|
Environmental Project no. 982, 2005 Quantification and Identification of Active Microorganisms in Microbial Plant Protection ProductsContents1 Microbial plant protection products marketed in Denmark 2 Investigation of microbial plant protection products
5 Conclusions and perspectives 6 Postscript by the Danish Environmental Protection Agency APPENDIX A: Separation of Bacillus thuringiensis spores in microbial plant protection products APPENDIX B: Identification schemes from Deutsche Sammlung von Mikroorganismen und Zellkulturen PrefaceThe present report is the product of a project financed by the Danish Environmental Protection Agency (Danish EPA) entitled "Quantification and identification of active micro-organisms in microbial plant protection products". The project was initiated in January 2004 and editing of this report was finalized in September 2004. The project was conducted by Department of Environmental Chemistry and Microbiology, National Environmental Research Institute (NERI), Roskilde with laboratory aid by technician Anne Grethe Holm-Jensen and consultancy by senior scientist Bjarne Munk Hansen. Karin Pedersen-Ulrich, Chemical Inspection Service, collected the microbial plant protection products from the national distributors BioProduction, Borregaard Bioplant ApS, Cillus A/S, DDH (Hedeselskabet), and Garta. The steering committee members were Jørn Kirkegård, (head), Anita Fjelsted, both Danish EPA, professor Jørgen Eilenberg and associate professor Ole Nybroe, both the Royal Veterinary and Agricultural University of Copenhagen, and senior scientist Anne Winding, NERI. SummaryThis project investigates all Microbial Plant Protection Products (MPPP) that were on the Danish market in January 2004. These products are regulated by Statutory order no. 533 of 18 June 2003, which is based on EU Directive 91/414/EEC. The general objectives of the project were to check and evaluate the MPPP on the Danish market. Specific objectives were to verify the identity and quantity of the active micro-organisms, detect any microbial contamination and compare with information provided by the producers. During collection of the MPPP it was noticed that not all the label information requested for authorization was provided on the labels. There was a lack of batch number and date of production and expiry. The abundance of active micro-organisms in the MPPP was found to be within a comparable range of the abundance specified by the producer of all the products based on the bacteria Bacillus thuringiensis and Streptomyces griseoviridis. This was also the case for the products Binab TF WP and Rotstop with fungi as the active micro-organism. However, three products based on the fungi Trichoderma spp., two products based on Verticillium lecanii, and two products based on Beauveria bassiana contained significantly lower number of active micro-organisms than specified by the producers. The lower number might have implications for the risk assessment and especially for the efficacy evaluation of the products. The number of active micro-organisms was determined by counting colony-forming units (CFU). This method holds some drawbacks like incomplete separation of clumping spores prior to plate spreading and low culturability at the incubation conditions. Especially, separation of spores can be optimized further. However, the lower abundance can be true and be due to low viability or lower abundance of spores in the products. The identity of Bacillus thuringiensis as the active micro-organisms in Dipel ES, Bactimos L and Vectobac 12AS was verified by molecular analysis and production of crystalline inclusions containing d-endotoxin, which is the toxin responsible for the plant protection. Of the 63 isolates tested, all were determined to belong to the B. cereus subgroup of the genus Bacillus and 62 produced the crystals. The active micro-organism in Mycostop was identified as Streptomyces umbrinus and not S. griseoviridis as specified by the producer. The active fungus in Mycotal was identified as Lecanicillium muscarium and in Vertalec as L. longisporum. The active micro-organisms in these products have until now been known as Verticillium lecanii. However, a recent revision of this genus has led to segregation of the two strains into two new species. The active micro-organisms in the remaining MPPP based on fungi were identified to the species specified by the producers. Contaminating micro-organisms were not detected in any of the MPPP that are based on bacteria and neither in the fungal products: Binab TF WP, Mycotal, Vertalec, and BotaniGard ES and 22WP. In Rotstop and in one batch of Tri002 a relatively low number of contaminating bacteria was found (just above the detection limit). However, in five batches of three products based on Trichoderma spp.: Supresivit, Tri002 and Tri003, significant numbers of contaminating bacteria were found. In Supresivit it was well above the number expected from the information specified by the producer. For Tri002 and Tri003 the producer had specified a higher number of contaminating bacteria in the product. Although contaminating bacteria were indeed found here, the level was not as high as specified by the producer. The preparation of these MPPP may be conducted in ways that have a higher chance of including bacteria, e.g. by the use of clay materials in the formulations. The presence of bacteria in a product with fungi as the active micro-organism may represent a problem if the bacteria affect the efficacy and proliferation of the active micro-organism or if the bacteria have health or environmentally related risks. This project is the first conducted in Denmark with the aim of quantifying and identifying the active micro-organisms in all the MPPP on the Danish market. As far as we know it is also the first project of its kind being performed in the EU. The results have shown that the identities of the active micro-organisms in the products are affected by on-going changes in taxonomy of micro-organisms. Further, the identification depends on the details of taxonomic keys used and in particular the increased use of new molecular based