g |
| 52 | Te | Tellurium | < 3.4 | µg/g |
| 53 | I | Iodine | < 6.8 | µg/g |
| 55 | Cs | Cesium | < 11 | µg/g |
| 56 | Ba | Barium | 1176 | µg/g |
| 57 | La | Lanthanum | < 22 | µg/g |
| 58 | Ce | Cerium | < 31 | µg/g |
| 74 | W | Tungsten | < 5.3 | µg/g |
| 80 | Hg | Mercury | 2.7 | µg/g |
| 81 | Tl | Thallium | 2 | µg/g |
| 82 | Pb | Lead | 4.6 | µg/g |
| 83 | Bi | Bismuth | 1.4 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 4.5 | µg/g |
Sample 30B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 8.05 | % |
| 12 | Mg | Magnesium | 0.199 | % |
| 13 | Al | Aluminum | < 0.025 | % |
| 14 | Si | Silicon | < 0.014 | % |
| 15 | P | Phosphorus | 0.2454 | % |
| 16 | S | Sulfur | < 0.0091 | % |
| 17 | Cl | Chlorine | 74.92 | % |
| 19 | K | Potassium | < 0.027 | % |
| 20 | Ca | Calcium | < 0.011 | % |
| 22 | Ti | Titanium | 0.0438 | % |
| 23 | V | Vanadium | < 0.0069 | % |
| 24 | Cr | Chromium | 0.00085 | % |
| 25 | Mn | Manganese | 0.00157 | % |
| 26 | Fe | Iron | < 0.00026 | % |
| 27 | Co | Cobalt | < 2.1 | µg/g |
| 28 | Ni | Nickel | 8.2 | µg/g |
| 29 | Cu | Copper | < 0.5 | µg/g |
| 30 | Zn | Zinc | 285.5 | µg/g |
| 31 | Ga | Gallium | 1.9 | µg/g |
| 32 | Ge | Germanium | < 0.6 | µg/g |
| 33 | As | Arsenic | < 0.6 | µg/g |
| 34 | Se | Selenium | 1.1 | µg/g |
| 35 | Br | Bromine | 2.1 | µg/g |
| 37 | Rb | Rubidium | 1.2 | µg/g |
| 38 | Sr | Strontium | 20.2 | µg/g |
| 39 | Y | Yttrium | < 0.6 | µg/g |
| 40 | Zr | Zirconium | < 3.4 | µg/g |
| 41 | Nb | Niobium | < 2.2 | µg/g |
| 42 | Mo | Molybdenum | < 2.9 | µg/g |
| 47 | Ag | Silver | 37.6 | µg/g |
| 48 | Cd | Cadmium | 2.2 | µg/g |
| 49 | In | Indium | < 1.2 | µg/g |
| 50 | Sn | Tin | < 2.1 | µg/g |
| 51 | Sb | Antimony | < 2.5 | µg/g |
| 52 | Te | Tellurium | < 3.8 | µg/g |
| 53 | I | Iodine | < 7.0 | µg/g |
| 55 | Cs | Cesium | < 11 | µg/g |
| 56 | Ba | Barium | 514 | µg/g |
| 57 | La | Lanthanum | < 24 | µg/g |
| 58 | Ce | Cerium | < 31 | µg/g |
| 74 | W | Tungsten | < 4.1 | µg/g |
| 80 | Hg | Mercury | 1.6 | µg/g |
| 81 | Tl | Thallium | 1.9 | µg/g |
| 82 | Pb | Lead | 6 | µg/g |
| 83 | Bi | Bismuth | 2.1 | µg/g |
| 90 | Th | Thorium | < 0.7 | µg/g |
| 92 | U | Uranium | < 4.0 | µg/g |
Sample 31A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 2.82 | % |
| 12 | Mg | Magnesium | 0.301 | % |
| 13 | Al | Aluminum | 0.23 | % |
| 14 | Si | Silicon | 0.0925 | % |
| 15 | P | Phosphorus | 0.0824 | % |
| 16 | S | Sulfur | 0.0808 | % |
| 17 | Cl | Chlorine | 7.394 | % |
| 19 | K | Potassium | 0.0472 | % |
| 20 | Ca | Calcium | 9.606 | % |
| 22 | Ti | Titanium | 1.398 | % |
| 23 | V | Vanadium | 0.0137 | % |
| 24 | Cr | Chromium | 0.00522 | % |
| 25 | Mn | Manganese | 0.00288 | % |
| 26 | Fe | Iron | 0.01066 | % |
| 27 | Co | Cobalt | < 2.2 | µg/g |
| 28 | Ni | Nickel | 16.3 | µg/g |
| 29 | Cu | Copper | < 1.0 | µg/g |
| 30 | Zn | Zinc | 279.5 | µg/g |
| 31 | Ga | Gallium | 4.1 | µg/g |
| 32 | Ge | Germanium | < 0.6 | µg/g |
| 33 | As | Arsenic | 24.4 | µg/g |
| 34 | Se | Selenium | 0.7 | µg/g |
| 35 | Br | Bromine | 8.8 | µg/g |
| 37 | Rb | Rubidium | 1.5 | µg/g |
| 38 | Sr | Strontium | 235.5 | µg/g |
| 39 | Y | Yttrium | < 0.7 | µg/g |
| 40 | Zr | Zirconium | < 8.1 | µg/g |
| 41 | Nb | Niobium | 7.9 | µg/g |
| 42 | Mo | Molybdenum | < 2.7 | µg/g |
| 47 | Ag | Silver | 31.6 | µg/g |
| 48 | Cd | Cadmium | 389.3 | µg/g |
| 49 | In | Indium | < 1.7 | µg/g |
| 50 | Sn | Tin | < 3.0 | µg/g |
| 51 | Sb | Antimony | 41.9 | µg/g |
| 52 | Te | Tellurium | < 3.9 | µg/g |
| 53 | I | Iodine | < 7.1 | µg/g |
| 55 | Cs | Cesium | < 11 | µg/g |
| 56 | Ba | Barium | 660 | µg/g |
| 57 | La | Lanthanum | < 22 | µg/g |
| 58 | Ce | Cerium | < 31 | µg/g |
| 74 | W | Tungsten | < 4.7 | µg/g |
| 80 | Hg | Mercury | < 1.1 | µg/g |
| 81 | Tl | Thallium | < 1.7 | µg/g |
| 82 | Pb | Lead | 474.3 | µg/g |
| 83 | Bi | Bismuth | 0.9 | µg/g |
| 90 | Th | Thorium | < 1.1 | µg/g |
| 92 | U | Uranium | < 3.8 | µg/g |
Sample 31B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 5.58 | % |
| 12 | Mg | Magnesium | < 0.046 | % |
| 13 | Al | Aluminum | < 0.016 | % |
| 14 | Si | Silicon | < 0.0069 | % |
| 15 | P | Phosphorus | 0.0828 | % |
| 16 | S | Sulfur | < 0.0054 | % |
| 17 | Cl | Chlorine | 38.17 | % |
| 19 | K | Potassium | < 0.016 | % |
| 20 | Ca | Calcium | 0.016 | % |
| 22 | Ti | Titanium | 0.0169 | % |
| 23 | V | Vanadium | < 0.0036 | % |
| 24 | Cr | Chromium | 0.00067 | % |
| 25 | Mn | Manganese | 0.00174 | % |
| 26 | Fe | Iron | < 0.00018 | % |
| 27 | Co | Cobalt | 1.4 | µg/g |
