|
| Front page | | Contents | | Previous | | Next |
Degradation of Estrogens in Sewage Treatment Processes
4 Experimental Programme
4.1 Overview
The experimental programme performed in this project has aimed to describe the two main removal processes for estrogens related to the stage of sewage treatment being considered the most important,
namely sorption and degradation (stability) in the activated sludge treatment step.
Five estrogens were selected for the study:
- The two natural steroid estrogens estrone (E1) and 17β-estradiol (E2).
- The active ingredient in most contraceptive pills, 17α-ethinylestradiol (EE2).
- Two representatives of the two most abundant groups of conjugated estrogen metabolites: estrone-3-glucuronide (E1-3Glu) of the glucuronides and estrone-3-sulphate (E1-3Sul) of the sulphate esters.
Sorption: Only sorption of the free estrogens was measured, as the conjugated estrogens are more hydrophilic and thus are not believed to sorb to sludge to a great extent.
Stability in sterile water: Stability of all five estrogens was measured in sterile water as a control experiment (possible sorption to glass walls or other experimental artefacts) and to establish a baseline for
stability of estrogens. If estrogens degrade abiotically within a certain time, they are not likely to exist longer in any environment than this experiment shows.
Stability in activated sludge suspensions: Stability of five estrogens in activated sludge suspensions were tested under aerobic conditions. However, to reduce the number of samples that had to be
measured, experiments with addition of E1 were omitted. It is well known from other studies that in activated sludge E2 is oxidized very fast to E1. Therefore, the degradation of E1 could be followed in the
experiment where E2 was added.
Stability of the free estrogens with activated sludge suspensions were tested under anaerobic conditions. Again, the experiment with addition of E2 was used to measure degradation of E1. It was not
possible within the constraints set by time and resources to measure degradation of conjugated estrogens under anaerobic conditions. It is likely that aerobic or anaerobic conditions will not affect the
degradation of conjugated estrogens.
4.2 Activated sludge characteristics
4.2.1 Activated sludge used in the sorption experiments
The sludge used for the sorption experiment was settled activated sludge taken from Egå STP at 7.30 AM on the 26th of August 2003. Preliminary sorption experiments were carried out using sludge taken
from Lundtofte STP at 9 AM on the 15th of July 2003. Upon arrival at the laboratory about three or one hours later, respectively, the sludge was immediately washed three times using tap water (US EPA
standard, see Section 4.3.2) and centrifuged to remove dissolved molecules. The sludge was then freeze dried. The dried sludge was sterilized by heating at 103 °C for 120 min.
The total solids and volatile solids in the activated sludge suspension were measured according to Dansk Standard DS 204 (Dansk Standardiseringsråd, 1991). Further, the temperature was measured in the
tank of the STP from which the sludge was taken. The data from the characterisation of the collected sludge are shown in table 4-1.
Table 4-1
Characterisation parameters for the activated sludge used in all experiments.
| STP |
Experiment |
Date of
sample
collection |
Temperature
in activated
sludge tank
°C |
MLSS
g/L |
VSS
g/L |
DS
g/L |
Loss on
ignition
% |
Loss on
ignition
after
washing
% |
| Lundtofte |
Sorption |
26-8 2003 |
ND |
10.3 |
ND |
ND |
ND |
64.9±0.1 |
| Egå1 |
Sorption |
15-7 2003 |
18.7 |
4.6 |
3.4 |
5.4 |
67 |
74.2±0.1 |
| Egå2 |
Degradation |
6-10 2003 |
16.2 |
4.4 |
3.3 |
5.1 |
69 |
ND |
4.2.2 Activated sludge used in the degradation experiments
The sludge used for the biodegradation experiment was activated sludge taken from Egå STP at 7.30 AM on the 6th October 2003. On arrival at the laboratory about three hours later, the sludge was
immediately aerated until the total solids concentration was determined and the batch experiment reactors could be started. The batch reactors were started about 12 hours after the sludge was taken from
the activated sludge tank of the STP. The data from the characterisation of the collected sludge are shown in Table 4–1.