techniques such as DNA sequence homology for species affiliation. For some of the MPPP the abundance of the active micro-organisms were lower than specified by the producers. The occurrence of contaminating micro-organisms in some products based on Trichoderma spp. was higher than specified by the producers, which merits further attention. The method of quantification of active micro-organisms may be further optimized especially regarding separation of spores prior to plating. SammenfatningI dette projekt undersøges alle mikrobiologiske plantebeskyttelsesmidler (MPPP) der i januar 2004 blev markedsført i Danmark. Markedsføringen af MPPP er reguleret af Bekendtgørelse nr. 533 af d. 18. juni 2003, som er baseret på EU Direktiv 91/414/EEC. Projektets overordnede formål var at kontrollere og evaluere de MPPP, der var på det danske marked. Mere specifikt var formålet at verificere identiteten af de aktive mikroorganismer, kvantificere dem, detektere mikrobiologiske kontaminanter, samt sammenligne egne observationer med producenternes oplysninger. Under indsamlingen af MPPP blev det bemærket, at ikke alle de krævede etiket-informationer til godkendelse angående batchnummer, produktionsdato og udløbsdato fremgik af produkternes etiketter. Antallet af aktive mikroorganismer i MPPP var indenfor det samme område som antallet specificeret af producenterne i produkter baseret på bakterierne Bacillus thuringiensis og Streptomyces griseoviridis. Det samme var tilfældet for produkterne Binab TF WP og Rotstop, hvor svampe er den aktive mikroorganisme. I tre produkter baseret på Trichoderma spp., i to produkter baseret på Verticillium lecanii og i to produkter baseret på Beauveria bassiana var der et signifikant lavere antal af den aktive mikroorganisme end specificeret af producenten. Det lavere antal kan have implikationer for risikovurderingen og specielt for effektivitetsvurderingen af produkterne. Antallet af aktive mikroorganismer blev bestemt ved at tælle kolonidannende enheder (CFU). Denne metode har nogle ulemper, f.eks. ufuldstændig adskillelse af klumpede sporer før udpladning og lav dyrkbarhed ved inkubationsforholdene. Især adskillelse af sporer kan yderligere optimeres. Det lavere antal kan dog også være reelt og skyldes lav levedygtighed eller et lavt antal sporer i produkterne. Identiteten af Bacillus thuringiensis som den aktive mikroorganisme i Dipel ES, Bactimos L og Vectobac 12AS blev verificeret med molekylær analyse og produktion af krystaller med d-endotoxin, som er toksinet ansvarlig for plantebeskyttelsen. Af de 63 undersøgte isolater blev alle identificeret til at tilhøre B. cereus subgruppen af Bacillus slægten og 62 producerede krystaller. Den aktive mikroorganisme i Mycostop blev identificeret til Streptomyces umbrinus og ikke S. griseoviridis som angivet af producenten. Den aktive svamp i Mycotal blev identificeret til Lecanicillium muscarium, og i Vertalec til L. longisporum. De aktive mikroorganismer i disse produkter har indtil nu været kendt som Verticillium lecanii. En nylig revision af denne slægt har imidlertid medført en opdeling af disse to stammer i to nye arter. De aktive mikroorganismer i de øvrige MPPP baseret på svampe blev identificeret til de samme arter, som angivet af producenterne. Der blev ikke fundet kontaminerende mikroorganismer i nogen af produkterne baseret på bakterier eller i svampeprodukterne Binab TF WP, Mycotal, Vertalec og BotaniGard ES og 22WP. I Rotstop og i et parti af Tri002 blev der fundet et relativt lavt antal kontaminerende mikroorganismer, kun lige over detektionsgrænsen. I fire partier af tre produkter baseret på Trichoderma spp.: Supresivit, Tri002 og Tri003, blev der imidlertid fundet et signifikant antal kontaminerende bakterier. I Supresivit var det langt over det forventede antal ud fra producentens informationer. For Tri002 og Tri003 havde producenten specificeret et højere antal kontaminerende bakterier i produkterne. Så selvom der var mange kontaminerende bakterier i produkterne, var fundet dog lavere end angivet af producenten. Produkterne produceres muligvis på en måde, der giver en højere chance for at inkludere bakterier, f.eks. ved at bruge lermaterialer i formuleringerne. Forekomsten af bakterier i et produkt baseret på svampe som den aktive mikroorganisme kan udgøre et problem, hvis bakterierne påvirker effektiviteten og spredningen af den aktive mikroorganisme, eller hvis bakterierne udgør sundheds eller miljømæssige risici. Dette projekt er det første af sin art udført i Danmark med det formål at kvantificere og identificere de aktive mikroorganismer i MPPP på det danske marked. Endvidere er det så vidt vides det første af sin art udført i EU. Resultaterne har vist, at identiteten af de aktive mikroorganismer i produkterne er påvirket af kontinuerlige ændringer i mikroorganismers taksonomi. Derudover afhænger identifikationen af detaljeringsgraden af de anvendte taksonomiske identifikationsnøgler og især den øgede anvendelse af nye molekylære teknikker som DNA sekvens homologi for artsidentifikation. I nogle af de undersøgte produkter var antallet af de aktive mikroorganismer lavere end oplyst af producenterne. Forekomsten af kontaminerende mikroorganismer i nogle produkter baseret på Trichoderma spp. var højere end angivet af producenterne, hvilket bør få yderligere opmærksomhed. Metoden til kvantificering af aktive mikroorganismer kan yderligere optimeres, specielt angående adskillelse af sporer før pladespredning. 1 Microbial plant protection products marketed in Denmark1.1 RegulationIn Denmark the marketing of pesticides is regulated by Statutory order no. 533 of 18 June 2003 which is based on EU Directive 91/414/EEC. Microbial plant protection products (MPPP) are defined as products with a micro-organism (bacteria, fungi, protozoa, vira, and viroids) as the active ingredient. In the EU MPPP are evaluated and authorized by the national regulatory authorities prior to marketing. The products marketed in Denmark in January 2004 are listed in Table 1.1. Table 1.1: Microbial plant protection products on the market in Denmark in January 2004, the active micro-organisms and the producer.