| 28 | Ni | Nickel | 5.4 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 424.1 | µg/g |
| 31 | Ga | Gallium | 0.4 | µg/g |
| 32 | Ge | Germanium | < 0.4 | µg/g |
| 33 | As | Arsenic | < 0.4 | µg/g |
| 34 | Se | Selenium | 1 | µg/g |
| 35 | Br | Bromine | 2 | µg/g |
| 37 | Rb | Rubidium | 1.3 | µg/g |
| 38 | Sr | Strontium | 29.9 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 3 | µg/g |
| 41 | Nb | Niobium | 4.8 | µg/g |
| 42 | Mo | Molybdenum | < 2.6 | µg/g |
| 47 | Ag | Silver | 24.6 | µg/g |
| 48 | Cd | Cadmium | 358.7 | µg/g |
| 49 | In | Indium | < 1.9 | µg/g |
| 50 | Sn | Tin | < 2.3 | µg/g |
| 51 | Sb | Antimony | < 4.9 | µg/g |
| 52 | Te | Tellurium | < 3.7 | µg/g |
| 53 | I | Iodine | < 9.3 | µg/g |
| 55 | Cs | Cesium | < 14 | µg/g |
| 56 | Ba | Barium | 2413 | µg/g |
| 57 | La | Lanthanum | < 29 | µg/g |
| 58 | Ce | Cerium | < 38 | µg/g |
| 74 | W | Tungsten | < 3.7 | µg/g |
| 80 | Hg | Mercury | 2 | µg/g |
| 81 | Tl | Thallium | 1.7 | µg/g |
| 82 | Pb | Lead | 3.9 | µg/g |
| 83 | Bi | Bismuth | 1.3 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 4.4 | µg/g |
Sample 34
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 7.48 | % |
| 12 | Mg | Magnesium | 0.203 | % |
| 13 | Al | Aluminum | < 0.025 | % |
| 14 | Si | Silicon | < 0.012 | % |
| 15 | P | Phosphorus | 0.1224 | % |
| 16 | S | Sulfur | < 0.0085 | % |
| 17 | Cl | Chlorine | 50.32 | % |
| 19 | K | Potassium | < 0.019 | % |
| 20 | Ca | Calcium | < 0.0074 | % |
| 22 | Ti | Titanium | < 0.0052 | % |
| 23 | V | Vanadium | < 0.0040 | % |
| 24 | Cr | Chromium | 0.00129 | % |
| 25 | Mn | Manganese | 0.0032 | % |
| 26 | Fe | Iron | < 0.00028 | % |
| 27 | Co | Cobalt | < 2.0 | µg/g |
| 28 | Ni | Nickel | 6.3 | µg/g |
| 29 | Cu | Copper | 43 | µg/g |
| 30 | Zn | Zinc | 732.4 | µg/g |
| 31 | Ga | Gallium | < 0.8 | µg/g |
| 32 | Ge | Germanium | < 0.6 | µg/g |
| 33 | As | Arsenic | 2.9 | µg/g |
| 34 | Se | Selenium | < 0.4 | µg/g |
| 35 | Br | Bromine | 14.2 | µg/g |
| 37 | Rb | Rubidium | 2.1 | µg/g |
| 38 | Sr | Strontium | 64.8 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 10.2 | µg/g |
| 41 | Nb | Niobium | 13.3 | µg/g |
| 42 | Mo | Molybdenum | < 2.3 | µg/g |
| 47 | Ag | Silver | < 1.8 | µg/g |
| 48 | Cd | Cadmium | 256.3 | µg/g |
| 49 | In | Indium | < 1.5 | µg/g |
| 50 | Sn | Tin | < 2.1 | µg/g |
| 51 | Sb | Antimony | < 3.9 | µg/g |
| 52 | Te | Tellurium | < 2.9 | µg/g |
| 53 | I | Iodine | < 7.3 | µg/g |
| 55 | Cs | Cesium | 24.9 | µg/g |
| 56 | Ba | Barium | 4679 | µg/g |
| 57 | La | Lanthanum | < 21 | µg/g |
| 58 | Ce | Cerium | < 29 | µg/g |
| 74 | W | Tungsten | < 5.4 | µg/g |
| 80 | Hg | Mercury | 0.7 | µg/g |
| 81 | Tl | Thallium | 2 | µg/g |
| 82 | Pb | Lead | 7.7 | µg/g |
| 83 | Bi | Bismuth | 1.3 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 4.2 | µg/g |
Sample 35A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.56 | % |
| 12 | Mg | Magnesium | 0.095 | % |
| 13 | Al | Aluminum | 0.2601 | % |
| 14 | Si | Silicon | 0.646 | % |
| 15 | P | Phosphorus | 0.0181 | % |
| 16 | S | Sulfur | 0.1735 | % |
| 17 | Cl | Chlorine | 0.7738 | % |
| 19 | K | Potassium | < 0.0041 | % |
| 20 | Ca | Calcium | 1.919 | % |
| 22 | Ti | Titanium | 5.924 | % |
| 23 | V | Vanadium | < 0.0045 | % |
| 24 | Cr | Chromium | < 0.00064 | % |
| 25 | Mn | Manganese | 0.00269 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | < 1.2 | µg/g |
| 28 | Ni | Nickel | 2.8 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 247.6 | µg/g |
| 31 | Ga | Gallium | < 0.5 | µg/g |
| 32 | Ge | Germanium | 0.6 | µg/g |
| 33 | As | Arsenic | 4.1 | µg/g |
| 34 | Se | Selenium | 0.8 | µg/g |
| 35 | Br | Bromine | 5 | µg/g |
| 37 | Rb | Rubidium | 0.4 | µg/g |
| 38 | Sr | Strontium | 161.1 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 68.5 | µg/g |
| 41 | Nb | Niobium | 29.6 | µg/g |
| 42 | Mo | Molybdenum | 3.2 | µg/g |
| 47 | Ag | Silver | 2 | µg/g |
| 48 | Cd | Cadmium | 8.1 | µg/g |
| 49 | In | Indium | < 1.5 | µg/g |
| 50 | Sn | Tin | < 2.5 | µg/g |
| 51 | Sb | Antimony | 102 | µg/g |
| 52 | Te | Tellurium | 5.8 | µg/g |
| 53 | I | Iodine | 12.8 | µg/g |
| 55 | Cs | Cesium | < 15 | µg/g |
| 56 | Ba | Barium | 4748 | µg/g |
| 57 | La | Lanthanum | < 29 | µg/g |
| 58 | Ce | Cerium | < 42 | µg/g |
| 74 | W | Tungsten | < 2.8 | µg/g |
| 80 | Hg | Mercury | < 0.7 | µg/g |
| 81 | Tl | Thallium | 1.1 | µg/g |
| 82 | Pb | Lead | 6.1 | µg/g |
| 83 | Bi | Bismuth | 0.6 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 4.0 | µg/g |
Sample 37B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.91 | % |
