|
Arctic Mercury Releases Inventory 4 Discussion of major source categories4.1 Combustion of carbon fuels In this section the major source categories are discussed in more detail. For each category, the project data are analysed briefly, selected current release reduction initiatives are outlined, and possible options for further reductions are listed. Most of the options are well known in many of the Arctic countries, and are in many cases already under consideration, planning or implementation in one or more of the countries. This does, however, not make them less relevant in this presentation, as the results of this inventory confirms their relevance and stresses that the reduction measures should be pursued further, if mercury release reductions are wanted. Note that a broader overview of existing mercury release reduction plans/strategies in the Arctic Countries is given in section 6. 4.1 Combustion of carbon fuels4.1.1 Analysis of mercury releases from combustion of carbon fuelsAs shown in table 3-4, coal combustion remains the largest single atmospheric mercury release source in the Arctic countries. Contributions from the use of carbon fuels are recalled in table 4-1. The magnitude of mercury release from this source category is closely related to the consumption of electricity, the role of coal as a dominating fuel type, and the suitability of applied emission reduction systems for mercury retention in the exhaust gases from power production and other major coal consuming sectors. The atmospheric releases from the 5 largest coal combustion point sources (power plants) reported from the Arctic countries amounted to The 5 coal combustion facilities (power plants) emitting most mercury in the Arctic countries released about 0.8 metric tons/ year each (on average). Together, the 5 largest point sources emit about 3% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. Table 4-1 Reported atmospheric mercury releases from combustion and extraction of fossil fuels and bio-fuels (extracts from table 3-4); metric tons/year.
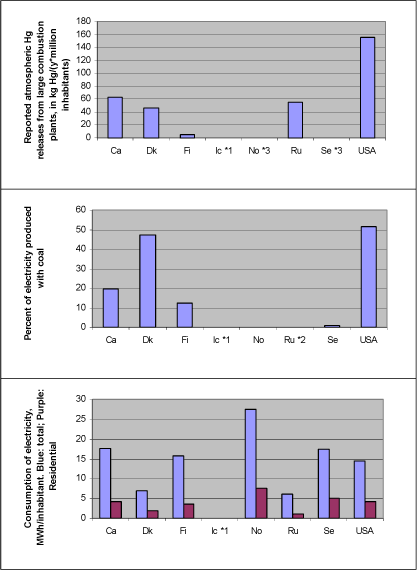
Electricity production with coal combustion Figure 4-1 illustrates reported atmospheric mercury releases from large power plants as related to percentage of produced electricity based on coal combustion. Additionally, consumption of electricity per inhabitant is shown, in total and for residential consumption only (the last thing as a possible indicator of personal use pattern, independent of industry use). The mercury release data are from the questionnaire responses and (ACAP, 2004), and all energy data are from the International Energy Agency's (IEA) reports "Energy Statistics of OECD Countries" and "Energy Statistics of Non-OECD Countries", both 2003 editions. The same data are presented in table 4-2. Note that all figures are subject to uncertainty and should be interpreted with caution. Uncertainties for release estimates from Denmark are presented in the Danish questionnaire response in appendices. No other countries reported quantitative uncertainties for submitted mercury data. Figure 4-1 Relations between reported atmospheric mercury releases from large power plants, dependence on coal for production of electricity, and consumption of electricity - total as well as residential only (Hg data from questionnaire responses and (ACAP, 2004); energy data from IEA, 2003 and 2003b)*4.
Notes: *1: Hg data from Iceland on coal does allow analysis of this aspect. *2: Data on percentage of electricity produced with coal from Yanin (2003). *3: Hg releases from small coal based power plants in Norway and Sweden were reported in another category, and are not included here (marginal releases) *4: Energy data for same year as reported Hg releases, except for USA for which reported atmospheric releases were from 1999, other releases for 2001, and energy data were for 2000. Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Table 4-2 Relations between reported atmospheric mercury releases from large power plants, dependence on coal for production of electricity, and consumption of electricity - total as well as residential only (Hg data from questionnaire responses and (ACAP, 2004); energy data from IEA, 2003a and 2003b)*4. Same data as in figure 4-1.
Notes: *1: Hg data from Iceland on coal did not allow analysis of this aspect. *2: Data on percentage of electricity produced with coal were not available for Russia. *3: Hg releases from small coal based power plants in Norway and Sweden were reported in another category, and are not included here (marginal releases). *4: Energy data for same year as reported Hg releases, except for USA for which reported atmospheric releases were from 1999, other releases for 2001, and energy data were for 2000 . Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Figure 4-1 and table 4-1 illustrate the following:
Of course any of the mentioned options for mercury release reductions have other adverse and positive effects that have to be taken into account. These include other types of environmental impacts, depletion of resources, and potential limitations on economic activity, among others. Other possible measures for mercury release reduction are mentioned below. National emission factors for coal use Another indicator for release reduction performance is the emission factor of mercury release per tonnage of coal used. Atmospheric mercury releases reported in the questionnaire responses are compared to national coal consumption data in table 4-3. For this comparison, mercury releases from large coal combustion plants (see definitions in the introduction to the ACAP mercury questionnaire in appendices) and other coal combustion/use were summed up (see table 4-3) and divided by the total consumption of coal of all types as reported by IEA (2003a; 2003b; for coal types included see table notes). For Denmark, Finland and USA, the same calculation was made for total reported mercury releases/outputs from coal use to all environmental media and by-products (includes Hg in deposition of residues and Hg in by-products for Denmark and Finland, but not for USA). The resulting calculated emission factors are shown in table 4-3. As shown, the calculated emission factors fall in the same range for all countries (except Norway) and are in line with emission factors for large power plants presented in the European EMEP/CORINAIR Emission Inventory Guidebook (2002) and by USEPA in AP 4-2 (1998). The calculated emission factors are, however, deemed too weakly documented here to allow strong comparisons between countries. The main confounding factor may be consumption of coal in sectors, for which mercury releases may not have been reported as assigned to coal use, even though coal contributed to the mercury outputs from these sectors. Coal related releases reported in this way do not contribute to the release category "other coal uses" included in the calculations, resulting in calculated emission factors which are lower than the true values. Probably, an important example of this could be coal (and coke) use in metallurgical production. This error could be of most significance for countries with relatively large consumption of coal in industries (this could be part of the reason for the deviation for Norway). Additionally, the reported releases may be associated with substantial uncertainties. For example for Denmark, the only country