1.2 BackgroundEarlier investigations at the Danish Institute of Agricultural Sciences (Løschenkohl et al. 2003) on seven MPPP have shown the number of the active micro-organism to deviate from the abundance indicated
by the producer. This included up to 24 times higher as well as 2.6 times lower numbers of the active micro-organism. The purity of the products was high and the active micro-organisms were reported as
identical to the producers' information. However, higher as well as lower abundance of the active micro-organisms will affect any human and animal risks associated with and the efficacy of the products,
respectively. A query among the regulatory authorities in nine EU member states (Belgium, Finland, France, Germany, Great Britain, Italy, The Netherlands, Sweden, and United Kingdom) regarding their experiences, if any, on control of quantity of active micro-organisms in MPPP on the market was performed. This gave no indication of previous investigations of this matter (Fjelsted 2004). The general opinion on the tolerance level of the abundance of active micro-organisms was that it would be acceptable with a deviation of approximately 5 times higher or lower than the abundance indicated by the producer. At present no routine control of marketed MPPP is taking place in Denmark or apparently in other EU member states. The results of this project will be the first control of identity, purity and quantity of the MPPP on the Danish market. Hence, the project will give an indication of the relevance of future routine inspections to control the quantity and purity of the active micro-organisms in MPPP. 1.3 ObjectivesThe general objectives of the project were to control and evaluate the active micro-organisms in the MPPP on the Danish market. Specific objectives were to
1.4 General methodologyThe objectives were fulfilled by quantification of the culturable number of active micro-organisms in 1-3 batches of each MPPP. The active micro-organisms were identified and detectable contaminating micro-organisms on the culturing media were noticed and quantified. The results were compared to the information provided by the producers either on the labels, in available information on the products, or in the data package sent with the applications for authorization to the Danish regulating authority. 2 Investigation of microbial plant protection products2.1 Collection of microbial plant protection productsThe Chemical Inspection Service in the Danish EPA collected the MPPP on the Danish market through two rounds of visits to the distributors at January 13 and January 30, 2004, respectively. One to three independent batch numbers were sampled for each product. 2.2 Quantification2.2.1 Colony-forming unitsBacillus thuringiensis From each batch of the B. thuringiensis (Bt) products triplicates of one ml were aseptically transferred to Blue Cap bottles and diluted 10 times with 9 ml phosphate buffer (pH 7.0). Five mg proteinase (proteinase K recombinant PCR Grade cat. no.745 723, Roche Diagnostics GmbH, Mannheim, Germany), 125 μl 10% SDS (w/v) (lauryl sulfate, SL-4390, Sigma Chemical Co., Steinheim, Germany) and 82.5 mg Dowex (50 Wx8, 44505, Fluka Chemie AG, Buchs, Schwitzerland) were added to one-ml samples, which were stirred for 3.5 hours at room temperature. Initial experiments had shown this treatment to give the best separation of the spores (appendix a). Ten-fold dilutions in phosphate buffer, pH 7.0, were spread plated on triplicate agar plates with 100 μl on each plate. The media used was Tryptic Soy Agar (TSA) which consists of 30 g l-1 Tryptic Soy Broth (02-200, Scharlau Chemie, Barcelona, Spain) solidified with agar (15 g l-1) and autoclaved at 121°C for 20 min. TSA is the media recommended by the producers. The importance of media composition was compared by culturing Vectobac 12, batch number 140046, on TSA as well as T3 media which is known for its ability to support growth of bacteria from the Bacillus cereus subgroup of the genus Bacillus (Travers et al. 1987). The number of CFU was enumerated after incubation of the agar plates at room temperature for 2 days. At the time of CFU counting, one colony was isolated from each counted plate, resulting in 9 individual colonies from each batch. In addition, 9 colonies were isolated from T3 media with Vectobac 12, batch number 140046. The colonies were further purified by re-streaking on TSA-plates and after re-growth cultured in 2 ml 1/10 Tryptic Soy Broth overnight. From 1.4 ml of this overnight culture, DNA was isolated by a freeze-thaw-boil method (Johnsen et al. 2002). The remaining 600 μl were stored at –80°C. Fungi and Streptomyces griseoviridis Fungal spores and hyphae and S. griseoviridis spores were extracted, separated and cultured on agar media as prescribed by the manufactures with modifications according to Table 2.1. The agar media used was 2% malt extract agar (MEA) (cat. no. 01-573 Scharlau Chemie, Barcelona, Spain) solidified with agar (15 g l-1) and potato dextrose agar (PDA) (cat. no. 01-483 Scharlau Chemie, Barcelona, Spain). Both agars were autoclaved at 121°C for 20 min. At the time of CFU counting, the morphology of the colonies was checked and representative colonies were isolated for identification. Table 2.1: Extraction, separation and culturing of BCA products with fungi and actinomycetes.
