[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for 2-Ethylhexyl acrylate, Propylene carbonate, Quaternary ammonium compounds, Triglycidyl isocyanurate, and Tripropyleneglycol diacrylate
Evaluation of health hazards by exposure to
Propylene carbonate
and estimation of a limit value in air.
Vibe Beltoft
Elsa Nielsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
1. General description
| Molecular formula: | C4H6O3 |
| Structural formula: | 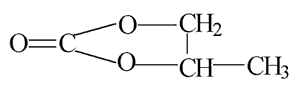 |
| Molecular weight: | 102.09 |
| CAS-no.: | 108-32-7 |
| Synonyms: | 4-methyl-1,3-dioxolan-2-one; dipropylene carbonate; 1,2-propanediolcarbonate; 1,2-PDC; cyclic methylethylene carbonate; cyclic propylene carbonate; cyclic 1,2-propylene carbonate; 1,2-propylene carbonate; propylene glycol cyclic carbonate; 4-methyl-2-oxo-1,3-dioxolane; carbonic acid, cyclic propylene ester. |
1.2 Physical / chemical properties
| Description: | Propylene carbonate is a colourless and odourless non-viscous liquid. |
| Purity: | - |
| Melting point: | -48.8 - -49.2 °C |
| Boiling point: | 241.7 - 243.4 °C |
| Density: | 1.189; 1.2069 g/ml (at 20°C) |
| Vapour pressure: | 0.03 mmHg (4 Pa) at 20°C |
| Concentration of saturated vapours: | 40 ppm (165 mg/m3) (calculated) |
| Vapour density: | - |
| Conversion factor: | 1 ppm = 4.17 mg/m3 20° C 1 mg/m3 = 0.236 ppm 1 atm |
| Flash point: | 135° C (open cup); 130-132° C |
| Flammable limits: | - |
| Autoignition temp.: | 510° C |
| Solubility: | Water: 83 g/l (at 20°C). Soluble in alcohol, ether, acetone, benzene, chloroform, ethyl acetate, carbon tetrachloride. |
| logPoctanol/water: | - |
| Henry’s constant: | - |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | Incompatible with strong oxidising agents, acid, bases, and reducing agents. |
| Odour threshold, air: | - |
| References: | CIR (1987), EPA (1998), HSDB (1998). |
Propylene carbonate is produced through the thermal cracking of saturated hydrocarbons. It can also be produced by the reaction of propylene oxide with carbon dioxide over a tetraethyl/ammonium bromide catalyst. (EPA 1998).
Propylene carbonate is used in CO2 recovery, in lithium batteries, in solvent extraction, plasticiser, organic synthesis, natural gas purification, synthetic fibre spinning solvent, and for the detection of N-containing drugs and their salts. (HSDB 1998).
Propylene carbonate is also used in painting and paint-stripping, to extraction of metals, and as a component of cooling agents and brake fluids. (EPA 1998).
It is also used in cosmetic products as a polar solvent at concentrations ranging from < 0.1 to 5%. In some products, up to 20% is used. (CIR 1987).
No data were found.
Air
No data were found.
Water
Propylene carbonate may decompose in aqueous solutions with the transient formation of propylene oxide. Based on the available physical and chemical properties, evaporation from water would be slow. (EPA 1998).
Soil
Leaching to water may occur, however, no data were available on soil to air or water to air mobility. Based on the available physical and chemical properties, evaporation from soil would be slow. (EPA 1998).
Bioaccumulation
No data were found.
Humans are exposed to propylene carbonate as cosmetic ingredient (up to 5%), and during work as a e.g. paint-stripping agent. (CIR 1987, EPA 1998).
2 Toxicokinetics
Inhalation
No data were found
Oral intake
No data were found
Dermal contact
A procedure to measure the steady-state rate of permeation of commercial solvents through living human skin was used on human female skin. The skin was removed from healthy females during plastic surgery of the breast. The samples were thinned by removing the dermal tissue from the epidermis and then stretched to a thickness of 300 to 600 m m. The permeability rate of propylene carbonate was reported to be 0.7 g/m2hour compared to a permeability rate for water of 24 g/m2h indicating that propylene carbonate is not readily absorbed through the skin. (Ursin et al. 1995 - quoted from EPA 1998).