reporting uncertainties on estimates, the rounded "best estimate" value of 0.3 metric tons mercury released, represents estimated releases to the atmosphere of 0.19-0.31 metric tons/year (calculations were based on non-rounded mean of the range). The uncertainties on the presented calculated emission factors could be minimised by further analysis of existing data. Table 4-3 Reported releases from coal use, to the atmosphere and to all media, respectively, coal consumption data, and roughly calculated emission factors. See discussion in text (Hg data from questionnaire responses and ACAP, 2004; coal consumption data from IEA, 2003 and 2003b). Cross media transfers A part of the picture, which is not reflected in the atmospheric release figures, is the deposition of solid exhaust gas cleaning residues and marketing of by-products with contents of mercury, as well as minor direct releases to aquatic environments. As indicated by the data for Denmark, Finland and the USA in table 4-3, the total outputs of mercury from coal combustion quite likely add up to about the double of the direct atmospheric releases. Secondary atmospheric and aquatic releases from residue deposits and by-products most likely add to the direct releases to these media, though this is a poorly understood and possibly underestimated source type. The only way to reduce total mercury outputs to all media, is to reduce coal consumption by reductions of energy demands, or by substituting for coal with less polluting energy production methods or fuels. 4.1.2 State of mercury release reductions from coal combustion in Arctic countriesEmission reduction systems Currently, release reduction systems retaining parts of the mercury inputs to power plants, are in place on a number of the larger plants in the Arctic countries. These are dust (PM) controls in combination with flue gas desulphurisation ("FGD")/de-nitrification. Existing FGD/PM systems demonstrate very varying efficiency in mercury retention (0-90%), but average retention rates generally fall in the interval of 30-80% of the mercury input, depending principally on coal types fired and FGD systems applied (Pacyna and Pacyna, 2000). An important feature of these systems are their potential for reductions of atmospheric releases of a number of priority pollutants, most particularly acidic and eutrophisating gasses and other heavy metals than mercury. Still, many large power plants in the Arctic countries only have dust controls. For example, about 75% of the power plants in USA currently have PM retention only. Generally dust controls are not considered efficient for mercury retention because of mercury's volatility and its tendency to exist as elemental mercury vapour in the combustion gasses. In a few recent examples, highly efficient fabric filters and electrostatic precipitators have, however, exhibited good mercury retention under specific conditions favouring oxidation and adsorption of mercury on particulate material (USEPA, 2002). Coal washing prior to combustion is used some places in the USA, and in Russia prior to coke production. The washing reduces sulphur contents in the coal, and also removes some of the mercury contents (on average about 20%). This technology reduces mercury inputs to the combustion processes, but transfers the mercury to liquid or solid residues which have to be managed properly to avoid releases. Coal substitution and reduction of energy consumption As mentioned above, particularly Norway and Sweden have very limited mercury releases from coal combustion because they rely on hydropower and nuclear power. Denmark has shifted some of its electricity production from coal to natural gas and wind power during the last decades, resulting in a drop in releases of mercury and other pollutants (Skaarup et al, 2003; Danish Energy Authority, 2003). Additionally, Denmark, and possibly also other Arctic countries, has attempted to reduce, or at least stabilise, energy consumption by awareness raising activities, implementation of cleaner technology, introduction of CO2-taxes etc. These initiatives have had good results in Denmark, showing quite stabile energy consumption in spite of economical growth (Danish Energy Authority, 2003). Reductions of CO2 releases as aimed at in the Kyoto Protocol of the Framework Convention on Climate Change may also have reducing effects on mercury releases due to its direct links to consumption of carbon fuels, of which coal is a dominating part. As of November 2003, the Kyoto protocol has been ratified by Canada, Denmark, Finland, Norway and Sweden, whereas Iceland is in accession (per 25/05/2002) and Russia has only signed the protocol so far (UNFCC, 2003). The government of USA has announced that it does not intend to ratify the protocol. 4.1.3 Options for further release reductions for coal combustionFor the majority of the coal consumption in the Arctic countries, the atmospheric mercury releases are relatively well documented and monitored, and strategy development and implementation for release reduction is ongoing (UNEP, 2002; USEPA-ORD, 2000; Skårup et al, 2003). Here, the speed and degree of implementation appears to be largely a question of political and financial priorities. As regards the situation in Russia, documentation, strategies and implementation are among the issues of this ACAP project, as well as in other on-going projects (Munthe et al., 2003; Pacyna, 2003; and possibly others). Atmospheric emission reduction systems These technologies transfer input mercury from air emissions to deposition/landfilling and releases to other media. A possible first step is to implement flue gas desulphurization (FGD) on the facilities that are only equipped with particle filters today. Such a step would reduce releases of several priority pollutants, including some reduction of mercury releases to the atmosphere. For recommendations as regards types of technologies see (USEPA, 2002). To further reduce atmospheric mercury releases, implementation of flue gas cleaning systems optimised for mercury capture will be an option in the near future. Such systems (for utility boilers) with higher retention rates, are under development (USEPA, 2002 and USEPA-ORD, 2004). Such systems can involve injection in the flue gas of sorbents capturing mercury and/or oxidising agents, which convert elemental mercury to oxidised forms that are better captured in particle filters and FGD systems. Also the so-called selective catalytic reduction (SRC) processes used for NOx-reduction may enhance mercury oxidation for some coal types. See detailed recommendations and cost estimates for a range of mercury emission reduction techniques in (USEPA, 2002). Another evolving technique is the addition of fixed carbon filters downstream to other flue gas cleaning systems. For the situation in the USA, USEPA-ORD (2004) projects the potentials shown in table 4.4 for different mercury release reductions options for coal fired power plants. The potentials and the time frames are based on the assumption that aggressive research, development and demonstration will be implemented. Table 4-4 Research, development and demonstration (RD&D) goals in the USA for projected cost-effective mercury removal capability (% of Hg input to combustion plant) for key coal type/control technology combinations, as projected by USEPA-ORD (2004), for detailing on background see the reference.
