Environmental Project no. 884, 2004
Substitution of cobalt driers and methyl ethyl ketoxime
Contents
- Screening
- Resultater fra den tekniske evaluering
- Miljø- og sundhedsmæssig vurdering
- Samlet vurdering
- 3.1 Chemical structure
- 3.2 Primary driers
- 3.3 Drying accelerators
- 3.4 Alternative driers
- 3.5 Secondary driers
- 3.6 Guidelines for use of driers
- 4.1 Function of anti-skinning agents
- 4.2 Types of anti-skinning agents
- 4.3 Alternative anti-skinning agents
5 Description of evaluated systems
6 Environmental and health screening
8 Results from technical evaluation
9 Environmental and health assessment
Preface
Due to environmental aspects as well as health concerns it would be desirable if cobalt driers and methyl ethyl ketoxime as well as hydroquinone could be avoided in coatings.
This project has therefore been undertaken to investigate the possibilities of substituting cobalt driers and methyl ethyl ketoxime in air-drying coatings. Alternatives for substitution of hydroquinone used as an antioxidant in printing inks have been investigated as well.
The project is financed by the Danish Environmental Protection Agency and the project partners are: The Association of Danish Paint and Varnish Industry (FDLF), EnPro and dk-TEKNIK ENERGY & ENVIRONMENT. Six coating manufactures and two printing ink manufactures have also participated in the project contributing with products as well as know-how. We thank Akzo Nobel Decorative Coatings A/S; Dyrup A/S; Beck & Jørgensen A/S; Flügger A/S; Junckers Industrier A/S; Teknos A/S; Akzo Nobel Inks A/S and Sun Chemical Ink A/S.
Manufacturers of driers and anti-skinning agents have contributed with samples and suggestions for substitution possibilities. We thank Surface Specialities Nordic A/S, OMG, Borchers GmbH, Sasol Servo BV and Elementis Specialities for supplying samples and technical know-how.
The technical evaluations were performed by Annette Jensen, Charlotte Pilemand and Eva Wallström – all from EnPro ApS, whereas the environmental and health assessment has been performed by Pia Brunn Poulsen and Leif Hoffmann, both dk-TEKNIK ENERGY & ENVIRONMENT. Chabanne Armand and Anette Harboe from the Association of Danish Paint and Varnish Industry have been project managers.
A steering committee was set up and four meetings have been held during the project period. The steering committee consisted of:
Kim Petersen Danish Environmental Protection Agency
Chabanne Armand The Association of Danish Paint and Varnish Industry (FDLF)
Anette Harboe The Association of Danish Paint and Varnish Industry (FDLF)
Leif Hoffmann dk-TEKNIK ENERGY & ENVIRONMENT
Pia Brunn Poulsen dk-TEKNIK ENERGY & ENVIRONMENT
Eva Wallström EnPro ApS
Charlotte Pilemand EnPro ApS
November 2003
Sammenfatning og konklusioner
Dette projekt er gennemført for at undersøge mulighederne for at erstatte koboltsikkativer og methyl ethyl ketoxim i lufttørrende malinger. En undersøgelse af alternativer til hydroquinon, som bruges i trykfarver, er ligeledes gennemført. Såvel den tekniske anvendelighed som miljø- og sundhedsprofil for alternativerne er blevet undersøgt og evalueret.
Antallet af mulige alternativer til koboltsikkativer er meget begrænset i dag. Der er indtil videre ikke blevet identificeret nogle ikke-metalliske forbindelser, som er i stand til at erstatte koboltsikkativer. Af metalliske forbindelser er det kun vanadium (V) og mangan (Mn), der har tilstrækkelig katalytisk effekt, til at blive betragtet som alternativer til kobolt (Co) sikkativer. I projektet er elleve sikkativprodukter blevet testet som erstatning for koboltsikkativer inden for et bredt spektrum af oxidativt tørrende produkter. Otte af sikkativerne er mangansikkativer, og tre er vanadiumsikkativer.
Antallet af mulige alternativer til methyl ethyl ketoxim og hydroquinon er også ret begrænset. To amin/amid-baserede anti-skind midler er blevet undersøgt sammen med to phenol-baserede produkter og acetone oxim. Vitamin E er også blevet inkluderet i testen, primært til brug i trykfarver.
Arbejdet med at finde egnede alternative sikkativsystemer, som er fri for kobolt, kan være ret omstændeligt, og da 17 produkter var inkluderet i testningen af alternativer, har det ikke været muligt at optimere hvert eneste produkt med hensyn til tørretid.
Screening
For at vurdere om alternativerne vil forbedre den generelle miljø- og sundhedsprofil for lufttørrende produkter, blev der gennemført en screening af alternative sikkativer og anti-skind midler. Alternativerne fik scorer for human toksicitet baseret på sikkerhedsdatablade og listen over farlige stoffer. Miljøscorer er kun blevet givet for meget få stoffer.
Screeningen viser, at sundhedsprofilen bliver forbedret, hvis koboltsikkativer kan erstattes med mangan- og vanadiumsikkativer. Dog indeholder nogle af de alternative sikkativer komponenter (organiske opløsningsmidler eller kompleksdannere) med uønskede sundheds- og/eller miljømæssige effekter. Ved substitution er det derfor nødvendigt at se på sikkativproduktet som helhed og ikke kun det aktive metal salt.
Ved betragtning af de aktive ingredienser i de alternative anti-skind midler, viser screeningen, at sundhedsprofilen på lufttørrende produkter kan forbedres ved at erstatte methyl ethyl ketoxim og hydroquinon med alternativerne, selv om et alternativ – acetone oxim –udelukkes ifølge screeningen. Også for anti-skind midlerne afhænger den totale sundhedsprofil for produktet af de organiske opløsningsmidler, som bliver brugt i produktet. Alligevel er den samlede profil bedre for alternativerne end for methyl ethyl ketoxim og hydroquinon.
Resultater fra den tekniske evaluering
Det samlede indtryk fra den tekniske evaluering er, at mangansikkativer i et vist omfang kan bruges som alternativer til koboltsikkativer. Vanadiumsikkativer er ikke egnede alternativer i de produkter, som er blevet undersøgt i dette projekt. Enten var tørringen utilstrækkelig, eller hvis tørring fandt sted, viste det sig, at malingsfilmen blev alt for blød sammenlignet med de originale malingsprodukter, der indeholder koboltsikkaktiv.
Baseret på de testede koncentrationer og kombinationer varierer den tekniske anvendelighed af de forskellige mangansikkativer en del fra et sikkativprodukt til et andet. Overordnet kan mangansikkativer med nogen succes bruges som alternativer til koboltsikkativer afhængig af såvel det specifikke mangansikkativ som af det specifikke produkt. Maling til gør-det-selv brug med talloliealkyder ser f.eks. ud til at være nemmere at kobolt substituere end maling med linolieakyder.
I de industrielle produkter var det generelt nemmere at erstatte kobolt end i gør-det-selv produkterne. Det skyldes formodentlig, de industrielle produkter enten indeholder modificerede alkyder eller blandinger af alkyder og ikke-oxidative tørrende bindere Mængden af oxidativt tørrende materiale er således relativt lavt i disse produkter. For trykfarverne var det muligt at opnå sammenlignelige tørretider og afsmitningseffekter i forhold til reference trykfarverne ved anvendelse af alternative Mn sikkativer.
Acetone oxim og de amin/amid baserede anti-skind midler ser ud til at forhindre overfladeskind på malinger i lukkede beholdere i et omfang, som er sammenlignelig med methyl ethyl ketoxim. Resultaterne fra skindtestene i åbne beholdere indikerer derimod, at amin/amid produkter kan have en negativ effekt på tørretiden sammenlignet med oximer. Dette bør undersøges nærmere.
Amin/amid-forbindelser virker til en vis grad som antioxidanter i trykfarver. Det samme gælder for vitamin E. Det bør undersøges, om alternativerne har en negativ indflydelse på afsmitningen.
Miljø- og sundhedsmæssig vurdering
En mere grundig vurdering af de miljø- og sundhedsmæssige effekter af alternativerne er udført. For sikkativerne er vurderingen udført på et mere generelt niveau – en vurdering af metalliske forbindelser – af to årsager: For det første er alle metal saltene i de undersøgte alternative ikke kendte pga. fortrolighed. For det andet findes der kun meget få eller ingen oplysninger om de miljø- og sundhedsmæssige forhold for specifikke metal salte.
Vurderingen af de alternative sikkativer viser, at sundhedsprofilen vil blive mindre negativ, hvis koboltsikkativerne bliver erstattet med mangan- eller vanadiumsikkativer, da kun koboltforbindelser er klassificerede med hensyn til kræftfremkaldende effekter hos mennesker. Gevinsten ved en substitution er dog ikke entydig, fordi både mangan- og vanadiumforbindelser har vist skadelige sundhedspåvirkninger (neurotoksiske påvirkninger).
Ligeledes vil miljøprofilen for lufttørrende produkter være mindre negativ, hvis koboltsikkativerne bliver erstattet med mangan- eller vanadiumsikkativer. Igen er forbedringen ikke tydeligt overbevisende, da alternativerne også er giftige overfor vandlevende organismer. Koboltforbindelser betragtes generelt som meget giftige overfor vandlevende organismer, hvorimod vanadiumforbindelser (vanadium pentaoxid) er giftige, og manganforbindelser betragtes som værende skadelige overfor vandlevende organismer.
Alt i alt vil den miljø- og sundhedsmæssige profil for lufttørrende produkter blive mindre negativ, hvis koboltsikkativer erstattes med mangan- eller vanadiumsikkativer, især da sikkativerne benyttes med de samme sekundære sikkativer i omtrent samme koncentrationer som koboltsikkativer.
For at forbedre den miljø- og sundhedsmæssige profil så meget som muligt bør der anvendes alternative sikkativer med den bedste profil med hensyn til organiske forbindelser og kompleksdannere, hvis det er teknisk muligt. Dette betyder, at sikkativer opløst i opløsningsmidler som for eksempel petroleumsdestillater og 2-ethylhexansyre bør undgås, og at sikkativer, der indeholder 2,2-bipyridyl som kompleksdanner, bør foretrækkes frem for de produkter, der indeholder 1,10-phenathrolin (pba. den nuværende klassificering). Da petroleumsdestillater i dag stadig findes i næsten alle sikkativprodukter, både primære og sekundære, er det umuligt at undgå dem helt, men produkter med et lavt indhold af petroleumsdestillater bør naturligvis foretrækkes. Ligeledes bør anti-skind midler med intet eller et lille indhold af petroleumsdestillater foretrækkes.
En vurdering af de organiske amin-forbindelser viser, at sundhedsprofilen ikke nødvendigvis bliver forbedret, hvis de eksisterende anti-skind midler bliver erstattet med de organiske amin-forbindelser. Den organiske amin-forbindelse er generelt mindre giftig og mindre irriterende end de eksisterende anti-skind midler, men oplysninger fra QSAR-studier antyder, at amin-forbindelsen kan være genotoksisk. Med hensyn til miljøet vil profilen dog forbedres, da amin-forbindelsen har en lav giftighed overfor vandlevende organismer.
En vurdering af vitamin E viser, at sundhedsprofilen på det lufttørrende produkt vil forbedres, hvis de eksisterende anti-skind midler erstattes med vitamin E. Vitamin E er grundlæggende ikke-toksisk og har vist både anti-mutagene og ikke-kræftfremkaldende virkninger. Der blev ikke fundet nogle miljøoplysninger for vitamin E.
Samlet vurdering
Konklusionen og kommentarerne, angivet i dette projekt, er kun gældende for de specifikke sikkativkombinationer og produkter, som er anvendt i den tekniske evaluering. Nogle generelle retningslinier kan der dog opstilles, og de opnåede resultater kan give en indikation til malings- og trykfarvefabrikanterne, om det på nuværende stadie, kan betale sig at erstatte koboltsikkativer i deres produkter. Før en eventuel substitution gennemføres, er det nødvendigt for fabrikanterne at efterprøve de opnåede resultater samt udføre nødvendige supplerende tests. Det er fabrikanternes eget ansvar at gennemføre en yderligere optimering af de alternative sikkativsystemer.
Med hensyn til at erstatte koboltsikkativer synes mangansikkativer at være lovende ud fra et teknisk synspunkt, da mangansikkativer på nuværende tidspunkt kan bruges som alternativer i visse produkter, men dets brugbarhed afhænger af det specifikke mangansikkativ samt af produkt et(bindertype etc.). Anvendelsen af vanadiumsikkativer som erstatning for kobolt ser ret begrænset ud på nuværende tidspunkt.
Sundheds- og miljøprofilen vil være mindre negativ, hvis koboltsikkativer erstattes med mangansikkativer, selv om manganforbindelser også har vist sundhedsskadelige effekter og er skadelige overfor vandlevende organismer. Med de oplysninger, der er tilgængelige på nuværende tidspunkt, er den sundheds- og miljømæssige profil for manganforbindelser dog bedre end profilen for koboltforbindelser. Den historiske arbejdsmiljømæssige manganeksponering indikerer, at sundhedsskadelige effekter først og fremmest er forbundet med intens eksponering over en lang tidsperiode. I malingens produktions- og brugsfase vil eksponeringen til sikkativproduktet være minimalt, hvorfor erstatning af koboltsikkativer med mangansikkativer kan anses som et skridt i den rigtige retning med hensyn til sundhedsprofilen for lufttørrende malinger og trykfarver.
Fra et teknisk synspunkt ser substitution af metyl ethyl ketoxim ud til at være ret begrænset. Det bør bemærkes, at alternativerne kun er blevet testet i én koncentration i hvert lufttørrende produkt, og en simpel optimering af koncentrationen kan føre til mere positive resultater. Det mest lovende alternativ er acetone oxim, som har en tvivlsom sundhedsprofil. For amin/amid forbindelserne, der havde en rimelig anti-skind effekt i lukkede beholdere, var der en stærk indikation af, at de vil påvirke tørretiden i større omfang end methyl ethyl ketoxim. Dette er ikke blevet bekræftet eksperimentelt. Vitamin E skal undersøges yderligere i lufttørrende malinger, før det kan fastslås, om det er et muligt alternativ til metyl ethyl ketoxim.
Med hensyn til trykfarver er en vis påvirkning af tørringen faktisk nødvendig for at opnå valsestabilitet i farven, hvorfor de mest flygtige alternativ, som f.eks. acetone oxim, ikke kan bruges. Både vitamin E og amin/amid-forbindelserne gav lovende resultater, men i den testede koncentration er de ikke nær så effektive antioxidanter som hydroquinon.
QSAR-studier indikerer, at fordelen ved at erstatte methyl ethyl ketoxim og hydroquinon med amin/amid-forbindelser kan være begrænset, fordi aminforbindelsen kan have genotoksisk effekt. Den bedste sundhedsprofil blev fundet for vitamin E, som praktisk taget er ugiftigt.
Da lufttørrende malingsprodukter vil være på markedet mange år fremover, vil der være brug for en fortsat udvikling af alternativerne. Brugen af anti-skind midler vil måske reduceres med tiden, da vandbaserede malinger vinder mere og mere frem på bekostning af de opløsningsmiddelbaserede produkter. Så længe der anvendes anti-skind midler vil det naturligvis være ønskeligt, hvis mere miljøvenlige og mindre sundhedsskadelige alternativer end methyl ethyl ketoxim og hydroquinon kunne blive tilgængelige.
Med hensyn til kobolt alternativer er der behov for at gøre dem anvendelige til at erstatte koboltsikkativer i et bredere spektrum af lufttørrende produkter. De indtryk der er opnået i dette projekt gennem kontakt til sikkativproducenterne er, at producenterne udfører en del forskning og udvikling inden for området, især med hensyn til at udvikle egnede mangansikkativer. Et virkeligt gennembrud, i det mindste fra et miljø- og sundhedsmæssigt synspunkt, ville være, hvis ikke-metalliske alternativer til koboltsikkativer kunne identificeres, men denne mulighed ligger måske langt ude i fremtiden.
Summary and conclusions
This project was undertaken to investigate the possibilities for substituting cobalt driers and methyl ethyl ketoxime in air-drying coatings. A search for alternatives to hydroquinone, used in printing inks, was performed as well. The efficiency as well as the environmental and health profile of the alternatives has been investigated and evaluated.
The number of alternatives to cobalt driers is quite limited at this stage. No non-metallic compounds, which are capable of substituting cobalt driers, have been identified so far. Of metallic compounds only vanadium (V) and manganese (Mn) possess enough catalytic effect at ambient conditions to be considered as alternatives to cobalt (Co) driers. Within the project eleven drier products have been tested as substitute to Co driers in a diversity of oxidative drying products. Eight of the driers are manganese driers and three are vanadium driers.
The number of alternatives to methyl ethyl ketoxime and hydroquinone is also quite limited. Two amino/amido based anti-skinning agents have been investigated along with two phenolic-based products, and acetone oxime. Vitamin E has also been included in the testing primarily for use in the printing inks.
The work finding a proper alternative cobalt free drier system can be laborious and as 17 products were included in the testing of the alternatives it has been impossible to work in depth with every single product optimising it with regard to drying time.
Screening
In order to determine if the alternatives will improve the overall environmental and health profile of air-drying products a screening of the alternative driers and anti-skinning agents was carried out. The alternatives were given human toxicity scores based on material safety data sheets and the list of dangerous substances. Environmental scores have only been given for very few substances.
The screening shows that if cobalt driers can be substituted with manganese and vanadium driers the health profile will improve. However, some alternative driers do contain components (organic solvents or drying accelerators) with undesirable health and/or environmental effects. In a substitution it is therefore necessary to look upon the entire drier product and not just the active metallic compound.
Only considering the active ingredients in the alternative anti-skinning agents the screening shows that the health profile of air-drying products can be improved by substituting methyl ethyl ketoxime and hydroquinone with the alternatives, although, one alternative – acetone oxime - is excluded when using the screening. However, also in this case the total health profile of the product depends on the organic solvents used in the products, but even so the profile is better than that of methyl ethyl ketoxime and hydroquinone.
Results from technical evaluation
The overall impression from the technical evaluation is that manganese driers to some extent can be used as alternatives to Co driers. The vanadium driers have not been suitable alternatives in any of the products in which they have been tested. Either did they give insufficient drying or if enough drying was induced the dry film turned out to be far too soft compared to the original products.
The efficiency of the various tested Mn driers differs quite a lot in the tested concentrations and combinations, but Mn driers can with some success be used as alternatives to Co driers depending on the specific Mn drier as well as the specific product. Do-it-yourself (DIY) coatings with tall oil alkyds seem for instance easier to substitute than coatings with linseed oil alkyds.
The industrial products were in general easier to Co substitute than the do-it-yourself products probably because these coatings either contain modified alkyds or blends of alkyds and non-oxidative drying binders, which means the amount of oxidative drying matter in the coatings is relatively low. With regard to the printing inks comparable drying time profiles and set-off effects to that of the reference inks were attainable with the alternative Mn driers.
Acetone oxime and the amino/amido based anti-skinning agents seem to prevent surface skinning on paints in closed containers to an extent, which is comparable to that of methyl ethyl ketoxime, but the results from the skinning test in open containers indicate that the amino/amido products might have a negative effect on drying time compared to the oximes. This needs to be investigated.
The amino/amido compounds do also to some extent work as antioxidants in the printing inks, so does vitamin E. It needs to be investigated if the alternatives have a negative influence on the set-off effect.
Environmental and health assessment
A more thorough assessment of the environmental and health effects of the alternatives has been carried out. For the driers, the assessment is carried out on a more general level – an assessment of the metal compounds – for two reasons. First of all, not every metal drier substance has been identified, because of confidentiality. Secondly, very few or no information on the environmental and health aspect of the specific substances was available.
The assessment of the alternative driers shows that the health profile will be less negative, if cobalt driers are substituted with manganese or vanadium driers, as only cobalt compounds are classified with regard to carcinogenic effects to humans. However, the effect is not unambiguous because both manganese and vanadium compounds have shown adverse health effects (neurotoxic effects).
Similarly, the environmental profile of air-drying products will be less negative if cobalt driers are substituted with manganese or vanadium driers. Again, the effect is not very obvious as the alternatives also are toxic to aquatic organisms. Cobalt compounds are in general considered to be very toxic to aquatic organisms whereas vanadium compounds (vanadium pentaoxide) are toxic and manganese compounds are regarded as harmful to aquatic organisms.
Overall, the environment and health profile of air-drying products will become less negative if cobalt driers are substituted with manganese or vanadium driers, especially as the driers are combined with the same secondary driers as cobalt driers and in the approximately same concentrations.
To improve the environmental and health profile as much as possible, alternative driers with the best profile of organic solvents and drying accelerators should be used if technically possible. This means that driers dissolved in solvents like petroleum distillates and 2-ethylhexanoic acid should be avoided, and that driers containing 2,2-bipyridyl as drying accelerator should be preferred to those containing 1,10-phenathroline (at present classification). As petroleum distillates today still are present in almost every drier product, both the primary and the secondary, it is impossible to avoid them completely, but products with a low content of petroleum distillates should of course be preferred. Similarly, anti-skinning agents with no or a low content of petroleum distillates should be preferred.
Assessment of the organic amino compounds shows that the health profile not necessarily will be improved if the existing anti-skinning agents are substituted with the organic amino compound. The organic amino compound is in general less toxic and less irritating than the existing anti-skinning agents, but information obtained from QSAR studies suggests that the amino compound may be genotoxic. However, with regard to the environment, the profile will improve as the amino compound has a low aquatic toxicity.
Assessment of vitamin E shows that the health profile of the air-drying product will be improved if the existing anti-skinning agents are substituted with vitamin E. Vitamin E is basically non-toxic and has shown both anti-mutagenic and anti-carcinogenic effects. No environmental information about the ecotoxicity of vitamin E was found.
Overall evaluation
The conclusion and comments made in this project can only account for the specific drier combinations and products used in the testing carried out in this project. However, some general guidelines could though be obtained and the achieved results can give some indications to the paint and ink manufacturers on whether at present it is worthwhile substituting Co driers in their air-drying product. Before substituting the manufacturers need to verify the results obtained during this project as well as perform any necessary complementary tests. Further optimising of the alternative drier systems also needs to be performed by the manufacturers.
With regard to substituting Co driers Mn driers are promising from a technical point of view as Mn driers at this point can be used as alternatives in some products, but its usability depends on the specific Mn drier as well as the product (binder type etc.). The use of V driers seems rather limited at this stage.
The health and environmental profile will be less negative, if cobalt driers are substituted with manganese driers even though manganese compounds also have shown adverse health effects and are harmful to aquatic organisms. However, with the information available at the moment the health and environmental profile of manganese compounds is better than the profile of cobalt compounds. The history of occupational manganese exposure furthermore indicates that the adverse health effects primarily are associated with intense exposure over a long period of time. In the production and use phase of paint, the exposure to the drier product will be at a minimum, for which reason a substitution of cobalt driers with manganese driers must be considered as a step in the right direction regarding the health profile of the air-drying products.
From a technical point of view the success in substituting methyl ethyl ketoxime seems rather limited. It should be noted that the alternatives have been tested in one concentration only in each air-drying paint product and a simple optimisation on the concentration might lead to more positive results. The most promising alternative is acetone oxime, which has a dubious health profile. For the amino/amido compounds, which have a reasonable anti-skinning effect in closed containers, there was a strong indication that they may influence more on the drying time than methyl ethyl ketoxime, but it has not been verified. Vitamin E needs to be investigated further in air-drying paints before it can be concluded whether it is a potential alternative to methyl ethyl ketoxime.
In the case of printing inks a certain influence on the drying is actually necessary to obtain duct stability of the ink, for which reason the most volatile alternative, e.g. acetone oxime, cannot be used. Both vitamin E and the amino/amido compounds gave promising results, but in the tested concentration they are not being as strong anti-oxidants as hydroquinone.
QSAR studies indicate that the benefit of substituting methyl ethyl ketoxime and hydroquinone with amino/amido compounds might be limited due to that they may have a genotoxic effect. The best health profile is found for vitamin E, which is practically non-toxic.
As air-drying products will remain on the coating market many years from now there is a need for continuous development of the alternatives. The use of anti-skinning agents might decrease in the future as waterborne coatings are used more and more in preference to solvent-borne. But as long as anti-skinning agents are used it would be desirable if more environmentally friendly and less harmful alternatives than methyl ethyl ketoxime and hydroquinone could be identified.
With regard to the alternative driers there is a need for making them capable of substituting Co driers in a broader spectrum of air-drying products. The impression gained during the project is that drier manufacturers actually do a lot of research and development within this area, especially with regard to Mn driers. A real break through, at least from an environmental and health point of view, would be if non-metallic alternatives could be identified. However, this might be a very distant prospect.
1 Introduction
Coatings containing drying oils and fatty acid derivatives from drying oil such as alkyds, epoxy esters and urethane alkyds dry by oxidative cross-linking. Such systems are called air-drying or oxidative drying coatings and do usually contain driers and anti-skinning agents. Most air-drying systems contain cobalt driers, often in combination with other metal driers. The most commonly used anti-skinning agents in paints are volatile oximes, methyl ethyl ketoxime being the most important, whereas hydroquinone is a commonly used anti-skinning agent (antioxidant) in printing inks.
Driers are used to accelerate the drying process of air-drying systems, whereas anti-skinning agents are added to prevent the coatings from skinning during storage in closed containers. In the case of printing inks the anti-skinning agents are used to prevent the ink from drying on the ink rollers.
Due to environmental aspects as well as health concerns it would be desirable if cobalt driers and methyl ethyl ketoxime as well as hydroquinone could be avoided in coatings. Cobalt driers can cause liver and kidney damage, dermatitis and are furthermore suspected to be carcinogenic to tissues and lungs. Methyl ethyl ketoxime is a sensibiliser and an irritant. Hydroquinone is an allergen, is possibly carcinogenic and mutagenic, and is very toxic to aquatic organisms.
The consumed amounts of driers and anti-skinning agents within the coating industry are directly related to the produced amount of air-drying coating systems. Alkyd coatings are the most important. Western Europe produces about 5 million tonnes of coatings (not including printing inks) per year, which accounts for approximately 22 % of the world production. In 1996 the European coating industry consumed 1.8 million tonnes of binders for the production of paints of which 25 % were alkyds. Round 90,000 tonnes of additives were used by the European paint industries in the same period. Of these approximately 26,000 tonnes (28.5 %) were driers and 6,300 tonnes (7.1 %) anti-skinning agents, /1/.
As the paint production in Denmark in 1996 was round 2.5 % of the total amount of produced paint in Western Europe, /1/, a rough estimate of the annual consumption of cobalt metal and methyl ethyl ketoxime in the Danish paint industry can be made. Assuming that between one third and one quarter of the total amount of driers are cobalt driers with an average cobalt content of 10 weight-% and assuming that 2.5 % of these driers are used in Denmark the annual consumption of cobalt metal within the Danish paint industry is approximately 16 to 22 tonnes.
The content of cobalt metal in a specific product depends on the amount of oxidative drying matter present in the product. The level of cobalt metal does in general rarely exceed 0.075 weight-% of the total product in an air-drying coating. The common level of cobalt metal in an air-drying coating is within the range of 0.03 to 0.05 weight-% of the total amount. Methyl ethyl ketoxime is commonly used in 0.3 to 0.7 weight-% of the total product in solvent-borne air-drying systems.
The annual consumption of anti-skinning agent is approximately 160 tonnes assuming that 2.5 % of the total amount is used in the Danish production. The major part of consumed anti-skinning agent will be methyl ethyl ketoxime. The estimated level of anti-skinning agents is probably too high as Denmark produces a relatively large amount of waterborne paints compared to the European average paint production. The level of used driers and anti-skinning agents today is expected to be comparable to the levels in 1996, /1/. The used amounts for the production of printing inks are not included in the above-mentioned numbers.
1.1 Project objects
The main objects of the project were to investigate what alternative cobalt free driers and alternative anti-skinning agents that are available on the market and to investigate and evaluate the efficiency as well as the environmental and health profile of these alternatives. The main focus has been on alternative products that are already commercially available today or will become commercially available in the near future. The alternatives have been tested in a diversity of oxidative drying products, which includes common do-it-yourself (DIY) products, industrial products and printing inks. Both solvent-borne and waterborne systems were included.
Paint and printing ink manufacturers have supplied 17 oxidative drying products for testing the technical efficiency of the alternatives. The products were selected so they cover the broadest possible product range of air-drying coatings based on the products made available from the involved manufacturers also taking into account that at least two products from each manufacturer had to be present according to the project description.
1.2 Search for alternatives
The number of alternatives to cobalt (Co) driers is quite limited at this stage. A preliminary search for alternatives was performed on the Internet and followed by direct enquiries at the drier manufacturers. The outcome of the search results is that only manganese and vanadium driers are potential alternatives to cobalt driers.
The drier manufacturers were found by the Internet search or by consulting available databases on raw material suppliers for the coating industry. Less than fifteen drier manufacturers were identified on the European market during the search. The manufacturers were addressed to clarify if they were marketing alternatives for cobalt driers and/or if they were working on developing alternatives.
The minor manufacturers, which responded, did in general at that stage not work in the area of developing alternatives. The major manufacturers did all have some activity in this area. Four manufacturers supplied samples of alternatives driers to the project work. The same four drier manufacturers do more or less cover the total supply for the entire Danish coating industry. Several manganese (Mn) and vanadium (V) based driers have been tested as alternatives to Co driers. The samples supplied were a mixture of already available drier products, either as commercial or trial products, and laboratory products. Most of the investigated drier products have during the project period been upgraded to either commercial or trial products.
A search for alternatives to methyl ethyl ketoxime and hydroquinone were performed in the same way, but only a limited number of suppliers responded to the enquiries. Three different manufacturers have supplied samples to the project. Acetone oxime, amino/amido based as well as phenolic based anti-skinning agents have been investigated as alternatives to methyl ethyl ketoxime and hydroquinone. As it was the case for driers the samples were covering both commercially available products as well as laboratory products. Vitamin E (á-tocopherol) was also included as a possible alternative, mainly for use in printing inks.
1.3 Project limitations
As a high number of products (17) were included for testing the alternatives it has not been possible to optimise every single system with regard to drying time. The obtained drying time results and complementary tests should therefore only be regarded as guidelines to the manufacturers on whether a specific drier is worthwhile testing in their product or not. The same accounts for the anti-skinning agents.
How successful the outcome of an environmental and health screening and assessment is depends on the available product information, the more information given the more reliable screenings and assessments can be performed. The information about the alternative driers and anti-skinning agents has in some cases been rather limited due to confidentiality. This especially accounts for the laboratory products, but even in some of the commercial products the exact identity of the active components is confidential. In a few cases the project group was told the identity of the active components in order to verify the classification of the substances and products.