| 12 | Mg | Magnesium | < 0.021 | % |
| 13 | Al | Aluminum | 0.2009 | % |
| 14 | Si | Silicon | < 0.0076 | % |
| 15 | P | Phosphorus | 3.016 | % |
| 16 | S | Sulfur | < 0.0019 | % |
| 17 | Cl | Chlorine | 2.403 | % |
| 19 | K | Potassium | < 0.0052 | % |
| 20 | Ca | Calcium | 0.02643 | % |
| 22 | Ti | Titanium | 0.1739 | % |
| 23 | V | Vanadium | < 0.00086 | % |
| 24 | Cr | Chromium | 0.00075 | % |
| 25 | Mn | Manganese | 0.00075 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | < 0.8 | µg/g |
| 28 | Ni | Nickel | 26.4 | µg/g |
| 29 | Cu | Copper | < 0.2 | µg/g |
| 30 | Zn | Zinc | 12.1 | µg/g |
| 31 | Ga | Gallium | 0.7 | µg/g |
| 32 | Ge | Germanium | < 0.2 | µg/g |
| 33 | As | Arsenic | < 0.3 | µg/g |
| 34 | Se | Selenium | 0.4 | µg/g |
| 35 | Br | Bromine | 16.3 | µg/g |
| 37 | Rb | Rubidium | 0.5 | µg/g |
| 38 | Sr | Strontium | 1.1 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 1.3 | µg/g |
| 41 | Nb | Niobium | < 1.0 | µg/g |
| 42 | Mo | Molybdenum | < 2.3 | µg/g |
| 47 | Ag | Silver | < 1.5 | µg/g |
| 48 | Cd | Cadmium | < 0.8 | µg/g |
| 49 | In | Indium | < 1.2 | µg/g |
| 50 | Sn | Tin | < 1.9 | µg/g |
| 51 | Sb | Antimony | 89.4 | µg/g |
| 52 | Te | Tellurium | < 3.5 | µg/g |
| 53 | I | Iodine | < 6.5 | µg/g |
| 55 | Cs | Cesium | < 11 | µg/g |
| 56 | Ba | Barium | < 16 | µg/g |
| 57 | La | Lanthanum | < 24 | µg/g |
| 58 | Ce | Cerium | < 33 | µg/g |
| 74 | W | Tungsten | < 1.6 | µg/g |
| 80 | Hg | Mercury | < 0.4 | µg/g |
| 81 | Tl | Thallium | 1.1 | µg/g |
| 82 | Pb | Lead | 2.3 | µg/g |
| 83 | Bi | Bismuth | 1.6 | µg/g |
| 90 | Th | Thorium | 1.3 | µg/g |
| 92 | U | Uranium | < 1.5 | µg/g |
Sample 38A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 2.12 | % |
| 12 | Mg | Magnesium | 0.1029 | % |
| 13 | Al | Aluminum | < 0.0079 | % |
| 14 | Si | Silicon | < 0.0031 | % |
| 15 | P | Phosphorus | 0.0282 | % |
| 16 | S | Sulfur | < 0.0026 | % |
| 17 | Cl | Chlorine | 8.382 | % |
| 19 | K | Potassium | < 0.0060 | % |
| 20 | Ca | Calcium | 2.448 | % |
| 22 | Ti | Titanium | 0.4632 | % |
| 23 | V | Vanadium | < 0.0028 | % |
| 24 | Cr | Chromium | 0.00067 | % |
| 25 | Mn | Manganese | 0.00119 | % |
| 26 | Fe | Iron | 0.00669 | % |
| 27 | Co | Cobalt | 0.8 | µg/g |
| 28 | Ni | Nickel | 3 | µg/g |
| 29 | Cu | Copper | < 0.4 | µg/g |
| 30 | Zn | Zinc | 128.3 | µg/g |
| 31 | Ga | Gallium | 0.3 | µg/g |
| 32 | Ge | Germanium | 0.5 | µg/g |
| 33 | As | Arsenic | 1.6 | µg/g |
| 34 | Se | Selenium | 0.5 | µg/g |
| 35 | Br | Bromine | 1.2 | µg/g |
| 37 | Rb | Rubidium | 1 | µg/g |
| 38 | Sr | Strontium | 60.3 | µg/g |
| 39 | Y | Yttrium | < 0.6 | µg/g |
| 40 | Zr | Zirconium | 5.9 | µg/g |
| 41 | Nb | Niobium | 23.8 | µg/g |
| 42 | Mo | Molybdenum | < 3.5 | µg/g |
| 47 | Ag | Silver | < 1.5 | µg/g |
| 48 | Cd | Cadmium | < 1.4 | µg/g |
| 49 | In | Indium | < 2.3 | µg/g |
| 50 | Sn | Tin | 1.9 | µg/g |
| 51 | Sb | Antimony | 158.5 | µg/g |
| 52 | Te | Tellurium | < 4.5 | µg/g |
| 53 | I | Iodine | < 9.1 | µg/g |
| 55 | Cs | Cesium | < 15 | µg/g |
| 56 | Ba | Barium | 648 | µg/g |
| 57 | La | Lanthanum | < 32 | µg/g |
| 58 | Ce | Cerium | < 49 | µg/g |
| 74 | W | Tungsten | < 1.6 | µg/g |
| 80 | Hg | Mercury | < 0.5 | µg/g |
| 81 | Tl | Thallium | 0.8 | µg/g |
| 82 | Pb | Lead | 29 | µg/g |
| 83 | Bi | Bismuth | 0.9 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 4.6 | µg/g |
Sample 38B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | < 0.026 | % |
| 12 | Mg | Magnesium | < 0.0065 | % |
| 13 | Al | Aluminum | 0.1068 | % |
| 14 | Si | Silicon | 0.0377 | % |
| 15 | P | Phosphorus | < 0.0050 | % |
| 16 | S | Sulfur | 0.01313 | % |
| 17 | Cl | Chlorine | 0.02306 | % |
| 19 | K | Potassium | < 0.0026 | % |
| 20 | Ca | Calcium | 0.03677 | % |
| 22 | Ti | Titanium | 0.3024 | % |
| 23 | V | Vanadium | 0.0018 | % |
| 24 | Cr | Chromium | < 0.00023 | % |
| 25 | Mn | Manganese | 0.00034 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | 0.7 | µg/g |
| 28 | Ni | Nickel | 6.2 | µg/g |
| 29 | Cu | Copper | < 0.2 | µg/g |
| 30 | Zn | Zinc | 4 | µg/g |
| 31 | Ga | Gallium | 0.5 | µg/g |
| 32 | Ge | Germanium | < 0.2 | µg/g |
| 33 | As | Arsenic | < 0.2 | µg/g |
| 34 | Se | Selenium | < 0.2 | µg/g |
| 35 | Br | Bromine | 1.9 | µg/g |
| 37 | Rb | Rubidium | 0.6 | µg/g |
| 38 | Sr | Strontium | 1.2 | µg/g |
| 39 | Y | Yttrium | 0.8 | µg/g |
| 40 | Zr | Zirconium | < 1.4 | µg/g |
| 41 | Nb | Niobium | < 1.3 | µg/g |
| 42 | Mo | Molybdenum | < 1.4 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | < 0.9 | µg/g |
