[Front page] [Contents] [Previous] [Next] |
Alternatives to brominated flame retardants
Appendix
The appendix contains the complete results of the data screening for the following compounds:
|
Compound |
CAS no. |
Pages |
|
115-86-6 |
21-30 |
|
|
1330-78-5 |
31-38 |
|
|
57583-54-7 |
39-44 |
|
|
20120-33-6 |
45-48 |
|
|
21645-51-2 |
49-56 |
|
|
1309-42-8 |
57-62 |
|
|
14728-39-9 and 68333-79-9 |
63-68 |
|
|
7723-14-0 |
69-76 |
|
|
1332-07-6 |
77-82 |
|
|
108-78-1 |
83-90 |
|
|
1309-64-4 |
91-98 |
|
|
Quinidine carbonate |
No CAS no. |
99 |
|
CAS number: 115-86-6
Data compilation, environmental and health screening |
|
Summary
Health: The available data indicate that TPP has a relatively low impact on health. In rare cases it can induce skin sensitisation and contact dermatitis in humans. Although TPP is a neurotoxin in animals, recent investigations indicate that TPP is not neurotoxic in humans, but persons with preexisting neuromuscular disorders may be at increased risk. Environment: The literature reviewed indicates that TPP is very toxic to algae, fish and some crustaceans (typical L(E)C50<1 mg/l). The compound is toxic to D. magna. NOEC data available for fish are in the range 0.014 - 0.23 mg/l. Bioaccumulation of this compound is high (>100). The available biodegradation data indicates that this compound is readily to inherently biodegradable under aerobic conditions. No data is available for anaerobic degradation. Mobility of TPP and its primary degradation product in soil is very low. |
|
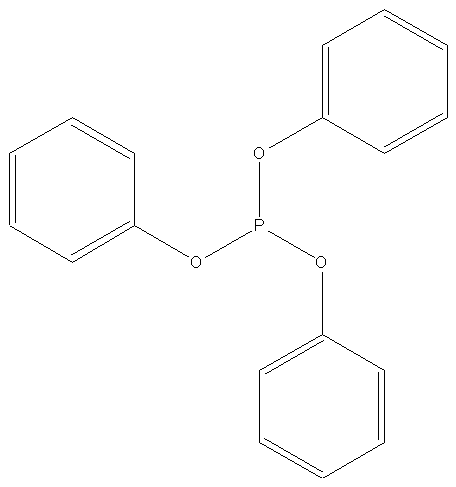
Triphenyl Phosphate |
||
|
Identification of the substance |
||
|
CAS No. |
115-86-6 |
|
|
EINECS No. |
204-112-2 |
|
|
IUPAC Name |
Triphenyl phosphate |
|
|
Synonyms |
Phenyl phosphate; TPP; Phosphoric acid triphenyl ester; triphenyl phosphoric acid ester; celluflex tpp |
|
|
Molecular Formula |
C18H15O4P |
|
|
Structual Formula |
|
|
|
Known Uses |
Fire-retarding agent, plasticizer for cellulose acetate and nitrocellulose |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None. |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
Colorless or white powder [2]. |
|
|
Molecular Weight (g/mol) |
326.28 |
|
|
Melting Point/range (° C) |
48 [1], 49-50 [2,3,4], 50 [5] |
|
|
Boiling Point/range (° C) |
370 [1], 220 [3] , 245 [3,4,5,6] |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
1 at 193.5 ° C [2,6,4] |
|
|
Density |
Specific gravity=1.268 g/cm3 at 60 °
C [5] |
|
|
Vapour Density (air=1) |
1.19 [2] |
|
|
Solubility (water) |
1.9 mg/l at 25 ° C [2,6,7] |
|
|
Partition coefficient (log Pow) |
2.62 [3] 4.59 [2,6,7] |
|
|
pKa |
Not applicable |
|
|
Flammability |
Nonflammable [2] |
|
|
Explosivity |
No relevant data found |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in Humans |
While a statistically significant reduction in red blood cell cholinesterase has been reported in some workers, there has been no evidence of neurological disease in workers in a TPP-manufacturing plant. There have been no reports of delayed neurotoxicity in cases of TPP poisoning. (10). |
|
|
Acute Toxicity |
||
|
Oral |
Oral-rat LD50: 3,800 mg/kg [2,4]. Oral-rat LD50: 3,500-10,000 mg/kg bw. [3,6]. Oral-mouse LD50:1,320 mg/kg [2,4,6]. Oral-mouse LD50: 1,300 mg/kg bw. [3]. LD50 White leghorn chicken oral > 5.0 g/kg [2]. |
|
|
Dermal |
Concerning dermal application one study indicates that the LD50 for rabbits is higher than 10.000 mg/kg and another that LD0 (no death) is higher than 7,900 mg/kg [2,3,6]. |
|
|
Inhalation |
No relevant data found |
|
|
Other Routes |
¨ Several studies concerning subcutaneous acute toxicity have been conducted. Some of the first studies were performed with TPP prepared from coal-tar sources containing neurotoxic impurities. Based on recent experimental data, it is concluded that TPP is not neurotoxic when it is administered subcutaneously [3]. Subcutaneous-monkey LDLo: 500 mg/kg [2,4,6]. Subcutaneous-cat LDLo: 300 mg/kg Subcutaneous-rat LD0: 3,000 mg/kg bw. [3]. Subcutaneous-guinea pig LD0: 3,000 mg/kg bw. [3]. |
|
|
Skin Irritation |
Based on 4 studies it is concluded that TPP is not irritating skin [3]. |
|
|
Eye Irritation |
100 mg TPP administered directly in the eye of rabbits cause minimal reversible irritation [3]. |
|
|
Irritation of Respiratory Tract |
No relevant data found |
|
|
Skin Sensitisation |
¨ Some people have been tested positive in TPP patch-tests [3] and one case of skin sensitisation has been recorded [2]. An allergic reaction in a 67-year old woman to spectacle frames containing triphenyl phosphate was reported. Patch tests with analytical grade triphenyl phosphate in that individual indicated a reaction at concentrations as low as 0.05%. [2]. |
|
|
Sensitisation by Inhalation |
No relevant data found |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in Humans |
¨ Numerous medical observations have been made on workmen employed for several (2-10) years in the factory where TPP was produced. No abnormal symptoms appear to have been observed, in particular no signs of neurotoxicity. Persons with preexisting neuromuscular disorders may be at increased risk. [2]. |
|
|
Oral |
Oral-rat NOAL: 1,900 mg/kg repeated dose [3]. Oral administration for 3 months to rats in doses of 1,800 mg/kg and 380 mg/kg caused no deaths, and it was concluded from the normal growth and cholinesterase activity that these doses have no cumulative toxic effects. [2]. When administered as repeated excessive doses orally, TPP can cause neurotoxic effects such as decreased cholinesterase activity [3]. Concerning neurotoxicity 3 studies have been conducted on hens and chickens with an exposure time of 5-6 days. Only one of these studies indicated signs of decreased colinesterase activity. The two others did not indicate signs of neurotoxicity [2,3]. |
|
|
Inhalation |
In workers engaged in the manufacture of aryl phosphates (including TPP and up to 20% triorthocresyl phosphate) and exposed to concentrations of aryl phosphates of 0.2 to 3.4 mg/m3. There was some inhibition of plasma cholinesterase, but no correlation between this effect and the degree of exposure or minor gastrointestinal or neuromuscular symptoms [2]. |
|
|
Dermal |
Contact dermatitis due to TPP has been described [10]. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
Triphenyl phosphate was tested for mutagenicity in the Salmonella/microsome preincubation assay using a protocol approved by the National Toxicology Program. Triphenyl phosphate was tested at doses of 0, 100, 333, 1000, 3333 and 10,000 ug/plate in four Salmonella typhimurium strains (TA98, TA100, TA1535, and TA1537) in the presence and absence of Aroclor-induced rat or hamster liver S9. Triphenyl phosphate was negative in these tests, and the highest ineffective dose level tested (not causing the formation of a precipitate) in any Salmonella tester strain was 1000 ug/plate. [2]. ¨ 4 AMES tests were negative [3] and WHO conclude that TPP is not mutagenic [10]. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer Review |
No IARC evaluation. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
One study concludes that TPP is not a development toxicant in rats [3]. The NOAEL on mothers and offspring from a 90-day rat study was terminated at 690 mg/kg per day [10]. |
|
|
Teratogenicity |
No relevant data found. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
TPP is poorly absorbed through the intact skin but readily through guinea pig skin [2]. Application of TPP on skin of rats as well as application of TPP in ethanol solution on skin of mice caused no skin irritation which leads to the conclusion that since cholinesterase is not inhibited after application, there is no dermal absorption. [2]. |
|
|
Ecotoxicity Data |
||
|
Algae |
Ankistrodesmus falcatus: Scenedesmus quadricauda Selenastrum capricornutum: |
|
|
Crustacean |
Daphnia magna Gammarus pseudolimnaeus: Mysidopsis bahia : |
|
|
Fish |
Carassius auratus (fw): Cyprinodon variegatus (fw): Lepomis macrochirus (fw): Menidia beryllina (sw): |
|
|
Oryzias latipes (fw): Onchorhynchus mykiis (fw): Pimephales promelas (fw) |
||
|
Fish |
Salmo gairdneri (fw) Rainbow trout (fw): |
|
|
Other aquatic organisms |
Chironomus tentans Chironomus riparius |
|
|
Bacteria |
Not available |
|
|
Environmental Fate |
||
|
BCF |
Oryzias latipes (fw) Phoxinus phoxinus (fw) Pimephales promelas (fw) Salmo gairdneri (fw): Oncorhynchus mykiis (fw): |
|
|
Aerobic biodegradation |
¨ Triphenyl phosphate biodegrades under aerobic conditions (half-life of 4 days or less) in water. However, biodegradation in benthic sediments is unclear. If released to soil, biodegradation will be the predominant fate process and aqueous hydrolysis may be important in alkaline soils. Biodegradation is expected to be the dominant fate process of triphenyl phosphate in soil; screening tests exhibited aerobic half-lives of about 4 days or less in natural waters [2]. Half-life in killifish=5h [3]. Half-life in goldfish>100h [3] ¨ Percent degraded (20d)=93.8 % (OECD
303A) [3] BOD7=61.9% BODth, adap. sludge inoc. [3] |
|
|
Anaerobic biodegradation |
No relevant data |
|
|
Metabolic pathway |
No relevant data |
|
|
Mobility |
¨ Koc=3,100 [3] Kd (silty clay)=21.52 [3] ¨ Mobility of TPP and its primary degradation product in soil was very low. It was strongly absorbed to the soil [3]. Kp=112 ± 26.8 [3] |
|
|
Conclusion |
||
|
Health |
The available data indicate that TPP has relatively low impact on health. TPP can induce skin sensitisation and contact demartitis in humans. Based on the available data TPP is not neurotoxic or mutagenic. Persons with preexisting neuromuscular disorders may be at increased risk. |
|
|
Environment |
The literature reviewed indicates that TPP is very toxic to algae, fish and some crustaceans (typical L(E)C50<1 mg/l). The compound is toxic to D. magna. NOEC data available for fish are in the range 0.014 - 0.23 mg/l. Bioaccumulation of this compound is high (BCF >100). The biodegradation data available indicates that this compound is readily to inherently biodegradable under aerobic conditions. No data is available for anaerobic degradation. Mobility of TPP and its primary degradation product in soil is very low. |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
3 |
IUCLID CD rom, European Commission, C 1996. |
|
|
4 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
5 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
6 |
RTECS. Online search December 1999. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
World Health Organization: IPCS Environmental Health Criteria 111 - Triphenyl Phosphate, Geneva, 1991. |
|
|
11 |
ECOTOX AQUIRE. ECOTOX database system. United States
Environmental Protection Agency. Online search December 1999.: |
|
|
CAS number: 1330-78-5
Data compilation, environmental and health screening
Summary
|
|
Health: In the available data there are indications that the investigated tricresyl phosphate is toxic by absorption through the skin. This substances seams not to be mutagenic or carcinogenic. Tricresyl phosphate might be connected with effects on the reproduction. This particular tricresyl phosphate has no classification. The main commercial product is a mixture of various isomers of tricresyl phosphates. Two other tricresyl phosphates (not 1330-78-5) are classified toxic or harmful. Environment: The available effect data originates from tests performed using either the pure compound or a formulation. The tests performed using the pure compound indicates that tricresyl phosphate are very toxic to fish and toxic to algae and crustaceans (L(E)C50 from <1 mg/l to 10 mg/l). Formulations are slightly less toxic, but typically in the 1-10 mg/l range. A study of long term acute and chronic effects in fish showed NOECs from 0.0001-0.00032 mg/l for a formulated product. Tricresyl phosphate bioaccumulates (BCF ranges from 165-281). Available screening studies suggest that aerobic biodegradation will occur at moderate to rapid rates with half-lives in the order of several days or less. The mobility in soil is presumably low. |