Another limiting factor in performing the environmental and health assessment is that the metallic salts in question are not very well documented with regard to environmental as well as health effects, for which reason a more general assessment had to be performed.
2 Air-drying systems
Coatings, which are able to dry by oxidative cross-linking, are classified as air-drying or oxidative drying coatings. Air-drying coating systems contain binders such as oils, alkyds and epoxy esters, which are all based on vegetable oils or vegetable oil derivatives. On a volume basis alkyds are far the most important of the air-drying binders.
2.1 Vegetable oils
Vegetable oil molecules are glycerides, which are constituted of glycerol backbones combined with different fatty acids. The majority of the molecules are triglycerides with only small proportions of mono- and diglycerides. The fatty acids present in vegetable oils have varying hydrocarbon chain lengths even within the same oil. The chain of a fatty acid does commonly contain an even number of carbon atoms ranging from 10 to 20 including the carbon atom in the acid group (-COOH). The chemical structures of vegetable oils, glycerol and fatty acids are schematically indicated in figure 2.1.
The fatty acids combined with glycerol determine the specific properties of vegetable oils and as the fatty acid combination differs from one type of oil to another so do the properties.
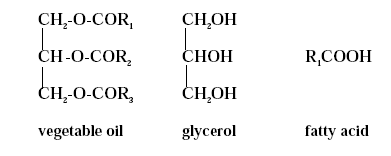
Figure 2.1 .
A vegetable oil is a triglyceride, consisting of glycerol and fatty acids. The fatty acids are symbolised by R1, R2 and
R3 indicating that vegetable oil contains fatty acids with different chain length. The fatty acids can either be
saturated or unsaturated.
The fatty acids can either be saturated containing no double bonds or they can be unsaturated containing one or more double bonds. The presence of double bonds makes the oils reactive as the double bonds are able to polymerise (cross-link) when exposed to oxygen. This ability to cross-link makes unsaturated oils able to form a solid, coherent and adherent film when spread on a surface and exposed to oxygen in the air.
The drying properties of oils depend on the degree of unsaturation. The more double bonds present in the oil the better the drying properties. Oils are usually classified as drying, semi-drying or non-drying oils according to their ability to dry when exposed to air. Over a period of time drying oils will form a tack free film, whereas semi-drying oils form films that will never become completely tack free. Non-drying oils are unable to react to form a cross-linked structure by oxidation as they mainly consist of saturated fatty acids, which have no drying properties. The non-drying oil types or derivatives of non-drying oils are therefore not being used for air-drying binders.
Semi-drying oils, such as soybean oil, sunflower oil, tall oil or safflower oil contain acids with only one or two double bonds. Semi-drying oils cannot be used unmodified in coatings. They are typically used for the manufacture of air-drying binder such as alkyds and epoxy esters.
Drying oils are oils with a high degree of unsaturation, as they consist of glycerides of fatty acids containing two or three double bonds. Linseed oil, tung oil, and oiticica oil are all classified as drying oils. Oils containing conjugated unsaturated acid show a much higher reactivity and better drying properties than oils only containing non-conjugated double bonds. Most of the oils are non-conjugated but tung oil and oiticica oil contain large amounts of fatty acids with conjugated double bonds.
Drying oils, especially the refined ones, are able to form films in their unmodified form but only very slowly. In most cases they are therefore modified to increase molecular weight and viscosity before using them in coatings to improve as well drying time as the overall film forming properties. An increased initial molecular weight means that less cross-linking is necessary to obtain a coherent film and therefore the drying time is reduced. The oils can be modified in several ways either by thermal treatments, which polymerise the oil molecules, or by chemical reaction polymerising the oil molecules with other compounds.
2.2 Drying mechanism
The drying mechanism for air-drying systems is described in general terms only as the drying mechanism of the process is very complex. Although the principal reactions involved in the oxidative cross-linking are known, the total mechanism is still not fully established. It is however accepted that the first steps in oxidative drying involve hydroperoxide formation. This initial peroxide formation is followed by decomposition of peroxides to form free radicals, which then initiate polymerisation, /2/3/4/. The chemical mechanisms presented are suggested in the open literature and they are largely based on work with model compounds, which may not always be easily related to the more complex polymer systems used in practice, /3/.
The simplest approach is to postulate oxygen attack at the site of the activated methylene, which is alpha to the double bond (C=C) involving the formation of allylic radicals obtained by hydrogen abstraction. This gives rise to peroxide formation. In the case of conjugated systems, such as tung oil, 1,4 cyclic peroxide is formed by oxygen addition, /2/3/4/.
Once, a peroxide has been formed it dissociates into free radicals, which enable a series of further reactions to take place. The peroxides decompose by dissociation of the O-O bonds leading to a variety of reaction products including intermolecular linkage and a cross-linked film is obtained. The polymerisation mechanism for non-conjugated fatty acids is presented below, /2/3/. The reactions are chain reactions which once started generate more and more free radicals and peroxides leading to auto-oxidation, /5/. The overall effect of the reactions is that the molecular size of the drying oil molecules is increased.
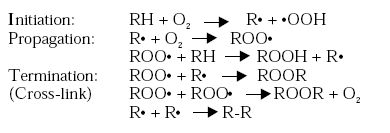
The termination reaction favours formation of polyperoxides, which subsequently decompose to polyethers. The probability of chain termination is rather high for which reason the length of the polymerised chains is relatively short, /2/3/. The rate of cross-linking does furthermore slow down as the cross-linking structure is built, due to oxygen penetration of the coating film being increasingly inhibited /4/. The cross-linking reactions will though continue very slowly within the dry coating film even years after application.
The process of oxidative polymerisation (cross-linking) is a rather slow process even for modified oil, as it normally takes from twelve to thirty-six hours to form a tack free film, /4/. Organic metal compounds, driers, can accelerate and modify these reactions. A coating that would take several days to dry will become tack free within a matter of hours when the proper driers are present in the coating systems.
During oxidation, a great number of by-products are formed, notably ketones and aldehydes. These oxidative by-products are responsible for the odour of oil containing systems, especially those containing drying oils or drying oil derivatives.
2.3 Air-drying coating/binder types
To give an impression of the diversity of air-drying binders, different vegetable oils and binders that are commonly used in air-drying coatings are described in brief in the following sections.
2.3.1 Vegetable oils
Vegetable oils have traditionally been used in a lot in paints, varnishes and printing inks because of their ability to cross-link. The oils are though commonly modified before using them in coatings to improve their drying properties. The most extensive use of vegetable oils within the coating industry is the manufacture of alkyd resins, ink vehicle systems and other synthetic resins for air-drying coatings, /3/.
2.3.1.1 Refined oils
Raw vegetable oils produced by expression or solvent extraction contain variable amounts of non-glyceride
impurities, such as free fatty acids, phospholipids, carbohydrates, sterols etc. For many applications, e.g. alkyd
manufacture, these impurities are undesirable as they may affect the drying properties and pigment wetting
capabilities of the oil, /3/4/. Raw oils are therefore rarely used directly in coating formulations. They are usually
refined by treatment with acid or alkali to precipitate the impurities. As the refined oils also have a relatively slow
drying speed they are often modified either by thermal treatment or chemical modification, or by blending them with
synthetic resins, /3/.
2.3.1.2 Polymerised and oxidised oils
A partly polymerising or oxidising of vegetable oils leads to an increase in the molecular weight. The oil thus has an
increased initial molecular weight and fewer cross-links are required to form a coherent film. The drying time of the
coating is thereby reduced, /3/.
Isomerised oil is obtained by heating oil with an aqueous alkali solution hereby increasing the extent of conjugation in unsaturated oils and making them more reactive and thereby improving their ability to cross-link when exposed to oxygen in air.
Oils polymerised by heating without the presence of accelerators are called heat-polymerised oils, heat-bodied oils or stand oils. Depending on the oil type the heating might be carried out in the presence of peroxides to improve the cross-linking. The heating is continued until the viscosity has increased to the desired value, /5/. In the case of highly conjugated oils the action of heat alone is sufficient to bring about a polymerisation. Even though the drying speed is increased, stand oils do still have a rather slow drying speed but their levelling properties are improved, which is also very important in many surface coating applications, /2/. Stand oils of drying oils can be used on their own in coatings or they can be used for further processing, for instance alkyd production. If the oils are heated and oxidised at the same time by blowing air through the oil they are called blown oils. The reaction may be catalysed by the addition of metal driers, /3/4/.
Boiled oils are produced from linseed oil using one or more driers. They are traditionally processed by controlled oxidation of raw linseed oils where metallic driers are used to accelerate the cross-linking. The oils are called boiled oils even though the cooking temperature is below the boiling and decomposition point. By proper control of the reaction, boiled oils with a wide range of viscosities can be obtained. Boiled oils are usually used in oil paints, enamels and oil-based primers. Today boiled oils are though often a simple blend of stand oils and driers, /6/7/.
2.3.1.3 Linseed oil
Linseed oil is one of the most widely used oils in air-drying coatings. Linseed oil contains a high proportion of
unsaturated linoleic and linolenic acids, which give the oil good air-drying properties. Linseed oil can be used on its
own in coatings, but it is extensively used for the production of air-drying alkyd resins and urethanated oils, /3/.
Coatings based on linseed oil tend to yellow with time, due to the presence of linolenic acid. The more linolenic acid
present the more prone will the dry coating film be to yellowing, especially in dark places. Coatings based on
linseed oils or linseed oil alkyds are therefore mainly for exterior use.
2.3.1.4 Tung oil (wood oil)
Around 80 % of the fatty acid content of tung oil is conjugated eleostearic acid, which gives tung oil rapid air-drying
properties. The surface drying of tung oil is actually so rapid that it often dries with a wrinkled surface, /6/. Tung oil
is therefore rarely used on its own. It is often used in combination with hard resins as phenolic resins, rosin esters or
alkyds in oil based printing inks.
2.3.1.5 Oiticica oil
Oiticica oil contains a high proportion of the conjugated licanic acid. The oil gives quick drying coating films with
good gloss and adhesion. The films are more brittle and have a greater tendency to yellow than those of tung oil and
therefore oiticica oil is often blended with soya bean oil to improve both colour and film flexibility. Oiticica oil is
used in printing inks that are required to dry on non-absorbent surfaces. It tends to be used interchangeably with
tung oil in ink formulations, /3/4/.
2.3.1.6 Dehydrated castor oil
Raw castor oil is a non-drying oil, but is possible to convert into an drying oil by removing the hydroxyl group from
the fatty acid together with a hydrogen atom from the neighbouring carbon atom. This yields conjugated fatty acids.
The reaction produces two isomers of linoleic acids, one non-conjugated and one conjugated in the ratio of about
3:1. The drying speed of dehydrated castor oil is somewhere in between the drying speed of semi-drying oils and
drying oils. Dehydrated castor oil is rarely used alone but is often used for the production of alkyds and epoxy ester
resins, /3/4/5/.
2.3.1.7 Soybean oil and sunflower oil
These oils are very similar in fatty acid composition and are often used interchangeably. They are semi-drying oils,
mainly used in their refined form and especially for alkyd manufacture, /3/. They have a pale colour, making them
suitable for use in white coating systems and varnishes, /4/.
2.3.1.8 Safflower oil
This oil contains a higher proportion of conjugated fatty acids than both soybean and sunflower oils and has better
drying characteristics, but still classified as semi-drying oil. Safflower is mainly used in its refined form and it is used
instead of soybean or sunflower oils where better drying is needed. Safflower oil does like sunflower and soybean
oil provides non-yellowing alkyds.
2.3.1.9 Tall oil
Tall oil is not a "true" vegetable oil as it is obtained as a by-product from wood pulp production, but as it contains
unsaturated fatty acids it is able to air-dry like vegetable oils. Tall oils are today widely used for the production of
alkyds.
2.3.2 Alkyd binders
Alkyd is one of the most used binder type within the European paint industry accounting for approximately 25 % of the total amount of consumed binders and at present they hold a majority share of the world market for non-aqueous binders, /1/3/.
Alkyd resins are short branched polyester chains containing fatty acids. They are condensation products of polyols, polybasic acids and vegetable oils or fatty acids. The properties and nature of the final alkyd are dependant on the quantity, type and nature of the modifying oil, fatty acid or acid anhydride used as well as the processing conditions. The presence of the oil provides alkyd binders with good pigment wetting properties and when the oil is unsaturated good air-drying properties are provided as well. The polyester chain gives hardness and durability to the film and improved drying speed, /5/. Alkyds may be further modified by reacting urethane, styrene, vinyl toluene or silicone groups into the alkyd binder to provide specific properties. Most widely used for the production of air-drying alkyds are linseed oil, soybean oil, tall oil, tung oil, and safflower oil. Dehydrated castor oil, linoleic acid and linolenic acid are also used in the production of alkyds, /4/.
Alkyds are classified as drying, semi-drying or non-drying dependent on the oil type used for manufacturing the alkyd. Alkyds containing more than 55 % w/w of oils are called long oil alkyds. Alkyds with oil content ranging from 45-55 % w/w are classified as medium oil alkyds whereas short oil alkyds contain less than 45 % w/w of vegetable oil, /3/4/7/. Short oil types dry fast by solvent evaporation but show limited cross-linking. Long oil alkyds dry slower, but their final durability is much better due to better cross-linking, /2/. Air-drying alkyds do therefore usually have an oil length greater than 45%, /5/.
The molecular weight of an alkyd is considerably higher than that of a vegetable oil, which means that fewer cross-links are required before a coherent film is formed. Alkyd binders do therefore dry much more rapidly than the corresponding vegetable oils. Addition of driers is though still needed to obtain a drying time, which is acceptable for commercial coating systems.
Alkyds are very versatile in use and can be used in several coating types such as paints, enamels, stains, varnishes, lacquers and printing inks. They can be utilised in a variety of applications both in decorative, industrial and speciality coatings. Oil inks are formulated almost exclusively from long oil alkyds, /3/.
2.3.2.1 High solids paints
Alkyd binders for high solids paints are similar to those for conventional organic solvent-borne systems but they
have a lower molecular weight. This makes it possible to formulate systems containing less amounts of volatile
organic solvents and yet having appropriate viscosity. Solvent free high solids systems can be formulated by using
reactive diluents.
2.3.2.2 Waterborne systems
Alkyd binders for waterborne systems are made either by converting the resin into an emulsion with the use of
emulsifiers or by incorporating water-soluble and cross-linking groups in the binder; e.g. carboxyl groups
neutralised with ammonia or reactive amines, /2/.
2.3.2.3 Modified Alkyd Resins
Alkyds can be modified to have properties ranging from fast drying hard coatings to slow drying, soft and flexible
films, /3/. The properties of alkyds are relatively easy tailored to specific needs, as there are several parameters
available for adjustments (chain length of fatty acids, degree of unsaturation, number of free OH groups, branching
etc.).
Modified alkyds are made by grafting vinyl monomers (styrene, vinyl toluene, methacrylates etc.), by radical mechanism onto unsaturated sites of the resin or by reacting free hydroxyl groups with silicone and isocyanates (urethane alkyds), /2/. Modified alkyds are widely used in applications where higher weather ability and durability, faster drying and higher gloss are desired than in conventional alkyd coatings, /1/. The higher average initial molecular weight in the modified alkyds means an improved speed of drying. This especially accounts the surface drying as the through-drying might take longer time due to the reduced levels of unsaturation in the alkyd, caused by the copolymerisation, /3/. The modified alkyds are primarily used in industrial coatings.
Polyamide modified alkyd resins (thixotropic alkyds) are made by chemical reaction with specially designed polyamide resins. This results in a jelly-like structured material, which breaks down under shear to a free flowing liquid. Once the shear is removed the resin re-sets to a jelly. These resins form the basis of non-drip or thixotropic paints. They are frequently used as blends, with unmodified alkyds or urethane modified alkyds, to impart structure. They are used in air-drying decorative paints, where their rheological properties make them attractive to users of do-it-yourself products, /3/.
2.3.3 Epoxy ester
The majority of epoxy ester resins are reaction products of an epoxy resin and a vegetable fatty acid combining the ease of handling of alkyds with some of the film properties of epoxy paint. Like alkyds, epoxy esters are characterised by oil length and oil type. All vegetable oils and fatty acids common to alkyd manufacture are also used in epoxy ester manufacture. Both air-drying and stoving types of epoxy esters are used commercially, /7/.
Although epoxy esters have similarities with conventional alkyds, they generally offer films with better colour, flexibility, adhesion and chemical resistance. Epoxy esters are less versatile in use than alkyds and more expensive, /7/.
3 Driers
The drying rate of air-drying systems is, as already mentioned, slow and even for reacted oils as alkyds the drying is too slow for commercial applications. The drying time is therefore commonly reduced, by adding metal driers to the system, as these catalyse and hereby accelerate the drying process. The drying time can hereby be reduced from days to hours. The presence of efficient driers is therefore essential for the drying of air-drying coating systems.
Different metal driers possess different drying properties as some metals have much more catalytic effect than others. Round 35 to 40 metals have been examined as possible driers, but less than twenty show worthwhile activity, /8/9/. Driers are commonly divided into two main classes according to their catalytic activity. Primary driers, which all possess some catalytic activity and secondary driers, which have no catalytic effect, when used on their own.
Some make a further division of the driers splitting up the secondary driers into through-driers and auxiliary driers due to different effects of these driers.
Primary driers can initiate and accelerate the oxidative drying process on their own, at least under certain conditions, but the strength of the catalytic activity varies within the group of conventional primary driers. At ambient conditions Co driers are the most active. As the secondary driers possess no catalytic effect, they will have no influence on the drying process if they are used on their own in air-drying systems, but combined with primary driers they become active enhancing the drying, especially the through-drying, and contribute to improved film properties as for instance gloss and film hardness.
Primary driers give drying on their own, but as they primarily promote the surface drying they are usually combined with one or two secondary driers to obtain the right balance of surface and through-drying and to obtain the right film properties.
Different metal driers are described shortly in the following paragraphs, /8/10/. All the presented metals are available as commercial driers, but some of them are used to a much larger extent than others. This especially accounts for cobalt, manganese, zirconium and calcium.
3.1 Chemical structure
Driers, which are also known as siccatives, are a group of metallic soaps containing either alkaline-earth metals or heavy metals combined with mono-basic carboxylic acids. They have the general formula (RCOO)xM where R represents an aliphatic or alicyclic hydrocarbon and M represents a metal with valence x. The acid, which is the anionic part of the metallic soap, can be varied, /11/. The presence of the acid secures adequate distribution of the metal throughout the coating medium due to their solubility in organic solvents and binders, /11/ . Naphtenic acid or octoates, especially the synthetic acids 2-ethyl hexanoic acids are commonly used today, /7/11/.
A drier product is besides the metallic salt also constituted of a solvent part. The drier component (the metallic salt) is dissolved or rather mixed into the solvent part, which acts as carrier medium. Today dearomatised hydrocarbons are typically used. Drier products with vegetable esters as carrier media have also become available. The fatty acid esters should have the advantage, besides being based on renewable resources, that it is able to cross-link with the coating film minimising the VOC contribution, /10/.
Different types of drier products based on the same metal are available as several parameters can be changed. The metal can be reacted with different acids. The active drier compound can be mixed into different carrier media and the same metal drier type is usually available in different concentrations. The typical metal content in a drier product is between 5 and 20 %, lead driers being an exception typical having a higher content.
Air-drying coatings usually contain a mixture of different driers and the coating manufacturers can either mix their own drier systems or they can purchase drier packages, which are commercial drier products combining two or more driers in one product.
3.2 Primary driers
Primary driers are also referred to as top driers, surface driers, oxidative driers or catalytic driers. Top driers and primary driers being the most used expressions. The main function of primary driers is to promote rapid surface drying of air-drying coatings. The driers do also, in varying degree, possess some through-drying properties. Primary driers are normally used in coatings in amounts varying from 0.005 to 0.2 % metal based on the solid binder or oil, /3/.
Cobalt (Co), manganese (Mn), cerium (Ce), iron (Fe) and vanadium (V) are five metals used for commercial top driers. Driers based on cobalt and manganese are the most commonly used.
The metal atom in the primary drier must be able to undergo oxidation from a lower state to a higher state with the fatty acid peroxides present in the system before the metal salt can act as a drier. In the case of cobalt the following mechanism has been proposed, /2/3/4/.
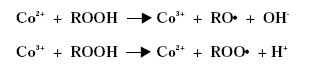
The driers have been shown to take up oxygen as follows, /2/:
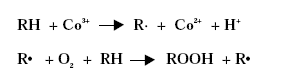
Driers have also been shown to act as oxygen carriers to initiate the radical formation, /3/4/:
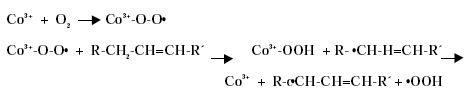
Primary driers catalyse the formation and/or decomposition of peroxides, which are formed by the reaction of oxygen with the air-drying binder or drying oil as described in a previous paragraph. Free radicals are formed and the formation of direct polymer to polymer cross-links (top drying) becomes possible. The reactions do also cause the formation of hydroxyl groups and carbonyl groups on the air-drying oil or binder, which are then available for the through-driers to form oxygen-metal-oxygen bridges (cross-links) between the polymer molecules, /10/.
Primary driers can for several reasons loose some of their activity before the cross-linking takes part and it inevitably results in prolonged drying time for the air-drying coating. The phenomenon is called loss-of-dry. The presence of pigments in the coating system can lead to a loss-of-dry as the driers within time are adsorbed on the surface of the pigment particles. Adsorption of driers on the pigments will have substantial effects on the drying, as the adsorbed driers no longer can participate in accelerating the drying process. This is especially a problem during prolonged storage. The loss-off-dry effect is most apparent for pigments with very high surface areas, such as carbon black. In waterborne systems the driers can be hydrolysed during storage or the active driers might form complexes with certain coating ingredients present in the systems. Both things lead to a loss-of-dry.
The loss-of-dry problems can usually be counteracted, at least to some extent, by using auxiliary driers or drying accelerators. Both types of compounds are described later.
Primary driers can also loose activity simply by change of oxidation state and it is recommended to add very active driers, as cobalt driers, as late in the manufacture process as possible.
3.2.1 Cobalt
Cobalt is far the most active of the five primary metal driers and therefore still the most important and widely used drier in air-drying coatings, solvent-borne as well as waterborne, despite the environmental and health drawbacks.
Cobalt driers give a rapid surface drying. Used alone or in relatively large amounts it may cause surface wrinkling. If a surface dry too rapidly the oxygen uptake is prohibited beneath the surface of the coating film. This leaves the coating mobile and soft right under the surface due to a low degree of cross-linking. Movements of the coating beneath the dry surface result in wrinkles in the film. To avoid a too rapid surface drying and to provide uniform drying cobalt is commonly used in combination with other metal driers, such as manganese, zirconium, rare earth metals and calcium, /12/. Cobalt driers can be used on its own in the waterborne system, but are most often combined with a drying accelerator.
Cobalt needs only to be added in very small amounts and does therefore tend to minimise discoloration compared to other drier metals. Cobalt does furthermore not discolour white coatings to the same extent as other driers since the deep blue colour of cobalt counteract the yellow of the oils and alkyd binders and thereby enhances the whiteness of the paint.
3.2.2 Manganese (traditional)
Manganese driers are some of the most important metal driers next to cobalt driers. Traditional manganese driers are medium in activity having both oxidising and polymerising properties, for which reason they promote both surface and through-drying /11/13/. Manganese is, in its traditional carboxylate form, used extensively in air-drying products, most commonly in combination with Co driers to enhance the through-drying of a coating. Used alone manganese driers have a tendency to produce hard brittle films.
Manganese has a relatively dark colour, which makes it most suitable for pigmented coatings as it tends to discolour light-coloured or clear coatings, /12/13/. Light-coloured manganese driers are though commercially available today, /10/. High atmospheric humidity may severely inhibit the efficiency of manganese, /13/.
A new generation of manganese driers has become available which possess more catalytic effect than the traditional type making them more suitable alternatives to Co driers. These driers are described in paragraph 3.4.
3.2.3 Vanadium
Vanadium driers provide both surface drying and through-drying of the coating film. According to some drier manufacturers it can be used as a substitute for other top driers, especially cobalt driers, /13/. Improved through-drying can be obtained by combining it with strontium, zinc or zirconium based driers. Vanadium driers can be used in solvent-borne air-drying coatings and for high solids paints. In its emulsifiable form it can be used for water-borne systems as well, /13/. Vanadium driers can cause discolouration of the film.
3.2.4 Iron
Iron is a primary drier which above all improves the through-drying. It exhibits very little drying at room temperature, for which reason the use in air-drying coatings is limited. It becomes a very efficient drier at high temperature and is therefore primarily used in stoving systems. Iron driers provide tough, durable, yet very flexible films with extremely good gloss. Iron driers are very dark in colour and have a severe tendency to yellow. Therefore they can only be used in dark coloured pigmented systems, /12/13/.
If iron driers are used in air-drying systems, they are particularly useful in eliminating after tack common to oxide pigmented paints and unprocessed fish oil compositions. Iron driers can also function as pigment wetting agents and help to obtain quicker and better grinds when used with carbon black pigments. They can also act as adhesion promoters in anti-corrosion coatings, /12/13/.
3.2.5 Cerium
Cerium driers are far less active than cobalt and manganese. At low temperatures (below 0ºC) or at very high atmospheric humidity cerium driers do though show higher efficacy than the other primary driers. Especially combined with cobalt they retain good drying properties even at low temperatures, /13/.
3.3 Drying accelerators
Drying accelerators or complexing agents are non-metallic compounds (organic ligands), which are able to increase the activity of primary drier metals causing a more rapid drying of the coating film. They function by complexing with the metal atoms by forming chelates, /10/14/.
Two different types of drying accelerators are used extensively commercially. These are 2,2'-bipyridyl and 1,10-phenanthroline. They are used in solvent-borne as well as waterborne air-drying systems.
In waterborne coatings, hydrolysis of the primary drier can lead to a loss-of-dry upon storage of the coating. Combining the primary driers with drying accelerators some protection from hydrolysis is obtained, /10/. Loss-of-dry due to adsorption of the metal drier on the pigment surface is also to some extent reduced by the use of drying accelerators, /10/.
3.4 Alternative driers
No non-metallic compounds with sufficient drying activity to substitute cobalt driers have to the best of our knowledge been identified so far and as the secondary driers possess no catalytic effect the alternatives to cobalt driers must necessarily be found within the group of primary driers. The substitution possibilities therefore seem quite limited.
Cerium and iron based driers have not been included in the testing as these driers are not efficient at ambient temperature. Cerium is efficient at low temperature and high humidity, /13/, whereas iron only becomes efficient at elevated temperature, /12/13/.
Vanadium driers provide both surface drying and through-drying of the coating film. Vanadium driers have been suggested to be useful substitutes for cobalt driers, /13/, and were therefore included in the evaluation.
Manganese driers are well-known driers, which in their most common form as Mn carboxylates generally possess far less catalytic effect than Co driers /12/, but the ability of drying accelerators/complexing agents to enhance the activity of the primary drier metal has in the recent years been utilised in developing new types of manganese driers. These driers, which typically are complexes of Mn carboxylates with chelating ligands as for instance bipyridene or phenanthroline, possess far more catalytic effect than the conventional types, /14/. Several Mn complex based driers have been included in the evaluation and tested as alternatives to Co driers.
Alternative cobalt free drier products based on both vanadium and manganese are commercial available.
3.5 Secondary driers
The primary driers, this accounts for both Co driers and their alternatives, need in most cases to be combined with secondary driers to obtain the right drying profile and film properties. Secondary driers are often divided further into through-driers and auxiliary driers according to the different effects of the driers.
Secondary driers are not able to start any cross-linking reactions on their own and can therefore only function in combination with primary driers. They play no part in the oxidation/reduction cycle as the primary driers do, but once electron-donating groups are present through-driers assist in the polymerisation process by the formation of coordination compounds with a consequent increase in the drying rate, /3/. Through-driers are also called cross-linking driers, polymerising driers or coordination driers. Eight different metals are used for commercial through-driers: zirconium (Zr), lanthanum (La), neodymium (Nd), aluminium (Al), bismuth (Bi), strontium (Sr), lead (Pb) and barium (Ba), /10/.
The other group of secondary driers are auxiliary driers, which are also referred to as promoters or coordination driers. Four metals are used for commercial auxiliary driers: potassium (K), lithium (Li), calcium (Ca) and zinc (Zn). The first three increase the rate of top drying, whereas zinc usually inhibits top drying, /10/. Auxiliary driers act as promoters for the primary driers and increase the rate of oxygen uptake in air-drying systems considerably, /3/. Besides promoting the through-drying they also improve the stability of drier systems by preventing loss-of-dry of the primary driers, /8/.
Secondary driers are normally used in amounts varying from 0.05 to 0.5 % metal based on the air-drying binder. The most commonly used secondary driers in conventional air-drying coating are Zr, Ca, Ba and Zn.
3.5.1 Zirconium
Zirconium driers strongly activate the primary driers thus promoting surface and through-drying. It is generally used in combination with cobalt, manganese and calcium and improves the through-drying primarily by the formation of coordination bonds, /12/13/.
Zirconium driers have been known for a long time, but gained its real popularity when legislation restricted the use of lead driers in many countries. Compared with other secondary driers zirconium has better properties in terms of colour, yellowing and stability, /13/. In combination with cobalt it is particularly suitable for use in light-coloured air-drying coatings and stoving systems. It is furthermore recommended for eliminating tack of certain tall-oil alkyd resins, /12/13/. Zirconium driers are the most widely used substitutes for lead driers as zirconium is less toxic than both lead and barium, /10/.
3.5.2 Aluminium
Aluminium driers are effective through-driers, which promote the cross-linking. In combination with primary driers they provide enhanced through-drying, better pigment wetting and dispersion, greater water resistance, higher gloss retention and less discoloration of air-drying systems. A limitation to the use of aluminium driers is that they are more resin specific and tend to build viscosity in systems with high acid number and/or hydroxyl number, /12/.
3.5.3 Rare earth metal driers
Rare earth driers, containing high levels of lanthanum, neodymium or cerium, promote polymerisation and through-drying. Rare earth driers are especially effective at low temperatures and high humidity conditions. They also contribute to improved gloss, /12/.
Rare earth driers are more active than lead or zirconium in oleoresinous and alkyd baking finishes, epoxy esters, styrenated alkyds and silicone formulations, /12/. Rare earths and aluminium are used in special formulations such as high solids paints, /12/.
3.5.4 Bismuth
Bismuth driers have a strong activating effect on cobalt driers improving the through-drying properties especially in extreme weather conditions as for instance high humidity. When combined with cobalt driers it improves the drying properties of conventional alkyd coatings, /13/.
