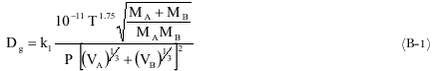Dry deposition and spray drift of pesticides to nearby water bodies
Appendix B. Viscosity of air and water and estimation of diffusivity of pesticides in air and waterDiffusivity of gaseous pesticides in airDiffusivity of pesticides in water Dynamic viscosity and kinematic viscosity of air Viscosity and kinematic viscosity of (sea)water References Diffusivity of gaseous pesticides in airThe diffusivity of a gas in air can be estimated by the method of Fuller, Schettler and Giddings (FSG method) (Lyman et al., 1982). It is most accurate for non-polar gases at low to moderate temperatures. Its accuracy is poorest for polar acids (12%) and glycols (12%) and minimal errors are found for aliphatics (4%) and aromatics (4%) (Lyman et al., 1982). The FSG method is based on the following equation:
where: The subscipts A and B in (B-1) refer to the air and the gaseous pesticide, respectively. MA is 28.97 g mol-1 and VA is 20.1× 10-6 m3 mol-1. VB can be estimated from the chemical structure of the molecule using the increments listed in Table 1:
where ni is the number of atoms of kind i and VB,i is the volume increment cause by atom i. A correction has to be made to the sum in case of aromatic and heterocyclic rings are present. Table 1.
Values in parentheses are based upon few data points (Fuller et al., 1966). For chemicals not listed in Table 1, VB can be estimated as 85-90% of the LeBas volume VB‘ . In the following calculations we have used VB = 0.875VB‘ . VB‘ can be found from Table 2.
The method mentioned above is somewhat complicated. A more simple method was developed here where the diffusivity at one temperature and pressure is estimated from the molecular weight alone. The idea behind this is that the molar volume is proportional to the molecular weight. First the diffusivity was calculated with the FSG method for 11 pesticides with different molecular weights between about 100 and 500 g mol-1. The following expression was found to give a good description for the relation between Dg and MB at 298.15 ° K and 1 atmosphere:
In this equation Dg,298.15, 1 is in m2 s-1 and MB is in g mol-1; k2 is a constant necessary to obtain the right dimensions. Its value is 1.0 and its dimension is
It would be possible to derive a slightly better fit with a more complicated relation than was used here. It was, however, decided that this was not necessary in view of the uncertainty in other factors in the model in which Dg is used. Dg can be calculated for other temperatures (and pressures) using the relation of B-1:
where Trefis 298.15° K and Pref is 1 atmosphere. Table 3 shows the results of both methods and their difference. The average absolute difference between the two methods is 5.0 %, which is very reasonable. The results are also shown in Figure 1. Table 3.
|
| molecular | Diffusivity | Diffusivity | % difference | ||
| weight | Hayduk and | simple method | |||
| (g mol-1) | Laudie method | (m2 s-1) | |||
| (m2 s-1) | |||||
| Ethylene dichloride | 99.0 | 1.04×10-9 | 9.60×10-10 | 7.9 | |
| Fenuron | 164.2 | 6.80×10-10 | 7.10×10-10 | -5.0 | |
| DNOC | 198.1 | 7.00×10-10 | 6.40×10-10 | 9.3 | |
| Ethylchlorate | 238.7 | 5.90×10-10 | 5.70×10-10 | 2.4 | |
| Bentazone | 240.3 | 6.05×10-10 | 5.70×10-10 | 5.8 | |
| Formethanate | 222.3 | 5.60×10-10 | 6.00×10-10 | -6.5 | |
| hydrochlorid | |||||
| Desmedipham | 300.3 | 5.00×10-10 | 5.00×10-10 | -1.0 | |
| DDT | 354.5 | 4.90×10-10 | 4.50×10-10 | 8.1 | |
| Dimethomorph | 387.9 | 4.30×10-10 | 4.30×10-10 | 0.2 | |
| Lactofen | 461.8 | 4.20×10-10 | 3.90×10-10 | 7.2 | |
| Deltamethrin | 505.2 | 4.10×10-10 | 3.70×10-10 | 9.6 | |
| Average absolute | 5.7 | ||||
| difference (%) |
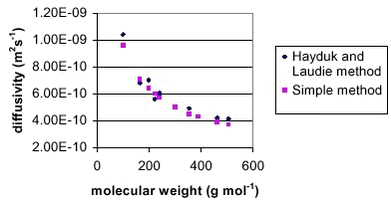
Figure 2. Comparison of the diffusivity in water calculated with the Hayduk and Laudie method and the simple method.