|
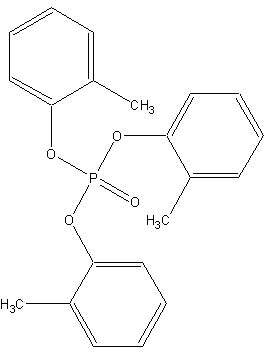
Tricresyl Phosphate |
|||
|
Identification of the substance |
|||
|
CAS No. |
1330-78-5 |
||
|
EINECS No. |
215-548-8 |
||
|
EINECS Name |
tris(methylphenyl) phosphate |
||
|
Synonyms |
Celluflex TPP; Disflamoll TP; Phosflex TPP; TPP; Trifenylfosfat (Czech); Triphenyl phosphate; Celluflex 179C; Cresyl phosphate; Disflamoll TKP; Durad; Flexol Plasticizer TCP; Fyrquel 150; IMOL S 140; Kronitex; Lindol; NCI-C61041; Phosflex 179A; Phosphate de tricresyle (French); Tricresilfosfati (Italian); Tricresylfosfaten (Dutch); Tricresyl phosphate; Trikresylfosfat (Czech); Trikresylphosphate (German); Tris(tolyloxy)phosphine oxide; Tritolylfosfat (Czech); Tritolyl phosphate [5] |
||
|
Molecular Formula |
C21H21O4P |
||
|
Structual Formula |
|
||
|
Known uses |
This compound is used as a plasticizer in vinyl plastics manufacturing, a flame-retardant, a solvent for nitrocellulose and in cellulose molding compositions. It is also used as an additive to extreme-pressure lubricants, as a nonflammable fluid in hydraulic systems, as a lead scavenger in gasoline, to sterilize certain surgical instruments, in polystyrene, in waterproofing, in common organic solvents and thinners, in linseed oil, in china wood oil and in castor oil. |
||
|
EU |
¨ Classification on annex 1 in Directive 67/548/EØF and its revisions: None, but within the family of tricresyl phosphates, two CAS No. (78-30-8 and 78-32-0) are classified toxic (T, N with R 39/23/24/25-51/53) and harmful (Xn;R21/22 N;R51/53) respectively. ¨ Commercial tricresyl phosphates normally contain a mixture of isomers of tricresyl phosphate which can have influence on the final classification. |
||
|
Physico-chemical Characteristics |
|||
|
Physical Form |
Practically colourless and odourless liquid [4]. |
||
|
Molecular Weight |
368.37 |
||
|
Melting Point/range (° C) |
-33 [8] |
||
|
Boiling Point/range (° C) |
420 [4], 265 [8] |
||
|
Decomposition Temperature (° C) |
No relevant data found |
||
|
Vapour Pressure (mm Hg(° C)) |
6´ 10-7 at 25 °
C, estimated [4] |
||
|
Density |
Density=1.16 [3], Specific gravity= 1.247g/cm3 |
||
|
Vapour Density (air=1) |
12.7 [1] |
||
|
Solubility (water) |
0.36 mg/l [4,8] |
||
|
Partition Coefficient (log Pow) |
5.11 [4,8] |
||
|
pKa |
No relevant data found |
||
|
Flammability |
No relevant data found |
||
|
Explosivity |
May burn, but does not ignite readily |
||
|
Oxidising properties |
No relevant data found |
||
|
Toxicological Data |
|||
|
Observation in humans |
Toxic by ingestion in humans [4]. |
||
|
Acute Toxicity |
|||
|
Oral |
Oral-rat LD50: 3,500 mg/kg [5]. Oral-rat LD50: 5,190 mg/kg [3]. Oral-muse LD50: 1,320 mg/kg [5]. Oral-mouse LD50: 3,900 mg/kg [3]. Oral-dog, adult LDLo: 500 mg/kg [3]. Oral-rabbit, adult LDLo: 100 mg/kg [3]. |
||
|
Dermal |
¨ Toxic by skin absorption [4]. Skin-rabbit LD50: >7,900 mg/kg [5]. Skin-guinea pig LD50: >4 gm/kg [5]. Skin-cat, adult LD50: 1,500 mg/kg [3]. |
||
|
Inhalation |
No relevant data found |
||
|
Other Routes |
No relevant data found |
||
|
Skin Irritation |
No relevant data found |
||
|
Eye Irritation |
No relevant data found |
||
|
Irritation of Respiratory Tract |
No relevant data found |
||
|
Skin Sensitisation |
No relevant data found |
||
|
Sensitisation by Inhalation |
No relevant data found |
||
|
Subchronic and Chronic Toxicity |
|||
|
Observation in humans |
No relevant data found |
||
|
Oral |
No relevant data found |
||
|
Inhalation |
No relevant data found |
||
|
Dermal |
Repeated dermal application of 128 mg/kg body weight of tricresyl phosphate every other day on up to 83 occasions on pig skin has been shown to produce total, irreversible paresis, but without development of the clinical signs associated with organophosphorus compound poisoning [4]. |
||
|
Genotoxicity and Carcinogenicity |
|||
|
Mutagenicity |
¨ Tricresyl phosphate was not mutagenic in Salmonella typhimurium strains TA98, TA100, TA1535, or TA1537, nor did it induce chromosomal aberrations or sister chromatid exchanges in cultured Chinese hamster ovary cells. These in vitro assays were all conducted with and without exogenous metabolic activation. [7]. |
||
|
Gene Mutation |
No relevant data found |
||
|
Chromosome Abnormalities |
No relevant data found |
||
|
Other Genotoxic Effects |
No relevant data found |
||
|
Cancer Review |
¨ In 2-year feeding studies there were no evidence of carcinogenic effects of tricresyl phosphate in male or female F344/N rats treated at 75, 150, or 300 ppm. There was no evidence of carcinogenic activity of tricresyl phosphate in male or female B6C3F1 mice treated at 60, 125, or 250 ppm. [7]. |
||
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
|||
|
Reproductive Toxicity |
¨ The reproductive effects of tricresyl phosphate (TCP) were investigated in Long Evans rats . Twelve male rats/dose group were given 0, 100, or 200 mg/kg TCP in corn oil (10 ml/kg body wt) by gavage once/day, 7 days/wk for 56 days prior to breeding and throughout the 10 day breeding period. Twenty four female rats/dose group received 0, 200, or 400 mg/kg TCP in corn oil (10 ml/kg body wt) for 14 days prior to breeding, and throughout breeding, gestation and lactation until the pups were weaned on day 21. |
||
|
Reproductive Toxicity cont. |
Control groups were given corn oil only. The results show that male rats treated with 200 mg/kg TCP had reduced sperm concentration, motility, and velocity (65, 4, and 5% of control, respectively). There was a dose-dependent increase in abnormal sperm morphology in both the 100 mg/kg and 200 mg/kg treated males. TCP did not have an adverse effect on mean testicular wt, but epididymal weights were reduced in the 200 mg/kg dose group males. The % of sperm-positive females for TCP-exposed pairs was not different from that of controls. [4]. |
||
|
Teratogenicity |
No relevant data found |
||
|
Other Toxicity Studies |
No relevant data found |
||
|
Toxicokinetics |
No relevant data found |
||
|
Ecotoxicity Data |
|||
|
Algae |
Anacystis aeruginosa Chlorella pyrenoidosa: Scenedesmus pannonicus: Selenastrum capricornutum: Stephanodiscus hantzschii: Euglena gracilis: |
||
|
Crustacean |
Daphnia magna: |
||
|
Fish |
Brachydanio rerio (fw): Gasterosteus aculeatus (fw): |
||
|
Fish cont. |
Gasterosteus aculeatus (fw): Ictalurus punctatus (fw): Jordanella floridae (fw): Lepomis macrochirus (fw): Menidia beryllina (fw): Oncorhynchus mykiss (fw): Oryzias latipes(fw): Perca flavescens (fw): Poecilia reticulata (fw): Poecilia reticulata (fw) cont.: |
||
|
Other aquatic organisms |
No relevant data found |
||
|
Bacteria |
No relevant data found |
||
|
Environmental Fate |
|||
|
BCF |
¨ BCF=165 (F) [10] |
||
|
Aerobic biodegradation |
¨ Available screening studies suggest that aerobic biodegradation will occur at moderate to rapid rates with half-lives in the order of several days or less [4] |
||
|
Anaerobic biodegradation |
Biodegradation under anaerobic conditions is unclear [4] |
||
|
Metabolic pathway |
No relevant data found. |
||
|
Mobility |
¨ Koc=7,700-79,000,
estimated [4] ¨ Kd=400 [4] |
||
|
Conclusion |
|||
|
Health |
The available data indicate that following repeated application tricresyl phosphate is toxic by absorption through the skin. Available data do not indicate mutagenic or carcinogenic effects of tricresyl phosphates. Tricresyl phosphate may cause effects on the reproduction. This particular tricresyl phosphate has no classification. The main commercial product is a mixture of various isomers of tricresyl phosphates. Two other tricresyl phosphates (not 1330-78-5) are classified toxic or harmful. |
||
|
Environment |
The available effect data originates from tests performed using either the pure compound or a formulation. The tests performed using the pure compound indicates that tricresyl phosphate are very toxic to fish and toxic to algae and crustaceans (L(E)C50 from <1 mg/l to 10 mg/l). Formulations are slightly less acutely toxic, but typically in the 1-10 mg/l range. A study of long term acute and chronic effects in fish showed NOECs from 0.0001-0.00032 mg/l for a formulated product. Tricresyl phosphate bioaccumulates (BCF ranges from 165-281). Available screening studies suggest that aerobic biodegradation will occur at moderate to rapid rates with half-lives in the order of several days or less. The mobility in soil is presumably low. |
||
|
References |
|||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
||
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
||
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
||
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
||
|
5 |
RTECS. Online search December 1999. |
||
|
6 |
IUCLID CD rom, European Commission, C 1996. |
||
|
7 |
Toxline. Online search December 1999. |
||
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
||
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
||
|
10 |
ECOTOX AQUIRE. ECOTOX database system. United States
Environmental Protection Agency. Online search December 1999.: |
||
|
Resorcinol bis(diphenyl phosphate)
CAS number: 57583-54-7
Data compilation, environmental and health screening |
|
Summary
Health: The health screening of the substance show an inadequate data set. In the reviewed studies there were no adverse effects on reproductive performance or fertility parameters associated with administration of the substance in the diet. In these studies the substance did not result in any biologically significant toxic or teratogenic effect in the foetuses. Environment: No data available. |
|
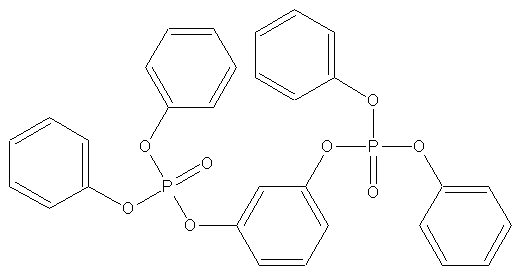
Resorcinol bis(diphenyl phosphate) |
||
|
Identification of the substance |
||
|
CAS No. |
57583-54-7 |
|
|
EINECS No. |
260-830-6 |
|
|
EINECS Name |
tetraphenyl m-phenylene bis(phosphate) |
|
|
Synonyms |
CRR-733S; Fyrolflex RDP; Mark PFK; Oligomeric phosphate ester; m-Phenylenebis(diphenyl phosphate); 1,3-Phenylene tetraphenyl phosphate; PMN 89-234; Resorcinol bis(diphenyl phosphate); Tetraphenylresorcinol diphosphate. |
|
|
Molecular Formula |
|
|
|
Structual Formula |
C30H24O8P2 |
|
|
Known Uses |
The substance is used as a flame retardant and is a component of certain plastics. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its updates: None |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
No relevant data found |
|
|
Molecular Weight |
No relevant data found |
|
|
Melting Point/range (° C) |
No relevant data found |
|
|
Boiling Point/range (° C) |
No relevant data found |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found |
|
|
Relative Density |
No relevant data found |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Solubility (water) |
No relevant data found |
|
|
Partition Coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
No relevant data found |
|
|
Explosivity |
No relevant data found |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in humans |
No relevant data found |
|
|
Acute Toxicity |
||
|
Oral |
Oral-rat LD50 >5 mg/kg (5). |
|
|
Dermal |
Skin-rat LD50 >2 mg/kg (5). |
|
|
Inhalation |
Inhalation-rat LC50 >4,860 mg/m3 (5). |
|
|
Other Routes |
No relevant data found. |
|
|
Skin Irritation |
No relevant data found. |
|
|
Eye Irritation |
No relevant data found. |
|
|
Irritation of Respiratory Tract |
No relevant data found. |
|
|
Skin Sensitisation |