No data were found.
No data were found.
3. Human toxicity
No data on short term toxicity are available except for studies on irritative effects.
In clinical studies, subjects were exposed to a wide range of cosmetic products containing from 0.5 to 10% propylene carbonate. In most of the studies, no skin irritation or sensitisation was noted. However, in one study (Hill Top Research 1977 - quoted from CIR 1987) of an antiperspirant (containing 2% propylene carbonate) using a modified Draize procedure, four subjects out of 51 developed skin erythema on intact sites and another four subjects developed erythema on abraded sites during the induction phase. In one study, moderate skin irritation was reported in subjects exposed to 20% solution of propylene carbonate. Occasionally other symptoms, e.g. hyperpigmentation, dryness, oedema and vesicles of the skin have been reported. (CIR 1987).
When five subjects were exposed to undiluted propylene carbonate applied to scarified skin once daily for three days, moderate skin irritation was observed; no evidence of phototoxicity was noted (CIR 1987).
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
No data were found.
4. Toxicity, animal data
Inhalation
"Concentrated vapours" (concentration not stated) for 8 hours was not lethal to six rats during a 14 days observation period. (Smyth et al. 1954 - quoted from CIR 1987).
Dogs, guinea pigs, and rats were exposed to an aerosol of propylene carbonate at a concentration of 2800 mg/m3 6 hours/day for 5 days/week for 21 days. The rats developed rhinorrhea and diarrhoea; no other toxicological effects were reported. (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Oral administration
Propylene carbonate was given by oral intubation in logarithmic doses to groups of five rats. Animals were observed for 14 days following the single oral dose. The LD50-value was reported to be 29.1 g/kg. (CIR 1987).
In mice, the oral LD50-value was reported to be 20.7 g/kg; no other data was reported (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Five male and female rats were administered undiluted propylene carbonate at a dose of 5 g/kg by oral gavage. They were observed for 14 days. Salivation was noted immediately after the single dose. No deaths and no lesions were observed at terminal necropsy. (Pharmakon Research International 1985 - quoted from CIR 1987).
Dermal contact irritation
Undiluted propylene carbonate was applied to the intact and abraded, clipped skin of rabbits (three males and three females). Skin responses were assessed at 24 and 72 hours after treatment. Very slight to well defined erythema and very slight oedema were noted at the 24 hours evaluation. All treated sites were normal at the 72 hours evaluation. The results indicated slight skin irritation. (Pharmakon Research International 1985 - quoted from CIR 1987).
Eye irritation
Undiluted propylene carbonate (0.1 ml, pH 8.82) was instilled into the right eye of three male and three female albino rabbits. Ocular irritation was reported to be only minimally; five had irritation of the conjunctivae only and one had irritation of the cornea, iris, and conjunctiva. (Pharmakon Research International 1985 - quoted from CIR 1987).
No ocular irritation was noted in six rabbits exposed to 10.5 or 17.5% propylene carbonate. (Kuramoto et al. 1972 - quoted from CIR 1987).
Instillation of 0.5 ml propylene carbonate into the conjunctival sac of the eyes of rabbits produced marked erythema of the conjunctivae, vascularisation of the sclera, and oedema of the lids and nictitating membrane within 24 hours; all eyes appeared normal by the 7th day. (Jefferson Chemical Company, Inc. - quoted from CIR 1987).
Inhalation
Rats (15/sex/group) were exposed to 100, 500, or 1000 mg/m3 propylene carbonate as aerosol for 90 days. No significant signs of toxicity were noted, except for some periocular swelling in the high and mid-dose groups. No systemic toxicity was reported.
An additional 20 rats per group were studied to investigate acute and subchronic neurotoxicity. For the acute study, rats received a single 6-hour exposure to propylene carbonate aerosol; no behavioural alterations were noted in any exposure groups at 1 hour and 24 hours after exposure. Standard neurobehavioral tests and motor activity were examined after 6 and 13 weeks; no behavioural alterations were noted in any of these exposure groups.