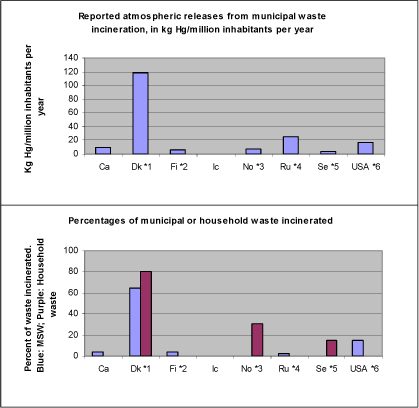
Coal washing Coal washing also transfers input mercury from air emissions to deposition/landfilling and releases to other media. In principle, a wider implementation of coal washing could reduce the atmospheric mercury releases from coal combustion. Like for other emission reduction systems this technique requires careful management of washing water and residues to avoid secondary mercury releases. Choice of energy sources As mentioned above, switching to other energy such as natural gas or renewable energy sources could reduce the mercury releases to all media. This would also imply reduced expenses for management of solid and liquid residues, because the total input of mercury is reduced. Mercury concentrations in coal vary, both between seams and within the same seam. Therefore, in principle, it could perhaps in some cases be possible to reduce mercury emissions by selecting coal with low mercury contents. Besides some practical problems that such an approach might imply, a main concern in this scenario is however that cheaper high mercury coal could be attractive for countries with poorer emission reduction systems, potentially resulting in a worse situation locally and no improvement globally. Reduction of energy consumption Reduction of energy consumption would lead to direct cuts in mercury releases to all media. From other research fields it is indicated that there is large potential for energy savings by a combination of implementation of more energy efficient appliances (light sources, motors, electronics etc.) and awareness raising activities and other incentives. 4.2 Primary metal extraction4.2.1 Analysis of mercury releases from primary metal extractionNon-ferrous metal extraction mobilises significant amounts of mercury due to it's extensive turnover of materials, its high operating temperatures and the fact that several metals are primarily produced from sulphidic ore with naturally elevated mercury concentration (gold, zinc, lead and copper ore). The sector is not well described as regards mercury releases to other media than the atmosphere, and total mobilisation (input) of mercury. The 5 non-ferrous metal extraction facilities emitting most mercury in the Arctic countries released more than 1 metric ton/year each, while the single largest point source in this category releases more than 6 metric tons/year. Together, the 5 largest point sources emit about 7% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. For the sector as such, most of the mobilised mercury is probably deposited on site or sold as by-product mercury. The major part of the recorded releases are emissions to the atmosphere, while minor releases to water and land are also recorded. Generally, mercury in extraction residues is not well unaccounted for in publicly available literature; some data for the Nordic countries are however given in (Huse et al., 1999), the questionnaire responses and (ACAP, 2004). As mentioned in section 3.2, the main contributions from the primary metal extraction sector in the Arctic countries are production of gold (mainly USA and Russia), zinc and zinc/copper (mainly Russia and Canada), and copper and copper/nickel (mainly Russia) (based on: Questionnaires from this study; Environment Canada, 2002; ACAP, 2004). As shown in table 3.4, the largest atmospheric mercury releases are reported for USA (12 metric tons/year), Russia (9.6 metric tons/year) and Canada (2.3 metric tons/year). Table 4-5 show, that as regards both atmospheric and total mercury releases in USA, gold extraction contribute with the largest releases, with copper and "other metals" (including zinc [3]) coming up next. Note particularly the reported releases from gold extraction to soil. To avoid misunderstandings, it should be mentioned that this mercury originates from mercury naturally present in the gold ore; the mercury amalgamation method used for artisanal (small scale) gold mining is not applied professionally in USA anymore. For Russia the largest contributions to atmospheric mercury releases are from nickel/copper extraction (5.3 metric tons/year) and zinc extraction (1.9 metric tons/year), while total mobilisation of mercury are roughly equal for zinc/lead extraction and nickel/copper extraction (estimated at app. 31 and 28 metric tons/year, respectively) (ACAP, 2004). It should be noted that these release estimates have not been confirmed with measurements. Gold extraction is also a significant mercury release source in Russia, and here a special factor is involved: The secondary gold extraction of older tailings containing large amounts of mercury from earlier extraction with the amalgamation method. This issue may warrant special attention. Gold extraction with the amalgamation method is illegal now in Russia, but may still take place (ACAP, 2004). For Canada, most of the atmospheric mercury releases from the sector are attributed to co-production of several non-ferrous metals in the questionnaire response (1.9 metric tons/year), while "other metals" contribute with 0.3 metric tons/year. A comprehensive report on emissions and pollution abatement in the sector describe that most of the atmospheric releases from this sector in Canada occur at a single combined copper/zinc smelter with less extensive emission reduction equipment on the copper production line (Environment Canada, 2002). Reported data on releases to other media and outputs are limited for this sector in the questionnaire response (and in the described report). Table 4-5 Reported mercury release data on primary non-ferrous metal production in USA (selection from table 3-8; data from questionnaire response from USA). Ferrous metal extraction Ferrous metal extraction also release mercury, but, as regards atmospheric emissions, generally in smaller amounts than from extraction of the dominant non-ferrous metals (Pacyna and Pacyna, 2000, ACAP, 2004). For Russia, the atmospheric releases from iron and steel production are estimated at 1.4 metric tons/year (ACAP, 2004). 4.2.2 State of release reductions in primary metal extraction in Arctic countriesFor non-ferrous metal extraction from sulphidic ore concentrates, the most important single factor influencing retention of atmospheric mercury releases is the presence of specific mercury removal steps in the exhaust gas lines. Sulphidic ore types are the most important virgin raw material for many of the non-ferrous metals (a major exception is aluminium). In the Arctic countries, such mercury removal steps appear to be present in most of the extraction facilities applying roasting, sintering and/or smelting of input ore (the process steps releasing most of the mercury present in the concentrates); (Environment Canada, 2002; European Commission, 2001; ACAP, 2004). The presence of a mercury removal step is likely partly driven by the technical need to purify the gases prior to the conversion of sulphur dioxide gases to sulphuric acid, and mercury removal is found in most extraction plants which are equipped with acid plants. The presence of a dedicated mercury removal step influences the distribution between output pathways considerably. Releases to the atmosphere are converted to marketed by-product outputs (mercury and its compounds) and releases to waste deposition, land and water. In case sulphuric acid is produced, mercury releases to sulphuric acid (a marketed by-product) will also be converted to other output pathways, if a mercury