No relevant data found. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No relevant data found. |
|
|
Oral |
FyrolflexQ RDP administered for more than 13 weeks and up to the entire life span (F1) resulted in increased liver weights with associated periportal hypertrophy. This change was considered an adaptive process associated with RDP metabolism in the liver. (7). |
|
|
Inhalation |
No relevant data found. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
No relevant data found. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer review |
No relevant data found. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
FyrolflexQ RDP was evaluated in a two-generation reproductive
study as part of a program to assess the overall toxicology of this
flame retardant. RDP was administered to male and female
Sprague-Dawley rats in the diet at concentrations of 1000, 10,000 or
20,000 ppm. The control group was given diet alone. |
|
|
Teratogenicity |
Groups of 27 sperm-positive New Zealand White rabbits (HRP, PA)
were administered graded concentrations of 50, 200 or 1000 mg/kg of
RDP in corn oil. A vehicle control group of equal size was
administered corn oil alone. Rabbits were dosed daily (1.5 mL/kg) on
gestation days 6-28 and sacrificed on gestation day 29. The fetuses
were removed by cesarian section and examined for gross external,
visceral, cephalic and skeletal anomalies. No treatment-related
clinical signs of toxicity were observed. No effects on maternal
food consumption, body weight, body weight gain, or on uterus, liver,
kidney and spleen weights were detected. Fetal viability and body
weight, as well as developmental endpoints were unaffected by the
treatment. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
No relevant data found. |
|
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found |
|
|
Crustacean |
No relevant data found |
|
|
Fish |
No relevant data found |
|
|
Bacteria |
No relevant data found |
|
|
Mobility |
No relevant data found |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found |
|
|
Aerobic biodegradation |
No relevant data found |
|
|
Anaerobic biodegradation |
No relevant data found |
|
|
Metabolic pathway |
No relevant data found |
|
|
Conclusion |
||
|
Health |
The available data are not sufficient to prepare a health screening of the substance. In the reviewed studies there were no adverse effects on reproductive performance or fertility parameters associated with administration of the substance in the diet. In these studies the substance did not result in any biologically significant toxic or teratogenic effect in the fetuses. |
|
|
Environment |
No data available. |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
Phosphonic acid, (2-((hydroxymethyl)carbanyl)ethyl)- dimethylester
CAS number: 20120-33-6
Data compilation, environmental and health screening |
||
|
Summary
Health: The available data are not sufficient to perform a health screening of the substance. One study reports an oral-LD50 which may indicate potential adverse acute effects at a dose of 13 mg/kg. Environment: The available data is not sufficient to make an environmental screening. Two data sets are available on the toxicity of a formulation to fish. The indication is that this compound is toxic to very toxic to fish. |
||
|
Phosphonic acid, (2-((hydroxymethyl)carbanyl)ethyl)- dimethyl ester |
||
|
Identification of the substance |
||
|
CAS No. |
20120-33-6 |
|
|
EINECS No. |
243-528-9 |
|
|
EINECS Name |
dimethyl [3-[(hydroxymethyl)amino]-3-oxopropyl]phosphonate |
|
|
Synonyms |
N-Methylol dimethylphosphonopropionamide; Phosphonic acid, (3-((hydroxymethyl)amino)-3-oxopropyl)-, dimethyl ester (9CI); Phosphonic acid, (2-((hydroxymethyl)carbanyl)ethyl)- dimethyl ester; Pyrovatex [5] |
|
|
Molecular Formula |
C6H14NO5P [5] |
|
|
Structural Formula |
No relevant data found. |
|
|
Known Uses |
No relevant data found. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None. |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
No relevant data found. |
|
|
Molecular Weight (g/mol) |
211.18 |
|
|
Melting Point/range (° C) |
No relevant data found. |
|
|
Boiling Point/range (° C) |
No relevant data found. |
|
|
Decomposition Temperature (° C) |
No relevant data found. |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found. |
|
|
Relative Density |
No relevant data found. |
|
|
Vapour Density (air=1) |
No relevant data found. |
|
|
Solubility (water) |
No relevant data found. |
|
|
Partition Coefficient (log Pow) |
No relevant data found. |
|
|
pKa |
No relevant data found. |
|
|
Flammability |
No relevant data found. |
|
|
Explosivity |
No relevant data found. |
|
|
Oxidising properties |
No relevant data found. |
|
|
Toxicological Data |
||
|
Observation in Humans |
No relevant data found. |
|
|
Acute Toxicity |
||
|
Oral |
¨ Oral-rat LD50: 13 mg/kg (5). |
|
|
Dermal |
No relevant data found. |
|
|
Inhalation |
No relevant data found. |
|
|
Other Routes |
No relevant data found. |
|
|
Skin Irritation |
No relevant data found. |
|
|
Eye Irritation |
No relevant data found. |
|
|
Irritation of Respiratory Tract |
No relevant data found. |
|
|
Skin Sensitisation |
No relevant data found. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in Humans |
No relevant data found. |
|
|
Oral |
No relevant data found. |
|
|
Inhalation |
No relevant data found. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
One study indicating that the substance has mutagenic effects is reported (5). |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer Review |
No relevant data found. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
No relevant data found. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
No relevant data found. |
|
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found. |
|
|
Crustacean |
No relevant data found. |
|
|
Fish |
Oncorhynchus mykiis (fw): |
|
|
Bacteria |
No relevant data found. |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found. |
|
|
Aerobic biodegradation |
No relevant data found. |
|
|
Anaerobic biodegradation |
No relevant data found. |
|
|
Metabolic pathway |
No relevant data found. |
|
|
Mobility |
No relevant data found. |
|
|
Conclusion |
||
|
Health |
The available data are not sufficient to make a health screening of the substance. One study reports an oral-LD50 which may indicate potential adverse acute effects at a dose of 13 mg/kg. |
|
|
Environment |
The available data is insufficient for an environmental screening. Only data available on the toxicity of a formulation to fish is available(LC50 = 0.56-1.14 ml/l). This indicates that the compound is very toxic to fish. |
|
|
References |
||
|
1 |
Chemfinder: |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
U.S. EPA ECOTOX Database system. AQUIRE On line search December
1999. |
|
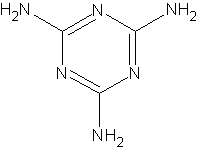
|
CAS number: 21645-51-2
Data compilation, environmental and health screening |
|
Summary
Health: Aluminium hydroxide is often an important source of aluminium in the body. Aluminium compounds can lead to deposition of aluminium in bones leading to decalcification. There are indications that aluminium compounds may lead to lung injuries. Most aluminium compounds may cause irritation of eyes and respiratory tract. Environment: Very few data was found on the compound Al(OH)3. Since the compound may dissociate in the environment a limited data set on the Al-ion is presented. The data on aluminium however indicates that this element is very toxic to fish and toxic to crustaceans. |
|
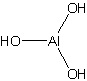
Aluminium trihydroxide |
||
|
Identification of the substance |
||
|
CAS No. |
21645-51-2 |
|
|
EINECS No. |
244-492-7 |
|
|
EINECS Name |
aluminium hydroxide |
|
|
Synonyms |
AF 260; Alcoa 331; Alcoa C 30BF; Alumigel; Alumina hydrated;
Alumina trihydrate; alpha-Alumina trihydrate; Aluminic acid;
aluminium hydroxide; aluminium hydrate; aluminium(III) hydroxide;
aluminium hydroxide gel; aluminium oxide trihydrate; aluminium
trihydrate; aluminium trihydroxide; Alusal; Amberol ST 140F;
Amphojel; BACO AF 260; British aluminium AF 260; C 31; C 33; C 31C;
C 4D; C 31F; C-31-F; C.I. 77002; GHA 331; GHA 332; H 46; Higilite;
Higilite H 32; Higilite H 42; Higilite H 31S; Hychol 705; Hydral
705; Hydral 710; Hydrated alumina; Liquigel; Martinal; P 30BF; PGA;
Trihydrated alumina; Trihydroxyaluminium [4] |
|
|
Molecular Formula |
Al(OH)3 |
|
|
Structural Formula |
|
|
|
Known uses |
Desiccant powder; in packaging materials; chemical intermediate; filler in paper, plastics, rubber, ceramics, in printing inks, lubricating compositions, detergents; iron-free aluminium and aluminium salts and cosmetics; glass additive to increase mechanical strength and resistance to thermal shock; in manufactures of activated alumina; flame retardants, for rubber reinforcing agent, paper coating; adsorbent; emulsifier; ion-exchanger, in chromatography; mordant in dyeing; filtering medium; waterproofing fabrics; used in pharmacy as the gel or dried gel. ¨ Aluminium hydroxide is sometimes used as an antidiarrheal agent, as a slow acting antacid and in protective dermatological pastes |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
White monoclinic crystals, white powder, pellets or granules [4]. |
|
|
Molecular Weight (g/mol) |
77.99 |
|
|
Melting Point/range (° C) |
300 [4] |
|
|
Boiling Point/range (° C) |
No relevant data found. |
|
|
Decomposition Temperature (° C) |
Ca. 150-220 °C decomposition to Al2O3 and H2O. |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found. |
|
|
Density |
Specific: Relative: |
|
|
Vapour Density (air=1) |
No relevant data found. |
|
|
Solubility (water) |
Insoluble in water [4] |
|
|
Partition Coefficient (log Pow) |
No relevant data found. |
|
|
pKa |
No relevant data found. |
|
|
Flammability |
No relevant data found. |
|
|
Explosivity |
Not explosive [6] |
|
|
Oxidising properties |
No oxidising properties [6] |
|
|
Toxicological Data |
||
|
Observation in humans |
¨ Aluminium hydroxide is one of the main sources to aluminium in the body. The implications of previous reports of elevated aluminium concentration in patients with Alzheimer's disease for the treatment of the disease are disused. At the present time there is no conclusive evidence that active attempts to alter aluminium concentration in diet or medicines produce any beneficial effect in Alzheimer's disease. [4]. |
|
|
Acute Toxicity |
||
|
Oral |
Because aluminium is only sparingly absorbed from the gut, LD50 values for aluminium ingestion are unavailable, since death occurs from intestinal blockage due to precipitated aluminium species rather than systemic aluminium toxicity [4]. The only LD50 value (>5000 mg/kg bw) found supports this [6]. Antacids including aluminium hydroxide may inhibit the gastrointestinal absorption of some beta-blockers [4]. |
|
|
Dermal |
No relevant data found. |
|
|
Inhalation |
Animal studies show that aluminium particles, in particular stamped aluminium powder, may cause fibrosis of the lung whereas particles of aluminium compounds appear to be less reactive [4]. ¨ On occasion workers chronically exposed to aluminium-containing dusts or fumes have developed severe pulmonary reactions [4]. |
|
|
Other Routes |
Aluminium salts are much more toxic intravenously than by mouth to animals [4]. |
|
|
Skin Irritation |
Not irritating [6]. |
|
|
Eye Irritation |
¨ One study indicates that aluminium hydroxide is not an eye irritant [6], but aluminium (dust or powder) is an eye irritant [4]. |
|
|
Irritation of Respiratory Tract |
¨ Aluminium (dust or powder) is a respiratory irritant [4]. Aluminium compounds appear to be less reactive [4]. |
|
|
Skin Sensitisation |