(Huntsman Corporation - quoted in EPA 1998).
Oral administration
Rats were given 1000, 3000, 5000 mg/kg/day propylene carbonate by gavage for 90 days. A recovery group was followed further from day 90 to day 118. No consistent dose-related findings were reported following necropsy or histopathological examination. (Huntsman Corporation - quoted in EPA 1998).
Dermal contact
To investigate dermal carcinogenicity, 50 ml propylene carbonate was applied twice a week to the shaved backs of 50 male mice for 104 weeks. A total of 10.4 ml was applied to each animal during the study. No deaths were observed, nor were any consistent body weight changes or significant dermal effects noted during the course of the study. (Huntsman Corporation - quoted in EPA 1998).
4.3 Reproductive and developmental effects
Twenty-seven dams (Sprague-Dawley rats) per group were orally exposed by gavage to 1000, 3000, and 5000 mg/kg/day propylene carbonate on days 6 through 15 of gestation. No developmental toxicity was observed at any dose. Maternal toxicity (decreased body weight gain and food consumption) was observed in high-dose dams. (Huntsman Corporation - quoted in EPA 1998).
4.4 Mutagenic and genotoxic effects
Propylene carbonate was evaluated for mutagenicity in Salmonella typhimurium, strains TA1535, TA1537, TA1538, TA98, TA100 with and without metabolic activation by liver homogenate. Doses of 50 to 5000 m g/plate were used. In the case of TA100, propylene carbonate showed some minor activity with and without activation at five doses, however, a dose-response relationship was not observed. (CIR 1987).
Propylene carbonate at five doses up to 4000 mg/plate was negative for genotoxicity in rat hepatocyte primary culture (CIR 1987).
To investigate dermal carcinogenicity, 50 ml propylene carbonate was applied twice a week to the shaved backs of 50 male mice for 104 weeks. A total of 10.4 ml was applied to each animal during the study. No tumours were noted during the course of the study. (Huntsman Corporation - quoted in EPA 1998).
5. Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
Propylene carbonate is classified for irritative effects (Xi;R36 - irritating to eyes) (MM 1997).
Cosmetics
As propylene carbonate is widely used in cosmetic products at concentrations from < 0.1% to 5%, it was concluded (based on clinical studies and animal studies) by the CIR panel (CIR 1987) that propylene carbonate is safe as a cosmetic ingredient in the present practices of use and concentrations.
EU
-
IARC/WHO
-
US-EPA
-
RD50
-
6. Summary
Description
Propylene carbonate is an odourless, clear liquid with a low vapour pressure (0.03 mmHg) and a high water solubility (83 g/l) at room temperature.
Environment
Only limited data are available. Based on the physical and chemical properties, evaporation from soil to water and air is considered to be slow.
Toxicokinetics
One study has reported that propylene carbonate is not readily absorbed through the skin. No other data have been found.
Human toxicity
No data on systemic toxicity have been found. In clinical studies, a 20% solution of propylene carbonate and undiluted propylene carbonate caused moderate skin irritation, whereas 5 and 10% aqueous solutions produced no skin irritation or sensitisation. However, in one study, subjects (4/51) developed skin erythema on intact sites and other four subjects developed erythema on abraded sites during the induction phase (2% solutions).
Animal toxicity
No deaths were observed in rats exposed to "concentrated vapours" for 8 hours. Dogs, guinea pigs, and rats exposed to propylene carbonate (aerosol) at 2800 mg/m3 6 hours/day for 5 days/week for 21 days developed rhinorrhea and diarrhoea; no other toxicological effects were reported. LD50-values was reported to be around 29 g/kg for rats and 20 g/kg for mice. Slight skin irritation were indicated in rabbits after undiluted propylene carbonate was applied to the intact skin, whereas marked irritation was observed following instillation in the eyes of rabbits.
No systemic effects were observed in rats exposed at concentrations of 100, 500 and
1000 mg/m3 propylene carbonate (aerosol) for 90 days; however, some periocular
swelling was noted at 500 and 1000 mg/m3; thus, a NOAEL of 100 mg/m3
is considered in this study.