a:: Malt Extract Agar b: Potato Dextrose Agar 2.2.2 Molecular identification of strains from the Bacillus cereus groupBt is a member of the B. cereus group, which is a subgroup of the genus Bacillus. Other known members of this group are B. cereus, B. anthracis, B. mycoides, B. weihenstephanensis, and B. pseudomycoides. The genetic similarity of the isolated Bt strains was studied by PCR amplification of the internally transcribed sequence (ITS) between 16S and 23S rDNA, which is a highly variable sequence. The primers used were ITS-16S-1392-S-15 and ITS-23S-206-A-21 (Willumsen et al. under revision) and they target conserved sequences in 16S and 23S rDNA, respectively. The method is referred to as PCR-ITS and the resulting PCR-ITS products were analyzed by electrophoresis in 2% agarose gels. 2.2.3 Formation of crystalline inclusions containing delta-endotoxin in the Bacillus thuringiensis culturesAll Bt strains with insecticidal activity forms crystalline inclusions containing d-endotoxin in sporulated culture (Hansen et al. 1998). Hence, the isolated strains were tested for formation of crystals by the following procedure: The isolates were grown on T3 agar media at 30°C for 3 days to ensure sporulation. The sporulated cell cultures were then examined for the distinct shape of the crystalline inclusions containing d-endotoxin by wet mount phase contrast microscopy at 1000 times magnification. 2.2.4 Identification of fungi and actinomycetesThe colony morphology of the fungi Trichoderma harzianum, T. polysporum, Verticillium lecanii, Phlebiopsis gigantea, Beauveria bassiana and Streptomyces griseoviridis was visually inspected after growth on agar plates. For microscopy, the fungi were grown underneath cover slips placed on top of agar blocs of ca. 1 cm2. The fungal hyphae, mycelia and spores adhering to the coverslips were mounted on slides and studied by 400 times magnification. Incubating the plates at 30°C and 35°C and morphological characteristics facilitated distinguishing between T. harzianum and T. polysporum. Identification of the fungi was based on Domsch et al. (1980), and a taxonomic key by McCray (2004) further identified the Trichoderma species. In addition, available information in the data package submitted with the applications for authorization of the respective products was used. One representative isolate of each fungal species and Streptomyces griseoviridis was identified by the identification service at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany (Appendix b). 2.3 Contaminating bacteriaWhenever bacterial colonies were encountered on agar plates inoculated with fungi, the number of bacterial CFU was determined. 3 Results
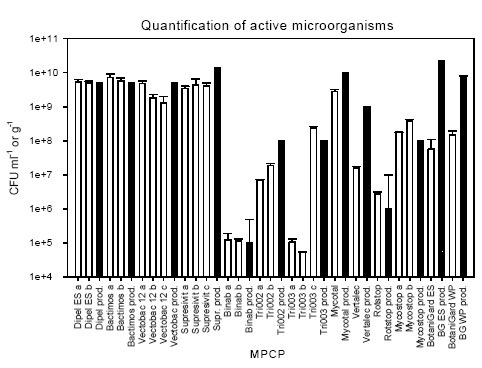
3.1 Available information on the microbial plant protection productsIt is noted that not all the collected MPPP was labeled with the required information regarding batch number and date of production and expiry (Table 3.1). One batch of Bactimos L and Supresivit, and both batches of Mycostop did not have any batch number. Bactimos L, Vectobac 12AS, and Tri002 had no date of expiry, while the date of expiry for Tri003 was indicated as one year after the date of purchase. This date does not take the storage time at the supplier into consideration. The date of expiry of Supresivit was 14 months after production date, which was not indicated. This makes the date of expiry of little value. Finally, for Dipel ES shelf life was 2 years. However, this information is of no use since no date of production is indicated. The number of active micro-organisms was not indicated on the product label of products based on Bacillus thuringiensis (Bt products). At these products the toxicity of the micro-organisms was indicated as International Toxic Units (ITU) (FAO 2004). However, the producer has subsequently informed us that the approximate CFU was 5 x 109 CFU ml-1. The producer of the products Tri002 and Tri003 has informed us that the products should contain 108 CFU g-1. 3.2 Quantification of active micro-organismsThe number of CFU of the active micro-organisms found in the various products is shown in Table 3.1 and Figure 3.1. All products based on Bt contained 1.29 – 7.31 x 109 CFU ml-1. The producer's information of abundance was 5 x 109 CFU ml-1, and some EU member states suggested an acceptable deviation of 5 times higher and lower abundance, which results in an acceptable range of abundance of 1 x 109 to 2.5 x 1010 CFU ml-1. Hence, the observed number was well within this range. The products Binab TF WP, Rotstop, and Mycostop also contained a number of active micro-organisms close to the number found in the producers' information. Of the four products based on the fungi Trichoderma spp., the abundance of active micro-organisms in Supresivit was 25-31 % of the indicated abundance. The abundance of active micro-organisms in Binab TF WP followed the specifications of the product and contained 1– 5 x 105 CFU g-1. The Tri002 and Tri003 products were expected to contain approximately 108 CFU g-1. However, we found a highly variable number of active micro-organisms in these products. Tri003 batch no. 0031 contained only 5.24 x 104 CFU g-1, while the other batch contained 2.43 x 108 CFU g-1. The Tri002 batches contained 0.7 – 1.9 x 107 CFU g-1. Hence, only one Tri003 batch contained a number of active micro-organisms close to the number specified by the producer. Table 3.1: The number of colony-forming units (CFU) of the active micro-organisms in the microbial plant protection products tested in this study. For comparison, the number of CFU or spores according to the producers' information is included.