3.5.5 Strontium
Strontium driers are a cost effective alternative to zirconium driers providing superior drying performance in low temperatures and high humidity conditions, /15/. Strontium driers are classified as non-toxic, /16/.
3.5.6 Lead
Lead driers are effective through-driers, which are almost always used in combination with cobalt and manganese to promote a uniform through-drying of coating films. In contrast to the primary driers lead affects the drying of the film throughout the entire film thickness and due to its superior polymerising effect lead ensures a thoroughly hardened film. Lead driers also improve flexibility, toughness and resistance properties of the coating film, /11/13/.
Lead is still a widely used drier even though its use becomes more and more restricted due to legislation. The use of lead driers is banned in Denmark due to its toxicity.
3.5.7 Barium
Barium driers promote the through-drying and improve gloss. Furthermore, Barium acts as a wetting agent for pigments and extenders and therefore prevents the adsorption of primary driers at the surface of the pigments. The stability of the drier system is thus increased even during prolonged storage. It is used as a substitute for lead driers, but has a relatively high acute toxicity, which to some extent prohibits its use, /13/. In connection with toys the use of barium is totally banned.
3.5.8 Lithium
Lithium driers improve the through-drying and hardness of air-drying coatings and reduce their tendency to wrinkle. The best results are obtained when used in combination with cobalt. Lithium driers are especially efficient in systems based on low molecular weight binders and are therefore excellent driers for high-solids paints, but the whiteness of long-oil alkyd paints may be influenced, /11/13/. Lithium driers further improve the storage stability and through-drying of water reducible alkyd dispersions, /13/.
3.5.9 Calcium
Calcium driers have a significant synergistic effect contributing to improving through-drying when combined with primary driers as cobalt and manganese, /13/. Calcium driers further help to improve hardness and gloss of the coating film. They also act as wetting agents and minimise loss-of-dry by being preferentially adsorbed at the surface of pigments, preventing the adsorption of primary driers. The drying stability of the system is hereby improved, even on prolonged storage, /11/. Salt formation might also be a contribution factor in the action of calcium as a stabilising drier. Calcium, which is a stronger base than other driers, would preferentially complex with acid groups, leaving the other driers free for catalytic activity in the system, /2/.
3.5.10 Zinc
The primary function of zinc driers is to keep the film open by retarding the surface drying, thereby allowing easy access of oxygen throughout the entire coating film for a prolonged period of time. This results in a better through-drying, a harder film and it prevents surface wrinkling. The drying time may, however, be increased slightly, /11/13/. Zinc is the best wetting agent of all the metal driers and when incorporated in a formulation at an early stage, it greatly reduces the mixing and grinding time of the formulation. Zinc driers have extremely light colours for which reason they can be used in relatively large amounts without discolouring the film. Zinc also improves gloss and has the additional property of counteracting mildew formation, /11/.
3.6 Guidelines for use of driers
The combination of driers as well as the optimum concentrations of driers varies from coating system to coating system, but some general recommendation can though be given. The most commonly used drier system is a combination of Co, Zr and Ca as this system in most cases, at least for conventional solvent-borne alkyd coatings, will give reasonable drying times.
High solids or coatings containing modified alkyds or non-alkyd binders often need other types of cobalt based drier system. The same accounts for waterborne coatings. Examples of cobalt based drier systems for different types of air-drying systems are given below. One example of a cobalt free drier system for high solids paints is given as well. The most commonly used cobalt drier system is presented in the first example. The concentrations given are metal concentrations on solid air-drying binder, /12/.
Conventional solvent-based alkyd coatings, /12/:
0.06 % Co + 0.3 % Zr + 0.2 % Ca
High solids, /12/:
0.04 % Co + 0.6 % Zr + 0.2 % drying accelerator
or
0.04 % Co + 0.3 % Nd + 0.2 % drying accelerator
or
0.04 % Co + 0.3 % Al
Coatings with non-alkyd binders, /12/:
0.04 % Mn + 0.04 Co + 0.2 % drying accelerator
or
0.04 % Mn + 0.2 % drying accelerator
Waterborne coatings, /12/:
0.1 % Co + 0.2 % drying accelerator
or
0.05 % Co + 0.05 % Mn + 0.2 % drying accelerator
The optimum concentration will vary from system to system, for which reason the above-mentioned examples only should be taken as guidelines. Other combination than the ones mentioned in the examples might also work perfectly well.
In table 3.1 ranges of commonly used concentration of selected driers are presented. The concentrations are given as metal content on solid air-drying binder in the coating system. The group of primary driers is placed at top of the table. The levels of used drier metals in the investigated alternative cobalt free drier systems are shown in chapter 8.
Table 3.1
Recommended level of metal content in weight-% based on amount
air-drying binder in the coating system /11/12/.
*Non-metallic compounds.
| Metal | % w/w on air-drying binder content |
| Cobalt | 0.01 – 0.2 |
| Manganese | 0.01 – 0.1 |
| Vanadium | 0.02 – 0.1 |
| Iron | 0.04 – 0.15 |
| Cerium | 0.05 – 0.3 |
| Zirconium | 0.1 – 1.0 |
| Aluminium | 0.2 – 1.0 |
| Rare earth | 0.1 – 0.3 |
| Strontium | 0.1 – 0.5 |
| Lead | 0.3 – 1.0 |
| Barium | 0.1 – 0.25 |
| Lithium | 0.01 – 0.05 |
| Calcium | 0.1 – 0.4 |
| Zinc | 0.05 – 0.25 |
| "Drying accelerators"* | 0.1 – 0.3 |
3.6.1 Driers for waterborne systems
In solvent-borne air-drying systems the same cobalt based drier will be able to function satisfactorily in many different coating types. This is not the case in waterborne systems, as the drier system is strongly dependent on the nature of each individual coating product, /10/. This is partly due to the large diversity of water-reducible binders and partly due to the presence of certain ingredients in some waterborne systems that may affect the driers.
The presence of large volumes of water changes the drying chemistry of air-drying binders. Water acts as a chain transfer agent in the free radical mechanism, which can slow the rate of the desired free radical reactions markedly, /10/. Therefore, large amounts of driers are needed in the waterborne systems. A cobalt content of 0.02-0.06% based on the solid binder is usually enough for most solvent-borne coatings to dry adequately, but in waterborne systems 0.1-0.15% cobalt are needed. Through-driers are less effective in waterborne coatings than in solvent-borne and in many systems no through-driers are needed at all. Manganese driers are though also effective in drying most alkyd emulsions, /10/.
Compared to solvent-borne air-drying systems it can be much more challenging to combine a drier system that works properly in waterborne systems. The presence of certain ingredients in waterborne coatings may affect the drying time. Especially ammonia, amines or phosphates could lead to a loss-of-drying of the primary drier, as these ingredients complex with cobalt metal and thereby reduce its drying activity. Additives having a negative charge might also influence the drying time, /10/.
Most of the mentioned problems can be counteracted, at least to some extent, by a proper choice of auxiliary drier and/or drying accelerator. Driers for specific use in waterborne systems are available, but driers intended for use in solvent-borne coatings can by proper dispersion often be used in waterborne systems as well.
4 Anti-skinning agents
The formation of surface skin on air-drying coatings results from the same drier catalysed oxidative polymerisation processes, which normally result in drying of the coating film. Anti-skinning agents are therefore added to prevent unintended skinning on paints and printing inks. The need for preventing skin formation might range from preventing in-can skinning during storage in closed container to preventing the ink from drying in the press duct. The former may be achieved by using volatile anti-skinning agents, whereas full overnight duct stability only can be achieved by using non-volatile anti-skinning agents, more often referred to as antioxidants, /17/.
In closed containers the oxidation and thereby the skin formation takes place due to the presence of air pillows between the coating surface and the closed lid. The skinning inevitably results in a loss of coating material and a possible contamination of the bulk. Anti-skinning agents are therefore added to solvent-borne air-drying coatings to prevent in-can skinning during storage by prohibiting the drier effect until application of the coating. Methyl ethyl ketoxime is far the most used anti-skinning agent in paints, where volatile types are preferred. In the printing inks where duct stability is the issue the non-volatile hydroquinone is used to a large extent. Printing inks are though also often sprayed with volatile anti-skinning agents on the surface of the ink before storing them in cans.
Only very small amounts of anti-skinning agents are needed to prevent skin formation. Typically a fraction of less than 1% of the total formulation is necessary, /4/17/18/. Anti-skinning agents are commonly only added to solvent-borne systems as the waterborne systems are not as prone to skinning.
4.1 Function of anti-skinning agents
Anti-skinning agents react with the free radicals formed during the oxidative polymerisation processes, as they are more readily oxidised than the drying oils or drying oil derivatives present in the coating. The anti-skinning agents hereby prevent the cross-linking from taking place, for which reason the drying of the coating is stopped, /19/. The volatile anti-skinning agent might also to some extend form complexes with the primary drier metal and hereby influence the activity of the drier system, /19/. The anti-skinning agents continue to act until all the molecules of the compound are exhausted.
The use of anti-skinning agents is always a compromise between preventing skinning and retaining an adequate drying potential of the coating after application. The cross-linking should be as slow as possible during storage and then regain its full drying potential as soon as possible after application. Care must be taken to ensure that the minimum amount of anti-skinning agents is used, especially for the non-volatile types as excessive amount might have significant effect on the drying of applied coating or printing ink, /4/. The activity of anti-skinning agents should therefore preferably come to an end immediately after application of the coating, /3/4/17/18/.
4.2 Types of anti-skinning agents
The most common types of anti-skinning agents are oximes, substituted phenols or quinones. Various napthols and aromatic amines are also sometimes used.
4.2.1 Volatile anti-skinning agents
Volatile anti-skinning agents are only effective where atmospheric contact is restricted, for which reason they are mainly used for preventing skinning in closed containers. They become ineffective in open or loosely closed containers. Due to the volatile nature they evaporate shortly after application having little or no effect on the drying of the coating. Volatile oximes are primarily used in paints, methyl ethyl ketoxime being far the most important.
Cyclohexanone oximes are also used from time to time, but mainly in printing inks as they are less volatile and therefore influence negatively on the drying time, /4/19/.
4.2.2 Non-volatile anti-skinning agents (antioxidants)
Non-volatile anti-skinning agents can either be substituted phenolics or quinines, /4/. Hydroquinone is a very strong antioxidant, which severely inhibits oxidation. Non-volatile anti-skinning agents might have a marked effect on the drying of the coating after application, even if only slight excess is used. They should therefore always be used with care. Non-volatile anti-skinning agents are mainly used in printing inks. Especially hydroquinone is used to induce overnight duct stability. Obviously, an ink containing a strong antioxidant will take longer to dry than if the antioxidant was not present, but as most/many inks are printed in very thin films on absorbent substrate the retarded drying time does commonly not lead to any severe set off effects.
4.3 Alternative anti-skinning agents
Amino/amido based compounds as well as phenolic based compounds have along with acetone oxime been suggested as possible alternatives to methyl ethyl ketoxime. The amino/amido based anti-skinning agents and the phenolic based have also been investigated as alternatives for hydroquinone. Vitamin E (á-tocopherol), which is a natural antioxidant, has also been included. It was mainly tested in the printing inks, but some linseed oil paint manufacturers actually use it in their paint products, /20/.
5 Description of evaluated systems
5.1 Products
Oxidative drying systems are available in a variety of products covering a broad range of application areas. The products included in this project for testing the alternative driers and anti-skinning agents have been chosen in such a manner that they cover the most relevant and broadest possible area of air-drying systems on basis of the coating systems made available from the participating manufacturers. The selection has been made on basis of the information the manufacturers have provided on their own systems.
Eight manufacturers have supplied products for the project within the area of do-it-yourself coating (also called decorative coating), industrial coating and printing inks. Two products from each coating and printing ink manufacturer had been included in the testing of the alternatives. The products included for testing have been made anonymous and it was agreed with the manufacturers which specific information that should be published and communicated. No additional information on the products can be given without the consensus of the manufacturers.
The products were typically received in pairs. One sample was the original product containing cobalt driers and methyl ethyl ketoxime (or hydroquinone in the case of printing inks), which from now on is referred to as reference products. The other sample was a product sample without any driers and anti-skinning agents.
5.1.1 Do-it-yourself products
Eight products within the area of do-it-yourself coatings (DIY coatings) were included to test the efficiency of the alternatives. All products are alkyd coatings mainly of the long oil alkyd type. The alkyds are either based on tall oil, linseed oil or soybean oil. A few products contain thixotropic alkyds (polyamide modified alkyds). Six of the products are wood stains and two are enamels. Two of the stains are waterborne products and two are high solids coatings.
All the solvent-borne products contain methyl ethyl ketoxime as anti-skinning agent. In most of the products, a Co/Ca/Zr drier system is used containing cobalt drier as primary drier and zirconium and calcium driers as secondary driers.
A few systems contain zinc driers instead of zirconium and one product used barium as an additional secondary drier. A general description of the do-it-yourself products is given in table 5.1.
Table 5.1
General descriptions of do-it-yourself products included in the project.
| Product code | DIY-P1 | DIY-P2 | DIY-P3 | DIY-P4 | DIY-P5 | DIY-P6 | DIY-P7 | DIY-P8 |
| Type | Stain | Enamel | Stain | Enamel | Stain | Paint | Stain | Stain |
| Thinner | Solvent | Solvent | Water | Solvent | Solvent | Water | Solvent | Solvent |
| Drier system | Co/Ca/ Zr |
Co/Ca/ Zr |
Co/Ba/ Zr |
Co/Ca/ Zr |
Co/Ca/ Zr |
Co/Ca/Ba/ Zn |
Co/Ca/ Zn |
Co/Ca/ Zn |
| Anti-skinning agent | MEKO* | MEKO* | None | MEKO* | MEKO* | None | MEKO* | MEKO* |
* Methyl ethyl ketoxime
5.1.2 Industrial products
Within the area of industrial coatings five products were included. Two of the products are alkyd primers, one waterborne and one solvent-borne. One product is an alkyd topcoat. Two of the products are urethane modified alkyd products, one wood primer and one wood lacquer.
The industrial products are more diverse in the use of driers than the do-it-yourself products, even though they basically use the same four metal driers. All the products except one contain cobalt driers. A general description of the industrial products is given in table 5.2. Product IND-P9 is included for substitution of methyl ethyl ketoxime. All the solvent-borne products contain methyl ethyl ketoxime.
Table 5.2
General descriptions of the industrial products included in the project.
| Product code | IND-P9 | IND-P10 | IND-P11 | IND-P12 | IND-P13 |
| Type | Primer | Lacquer | Topcoat | Primer | Primer |
| Thinner | Solvent | Solvent | Solvent | Solvent | Water |
| Binders | Oil modified urethane | Oil modified urethane | Modified alkyd + Blend of non-oxidative drying binders |
Modified alkyd +
Blend of non-oxidative drying binders |
Modified alkyd +
Blend of non-oxidative drying binders |
| Drier system | Ca/Mn | Co/Mn/Ca/Zr | Co/Zr/Zn | Co/Ca/Zr | Co + phenanthroline |
| Anti-skinning agent | MEKO* | MEKO* | MEKO* | MEKO* | None |
* Methyl ethyl ketoxime
5.1.3 Printing inks
Four printing inks (two cyan and two magenta) for sheet-fed offset were used for test of the efficiency of alternative driers and anti-skinning agents in printing inks. The inks are either based on blends of long oil alkyd, soybean oil and linseed oil or on blends of alkyd, linseed oil and wood oil (tung oil). All the original printing inks contain drier systems based on Co and conventional Mn driers. The antioxidant used is hydroquinone. Reference inks containing the original driers and antioxidants were used for comparative test.
Table 5.3
General descriptions of the printing inks included in the project.
| Product code | INK-P14 | INK-P15 | IN-P16 | INk-P17 |
| Type | Sheet-fed | Sheet-fed | Sheet-fed | Sheet-fed |
| Colour | Cyan | Cyan | Magenta | Magenta |
| Binders | Linseed oil alkyds | Linseed oil alkyds | Linseed oil alkyds Linseed oil |
Alkyds
Linseed oil Wood oil |
| Thinner | Vegetable oils | Mineral oils | Mineral oils | Vegetable oils |
| Drier system | Co/Mn | Co/Mn | Co/Mn | Co/Mn |
| Anti-skinning agent | Hydroquinone | Hydroquinone | Hydroquinone | Hydroquinone |
5.2 Alternative Cobalt free drier systems
5.2.1 Alternatives to Co-driers
During the search for alternatives available on the market at present only manganese and vanadium came up as alternatives to Co driers. Various driers of both metal types have been investigated and evaluated. Of these 8 were manganese driers and 3 were vanadium driers. As the intention of the project was to investigate if alternatives to cobalt driers exist and not to promote one commercial drier product in preference to another the driers have been coded. The commercial name of the driers will though be made available on request from the participating paint and ink manufacturers and other interested parties. The investigated alternative driers are described in table 5.4.
Table 5.4
The investigated alternatives to cobalt driers. It is indicated if a drying accelerator is build into the drier product and
if the drier product is made for waterborne systems only. Furthermore is it indicated in how many systems the driers
have been tested in.
*Used as a second primary drier, not as an alternative to cobalt driers.
| Code | Metal | Drying accelerator | Specific for waterborne | Numbers of systems | ||
| DIY | Industrial | Printing ink | ||||
| Mn Traditionel |
Mn | No | No | 3 | 0 | 4* |
| Mn1 | Mn | Yes | No | 8 | 4 | 4 |
| Mn2 | Mn | Yes | No | 6 | 3 | 4 |
| Mn3 | Mn | Yes | No | 5 | 3 | 2 |
| Mn4 | Mn | Yes | No | 8 | 4 | 1 |
| Mn5(w) | Mn | Yes | Yes | 2 | 1 | 0 |
| Mn6(w) | Mn | No | Yes | 1 | 0 | 0 |
| Mn7 | Mn | Yes | No | 0 | 0 | 0 |
| V1 | V | (No) | No | 5 | 0 | 2 |
| V2 | V | No | No | 6 | 0 | 0 |
| V3(w) | V | ? | Yes | 2 | 0 | 0 |
Mn traditional is a traditional manganese carboxylate drier, which needs to be combined with a drying accelerator (2,2-bipyridyl) to increase its catalytic activity to become a potential alternative to cobalt driers. In the case of the printing ink, Mn traditional was not used as an alternative to Co driers, but as a second primary drier.
The drier coded Mn1 is developed primarily for use in waterborne systems, but was suggested as a Co alternative in both solvent-borne and waterborne systems even though the drier manufacturer has a similar drier product intended for solvent-borne systems, namely Mn7. That is mainly due to Mn1 having a better environmental profile than the solvent-borne alternative and due to the fact that Mn1 actually performs well in solvent-borne systems.
Mn2, Mn3 and Mn4 are manganese based driers intended for use in solvent-borne oxidative drying coatings, but they can also be used in waterborne systems. Mn1, Mn2, Mn3, Mn4 and Mn7 contain all a drying accelerator (complexing agent). Mn1, Mn2, Mn3 and Mn4 have more or less all been tested in all the air-drying systems included in the project. Table 5.4 also gives an indication of how many products the specific alternatives have been tested in for each of the three product groups.
Mn4 was included late in the project compared to the other manganese driers. Mn7 has only been included in the environmental and health screening, not in the technical evaluation. Mn5(w) and Mn6(w) are manganese based driers developed specifically for waterborne systems, both containing drying accelerators. These driers have only been tested in the waterborne systems. All the tested Mn dries are commercially available either as commodity driers or at least as trial products.
V1 and V2 are commercially available vanadium based driers for solvent-borne coatings. V2 is primarily intended for use in high solids paints, which have a relatively low solvent content. After the technical evaluations had ended it was realised, according to the manufacturer descriptions that V2 can be combined with a drying accelerator to achieve optimal catalytic effect. This was not done in this project.
In the case of V1 it has not been indicated by the manufacturer that a drying accelerator should be used in combination with it. No information has been given if the drier product, V1, contains a drying accelerator. V3(w) is a laboratory product made specifically for waterborne systems and has only been tested in these. As it is a laboratory product the composition is confidential, for which reason it is unknown if a drying accelerator is built into the drier product.
Vanadium driers have only been tested in the do-it-yourself products and in two printing inks. In the case of printing ink only one vanadium based drier system was tested and as no significant drying was induced no further testing was performed with vanadium driers. The vanadium driers were not tested in the industrial products as the drier manufacturers beforehand had indicated that manganese driers are expected to perform best in these products.
The alternative primary driers need in most cases, just like cobalt driers, to be combined with other driers to enhance through-drying as well as other film properties. These driers are described in brief in the following section. A listing of these driers can be seen in table 6.1. All the presented driers, the cobalt drier, the alternatives and the secondary driers have all, if possible, been screened with regard to environmental and health effects before they were included in the technical evaluation performed.
5.2.2 Other driers
Cobalt driers were included for comparison in the environmental and health screening. A Co drier has been used for making cobalt based drier systems for the printing ink samples used in the test of the alternative anti-skinning agents.
Zirkonium (Zr), barium (Ba), bismuth (Bi), strontium (Sr) and aluminium (Al) are all metals used for through-driers. Zr driers have been present in most of the tested alternative drier systems. Bi, Sr and Ba driers have been tested in a few products, where they have been combined with a few of the alternative driers. Only the Ba drier turned out to be useful in the drier combinations investigated in this project. The Al drier has not been tested in any of the alternative drier systems.
Calcium (Ca) driers are used in almost every tested alternative system, at least the solvent-borne ones. Zinc (Zn) and potassium (K) driers have been included in the testing as well. The potassium drier has only been tested in alternative drier systems in one product and without any successful outcome. Zn has been tested in several alternative drier systems combined with different Mn driers.
5.3 Alternative anti-skinning agents
Six alternative anti-skinning agents have been received. Five are commercial or trial products and one is a laboratory product. One of the alternatives was intended for printing inks only. The other alternatives have been tested both in paints and printing inks.
Two of the alternatives are amino/amido based compounds and two are phenolic based compounds. The last alternative is acetone oxime, which so far is not labelled as strictly as methyl ethyl ketoxime. Vitamin E has furthermore been tested as an alternative anti-skinning agent in some of the products, mainly the printing inks.
Methyl ethyl ketoxime and a hydroquinone product have also been included, both in the skinning test and in the drying time test, as reference compounds. The alternatives are presented in table 6.2 in next chapter. As it was the case with the driers the anti-skinning agents are not presented with their commercial product names. The alternative anti-skinning agents were also screened before including them in the technical evaluation.
6 Environmental and health screening
An environmental and health screening has been performed of the existing and alternative driers and anti-skinning agents in order to learn more about which alternatives that may or may not be relevant from a health and environmental point of view. Alternatives with an inferior environment and health profile in the screening will not be worth testing as the goal is to find more environmental and health friendly alternative primary driers and anti-skinning agents.
The environmental and health screening of cobalt driers and alternatives, and hydroquinone, methyl ethyl ketoxime and alternatives, has been performed based on the classification of the substances combined with ready available information, /21/. The risk phrases for ecotoxicity as well as human toxicity or the corresponding criteria are used in the allocation of an environmental and human score to the substances. The risk phrases can be used as the following examples illustrate:
| Aquatic ecotoxicity | Score 4:
R50 (very toxic) |
Score 2:
R51 (toxic) |
Score 1:
R52 (harmful) |
| Human toxicity | Score 8:
R26-27-28 (very toxic) R45 (may cause cancer) |
Score 4:
R23-24-25 (toxic) |
Score 1:
R20-21-22 (harmful) R38-37-38 (irritating) |
In the screening it is not only relevant to look upon the active ingredient of the drier products and anti-skinning agents. It is also necessary to include an assessment of the other components such as organic solvents and drying accelerators, as the purpose is to improve the entire profile of the products.
The active ingredient (i.e. the metallic component for the driers) is dissolved in one or more organic solvents. The solvents are also scored for their human toxicological effects and will be included in the evaluation of the different drier products and the different anti-skinning agents.
Some of the alternative drier products also contain a drying accelerator to enhance the catalytic effect of the drier. These drying accelerators are also scored for their human toxicological effects.
Only very few of the substances in the drier products or in the anti-skinning agents are given an environmental score as only few substances are classified due to environmental hazards.
6.1 Screening of the drier products
The results of the screening are presented in Table 6.1 The screening has predominantly been based on information from the suppliers presented in material safety data sheets, /22/. Where readily available information was available on specific substances (and where the substances were specified with CAS-numbers), this information has been included in the screening as well. This especially applies for the cobalt compounds, which are considered possible carcinogens by IARC, /23/.
The classification applied to the organic solvents (the petroleum distillates) has also been checked in the "European list of dangerous substances", /24/. In case of deviation from the classification given in the MSDS the classification is presented as a note to the table and the score is presented in brackets.
Table 6.1
Environmental
and health
screening of
driers.
| Codes | Substance | Metal | % | Classification / Labelling | Health score | Environ-mental score |
| Co | Cobalt 2-ethylhexanoate | Co | 40-70 | Xi R38 | 81 | |
| Naphtha (petroleum), hydrated heavy4 |
Xn R65 | 1 (8) | ||||
| Mn | Manganese 2-ethylhexanoate | Mn | 30-60 | Xi R38 | 1 | |
| Traditional | Naphtha (petroleum), hydrated heavy4 |
40-70 | Xn R65,66 | 1 (8) | ||
| 2-(2-ethoxyethoxy) ethanol |
0-6 | Xi R36 | 1 | |||
| Mn1 | Manganese compound3 | Mn | 2.5-10 | ? | ? | |
| (2-methoxymethylethoxy) propanol9 |
50-100 | Not classified | 0 (4) | |||
| n-Butanol | 2.5-10 | Xn R10-22-37/38-41-67 | 4 | |||
| 1,10-phenanthroline | 2.5-10 | T, N R25-50/53 | 4 | 4 | ||
| Diethylenglycol monobutyl ether |
2.5-10 | Xi R36 | 1 | |||
| 2-ethylhexanoic acid | <2.5 | Xn R63 | 8 | |||
| White spirit5 | <2.5 | R10, Xn R65 | 1 (8) | |||
| Mn2 | Manganese salt of C6-19 branched fatty acid and naphthenic acid3 |
Mn | <43 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<48 | Xn R65,66 | 1 (8) | |||
| Amino complexing agent | <2.6 | Xn R20/21/22 | 1 | |||
| Mn3 | Manganese salt of C6-19 branched fatty acid3 |
Mn | <52 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<44.5 | Xn R65,66 | 1 (8) | |||
| Amino complexing agent3 | <10 | Xn R20/21/22 | 1 | |||
| Mn4 | Manganese salt of C6-19 branched fatty acid |
Mn | 10-25 | Xi R38 | 1 | |
| (2-methoxymethylethoxy) propanol9 |
50-100 | Not classified | 0 (4) | |||
| Naphtha (petroleum), hydrated heavy4 |
2.5-10 | Xn R65,66 | 1 (8) | |||
| 2,2'-bipyridyl11 | 2.5-10 | Xn R20/21/22 | 1 (0) | |||
| 2-(2-butoxyethoxy)ethanol | <2.5 | Xi R36 | 1 | |||
| Mn5(w) | Manganese salt of C6-19 branched fatty acid3 |
Mn | <47 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<9 | Xn R65,66 | 1 (8) | |||
| Amino complexing agent3 | <2.6 | Xn R20/21/22 | 1 | |||
| 2-methylpentane-2,4-diol | <4 | Xi R36/38 | 1 | |||
| Highly refined mineral oil | <12 | - | 0 | |||
| Ethoxylated alcohols, C12-14 | <11.5 | Xi R41, N50 | 4 | 4 | ||
| 2-(2-butoxyethoxy) ethanol |
<1 | Xi R36 | 1 | |||
| Mn6(w) | Manganese dipropionate Manganese(II) isooctanoate Manganese isononate |
Mn Mn Mn |
10-30 10-30 10-30 |
Xi R38 Xi R38 Xi R38 |
1 1 1 |
|
| White spirit5 | 10-30 | R10, Xn R65 | 1 (8) | |||
| 2-butoxyethanol | 5-15 | Xn R20/21/22, Xi R36/38 | 1 | |||
| 2,2'-bipyridyl11 | 1-10 | T, R25, Xn R21, R52/53 | 4 (0) | 1 | ||
| Mn7 | Manganese 2-ethylhexanoate | Mn | 2.5-10 | Not mentioned10 | (1) | |
| Xylene, mixture of isomers | 25-50 | Xn R10, Xi R20/21,38 | 1 | |||
| n-butanol | 25-50 | Xn R10-22-37/38-41-67 | 4 | |||
| White spirit5 | 10-25 | Xn R10-65 | 1 (8) | |||
| Ethylbenzene | 2.5-10 | F, Xn R11-20 | 1 | |||
| 1,10-phenanthroline | 2.5-10 | T, N R25-50/53 | 4 | 4 | ||
| 2-ethylhexanoic acid | <2.5 | Xn R63 | 8 | |||
| V1 | Vanadium organophosphate | V | <59.5 | Xn R22 | 1 | |
| Glycolether | ?? | ? | ||||
| V2 | Vanadium neodecanoate Potassium 2-ethylhenanoate |
V K |
10-30 10-30 |
Xi R38 Xi R38 |
1 1 |
|
| White spirit5 | 30-50 | R10, Xn R65 | 1 (8) | |||
| Neodecanoic acid | 10-20 | Not classified | 0 | |||
| 2-(2-butoxyethoxy)ethanol | 1-10 | Xi R36 | 1 | |||
| Diethylenglycol | 1-10 | Xi R22 | 1 | |||
| V3(w) | Confidential | V | ?? | ? | ||
| Drying
accelerator |
2-butoxyethanol | None8 | 78 | Xn R20/21/22, Xi R36/38 | 1 | |
| 2,2'-bipyridyl11 | 19 | T, R25, Xn R21, R52/53 | 4 (0) | 1 | ||
| Water | 3 | Not classified | 0 | |||
| Al | Aluminium complex, organic3 | Al | 80-90 | R10, Xi R41 | 4 | |
| Solvent naphtha (petroleum), medium heavy, aliphatic hydrocarbons6,7 | 20-50 | Xn R65 | 1 (4) | |||
| Ca | Calcium octoate2 | Ca | <42 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 | <57.5 | Xn R65,66 | 1 (8) | |||
| 2-(2-butoxyethoxy)ethanol | <3.5 | Xi R36 | 1 | |||
| Ba | Barium salt of C6-12 fatty acid | Ba | <40 | Xn R20/22 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<44.5 | Xn R65,66 | 1 (8) | |||
| 2-ethylhexanoic acid | <15.5 | Xn R63 | 8 | |||
| 2-methylpentane-2,4-diol | <2.5 | Xi R36/38 | 1 | |||
| 2-(2-butoxyethoxy)ethanol | <2 | Xi R36 | 1 | |||
| Bi | Bismuth salt of C8 carboxylic acid3 | Bi | 50-100 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
5-25 | Xn R65,66 | 1 (8) | |||
| K | Potassium octoate2 | K | <72 | Xi R38 | 1 | |
| 2-(2-butoxyethoxy) ethanol |
<26.5 | Xi R36 | 1 | |||
| C3-24 fatty acid | <5.5 | Xn R22,36 | 1 | |||
| Sr | Strontium(II)octanoate | Sr | <4 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<38 | Xn R65,66 | 1 (8) | |||
| C3-24 fatty acid | <18.5 | Xn R22,36 | 1 | |||
| 2-(2-butoxyethoxy) ethanol |
<3.5 | Xi R36 | 1 | |||
| Zn | Zinc 2-ethylhexanoate | Zn | 30-60 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
40-70 | Xn R65,66 | 1 (8) | |||
| 2-(2-ethoxyethoxy) ethanol12 |
0-5 | Xi R36 | 1 (0) | |||
| Zr | Zirconium octoate2 | Zr | <46 | Xi R38 | 1 | |
| Naphtha (petroleum), hydrated heavy4 |
<55 | Xn R65,66 | 1 (8) | |||
| 2-(2-butoxyethoxy) ethanol |
<2.5 | Xi R36 | 1 |
1. Dermatitis of allergenic type. Indication of a carcinogenic effect in human populations. IARC: Possibly carcinogenic to humans (Group 2B), /23/.