Not sensitising [6]. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
There has been and still is much dispute about aluminium's influence on CNS, e.g. the development of Alzheimer syndrome. Although aluminium is common in nature, and the exposure therefore rather comprehensive, the amount found in humans is rather limited [6]. |
|
|
Oral |
Severe aluminium intoxication following oral administration of aluminium hydroxide, chloride, or sulphate to rats is characterised by anorexia or death [4]. The effects of dietary administration of aluminium hydroxide were examined in male Sprague-Dawley rats. Groups of 25 rats were fed a diet containing 14,470 ppm aluminium hydroxide or a control diet for 28 days. The mean daily aluminium dose was calculated as 302 mg/kg body weight/day. Dietary administration of aluminium hydroxide did not induce any signs of toxicity. Clinical observations during the 28-day treatment period and the recovery phase were similar in control and treated rats. There were no significant changes in haematology, clinical chemistry parameters, or organ weights. Histopathological examination of tissues revealed no treatment-related changes. Ingestion of aluminium hydroxide caused no significant deposition of Al in bone samples. [4]. |
|
|
Inhalation |
No relevant data found. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
¨ Aluminium compounds have been evaluated as non-mutagenic by most standard methods of mutagenic assays. [4]. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer review |
No relevant data found. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
¨ In one study, concentrations of aluminium ranging from 500 to 1,000 ug/g body weight were added to the diets of pregnant rats from day 6 to day 19 of gestation, when the fetuses were removed by Caesarean section. Aluminium in the diet did not affect embryo or fetal mortality rate, litter size, fetal body weight, or length. [4]. ¨ 5-16 days' exposure of mice did not lead to material toxicity, embryo/fetal toxicity or teratogenicity [6]. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
Aluminium salts are absorbed in small amounts from the digestive tract and can be deposited in bones [4]. |
|
|
Aluminium hydroxide or oxide is slowly solubilised in the stomach and reacts with hydrochloric acid to form aluminium chloride and water. About 17-30% of the aluminium chloride formed is absorbed and rapidly excreted by the kidneys in patients with normal renal functions. In vitro studies indicate that aluminium hydroxide binds salts with an affinity and capacity similar to that of cholestyramine. Calcium and aluminium salts decrease the absorption of fluoride from the intestinal tract. In studies of humans, Spencer and co-workers demonstrated that ingestion of antacids containing aluminium hydroxide increased fecal excretion of fluoride by as much as 12 times, resulting in decreased absorption and lowered plasma levels of fluoride. [4]. |
||
|
Adults (with renal failure), who ingested 1.5 to 3.0 g aluminium hydroxide per day for 20 to 32 days, absorbed between 100 and 568 mg aluminium per day (7-19% of the dose). Thus, it is quite clear that the administration of large doses of aluminium result in significant systemic absorption of the metal. [4]. |
||
|
Toxicokinetics |
Aluminium hydroxide and aluminium phosphate are some of the least soluble aluminium salts, but both compounds are sources of aluminium exposure. In metabolic studies on six patients, 12% of an oral load of aluminium in the form of a hydroxide was retained, but absorption was not calculated. At least 50% of serum aluminium is bound to proteins, which include both albuminand transferrin. |
|
|
Most of the tissue aluminium stores (about 30-50 mg) reside in bone. Current data indicate that biliary excretion is the major route of excretion, but renal elimination appears more important after large aluminium loads. [4]. Studies have shown that normal persons who consume one of several aluminium salts (eg, hydroxide or carbonate), but not aluminium phosphate readily absorb aluminium from the gastrointestinal tract. [4]. |
||
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found. |
|
|
Crustacean |
Daphnia magna: ¨ A search in [10] on Al resulted in
several values on Daphnia magna and Daphnia pulex
(range): |
|
|
Fish |
Leuciscus idus (fw): ¨ A search in [10] on Al resulted in
one value on Oncorhynchus mykiis: |
|
|
Bacteria |
¨ Pseudomonas putida: |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found. |
|
|
Aerobic biodegradation |
Not relevant for metals. |
|
|
Anaerobic biodegradation |
No relevant for metals. |
|
|
Metabolic pathway |
No relevant data found. |
|
|
Mobility |
No relevant data found. |
|
|
Conclusion |
||
|
Health |
Aluminium hydroxide is often an important source of aluminium in the body. Oral ingestion of aluminium compounds can lead to deposition of aluminium in bones. Epidemiological studies indicate that that aluminium compounds may lead to lung injuries. Most aluminium compounds may cause irritation of eyes and respiratory tract. Aluminium compounds have been evaluated as non-mutagenic by most standard methods of mutagenic assays. Aluminium in the diet did not affect a number of teratogenic parameters in mice or rats (dose 500-1000 ug/g). |
|
|
Environment |
Very few data was found on the compound Al(OH)3. Since the compound may dissociate in the environment, a limited data set on the Al-ion is presented. The data on aluminium indicates that this element is very toxic to fish and toxic to crustaceans. |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
ECOTOX AQUIRE. ECOTOX database system. United States
Environmental Protection Agency. Online search December 1999.: |
|
|
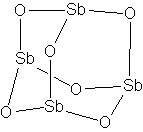
CAS number: 1309-42-8
Data compilation, environmental and health screening |
|
Summary
Health: Only few data are reported on the substance (acute oral toxicity in rat and mouse 8,500 mg/kg). Magnesium is used in pharmaceuticals and food, and short term human exposure to the substance in small quantities is assumed not to affect human health adversely. Repeated or prolonged human exposure to larger quantities of the substance may imply impact on human health, such as malaise and general irritation of skin and respiratory tract. Effects on the central nerve system (CNS) associated with long term exposure and large doses) can not be excluded. Repeated or prolonged human exposure to larger quantities of the substance may imply adverse impact on human health, such as general irritation and malaise. Environment: Very few data was found on the compound Mg(OH)2. The compound may dissociate in the environment in Mg-ion and hydroxide. Magnesium is an essential element in many organisms. One LC50 was identified: 64.7 mg/l, which indicates that magnesium is harmful to crustaceans. |
|
Magnesium hydroxide |
||
|
Identification of the substance |
||
|
CAS No. |
1309-42-8 |
|
|
EINECS No. |
215-170-3 |
|
|
EINECS Name |
Magnesium hydroxide |
|
|
Synonyms |
Magnesium hydrate; Milk of magnesia; Magnesia; Magnesium dihydroxide; Gastrobrom; Gastrogel; Mylanta |
|
|
Molecular Formula |
H2MgO2 |
|
|
Structural Formula |
|
|
|
Known Uses |
Antacid in medicine, alkaline buffer and chemical thickener in food, ingredient in pharmaceuticals, cosmetics, toothpaste, rubber, plastics and adhesives industries. Additive in fuel oil. Chemical intermediate in the production of magnesium chloride and magnesium carbonate. Raw material for the production of magnesium metal. In sugar refining, uranium processing and denetrification. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None. Labelling of metals not developed. |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
White powder [3] |
|
|
Molecular Weight |
58.33 |
|
|
Melting Point/range (° C) |
350 [4] |
|
|
Boiling Point/range (° C) |
No relevant data found |
|
|
Decomposition Temperature (° C) |
350 [3] |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found |
|
|
Density |
Specific gravity=1.573 at 14 ° C [4] |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Conversion Factor |
No relevant data found |
|
|
Solubility (water) |
9 mg/l at 18 ° C [3] |
|
|
Partition coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
No relevant data found |
|
|
Explosivity |
No relevant data found |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in Humans |
¨ The substance is used for
medication mainly as an antacid but also as antidote for poisoning. ¨ Magnesium hydroxide is a general food additive [10,11]. |
|
|
Acute Toxicity |
||
|
Oral |
Intoxication of humans occurring after oral administration of magnesium salts is rare, but may happen in the face of renal impairment [10]. ¨ Oral-rat LD50: 8.500 mg/kg [5]. ¨ Oral-mouse LD50: 8.500 mg/kg [5]. Human ingestion of quantities above normal content in food may be connected with nausea, vomiting and diarrhoea. |
|
|
Dermal |
Relevant data not found. |
|
|
Inhalation |
No relevant experimental data reported, but according to suppliers of the substance it may irritate the respiratory tract on prolonged or repeated contact [11]. |
|
|
Other Routes |
Relevant data not found. |
|
|
Skin Irritation |
¨ No relevant experimental data reported, but according to suppliers of the substance it is indicated that repeated or prolonged contact may cause irritation [11]. |
|
|
Eye Irritation |
¨ No relevant experimental data reported, but according to suppliers of the substance it may irritate or injure the eye [11]. |
|
|
Irritation of Respiratory Tract |
¨ No relevant experimental data reported, but according to suppliers of the substance it may irritate the respiratory tract on prolonged or repeated contact [11]. |
|
|
Skin Sensitisation |
Relevant data not found. |
|
|
Sensitisation by Inhalation |
Relevant data not found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
Intoxication of humans is rare but may happen in case of renal impairment [10]. |
|
|
Oral |
¨ Oral administration of large quantities of magnesium salts may cause central nerve system (CNS) depression [12]. |
|
|
Inhalation |
¨ No relevant experimental data is reported. According to the supplier inhalation of mineral dust over long periods of time may cause industrial bronchitis, reduce breathing capacity and lead to increased susceptibility to other lung diseases [11]. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
No relevant data found. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer review |
¨ No IARC evaluation. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
No relevant data found. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
No relevant data found. |
|
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found. |
|
|
Crustacean |
No relevant data found. Gammarus lacustris (data for Mg) |
|
|
Fish |
No relevant data found |
|
|
Bacteria |
No relevant data found |
|
|
Environmental Fate |
||
|
BCF |
Essential element, not relevant |
|
|
Aerobic biodegradation |
Not relevant for inorganic compounds |
|
|
Anaerobic biodegradation |
Not relevant for inorganic compounds |
|
|
Metabolic pathway |
Essential element. |
|
|
Mobility |
No relevant data found |
|
|
Conclusion |
||
|
Health |
Only few data are reported on the substance (acute oral toxicity in rat and mouse 8,500 mg/kg). Magnesium is used in pharmaceuticals and food, and short term human exposure to the substance in small quantities is assumed not to affect human health adversely. Repeated or prolonged human exposure to larger quantities of the substance may imply impact on human health, such as malaise and general irritation of skin and respiratory tract. Effects on the central nerve system (CNS) associated with long term exposure and large doses) can not be excluded. |