*: data available from the data package submitted to the Danish EPA na: not available
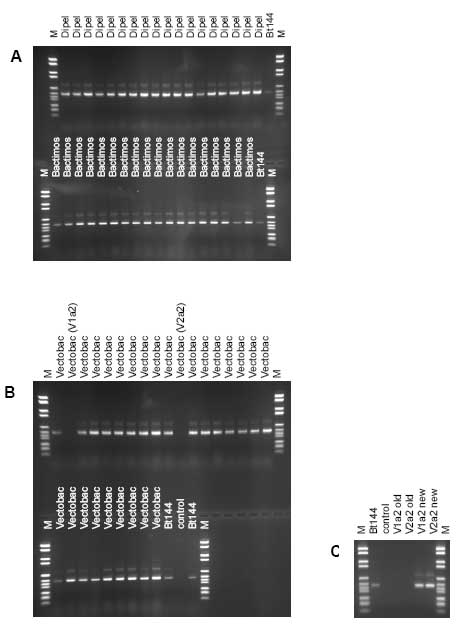
Figure 3.1: The number of active micro-organisms quantified in the products (open bars) and the number of active micro-organisms in the products as informed by the producers (hatched bars). a, b, and c denotes replicate batches of the products. Error bars show standard deviation for the tested products. For the producers' information the error bars indicate the upper limit of abundance and the bar the lower limit. Mycotal contained slightly below the expected number of active micro-organisms, while Vertalec contained a tenth of the number specified. BotaniGard ES and 22WP with the fungi Beauveria bassiana contained only 0.25% and 0.4%, respectively, of the number indicated on the container and 1-2% of the number indicated in the data package submitted with the application. 3.3 Identification of Bacillus thuringiensisThe morphology and visual appearance of the bacterial colonies on the agar plates were similar for all colonies. 3.3.1 Molecular analysisThe results of the molecular analysis by PCR-ITS showed all 63 Bt isolates to have identical band patterns and share band pattern with the reference strain B. thuringiensis subsp. kurstaki HD1 (Bt144), which is a strong indication that they belong to the same phylogenetic group (Figure 3.2A-C). Two isolates from Vectobac 12AS (V1a2 and V2a2) did not show any bands on the first gel (Figure 3.2B). However, after DNA extraction from another overnight culture of the isolates, PCR-ITS followed by gel analysis showed bands (Figure 3.2C). 3.3.2 Crystalline inclusions containing delta-endotoxinOf the 63 isolates tested only one isolate from Bactimos L, batch number 105-421-N9, did not contain crystals. The remaining eight isolates from this batch did contain crystals. PCR-RAPD analysis enabling differentiation between variants of Bt was performed on this isolate according to Hansen et al. (1998) and showed that the isolate was indeed a B. thuringiensis subsp. israelensis (Hansen 2004). 3.4 Identification of fungi and actinomycetesThe active micro-organism in Mycostop, supposed to be Streptomyces griseoviridis, was identified by DSMZ to be Streptomyces umbrinus (Appendix b). This identification was based on a sequence similarity of a 500-nucleotide long variable part of 16S rDNA of 99.5% and good correlation in fatty acid markers, in addition to results of other markers and physiological tests. Differences in identification results can be related to the identification keys used and the tests performed. However, the DNA sequence homology is considered as one of the most important characters for identification of bacteria. The active micro-organism in Mycotal was identified to be a Lecanicillium muscarium, and the active micro-organism in Vertalec was identified to be a Lecanicillium longisporum. According to the producer's information the active micro-organisms in these two products were two different strains of Verticillium lecanii. However, in 2001 the taxonomy of this genus was revised and the two strains segregated into two new species (Zare and Gams 2001). This taxonomy is primarily based on morphological and phenotypic characters (Appendix b). The remaining fungal isolates were identical to the species specified by the producers (Appendix b).
Figure 3.2: Band patterns of the products of polymerase chain reaction of the internally transcribed sequence (PCR-ITS) of DNA from bacterial isolates from the Bt products after electrophoresis in 2% agarose gels. Bt144: Bacillus thuringiensis subsp. kurstaki HD1; M: molecular weight marker; control: no added DNA template to PCR. A: In the upper panel 18 isolates of Dipel ES, in the lower panel 18 isolates of Bactimos L. B: In the upper panel 18 isolates of Vectobac 12AS, in the lower panel nine isolates of Vectobac 12AS. C: PCR-ITS products of the Vectobac 12AS isolates that did not yield a product in gel B. Shown are the old PCR-ITS products not yielding any bands (also shown in gel B) and the new PCR-ITS products yielding bands of V1a2 and V2a2. 3.5 Detection of contaminating bacteriaThe experiments were not designed to detect contaminating bacteria but to enumerate the active micro-organisms. As such the level of detection was rather high (2.8 x 102 – 1.1 x 107 CFU g-1 or ml-1) and depending on the number of the active micro-organism. Additionally, the media used was optimal for the active micro-organism and not necessarily for contaminating bacteria. In spite of this, contaminating bacteria were found in 7 batches of fungal-based MPPP (Table 3.2). Significant numbers of contaminating bacteria were found in Supresivit (all three batches), Tri002 (batch no. 0402) and Tri003 (batch no. 0031). Furthermore, few bacteria (1 to 12 per plate) were found in Tri002 (batch no. 0326) and in Rotstop. No contaminating fungi or bacteria were detected in the Bt products Dipel ES, Bactimos L, and Vectobac 12AS, the actinomycete product Mycostop, and in the fungal products Binab TF WP, Mycotal, Vertalec, and BotaniGard ES and 22WP. Table 3.2: The abundance of contaminating bacteria in the tested microbial plant protection products is indicated as the number of colony-forming units (CFU). When available, the producers' information of the number of CFU or spores of other micro-organisms than the active micro-organism is shown.