2. Octanoate?.
3. Confidential.
4. Carc2;R45 Xn;R65 according to the List of dangerous substances /24/. The difference in classification for the substance is due to different MSDS's from different suppliers, and may also be due to how updated the MSDS's are.
5. Carc2;R45 R10 Xn;R48/20-65 according to the List of dangerous substances /24/.
6. R10 Xn;R48/20-65 according to the List of dangerous substances /24/.
7. The substance is adopted at the List of undesirable substances, /25/.
8. The product is drying accelerator.
9. QSAR models indicate that the solvent can produce airway allergy, /26/. Not classified according to the list of dangerous substances /24/.
10. Classification not mentioned on the American MSDS – but probably Xi, R38.
11. At present not classified according to the List of dangerous substances /24/. The difference in classification for the substance is due to different MSDS's from different suppliers, and may also be due to how updated the MSDS's are.
12. At present not classified according to the List of dangerous substances /24/.
Xi Irritant.
Xn Harmful.
N Environmental dangerous
R10 Flammable
R20/22 Harmful by inhalation or if swallowed.
R20/21/22 Harmful by inhalation, in contact with skin or if swallowed.
R21 Harmful in contact with skin.
R22 Harmful if swallowed.
R36 Irritating to eyes.
R36/38 Irritating to eyes and skin.
R36/37/38 Irritating to eyes, the respiratory system and to the skin.
R38 Irritating to the skin.
R40 Limited evidence of a carcinogenic effect.
R41 Risk of serious damage to the eyes.
R45 May cause cancer.
R48 Danger of serious damage to health by prolonged exposure.
R48/20 Harmful: danger of serious damage to health by prolonged exposure through inhalation.
R48/20/22. Harmful: danger of serious damage to health by prolonged exposure through inhalation or if swallowed.
R50/53 Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
R63 Possible risk of harm to unborn child.
R65 Harmful: may cause lung damage if swallowed.
R66 Repeated exposure may cause skin dryness or cracking.
R67 Vapours may cause drowsiness and dizziness.
6.1.1 Evaluation of the driers
The drier included in the screening is the cobalt drier:
- Cobalt 2-ethylhexanoate
and alternative driers:
- Manganese compounds (e.g. manganese 2-ethylhexanoate)
- Vanadium compounds (e.g. vanadium organophosphate and vanadium neodecanoate)
As primary driers rarely are used on their own a number of secondary driers are also included in the screening.
A preliminary search for toxicological information has been done for the active substances in the driers. Only cobalt 2-ethylhexanoate has been found explicit in the common databases, but the information is sparse.
Cobalt 2-ethylhexanoate has been scored 8 due to indication of carcinogenic effect in human populations. IARC (International Agency for Research on Cancer) evaluate cobalt and cobalt compounds as possibly carcinogenic to humans (Group 2B), which cause the cobalt compounds to end up with a health score of 8, /23/. Furthermore the substance can result in dermatitis of the allergenic type. According to the MSDS cobalt 2-ethylhexanoate is classified as Xi, R38 (Irritating to skin), resulting in a score 1. Although the MSDS indicate a score 1 for the cobalt compound, the information found in the literature has been used in the screening.
The manganese driers are classified Xi, R38 (Irritating to skin), resulting in a score 1. The rest of the alternative driers are classified as Xi, R38 (Irritating to skin) or Xn, R22 (Harmful if swallowed), resulting in a score 1. For a single drier product the content is confidential, therefore no classification or health score is given.
Of the alternative secondary driers the aluminium complex is scored 4 due to the potential risk for eye irritation (a classification of Xi, R41 (Risk of serious damage to eyes).
Evaluation of the primary driers by using the screening method excludes the cobalt drier. Of secondary driers the aluminium complex is excluded.
6.1.2 Evaluation of the organic solvents
The organic solvents can be divided in two groups: petroleum distillates (more or less well defined substances/group of substances) and specific substances. The petroleum distillates are present in most of the drier products whereas the other substances are present in one or more of the drier products.
The petroleum distillates are:
| • Naphtha (petroleum) – hydrated heavy | (CAS-no. 64742-48-9) |
| • White spirit (Stoddard solvent) | (CAS-no. 8052-41-3) |
| • Solvent naphtha (petroleum) medium heavy aliphatic hydrocarbon | (CAS-no. 64742-88-7) |
| • Highly refined mineral oil | (CAS-no. 8042-47-5) |
The petroleum distillates (with the exception of highly refined mineral oil) are classified as Xn, R65 (Harmful: may cause lung damage if swallowed) and/or R66 (Repeated exposure may cause skin dryness or cracking), depending on the supplier, leading to score 1. According to the "European list of dangerous substances", /24/ these substances are classified as T, R45 (carc2; May cause cancer) or Xn, R48/20 (Danger of serious damage to health by prolonged exposure via inhalation) leading to score 8 and 4 respectively. The petroleum fraction "highly refined mineral oil" has no classification and hence a score of 0.
The specific substances are:
| • (2-Methoxymethylethoxy)propanol | (CAS-no. 34590-94-8) |
| • Neodecanoic acid | (CAS-no. 26896-20-8) |
| • 2-(2-Ethoxyethoxy)ethanol | (CAS-no. 111-90-0) |
| • 2-(2-Butoxyethoxy)ethanol | (CAS-no. 112-34-5) |
| • 2-Methylpentane-2,4-diol | (CAS-no. 107-41-5) |
| • 2-Butoxyethanol | (CAS-no. 111-76-2) |
| • Diethylenglycol | (CAS-no. 111-46-6) |
| • Xylene | (CAS-no. 1330-20-7) |
| • Ethylbenzene | (CAS-no. 100-41-4) |
| • n-butanol | (CAS-no. 71-36-3) |
| • 2-Ethylhexanoic acid | (CAS-no. 149-57-5) |
The first two mentioned substances (2-Methoxymethylethoxy)propanol (dipropylen glycol monoethyl ether) and neodecanoic acid are scored 0 because of no classification. However, use of QSAR models indicates that the solvent (2-Methoxymethylethoxy)propanol can produce airway allergy, but not in normal use. Furthermore, one of the known metabolites of the solvent is considered as genotoxic. QSAR models indicate that the solvent probably do not have any effects on the external environment /26/.
The next 7 substances are all scored 1 whereas n-butanol is scored 4 because of the classification R41 (Risk of serious damage to eyes). Finally, the last mentioned substance 2-ethylhexanoic acid are classified as Xn R63 (Possible risk of harm to unborn child) leading to a score 8.
In addition to petroleum distillates and specific substances a number of unspecified substances are present:
- Fatty acid ester (not further specified)
- C3-24 Fatty acid
- Glycolether
- Alcohols, C12-14, ethoxylated
The first three mentioned substances are scored 1 based on the classification given on the MSDS for the actual drier products. Ethoxylated alcohols, C12-14 is scored 4 because of the classification R41 (Risk of serious damage to eyes). Furthermore, the ethoxylated alcohols are as the only solvent given an environmental score of 4, because of the labelling N R50 ("Very toxic to aquatic organisms").
Evaluation of the organic solvents by using the screening method excludes the petroleum distillates, the solvent 2-ethylhexanoic acid, and the ethoxylated alcohols, C12-14.
6.1.3 Evaluation of the drying accelerators
Drying accelerators are used in some of the alternative driers to enhance the drying abilities. The drying accelerators used are:
| • 2,2'-Bipyridyl | (CAS-no. 366-18-7) |
| • 1,10-phenathroline | (CAS-no. 66-71-7) |
| • Amino complexing agent | (CAS-no. confidential) |
2,2'-Bipyridyl is one of the substances that differs in classification depending on the supplier. 2,2'-Bipyridyl is scored 1 due to a classification of Xn R20/21/22 (harmful by inhalation, in contact with skin or if swallowed) at one supplier, but scored 4 due to a classification of T, R25, Xn R21 (toxic if swallowed, harmful in contact with skin) at another supplier. However, 2,2'-Bipyridyl is at present not on the list of dangerous substances /24/.
1,10-phenathroline is scored 4 because of the classification T, R25 (toxic if swallowed). The amino complexing agent is confidential and is found in two alternative driers. In both cases the amino complexing agent is scored 1 because of a classification of Xn R20/21/22 (harmful by inhalation, in contact with skin or if swallowed).
Two of the substances have an environmental classification. The solvent 1,10-phenathroline is classified as N, R50/53 "Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment" resulting in the highest environmental score – a score 4. 2,2'-Bipyridyl is by one supplier classified as R52/53 (harmful to aquatic organisms, may cause adverse long term effects in the aquatic environment) resulting in an environmental score of 1.
Evaluation of the drying accelerators by using the screening method excludes 1,10-phenathroline.
6.1.4 Overall evaluation of the drier products
The screening of the driers shows that if the primary cobalt driers can be substituted with the primary driers manganese or vanadium, the health profile will improve just taking the metal component into account.
Some of the alternative drier products do contain components (organic solvents or drying accelerators) with undesirable health and/or environmental effects. In a substitution it is therefore necessary to look upon the entire product and not just the active metallic compound. However, this is not only valid for the alternative drier products as also the existing cobalt driers contain solvents with undesirable health effects.
Based on the conducted screening it is recommended to avoid the following drier products:
- The drier products based on cobalt
- The drier product containing 2-ethylhexanoic acid
- The drier products containing petroleum distillates or at least systems with high content of petroleum distillate
- The drier products containing the drying accelerator 1,10-phenathroline
- The drier products containing ethoxylated alcohols, C12-14
The screening also shows that of the secondary driers the aluminium driers should be avoided.
To improve the environment and health profile of air-drying products as much as possible, alternative driers with the best profile of organic solvents and drying accelerators should be used if technical possible. This means that driers dissolved in solvents like petroleum distillates and 2-ethylhexanoic acid should be avoided, and that driers containing 2,2-bipyridyl as drying accelerator should be preferred to those containing 1,10-phenathroline (at present classification). Of the mentioned undesirable substances the petroleum distillates are used in the largest amount in the drying compounds. The petroleum distillates content varies between 2.5 and 70% (mostly between 30-50%), whereas 2-ethylhexanoic acid and 1,10-phenathroline are used in smaller concentrations of <2.5% and 2.5-10% respectively. The petroleum distillates are therefore the far most important substances to avoid in driers. As petroleum distillates today still are present in almost every drier product, both the primary and the secondary, it is impossible to avoid them completely, but products with a low content of petroleum distillates should of course be preferred.
Overall, the screening of the drier products shows that a substitution of the primary drier cobalt with manganese or vanadium will improve the health profile of the drier product. However, in spite of the fact that some alternative drier products do contain constituents, which have undesirable health and/or environmental effects, it is relevant to test them in order to learn if they in practise will be alternatives to the existing cobalt driers, as they overall have a better score by use of the screening method. Only one manganese drier, Mn7, was not included in the testing as one drier, Mn1, with a better profile was available from the same manufacturer.
Furthermore, it should be mentioned, that some of the constituents in the alternative drier products were not available until the very end of the project, and therefore some alternatives with a more negative health profile also has been tested in the project.
6.2 Screening of the anti-skinning agents / antioxidants
The results of the screening are presented in Table 6.2. The screening has been based on information from the suppliers presented in material safety data sheets, /22/.
For two of the alternative anti-skinning agents confidential information has been received from the supplier to be able to verify the classification from the MSDS.
Table 6.2
Environmental and health screening of anti-skinning agents.
| Codes | Substance | % | Classification / Labelling | Health score | Environ.mental score |
| Hydroquinone | Hydroquinone | Xn;R22 Carc3;R40 Xi;R41 R43 Mut3;R68 N;R50 | 8 | 4 | |
| MEKO | Butanone oxime (methyl ethyl ketoxime)1 | Xn R21, 40, 41, 43 | 8 | ||
| Acetone oxime | Acetone oxime | 10-25 | Xn R22 R48/20/22 | 4 | |
| (2-methoxymethylethoxy)propanol | 50-100 | not classified | 0 | ||
| Amino/amido no. 1 | Amino organic compound2 | 4-20 | R10, Xn R20/21, R36/37/38 | 1 | |
| Amido organic compound3 | 2-12 | Xn R20/21/22 | 1 | ||
| Glycols (confidential) | ? | not classified | 0 | ||
| Amino/amido no. 2 | Amino organic compound2 | 4-20 | R10, Xn R20/21, R36/37/38 | 1 | |
| Amido organic compound3 | 2-12 | Xn R20/21/22 | 1 | ||
| Naphtha (petroleum), hydrated heavy4 | 65-87 | Xn R65,66 | 1 (8) | ||
| 1-butoxy-2-propanol | <6 | Xi R36/38 | 1 | ||
| Phenolic no. 1 | ?? | ?? | |||
| Phenolic no. 2 | Terpenes and terpenoids, turpentine-oil, a-pinene fraction | <9.6 | R10, Xn R20/21/22, R36 | 1 | - |
| Naphtha (petroleum), hydrodesulfurized heavy | <26.5 | Xn R10 R48/20, R65/66/67 N R51/53 | 4 (8) | 2 | |
| p-tert-butylphenol5 | <20.7 | Xi R36/37/38 | 1 (0) | - | |
| 1-methoxy-2-propanol | <13 | R10 | 0 | - | |
| ?? | ?? | not classified | 0 | - | |
| Vitamin E | Vitamin E (DL-alpha-tocopherol) |
1. Xn;R21 Carc3;R40 Xi;R41 R43 according to /24/.
2. No classification according to Danish EPA (2002). Self-classification of Xn, R22 according to /27/.
3. No classification according to /24/.
4. Carc2;R45 Xn;R65 according to /24/.
5. At present not classified according to the List of dangerous substances /24/.
Xi Irritant.
Xn Harmful.
R10 Flammable
R20/22 Harmful by inhalation or if swallowed.
R20/21/22 Harmful by inhalation, in contact with skin or if swallowed.
R21 Harmful in contact with skin.
R22 Harmful if swallowed.
R36 Irritating to eyes.
R36/37/38 Irritating to eyes, the respiratory system and to the skin.
R38 Irritating to the skin.
R40 Limited evidence of a carcinogenic effect.
R41 Risk of serious damage to the eyes.
R43 May cause sensitisation by skin contact.
R45 May cause cancer.
R48/20 Harmful: danger of serious damage to health by prolonged exposure through inhalation.
R48/20/22. Danger of serious damage to health by prolonged exposure via inhalation or if swallowed.
R50 Very toxic to aquatic organisms.
R65 Harmful: may cause lung damage if swallowed
R66 Repeated exposure may cause skin dryness or cracking.
R67 Vapours may cause drowsiness and dizziness.
R68 May cause irreversible damage to health.
6.2.1 Evaluation of the anti-skinning agents
The anti-skinning agents included in the screening are:
- Methyl ethyl ketoxime
- Hydroquinone
and alternative anti-skinning agents:
- Acetone oxime
- Amino/amido organic compounds
- Terpenes and terpenoids, turpentine-oil, a-pinene fraction
- Vitamin E
Methyl ethyl ketoxime (butanone oxime) has been scored 8 due to possible risk of irreversible effects (Carc3; R40).
The alternative anti-skinning agents are scored 1 except acetone oxime that is scored 4 due to the classification R48/20/22 (Danger of serious damage to health by prolonged exposure via inhalation or if swallowed).
The amino/amido organic compounds are scored 1 according to the classification Xn R20/21, R36/37/38 ("Harmful by inhalation and in contact with skin" and "Irritating to eyes, respiratory system and skin") or the classification Xn R20/21/22 ("Harmful by inhalation, in contact with skin or if swallowed") on the MSDS. Confidential information received from the supplier confirms that this score given is the maximum score for the compounds. Neither the amino nor the amido organic compound is classified according to the list of dangerous substances, /24/.
Since the testing phase of this project the formulation of the products containing the amino and amido organic compound has been changed. The tests performed were on the amino/amido organic compound. Now, however, the amido compound is no longer included in the formulation of the anti-skinning agent, as a better working combination has been found.
Due to the present scores the amino/amido organic compound as well as the terpenes etc. can be recommended in preference to hydroquinone, methyl ethyl ketoxime and acetone oxime.
6.2.2 Evaluation of the organic solvents
The organic solvents can be divided in two groups: petroleum distillates and specific substances. The petroleum distillates are:
| • Naphtha (petroleum) – hydrated heavy | (CAS-no. 64742-48-9) |
| • Naphtha (petroleum) – hydrodesulfurized heavy | (CAS-no. 64742-82-1) |
The petroleum distillates are scored 1 and 4 based on classification as Xn R65/66 (Harmful: may cause lung damage if swallowed and Repeated exposure may cause skin dryness or cracking) and Xn R48/20 (Danger of serious damage to health by prolonged exposure by inhalation). According to the "European list of dangerous substances" /24/ these petroleum distillates are classified as carc2; R45 Xn; R65 leading to a score 8.
The specific substances are:
| • (2-Methoxymethylethoxy)propanol | (CAS-no. 34590-94-8) |
| • 1-Butoxy-2-propanol | (CAS-no. 5131-66-8) |
| • 1-Methoxy-2-propanol | (CAS-no. 107-98-2) |
| • p-tert-Butylphenol | (CAS-no. 98-54-4) |
These substances are scored 1 due to classification as Xi R36/37/38 (Irritating to eyes, the respiratory system and to the skin) except (2-methoxymethylethoxy)propanol and 1-methoxy-2-propanol which are scored 0. However, p-tert-Butylphenol is at present not classified according to the list of dangerous substances /24/.
(2-Methoxymethylethoxy)propanol (dipropylen glycol monoethyl ether) is scored 0 because of no classification. However, use of QSAR models indicates that this solvent can produce airway allergy, but not in normal use. Furthermore, one of the known metabolites of the substance is considered as genotoxic. The QSAR models indicate that the substance probably does not have any effects on the external environment, /26/.
Furthermore unspecified glycols are present in one of the anti-skinning agent systems. Based on the confidential information received from the producer none of the glycols has to be classified and is therefore scored 0.
6.2.3 Overall evaluation of the anti-skinning agent systems
The screening of the anti-skinning agents shows that when only considering the active ingredients in the alternatives, the health profile of air-drying products can be improved by substituting methyl ethyl ketoxime and hydroquinone with the alternatives.
Some of the alternative anti-skinning agents contain organic solvents with undesirable health and/or environmental effects, and the total health profile of the product depends of course also on the organic solvents used in the product. However, even so, the profile is better than that of methyl ethyl ketoxime and hydroquinone.
Based on the conducted screening the following anti-skinning agent systems are recommended to be avoided:
- The anti-skinning agent system based on hydroquinone
- The anti-skinning agent system based on methyl ethyl ketoxime
- The anti-skinning agent system based on acetone oxime
- The anti-skinning agent system containing petroleum distillates or at least systems with high content of petroleum distillate (e.g. the amino/amido compound dissolved in petroleum distillates)
The two anti-skinning agent systems based amino/amido organic compounds have the same classification as far as regarding the anti-skinning agent. However, the amino/amido compounds dissolved in glycol are preferred in preference to the system dissolved in petroleum distillate.
It should be mentioned, that some of the constituents in the alternative anti-skinning agents were not available until the very end of the project, for which reason some alternatives with a more negative health profile also have been tested in the project. Actually, for one anti-skinning agent no identity of the constituents was available for the project. The anti-skinning agent is, however, tested anyway as the total number of alternatives is limited.
7 Technical evaluation
The testing of air-drying coating systems with alternative driers and anti-skinning agents has been performed at several stages. First, initial drying time tests were performed for the do-it-yourself (DIY) products. The experiences from these tests were used in the initial drying time test for the industrial products, which in general meant that the number of initial drying time tests for the industrial products was considerable lower than for the DIY products.
After the initial drying time test the most promising alternative drier systems were chosen for further testing of stability, viscosity, film hardness, gloss and yellowing. This accounts for both DIY and industrial products. The results obtained are used to evaluate the possibility to substitute Co driers in the specific air-drying products.
One alternative Co free drier system was chosen for each product to be used for the skinning test, except for the printing inks where cobalt based drier systems were used when testing the efficacy of the alternative anti-skinning agents.
The alternative anti-skinning agents have in most cases only been tested in a single concentration, as it was not intended to optimise the different systems. The tests were only performed to get an indication of whether an alternative anti-skinning agent can be expected to work properly or not in a specific product. Increasing the concentration of anti-skinning agents would give a better protection against skinning, but higher concentrations will inevitable mean longer drying times. No drying times and set-off effects have been investigated with the alternative anti-skinning agents.
The printing inks differ as product type from the DIY and industrial products for which reason they needed to be tested in another way. In the DIY and industrial products the alternative anti-skinning agents need to prevent in-can skinning, whereas in the printing ink they need to induce duct stability for an extended time period. For the printing inks only drying time profiles and the set-off effect were investigated along with the auto-oxidation temperature and duct stability.
All tests in the technical evaluation of the alternatives have been performed as comparative tests, meaning that the reference product containing the original Co based drier system and methyl ethyl ketoxime, or hydroquinone in the case of printing inks, was always included in the testing.
7.1 Do-It-Yourself and industrial products
7.1.1 Procedure for technical evaluation
The first step in finding an alternative drier to Co-driers was to investigate the drying profile of samples, containing alternative drier systems, and compare them to the reference products. The testing was performed by means of a drying time recorder.
The first test series was only meant to be a screening to verify whether the alternative driers seemed to work in the respective products or not. The pre-selection of alternative drier systems were therefore performed on systems without anti-skinning agents. The alternative driers were tested in different concentrations and combinations with secondary driers for each product. The tested drier systems (usually Ca and Zr and the Co alternative) have in most cases been based on suggestions from the drier manufacturers. If insufficient drying times were obtained the drier concentrations/combinations were adjusted and the new system was tested. For some of the products many adjustments and initial drying time tests were necessary before some reasonable alternative drier systems (if any) were obtained for further testing.
In the next step the most promising drier compositions were tested on the drying time recorder again. This time anti-skinning agent, preferable methyl ethyl ketoxime, was added to the solvent-borne systems to make the drying profile even more comparable to the reference product. No such testing was performed for the waterborne products.
On basis of the drying time test in the second step between 0 and 5 of the most promising alternative Co free drier systems were selected for each product for further testing. Viscosity, pendulum hardness and gloss were measured and compared with the properties of the reference products. If reasonable drying times and film properties were obtained it was investigated how the samples were influenced by storage at 40ºC for two week. Storage at elevated temperature induces an accelerated ageing in the samples and to some extent simulates prolonged storage and an increase in drying time after storage indicates a loss-of-dry of the drier systems.
After storage the samples were evaluated. If severe phase separation or sedimentation was observed for the alternative systems and this was not the case for the reference product, the alternative system was rejected, especially if it was not possible to easily mix the sample again by stirring.
Table 7.1.
Methods used for evaluating the efficiency of the alternative driers in different oxidative drying products.
| Test | Apparatus | Standard method | Comment |
| Drying time | Straight-line drying time recorder | ASTM D5895 | 23ºC and 50 % RH 80 my wet film |
| Stability | Oven | Own method | 2 weeks at 40ºC |
| Viscosity | Bohlin VOR | ISO 3219 | Measured at different shear rates at 23ºC |
| Pendulum hardness | König Albert | ISO 1522 | 23ºC and 50 % RH |
| Gloss | Picogloss 503 | ISO 2813 | 23ºC and 50 % RH |
| Yellowing | - | Own method | 1 month in a dark cupboard |
| Water resistance | - | ISO 2812-1 | 23ºC and 50 % RH |
Properties as viscosity, gloss and hardness of the dry film were measured before and after storage at 40ºC to verify how much the accelerated ageing had altered the products and to evaluate how comparable the alternative system was with the reference products. The drying time profile was also evaluated again after storage. In table 7.1 an overview is given of the methods used for the technical evaluation of coatings containing the Co free alternative drier systems. The overall process of the technical evaluation is described in figure 7.1.
All tests were performed as comparative tests meaning that the reference product containing the original Co based drier system and methyl ethyl ketoxime was always included in the testing.
For each solvent-borne coating product the most promising alternative system was chosen for the skin formation test.
Figure 7.1.
Schematic overview of the technical evaluation and selection process of Co free drier alternatives for a
do-it-yourself product or an industrial product. Any test results obtained with the alternative systems have always
been compared to those of the reference coating containing Co drier.
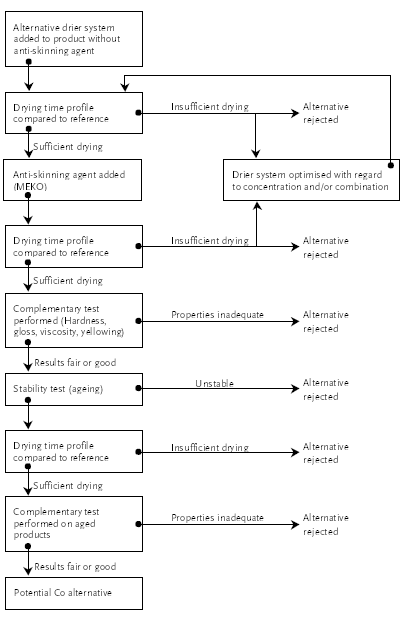
7.1.2 Preparation of test systems
The respective driers were added to 100 g of product sample (in some cases only 25 or 50 g of product sample was used). A balance capable of weighing to the nearest 0.001 g was used and the exact amount added of each drier was noted. The numbers of driers added to a system range from one to four. In most cases three different driers were added, a primary drier (the alternative to Co driers), a through-drier and an auxiliary drier.
Through-driers and auxiliary driers were added first. The sample was then shaken mechanically or stirred thoroughly by hand for about 5-15 minutes. The primary drier was then added and the sample was shaken or stirred again to ensure a uniform distribution of the driers in the product.
If an anti-skinning agent should be present in the system, it was added after the driers. The exact amount of anti-skinning agent was noted as well.
The sample rested for at least 20 – 24 h in a tight closed can at 23 2ºC and 50 5% relative humidity before any testing was performed.
7.1.3 Used methods
7.1.3.1 Drying time profile
The drying time profile of the coating samples has been evaluated with a straight-line drying time recorder used in
accordance with ASTM D 5895 "Measuring Times of Drying or Curing during Film Formation of Organic
Coatings Using Mechanical Recorders".
The samples were applied on glass plates in a well-defined film thickness using an adjustable cube applicator with 5 buckets. The DIY and industrial products were applied 80 m wet whereas the printing inks have been applied 30 m wet.
The stylus of the drying time recorder was immediately after application lowered down into the wet film. The stylus then moved across the sample on the glass plate with a constant and well-defined velocity. The measurements were performed at 23 2C and at a relative humidity of 50 5%. The drying time measurement can range from 1 hour to 50 hours depending on what stylus velocity that is chosen. All do-it-yourself products have been tested over a period of 20 hours. The industrial products have been tested over period of 2 to 20 hours depending on the product. The printing inks have been tested for periods of 50 hours.
Afterwards the track left of the stylus on the film was evaluated. The appearance of the track depends on how dry the film is. The drying of the film is divided into following stages:
Stage 1: Set-to-touch
The set-to-touch condition is reached when the film has solidified sufficiently that it no longer flows or sticks to
objects that lightly touch it. The coating keeps flowing together until the set-to-touch time is reached. A
pear-shaped depression appears in the film and the stylus begins to leave a visible trace in the film.
Stage 2: Tack-free time
After the set-to-touch has been reached the coating begins to polymerise for which reason the stylus leaves a visible
line in the coating film. The tack-free condition is reached when the film surface has dried to an extent where the
film no longer adheres to light objects placed on it. The continuous track of the stylus in the film stops and the stylus
starts to tear the film or leave discontinuous cutting of the film.
Stage 3: Dry-hard time
The dry-hard condition is reached when the drying has proceeded to an extent where the film is not displaced or
that no noticeable marks are left on the film when influenced with a relatively strong pressure. The stylus stops
tearing or cutting the film, but leaves a visible trace on the film.
Stage 4: Dry-through time
The dry-through condition is reached when the film has solidified to an extent where a large twisting force can be
applied without distorting the film. The stylus leaves no longer any visible trace on the film.
The drying time test has in general not been performed in duplicate due to the huge amount of tests performed. The drying times of samples containing alternative Co-free drier systems were always compared with the drying time of the reference product.
7.1.3.2 Hardness (elasticity) of dry film
The hardness of the dry film has been measured with a König Albert Pendulum according to ISO 1522-73
"Pendulum damping test". The hardness of the dry film is determined by registering the time (the number of
pendulum swings) it takes before the amplitude of the pendulum is damped to a certain extent. The more swings
observed the harder is the film.
The samples have been applied on glass plates with a baker applicator with a gap size of 90 µm for the solvent-borne products and 120 µm for waterborne products. The samples were after application stored at 23 2C and at a relative humidity of 50 5%. The hardness of the dry film has been determined after different periods of drying time.
Testing of the film hardness of a product series was continued until the number of swings did not change significantly from one drying period to another. The gap between two such periods should be at least five to seven days. The hardness of the dry film of the products containing the alternative drier systems was always compared with the film hardness of the reference product.
7.1.3.3 Gloss
The gloss of the coating systems is measured on the same samples as the film hardness. The measurements have
been performed with a Picogloss gloss meter model 503 according to DS/EN ISO 2813 "Determination of
specular gloss of non-metallic paint films at 20º, 60º and 85º". The measurements have mainly been performed with
the 60º geometry.