removal step is present. Most or all non-ferrous metal extraction plants applying heating in the initial process steps also have exhaust gas particle filters (cyclones, wet scrubbers, ESPs and/or fabric filters), which may also reduce atmospheric mercury releases somewhat and convert the retained parts of the mercury to solid, suspended and/or liquid residues. Particle filters generally only have limited retention efficiencies on mercury, because major parts exist as elemental mercury gas in the exhaust gasses. Some non-ferrous metal extraction plants in the Arctic countries employ so-called direct leach processes, in which the sulphur contents (and mercury with it) are not driven of the concentrates with high temperature processes prior to extraction in aquatic solutions/suspensions. With direct leaching, most of the mercury follows the wet extraction residues, of which some are treated to extract the marketable mercury, and which require careful handling to avoid further releases. Extraction plants employing direct leaching may have very limited atmospheric mercury releases, as is for example the case in Finland (Finnish response to questionnaire and Fugleberg, 1999). Waste waters from different process steps can contain mercury and must be treated carefully to avoid or minimise releases to aquatic environments. Release reductions in the Canadian base metal sector As an example, significant reductions of atmospheric mercury releases have taken place in Canada through reductions efforts over the last 15 years (or more). Atmospheric mercury releases dropped from 27 metric tons/year in 1988, to 10 metric tons in 1993 and 2 metric tons in 2000 (Environment Canada, 2002). Mercury releases from waste rock and tailings The waste rock and tailings from non-ferrous metal extraction may - just like the produced concentrates contain trace amounts of mercury. This material is much more susceptible to weathering due to the reduced particle sizes and higher accessibility for air and precipitation. For sulphicid ores, this weathering liberates and oxidizes the contained sulphur and produce sulphuric acid. The acid renders mercury and other constituents more soluble and thus increases leaching of the metal to the environment manifold as compared to the untouched mineral deposit. This process is called "acid rock drainage" (or ARD) and is considered a serious environment risk (European Commission, 2003). The questionnaire responses of this study give a few data on releases to soil which most likely relate to extraction residues. It is not known if reported releases to air and water include secondary releases from extraction residues. Otherwise, quantitative data on release of mercury from waste rock and mining tailings to air, water and land has not been identified in recent data compilations. This release source could potentially be significant, because even moderate mercury concentrations in the material may render substantial mercury amounts mobile because of the enormous amounts of materials handled in mining operations. 4.2.3 Options for further release reductions in the primary metal extraction sectorAtmospheric release reduction measures As primary metal extraction contributes with 25 out of a total of 157 metric tons of reported mercury releases to the atmosphere per year in the Arctic countries, further release reductions may be necessary in this sector if overall reductions are desired. As regards atmospheric releases, a general recommendation would be to raise the remaining facilities to the emission retention levels attained in many facilities today ("best practices"/best available technologies). Such actions would include, among others, establishing high efficiency mercury removal steps in all facilities, or convert production to the direct leach process. All such improvements could be based on existing, industrially mature technologies. Detailed recommendations are presented in for example the report "Multi-pollutant Emission Reduction Analysis Foundation (MERAF) for the Base Metal Smelting Sector" (includes also examples of economic estimates for reduction actions; Environment Canada, 2002), and in "Integrated pollution prevention and control (IPPC) - Reference document on best available techniques in the non ferrous metals industry" (European Commission, 2001). Both reports are available on the Internet (see the reference list for web-links). Releases to other media, and releases from extraction residues From publicly available literature, it could appear as if mercury releases from primary metal extraction to other media than the atmosphere may be less in focus as regards release reductions. If this is the case, it may be a field where further release reductions could be attained. This also includes management of extraction residues. It seams that secondary releases of mercury to all media - also the atmosphere - from deposition of extraction residues are often not accounted for or described with any detail. Secondary gold extraction from old amalgam tailings in Russia Because of the potentially large amounts of mercury involved and the risks of mobilising mercury while disturbing (excavating etc.) the tailings deposits, prudent precautions should made to avoid provoking substantial mercury releases to all media. A deeper analysis of this problem is not possible for this report. Improvement of mercury data base to enhance management possibilities Improvements to the data base on releases to other media than the atmosphere, as well as releases to all media from extraction residues, seams warranted in order to enhance possibilities for quantifying and managing these releases in a national and global perspective, as well as improving the basis for quantifying the relative importance of other mercury release sources for management purposes; preferably on a mass balance basis describing dependent inputs and outputs of mercury with all fluxes/pathways. Reduction of mercury inputs Mercury concentrations in non-ferrous metal ores and concentrates vary considerably. Therefore, in principle, it should be possible to reduce mercury emissions by selecting raw materials with low mercury contents. In practice however, it may be difficult, and there is also a risk of such a scenario, that cheaper high mercury concentrates are attractive for industry in countries with poorer emission reduction systems and poorer regulation, potentially resulting in a worse situation locally and no improvement globally. 4.3 Waste treatmentAs shown in table 3-4 in section 3.2.3, incineration of municipal waste and hazardous/medical wastes are large sources to atmospheric mercury releases within this category. As regards releases to other media, Denmark, Finland and USA has submitted the most comprehensive data sets. When considering reported data for all media (tables 3-5 to 3-7), waste water systems is also a major source in these countries. Other significant release categories, when considering reported data across all media, are the mixed categories "other waste treatment" (including in questionnaire responses for example "other incinerators", switches, electronics, contaminated soil) and "recycling of other materials" (including for example steel and non-ferrous metal recycling). As a whole, these source types include all or most of the mercury flow through society with products (consumer and industrial products); both products where mercury is used intentionally, and high volume products with trace concentrations of mercury. The 4 waste incineration facilities emitting most mercury in the Arctic countries released more than 0.7 metric ton/year each (on average). Together, the 4 largest point sources emit about 2% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. Chlor-alkali production with mercury technology poses special problems as regards mercury containing waste; see section 4.4 on this issue. 