4.3 Sorption experiments
In the experiment, the sorption equilibrium between sludge and water was allowed to adjust. A range of estrogen concentrations were tested.
The water used was similar to the artificial sewage described by Nyholm et al. (1996) except that the organic carbon sources were omitted to avoid risk of excessive bacterial growth.
4.3.1 Sorption kinetics
An experiment was carried out to determine the minimal time for establishing equilibrium between the water phase and solid phase (activated sludge). Activated sludge from Egå STP was used for this
experiment. The experiment design was based on determining water concentrations at different times after a 1 g/L sludge suspension was spiked with 400 ng/L of each steroid estrogen. Based on this
experiment the equilibration time for the experiment was set to 3 hours. This experiment was not performed on sludge from Lundtofte STP. Instead, the sorption experiment was allowed to equilibrate for 6 h
in experiments with sludge from Lundtofte SPT.
4.3.2 Sorption isotherm
It was intended to perform the sorption isotherm experiment as suggested in the US EPA standard (US EPA, 1996a; US EPA, 1996b). This standard suggests that a range of equilibrium concentrations are
produced by adding different amounts of sludge to bottles with a fixed concentration of the compound. The reduction in aqueous concentration of the measured compound provided by the sorption process
is used to establish the different equilibrium concentrations needed to experimentally determine a sorption isotherm. This experimental design was attempted with sludge from Lundtofte STP varying in
concentrations from 0.1 g/L to 5 g/L. However, it was found that aqueous equilibrium concentrations varied relatively little (less than two decades). Given the normal analytical random error and additional
variation caused by inhomogeneity of the sludge, the isotherms were poorly described.
It was therefore decided to use a modified experimental design still based on varying the ratios of compound concentration to sludge concentration as prescribed in the standard. We used different
concentrations of steroid estrogens covering four decades spaced equally on a logarithmic scale and equilibrated with a fixed sludge concentration of 1.0 g/L. Since the analytical method was not able to
quantitate the estrogens precisely over 4 decades of concentrations, different sample volumes were extracted ranging from 1 to 1000 ml. The volumes and starting concentrations used for the experiments are
shown in table 4-2.
The sorption test was performed at 16 °C using 100 ml, 500 ml or 1000 ml bluecap®-bottles in a laboratory shaker. The modified experimental design reduced the random variation compared to the scale
of the isotherms and provided a more precise determination of isotherms.
Table 4-2
Sample volumes and initial concentrations used for the different concentration replicates in the experiments for determining the sorption isotherms of E1, E2 and EE2. CO is the nominal starting concentration of each steroid estrogen. One replicate was made for each steroid estrogen at each concentration level.
| Lundtofte STP |
|
Egå STP |
Sample
volume |
CO |
Sample
volume |
CO |
| mL |
ng/L |
mL |
ng/L |
| 1 |
44272 |
1 |
47434 |
| 5 |
4427 |
5 |
15000 |
| 10 |
1400 |
10 |
4743 |
| 25 |
443 |
25 |
1500 |
| 70 |
140 |
70 |
474 |
| 200 |
44 |
200 |
150 |
| 950 |
14 |
950 |
47 |
| 950 |
8 |
950 |
15 |
4.3.3 Analysis
Sample volumes varied from 1 ml to 950 ml to allow exact quantification of a wide range of concentrations. Samples were filtered before extraction by solid phase extraction (SPE) and analysed as
described in Section 4.6.
4.4 Study of abiotic degradation in water
The stability of estrone (E1), 17β-estradiol (E2), 17α-ethinylestradiol (EE2), estrone-3glucuronide (E1-3Glu) and estrone-3sulphate (E1-3Sul) was followed for 4 or 6 days in a sterile mineral medium.
