Analysis of chemical substances in balloons
5 Screening analysis by TLC
5.1 Sample preparation and method of analysis
5.1.1 Introduction
Thin layer chromatography (TLC) is a preferred chromatographic method for determination of the accelerators used by vulcanisation of rubber, cured as well as uncured. Methods for determination of the applied accelerators are i.a. standardised in ISO 10398 ”Rubber – Identification of accelerators in cured and uncured compounds” (1998) and ”Kunststoffe im Lebensmittelverkehr” XXI BII, 2.5.
An important reason for using TLC for analysis of accelerators in rubber is that the accelerators are being decomposed at higher temperatures and thus cannot directly be analysed by gas chromatography. Moreover it is common to use zinc salts of dithiocarbamine acids, which makes the HPLC less attractive as analysis method. Finally, it is possible to spray TLC plates with reagents which yield colour according to the applied chemical substance class.
5.1.2 Sample preparation
The extraction of accelerators is made by weighing out 3 g shredded balloon and extraction with 50 ml dichlormethane 1 hour in ultrasonic bath. The solvent is decanted and flushed with 25 ml dichlormethane. The procedure is repeated and the extracts are collected and evaporated. The evaporation residue is dissolved in 2 ml dichlormethane for the TLC analysis. The amount of evaporation residue can be seen from the below table.
Table 5.1 Evaporation residue from the dichlormethane extraction
| Balloon nr. | 1 | 3 | 6 | 8 | 10 | 11 | 13 | 14 | 15 | 16 | 17 | 20 |
| %w/w | 4.9 | 4.9 | 3.2 | 2.7 | 4.2 | 3.0 | 3.3 | 3.7 | 5.0 | 5.7 | 2.9 | 4.0 |
5.1.3 The TLC-analysis
As developing solvent a mixture of hexane, toluene and methanol in the proportions 30:58:12 was chosen.
For the visualisation UV-light, 1 % cuprisulphate solution and iodine vapours were used.
Silica TLC-plates from Merck (Article 1.11798) 20 cm x 20 cm Silica 60 F 254 with concentration zone were used. 5 µl of the concentrated sample extracts and produces standards were added. The elution of the applied samples and standard substances took place over a distance of 12 cm from the start zone.
The reference substances listed in the table were used in concentrations of 1 %. The references were dissolved in dichloromethane, acetone or a mixture of these.
Table 5-2 Reference substances used for for the TLC-screening.
| Reference substance | Abbr. | CAS nr. |
| 4,4-Dithiodimorpholine | DTDM | 103-34-4 |
| Dibenzothiazolyl disulfide | MBTS | 120-78-5 |
| 2-Mercaptobenzothiazole | MBT | 149-30-4 |
| N-Morpholinyl-2-benzothiozole sulfenamide | MBS | 102-77-2 |
| Tetramethyl thiurammonosulfide | TMTM | 97-74-5 |
| Zinc dibenzyl dithiocarbamate | ZBEC | 14726-36-4 |
| Zinc dibutyl dithiocarbamate | ZDBC | 136-23-2 |
| Zinc diethyl dithiocarbamate | ZDEC | 14324-55-1 |
| Zinc dimethyl dithiocarbamate | ZDMC | 137-30-4 |
5.1.4 Results from the TLC-screenings
Table 5-3 Survey scheme TLC-screening
| Balloon no. | Result |
| 1 | ZDBC, (ZBEC), ZDMC |
| 3 | ZDBC (ZBEC), ZDMC |
| 6 | ZDBC (ZBEC),ZDMC |
| 8 | ZDBC (ZBEC) |
| 10 | ZDBC (ZBEC) |
| 11 | ZDBC (ZBEC) |
| 13 | ZDBC (ZBEC), ZDMC |
| 14 | ZDBC (ZBEC), ZDMC |
| 15 | ZDBC (ZBEC) |
| 16 | ZDBC (ZBEC) |
| 17 | ZDBC (ZBEC) |
| 20 | ZDBC (ZBEC),ZDMC |
In the chromatographic system it is not possible to differ between zinc salts of dibenzyldithiocarbamate (ZBEC) and of dibutyldithiocarbamate ZDBC), as they have the same Rf-value (the Rf-value is obtained by dividing the position of the substance in the chromatogram by the total possible distance (12 cm).
The literature (ref.-1) states that it will most likely be ZDBC based on either di-n-butylamine or di-isobutylamine.
According to FDA (Food and Drug Administration, USA) ZDBC may be used in amounts of up to 1.5 % in products intended for repeated contact with foodstuffs. Similar limits are stated in Kunststoffe im Lebensmittelverkehr, i.e. 1.2 % for zinc-dialkyldithiocarbamate accelerators.
The TLC screening cannot with certainty verify the presence of any of the other reference substances used at the screening. This applies for 2-MBT and MBS and for thiuram mono- and disulfides based on dimethyl-, diethyl- and dibutylamine.
Examples of TLC chromatograms are shown below:
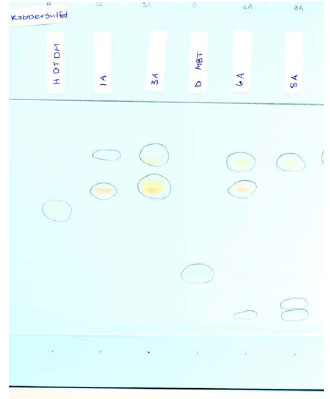
Fig. 5.1 TLC of balloon nos. 1, 3, 6, and 8. Visualisation by cupri sulphate
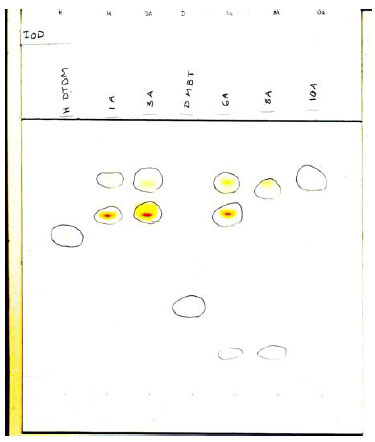
Fig. 5.2 TLC of extract from balloon 1, 3, 6, 8, and 10. Visualisation by iodine vapour
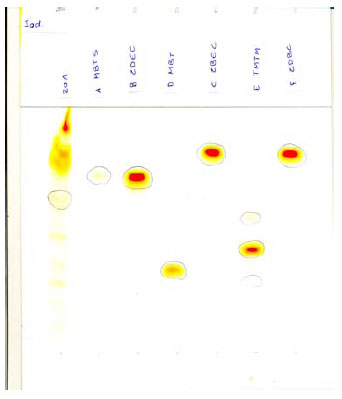
Fig. 5.3 TLC of balloon no.. 20 and a number of reference substances. Visualisation iodine.
There are individual differences in the TLC chromatograms for the selected balloons. Partly, colorants were detected in some of them, partly, chemical substances were detected with a relatively low Rf-value, and partly a colour reaction occurred after the copper sulphate visualisation which seems to indicate the presence of a substance with the same value as the zinc salt of dimethyldithiocarbamate (balloon nos. 1, 3, and 6), which gives quite another colour tone (reddish instead of yellow-green).
Version 1.0 December 2007, © Danish Environmental Protection Agency