4.3.1 Analysis of mercury releases from waste treatmentReported atmospheric mercury releases from municipal waste incineration per million inhabitants, and percent of general waste types incinerated are shown in figure 4-2 and table 4-6 below. Note that the percentage data have different basis according to available knowledge of waste types included. The figures show the expected relationship between dependency on waste incineration and mercury releases - high volumes of waste incinerated yield high mercury releases. Because of the modest efficiency for mercury retention of most emission reduction systems currently applied, this affect the atmospheric release figures directly. Denmark has rather high mercury releases per inhabitant from waste incineration in spite of decades of separate collection of mercury-containing waste. Reported mercury releases versus incinerated waste amounts are described further below. Table 4-6 also show reported releases from incineration of hazardous/medical waste, because this also describe aspects of how much mercury is flowing through society in products, and how this mercury is handled in the national waste treatment setups. Procedures of collection and treatment of hazardous waste influence the fate of mercury outputs from society's product usage quite strongly. As for the Danish situation for example, collection of waste products with high mercury contents (dental amalgam, thermometers, button cell batteries, manometers, blood pressure gauges etc.) has been going on for some decades, and the collected mercury waste has been recycled or deposited under special conditions. Yet, un-collected mercury-containing waste still follows other general waste to incineration due to the general waste handling priorities. In comparison, mercury releases from incineration of hazardous/medical waste seem to be relatively high in Canada and the USA. Figure 4-2 Reported atmospheric mercury releases from municipal waste incineration (in kg Hg/million inhabitants), and percent of general waste types incinerated. Note that the percentage data have different basis according to available knowledge of waste types included (see table notes below).
Notes: Please see identical notes to table 4-6. Table 4-6 Reported atmospheric mercury releases from municipal waste incineration (in kg Hg/million inhabitants), and percent of general waste types incinerated. Note that the percentage data have different basis according to available knowledge of waste types included (see table notes).*7.
Notes: *1: Percentage for MSW based on total waste amounts minus recycled construction wastes and composted gardening wastes (DEPA, 2003). Percentage for household wastes also from (DEPA, 2003). Uncertainties on atm. releases +/- 60% of mean (questionnaire response from DK). *2: Releases were unusually high in 2000; mean releases 1995-2001 was about 1/3. Release estimates are worst case and do not take effects of emission reduction systems into account (Questionnaire response from FI). *3: Percentage based on numbers for household wastes from 2002 (Sleire, 2003). *4: Based on (ACAP, 2004); uncertainty on waste definitions for percentage number. *5: Percentages are based on household waste data from (RFV, 2003). *6: Based on waste data from (Durkee, 2003) and waste definitions from www.epa.org/osw. *7: Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Most of the remaining product wastes from the Arctic countries' societies are landfilled (Skaarup et al., 2003; Questionnaire response from FI; Sleire, 2003; ACAP, 2004; RFV, 2003; Durkee, 2003). Figures on reported current mercury releases from landfills are low and few (see table 3-4, and national overview tables in section 3.3). As mentioned for other waste deposition, quantification of releases from deposition of mercury-bearing wastes is not as advanced as for direct atmospheric releases. Also, releases from waste deposition happen slowly over decades, centuries and more; and through occurrences less predictable in time such as excavation activities or other disturbances of the waste deposits. However, waste deposition may constitute a delay of the releases of the mercury in wastes (and thereby sometimes lower current mercury concentrations in the local environment), when compared to waste incineration with today's atmospheric emission reduction systems. Many of the products intentionally containing mercury are internationally traded, and would therefore be expected to be relatively uniform as regards mercury in (western) market economies; any major differences would be due to national trade restrictions and possibly also to some degree consumer's/user's preferences. From the history of mercury use reductions in Denmark, including trade bans, special waste collection, public awareness activities etc., it is deemed highly unlikely that waste in Denmark contain more mercury than average on western markets. This could indicate that mercury amounts similar to those found in waste in Denmark (sum of all releases/deposition from all waste types) per inhabitant would be expected in the other Arctic countries as well, but here more of it is deposited (and therefore potentially more difficult to quantify). An exception could be Sweden, where the "detoxification of society", that is: eliminating old mercury wastes accumulated in society , has been more systematic and comprehensive than for example in Denmark (see von Rein and Hylander, 2000). Note that all figures are subject to uncertainty and should be interpreted with caution. Uncertainties for release estimates from Denmark are presented in the Danish questionnaire response in appendices. No other countries reported uncertainties on figures. National emission factors for waste incineration In table 4-7, calculated emission factors in g mercury released per metric ton of waste incinerated are presented for the countries which have reported the involved data. The calculated factors are very dependent on both the quality of the reported release data, and of which types of wastes are included under the "general/municipal waste" parameter. As can be seen from the table notes, it has not been possible with the available data to calculate all the countries' factors with the same waste definition basis. This naturally introduces a significant uncertainty in any comparison between the countries. The atmospheric emission factors do, however, appear relatively uniform, except for Norway and Sweden, for which the calculated factors are an order of magnitude lower than for Canada, Finland, Denmark and USA. Only Denmark reported the necessary data to calculate a total release factor per ton of waste incinerated; with the rounded "best estimates" presented in the table, the total releases from municipal waste incineration amounts to about five times the reported atmospheric releases. The uncertainties on the presented calculated emission factors could be minimised by further analysis of existing data. Table 4-7 Calculated emission factors for incineration of general/municipal waste. See table notes.