7.1.3.4 Viscosity
The reference coating products as well as the coating samples containing the selected alternative drier systems have
been characterised by measuring the viscosity of the samples on a rotational visco.me.ter with defined shear rate.
A Bohlin VOR Rheometer with C 25 geometry and different torque bars was used for the measurements. The
viscosity was measured over a large span of shear rates. The measurements have been performed in accordance
with ISO 3219 "Determination of the rheological behaviour of paint and printing inks using a rotational viscometer
with defined shear rate".
7.1.3.5 Yellowing
The samples were applied on Hostaphan PE sheets with a baker applicator with a gap size of 90 µm for
solvent-borne systems and 120 µm for the waterborne systems. The applied film on Hostaphan was after 24 h of
drying cut into two equally sized pieces. One piece was kept in natural daylight (also direct sunlight) for a month.
The other piece was stored in a cupboard in complete darkness for the same time period.
After a month the two pieces were compared according to ISO 3668 "Visual comparison of the colour of paints". The colour of the two samples was compared in artificial daylight in a colour-matching booth. The observer judged the degree of yellowing of the sample kept in dark by comparing it to the sample kept in natural daylight. The observer judged the yellowing of the film by following ranking:
| Ranking | Comment |
| 0 | No difference in colour can be observed |
| 1 | A faint difference in colour is visible from one angle |
| 2 | A small difference in colour is visible from more than one angle |
| 3 | A difference in colour is easily observed from more than one angle |
| 4 | Server yellowing and/or pronounced difference in colour |
It has to be noted that the judging is subjective but it gives an indication of the tendency that the different systems have to yellowing in dark places. The same person has evaluated all samples.
For each samples series (each air-drying coating product) the reference product (containing the original Co-drier system) was included to verify whether the samples with the alternative drier systems were more prone to yellowing or not.
7.1.3.6 Stability/ageing
After preparation the samples were stored at ambient temperature for at least 24 hours before storage at increased
temperature.
All samples were evaluated concerning appearance in the container and the viscosity was measured prior to placement of the samples in the oven at 40ºC for 2 weeks. The reference product of each product series was also stored at 40ºC for 2 weeks. When comparing the aged alternative with the aged reference product one should be aware of that most of the reference products already have been stored between ½ and 1½ year at ambient temperature before the stability/ageing test was performed.
After storage the appearance was evaluated again. If severe phase separation or sedimentation was observed for the alternative systems and this was not the case for the reference product, the alternative system was rejected, especially if it was not possible to easily mix the sample again by stirring. If a sample containing an alternative driers system passed the evaluation, the properties investigated before storage (drying time, viscosity, film hardness and gloss) were investigated again.
7.1.3.7 Water resistance
Some of the industrial products were also tested for early water resistance according to ISO 2812, "Determination
of resistance to liquids – Method 2".
The products were applied on glass panel in wet film thickness ranging from 90 – 120 µm depending on the dry matter of the respective products. The applied systems were drying for 24 hours at 23 ± 2ºC and a relative humidity of 50 ± 5 % before the test for water resistance was performed.
Absorbent material of the dimension 20 x 20 mm was soaked with water. Excess water was allowed to drip of before placing the wet material on the film surface. The wet material was then covered by watch-glasses. The wet materials were left at three different spots for periods of 1, 3, and 8 hours respectively. It was evaluated, if water affected the film and whether the alternative system was affected more or less than the reference product.
7.1.3.8 Test of skin formation
Only one drier system was chosen for each product. A large sample (200 g) was prepared containing the selected
alternative drier system. Different anti-skinning agents were than added to 20 g of the basis sample.
15 g of the each sample is stored in closed PE containers. The sample was inspected every second day by opening the lid and noting if the sample had skin on the surface. 5 g of same samples was kept in an open container. These were also investigated for skinning every second day.
7.2 Printing Ink
The printing inks have been evaluated in a slightly different way than the paint products. For the printing inks it is essential that the ink film does not have a high extent of set-off. After performing a number of drying time measurements one alternative drier system was selected for each ink and the ink was tested for set-off.
7.2.1 Sample preparation
7.2.1.1 Driers
The respective driers were added to 10 g of sample. A balance capable of weighing to the nearest 0.001 g was
used and the exact amount added of each drier was noted. The number of driers used in the printing ink systems
ranged from one to three. In most cases two driers were used. The driers were mixed in the sample one at the time,
ending with the most active drier. The sample was stirred by hand. The samples rested for 20-24 h in a tight closed
can at 23 2ºC and 50 5% relative humidity before the drying test was performed.
7.2.1.2 Anti-skinning agents
Ink samples containing the original cobalt based drier system were added the alternative anti-skinning
agents/anti-oxidant in one or two concentrations.
7.2.2 Used methods
7.2.2.1 Drying time
See description in paragraph 7.1.3.1 as the drying time measurements are performed in the same way for paint
products and printing inks.
7.2.2.2 Set-off test
Set-off effects have been evaluated using an IGT-tester, which is an apparatus for applying ink with a well-defined
film thickness on paper. These test prints can be tested for set-off by running a paper against the print after different
periods of drying. The set-off was evaluated by measuring the density of the printing ink on the set-off paper. The
higher the density was the more set-off effect. The set-off was compared to that of the reference inks.
7.2.2.3 Auto-oxidation temperature
5 to 10 mg of sample is placed in an open 40 l aluminium crucible. The sample is then scanned by means of
differential scanning calorimetry (DSC) in a temperature interval of ranging from 50 – 300 C. The heating rate is
10C/min. Air is used as a carrier gas with a flow of 100 cm3/min.
An exothermic raise in the DSC curves is observed due to oxidation in the sample. The auto-oxidation temperature is taken as the intersection of the extrapolated baseline and the tangent to the exothermic raise. The determinations are performed in duplicate.
Measurements were performed on samples without any anti-skinning agents and on samples containing different anti-skinning agents. This method is a qualitative method and can only be used to compare the auto-oxidation temperatures of samples that belong to the same product series. The higher the auto-oxidation temperature the more efficient is the anti-skinning agent/anti-oxidant assumed to be.
7.2.2.4 The roller/duct stability
The roller/duct stability of printing inks containing alternative antioxidant has been investigated by performing drying
time measurements on a drying time recorder. The measurements were performed as described in paragraph
7.1.3.1
8 Results from technical evaluation
The results obtained in the technical evaluation of the alternative driers and anti-skinning agents in different air-drying coating systems were used to evaluate if there from a technical point of view, at present, exist any proper substitution alternatives to cobalt driers, to methyl ethyl ketoxime and to hydroquinone respectively.
Only the overall results/conclusions from the testing/evaluation are presented.
Running eight do-it-yourself products and four industrial coatings through the evaluation procedure described in figure 7.1 and section 7.1 and testing four printing inks according to the procedure in section 7.2 generates a high number of data, which is rather difficult to present in a clear and well-arranged way.
For one product, DIY-P3, which is a waterborne stain, all the generated data from the project is presented in Appendix A – Chapter 1 to give an overview of the outcome of running one product through the technical evaluation procedure. The example given is for an air-drying product where the possibility of substituting Co driers seems rather promising. For the other air-drying products only the number of tests performed during the technical evaluation procedure is presented in Appendix A – Chapter 2. All obtained results have been communicated to the respective manufacturers on their own products.
As the technical evaluation has been so intensive on laboratory testing, it is quite obvious that it has been impossible to work in-depth with every single system within the frame of this project optimising it completely with regard to drying time. Even though alternative drier systems with potential to substitute Co driers in specific products have been identified, the products are only developed to a certain extent due to the limited time. The manufacturers need to continue the work optimising their own products if it seems worthwhile doing so. They also need to verify the obtained results as well as perform necessary supplementary tests before they carry out any substitution in their products. The results presented are therefore only meant as guidelines if Co free alternative driers seem to be worthwhile testing in a specific type of air-drying product.
The alternative anti-skinning agents were tested in one concentration only in each of the investigated products, for which reason the results can only be taken as guidelines on whether a specific anti-skinning agent can be expected to work in a specific product or not. As it is the case with the alternative driers the manufacturers need to verify the results themselves and to perform any necessary optimising.
8.1 Substitution of cobalt driers
The results from the technical evaluation were used to evaluate if appropriate alternatives to Co driers exist for the respective oxidative drying products included in the project. The tested drier combinations for the specific products containing the alternatives were as a starting point based on suggestions from the drier manufacturers. During the initial drying time tests the drier combinations were adjusted with regard to concentrations and combinations if necessary.
Table 8.1 gives an overview of the most common concentrations and combinations that the alternatives have been tested in. Combinations containing other secondary driers have also been tested in a limited number, and with limited success.
The concentrations of the alternative driers are, where possible, given as concentration metal on solid oxidative drying binder present in the coating product. Otherwise the concentration is given as the total drier product on the solid oxidative drying matter. If an alternative has been identified as a potential Co substitute in a specific product, they are in most cases used in combination 1 (see table 8.1). In a few cases they are used in combination 2 or combination 3 (see table 8.1).
Table 8.1.
The concentrations and combinations the alternative driers typically have been used in when tested in DIY and
industrial air-drying coatings.
| Drier code of primary drier | Concentration of metal on solid binder
(% w/w) |
Combinations of secondary driers | ||
| 1 | 2 | 3 | ||
| Mn traditional# | 0.6 –0.8 | Ca (0.04 – 0.3)
Zr (0.10 – 0.30) DA (0.25 – 1.25)# |
Ca (0.04 – 0.3)
Bi (0.60 – 0.70) DA (0.25 – 1.25)# |
|
| Mn1 | 0.05 – 0.15 | Ca (0.04 – 0.3)
Zr (0.10 – 0.30) |
Ba (0.40 – 0.60)
Zr (0.10 – 0.30) |
Ca (0.05 – 0.30) |
| Mn2 | 3 – 8 ## | Ca (0.05 – 0.30) | Ca (0.04 – 0.30)
Zr (0.10 – 0.30) |
Without secondary drier |
| Mn3 | 3 – 8 ## | Ca (0.05 – 0.30) | Ca (0.04 – 0.30)
Zr (0.10 – 0.30) |
Without secondary drier |
| Mn4 | 0.03 – 0.11 | Without secondary driers | Ca (0.05 – 0.30) | Ca (0.04 – 0.30)
Zr (0.10 – 0.30) |
| Mn5(w) | 3 – 5 ## | Without secondary driers | ||
| Mn6(w) | 0.05 – 0.2 | Ca (0.04 – 0.3)
Zr (0.20 – 0.40) DA (0.4 – 0.6)# |
||
| V1 | 0.08 – 0.11 | Ca (0.04 – 0.30)
Zr (0.10 – 0.30) |
Ca (0.04 – 0.30)
Sr (0.20 – 0.40) |
|
| V2 | 0.06 – 0.1 | Ca (0.04 – 0.30)
Zr (0.10 – 0.30) |
Ca (0.04 – 0.30)
Bi (0.60 – 0.70) |
|
| V3(w) | 0.05 - 0.09 | Ba (0.30 – 0.60) | K (0.30 – 0.40) | |
# DA = Drying accelerator (2,2-bipyridyl product)
## Concentration of drier product on solid binder
8.1.1 Overall results for do-it-yourself products
Eight different do-it-yourself products were included in the project in which the different alternative driers have been tested. In some products it was considerable more difficult to substitute the cobalt driers than in others. If a high number of sample preparations and drying time tests was needed it is an indication of the product being particularly difficult to Co substitute (See table 8.2 and appendix A - Chapter 2). This especially accounts DIY-P1, DIY-P2, DIY-P4, DIY-P6 and DIY-P8.
In table 8.2 it is indicated for each of the air-drying do-it-your-self products, which drier that seems to be the most promising Co alternative on basis of the investigated drier combination and concentrations. For some products it is indicated that further optimising MAYBE necessary. This classification has only been given to those alternatives, which is comparable to or even better than the reference product for almost every tested property.
Table 8.2
The most promising Co alternatives are listed and it is indicated if further optimising of the alternative drier systems
is needed. If no other indication is given the alternatives listed have been used in combination 1 (See table 8.1)
| Co substitution results | ||||||||
| Product | DIY-P1 | DIY -P2 | DIY -P3 | DIY -P4 | DIY-P5 | DIY-P6 | DIY-P7 | DIY -P8 |
| Oil type in alkyd | Linseed + Soya | Soya + ? | Tall | Linseed | Tall | Tall | Tall | Tall |
| Number of tested systems | 18 | 19 | 18 | 34 | 18 | > 40 | 8 | 38 |
| Number of promising systems | 1 | (1) | 1 | (1) | 5 | 0 | 1 | 0 |
| Further optimising needed | YES | YES | MAYBE | YES | MAYBE | YES | YES | YES |
| Most promising alternative drier | Mn1 | (Mn1) | Mn1* | (Mn1/Mn4*) | Mn1/Mn3/ Mn4* | None | Mn1 | (Mn1) |
| Other potential alternative driers | Mn4 | Mn2/ Mn4** | (Mn5(w)) | Mn2/V1 | None | Mn4 | ||
* The Alternative has been used in combination 2 (See table 8.1)
** The alternative has been used in combination 3 (See table 8.1)
If the most promising drier is given in brackets it indicates that the drier was promising in the screening, but turned out to be inferior compared to the reference in the further testing. As these driers in most cases in fact induced comparable drying with the reference in the initial testing, it might be a matter of optimising the system. For instance by increasing the amount of the calcium drier to obtain a more stable drier system and hereby reducing the loss-of-dry. The same comments account for the results shown for the industrial products in table 8.3.
The alternatives in table 8.2 have been chosen by evaluating and comparing the drying times with those of the reference product. The film properties, hardness, gloss and yellowing in dark places of the alternative systems have also been compared to those of the reference products before selecting the most promising alternative driers for the respective products. In Appendix A - Chapter 3 the drying times of the alternative systems are compared with the reference products. This accounts for drying times obtained both before and after storing the samples at elevated temperatures. A comparison of film hardness, gloss, viscosity and yellowing can also be seen in Appendix A – Chapter 3. Only results from the most promising alternatives are shown together with results obtained for the references.
In table 8.2 other potential alternative driers are listed as well. In general these alternative drier systems have not been included in the stability/ageing test due to having inferior drying profiles in the initial testing compared to those alternatives chosen for further testing. However, this fact should not exclude them totally as potential alternatives as there is no knowledge on how they would perform after an induced ageing. With regard to Mn4, which was received late in the technical evaluation work, it has not been possible due to the late receipt to include it in the stability test for every product, even though it gave promising drying times in the initial testing. The same comments account for the results shown for the industrial products in table 8.3.
In most cases none of the vanadium driers gave sufficient drying in the tested concentrations and combinations. If sufficient drying was actually induced by the vanadium drier system the coating film became much too soft. As it can be seen in table 8.2 there is only one case where vanadium has been pointed out as "other potential" alternative, but it was not selected for further testing due to a soft coating film.
Mn1 seems to be the alternative drier, which is most useful for all-round purposes in conventional do-it-yourself coatings. Mn1 is among the most potential alternative drier for every product except DIY-P6 where the substitution totally failed.
Mn4 was included rather late in the testing process, for which reason drier systems containing Mn4 might not have been optimised to the same degree as for the other alternatives. In several cases it has been tested without secondary driers. Improved drying and film properties would most likely be obtainable by using the right combination with Zr and Ca driers. It was realised during the project that the concentration should rather be lowered than increased when optimising a drier system containing Mn4. This should be borne in mind when reading the used concentration in table 8.1 where the optimum concentrations of Mn4 most likely have to be found in the low range.
Mn2 and Mn3 are only among the most promising and other potential alternative driers in a few cases. It could be an indication of that these driers are not as efficient for air-drying do-it-yourself products as Mn1 and Mn4, or they might be more difficult to dose. Mn2 and Mn3 are more useful in the case of industrial products, which could indicate that these driers are more efficient for speciality products.
The driers made specifically for waterborne systems, Mn5(w) and Mn6(w) could be used in DIY-P3 with some success. Both Mn5(w) and Mn6(w) gave reasonable drying times in the initial test. Mn5(w) was slightly better than Mn6(w), but as one of the other alternatives, Mn1, gave a much faster drying in the initial test, Mn5(w) and Mn6(w) were excluded for further testing.
Mn traditional, the conventional manganese carboxylate drier, was only tested in a few do-it-yourself products without much success and it seems like Mn traditional has been tested in concentration that are 10 times higher than it should have been. The reason why Mn traditional was dropped in the rest of the products was though mainly that the addition of a drying accelerator induced another adjustable parameter in the alternative drier systems compared to the systems using driers where the drying accelerator is built into the drier product. More adjustable concentrations in a system often lead to an increase in the number of systems that needs to be investigated before proper drying is obtained.
All the alternative manganese driers tend to build in viscosity in the products. As no vanadium driers were selected for further testing their tendency to build up viscosity is not known.
Alternative drier systems with some potential to substitute cobalt driers were identified in case of DIY-P2, DIY-P4 and DIY-P8, but a relatively high extent of loss-of-dry was experienced after storage of the samples at elevated temperature, as the drying times were increased considerably. In the case of DIY-P1 and DIY-P7 substitution was possible with some success and for DIY-P3 and DIY-P5 the substitutions came out quite successfully. No potential alternative drier system was identified in the case of DIY-P6.
At present it seems not worthwhile substituting cobalt driers with vanadium driers from a technical point of view. To obtain proper drying as well as proper film hardness a manganese alternative should be used in preference to a vanadium alternative. This conclusion is of course only valid on basis of the drier systems and products tested in this project. As the success of substituting cobalt driers seems very binder and product specific, vanadium driers might work in other systems.
In the used concentration and combinations it was experienced that the various manganese driers differ quite a lot in drying efficiency in the specific products. Products containing binders based on linseed oil seem in general more difficult to Co-substitute than those products containing binders based on tall oil. The higher the amount of oxidative drying matter in a product the more difficult the substitution seems to become. As only two waterborne products, DIY-P3 and DIY-P6, were included it is difficult to draw any conclusion on what influence the thinner has on the substitution process. The overall conclusion is though that Co substitution seems possible in certain cases depending on the specific alternative driers as well as the specific product.
In the cases where the substitution success was rather limited due to a high extent of loss-of-dry after the stability tests it might be a question of optimising the drier system with regard to the auxiliary drier (e.g. increasing the content of calcium driers). Within the project frame it has not been possible to change the drier systems after the stability test had been performed. As the optimum proportion between manganese drier and drying accelerator also might differ from one type of binder to another it could in certain cases be worthwhile using a traditional manganese drier combined with a drying accelerator, which makes the proportion adjustable.
8.1.2 Overall results for the industrial products
As the group of do-it-yourself products was tested and investigated before the group of industrial products, some of the prior experiences could be used, for which reason fewer drier combinations and fewer tests in general were needed for the industrial products. The number of tested systems is given in table 8.3. In Appendix A – Chapter 2 an overview of all performed tests for the industrial products in connection with substituting cobalt driers and methyl ethyl ketoxime is given.
In table 8.3 it is indicated for each of the included air-drying industrial products, which drier that seems to be the most promising Co alternative if any on basis of the investigated drier combination and concentrations. Other potential alternative driers are listed as well. For some products it is indicated that further optimising MAYBE necessary. This classification has only been given to those alternatives, which is comparable to or even better than the reference product for almost every tested property. In table 8.3 the number of tested drier systems is given. In general, the higher the number of tested drier systems the more difficult the substitution was.
Table 8.3
The most promising Co alternatives are listed and it is indicated if further optimising of the alternative drier systems
is needed.
| Results from substitution of Co driers | ||||
| IND-P10 | IND-P11 | IND-P12 | IND-P13 | |
| Coating type | Lacquer | Topcoat | Primer | Primer |
| Number of tested systems | 25 | 20 | 12 | 13 |
| Number of promising systems | (1/2) | 0 | 2 | 3 |
| Further optimising needed | YES | YES | MAYBE | MAYBE |
| Most promising alternative drier | Mn1 /Mn2 | (Mn4) | Mn4/Mn3/ (Mn2) |
Mn1/Mn4/ Mn5(w) |
| Other potential alternative driers | Mn4* | Mn1 | Mn1 | |
* The Alternative has been used in combination 2 (See table 8.1)
As it can be seen from the number of sample preparation IND-P10 and IND-P11 were more difficult to Co substitute than IND-P12 and IND-P13, even though potential alternative drier systems were identified for all four products. In the case of IND-P10 a too high extent of loss-of-dry was observed after storing the samples with alternative drier systems at elevated temperatures, even though the drying times were still quite low. The very fast drying is very essential to IND-P10. The initial film hardness of the samples with the alternative drier systems was also too low.
In the case of IND-P11 the film hardness of the samples with alternative drier systems were far too low. This problem might be overcome by adding Zn as an additional drier. Loss-of-dry after storage at elevated temperature was also observed.
In IND-P12 and IND-P13 the cobalt driers were substituted with much higher success than in IND-P10 and IND-P11, the alternative systems having comparable drying time with the reference products, even after the samples had been stored at elevated temperature. In the case of IND-P12 three different manganese driers, Mn2, Mn3 and Mn4, can be used as Co substitute. All of them give higher film hardness and gloss, comparable or improved yellowing properties and comparable water resistance with reference product. Only in one case, inferior water resistance was observed.
Three driers, Mn1, Mn4 and Mn5(w) could be used with success for substituting cobalt driers in IND-P13, but in this case, film properties as film hardness, gloss and water resistance were slightly inferior to the reference in most cases. All three alternative systems were comparable to the reference with regard to yellowing.
As it was the case with the do-it-yourself products the alternatives driers shown in table 8.3 have been chosen by evaluating and comparing the drying times and other film properties with those of the reference product. These comparisons are shown in appendix A - Chapter 3 as well. Only results from the most promising alternatives are shown together with results obtained for the references.
Vanadium driers were not tested in the industrial products. All manganese alternatives included in the evaluation are represented within the group of most promising alternatives, for which reason it seems as if the industrial products in fact are easier to Co substitute than the do-it-yourself products. That is probably due to the binders. The do-it-yourself products contain common alkyds, whereas the industrial products contain either modified alkyds or alkyd blended with non-oxidative drying binders, and therefore the amount of oxidative drying matter is low compared to the do-it-yourself products. The industrial products therefore have a better drying profile even without the presence of driers.
8.1.3 Overall results for printing inks
The product group of printing inks differs quite a lot from the other air-drying products investigated. They are typically applied (printed) in a very thin layer and often at absorbent substrate, and therefore they show very little set off even though the drying times are much longer than those of the paints. The need for duct stability is one of the reasons to the longer drying time.
Therefore the printing inks, also without hydroquinone, have relatively long drying times when tested on a drying time recorder, even though they were applied in a much thinner film compared to the paint products. In the case of INK-P16 and INK-P17 the drying time measurements were performed on samples without hydroquinone. This accounts for both samples with alternative drier systems as well as the reference inks.
Mn1 combined with Mn traditional gave comparable drying results to the reference in all four sheet-fed printing inks. Both Mn1 and Mn traditional are used in rather high concentrations, Mn1 being added in concentrations ranging from 0.32 – 0.45% Mn metal on oxidative drying matter and Mn traditional was added in concentration corresponding to 0.5 – 0.8% Mn metal. In Appendix A – Chapter 3 the drying time results are presented for one alternative drier system for each ink and compared to the reference inks.
Mn4 has been tested in combination with Mn traditional. In one ink Mn4 was added in concentrations of 0.03 – 0.07% Mn metal on oxidative drying matter in combination with Mn traditional added in 0.5% Mn metal on oxidative drying matter. The system gives comparable or even better drying than the reference ink. Set-off was not investigated. The drying result for Mn4 used in INK-P15 is shown in Appendix A – Chapter 3.
Mn2 and Mn3 did not induce sufficient drying in the inks in the tested concentration of 5.0 – 13.5% [1] of total drier product on oxidative drying matter. One vanadium alternative was tested in a single drier system in INK-P14 and INK-P15, but as no drying was induced it was not tested further.
The number of tested systems in INK-P16 and INK-P17 is much lower than in INK-P14 and INK-P15 (see Appendix A – Chapter 2) as only the two most promising alternatives, Mn1 and Mn2 from the testing of INK-P14 and INK-P15 were investigated. Mn4 has only been tested in INK-P15 due to late receipt of this drier, but it seems as a potential alternative to cobalt driers in inks.
Only one drier system for each printing ink was chosen for testing the set-off effects. The set off effect of the inks containing the alternative driers was compared to those of the reference inks and in most cases the set-off effect was comparable to the reference ink.
It seems possible to substitute cobalt driers in sheet-fed printing inks, but the test results obtained for the drying times seem less significant than for the paint products. Probably due to the very thick printing inks are difficult to apply in even layers for the drying time tests and larger variations are therefore observed making them more difficult to evaluate. However, it might in fact be easier to substitute cobalt driers in this product group than the others. Less surface drying is needed for the printing inks and if the alternative drier system induces less drying than the cobalt based it is possible to reduce the amount of antioxidant (hydroquinone) present in the printing ink.
8.2 Alternative anti-skinning agents
To get an indication of whether the alternative anti-skinning agents can be expected to work in an air-drying product or not it has been investigated how effective the different alternatives prevent skinning from occurring in open and closed containers for the do-it-yourself and industrial products. The testing has been performed in accordance with the description given in 7.1.3.8.
In the printing inks the alternatives have been evaluated by measuring auto-oxidation temperature and by investigating duct stability as described in 7.2.2.3 and 7.2.2.4. The original cobalt based drier systems were used in the inks.
8.2.1 DIY and Industrial products
The anti-skinning agents have been tested in one concentration only in each product. These concentrations are listed in table 8.4. The concentrations listed are the concentration of the entire anti-skinning product, not just the active substances. All alternatives, except acetone oxime, were tested in approximately the same concentration in all air-drying products, no matter what the original methyl ethyl ketoxime concentration had been. This means that they are added in a higher concentration than methyl ethyl ketoxime in some products.
The samples within a product series contain the same alternative drier system, chosen from the technical evaluation of the alternative driers. The manufacturer supplied IND-P9 with its original drier system.
In table 8.5 and 8.6 it is shown for how many days the different anti-skinning agents are able to prevent skinning on the surface of the different air-drying products in closed and open containers respectively.
Table 8.4
The used concentrations of the tested anti-skinning agents in
do-it-yourself and industrial products.
| Anti-skinning agent | Concentration in product (% w/w) |
| MEKO | As in reference product |
| Acetone oxime | 4 x MEKO concentration |
| Amino/Amido no 1 and 2 | 0.25 – 0.4 |
| Phenolic no 1 | 0.25 – 0.4 |
| Phenolic no 2 | 0.25 – 0.4 |
| Vitamin E | 0.4 – 2.5 |
The results of each anti-skinning agent should be compared both with the results obtained for the product without anti-skinning agent as well as the product containing methyl ethyl ketoxime. As DIY-P8 had a high extent of loss-of-dry after the ageing test, this product was not included in the test of anti-skinning agent. Due to a limited amount of sample DIY-P4 was only tested with regard to skinning in closed containers.
Table 8.5
Number of days before skinning is observed in the DIY and industrial products for sample stored in closed
containers at 23C and 50 % relative humidity.
| Number of days before skinning occurs | |||||||||
| Anti-skinning agent | DIY-P1 | DIY-P2 | DIY-P4 | DIY-P5 | DIY-P7 | IND-P9 | IND-P10 | IND-P11 | IND-P12 |
| None | 2 | 2 | 1 | 2 | 2 | 4 | 2 | 2 | 7 |
| Methyl ethyl ketoxime | 49 | 11 | 4-27 | 16 | 40* | > 37 | 7 (14) | > 30 | 17 |
| Acetone oxime | > 49 | 11 | > 35 | 16 | 35* | > 37 | 7 (10) | > 30 | 17 |
| Amino/amido no 1 | 8 | 4 | > 35 | 16 | 30* | > 37 | 7 | > 30 | 27 |
| Amino/amido no 2 | 8 | 2 | > 35 | 16 | 30* | > 37 | 4 | > 30 | 20 |
| Phenolic no 1 | 2 | 2 | 1-4 | 7 | 2 | > 37 | 2 | 2 | 7 |
| Phenolic no 2 | 2 | 2 | 1-4 | 9 | 2 | > 37 | 2 | 4 | 7 |
| Vitamin E | 1-4 | > 37 | |||||||
* All systems separate within 4 days
Tests performed in closed containers show that acetone oxime is the anti-skinning agent, which is most comparable to methyl ethyl ketoxime, but amino/amido based anti-skinning agents also give comparable or better results in several cases. The phenolic based anti-skinning agents do not work in the tested concentrations. Vitamin E has only been tested in product IND-P9 in three concentration, 0.5, 1.0 and 2.0% respectively and in one concentration in DIY-P4 (0.35%). Vitamin E is not efficient in DIY-P4 and in IND-P9 it is impossible to differentiate between the different anti-skinning agents.
The results from the open containers show that the amino/amido compounds in most cases are comparable to or more efficient than methyl ethyl ketoxime in preventing skin formation in this situation. This could strongly indicate that these anti-skinning agents would have a more negative effect on the drying times than the oximes. Vitamin E prevents the skinning to some extent in product IND-P9, but is also added in rather high concentrations.
The results from the skinning tests can also be used to indicate whether more or less of a specific alternative anti-skinning agent is needed to get comparable results with the reference. Further optimisation of the concentrations is most likely necessary. For some anti-skinning agents a higher concentration would properly give better results with regard to prevent the skinning. On the other hand higher concentrations of anti-skinning agents would inevitable influence on the drying times. The effect was more pronounced for the less volatile alternatives.
Table 8. 6
Number of days before skinning is observed in the DIY and industrial products for sample stored in open
containers at 23C and 50 % relative humidity.
| Number of days before skinning occurs | ||||||||
| Anti-skinning agent | DIY-P1 | DIY-P2 | DIY-P5 | DIY-P7 | IND-P9 | IND-P10 | IND-P11 | IND-P12 |
| None | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Methyl ethyl ketoxime | 4 | 4 | 7 | 7 | 7 | 4 | 7 | 2 |
| Acetone oxime | 4 | 4 | 4 | 4 | 4 | 2 | 7 | 2 |
| Amino/amido no 1 | 4 | 2 | 14 | 17 | 7 | 2 | 11 | 2 |
| Amino/amido no 2 | 4 | 2 | 116 | 17 | 7 | 2 | 14 | 2 |
| Phenolic no 1 | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 |
| Phenolic no 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Vitamin E | 7/4/2 | |||||||
The skin formation tests have only been performed with one concentration of the respective anti-skinning agent and the effect of the anti-skinning agents on the drying time has not been investigated. The obtained results should therefore only be used as guidance to the paint manufacturers on whether it is worthwhile trying to substitute methyl ethyl ketoxime in their products or not.