| 49 | In | Indium | < 1.2 | µg/g |
| 50 | Sn | Tin | < 1.5 | µg/g |
| 51 | Sb | Antimony | 198.4 | µg/g |
| 52 | Te | Tellurium | < 2.6 | µg/g |
| 53 | I | Iodine | < 4.4 | µg/g |
| 55 | Cs | Cesium | < 7.2 | µg/g |
| 56 | Ba | Barium | < 11 | µg/g |
| 57 | La | Lanthanum | < 15 | µg/g |
| 58 | Ce | Cerium | < 23 | µg/g |
| 74 | W | Tungsten | 0.4 | µg/g |
| 80 | Hg | Mercury | < 0.3 | µg/g |
| 81 | Tl | Thallium | < 0.3 | µg/g |
| 82 | Pb | Lead | 1.5 | µg/g |
| 83 | Bi | Bismuth | 0.7 | µg/g |
| 90 | Th | Thorium | 1.2 | µg/g |
| 92 | U | Uranium | < 1.7 | µg/g |
Sample 38C
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 5.78 | % |
| 12 | Mg | Magnesium | 0.228 | % |
| 13 | Al | Aluminum | 0.287 | % |
| 14 | Si | Silicon | 0.278 | % |
| 15 | P | Phosphorus | 0.0704 | % |
| 16 | S | Sulfur | 0.1666 | % |
| 17 | Cl | Chlorine | 16.47 | % |
| 19 | K | Potassium | 0.0205 | % |
| 20 | Ca | Calcium | 8.242 | % |
| 22 | Ti | Titanium | 3.509 | % |
| 23 | V | Vanadium | < 0.0093 | % |
| 24 | Cr | Chromium | 0.01215 | % |
| 25 | Mn | Manganese | 0.00093 | % |
| 26 | Fe | Iron | < 0.00022 | % |
| 27 | Co | Cobalt | 1.8 | µg/g |
| 28 | Ni | Nickel | 7.1 | µg/g |
| 29 | Cu | Copper | < 0.7 | µg/g |
| 30 | Zn | Zinc | 484.2 | µg/g |
| 31 | Ga | Gallium | 3.3 | µg/g |
| 32 | Ge | Germanium | < 0.5 | µg/g |
| 33 | As | Arsenic | 6.7 | µg/g |
| 34 | Se | Selenium | 0.9 | µg/g |
| 35 | Br | Bromine | 0.8 | µg/g |
| 37 | Rb | Rubidium | 0.9 | µg/g |
| 38 | Sr | Strontium | 46.4 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 2.8 | µg/g |
| 41 | Nb | Niobium | 4.5 | µg/g |
| 42 | Mo | Molybdenum | < 1.4 | µg/g |
| 47 | Ag | Silver | < 0.7 | µg/g |
| 48 | Cd | Cadmium | 0.5 | µg/g |
| 49 | In | Indium | < 0.6 | µg/g |
| 50 | Sn | Tin | 0.9 | µg/g |
| 51 | Sb | Antimony | 13 | µg/g |
| 52 | Te | Tellurium | < 1.3 | µg/g |
| 53 | I | Iodine | < 2.5 | µg/g |
| 55 | Cs | Cesium | < 3.8 | µg/g |
| 56 | Ba | Barium | < 5.5 | µg/g |
| 57 | La | Lanthanum | < 7.9 | µg/g |
| 58 | Ce | Cerium | < 11 | µg/g |
| 74 | W | Tungsten | < 4.9 | µg/g |
| 80 | Hg | Mercury | < 0.8 | µg/g |
| 81 | Tl | Thallium | 1.6 | µg/g |
| 82 | Pb | Lead | 93.9 | µg/g |
| 83 | Bi | Bismuth | 1 | µg/g |
| 90 | Th | Thorium | < 0.7 | µg/g |
| 92 | U | Uranium | < 2.0 | µg/g |
Sample 39A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.259 | % |
| 12 | Mg | Magnesium | 0.0293 | % |
| 13 | Al | Aluminum | 0.1796 | % |
| 14 | Si | Silicon | 0.03137 | % |
| 15 | P | Phosphorus | < 0.0050 | % |
| 16 | S | Sulfur | 0.01815 | % |
| 17 | Cl | Chlorine | 0.4745 | % |
| 19 | K | Potassium | < 0.0014 | % |
| 20 | Ca | Calcium | 0.01389 | % |
| 22 | Ti | Titanium | 1.37 | % |
| 23 | V | Vanadium | < 0.0011 | % |
| 24 | Cr | Chromium | < 0.0015 | % |
| 25 | Mn | Manganese | < 0.0010 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | 0.4 | µg/g |
| 28 | Ni | Nickel | 8.8 | µg/g |
| 29 | Cu | Copper | < 0.2 | µg/g |
| 30 | Zn | Zinc | 21.2 | µg/g |
| 31 | Ga | Gallium | 0.7 | µg/g |
| 32 | Ge | Germanium | < 0.2 | µg/g |
| 33 | As | Arsenic | < 0.3 | µg/g |
| 34 | Se | Selenium | 0.3 | µg/g |
| 35 | Br | Bromine | 16.8 | µg/g |
| 37 | Rb | Rubidium | 0.4 | µg/g |
| 38 | Sr | Strontium | 1.2 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 2.3 | µg/g |
| 41 | Nb | Niobium | 6.3 | µg/g |
| 42 | Mo | Molybdenum | < 2.3 | µg/g |
| 47 | Ag | Silver | < 1.2 | µg/g |
| 48 | Cd | Cadmium | 1.3 | µg/g |
| 49 | In | Indium | < 1.4 | µg/g |
| 50 | Sn | Tin | 11.3 | µg/g |
| 51 | Sb | Antimony | 5.7 | µg/g |
| 52 | Te | Tellurium | < 3.4 | µg/g |
| 53 | I | Iodine | < 6.9 | µg/g |
| 55 | Cs | Cesium | < 11 | µg/g |
| 56 | Ba | Barium | < 16 | µg/g |
| 57 | La | Lanthanum | < 25 | µg/g |
| 58 | Ce | Cerium | < 37 | µg/g |
| 74 | W | Tungsten | < 0.9 | µg/g |
| 80 | Hg | Mercury | < 0.3 | µg/g |
| 81 | Tl | Thallium | 0.8 | µg/g |
| 82 | Pb | Lead | 2.4 | µg/g |
| 83 | Bi | Bismuth | 1 | µg/g |
| 90 | Th | Thorium | < 0.4 | µg/g |
| 92 | U | Uranium | < 2.3 | µg/g |
Sample 39B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 1.05 | % |
| 12 | Mg | Magnesium | < 0.037 | % |
| 13 | Al | Aluminum | 0.332 | % |
| 14 | Si | Silicon | 1.694 | % |
| 15 | P | Phosphorus | < 0.0032 | % |
| 16 | S | Sulfur | 0.1865 | % |
| 17 | Cl | Chlorine | 3.302 | % |
| 19 | K | Potassium | < 0.0079 | % |
| 20 | Ca | Calcium | 2.426 | % |
| 22 | Ti | Titanium | 9.144 | % |
| 23 | V | Vanadium | < 0.0078 | % |
| 24 | Cr | Chromium | < 0.00077 | % |
| 25 | Mn | Manganese | 0.00192 | % |