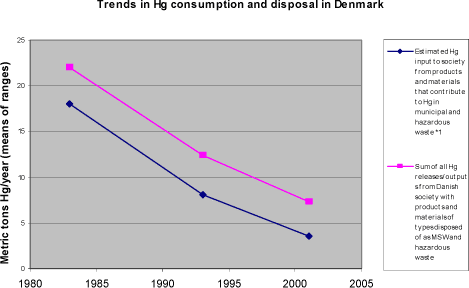
Notes: *1: Percentage for MSW based on total waste amounts minus recycled construction wastes and composted gardening wastes (DEPA, 2003). Percentage for household wastes also from (DEPA, 2003). Uncertainties on atm. releases +/- 60% of mean (questionnaire response from DK). *2: Releases were unusually high in 2000; mean releases 1995-2001 was about 1/3. Release estimates are worst case and do not take effects of emission reduction systems into account (Questionnaire response from FI). *3: Percentage based on figures for household wastes from 2002 (Sleire, 2003). *4: Based on (ACAP, 2004); uncertainty on waste definitions for percentage number. *5: Percentages are based on household waste data from (RFV, 2003). *6: Based on waste data from (Durkee, 2003) and waste definitions from www.epa.org/osw. *7: The differences and uncertainties in definitions of waste categories affect these calculations considerably. As marked in other notes, waste amounts are presented as "MSW" (= municipal solid waste) for some countries and as "household waste" for other countries, and even with this distinction, there may be differences in waste types included in the categories. Time trends in mercury disposal To evaluate the needs for mercury release reductions for society's waste treatment, it is useful to consider the trends in mercury consumption versus mercury in disposed off wastes. These data are shown for Denmark, as an example, in figure 4-3 and table 4-8. Similar consumption trends have been observed in USA, Sweden and Norway for example (Maag et al., 2002). Reduced mercury consumption has been seen generally in the West and the recent mercury release inventory for Russia of this project also shows a significant decrease (ACAP, 2004). In such a scenario, the corresponding decrease in mercury disposal is delayed a number of years due to product life time and time span from dysfunction of the product till it reaches the waste treatment facilities where it can be monitored (typically waste incineration and treatment facilities for hazardous waste). For Denmark, the current average delay for mercury in products is about seven years, as shown in figure 4-3. This delay varies strongly between products however, depending on their characteristics and users; for mercury containing alkaline button cell batteries it would only be a few years, while dental amalgam fillings blood pressure gauges, or fever thermometers used privately, may work for a decade or more. Furthermore, the mercury disposal curve would be expected to flatten out as the consumption approaches trace levels, because the last spent mercury-containing products are only found and disposed of slowly. The latest findings for Denmark indicate that perhaps this "detoxification" of taking old mercury products out of the cycling in society will continue longer than previously expected (Skaarup et al., 2003). Figure 4-3 Time trends in consumption and disposal of mercury with products that are disposed of as municipal and hazardous/medical waste in Denmark *3 (data from Skaarup et al, 2003; see notes under for table below).
Table 4-8 Time trends in consumption and disposal of mercury with products that are disposed of as municipal and hazardous/medical waste in Denmark (data from Skaarup et al, 2003)*3.
Notes: *1: Includes all consumption of intentional Hg uses + mobilisation of Hg impurities in "other materials in municipal waste" (packaging, food leftovers etc., estimated at 0.76-3.1 metric tons/year in 2000/2001). *2: Releases during use contribute with small amounts only, see table 3-6. *3: The true time gap between consumption and disposal may deviate slightly from the shown, because estimates of disposals are not completely independent of consumption figures, due to estimation procedures and lack of data to minimise uncertainties on cross-checking balances. 4.3.2 State of mercury release reductions in waste treatment in Arctic countriesEmission reduction systems As mentioned above, most emission reduction systems for exhaust gases from waste incineration are not optimised for mercury retention. Part of the mercury in the exhaust gas, especially gaseous elemental mercury, is not retained well by particle filters and acidic gas retention systems. Therefore, incineration currently result in an undesired enhanced spreading of the mercury in the waste, rather than preventing or delaying releases to the environment. This is well known, and has been one of the reasons for example Denmark's works on minimising the mercury input to waste by restricting trade of products with intentional mercury contents and enhancing separate waste collection. The recently initiated implementation of carbon injection/carbon filters in some Arctic countries on waste incinerators (mainly driven by dioxin reduction needs) have the potential for improving this situation. Though still significant, mercury releases from waste incineration has declined during the last decades as a result of various reduction efforts as described in this section. For example, most of the reductions in national mercury releases to the atmosphere in the USA since the early 1990's have been made in the waste incineration sector. As the case is for direct landfilling of waste practised in many countries, the part of the mercury that is retained from waste incineration with incineration residues still needs safe deposition and careful management for centuries to avoid unacceptable secondary releases from the residue deposits. Waste incineration residues are sometimes deposited in safer deposits than general waste due to the concentrated toxic constituents. The situation for waste water treatment (also considered waste treatment here) is in principle worse. Most of the mercury lead to waste water treatment ultimately ends up directly in the environment. Even modern waste water treatment plants only retain parts of mercury in the waste water (roughly about half in Denmark), that is: a significant part is released to aquatic environments after the treatment. The mercury that is retained ends up in the sewage sludge, of which much is applied directly on farm land as nutrition, and therefore also adds to the mercury pool that may affect humans and the environment (the remainder is incinerated or deposited). Mercury releases to waste water appear to be smaller than atmospheric releases in the countries which reported these data, but even the "basic" releases from dental clinics may be worth keeping under observation. The release estimates from Denmark indicate that this may be an underestimated release source in the other Arctic countries. Amalgam filters for dental clinics with very high retention rates exist and are widely used in Denmark and probably also in other Arctic countries, yet they are not used by all clinics as their use is not always mandatory (regulated locally in Denmark). Also for hazardous waste the situation as regards incinerated or deposited waste described above applies in principle. Waste products with high mercury contents can to some extent be collected separately to minimize their entry into the general waste treatment. This is done in many countries today. Generally however, such separate collection depends on consumers'/industry's own ability to identify these products, and their motivation to store and deliver them separately for waste treatment. Experience has proven that such collection schemes have some success, but that substantial parts of the disposed products are not collected, but end up in general waste (see for example Hansen and Hansen, 2003, and Skaarup et al., 2003). An additional aspect is the recycling of mercury from separately collected waste with high mercury contents, including virtually pure metallic mercury from spent manometers, barometers, switches etc. As mercury demand is