4.4.1 Experiments
One experiment was made for each chemical except EE2 for which the experiment was made in quadruplicate (in order to determine the variability between reactors rather than studying this substance in
particular detail). For E1, E2 and EE2 the recovery of a 500 ng/L starting concentration was measured. In the experiment with E1, E2 was also measured and likewise E1 was measured in the experiment
with E2 since E1 and E2 can be transformed to each other by simple oxidation or reduction reactions. Since an analytical method was not available for analysing estrogen conjugates E1 was measured in
experiments with E1-3Glu and E1-3Sul. The main degradation reaction for these two conjugates is expected to be hydrolysis which forms E1. In these experiments the starting concentration was 500 ng/L
E1 equivalents.
4.4.2 Experimental procedure
Experiments were performed in 1 l glass reactors as described by Nyholm et al. (1996) using phosphate buffered water adjusted to pH = 7.1±0.1. The experiment took place in a thermostatic room at a
temperature of 16 °C. Stirring of the reactors was done by bubbling air at a rate of 15 l/h. Stock solutions of the test compounds were made in methanol and addition was done with 150 µl methanol solution
per reactor. Samples were taken after 5 min, 1 h, 12 h, 24 h, 48 h, 72 h and 96 h. The two compounds expected to be most persistent with activated sludge, E1 and E1-Sul, were also sampled after 144 h.
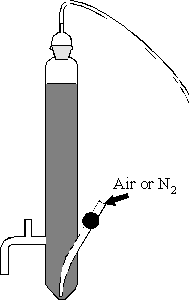
Figure 4-1
Schematic drawing of the glass reactor used for all degradation experiments.
4.4.3 Analysis
Samples of 100 ml were extracted with SPE and analysed as described in Section 4.6. No filtering or clean-up was used.
4.5 Study of degradation in activated sludge
The degradation of E2 and EE2 were investigated in diluted activated sludge with oxygen and nitrate as oxidation source. E1-3Glu and E1-3Sul were investigated in diluted activated sludge only with oxygen.
4.5.1 Experiments
One experiment was made for each chemical except EE2 in aerobic reactors for which the experiment was made in triplicate (to determine variability between the reactors).
For E2 and EE2 the recovery using a 500 ng/L starting concentration was determined. In the experiment with E2, E1 was also measured since E2 can be transformed to E1 by a simple reduction reaction.
Since an analytical method was not available for analysing estrogen conjugates E1 was measured in experiments with E1-3Glu and E1-3Sul. The main degradation reaction for these two conjugates is
expected to be hydrolysis which forms E1. In these experiments the starting concentration was 500 ng/L E1 equivalents.
4.5.2 Experimental procedure
Experiments were performed in 1 l glass reactors with 0.50 g/L of activated sludge and 1.00 ml phosphate buffered mineral media (Ca2+, Mg2+, Na+, Cl-, SO42-) at 16 °C with pH = 7.8±1.0. Reactors
were fed with OECD artificial peptone sewage with modifications according to Nyholm et al. (1996) at a starting concentration of 100 mg/L BOD. Each day the BOD was replenished with further 25 mg/L.
The activated sludge was added 12 h prior to initiating the experiment started to give the sludge bacteria time to adapt to the oxidation sources (O2 or NO3-) in the reactors. Samples were taken after 3 min,
6 min, 20 min, 40 min, 90 min, 3 h, 6 h, 12 h, 24 h, 48 h and 72 h. Further, six blank samples and six control samples were taken. Control samples were samples of sludge suspensions taken from the
reactors before addition of test compounds, which was stabilised according to the analytical procedure to stop biodegradation activity (pH adjusted to pH = 3), before addition of test compound at the
concentration used in the degradation experiment.
4.5.2.1 Stability experiment in aerobic activated sludge
In reactors with oxygen as oxidation source stirring was done by bubbling air at a rate of 15 l/h resulting in a dissolved oxygen concentration of 7.25 mg/L.