| 26 | Fe | Iron | < 0.00022 | % |
| 27 | Co | Cobalt | < 1.8 | µg/g |
| 28 | Ni | Nickel | 6.2 | µg/g |
| 29 | Cu | Copper | < 0.7 | µg/g |
| 30 | Zn | Zinc | 1007 | µg/g |
| 31 | Ga | Gallium | 0.4 | µg/g |
| 32 | Ge | Germanium | 0.7 | µg/g |
| 33 | As | Arsenic | 0.7 | µg/g |
| 34 | Se | Selenium | 0.5 | µg/g |
| 35 | Br | Bromine | 4 | µg/g |
| 37 | Rb | Rubidium | 0.8 | µg/g |
| 38 | Sr | Strontium | 107.1 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 8.8 | µg/g |
| 41 | Nb | Niobium | 18.5 | µg/g |
| 42 | Mo | Molybdenum | < 1.9 | µg/g |
| 47 | Ag | Silver | < 1.4 | µg/g |
| 48 | Cd | Cadmium | 1 | µg/g |
| 49 | In | Indium | < 1.4 | µg/g |
| 50 | Sn | Tin | 3.5 | µg/g |
| 51 | Sb | Antimony | 28.3 | µg/g |
| 52 | Te | Tellurium | < 3.1 | µg/g |
| 53 | I | Iodine | < 6.2 | µg/g |
| 55 | Cs | Cesium | < 10 | µg/g |
| 56 | Ba | Barium | 1155 | µg/g |
| 57 | La | Lanthanum | < 21 | µg/g |
| 58 | Ce | Cerium | < 31 | µg/g |
| 74 | W | Tungsten | < 6.7 | µg/g |
| 80 | Hg | Mercury | < 0.8 | µg/g |
| 81 | Tl | Thallium | 0.9 | µg/g |
| 82 | Pb | Lead | 6.5 | µg/g |
| 83 | Bi | Bismuth | 1.3 | µg/g |
| 90 | Th | Thorium | < 0.6 | µg/g |
| 92 | U | Uranium | < 3.0 | µg/g |
Sample 39C
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 5.35 | % |
| 12 | Mg | Magnesium | 0.0817 | % |
| 13 | Al | Aluminum | < 0.020 | % |
| 14 | Si | Silicon | < 0.0084 | % |
| 15 | P | Phosphorus | 0.1201 | % |
| 16 | S | Sulfur | < 0.0065 | % |
| 17 | Cl | Chlorine | 30.23 | % |
| 19 | K | Potassium | < 0.012 | % |
| 20 | Ca | Calcium | < 0.0041 | % |
| 22 | Ti | Titanium | < 0.0035 | % |
| 23 | V | Vanadium | < 0.0026 | % |
| 24 | Cr | Chromium | < 0.00045 | % |
| 25 | Mn | Manganese | 0.00065 | % |
| 26 | Fe | Iron | < 0.00018 | % |
| 27 | Co | Cobalt | 1 | µg/g |
| 28 | Ni | Nickel | 5.1 | µg/g |
| 29 | Cu | Copper | < 0.5 | µg/g |
| 30 | Zn | Zinc | 131.4 | µg/g |
| 31 | Ga | Gallium | 1 | µg/g |
| 32 | Ge | Germanium | < 0.3 | µg/g |
| 33 | As | Arsenic | < 0.4 | µg/g |
| 34 | Se | Selenium | 0.6 | µg/g |
| 35 | Br | Bromine | 1 | µg/g |
| 37 | Rb | Rubidium | 0.9 | µg/g |
| 38 | Sr | Strontium | 8.1 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 69.4 | µg/g |
| 41 | Nb | Niobium | 15.7 | µg/g |
| 42 | Mo | Molybdenum | < 3.4 | µg/g |
| 47 | Ag | Silver | < 1.8 | µg/g |
| 48 | Cd | Cadmium | < 1.5 | µg/g |
| 49 | In | Indium | < 1.6 | µg/g |
| 50 | Sn | Tin | < 3.0 | µg/g |
| 51 | Sb | Antimony | < 3.0 | µg/g |
| 52 | Te | Tellurium | < 4.5 | µg/g |
| 53 | I | Iodine | < 9.2 | µg/g |
| 55 | Cs | Cesium | < 16 | µg/g |
| 56 | Ba | Barium | 869 | µg/g |
| 57 | La | Lanthanum | < 35 | µg/g |
| 58 | Ce | Cerium | < 49 | µg/g |
| 74 | W | Tungsten | < 2.2 | µg/g |
| 80 | Hg | Mercury | 0.9 | µg/g |
| 81 | Tl | Thallium | 1.2 | µg/g |
| 82 | Pb | Lead | 3.8 | µg/g |
| 83 | Bi | Bismuth | < 0.6 | µg/g |
| 90 | Th | Thorium | 0.8 | µg/g |
| 92 | U | Uranium | < 4.4 | µg/g |
Sample 40A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.337 | % |
| 12 | Mg | Magnesium | 0.0361 | % |
| 13 | Al | Aluminum | 0.1066 | % |
| 14 | Si | Silicon | 0.0562 | % |
| 15 | P | Phosphorus | < 0.0013 | % |
| 16 | S | Sulfur | 0.03931 | % |
| 17 | Cl | Chlorine | 0.951 | % |
| 19 | K | Potassium | 0.0085 | % |
| 20 | Ca | Calcium | 1.992 | % |
| 22 | Ti | Titanium | 0.3367 | % |
| 23 | V | Vanadium | 0.00147 | % |
| 24 | Cr | Chromium | 0.00039 | % |
| 25 | Mn | Manganese | 0.00063 | % |
| 26 | Fe | Iron | 0.00206 | % |
| 27 | Co | Cobalt | 1.1 | µg/g |
| 28 | Ni | Nickel | 3.9 | µg/g |
| 29 | Cu | Copper | < 0.4 | µg/g |
| 30 | Zn | Zinc | 25.1 | µg/g |
| 31 | Ga | Gallium | < 1.2 | µg/g |
| 32 | Ge | Germanium | < 0.4 | µg/g |
| 33 | As | Arsenic | 14.3 | µg/g |
| 34 | Se | Selenium | < 0.5 | µg/g |
| 35 | Br | Bromine | 17.7 | µg/g |
| 37 | Rb | Rubidium | 1.4 | µg/g |
| 38 | Sr | Strontium | 67.7 | µg/g |
| 39 | Y | Yttrium | < 0.8 | µg/g |
| 40 | Zr | Zirconium | < 4.8 | µg/g |
| 41 | Nb | Niobium | 3.7 | µg/g |
| 42 | Mo | Molybdenum | < 3.2 | µg/g |
| 47 | Ag | Silver | < 1.9 | µg/g |
| 48 | Cd | Cadmium | 11.9 | µg/g |
| 49 | In | Indium | < 1.5 | µg/g |
| 50 | Sn | Tin | 2 | µg/g |
| 51 | Sb | Antimony | 50.7 | µg/g |
| 52 | Te | Tellurium | < 4.8 | µg/g |
| 53 | I | Iodine | < 9.3 | µg/g |
| 55 | Cs | Cesium | < 15 | µg/g |
| 56 | Ba | Barium | 1534 | µg/g |
| 57 | La | Lanthanum | < 32 | µg/g |
| 58 | Ce | Cerium | < 46 | µg/g |
| 74 | W | Tungsten | < 1.4 | µg/g |
| 80 | Hg | Mercury | < 0.8 | µg/g |
| 81 | Tl | Thallium | < 1.5 | µg/g |