|
|
Environment |
Very few data was found on the compound Mg(OH)2. The compound may dissociate in the environment. Magnesium is an essential element in many organisms. One LC50 was identified: 64.7 mg/l, which indicates that magnesium is harmful to crustaceans. |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
Casarett and Doull: TOXICOLOGY - The Basic Science of Poisons, fourth edition, Pergamon Press, New York, 1991. |
|
|
11 |
Supplier MSDS: |
|
|
12 |
Supplier MSDS |
|
|
13 |
ECOTOX AQUIRE. ECOTOX database system. United States Environmental Protection Agency. Online search December 1999: http://www.epa.gov/ecotox/ecotox_home.htm |
|
|
CAS number: 68333-79-9
Data compilation, environmental and health screening |
|
Summary
Health: No relevant data available. Environment: The available data indicates that this substance may be harmful to crustaceans and possibly toxic to algae (the latter is based on a test with a formulated product). |
|
Ammonium Polyphosphate |
||
|
Identification of the substance |
||
|
CAS No. |
68333-79-9 |
|
|
EINECS No. |
269-789-9 |
|
|
EINECS Name |
Polyphosphoric acids, ammonium salts |
|
|
Synonyms |
No relevant data found |
|
|
Molecular Formula |
No relevant data found |
|
|
Structual Formula |
No relevant data found |
|
|
Known Uses |
In fire-retardant intumescent paints, mastics, and polymers. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
No relevant data found |
|
|
Molecular Weight |
No relevant data found |
|
|
Melting Point/range (° C) |
No relevant data found |
|
|
Boiling Point/range (° C) |
No relevant data found |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found |
|
|
Relative Density |
No relevant data found |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Solubility (water) |
No relevant data found |
|
|
Partition Coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
No relevant data found |
|
|
Explosivity |
No relevant data found |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in humans |
No relevant data found. |
|
|
Acute Toxicity |
||
|
Oral |
No relevant data found. |
|
|
Dermal |
No relevant data found. |
|
|
Inhalation |
No relevant data found. |
|
|
Other Routes |
No relevant data found. |
|
|
Skin Irritation |
No relevant data found. |
|
|
Eye Irritation |
No relevant data found. |
|
|
Irritation of Respiratory Tract |
No relevant data found. |
|
|
Skin Sensitisation |
No relevant data found. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No relevant data found. |
|
|
Oral |
No relevant data found. |
|
|
Inhalation |
No relevant data found. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
No relevant data found. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer review |
No relevant data found. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
No relevant data found. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
No relevant data found. |
|
|
Ecotoxicity Data |
||
|
Algae |
Selenastrum capricornutum (fw) |
|
|
Crustacean |
Daphnia magna Two values are available on a formulation (FIRE-TROL LCG-R) EC50(48h)= 813-848 mg/l [10]. |
|
|
Fish |
Oncorhynchus mykiis (fw): Several values are available on a formulation (FIRE-TROL LCG-R) Low range LC50(96h)= 872-910 mg/l [10]. |
|
|
Bacteria |
No relevant data found |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found |
|
|
Aerobic biodegradation |
No relevant data found |
|
|
Anaerobic biodegradation |
No relevant data found |
|
|
Metabolic pathway |
No relevant data found |
|
|
Conclusion |
||
|
Health |
The available data are not sufficient to make a health screening. |
|
|
Environment |
The available data indicates that this substance may be harmful to crustaceans and possibly toxic to the algae (the latter is based on a test with a formulated product). |
|
|
Mobility |
No relevant data found |
|
|
References |
||
|
1 |
Chemfinder: |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
ECOTOX AQUIRE. ECOTOX database system. United States Environmental Protection Agency. Online search December 1999: http://www.epa.gov/ecotox/ecotox_home.htm |
|
|
CAS number: 7723-14-0
Data compilation, environmental and health screening |
|
Summary Remark Phosphorus exists in many allotropic forms (same molecular structure, different crystal lattice structure). Most data on phosphorus are on the white and yellow forms. Red phosphorus is prepared by heating white phosphorus to 270-300 ° C in the absence of air. The red form of phosphorus is much less reactive than the white and presumably also the yellow form. Health Red phosphorus is often contaminated with white and yellow phosphorus. Therefore information of the two other allotropic forms included. Red phosphorus is not absorbed very well. Lethal dose in mammals 1.4 - 4.8 mg/kg bw (allotropic form not specified). Pure red phosphorus is apparently less harmful than the two other allotropic forms. Inhalation of 4.3 mg/l red phosphorus (1h) or 1.5 mg/l (4 h) killed 9 respectively 2 of 10 rats. The substance is classified as highly flammable and may explode when exposed to heat or by chemical reaction with oxidisers. Red phosphorus can also react with reducing materials and represent a moderate explosion hazard by chemical reaction or on contact with organic materials. It reacts with oxygen and water vapour to produce the toxic phosphine. The abiotic degradation of yellow phosphorus proceeds at ambient temperature. Environment No ecotoxicological data on red phosphorus were located. Yellow phosphorus is very toxic to crustaceans and fish in standard tests (L(E)C50 down to 0.011 mg/l and 0.018 mg/l, respectively)´ |
|
Red Phosphorus |
||
|
Identification of the substance |
||
|
CAS No. |
7723-14-0 (this CAS No. is covering all allotropic forms of elementary phosphorus: red, yellow, white etc.). |
|
|
EINECS No. |
231-768-7 |
|
|
EINECS Name |
Phosphorus |
|
|
Synonyms |
Red phosphorus; Phosphorus, red, amorphous; Phosphorus (yellow); yellow phosphorus; elemental white phosphorus; Exolit-LPKN-WP; Exolit VPK-n 361, Fosforo-Bianco- (Italian), phosphorus, amorphous, red; Rat nip; Bonide Blue Death Rat Killer; White phosphorus; Phosphorus (white); Phosphorus-31; *; *Phosphorus,-white,-molten-, Phosphore-Blanc (French), Phosphorus- (red), Phosphorus (yellow or white); Phosphorus atom; Phosphorous, Yellow/White Black-Phosphorus; *Bonide-Blue-Death-Rat-Killer; Gelber-Phosphor- (German), Rat-Nip, Red-Phosphorus, Tetrafosfor (Dutch), Violet-Phosphorus, Weiss-Phosphor- (German), White-Phosphorus; Yellow-Phosphorus |
|
|
Molecular Formula |
P4 (chain) |
|
|
Structual Formula |
|
|
|
Known uses |
Manufacture of phosphoric acid and other phosphorus compounds, phosphor bronzes, metallic phosphides, additive to semiconductors, electroluminescent coatings, striking surfaces for matches, fertilizers and as flame retardants in polymers. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: F;R11 R16 (Highly flammable; Explosive when mixed with oxidising substances). |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
Exists in three main allotropic forms: white, black, and red. The
same liquid is obtained from all forms on melting. |
|
|
Molecular Weight (g/mol) |
Depending on crystal structure (number of P4 in chains) |
|
|
Melting Point/range (° C) |
>590 [6] |
|
|
Boiling Point/range (° C) |
>400 [6] |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
0.026 at 20 ° C [4] |
|
|
Density |
Density=2.2 g/cm3 [6] |
|
|
Vapour Density (air=1) |
4.77 [4] |
|
|
Solubility (water) |
5 mg/l at 35 ° C [6] |
|
|
Partition Coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
Large quantities of red phosphorus ignite spontaneously and on exposure to oxidising materials [2] |
|
|
Explosivity |
No relevant data found |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in humans |
One case of phosphorus poisoning (effect not specified) in an 18-month old male is reported [4]. ¨ The lowest lethal dose referred to in humans is found to be 1.4 mg/kg [6]. |
|
|
Acute Toxicity |
||
|
Oral |
¨ LD50 Rat oral 3.03 mg/kg, allotropic form not specified [4, 6]. ¨ LD50 Mouse oral 4.82 mg/kg, allotropic form not specified [4, 6]. Elemental red phosphorus is non-volatile, insoluble and thus non-toxic when ingested, unless it is contaminated with traces of yellow phosphorus. Ingestion of such a mixture produces a sensation of warmth or a burning pain in the throat connected to an intense thirst, vomiting, diarrhoea or severe abdominal pain. Worst cases may be severe enough to cause death in 24 to 48 hours, possible because of hepatic failure, central nervous system damage or renal insufficiency [4]. Elemental yellow phosphorus is highly toxic. The acute fatal dose in adults is 15 to 100 mg (1 mg/kg), although survival has occurred after ingestion exceeding 1 g. The fatality rate varies between 20% and 50%. [4]. White phosphorus is extremely poisonous and can cause "phossy jaw", a disease caused by phosphorus fumes that are inhaled or absorbed through cavities in the teeth and then attack and destroy bones, particularly the jaw bone. Phossy jaw is usually fatal. [4]. |
|
|
Oral cont. |
The mortality rate of acute phosphorus (allotropic form not
specified) poisoning is approximately 25% for victims who had early
symptoms of nausea and vomiting, nearly 50% when both
gastrointestinal and CNS symptoms were present, and almost 75% when
the first manifestation of poisoning was restlessness, irritability,
or coma. Most likely this difference in survival rates reflects the
interval between time of ingestion and treatment. |
|
|
Dermal |
¨ LD50 for dermal application on rats is found to be 100 mg/kg bw, allotropic form not specified [6]. ¨ A skin contact produces painful penetrating of second and third degree burns, which heal slowly [4]. |
|
|
Inhalation |
¨ 9 out of 10 rats died when exposed for 1 hour to 4.3 mg/l red phosphorus, and 2 out of 10 died when exposed to 1.5 mg/l for 4 hours [6]. Inhalation of phosphorus vapour (allotropic form not specified) by rabbits for 30 min daily at a concentration of 150-160 mg/m3 led to decreased haemoglobin counts [4]. Rabbits and rats were exposed to single doses of smoke from pyrotechnic mixtures containing red phosphorus. The survivors were observed for up to 14 days. Most of the histological changes observed were found in the respiratory tract, including abnormalities in the alveolitis and, in a few cases, frank pneumonia. [4]. Mice and rats were exposed to the smoke produced by ignition of a red phosphorus pyrotechnic composition, 1 hour/day, 5 days/week, at two different dose levels, together with controls. The mice received 180 exposures, while the rats received 200 exposures. Guinea pigs also underwent 200 exposures at the lower concentration, but all animals exposed at the higher concentration died during or immediately after the first dose. Growth of the test groups of mice and rats was depressed during the exposure period. Organ specific toxicity appeared not to be present in rats and was generally confined to the respiratory tract of the mice and the guinea pigs. [4]. |
|
|
Other Routes |
Rats injected subcutaneously with 0.05 mg/kg of yellow phosphorus per day developed bone changes after administration of 50 mg. [4]. Subcutaneous injection of 0.2-0.4 mg/kg/day (allotropic form not specified) in dogs caused delayed deaths within a few days [4]. |
|
|
Skin Irritation |
The substance (allotropic form not specified) is corrosive [6]. |
|
|
Eye Irritation |
The substance (allotropic form not specified) irritates eyes [4]. |
|
|
Irritation of Respiratory Tract |