8.2.2 Printing inks
The efficiency of anti-skinning agents/antioxidant within printing inks was investigated by measuring the auto-oxidation temperature of ink samples containing different anti-skinning agents. The testing has been performed as described in 7.2.2.3.
The more the auto-oxidation temperature is increased compared to the ink without any antioxidant the more efficient can one expects the antioxidant to be. The obtained results for two sheet-feed inks are given in table 8.7. The concentrations given are for the entire anti-skin product, not just the active substances.
Compared to the inks without anti-skinning agents, all the investigated anti-skinning agents, except methyl ethyl ketoxime and phenolic type 2, have an effect on the auto-oxidation temperature. Hydroquinone, which is the antioxidant commonly used in printing ink, has the strongest effect. In the tested concentration (0.8 % w/w) it gives higher auto-oxidation temperature than the reference in the tested concentration. This was expected as the references only contain approximately 0.2 % w/w hydroquinone.
Vitamin E gives in concentrations of 2.5 % w/w some reasonable results, which are comparable to the reference inks. The effect on the auto-oxidation temperature is not as distinct for the amino/amido based compounds, but they show an effect, which probably could be increased by increasing the amount of anti-skinning agents.
Table 8.7
The measured auto-oxidation temperatures of two inks containing different anti-skinning agents.
| Anti-skinning agent | Concentration
(% w/w on total) |
Auto-oxidation temperature (ºC) | |
| INK-P16 | INK-P17 | ||
| Reference ink | 131 | 114 | |
| None | 82 | 69# | |
| Hydroquinone | 0,8 | 129 | 137 |
| Hydroquinone | 1,6 | 161 | 171 |
| E-vitamin | 1,25 | 109 | 90 |
| E-vitamin | 2,5 | 126 | 100 |
| Amino/amido No 1 | 1 | 96 | 95 |
| Amino/amido No 2 | 1 | 96 | 96 |
| Phenolic type No1 | 1,7 | 87 | 65# |
| Phenolic type No1 | 3,4 | 89 | 67# |
| Phenolic type No2 | 1 | 83 | |
| MEKO | 1 | 81 | |
# No distinct auto-oxidation.
An investigation of the duct stability was performed on a drying time recorder. The drying time results obtained for samples with different alternative anti-skinning agents are given in table 8.8. The samples with 0.4 % hydroquinone are used as reference. All inks contain the original cobalt drier systems. The concentrations given are the concentration of the entire anti-skinning product and not just of the active substances. The compositions of the alternatives can be found in table 6.2. The used hydroquinone product contains 49 weight-% active substance.
If an ink sample with an alternative anti-skinning agent dries faster than the ink sample with hydroquinone, the alternative is not expected to prevent drying in the ducts to the same extent as the reference ink.
Vitamin E is used in reduced amount compared to the auto-oxidation tests, whereas the amino/amido compounds are used in increased concentrations. In the tested concentration amino/amido no. 1 and no. 2 seem to give the best protection against drying, amino/amido no. 2 being slightly better than no. 1.
Vitamin E also protects from drying, but has lower set-to-touch and tack-free times than both the amino/amido compounds and hydroquinone. For INK-P16 vitamin E seems as efficient as the amino compounds.
Table 8.8.
test of duct stability. Investigated by comparing drying times of the ink samples at a drying time recorder.
| Anti-skinning agent | Conc. (%) | Drying times (h) | ||||
| Ink sample | Set-to-touch | Tack-free | Dry-through | Dry-hard | ||
| INK-P14-1 | Hydroquinone | 0,25 | 24,2 | 33,8 | 39,6 | 47,3 |
| INK-P14-4 | Vitamin E | 2,0 | 12,1 | 18,3 | 33,3 | 47,1 |
| INK-P14-6 | Amino/amido no. 1 | 2,0 | 29,6 | 35,8 | 39,6 | > 50 |
| INK-P14-8 | Amino/amido no. 2 | 2,0 | 30,0 | 36,7 | 40,4 | > 50 |
| INK-P15-1 | Hydroquinone | 0,25 | 36,3 | > 50 | > 50 | > 50 |
| INK-P15-4 | Vitamin E | 2,0 | 11,7 | 17,1 | 25,0 | 32,5 |
| INK-P15-6 | Amino/amido no. 1 | 2,0 | 24,2 | 27,1 | 36,7 | 39,2 |
| INK-P15-8 | Amino/amido no. 2 | 2,0 | 24,2 | 26,7 | 35,4 | 38,3 |
| INK-P16-1 | Hydroquinone | 0,4 | > 78 | > 78 | > 78 | > 78 |
| INK-P16-4 | Vitamin E | 2,0 | 33,8 | 47,6 | > 78 | > 78 |
| INK-P16-6 | Amino/amido no. 1 | 2,0 | < 28 | 45,5 | > 78 | > 78 |
| INK-P16-8 | Amino/amido no. 2 | 2,0 | 36,3 | 45,9 | > 78 | > 78 |
| INK-P17-1 | Hydroquinone | 0,4 | > 50 | > 50 | > 50 | > 50 |
| INK-P17-4 | Vitamin E | 2,0 | 10,8 | 12,1 | 37,5 | 37,5 |
| INK-P17-6 | Amino/amido no. 1 | 2,0 | 14,2 | 19,6 | 43,3 | 43,3 |
| INK-P17-8 | Amino/amido no. 2 | 2,0 | 20,0 | 20,8 | 40,8 | > 50 |
The alternatives are only comparable or slightly better in the case of INK-P14 even though they are used in rather high concentration. This indicates that they are not as efficient antioxidant as hydroquinone, but that they might work in the printing inks. The influence on the set-off effect of the different alternative anti-skinning agents has not been investigated.
9 Environmental and health assessment
The environmental and health assessment of driers as well as anti-skinning agents will be done on groups of substances rather than single substances as specific information is very sparse. In some cases it has even been impossible to obtain information about the exact identity of the substances from manufacturers. In general, environmental and health information about specific substances is limited.
The groups of substances to be evaluated are selected based on the screening and on the results of the technical testing of the identified alternatives. Constituents of the most promising alternative driers and anti-skinning agents are included in the environmental and health assessment in order to assess the overall improvements of the environment and health profiles of air-drying products in connection with a substitution.
If substances are excluded on the basis of the screening, there is no need for a more thorough assessment. However, some exceptions are made: The existing driers (cobalt driers) and anti-skinning agents (methyl ethyl ketoxime and hydroquinone) are all included in the environment and health assessment in order to compare their environment and health effects with the alternatives.
The petroleum distillates are selected for evaluation as they are present in most of the drier products and the anti-skinning agents in considerable amounts and they are often classified due to health and environmental effects.
The groups to be evaluated are:
| Driers | Anti-skin agents | Solvents | |
| Existing substances | • Cobalt driers / cobalt compounds | • Methyl ethyl ketoxime •Hydroquinone |
• Petroleum distillates |
| Alternatives | • Manganese driers / manganese compounds • Vanadium driers / vanadium compounds |
• Organic amino anti-skinning agents • Vitamin E |
As far as specific information about the tested substances is available this information is included in the evaluation.
9.1 Driers
The driers to be evaluated are the existing cobalt driers, and the alternatives manganese and vanadium driers.
9.1.1 Cobalt driers
Cobalt paint driers are typically either
- Cobalt naphthenate (CAS-no. 61789-51-3) or
- Cobalt 2-ethyl hexanoate (CAS-no. 136-52-7).
Cobalt compounds in general are also included in the assessment of the ecotoxic and toxic effects of cobalt driers because the information on the specific cobalt driers is limited.
9.1.1.1 Cobalt naphthenate
Cobalt(II) naphthenate (CAS-no. 61789-51-3) is a cobalt salt of naphthenic acid, which is a mixture of
cycloalkanic acids. Cobalt naphtenate is insoluble in water, but soluble in alcohol, ether and oils, /28/
A study has shown that the absorption of cobalt naphthenate after oral ingestion is limited in rats – up to 73% of the dose was excreted in the faeces. Similarly, experiments have shown that the skin absorption of cobalt napthenate in rats is minimal, /28/.
The acute toxicity of cobalt naphthenate is very low (oral LD50 of 3900 mg/kg in rats), which may be because of the low solubility causing a low absorption, /28/.
Aerosols of cobalt naphthenate are irritating to the eyes and the respiratory tract. Repeated or prolonged contact may develop lung or skin allergy, /22/28/.
IARC (International Agency for Research on Cancer) classifies cobalt and cobalt compounds as a group 2B substance i.e. the substance is possibly carcinogenic to humans. A single Polish study has shown development of tumours in rabbits and mice following cobalt naphthenate exposure, but the study was found to be inadequate by IARC, /28/. IARC concludes that there is inadequate evidence for the carcinogenicity of cobalt naphthenate, /23/.
No data on ecotoxicity was found for this specific substance.
9.1.1.2 Cobalt 2-ethylhexanoate
The information about cobalt 2-ethylhexanoate is very limited. Most of the information refers to cobalt compounds
in general.
Cobalt 2-ethylhexanoate may cause irritation of the skin. Prolonged or repeated contact may cause dermatitis of the allergic type, /22/. Furthermore, cobalt 2-ethylhexanoate is found to damage kidneys, /29/.
No data on environmental fate or toxicity was found for this specific substance.
9.1.1.3 Cobalt compounds
Cobalt is, as Vitamin B12 (cyanocobalamin), an essential trace element in humans and animals. Vitamin B12 acts as
a coenzyme in many enzymatic reactions. Cobalt exists in the valence states –1, 0, +1, +2, +3, +4, and +5, where
0, +2 and +3 are the most common, and cobalt +2 the most stable. The two typical cobalt paint driers cobalt
2-ethyl hexanoate and cobalt naphthenate are both cobalt (II) compounds, /30/.
Results indicate that only a portion (probably less than 50%) of ingested cobalt will be absorbed. Insoluble cobalt compounds are in general absorbed much less than soluble cobalt compounds, /28/. Inhaled cobalt particles are deposited in the lungs and subsequently absorbed. Dermal absorption of cobalt compounds depends greatly on whether the skin is intact or damaged. Studies show that absorption through intact skin is very small (below 1%), while absorption through abraded skin can be as high as 80% three hours after exposure (measured for cobalt chloride). Absorbed cobalt is transported throughout the body in the blood, with largest levels found in liver, followed by the kidney, /30/.
Oral LD50 values for different cobalt compounds (cobalt chloride, cobalt oxide, cobalt sulphate, cobalt acetate, cobalt bromide) indicate that cobalt compounds are toxic by ingestion, /30/.
The primary targets following acute exposures to cobalt are in humans the respiratory system (inhalation exposure), the thymus (oral exposure), and the immunological system (dermal exposure). The effects of chronic occupational exposure to cobalt compounds on the respiratory system are well documented, and include effects like respiratory irritation, wheezing, asthma, and pneumonia. Furthermore, effects on the nervous system, including memory loss, nerve deafness, and a decreased visual activity, have been reported for occupational exposure to cobalt. Ingestion of cobalt can cause nausea, vomiting, and diarrhea, but has also resulted in e.g. respiratory, cardiovascular, muscoskeletal, haematological, and body weight effects, /29/30/.
Irritation of the skin is a common result of dermal exposure to cobalt in humans. This has been verified in a large number of studies. Contact allergy has been reported, as well as allergic dermatitis following oral exposure to cobalt. The studies indicate that cobalt is a sensitizer, /30/.
Soluble cobalt salts are shown to interfere adversely with cell division, to introduce chromosome aberrations in plants and to be mutagenic to some cultured animal cells. However, in other test systems cobalt compounds are non-mutagenic, co-mutagenic or anti-mutagenic. The conclusion is that cobalt salts are slightly genotoxic, /31/.
Jensen and Tüchsen, /31/, concluded that there seems to be sufficient evidence that cobalt, and soluble as well as insoluble cobalt compounds are carcinogens in animal experiments, and that data for evaluation of human cancer risk is insufficient, but indicates a carcinogenic potential in humans. The material available indicates that it is the cobalt metal itself, which is the problem with regard to cancer. The results of several studies suggest a relationship between occupational cobalt exposure and excess lung cancer mortality, /29/30/. On the basis of the available material IARC classifies cobalt and cobalt compounds as a group 2B substance i.e. the substance is possibly carcinogenic to humans, /23/. Cobalt compounds in general are also adapted on the Danish list of carcinogenic substances, /32/.
Inhalation and oral studies in male animals have demonstrated adverse effects on reproductive organs, /30/.
Cobalt released into water may absorb to particles in the water or sediment, or remain in the water in ionic form. Cobalt deposited on soil is often strongly attached to soil particles, and will not travel very far into the ground. The specific fate of cobalt will depend on different chemical factors; however, ultimately most cobalt ends up in soil or sediment, /30/.
The information on the environmental fate and toxicity of cobalt compounds in general is sparse. Data for cobalt suggest a low ability to bioconcentrate in aquatic organisms (BCF below 100). The very few LC50 values found for cobalt indicate that cobalt is toxic or very toxic to aquatic organisms. (LC50 (28 days) for rainbow trout found between 0.2 and 15.6 mg/l), /33/. The Nordic list of suggestions for environmental classification classifies cobalt as "may cause long-term adverse effects in the aquatic environment" (R53). Different cobalt compounds like cobalt chloride, cobalt sulphide, cobalt oxide, and cobalt sulphate are all classified by the Nordic Council of Ministers as "very toxic to aquatic organisms" and "may cause long-term adverse effects in the aquatic environment", /34/.
9.1.2 Manganese driers
Manganese driers used in this project as alternatives to cobalt driers are:
- Manganese 2-ethylhexanoate (CAS-no. 13434-24-7) - Xi R38 [2]
- Manganese salt (type and CAS-no. confidential) – classification not known
- Manganese salt of C6-19 branched fatty acid and naphthenic acid (not further specified, CAS-no. confidential) – Xi R38
- Manganese dipropionate (CAS-no. 21129-18-0) – Xi R38
- Manganese (II) isooctanoate (CAS-no. 37449-19-7) – Xi R38
- Manganese isononate (CAS-no. 29826-51-5) – Xi R38
- Manganese compound (confidential) – classification not known
No environmental or health information was found for these specific substances, therefore manganese compounds are described in general.
9.1.2.1 Manganese compounds
Manganese is an essential trace element in humans that plays a role e.g. in bone mineralization, protein and energy
metabolism, and cellular protection from damaging free radical species. Manganese deficiency can lead to slowed
blood clotting, skin problems, changes in hair colour, lowered cholesterol level, and other alterations in metabolism.
However, exposure to high levels of manganese via inhalation or ingestion is toxic, and can cause adverse health
effects, /35/36/.
Manganese exists in different oxidation states from –3 to +7, the most common being +4, +3, and +2, both in the environment and in the workplace. In living systems, manganese is found in the +2 valence as an essential element. Most manganese compounds seem to cause the same effects, although it is unknown whether exposure to different manganese compounds results in slight differences in adverse effect. Mn2+ is in general considered to be more toxic than Mn3+, /29/36/37/.
The primary route of absorption of manganese in the human body is via inhalation. The dermal route does not appear to be of significant concern. Given comparable doses, more manganese reaches the brain following inhalation than following ingestion, and most health effects are associated with chronic inhalation exposure, /35/. Once absorbed, manganese is transported to organs like the liver and pancreas where it is rapidly concentrated. Accumulation of manganese in the central nervous system (CNS) occurs more slowly. Manganese does not undergo metabolism in the human body, /37/
For some organic manganese compounds irritation of skin may occur by contact with skin, and some organic manganese compounds have been found to cause allergic contact dermatitis, /36/.
Acute inhalation exposure to high concentration of manganese dust can cause an inflammatory response in the lung, which over time can result in impaired lung function. This lung toxicity results in symptoms like increased incidence of colds, bronchitis and pneumonia, /35/37/.
In chronic inhalation exposure to manganese, the main organ systems affected are the lungs, the CNS, and reproductive system, although effects on other organ systems also have been observed, /35/.
The primary effect of manganese toxicity from inhalation exposure in humans is symptoms of CNS toxicity. Prolonged inhalation of elevated manganese concentrations result in chronic manganese neurotoxicity – a Parkinsonism-like disease called manganism. Initial symptoms of manganism are usually general feelings of weakness, headache, muscle pain, insomnia, nervousness, irritability, speech disturbances and memory loss. Removal of the affected person from the manganese source usually results in reversal of most of the symptoms. However, a continued chronic exposure can result in motor difficulties, clumsiness, muscle cramps, tremors – symptoms similar to Parkinsons disease. The later stages of manganese toxicity are irreversible even though manganese concentrations in the tissues decrease to normal levels upon removal from the manganese source, /36/37/38/.
Manganese can result in reproductive effects, such as decreased libido, impotence, and decreased fertility in men by chronic inhalation exposure. No information is available on the reproductive effects in women, /35/.
In vitro studies show that some chemical forms of manganese (manganese chloride, manganese sulphate) have mutagenic potential. However, in vivo studies are inconsistent, therefore no overall conclusion can be made about the possible genotoxic hazard to humans, /35/. Equally, information about the carcinogenic potential of manganese is limited. U.S. EPA assesses manganese as not classifiable as to human carcinogenicity based on no evidence in humans and inadequate evidence in animals, /36/37/.
In 1995 a Danish occupational medicine clinic investigated the relationship between working with manganese and serious diseases at a steel rolling mill (Stålvalseværket) in Denmark. A clear connection between working with manganese and serious diseases as trembling and lapse of memory was found, and resulted in a strong suspicion that manganese can result in brain damage. The investigation showed that the group of people working with manganese dust was hit the hardest, /38/.
Today, about 50 people in Denmark have received financial compensation for their occupational injury because of working with manganese, and at least the same number of people is waiting for a decision in their occupational injury case. Most of them are not able to work because of their illness, /38/.
Other international investigations have shown that not all people working with manganese are getting sick. Experts agree on the fact that manganese is a hazardous substance, but knowledge of what makes the metal hazardous is lacking. Investigations suggest that inhaled manganese dust will enter the blood stream, enter the brain and cause serious damage. However, it may also be manganese in combination with another metal like lead that is problematic. More research on this field is necessary, /38/. A study that will investigate the mechanism for neurotoxic effect of manganese and the combination effects of manganese has been initiated in Denmark. No results are ready so far as the study is due during 2004.
In the external environment, the oxidation states +2, +3 and +4 are the most stable. Manganese +2 is the most stable oxidation state in water, while manganese +3 and +4 compounds are immobile solids that may be reduced to the soluble manganese +2 by organic matter. Manganese +2 compounds are relatively mobile, as they do not strongly complex to soil and organic matter, and may therefore potentially leach into surface and groundwater. Manganese compounds are not expected to volatilise from water or moist soil surfaces, /29/.
With regard to the aquatic toxicity of manganese, the LC50 (96 h) values for manganese and manganese +2 that are found, leads to a classification of harmful to aquatic organisms or no environmental classification. (Manganese: crayfish 28-51 mg/l; manganese (II) sulphate: fathead minnow 24-37 mg/l, longfin dace 100-169 mg/l), /33/. The Nordic list of suggestions for environmental classification classify manganese (II) sulphate as toxic to aquatic organisms, and may cause long-term adverse effects in the aquatic environment (N, R51, R53), /34/.
Manganese accumulates (up to a factor 40.000) in different kinds of plants, and in some mussels and other marine invertebrates. However, manganese compounds do not bioconcentrate in humans and animals, /29/. A bioconcentration factor (BCF) for different manganese compounds is found between 3 and 61 for different fish (starfish: BCF = 3-61, fathead minnow: BCF = 23, and brown trout: BCF = 18), /33/.
9.1.3 Vanadium driers
Vanadium driers used in this project as alternatives to cobalt driers are:
- Vanadium organophosphate (CAS-no. confidential) - Xn R22 [3]
- Vanadium compound (confidential) – classification?
- Vanadium neodecanoate (CAS-no. 60451-07-2) – Xi R38 [4]
No environmental or health information was found for these specific substances, therefore vanadium compounds are described in general.
9.1.3.1 Vanadium compounds
In general little information about vanadium compounds exists. The information is primarily on the vanadium metal
or inorganic compounds – primarily vanadium pentaoxide.
Vanadium metal has a low toxicity, and does not seem to be dangerous for human health. Pentavalent vanadium and vanadates are the most toxic vanadium substances, /29/39/
Vanadium compounds are in general poorly absorbed when ingested, but are more easily absorbed through the lungs, /40/. The toxic effects are therefore to some extent limited to the respiratory system. Effects like coughing, difficulty in breathing, pneumonia and bronchitis are observed by exposure in industry, /40/41/. Vanadium compounds may cause CNS-toxicity. Effects, like tremors, headaches, tinnitus and changes in the mental status, have been observed, /29/41/.
Vanadium is skin irritant, and vanadium dust (usually vanadium pentaoxide) is severely irritating to eyes, nose, throat and respiratory tract, /29/42/. Still, no clear information is available from animal studies with regard to the potential of vanadium compounds to produce skin or eye irritation or skin sensitisation, /42/.
Some vanadium compounds (primarily inorganic vanadium compounds, e.g. vanadium pentaoxide) have long-term effects and are carcinogenic, mutagenic and have reproductive effects, /39/. Inhalation studies of vanadium pentaoxide in rats and mice show some evidence of carcinogenic activity in rats, and clear evidence of carcinogenic activity in mice, /43/. Furthermore, there is evidence that tetravalent vanadium has the ability to cross the placental barrier to the foetus, /42/, and studies with female rats indicate a possible teratogenic effect. Vanadium pentooxide has to be classified as mutagenic (Mut3) and reprotoxic (Rep3) according to the list of dangerous substances, /24/.
The environmental fate of vanadium is characterised by the fact that a large part is absorbed by organic material, e.g. the sediment. Dissolved in water vanadium will oxidise to the pentavalent state. Vanadium pentaoxide is classified as toxic for aquatic organisms. /39/. Acute LC50 values for aquatic organisms range from 0.2 to about 120 mg/litre, with the majority lying between 1 and 12 mg/litre, /42/. The Nordic list of suggestions for environmental classification classify vanadium pentaoxide as toxic to aquatic organisms, and may cause long-term adverse effects in the aquatic environment (N, R51, R53) as the substance is assessed not to be readily biodegradable, /34/.
A few bioconcentration factors are found: 50 to 600 for fish, and factors 400 and 1900 for phyto- and zooplankton, indicating an ability to accumulate, /29/. However, there is no evidence of accumulation in food chains in marine organisms, /42/.
A recent study by the Danish EPA, /39/, has investigated the present knowledge of a number of "second rank" elements – including vanadium - with regard to use pattern and consumption in Denmark, dispersal into and behaviour in the environment, hazards to human health and potential effects in the environment. The conclusion of the study – with regard to vanadium – is that if the inherent toxicological and ecotoxicological properties of the investigated elements are combined with consumption, use pattern and risk of dispersal into the environment, vanadium is one of the elements that are assessed to be the most critical among the second rank elements at present.
The study concludes that vanadium can be described as a substance that is toxic for aquatic organisms. However, in Denmark, concentrations of vanadium in the environment are primarily found in solid waste, and will therefore not have a significant effect on the aquatic environment. If vanadium driers are to substitute cobalt driers in paints, the vanadium discharged to the aquatic environment will increase, because of discharge of paint residues to the aquatic environment.
9.1.4 Evaluation of the driers
The driers included in the environmental and health assessment are the existing cobalt driers (cobalt naphtenate and cobalt 2-ethylhexanoate) and different manganese and vanadium driers as alternatives. Examples of manganese and vanadium driers are listed above (see the sections "Manganese driers" and "Vanadium driers"). However, some of the alternative driers are confidential, and for all alternatives apply that no specific environmental or health information was found. Therefore, the environmental and health assessment of the driers is carried out on the basis of the group of substances.
Cobalt, manganese and vanadium compounds have in common that the oral and dermal absorption of the substances is low – absorption is more or less limited to absorption by inhalation. Cobalt, manganese and vanadium compounds therefore show some of the same effects, e.g. pneumonia-like symptoms and CNS-damage. Manganese exposure may lead to Parkinsonism-like disease.
Cobalt compounds may lead to allergic contact dermatitis, and the same effect has been seen for some organic manganese compounds. However, for vanadium compounds no clear information is available.
Cobalt compounds are in general found to be possibly carcinogenic to humans (group 2B). For manganese compounds no evidence of carcinogenic effects exists for humans, and the evidence in animals is inadequate. For vanadium carcinogenic effects have been shown, but vanadium compounds are not classified due to its carcinogenicity to humans.
Mutagenic and reprotoxic effects are seen for all three metal compounds. However, mutagenic studies for manganese compounds are inconsistent, and the mutagenic and reprotoxic effects for vanadium are primarily seen for vanadium pentaoxide.
The health profile for air-drying products will be less negative, if cobalt driers are substituted with manganese or vanadium driers, as only cobalt compounds are classified with regard to the carcinogenic effects to humans. The effect is not very obvious because both manganese and vanadium compounds have shown adverse health effects. The adverse effects for vanadium compounds are, though, primarily found for vanadium pentaoxide. Health effects of other vanadium compounds are very sparse. The history of occupational manganese exposure indicates that the adverse health effects primarily are associated with intense exposure over a long period of time.
With regard to ecotoxicity of the driers, the environmental profile will also be less negative, if cobalt driers are substituted with manganese or vanadium driers. Cobalt driers are in general regarded as very toxic or toxic to aquatic organisms, whereas vanadium compounds (vanadium pentaoxide) are toxic and manganese compounds are regarded as harmful to aquatic organisms.
Overall, the environment and health profile of air-drying products will become less negative if cobalt driers are substituted with manganese or vanadium driers, especially as the driers are combined with the same secondary driers as cobalt driers and in approximately the same concentrations. However, this evaluation is based on information about the group of substances alone, as no specific information was found on the alternative manganese or vanadium driers.
9.2 Anti-skinning agents / antioxidants
The anti-skinning agents to be evaluated are the existing anti-skinning agents – methyl ethyl ketoxime and hydroquinone, and the alternatives amino/amido anti-skinning agents and vitamin E.
9.2.1 Methyl ethyl ketoxime
Methyl ethyl ketoxime (CAS-no. 96-29-7) is hazardous by ingestion, and is also harmful by inhalation and in contact with skin, as the substance is readily absorbed through the skin. The substance is a severe eye irritant and is also irritating to the skin, /44/.
Methyl ethyl ketoxime may cause sensitization by skin contact, and is capable of causing allergic skin reactions. The substance has to be labelled with the risk phrases R41 "Risk of serious damage to the eyes" and R43 "May cause sensitisation by skin contact", /24/.
Mutagenic effects have been observed for methyl ethyl ketoxime as well as tumorigenic effects. Methyl ethyl ketoxime is considered to be carcinogenic according to the criteria set by RTECS, /44/. Methyl ethyl ketoxime must be labelled with "Carc Cat. 3" and the risk phrase R40 "Limited evidence of a carcinogenic effect", /24/.
Methyl ethyl ketoxime is not found to be toxic for aquatic organisms. (LC50 96 h values for fathead minnow are found between 777 and 914 mg/l), /33/. According to the Nordic list of suggestions for environmental classification methyl ethyl ketoxime is not likely to bioconcentrate in aquatic organisms (Log POW = 0.65 and BCF = 6), /34/. No data on the biodegradability of methyl ethyl ketoxime was found.
9.2.2 Hydroquinone
Hydroquinone (CAS-no. 123-31-9) is rapidly and extensively absorbed from the gut and trachea of animals. Absorption via skin is slower. Hydroquinone is distributed rapidly and widely among tissues, but hydroquinone and its metabolites are excreted rapid – primarily via the urine. Hydroquinone and its derivatives react with different biological compounds and have effects on cellular metabolism, /45/.
Hydroquinone is hazardous by ingestion, and may actually be fatal if swallowed, /44/. The major signs of hydroquinone poisoning are dark urine, vomiting, abdominal pain, tremors, convulsions and coma, /45/. The substance is harmful if inhaled, and is toxic to lungs, the central nervous system and the mucous membranes. Acute high-level exposure to hydroquinone causes severe effects on the CNS including, tremor, coma, convulsions and death, /45/. Repeated or prolonged exposure to the substance can produce target organ damage.
Hydroquinone is hazardous in case of skin contact, as the substance can be absorbed through the skin. The substance causes severe skin and eye irritation, and may cause allergic skin reactions. The substance is reported to be an active allergen, /29/44/. Hydroquinone must be labelled with the risk phrases R41 "Risk of serious damage to the eyes" and R43 "May cause sensitisation by skin contact", /24/.
Based on animal studies hydroquinone may cause cancer /29/. However, these findings are limited and IARC (International Agency for Research on Cancer) classifies hydroquinone in group 3 i.e. not classifiable as to its carcinogenicity to humans, because of inadequate evidence in humans for the carcinogenicity, /46/. Hydroquinone must be labelled with "Carc Cat. 3" and the risk phrases R40 "Limited evidence of a carcinogenic effect" and R68 "Possible risk of irreversible effects", as well as "Mut Cat. 3" as it is suspected to be a human mutagen, /24/. Gene mutations and DNA damages have been demonstrated in test tube experiments, /45/.
Hydroquinone is highly soluble in water, and will mainly be distributed to the water compartment when released into the environment /45/. Hydroquinone is very toxic to aquatic organisms (LC50 96 h for fathead minnow 0.05 – 0.4 mg/l) /33/, but may be readily biodegrade and is not likely to bioconcentrate (Log POW = 0.59 and BCF = 40), /34/45/. However, the products of degradation are as toxic as the original products. Hydroquinone must be labelled with the risk phrase R50 "Very toxic to aquatic organisms", /24/.
9.2.3 Organic amino compound
Two anti-skinning agents (trial products) with an organic amino and amido compound as the active ingredients have been tested. The test product received contained both an organic amido compound and an organic amino compound. However, the formulation of these anti-skinning agents has been changed since the testing, resulting in the amido compound being excluded from the formulation.
The identity of the compounds is confidential. However, the project group was told the identity of the compounds in return for keeping the identity as a secret. This is the reason why no exact references will be given in this section, as the references may reveal the identity of the substance. Only the amino compound will be described in this section, as the amido compound is no longer relevant after exclusion from the formulation.
The data on the toxicity and ecotoxicity of the amino compound is found in several MSDS's found by use of the Internet, and databases like TOXNET, /47/, and RTECS. However, toxicity and ecotoxicity information about the specific substance is limited.
A couple of MSDS's found by use of the Internet classify the pure organic amino compound in different ways, either as Xn R20/21, Xn R10-20/21-36, Xn R10-20/21-36/37/38-40 or as Xn R10-20/21-36/37/38-40-65 [5].