falling, the desirability of mercury recycling will change due to risks of over-saturating the market, potentially resulting in increased releases to the environment (see discussion of this aspect in the Global Mercury Assessment: UNEP, 2002). Additionally, mercury recycling plants are also sources of direct releases to the environment. Sweden has taken the radical step of preventing collected waste mercury from being marketed and decisions for final disposal in a high safety deep rock mercury waste deposit is pending. Sales of mercury from US government stocks have been suspended for similar reasons since 1994. Reduction of mercury input to wastes In many of the Arctic countries efforts have been made to reduce consumption of many "non-essential" products with intentional mercury contents and some countries also have limits to allowable mercury contents in high volume materials such as packaging which also contribute to mercury inputs to waste systems. These efforts have produced results in the form of reduced mercury releases from waste treatment. Yet, the reported mercury releases from Arctic countries indicate that these reductions may still not be enough to reach environmentally sustainable levels. This may have several causes. The consumption of mercury for intentional use is still to high and can be lowered further (alternatives are available for most product uses). The delay in disposal of mercury-containing products is long, and even products which are not sold anymore may continue to be lead to waste treatment for a couple of decades. For brief, but good, overviews of trends in the release situation for waste treatment in Finland, see the "Release trends" sheet in the Finnish response to the questionnaire in appendices. 4.3.3 Options for future release reduction in waste treatmentReductions of mercury releases from waste treatment - that is: from mercury in products and materials - seems to be unavoidable if significant decreases in overall mercury releases to the atmosphere (or in total) are desired. Even in a scenario where mercury use in products was limited to the strictly essential, the disposal of older mercury-containing products would continue for some time - most likely at least two decades. And after that time, a certain mercury input to waste treatment would still take place due to trace level mercury impurities in high volume materials. Landfilling of waste with elevated mercury contents - including waste incineration residues - could impose a burden of continued landfill management and risks of adverse environmental impacts on future generations. But as mentioned above, provided the landfill is secured with lining to prevent ground water contamination, it does not invoke immediate long-range distribution of mercury from the waste, as waste incineration may do. The following options could be considered:
Denmark, Finland, Iceland, Norway and Sweden has earlier explored the possibilities for common strategies for - and handling of - mercury containing wastes in a process under the Nordic Council of Ministers (Endre et al., 1999). 4.4 Chlor-alkali productionChlor-alkali production with mercury technology range among the moderate size mercury release sources, as regards recorded atmospheric releases seen in an overview perspective for the eight Arctic countries (7 metric tons/year of total of 157 metric tons for atmospheric releases). These releases are however produced by relatively few plants with mercury technology, as many plants have converted to other technologies or closed down. This means that the individual plants are major point sources. The 5 chlor-alkali facilities emitting most mercury in the Arctic countries released about 0.6 metric ton/year each (on average). Together, the 5 largest point sources emit about 2% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. The mercury balance for the remaining 4 mercury-based chlor-alkali production plants in Russia is presented in table 4-9 (from ACAP, 2004). Table 4-9 Mercury balance for chlor-alkali plants in the Russian Federation in 2002 (from ACAP, 2004).
Notes: * to water system (ponds-evaporators). *1 Purchased mercury amounts may differ from consumption in the year in same year due to internal mercury stock changes. Furthermore, a regularly encountered problem in assessing releases from these facilities is, that their material balances do not fit and substantial mercury amounts used cannot be accounted for by recorded emissions and disposals (UNEP, 2002; Sznopek and Goonan, 2000; ACAP, 2004). Parts of these releases may be accumulated in piping, equipment, building materials and in the ground under and around the production plant sites, and parts may possibly be emitted in ways that are not detected by the monitoring activities carried out. It could be feared that many old chlor-alkali plants (including shut down plants) are sites with severe contamination, posing great challenges and requiring large costs when they are decommissioned or clean-up is initiated for other reasons. These sites pose local mercury contamination risks and may possibly add to present and future regional releases due to evaporation of mercury. Data on a number of shut down chlor-alkali production sites in the Russian Federation are given in table 4-10. Table 4-10 Mercury in soils, waste dumps and water bodies by shot down enterprises in the Russian Federation (from ACAP, 2004).
Chlor-alkali production with mercury technology is considered an obsolete technology, even by the industry it self today, and besides its contamination problems, it drives substantial parts of the global mercury trade and recycling, and thereby increases risks also of other mercury releases in the cycle. The market shares for chloride produced with mercury technology have steadily been decreasing during the last years due to conversions and shut-downs. In line with OSPAR decisions, it could be considered to further stimulate conversion to available mercury-free technologies, and diligent clean-up on the contaminated sites. See the Global Mercury Assessment (UNEP, 2002) for further discussion of mercury use in chlor-alkali production. 4.5 Other selected release sourcesThis section gives some comments additional, selected source types. A detailed discussion of all source types is not given here. For description of other mercury release sources and options for their reduction, see the Global Mercury Assessment (UNEP, 2002). 4.5.1 Mercury contatination from gold extraction in RussiaThe following paragraphs are extracts from the text of Laperdina in (ACAP, 2004). For further description, see that report. Mercury contamination of traditional gold mining areas of Russia, as in all gold-mining areas of the world, is very urgent and poorly known problem. Scope of Hg contamination and its effect in different territories are not thoroughly investigated and require complex expensive research. However, it can be stated with certainty, that all traditional gold mining areas shown in Figure 3.3 have different extent of mercury contamination, which is not localized as a rule. With the introduction of the effective gold mining technologies , the same sites of rich placer deposits were repeatedly washed up again, with subsequent mixing of mercury-containing dredges and hydraulic monitors dumps with the washed-out rocks, which resulted in their distribution all-over the bigger territory. The point sources include abandoned and operating tailing dumps of extracting and concentrating plants, gold-receiving offices. The industrial and residential areas of old gold-mining enterprises are often either transferred from the worked-out territories or gradually destroyed. Restoration and conservation of the contaminated gold-mining sites have not been planned and carried out earlier, therefore the destroyed tailing dumps and exhaust schliches with high Hg content cause the severe environmental pollution. As the location of the old placer gold mining sites can not always be found based on historical records, the assessment of mercury contamination of the traditional gold mining areas requires conduction of the expensive field and desk studies. The local, but isolated from the gold-mining areas, sources of mercury contamination are the refining plants. In present, there are five main sources of mercury release from gold mining activities, quantitative characteristics of which depend on deposit type and gold reserves, duration and intensity of the deposit mining and mercury use in technological operations:
Re-processing of secondary industrial placers The extended re-processing of secondary industrial placers, as well as processing of tailings and schlich concentrates of ore and placer gold have lead to extraction of Hg buried in dumps, pits, , its conversion into the active migrating state and release to the environment with atmospheric emissions (thermal treatment of concentrates, mercury degassing from dumps etc.) and wastewater discharges. The licensing agreement on mining of such placers doesn't take into consideration a high industrial Hg content in the processed sands, and therefore the dissemination and extension of mercury contamination scope is not controlled. In spite of the currently developed and applied technologies of industrial feedstock processing with extraction of both gold and mercury, small scale enterprises with low revenues will likely to use cheaper technologies with only gold extraction, i.e. use burning of the amalgamated gold without Hg vapors condensation at the final phase. In case the environmental control over licensing and further mining of such gold- and mercury-bearing secondary industrial deposits is not strengthened, a half of mercury presently contained in dumps and wastes (3,000-6,000 t) is supposed to be released gradually to the atmosphere and water bodies. The scarce data available indicates, that a share of secondary industrial gold for various regions constitutes 1-5% of the total amount of the gold extracted. In general, a share of technogenic gold in Russia can be approximately estimated as 2-4 %, therefore the amount of secondary industrial gold extracted in 2001 may be equal to about 2,800-5,600 kg. Taking into account the average content of gold in the industrial wastes equal to 350 mg/m³ , the volume of re-processed industrial wastes can be estimated as the following: 2,800 kg : 350 mg/m³ = 8 million m³; 5,600 kg : 350 mg/m³ = 16 million m³. Given the amount of the re-processed industrial wastes as 8-16 mil. m³ and average Hg content as 0.2-0.5 g/m³, the total share of industrial mercury in this volume might make up from 2 to 8 t. About 15-20 % of this amount could have been utilized using modern technologies, however the basic amount of previously accumulated industrial mercury (approx. from 1.5 to 6.5 t) could be released in 2001 in the gold-mining sites and surrounding environments. 4.5.2 Oil and gas extractionA source type which has gained growing attention in the last years is the extraction of oil and gas. This source is generally poorly described as regards mercury releases. Mercury concentrations in oil and gas extraction fields vary quite strongly, and may be low in some countries. But for Russia, for example, the available data indicate that oil and gas extraction are major mercury release sources. As described by ACAP, 2004 the fate of this mercury is not clear. Oil and gas extraction activities may be worth more attention as regards mercury releases, and additional compilation of mercury mobilisation and release data on these sources may be warranted. 4.5.3 Dental amalgamDental amalgam is only large intentional use of mercury remaining in Denmark, and it constitutes significant release contributions to both waste water and waste treatment. Also in other Arctic countries, this may be a significant use of mercury, but it seems that it may possibly given less attention in some countries. The alternative filling materials have gained increased market shares, but in are still not deemed adequate for all filling types by the dental safety authorities in for example Denmark and Sweden. Both countries' environmental authorities are ready to promote full substitution when the health authorities find available alternatives adequate. In Sweden, to enhance the substitution, the public health subsidiary for amalgam fillings has been cancelled, whereas use of alternative fillings still gets financial support. In Denmark, the situation is that both filling types get the same subsidiary, and this enhances the use of amalgam fillings because they are cheaper (shorter dentist working time). As regards aquatic releases, high efficiency filters exist, which capture close to 100% of the mercury losses to the drain, but these filters are not compulsory in all countries. 4.5.4 Laboratory reagentsStill, a few types of laboratory standard analyses are involving the prescribed use of mercury compounds, COD analysis used to quantify organic substances (oxygen demand) in waste water is an example. Relevant alternative reagents and standard analyses are readily available, and international co-operation would enhance possibilities for a more expedite substitution, by involving the standardisation organisations, underlining the arguments for substitution, and stimulating substitution through changes in the regulation and practises of national and local environmental authorities. Nitrogen analysis with the Kjeldal method is also used in environmental control activities, and also for this analysis, the public environmental authorities themselves hold the keys to mercury substitution. 4.5.5 "Least essential uses" elimination procedureAs a possible activity of the Arctic countries' work with a mercury strategy framework, a process of identifying common positions on "least essential" mercury uses and developing concrete, non-binding recommendation on substitution could be initiated. Even though it is well known that mercury-free alternatives exist for many intentional mercury uses, a process of pointing out uses which could be eliminated, and proposing time schedules for their elimination according to the countries' priorities, could possibly stimulate substitution. Such detailed recommendations could be a concrete measure to enhance implementation of the already agreed goals of elimination/reduction of "priority hazardous substances" (OSPAR, HELCOM and EU goals). For some individual mercury uses, such recommendations have - for example - been developed under auspices of HELCOM (see http://www.helcom.fi/recommendations/reclist.html). Another example is the Danish mercury trade ban legislation which gives a general trade ban and detailed lists of uses which are exempted until specific dates, or for some uses until further notice. 4.5.6 Other mercury release sourcesA number of other mercury release sources exist and are reported from the countries to this project. A detailed description of every source can however not be given in this report. For listing of more source types please see the Global Mercury Assessment (UNEP, 2002) and the documents cited there (among many others). Footnotes[2] Capacity values have been obtained from EMF controls available in “EPA's 2003 Clear Skies Act parsed file for 2010” available at http://www.epa.gov/airmarkets/epaipm/results2003.html. The capacity values have been rounded to the nearest whole number. [3] Because the U.S. used Standard Industrial Codes (SIC codes) and there is no code for primary zinc (Zn) smelting, emissions from this source category for the U.S. submission to this project are included in the “Other Primary Extraction of Metals” category in the ACAP mercury questionnaire.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||