bd: below detection level na: not available 4 Discussion
4.1 Quantification of active micro-organisms and comparison to available information on abundance4.1.1 Bacillus thuringiensisWithin an acceptable range, all the MPPP based on Bt contained the number of micro-organisms specified by the producers. However, Løschenkohl et al. (2003) reported both lower (2.6 x 108 CFU ml-1 for Bactimos) and higher (1.1 x 1010 CFU ml-1 for Vectobac 12 and 2.2 x 1010 ml-1 for Dipel) number of active micro-organisms. This could indicate variation in the products over time or problems with the technique of quantification. For the Bt products the toxicity was indicated as International Toxic Unit (ITU), which FAO and WHO recommend. According to FAO (2004), toxicity is determined by bioassays of bacterial preparations of Bt against mosquito larvae using internationally recognized reference powders. Three standard powders have been used for testing B. thuringiensis subsp. israelensis (Bti): IPS78, IPS82, and HD968 (Glare and O'Callaghan 2000). In the bioassay, the Bti products are tested against the Bti reference powder, using early fourth-instar larvae of Aedes aegypti (strain Bora Bora). The toxicity of IPS82 and HD968 has an arbitrarily toxicity of 15,000 ITU mg-1 powder and 4740 ITU mg-1 powder, respectively, against this insect strain (FAO 2004, Glare and O'Callaghan 2000). When the active micro-organism is a B. thuringiensis subsp. kurstaki (Btk) it should be tested against the reference strain Btk HD1 (Glare and O'Callaghan 2000), using neonate larvae of the cabbage looper Trichoplusia ni. A significant problem exists with the determination of ITU of a product as the supply of reference powders eventually is exhausted. For example, the Pasteur Institute, Paris, France, made IPS82 available. The Pasteur Institute has, however, scaled down these activities and the reference is no longer available. FAO recommends the tests to be conducted by independent laboratories and the test results as well as the reference powders made available. Due to the present need of references, activities are in progress in the international community of producers and scientists to make new reference strains and powders available (Hansen 2004). The toxicity test with bioassays using reference powder and living insects has received criticism. Toxicity is influenced by product particle size (Skovmand et al. 1997). Additionally, the larval stage, the food fed to the larvae prior and during the bioassay, temperature, and light regime affect the results of the bioassay (Skovmand et al. 1998). 4.1.2 Fungi and actinomycetesIn seven products out of the nine MPPP based on fungi and actinomycetes that we tested, we found a lower abundance of active micro-organisms than indicated by the producers (Table 3.1). The lower abundance of active micro-organisms detected can be due to methodological problems such as:
The fungal MPPP were all treated as prescribed by the producers (Table 2.1), but still incomplete separation of spores might have lowered the CFU. The extraction and separation of Bt was optimized, but still clumping was observed (Appendix a). Hence, incomplete separation of the spores is indeed likely. This does not, however, explain the difference between batches, which most likely is not due to methodological problems but reflect real differences in abundance and culturability of the active micro-organisms. The lower abundance observed might be a problem for the efficacy of the products when used. For routine control of the quantity of active micro-organisms of both bacteria and fungi, separation of spores prior to CFU determination should be optimized by testing combinations of separation treatment. A few were tested on Bt spores (Appendix a), and this gave some indications of the achievements possible. However, it was not tested on MPPP with fungi as the active micro-organism. Direct counting of spores in the microscope circumvents the problem of incomplete spore separation. However, direct counting does not tell us anything about the viability of the spores and hence their potential survival, activity and efficacy upon use. 4.2 Identification of active micro-organismsAll 63 putative Bt isolates were identified as B. cereus group members by PCR-ITS. Upon microscopic examination only one of the isolates did not produce the crystalline inclusions containing d-endotoxin, while the other 62 did and as such were identified as Bt. Probably, the isolate not producing the crystals either did not express the toxin genes or had lost the plasmid carrying the toxin genes. Either way, it has lost its virulence at the present conditions. However, this is not anticipated to cause any relevant change in the efficacy. It does, though, highlight the chance of loss of virulence of the active micro-organisms. As the d-endotoxin production can be a metabolic burden and as the genes are positioned on a plasmid in Bt, and as such has a higher chance of being lost, this loss of virulence should be considered in risk assessment. The active micro-organism in Mycostop was identified as Streptomyces umbrinus and not S. griseoviridis as stated by the producer. According to DSMZ, possible reasons for this difference in identification can be due to incompleteness of the identification keys. Additionally and most importantly, the identification to S. umbrinus is to a large extent based on DNA sequence homology, which is considered as one of the most important features for identification. The active micro-organisms in Mycotal and Vertalec were not identified to Verticillium lecanii as expected based on the producer's information, but rather to Lecanicillium muscarium and L. longisporum, respectively. The reason for this is probably a revision in 2001 of the genus. However, it reflects the dynamic nature of microbial taxonomy that is still changing and developing. The remaining fungal MPPP were identified to the expected species. The active micro-organisms were identified to species level but not to strain or isolate level. Often the plant protecting activity is strain related and the best control of the MPPP would have included identification to strain level, if possible, and tests of plant protecting efficacy. For the Bt products the toxicity was made likely by detecting the presence of the crystalline inclusions containing d-endotoxin. For the streptomycete and the fungi no such tests of toxicity were performed. For risk assessment these examples of changes in the species affiliation of active micro-organisms in Mycostop, Mycotal and Vertalec accentuate the necessity to use the exact same strain for experimental evaluation of risks. It also raises questions about the use of the principle of familiarity. If the risk assessment of a micro-organism is based on experience and results of closely related micro-organisms, then a change in identity of the micro-organism or segregation into several species could lead to a need of a re-evaluation of the risk assessment. 