Inhalation of more than 20 ppm phosphorus (allotropic form not specified) vapours by rats (7 hours/day, 5 days/week) resulted in severe respiratory irritation [4]. |
|
|
Skin Sensitisation |
No relevant data found. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No relevant data found. |
|
|
Oral |
The most important manifestation of chronic phosphorus (allotropic form not specified) poisoning is osteomyelitis of the jaw bones ("phossy jaw"), which commonly begins as a dental disturbance. [4]. |
|
|
Inhalation |
One inhalation study with an exposure period of 1-4 days and a dose level from 0 to 5.9 mg/kg bw (allotropic form not specified ) showed for highest dose group minor effects on the respiratory system, such as irritation in the nose. [6]. |
|
|
Dermal |
No relevant data found. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
No relevant data found. |
|
|
Gene Mutation |
No relevant data found. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer review |
No relevant data found. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
Two studies on oral ingestion of yellow phosphorus in rats showed no signs of teratogenicity [6]. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
Elemental yellow phosphorus is well absorbed from the skin and
gastrointestinal tract. The lung and gut excrete yellow phosphorus,
but little elimination occurs via the kidneys. In the body, phosphorus (allotropic form not specified) is converted to phosphates. It appears that it is metabolized to hypophosphoric acid via oxidation. [4]. |
|
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found |
|
|
Crustacean |
Yellow phosphorus: |
|
|
Crustacean cont. |
Yellow phosphorus: Chironomus tentans: Asellus militaris: A search in [9] resulted in several values on crustaceans. The allotropic form of phosphorus was however not specified for any of these values. |
|
|
Fish |
Yellow phosphorus: Lepomis macrochirus (fw): Onchorhynchus mykiis (fw): Pimephales promelas (fw): A search in [9] resulted in several values on fish. The allotropic form of phosphorus was however not specified for any of these values. The data therefore contains no useable information. |
|
|
Bacteria |
No relevant data found |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found. |
|
|
Aerobic biodegradation |
No relevant data found. |
|
|
Anaerobic biodegradation |
No relevant data found. |
|
|
Metabolic pathway |
No relevant data found. |
|
|
Abiotic transformation |
¨ Yellow phosphorus: |
|
|
Mobility |
No relevant data found |
|
|
Conclusion |
||
|
Health |
Red phosphorus is often contaminated with white and yellow phosphorus. Therefore information of the two other allotropic forms included. Red phosphorus is not absorbed very well. Lethal dose in mammals 1.4 - 4.8 mg/kg bw (allotropic form not specified). Pure red phosphorus is apparently less harmful than the two other allotropic forms. Inhalation of 4.3 mg/l red phosphorus (1h) or 1.5 mg/l (4 h) killed 9 respectively 2 of 10 rats. The substance is classified as highly flammable and may explode when exposed to heat or by chemical reaction with oxidisers. Red phosphorus can also react with reducing materials and represent a moderate explosion hazard by chemical reaction or on contact with organic materials. It reacts with oxygen and water vapour to produce the toxic phosphine. The abiotic degradation of yellow phosphorus proceeds at ambient temperature. |
|
|
Environment |
No ecotoxicological data on red phosphorus were located. Yellow phosphorus is very toxic to crustaceans and fish in standard tests (down to 0.011 mg/l and 0.018 mg/l, respectively) |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
8 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
9 |
ECOTOX AQUIRE. ECOTOX database system. United States Environmental Protection Agency. Online search December 1999: http://www.epa.gov/ecotox/ecotox_home.htm |
|
|
Zinc Borate
CAS number: 1332-07-6
Data compilation, environmental and health screening |
|
Summary
Health: There is not sufficient data to make a complete health screening of zinc borate. Boric acid can be formed, if zinc borate gets in contact with water, e.g. body fluids. By skin contact there is a risk of formation of boric acid which can irritate skin and eyes. Boric acid is suspected of having effects on the unborn child. Inhalation of zinc borate dust may cause irritation of the respiratory tract. Environment: No data was found on the compound ZnO(B2O3)2. Using data disodium tetraborate (CAS number:1330-43-4) is not harmful to crustaceans or fish based on a limited data set. The Zn-ion is very toxic in aquatic standard test (acute effects < 1 mg/l). This approach is based on the assumption that the total toxicity of zinc borate originates from the boric acid and zinc ion formed upon dissolution. |
|
Zinc Borate |
||
|
Identification of the substance |
||
|
CAS No. |
1332-07-6 |
|
|
EINECS No. |
215-566-6 |
|
|
EINECS Name |
Boric acid, zinc salt |
|
|
Synonyms |
Borax-2335-, Boric-acid,-zinc-salt-, ZB-112-, ZB-237-, ZN-100- |
|
|
Molecular Formula |
ZnO(B2O3)2 |
|
|
Structural Formula |
No data |
|
|
Known Uses |
Antimicrobial in cosmetics [10,11]. Fire retardant for PVC, cellulose and unsaturated halogenated polyesters; fireproofing textiles; synergist with antimony oxide and aluminium trihydrate [4]. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None. Denmark has proposed to EU that boric acid should be classified as Rep3;R63 - possible risk of harm to the unborn child. |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
White, amorphous powder [4] |
|
|
Molecular Weight |
383.41 [4] |
|
|
Melting Point/range (° C) |
980 ° C |
|
|
Boiling Point/range (° C) |
No relevant data found |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
No relevant data found |
|
|
Relative Density |
3.64 and 4.22 [4] |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Conversion Factor |
No relevant data found |
|
|
Solubility (water) |
¨ Slightly soluble in water, 0.3% (3 g/l) in water at 20 ° C [4]. The solubility of the Na-salt of boric acid in water at 20° C is 47.1 g/l. |
|
|
Partition Coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
May burn, but does not ignite readily |
|
|
Explosivity |
May polymerise explosively when heated. |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in humans |
No relevant data found for Zinc Borate. |
|
|
Acute Toxicity |
||
|
Oral |
No acute toxicity on Zinc Borate. Zinc toxicity from ingestion is uncommon but gastrointestinal distress and diarrhea have been reported. Human ingestion of 12 g of elemental zinc over a two-day period did not lead to any evidence of hematological, hepatic or renal toxicity [12]. The LD50 for Boric Acid seams be between 2,000- and 3,500 mg/kg. Studies have indicated that the substance may cause effects on the Central Nerve System (CNS). |
|
|
Dermal |
No relevant data found for Zinc Borate. |
|
|
Inhalation |
Inhalation of dust may irritate nose and throat [4]. |
|
|
Other Routes |
No relevant data found for Zinc Borate. |
|
|
Skin Irritation |
¨ Contact with skin causes irritation [4]. |
|
|
Eye Irritation |
¨ Contact with eyes causes irritation [4]. |
|
|
Irritation of Respiratory Tract |
No relevant data found for Zinc Borate. |
|
|
Skin Sensitisation |
No relevant data found for Zinc Borate. |
|
|
Sensitisation by Inhalation |
No relevant data found for Zinc Borate. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No relevant data found for Zinc Borate. |
|
|
Oral |
No relevant data found for Zinc Borate. |
|
|
Inhalation |
No relevant data found for Zinc Borate. |
|
|
Dermal |
No relevant data found for Zinc Borate. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
No relevant data found for Zinc Borate. |
|
|
Gene Mutation |
No relevant data found for Zinc Borate. |
|
|
Chromosome Abnormalities |
No relevant data found for Zinc Borate. |
|
|
Other Genotoxic Effects |
No relevant data found for Zinc Borate. |
|
|
Cancer review |
No relevant data found for Zinc Borate. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found for Zinc Borate. |
|
|
Teratogenicity |
Boric Acid is proposed to be classified in EU because of a possible risk of harm to the unborn child. |
|
|
Other Toxicity Studies |
No relevant data found for Zinc Borate. |
|
|
Toxicokinetics |
No relevant data found for Zinc Borate but Zinc itself does not accumulate from continued exposure [12]. |
|
|
Ecotoxicity Data |
||
|
Algae |
No relevant data found Zinc: |
|
|
Crustacean |
Disodium tetraborate: Zinc: |
|
|
Fish |
Disodium tetraborate: Lepomis macrochirus (fw): Zinc: |
|
|
Bacteria |
No relevant data found |
|
|
Other aquatic organisms |
Disodium tetraborate: Zinc: |
|
|
Environmental Fate |
||
|
BCF |
No relevant data found |
|
|
Aerobic biodegradation |
No relevant data found |
|
|
Anaerobic biodegradation |
No relevant data found |
|
|
Metabolic pathway |
No relevant data found |
|
|
Mobility |
No relevant data found |
|
|
Conclusion |
||
|
Health |
There is not sufficient data to make a health screening of zinc borate. Boric acid can be formed, if zinc borate gets in contact with water, e.g. body fluids. The solubility of zinc borate is less than 10% of the solubility of disodium tetraborate. By skin contact there is a risk of formation of boric acid which can irritate skin and eyes. Boric acid is suspected of having effects on the unborn child. Inhalation of zinc borate dust may cause irritation of the respiratory tract. |
|
|
Environment |
No data was found on the compound ZnO(B2O3)2. Disodium tetraborate (CAS number 1330-43-4) is not harmful to crustaceans or fish based on a limited data set. The Zn-ion is toxic to very toxic standard test with crustaceans and fish (acute effects 10 to < 1 mg/l). This approach is based on the assumption that the total toxicity of zinc borate originates from the boric acid and zinc ion formed upon dissolution. |
|
|
References |
||
|
1 |
Chemfinder: http://www.chemfinder.com/cgi-win/cfserver.exe/ |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
http://www.cosmetic-world.com/inci/InciAZI.htm |
|
|
11 |
http://www3.is.eudra.org/INCI/InciAZI.htm |
|
|
12 |
Casarett and Doull: TOXICOLOGY - The basic Science of Poisons, fourth edition, Pergamon Press, USA, 1991. |
|
|
13 |
ECOTOX AQUIRE. ECOTOX database system. United States Environmental Protection Agency. Online search December 1999: http://www.epa.gov/ecotox/ecotox_home.htm |
|
|
CAS number: 108-78-1
Data compilation, environmental and health screening |
|
Summary
Health: Melamine seems to be only mildly toxic if ingested by animals. There is not sufficient data to predict acute toxicity from dermal application in humans. The available test data do not show evidence of irritation, cancer induction or mutageneity by melamine. Based on animal tests it seems there is a risk of formation of stones in the urinary bladder. Dermatitis has been reported from melamine formaldehyde resins and glues. Probably these cases were chiefly due to formaldehyde or intermediate reaction products of formaldehyde. The LD50 for application of melamine on rabbit skin is found in one study to be slightly larger than 1 mg/kg (1 mg/kg implicates a high risk of adverse effects on skin of humans). Environment: The reviewed limited toxicity data show little aquatic toxicity of melamine. A 96h algae EC50 is 940 mg/l, a 21d NOEC for Daphnia 18 mg/l. The available BCF and the pKa values indicate that the bioaccumulation of this compound is low in the natural pH range (pH 6-8). The available biodegradation data indicates that this compound is persistent both under aerobic and anaerobic conditions. |
|
Melamine |
||
|
Identification of the substance |
||
|
CAS No. |
108-78-1 |
|
|
EINECS No. |
203-615-4 |
|
|
EINECS Name |