4.5.2.2 Stability experiment in denitrifying activated sludge
In reactors with nitrate as oxidation source stirring was done by bubbling nitrogen at a rate of 15 l/h. Nitrate was added at the beginning of the experiment to a concentration of 20 mg-N/l. During the
experiment nitrate was measured and replenished at least twice daily.
4.5.3 Analysis
Samples of 100 ml were extracted with SPE and analysed as described in Section 4.6. The particles in the sample was loaded on top of the cartridge rather than removed by filtration before the extraction
(as was done in the sorption experiment). This made it possible to elute the particle bound steroid estrogens together with the steroid estrogens on the SPE column. This was done in order to extract all
estrogens present in the sample rather than only the estrogens dissolved in the water phase.
4.5.4 Batch reactor characterisation
The chemical environment in the batch reactor was characterised by measuring O2 and pH with selective electrodes and nitrate with a colorimetric test kit (see appendix 1 for details on instrumentation)
several times a day during the experiments.
The stability of the biodegradation activity in the sludge slurry over the experimental period was further estimated by measuring the cumulative biological oxygen demand (BOD) over 24 h in samples taken
from reactors with both types of RedOx regimes every day of the experiment.
4.6 Chemical analysis
4.6.1 Analytical procedures
4.6.1.1 Sorption experiment
Samples from the sorption experiment were separated from the sludge by filtration over a GF/C filter (47 mm diameter, Whatman International Ltd, Maidstone, England) or by centrifugation depending on
what was most convenient for the size of sample. Each water sample was acidified to pH = 3 using sulphuric acid and spiked with 250 ng E2-acetate, which functions as the extraction pseudo standard. All
samples were extracted onto the 500 mg version of the BondElut C18 solid phase extraction cartridge (Varian, USA). The cartridge was then freeze dried. The estrogens were eluted from the cartridge with
5 ml acetone. This extract was evaporated and reconstituted in 200 µl H2O:MeOH (35:65), which was transferred to the analysis.
4.6.1.2 Abiotic stability experiment
Samples from the abiotic stability experiment were treated exactly as samples for the sorption experiment except that no solids had to be removed.
4.6.1.3 Activated sludge stability experiments
Each sample from the sludge biodegradation experiment were acidified to pH = 3 exactly at the time the sample was taken, to stop the biodegradation processes. Samples were spiked with 100 ng of
deuterated standards (d4-E1, d5-E2 and d5-EE2) as extraction standards. The sample containing both water and sludge were then loaded onto a solid phase extraction cartridge. The particles in the sample
was loaded on top of the cartridge rather than removed by filtration before extraction according to a method described by Ternes et al. (1999). This was done in order to extract all estrogens present in the
sample rather than only the estrogens dissolved in the water phase. In this experiment the 500 mg version of the ISOLUTE C18 solid phase extraction cartridge (ICT, Germany), was used rather than the
cartridge from Varian, as the ICT cartridge was previously evaluated for this purpose (Joss et al. 2003). Then all the water had been drawn through the cartridge the sludge was left on top of the column. The
cartridge was then dried by freeze drying. The estrogens were eluted from the cartridge with 2 x 2.5 ml acetone. This extract was evaporated and reconstituted in 200 µl acetone:hexane 35:65. The reduced
extract was purified by passing in through a custom made 1 g silica gel column with 5 ml acetone:hexane 35:65 mixture (Andersen et al., 2003). The clean-up removed most of the coloured matrix from the
extract. This eluate was evaporated and reconstituted in 200 µl H2O:MeOH 35:65, which was transferred to the analysis.
In the method evaluation of the analytical procedure for this sample type it was found that the use of silica gel clean-up was essential for being able to detect the estrogens in this matrix rich sample type. Even
after the clean-up some parts of the matrix were still left and made the ionisation efficiency for the MS detector unstable. This problem could be satisfactory solved by using deuterated standards of each of
the analytes. As the deuterated standards were chemically exactly like the analyte they represent the variation in ionisation efficiency was exactly matched by the same variation in the ionisation of the
standard.