4.3 Contaminating bacteriaIn the data packages submitted to the Danish EPA the producers have included information on the level of microbial contaminants expected to be present in their products (Table 3.2). In the data package submitted to the Danish EPA on Supresivit it is recommended to include the antibiotic chloramphenicol in the media when enumerating the active micro-organism (Trichoderma harzianum). As chloramphenicol inhibits bacterial growth, this is a clear indication that some bacteria are expected to be present. According to the submitted data a concentration of contaminating bacteria of 50 CFU g-1 is expected. The number found in the present study was several magnitudes higher (2-13 x 108 CFU g-1). From information supplied by the producer of Tri002 and Tri003, it appears that T. harzianum is added to inert montmorillonite clay, which harbors an average of 4.1 x 107 CFU g-1 bacteria and an average of 4.1 x 107 CFU g-1 fungi. Hence, it is expected that the end product also contain contaminating bacteria and fungi, which was also reported by the producer. The number of CFU found in this study was actually well below the number reported by the producer. In a study submitted by the producer, no pathogenic bacteria (E. coli, Staphylococcus, H. influenza, S. pneumonia, Vibrio sp., Yersinia sp., Salmonella, Shigella, Bacillus, and Campylobacter) were, however, found in Tri002 and Tri003 (Stasz and Hayes 1997). Batch no. 0031 of Tri003 only harbored 5.24 x 104 CFU g-1 of the active micro-organism and at the same time a similar number of contaminating bacteria (3.78 x 104 CFU g-1). This could indicate that something has gone wrong, either in the production of this batch or during storage. In the Tri002 batch no. 0326 and Rotstop the number of contaminating bacteria were just above the detection limit. The presence of contaminating bacteria can be a disadvantage for the product. The mode of action and efficacy of the MPPP become uncertain when other micro-organisms are present. Large quantities of contaminating organisms will cause difficulties in the risk assessment, as these micro-organisms should be taken into consideration. Especially, when the number of both the active micro-organism and the number of contaminating bacteria fluctuate between batches, as was the case with Tri003, the risks and efficacy of the MPPP is difficult to evaluate. Obviously, the presence of human, animal and plant pathogenic micro-organisms will be of greatest concern. For several of the products it is reported that no pathogenic bacteria are present. In order to clarify whether the isolated bacteria found in the fungal MPPP are pathogenic, further testing has to be performed, which is beyond the scope of this project. 5 Conclusions and perspectivesQuantification of the active micro-organisms in the 13 MPPP on the market in Denmark in January 2004 generally revealed the abundance of active micro-organisms to be within a comparable range of the abundance specified in all the products based on the bacteria Bacillus thuringiensis and Streptomyces griseoviridis and in the products Binab TF WP and Rotstop with fungi as the active micro-organism. However, three products based on the fungi Trichoderma spp., two products based on Verticillium lecanii, and two products based on Beauveria bassiana contained significantly lower number of active micro-organisms than specified by the producers. The lower number might have implications for the general evaluation and especially for the evaluation of efficacy of the products. The number of active micro-organisms was determined by CFU counting and this method holds some drawbacks like incomplete separation of clumping spores prior to plate spreading and low culturability at the incubation conditions. Especially, separation of spores can be optimized further. However, the lower abundance can be real and due to low viability or lower abundance of spores in the products. Molecular analysis and production of crystalline inclusions containing d-endotoxin verified the identity of Bacillus thuringiensis as the active micro-organisms in Dipel ES, Bactimos L and Vectobac 12AS. Of the 63 isolates tested, all were determined to belong to the B. cereus group and 62 produced the crystals. The active micro-organism in Mycostop was identified as Streptomyces umbrinus and not S. griseoviridis as stated by the producer. The active micro-organisms in Mycotal and Vertalec were identified as Lecanicillium muscarium and L. longisporum, respectively, and not Verticillium lecanii as stated by the producer. The active micro-organisms in the remaining MPPP were identified to the species specified by the producers. These changes in species identity accentuate the necessity to use the same strain for risk evaluation. Contaminating micro-organisms were not detected in any of the MPPP based on bacteria and in the products: Binab TF WP, Mycotal, Vertalec, and BotaniGard ES and 22WP. In Rotstop and in one batch of Tri002 low numbers, just above the detection limit, of contaminating bacteria were found. However, in five batches of three products based on Trichoderma spp.: Supresivit, Tri002 and Tri003, significant numbers of contaminating bacteria were found. For Supresivit it was well above the number expected from the information specified by the producer, while for the Tri002 and Tri003, the producer specified a higher number of contaminating bacteria in the product and carrying material than was actually found in this study. The preparation of these MPPP may be conducted in ways that have a higher chance of including bacteria, e.g. by the use of clay materials in the formulations. The presence of bacteria in a product with fungi as the active micro-organism may represent a problem if the bacteria affect the efficacy and proliferation of the active micro-organism or if the bacteria have health or environmentally related risks. This project is the first conducted in Denmark with the aim of quantifying and identifying the active micro-organisms in all the MPPP on the Danish market. Not all containers of the collected MPPP were labelled with the information required for authorization for marketing. The missing information included batch number and date of production and expiry. The results have shown that the identity of the micro-organisms are affected by the changing taxonomy of micro-organisms, including details of taxonomic keys used and the use of new molecular based techniques such as DNA sequence homology for species affiliation. For some of the MPPP the abundance of the active micro-organisms and the occurrence of contaminating micro-organisms deviated from the producers' information, which merits further attention. The method of quantification of active micro-organisms may be further optimized, especially regarding separation of spores prior to plating. 