The organic amino compound is not on the list of hazardous substances, /24/. However, the amino compound is adopted on the advisory list for self-classification of dangerous substances, that the Danish EPA has prepared on the basis of predictions by computer models (QSAR models - Quantitative Structure-Activity Relationship). On this list of self-classification the organic amino compound is given the recommended classification Xn R22 (Harmful if swallowed), /27/.
The MSDS's denote an oral LD50 value in rats of about 2200 mg/kg, indicating that the amino compound is slightly toxic to rats. RTECS (The Registry of Toxic Effects of Chemical Substances) lists that the lowest published lethal dose by oral intake for rats is about 1600 mg/kg.
Use of QSAR models indicates that the amino compound have moderate skin penetrability, and that the vapour pressure of the amino compound may cause concern, /26/.
Two of the MSDS's indicate that the amino compound may be a possible sensitiser. An early study has shown an allergic skin reaction in guinea pigs following repeated skin application, but in a more recent study no skin allergy was observed. Use of QSAR models confirms that the amino compound may cause skin allergic reactions, /26/.
One of the MSDS's states that the amino compound may possibly be a mutagen. Information about mutagenicity studies found on TOXNET, /47/, and in RTECS for the substance shows a positive test for gene mutation and DNA effects. However, a number of other mutation tests are negative or show no conclusion.
According to one of the MSDS's there is limited evidence of carcinogenic effects of the amino compound. The National Toxicology Program, /44/, has nominated the substance for toxicological evaluation for carcinogenic effects, because of lack of carcinogenicity data. Use of QSAR models shows a possible indication of carcinogenic effect in female rats, /26/. However, no information about the carcinogenic effect is available at present.
Studies on reproductive effects are negative. No birth defects were observed in rats following oral exposure and in mice after inhalation during pregnancy, even at dosages, which produced adverse effects on the mothers.
Only very sparse information is available on the environmental toxicity of the amino compound and the results regarding biodegradability are inconclusive.
With regard to aquatic toxicity LC50 values of 130 and 150 mg/l (for guppies) are found for the substance, i.e. a fairly low aquatic toxicity, resulting in no environmental classification. Use of QSAR models does not indicate that the amino compound is toxic to aquatic organisms, /26/.
9.2.4 Vitamin E
Vitamin E (CAS-no. 59-02-9) is a yellow viscous fat-soluble oil that exists in eight different forms. Alpha-tocopherol, which is the form used in this project as alternative anti-skinning agent, is the most active form of vitamin E in humans, and is a POWerful biological antioxidant.
Vitamin E is an essential vitamin, which we daily get via food (e.g. nuts and green leafy vegetables). Vitamin E is vital for protecting the nerve and muscle cell function. Vitamin E deficiency is rare in humans, but can cause symptoms like dry skin, eczema, decreased clotting time and easy bruising, /48/.
Vitamin E is usually non-toxic. Daily intake of vitamin E supplements (e.g. 200 to 600 mg vitamin E) is considered to be safe and unlikely to cause adverse side effects. However, intakes of large doses have been known to cause effects like nausea, diarrhea, fatigue, headache, rash and abdominal pain, but is rare, /29/48/.
A Canadian experiment with mice showed that vitamin E might reduce the average mutation frequency in tumour cells by up to 84%. The experiment suggests that vitamin E acts to protect cells against the effects of free radicals (potentially damaging by-product of metabolism). Free radicals can cause cell damage that may lead to the development of cancer. In summary the results suggest that vitamin E may exert antimutagenic/anticancer properties, /49/.
Other studies show that vitamin E protects against prostate, stomach and colon cancer, /50/51/, reduce skin tumour incidence, /52/, and that Vitamin E may protect the liver and the rest of the body against environmental pollutants such as ozone and other constituents of smog, /48/.
A few animal experiments on reproduction and teratogenicity have been carried out, and vitamin E shows no effect, /29/52/.
An experiment with mice indicates that the skin sensitivity commonly associated with UVB induced sunburn ise significantly reduced by topical application of tocopherol acetate, even after the exposure has occurred. Vitamin E has therefore been prescribed for therapeutic use on inflammatory skin disorders. In contrast, topical application of vitamin E on human skin has caused inflammation of the surface of the skin (contact dermatitis), and allergic reactions to creams containing vitamin E have been seen in patch tests, /29/. Furthermore, adults have developed skin rashes when given a high dose of Vitamin E (2 to 3 g/day) over a period. However, the reported effects of vitamin E on human skin are very rare, /29/.
No information on environmental fate and toxicity was found for vitamin E.
9.2.5 Evaluation of the anti-skinning agents/antioxidants
The anti-skinning agents included in the environmental and health assessment are the existing substances methyl ethyl ketoxime and hydroquinone and the alternatives vitamin E and a confidential organic amino compound. The evaluation has been carried out for the specific substances as the confidential identity of the organic amino compound was revealed to the project group. However, specific information was sparse.
The existing anti-skinning agents (methyl ethyl ketoxime and hydroquinone) are both hazardous substances by ingestion and harmful by inhalation. Both substances are severe eye and skin irritants and may produce allergic effects. They should both be labelled carcinogenic cat.3, i.e. limited evidence of carcinogenic effects. Hydroquinone is furthermore a suspected mutagen, whereas mutagenic effects have been observed for methyl ethyl ketoxime.
The health profile of air-drying products will not necessarily be improved if the existing agents are substituted with the organic amino compound. The organic amino compound is less toxic (harmful by ingestion and inhalation) and less irritating than the existing anti-skinning agents, and studies on skin allergy are mixed (positive and negative results). However, some of the MSDS's found for the amino compound, classify the amino compound as "limited evidence for carcinogenic effect", and information available from RTECS suggests that the organic amino compound may be genotoxic.
The health profile will be improved if the existing anti-skinning agents are substituted with vitamin E. Vitamin E is basically non-toxic, has shown both anti-mutagenic and anti-carcinogenic effects, and has been used for therapeutic use on inflammatory skin disorders – even though allergic reactions have been seen.
With regard to ecotoxicity hydroquinone is a problem. Hydroquinone is very toxic to aquatic organisms, whereas methyl ethyl ketoxime is not. Of the alternatives, the organic amino compound has a better environmental profile than hydroquinone. The amino compound has a low aquatic toxicity. No information about the ecotoxicity of vitamin E was found.
9.3 Petroleum distillates
The petroleum distillates are present in most of the drier products and the anti-skinning agents in considerable amounts. The petroleum distillates used are different types of white spirit, se Table 9.1.
According to the "European list of dangerous substances", /24/, most of the petroleum distillates are classified as carc2; R45 Xn (Harmful: may cause cancer); R65 (Harmful: may cause lung damage if swallowed).
Table 9.1
The petroleum distillates used in the drying and anti-skin alternatives
| Name | CAS-no. | Used in | Classification1 |
| Naphta (petroleum) – hydrated heavy | 64742-48-9 | Several drier products and one anti-skinning agent | Carc 2, R45 Xn 65 |
| White spirit (Stoddard solvent) | 8052-41-3 | A few drier products | Carc 2, R45 Xn R48/20-65 |
| Solvent naphta (petroleum) medium heavy aliphatic hydrocarbon | 64742-88-7 | One drier product | Xn R48/20-65 |
| Naphta (petroleum) – hydrodesulfurized heavy | 64742-82-1 | One anti-skinning agent | Carc 2, R45, Xn R65 |
1 Classification according to the list of dangerous substances, /24/.
Xn Harmful.
R45 May cause cancer.
R48/20 Harmful: danger of serious damage to health by prolonged exposure through inhalation.
R65 Harmful: may cause lung damage if swallowed.
The primary route of exposure to white spirit is by inhalation of vapours. White spirit vapour is readily absorbed by inhalation and distributed from the blood to other tissues and fat in the human body. Sparse information on the elimination of white spirit exists, but the elimination rate seems to be slow.
In general, white spirit has a low acute toxicity by inhalation, ingestion and by absorption through skin. Studies show that the central nervous system (CNS), respiratory system, liver and kidney generally are the targets for white spirit toxicity.
Acute CNS symptoms such as headache, "drunkenness", dizziness and fatigue have been reported in several cases of occupational exposure. The effects of long-term exposure to white spirit are shown by painter's syndrome. Memory impairment, fatigue, irritability, dizziness, impaired concentration, headache, anxiety and apathy have been the long-term effect of white spirit.
White spirit is a slight to moderate skin irritant, and eye irritation has been reported in connection with acute exposure to white spirit, /53/.
IARC (International Agency for Research on Cancer) evaluates residual (heavy) fuel oil as "possibly carcinogenic to humans (group 2B)" because there is found sufficient evidence for the carcinogenicity in experimental animals of residual (heavy) fuel oils, /54/. For painters, evidence has been found of increased cancer risks, particular in the lung and bladder, /53/.
No conclusive findings exist on the reproductive effects in humans for white spirit. Still, studies suggest that parental exposure to solvents may have an unwanted effect on the offspring. However, there is no adequately reported information directly related to white spirit, /53/.
When released in the environment, the lower molecule weight alkanes and aromatics of the white spirit will evaporate and undergo photodegradation in the atmosphere. The higher molecule weight alkanes tends to be sorbed to organic matter in soil and water, /53/.
Biodegradation of white spirit is expected to be fairly quick (90% reduction in soil concentration over a four month period), /53/.
Only few studies on aquatic toxicity of white spirit are available. These findings indicate that white spirit is moderately toxic to aquatic organisms (LC50 (96-h) values range from 0.5 to 5 mg/litre), /53/. However, because of the volatility and the low bioavailability of its constituents following sorption to soil/sediment, white spirit, although it is moderately toxic to aquatic organisms, is unlikely to present significant hazards to the environment.
9.3.1 Evaluation of petroleum distillates
The petroleum distillates are used in both driers and anti-skinning agents in considerable amounts. The petroleum distillates have, as described above, several adverse effects on human health – the worst being possibly carcinogenic, and are moderately toxic to aquatic organisms.
To improve the overall profile of the driers and anti-skinning agents, it is therefore necessary to use other organic solvents, with a better health profile, for dissolving the driers and the anti-skinning agents, or at least to use as low a content of the petroleum distillates as possible.
10 Discussion
The number of alternatives to cobalt driers is quite limited at this stage.
No non-metallic compounds, which are capable of substituting cobalt driers, have been identified so far. As it is essential that the alternatives possess some catalytic effect it narrows the substitution possibilities to the primary driers. Of these only vanadium and manganese possess enough catalytic effect at ambient conditions to be considered as alternatives to Co driers.
As drier products can vary due to differences in the used metallic salts, solvent and drying accelerator, eleven drier products have been investigated during the project, even though only two types of metals are in question as alternative. Of these are eight manganese driers and three are vanadium driers.
Between four and six alternative driers were tested in each product. In most cases they needed to be combined with one to two secondary driers. As the concentration of the driers also is important it can lead to a relatively high number of drier systems that need to be tested before a well functioning drier system is found. As 17 products were included in the testing of the alternatives it has been impossible to work in depth with every single product, optimising it with regard to drying time.
The number of alternatives to methyl ethyl ketoxime and hydroquinone is also quite limited. Two amino/amido based anti-skinning agents have been investigated along with two phenolic based products and acetone oxime. Vitamin E has also been included in the testing primarily for use in the printing inks.
Several of the included alternatives, this accounts for both driers and anti-skinning agents, were either trial products or even still laboratory products at the project start, for which reason there was a risk of the products being changed during the evaluation period. This turned out to be the case for a few products).
10.1 Overall evaluation
The conclusion and comments made in this project can only account for the specific drier combinations and products used in the testing carried out in this project. However, some general guidelines can though be obtained and the achieved results can give some indication to the paint and ink manufacturers on whether it at present is worthwhile substituting Co driers in their air-drying products. Before substituting, the manufacturers need to verify the results obtained during this project as well as perform any necessary complementary tests. Further optimising of the alternative drier systems need also to be performed by the manufacturers.
The overall impression from the results of this project is that manganese driers are the best suggestion as alternative to the Co driers. Mn driers can be used as alternatives to Co driers in some products, but its usability depends on the specific Mn drier as well as the product (binder type etc.). Vanadium driers cannot generally speaking at this stage be regarded as suitable Co alternatives. Earlier studies have also concluded that V driers are not proper alternatives to Co driers, /14/.
The health and environmental profile will be less negative, if cobalt driers are substituted with manganese driers even though the manganese compounds also have shown adverse health effects and are harmful to aquatic organisms. However, with the information available at the moment the health and environmental profile of manganese compounds is better than the profile for cobalt compounds.
The history of occupational manganese exposure indicates that the adverse health effects primarily are associated with intense exposure for a long period of time. In the production and use phase of paint, the exposure to the drier product will be at a minimum, and therefore a substitution of cobalt driers with manganese driers must be considered as a step in the right direction regarding the health profile of the air-drying products.
In this evaluation it is necessary to remember that the content of drier metal in air-drying coatings generally lies within the range of 0.03 to 0.05 weight-% of the total amount of coating. The human exposure to the drier metals is therefore practically insignificant during the application phase of the coatings. The exposure is potentially more concentrated and more significant in the production of the driers and in the production of coatings.
To improve the environment and health profile as much as possible, alternative driers with the best profile of organic solvents and drying accelerators should be used if technical possible. This means that driers dissolved in solvents like petroleum distillates and 2-ethylhexanoic acid should be avoided, and that driers containing 2,2-bipyridyl as drying accelerator should be preferred to those containing 1,10-phenathroline (at present classification). As petroleum distillates today still are present in almost every drier product, both the primary and the secondary, it is impossible to avoid them completely, but products with a low content of petroleum distillates should of course be preferred.
From a technical point of view the success in substituting methyl ethyl ketoxime seems rather limited. However, the alternatives have been tested in one concentration only in each air-drying paint product and a simple optimization on the concentration might lead to more positive results. The most promising alternative is acetone oxime, which has a dubious health profile. For the amino/amido compounds, which have a reasonable anti-skinning effect in closed containers, there was a strong indication that they may influence more on the drying time than methyl ethyl ketoxime, but it has not been verified. Vitamin E needs to be investigated further in air-drying paints before it can be concluded whether it is a potential alternative to methyl ethyl ketoxime.
In the case of printing inks a certain influence on the drying is actually necessary to obtain duct stability of the ink, and therefore the most volatile alternative, e.g. acetone oxime, cannot be used. Both vitamin E and the amino/amido compounds gave promising results, but in the tested concentration they are not as strong anti-oxidants as hydroquinone, as much higher concentrations are needed for the alternative products than for the hydroquinone product to induce the wanted delay of the drying of the printing inks.
QSAR studies indicate that benefit of substituting methyl ethyl ketoxime and hydroquinone with amino/amido compounds might be limited due to that they may have a genotoxic effect. The best health profile is found for vitamin E, which is practically non-toxic.
As for the driers, the anti-skinning agents are used in small amounts in the coatings. Methyl ethyl ketoxime is for example used in 0.3 to 0.7 weight-% of the total amount in solvent-borne air-drying coatings. Hence, the exposure to anti-skinning agents during the application phase of the coatings is practically insignificant, even though the anti-skinning agent used is volatile. The exposure is potentially more concentrated and more significant in the production of the anti-skinning agents and in the production of the coatings, where the production, however, usually takes place in closed systems with a minimum of exposure.
To improve the environment and health profile as much as possible, alternative anti-skinning agents with the best profile of organic solvents should be used if technical possible. This means that anti-skinning agents with no or a low content of petroleum distillates should be preferred.
In conclusion it can be said that both alternative driers and anti-skinning agents with a better environmental and health profile than the existing alternatives are available.
10.2 Future prospects
In the future there will still be a need for using driers and anti-skinning agents within the coating industry and this especially accounts for the driers as they are used both in waterborne as well as solvent-borne systems. Alkyd binders will still be among the most used binders despite the environmental pressure, partly due to their cost-effectiveness and partly due to a continuous development of new alkyd binder types, which can be used in more environmentally friendly systems.
Therefore there will be a continuous need for developing proper alternatives to cobalt driers, which at this stage means to develop manganese and vanadium driers further. Manganese driers can at this point be used as alternatives in some products, but it would be an advantage if they could be improved even further. The impression is that drier manufacturers actually do a lot of research and development within this area, especially with regard to manganese driers.
A real break through, at least from an environmental and health point of view, would be if non-metallic alternatives could be identified. However, this might be a very distant prospect.
11 References
/1/ IRL (Information Research Limited); "A profile of the European paint industry"; 12th edition; June 1998.
/2/ Tuck N.; "Waterborne and Solvent Based Alkyds and their End User Application"; Surface Coating Technology – Volume VI; SITA Technology/Wiley 2000.
/3/ Oldring P & Hayward G; "Resisn for Surface Coatings"; Surface Coating Technology – Volume I; SITA Technology 1995.
/4/ Owen D.J.; "Printing Inks for Litography"; SITA Technology 1990.
/5/ Wicks Z. W.; "Film Formation"; Federation series on coatings technology, 1986.
/6/ Lambourne, R. "Paint and Surface Coatings"; Ellis Horwood Limited 1987.
/7/ Oldring P. & Hayward G.; " Resins for Surface Coatings"; Surface Coating Technology – Volume II; SITA Technology 1995.
/8/ Information from Elementis Specialities; "Dapro 5005 & 7007 – Why an alternative to cobalt driers?"; Received 2002.
/9/ DRI-RX 19 LC-ETM : The chelator/accelerator for driers for coatings and inks. TECH Solutions, OMG, Issue 04/98.
/10/ Wiskemann R.; "Development in Drier Technology for Air Drying Waterborne Coatings"; Färg och Lack Scandinavia, no. 5, p. 4-9; 2000.
/11/ Internet information from CONDEA Servo available at www.servo.nl/performance/coating/index.htm (18-06-2001)
/12/ Information from OMG available at www.omgi.com (25-05-2001).
/13/ Information from Borchers available at www.borcher-additive.de (18-06-2001).
/14/ Bielemans J.H.; "Progress in the Development of Cobalt-free Drier systems"; Macromol. Symp. 187, 811-821, 2002.
/15/ European Coatings Net: "Non-toxic strontiumbased drier" available at www.coatings.de (13-12-2001) .
/16/ Information available at www.soctec.ro/English/Products/mcarbox-prop.htm (20-02-2002).
/17/ Leach, R.H.; "The Printing Ink Manual"; Fourth edition; Van Nostrand Reinhold (International); 1988.
/18/ Todd, R.E.; "Printing Inks – Formulation principles, manufacture and quality control testing procedures"; Pira Printing Ink Guide Series, 1994.
/19/ Sasol Servo BV; "Recent Developments in Anti-skinning Agents for Air-drying Paints" Report 6.01E; 2003.
/20/ Eggers U.; "Vegetable oils provide new functionalities for decorative paints"; Proceedings from CTVO-net Workshop on Decorative Paints and Printing Inks, Bedfordshire, UK, June 1999.
/21/ Wenzel H, Hauschild M, Alting L (1997). Environmental assessment of products. Volume 1: Methodology, tools and case studies in product development. London: Chapman & Hall.
/22/ Information found on different Material Safety Data Sheets for the specific substances.
/23/ Cobalt and Cobalt Compounds. Volume 52, p. 363, IARC (International Agency for Research on Cancer), 1991.
/24/ List of dangerous substances. 3rd June 2002. Available at the Danish EPA. http://www.mst.dk
/25/ List of undesirable substances 2000. An advisory list of chemicals, the use of which should be reduced or stopped in the long term. Environmental Review No. 9 2000. Copenhagen: Danish Environmental Protection Agency.
/26/ QSAR models used on specific substances. Carried out by the Danish EPA, 2003.
/27/ Report on the Advisory list for self-classification of dangerous substances. Environmental Project No. 636, Miljøstyrelsen (Danish EPA), 2001.
/28/ Hazard assessment of cobalt(II) naphthenate. Brief evaluation for Ecolabelling Denmark. Allan Astrup Jensen, dk-TEKNIK ENERGY & ENVIRONMENT, February 2000.
/29/ Search results using Hazardous Substances Data Bank (HSDB) at http://toxnet.nlm.nih.gov/. Searches performed 2003.
/30/ Draft Toxicological Profile for Cobalt. U.S. Department of Health and Human Services. Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR), September 2001. Draft for Public Comment.
/31/ Cobalt exposure and cancer risk. Critical review in Toxicology Volume 20 Issue 6:427-437, Jensen AA, Tüchsen F, 1990.
/32/ Arbejdstilsynet (2002). At-vejledning C.0.1. Grænseværdier for stoffer og materialer, October 2002. Title in English: "Threshold limit values for substances and materials", The Danish Labour Inspectorate.
/33/ Search result using U.S. Environmental Protection Agency ECOTOX Database at http://www.epa.gov/ecotox/. Searches performed 2003.
/34/ Search results using the N-class database on Environmental Hazard Classification - found at http://www.kemi.se/nclass/default.asp. Nordic Council of Ministers in collaboration with European Chemicals Bureau. April 2002.
/35/ Manganese and its compounds. Consice International Chemical Assessment Document 12, World Health Organization (WHO), 1999.
/36/ Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR), September 2000.
/37/ Toxicity summary for manganese. Chemical Hazard Evaluation Group, Biomedical and Environmental Information Analysis Section, Health Sciences Research Division, Oak Ridge National Laboratory, Francis, AA et al, July 1995.
/38/ A series of articles in the Danish magazine "Arbejdsmiljø", No. 2, 3, 8 and 12, 2002. No. 2, 2002: Arbejdsmedicinere vil til bunds i mangan-problemer. No. 3, 2002: Frederiksværk betaler høj pris for manganskader. No. 8, 2002: Fortsat usikkerhed om mangan. No. 12, 2002: Jernladyen fra Stålvalseværket.
/39/ Grundstofferne i 2. geled – et miljøproblem nu eller i fremover ? Miljøprojekt nr. 700, 2002, Miljøstyrelsen. Title in English: The elements in the second rank – an environmental problem now or in the future?
/40/ Toxicity summary for Vanadium and Vanadium Compounds. Chemical Hazard Evaluation and Communication Group, Biomedical and Environmental Information Analysis Section, Health and Safety Research Division, Oak Ridge National Laboratory, Opresko, M.D., December 1991.
/41/ Toxicological profile for vanadium and compounds. Agency for Toxic Substances and Disease Registry, U.S. Public Health Service, July 1992.
/42/ Vanadium Pentaoxide and other Inorganic Vanadium Compounds. Concise International Chemical Assessment Document 29, World Health Organization (WHO), 2001.
/43/ NTP Technical Report on the Toxicology and Carcinogenesis studies of Vanadium Pentaoxide in F344/N Rats and B63F1 Mice. NTP TR 507, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, December 2002.
/44/ Search result at National Toxicology Program (NTP), National Institute of Environmental Health Sciences (NIEHS). http://ntp-server.niehs.nih.gov/.
/45/ Hydroquinone. International Programme on Chemical Safety (IPSC), Environmental health criteria 157, World Health Organization, 1994.
/46/ Hydroquinonoe. Volume 71, p. 691, IARC (International Agency for Research on Cancer), 1999.
/47/ Search result at Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?IRIS
/48/ Vitamin E, Integrative Mecial Arts Group, Inc., IBISmedical.com, 1998-2000. www.IBISmedical.com
/49/ Effect of Dietry Vitamin E on Spontaneous or Nitric Oxide Donor-Induced Mutations in a Mouse Tumor Model. Journal of the National Cancer Institute. Vol. 92, pp. 1429-33, Sandhu, J. et al, 2000.
/50/ Vitamin Supplements and Cancer Risk: The Epidemiologic Evidence. Cancer Causes and Control. Vol. 8, pp. 786-802, Patterson, R. et al, 1997.
/51/ Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Trial, National Cancer Institute, 22 July 2003. Found as article 03377 at www.charitywire.com.
/52/ Alpha-tocopherol, WHO Food Additive Series 21. International Programme on Chemical Safety (IPCS). Found at http://www.inchem.org/pages/search.html.
/53/ White Spirit (Stoddard Solvent). International Programme on Chemical Safety (IPCS), Environmental Health Criteria 187, World Health Organization, 1996.
/54/ Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels, Volume 45, IARC (International Agency for Research on Cancer), 1989.
1 Technical evaluation example
The example shown is the outcome of running one product, DIY-3, through the technical evaluation procedure described in section 7.1 and shown in figure 7.1. All the obtained data during the technical evaluation are shown in the next sections.
The substitution of the cobalt drier system in DIY-P3 was less difficult than for most of the other air-drying do-it-yourself products, for which reason the technical evaluation in the many other cases has been even more data intensive than shown in this example. 6 different alternative driers have been tested in DIY-3 and the obtained results are shown in the following sections.
1.1 Product description
DIY-3 is a waterborne stain based on tall oil alkyd with an original drier system consisting of Cobalt, barium and zirconium (a Co/Ba/Zr system). As DIY-P3 is waterborne it contains no methyl ethyl ketoxime. DIY-P3 belongs to the group of do-it-yourself products.
1.2 Alternative drier systems
19 samples of DIY-P3 with alternative drier systems were investigated. The drier systems are presented in table A.1.1. Some of the drier systems are more or less identical, due to the system has been tested once or twice in the initial stage and maybe once in the further testing.
Table A.1.1
Drier systems investigated in do-it-yourself product, DIY-P3. The concentrations are given as metal on solid
Air-drying binder, except For Mn1 and Mn5(w) where the concentrations are given as drier product on solid
air-drying binder.
| Drier (% w/w) | |||||||
| Sample | Alternative Code |
Alternative | Ca | Ba | Zr | K | Drying accelerator |
| DIY-P3-1 | Mn1 | 1,14 | 0,57 | 0,24 | |||
| DIY-P3-8 | Mn1 | 1,05 | 0,55 | 0,25 | |||
| DIY-P3-13 | Mn1 | 1,08 | 0,58 | 0,24 | |||
| DIY-P3-9 | Mn4 | 0,05 | |||||
| DIY-P3-10 | Mn4 | 0,09 | |||||
| DIY-P3-2 | Mn5(w) | 5,07 | |||||
| DIY-P3-6 | Mn5(w) | 3,07 | |||||
| DIY-P3-7 | Mn5(w) | 4,17 | |||||
| DIY-P3-3 | Mn6(w) | 0,21 | 0,22 | 0,21 | 0,51 | ||
| DIY-P3-4 | Mn6(w) | 0,44 | 0,22 | 0,25 | 0,44 | ||
| DIY-P3-5 | Mn6(w) | 0,09 | 0,22 | 0,41 | 0,47 | ||
| DIY-P3-14 | V3(w) | 0,07 | 0,36 | ||||
| DIY-P3-15 | V3(w) | 0,05 | 0,36 | ||||
| DIY-P3-16 | V3(w) | 0,07 | 0,32 | ||||
| DIY-P3-17 | V3(w) | 0,05 | 0,32 | ||||
| DIY-P3-18 | V3(w) | 0,06 | 0,55 | ||||
| DIY-P3-19 | V3(w) | 0,09 | 0,56 | ||||
| DIY-P3-20 | V3(w) | 0,08 | 0,36 | ||||
| DIY-P3-21 | V2 | 0,06 | 0,56 | ||||
1.3 Initial drying time test
Drying times of the samples present in table A.1.1 were investigated by means of a straight-line drying time recorder. Due to the large number of drying time tests performed within the experimental work only a few determinations have been made. The drying profiles of the samples are described through four different drying stages, which are described in 7.1.3.1. The drying profile of the reference product DIY-P3 has also been investigated for comparison.
The drying time profiles of an aged alternative sample, DIY-P3-13 aged and the aged reference are included in the table as well.
Table A.1.2. Drying times at different stages obtained on a straight-line drying time recorder. For the alternative systems and for the aged reference the results are single determination. For the reference product the results are an average of 8 determinations.
| Drying time at different stages (h) | |||||
| Sample | Alternative | Set-to- touch |
Tack- free |
Dry- hard |
Dry- through |
| Reference | 0,3 | 2,1 | 10,2 | > 20 | |
| Reference aged | 0,2 | 1,3 | 17,7 | > 20 | |
| DIY-P3-1 | Mn1 | 0,3 | - | 5,3 | > 20 |
| DIY-P3-8 | Mn1 | 0,2 | 2,8 | 5,8 | > 20 |
| DIY-P3-13 | Mn1 | 0,3 | 0,7 | 5,5 | 8,8 |
| DIY-P3-13 aged | Mn1 | 0,2 | 1,3 | 14,3 | 15,2 |
| DIY-P3-9 | Mn4 | 0,3 | ? | ? | > 20 |
| DIY-P3-10 | Mn4 | 0,3 | - | 18,5 | > 20 |
| DIY-P3-2 | Mn5(w) | 0,3 | - | 17,3 | > 20 |
| DIY-P3-6 | Mn5(w) | 0,2 | 2,6 | 13,5 | > 20 |
| DIY-P3-7 | Mn5(w) | 0,2 | 2,6 | (10,1) | > 20 |
| DIY-P3-3 | Mn6(w) | 0,3 | - | 10,7 | > 20 |
| DIY-P3-4 | Mn6(w) | 0,3 | - | 17,7 | > 20 |
| DIY-P3-5 | Mn6(w) | 0,2 | 2,3 | (12,9) | > 20 |
| DIY-P3-14 | V3(w) | 0,2 | 2,8 | 18,7 | > 20 |
| DIY-P3-15 | V3(w) | 0,2 | 2,8 | (18,8) | > 20 |
| DIY-P3-16 | V3(w) | 0,3 | 2,8 | 19,2 | > 20 |
| DIY-P3-17 | V3(w) | 0,3 | 2,8 | 19,2 | > 20 |
| DIY-P3-18 | V3(w) | 0,2 | 2,2 | ? | ? |
| DIY-P3-19 | V3(w) | 0,3 | (6,6) | > 20 | > 20 |
| DIY-P3-20 | V3(w) | 0,3 | 3,5 | (16,2) | > 20 |
| DIY-P3-21 | V2 | 0,2 | 1,7 | ? | ? |
1.4 Further testing
As DIY-P3 is waterborne no anti-skinning agent is present in the product. Investigation of the most promising drier systems with an anti-skinning agent is therefore omitted in this case. The complementary tests and stability tests were made on a system chosen directly from the initial drying time test.
1.4.1 Stability test/ageing
Sample DIY-P3-13 was selected for the stability test, as it had the most promising drying time profile in the initial testing (the composition of DIY-P3-13 is identical with DIY-P3-1 and DIY-P3-8).
Both sample DIY-P3-13 and the reference product were stored at 40 ºC for 2 weeks. Both samples had changed slightly to a more "creamy" consistency. DIY-P3-13 had a slight reddish discolouration after storage.