| 82 | Pb | Lead | 740.4 | µg/g |
| 83 | Bi | Bismuth | < 1.5 | µg/g |
| 90 | Th | Thorium | 4 | µg/g |
| 92 | U | Uranium | < 2.1 | µg/g |
Sample 40B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 4.43 | % |
| 12 | Mg | Magnesium | 0.0514 | % |
| 13 | Al | Aluminum | < 0.015 | % |
| 14 | Si | Silicon | < 0.0064 | % |
| 15 | P | Phosphorus | 0.0949 | % |
| 16 | S | Sulfur | 0.00512 | % |
| 17 | Cl | Chlorine | 43.06 | % |
| 19 | K | Potassium | 0.07 | % |
| 20 | Ca | Calcium | < 0.0069 | % |
| 22 | Ti | Titanium | 0.00337 | % |
| 23 | V | Vanadium | < 0.0039 | % |
| 24 | Cr | Chromium | < 0.00077 | % |
| 25 | Mn | Manganese | < 0.00051 | % |
| 26 | Fe | Iron | < 0.00027 | % |
| 27 | Co | Cobalt | < 1.8 | µg/g |
| 28 | Ni | Nickel | 5 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 46.6 | µg/g |
| 31 | Ga | Gallium | 1.9 | µg/g |
| 32 | Ge | Germanium | 0.5 | µg/g |
| 33 | As | Arsenic | < 0.5 | µg/g |
| 34 | Se | Selenium | 1.6 | µg/g |
| 35 | Br | Bromine | 7.9 | µg/g |
| 37 | Rb | Rubidium | 1.4 | µg/g |
| 38 | Sr | Strontium | 6.7 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | < 1.5 | µg/g |
| 41 | Nb | Niobium | < 1.5 | µg/g |
| 42 | Mo | Molybdenum | 2.2 | µg/g |
| 47 | Ag | Silver | < 1.2 | µg/g |
| 48 | Cd | Cadmium | 393.7 | µg/g |
| 49 | In | Indium | < 0.8 | µg/g |
| 50 | Sn | Tin | 3.1 | µg/g |
| 51 | Sb | Antimony | < 2.4 | µg/g |
| 52 | Te | Tellurium | < 1.4 | µg/g |
| 53 | I | Iodine | < 2.6 | µg/g |
| 55 | Cs | Cesium | < 4.0 | µg/g |
| 56 | Ba | Barium | 414.5 | µg/g |
| 57 | La | Lanthanum | < 8.3 | µg/g |
| 58 | Ce | Cerium | < 11 | µg/g |
| 74 | W | Tungsten | < 2.2 | µg/g |
| 80 | Hg | Mercury | 1 | µg/g |
| 81 | Tl | Thallium | 1.9 | µg/g |
| 82 | Pb | Lead | 4.3 | µg/g |
| 83 | Bi | Bismuth | 1.4 | µg/g |
| 90 | Th | Thorium | 4.1 | µg/g |
| 92 | U | Uranium | < 2.9 | µg/g |
Sample 40C
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 1.5 | % |
| 12 | Mg | Magnesium | < 0.028 | % |
| 13 | Al | Aluminum | 0.1295 | % |
| 14 | Si | Silicon | 0.0914 | % |
| 15 | P | Phosphorus | 0.0338 | % |
| 16 | S | Sulfur | 0.0668 | % |
| 17 | Cl | Chlorine | 2.819 | % |
| 19 | K | Potassium | < 0.0089 | % |
| 20 | Ca | Calcium | 9.368 | % |
| 22 | Ti | Titanium | 1.145 | % |
| 23 | V | Vanadium | < 0.0028 | % |
| 24 | Cr | Chromium | 0.02621 | % |
| 25 | Mn | Manganese | 0.00168 | % |
| 26 | Fe | Iron | < 0.00019 | % |
| 27 | Co | Cobalt | < 1.7 | µg/g |
| 28 | Ni | Nickel | 5.5 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 428.5 | µg/g |
| 31 | Ga | Gallium | < 2.5 | µg/g |
| 32 | Ge | Germanium | < 0.7 | µg/g |
| 33 | As | Arsenic | 9.5 | µg/g |
| 34 | Se | Selenium | < 0.8 | µg/g |
| 35 | Br | Bromine | 2.6 | µg/g |
| 37 | Rb | Rubidium | 3.1 | µg/g |
| 38 | Sr | Strontium | 143.8 | µg/g |
| 39 | Y | Yttrium | < 1.2 | µg/g |
| 40 | Zr | Zirconium | < 6.9 | µg/g |
| 41 | Nb | Niobium | 6.5 | µg/g |
| 42 | Mo | Molybdenum | 4.5 | µg/g |
| 47 | Ag | Silver | < 2.1 | µg/g |
| 48 | Cd | Cadmium | 375.2 | µg/g |
| 49 | In | Indium | 6.8 | µg/g |
| 50 | Sn | Tin | < 3.0 | µg/g |
| 51 | Sb | Antimony | 170.8 | µg/g |
| 52 | Te | Tellurium | < 4.0 | µg/g |
| 53 | I | Iodine | < 8.6 | µg/g |
| 55 | Cs | Cesium | 27.4 | µg/g |
| 56 | Ba | Barium | 2501 | µg/g |
| 57 | La | Lanthanum | < 27 | µg/g |
| 58 | Ce | Cerium | < 39 | µg/g |
| 74 | W | Tungsten | < 4.6 | µg/g |
| 80 | Hg | Mercury | < 1.4 | µg/g |
| 81 | Tl | Thallium | < 3.1 | µg/g |
| 82 | Pb | Lead | 2427 | µg/g |
| 83 | Bi | Bismuth | < 2.8 | µg/g |
| 90 | Th | Thorium | 12.1 | µg/g |
| 92 | U | Uranium | < 3.9 | µg/g |
Sample 42A
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | < 0.064 | % |
| 12 | Mg | Magnesium | < 0.014 | % |
| 13 | Al | Aluminum | 0.1111 | % |
| 14 | Si | Silicon | 0.0478 | % |
| 15 | P | Phosphorus | < 0.0015 | % |
| 16 | S | Sulfur | 0.2522 | % |
| 17 | Cl | Chlorine | 0.7282 | % |
| 19 | K | Potassium | 0.0265 | % |
| 20 | Ca | Calcium | 0.8723 | % |
| 22 | Ti | Titanium | 0.1431 | % |
| 23 | V | Vanadium | < 0.00065 | % |
| 24 | Cr | Chromium | 0.0538 | % |
| 25 | Mn | Manganese | < 0.00051 | % |
| 26 | Fe | Iron | 0.01166 | % |
| 27 | Co | Cobalt | 0.9 | µg/g |
| 28 | Ni | Nickel | 3.3 | µg/g |
| 29 | Cu | Copper | 59.6 | µg/g |
| 30 | Zn | Zinc | 173.9 | µg/g |
| 31 | Ga | Gallium | < 3.2 | µg/g |
| 32 | Ge | Germanium | < 0.9 | µg/g |
| 33 | As | Arsenic | < 12 | µg/g |
| 34 | Se | Selenium | < 1.1 | µg/g |
| 35 | Br | Bromine | 62.7 | µg/g |
| 37 | Rb | Rubidium | 4.5 | µg/g |
| 38 | Sr | Strontium | 30.6 | µg/g |