4.6.2 Quantification standards and control samples
For each sample series a range of concentrations of steroid estrogens and extraction standards spiked to samples of drinking water was used to create a standard curve. These spiked samples were
extracted and cleaned with the same procedure as the real samples. Thus all systematic losses in the extraction and clean-up procedure for the samples were repeated on the standards and, hence, the effects
will cancel each other. Further, each sample series included blank samples and spike recovery samples.
The extraction standards (E2-acetate (AE2) and deuterated standards) were used in an in-method statistical control system as described by Ternes et al. (2002).
4.6.3 Instrumentation for quantification
Quantification of steroid estrogens concentrations in the extracts was done by LC-triplequadropole-MS-MS. The Sciex API 3000 LC-triplequad-MS-MS was used with an APPI (Atmospheric Pressure
Photo Ionisation) ion source.
Chromatographic separation of the analytics before the quantification by MS-MS was done with a HPLC system (Agilent 1100 Series) using a C18 XTerra®RP18 3,5m, 3.0 x 150mm column and gradient
elution by H2O:MeOH from 35:65 to 0:100. The HPLC had a total run time of 60 min and retention times as shown in Table 4-3.
Table 4-3
Retention times and m/Z for mother and daughter ions used for quantification of the steroid estrogens.
| |
Retention time
(min) |
Mother ion
(m/Z) |
Daughter ion 1
(m/Z) |
Daughter ion 2
(m/Z) |
| E1 |
16.12 |
271.4 |
157.1 |
159.3 |
| E2 |
19.03 |
255.2 |
133.2 |
159.3 |
| EE2 |
19.73 |
279.4 |
133.3 |
159.3 |
| AE2 |
32.52 |
255.2 |
133.2 |
159.3 |
| d4-E1 |
16.12 |
275.4 |
257.2 |
161.2 |
| d5-E2 |
19.03 |
260.3 |
161.1 |
162.1 |
| d5-EE2 |
19.73 |
283.3 |
161.2 |
135.2 |
4.6.4 MS-MS parameters
For each steroid estrogen a characteristic mother ion formed from the molecule in the ionisation head of the MS-MS is selected by the mass detector. This ion is fragmented in the MS-MS and two
characteristic daughter ions formed in the fragmentation process are selected for identification and quantification (see Figure 4-2 and Table 4-3).
Click here to see Figure 4-2.
Only when both ions were present in the correct ratio between the two, the signal could be used for quantification. A high certainty for quantification of the correct analyte is inherent to the chosen
quantification method. Each analyte had to elute at the correct retention time in the LC system. Then the ionisation should produce an ion with a correct mass, and fragmentation of this ion should produce
two new ions with correct masses.
4.7 Data treatment for stability experiments
Each concentration profile obtained in the abiotic and activated sludge stability experiment was fitted to first order reaction expressions as shown in Box 1 (Figure 4-3). This was done by integrating the
expression with appropriate limits and fit the resulting expression to the observed concentration profiles.
Since E1 and E2 are degraded very fast in this experimental setup, it is difficult to measure the degradation rate precisely. Therefore, the net loss of the steroid estrogen structure was also determined by
fitting the first order degradation expression to the sum of the concentrations of E1 and E2. This should be interpreted as the effective rate constant for loss of the estrogenic structure.
Box 1: Fitting formulas
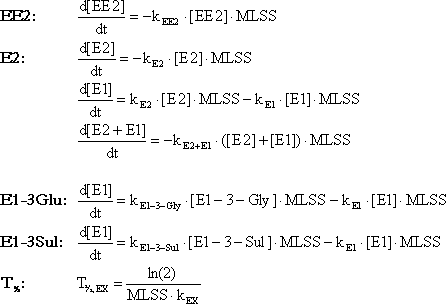
Figure 4-3
Box 1: Fitting formulas for first order reaction rate constants and half-lives.
T½, EX and kEX is the half-life and first order reaction constant, respectively, for any of the degradation reactions.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 November 2004, © Danish Environmental Protection Agency
|