1.4.2 Viscosity
The viscosity has been measured as described in section 7.1.3.4. The viscosity measured at different shear rates at 23ºC can be seen in figure A.1 and A.2. Figure A.1 compares the viscosity of the DIY-P3 reference and DIY-P3-13 before the samples are stored at elevated temperature for 2 weeks. Figure A.2 compares the viscosity of the samples after they have been aged at elevated temperature. The results on which the viscosity curves are based are given in table A.1.3.
Table A.1.3
Measured viscosities of DIY-P3 samples at different sheer rates at 23ºC.
| Viscosity (mPa s) | |||||
| 0,09 s -1 | 0,023 s-1 | 0,23 s-1</td> | 0,023 s-1 | 0,09 s-1 | |
| DIY-P3-REF | 373500 | 248500 | 54550 | 225500 | 364000 |
| DIY-P3-13 | 376000 | 290500 | 77950 | 314500 | 460500 |
| DIY-P3-REF (aged) | 376000 | 264000 | 54900 | 260000 | 459000 |
| PIY-P13-13 (aged) | 482500 | 310500 | 65100 | 355500 | 573500 |
DIY-P3-13 has a slightly higher viscosity than the reference product. The viscosity increases during storage for both DIY-P3-13 and the reference product. The increase is slightly more noticeable for DIY-P3-13.
Figure a.1.1
Viscosity of DIY-P3-13 and The reference product, DIY-P3-REF measured at different shear rates at 23ºC. The
viscosity is measured before the samples have been aged.
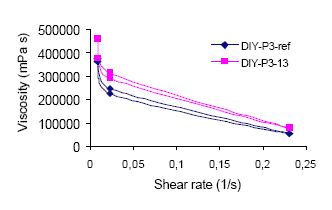
Figure a.1.2
Viscosity of DIY-P3-13 and The reference product, DIY-P3-REF measured at different shear rates at 23ºC. The
viscosity is measured after the samples have been aged.
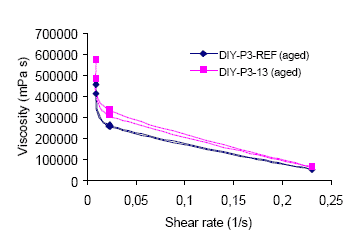
1.4.3 Pendulum hardness
The hardness of the dry coating films was investigated by measuring the pendulum hardness of the films as described in section 7.1.3.2.
The film hardness was determined for aged samples of DIY-P3 and the reference product of DIY-P3. A non-aged sample of the reference was also included for comparison. The obtained film hardness is presented in table A.1.4. The results are also shown in figure A.1.3.
table a.1.4
pendulum hardness after different drying periods For DIY-P3 samples that have been aged. A non-aged reference
sample was also included. The results are averages of three measurements.
| Drying time (h) | Number of swings | ||
| DIY-P3-REF | DIY-P3-REF (aged) | DIY-P3-13 (aged) | |
| 20 | 7 | 5 | 5 |
| 44 | 9 | 8 | 8 |
| 117 | 9 | 10 | 9 |
| 312 | 11 | 11 | 11 |
| 455 | 13 | 13 | 14 |
| 624 | 13 | 13 | 13 |
The film hardness of DIY-P3-13 is comparable to the reference. Both the aged reference and the aged sample of DIY-P3-13 have a slightly softer film than the non-aged reference at the very start, but otherwise the hardness is more or less identical for the three samples.
Figure a.1.3
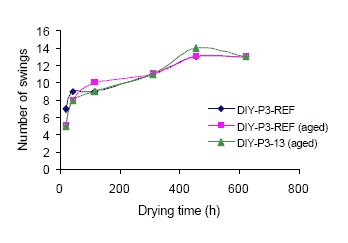
The film of DIY-P3 is relatively soft, as low numbers of pendulum swing have been observed. This accounts both for the reference as well as the sample with the alternative drier system.
1.4.4 Gloss
The aged sample DIY-P3-13 has about the same gloss as the aged reference at 60ºC. The value being 47 compared to 53. The values are measured at 60º and are averages of three measurements.
1.4.5 Yellowing
DIY-P3-13 is comparable to the reference with regard to yellowing in dark places, as both samples got the ranking 2 – a small difference in colour is visible from more than one angle.
1.4.6 Drying time
DIY-P3-13 (aged) has a better drying profile than the aged reference product. See table A.1.2 where the results have been included. The alternative and the reference have comparable set-to-touch and tack–free times, whereas the alternative, DIY-P3-13 has a faster dry-hard and dry-through.
It should though be born in mind that the reference already had been stored for more than a year before it was aged at elevated temperature together with the alternative. It could therefore be argued that the aged alternative rather should be compared to the non-aged reference, but even so the DIY-P3-13 has comparable drying profile with the reference. It takes longer before the aged alternative reaches the dry-hard condition. However, in return it reaches the tack-free and dry-through conditions faster.
2 Performed test for all products
An overview of all the tests performed within this project during testing and evaluation of alternative driers and anti-skinning agent is given below. The tests have been performed in accordance with the technical evaluation procedures described in chapter 7.
2.1 Do-it-yourself products
Table A.2.1 describes the number of tests performed in connection with the investigation of alternatives to cobalt driers and methyl ethyl ketoxime in the do-it-yourself products.
Table A.2.1
Test performed in connection with substitution of Co driers and methyl ethyl ketoxime in do-it-yourself (DIY)
products.
| Driers | Number of tests performed | ||||||||
| Product | DIY-P1 | DIY-P2 | DIY-P3 | DIY-P4 | DIY-P5 | DIY-P6 | DIY-P7 | DIY-P8 | Total |
| Sample preparation | 22 | 27 | 19 | 36 | 23 | 40 | 16 | 39 | 222 |
| Drying time | 44 | 51 | 29 | 63 | 39 | 45 | 27 | 54 | 352 |
| Pendulum Hardness | 29 | 106 | 42 | 25 | 72 | 20 | 30 | 24 | 348 |
| Gloss | 5 | 7 | 5 | 7 | 9 | 4 | 6 | 6 | 49 |
| Viscosity | 4 | 6 | 4 | 6 | 8 | 0 | 4 | 6 | 38 |
| Stability | 2 | 3 | 2 | 3 | 3 | 0 | 2 | 3 | 18 |
| Yellowing | 2 | 3 | 2 | 3 | 7 | 0 | 2 | 2 | 21 |
| Anti-skinning agents | Number of tests performed | ||||||||
| Sample preparation | 7 | 7 | 0 | 8 | 7 | 0 | 7 | 0 | 36 |
| Skinning test | 14 | 14 | 0 | 8 | 14 | 0 | 14 | 0 | 64 |
A high number of sample preparations and drying time tests is in general an indication of that the product has been particularly difficult to Co substitute. This especially accounts DIY-P1, DIY-P2, DIY-P4, DIY-P6 and DIY-P8. No potential alternative drier system was identified in the case of DIY-P6. Alternative drier systems with some potential to substitute cobalt driers were identified in case of DIY-P1, DIY-P2, DIY-P4 and DIY-P8, but in most cases, except DIY-P1, a relatively high loss-of-dry was experienced after storage of the sample at elevated temperature, as the drying times were increased considerably.
In the case of DIY-P1 and DIY-P7 substitution was possible with some success and for DIY-P3 and DIY-P5 the substitutions came out quite successfully.
The most promising drier system for each solvent-borne product was chosen for use in the samples when performing the skinning tests. The tests have been performed for one concentration only of each alternative anti-skinning agent in each product. As DIY-P8 had an extent loss-of-dry after the ageing test, this product was not included in the test of anti-skinning agent. Due to a limited amount of sample DIY-P4 was only tested with regard to skinning in closed containers.
2.2 Industrial products
As the group of do-it-yourself products was tested and investigated before the group of industrial products, some of the experiences could be used within this product group, and therefore fewer drier combinations and fewer tests in general were needed for the industrial products. Table A.2.2 describes the number of tests performed in connection with the investigation of alternatives to cobalt driers and methyl ethyl ketoxime in industrial air-drying products. IND-P9 is already a cobalt-free product and has only been included for testing the alternative anti-skinning agents.
Table A.2.2
Tests performed in connection with substitution of Co driers and methyl ethyl ketoxime in industrial products.
| Driers | Number of tests performed | |||||
| Product | IND-P9 | IND-P10 | IND-P11 | IND-P12 | IND-P13 | Total |
| Sample preparation | - | 28 | 22 | 15 | 16 | 81 |
| Drying time | - | 40 | 34 | 28 | 26 | 128 |
| Pendulum Hardness | - | 129 | 54 | 104 | 79 | 366 |
| Gloss | - | 5 | 9 | 9 | 5 | 28 |
| Viscosity | - | 6 | 8 | 8 | 8 | 30 |
| Stability | - | 3 | 4 | 4 | 4 | 15 |
| Yellowing | - | 3 | 4 | 4 | 4 | 15 |
| Water resistance | - | 0 | 4 | 5 | 5 | 14 |
| Anti-skinning agents | Number of tests performed | |||||
| Sample preparation | 10 | 7 | 7 | 7 | 0 | 31 |
| Skinning test | 20 | 14 | 7 | 14 | 0 | 55 |
As it can be seen from the number of sample preparations of drier systems and drying time tests, IND-P10 and IND-P11 were more difficult to Co substitute than IND-P12 and IND-P13. Potential alternative drier systems were identified for all four products, but in the case of IND-P10 a too high extent of loss-of-dry was observed even though the drying times were quite low, as IND-P10 needs to be a very fast drying industrial coating. Even though the film hardness of the alternative systems with time becomes comparable with those of the reference product, the initial film hardness was too low.
In the case of IND-P11 the film hardness of the samples with alternative drier systems were far too low. This problem might be overcome by adding Zn as an additional drier. Loss-of-dry after storage at elevated temperature was also seen.
In IND-P12 and IND-P13 the cobalt driers were substituted with much higher success having comparable drying time with the reference products even after the samples had been stored at elevated temperature. In the case of IND-P12 three different manganese driers can be used as Co substitute all of them giving higher film hardness and gloss, comparable or improved yellowing properties and comparable water resistance with reference product. Only in one case, inferior water resistance was observed.
Also three driers could with success be used for substitution of Co in IND-P13, but in this case, the other film properties as film hardness, gloss and water resistance were slightly inferior to the reference in most cases. All three alternative systems were comparable to the reference with regard to yellowing.
The most promising drier system for each solvent-borne product was chosen to be used in the samples when performing the skinning tests. The tests have been performed for one concentration only of each alternative anti-skinning agent in each product. IND-P9 was supplied by the manufacturer with its original drier system.
2.3 Printing inks
The product group of printing inks differs quite a lot from the other investigated air-drying products. They are typically used in a very thin film. Therefore they, also without hydroquinone, have relatively long drying times when tested on a drying time recorder, even though they are applied in a much thinner film than the paint products.
Table A.2.3 describes the number of tests performed in connection with the investigation of alternatives to cobalt driers and hydroquinone in sheet-fed printing inks.
Table a.2.3
Tests performed in connection with substitution of Co driers and hydroquinone in Sheet-fed printing inks.
| Driers | Number of tests performed | ||||
| Product | INK-P14 | INK-P15 | INK-P16 | INK-P17 | Total |
| Sample preparation | 24 | 39 | 8 | 8 | 79 |
| Drying time | 31 | 49 | 10 | 10 | 100 |
| Set off | 2 | 2 | 2 | 2 | 8 |
| Anti-skinning agents | Number of tests performed | ||||
| Auto-oxidation | 0 | 0 | 13 | 10 | 23 |
| Duct stability | 10 | 10 | 10 | 10 | 40 |
The number of tested systems in INK-P16 and INK-P17 is much lower than in INK-P14 and INK-P15 as only the two most promising alternatives, Mn1 and Mn2 from the testing of INK-P14 and INK-P15 were investigated. Mn4 has only been tested in INK-P15 due to late receipt of this drier, but it seems useful.
Only one drier system for each printing ink was chosen for the test of the set off effects. The set off effect of the inks containing the alternative driers was compared to those of the reference inks.
The auto-oxidation measurements and test of duct were performed on samples containing original cobalt based drier systems.
3 Comparison of drying times and film properties
The efficiency of the alternative driers has all been evaluated by measuring drying times of the samples containing the alternative drier systems and comparing them to the drying times of the respective reference products.
More than 350 drying time measurements have been performed during the project to identify the most promising cobalt substitute for each product included in the technical evaluation. The measurements are performed in accordance with the description in section 7.1.3.1.
On basis of the initial drying time tests between 0 and 5 alternative drier systems were chosen for further testing for each product. If no alternative drier systems were identified in the initial stage, as it e.g. is the case for DIY-P6, no further testing was performed on the product.
In table A.3.1 and A.3.2 the presented results have been obtained from testing the alternative driers in do-it-yourself products. The drying times of the respective reference product are presented as well for comparison. Only drying times for the most promising alternative driers are shown. The used drier combinations and drier concentrations are given as well. The systems having a cobalt drier are the reference products.
Table a.3.1
Drying time results before the product samples have been aged. The drying time profile is given as the drying times
at different stages. See description of stages in section 7.1.3.1.
| Drying time at different stages of the drying (h) | ||||||
| Product | Primary drier# |
Secondary driers |
Set-to- touch |
Tack- free |
Dry- hard |
Dry- through |
| DIY-P1 | Co | Ca Zr |
1,8 | 3,4 | 4,3 | 7,4 |
| Mn1 (1,84) | Ca (0,17) Zr (0,1) |
2,3 | 3,7 | 4,7 | 6,3 | |
| Mn4 (0,06) | None | 1,8 | 5,5 | 6,0 | 9,2 | |
| DIY-P2 | Co | Ca Zr |
0,7 | 2,9 | 14,1 | > 20 |
| Mn1 (1,10) | Ca (0,21) Zr (0,3) |
0,8 | 3,3 | 19,1 | 19,1 | |
| Mn2 (7,53) | Ca (0,3) | 0,8 | 11,7 | 14,2 | 14,2 | |
| Mn4 (0,06) | Ca (0,1) Zr (0,31) |
0,8 | 3,6 | 13,4 | > 20 | |
| DIY-P3 | Co | Ba Zr |
0,3 | 2,1 | 10,2 | > 20 |
| Mn1 (1,08) | Ba (0,58) Zr (0,24) |
0,3 | 0,7 | 5,5 | 8,8 | |
| Mn5(w) (3,07) | None | 0,3 | 2,6 | 13,3 | > 20 | |
| DIY-P4 | Co | Ca Zr |
0,5 | 7,8 | 10,7 | > 20 |
| Mn1 (2,07) | Ca (0,2) Zr (0,15) |
0,4 | 2,6 | 18,8 | 18,8 | |
| Mn4 (0,08) | Ca (0,05) | 0,4 | 5,3 | 14,2 | > 20 | |
| DIY-P5 | C0 | Ca Zr |
2,7 | 11,0 | 14,3 | - |
| Mn1 (2,99) | Ca (0,2) Zr (0,1) |
2,3 | 3,5 | 3,8 | 4,4 | |
| Mn2 (7,82) | Ca (0,3) | 2,4 | 7,7 | 10,3 | > 20 | |
| Mn3 (6,12) | Ca (0,33) | 2,2 | 11,2 | 12,3 | 13,2 | |
| Mn4 (0,11) | None | 2,8 | 4,3 | 7,7 | 7,7 | |
| DIY-p7 | C0 | Ca Zn |
1,2 | 3,3 | 4,3 | 5,5 |
| Mn1 (3,01) | Ca (0,16) Zr (0,05) |
1,5 | 6,7 | 7,8 | 9 | |
| DIY-P8 | Co | Ca Zn |
1,8 | 4,2 | 5,5 | 6,9 |
| Mn1 (1,65) | Ca (0,16) Zr (0,04) |
0,9 | 9,8 | 11,5 | 12,4 | |
| Mn4 (0,08) | None | 0,8 | 7,3 | 8,5 | 9,2 | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
The results in table A.3.1 are for samples before they have been aged at elevated temperature, whereas table A.3.2 shows the results obtained after the ageing. In all solvent-borne products an anti-skinning agent has been added in the same concentration as in the corresponding reference products.
Table a.3.2
Drying time results after the product samples have been aged. The drying time profile is given as the drying times at
different stages. See description of stages in section 7.1.3.1.
| Drying time at different stages of drying (h) | ||||||
| Product | Primary drier# |
Secondary driers |
Set-to- touch |
Tack- free |
Dry- hard |
Dry- through |
| DIY-P1 | Co | Ca Zr |
1,5 | 3,9 | 4,7 | 6,5 |
| Mn1 (1,84) | Ca (0,17) Zr (0,1) |
1,2 | 11,3 | 14,3 | 15,7 | |
| Mn4 (0,06) | None | - | - | - | - | |
| DIY-P2 | Co | Ca Zr |
0,5 | 4,8 | 17,8 | 17,8 |
| Mn1 (1,10) | Ca (0,21) Zr (0,3) |
0,4 | 9,7 | 17,8 | 17,8 | |
| Mn2 (7,53) | Ca (0,3) | 0,4 | 16,3 | > 20 | > 20 | |
| Mn4 (0,06) | Ca (0,1) Zr (0,31) |
- | - | - | - | |
| DIY-P3 | Co | Ba Zr |
0,3 | 1,3 | 17,7 | > 20 |
| Mn1 (1,08) | Ba (0,58) Zr (0,24) |
0,3 | 1,3 | 14,3 | 15,2 | |
| Mn5(w) (3,07) | None | - | - | - | - | |
| DIY-P4 | Co | Ca Zr |
0,6 | 12,0 | 15,0 | 19,0 |
| Mn1 (2,07) | Ca (0,2) Zr (0,15) |
0,5 | 9,0 | 14,3 | > 20 | |
| Mn4 (0,08) | Ca (0,05) | 0,6 | 7,5 | 16,8 | > 20 | |
| DIY-P5 | C0 | Ca Zr |
2,8 | > 20 | > 20 | > 20 |
| Mn1 (2,99) | Ca (0,2) Zr (0,1) |
2,3 | 18,3 | > 20 | > 20 | |
| Mn2 (7,82) | Ca (0,3) | 2,o | > 20 | > 20 | > 20 | |
| Mn3 (6,12) | Ca (0,33) | 2,0 | > 20 | > 20 | > 20 | |
| Mn4 (0,11) | None | 2,8 | 18,8 | > 20 | > 20 | |
| DIY-p7 | C0 | Ca Zn |
1,2 | 3,3 | 4,6 | 4,9 |
| Mn1 (3,01) | Ca (0,16) Zr (0,05) |
1,3 | 6,0 | 7,8 | 8,5 | |
| DIY-P8 | Co | Ca Zn |
4,0 | 6,5 | 7,9 | 9,2 |
| Mn1 (1,65) | Ca (0,16) Zr (0,04) |
1,5 | > 20 | > 20 | > 20 | |
| Mn4 (0,08) | None | - | - | - | - | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
In the same way selected drying times are obtained from testing of the alternative driers in the industrial product presented in table A.3.3 and A.3.4.
Table a.3.3
Drying time results before the product samples have been aged. The drying time profile is given as the drying times
at different stages. See description of stages in section 7.1.3.1.
| Drying time at different stages of drying (h) | ||||||
| Product | Primary drier# |
Secondary driers |
Set-to- touch |
Tack- free |
Dry- hard |
Dry- through |
| IND-P10 | Co | Mn Ca Zr |
0,4 | 1,4 | 2,0 | 2,6 |
| Mn1 (2,67 ) | Ca (0,08) Zr (0,1) |
0,4 | 2,1 | 2,5 | > 5 | |
| Mn2 (4,44) | Ca (0,06) | 0,4 | 2,3 | 3,5 | (3.8) | |
| Mn4 (0,06 ) | None | 0,3 | 1,5 | 2,0 | 2,2 | |
| IND-P11 | Co | Zn Zr |
0,3 | 3,7 | 5,2 | 11,2 |
| Mn1 ( 2,57) | Ca (0,1) Zr (0,19 |
0,3 | 3,7 | 5,5 | > 20 | |
| Mn4 ( 0,08) | Ca (0,1) Zr (0,2) |
0,3 | 3,1 | 5,4 | > 20 | |
| IND-P12 | Co | Ca Zr |
0,1 | 0,1 | 1,1 | > 5 |
| Mn1 (2,72) | Ca (0,12) Zr (0,14) |
- | 0,06 | 0,5 | > 1 | |
| Mn2 (3,56) | Ca (0,11) | - | 0,06 | 0,2 | > 5 | |
| Mn3 (3,08) | Ca (0,10) | - | 0,07 | 0,15 | > 5 | |
| Mn4 ( 0,05) | None | - | 0,07 | 0,2 | >5 | |
| IND-P13 | Co | phenanthroline | 0,09 | 0,12 | 0,36 | > 5 |
| Mn1 ( 1,71) | Ca (0,1 ) | - | 0,12 | 0,28 | > 5 | |
| Mn4 (0,08) | None | - | 0,1 | 0,23 | > 5 | |
| Mn5(w) (2,39) | None | - | 0,13 | 0,27 | > 5 | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
Table a.3.4
Drying time results after the product samples have been aged. The drying time profile is given as the drying times at
different stages. See description of stages in section 7.1.3.1.
| Drying time at different stages (h) | ||||||
| Product | Primary drier# |
Secondary driers |
Set-to- touch |
Tack- free |
Dry- hard |
Dry- through |
| IND-P10 | Co | Mn Ca Zr |
0,2 | 1,6 | 1,8 | > 5 |
| Mn1 (2,67 ) | Ca (0,08) Zr (0,1) |
0,2 | 4,1 | 4,7 | > 5 | |
| Mn2 (4,44) | Ca (0,06) | 0,2 | > 5 | > 5 | > 5 | |
| Mn4 (0,06 ) | None | - | - | - | - | |
| IND-P11 | Co | Zn Zr |
1,7 | 3,4 | 4,4 | 5,5 |
| Mn1 ( 2,57) | Ca (0,1) Zr (0,19 |
1,7 | 10,3 | 13,2 | 14,0 | |
| Mn4 ( 0,08) | Ca (0,1) Zr (0,2) |
1,7 | 7,0 | 8,3 | 13,0 | |
| IND-P12 | Co | Ca Zr |
0,08 | - | 1,6 | > 5 |
| Mn1 (2,72) | Ca (0,12) Zr (0,14) |
- | - | - | - | |
| Mn2 (3,56) | Ca (0,11) | - | - | 1,6 | > 5 | |
| Mn3 (3,08) | Ca (0,10) | 0,08 | - | 0,6 | > 5 | |
| Mn4 ( 0,05) | None | 0,08 | - | 1,7 | >5 | |
| IND-P13 | Co | phenanthroline | 0,13 | - | 0,35 | > 5 |
| Mn1 ( 1,71) | Ca (0,1 ) | 0,13 | - | 0,35 | > 5 | |
| Mn4 (0,08) | None | 0,10 | - | 0,30 | > 5 | |
| Mn5(w) (2,39) | None | 01,2 | - | 0,33 | > 5 | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
In table A.3.5 drying time results for printing inks are shown. Again only the most promising drier systems have been included in the table. The inks with the cobalt drier system are the references.
Table a.3.5
Drying time results obtained for products in the group of printing inks. The drying times of the reference product are
given as well. The drying time profile is given as the drying times at different stages. See description is section
7.1.3.1.
| Drying time at different stages of drying (h) | ||||||
| Product | Primary drier# | Secondary driers |
Set-to-touch | Tack-free | Dry-hard | Dry- through |
| INK-P14 | Co | Mn traditional | 28,3 | 31,3 | > 50 | > 50 |
| Mn1 (5,7) | Mn traditional (0,8) |
29,2 | 35,0 | > 50 | > 50 | |
| INK-P15 | Co | Mn traditional | 14,6 | 36,3 | 43,3 | 44,2 |
| Mn1 (7,1) | Mn traditional (0,5) |
22,1 | 36,7 | 41,7 | 41,7 | |
| Mn4 (0,07) | Mn traditional (0,53) | 23,3 | 25,4 | 31,3 | 31,3 | |
| INK-P16 | Co | Mn traditional | 16,3 | 26,7 | > 50 | > 50 |
| Mn1 (7,8) | Mn traditional (0,8) |
11,3 | 19,2 | > 50 | > 50 | |
| INK-P17 | Co | Mn traditional | 4,6 | 5,8 | 35,4 | 35,4 |
| Mn1 (6,8) | Mn traditional (0,8) |
3,1 | 4,2 | 8,6 | > 50 | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
The drying time tests were performed without any anti-skinning in the case of INK-P16 and INK-P17 both for the samples with alternative drier systems and for the corresponding reference. On basis of the obtained drying time one drier system for each printing ink was chosen for test of set-off effect. The results of testing the inks with regard to set-off effects can be seen in table A.3.6. The higher the measured density on the set-off paper the more set-off the ink has. In general the set-off effects of the alternatives are comparable with the reference.
Table a.3.6
Set-off effects of printing inks containing alternative drier systems, and of reference inks. Obtained by measuring the
density of the set-off on paper.
| Density | ||||||
| Product | Primary drier# |
Secondary driers |
30 s | 5 min | 10 min | 30 min |
| INK-P14 | Co | Mn traditional | 0,24 | 0,002 | 0,001 | |
| Mn1 (5,7) | Mn traditional (0,8) |
0,17 | 0,005 | 0,004 | ||
| INK-P15 | Co | Mn traditional | 0,13 | 0,01 | 0,01 | 0,004 |
| Mn1 (7,1) | Mn traditional (0,5) |
0,14 | 0,02 | 0,01 | 0,007 | |
| INK-P16 | Co | Mn traditional | 0,05 | 0,004 | 0,002 | 0,000 |
| Mn1 (7,8) | Mn traditional (0,8) |
0,13 | - | 0,005 | 0,000 | |
| INK-P17 | Co | Mn traditional | 0,19 | 0,012 | 0,006 | 0,006 |
| Mn1 (6,8) | Mn traditional (0,8) |
0,16 | 0,013 | 0,009 | 0,005 | |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
After ageing the samples at 40ºC for 2 weeks the number of promising drier systems was reduced even further for some products, mainly due to loss-of dry. The loss-of-dry can be observed by comparing the drying times of the respective product before and after the ageing.
In the case of do-it-yourself products and industrial products film properties, film hardness, gloss, and yellowing in dark places were also investigated to evaluate the efficiency of the alternative driers. Some of the industrial products were further investigated with regard to early water resistance. The investigations have been performed as described in various sections in chapter 7.
The results obtained are presented in table A.3.7 for both do-it-yourself and industrial products. Only the most promising alternatives after the ageing are included in the table. The results for the aged reference products are included as well.
Table a.3.7
Film properties of product samples that have been aged. The properties of the aged reference product are given as
well.
| Product | Primary drier# |
Secondary driers |
Gloss (º) | Hardness (Number of swings) |
Yellowing |
| DIY-P1 | Co | Ca Zr |
40 | 24 | 1 |
| Mn1 (1,84) | Ca (0,17) Zr (0,1) |
44 | 20 | 3-4 | |
| DIY-P2 | Co | Ca Zr |
47 | 41 | 2 |
| Mn1 (1,10) | Ca (0,21) Zr (0,3) |
57 | 28 | 2 | |
| Mn2 (7,53) | Ca (0,3) | 63 | 34 | 3 | |
| DIY-P3 | Co | Ba Zr |
53 | 13 | 2 |
| Mn1 (1,08) | Ba (0,58) Zr (0,24) |
47 | 13 | 2 | |
| DIY-P4 | Co | Ca Zr |
69 | 24 | 2 |
| Mn1 (2,07) | Ca (0,2) Zr (0,15) |
88 | 19 | 3 | |
| Mn4 (0,08) | Ca (0,05) | 83 | 16 | 4 | |
| DIY-P5 | C0 | Ca Zr |
101 | 25 | 0 |
| Mn1 (2,99) | Ca (0,2) Zr (0,1) |
101 | 23 | 0 | |
| Mn3 (6,12) | Ca (0,33) | 96 | 30 | 0 | |
| Mn4 (0,11) | None | 101 | 20 | 0 | |
| DIY-p7 | C0 | Ca Zn |
65 | 11 | 0 |
| Mn1 (3,01) | Ca (0,16) Zr (0,05) |
77 | 14 | 0-2 | |
| DIY-P8 | Co | Ca Zn |
- | - | 1 |
| Mn1 (1,65) | Ca (0,16) Zr (0,04) |
- | - | 0 | |
| IND-P10 | Co | Mn, Ca, Zr | 34 | 41 | 2 |
| Mn1 (2,67 ) | Ca (0,08), Zr (0,1) | 49 | 36 | 2 | |
| Mn2 (4,44) | Ca (0,06) | 45 | 43 | 2 | |
| IND-P11 | Co | Zn, Zr | 117 | 63 | 1 |
| Mn1 ( 2,57) | Ca (0,1), Zr (0,19 | 126 | 27 | 0/1 | |
| Mn4 ( 0,08) | Ca (0,1), Zr (0,2) | 112 | 26 | 1 | |
| IND-P12 | Co | Ca, Zr | 6 | 58 | 1 |
| Mn2 (3,56) | Ca (0,11) | 5 | 58 | 0 | |
| Mn3 (3,08) | Ca (0,10) | 5 | 59 | 0 | |
| Mn4 ( 0,05) | None | 5 | 61 | 1 | |
| IND-P13 | Co | phenanthroline | 9 | 34 | 0 |
| Mn1 ( 1,71) | Ca (0,1 ) | 8 | 33 | 0 | |
| Mn4 (0,08) | None | 7 | 32 | 0 | |
| Mn5(w) (2,39) | None | 8 | 27 | 0 |
# Concentration of Mn1, Mn2, Mn3, and Mn5(w) is given as concentration of the total drier product on solid air-drying binder in the product. In the case of Mn4 and the secondary driers the concentration is given as metal on the solid air-drying binder.
Both the drying time and the investigated film properties have been taken into account when suggestions for the most promising alternative drier systems have been made for each product. These selections can be seen in chapter 8.
Footnotes
[1] The reason for these percentages being much higher compared to Mn1 and Mn4 is that they are given as total drier product and not as metal concentration.
[2] Xi – Irritating substances, R38 – Irritating to skin.
[3] Xn – Harmful substances, R22 – Harmful if swallowed.
[4] Xi – Irritating substances, R38 – Irritating to skin.
[5] R10 – Flammable. R20/21 – Harmful by inhalation and in contact with skin. R36 – Irritating to eyes. R36/37/38 – Irritating to eyes, respiratory system and skin. R40 - Limited evidence of carcinogenic effect. R65 – Harmful: may cause lung damage if swallowed.
Version 1.0 December 2003, © Danish Environmental Protection Agency