Melamine |
|
|
Synonyms |
Cymel; 1,3,5-Triazine-2,4,6-triamine; cyanuramide; cyanuric triamide; triaminotriazine; 2,4,6-triamino-1,3,5-triazine; cyanurotriamide; cyanurotriamine; 2,4,6-triamino-s-triazine; s-triaminotriazine; 1,3,5-triazine-2,4,6(1H,3H,5H)triimine; 2,4,6-triamino sym-triazine; AERO; hicophor pr; isomelamine; teoharn; theoharn; virset 656-4; Sym Triaminotriazine |
|
|
Molecular Formula |
C3H6N6 |
|
|
Structural Formula |
|
|
|
Known Uses |
Melamine resins, organic synthesis, leather tanning, melamine resin [2,6]. Food additive [3,4]. Production of high pressure laminate resins, moulding compounds, surface coating, textile and paper treating resins, adhesive resins for gluing lumber and as a flame retardant [4,6]. Production of paint and lacquers [6]. |
|
|
IUCLID |
No data (will reportedly be included in new IUCLID version) |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: None. |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
Monoclinic prisms, colourless or white crystals [4]. |
|
|
Molecular Weight (g/mole) |
126.13 |
|
|
Melting Point/range (° C) |
345 [1], 354 [2], 350 [6] |
|
|
Boiling Point/range (° C) |
Sublimes [1,3] |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg at ° C) |
50 at 315°C [3,4] and 3.6x10-10 at 20 ° C [4] |
|
|
Density |
Specific gravity=1.573 g/cm3at 14 °
C [1] |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Solubility (water) |
3.2 g/l at 20 ° C [6] |
|
|
Partition Coefficient (log Pow) |
-1.14 at 25 ° C (OECD 107) [6]. |
|
|
pKa |
pKa» 5.00 [5,8] |
|
|
Flammability |
Not flammable [6] |
|
|
Explosivity |
Not explosive [6] |
|
|
Oxidising Properties |
No oxidising properties [6] |
|
|
Toxicological Data |
||
|
Observation in Humans |
It is reported that: ¨ Workers engaged in the production of melamine-formaldehyde products had dermatoses. This effect is assessed to be due to irritation [6]. ¨ Workers engaged in the production of melamine and dicyanid-diamide showed symptoms of allergic dermatitis [6]. ¨ Some workers suffered dermatitis on areas of exposed skin in production of fiber-resin composite by impregnation of cellulose fibers with phenol-formaldehyde and melamine-formaldehyde resins [7]. |
|
|
Acute Toxicity |
||
|
Oral |
No data are available on the acute effects of melamine in humans [7]. Oral-rat LD50: 3,100-3,800 mg/kg [6]. Oral-rat (male) LD50: 3,200 mg/kg [3,4]. Oral-rat (female) LD50: 3,800 mg/kg [4]. Oral-mouse LD50: 4,550 mg/kg [6]. Oral-mouse (male) LD50: 3,296 mg/kg [3,4,6]. Oral-mouse (female) LD50: 7,000 mg/kg [4,6]. |
|
|
Dermal |
¨ One study has found a very low value for "Skin-rabbit LD50". This value is just above >1mg/kg. [5]. |
|
|
Inhalation |
Without specifying the time of exposure inhalation-rat LC50 is referred to be 3,248 mg/m3 [5]. |
|
|
Other Routes |
No relevant data found. |
|
|
Skin Irritation |
Melamine is not irritating in Guinea Pigs at solutions of 1% in water [6]. |
|
|
Eye Irritation |
Eyes-rabbit, adult 500 mg/24h [5]. Not irritating [6]. |
|
|
Irritation of Respiratory Tract |
No relevant data found. |
|
|
Skin Sensitisation |
Not a sensitiser [6]. |
|
|
Sensitisation by Inhalation |
No relevant data found. |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No data are available on the subchronic effects of melamine in humans [7]. |
|
|
Oral |
Chronic feeding tests have been carried out on rats over a period of 2 years at a dietary level of 1,000 ppm and on dogs for 1 year at a level of 30,000 ppm. Throughout the study, the general health was not significantly different from that of the controls. At these levels microscopic examination of the tissues revealed no abnormality attributable to the feeding of Melamine. [4]. Body weight gain was depressed in males receiving 6,000 and 12,000 ppm but not in females [6]. When rats where fed with Melamine at a 1.0% level (10,000 ppm) over their life-span, bladder stones with benign papillomata were found in about 1/3 of the animals. These papillomata are interpreted as a typical response of the rat's bladder mucosa to the presence of a foreign body. No disturbance of the nutrition or the general healthy appearance of these animals was noted. [4]. In 2 studies on B6C3F mice and 2 on Fischer 344/N rats effects on bladder after 14 days exposure have been studied. In these studies stones were found in the urinary bladders of most male rats, as a dose-related incidence, and in the bladders of some female rats receiving 15,000 mg/kg or more. Bladder stones were observed in both male and female mice receiving 12,000 mg/kg or more [4,6]. |
|
|
Inhalation |
No relevant data found. |
|
|
Dermal |
¨ Dermatitis has been reported from melamine formaldehyde resins and glues. Probably these cases were chiefly due to formaldehyde or intermediate reaction products of formaldehyde. |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
Oral-rat TDLo: 195 g/kg/2Y [3,5]. Melamine (tested up to 5,550 m g/plate) was not mutagenic to Salmonella typhimurium TA1535, TA1537, TA98 or TA100 in the presence or absence of a metabolic system (S9) from the liver of Aroclor-induced rats or hamsters. [4]. |
|
|
Gene Mutation |
4 Ames test studies were negative in Salmonella typhimurium at concentrations of 0.1-5,000 m g/plate [6]. |
|
|
Chromosome Abnormalities |
No relevant data found. |
|
|
Other Genotoxic Effects |
No relevant data found. |
|
|
Cancer Review |
Inadequate evidence of carcinogenicity in animals. ¨ IARC Cancer Review: Group 3: No data available in humans. The agent is not classifiable as to its carcinogenicity to humans [3,4]. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
No relevant data found. |
|
|
Teratogenicity |
No toxic effect or gross malformation was found in fetuses of pregnant rats injected intraperitoneally with 70 mg/kg bw melamine on gestation days 5 and 6, 8 and 9 or 12 and 13 [4]. |
|
|
Other Toxicity Studies |
No relevant data found. |
|
|
Toxicokinetics |
No relevant data found. |
|
|
Ecotoxicity Data |
||
|
Algae
|
Scenedesmus pannonicus: |
|
|
Crustacean |
Daphnia magna: |
|
|
Fish |
Leuciscus idus (fw): Poecilia reticulata (fw): Jordanella floridae (fw): |
|
|
Bacteria |
Nitrosomonas sp. (inhib. of
ammonium-oxidation): Pseudomonas putida (DIN 38412, part 27): Sludge (ISO 8192) |
|
|
Environmental Fate |
||
|
BCF |
¨ BCF »
15, pH unknown) [6] |
|
|
Aerobic biodegradation |
BOD5 (20 ° C)=0% BODTh,
inoc. unknown [6] ¨ Percent degraded (14 d)<30 %, MITI test [6] Percent degraded (20 d)<20 % TOC, adap. sludge inoc. [6]. ¨ A standard 5 days BOD test of melamine resulted in almost no biochemical oxygen demand. Based on the five day BOD data the author considered melamine to be non-biodegradable [5]. |
|
|
Anaerobic biodegradation |
¨ After up to 28 weeks incubation a nitrification of 0-8.9 % was observed in field study in silty clay loam at a test compound concentration of 0.2 mg/g at 32 ° C [6]. |
|
|
Metabolic pathway |
Pure culture studies of 3 mM melamine samples indicated the degradation pathway of melamine involves the conversion of melamine to ammeline and eventually cyanuric acid. [5] |
|
|
Mobility |
Koc=51 , estimated value, pH unknown [8] Adsorption of melamine to suspended clay sediment was reported from pH 1 to 6.5, with a maximum absorption of 5.00´ 10-4 mols/g at pH 4.0 [4]. |
|
|
Conclusion |
||
|
Health |
The available data lead to the conclusion that melamine seems to be only mild toxic ingested by animals (LD50 >3,000 mg/kg). There is not sufficient data to predict acute toxicity from dermal application in humans. The available data does not show evidence of cancer induction by melamine. Based on animal tests it seems that there is a risk of formation of stones in the urinary bladder. Dermatitis has been reported from melamine formaldehyde resins and glues. Probably these cases were chiefly due to formaldehyde or intermediate reaction products of formaldehyde. The LD50 for application of melamine on rabbit skin is found in one study to be slightly larger than 1 mg/kg (1 mg/kg implicates a high risk of adverse effects on skin of humans). |
|
|
Environment |
The reviewed literature indicates that show little aquatic toxicity. A 96h algae EC50 is 940 mg/l, a 21d NOEC for Daphnia 18 mg/l. From the available BCF and the pKa values indications are available that the bioaccumulation of this compound is low in the natural pH range (pH 6-8). The biodegradation data available indicates that this compound is persistent both under aerobic and anaerobic conditions. |
|
|
References |
||
|
1 |
Chemfinder: |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline: Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
CAS number: 1309-64-4
Data compilation, environmental and health screening |
|
Summary Remark: Antimony trioxide is used in combination with other flame retardants. Health: Antimony trioxide is in the EU classified as "Harmful (Xn)" and must be labelled with the risk-phrase "Possible risk of irreversible effects" (R40) as a possible carcinogen. There are epidemiological indications that antimony trioxide causes dermatitis and has an impact on reproduction in female workers. The substance is reportedly teratogenic in rats. Data from animal experiments seem to indicate that females are more sensitive concerning developing lung eoplasms than males. The overall evaluation from IARC is: ‘Antimony trioxide is probably carcinogenic to humans’. Environment: The toxicity of the substance to algae ranges from harmful to very toxic (EC50 <1 to 67 mg/l). The majority of data is < 1 mg/l. To crustaceans the substance is harmful (EC50 <100 mg/l). Weight-of-evidence indicates that the substance is not harmful to fish. |
|
Antimony trioxide |
||
|
Identification of the substance |
||
|
CAS No. |
1309-64-4 |
|
|
EINECS No. |
215-175-0 |
|
|
EINECS Name |
Diantimony trioxide |
|
|
Synonyms |
Antimony (III) oxide; antimony white; bianitmony trioxide; flowers of antimony; antimonius oxide; antimony peroxide; antimony sesquioxide; antimony oxide; diantimony trioxide; senarmontite; exitelite; weisspiessglanz; A1530; A1582; a1588 lp; AP 50; chemetron fire shield; ci 77052; ci pigment white 11; dechlorane a-o; nyacol a 1530; thermoguard b; thermoguard s; timonox |
|
|
Molecular formula |
Sb4O6 |
|
|
Structual Formula |
|
|
|
Known uses |
Flameproofing of textiles, paper, and plastics (polyvinyl chloride); paint pigments; ceramic opacifier; catalyst; intermediate; staining iron and copper; phosphors; mordant; glass decolorizer [2]. |
|
|
EU |
Classification on annex 1 in Directive 67/548/EØF and its revisions: Carc3;R40 (labelling: Xn; R40) |
|
|
Physico-chemical Characteristics |
||
|
Physical Form |
White, odourless, crystalline powder [2], white cubes [3] |
|
|
Molecular Weight (g/mol) |
583.04 |
|
|
Melting Point/range (° C) |
655 [2], 655 [4], 656 [5], |
|
|
Boiling Point/range (° C) |
1,425 ° C [4], 1,550 ° C at 1,000 hPa [6], 1,550 ° C [3]. |
|
|
Decomposition Temperature (° C) |
No relevant data found |
|
|
Vapour Pressure (mm Hg(° C)) |
1 at 574 ° C [4] |
|
|
Density |
Specific gravity=5.2 g/cm3 (Senarmonite) [2] |
|
|
Vapour Density (air=1) |
No relevant data found |
|
|
Solubility (water) |
Insoluble in water [3], |
|
|
Partition Coefficient (log Pow) |
No relevant data found |
|
|
pKa |
No relevant data found |
|
|
Flammability |
Flammable when exposed to heat or flame [3]. |
|
|
Explosivity |
Moderately explosive when shocked [3] |
|
|
Oxidising properties |
No relevant data found |
|
|
Toxicological Data |
||
|
Observation in humans |