Figur 2 Sammenligning af diffusionskoefficienten i vand beregnet med Hayduk og Laudie metoden og den simple metode.
Dynamic viscosity and kinematic viscosity of air
The kinematic viscosity of air is a function of the dynamic viscosity of air and the density of air. For that reason the density of dry air has to be calculated (Pruppacher and Klett, 1997):
![]()
where:
ρa = density of dry air (kg m-3)
Mda = molecular weight dry air (28.9644 g m-2)
P = pressure (atm)
R = gas constant (8.2057× 10-5 atm m3 mol-1 K-1)
T = temperature (K)
The dynamic viscosity of air between -50° C and +50° C is given by (Pruppacher and Klett, 1997):
![]()
where:
ηa = dynamic viscosity of the air (kg m-1 s-1)
t = temperature (° C)
The kinematic viscosity of air νa (m2 s-1) is:
Viscosity and kinematic viscosity of (sea)water
The kinematic viscosity of (sea) water at 1 atmosphere is a function of the viscosity of water, but also of the density of water and for seawater also of the salinity. For that reason the density of (sea) water has to be calculated. The density of pure (fresh) is (kg m-3) (Sündermann, 1986):

where ρw is the density of pure (fresh) water (kg m-3) and t is the temperature (° C).
The density of seawater at 1 atmosphere as a function of the temperature and the salinity is now given by (Sündermann, 1986):
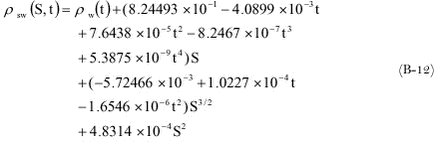
The above equation is valid for salinities between 0 and 42 pro mille and a temperature between –2 and +40 ° C.
The viscosity of pure water is given by (Sündermann, 1986):
![]()
where t is the temperature (°C) and ηw(20) = 1.002×10-3 (kg m-1 s-1). This equation is valid for 1 atmosphere and a temperature range of 10 to 70 °C.
The viscosity of sea water at 1 atmosphere can be obtained from (Sündermann, 1986):
In this equation ηsw(S,t) is the dynamic viscosity of sea water at 1 atmosphere at salinity S (pro mille) and temperature t(° C). Clv is the volume chlorinity, which is related to salinity by (Sündermann, 1986):
![]()
This is valid for salinities between 0 and 40 pro mille and temperatures between 5 and 25 °C.
The kinematic viscosity of (sea) water at 1 atmosphere νsw (m2 s-1) is defined by:
![]()
Note: the equations for sea water can also be used to calculate the kinematic viscosity of freshwater by putting a value of 0 for the salinity in the equations.
References
Fuller, E.N., Schettler, P.D., Giddings, J.C. (1966) A new method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 58, 19-27.
Lyman, W.J., Reehl, W.F., Rosenblatt, D.H. (eds.) (1990) Handbook of chemical property estimation methods, American Chemical Society, Washington DC, USA.
Pruppacher, H.R., Klett, J.D. (1997) Microphysics of clouds and precipitation. Kluwer, Dordrecht, The Netherlands.
Reid, R.C., Prausnitz, J.M., Sherwood, T.K. (1977) The properties of gases and liquids, 3rd. edition, McGraw-Hill, New York.
Sündermann, J. (ed.) (1986) Landolt-Börnstein, Numerical data and functional relationships in science and technology. New series. Group V: Geophysics and space research, Vol. 3: Oceanography, subvolume a. Springer, Berlin, Germany.