| 39 | Y | Yttrium | < 1.8 | µg/g |
| 40 | Zr | Zirconium | < 6.0 | µg/g |
| 41 | Nb | Niobium | < 2.5 | µg/g |
| 42 | Mo | Molybdenum | 36.8 | µg/g |
| 47 | Ag | Silver | < 1.5 | µg/g |
| 48 | Cd | Cadmium | 15.8 | µg/g |
| 49 | In | Indium | < 3.8 | µg/g |
| 50 | Sn | Tin | 24.5 | µg/g |
| 51 | Sb | Antimony | 2302 | µg/g |
| 52 | Te | Tellurium | < 4.5 | µg/g |
| 53 | I | Iodine | < 9.0 | µg/g |
| 55 | Cs | Cesium | < 14 | µg/g |
| 56 | Ba | Barium | 1042 | µg/g |
| 57 | La | Lanthanum | < 29 | µg/g |
| 58 | Ce | Cerium | < 41 | µg/g |
| 74 | W | Tungsten | < 3.2 | µg/g |
| 80 | Hg | Mercury | < 1.7 | µg/g |
| 81 | Tl | Thallium | < 4.0 | µg/g |
| 82 | Pb | Lead | 4682 | µg/g |
| 83 | Bi | Bismuth | < 3.9 | µg/g |
| 90 | Th | Thorium | 29.9 | µg/g |
| 92 | U | Uranium | < 3.6 | µg/g |
Sample 42B
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 0.159 | % |
| 12 | Mg | Magnesium | < 0.013 | % |
| 13 | Al | Aluminum | 0.2736 | % |
| 14 | Si | Silicon | 0.0566 | % |
| 15 | P | Phosphorus | 0.01925 | % |
| 16 | S | Sulfur | 0.04555 | % |
| 17 | Cl | Chlorine | 0.1904 | % |
| 19 | K | Potassium | < 0.0045 | % |
| 20 | Ca | Calcium | 0.02929 | % |
| 22 | Ti | Titanium | 0.5351 | % |
| 23 | V | Vanadium | 0.00215 | % |
| 24 | Cr | Chromium | 0.00043 | % |
| 25 | Mn | Manganese | 0.00086 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | < 0.7 | µg/g |
| 28 | Ni | Nickel | 15.1 | µg/g |
| 29 | Cu | Copper | < 0.3 | µg/g |
| 30 | Zn | Zinc | 3.2 | µg/g |
| 31 | Ga | Gallium | 0.7 | µg/g |
| 32 | Ge | Germanium | < 0.3 | µg/g |
| 33 | As | Arsenic | < 0.3 | µg/g |
| 34 | Se | Selenium | < 0.4 | µg/g |
| 35 | Br | Bromine | 882.3 | µg/g |
| 37 | Rb | Rubidium | < 1.2 | µg/g |
| 38 | Sr | Strontium | 1.3 | µg/g |
| 39 | Y | Yttrium | 0.9 | µg/g |
| 40 | Zr | Zirconium | < 1.6 | µg/g |
| 41 | Nb | Niobium | 3 | µg/g |
| 42 | Mo | Molybdenum | < 1.2 | µg/g |
| 47 | Ag | Silver | < 0.8 | µg/g |
| 48 | Cd | Cadmium | 0.5 | µg/g |
| 49 | In | Indium | < 1.0 | µg/g |
| 50 | Sn | Tin | < 1.6 | µg/g |
| 51 | Sb | Antimony | 95.7 | µg/g |
| 52 | Te | Tellurium | < 2.0 | µg/g |
| 53 | I | Iodine | < 3.9 | µg/g |
| 55 | Cs | Cesium | < 6.5 | µg/g |
| 56 | Ba | Barium | < 10 | µg/g |
| 57 | La | Lanthanum | < 14 | µg/g |
| 58 | Ce | Cerium | < 20 | µg/g |
| 74 | W | Tungsten | 1.5 | µg/g |
| 80 | Hg | Mercury | < 0.5 | µg/g |
| 81 | Tl | Thallium | 1.7 | µg/g |
| 82 | Pb | Lead | 4 | µg/g |
| 83 | Bi | Bismuth | < 0.5 | µg/g |
| 90 | Th | Thorium | < 0.7 | µg/g |
| 92 | U | Uranium | < 1.2 | µg/g |
Sample 42C
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | 12.4 | % |
| 12 | Mg | Magnesium | 0.171 | % |
| 13 | Al | Aluminum | < 0.041 | % |
| 14 | Si | Silicon | < 0.017 | % |
| 15 | P | Phosphorus | 0.3611 | % |
| 16 | S | Sulfur | < 0.013 | % |
| 17 | Cl | Chlorine | 44.48 | % |
| 19 | K | Potassium | < 0.017 | % |
| 20 | Ca | Calcium | 0.0312 | % |
| 22 | Ti | Titanium | 0.0397 | % |
| 23 | V | Vanadium | < 0.0035 | % |
| 24 | Cr | Chromium | 0.00092 | % |
| 25 | Mn | Manganese | 0.00103 | % |
| 26 | Fe | Iron | < 0.00026 | % |
| 27 | Co | Cobalt | < 1.8 | µg/g |
| 28 | Ni | Nickel | 7.7 | µg/g |
| 29 | Cu | Copper | < 0.6 | µg/g |
| 30 | Zn | Zinc | 337.1 | µg/g |
| 31 | Ga | Gallium | 1.9 | µg/g |
| 32 | Ge | Germanium | < 0.6 | µg/g |
| 33 | As | Arsenic | < 0.5 | µg/g |
| 34 | Se | Selenium | 0.7 | µg/g |
| 35 | Br | Bromine | 2.4 | µg/g |
| 37 | Rb | Rubidium | 1 | µg/g |
| 38 | Sr | Strontium | 25.2 | µg/g |
| 39 | Y | Yttrium | < 0.5 | µg/g |
| 40 | Zr | Zirconium | 10.4 | µg/g |
| 41 | Nb | Niobium | < 1.6 | µg/g |
| 42 | Mo | Molybdenum | < 1.6 | µg/g |
| 47 | Ag | Silver | < 0.9 | µg/g |
| 48 | Cd | Cadmium | 0.3 | µg/g |
| 49 | In | Indium | < 0.8 | µg/g |
| 50 | Sn | Tin | < 1.3 | µg/g |
| 51 | Sb | Antimony | < 1.3 | µg/g |
| 52 | Te | Tellurium | < 2.0 | µg/g |
| 53 | I | Iodine | < 4.0 | µg/g |
| 55 | Cs | Cesium | < 6.2 | µg/g |
| 56 | Ba | Barium | 649.2 | µg/g |
| 57 | La | Lanthanum | < 12 | µg/g |
| 58 | Ce | Cerium | < 17 | µg/g |
| 74 | W | Tungsten | < 4.5 | µg/g |
| 80 | Hg | Mercury | < 0.9 | µg/g |
| 81 | Tl | Thallium | 1.4 | µg/g |
| 82 | Pb | Lead | 3.7 | µg/g |
| 83 | Bi | Bismuth | 0.8 | µg/g |
| 90 | Th | Thorium | 0.9 | µg/g |
| 92 | U | Uranium | < 2.5 | µg/g |
Sample 42D
| Z | Symbol | Element | Concentration | |
| 11 | Na | Sodium | < 0.024 | % |
| 12 | Mg | Magnesium | < 0.0060 | % |
| 13 | Al | Aluminum | 0.1026 | % |
| 14 | Si | Silicon | 0.0515 | % |
| 15 | P | Phosphorus | 0.00641 | % |