6 Postscript by the Danish Environmental Protection AgencyBy Anita Fjelsted, Danish EPA. The Danish EPA acknowledges that the microbial plant protection products contain living micro-organisms, and hence the content of viable micro-organisms may decrease during the period from the date of production until expiry. However, it is the producers' responsibility to make sure that the level and the efficacy that can be expected when the grower applies the product in the field are relatively constant during the period until the expiry. And of course, batch number, expiry date and information regarding storage condition always need to be included on the label of all these products. If the products need to be stored under certain conditions in order to keep viability and reduce the growth of contaminating bacteria (e.g. low temperature) it is the producers' and distributors' responsibility that this information is communicated to the end users. When evaluating and authorizing chemical pesticides in the EU a certain level of maximum deviation of content of the active ingredient is allowed. However, such fixed deviations are not established for microbial plant protection products. As mentioned already, this item has briefly been discussed among a few EU regulatory authorities. It was suggested by a couple of member states that a margin of maximum five-fold deviation should be accepted. As a follow up on this report, the Danish EPA would like to initiate a discussion at the EU level, in order to establish an agreement on an acceptable level of deviation in the amount of CFU in the microbial plant protection products. The Danish EPA will contact the producers who did not seem to have a constant and satisfactory level of active micro-organisms in their products. We will ask for explanation and for data proving that this large deviation is unlikely to occur in the future. The Agency will also look further into the question regarding what level of contaminating bacteria seems acceptable in microbial plant protection products. The relevant producers will be contacted by the Agency in order to require further information with the aim of assessing the potential risks connected with the presence of high levels of contaminating bacteria in these products. The Danish EPA will in this way make sure to follow up on the outcome of this project. It will be the producers' responsibility to follow up by informing the Danish EPA on improved production methods and storage facilities as well as fulfillment of label requirements. The producer of the product Mycostop containing the active micro-organism Streptomyces griseoviridis which has now been determined as S. umbrinus has been asked to investigate the taxonomic affiliation of the organisms further. The new affiliation of Verticillium lecanii is not expected to have an effect on the evaluation of products based on this species. The Danish EPA does not foresee the project presented in this report as resulting in a need for continuous and regular control of the microbial plant protection products on the Danish market. However, the products that have shown large deviations compared with the expected content may be collected and checked again in the near future. 7 ReferencesDomsch, KH, Gams, W, Anderson, T-H. 1980. Compendium on soil fungi. AP London. FAO 2004. http://www.fao.org/WAICENT/FAOINFO/AGRICULT/AGP/AGPP/ Fjelsted, A. 2004. Danish Environmental Protection Agency. pers. com. Glare, TR, O'Callaghan, M. 2000. Bacillus thuringiensis: Biology, Ecology and Safety. Wiley and Sons, Ltd., West Sussex, UK. Hansen, BM. 2004. National Environmental Research Institute. pers. com. Hansen, BM, Damgaard, PH, Eilenberg, J, Pedersen, JC. 1998. Molecular and phenotypic characterization of Bacillus thuringiensis isolated from leaves and insects. J. Inv. Pathol. 71:106-114. Johnsen, A, Winding, A, Karlson, U, Roslev, P. 2002. Linking of micro-organisms to phenanthrene metabolism in soil by analysis of 13C-labelled cell lipids. Appl. Env. Microbiol. 68:6106-6113. Løschenkohl, B, Thygesen, K, Nielsen, SL. 2003. Måling af bioaerosoler under udbringning af mikrobiologiske bekæmpelsesmidler og ved efterfølgende arbejdsprocesser i potteplanter. Danish EPA Bekæmpelsesmiddelforskning, nr. 79 2003. (in Danish). McCray, E. 2004. http://nt.ars-grin.gov/taxadescriptions/keys (28/04/2004) maintained by Systematic Botany and Mycology Laboratory, Agricultural Research Service, U.S. Department of Agriculture. Skovmand, O, Høgh, D, Pedersen, HS, Rasmussen, T. 1997. Parameters influencing potency of Bacillus thuringiensis var. israliensis products. J. Economic Entomology 90:361-369. Skovmand, O, Thiery, I, Benzon, GL, Sinegre, G, Monteny, N, Becker, N. 1998. Potency of products based on Bacillus thuringiensis var. israliensis: Interlaboratory variations. J. American Mosquito Control Ass. 14:298-304. Stasz, TE, Hayes, C. 1997. Test for microbial contaminants in end-use formulations. BioWorks, Inc. New York. Travers, RS, Martin, PAW, Reichelderfer, CF. 1987. Selective process for efficient isolation of soil Bacillus spp. Appl. Env. Microbiol. 53:1263-1266. Willumsen, PA, Johansen, JE, Karlson, U, Hansen, BM. Under revision after submission to Appl. Microbiol. Biotech. Isolation and taxonomic affiliation of N-heterocyclic aromatic hydrocarbon-transforming bacteria. Zare, R, Gams, W. 2001. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73:1-50. Appendix A Separation of Bacillus thuringiensis spores in microbial plant protection productsThe separation of Bt spores prior to spread plating was optimized through testing of different methods (Table a.1), using chemical, enzymatic and physical treatments. One ml of a ten-fold dilution of the Bt product Bactimos L was tested for each treatment. The number of individual and aggregated spores as well as the number of CFU determined after the separation treatments showed that the treatment with proteinase, SDS and Dowex followed by mixing yielded the highest fraction of individual cells and the highest number of CFU (Table a.2). Subsequently, this treatment was applied to all the Bt products. Table a.1: Treatments for separation of Bt spores prior to CFU counts
+: treatment added; -: treatment not added Table a.2: Number of individual and aggregated spores and CFU
nd: not determined Appendix B Identification schemes from Deutsche Sammlung von Mikroorganismen und Zellkulturen
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||