Fifty-one workers (ages 31-54, mean 45.23 years) in an antimony melting plant (worked 9-31 years, mean 17.91 years), were exposed to airborne dust containing up to 88% antimony trioxide and the remainder, antimony pentoxide. Pneumoconiotic changes were seen in the lungs after 1 decade of employment. No systemic changes were seen except for "antimony dermatosis". No massive lung fibrosis was noted. 32 of the 51 exposed developed antimony dermatosis. 35% developed upper airway inflammation, 37% developed chronic bronchitis. [4]. ¨ Female workers exposed to antimony aerosols in an antimony plant experienced a greater incidence of spontaneous abortions than did a control group of unexposed working women. There were higher rates of spontaneous late abortions (12.5 versus 4.1 percent), premature births (3.4 versus 1.2 percent), and gynaecological problems (77.5 versus 56 percent) among female metallurgical workers exposed to antimony aerosols. Antimony concentrations were not specified, but air samples reportedly contained metallic dust, antimony trioxide, and pentasulfide. Weights of the offspring began to lag behind those of control babies at 3 months, and were significantly reduced at 1 year. Blood antimony levels were 10 times those of a corresponding unexposed group of women, and average urinary antimony levels ranged from 2.1 to 2.9 mg versus none detected in the controls. Some values among the 318 tested reached 18.2 mg. Antimony in breast milk was 3.3 +/- 2 mg/l; in placentaltissue, 3.2 to 12.6 mg; in amniotic fluid, 6.2 +/ - 2.8 mg; and in blood 6.3 +/- 3 mg. [4]. Heavy exposure resulted in symptoms such as abdominal cramps, nausea, vomiting, diarrhea, metallic taste, and dyspnea. [4]. Ingestion in humans can cause irritation of the mouth, nose, stomach, and intestines; vomiting, purging with bloody stools; slow pulse, and low blood pressure; slow, shallow breathing; coma, and convulsions sometimes followed by death [4]. |
|
|
Acute Toxicity |
||
|
Oral |
LD50 Rat oral larger than 34,600 mg/kg [4,5,6]. LD50 Rabbit percutaneous larger than 2,000 mg/kg [4]. Rabbits fed daily up to 150 mg/kg for 4 weeks showed no pathologic changes [4]. |
|
|
Dermal |
Skin-rabbit LDLo: 2 mg/kg. |
|
|
Inhalation |
Inhalation in humans causes inflammation of upper and lower respiratory tract [4]. Rats and rabbits exposed to antimony trioxide (90-125 mg antimony trioxide/m3 during 100 h/month) for periods of up to 14 months, developed in addition to pneumonitis, also lipoid pneumonia, fibrous thickening of alveolar walls, and focal fibrosis. Rabbits appeared to be more susceptible than rats. [4]. Guinea pigs exposed to a dust concentration of antimony trioxide of 45.4 mg/m3 of air, for 2 h daily 7 days a week for the first 3 weeks, later for 3 h daily; which corresponded to an estimated daily retention of 1.6 mg. All the animals showed extensive interstitial pneumonitis, and 4 died during the period of exposure. No cardiac lesions, as evidenced by the electrocardiogram, were observed. Fatty degeneration of the liver in 11 out of 15 guinea pigs having 138 or more hours of exposure was recorded. The blood picture showed a decrease in total white cells. [4]. |
|
|
Other Routes |
No relevant data found |
|
|
Skin Irritation |
Contact with skin caused dermatitis [4], and the substance is regarded as moderately irritating [6]. |
|
|
Eye Irritation |
Contact with eyes causes conjunctivitis [4]. |
|
|
Irritation of Respiratory Tract |
Ingestion can cause irritation of the mouth and nose [4]. |
|
|
Skin Sensitisation |
No relevant data found |
|
|
Sensitisation by Inhalation |
No relevant data found |
|
|
Subchronic and Chronic Toxicity |
||
|
Observation in humans |
No relevant data found |
|
|
Oral |
No relevant data found |
|
|
Inhalation |
Three groups of 8 month old Wistar derived rats (90 males and 90 females per group) were exposed by inhalation to either antimony trioxide (time-weighted average (TWA) 45 mg/m3), antimony ore concentrate (TWA 36 +40 mg/m3), or filtered air (controls) for 7 h/day, 5 day/wk, for up to 52 weeks and sacrificed 20 weeks after terminating exposures. The concentration of antimony (Sb) in the lung of male rats (38,300 ug Sb/g) exposed to antimony trioxide was significantly larger than that in female rats (25,000 ug/g) exposed to antimony trioxide. The lung of both male and female rats exposed to antimony trioxide contained significantly more Sb than the lungs of males and females exposed to Sb ore (approximately 5 times larger). ¨ The most significant findings were the presence of lung neoplasms in 27% of females exposed to antimony trioxide and 25% of females exposed to Sb ore concentrate. None of the male rats in any group or the female controls developed lung eoplasms. [4]. |
|
|
Dermal |
No relevant data found |
|
|
Genotoxicity and Carcinogenicity |
||
|
Mutagenicity |
Antimony trioxide produced differential killing in DNA repair-proficient compared to repair-deficient strains of Bacillus subtilis. In a spot test it was not mutagenic to Escherichia coli B/rWP2 or to Salmonella typhimurium TA1535, TA1537, TA1538, TA98 or TA100 (details not given). [4]. In general the substance is less mutagenic than many other metals such as As, Cr and Ni [7]. |
|
|
Gene Mutation |
No relevant data found |
|
|
Chromosome Abnormalities |
No relevant data found |
|
|
Other Genotoxic Effects |
Antimony trioxide is not genotoxic in vivo and does not present a genotoxic hazard to humans [7]. |
|
|
Cancer review |
¨ There is inadequate evidence for the carcinogenicity of antimony trioxide in humans. There is sufficient evidence for the carcinogenicity of antimony trioxide in experimental animals. The overall evaluation is therefore: ‘Antimony trioxide is probably carcinogenic to humans’ (IARC: Group 2B)". [4]. |
|
|
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
||
|
Reproductive Toxicity |
Female rats were exposed by inhalation for 4 hours per day for 1.5-2 months to 0 or 250 mg/m3 antimony trioxide. They were then mated, and exposure continued until days 3-5 before expected delivery. Pregnancy was obtained in 16/24 treated females and in 10/10 controls. Litter size and weight of offspring at birth and weaning were not altered by exposure to antimony trioxide. [4]. |
|
|
Teratogenicity |
¨ Pregnant female rats (six to seven per group) were exposed by inhalation to 0, 0.027, 0.082 or 0.27 mg/m3 antimony trioxide for 24 hours per day for 21 days. Fetal growth and viability were assessed at the end of gestation. Maternal body weight gain was not affected by exposure, but at the high-dose level increased pre- and postimplantation death of embryos was observed. At the mid-dose level, preimplantation loss and fetal growth retardation were evident. [4]. |
|
|
Other Toxicity Studies |
No relevant data found |
|
|
Toxicokinetics |
There is no particular difference in tissue distribution between rabbit and rat, when the animals were fed with 2 percent Sb2O3 in a casein diet. Mean amounts of the antimony ranged from 6.7 to 88 ug/g of tissue in seven samples analyzed. The largest amounts were in the thyroid and adrenal glands, spleen, liver, lung, heart, and kidney. [4]. When rats were administered single oral doses of 200 mg antimony trioxide in 5 ml of water, only 3.24% of the dose was eliminated in the urine. Levels in the feces were not measured. After administration of antimony trioxide in the diet at a concentration of 2% for 8 months, antimony excretion in the feces was much larger than in the urine. [4]. After administration of 2% antimony trioxide to rats in the diet for eight months, very high levels were found in the thyroid, while retention was much lower (in decreasing order) in the liver, spleen, kidney, heart and lungs. After administration of 1% antimony trioxide to rats in the diet for 12 weeks, the highest antimony concentrations were found (in decreasing order) in the blood, spleen, lungs, kidneys, hair, liver and heart; 12 weeks after the end of treatment, levels in the blood, lungs and kidneys had decreased to about 50%, but the spleen still contained about 75% of the concentration observed at the termination of exposure. [4]. Part of the intravenously administered antimony salts is absorbed by erythrocytes, and the rest is distributed to other tissues, predominantly the liver, adrenals, spleen, and thyroid. In rats, trivalent antimony is absorbed by erythrocytes, distributed to other tissues, and retained in the liver for a short time before it is gradually excreted in feces. [4]. |
|
|
Ecotoxicity Data |
||
|
Algae |
Selenastrum capricornutum: |
|
|
Crustacean |
Daphnia magna: |
|
|
Fish |
Brachydanio rerio (fw): Fundulus heteroclitus (sw): Lepomis macrochirus (fw): Pimephales promelas (fw): |
|
|
Other aquatic organisms |
Tubifex tubifex (fw): |
|
|
Bacteria |
Pseudomonas putida: |
|
|
Environmental Fate |
||
|
BCF |
Antimony is possibly an essential element [1] |
|
|
Aerobic biodegradation |
Not relevant, inorganic compound |
|
|
Anaerobic biodegradation |
Not relevant, inorganic compound |
|
|
Metabolic pathway |
No relevant data found |
|
|
Other biotic transformation |
Pure cultural study using Stibiobacter senarmontii, an autotrophic bacterium isolated from antimony ore samples, demonstrated that biological transformation of antimony oxides in the environment could be possible. The bacteria were grown in a mineral medium containing antimony trioxide and oxidised the chemical (antimony trioxide) at rates of 45.5-51.6 mg/month for senarmonite (cubic) and 13.5-19.3 mg/month for valentinite (rhombic). Little antimony trioxide oxidation occurred in the sterile medium. [4] |
|
|
Mobility |
No relevant data found |
|
|
Conclusion |
||
|
Health |
The overall evaluation from IARC is: ‘Antimony trioxide is probably carcinogenic to humans’. Dermatitis and teratogenic effects are observed in animal experiments. Animal experiments also indicate that females are more sensitive with respect to developing lung neoplasms than males. |
|
|
Environment |
The toxicity of the substance to algae ranges from harmful to very toxic (EC50 <1 to 67 mg/l). The majority of data is < 1 mg/l. The available data on crustaceans indicates that the substance is harmful to crustaceans. Weight-of-evidence indicates that the substance is not harmful to fish. |
|
|
References |
||
|
1 |
Chemfinder: |
|
|
2 |
HAWLEY'S CONDENSED CHEMICAL DICTIONARY. Twelfth Edition. Revised by Richard J. Lewis, Sr. CD-rom. Van Nostrand Reinhold Company, New York, 1994. |
|
|
3 |
SAX'S DANGEROUS PROPERTIES OF INDUSTRIAL MATERIALS Eighth Edition on CD-rom. Revised by Richard J. Lewis, Sr. Van Nostrand Reinhold Company, New York, 1994. |
|
|
4 |
Hazardous Substance Data Bank (HSDB). HSDB ACCESSION NUMBER: 2648. UPDATE CODE: 199905. SRP REVIEW DATE: Reviewed by SRP on 1/23/1997. Online search December 1999. |
|
|
5 |
RTECS. Online search December 1999. |
|
|
6 |
IUCLID CD rom, European Commission, C 1996. |
|
|
7 |
Toxline. Online search December 1999. |
|
|
8 |
Environmental Fate Database - CHEMFATE (SRC/Procter and Gamble/EPA). |
|
|
9 |
Environmental Fate Database - BIODEG (SRC/Procter and Gamble/EPA) |
|
|
10 |
U.S. EPA ECOTOX Database system. AQUIRE On line search December
1999. |
|
|
CAS number: not available
Data compilation, environmental and health screening |
|
Summary Remark The lack of data on the compound selected for screening led to the tentative use of available quinidine sulfate test data for the estimation of the toxicity of the quinidine carbonate. Only the summary page is presented. Health Environment The toxicity of quinidine carbonate may tentatively be estimated
from the following data on quinidine sulfate: Daphina magna: The toxicity of quinidine carbonate estimated from the toxicity of quinidine sulfate indicates that quinidine carbonate could be harmful to crustaceans. |
[Front page] [Contents] [Previous] [Next] [Top] |