| 16 | S | Sulfur | 0.0302 | % |
| 17 | Cl | Chlorine | 0.06387 | % |
| 19 | K | Potassium | 0.0111 | % |
| 20 | Ca | Calcium | 0.1413 | % |
| 22 | Ti | Titanium | 0.01255 | % |
| 23 | V | Vanadium | 0.00118 | % |
| 24 | Cr | Chromium | < 0.00034 | % |
| 25 | Mn | Manganese | 0.00063 | % |
| 26 | Fe | Iron | < 0.0015 | % |
| 27 | Co | Cobalt | 0.4 | µg/g |
| 28 | Ni | Nickel | 11.1 | µg/g |
| 29 | Cu | Copper | < 0.2 | µg/g |
| 30 | Zn | Zinc | 3.7 | µg/g |
| 31 | Ga | Gallium | 0.7 | µg/g |
| 32 | Ge | Germanium | < 0.2 | µg/g |
| 33 | As | Arsenic | < 0.2 | µg/g |
| 34 | Se | Selenium | 0.4 | µg/g |
| 35 | Br | Bromine | 1.7 | µg/g |
| 37 | Rb | Rubidium | 0.2 | µg/g |
| 38 | Sr | Strontium | 155.2 | µg/g |
| 39 | Y | Yttrium | < 0.3 | µg/g |
| 40 | Zr | Zirconium | < 3.2 | µg/g |
| 41 | Nb | Niobium | < 0.8 | µg/g |
| 42 | Mo | Molybdenum | < 1.1 | µg/g |
| 47 | Ag | Silver | < 0.5 | µg/g |
| 48 | Cd | Cadmium | < 0.6 | µg/g |
| 49 | In | Indium | < 0.6 | µg/g |
| 50 | Sn | Tin | 0.3 | µg/g |
| 51 | Sb | Antimony | < 1.0 | µg/g |
| 52 | Te | Tellurium | < 1.7 | µg/g |
| 53 | I | Iodine | < 3.5 | µg/g |
| 55 | Cs | Cesium | < 5.5 | µg/g |
| 56 | Ba | Barium | 113.6 | µg/g |
| 57 | La | Lanthanum | < 12 | µg/g |
| 58 | Ce | Cerium | < 17 | µg/g |
| 74 | W | Tungsten | 3.2 | µg/g |
| 80 | Hg | Mercury | < 0.4 | µg/g |
| 81 | Tl | Thallium | 0.7 | µg/g |
| 82 | Pb | Lead | 1.9 | µg/g |
| 83 | Bi | Bismuth | < 0.3 | µg/g |
| 90 | Th | Thorium | < 0.4 | µg/g |
| 92 | U | Uranium | < 0.9 | µg/g |
Appendix F ICP analysis results
| Cr | As | Se | Cd | Sb | Ba | Hg | Pb | |
| µg/l | µg/l | µg/l | µg/l | µg/l | mg/l | µg/l | µg/l | |
| 31 A | 7.4 | 1.7 | 49 | 1.4 | 12 | 1.1 | <0.1 | 0.64 |
| 31 B | 4.9 | 0.35 | 57 | 33 | <0.1 | 0.11 | <0.1 | 0.86 |
| 34 | 7.0 | 12 | 62 | 0.19 | 0.1 | 1.6 | <0.1 | 0.62 |
| 35 A | 6.8 | 2.9 | 63 | 1.8 | 1.5 | 1.3 | <0.1 | 0.31 |
| 37 B | 17 | 1.4 | 75 | 2.2 | 43 | 0.087 | <0.1 | 21 |
| 38 A | 9.1 | 0.52 | 63 | 0.030 | 0.82 | 0.75 | <0.1 | <0.1 |
| 38 B | 11 | 1.1 | 60 | 0.13 | 20 | 0.043 | <0.1 | 0.12 |
| 38 C | 19 | 1.0 | 58 | 0.50 | 5.3 | 0.026 | <0.1 | 1.3 |
| 39 B | 7.3 | 0.89 | 56 | 0.064 | 4.5 | 0.45 | <0.1 | 1.5 |
| 39 C | 10 | 2.8 | 56 | 0.18 | <0.1 | 0.075 | <0.1 | 0.48 |
| 40 A | 10 | 1.4 | 59 | 0.16 | 27 | 0.22 | <0.1 | 0.50 |
| 40 B | 6.4 | 0.60 | 63 | 13 | 48 | 0.047 | <0.1 | 0.63 |
| 40 C | 86 | 1.9 | 51 | 39 | <0.1 | 0.80 | <0.1 | 14 |
| 42 A | 41 | 17 | 50 | 1.7 | 5.8 | 0.16 | <0.1 | 88 |
| 42 B | 9.8 | 3.0 | 52 | 0.10 | <0.1 | 0.045 | <0.1 | 18 |
| 42 C | 7.2 | 1.1 | 58 | 1.4 | 3.0 | 0.14 | <0.1 | 6.5 |
| GV | 60 mg/kg | 25 mg/kg | 500 mg/kg | 75 mg/kg | 60 mg/kg | 1000 mg/kg | 60 mg/kg | 90 mg/kg |
Appendix G PFOS analysis results
| Concentrations: ng/g textile | ||||||||||
| Substance | 35A | 38A | 39A | 39B | 40A | 41A | 41B | 42A | 42B | 42C |
| PFOS | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 |
| PFOSA | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 |
| PFBS | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 | <75 |
| PFHXS | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| PFOA | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| PFNA | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 | <40 |
| PFDA | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 | <80 |
| PFUnA | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 | <110 |
Footnotes
[1] Rep2 substances are substances which ought to be considered as impairing to human fertility or causing damages to the unborn child. Sufficient documentation is available to have a strong assumption that the human exposure to the substance can result in a reduced fertility or can result in damages to the unborn child. (Stat. Ord. 329, 2002)
[2] CARC2 substances are substances which ought to be regarded as carcinogenic to humans. Sufficient documentation is available to have a strong assumption that the effect of the substance on humans may cause cancer (Stat. Ord. 329, 2002).
[3] Substances in this carcinogenic group are substances giving cause for concern as they may be carcinogenic to human. But for these substances no sufficient information is available to conduct a satisfactory assessment (Stat. Ord. 329, 2002).
[4] Substances in this carcinogenic group are substances giving cause for concern as they may be carcinogenic to human. But for these substances no sufficient information is available to conduct a satisfactory assessment (Stat. Ord. 329, 2002).
Version 1.0 August 2007, © Danish Environmental Protection Agency