Likewise, no effects were seen in rats following oral administration at doses up to 5000
mg/kg/day for 90 days or in mice following dermal application for 104 weeks.
Reproductive and developmental effects
No human data have been found.
No developmental toxicity was observed in rats following oral doses of 1000, 3000 and 5000 mg/kg/day during gestation days 6 through15; maternal toxicity (decreased body weight gain and food consumption) was noted at 5000 mg/kg.
Mutagenic and genotoxic effects
Propylene carbonate was negative when tested in Salmonella typhimurium (strains TA1535, TA1537, TA1538, TA98) with and without metabolic activation whereas strain TA100 showed minor activity although not in a dose related manner. Propylene carbonate was negative in rat hepatocyte primary culture.
Carcinogenicity
No human data have found.
In a 2-year dermal mice study, no tumours were observed.
7. Evaluation
The available data on health effects of propylene carbonate in humans are limited to data on irritative effects. Following application to the skin of undiluted propylene carbonate (one study) or of a 20% solution (one study), a moderate skin irritation was observed; however, no irritative effects were observed following application of solutions containing up to 10% propylene carbonate, One study using a modified Draize procedure has reported that 4/51 subjects developed skin erythema on intact sites and other four subjects developed erythema on abraded sites during the induction phase (2% propylene carbonate).
Propylene carbonate is of low acute toxicity in experimental animal following a single oral administration; no data have been found for inhalation exposure.
Following repeated inhalation exposure to propylene carbonate as aerosol, no systemic effects were observed following 21 days (dogs, guinea pigs, and rats - 2800 mg/m3) or 90 days (rats - up 1000 mg/m3); local effects in form of rhinorrhea and diarrhoea (21-day study) and some periocular swelling (90-day study) were reported. Likewise, no effects were seen in rats following oral administration (up to 5000 mg/kg/day) for 90 days or in mice following dermal application for 104 weeks. As diarrhoea was not reported in the oral study, the relevance of this finding is uncertain.
No reproductive toxicity studies have been found. In the only developmental study, no developmental toxicity including teratogenicity was observed in rats even when tested at dose levels giving rise to maternal toxicity.
The mutagenicity and genotoxicity tests available do not indicate that propylene carbonate is a genotoxic substance. No tumours were observed in the dermal carcinogenicity study on mice, the only study available.
The available studies indicate that exposure to propylene carbonate does not result in systemic toxicity even when tested at high dose levels by inhalation or oral administration. Thus, the critical effects following exposure to propylene carbonate are considered to be the local effects observed in the inhalation studies, effects which are probably related to the irritative properties of propylene carbonate. For the estimation of a limit value in air, a NOAEL of 100 mg/m3 is considered for local effects (periocular swelling) observed in the 90-day rat study.
8. Limit value in air
The limit value is calculated based on a NOAEL of 100 mg/m3 for local effects (periocular swelling) in rats exposed to propylene carbonate for 90 days.
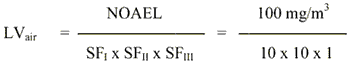 |
= 1 mg/m3
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 1 because of using a NOAEL for local effects and as no systemic effects have been observed even following administration of high doses.
9. C-value
A limit value of 1 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990). A C-value of 1 mg/m3 and placing in Main Group 2 is proposed.
C-value
1 mg/m3, Main Group 2.
10. References
EPA (1998). Environmental profile for propylene carbonate. EPA/600/R-98/068. National risk management research laboratory office of research and development. U.S. environmental protection agency. Cincinnati.
CIR (1987). Final report on the safety assessment of propylene carbonate (1987). J Amer Coll Toxicol 6, 23-51.
HSDB (1998). Propylene carbonate. In: Hazardous Substances Data Base.
MM (1997). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
MST (1990). Begrænsning af luftforurening fra virksomheder. Vejledning fra Miljøstyrelsen nr. 6 1990.
[Front page] [Contents] [Previous] [Next] [Top] |